- 1Key Laboratory of Freshwater Fish Reproduction and Development, Ministry of Education, College of Fisheries, Southwest University, Chongqing, China
- 2Chongqing Aquatic Science and Technology Innovation Alliance, Chongqing, China
- 3Guangzhou A Share Aquatic Science and Technology Co., Ltd, Guangzhou, China
A 70-day experiment was conducted to investigate the effects of mulberry leaf extract (MLE) on growth, proximate compositions, antioxidant and plasma biochemical parameters, and indices of non-specific immunity of largemouth bass (Micropterus salmoides) fed a high-starch diet. Two hundred eighty largemouth bass (initial body weight, 68.00 ± 0.19 g) were randomly fed seven diets: basal-starch diet (BSD; 8.88% starch), high-starch diet (HSD; 11.42% starch), and HSD diets supplemented with 0.05%, 0.10%, 0.20%, 0.50%, and 1.00% MLE (MLE1, MLE2, MLE3, MLE4, and MLE5, respectively). The results indicated that HSD and MLE did not significantly affect the growth performance of largemouth bass during the experimental period compared with that of the BSD, but the supplementation with more than 0.20% (MLE3, MLE4, and MLE5) MLE significantly decreased the hepatosomatic index (HSI) values, and 1.00% (MLE5) MLE significantly decreased the viscerosomatic index (VSI) values. The whole-body moisture of fish fed an HSD decreased significantly, while the whole-body lipid content increased significantly in the HSD group compared with the BSD group. Compared with HSD, MLE supplementation significantly decreased the moisture and lipid contents in the whole body. Supplementation with more than 0.20% MLE (MLE3, MLE4, and MLE5) significantly decreased the moisture content in the muscle. Supplementation with 1.00% MLE significantly decreased the content of hepatic and muscle glycogen. The malondialdehyde (MDA) content of the HSD group was significantly increased compared to that of the BSD group, whereas more than 0.10% (MLE2, MLE3, MLE4, and MLE5) MLE significantly decreased the MDA content. Additionally, the total antioxidant capacity (T-AOC), catalase (CAT), and glutathione peroxidase (GSH-Px) activities of MLE5 were significantly higher than those of the HSD group. The complement-3 (C3) content and globulin (GLB) in the plasma of the HSD group were significantly lower than those of the BSD group. Plasma C3 levels in the MLE3, MLE4, and MLE5 groups were significantly higher than those in the HSD group. In addition, glucose (GLU) levels in the MLE3, MLE4, and MLE5 groups were significantly lower than those in the HSD group. Supplementation with 0.50% (MLE4) MLE significantly increased the lysozyme (LYZ) content and decreased the activities of alanine transaminase (ALT) and aspartate transaminase (AST). Supplementation with 1.00% MLE significantly increased complement-4 (C4) and GLB contents and alkaline phosphatase (ALP) activity. Overall, these findings suggest that MLE could improve antioxidant capacity, immune function, and glycolipid metabolism, thereby alleviating the negative effects of a high-starch diet in M. salmoides.
Introduction
Dietary starch is the cheapest and major source of energy in aquafeed, and it improves the efficiency of dietary protein and lipid utilization (Enes et al., 2009; Cui et al., 2010). Dietary starch is beneficial for the physical quality of feed (Sørensen et al., 2010). However, the application of starch is limited to most aquatic species, particularly carnivorous fishes (Taj et al., 2020). The largemouth bass (Micropterus salmoides) is a typical carnivorous fish and a major aquaculture freshwater species in China. The annual production of M. salmoides was estimated to have reached 619,000 tons in 2020. Numerous studies have evaluated the M. salmoides intake of dietary starch that exceeds 10%, which significantly impairs the antioxidant capacity and reduces the non-specific immunity, thus resulting in poor fish health and lower growth performance (Zhou et al., 2014; Ma et al., 2019; Li S et al., 2020). Long-term intake of a high-starch diet (HSD) can lead to disorders in glucolipid metabolism, non-specific immunity, and antioxidant capacity, which in turn compromises growth (Lee, 2002; Li et al., 2012). The addition of some functional additives to aquafeeds is an effective approach to prevent the detrimental effects of an HSD in fish. For example, berberine supplementation in an HSD can reduce hepatic lipid accumulation in black sea bream and improve liver health (Wang et al., 2020). Nicotinamide benefits the glucose and lipid metabolism of blunt snout bream (Megalobrama amblycephala) fed a high-carbohydrate diet (Shi et al., 2020). Bile acids supplementation can significantly improve growth performance and enhance liver function and immunity in M. salmoides fed a high-starch diet (Guo et al., 2020).
Mulberry leaves have been used in Chinese medicine for liver improvement as well as antihyperlipidemic and antihyperglycemic effects (El-Beshbishy et al., 2006; Kimura et al., 2007). Some studies have shown that mulberry contains abundant alkaloids, flavonoids, polysaccharides, phenols, and other active substances (Wang et al., 2010; Ou-yang et al., 2013; Gryn Rynko et al., 2016) such as 1-deoxynojirimycin, which is an alkaloid that can suppress the postprandial increases in plasma glucose (Wang et al., 2018) and reduce the α-glucosidase activity in humans (Kimura, 2011). Bioactive substances in mulberries have been shown to modulate glucose metabolism by correcting hyperglycemia, improving antioxidant status, and increasing insulin secretion in rats (Jeszka Skowron et al., 2014). To date, only a few studies on mulberry leaf extract (MLE) in aquaculture have been reported. Dietary MLE can improve the growth performance, feed utilization, digestive capacity, and hepatic antioxidant status of the Chinese giant salamander (Li Z et al., 2020). Mulberry leaf extract can also alleviate Aeromonas hydrophila infection of African catfish (Clarias gariepinus) (Sheikhlar et al., 2014; Sheikhlar et al., 2017). However, MLE supplementation has not been reported for M. salmoides. Thus, the aim of this study was to determine the effects of MLE supplementation in a high-starch diet on growth performance, antioxidant capacity, and immune parameters in M. salmoides.
Materials and methods
Preparation of mulberry leaf extract
First, the mulberry leaves were crushed, ground, and sieved through a 50-μm mesh. Then, mulberry leaf powder was placed in a 70% ethanol solution (v/v) on an ultrasonic frequency table at 100 kHz for 20 min at room temperature (ultrasound-assisted extraction). After the ultrasonic extraction, filtration was performed using a Boucher funnel. The extracted liquid was freeze-dried to obtain the MLE that was used for diet preparation.
Experimental diets and experimental procedure
Mulberry leaf extract was supplemented to formulate seven experimental diets, including a control diet (BSD; 8.88% starch), HSD (11.42% starch), and HSD diets supplemented with 0.05%, 0.10%, 0.20%, 0.50%, and 1.00% MLE (MLE1, MLE2, MLE3, MLE4, and MLE5, respectively) (Table 1). All ingredients were ground and sieved through a 60-μm mesh before final mixing using a commercial food mixer and then mixed with oils. Then, 20% water was added to the mixture. The mixture was then pelleted (without injected steam) using a pellet machine (Valva-60; Guangzhou Weilawei Machinery Co., Ltd., Guangzhou, China), and the pellets were dried in a ventilated oven at 85°C for 30 min. After drying, diets were stored in sealed plastic bags at −20°C until use.
One thousand M. salmoides specimens were purchased from a commercial fish hatchery in Guangzhou, Guangdong Province, China. After acclimatization with commercial feed (Guangdong Junyou Feed Co., Ltd., Guangdong, China) for 14 days, the specimens were fasted for 24 h, then a total of 280 healthy M. salmoides (68.00 ± 0.19 g) were randomly distributed to 28 round plastic drums (237 L, 10 fish per drum) connected to a recirculating aquaculture system. Fish in each tank were randomly assigned to one of the seven experimental diets. Each diet was tested in four tanks. All fish were fed a certain proportion of their respective fish weights twice daily at 08:00 and 16:00, and feed consumption was recorded daily. The proportion of feeding was adjusted according to feeding conditions. During the 10-week feeding trial, the water temperature was kept at 24°C–28°C, dissolved oxygen was >6.8 mg/L, ammonia-nitrogen was<0.45 mg/L, and pH was between 7.5 and 8.0. The photoperiod was maintained at 12 h:12 h (light:dark).
Sample collection
At the end of the feeding trial, all fish were fasted for approximately 24 h and then anesthetized with MS-222 (50 mg/L water), counted, and weighed. One fish per drum was randomly sampled and stored at −20°C for the body proximate analysis. Five fish per drum were used for the collection of blood samples using heparinized syringes after measuring the body weight and length. The fish were then dissected to obtain the viscera, liver, intestinal fat, and dorsal muscles. The viscera, liver, and intestinal fat weights of the five fish were measured to calculate the viscerosomatic (VSI), hepatosomatic (HSI), and intestinal fat (IFI) index values, respectively. In addition, the apparent condition of the liver was recorded and classified as normal or abnormal. The blood samples were centrifuged at 3,000 × g for 10 min (4°C) to obtain the plasma samples and stored at −80°C until used. Liver and dorsal muscles were immediately frozen in liquid nitrogen and stored at −80°C until analysis.
Chemical analysis
The proximate composition of feed ingredients, whole fish, and muscle was analyzed using the standard methods reported by the Association of Official Analytical Chemists (AOAC) (AOAC, 1995). Moisture was evaluated by oven drying at 105°C to a constant weight. Crude protein (N × 6.25) was determined according to the Kjeldahl method using the Kjeltec system (Kjeltec 8400, FOSS, Denmark). Crude lipid was quantified via ether extraction using a Soxhlet apparatus. Ash was detected using a muffle furnace at 550°C for 12 h. Glycogen in the liver and muscle samples was determined using a commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer’s protocol.
Activity quantification of antioxidant enzymes
Liver catalase (CAT), malondialdehyde (MDA), total antioxidant capacity (T-AOC), and glutathione peroxidase (GSH-Px) levels were measured using a commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer’s protocol.
Non-specific immune indices and plasma biochemical parameters
Plasma glucose (GLU), globulin (GLB), total triglyceride (TG), total cholesterol (TC), alanine transaminase (ALT), aspartate transaminase (AST), and alkaline phosphatase (ALP) levels were determined using an automatic biochemical analyzer (DT480, Dotopmed, Beijing, China). Plasma complement-3 (C3) and complement-4 (C4) levels were measured using commercially customized ELISA kits for fish according to the manufacturer’s protocols (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China). Lysozyme (LYZ) activity was measured using a commercial kit (Shanghai Enzyme-linked Biotechnology Co., Ltd.).
Calculations and statistical analysis
where N0 is the mean of the initial number of fish in each drum, and Nt is the mean final number of fish in each drum; Wt and W0 represent the final and initial body weights (g), respectively; t is the experimental duration in days; Df is the dry diet intake of each drum, WL is the liver weight of the fish, Wv is the viscerosomatic weight of the fish, and WIF is the mean intestinal fat content.
All data are presented as the mean ± standard error and were statistically analyzed using SPSS (version 26.0) after Tukey’s test. All data were subjected to a one-way ANOVA. The level of significance was set at p< 0.05.
Results
Feed utilization and fish growth
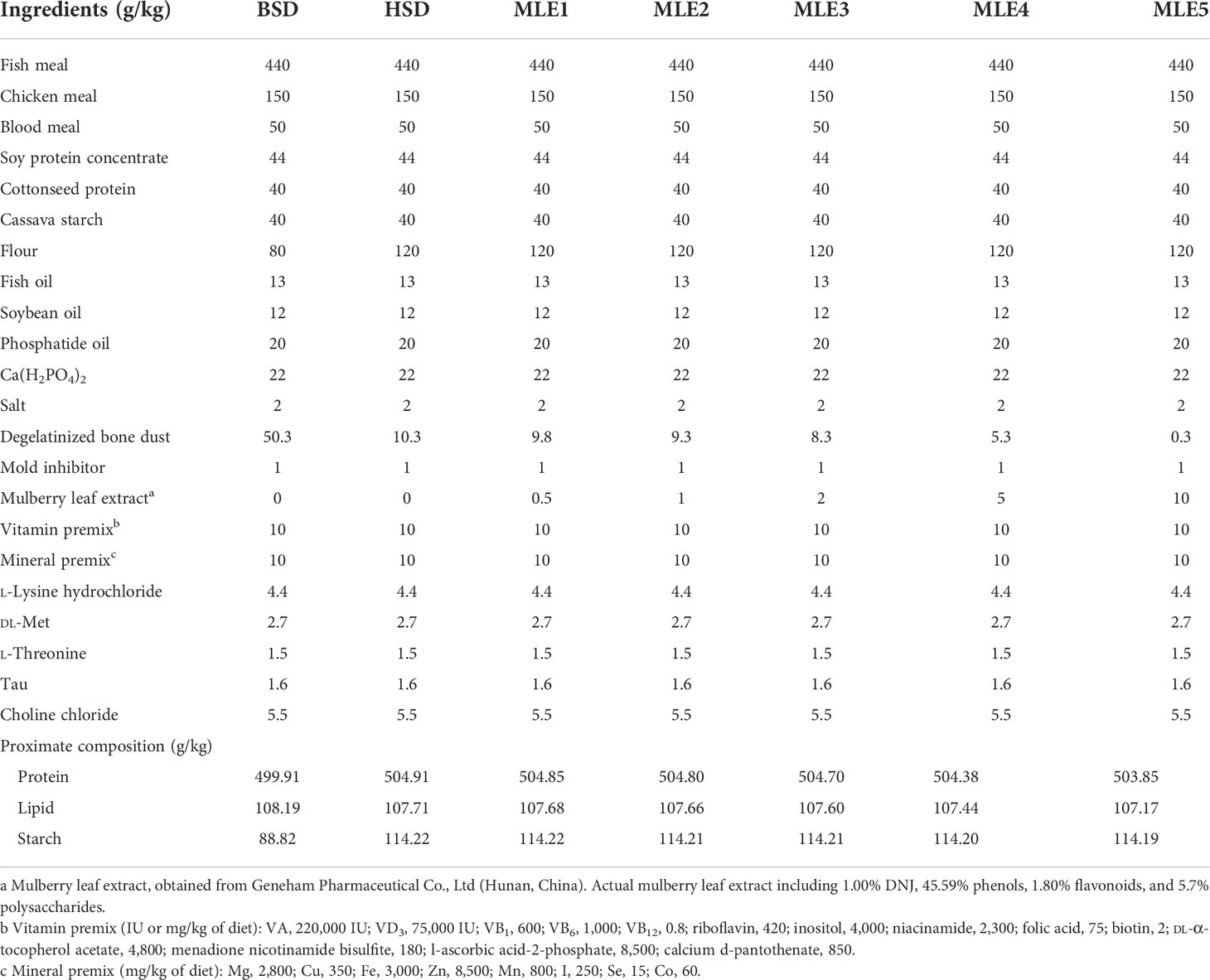
The feed utilization and growth rate of the fish fed various diets supplemented with MLE are summarized in Table 2. No significant differences in WG, SGR, FI, or IFI were observed among any of the experimental treatments (p > 0.05). However, weight gain was lower (7.12%) in the HSD group compared to the BSD group. For HSD, the MLE1, MLE2, and MLE5 groups showed an improved weight gain of 6.67%, 4.00%, and 7.59%, respectively. The MLE2–MLE5 groups showed significantly lower HSI values than HSD (p< 0.05), but no significant differences were observed between the BSD and HSD (p > 0.05). The HSI values of the MLE3, MLE4, and MLE5 groups were significantly lower than those of the BSD group (p< 0.05). The VSI values of the HSD group showed no significant differences compared to those of the BSD group (p > 0.05). The VSI value of the MLE5 group was significantly lower than that of the BSD group (p< 0.05). The percentage of the normal liver in HSD is the lowest (34.38%). The percentage of the normal liver in BSD is only 53.13%. In addition, the percentage of normal lives in MLE2, MLE3, MLE4, and MLE5 is higher than in BSD.
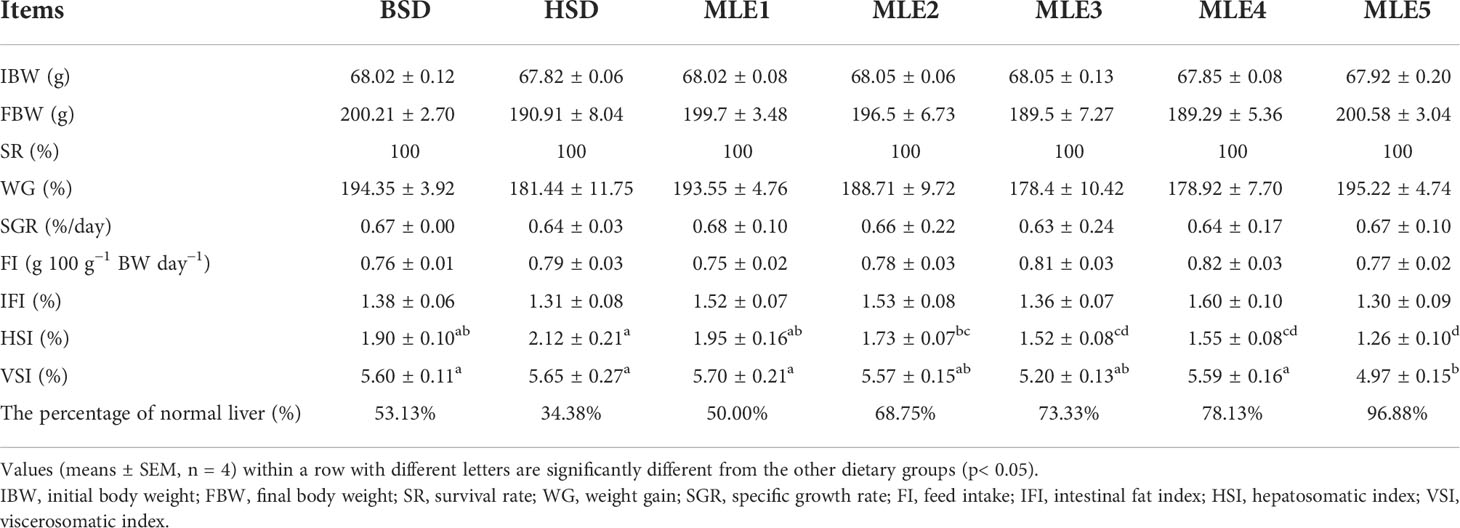
Table 2 Effects of mulberry leaf extract on growth performance and feed utilization of Micropterus salmoides fed high-starch diet diets.
Proximate compositions
The proximate compositions of the fish fed various diets supplemented with MLE are summarized in Table 3. In this study, no significant differences were found in the body protein and ash among the dietary treatments (p > 0.05). However, the whole-body moisture of the HSD group was significantly lower than that of the BSD group (p< 0.05). Body moisture decreased significantly as the MLE levels increased (p< 0.05), and the body moisture content of the MLE5 group was significantly lower than that of the BSD group (p< 0.05). Body lipid levels were significantly higher in the HSD group than that in other groups (p< 0.05). The protein, lipid, and ash contents in the muscle did not show any statistical differences among dietary treatments (p > 0.05). The muscle moisture of the BSD and HSD groups was significantly higher than that of the MLE3, MLE4, and MLE5 groups (p< 0.05). There were no significant differences in hepatic and muscle glycogen contents between the HSD and BSD groups (p > 0.05). However, MLE decreased the content of hepatic and muscle glycogen, and a significant decrease was observed in the MLE5 group (p< 0.05).
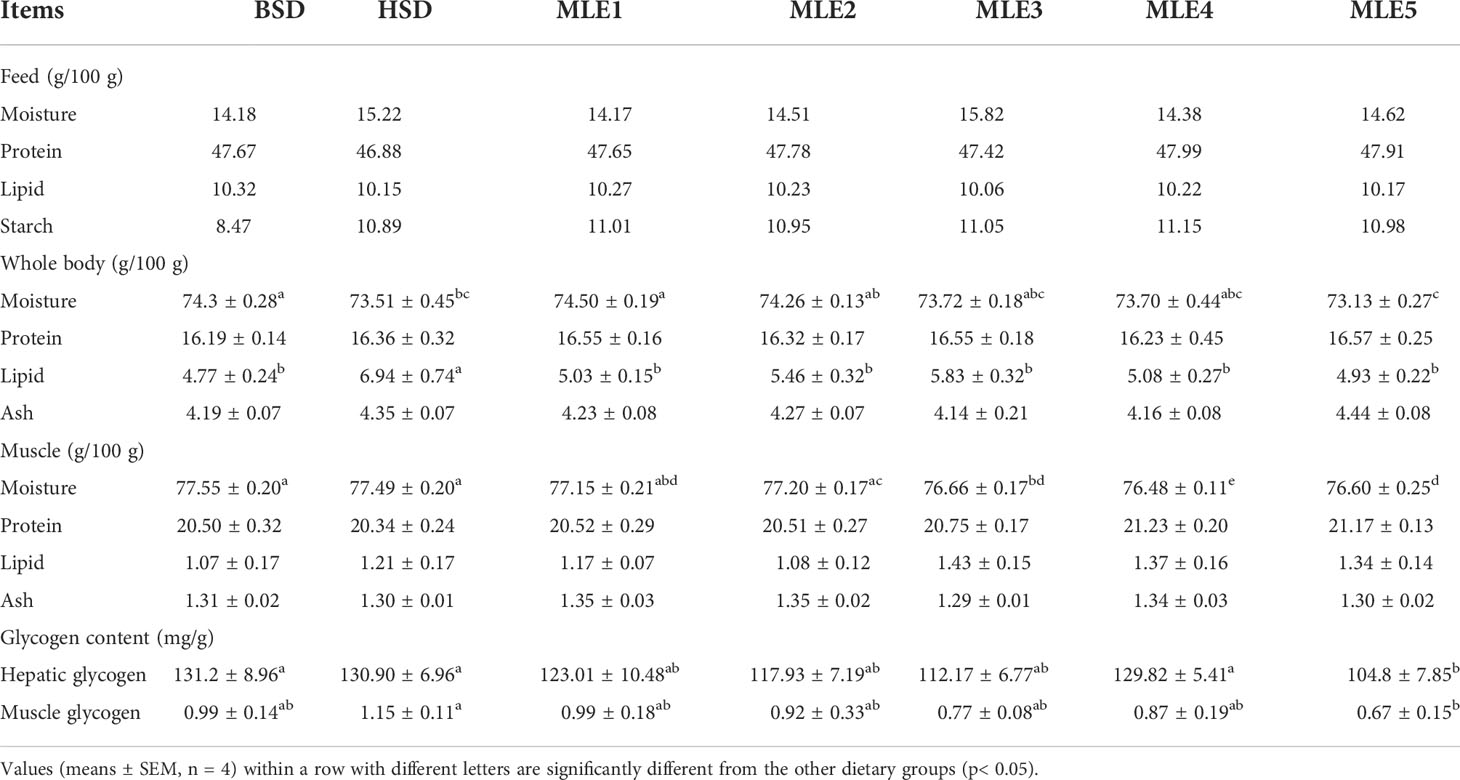
Table 3 Effects of mulberry leaf extract on experimental diets and proximate compositions of Micropterus salmoides fed high-starch diet diets.
Liver antioxidant indices
The activity quantification of antioxidant enzymes in the fish fed various diets supplemented with MLE is summarized in Figure 1. The hepatic MDA content in M. salmoides in the HSD group was significantly higher than that in the BSD group (p< 0.05), and the hepatic MDA content in M. salmoides in the MLE2, MLE3, MLE4, and MLE5 groups was significantly lower than that in the HSD group (p< 0.05); however, no significant difference was found in the MDA content among the MLE2, MLE3, MLE4, MLE5, and BSD groups (p > 0.05). The T-AOC, CAT, and GSH-PX of the HSD group showed no significant differences compared to those of the BSD group (p > 0.05). MLE improved the activities of T-AOC, CAT, and GSH-PX. In addition, fish from the MLE5 group had significantly higher T-AOC, CAT, and GSH-PX activities than those in the HSD group (p< 0.05).
Figure 1 Effects of mulberry leaf extract on antioxidant enzymes of Micropterus salmoides fed high-starch diet diets. Values in each column with different superscripts have significant differences (p< 0.05).
Non-specific immune indices and plasma biochemical parameters
The non-specific immune indices and plasma biochemical parameters of the fish fed various diets supplemented with MLE are summarized in Figure 2. The lowest plasma LYZ, C3, C4, and GLB contents and the highest plasma ALT, AST, GLU, TG, and TC contents were observed in the HSD group, and C3 and GLB were significantly different from those of the BSD group (p< 0.05). Compared with the HSD group, adding 0.10%–1.0% (MLE2, MLE3, MLE4, and MLE5) MLE significantly decreased the activity of ALT (p< 0.05). Adding more than 0.20% (MLE3, MLE4, and MLE5) MLE significantly decreased the GLU content and increased the C3 content (p< 0.05). Adding more than 0.50% (MLE4 and MLE5) MLE significantly decreased AST activity and TC content and significantly increased LYZ content (p< 0.05). The addition of 1.00% (MLE5) MLE significantly increased the C4 and GLB contents and ALP activity (p< 0.05).
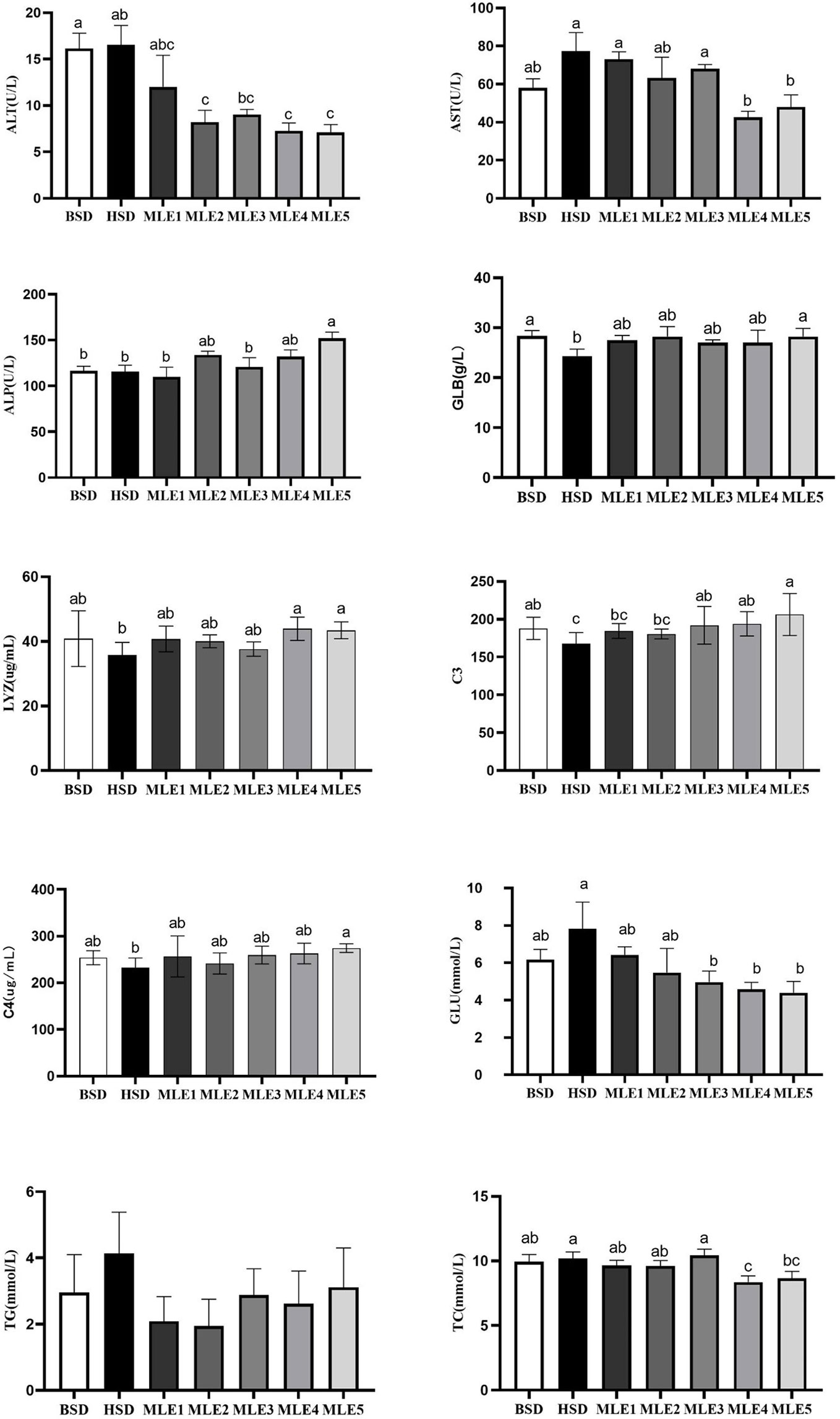
Figure 2 Effects of mulberry leaf extract on non-specific immune indices and plasma biochemical parameters of Micropterus salmoides fed high-starch diet diets. Values in each column with different superscripts have significant differences (p< 0.05).
Discussion
Carbohydrates are an important energy source for vertebrates, and starch is one of the most common carbohydrate sources in fish feed. Scientific evidence has recently indicated that the source and level of starch play a decisive role in fish growth (Xia et al., 2018; Li et al., 2019; Zhao et al., 2020). M. salmoides is a carnivorous fish, and the level of starch in its feed requires serious consideration. Recent studies have shown that the growth performance of M. salmoides is impaired when its dietary starch content is >10% (Lin et al., 2018; Ma et al., 2019; Zhang et al., 2020a; b). This study showed that M. salmoides fed with 8.88% and 11.42% dietary starch had no significant influence, indicating that this range of starch (8.88%–11.42%) content can be tolerated by M. salmoides in the short-term without a visible reduction in production performance.
The viscera are crucial for fish metabolism. The VSI and HIS values of the HSD group showed no significant differences compared with those of the BSD group after the 70-day feeding trial, which was similar to a previous report (Zhang et al., 2020b). Zhao et al. (2020) demonstrated that VSI values are not significantly affected by feeding juvenile golden pompano (Trachinotus ovatus) different levels of corn starch, and HSI values were significantly higher when the corn starch level was >20%. Zhou et al. (2015) also reported that the HSI values of fish fed a 22.4% carbohydrate diet were significantly higher than those of fish fed a 0%–11.2% carbohydrate diet. Modern pharmacology shows that MLE contains polysaccharides, flavonoids, alkaloids, and other active ingredients (Sánchez-Salcedo et al., 2015; Yuan et al., 2015), and previous studies have shown that MLE protects the liver by regulating glucose and lipid metabolism (Chang et al., 2013; Ou et al., 2013; Sheikhlar et al., 2017). This study showed that M. salmoides fed a high-starch diet supplemented with > 0.1% MLE had lower HSI values than fish fed a high-starch diet alone, which indicates that MLE may be conducive to liver health. Therefore, MLE could repair and improve liver function in fish fed a high-starch diet.
Excessive dietary starch levels can lead to excessive glycogen and lipid deposition (Lin et al., 2018; Ma et al., 2019; Zhang et al., 2020b). After the 70-day feeding trial, whole-body lipid levels were significantly higher in the HSD group than in the BSD group. This is in agreement with previous studies on the golden pompano (Zhou et al., 2015), blunt snout bream (Xia et al., 2018), and grass carp (Ctenopharyngodon idella) (Tian et al., 2011). However, hepatic and muscle glycogen contents in this study were not correlated with dietary starch content. It has been reported that hepatic and muscle glycogen contents were significantly affected when dietary starch content was >15% in M. salmoides (Ma et al., 2019). The possible reason may relate to the starch levels in the diet. The results showed that M. salmoides in the MLE5 group had significant decreases in hepatic and muscle glycogen contents. Dietary MLE significantly decreased the whole-body lipid content. Jeszka Skowron et al. (2014) found that bioactive substances in mulberry leaves regulate glucose metabolism by correcting hyperglycemia and increasing insulin secretion in the streptozotocin-induced non-obese diabetic rat model. Dietary MLE has also been reported to inhibit lipid accumulation by reducing lipogenesis and promoting hepatic lipid clearance (Chang et al., 2013). Moreover, Hou et al. (2019) found that mulberry leaf meal reduced liver lipid content by suppressing the isolation and proliferation of adipocytes. In this experiment, dietary MLE lowered the contents of GLU, TG, and TC of M. salmoides fed a high-starch diet. Therefore, high starch diet supplemented with MLE decreased lipid and glycogen deposition by enhancing glucose and lipid metabolism. In addition, the whole-body moisture and muscle were significantly decreased in the MLE groups, which was consistent with results previously obtained in the Chinese giant salamander (Andrias davidianus) (Li Z et al., 2020).
Antioxidant enzyme systems are an important defense mechanism of organisms, which can reduce peroxide in the body into less harmful substances (Bogdan et al., 2000). A previous study indicated that hyperglycemia after consuming a high-starch diet is associated with oxidative stress (Rains and Jain, 2011). MDA is the final product of lipid peroxidation and reflects the degree of lipid peroxidation (Koruk et al., 2004). SOD, GSH-Px, and CAT are three important members of the antioxidant system that work against the formation of reactive oxygen species (ROS), protect cell membranes and intracellular nucleic acids, and reflect the growth and development of the organism, changes in the metabolic state in vivo, and environmental stress (Zimmermann et al., 1973; Holmblad and Söderhäll, 1999). Additionally, T-AOC is the main index used to determine the total antioxidant level of an organism, which reflects a compensatory mechanism of the organism under the stimulation of oxidative stress (Decker et al., 2000). This study suggests that significantly higher MDA levels might be induced in M. salmoides fed a high-starch diet but with no significant effect on the activities of SOD, GSH-Px, CAT, and T-AOC. Some recent studies have indicated that oxidative stress is not directly related to dietary starch (Wang et al., 2014) and that appropriate starch levels can improve antioxidant capacity (Wu et al., 2015). However, excess starch can induce strong oxidative stress in fish (Zhou et al., 2013; Zhao et al., 2020). A previous study indicated that 5% and 10% starch diets had no negative effects on M. salmoides (Lin et al., 2018). Zhang et al., (2020a) also found that suitable dietary starch levels (0–100 g/kg) had no negative effect on antioxidant capacity. In this study, the higher MDA content in the HSD group may indicate fatty liver injury. Mulberry leaf extract contains enriched polyphenolics, flavone, and 1-deoxynojirimycin, which has been shown to scavenge free radicals (Radojković et al., 2012) and enhance antioxidant enzyme activities in mammals (Bae et al., 2013; Lee et al., 2016). In addition, the antioxidative effects of MLE have been demonstrated in African catfishes (Sheikhlar et al., 2017). In this study, significantly higher antioxidant capacities of M. salmoides were observed after 1.0% MLE was added to the high-starch diet. Thus, MLE improved liver function by suppressing oxidative stress.
Hematological parameters are vital physiological indicators that reflect the metabolic and physiological states of the body; therefore, they are beneficial for disease diagnosis (Ahmdifar et al., 2011). Lysozyme activity is an important immune parameter that protects against microorganisms (Jiang et al., 2009). Complement is also an important component of non-specific immunity, which mediates inflammatory and immune responses (Han and Ulevitch, 2005; Boshra et al., 2006). The inhibition of lysozyme and complement activity has been confirmed in some fish fed a high-starch diet (Wu et al., 2015; Xia et al., 2018; Li S et al., 2020). In this study, the lysozyme and C3 content in the HSD group were lower than those in the BSD group, and C4 was significantly lower. These results suggested that starch levels could impact the immune system. Several studies have suggested that MLE has anti-bacterial and anti-inflammatory effects (Wang et al., 2009; Forato Anhê et al., 2014). In addition, MLE can improve hepatic injury and inflammation induced by a high-sugar and high-fat diet through various pathways (Ou et al., 2013; Park et al., 2013). In the experiment, MLE supplementation in a high-starch diet enhanced the non-specific immunity of M. salmoides. AST and ALT are two crucial aminotransferases that mainly exist in cells and are rarely present in the plasma. The activities of AST and ALT were higher in cardiomyocytes and hepatocytes than those in other organs. Therefore, the activities of AST and ALT in plasma can reflect the health status of the liver and heart (Cho et al., 1994; Liu et al., 2010). ALP is a hallmark enzyme of lysosomal integrity, is involved in the transfer and metabolism of phosphoric groups in organisms, and plays a crucial role in immunity and growth (Oner et al., 2008; Yan et al., 2014). In this study, no differences were observed in plasma levels of ALT, AST, and ALP between the HSD and BSD groups. However, there was approximately 46.87% observable liver damage in the BSD group during sample collection. Thus, M. salmoides could not adapt to an 8.88% starch-formulated diet. Alternatively, formulated diets of M. salmoides must be optimized (Huang et al., 2017; Ma et al., 2019; Ma et al., 2020). In this study, the plasma ALT and AST activities in the MLE5 group were significantly lower than those in the HSD group, indicating that 1.0% MLE can alleviate liver damage of M. salmoides fed a high-starch diet. GLB is an important part of non-specific immunity. The plasma GLB level in the MLE5 group was significantly higher than that in the HSD group. This result indicated that mulberry leaf extract improved the non-specific immunity of M. salmoides fed a high-starch diet. The dietary starch level markedly affects the metabolism of carbohydrates and lipids. In this experiment, increased levels of GLU, TG, and TC were observed in the HSD group compared to the BSD group. Similar results have been observed in the blunt snout bream, golden pompano, and M. salmoides (Xia et al., 2018; Zhang et al., 2020a; Zhao et al., 2020). Mulberry leaf extract contains DNJ as a competitive inhibitor, which improves control and decreases plasma glucose content in mammals (Shang et al., 2012; Wang et al., 2018). In addition, mulberry leaf extract inhibits lipid accumulation by reducing lipogenesis and promoting hepatic lipid clearance (Chang et al., 2013). In this experiment, dietary MLE lowered the contents of GLU, TG, and TC in this study. These results suggest that MLE can improve the transport of glucose and lipids in the liver of M. salmoides fed a high-starch diet.
Conclusion
In conclusion, no significant difference in growth in the 8.88% starch level was observed, whereas it significantly affected proximate compositions, liver antioxidant activity, and non-specific immunity in M. salmoides. Dietary supplementation with 1.0% MLE and 11.42% starch decreased the moisture, lipids, and glycogen content in the body. Moreover, MLE improved immune and liver function. This study shows that MLE has positive effects on the health of M. salmoides when combined with an 11.42% starch diet. Mulberry leaf extract may also play a protective role by regulating glycolipid metabolism. The underlying mechanisms of MLE on glycolipid metabolism in M. salmoides warrant further exploration.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by The Animal Ethics Committee of Southwest University.
Author contributions
JT: methodology and design. LH: methodology and investigation. HJ: design. LZ, LY, JH, ZX and KZ: carried out the chemical analysis. SW and HM: carried out the data analysis and statistical analysis. YH and YHJ: supervision and project administration. All authors contributed to the article and approved the submitted version.
Funding
The authors declare that this study received funding from Guangzhou A Share Aquatic Science and Technology Co. LTD.. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of interest
Author HJ is employed by Guangzhou A Share Aquatic Science and Technology Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmdifar E., Akrami R., Ghelichi A., Mohammadi Zarejabad A. (2011). Effects of different dietary prebiotic inulin levels on blood serum enzymes, hematologic, and biochemical parameters of great sturgeon (Huso huso) juveniles. Comp. Clin. Pathol. 20, 447–451. doi: 10.1007/s00580-010-1017-2
AOAC (1995). Official methods of analysis of AOAC 16th edn. Assoc.Off. anal. chem (Washington, DC, USA: Association of Official Analytical Chemists).
Bae W. J., Ha U. S., Bae J. H., Choi Y. S., Kim S. J., Cho H. J., et al. (2013). 445 protective effect of cyanidin-3-O-beta-D-glucopyranoside fraction from mulberry fruit pigment against oidative damage in streptozotocin-induced diabetic rat bladder. Eur. Urol. Suppl. 28th Annu. Congress Eur. Assoc. Urol. Abstracts 12, e445. doi: 10.1016/S1569-9056(13)60929-X
Bogdan C., Röllinghoff M., Diefenbach A. (2000). Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr. Opin. Immunol. 12, 64–76. doi: 10.1016/S0952-7915(99)00052-7
Boshra H., Li J., Sunyer J. O. (2006). Recent advances on the complement system of teleost fish. Fish Shellfish Immunol. Rev. Fish Immunol. 20, 239–262. doi: 10.1016/j.fsi.2005.04.004
Chang J.-J., Hsu M.-J., Huang H.-P., Dai-Jung C., Chang Y.-C., Wang C.-J. (2013). Mulberry anthocyanins inhibit oleic acid induced lipid accumulation by reduction of lipogenesis and promotion of hepatic lipid clearance. J. Agric. Food Chem. 61 (25), 6069–6076. doi: 10.1021/jf401171k
Cho Y. J., Kim Y. Y., Lee N. G., Choi Y. J. (1994). Basic studies on developing equipment for waterless transportation of live fish. Bull. Kor Fish Soc. 27, 501–508.
Cui X.-J., Zhou Q.-C., Liang H.-O., Yang J., Zhao L.-M. (2010). Effects of dietary carbohydrate sources on the growth performance and hepatic carbohydrate metabolic enzyme activities of juvenile cobia (Rachycentron canadum linnaeus.). Aquac. Res. 42, 99–107. doi: 10.1111/j.1365-2109.2010.02574.x
Decker E., Livisay S. A., Zhou S. (2000). Mechanisms of endogenous skeletal muscle antioxidants: Chemical and physical aspects. Antioxid. Muscle Foods Nutr. Strateg. Improve Qual. 2000, 25–60.
El-Beshbishy H. A., Singab A. N. B., Sinkkonen J., Pihlaja K. (2006). Hypolipidemic and antioxidant effects of Morus alba L. (Egyptian mulberry) root bark fractions supplementation in cholesterol-fed rats. Life Sci. 78, 2724–2733. doi: 10.1016/j.lfs.2005.10.010
Enes P., Panserat S., Kaushik S., Oliva Teles A. (2009). Nutritional regulation of hepatic glucose metabolism in fish. Fish Physiol. Biochem. 35, 519–539. doi: 10.1007/s10695-008-9259-5
Forato Anhê F., Roy D., Pilon G., Dudonné S., Matamoros S., Varin T., et al. (2014). A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased akkermansia spp. population in the gut microbiota of mice. Gut 64 (6), 872–883. doi: 10.1136/gutjnl-2014-307142
Gryn Rynko A., Bazylak G., Olszewska-Slonina D. (2016). New potential phytotherapeutics obtained from white mulberry (Morus alba l.) leaves. Biomed. Pharmacother. 84, 628–636. doi: 10.1016/j.biopha.2016.09.081
Guo J. L., Kuang W. M., Zhong Y. F., Chen Y. J., Lin S. M. (2020). Effects of supplemental dietary bile acids on growth, liver function and immunity of juvenile largemouth bass(Micropterus salmoides)fed high-starch diet - ScienceDirect. Fish Shellfish Immunol. 97, 602–607. doi: 10.1016/j.fsi.2019.12.087
Han J., Ulevitch R. J. (2005). Limiting inflammatory responses during activation of innate immunity. Nat. Immunol. 6, 1198–1205. doi: 10.1038/ni1274
Holmblad T., Söderhäll K. (1999). Cell adhesion molecules and antioxidative enzymes in a crustacean, possible role in immunity. Aquaculture 172, 111–123. doi: 10.1016/S0044-8486(98)00446-3
Hou Q., Thidza I., Miao L., Dong Z., Pan W., Zhu W., et al. (2019). Lipid metabolism responses of common carp ( cyprinus carpio l . ) to mulberry leaf meal in diet. Aquac. Res. 51 (2), 719–727. doi: 10.1111/are.14422
Huang D., Wu Y., Lin Y., Chen J., Karrow N., Ren X., et al. (2017). Dietary protein and lipid requirements for juvenile largemouth bass, micropterus salmoides. J. World Aquac. Soc 48 (5), 782–790. doi: 10.1111/jwas.12417
Jeszka Skowron M., Flaczyk E., Jeszka J., Krejpcio Z., Król E., Buchowski M. S. (2014). Mulberry leaf extract intake reduces hyperglycaemia in streptozotocin (STZ)-induced diabetic rats fed high-fat diet. J. Funct. Foods 8, 9–17. doi: 10.1016/j.jff.2014.02.018
Jiang W.-D., Feng L., Liu Y., Jiang J., Zhou X.-Q. (2009). Myo-inositol prevents oxidative damage, inhibits oxygen radical generation and increases antioxidant enzyme activities of juvenile jian carp (Cyprinus carpio var. Jian). Aquac. Res. 40, 1770–1776. doi: 10.1111/j.1365-2109.2009.02283.x
Kimura T. (2011). “Development of mulberry leaf extract for suppressing postprandial blood glucose elevation,” in Hypoglycemia - causes and occurrences. Ed. Rigobelo E. (Rijeka: InTech). doi: 10.5772/24893
Kimura T., Nakagawa K., Kubota H., Kojima Y., Goto Y., Yamagishi K., et al. (2007). Food-grade mulberry powder enriched with 1-deoxynojirimycin suppresses the elevation of postprandial blood glucose in humans. J. Agric. Food Chem. 55, 5869–5874. doi: 10.1021/jf062680g
Koruk M., Taysi S., Savas M. C., Yilmaz O., Akcay F., Karakok M. (2004). Oxidative stress and enzymatic antioxidant status in patients with nonalcoholic steatohepatitis. Ann. Clin. Lab. Sci. 34, 57–62.
Lee S. M. (2002). Apparent digestibility coefficients of various feed ingredients for juvenile and grower rockfish (Sebastes schlegeli). Aquaculture 207(1), 79–95.
Lee Y. R., Hsu J. D., Lin W. L., Kao S. H., Wang C. J. (2016). Upregulation of caveolin-1 by mulberry leaf extract and its major components chlorogenic acid derivatives attenuates alcoholic steatohepatitis via inhibition of oxidative stress. Food Funct. 8 (1), 397–405. doi: 10.1039/C6FO01539E
Li Z., Chen X., Chen Y., Li W., Feng Q., Zhang H., et al. (2020). Effects of dietary mulberry leaf extract on the growth, gastrointestinal, hepatic functions of Chinese giant salamander (Andrias davidianus). Aquac. Res. 51, 2613–2623. doi: 10.1111/are.14639
Li S., Li Z., Zhang J., Sang C., Chen N. (2019). The impacts of dietary carbohydrate levels on growth performance, feed utilization, glycogen accumulation and hepatic glucose metabolism in hybrid grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂). Aquaculture 512, 734351. doi: 10.1016/j.aquaculture.2019.734351
Lin S. M., Shi C. M., Mu M. M., Chen Y. J., Luo L. (2018). Effect of high dietary starch levels on growth, hepatic glucose metabolism, oxidative status and immune response of juvenile largemouth bass, micropterus salmoides. Fish Shellfish Immunol. 78, 121–126. doi: 10.1016/j.fsi.2018.04.046
Liu B., Xie J., Ge X., Xu P., Wang A., He Y., et al. (2010). Effects of anthraquinone extract from rheum officinale bail on the growth performance and physiological responses of macrobrachium rosenbergii under high temperature stress. Fish Shellfish Immunol. 29, 49–57. doi: 10.1016/j.fsi.2010.02.018
Li S., Wang A., Li Z., Zhang J., Sang C., Chen N. (2020). Antioxidant defenses and non-specific immunity at enzymatic and transcriptional levels in response to dietary carbohydrate in a typical carnivorous fish, hybrid grouper (Epinephelus fuscoguttatus ♀ × e. lanceolatus ♂). Fish Shellfish Immunol. 100, 109–116. doi: 10.1016/j.fsi.2020.03.015
Li X. T., Yong J. L., Hui J. Y., Gui Y. L., Jin N. (2012). Effects of different dietary wheat starch levels on growth, feed efficiency and digestibility in grass carp (Ctenopharyngodon idella). Aquac. Int. 20, 283–293. doi: 10.1007/s10499-011-9456-6
Ma D., Fan J., Huaping Z., Su H., Jiang P. (2020). Histologic examination and transcriptome analysis uncovered liver damage in largemouth bass from formulated diets. Aquaculture 526, 735329. doi: 10.1016/j.aquaculture.2020.735329
Ma H. J., Mou M. M., Pu D. C., Lin S. M., Chen Y. J., Luo L. (2019). Effect of dietary starch level on growth, metabolism enzyme and oxidative status of juvenile largemouth bass, micropterus salmoides. Aquaculture 498, 482–487. doi: 10.1016/j.aquaculture.2018.07.039
Oner M., Atli G., Canli M. (2008). Changes in serum biochemical parameters of fresh water fish oreochromis niloticus following prolonged metal (Ag cd cr Cu zn) exposures. Environ. Toxicol. Chem. SETAC 27, 360–366. doi: 10.1897/07-281R.1
Ou T. T., Kuo C. Y., Chyau C. C., Lee H. J., Peng J. S., Wang C. J. (2013). Improvement of lipopolysaccharide-induced hepatic injuries and inflammation with mulberry extracts. J. Sci. Food Agric. 93 (8), 1880–1886. doi: 10.1002/jsfa.5984
Ou-yang Z., Cao X., Wei Y., Zhang W. W. Q., Zhao M., Duan J. (2013). Pharmacokinetic study of rutin and quercetin in rats after oral administration of total flavones of mulberry leaf extract. Rev. Bras. Farmacogn. 23, 776–782. doi: 10.1590/S0102-695X2013000500009
Park E., Lee S. M., Lee J.e., Kim J. H. (2013). Anti-inflammatory activity of mulberry leaf extract through inhibition of NF-κB. J. Funct. Foods 5, 178–186. doi: 10.1016/j.jff.2012.10.002
Radojković M., Zekovic Z., Vidovic S., Kocar D., Maskovic P. (2012). Free radical scavenging activity, total phenolic and flavonoid contents of mulberry (Morus spp. L. Moraceae) extracts. Hem. Ind. 66, 547–552. doi: 10.2298/HEMIND111111002R
Rains J. L., Jain S. K. (2011). Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 50, 567–575. doi: 10.1016/j.freeradbiomed.2010.12.006
Sørensen M., Nguyen G., Storebakken T., Øverland M. (2010). Starch source, screw configuration and injection of steam into the barrel affect the physical quality of extruded fish feed. Aquac. Res. 41, 419–432. doi: 10.1111/j.1365-2109.2009.02346.x
Sánchez-Salcedo E., Mena P., Cristina G.-V., Nicolás J. J., Hernandez F. (2015). Phytochemical evaluation of white (Morus alba l.) and black (Morus nigra l.) mulberry fruits, a starting point for the assessment of their beneficial properties. J. Funct. Foods 12, 399–408. doi: 10.1016/j.jff.2014.12.010
Shang Q., Xiang J. F., Tang Y. L. (2012). Screening α-glucosidase inhibitors from mulberry extracts via DOSY and relaxation-edited NNR. Talanta 97, 362–367. doi: 10.1016/j.talanta.2012.04.046
Sheikhlar A., Alimon A. R., Daud H., Saad C. R., Webster C. D., Meng G. Y., et al. (2014). White mulberry ( morus alba ) foliage methanolic extract can alleviate aeromonas hydrophila infection in African catfish ( clarias gariepinus ). Sci. World J. 48 (8), 4409–4419. doi: 10.1155/2014/592709
Sheikhlar A., Goh Y., Alimon R., Ebrahimi M. (2017). Antioxidative effects of mulberry foliage extract in African catfish diet. Aquac. Res. 48 (8), 4409–4419. doi: 10.1111/are.13266
Shi H., Li X., Xu C., Zhang D., Zhang L., Xia S., et al. (2020). Nicotinamide improves the growth performance, intermediary metabolism and glucose homeostasis of blunt snout bream megalobrama amblycephala fed high-carbohydrate diets. Aquac. Nutr. 26, 1311–1328. doi: 10.1111/anu.13088
Taj S., Irm M., Jin M., Timothée A. H. J., Xin C., Zhou Q. (2020). Carbohydrate utilization in black seabream: Effects of the carbohydrate sources on growth, insulin signalling pathway and hepatic glucose metabolism. Aquac. Nutr 26 (6), 2102–2114. doi: 10.1111/anu.13150
Tian L., Liu Y., Yang H., Liang G., Niu J. (2011). Effects of different dietary wheat starch levels on growth, feed efficiency and digestibility in grass carp ( ctenopharyngodon idella ). Aquac. Int. 20 (2), 283–293. doi: 10.1007/s10499-011-9456-6
Wang F., Li J., Jiang Y. (2009). Polysaccharides from mulberry leaf in relation to their antioxidant activity and antibacterial ability. J. Food Process Eng. 33, 39–50. doi: 10.1111/j.1745-4530.2008.00258.x
Wang F., Li J., Jiang Y. (2010). Polysaccharides from mulberry leaf in relation to their antioxidant activity and antibacterial ability. J. Food Process Eng. 33, 39–50. doi: 10.1111/j.1745-4530.2008.00258.x
Wang R., Li Y., Mu W., Li Z., Sun J., Wang B., et al. (2018). Mulberry leaf extract reduces the glycemic indexes of four common dietary carbohydrates. Medicine (Baltimore) 97 (34), e11996. doi: 10.1097/MD.0000000000011996
Wang L. N., Liu W. B., Lu K. L., Xu W. N., Cai D. S., Zhang C.-N., et al. (2014). Effects of dietary carbohydrate/lipid ratios on non-specific immune responses, oxidative status and liver histology of juvenile yellow catfish pelteobagrus fulvidraco. Aquaculture 426–427, 41–48. doi: 10.1016/j.aquaculture.2014.01.022
Wang L., Sun Y., Xu B., Sagada G., Chen K., Xiao J., et al. (2020). Effects of berberine supplementation in high starch diet on growth performance, antioxidative status, immune parameters and ammonia stress response of fingerling black sea bream (Acanthopagrus schlegelii). Aquaculture 527, 735473. doi: 10.1016/j.aquaculture.2020.735473
Wu C., Ye J., Gao J., Chen L., Lu Z. (2015). The effects of dietary carbohydrate on the growth, antioxidant capacities, innate immune responses and pathogen resistance of juvenile black carp mylopharyngodon piceus. Fish Shellfish Immunol. 49, 132–142. doi: 10.1016/j.fsi.2015.12.030
Xia S., Li X., Abasubong K. P., Xu C., Shi H., Liu W., et al. (2018). Effects of dietary glucose and starch levels on the growth, apparent digestibility, and skin-associated mucosal non-specific immune parameters in juvenile blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol. 79, 193–201. doi: 10.1016/j.fsi.2018.05.001
Yan F., Tian X., Dong S., Fang Z., Yang G. (2014). Growth performance, immune response, and disease resistance against vibrio splendidus infection in juvenile sea cucumber apostichopus japonicus fed a supplementary diet of the potential probiotic paracoccus marcusii DB11. Aquaculture 420–421, 105–111. doi: 10.1016/j.aquaculture.2013.10.045
Yuan Q., Xie Y., Wang W., Yan Y., Ye H., Jabbar D. (2015). Extraction optimization, characterization and antioxidant activity in vitro of polysaccharides from mulberry (Morus alba l.) leaves. Carbohydr. Polym. 128, 52–62. doi: 10.1016/j.carbpol.2015.04.028
Zhang Y.-M., Guo T., Liu Z., Fang H., Zheng L., Xie J., et al. (2020a). High dietary starch inclusion impairs growth and antioxidant status, and alters liver organization and intestinal microbiota in largemouth bass micropterus salmoides. Aquac. Nutr. 26 (5), 1806–1821. doi: 10.1111/anu.13131
Zhang Y., Xie S., Wei H., Zheng L., Liu Z., Fang H., et al. (2020b). High dietary starch impaired growth performance, liver histology and hepatic glucose metabolism of juvenile largemouth bass, micropterus salmoides. Aquac. Nutr. 26. doi: 10.1111/anu.13066
Zhao W., Xie J., Fang H.-H., Liu Y.-J., Tian L.-X., Niu J. (2020). Effects of corn starch level on growth performance, antioxidant capacity, gut morphology and intestinal microflora of juvenile golden pompano, trachinotus ovatus. Aquaculture 524, 735197. doi: 10.1016/j.aquaculture.2020.735197
Zhou C., Bo L., Ge X., Xie J., Xu P. (2013). Effect of dietary carbohydrate on the growth performance, immune response, hepatic antioxidant abilities and heat shock protein 70 expression of wuchang bream, megalobrama amblycephala. J. Appl. Ichthyol. 29 (6), 1348–1356. doi: 10.1111/jai.12264
Zhou C., Ge X., Lin H., Niu J. (2014). Effect of dietary carbohydrate on non-specific immune response, hepatic antioxidative abilities and disease resistance of juvenile golden pompano (Trachinotus ovatus). Fish Shellfish Immunol. 41, 183–190. doi: 10.1016/j.fsi.2014.08.024
Zhou C., Ge X., Niu J., Lin H., Huang Z., Tan X. (2015). Effect of dietary carbohydrate levels on growth performance, body composition, intestinal and hepatic enzyme activities, and growth hormone gene expression of juvenile golden pompano, trachinotus ovatus. Aquaculture 437, 390–397. doi: 10.1016/j.aquaculture.2014.12.016
Keywords: mulberry leaf extract, growth performance, antioxidant, nonspecific immune indices, Micropterus salmoides
Citation: Tingsen J, Hui L, Junwa H, Zhe L, Yu L, Honghao J, Xinxi Z, Zhenlin K, Wenbo S, Mengdan H, Huijun Y and Hua Y (2022) Mulberry leaf extract improves non-specific immunity and antioxidant capacity of largemouth bass (Micropterus salmoides) fed a high-starch diet. Front. Mar. Sci. 9:1029360. doi: 10.3389/fmars.2022.1029360
Received: 27 August 2022; Accepted: 18 October 2022;
Published: 11 November 2022.
Edited by:
Jianchun Shao, Fujian Agriculture and Forestry University, ChinaReviewed by:
Guoxing Nie, Henan Normal University, ChinaYu Hong Liu, Guangdong Ocean University, China
Copyright © 2022 Tingsen, Hui, Junwa, Zhe, Yu, Honghao, Xinxi, Zhenlin, Wenbo, Mengdan, Huijun and Hua. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Huijun, eWFuZ2hqOTNAMjYzLm5ldA==; Ye Hua, eWhsaDIwMDBAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Jing Tingsen
Jing Tingsen Luo Hui
Luo Hui Huang Junwa3†
Huang Junwa3† Li Zhe
Li Zhe