- 1International Research Center for Marine Biosciences, Ministry of Science and Technology, Shanghai Ocean University, Shanghai, China
- 2Key Laboratory of Exploration and Utilization of Aquatic Genetic Resources, Ministry of Education, Shanghai Ocean University, Shanghai, China
- 3Guangxi Key Laboratory of Beibu Gulf Marine Biodiversity Conservation, College of Marine Science, Beibu Gulf University, Qinzhou City, China
- 4Institute of Tropical Aquaculture and Fisheries, Universiti Malaysia Terengganu, Kuala Nerus, Malaysia
Juvenile tri-spine horseshoe crabs (Tachypleus tridentatus) were exposed to determine the effects of single and combined stresses of polystyrene nanoplastics (nano-PS) and heavy metal (Cu2+) on antioxidant enzyme parameters. The juveniles were exposed to a 21-day 100-nm polystyrene concentration (104 particles l-1) and a concentration of Cu2+ (10 µg l-1) followed by a recovery period of 7 days. The in vivo antioxidant activity for whole horseshoe crab was analyzed. The results revealed that all antioxidant parameters, i.e., superoxide dismutase (SOD), catalase (CAT), glutathione (GSH), malondialdehyde (MDA), and lipid peroxidation (LPO), showed both increased and decreased levels in different experimental groups of horseshoe crabs having different experimental conditions compared to the control group at three time points, i.e., on days 7, 14, and 21. Similarly during the recovery period, SOD, CAT, and MDA showed decreased levels in all experimental groups, while GSH and LPO showed increased levels in all experimental groups of horseshoe crabs under the influence of different experimental conditions of nanoplastics and heavy metals compared to the control group on day 28. These results showed that the exposure of nano-PS and Cu2+ had precise effects on juvenile horseshoe crabs. Integrated biomarker responses showed that nano-PS and Cu2+ had adverse effects on juvenile horseshoe crabs. By principal component analysis, the potentially toxic effects of nano-PS and Cu2+ on horseshoe crabs were obtained.
Introduction
Horseshoe crabs belong to arthropods of the family Limulidae, suborder Xiphosurida, and order Xiphosura. Only four species belong to three genera that exist in the world, namely, Limulus polyphemus, Tachypleus tridentatus, Tachypleus gigas, and Carcinoscorpius rotundicauda. Rudkin and Young (2009) reported horseshoe crabs with an ancestry dating to the late Ordovician Period, approximately 445 million years ago, and thereby known as “living fossils”. Tachypleus tridentatus and C. rotundicauda can be found in the Beibu Gulf, southern China (Obst et al., 2012; Hu et al., 2015a). Horseshoe crabs play an important ecological role in supporting other species in the food web. They have a larger adult body size as compared to many other invertebrates on the shores; due to this, horseshoe crabs can be attributed as an indicator species to reflect the health of coastal shore systems (Chen et al., 2004). However, environmental pollution and shoreline habitat destruction are causing the population depletion of horseshoe crabs (Jackson and Nordstrom, 2009; Shin et al., 2009). A field survey was conducted in 2019 to study the depletion of horseshoe crab populations from different sites around the northern Beibu Gulf, China, where they revealed that the main reasons were unsustainable fishing practices and anthropogenic activities (Liao et al., 2019). Greater anthropogenic activities, including fishery and mariculture, have contributed to a huge sum of microplastics within the South China Sea (Wang et al., 2018; Li et al., 2020).
More than 300 million tons of plastic items are globally manufactured every year, of which around 10% goes into the sea (Jambeck et al., 2015; Thompson, 2015). China is regarded as one of the biggest nations, known for plastic production and consumption in the world (Fu and Wang, 2019). Each year, a colossal number of microplastics (MPs) enter the ocean through anthropogenic activities (Su et al., 2022). A material known to be non-toxic in bulk can be toxic at the nanometer scale due to its characteristic properties (Karlsson et al., 2009). MPs have unevenly chemically active surfaces and large surface areas. Due to these properties, MPs absorb large varieties of hazardous materials including heavy metals from the surrounding environment and transfer them along the food webs resulting in potential hazards to organisms (Tang et al., 2018; Hüffer et al., 2018; Liu et al., 2020; Zhou et al., 2020). Laboratory experiments have proved that MPs have strong negative effects on invertebrates and fishes (Duis and Coors, 2016). Polyethylene (PE), polypropylene (PP), polystyrene (PS), polyamides (PA), and polyvinyl chloride (PVC) have caused physical damage and adverse effects on the intestine, enterocytes, digestive tracts, and liver of zebrafish (Danio rerio) (Lei et al., 2018; Capó et al., 2021). Endocrine disorders, immune responses, oxidative stress, and alterations in gene expression have been reported in organisms as a result of different kinds of MPs (Qiao et al., 2019). Exposure to PE, PP, and PVC induced behavioral changes and decreased the reproductive and survival rates in marine crustaceans, copepods, shrimp, and fish (Yin et al., 2019; Costa et al., 2020; Wang et al., 2020).
Among the absorbed toxic contaminants on MP, heavy metals are the major toxic contaminants of inorganic nature (Khalid et al., 2018). Excessive amounts of heavy metals can exert adverse effects on soil and aquatic ecosystems, can reduce the growth and activity of organisms, and through the food chain can cause threats to human health and downstream animals (Cao et al., 2021). In an experiment, original and aged polyethylene particles were used to adsorb metal ions of different heavy metals in freshwater; it was found that the main factor affecting the adsorption was different in ion concentration between the liquid phase (metal solution) and the solid phase (PE particles), where the metal ion concentration eventually reached equilibrium (Turner and Holmes, 2015). Due to weathering and oxidation, the surface morphology of the particles changed, which made it easy to obtain electric charge and to adsorb metal ions to achieve charge balance. On account of the adsorption of harmful chemicals, ingestion of contaminated MPs by marine fish causes liver inflammation, oxidative stress, and other pathological reactions (Rochman et al., 2013). A few years back, some researchers also observed activated antioxidant enzymes in Nile tilapia (Oreochromis niloticus), during 14 days of exposure to MPs (Ding et al., 2018). It was noted that polystyrene microplastic accumulation induced disturbed lipid and energy metabolism and liver oxidative stress in zebrafish (Danio rerio) (Lu et al., 2016).
The Beibu Gulf in the South China Sea is an important passageway between China and the Association of Southeast Asian Nations (ASEAN). Various kinds of industries have been developing in the Beibu Gulf. This has caused more serious heavy metal pollution besides improving the local economy (Lao et al., 2019). The sample collected in recent years indicated a higher concentration of Cu than before, particularly in areas where oil production occurs (Chen et al., 2018; Lao et al., 2019). Some areas have a higher concentration of heavy metals and are not suitable for mariculture (Gu et al., 2015; Gu et al., 2018). Previous research studies have proven that copper has a role in the physiological and biochemical processes of organisms, for example, cell growth, development, mitochondrial respiration, and the antioxidant defense system (Tao and Gitlin, 2003). Similarly, copper is an essential element in the metabolism of arthropods and is needed for the synthesis of many important enzymes, such as superoxide dismutase, phenoloxidase, and hemocyanin, but excessive copper has its detrimental effects (Schmidt et al., 2021). Excessive ingestion of copper can put ecosystems and human food safety in danger (Schmidt et al., 2021). When the amount of copper exceeded the amount required by cells, copper could cause oxidative stress (Guo et al., 2017). Moreover, copper stress had varying effects on immune-related factors such as alkaline phosphatase (AKP), acid phosphatase (ACP), and phenoloxidase (PO), which are important components in the immune defense system of organisms (Wei and Yang, 2016).
The toxicity of nanoplastics and heavy metals has not been studied on a wider spectrum. Furthermore, no data are addressing the combined toxicity of nanoplastics and heavy metals to date. Therefore, it is worth inspecting whether these two pollutants have ecological effects after the short-term exposure to marine organisms. This study aims to observe the antioxidant activity indexes (SOD, CAT, MDA, GSH, LPO activity) in juvenile horseshoe crabs after exposure to polystyrene nanoplastics in the combination of heavy metal (Cu2+).
Material and methods
Experimental animals
In this study, juvenile T. tridentatus were artificially bred at Shanghai Ocean University, China. Its body weight was 0.0242 g ± 0.0064 g and body length 0.622 cm ± 0.131 cm. The juveniles were placed in dechlorinated seawater with a salinity of 28 ± 0.5 PSU and a pH of 8.1 ± 0.1. Juvenile horseshoe crabs were acclimatized for a week in the laboratory of Shanghai Ocean University with continuous air access for 24 h. Every day, a multiparameter instrument (model 5200A, YSI, USA) was used to measure the water quality in all the glass tanks, and the water temperature was (24 ± 1) °C; The photoperiod was 12 h of light and 12 h of darkness..
The experimental materials
Polystyrene microspheres (100 nm) with fluorescent dyes were purchased from Tianjin Beisler Chromatography Technology Development Center, China. Anhydrous Cu2+ was purchased from Shanghai Xianding Biotechnology Co., Ltd. (CAS: 7758-98-7; purity 99%). Enzyme activity assay kits were purchased from Nanjing Jiancheng Bioengineering Institute.
Experimental design
For this study, four different tanks of 4-l volume were used for exposure. Continuous water circulation was enabled using an oxygen pump. For each of the four groups, 100 individuals were kept in a tank. Each tank contained dechlorinated seawater with a salinity of 28 ± 0.5 PSU, pH of 8.1 ± 0.1, and water temperature of 24 ± 1°C. Juvenile horseshoe crabs were divided into four treatment groups labeled A, B, C, and D. Group A was fed only freshly hatched Artemia, group B was fed nanopolystyrene (nano-PS 104 particles l-1)-attached Artemia, group C was fed copper (Cu2+ 10 µg l-1)-attached Artemia, and group D was fed a bait of a mixture of heavy metals and nanoplastics (10 µg l-1) prepared according to the method of Jinhui et al. (2019).
In preparation for the nanoplastic bait, the heavy-metal-saturated nanoplastics were collected by centrifugation at 2,500 rpm and 20 mg of nano-PS was added to 10 g Artemia. After mixing, the nanoplastic bait was poured into the glass aquarium containing 100 individuals of juvenile horseshoe crabs and 4 l of filtered seawater at 24 ± 1°C temperature. Water renewal was conducted every 2 days. Horseshoe crabs were fed daily at 5:00 p.m., and each bait was prepared 2 h before feeding. Samples were taken on days 7, 14, 21, and 28. The duration of the experiment was 21 days followed by 7 days of recovery period with a water temperature of 24 ± 1°C and dechlorinated seawater with a salinity of 28 ± 0.5 PSU and a pH of 8.1 ± 0.1.
Sample preparation for antioxidant activity
After 21 days of exposure followed by a 7-day recovery period, 10 horseshoe crabs from each exposed group were collected, and excessive limbs and carapace were removed to obtain tissue samples. Physiological saline was added (saline weight: tissue mass, 9:1) to samples under ice bath conditions. The tissues were then homogenized in a centrifuge tube (6,000 r/min; four times; 20 s each) to obtain a 10% tissue homogenate solution. The solution was centrifuged for 10 min at 3,000 rpm at 4°C, and the SOD, CAT, GSH, and LPO activities and MDA content were determined in the supernatant using recommended kits, according to the manufacturer’s instructions.
Integrated biomarker response analysis
To determine the stress levels in each treatment group, the integrated biomarker response (IBR) method was used to integrate all the measured biomarkers (Beliaeff and Burgeot, 2002). In this study, the IBR method was used to indicate the stress levels in juvenile horseshoe crabs after 21 days of exposure, followed by a 7-day recovery period for nano-PS and Cu2+ in four different groups. It followed the detailed instructions provided by Beliaeff and Burgeot (2002).
Primarily for biomarkers, the mean value and standard deviation of all groups in a comparison with the whole experiment were calculated. The calculation of Y was conducted as Y = (X - m)/s, where Y is the value of each standard biomarker, X is the response of the biomarker, m is the mean value of the biomarker, and s is the standard deviation. Then, the treatment biomarker score S was calculated as S = Y + |Min|, where S ≥ 0 and |Min| is the absolute minimum value of Y for each biomarker response. The acquired values of score S were used to plot radar graphs. Lastly, IBR values were procured as the sum of the triangular regions defined by k-standardized biomarkers
Where Ai = Si × Si+1 × Sin(2π/k)/2.
Data analysis
All data were processed through Statistical Package for Social Sciences (SPSS) software. Before analysis, Levene’s test was used to test the homogeneity of the variance and the Shapiro–Wilk test was used to test the normality of the data. Then, the effects of MPs and heavy metals were determined through one-way ANOVA followed by Tukey’s HSD tests. The results were presented as means ± SD, and p< 0.05 was considered a significant difference between the treatments. Principal component analysis was executed for all biochemical parameters using XLSTAT® 2014.
Results
On day 7, the nanoplastic (nano-PS 104 particles l-1)-exposed group showed a significant reduction in SOD activity compared to the control. The heavy metal (Cu2+ 10 µg l-1)-exposed group showed a significant increase in SOD activity compared to the control. Similarly, nano-PS+Cu2+ treatment induced significantly higher SOD activity compared to control in experimental horseshoe crabs. On day 14, all the groups showed higher SOD activities compared to the control except the Cu2+-exposed group, which showed a decreased SOD activity compared to the control group. On day 21, all the groups showed a significant reduction in SOD activities compared to the control. During the recovery period, the Cu2+- and nano-PS+Cu2+-exposed groups showed a significant reduction in SOD activities compared to the control (Figure 1).
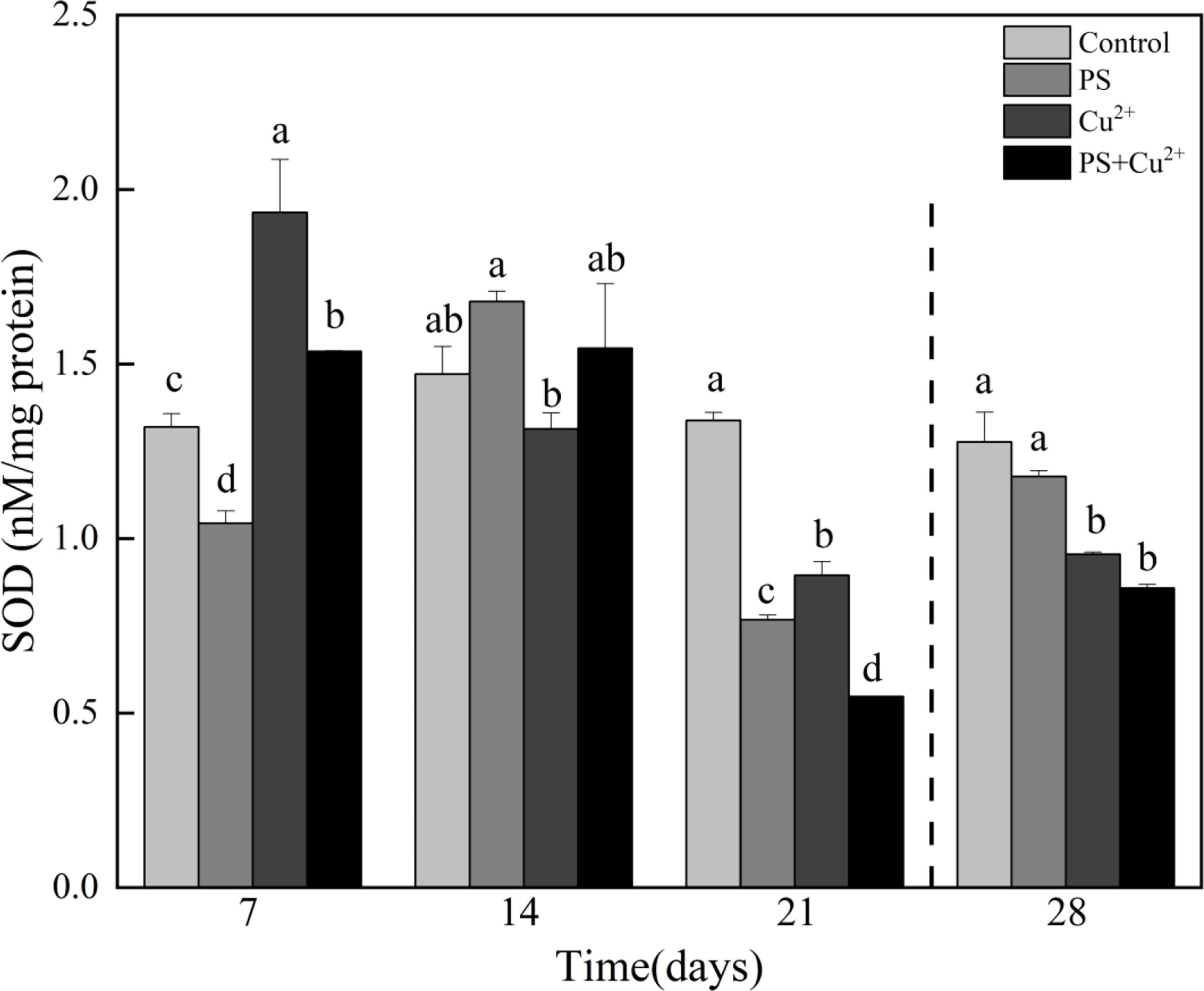
Figure 1 Superoxide dismutase (SOD) activities in whole tissues of Tachypleus tridentatus exposed to 100-nm micro-PS (104 particles l-1), Cu2+ (10 µg l-1), and a combination of PS and Cu2+ at days 7, 14, and 21, allowed to recover for 7 days (28 days). Data are provided as mean values ± standard deviations. The superscripts of different lowercase letters indicate significant differences (p < 0.05) between the four groups.
All the nano-PS-, Cu2+-, and nano-PS+Cu2+-exposed groups showed no significant effects on CAT activities compared to the control on day 7. Only the nano-PS-exposed group induced higher CAT activities in horseshoe crabs on day 7 compared to the control group. On day 14, both nano-PS- and Cu2+-exposed groups showed decreased CAT activities compared to the control, while the nano-PS+Cu2+-group showed higher CAT activities compared to the control. Similarly on day 21, the nano-PS-exposed group showed significantly higher activities compared to the control, and the Cu2+- and nano-PS+Cu2+-exposed groups showed significantly lower activities compared to the control. During the recovery period, all the groups showed a significant reduction in CAT activities compared to the control (Figure 2).
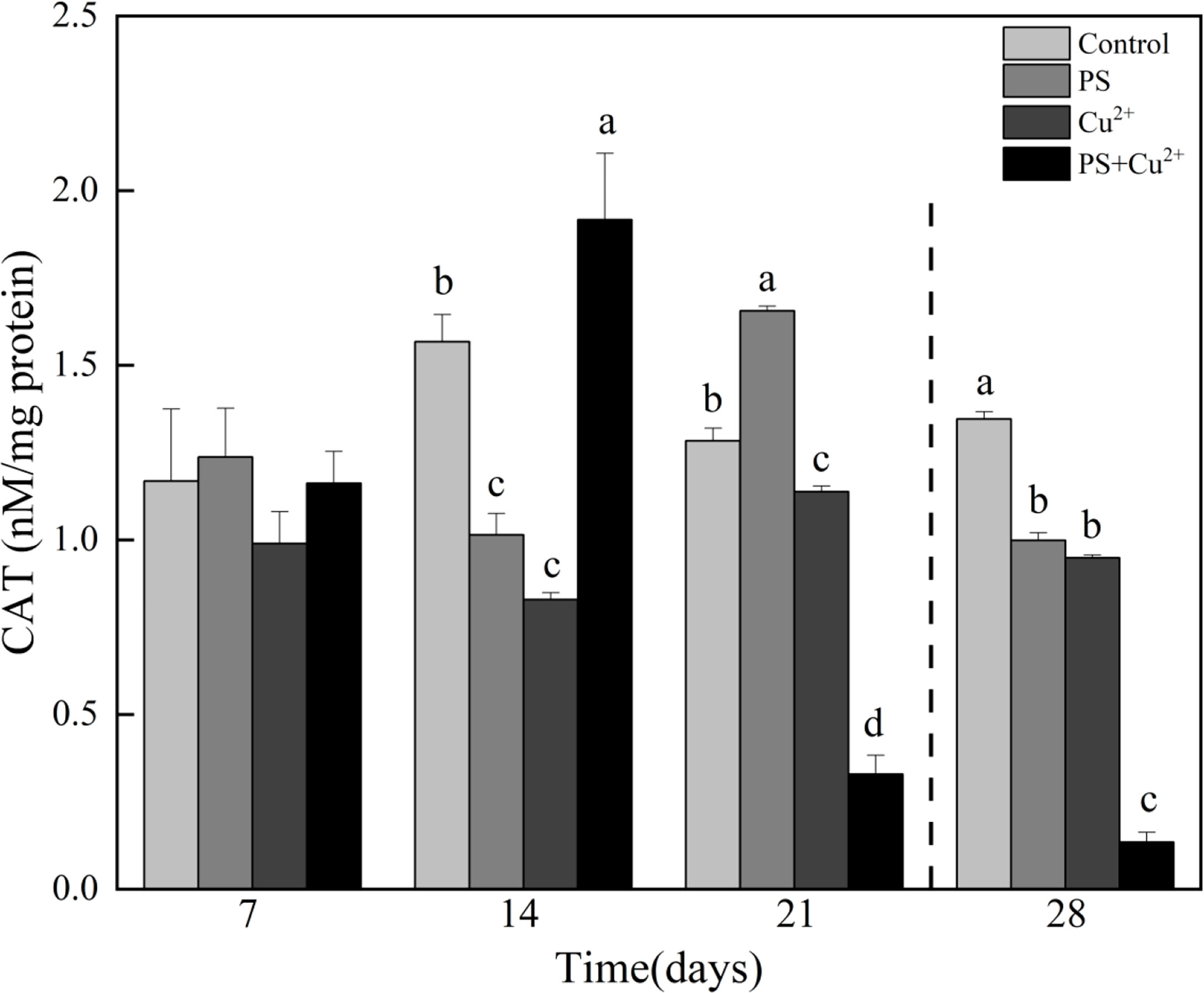
Figure 2 Catalase (CAT) activities in whole tissues of Tachypleus tridentatus exposed to 100-nm micro-PS (104 particles l-1), Cu2+ (10 µg l-1), and a combination of PS and Cu2+ at days 7, 14, and 21, allowed to recover for 7 days (28 days). Data are shown as mean values ± standard deviations. The superscripts of different lowercase letters indicate significant differences (p < 0.05) between the four groups.
The horseshoe crab groups that were subjected to Cu2+ and nano-PS+Cu2+ showed significantly higher MDA levels compared to the control on day 7. The nano-PS-exposed group showed no significant effects on MDA level on day 7. On day 14, the nano-PS- and Cu2+-exposed groups showed higher MDA levels compared to the control, while the nano-PS+Cu2+ group showed lower MDA levels compared to the control. All the groups showed no significant effects on MDA levels compared to the control on day 14. On day 21, all the groups subjected to different treatments showed lower MDA levels compared to the control. During the recovery period, significantly lower MDA levels were noted in all experimental treatment groups compared to the control (Figure 3).
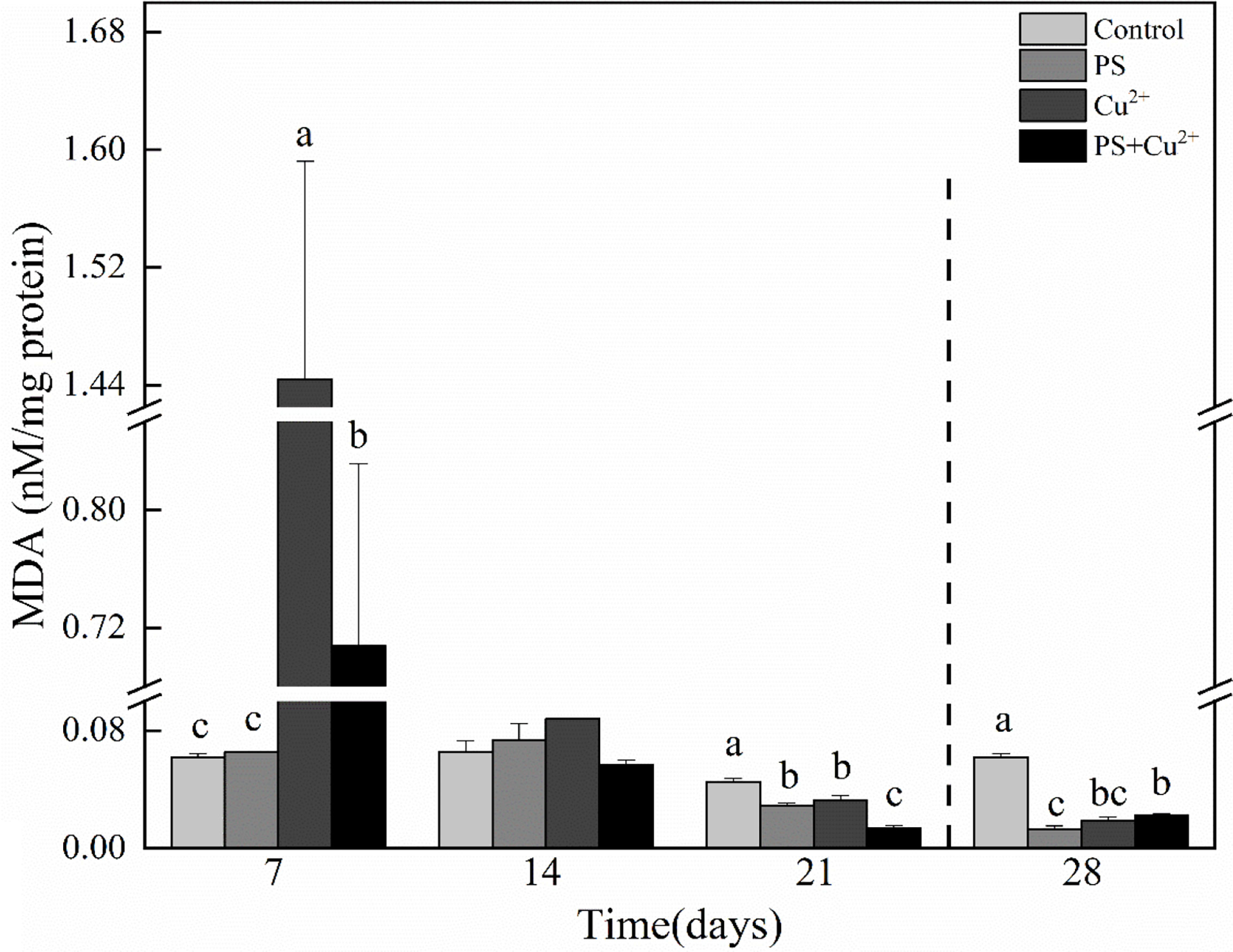
Figure 3 Malondialdehyde (MDA) activities in whole tissues of Tachypleus tridentatus exposed to 100-nm micro-PS (104 particles l-1), Cu2+ (10 µg l-1), and a combination of PS and Cu2+ at days 7, 14, and 21, allowed to recover for 7 days (28 days). Data are presented as mean values ± standard deviations. The superscripts of different lowercase letters indicate significant differences (p < 0.05) between the four groups.
Compared with the control group on day 7, GSH levels were decreased in the nano-PS- and nano-PS+Cu2+-exposed groups. On days 14 and 21, all the groups having different experimental conditions showed lower GSH levels compared to the control group. During the recovery period, there were no significant effects on GSH levels compared to the control (Figure 4).
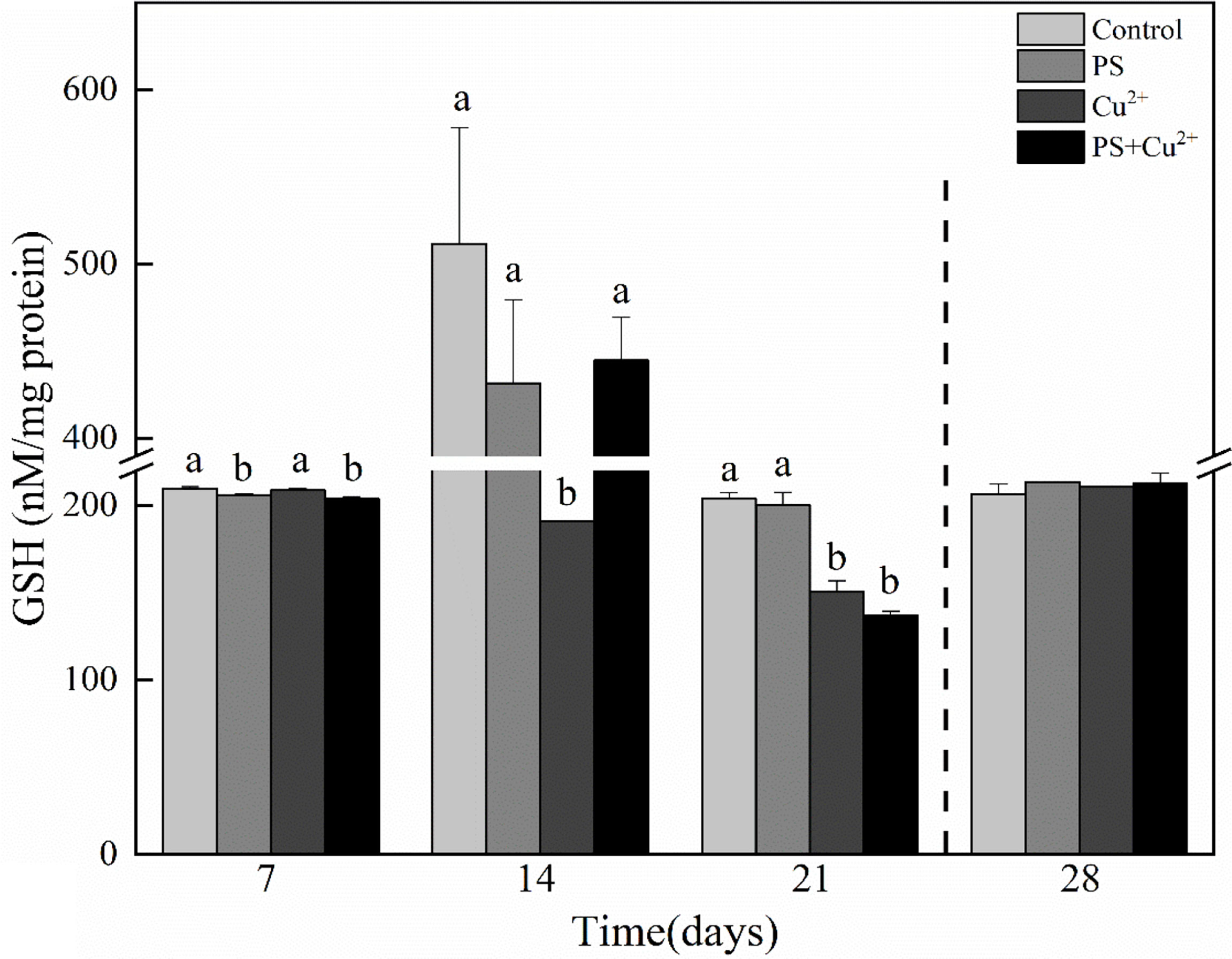
Figure 4 Glutathione (GSH) activities in whole tissues of Tachypleus tridentatus exposed to 100-nm micro-PS (104 particles l-1), Cu2+ (10 µg l-1), and a combination of PS and Cu2+ at days 7, 14, and 21, allowed to recover for 7 days (28 days). Data are given as mean values ± standard deviations. The superscripts of different lowercase letters indicate significant differences (p < 0.05) between the four groups.
On day 7, all the groups having different experimental treatments showed lower LPO levels compared to the control. The nano-PS- and nano-PS+Cu2+-exposed groups showed significant effects on day 7. All the treatments on days 14 and 21 showed no significant effects on the LPO levels compared to the control. The nano-PS- and nano-PS+Cu2+-exposed groups showed higher LPO levels compared to the control while the Cu2+-exposed group showed lower LPO levels compared to the control on day 14. All the experimental treatments at day 21 showed lower LPO levels compared to the control. At the recovery period, all the experimental groups of horseshoe crabs having different experimental conditions showed higher LPO levels compared to the control (Figure 5).
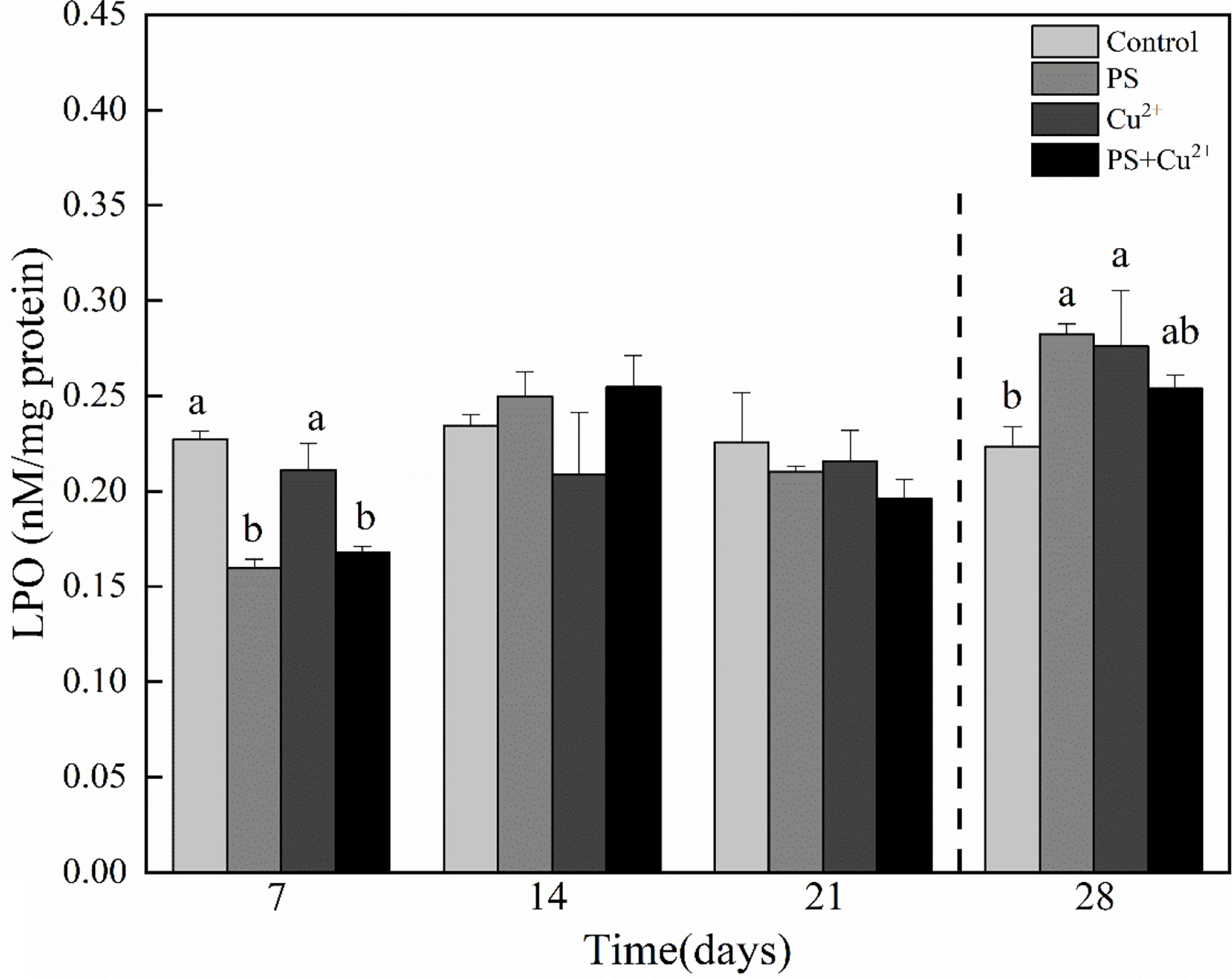
Figure 5 Lipid peroxidation (LPO) activities in whole tissues of Tachypleus tridentatus exposed to 100-nm micro-PS (104 particles l-1), Cu2+ (10 µg l-1), and a combination of PS and Cu2+ at days 7, 14, and 21, allowed to recover for 7 days (28 days). Data are given as mean values ± standard deviations. The superscripts of different lowercase letters indicate significant differences (p < 0.05) between the four groups.
IBR values were computed from the standardized data of six different biomarkers in juvenile horseshoe crabs exposed to microplastics and heavy metals after 21 days of exposure and a recovery period of 7 days (Figure 6). The graphs represent the stress levels of antioxidant enzymes of juvenile horseshoe crabs under the influence of microplastics and heavy metals. The obtained IBR values ranged from 2.325 (group 2) to 12.840 (group 3) on day 7 (Figure 6A), from 4.376 (group 3) to 12.957 (group 4) on day 14 (Figure 6B), from 3.254 (group 4) to 7. 350 (group 2) on day 21 (Figure 6C), and from 4.092 (group 4) to 7.655 (group 3) on day 28 (Figure 6D). Figure 6E shows the stress level of all groups of horseshoe crabs exposed to different experimental conditions throughout the whole experimental period. The highest IBR value 12.957 was observed in the co-exposed (nano-PS + Cu2+) group on day 14, and the lowest value 2.325 was noted in the microplastic (nano-PS)-exposed group on day 7. The PCA of the effects of nano-PS and Cu2+ on antioxidant activity (biochemical indicators) showed that 35.07% of the total variance was accounted by PC1 (Figure 7). This axis represents the specific reaction of microplastics and heavy metal exposure which separated the control group and the nano-PS- (104 particles l-1), Cu2+- (10 µg l-1), and co-exposed (nano-PS + Cu2+) groups.
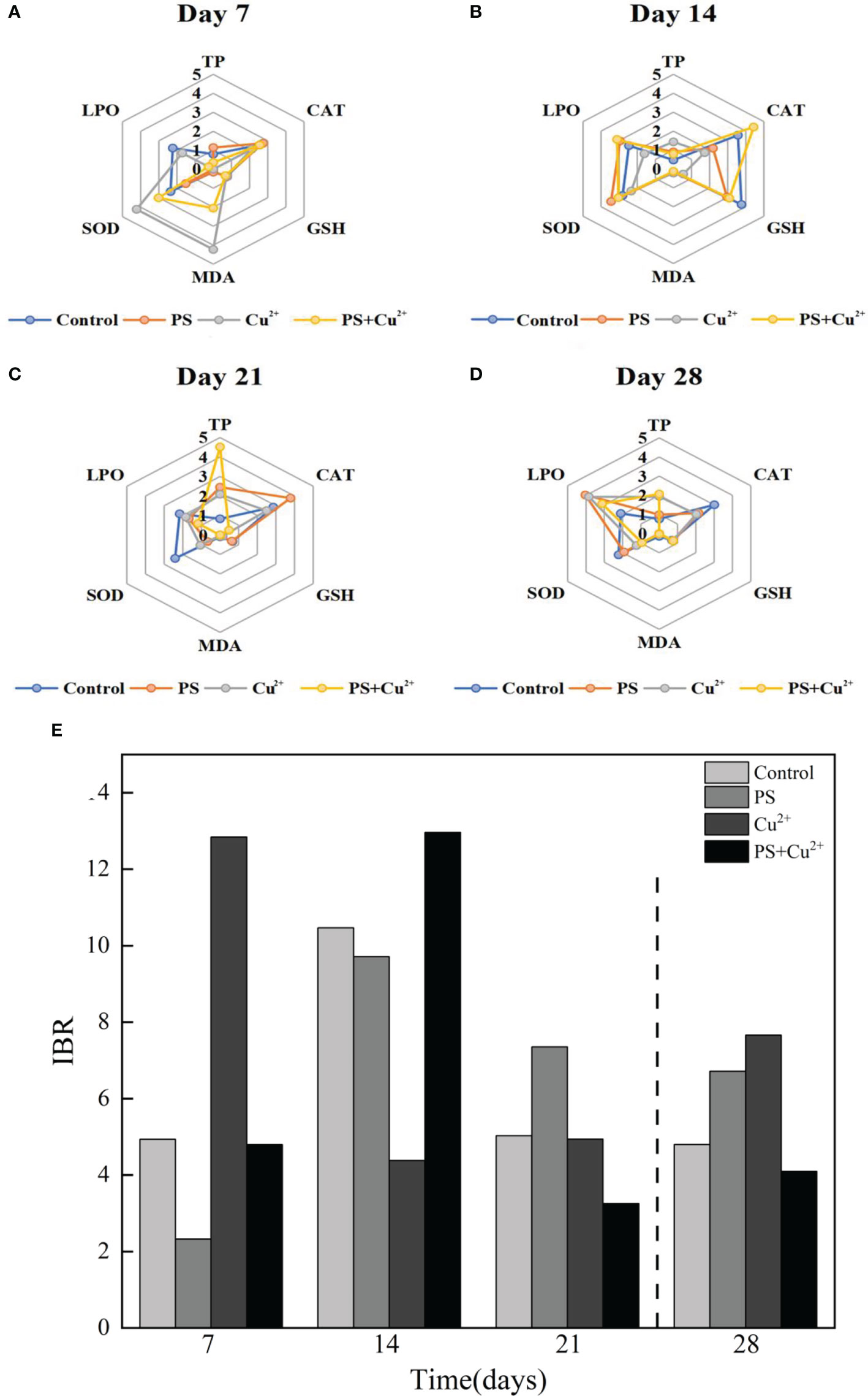
Figure 6 (A–D) represent radar plots of IBR for different treatments of experimental juvenile horseshoe crabs on days 7, 14, 21, and 28, respectively, showing the stress levels as indicated by antioxidant parameter levels in the graphs. (E) represents the stress levels presented by different groups of experimental horseshoe crabs throughout the experimental period.
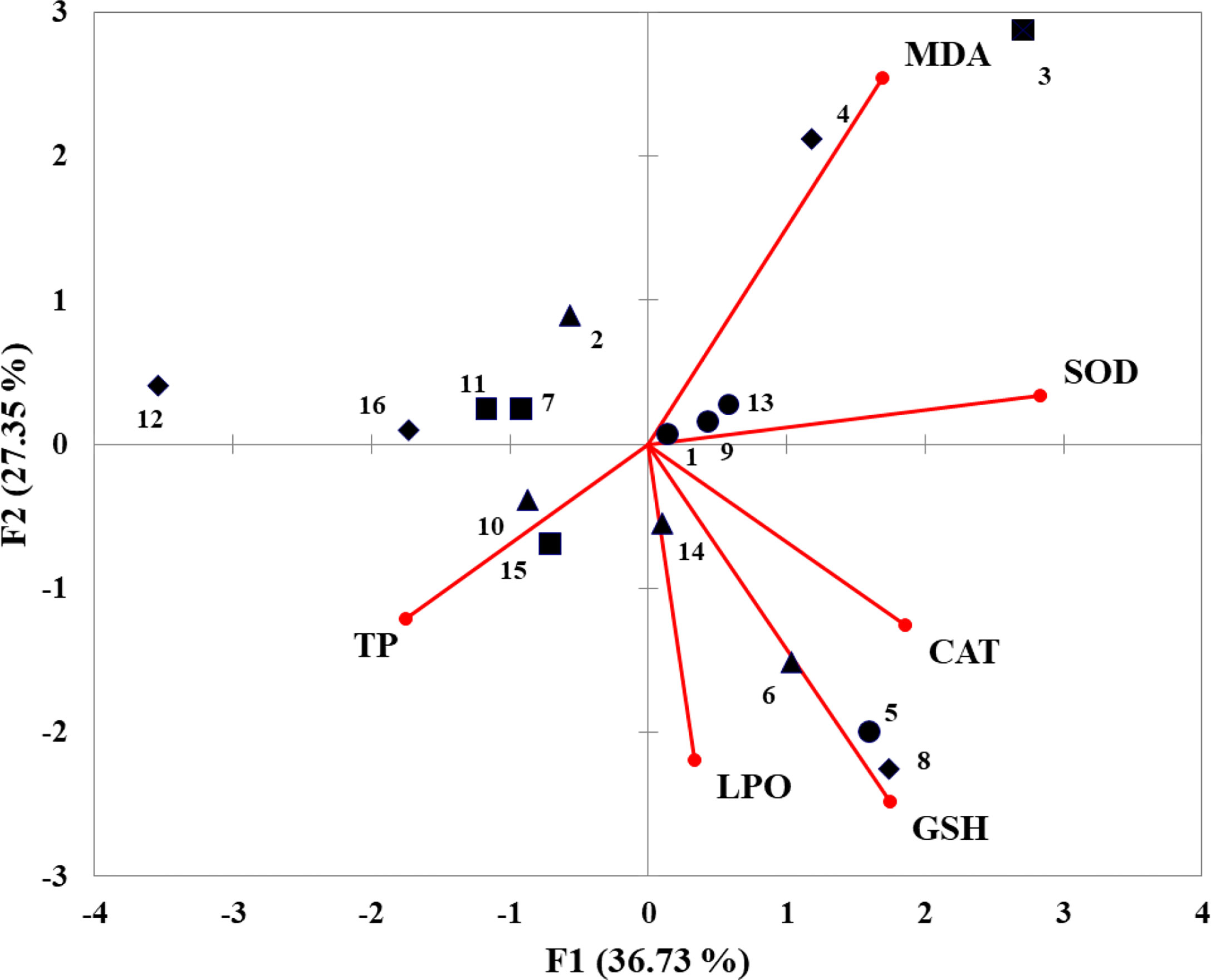
Figure 7 Biplot of principal component analysis with all measured variables (TP, SOD, CAT, MDA, GSH, LPO) at five different time points (days 7, 14, 21, and 28) in five different treatments (●artemia only with 0 conc., ▲nano-PS 104 particles l-1 conc., ■Cu2+ 10 µg l-1 conc., ♦ nano-PS + Cu2+).
Discussion
Previous studies have shown that toxins in oceanic water can be toxic to organisms by stimulating the generation of reactive oxygen species (ROS) (Sussarellu et al., 2016) as well as by producing oxidative stress (Livingstone, 2001; Lushchak, 2011). SOD, CAT, and GSH can serve as sensitive biomarkers of environmental pollution in aquatic organisms. SOD is the first and foremost defense line in antioxidant systems, which effectively scavenges reactive oxygen species in living organisms (Tomanek et al., 2011). The SOD enzyme converts the superoxide radical anion (O−2) into H2O2 which then converts into harmless forms O2 and H2O. In the current study, the activity level of SOD was elevated in the presence of the nano-PS-, Cu2+-, and co-exposed groups of juvenile horseshoe crabs as compared to the control, indicating the activation of the oxidant defensive system. This study is consistent with previous studies on D. rerio and E. sinensis (Lu et al., 2016; Yu et al., 2018). However, over a 21-day exposure, pollutant-exposed juveniles had a lower SOD activity as compared to the control group. This might be due to damage to the immune system of juveniles due to continuation of stress for a relatively long time. Similarly, decreased SOD activities were noted during the recovery period. It may be because animals like horseshoe crabs which have a strong immune defense system were unable to recover completely from such a stressful situation. Likewise, CAT activity has also increased, destroying H2O2 which could otherwise penetrate through the biomembranes and may inactivate several enzymes (Vutukuru et al., 2006). CAT plays an important role in the defense against oxidative stress through the removal of H2O2 which is the major precursor of hydroxyl radical (Soldatov et al., 2013). On day 14, a significant increase in CAT activity levels in the co-exposed group was noted as compared to the control group, indicating that two pollutants together were inducing the generation of ROS. The factor behind this increase might be time-dependent because on days 21 and 28 (recovery period), the SOD activity was at the lowest levels. Other groups including the nano-PS- and Cu2+-exposed groups had lower CAT activity levels than the control group. Previous studies showed an increase in CAT activity in the clam S. plana exposed to polystyrene MPs but also an inhibition in the digestive gland of the marine mussels M. galloprovincialis and M. edulis (Avio et al., 2015; Paul-Pont et al., 2016; Ribeiro et al., 2017). The results of this study coincide with our findings. During the recovery period, both SOD and CAT activities decreased significantly indicating the inhibition of these enzymes and it was extremely low in the co-exposed group. The probable reason for this decrease could be the exhaustion of energy caused by oxidative stress (Yu et al., 2018).
Excessive production of ROS can induce lipid peroxidation which causes damage to cells and cell membranes. There was an increase observed in the LPO activity level in some experimental groups of horseshoe crabs. Similarly, reduced activity levels were observed in some pollutant-exposed groups. Crustaceans are considered a rich source of PUFA. The heavy metal (Cu2+) exposed herein is redox active, accumulated significantly in horseshoe crab tissues, and could contribute to the generation of ROS which is responsible for damaging lipids, proteins, and DNA (Lesser, 2006). In the recovery period, elevations in LPO levels were observed in all pollutant-exposed groups as compared to the control group indicating the presence of ROS even when there was no exposure. Lipid peroxidation (LPO) reactions are generally free radical-driven chain reactions in which one radical can induce the oxidation of a comparatively large number of substrate molecules, which are represented by polyunsaturated fatty acids (PUFA) (Porter, 1984). For LPO enzyme activity, increased reactive oxygen species react with polyunsaturated fatty acids which induce the release of toxic and reactive aldehyde metabolites, such as MDA. This study is consistent with Rangasamy et al. (2022). MDA is the end product of LPO, engendered by free radical damage of unsaturated fatty acids which can cross-link with DNA, interrupting protein activity, resulting in DNA damage, and inducing gene mutations. A concentration of MDA can be used to indicate oxidative stress to measure the endogenic oxidative state of the body (Li et al., 2016). MDA exhibited a huge increase on day 7 in some experimental groups, indicating that heavy metal Cu2+ and nano-PS have a strong influence on the antioxidant parameters in the form of a single toxicant as well as in combination. This study is consistent with previous studies (Wang et al., 2010; Jinhui et al., 2019). Moreover, during the recovery period, inhibition of MDA activity was observed in all experimental treatments. GSH is used by GPx as a cofactor to convert H2O2 into water and alcohol. Animal GPx can act as a prooxidant that removes harmful peroxide metabolites in cells (Hu et al., 2015a; Peng et al., 2017). From the results, it was concluded that microplastics and heavy metals have negative impacts on the antioxidant parameters of horseshoe crabs. Javed et al. (2017) observed lower levels of GSH during exposure as compared to reference fish. In the current study, some experimental groups had lower GSH levels than the control group during exposure, which may be due to energy exhaustion due to excessive stress that ultimately results in the inhibition of antioxidant activities in the horseshoe crabs. During the recovery period, juveniles were able to successfully recover according to the control group which indicated that horseshoe crabs regained fitness.
IBR indices in animals and plants were applied in both laboratory experiments (Bertrand et al., 2016; Bertrand et al., 2017) and field studies (Santos et al., 2016), but the modes of toxicities have merely been studied. To evaluate contaminated sites, IBR indices were developed for immunotoxical biomarkers in mussels (Auffret et al., 2006). In the present study, IBR indices allowed us to observe the potential effects of nano-PS and Cu2+ in juvenile horseshoe crabs during 21 days of exposure and 7 days of purification period. Differences in the biomarkers were observed among different experimental groups of horseshoe crabs. As indicated by the IBR values, a non-linear increase or decrease in stress response over time was observed between the different experimental groups of limulus compared to the control group. The IBR results coincide with the findings of Todgham and Stillman (2013), who stated that external environmental stressors, for example, chemical pollutants including metals and various organic compounds, can act independently or may exert antagonistic and synergistic effects on an organism’s physiological performances. Similarly, Madeira et al. (2016) reported an increase in IBR values in the gills of clownfish as a result of an environmental stressor (elevated temperature) on days 7 and 14 of the 28 days of experiment but noted the lower IBR values on day 21 of the same experiment. The increase in IBR values may be due to the activation of the antioxidant defense system of horseshoe crabs in response to stress conditions as stated by Lushchak et al. (2011) that environmental stressors like chemical pollutants and various organic compounds can bring about the increase in ROS generation which provokes the antioxidant defense system of organisms. A reduction in IBR values compared to the control can be a result of exhaustion of cellular defense mechanisms (Madeira et al., 2016). Our results are similar to Huang et al.’s (2018) results reporting that nano-TiO2 caused adverse effects on juvenile flounders.
According to the results, juvenile horseshoe crabs were exposed to a polystyrene concentration (104 particles l-1) and a concentration of Cu2+ (10 µg l-1) for 21 days and a purification period of 7 days and suffered from higher levels of stress in contrast to the control group. Apart from it, the obtained values highlighted the capacity of the experimental juvenile horseshoe crabs to respond to environmental pollutants, supporting the fact that horseshoe crabs can act as a bioindicator of microplastic and heavy metal pollution, at least at the range of nano-PS (104 particles l-1) and Cu2+ (10 µg l-1). Taking into account the standardized value (S), it is possible to identify which biomarker had the most important weight on final IBR values for the tested conditions. Among the five biomarkers, SOD and MDA showed the highest values on day 7 in the only microplastic-exposed group and CAT was the highest in the co-exposed group on day 14, which indicates the stress levels in horseshoe crabs toward experimental stressors. However, 21 days of caused stress was significant that juvenile horseshoe crabs were unable to recover completely in a 7-day recovery period, despite the fact that they have a stronger immune responsive system. The PCA biplot suggested that all enzymes were influenced by exposure, trying to counteract the effects of heavy metals and microplastics to reduce the oxidative stress. A positive relationship was observed between all enzymes which correlated with the results of Hu et al. (2015b).
This study is consistent with Wen et al. (2018), who explained that heavy metals and microplastics have adverse effects on antioxidant activity. They found that when Cd and MPs were co-applied to Amazon discus fish, a severe oxidative stress response and an innate immune defense were generated compared to the administration of a single poison. Elevations in antioxidant enzyme activities in living organisms exposed to fraught conditions indicate their ability to tolerate environmental stressors (Vosloo et al., 2013). This study provides evidence that environmental microplastics, mainly nano-PS combined with a heavy metal (Cu2+), can affect the antioxidant activities of the assessed species. In this study, Cu2+ + nano-PS caused more severe oxidative stress in combination in the co-exposed group. However, a study showed that the combination of MPs and heavy metals can induce hippocampal oxidative damage and increase mortality, but this effect was mainly caused by heavy metals, not MPs (Jinhui et al., 2019). Contrarily in this experiment, nano-PS as a single pollutant also caused oxidative stress. Heavy metal and nanoplastic induced oxidative stress independently as well as in combination. So, it can be said that in combination they made the damage more severe than a single pollutant.
Conclusion
In the present study, juvenile T. tridentatus were exposed to nanoplastics and heavy metals in combination as well as individually. Both nanoplastics and heavy metals induce the antioxidant response of juvenile tri-spine horseshoe crabs. Integrated biomarker responses indicated that heavy metals and microplastics in combination caused more severe oxidative stress than as a single pollutant, highlighting the interaction between nano-sized plastics and heavy metals.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
MH and KK contributed to the conception and design of the study. IA and YS performed the statistical analysis. IA, YS, and FK wrote the first draft of the manuscript. CZ, KT, KW, and YW wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study was supported by a research grant from the National Natural Science Foundation of China (31872587).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Auffret M., Rousseau S., Boutet I., Tanguy A., Baron J., Moraga D., et al. (2006). A multiparametric approach for monitoring immunotoxic responses in mussels from contaminated sites in Western mediterranea. Ecotoxicol. Environ. Safety 63, 393–405. doi: 10.1016/j.ecoenv.2005.10.016
Avio C. G., Gorbi S., Milan M., Benedetti M., Fattorini D., d'Errico G., et al. (2015). Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 198, 211–222. doi: 10.1016/j.envpol.2014.12.021
Beliaeff B., Burgeot T. (2002). Integrated biomarker response: A useful tool for ecological risk assessment. Environ. Toxicol. Chem. 21, 1316–1322. doi: 10.1002/etc.5620210629
Bertrand L., Asis R., Monferrán M. V., Amé M. V. (2016). Bioaccumulation and biochemical response in south American native species exposed to zinc: Boosted regression trees as novel tool for biomarkers selection. Ecol. Indicators 67, 769–778. doi: 10.1016/j.ecolind.2016.03.048
Bertrand L., Marino D. J., Monferrán M. V., Amé M. V. (2017). Can a low concentration of an organophosphate insecticide cause negative effects on an aquatic macrophyte? exposure of potamogeton pusillus at environmentally relevant chlorpyrifos concentrations. Environ. Exp. Botany 138, 139–147. doi: 10.1016/j.envexpbot.2017.03.006
Cao Y., Zhao M., Ma X., Song Y., Zuo S., Li H., et al. (2021). A critical review on the interactions of microplastics with heavy metals: Mechanism and their combined effect on organisms and humans. Sci. Total Environ. 788, 147620. doi: 10.1016/j.scitotenv.2021.147620
Capó X., Company J. J., Alomar C., Compa M., Sureda A., Grau A., et al. (2021). Long-term exposure to virgin and seawater exposed microplastic enriched-diet causes liver oxidative stress and inflammation in gilthead seabream sparus aurata, Linnaeus 1758. Sci. Total Environ. 767, 144976. doi: 10.1016/j.scitotenv.2021.144976
Chen F., Lin J., Qian B., Wu Z., Huang P., Chen K., et al. (2018). Geochemical assessment and spatial analysis of heavy metals in the surface sediments in the Eastern beibu gulf: A reflection on the industrial development of the south China coast. Int. J. Environ. Res. Public Health 15, 496. doi: 10.3390/ijerph15030496
Chen C.-P., Yeh H.-Y., Lin P.-F. (2004). Conservation of the horseshoe crab at kinmen, Taiwan: strategies and practices. Biodiversity Conserv. 13, 1889–1904. doi: 10.1023/B:BIOC.0000035868.11083.84
Costa E., Gambardella C., Piazza V., Vassalli M., Sbrana F., Lavorano S., et al. (2020). Microplastics ingestion in the ephyra stage of aurelia sp. triggers acute and behavioral responses. Ecotoxicol. Environ. Safety 189, 109983. doi: 10.1016/j.ecoenv.2019.109983
Ding J., Zhang S., Razanajatovo R. M., Zou H., Zhu W. (2018). Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 238, 1–9. doi: 10.1016/j.envpol.2018.03.001
Duis K., Coors A. (2016). Microplastics in the aquatic and terrestrial environment: sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Europe 28, 2. doi: 10.1186/s12302-015-0069-y
Fu Z., Wang J. (2019). Current practices and future perspectives of microplastic pollution in freshwater ecosystems in China. Sci. Total Environ. 691, 697–712. doi: 10.1016/j.scitotenv.2019.07.167
Gu Y. G., Huang H. H., Liu Y., Gong X. Y., Liao X. L. (2018). Non-metric multidimensional scaling and human risks of heavy metal concentrations in wild marine organisms from the maowei Sea, the beibu gulf, south China Sea. Environ. Toxicol. Pharmacol. 59, 119–124. doi: 10.1016/j.etap.2018.03.002
Gu Y. G., Lin Q., Yu Z. L., Wang X. N., Ke C. L., Ning J. J. (2015). Speciation and risk of heavy metals in sediments and human health implications of heavy metals in edible nekton in beibu gulf, China: A case study of qinzhou bay. Mar. pollut. Bull. 101, 852–859. doi: 10.1016/j.marpolbul.2015.11.019
Guo H., Li K., Wang W., Wang C., Shen Y. (2017). Effects of copper on hemocyte apoptosis, ROS production, and gene expression in white shrimp litopenaeus vannamei. Biol. Trace Element Res. 179, 318–326. doi: 10.1007/s12011-017-0974-6
Huang X., Lan Y., Liu Z., Huang W., Guo Q., Liu L., et al. (2018). Salinity mediates the toxic effect of nano-TiO2 on the juvenile olive flounder paralichthys olivaceus. Sci. Total Environ. 640, 726–735. doi: 10.1016/j.scitotenv.2018.05.350
Hüffer T., Weniger A.-K., Hofmann T. (2018). Sorption of organic compounds by aged polystyrene microplastic particles. Environ. Pollut. 236, 218–225. doi: 10.1016/j.envpol.2018.01.022
Hu M., Kwan B. K. Y., Wang Y., Cheung S. G., Shin P. K. S. (2015a). “Population structure and growth of juvenile horseshoe crabs tachypleus tridentatus and carcinoscorpius rotundicauda (Xiphosura) in southern China,” in Changing global perspectives on horseshoe crab biology, conservation and management. Eds. Carmichael R. H., Botton M. L., Shin P. K. S., Cheung S. G. (Cham: Springer International Publishing), 167–180. doi: 10.1007/978-3-319-19542-1_8
Hu M., Li L., Sui Y., Li J., Wang Y., Lu W., et al. (2015b). Effect of pH and temperature on antioxidant responses of the thick shell mussel mytilus coruscus. Fish Shellfish Immunol. 46, 573–583. doi: 10.1016/j.fsi.2015.07.025
Jackson N. L., Nordstrom K. F. (2009). “Strategies to conserve and enhance sandy barrier habitat for horseshoe crabs (Limulus polyphemus) on developed shorelines in Delaware bay, United States”, in Biology and conservation of horseshoe crabs. Eds. Tanacredi J., Botton M., Smith D. (Boston, MA: Springer US), 399–416. doi: 10.1007/978-0-387-89959-6_25
Jambeck J. R., Geyer R., Wilcox C., Siegler Theodore R., Perryman M., Andrady A., et al. (2015). Plastic waste inputs from land into the ocean. Science 347, 768–771. doi: 10.1126/science.1260352
Javed M., Ahmad M. I., Usmani N., Ahmad M. (2017). Multiple biomarker responses (serum biochemistry, oxidative stress, genotoxicity and histopathology) in channa punctatus exposed to heavy metal loaded waste water. Sci. Rep. 7, 1675. doi: 10.1038/s41598-017-01749-6
Jinhui S., Sudong X., Yan N., Xia P., Jiahao Q., Yongjian X. (2019). Effects of microplastics and attached heavy metals on growth, immunity, and heavy metal accumulation in the yellow seahorse, hippocampus kuda bleeker. Mar. pollut. Bull. 149, 110510. doi: 10.1016/j.marpolbul.2019.110510
Karlsson H. L., Gustafsson J., Cronholm P., Möller L. (2009). Size-dependent toxicity of metal oxide particles–a comparison between nano- and micrometer size. Toxicol. Lett. 188, 112–118. doi: 10.1016/j.toxlet.2009.03.014
Khalid N., Hussain M., Young H. S., Boyce B., Aqeel M., Noman A. (2018). Effects of road proximity on heavy metal concentrations in soils and common roadside plants in southern California. Environ. Sci. pollut. Res. 25, 35257–35265. doi: 10.1007/s11356-018-3218-1
Lao Q., Su Q., Liu G., Shen Y., Chen F., Lei X., et al. (2019). Spatial distribution of and historical changes in heavy metals in the surface seawater and sediments of the beibu gulf, China. Mar. pollut. Bull. 146, 427–434. doi: 10.1016/j.marpolbul.2019.06.080
Lei L., Wu S., Lu S., Liu M., Song Y., Fu Z., et al. (2018). Microplastic particles cause intestinal damage and other adverse effects in zebrafish danio rerio and nematode caenorhabditis elegans. Sci. Total Environ. 619-620, 1–8. doi: 10.1016/j.scitotenv.2017.11.103
Lesser M. P. (2006). Oxidative stress in marine environments: biochemistry and physiological ecology. Annu. Rev. Of Physiol. 68, 253–278. doi: 10.1146/annurev.physiol.68.040104.110001
Liao Y., Hsieh H.-L., Xu S., Zhong Q., Lei J., Liang M., et al. (2019). Wisdom of crowds reveals decline of Asian horseshoe crabs in beibu gulf, China. Oryx 53, 222–229. doi: 10.1017/S003060531700117X
Li Y., Lu Z., Zheng H., Wang J., Chen C. (2020). Microplastics in surface water and sediments of chongming island in the Yangtze estuary, China. Environ. Sci. Europe 32, 15. doi: 10.1186/s12302-020-0297-7
Liu P., Lu K., Li J., Wu X., Qian L., Wang M., et al. (2020). Effect of aging on adsorption behavior of polystyrene microplastics for pharmaceuticals: Adsorption mechanism and role of aging intermediates. J. Hazardous Mater. 384, 121193. doi: 10.1016/j.jhazmat.2019.121193
Livingstone D. R. (2001). Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar. pollut. Bull. 42, 656–666. doi: 10.1016/S0025-326X(01)00060-1
Li Y., Wei L., Cao J., Qiu L., Jiang X., Li P., et al. (2016). Oxidative stress, DNA damage and antioxidant enzyme activities in the pacific white shrimp (Litopenaeus vannamei) when exposed to hypoxia and reoxygenation. Chemosphere 144, 234–240. doi: 10.1016/j.chemosphere.2015.08.051
Lushchak V. I. (2011). Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 101, 13–30. doi: 10.1016/j.aquatox.2010.10.006
Lushchak V. I., Semchyshyn H. M., Lushchak O. V. (2011). “The classic methods to measure oxidative damage: lipid peroxides, thiobarbituric-acid reactive substances, and protein carbonyls”, in Oxidative Stress in Aquatic Ecosystems. Eds. Abele D., Vázquez-Medina J. P., Zenteno-Savín T.. (Hoboken, NJ: Wiley), 420–431. doi: 10.1002/9781444345988.ch32
Lu Y., Zhang Y., Deng Y., Jiang W., Zhao Y., Geng J., et al. (2016). Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Technol. 50, 4054–4060. doi: 10.1021/acs.est.6b00183
Madeira C., Madeira D., Diniz M. S., Cabral H. N., Vinagre C. (2016). Thermal acclimation in clownfish: an integrated biomarker response and multi-tissue experimental approach. Ecol. Indic. 71, 280–292. doi: 10.1016/j.ecolind.2016.07.009
Obst M., Faurby S., Bussarawit S., Funch P. (2012). Molecular phylogeny of extant horseshoe crabs (Xiphosura, limulidae) indicates paleogene diversification of Asian species. Mol. Phylogenet. Evol. 62, 21–26. doi: 10.1016/j.ympev.2011.08.025
Paul-Pont I., Lacroix C., González Fernández C., Hégaret H., Lambert C., Le Goïc N., et al. (2016). Exposure of marine mussels mytilus spp. to polystyrene microplastics: Toxicity and influence on fluoranthene bioaccumulation. Environ. Pollut. 216, 724–737. doi: 10.1016/j.envpol.2016.06.039
Peng X., Shang G., Wang W., Chen X., Lou Q., Zhai G., et al. (2017). Fatty acid oxidation in zebrafish adipose tissue is promoted by 1α,25(OH)2D3. Cell Rep. 19, 1444–1455. doi: 10.1016/j.celrep.2017.04.066
Porter N. A. (1984). Chemistry of lipid peroxidation. Methods Enzymol. 105, 273–282. doi: 10.1016/S0076-6879(84)05035-7
Qiao R., Sheng C., Lu Y., Zhang Y., Ren H., Lemos B. (2019). Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 662, 246–253. doi: 10.1016/j.scitotenv.2019.01.245
Rangasamy B., Malafaia G., Maheswaran R. (2022). Evaluation of antioxidant response and na+-K+-ATPase activity in zebrafish exposed to polyethylene microplastics: Shedding light on a physiological adaptation. J. Hazardous Mater. 426, 127789. doi: 10.1016/j.jhazmat.2021.127789
Ribeiro F., Garcia A. R., Pereira B. P., Fonseca M., Mestre N. C., Fonseca T. G., et al. (2017). Microplastics effects in scrobicularia plana. Mar. pollut. Bull. 122, 379–391. doi: 10.1016/j.marpolbul.2017.06.078
Rochman C. M., Hoh E., Kurobe T., Teh S. J. (2013). Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 3, 3263. doi: 10.1038/srep03263
Santos R., Joyeux A., Palluel O., Palos-Ladeiro M., Besnard A., Blanchard C., et al. (2016). Characterization of a genotoxicity biomarker in three-spined stickleback (Gasterosteus aculeatus l.): Biotic variability and integration in a battery of biomarkers for environmental monitoring. Environ. Toxicol. 31, 415–426. doi: 10.1002/tox.22055
Schmidt L., Novo D. R., Druzian G. T., Landero J. A., Caruso J., Mesko M. F., et al. (2021). Influence of culinary treatment on the concentration and on the bioavailability of cadmium, chromium, copper, and lead in seafood. J. Trace Elements Med. Biol. 65, 126717. doi: 10.1016/j.jtemb.2021.126717
Shin P. K. S., Li H., Cheung S. G. (2009). “Horseshoe crabs in Hong Kong: Current population status and human exploitation,” in Biology and conservation of horseshoe crabs. Eds. Tanacredi J., Botton M., Smith D. (Boston, MA: Springer), 347–360. doi: 10.1007/978-0-387-89959-6_22
Soldatov A. A., Gostiukhina O. L., Borodina A. V., Golovina I. V. (2013). Qualitative composition of carotenoids, catalase and superoxide dismutase activities in tissues of bivalve mollusc anadara inaequivalvis (Bruguiere 1789). J. Evol. Biochem. Physiol. 49, 389–398. doi: 10.1134/S0022093013040026
Sussarellu R., Suquet M., Thomas Y., Lambert C., Fabioux C., Pernet M. E., et al. (2016). Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. U. States A. 113, 2430–2435. doi: 10.1073/pnas.1519019113
Su L., Xiong X., Zhang Y., Wu C., Xu X., Sun C., et al. (2022). Global transportation of plastics and microplastics: A critical review of pathways and influences. Sci. Total Environ. 831, 154884. doi: 10.1016/j.scitotenv.2022.154884
Tang G., Liu M., Zhou Q., He H., Chen K., Zhang H., et al. (2018). Microplastics and polycyclic aromatic hydrocarbons (PAHs) in xiamen coastal areas: Implications for anthropogenic impacts. Sci. Total Environ. 634, 811–820. doi: 10.1016/j.scitotenv.2018.03.336
Tao T. Y., Gitlin J. D. (2003). Hepatic copper metabolism: insights from genetic disease. Hepatology 37, 1241–1247. doi: 10.1053/jhep.2003.50281
Thompson R. C. (2015). “Microplastics in the marine environment: sources, consequences and solutions,” in Marine anthropogenic litter. Eds. Bergmann M., Gutow L., Klages M. (Cham: Springer International Publishing), 185–200. doi: 10.1007/978-3-319-16510-3_7
Todgham A. E., Stillman J. H. (2013). Physiological responses to shifts in multiple environmental stressors: Relevance in a changing world. Integr. Comp. Biol. 53, 539–544. doi: 10.1093/icb/ict086
Tomanek L., Zuzow M. J., Ivanina A. V., Beniash E., Sokolova I. M. (2011). Proteomic response to elevated PCO2 level in eastern oysters, crassostrea virginica: Evidence for oxidative stress. J. Exp. Biol. 214, 1836–1844. doi: 10.1242/jeb.055475
Turner A., Holmes L. (2015). Adsorption of trace metals by microplastic pellets in fresh water. Environ. Chem. 12, 600–610. doi: 10.1071/EN14143
Vosloo D., Rensburg L., Vosloo A. (2013). Oxidative stress in abalone: The role of temperature, oxygen and l-proline supplementation. Aquaculture 416-417, 265–271. doi: 10.1016/j.aquaculture.2013.09.031
Vutukuru S. S., Chintada S., Radha Madhavi K., Venkateswara Rao J., Anjaneyulu Y. (2006). Acute effects of copper on superoxide dismutase, catalase and lipid peroxidation in the freshwater teleost fish, esomus danricus. Fish Physiol. Biochem. 32, 221–229. doi: 10.1007/s10695-006-9004-x
Wang Q., Fan C. P., Chen R. C. (2010). Effects of three typical sulfonamides on GST activity and MDA content in liver tissue of oreochromis niloticus. Ecol. Environ. Sci. 19, 1014–1019. doi: 10.16258/j.cnki.1674-5906.2010.05.002
Wang X., Liu L., Zheng H., Wang M., Fu Y., Luo X., et al. (2020). Polystyrene microplastics impaired the feeding and swimming behavior of mysid shrimp neomysis japonica. Mar. pollut. Bull. 150, 110660. doi: 10.1016/j.marpolbul.2019.110660
Wang T., Zou X., Li B., Yao Y., Li J., Hui H., et al. (2018). Microplastics in a wind farm area: A case study at the rudong offshore wind farm, yellow Sea, China. Mar. pollut. Bull. 128, 466–474. doi: 10.1016/j.marpolbul.2018.01.050
Wei K., Yang J. (2016). Copper-induced oxidative damage to the prophenoloxidase-activating system in the freshwater crayfish procambarus clarkii. Fish Shellfish Immunol. 52, 221–229. doi: 10.1016/j.fsi.2016.03.151
Wen B., Jin S. R., Chen Z. Z., Gao J. Z., Liu Y. N., Liu J. H., et al. (2018). Single and combined effects of microplastics and cadmium on the cadmium accumulation, antioxidant defence and innate immunity of the discus fish (Symphysodon aequifasciatus). Environ. Pollut. 243, 462–471. doi: 10.1016/j.envpol.2018.09.029
Yin L., Liu H., Cui H., Chen B., Li L., Wu F. (2019). Impacts of polystyrene microplastics on the behavior and metabolism in a marine demersal teleost, black rockfish (Sebastes schlegelii). J. Hazardous Mater. 380, 120861. doi: 10.1016/j.jhazmat.2019.120861
Yu P., Liu Z., Wu D., Chen M., Lv W., Zhao Y. (2018). Accumulation of polystyrene microplastics in juvenile eriocheir sinensis and oxidative stress effects in the liver. Aquat. Toxicol. 200, 28–36. doi: 10.1016/j.aquatox.2018.04.015
Keywords: horseshoe crab, nanoplastic, heavy metal, antioxidant enzyme activity, physiology
Citation: Arif I, Shang Y, Zhang C, Khan FU, Tan KA, Waiho K, Wang Y, Kwan KY and Hu M (2022) Combined effects of nanoplastics and heavy metal on antioxidant parameters of juvenile tri-spine horseshoe crabs. Front. Mar. Sci. 9:1005820. doi: 10.3389/fmars.2022.1005820
Received: 28 July 2022; Accepted: 15 August 2022;
Published: 02 September 2022.
Edited by:
Xiaoshou Liu, Ocean University of China, ChinaReviewed by:
Zhihua Feng, Jiangsu Ocean University, ChinaBin Xia, Yellow Sea Fisheries Research Institute (CAFS), China
Copyright © 2022 Arif, Shang, Zhang, Khan, Tan, Waiho, Wang, Kwan and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Menghong Hu, bWhodUBzaG91LmVkdS5jbg==; Kit Yue Kwan, a2l0eXVla3dhbkBiYmd1LmVkdS5jbg==
†These authors have contributed equally to this work
 Iqra Arif1,2†
Iqra Arif1,2† Yueyong Shang
Yueyong Shang Kian Ann Tan
Kian Ann Tan Khor Waiho
Khor Waiho Youji Wang
Youji Wang Kit Yue Kwan
Kit Yue Kwan Menghong Hu
Menghong Hu