- Department of Life and Environmental Sciences, University of Cagliari, Cagliari, Italy
Introduction: Holothuria tubulosa is one of the most common sea cucumbers in the Mediterranean Sea, generally associated with organically enriched coastal sediments and seagrass beds. As a deposit-feeder, it is responsible for strong bioturbation processes and plays a putative key role in sedimentary carbon cycling and benthic trophodynamics. With the aim of exploring the potential use of holothuroids as a tool for remediating eutrophicated sediments, we investigated the effects of H. tubulosa on sedimentary organic matter quantity, biochemical composition, and nutritional quality.
Methods: Holothuroids and associated samples of ambient sediments were collected in two sites located in the Central-Western Mediterranean Sea (Sardinia, Italy) and characterized by different trophic status backgrounds: the site of Oristano characterized by sandy-muddy sediments and the presence of mariculture plants (ranked as meso-eutrophic) and the site of Teulada characterized by sandy sediments and Posidonia oceanica meadows (ranked as oligo-mesotrophic). We compared the biochemical composition (proteins, carbohydrates, lipids) of ambient sediment vs sea cucumbers feces and the sedimentary protein content vs protein content in the sediments retrieved in different gut sections (esophagus, mid gut, end gut) of the holothuroid.
Results: Our results reveal that holothuroids feeding on meso-eutrophic sediments can increase protein (1.5 times) and lipid (1.3 times) content through their defecation, thus making these substrates a more labile food source for other benthic organisms. We report here that H. tubulosa feeding on meso-eutrophic sediment is most likely able to actively select particles rich in labile organic matter with buccal tentacles, as revealed by the protein content in the esophagus that is up to 2-folds higher than that in the source sediment. According to the inverse relationship between assimilation rates and availability of organic substrates and the optimal foraging theory, H. tubulosa feeding on oligo-mesotrophic sediments showed potential assimilation of proteins ca. 25% higher than that of specimens feeding on meso-eutrophic sediments.
Discussion: Our results reveal that H. tubulosa feeding on meso-eutrophic sediments can profoundly influence the benthic trophic status, specifically modifying the biochemical composition and nutritional quality of organic matter, thus paving the way to its possible use in bioremediation actions of eutrophicated sediments and in Integrated Multi-Trophic Aquaculture systems.
Introduction
Bioturbation mediated by benthic marine deposit-feeder plays a key role in carbon biogeochemical cycling from coastal to hadal depths (Uthicke and Karez, 1999; Roberts et al., 2000; Lohrer et al., 2004; Slater and Carton, 2009; Amaro et al., 2010; Slater et al., 2011a; Purcell et al., 2016). Bioturbation can indeed influence sediment permeability, chemical gradients in pore waters and sedimentary organic matter (OM) degradation rates (Reise, 2002; Lohrer et al., 2004; Solan et al., 2004; Meysman et al., 2006a; Meysman et al., 2006b; Schenone et al., 2019). Among the most effective bioturbators, sea cucumbers (Phylum Echinodermata), common members of marine benthic communities in world oceans at all depths, count more than 1,500 species (Horton et al., 2018). Deposit-feeding sea cucumbers, ingesting the whole sediment, and feeding on sedimentary OM, can rework large amounts of sediments (5.9 – 12.9 kg dw m-2 yr-1) (Coulon and Jangoux, 1993; Uthicke and Karez, 1999; Mangion et al., 2004, Hartati et al., 2020), thus contributing to the redistribution and remineralization of sedimentary organic loads from hadal depth to coastal areas and coral reefs (Amaro et al., 2010; MacTavish et al., 2012; Purcell et al., 2016; Wolfe et al., 2017; Yamazaki et al., 2019).
Bioturbation caused by sea cucumbers extends from the upper layer of the seafloor to several centimeters’ depth in the sediment, depending on the variable ability of the different species to dig into the sediment and their borrowing habits (Mercier et al., 1999; Roberts et al., 2000; Amaro et al., 2010; Ramón et al., 2019). During feeding, sediment particles are captured by the sea cucumber with the tentacles and released into the pharynx. Ingested particles are then mixed with the digestive enzymes and, once compressed into a plug that moves along the digestive system are released through the cloaca (Zamora and Jeffs, 2011). Thus, deposit-feeding sea cumbers, with their peculiar feeding activity, can reduce sedimentary OM loads (Slater and Carton, 2009; MacDonald et al., 2013; Neofitou et al., 2019). For this reason, sea cucumbers are considered one of the best candidates for Integrated Multi-Trophic Aquaculture (IMTA) practices (MacDonald et al., 2013; Purcell et al.,2014; Cubillo et al., 2016; Grosso et al., 2021). IMTA, in fact, can transform mariculture potential wastes (e.g., uneaten food and fish feces) into food sources for other reared species positioned at lower trophic levels (Zhou et al., 2006; Slater and Carton, 2007; Slater and Carton, 2009; Slater et al., 2009; Zamora and Jeffs, 2011; Zamora and Jeffs, 2012; Yuan et al., 2013; Lamprianidou et al., 2015; Shpigel et al., 2018). This sort of circular reuse of mariculture waste for producing extra commercial biomass could help mitigate the well-documented impacts of marine aquaculture on the trophic status, biodiversity and functioning of coastal marine ecosystems (Kalantzi and Karakassis, 2006; Pusceddu et al., 2007; Holmer et al., 2008; Holmerm, 2010; Mirto et al., 2010).
So far, sea cucumbers used in IMTA and co-culture trials include high commercially valuable species such as Apostichopus japonicus Selenka, 1867 (Zhou et al., 2006; Yuan et al., 2013; Kim et al., 2015), Australostichopus mollis Hutton, 1872 (Slater and Carton, 2007; Slater and Carton, 2009; Slater et al., 2009; Zamora and Jeffs, 2011; Zamora and Jeffs, 2012), Holothuria scabra Jaeger, 1833 (Mathieu-Resuge et al., 2020), Parastichopus californicus Stimpson, 1857 (Paltzat et al., 2008) and Holothuria tubulosa Gmelin 1788 (Grosso et al., 2021). Recent experiments have shown that the Mediterranean Sea cucumber Holothuria tubulosa grows faster beneath finfish cages than in mariculture-free areas (Tolon et al., 2017). In another study, it was shown that the monthly reduction of organic carbon (OC) caused by H. tubulosa (ca. 10 ind m-2) feeding in sediments beneath seabream and seabass cages has been estimated to account up to 62% (Neofitou et al., 2019).
Moreover, sea cucumbers have a high commercial value and are currently overexploited worldwide (Conand, 2006; Anderson et al., 2011a, b; Bordbar et al., 2011; Purcell et al., 2011; Conand et al., 2014; González-Wangüemert et al., 2018). Indeed, holothuroid fisheries have rapidly expanded in the last three decades, because of the increasing demand for food in international markets and biomedical research programs (Bordbar et al., 2011). Holothuroids, in fact, are a traditional food in China and other eastern regions, where they are considered gourmet and luxury seafood (Wen et al., 2010; Yang and Bai, 2015), and sold at prices as high as up to ca. 3000 US$ per dried kg (Purcell et al., 2012; Purcell et al., 2014). The large market demand for these animals has led to overfishing in worldwide oceans and seas, including the Mediterranean. Consequently, 16 species have been classified as threatened with extinction based on standard IUCN methodology (Conand et al., 2014) and 4 species are included in the Convention on International Trade in Endangered Species list (CITES, 2022). In this sense, restocking and stock enhancement practices of holothuroids, eventually also including hatchery production of juveniles could support their protection.
Organic carbon in marine sediments is made of a variable combination of molecules that are differently prone to a rapid utilization by benthic detritivores (Pusceddu et al., 2009). The total organic C content of the sediments is, in this sense, a rather weak descriptor of the potential amount of food for the benthos. Since total organic C content does not discriminate between refractory vs. labile fractions, its biopolymeric fraction (the sum of protein, carbohydrate and lipid C equivalents, sensu Fabiano et al., 1995) is currently used as a more reliable descriptor of the benthic trophic status, namely the availability of food available for the benthos in a variety of wetlands, coastal and even deep-sea sedimentary environments (Pusceddu et al., 2009; Pusceddu et al., 2010; Pusceddu et al., 2011; Dell’Anno et al., 2013). Nonetheless, studies dealing with the role of holothuroids on sedimentary organic matter dynamics have almost exclusively dealt with the mere measurement of total organic C only, with a very few exceptions (Amaro et al., 2010).
With an eye to the potential use of H. tubulosa for abating, also in an IMTA perspective, the consequences of benthic eutrophication, we investigated differences in quantity and biochemical composition of sedimentary OM and biochemical composition of H. tubulosa feces, along with changes in protein loads of ingested sediment in the different digestive tracts of H. tubulosa. More specifically, we posed the following questions: 1) does H. tubulosa influence sedimentary OM biochemical composition, and nutritional quality and, thus, the benthic trophic status? 2) does H. tubulosa has a specific influence on food availability of sedimentary proteins, supposed to represent the most labile fraction of OM? To answer these questions, we conducted experiments aimed at testing the following null hypotheses: 1) the biochemical composition of sediment OM hosting H. tubulosa is not different from that of sea cucumbers feces; 2) the protein loads of the sediment ingested by sea cucumbers do not vary among the digestive tracts and the feces.
Materials and methods
Model species, study sites and sampling strategy
Our study is focused on the sea cucumber Holothuria tubulosa Gmelin 1788, one of the most common and most exploited native species across the coastal Mediterranean Sea, where it can be pre-eminently found in organically enriched bottoms and seagrass beds (Tortonese, 1965; Bulteel et al., 1992; Koukouras et al., 2007; Costa et al., 2014; González-Wangüemert et al., 2014; González-Wangüemert et al., 2015; González-Wangüemert et al., 2018; Pasquini et al., 2021; Pasquini et al., 2022). Fishery of H. tubulosa is banned by the Italian Government that precautionarily prohibited its harvesting since 2018 (Ministerial decree 156/2018; Pasquini et al., 2021). In other areas of the Mediterranean, such as in Turkey, the fishery of H. tubulosa is governed since 2007 (González-Wangüemert et al., 2018; Dereli and Aydın, 2021).
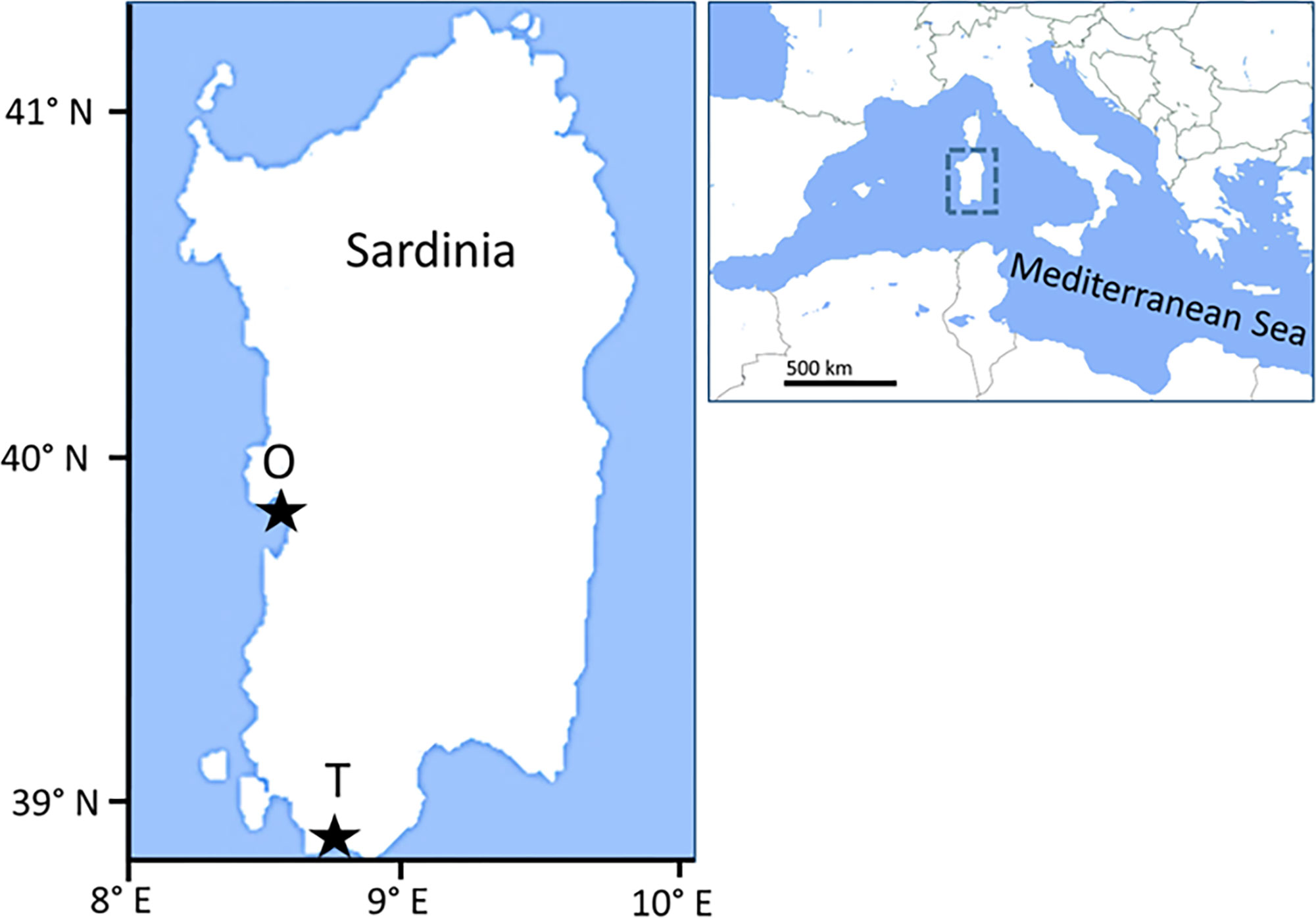
H. tubulosa specimens and sediments were collected from two sites 80 nautical miles apart, in the Gulf of Teulada and the Gulf of Oristano, respectively (Sardinia, W Mediterranean Sea). Sediment grain size was determined by gravimetry after organic matter digestion with H2O2 and mesh sieving, using 63, 125, 250, 500, and 1000 μm meshes ICRAM, 2001). The sediments of the Oristano site (39°52’50’’N; 8°28’54’’E) were characterized by the dominance of sands (63-1000 µm; 75%), followed by mud (<63 µm; 14%) and gravel (>1000 µm; 11%) and by the presence of a nearby offshore mariculture fish farming plant for seabream and shi drum. The Teulada site (38°55’46’’N; 8°43’17’’E) was characterized by the dominance of sandy sediments (74%), followed by gravel (24%), and mud (1%), and by the presence of wide Posidonia oceanica meadows interspersed with unvegetated sediments (Figure 1).
At both sites, holothuroid and sediment samples were collected by SCUBA divers at depths between 3 and 7 m. In order to include environmental conditions spanning from the worst conditions (in winter, January) to the most favorable ones (in spring and, to a lesser extent, in summer: May, June, July), data were collected in April, June, and August 2019 and January, May, and July 2020. At each site and on each sampling date, three metal frames 60x60 cm (sampling unit n=3) were placed on the sea floor having a single sea cucumber in the central sub-square (20x20 cm). From each sampling unit (60x60 frame), three sediment samples were obtained by scraping ca. 50 mL from the top 2 cm of the sea floor with a Falcon-type. One tube was collected from the central sub-square, in which the sea cucumber occurs, and two from other two random sub-squares (n=3 for each sampling unit), resulting in a total of 9 sediment samples per each site at each sampling date (Moccia et al., 2019) (Supplementary Figure 1). All sea cucumbers (one per each 60x60 cm squared frame, n=3) were placed separately into 3-L plastic bag filled with seawater collected in situ and maintained in a cooler box (at in situ temperature) during transportation to the laboratory (within 1.5 h from sampling). We notice here that the choice of sampling only one specimen per squared frame, despite the official authorization (see the acknowledgments for more details) was dictated by the need of limiting as much as possible the pressure on a locally protected species. Once in the laboratory, sea cucumbers were singularly placed in a tank filled with in situ seawater (15-L) kept at in situ temperature and salinity, deprived of sediment. The sea cucumbers feces were collected every 6-8 hours and until the complete evacuation of the digestive tract (usually within 3 days). Feces from each specimen were pooled together to ensure the storage of a sufficient amount of feces for the subsequent biochemical analyses. The sediments and the feces were stored at -20°C until the subsequent analyses.
Additional (2-3) sea cucumber specimens were collected at both sites in June 2019 and used for the analysis of the protein content of the sediment within the digestive tract. Sea cucumbers were weighed (± 0.1 g) before dissection and the sediments present in the intestine esophagus (ESO), mid-gut (MID), and end gut (END) were carefully collected to avoid contaminations from the digestive tissue (Amaro et al., 2010) and stored at -20°C until analysis. As for above, we sampled a low number of experimental individuals to limit as much as possible the pressure on a locally protected species.
Biochemical composition of field sediment, holothuroids’ feces, and sediment from the holothuroids’ digestive tracts
Protein, carbohydrate, and lipid contents were determined spectrophotometrically (Danovaro, 2010). Proteins were determined according to Hartree (1972), as modified by Lowry et al. (1951) and Rice (1982) using the Folin-Ciocalteau reagent in a basic environment and expressed as bovine serum albumin equivalents. The procedure proposed by Gerchakov and Hatcher (1972), based on the phenol and concentrated sulfuric acid reaction with saccharides, was used to determine carbohydrates, then expressed as D (+) Glucose equivalents. Lipids, after extraction in chloroform: methanol (1:1, vol:vol) (Bligh and Dyer, 1959), and evaporation in a dry hot bath at 80 to 100°C for 20 min, were determined after the sulfuric acid carbonization procedure (Marsh and Weinstein, 1966) and expressed as tripalmitin equivalents. For each biochemical assay, blanks were obtained using pre-calcinated sediments (450°C for 4 h). Protein, carbohydrate, and lipid concentrations were converted into C equivalents using the conversion factors 0.49, 0.40, and 0.75 mgC mg-1, respectively, obtained from the C contents of the respective standard molecules (albumin, glucose and tripalmitin, respectively), and their sum was reported as the biopolymeric C (BPC) (Fabiano et al., 1995).
The percentage of proteins potential digestion was calculated as follows:
where:
PD = potential proteins’ digestion
ESO PRT= protein content (mg g-1) in the sediment in the esophagus
END PRT= protein content (mg g-1) in the sediment in the end gut
Chloroplastic pigments (chlorophyll-a and phaeopigments) in the ambient sediment were analysed fluorometrically according to Lorenzen and Jeffrey (1980). Pigments were extracted with 90% acetone (24 h in the dark at 4°C). After centrifugation (800 × g), the supernatant was used to determine the chlorophyll-a and, after acidification with 0.1 N HCl, to estimate the phaeopigments (Danovaro, 2010). The total algal C contribution (sum of chlorophyll-a and phaeopigments) to BPC was calculated as the percentage of phytopigment-to-BPC concentrations after converting the total phytopigment concentrations into C equivalents using a mean value of 40 mgC mg–1 (Pusceddu et al., 2010). Although the C: Chla can vary from 10 to 100 (on average 35 for phytoplankton) (Cloern et al., 1995), we used the conversion factor proposed in Pusceddu et al. (2010) to allow comparability with other studies carried out in a variety of other shallow coastal aquatic environments (Pusceddu et al., 2009).
Statistical analyses
Differences in quantity and biochemical composition (protein, carbohydrates, lipids, chlorophyll-a, phaeopigments) of ambient sediments were investigated using Site (Si; 2 fixed levels: Oristano vs. Teulada) and Month (Mo; 6 fixed levels: April, June, August 2019, and January, May, July 2020) as orthogonal sources of variance, with n=9 for the combination of factors. Differences in OM quantity and biochemical composition in terms of protein, carbohydrates, lipids between sediment and holothuroid feces were investigated separately for the two sites (Oristano and Teulada) using Matrix (Ma; 2 fixed levels: sediment vs. feces) and Month (Mo; 6 fixed levels: April, June, August 2019, and January, May, July 2020) as orthogonal sources of variance. In both designs, as any sampled sediment portion around H. tubulosa specimens could have been visited by holothuroids before sampling, we chose to consider as independent replicates of ambient sediments either sediments without or with (in the center of the large frame) holothuroids. This choice generated an unbalanced design, which biases are, however, counterbalanced by the use of PERMANOVA, which is extended to accommodate, apart random effects, hierarchical models, mixed models, quantitative covariates, repeated measures, also unbalanced and/or asymmetrical designs (Anderson, 2017). Differences in protein content among sediment, sediment in the gut (three sectors: esophagus, mid gut, and end gut) and feces were assessed by means of univariate PERMANOVA tests using the matrix (5 fixed levels: ambient sediment, esophagus, mid gut, end gut and feces) as the sole source of variation.
In all designs, differences were assessed with permutational analyses of variance (PERMANOVA), in both univariate and multivariate contexts. PERMANOVA is a semiparametric method described as a geometric partitioning of multivariate variation in the space of a chosen dissimilarity measure according to a given ANOVA design, with p-values obtained using appropriate distribution‐free permutation techniques (Anderson, 2017). PERMANOVA on one response variable using Euclidean distance yields the classical univariate F statistic, so that it can also be used to do univariate ANOVA, but where p values are obtained by permutation (Anderson and Millar, 2004), thus avoiding the assumption of normality (Anderson, 2017). PERMANOVA tests were carried out on Euclidean distance-based resemblance matrixes of normalized data, using 999 random permutations of the appropriate units. When significant differences were observed, pairwise tests were also carried out to ascertain patterns of differences among treatments and/or sampling times.
Multivariate differences in sedimentary OM biochemical composition (in terms of protein, carbohydrate, lipid and phytopigment contents) between sites and months were visualized with a biplot after a canonical analysis of the principal coordinates (CAP) (Anderson and Willis, 2003). CAP allows identifying an axis through the multivariate cloud of points that is best at separating a priori groups. The motivation for the CAP routine arose as sometimes there are real differences among a priori groups in multivariate space that cannot be easily seen in an unconstrained ordination (e.g., PCA or MDS plots; Anderson and Gorley, 2008). All the statistical analyses were performed using the routines included in the PRIMER 7+ software (Anderson et al., 2008).
Results
Quantity and biochemical composition of organic matter in field sediments
Overall, contents of all organic compounds, but carbohydrates, differed significantly between sites (Supplementary Table 1), with values in the muddy organically enriched sediments at Oristano significantly higher (3-5 times) than those in the sandy poorer sediments at Teulada (Supplementary Figure 2). In both sites, significant temporal variations, though not consistent for the different classes of organic compounds, and significant effects of the Si×Mo interaction occurred only for protein, lipid, and chlorophyll-a contents. Results of the pairwise tests conducted to ascertain temporal differences are reported in Supplementary Table 2 and illustrated in Supplementary Figure 2.
In organically enriched sediments at Oristano, seasonal differences in the sedimentary protein contents varied between years: in 2019 values in spring (April) and early summer (June) were generally lower (1.5-2.9 times) than those in all other months, whereas in 2020 values in May were ca. 1.4 times higher than those in July. The lipid contents in this site, were significantly higher (3.5 – 5.4 times) in April 2019 than those in all other months, both in 2019 and 2020. The chlorophyll-a content of Oristano sediments in April was 1.7 times higher than those in January 2020 and 1.5 times lower than those in May 2020. Moreover, chlorophyll-a contents of Oristano sediments in June 2019 and January 2020 were significantly lower (1.5-3 times) than those in August 2019, May 2020, and July 2020 (Supplementary Figure 2).
In the poorer Teulada sediments, the sedimentary protein contents varied significantly among sampling periods, with values in summer months (June, August) ca. 3-10 times higher than those in spring-winter ones (April, January), respectively of the study year. The lowest sedimentary lipid content was observed in spring 2019 (April), when values were 1.8 times lower than those in January 2020 and 6.5 times lower than those in June 2019. Lipid content in June 2019 was also significantly higher (ca. 3.3 times) than that in August 2019. Temporal variability of the chlorophyll-a contents in the poorer Teulada sediments was much less pronounced than in that in the organically enriched sediments in Oristano, with values in Summer 2019 (August) 2.5 times higher than those in Spring (April 2019) and Winter (January 2020). Phaeopigment contents differed significantly only between sites, with values in the richer Oristano sediments up to 7.7 times higher than those in the poorer Teulada ones (Supplementary Figure 2).
The biochemical composition of sediments differed spatially and temporally, as found in sedimentary OM levels (Supplementary Table 3). The pairwise tests revealed that significant differences occurred among sites in all the sampling months, but May and June (Supplementary Table 4A) and that in both sites the biochemical composition of sedimentary OM varied with time. More specifically, in the organically enriched sediments in Oristano proteins (46 – 52%) were the dominant class of organic compounds followed by carbohydrates (34 – 40%) and lipids (9 – 14%) in almost all samplings, with exceptions in April and June 2019, when carbohydrates were dominant class (54-56%) followed by lipids and proteins (April 2019, 32% and 11%, respectively; June 2019, 11% and 35%, respectively) (Supplementary Figure 3).
The biochemical composition of sediments from the poorer Teulada site was more variable (Figure 2) and significant differences among sampling occasions were observed between January 2020 vs April, June, and August 2019; and between April vs August 2019 (Supplementary Table 4B). Sediments collected in 2019 and in July 2020 were characterized by the dominance of carbohydrates (45 – 61%), followed by proteins (28 – 42%) and lipids (10 -17%); sediments collected in January (2020) showed a dominance of proteins (39%) and similar contributions of lipids and carbohydrates (32 and 29% respectively). The sediments collected in May 2020 were characterized by the dominance of proteins (46%), followed by carbohydrates (41%) and lipids (13%) (Supplementary Figure 3).
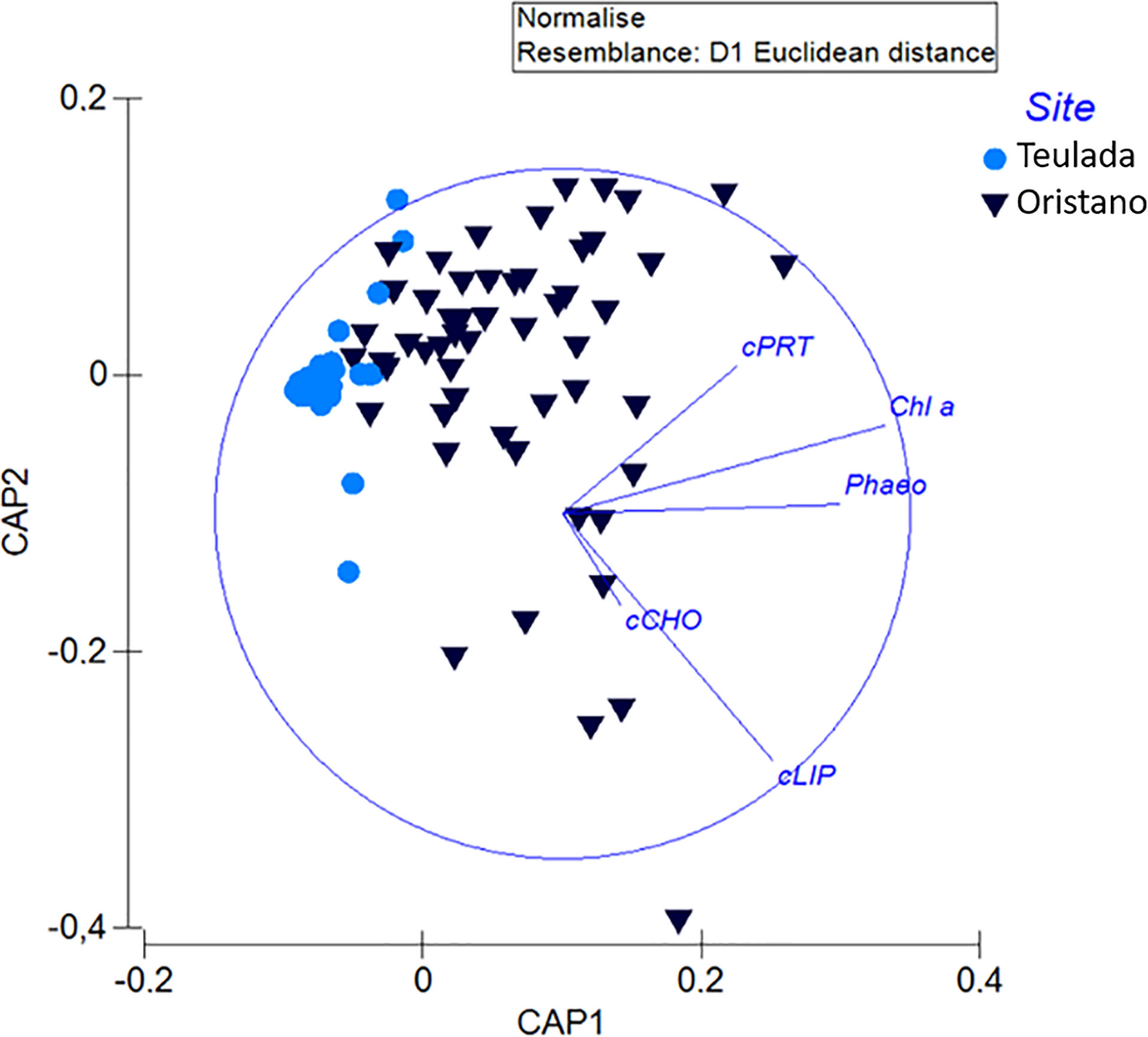
Figure 2 The biplot produced after canonical analysis of the principal coordinates (CAP) showing variations in the biochemical composition of sedimentary organic matter (chlorophyll: Chl a; phaeopigments: Phaeo; and proteins: cPRT; carbohydrates: cCHO; lipids: cLIP, converted in carbon equivalent) between the organically enriched site of Oristano and the poorer site of Teulada.
Effect of sea cucumbers on quantity and composition of the sedimentary organic matter
The effect of the sea cucumbers’ feeding was considered separately for each site. In the organically enriched Oristano site (putatively impacted by mariculture effluents), protein contents were affected by a significant effect of the Mo×Ma interaction (Table 1A). More specifically, the results of the post-hoc tests revealed that the protein content of feces was significantly higher than that in the surrounding sediment in June 2019 (2.4 times) and in July 2020 (1.9 times) (Table 2; Figure 3A). In the poorer Teulada site (mariculture-free sediments) no significant differences between the sediment and the feces were observed (Table 1B; Figure 3B), but differences occurred only among months (Supplementary Table 5). Carbohydrate content of sediment and feces did not vary between the two matrixes in each of the sampling dates in both sites (Tables 1A, B and Figures 3C, D).
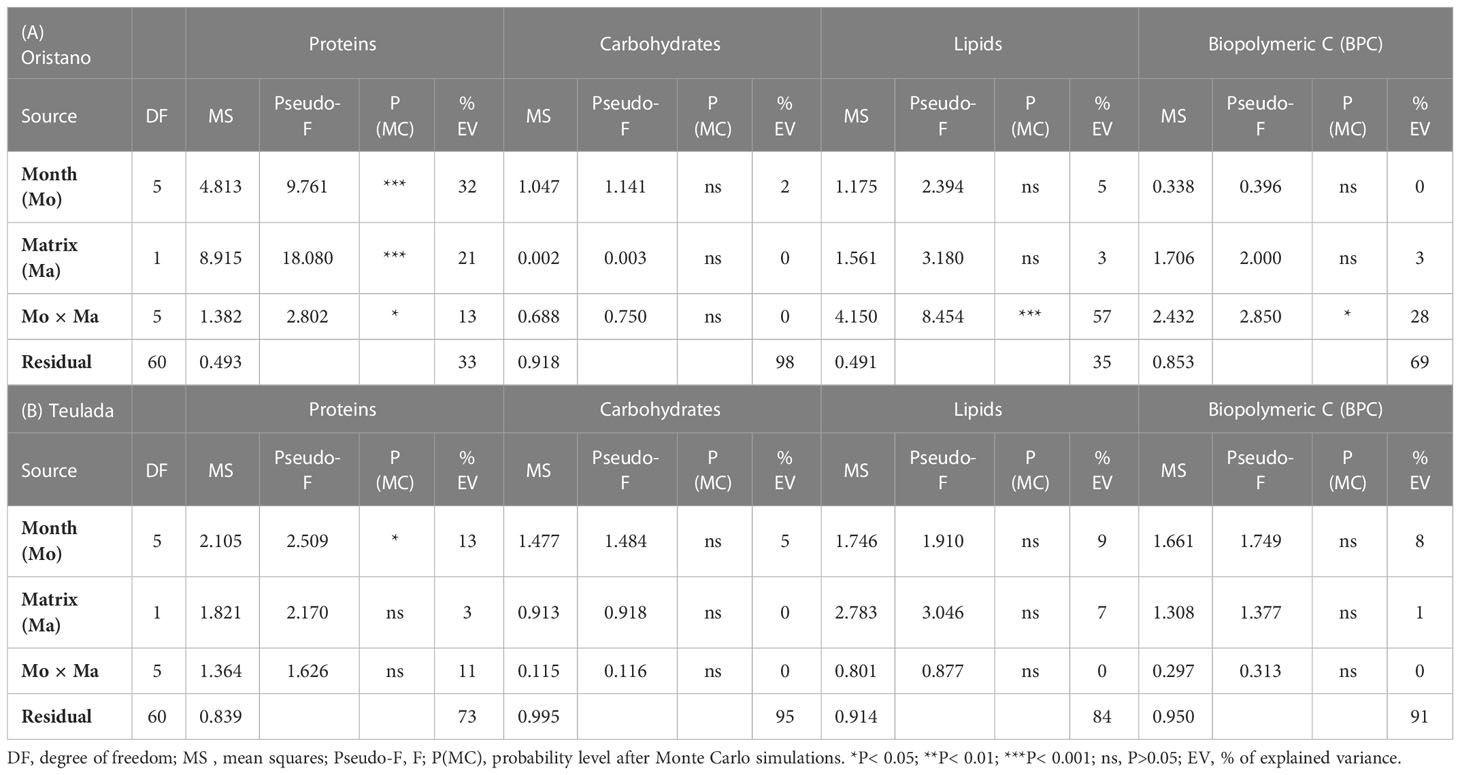
Table 1 Results of univariate PERMANOVA testing for the effects of Months (Mo) and Matrices (Ma) and their interaction on protein, carbohydrate, lipid and BPC contents in sediments and feces from the two sites of Oristano (A) and Teulada (B).
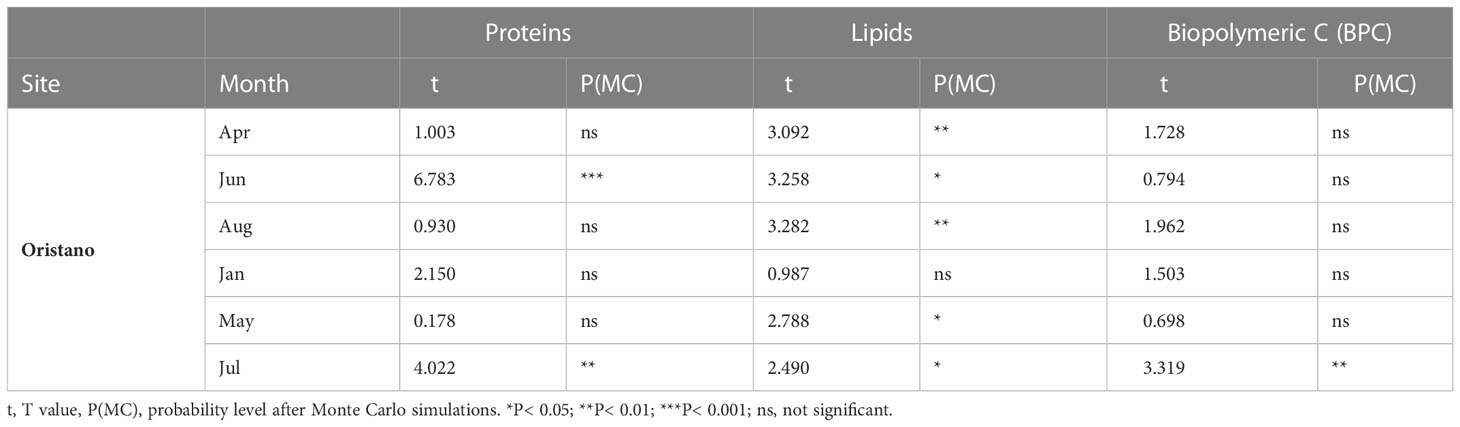
Table 2 Results of the pairwise comparisons contrasting proteins and lipid contents between sediment and feces in the meso-eutrophic site (Oristano) in all sampling dates.
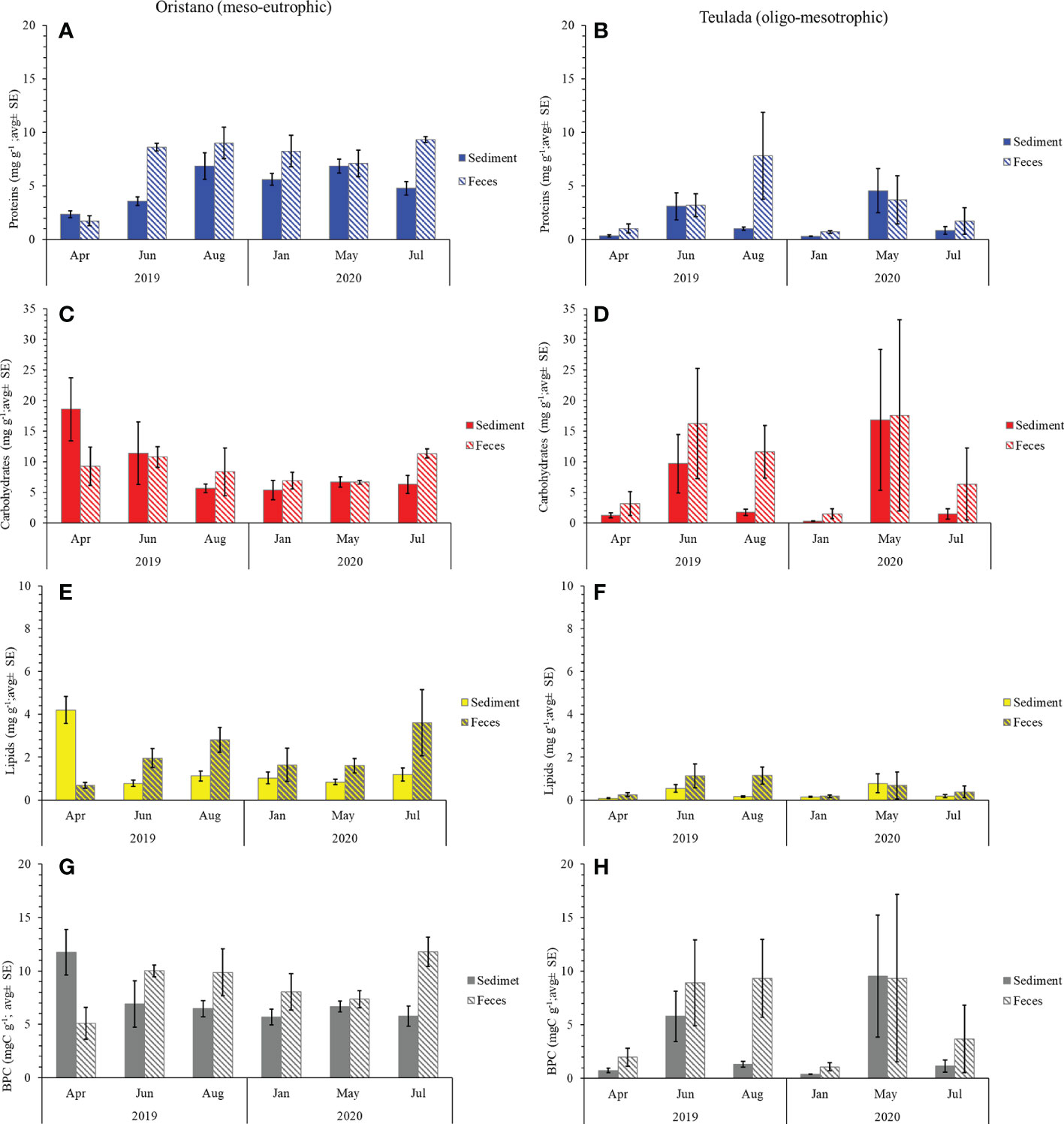
Figure 3 Protein (A, B), carbohydrate (C, D), lipid (E, F), and BPC (G, H) contents in sediment and feces from Oristano (organically enriched site) and Teulada (poorer site) during the study period. Error bars indicate standard error (sediments n=9; feces n=3).
In the organically enriched Oristano site the lipid content was affected by a significant Mo×Ma interaction (Table 1A). In more details, the results of the post-hoc tests revealed that feces were strongly depleted (-83%) in lipids when compared with the surrounding sediment only in April 2019, whereas in all other sampling months, lipid contents in the feces were significantly higher (by 48 - 67%) than those in the surrounding sediment, with the exception for January 2020, when no significant differences were observed (Table 2 and Figure 3E). In the poorer Teulada site, the lipid content did not vary significantly between the matrices in each of the sampling dates (Table 1B and Figure 3F). The biopolymeric C contents were affected by a significant Mo×Ma interaction only in the organically enriched Oristano site (Table 1A), where the post-hoc test revealed that differences in biopolymeric contents between sediments and feces were significant only in summer 2020 (July), with values in feces 2 times higher than those in the sediment (Table 2 and Figure 3G). In the poorer Teulada site biopolymeric C contents did not vary among matrices in any of the sampling periods (Table 1B and Figure 3H).
The biochemical composition of OM was affected by a significant interaction of the factors Mo×Ma only in the organically enriched Oristano site (Table 3A). More in details, differences in OM composition between sediment and feces in Oristano occurred only in April 2019, when carbohydrate and protein contributions to biopolymeric C were higher in feces than in the sediments, at the expense of lipids, and in Jul-Aug 2020 because of a higher lipid contribution in feces, at the expenses of proteins (Table 3B; Figure 4A). In the poorer Teulada, the biochemical composition did not vary between sediment and feces in all sampling periods (Table 3B; Figure 4B).
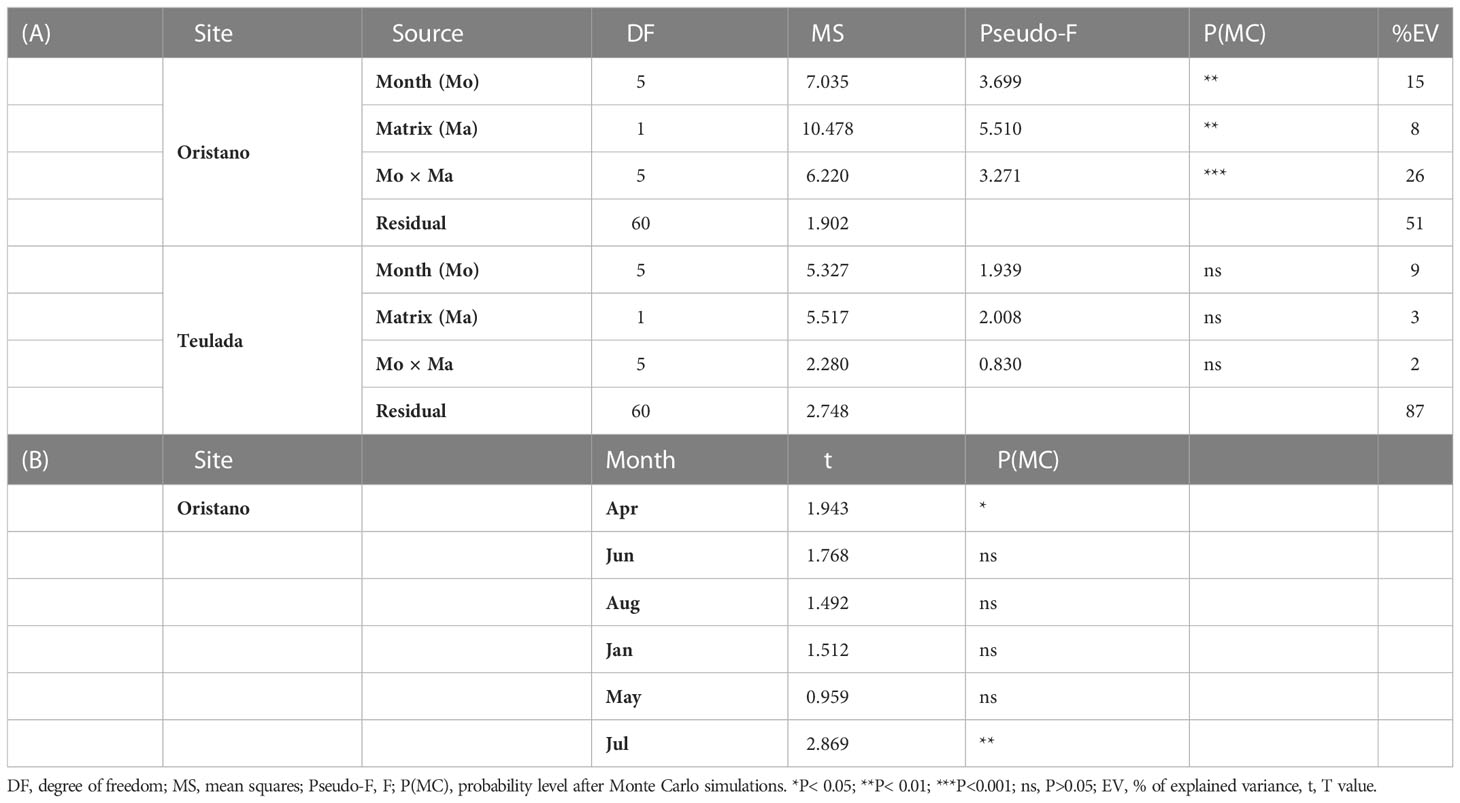
Table 3 Results of multivariate PERMANOVA testing for the effects of Month, Matrices and their interactions on the biochemical composition of OM in two study sites (A); and results of the pairwise comparisons testing for differences in the biochemical composition of OM between sediment and feces in the meso-eutrophic site (Oristano) during the study period (B).
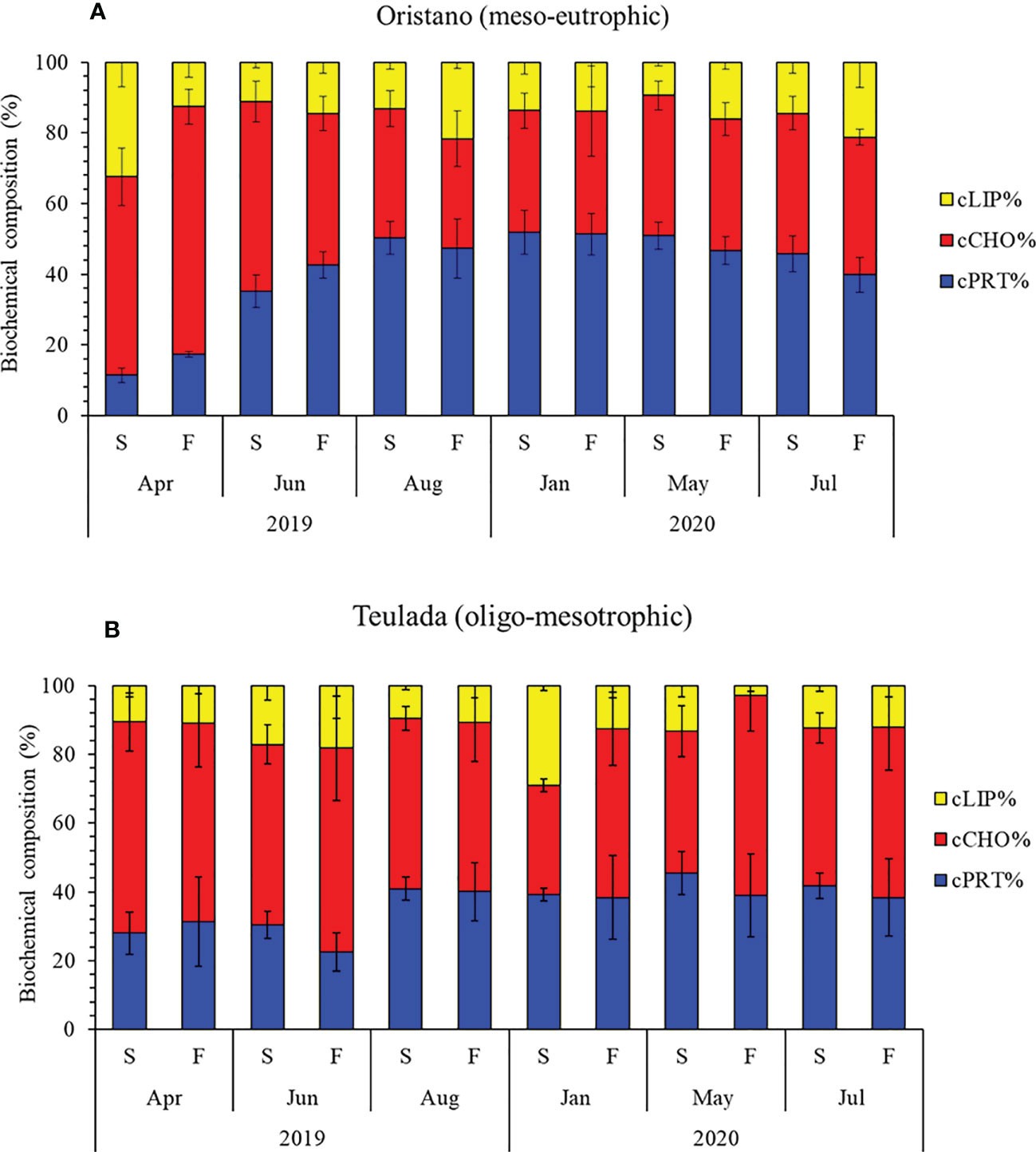
Figure 4 Variations in the percentage contribution of proteins, carbohydrates, and lipids to the BPC in sediments (S) and feces (F) in the organically enriched site of Oristano (A) and in the poorer site of Teulada (B). Error bars indicate the standard error (sediments n=9; feces n=3). cLIP, lipid; cCHO, carbohydrate; cPRT, protein.
Proteins quantity in food sources, holothuroids’ digestive tracts and feces
The results revealed that the protein content differed significantly among the different matrices (ambient sediment, digestive tracts, and feces) only in the organically enriched Oristano site (Table 4A), where proteins’ content of sediment in the esophagus was ca. 2 times higher than that in the ambient sediment, and slightly decreased along the other digestive tracts (Table 4B; Figure 5). The estimated digestion rate of proteins along the digestive tracts of H. tubulosa (Figure 5A) was slightly higher (40%) in specimens from the poorer Teulada site than that in specimens from the organically enriched Oristano site (30%). As a result, the amounts of proteins accumulated in the esophagus in specimens from Oristano are so high that feces are enriched in proteins when compared to the ambient sediment, whereas in the poorer Teulada site feces have protein contents similar to those measured in the ambient sediment (Figures 5B, C).
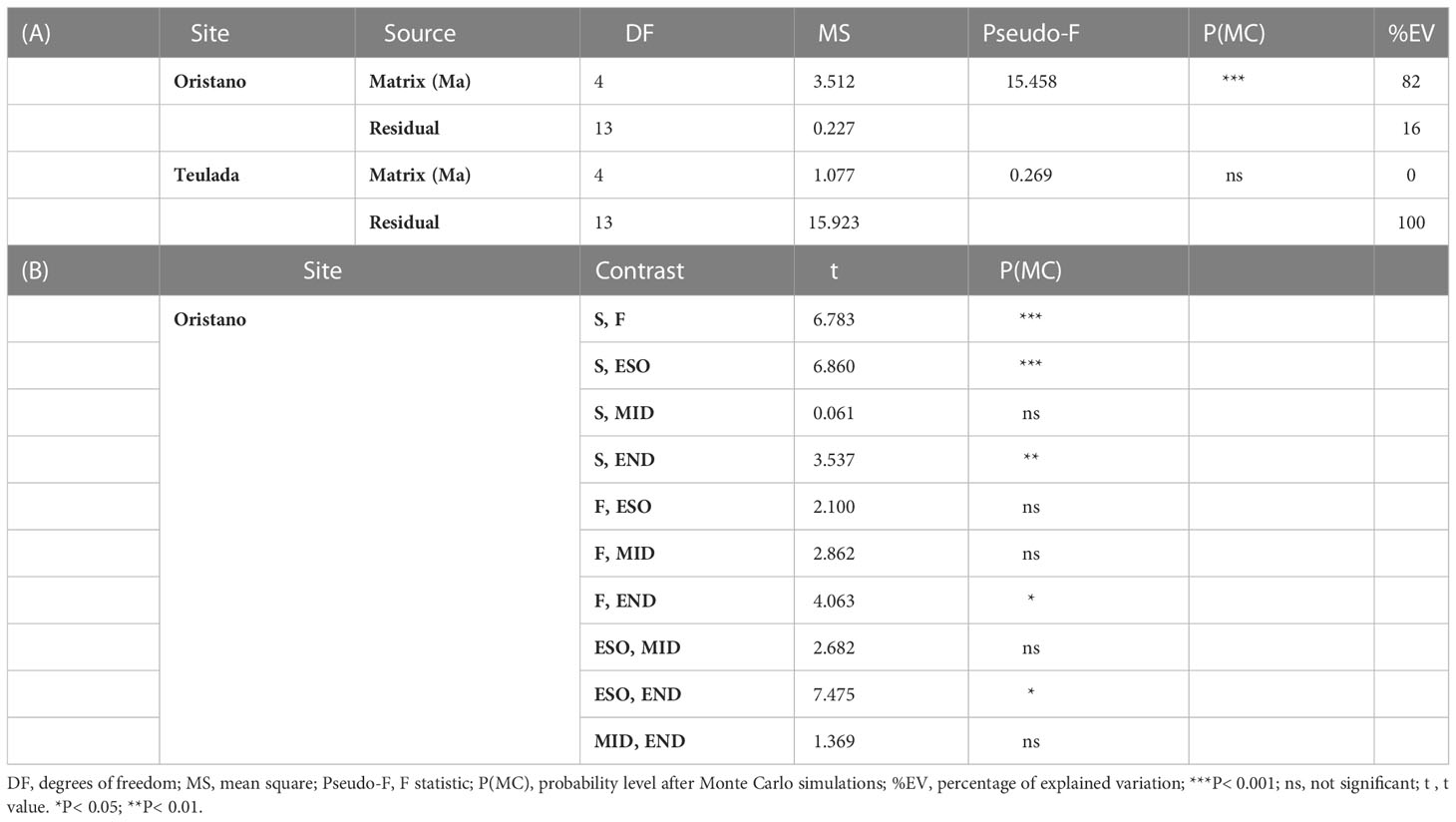
Table 4 Results of the univariate PERMANOVA testing differences in the protein content of the source sediment, digestive tract and feces of H. tubulosa in the two study sites (A); and results of the pairwise comparison testing for difference in protein contents in the source sediment (S), digestive tracts (esophagus = ESO; mid gut = MID; end gut = END) and feces (F) in the meso-eutrophic site (Oristano) (B).
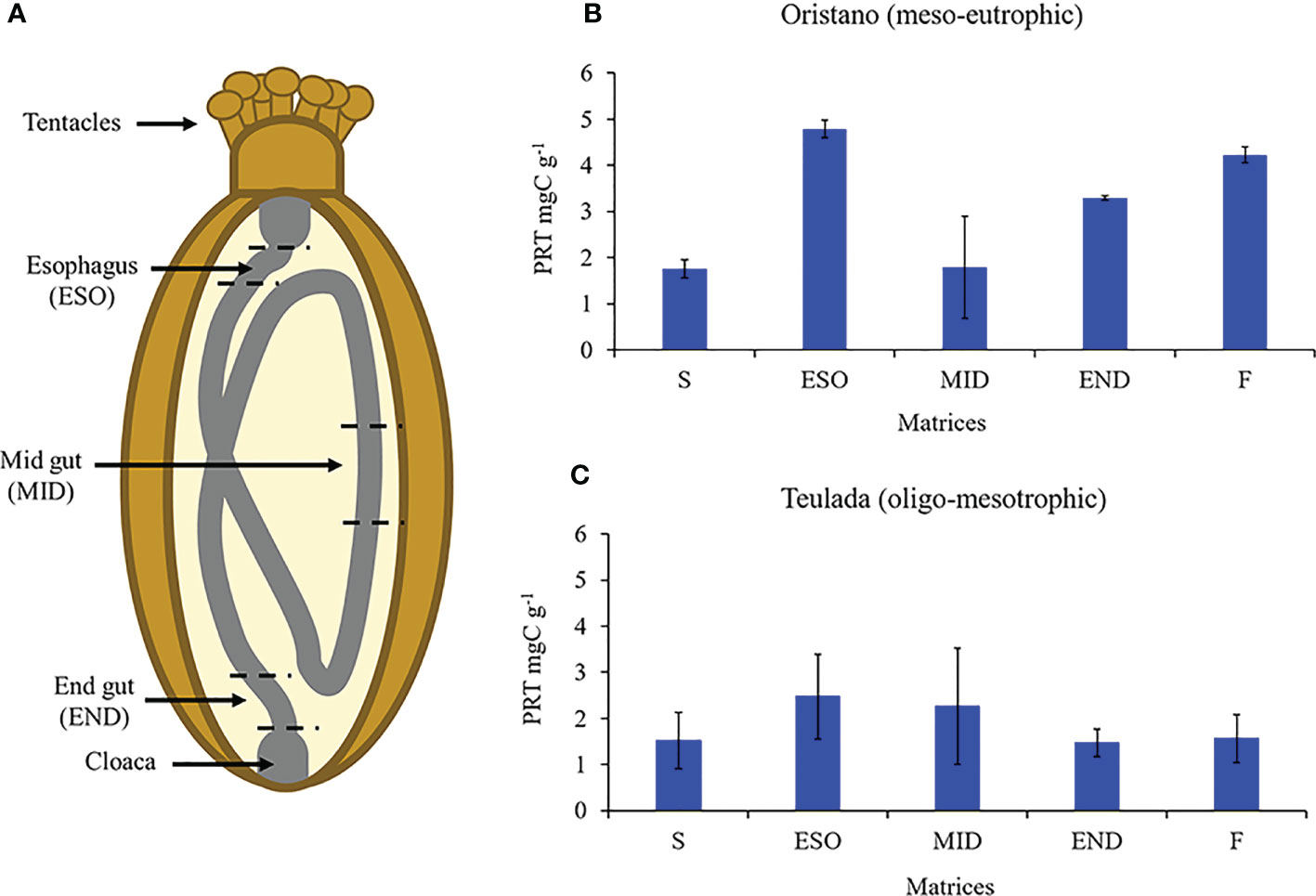
Figure 5 Schematic representation of sea cucumbers’ digestive tract (A). Protein contents (PRT) of source sediment (S), sediment in the esophagus (ESO), mid gut (MID), and end gut (END) and feces (F) of holothurians from the meso-eutrophic site (Oristano, B) and from the oligo-mesotrophic site (Teulada, C). Error bars indicate standard error (n=9 S; n=3 ESO; MID; END; F).
Discussion
Trophic status of ambient sediments
We report here that, as expected, the two investigated sites were characterized by well-delineated differences in benthic trophic status, as assessed in terms of quantity and composition of sedimentary OM. The sandy-mud sediments of the Oristano site, influenced by putative organic inputs released by a close mariculture plant, were in fact characterized by biopolymeric C contents (dominated by proteins) generally > 3 mgC g-1 and by a limited temporal variability of either quantity or composition of sedimentary OM. The sandy sediments of the Teulada site were characterized by biopolymeric C contents varying from<1 mgC g-1 in Winter and early Spring to 1-3 (or higher) mgCg-1 in Summer, and a much wider temporal variability of OM biochemical composition (though generally characterized by the dominance of carbohydrates). Overall, chlorophyll-a contents and the algal contribution to biopolymeric C (estimated as the percentage fraction of total phytopigments transformed in C equivalents over biopolymeric C contents) were higher in the Oristano than in the Teulada sediments. Meso-eutrophic sediments are typically characterized by biopolymeric C contents > 3 mgC g-1 (Pusceddu et al., 2011) and a general dominance of proteins over carbohydrates (Pusceddu et al., 2009; Pusceddu et al., 2011), which makes them a more labile source of food for benthic (especially deposit-feeders) consumers (Pusceddu et al., 2009). According to this, we can rank the sediments from Oristano as meso-eutrophic, and those from Teulada as oligo-mesotrophic. Moreover, the contribution of total phytopigments (as a proxy of OM of primary origin) to biopolymeric C was slightly higher in Oristano (ca. 14 ± 4%) than in Teulada (ca. 10 ± 4%). Since the higher the algal fraction of biopolymeric C, the higher the lability of sediment OM for benthic consumers (Pusceddu et al., 2003), altogether our results indicate that the two sites offered totally different trophic conditions (i.e., food availability levels) to the holothuroids.
Effects of H. tubulosa on the benthic trophic status
Deposit-feeders sea cucumbers are generally associated with soft organic-rich bottoms, where they rework the upper sediment layers feeding on the most readily utilizable OM (Yingst, 1982; Roberts et al., 2000; Purcell et al., 2016). The studies conducted so far on the feeding behavior of coastal sea cucumbers investigated the fate of total organic matter (TOM, as obtained by the loss on ignition technique) and/or total organic carbon (TOC), without considering the biochemical composition and the biopolymeric C content of the sediments. Both TOM and TOC include any organic molecule and do not allow a distinction between labile (i.e., nutritionally available) vs. refractory fractions of OM (Pusceddu et al., 2009). In this regard, most recently the biochemical gross composition of sedimentary OM (in terms of protein, carbohydrate, and lipid contents) has been used as a proxy of OM availability for benthic consumers (Danovaro et al., 2001; Gambi et al., 2014; Gambi et al., 2017).
To shed light on the potential role of H. tubulosa on the benthic trophic status, we investigated differences in quantity and biochemical composition of ambient sediments and sea cucumbers’ feces in two distinct sites characterized by the above-described different background trophic status. Although the analysis refers to a relatively limited number of individuals in each sampling unit, our data suggest that the feces produced by H. tubulosa feeding on sediment from the less productive “oligo-mesotrophic” site showed the same OM contents as the ambient sediment. Instead, sea cucumbers feeding on richer (meso-eutrophic) sediments (Oristano) produced, especially in Summer, feces with OM contents significantly higher than those of the surrounding sediment. These results are consistent with previous studies conducted by comparing when OM contents in sediment and feces of other holothuroids (Mercier et al., 1999). These results are apparently in contrast with those from previous studies showing that holothuroids feeding can abate OM sedimentary contents on long time scales (Paltzat et al., 2008; Slater and Carton, 2009; MacDonald et al., 2013; Neofitou et al., 2019). At a first glance, such a discrepancy could be related to the different proxy of OM quantity used in this study (biopolymeric C) and that (TOM) used in previous studies. Although we did not estimate TOM contents in the sediment and the feces, such discrepancy suggests that H. tubulosa consumes TOM, but releases feces enriched in biopolymeric compounds. Since biopolymeric C is assumed to represent the most labile fraction of OM, our results suggest that other mechanisms, besides nutrition and assimilation, could be responsible for increasing BPC contents in feces. For instance, based on the results of previous studies which identified meiofauna as a food source for holothuroids (Wolfe and Byrne, 2017; Wolfe et al., 2018; Wolfe et al., 2021), we cannot exclude that some of the observed patterns could be also due to the different intake of meiofauna in the two different environmental assets. Moreover, we have to consider that seasonal variations in the intake of organic C by holothuroids, caused, for instance, by aestivation in Summer and hibernation in Winter as observed in other temperate species (e.g., A. japonicus; Ji et al., 2008; Yu et al., 2014) could cause variations in their behavior and effects on sedimentary OM. Nevertheless, nor previous studies (Günay et al., 2019) nor our study have observed this in H. tubulosa.
We notice also that the overall increase in BPC contents of feces produced by holothuroids feeding on OM-rich sediments (Oristano), was accompanied by an increase of the most labile components of OM (i.e., proteins and lipids). Since both proteins and lipids are high-energy compounds putatively used by holothuroids for their somatic growth and gonad development, respectively (Yang et al., 2006; Slater et al., 2011b), their enrichment in the feces appears counterintuitive. Whatever the potential explanations (see below), this result first indicates that feces released by holothuroids during their feeding can enhance locally and temporarily the nutritional quality of the ambient sediment. Since this enrichment process is not detected in the more oligotrophic site, we contend that the efficacy of sea cucumbers as bioreactors (whatever the effect on the sediment trophic status) is most likely modulated by the actual quantity and nutritional quality of the sedimentary OM.
Fate of proteins ingested by H. tubulosa: Role of food particle selection and estimate of potential assimilation of proteins
As delineated above, the protein content in H. tubulosa feces collected from the meso-eutrophic site (Oristano) is higher than that in the ambient sediment. This apparently counterintuitive result could be caused by the peculiar feeding behavior of the sea cucumbers and, to shed light on the mechanism behind such a phenomenon, we compared protein contents in ambient sediment with those in sediment remains within the holothuroids’ gut and feces from the two sites. The protein content in the esophagus of H. tubulosa is significantly higher than that in the source sediment only in specimens collected from the meso-eutrophic site (Oristano), suggesting, in this trophic asset, the presence of a possible accumulation or a concentration mechanism of proteins in the esophagus. This result is consistent with the results reported on the protein contents of the deep-sea holothuroid Molpadia musculus gut, in which the concentration factor was estimated to be 3.1 (Amaro et al., 2010). Other studies, based on total N or total OM contents reported a similar concentration mechanism in Australostichopus mollis (Slater et al., 2011a, b; Zamora and Jeffs, 2011) and Parastichopus californicus (Paltzat et al., 2008). The increase in proteins (or total OM) contents in sediment remains retrieved from the esophagus can be explained by the presence of a selection mechanism of protein-rich particles ingested by holothuroids. Several studies have indeed proven that holothuroids, rather than simply packing up sediment particles with mucus and digestive fluids, can select, possibly by chemo-selection mechanisms, food particles with their tentacles (Uthicke and Karez, 1999; Roberts et al., 2000; Dar and Ahmad, 2006; Paltzat et al., 2008; Slater et al., 2011a; Navarro et al., 2013; Ramón et al., 2019; Hartati et al., 2020). However, this hypothesis is not supported everywhere as, in our study, the concentration mechanism was reported only for holothuroids from the OM-enriched sediments (Oristano). Nevertheless, we must notice that the protein and lipid enrichment of gut sediments and feces of holothuroids, when compared to ambient sediments, could be also the result of the presence of mucus coating the feces before their release (Mercier et al., 1999; Slater et al., 2011b). Based on our sampling approach, we cannot exclude this, but we notice here that such enrichment is documented only from OM-enriched sediments, giving room to consider this process the result of mixed effects associated with either the physiology of feces production or with the process of food particles selection.
The apparently different behavior of holothuroids feeding on sediments with different OM contents could be related to the different sediment grain size and organic nutritional quality observed in the two sites. Sediments from the meso-eutrophic site (Oristano) show a fraction of mud and fine sand, including the particles typically selected with buccal tentacles (Mezali and Soualili, 2013), higher than that in the oligo-mesotrophic site (Teulada). Moreover, we report here also that the estimated protein assimilation rate of holothuroids from the oligo-mesotrophic site (ca. 40%) is higher than that (ca. 30%) of holothuroids from the meso-eutrophic one. This result fits the known inverse relationship between assimilation rates and availability of organic substrates (Roberts et al., 2000; Zamora and Jeffs, 2011) and the optimal foraging theory, which foresees that consumers tend to optimize energy intake by means of a trade-off between the quantity of energy necessary for survival and that spent to acquire it (Stephens and Krebs, 1986). Our contention, indeed, corroborated by the finding of higher defecation rates of the holothuroid Thelenota anax in sediments with lower organic matter levels (Hammond et al., 2020), suggests that feces produced by holothuroids in oligo-mesotrophic bottoms are poorer in protein contents than those from meso-eutrophic sediments because of their larger needs of accumulating energy from a poorer food source. Although on a merely speculative basis, due to the restricted number of specimens considered here, our results support the hypothesis that the sea cucumber H. tubulosa can select food particles from the surrounding sediment and concentrate them in the esophagus, but also that the potential assimilation can be strongly dependent upon the relative amount of available food and its nutritional quality.
Protecting and harnessing sea cucumbers’ environmental services
With an eye to the possible use of sea cucumbers as a bioremediation tool of eutrophicated sediments, since proteins are N-rich compounds, and N is the most limiting factor for heterotrophic nutrition (Dell’Anno et al., 2002; Pusceddu et al., 2009), we conclude that the availability of protein-enriched feces could foster and facilitate the transfer of energy towards higher trophic levels, thus limiting benthic eutrophication (i.e., the accumulation of huge amounts of labile organic C in sediments below fish cages; Pusceddu et al., 2007). In this sense, H. tubulosa, by accelerating the detritus cycling, especially in OM-enriched sea floors, could be also potentially used to condition the benthic trophic status for additional components (including reared species, such as small scavenging crustaceans) of the ‘manipulated’ food webs in Integrated Multi-Trophic Aquaculture (IMTA) (Neofitou et al., 2019). Encouraging results from recent studies on breeding Mediterranean holothuroids, including H. tubulosa, pave the way for the possibility of using captive-bred specimens as tools for bioremediation and IMTA purposes, at the same time limiting the exploitation of this protected species (Rakaj et al., 2018; Pasquini, 2022).
Conclusions
H. tubulosa is a “continuous” feeder that can ingest and rework large amounts of sediment and can have a role on the recycling of seagrass detritus in Posidonia oceanica meadows (Costa et al., 2014; Boncagni et al., 2019). Here we showed that the sea cucumber H. tubulosa can influence the benthic trophic status of marine sediments, specifically by modifying the quantity, biochemical composition, and nutritional quality of sedimentary OM. Our results proved also that H. tubulosa influences particularly the sedimentary contents of the most labile molecules (i.e., proteins and lipids) and that, by producing protein- and lipid-enriched feces (especially in meso-eutrophic assets), can contribute making labile substrates more available for higher trophic levels.
Holothuroid fisheries had rapidly grown and expanded in the last three decades and large market demand for these animals has led to overfishing in worldwide oceans and seas, including the Mediterranean. Since our results have shown that holothuroids play an important role in conditioning sediment OM content, possibly interacting with species from different trophic levels, we anticipate that their overfishing, besides posing their populations at risk, might have severe consequences on the structure of benthic communities and ecosystem functioning.
Based on the changes in protein concentration of sediment within the digestive tract we also provided elements in support of the hypothesis of an active selection of labile food particles by holothuroids and that this mechanism could be dependent upon sediment grain size and the actual bioavailability of organic substrates. Finally, based on our results, we conclude that, since the ‘bioreactor’ efficacy of H. tubulosa can vary according to the OM quantity biochemical composition and nutritional quality of the ambient sediment, their use in Integrated Multi-trophic Aquaculture and bioremediation actions should be modulated accordingly.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
VP, AP, and PA conceived and planned the experiments. VP, AG and DM carried out the sampling and laboratory analyses, VP and AP carried out the statistical analyses. All authors contributed to the interpretation of the results. VP and PA took the lead in writing the manuscript followed by extensive revision by AP to achieve the final version. All authors provided critical feedback and helped shape the research, analyses, and manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study has been carried out in the framework of the projects “Marine habitats restoration in a climate change-impaired Mediterranean Sea [MAHRES]”, funded by the Ministero dell’Università e della Ricerca under the PRIN 2017 call (Protocol: 2017MHHWBN; CUPF74I19001320001), and “Innovative species of commercial interest for Sardinian aquaculture: development of experimental protocols for the breeding of sea cucumbers, (project n.1/INA/2.47/2017)” funded by the European Maritime and Fisheries Fund (EMFF) Programme 2014/2020, Measure:2.47 – Innovation; and the EU co-founded project “InEVal: Increasing Echinoderm Value Chains” (grant n. ID 101 InEVal) funded by ERA-NET BlueBio programme.
Acknowledgments
The authors wish to express their gratitude to the Marine Protected Area Penisola del Sinis – Isola di Mal di Ventre in the people of the director Ing. Massimo Marras and Dr. Roberto Brundu; Dr. Gaspare Barbera technical manager of the L.P.A. Group fish farm and the Marina di Teulada in the person of Mrs. Barbara Lai, for the logistic support in the field sampling. We are thankful to Dr. Marco Secci and Mr. Marco Maxia (Agris Sardinia Agency) for their help in the field and sampling activities. The collection of sea cucumbers was authorized with Scientific Research Permit for echinoderms by Regione Autonoma della Sardegna (Prot. N. 6261, 03/05/2018; Prot. N. 1845, 06/02/2019; Prot. N. 20735, 28/11/2019).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.1010014/full#supplementary-material
References
Amaro T., Bianchelli S., Billett D. S. M., Cunha M. R., Pusceddu A., Danovaro R. (2010). The trophic biology of the holothurian Molpadia musculus: Implications for organic matter cycling and ecosystem functioning in a deep submarine canyon. Biogeosciences 7, 2419–2432. doi: 10.5194/bg-7-2419-2010
Anderson M. J. (2017). “Permutational multivariate analysis of variance (PERMANOVA),” in Wiley StatsRef: Statistics reference online. Eds. Balakrishnan N., Colton T., Everitt B., Piegorsch W., Ruggeri F., Teugels J. L. (Wiley), 1–15.
Anderson S. C., Flemming J. M., Watson R., Lotze H. K. (2011a). Rapid global expansion of invertebrate fisheries: Trends, drivers, and ecosystem effects. PloS One 6 (3), 1–9. doi: 10.1371/journal.pone.0014735
Anderson S. C., Flemming J. M., Watson R., Lotze H. K. (2011b). Serial exploitation of global sea cucumber fisheries. Fish. Fish. 12, 317–339. doi: 10.1111/j.1467-2979.2010.00397.x
Anderson M. J., Gorley R. N., Clarke K. R. (2008). PERMANOVA+ for PRIMER: Guide to software and statistical methods. 1st ed (Plymouth, UK: PRIMER-E).
Anderson M. J., Millar R. B. (2004). Spatial variation and effects of habitat on temperate reef fish assemblages in northeastern new Zealand. J. Exp. Mar. Biol. Ecol. 305, 191–221. doi: 10.1016/j.jembe.2003.12.011
Anderson M. J., Willis T. J. (2003). Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology 84, 511–525. doi: 10.1890/0012-9658(2003)084[0511:CAOPCA]2.0.CO;2
Bligh E. G., Dyer W. J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917. doi: 10.1139/o59-099
Boncagni P., Rakaj A., Fianchini A., Vizzini S. (2019). Preferential assimilation of seagrass detritus by two coexisting Mediterranean sea cucumbers: Holothuria polii and. Holothuria tubulosa. Estuar. Coast. 231, 106464. doi: 10.1016/j.ecss.2019.106464
Bordbar S., Anwar F., Saari N. (2011). High-value components and bioactives from sea cucumbers for functional food - a review. Mar. Drugs 9 (10), 1761–1805. doi: 10.3390/md9101761
Bulteel P., Jangoux M., Coulon P. (1992). Biometry, bathymetric distribution, and reproductive cycle of the holothuroid Holothuria tubulosa (Echinodermata) from Mediterranean Sea grass beds. Mar. Ecol. 13, 53–62. doi: 10.1111/j.1439-0485.1992.tb00339.x
CITES (2022) Checklist of CITES species. Available at: https://checklist.cites.org (Accessed December 14, 2020).
Cloern J. E., Grenz C., Vidergar-Lucas L. (1995). An empirical model of the phytoplankton chlorophyll: Carbon ratio-the conversion factor between productivity and growth rate. Limnol. Oceanogr. 40, 1313–1321. doi: 10.4319/lo.1995.40.7.1313
Conand C. (2006). “Sea Cucumber biology, taxonomy, distribution and conservation status,” in Proceedings of the CITES workshop on the conservation of sea cucumbers in the families holothuriidae and stichopodidae. Ed. Bruckner A. W. (Silver Spring, MD: NOAA Technical Memorandum NMFSOPR 34), 33–50.
Conand C., Polidoro B., Mercier A., Gamboa R., Hamel J.-F., Purcell S. (2014). The IUCN red list assessment of aspidochirotid sea cucumbers and its implications. SPC Beche-de-mer Inf. Bull. 34, 3–7.
Costa V., Mazzola A., Vizzini S. (2014). Holothuria tubulosa gmelin 1791 (Holothuroidea, Echinodermata) enhances organic matter recycling in Posidonia oceanica meadows. J. Exp. Mar. Bio. Ecol. 461, 226–232. doi: 10.1016/j.jembe.2014.08.008
Coulon P., Jangoux M. (1993). Feeding rate and sediment reworking by the holothuroid Holothuria tubulosa (Echinodermata) in a Mediterranean seagrass bed off ischia island, Italy. Mar. Ecol. Prog. Ser. 92, 201–204. doi: 10.3354/meps092201
Cubillo A. M., Ferreira J. G., Robinson S. M. C., Pearce C. M., Corner R. A., Johansen J. (2016). Role of deposit feeders in integrated multi-trophic aquaculture – a model analysis. Aquaculture 453, 54–66. doi: 10.1016/j.aquaculture.2015.11.031
Danovaro R. (2010). Methods for the study of deep-sea sediments, their functioning and biodiversity (Boca Raton: CRC Press, Taylor & Francis Group).
Danovaro R., Dell’Anno A., Fabiano M. (2001). Bioavailability of organic matter in the sediments of the porcupine abyssal plain, northeastern Atlantic. Mar. Ecol. Prog. Ser. 220, 25–32. doi: 10.3354/meps220025
Dar M. A., Ahmad H. O. (2006). The feeding selectivity and ecological role of shallow water holothurians in the red Sea. SPC Beche-de-mer Inf. Bull. 24, 11–21.
Dell’Anno A., Mei M. L., Pusceddu A., Danovaro R. (2002). Assessing the trophic state and eutrophication of coastal marine systems: a new approach based on the biochemical composition of sediment organic matter. Mar. pollut. Bull. 44, 611–622. doi: 10.1016/s0025-326x(01)00302-2
Dell’Anno A., Pusceddu A., Corinaldesi C., Canals M., Heussner S., Thomsen L., et al. (2013). Trophic state of benthic deep-sea ecosystems from two different continental margins off Iberia. Biogeosciences 10, 2945–2957. doi: 10.5194/bg-10-2945-2013
Dereli H., Aydın M. (2021). Sea Cucumber fishery in Turkey: Management regulations and their efficiency. Reg. Stud. Mar. Sci. 41, 101551. doi: 10.1016/j.rsma.2020.101551
Fabiano M., Danovaro R., Fraschetti S. (1995). A three-year time series of elemental and biochemical composition of organic matter in subtidal sandy sediments of the ligurian Sea (Northwestern Mediterranean). Cont. Shelf Res. 15, 1453–1469. doi: 10.1016/0278-4343(94)00088-5
Gambi C., Corinaldesi C., Dell’Anno A., Pusceddu A., D’Onghia G., Covazzi-Harriague A., et al. (2017). Functional response to food limitation can reduce the impact of global change in the deep-sea benthos. Global. Ecol. Biogeogr. 26 (9), 1008–1021. doi: 10.1111/geb.12608
Gambi C., Pusceddu A., Benedetti-Cecchi L., Danovaro R. (2014). Species richness, species turnover and functional diversity in nematodes of the deep Mediterranean Sea: Searching for drivers at different spatial scales. Global. Ecol. Biogeogr. 23 (1), 24–39. doi: 10.1111/geb.12094
Gerchakov S. M., Hatcher P. G. (1972). Improved technique for analysis of carbohydrates in sediments. Limnol. Oceanogr. 17, 938–943. doi: 10.4319/lo.1972.17.6.0938
González-Wangüemert M., Aydin M., Conand C. (2014). Assessment of sea cucumber populations from the Aegean Sea (Turkey): first insights to sustainable management of new fisheries. Ocean Coast. Manage. 92, 87–94. doi: 10.1016/j.ocecoaman.2014.02.014
González-Wangüemert M., Domínguez-Godino J. A., Cánovas F. (2018). The fast development of sea cucumber fisheries in the Mediterranean and NE Atlantic waters: from a new marine resource to its over-exploitation. Ocean Coast. Manage. 151, 165–177. doi: 10.1016/J.OCECOAMAN.2017.10.002
González-Wangüemert M., Valente S., Aydin M. (2015). Effects of fishery protection on biometry and genetic structure of two target sea cucumber species from the Mediterranean Sea. Hydrobiologia 743, 65–74. doi: 10.1007/s10750-014-2006-2
Grosso L., Rakaj A., Fianchini A., Morroni L., Cataudella S., Scardi M. (2021). Integrated multi-trophic aquaculture (IMTA) system combining the sea urchin Paracentrotus lividus, as primary species, and the sea cucumber Holothuria tubulosa as extractive species. aquaculture. Aquaculture 534, 736268. doi: 10.1016/j.aquaculture.2020.736268
Günay D., Emiroğlu D., Suzer C. (2019). Seasonal variations of digestive enzymes in sea cucumbers (Holothuria tubulosa, G. 1788) under culture conditions. J. Exp. Zool. 333 (3), 144–150. doi: 10.1002/jez.2336
Hammond A. R., Meyers L., Purcell S. W. (2020). Not so sluggish: movement and sediment turnover of the world’s heaviest holothuroid, Thelenota anax. Mar. Biol. 167 (5), 1–9. doi: 10.13057/biodiv/d210552
Hartree E. F. (1972). Determination of proteins: A modification of the lowry method that gives a linear photometric response. Anal. Biochem. 48, 422–427. doi: 10.1016/0003-2697(72)90094-2
Hartati R., Zainuri M, Ambariyanto A, Widianingsih W (2020). Feeding selectivity of Holothuria atra in different micro-habitat in Panjang Island, Jepara (Java, Indonesia). Biodiversitas 21, 2233–2239. doi: 10.13057/biodiv/d210552
Holmer M., Argyrou M., Dalsgaard T., Danovaro R., Diaz-Almela E., Duarte C. M., et al. (2008). Effects of fish farm waste on Posidonia oceanica meadows: Synthesis and provision of monitoring and management tools. Mar. Poll. Bull. 56 (9), 1618–1629. doi: 10.1016/j.marpolbul.2008.05.020
Holmerm M. (2010). Environmental issues of fish farming in offshore waters: Perspectives, concerns and research needs. Aquac. Env. Int. 1, 57–70. doi: 10.3354/aei00007
Horton T., Kroh A., Ahyong S., Bailly N., Boyko C. B., Brandão S. N., et al. (2018). World register of marine species (WoRMS). Available at: https://www.marinespecies.org/imis.php?module=ref&refid=291592.
ICRAM (2001). Sedimenti, scheda 3: Analisi delle caratteristiche granulometriche (Roma, Italy: Italian Ministry for Environment), 122.
Ji T., Dong Y., Dong S. (2008). Growth and physiological responses in the sea cucumber, Apostichopus japonicus selenka: Aestivation and temperature. Aquaculture 283, 180–187. doi: 10.1016/j.aquaculture.2008.07.006
Kalantzi I., Karakassis I. (2006). Benthic impacts of fish farming: Meta-analysis of community and geochemical data. Mar. Poll. Bull. 52, 484–493. doi: 10.1016/j.marpolbul.2005.09.034
Kim T., Yoon H. S., Shin S., Oh. M. H., Kwon I., Lee J., et al. (2015). Physical and biological evaluation of co-culture cage systems for grow-out of juvenile abalone, Haliotis discus hannai, with juvenile sea cucumber, Apostichopus japonicus (Selenka), with CFD analysis and indoor seawater tanks. Aquaculture 447, 86–101. doi: 10.1016/j.aquaculture.2014.07.001
Koukouras A., Sinis A. I., Bobori D., Kazantzidis S., Kitsos M. S. (2007). The echinoderm (Deuterostomia) fauna of the Aegean Sea, and comparison with those of the neighbouring seas. J. Biol. Res. 7, 67–92.
Lamprianidou F., Telfer T., Ross L. G. (2015). A model for optimization of the productivity and bioremediation efficiency of marine integrated multitrophic aquaculture. estuar. Coast. Shelf Sci. 164, 253–264. doi: 10.1016/j.ecss.2015.07.045
Lohrer A. M., Thrush S. F., Gibbs M. M. (2004). Bioturbators enhance ecosystem function through complex biogeochemical interactions. Nature 431 (7012), 1092–1095. doi: 10.1038/nature03042
Lorenzen C., Jeffrey J. (1980). Determination of chlorophyll in sea water. UNESCO. Tech. Papers Mar. Sci. 35, 1–20.
Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951). Protein measurement with the folin phenol reagent. J. Biol. Chem. 193, 265–275. doi: 10.1016/S0021-9258(19)52451-6
MacDonald C. L. E., Stead S. M., Slater M. J. (2013). Consumption and remediation of European seabass (Dicentrarchus labrax) waste by the sea cucumber Holothuria forskali. Aquac. Int. 21, 1279–1290. doi: 10.1007/s10499-013-9629-6
MacTavish T., Stenton-Dozey J., Vopel K., Savage C. (2012). Deposit-feeding sea cucumbers enhance mineralization and nutrient cycling in organically enriched coastal sediments. PloS One 7 (11), 50031. doi: 10.1371/journal.pone.0050031
Mangion P., Taddei D., Conand C., Frouin P. (2004). “Feeding rate and impact of sediment reworking by two deposit feeders holothuria leucospilota and holothuria atra on fringing reef (Reunion island, Indian ocean),” in Echinoderms: München. Eds. Heinzeller T., Nebelsick J. H. (London: Taylor and Francis), 311–317.
Marsh J. B., Weinstein W. J. (1966). A simple charring method for determination of lipids. J. Lipid Res. 7, 574–576. doi: 10.1016/S0022-2275(20)39274-9
Mathieu-Resuge M., Le Grand F., Schaal G., Kraffe E., Lorrain A., Letourneur Y., et al. (2020). Assimilation of shrimp farm sediment by Holothuria scabra: A coupled fatty acid and stable isotope approach. Aquat. Living Resour. 33, 1–12. doi: 10.1051/alr/2020004
Mercier A., Battaglene S. C., Hamel J.-F. (1999). Daily burrowing cycle and feeding activity of juvenile sea cucumbers Holothuria scabra in response to environmental factors. J. Exp. Mar. Biol. Ecol. 239, 125–156. doi: 10.1016/S0022-0981(99)00034-9
Meysman F. J. R., Galaktionov O. S., Gribsholt B., Middelburg J. J. (2006a). Bioirrigation in permeable sediments: advective pore-water transport induced by burrow ventilation. Limnol. Oceanogr. 51, 142–156. doi: 10.4319/lo.2006.51.1.0142
Meysman F. J. R., Middelburg J. J., Heip C. H. R. (2006b). Bioturbation: a fresh look at darwin’s last idea. Trend. Ecol. Evol. 21 (12), 688–695. doi: 10.1016/j.tree.2006.08.002
Mezali K., Soualili D. L. (2013). The ability of holothurians to select sediment particles and organic matter. SPC Beche-de-mer Inf. Bull. 33, 38–43.
Mirto S., Bianchelli S., Gambi C., Krzelj M., Pusceddu A., Scopa M., et al. (2010). Fish-farm impact on metazoan meiofauna in the Mediterranean Sea: Analysis of regional vs. habitat effects. Mar. Env. Res. 69, 38–47. doi: 10.1016/j.marenvres.2009.07.005
Moccia D., Cau A., Meloni M. C., Pusceddu A. (2019). Small-scale distribution of metazoan meiofauna and sedimentary organic matter in subtidal sandy sediments (Mediterranean Sea). Adv. Oceanogr. Limnol. 10 (1), 57–66. doi: 10.4081/aiol.2019.8169
Navarro P. G., García-Sanz S., Barrio J. M., Tuya F. (2013). Feeding and movement patterns of the sea cucumber Holothuria sanctori. Mar. Biol. 160, 2957–2966. doi: 10.1007/s00227-013-2286-5
Neofitou N., Lolas A., Ballios I., Skordas K., Tziantziou L., Vafidis D. (2019). Contribution of sea cucumber Holothuria tubulosa on organic load reduction from fish farming operation. Aquaculture 501, 97–103. doi: 10.1016/j.aquaculture.2018.10.071
Paltzat D. L., Pearce C. M., Barnes P. A., McKinley R. S. (2008). Growth and production of California sea cucumbers (Parastichopus californicus stimpson) co-cultured with suspended pacific oysters (Crassostrea gigas thunberg). Aquaculture 275, 124–137. doi: 10.1016/j.aquaculture.2007.12.014
Pasquini V. (2022). Reproductive biology and ecology of the sea cucumber holothuria tubulosa gmelin 1788 (Italy: University of Cagliari), 147. PhD thesis.
Pasquini V., Giglioli A. A., Pusceddu A., Addis P. (2021). Biology, ecology and management perspectives of overexploited deposit-feeders sea cucumbers, with focus on Holothuria tubulosa (Gmelin 1788). Adv. Oceanogr. Limnol. 12 (2). doi: 10.4081/aiol.2021.9995
Pasquini V., Porcu C., Marongiu F. M., Follesa M. C., Giglioli A. A., Addis P. (2022). New insights upon the reproductive biology of the sea cucumber Holothuria tubulosa (Echinodermata, holothuroidea) in the Mediterranean: Implications for management and domestication. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.1029147
Purcell S. W. (2014). Value, market preferences and trade of beche-De-Mer from pacific island Sea cucumbers. PloS One 9 (4), 95075. doi: 10.1371/journal.pone.0095075
Purcell S. W., Conand C., Uthicke S., Byrne M. (2016). Ecological roles of exploited sea cucumbers. Oceanogr. Mar. Biol. Annu. Rev. 54, 367–386. doi: 10.1201/9781315368597-8
Purcell S. W., Mercier A., Conand S., Hamel J. F., Toral- Granda M. V., Lovatelli A., et al. (2011). Sea Cucumber fisheries: Global analysis of stocks, management measures and drivers of overfishing. Fish. Fish. 14, 34–59. doi: 10.1111/j.1467-2979.2011.00443.x
Purcell S. W., Hair C. A., Mills D. J. (2012). Sea Cucumber culture, farming and sea ranching in the tropics: Progress, problems and opportunities. Aquaculture 368-369, 68–81. doi: 10.1016/j.aquaculture.2012.08.053
Pusceddu A., Bianchelli S., Canals M., Sanchez-Vidal A., Durrieu De Madron X., Heussner S., et al. (2010). Organic matter in sediments of canyons and open slopes of the Portuguese, Catalan, southern Adriatic and Cretan Sea margins. Deep Sea Res. Part I Oceanogr. Res. 57, 441–457. doi: 10.1016/j.dsr.2009.11.008
Pusceddu A., Bianchelli S., Gambi C., Danovaro R. (2011). Assessment of benthic trophic status of marine coastal ecosystems: Significance of meiofaunal rare taxa. Estuar. Coast. Shelf Sci. 93, 420–430. doi: 10.1016/j.ecss.2011.05.012
Pusceddu A., Dell’Anno A., Danovaro R., Manini E., Sarà G., Fabiano M. (2003). Enzymatically hydrolyzable protein and carbohydrate sedimentary pools as indicators of the trophic state of ‘detritus sink’ systems: a case study in a Mediterranean coastal lagoon. Estuaries 26, 641–650. doi: 10.1007/BF02711976
Pusceddu A., Dell’Anno A., Fabiano M., Danovaro R. (2009). Quantity, biochemical composition and bioavailability of sediment organic matter as complementary signatures of benthic trophic status. Mar. Ecol. Prog. Ser. 375, 41–52. doi: 10.3354/meps07735
Pusceddu A., Fraschetti S., Mirto S., Holmer M., Danovaro R. (2007). Effects of intensive mariculture on sediment biochemistry. Ecol. Appl. 17 (5), 1366–1378. doi: 10.1890/06-2028.1
Rakaj A., Fianchini A., Boncagni P., Lovatelli A., Scardi M., Cataudella S. (2018). Spawning and rearing of Holothuria tubulosa: a new candidate for aquaculture in the Mediterranean region. Aquacult. Res. 49, 557–568. doi: 10.1111/are.13487
Ramón M., Simarro G., Galimany E., Lleonart J. (2019). Evaluation of sediment particle size selection during feeding by the holothurian Parastichopus regalis (Cuvier 1817). Reg. Stud. Mar. Sci. 31, 100763. doi: 10.1016/j.rsma.2019.100763
Reise K. (2002). Sediment mediated species interactions in coastal waters. J. Sea Res. 48 (2), 127–141. doi: 10.1016/S1385-1101(02)00150-8
Rice D. L. (1982). The detritus nitrogen problem: New observations and perspectives from organic geochemistry. Mar. Ecol. Prog. Ser. 9, 153–162. doi: 10.3354/meps009153
Roberts D., Gebruk A. V., Levin V., Manship B. A. D. (2000). Feeding and digestive strategies in deposit-feeding holothurians. Oceanogr. Mar. Biol. Ann. Rev. 38, 257–310.
Schenone S., O’Meara T., Thrush S. F. (2019). Non-linear effects of macrofauna functional trait interactions on biogeochemical fluxes in marine sediments change with environmental stress. Mar. Ecol. Progr. Ser. 624, 13–21. doi: 10.3354/meps13041
Shpigel M., Shauli L., Odintsov V., Ben-Ezra D., Neori A., Guttman L. (2018). The sea urchin, Paracentrotus lividus, in an integrated multi-trophic aquaculture (IMTA) system with fish (Sparus aurata) and seaweed (Ulva lactuca): Nitrogen partitioning and proportional configuration. Aquaculture 490, 260–269. doi: 10.1016/j.aquaculture.2018.02.051
Slater M. J., Carton A. G. (2007). Survivorship and growth of the sea cucumber Australostichopus (Stichopus) mollis (Hutton 1872) in polyculture trials with green-lipped mussel farms. Aquaculture 272, 389–398. doi: 10.1016/j.aquaculture.2007.07.230
Slater M. J., Carton A. G. (2009). Effect of sea cucumber (Australostichopus mollis) grazing on coastal sediments impacted by mussel farm deposition. Mar. pollut. Bull. 58 (8), 1123–1129. doi: 10.1016/j.marpolbul.2009.04.008
Slater M. J., Jeffs A. G., Carton A. G. (2009). The use of the waste from green-lipped mussels as a food source for juvenile sea cucumber, Australostichopus mollis. Aquaculture 292 (3–4), 219–224. doi: 10.1016/j.aquaculture.2009.04.027
Slater M. J., Jeffs A. G., Sewell M. A. (2011a). Selective movement and deposit-feeding in juvenile sea cucumber, Australostichopus mollis determined in situ and in the laboratory. J. Exp. Mar. Bio. Ecol. 409 (1-2), 315–323. doi: 10.1016/j.jembe.2011.09.010
Slater M. J., Lassudrie M., Jeffs A. G. (2011b). Method for determining apparent digestibility of carbohydrate and protein sources for artificial diets for juvenile sea cucumber, Australostichopus mollis. J. World Aquacult. Soc 42 (5), 714–725. doi: 10.1111/j.1749-7345.2011.00510.x
Solan M., Cardinale B. J., Downing A. L., Engelhardt K. A. M., Ruesink J. L., Srivastava D. S. (2004). Extinction and ecosystem function in the marine benthos. Science 306 (5699), 1177–1180. doi: 10.1126/science.1103960
Stephens D. W., Krebs J. R. (1986). Foraging theory (Princeton, NJ, USA: Princeton University Press).
Tolon M. T., Emiroglu D., Gunay D., Ozgul A. (2017). Sea Cucumber (Holothuria tubulosa gmelin 1790) culture under marine fish net cages for potential use in integrated multitrophic aquaculture (IMTA). Indian J. Geo. Mar. Sci. 46, 749–756.
Tortonese E. (1965). Fauna d’Italia, Echinodermata (Echinodermata. Fauna d’Italia: Ed. Calderini, Bologna.), 419.
Uthicke S., Karez R. (1999). Sediment patch selectivity in tropical sea cucumbers (Holothuroidea: Aspidochirotida) analysed with multiple choice experiments. J. Exp. Mar. Biol. Ecol. 236, 69–87. doi: 10.1016/S0022-0981(98)00190-7
Wen J., Hu C., Fan S. (2010). Chemical composition and nutritional quality of sea cucumbers. J. Sci. Food Agri. 90, 2469–2474. doi: 10.1002/jsfa.4108
Wolfe K., Byrne M. (2017). Biology and ecology of the vulnerable holothuroid, Stichopus herrmanni, on a high-latitude coral reef on the great barrier reef. Coral Reefs. 36 (4), 1143–1156. doi: 10.1007/s00338-017-1606-5
Wolfe K., Deaker D. J., Graba-Landry A., Champion C., Dove S., Lee R., et al. (2021). Current and future trophic interactions in tropical shallow-reef lagoon habitats. Coral Reefs. 40 (1), 83–96. doi: 10.1007/s00338-020-02017-2
Wolfe K., Vidal-Ramirez F., Dove S., Deaker D., Byrne M. (2018). Altered sediment biota and lagoon habitat carbonate dynamics due to sea cucumber bioturbation in a high-pCO2 environment. Glob. Change Biol. 24 (1), 465–480. doi: 10.1111/gcb.13826
Yamazaki Y., Sakai Y., Mino S., Suda W., Hattori M., Meirelles P. M., et al. (2019). Repeated selective enrichment process of sediment microbiota occurred in sea cucumber guts. Environ. Microbiol. Rep. 11 (6), 797–807. doi: 10.1111/1758-2229.12791
Yang H., Zhou Y., Zhang T., Yuan X., Li X., Liu Y., et al. (2006). Metabolic characteristics of sea cucumber, Apostichopus japonicus (Selenka) during aestivation. J. Exp. Mar. Biol. Ecol. 330, 505–510. doi: 10.1016/j.jembe.2005.09.010
Yang H., Bai Y. (2015). “Apostichopus japonicus in the life of Chinese people”. in The Sea cucumber Apostichopus japonicus. history, biology, and aquaculture. Eds. Yang H., Hamel J. F., Mercier A. (Academic Press), 1–24.
Yingst J. Y. (1982). Factors influencing rates of sediment ingestion by Parastichopus parvimensis (Clark), an epibenthic deposit-feeding holothurian. Estuar. Coast. Shelf Sci. 14, 119–134. doi: 10.1016/S0302-3524(82)80040-6
Yuan X., Yang H., Meng L., Wang L., Li Y. (2013). Impacts of temperature on the scavenging efficiency by the deposit-feeding holothurian Apostichopus japonicus on a simulated organic pollutant in the bivalve-macroalage polyculture from the perspective of nutrient budgets. Aquaculture 406–407, 97–104. doi: 10.1016/j.aquaculture.2013.05.009
Yu Z., Zhou Y., Yang H., Ma Y., Hu C. (2014). Survival, growth, food availability and assimilation efficiency of the sea cucumber Apostichopus japonicus bottom-cultured under a fish farmin southern China. Aquaculture, 426–427, 238–248. doi: 10.1016/j.aquaculture.2014.02.013
Zamora L. N., Jeffs A. G. (2011). Feeding, selection, digestion and absorption of the organic matter from mussel waste by juveniles of the deposit-feeding sea cucumber, Australostichopus mollis. Aquaculture 317 (1–4), 223–228. doi: 10.1016/j.aquaculture.2011.04.011
Zamora L. N., Jeffs A. G. (2012). The ability of the deposit-feeding sea cucumber Australostichopus mollis to use natural variation in the biodeposits beneath mussel farms. Aquaculture 326–329, 116–122. doi: 10.1016/j.aquaculture.2011.11.015
Keywords: sea cucumbers, Holothuria tubulosa, sediment organic matter, benthic trophodynamics, bioturbation
Citation: Pasquini V, Addis P, Giglioli AA, Moccia D and Pusceddu A (2023) Outcomes of feeding activity of the sea cucumber Holothuria tubulosa on quantity, biochemical composition, and nutritional quality of sedimentary organic matter. Front. Mar. Sci. 9:1010014. doi: 10.3389/fmars.2022.1010014
Received: 02 August 2022; Accepted: 29 December 2022;
Published: 26 January 2023.
Edited by:
Annie Mercier, Memorial University of Newfoundland, CanadaReviewed by:
Kennedy Wolfe, The University of Queensland, AustraliaLeo Zamora, Cawthron Institute, New Zealand
Copyright © 2023 Pasquini, Addis, Giglioli, Moccia and Pusceddu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Viviana Pasquini, dml2aWFuYS5wYXNxdWluaUB1bmljYS5pdA==
†These authors have contributed equally to this work
 Viviana Pasquini
Viviana Pasquini Pierantonio Addis
Pierantonio Addis Ambra Angelica Giglioli
Ambra Angelica Giglioli Davide Moccia
Davide Moccia Antonio Pusceddu
Antonio Pusceddu