- 1Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand
- 2Department of Pharmaceutical Science, Faculty of Pharmacy, Chiang Mai University, Chiang Mai, Thailand
- 3Environmental Science Research Centre, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand
- 4Research Center in Bioresources for Agriculture, Industry and Medicine, Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand
Almost all of the current hair dye products today contain synthetic chemicals which may cause allergic reactions in some users. Phycocyanin (PC), a non-toxic cyanobacterial pigment, has been used in the food and cosmetics sectors. There are however, been a few reports on the application of phycocyanin as a hair colorant. This study aimed to assess the biological qualities of phycocyanin for use in natural hair dye product. Phycocyanin was tested for use against anti skin-pathogen (Staphylococcus aureus ATCC 25923, Staphylococcus epidermidis ATCC 14990, Methicillin-resistant Staphylococcus aureus (MRSA) DMST 20625, Propionibacterium acnes DMST 14916, Candida albicans DMST 21424, and Malassezia furfur M21), cytotoxicity of human immortalized keratinocyte (HaCaT) cells and tested for color fastness when used as a shampoo wash. According to the findings, Arthrospira (Spirulina) platensis phycocyanin has not shown the potential for use against anti-skin pathogenic microorganisms. While testing phycocyanin at the maximum doses of 2.5 mg/mL, the cytotoxicity test revealed that it is not hazardous to HaCaT cells. Bleached hair was dyed with a mixture of phycocyanin, natural developers, and mordants. A chroma meter was used to monitor color changes after shampoo washing. The findings revealed that phycocyanin has dyeability potential. 50% of the dyed hair color remained after 5 shampoo washes. The stability and color degradation of phycocyanin in hair dye powder formulation demonstrated good physical stability along with four cycles of heating/cooling. As a result, we can see that this pigment has the potential to be used as an active ingredient in natural hair dyes.
1 Introduction
Hair dyeing products are currently in high demand; they are mostly used to conceal gray hair or modify the color of hair (Guerra-Tapia and Gonzalez-Guerra, 2014). However, chemical oxidation dyeing generally requires the use of a strong base and oxidants, which can cause serious hair damage. Worldwide, highly sensitizing chemicals like para-phenylenediamine (PPD) and its derivatives are the most common causes of allergic contact dermatitis (Guerra-Tapia and Gonzalez-Guerra, 2014; Antelmi et al., 2017). In a study of 3,000 patients from 12 dermatological clinics in Denmark, it was discovered that 4.5% of patients tested positive for PPD, 2.8% for toluene-2, 5-diamine (PTD), 1.8% for p-aminophenol, 1% for m-aminophenol, and 0.1% for resorcinol (Antelmi et al., 2017). The primary patch test screening substance for contact allergies to hair dye is p-phenylenediamine. PPD contact allergy is very common on all continents. In the European population, PPD patch test positive rates ranged from 12.7% to 25.4%. Clinical relevance between hair dye use and allergy was about 23–73% in Asia, which was higher than in Europe (Krasteva et al., 2009). More research has revealed that certain hair dye components are more likely to include possibly carcinogenic ingredients and may induce contact allergies (Perveen et al., 2017), whereas natural dyes are more environmentally friendly, biodegradable, less allergic, and less toxic (Ali et al., 2009). Consumers are becoming more interested in using natural hair dyeing products as a result of a growing global awareness of the importance of using environmentally friendly products (Boonsong et al., 2012). Therefore, the development of new and novel natural based hair dyes is one of commercial interest.
Previous reports have described the use of plant pigment extracts in natural hair dye applications. For instance, Lawsonia inermis (Henna), Indigofera tinctoria (Indigo), Emblica officialis (Amla), Hibiscus rosasinensis (Shoe flower), and Juglans nigra (walnut hull) have also been documented in literature for use as natural hair dye sources (Shin and Lee, 2006; Komboonchoo and Bechtold, 2009). Thai plants, including Sappan tree, False daisy, Thao yanang, Kae lae, and turmeric extracts are yellowish, brownish, and greenish color. These extracts were applied for hair dye when mixed with natural developer and metal as a mordant agent. The appearance color gave a dark reddish-brown to orangish-brown color (Boonsong et al., 2012). Black beans, one of the highly anthocyanin contents, could be used in food and consumer products as well as hair dye colorant when extracted with hydrochloric ethanol and the color of dyed hair was maintained through four washings (Inman et al., 2020). Moreover, the fruit skin waste of blackcurrant from the pressing industry has potential in hair dye after acidified extraction and purification with a solid stage. The results of blackcurrant hair dye were stable to multiple washes (Rose et al., 2018). There is a patent for the use of spirulina extract as a colorant in hair dye applications (Zirwan and Kleen, 2009). These formulations include an oxidizing agent composition that includes at least one chemical oxidizing agent, particularly hydrogen peroxide. However, it has been shown that some hair dyes can cause increased skin irritation when used with hydrogen peroxide (Park et al., 2018).
Weak color and challenges with long-term deposition are difficulties when hair dyeing using natural dyes. This is because natural dyes are unable to permeate the hair deeply enough to protect the dyed hair from washing or fading (Inman et al., 2020). Some additives such as developer and mordant, can enhance the dyeability of hair. The dye molecules can penetrate deeper into the hair shaft and form bonds with hair proteins because the developer can dissolve chemical bonds and open the hair cuticle (Burkinshaw and Kumar, 2009). Hydrogen peroxide, the most frequently used developer or oxidizing ingredient for hair dye, might cause an allergic reaction (Harrison and Sinclair, 2003). Ascorbic acid, lime juice, and lemon juice have all been traditionally used as natural developers in hair products for purposes such as permanent waving and hair bleaching and dyeing. Likewise, mordanting promotes color fastness and fixes dye in hair fibers. The most popular metal salts used as mordant agents are ferrous sulfate, copper sulfate, and alum, although these substances are considered metal pollution sources (Beiki et al., 2018). Oxalic acid and tea water extract (tannin), which have the added benefit of being safe for human health, have also been employed as natural mordants for dyeing.
Cyanobacterial pigments such as carotenoids, chlorophylls, and phycobiliproteins have recently been applied in the manufacture of healthy products because of their pharmaceutical and fluorescent properties. Arthrospira (Spirulina) platensis is a helicoidal, filamentous blue-green cyanobacterium in the Oscillatoriaceae family. Arthrospira (Spirulina) platensis has long been used as a supplement in human and animal food, either as a health drink or in tablet form, due to its nutritional content (Flores et al., 2003). Arthrospira (Spirulina) platensis can be found in both freshwater and marine environments (Vonshak, 1997). Although Arthrospira (Spirulina) platensis cannot grow naturally in salt water, earlier reports indicate that with an adequate supply of bicarbonate, nitrogen, and phosphorus, as well as an appropriate pH and salinity, Arthrospira (Spirulina) platensis species can be produced in seawater (Materassi et al., 1984). Growing Arthrospira (Spirulina) platensis in natural seawater has economic benefits such as lower production costs due to reduced use of expensive potable water, which in turn could lower costs on an industrial scale for commercialization.
Phycocyanin (PC) can be extracted from Arthrospira (Spirulina) platensis, a water-soluble pigment protein complex from the light harvesting phycobiliprotein family, which is ideally suited as a fluorescent reagent for immunological analysis due to its broad excitation spectrum (Kuddus et al., 2013). Phycocyanin is an antenna pigment found in cyanobacteria and eukaryotic algae, consist with apoprotein conjugated with a linear tetrapyrrole (phycocyanobilin) by covalent bond (Padyana et al., 2001), that absorbs light energy and effectively transmits it to a reaction center containing chlorophyll to improve photosynthesis efficiency by collecting light energy at wavelengths that chlorophyll absorbs poorly (Glazer, 1982). Presently, phycocyanin is a natural dye that has replaced many synthetic dyes in food and cosmetics as it contains numerous bioactive compounds which have the potential to be used as some medicinal additives. Based on a number of experimental findings, PC is used as an adjuvant because of its several beneficial properties, including reactive oxygen species (ROS) inhibitory effects and it’s antimicrobial properties (Romay and Gonzalez, 2000). Furthermore, it has the capacity to slow down inflammatory processes (Reddy et al., 2000), including a decrease in nuclear factor activation in RAW 264.7 macrophages, which attenuated lipopolysaccharide-induced iNOS expression and TNF production. In vitro evidence of the suppression of COX-2 activity (Cherng et al., 2007).
However, the application of PC on hair dyeing colorants is yet to been fully investigated. Thus, the research aimed to study the feasibility of using phycocyanin as an alternative hair dye. For this, phycocyanin was evaluated for anti skin-pathogenic activities, as well as toxicity test of human immortalized keratinocyte cells (HaCaT), and the formulation of the hair dye using natural developers and mordants. Additionally, the dyeability of dyed hair after washing with shampoo was examined.
2 Materials and methods
2.1 Extraction of phycocyanin
Phycocyanin extraction was carried out by using a slightly modified version of the freezing and thawing method (Takano et al., 1973). First, two grams of dried Arthrospira (Spirulina) platensis biomass powder was suspended in 40 mL of sterilized distilled water. The cells were lysed freezing at -20°C for 4 h and thawing at 37°C for 30 min. This process was repeated 25 times. Cell debris was removed by centrifugation at 2800 rpm and 4°C for 10 min. After that, the solvent was removed from pigment extracts using a rotary evaporator and dehydration with a lyophilizer.
2.2 Determination of bioactivity against skin pathogenic microorganisms
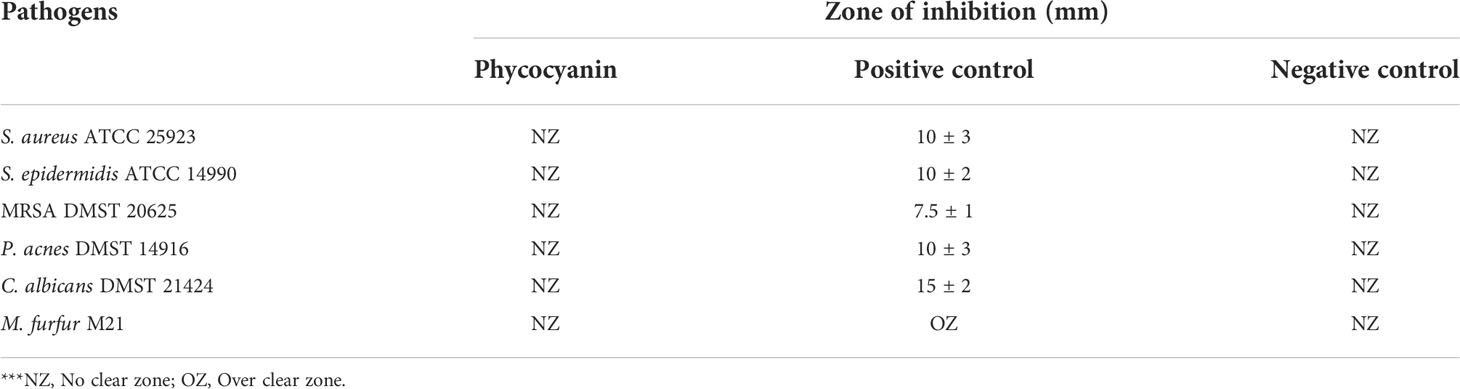
The phycocyanin was tested for antibacterial activity against six skin pathogenic microorganisms; Staphylococcus aureus ATCC 25923, Staphylococcus epidermidis ATCC 14990, Methicillin-resistant Staphylococcus aureus (MRSA) DMST 20625, Propionibacterium acnes DMST 14916, Candida albicans DMST 21424, and Malassezia furfur M21 in agreement with the agar well diffusion method described by (Balouiri et al., 2016 and Osman et al., 2015) with some modifications. The tested microorganisms were obtained from the SCB 2711 Microbiology Laboratory, Department of Biology, Faculty of Science, Chiang Mai University excluding Malassezia furfur M21 which was obtained from the Department of Microbiology, Faculty of Medicine, Chiang Mai University. Each species of bacteria was inoculated with a single colony that was transferred into a 10 mL of sterile culture broth (Mueller Hinton broth (MHB) for S. aureus ATCC 25923, S. epidermidis ATCC 14990, and MRSA DMST 20625; Brain Heart Infusion Broth (BHI) for P. acnes DMST 14916; Sabouraud Dextrose broth (SDB) for C. albicans DMST 21424; and Dixon broth for M. furfur M21) using a sterile loop. The broth cultures were incubated at 37°C for 24 h excluding P. acnes which was incubated at 37°C for 72 h in an anaerobic jar. The grown bacteria were adjusted to 0.5 McFarland standard turbidity and spread on agar plates using sterile cotton swabs then 5 wells were made using sterilized micropipette tips. In each well, 50 µL of 25 mg/mL phycocyanin (1.25 mg/well) was loaded including positive and negative control. After incubation at suitable conditions for each microorganism, a clear zone of inhibition (diameter) was measured in millimeters (mm). Treatments using sterilized distilled water as a negative control and antibiotic as a positive control (1 mg/mL Gentamycin for S. aureus ATCC 25923, S. epidermidis ATCC 14990, and P. acnes DMST 14916; 1mg/mL Doxycycline for MRSA DMST 20625; 0.5 mg/mL Nystatin for C. albicans; and 10 mg/mL Ketoconazole for M. furfur M21) were performed. Each experiment was performed three times.
2.3 Cytotoxicity
The MTT assay was used to assess the cytotoxicity of phycocyanin on human immortalized keratinocyte (HaCaT) as described by (Lee et al., 2013 and Imbimbo et al., 2019) with slight modification. HaCaT cells (CLS Dell Lines Service, 300493) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (GibcoTM) with 10% fetal bovine serum (GibcoTM), in a 5% CO2 humidified atmosphere at 37°C. HaCaT cells were seeded in 96 well plates with a density of 2 × 104 cells/well and incubated for 1 day in a 5% CO2 incubator at 37°C. Approximately 24 h after seeding, phycocyanin in deionized water was diluted with serum-free DMEM media (0.005-2.5 mg/mL) and then treated in HaCaT cells. The control was treated with serum-free DMEM media. Three days later, 30 µL of MTT (2 mg/mL in PBS, pH 7.4) (Bio Basic Inc., Markham, ON, Canada) was added to each well and the mixture was further incubated for 4 h. The formazan crystals were then solubilized with dimethyl sulfoxide (DMSO) (PanReac AppliChem) and the absorbance at 540 nm and 630 nm were determined using a microplate spectrophotometer (Biochrom, UK). Each experimental value was the mean value from three wells. Cell survival was expressed as the percentage of viable cells in the presence of the phycocyanin concentration compared with the control.
2.4 Phycocyanin hair dye formulation and color fastness with shampoo wash
2.4.1 Hair sample preparation
Black human hair for dye testing was purchased from a local salon in Chiang Mai, Thailand. Bleaching was done to lighten the hair so that color changes could be observed more clearly while using natural dyes. The hair was shampooed, air-dried, and then bleached using 6%(v/v) hydrogen peroxide (H2O2) mixed with 30% (w/v) potassium persulfate (K2S2O8) for 10 min. The proportion of hair sample to bleaching agent was 1:25 (w/v). The bleached black hair turned blonde. After that, the bleached hair was bundled the rubber, approximately 2 cm long and 0.2 g for each sample. It was then kept at 25°C ± 3 with a relative humidity of 70 ± 5% (Boonsong et al., 2012). The bleached hair was color assessed and used as a reference before being dyed with the new formula of phycocyanin hair dye. The color of the hair dyed sample was recorded in terms of CIELAB color system, which are internationally recognized as the standard method (Lozano et al., 2017).
2.4.2 Ingredients preparation
Hair dye consists of three components: colorant, developer, and mordants. Phycocyanin extracted from Arthrospira (Spirulina) platensis was using as a coloring agent. Hydrogen peroxide (H2O2) (The United Drug (1996) Co., Ltd.), ascorbic acid (Northeast Pharmaceutical Group Co., Ltd.), and 5% Chamomile tea (dried Chamomile flowers from a local market in Chiang Mai, Thailand) were used as developers. Alum (Ward william), 5% Assum tea (dried Assum tea leaves from a local market in Chiang Mai, Thailand), Arabic gum (Union Science Co., Ltd.), and Aloe vera extract (fresh Aloe vera leaves from a local market in Chiang Mai, Thailand) were used as mordants. 5% Chamomile tea and 5% Assum tea were freshly prepared. Briefly, 5 g of dried Chamomile flowers or dried Assum tea leaves were stirred and boiled with distilled water for 30 min, then cooled down at room temperature and the extracted was poured through a filter cloth (Boonsong et al., 2012). The Aloe vera extract was prepared by washing thoroughly and the outer green surface was peeled off and rinsed the gel twice to three times. Filtration was performed on 100 g of the Aloe vera gel was crushed to a semi-solid consistency which was subjected to filtration. The filtrate was subjected to evaporation to 1/10th of its volume under a controlled temperature (60°C), and stored in a clean container and kept at -20°C until ready for use (Packianathan and Karumbayaram, 2010).
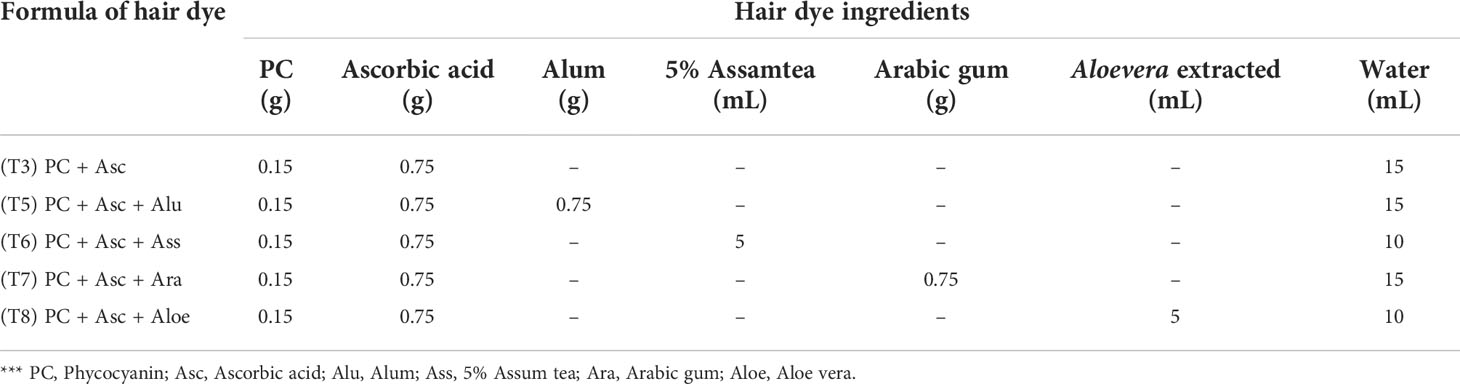
The formulation of hair dye is designed followed by previous of natural hair dye (Boonsong et al., 2012) with some modifications. Phycocyanin’s specific volume is based on its maximal solubility in water. In order to establish a developer for a new formula, phycocyanin hair dye containing the ingredients mentioned in Table 1 was initially produced.
Then, the hair dye mixtures were applied to the bleached hair for 1.30 h before being thoroughly cleansed with water and shampoo. The samples were air dried at 70 ± 5% RH. The color appearance of dyed hair was observed by the naked eyes and tested for the color coordinate values measured by a Chroma Meter (MINOLTA CR-300, JAPAN) as follows.
Where ΔL* is difference in lightness/darkness value (0-100; black to white), Δa* is differenced on red/green axis (-60 to +60; green to red), Δb* is differenced on yellow/blue axis (-60 to +60; blue to yellow), and ΔE*ab is total color difference values.
The best formula for developer identification was used to identify the mordant list in Table 2. After that, the hair dye mixtures were applied. The technique was completed as described previously. The concentration of mordant was listed in Table 3. Finally, the color fastness of shampoo wash was measured by comparing the total color difference values after repeating the shampoo wash at the first, fifth, and tenth time.
2.5 Stability testing and color degradation of phycocyanin in hair dye formulation
2.5.1 Stability test
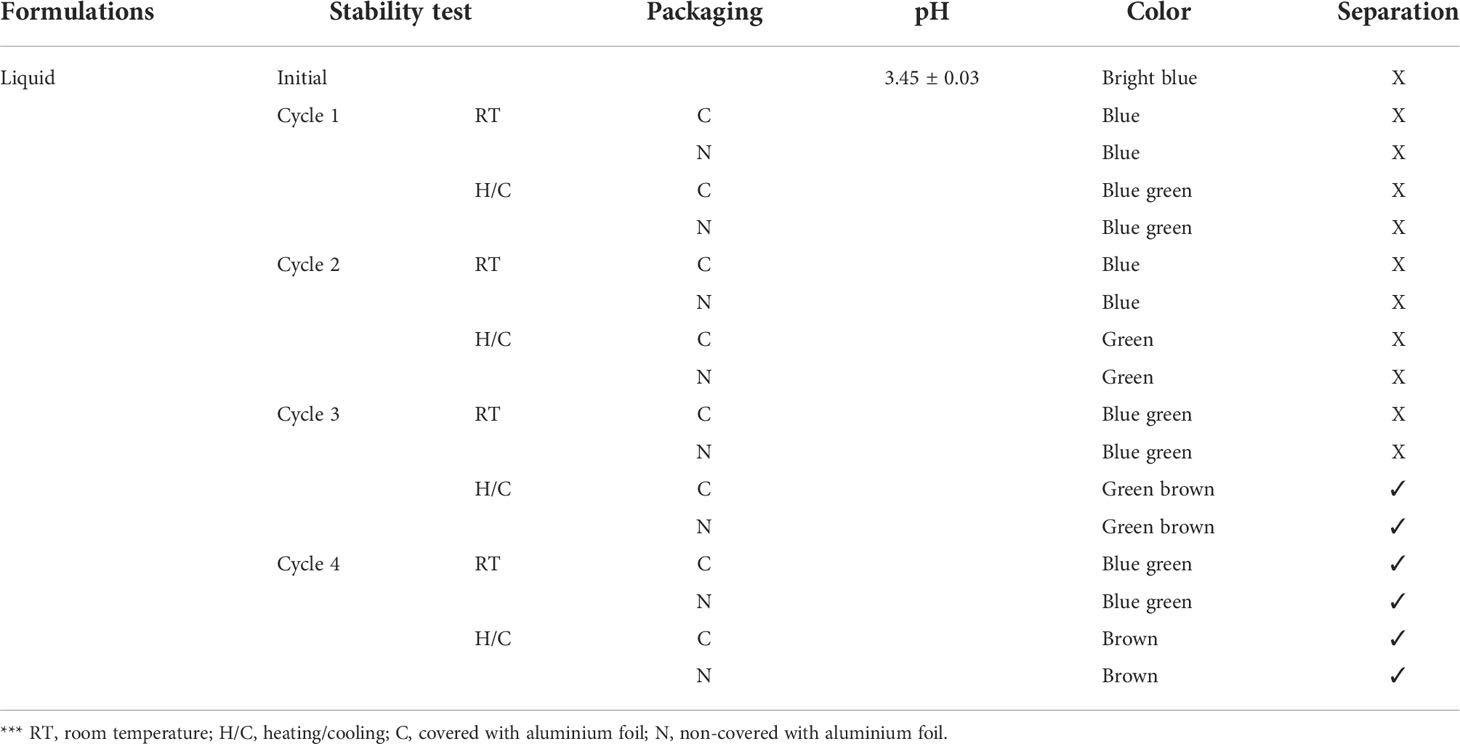
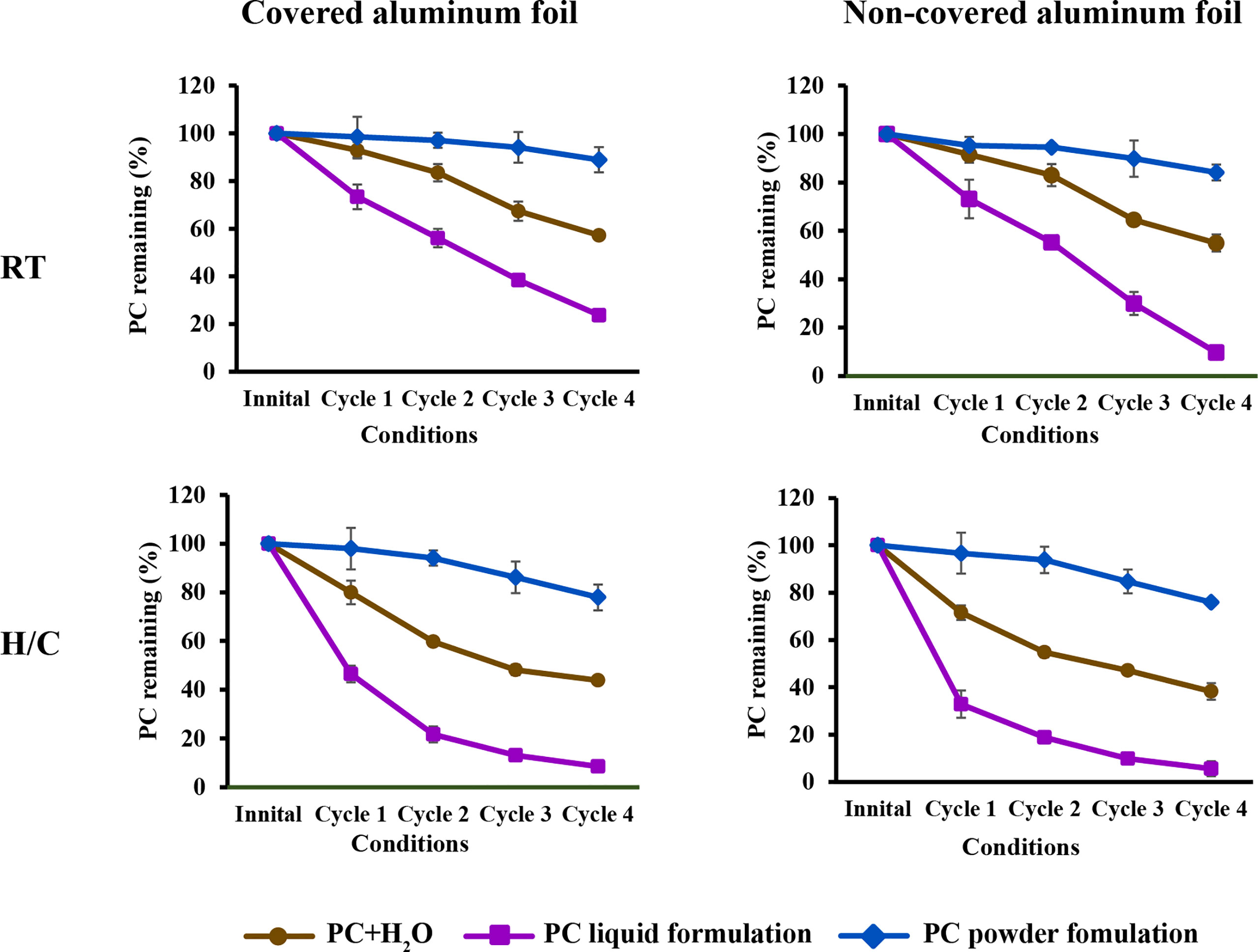
The stability of the phycocyanin hair dye formulation was investigated in liquid formulations (ready to use) and powder formulations (fresh mixing before use). They were kept in microcentrifuge tube and collected each formulation by covered with aluminium foil and non-covered with aluminium foil. After that incubated at room temperature and accelerated stability testing. The test was also performed in four cycles of the heating/cooling method (45°C, 48 h alternated with 4°C, 48 h for 1 cycle). The formulation’s appearance, including any variations in pH, color and phase separation, was examined (Whangsomnuek et al., 2019).
2.5.2 Color degradation
The color degradation of phycocyanin present in the hair dye formulation (liquid/powder) during storage was estimated in terms of the percentage of phycocyanin. The water soluble phycocyanin was collected in same condition and estimated the percentage of phycocyanin remaining to demonstrate the trend of color degradation in neutral solution. The phycocyanin concentration in the hair dye formulation was calculated using absorbance at 615 nm and 625 nm using a microplate spectrophotometer (Biochrom, UK) with the simultaneous equations of Bennett and Bogorad (1973).
The remaining phycocyanin quantity was calculated and compared with that of the initial concentration.
2.6 Statistical analysis
Three replicate analyses were performed, and the average results (mean standard deviation) were reported. Difference in significance values between treatments were assessed by one-way ANOVA using Duncan’s multiple range tests (p< 0.05). The SPSS statistical program (SPSS version 28.0 for Windows, SPSS Inc., Chicago, IL, https://www.ibm.com/products/spss-statistics) was used for the statistical study.
3 Results
3.1 Extraction of phycocyanin
The appearance of phycocyanin after lyophilization shows a bright blue color and fine powder that resembles flour (Figure 1).
3.2 Determination of bioactivity against skin pathogenic microorganisms
Phycocyanin did not show a clear zone on the surface of culture plates when tested for skin pathogenic microorganisms, as shown in Table 4.
3.3 Cytotoxicity
The in vitro cytotoxicity of phycocyanin was investigated in HaCaT cells at various concentrations from 2.5 mg/mL then two-fold dilution was performed 4 times. As demonstrated in Figure 2, phycocyanin was shown to be highly safe and non-toxic to the HaCaT cells at concentration that used from 0.156-2.5 mg/mL.
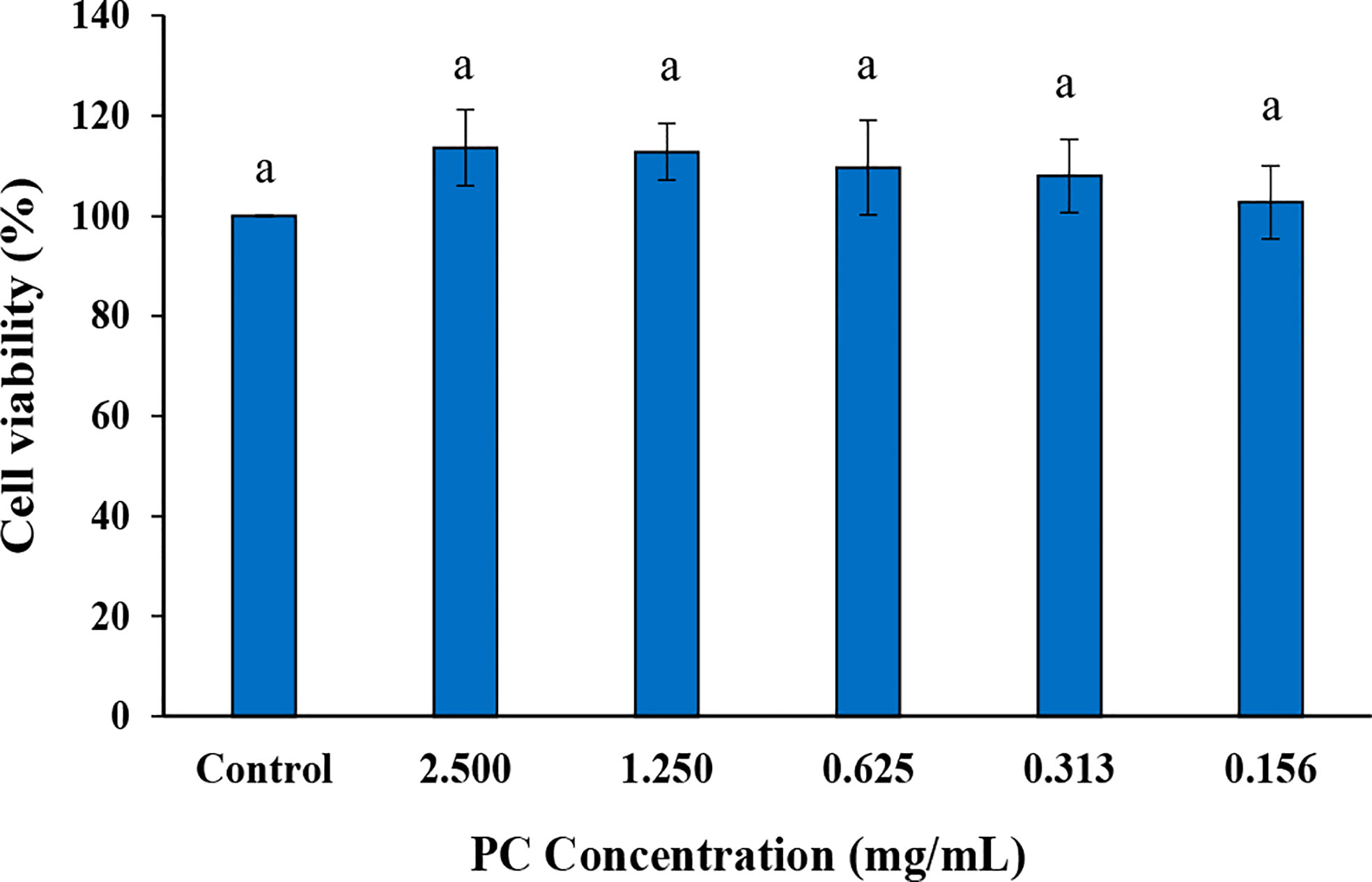
Figure 2 MTT cell viability assay of HaCaT cell treated with various concentrations of phycocyanin (PC). a,b,c Means followed by a different letter are significantly different in relation to Duncan’s Multiple Range Test (p < 0.05) of (%) cell viability.
3.4 Phycocyanin hair dye formulation and color fastness with shampoo wash
As shown in Figure 3, the color coordinate values (L*, a*, and b*) were significantly affected by the interaction of the phycocyanin hair dye compared with the control. T1; The phycocyanin powder was dissolved in water. It was discovered that the phycocyanin could make the hair slightly darker and increase the greenness and blueness of the dyed hair, as shown in Figure 4B. T2; 2%(v/v) of H2O2 was used as a dissolving agent and chemical developing agent in this formula. The color coordinate values of T2 demonstrated that this formula could increase the lightness, greenness, and blueness of dyed hair, as illustrated in Figure 4C. T3; 5%(w/v) of ascorbic acid was used as a dissolving and natural developing agent. This formula could make the hair a little darker than the control, with a considerable increase in greenness and blueness, as well as the highest difference in appearance as shown in Figure 4D. T4; Chamomile tea at 5%(v/v) was used as a dissolving and developing agent. The color coordinate values demonstrated that it could lighten the hair and also increased the value of greenness and blueness, Figure 4E. However, the total color difference was not significantly different from the formulas T1, T2.
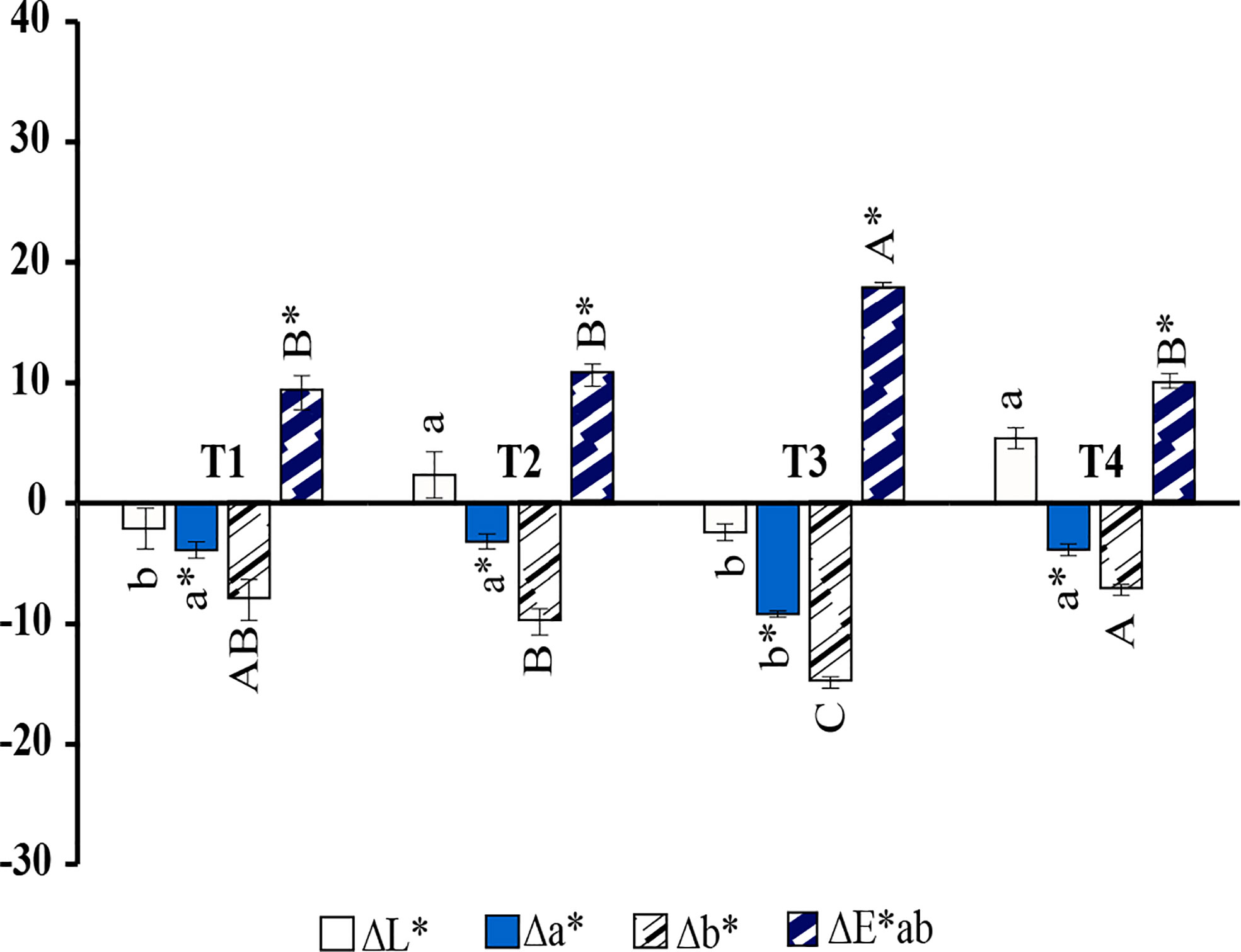
Figure 3 Different in color coordinate values compared with the bleached hair (control); lightness values (∆L*), redness (∆a*), yellowness (∆b*), and total difference (DE*ab) of hair dyed with (T1) PC + water, (T2) PC + 2% H2O2, (T3) PC + 5% ascorbic acid, (T4) PC + 5% Chamomile.a,b,c Means followed by a different letter are significantly different in relation to Duncan’s Multiple Range Test (p < 0.05) of lightness values (∆L*), a*,b*,c* means followed by a different letter are significantly different in relation to Duncan’s Multiple Range Test (p < 0.05) of redness (∆a*), A,B,C means followed by a different letter are significantly different in relation to Duncan’s Multiple Range Test (p < 0.05) of yellowness (∆b*), and A*,B*,C* means followed by a different letter are significantly different in relation to Duncan’s Multiple Range Test (p < 0.05) of total difference (∆E*ab).
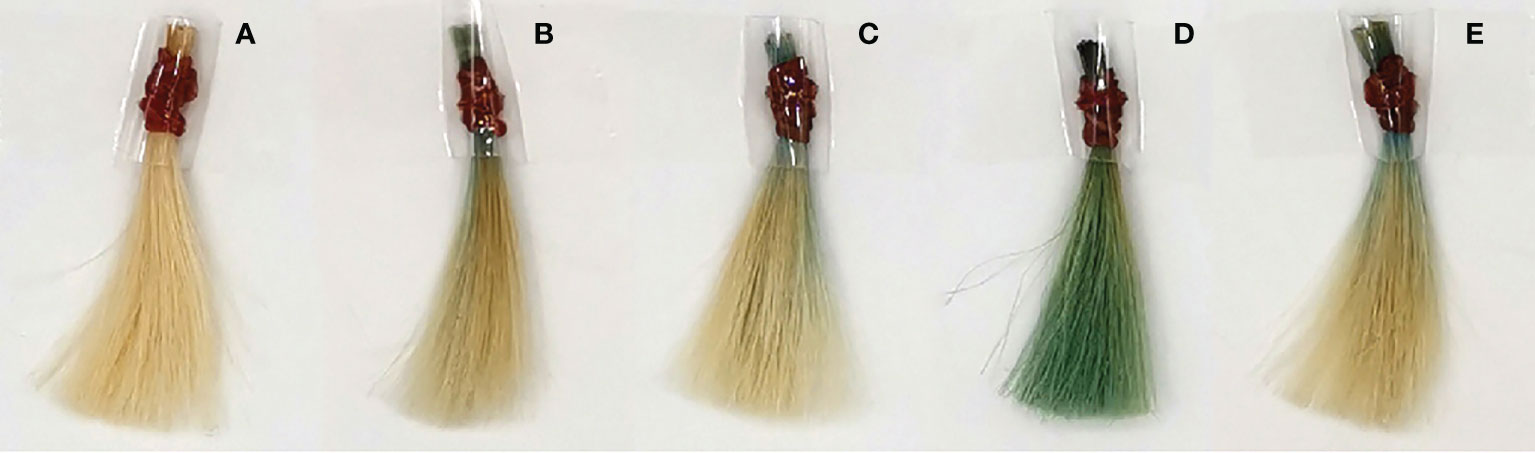
Figure 4 Hair samples after dyeing with phycocyanin and some developer. (A) bleached hair, (B) PC + water, (C) PC + 2% H2O2, (D) PC + 5% ascorbic acid, (E) PC + 5% Chamomile tea.
The color coordinate value and the color appearance of hair of the hair dyed with formula T3 show the highest significance compared to other formulas. To improve the phycocyanin hair dye, ascorbic acid was selected as the mordant in the next experiment.
Figure 5 shows the results of mordant when phycocyanin was used as a dye and ascorbic acid was used as a developing agent in the hair dye formula. T5; 5%(w/v) of alum was used as the mordant, which lightened the color and had a minor effect on the redness, but also slightly increased in the blueness value, as shown in Figure 6B. T6; 1.6%(W/V) of Assum tea could darkened the hue and enhanced greenness, blueness, and the effects were similar in formulas T7; 5%(w/v) of Arabic gum, and T8; 1.6%(W/V) of Aloe vera, which is shown in Figures 6C–E. However, when observing a higher Δb*, which represents the difference on the yellow/blue axis, formula T7 showed increased blueness. As a result, Arabic gum was chosen and optimized for a concentration in the next experiment.
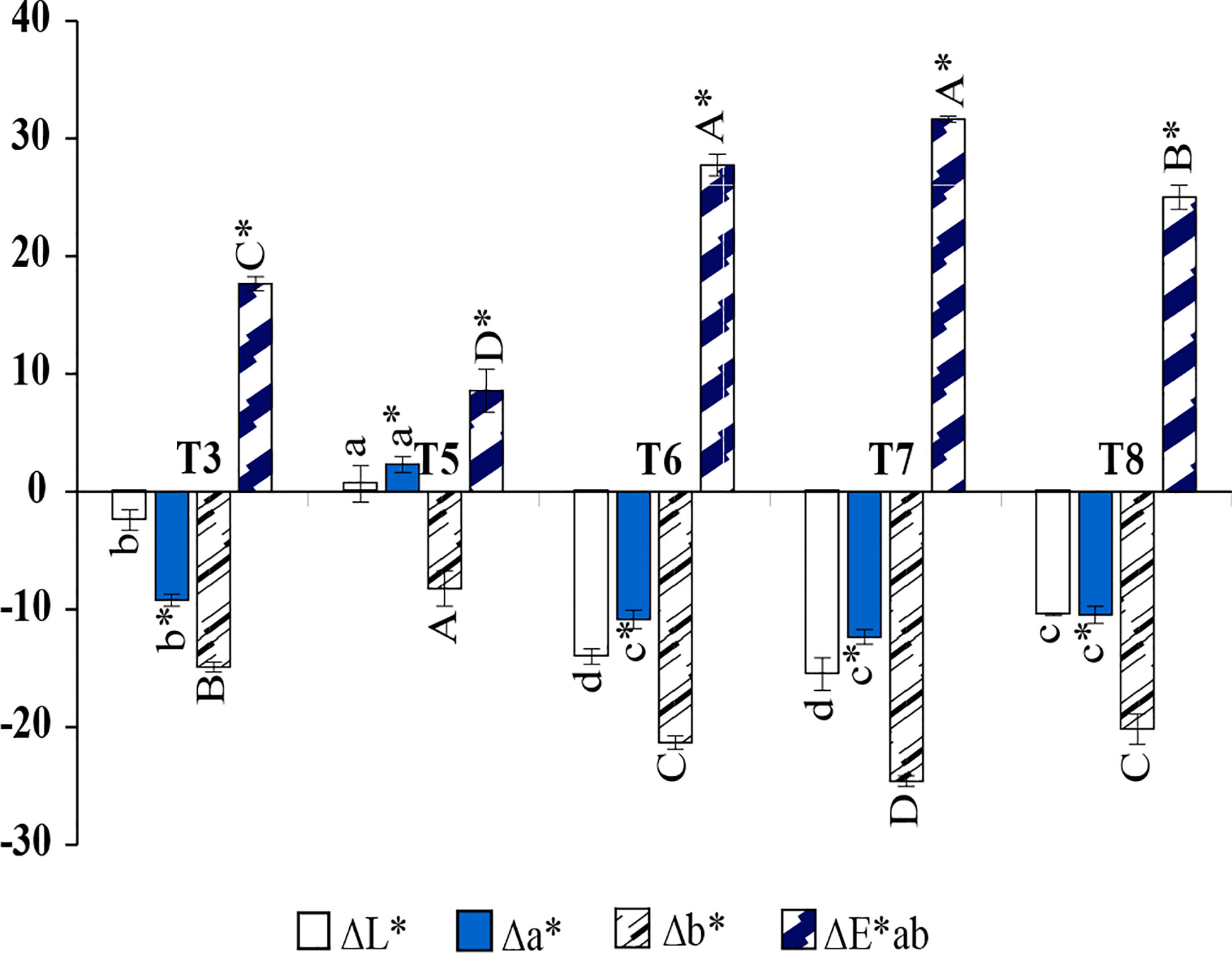
Figure 5 Different in color coordinate values compared with the bleached hair (control); lightness values (∆L*), redness (∆a*), yellowness (∆b*), and total difference (∆E*ab) of hair dyed with (T3) PC + Asc, (T5) PC + Asc + Alu, (T6) PC + Asc + Ass, (T7) PC + Asc + Ara, (T8) PC + Asc + Aloe. a,b,c Means followed by a different letter are significantly different in relation to Duncan’s Multiple Range Test (p < 0.05) of lightness values (∆L*), a*,b*,c* means followed by a different letter are significantly different in relation to Duncan’s Multiple Range Test (p < 0.05) of redness (∆a*), A,B,C means followed by a different letter are significantly different in relation to Duncan’s Multiple Range Test (p < 0.05) of yellowness (∆b*), and A*,B*,C* means followed by a different letter are significantly different in relation to Duncan’s Multiple Range Test (p < 0.05) of total difference (∆E*ab).
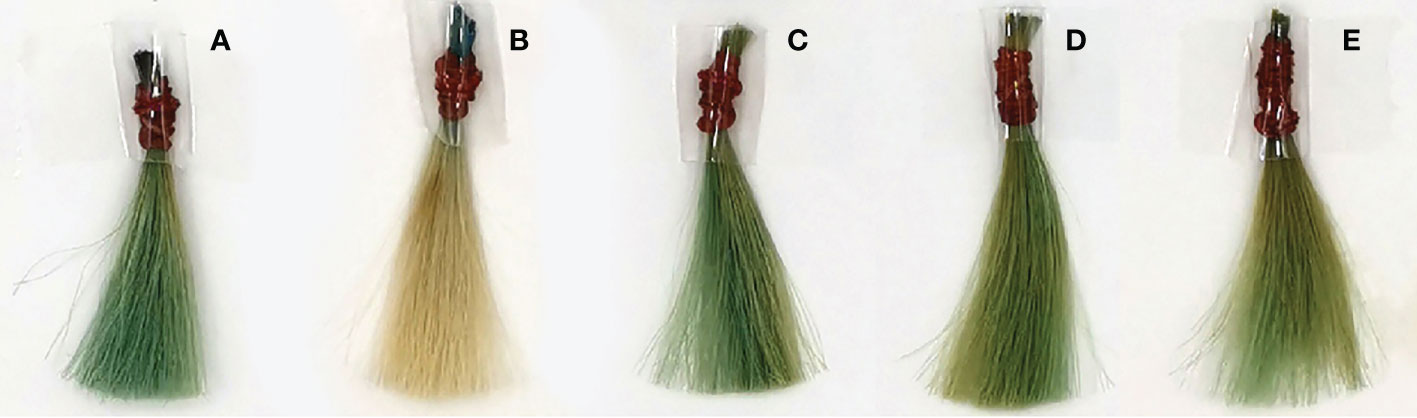
Figure 6 Hair samples after dyeing with phycocyanin ascorbic acid and some mordant. (A) PC + Asc, (B) PC + Asc + Alu, (C) PC + Asc + Ass, (D) PC + Asc + Ara, (E) PC + Asc + Aloe.
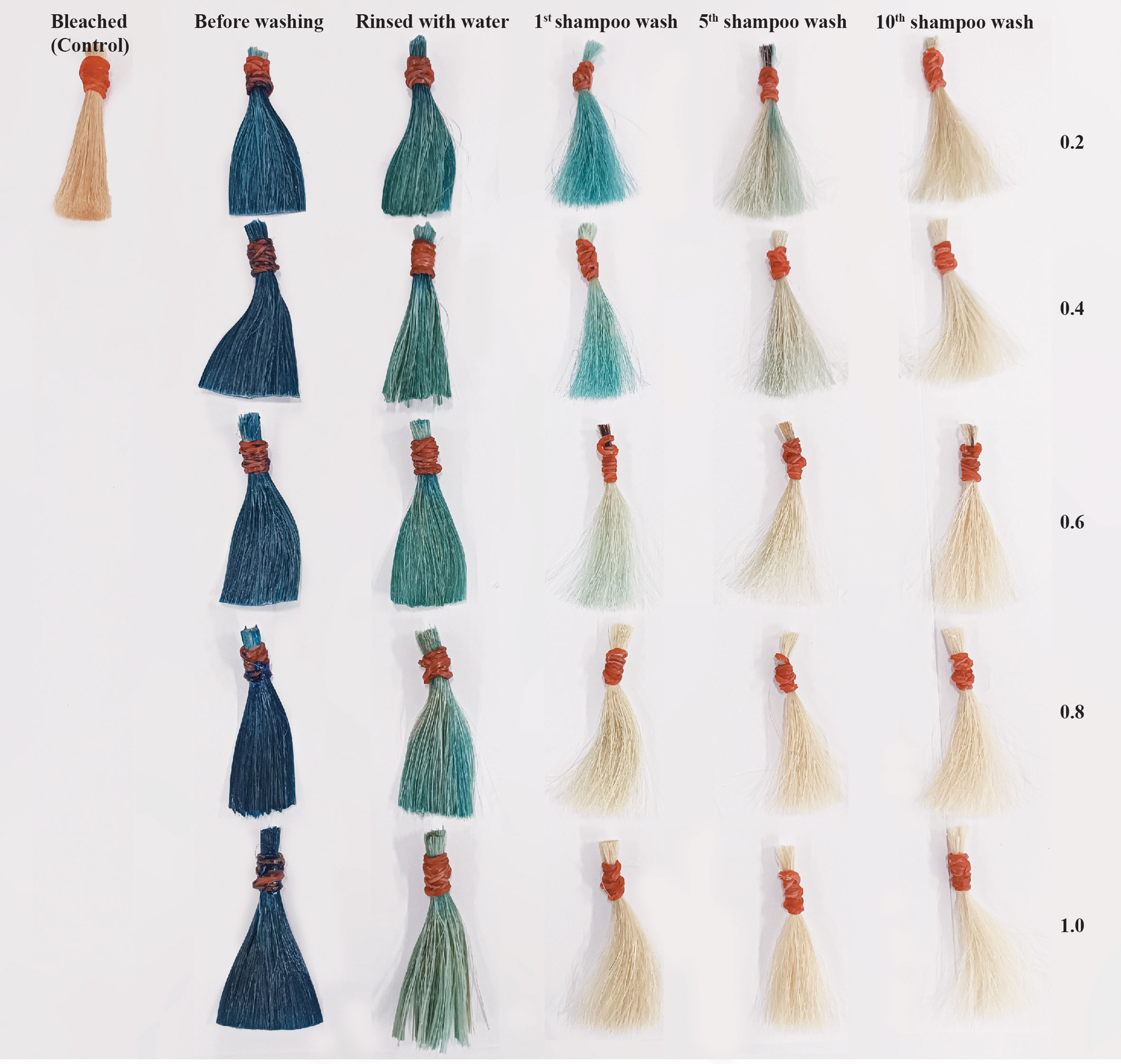
Figure 7 shows the results of various Arabic gum concentrations. The color difference is demonstrated in terms of ΔE*ab. The strongest color, which appeared on dyed hair in the first shampoo wash was equal to 42 when 0.2 g/mL of Arabic gum was added, and slightly dropped to 40 when added 0.4 g/mL, but moiety decreased when 0.6 g/mL of Arabic gum was added and gradually decreased as the concentration of Arabic gum was increased.
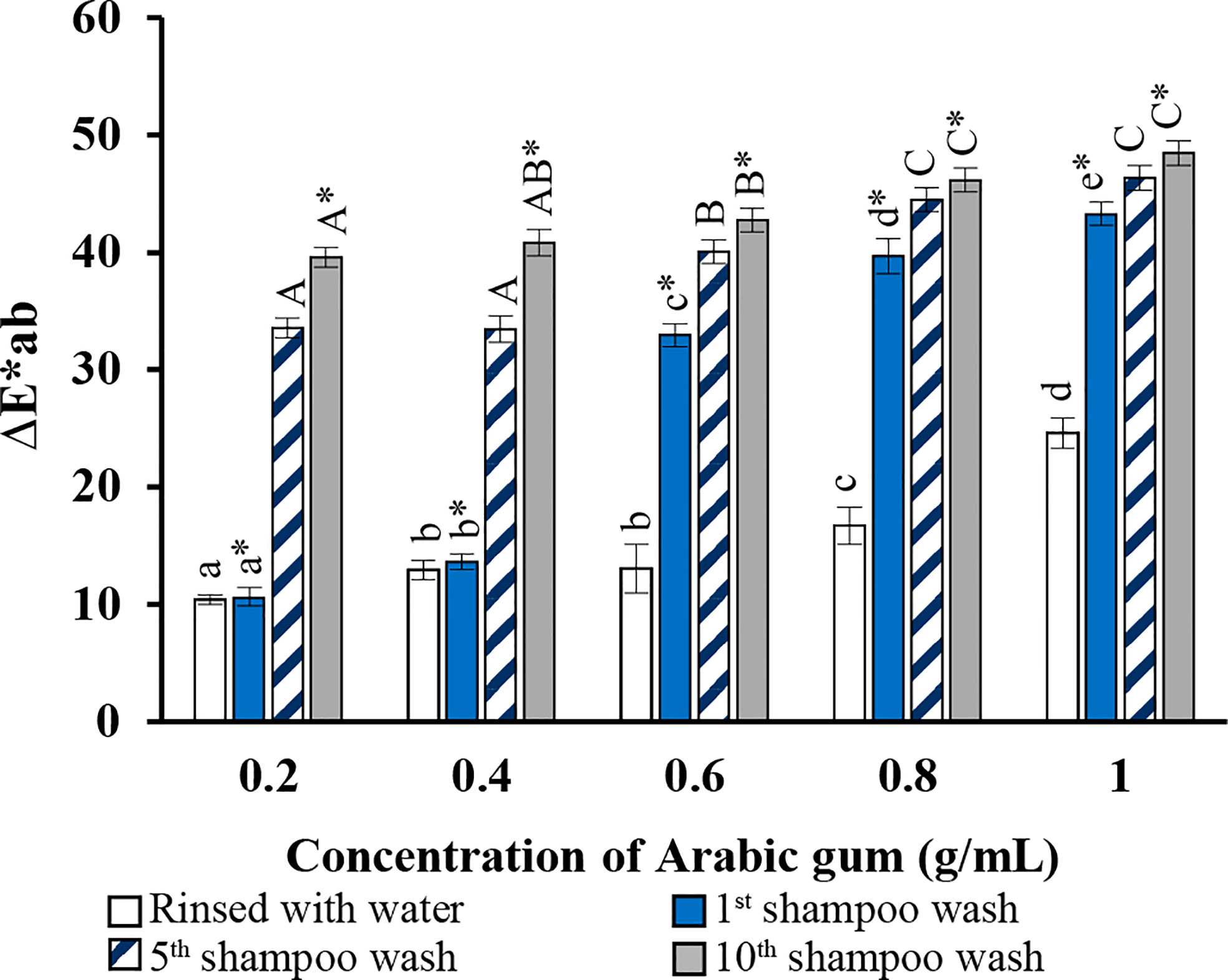
Figure 7 Total different color (∆E*ab) values of hair dyed compared with the dyed hair before washing. a,b,c Means followed by a different letter are significantly different in relation to Duncan’s Multiple Range Test (p < 0.05) of lightness values (∆L*), a*,b*,c* means followed by a different letter are significantly different in relation to Duncan’s Multiple Range Test (p < 0.05) of redness (∆a*), A,B,C means followed by a different letter are significantly different in relation to Duncan’s Multiple Range Test (p < 0.05) of yellowness (∆b*), and A*,B*,C* means followed by a different letter are significantly different in relation to Duncan’s Multiple Range Test (p < 0.05) of total difference (∆E*ab).
The different color box in Figure 7 represents how the dyed hair changes color after the first, fifth, and tenth shampoo washes. The purpose of this test was to examine the color fastness of dyed hair. The results of hair dye using the mordant in concentration at 0.2 and 0.4 g/mL shows highly significant change in color in first shampoo wash and half elimination after the fifth shampoo wash, and a slight decrease after the tenth shampoo wash. The total difference in color after the first shampoo wash was less than two concentrations before, and somewhat decreased when shampoo wash was repeated while using higher concentrations of mordant (0.6, 0.8, and 1 g/mL). A photograph showing the most change using the dye in this study after washing is shown in Figure 8.
3.5 Stability testing and color degradation of phycocyanin in hair dye formulation
The stability testing of liquid formulations under room temperature and accelerated conditions showed that the pH of the hair dye formulation did not change after testing. Other appearances, such as the color of liquid formulations, dramatically changed from bright blue to brown. The formulations were separated after heating/cooling cycle 3, as shown in Table 5. This result was different from the stability testing of powder formulations, which did not change when visually observed.
The color degradation of phycocyanin present in the hair dye formulation is presented as a percentage of phycocyanin remaining in Figure 9. The phycocyanin in water and formulation decreased in all storage conditions. The phycocyanin that was soluble in water tended to decrease linearly when incubated at room temperature and through a heating/cooling cycle. The phycocyanin concentration remained about 60% and 40% after four cycles (16 days). The amount of phycocyanin in liquid formulations declined linearly when incubated at room temperature, although this decreased considerably after the first heating/cooling cycle and continued to decrease to less than 10% after the fourth. The phycocyanin concentration in powder formulations was more stable than before in all conditions. There was very little change up to the second cycle, then gradually decreased, but the phycocyanin concentration remained above 75%.
4 Discussion
According to the research, concentration of phycocyanin at 25 mg/mL which was equal to the concentration used to formulate the hair dye was studied. Crude phycocyanin obtained from Arthrospira (Spirulina) platensis has no effect on skin pathogenic microorganisms in this study. However, previous studies have been reported the anti-microbial effect of phycocyanin extracted from Arthrospira (Spirulina) platensis. Crude phycocyanin extracted from Arthrospira (Spirulina) platensis isolated from the extreme haloalkakine environment of Lonar Crater Lake has an antibacterial potency against E. coli, S. aureus, S. typhi, Shigella spp., and P. aeuroginosa. However, fungi were found to be not affected (Mohite et al., 2015). Arthrospira (Spirulina) platensis phycocyanin extracted by enzymatic (lysozyme) digestion process and partial purified by ammonium sulphate precipitation showed the antimicrobial activities against L. monocytogenes, S. aureus, S. iniae, Y. ruckeri, and E. coli (Safari et al., 2020). The phycocyanin extracts used in this investigation were not purified so the active ingredient compound may not be concentrated enough to inhibit skin pathogenic microorganisms. The phycocyanin extracts have no inhibitory effect on the growth of M. furfur M21 because Malassezia species are lipid-dependent fungi which require external lipid sources for growth (Juntachai, 2018). The tested extract is a water-soluble protein (Kuddus et al., 2013). Therefore, the infection was not inhibited.
The in vitro cytotoxicity test is a general approach for assessing the toxicity of chemical and biological compounds in a laboratory. Moreover, the type of selected cell line depends on the investigation purpose. HaCaT cells is a spontaneously immortalized human keratinocyte cell line that has been widely used in skin biology and differentiation research (Wilson, 2013). The biocompatibility of Arthrospira (Spirulina) platensis commercial PC with HaCaT cell line was previously documented using a low concentration range of 5-80 µg/mL and it also offered UVB protection, reduced wrinkle formation, restored the physical barrier function of skin, and suppressed oxidative stress (Jang and Kim, 2021). Alternatively, the phycocyanin that was extracted from Anabaena cylindrica exhibited some toxicity to HaCaT cells and especially evident in the purified phycocyanin extracts (Macário et al., 2022). In this study, phycocyanin was used as a colorant in the hair dye formula at high concentration. The cytotoxicity of phycocyanin at various concentrations, from 0.156 mg/mL to 2.5 mg/mL, was examined in HaCaT cells. The result demonstrated that the percentage of HaCaT cell viability did not decrease comparing with the control for all of the phycocyanin concentrations. This could mean that certain pigment is not harmful to human skin. This is in accordance with the previous studies which demonstrated that the phycocyanin extracted from Arthrospira (Spirulina) platensis is non-toxic to HaCaT cells (Jang and Kim, 2021).
Hair dyes are classified as temporary, semipermanent, and permanent based on their capacity to penetrate the surface of deeper parts of the hair shaft and the longevity of the color after washing (Guerra-Tapia and Gonzalez-Guerra, 2014). Temporary dyes cause less damage to the hair than the other two types and do not reach the cortex layer. The intensity of these colors is only suitable for 1 shampoo wash (Sargsyan et al., 2021). Natural dyes function as similarly to temporary hair dye. They have weak color and long-term deposition challenges, so they are unable to penetrate deeply enough into the hair to prevent the dyed hair from washing or fading (Inman et al., 2020). Hair dyeability can be improved by adding ingredients like a developer which can open the hair cuticle and disrupt chemical connections allowing dye molecules to go deeper into the hair shaft and interact with hair proteins. Furthermore, mordant fixes the dye in hair fibers and improves hair color fastness (Boonsong et al., 2012).
In this study, phycocyanin dissolved in water made the color of the hair slightly darker and increase the greenness and blueness a bit compared with control (Figure 4A), as shown in Figure 4B. It is possible to presume that some molecules of phycocyanin that had entered and adhered to the hair fiber due to some functional groups of phycocyanin could be bonded to a cationic polymer with the hair cuticle (Guerra-Tapia and Gonzalez-Guerra, 2014). Additionally, the phycocyanin protein function might be conjugated with a keratin protein function in hair, resulting in a fusion insoluble protein (Tinoco et al., 2019). It may be argued that phycocyanin protein has the ability to function as both a coloring agent and a mordanting agent in this hair dye formula. Hydrogen peroxide, ascorbic acid, and Chamomile tea were used as developers. Hair dye mixed with ascorbic acid as a natural developer, had the highest amount of total color different (ΔE*ab), as shown in Figure 3, and the appearance of dyed hair is presented in Figure 4D. This is consistent with the previous studies (Boonsong et al., 2012). Ascorbic acid could break some hydrogen and ionic bonds in the hair structure. In addition, a few disulfide bonds in the hair structure might also be broken in an acidic solution (Rinzler, 1982), and reduced to be sulfhydryl groups. As a result, the phycocyanin’s reactive functional groups, such as methyl, carboxyl, and hydroxyl, could bind to sulfhydryl groups by covalent of ionic bonds with the amino groups of hair keratin (Burkinshaw and Kumar, 2009). For the reason that apoproteins in phycocyanin were consequently denatured by the acidic conditions caused by ascorbic acid (Li et al., 2021). The acid-induced denaturation is usually accompanied by changes in its blue hue fading, so the color of hair dyed with phycocyanin and ascorbic acid revealed a greenness more than blue, which is the color of phycocyanobilin.
The following mordants for phycocyanin and ascorbic acid were identified as appropriate; alum, Assum tea, Arabic gum, and Aloe vera as shown in Figure 6. In addition to the formulation T5, the results showed that nearly all formulations improved the appearance of dyed hair that was dyed. Alum, metal ion, is able to contact the functional groups of phycocyanin protein and precipitate (Boonsong et al., 2012) so that the formulation T5 fails to dye, as Figure 6B. Arabic gum performed the best in this experiment (Figure 5). Arabic gum or acacia gum is a polysaccharide that is widely used as an important naturally occurring oil in water emulsifiers as a suspending agent and thickener in food and in pharmaceutical industries. The USFDA (U.S. Food and Drug administration) classifies it as Generally Recognized as Safe (Barak et al., 2020). This gum’s thickening properties boosted dyeability by increasing the contact surface area between hair and pigment. The highly polymerized polysaccharides offered strong affinities to the phycocyanin molecules through both electrostatic and hydrophobic interactions, according to characterizations and mechanistic studies (Li et al., 2021). However, the color of the dyed hair decreased with the increasing concentration of Arabic gum. This is because the dye molecule infiltration on the hair surface was troubled by the over sticky formula.
As shown in Figure 8, the dyed hair was able to appear vivid blue. The color fastness was repeated 10 times. Additionally, L*, a*, and b* were recorded and demonstrated in terms of ΔE*ab before washing, rinsed with water, and washing with shampoo at the first, fifth, and tenth. The color appearance of dyed hair was able to persist through shampooing until 5 times when visually observed. The type of hair dye can be divided according to the color durability after the application on hair strands: temporary (the color leaving the hair after the first shampoo wash), semi-permanent (washing off thoroughly in 4–12 shampoo washes), and permanent (lasting for up to 28 shampoo washes) (Da França et al., 2015). The phycocyanin’s capacity for color staining in this developed hair dye formulation could be categorized as a semi-permanent hair dye. This result resembled other earlier documented. The use of anthocyanin derived from black beans as an alternative hair dye and the wash fastness shown the monitored color were significantly different at least four shampooing (Inman et al., 2020). However, the color fastness of dyed hair is less compared to studies utilizing natural dyes from several Thai plants and ferrous sulfate as a mordant, which showed slight fading of the color in dyed hair until fifteen shampoo washings (Boonsong et al., 2012). The results also demonstrate that hair colored with those extracts had a darker and more color fastness property. Due to the fact that ferrous sulfate (metal ions) frequently forms a complex with the hair fiber and the electron-donating chromophores of natural pigments (Burkinshaw and Kumar, 2009). The dyeing time in the experiment is 1.30 hours. This may take longer than the average amount of time for hair dyes to be marketed, which generally suggests dying hair for 45-60 min and may need more time if you want a brighter effect. Since every ingredient of this formulation is natural, it has little effect on the dyeing process. Moreover, some previous studies (Inman et al., 2020) that used the color from black bean and took 45 min for dyeing time and (Gargote, 2012) took 2 hours for dyeing hair, the colorant from plant extract, showed more durability time than the less time. It may be assumed that adhesion of the natural dye to the hair enhances the contact time of the extract, enabling penetration of the colorant molecules into the hair cuticle and partially diffusing throughout the cortex.
The stability and color degradation of phycocyanin hair dye powder formulation kept at room temperature and covered with aluminum foil showed the best stability in this study. The acidified condition and high temperature were the main reasons for phycocyanin denaturation, which cause a fading rapidly (Zhang et al., 2020). The thermodynamic stability of an enzyme is significantly influenced by its solution state. Dried proteins contain a very small amount of water, they are typically more stable and some of them can be kept in dark environments for several months or even over a year without losing their activity (Plou et al., 1998). Thus, this study advises that to use phycocyanin as hair dye application were keeping it at a normal or low temperature in dark packaging and mixing with water before use.
5 Conclusion
Phycocyanin extracted from Arthrospira (Spirulina) platensis has the potential to be used as a hair dye with no toxicity to HaCaT cells. The best developer was ascorbic acid and the best mordant was Arabic gum. The aim of further study will be aimed to improve the dye ability and stability for commercial products.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
OK: conceptualization, methodology, formal analysis, resources, investigation, and writing-original draft. YT: resources, writing-review, and editing. HP: writing-review and editing. RK: writing-review and editing. WP-a: writing-review and editing. JP: writing-review and editing. CP: supervision, conceptualization, project administration, funding acquisition, resources, and writing-review and editing. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by National Research Council of Thailand (NRCT) and the Research and Researchers for Industries (RRi), grant number PHD61I0009 and partially supported by Chiang Mai University, and the Graduate School of Chiang Mai University.
Acknowledgments
I would like to thank Dr. Thida Kaewkod and Miss Kanok-orn Mayer for their assistance with the cytotoxicity assay. I also thank Assoc. Prof. Dr. Itthayakorn Promputtha for providing microorganisms in this research. Thank you, Mr. Andrew John Semansco, for proofreading. We appreciate the funds provided for this effort by the National Research Council of Thailand (NRCT) and the Research and Researchers for Industries (RRi), grant number PHD61I0009. This research was partially supported by Research Center in Bioresources for Agriculture, Industry and Medicine, Chiang Mai University, and the Graduate School of Chiang Mai University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ali S., Hussain T., Nawaz R. (2009). Optimization of alkaline extraction of natural dye from henna leaves and its dyeing on cotton by exhaust method. J. Clean. Prod. 17 (1), 61–66. doi: 10.1016/j.jclepro.2008.03.002
Antelmi A., Bruze M., Zimerson E., Engfeldt M., Young E., Persson L., et al. (2017). Evaluation of concordance between labelling and content of 52 hair dye products: overview of the market of oxidative hair dye. Eur. J. Dermatol. 27 (2), 123–131. doi: 10.1684/ejd.2016.2934
Balouiri M., Sadiki M., Ibnsouda S. K. (2016). Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 6 (2), 71–79. doi: 10.1016/j.jpha.2015.11.005
Barak S., Mudgil D., Taneja S. (2020). Exudate gums: chemistry, properties and food applications–a review. J. Sci. Food Agric. 100 (7), 2828–2835. doi: 10.1002/jsfa.10302
Beiki T., Najafpour G. D., Hosseini M. (2018). Evaluation of antimicrobial and dyeing properties of walnut (Juglans regia l.) green husk extract for cosmetics. Color. Technol. 134 (1), 71–81. doi: 10.1111/cote.12322
Bennett A., Bogorad L. (1973). Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 58 (2), 419–435. doi: 10.1083/jcb.58.2.419
Boonsong P., Laohakunjit N., Kerdchoechuen O. (2012). Natural pigments from six species of Thai plants extracted by water for hair dyeing product application. J. Clean. Prod. 37, 93–106. doi: 10.1016/j.jclepro.2012.06.013
Burkinshaw S., Kumar N. (2009). The mordant dyeing of wool using tannic acid and FeSO4, part 1: Initial findings. Dyes Pigments 80 (1), 53–60. doi: 10.1016/j.dyepig.2008.05.008
Cherng S.-C., Cheng S.-N., Tarn A., Chou T.-C. (2007). Anti-inflammatory activity of c-phycocyanin in lipopolysaccharide-stimulated RAW 264.7 macrophages. Life Sci. 81 (19-20), 1431–1435. doi: 10.1016/j.lfs.2007.09.009
Da França S. A., Dario M. F., Esteves V. B., Baby A. R., Velasco M. V. R. (2015). Types of hair dye and their mechanisms of action. Cosmetics 2 (2), 110–126. doi: 10.3390/cosmetics2020110
Flores L. E. R., Madrigal-Bujaidar E., Salazar M. A., Chamorro G. (2003). Anticlastogenic effect of Spirulina maxima extract on the micronuclei induced by maleic hydrazide in tradescantia. Life Sci. 72 (12), 1345–1351. doi: 10.1016/S0024-3205(02)02412-8
Glazer A. N. (1982). Phycobilisomes: structure and dynamics. Annu. Rev. Microbiol. 36 (1), 173–198. doi: 10.1146/annurev.mi.36.100182.001133
Guerra-Tapia A., Gonzalez-Guerra E. (2014). Hair cosmetics: dyes. Actas Dermo-Sifiliográficas (English Edition) 105 (9), 833–839. doi: 10.1016/j.adengl.2014.02.003
Harrison S., Sinclair R. (2003). Hair colouring, permanent styling and hair structure. J. Cosmetic Dermatol. 2 (3-4), 180–185. doi: 10.1111/j.1473-2130.2004.00064.x
Imbimbo P., Romanucci V., Pollio A., Fontanarosa C., Amoresano A., Zarrelli A. (2019). A cascade extraction of active phycocyanin and fatty acids from Galdieria phlegrea. Appl. Microbiol. Biotechnol. 103 (23), 9455–9464. doi: 10.1007/s00253-019-10154-0
Inman C., Lourith N., Kanlayavattanakul M. (2020). Alternative application approach on black bean: hair coloring product. Chem. Biol. Technol. Agric. 7 (1), 1–7. doi: 10.1186/s40538-019-0163-2
Jang Y. A., Kim B. (2021). Protective effect of spirulina-derived c-phycocyanin against ultraviolet b-induced damage in HaCaT cells. Medicina 57 (3), 273. doi: 10.3390/medicina57030273
Juntachai W. (2018). Effect of tween 60 and tween 80 on the growth of Malassezia species. Acta Microbiol. Hell. 63 (2/3), 119–128.
Komboonchoo S., Bechtold T. (2009). Natural dyeing of wool and hair with indigo carmine (CI natural blue 2), a renewable resource based blue dye. J. Clean. Prod. 17 (16), 1487–1493. doi: 10.1016/j.jclepro.2009.05.007
Krasteva M., Bons B., Ryan C., Gerberick F. G. (2009). Consumer allergy to oxidative hair coloring products: epidemiologic data in the literature. Dermatitis 20 (3), 123–141. doi: 10.2310/6620.2009.08089
Kuddus M., Singh P., Thomas G., Al-Hazimi A. (2013). Recent developments in production and biotechnological applications of c-phycocyanin. BioMed. Res. Int. 2013:742859. doi: 10.1155/2013/742859
Lee H. Y., Jeong Y. I., Kim D. H., Choi K. C. (2013). Permanent hair dye-incorporated hyaluronic acid nanoparticles. J. Microencapsulation 30 (2), 189–197. doi: 10.3109/02652048.2012.714412
Li Y., Zhang Z., Abbaspourrad A. (2021). Improved thermal stability of phycocyanin under acidic conditions by forming soluble complexes with polysaccharides. Food Hydrocolloids 119, 106852. doi: 10.1016/j.foodhyd.2021.106852
Lozano I., Saunier J., Panhard S., Loussouarn G. (2017). The diversity of the human hair colour assessed by visual scales and instrumental measurements. a worldwide survey. Int. J. Cosmetic Sci. 39 (1), 101–107. doi: 10.1111/ics.12359
Macário I. P., Veloso T., Fernandes A. P., Martins M., Dias T. R., Oliveira H. (2022). Blue is not enough: biological activities of c-phycocyanin extracts from Anabaena cylindrica. J. Chem. Technol. Biotechnol. 97 (5), 1171–1179. doi: 10.1002/jctb.7001
Materassi R., Tredici M., Balloni W. (1984). Spirulina culture in sea-water. Appl. Microbiol. Biotechnol. 19 (6), 384–386. doi: 10.1007/BF00454374
Mohite Y., Shrivastava N., Sahu D. (2015). Antimicrobial activity of c-phycocyanin from Arthrospira platensis isolated from extreme haloalkaline environment of lonar lake. J. Environ. Sci. Toxicol. Food Technol. 1 (4), 40–45.
Osman A., Salama A., Ghany A. A., Sitohy M. (2015). Antibacterial activity and mechanism of action of phycocyanin extracted from an Egyptian strain of Anabaena oryzae SOS13. Zagazig J. Agric. Biochem. It’s Appl. 42, 30–321.
Packianathan N., Karumbayaram S. (2010). Formulation and evaluation of herbal hair dye: an ecofriendly process. J. Pharm. Sci. Res. 2 (10), 648.
Padyana A. K., Bhat V. B., Madyastha K. M., Rajashankar K. R., Ramakumar S. (2001). Crystal structure of a light-harvesting protein c-phycocyanin from spirulina platensis. Biochem. Biophys. Res. Commun. 282 (4), 893–898. doi: 10.1006/bbrc.2001.4663
Park H., Hwang J.-h., Han J.-S., Lee B.-S., Kim Y.-B., Joo K.-M., et al. (2018). Skin irritation and sensitization potential of oxidative hair dye substances evaluated with in vitro, in chemico and in silico test methods. Food Chem. Toxicol. 121, 360–366. doi: 10.1016/j.fct.2018.09.017
Perveen I., Sehar S., Naz I., Ahmed S. (2017). P73: Contact allergy to hair dyes (p-phenylenediamine): A preliminary report. Internal Med. J. 47, 26–27. doi: 10.1111/imj.73_13578
Plou F. J., Iborra J. L., Halling P. J. (1998). Stability and stabilization of biocatalysts (Eastbourne CPI Antony Rowe: Elsevier).
Reddy C. M., Bhat V. B., Kiranmai G., Reddy M. N., Reddanna P., Madyastha K. (2000). Selective inhibition of cyclooxygenase-2 by c-phycocyanin, a biliprotein from Spirulina platensis. Biochem. Biophys. Res. Commun. 277 (3), 599–603. doi: 10.1006/bbrc.2000.3725
Romay C., Gonzalez R. (2000). Phycocyanin is an antioxidant protector of human erythrocytes against lysis by peroxyl radicals. J. Pharm. Pharmacol. 52 (4), 367–368. doi: 10.1211/0022357001774093
Rose P. M., Cantrill V., Benohoud M., Tidder A., Rayner C. M., Blackburn R. S. (2018). Application of anthocyanins from blackcurrant (Ribes nigrum l.) fruit waste as renewable hair dyes. J. Agric. Food Chem. 66 (26), 6790–6798. doi: 10.1021/acs.jafc.8b01044
Safari R., Raftani Amiri Z., Esmaeilzadeh Kenari R. (2020). Antioxidant and antibacterial activities of c-phycocyanin from common name Spirulina platensis. Iranian J. Fish. Sci. 19 (4), 1911–1927.
Sargsyan L., Hippe T., Manneck H., Vill V. (2021). Tannin-mordant coloration with matcha (Camelia sinensis) and iron (II)-lactate on human hair tresses. Molecules 26 (4), 829. doi: 10.3390/molecules26040829
Shin Y. S., Lee S. H. (2006). Natural dyeing of hair using juglone. J. Korean Soc. Clothing Textiles 30 (12), 1708–1713.
Takano M., Sado J.-I., Ogawa T., Terui G. (1973). Freezing and freeze-drying of Spirulina platensis. Cryobiology 10 (5), 440–444. doi: 10.1016/0011-2240(73)90073-4
Tinoco A., Antunes E., Martins M., Gonçalves F., Gomes A. C., Silva C. (2019). Fusion proteins with chromogenic and keratin binding modules. Sci. Rep. 9 (1), 1–13. doi: 10.1038/s41598-019-50283-0
Vonshak A. (1997). Spirulina platensis arthrospira: physiology, cell-biology and biotechnology (New York: CRC press).
Whangsomnuek N., Mungmai L., Mengamphan K., Amornlerdpison D. (2019). Efficiency of skin whitening cream containing Etlingera elatior flower and leaf extracts in volunteers. Cosmetics 6 (3), 39. doi: 10.3390/cosmetics6030039
Wilson V. G. (2013). “Growth and differentiation of HaCaT keratinocytes,” in Epidermal cells (New York: Springer), 33–41. doi: 10.1007/7651_2013_42
Zhang Z., Li Y., Abbaspourrad A. (2020). Improvement of the colloidal stability of phycocyanin in acidified conditions using whey protein-phycocyanin interactions. Food Hydrocolloids 105, 105747. doi: 10.1016/j.foodhyd.2020.105747
Keywords: pigment, phycobiliprotein, mordant, skin-pathogen, cyanobacteria
Citation: Kraseasintra, Tragoolpua, Pandith, Khonkarn, Pathom-aree, Pekkoh and Pumas (2022) Application of phycocyanin from Arthrospira (Spirulina) platensis as a hair dye. Front. Mar. Sci. 9:1024988. doi: 10.3389/fmars.2022.1024988
Received: 22 August 2022; Accepted: 18 October 2022;
Published: 01 November 2022.
Edited by:
Nitin Trivedi, Institute of Chemical Technology, IndiaReviewed by:
Shailesh Kumar Patidar, Central University of Rajasthan, IndiaSourish Bhattacharya, Central Salt & Marine Chemicals Research Institute (CSIR), India
Jungsoon Lee, Chungnam National University, South Korea
Copyright © 2022 Kraseasintra, Tragoolpua, Pandith, Khonkarn, Pathom-aree, Pekkoh and Pumas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chayakorn Pumas, Y2hheWFrb3JuLnB1bWFzQGdtYWlsLmNvbQ==
 Oranit Kraseasintra
Oranit Kraseasintra Yingmanee Tragoolpua1
Yingmanee Tragoolpua1 Hataichanok Pandith
Hataichanok Pandith Wasu Pathom-aree
Wasu Pathom-aree Jeeraporn Pekkoh
Jeeraporn Pekkoh Chayakorn Pumas
Chayakorn Pumas