- 1Department of Veterinary Medicine, Peoples’ Friendship University of Russia (RUDN University), Moscow, Russia
- 2Inland Waters Aquatics Resources Research Center, Iranian Fisheries Sciences Research Institute, Agricultural Research, Education and Extension Organization, Gorgan, Iran
- 3Faculty of Life Sciences and Biotechnology, Shahid Beheshti University, Tehran, Iran
The aims of the present study were to assess the effects of Hyssop, Hyssopus officinalis, methanolic extract (HE) on growth performance, hepatic oxidative status, humoral and intestinal immunity, and intestinal bacteria of rainbow trout, Oncorhynchus mykiss. Fish were allocated into twelve tanks for four treatments, receiving diets containing 0, 100, 250, and 500 mg/kg HE for eight weeks. The results showed that dietary HE supplementation induced no significant differences in the growth performance, feed efficiency, and hematological parameters (P > 0.05). HE supplementation significantly increased total leukocyte count and the highest count was observed in 250 mg/kg HE treatment (P < 0.001). Fish in 250 and 500 mg/kg HE treatments exhibited significantly lower lymphocyte (P = 0.001) and higher neutrophil (P = 0.002) percentages; the former exhibited a significantly higher monocyte percentage (P = 0.021). Hepatic superoxide dismutase (100 and 250 mg/kg HE; P < 0.001), glutathione peroxidase (100 and 250 mg/kg HE; P = 0.001), glutathione reducatse (all HE treatments; P < 0.001), and reduced glutathione (250 mg/kg HE; P = 0.046) significantly increased, whereas hepatic malondialdehyde levels (250 and 500 mg/kg HE; P = 0.007) significantly decreased in HE-treated fish. Plasma total protein, albumin, globulin, lysozyme, and alternative complement significantly increased in 250 and 500 mg/kg HE treatments and plasma total Ig significantly increased in 250 mg/kg HE treatment. Quantitative real time PCR found no Streptococcus iniae, Lactococcus garvieae, Aeromonas hydrophila, Yersinia ruckeri, and Vibrio anguillarum in the fish intestines in any treatments. Lactobacillus sp. was detected in the fish intestinal samples, but there were no significant differences among the treatments (P = 0.352). Intestinal defensin (P = 0.044) and interleukin-1 beta (P = 0.0.035) expressions were significantly up-regulated in 100 mg/kg HE; intestinal interleukin-10 (P < 0.001) and tumor necrosis factor-alpha (P < 0.001) expressions were significantly up-regulated in 100 and 500 mg/kg HE; whereas, intestinal interleukin-6 expression was significantly (P = 0.009) up-regulated in 250 mg/kg HE treatments. It is concluded that HE is able to stimulate humoral and intestinal immune responses and hepatic antioxidant capacity. HE effective concentration in rainbow trout may be in the range of 100-250 mg/kg.
Introduction
The rapid growth in global demand for aquatic products has resulted in shifting the rearing strategy form the traditional extensive to a modern intensive one (Harikrishnan et al., 2021). Rearing fish at high intensities increases annual yield of a farm and yield per unit of space, but has certain negative drawbacks. Physiological stress due to crowding and water quality deterioration due to elevation in fish wastes are crucial threats in the modern aquaculture industry that decrease fish growth rate, health, and disease resistance (Martos-Sitcha et al., 2020). Under such conditions, opportunistic pathogens may cause disease outbreak and economic loss. Chemical drugs and antibiotics have been extensively used to control disease outbreaks in aquaculture, but environmental concerns and rise of resistance pathogens oriented the global authorities to restrict the use of chemical drugs and antibiotics (Serrano, 2005), focusing on improving fish health and disease resistance. One technique used to boost fish immunity and health is dietary supplementation with different feed additives including herbal additives (Lee et al., 2015). Herbal additives are known for their growth-promoting, immunostimulant, antioxidant, and anti-microbial properties and dietary supplementation with these additives has been found to boost host immunity and health (Hoseinifar et al., 2020a; Elumalai et al., 2021).
Plants are rich sources of antioxidant compounds; as a result, one of the main benefits of dietary herbal additives is the improvement of the antioxidant system and a reduction of oxidative stress (Mohiseni, 2017; Abdel-Latif et al., 2020a; Abdel-Latif et al., 2020b). The liver has high metabolic rate and is the main organ of detoxification; hence, it possesses high antioxidant capacities (Hoseini et al., 2022b). Modern aquaculture is accompanied by various stressors such as high stocking density and inferior water quality that induce oxidative conditions in fish; so that dietary herbal additives may benefit the host by suppressing such drawbacks. Studies have shown that dietary herbal additives improve hepatic antioxidant enzymes’ activities and suppress oxidative stress (Hamed et al., 2021; Adeyemi et al., 2022; He et al., 2022).
Use of medicinal herbs as feed additives also maintains healthy intestinal microflora by dominating beneficial bacteria and limiting harmful bacteria populations, thereby improving immune status (Ganguly and Prasad, 2012; Foysal et al., 2019; Ashry et al., 2021). For example, Lactobacillus sp. are known for their health benefits in fish and herbal feed additives have been found to increase their proportion in fish gut (Adel et al., 2015; Adel et al., 2016). Besides, herbal additives have critical roles in intestinal immunity by stimulating transcriptions of various genes including cytokines and antimicrobial peptide (Gora et al., 2018; Sun et al., 2018; Hoseinifar et al., 2020c; Bilen et al., 2021); thereby these additives increase disease resistance (Adeshina et al., 2021; Lumsangkul et al., 2022).
Although various plants have been used as feed additives in aquaculture (Hoseinifar et al., 2020a; Elumalai et al., 2021), there are still other plants that need to be studied in this field. Hyssop, (Hyssopus officinalis) is a popular medicinal, aromatic, and culinary herb. It is one of the most important medicinal plants, grown in central and southern Europe, such as Russia, Spain, France, and Italy (Omidbaigi, 2005). Hyssop extract has been studied for several biological and pharmaceutical applications (Fathiazad et al., 2011). It has been shown that Hyssop has antioxidant (Kizil et al., 2010; Rezaei Savadkouhi et al., 2020) and immune-boosting (Akbarizadeh et al., 2020) properties, and antagonistic effects on harmful bacteria and fungi (Kizil et al., 2010; Michalczyk et al., 2012). Despite such potentials, there are no data about the benefits of Hyssop extract in fish, indicating the need for further studies.
Rainbow trout (Oncorhynchus mykiss) is one of most important aquaculture fish species worldwide and intensification of this species can lead to a remarkable increase in stress-related infectious diseases. Among the opportunistic bacterial pathogens, Streptococcus iniae (Lahav et al., 2004), Lactococcus garvieae (Pérez-Sánchez et al., 2011), Aeromonas hydrophila (LaPatra et al., 2010), Yersinia ruckeri (Raida and Buchmann, 2008), and Vibrio anguillarum (Croxatto et al., 2007) have been commonly reported to induce disease in rainbow trout. Research has shown that dietary herbal additives are capable of increasing rainbow trout immunity and antioxidant strength and resistance of the fish to these bacteria (Nya and Austin, 2011; Baba et al., 2015; Terzİoğlu and Dİler, 2016; Saeidi Asl et al., 2017; Rufchaei et al., 2020). However, there are no data available regarding the use of methanolic extract of Hyssop (HE) on this species. Hence, the current study was performed to evaluate the impact of dietary methanolic extract of Hyssop on growth performance, antioxidant, and immunological parameters, and gut bacterial populations of rainbow trout.
Materials and methods
Herbal material and extraction
Hyssop flowers were dried by a fan in the shade (24 h), and crushed by a mill. One hundred grams of the crushed materials were mixed with 1 L of methanol and kept at room temperature for 48 h with occasional shaking. After that, the mixture was passed through a mesh (500 µ) followed by filter paper (Whatman No. 1). The resultant solution was then dried at 37°C for 48 h. The residual was collected and preserved in a capped bottle at 4°C. To explore the composition of HE, 1 g of the dried HE was dissolved in methanol and used for GC-MS analysis (supplementary material).
Preparation of diets
HE was added to the diets at 100, 250, and 500 mg/kg. A control (CTL) diet without HE supplementation was included. The composition of the diets are presented in Table 1. Five grams of the dried HE were mixed with 10 mL methanol and the resultant mixture was set to 80 mL by adding distilled water. Then, 1.6, 4, and 8 mL of the mixture were added to diets to have final HE levels of 100, 250, and 500 mg per kg diet. The feedstuffs were finely milled, mixed and moisturized by adding a desired amount of water. The resultant paste was pelleted using a meat-grinder and dried against a fan blower. Standard protocols were followed to determine dietary chemical compositions (AOAC, 2005).
Experimental protocol
Two hundred rainbow trout (~ 60 g) were purchased and transported to a laboratory, where they were kept in a 1500-L tank and fed the CTL diet for one week. After that, 144 fish with similar size and without external abnormalities/lesions were allocated to twelve plastic tanks (150 L water) at the density of 12 fish per tank. The tanks were assigned to four treatments called: CTL (fed CTL diet), 100E (fed diet supplemented with 100 mg/kg HE), 250E (fed diet supplemented with 250 mg/kg HE), and 500E (fed diet supplemented with 500 mg/kg HE). The fish were fed daily based on 3% of biomass, divided to two meals. The feed amounts were corrected every other week, after measuring the tanks’ biomasses. Water temperature, dissolved oxygen, pH, and total ammonia nitrogen were 13.1 ± 0.07°C, 8.55 ± 0.40 mg/L, 7.77 ± 0.31, and 0.12 ± 0.02 mg/L [measured by Hach Co. (Colorado, USA) Digital probe and kits]. After 8 weeks of rearing, the fish growth performance and feed efficiency were determined and the blood, hepatic, and intestine samples were collected for further analysis.
Blood sampling and analysis
Two fish were caught from each tank and anesthetized in a eugenol bath (100 µL/L). Blood samples (1.5 mL) were taken from the fish caudal vein using heparinized syringes and divided into two aliquots; one for hematological analysis and the other for plasma separation. Plasma separation was done by centrifugation at 4°C (3000 g; 7 min) and the obtained materials were kept at -70°C until analysis.
The blood erythrocyte (RBC) and leukocyte (WBC) counting was done using the dacie diluting solution based on Dacie and Lewis (1996). Hemoglobin levels were measured using a commercial kit (Zistchem Co., Tehran, Iran) and a spectrophotometer; whereas, hematocrit percentages were calculated by micro-centrifuging according to Dacie and Lewis (1996). Mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) were calculated based on Blaxhall (1972). Differential leukocyte count was performed after preparing the blood smear and staining with Giemsa according to Sheikh and Ahmed (2020).
Plasma total protein and albumin were measured using commercial kits and a spectrophotometer. Plasma globulin levels were measured by subtracting total protein and albumin levels. Plasma lysozyme activity was determined based on lysis rate of Micrococcus luteus over 5 min at 550 nm, as suggested by Ellis (1990). Briefly, 30 µL of the samples were mixed 1 mL of the bacterial suspension (made in 0.05 M phosphate buffer, pH 6.2) and a decrease in absorbance was recorded over 5 min. Each 0.001 decrease in absorbance per min was considered as one unit of lysozyme. Plasma alternative complement activity (ACH50) was determined based on hemolytic activity against sheep erythrocyte as described by Yano (1992). Briefly, the plasma samples were diluted by 0.303-10%. Hemolytic activity of each dilution was determined against sheep erythrocyte for 2 h, and the reaction medium consisted of veronal buffer containing magnesium and gelatin. Plasma total immunoglobulin (Ig) levels were determined after precipitation with polyethylene glycol according to Siwicki and Anderson (1993). Briefly, 100 µL of the plasma samples were mixed with equal volume of polyethylene glycol 12% and shaken for 2 h. Then, the mixture was centrifuged for Ig precipitation. Difference in the total protein before and after precipitation was considered as total Ig level.
Hepatic sampling and analysis
Hepatic samples were taken from the same fish that the blood samples were collected from. A piece of ~1 g was dissected from each fish liver and immediately frozen in liquid nitrogen. Then, the samples were homogenized in five volumes of phosphate buffer (pH 7.0), centrifuged at 4°C (9500 g; 15 min), and the supernatants were collected in separate tubes.
Hepatic antioxidant parameters were determined using commercial kits provided by Zellbio Co. (Deutschland, Germany) as validated for this species (Taheri Mirghaed et al., 2020). Superoxide dismutase (SOD) activity was determined based on the reduction rate of the cytochrome C. Glutathione peroxidase (GPx), glutathione reductase (GR), and reduced glutathione (GSH) were determined based on the conversion rate of GSH to glutathione disulfide. Malondialdehyde (MDA) content was determined based on the reaction with thiobarbituric acid at 95°C.
Intestinal sampling and analysis
After dissecting the hepatic samples, the fish intestine samples (the posterior parts) were dissected and frozen in liquid nitrogen. The intestinal sample of one fish per tank was used for gene expression analysis and the sample of the other fish was used for Lactobacillus sp., Streptococcus iniae, Lactococcus garvieae, Aeromonas hydrophila, Yersinia ruckeri, and Vibrio anguillarum populations examination.
For gene expression analysis, RNA was extracted from the intestinal samples using a commercial kit (Denazist Co., Tehran, Iran). DNAse I (Thermo Fisher Scientific, Waltham, MA, USA) treatment was applied to avoid the product contamination. cDNA was synthetized using a commercial kit (SMOBIO Technology Co.; Hsinchu City 30075, Taiwan). Relative gene expression was assessed by qRT-PCR method using an apparatus provided by Applied Bioscience (USA). Amplification of the target genes along with a reference gene (gadph) was performed using specific primers (Table 2) and SYBR Green (Ampliqon A/S, Stenhuggervej 22, 5230 Odense M, Denmark) kit. Relative gene expressions were calculated based on ΔΔCt method (Livak and Schmittgen, 2001).
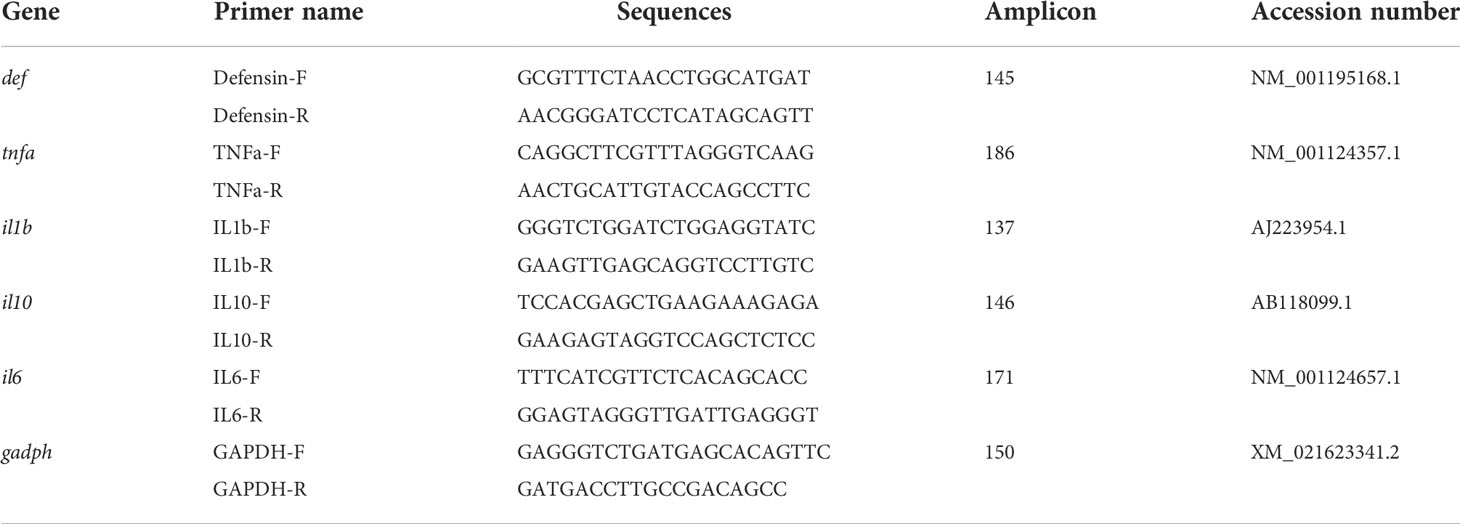
Table 2 Sequence, amplicon, and accession number of specific primers used for intestinal transcriptomic analysis.
Populations of Lactobacillus sp., S. iniae, L. garvieae, A. hydrophila, Y. ruckeri, and V. anguillarum were examined in the intestinal samples. Procedures of the sample digestion and DNA extraction (phenol-chloroform using washing kit provided by GeneAll Co., Seoul, Korea) have been described before (Hoseini et al., 2022a). The bacterial populations were determined based on total bacteria in the samples. Specific primers for the target bacteria groups are presented in Table 3. The universal 16s primer was used for total bacteria population examination. qRT-PCR was used to amplified the target genes and bacterial population was calculated based on ΔΔCt method (Hoseini et al., 2022a).
Statistical analysis
Means of the treatments were compared using one-way ANOVA and Duncan tests, except percentile data (leukocyte differential counts) that were analyzed by Kruskal-Wallis and Mann-Whitney U tests. For ANOVA execution, the data were first subjected to Shapiro-Wilk and Levene tests aiming at confirming normal distribution and variance homogeneity, respectively. Accordingly, Lactobacillus sp., il10 and tnfa data were log-transformed as they failed to meet the ANOVA assumptions. SPSS v.22 was used for analysis and significance has been checked at α = 0.05.
Results
No fish mortality was observed during the study. There were no significant differences in final weight (P = 0.774), weight gain (P = 0.838), SGR (P = 0.841), and FCR (P = 0.701) among the treatments (Table 4).
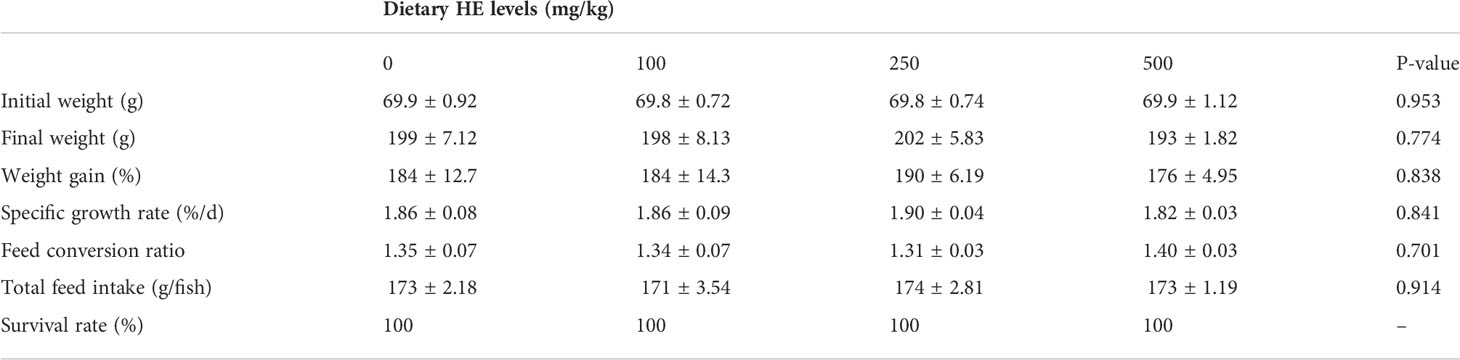
Table 4 Growth performance and feed efficiency of rainbow trout fed diets supplemented with 0-500 mg/kg HE over eight weeks (mean ± SE; n = 3).
RBC (P = 0.970), hemoglobin (P = 0.849), hematocrit (P = 0.981), MCV (P = 0.916), MCH (P = 0.093), and MCHC (P = 0.233) exhibited no significant differences among the treatments (Table 5).
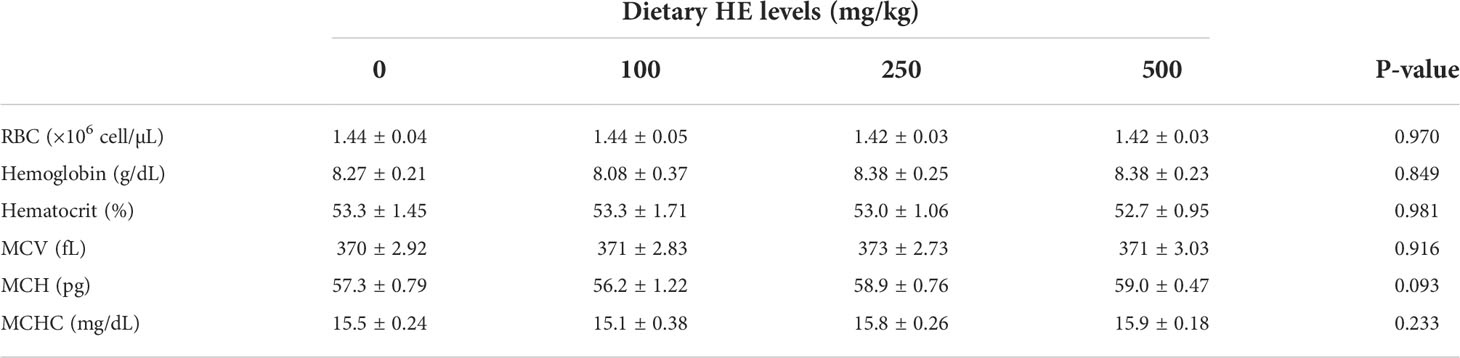
Table 5 Hematological parameters of rainbow trout fed diets supplemented with 0-500 mg/kg HE over eight weeks (mean ± SE; n = 6).
Hepatic antioxidant parameters are shown in Figure 1. HE supplementation significantly affected hepatic SOD (P < 0.001), GPx (P = 0.001), GR (P < 0.001), GSH (P = 0.046), and MDA (P = 0.007). Hepatic SOD and GPx activities in 100E and 250E treatments were significantly higher than in the CTL treatment. All HE-treated fish exhibited significantly higher GR activities, compared to CTL treatment and the highest activity was observed in the 100E treatment. Hepatic GSH level significantly increased in 250E treatment, compared to CTL; whereas, both 250E and 500E treatments exhibited significantly lower MDA levels, compared to CTL.
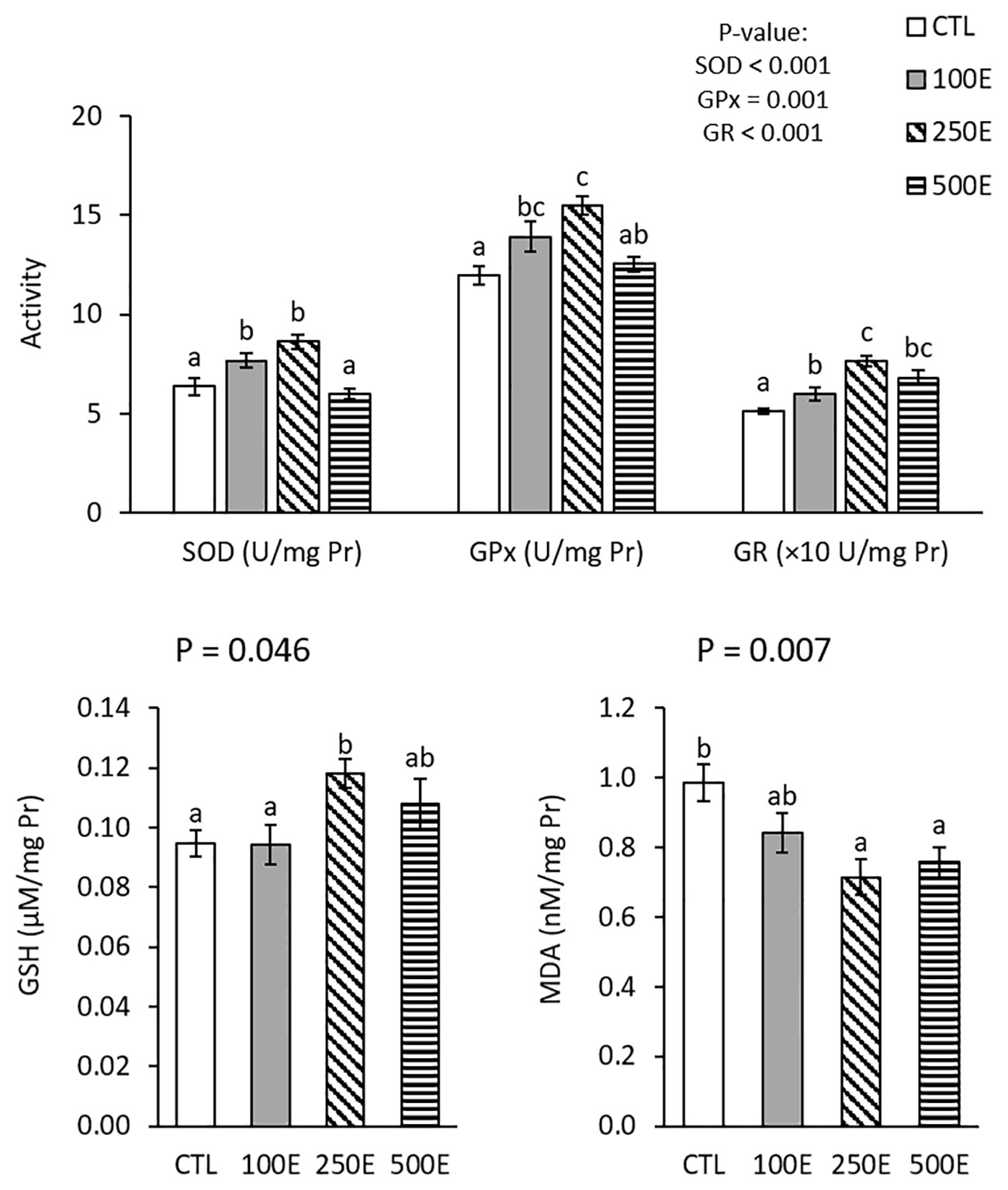
Figure 1 Hepatic antioxidant parameters (mean ± SE) of rainbow trout fed diets supplemented with 0-500 mg/kg HE over eight weeks. Different letters above the bars indicate significant differences among the treatments (Duncan test; n = 6).
All HE-treated fish exhibited significant increases in WBC, compared to CTL, and the highest level was related to 250E (Figure 2). Lymphocyte percentage decreased as neutrophil percentage increased in 250E and 500E treatments, compared to CTL (Figure 2). There were no significant differences in eosinophil percentages among the treatments, however, monocyte percentage exhibited a significant elevation in 250E, compared to CTL treatment (Figure 2).
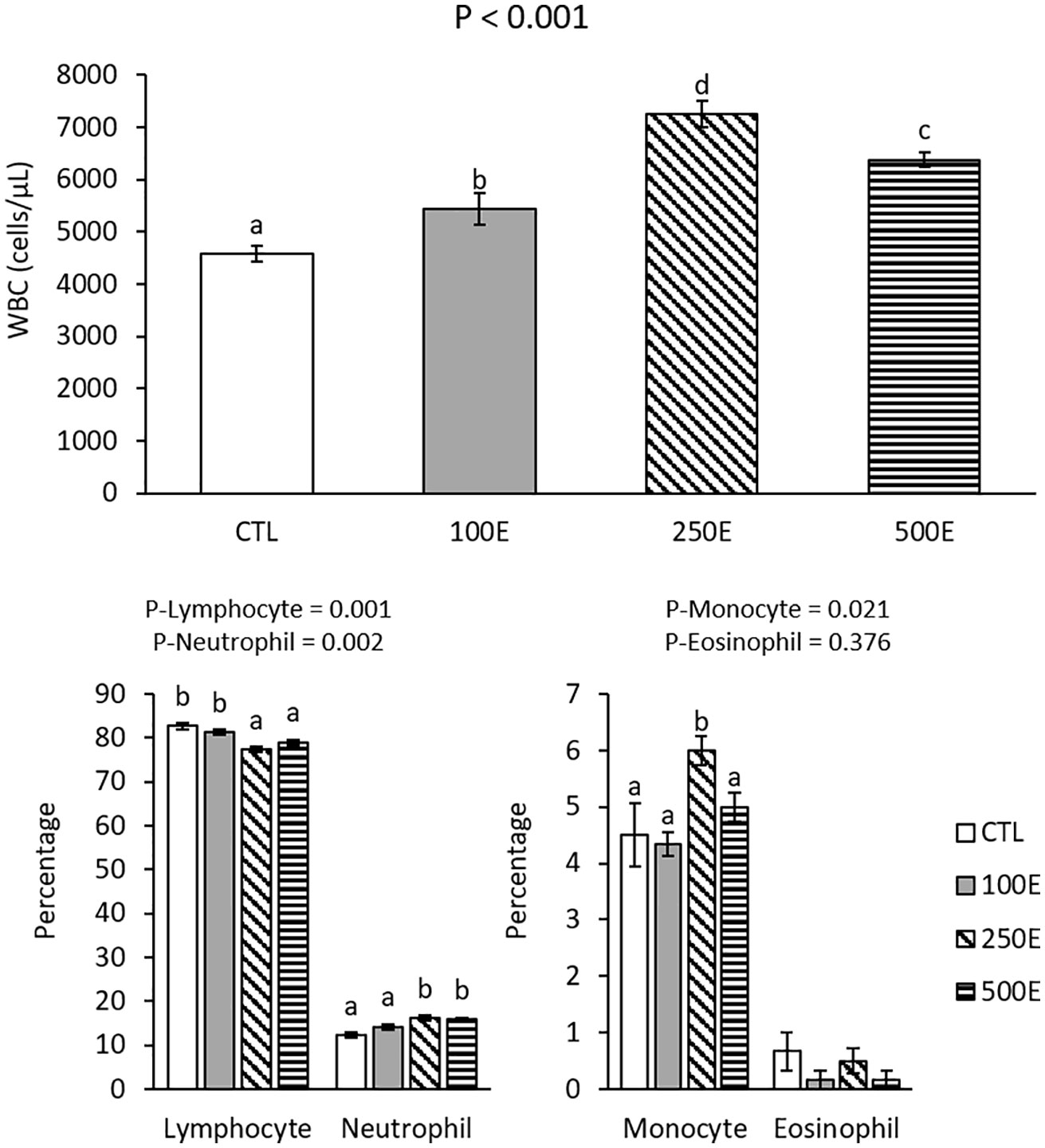
Figure 2 WBC and differential leukocyte count (mean ± SE) of rainbow trout fed diets supplemented with 0-500 mg/kg HE over eight weeks. Different letters above the bars indicate significant differences among the treatments (Duncan test for WBC and Mann-Whitney U test for differential counts; n = 6).
Plasma total protein, albumin, globulin, lysozyme, ACH50, and total Ig showed significant differences among the treatments (Figure 3). Plasma total protein, albumin, lysozyme, and ACH50 in 100E and 250E treatments were significantly higher than CTL and 250E showed the highest levels in the case of plasma lysozyme and ACH50. Plasma globulin and total Ig in 250E treatment was significantly higher than CTL treatment.
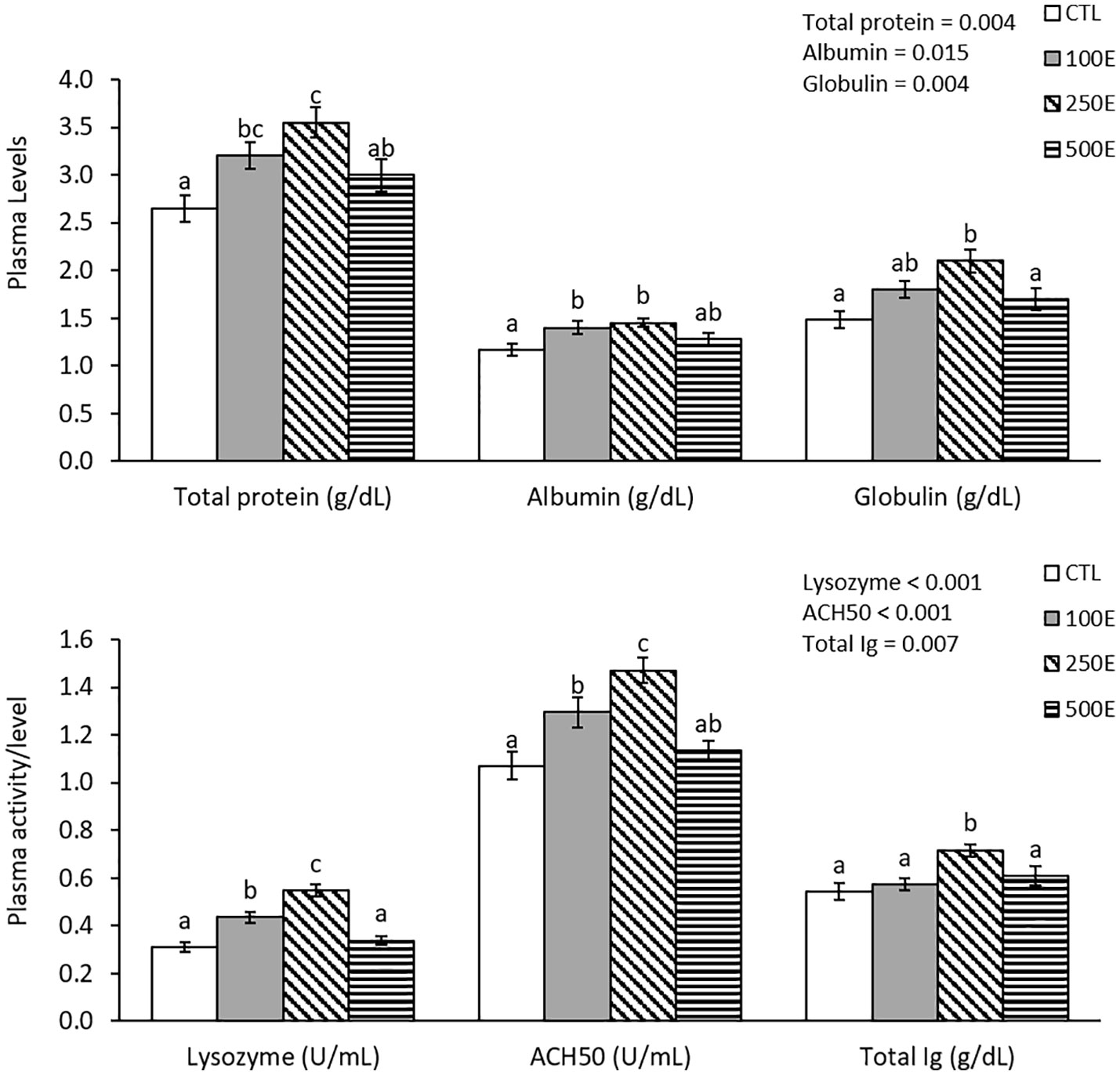
Figure 3 Blood plasma immunological parameters (mean ± SE) of rainbow trout fed diets supplemented with 0-500 mg/kg HE over eight weeks. Different letters above the bars indicate significant differences among the treatments (Duncan test; n = 6).
Among the bacterial groups examined, only Lactobacillus sp. was detected in the samples (Figure 4). However, HE treatment had no significant effects on Lactobacillus sp. population (P = 0.352)
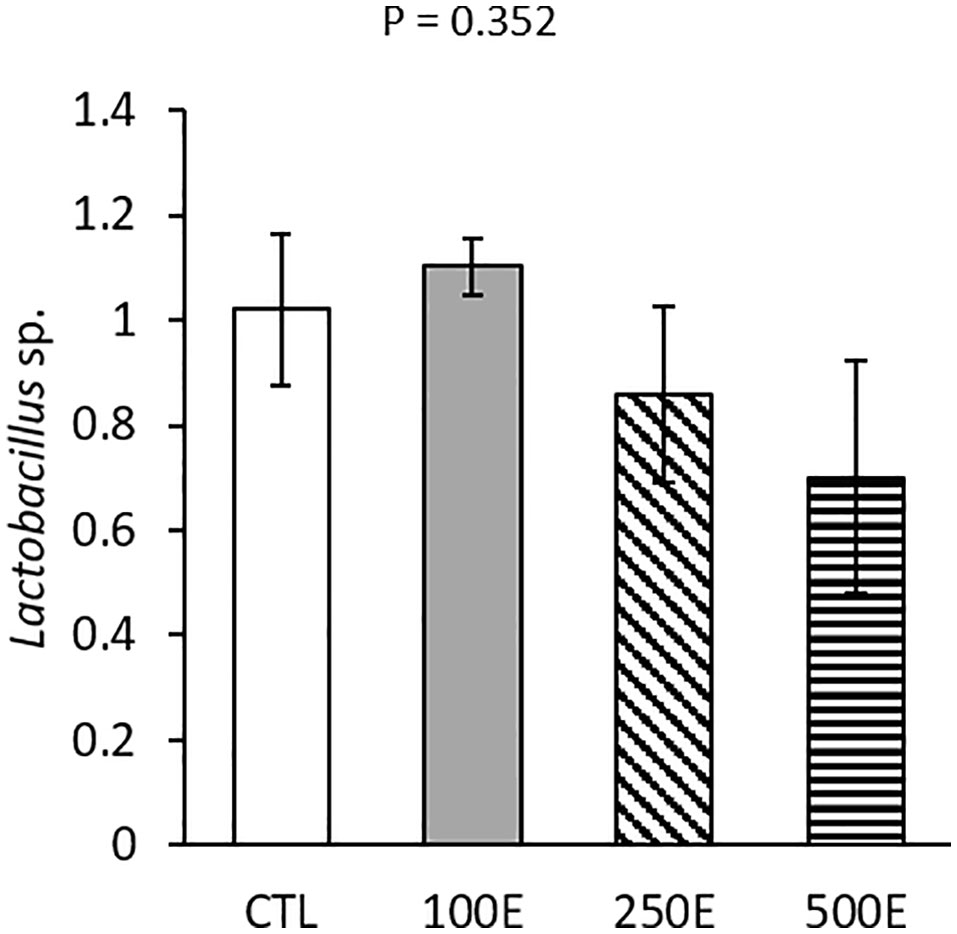
Figure 4 Intestinal Lactobacillus sp. population (mean ± SE) of rainbow trout fed diets supplemented with 0-500 mg/kg HE over eight weeks (n = 3).
Dietary HE supplementation significantly affected the intestinal def (P = 0.044), il1b (P = 0.0.035), il10 (P < 0.001), il6 (P = 0.009), and tnfa (P < 0.001) expression (Figure 5). Expressions of def and il1b were up-regulated in 100E treatment, compared to CTL. Intestinal expressions of il10 and tnfa were up-regulated in 100E and 500E treatments, compared to CTL. The highest expressions of il10 and tnfa were observed in 500E and 100E treatments, respectively. Expression of the intestinal il6 was significantly up-regulated in 250E treatment, compared to CTL.

Figure 5 Intestinal gene expressions (mean ± SE) of rainbow trout fed diets supplemented with 0-500 mg/kg HE over eight weeks. Different letters above the bars indicate significant differences among the treatments (Duncan test; n = 3).
Discussion
Intensification of aquaculture has led to several issues including poor fish growth and health. This has made it hard for fish farmers to turn the biological benefits associated with intensive farming systems into economical gain. In addition, the usage of chemotherapeutic drugs to maintain fish growth and health seems to be less production-oriented, hence, unsustainable (Gabriel, 2019). Thus, development of aquaculture should also rely on environmentally friendly and sustainable practices. Recently, much attention has been paid to the use of herbal additives as alternatives to chemical agents, with an aim of increasing yield in aquaculture (Gabriel, 2019). Growth-promoting effects of dietary herbal additives relate to improvements in digestion and absorption of nutrients (Bilen et al., 2020; Xu et al., 2020; Phukan et al., 2022; Wangkahart et al., 2022) and/or stimulation of somatotropic pathways in the fish (Midhun et al., 2016; Zemheri-Navruz et al., 2020). Therefore, it is speculated that HE might fail to have such effects on rainbow trout under the conditions of the present study. Similarly, other herbal additives have failed to improve growth performance in rainbow trout (Hernández et al., 2016; Baba et al., 2018).
Living cells are under constant threat of oxidation by pro-oxidants that are normally produced during the cell respiration or as a result of adverse ambient conditions. The antioxidant enzymes (SOD, CAT, GPx, and GR) protect cells from oxidative damage (Yousefi et al., 2020) and it is one of the most crucial benefits of using plant extracts in fish diets. Plant extracts have antioxidant capacity and high capability to donate hydrogen atoms to electrons and free electrons and this property is due to the existence of phenolic compounds in herbs (Jeney et al., 2015). Moreover, plant extracts have been found to stimulate the activity of the antioxidant enzymes in fish, although the exact underlying mechanisms are not clear yet (Mohiseni, 2017). These capabilities make plant extract supplementation one of the functional approaches to reduce oxidative stress in animals. SOD is an early-functioning antioxidant enzyme that neutralizes superoxide ions and has a crucial role in detoxifying the respiration-produced superoxide ion in living cells (Shi et al., 2022). GPx is responsible for neutralizing hydrogen peroxide using GSH as a co-factor. After neutralizing hydrogen peroxide, GSH is oxidized and loses its function. GR reduces the oxidized glutathione to GSH, which can be used again in hydrogen peroxide neutralization (Rocha-Santos et al., 2018). According to the present results, the lowest lipid peroxidation was observed in 250E and 500E treatments. This could be due to improved antioxidant enzymes’ activity and radical scavenging activity of HE in 250E treatment (as evidenced by high GSH reserves). However, lower lipid peroxidation in 500E suggests radical scavenging activity of HE is the main reason for mitigation of lipid peroxidation. This is supported by the results of 100E treatment, where improvement in the activity of the antioxidant enzymes failed to mitigate lipid peroxidation. Supporting this, radical scavenging activity of HE has been reported under in vitro conditions (Soleimani et al., 2011; Alinezhad et al., 2012).
Hematological parameters provide valuable information about fish health. Studies on different fish species have indicated herbal supplements such as Spirulina platensis (Adel et al., 2016), garlic (Nya and Austin, 2011), and Echinacea purpurea (Oskoii et al., 2012) have hematopoietic effects in fish and increase blood RBC, hematocrit, and hemoglobin. However, in the present study, RBC, hemoglobin, hematocrit, MCV, MCH, and MCHC exhibited almost no change among the different treatments suggesting no hematopoietic ability of HE in this fish species, which is similar to the findings in previous studies on other herbal supplements (Adel et al., 2015; Soltanian and Fereidouni, 2016).
Total blood leukocytes are known as one of the most important cellular defenses in fish (Newaj-Fyzul and Austin, 2015). Differential leukocyte counting is a reliable method of assessing fish health and cellular immunity (Machado et al., 2015). Depending on type, leukocytes have various roles in host defense. Lymphocytes are the most abundant blood leukocyte and have roles in adaptive immunity (antibody production) and killing tumor cells (Shoemaker et al., 2015). Neutrophils are the next abundant leukocytes that are responsible for early responses to infection; they are phagocytic and involve in respiratory burst activity and lysozyme production (Shoemaker et al., 2015). Monocytes reside in the blood or migrate to the body tissues and transform to macrophages to find and destroy germs (viruses, bacteria, fungi, and protozoa) (Shoemaker et al., 2015). According to the present results, HE administration shifted leukocyte composition from late-response (lymphocyte) to early-response (phagocytes; i.e. neutrophils and monocytes). Such changes in leukocyte composition have been previously observed, when fish fed supplemented diets and helped the fish to resist against a pathogenic challenge (Lin et al., 2012). Similar results have been obtained, when rainbow trout was treated with dietary Ginger, Zingiber officinale; where increase in neutrophil and monocyte proportions and decrease in lymphocyte proportion after ginger administration helped the fish to better resist against A. hydrophila infection (Nya and Austin, 2009b). A higher neutrophil percentage (with no changes in lymphocyte and monocyte percentages) after oral administration of Azadirachta indica leaves has resulted in maximum survival in Lates calcarifer challenged with Vibrio harveyi (Talpur and Ikhwanuddin, 2013).
The non-specific immune system of fish is an important defense line against invading pathogens. Plasma protein levels and fractions are indicators of fish health as most of them are produced in the liver. Healthier liver and/or good rearing conditions may lead to higher plasma protein levels (Hoseini and Tarkhani, 2013; Sayed and Hamed, 2017). Ig encompass a fraction of plasma protein and it has been found that basal plasma Ig may be sensitive to dietary supplementation (Jinendiran et al., 2019; Lim et al., 2019). Complement proteins are another fraction of plasma protein that are produced in the liver and have different immune roles such as cell lysis and opsonization (Holland and Lambris, 2002). According to the present results, HE supplementation seems to improve hepatic health, which leads to higher protein synthesis and innate immunity improvement. The increased levels of complement, Ig, albumin, and globulins in fish are linked with a strong innate immune response and studies on fish have shown dietary herbal supplementation increases in plasma proteins, Ig, and complement that leads to higher disease resistance (Nya and Austin, 2009a; Nya and Austin, 2009b; Nya and Austin, 2011; Talpur and Ikhwanuddin, 2013; Talpur et al., 2013). Lysozyme is another immune-related enzyme that acts as a bactericidal agent (Zhang et al., 2018). Lysozyme is produced by neutrophil and the increase in the plasma lysozyme in the present study may be due to a higher number of neutrophil and/or lysozyme production by these cells. Other studies have found an increase in plasma lysozyme activity after herbal treatment led to higher disease resistance in fish (Nya and Austin, 2009a; Nya and Austin, 2009b; Nya and Austin, 2011).
The fish intestine is a unique organ that plays important roles in digestion/absorption and immunity (Hoseini et al., 2021). The fish intestine is a site of various microbial populations, which can alter the host immunity (Hoseinifar et al., 2019). A healthy intestine is characterized by low density of harmful microbes and high density of beneficial ones (Hoseinifar et al., 2019). In this study, none of the tested pathogenic bacteria were detected in the samples, suggesting that the fishes’ intestines were in good health. Moreover, Lactobacillus sp. was detected in the samples, which was in line with the previous studies on rainbow trout (Wang et al., 2020; Hoseini et al., 2022a). However, HE induced no significant changes in Lactobacillus sp. population, which is not in line with studies evaluating other herbal additives in fish (Adel et al., 2015; Adel et al., 2016; Meng et al., 2019). Such variations in the results can be due to the differences in fish species and feed additives.
Defensin is an antimicrobial peptide, known for its roles in combating bacteria, fungi, and viruses (Guo et al., 2012). It is found in fish mucosal tissues such as intestine (van der Marel et al., 2012). Similar to the present results, intestinal def expression has been found elevated in different fish following dietary additive supplementations such as Sargassum wightii extract (Gora et al., 2018), mannan oligosaccharide (Fernández-Montero et al., 2019), microalgae mixture (Cerezuela et al., 2012). Such an up-regulation in the intestinal def expression may help the fish to resist against bacterial and viral disease, as Casadei et al. (2009) have shown bacterial and viral stimulation significantly up-regulates intestinal def expression rainbow trout.
Cytokines have numerous roles in inflammation and immune responses. il1b is a pro-inflammatory cytokine with wide physiological roles, including increasing the number of macrophages and phagocytosis (Hong et al., 2003), antibody production (Taechavasonyoo et al., 2013) and lysozyme activity (Hong et al., 2003). It also stimulates tnfa expression (Bo et al., 2015), which is involved in increased phagocytosis, inhibiting bacterial growth in infected macrophages, and necrosis of tumor cells (Zou and Secombes, 2016). il6 is another cytokine with both anti-inflammatory and pro-inflammatory roles, which is involved in macrophage growth and expression of antimicrobial peptides (Zou and Secombes, 2016). On the other hand, il10 is an anti-inflammatory cytokine that suppresses the immune responses in fish (Piazzon et al., 2015). Intestinal cytokines have an important roles in fish immune defense. Herbal additives potentiate to alter cytokine expression in fish intestine (Hoseinifar et al., 2020b; Hoseinifar et al., 2020d). It has been demonstrated that up-regulation in the intestinal pro-inflammatory cytokines following herbal additive administration has been accompanied by better disease resistance after a pathogenic challenge (Noor-Ul et al., 2020; Bilen et al., 2021; Ibrahim et al., 2021). The present results suggest that 100 mg/kg HE induces several immune genes in the fish intestine, which may be interpreted as improved intestinal health. However, it is not clear why il6 patterns differed from those of il1b and tnfa, based on the present data. It is also speculated that il10 up-regulation in 100 mg/kg HE treatment is a protective response to up-regulation of il1b and tnfa to protect the host from negative consequences of inflammation. But, higher up-regulation in il10 gene expression in 500 mg/kg HE may be an indication of immunosuppression. Moreover, the patterns of blood, hepatic, and intestinal responses to HE must be considered, as HE concentration is a determinant of its effects on different tissues.
It is concluded that dietary HE supplementation has no growth-promoting effects in rainbow trout. However, HE is able to stimulate humoral and intestinal immune responses and hepatic antioxidant capacity. However, effective concentration of HE must be selected with caution, as its benefits were observed at different concentrations, based on the fish tissue examined. Therefore, HE effective concentration in rainbow trout may be in the range of 100-250 mg/kg.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Peoples’ Friendship University of Russia (RUDN University) ethical committee (EC1/351, 05/06/2021).
Author contributions
MY: conceptualization, supervision, grant acquisition, methodology, and editing. SMH: conceptualization, supervision, methodology, and drafting. BA: conceptualization, study design, and drafting. YV: conceptualization and data analysis. EK: conceptualization and study design. NR: methodology, and data analysis. All authors contributed to the article and approved the submitted version.
Funding
This publication has been supported by the RUDN University Scientific Projects Grant System, project № “202196-2-174”.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Latif H. M., Abdel-Tawwab M., Khafaga A. F., Dawood M. A. (2020a). Dietary oregano essential oil improved antioxidative status, immune-related genes, and resistance of common carp (Cyprinus carpio l.) to Aeromonas hydrophila infection. Fish. Shellfish Immunol. 104, 1–7. doi: 10.1016/j.fsi.2020.05.056
Abdel-Latif H. M., Abdel-Tawwab M., Khafaga A. F., Dawood M. A. (2020b). Dietary oregano essential oil improved the growth performance via enhancing the intestinal morphometry and hepato-renal functions of common carp (Cyprinus carpio l.) fingerlings. Aquaculture. 526, 735432. doi: 10.1016/j.aquaculture.2020.735432
Adel M., Safari R., Pourgholam R., Zorriehzahra J., Esteban M. Á. (2015). Dietary peppermint (Mentha piperita) extracts promote growth performance and increase the main humoral immune parameters (both at mucosal and systemic level) of Caspian brown trout (Salmo trutta caspius Kessler 1877). Fish. Shellfish Immunol. 47, 623–629. doi: 10.1016/j.fsi.2015.10.005
Adel M., Yeganeh S., Dadar M., Sakai M., Dawood M. A. O. (2016). Effects of dietary Spirulina platensis on growth performance, humoral and mucosal immune responses and disease resistance in juvenile great sturgeon (Huso huso Linnaeus 1754). Fish. Shellfish Immunol. 56, 436–444. doi: 10.1016/j.fsi.2016.08.003
Adeshina I., Abdel-Tawwab M., Tijjani Z. A., Tiamiyu L. O., Jahanbakhshi A. (2021). Dietary Tridax procumbens leaves extract stimulated growth, antioxidants, immunity, and resistance of Nile tilapia, Oreochromis niloticus, to monogenean parasitic infection. Aquaculture 532, 736047. doi: 10.1016/j.aquaculture.2020.736047
Adeyemi K. D., Oludemokun O. O., Zubair M. F., Atolani O., Ibrahim S. O. (2022). Influence of dietary Plukenetia conophora seed on growth performance, hepatic antioxidant status, blood chemistry, fillet quality, fatty acid, and oxidative stability of Clarias gariepinus. Anim. feed Sci. Technol. 288, 115298. doi: 10.1016/j.anifeedsci.2022.115298
Akbarizadeh N., Khatibjoo A., Varmaghany S., Jafari H., Shokri A. (2020). Effect of Hyssopus officinalis, virginiamycine and aspirine on growth performance, immunity, and ascites indexes of broiler chicken under cold stress. Anim. prod. 22, 289–299. doi: 10.22059/jap.2020.293770.623478
Alinezhad H., Baharfar R., Zare M., Azimi R., Nabavi S. F., Nabavi S. M. (2012). Biological activities of ethyl acetate extract of different parts of Hyssopus angustifolius. pharm. Biol 50, 1062–1066. doi: 10.3109/13880209.2012.655859
AOAC (2005). Official methods of analysis of the association of official analytical chemists. International, 18th Ed. (Washington, DC, USA).
Ashry A. M., Hassan A. M., Habiba M. M., El-Zayat A., El-Sharnouby M. E., Sewilam H., et al. (2021). The impact of dietary curcumin on the growth performance, intestinal antibacterial capacity, and haemato-biochemical parameters of gilthead seabream (Sparus aurata). Animals 11, 1779. doi: 10.3390/ani11061779
Baba E., Acar Ü., Yılmaz S., Zemheri F., Ergün S. (2018). Dietary olive leaf (Olea europea l.) extract alters some immune gene expression levels and disease resistance to Yersinia ruckeri infection in rainbow trout Oncorhynchus mykiss. Fish. Shellfish Immunol. 79, 28–33. doi: 10.1016/j.fsi.2018.04.063
Baba E., Uluköy G., Öntaş C. (2015). Effects of feed supplemented with Lentinula edodes mushroom extract on the immune response of rainbow trout, Oncorhynchus mykiss, and disease resistance against Lactococcus garvieae. Aquaculture 448, 476–482. doi: 10.1016/j.aquaculture.2015.04.031
Bastardo A., Ravelo C., Romalde J. L. (2012). Highly sensitive detection and quantification of the pathogen Yersinia ruckeri in fish tissues by using real-time PCR. Appl. Microbiol. Biotechnol. 96, 511–520. doi: 10.1007/s00253-012-4328-1
Bilen S., Altief T. A. S., Özdemir K. Y., Salem M. O. A., Terzi E., Güney K. (2020). Effect of lemon balm (Melissa officinalis) extract on growth performance, digestive and antioxidant enzyme activities, and immune responses in rainbow trout (Oncorhynchus mykiss). Fish. Physiol. Biochem. 46, 471–481. doi: 10.1007/s10695-019-00737-z
Bilen S., Mohamed Ali G. A., Amhamed I. D., Almabrok A. A. (2021). Modulatory effects of laurel-leaf cistus (Cistus laurifolius) ethanolic extract on innate immune responses and disease resistance in common carp (Cyprinus carpio). Fish. Shellfish Immunol. 116, 98–106. doi: 10.1016/j.fsi.2021.07.001
Blaxhall P. C. (1972). The haematological assessment of the health of freshwater fish. J. Fish. Biol. 4, 593–604. doi: 10.1111/j.1095-8649.1972.tb05704.x
Bo Y.-X., Song X.-H., Wu K., Hu B., Sun B.-Y., Liu Z.-J., et al. (2015). Characterization of interleukin-1β as a proinflammatory cytokine in grass carp (Ctenopharyngodon idella). Fish. Shellfish Immunol. 46, 584–595. doi: 10.1016/j.fsi.2015.07.024
Casadei E., Wang T., Zou J., González Vecino J. L., Wadsworth S., Secombes C. J. (2009). Characterization of three novel β-defensin antimicrobial peptides in rainbow trout (Oncorhynchus mykiss). Mol. Immunol. 46, 3358–3366. doi: 10.1016/j.molimm.2009.07.018
Cerezuela R., Guardiola F. A., Meseguer J., Esteban M.Á. (2012). Enrichment of gilthead seabream (Sparus aurata l.) diet with microalgae: effects on the immune system. Fish. Physiol. Biochem. 38, 1729–1739. doi: 10.1007/s10695-012-9670-9
Chapela M.-J., Ferreira M., Ruiz-Cruz A., Martin-Varela I., Fernández-Casal J., Garrido-Maestu A. (2018b). Application of real-time PCR for early diagnosis of diseases caused by aeromonas salmonicida, vibrio anguillarum, and Tenacibaculum maritimum in turbot: A field study. J. Appl. aquacult. 30, 76–89. doi: 10.1080/10454438.2017.1406419
Chapela M.-J., Ferreira M., Varela C., Arregui L., Garrido-Maestu A. (2018a). Development of a multiplex real-time PCR method for early diagnosis of three bacterial diseases in fish: A real-case study in trout aquaculture. Aquaculture 496, 255–261. doi: 10.1016/j.aquaculture.2018.07.003
Croxatto A., Lauritz J., Chen C., Milton D. L. (2007). Vibrio anguillarum colonization of rainbow trout integument requires a DNA locus involved in exopolysaccharide transport and biosynthesis. Environ. Microbiol. 9, 370–382. doi: 10.1111/j.1462-2920.2006.01147.x
Ellis A. E. (1990). “Lysozyme assays,” in Techniques in fish immunology. Ed. J.S. S. (Fair Haven: SOS publication), 101–103.
Elumalai P., Kurian A., Lakshmi S., Faggio C., Esteban M. A., Ringø E. (2021). Herbal immunomodulators in aquaculture. Rev. fish. Sci. aquacult. 29, 33–57. doi: 10.1080/23308249.2020.1779651
Fathiazad F., Mazandarani M., Hamedeyazdan S. (2011). Phytochemical analysis and antioxidant activity of Hyssopus officinalis l. from Iran. Adv. Pharm. Bull. 1, 63–67. doi: 10.5681/apb.2011.009
Fernández-Montero Á., Torrecillas S., Izquierdo M., Caballero M. J., Milne D. J., Secombes C. J., et al. (2019). Increased parasite resistance of greater amberjack (Seriola dumerili risso 1810) juveniles fed a cMOS supplemented diet is associated with upregulation of a discrete set of immune genes in mucosal tissues. Fish. Shellfish Immunol. 86, 35–45. doi: 10.1016/j.fsi.2018.10.034
Foysal M. J., Alam M., Momtaz F., Chaklader M. R., Siddik M. A. B., Cole A., et al. (2019). Dietary supplementation of garlic (Allium sativum) modulates gut microbiota and health status of tilapia (Oreochromis niloticus) against Streptococcus iniae infection. Aquac. Res. 50, 2107–2116. doi: 10.1111/are.14088
Frank J. A., Reich C. I., Sharma S., Weisbaum J. S., Wilson B. A., Olsen G. J. (2008). Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. Environ. Microbiol. 74, 2461–2470. doi: 10.1128/AEM.02272-07
Gabriel N. N. (2019). Review on the progress in the role of herbal extracts in tilapia culture. Cogent. Food Agric. 5, 1619651. doi: 10.1080/23311932.2019.1619651
Ganguly S., Prasad A. (2012). Microflora in fish digestive tract plays significant role in digestion and metabolism. Rev. Fish. Biol. Fish. 22, 11–16. doi: 10.1007/s11160-011-9214-x
Gora A. H., Sahu N. P., Sahoo S., Rehman S., Dar S. A., Ahmad I., et al. (2018). Effect of dietary Sargassum wightii and its fucoidan-rich extract on growth, immunity, disease resistance and antimicrobial peptide gene expression in Labeo rohita. Int. Aquat. Res. 10, 115–131. doi: 10.1007/s40071-018-0193-6
Guo M., Wei J., Huang X., Huang Y., Qin Q. (2012). Antiviral effects of β-defensin derived from orange-spotted grouper (Epinephelus coioides). Fish. Shellfish Immunol. 32, 828–838. doi: 10.1016/j.fsi.2012.02.005
Hamed H. S., Ismal S. M., Faggio C. (2021). Effect of allicin on antioxidant defense system, and immune response after carbofuran exposure in Nile tilapia, Oreochromis niloticus. comparative biochemistry and physiology part c. Toxicol. Pharmacol. 240, 108919. doi: 10.1016/j.cbpc.2020.108919
Harikrishnan R., Devi G., Van Doan H., Balasundaram C., Thamizharasan S., Hoseinifar S. H., et al. (2021). Effect of diet enriched with agaricus bisporus polysaccharides (ABPs) on antioxidant property, innate-adaptive immune response and pro-anti inflammatory genes expression in Ctenopharyngodon idella against Aeromonas hydrophila. Fish. Shellfish Immunol. 114, 238–252. doi: 10.1016/j.fsi.2021.04.025
Hernández A. J., Romero A., Gonzalez-Stegmaier R., Dantagnan P. (2016). The effects of supplemented diets with a phytopharmaceutical preparation from herbal and macroalgal origin on disease resistance in rainbow trout against Piscirickettsia salmonis. Aquaculture 454, 109–117. doi: 10.1016/j.aquaculture.2015.12.016
He G., Sun H., Liao R., Wei Y., Zhang T., Chen Y., et al. (2022). Effects of herbal extracts (Foeniculum vulgare and Artemisia annua) on growth, liver antioxidant capacity, intestinal morphology and microorganism of juvenile largemouth bass. Micropterus salmoides. Aquacult. Rep. 23, 101081. doi: 10.1016/j.aqrep.2022.101081
Holland M. C. H., Lambris J. D. (2002). The complement system in teleosts. Fish. Shellfish Immunol. 12, 399–420. doi: 10.1006/fsim.2001.0408
Hong S., Peddie S., Campos-Pérez J. J., Zou J., Secombes C. J. (2003). The effect of intraperitoneally administered recombinant IL-1β on immune parameters and resistance to Aeromonas salmonicida in the rainbow trout (Oncorhynchus mykiss). Dev. Comp. Immunol. 27, 801–812. doi: 10.1016/S0145-305X(03)00056-9
Hoseinifar S. H., Shakouri M., Van Doan H., Shafiei S., Yousefi M., Raeisi M., et al. (2020b). Dietary supplementation of lemon verbena (Aloysia citrodora) improved immunity, immune-related genes expression and antioxidant enzymes in rainbow trout (Oncorrhyncus mykiss). Fish. Shellfish Immunol. 99, 379–385. doi: 10.1016/j.fsi.2020.02.006
Hoseinifar S. H., Shakouri M., Yousefi S., Van Doan H., Shafiei S., Yousefi M., et al. (2020c). Humoral and skin mucosal immune parameters, intestinal immune related genes and antioxidant defense in rainbow trout (Oncorhynchus mykiss) fed dietary olive (Olea europea l.) waste. Fish. Shellfish Immunol. 100, 171–178. doi: 10.1016/j.fsi.2020.02.067
Hoseinifar S. H., Shakouri M., Yousefi S., Van Doan H., Shafiei S., Yousefi M., et al. (2020d). Humoral and skin mucosal immune parameters, intestinal immune related genes expression and antioxidant defense in rainbow trout (Oncorhynchus mykiss) fed olive (Olea europea l.) waste. Fish. Shellfish Immunol. 100, 171–178. doi: 10.1016/j.fsi.2020.02.067
Hoseinifar S. H., Sun Y.-Z., Zhou Z., Van Doan H., Davies S. J., Harikrishnan R. (2020a). Boosting immune function and disease bio-control through environment-friendly and sustainable approaches in finfish aquaculture: herbal therapy scenarios. Rev. Fish. Sci. Aquacult. 28, 1–19. doi: 10.1080/23308249.2020.1731420
Hoseinifar S. H., Van Doan H., Dadar M., Ringø E., Harikrishnan R. (2019). “Feed additives, gut microbiota, and health in finfish aquaculture,” in Microbial communities in aquaculture ecosystems: Improving productivity and sustainability. Ed. Derome N. (Cham: Springer International Publishing), 121–142.
Hoseini S. M., Khosraviani K., Hosseinpour Delavar F., Arghideh M., Zavvar F., Hoseinifar S. H., et al. (2022b). Hepatic transcriptomic and histopathological responses of common carp, Cyprinus carpio, to copper and microplastic exposure. Mar. Pollut. Bull. 175, 113401. doi: 10.1016/j.marpolbul.2022.113401
Hoseini S. M., Rajabiesterabadi H., Abbasi M., Khosraviani K., Hoseinifar S. H., Van Doan H. (2022a). Modulation of humoral immunological and antioxidant responses and gut bacterial community and gene expression in rainbow trout, Oncorhynchus mykiss, by dietary lactic acid supplementation. Fish. Shellfish Immunol. 125, 26–34. doi: 10.1016/j.fsi.2022.04.038
Hoseini S. M., Sinha R., Fazel A., Khosraviani K., Hosseinpour Delavar F., Arghideh M., et al. (2021). Histopathological damage and stress- and immune-related genes’ expression in the intestine of common carp, Cyprinus carpio exposed to copper and polyvinyl chloride microparticle. J. Exp. Zool. Part A: Ecol. Integr. Physiol. 337, 181–190. doi: 10.1002/jez.2555
Hoseini S. M., Tarkhani R. (2013). Effect of short-term treatment with potassium permanganate on stress markers and blood biochemistry in goldfish Carassius auratus. Aquac. Res 44, 869–875. doi: 10.1111/j.1365-2109.2012.03091.x
Ibrahim D., Kishawy A. T. Y., Khater S. I., Khalifa E., Ismail T. A., Mohammed H. A., et al. (2021). Interactive effects of dietary quercetin nanoparticles on growth, flesh antioxidant capacity and transcription of cytokines and Aeromonas hydrophila quorum sensing orchestrating genes in Nile tilapia (Oreochromis niloticus). Fish. Shellfish Immunol. 119, 478–489. doi: 10.1016/j.fsi.2021.10.034
Jeney G., De Wet L., Jeney Z., Yin G. (2015). “Plant extracts,” in Dietary nutrients, additives, and fish health. Eds. Lee C.-S., Lim C., Webster C. D. (NJ, USA: Wiley-Blackwell), 321–333.
Jinendiran S., Nathan A. A., Ramesh D., Vaseeharan B., Sivakumar N. (2019). Modulation of innate immunity, expression of cytokine genes and disease resistance against Aeromonas hydrophila infection in goldfish (Carassius auratus) by dietary supplementation with exiguobacterium acetylicum S01. Fish. Shellfish Immunol. 84, 458–469. doi: 10.1016/j.fsi.2018.10.026
Kizil S., HaŞİMİ N., Tolan V., KilinÇ E., KarataŞ H. (2010). Chemical composition, antimicrobial and antioxidant activities of hyssop (Hyssopus officinalis l.) essential oil. Notulae botanicae horti. agrobot. cluj-napoca 38, 99–103. doi: 10.15835/nbha3834788
Lahav D., Eyngor M., Hurvitz A., Ghittino C., Lublin A., Eldar A. (2004). Streptococcus iniae type II infections in rainbow trout Oncorhynchus mykiss. dis. Aquat. Organ. 62, 177–180. doi: 10.3354/dao062177
LaPatra S. E., Plant K. P., Alcorn S., Ostland V., Winton J. (2010). An experimental vaccine against Aeromonas hydrophila can induce protection in rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish. Dis. 33, 143–151. doi: 10.1111/j.1365-2761.2009.01098.x
Lee C.-S., Lim C., Webster C. D. (2015). Dietary nutrients, additives, and fish health (NJ, USA: Wiley-Blackwell).
Lim K. C., Yusoff F. M., Shariff M., Kamarudin M. S., Nagao N. (2019). Dietary supplementation of astaxanthin enhances hemato-biochemistry and innate immunity of Asian seabass, Lates calcarifer (Bloch 1790). Aquaculture 512, 734339. doi: 10.1016/j.aquaculture.2019.734339
Lin S., Mao S., Guan Y., Lin X., Luo L. (2012). Dietary administration of chitooligosaccharides to enhance growth, innate immune response and disease resistance of Trachinotus ovatus. Fish. Shellfish Immunol. 32, 909–913. doi: 10.1016/j.fsi.2012.02.019
Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2– ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Lumsangkul C., Vu Linh N., Chaiwan F., Abdel-Tawwab M., Dawood M. A. O., Faggio C., et al. (2022). Dietary treatment of Nile tilapia (Oreochromis niloticus) with aquatic fern (Azolla caroliniana) improves growth performance, immunological response, and disease resistance against Streptococcus agalactiae cultured in bio-floc system. Aquacult. Rep. 24, 101114. doi: 10.1016/j.aqrep.2022.101114
Machado M., Azeredo R., Díaz-Rosales P., Afonso A., Peres H., Oliva-Teles A., et al. (2015). Dietary tryptophan and methionine as modulators of European seabass (Dicentrarchus labrax) immune status and inflammatory response. Fish. Shellfish Immunol. 42, 353–362. doi: 10.1016/j.fsi.2014.11.024
Martos-Sitcha J. A., Mancera J. M., Prunet P., Magnoni L. J. (2020). Editorial: Welfare and stressors in fish: Challenges facing aquaculture. Front. Physiol. 11. doi: 10.3389/fphys.2020.00162
Meng X., Hu W., Wu S., Zhu Z., Lu R., Yang G., et al. (2019). Chinese Yam peel enhances the immunity of the common carp (Cyprinus carpio l.) by improving the gut defence barrier and modulating the intestinal microflora. Fish. Shellfish Immunol. 95, 528–537. doi: 10.1016/j.fsi.2019.10.066
Michalczyk M., Macura R., Tesarowicz I., Banaś J. (2012). Effect of adding essential oils of coriander (Coriandrum sativum l.) and hyssop (Hyssopus officinalis l.) on the shelf life of ground beef. Meat Sci. 90, 842–850. doi: 10.1016/j.meatsci.2011.11.026
Midhun S. J., Arun D., Edatt L., Sruthi M. V., Thushara V. V., Oommen O. V., et al. (2016). Modulation of digestive enzymes, GH, IGF-1 and IGF-2 genes in the teleost, tilapia (Oreochromis mossambicus) by dietary curcumin. Aquac. Int. 24, 1277–1286. doi: 10.1007/s10499-016-9984-1
Mohiseni M. (2017). Medicinal herbs, strong source of antioxidant in aquaculture: a mini review. Modern Appl. Pharm. Pharmacol. 1, 1–5. doi: 10.31031/MAPP.2017.01.000504
Newaj-Fyzul A., Austin B. (2015). Probiotics, immunostimulants, plant products and oral vaccines, and their role as feed supplements in the control of bacterial fish diseases. J. Fish. Dis. 38, 937–955. doi: 10.1111/jfd.12313
Noor-Ul H., Haokun L., Junyan J., Xiaoming Z., Dong H., Yunxia Y., et al. (2020). Dietary supplementation of Geotrichum candidum improves growth, gut microbiota, immune-related gene expression and disease resistance in gibel carp CAS III (Carassius auratus gibelio). Fish. Shellfish Immunol. 99, 144–153. doi: 10.1016/j.fsi.2020.02.001
Nya E. J., Austin B. (2009a). Use of garlic, Allium sativum, to control Aeromonas hydrophila infection in rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish. Dis. 32, 963–970. doi: 10.1111/j.1365-2761.2009.01100.x
Nya E. J., Austin B. (2009b). Use of dietary ginger, Zingiber officinale Roscoe, as an immunostimulant to control Aeromonas hydrophila infections in rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish. Dis. 32, 971–977. doi: 10.1111/j.1365-2761.2009.01101.x
Nya E. J., Austin B. (2011). Development of immunity in rainbow trout (Oncorhynchus mykiss, walbaum) to aeromonas hydrophila after the dietary application of garlic. Fish. Shellfish Immunol. 30, 845–850. doi: 10.1016/j.fsi.2011.01.008
Oskoii S. B., Kohyani A. T., Parseh A., Salati A. P., Sadeghi E. (2012). Effects of dietary administration of Echinacea purpurea on growth indices and biochemical and hematological indices in rainbow trout (Oncorhynchus mykiss) fingerlings. Fish. Physiol. Biochem. 38, 1029–1034. doi: 10.1007/s10695-011-9587-8
Pérez-Sánchez T., Balcázar J. L., Merrifield D. L., Carnevali O., Gioacchini G., de Blas I., et al. (2011). Expression of immune-related genes in rainbow trout (Oncorhynchus mykiss) induced by probiotic bacteria during Lactococcus garvieae infection. Fish. Shellfish Immunol. 31, 196–201. doi: 10.1016/j.fsi.2011.05.005
Phukan B., Talukdar A., Kalita R., Nath B. B., Sharma N., Mir I. N., et al. (2022). Effects of dietary Leucas aspera levels on growth performance, nutrient utilization, digestive enzymes and physio-metabolic and health status of bagrid catfish, Rita rita (Hamilton 1822). Aquac. Res. 53, 22–35. doi: 10.1111/are.15549
Piazzon M. C., Savelkoul H. F. J., Pietretti D., Wiegertjes G. F., Forlenza M. (2015). Carp Il10 has anti-inflammatory activities on phagocytes, promotes proliferation of memory t cells, and regulates b cell differentiation and antibody secretion. J. Immunol. 194, 187. doi: 10.4049/jimmunol.1402093
Raida M. K., Buchmann K. (2008). Development of adaptive immunity in rainbow trout, Oncorhynchus mykiss (Walbaum) surviving an infection with Yersinia ruckeri. Fish. Shellfish Immunol. 25, 533–541. doi: 10.1016/j.fsi.2008.07.008
Rezaei Savadkouhi N., Ariaii P., Charmchian Langerodi M. (2020). The effect of encapsulated plant extract of hyssop (Hyssopus officinalis l.) in biopolymer nanoemulsions of lepidium perfoliatum and Orchis mascula on controlling oxidative stability of soybean oil. Food Sci. Nutr. 8, 1264–1271. doi: 10.1002/fsn3.1415
Rocha-Santos C., Bastos F. F., Dantas R. F., Hauser-Davis R. A., Rodrigues L. C., Cunha Bastos V. L. F., et al. (2018). Glutathione peroxidase and glutathione s-transferase in blood and liver from a hypoxia-tolerant fish under oxygen deprivation. Ecotoxicol. Environ. Saf. 163, 604–611. doi: 10.1016/j.ecoenv.2018.06.089
Rufchaei R., Mirvaghefi A., Hoseinifar S. H., Valipour A., Nedaei S. (2020). Effects of dietary administration of water hyacinth (Eichhornia crassipes) leaves extracts on innate immune parameters, antioxidant defence and disease resistance in rainbow trout (Oncorhynchus mykiss). Aquaculture 515, 734533. doi: 10.1016/j.aquaculture.2019.734533
Saeidi Asl M. R., Adel M., Caipang C. M. A., Dawood M. A. (2017). Immunological responses and disease resistance of rainbow trout (Oncorhynchus mykiss) juveniles following dietary administration of stinging nettle (Urtica dioica). Fish. Shellfish Immunol. 71, 230–238. doi: 10.1016/j.fsi.2017.10.016
Sayed A.E.-D.H., Hamed H. S. (2017). Induction of apoptosis and DNA damage by 4-nonylphenol in African catfish (Clarias gariepinus) and the antioxidant role of Cydonia oblonga. ecotoxicol. Environ. Saf. 139, 97–101. doi: 10.1016/j.ecoenv.2017.01.024
Serrano P. H. (2005). Responsible use of antibiotics in aquaculture (Roma, Italy: Food & Agriculture Org.).
Sheikh Z. A., Ahmed I. (2020). Effect of sex on hematology, morphology and blood cell characteristics of Schizothorax niger. comp. Clin. Path. 29, 1069–1078. doi: 10.1007/s00580-020-03153-5
Shi Q., Xiong X., Wen Z., Qin C., Li R., Zhang Z., et al. (2022). Cu/Zn superoxide dismutase and catalase of Yangtze sturgeon, Acipenser dabryanus: Molecular cloning, tissue distribution and response to fasting and refeeding. Fishes 7, 35. doi: 10.3390/fishes7010035
Shoemaker C., Xu D.-H., LaFrentz B., LaPatra S. (2015). “Overview of fish immune system and infectious diseases,” in Dietary nutrients, additives, and fish health. Eds. Lee C.-S., Lim C., Gatlin III, D., Webster C. D. (NJ, USA: Wiley-Blackwell), 1–24.
Siwicki A., Anderson D. (1993). “Nonspecific defense mechanisms assay in fish: II. potential killing activity of neutrophils and macrophages, lysozyme activity in serum and organs and total immunoglobulin level in serum,” in Fish disease diagnosis and prevention methods. Eds. Siwicki A., Anderson D., Waluga J. (Olsztyn, Poland: Wydawnictwo Instytutu Rybactwa Srodladowego), 105–112.
Soleimani H., Barzegar M., Sahari M. A., Naghdi Badi H. (2011). An investigation on the antioxidant activities of Hyssopus officinalis l. and Echinacea purpurea l. plant extracts in oil model system. J. med. Plants 10, 61–72. doi: 20.1001.1.2717204.2011.10.37.8.9
Soltanian S., Fereidouni M. S. (2016). Effect of henna (Lawsonia inermis) extract on the immunity and survival of common carp, Cyprinus carpio infected with Aeromonas hydrophila. Int. Aquat. Res. 8, 247–261. doi: 10.1007/s40071-016-0141-2
Sun Z., Tan X., Ye H., Zou C., Ye C., Wang A. (2018). Effects of dietary Panax notoginseng extract on growth performance, fish composition, immune responses, intestinal histology and immune related genes expression of hybrid grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀) fed high lipid diets. Fish. Shellfish Immunol. 73, 234–244. doi: 10.1016/j.fsi.2017.11.007
Taechavasonyoo A., Hirono I., Kondo H. (2013). The immune-adjuvant effect of Japanese flounder Paralichthys olivaceus IL-1β. Dev. Comp. Immunol. 41, 564–568. doi: 10.1016/j.dci.2013.07.003
Taheri Mirghaed A., Hoseini S. M., Hoseinifar S. H., Van Doan H. (2020). Effects of dietary thyme (Zataria multiflora) extract on antioxidant and immunological responses and immune-related gene expression of rainbow trout (Oncorhynchus mykiss) juveniles. Fish. Shellfish Immunol. 106, 502–509. doi: 10.1016/j.fsi.2020.08.002
Talpur A. D., Ikhwanuddin M. (2013). Azadirachta indica (neem) leaf dietary effects on the immunity response and disease resistance of Asian seabass, Lates calcarifer challenged with Vibrio harveyi. Fish. Shellfish Immunol. 34, 254–264. doi: 10.1016/j.fsi.2012.11.003
Talpur A. D., Ikhwanuddin M., Bolong A.-M. A. (2013). Nutritional effects of ginger (Zingiber officinale Roscoe) on immune response of Asian sea bass, Lates calcarifer (Bloch) and disease resistance against Vibrio harveyi. Aquaculture 400, 46–52. doi: 10.1016/j.aquaculture.2013.02.043
Terzİoğlu S., Dİler Ö. (2016). Effect of dietary sage (Salvia officinalis l.), licorice root (Glycyrrhize glabra l.), blueberry (Vaccinium myrtillus l.) and echinaceae (Echinacea angustifolia hell) on nonspecific immunity and resistance to Vibrio anguillarum infection in rainbow trout,(Oncorhynchus mykiss). Süleyman Demirel Üniversitesi Eğirdir Su Ürünleri Fakültesi Dergisi. 12, 110–118. doi: 10.22392/egirdir.284921
Torres-Corral Y., Santos Y. (2021). Development of a real-time PCR assay for detection and quantification of Streptococcus iniae using the lactate permease gene. J. Fish. Dis. 44, 53–61. doi: 10.1111/jfd.13267
Tsai Y.-H., Chen P.-H., Yu P.-A., Chen C.-L., Kuo L. T., Huang K.-C. (2019). A multiplex PCR assay for detection of vibrio vulnificus, aeromonas hydrophila, methicillin-resistant Staphylococcus aureus, Streptococcus pyogenes, and Streptococcus agalactiae from the isolates of patients with necrotizing fasciitis. Int. J. Infect. Dis. 81, 73–80. doi: 10.1016/j.ijid.2019.01.037
van der Marel M., Adamek M., Gonzalez S. F., Frost P., Rombout J. H., Wiegertjes G. F., et al. (2012). Molecular cloning and expression of two β-defensin and two mucin genes in common carp (Cyprinus carpio l.) and their up-regulation after β-glucan feeding. Fish. Shellfish Immunol. 32, 494–501. doi: 10.1016/j.fsi.2011.12.008
Wangkahart E., Wachiraamonloed S., Lee P.-T., Subramani P. A., Qi Z., Wang B. (2022). Impacts of Aegle marmelos fruit extract as a medicinal herb on growth performance, antioxidant and immune responses, digestive enzymes, and disease resistance against Streptococcus agalactiae in Nile tilapia (Oreochromis niloticus). Fish. Shellfish Immunol. 120, 402–410. doi: 10.1016/j.fsi.2021.11.015
Wang F., Liu H., Liu F., Chen W. (2020). Effects of Chinese yam (Dioscorea oppositifolia l.) dietary supplementation on intestinal microflora, digestive enzyme activity and immunity in rainbow trout (Oncorhynchus mykiss). Aquac. Res. 51, 4698–4712. doi: 10.1111/are.14815
Wei H. C., Xing S. J., Chen P., Wu X. F., Gu X., Luo L., et al. (2020). Plant protein diet-induced hypoimmunity by affecting the spiral valve intestinal microbiota and bile acid enterohepatic circulation in amur sturgeon (Acipenser schrenckii). Fish. Shellfish Immunol. 106, 421–430. doi: 10.1016/j.fsi.2020.08.025
Xu A., Shang-Guan J., Li Z., Gao Z., Huang Y. C., Chen Q. (2020). Effects of dietary Chinese herbal medicines mixture on feeding attraction activity, growth performance, nonspecific immunity and digestive enzyme activity of Japanese seabass (Lateolabrax japonicus). Aquacult. Rep. 17, 100304. doi: 10.1016/j.aqrep.2020.100304
Yano T. (1992). “Assays of hemolytic complement activity,” in Techniques in fish immunology. Ed. Stolen J. S. (Fair haven: SOS publication), 131–141.
Yousefi M., Adineh H., Reverter M., Hamidi M. K., Vatnikov Y. A., Kulikov E. V., et al. (2020). Protective effects of black seed (Nigella sativa) diet supplementation in common carp (Cyprinus carpio) against immune depression, oxidative stress and metabolism dysfunction induced by glyphosate. Fish. Shellfish Immunol. 109, 12–19. doi: 10.1016/j.fsi.2020.11.032
Zemheri-Navruz F., Acar Ü., Yılmaz S. (2020). Dietary supplementation of olive leaf extract enhances growth performance, digestive enzyme activity and growth related genes expression in common carp Cyprinus carpio. Gen. Comp. Endocrinol. 296, 113541. doi: 10.1016/j.ygcen.2020.113541
Zhang S., Xu Q., Boscari E., Du H., Qi Z., Li Y., et al. (2018). Characterization and expression analysis of g- and c-type lysozymes in dabry’s sturgeon (Acipenser dabryanus). Fish. Shellfish Immunol. 76, 260–265. doi: 10.1016/j.fsi.2018.03.006
Keywords: herbal additives, fish nutrition, intestinal health, intestinal genes, hepatic antioxidant capacity
Citation: Yousefi M, Hoseini SM, Abtahi B, Vatnikov YA, Kulikov EV and Rodionova NY (2022) Effects of dietary methanolic extract of hyssop, Hyssopus officinalis, on growth performance, hepatic antioxidant, humoral and intestinal immunity, and intestinal bacteria of rainbow trout, Oncorhynchus mykiss. Front. Mar. Sci. 9:1026651. doi: 10.3389/fmars.2022.1026651
Received: 24 August 2022; Accepted: 14 September 2022;
Published: 31 October 2022.
Edited by:
Miquel Planas, Spanish National Research Council (CSIC), SpainReviewed by:
Mohsen Abdel-Tawwab, Agricultural Research Center, EgyptHamed Paknejad, Gorgan University of Agricultural Sciences and Natural Resources, Iran
Copyright © 2022 Yousefi, Hoseini, Abtahi, Vatnikov, Kulikov and Rodionova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Morteza Yousefi, bXlvdXNlZmk4MUBnbWFpbC5jb20=; Seyyed Morteza Hoseini, c2V5eWVkbW9ydGV6YS5ob3NlaW5pQGdtYWlsLmNvbQ==
 Morteza Yousefi
Morteza Yousefi Seyyed Morteza Hoseini
Seyyed Morteza Hoseini Behrooz Abtahi3
Behrooz Abtahi3
