- 1Department of Biology, University of Bari Aldo Moro, Bari, Italy
- 2Consorzio Nazionale Interuniversitario per le Scienze del Mare (CoNISMa), Piazzale Flaminio, Roma, Italy
- 3Department of Economics and Finance, University of Bari Aldo Moro, Bari, Italy
Deep-sea communities are impacted by several anthropogenic activities, such as fisheries, which still remain one of the most damaging on the marine environments in terms of biodiversity loss and habitat degradation. The north-western Ionian Sea (Central Mediterranean) experienced long-standing trawl fishing activity with the exploitation of deep-sea demersal resources. The integrate analysis with data of both benthic, demersal and benthopelagic species collected during a time series of experimental trawl survey, yearly carried out in the Ionian basin down to 800 m in depth, allowed to asses the current status of the Ionian deep-sea faunal assemblages and their potential changes overtime. Multivariate analysis and univariate indices, modelled using Generalized Additive Model (GAM) framework, confirm a depth zonation pattern of deep-sea faunal assemblages in the study area, with the presence of two distinct epibathyal and mesobathyal groups. GAM also highlighted a temporal effect on the biodiversity indices, with significant negative trends of diversity and evenness indices as well as a significant increasing of dominance one, over the study period 2012-2020. The benthic community is characterised by more generalist species and a simplified structure, with a severe depletion in habitat-former taxa whereas the demersal and benthopelagic fauna of the Ionian Sea show a general stability in the overall structure if compared with previous studies lasting over two decades. The presence of complex and heterogenous habitats unsuitable for trawl, like cold-water coral communities and canyon systems, provide Essential Fish Habitats (EFHs) for commercial species, acting as potential renewal areas for exploited resources in the neighbouring fishing grounds. These findings encourage an ecosystem-based management including spatial considerations for the objectives of biodiversity conservation combined with those of management of fishery resources.
Introduction
Different anthropogenic activities, including fisheries, are accelerating pressures on deep-sea communities, leading to the degradation of benthic and pelagic environments, with global impacts on deeper ecosystems (Halpern et al., 2007; Armstrong et al., 2012; Thurber et al., 2014; Danovaro et al., 2020), particularly in terms of species loss and habitat damages (Danovaro et al., 2008; Mengerink et al., 2014). Bottom trawling is the most widespread source of anthropogenic disturbance on the sea bottom. It is generally considered as a low-selective fishing gears, with removal of large parts of demersal/benthic communities, including Vulnerable Marine Ecosystem (VME) indicator taxa (sensu GFCM, 2017) and species with key roles in the marine system (Hall et al., 2000; Hiddink et al., 2017; Carpentieri et al., 2021). Deep-sea fisheries have a recognized capacity to remove slow-growing, long-lived species and many habitat-forming organisms from the seafloor. As a result, profound changes can occur in habitat properties as well as in the ecosystem structure and functioning (Danovaro et al., 2020; de Juan et al., 2020). The effects of bottom trawling can be seen particularly in benthic assemblages, where the decrease in biomass and diversity may lead to a decline of their complexity (Stamouli et al., 2022), resulting more severe in deeper areas of the upper continental slope (Ramirez-Llodra et al., 2011; Puig et al., 2012). Therefore, development of new fisheries, conservation, and ecosystem-based management strategies requires assessments of the distribution and the relative status of both benthic and demersal assemblages (Hiddink et al., 2017), as well as the evaluation of their changes over time.
Trawl surveys can provide valuable information in terms of quantitative abundance and biological parameters of demersal species. Indeed, trawl surveys have gained more attention during the last decades as a primary tool for the assessment process of the demersal and benthic communities (e.g. Cotter et al., 2009; Spedicato et al., 2019).
In the Mediterranean Sea, data on the demersal assemblages are systematically collected in the framework of the Mediterranean Trawl Surveys (MEDITS) program (Bertrand et al., 2002) since 1994 as a European Commission–funded project, and from 2002 within the European Data Collection Framework (DCF). This program is carried out yearly with the main goal to assess the demersal resources by means of population and community indicators. The EU Common Fishery Policy (CFP) for fishery management adopted a strategy of ecosystem-based fishery management (EBFM) with the overall objective of sustaining healthy marine ecosystems and the fisheries they support. This implies sustainable management not only of the commercial stocks but also of the whole ecosystem which supports their production (Maiorano et al., 2022). Consequently, more emphasis was directed towards addressing ecosystem questions during the last few years and MEDITS’ target was extended from the population of demersal species to the whole community. Since 2012, the sampled taxonomic categories have been expanded, including the non-commercial benthos in the target species, to provide new information linked to the EU Marine Strategy Framework Directive (MSFD; Directive 2008/56/EC) and to the ecosystem approach to fishery and EU Marine Spatial Planning Directive (MSP; Directive 2014/89/EU) (Spedicato et al., 2019). Accordingly, the systematic collection of data on all sampled species by the experimental MEDITS surveys provides a good opportunity for understanding the demersal community as a whole, including benthic species, as well as to detect distribution patterns and potential significant changes over a wide spatial and temporal scale. Despite the bottom trawl is not the best sampler for exploring the benthic communities, the use of systematic experimental trawl surveys could be useful to collect data on the presence and distribution of benthic species. Indeed, these species are generally not available in large and consistent time series (e.g. Terribile et al., 2016; Lauria et al., 2017; Chimienti et al., 2018; Carbonara et al., 2020; de Juan et al., 2020).
In the north-western Ionian Sea (central Mediterranean), knowledge on the distribution and abundance of the deep-sea demersal fauna on the trawlable bottoms comes from systematic surveys of the demersal resources carried out since 1985 (e.g. D’Onghia et al., 1998b; D’Onghia et al., 2003; Capezzuto et al., 2010; Maiorano et al., 2010; Carlucci et al., 2018). Moreover, portions of the Ionian faunal assemblages distributed down to 800 m were described in few studies, reporting the composition of ichthyofauna (Matarrese et al., 1996; Sion et al., 2000) and teuthofauna (Tursi and D’Onghia, 1992; D’Onghia et al., 1995; Maiorano et al., 1999; Battista et al., 2011) throughout the investigated area and period. Deepest fauna assemblages in the Ionian Sea were studied within the framework of the international DESEAS project carried out in three Mediterranean areas (Sardà et al., 2004). Their structure, between 600 and 4000 m in depth, was described for cartilaginous, teleost fish and decapod crustaceans separately (Company et al., 2004; D’Onghia et al., 2004; Sion et al., 2004).
On the contrary, a systematic investigation of the benthic community structure and its large-scale spatial distribution on the trawlable deep-sea bottoms of the north-western Ionian Sea has not been carried out so far. Only a few, local studies have highlighted the occurrence of VME indicator taxa on the deep, Ionian soft bottoms, including large sea pen aggregations along the Italian coast (Mastrototaro et al., 2013). Predictive models had also shown the potential presence of deep-sea pennatulaceans (Bastari et al., 2018) and Isidella elongata (Carbonara et al., 2020) in different areas of the basin, although hard-bottom assemblages have been historically studied more in detail.
The large-scale distribution pattern of megafauna communities along the Mediterranean trawlable middle slope (Fernandez-Arcaya et al., 2019) as well as the large spatio-temporal patterns of demersal fish assemblages in the northern Mediterranean (Mérigot et al., 2019) were recently explored, but no detailed information and high-spatial resolution were reported for the Ionian basin. A previous study around the Maltese island (Central Mediterranean) suggests that part of the faunal assemblages cannot be considered independently, and that benthic and demersal species are strictly linked in the structuring of demersal communities (Terribile et al., 2016).
To date, no comprehensive study on the characterization of deep-sea assemblages as a whole is still available for the north-western Ionian Sea.
Thus, the aim of this study is to assess the current status of the Ionian deep-sea faunal assemblages impacted by trawl fishing and their potential changes over time, using an integrate analysis with data of both benthic, benthopelagic and demersal species. The characterization of deep-sea exploited community with species of distinctive habitus and domain could allow to better understand the role of different species in structuring the demersal assemblages and to highlight the eventual changes induced by fishery exploitation, in order to promote an ecosystem-based management including spatial considerations for a future Marine Spatial Planning.
Material and methods
Study area
The north-western Ionian Sea (central Mediterranean) corresponds to Geographical Sub-Area (GSA, sensu FAO-GFCM) 19, between Cape Otranto (Apulian) (40°C 06′N 18°C 31′E) and Cape Passero (Sicily) (36°C 41′N 15°C 10′E), along a coastline of about 1000 km (Figure 1). This area is characterized by a complex geomorphology that results in different features between the eastern and western sectors divided by the Taranto Valley, a large submarine canyon over 2200 m depth (Rossi and Gabbianelli, 1978). In the eastern sector, corresponding to the Apulian region, the continental shelf is wide and abrasion terraces and bioclastic calcareous deposits with several coral rocks are distributed from the shallowest to the deepest grounds. Clustered and isolated mound-like features are located between 400 and 1000 m in depth within a broad area (Bargain et al., 2017).
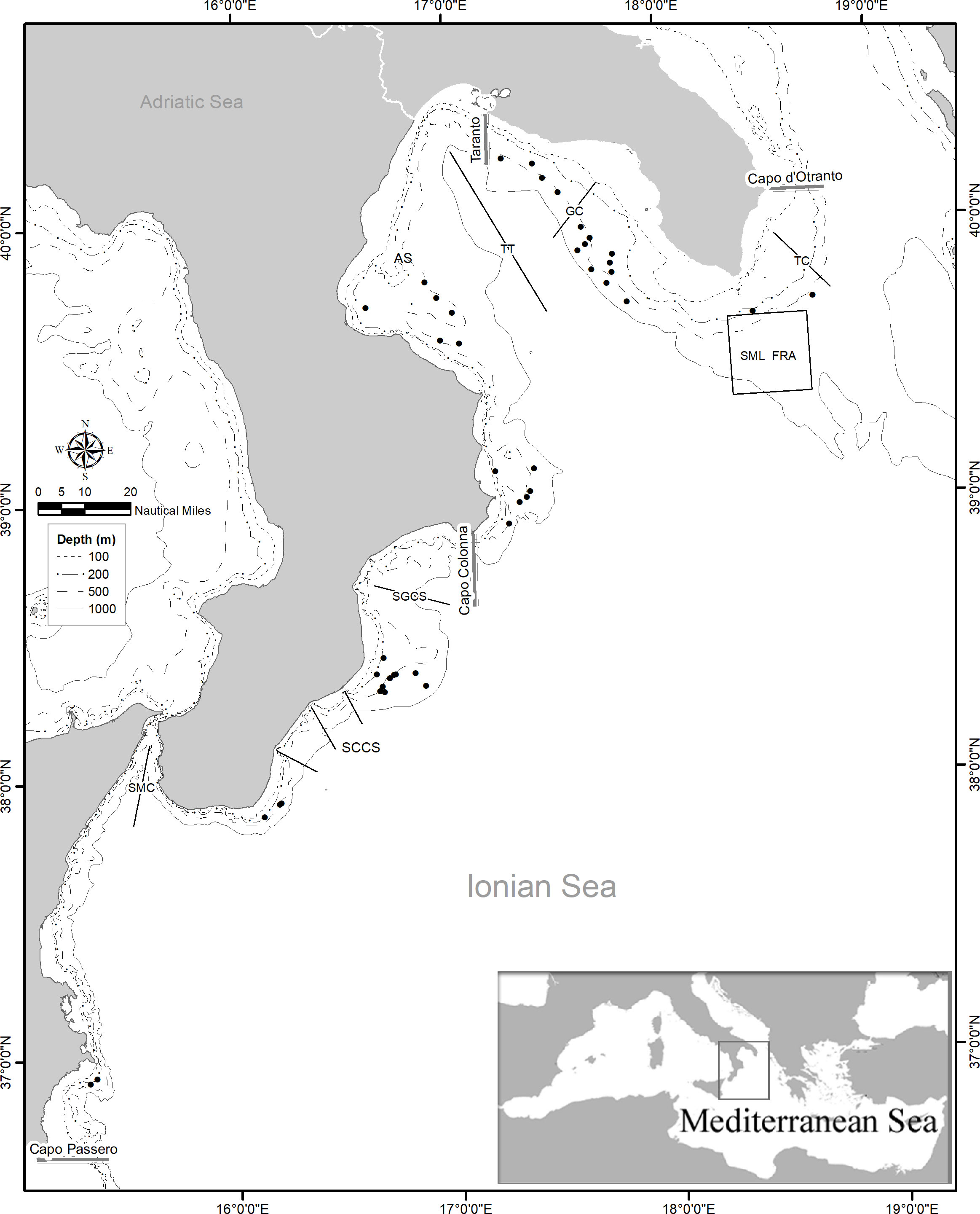
Figure 1 Map of the study area in the northwestern Ionian Sea, with indication of the sampling hauls (black circle) performed by year from 2012 to 2020 and topographic features. SML FRA, Santa Maria di Leuca Fisheries Restricted Area; TC, Tricase Canyon; GC, Gallipoli Canyon; TT, Taranto Trench; AS, Amendolara Shoal; SGCS, Squillace Gulf Canyon System; SCCS, South Calabria Canyon System; SMC, Strait of Messina Canyon.
In the western area, the Calabrian shelf platform is very narrow and shaped by active submarine canyons, that provide a direct pathway for sediment transport and dense water cascading from coastal to deep waters, playing an important role in the functioning of the deep-sea ecosystems and as refuge areas for many demersal species (Pierdomenico et al., 2019).
The geomorphological diversity of the basin is reflected in different habitat distribution on the shallow and deep bottoms affecting the abundances of the megafauna in the area (D’Onghia et al., 1998b; Capezzuto et al., 2019; Maiorano et al., 2010; D’Onghia et al., 2011; Capezzuto et al., 2018; Ricci et al., 2022).
On the deep-sea grounds of the north-western Ionian Sea a few, unquantified soft-bottom habitats are patchy present, mostly structured by monospecific aggregations of octocorals (e.g. Chimienti et al., 2018), although the available information is very little. The area has been better assessed in terms of hard-bottom communities, with the remarkable presence of cold-water coral (CWC) communities down to more than 1100 m depth (Chimienti et al., 2019b; D’Onghia, 2019 and references therein). Few miles off Cape of SML (Italy), at depths between 300 and 1100 m in depth, there is the Santa Maria di Leuca (SML) CWC province that host a huge bank of Madrepora oculata and Lophelia pertusa (Desmophyllum pertusum) (Tursi et al., 2004; Mastrototaro et al., 2010). Considering its well documented role as biodiversity hot spot, refuge area and Essential Fish Habitat (EFH) for many commercial species, a Fisheries Restricted Area (FRA) were established for the SML CWC province (D’Onghia, 2019 and references therein).
All these communities are supported by a complex system of water circulation, as the basin receives Modified Atlantic Water (MAW) from the western Mediterranean through the Sicilian Channel (Theocharis et al., 1993), as well as Levantine Intermediate Waters (LIW) with variable salinity and temperature values between the southern and northern Ionian. This system shows reversals of the northern Ionian Gyre (NIG) direction affected by Bimodal Oscillating System (BiOS) (Ricci et al., 2022). The NIG inversion from anticyclonic to cyclonic, and vice versa, is mainly influenced by the inlet of MAW eastward, and salty LIW westward (Civitarese et al., 2010; Liu et al., 2021). In addition, these oscillations are also influenced by the cold and dense deep-water masses of the Adriatic Sea flowing in the Ionian Sea through the Otranto Channel (Menna et al., 2019; Ricci et al., 2022).
In the north-western Ionian Sea, fishing activities occur from coastal waters to about 800 m. Gallipoli, Taranto, Corigliano Calabro, Crotone, Roccella Jonica and Reggio Calabria host the most important fishing fleets, although with a different distribution of the fishing effort (Maiorano et al., 2010). In the GSA 19, the fishery is characterized by a lower number and percentage of trawlers than small-scale fleet; however, with a high percentage in terms of gross tonnage and engine power of the total fleet. The fleet is almost equally distributed along the three sectors of Apulia, Calabria and Sicily although with differences in the fleet segmentation: trawlers are more important in the Apulian sector whereas the longline and polyvalent vessels characterize the Sicilian fishery (Maiorano et al., 2019). Data coming from the European Fishing Fleet Register (http://ec.europa.eu/fisheries/fleet/index.cfm), showed a significant decreasing trend of the Fishing Effort (FE) in the overall north-western Ionian, in terms of number (N) of bottom trawlers (OTB), over the period 1995-2020. Considering three geographical sub-areas (Apulia, northern and southern Calabria), the highest FE by bottom trawlers was observed in the Apulia sub-area, with a highly significant decrease (ρ=-0.961; p<0.0001) over the period 1995-2020. A highly significant decrease (ρ=-0.947; p<0.0001) of OTB fishing effort was detected in the northern Calabria whereas the lowest FE with no significant trend (ρ=-0.002; p>0.05) over time was detected in the southern Calabria.
The most important demersal resources on the deep-sea bottoms of the north-western Ionian Sea are the European hake (Merluccius merluccius), the deep-water rose shrimp (Parapenaeus longirostris) and the Norway lobster (Nephrops norvegicus) over a wide bathymetric range and the deep-water red shrimps (Aristeus antennatus and Aristaeomorpha foliacea) on the slope (Maiorano et al., 2010; Maiorano et al., 2019).
Data collection
Data were collected during the Mediterranean Trawl Surveys (MEDITS) program (Bertrand et al., 2002), to date included in the EU Data Collection Framework (Spedicato et al., 2019). The MEDITS surveys are carried out in Mediterranean from late spring to summer every year, according to a standardized protocol which includes gear characteristics, haul duration and sampling procedures. A depth-stratified random design was adopted, with haul allocation being proportional to the surface of depth strata (10-50, 51-100, 101-200, 201-500 and 501-800 m), from 10 to 800 m depth (Spedicato et al., 2019). Data used in the present study come from nine experimental surveys carried out from 2012 to 2020 in the north-western Ionian Sea where 43 hauls were yearly performed on the deep-sea trawlable bottoms, from 200 to 800 m in depth, for a total number of 387 hauls. The experimental bottom trawl GOC 73 (Bertrand et al., 2002) was used for sampling, with a vertical opening larger than that of the most common professional gears and the stretched mesh size at codend of 20 mm to also allow the catch of juveniles of many species. Haul performance and the trawl net geometry were monitored using the SCANMAR system and the horizontal opening on each haul was used to standardize abundance and biomass of catches in relation to the swept area.
All the species larger than 1 cm caught during the survey were identified at the lowest possible taxonomic level. Then the total weight and number of individuals were recorded for the main target categories (Cephalopoda, Crustacea Decapoda, Chondrichthyes and Osteichthyes); moreover, the total weight for all collected benthic species was recorded according to the MEDITS protocol (Spedicato et al., 2019).
Data processing
The standardized abundances in weight (expressed as biomass kg/km2) and number, when possible (expressed as density N/km2), were computed for all the species caught on deep muddy bottoms (200-800 m) of the north-western Ionian Sea, and the frequency of occurrence (Foc) of each species was computed as the percentage of the positive hauls on the total hauls.
All sampled species were classified for their characteristic domain and behaviors according to the available database FishBase (Froese and Pauly, 2021) and SeaLife (Palomares and Pauly, 2021), as well as to the knowledge of experts as: strictly benthic (BE, sessile and sedentary), demersal (DE), benthopelagic (BP) and pelagic. Accordingly, all pelagic species and those species with Foc less than 1% were removed from further analysis.
Multivariate analyses were subsequently carried out to better define the composition of species assemblages and to investigate differences in assemblage structure in terms of taxonomic composition and species abundance. Data on the matrix of abundance per species–hauls were compiled using a fourth-root transformation in order to minimize the importance of highly-abundant species (Legendre and Legendre, 1998). Multivariate analysis of the species-hauls matrix was performed by means of non-metric multidimensional scaling (nMDS), based on the Bray-Curtis similarity index. The ordination of hauls in the MDS was explored using two factors to aggregate the hauls in common groups. In particular, two bathymetric layers (200-400 m of depth, epibathyal assemblage, and 400-800 m of depth, mesobathyal assemblage), and three geographical sub-areas (Apulian, north Calabria and southern Calabria) were used as factors. The analysis of similarities (ANOSIM) was performed to test the differences between the groups of the species–hauls. The Similarity Percentages (SIMPER) analysis was applied to characterize by species each faunal assemblage and to establish which species contribute to the measures of similarity between each haul (Clarke and Warwick, 2001). Moreover, in order to explain the percentage of total variation in the assemblage structure, the spatial ordination of the deep-sea assemblages was plotted by means of the unconstrained ordination Principal Coordinate (PCO) analysis based on Bray–Curtis similarity applied to the selected species-hauls matrix. Species correlated to the main PCO axes were plotted using the Spearman correlation. All the above-mentioned analyses were conducted using the PRIMER v6.1 software (Clarke and Warwick, 2001).
A combined indicator of species abundance and frequency of occurrence, the Indicator Value (IndVal) index (Dufreêne and Legendre, 1997), was assigned to each species in each group previously identified in the multivariate analysis in order to identify indicator species of each obtained assemblage, according to the following equation:
The IndVal combines the specificity (A) of a species i in a sites group j (its relative abundance), with the species fidelity (B) to the sample sites (relative frequency of occurrence of the species within a given group of observations) (Dufreêne and Legendre, 1997; De Caíceres and Legendre, 2009). Species with an IndVal greater than or equal to 25 were considered the indicator species of each group identified within the overall faunal assemblage (Dufreêne and Legendre, 1997). According to Dufreêne and Legendre (1997), the highest IndVal value (IndVal Max) showed by each indicator species across all divisions of the sites groups was calculated, identifying ubiquitous and characteristic species. The former species are indicators of different sites groups, indicating a broad niche breadth of the species, while characteristic species are indicators of only one group of stations, with a smaller niche breadth. The IndVal analysis was conducted within the assemblages of the north-western Ionian Sea identified by multivariate analysis, considering the division of sites (hauls) in two bathymetric layers, as well as in three geographical sub-areas.
All these procedures were repeated starting with the dataset of BE, DE, and BP species, and then with sub-sets of data consisting of BE species only or DE+BE species, in order to assess the contribution of species with different habitus to the structure of the deep-sea assemblages.
Finally, to evaluate the biodiversity within each identified group/assemblage, the univariate ecological indices of Margalef richness (d), Shannon-Wiener diversity (H’), Pielou’s evenness (J) and Simpson (Λ) (Magurran, 1991) were computed for each sampling haul using standardized abundance data (N/km2) of demersal, benthopelagic and vagile benthic species and relative boxplots of each index were produced. Each computed index was modeled using the Generalized Additive Models (GAMs) (Wood, 2017). Specifically, we assumed that the mean of each response depends on an additive predictor through a link function chosen accordingly to the response distributional assumption (i.e. Gaussian distribution for Margalef richness (d) and Gamma distributions, with logarithmic link, for positive not symmetric distributions of the other indices). As an extension of Generalized Linear Models (GLM), the GAM framework allows for penalized estimation of smooth terms using shape functions to capture potential not-linear relationships between response and independent variables. Thus, we explored the possibility to model each ecological index as a function of the temporal trend and the effect of depth considering both linear and smooth effects, estimated using spline basis functions (Ruppert et al., 2003). For each index, alternative models with different type of effects were compared using the Analysis Of Variance (ANOVA). Moreover, the linear effect of the geographical sub-area on responses was investigated.
Results
Faunal assemblages’ structure
Throughout the study period 2012-2020, 288 species were recorded on the deep-sea bottoms between 200 and 800 in depth in the north-western Ionian Sea. Of these, 118 taxa were strictly benthic, 110 were demersal and 60 benthopelagic. Osteichthyes was the dominant taxonomic group with 105 species and other main taxonomic groups were Crustacea Decapoda (58 species), Cephalopoda (31 species), Echinodermata (27 species), Cnidaria (15 species) and Chondrichthyes (14 species). Few species were collected in the other taxonomic groups (Figure 2).
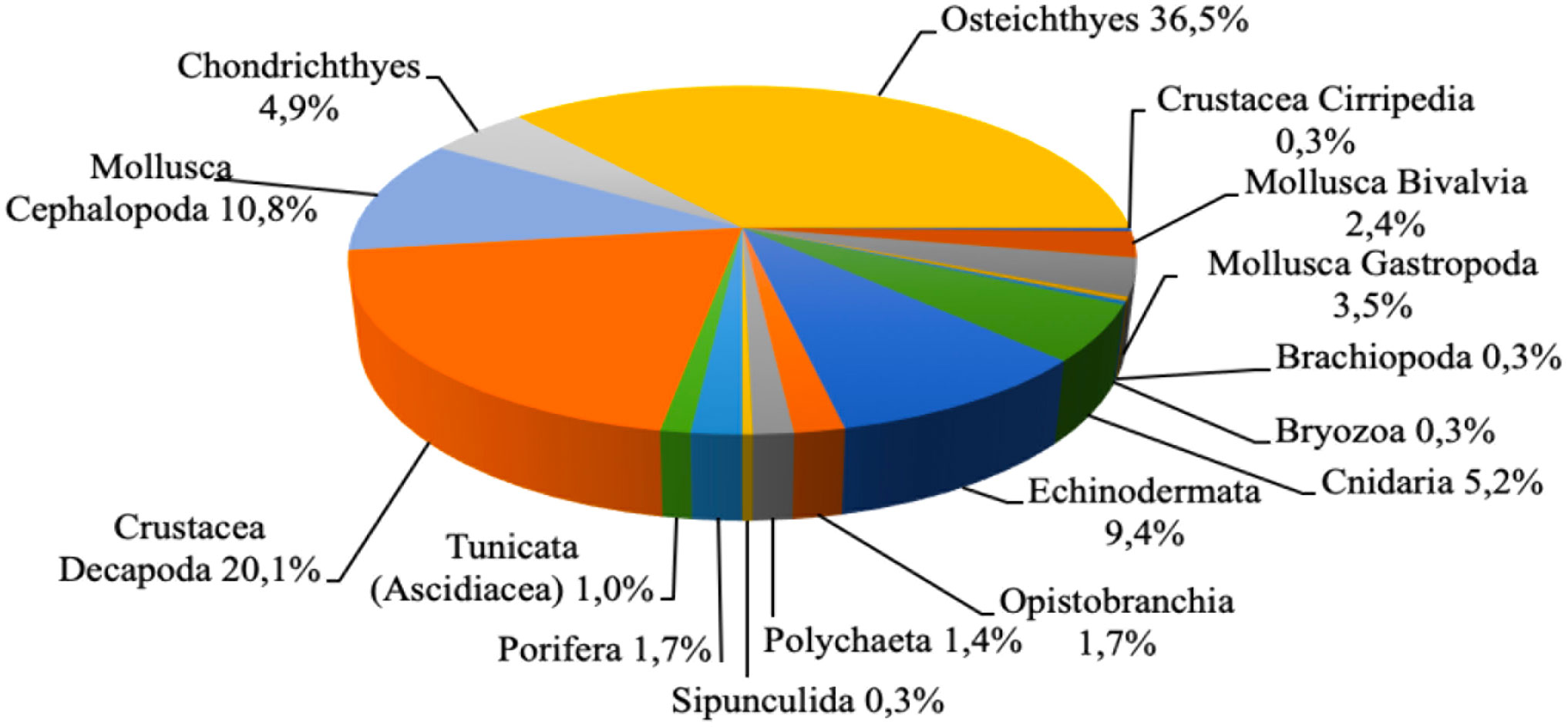
Figure 2 Percentage composition of species by taxonomic groups identified in the deep-sea assemblage of the north-western Ionian Sea from 2012 to 2020.
Considering all benthic taxa identified at species level (100) and selecting from other domains only those with Foc equal or higher than 1% (88 from demersal and 45 from benthopelagic species), a total of 233 species were considered in the multivariate analysis, using three sets of data. The first sub-set with strictly benthic only (both sessile and sedentary; BE), the second one adding demersal (DE) and the last whole dataset with also benthopelagic (BP) species.
The nMDS plots indicated a separation in two stations groups related to depth range of 200–400 m and 400–800 m, both considering only BE species and adding DE and BP ones (Figure S1). The ANOSIM test confirmed significant differences between groups along with increasing number of species considered, from the lower value for the strictly benthic species (Global R=0.495; p-level=0.01) to the higher one with BE+DE species (Global R=0.906; p-level=0.01) or with BE+DE+BP species (Global R=0.919; p-level=0.01). The ordination of the data showed no clear separation or grouping in nMDS plots with year and geographic sub-areas.
SIMPER results on the benthic species showed an average similarity of 27.74% in the upper slope group (200–400 m), characterized by the presence of the crab Macropipus tuberculatus and the royal sea cucumber Parastichopus regalis (Table S1). An average similarity of 31.37% was detected in the second deeper group (400–800 m), characterized by the dominance of the blind lobster Polycheles typhlops (Table S1). SIMPER computed with benthic and demersal species indicated a higher similarity within each group with an average value of 43.87% in the first and 50.85% in the second one. The main representative species on the upper epibathyal group were the deep-water rose shrimp P. longirostris, the European hake M. merluccius and the shortnose greeneye Chlorophthalmus agassizi, whereas a comparable percentage contribution to the similarity within the deeper mesobathyal group was observed for different species (Table S1). SIMPER computed with the BE+DE+BP species exhibited comparable results, with an average similarity of 43.19% and 48.60% in the epibathyal and mesobathyal groups respectively. The same representative species were detected in the upper group, with a further contribution of the broadtail shortfin squid Illex coindetii. Comparable percentage contributions to the similarity were observed within the mesobathyal group for different species, with the further presence of Hoplostethus mediterraneus within the main representative species (Table S1).
The PCO plots showed a pattern with two distinct groups of samples in all analysis performed with BE species only, BE+DE species and BE+DE+BP species, but with different percentages of explained variance (Figure 3). The first two axes explained 31.6% of the total variance considering only BE species with Echinus melo and P. regalis as the main correlated species to the first two axes in the 200-400 m hauls and P. typhlops in the 400-800 m group (Figure 3A). As for the nMDS, the addition of DE and BP species improved the results, with a percentage of the explained total variance of 48.6% for BE+DE species (Figure 3B) and a percentage of 46.4% for BE+DE+BP (Figure 3C). In the case of BE+DE, the main correlated species to the first two axes were P. longirostris, M. merluccius and C. agassizi on the upper slope (200-400 m) but also the teleost fish Macroramphosus scolopax and Glossanodon leioglossus seem to be representative of this group. The deep-sea red shrimps A. antennatus and A. foliacea together with the golden shrimp Plesionika martia and the macrourids Nezumia sclerorhynchus and Hymenocephalus italicus were the main correlated species in the deeper group (400-800 m) (Figure 3B). Considering BE+DE+BP species, the PCO plot showed almost similar results, with the same most correlated species to the first two axes in both depth groups, but with a further relevant contribution of H. mediterraneus on the mesobathyal group (Figure 3C).
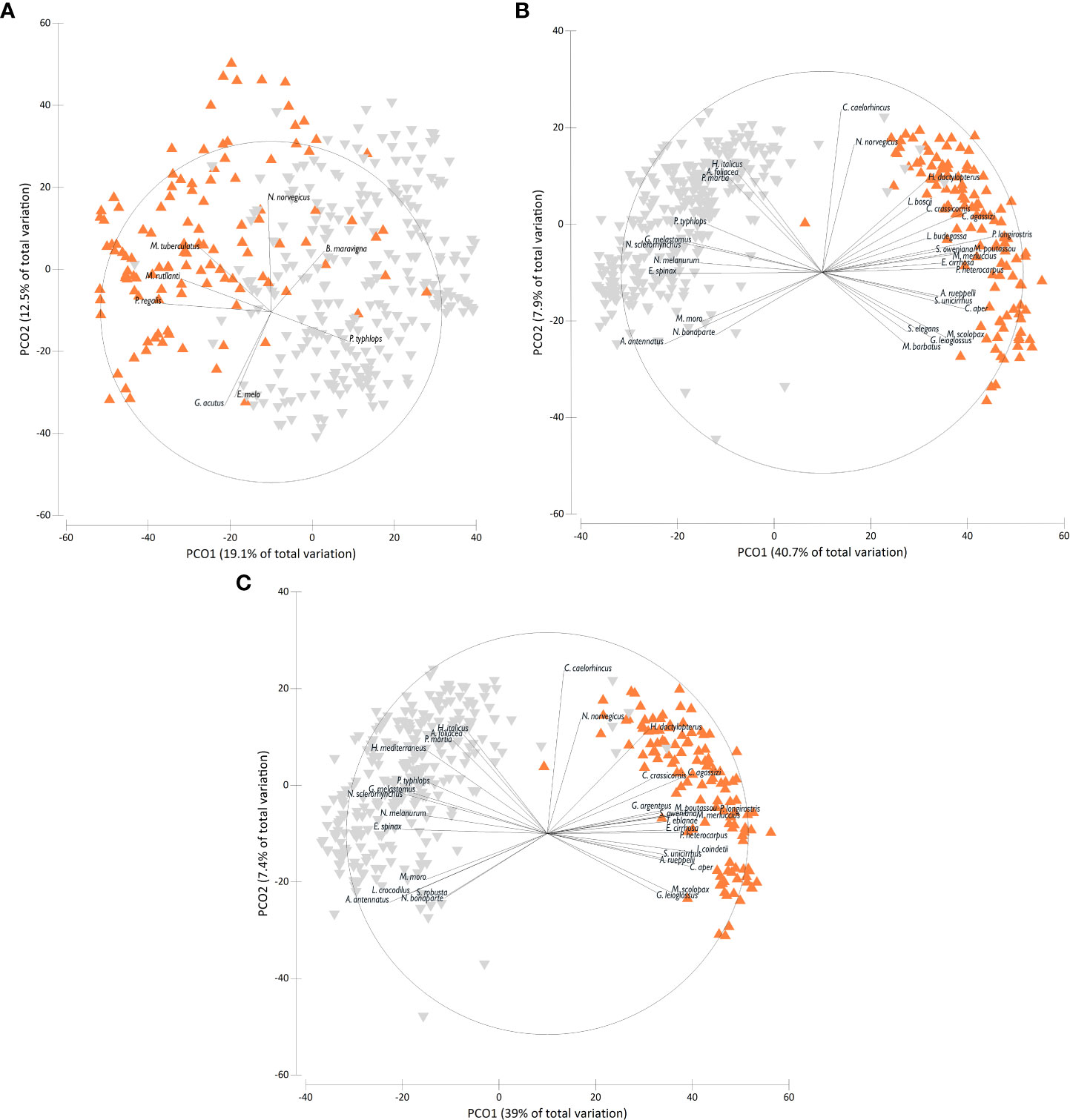
Figure 3 Principal Coordinate (PCO) analysis ordination plot based on Bray–Curtis similarity applied to the species-stations matrix of the deep-sea assemblage structure classified according to the two depth groups (orange = 200-400 m and grey = 400-800 m) in the north-western Ionian Sea for: (A) Benthic species only, (B) Benthic and demersal species, (C) Benthic, demersal and benthopelagic species.
In order to identify key representative species in each cluster, the taxa were also ranked by means of an indicator value index (IndVal) on the biomass data and the frequency of occurrence. The analysis confirmed the SIMPER results for both benthic and benthic-demersal assemblages, but also highlighted the contributions of other characteristic species in the clusters previously identified in the benthic-demersal assemblages. In particular, the crustaceans Aegeon lacazei and Plesionika edwardsii, the cephalopods Rossia macrosoma, Rondeletiola minor and Sepia elegans, the teleost fish Trigla lyra and Mullus surmuletus and the anemone Actinauge richardi were added to the important species in the epibathyal assemblage while the shrimp Plesionika acanthonotus and the fish Notacanthus bonaparte contributed to the deeper one (Figure 4).

Figure 4 Characteristic species of the deep-sea assemblages (benthic on the left; benthic and demersal on the right) identified by means of the Individual Value index (IndVal) in each group, with indication of specifity column (A) and fidelity column (B). Only species with IndVal >25 are listed.
The identification of key representative species was also performed distinctly in each sub-area, although no clear separation or grouping with sub-areas was observed in nMDS and PCO plots. A different number of characteristic species was detected in the three areas, with distinctive contribution of strictly benthic species (Figure S2). In particular, the sea urchins E. melo and Gracilechinus acutus resulted with the highest IndVal in both Apulian and northern Calabrian areas in the overall depth range 200-800 m together with the anemone Adamsia palliata in the Apulian assemblage and the gastropod Galeodea echinophora in northern Calabrian one. All these benthic species seem to be ubiquitarian in these two subareas whereas P. regalis, M. tuberculatus and Munida speciosa characterised the epibathyal assemblages of all sub-areas together with A. richardi, Liocarcinus depurator and Astropecten irregularis in the northern Calabria (Figure S2). B. maravigna resulted as characteristic benthic species in the deeper assemblages (400-800 m) in all investigated sub-areas with Pagurus alatus in the Apulian area, A. palliata in northern Calabrian and Paromola cuvieri in the southern Calabrian one (Figure S2). Concerning the demersal species, P. longirostris resulted the main characteristic species in epibathyal assemblages of all sub-areas, while C. agassizi and P. heterocarpus in Apulian and northern Calabrian and M. merluccius in the southern Calabrian. Nezumia sclerorhynchus characterized the deeper assemblages in all investigated sub-areas followed by P. martia and P. typhlops in both southern and northern Calabrian areas and Galeus melastomus in the Apulian one.
The blue-and-red shrimp A. antennatus resulted more characteristic of the Apulian and northern Calabrian deeper assemblages while the red shrimp A. foliacea showed the highest IndVal in the southern Calabrian area.
Assemblage biodiversity
The univariate ecological indices computed using the density (N/km2) of strictly benthic, demersal and benthopelagic species collected in the overall depth range of 200–800 m of the north-western Ionian Sea from 2012 to 2020, presented different patterns (Figure 5). The estimates of linear and smooth effects involved with the distribution of the features of the four ecological indices are reported in Table 1. Results showed the best fit in terms of type of effects for each considered response. The time evolution of biodiversity over the years was shown considering the estimated highly significant effect of the temporal component on H’, J and λ indices. In particular, significant negative linear trend was found for both the Shannon-Wiener diversity (H’) and the Pielou’s evenness (J) indices. For the Simpson (λ) index, the best model suggests an increasing temporal effect, basically linear. Not significant effect of temporal component was instead detected for Margalef richness (d) index.
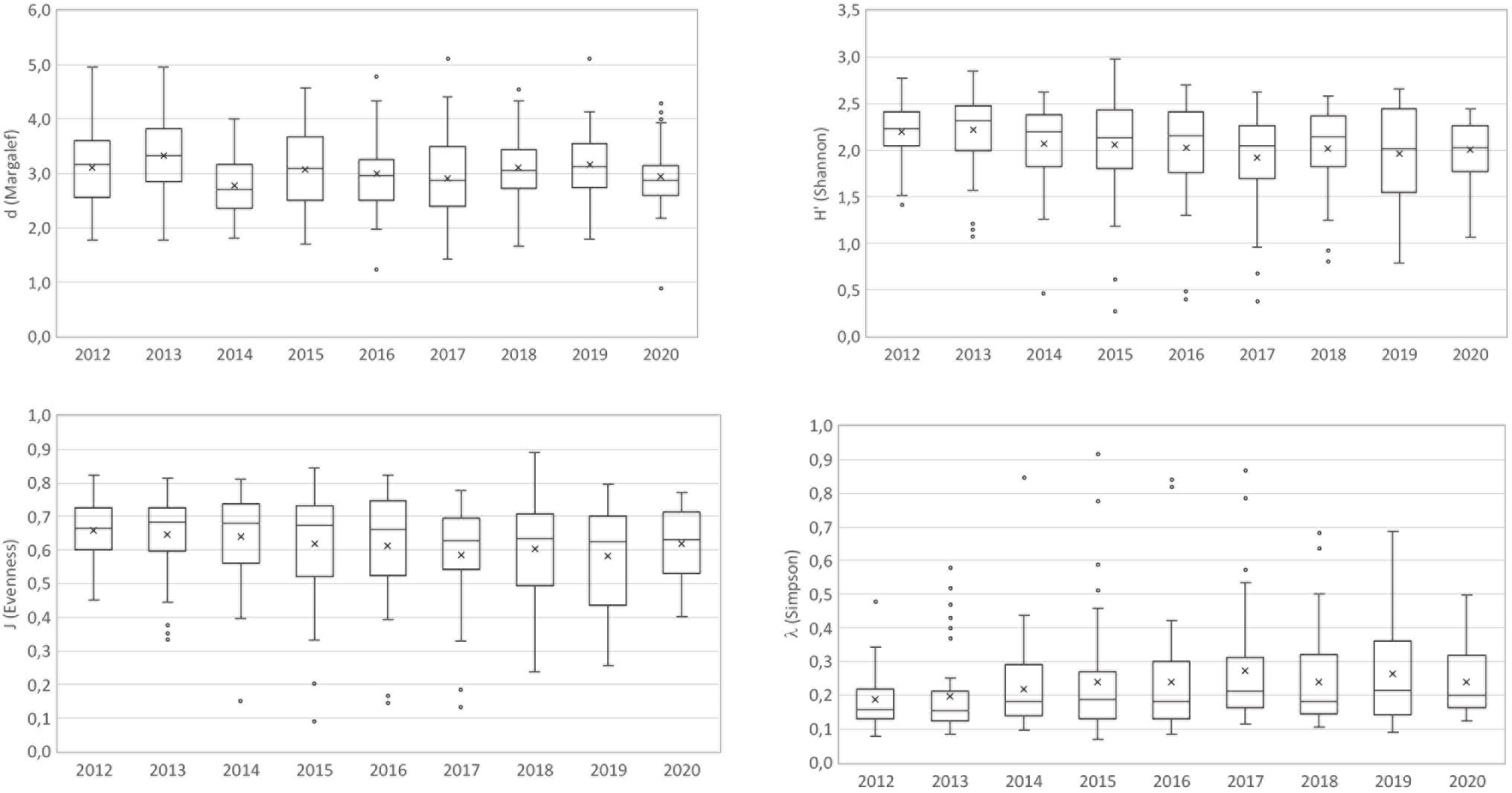
Figure 5 Boxplots of species richness (Margalef, d), diversity (Shannon-Wiener, H’), evenness (Pielou, J) and dominance (Simpson, Λ) computed by year for the deep-sea assemblages of the northwestern Ionian Sea.
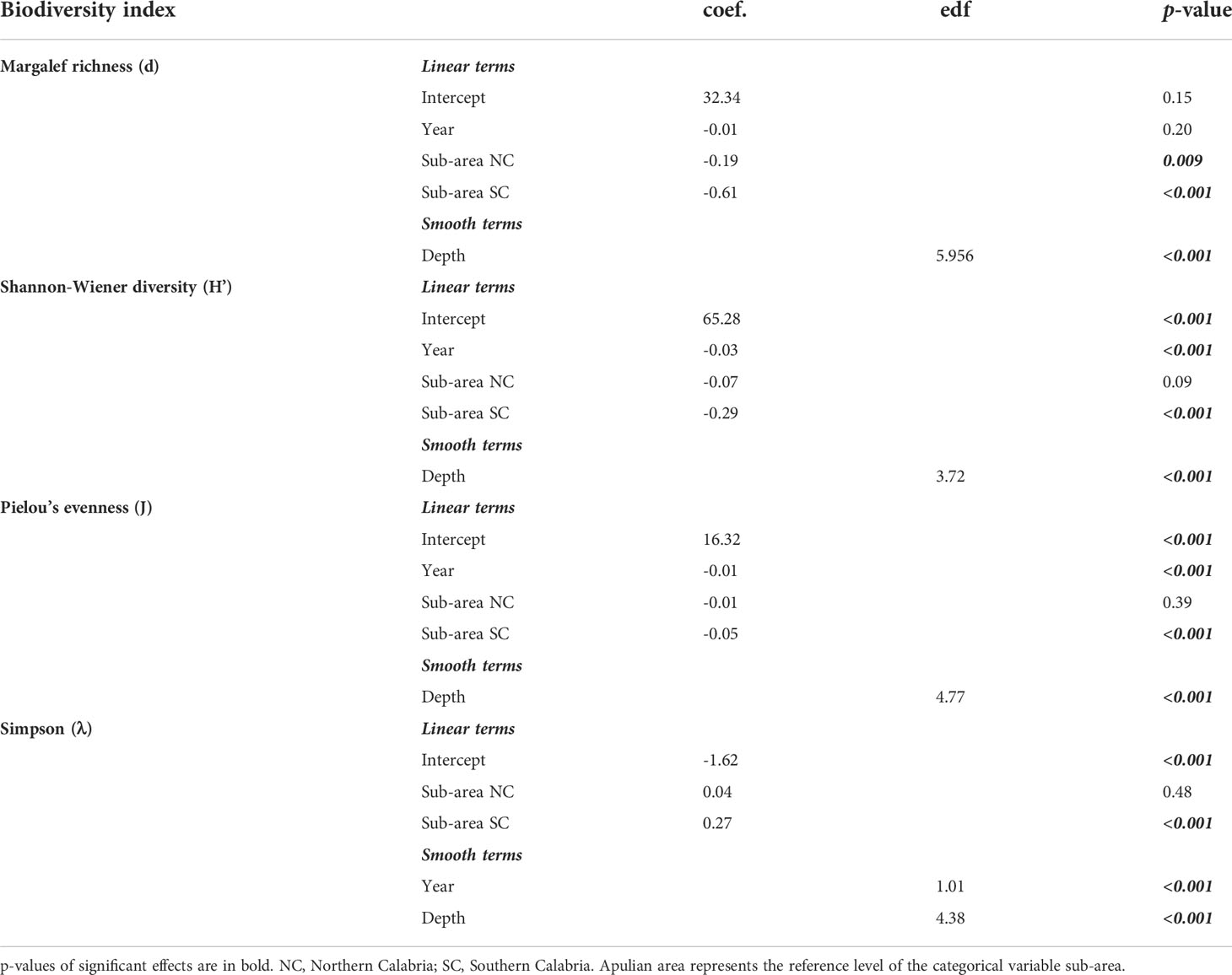
Table 1 GAMs estimates for biodiversity indices considering linear (coef.) and smooth effects (estimated degrees of freedom, edf) of temporal, geographical sub-area and depth.
GAM estimates indicated the not linear significant effect of depth covariate on each biodiversity index (Table 1, Figure 6). A similar increasing behavior up to 500-600 m was detected for H’ and J indices. The significant smooth effect of depth on d index suggested a hump-shaped pattern with a richness peak at intermediate investigated depths between 300 and 500 m whereas λ decreased with depths within 200-500 m depth range. Finally, significant linear effects of the geographical sub-area on the distribution of ecological indices were found with a significant increase moving northward (from the southern Calabria to the Apulian areas), except for the dominance index showing an inverse decreasing pattern with significantly higher values in the southernmost Calabrian area (Table 1).
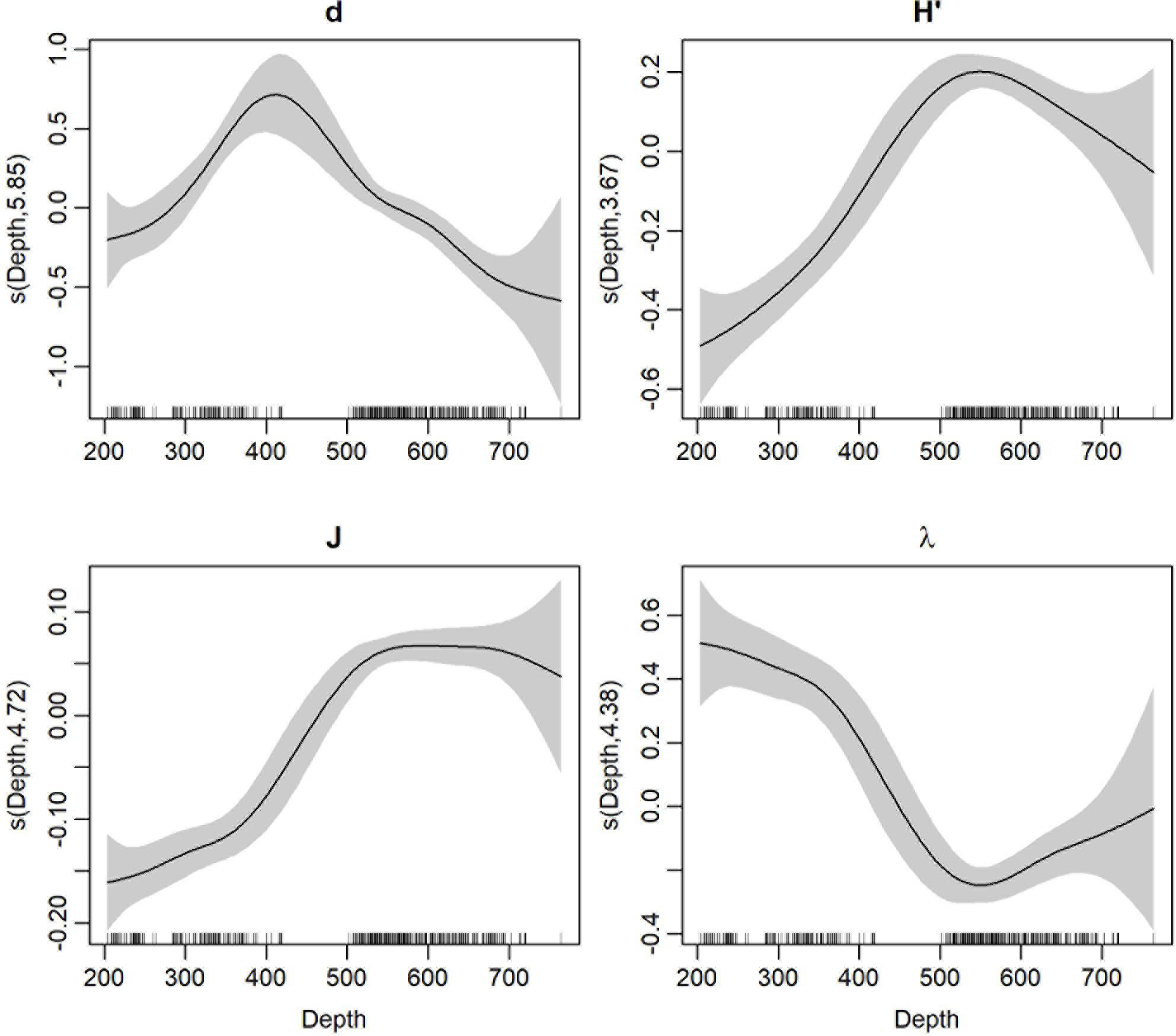
Figure 6 Fitted depth smooth effects for ecological indices. Tick marks on the x-axis are the observed data; y-axis represents the smooth effects with estimated degrees of freedom. Grey area corresponds to 95% confidence bands for smooths. d, Margalef richness; H’Shannon-Wiener diversity, Shannon-Wiener, J, Pielou evenness, Λ, Simpson dominance.
Discussion
The integrated analysis of all benthic, demersal and benthopelagic species sampled by the experimental trawls allowed to characterize the deep-sea faunal assemblages of the north-western Ionian Sea, as well as to detect their current status on the soft bottoms historically impacted by fishing.
The results confirm depth as the main factor influencing and structuring the deep faunal assemblages in the study area, as already reported in previous studies on the demersal assemblages in the Ionian Italian area (e.g. D’Onghia et al., 1998b; D’Onghia et al., 2003; Capezzuto et al., 2010; Carlucci et al., 2018). Depth seems to play a key role in the group differentiation, indicating the presence of two distinct bathyal faunal assemblages in the Ionian Sea, with a transition from epibathyal to mesobathyal groups on around 400 m in depth, as documented in the study area by (D’Onghia et al., 1998b; D’Onghia et al., 2003) many years ago. This depth-related pattern is largely reported in several previous studies on demersal assemblages in other Mediterranean areas (e.g. Ungaro et al., 1998; Colloca et al., 2003; Massutií et al., 2004; Gaertner et al., 2005; Politou et al., 2008; García-Rodríguez et al., 2011; Terribile et al., 2016; Lauria et al., 2020). The present findings also support the results of recent investigations at a large-scale distribution in the Mediterranean, where the demersal fish assemblages resulted strongly organized along a depth gradient (Farriols et al., 2019 and references therein). Moreover, significant changes in the mega-faunal assemblage with depth have been also documented outside the Mediterranean, from the northwest Atlantic (Hargrave et al., 2004; Kenchington et al., 2014) to the Canadian Pacific, Arctic and Atlantic Oceans (Wei et al., 2020), generally with biodiversity peaks near shelf breaks. These observations are aligned with the general idea that the physical environment likely dominates in structuring deep-sea communities, with respect to competition (Ashford et al., 2018). Indeed, physical and biotic factors, such as light intensity, food availability and temperature, change with depth and they may interact to influence local patterns of demersal assemblage structure, also affected by trawl fishing. Particularly, temperature and trawl fishing intensity, strongly influence species occurrence and diversity of megabenthic assemblages from mesophotic to deep-sea areas (Ashford et al., 2018; Chimienti et al., 2021; Morato et al., 2020; Morato et al., 2021).
In the present study, the identified upper and middle slope groups are better depicted with the contribution of the strictly benthic species, analysed for the first time in this area together with demersal and benthopelagic fauna.
All the analysis highlighted the distinctive role of benthic megafauna in characterizing the epibathyal and mesobathyal assemblages of the Ionian Sea. The portunid crab M. tuberculatus, the squat lobster M. speciosa and the sea cucumber P. regalis resulted the main indicator benthic species on the upper slope of the overall study area. Their role was confirmed by the ranking analysis in each Ionian sub-area. The widespread occurrence and increasing abundance of M. speciosa (previously reported as Munida rutllanti) on the upper slope of the Ionian Sea dates back to 2000 when its fast spreading throughout the study area was probably favoured by variations in environmental parameters occurred in the last decades (Maiorano et al., 2013). The blind lobster P. typhlops characterized the mesobathyal benthic assemblage of the Ionian Sea and also resulted an indicator species in the deeper assemblages of both Calabrian sub-areas. The species traditionally occurred on the slope of the Ionian basin as a by-catch species of the deep-water trawling (Maiorano et al., 1998) and one of the main species on the Ionian middle slope (D’Onghia et al., 1998b; D’Onghia et al., 2003; Company et al., 2004). The sea urchin E. melo seems to be a more ubiquitarian species, with the maximum IndVal in the overall bathymetric range 200-800 m.
The dominance of these mobile scavengers as characteristic species, together with the low presence of sessile invertebrates highly vulnerable to trawling, generally characterize the soft-bottom benthic assemblages impacted by trawl fishing activity (e.g. Jennings et al., 1999; Thrush and Dayton, 2002; de Juan et al., 2020). Trawling generally produces the replacement of vulnerable organisms (e.g., sessile cnidarians, sponges and bivalves) by taxa with a higher ability to tolerate the prolonged impact by fishing (e.g., echinoderms, small swimming crabs, polychaetes) (de Juan et al., 2011; García-Rodríguez et al., 2011). The decrease of habitat-forming and low resilient fauna can deplete the whole community structure with a decline in their complexity (Stamouli et al., 2022).
Concerning the demersal and benthopelagic fauna, the epibathyal and bathyal groups are still characterized by almost the same species identified in the last decades. The deep-water rose shrimp and the European hake are the most abundant species on the upper slope while the blue-and-red shrimp A. antennatus, the fish H. mediterraneus and different macrourids on the middle slope. The ranking analysis by IndVal highlighted the role of the deep-water rose shrimp P. longirostris in characterizing the upper slope assemblage together with C. agassizi. The macrourid N. sclerorhynchus and the golden shrimp P. martia result the main distinctive species in the middle slope assemblages whereas the other macrourids seem to be more ubiquitarian species. Most species of the Plesionika genus, and especially P. martia, are widely distributed in the Ionian area, particularly on the deep-sea grounds of the Calabrian coast where they spent different phases of their life cycle (Maiorano et al., 2002; Maiorano et al., 2010).
The depth zonation pattern of deep-sea assemblages detected in the study area is also supported by univariate diversity indices that showed a nonlinear and highly significant relationship with depth. This result is consistent with the expected depth patterns of species richness, described by hump-shaped curves with a diversity peak at intermediate bathymetric level explored, although different faunal components display different spatial patterns with increasing depth (Danovaro et al., 2010). The lower diversity and evenness detected in the upper slope seem to be more correlated to its higher dominance index than to the species richness. Indeed, higher species richness combined with higher dominance can explain the lower diversity detected in the exploited epibathyal hauls. Such a result can be due to the relevant biomass of non-commercial species, such as Capros aper, M. scolopax and G. leioglossus, observed in the experimental catch of last years. Accordingly, trawled assemblages in the upper slope resulted dominated by such small-sized and fast-growing species or by other resilient and opportunistic species like the deep water rose shrimp P. longirostris. Dominance of high resilient species is a community response to the bottom trawling as an effect of adaptation to persistent environmental impacts due to fishing which tends to remove large individuals of slow-growing species and could generate loss of ecosystem functions in the long term (Jennings et al., 1999; Duarte et al., 2008; de Juan et al., 2020). Thus, fishing activity can modulate the deep-sea communities, particularly in highly impacted areas like the upper slope.
Despite the reduction of fishing effort detected in the Ionian area over the period 1995-2020 (Maiorano et al., 2022), a highly significant effect of the temporal component was generally detected on the biodiversity pattern of the assemblages. On the overall depth range explored in Ionian area, a low but significant decrease of diversity and evenness indices together with an increasing dominance were observed over the study period 2012-2020. No significant changes were detected for richness index which fluctuated around relatively high values, but generally lower than in the western Mediterranean. This appears to be consistent with the general pattern of regional diversity in the Mediterranean (Coll et al., 2010), and recently detected on both demersal fish assemblages (Granger et al., 2015; Farriols et al., 2017) and strictly benthic fauna (Stamouli et al., 2022).
Fishery expansion to deeper waters is exploiting the last refugees for most fish species and affecting highly vulnerable species with little resilience to overexploitation (Watson and Morato, 2013, Morato et al., 2020).
The complex hydrography and geomorphology of the north-western Ionian Sea determine the presence of different habitats that provide a complex system of environmental patches, reflected in the species richness and biodiversity of this basin (Maiorano et al., 2010). The presence of heterogeneous and complex habitats, like canyons, banks and rocky bottoms on the Ionian slope, can guarantee hotspots of biodiversity, hosting a variety of species, as well as a refuge area for commercial species exploited in adjacent areas (Fernandez-Arcaya et al., 2017; D’Onghia, 2019; Rueda et al., 2019). The different distribution of these habitats along the Ionian basin could explain the geographical pattern of biodiversity in the three geographical sub-areas (Apulian, north Calabria and southern Calabria). Despite the greatest fishing effort by trawl described in the Apulian area, a general latitudinal increasing of biodiversity and decreasing of dominance was detected from the southern Calabria to the northern Apulia, where the highest values of diversity and evenness indices were showed on the overall depth range 200-800 m. The presence of the SML CWC province, unsuitable for trawl and established as Fisheries Restricted Area, could support the highest diversity and lowest dominance of small-sized species detected in the Apulian area. Many demersal and benthopelagic organisms can feed, grow and spawn there, reaching greater biomass and size than in adjacent exploited areas (D’Onghia et al., 2010; Capezzuto et al., 2018; D’Onghia, 2019). Despite the historical decline of the cartilaginous fishes (Damalas et al., 2015), widely recognized to be negatively affected by fishing, the black mouth catshark G. melastomus and the velvet belly Etmopterus spinax resulted among the main indicator species of the mesobathyal group in the Apulian area. Indeed, for several characteristic fish species of the Apulian deep-sea assemblages, such as Phycis blennioids, Pagellus bogaraveo and Helicolenus dactylopterus on the upper slope and G. melastomus on the deeper one, the presence of reproductive individuals has been previously reported in SML CWC province (Capezzuto et al., 2018). A remarkable abundance of juveniles of E. spinax, M. merluccius, P. blennoides and H. dactylopterus was also recorded in this CWC province (D’Onghia et al., 2010). EFHs are defined as habitats essential to the ecological requirements for critical life history stages of exploited species (GFCM, 2018). CWC communities may provide EFH for commercial species and therefore they act as potential renewal areas for exploited resources in the neighbouring fishing grounds.
Moreover, the geographic pattern of different indicator species detected in the three sub-areas supports previous studies on the geographic characterization of demersal resources in the Ionian Sea. The blue-and-red shrimp A. antennatus mostly characterizes the deeper assemblages of the Apulian and northern Calabrian where it already showed higher abundance and larger sizes more than two decades ago (D’Onghia et al., 1998a) while A. foliacea results more characteristic in the southern Calabrian area. Indeed, the giant red shrimp is more abundant in the central and eastern basins, particularly in the nearby Strait of Sicily (e.g. D’Onghia et al., 1998a; Cau et al., 2002; Politou et al., 2004; Palmas et al., 2017; Guijarro et al., 2019). The pandalid P. martia appears as indicator species on the middle slope of all sub-areas but with the highest ranking IndVal in the south Calabria where the species generally occurred with the highest abundance within the Ionian area (Maiorano et al., 2002). Its occurrence in the canyon system along the south Calabrian margin, as one of the most common species in the slope assemblage, was also revealed using Remotely Operated Vehicle by Pierdomenico et al. (2019).
The present study provides information on the deep-sea faunal assemblages of the north-western Ionian Sea as a whole for the first time. The integrated quantitative analysis of benthic, demersal and benthopelagic fauna down to 800 m in depth highlighted the importance of all components, differently impacted by trawl fishing, in assessing the current status of the Ionian deep-sea assemblages. The megabenthic fauna of the north-western Ionian Sea seems to be characterised by a simplified structure, with the generalist species resulting more abundant than the engineering and habitat-former taxa, highly vulnerable to the trawl fishing. For instance, the bamboo coral I. elongata was recently found in the study areas with very few colonies (Carbonara et al., 2020) and it resulted already absent in the Apulian area twenty years ago (D’Onghia et al., 2003), confirming the global condition of this critically endangered species. In fact, it was once considered one of the most common coral bycatch, while currently it persists only in a few, undisturbed areas of the Mediterranean Sea (Carpine, 1970; Mastrototaro et al., 2017; Carbonara et al., 2020). Indeed, the co-occurrence of I. elongata with the valuable aristeid shrimps, made its populations highly affected by bottom-fishing activities also in the central Mediterranean (Lauria et al., 2017; Carbonara et al., 2020). This is especially true in the north-western Ionian, where the trawl fishing is concentrated on the slope, with A. antennatus and A. foliacea as the main target species (D’Onghia et al., 2005; Carlucci et al., 2006; Maiorano et al., 2010; Russo et al., 2017).
As for I. elongata, deep-sea pennatulacean aggregations are also affected by intense destructive trawl fishing and the formerly common facies of Funiculina quadrangularis are almost completely missing from many Mediterranean areas, including Ionian Sea, due to trawl fishing (D’Onghia et al., 2003; Lauria et al., 2017; Chimienti et al., 2019a and reference therein). Otherwise, sedentary benthic species seem to be more resilient to the effects of trawling disturbance, e.g. starfish and crabs (de Juan et al., 2007; Mangano et al., 2013). Mobile scavengers and opportunistic taxa as the starfish A. irregularis, the decapod P. typhlops and the portunid crabs L. depurator and M. tuberculatus resulted abundant in the overall Ionian and they are generally higher in areas subjected to high levels of fishing intensity. All these species are characterized by common features, such as their feeding habits or their ability to regenerate body tissues damaged by fishing (Ramsay et al., 2001; Mangano et al., 2013).
Despite so strong changes in the strictly benthic assemblage, the demersal and benthopelagic fauna of the Ionian Sea show a general stability in the overall structure and depth-related zonation if compared with previous studies lasting over two decades (D’Onghia et al., 1998b; D’Onghia et al., 2003; Capezzuto et al., 2010; Maiorano et al., 2010). Some commercial deep-sea species, namely A. foliacea, N. norvegicus, P. longirostris, M. merluccius and P. blennoides fluctuated over time in their abundance, either correlated to both climatic indices and fishing pressure (Maiorano et al., 2010; D’Onghia et al., 2012). Moreover, an uncommon decrease in the abundance of A. antennatus and an inverted ratio between the two red shrimps was recorded during the period 2002-2004 (Carlucci et al., 2007; Capezzuto et al., 2010) as a consequence of changes in the thermohaline circulation derived from the “transient” phenomenon (Klein et al., 1999; Manca et al., 2002).
Notwithstanding the prolonged exploitation of the deep-sea resources in the Ionian Sea, significant increases in abundances have been observed for P. longirostris and A. foliacea in the last years, probably related both to the reduction of fishing effort and the increase of bottom temperature detected over the period 1995-2020 (Maiorano et al., 2022).
Some deep-sea resources can be also supported by the ‘refuge effect’ of the submarine canyons distributed along the continental slope of the north-western Ionian Sea, which provide high productivity and greater food availability (Sabatini et al., 2007; Kapiris and Thessalou-Legaki, 2009). Indeed, submarine canyons affect the structure and composition of the deep macrofaunal community, influencing sediment and nutrient transport processes as well as plankton production, which is the main food resource for the benthopelagic and demersal fauna (Fernandez-Arcaya et al., 2017; Fanelli et al., 2018; Pierdomenico et al., 2019; Ricci et al., 2019; Sion et al., 2019). The presence of submarine canyons seems also to support the stability of the Calabrian food web and the maintenance of the biomass structure in this ecosystem, probably due to a refuge effect for several commercial and non-commercial species (Ricci et al., 2022).
Conclusions
Increasing interest in deep-sea exploitation generates an urgent need to increase ecological knowledge at proper spatial and temporal scales (Danovaro et al., 2020). The central Mediterranean is an area with complex hydrology and geomorphology, rich in biodiversity and fishery resources requiring greater knowledge and continuous monitoring of sensitive habitats and VMEs as well as their associated biotic components (IUCN, 2019; Otero, 2019; Maiorano et al., 2022). The knowledge accumulated over a long term of research in the north-western Ionian Sea allowed to track small-scale variability in the status of the deep-sea assemblages as a whole. The assemblages appear to be almost stable over time, particularly for the demersal and benthopelagic components whereas a more severe impact has been detected for the benthic fauna, mostly depleted of its engineering and habitat-former species by trawl fishing activity. Complex and heterogenous habitats, such as CWC communities and canyons system, can provide EFH for commercial fish and invertebrates. The implementation of a network of protected or fishing restricted areas represents a fundamental measure to guarantee protection of sensitive sites and VMEs in the Mediterranean Sea that would satisfy both conservation of vulnerable species and habitats and management of fishery resources according to the Ecosystem Approach to Fisheries (Capezzuto et al., 2018; Maiorano et al., 2022).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
All specimens analysed in this study were collected from the fishery (Data Collection Framework [DCF]; EU Reg. 199/2008). Therefore, this study does not comply with the European Commission recommendations (Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010) or with Italian National Law (Decree Law n. 26 of 4 March 2014) on the protection of animals used for scientific experiment.
Author contributions
Conceptualization, PM and GD. Methodology, PR, FM, PM. Formal analysis, PR, PM, FM, CC. Data investigation and sampling design, GD, PM, GC, FM, PR. Writing—original draft preparation, PM, PR, GD. Writing—review and editing, GD, PM, PR, GC, FM, CC. Supervision, PM, GD. All authors contributed to the article and approved the submitted version.
Funding
The data used in this study were collected under the Data Collection Framework (DCF), supported by the Italian Ministry of Agriculture, Food and Forestry Policy (MiPAAF) and by the European Commission (EU Regulations 1004/2017).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.1007671/full#supplementary-material
References
Armstrong C. W., Foley N. S., Tinch R., van den Hove S. (2012). Services from the deep: Steps towards valuation of deep-Sea goods and services. Ecosyst. Serv. 2, 2–13. doi: 10.1016/j.ecoser.2012.07.001
Ashford Oliver S., Kenny Andrew J., Barrio Froján Christopher R. S., Bonsall Michael B., Horton T., Brandt A., et al. (2018). Phylogenetic and functional evidence suggests that deep-ocean ecosystems are highly sensitive to environmental change and direct human disturbance. Proc. R. Soc B. 285, 20180923. doi: 10.1098/rspb.2018.0923
Bargain A., Marchese F., Savini A., Taviani M., Fabri M. C. (2017). Santa Maria di leuca province (Mediterranean sea): Identification of suitable mounds for cold-water coral settlement using geomorphometric proxies and maxent methods. Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.0033
Bastari A., Pica D., Ferretti F., Micheli F., Cerrano C. (2018). Sea Pens in the Mediterranean Sea: Habitat suitability and opportunities for ecosystem recovery. ICES J. Mar. Sci. 75 (5), 1722–1732. doi: 10.1093/icesjms/fsy010
Battista D., Capezzuto F., Indennidate A., Panza M., Maiorano P. (2011). Variazioni temporali nelle abbondanze della teutofauna del mar ionio nord-occidentale. Biol. Mar. Mediterr. 18 (1), 330–331.
Bertrand J. A., Gil de Sola L., Papaconstantinou C., Relini G., Souplet A. (2002). The general specifications of the MEDITS surveys. Sci. Mar. 66 (2), 9–17. doi: 10.3989/scimar.2002.66s29
Capezzuto F., Ancona F., Carlucci R., Carluccio A., Cornacchia L., Maiorano P., et al. (2018). Cold-water coral communities in the central Mediterranean: Aspects on megafauna diversity, fishery resources and conservation perspectives. Rend. Lincei Sci. Fish. Nat. 29, 589–597. doi: 10.1007/s12210-018-0724-5
Capezzuto F., Calculli C., Carlucci R., Carluccio A., Maiorano P., Pollice A., et al. (2019). Revealing the coral habitat effect on benthopelagic fauna diversity in the Santa maria di leuca cold-water coral province using different devices and Bayesian hierarchical modelling. Aquat. Conserv: Mar. Freshw. Ecosyst. 29, 1608–1622. doi: 10.1002/aqc.3144
Capezzuto F., Carlucci R., Maiorano P., Sion L., Giove A., Indennidate A., et al. (2010). The bathyal bentopelagic fauna in the north-Western Ionian Sea: Structure, patterns and interactions. Chem. Ecol. 26, 199–217. doi: 10.1080/02757541003639188
Carbonara P., Zupa W., Follesa M. C., Cau A., Capezzuto F., Chimienti G., et al. (2020). Exploring a deep-Sea vulnerable marine ecosystem: Isidella elongata (Esper 1788) species assemblages in the Western and central Mediterranean. Deep Sea Res. Part I: Oceanographic Res. Papers 166, 103406. doi: 10.1016/j.dsr.2020.103406
Carlucci R., Bandelj V., Ricci P., Capezzuto F., Sion L., Maiorano P., et al. (2018). Exploring spatio-temporal changes of the demersal and benthopelagic assemblages of the north-western Ionian Sea (Central Mediterranean Sea). Mar. Ecol 598, 1–19. doi: 10.3354/meps12613
Carlucci R., D’Onghia G., Maiorano P., Sion L., Capezzuto F., Matarrese A., et al. (2007). Abundance and size fluctuations in the deep-water shrimps Aristaeomorpha foliacea (Risso 1827) and Aristeus antennatus (Risso 1819) in the north-western Ionian Sea (Mediterranean Sea). Rapp. Commun. Int. Mer Médit. 38, 445.
Carlucci R., D’Onghia G., Sion L., Maiorano P., Tursi A., Thessalou-Legaki M. (2006). “Selectivity parameters and size at first maturity in deep-water shrimps, aristaeomorpha foliacea (Risso 1827) and aristeus antennatus (Risso 1816), from the north-western Ionian Sea (Mediterranean Sea),” in Hydrobiologia (Netherlands). Ed. Thessalou-Legaki M. 557, 145–154. Issues of Decapod Crustacean Biology. doi: 10.1007/s10750-005-1317-8
Carpentieri P., Nastasi A., Sessa M., Srour A. (2021). “Incidental catch of vulnerable species in Mediterranean and black Sea fisheries – a review,” in General fisheries commission for the mediterranean. studies and reviews. (Rome: FAO). 101. doi: 10.4060/cb5405en
Carpine C. (1970). Ecologie de l’étage bathyal dans la méditerranée occidentale. Mém Inst Océanogr Monaco 2, 1–146.
Cau A. A., Carbonell A., Follesa M. C., Mannini A., Norrito G., Orsi-Relini L., et al. (2002). MEDITS-based information on the deep-water red shrimps Aristaeomorpha foliacea and Aristeus antennatus (Crustacea: Decapoda: Aristeidae). Scientia Marina 66 (Suppl. 2), 103–124. doi: 10.3989/scimar.2002.66s2103
Chimienti G., Angeletti L., Rizzo L., Tursi A. (2018). ROV vs trawling approaches in the study of benthic communities: the case of Pennatula rubra (Cnidaria: Pennatulacea). J. Mar. Biol. Assoc. United Kingdom 98 (8), 1859–1869. doi: 10.1017/S0025315418000851
Chimienti G., Bo M., Taviani M., Mastrototaro F. (2019a). Occurrence and biogeography of Mediterranean cold-water corals. Mediterranean cold-water corals: Past, present and future. Springer Chapter 19, 213–243. doi: 10.1007/978-3-319-91608-8_19
Chimienti G., De Padova D., Adamo M., Mossa M., Bottalico A., Lisco A., et al. (2021). Effects of global warming on Mediterranean coral forests. Sci. Rep. 11, 20703. doi: 10.1038/s41598-021-00162-4
Chimienti G., Mastrototaro F., D’Onghia G. (2019b). Mesophotic and deep-Sea vulnerable coral habitats of the Mediterranean Sea: Overview and conservation perspectives. the benthos zone. IntechOpen, 1–20. doi: 10.5772/intechopen.90024
Civitarese G., Lipizer M., Eusebi Borzelli G. L. (2010). On the impact of the bimodal oscillating system (BiOS) on the biogeochemistry and biology of the Adriatic and Ionian seas (Eastern Mediterranean). Biogeosciences 7 (12), 3987–3997. doi: 10.5194/bg-7-3987-2010
Clarke K. R., Warwick R. M. (2001). Change in marine communities: an approach to statistical analysis and interpretation. Plymouth: PRIMER-E. 1–176
Coll M., Piroddi C., Steenbeek J., et al (2010). The Biodiversity of the Mediterranean Sea: Estimates, Patterns, and Threats. PLoS ONE 5: e11842. doi: 10.1371/journal.pone.0011842
Colloca F., Cardinale M., Belluscio A., Ardizzone G. (2003). Pattern of distribution and diversity of demersal assemblages in the central Mediterranean Sea. Estuar. Coast. Shelf Sci. 56 (3), 469–80. doi: 10.1016/S0272-7714(02)00196-8
Company J. B., Maiorano P., Tselespides A., Politou C. Y., Plaity W., Rotllant G., et al. (2004). Deep-Sea decapod crustaceans in the Western and central Mediterranean Sea: Preliminary aspects of species distribution, biomass and population structure. Sci. Mar. 68 (3), 73–86. doi: 10.3989/scimar.2004.68s373
Cotter J., Mesnil B., Witthames P., Parker-Humphreys M.. (2009b). Notes on nine biological indicators estimable from trawl surveys with an illustrative assessment for north Sea cod. Aquat. Living Resour. 22, 135–153. doi: 10.1051/alr/2009016
Damalas D., Maravelias C. D., Osio G. C., Maynou F. X., Sbrana M., Sartor P., et al. (2015). Historical discarding in Mediterranean fisheries: a fishers’ perception. ICES J. Mar. Sci. 72, 2600–2608. doi: 10.1093/icesjms/fsv141
Danovaro R., Company J. B., Corinaldesi C., D’Onghia G., Galil B., Gambi C., et al. (2010). Deep-Sea biodiversity in the Mediterranean Sea: The known, the unknown, and the unknowable. PLoS One 5 (8), e11832. doi: 10.1371/journal.pone.0011832
Danovaro R., Company J.B., Corinaldesi C., D'Onghia G. alil B., Gambi C.. (2008). Exponential decline of deep-Sea ecosystem functioning linked to benthic biodiversity loss. Curr. Biol. 18, 1–8. doi: 10.1016/j.cub.2007.11.056
Danovaro R., Fanelli E., Aguzzi J., Billett D., Carugati L., Corinaldesi C., et al. (2020). Ecological variables for developing a global deep-ocean monitoring and conservation strategy. Nat. Ecol. Evol. 4, 181–192. doi: 10.1038/s41559-019-1091-z
De Caíceres M., Legendre P. (2009). Associations between species and groups of sites: Indices and statistical inference. Ecology 90, 3566–3574. doi: 10.1890/08-1823.1
de Juan S., Hinz H., Sartor P., Vitale S., Bentes L., Bellido J. M., et al. (2020). Vulnerability of demersal fish assemblages to trawling activities: A traits-based index. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00044
de Juan S., Demestre M., Sanchez P. (2011). Exploring the degree of trawling disturbance by the analysis of benthic communities ranging from a heavily exploited fishing ground to an undisturbed area in the NW Mediterranean. Sci. Mar. 75 (3), 507–16. doi: 10.3989/scimar.2011.75n3507
de Juan S., Thrush S. F., Demestre M. (2007). Functional changes as indicators of trawling disturbance on a benthic community located in a fishing ground (NW Mediterranean Sea). Mar. Ecol. Prog. Ser. 334, 117–29. doi: 10.3354/meps334117
D’Onghia G., Giove A., Maiorano P., Carlucci R., Minerva M., Capezzuto et al. F., et al (2012). Exploring relationships between demersal resources and environmental factors in the Ionian Sea (Central Mediterranean). Journal of Marine Biology. 2012, 12. doi: 10.1155/2012/279406
D’Onghia G. (2019). “Cold-water corals as shelter, feeding and life-history critical habitats for fish species: Ecological interactions and fishing impact,” in In Mediterranean cold-water corals: Past, present and future (Switzerland: Springer), 335–356. doi: 10.1007/978-3-319-91608-8_30
D’Onghia G., Capezzuto F., Mytilineou C. H., Maiorano P., Kapiris K., Carlucci R., et al. (2005). Comparison of the population structure and dynamics of Aristeus antennatus (Risso 1816) between exploited and unexploited areas in the Mediterranean Sea. Fish. Res. 76, 22–38. doi: 10.1016/j.fishres.2005.05.007
D’Onghia G., Indennidate A., Giove A., Savini A., Capezzuto F., Sion L., et al. (2011). Distribution and behaviour of deep-Sea benthopelagic fauna observed using towed cameras in the Santa maria di leuca cold-water coral province. Mar. Ecol. Prog. Ser. 443, 95–110. doi: 10.3354/meps09432
D’Onghia G., Lloris D., Politou C.-Y., Sion L., Dokos J. (2004). New records of deep-water teleost fishes in the Balearic Sea and Ionian Sea (Mediterranean Sea). Sci. Mar. 68 (Suppl. 3), 171–183.
D’Onghia G., Maiorano P., Sion L., Giove A., Capezzuto F., Carlucci R., et al. (2010). Effects of deep-water coral banks on the abundance and size structure of the megafauna in the Mediterranean Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 57 (5), 397–411. doi: 10.1016/j.dsr2.2009.08.022
D’Onghia G., Mastrototaro F., Matarrese A., Politou C. Y., Mytilineou C. (2003). Biodiversity of the upper slope demersal community in the Eastern Mediterranean: Preliminary comparison between two areas with and without trawl fishing. J. Northwest Atl Fish Sci. 31, 263–273. doi: 10.2960/J.v31.a20
D’Onghia G., Matarrese A., Tursi A., Maiorano P., Panetta P. (1995). Osservazioni sulla teuthofauna epi e meso-batiale nel mediterraneo orientale (Mar jonio e mar egeo). Biol. Mar. Mediterr. 2 (2), 199–204.
D’Onghia G., Tursi A., Maiorano P., Matarrese A., Panza M. (1998b). Demersal fish assemblages from the bathyal grounds of the Ionian Sea (Middle-Eastern Mediterranean). Ital. J. Zool. 65, 287–292. doi: 10.1080/11250009809386834
D’Onghia G., Tursi A., Maiorano P., Panza M. (1998a). Caratterizzazione geografica dello stock di Aristeus antennatus (Risso 1816) (Crustacea, decapoda) nel mar ionio settentrionale. Biol. Mar. Medit. 5 (2), 239–251.
Duarte C. M., Conley D. J., Carstensen J., Sánchez-Camacho M. (2008). Return to Neverland: Shifting Baselines Affect Eutrophication Restoration Targets. Estuaries and Coasts 32, 29–36. doi: 10.1007/s12237-008-9111-2
Dufreêne M., Legendre P. (1997). Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol. Monogr. 67 (3), 345–366. doi: 10.2307/2963459
Fanelli E., Bianchelli S., Danovaro R. (2018). Deep-sea mobile megafauna of Mediterranean submarine canyons and open slopes: analysis of spatial and bathymetric gradients. ProgOceanogr 168, 23–24. doi: 10.1016/j.pocean.2018.09.010
Farriols M. T., Ordines F., Carbonara P., Casciaro L., Di Lorenzo M., Esteban A. (2019). Spatio-temporal trends in diversity of demersal fish assemblages in the Mediterranean. Sci. Mar. 83S1, 189–206. doi: 10.3989/scimar.04977.13A
Farriols M. T., Ordines F., Somerfield P. J. (2017). Bottom trawl impacts on Mediterranean demersal fish diversity: Not so obvious or are we too late? Cont. Shelf Res. 137, 84–102. doi: 10.1016/j.csr.2016.11.011.
Fernandez-Arcaya U., Bitetto I., Esteban A., Farriols M. T., Garciía-Ruiz C., Gil de Sola L., et al. (2019). Large-Scale distribution of a deep-Sea megafauna community along Mediterranean trawlable grounds. Sci. Mar. 83S1 175–187. doi: 10.3989/scimar.04852.14A
Fernandez-Arcaya U., Ramirez-Llodra E., Aguzzi J., Allcock A. L., Davies J. S., Dissanayake A., et al. (2017). Ecological role of submarine canyons and need for canyon conservation: A review. Front. Mar. Sci. 4, 5. doi: 10.3389/fmars.2017.00005
Froese R., Pauly D. (2021). FishBase (Publication: World Wide Web Electronic). Available at: www.fishbase.org.
Gaertner J.C., Bertrand J.A., De Sola L.G., et al (2005). Large spatial scale variation of demersal fish assemblage structure on the continental shelf of the NW Mediterranean Sea. Mar. Ecol. Prog. Ser. 297, 245–57. doi: 10.3354/meps297245
Garciía-Rodriíguez M., Abelló P., Fernández A., Esteban A. (2011). Demersal assemblages on the soft bottoms off the catalan-levante coast of the Spanish Mediterranean. J. Mar. Biol. 1–16. doi: 10.1155/2011/976396
GFCM (2017). Report of the first meeting of the working group on vulnerable marine ecosystems (WGVME) (Malaga, Spain: FAO-GFCM). Available at: http://www.fao.org/gfcm/technical-meetings/detail/en/c/885358/.
GFCM (2018) GFCM data collection reference framework (DCRF). version: 20.1. Available at: http://www.fao.org/gfcm/data/dcrf/en/.
Granger V., Fromentin J.-M., Bez N., et al (2015). Large-scale spatio-temporal monitoring highlights hotspots of demersal fish diversity in the Mediterranean Sea. Prog. Oceanogr. 130, 65–74. doi: 10.1016/j.pocean.2014.10.002
Guijarro B., Bitetto I., D’Onghia G., Follesa M. C., Kapiris K., Mannini A., et al. (2019). Spatial and temporal patterns in the Mediterranean populations of Aristaeomorpha foliacea and Aristeus antennatus (Crustacea: Decapoda: Aristeidae) based on the MEDITS surveys. Scientia Marina 83 (S1), 57–70. doi: 10.3989/scimar.05012.04
Hall M. A., Alverson D. L., Metuzals K. I. (2000). By-catch: Problems and solutions. Mar. pollut. Bull. 41, 204–219. doi: 10.1016/S0025-326X(00)00111-9
Halpern B. S., Selkoe K. A., Micheli F., Kappel C. V. (2007). Evaluating and ranking the vulnerability of global marine ecosystems to anthropogenic threats. Conserv. Biol. 21, 1301–1315. doi: 10.1111/j.1523-1739.2007.00752.x
Hargrave B. T., Kostylev V. E., Hawkin C. M. (2004). Benthic epifauna assemblages, biomass and respiration in the gully region on the scotian shelf, NW Atlantic ocean. Mar. Ecol. Prog. Ser. 270, 55–70. doi: 10.3354/meps270055
Hiddink J. G., Jennings S., Sciberras M., Szostek C. L., Hughes K. M., Ellis N., et al. (2017). Global analysis of depletion and recovery of seabed biota after bottom trawling disturbances. Proc. Natl. Acad. Sci. U.S.A. 114, 8301–8306. doi: 10.1073/pnas.1618858114
IUCN (2019). Thematic report – conservation overview of Mediterranean deep-Sea biodiversity: A strategic assessment (IUCN Gland, Switzerland and Malaga, Spain: 122).
Jennings S., Greenstreet S. P., Reynolds J. D. (1999). Structural change in an exploited fish community: a consequence of differential fishing effects on species with contrasting life histories. J. Anim. Ecol. 68, 617–627. doi: 10.1046/j.1365-2656.1999.00312.x
Kapiris K., Thessalou-Legaki M. (2009). Comparative reproduction aspects of the deep-water shrimps Aristaeomorpha foliacea and Aristeus antennatus (Decapoda, aristeidae) in the Greek Ionian Sea (Eastern Mediterranean). J. Zoology Article, 2009 9, 979512.
Kenchington E. L., Cogswell A. T., MacIsaac K. G., Beazley L., Law B. A., Kenchington T. J. (2014). Limited depth zonation among bathyal epibenthic megafauna of the gully submarine canyon, northwest Atlantic. Deep Sea Res. Part II: Topical Stud. Oceanography 104, 67–82. doi: 10.1016/j.dsr2.2013.08.016
Klein B., Roether W., Manca B. B., Bregant D., Beitzel V., Kovacevic V., et al. (1999). The large deep water transient in the Eastern Mediterranean. Deep Sea Res. I 371, 371–414. doi: 10.1016/S0967-0637(98)00075-2
Lauria V., Garofalo G., Fiorentino F., Massi D., Milisenda G., Piraino S. (2017). Species distribution models of two critically endangered deep-Sea octocorals reveal fishing impacts on vulnerable marine ecosystems in central Mediterranean Sea. Sci. Rep. 7, 1–14. doi: 10.1038/s41598-017-08386-z
Lauria V., Gristina M., Fiorentino F., Attrill M. J., Garofalo G. (2020). Spatial management units as an ecosystem-based approach for managing bottom-towed fisheries in the central Mediterranean Sea. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00233
Liu F., Mikolajewicz U., Six K. D. (2021). Drivers of the decadal variability of the north Ionian gyre upper layer circulation during 1910–2010: A regional modelling study. Clim Dyn. 58, 2065–2077. doi: 10.1007/s00382-021-05714-y
Magurran A. E. (1991). Ecological diversity and its measurement, London, united kingdom. Croom Helm 179, 1–178.
Maiorano P., Capezzuto F., Carluccio A., Calculli C., Cipriano G., Carlucci R., et al. (2022). Food from the depths of the Mediterranean: The role of habitats, changes in the Sea-bottom temperature and fishing pressure. Foods 11, 1–32. doi: 10.3390/foods11101420
Maiorano P., Capezzuto F., Sion L., D’Onghia G., Tursi A. (2013). Spatio-temporal changes of Munida rutllanti zariquiey-Alvarez 1952 (Decapoda: Galatheidae) in the north-Western Ionian Sea (Central Mediterranean). Mediterr. Mar. Sci. 14, 42–48. doi: 10.12681/mms.619
Maiorano P., D’Onghia G., Capezzuto F., Sion L. (2002). Life-history traits of plesionika martia (Decapoda: Caridea) from the eastern–central Mediterranean Sea. Mar. Biol. 141, 527–539. doi: 10.1007/s00227-002-0851-4
Maiorano P., Mastrototaro F., Casamassima F., Panetta P. (1999). Analisi comparativa della teutofauna catturata con due differenti reti a strascico. Biol. Mar. Mediterr. 6 (1), 579–583.
Maiorano P., Pastore M., D’Onghia G., Latorre F. (1998). Note on the population structure and reproduction of Polycheles typhlops (Heller 1862) (Decapoda: Polychelidae) on the upper slope of the Ionian Sea. J. Natural History 32, 1609–1618. doi: 10.1080/00222939800771141
Maiorano P., Sabatella R. F., Marzocchi B. M. (. (2019). Annuario sullo stato delle risorse e sulle strutture produttive dei mari italiani Vol. , 432 (Genova, Italy: SIBM).
Maiorano P., Sion L., Carlucci R., Capezzuto F., Giove A., Costantino G., et al. (2010). The demersal faunal assemblage of the north-Western Ionian Sea (Central mediterranean): Current knowledge and perspectives. Chem. Ecol. 26, 219–240. doi: 10.1080/02757541003693987
Manca B. B., Ursella L., Scorazzato P. (2002). New development of eastern Mediterranean circulation based on hydrological observations and current measurements. Mar. Ecol. Prog. Ser. 23, 237–257. doi: 10.1111/j.1439-0485.2002.tb00023.x
Mangano M. C., Kaiser M. J., Porporato E. M., Spanoò N. (2013). Evidence of trawl disturbance on mega-epibenthic communities in the southern tyrrhenian Sea. Mar. Ecol. Prog. Ser. 475, 101–117. doi: 10.3354/meps10115
Massutí E., Gordon J. D., Morata J., Swan S.C., Stefanescu C., Merrett N. R. (2004). Mediterranean and Atlantic deep-sea fish assemblages: differences in biomass composition and size-related structure. Sci. Mar. 68 (S3), 101–15.
Mastrototaro F., Chimienti G., Acosta J., Blanco J., Garcia S., Rivera J., et al. (2017). Isidella elongata (Cnidaria: Alcyonacea) facies in the western Mediterranean Sea: visual surveys and descriptions of its ecological role. Eur. Zool J. 84, 209–225. doi: 10.1080/24750263.2017.1315745
Mastrototaro F., Maiorano P., Vertino A., Battista D., Indennidate A., Savini A., et al. (2013). A facies of Kophobelemnon (Cnidaria, octocorallia) from Santa maria di leuca coral province (Mediterranean Sea). Mar. Ecol. 34, 313–320. doi: 10.1111/maec.12017
Mastrototaro F., D’Onghia G., Corriero G., Matarrese A., Maiorano P., Panetta et al. P., et al (2010). Biodiversity of the White Coral Bank off Cape Santa Maria Di Leuca (Mediterranean Sea): An Update. Deep-Sea Res Part II: Top. Stud. Oceanogr. 57, 412–30, doi: 10.1016/j.dsr2.2009.08.021
Matarrese A., D’Onghia G., Tursi A., Basanisi M. (1996). New information on the ichthyofauna of the south-Eastern Italian coasts (Ionian Sea). Cybium 20 (2), 197–211.
Meírigot B., Gaertner J.-C., Brind’Amour A., Carbonara P., Esteban A., Garcia-Ruiz C. (2019). Stability of the relationships among demersal fish assemblages and environmental-trawling drivers at Large spatio-temporal scales in the northern Mediterranean Sea. Sci. Mar. 83S1, 153–163. doi: 10.3989/scimar.04954.30A
Mengerink K. J., Van Dover C. L., Ardron J., Baker M., Escobar-Briones E.. (2014). A call for deep-ocean stewardship. Science 344, 696–698. doi: 10.1126/science.1251458
Menna M., Suarez N. R., Civitarese G., Gacčicí M., Rubino A., Poulain P. M. (2019). Decadal variations of circulation in the central Mediterranean and its interactions with mesoscale gyres. Deep Sea Res. II 164, 14–24. doi: 10.1016/j.dsr2.2019.02.004
Morato T., González-Irusta J. M., Dominguez-Carrió C., Wei C. L., Davies A., Sweetman A.K., et al. (2020). Climate-induced changes in the suitable habitat of cold-water corals and commercially important deep-sea fishes in the north Atlantic. Global Change Biol. 26, 2181–2202. doi: 10.1111/gcb.14996
Morato T., Pham C. K., Fauconnet L., Taranto G. H., Chimienti G., Cordes E., et al. (2021). North Atlantic basin-scale multi-criteria assessment database to inform effective management and protection of vulnerable marine ecosystems. Front. Mar. Sci. 8, 637078. doi: 10.3389/fmars.2021.637078
Otero del M., Marin P. (2019). Conservation of cold-water corals in the Mediterranean: Current status and future prospects for improvement. In Mediterr. Cold-Water Corals: Past Present Future; Springer; pp, 535–545. doi: 10.1007/978-3-319-91608-8_46
Palmas F., Olita A., Addis P., Sorgente R., Sabatini A. (2017). Modelling giant red shrimp larval dispersal in the sardinian seas: Density and connectivity scenarios. Fisheries Oceanography 26 (3), 364–378. doi: 10.1111/fog.12199
Palomares M. L. D., Pauly D. (2021). SeaLifeBase (USA: World Wide Web Electronic Publication). Available at: www.sealifebase.org.
Pierdomenico M., Cardone F., Carluccio A., Casalbore D., Chiocci F., Maiorano P., et al. (2019). Megafauna distribution along active submarine canyons of the central Mediterranean: Relationships with environmental variables. Prog. Oceanogr. 171, 49–69. doi: 10.1016/j.pocean.2018.12.015
Politou C.Y., Mytilineou C., D’Onghia G., Dokos J. (2008). Demersal faunal assemblages in the deep waters of the eastern Ionian Sea. J. Nat. Hist. 42 (5–8), 661–72. doi: 10.1080/00222930701835613
Politou C. Y., Kapiris K., Maiorano P., Capezzuto F., Dokos J. (2004). Deep-sea Mediterranean biology: the case of Aristaeomorpha foliacea (Risso 1827) (Crustacea: Decapoda: Aristeidae). Sci. Mar. 68 (Suppl. 3), 129–139.
Puig P., Canals M., Company J. B., Martiín J., Amblas D. (2012). Ploughing the deep-Sea floor. Nature 489, 286–290. doi: 10.1038/nature11410
Ramirez Llodra E., Tyler P. A., Baker M. C., Bergstad O. A., Clark M., Escobar E., et al. (2011). Man and the last great wilderness: Human impact on the deep Sea. PLoS One 6 (8), e22588. doi: 10.1371/journal.pone.0022588
Ramsay K., Kaiser M. J., Richardson C. A. (2001). Invest in arms: The behavioural and energetic implications of multiple autotomy in starfish. Behav. Ecol. Sociobiol 50, 360–365. doi: 10.1007/s002650100372
Ricci P., Carlucci R., Capezzuto F., Carluccio A., Cipriano G., D’Onghia G., et al. (2022). Contribution of intermediate and high trophic level species to benthic-pelagic coupling: Insights from modelling analysis. Front. Mar. Sci. 9, 10198–212. doi: 10.3389/fmars.2022.887464
Ricci P., Libralato S., Capezzuto F., D’Onghia G., Maiorano P., Sion L., et al. (2019). Ecosystem functioning of two marine food webs in the north-western Ionian Sea (Central Mediterranean Sea). Ecol. Evol. doi: 10.1002/ece3.5527
Rossi S., Gabbianelli G. (1978). Geomorfologia del golfo di taranto. Boll. Soc Geol. It. 97, 423–437.
Rueda J. L., Urra J., Aguilar R., Angeletti L., Bo M., García-Ruiz C., et al. (2019). Cold-water coral associated fauna in the Mediterranean Sea and adjacent areas. in Mediterranean cold-water corals: past, present and future;. Springer, 295–333. doi: 10.1007/978-3-319-91608-8_29
Ruppert D., Wand M. P., Carroll R. J. (2003). Semiparametric regression (USA: Cambridge University Press).
Russo T., Bitetto I., Carbonara C., Carlucci R., D’Andrea L., Facchini M. T., et al. (2017). A holistic approach to fishery management: evidence and insights from a central mediterranean case study (western Ionian Sea). Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00193
Sabatini A., Follesa M. C., Locci I., Pendugiu A. A., Pesci P., Cau A. (2007). “Assemblages in a submarine canyon: influence of depth and time,” in In biodiversity in enclosed seas and artificial marine habitats (Dordrecht: Springer), 265–271).
Sardaí F., Calafat A., Flexas M., Tselepides A., Canals M., Espino M. (2004). An introduction to Mediterranean deep-Sea biology, in Mediterranean deep-Sea biology. Sci. Mar. 68 (Suppl. 3), 7–38.
Sion L., Bozzano A., D’Onghia G., Capezzuto F., Panza M., et al. (2004). Chondrichthyes Species in deep waters of the Mediterranean Sea. Sci. Mar. 68, 153–162. doi: 10.3989/scimar.2004.68s3153
Sion L., Calculli C., Capezzuto F., Carlucci R., Carluccio A., Cornacchia L., et al. (2019). Does the bari canyon (Central Mediterranean) influence the fish distribution and abundance? Prog. Oceanography 170, 81–92. doi: 10.1016/j.pocean.2018.10.015
Sion L., D’Onghia G., Basanisi M., Panza M. (2000). Distribuzione dei selaci sui fondi strascicabili del mar ionio nord-occidentale. Biol. Mar. Medit. 7 (1), 455–460.
Spedicato M. T., Massutií E., Meírigot B., Tserpes G., Jadaud A., Relini G. (2019). The MEDITS trawl survey specifications in an ecosystem approach to fishery management. Sci. Mar. 83S1, 9–20. doi: 10.3989/scimar.04915.11X
Stamouli C., Zenetos A., Kallianiotis A., Voultsiadou E. (2022). Megabenthic invertebrate diversity in Mediterranean trawlable soft bottoms: a synthesis of current knowledge. Mediterr. Mar. Sci. 23 (3), 447–459. doi: 10.12681/mms.29165
Theocharis A., Georgopoulos D., Lascaratos A., Nittis K. (1993). Water masses and circulation in the Central region of the Eastern Mediterranean: Eastern Ionian, South Aegean and Northwest Levantine, 1986-1987. Deep-Sea Res I 40 (6), 1121–42. doi: 10.1016/0967-0645(93)90064-T
Terribile K., Evans J., Knittweis L., Schembri P. J. (2016). Maximising MEDITS: Using data collected from trawl surveys to characterise the benthic and demersal assemblages of the circalittoral and deeper waters around the Maltese islands (Central mediterranean). regional studies in marine. Science 3, 163–175. doi: 10.1016/j.rsma.2015.07.006
Thrush S. F., Dayton P. K. (2002). Disturbance to marine benthic habitats by trawling and dredging: Implications for marine biodiversity. Annu. Rev. Ecol. Evol. Syst. 33, 449–473. doi: 10.1146/annurev.ecolsys.33.010802.150515
Thurber A. R., Sweetman A. K., Narayanaswamy B. E., Jones D. O.B., Ingels J., Hansman R. L. (2014). Ecosystem function and services provided by the deep Sea. Biogeosciences 11, 3941–3963. doi: 10.5194/bg-11-3941-2014
Tursi A., Mastrototaro F., Matarrese A., Maiorano P., D’Onghia G. (2004). Biodiversity of the White Coral Reefs in the Ionian Sea (Central Mediterranean). Chem Ecol 20, 107–16, doi: 10.1080/02757540310001629170
Tursi A., D’Onghia G. (1992). Cephalopods of the Ionian Sea (Mediterranean Sea). Oebalia XVIII N.S., 25–43.
Ungaro N., Marano G., Vlora A., Martino M. (1998). Space-time variations of demersal fish assemblages in southwestern Adriatic Sea. Vie Milieu. 48, 191–201.
Watson R. A., Morato T. (2013). Fishing down the deep: Accounting for within-species changes in depth of fishing. Fisheries Res. 140, 63–65. doi: 10.1016/j.fishres.2012.12.004
Wei C.-L., Cusson M., Archambault P., Belley R., Brown T., Burd B. J. (2020). Seafloor biodiversity of canada’s three oceans: Patterns, hotspots and potential drivers. Divers. Distrib. 26, 226–241. doi: 10.1111/ddi.13013
Keywords: deep-sea, megafauna, community, biodiversity, Mediterranean
Citation: Maiorano P, Ricci P, Chimienti G, Calculli C, Mastrototaro F and D’Onghia G (2022) Deep-water species assemblages on the trawlable bottoms of the Central Mediterranean: Changes or not over time? Front. Mar. Sci. 9:1007671. doi: 10.3389/fmars.2022.1007671
Received: 30 July 2022; Accepted: 27 September 2022;
Published: 14 October 2022.
Edited by:
Sergio Stefanni, Zoological Station Anton Dohrn, ItalyReviewed by:
Frine Cardone, Anton Dohrn Zoological Station, ItalyFrancis Neat, World Maritime University, Sweden
Copyright © 2022 Maiorano, Ricci, Chimienti, Calculli, Mastrototaro and D’Onghia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pasquale Ricci, pasquale.ricci@uniba.it
 Porzia Maiorano
Porzia Maiorano Pasquale Ricci
Pasquale Ricci Giovanni Chimienti
Giovanni Chimienti Crescenza Calculli3
Crescenza Calculli3 Francesco Mastrototaro
Francesco Mastrototaro Gianfranco D’Onghia
Gianfranco D’Onghia