- 1National Demonstration Center for Experimental Fisheries Science Education, Shanghai Ocean University, Shanghai, China
- 2International Research Center for Marine Biosciences, Ministry of Science and Technology, Shanghai Ocean University, Shanghai, China
- 3Shanghai Collaborative Innovation Center for Cultivating Elite Breeds and Green-Culture of Aquaculture Animals, Shanghai Ocean University, Shanghai, China
Low-frequency noise has become a marine pollutant that cannot be ignored, but most studies have focused on the behavioral and physiological effects on marine vertebrates, with few studies in marine mollusks. Therefore, sea slug was used in this study to investigate the effect of low-frequency noise on its physiological aspects. This experiment was designed with different low-frequency noise (0, 100, 300, and 500 Hz) and different stimulation times (0, 6, and 12 h) to measure superoxide dismutase (SOD), malondialdehyde (MDA), and catalase (CAT) activities in hemolymph and transcriptomics in the control (C) and 6 and 12 h groups (L1 and L2) with 500 Hz noise. The results showed a positive correlation between antioxidant enzyme activity and low-frequency noise frequency (P < 0.05) and no correlation with time (P > 0.05). In central nervous system (CNS) transcriptomics, 2,460 and 3,268 genes had upregulated expression and 2,765 and 2,783 genes had downregulated expression in the L1 and L2 groups, respectively, compared to the C group. According to Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis, low-frequency noise mainly affects signaling pathways such as cytokine-cytokine receptor interaction, the FoxO signaling pathway, natural killer cell-mediated cytotoxicity, apoptosis immune-related pathways, and energy metabolic pathways such as glycolysis, the TCA cycle, and glycerophospholipid metabolism, as well as neurological pathways such as GABAergic synapses, the synaptic vesicle cycle, amyotrophic lateral sclerosis (ALS) and other neurological pathways. This study would provide valuable reference information on the potential response of mollusks to low-frequency noise stress.
Introduction
Since the 21st century, noise from marine activities such as ships, port construction, and wind farms has continued to grow and has been a non-negligible source of pollution in the ocean (Dolman et al., 2011; Simmonds et al., 2014). Compared with the high-frequency noise generated by sonar and echosounders, the stable low-frequency noise generated by ships, wind turbines and offshore facilities travels farther and decays more slowly, causing more damage to marine organisms and lasting longer (Codarin et al., 2009). Ilyina et al. (2010) predicted that there will be a significant increase in low-frequency noise by 60% in 2100 compared to today. Therefore, elucidation of the biological effects of low-frequency noise on marine animals can help us more comprehensively understand marine noise pollution.
Studies have shown that low-frequency noise caused physiological damage to auditory organs, auditory masking, reduced metabolism and immunity, and restricted reproduction and development in marine animals, resulting in significant effects on communication, feeding, escape from enemies, and population distribution (De Soto et al., 2013; Solé et al., 2013; Wale et al., 2013; Ruiz-Ruiz et al., 2019). In recent years, marine low-frequency noise has received attention from researchers, but most studies have focused on vertebrates such as fish and marine mammals, and few have focused on invertebrates (de Soto, 2016). Invertebrates are abundant and widespread in marine ecosystems, and thus, they are often used as good bioindicators of the effects of environmental change and more sensitive to low-frequency sounds than vertebrates (Tidau and Briffa, 2016). Therefore, it is particularly important to evaluate the impacts of low-frequency noise on these organisms and the ecosystems in which they live.
Sea slug (Onchidium reevesii) is a representative of the aquatic to terrestrial evolution of mollusks, which lives in the intertidal high tide zone, and it is considered an indicator species for pollution monitoring (Zhang et al., 2019). Furthermore, this organism is a low-fat, low-protein animal and rich in essential amino acids and mineral elements, which has important economic and medicinal value (Huang and Wang, 2008). We has proved previously that low-frequency noise affects the behavior and physiology of sea slugs. When exposed to low-frequency noise, sea slugs move away from the noise source, and the activity of antioxidant enzymes in hemolymph is positively correlated with noise frequency. The nervous system is the primary regulatory system in the body, by which the organism generates rapid and accurate responses to internal and external environmental stimuli (Van Damme et al., 2021). The central nervous system (CNS) is the basis for adaptive behavior in response to external environmental stimuli and is important for the survival and reproduction of animals in their environment (Gattoni and Bernocchi, 2019). In recent years, stress response mechanisms regulated by the neuroendocrine system have attracted more attentions. Much evidence has proven that the neuroendocrine system is indispensable in responding to various environmental stressors by regulating immune activity, energy allocation, growth, and exercise (Lacoste et al., 2001; Lubawy et al., 2020). Kight and Swaddle (2011) indicated that the neuroendocrine response for noise is highly plastic. Moreover, the central nervous system of mollusks is structurally simple, easily separable, and highly conserved throughout their evolutionary history. Therefore, the CNS is a suitable organ for exploring the effect of low-frequency noise on sea slugs. The hemolymph circulates continuously and flows through all vital organs, which penetrates into all tissues, participates in the body metabolism, regulates and maintains the balance of functional activities. If the organism is under adversity, the dynamic balance of reactive oxygen species (ROS) production and scavenging is disrupted, leading to the accumulation of ROS in the body. For survival, the animals form enzymatic and non-enzymatic antioxidant defense systems to scavenge excess reactive oxygen species. This change is conveyed by the hemolymph, so checking the hemolymph index is the most intuitive indicator of the body’s status.
Transcriptomic analysis evaluates genome-wide expression and is a very powerful research tool for elucidating the physiological response of animals to environmental changes. RNA-seq sequencing has thus far been widely used to study the physiological changes of invertebrates caused by environmental stimuli such as cadmium exposure (Gu et al., 2019), light stimulation (Li et al., 2020), and salinity changes (Chen et al., 2019), but it has not been applied to the effects of low-frequency noise on mollusks. Thus, sea slugs were exposed to low-frequency noise in this study, and the potential molecular mechanism of the stress response caused by low-frequency noise on sea slugs was investigated by high-throughput sequencing of the central nerve, which provides a theoretical basis for the study of marine noise pollution in mollusks.
Materials and Methods
Study Species
Sea slugs were collected from the East China Sea beach (Shanghai, China), and were temporarily reared in the shellfish laboratory of Shanghai Ocean University. The temporary rearing method referred to the study of Heding et al. (2004). According to the experimental design, 54 animals with a mean weight of 13.0 ± 1.5 g were selected for the study.
Experimental Setup and Protocol
The noise generator device is shown in Figure 1. The device consisted of a frequency signal generator (SA-SG030), a power amplifier (SA-PA010 100 W) and a noise amplifier (SA-JZ005). The experiment was conducted in a 3-m-long, 0.6-m-wide, and 0.5-m-high tank with a 15-cm-thick marine mud layer at the bottom of the tank. The sound level meter was placed on the wall of the tank to determine the decibel value of the generated noise. According to our measurements of the sound pressure level inside the tank before the experiment, there was no significant difference in the sound pressure level inside the tank with an error range of 3 dB. A thermometer and hygrometer were placed on the wall of the chamber to monitor the temperature and relative humidity (26°C and 90%) inside the chamber.
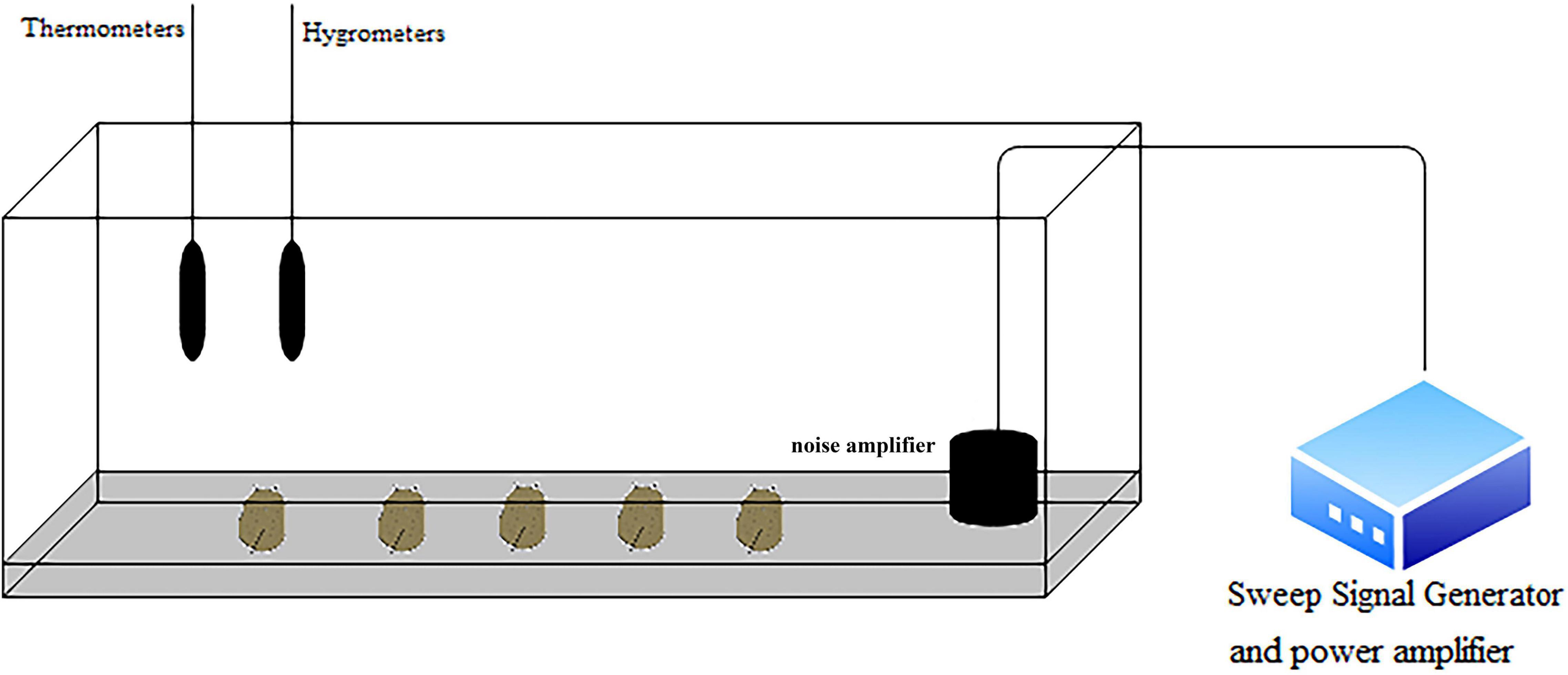
Figure 1. Schematic diagram of the experimental device. The sea slug is at the bottom of the tank. The noise amplifier is located on the right side of the tank. The thermometer and hygrometer are placed on the wall of the tank. The signal generator and power amplifier are located outside the tank.
The animals were stimulated with sinusoidal waves at frequencies of 100, 300, and 500 Hz (sound pressure levels of 80–107 dB) for 6 and 12 h. The groups were named L1 and L2. Both the control and experimental groups were subjected to the same conditions. Nine biological replicates were used for each experimental group. All the procedures were strictly carried out in accordance with the Regulations of the Experimental Animal Ethics Committee of Shanghai Ocean University.
Measurement of Enzymatic Activity
Superoxide dismutase (SOD), malondialdehyde (MDA), and catalase (CAT) in hemolymph were measured by following the manufacturer’s instructions for commercial kits (Nanjing Jiancheng Bioengineering Institute, China) and assessed on an enzyme-labeled instrument (Synergy 2, United States).
Transcriptome Analysis of Sea Slugs With Low-Frequency Noise Exposure
Based on the above results of immune-related enzyme activity, three groups, e.g., the unexposed (C) and 500 Hz-exposed groups (L1 and L2), were chosen for transcriptome analysis to further illustrate the effect and related mechanism.
RNA Extraction, Library Construction, and Sequencing
Total CNS RNA was extracted according to the mirVana™ miRNA Isolation Kit instructions (Thermo, Waltham, MA, United States), and then, the OD260/280 value was tested for total RNA integrity using a 1% agarose gel. The samples with an RNA Integrity Number (RIN) ≥ 7 was subjected to subsequent analysis. The libraries were constructed using the TruSeq Stranded mRNA LT Sample Prep Kit (Illumina, San Diego, CA, United States) according to the manufacturer’s instructions. Then, these libraries were sequenced on the Illumina sequencing platform (HiSeq™ 2500), and 125 bp/150 bp paired-end reads were generated.
Quality Control and de novo Assembly
Transcriptome sequencing and analysis were conducted by OE Biotech Co., Ltd. (Shanghai, China). Raw data (raw reads) were processed using Trimmomatic. The reads containing poly-N and the low-quality reads were removed to obtain clean reads. After removal of adaptor and low-quality sequences, the clean reads were assembled into expressed sequence tag clusters (contigs) and de novo assembled into transcripts by using Trinity (version: 2.4) in the paired-end method. The longest transcript was chosen as a unigene based on the similarity and length of a sequence for subsequent analysis.
Functional Annotation, Analysis of Differentially Expressed Unigenes, Cluster Analysis, and Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis
The function of the unigenes was annotated by alignment of the unigenes with the NCBI non-redundant (NR), SwissProt, and Clusters of Orthologous Groups for eukaryotic complete genomes (KOG) databases using Blastx with a threshold E-value of 10–5. The proteins with the highest hits to the unigenes were used to assign functional annotations. Based on the SwissProt annotation, Gene Ontology (GO) classification was performed by mapping the relation between SwissProt and GO terms. The unigenes were mapped to the Kyoto Encyclopedia of Genes and Genomes (KEGG) database to annotate their potential metabolic pathways.
FPKM and read count values of each unigene were calculated using Bowtie2 and eXpress [7]. DEGs were identified using the DESeq (2012) functions estimateSizeFactors and nbinomTest. A P-value < 0.05 and fold change > 2 or fold change < 0.5 were set as the thresholds for significantly differential expression. Hierarchical cluster analysis of DEGs was performed to explore transcript expression patterns. GO enrichment and KEGG pathway enrichment analyses of DEGs were performed using R based on the hypergeometric distribution.
qRT-PCR Analysis
Sea slug CNS RNA was extracted with Total RNA Extraction Reagent (Nanjing Novozymes Biotechnology Co., Ltd.), and cDNA first strand synthesis was performed with a HiScript Q RT SuperMix for qPCR (+gDNA wiper) kit (Nanjing Novozymes Biotechnology Co., Ltd.) for cDNA first strand synthesis.
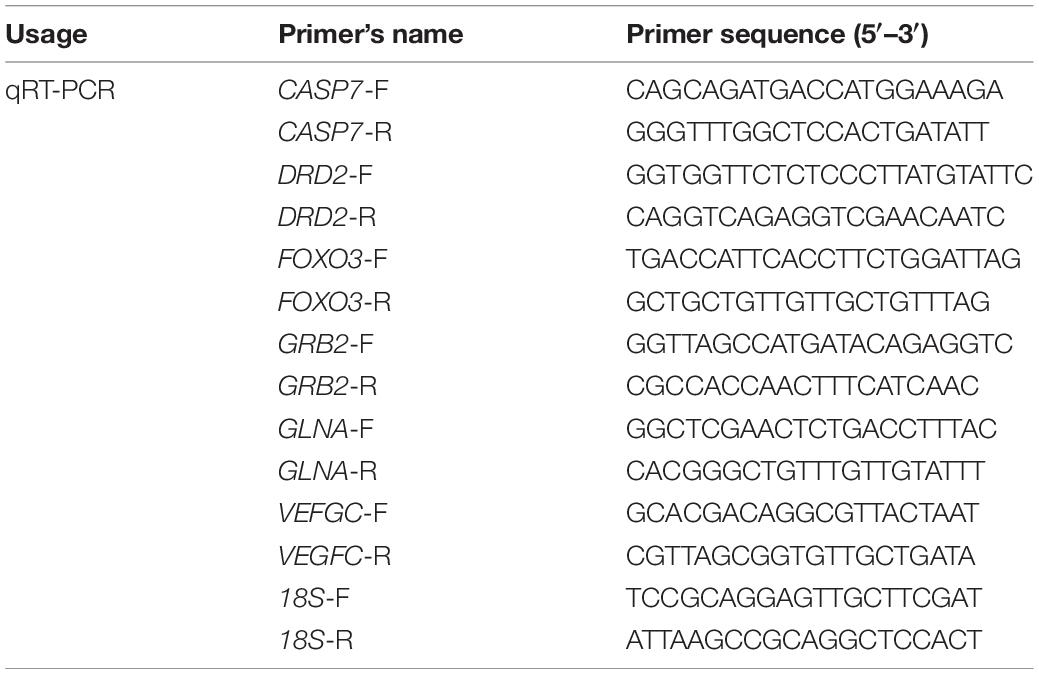
The ORF sequences of the genes were obtained from the CNS transcriptome database, and fluorescent quantitative primers were designed using Primer Premier 5 software. The 18S gene was selected as the internal reference and is listed in Table 1. The obtained cDNA was used as a template for fluorescence quantification of the gene using the Cham Q TM SYBR® qPCR Master Mix kit (Nanjing Novozymes Biotechnology Co., Ltd.).
The relative expression of the target gene in the CNS was calculated using the method of comparing CT values. The difference between the CT value of the target gene and the CT value of the internal reference gene was calculated as ΔCT, and the difference between the CT value of the target gene of the experimental group and the CT value of the target gene of the control group was calculated as ΔCT. Gene expression levels were analyzed using the following equation: 2–ΔΔ CT = 2–[(CT, target – CT, 18S)experimental group – (CT, target –CT, 18S) control group]. Six biological replicates were used for qRT-PCR analysis. The amount of change in gene expression in all samples was relative to that of the control group.
Statistical Analysis
All data are expressed as the mean ± SD. Statistical analysis was performed using SPSS 22.0 software. Two-way ANOVA and Duncan’s multiple range test were used. P < 0.05 was considered significant. Differences between the experimental and control groups were compared using chi-squared tests.
Results
Analysis of Enzyme Activity in Hemolymph
As shown in Figure 2, the enzyme activities of SOD, CAT, and MDA showed an increasing trend with increasing frequency; the highest enzyme activity was observed at 500 Hz, and the difference between the groups was significant (P < 0.05). The SOD, CAT, and MDA activities were significantly higher (P < 0.05) than those of the unexposed group at both frequencies, except for 100 Hz. Two-way analysis of variance showed that there is no significant interaction between low-frequency noise frequency and time on the production of SOD, CAT, and MDA in hemolymph (Supplementary Table 1).
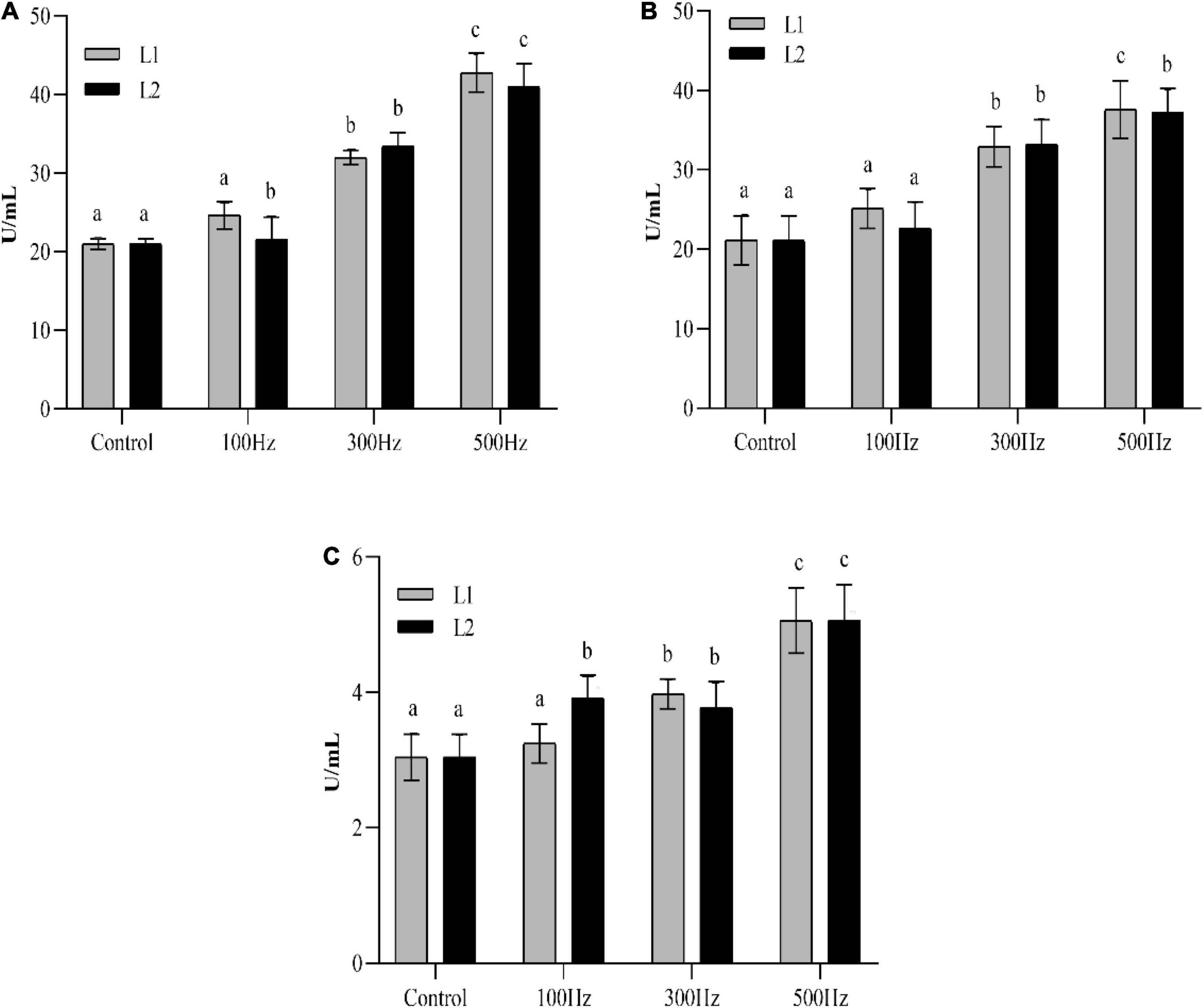
Figure 2. Changes in enzyme activities in the hemolymph of sea slugs exposed to different frequencies of low-frequency noise. SOD (A) superoxide dismutase; CAT (B) catalase; MDA (C) malondialdehyde. Data are shown as the mean ± SE of n = 9. Lowercases indicate statistically significant differences between experimental groups at the same time point (P < 0.05).
Sequencing and Assembly of Transcripts
We have the transcriptome sequencing of 9 samples, and a total of 59.97 G of clean data were obtained. The effective data volume of each sample was 6.06–7.12 G, and the distribution of Q30 bases was 92.42–94.63%, with an average GC content of 40.18% (Supplementary Table 2). Since genomic data were not available for sea slug, de novo splicing was performed without relying on the reference genome to produce 41,454 unigene entries with a total length of 73,034,255 bp and an average length of 1,066.1 bp (Supplementary Table 3).
Annotation of Transcripts
We used Diamond software to compare the annotations of the NR, Swiss-Prot, KEGG, KOG, eggNOG, GO, and Pfam databases and took the annotations with e < 1e–5 to filter the proteins with the highest sequence similarity, resulting in 17,325 (25.29%), 11,717 (17.10%), 8,688 (12.68%), 10,046 (14.66%), 12,769 (18.64%), 10,829 (15.81%), and 10, 636 (15.53%) annotations, respectively (Supplementary Table 4).
In the KOG analysis, 41,454 unigenes were annotated to 25 categories, with the most enriched unigenes in general function prediction only (R), followed in order by signal transduction mechanisms (T), secondary metabolite biosynthesis, transport and catabolism (O), function unknown (S), intracellular trafficking, secretion, vesicular transport (U), translation, ribosomal structure and biogenesis (J), transcription (K), and cytoskeleton (Z) (Figure 3).
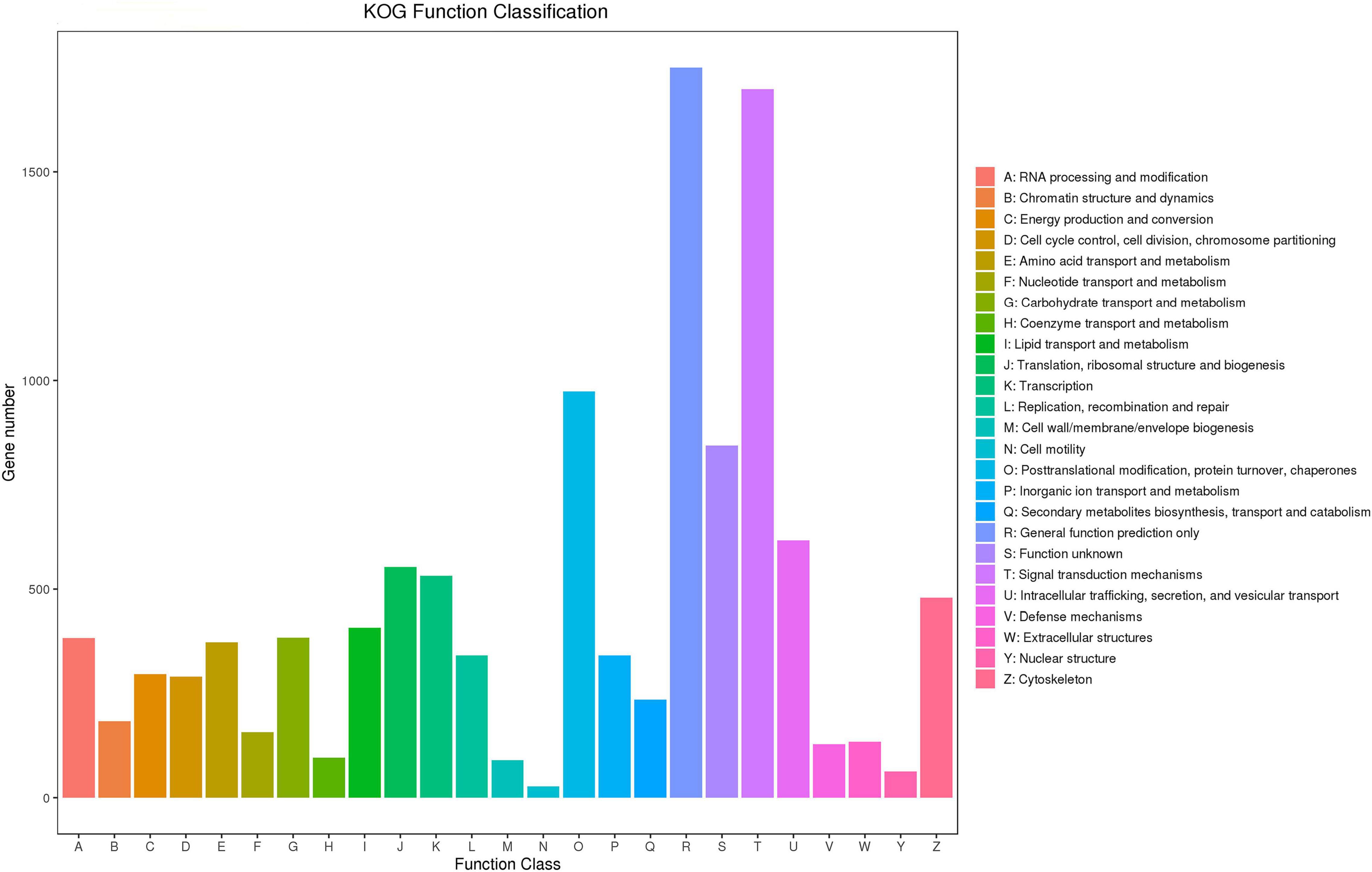
Figure 3. KOG functional classification diagram. The horizontal axis indicates the KOG functional classification, and the vertical axis indicates the number of genes.
In the GO analysis, all unigenes were annotated to three major categories, cellular component, molecular function and biological process, and then subdivided into 55 subcategories. In the biological processes category, cellular process, metabolic process, biological regulation, regulation of biological process and response to stimulus had the most enriched unigenes. In the cellular component category, cell, cell part, organelle, organelle part, and membrane were enriched in the largest proportion of unigenes. In molecular function, binding, catalytic activity, and transporter activity enriched in unigenes accounted for a larger proportion (Supplementary Figure 1).
In the KEGG analysis, all unigenes were annotated to the six major categories of cellular processes, environmental information processing, metabolism, organismal systems and human diseases, in which the number of genes in categories such as signal transduction, translation, cancer, endocrine system, folding, sorting and degradation, carbohydrate metabolism and immune system was relatively large (Supplementary Figure 2).
Gene Ontology Enrichment Analysis of Differentially Expressed Unigenes
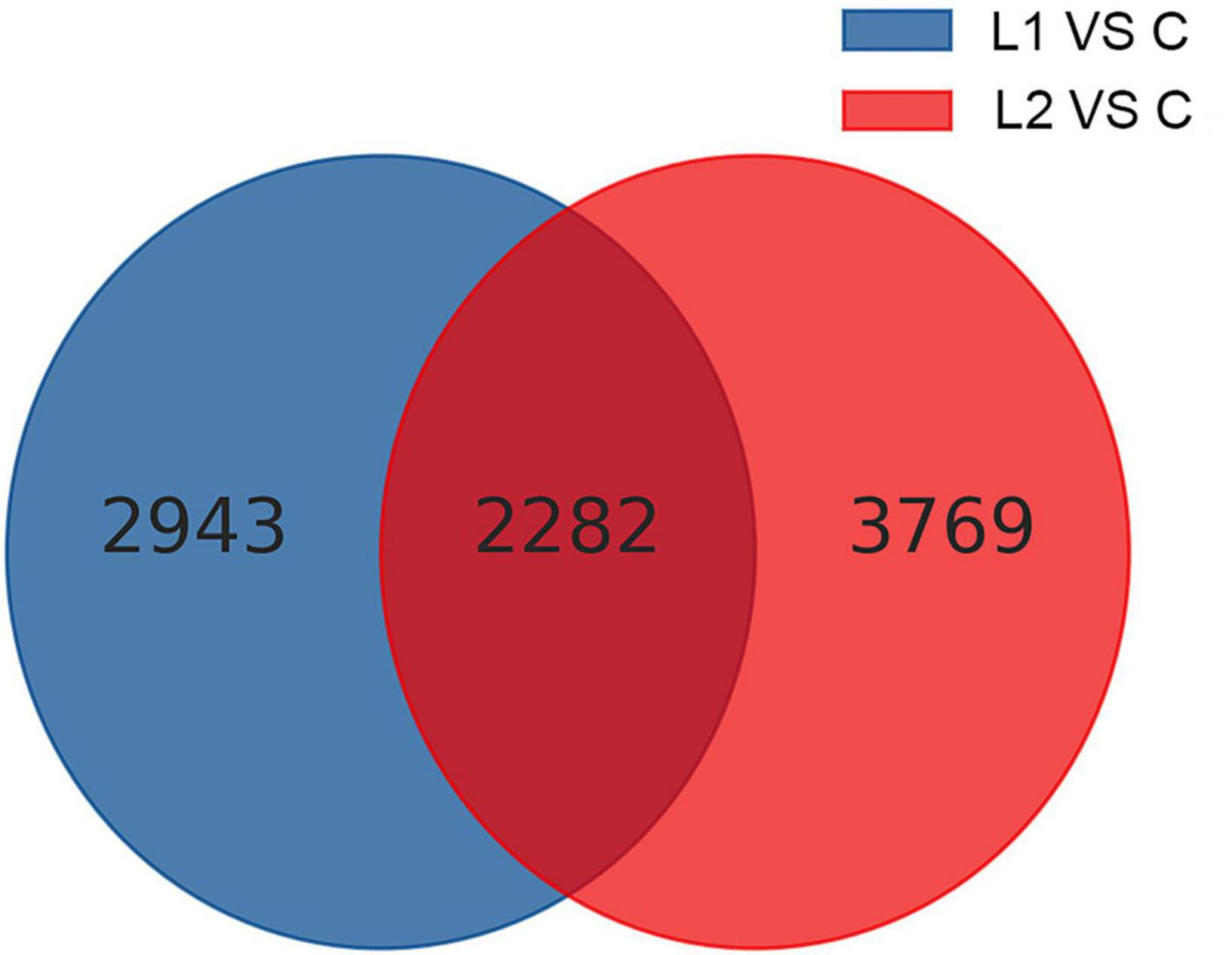
Differential gene analysis of the sample genes showed that 2,460 genes were upregulated and 2,765 genes were downregulated in Group L1, and 3,268 genes were upregulated and 2,783 genes were downregulated in Group L2 when compared to those of Group C (Figure 4). The statistics of the number of common and unique differentially expressed genes between the L groups are shown in Figure 5. There were 2,282 differentially expressed genes in the L1 and L2 groups, 2,943 differentially expressed genes unique to the L1 group and 3,769 differentially expressed genes unique to the L2 group. After the differentially expressed unigenes were obtained, GO enrichment analysis was performed on the differentially expressed unigenes to characterize their function, and the distribution of these genes and all genes at GO level 2 was compared as follows (Supplementary Figure 3). Compared to Group C, Groups L1 and L2 had the most differentially expressed genes in biological regulation, cellular process, and metabolic process in the biological process category. In the cellular component category, the number of differentially expressed genes in cells, cell parts, and organelles was relatively large. In molecular function, differentially expressed genes in binding and catalytic activity were the most abundant.
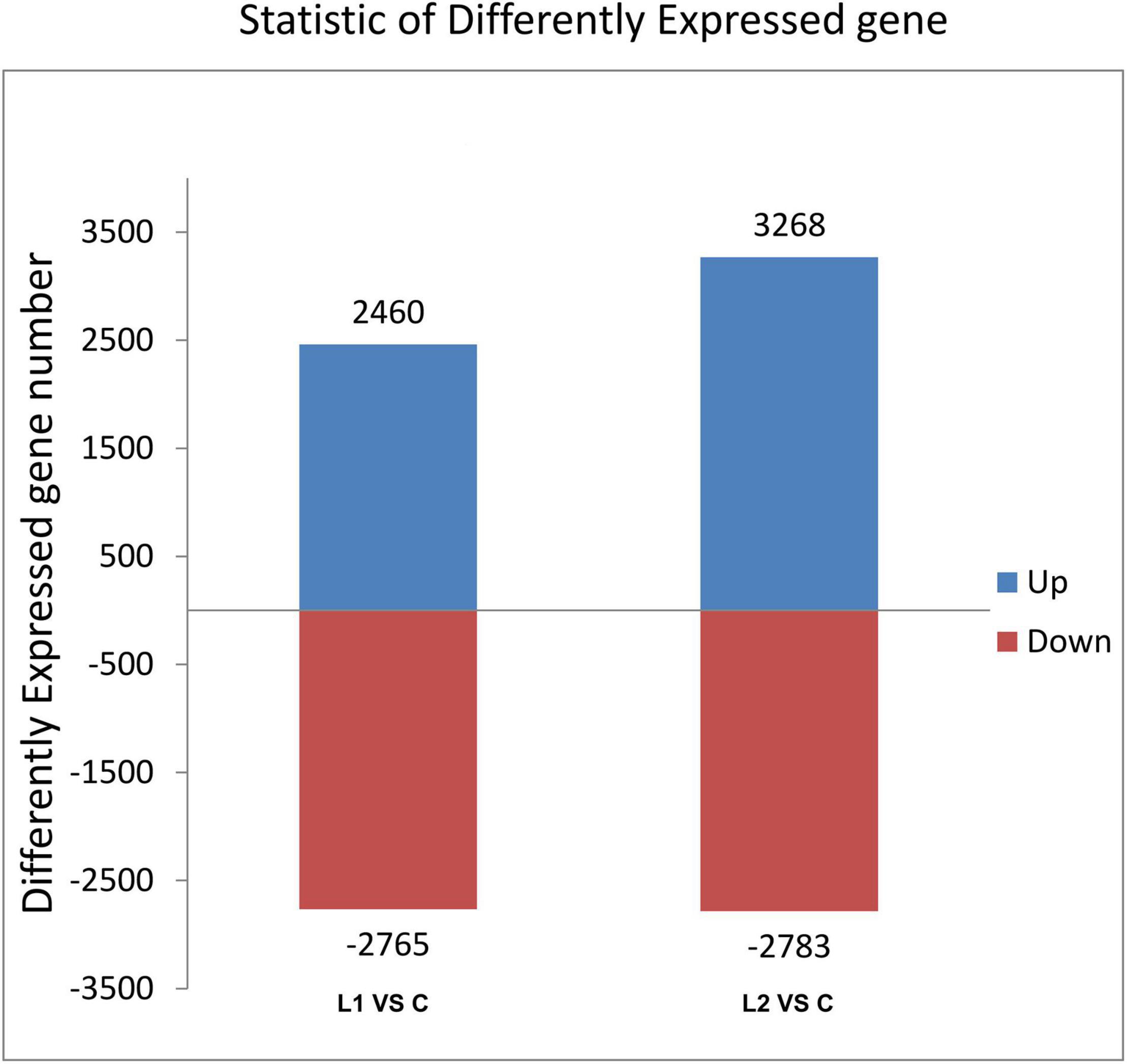
Figure 4. Histogram of differentially expressed gene statistics. The horizontal axis is each comparison group; the vertical axis is the number of differentially expressed genes in the comparison groups, where up is the number of significant genes with upregulated expression and down is the number of significant genes with downregulated expression.
Kyoto Encyclopedia of Genes and Genomes Enrichment Analysis of Differentially Expressed Unigenes
In this study, compared to those in Group C, the L1 and L2 differentially expressed genes were enriched in 287 and 284 KEGG pathways, respectively. The upregulated differentially expressed genes were enriched in 161 and 189 KEGG pathways, respectively. The downregulated differentially expressed genes were enriched in 274 and 280 KEGG pathways, respectively (Supplementary Table 5). According to the functional classification, the distribution of differentially expressed genes and all genes at the KEGG level 2 were compared as follows (Figure 6). Compared to Group C, Groups L1 and L2 had the most DEGs for signal transduction in environmental information processing (104 and 151). In cellular processes, transport and catabolism had the highest number of DEGS (46 and 68). Most DEGs were involved in folding, sorting and degradation (26 and 49) in genetic information processing. In human diseases, infectious diseases had the most DEGs (57 and 87). In metabolism, carbohydrate metabolism had the highest number of DEGS (49 and 52). In organismal systems, the endocrine system had the highest number of DEGS (63 and 88).
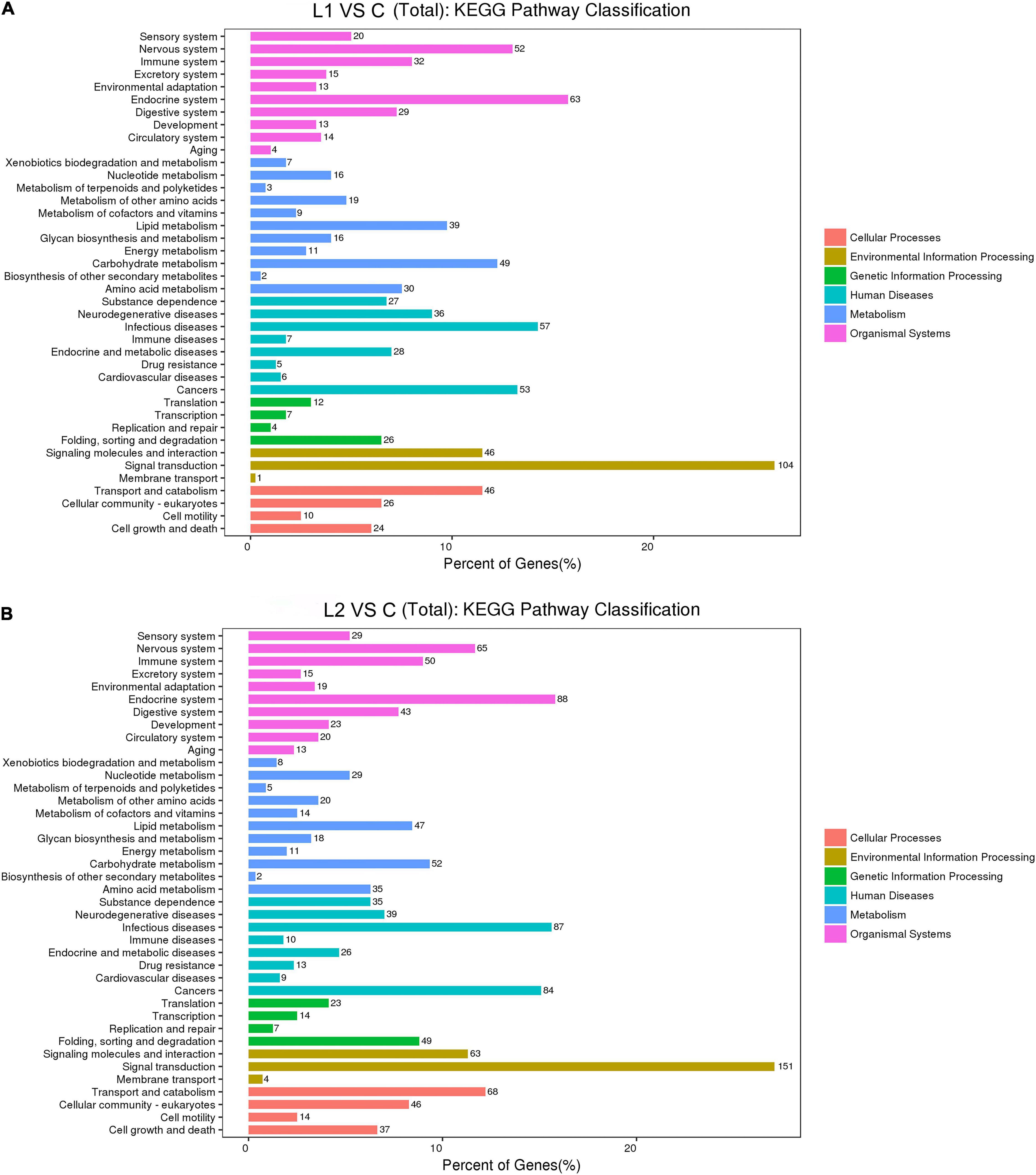
Figure 6. Comparison of the distribution of differentially expressed genes and all genes in KEGG level 2 in L1 (A) and L2 (B) groups compared to the C group. The horizontal axis is the ratio (%) of the genes annotated to each level 2 pathway (differentially expressed genes) to the total number of all genes annotated to the KEGG pathway (differentially expressed genes), the vertical axis indicates the name of the level 2 pathway, and the number on the right of the bar represents the number of differentially expressed genes annotated to that level 2 pathway.
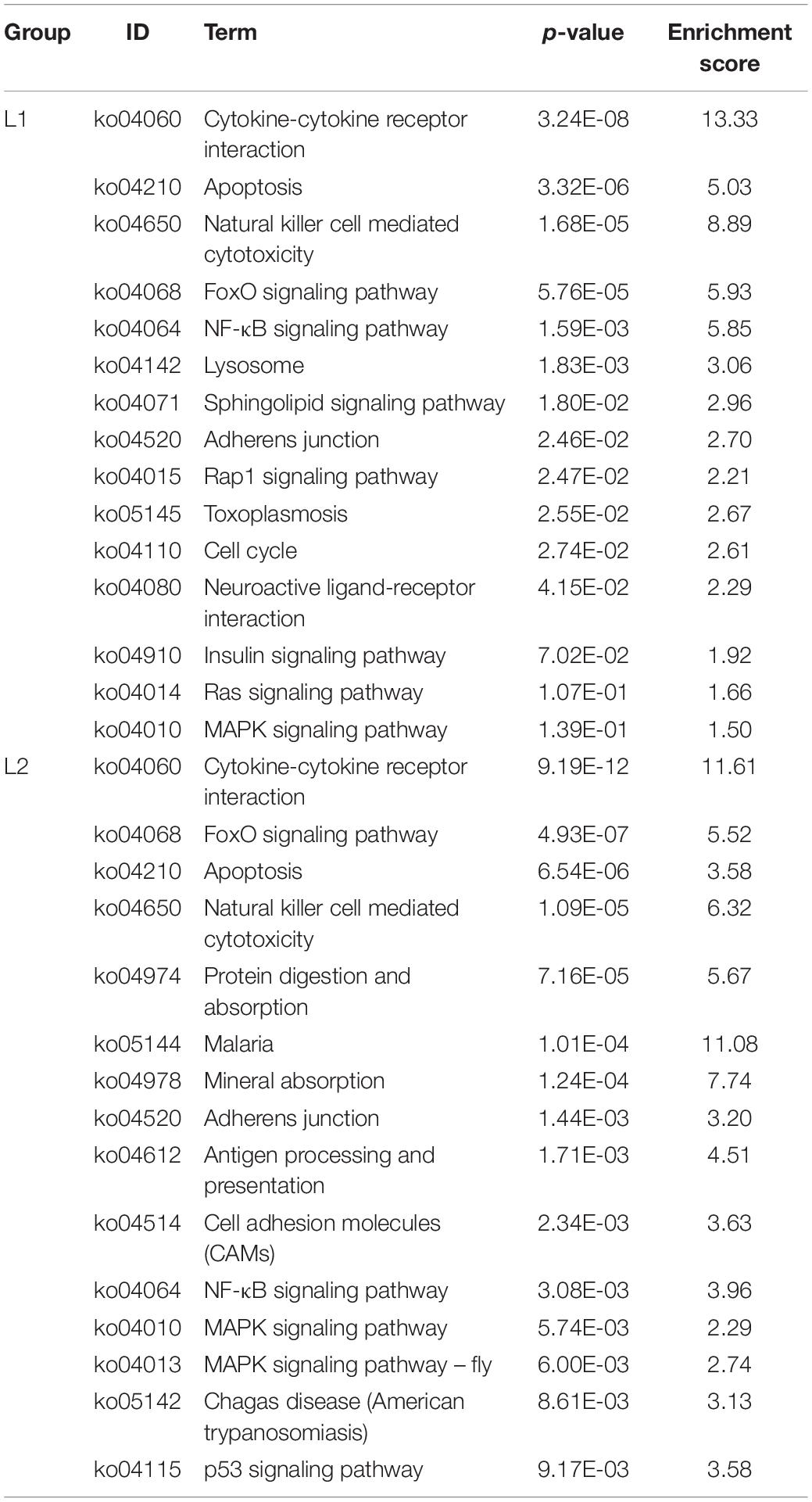
We list here the top 15 most enriched up- and downregulated pathways (Tables 2, 3). Compared to Group C, 10 of the most enriched pathways in the L1 DEGs group were involved in signaling, and 5 upregulated pathways were involved in the immune system and apoptosis. The downregulated pathways were nine involved in metabolism, two in the immune system, and one and three in signaling and the nervous system, respectively.
Compared to the C group, the L2 group showed similar enrichment pathways but with different ratios (Tables 2, 3). There were seven pathways involved in signaling and four pathways involved in the immune system among the upregulated pathways, and two pathways were involved in the digestive system. Six of the downregulated pathways were involved in metabolism, three involved in signaling, and six involved in the nervous system.
Validation of the RNA-Seq Data by qRT-PCR
Six genes related to signal transduction, immunity and metabolism were validated by qRT-PCR (Table 1). After the results were obtained with the 2–ΔΔCT algorithm, they were compared with the RNA-seq expression profile (Figure 7). The amplification efficiency of all primers was above 97%. The results showed that the findings obtained by qRT-PCR were consistent with the RNA-seq data, which confirmed the reliability of the RNA-seq data.
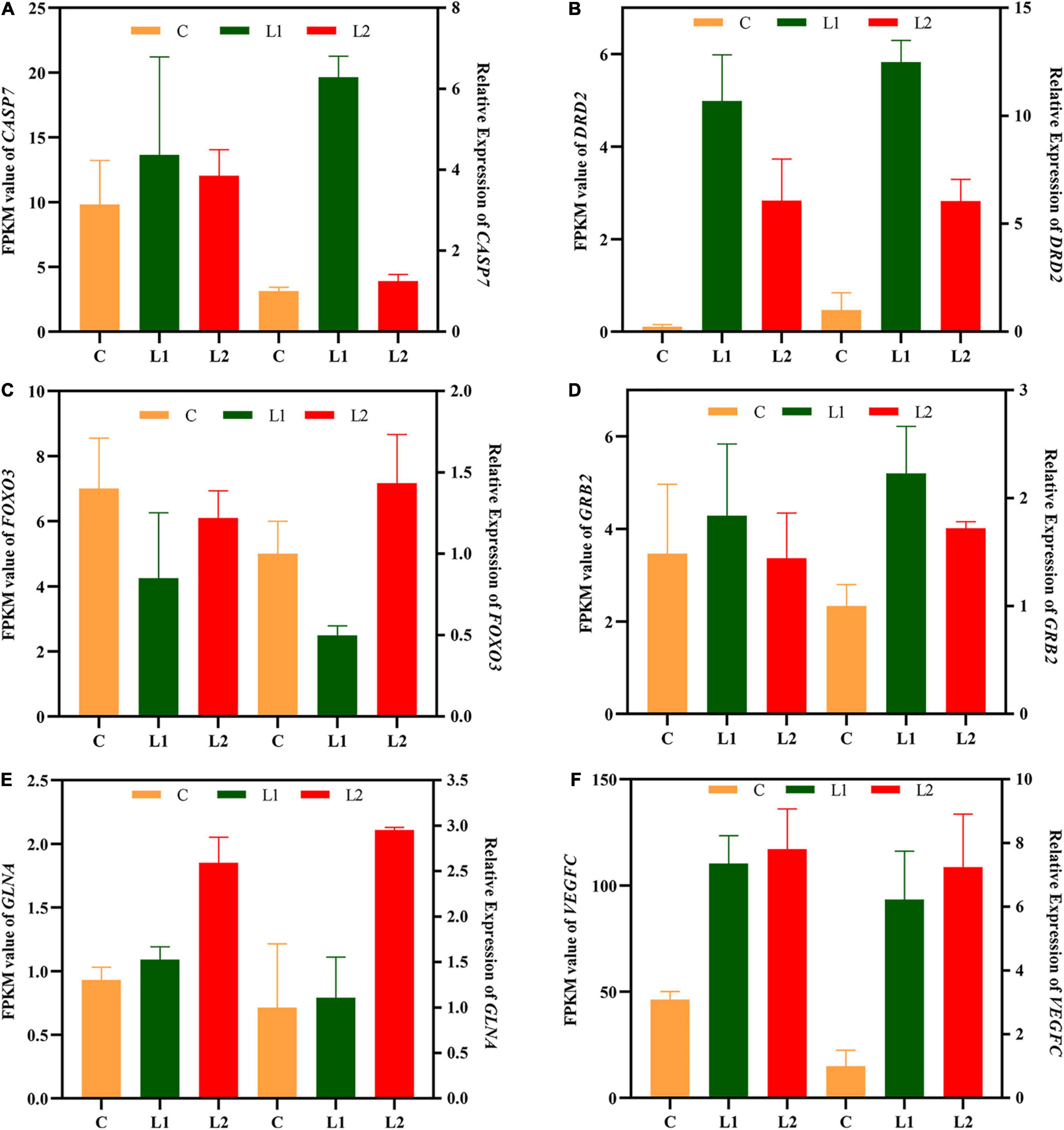
Figure 7. Validation of central nervous system DEGs profiles by qRT-PCR. The x-axis indicates the different experimental groups. The left y-axis indicates the FPKM value of RNA-seq (n = 3), and the right y-axis indicates the relative expression of 6 genes (n = 6) in Group L vs. Group C. All data are expressed as the mean ± standard deviation. CASP7 (A), caspase 7; DRD2 (B), dopamine receptor D2; FOXO3 (C), forkhead box O3; GRB2 (D), growth factor receptor bound protein 2; GLNA (E), glutamine synthetase; VEGFC (F), vascular endothelial growth factor C.
Discussion
Effects of Low-Frequency Noise on the Antioxidant Index of Sea Slug Hemolymph
Hemolymph plays a vital role in the life activities of mollusks and is the first line of antibacterial and antiviral defense for mollusks. Under stress by the external environment, homeostasis in mollusks can be altered or lost, affecting their growth and activity (Haider et al., 2020). Proteins, carbohydrates, lipids and enzymes in the hemolymph are essential components of mollusks that regulate basic life activities such as osmotic pressure, immune response, and metabolism in the body environment (Bislimi et al., 2013; Lu et al., 2015). Physiologically, SOD, CAT, and MDA are valuable indicators that are widely used to determine the responses of invertebrates to external stressors (Giarratano et al., 2014; Xu et al., 2018). Noise can lead to oxidative stress and activate antioxidant systems in animals, which eliminates excess reactive oxygen species (ROS) from the body (Chang et al., 2018; Woo et al., 2021). Both SOD and CAT are antioxidant defense enzymes that are involved in regulating the antioxidant system, scavenging excess free radicals and protecting cells from ROS damage (Li et al., 2021). MDA is an indicator of the degree of lipid peroxidation in the cell membrane (Xu et al., 2018). Exposure of mussels (Mytilus edulis) to anthropogenic noise revealed lipid peroxidation in gill epithelial cells and DNA damage in blood cells, indicating a negative effect of anthropogenic noise on mussels (Wale et al., 2019). Total oxidant status (TOS) and total antioxidant capacity (TAC) levels were increased in the blood of gilthead sea bream (Sparus aurata) juveniles exposed to offshore culture noise. These results implied that ROS was produced in juvenile fish under the influence of noise, which caused a stress response in juvenile fish (Filiciotto et al., 2017). In this study, the levels of SOD, CAT, and MDA were significantly higher in the L1 and L2 groups than in the C group, indicating that low-frequency noise can stimulate the antioxidant system of the body. The changes in the SOD, CAT, and MDA contents in the L group were positively correlated with the frequency of low-frequency noise. These results indicated that the higher the frequency is, the more ROS are produced in vivo, the stronger the oxidative stress response, and the stronger the stress response of sea slug to low-frequency noise. In our study, there was no significant difference in SOD, CAT, and MDA contents between L1 and L2 groups, indicating that hemolymph antioxidant indexes did not correlate with time. Despite the long-term nature of environmental noise disturbances, animals may adapt to stressors over time. This indicates habituation. Cortisol levels rise during the first 30 min of noise exposure in Eurasian perch (Perca fluviatilis), but cortisol in Eurasian perch exposed to noise repeatedly over a long period of time was not significantly different from the control group, indicating that repeat exposure to noise causes the fish to become habituated (Johansson et al., 2016). Exposure of European perch (Dicentrarchus labrax) to noise revealed that the experimental group exhibited habituation of swimming depth (Neo et al., 2018). At present, we do not have further verification of this conjecture. It remains to be seen whether sea slug will be found to have similar patterns of habituation and adaptation in response to noise stress.
Effects of Low-Frequency Noise on the Antioxidant Capacity
Environmental stresses such as osmotic imbalance and heat stress can lead to excessive cellular production of ROS, which can cause oxidative stress in organisms. ROS is considered as important signaling molecules that regulate a wide range of signaling pathways through interactions with key signaling molecules to influence various cellular processes to adapt to changing environments. Studies have shown that ROS play an important role in regulating cell signaling pathways such as proliferation, metabolism, differentiation and apoptosis (Choi et al., 2005; Fiol and Kültz, 2007). To date, studies of ocean noise effects on aquatic animals have mainly focused on behavior and physiology, and information on the mechanisms of perceiving noise signals and intracellular responses is lacking. In this study, we found that the most enriched antioxidant-mediated pathways in the L1 and L2 groups were cytokine-cytokine receptor interaction (ko04060), FoxO signaling pathway (ko04068), and MAPK signaling pathway (ko04010). Cytokines are key regulators of adaptive immunity in animals that regulate cellular responses to stress and are involved in immune, inflammatory, cellular growth and cellular differentiation processes, resulting in restoration of body homeostasis and repair of damage (Plata-Salamán, 1998). Oxidative stress was shown to induce a variety of inflammatory cytokines (Vlahopoulos et al., 1999). Vlahopoulos et al. (1999) showed that ROS in U937 human histiocytoma lymphoma cells lead to the production of IL-8 (interleukin-8) by TNFα (tumor necrosis factor α) through activation of the NF-κB pathway. In cells cultured with high glucose, ROS activate the transcription factor NF-κB, which leads to an elevation of the cytokine TNFα (Guha et al., 2000). The Asian clam (Corbicula fluminea) showed upregulated expression of the cytokines IL-1β (interleukin 1 beta), IL-8, IL-17 (interleukin 17) and TNF-α associated with inflammatory responses in digestive glands and gills after ammonia stress, indicating that the Asian clam was stimulated by damage and that the organism cleared or limited harmful substances through inflammatory responses (Zhang et al., 2020). FOXO is a key growth factor and stress-regulated transcription factor involved in physiological processes such as apoptosis, cell proliferation, DNA damage and repair, and resistance to oxidative stress (Dos Santos et al., 2020). FOXOs are regulated not only by PKB, JNK, and AMPK but also by post-translational modifications such as phosphorylation, acetylation, methylation and ubiquitination (Farhan et al., 2017). FOXO can enhance the expression of the antioxidant defense enzyme SOD, which alleviates damage from oxidative stress (Kops et al., 2002). The mRNA expression of Jnk, Foxo3a, and Puma genes in gills was found to increase with time in cadmium-treated carp. Moreover, the levels of oxidants such as H2O2 and MDA in the gills increased, causing oxidative stress in the gills. The results implied that cadmium-induced oxidative stress caused apoptosis in gill cells (Chen et al., 2021). The expression of the Foxo and Gadd45 genes in the liver of grass carp was upregulated after exposure to high ammonia, leading to an increase in their antioxidant enzyme activity and the scavenging of ROS in vivo, thereby enhancing resistance to oxidative stress (Jin et al., 2017). The MAPK pathway is a crucial signal transducer widely present in eukaryotes and prokaryotes that is involved in many physiological processes, such as cell proliferation and differentiation, apoptosis, immunity, and oxidative stress (Roux and Blenis, 2004). In invertebrates, various environmental stimuli activate the MAPK pathway and act as key stress response regulators (Qu et al., 2016; Wang et al., 2016). The MAPK pathway is one of the many pathways affected by ROS. ROS activate the MAPK pathway by mediating cGMP-dependent protein kinase (PKG) (Hofmann et al., 2006). The expression of p38-MAPK in the gills of purple mussels (Mytilus galloprovincialis) was significantly increased under cold stress, which indicated that p38-MAPK plays an important role in the early stages of stress (Wang et al., 2018). Exposure to microplastics increased the expression of p38 in the hepatopancreas of Eriocheir sinensis, which indicated that microplastics activated the expression of p38, leading to enhanced cellular oxidative stress (Yu et al., 2018). In this study, the L1 and L2 groups had the highest number of DEGs with enhanced cytokines and FOXO and MAPK pathways. This result indicated that low-frequency noise may have led to a change in the balance between antioxidants and ROS in sea slug, which generated oxidative stress. Cells must activate the expression of a number of cytokines and transcription factors to reconstitute redox homeostasis as a result of oxidative stress. These results are also consistent with our measurements of SOD, CAT, and MDA in hemolymph, where low-frequency noise led to oxidative stress in sea slugs.
Effects of Low-Frequency Noise on the Immune System
The immunity of animals is significantly negatively affected under the stress of external environmental factors such as temperature (Huang et al., 2011), heavy metals (Ali et al., 2014), salinity (Lin et al., 2012), etc. It has been shown that anthropogenic noise also poses a significant challenge on shellfish immunity (Wale et al., 2016). However, shellfish do not have specific immunity, and they rely on innate immunity to defend themselves against pathogens. Innate immune defenses in shellfish play a pivotal role in normal physiological activity. The importance of the intertidal zone to the material and energy cycles of marine ecosystems is well known, especially the importance and contribution of intertidal organisms to communities, ecosystems and ecological processes. However, the biomass of parasitoids in intertidal ecosystems may be substantial, more than the biomass of top predators and major free-living communities (Kuris et al., 2008). Thus, it is necessary to assess the impact of parasites on these intertidal organisms. Perkinsus, Haplosporidium, Marteilia, and Bonamia have all been identified as major parasites responsible for significant mortality in wild and farmed shellfish (Marquis et al., 2015). Plasmodium spores were found in the digestive glands of mussels in Sivuchya Bay. This phenomenon caused lysis of connective tissue and necrosis of muscle fibers. The authors also found that the rate of infection in mussels was related to the level of pollution in the bay (Usheva et al., 2006). Masud et al. (2020) found a significantly higher infection rate in the acute noise group than in the control group by establishing a host-parasite model (guppy-Gyrodactylus turnbull). The chronic noise group had the lowest infection rate, but the mortality rate was significantly higher than that of the acute noise group and the control group. This finding indicates that noise can aggravate the harmful effects of parasites on fish. In this study, DEGs were significantly upregulated in parasite-associated pathways (ko05145, ko05144, and ko05142) in the L1 and L2 groups, indicating that low-frequency noise may have increased the infection rate of sea slug parasites.
A study has shown that noise can reduce the immunity of marine organisms (Celi et al., 2015; Vazzana et al., 2020). The total number of blood cells decreased by 60% when lobsters were exposed to seismic airgun noise, revealing that the immunity of lobsters was affected by noise (Fitzgibbon et al., 2017). In this study, the immune pathways with the highest DEG enrichment in the L1 and L2 groups were natural killer cell-mediated cytotoxicity (ko04650) and the NF-κB signaling pathway (ko04064). Natural killer cells, also known as “natural killers,” are critical non-specific immune lymphocytes that do not need to be activated and can respond quickly to kill harmful cells (Vivier et al., 2011). We found that the top gene with upregulated expression of this pathway is TRAIL, which is a ligand protein that induces apoptosis. TRAIL can induce a cellular response to stress through JNK activation of the transcription factors NF-κB and MAPK (Schaefer et al., 2007). In our study, the trend of upregulation of the NF-κB pathway and the MAPK pathway under the stress of low-frequency noise was also evident. This result indicated that low-frequency noise might upregulate the expression of the sea slug TRAIL gene, which then activates the expression of these two pathways to play a role in the immune response under low-frequency noise stress. The NF-κB pathway plays an important role in innate immunity because it is conserved in evolutionary history (Hatada et al., 2000). Infection of healthy Cyclina sinensis with pathogens resulted in higher NF-κB expression, indicating that NF-κB may be involved in its innate immune pathway (Gao et al., 2016). In addition, an enrichment of DEGs enhanced in the apoptotic signaling pathway (ko04210) was also significant in the study. Apoptosis is important for the function of the molluscan immune system, which clears damaged, malignant and infected cells when stress signals are received. When mollusks are exposed to contaminants, both tissues and cells of the immune system are affected. Cd2+ could induce apoptosis of oyster blood cells, resulting in reduced disease resistance and increased opportunistic infections (Sokolova et al., 2004). The exposure of Pacific oysters (Crassostrea gigas) to Alexandrium catenella resulted in a significant increase in the number of apoptotic cells and a significant upregulation of the expression of proapoptotic genes (Bax and Bax-like), indicating that toxic algae affected the immune system of the oyster (Medhioub et al., 2013). A study has shown that excess ROS can induce apoptosis (Buttke and Sandstrom, 1994). Based on our results on SOD, CAT, and MDA in hemolymph, excess ROS may also be produced in CNS cells under the stress of low-frequency noise, which induced apoptosis and challenged the immune system of sea slug.
Effects of Low-Frequency Noise on the Central Nervous System
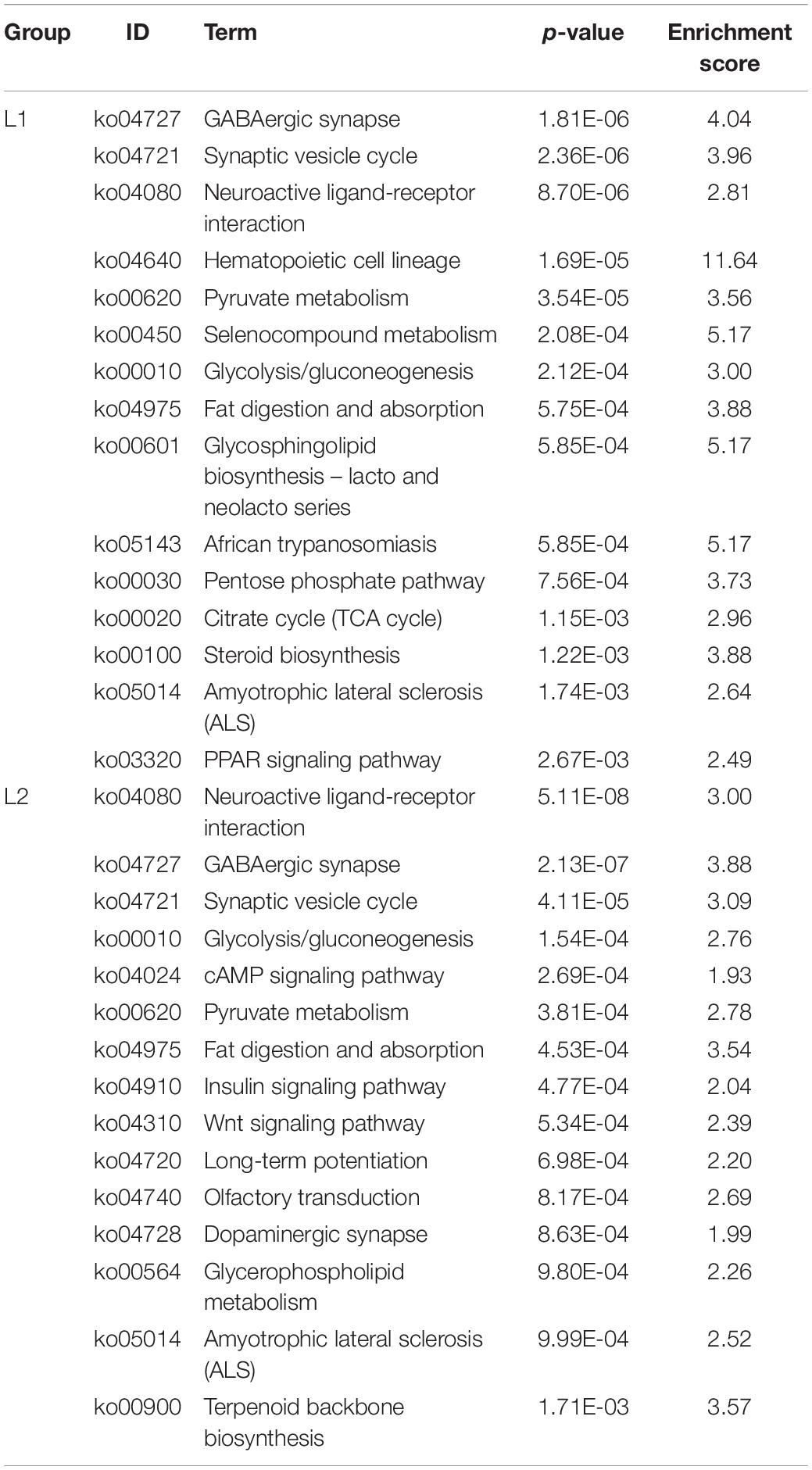
Intertidal marine organisms live in a complex and variable environment that requires a very specific homeostatic system to regulate internal metabolism. The central nervous system of mollusks is relatively simple in structure. Moreover, it shares a common strategy for processing information with the vertebrate nervous system, which is structurally composed of neurons and glial cells. Many studies have used mollusks, such as the California sea hare (Aplysia californica) (Moroz et al., 2006) and the sea cucumber (Holothuria glaberrima) (San Miguel-Ruiz et al., 2009), as good models for neuroscience. In this study, we found 9 of the top 15 most enriched pathways in the L1 group regarding gluconeogenesis and lipid metabolism were significantly downregulated, and 6 were found in the L2 group (Table 3). Exposure of Sinonovacula constricta to anthropogenic noise revealed that the expression of 10 genes involved in glycolysis, fatty acid synthesis, tryptophan metabolism, and tricarboxylic acid cycle pathways was inhibited at sound intensities of ∼100 dB re 1 μPa, indicating that anthropogenic noise negatively affects the metabolism of S. constricta (Peng et al., 2016). Exposing mice to noise resulted in reduced methylation of BDNF genes in the brain, and there was a strong correlation between this phenomenon and reduced body weight in mice, suggesting that noise may have had a negative effect on metabolism in mice (Guo et al., 2017). In addition, we found that 4 neurologically related pathways in the top 15 were downregulated in the L1 group and 6 in the L2 group (Table 3). The CNS is a heavily energy-dependent tissue, and studies have found glucose metabolic disturbances in neurological disorders such as Alzheimer’s disease (AD) and amnestic mild cognitive impairment (aMCI) (Neth and Craft, 2017; Weise et al., 2018). However, low glucose utilization rates lead to insufficient ATP production, which in turn leads to oxidative damage. Oxidative damage can cause a decrease in the activity of glucose-metabolizing enzymes (Butterfield and Boyd-Kimball, 2018). When A. californica was used as a model to explore whether aging was caused by neuronal damage, decreased expression of pathways involved in energy metabolism and the nervous system and increased expression of stress pathways suggested that cognitive impairment was caused by a decline in neural metabolism (Kron et al., 2020). This finding is consistent with our results. Based on previous results, upregulation of the FOXO pathway and MAPK pathway alleviates oxidative stress. Moreover, it has been shown that damage to the equilibrium capsule occurs in Mediterranean squid (Illex coindetii) and European squid (Loligo vulgaris) when exposed to anthropogenic noise (Solé et al., 2013). Glucose metabolism and lipid metabolic pathways were significantly downregulated, indicating that low-frequency noise may have caused damage to the CNS in sea slug. Whether the damage to the CNS of sea slug truly occurred under the stimulation of low-frequency noise will be verified in more depth in our subsequent study.
Conclusion
In this study, the results showed that low-frequency noise induced oxidative stress in sea slugs by affecting the hemolymph antioxidant enzyme index. Moreover, it is possible that cytokine-cytokine receptor interactions, the FoxO signaling pathway, the MAPK signaling pathway, natural killer cell-mediated cytotoxicity, the NF-κB signaling pathway, glycolysis, the citrate cycle (TCA cycle) and amyotrophic lateral sclerosis (ALS) significantly affect sea slug oxidative stress, the immune system, apoptosis, energy metabolism and the nervous system. These results increased our knowledge of events at the molecular level under low-frequency noise stress and revealed the overall response mechanism of mollusks under low-frequency noise stress.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/Traces/study/?acc=PRJNA776733.
Ethics Statement
The animal study was reviewed and approved by The Experimental Animal Ethics Committee of Shanghai Ocean University.
Author Contributions
ZT: designer of this experiments and the executor of the experimental study, completing the data analysis, and writing the first draft of the manuscript. LT, XZ, and JJ: participate in sample collection and processing. HS: conceptualizer and leader of the project. All authors contributed to the article and approved the submitted version.
Funding
Funding for this study was provided by Shanghai Collaborative Innovation Center for Cultivating Elite Breeds and Green-culture of Aquaculture Animals (A1-3605-21-000202) and the Capacity Enhancement of Aquatic Germplasm Resources Research and Support Platform of Shanghai Ocean University (A1-3201-20-300206).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The RNA-seq analysis part was done by Shanghai OE Biomedical Technology Co., Ltd. Thanks to Hang Yang (Shanghai Ocean University) and Xiaoying Cao (Xiamen University) for them help in this research.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.807489/full#supplementary-material
References
Ali, A. S., Us, S. A., and Ahmad, R. (2014). Effect of different heavy metal pollution on fish. Res. J. Chem. Environ. Sci. 2, 74–79.
Bislimi, K., Behluli, A., Halili, J., Mazreku, I., Osmani, F., and Halili, F. (2013). Comparative analysis of some biochemical parameters in hemolymph of garden snail (Helix pomatia L.) of the Kastriot and Ferizaj regions, Kosovo. Int. J. Eng. Appl. Sci. 4:8269.
Butterfield, D. A., and Boyd-Kimball, D. (2018). Oxidative stress, amyloid-β peptide, and altered key molecular pathways in the pathogenesis and progression of Alzheimer’s disease. J. Alzheimers Dis. 62, 1345–1367. doi: 10.3233/JAD-170543
Buttke, T. M., and Sandstrom, P. A. (1994). Oxidative stress as a mediator of apoptosis. Immunol. Today. 15, 7–10. doi: 10.1016/0167-5699(94)90018-3
Celi, M., Filiciotto, F., Vazzana, M., Arizza, V., Maccarrone, V., Ceraulo, M., et al. (2015). Shipping noise affecting immune responses of European spiny lobster (Palinurus elephas). Can. J. Zool. 93, 113–121. doi: 10.1139/cjz-2014-0219
Chang, H.-Y., Lin, T.-H., Anraku, K., and Shao, Y. (2018). The effects of continuous acoustic stress on ROS levels and antioxidant-related gene expression in the Black Porgy (Acanthopagrus schlegelii). Zool. Stud. 57:e59. doi: 10.6620/ZS.2018.57-59
Chen, J., Chen, D., Li, J., Liu, Y., Gu, X., and Teng, X. (2021). Cadmium-induced oxidative stress and immunosuppression mediated mitochondrial apoptosis via JNK-FoxO3a-PUMA pathway in common carp (Cyprinus carpio L.) gills. Aquat. Toxicol. 233:105775. doi: 10.1016/j.aquatox.2021.105775
Chen, X., Chen, J., Shen, Y., Bi, Y., Hou, W., Pan, G., et al. (2019). Transcriptional responses to low-salinity stress in the gills of adult female Portunus trituberculatus. Comp. Biochem. Physiol. Part D Genomics Proteomics 29, 86–94. doi: 10.1016/j.cbd.2018.11.001
Choi, K., Mollapour, E., and Shears, S. B. (2005). Signal transduction during environmental stress: insP8 operates within highly restricted contexts. Cell Signal. 17, 1533–1541. doi: 10.1016/j.cellsig.2005.03.021
Codarin, A., Wysocki, L. E., Ladich, F., and Picciulin, M. (2009). Effects of ambient and boat noise on hearing and communication in three fish species living in a marine protected area (Miramare. Italy). Mar. Pollut. Bull. 58, 1880–1887.
de Soto, N. A. (2016). “Peer-reviewed studies on the effects of anthropogenic noise on marine invertebrates: from scallop larvae to giant squid,” in The Effects of Noise on Aquatic Life II. Advances in Experimental Medicine and Biology, Vol. 875, eds A. Popper and A. Hawkins (New York, NY: Springer), 17–26.
De Soto, N. A., Delorme, N., Atkins, J., Howard, S., Williams, J., and Johnson, M. (2013). Anthropogenic noise causes body malformations and delays development in marine larvae. Sci. Rep. 3:2831. doi: 10.1038/srep02831
Dolman, S. J., Evans, P. G., Notarbartolo-Di-Sciara, G., and Frisch, H. (2011). Active sonar, beaked whales and European regional policy. Mar. Pollut. Bull. 63, 27–34. doi: 10.1016/j.marpolbul.2010.03.034
Dos Santos, M. M., De Macedo, G. T., Prestes, A. S., Ecker, A., Mueller, T. E., Leitemperger, J., et al. (2020). Modulation of redox and insulin signaling underlie the anti-hyperglycemic and antioxidant effects of diphenyl diselenide in zebrafish. Free Radic. Biol. Med. 158, 20–31. doi: 10.1016/j.freeradbiomed.2020.06.002
Farhan, M., Wang, H., Gaur, U., Little, P. J., Xu, J., and Zheng, W. (2017). FOXO signaling pathways as therapeutic targets in cancer. Int. J. Biol. Sci. 13:815. doi: 10.7150/ijbs.20052
Filiciotto, F., Cecchini, S., Buscaino, G., Maccarrone, V., Piccione, G., and Fazio, F. (2017). Impact of aquatic acoustic noise on oxidative status and some immune parameters in gilthead sea bream Sparus aurata (Linnaeus, 1758) juveniles. Aquac. Res. 48, 1895–1903. doi: 10.1111/are.13027
Fiol, D. F., and Kültz, D. (2007). Osmotic stress sensing and signaling in fishes. FEBS J. 274, 5790–5798. doi: 10.1111/j.1742-4658.2007.06099.x
Fitzgibbon, Q. P., Day, R. D., Mccauley, R. D., Simon, C. J., and Semmens, J. M. (2017). The impact of seismic air gun exposure on the haemolymph physiology and nutritional condition of spiny lobster, Jasus edwardsii. Mar. Pollut. Bull. 125, 146–156. doi: 10.1016/j.marpolbul.2017.08.004
Gao, S., Ren, Y., Zhang, H., Pan, B., and Gao, H. (2016). Identification and expression analysis of IκB and NF-κB genes from Cyclina sinensis. Fish Shellfish Immunol. 56, 427–435. doi: 10.1016/j.fsi.2016.07.035
Gattoni, G., and Bernocchi, G. (2019). Calcium-binding proteins in the nervous system during hibernation: neuroprotective strategies in hypometabolic conditions? Int. J. Mol. Sci. 20:2364. doi: 10.3390/ijms20092364
Giarratano, E., Gil, M. N., Malanga, G. J. E., and Safety, E. (2014). Biomarkers of environmental stress in gills of ribbed mussel Aulacomya atra atra (Nuevo Gulf, Northern Patagonia). Ecotoxicol. Environ. Saf. 107, 111–119. doi: 10.1016/j.ecoenv.2014.05.003
Gu, B., Yang, T., Liu, X., and Shen, H. (2019). Transcriptomic analysis of the onchidium reevesii central nervous system in response to cadmium. Front. Mar. Sci. 6:547. doi: 10.3389/fmars.2019.00547
Guha, M., Bai, W., Nadler, J. L., and Natarajan, R. (2000). Molecular mechanisms of tumor necrosis factor α gene expression in monocytic cells via hyperglycemia-induced oxidant stress-dependent and-independent pathways. J. Biol. Chem. 275, 17728–17739. doi: 10.1074/jbc.275.23.17728
Guo, L., Li, P.-H., Li, H., Colicino, E., Colicino, S., Wen, Y., et al. (2017). Effects of environmental noise exposure on DNA methylation in the brain and metabolic health. Environ. Res. 153, 73–82. doi: 10.1016/j.envres.2016.11.017
Haider, F., Falfushynska, H. I., Timm, S., and Sokolova, I. M. (2020). Effects of hypoxia and reoxygenation on intermediary metabolite homeostasis of marine bivalves Mytilus edulis and Crassostrea gigas. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 242:110657. doi: 10.1016/j.cbpa.2020.110657
Hatada, E. N., Krappmann, D., and Scheidereit, C. (2000). NF-κB and the innate immune response. Curr. Opin. Immunol. 12, 52–58. doi: 10.1016/S0952-7915(99)00050-3
Heding, S., Hanchun, C., Xianlong, C., Huawei, S., Xueming, H., and Huajun, X. (2004). Preliminary studies on the absorption rates and the feeding effects of different diets on sea-slug Onchidium sp. J. Ocean Univ. Shanghai 13, 293–297.
Hofmann, F., Feil, R., Kleppisch, T., and Schlossmann, J. (2006). Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol. Rev. 86, 1–23. doi: 10.1152/physrev.00015.2005
Huang, J. T., and Wang, A. M. (2008). Determination of the nutrients of Onchidium struma and evaluation of its quality. Mar. Fish 32, 29–35.
Huang, Z. H., Ma, A. J., and Wang, X. A. (2011). The immune response of turbot, Scophthalmus maximus (L.), skin to high water temperature. J. Fish Dis. 34, 619–627. doi: 10.1111/j.1365-2761.2011.01275.x
Ilyina, T., Zeebe, R. E., and Brewer, P. G. (2010). Future ocean increasingly transparent to low-frequency sound owing to carbon dioxide emissions. Nat. Geosci. 3, 18–22. doi: 10.1038/ngeo719
Jin, J., Wang, Y., Wu, Z., Hergazy, A., Lan, J., Zhao, L., et al. (2017). Transcriptomic analysis of liver from grass carp (Ctenopharyngodon idellus) exposed to high environmental ammonia reveals the activation of antioxidant and apoptosis pathways. Fish Shellfish Immunol. 63, 444–451. doi: 10.1016/j.fsi.2017.02.037
Johansson, K., Sigray, P., Backström, T., and Magnhagen, C. (2016). “Stress response and habituation to motorboat noise in two coastal fish species in the bothnian sea,” in The Effects of Noise on Aquatic Life II. Advances in Experimental Medicine and Biology, eds A. Popper and A. Hawkins (New York, NY: Springer), 875. doi: 10.1007/978-1-4939-2981-8_6
Kight, C. R., and Swaddle, J. P. (2011). How and why environmental noise impacts animals: an integrative, mechanistic review. Ecol. Lett. 14, 1052–1061.
Kops, G. J., Dansen, T. B., Polderman, P. E., Saarloos, I., Wirtz, K. W., Coffer, P. J., et al. (2002). Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419, 316–321. doi: 10.1038/nature01036
Kron, N. S., Schmale, M. C., and Fieber, L. A. (2020). Changes in metabolism and proteostasis drive aging phenotype in aplysia californica sensory neurons. Front. Aging Neurosci. 12:573764. doi: 10.3389/fnagi.2020.573764
Kuris, A. M., Hechinger, R. F., Shaw, J. C., Whitney, K. L., Aguirre-Macedo, L., Boch, C. A., et al. (2008). Ecosystem energetic implications of parasite and free-living biomass in three estuaries. Nature 454, 515–518. doi: 10.1038/nature06970
Lacoste, A., Jalabert, F., Malham, S. K., Cueff, A., and Poulet, S. A. (2001). Stress and stress-induced neuroendocrine changes increase the susceptibility of juvenile oysters (Crassostrea gigas) to Vibrio splendidus. Appl. Environ. Microbiol. 67, 2304–2309. doi: 10.1128/AEM.67.5.2304-2309.2001
Li, N., Zhou, J., Wang, H., Mu, C., Shi, C., Liu, L., et al. (2020). Transcriptome analysis of genes and pathways associated with metabolism in Scylla paramamosain under different light intensities during indoor overwintering. BMC Genomics 21:775. doi: 10.1186/s12864-020-07190-w
Li, P., Li, Z. H., and Wu, Y. (2021). Interactive effects of temperature and mercury exposure on the stress-related responses in the freshwater fish Ctenopharyngodon idella. Aquac. Res. 52, 2070–2077. doi: 10.1111/are.15058
Lin, Y.-C., Chen, J.-C., Li, C.-C., Morni, W. Z. W., Suhaili, A. S. N., Kuo, Y.-H., et al. (2012). Modulation of the innate immune system in white shrimp Litopenaeus vannamei following long-term low salinity exposure. Fish Shellfish Immunol. 33, 324–331. doi: 10.1016/j.fsi.2012.05.006
Lu, Y., Wang, F., and Dong, S. (2015). Energy response of swimming crab Portunus trituberculatus to thermal variation: implication for crab transport method. Aquaculture 441, 64–71. doi: 10.1016/j.aquaculture.2015.02.022
Lubawy, J., Urbański, A., Colinet, H., Pflueger, H. J., and Marciniak, P. (2020). Role of the insect neuroendocrine system in the response to cold stress. Front. Physiol. 11:376. doi: 10.3389/fphys.2020.00376
Marquis, N. D., Record, N. R., and Robledo, J. A. F. (2015). Survey for protozoan parasites in Eastern oysters (Crassostrea virginica) from the Gulf of Maine using PCR-based assays. Parasitol. Int. 64, 299–302. doi: 10.1016/j.parint.2015.04.001
Masud, N., Hayes, L., Crivelli, D., Grigg, S., and Cable, J. (2020). Noise pollution: acute noise exposure increases susceptibility to disease and chronic exposure reduces host survival. R. Soc. Open Sci. 7:200172. doi: 10.1098/rsos.200172
Medhioub, W., Ramondenc, S., Vanhove, A. S., Vergnes, A., Masseret, E., Savar, V., et al. (2013). Exposure to the neurotoxic dinoflagellate, Alexandrium catenella, induces apoptosis of the hemocytes of the oyster, Crassostrea gigas. Mar. Drugs 11, 4799–4814. doi: 10.3390/md11124799
Moroz, L. L., Edwards, J. R., Puthanveettil, S. V., Kohn, A. B., Ha, T., Heyland, A., et al. (2006). Neuronal transcriptome of Aplysia: neuronal compartments and circuitry. Cell 127, 1453–1467. doi: 10.1016/j.cell.2006.09.052
Neo, Y. Y., Hubert, J., Bolle, L. J., Winter, H. V., and Slabbekoorn, H. (2018). European seabass respond more strongly to noise exposure at night and habituate over repeated trials of sound exposure. Environ. Pollut. 239, 367–374. doi: 10.1016/j.envpol.2018.04.018
Neth, B. J., and Craft, S. (2017). Insulin resistance and Alzheimer’s disease: bioenergetic linkages. Front. Aging Neurosci. 9:345. doi: 10.3389/fnagi.2017.00345
Peng, C., Zhao, X., Liu, S., Shi, W., Han, Y., Guo, C., et al. (2016). Effects of anthropogenic sound on digging behavior, metabolism, Ca2+/Mg2+ ATPase activity, and metabolism-related gene expression of the bivalve Sinonovacula constricta. Sci. Rep. 6:24266. doi: 10.1038/srep24266
Plata-Salamán, C. R. (1998). Cytokine-induced anorexia: behavioral, cellular, and molecular mechanismsa. Ann. N. Y. Acad. Sci. 856, 160–170.
Qu, F., Xiang, Z., Zhang, Y., Li, J., Xiao, S., Zhang, Y., et al. (2016). A novel p38 MAPK indentified from Crassostrea hongkongensis and its involvement in host response to immune challenges. Mol. Immunol. 79, 113–124. doi: 10.1016/j.molimm.2016.10.001
Roux, P. P., and Blenis, J. (2004). ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 68, 320–344. doi: 10.1128/MMBR.68.2.320-344.2004
Ruiz-Ruiz, P. A., Hinojosa, I. A., Urzua, A., and Urbina, M. A. (2019). Anthropogenic noise disrupts mating behavior and metabolic rate in a marine invertebrate. J. Acoust. Soc. Am. Proc. Mtgs. Acoust. 37:040006. doi: 10.1121/2.0001302
San Miguel-Ruiz, J. E., Maldonado-Soto, A. R., and García-Arrarás, J. E. (2009). Regeneration of the radial nerve cord in the sea cucumber Holothuria glaberrima. BMC Dev. Biol. 9:3. doi: 10.1186/1471-213X-9-3
Schaefer, U., Voloshanenko, O., Willen, D., and Walczak, H. (2007). TRAIL: a multifunctional cytokine. Front. Biosci. 12:3813–3824. doi: 10.2741/2354
Simmonds, M. P., Dolman, S. J., Jasny, M., Parsons, E., Weilgart, L., Wright, A. J., et al. (2014). Marine noise pollution-increasing recognition but need for more practical action. J. Ocean Technol. 9, 71–90.
Sokolova, I., Evans, S., and Hughes, F. (2004). Cadmium-induced apoptosis in oyster hemocytes involves disturbance of cellular energy balance but no mitochondrial permeability transition. J. Exp. Biol. 207, 3369–3380. doi: 10.1242/jeb.01152
Solé, M., Lenoir, M., Durfort, M., López-Bejar, M., Lombarte, A., and André, M. (2013). Ultrastructural damage of Loligo vulgaris and Illex coindetii statocysts after low frequency sound exposure. PLoS One 8:e78825. doi: 10.1371/journal.pone.0078825
Tidau, S., and Briffa, M. (2016). Review on behavioral impacts of aquatic noise on crustaceans. J. Acoust. Soc. Am. Proc. Mtgs. Acoust. 27:010028. doi: 10.1121/2.0000302
Usheva, L., Vaschenko, M., and Durkina, V. (2006). Histopathology of the digestive gland of the bivalve mollusk Crenomytilus grayanus (Dunker, 1853) from southwestern Peter the Great Bay, Sea of Japan. Russ. J. Mar. Biol. 32, 166–172. doi: 10.1134/S1063074006030047
Van Damme, S., De Fruyt, N., Watteyne, J., Kenis, S., Peymen, K., Schoofs, L., et al. (2021). Neuromodulatory pathways in learning and memory: lessons from invertebrates. J. Neuroendocrinol. 33:e12911. doi: 10.1111/jne.12911
Vazzana, M., Ceraulo, M., Mauro, M., Papale, E., Dioguardi, M., Mazzola, S., et al. (2020). Effects of acoustic stimulation on biochemical parameters in the digestive gland of Mediterranean mussel Mytilus galloprovincialis (Lamarck, 1819). J. Acoust. Soc. Am. 147, 2414–2422. doi: 10.1121/10.0001034
Vivier, E., Raulet, D. H., Moretta, A., Caligiuri, M. A., Zitvogel, L., Lanier, L. L., et al. (2011). Innate or adaptive immunity? The example of natural killer cells. Science 331, 44–49. doi: 10.1126/science.1198687
Vlahopoulos, S., Boldogh, I., Casola, A., and Brasier, A. R. (1999). Nuclear Factor-κB–dependent induction of interleukin-8 gene expression by tumor necrosis factor α: evidence for an antioxidant sensitive activating pathway distinct from nuclear translocation. Blood 94, 1878–1889.
Wale, M., Briers, R., Bryson, D., Hartl, M., and Diele, K. (2016). “The effects of anthropogenic noise playbacks on the blue mussel Mytilus edulis (annual science meeting),” in Proceedings of the Abstract presented at 4th International Conference on The Effects of Noise on Aquatic Life, Dublin.
Wale, M. A., Briers, R. A., Hartl, M. G., Bryson, D., and Diele, K. (2019). From DNA to ecological performance: effects of anthropogenic noise on a reef-building mussel. Sci. Total Environ. 689, 126–132. doi: 10.1016/j.scitotenv.2019.06.380
Wale, M. A., Simpson, S. D., and Radford, A. N. (2013). Noise negatively affects foraging and antipredator behaviour in shore crabs. Anim. Behav. 86, 111–118. doi: 10.1016/j.anbehav.2013.05.001
Wang, J., Ren, R. M., and Yao, C. L. (2018). Oxidative stress responses of Mytilus galloprovincialis to acute cold and heat during air exposure. J. Molluscan Stud. 84, 285–292. doi: 10.1093/mollus/eyy027
Wang, Z., Wang, B., Chen, G., Jian, J., Lu, Y., Xu, Y., et al. (2016). Transcriptome analysis of the pearl oyster (Pinctada fucata) hemocytes in response to Vibrio alginolyticus infection. Gene. 575, 421–428. doi: 10.1016/j.gene.2015.09.014
Weise, C. M., Chen, K., Chen, Y., Kuang, X., Savage, C. R., Reiman, E. M., et al. (2018). Left lateralized cerebral glucose metabolism declines in amyloid-β positive persons with mild cognitive impairment. Neuroimage Clin. 20, 286–296. doi: 10.1016/j.nicl.2018.07.016
Woo, H., Kim, M. K., Park, S., Han, S. H., Shin, H. C., Kim, B. G., et al. (2021). Effect of Phlorofucofuroeckol A and dieckol extracted from ecklonia cava on noise-induced hearing loss in a mouse model. Mar. Drugs 19:443. doi: 10.3390/md19080443
Xu, K., Tang, Z., Liu, S., Xia, H., Liu, L., Wang, Z., et al. (2018). Effects of low concentrations copper on antioxidant responses, DNA damage and genotoxicity in thick shell mussel Mytilus coruscus. Fish Shellfish Immunol. 82, 77–83. doi: 10.1016/j.fsi.2018.08.016
Yu, P., Liu, Z., Wu, D., Chen, M., Lv, W., and Zhao, Y. (2018). Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat. Toxicol. 200, 28–36. doi: 10.1016/j.aquatox.2018.04.015
Zhang, M., He, P., Liu, P., Shen, Y., Qiao, G., Li, Q., et al. (2019). Acute toxicity of heavy metals to Onchidium struma under different salinities. Int. J. Agric. Biol. 21, 307–313. doi: 10.17957/IJAB/15.0895
Keywords: Onchidium reevesii, low-frequency noise, transcriptome analysis, mollusk, immune response, oxidative stress
Citation: Tu Z, Tang L, Zhang X, Jia J and Shen H (2021) Transcriptome Analysis of the Central Nervous System of Sea Slug (Onchidium reevesii) Exposed to Low-Frequency Noise. Front. Mar. Sci. 8:807489. doi: 10.3389/fmars.2021.807489
Received: 02 November 2021; Accepted: 03 December 2021;
Published: 29 December 2021.
Edited by:
Li Zhang, South China Sea Institute of Oceanology, Chinese Academy of Sciences (CAS), ChinaCopyright © 2021 Tu, Tang, Zhang, Jia and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heding Shen, aGRzaGVuQHNob3UuZWR1LmNu
 Zhihan Tu
Zhihan Tu Liusiqiao Tang1,2,3
Liusiqiao Tang1,2,3 Heding Shen
Heding Shen