
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 10 October 2022
Sec. Marine Biology
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.1040466
This article is part of the Research Topic Integration of Development, Physiology and Responses to Environmental Change in Aquatic Invertebrates View all 13 articles
Mussel is an economically and ecologically important species widely distributed throughout the world. The mussel adheres to the attachment substrate by secreting byssus external to the body. Various environmental and biological factors influence the process of byssus secretion, and the present study investigated the effect of starvation on byssal secretion in the hard-shelled mussel Mytilus coruscus. Histological changes in mussel foot secretory glands and gene expression of mussel foot proteins were also determined. The experimental setup consisted of starvation treatments for 7, 14 and 21 days, and the control groups. The results showed that the number of produced byssus was higher in the starvation group compared to the control (CTR) group, and the starvation group had a significantly higher of byssal shedding number from 6 days of starvation treatment onwards (p < 0.05). The byssal thread diameter was significantly reduced in all starvation treatment groups (p < 0.05). However, starvation treatment had no effect on the length of the byssal thread (p > 0.05). After 21 days of starvation treatment, the byssal thread volume was significantly lower than that of the CTR group (p < 0.05). A significant decrease in the breaking force of the byssal thread was observed after 14 and 21 days of starvation treatment (p < 0.05), along with an upward shift in the breakpoints. Starvation treatment significantly reduced the percentage of foot secretory glands area to total tissue (p < 0.05). The expression of the mussel foot protein genes (Mcfp-1P and Mcfp-1T) was significantly up-regulated at 7 days of starvation treatment (p < 0.05). These findings reveal that starvation weakens byssal thread performance by influencing mussel foot secretory glands, which increases the dislodgment risks of suspended-cultured mussels.
As benthic organisms, mytilid mussels are ecologically and economically important species in the coastal marine food webs (Fitzgerald-Dehoog et al., 2012). Mussels generally aggregate on the sea floor and rocky shore mussel beds, forming dense mussel beds, and are thought to support a diverse community structure. (Buschbaum et al., 2009). Mussels are intermediate between primary producers and higher consumers in the marine ecosystem, transferring energy and nutrients to higher levels of the food web (Harris and Carrington, 2020). In addition, mussels have been extensively farmed and represent a key economic species, providing high-quality protein and a wide range of vitamins and minerals (Buck et al., 2010; Suplicy, 2020). According to data from FAO, world aquaculture production of mussels reached 2.1 million tons (worth $4.5 billion) in 2018 (FAO, 2020).
Marine mussels produce the byssal threads that act as anchors, allowing them to be firmly attached to ropes or other natural substrates (O’Donnell et al., 2013). The mussel foot is comprised of secretory glands in which the thread proteins are stored (Demartini et al., 2017). Foot glands secrete a variety of mussel foot proteins (mfps) into the foot groove by exocrine secretion and solidify in vitro after a series of reactions to form stiff, stretchable threads (Pujol, 1967; Priemel et al., 2017). Generally, a byssal thread can be subdivided into four distinct regions: the stem, the plaque, and the proximal and distal portions of the thread (Harrington and Waite, 2007). The stem attaches the thread to the mussel tissue, with a corrugated proximal portion near the stem and a smoother distal portion (Lucas et al., 2002; Harrington and Waite, 2007). The plaque contains a series of adhesive foot proteins enriched in 3,4-dihydroxyphenyl-L-alanine (DOPA) that ensure the ability to attach to the substrate (Lin et al., 2007).
The adhesion strength of mussels depends on the number and the mechanical performance of the byssal threads (Newcomb et al., 2019). The physiological condition of mussels is influenced by biological and environmental factors that can alter the production of byssal threads and the strength of attachment to the substratum (Lachance et al., 2008; Carrington et al., 2015). Previous studies have demonstrated that changes in seawater temperature, pH, current, food availability and hypoxia can affect the secretion of byssus (Babarro et al., 2008; Fitzgerald-Dehoog et al., 2012; Garner and Litvaitis, 2013; O’Donnell et al., 2013; Sui et al., 2015; Clements et al., 2018; George and Carrington, 2018). The production of byssal threads costs 2-8%, or even up to 47% of the daily mussel’s energy budget (Roberts et al., 2021). Food supply determines the amount of energy budget available to organisms, reflecting the partitioning energy among reproduction, growth and maintenance functions (Sarà et al., 2014). Food availability plays an important role in buffering the energy expenditure caused by byssus secretion (Carrington et al., 2015). Energy balance can be disturbed when less food is available, which may result in less energy being allocated to mussel byssus secretion (Clarke, 1999; Babarro et al., 2008; Shang et al., 2021). Reduced feeding or starvation can compromise the energy balance, resulting in less energy allocation to mussel byssus secretion (Clarke, 1999; Babarro et al., 2008). Mussels located close to the rope are within the mussel aggregation layer and may not have access to enough food or endure starvation during periods when algae levels are low.
The hard-shelled mussel (Mytilus coruscus) is a major cultured bivalve in China. In recent years, the sea area dedicated to the aquaculture of M. coruscus has expanded rapidly on the coast of Shengsi Island. Increased cultured mussel density impedes the food available to mussels. It leads to a reduction in the somatic mass of individual mussels and elevated mussel dislodgment risk. The present study aims to explore the effects of starvation on the byssus secretion of the mussel M. coruscus. We observed byssus secretion and shedding number and measured byssal breaking force, byssal thread length, and diameter. Changes in foot glands area and expression levels of byssus protein genes were determined. The evidence obtained in this study can reveal the effect of starvation stress on mussel byssus secretion, providing a theoretical basis and data support for the study of mussel aquaculture and benthic ecology.
Adult mussels (M. coruscus) (shell length: 9.1 ± 0.4 cm) were obtained in November 2020 from Gouqi Island (30°73′N; 122°77′E), Zhoushan, Zhejiang Province, China. Upon arrival in the laboratory, the mussels’ surface attachments were removed, and the byssus was cut off along the edge of the shell, and then the mussels were acclimated in a polycarbonate tank with 10 L of seawater at a salinity of 30 for two weeks at 21°C. The seawater was changed every day. The mussels were fed with a mixture of microalgae Platymonas helgolandica var. tsingtaoensis and Isochrysis zhanjiangensis twice a day at concentrations of 5×106 cells/mL and 1.5×106 cells/mL, respectively. All procedures for mussel acclimation and experimentation were authorized by the Animal Ethics committee of Shanghai Ocean University with the registration number of SHOU-DW-2021-011.
The experimental setup consisted of a starvation treatment for 21 days and a control group (CTR). Additional starvation treatment groups for 7 and 14 days were carried out, which were only for mussel foot sampling. The starvation-treated mussels were kept without feeding. The control mussels were fed as described above. Twenty-eight mussels from each treatment were evenly distributed in four polycarbonate tanks containing 10 L of seawater, with seven mussels in each tank. The seawater was changed daily during the experiment. The numbers of byssus secretion and shedding were recorded daily. At the end of the experiment, byssus and foot samples were collected.
The parameters of byssal threads were determined as previously described (Li et al., 2020). Briefly, the diameter of the byssal thread was measured with an ocular micrometer under a stereo microscope (SZX2, Olympus, Japan). The proximal, intermediate, and distal plaques (near the adhesive plaque) of the byssus were used to calculate the mean diameter of a byssal thread. Byssal thread length was determined from the proximal region of the thread to the adhesive plaque with a vernier caliper. The volume of the byssal thread was calculated on the assumption that the byssal thread is a cylindrical tube using the formula: V =πr2 × l, where r = byssal thread radius and l = byssal thread length. A digital force gauge installed on a testing frame (HLB, Handpi, China) was used to measure the byssal breaking force. The thread was expanded at a rate of 10 mm min-1 until it broke, and the breaking force were recorded. The breaking point of each thread was also determined based on measuring the length of two threads that came from a whole thread after the breaking force measurement. The value of the byssal breaking force was calculated based on a total of 405, 512 and 509 measurements from 7, 14 and 21 days of treatment groups and presented in Newton (N).
The foot samples of M. coruscus were fixed in Bouin’s Fixative (Phygene Biotechnology Co., Ltd, Fuzhou, China) for 12 h at 4°C. The fixed samples were then cleaned with 30%, 50% and 70% ethanol for 30, 20 and 20 minutes, respectively. Samples were dehydrated in a graduated series of ethanol (50%, 70%, 90% and 100% for 45 min, respectively). After dehydration, the samples were cleaned and waxed with the steps of alcohol and xylene (1:1) for 40 min, xylene twice for 15 min each, xylene and paraffin wax (1:1) for 50 min at 60°C and paraffin wax for 3 h at 60°C, followed by embedding in paraffin blocks. Paraffin-embedded samples were cut at a thickness of 5 μm on a microtome (Leica, RM2245, Germany). For the histological analysis, the sections were stained with Harris hematoxylin and eosin (BBI, Shanghai, China), dipped for 5 min in 95% ethanol, two times 5 min in 100% ethanol, and two times 5 min in xylene. Finally, the sections were covered with neutral balsam (Solarbio, Beijing, China), and photographed using the Axio Imager M2 microscope (Zeiss, Germany). The foot glands area was determined using FIJI software. For each treatment group, nine replicates were performed.
Total RNA was extracted from the mussel foot samples using RNAiso Plus reagent and following the manufacturer’s instructions (Takara, Japan). The concentration of the extracted total RNA was assessed using a NanoDrop spectrophotometer (ND-2000, NanoDrop Technologies, USA). A 1% agarose gel was used to assess the quality of total RNA by electrophoresis. A volume of 10 μL of the mixture containing 1 μL gDNA Eraser, 500 ng total RNA, 2 μL 5× gDNA Eraser buffer and RNase-free dH2O was used to eliminate genomic DNA with the PrimeScript™ RT kit (Takara, China). For cDNA synthesis, 10 μL volume of the mixture was added to a final volume of 20 μL containing 4 μL 5× PrimeScript Buffer 2, 1 μL RT Primer Mix, 1 μL PrimeScript RT Enzyme Mix I, and 4 μL RNase Free dH2O. The reactions were performed at 37°C for 15 min and followed by 5 sec at 87°C.
Three genes (Mcfp-1P, Mcfp-1T and Mccol2) implicated in byssus formation were analyzed by qPCR. The elongation factor 1α (EF-1α) of M. coruscus was used as the reference gene for normalization. Primer 6.0 software was utilized to design specific primers (Table 1). qPCR analysis was performed with four biological replicates/treatment in 96 multi-well plates using a LightCycler 960 (Roche). PCR amplicons were sequenced to confirm their identity and were used as standards ranging from 107 - 101 DNA copies of the target amplicon for absolute quantification. qPCR reaction contained 1 μL cDNA, 0.3 μL of each of the primers (10 μM), 5 μL of 2 × FastStart Essential DNA Green Master (Roche) and sigma water to reach a final reaction volume of 10 μL with the following amplification protocol: 10 min at 95°C followed by 45 cycles of 10 s at 95°C and 10 s at 57°C. Melting curve analysis verified a single reaction peak and the standard curve confirmed the amplification efficiency of the primers (90%-110%). As previously mentioned, absolute quantification was used to calculate the relative mRNA expression (Li et al., 2019a).
JMP™ software (Version 10.0.0) was used to analyze the data. The Shapiro-Wilk and O’Brien tests were first used to determine the normality and homogeneity of all data. The Student’s t-test was performed when data conformed to normal distribution and homogeneity. Alternatively, the data were analyzed by the Wilcoxon test when data do not meet the requirements of normality and homogeneity. Differences were considered significant at p < 0.05.
The results of the secretion and shedding of byssal threads for 21 days of starvation are shown in Figure 1. A significantly higher number of byssal threads secretion was observed in the starvation group than in the control group from day 3 to day 8 (p < 0.05, Figure 1A). The shedding number of byssal threads significantly increased in the starvation group compared to the CTR group after six days of treatment (p < 0.05, Figure 1B).
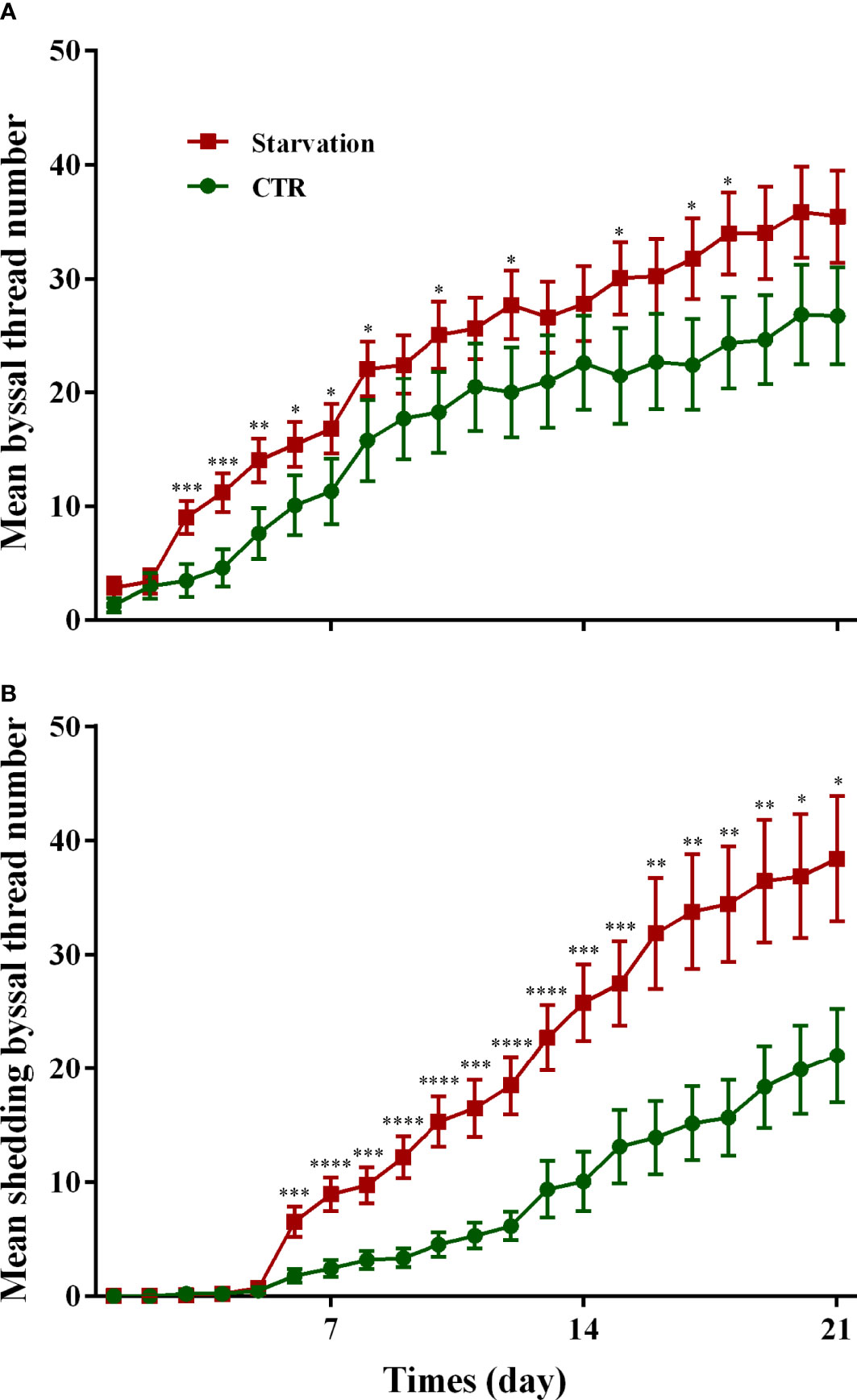
Figure 1 Effects of 21 days of starvation on the (A) secreted byssal thread number and (B) byssal shedding number in M. coruscus. CTR: control group. Asterisks indicate significant differences between starvation groups and CTR groups. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
After 7, 14 and 21 days of starvation treatment, byssal thread diameter was significantly reduced in the starvation group compared to the CTR group (p < 0.05, Figure 2A). However, starvation treatment had no effect on byssal thread length (p > 0.05, Figure 2B). The volume of the byssal thread was significantly lower than the CTR group after 21 days of starvation treatment (p < 0.05, Figure 2C).
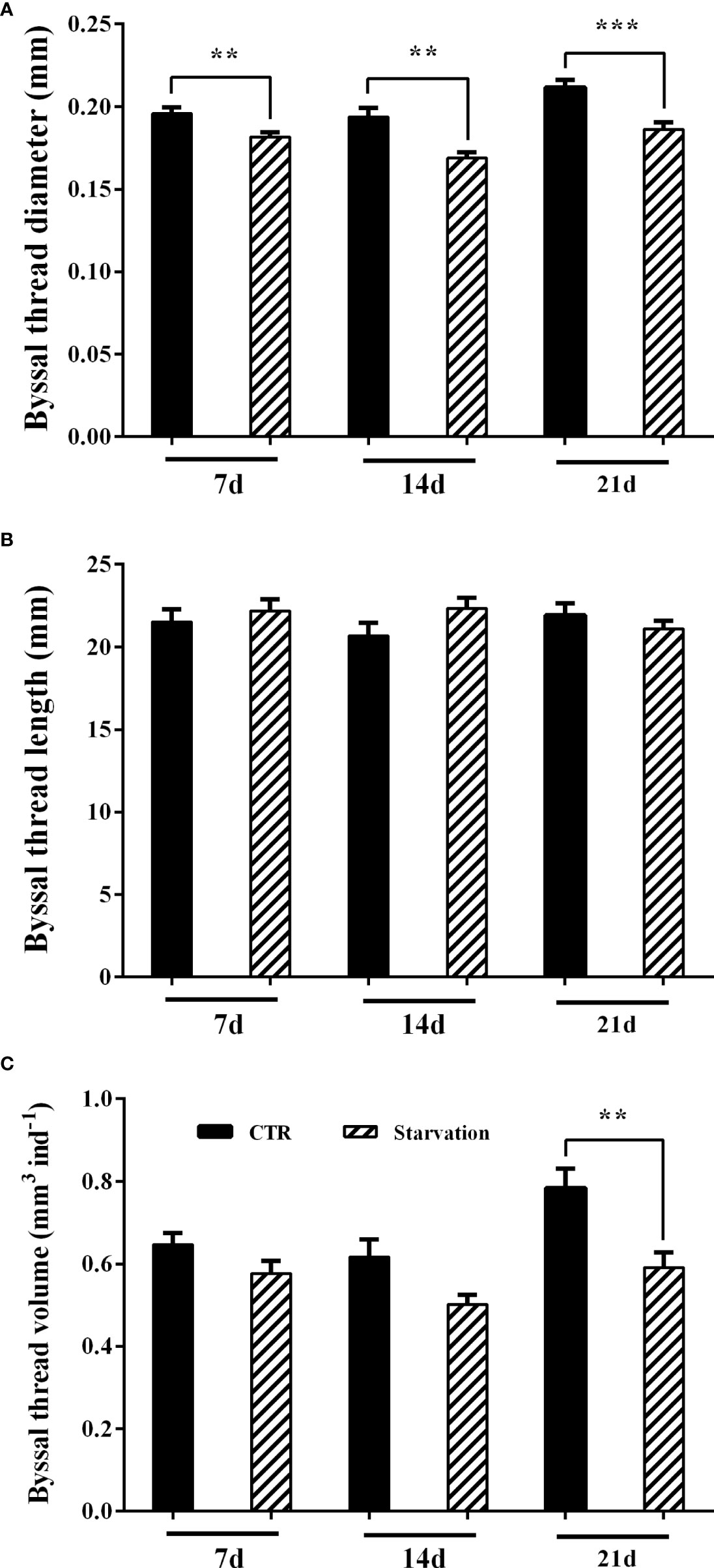
Figure 2 Effects of 21 days of starvation on (A) byssal thread diameter, (B) byssal thread length and (C) the cumulative byssal thread volume in M. coruscus. Asterisks indicate significant differences between starvation groups and CTR groups. **p < 0.01; ***p < 0.001.
The breaking force of the byssal thread significantly decreased in 14 and 21 days of treatment groups compared to the CTR group (p < 0.05, Figure 3A). The failure location of threads was identified to understand the weakest part in the byssal threads (Figure 3B). The breakpoints of the byssal threads mainly occurred in the middle of the thread, representing 54.4% (7d, CTR), 54.11% (7d, starvation), 40.13% (14d, CTR), 41.04% (14d, starvation), 45.53% (21d, CTR) and 50.41% (21d, starvation) (Figure 3B). Increased breakpoints in the upper part of the thread were observed on 14 and 21 days of starvation treatment compared to the CTR group (Figure 3B).
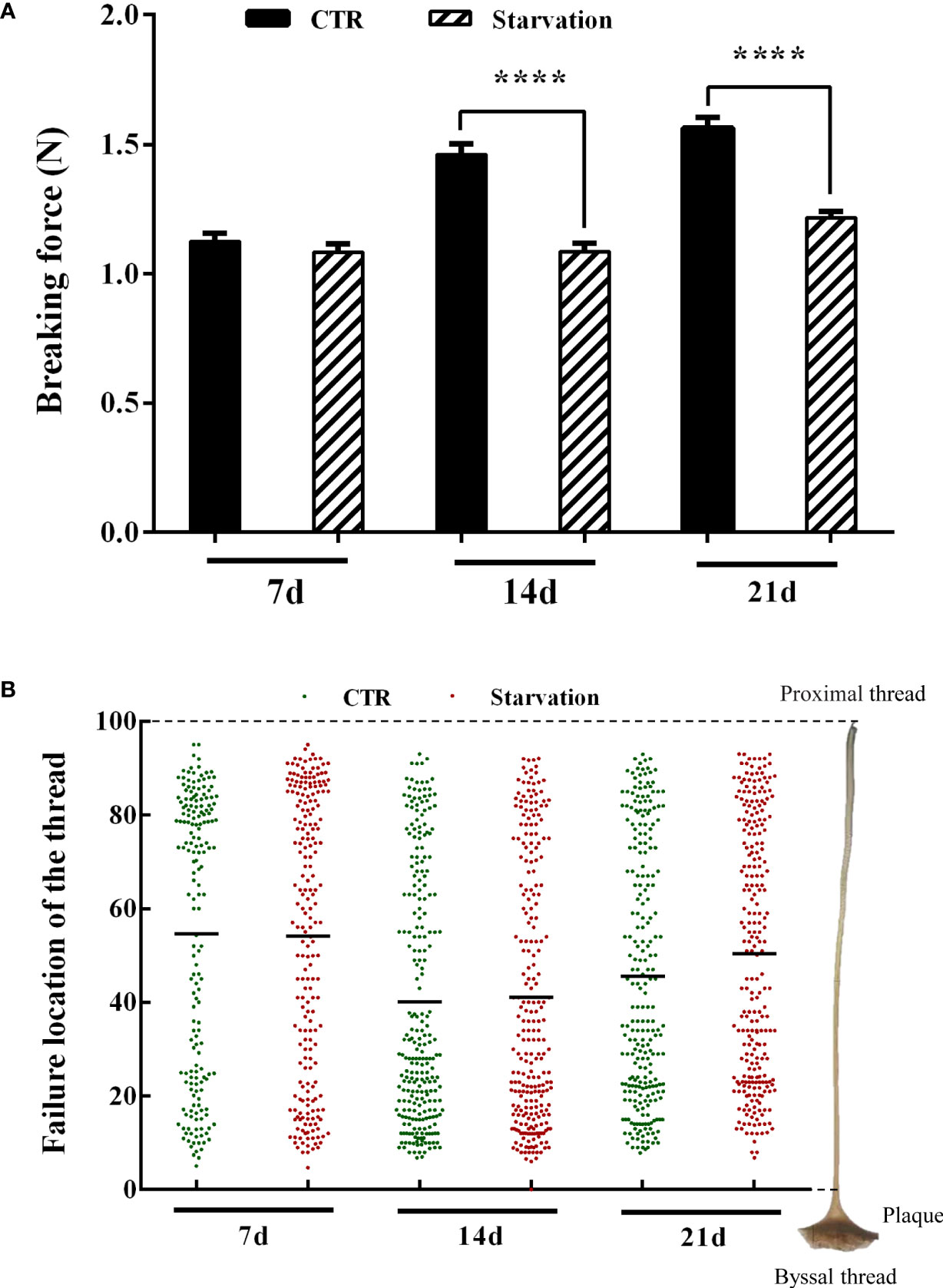
Figure 3 Effects of 21 days of starvation on (A) breaking force of the byssal thread and (B) failure location in the mussel M. coruscus. Each green dot and the red dot represent a thread breaking force test measurement. Each green dot and the red dot on the Y-axis corresponds to the breakpoints of a single byssal thread on the right side of the diagram. The value 100 on the Y-axis represents the proximal region of the thread, and the value 0 on Y-axis represents the distal region of the thread (close to the plaque). The amount of byssal breaking force measurements was as follows: 184 (7d, CTR), 221 (7d, starvation), 256 (14d, CTR), 256 (14d, starvation), 253 (21d, CTR) and 256 (21d, starvation). Asterisks indicate significant differences between starvation groups and CTR groups. ****p < 0.0001.
The effect of starvation on the foot tissues of M. coruscus is shown in Figure 4. The foot was oval in the transverse section. A foot groove (fg) was situated in the middle of the ventral surface (Figure 4). The epidermis of the foot had “creases” that were depressed inward (Figure 4). The foot glands were located close to the foot groove with purplish red color (Figure 4). The muscles of the mussel foot were located at the periphery of the glands, away from the foot groove side, and the whole muscles were pink in color (Figure 4). The proportion of foot glands area versus total foot tissue was analyzed and the results show that starvation treatment for 7, 14 and 21 days significantly reduced the percentage of foot secretory glands area relative to CTR groups (p < 0.05, Figure 4H).
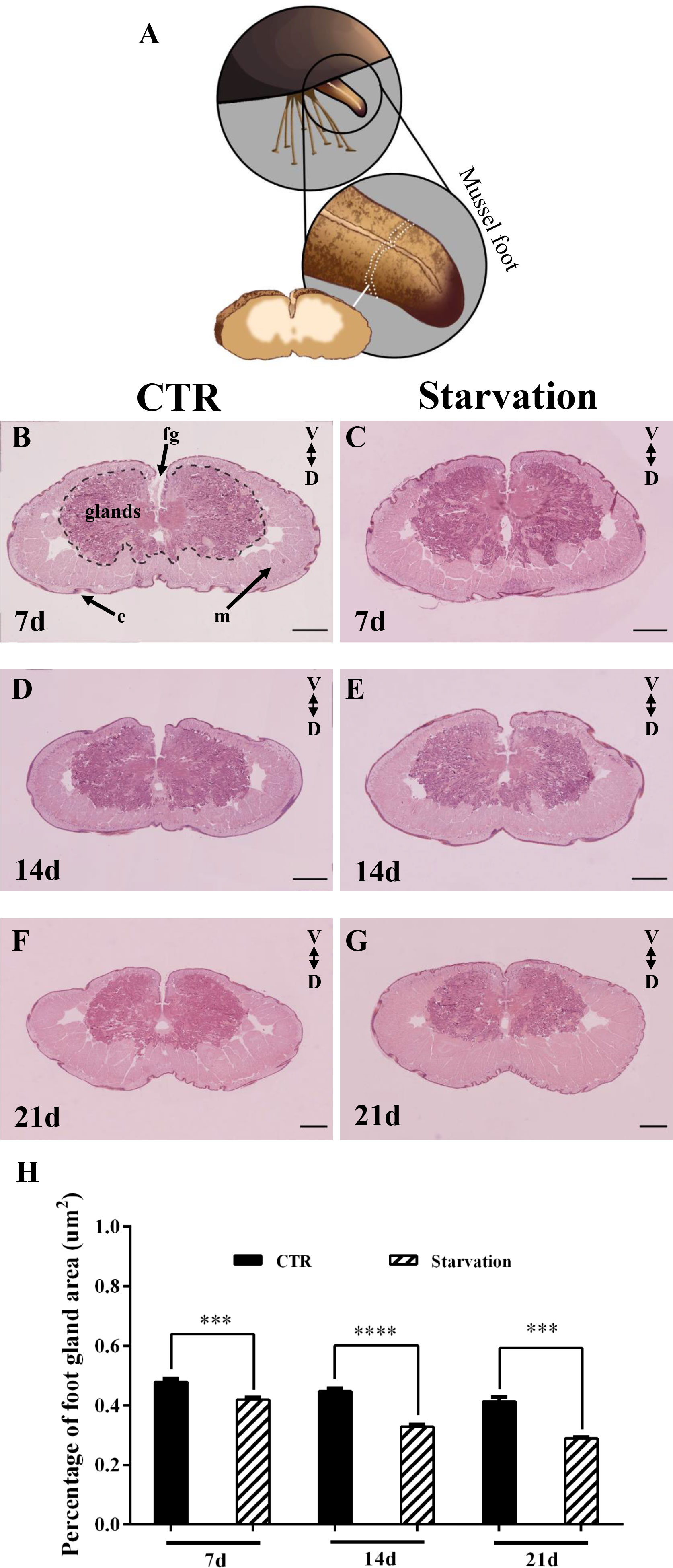
Figure 4 The foot of M. coruscus stained with hematoxylin and eosin (H&E). (A) A schematic depiction of foot section sampling. (B) 7d CTR, (C) 7d starvation, (D) 14d CTR, (E) 14d starvation, (F) 21d CTR, (G) 21d starvation. (H) The percentage of foot gland area versus total foot tissue. CTR: control group; fg: foot groove; m: muscle; e: epidermis; V: ventral; D: dorsal. Scale bar = 500 μm. Asterisks indicate significant differences between starvation groups and CTR groups. ***p < 0.001; ****p < 0.0001.
As shown in Figure 5, the gene expression of Mcfp-1P and Mcfp-1T in foot tissue was affected by starvation stress. The transcript abundance of Mcfp-1P and Mcfp-1T was only significantly up-regulated after 7 days of starvation (p < 0.05, Figures 5A, B), while no effect was observed on 14 and 21 days (p > 0.05, Figures 5A, B). There was no significant difference in Mccol2 transcript abundance in the starvation group relative to the CTR group (p > 0.05, Figure 5C).
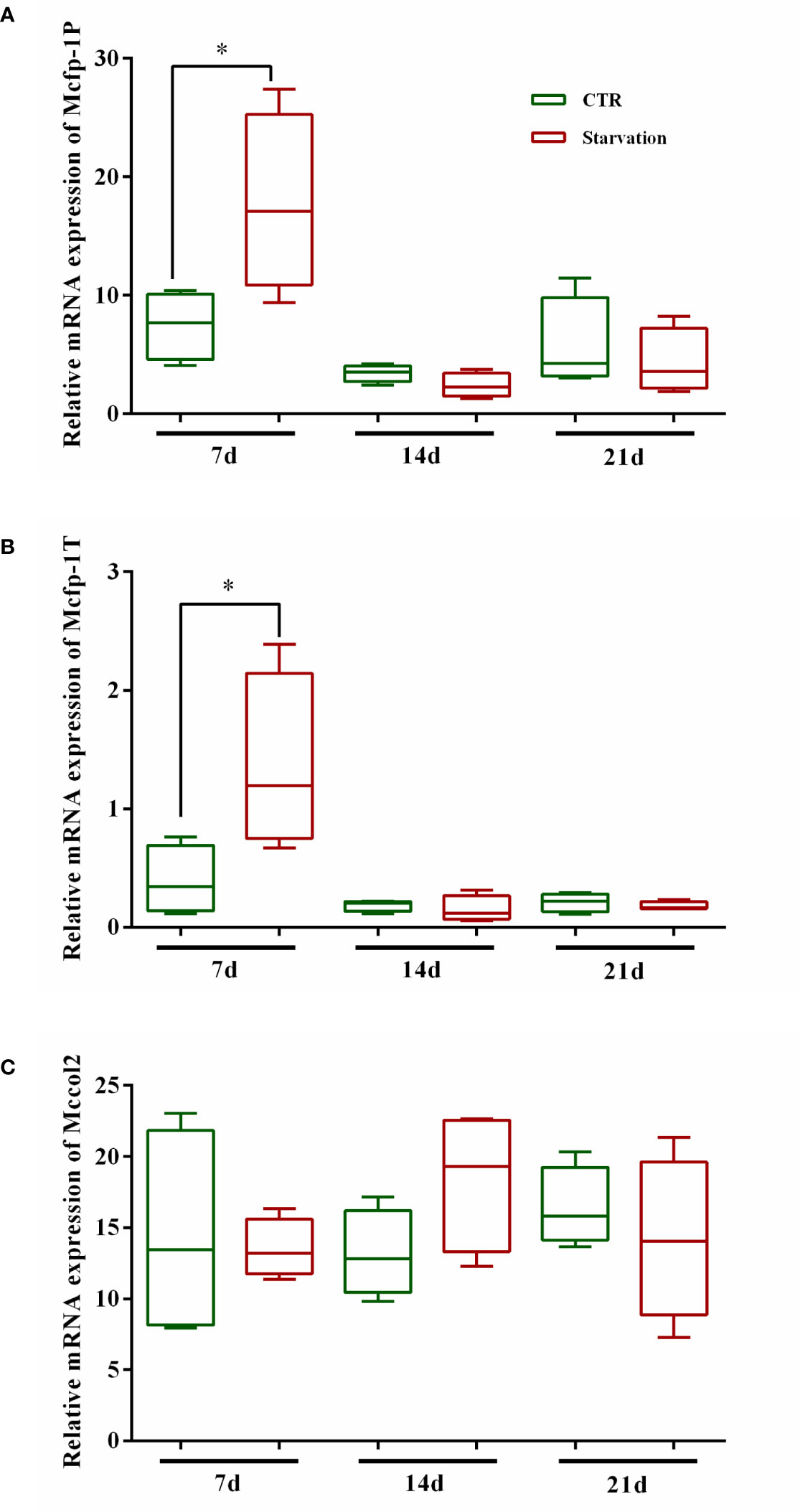
Figure 5 Effects of 21 days of starvation on gene expressions of mfps in foot tissue of M. coruscus. Mcfp-1P: adhesive plaque matrix protein; Mccol2: byssus collagen protein 2; Mcfp-1T: thread protein. CTR: control group. The results of statistical significance of the starvation groups compared to the control group were marked with asterisks above the columns. *p < 0.05.
The secretion of the byssus is a biologically and chemically process by which the mussel anchors itself to the substrate to resist the impact of enormous waves and predators (Carrington, 2002; Li et al., 2020). Food limitation constrains the energy available to mussels, which may increase the risk of promoting mussel detachment (Carrington et al., 2015). The results of this study show that starvation affects byssal production (including byssus number, diameter, and the cumulative byssal thread volume), the number of byssus shedding number, and the mechanical performance of byssal threads (breaking force and failure location). Furthermore, we showed that starvation alters the mussel foot secretory glands area and the expression level of foot protein genes.
Byssal threads are the fibrous holdfast for mussel attachment, and unfavorable conditions such as reduced wind-driven water movement in summer restrict food delivery to the innermost layers of mussel aggregations, resulting in weaker attachment and promoting detachment (Carrington et al., 2015). In the present study, we found that starvation-treated mussels at 21°C did not show a reduction of byssus secretion number, and even the number of byssus was observed to exceed that of the CTR group. This result is contrary to previous results showing that hard-shelled mussels (M. coruscus) starved for 21 days at 26°C reduced byssus production (Shang et al., 2021). This discrepancy in byssus secretion might be attributed to the variation of temperature and the experimental body size of mussels. Temperature is one of the most critical environmental factors affecting mussel physiology, controlling many biological and chemical processes (Zippay and Helmuth, 2012). Moreover, high temperatures inhibited mussel byssus production (Young, 1985; Newcomb et al., 2019; Li et al., 2020). In addition, the experimental body size of mussels could be another reason for the contrary results. The mussels with a shell length of 9.1 ± 0.4 cm were used in this study, which was larger than the mussels (shell length of 7.5 ± 0.2 cm) used in a previous study (Shang et al., 2021). Larger mussels may have more energy reserves to ensure their byssus production under starvation (Babarro et al., 2008).
Mussels undergo migration under unfavorable conditions, which can be reflected in the secretion and shedding of byssus (Côté and Jelnikar, 1999; Carrington et al., 2015; Li et al., 2015). Passive migration can be seen as a form of self-protection for mussels when they encounter unfavorable environments, dislodging themselves from the substrate to find suitable habitats (Hunt and Scheibling, 2001; Duchini et al., 2015; Iwasaki, 2015). Hard-shelled mussels (M. coruscus) are more prone to shedding byssus under high temperatures, low pH, and predator presence (Li et al., 2015; Li et al., 2020). In the present study, we found that starvation led to a significant increase in the number of byssus shedding. This could be an adaptive response to passive migration in a hostile environment (food scarcity), where mussels may release themselves from attachments and move.
Unfavorable environmental stresses may compromise the integrity of the byssal thread structure and ultimately weaken the attachment (Carrington et al., 2015; Li et al., 2017; Clements et al., 2018; Li et al., 2020). In this study, the starvation treatment decreased the byssal thread diameter and byssal breaking force, which undermined the byssal attachment strength in starved mussels. These results were consistent with a previous study showing decreased byssal attachment strength in non-feeding M. galloprovincialis (Babarro et al., 2008). The process of byssus formation may consume a large portion of the mussel’s energy budget (Li et al., 2017). Under starvation stress, insufficient energy intake may negatively affect protein synthesis in mussels, leading to thinner byssal thread and reduced breaking force, and these directly lead to weakened attachment strength, increasing the risk of mussels detaching from the attachment substrate. The weakest part of the thread might be an important factor that determines the strength of mussel attachment. We found that the mean value of the failure location on day 7 was mainly found in the upper part of the thread, while the breakpoints mainly occurred in the lower part of the thread due to increased breakpoints after 14 and 21 days of starvation treatment. However, after 14 and 21 days of starvation treatment, the mean value of the failure location increased due to the increase of break points in the upper part of the threads. Reduced byssal breaking force and shifted failure location both lead to reduced adhesion (Li et al., 2020). Similar results suggest that changes in the location of byssal thread breakage are also an indication of environmental stress in mussels (O’Donnell et al., 2013; Li et al., 2019b; Li et al., 2020). Considering that byssus is formed by proteins that secrete proteins, it can be hypothesized that starvation may regulate byssus performance by affecting the synthesis and secretion of proteins in the mussel foot.
Many animals suffer from stress due to food scarcity in their natural environment, and though some can tolerate such conditions, they still respond physiologically (Sánchez-Paz et al., 2008; Watts et al., 2014). For example, chronic starvation led to changes in the activity of some key enzymes in the digestive glands of redclaw crayfish (Cherax quadricarinatus), and histological analysis showed structural alterations (Sacristán et al., 2016). There was a significant difference in the size of the digestive glands between mussels (M. galloprovincialis) fed more food per day and those fed less food per day (Albentosa et al., 2012). Pacific oysters (Crassostrea gigas) differed significantly in the wet weight of digestive glands when fed low and high rations (Huvet et al., 2003). In this study, we found that starvation resulted in a decrease in the percentage of foot secretory glands area to total foot tissue. Therefore, the reduction in the foot secretory glands area observed in this study might be due to the atrophy of mussel foot secretory glands caused by starvation. The mussel foot tissue consists mainly of muscles and secretory glands (collagen, enzyme and phenolic glands) that are responsible for the synthesis and storage of the molecular components of the byssus (Waite, 1992; Waite, 2017). The mussel foot secretory glands contain large amounts of endoplasmic reticulum, ribosomes and secretory vesicles containing proteins essential for byssus production (Tamarin and Keller, 1972; Priemel et al., 2017). Given the close association between byssus production and foot secretory glands, it is hypothesized that foot secretory glands atrophy leads to abnormal gland function, further impacting the structure and strength of the byssus.
Various molecular precursor proteins that form the byssus are assembled in the foot, and the increased expression of the mussel foot protein genes promotes the accumulation of mussel foot proteins, which is essential for byssus production (Waite, 2017; Li et al., 2020). Mcfp-1P and Mcfp-1T are located in the plaque and thread of the byssus, respectively (Inoue et al., 1996; Liao et al., 2012). In this study, we found that starvation treatment for 7 days increased Mcfp-1P and Mcfp-1T mRNA expression levels. The up-regulation of Mcfp-1P and Mcfp-1T mRNA expression corresponded with the increase in the number of byssuses reflected the involvement of mussel foot proteins in byssus synthesis. However, after 14 days, the mRNA expression of Mcfp-1P and Mcfp-1T in control and starvation mussels showed no difference, although the number of byssuses was higher in the starved group than in the control group. Thus, suggesting that Mcfp-1P and Mcfp-1T are not the most critical element in the production of byssus.
The present study shows that starvation increases the number of byssus secretions in mussels, but also increases the number of shed byssus and weakens byssus performance. Furthermore, starvation treatment reduced the percentage of mussel foot glands area to total foot tissue which might be a cause for the weakened byssal thread strength. Food limitation might alter the mussel foot gland’s physiology, which needs further investigation. Food scarcity has potentially adverse effects on the byssus production of mussels in a variety of habitats, which can increase the dislodgment risks of suspended-cultured mussels.
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding authors.
YZ: Investigation, Data curation, Formal analysis, Writing – original draft review and editing. Y-MY: Investigation, Data curation, Formal analysis, Writing – original draft. Y-FX: Investigation, Writing – original draft. Y-QW: Investigation, Data curation. XS: Investigation. G-HZ: Investigation, Conceptualization, Writing - review and editing. Y-FL: Conceptualization, Supervision, Writing – original draft, Writing – review and editing, Funding acquisition. All authors contributed to the article and approved the submitted version.
The authors acknowledge grants from the National Natural Science Foundation of China (No. 32172992) and Science and technology innovation action plan (19590750500).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Albentosa M., Sánchez-Hernández M., Campillo J. A., Moyano F. J. (2012). Relationship between physiological measurements (SFG-scope for growth-) and the functionality of the digestive gland in Mytilus galloprovincialis. Comp. Biochem. Physiol. Mol. Integr. Physiol. 163 (3-4), 286–295. doi: 10.1016/j.cbpa.2012.07.019
Babarro J. M. F., Reiriz M. J. F., Labarta U. (2008). Secretion of byssal threads and attachment strength of mytilus galloprovincialis: The influence of size and food availability. J. Mar. Biol. Assco UK. 88, 783–791. doi: 10.1017/S0025315408001367
Buck B. H., Ebeling M. W., Michler-Cieluch T. (2010). Mussel cultivation as a co-use in offshore wind farms: Potential and economic feasibility. Aquacult. Econ. Manage. 14 (4), 255–281. doi: 10.1080/13657305.2010.526018
Buschbaum C., Dittmann S., Hong J. S., Hwang I. S., Strasser M., Thiel M., et al. (2009). Mytilid mussels: Global habitat engineers in coastal sediments. Helgoland Mar. Re. 63 (1), 47–58. doi: 10.1007/s10152-008-0139-2
Carrington E. (2002). Seasonal variation in the attachment strength of blue mussels: Causes and consequences. Limnol. Oceanogr. 47 (6), 1723–1733. doi: 10.4319/lo.2002.47.6.1723
Carrington E., Waite J. H., Sarà G., Sebens K. P. (2015). Mussels as a model system for integrative ecomechanics. Annu. Rev. Mar. Sci. 7, 443–469. doi: 10.1146/annurev-marine-010213-135049
Clarke M. (1999). The effect of food availability on byssogenesis by the zebra mussel (Dreissena polymorpha pallas). J. Mollus. Stu. 65 (3), 327–333. doi: 10.1093/mollus/65.3.327
Clements J. C., Hicks C., Tremblay R., Comeau L. A. (2018). Elevated seawater temperature, not pCO2, negatively affects post-spawning adult mussels (Mytilus edulis) under food limitation. Conserv. Physiol. 6 (1), cox078. doi: 10.1093/conphys/cox078
Côté I. M., Jelnikar E. (1999). Predator-induced clumping behaviour in mussels (Mytilus edulis Linnaeus). J. Exp. Mar. Biol. Ecol. 235 (2), 201–211. doi: 10.1016/S0022-0981(98)00155-5
Demartini D. G., Errico J. M., Sjoestroem S., Fenster A., Waite J. H. (2017). A cohort of new adhesive proteins identified from transcriptomic analysis of mussel foot glands. J. R. Soc Interface. 14 (131), 20170151. doi: 10.1098/rsif.2017.0151
Duchini D., Boltovskoy D., Sylvester F. (2015). Detachment, displacement and reattachment activity in a freshwater byssate mussel (Limnoperna fortunei): the effects of light, temperature and substratum orientation. Biofouling 31 (7), 599–611. doi: 10.1080/08927014.2015.1080251
Fitzgerald-Dehoog L., Browning J., Allen B. J. (2012). Food and heat stress in the California mussel: Evidence for an energetic trade-off between survival and growth. Biol. Bull-US. 223 (2), 205–216. doi: 10.1086/BBLv223n2p205
Garner Y. L., Litvaitis M. K. (2013). Effects of wave exposure, temperature and epibiont fouling on byssal thread production and growth in the blue mussel, mytilus edulis, in the gulf of Maine. J. Exp. Mar. Biol. Ecol. 446, 52–56. doi: 10.1016/j.jembe.2013.05.001
George M. N., Carrington E. (2018). Environmental post-processing increases the adhesion strength of mussel byssus adhesive. Biofouling 34, 388–397. doi: 10.1080/08927014.2018.1453927
Harrington M. J., Waite J. H. (2007). Holdfast heroics: comparing the molecular and mechanical properties of mytilus californianus byssal threads. J. Exp. Biol. 210 (24), 4307–4318. doi: 10.1242/jeb.009753
Harris L. S., Carrington E. (2020). Impacts of microplastic vs. natural abiotic particles on the clearance rate of a marine mussel. Limnol. Oceanogr. Lett. 5 (1), 66–73. doi: 10.1002/lol2.10120
Hunt H. L., Scheibling R. E. (2001). Patch dynamics of mussels on rocky shores: integrating process to understand pattern. Ecology 82 (11), 3213–3231. doi: 10.1890/0012-9658(2001)082[3213:PDOMOR]2.0.CO;2
Huvet A., Daniel J. Y., Quéré C., Dubois S., Prudence M., Van Wormhoudt A., et al. (2003). Tissue expression of two α-amylase genes in the pacific oyster Crassostrea gigas. effects of two different food rations. Aquaculture 228 (1-4), 321–333. doi: 10.1016/S0044-8486(03)00323-5
Inoue K., Takeuchi Y., Takeyama S., Yamaha E., Yamazaki F., Odo S., et al. (1996). Adhesive protein cDNA sequence of the mussel mytilus coruscus and its evolutionary implications. J. Mol. Evol. 43, 348–356. doi: 10.1007/BF02339009
Iwasaki K. (2015). Behavior and taxis of young and adult limnoperna fortunei. Springer Cham. 2015, 249–260. doi: 10.1007/978-3-319-13494-9_14
Lachance A., Myrand B., Tremblay R., Koutitonsky V., Carrington E. (2008). Biotic and abiotic factors influencing attachment strength of blue mussels mytilus edulis in suspended culture. Aquat. Biol. 2, 119–129. doi: 10.3354/ab00041
Liao Z., Li N. N., Wang X. C., Sun J. J., Shen W., Fan M. H., et al. (2012). Identification of novel foot proteins from mytilus coruscus byssus through proteomic analysis. Chin. J. Biochem. Mol. Biol. 28, 870–878. doi: 10.13865/j.cnki.cjbmb.2012.09.016
Li Y. F., Canário A. V. M., Power D. M., Campinho M. A. (2019a). Ioxynil and diethylstilbestrol disrupt vascular and heart development in zebrafish. Environ. Int. 124, 511–520. doi: 10.1016/j.envint.2019.01.009
Li S. G., Chen Y. Y., Gao Y. C., Xia Z. Q., Zhan A. B. (2019b). Chemical oxidants affect byssus adhesion in the highly invasive fouling mussel Limnoperna fortunei. Sci. Total Environ. 646, 1367–1375. doi: 10.1016/j.scitotenv.2018.07.434
Li S. G., Liu C., Zhan A. B., Xie L. P., Zhang R. Q. (2017). Influencing mechanism of ocean acidification on byssus performance in the pearl oyster pinctada fucata. Environ. Sci. Technol. 51 (13), 7696–7706. doi: 10.1021/acs.est.7b02132
Li L. S., Lu W. Q., Sui Y. M., Wang Y. J., Gul Y., Dupont S. (2015). Conflicting effects of predator cue and ocean acidification on the mussel mytilus coruscus byssus production. J. Shellfish Res. 34 (2), 393–400. doi: 10.2983/035.034.0222
Li Y. F., Yang X. Y., Cheng Z. Y., Wang L. Y., Wang W. X., Liang X., et al. (2020). Near-future levels of ocean temperature weaken the byssus production and performance of the mussel mytilus coruscus. Sci. Total Environ. 733, 139347. doi: 10.1016/j.scitotenv.2020.139347
Lin Q., Gourdon D., Sun C., Holten-Andersen N., Anderson T. H., Waite J. H., et al. (2007). Adhesion mechanisms of the mussel foot proteins mfp-1 and mfp-3. PNAS 104 (10), 3782–3786. doi: 10.1073/pnas.0607852104
Lucas J. M., Vaccaro E., Waite J. H. (2002). A molecular, morphometric and mechanical comparison of the structural elements of byssus from mytilus edulis and mytilus galloprovincialis. J. Exp. Biol. 205 (12), 1807–1817. doi: 10.1242/jeb.205.12.1807
Newcomb L. A., George M. N., O’Donnell M. J., Carrington E. (2019). Only as strong as the weakest link: Structural analysis of the combined effects of elevated temperature and pCO2 on mussel attachment. Conserv. Physiol. 7 (1), coz068. doi: 10.1093/conphys/coz068
O’Donnell M. J., George M. N., Carrington E. (2013). Mussel byssus attachment weakened by ocean acidification. Nat. Clim. Change 3, 587–590. doi: 10.1038/NCLIMATE1846
Priemel T., Degtyar E., Dean M. N., Harrington M. J. (2017). Rapid self-assembly of complex biomolecular architectures during mussel byssus biofabrication. Nat. Commun. 8 (1), 1–12. doi: 10.1038/ncomms14539
Pujol J. P. (1967). Formation of the byssus in the common mussel (Mytilus edulis l.). Nature 214 (5084), 204–205. doi: 10.1038/214204a0
Roberts E. A., Newcomb L. A., McCartha M. M., Harrington K. J., LaFramboise S. A., Carrington E., et al. (2021). Resource allocation to a structural biomaterial: Induced production of byssal threads decreases growth of a marine mussel. Funct. Ecol. 35 (6), 1222–1239. doi: 10.1111/1365-2435.13788
Sacristán H. J., Ansaldo M., Franco-Tadic L. M., Fernández Gimenez A. V., López Greco L. S. (2016). Long-term starvation and posterior feeding effects on biochemical and physiological responses of midgut gland of Cherax quadricarinatus juveniles (Parastacidae). PloS One 11 (3), e0150854. doi: 10.1371/journal.pone.0150854
Sánchez-Paz A., Soñanez-Organis J. G., Peregrino-Uriarte A. B., Muhlia-Almazán A., Yepiz-Plascencia G. (2008). Response of the phosphofructokinase and pyruvate kinase genes expressed in the midgut gland of the pacific white shrimp Litopenaeus vannamei during short-term starvation. J. Exp. Mar. Biol. Ecol. 362 (2), 79–89. doi: 10.1016/j.jembe.2008.06.002
Sarà G., Rinaldi A., Montalto V. (2014). Thinking beyond organism energy use: a trait-based bioenergetic mechanistic approach for predictions of life history traits in marine organisms. Mar. Ecol. 35, 506–515. doi: 10.1111/MAEC.12106
Shang Y., Gu H., Li S., Chang X., Sokolova I., Fang J. K., et al. (2021). Microplastics and food shortage impair the byssal attachment of thick-shelled mussel mytilus coruscus. Mar. Environ. Res. 171, 105455. doi: 10.1016/j.marenvres.2021.105455
Sui Y., Hu M., Huang X., Wang Y., Lu W. (2015). Anti-predatory responses of the thick shell mussel mytilus coruscus exposed to seawater acidification and hypoxia. Mar. Environ. Res. 109, 159–167. doi: 10.1016/j.marenvres.2015.07.008
Suplicy F. M. (2020). A review of the multiple benefits of mussel farming. Rev. Aquacult. 12 (1), 204–223. doi: 10.1111/raq.12313
Tamarin A., Keller P. J. (1972). An ultrastructutal study of the byssal thread forming system in mytilus. J. Ultrastruct. Res. 40 (3-4), 401–416. doi: 10.1016/S0022-5320(72)90110-4
Waite J. H. (1992). “The formation of mussel byssus: anatomy of a natural manufacturing process,” in Structure, cellular synthesis and assembly of biopolymers. results and problems in cell differentiation. Ed. Case S. T. (Berlin, Heidelberg: Springer), 27–54. doi: 10.1007/978-3-540-47207-0_2
Waite J. H. (2017). Mussel adhesion–essential footwork. J. Exp. Biol. 220 (4), 517–530. doi: 10.1242/jeb.134056
Watts A. J. R., McGill R. A. R., Albalat A., Neil D. M. (2014). Biophysical and biochemical changes occur in Nephrops norvegicus during starvation. J. Exp. Mar. Biol. Ecol. 457, 81–89. doi: 10.1016/j.jembe.2014.03.020
Young G. A. (1985). Byssus-thread formation by the mussel mytilus edulis: effects of environmental factors. Mar. Ecol. Prog. Ser. 24, 261–271. doi: 10.3354/meps024261
Keywords: starvation, byssal threads, mussel foot secretory glands, mussel foot protein, Mytilus coruscus
Citation: Zheng Y, Yang Y-M, Xu Y-F, Wang Y-Q, Shi X, Zheng G-H and Li Y-F (2022) Starvation shrinks the mussel foot secretory glands and impairs the byssal attachment. Front. Mar. Sci. 9:1040466. doi: 10.3389/fmars.2022.1040466
Received: 09 September 2022; Accepted: 27 September 2022;
Published: 10 October 2022.
Edited by:
Zhiguo Dong, Jiangsu Ocean University, ChinaReviewed by:
Liqiang Zhao, Guangdong Ocean University, ChinaCopyright © 2022 Zheng, Yang, Xu, Wang, Shi, Zheng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gao-Hai Zheng, eWl4aWFvbG91QDE2My5jb20=; Yi-Feng Li, eWlmZW5nbGlAc2hvdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.