- Møreforsking AS, Ålesund, Norway
The deposit feeding sea cucumber Parastichopus tremulus is an underutilised resource in North Atlantic waters. Geographically it is distributed from the Barents Sea in the north to the Canary Islands in the south. At present performance of P. tremulus in aquaculture is largely unknown. Species and stage specific biological knowledge gaps need to be filled for a potential industry to develop, and feeds that support growth needs special attention. Particulate matter (sludge) from fish farms is an unutilised resource that has potential as ingredient in feeds for sea cucumbers, which would help to reduce the environmental footprint of P. tremulus aquaculture production. The suitability of salmon sludge as a feed ingredient is unknown. Feeds using dried salmon freshwater sludge (50% and 75% volume ratios) or seaweed powder (Sargassum spp. 25%, 50% and 75% volume ratios) were compared in this study. Feed mixes with different ratios of ingredients and sand (0.6-1 mm) were given in excess (50% wet weight/wet weight animal/week) to adult P. tremulus. Daily feed intake was estimated by measuring daily faeces production rate. Each animal was given all feeds sequentially, and faeces collected for a ten-day period. Absorption efficiencies were estimated based on analysis of organic matter content in feed and faeces. Large variations were found in feed intake, both between individuals and between days. Our results indicated that P. tremulus showed a higher intake of feeds containing seaweed, with a trend of higher intake with increasing seaweed content. Absorption efficiency estimates of seaweed-based feeds ranged from -337 to 73.7%. P. tremulus showed a preferential selection of organic particles in the feed with lowest content of seaweed. Absorption efficiency of feeds containing sludge (2.5 – 58.3%) was comparable to that of feeds containing seaweed, however, feed intake of sludge-based feeds was significantly lower than that of the seaweed-based feeds and resulted in large variation in estimates. The results suggest that salmon freshwater sludge could have a potential future use as an ingredient in sustainable feeds for P. tremulus, but that optimisation of feed formulations need to be studied further.
1 Introduction
Knowledge gaps regarding species specific biology of cold-water sea cucumbers are current obstacles to the development of an aquaculture industry of emerging species from the North Atlantic. Especially feeding in captivity that support growth and development during the entire life cycle is a field that needs substantial attention for achieving successful and healthy production. The red sea cucumber, Parastichopus tremulus, is a species which has drawn increased attention from fishers, farmers, manufacturers, product developers, and trade actors during the last decade (Kjerstad et al., 2015; Landes et al., 2019; Schagerström et al., 2022). Being a cold-water species, P. tremulus could physiologically be suited for cultivation all along the Norwegian coast as it inhabits the entire geographical range from south to north. The wider distribution of this species in the Eastern Atlantic is from the Barents Sea in the north to the Canary Islands in the south, and west to Iceland and off the western coast of Ireland (Madsen and Hansen, 1994; Christophersen et al., 2021). There is currently no regulated fishery of P. tremulus, meaning the sea cucumbers are only available as bycatch from European trawl (shrimp and fish) and pot (Norway lobster) fisheries. Aquaculture has therefore been proposed as an alternative way to produce the species, but at present performance of P. tremulus in aquaculture is unknown.
Feeding behaviour of sea cucumbers (holothuroids) differ according to habitat and nutrient availability. Suspension-feeding species, such as e.g. Cucumaria frondosa ingest mainly phytoplankton and particles from the surrounding water, whereas deposit-feeding species, to which P. tremulus belongs, extract nutrients from the sediment and live on a diet that includes microorganisms, organic detritus, silica fragments and faeces (Yingst, 1976; Hauksson, 1979; Moriarty, 1982; Hudson et al., 2004). Sea cucumbers inhabit all oceans and live in a wide range of depths and on a variety of substrates including gravel, corals, and rocks, but mainly on softer bottoms (Purcell et al., 2012), which is also the case for P. tremulus (Christophersen et al., 2021). Most commercial species are deposit feeders having peltate tentacles that extends to acquire mainly particulate matter (Roberts et al., 2000; Purcell et al., 2012). The mouth is directed ventrally, and nutrients are extracted from organic matter within the upper benthic sediment layer, thus contributing to oxygenating and nutrient upcycling through the food chain. Holothurians are also shown to play important ecological roles in coral reef nutrient cycles (Uthicke and Klumpp, 1998). Several species of deposit-feeding sea cucumbers show active selection of organic matter from sediment, such as Apostichopus californicus (Paltzat et al., 2008) and Australostichopus mollis (Slater and Carton, 2009; Slater and Carton, 2010). In the studies of Hauksson (1977); Hauksson (1979) it was also found that P. tremulus has the ability to be selective in its feeding, and select faecal pellets and other sediment particles, which are richer in organic material than the general surface sediment. In nature the feeding pattern may be seasonal and related to availability of food items (David and MacDonald, 2002; Dar and Ahmad, 2006; Xu et al., 2015).
Growth relates to availability of nutritional food, and the dietary needs of sea cucumbers differ between species and the different life stages (Yang et al., 2015). Adult specimens of deposit-feeding sea cucumbers are benthic, but similar to other echinoderms they develop through several pelagic larval stages before metamorphosis and settlement. Depending on the species, the larvae exhibit different nutritional strategies (McEdward and Miner, 2001): either planktotrophic (external feeding) or lecithotrophic (relying on internal energy stores). During the post-larval and settlement phase, dietary requirements change to be more similar to that of the adults (Cameron and Fankboner, 1984). Successful gonad maturation most likely is influenced by both nutritional and environmental conditions (Whitefield et al., 2018) and thus broodstock feed for aquaculture may require optimised diets designed for this purpose. It has been shown that when given a choice of diets, Apostichopus japonicus show selective behaviour and that preference may change over time (Xia et al., 2012). Jespersen and Lützen (1971) suggested that P. tremulus in Norwegian waters cease to feed from late autumn and during winter, which is the period of low primary production. Understanding the feeding habit of sea cucumbers is thus of great importance when developing relevant aquaculture technology and artificial feeds.
Feeding studies on sea cucumbers in culture indicate that well-balanced diets need to be rich in lipids, essential fatty acids and carotenoids, especially if vitellogenesis and reproduction are to be promoted (Giraspy and Ivy, 2008; Gianasi et al., 2017; Whitefield et al., 2018). Many of these compounds are common ingredients in aquaculture feeds developed for other species, and it has therefore been postulated that particulate waste from fed fish aquaculture could be repurposed as feed or feed ingredients for deposit-feeding species such as sea cucumbers and polychaetes and thus improve the overall sustainability of current aquaculture practice. As an example, the land-based salmon smolt production industry in Norway alone generated more than 10 000 tonnes of sludge (dry matter) in 2017 (Aas and Åsgård, 2017). This nutrient-rich side stream consists of uneaten feed and faeces, of which only a minor fraction is collected and used for low-value applications. There is an increased interest in valorising this side stream for e.g. sustainable energy or feed production. Since deposit feeding species such as P. tremulus are able to ingest and utilize nutrients in particulate organic matter, they have been suggested as candidate species for inclusion in integrated multi-trophic aquaculture (IMTA) systems. At present no information exists on the feed preference and nutritional metabolism of P. tremulus in culture, and only a few studies have investigated the efficiency of P. tremulus in extracting and utilizing organic matter from marine sediments (Hauksson, 1977; Hauksson, 1979).
Macroalgae are among the most common feed ingredients used in Chinese sea cucumber aquaculture (Song et al., 2017), and brown algae are generally regarded as high-quality food sources for temperate sea cucumber species (Liu et al., 2010; Song et al., 2017). Green and red algae most probably are not ingested by sea cucumbers as shown in e.g. Holothuria forskali (David et al., 2020). In farming of the sea cucumber A. japonicus the commercially available seaweed Sargassum spp. is commonly used as an ingredient in feed. There are, few, if any, data published on the suitability of Sargassum spp. in feeds for cold-water species, and this study is to our knowledge the first to provide data on the use of Sargassum spp. as feed for P. tremulus.
The goal of this study was to obtain new information about P. tremulus feeding to further the aquaculture development of this species. With this in mind, we wanted to evaluate the feasibility of using either Sargassum spp. or dried sludge originating from Atlantic salmon (Salmo salar L.) aquaculture as feed ingredients for P. tremulus. By composing a series of feeds that ranged in organic content from values close to that of natural sediment to that of high-energy feeds used in aquaculture of other sea cucumber species, we aimed at assessing whether there was an upper limit to the content of organic matter that could be utilized by the sea cucumber. Feed intake and absorption efficiency were measured using faeces production as an indirect indicator of feed consumption. Based on the responses, the two feed sources were compared to evaluate attractivity and suitability of sludge and Sargassum as ingredients for P. tremulus feed purposes.
2 Materials and methods
2.1 Sea cucumbers and holding conditions
Sea cucumbers were selected from a larger batch of adult P. tremulus caught in shrimp trawls during autumn-winter 2019-2020 in the Vigra fjord outside of Ålesund in western Norway (62°N-6°E) and kept in flow-through holding tanks supplied with untreated seawater from 40 m depth until experimental start in May 2020. Length and total wet weight (WW) of the sea cucumbers ranged from 118 to 220 mm and from 39 to 288 g, respectively. Two feeding experiments (Experiment 1 and Experiment 2) were performed, with the first starting in May and the second in October. During the experimental periods, the sea cucumbers were held individually in flow-through 25 L glass aquaria (40 x 25 x 25 cm) supplied with filtrated (1 µm) and UV-treated seawater at a rate of 23.0 ± 0.3 L h -1. Water temperature ranged between ~ 9-13°C and was logged every 15 min in all aquaria using HOBO TidBit v2 UTBI-001 loggers (Onset Computer Corporation, Bourne, MA, USA).
Length and weight were measured at the start and at the end of each feeding experiment. Before each measurement, sea cucumbers were starved for at least 72 hours to ensure emptying of the gut. Body length (mouth to anus) was measured to the nearest 1 mm using a measuring board and total WW to the nearest 0.1 g using an electronic scale (SKX2202, Ohaus GmbH, Nänikon, Switzerland).
2.2 Feed ingredients and composition
Commercially available powdered Sargassum thunbergii (Fuzhou Wonderful Biological Technology Co., Ltd., Fujian, China) was acquired and subsequently stored under dry conditions at ambient temperature until feed preparation. Aquaculture sludge was obtained from a freshwater land-based RAS facility (MOWI Steinsvik, Volda, Norway) producing Atlantic salmon (Salmo salar L.) smolts. The sludge had been dewatered using a drum filter and dried using an oil-heated drum dryer (Scanship AS, Tønsberg, Norge) to a final dry matter content of 85-90% and was stored at ambient temperature until receipt. Sludge particle size was standardized to <1 mm by sifting through a 1-mm stainless steel sieve (wired mesh) and was subsequently stored at -20°C until feed preparation. The organic feed ingredients, Sargassum powder and sludge, were analysed for macronutrient composition and energy content calculated (Table 1). Protein was determined as nitrogen (N) in ground freeze-dried samples using a CHNS–O elemental combustion system (Costech Instruments ECS 4010) at a temperature of approximately 1000°C, where the sample N is converted to N gas/oxides. Results were expressed in g N per 100 g of dried sample and a N-to-protein conversion factor of 6.25 was used. Fatty acids (lipid) were analysed according to ISO 12966-2 and ISO 5509, and carbohydrate content was calculated as the difference between total weight subtracted water, protein, lipid, and ash. For each feed formulation, macronutrient content in Table 2 was calculated from the known composition of each separate feed ingredient (Table 1).
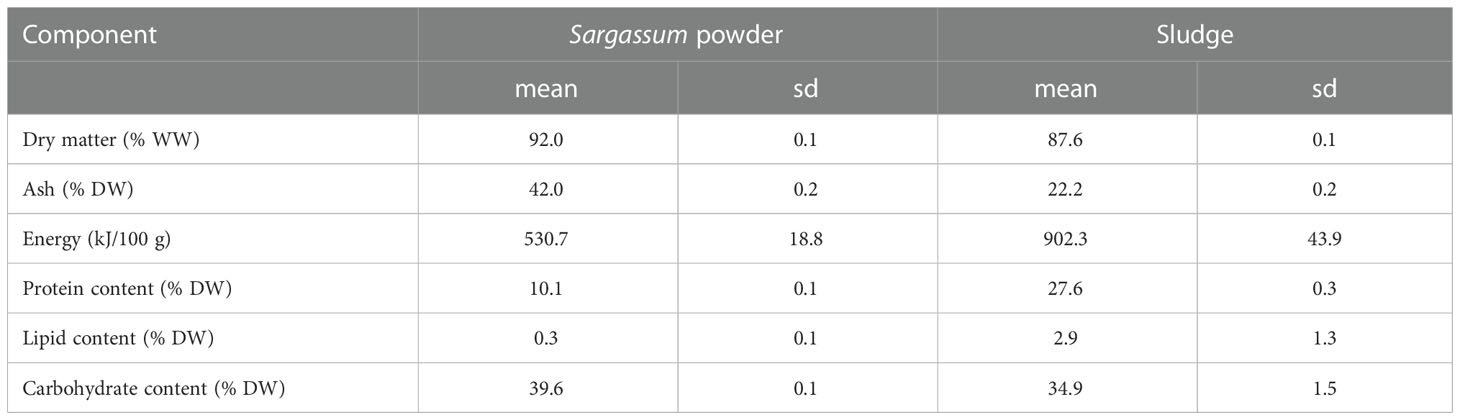
Table 1 Macronutrient composition, dry matter, ash and energy content of the experimental feed ingredients (n=3, sd=standard deviation).
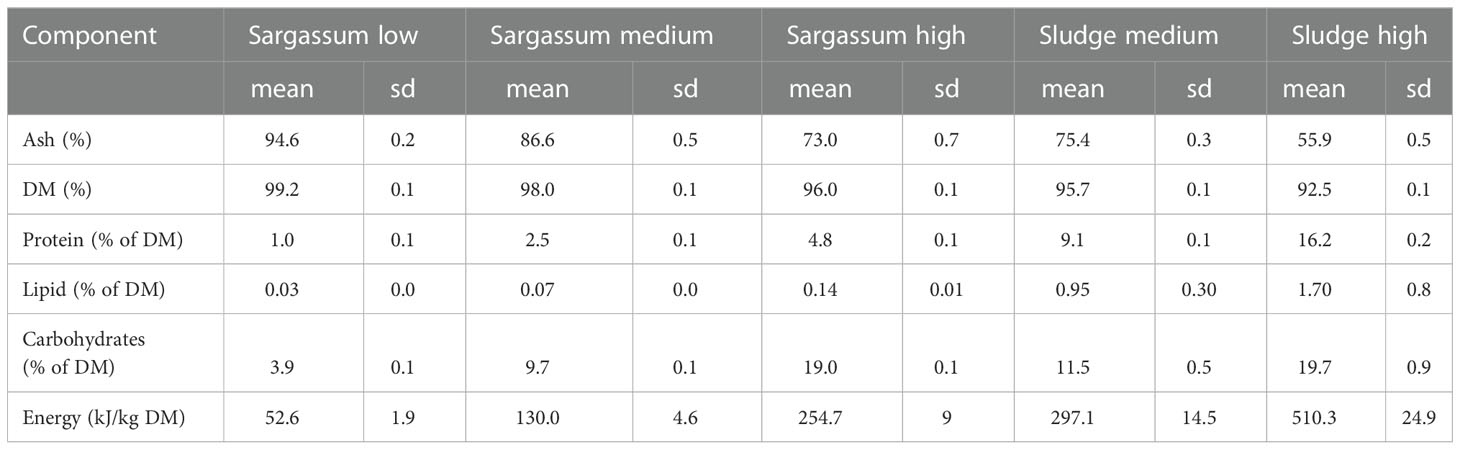
Table 2 Ash, dry matter (DM), macronutrient composition and energy content of the experimental feeds (n=3, sd=standard deviation) used in Experiment 1 and Experiment 2.
To prepare the experimental feeds, each organic substrate was mixed with sand of grain size 0.6-1 mm. To achieve this range in grain size, commercially dried sand with a grain size 0-2 mm (Adda Byggkjemi AS, Drammen, Norway) was sifted through stainless steel sieves with 1 mm and 0.6 mm wired mesh size and the fraction retained on the 0.6 mm mesh was used. Each feed ingredient was mixed with sand and added filtered fresh water until they had a low moisture mash consistency suitable for deployment in water. Water content thus varied between the experimental feeds depending on the proportion of ingredients used. Due to the physical properties of the sludge and its high water solubility observed when submerged in seawater, feeds that contained sludge were added guar gum as a binder at 1% of feed dry weight (DW) to maintain their integrity (based on Won et al., 2018).
2.3 Experimental setup
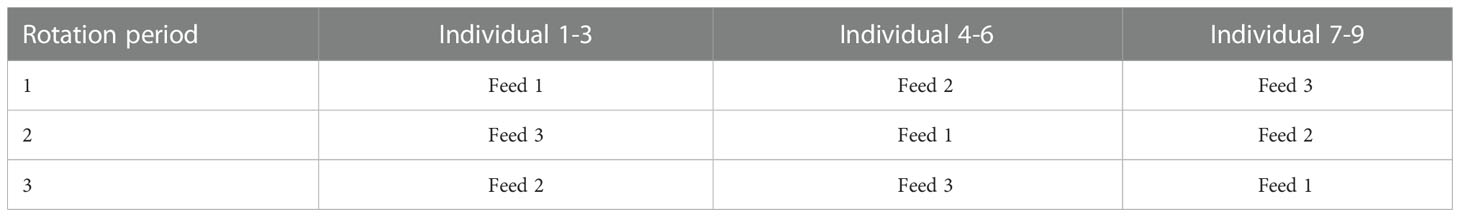
Each feeding experiment was carried out using a 3x3 setup, where three individuals were given the same feed in triplicate, hence a total of 9 individually held sea cucumbers were used. During each of two experimental periods (May and October 2020) the sea cucumbers were given three experimental feeds in a rotating experimental design that presented all individuals with all feeds (Table 3). In Experiment 1, sea cucumbers were fed experimental feeds with three different ratios (volume:volume) of seaweed powder and sand: 25:75 (low), 50:50 (medium) and 75:25 (high). The lower range of Sargassum content in the experimental feeds was selected based on organic content reported in sediment samples from natural habitats of P. tremulus in Norway (Hauksson, 1979; Sargent et al., 1983), and the upper range was based on Liu et al. (2010), who used a mixture of 80% Sargassum thunbergii powder and 20% yellow soil in culture of A. japonicus.
In Experiment 2, the Sargassum:sand feed (50:50) was repeated as a reference feed and compared to two additional feeds that were formulated from dried aquaculture sludge in medium (50:50) and high (75:25) volume ratios. The composition of the individual feed ingredients is given in Table 1, and for the experimental feeds in Table 2. To make sure feed intake was not limited by the amount of feed available, a high feeding rate (50% WW of feed per WW body weight per rotational period) was chosen. Feed was deposited in one portion at the centre of the floor of each aquarium at the start of each feeding period.
Each animal was presented with each feed for 7 days, after which the remaining feed was removed from the tank, and the animals starved for 72 h before the entire sequence was repeated with a new feed. Faeces was collected daily from each tank using a pipette throughout the entire 10-day period. Only particles that could visually be identified as faecal pellets were collected. Samples of faeces and feed were immediately frozen and stored at -20°C until processing. Particulate matter of faeces and feed was retained by filtering the samples through pre-weighed Whatman GF/C RTU glass microfiber filter of 1.2 µm particle retention size (47 mm, GE Healthcare Life Sciences, Buckinghamshire, UK). Any remaining salt was removed by rinsing the filter with 50 mL distilled water. All samples of feed and faeces were analysed for organic matter in terms of ash-free dry weight (AFDW), which was determined as the difference between DW after drying at 60°C for 48 h, and ash weight after combustion at 490°C for 6 hours.
2.4 Calculations and statistical analyses
To standardize measurements, daily faeces production rates were corrected for WW of each animal at the start of the experiment and converted to DW feed per WW initial body weight (mg g-1 day-1), according to Zamora and Jeffs (2011). Faeces production was used as an indirect indicator of feed consumption, and daily feed intake was estimated from faeces production rate by relating the ash weight in the faeces collected to the ash content in each respective feed, assuming that mineral absorption from ash was minimal and negligible during the time it passed through the intestine.
The performance of each feed was evaluated by calculating the absorption efficiency (AE), as the difference in organic content (AFDW) of the feed and the collected faeces (Conover, 1966):
AE (%) = [(AFDWfeed −AFDWfaeces/(1 −AFDWfaeces) × AFDWfeed] ×100, where AFDWfeed and AFDWfaeces is the ash-free dry weight of feed and faeces, respectively.
Both feed intake and AE calculations assumed that the inorganic (ash) fraction was indigestible and unaffected by passage through the digestive tract of P. tremulus.
Weight gain (% per 34 days) and specific growth rate (SGR) were calculated for pooled WW data of the 9 individuals over the period of each experiment to investigate the capacity to grow in captivity:
Weight gain (%) = (final weight–initial weight)/initial weight) x 100
SGR (% d−1) = 100 × (ln (WWfinal) – ln (WWinitial))/# days of experimental period x 100
For statistical analysis, the R package version 4.1.3 (R Core Team, 2022) was used. Data were tested for normality using the Shapiro-Wilks test and for heterogeneity of variance using the Levene’s test. For data that were normally distributed, one- or two-way analysis of variance (ANOVA) was used, followed by a post hoc Tukey HSD test. For non-normal data that showed homogenous variance, a non-parametric Kruskal-Wallis test was used.
3 Results
The average seawater temperature in the aquaria (n=9) was 9.5 ± 0.2°C for Experiment 1 in May-June and 12.5 ± 0.5°C for Experiment 2 in October. The maximum daily difference in temperature between aquaria never exceeded 0.5°C. There was a slight temperature increase registered (0.5°C) during both experimental periods, but halfway in Experiment 2 a temperature peak (13.5°C) was registered. The sea cucumbers showed a net positive weight gain (5.9% and 6.8%) from start to end of both the experimental periods after being fed the three different diets. WW increased from 127 g to 134 g, and from 98 g to 104 g in Experiment 1 and 2, respectively, corresponding to daily specific growth rates (SGR) of 0.17 and 0.19% d-1.
3.1 Feed intake
The feeding ratio used (50% WW) was found to be sufficient for the animals to feed to satiation in both experiments, as none of the animals were found to have consumed their given feed ration at the end of each rotational period. Faecal production was intermittent, as not all animals produced faeces every day, and was in general found to be highly variable, both between individuals and between days. The average feed intake for each rotational period (10 days) was used to compare attractivity of each of the feeds. There was a significant linear relationship between feed intake and initial WW of the animal for both the Sargassum medium (p<0.0001) and Sargassum high feeds (p=0.0035) in Experiment 1, whereas in Experiment 2, no such relationship was found.
In Experiment 1, feed intake was found to be significantly affected by feed type (p < 0.005) and there was a trend of higher feed intake with increasing proportion of Sargassum powder inclusion in the feed. The feed with the lowest content of Sargassum, was associated with a significantly lower feed intake (p < 0.001), whereas no significant difference was found in feed intake between animals given feeds with either medium or high content of Sargassum (Figure 1).
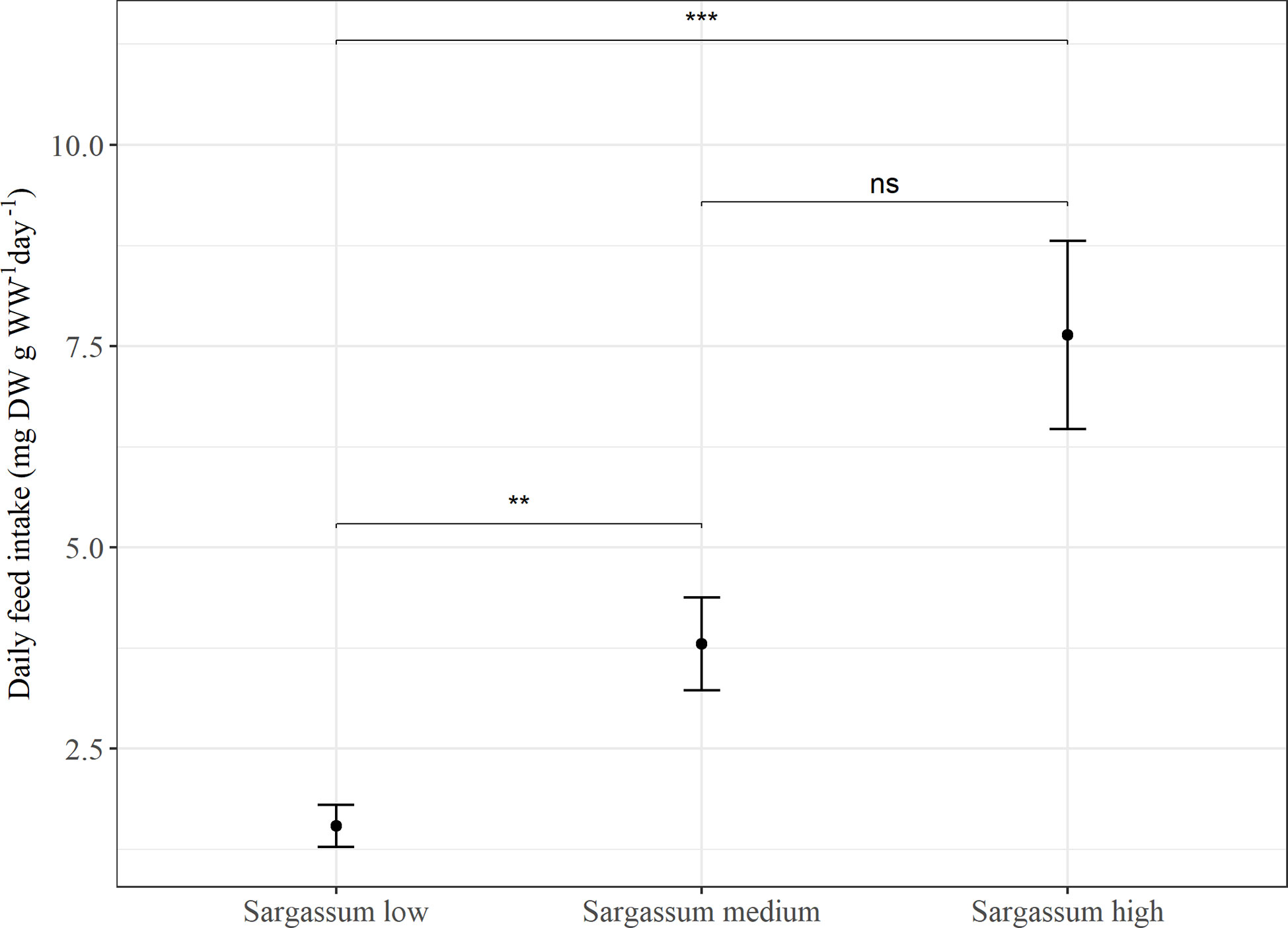
Figure 1 Average daily feed intake (mg DW g WW-1 day-1) in Experiment 1. P. tremulus (n=9) given feeds with three different levels of Sargassum powder (low: 25%, medium: 50% and high: 75% v:v) over a 10-day period. Error bars show standard error and significant differences between feeds are indicated with asterisks (ns, not significant; **: p≤0.01; ***: p≤0.001).
Overall, the average daily feed intake was lower in Experiment 2 than in Experiment 1. In Experiment 2 (Figure 2), the sea cucumbers showed a higher daily feed intake when the Sargassum reference feed was given (p < 0.00001), whereas feed intake was similar (p > 0.05) and close to zero for both sludge feeds. When comparing feed intake of the reference feed in both experiments, intake was significantly higher in Experiment 2 than in Experiment 1 (p < 0.002).
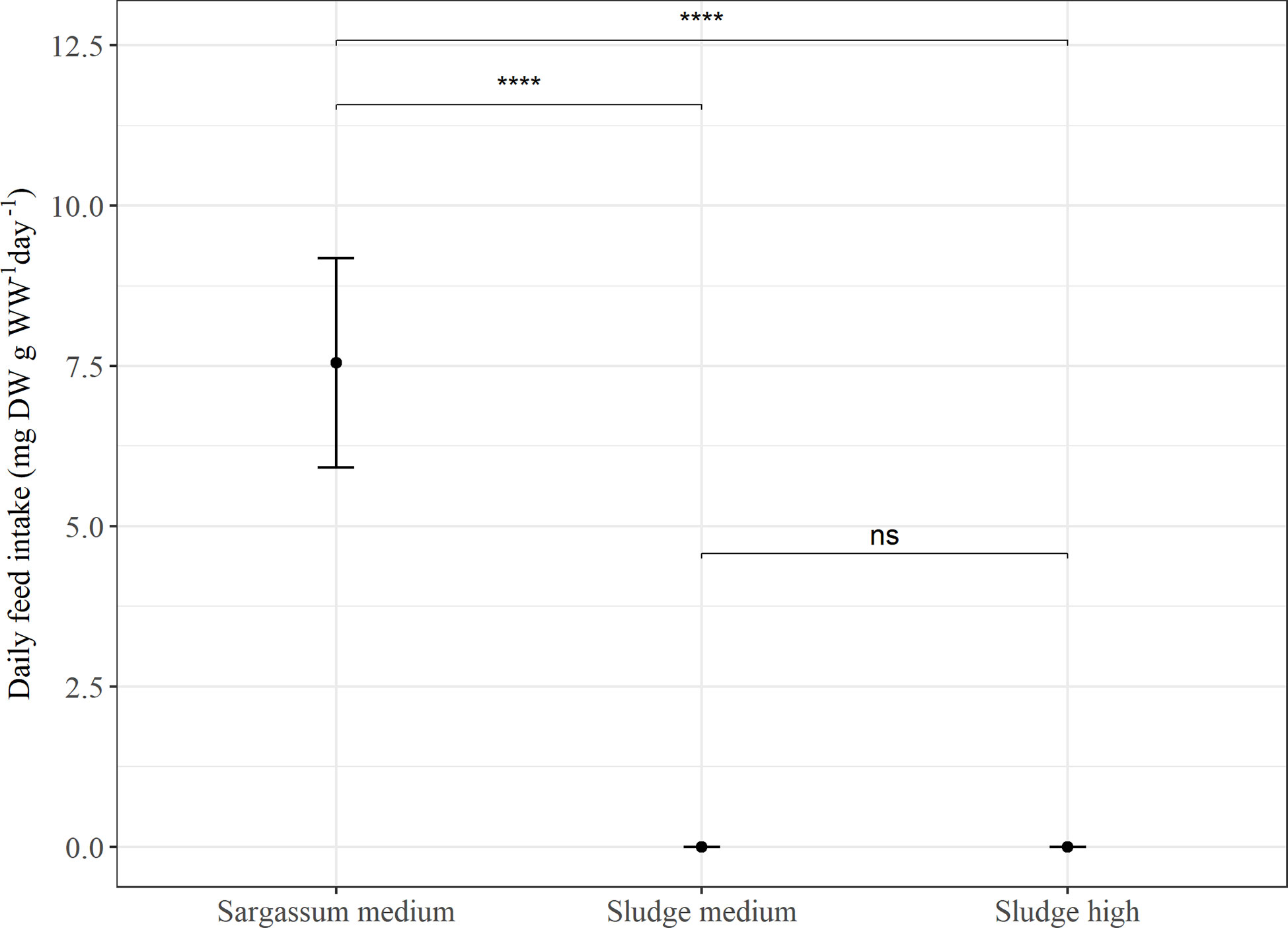
Figure 2 Average daily feed intake (mg DW g WW-1 day-1) in Experiment 2. P. tremulus (n=9) given feeds with either Sargassum powder (Sargassum medium: 50% v:v) or aquaculture sludge (Sludge medium: 50% and Sludge high: 75% v:v) over a 10-day period. Error bars show standard error and significant differences between feeds are indicated with asterisks (ns, not significant; ****: p≤0.0001).
3.2 Absorption efficiency
The organic content of each experimental feed and that of the respective collected faeces is given in Table 4. In Experiment 1, there was a strong inverse relationship between organic content in the feed given and that of the resulting faeces (r=0.90), whereas this was not the case in Experiment 2. The variation in faeces organic matter was high, both between individuals and between days.
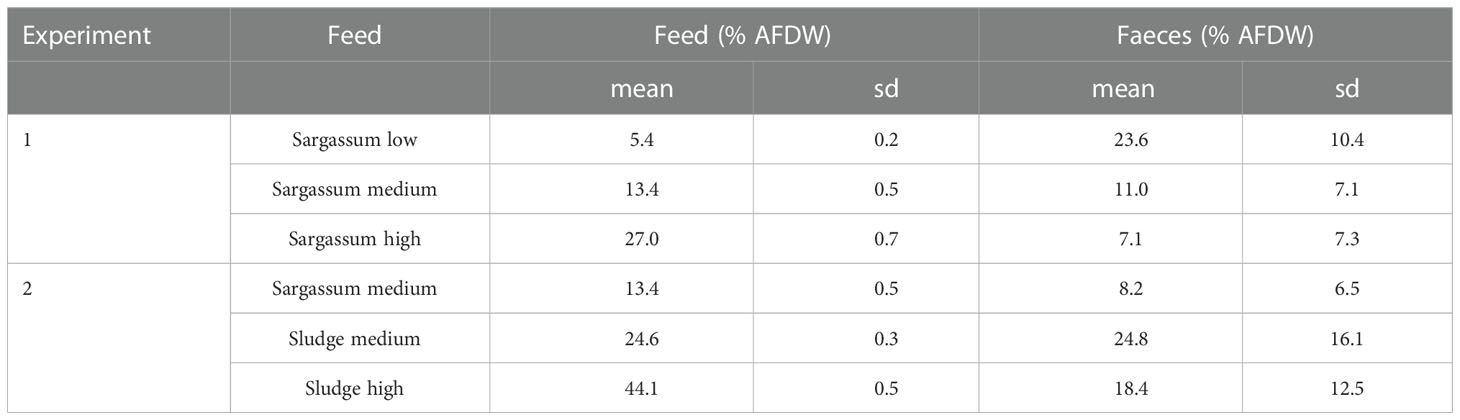
Table 4 Content of organic matter (% ash-free dry weight, AFDW) in experimental feeds and faeces from Parastichopus tremulus (sd, standard deviation).
Absorption efficiency (AE) estimates from feeding the Sargassum based feeds in Experiment 1 (Figure 3) found significant differences between all three feed types (p < 0.0001), and there was a trend of increasing AE with increasing Sargassum content. However, whereas estimates were consistently low or negative for the feed with the lowest Sargassum content (-337 ± 194%), they were on average positive for the feeds containing 50% and 75% of Sargassum powder (medium: 18.2 ± 52.7% and high: 73.7 ± 27.1%). The negative absorption efficiency for the Sargassum low feed is the result of a higher content of organic matter in faeces than in the feed given. On average, the faeces collected showed a more than 4 times higher organic content than the feed (23.6 ± 10.4% vs 5.4 ± 0.2%, see Table 4). For all other feeds, organic content in faeces was lower than that of the feed (Table 4).
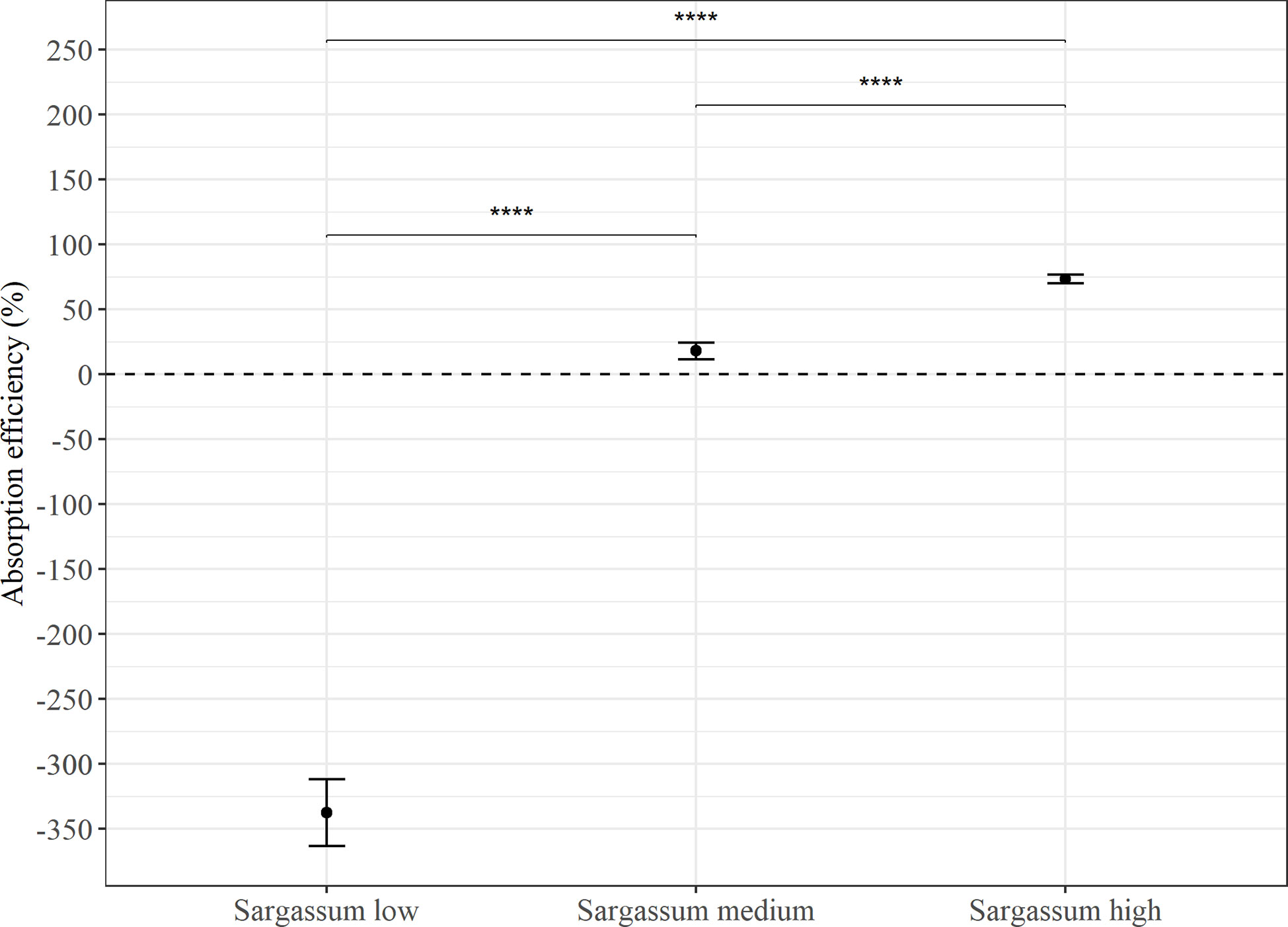
Figure 3 Average absorption efficiency (%) in P. tremulus (n=9) given feeds with three different levels of Sargassum powder (low: 25%, medium: 50% and high: 75% v:v) over a 10-day period in Experiment 1. Error bars show standard error (se) and significant differences between feeds are indicated with asterisks (****: p≤0.0001).
In Experiment 2 (Figure 4), the feed with 50% aquaculture sludge on average showed a significantly lower AE than both the reference feed (p < 0.05) and the feed with 75% sludge (p < 0.00001). However, this estimate showed a large standard deviation (2.5 ± 64.7%), with several measurements of negative AE values. There was no significant difference in AE between the reference Sargassum feed (39.0 ± 48.2%) and the feed containing 75% sludge (58.3 ± 28.3%).
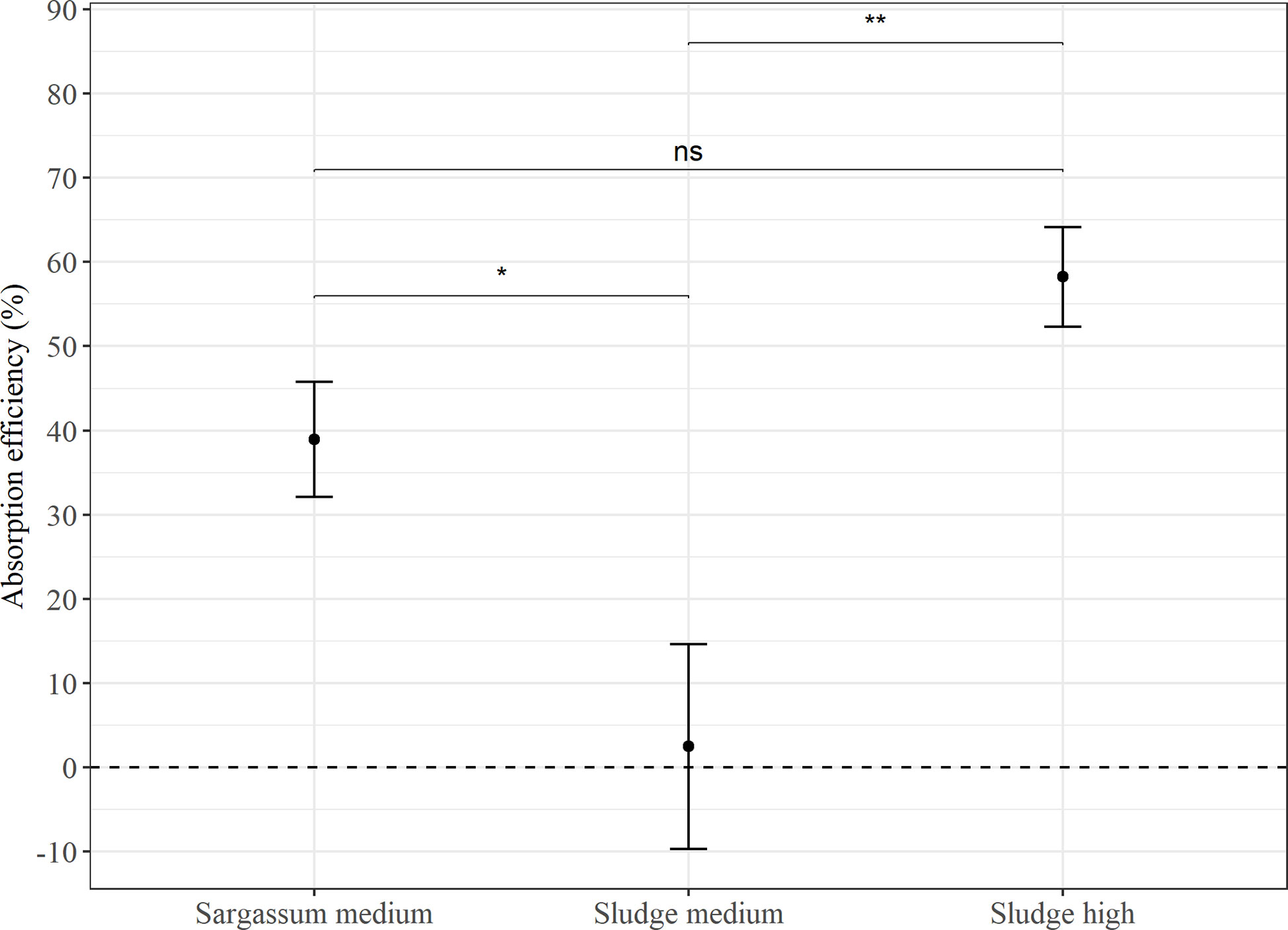
Figure 4 Average absorption efficiency (%) in P. tremulus (n=9) given feeds with either Sargassum powder (Sargassum medium: 50% v:v) or aquaculture sludge (Sludge medium: 50% and Sludge high: 75% v:v) over a 10-day period in Experiment 2. Error bars show standard error and significant differences between feeds are indicated with asterisks (ns, not significant; *: p ≤ 0.05; **: p≤0.01).
4 Discussion
4.1 Feed intake
All individuals consumed all the diets offered in Experiment 1. On the other hand, not all individuals consumed all the diets offered in Experiment 2. This was particularly apparent for the sludge-based feeds, indicating a degree of aversion in most individuals towards these feed formulations. The assumption of increased feed intake with increasing body size was only partly supported by our results as significant relationships between feed intake and sea cucumber WW was found for the Sargassum medium and high feeds in Experiment 1 only. The wide size range of experimental sea cucumbers used (39-288 g) and the intermittent faeces production, may have influenced the results. Previous studies found increased food consumption rates in A. japonicus with increasing body weights of 43, 84 and 147 g as well as with increasing temperatures of 10, 15 and 20°C (Yang et al., 2005). Sun et al. (2015) found significantly lower faeces production rate in small A. japonicus (18 g) than in sea cucumbers of 40 g and 89 g, explained by less developed tentacles in the smallest size group. Yang et al. (2005) also observed day-to-day variation in consumption by the different sea cucumbers and that some individuals ceased feeding during the experimental period. This is in line with our findings, where certain individuals showed several days without faeces production. We found indications of increased feed intake with higher water temperature for some individuals when comparing intake of the Sargassum reference feed between May-June (Experiment 1, 9.5°C) and Oct-Nov (Experiment 2, 12.5°C), although this difference was not significant overall (p=0.62). Jespersen and Lützen (1971) observed a seasonal feeding pattern in nature, with a higher incidence of P. tremulus specimens lacking intestines from the end of October to January in the Oslo fjord in southern Norway, postulating that animals could cease feeding due to a naturally lower nutrient availability during winter months.
Whereas species such as A. japonicus show distinct nocturnal feeding behaviour, with higher ingestion and faeces production at night, observed by Sun et al. (2015) in different size groups of A. japonicus (18, 40 and 89 g), this seems not to be the case for P. tremulus (Jespersen and Lützen, 1971; Hauksson, 1977; Hauksson, 1979). During our experiments, the sea cucumbers were mainly kept under dark conditions and light was only switched on during faeces collection and feeding. Since no video monitoring was employed, we can neither confirm nor refute this hypothesis, but considering that P. tremulus inhabits deeper waters than A. japonicus (our specimens were collected at about 150 m depth), diurnal light cycles may not be important in influencing feeding behaviour in this species. Jespersen and Lützen (1971) indicated that P. tremulus in the Oslo fjord continually fed through night and day and that for animals dredged in deep water, no seasonal variation in feeding was indicated. Based on the irregular frequency of production of faecal pellets, the sea cucumbers in our aquaria did not seem to show continuous feeding, but rather large individual and day-to-day variation. Discontinuous defaecation suggests an irregular feeding pattern. Hauksson (1979) estimated that 50% of the gut content is voided at each defaecation event and indicated that the time between each defaecation may vary depending on season or experimental conditions.
The amount of faeces production measured in our study was only partly related to the size of the individual. We also observed large differences in feed intake between the sea cucumbers given the same feed. This is difficult to explain without more detailed investigation of metabolic parameters, which was outside the scope of this study. Other factors related to holding conditions may have an effect on both growth and feed uptake, such as the use of bottom substrate in the tank and the ratio of mud to sand in the feed (Hou et al., 2017).
Feed intake and the absorption efficiency measured in our experiments may have been influenced by the particle size of the organic as well as the inorganic particles (sand). In analyses of intestinal content from P. tremulus feeding at 374-505 m depth, about 80% of the particles ingested were <200 µm in size, whereas very few particles were >1 mm (Hudson et al., 2004). We reasoned that the use of sand of grain size 0.6-1 mm as filler in our diets was adequate due to the relatively large size of our sea cucumbers (mean initial WW 127 g) and the upper limit of grain size of 1 mm for the sand and the sludge ingredients was justified by Hudson et al. (2004). As long as grain size was similar (< 0.15 mm), the origin of particles (sea mud vs yellow soil) did not seem to affect growth in A. japonicus (Liu et al., 2010).
P. tremulus are reported to be fully exposed on the substratum in nature and do not bury themselves into the sediment (Jespersen and Lützen, 1971). This has been confirmed in aquarium and cage studies (Hauksson, 1977), indicating that feeding in tanks and aquaria can be similar to feeding on the upper sediment layer in nature. Fine branched tentacles that extend from the oral crown are used for ingestion of food particles. From videos recorded by underwater Remote Operated Vehicles (ROV), Hudson et al. (2004) observed that about half of the 20 tentacles were in contact with the seafloor and actively feeding at any one time. The assumption by Jespersen and Lützen (1971) that P. tremulus is non-selective in its feeding habits has been contradicted in later studies (Hauksson, 1979; Hudson et al., 2004). Our results seem to suggest that P. tremulus exhibits a selective feeding behaviour when organic matter content of the feed is low. Organic matter content in faeces was more than 400% higher than that of the feed when feeding on the feed with the lowest content of organic matter (Sargassum low, 5.4%). Zamora and Jeffs (2011) also found that selection efficiency in A. mollis (the accumulation of organic matter in the foregut) increased with decreasing organic matter content (TOM) in the feed and was higher for feeds with 1% and 4% TOM than that of feeds containing 12% and 20% TOM.
The fact that the sea cucumbers gained weight during the time of the experiments, combined with absorption efficiency estimates suggests that P. tremulus was able to assimilate at least part of the nutrients from the formulated feeds used in this study. The SGRs measured in this experiment were slightly lower than growth rates of smaller P. tremulus of about 15 g fed a diet identical to the Sargassum medium diet from May to July (0.2 vs. 0.2-0.3% d-1), but higher than growth rates obtained for the same individuals later in the autumn (Christophersen and Sunde, 2022). These values are in line with SGR reported of juvenile A. californicus (0.267% d-1) based on measurements for one year (Hannah et al., 2012). Domínguez-Godino and González-Wangüemert (2019) reported SGR 0.2% d-1for adult Holothuria arguinensis based on eviscerated body weight. Gianasi et al. (2017) reported SGR based on length and immersed weight within the same range for C. frondosa, and SGR 0.4-1.1% d-1 are reported for juvenile A. japonicus based on dry body weight (Ru et al., 2019). Since we based the SGR on WW, the amount of coelomic fluid and intestines may have influenced the results. However, we have previously documented a high correlation (r = 0.84) between total and body wall WW in P. tremulus from western Norway (Christophersen et al., 2021).
The possible aversion for sludge-based feeds observed in Experiment 2, could have partly been due to the short duration of the experiment. Xia et al. (2012) found that A. japonicus in a feed selection experiment changed their feed preference for Sargassum spp. from rejection to somewhat preferred over a 30-day period, and it is possible feed intake could increase if the animals had been given these feeds over a longer period of time.
4.2 Diet composition and nutritional value
Well balanced diets are prerequisites for capture-based or land-based production of sea cucumbers to maintain physiological properties and enhance growth and nutritional quality of the animals. No information is currently available on what constitutes an optimum diet for P. tremulus. In its natural habitat, the deposit feeding P. tremulus encounters and ingests substrate of variable quality of which only a small fraction will be assimilated. In general, the digestive physiology of deposit-feeding sea cucumbers is adapted to ingestion of nutrient-poor sediment and only occasionally will they encounter nutrient-rich environments. It is therefore of interest to evaluate to what extent P. tremulus is capable of absorbing nutrient-rich feeds.
The experimental diets used in this study ranged from 5.4 – 44.1% in organic content (AFDW, Table 4). For comparison, published values from marine sediments in typical Norwegian fjord systems show a wide range, from 9.3% organic matter in Balsfjord in northern Norway (Sargent et al., 1983), to 0.91-1.36% in the Raune fjord in southwestern Norway (Hauksson, 1979). In areas of intensive aquaculture activity, higher sediment organic matter concentrations could be expected. For instance, Yu et al. (2014) reported 15% organic matter in sediments collected in traps below fish cages in China, 25% higher than that of a reference site, whereas Paltzat et al. (2008) reported 5.9% in sediment samples collected from a suspended culture Pacific oyster farm in Canada. Macronutrient composition of the experimental diets varied from 1 – 16.2% protein, from 3.9 – 19.7% carbohydrates and from 0.03 – 1.70% lipids (Table 3). The capacity and ability of P. tremulus to digest these various macronutrients is unknown, but based on studies of other deposit-feeding sea cucumbers fed formulated feeds, it can be elucidated that the majority of nutritional energy that is ingested most likely will be lost through faeces. For instance, Yuan et al. (2006) estimated energy loss through faeces in A. japonicus with positive SGR to be between 55.6–67.1% at temperatures of ~13 – 20°C, and that energy deposited in growth was low (5.5–7.9%) when fed diets prepared from dried bivalve faeces and powdered algae. Israel et al. (2019) found that 24.1% of feed energy was digested by the Mediterranean deposit-feeding holothuroid Actinopyga bannworthi when fed pellets prepared from particulate gilthead seabream (Sparus aurata) waste. In the same study, apparent digestibility coefficients of protein and lipid was 16.6% and 45.9%, respectively, whereas that of organic matter was 26.7%. Formulation of feeds and selecting an appropriate feeding regime is therefore crucial in order to maximise ingestion and absorption of nutrients (Xia et al., 2017). The use of the right kind of sea mud or sand as feed ingredients might be particularly important for improving nutrient absorption by both regulating the residence time of feed in the digestive tract of the sea cucumber and diluting feed nutrients, leading to better growth performance (Shi et al., 2015).
Nutrition research on commercially farmed A. japonicus has found that optimal protein content in artificial feeds for this species lies between 16-21% (Sun et al., 2004), with slightly higher optimum dietary protein requirements for juveniles at about 18-24% (Zhu et al., 2005). Similarly, lipid requirements of juvenile A. japonicus lie around 5% (Zhu et al., 2005). Seo and Lee (2011) found that feeds with a protein content of either 20% and 40% and a lipid content of 2% gave the highest growth in juveniles from the same species. Our sludge formulated feeds had a similar protein (9.1-16.2%) and lipid (0.95-1.70%) content, but since feed intake was low during our experiment, AE could not be fully assessed in this study. The experimental feeds were not prepared as extruded pellets, but instead were given as a “wet feed”. This allowed the animals to selectively feed on organic particles. We did not add inorganic digestibility markers such as chromic oxide Cr2O3 (Liu et al., 2009; Israel et al., 2019), but instead assumed that absorption of minerals from ash were negligible and that the ash content could be considered as a digestibility marker.
Assimilation efficiencies (AE) of the medium and high Sargassum feeds as well as the sludge high feed were close to the range of values reported from A. californicus feeding on substrate with high organic matter (Ahlgren, 1998; Paltzat et al., 2008). However, these studies utilized a different methodology, where AE was calculated from the difference in organic matter between foregut and hindgut content in dissected animals. Hauksson (1979), using a similar method, found an average AE of around 27% in P. tremulus feeding on natural sediment low in organic matter. Ahlgren (1998) estimated an organic matter AE of around 15% for the related cold-water species A. californicus feeding on marine sediment low in organic matter. but found a more than 3 times higher AE (50%) when animals fed on salmon net pen fouling debris with a much higher organic matter content. This demonstrated that the animals were able to more efficiently absorb nutrients from a higher energy substrate. Similarly, an absorption of 40.4% of organic matter was found in A. californicus feeding on sediments with a high organic content located under Pacific oyster (Crassostrea gigas) suspended culture (Paltzat et al., 2008).
Factors that may have influenced our AE estimates include dissolving of the organic fraction of both feed and faecal pellets in the surrounding water. For the feed, leakage of nutrients was attempted minimized by the addition of binder (guar gum, 1%), whereas collection of faeces at 24 h intervals minimized the time the faeces was exposed to water. A defecation rate of ~24 h was reported by Hudson et al. (2004) who studied the movement of trace particles through the intestine of P. tremulus held in sea cages, and Hauksson (1979) similarly reported about 20 h for P. tremulus fed a typical sediment from a Norwegian fjord. A higher defecation rate was reported by Jespersen and Lützen (1971) who noted that harvested sea cucumbers had emptied their intestines after about 8–10 h when held in aquaria. Higher values of AFDW in faeces than in the feed, as measured when animals were given the Sargassum low feed, suggests that nutrient leakage from faeces probably was low, at least for this feed type. However, the low AE measured for the 50% sludge feed could indicate that leakage from feeds prepared from sludge could be higher. Further studies ought to investigate this aspect in more detail if aquaculture sludge is to be used in feed formulation for sea cucumber.
In our study, we offered diets consisting of one organic component mixed with dry sand of grain size 0.6-1 mm in aquaria with no bottom sediment added. In order to ensure only the feed given was ingested, bottom sediment was not used, although some studies suggest this can promote growth. Different types of bottom substrate are recommended for cultivating the species A. japonicus (Liu et al., 2010; Hou et al., 2017), and a heterogenous substrate layer may increase the availability for organic matter and facilitate feed intake and enhance growth accordingly. The mean ingestion rate did not differ significantly, but the variability in food intake was lowered between A. mollis individuals given feed containing 20% organic matter compared to feed containing 1-12% (Zamora and Jeffs, 2011). We observed very random movements of the different individuals as they were exploring the aquaria side surfaces as well as moving to and from the feed portion during the feeding periods. In a study on the sea cucumber A. californicus it was shown that movement was more random on sediment with high level of organic matter than on sediment with a low level of organic matter, but that hard vertical surfaces were favoured independent of organic content in the substrate (van Dam-Bates et al., 2016). This indicates that a high content of organic matter alone is not sufficient to make the sea cucumbers feed continuously in captivity.
Feeds based on single macroalgal ingredients such as Sargassum spp. may not be optimal as sea cucumber feed, and are often lacking in specific nutrients, such as omega-3 fatty acids (Feng et al., 2016). For instance, nutrient absorption in A. japonicus was improved when macroalgal diets were supplied with benthic microalgae (Gao et al., 2011), and Yuan et al. (2006) found that growth of juvenile A. japonicus was improved when powdered fermented algae diets were supplemented with dried bivalve faeces. Fermentation of macroalgal residues with probiotic bacteria also improved growth rates, digestive enzyme activities and intestinal microbiota in A. japonicus (Wang et al., 2020). The addition of aquaculture sludge (with a higher lipid content and higher omega-3/omega-6 fatty acid ratios) to sea cucumber feed formulations could help make up for some of the nutritional deficiencies of macroalgae.
These first preliminary results indicate that attractivity and digestibility of Sargassum spp. is high in P. tremulus, but that long-term studies of growth rates and feed conversion will have to be conducted to evaluate the nutritional and growth-promoting properties of Sargassum as an ingredient in formulated feeds for P. tremulus. Dried aquaculture sludge from Norwegian freshwater salmon production has a macronutrient composition that could be beneficial for inclusion in sea cucumber formulated feeds, but work remains to increase palatability and optimise incorporation of these ingredients in sea cucumber feeds.
4.3 Aquaculture sustainability
Due to their unique ability to feed on particulate matter and their high value in the seafood market, sea cucumbers have been considered prime candidates for inclusion in integrated multi-trophic aquaculture (IMTA) systems, by adding value and utilizing particulate matter released from finfish aquaculture (Tolon et al., 2017; Zamora et al., 2018; Israel et al., 2019). The Norwegian aquaculture industry which is dominated by Atlantic salmon (Salmo salar), releases large amount of valuable nutrients in sludge each year. This represents an underutilized resource that if not used may result in environmental overload. National environmental regulations require that at least 50% of the particulate matter discharged from land-based farms is collected. Attempts have been made at utilizing sludge for biogas (energy) production, but currently most of the collected sludge is sold as a low-valued fertilizer product. Bioconversion of particulate organic matter (sludge) which are residuals from the large Atlantic salmon production in Norway into sea cucumber feed will contribute to the sustainability of both productions. At present, current legislation in the EU/EAA area prohibits the use of waste (faeces) as feed for other food producing organisms.
The potential of P. tremulus as an aquaculture species, however, depend on a range of factors such as control of reproduction, growth rate and feed conversion efficiency in culture (Landes et al., 2019). Studies on other sea cucumber species such as Holothuria scabra has shown that aquaculture waste products can be used as feed in integrated sea cucumber aquaculture systems (Robinson et al., 2019). To fully evaluate the prospects of P. tremulus as an IMTA candidate species for Norway, it would be necessary to evaluate further the performance and growth when fed different feed sources, and also investigate the possibility of further improvement of sludge by microbiological pre-processing to achieve increased digestibility and/or probiotic function (Bao et al., 2017; Wang et al., 2020).
Our study is the first step towards investigating if particular waste (sludge) from the local Atlantic salmon industry could be part of the diet for a potential sea cucumber aquaculture industry in Norway. Our results indicate that sludge could have a use in formulation of sea cucumber feeds, and that it could contribute towards establishing a sustainable value chain. However, the combination of ingredients needs to be optimised through further studies on feed formulation. In China there is also an urgent need to find substitutes for Sargassum spp. due to the expansion of semi-intensive sea cucumber farming (Liu et al., 2010; Song et al., 2017), suggesting that re-use of aquaculture waste could have a similar potential in other countries.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
GC and JS contributed equally to conception and design of the study, performance of experimental work, data processing and writing of the submitted manuscript. JS performed the statistical analysis. All authors contributed to the article and approved the submitted version.
Funding
The work has been funded by the Research Council of Norway – SANOCEAN (project no. 288536), and the Møre og Romsdal County Municipality - marint miljø og verdiskapingsfond (grant no. 2018-0150).
Acknowledgments
The authors wish to thank MOWI Steinsvik in Volda, Norway for supplying the dried aquaculture sludge used in this experiment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aas T. S., Åsgård T. (2017). Estimated content of nutrients and energy in feed spill and faeces in Norwegian salmon culture. Nofima Rep. (in Norwegian). Report no. 18/2017, 8 pages.
Ahlgren M. O. (1998). Consumption and assimilation of salmon net pen fouling debris by the red sea cucumber Parastichopus californicus: implications for polyculture. J. World Aquaculture Soc. 29 (2), 133–139. doi: 10.1111/j.1749-7345.1998.tb00972.x
Bao N., Ren T., Han Y., Wang F., Chen F., Jiang Z. (2017). Alteration of growth, intestinal lactobacillus, selected immune and digestive enzyme activities in juvenile sea cucumber Apostichopus japonicus, fed dietary multiple probiotics. Aquaculture Int. 25 (5), 1721–1731. doi: 10.1007/s10499-017-0148-8
Cameron J. L., Fankboner P. V. (1984). Tentacle structure and feeding processes in life stages of the commercial sea cucumber Parastichopus californicus (Stimpson). J. Exp. Mar. Biol. Ecol. 81 (2), 193–209. doi: 10.1016/0022-0981(84)90006-6
Christophersen G., Bakke S., Sunde J. (2021). Norwegian Red sea cucumber (Parastichopus tremulus) fishery and aquaculture north of 60°N latitude: Feasible or fictional? SPC Beche-de-mer Inf. Bull. 41, 25–36.
Christophersen G., Sunde J. (2022). The challenge of economically viable aquaculture of the cold-water sea cucumber Parastichopus tremulus in Norway. SPC Beche-de-mer Inf. Bull. 42, 65–72.
Conover R. J. (1966). Assimilation of organic matter by zooplankton. Limnology Oceanography 11, 338–345. doi: 10.4319/lo.1966.11.3.0338
Dar M. A., Ahmad H. O. (2006). The feeding selectivity and ecological role of shallow water holothurians in the red Sea. Bêche-de-mer Inf. Bull. 24, 11–21.
David F., Hubas C., Laguerre H., Badou A., Herault G., Bordelet T., et al. (2020). Food sources, digestive efficiency and resource allocation in the sea cucumber Holothuria forskali (Echinodermata: Holothuroidea): Insights from pigments and fatty acids. Aquaculture Nutr. 26 (5), 1568–1583. doi: 10.1111/anu.13103
David V. M. M., MacDonald B. A. (2002). Seasonal biochemical composition of tissues from Cucumaria frondosa collected in the bay of fundy, Canada: feeding activity and reproduction. J. Mar. Biol. Assoc. United Kingdom 82 (1), 141–147. doi: 10.1017/S0025315402005258
Domínguez-Godino J. A., González-Wangüemert M. (2019). Assessment of Holothuria arguinensis feeding rate, growth and absorption efficiency under aquaculture conditions. New Z. J. Mar. Freshw. Res. 53 (1), 60–76. doi: 10.1080/00288330.2018.1480499
Feng J., Anisuzzaman M., U-Cheol J., Jong-Kuk C., Hak-Sun Y., Seung-Wan K., et al. (2016). Comparison of fatty acid composition of wild and cultured sea cucumber Apostichopus japonicus. Korean J. Fisheries Aquat. Sci. 49, 474–485. doi: 10.5657/KFAS.2016.0474
Gao Q.-F., Wang Y., Dong S., Sun Z., Wang F. (2011). Absorption of different food sources by sea cucumber Apostichopus japonicus (Selenka) (Echinodermata: Holothuroidea): Evidence from carbon stable isotope. Aquaculture 319 (1-2), 272–276. doi: 10.1016/j.aquaculture.2011.06.051
Gianasi B. L., Parrish C. C., Hamel J. F., Mercier A. (2017). Influence of diet on growth, reproduction and lipid and fatty acid composition in the sea cucumber Cucumaria frondosa. Aquaculture Res. 48 (7), 3413–3432. doi: 10.1111/are.13168
Giraspy D. A. B., Ivy G. (2008). The influence of commercial diets on growth and survival in the commercially important sea cucumber Holothuria scabra var. versicolor (Conand 1986) (Echinodermata: Holothuroidea). SPC beche-de-mer Inf. Bull. 28, 46–52.
Hannah L., Duprey N., Blackburn J., Hand C. M., Christopher M. (2012). Growth rate of the California sea cucumber Parastichopus californicus: Measurement accuracy and relationships between size and weight metrics. North Am. J. Fisheries Manage. 32, 167–176. doi: 10.1080/02755947.2012.663455
Hauksson E. (1977). Ernæringsøkologiske undersøkelser av Stichopus tremulus (Gunnerus), en detritus-etende holothuroid (Bergen, Norway: University of Bergen). M.Sc. thesis (in Norwegian).
Hauksson E. (1979). Feeding biology of Stichopus tremulus, a deposit-feeding holothurian. Sarsia 64 (3), 155–160. doi: 10.1080/00364827.1979.10411376
Hou Y. R., Sun Y. J., Gao Q. F., Dong S. L., Wen B., Yu H. B. (2017). Optimal mud to sand ratios in sediment for sea cucumber aquaculture as revealed by carbon stable isotopes. Aquaculture Environ. Interact. 9, 281–291. doi: 10.3354/aei00228
Hudson I. R., Wigham B. D., Tyler P. A. (2004). The feeding behaviour of a deep-sea holothurian, Stichopus tremulus (Gunnerus) based on in situ observations and experiments using a remotely operated vehicle. J. Exp. Mar. Biol. Ecol. 301 (1), 75–91. doi: 10.1016/j.jembe.2003.09.015
Israel D., Lupatsch I., Angel D. L. (2019). Testing the digestibility of seabream wastes in three candidates for integrated multi-trophic aquaculture: Grey mullet, sea urchin and sea cucumber. Aquaculture 510, 364–370. doi: 10.1016/j.aquaculture.2019.06.003
Jespersen A., Lützen J. (1971). On the ecology of the aspidochirote sea cucumber Stichopus tremulus (Gunnerus). Norwegian J. Zoology 19, 117–132.
Kjerstad M., Ringvold H., Søvik G., Knott K., Thangstad T. (2015). “Preliminary study on the utilisation of Norwegian red sea cucumber, Parastichopus tremulus (Gunnerus 1767) (Holothuroidea, Echinodermata), from Norwegian waters: resource, biology and market. pp. 109–132,” in Blue bio-resources. Eds. Gundersen A. C., Velle L. G. (Norway: Orkana Akademisk).
Landes A. M., Sunde J., Christophersen G. (2019). “Atlantic Sea cucumber species in the spotlight – prospects for Norwegian aquaculture. pp 19-49,” in International perspectives on regional research and practice. Eds. Akslen-Hoel L. K., Egilsson B. (Norway: Orkana Akademisk).
Liu Y., Dong S., Tian X., Wang F., Gao Q. (2010). The effect of different macroalgae on the growth of sea cucumbers (Apostichopus japonicus selenka). Aquaculture Res. 41 (11), e881–e885. doi: 10.1111/j.1365-2109.2010.02582.x
Liu Y., Dong S., Tian X., Wang F., Gao Q. (2009). Effects of dietary sea mud and yellow soil on growth and energy budget of the sea cucumber Apostichopus japonicus (Selenka). Aquaculture 286 (3-4), 266–270. doi: 10.1016/j.aquaculture.2008.09.029
Madsen F. J., Hansen B. (1994). “Echinodermata, Holothurioidea,” in Marine invertebrates of Scandinavia number 9 (Oslo: Scandinavian University press).
McEdward L. R., Miner B. G. (2001). Larval and life-cycle patterns in echinoderms. Can. J. Zoology 79 (7), 1125–1170. doi: 10.1139/z00-218
Moriarty D. J. W. (1982). Feeding of Holothuria atra and Stichopus chloronotus on bacteria, organic carbon and organic nitrogen in sediments of the great barrier reef. Mar. Freshw. Res. 33 (2), 255–263. doi: 10.1071/MF9820255
Paltzat D. L., Pearce C. M., Barnes P. A., McKinley R. S. (2008). Growth and production of California sea cucumbers (Parastichopus californicus stimpson) co-cultured with suspended pacific oysters (Crassostrea gigas thunberg). Aquaculture 275 (1-4), 124–137. doi: 10.1016/j.aquaculture.2007.12.014
Purcell S. W., Samyn Y., Conand C. (2012). Commercially important sea cucumbers of the world. FAO species catalogue for fishery purposes. no. 6 (Rome: FAO), 150 pp. 30 colour plates.
R Core Team (2022). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org/.
Roberts D., Gebruk A., Levin V., Manship B. A. D. (2000). Feeding and digestive strategies in deposit-feeding holothurians. Oceanography Mar. Biology: an Annu. Rev. 38, 257–310.
Robinson G., Caldwell G. S., Jones C. L. W., Stead S. M. (2019). The effect of resource quality on the growth of Holothuria scabra during aquaculture waste bioremediation. Aquaculture 499, 101–108. doi: 10.1016/j.aquaculture.2018.09.024
Ru X., Zhang L., Li X., Liu S., Yang H. (2019). Development strategies for the sea cucumber industry in China. J. Oceanology Limnology 37 (1), 300–312. doi: 10.1007/s00343-019-7344-5
Sargent J. R., Hopkins C. C. E., Seiring J. V., Youngson A. (1983). Partial characterization of organic material in surface sediments from Balsfjorden, northern Norway, in relation to its origin and nutritional value for sediment-ingesting animals. Mar. Biol. 76 (1), 87–94. doi: 10.1007/BF00393059
Schagerström E., Christophersen G., Sunde J., Bakke S., Matusse N. R., Dupont S., et al. (2022). Controlled spawning and rearing of the sea cucumber, Parastichopus tremulus. J. World Aquaculture Soc. 53 (1), 224–240. doi: 10.1111/jwas.12816
Seo J. Y., Lee S. M. (2011). Optimum dietary protein and lipid levels for growth of juvenile sea cucumber Apostichopus japonicus. Aquaculture Nutr. 17, e56–e61. doi: 10.1111/j.1365-2095.2009.00728.x
Shi C., Dong S., Wang F., Gao Q., Tian X. (2015). Effects of the sizes of mud or sand particles in feed on growth and energy budgets of young sea cucumber (Apostichopus japonicus). Aquaculture 440, 6–11. doi: 10.1016/j.aquaculture.2015.01.028
Slater M. J., Carton A. G. (2009). Effect of sea cucumber (Australostichopus mollis) grazing on coastal sediments impacted by mussel farm deposition. Mar. pollut. Bull. 58, 1 123–1 129. doi: 10.1016/j.marpolbul.2009.04.008
Slater M. J., Carton A. G. (2010). Sea cucumber habitat differentiation and site retention as determined by intraspecific stable isotope variation. Aquaculture Res. 41, e695–e702. doi: 10.1111/j.1365-2109.2010.02607.x
Song X. Y., Xu Q., Zhou Y., Lin C. G., Yang H. S. (2017). Growth, feed utilization and energy budgets of the sea cucumber Apostichopus japonicus with different diets containing the green tide macroalgae Chaetomorpha linum and the seagrass. Zostera marina. Aquaculture 470, 157–163. doi: 10.1016/j.aquaculture.2016.12.035
Sun H., Liang M., Yan J., Chen B. (2004). “Nutrient requirements and growth of the sea cucumber, Apostichopus japonicus,” in Advances in sea cucumber aquaculture and management, vol. 463 . Eds. Lovatelli A., Conand C., Purcell S., Uthicke S., Hamel J.-F., Mercier A. (Rome, Italy: FAO Fisheries Technical Paper), 327–331.
Sun J., Zhang L., Pan Y., Lin C., Wang F., Kan R., et al. (2015). Feeding behavior and digestive physiology in sea cucumber Apostichopus japonicus. Physiol. Behav. 139, 336–343. doi: 10.1016/j.physbeh.2014.11.051
Tolon T., Emiroglu D., Gunay D., Ozgul A. (2017). Sea cucumber (Holothuria tubulosa gmelin 1790) culture under marine fish net cages for potential use in integrated multi-trophic aquaculture (IMTA). Indian J. Geo Mar. Sci. 46 (4), 749–756.
Uthicke S., Klumpp D. W. (1998). Microphytobenthos community production at a near-shore coral reef: seasonal variation and response to ammonium recycled by holothurians. Mar. Ecol. Prog. Ser. 169, 1–11. doi: 10.3354/meps169001
van Dam-Bates P., Curtis D. L., Cowen L. L., Cross S. F., Pearce C. M. (2016). Assessing movement of the California sea cucumber Parastichopus californicus in response to organically enriched areas typical of aquaculture sites. Aquaculture Environ. Interact. 8, 67–76. doi: 10.3354/aei00156
Wang G., Meng Z., Chen L., Jiang J., Feng Y., Zhang B. (2020). Effects of kelp residues fermented with probiotics on the culture of sea cucumber, Apostichopus japonicus. Aquaculture Res. 51 (3), 1133–1142. doi: 10.1111/are.14460
Whitefield C. R., Oliveira A. C., Hardy S. M. (2018). Composition of phytodetrital food resources affects reproductive success in the deposit-feeding sea cucumber, Parastichopus californicus (Stimpson 1857). J. Exp. Mar. Biol. Ecol. 500, 1–11. doi: 10.1016/j.jembe.2017.12.004
Won S., Hamidoghli A., Lee J., Bae J., Bai S. C. (2018). Effects of three different dietary binders on juvenile sea cucumber, Apostichopus japonicus. Turkish J. Fisheries Aquat. Sci. 18 (7), 913–920. doi: 10.4194/1303-2712-v18_7_09
Xia B., Ren Y., Wang J., Sun Y., Zhang Z. (2017). Effects of feeding frequency and density on growth, energy budget and physiological performance of sea cucumber Apostichopus japonicus (Selenka). Aquaculture 466, 26–32. doi: 10.1016/j.aquaculture.2016.09.039
Xia S., Zhao P., Chen K., Li Y., Liu S., Zhang L., et al. (2012). Feeding preferences of the sea cucumber Apostichopus japonicus (Selenka) on various seaweed diets. Aquaculture 344, 205–209. doi: 10.1016/j.aquaculture.2012.03.022
Xu Q., Hamel J.-F., Mercier A. (2015). “Chapter 10. feeding, digestion, nutritional physiology, and bioenergetic,” in The Sea cucumber Apostichopus japonicus. history, biology and aquaculture. Eds. Yang H., Hamel J.-F., Mercier A. (Oxford, UK: Academic Press), 153–176. doi: 10.1016/B978-0-12-799953-1.00010-6
Yang H., Hamel J.-F., Mercier A. (Eds.) (2015). The Sea cucumber Apostichopus japonicus. history, biology and aquaculture (Oxford, UK: Academic Press). doi: 10.1016/B978-0-12-799953-1.00002-7
Yang H., Yuan X., Zhou Y., Mao Y., Zhang T., Liu Y. (2005). Effects of body size and water temperature on food consumption and growth in the sea cucumber Apostichopus japonicus (Selenka) with special reference to aestivation. Aquaculture Res. (Oxford: UK) 36 (11), 1085–1092. doi: 10.1111/j.1365-2109.2005.01325.x
Yingst J. Y. (1976). The utilization of organic matter in shallow marine sediments by an epibenthic deposit-feeding holothurian. J. Exp. Mar. Biol. Ecol. 23 (1), 55–69. doi: 10.1016/0022-0981(76)90085-X
Yuan X., Yang H., Zhou Y., Mao Y., Zhang T., Liu Y. (2006). The influence of diets containing dried bivalve feces and/or powdered algae on growth and energy distribution in sea cucumber Apostichopus japonicus (Selenka) (Echinodermata: Holothuroidea). Aquaculture 256 (1-4), 457–467. doi: 10.1016/j.aquaculture.2006.01.029
Yu Z., Zhou Y., Yang H., Hu C. (2014). Survival, growth, food availability and assimilation efficiency of the sea cucumber Apostichopus japonicus bottom-cultured under a fish farm in southern China. Aquaculture 426, 238–248. doi: 10.1016/j.aquaculture.2014.02.013
Zamora L. N., Jeffs A. G. (2011). Feeding, selection, digestion and absorption of the organic matter from mussel waste by juveniles of the deposit-feeding sea cucumber, Australostichopus mollis. Aquaculture 317 (1-4), 223–228. doi: 10.1016/j.aquaculture.2011.04.011
Zamora L. N., Yuan X., Carton A. G., Slater M. J. (2018). Role of deposit-feeding sea cucumbers in integrated multitrophic aquaculture: progress, problems, potential and future challenges. Rev. Aquaculture 10 (1), 57–74. doi: 10.1111/raq.12147
Keywords: aquaculture, feed intake, IMTA, parastichopus tremulus, sargassum, sea cucumber, sludge, absorption efficiency
Citation: Sunde J and Christophersen G (2023) Appetite in captivity - feeding studies of the red sea cucumber Parastichopus tremulus. Front. Mar. Sci. 9:1052968. doi: 10.3389/fmars.2022.1052968
Received: 24 September 2022; Accepted: 23 December 2022;
Published: 12 January 2023.
Edited by:
Libin Zhang, Institute of Oceanology (CAS), ChinaReviewed by:
Georgina Robinson, Scottish Association For Marine Science, United KingdomIngrid Lupatsch, AB Agri Ltd, United Kingdom
Copyright © 2023 Sunde and Christophersen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gyda Christophersen, Z3lkYS5jaHJpc3RvcGhlcnNlbkBtb3JlZm9yc2tpbmcubm8=
 Jan Sunde
Jan Sunde Gyda Christophersen
Gyda Christophersen