- 1Guangdong Provincial Key Laboratory of Animal Molecular Design and Precise Breeding, School of Life Science and Engineering, Foshan University, Foshan, China
- 2Laboratory of Tropical Marine Bio-resources and Ecology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou, China
- 3Department of Animal science, Faculty of Science and Technology, Muban Chombueng Rajabhat University, Ratchaburi, Thailand
The trial was conducted to investigate the effects of limonene, allicin and betaine supplementation in low fish meal (FM) diet on growth performance, antioxidant capacity, meat quality and intestinal health in largemouth bass (M. salmoides). The biting-balls test and feeding trial were successively conducted. For the one, the results of the biting-ball test showed that with the increase of the concentration of the three attractants, the attracting effect firstly increased, then decreased, and the effect reached maximum at 0.2% concentration. (P < 0.05). Further, a 9-week feeding trial was conducted using five diets, including a basal diet with 30% and 40% fish meal without attractant, 30% fish meal supplemented with 0.2% limonene, 0.2% allicin or 0.2% betaine (the diets were named FM30, FM40, FM30 + L, FM30 + A, FM30 + B, respectively). The results demonstrated that adding limonene, allicin and betaine at concentration of 0.2% to the low fish meal feed could improve final body weight, weight gain rate, and specific growth rate of M. salmoides but only in 4 weeks (P > 0.05). Besides, dietary supplementation with attractants could significantly reduce the content of MDA in serum and liver, and increase the activity of GSH in liver (P < 0.05). Compared with FM30 group, the supplementation with limonene, allicin or betaine diet had higher pH, redness (a*), yellowness (b*) (P > 0.05), and lower refrigeration loss, cooking loss values (P < 0.05). Furthermore, supplementation with attractants groups had higher values for villus height, lamina propria, crypt depth, submucous layer, and serous layer (P < 0.05). Taken together, these results indicated that limonene, allicin and betaine had a time effect on the growth performance, and could improve antioxidant capacity, meat quality and intestinal health of M. salmoide.
Introduction
Fish meal is the preferred protein source for manufacturing aquafeed due to its nutritional contents, such as protein, fatty acids, and amino acid profile, as well as its excellent digestibility and palatability (Niu et al., 2020). However, resource depletion and rising prices seriously limited the use of fish meal in aquaculture (Li X. et al., 2021). Earlier, a number of studies conducted on various fish species demonstrated that low-fishmeal (LFM) diets can lead to poor feed palatability, decrease food intake and reduce the growth performance. For instance, olive flounder (Paralichthys olivaceus) (Niu et al., 2019), rainbow trout (Oncorhynchus mykiss) (Lazzarotto et al., 2018), Nile Tilapia (Oreochromis niloticus) (Wattanakul et al., 2019), Japanese seabass (Lateolabrax japonicu) (Rahimnejad et al., 2019). While, the attractants such as L-amino acids, taurine, betaine, glycine, fish meal, earthworms, Chinese herbs, and herbal extracts (Lunger et al., 2007; Shamushaki et al., 2007; Pu et al., 2017; Rufchaei et al., 2019; Xu et al., 2020) supplementation in LFM diets were considered as one of the most effective and reliable ways to improve the feed palatability (Hirt-Chabbert et al., 2012; Dar et al., 2019). But, it was also found that such odorants supplementation in fish feeds could affect the foraging behaviors of some species (Schmachtenberg, 2015). Therefore, the formulation of fish feeds using plants with distinct smells merits investigation to discover beneficial effects on feeding attractant activity.
Limonene is an aromatic compound in essential oils, commonly used food additive obtained from oranges, grapefruits, and lemons (Cicero et al., 2015; Giarratana et al., 2016; Ravichandran et al., 2018). It has been reported that limonene has with a variety of beneficial impact including growth improvement (Kesbiç et al., 2019), nutrient absorption (Aanyu et al., 2018), antioxidant enzymatic activity (Djenane, 2015), and can also improve the specific immunity (de Souza et al., 2019; Han et al., 2019). Similarly, allicin is an important biologically active sulfur containing organic compound extracted from the bulbs of garlic (Huang et al., 2020). Currently, various studies have shown that allicin could improve the growth performance (Lee et al., 2014; Ajiboye et al., 2016), reduce oxidative stress (Abdel-Daim et al., 2015), strengthen immunity (Hamed et al., 2021) as well as improve meat quality (Kaswinarni, 2015) of fish. And it has been found that allicin could promote the daily feed intake of many fish such as Litopenaeus vannamei (Samadi et al., 2016), common carp (Cyprinus carpio L) (Mohammad, 2020), Nile Tilapia (Oreochromis niloticus) (Soltan and Amal Elfeky, 2016), benni fish (Mesopotamichthys sharpeyi) (Milad Maniat et al., 2014) and African catfish (Clarias gariepinus) (Gabriel et al., 2019). In addition, diet replenished with allicin improved the survival and growth of large yellow croaker (Larimichthys crocea) larvae probably by promoting the intestinal development, alleviating inflammation and enhancing appetite (Huang et al., 2020). Betaine, a stable and non-toxic natural substance, is mainly extracted from the processing of sugar beet (Zhao et al., 2018) and was observed to improve growth performance, health status, feed digestibility, as well as flesh quality and the immune status of fish (Hirt-Chabbert et al., 2012; Pinedo-Gil et al., 2017; Ismail et al., 2020; Sun et al., 2020). It has been proven that betaine could act as a feed attractant and appetizer through stimulating the olfactory bulb, leading to increase the feed intake, which minimize the feed wastage and water pollution (Danaceau and Lucero, 2000).
In China, largemouth bass (Micropterus salmoides) typically a freshwater carnivore fish traditionally been cultured due to high commercial values and over the past decade its production has expanded over 600,000 tons because of its suitability for aquaculture, marketability, and high nutritional value (China Fishery Statistics Yearbook 2020). So far, there are no comprehensive studies have been reported though using betaine, limonene and allicin as a natural attractant in largemouth bass fed low fishmeal diets. Thus, the current study aimed to evaluate the effects of three herbal extracts on feed intake, growth performance, antioxidant capability, meat quality and intestinal health for largemouth bass supplemented low fishmeal diets.
Materials and methods
The biting-balls test
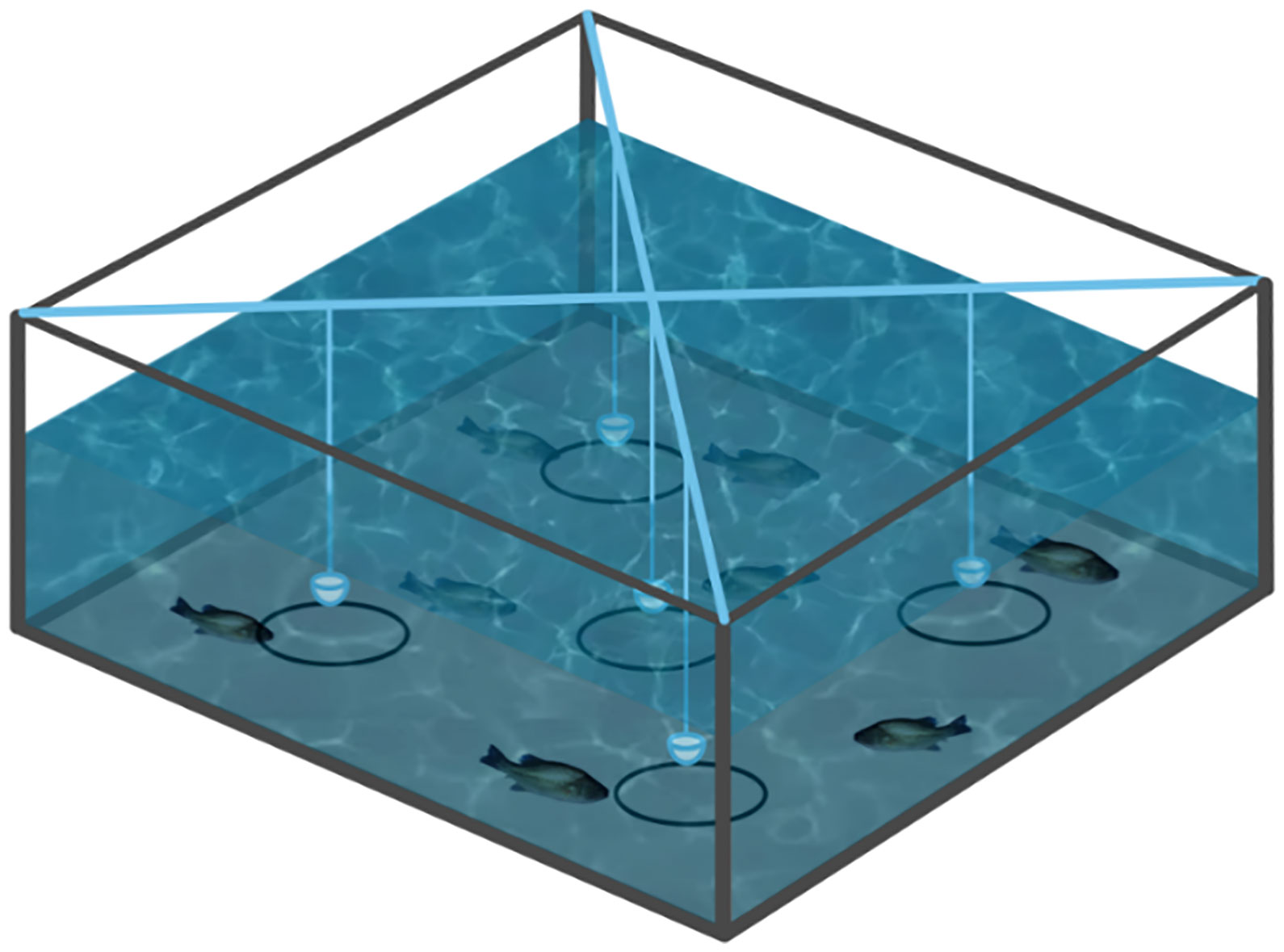
A biting-ball test device was prepared as reported previously and the schematics was shown in Figure 1 (Yu et al., 2021). A total of 150 fishes were placed into 3 tanks evenly [(150 × 150 × 60 cm) (height × width × length)], supplied with dechlorinated water. The water depth was maintained at 40 cm during the experiment and the experiment was carried out twice a day at 8:30 and 17:00 for three days. Five different concentrations (0.0%, 0.1%, 0.2%, 0.6% and 1.0%) solution of limonene, allicin and betaine were prepared and stored at 4°C, then injected into a cotton ball and wrapped with gauze, respectively. The biting-ball was fixed with iron wire and submerged 5 cm under the water’s surface to allow the fish to touch or bite it. In addition, a 10 cm diameter circle was drawn at the tank’s bottom as an effective region based on the center of the biting ball. The mobile phone recorded the number of bites of each bait ball and entries into the effective region within 10 minutes in order to determine the proper concentration of limonene, allicin, and betaine.
Experimental design and diet preparation
Five experimental diets were formulated and the formulation, and proximate composition of the experimental diets are presented in Table 1. The basal diet was prepared with fish meal, soybean meal and peanut meal as the main protein source, and fish oil, and wheat flour as the main lipid and carbohydrate source respectively. According to the results in the biting-balls test, we selected the same concentration (0.2%) of allicin, betaine, limonene for further experiments. All the five test diets were designed as follows: (1) the normal fishmeal group (FM 40); (2) the low fishmeal group (FM 30); (3) the low fishmeal diet supplemented with 0.2% limonene (FM30 + L); (4) the low fishmeal supplemented with 0.2% allicin (FM30 + A); (5) the low fish meal supplemented with 0.2% betaine (FM30 + B) as presented Table 1. Crystalline amino acids (lysine, methionine) also were added to the diet to balance the dietary amino acid requirements in low fish meal diets. All dry ingredients were mixed thoroughly, and then oil and water were added. The mixture was extruded as an expanded particle diet (diameter of 1.5 mm) using a DS32-II type two-screw extruder (Guangzhou Vilavi Mechanical Equipment Co., Ltd.) after water addition, then air-dried and stored at -20°C until use.
Feeding trial and experimental conditions
M. salmoides were obtained from Guangdong Ho’s Aquatic Products Co., Ltd. (Guangdong, China) and cultured in recirculating water system in Foshan University. Throughout the experiment, water temperature, pH, NH4+, nitrite, nitrate and dissolved O2 in water were maintained at 24-30°C, 6.5-7.5, < 1 mg/L, < 1 mg/L, < 20 ppm, and > 6 mg/L, respectively. After acclimation for 2 weeks, a total of 600 fish with similar body weight (mean initial weight 6.26 ± 0.01 g) were randomly assigned into 20 tanks. Each group contained four replicate tanks (30 fish/tank). All groups were fed two times per day at 8:30 and 17:00. The weight of the fish in each tank was recorded at fourth and sixth week.
Sample collection
After fasting for 24 h, fishes were anaesthetized with buffered MS-222, and the fishes in each tank were weighed to evaluate the growth performance parameters. Three whole fishes from each tank were sampled and stored at -20°C for subsequent proximate composition analysis. Blood was collected from the caudal vein of eleven fishes of each tank and blood samples were centrifuged (3000 r/min, 15 min) at 4°C, and the supernatant (serum) was stored at -80°C for further analysis. The livers and intestines of five fish per tank were collected and used for histopathological and enzyme activity analyses. Similarly, the dorsal muscles of six fish/tank were collected for flesh quality parameters analysis.
Enzyme assays
The collected livers were centrifuged for 10 min (2000 r/min, 4°C) before collecting the supernatant and then kept at -80°C. The supernatant of livers and serum were used to determine the superoxide dismutase (SOD) (determined by AST-1 method), malondialdehyde (MDA) (determined by thiobarbituric acid (TBA) test method), catalase (CAT) (determined by ammonium molybdenum acid method), glutathione (GSH) (determined by microplate method) and total protein (TP) (determined by coomassie blue staining) using the kits purchased from Nanjing Jiancheng Bioengineering Institute, China. All the analyses were performed according to the instructions of the manufacturer.
Muscle quality measurement
The muscle quality related parameters including the pH, lightness (L*), redness (a*), yellowness (b*) and water holding capacity (included thawing loss, refrigeration loss, centrifugal loss, cooking loss, drop loss and pressure loss) of dorsal muscle were measured as earlier been reported by Caimi et al. (Caimi et al., 2021). The L*, a* and b* of muscle were analyses using colorimeter (SCQ-1A Tenovo International Co., Limited) while, muscle pH was measured with a direct pH meter (accurate to 0.01, pH star, Mets, Germany).
Intestinal morphology analysis
The whole intestines were fixed in 4% paraformaldehyde, dehydrated in a graded alcohol series, cleared in xylol, embedded in paraffin, sectioned at 5 μm thickness, and hematoxylin and eosin (H&E) staining were performed. Lastly, the stained sections were observed under the microscope camera NLCD 500 (Nanjing China). Image J software (W. Rasband, NIH, USA) was used to measure the villi height (VH), villi width (VW), muscle thickness (ML), lamina propria (LP), crypt depth (CD), submucous layer (SML) and serous layer (SL).
Calculation and statistical method
Growth performance of M. salmoides was calculated as follows:
Final body weight (FBW) = the weight of fish in the tank/the number of fish in the tank;
Weight gain rate (WGR, %) = 100 × (final body weight-initial body weight)/initial body weight;
Daily feed intake (DFI, g/fish) = (amount of feed consumed by all fish in a tank/(days of the experiment × (IBW+FBW)/2) ×100%);
Specific growth rate (SGR, %/d) = 100 × (Ln final body weight-Ln initial body weight)/days of the experiment;
Feed conversion ratio (FCR) = feed intake/body weight gain;
Survival rate (SR, %) = 100 × (final number of fish)/(initial number of fish);
Condition factor (CF, g/cm3) = 100 × body wet weight (g)/body length (cm)3;
Hepatosomatic index (HSI, %) = 100 × (liver weight/whole body weight);
Viscerosomatic index (VSI, %) = 100 × (viscera weight/whole body weight);
Intestinal index (ISI, %) = 100 × (intestine weight/whole body weight);
Intestinal length index (ILI, %) = 100 × (intestine length/body length).
All the data were statistically analyzed by using SPSS 26.0 (SPSS Inc., Michigan Avenue, Chicago, IL, USA). One-way ANOVA followed by Duncan’s multiple range tests was used and all the results were presented as means ± S.E.M (standard error of the mean). Whereas, the values of P ≤ 0.05 were considered as level of significance.
Results
The biting-balls test
It has been observed that at 8:00, only the effect of 0.2% allicin and betaine as food attractants was significantly higher than that of the 0.0% group (P < 0.05). While at 17:00, all the three food attractants (limonene, allicin, and betaine) with 0.2% concentration have a substantially higher effect than that of the 0.0% group (P < 0.05). Furthermore, limonene, allicin, and betaine as a food attractant with different concentrations 0.0%, 0.1%, 0.2%, 0.6%, and 1.0% are given in Table 2.
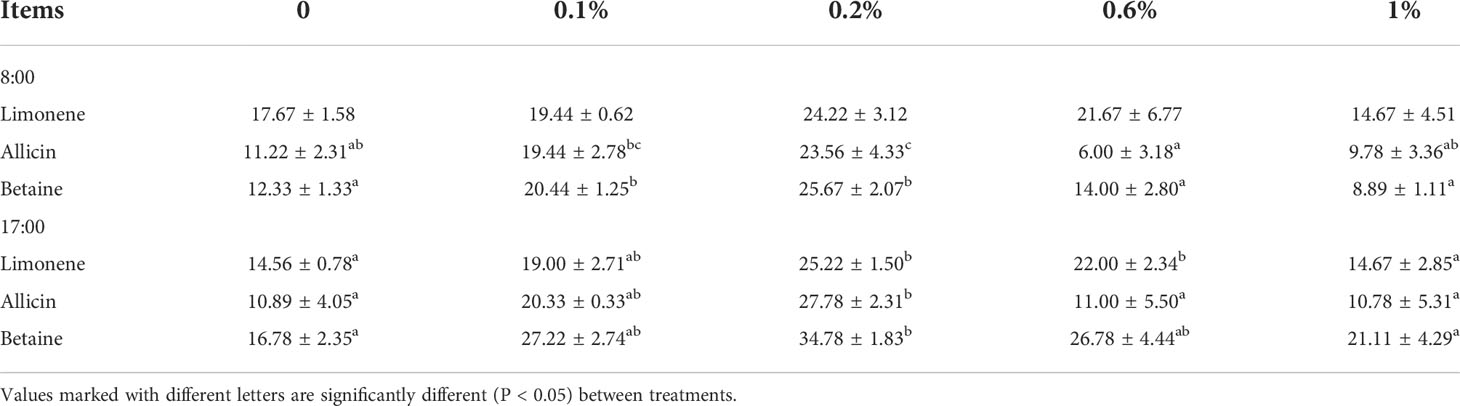
Table 2 The effects of different concentrations of limonene, allicin and betaine on attracting of M. salmoides at 8:00 and 17:00.
Growth performance
Similarly, the growth performance, feed utilization and biometric indices were also evaluated and are presented in Table 3. At 4th week the group fed with FM40 diet presented significantly higher FBW, WGR, and SGR than the group fed with FM30 diet (P < 0.05), meanwhile, no difference was observed for FBW, WGR, or SGR among all the attractant groups (P > 0.05). Although, both the FM30 and FM40 groups at 6th week exhibited an insignificant (P > 0.05) differences for DFI and FCR whereas, at 6th week the DFI in FM30 + A group was significantly higher than that of the FM30 group (P < 0.05). Additionally, no significant difference was observed for FBW, WGR, and SGR among the experimental groups (P > 0.05) at 9th week.
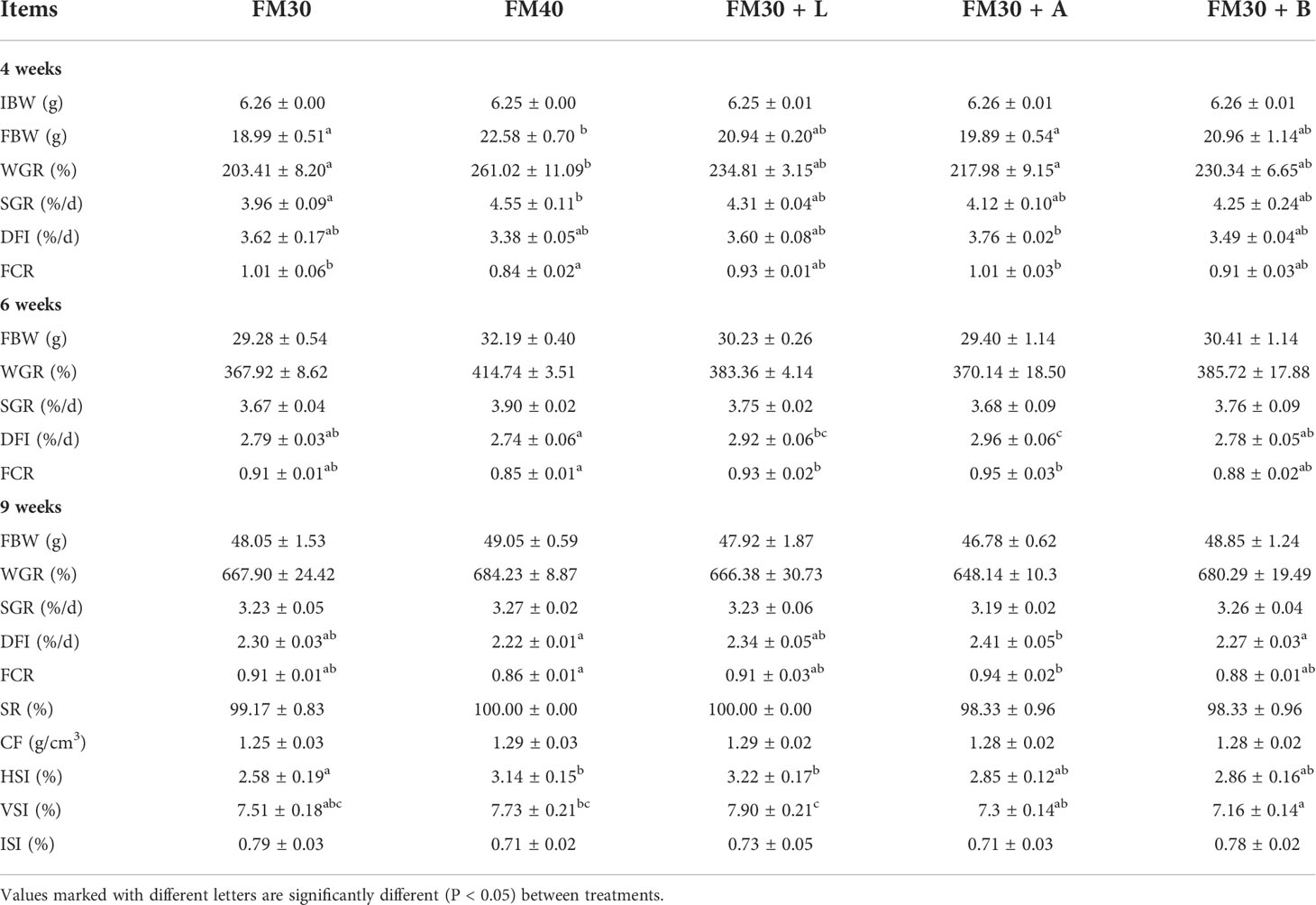
Table 3 Effects of limonene, allicin and betaine on growth performance of M. salmoides for 4, 6 and 9 weeks.
Furthermore, the CF, ISI and ILI was not changed among the experimental groups after 9 weeks (P > 0.05). Besides, the FM30 + L diet group had significantly higher levels of HSI than the FM30 diet group (P < 0.05) after 9 weeks, but there was no significant difference in HSI between the FM40 and the supplementation with limonene, allicin or betaine groups (P > 0.05).
Whole-body and muscle chemical composition
All the dietary treatments had an insignificant (P > 0.05) effect on the contents of the crude protein, crude lipid, and moisture levels of the whole body and muscle mass. However, the contents of the crude ash in FM30, FM30 + L, FM30 + A and FM30 + B groups were lower than that in FM40 group to varying degrees, and the FM30 + B group was significantly lower than that in FM40 group. The results of the whole body and muscle composition analysis are depicted in Tables 4, 5.
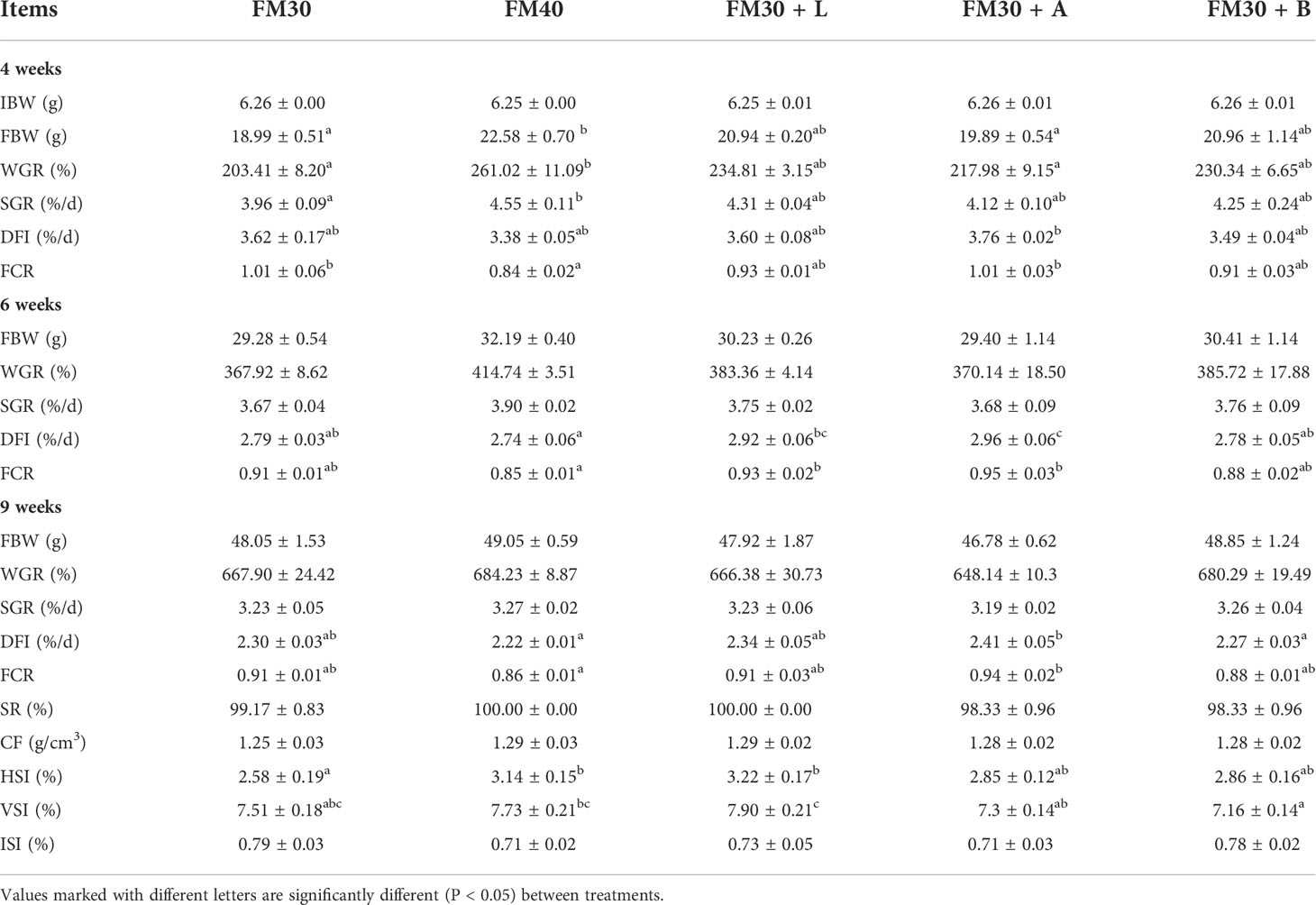
Table 4 Effects of limonene, allicin and betaine on whole-body composition (dry-weight basis) of M. salmoides for 9 weeks.
Table 5 Effects of limonene, allicin and betaine on muscle composition (dry-weight basis) of M. salmoides for 9 weeks.
Liver antioxidant capability
A significantly higher contents of the MDA were detected in FM30 diet group (P < 0.05) as compared to the FM30 + L and FM30 + B groups, while no change in MDA contents were observed between the FM40 group and the supplementation with limonene, allicin or betaine groups (P > 0.05) as shown in Figure 2A. Similarly, the GSH contents in FM30 + A and FM30 + B diet groups were significantly higher than that of the FM30 diet group (P < 0.05), but insignificant difference was perceived between the FM40 group and the supplementation with limonene, allicin or betaine group (P > 0.05) (Figure 2B). In addition, among all the groups (P > 0.05) the activity of SOD was not differ (Figure 2C). Moreover, the CAT activity of the FM30 + L, FM30 + A and the FM30 + B group were significantly lower than that of the FM30 and the FM40 groups (P > 0.05) as presented in Figure 2D.
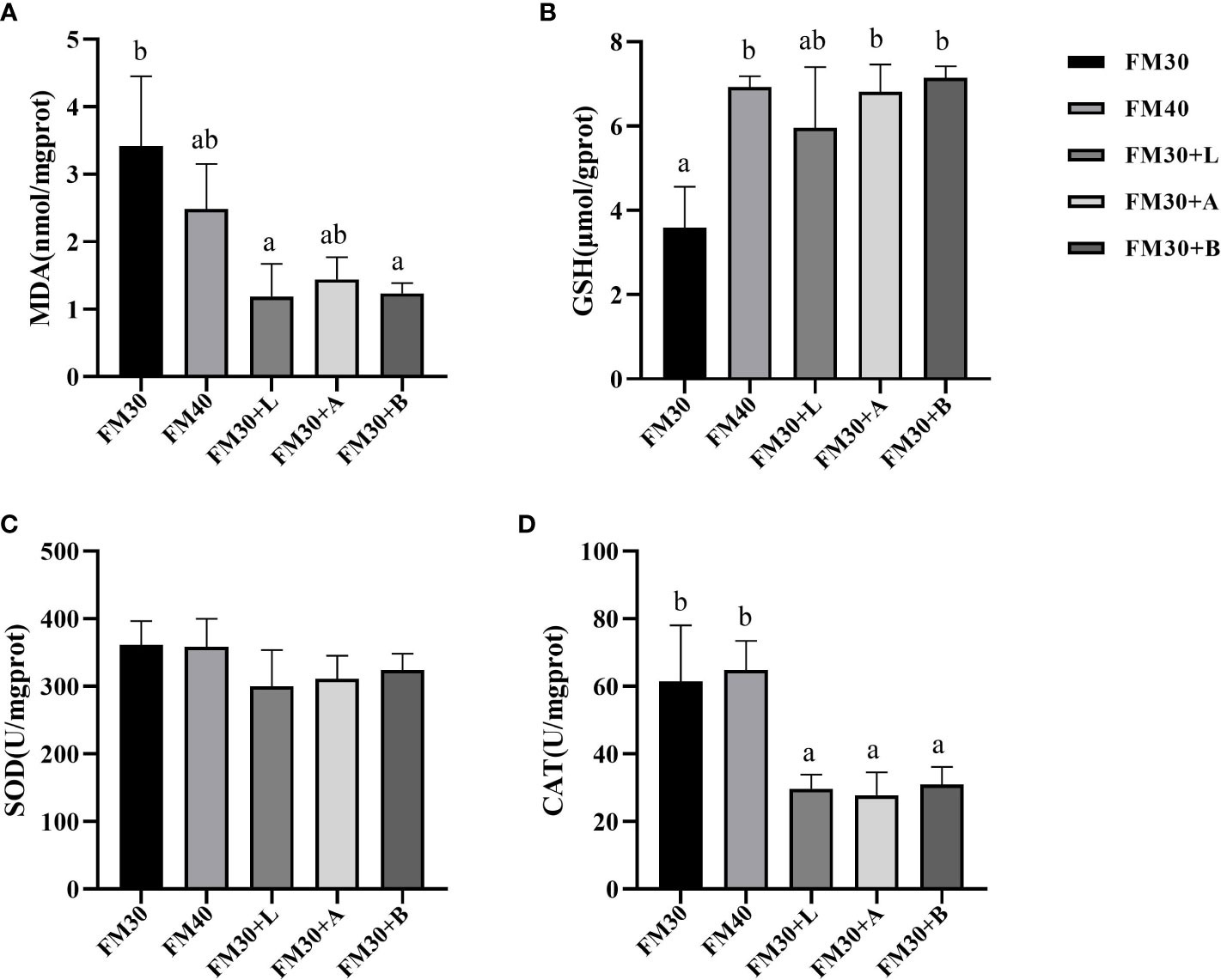
Figure 2 Effects of limonene, allicin and betaine on liver antioxidant capability of M. salmoides for 9 weeks. (A) Malondialdehyde (MDA); (B) glutathione (GSH); (C) superoxide dismutase (SOD); (D) catalase (CAT). Values (mean ± standard error of the mean, SEM) in bars that have the same letter are not significantly different (P > 0.05) between treatments.
Serum antioxidant capacity
The contents of MDA in FM30 + A group was significantly lower than that of the FM30 group (P < 0.05), while was not differ between the FM40 group and supplementation with limonene, allicin or betaine group (P > 0.05) (Figure 3A). Similarly, the GSH contents in FM30 + A and FM30 + B groups were markedly lower than the FM40 group (P < 0.05) (Figure 3B). Moreover, the FM30 + A group had lower SOD activity compared to other groups (P < 0.05) (Figure 3C), however, no difference has been observed for CAT activity among all the groups (P > 0.05) (Figure 3D).
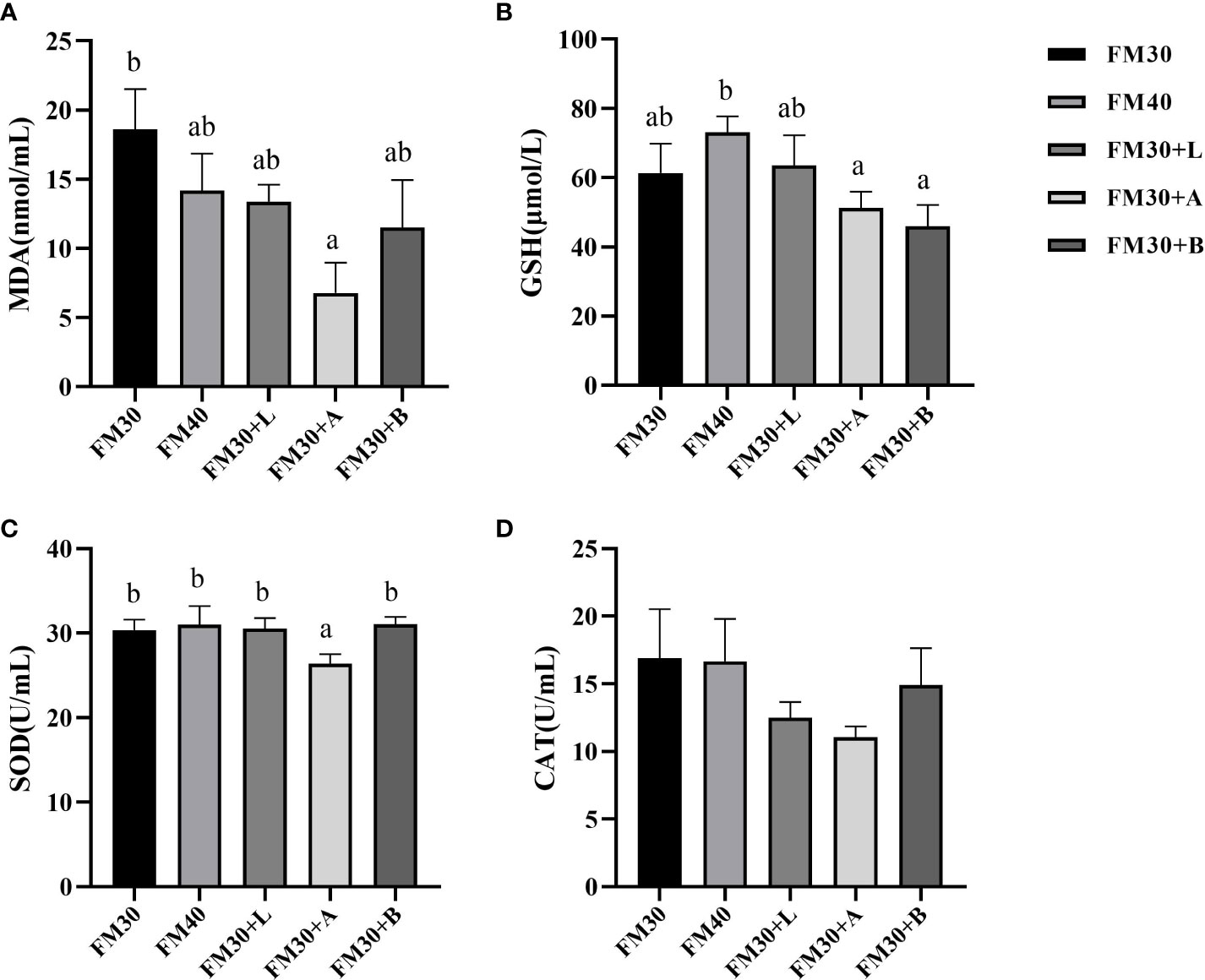
Figure 3 Effects of limonene, allicin and betaine on serum antioxidant capability of M. salmoides for 9 weeks. (A) Malondialdehyde (MDA); (B) glutathione (GSH); (C) superoxide dismutase (SOD); (D) catalase (CAT). Values (mean ± standard error of the mean, SEM) in bars that have the same letter are not significantly different (P > 0.05) between treatments.
Meat quality
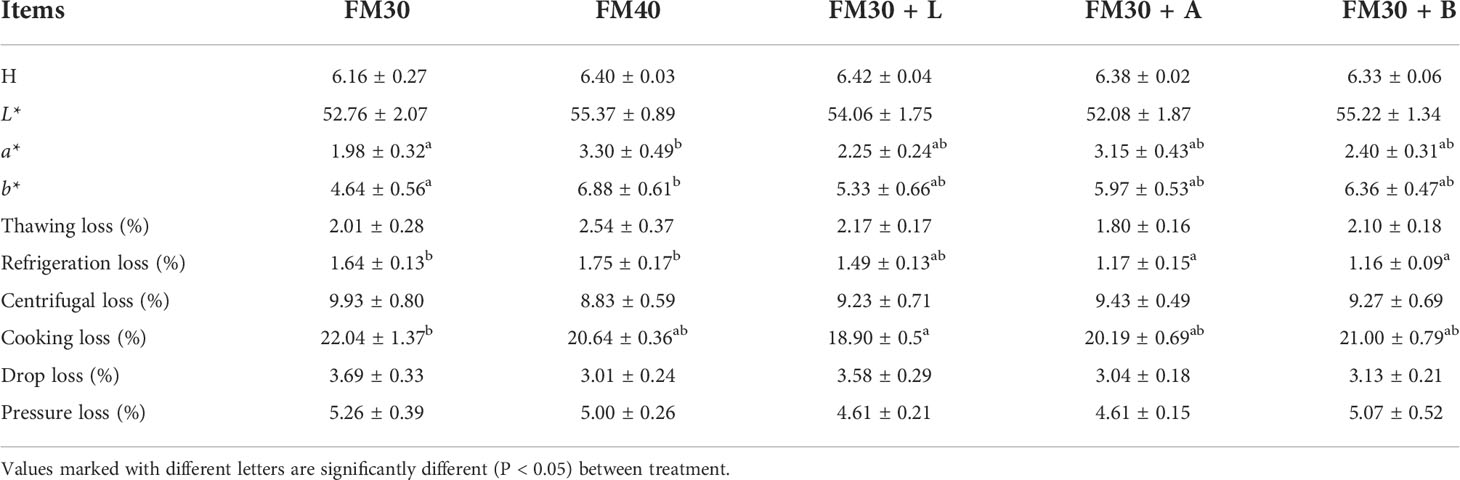
As shown in Table 6, the pH, L*, thawing loss, centrifugal loss, drop loss and pressure loss were not differ among all the experimental groups (P > 0.05), while, the a* and b* of FM40 group was significantly higher than that of the FM30 group (P < 0.05), but no difference was there between the FM40 group and the supplementation with limonene, allicin or betaine groups (P > 0.05). The refrigeration loss of the FM30 + A and FM30 + B diets were significantly lower than that of the FM30 and FM40 groups (P < 0.05). The cooking loss of the FM30 + L group was also lower than that of the FM30 group (P < 0.05).
Intestinal morphology
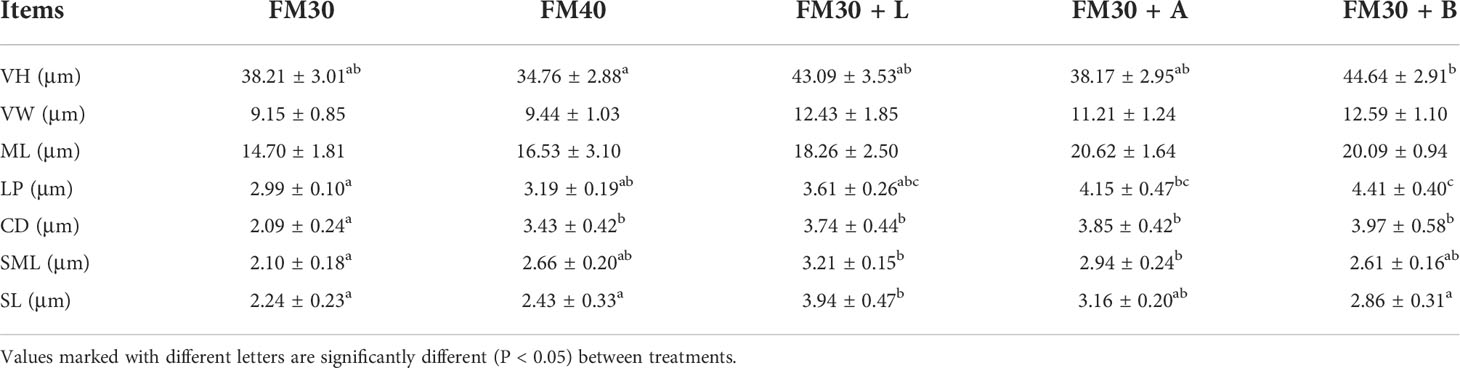
The intestinal morphology showed that the villi of FM30 group were injured and broken, and the thickness of the small intestinal wall was heterogeneous as depicted in Figure 4A. The FM30 + B group had significantly higher number of villi compared with the FM40 group (P < 0.05) (Figure 4B; Table 7) But the width and muscular layer of fishes’villus were not differ in all groups (P > 0.05). The lamina propria of FM30 + A and FM30 + B groups were significantly higher compared to the FM30 group (P < 0.05) (Table 7). Meanwhile, FM40, FM30 + L, FM30 + A and FM30 +B groups had significantly deeper crypt depth compared with FM30 group (P < 0.05) (Figures 4C–E). The submucous layer value of FM30 + L and FM30 + A group were significantly higher than FM30 group (P < 0.05). while, the serous layer of FM30 + L group was significantly thicker than FM30, FM40 and FM30 + B groups (P < 0.05). The villus height, villus width, muscular layer, lamina propria, crypt depth, and submucous layer values of fishes was not varied in limonene-, allicin- or betaine-supplementation groups (P > 0.05).
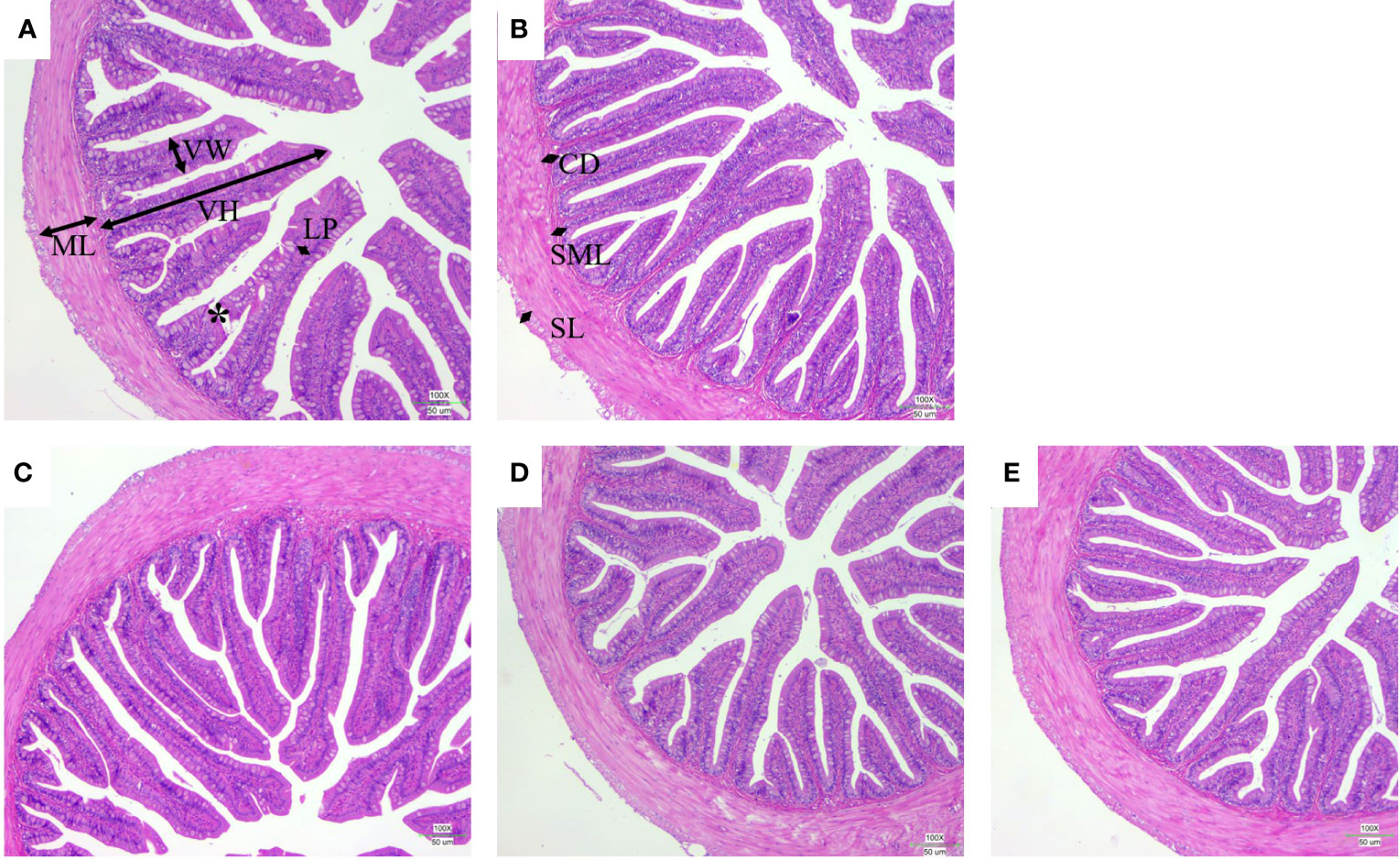
Figure 4 Effects of limonene, allicin and betaine on intestinal morphology of M. salmoides for 9 weeks. CD, crypt depth; LP, lamina propria; ML, muscular layer; SL, serous layer; SML, submucous layer; VH, villus height; VW, villus width. Scale bars = 50 μm. (A) FM30; (B) FM40; (C) FM30 + L; (D) FM30 + A; (E) FM30 + B.
Discussion
In aquaculture, the commercial bait is composed of food-based basic materials and attractants, among which the attractants play a decisive role in the entire bait due to their characteristic flavour (Yu et al., 2021). Simultaneously, fish predominantly rely on their olfaction for a variety of fundamental behaviors such as foraging (Volz et al., 2020), and food attractants that could stimulate the olfactory receptors (Wang et al., 2021). Limonene is a translucent liquid with pleasant lemon-like odor (Ibáñez et al., 2020), allicin is the compound responsible for garlic’s pungent odor (Borlinghaus et al., 2014), and betaine is a flavor enhancer used to reduce bitterness and imparting optimal sweetness and umami to food (Tu et al., 2020). Based on these theories, we designed this biting-balls test, it showed that these three compounds limonene, allicin and betaine do have positive impact on food consumption. And it is consistent with the results of our biting-balls test, indicating that limonene, allicin and betaine could stimulate the smell or taste receptors of M. salmoides and had a strong attraction effect (Reyes-Camacho et al., 2021). In addition, the attraction effects of different food attractants varied, which could be attributed to differences in the number of olfactory receptor genes responsible for detecting different odor molecules (Liu et al., 2021), resulting in different sensations or recognition capacities of olfactory and taste receptors to attractants in M. salmoides. In this study, when the concentrations of limonene, allicin and betaine were higher than 0.2%, the attraction effects on M. salmoides was gradually weakened, even lower than that of the control group. It might be because high concentrations of limonene, allicin and betaine were beyond the tolerance range of M. salmoides.
Interestingly, a gradual decrease in specific growth rate and daily feed intake was observed from 4th to 9th weeks, and the variations in growth performance progressively became inconspicuous among all the experimental groups. We speculate that M. salmoides might adapt to the taste and texture of the different diets with the extension of the feeding time, similar phenomenon was also reflected in previous researches (Tian et al., 2016; Lazado et al., 2019; Le et al., 2020; Martchenko et al., 2021). In this study, the crude protein, crude lipid and moisture of whole body and muscle did not differ among all the dietary treatments. The content of crude protein and moisture in whole fish and muscle of M. salmoides in the FM30 + L, FM30 + A and FM30 + B groups were closer to those of the FM40 group. Various factors have contributed to the nutritional composition of fish, such as genetic factors (Cai et al., 2021), water environment (Mohanty et al., 2019; Byrd et al., 2020), and season (Duarte et al., 2022), while the most important factor is the feed nutrition (Khalili Tilami and Sampels, 2017). At present, there have been many studies shown that supplementation of attractants, such as betaine (Yeşilayer and Kaymak, 2020), squid hydrolysate and squid meal (Novriadi et al., 2017), red seaweed eucheuma denticulatum (Eucheuma denticulatum) (Ragaza et al., 2015), taurine (Nguyen et al., 2020) to a low-fishmeal diet had no significant effect on fish chemical composition. However, the contents of the crude ash in FM30, FM30 + L, FM30 + A and FM30 + B groups were lower than that in FM40 group to varying degrees, and the FM30 + B group was significantly lower than that in FM40 group. Similar results were also shown in the study of Nile tilapia (Oreochromis niloticus) (Ahmad and Abdel-Tawwab, 2011) and juvenile tinfoil barb (Barbonymus schwanenfeldii, Bleeker 1853) (Nafees et al., 2022). In addition, previous study have shown that the crude ash content of fish decreases with the prolongation of starvation time, (Abdel-Tawwab et al., 2006), thus, we speculate that largemouth bass fed a low fish meal diet were starved more quickly and fasted for longer, resulting in differences in the crude ash content of the whole fish. Thus, it has been perceived that growth performance, whole-body and muscle chemical composition as well as health parameters were not negatively affected by limonene, allicin, and betaine, even almost similar to FM40 group.
The SOD and CAT are typical antioxidant enzymes found in fish serum or liver that can prevent organisms from being harmed by reactive oxygen species (ROS), which can cause a variety of disorders by attacking macromolecules (Balaban et al., 2005). GSH is the most prominent non-enzymatic antioxidant in fish, as well as a free radical scavenger and detoxifier (Chen et al., 2015). MDA, a byproduct of lipid peroxidation that can interact with the free amino groups in protein causing cell damage (Xiao et al., 2022), and the contents of MDA in the fish liver can reflect the severity of the free radical attack on the liver or body cells (Całyniuk et al., 2016). Previous studies have demonstrated that limonene, allicin and betaine could improve the antioxidant capacity of fish (Abdel-Tawwab et al., 2021; Dong et al., 2021; Hamed et al., 2021; Ajiboye et al., 2016; Lopes et al., 2019; Lopes et al., 2020; Mohseni et al., 2021). In this study, the GSH and MDA contents variations in the liver as well as the MDA contents in the serum demonstrated the antioxidant effect of the aforesaid three attractants. On the contrary, the CAT activity in the liver and the GSH content as well as the SOD activity in the serum decreased in different group. This was because limonene, allicin and betaine could significantly reduce the oxidative stress damage, resulting in low concentration of catalytic substrates-free radicals, and SOD being unable to perform disproportionation reaction, whereas the CAT activity dropped as SOD activity declined. Furthermore, there were no significant differences between SOD in the liver and CAT in the serum, which might be because various antioxidant enzymes compete to respond with different degrees of antioxidative stress (Wang et al., 2019). Thus, it has been demonstrated that limonene, allicin, and betaine in a low fish meal diet may considerably minimize the degree of oxidative damage to body cells and our results are in line with the previous studies conducted on fruit fly (Drosophila melanogaster) (Nagpal and Abraham, 2017), Nile tilapia (Oreochromis niloticus) (Hamed et al., 2021), male rats (Rattus norvegicus) (Li et al., 2021b), broilers (Chen et al., 2021) and rats (Shan et al., 2021). These results indicated that limonene, allicin, and betaine might enhance M. salmoides’ antioxidant capability by enhancing antioxidant enzymes and decreasing MDA levels.
Meat quality is an important feature for producers and consumers. In addition to sensory attributes (color, juiciness, and flavor) (Oliveira et al., 2017), the meat quality is reflected in its physicochemical parameters, such as WHC, pH, and nutrient composition (Maltin et al., 2007). The pH is one of the most important factors affecting many meat quality attributes, such as meat color, tenderness, the WHC and other characteristics of muscle (Cao et al., 2012). The fish meat tenderness decreased as the pH decline. Furthermore, fish color is one of the major criteria for determining freshness, with a significant influence on customer purchasing decisions (Truong et al., 2014). In this study, the pH of supplementation groups was all higher than that of the FM30 group, indicating that limonene, allicin and betaine could effectively maintain the relatively high pH in a short time, and improved the tenderness. Moreover, the a* and b* of fish fed the FM40 diet were significantly higher than that of fish fed the FM30 diet, whereas the a* and b* did not differ between the FM40 group and the supplementation groups, indicating that the low fish meal diet could affect the body color. However, limonene, allicin and betaine supplementation in low fish meal diet could restore a* and b* indices to the level of the normal fish meal diet, which were consistent with the earlier studies conducted on pigs (Lan et al., 2017) and chicks (Attia et al., 2009). Thus, we speculated that these are because of the antioxidant and antibacterial activity of the limonene and allicin (Bacanli et al., 2015; Costa et al., 2019; Dwivedi et al., 2019; Li D. et al., 2021). Limonene and allicin reduced the oxidation, degeneration and acidification rate of muscle, while increasing the pH. Betaine might improve the muscle pH by altering the anaerobic glycolysis and antioxidant capacity of muscle (Chen et al., 2020).
Water-holding capacity (WHC) is of great significance to the physical form, flavor and color of muscle, which can be evaluated by thawing, refrigeration, centrifugal, cooking, drop and pressure loss. Our results showed that the refrigeration loss of fish fed FM30 + A and FM30 + B diets were significantly lower than that of fish fed FM30 and FM40 diets, and the cooking loss of fish fed FM30 + L diet was lower than that of fish fed FM30 diet, indicating that limonene, allicin and betaine could improve the flesh WHC. Earlier it has been illustrated that muscle WHC was positively correlated with the MDA content (Datta et al., 2015). Furthermore, studies have also been documented that the WHC of muscle is closely related to pH and decline in pH decreases results in lower electrostatic strength of muscle protein, which dipping the interaction between charges and the gap between myoprotein fiber and actin fiber. Water permeates from myofibrils to sarcoplasma and further into the extracellular space of muscle, resulting in increased water loss and lower WHC (Huff-Lonergan and Lonergan, 2005). In the study, the MDA content in the liver or serum in the supplementation groups were significantly lower than that of the FM30 group, and the pH was higher, indicating that limonene, allicin and betaine might improve the WHC by reducing the oxidative damage and increasing pH. Furthermore, the WHC had the highest cooking loss due to denaturation of muscle protein causing myofibril contraction, exposing more hydrophobic groups, and increasing water fluidity and eventually the water loss (Wang K. et al., 2020).
Intestinal health and integrity are directly connected to the precise fish physiological processes since it is a vital organ for nutrition absorption and utilization (Wang J. et al., 2020). In fish, intestinal villi are an important site for the secretion of digestive enzymes and nutrient absorption, therefore, the villus with regular shape and complete structure are the basic conditions to ensure fish intestinal health (Torrecillas et al., 2019; Yuan et al., 2019; Li W. et al., 2021). Crypt depth influence the process of migration, development and differentiation of tiny intestinal cells, hence influencing the process of digestion and absorption. Besides, the force of intestinal peristalsis generated from the contraction of smooth muscle, as the thickness of muscular layer increases, more will be the intestinal peristalsis which could improve the digestibility and absorption of the intestine. Previous studies have reported that allicin can improve digestion (Yan and Kim, 2013), intestinal microbiota and increase the beneficial microbiota of animals (Zhang et al., 2020; Guillamon et al., 2021). Furthermore, studies have also shown that betaine could improve intestinal barrier function (Shakeri et al., 2019) (Alhotan et al., 2021). In this study, compared with the FM30 group, lamina propria, muscular layer, serous layer, submucous layer, villus height, villus width and crypt depth of the supplementation groups were significantly higher, indicating that limonene, allicin and betaine could promote the development of intestinal villi and improve the structure of M. salmoides digestive tract by increasing the villi height, villi width, crypt depth and muscle thickness. However, further research is needed on whether limonene, allicin, and betaine improve intestinal structure by altering the intestinal microbiota of M. salmoides.
Conclusion
In conclusion, the results showed that the optimum attractant concentration of limonene, allicin and betaine was 0.2%. Adding limonene, allicin and betaine at concentration of 0.2% to the low fish meal feed could improve growth performance (increased final body weight, weight gain rate, and specific growth rate) of M. salmoides but only in 4 weeks. In addition, in 9th week, supplementation of limonene, allicin and betaine with concentration of 0.2% in low fishmeal feed improved antioxidant capacity in liver and serum (reduced the MDA contents.), meat quality (increased pH, a*, and b* values and decreased refrigeration loss, cooking loss values in the muscle) and intestinal morphology (increased villus height, lamina propria, crypt depth, submucous layer, and serous layer) of M. salmoides. Therefore, limonene, allicin and betaine may be recommended as promising attractants in the compound feed of M. salmoides and can alleviate the current shortage of fishmeal to a certain degree.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
All the experimental procedures including the animal experimentation were approved by the animal research committees of Foshan University Animal Ethics Committee (approval number: 2020056).
Author contributions
YuhuaY: conceptualization, data curation, writing - original draft. MC and XB: conducting a research and investigation process, specifically performing the experiments, or data/evidence collection. YingyY: conceptualization, supervision, methodology, writing - review & editing. WS: formulation or evolution of overarching research goals and aims. YL and YingY: writing - review & editing. HY: writing - review & editing. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the fund of The Guangdong Provincial Key Laboratory of Animal Molecular Design and Precise Breeding (2019B030301010), The Guangdong Basic and Applied Basic Research Foundation (2019A1515110068) and Key-Area Research and Development Program of Guangdong Province (2019B110209005).
Acknowledgments
This is a short text to acknowledge the contributions of specific colleagues, institutions, or agencies that aided the efforts of the authors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aanyu M., Betancor M. B., Monroig O. (2018). Effects of dietary limonene and thymol on the growth and nutritional physiology of Nile tilapia ( Oreochromis niloticus ). Aquaculture 488, 217–226. doi: 10.1016/j.aquaculture.2018.01.036
Abdel-Daim M. M., Abdelkhalek N. K., Hassan A. M. (2015). Antagonistic activity of dietary allicin against deltamethrin-induced oxidative damage in freshwater Nile tilapia; Oreochromis niloticus. Ecotoxicol. Environ. Saf. 111, 146–152. doi: 10.1016/j.ecoenv.2014.10.019
Abdel-Tawwab M., Khalil R. H., Diab A. M., Khallaf M. A., Abdel-Razek N., Abdel-Latif H. M. R., et al. (2021). Dietary garlic and chitosan enhanced the antioxidant capacity, immunity, and modulated the transcription of HSP70 and cytokine genes in zearalenone-intoxicated European seabass. Fish Shellfish Immunol. 113, 35–41. doi: 10.1016/j.fsi.2021.03.012
Abdel-Tawwab M., Khattab Y. A. E., Ahmad M. H., Shalaby A. M. E. (2006). Compensatory growth, feed utilization, whole-body composition, and hematological changes in starved juvenile Nile Tilapia,Oreochromis niloticus (L.). J. Appl. Aquacult. 18, 17–36. doi: 10.1300/J028v18n03_02
Ahmad M. H., Abdel-Tawwab M. (2011). The use of caraway seed meal as a feed additive in fish diets: Growth performance, feed utilization, and whole-body composition of Nile tilapia, oreochromis niloticus (L.) fingerlings. Aquaculture 314, 110–114. doi: 10.1016/j.aquaculture.2011.01.030
Ajiboye O. O., Yakubu A. F., Simpa J. O., Balogun S. A. (2016). Effect of garlic-supplemented diets on growth response, survival, nutrient utilization and body composition of monosex tilapia zillii. World J. Fish Mar. Sci. 8 (2), 115–122. doi: 10.5829/idosi.wjfms.2016.8.2.103110
Alhotan R. A., Al Sulaiman A. R., Alharthi A. S., Abudabos A. M. (2021). Protective influence of betaine on intestinal health by regulating inflammation and improving barrier function in broilers under heat stress. Poult. Sci. 100, 101337. doi: 10.1016/j.psj.2021.101337
Attia Y. A., Hassan R. A., Qota E. M. (2009). Recovery from adverse effects of heat stress on slow-growing chicks in the tropics 1: Effect of ascorbic acid and different levels of betaine. Trop. Anim. Health Prod. 41, 807–818. doi: 10.1007/s11250-008-9256-9
Bacanli M., Basaran A. A., Basaran N. (2015). The antioxidant and antigenotoxic properties of citrus phenolics limonene and naringin. Food Chem. Toxicol. 81, 160–170. doi: 10.1016/j.fct.2015.04.015
Balaban R. S., Nemoto S., Finkel T. (2005). Mitochondria, oxidants, and aging. Cell 120, 483–495. doi: 10.1016/j.cell.2005.02.001
Borlinghaus J., Albrecht F., Gruhlke M. C., Nwachukwu I. D., Slusarenko A. J. (2014). Allicin: chemistry and biological properties. Molecules 19, 12591–12618. doi: 10.3390/molecules190812591
Byrd K. A., Thilsted S. H., Fiorella K. J. (2020). Fish nutrient composition: a review of global data from poorly assessed inland and marine species. Public Health Nutr. 24, 476–486. doi: 10.1017/S1368980020003857
Caimi C., Biasato I., Chemello G., Oddon S. B., Lussiana C., Malfatto V. M., et al. (2021). Dietary inclusion of a partially defatted black soldier fly (Hermetia illucens) larva meal in low fishmeal-based diets for rainbow trout (Oncorhynchus mykiss). J. Anim. Sci. Biotechnol. 12, 50. doi: 10.1186/s40104-021-00575-1
Cai L., Tong F., Tang T., Ao Z., Wei Z., Yang F., et al. (2021). Comparative evaluation of nutritional value and flavor quality of muscle in triploid and diploid common carp: Application of genetic improvement in fish quality. Aquaculture 541, 736780–736789. doi: 10.1016/j.aquaculture.2021.736780
Całyniuk B., Grochowska-Niedworok E., Walkiewicz K., Kawecka S., Popiołek E., Fatyga E. (2016). Malondialdehyde (MDA) – product of lipid peroxidation as marker of homeostasis disorders and aging. Annales Academiae Medicae Silesiensis 70, 224–228. doi: 10.18794/aams/65697
Cao F. L., Zhang X. H., Yu W. W., Zhao L. G., Wang T. (2012). Effect of feeding fermented ginkgo biloba leaves on growth performance, meat quality, and lipid metabolism in broilers. Poult. Sci. 91, 1210–1221. doi: 10.3382/ps.2011-01886
Chen B., Lu Y., Chen Y., Cheng J. (2015). The role of Nrf2 in oxidative stress-induced endothelial injuries. J. Endocrinol. 225, R83–R99. doi: 10.1530/JOE-14-0662
Chen R., Wen C., Gu Y., Wang C., Chen Y., Zhuang S., et al. (2020). Dietary betaine supplementation improves meat quality of transported broilers through altering muscle anaerobic glycolysis and antioxidant capacity. J. Sci. Food Agric. 100, 2656–2663. doi: 10.1002/jsfa.10296
Chen R., Yang M., Song Y. D., Wang R. X., Wen C., Liu Q., et al. (2021). Effect of anhydrous betaine and hydrochloride betaine on growth performance, meat quality, postmortem glycolysis, and antioxidant capacity of broilers. Poult. Sci. 101, 101687. doi: 10.1016/j.psj.2021.101687
Cicero N., Corsaro C., Salvo A., Vasi S., Giofre S. V., Ferrantelli V., et al. (2015). The metabolic profile of lemon juice by proton HR-MAS NMR: the case of the PGI interdonato lemon of Messina. Nat. Prod. Res. 29, 1894–1902. doi: 10.1080/14786419.2015.1012166
Costa M. D. S., Rocha J. E., Campina F. F., Silva A. R. P., Da Cruz R. P., Pereira R. L. S., et al. (2019). Comparative analysis of the antibacterial and drug-modulatory effect of d-limonene alone and complexed with β-cyclodextrin. Eur. J. Pharm. Sci. 128, 158–161. doi: 10.1016/j.ejps.2018.11.036
Danaceau J. P., Lucero M. T. (2000). Mixture interactions of glutamate and betaine in single squid olfactory neurons. J. Comp. Physiol. A 186, 11. doi: 10.1007/s003590050007
Dar S. A., Srivastava P. P., Varghese T., Gupta S., Krishna G., Nuzaiba P. M., et al. (2019). Expression of growth and hunger related genes and physio-biochemical responses in labeo rohita (Hamilto1822) fed with lysine and betaine. Cell Physiol. Biochem. 53, 851–864. doi: 10.33594/000000177
Datta P. K., Zhao H.-F., Feng L., Jiang W.-D., Liu Y., Jiang J., et al. (2015). Flesh shear force, cooking loss, muscle antioxidant status and relative expression of signaling molecules (Nrf2, Keap1, TOR, and CK2) and their target genes in young grass carp (Ctenopharyngodon idella) muscle fed with graded levels of choline. PloS One 10 (11), e0142915. doi: 10.1371/journal.pone.0142915
de Souza M. C., Vieira A. J., Beserra F. P., Pellizzon C. H., Nobrega R. H., Rozza A. L. (2019). Gastroprotective effect of limonene in rats: Influence on oxidative stress, inflammation and gene expression. Phytomedicine 53, 37–42. doi: 10.1016/j.phymed.2018.09.027
Djenane D. (2015). Chemical profile, antibacterial and antioxidant activity of Algerian citrus essential oils and their application in sardina pilchardus. Foods 4, 208–228. doi: 10.3390/foods4020208
Dong X., Qin W., Fu Y., Ji P., Wang J., Du X., et al. (2021). Effects of dietary betaine on cholesterol metabolism and hepatopancreas function in gibel carp (Carassius gibelio) fed with a high-fat diet. Aquacult. Nutr. 27, 1789–1797. doi: 10.1111/anu.13316
Duarte A. M., Silva F., Mendes S., Pinto F. R., Barroso S., Silva E., et al. (2022). Seasonal study of the nutritional composition of unexploited and low commercial value fish species from the Portuguese coast. Food Science & Nutrition 00, 1–12. doi: 10.1002/fsn3.2937
Dwivedi V. P., Bhattacharya D., Singh M., Bhaskar A., Kumar S., Fatima S., et al. (2019). Allicin enhances antimicrobial activity of macrophages during Mycobacterium tuberculosis infection. J Ethnopharmacol 243:111634. doi: 10.1016/j.jep.2018.12.008
Gabriel N. N., Wilhelm M. R., Habte-Tsion H.-M., Chimwamurombe P., Omoregie E. (2019). Dietary garlic (Allium sativum) crude polysaccharides supplementation on growth, haematological parameters, whole body composition and survival at low water pH challenge in African catfish (Clarias gariepinus) juveniles. Scientific African 5, 128–138. doi: 10.1016/j.sciaf.2019.e00128
Giarratana F., Muscolino D., Beninati C., Ziino G., Giuffrida A., Panebianco A. (2016). Activity of R (+) limonene on the maximum growth rate of fish spoilage organisms and related effects on shelf-life prolongation of fresh gilthead sea bream fillets. Int J Food Microbiol 237:109–113. doi: 10.1016/j.ijfoodmicro.2016.08.023
Guillamon E., Andreo-Martinez P., Mut-Salud N., Fonolla J., Banos A. (2021). Beneficial Effects of Organosulfur Compounds from Allium cepa on Gut Health: A Systematic Review. Foods 10 (8), 1680–1696. doi: 10.3390/foods10081680
Hamed H. S., Ismal S. M., Faggio C. (2021). Effect of allicin on antioxidant defense system, and immune response after carbofuran exposure in Nile tilapia, Oreochromis niloticus. Comp Biochem Physiol C Toxicol Pharmacol 240:108919–108925. doi: 10.1016/j.cbpc.2020.108919
Han Y., Sun Z., Chen W. (2019). Antimicrobial Susceptibility and Antibacterial Mechanism of Limonene against Listeria monocytogenes. Molecules 25 (1): 33–47. doi: 10.3390/molecules25010033
Hirt-Chabbert J., Skalli A., Young O., Gisbert E. (2012). Effects of feeding stimulants on the feed consumption, growth and survival at glass eel and elver stages in the European eel (Anguilla anguilla). Aquaculture Nutrition 18: 152–166. doi: 10.1111/j.1365-2095.2011.00883.x
Huang W., Yao C., Liu Y., Xu N., Yin Z., Xu W., et al. (2020). Dietary Allicin Improved the Survival and Growth of Large Yellow Croaker (Larimichthys crocea) Larvae via Promoting Intestinal Development, Alleviating Inflammation and Enhancing Appetite. Front Physiol 11, 587674–587686. doi: 10.3389/fphys.2020.587674
Huff-Lonergan E., Lonergan S. M. (2005). Mechanisms of water-holding capacity of 559 meat: The role of postmortem biochemical and structural changes. Meat Sci 71 (1):194–204. doi: 10.1016/j.meatsci.2005.04.022
Ibáñez M. D., Sanchez-Ballester N. M., Blázquez M. A. (2020). Encapsulated Limonene: A Pleasant Lemon-Like Aroma with Promising Application in the Agri-Food Industry. A Review. Molecules 25 (11):2598–2617. doi: 10.3390/molecules25112598
Ismail T., Hegazi E., Dawood M. A.O., Nassef E., Bakr A., Paray B. A., et al. (2020). Using of betaine to replace fish meal with soybean or/and corn gluten meal in nile tilapia (Oreochromis niloticus) diets: Histomorphology, growth, fatty acid, and glucose-related gene expression traits. Aquaculture Reports 17: 100376–100383. doi: 10.1016/j.aqrep.2020.100376
Kaswinarni F. (2015). Aspek gizi, mikrobiologis, dan organoleptik tempura ikan rucah dengan berbagai konsentrasi bawang putih (Allium sativum). Pros Sem Nasmasy Biodiv Indon 1 (1), 127–130. doi: 10.13057/psnmbi/m010121
Kesbiç O. S., Acar Ü., Yilmaz S., Aydin Ö. D. (2019). Effects of bergamot (Citrus bergamia) peel oil-supplemented diets on growth performance, haematology and serum biochemical parameters of Nile tilapia (Oreochromis niloticus). Fish Physiol. Biochem. 46, 103–110. doi: 10.1007/s10695-019-00700-y
Khalili Tilami S., Sampels S. (2017). Nutritional value of fish: Lipids, proteins, vitamins, and minerals. Rev. Fish. Sci. Aquacult. 26, 243–253. doi: 10.1080/23308249.2017.1399104
Lan R. X., Park J. W., Lee D. W., Kim I. H. (2017). Effects of astragalus membranaceus, codonopsis pilosula and allicin mixture on growth performance, nutrient digestibility, faecal microbial shedding, immune response and meat quality in finishing pigs. J. Anim. Physiol. Anim. Nutr. (Berl) 101, 1122–1129. doi: 10.1111/jpn.12625
Lazado C. C., Nayak S., Khozin-Goldberg I., Zilberg D. (2019). The gut mucosal barrier of zebrafish (Danio rerio) responds to the time-restricted delivery of lobosphaera incisa-enriched diets. Fish Shellfish Immunol. 89, 368–377. doi: 10.1016/j.fsi.2019.04.012
Lazzarotto V., Medale F., Larroquet L., Corraze G. (2018). Long-term dietary replacement of fishmeal and fish oil in diets for rainbow trout (Oncorhynchus mykiss): Effects on growth, whole body fatty acids and intestinal and hepatic gene expression. PloS One 13, e0190730. doi: 10.1371/journal.pone.0190730
Lee D. H., Lim S. R., Han J. J., Lee S. W., Ra C. S., Kim J. D. (2014). Effects of dietary garlic powder on growth, feed utilization and whole body composition changes in fingerling sterlet sturgeon, acipenser ruthenus. Asian-Australas J. Anim. Sci. 27, 1303–1310. doi: 10.5713/ajas.2014.14087
Le D., Nguyen P., Nguyen D., Dierckens K., Boon N., Lacoere T., et al. (2020). Gut microbiota of migrating wild rabbit fish (Siganus guttatus) larvae have low spatial and temporal variability. Microb. Ecol. 79, 539–551. doi: 10.1007/s00248-019-01436-1
Li D., Liang H., Li Y., Zhang J., Qiao L., Luo H. (2021a). Allicin alleviates lead-induced bone loss by preventing oxidative stress and osteoclastogenesis Via SIRT1/FOXO1 pathway in mice. Biol. Trace Elem. Res. 199, 237–243. doi: 10.1007/s12011-020-02136-5
Li M., Ning J., Huang H., Jiang S., Zhuo D. (2021b). Allicin protects against renal ischemia-reperfusion injury by attenuating oxidative stress and apoptosis. Int. Urol. Nephrol 54, 1761–1768. doi: 10.1007/s11255-021-03014-2
Li W., Liu B., Liu Z., Yin Y., Xu G., Han M., et al. (2021c). Effect of dietary histamine on intestinal morphology, inflammatory status, and gut microbiota in yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 117, 95–103. doi: 10.1016/j.fsi.2021.07.017
Li X., Zheng S., Ma X., Cheng K., Wu G. (2021d). Use of alternative protein sources for fishmeal replacement in the diet of largemouth bass (Micropterus salmoides). part I: effects of poultry by-product meal and soybean meal on growth, feed utilization, and health. Amino Acids 53, 33–47. doi: 10.1007/s00726-020-02920-6
Liu H., Chen C., Lv M., Liu N., Hu Y., Zhang H., et al. (2021). A chromosome-level assembly of blunt snout bream (Megalobrama amblycephala) genome reveals an expansion of olfactory receptor genes in freshwater fish. Mol. Biol. Evol. 38, 4238–4251. doi: 10.1093/molbev/msab152
Lopes J. M., de Freitas Souza C., Saccol E. M. H., Pavanato M. A., Antoniazzi A., Rovani M. T., et al. (2019). Citrus x aurantium essential oil as feed additive improved growth performance, survival, metabolic, and oxidative parameters of silver catfish (Rhamdia quelen). Aquacult. Nutr. 25, 310–318. doi: 10.1111/anu.12854
Lopes J. M., Marques N. C., Santos M., Souza C. F., Baldissera M. D., Carvalho R. C., et al. (2020). Dietary limonCitrus × latifoliafruit peel essential oil improves antioxidant capacity of tambaqui (Colossoma macropomum) juveniles. Aquacult. Res. 51, 4852–4862. doi: 10.1111/are.14771
Lunger A. N., McLean E., Gaylord T. G., Kuhn D., Craig S. R. (2007). Taurine supplementation to alternative dietary proteins used in fish meal replacement enhances growth of juvenile cobia (Rachycentron canadum). Aquaculture 271, 401–410. doi: 10.1016/j.aquaculture.2007.07.006
Maltin C., Balcerzak D., Tilley R., Delday M. (2007). Determinants of meat quality: tenderness. Proc. Nutr. Soc. 62, 337–347. doi: 10.1079/PNS2003248
Martchenko S. E., Prescott D., Martchenko A., Sweeney M. E., Philpott D. J., Brubaker P. L. (2021). Diurnal changes in the murine small intestine are disrupted by obesogenic Western diet feeding and microbial dysbiosis. Sci. Rep. 11, 20571. doi: 10.1038/s41598-021-98986-7
Milad Maniat N. G. A. U., Ahvaz I., Rajabzadeh Ghatrami E. (2014). Effect of garlic on growth performance and body composition of benni fish (Mesopotamichthys sharpeyi). Int. J. Biosci. (IJB) 5, 269–277. doi: 10.12692/ijb/5.4.269-277
Mohammad M. A. (2020). Influence of different levals of garlic allium sativum powder additions on growth promoter and biochemical composition of common carp cyprinus carpiol. Plant Arch. 20 (2), 7321–7326.
Mohanty B. P., Mahanty A., Ganguly S., Mitra T., Karunakaran D., Anandan R. (2019). Nutritional composition of food fishes and their importance in providing food and nutritional security. Food Chem. 293, 561–570. doi: 10.1016/j.foodchem.2017.11.039
Mohseni M., Saltanat N. L., Rastravan M. E., Golalipour Y. (2021). Effects of betaine supplementation in plant-protein-based diets on growth performance, haemato-immunological parameters, antioxidant status and digestive enzyme activities of juvenile Caspian trout (Salmo trutta, kessler 1877). Aquacult. Nutr. 27, 2132–2141. doi: 10.1111/anu.13348
Nafees M. S. M., Kamarudin M. S., Karim M., Hassan M. Z., de Cruz C. R. (2022). Effects of dietary starch sources on growth, nutrient utilization and liver histology of juvenile tinfoil barb (Barbonymus schwanenfeldii, bleeker 1853). Aquacult. Rep. 23, 101069–101080. doi: 10.1016/j.aqrep.2022.101069
Nagpal I., Abraham S. K. (2017). Ameliorative effects of gallic acid, quercetin and limonene on urethane-induced genotoxicity and oxidative stress in drosophila melanogaster. Toxicol. Mech. Methods 27, 286–292. doi: 10.1080/15376516.2016.1278294
Nguyen H. P., Do T. V., Tran H. D. (2020). Dietary replacement of fish meal by defatted and fermented soybean meals with taurine supplementation for pompano fish: effects on growth performance, nutrient digestibility, and biological parameters in a long-term feeding period. J. Anim. Sci. 98 (12), 1–9. doi: 10.1093/jas/skaa367
Niu K. M., Khosravi S., Kothari D., Lee W. D., Lim J. M., Lee B. J., et al. (2019). Effects of dietary multi-strain probiotics supplementation in a low fishmeal diet on growth performance, nutrient utilization, proximate composition, immune parameters, and gut microbiota of juvenile olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 93, 258–268. doi: 10.1016/j.fsi.2019.07.056
Niu K. M., Lee B. J., Kothari D., Lee W. D., Hur S. W., Lim S. G., et al. (2020). Dietary effect of low fish meal aquafeed on gut microbiota in olive flounder (Paralichthys olivaceus) at different growth stages. Microbiologyopen 9, e992. doi: 10.1002/mbo3.992
Novriadi R., Spangler E., Rhodes M., Hanson T., Allen Davis D. (2017). Effects of various levels of squid hydrolysate and squid meal supplementation with enzyme-treated soy on growth performance, body composition, serum biochemistry and histology of Florida pompano trachinotus carolinus. Aquaculture 481, 85–93. doi: 10.1016/j.aquaculture.2017.08.032
Oliveira F., Neto O. C., Santos L., Ferreira E. H. R., Rosenthal A. (2017). Effect of high pressure on fish meat quality – a review. Trends Food Sci. Technol. 66, 1–19. doi: 10.1016/j.tifs.2017.04.014
Pinedo-Gil J., Tomas-Vidal A., Jover-Cerda M., Tomas-Almenar C., Sanz-Calvo M. A., Martin-Diana A. B. (2017). Red beet and betaine as ingredients in diets of rainbow trout (Oncorhynchus mykiss): effects on growth performance, nutrient retention and flesh quality. Arch. Anim. Nutr. 71, 486–505. doi: 10.1080/1745039X.2017.1391503
Pu H., Li X., Du Q., Cui H., Xu Y. (2017). Research progress in the application of Chinese herbal medicines in aquaculture: A review. Engineering 3, 731–737. doi: 10.1016/J.ENG.2017.03.017
Ragaza J. A., Koshio S., Mamauag R. E., Ishikawa M., Yokoyama S., Villamor S. S. (2015). Dietary supplemental effects of red seaweed eucheuma denticulatumon growth performance, carcass composition and blood chemistry of juvenile Japanese flounder,Paralichthys olivaceus. Aquacult. Res. 46, 647–657. doi: 10.1111/are.12211
Rahimnejad S., Lu K., Wang L., Song K., Mai K., Davis D. A., et al. (2019). Replacement of fish meal with bacillus pumillus SE5 and pseudozyma aphidis ZR1 fermented soybean meal in diets for Japanese seabass (Lateolabrax japonicus). Fish Shellfish Immunol. 84, 987–997. doi: 10.1016/j.fsi.2018.11.009
Ravichandran C., Badgujar P. C., Gundev P., Upadhyay A. (2018). Review of toxicological assessment of d-limonene, a food and cosmetics additive. Food Chem. Toxicol. 120, 668–680. doi: 10.1016/j.fct.2018.07.052
Reyes-Camacho D., Perez J. F., Vinyeta E., Aumiller T., van der Klis J. D., Sola-Oriol D. (2021). Prenatal exposure to innately preferred d-limonene and trans-anethole does not overcome innate aversion to eucalyptol, affecting growth performance of weanling piglets. Anim. (Basel) 11 (7), 2062–2073. doi: 10.3390/ani11072062
Rufchaei R., Hoseinifar S. H., Nedaei S., Bagheri T., Ashouri G., Van Doan H. (2019). Non-specific immune responses, stress resistance and growth performance of Caspian roach (Rutilus caspicus) fed diet supplemented with earthworm (Eisenia foetida) extract. Aquaculture 511, 734275–734280. doi: 10.1016/j.aquaculture.2019.734275
Samadi L. Z., Nasim, Mousavi S. M., Zakeri M. (2016). Effect of dietary garlic extract on growth, feeding parameters, hematological indices and body composition of litopenaeus vannamei. Journal of the Persian Gulf (Marine Science) 24, 29–41.
Schmachtenberg J. n. V. s. J. s. O. D. P. O. (2015). Analysis of olfactory sensitivity in rainbow trout (Oncorhynchus mykiss) reveals their ability to detect lacticacid, pyruvic acid and four B vitamins. Fish Physiol. Biochem. 41, 879–885. doi: 10.1007/s10695-015-0054-9
Shakeri M., Cottrell J. J., Wilkinson S., Zhao W., Le H. H., McQuade R., et al. (2019). Dietary betaine improves intestinal barrier function and ameliorates the impact of heat stress in multiple vital organs as measured by Evans blue dye in broiler chickens. Anim. (Basel) 10 (1), 38–51. doi: 10.3390/ani10010038
Shamushaki V. A. J., Kasumyan A. O., Abedian A., Abtahi B. (2007). Behavioural responses of the Persian sturgeon (Acipenser persicus) juveniles to free amino acid solutions. Mar. Freshw. Behav. Physiol. 40, 219–224. doi: 10.1080/10236240701602184
Shan Y., Chen D., Hu B., Xu G., Li W., Jin Y., et al. (2021). Allicin ameliorates renal ischemia/reperfusion injury via inhibition of oxidative stress and inflammation in rats. BioMed. Pharmacother. 142, 112077. doi: 10.1016/j.biopha.2021.112077
Soltan M. A., Amal Elfeky I. M. F. (2016). Growth and feed utilization of Nile tilapia, oreochromis niloticus fed diets containing probiotic. Glob. Vet. 17 (5), 442–450. doi: 10.5829/idosi.gv.2016.442.450
Sun H., Jiang W.-D., Wu P., Liu Y., Jiang J., Yang Q.-H., et al. (2020). Betaine supplementations enhance the intestinal immunity of on-growing grass carp (Ctenopharyngodon idella): Partly related to TOR and NF-κB signaling pathways. Aquaculture 518, 734846–734863. doi: 10.1016/j.aquaculture.2019.734846
Tian J. J., Lei C. X., Ji H., Chen L. Q., Du Z. Y. (2016). Dietary arachidonic acid has a time-dependent differential impact on adipogenesis modulated via COX and LOX pathways in grass carp ctenopharyngodon idellus. Lipids 51, 1325–1338. doi: 10.1007/s11745-016-4205-2
Torrecillas S., Terova G., Makol A., Serradell A., Valdenegro V., Gini E., et al. (2019). Dietary phytogenics and galactomannan oligosaccharides in low fish meal and fish oil-based diets for European sea bass (Dicentrarchus labrax) juveniles: Effects on gut health and implications on in vivo gut bacterial translocation. PloS One 14, e0222063. doi: 10.1371/journal.pone.0222063
Truong B. Q., Buckow R., Stathopoulos C. E., Nguyen M. H. (2014). Advances in high-pressure processing of fish muscles. Food Eng. Rev. 7, 109–129. doi: 10.1007/s12393-014-9084-9
Tu L., Wu X., Wang X., Shi W. (2020). Effects of fish oil replacement by blending vegetable oils in fattening diets on nonvolatile taste substances of swimming crab (Portunus trituberculatus). J. Food Biochem. 44 (9), 13345–13355. doi: 10.1111/jfbc.13345
Volz S. N., Hausen J., Nachev M., Ottermanns R., Schiwy S., Hollert H. (2020). Short exposure to cadmium disrupts the olfactory system of zebrafish (Danio rerio) - relating altered gene expression in the olfactory organ to behavioral deficits. Aquat. Toxicol. 226, 105555. doi: 10.1016/j.aquatox.2020.105555
Wang H., Chen L., Dong C., Chen B., Li B., Li X., et al. (2021). Genome-wide identification and characterization of olfactory receptor genes in common carp (Cyprinus carpio). Gene 777, 145468. doi: 10.1016/j.gene.2021.145468
Wang J., Liang D., Yang Q., Tan B., Dong X., Chi S., et al. (2020). The effect of partial replacement of fish meal by soy protein concentrate on growth performance, immune responses, gut morphology and intestinal inflammation for juvenile hybrid grouper (Epinephelus fuscoguttatus female symbol x epinephelus lanceolatus male symbol). Fish Shellfish Immunol. 98, 619–631. doi: 10.1016/j.fsi.2019.10.025
Wang K., Lin X., Zhao W., Fan X., Yu W., Ma Z., et al. (2020). Low-temperature steaming improves eating quality of whitefish. J. Texture Stud. 51, 830–840. doi: 10.1111/jtxs.12540
Wang L., Zhang W., Gladstone S., Ng W.-K., Zhang J., Shao Q. (2019). Effects of isoenergetic diets with varying protein and lipid levels on the growth, feed utilization, metabolic enzymes activities, antioxidative status and serum biochemical parameters of black sea bream (Acanthopagrus schlegelii). Aquaculture 513, 734397–7343106. doi: 10.1016/j.aquaculture.2019.734397
Wattanakul U., Wattanakul W., Thongprajukaew K. (2019). Optimal replacement of fish meal protein by stick water in diet of sex-reversed Nile tilapia (Oreochromis niloticus). Anim. (Basel) 9 (8), 521–532. doi: 10.3390/ani9080521
Xiao Z., Yu X., Zhang S., Liang A. (2022). The expression levels and significance of GSH, MDA, SOD, and 8-OHdG in osteochondral defects of rabbit knee joints. BioMed. Res. Int. 2022, 6916179. doi: 10.1155/2022/6916179
Xu A., Shang-Guan J., Li Z., Gao Z., Huang Y. C., Chen Q. (2020). Effects of dietary Chinese herbal medicines mixture on feeding attraction activity, growth performance, nonspecific immunity and digestive enzyme activity of Japanese seabass (Lateolabrax japonicus). Aquacult. Rep. 26 (2), 390–399. doi: 10.1016/j.aqrep.2020.100304
Yan L., Kim I. H. (2013). Effects of dietary supplementation of fermented garlic powder on growth performance, apparent total tract digestibility, blood characteristics and faecal microbial concentration in weanling pigs. J. Anim. Physiol. Anim. Nutr. (Berl) 97, 457–464. doi: 10.1111/j.1439-0396.2012.01286.x
Yeşilayer N., Kaymak I. E. (2020). Effect of partial replacement of dietary fish meal by soybean meal with betaine attractant supplementation on growth performance and fatty acid profiles of juvenile rainbow trout (Oncorhynchus mykiss). Aquacult. Res. 51, 1533–1541. doi: 10.1111/are.14501
Yuan X. Y., Jiang G. Z., Wang C. C., Abasubong K. P., Zou Q., Zhou Y. Y., et al. (2019). Effects of partial replacement of fish meal by yeast hydrolysate on antioxidant capability, intestinal morphology, and inflammation-related gene expression of juvenile jian carp (Cyprinus carpio var. Jian) Fish Physiol. Biochem. 45, 187–197. doi: 10.1007/s10695-018-0552-7
Yu H., Wang X., Kong F., Song X., Tan Q. (2021). The attractive effects of amino acids and some classical substances on grass carp (Ctenopharyngodon idellus). Fish Physiol. Biochem. 47, 1489–1505. doi: 10.1007/s10695-021-00990-1
Zhang C., He X., Sheng Y., Yang C., Xu J., Zheng S., et al. (2020). Allicin-induced host-gut microbe interactions improves energy homeostasis. FASEB J. 34, 10682–10698. doi: 10.1096/fj.202001007R
Keywords: feed attractants, largemouth bass, meat quality, physiological biochemistry, intestinal health
Citation: Yue Y, Chen M, Bao X, Yu Y, Shi W, Kumkhong S, Liu Y, Yang Y and Yu H (2022) Effects of three feed attractants on the growth performance and meat quality of the largemouth bass (Micropterus salmoides). Front. Mar. Sci. 9:1029969. doi: 10.3389/fmars.2022.1029969
Received: 28 August 2022; Accepted: 20 September 2022;
Published: 06 October 2022.
Edited by:
Jianchun Shao, Fujian Agriculture and Forestry University, ChinaReviewed by:
Li Gong, Zhejiang Ocean University, ChinaHouguo Xu, Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences (CAFS), China
Copyright © 2022 Yue, Chen, Bao, Yu, Shi, Kumkhong, Liu, Yang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingying Yu, eXV5eTk5OUB5ZWFoLm5l; Hui Yu, NDY1NTQ4OTZAcXEuY29t
 Yuhua Yue
Yuhua Yue