- 1Veterinary Department, Mundomar, Benidorm, Spain
- 2Department of Pharmacology and Toxicology, Faculty of Veterinary Medicine, Complutense University of Madrid, Madrid, Spain
- 3Experimental Zooprophylactic Institute for Piedmont, Liguria and Valle d’Aosta (IZSTO), Turin, Italy
- 4Dipartimento di Scienze Fisiche, della Terra e dell’Ambiente, University of Siena, Siena, Italy
- 5Centro Interuniversitario di Ricerca sui Cetacei (CIRCE), University of Siena, Siena, Italy
- 6Dipartimento di Scienze della Terra, dell’Ambiente e della Vita, Università di Genova, Genoa, Italy
- 7ASL 1 Sistema Sanitario Regione Liguria - Imperia, Bussana di Sanremo, Italy
- 8CIMA foundation, Savona, Italy
- 9Delfini del Ponente, Imperia, Italy
Information regarding bacterial diseases in Cuvier’s beaked whale (CBW, Ziphius cavirostris) is scattered and mostly incomplete. This report describes a case of septicemia by Morganella morganii in a juvenile male CBW with concurrent renal crassicaudiasis. The animal stranded along the Ligurian coastline (Italy) and underwent a systematic post-mortem examination to determine the cause of death. Histopathology showed lesions consistent with a septicemic infection, severe meningoencephalitis, and renal crassicaudiasis. An M. morganii alpha-hemolytic strain was isolated in pure culture from liver, lung, prescapular lymph node, spleen, hepatic and renal abscesses, and central nervous system (CNS). The antimicrobial susceptibility profile of the strain was evaluated with the minimum inhibitory concentrations (MICs) method and reduced susceptibility to Trimethoprim-Sulfamethoxazole is reported. Crassicauda sp. nematodes were retrieved from both kidneys. No other pathogens were detected by immunohistochemistry, serology, or biomolecular analyses. Toxicological investigations detected high concentrations of immunosuppressant pollutants in the blubber. The chronic parasitic infestation and the toxic effects of xenobiotics likely compromised the animal's health, predisposing it to an opportunistic bacterial infection. To our knowledge, this is the first description of M. morganii septicemia with CNS involvement in a wild cetacean.
Introduction
The Ligurian sea is listed as a high-density area for Cuvier’s beaked whales CBW (Ziphius cavirostris), the only commonly-observed beaked whale in the Mediterranean Sea (Podestà et al., 2006). CBW’s Mediterranean sub-population is listed as “Vulnerable” on the International Union for the Conservation of Nature (IUCN) Red List (Cañadas and Notarbartolo di Sciara, 2018). Being an elusive, deep diver predator, this odontocete is difficult to study in the field and most of the available biological data derive from strandings (Podestà et al., 2006; Heyning and Mead, 2009; Podestà et al., 2016; Carlucci et al., 2020).
Infectious diseases reported in stranded CBW include viral infection by alpha herpesvirus (Arbelo et al., 2010) or morbillivirus (Centelleghe et al., 2017; Felipe-Jiménez et al., 2022), crassicaudiasis (Díaz-Delgado et al., 2016; Febronio et al., 2021), and bacterial diseases (Alstrup et al., 2021; Febronio et al., 2021), including septicemia by Citrobacter freundii (Fernández et al., 2011).
Parasitic disease due to Crassicauda spp. has been increasingly reported as a significant cause of death in beaked whales (Díaz-Delgado et al., 2016; Febronio et al., 2021; Jerdy et al., 2022) and other cetaceans (Balbuena and Simpkin, 2014) worldwide. For most of the 14 species of the genus, life cycle and transmission are still unclear. Since other marine spirurids usually require intermediate hosts (Anderson, 1988), an indirect cycle involving crustaceans, cephalopods and fishes has been speculated (Lambertsen, 1986; Marcer et al., 2019). However direct transmission cannot be discarded as larvae and/or eggs have been reported in milk (Geraci et al., 2011) and urine (Lambertsen, 1986; Febronio et al., 2021) and transplacental infection has been documented as well (Lambertsen, 1986; Suárez-Santana et al., 2018). These nematodes show different tissue tropisms (urogenital, vascular, integumentary, respiratory) and pathogenic potential, playing a regulatory role in some marine mammal populations (Balbuena and Simpkin, 2014). Crassicauda boopis causes severe renal lesions in baleen whales (Lambertsen, 1986; Marcer et al., 2019), C. grampicola is associated with moderate to severe sinusitis in Risso’s dolphins (G. griseus) (Cuvertoret-Sanz et al., 2020) whereas C. anthonyi and C. magna migration produces verminous arteritis and chronic renal disease in beaked whales (Díaz-Delgado et al., 2016; Febronio et al., 2021; Jerdy et al., 2022). Moreover, several species of crassicaudid nematodes, including C. grampicola (Geraci et al., 2011) and C. fuelleborni (Kot et al., 2022) produce parasitic mastitis impacting the reproductive success of endangered cetaceans.
Furthermore, a chronic parasitic infestation may favor infection by opportunistic bacteria, such as members of the family Enterobacteriaceae (Paterson and Mathers, 2020), disrupting tissue integrity, translocating pathogens, and/or modulating the immune system of the host (Ashour and Othman, 2020).
Morganella morganii is a gram-negative bacillus, belonging to the Enterobacteriaceae family, found in the environment and the digestive tract of humans and animals. It is considered an unusual opportunistic pathogen in animals and nosocomial infections, frequently isolated from the urinary tract or skin wounds (Liu et al., 2016). Its zoonotic potential should not be underestimated, especially in hosts with compromised health status, considering the relatively high mortality rate in human hospitals and the emergence of virulent antimicrobial resistance (AMR) strains.
M. morganii has been identified as a pathogen in ocular lesions in pinnipeds (Thornton et al., 1998), and in two bottlenose dolphins (Tursiops truncatus) under human care (Elfadl et al., 2017; Sánchez Contreras and Biancani, 2021). In both cases, the strain exhibited resistance against several antibiotic classes, including new-generation cephalosporins, and in one case was linked to a fatal infection, causing fibrino-hemorrhagic bronchopneumonia and septicemia.
In cetaceans, higher concentrations of M. morganii were cultured from diseased or stranded animals compared with the relatively low numbers isolated from their free-ranging counterparts (Martineau et al., 2003; Buck et al., 2006). Moreover, the presence of high numbers of opportunistic pathogens, including M. morganii has been linked to immunosuppression in beluga whales (Delphinapterus leucas) living in the polluted waters of the Saint Lawrence Estuary (SLE) in Canada (Martineau et al., 2003).
Here we describe the findings of the post-mortem examination and the advanced diagnostic investigations performed on a juvenile male CBW stranded in Italy to determine the cause of death. A case of septicemia caused by M. morganii with concurrent renal crassicaudiasis is reported in a threatened cetacean inhabiting the highly polluted waters of the Mediterranean Sea. This study provides valuable information for the conservation of this species and strengthens the role of marine mammals as sentinels for human and ecosystem health (Bossart, 2011).
Materials and methods
Post mortem examination
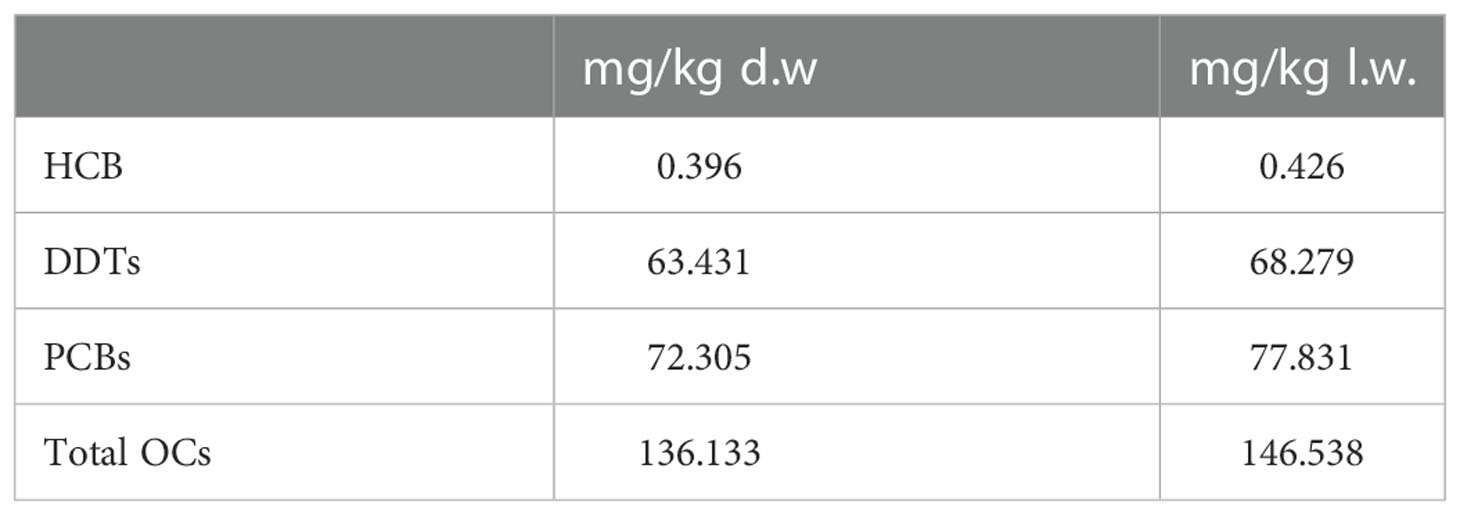
On October, 3rd 2020, a CBW (IZS number 70726/20) stranded in Sanremo (Italy), along the Ligurian coastline of the Pelagos Sanctuary. A complete field post-mortem examination was performed according to standard protocols (Geraci and Lounsbury, 2005). The animal was a juvenile-subadult male of 498 cm (total length, TL) in good nutritional status, and in moderate decomposition (code 3 – Figure 1A). Blubber thickness was 6 cm. This animal did not display any evidence of interaction with fishing activities, and the stomach content was scarce, consisting of a few, highly-digested cephalopod beaks and lenses. Photo-identification (ID) analysis resulted in a positive match of an individual that was photo-identified in the Ligurian Sea in 2017 and 2018 (CIMA Foundation database, ID: 31705302). Age at first identification was estimated to be 3 or 4yo (weaned, totally brown colored, poorly marked). Photo-ID life history data and natural markings (from marking gain rate; Rosso et al., 2011) suggested an estimated age at death of about seven years.
Figure 1 (A) Cuvier’s beaked whale carcass before retrieval. (B) Right atrioventricular valve - fibrinous valvular endocarditis. (C) Adult Crassicauda spp. nematodes retrieved from the renal parenchyma for parasitological analysis.
The necropsy was performed in the field under high environmental temperatures and, due to logistic issues, there was a significant delay between carcass retrieval, visual evaluation of the organs, and sampling. The tissue samples of all the major organs and lesions were collected and split into aliquots for subsequent analyses as previously described (Giorda et al., 2021). Blood serum, aqueous humor, and cerebrospinal fluid (CSF) were collected and kept frozen at -20°C for serological investigations. Parasites were collected in 70% ethanol and morphologically identified according to taxonomic criteria proposed by Anderson et al., 2009.
Histopathology and immunohistochemistry
Representative tissues (brain, tonsils, lung, prescapular and tracheobronchial lymph nodes, heart, liver, spleen, pancreas, intestine, skeletal muscle, skin, kidney, urinary bladder, adrenal gland, mesenteric artery, and reproductive system) were collected and fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 4 ± 2 μm, stained with hematoxylin and eosin (H&E) and examined through a light microscope.
Immunohistochemistry (IHC) for Morbillivirus was performed on tissue sections using a monoclonal anti-Canine Distemper Virus (CDV) antibody (VMRD) (Di Guardo et al., 2010). Toxoplasma gondii IHC was carried out on the brain tissues, using a polyclonal serum of caprine origin (VMRD) (Di Guardo et al., 2010).
Microbiology
Tissue samples including brain, lung, lymph nodes, liver, and spleen were processed for standard aerobic, anaerobic, and microaerobic (5% CO2) bacterial culture and identification, by biochemical analyses (VITEK® MS, bioMérieux SA, Marcy l’Etoile, France) and matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF MS, Bruker Daltonics, Bremen, Germany). Following international recommendations (World Organisation for Animal Health (WOAH), 2018), samples from target tissues underwent specific bacteriological procedures to screen for Salmonella spp., Listeria spp., and Brucella spp.
Urease test on M. morganii grown on Blood Agar Base (Liofilchem srl, Roseto degli Abruzzi, Teramo, Italy) was performed according to the manufacturer’s instruction (Microbiol srl, UTA, Cagliari, Italy). Intermediate checks of the vials occurred at 30 minutes, 60 minutes, and 4 hours.
To detect and evaluate biofilm formation by M.morganii, the Safranin Staining (SS) was performed as previously described (Ceri et al., 2001; Olson et al., 2002). Briefly, the bacteria were grown into Tryptone Soy Broth (Microbiol srl, UTA, Cagliari, Italy) added with 2% Glucose (Microbiol srl, UTA, Cagliari, Italy) aerobically at 37°C overnight, then resuspended in the same medium at a bacterial concentration of 106 CFU/ml and 2ml were dispensed in a 24-wells plate. The plate was incubated aerobically at 37°C overnight. The wells were washed three times with Phosphate Buffered Saline (PBS, Sigma-Aldrich Merck KGaA, Darmstadt, Germany) and then stained with 2 ml of Safranin 1% (Merck KGaA, Darmstadt, Germany) for 1 minute, due to its affinity to biofilm matrix components (polysaccharides, proteins, lipids, and nucleic acids). After overnight incubation aerobically at 37°C, the wells were washed with Acetic Acid 30% (CH3COOH, Sigma-Aldrich Merck KGaA, Darmstadt, Germany) to remove biofilm and 200 μL of well content were dispensed in triplicate into 96 wells-microplate and read spectrophotometrically at a wavelength of 492 nm. Pseudomonas aeruginosa ATCC27853 and Escherichia coli ATCC 25922 were used as positive control (strong biofilm former) and negative control (no biofilm former), respectively.
Antibiotic susceptibility testing
Antibiotic susceptibility of the cryopreserved M. morganii strain was tested using the minimum inhibitory concentrations (MICs) method according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) (CLSI, 2018b). Quality controls of the plates used for MIC were performed according to Table 5 of the CLSI VET01S supplement (CLSI, 2020). MIC breakpoints (expressed in μg/mL) were evaluated and interpretative criteria were retrieved from both human (CLSI, 2018b; CLSI, 2018a) and veterinary CLSI Standards (CLSI, 2020) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (The European Committee on Antimicrobial Susceptibility Testing, 2022b).
M. morganii strain was tested with cefotaxime for evaluating the synergistic effects when combined with clavulanic acid, which inhibits ESBL β-lactamases, and cloxacillin, which inhibits AmpC β-lactamases. The Combination Disc Tests (Liofilchem srl, Roseto degli Abruzzi, Italy) are carried out using a 10mm paper disk containing cefotaxime alone or in combination with clavulanic acid, cloxacillin, or both of these inhibitors. The inhibition zone around the cefotaxime disc combined with inhibitors is compared with the zone around the disc without the inhibitors according to EUCAST guidelines (The European Committee on Antimicrobial Susceptibility Testing, 2017).
MIC values were also compared with EUCAST MIC distributions based on collated data from an increasing total of more than 30 000 MIC distributions from worldwide sources (The European Committee on Antimicrobial Susceptibility Testing, 2022a).
When available on the EUCAST website, Epidemiological Cut-off values (ECOFFs) that distinguish microorganisms without (wild type) and with (non-wild type) phenotypically detectable acquired resistance mechanisms to a specific pharmacological active substance, were reported and compared with the values obtained from M. morganii MIC tests.
Toxicology
Polychlorinated biphenyls (PCBs), hexachlorobenzene (HCB), and dichlorodiphenyltrichloroethanes (DDTs) were measured in blubber. Data were expressed in mg/kg dry weight (d.w) or mg/kg lipid weight (l.w) if the Extracted Organic Material percentage (MOE%) was considered. Measurements were made according to the Environmental Protection Agency method 8081/8082, with modifications (Marsili and Focardi, 1997). To assess the potential toxicological impact of these POP concentrations in the specimen, two different threshold limits proposed in the literature for PCBs were considered. Kannan et al. (2000) and Jepson et al. (2005) proposed the value of 17.0 mg/kg l.w. of ΣPCB in blubber as the tolerance threshold for no deleterious effects in marine mammals; Helle et al. (1976) and Jepson et al. (2016) prefer a much higher value (41 mg/kg l.w. ΣPCB in blubber) as a toxicity threshold for reproductive impairment in Baltic ringed seals (Pusa hispida).
Molecular and serological investigations
Molecular detection of Dolphin Morbillivirus (DMV) (Verna et al., 2017), Herpesvirus (HV) (VanDevanter et al., 1996), T. gondii (Vitale et al., 2013), Brucella spp. (Baily et al., 1992) and Photobacterium damselae sub. damselae (Osorio et al., 2000) was routinely performed on target tissues.
Serological investigations to screen for the presence of specific antibodies against DMV, Brucella spp. and T. gondii (Di Guardo et al., 2010) were performed on serum, CSF, and aqueous humor.
To corroborate bacterial ID obtained by MALDI-TOF MS, the MicroSEQ™ 500 16S rDNA PCR Kit (Thermo Fisher Scientific Inc., Waltham, USA) was used for the amplification of the first 500 base pairs(bp) of the 16S ribosomal RNA gene (rDNA) of the M. morganii isolate. DNA extracts were made from pure cultures of M. morganii by thermal lyses in PrepMan™ Ultra solution (Thermo Fisher Scientific Inc., Waltham, USA) following protocol for gram-negative bacteria described by the manufacturer. Positive samples were subjected to Sanger sequencing reaction using the MicroSEQ™ 500 16S rDNA Sequencing Kit (Thermo Fisher Scientific Inc., Waltham, USA). Sequencing products were analyzed on the Applied Biosystems® Sanger Sequencing 3500 Series Genetic Analyzers (Thermo Fisher Scientific Inc., Waltham, USA). Electropherograms were processed with the Bioedit 7.2.5 software (Hall, T.A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids. Symp. Ser. 41:95-98.) and the sequences (forward and reverse) were aligned to obtain a consensus sequence. The consensus sequence was uploaded to the GenBank® server and compared with available sequences retrieved from the National Center for Biotechnology Information (NCBI) database through the Basic Local Alignment Search Tool (BLAST, http://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 10/08/2022)
Results
Post-mortem findings
The most significant gross findings at necropsy were observed in the vascular system and the kidneys. The vascular wall of the mesenteric arteries and their branches was hard, thickened with endothelial intraluminal pale-yellow plaques of fibrinous-purulent material, partly calcified. Greyish-yellow fibrinous deposits were attached to the right atrioventricular valve leaflets (fibrinous valvular endocarditis - Figure 1B). Light discoloration of the left myocardium was also observed. Multifocal pyogranulomatous lesions were observed in both kidneys, heavily parasitized by Crassicauda sp. nematodes. Multifocal abscess-like lesions of approximately 3 cm in diameter were observed in the perisplenic and perihepatic areas. Red discoloration of the blubber was reported in the cranio-dorsal region and in the melon, alongside multiple, light yellow, abscess lesions of about 0.5 cm in diameter. Upon the opening of the skull, meningeal fibrosis and hemorrhagic CSF were observed.
Renal nematodes were identified as Crassicauda sp. according to the morphometrics of the cephalic and terminal portions of adult parasites (Figure 1C).
Although specific investigations could not be performed, there was no evidence of gas emboli in the tissues examined, as a consequence of gas and fat embolic syndrome, known to affect this species in particular circumstances, such as military exercises (Fernández et al., 2005).
Histopathology and immunohistochemistry
Histologically, a mixed inflammatory infiltrate associated with foci of necrosis, partly mineralized, was observed mostly in the tunica media of arterial vessels. In the SNC, severe pyogranulomatous encephalitis associated with mild non-suppurative meningitis was diagnosed (Figure 2D). In all SNC areas, perivascular cuffings and vasculitis were observed (Figures 2A, B). Microabcesses, granulomas, and a mixed inflammatory infiltrate invaded the cerebral parenchyma (Figure 2C).
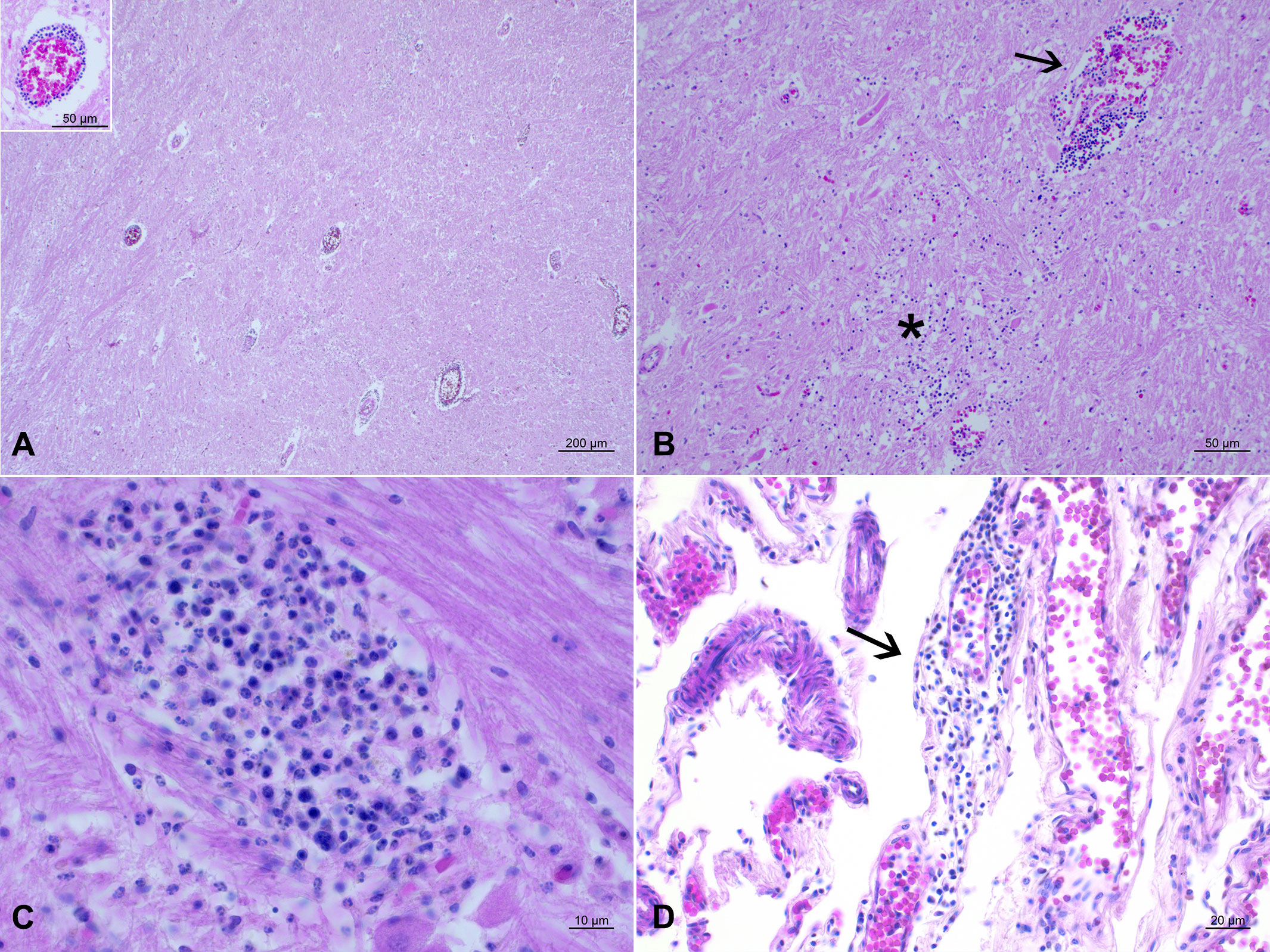
Figure 2 Neuropathological lesions observed in the stranded Cuvier’s beaked whale. HE. (A) Thalamus. Severe encephalitis with several perivascular mononuclear cuffings. Inset: perivascular cuffing characterized by mononuclear cells infiltration. (B) Pons. Vasculitis with damage to the vessel wall causing leakage of red blood cells with formation of perivascular hemorrhages and presence of mononuclear inflammatory infiltration around it (arrow) and invading the underlying neuroparenchyma (asterisk). (C) Pons. Small granuloma, as an outcome of a microabscess, constituted of macrophages, lymphocytes, and plasma cells. (D) Parietal cortex. Focal and mild non-suppurative meningitis (arrow).
Multiple hemorrhages were scattered in the pulmonary parenchyma. The bronchial submucosa was filled with a mixed inflammatory infiltrate and the associated blood vessels were congested. In the mucosa of the first gastric chamber, foci of pyogranulomatous inflammation (gastritis) were observed multifocally. In the spleen and lymph nodes, lymphoid follicles were depleted and surrounded by multifocal hemorrhages. In all these organs, lesions were described alongside changes compatible with mild to moderate autolysis.
Several other internal organs presented with a severe grade of autolysis due to the delay in sampling and unfavorable environmental conditions. Heart, liver, kidney, and urinary bladder could not be evaluated due to advanced autolysis.
Immunohistochemical investigations against DMV and T. gondii were negative in all tissues examined.
Microbiology
M. morganii alpha-hemolytic strain was retrieved in pure culture from liver, lung, prescapular lymph node, spleen, hepatic and renal abscesses, and CNS. Pasteurella canis was also isolated from the lungs. There was no evidence of growth for Salmonella sp., Listeria spp., and Brucella spp.
Urease test performed on freshly cultured M. morganii strains gave positive results in less than 30 minutes. SS test revealed that the isolated M. morganii strain was a weak biofilm former (M. morganii OD Mean = 0.082; Negative control OD Mean = 0.057; Positive control OD Mean = 0.273).
Antibiotic susceptibility testing
The results of the MIC test performed on the M. morganii isolate, including both CLSI and EUCAST interpretative criteria (clinical breakpoints), are shown in Table SM1. The obtained MIC values were compared to the ECOFFs (and/or TECOFFs) available on the EUCAST site (“European Committee on Antimicrobial Susceptibility Testing. Data from the EUCAST MIC distribution website, last accessed 11/09/2022”. http://www.eucast.org).
The isolate showed natural resistance to the fixed-dose association Ampicillin-Sulbactam and Amoxicillin-Clavulanic Acid, Oxacillin, Ampicillin, Amoxicillin, most of the first- and second-generation Cephalosporins (Cefazolin, Cefalothin, Cefalexin), Macrolides, Lincosamides, Tetracycline, Nitrofurantoin and Colistin as reported in Morganellaceae. Combination Disc Tests for evaluation of Extended-spectrum β-lactamases (ESBLs) and/or AmpC β-lactamase producers gave negative results.
Based on ECOFF and TECOFF records on the EUCAST website, the strain exhibited MIC values suggestive of a non-wild type M. morganii for Trimethoprim-Sulfamethoxazole.
Biomolecular and serological investigations
The biomolecular analysis did not detect any DVM, Herpesvirus, T. gondii, and PDD DNA in target organs. Serological screening for the presence of DMV, Brucella spp, and T. gondii-specific antibodies retrieved negative results.
Amplification of the 16S rDNA region yielded an amplicon of approximately 530 bp. Sequence alignment with the BLAST tool allowed the identification of M. morganii species with homology greater than 99% (99,25%) with the M. morganii strain DG56-16 chromosome (accession number CP032295.1). The new sequence was deposited in GenBank® with the accession number OQ096688.
Toxicology
Blubber concentrations of organochlorine (OC) pollutants are reported in Table 1. MOE% was 92,9%. The blubber levels of organochlorine contaminants, in particularly polychlorinated biphenyls (PCBs) and dichlorodiphenyl dichloroethane and related compounds (DDTs), were extremely high reaching concentrations of 77.831 mg/kg l.w and 68.279 mg/kg l.w respectively,
Discussion
M. morganii septicemia
We diagnosed the stranded cetacean with a septicemic infection by M. morganii. Our diagnosis is supported by the systemic isolation of the bacteria in all major organs and by both macroscopic and microscopic lesions suggestive of septicemia such as multifocal hemorrhages, multifocal abscesses and granulomas, severe pyogranulomatous encephalitis, and endocarditis.
In human medicine, bacteremia and sepsis are quite common features (Bandy, 2020) of M. morganii infections whereas CNS involvement is rare. When the pathogen succeeds in trespassing the hematoencephalic barrier, meningitis and parenchymal abscesses are the main lesions observed (Abdalla et al., 2006). In animals, to date, this bacterium has been mostly associated with respiratory pathologies (Elfadl et al., 2017) and there are no reports of CNS involvement, unlike in this CBW that suffered from both meningitis and encephalitis.
All of these pathological conditions are reported more frequently in patients with underlying diseases and/or immunosuppression (Bandy, 2020). Urinary tract infections (UTIs) are frequently the original foci of the bacteremia, as biofilm formation and bacterial growth in the urinary tract are enhanced by the urease activity of this organism. Other typical sources of bacteremia in humans are soft tissues and hepatobiliary tract infections (Liu et al., 2016).
In this CBW, dermatological disease or deep wounds were not observed. Gross hepatobiliary abnormalities were not reported either, even though autolysis prevented exhaustive histopathological examination. Therefore, we hypothesize that renal crassicaudiasis and a high burden of organic pollutants have been the most likely predisposing factors for an infection by M. morganii.
A parasitized organ represents an optimal pabulum for bacterial growth. For instance, Suárez-Santana et al. (2018) described septic prostatitis and ascending cystitis in two spotted dolphins (Stenella frontalis) with heavy prostatic and urethral Crassicauda infestation, speculating that nematode parasitism might have favored bacterial colonization. Furthermore, our isolate was phenotypically capable of urease and biofilm production, and alpha-hemolytic strains produce a potent hemolysin with hemolytic and leucocidal properties similar to E.coli ones (Eberspacher et al., 1990). However, since autolysis did not allow to confirm or exclude a UTI, it is difficult to determine whether M. morganii retrogradely colonized the kidney via the lower urinary tract or if the pathogen could have been translocated during the parasitic migration from the intestine.
Interestingly, another opportunistic pathogen, P. canis, was isolated from the lung. This bacterium is also part of the microbiota of the oral and upper respiratory tract mucosa of animals, especially carnivores (biovar 1) and ruminants (biovar 2), and it has been rarely reported as a cause of pneumonia in patients with lung disease or immunodeficiency (Arun et al., 2019). P. canis could have overgrown on existing pulmonary lesions observed in this CBW, worsening the respiratory competence of the animal.
The health status of the animal was also compromised by a high burden of organic pollutants with demonstrated detrimental effects on the immune system (Marsili et al., 2004; Marsili et al., 2019; Centelleghe et al., 2019). Blubber concentrations of organochlorine compounds (OCs), particularly DDTs and PCBs, were very high both compared to the values found in other CBWs sampled in the Mediterranean basin (Hatzianestis et al., 1998; Baini et al., 2020) and compared to those measured in BWs from other parts of the world (Knap and Jickells, 1983; Bachman et al., 2014). The blubber PCBs concentrations in the studied specimen of Cuvier’s beaked (77.8 mg/kg l.w.) largely exceeded the PCBs toxicity thresholds reported (Kannan et al., 2000; Jepson et al., 2005; Jepson et al., 2016).
A chronic parasitic infestation and the toxic effects of immunosuppressant xenobiotics represented for this CWB a lethal combination that made the animal poorly immunocompetent, as confirmed histologically by the severe lymphoid depletion, and prone to succumb to a non-negligent opportunistic pathogen as M. morganii (Liu et al., 2016).
Antimicrobial susceptibility of the M. morganii isolate
Since the isolation of antibacterial-resistant (ABR) strains is also increasing in marine animals (Blasi et al., 2020), we performed additional testing to evaluate the antimicrobial susceptibility profile of our strain.
The isolate was confirmed to be sensitive to the antibiotics normally used to treat M. morganii infection: aminoglycosides, third- and fourth-generation cephalosporins, carbapenems, quinolones, chloramphenicol, sulfonamides and their association with trimethoprim.
Differently from our case, resistance to third- and fourth-generation cephalosporins has been previously observed in two M. morganii strains cultured from captive cetaceans lesions (Park et al., 2020; Sánchez Contreras and Biancani, 2021). However, ESBLs production was not demonstrated in these cases whereas is increasingly reported in strains related to nosocomial infections. ESBLs and AmpC production has been observed in most Enterobacteriaceae species, particularly E. coli and Klebsiella pnuemoniae (Sheng et al., 2013), and can be transferred to other bacterial species by horizontal gene transfer. Furthermore, apart from being plasmid-mediated, AmpC production can derive from the deregulation of natural genetically-encoded β-lactamase enzymes in response to antibiotic exposure (Mizrahi et al., 2020). As expected in a free-ranging wildlife species, our strain did not show any AmpC enzyme production since it is unlikely for a wild CBW to be directly exposed to β-lactam antibiotics.
Nevertheless, the natural wide distribution of M. morganii could be the direct consequence of its adaptability to the environment. Several mechanisms are involved in acquiring antibiotic resistance, even if, often, adaptive resistance is a reversible reply of the bacteria to gradual antibiotic increases (Liu et al., 2016).
On the other hand, the MIC value (1mg/L) that was observed for Trimetroprim-Sulfamethoxazole exceeded the (T)ECOFF value proposed by EUCAST of 0,5 mg/L to distinguish between wild and non-wild type microorganisms.
As occurred for Acinetobacter species exhibiting the highest abundance of sulphonamide-resistant strains (Xiong et al., 2015) or for bacteria isolated from fluvial sediment samples in India and Spain (Kristiansson et al., 2011; Marti et al., 2013), also for M. morganii, the relatively low but constant environmental concentrations of sulpha antibiotics could explain the elevation of the MIC of our strains versus Sulfamethoxazole-Trimethoprim and leading to classify it as a “non-Wild Type” strain.
Wastewaters, frequently insufficiently treated, produced in livestock and poultry breeding, aquaculture, and hospitals (Zhou et al., 2022) are one of the main sources of subinhibitory concentrations of antibiotics, favoring the rise and spreading of ABR bacteria and resistance genes (Lépesová et al., 2019).
Moreover, sulphonamides, apart from being widely used in human and veterinary medicine, including aquatic farmed species, exhibit strong hydrophilicity and easily persist in freshwater (Pruden et al., 2012; Danner et al., 2019) and marine ecosystems where they can exert acute or chronic toxic effects to a wide range of organisms, from algae to fishes (Zhou et al., 2022).
Sulfonamides toxicity has been proven in marine fishes (Zhou et al., 2022) but no ecotoxicological studies have been conducted on marine mammals regarding these antimicrobials. Moreover, the health status of marine species is not impacted just by chronic exposure to environmental levels of antibiotics. Still, it is threatened by other contaminants such as heavy metals, microplastics, OCs, and other chemicals.
In this scenario, the data reported in this study may contribute to the understanding of the combined effects of toxic marine pollutants and pathogens on the health status of an endangered species, such as the CWB, that is challenging to study at sea.
Conclusion
Information regarding bacterial infections in CBW is scattered and mostly incomplete, with no antimicrobial susceptibility testing reported before (Fernández et al., 2011; Alstrup et al., 2021; Febronio et al., 2021). Moreover, detailed and conclusive necropsies are rare for this species in the Mediterranean region (Podestà et al., 2016). In this report, we present the results of systematic post-mortem investigations stressing their importance to fill knowledge gaps and identify both non-anthropogenic and anthropogenic threats for cetaceans, especially for the elusive ones such as the beaked whales. This study expands information on infectious diseases and chemical contaminants affecting marine mammal health and gives new insights into the interaction between pathogens, host, and environment, whose understanding is still scarce to date (Di Guardo et al., 2018) and contributes to better conservation strategies for marine animals.
To our knowledge, this is the first description of M. morganii septicemia with CNS involvement in a wild cetacean. M. morganii can represent a threat to marine mammals, especially when they are immunocompromised and inhabit highly polluted environments, such as CBWs in the Mediterranean Sea (Baini et al., 2020). As occurred in terrestrial animals (Franzoni et al., 2022), further research is advised to investigate the cumulative and synergic effect of antibiotics and other contaminants on aquatic species. Although the isolate did not show any antibiotic resistance “sensu stricto”, a higher threshold of sensitivity for Trimethoprim-Sulfamethoxazole is reported in this study consisting of the first report of a non-wild M. morganii strain in a Mediterranean Cuvier’s beaked whale.
In addition, since adaptive resistance has been well established in Enterobacteriaceae (E. coli, S. enterica), while further investigation for M. morganii is suggested (Liu et al., 2016), this report should be the door opener for these studies both in human as well as in veterinary medicine in a One Health approach.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors. The sequence generated in the present study was submitted to the GenBank® database with the accession number OQ096688.
Ethics statement
Ethical review and approval was not required for the animal study because the investigations were performed on a dead stranded cetacean. No live animals were involved.
Author contributions
Conceptualization, UR-C, CG, FG. Acquisition of data, VM, TA, LM, KV, BI, CM, RZ, RB, AD, FGa, EB, AP, MR, DA. Methodology, UR-C, SZ, TA, FG. Data curation, UR-C, SZ, CG, FG. Software, FG. Supervision, CG, FG. Manuscript drafting, UR-C, SZ. Funding acquisition, CC. Writing review and editing, CC, FG All authors reviewed and agreed on the current version of the manuscript.
Funding
This research was funded by the Italian Ministry of Health [Ricerca Corrente 2019 IZS PLV 05/19 and Ricerca Corrente 2021 IZS PLV 06/21].
Acknowledgments
The authors are grateful to Sabina Airoldi, Aurelie Moulins and Walter Mignone for their assistance during necropsy procedures.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.1058724/full#supplementary-material
Supplementary Table 1 | Results of the MIC test performed on the M. morganii isolate, including CLSI and EUCAST interpretative criteria (clinical breakpoints). When ECOFF (and TECOFF) were available, they were inserted and compared with the MIC values of the tested strain.
References
Abdalla J., Saad M., Samnani I., Lee P., Moorman J. (2006). Central nervous system infection caused by morganella morganii. Am. J. Med. Sci. 331, 44–47. doi: 10.1097/00000441-200601000-00013
Alstrup A. K. O., Thostesen C. B., Madsen P. T., Petersen H. H., Jensen T. K., Olsen M. T., et al. (2021). First stranding of cuvier’s beaked whale (Ziphius cavirostris) on the Danish north Sea coast. Aquat. Mamm. 47, 303–310. doi: 10.1578/AM.47.3.2021.303
Anderson R. C. (1988). Nematode transmission patterns. J. Parasitol. 30–45 (16 pages). doi: 10.2307/3282477
Anderson R. C., Chabaud A. G., Willmott S. (2009). Keys to the nematode parasites of vertebrates: archival volume. Eds. Anderson R. C., Chabaud A. G., Willmott S. (Wallingford (UK: CABI). doi: 10.1079/9781845935726.0000
Arbelo M., Sierra E., Esperón F., Watanabe T. T. N. N., Bellière E. N., De Los Monteros A. E., et al. (2010). Herpesvirus infection with severe lymphoid necrosis affecting a beaked whale stranded in the canary islands. Dis. Aquat. Organ. 89, 261–264. doi: 10.3354/dao02208
Arun M., Bhat G., Acharya V., Shenoy S. (2019). Varied clinical spectrum of a rare entity pneumonia-pasteurella canis: Case series and review of the literature. J. Clin. Diagn. Res. 13, 1–4. doi: 10.7860/jcdr/2019/40234.12656
Ashour D. S., Othman A. A. (2020). Parasite–bacteria interrelationship. Parasitol. Res. 2020. 11910. 119, 3145–3164. doi: 10.1007/S00436-020-06804-2
Bachman M. J., Keller J. M., West K. L., Jensen B. A. (2014). Persistent organic pollutant concentrations in blubber of 16 species of cetaceans stranded in the pacific islands from 1997 through 2011. Sci. Total. Environ. 488–489, 115–123. doi: 10.1016/j.scitotenv.2014.04.073
Baily G. G., Krahn J. B., Drasar B. S., Stoker N. G. (1992). Detection of brucella melitensis and brucella abortus by DNA amplification. J. Trop. Med. Hyg. 95, 271–275.
Baini M., Panti C., Fossi M. C., Tepsich P., Jiménez B., Coomber F., et al. (2020). First assessment of POPs and cytochrome P450 expression in cuvier’s beaked whales (Ziphius cavirostris) skin biopsies from the Mediterranean Sea Sci. Rep. 10, 21891. doi: 10.1038/s41598-020-78962-3
Balbuena J. A., Simpkin A. (2014). Role of crassicauda sp. in natural mortality of pantropical spotted dolphins stenella attenuata: a reassessment. Dis. Aquat. Organ. 108, 83–89. doi: 10.3354/DAO02694
Bandy A. (2020). Ringing bells: Morganella morganii fights for recognition. Public Health 182, 45–50. doi: 10.1016/j.puhe.2020.01.016
Blasi M. F., Migliore L., Mattei D., Rotini A., Thaller M. C., Alduina R. (2020). Antibiotic resistance of gram-negative bacteria from wild captured loggerhead Sea turtles. Antibiot. 9, 162. doi: 10.3390/ANTIBIOTICS9040162
Bossart G. D. (2011). Marine mammals as sentinel species for oceans and human health. Vet. Pathol. 48, 676–690. doi: 10.1177/0300985810388525
Buck J. D., Wells R. S., Rhinehart H. L., Hansen L. J. (2006). Aerobic microorganisms associated with free-ranging bottlenose dolphins in coastal gulf of Mexico and Atlantic ocean waters. J. Wildl. Dis. 42, 536–544. doi: 10.7589/0090-3558-42.3.536
Cañadas A., Notarbartolo di Sciara G. (2018) Ziphius cavirostris Mediterranean subpopulation (Cuvier’s beaked whale). IUCN red list threat. species. Available at: https://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T16381144A199549199.en (Accessed September 27, 2022).
Carlucci R., Cipriano G., Santacesaria F. C., Ricci P., Maglietta R., Petrella A., et al. (2020). Exploring data from an individual stranding of a cuvier’s beaked whale in the gulf of taranto (Northern Ionian Sea, central-eastern Mediterranean Sea). J. Exp. Mar. Bio. Ecol. 533, 151473. doi: 10.1016/J.JEMBE.2020.151473
Centelleghe C., Beffagna G., Palmisano G., Franzo G., Casalone C., Pautasso A., et al. (2017). Dolphin morbillivirus in a cuvier’s beaked whale (Ziphius cavirostris), Italy. Front. Microbiol. 8. doi: 10.3389/FMICB.2017.00111
Centelleghe C., Da Dalt L., Marsili L., Zanetti R., Fernandez A., Arbelo M., et al. (2019). Insights into dolphins’ immunology: Immuno-phenotypic study on mediterranean and atlantic stranded cetaceans. Front. Immunol. 10. doi: 10.3389/FIMMU.2019.00888/BIBTEX
Ceri H., Olson M., Morck D., Storey D., Read R., Buret A., et al. (2001). The MBEC assay system: multiple equivalent biofilms for antibiotic and biocide susceptibility testing. Methods Enzymol. 337, 377–385. doi: 10.1016/S0076-6879(01)37026-X
CLSI (2018a). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically (USA: CLSI Standard M07).
CLSI (2018b). Performance standards for antimicrobial disk susceptibility Tests.CLSI standard M02. 13th ed (USA: Clinical and Laboratory Standards Institute (CLSI).
CLSI (2020). Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from Animals.Supplement VET01S. 5th ed (USA: Clinical and Laboratory Standards Institute (CLSI).
Cuvertoret-Sanz M., López-Figueroa C., O’Byrne A., Canturri A., Martí-Garcia B., Pintado E., et al. (2020). Causes of cetacean stranding and death on the catalonian coast (western Mediterranean Sea), 2012-2019. Dis. Aquat. Organ. 142, 239–253. doi: 10.3354/DAO03550
Danner M. C., Robertson A., Behrends V., Reiss J. (2019). Antibiotic pollution in surface fresh waters: Occurrence and effects. Sci. Total. Environ. 664, 793–804. doi: 10.1016/j.scitotenv.2019.01.406
Díaz-Delgado J., Fernández A., Xuriach A., Sierra E., Bernaldo de Quirós Y., Mompeo B., et al. (2016). Verminous arteritis due to crassicauda sp. in cuvier’s beaked whales (Ziphius cavirostris). Vet. Pathol. 53, 1233–1240. doi: 10.1177/0300985816642228
Di Guardo G., Centelleghe C., Mazzariol S. (2018). Cetacean host-pathogen interaction(s): Critical knowledge gaps. Front. Immunol. 92815. doi: 10.3389/FIMMU.2018.02815
Di Guardo G., Proietto U., Di Francesco C. E., Marsilio F., Zaccaroni A., Scaravelli D., et al. (2010). Cerebral toxoplasmosis in striped dolphins (Stenella coeruleoalba) stranded along the ligurian Sea coast of Italy. Vet. Pathol. 47, 245–253. doi: 10.1177/0300985809358036
Eberspacher B., Hugo F., Pohl M., Bhakdi S. (1990). Functional similarity between the haemolysins of escherichia coli and morganella morganii. J. Med. Microbiol. 33, 165–170. doi: 10.1099/00222615-33-3-165
Elfadl A. K., Lee S. W., Kim J. H., Lee K. L., Ullah H. M. A., Chung M. J., et al. (2017). Fatal fibrino-hemorrhagic bronchopneumonia associated with morganella morganii in a bottlenose dolphin: A case report. Dis. Aquat. Organ. 127, 41–47. doi: 10.3354/dao03184
Febronio A. M. B., Boos G. S., Batista R. L. G., Amorim D. B., Guimarães J. P., Bianchi M. V., et al. (2021). Crassicaudiasis in three geographically and chronologically distant cuvier’s beaked whales (Ziphius cavirostris) stranded off Brazil. Int. J. Parasitol. Parasites Wildl. 16, 262–269. doi: 10.1016/j.ijppaw.2021.10.010
Felipe-Jiménez I., Fernández A., Arbelo M., Segura-Göthlin S., Colom-Rivero A., Suárez-Santana C. M., et al. (2022). Molecular diagnosis of cetacean morbillivirus in beaked whales stranded in the canary island (1999–2017). Vet. Sci. 9 (3), p.121. doi: 10.3390/VETSCI9030121
Fernández A., Edwards J. F., Rodríguez F., Espinosa De Los Monteros A., Herráez P., Castro P., et al. (2005). “’Gas and fat embolic syndrome’” involving a mass stranding of beaked whales (Family ziphiidae) exposed to anthropogenic sonar signals. Vet. Pathol. 42, 446–457. doi: 10.1354/vp.42-4-446
Fernández A., Vela A. I., Andrada M., Herraez P., Díaz-Delgado J., Domínguez L., et al. (2011). Citrobacter freundii septicemia in a stranded newborn cuvier’s beaked whale (Ziphius cavirostris). J. Wildl. Dis. 47, 1043–1046. doi: 10.7589/0090-3558-47.4.1043
Franzoni G., Ciccotelli V., Masiello L., De Ciucis C. G., Anfossi A. G., Vivaldi B., et al. (2022). Cadmium and wild boar: Environmental exposure and immunological impact on macrophages. Toxicol. Rep. 9, 171–180. doi: 10.1016/J.TOXREP.2022.01.009
Geraci J. R., Dailey M. D., Aubin St. D.J. (2011). Parasitic mastitis in the Atlantic white-sided dolphin, lagenorhynchus acutus, as a probable factor in herd productivity. Journal of the Fisheries Board of Canada 35, 1350–1355. doi: 10.1139/F78-210
Geraci J. R., Lounsbury V. J. (2005). Marine mammals ashore: A field guide for strandings. second., ed (Baltimore,MD: National Aquarium in Baltimore).
Giorda F., Romani-Cremaschi U., Marsh A. E., Grattarola C., Iulini B., Pautasso A., et al. (2021). Evidence for unknown sarcocystis-like infection in stranded striped dolphins (Stenella coeruleoalba) from the ligurian Sea, Italy. Animals 11 (5), 1201. doi: 10.3390/ani11051201
Hatzianestis J., Georgakopoulou-Gregoriadou E., Frantzis A. (1998). Organochlorine levels in cuvier’s beaked whales from the Ionian Sea, hellas. Fresenius. Environ. Bull. 7, 345–350.
Helle E., Olsson M., Jensen S. (1976). DDT And PCB levels and reproduction in ringed seal from the bothnian bay. Ambio 5, 188–189.
Heyning J. E., Mead J. G. (2009). ““Cuvier’s beaked whale: Ziphius cavirostris,”,” in Encyclopedia of marine mammals. Eds. Perrin W., Wursig B., Thewissen J. (San Diego: Academic Press), 294–295. doi: 10.1016/B978-0-12-373553-9.00069-9
Jepson P. D., Bennett P. M., Deaville R., Allchin C. R., Baker J. R., Law R. J. (2005). Relationships between polychlorinated biphenyls and health status in harbor porpoises (Phocoena phocoena) stranded in the united kingdom. Environ. Toxicol. Chem. 24(1), 238–248. doi: 10.1897/03-663.1
Jepson P. D., Deaville R., Barber J. L., Aguilar À., Borrell A., Murphy S., et al. (2016). PCB Pollution continues to impact populations of orcas and other dolphins in European waters. Sci. Rep. 6. doi: 10.1038/SREP18573
Jerdy H., Werneck M., Barbosa L., Hauser-Davis R. A., De-Oliveira-Nogueira C. H., da Silveira L. S. (2022). First report on phyllobothrium delphini infection and crassicauda sp. parasitism resulting in osseous metaplasia in a cuvier’s beaked whale (Ziphius cavirostris) from the Brazilian region. Int. J. Parasitol. Parasites Wildl. 17, 60–64. doi: 10.1016/J.IJPPAW.2021.12.005
Kannan K., Blankenship A. L., Jones P. D., Giesy J. P. (2000). Toxicity reference values for the toxic effects of polychlorinated biphenyls to aquatic mammals. Hum. Ecol. Risk Assess. Int. J. 6, 181–201. doi: 10.1080/10807030091124491
Knap A. H., Jickells T. D. (1983). Trace metals and organochlorines in the goosebeaked whale. Mar. pollut. Bull. 14, 271–274. doi: 10.1016/0025-326X(83)90172-8
Kot B. C. W., Ho H. H. N., Leung E. K. C., Chung T. Y. T., Tsui H. C. L. (2022). Characterisation of crassicauda fuelleborni nematode infection in indo-pacific finless porpoises (Neophocaena phocaenoides) using postmortem computed tomography. Int. J. Parasitol. Parasites Wildl. 18, 68–75. doi: 10.1016/J.IJPPAW.2022.04.005
Kristiansson E., Fick J., Janzon A., Grabic R., Rutgersson C., Weijdegård B., et al. (2011). Pyrosequencing of antibiotic-contaminated river sediments reveals high levels of resistance and gene transfer elements. PloS One 6, e17038. doi: 10.1371/JOURNAL.PONE.0017038
Lambertsen R. H. (1986). Disease of the common fin whale (Balaenoptera physalus): crassicaudiosis of the urinary system. J. Mammal. 67, 353–366. doi: 10.2307/1380889
Lépesová K., Olejníková P., Mackuľak T., Tichý J., Birošová L. (2019). Annual changes in the occurrence of antibiotic-resistant coliform bacteria and enterococci in municipal wastewater. Environ. Sci. pollut. Res. 26, 18470–18483. doi: 10.1007/s11356-019-05240-9
Liu H., Zhu J., Hu Q., Rao X. (2016). Morganella morganii, a non-negligent opportunistic pathogen. Int. J. Infect. Dis. 50, 10–17. doi: 10.1016/j.ijid.2016.07.006
Marcer F., Marchiori E., Centelleghe C., Ajzenberg D., Gustinelli A., Meroni V., et al. (2019). Parasitological and pathological findings in fin whales balaenoptera physalus stranded along Italian coastlines. Dis. Aquat. Organ. 133, 25–37. doi: 10.3354/dao03327
Marsili L., D’Agostino A., Bucalossi D., Malatesta T., Fossi M. C. (2004). Theoretical models to evaluate hazard due to organochlorine compounds (OCs) in Mediterranean striped dolphin (Stenella coeruleoalba). Chemosphere 56, 791–801. doi: 10.1016/j.chemosphere.2004.03.014
Marsili L., Di Guardo G., Mazzariol S., Casini S. (2019). Insights into cetacean immunology: Do ecological and biological factors make the difference? Front. Immunol. 10. doi: 10.3389/FIMMU.2019.01219/XML/NLM
Marsili L., Focardi S. (1997). Chlorinated hydrocarbon (HCB, DDTs and PCBs) levels in cetaceans stranded along the Italian coasts: An overview. Environ. Monit. Assess. 45, 129–180. doi: 10.1023/A:1005786627533
Marti E., Jofre J., Balcazar J. L. (2013). Prevalence of antibiotic resistance genes and bacterial community composition in a river influenced by a wastewater treatment plant. PloS One 8, e78906. doi: 10.1371/JOURNAL.PONE.0078906
Martineau D., Mikaelian I., Lapointe J.-M., Labelle P., Higgins R. (2003). ““Pathology of cetaceans. a case study: Beluga from the st. Lawrence estuary,”,” in Toxicology of marine mammals (London: Taylor & Francis), 333–380. Available at: https://www.taylorfrancis.com/chapters/edit/10.1201/9780203165577-18/pathology-cetaceans-case-study-beluga-st-lawrence-estuary-daniel-martineau-igor-mikaelian-jean-martin-lapointe-philippe-labelle-robert-higgins.
Mizrahi A., Delerue T., Morel H., Le Monnier A., Carbonnelle E., Pilmis B., et al. (2020). Infections caused by naturally AmpC-producing enterobacteriaceae: Can we use third-generation cephalosporins? a narrative review. Int. J. Antimicrob. Agents 55. doi: 10.1016/J.IJANTIMICAG.2019.10.015
Olson M. E., Ceri H., Morck D. W., Buret A. G., Read R. R. (2002). Biofilm bacteria: formation and comparative susceptibility to antibiotics. Can. J. Vet. Res. 66, 86–92.
Osorio C. R., Toranzo A. E., Romalde J. L., Barja J. L. (2000). Multiplex PCR assay for ureC and 16S rRNA genes clearly discriminates between both subspecies of photobacterium damselae. Dis. Aquat. Organ. 40, 177–183. doi: 10.3354/DAO040177
Park S. Y., Lee K., Cho Y., Lim S. R., Kwon H., Han J. E., et al. (2020). Emergence of third-generation cephalosporin-resistant morganella morganii in a captive breeding dolphin in south Korea. Animals 10, 1–7. doi: 10.3390/ani10112052
Paterson D. L., Mathers A. J. (2020). ““Infections due to other members of the enterobacteriaceae, including management of multidrug-resistant strains - ClinicalKey,”,” in Goldman-Cecil Medicine. Eds. Goldman L., Schafer A. (USA: Elsevier), 1927–1930.
Podestà M., Azzellino A., Cañadas A., Frantzis A., Moulins A., Rosso M., et al. (2016). Cuvier’s beaked whale, ziphius cavirostris, distribution and occurrence in the Mediterranean Sea: High-use areas and conservation threats. Adv. Mar. Biol. 75, 103–140. doi: 10.1016/BS.AMB.2016.07.007
Podestà M., D’Amico A., Pavan G., Drougas A., Komnenou A., Portunato N. (2006). A review of cuvier’s beaked whale strandings in the Mediterranean Sea. J. Cetacean. Res. Manage. 7, 251–261.
Pruden A., Arabi M., Storteboom H. N. (2012). Correlation between upstream human activities and riverine antibiotic resistance genes. Environ. Sci. Technol. 46, 11541–11549. doi: 10.1021/es302657r
Rosso M., Ballardini M., Moulins A., Würtz M., et al. (2011). Natural markings of Cuvier's beaked whale Ziphius cavirostris in the Mediterranean Sea. African Journal of Marine Science 33 (1), 45–57. doi: 10.2989/1814232X.2011.572336
Sánchez Contreras G. J., Biancani B. (2021). Third- and fourth-generation cephalosporin resistant morganella morganii associated to an abscess on the perineum of a Male bottlenose dolphin (Tursiops truncatus). Aquat. Mamm. 47, 530–539. doi: 10.1578/am.47.6.2021.530
Sheng W. H., Badal R. E., Hsueh P. R. (2013). Distribution of extended-spectrum β-lactamases, AmpC β-lactamases, and carbapenemases among enterobacteriaceae isolates causing intra-abdominal infections in the Asia-pacific region: results of the study for monitoring antimicrobial resistance trends (SMART). Antimicrob. Agents Chemother. 57, 2981–2988. doi: 10.1128/AAC.00971-12
Suárez-Santana C. M., Sierra E., Díaz-Delgado J., Zucca D., de Quirós Y. B., Puig-Lozano R., et al. (2018). Prostatic Lesions in Odontocete Cetaceans. Veterinary Pathology 55 (3), 466–472. doi: 10.1177/0300985818755252
The European Committee on Antimicrobial Susceptibility Testing (2017). EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance, version 2.0. (EUCAST).
The European Committee on Antimicrobial Susceptibility Testing (2022a) Antimicrobial wild type distributions of microorganisms. MIC distributions for morganella morganii. Available at: https://mic.eucast.org/search/?search%5Bmethod%5D=mic&search%5Bantibiotic%5D=-1&search%5Bspecies%5D=324&search%5Bdisk_content%5D=-1&search%5Blimit%5D=50 (Accessed September 12, 2022).
The European Committee on Antimicrobial Susceptibility Testing (2022b) Breakpoint tables for interpretation of MICs and zone diameters, version 12.0. Available at: http://www.eucast.org/clinical_breakpoints/ (Accessed August 8, 2022).
Thornton S. M., Nolan S., Gulland F. M. D. (1998). Bacterial isolates from California Sea lions (Zalophus californianus), harbor seals (Phoca vitulina), and northern elephant seals (Mirounga angustirostris) admitted to a rehabilitation center along the central California coas-1995. J. Zoo Wildl. Med. 29, 171–176.
VanDevanter D. R., Warrener P., Bennett L., Schultz E. R., Coulter S., Garber R. L., et al. (1996). Detection and analysis of diverse herpesviral species by consensus primer PCR. J. Clin. Microbiol. 34, 1666–1671. doi: 10.1128/JCM.34.7.1666-1671.1996
Verna F., Giorda F., Miceli I., Rizzo G., Pautasso A., Romano A., et al. (2017). Detection of morbillivirus infection by RT-PCR RFLP analysis in cetaceans and carnivores. J. Virol. Methods 247, 22–27. doi: 10.1016/j.jviromet.2017.05.009
Vitale M., Galluzzo P., Currò V., Gozdzik K., Schillaci D., Di Marco Lo Presti V. (2013). A high sensitive nested PCR for toxoplasma gondii detection in animal and food samples. J. Microb. Biochem. Technol. 05, 39–41. doi: 10.4172/1948-5948.1000097
World Organisation for Animal Health (WOAH) (2018). Manual of diagnostic tests and vaccines for terrestrial animals (Terrestrial manual). 8th ed. (Paris, France: WOAH).
Xiong W., Sun Y., Zhang T., Ding X., Li Y., Wang M., et al. (2015). Antibiotics, antibiotic resistance genes, and bacterial community composition in fresh water aquaculture environment in China. Microb. Ecol. 70, 425–432. doi: 10.1007/S00248-015-0583-X
Keywords: Cuvier’s beaked whale, Morganella morganii, Crassicauda, antimicrobial resistance, septicemia, pollutants(environmental)
Citation: Romani-Cremaschi U, Zoppi S, Mattioda V, Audino T, Marsili L, Varello K, Iulini B, Marra C, Zoccola R, Battistini R, Dondo A, Garibaldi F, Berio E, Pautasso A, Rosso M, Ascheri D, Casalone C, Grattarola C and Giorda F (2023) Morganella morganii septicemia and concurrent renal crassicaudiasis in a Cuvier’s beaked whale (Ziphius cavirostris) stranded in Italy. Front. Mar. Sci. 9:1058724. doi: 10.3389/fmars.2022.1058724
Received: 30 September 2022; Accepted: 30 December 2022;
Published: 19 January 2023.
Edited by:
Roberto Carlucci, University of Bari Aldo Moro, ItalyReviewed by:
Salvatore Siciliano, Escola Nacional de Saúde Pública Sergio Arouca, Fundação Oswaldo Cruz (Fiocruz), BrazilGuido Pietroluongo, University of Padua, Italy
Copyright © 2023 Romani-Cremaschi, Zoppi, Mattioda, Audino, Marsili, Varello, Iulini, Marra, Zoccola, Battistini, Dondo, Garibaldi, Berio, Pautasso, Rosso, Ascheri, Casalone, Grattarola and Giorda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simona Zoppi, c2ltb25hLnpvcHBpQGl6c3RvLml0; Carla Grattarola, Y2FybGEuZ3JhdHRhcm9sYUBpenN0by5pdA==
 Umberto Romani-Cremaschi
Umberto Romani-Cremaschi Simona Zoppi
Simona Zoppi Virginia Mattioda
Virginia Mattioda Tania Audino3
Tania Audino3 Letizia Marsili
Letizia Marsili Katia Varello
Katia Varello Roberto Zoccola
Roberto Zoccola Roberta Battistini
Roberta Battistini Alessandro Dondo
Alessandro Dondo Fulvio Garibaldi
Fulvio Garibaldi Massimiliano Rosso
Massimiliano Rosso Davide Ascheri
Davide Ascheri Cristina Casalone
Cristina Casalone Carla Grattarola
Carla Grattarola Federica Giorda
Federica Giorda