- 1Cooperative Institute for Climate, Ocean, and Ecosystem Studies, University of Washington, Seattle, WA, United States
- 2Marine Mammal Laboratory, Alaska Fisheries Science Center, National Oceanic and Atmospheric Administration (NOAA) Fisheries, Seattle, WA, United States
- 3School of Fisheries and Aquatic Sciences, University of Washington, Seattle, WA, United States
- 4Nicholas School of the Environment, Duke University, Durham, NC, United States
We delineated and scored Biologically Important Areas (BIAs) for cetaceans in the Aleutian Islands and Bering Sea region. BIAs represent areas and times in which cetaceans are known to concentrate for activities related to reproduction, feeding, and migration, and also the known ranges of small and resident populations. This effort, the second led by the National Oceanic and Atmospheric Administration (NOAA), uses structured elicitation principles to build upon the first version of NOAA’s BIAs (BIA I) for cetaceans. Supporting evidence for BIA II came from aerial-, land-, and vessel-based surveys; satellite-tagging data; passive acoustic monitoring; Indigenous knowledge; photo-identification data; whaling data, including stomach and fecal contents; prey studies; and genetics. In addition to narratives, maps, and metadata tables, the BIA II products incorporate a scoring and labeling system, which will improve their utility and interpretability. BIAs are compilations of the best available science and have no inherent regulatory authority. They have been used by NOAA, other federal agencies, and the public to support planning and marine mammal impact assessments, and to inform the development of conservation measures for cetaceans. In the Aleutian Islands and Bering Sea region, a total of 19 BIAs were identified, delineated, and scored for seven species, including bowhead, North Pacific right, gray, humpback, fin, and sperm whales, and belugas. These include one hierarchical BIA for belugas that consists of one localized “child” BIA within an overarching “parent” BIA. There were 15 feeding, 3 migratory, and 1 small and resident population BIAs; no reproductive BIAs were identified. In some instances, information existed about a species’ use of a particular area and time, but the information was insufficient to confidently delineate the candidate BIA; in those cases, the candidate BIA was added to a watch list. A total of 22 watch list areas were identified and delineated for 10 species, including all species mentioned above and minke whales, harbor porpoises, and Dall’s porpoises. There were 15 feeding, 4 migratory, 2 reproductive, and 1 small and resident population watch list areas. Some BIAs and watch list areas were transboundary between the Aleutian Islands and Bering Sea region and the Arctic region.
1. Introduction
Cetacean seasonal distributions in the Aleutian Islands and Bering Sea overlap with numerous anthropogenic activities, including commercial fisheries, shipping, recreational activities, and subsistence activities, which are all increasingly influenced by climate change. The ecosystems in the region are changing rapidly due to the warming climate, highlighted recently by the unprecedented lack of sea ice over the eastern Bering Sea shelf during the winters of 2017/18 and 2018/19, with little or no cold bottom water formation (Siddon and Zador, 2018; Siddon and Zador, 2019; Stabeno and Bell, 2019).
Many cetacean species are found in the Aleutian Islands and Bering Sea region, including mysticetes, or baleen whales, such as bowhead (Balaena mysticetus), North Pacific right (Eubalaena japonica), gray (Eschrichtius robustus), humpback (Megaptera novaeangliae), fin (Balaenoptera physalus), and minke (Balaenoptera acutorostrata) whales. Mysticetes feed primarily on zooplankton and small schooling fish. Also present in the region are odontocetes, or toothed whales, such as belugas (Delphinapterus leucas), harbor (Phocoena phocoena) and Dall’s (Phocoenoides dalli) porpoises, killer (Orcinus orca) and sperm (Physeter microcephalus) whales, and Pacific white-sided dolphins (Lagenorhynchus obliquidens). Odontocetes feed on fish, squid, crustaceans, and other marine mammals.
As the climate continues to warm and the annual open water (ice-free) period continues to lengthen, there will be more human interest in the Bering Sea and Arctic regions, and anthropogenic stressors are expected to increase in magnitude, space, and time (Huntington et al., 2015; Aksenov et al., 2017). These stressors may affect marine mammals by disrupting behavior (e.g., migrating, feeding, breeding, resting); increasing environmental and noise pollution, which can mask communication and lead to elevated stress levels; increase risk of ship strike; degrade habitat; and introduce non-native species (Huntington, 2009; Moore et al., 2012; Rolland et al., 2012; Blackwell et al., 2013; Reeves et al., 2014; Huntington et al., 2015; Silber et al., 2021). These issues are particularly concerning for the Bering Strait area because it is narrow (85 km), shallow (50 m), and the only gateway into the Pacific Arctic for all ships and migratory marine mammals. We are presently in a critical time for making conservation and management decisions that affect the Bering Sea and Aleutian Islands.
To inform impact assessment and place-based marine conservation and management efforts in the region, we identified and scored biologically important areas (BIAs) for cetaceans in marine waters surrounding the U.S. as part of a nationwide process led by the National Oceanic and Atmospheric Administration (NOAA). BIAs represent places and periods (months or seasons) that are important to cetacean species, stocks, or populations for feeding, migrating, or activities related to reproduction (Ferguson et al., 2015; Harrison et al. in review). BIAs may also be defined to encompass the range or core areas of small and resident populations. BIAs are compilations of the best available information and have no inherent or direct regulatory power. They have been used by NOAA, other federal agencies, and the public to support planning and marine mammal impact assessments, and to inform the development of conservation measures for cetaceans. This effort builds on NOAA’s inaugural BIA process (BIA I; Van Parijs et al., 2015) by revising existing BIAs (Ferguson et al., 2015) and creating new BIAs (BIA II) based on new information, and by scoring each BIA based on intensity of use, data support, spatiotemporal variability, and boundary certainty (Harrison et al. in review).
The ecosystems in the Aleutian Islands and Bering Sea region are spatially heterogeneous and temporally dynamic. Here, we present a synopsis of this ecological variability because it shapes the spatiotemporal variability in cetacean distribution, density, and habitat upon which this BIA assessment was based.
The Aleutian Islands and Bering Sea region (Figure 1) is a highly productive marine ecosystem (Stabeno et al., 2005; Grebmeier et al., 2006; Grebmeier, 2012) and contains the largest fishery in the U.S. (Liddel and Yencho, 2021). In the Aleutian Islands, currents (Figure 1) flow through the passes of the Aleutian archipelago, bringing nutrient-rich water, with net flow going northward particularly east of Samalga Pass (Ladd et al., 2005; Stabeno et al., 2005). The waters mix while flowing through the passes, but become stratified north of the passes, concentrating nutrients in the euphotic zone and leading to enhanced productivity (Ladd et al., 2005; Stabeno et al., 2005).
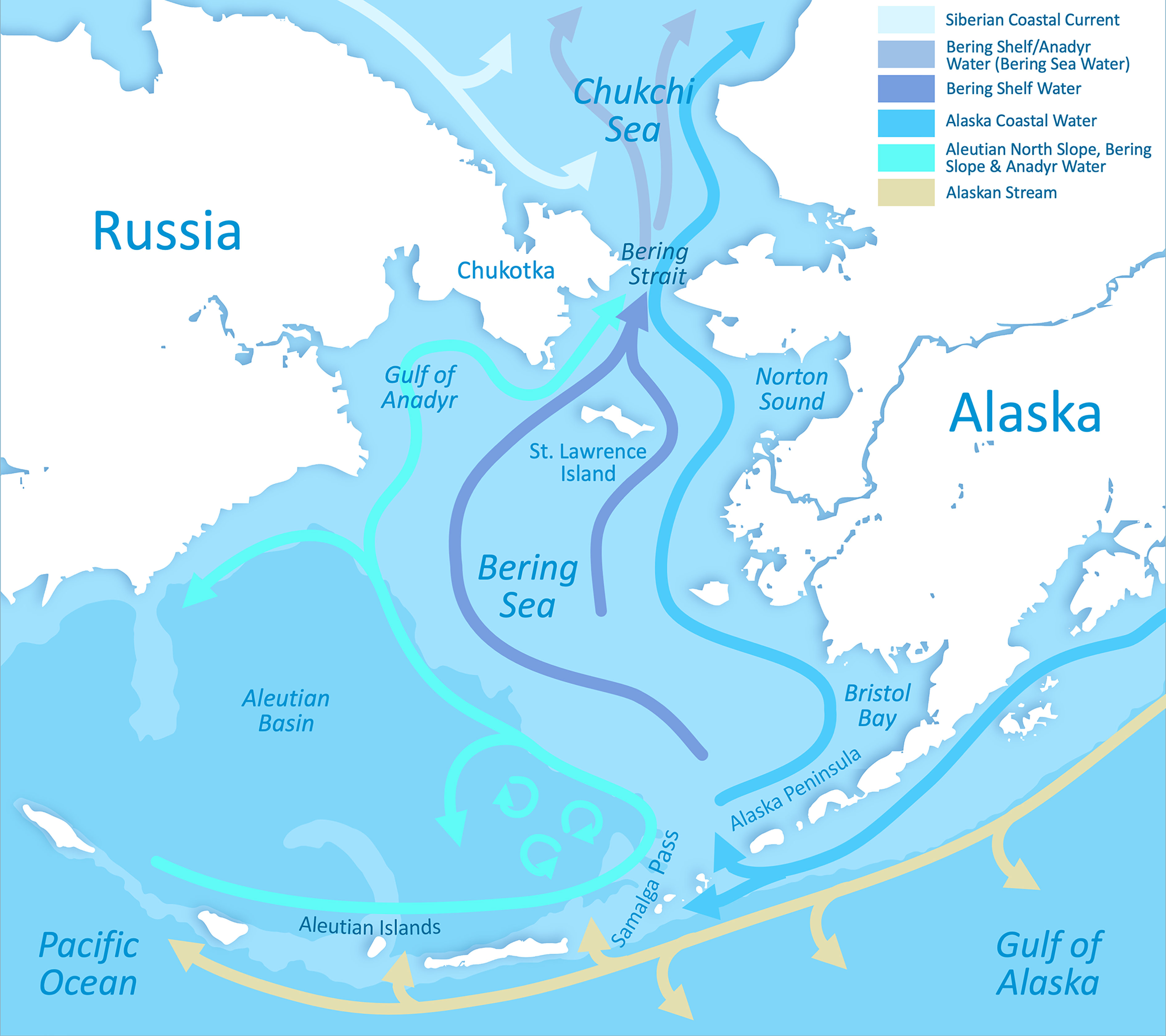
Figure 1 Aleutian Islands and Bering Sea region, showing water masses and prevailing direction of flow.
The deep Aleutian Basin encompasses the western and southern Bering Sea. The eastern and northern Bering Sea comprise a broad continental shelf. In the Bering Sea, the shelf break between the Aleutian Basin and the continental shelf has high primary and secondary productivity, with upwelling bringing nutrients into the euphotic zone on the eastern Bering Sea shelf, and influencing planktonic and benthic food webs and sediment community dynamics on the shelf (Springer et al., 1996; Grebmeier et al., 2006; Grebmeier et al., 2018).
The eastern Bering Sea shelf may be divided into coastal (shore to 50 m), middle (50-100 m), and outer (100-180 m) domains, which are separated by oceanographic fronts. The locations of the fronts vary, but are generally near the 50- and 100-m isobaths (Coachman, 1986; Kachel et al., 2002). On the middle shelf, the cold pool that usually forms in the bottom water layer from winter sea ice formation separates the pelagic-dominated ecosystem of the southern Bering Sea from the benthic-dominated ecosystem of the northern Bering Sea (Kachel et al., 2002; Stabeno et al., 2012). The cold pool acts as a barrier for subarctic crab and fish species, historically keeping them in the outer domain (Wyllie-Echeverria and Wooster, 1998; Stabeno et al., 2012); however, there appears to be a northward shift in the cold water temperature barrier in recent decades, with potential to restructure the ecosystem (Grebmeier et al., 2006; Grebmeier, 2012; Grebmeier et al., 2018; Stabeno and Bell, 2019; Stevenson and Lauth, 2019; Huntington et al., 2020).
In the northern Bering Sea, three water masses (Figure 1) flow through Bering Strait in a generally northward direction (Grebmeier et al., 1988; Woodgate and Aagaard, 2005; Grebmeier et al., 2006; Woodgate et al., 2015). These water masses include the nutrient-rich Anadyr Water on the western side of the northern Bering Sea and Chukchi Sea, and the fresh and nutrient-limited Alaska Coastal Water on the eastern side (Grebmeier et al., 1988; Weingartner et al., 2005; Grebmeier et al., 2006; Woodgate et al., 2015). The third Bering Strait water mass is the Bering Shelf-Anadyr Water, consisting of intermediate salinity Bering Shelf Water mixed with Anadyr Water (Grebmeier et al., 1988; Grebmeier et al., 2006). Transport of Pacific Water through Bering Strait peaks in summer. Currents are important sources of nutrients, heat, freshwater, organic carbon, and plankton for Arctic ecosystems, which provide foraging opportunities for seabirds and marine mammals (e.g., Piatt and Springer, 2003; Bluhm et al., 2007; Ashjian et al., 2010). Current advection and velocity to the Arctic affect organic carbon cycling, sediment structure, and pelagic-benthic coupling (Grebmeier et al., 2006; Grebmeier et al., 2015; Pisareva et al., 2015; Woodgate et al., 2015; Grebmeier et al., 2018; Moore et al., 2018).
The marine environment of the Aleutian Islands and Bering Sea region is highly seasonal. Sea ice covers the Bering Sea shelf in winter; the southerly extent can vary by >100 km per year (Stabeno et al., 2012), with maximum ice extent occurring in March, and no ice during summer or fall. Arctic and subarctic sea ice extent, volume, and duration are declining with warming ocean temperatures and changing wind patterns, resulting in sea ice forming later in the season and melting earlier in the season (Maslanik et al., 2011; Stroeve et al., 2012; Stroeve et al., 2014; Frey et al., 2015; Wood et al., 2015; Siddon and Zador, 2018). Some models predict a seasonal sea-ice-free state in the Pacific Arctic by 2040 (Wang and Overland, 2012; Koenigk et al., 2013; Wang and Overland, 2015; Wang et al., 2018; Guarino et al., 2020). This reduction in sea ice and warming temperatures will affect oceanic circulation and water column processes that influence primary productivity, benthic faunal biomass and composition, and ecosystem processes, and will have profound impacts throughout the Bering Sea region, possibly shifting the benthic-dominated system of the northern Bering and Chukchi seas to a pelagic-dominated system (Grebmeier et al., 2006; Grebmeier et al., 2018).
Sea ice and the cold pool are extremely important to this region’s ecosystems. In the Bering Sea, the cold pool is defined as cold bottom water <2°C that persists from winter sea ice (Stabeno et al., 2012). Melted sea ice provides freshwater, contributing to water column stratification and allowing the cold pool to form, with cascading effects on the timing of the spring phytoplankton bloom (the base of the marine food web) and the distributions and densities of species across a range of taxa and trophic levels. Documented effects of the lack of sea ice in winters 2017/18 and 2018/19 were numerous. In 2018, the spring phytoplankton bloom in the northern Bering Sea was delayed by one month (Siddon and Zador, 2018). Species of large, lipid-rich zooplankton (>2 mm, e.g., Calanus spp., Neocalanus spp.) and euphausiids (>15 mm, e.g., Thysanoessa spp.) exhibited low abundances in 2018 and 2019; in contrast, there were relatively high abundances of small zooplankton (≤2mm, e.g., Acartia spp., Pseudocalanus spp., Oithona spp.), which represent only small packages of energy for higher trophic level predators (Siddon and Zador, 2018; Siddon and Zador, 2019). Large numbers of adult Pacific cod (Gadus microcephalus) and pollock (Gadus chalcogrammus) were found farther north than usual in the northern Bering Sea in 2017-19 (Siddon and Zador, 2018; Eisner et al., 2020). Seabird die-offs and near complete reproductive failure occurred at breeding colonies in the northern Bering Sea in 2018 (Siddon and Zador, 2018; Romano et al., 2020; Will et al., 2020). An ice seal Unusual Mortality Event (UME) was declared in 2019 for bearded (Erignathus barbatus), ringed (Pusa hispida), and spotted (Phoca largha) seals in the Bering and Chukchi seas due to elevated strandings beginning in June 2018. Although the cause of the ice seal UME has not yet been determined, the loss of spring sea ice habitat for pupping and nursing and reduced prey increased mortality and resulted in a decline in body condition of these seals (Siddon and Zador, 2018; Siddon and Zador, 2019; Huntington et al., 2020; https://www.fisheries.noaa.gov/alaska/marine-life-distress/2018-2021-ice-seal-unusual-mortality-event-alaska). In 2019, a gray whale UME was declared due to the elevated number of gray whales that stranded along the west coast of North America; the cause of this UME has yet to be determined as of April 2022 (Siddon, 2020; https://www.fisheries.noaa.gov/national/marine-life-distress/2019-2021-gray-whale-unusual-mortality-event-along-west-coast-and).
The Aleutian Islands and Bering Sea region has few villages along the coastline and is a difficult and challenging place to study cetaceans. The region is vast, most of it is very remote, and the environment is harsh. The region can experience severe storms. The northern portion of the Bering Sea receives little daylight during the winter and is covered by sea ice into the spring. Cetacean studies are often funded by the oil and gas industry or U.S. Navy when they are working or conducting exercises in areas that overlap with cetacean presence; however, there have been few of these activities in the Aleutian Islands and Bering Sea region in recent years, resulting in relatively few cetacean studies. Within the region, the Bering Strait area has not had as much cetacean or cetacean prey research done as the southeastern Bering Sea due to commercial fisheries research in the latter. The Aleutian Islands and Bering Sea region has not had as much cetacean research as the Arctic region due to oil and gas-funded work in the latter. For these reasons, in some cases there is little information to inform the BIA II assessment for this region.
The goals of this manuscript are to provide insight into the processes used to delineate and score BIAs in the Aleutian Islands and Bering Sea region and a summary of the results. The objectives are to:
● Present detailed information on the data sources and decision-making processes used to delineate and score BIAs in this region. (See Harrison et al. in review for the detailed and comprehensive BIA delineation and scoring protocols for all regions.)
● Summarize all BIAs for the region by BIA type, species, scores, and summary statistics.
● Present three example BIAs which span a range of BIA types, intensities, information availability, and spatiotemporal variability, detailing the information used to assess BIA status, the process used to delineate the BIA in space and time, and the scoring decisions.
● List the region’s watch list areas. In some instances, information existed about a species’ use of a particular area and time, but was insufficient to confidently delineate the candidate BIA; in those cases, the candidate BIA was added to a watch list.
● Provide recommendations to facilitate future conservation and management efforts in the region.
It was not practical to include details on every BIA in the manuscript; rather, information and shapefiles for all BIAs can be found in the Supplementary Information Descriptions available via the BIA website (https://oceannoise.noaa.gov/biologically-important-areas).
2. Methods
BIAs for all seven regions around the U.S. were consistently delineated, scored, and labeled using the methodology described in the Introductory chapter included in this special edition (Harrison et al. in review). Additionally, Harrison et al. (in review) highlights the changes in BIA II since Van Parijs et al. (2015), describes the intended use of the BIAs, and specifically addresses common mischaracterizations of the BIA I products to try to eliminate inappropriate use of BIAs in the future. Fundamentally, BIAs are compilations of the best available information and have no inherent or direct regulatory power. We provide a brief overview of the methods outlined in Harrison et al. (in review) below.
The BIA II effort applied principles of expert elicitation in a structured manner to identify, delineate, and score BIAs to ensure that information that was not incorporated during BIA I (e.g., Indigenous knowledge, local knowledge, or community science) was included. Expert elicitation is a formal, structured process for obtaining experts’ opinions and knowledge to help inform decision-making, particularly in an information-limited situation. During an introductory workshop between the BIA II Working Group (WG) leads, NOAA and Navy project sponsors, regional leads with cetacean expertise, cetacean Subject Matter Experts (SME), and other interested parties, the WG presented an overview of the purpose and BIA delineation and draft scoring protocols. Workshop participants were encouraged to provide targeted input to help finalize scoring and labeling protocols. Based on feedback from workshop participants, WG leads revised the scoring and labeling protocols and subsequently met with regional leads and SMEs to present the protocols in a comprehensive, step-by-step manner. An individual with extensive experience in structured expert elicitation facilitated these early meetings to ensure a shared understanding of the scoring and labeling protocols across regional leads and SMEs. Regional check-in meetings were held with regional and WG leads and available SMEs to answer questions and provide clarity. To promote consistency, notes from regional check-in meetings were shared across regions. In a few instances, protocols were revised to address issues that arose in practice. Additional details on expert elicitation are included in Harrison et al. (in review).
Information for all cetacean species occurring in the Aleutian Islands and Bering Sea region was evaluated by the regional lead and SMEs. The regional lead oversaw the identification, delineation, and scoring of BIAs and engaged with SMEs as needed to ensure all available information and necessary expertise were included for all cetacean taxa. Each BIA was delineated only for times and areas for which direct information exists on a particular cetacean species, population, or stock. Any reliable published or unpublished information from scientific research, Indigenous or local knowledge, or community science, including both data and personal observations, were considered valid. Four types of BIAs were defined (Table 1): feeding areas (F-BIAs), reproductive areas (R-BIAs), migratory routes (M-BIAs), and small and resident populations (S-BIAs). F-BIAs, R-BIAs, and M-BIAs indicate where a substantial portion of a species “preferentially feeds”; “selectively mates, gives birth, or is found with neonates or calves”; or within which “a substantial portion” is known to migrate, and likely include less than 100% of the area and time in which the associated activity occurs. In contrast, BIA boundaries for small and resident populations aim to include 100% of the population. Geographic boundaries were delineated using a variety methods, such as geographic features (isobaths, boundaries of bays or inlets, etc.), distances to geographic features, hydrographic features, minimum convex polygons around observation points (e.g., sightings, acoustic detections, or satellite tag locations), and polygons surrounding a certain percentage of individuals engaged in a specific activity. The polygons were made as detailed and specific as possible, and depended on the quantity and quality of available information. Intentional “buffers” or other “precautionary” additions of area or time were not allowed. Similarly, predictions of potential habitat alone were insufficient to support BIA delineation. BIAs were delineated within U.S. waters; however, the BIA was not truncated if it extended past the U.S. Exclusive Economic Zone (EEZ). When a BIA spanned more than one region, region leads worked together to delineate and score the BIA as a “transboundary” BIA. Transboundary BIAs are included in only one region’s metadata, generally the region containing the larger area of the BIA.
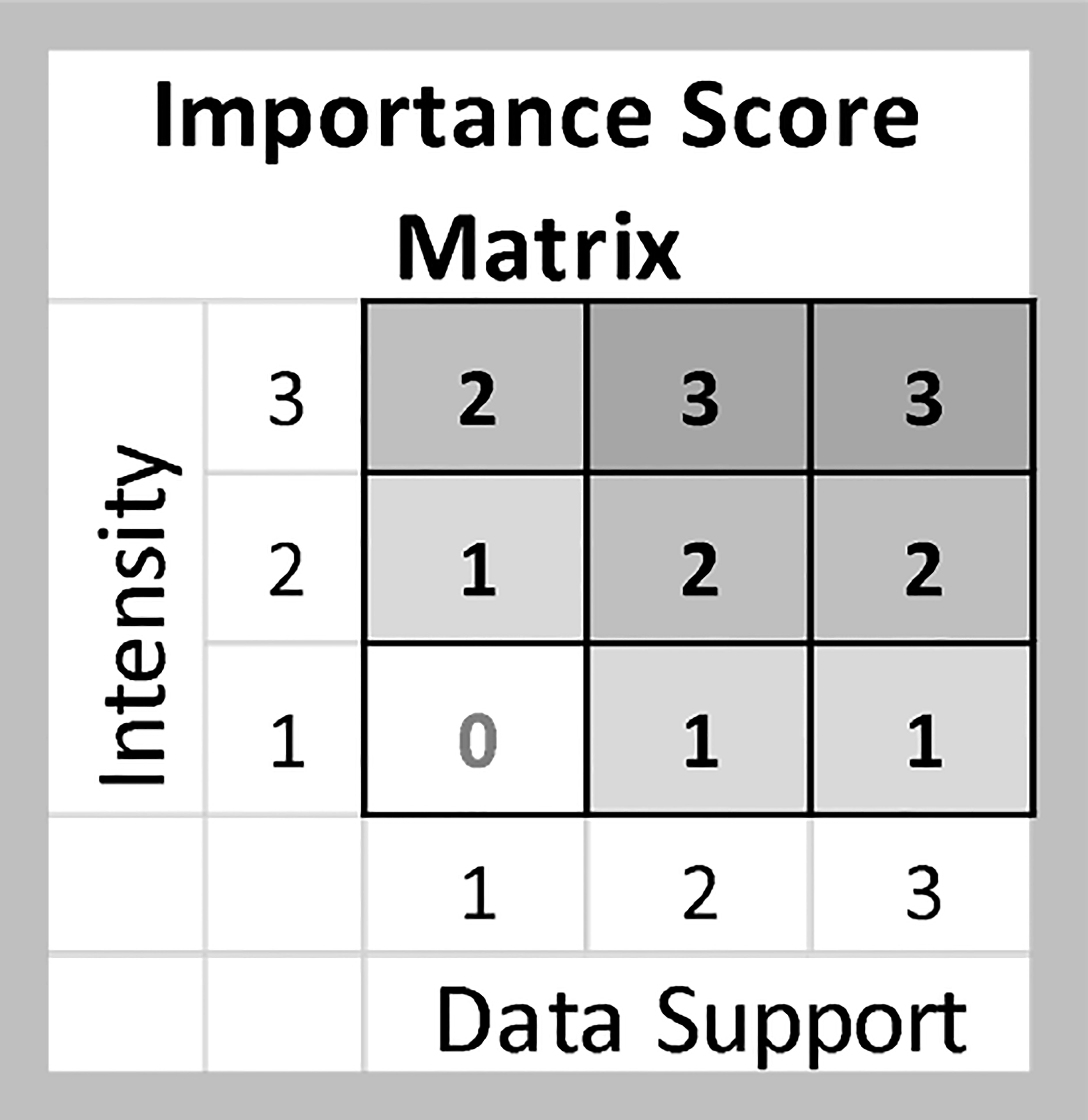
All candidate BIAs were scored and labeled using five metrics: Intensity, Data Support, Importance, Boundary Certainty, and Spatiotemporal Variability (Table 2). All scoring metrics except Spatiotemporal Variability were assigned an integer value ranging from 1 (“low”) to 3 (“high”). For each candidate BIA, Intensity and Data Support were independently scored using scoring rules specific to each BIA type. Boundary Certainty and Spatiotemporal Variability were assigned to each BIA using the same rules across BIA types, and independent of the Intensity and Data Support scores.
The Intensity score indicates the comparative significance of an area to a species in the context of the BIA type definition and the species’ range and size. This score considers factors such as abundance, density, spatial or temporal extent of use, and proportions, rates, or frequencies of relevant processes (e.g., proportion of the population that uses a migratory corridor; biomass of prey consumed per day; annual use). Intensity was scored entirely quantitatively for S-BIAs and entirely qualitatively for F-BIAs and R-BIAs. Experts could use either an entirely qualitative or partially quantitative approach for M-BIAs. Quantitative scoring criteria can be found in Harrison et al. (in review).
The Data Support score is intended to distinguish meaningful differences in the information used to support the identification of and score for the BIA. The scoring included consideration of four factors: information type, sample size, and quality and uncertainty of supporting information. To score Data Support, the available information is variable enough and presents enough possible permutations of type, sample size, quality, and uncertainty that a strict quantitative scoring system (e.g., matrix) would be challenging to construct; therefore, a qualitative approach was applied.
The Intensity and Data Support scores were combined to determine an overall Importance score using a single Importance score matrix (Figure 2) for all BIA types. Candidate BIAs with an Importance score of 0 were added to a watch list of areas for future consideration, but were not included as BIAs.
Boundary Certainty describes the degree of confidence in the location and timing of the BIA boundary. The score incorporates information about the factors that define the boundary and certainty regarding the size, location, and period of occupancy of the BIA.
Spatiotemporal variability among different areas exists across a continuum. The geographic location of some BIAs may be known, or be highly likely, to vary with time according to some periodicity (i.e., inter-annual, inter-decadal, etc.); however, in this BIA II effort, Spatiotemporal Variability was characterized using one of three descriptors: static, ephemeral, or dynamic.
The definition of a BIA unit was expanded for this BIA II process. In the simplest case, a BIA unit corresponds to a single polygon and one continuous period within which a species engages in a particular biologically important activity, or it corresponds to the range of a small and resident population. However, it is possible that multiple polygons of the same type of BIA for a species could exist in a single region and period. In that case, a cluster of BIA polygons could be delineated, scored, and labeled as a single unit, regardless of whether they share common boundaries, as long as the scores for all metrics were identical across all polygons in the cluster. Another new feature of this BIA II process was the option to identify “hierarchical” BIAs for cases in which high-resolution information are available and it is appropriate and helpful to reflect a gradation in animal use (Intensity), available information (Data Support), Boundary Certainty, or ecological characteristics (Spatiotemporal Variability) across a broader area. For example, in some cases data may support a single core area (a “child” BIA) identified within the larger “parent” BIA. In other cases, one or more clusters of identically scored polygons may appropriately be identified as child BIAs within a larger parent BIA. For R-, F-, and M-BIAs, the Intensity score for the parent BIA must be less than the highest Intensity score among the child BIAs. For S-BIAs, when hierarchical scoring is used to identify core habitat within the population’s range, the Intensity score may be the same for the core habitat (the child BIA) and the overall range (the parent BIA), as S-BIAs have quantitative scoring protocols and the parent BIA could score a 3.
Because ecosystems in the Aleutian Islands and Bering Sea region are experiencing alterations due to climate change, we did not think it appropriate to base BIAs on data that are several decades old; therefore, the oldest data considered for this BIA assessment were from ~1999-2000. The exceptions to this are two gray whale M-BIAs. We expect the spatiotemporal boundaries for migration to be less likely to change over time than for feeding or activities related to reproduction. Gray whale M-BIAs are based primarily on data from the 1970s and 80s due to lack of more recent studies or information.
3. Regional summary
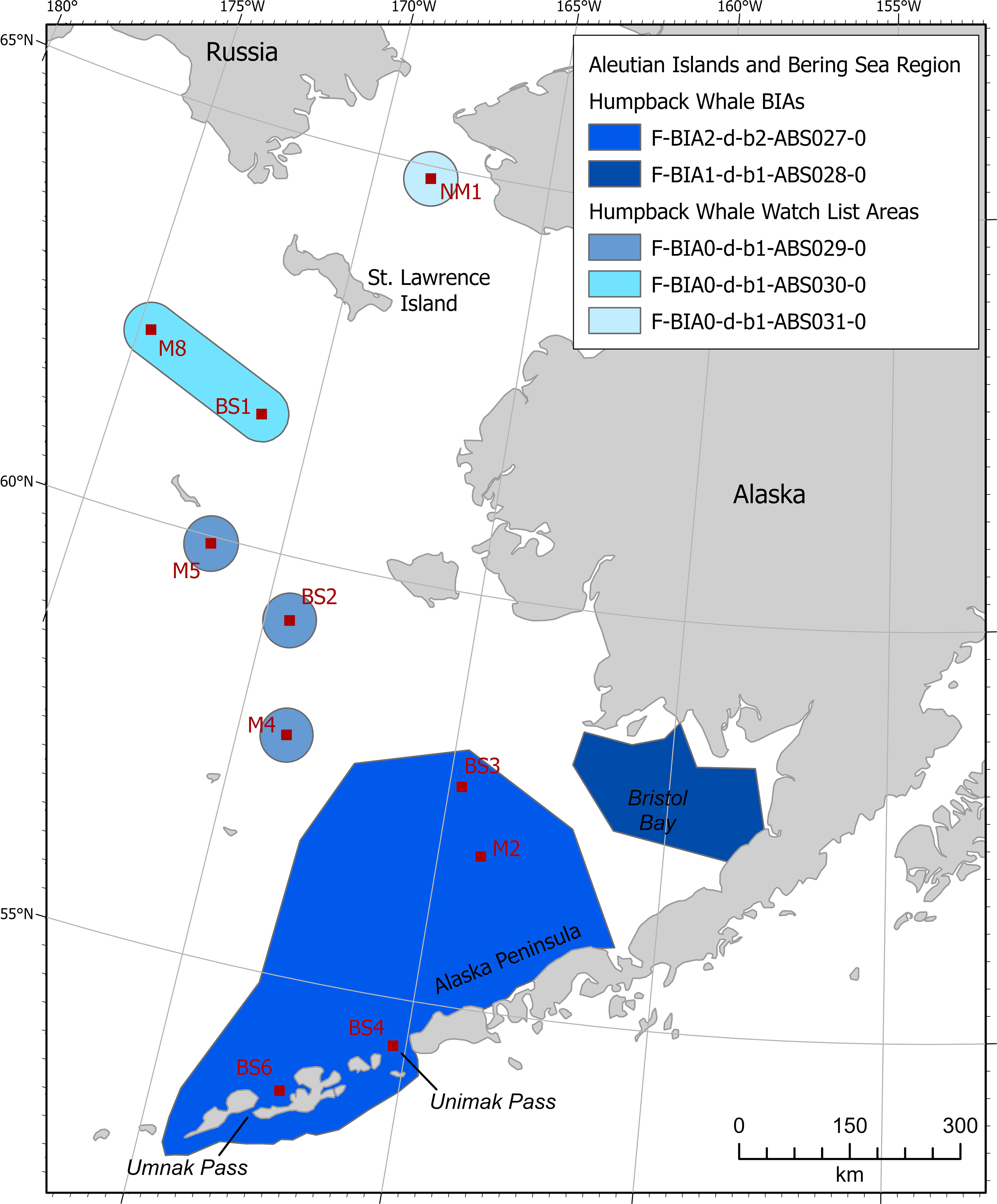
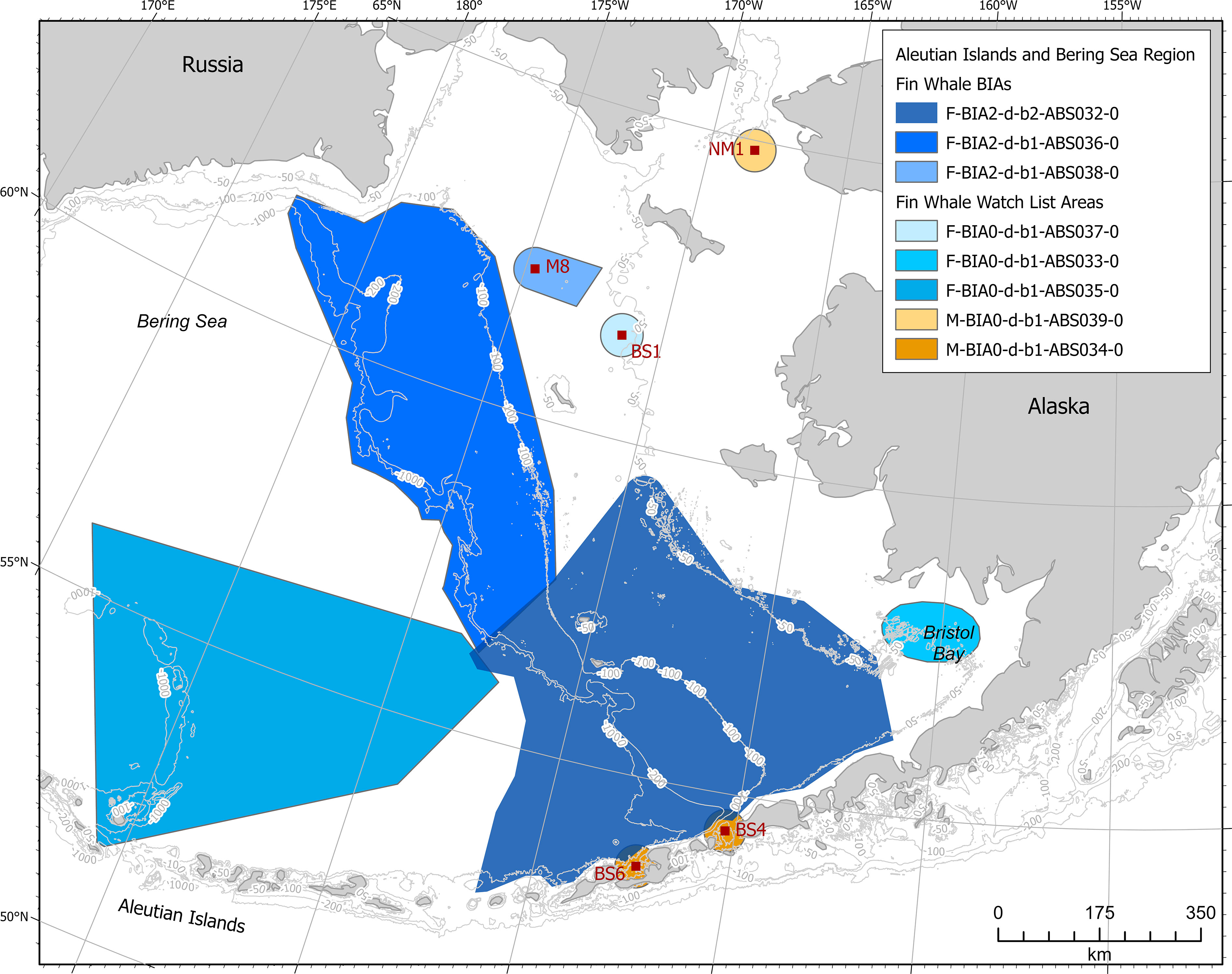
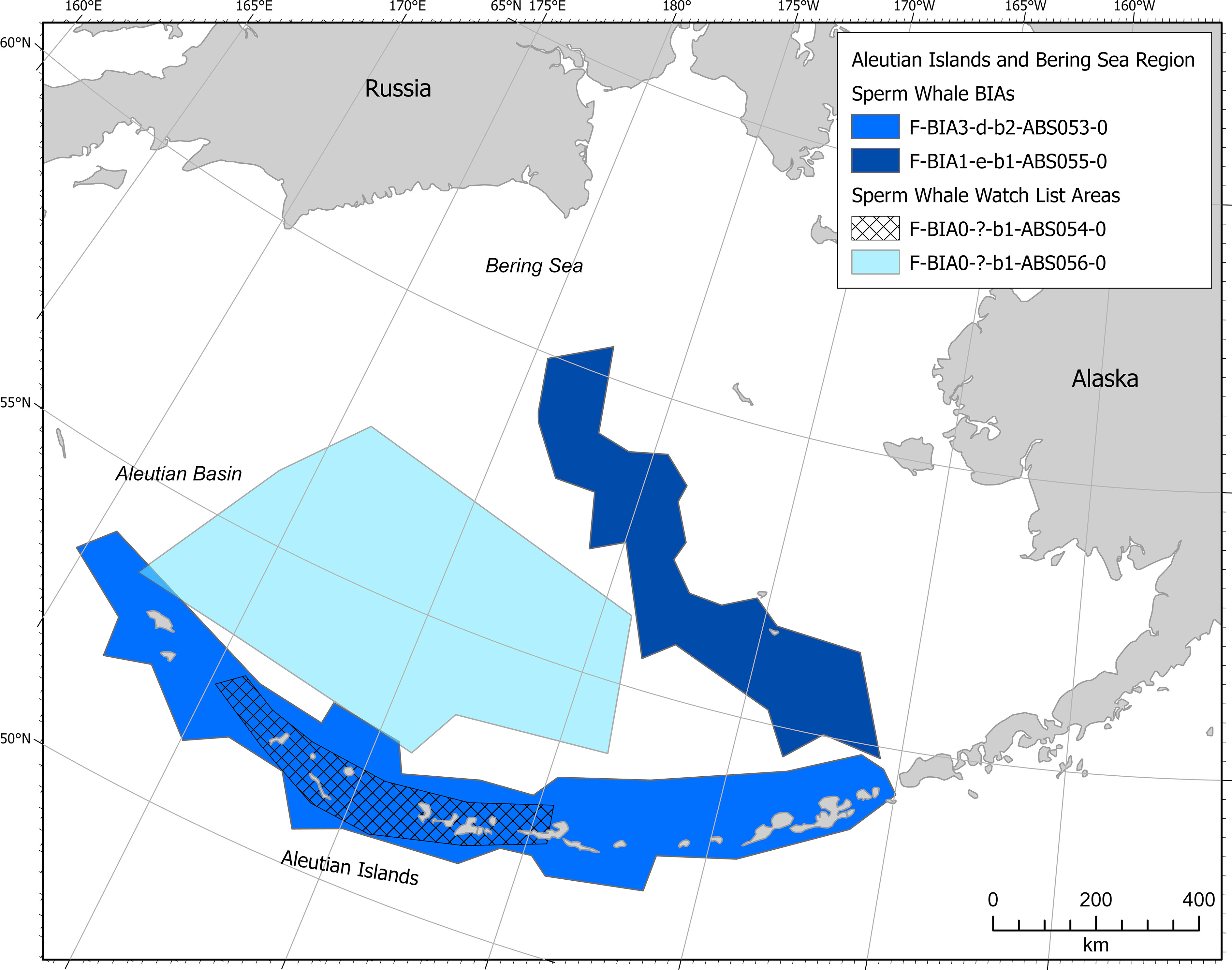
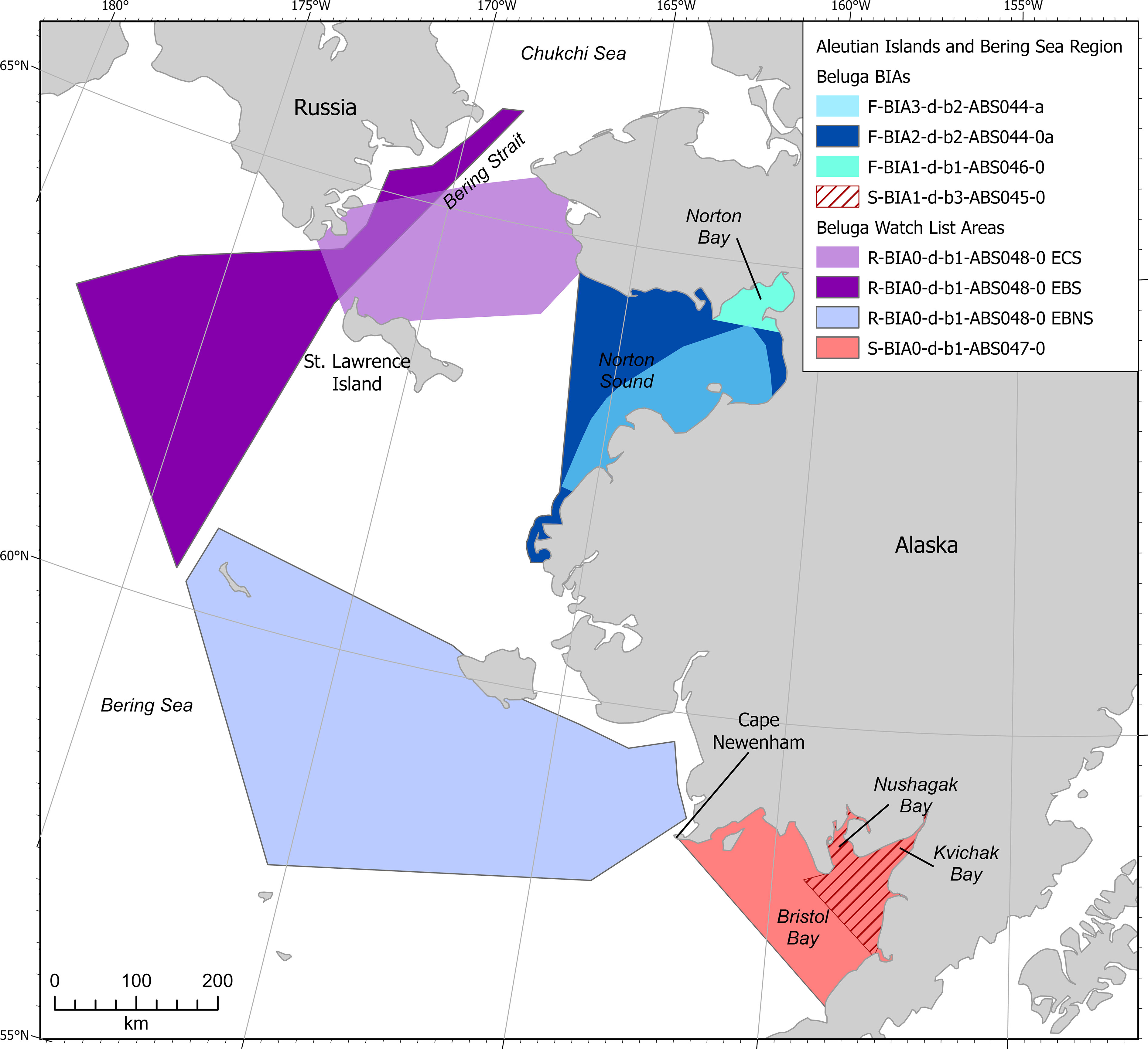
In the Aleutian Islands and Bering Sea region, a total of 19 BIAs were identified and delineated for seven species, including bowhead, gray, humpback, fin, North Pacific right, and sperm whales and belugas (Figures 3–9, Table 3). This includes one hierarchical BIA for belugas that consists of one parent and one child BIA. There are 15 feeding, 3 migratory, and 1 small and resident population BIAs; no reproductive BIAs were identified. The seasonality of BIA types is depicted in Figure 10. A summary of scores per scoring metric and summary statistics by species and BIA types can be found in Supplementary Tables 1, 2, respectively.

Table 3 Aleutian Island and Bering Sea region BIAs by species and BIA type, with general locations, scores, and designated months.
One transboundary BIA was included in the Aleutian Islands and Bering Sea region: a bowhead whale feeding BIA that extends into the Arctic region along the north coast of the Chukotka Peninsula. Within the Arctic region, six transboundary BIAs were created that extend into the Aleutian Islands and Bering Sea region (Clarke et al. in review). These include two bowhead whale spring migratory BIAs that begin in the Bering Sea and extend north to the Chukchi and Beaufort seas; one bowhead whale feeding BIA along the Chukotka Peninsula that extends south of Bering Strait; one gray whale feeding BIA along the Chukotka Peninsula that extends north and south of Bering Strait; one Eastern Chukchi Sea beluga fall migratory BIA north and south of Bering Strait; and one Beaufort Sea beluga spring migratory BIA that begins south of Bering Strait and extends north (Clarke et al. in review).
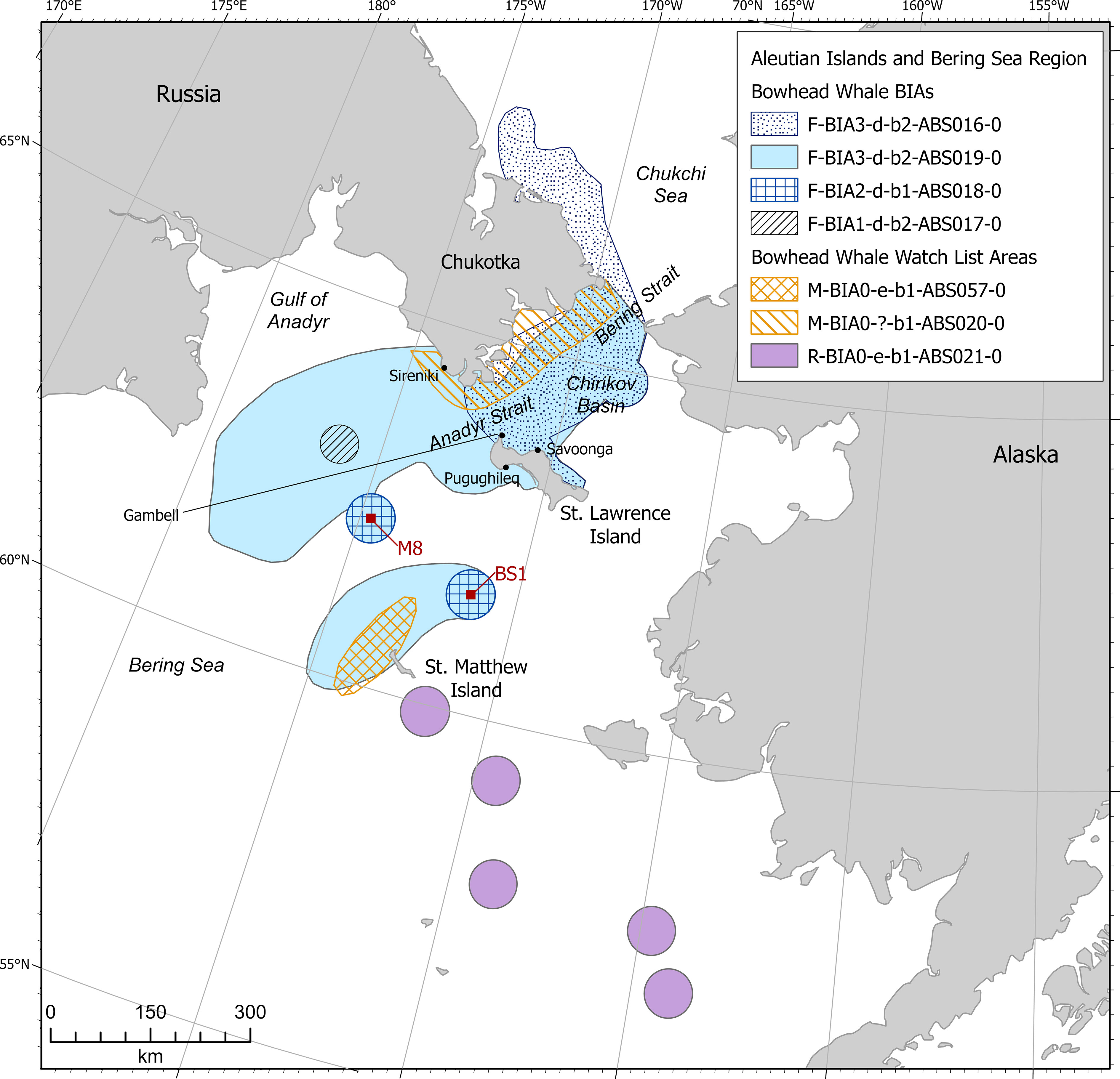
The Bering-Chukchi-Beaufort Seas (BCB) stock of bowhead whales were included in four feeding BIAs (Figure 3). These included three individual BIAs in December: 1) north and east of the Chukotka Peninsula, Bering and Anadyr straits, Chirikov Basin, and St. Lawrence Island; 2) the Gulf of Anadyr; and 3) at moorings in the northern Bering Sea. The fourth BIA was defined for January-April in Bering and Anadyr straits, Chirikov Basin, Gulf of Anadyr, St. Lawrence Island, and near St. Matthew Island. Three of the BIAs cross international boundaries with Russia.
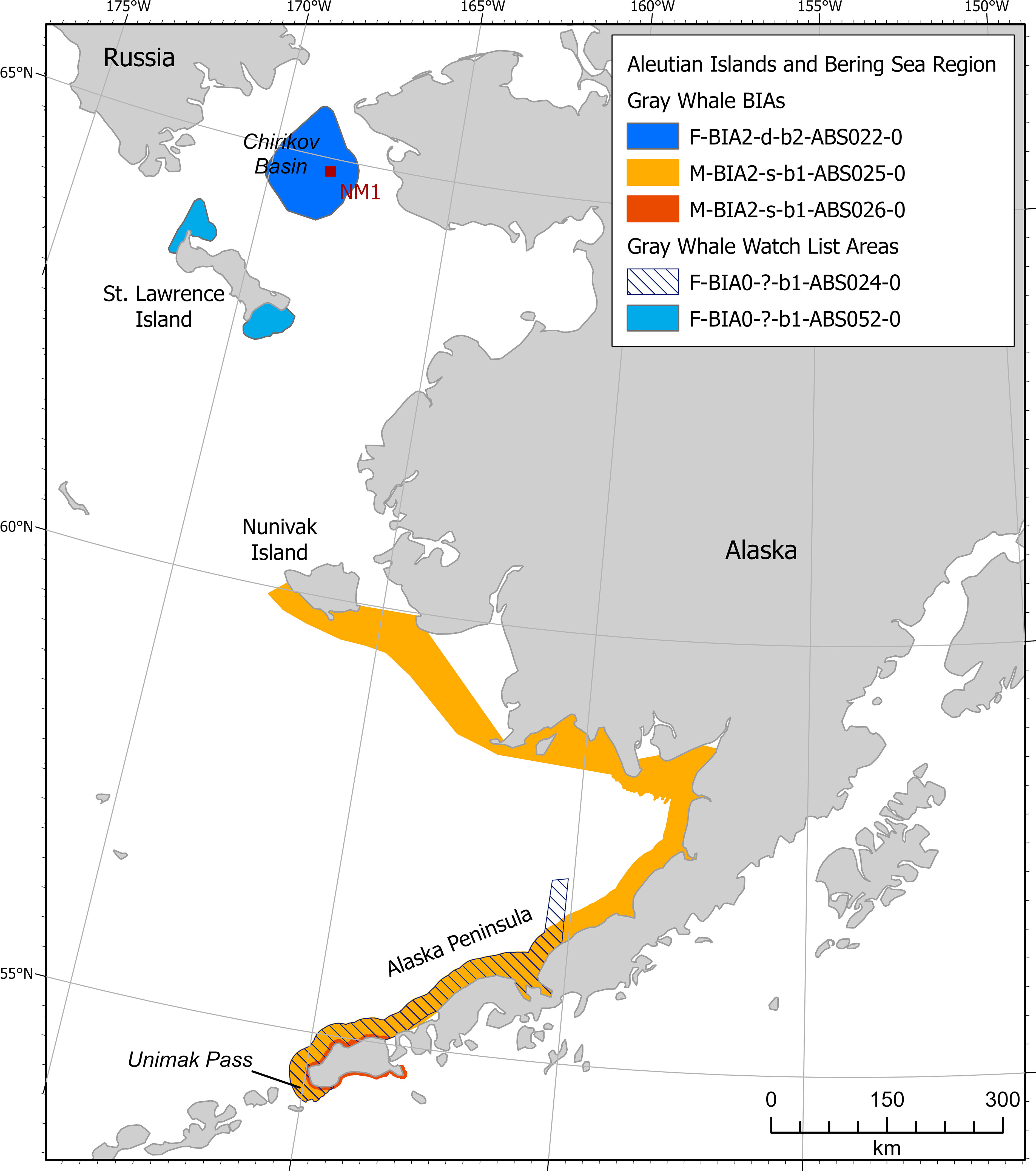
Gray whales of the Eastern North Pacific stock were included in one feeding BIA and two migratory BIAs (Figure 4). The feeding BIA is in the Chirikov Basin during late May-December. A northbound migratory BIA encompasses Unimak Pass to Nunivak Island during April-June and a southbound migratory BIA is described near Unimak Pass in November-January.
Humpback whales that occur in the Aleutian Islands and Bering Sea region can be from either the Western North Pacific or Central North Pacific stock, though these stock designations are currently being revised. Three U.S. Endangered Species Act Distinct Population Segments (DPS) occur in the Aleutian Islands and Bering Sea region; these include the Western North Pacific, Hawaii, and Mexico DPSs (Federal Register, 2016). Two feeding BIAs were defined for humpback whales (Figure 5). One BIA is located in Unimak and Umnak passes and in the North Pacific right whale critical habitat area in May-January, and the other is in Bristol Bay in June-September.
Fin whales of the Northeast Pacific stock were included in three feeding BIAs (Figure 6). One of these is located in the southeast Bering Sea for May-February, one is in the western-central Bering Sea in June-August and crosses international boundaries with Russia, and one is near a mooring in the northern Bering Sea in July-January.
North Pacific right whales of the Eastern North Pacific stock were included in one feeding BIA in the North Pacific right whale critical habitat in June-January, and one migratory BIA was identified in Unimak Pass year-round (Figure 7).
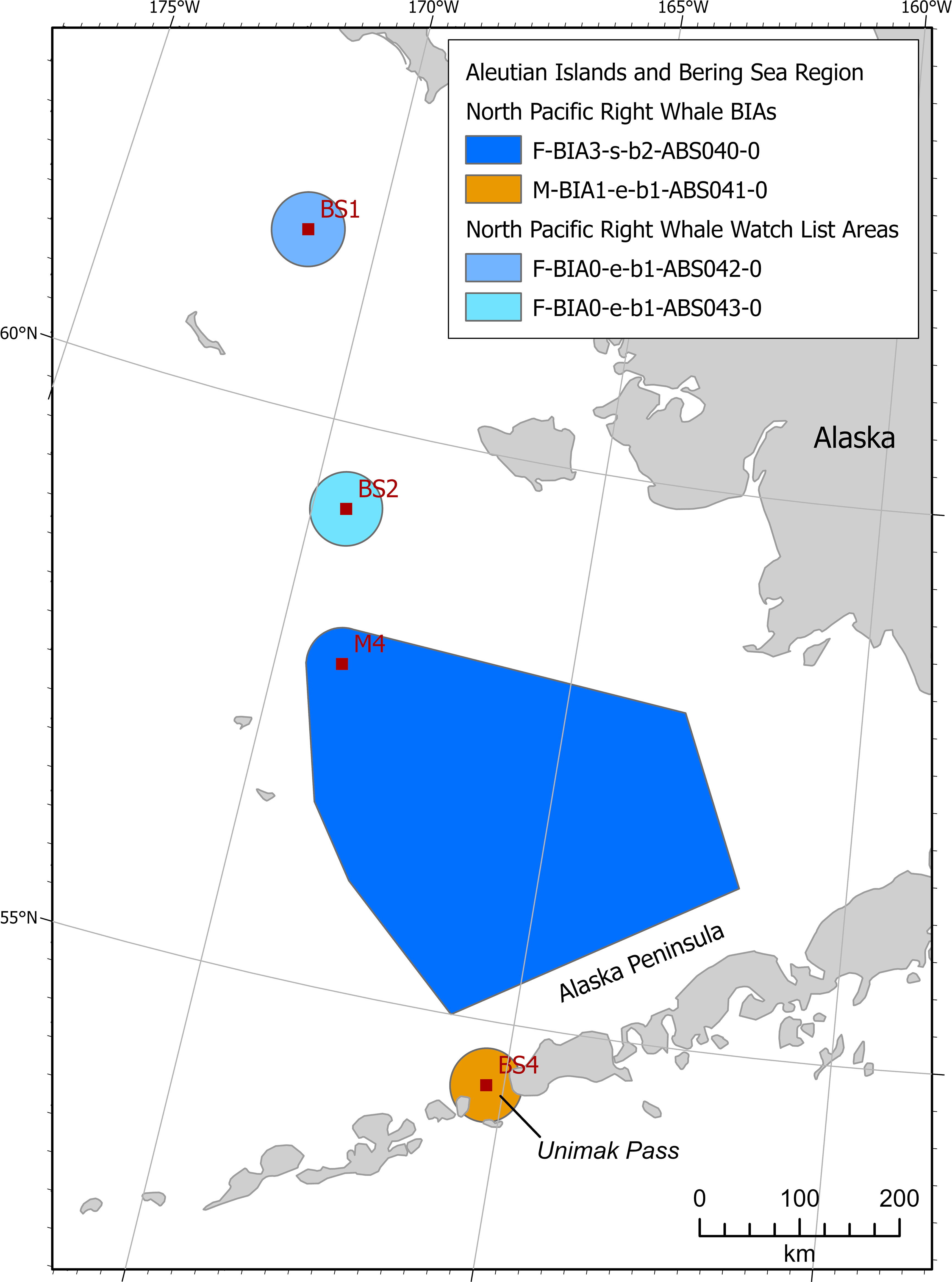
Figure 7 Aleutian Islands and Bering Sea region North Pacific right whale BIAs and watch list areas.
Sperm whales of the North Pacific stock were included in two feeding BIAs (Figure 8). One BIA is located along the Aleutian Islands in April-September and the other BIA is located along the Bering Sea slope in May-September.
Belugas of the Eastern Bering Sea stock were included in two feeding BIAs and belugas of the Bristol Bay stock were included in one small and resident population BIA (Figure 9). One of the feeding BIAs was delineated as a hierarchical BIA with one parent and one child BIA, and is located in Norton Sound in May-November. The child BIA encompasses an especially high density area where belugas congregate to feed. The second feeding BIA is non-hierarchical and is located in Norton Bay in April-May and August-October. The small and resident population BIA is for belugas of the Bristol Bay stock in Nushagak and Kvichak bays in mid-April-mid-December.
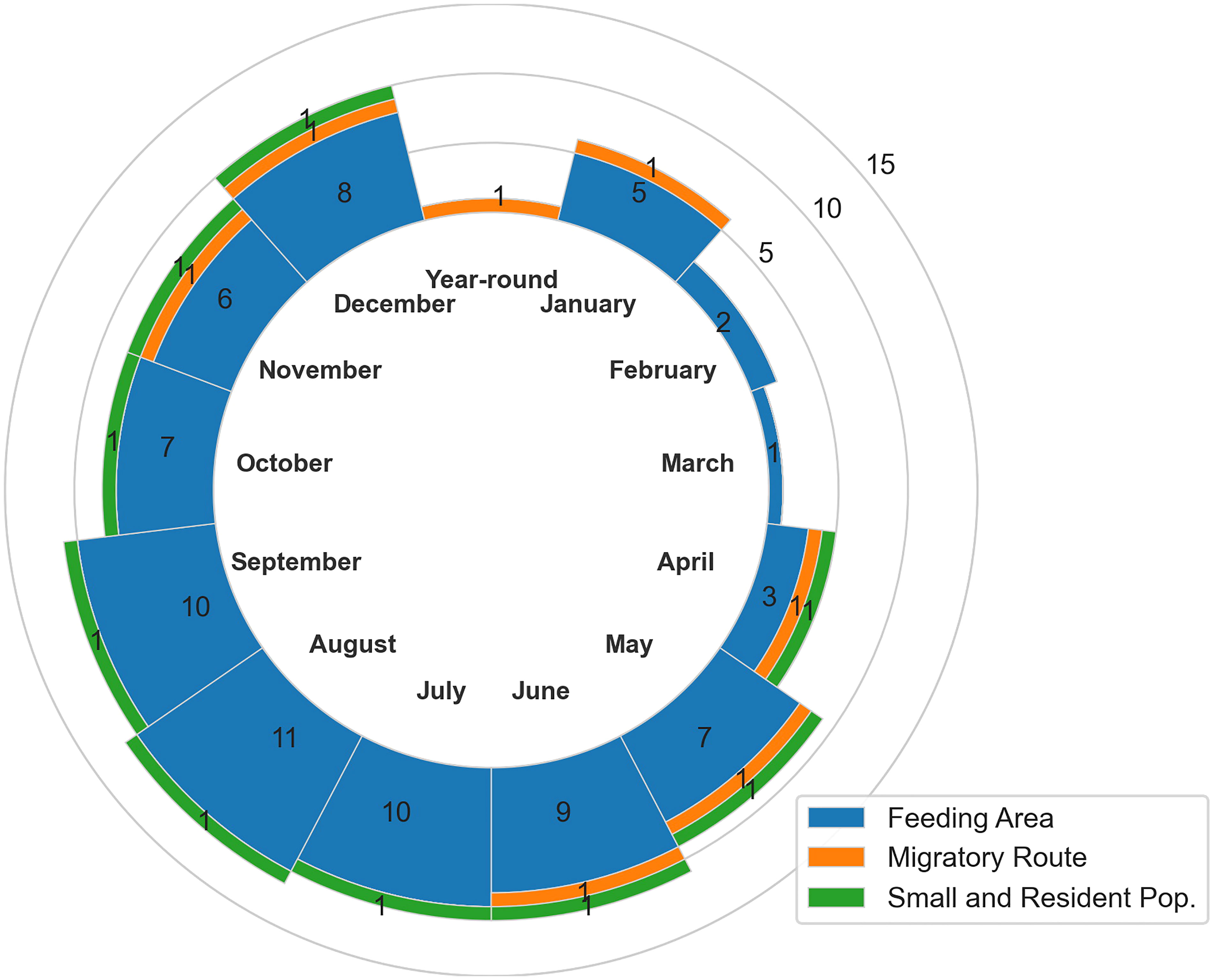
Figure 10 Aleutian Islands and Bering Sea region seasonality of BIAs by month and BIA type. The number of BIAs includes the hierarchical BIA parent only and does not include the child BIA.
4. Detailed BIA examples
Three detailed BIA case studies are provided below. For each case study, we present the life history and background information for the species, the available information sources that were used to assess candidate BIAs, the process used to delineate the BIA in space and time, and details of how each score was determined. Comprehensive metadata for every BIA is available in the Supplementary Information and BIA website.
4.1. Example 1: Bowhead whale F-BIA in the Bering and Anadyr straits, Chukotka, Gulf of Anadyr, and St. Lawrence and St. Matthew islands region, in January – April.
Importance: 3; Intensity: 3; Data Support: 2; Spatiotemporal Variability: d; Boundary Certainty: 2
F-BIA3-d-b2-ABS019-0 (Figure 11)
4.1.1. Life history and background information
Bowhead whales are endemic to the Arctic, living in and near Arctic seas year-round. They are currently listed as endangered on the U.S. Endangered Species List. The majority of BCB bowhead whales winter in the Bering Sea over the continental shelf and north of the sea ice edge (Citta et al., 2021). In spring, they migrate through the Chukchi Sea to summertime foraging areas in the Beaufort Sea and Amundsen Gulf (Citta et al., 2021). In autumn, they migrate back through the western Beaufort Sea, stopping to forage when oceanographic conditions are conducive to aggregating prey (Citta et al., 2021; Ferguson et al., 2021), through the Chukchi Sea, along the Chukotka coast where they also spend time feeding (Moore and Reeves, 1993; Moore et al., 1995), and back to the Bering Sea. Some BCB bowhead whales deviate from the stereotypical migration described above. For instance, bowhead whales were documented overwintering in the southern Chukchi Sea during winter 2017-2018 by two satellite tagged whales (Moore et al., 2021) and from moored passive acoustics (Alaska Fisheries Science Center, 2021), although the number of whales present is unknown. Indigenous knowledge, aboriginal subsistence whaling data, stable isotope analysis, and bowhead whale satellite telemetry data indicate bowhead whales also feed while on their wintering grounds in the Bering Sea (Schell and Saupe, 1993; Noongwook et al., 2007; Sheffield and George, 2013; Citta et al., 2015; Citta et al., 2021; Sheffield and George, 2021).
Bowhead whales are filter feeders and use their baleen to strain zooplankton, namely calanoid copepods, euphausiids, mysids, and amphipods (Lowry, 1993, Lowry et al., 2004; Moore et al., 2010; Sheffield and George, 2021). They need dense prey patches on productive foraging grounds to meet their energetic requirements (Lowry, 1993).
4.1.2. Information sources
Information supporting this bowhead whale F-BIA included Indigenous knowledge, satellite telemetry, visual, and acoustic data.
Satellite tags were deployed on a total of 77 bowhead whales from 2006-2018 near Point Barrow and St. Lawrence Island, U.S., and Tuktoyaktuk, Herschel Island, and Shingle Point, Canada (Citta et al., 2021). The average tag transmitted for 167 days, although 10 tags lasted more than 365 days (Citta et al., 2021). The following maps showing the highest densities of bowhead whale distribution in the Bering, Chukchi, and Beaufort seas were available: 1) per month for 2006-2019, utilizing data from 77 tagged bowhead whales (Citta et al., 2021); 2) per season, December-April and May-November, for 2006-2015, utilizing data from 46 tagged bowhead whales (Citta et al., 2018a); and 3) year-round, May 2006 – December 2012, utilizing data from 54 tagged bowhead whales (Citta et al., 2015). In the Bering Sea, tagged bowhead whales began traveling through Bering Strait during their southern migration in November. The tagged whales occurred in high densities in the region during two periods: 1) from December through April, ranging from Bering Strait through Anadyr Strait and in the Gulf of Anadyr; and 2) January through April, near St. Matthew Island (Citta et al., 2021). Dive data from tagged bowhead whales in the Gulf of Anadyr and Anadyr Strait indicated half of the whales’ time was spent at the seafloor, more so than at other depths (Citta et al., 2015; Citta et al., 2021). Two-thirds of their dives were square-shaped, where the majority of the dive duration is spent at the maximum depth of the dive (Citta et al., 2021). These bowhead whales were presumed feeding on overwintering copepods and possibly euphausiids that were in diapause and aggregating near the seafloor, where a subsurface front between cold Anadyr Water and warmer Bering Shelf Water and a strong thermocline exist (Citta et al., 2015; Citta et al., 2021). The bowhead whale spring migration corresponded with copepods ending diapause, rising from the seafloor, and dispersing (Citta et al., 2015; Citta et al., 2021).
Indigenous knowledge from St. Lawrence Island, including firsthand knowledge dating to the 1940s and earlier, indicated that bowhead whales became more abundant around St. Lawrence Island in winter. Bowhead whales feed along the north coast of the island in December-January, are seen near Gambell in December-February, and feed in spring (March and April) near Pugughileq (Southwest Cape) (Noongwook et al., 2007).
Oceana and Kawerak, Inc (2014) used the satellite tag data and Indigenous knowledge described above in a synthesis of available information sources for the Bering Strait and St. Lawrence Island region to map subsistence use (40-km buffers around whaling communities and camps) near Gambell and Savoonga in late fall and winter, and near Gambell and Pugughileq in spring. They mapped high concentration areas of bowhead whales during winter along the Chukotka, Russia, coast on the western side of Bering Strait, and between St. Lawrence Island and Chukotka. During spring, high concentration areas are shown between St. Lawrence Island and Chukotka (Oceana and Kawerak, Inc, 2014).
Stomach and fecal samples (n >15) were collected from bowhead whales taken in aboriginal subsistence hunts in the St. Lawrence Island area from November through April, 2007-2017 (Sheffield and George, 2013; Sheffield and George, 2021). These samples indicated that bowhead whales were feeding on copepods, mysids, euphausiids, shrimp, clams, and amphipods (Sheffield and George, 2013; Sheffield and George, 2021).
Shore-based counts and experienced hunter observations of migrating bowhead whales were conducted at Sireniki, on the Chukotka Peninsula, Russia, in April 1999-2001 (Melnikov et al., 2004). During the Sireniki counts, typically 1-5 bowhead whales were sighted per day, though sometimes large groups of up to 27 whales were sighted in a single day (Melnikov et al., 2004). Some whales were reported to be milling and lingering in place for long periods, indicative of feeding behavior (Melnikov et al., 2004).
Acoustic data were obtained from several moored acoustic recorders. Two recorders were deployed from September 2011 through September 2018, (BS1, between St. Lawrence and St. Matthew islands and M8, outside the Gulf of Anadyr). One recorder was deployed from September 2012 through September 2018 (NM1, Chirikov Basin). These three moorings had an ~30% duty cycle and 16 kHz sampling rate (Alaska Fisheries Science Center, 2021). Bowhead whales were present at each mooring in January-April each year, 2012-2018 (Alaska Fisheries Science Center, 2021).
Three additional acoustic recorders had an ~10-20% duty cycle and 16-48 kHz sampling rate and were deployed in Bering Strait and near Gambell and Savoonga (Chou et al., 2019). The Bering Strait mooring was deployed from September 2012 to May 2013 and recorded bowhead whale calls from November to January and mid-March to April (Chou et al., 2019). The Gambell mooring was deployed from October 2014 to July 2016 and recorded bowhead whale detections from December to April (Chou et al., 2019). The Savoonga mooring was deployed from October 2014 to June 2015 and recorded bowhead whale detections from December to April (Chou et al., 2019).
Data not included in this BIA are Aerial Surveys of Arctic Marine Mammals (https://www.fisheries.noaa.gov/alaska/marine-mammal-protection/aerial-surveys-arctic-marine-mammals) line-transect data in the Chirikov Basin and near St Lawrence Island, April through May, 1980-84. These data are ~40 years old, and considerable changes to the northern Bering Sea ecosystem due to climate change (e.g., Grebmeier et al., 2018; Huntington et al., 2020), indicate this is likely an unreliable dataset for evaluating current bowhead whale spatiotemporal distribution.
4.1.3. BIA space and time delineation process
All information sources described above indicate that bowhead whales are present and feeding in high densities from January through April, from Bering Strait through Anadyr Strait, near St. Lawrence Island, the Gulf of Anadyr, and St. Matthew Island (Figure 11). Polygons in this F-BIA are based on the satellite tag 50% density contour from (Citta et al. (2021), Fig. 4.4, January through April, 27-32 satellite tags were transmitting during January-April); St. Lawrence Island Indigenous knowledge (Noongwook et al., 2007; Oceana and Kawerak, Inc, 2014, the “Concentration” area); the Sireniki Chukotka shore station (Melnikov et al., 2004); a 37-km radius around acoustic moorings BS1, M8, and NM1 (based on an average detection range of 28-37 km per mooring), and a 20-km radius to the south of the Gambell mooring (Chou et al., 2019). The detection range for the Gambell mooring is reported as several tens of kilometers; we extended the radius around that mooring only 20 km south because bowhead whale direction from the mooring cannot be determined; additionally, from satellite tag data, we expected higher densities of bowhead whales to be north (not south) of the Gambell mooring in December.
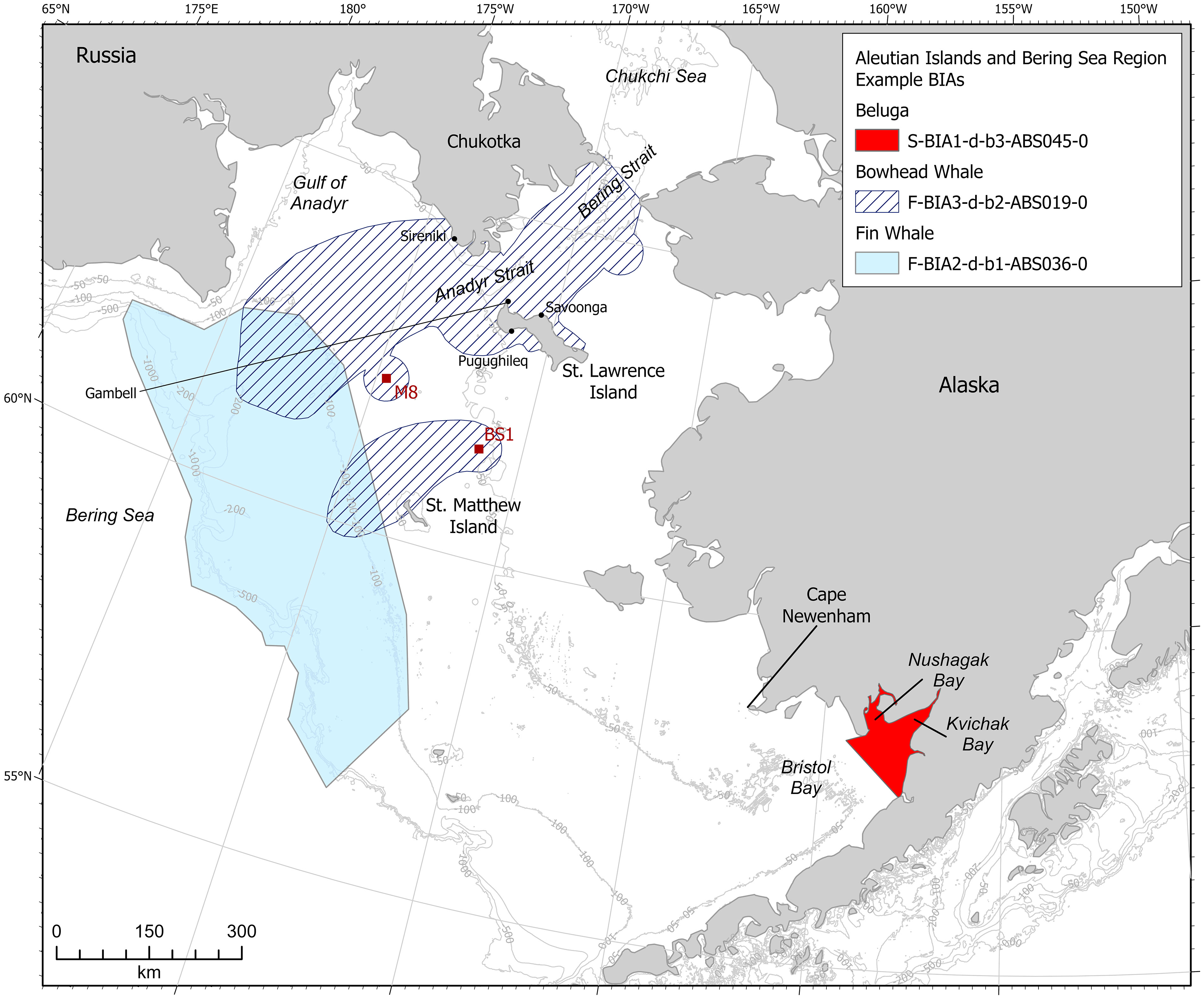
Figure 11 Aleutian Islands and Bering Sea region BIAs for bowhead whale, beluga, and fin whale that were detailed in case studies.
Bowhead whales are also present in high densities in some but not all of the same areas as this F-BIA in December. High densities of bowhead whales have been documented feeding in December on the northern side of the Chukotka Peninsula, but are not present in that area in January. Bowhead whales were not documented in high densities near the Gulf of Anadyr or St. Matthew Island in December. For these reasons, the December F-BIA could not be combined with the January-April F-BIA, and separate F-BIAs were delineated for December.
4.1.4. Score determination
We scored Intensity high (3). Data presented above indicate bowhead whale feeding in this BIA is substantial. Satellite tag data indicate consistent bowhead whale use in these areas of the Bering Sea from January through April. From satellite tag dive data, we can infer bowhead whales are feeding while on their winter grounds. St. Lawrence Island Indigenous knowledge and subsistence whaling data, including bowhead whale stomach and fecal contents, indicate bowhead whales have been feeding there for generations or longer and that their presence in winter has become more abundant. Passive acoustic moorings show a strong annual bowhead whale presence. In the Arctic region, bowhead whale F-BIAs in the eastern Beaufort Sea in summer also scored high (3) Intensity (Clarke et al. in review). These common Intensity scores do not imply that bowhead whale feeding in these F-BIAs are perfectly equal; rather, high densities of bowhead whales are feeding consistently in these areas during the designated BIA time frames, and the coarseness of the numeric scale used to score BIAs resulted in these BIAs having the same Intensity score.
We scored Data Support moderate (2). The satellite tag data are recent. However, while we assume they are representative of the population, 27-32 tags per month out of a population numbering ~16,000-17,000 (Givens et al., 2016; Givens et al., 2021) is a small proportion of the population. Satellite tag data are important because feeding behavior can be inferred from a combination of satellite tag dive behavior and zooplankton data. It is difficult to factor in acoustic data because they provide information on presence only, not behavior, density, or abundance; however, the acoustic presence is substantial. The other data sets are for smaller regions within the overall F-BIA (St. Lawrence Island & Chukotka shore station), but Indigenous knowledge is important because St. Lawrence Island residents have generations of experiential knowledge of their area.
We scored Spatiotemporal Variability as dynamic. Some of the information sources (satellite tag, Indigenous knowledge) did not provide information by year, so it is difficult to infer the level of temporal variability in these F-BIAs. Bowhead whales tend to stay north of the sea ice edge, which varies in space and time by year. Bowhead whales also tend to feed where there are dense aggregations of prey, and dynamic oceanographic factors such as currents, winds, fronts, and upwelling will affect if, when, where, and how dense those prey aggregations form.
We scored Boundary Certainty as moderate (2). Satellite tag data indicate where tagged bowhead whales are feeding during January to April. St. Lawrence Island Indigenous knowledge and subsistence whaling data go back for generations, but represent a small area within the F-BIA. The Chukotka data spanned only three years and are from 20 years ago. Acoustic data provided presence only near the moorings, not the entire F-BIA. During recent extreme winter sea ice loss in the Bering Sea, tagged bowhead whales shifted their range northward, never moving south of the marginal ice edge in winter 2018-19 (Citta et al., 2021), and were documented overwintering in the southern Chukchi Sea in winter 2017-18 by two tagged whales (Moore et al., 2021) and from passive acoustic detections (Alaska Fisheries Science Center, 2021).
4.2. Example 2: Beluga S-BIA in Bristol Bay, in mid-April – mid-December.
Importance: 1; Intensity: 1; Data Support: 3; Spatiotemporal Variability: d; Boundary Certainty: 3
S-BIA1-d-b3-ABS045-0 (Figure 11)
4.2.1. Life history and background information
Belugas are small (3-5 m long) odontocetes that are found in Arctic and sub-Arctic seas. Belugas are circumpolar, but occur in distinct populations; some of these populations migrate across vast ranges, and some are residents of a particular bay or inlet. In the Bering Sea, there are five beluga stocks that winter in discrete areas that are mostly exclusive to each other (Citta et al., 2016a). The Bristol Bay, Alaska, population of belugas is considered resident to Bristol Bay. Bristol Bay is an estuarine system and home to the largest commercial sockeye salmon fishery in the world (Tiernan et al., 2021). Research on belugas in Bristol Bay began in the 1950s (Heintzleman et al., 1955) and is ongoing, with multiple objectives to assess the following: whether belugas consume enough salmon to cause salmon stocks to decline; whether there is incidental mortality of belugas in the commercial salmon fisheries; potential impacts of proposed mining within the headwaters of Bristol Bay rivers; potential impacts of proposed oil and gas exploration and development in the Bering Sea; and population abundance, which is needed to sustainably manage the aboriginal subsistence hunt.
Bristol Bay belugas spend late spring, summer, and fall in smaller bays within the greater Bristol Bay region, including Nushagak and Kvichak bays, and associated river mouths and tributaries where they concentrate to feed on salmon and smelt fish runs migrating upriver to spawn (Citta et al., 2016b; Citta et al., 2018a; Citta et al., 2018b; Citta et al., 2019; Lowry et al., 2019). In winter, when sea ice forms in the inner bays, belugas disperse into the greater Bristol Bay region (Citta et al., 2016a; Citta et al., 2016b; Citta et al., 2018a; Lowry et al., 2019).
4.2.2. Information sources
Data Support for this beluga S-BIA included satellite telemetry, aerial survey, and genetic mark-recapture data that documented thousands of belugas in Nushagak and Kvichak bays in summer.
A total of 40 belugas were outfitted with satellite tags from 2002-2013 (Citta et al., 2016a; Citta et al., 2016b; Citta et al., 2018a; Lowry et al., 2019). These telemetry data documented Bristol Bay belugas in Nushagak and Kvichak bays from spring (16 April) through late fall/early winter (15 December). In spring (16 April – 22 June), ice in the river breaks up, rainbow smelt (Osmerus mordax) migrate upriver to spawn, and salmon smolt (Oncorhynchus spp.) begin out-migrating from rivers to the ocean. In summer (23 June – 23 July), adult sockeye salmon (Oncorhynchus nerka) begin spawning in the rivers. In fall (24 July – 31 August), pink (Oncorhynchus gorbuscha) and coho (Oncorhynchus kisutch) salmon begin spawning. In the “post-salmon season” (1 September – 15 December), most salmon runs have ended, though belugas are still present in the inner bays (Citta et al., 2016a; Citta et al., 2016b; Citta et al., 2018a; Lowry et al., 2019). In winter (15 December – 15 April), belugas have been documented farther out in Bristol Bay, though never farther than Cape Newenham, Alaska, south of the southern sea ice edge, or leaving Bristol Bay (Citta et al., 2016a; Citta et al., 2016b; Citta et al., 2018a; Lowry et al., 2019). Belugas likely move out of inner bays in winter because the rivers freeze and sea ice moves into the inner bays, potentially posing a risk of ice entrapment to belugas, and there is likely less beluga prey in the area.
Aerial line-transect surveys were conducted in July 2016 in Nushagak and Kvichak bays (Citta et al., 2019). Previous aerial surveys were also conducted in 1993, 1994, 1999, 2000, 2004, and 2005 (Lowry et al., 2008). In 2016, nine surveys were flown, with 484-1,024 belugas sighted per survey. The 2016 survey resulted in a population estimate of 2,040 (CV =0.22, 95% CI=1,541–2,702); this is the most recent population estimate (Citta et al., 2019).
Genetic mark-recapture studies were conducted in 2002-2011 (Citta et al., 2018b). Skin biopsies were collected from 516 belugas (468 from Kvichak Bay and 48 from Nushagak Bay), and there were 85 recapture events in separate years from 75 belugas, resulting in a population estimate of 1,928 (95% CI = 1,611–2,337) (Citta et al., 2018b). The authors note this should be considered a minimum estimate because it was likely that some belugas did not enter the study area during the sampling time (Citta et al., 2018b).
Information on beluga distribution, abundance, and movements in western Alaska through the 1980s (Seaman et al., 1985; Frost and Lowry, 1990) was not directly incorporated in this BIA assessment due to the availability of more recent information. The general beluga distribution and temporal movements inferred from the historical data are similar to recent data.
4.2.3. BIA space and time delineation process
Satellite tag, aerial survey, and genetic mark-recapture data described above indicate that this is a small and resident population of belugas concentrated in high densities in Nushagak and Kvichak bays from mid-April through mid-December (Figure 11). The boundary of this S-BIA is based on satellite tag data, particularly the tags per season as outlined in Citta et al. (2016b) and Lowry et al. (2019), using 100% of satellite tag locations, and aerial survey sightings (Citta et al., 2019).
From mid-December through mid-April, belugas are distributed farther out in Bristol Bay, and a winter small and resident population watch list area was created for them. That watch list area cannot be combined with this S-BIA into a year-round S-BIA because the Intensity and Data Support scores are different due to the difference in geographic range that belugas cover in the different seasons and differences in Data Support (see Intensity and Data Support sections below for more details).
4.2.4. Score determination
We scored Intensity low (1) based on an abundance score of 1 and geographic range score of 2. S-BIA Intensity is scored quantitatively based on abundance and range size (Harrison et al. in review). For abundance, this S-BIA scores low (1), in the abundance range of 501-2,000. The most recent Bristol Bay beluga abundance estimate from aerial surveys in 2016 is 2,040 individuals (Citta et al., 2019). Although that is slightly higher than the maximum of 2,000 for small and resident BIA classification, the lower bound of the 95% confidence interval on the abundance estimate is 1,541 individuals (the upper bound is 2,702 individuals). The previous abundance estimate from genetic mark-recapture methods in 2002-2011 was a minimum estimate of 1,928, with a confidence interval of 1,611–2,337 (Citta et al., 2018b). The estimated trend in abundance from aerial surveys in 1993-2005 was 4.8% increase per year over the 12-year period; however, the 2016 survey produced an estimate similar to that in 2005, suggesting the population has been stable in recent years and is not significantly increasing (Citta et al., 2019). Given the uncertainty around the most recent point estimate of 2,040, and the lower 95% confidence bound of 1,541, we consider Bristol Bay belugas to be a small and resident population.
For geographic range size, this S-BIA scores moderate (2: 2,001-10,000 km2) because the S-BIA polygon is 6,932 km2.
Data Support scored high (3) because there are ample recent satellite tag, aerial survey, and genetic mark-recapture data for this population. Satellite tag data spanned 12 years, with a relatively high number of belugas tagged (n=40). Aerial surveys were flown in 2016 and also previously in 1993, 1994, 1999, 2000, 2004, and 2005; during the 2016 surveys, many belugas were sighted on each survey (n=484-1,024). A large number of biopsies (n=516) were collected over 10 years.
Spatiotemporal Variability was scored dynamic. Bristol Bay beluga distribution and density are dependent on prey distribution and density, namely salmon and smelt runs. In spring, the timing of these runs vary by ~2-3 weeks each year depending on ice melt in the rivers. From 2002 to 2009, the first date each year that belugas were reported in the Naknek River ranged between 10-29 April. The freeze-up timing of the rivers and bays, when belugas likely start moving out of inner bays, is also highly variable per year.
Boundary Certainty scored high (3) based on high Data Support, particularly satellite tag data that provide tracks of animals wherever they go, and aerial survey data, which cover broad areas. Additionally, Bristol Bay beluga distribution has not changed perceptibly from the decades of data available prior to the data used in this BIA assessment.
The winter small and resident population watch list area (S-BIA0-d-b1-ABS047-0) cannot be combined with this S-BIA due to geographic range scoring. In winter, when beluga distribution expands into greater Bristol Bay, the range is >30,000 km2, resulting in a range score of 1. Furthermore, Data Support for the winter small and resident population watch list area (S-BIA0-d-b1-ABS047-0) consists of only satellite tag data, so it scored low (1), resulting in an Importance score of 0.
4.3. Example 3: Fin whale F-BIA in the western-central Bering Sea, in June – August.
Importance: 2; Intensity: 3; Data Support: 1; Spatiotemporal Variability: d; Boundary Certainty: 1
F-BIA2-d-b1-ABS036-0 (Figure 11)
4.3.1. Life history and background information
Fin whales are the second largest baleen whale and are found in all of the world’s oceans. They were hunted extensively by commercial whalers and are currently listed as endangered on the U.S. Endangered Species List. Fin whales have been documented in the Aleutian Islands and Bering Sea region year-round (Alaska Fisheries Science Center, 2021), although it is unknown whether any individuals remain year-round. There is evidence of seasonal movements within the region (Mizroch et al., 2009; Clapham et al., 2012; Muto et al., 2021), and some fin whales migrate through Bering Strait to feed in the Chukchi Sea in summer and fall (Clarke et al., 2013; Brower et al., 2018; Alaska Fisheries Science Center, 2021, Clarke et al., 2020). However, current information on fin whales in the region is sparse, particularly in the northern and western Bering Sea and off the continental shelf (Muto et al., 2021) and is not enough to fully describe seasonal movements. Fin whales that occur in the Bering Sea and near the Aleutian Islands are considered to be from the Northeast Pacific stock; however, it is possible that multiple stocks occur in the Bering Sea, but data are lacking to determine this (Muto et al., 2021).
Fin whales feed on small schooling fishes, squid, and crustaceans, including copepods and krill. In the eastern Bering Sea, fin whales historically consumed euphausiids of the genus Thysanoessa when over the continental shelf, and copepods of the genus Calanus in waters beyond the slope (Thompson, 1940; Nemoto, 1957; Nemoto, 1959). Fish, particularly capelin (Mallotus villosus) and juvenile pollock (Gadus chalcogrammus) were consumed over the Bering Sea shelf north of 58°N in years with low euphausiid abundance (Nemoto, 1957; Nemoto, 1959).
4.3.2. Information sources
Shipboard line-transect surveys for cetaceans were conducted during echo-integration trawl surveys for walleye pollock on the eastern Bering Sea shelf in June-July of 2002 (a warm year) and 2008 and 2010 (cold years) (Friday et al., 2013). The study area included three oceanographic domains separated by two fronts: 1) coastal domain, shore to inner front; 2) middle shelf domain, inner front to middle front; and 3) outer domain, middle front to the western edge of the study area on the eastern edge of the continental shelf (Friday et al., 2013). Locations of the fronts vary, but are generally near the 50- and 100-m isobaths, which were used to demarcate the domains in Friday et al. (2013). A salinity front also separated the outer shelf domain from the shelf break (200 m). Friday et al. (2013) combined the outer shelf domain and shelf break as the “outer stratum” because zooplankton communities in the two areas were similar and only the eastern edge of the slope was sampled (Friday et al., 2013).
Fin whales were broadly distributed in the outer stratum in 2008 and 2010, and sighting numbers were higher than in 2002 when sightings were few and found in low densities (Friday et al., 2013). Fin whale density in 2008 and 2010 was highest in the outer stratum; within the outer stratum, density was higher in the Russian section than the U.S. section (Friday et al., 2013). In 2002, density was higher in the coastal domain than the middle or outer domain; the Russian section was not surveyed (Friday et al., 2013). Fin whale abundance in all three years in U.S. waters was highest in the outer stratum; abundance estimates in 2010 (n=911) and 2008 (n=802) were higher than 2002 (n=295) (Friday et al., 2013).
Zerbini et al. (2016) used the 2008 and 2010 fin whale data from Friday et al. (2013) and fisheries data to provide a habitat baseline for fin whales based on a quantitative assessment of the relationship between fin whale abundance, environmental variables, and density of euphausiids and age-1 pollock. Modeling results indicated fin whale abundance increased at higher euphausiid biomasses and near the shelf edge at the 200-m isobath. Zerbini et al. (2016) did not find a relationship between fin whales and age-1 pollock, which is expected if fin whales are consuming pollock only in years with low euphausiid abundance (Nemoto, 1957; Nemoto, 1959).
Shipboard line-transect surveys were also conducted in the central Bering Sea in July-September 2018 (Matsuoka et al., 2019). There were 102 sightings of 153 fin whales and several acoustic detections, though most of these sightings and acoustic detections were in the south-central Bering Sea (Matsuoka et al., 2019).
Non-systematic vessel surveys occurred along the shelf break in the southeastern and central Bering Sea in July-August 2002 (LeDuc, 2004). There were 8 sightings of 20 fin whales in the western-central Bering Sea.
Data not included in this assessment are cetacean line-transect data from the pollock trawl surveys of the 1990s (Friday et al., 2012) because those data were > 20 years old and not considered representative of present conditions.
4.3.3. BIA space and time delineation process
The line-transect and non-systematic vessel sighting and acoustic data, and quantitative modeling of fin whales and their relationship to their prey, indicate that fin whales are present in high densities in the western-central Bering Sea on the continental shelf in the outer domain from the 100-m isobath to the eastern edge of the continental slope (Figure 11). The F-BIA polygon is based on fin whale sightings and acoustic detections from line-transect and non-systematic vessel surveys. Along the north side, the polygon follows just shallow of the 100-m isobath, to incorporate all of the outer domain. Along the south side, the polygon runs just deeper than the 1000-m isobath to incorporate the eastern edge of the continental shelf where the vessel line-transect study area ended (Friday et al., 2013).
4.3.4. Score determination
Intensity scored high (3). Zerbini et al.'s (2016) spatially-explicit density model suggests this is likely an important feeding area for fin whales. The model results show a higher abundance of fin whales in this F-BIA than farther to the southeast, in the southeastern Bering Sea.
Data Support scored low (1). The majority of data supporting this F-BIA (Friday et al., 2013; Zerbini et al., 2016) includes only two months (in three different years) of surveys; other surveys (LeDuc, 2004; Matsuoka et al., 2019) covered only the southwestern edge of this F-BIA. All of these sources are at least 10 years old.
Spatiotemporal Variability scored dynamic. Fin whale prey availability on the eastern Bering Sea shelf is affected by the prevailing temperature regime. The Bering Sea shelf underwent various temperature regime shifts with high interannual variability until 2000, followed by a warm period from 2001-2005, and a cold period from 2006-2013, and another warm period beginning in 2014 that is greater in magnitude and duration than that of the early 2000s (Siddon and Zador, 2017; Siddon, 2020). Sea ice cover on the eastern Bering Sea shelf determines whether it will be a warm or cold year in that region. The minimum southerly extent of sea ice can vary by 100 km each year and is affected by prevailing winds and ocean currents, particularly in spring (Stabeno et al., 2012). Winds and ocean currents also affect the location of fronts that separate the coastal, middle, and outer domains.
Boundary Certainty scored low (1). These boundaries are uncertain because of the limited spatial and temporal extent of the data. Fin whales may be present longer than the three months during which surveys were conducted and in an area greater than what was surveyed. For example, at passive acoustic mooring M8 (see ABS region fin whale F-BIA M8 Mooring, “F-BIA2-d-b1-ABS038-0”), located just northeast of this F-BIA, there were high detections of fin whale calls from July to January in each year, 2010-2018 (Alaska Fisheries Science Center, 2021). That F-BIA could not be combined with this one because that F-BIA extends to January. Another source of uncertainty is that fin whale abundance was higher in the cold years of 2008 and 2010 than in the warm year of 2002. Cetacean line-transect surveys conducted on the Bering Sea middle shelf and outer stratum in 1999, a cold year, also indicated higher fin whale abundance compared to the warm year of 2002 (Friday et al., 2012; Stabeno et al., 2012). Adult and juvenile euphausiids and Calanus spp. increased in biomass, and recruitment of pollock increased, on the southeastern Bering Sea shelf during cold years, indicating that fin whale abundance may be linked to prey availability (Stabeno et al., 2012). With the extensive warming that the Bering Sea has been undergoing, particularly since the extreme loss of winter sea ice in 2017-18, it is possible that fin whales’ and their prey’s spatiotemporal distribution and density could be changing.
5. Watch list areas
A total of 22 watch list areas were identified and delineated for 10 species, including bowhead, gray, humpback, fin, minke, North Pacific right, and sperm whales; belugas; and harbor and Dall’s porpoises (Figures 3–9, 12; Supplementary Table 3). There are 15 feeding, 4 migratory, 2 reproductive, and 1 small and resident population watch list areas. One minke whale feeding watch list area is transboundary with the Arctic region. All watch list areas scored the lowest score of 1 in both the Intensity and Data Support categories, which resulted in Importance scores of 0. All watch list areas also received the lowest score for Boundary Certainty due to lack of information. For Spatiotemporal Variability, 13 watch list areas scored dynamic, 4 ephemeral, and 0 static, and 5 watch list areas received no Spatiotemporal Variability score because there was not enough information to determine a score.
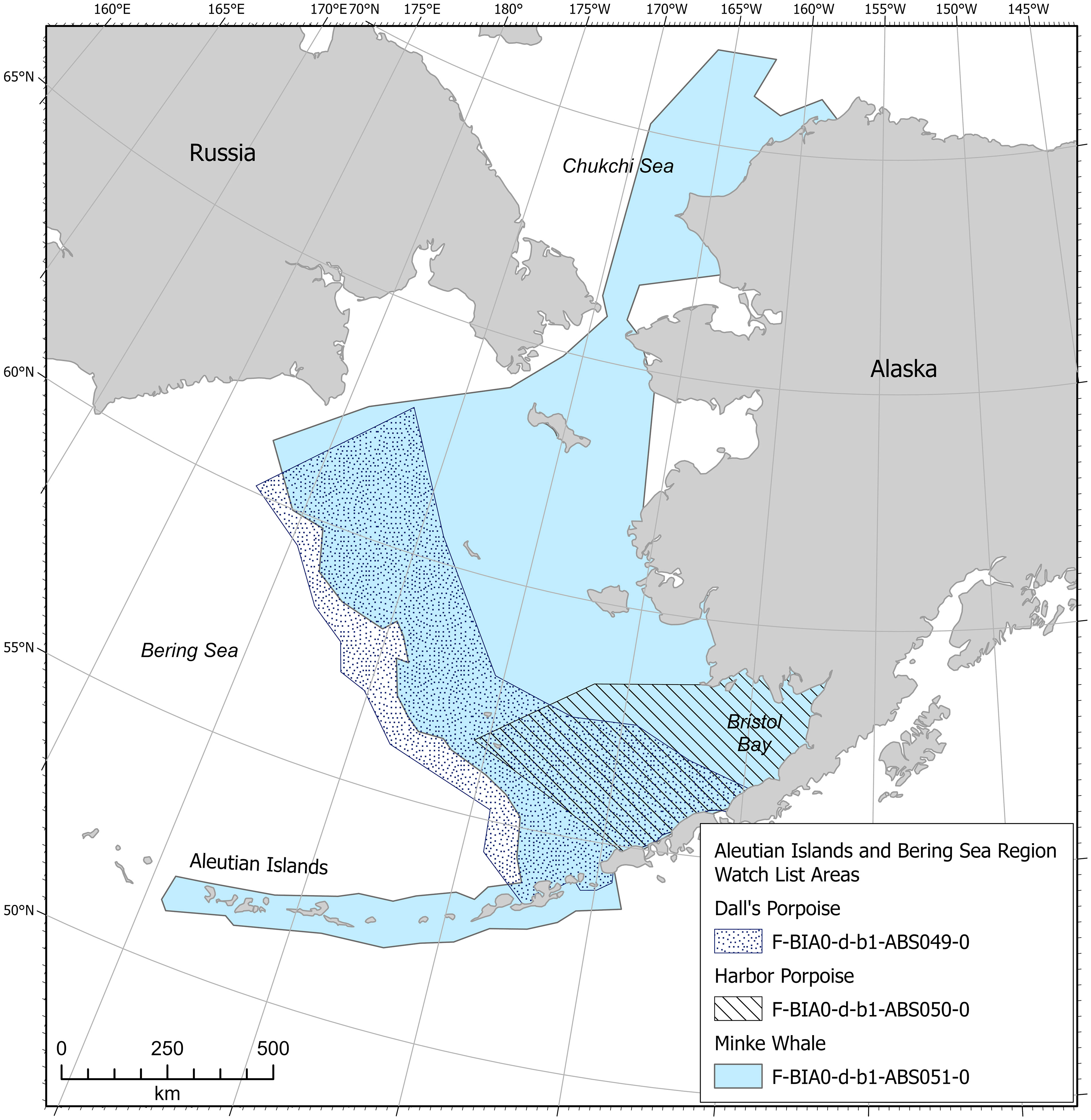
Figure 12 Aleutian Islands and Bering Sea region minke whale, harbor porpoise, and Dall’s porpoise watch list areas.
The species represented in the watch list areas for the Aleutian Islands and Bering Sea region included all seven species for which BIAs were created, plus three additional species. This region is data poor, with significant areas and periods that have had no dedicated cetacean surveys conducted in recent years. Throughout the region, there is sparse information on cetacean presence, density, and behavior. To better evaluate these watch list areas, more and current data are needed in all months and in all areas of the Aleutian Islands and Bering Sea region. Shipboard line-transect surveys of the vast Bering Sea would help obtain spatiotemporal distribution and density of all cetaceans in the area. Aerial line-transect surveys collecting data on all cetaceans could be conducted in smaller regions such as Bristol Bay. Satellite tag deployments on additional species would provide important data on migration timing and habitat use. Experienced and dedicated marine mammal observers could be included on other research cruises that operate in the area to obtain opportunistic sighting data. Finally, unmanned aerial systems could be used in remote areas to collect visual data. Regions that are expected to be used most heavily by humans with potential for anthropogenic impact to cetaceans, such as the Bering Strait and proposed shipping lanes, would benefit from focused survey effort to obtain baseline cetacean distribution, density, and abundance data.
6. Conclusions and recommendations
During the BIA II process, 19 BIAs were identified and delineated in the Aleutian Islands and Bering Sea region, including: feeding areas for bowhead, gray, humpback, fin, North Pacific right, and sperm whales, and belugas; migratory routes for gray and North Pacific right whales; and a small and resident population of belugas. In addition, 22 watch list areas were identified and delineated, including: feeding areas for gray, humpback, fin, minke, North Pacific right, and sperm whales, and harbor and Dall’s porpoises; migratory routes for bowhead and fin whales, reproductive areas for bowhead whales and belugas; and a small and resident population of belugas.
With the addition of information on cetaceans that was made available after the BIA I effort, we were able to better delineate BIA areas and time periods to reflect current cetacean use of this region, at times expanding the time period into winter months due to the addition of year-round acoustic data. Expanding BIAs beyond the U.S. EEZ into adjacent nation EEZs (e.g., Russia) and into international waters allowed for a more accurate account of the areas that cetaceans are using.
The Aleutian Islands and Bering Sea region is vast, remote, harsh, and difficult to study; consequently, spatiotemporal information on cetacean distribution, density, and habitat use is relatively limited. For this BIA II assessment, we considered all available information and incorporated all that was relevant. However, there may be areas and times where a species occurs in high density and engages in biologically important activities that were not identified here due to lack of available information. A portion of the seasonality of BIAs in the Aleutian Islands and Bering Sea region is depicted in Figure 10. Few BIAs were identified in the late winter/early spring months, which may be partly due to limited information during those months.
Too little information was available to consider creating even watch list areas for a number of additional cetacean species that inhabit the Aleutian Islands and Bering Sea region. These species include killer, sei (Balaenoptera borealis), Baird’s beaked (Berardius bairdii), and Stejneger’s beaked (Mesoplodon stejnegeri) whales, and Pacific white-sided dolphins. In future BIA assessments, information for these species should be evaluated to determine whether sufficient new information is available to delineate and score BIAs.
Climate change continues to cause rapid changes in the ecosystems of the Aleutian Islands and Bering Sea. Some species are expanding their ranges northward (Brower et al., 2018; Siddon and Zador, 2018; Eisner et al., 2020), unusual mortality events have occurred (Siddon and Zador, 2018; Siddon and Zador, 2019; Huntington et al., 2020; Siddon, 2020), and there is the potential for a complete restructuring of the northern Bering Sea from a benthic-dominated to a pelagic-dominated system (Grebmeier et al., 2006; Grebmeier et al., 2018). These, and many other changes, suggest that BIAs be reassessed and updated every 4-5 years.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: AFSC/MML: Acoustics long-term passive monitoring using moored autonomous recorders in the Bering, Chukchi, and Western Beaufort Seas, 2007-2021: https://www.fisheries.noaa.gov/inport/item/17343 Distributed Biological Observatory: https://arcticdata.io doi:10.18739/A26T0GX06.
Author contributions
MF, JC, and AB identified and compiled available information sources. MF, as part of the BIA planning team, provided guidance on BIA delineation and scoring methods. AB delineated and scored BIAs, with guidance and input from MF and JC. AB wrote the manuscript with contributions by MF and review by MF and JC. EF created the BIA Tool website and summary tables. SD created BIA maps. All authors contributed to the article and approved the submitted version.
Acknowledgments
We are grateful to regional experts that provided information and reviews for the Aleutian Islands and Bering Sea region: Jessica Crance, Sue Moore, Gay Sheffield, Kim Shelden, Kate Stafford, and Paul Wade. The BIA II Working Group leads: Corrie Curtice, Jolie Harrison, Leslie New, and Sofie Van Parijs provided direction and guidance throughout the entire BIA II process. Paul Irvin of the Alaska Fisheries Science Center Graphics Dept. created Figure 1.
Funding
This work was completed through a collaboration between NOAA and the U.S. Navy to better describe areas and time periods in which cetacean populations are known to concentrate for breeding, feeding, and migration, and areas within which small and resident populations occupy a limited geographic extent.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.1055398/full#supplementary-material
References
Aksenov Y., Popova E. E., Yool A., Nurser A. J. G., Williams T. D., Bertino L., et al. (2017). On the future navigability of Arctic sea routes: High-resolution projections of the Arctic Ocean and sea ice. Mar. Policy. 75, 300–317.
Alaska Fisheries Science Center (2021) AFSC/NMML: Acoustics long-term passive monitoring using moored autonomous recorders in the Bering, Chukchi, and Western Beaufort Seas 2007-2012. Available at: https://www.fisheries.noaa.gov/inport/item/17343.
Ashjian C. A., Braund S. R., Campbell R. G., George J. C., Kruse J., Maslowski W., et al. (2010). Climate variability, oceanography, bowhead whale distribution, and iñupiat subsistence whaling near Barrow, Alaska. Arctic. 63, 179–194.
Blackwell S. B., Nations C. S., McDonald T. L., Greene C. R. Jr., Thode A. M., Guerra M., et al. (2013). Effects of airgun sounds on bowhead whale calling rates in the alaskan Beaufort Sea. Mar. Mamm. Sci. 29:4, E342–E365.
Bluhm B. A., Coyle K. O., Konar B., Highsmith R. (2007). High gray whale relative abundances associated with an oceanographic front in the south-central chukchi Sea. Deep-Sea Res. Pt. II. 54, 2919–2933.
Brower A. A., Clarke J. T., Ferguson M. C. (2018). Increased sightings of subarctic cetaceans in the eastern Chukchi Sea 2008-2016: population recovery, response to climate change, or increased survey effort? Polar Biol. 41, 1033–1039. doi: 10.1007/s00300-018-2257-x
Chou E., Antunes R., Sardelis S., Stafford K., West L., Spagnoli C., et al. (2019). Seasonal variation in Arctic marine mammal acoustic detection in the northern Bering Sea. Mar. Mamm. Sci. 36:2, 522–547. doi: 10.1111/mms.12658
Citta J. J., Frost K. J., Quakenbush L. (2019). Aerial surveys of Bristol bay beluga whales, Delphinapterus leucas, in 2016. Mar. Fish. Rev. 81:3-4, 98–104. doi: 10.7755/MFR.81.3–4.5
Citta J. J., Lowry L. F., Quakenbush L. T., Kelly B. P., Fischbach A. S., London J. M., et al. (2018a). A multi-species synthesis of satellite telemetry data in the Pacific Arctic, (1987-2015): Overlap of marine mammal distributions and core use areas. Deep-Sea Res. Pt. II 152, 132–153.
Citta J. J., O’Corry-Crowe G., Quakenbush L. T., Bryan A. L., Ferrer T., Hobbs R. C., et al. (2018b). Assessing the abundance of Bristol bay belugas with genetic mark-recapture methods. Mar. Mamm. Sci. 34:3, 666–686. doi: 10.1111/mms.12472
Citta J. J., Quakenbush L. T., Frost K. J., Lowry L. (2016b). Movements of beluga whales (Delphinapterus leucas) in Bristol bay, Alaska. Mar. Mamm. Sci. 32:4, 1272–1298. doi: 10.1111/mms.12337
Citta J. J., Quakenbush L., George J. C. (2021). “Distribution and behavior of Bering-Chukchi-Beaufort bowhead whales as inferred by telemetry,” in The bowhead whale balaena mysticetus: Biology and human interactions. Eds. George J. C., Thewissen J. G. M. (London, United Kingdom: Academic Press), 31–51.
Citta J. J., Quakenbush L. T., Okkonen S. R., Druckenmiller M. L., Maslowski W., Clement-Kinney J., et al. (2015). Ecological characteristics of core areas used by Bering-Chukchi-Beaufort (BCB) bowhead whales 2006-2012. Progr. Oceanogr. 136, 201–222.
Citta J. J., Richard P., Lowry L. F., O’Corry-Crowe G., Marcoux M., Suydam R., et al. (2016a). Satellite telemetry reveals population specific winter ranges of beluga whales in the Bering Sea. Mar. Mamm. Sci. 33:1, 236–250. doi: 10.1111/mms.12357
Clapham P. J., Kennedy A. S., Rone B. K., Zerbini A. N., Crance J. L., Berchok C. L. (2012). North pacific right whales (Eubalaena japonica) in the southeastern Bering Sea (Final report, OCS study BOEM 2012-074) (Seattle, WA: National Marine Mammal Laboratory, Alaska Fisheries Science Center, National Oceanic and Atmospheric Administration).
Clarke J. T., Brower A. A., Ferguson M. C., Willoughby A. L., Rotrock A. D. (2020). Distribution and relative abundance of marine mammals in the eastern Chukchi Sea, eastern and western Beaufort Sea, and Amundsen Gulf 2019. Annual Report (Seattle, WA, United States: Marine Mammal Laboratory, Alaska Fisheries Science Center, NMFS, NOAA), 603 pp.
Clarke J. T., Ferguson M. C., Brower A. A., Fujioka E., DeLand S. Biologically important areas II for cetaceans in the Arctic region. Front. Mar. Sci. (in review).
Clarke J. T., Stafford K., Moore S. E., Rone B., Aerts L., Crance J. (2013). Subarctic cetaceans in the southern chukchi Sea: Evidence of recovery or response to a changing ecosystem. Oceanogr 26:4, 136–149. doi: 10.5670/oceanog.2013.81
Coachman L. K. (1986). Circulation, water masses, and fluxes on the southeastern Bering Sea shelf. Continent. Shelf Res. 5:1–2, 23–108. doi: 10.1016/0278-4343(86)90011-7
Eisner L. B., Zuenko Y. I., Basyuk E. O., Britt L. L., Duffy-Anderson J. T., Kotwicki S., et al. (2020). Environmental impacts on walleye pollock (Gadus chalcogrammus) distribution across the Bering Sea shelf. Deep-Sea Res. Pt. II. 181-182, 104881. doi: 10.1016/j.dsr2.2020.104881
Federal Register (2016). Endangered and threatened species; identification of 14 distinct population segments of the humpback whale (Megaptera novaeangliae) and revision of species-wide listing. (U.S. National Oceanic and Atmospheric Administration: Federal Register) 81 (174), 62260–62319.
Ferguson M. C., Clarke J. T., Brower A. A., Willoughby A. L., Okkonen S. R. (2021). “Ecological variation in the western Beaufort Sea,” in The bowhead whale balaena mysticetus: Biology and human interactions. Eds. George J. C., Thewissen J. G. M. (London, United Kingdom: Academic Press), 365–379.
Ferguson M. C., Waite J. M., Curtice C., Clarke J. T., Harrison J. (2015). “Biologically important areas for cetaceans within U.S. waters – Aleutian islands and Bering Sea region,” in Biologically important areas for cetaceans within U.S. waters, vol. 41:1 . Eds. Van Parijs S. M., Curtice C., Ferguson M. C.. Aquat. Mamm. 41 (1), 79–93.
Frey K. E., Moore G. W. K., Cooper L. W., Grebmeier J. M. (2015). Divergent patterns of recent sea ice cover across the Bering, chukchi and Beaufort seas of the pacific Arctic region. Prog. Oceanogr 136, 32–49. doi: 10.1016/j.pocean.2015.05.009
Friday N. A., Waite J. M., Zerbini A. N., Moore S. E. (2012). Cetacean distribution and abundance in relation to oceanographic domains on the eastern Bering Sea shelf: 1999-2004. Deep-Sea Res. Pt. II. 65-70, 260–272.
Friday N. A., Zerbini A. N., Waite J. M., Moore S. E., Clapham P. J. (2013). Cetacean distribution and abundance in relation to oceanographic domains on the eastern Bering Sea shelf, June and July of 2002, 2008, and 2010. Deep-Sea Res. Pt. II 94, 244–256. doi: 10.1016/j.dsr2.2013.03.011
Frost K. J., Lowry L. F. (1990). Distribution, abundance and movements of beluga whales, Delphinapterus leucas, in coastal waters of western Alaska. Fish. Aquat. Sci. 224, 39–57.
Givens G. H., Edmondson S. L., George J. C., Suydam R., Charif R. A., Rahaman A., et al. (2016). Horvitz-Thompson whale abundance estimation adjusting for uncertain recapture, temporal availability variation, and intermittent effort. Environmetrics 27, 134–146. doi: 10.1002/env.2379
Givens G. H., George J. C., Suydam R., Tudor B., Von Duyke A., Person B., et al. (2021). Correcting the 2019 survey abundance of Bering-Chukchi-Beaufort seas bowhead whales for disturbance from powered skiffs. Paper SC/68C/ASI/01 presented to the IWC Scientific Committee, [virtual meeting] 18 pp.
Grebmeier J. M. (2012). Shifting patterns of life in the pacific Arctic and sub-Arctic seas. Annu. Rev. Mar. Sci. 4, 63–78. doi: 10.1146/annurev-marine-120710-100926
Grebmeier J. M., Bluhm B. A., Cooper L. W., Danielson S. L., Arrigo K. R., Blanchard A. L., et al. (2015). Ecosystem characteristics and processes facilitating persistent macrobenthic biomass hotspots and associated benthivory in the pacific Arctic. Prog. Oceanogr. 136, 92–114. doi: 10.1016/j.pocean.2015.05.006
Grebmeier J. M., Cooper L. W., Feder H. M., Sirenko B. I. (2006). Ecosystem dynamics of the pacific-influenced northern Bering and chukchi seas in the amerasian Arctic. Prog. Oceanogr. 71, 331–361. doi: 10.1016/j.pocean.2006.10.001
Grebmeier J. M., Frey K. E., Cooper L. W., Kędra M. (2018). Trends in benthic macrofaunal populations, seasonal sea ice persistence, and bottom water temperatures in the Bering strait region. Oceanogr. 31:2, 136–151. doi: 10.5670/oceanog.2018.224
Grebmeier J. M., McRoy C. P., Feder H. M. (1988). Pelagic-benthic coupling on the shelf of the northern Bering and chukchi seas. i. food supply source and benthic biomass. Mar. Ecol. Prog. Ser. 48, 57–67.
Guarino M. V., Sime L. C., Schroeder D., Malmierca-Vallet I., Rosenblum E., Ringer M., et al. (2020). Sea-Ice-free Arctic during the last interglacial supports fast future loss. Nat. Clim. Change. 10, 928–932.
Harrison J., Ferguson M. C., New L., DeLand S., Fujioka E., Curtice C., et al. Biologically important areas for cetaceans: Updates and the application of a new scoring system. Front. Mar. Sci. (in review).
Heintzleman B. F., Wakefield J. H., Anderson C. L. (1955). 1955 annual report no. 7. (Juneau, Alaska: Alaska fisheries board and Alaska department of fisheries), 1–152. Available at: https://www.arlis.org/docs/vol1/A/31110164etc/31110164etc-1955.pdf.
Huntington H. P. (2009). A preliminary assessment of threats to arctic marine mammals and their conservation in the coming decades. Mar. Policy. 33, 77–82.
Huntington H. P., Daniel R., Hartsig A., Harun K., Heiman M., Meehan R., et al. (2015). Vessels, risks, and rules: planning for safe shipping in Bering strait. Mar. Policy. 51, 119–127.
Huntington H. P., Danielson S. L., Wiese F. K., Baker M., Boveng P., Citta J. J., et al. (2020). Evidence suggests potential transformation of the pacific Arctic ecosystem is underway. Nat. Clim. Change. 10, 342–348. doi: 10.1038/s41558-020-0695-2
Kachel N. B., Hunt G. L. Jr., Salo S. A., Schumacher J. D., Stabeno P. J., Whitledge T. E. (2002). Characteristics and variability of the inner front of the southeastern Bering Sea. Deep-Sea Res. Pt. II. 49, 5889–5909.
Koenigk T., Brodeau L., Grand Graversen R., Karlsson J., Svensson G., Tjernstrom M., et al. (2013). Arctic Climate change in 21st century CMIP5 simulations with EC-earth. Clim. Dyn. 40, 2719–2743.
Ladd C., Hunt G. L. Jr., Mordy C. W., Salo S., Stabeno P. J. (2005). Marine environment of the central and eastern Aleutian islands. Fish. Oceanogr. 14:Suppl.1, 22–38.
LeDuc R. G. (2004). Report of the results of the 2002 survey for north pacific right whales (Washington, DC: National Oceanic and Atmospheric Administration).
Liddel M., Yencho M. (2021) Fisheries of the united state. Available at: https://www.fisheries.noaa.gov/national/sustainable-fisheries/fisheries-united-states.
Lowry L. F. (1993). “Foods and feeding ecology,” in The bowhead whale, spec. publ. 2. Eds. Burns J. J., Montague J. J., Cowles C. J. (Lawrence, KS: Society for Marine Mammalogy), 201–238.
Lowry L. F., Citta J. J., O'Corry-Crowe G., Quakenbush L. T., Frost K. J., Suydam R., et al. (2019). Distribution, abundance, harvest, and status of western Alaska beluga whale, Delphinapterus leucas, stocks. Mar. Fish. Rev. 81:3-4, 54–71. doi: 10.7755/MFR.81.3–4.2
Lowry L. F., Frost K. J., Zerbini A., DeMaster D., Reeves R. (2008). Trend in aerial counts of beluga or white whales (Delphinapterus leucas) in Bristol bay, alaska 1993-2005. J. Cetacean Res. Manage 10:3, 201–207.
Lowry L. F., Sheffield G., George J. C. (2004). Bowhead whale feeding in the alaskan Beaufort Sea, based on stomach content analysis. J. Cetacean Res. Manage 6:3, 215–223.
Maslanik J., Stroeve J., Fowler C., Emery W. (2011). Distribution and trends in Arctic sea ice age through spring 2011. Geophys. Res. Lett. 38, L13502. doi: 10.1029/2011GL047735
Matsuoka K., Crance J., James A., Yoshimura I., Kasai H. (2019). Cruise report of the 2018 IWC-pacific ocean whale and ecosystem research (IWC-POWER) (Nairobi, Kenya: Report to the Int. Whaling Comm.). paper SC/68A/ASI/04.
Melnikov V. V., Litovka D. I., Zagrebin L. A., Zelensky G. M., Ainana L. I. (2004). Shore-based counts of bowhead whales along the chukotka peninsula in may and June 1999-2001. Arctic 57:3, 290–298.
Mizroch S. A., Rice D. W., Zwiefelhofer D., Waite J., Perryman W. L. (2009). Distribution and movements of fin whales in the north pacific ocean. Mammal. Rev. 39:3, 193–227.
Moore S. E., George J. C., Coyle K. O., Weingartner T. J. (1995). Bowhead whales along the chukotka coast in autumn. Arctic 48:2, 155–160.
Moore S. E., George J. C., Reeves R. R. (2021). “Bowhead whale ecology in changing high-latitude ecosystems,” in The bowhead whale balaena mysticetus: Biology and human interactions. Eds. George J. C., Thewissen J. G. M. (London, United Kingdom: Academic Press), 417–427.
Moore S. E., George J. C., Sheffield G., Bacon J., Ashjian C. J. (2010). Bowhead whale distribution and feeding near Barrow, Alaska, in late summer 2005-06. Arctic 63:2, 195–205.
Moore S. E., Reeves R. R. (1993). “Distribution and movement,” in The bowhead whale, spec. publ. 2. Eds. Burns J. J., Montague J. J., Cowles C. J. (Lawrence, KS: Society for Marine Mammalogy), 313–386.
Moore S. E., Reeves R. R., Southall B. L., Ragen T. J., Suydam R. S., Clarke C. W. (2012). A new framework for assessing the effects of anthropogenic sound on marine mammals in a rapidly changing Arctic. BioSci. 62, 289–295.
Moore S. E., Stabeno P. J., Grebmeier J. M., Okkonen S. R. (2018). The Arctic marine pulses model: linking annual oceanographic processes to contiguous ecological domains in the pacific Arctic. Deep-Sea Res. 152, 8–21.
Muto M. M., Helker V. T., Delean B. J., Young N. C., Freed J. C., Angliss R. P., et al. (2021). Alaska Marine mammal stock assessment (Seattle, WA, United States: NOAA Tech. Memo. NMFS-AFSC-421), 398 p. U.S. Dep. Commer.
Nemoto T. (1957). Food habits of baleen whales in the north pacific. Sci. Rep. Whales Res. Inst. 12, 33–89.
Nemoto T. (1959). Food of baleen whales with reference to whale movements. Sci. Rep. Whales Res. Inst. 14, 149–290.
Noongwook G., Huntington H. ,. P., George J. C. (2007). Traditional knowledge of the bowhead whale (Balaena mysticetus) around st. Lawrence island, Alaska. Arctic. 60, 1, 47–1, 54. the Native village of Savoonga, the Native village of Gambell.
Oceana and Kawerak, Inc (2014). Bering Strait marine life and subsistence use data synthesis (Washington D.C. and Nome, Alaska, United States: Kawerak Incorporated), 1–499. Available at: https://oceana.org/publications/reports/the-bering-strait-marine-life-and-subsistence-data-synthesis.
Piatt J. F., Springer A. M. (2003). Advection, pelagic food webs and the biogeography of seabirds in beringia. Mar. Ornithol. 31, 141–154.
Pisareva M. N., Pickart R. S., Iken K., Ershova E. A., Grebmeier J. M., Cooper L. W., et al. (2015). The relationship between patterns of benthic fauna and zooplankton in the chukchi Sea and physical forcing. Oceanogr. 28, 68–83. doi: 10.5670/oceanog.2015.58
Reeves R. R., Ewins P. J., Agbayani S., Heide-Jorgensen M. P., Kovacs K. M., Lydersen C., et al. (2014). Distribution of endemic cetaceans in relation to hydrocarbon development and commercial shipping in a warming Arctic. Mar. Policy. 44, 375–389.
Rolland R. M., Parks S. E., Hunt K. E., Castellote M., Corkeron P. J., Nowacek D. P., et al. (2012). Evidence that ship noise increases stress in right whales. Proc. R. Soc B. Biol. Sci. 279, 2363–2368.
Romano M. D., Renner H. M., Kuletz K. J., Parrish J. K., Jones T., Burgess H. K., et al. (2020). Die–offs, reproductive failure, and changing at–sea abundance of murres in the Bering and chukchi seas in 2018. Deep-Sea Res. Pt. II. 181-182, 104877. doi: 10.1016/j.dsr2.2020.104877
Schell D. M., Saupe S. M. (1993). “Feeding and growth as indicated by stable isotopes,” in The bowhead whale, spec. publ. 2. Eds. Burns J. J., Montague J. J., Cowles C. J. (Lawrence, KS: Society for Marine Mammalogy), 491–509.
Seaman G., Lowry L. F., Frost K. J. (1985). Investigation of belukha whales in coastal waters of western and northern alaska. i. distribution, abundance, and movements (Washington, DC: U.S. Department of Commerce, National Oceanic and Atmospheric Administration).
Sheffield G., George J. C. (2013). “Diet studies,” in Bowhead whale feeding ecology study (BOWFEST) in the Western Beaufort Sea. Eds. Shelden K. E. W., Mocklin J. A. (Seattle, WA: NOAA), 245–269. Available at: http://www.afsc.noaa.gov/nmml/cetacean/bwasp/bowfest.php. Final Report, OCS Study BOEM 2013. National Marine Mammal Laboratory, Alaska Fisheries Science Center, NMFS.
Sheffield G., George J. C. (2021). “Diet and prey,” in The bowhead whale balaena mysticetus: Biology and human interactions. Eds. George J. C., Thewissen J. G. M. (London, United Kingdom: Academic Press), 429–455.
Siddon E. (2020). Ecosystem status report 2020 for the Eastern Bering Sea, stock assessment and fishery evaluation report (Anchorage, AK: North Pacific Fishery Management Council). 1007 West Third, Suite 40099501.
Siddon E., Zador S. (2017). Ecosystem considerations 2017: Status of the Eastern Bering Sea marine ecosystem, stock assessment and fishery evaluation report (Anchorage, AK: North Pacific Fishery Management Council). 605 W 4th Ave, Suite 30699501.
Siddon E., Zador S. (2018). Ecosystem status report 2018: Eastern Bering Sea, stock assessment and fishery evaluation report (Anchorage, AK: North Pacific Fishery Management Council). 605 W 4th Ave, Suite 30699501.
Siddon E., Zador S. (2019). Ecosystem status report 2019: Eastern Bering Sea, stock assessment and fishery evaluation report (Anchorage, AK: North Pacific Fishery Management Council). 605 W 4th Ave, Suite 30699501.
Silber G. K., Weller D. W., Reeves R. R., Adams J. D., Moore T. J. (2021). Co-Occurrence of gray whales and vessel traffic in the north pacific ocean. Endang. Species. Res. 44, 177–201. doi: 10.3354/esr01093
Springer A. M., McRoy C. P., Flint M. V. (1996). The Bering Sea green belt: shelf-edge processes and ecosystem production. Fish. Oceanogr. 5:3/4, 205–233.
Stabeno P. J., Bell S. W. (2019). Extreme conditions in the Bering Sea, 2017–2018): record-breaking low sea-ice extent. geophys. Res. Lett. 46, 8952–8959. doi: 10.1029/2019GL083816
Stabeno P. J., Kachel D. G., Kachel N. B., Sullivan M. E. (2005). Observations from moorings in the Aleutian passes: temperature, salinity and transport. Fish. Oceanogr 14:Suppl.1, 39–54.
Stabeno P. J., Kachel N. B., Moore S. E., Napp J. M., Sigler M., Yamaguchi A., et al. (2012). Comparison of warm and cold years on the southeastern Bering Sea shelf and some implications for the ecosystem. Deep-Sea Res. Pt. II 65-70, 31–45. doi: 10.1016/j.dsr2.2012.02.020
Stevenson D. E., Lauth R. R. (2019). Bottom trawl surveys in the northern Bering Sea indicate recent shifts in the distribution of marine species. Polar Biol. 42, 407–421. doi: 10.1007/s00300-018-2431-1
Stroeve J. C., Markus T., Boisvert L., Miller J., Barrett A. (2014). Changes in Arctic melt season and implications for sea ice loss, geophys. Res. Lett. 41, 1216–1225. doi: 10.1002/2013GL058951
Stroeve J. C., Serreze M. C., Holland M. M., Kay J. E., Malanik J., Barrett A. P. (2012). The Arctic's rapidly shrinking sea ice cover: A research synthesis. Clim. Change. 110, 1005–1027. doi: 10.1007/s10584-011-0101-1
Thompson R. J. (1940). Analysis of stomach contents of whales taken during the years 1937 and 1938 from the north pacific (Seattle: Univ. of Wash.).
Tiernan A., Elison T., Sands T., Head J., Vega S., Neufeld G. (2021). 2020 Bristol bay annual management report (Anchorage: Alaska Department of Fish and Game). Available at: https://www.adfg.alaska.gov/FedAidPDFs/FMR21-16.pdf.
Van Parijs S. M., Curtice C., Ferguson M. C. (2015). Biologically important areas for cetaceans within U.S. waters. Aquat. Mamm. 41:1, 128. (Special Issue).
Wang M., Overland J. E. (2012). A sea-ice free summer Arctic within 30 years-an update from CMIP5 models. Geophys. Res. Lett. 39, L18501. doi: 10.1029/2012GL052868
Wang M., Overland J. E. (2015). Projected future duration of the sea-ice-free season in the alaskan Arctic. Prog. Oceanogr. 136, 50–59.
Wang M., Yang Q., Overland J. E., Stabeno P. J. (2018). Sea-Ice cover timing in the pacific Arctic: The present and projections to midcentury by selected CMIP5 models. Deep-Sea Res. Pt. II 152, 22–34. doi: 10.1016/j.dsr2.2017.11.017
Weingartner T. J., Danielson S. L., Royer T. C. (2005). Freshwater variability and predictability in the Alaska coastal current. Deep-Sea Res. Pt. II. 52, 169–191. doi: 10.1016/j.dsr2.2004.09.030
Will A., Takahashi A., Thiebot J. B., Martinez A., Kitaiskaia E., Britt L., et al. (2020). The breeding seabird community reveals that recent sea ice loss is the pacific Arctic does not benefit piscivores and is detrimental to planktivores. Deep-Sea Res. Pt. II. 181-182, 104902. doi: 10.1016/j.dsr2.2020.104902
Wood K. R., Bond N. A., Danielson S. L., Overland J. E., Salo S. A., Stabeno P. J., et al. (2015). A decade of environmental change in the pacific Arctic region. Prog. Oceanogr. 136, 12–31. doi: 10.1016/j.pocean.2015.05.005
Woodgate R. A., Aagaard K. (2005). Monthly temperature, salinity, and transport variability of the Bering strait through flow. Geophys. Res. Lett. 32, L04601. doi: 10.1029/2004GL021880
Woodgate R. A., Stafford K. M., Prahl F. G. (2015). A synthesis of year-round interdisciplinary mooring measurements in Bering Strait, (1990–2014) and the RUSALCA Years, (2004–2011). Oceanogr. 28, 39–59.
Wyllie-Echeverria T., Wooster W. S. (1998). Year-to-year variations in Bering Sea ice cover and some consequences for fish distribution. fish. Oceanogr. 7:2, 159–170. doi: 10.1046/j.1365-2419.1998.00058.x
Keywords: Bering Sea, Aleutian Islands, Alaska, feeding area, migratory corridor, small and resident population, cetaceans
Citation: Brower A, Ferguson M, Clarke J, Fujioka E and DeLand S (2022) Biologically Important Areas II for cetaceans within U.S. and adjacent waters – Aleutian Islands and Bering Sea Region. Front. Mar. Sci. 9:1055398. doi: 10.3389/fmars.2022.1055398
Received: 27 September 2022; Accepted: 16 November 2022;
Published: 06 December 2022.
Edited by:
Sofie Van Parijs, Northeast Fisheries Science Center (NOAA), United StatesReviewed by:
Desray Reeb, United States Department of the Interior, United StatesBrandon L Southall, Southall Environmental Associates, Inc., United States
Copyright © 2022 Brower, Ferguson, Clarke, Fujioka and DeLand. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amelia Brower, YW1lbGlhLmJyb3dlckBub2FhLmdvdg==
 Amelia Brower
Amelia Brower Megan Ferguson
Megan Ferguson Janet Clarke
Janet Clarke Ei Fujioka
Ei Fujioka Sarah DeLand
Sarah DeLand