- 1Laboratory of Marine Biology, Faculty of Agriculture, Kyushu University, Fukuoka, Japan
- 2Karatsu Satellite of Aqua-Bioresource Innovation Center, Kyushu University, Karatsu, Japan
The gonadal sexual fate of vertebrates is either defined by genetics or environment, or a combination of both factors. Interestingly, in sequential hermaphroditism, the animal can undergo natural sex changes from female-to-male, male-to-female, and bidirectional way throughout their lives. This change exhibits the process which shifts between oogenesis and spermatogenesis and is regarded as an ideal instance of sexual plasticity. To develop the experimental model for studying the sexual plasticity of protogynous fish, the social conditions that induce sex changes were defined in wrasse, Pseudolabrus sieboldi. When six females were kept together in a tank, the largest female became a male, whereas a similar conversion did not occur when only two females were present in a tank. A semi-gonadectomy analysis developed in the present study verified the direct relationship between gonadal sex and body coloration. In P. sieboldi, the sex change is controlled by the relative body size of an individual within a group, rather than by absolute body size. When six females were kept in smaller sized tanks, delayed sex change or unchanged individuals was observed. Overall, more than 90% of the largest females demonstrated sex change after being housed with five smaller females in different sizes of tanks ranging from 80 to 500 L. Furthermore, the experiment using a transparent barrier suggested that visual stimuli are one of the major cues to initiate sex change. Our findings on the laboratory conditions leading to the initiation of sex change in wrasse suggest the usefulness of this species as a model organism for comparative studies in molecular, cellular, and physiological mechanisms of sexual plasticity, as well as on social and reproductive behaviors.
1. Introduction
In vertebrates, sex is often determined by genetics, environment, or a combination of both factors. The primordial gonad either differentiates as an ovary or a testis according to their sex, and this gonad undergoes active maintenance to suppress the opposite sex throughout adulthood (Capel, 2017; Nagahama et al., 2021; Stöck et al., 2021). Notably, most fish species are gonochoristic (i.e., males always remain as males and females always remain as females) in nature and retain the same sex throughout their lifespans. However, hermaphroditism, harboring both male and female gametes at some point in their life, has been documented in about 6% of all teleost families occupying nearly 2% of all teleost species (Sadovy de Mitcheson and Liu, 2008; Kuwamura et al., 2020; Pla et al., 2021). Sequential hermaphrodites have the ability to undergo natural sex change from female to male (e.g. Asian sheepshead wrasse), male to female (e.g. Australian barramundi, gilthead seabream and the black porgy), and/or bidirectional (e.g. coral gobies) way during their lives. This change exhibits the process which shifts between oogenesis and spermatogenesis and is generally accompanied by the transformation of gonadal structure between ovarian and testicular tissues. Concurrently, change in mating behavior and morphology, such as body shapes and coloration, have been observed in various sex-changing species. Thus, sequential hermaphroditism has been regarded as an ideal coordinated example of sexual plasticity in the gonad, brain, and other morphological characteristics.
Numerous attempts have been made to reveal the physiological, molecular, and cellular control of sex change in hermaphrodite fish (Guiguen et al., 2010; Todd et al., 2016; Nagahama et al., 2021). Among these fish, several species undergo socially controlled sex change which is brought about by the transformation sequences in the gonad and brain (e.g., Wrasses: Todd et al., 2019; Goikoetxea et al., 2021; Groupers: Han et al., 2018; Gobies: Kobayashi et al., 2009; Black et al., 2011). Interspecies differences in transformation mechanisms, e.g., direction, timing, pattern of gonadal transformation, scarcity of specimen, and tedious process of captive sex change, limit the animal for such socially controlled natural sex change research. Notably, wrasse, Pseudolabrus sieboldi, is a diandric protogynous fish species, which typically exhibits primary males and sex-changed males, i.e., the secondary males derived from females. Females change sex by social condition and become territorial males with alteration of body coloration from the initial phase (IP) to the terminal phase (TP) (Nakazono, 1979). The primary males also change body coloration from IP to TP when they become territorial males. Additionally, the change in body coloration from IP female to TP secondary male and the correlation between the gonadal stage and body coloration has already been demonstrated and quantified (Ohta et al., 2008; Ohta et al., 2012). Moreover, sex change in captivity has been observed when six females were kept together in a tank (Ohta et al., 2003). Thus, this species holds the potential to be used as a unique model for studying the social control of sexual plasticity and its physiological mechanisms. However, the social cue to induce sex change in the captive condition has not yet been clearly defined.
In this study, we have demonstrated the social conditions required for inducing sex change in captivity. The conditions related to absolute body size and aquarium capacity were essentially analyzed to document the process of gonadal changes. A semi-gonadectomy analysis was also developed to evaluate the relationship between the gonad and change in body color within the same individual. Additionally, visual cues acted as one of the major cues to initiate sex change. The results propose that wrasse (P. sieboldi) holds the potential to zeitgebers as a new experimental model system for sexual plasticity studies of the gonad and other sex-linked characteristics which are controlled by the social environment.
2. Materials and methods
2.1. Fish and sampling
Fish were obtained by hook and line along the coast of Tsuyazaki (Fukuoka, Japan) near the Fisheries Institute of Kyushu University (33.8°N, 130.4°E; Fukuoka, Japan) and transported to 1,000 L stock tanks in the laboratory. In each tank, 10 terminal-phase (TP) fish and 20 initial-phase (IP) fish were kept together. At the start of the experiment, the fish were anesthetized using 50 ppm ethyl-4-aminobenzoate (Sigma Aldrich, Japan) and photographed as described in our previous study (Ohta et al., 2008). Upon recovery from anesthetization, the fish were divided into singles, pairs, or groups based on the total length and coloration, and subsequently transferred to black color tanks corresponding to the different experiments, as described below. After the completion of the experiment, fish were again anesthetized and photographed, and the gonads were dissected and sexed accordingly for histological analysis. It is of importance to note that, if an IP male was present in the tank, the group was eliminated from further analysis. Similarly, if some fish died, then the whole group was omitted from the experiment. All experimental protocols herein used were approved by the Institutional Animal Care and Use Committee of Kyushu University.
2.1.1. Experiment 1
To investigate the social condition and the seasonality of sex change in P. sieboldi, the following experiments were conducted during the pre-spawning (July to August) and post-spawning (December to March) seasons. One IP individual (>140 mm in total length) and five IP individuals (<130 mm) were placed in a 500 L tank, with or without a TP male. The TP male, when present, was the largest in the group. Five tanks were prepared for each season. For the experiments conducted during the spawning and post-spawning seasons, the largest female in each group was sexed based on the spawning characteristics before the start of the experiment. During the pre-spawning season experiments, the fish were maintained in the tanks for 30 days, while for the experiments conducted during the post-spawning season, the fish were reared for 120 days, owing to the relatively slower gonadal changes brought about by the low water temperature.
2.1.2. Experiment 2
To evaluate the gonadal alterations directly within the same individual, a semi-gonadectomy was performed before the induction of sex change during the pre-spawning season. P. sieboldi possesses a pair of gonads at the dorsal side of the body cavity, and upon gonadal sex change, no differences were observed between the two gonads. After anesthetizing with 50 ppm of benzocaine, laparotomy was performed by making an incision using a surgical knife in the ventral area between the pelvic fins and the anus. The left side of the gonad was excised using forceps and scissors, without disturbing the intestine and other tissues. The incised area was then sutured with a 3-0 silk suture. The fish were disinfected with 0.01% sodium nifurstyrenate (Ueno Food Techno Industry, Ltd., Tokyo, Japan) for 2 h and then placed in a 500 L tank containing five smaller IP fish. Five such replicate tanks were prepared.
2.1.3. Experiment 3
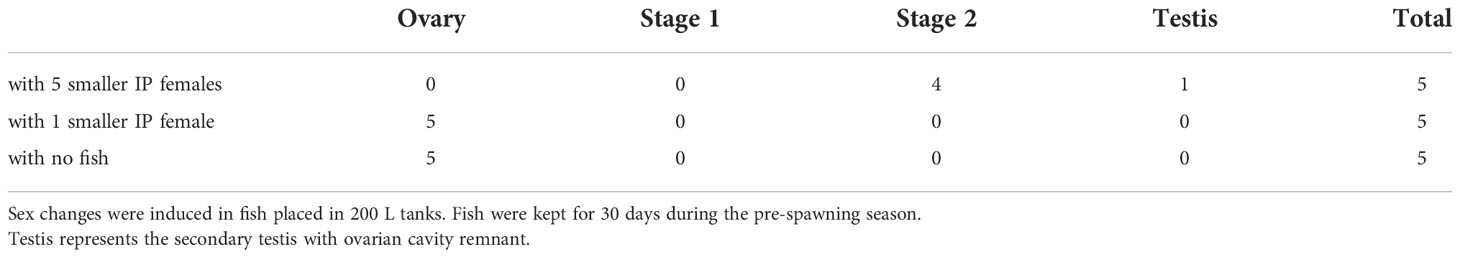
To test the occurrence of sex change in smaller tank sizes and to define the number of smaller IP conspecifics for the sex change of larger IP females, one IP fish (>140 mm in length) was housed in a 200 L tank with 0, 1, or 5 smaller IP fish (<130 mm in length) for 30 days, during the pre-spawning season (July to August).
2.1.4. Experiment 4
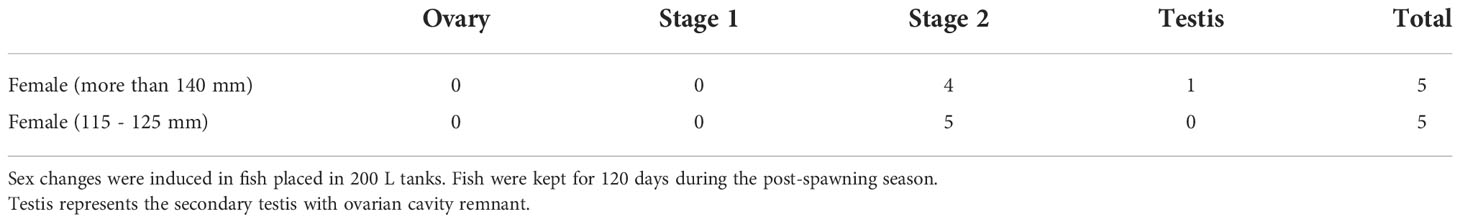
To determine whether the induction of a sex change is linked to social relationships rather than to absolute body length, one small (115–125 mm) IP female was placed in a 200 L tank together with five smaller (<105 mm) IP females. The IP females were selected based on their spawning activity with a larger TP male during the spawning season. After the spawning season, five tanks were prepared as described above (Experiment 3) and maintained for 120 days during the post-spawning season (December to March).
2.1.5. Experiment 5
To understand the effect of a small-sized tank on the sex change of P. sieboldi, one IP fish of >140 mm in length was kept in an 80 L tank together with five smaller IP fish (<130 mm in length) for 3, 7, 21, or 28 days during the pre-spawning season (July to August). The time course of the sex change was also observed. Eight replicate tanks were prepared for each time point.
2.1.6. Experiment 6
To comprehend whether physical interaction is involved in the sex change process, an IP female was separated from five smaller females by a clear rectangle barrier in an 80 L tank. The barrier allowed only visual interactions, and all physical interactions were avoided. Water flow was adjusted to flow from the region of the largest female to the area of smaller females. Eight such replicate tanks were prepared and maintained for 30 days during the post-spawning season.
The conditions of the experiments are illustrated and summarized in Figure 1.
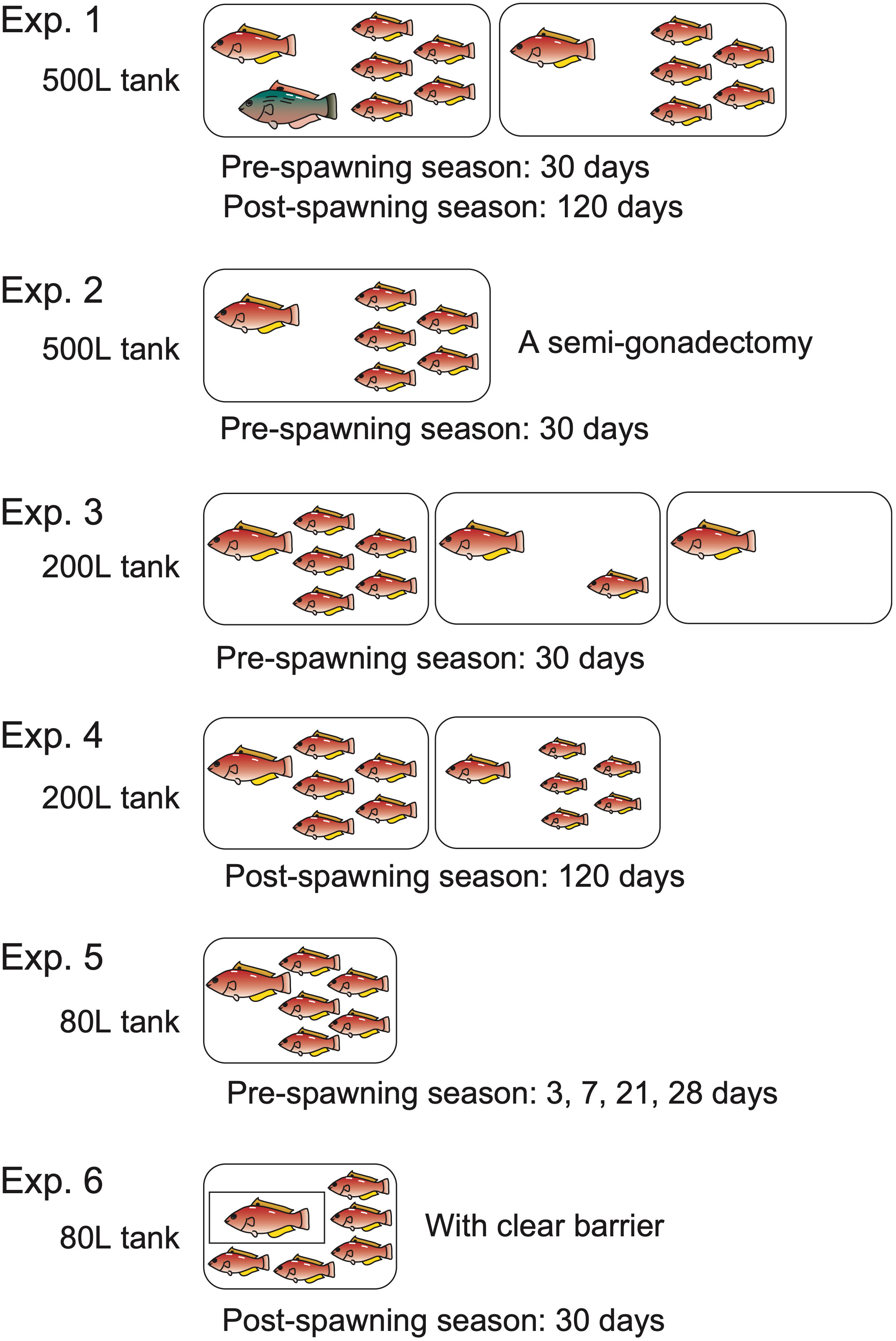
Figure 1 The experimental conditions in this study are summarized. In Experiment 1, one IP individual and five smaller IP individuals were placed in a 500 L tank, with or without a TP male. The fish were maintained for 30 days during the pre-spawning season, while, on the other hand, the fish were reared for 120 days during the post-spawning season. In Experiment 2, a semi-gonadectomy was performed before the induction of sex change during the pre-spawning season and placed in a 500 L tank containing five smaller IP fish. In Experiment 3, one IP fish was housed in a 200 L tank with zero, one, or five smaller IP fish for 30 days during the pre-spawning season. In Experiment 4, one small IP female was placed in a 200 L tank together with five smaller IP females and maintained for 120 days during the post-spawning season. In Experiment 5, one IP fish was kept in an 80 L tank together with five smaller IP fish for 3, 7, 21, or 28 days during the pre-spawning season. In Experiment 6, an IP female was separated from five smaller females by a clear rectangle barrier in an 80 L tank and maintained for 30 days during the post-spawning season.
2.2. Gonadal histology
Gonads were fixed overnight in Bouin’s solution, dehydrated, and embedded in Technovit 7100 resin (Kulzer, Wehrheim, Germany). For light microscopy, 4-μm-thick sections were cut and stained with 1% toluidine blue. The oocytes and testicular germ cells were classified according to the developmental stages (Ohta et al., 2008). Stage-1 gonads contained the cysts of type B spermatogonia and spermatocytes. The cysts appeared sporadically, but ubiquitously, among the oocytes. Stage-2 gonads contained spermatids and/or spermatozoa with the oocytes. The secondary testes, characteristic of sex-changed females, were distinguished from the primary testes of IP males by the presence of a remnant ovarian cavity, ovarian wall, or peripherally located sperm duct (Matsuyama et al., 1997).
2.3. Body coloration
Briefly, fish were anesthetized and placed in a transparent glass aquarium filled with seawater. A digital camera (C-920; Olympus, Tokyo, Japan) was fixed on a camera stand (CS-5; LPL SHOJI K.K., Tokyo, Japan) in a dark room. The left-lateral view of each fish was digitally photographed, together with a color separation guide (Kodak, Rochester, NY, USA), and the images were analyzed using Adobe Photoshop (Adobe Systems Inc., San Jose, CA, USA). To equalize the color among the images, the RGB (red, green, and blue) values of the respective black and white squares of the color separation guide in each picture were adjusted to R = 5, G = 5, B = 5 and R = 245, G = 245, B = 245. In this equalization process, the hue values of the red and yellow squares of the color separation guide were always maintained at 0° and 55°, respectively. Subsequently, the hue value of the area of the anal fin between the third spine and the first ray were measured. Our previous study demonstrated that females have a yellowish anal fin whose hue value ranges from 40° to 55°, whereas the anal fin of the secondary (TP) males exhibited a reddish hue ranging less than 25°.
2.4. Statistical analysis
Statistical analyses were carried out using GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA). In Experiment 3, the difference in the hue values between the start and the end of the experimental period were compared using paired t-test.
3. Results
3.1. Experiment 1: Occurrence of sex change without a larger TP male
When a larger IP female was placed in a 500 L tank with five smaller IP females, sex change was observed in both the pre- and post-spawning season, especially in the absence of a TP male. During the pre- and post-spawning seasons, the largest fish had stage 2 inter-sexual gonads and/or secondary testes (Table 1), along with the change in body coloration. Specifically, the hue of the anal fin changed from yellow to red, typical of the change in body coloration that occurs during the IP to TP transition (Figures 2, 3). However, when a larger TP male was present in the group, neither gonadal nor body coloration changed in the largest female.
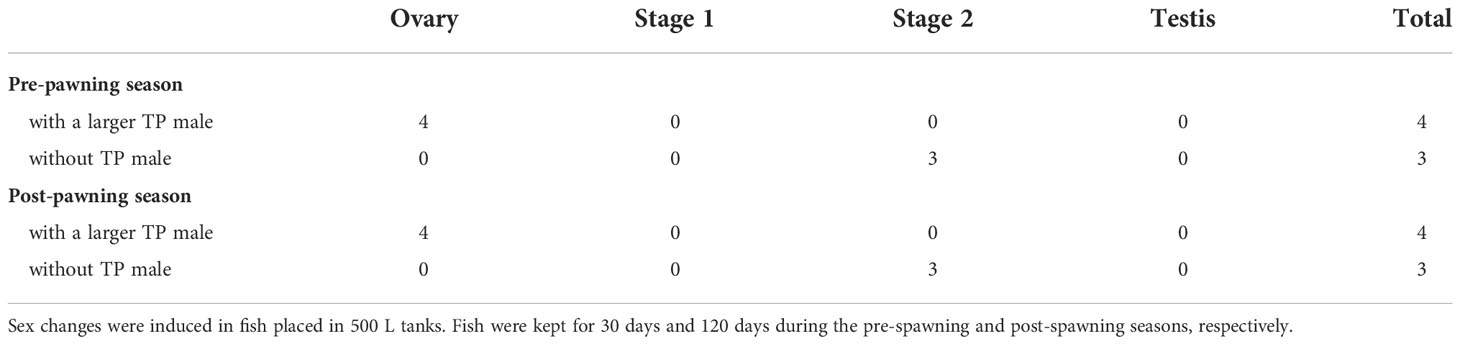
Table 1 Gonadal stages of IP fish kept with five smaller IP females during the pre-spawning and post-spawning seasons.

Figure 2 Alterations in the body coloration of P. sieboldi after the induction of a sex change. Images from (A) the beginning and (B) the end of the experiment are shown. Sex changes were induced in fish placed in a 500 L tank during the post-spawning season. Bar length, 10 mm.
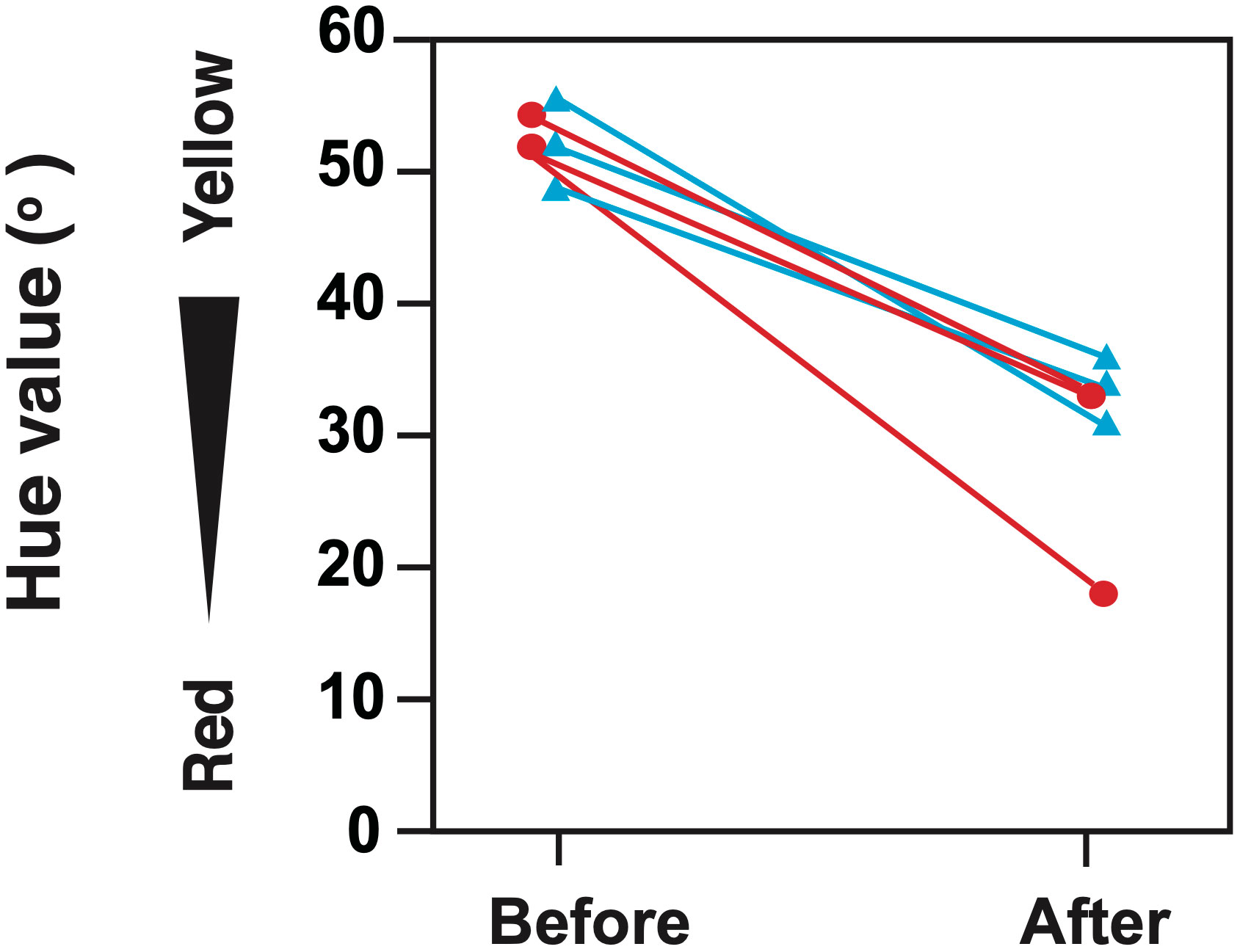
Figure 3 Switch in the hue values of the anal fin of P. sieboldi after the induction of a sex change during different seasons. Sex changes were induced during the pre-spawning (30 days; circles) and post-spawning (120 days; triangles) seasons in fish placed in 500 L tanks (n = 3).
3.2. Experiment 2: Gonadal transition linked to body color change
A semi-gonadectomy analysis verified the direct relationship between the gonadal sex and body coloration during the pre-spawning season. While the gonads of the treated fish at the start of the experiment were ovaries, only the larger female exhibited stage 2 inter-sexual gonads after the experimental period, during which the treated female was placed together with five smaller females (Figure 4). The gonad showed cysts of sperm along with degenerating oocytes. The coloration of the anal fin of these fish also changed during the IP to TP transition (Figure 5). On the contrary, the smaller semi-gonadectomized fish did not show any signs of gonadal sex change.
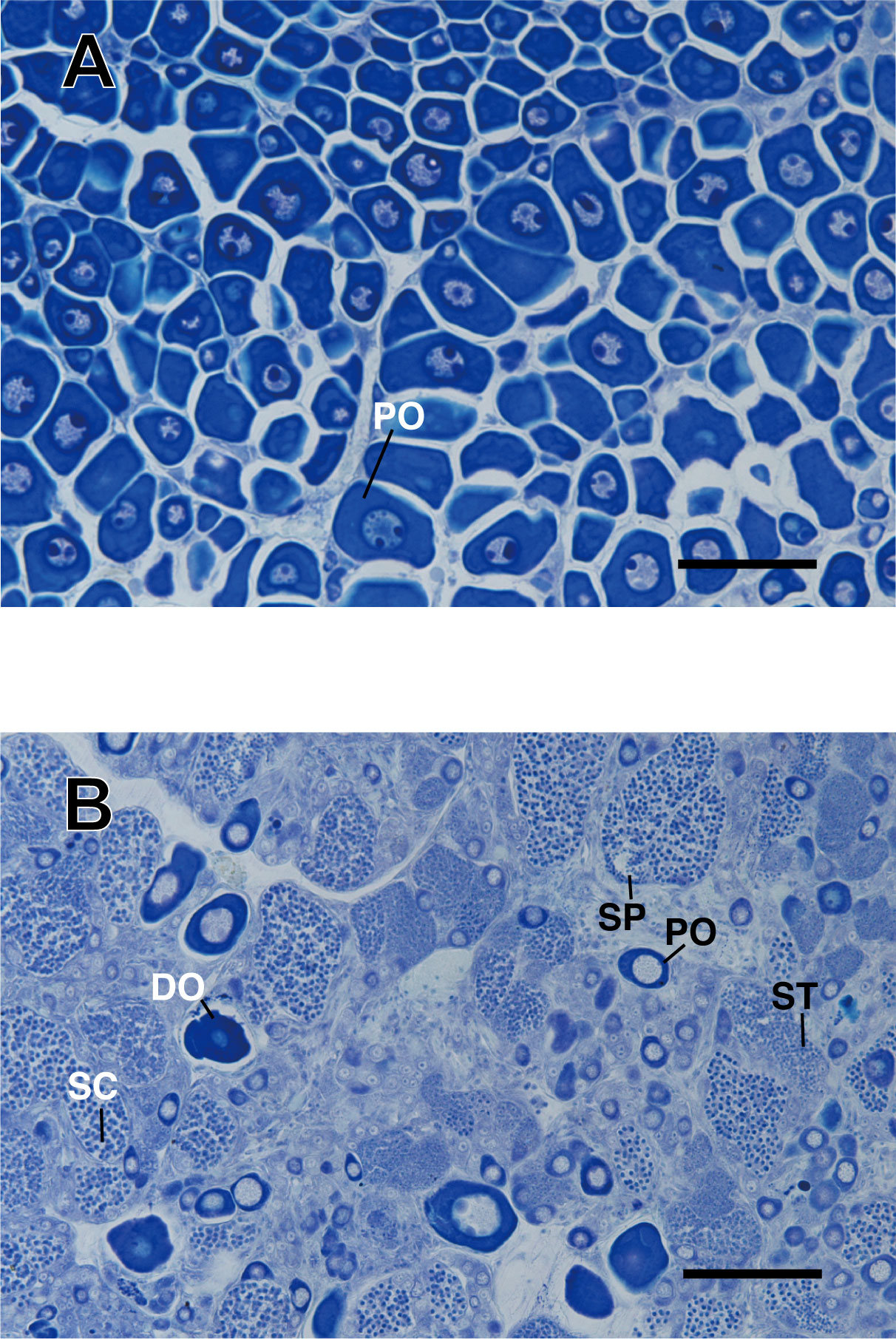
Figure 4 Conversion in the gonadal histology of the semi-gonadectomized P. sieboldi. Histological changes were evaluated in semi-gonadectomized fish, prior to induction of sex change. Images from (A) the beginning and (B) the end of the experiment are shown. Sex changes were induced in fish after semi-gonadectomy and placed in a 500 L tank during the pre-spawning season. Black arrow; sperm, white arrow; oocyte. PO, perinuclear oocyte; DO, degenerated oocyte; SC, spermatocytes; ST, spermatid;, SP, sperm; Bar length, 100 mm.

Figure 5 Changes in the anal fin coloration in the semi-gonadectomized P. sieboldi. Images from (A) the beginning and (B) the end of the experiment are presented. Sex changes were induced in fish after semi-gonadectomy and placed in a 500 L tank during the pre-spawning season.
3.3. Experiment 3: Harem is mandatory for sex change
When a large female was housed together with five smaller females in a 200 L tank during the pre-spawning season, the body color of the larger female transmuted (p < 0.001) (Figure 6). The coloration of the larger fish changed significantly when housed with five smaller females. After the experimental period, the gonads of the larger fish that had undergone a color change depicted either stage 2 inter-sexual gonads or secondary testis developmental phase (Table 2). Only, 20% of the larger female kept with 5 IP males showcased a complete sex change, while the remaining 80% of the larger female fish were on the verge of sex change and harbored stage 2 gonad. Interestingly, this phenomenon was not observed when the larger female was paired with one IP female or kept alone.
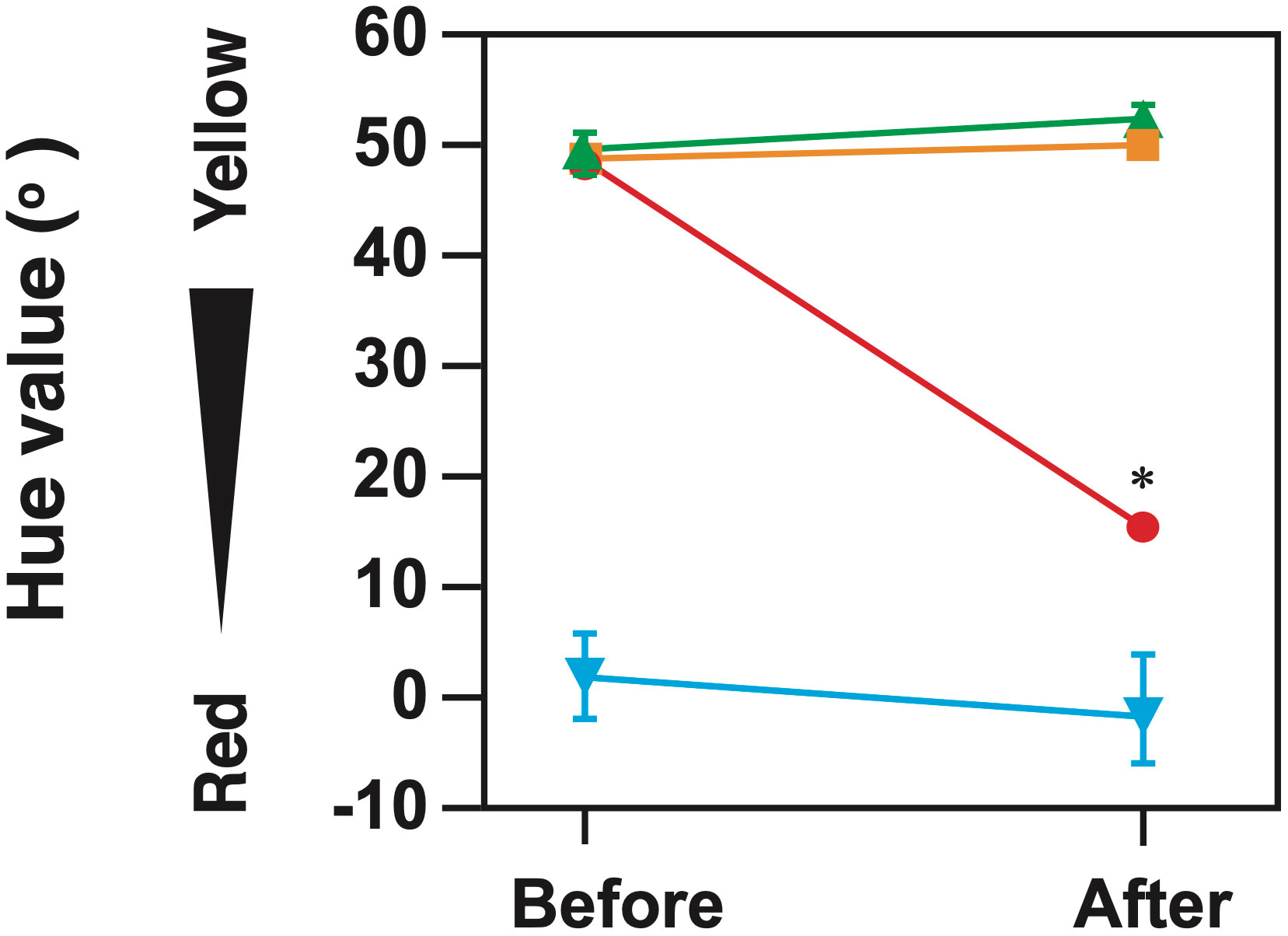
Figure 6 Commutation in the hue of the anal fin of P. sieboldi after the induction of a sex change. Circles, the largest female housed with five smaller females; squares, a larger female with a smaller female; triangles, a female with no other individuals; inverted triangles, a male with a female during the same period as a reference for TP coloration. Sex changes were induced during the pre-spawning season in fish placed in 200 L tanks. Each value represents the mean ± SEM of five replicates. Asterisks indicate a significant difference between the start and end of the experimental period (p < 0.001).
3.4. Experiment 4: Size matters
To investigate whether sex change occurs in small females, a female of 115–125 mm in length was kept together with five smaller (<105 mm) females during the post-spawning season. Similar to the results of experiment 2, the gonads of these females were either stage 2 inter-sexual gonads (80%) or secondary testis (20%) (Table 3). The body coloration of these fish showed substantial alterations, consistent with the IP to TP transition during development (data not shown).
3.5. Experiment 5: Crowding affects sexual change
Similar to 200 L and 500 L tank experiments, sex change was also observed in the fish kept in 80 L tanks. However, the number of sex-changed fish varied (Table 4) (Figure 7). After three days, the gonads of five fish had ovaries while one fish (16.7%) had stage 1 intersex gonads. Within a week, the number of fish with stage 1 gonads increased to five (83.3%) out of six. At 21 days, both stage 1 and 2 intersex gonads were observed in three (42.9%) fish per stage. By 28 days, out of the total of eight fish, two (25%) and five (62.5%) fish had stage 1 and 2 intersex gonads, respectively. Interestingly, one remained sexually unchanged at 7, 21, and 28 days.

Table 4 Time course of the occurrence of intersex gonad in IP fish kept with five smaller IP females.
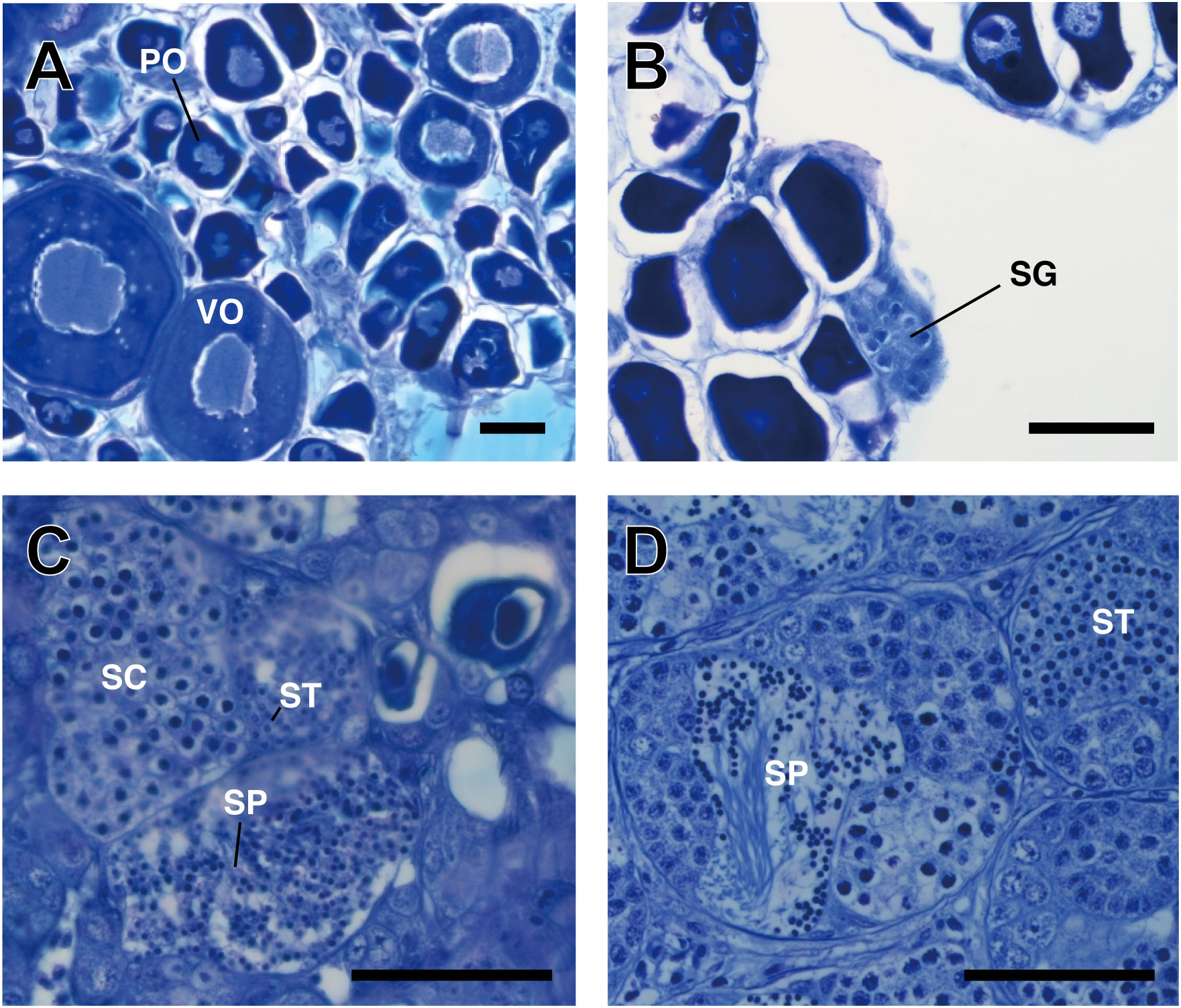
Figure 7 Modification in the gonadal histology of P. sieboldi after the induction of a sex change. (A) ovary; (B) stage 1 gonad after 7 days; (C) stage 2 gonad after 28 days. As a reference, the testis of TP male is shown (D). Sex changes were induced during the pre-spawning season in fish placed in an 80 L tank. Black arrow; spermatogonia; white arrow; sperm. VO, vitellogenic oocyte, PO, perinuclear oocyte, SG, spermatogonia, SC spermatocytes, ST spermatids, SP sperm; bar length, 50 mm.
3.6. Experiment 6: Importance of physical interaction
Physical interaction between an IP female and five smaller females was prohibited by separating the fish using a clear glass barrier in an 80 L tank. After the experimental period of 30 days, the gonads of four fish had ovaries (50%) while four fish (50%) showed stage 1 inter-sexual gonads (Table 5). Stage 2 intersex gonad and testis were not observed probably due to slower progression of sex change in the post-spawning season compared to the pre-spawning season.
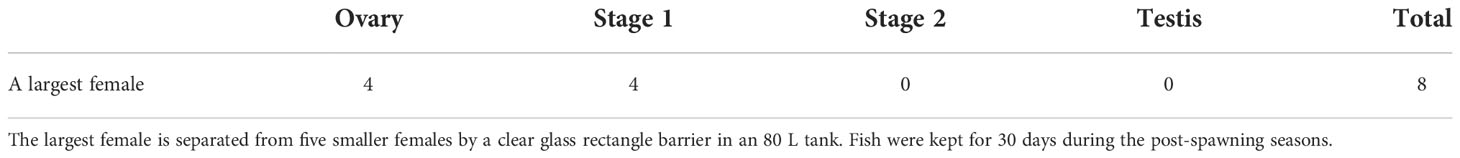
Table 5 The occurrence of the intersex gonad in IP fish by prohibiting physical interaction with 5 smaller females.
4. Discussion
In this study, we have elucidated the social condition (crowding, size, sexual status, etc.) of sex change of wrasse, P. sieboldi, in captivity. When six IP fish were kept in an 80–500 L tank for more than 21 days, 94% of the largest females experienced some degree of sex change. Such a high occurrence of sex change in captivity makes this species a novel and unique experimental model system for the study of sexual plasticity in gonads and other sex-linked characteristics that are controlled by the social environment. Moreover, a semi-gonadectomy analysis developed in this study might suggest a direct relationship between the gonadal sex/circulating sex steroid and body coloration. To our knowledge, this is the first report to trace the gonadal sex change with body color within the same individual, thus clarifying the physiological relationship between the gonad and body coloration.
P. sieboldi has the aptness to change sex in a laboratory setting in response to the social conditions present in the tank in which they are housed. This is consistent with the field observation that P. sieboldi undergoes protogynous sex change under social relationships (Nakazono, 1979). The present data also shows that the sex change of P. sieboldi preferentially occurs outside the spawning season, which is consistent with the field data of our previous study (Ohta et al., 2008). Similar to some hermaphrodite species (e.g., Thalassoma duperrey, Trimma okinawae, Gobiodon spp., Pseudochromis spp., Lythrypnus dalli), P. sieboldi also undergoes sex change in an aquarium when no territorial male is present (Ross et al., 1983; Sunobe and Nakazono, 1993; Nakashima et al., 1996; Wittenrich and Munday, 2005; Lorenzi et al., 2006), or in the presence of a smaller conspecific (Ross et al., 1983; Lutnesky, 1994.). Interestingly, unlike the saddle wrasse, Thalassoma duperrey, in which the sex change occurs in the larger female in a pair of females (Ross et al., 1983), the larger female of P. sieboldi within a pair of females does not undergo a sex change. By increasing the number of smaller females in a tank to six, we were able to trigger sex change in the largest female in the group. It could be related to the differences in their habitat and life history. P. sieboldi is found in rocky water of temperate areas whereas T. duperrey inhabits the coral reef of tropical water. As seen in the current data, the period of gonadal transition differs between the pre-spawning season (summer to autumn) and the post-spawning season in winter, indicating that temperature influences the time required for complete sex change. In both species, TP males defended their territory and spawned with a plural number of females. Therefore, our data was consistent with the size-advantage model proposing that individuals should switch sex when size-mediated reproductive success increases at a greater rate for the second sex than for the first sex (Ghiselin, 1969; Warner, 1975). Though it is prevalent that size, crowding, and surrounding environments are major drivers for sex change, we found that some females were phenotypically resilient after the experimental periods, which suggests that some other social and physiological conditions might be in play. In this regard, in a recent investigation using protandrous clown fish, it was found that rapid and complex genomic responses in the brain, involving numerous well-known and novel genes, transmitted the molecular signal to the gonad and the aftermath was sex change (Casas et al., 2016).
In T. duperrey, sex change was initiated in response to visual stimuli when a larger individual was placed next to a smaller one but separated by a barrier to prevent physical and olfactory interactions (Ross et al., 1983). Unlike T. duperrey, P. sieboldi does not change sex when presented as a member of a pair. In our experiment, upon separating a larger individual from five smaller individuals using a transparent barrier, only 50% of individuals underwent sex change with a stage 1 intersexual gonad, thus confirming that visual stimuli might be a major cue to initiate sex change. This, along with the fact that some females are more plastic than others and are more prone to sex change suggests that visual cues mostly attain the driver seat and initiate the sex change. On the other hand, the remaining 50% of individuals that did not change sex point towards some unknown factor(s), i.e., pheromonal and/or physical interactions that might be playing a secondary role during sex change. It is noteworthy to mention that physical contact is primarily important for the initiation of sex change. A recent study on the orange-spotted grouper Epinephelus coioides has demonstrated that physical interactions between females in a social group are crucial for the initiation and completion of sex change (Chen et al., 2020; Chen et al., 2021). Information on the stimuli for the initiation of sex change would be instructive to understand the adaptive significance of sex change in P. sieboldi as well as recognizing the mechanism of their social environment.
Our results also determine the sex change potential of small IP females. When kept with five other smaller females, a small (total length: 115–125 mm) IP female showed the potential to change into a TP male. So, it can be stated that the sex change in P. sieboldi is linked to relative body size instead of absolute body size. In the bluehead wrasse T. bifasciatum, the development of a primary male from a juvenile is socially biased, as an individual still in the juvenile stage could undergo a female-to-male sex change (Munday et al., 2006). Thus, in T. bifasciatum, the potential for a socially controlled sex change may already be present during the early stages of development and is maintained until the end of the juvenile period. It is still unknown when P. sieboldi acquires the sex change potential in response to social conditions during its life span. This sex-changing ability might be associated with the sexual development and plasticity of the brain, and future studies are needed in this direction to confirm such speculations.
In terms of tank size, all the larger females in 200 and 500 L tanks changed into males. On the other hand, in the 80 L tank, a delay in gonadal sex change was observed in two individuals among the eight larger females, and one individual did not change sex after 28 days although the other five individuals showed advanced stages of gonadal sex change, like the 200 and 500 L tanks. This indicates that individual differences might play an important role in the initiation and the completion of sex change. Another possibility would be that the largest females in the 80 L tank may have been experiencing some stress due to the relatively high density and limited space of habitat. It would be interesting to study whether crowding could altogether halt sex change or eventually help the sex-changing female to revert to its original female state. Notably, it is hypothesized that dominant males in social groups of protogynous species are responsible for inhibiting the socially induced sex change of subordinate females through their aggressive behavior, and the release from this high stress (i.e., drop in cortisol) could trigger the sex change of the alpha female, which would, in turn, keep the cortisol levels of the rest of the females elevated (Perry and Grober, 2003; Goikoetxea et al., 2017; Iger et al., 1995; Overli et al., 1999; Hattori et al., 2009; Yamaguchi et al., 2010; Hayashi et al., 2010; Iwata et al., 2012; Nozu and Nakamura, 2015; Goikoetxea et al., 2021; Gozdowska et al., 2022). We also found a significant increase in the occurrence of stage-1 sex change fish, immediately (within 7 days) after the removal of the males from the group, which largely supports the abovementioned cortisol-based sex change mechanisms. Cortisol is the major hormone that is upregulated by various environmental stressors including the change in water temperature (Goikoetxea et al., 2021). Contrarily, thermal inhibition of reproduction has been reported in several fish species (Pankhurst and Munday, 2011; Servili et al., 2020). Therefore, the determination of the role of cortisol in the sex change of P. sieboldi will help to evaluate the effect of climatic change on the reproductive abilities of a hermaphrodite fish.
In summary, we have established the model system of P. sieboldi to induce the socially controlled sex change in captivity and evaluated the direct relationship between gonad and body coloration in association with sex change. This study also sheds light on the effect of various environmental and behavioral factors on the sexual plasticity and subsequent reproductive capacity of fish. Thus, P. sieboldi holds the potential to be a very good candidate species to comprehend the intricacies underlying the molecular and physiological mechanisms of vertebrate sexual plasticity.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by Institutional Animal Care and Use Committee of the Kyushu University.
Author contributions
KO, YY, TC, and MM designed experiments and wrote the manuscript. YY, SH, MH, SM, and AY performed the experiments and analyzed the data. KO and TT developed and performed a semi-gonadectomy experiment. All authors contributed to the article and approved the submitted version.
Funding
JSPS KAKENHI grant numbers JP16H04981, JP19H03049,JP22H00386, and JP22K05832.
Acknowledgments
We extend our sincere thanks to Shione Taminato, Kodai Mizumura, and the staff of the Fishery Research Laboratory of Kyushu University for their assistance during the experiments. This work was supported by JSPS KAKENHI grant numbers 16H04981, 19H03049, and 22H00386.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Black P., Balthazart B., Baillien M., Grober M. S. (2011). Rapid increase in aggressive behavior precedes the decrease in brain aromatase activity during socially mediated sex change in lythrypnus dalli. Gen. Comp. Endocrinol. 170, 119–124. doi: 10.1016/j.ygcen.2010.09.019
Capel B. (2017). Vertebrate sex determination: Evolutionary plasticity of a fundamental switch, nat. Rev. Genet. 18, 675–689. doi: 10.1038/nrg.2017.60
Casas L, Saborido-Rey F, Ryu T, Michell C, Ravasi T, Irigoien X. Sex Change in Clownfish: Molecular Insights from Transcriptome Analysis. Sci Rep. (2016) 17(6):35461. doi: 10.1038/srep35461
Chen J., Chen H., Peng C., Ye Z., Zhao M., Xiao L., et al. (2020). A highly efficient method. of inducing sex change using social control in the protogynous orange-spotted grouper (Epinephelus coioides). Aquaculture 517. doi: 10.1016/j.aquaculture.2019.734787
Chen J., Peng C., Huang J., Shi H., Xiao L., Tang L., et al. (2021). Physical interactions facilitate sex change in the protogynous orange-spotted grouper, epinephelus coioides. J. Fish Biol. 98, 1308–1320. doi: 10.1111/jfb.14663
Ghiselin M. T. (1969). The evolution of hermaphroditism among animals. Q. Rev. Biol. 44, 189–208. doi: 10.1086/406066
Goikoetxea A., Muncaster S., Todd E. V., Lokman P. M., Robertson H. A., De Farias e Moraes C. E., et al. (2021). A new experimental model for the investigation of sequential hermaphroditism. Sci. Rep. 11, 1–14. doi: 10.1038/s41598-021-02063-y
Goikoetxea A., Sadoul B., Blondeau-Bidet E., Aerts J., Blanc M., Parrinello H., et al. (2021). Genetic pathways underpinning hormonal stress responses in fish exposed to short- and long-term warm ocean temperatures. Ecol. Indic. 120. doi: 10.1016/j.ecolind.2020.106937
Goikoetxea A., Todd E. V., Gemmell N. J. (2017). Stress and sex: Does cortisol mediate sex change in fish? Reproduction 154, 149–160. doi: 10.1530/REP-17-0408
Gozdowska M., Sokołowska E., Pomianowski K., Kulczykowska K. (2022). Melatonin and cortisol as components of the cutaneous stress response system in fish: Response to oxidative stress. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 268, 111207. doi: 10.1016/j.cbpa.2022.111207
Guiguen Y., Fostier A., Piferrer F., Chang C. F. (2010). Ovarian aromatase and estrogens: a pivotal role for gonadal sex differentiation and sex change in fish. Gen. Comp. Endocrinol. 165, 352–366. doi: 10.1016/j.ygcen.2009.03.002
Han Y., Peng C., Wang L., Guo J., Lu M., Chen J., et al. (2018). Female-to-male sex reversal in orange-spotted grouper (Epinephelus coioides) caused by overexpressing of amh in vivo. Biol. Reprod. 99, 1205–1215. doi: 10.1093/biolre/ioy157
Hattori R. S., Fernandino J. I., Kishil A., Kimura H., Kinno T., Oura M., et al. (2009). Cortisol-induced masculinization: Does thermal stress affect gonadal fate in pejerrey, a teleost fish with temperature-dependent sex determination? PloS One 4 (8). doi: 10.1371/journal.pone.0006548
Hayashi Y., Kobira H., Yamaguchi T., Shiraishi E., Yazawa T., Hirai T., et al. (2010). High temperature causes masculinization of genetically female medaka by elevation of cortisol. Mol. Reprod. Dev. 77, 679–686. doi: 10.1002/mrd.21203
Iger Y., Balm P. H., Jenner H. A., Wendelaar Bonga S. E. (1995). Cortisol induces stress-related changes in the skin of rainbow trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 97, 188–198. doi: 10.1006/gcen.1995.1018
Iwata E., Mikami K., Manbo J., Moriya-Ito K., Sasaki H. (2012). Social interaction influences blood cortisol values and brain aromatase genes in the protandrous false clown anemonefish, amphiprion ocellaris. Zool. Sci. 29, 849–855. doi: 10.2108/zsj.29.849
Kobayashi Y., Nakamura M., Sunobe T., Usami T., Kobayashi T., Manabe H., et al. (2009). Sex change in the gobiid fish is mediated through rapid switching of gonadotropin receptors from ovarian to testicular portion or vice versa. Endocrinology 150, 1503–1511. doi: 10.1210/en.2008-0569
Kuwamura T., Sunobe T., Sakai Y., Kadota T., Sawada K. (2020). Hermaphroditism in fishes: an annotated list of species, phylogeny, and mating system. Ichthyol. Res. 67, 341–360. doi: 10.1007/s10228-020-00754-6
Lorenzi V., Earley R. L., Grober M. S. (2006). Preventing behavioral interactions with a male facilitates sex change in female blue banded gobies, lythrypnus dalli. Behav. Ecol. Sociobiol. 59 (6), 715–722. doi: 10.1007/s00265-005-0101-0
Lutnesky M. M. F. (1994). Density-dependent protogynous sex change in territorial-haremic fishes: Models and evidence. Behav. Ecol. 5, 375–383. doi: 10.1093/beheco/5.4.375
Matsuyama M., Morita S., Hamaji N., Kashiwagi M., Ohta K., Nagahama Y. (1997). Diurnal spermatogenesis and spawning in the secondary male of a protogynous wrasse, pseudolabrus japonicus (teleostei, labridae). Zool. Sci. 14, 1001–1008. doi: 10.2108/zsj.14.1001
Munday P. L., Wilson White J., Warner R. R. (2006). A social basis for the development of primary males in a sex-changing fish. Proc. Biol. Sci. 273, 2845–2851. doi: 10.1098/rspb.2006.3666
Nagahama Y., Chakraborty T., Paul-Prasanth B., Ohta K., Nakamura M. (2021). Sex determination, gonadal sex differentiation, and plasticity in vertebrate species. Physiol. Rev. 101, 1237–1308. doi: 10.1152/physrev.00044.2019
Nakashima Y., Kuwamura T., Yogo Y. (1996). Both-ways sex change in monogamous coral gobies, gobiodon spp. Environ. Biol. Fishes 46, 281–288. doi: 10.1007/BF00005004
Nakazono A. (1979). Studies on the sexual reversal and spawning behavior of the five species of Japanese labrid fishes. Rep. Fish Res. Lab. Kyushu Univ. 4, 1–64.
Nozu R., Nakamura M. (2015). Cortisol administration induces sex change from ovary to testis in the protogynous wrasse, halichoeres trimaculatus. Sex Dev. 9, 118–124. doi: 10.1159/000373902
Ohta K., Hirano M., Mine T., Mizutani H., Yamaguchi A., Matsuyama M. (2008). Body color change and serum steroid hormone levels throughout the process of sex change in the adult wrasse, pseudolabrus sieboldi. Mar. Biol. 153, 843–852. doi: 10.1007/s00227-007-0856-0
Ohta K., Sakai M., Sundaray J. K., Kitano T., Takeda T., Yamaguchi A., et al. (2012). Bidirectional sex change induced by sex steroid implantation in the hermaphrodite fish, pseudolabrus sieboldi. J. Exp. Zool. A Ecol. Genet. Physiol. 317, 552–560. doi: 10.1002/jez.1747
Ohta K., Sundaray J. K., Okida T., Sakai M., Kitano T., Yamaguchi A., et al. (2003). Bi-directional sex change and its steroidogenesis in the wrasse, pseudolabrus sieboldi. Fish Physiol. Biochem. 28, 173–174. doi: 10.1023/B:FISH.0000030517.06738.e7
Overli O., Olsen R. E., Lovik F., Ringo E. (1999). Dominance hierarchies in Arctic charr, salvelinus alpinus l.: differential cortisol profiles of dominant and subordinate individuals after handling stress. Aquac. Res. 30, 259–264. doi: 10.1046/j.1365-2109.1999.00322.x
Pankhurst N. W., Munday P. L. (2011). Effects of climate change on fish reproduction and early life history stages. Mar. Freshw. Res. 62 (9), 1015–1026. doi: 10.1071/MF10269
Perry A. N., Grober M. S. (2003). A model for social control of sex change: Interactions of behavior, neuropeptides, glucocorticoids, and sex steroids. Horm. Behav. 43 (1), 31–38. doi: 10.1016/s0018-506x(02)00036-3
Pla S., Maynou F., Piferrer F. (2021). Hermaphroditism in fish: Incidence, distribution and associations with abiotic environmental factors. Rev. Fish Biol. Fish. 31 (4), 935–955. doi: 10.1007/s11160-021-09681-9
Ross R. M., Losey G. S., Diamond M. (1983). Sex change in a coral-reef fish: dependence of stimulation and inhibition on relative size. Science 221, 574–575. doi: 10.1126/science.221.4610.574
Sadovy de Mitcheson Y., Liu M. (2008). Functional hermaphroditism in teleosts. Fish 9 (1), 1–43. doi: 10.1111/j.1467-2979.2007.00266.x
Servili A., Canario A. V., Mouchel O., Antonio Muñoz-Cueto J. (2020). Climate change impacts on fish reproduction are mediated at multiple levels of the brain-pituitary-gonad axis. Gen. Comp. Endocrinol. 291, 113439. doi: 10.1016/j.ygcen.2020.113439
Stöck M., Kratochvíl L., Kuhl H., Rovatsos M., Evans B. J., Suh A., et al. (2021). A brief review of vertebrate sex evolution with a pledge for integrative research: Towards “sexomics. Philos. Trans. R. Soc Lond. B Biol. Sci. 376 (1832). doi: 10.1098/rstb.2020.0426
Sunobe T., Nakazono A. (1993). Sex change in both directions by alteration of social dominance in trimma okinawae (Pisces: Gobiidae). Ethology 94, 339–345. doi: 10.1111/j.1439-0310.1993.tb00450.x
Todd E. V., Liu H., Muncaster S., Gemmell N. J. (2016). Bending genders: The biology of natural sex change in fish. Sex Dev. 10 (5–6), 223–241. doi: 10.1159/000449297
Todd E. V., Ortega-Recalde O., Liu H., Lamm M. S., Rutherford K. M., Cross H., et al. (2019). Stress, novel sex genes, and epigenetic reprogramming. orchestrate socially controlled sex change. Sci. Adv. 5 (7), 1–15. doi: 10.1126/sciadv.aaw7006
Warner R. R. (1975). The adaptive significance of sequential hermaphroditism in animals. Am. Nat. 109, 61–82. doi: 10.1086/282974
Wittenrich M. L., Munday P. L. (2005). Bi-directional sex change in coral reef fishes from the. family pseudochromidae: An experimental evaluation. Zool. Sci. 22, 797–803. doi: 10.2108/zsj.22.797
Keywords: sex change, sequential hermaphrodite fish, social condition, body color, sexual plasticity, wrasse, Pseudolabrus sieboldi
Citation: Chakraborty T, Yamamoto Y, Hanai S, Hirano M, Mohapatra S, Yamaguchi A, Takeda T, Matsuyama M and Ohta K (2022) Divulging the social sex change mechanism in a unique model system for studying the sexual plasticity of protogynous hermaphrodite fish, three bamboo leaf wrasse (Pseudolabrus sieboldi). Front. Mar. Sci. 9:1048506. doi: 10.3389/fmars.2022.1048506
Received: 19 September 2022; Accepted: 29 November 2022;
Published: 16 December 2022.
Edited by:
Vikash Kumar, Central Inland Fisheries Research Institute (ICAR), IndiaReviewed by:
Jinxiang Liu, Ocean University of China, ChinaAmit Kumar Sinha, University of Arkansas at Pine Bluff, United States
Jitendra Kumar Sundaray, Central Institute of Freshwater Aquaculture (ICAR), India
Copyright © 2022 Chakraborty, Yamamoto, Hanai, Hirano, Mohapatra, Yamaguchi, Takeda, Matsuyama and Ohta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kohei Ohta, a19vaHRhQGFnci5reXVzaHUtdS5hYy5qcA==; Tapas Chakraborty, dGFwYXNfY2hAYWdyLmt5dXNodS11LmFjLmpw
†These authors have contributed equally to this work and share co-first authorship
 Tapas Chakraborty
Tapas Chakraborty Yume Yamamoto1†
Yume Yamamoto1† Sipra Mohapatra
Sipra Mohapatra Akihiko Yamaguchi
Akihiko Yamaguchi Michiya Matsuyama
Michiya Matsuyama Kohei Ohta
Kohei Ohta