
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 31 January 2022
Sec. Marine Evolutionary Biology, Biogeography and Species Diversity
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.800250
Understanding the diversity and ecology of deep-reef fishes is challenging. Due to intensive and widely dispersed sampling, the Greater Caribbean (GC) fauna of species found on shallow reefs is much better characterized than the fauna of deep-reef species restricted to mesophotic (40–130 m) and rariphotic (130–300 m) depths. Our knowledge about deep-reef fishes is based on ship-board sampling and the recent use of rebreather diving, remotely operated vehicles (ROVs), baited remote underwater videos, and crewed submersibles. Submersible research on GC deep-reef fishes began in the 1960s and has flourished over the last decade through research by the Smithsonian Institution’s Deep Reef Observation Project (DROP). Here we quantify the contribution of submersible research, particularly the surge by DROP, to our understanding of the diversity of the deep-reef fish fauna of the GC. We compared shallow- and deep-reef fish faunas of three GC sites subjected to DROP research to faunas of three sites without such research. DROP increased the size of the deep faunas at three islands ∼9-fold, and they have deep-reef faunas ∼2–4 times the size of those of the other three sites. Those deep-reef faunas have high proportions of small cryptobenthic fishes, which also represent a major component of shallow faunas. That research increased the rate of discovery (collection) of new species of deep-reef fishes ∼6-fold and accounts for 31% of the deep-reef species first discovered within the GC. Substantial numbers of new species at each of the three DROP islands were not found at the other two. This indicates that other parts of the GC likely harbor many undetected deep-reef fishes, and that the size of the deep-reef fauna of the GC is significantly underestimated. These results show that small research submersibles are versatile, highly productive tools for deep-reef studies. They allow long-duration dives at any depth, while offering unparalleled views of their surroundings to study the ecology of deep-reef fishes (e.g., DROP’s definition of the rariphotic assemblage from fish depth distributions). Submersibles can efficiently collect reef fishes of a broad range of taxa, ecotypes and sizes, leading to a more comprehensive understanding of the regional GC deep-reef fish fauna.
Obtaining comprehensive information on the diversity, geographical distributions, and ecology of fishes that live on tropical deep reefs is not an easy task and is one that has barely begun in the great majority of biogeographic regions. While many species of reef fishes commonly encountered on shallow reefs are restricted to those habitats, others commonly extend their depth ranges into or live entirely within habitats far deeper than are readily accessible by divers using open-circuit SCUBA. Further, many species of fishes that belong to families and genera typically found on shallow reefs are restricted to deep reefs, which harbor fish assemblages with different taxonomic and ecological properties to those on shallow reefs (Pyle, 2000; Pinheiro et al., 2016, 2020; Baldwin et al., 2018; Goodbody-Gringley et al., 2019; Stefanoudis et al., 2019). How much undiscovered diversity there is among the faunas of deep-reef fishes in different parts of the tropics is far from clear, although it evidently is substantial (Pyle, 2000; Baker et al., 2016).
Currently, four techniques are widely used throughout the tropics to study deep-reef fishes: crewed submersibles, remotely operated vehicles (ROVs), Closed-Circuit Rebreathers (CCR) used by technical divers and baited remote underwater videos (BRUVs), with submersibles having the longest history of such usage. Those techniques each have advantages and limitations in terms of cost, logistical complexity, mobility, the depths and duration of dives they support, and the types and efficiency of information collection they allow (see section “Discussion”).
The Smithsonian Institution’s (SI) research on deep reefs in the Greater Caribbean using submersibles began with the Deep Diver in the late 1960s. Subsequently, SI owned the Johnson Sealink Submersible JSL-1 between 1971 and 73, until that program was transferred to the Harbor Branch Oceanographic Institute (HBOI). SI research with Deep Diver in the Bahamas included the first collection of type specimens of a new species of deep-reef fish by a submersible anywhere in the world (Gramma linki by Starck and Colin, 1978). SI continued studies of deep-living invertebrates using the JSLs through the late 1980s. After a 40 year hiatus, SI-sponsored research on deep-reef fishes using submersibles was renewed during the 2010s through the DROP (Deep Reef Observation Project) research program. DROP has used the privately owned submersibles Curasub at Curacao, Bonaire, Dominica and Sint Eustatius (Statia), and Idabel at Roatan. Curasub can accommodate a crew of five, including the pilot, with two observers at the front dome. It has an operating depth limit of 310 m, with dives up to 7.7 h duration. Idabel supports a pilot, plus two observers at the front dome. It has an operating depth limit of 915 m and can make dives up to 17 h duration. Both those submersibles were equipped with fish-catching devices that inject anesthetic into the substratum and use a suction tube to deposit them into a chamber on the submersible (see Figure 1), from which they are retrieved at the end of a dive for study by DROP fish biologists.
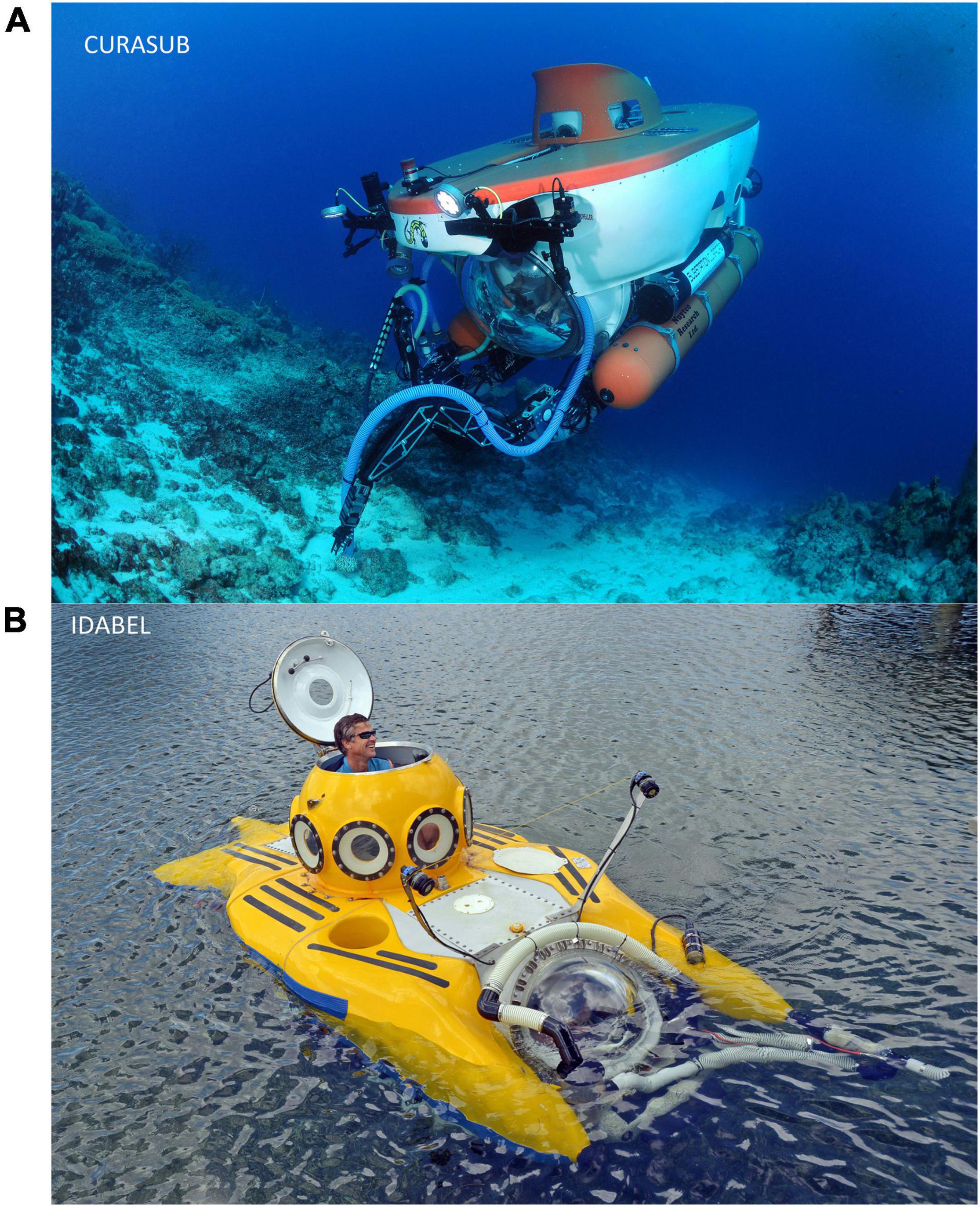
Figure 1. The two submersibles used by the Deep Reef Observation Project (DROP) to conduct the research and collections described in this paper. (A) Curasub. The S shaped suction tube of that submersibles fish-catching system is visible on the near front side of the vessel, with the slender S shaped tube that delivers anesthetic in the background. Photo: Barry Brown. (B) Idabel. Idabel was temporarily equipped with a fish collection system equivalent to that on Curasub during DROP research. That system is visible as the cluster of white tubing around the front dome and extending forward of the far front side of the submersible, with the owner and pilot Karl Stanley, maneuvering Idabel on the surface. Photo: DR.
In this paper we provide an overview of the contribution of submersible research, particularly that by DROP, to knowledge of the diversity of deep-reef fishes in the GC. Specifically, we (i) compare variation in the taxonomic structure of local shallow and deep faunas at six well-studied sites that vary in levels of submersible research, and (ii) examine the contribution of submersible research to the discovery and description of new species of deep-reef fishes. There has been extensive documentation of the faunas of shallow-water reef fishes at all six sites, and three of those have been subject to DROP submersible research that involved targeted collections of deep-reef fishes to provide information on their identity. That research also involved surveys of depth distributions of members of deep-reef fish assemblages, i.e., variation in abundance of individual species across different strata of their depth ranges. Cryptobenthic reef fishes, particularly small species, represent a major component of both local and regional reef-fish faunas throughout the tropics (Depczynski and Bellwood, 2003; Brandl et al., 2018). Such fishes cannot be thoroughly documented by visual surveys alone due to their cryptic form, often small size, coloration and behavior, and their use of inaccessible microhabitats (Ackerman and Bellwood, 2000; Smith-Vaniz et al., 2006; Alzate et al., 2014). Hence visual surveys by ROVs, BRUVs, submersibles and CCR can be expected to report fewer cryptobenthic species, particularly small species, than do surveys that also involve intensive collecting of such species using ichthyocides or anesthetics to extract them from hiding. Until the present series of DROP studies this difference has not been effectively documented. Collectively these comparisons allow us to assess the contributions of submersible research by DROP and others to understanding of the diversity, ecology and biogeography of deep-reef fishes in the Greater Caribbean (GC).
In the Greater Caribbean, coral- and rocky reefs down to depths of ∼250–300 m have reef-fish faunas dominated by members of typical shallow-reef families of bony fishes. As it currently stands this fauna includes 992 species belonging to 342 genera in 84 families (Robertson and Tornabene, 2021; Supplementary File 1 and Supplementary Table 1). We divided those species into two groups: shallow-reef and deep-reef forms based on their depth ranges relative to the 40 m line below which deep-reef habitats occur. In this paper we include among the 850 shallow-reef species not only those restricted to depths above 40 m but also others that are r found at depths above and below 40 m, sometimes well below that depth. Another 142 species in those same families we classify as deep-reef fishes because they are restricted entirely or largely to depths below 40 m depth (see Robertson and Tornabene, 2021 for an updated regional inventory used here). These reef-associated fishes include not only benthic and demersal species found on hard reef substrata, but also pelagic fishes and benthic and demersal species that live on soft bottoms within and immediately around the fringes of reefs. Those pelagic fishes facultatively associate with reefs but do not rely on them for shelter as they live in the water column. Benthic species are restricted to using benthic habitats (e.g., flat-fishes, eels, most gobies), while demersal species regularly use the water column as well as the substratum (e.g., snappers, grunts, and wrasses). Two further groups considered here are Cryptobenthic species and Core Cryptobenthic Reef-Fish Families (CCRFs). Cryptobenthic species are visually cryptic and typically small. CCRFs are taxonomic families that contain many small cryptobenthic species. Supplementary File 1 contains a more detailed description of characteristics of that regional fauna as treated here.
The six sites included in this study are Alligator Reef in the Florida Keys, Bermuda, Curacao, Roatan, Sint Eustatius (Statia), and St. Croix. DROP research was conducted at Curacao, Roatan, and Statia. All six sites have well characterized shallow reef-fish faunas that have been extensively studied using SCUBA investigation. All except Alligator are individual islands scattered between the northern and southern limits of the Greater Caribbean, which extends from North Carolina and Bermuda south through the Gulf of Mexico, the West Indies and Caribbean to ∼Guyana. Supplementary File 2 contains detailed description of the islands, their environments and the research that led to the construction of a checklist of reef-associated fishes at each, including that on deep-reef fishes using submersibles, ROVs, CCRs, and BRUVs. Table 1 contains a summary of such information, as well as a summary of the level of usage of the ichthyocide Rotenone to extract shallow cryptobenthic fishes from their hiding places and elucidate their diversity.

Table 1. Characteristics of the six study sites and research activities on deep-reef fishes at each.
We characterized the history of the contributions of different collecting methods toward the discovery and eventual description of new species of deep-reef fishes. To do this we recorded the year of collection of the oldest specimen in the type series (i.e., the initial discovery) of each species of deep-reef fish. For each species we also noted whether the type series consisted of or included specimens collected by submersibles or was comprised solely of specimens collect by other means (SCUBA, CCR, trawls, dredges, traps, stomach contents etc.). Since, in many cases information on the collection year is lacking for specimens collected during the nineteenth century we used the date of description rather than collection for all species named before 1900. Information on the collection date is also lacking for two species collected during the early twentieth century, and we used the description date for those as well (see Supplementary Table 3). Our tally of those deep-reef fishes includes 18 species collected by DROP that have yet to be described: 10 species of Gobiidae, two of Grammatidae, three of Serranidae, and three of Labridae.
The number of species in the regional reef-fish fauna currently stands at 992, belonging to 342 genera and 84 families (Supplementary Table 1; and see Robertson and Tornabene, 2021). Less than 10% of those are pelagic species, only one of which, Seriola fasciata, we classify as a deep-reef species. Demersal species constitute only about a third of the non-pelagics, and benthic species are by far the largest group, at almost 2/3 of the non-pelagics. The vast majority (almost 99%) of benthic species are cryptobenthic, and a little more than 40% of all non-pelagic species are small cryptobenthics. Almost half the non-pelagic species belong to Core Cryptobenthic Reef Fish (CCRF) families (as defined by Brandl et al., 2018; see Supplementary File 1).
Shallow forms (including pelagics) are represented by 850 species from 301 genera and 82 families. They constitute 85% of the non-pelagic species, and proportions of the different subgroups of such species (demersal, benthic, cryptobenthic, and CCRF) are mirrored in the entire fauna.
Deep non-pelagic forms include 141 species from 67 genera and 26 families and constitute 14.3% of the entire fauna and 15.4% of the non-pelagic sector. Deep species are represented in 20% of the genera and 37% of the families. Among those genera, 39 (11.4%) of the total have no shallow members, 28 (8.2%) have both and the remaining 80.3% have only shallow members. Those 39 exclusively deep-reef genera represent 58% of the genera that have deep species among their members.
Collectively the six sites harbor the vast majority of GC reef fishes, almost 80% of the total and at least 70% of each of the different ecotypic subgroups (Supplementary Table 1). They also hold similarly high percentages of all ecotypes of shallow species, and slightly lower percentages of different ecotypes of deep species. In all three cases (total, shallow and deep faunas) the combined fauna includes a higher percentage of the regional demersal fauna that for equivalent benthic subgroups.
The faunas of the six sites vary 1.5-fold in size and include 35–53% of the entire regional fauna, 47–67% of the regional pelagic species, 51–68% of the regional demersal species, and 23–45% of the regional benthic species (Supplementary Table 1). The smallest fauna is at Statia, the second smallest at Bermuda and the largest at Curacao. The sizes of the entire non-pelagic faunas and of the shallow-reef fish faunas of the four sites other than Bermuda and Statia are within 10% of each other (Figure 2 and Supplementary Table 1).
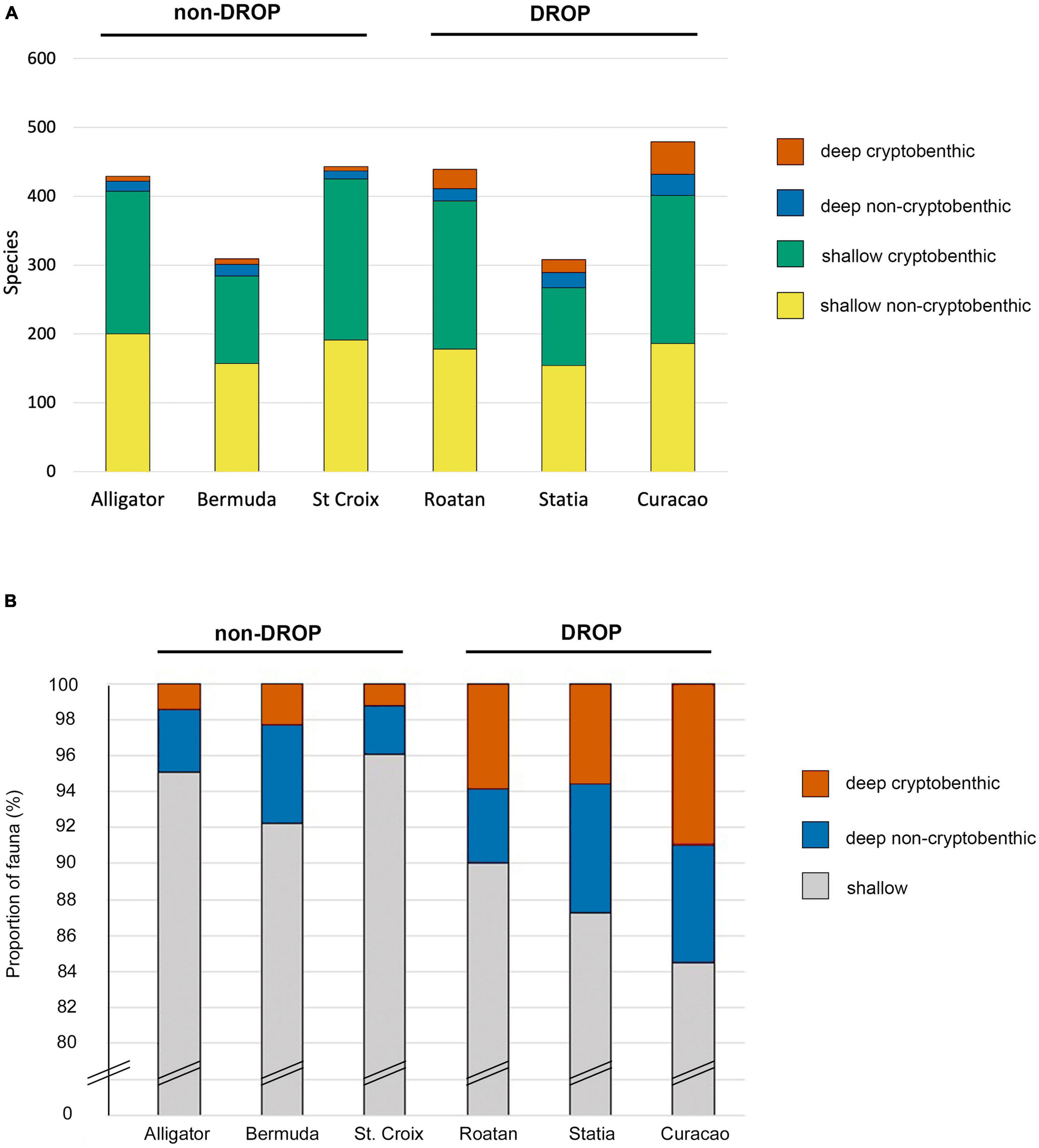
Figure 2. Abundance of deep and shallow cryptobenthic fishes in the faunas of six sites. (A) Numbers of species of cryptobenthic and other non-pelagic species in the shallow and deep sectors of the faunas. (B) Relative abundance of cryptobenthic vs. other non-pelagic species in the deep faunas of six islands. Data in Supplementary Table 1.
As with the regional fauna most species in each of the six site faunas are shallow forms (Figure 2 and Supplementary Table 1). The sizes of the shallow faunas at two sites lacking DROP research (Alligator and St Croix) were slightly higher than at any site with such research. In contrast, the deep-reef faunas of the three DROP sites were 1.6–4.3 times the absolute size and 2–4 times the relative size of those at the non-DROP sites (Figure 2 and Supplementary Table 1) and included 29–55% of the regional deep-reef fauna. Statia, with the smallest total and shallow faunas, had a deep fauna much larger than that of each of the three sites that lacked DROP research.
The relative proportions of different subgroups of deep species (demersal, benthic, cryptobenthic, CCRF) parallel those among shallow species (Supplementary Table 1). Compared to the regional fauna the faunas of the six sites have marginally higher percentages of pelagic species, and marginally lower percentages of non-pelagics (Supplementary Table 1). Relative to the regional fauna of non-pelagics the six sites have higher percentages of demersal species, and lower percentages of benthic, cryptobenthic, small cryptobenthic, and CCRF species (Supplementary Table 1).
The range of variation in relative abundances of the different ecotypes of species in the entire (shallow + deep) faunas of the DROP sites is similar to that among the non-DROP sites. However, the percentages of demersal species are highest and the percentages of benthic species and of each benthic subgroup are lowest at the two sites with the smallest faunas: Bermuda and Statia (Supplementary Table 1).
There are marked differences in the deep, non-pelagic faunas of the sites with DROP research vs. those without (Figure 2 and Supplementary Table 1). The percentage of the site-fauna represented by deep fishes is 1.3–3.8 times higher in the three DROP sites than the non-DROP sites. The deep-reef component is smallest at the two non-DROP reefs (Alligator and St. Croix) with the least amounts of submersible research. The three non-DROP sites have distinctly higher proportions of demersal species in their deep faunas than do either the DROP sites or the regional fauna. Further, the deep faunas of the three DROP sites have proportions of benthic, cryptobenthic and CCRF species similar to the regional fauna, proportions substantially higher than at the three non-DROP sites (Supplementary Table 1). Small cryptobenthic and CCRF species make up large proportions of the deep fauna at DROP sites but are almost absent from the non-DROP sites (Supplementary Table 1.) The rankings of the different ecotypic categories of deep non-pelagics are also much more similar to the regional pattern at the DROP sites than at the non-DROP sites (Supplementary Table 1).
There has been no ichthyological research using submersibles, ROVs or CCRs at Alligator Reef. Fifteen (68%) of the 22 deep-reef fishes known at that reef are demersal, with only one small cryptobenthic species.
Submersible research between 1983 and 2016 at Bermuda (Smith-Vaniz et al., 1999; Goodbody-Gringley et al., 2019; Stefanoudis et al., 2019), although not specifically aimed at species discovery, recorded three shallow species on deep reefs and 10 of the 25 known deep-reef species. Nine of the latter were new records, seven found in 1983–1997 and two in 2016. Three CCR studies in the 2010s (Pinheiro et al., 2016; Goodbody-Gringley et al., 2019; Stefanoudis et al., 2019) recorded 50 shallow species below ∼40 m and three deep-reef species, none of them new records. Thus, most information from the CCR studies was on shallow species that ranged into deep-reef waters below 40 m. Due to data-collection aims those CCR records also were heavily skewed toward demersal, readily visible species at all study depths and included very few cryptobenthic and small species. In contrast, benthic species represent almost half the non-pelagic shallow species known from Bermuda.
Two JSL-II submersible dives in 1985 at 30–600 m (Nelson and Appeldoorn, 1985; García-Sais, 2005) produced records of two of 19 known deep species, both of them new records, and 26 shallow species. Thus, most submersible information is related to shallow species that extend their depth ranges down into deep-reef habitats.
Pinheiro et al.’s (2016) CCR work, which reached substantially greater depths than at Bermuda (130 m vs. 80 m), recorded 55 shallow species and 10 deep species, 12.8% of the known deep species in the fauna. Those CCR records were heavily skewed toward demersal, readily visible forms of both shallow and deep species, whereas benthic and cryptobenthic species constitute well over half of the known shallow and deep species at that island. Curasub dives at Curacao by DROP produced records (most with specimens or photographs acting as vouchers) of 92.3% of the 78 deep-reef fishes known from Curacao. Curasub records of shallow species on deep reefs were similar in number to the CCR results and also skewed toward demersal species (70%), although not as strongly as in the CCR study (89%).
DROP (Idabel) dives at Roatan produced records of 66 species of shallow fishes and 41 deep species, 17 and 89%, respectively, of species in those groups known from Roatan. At Statia, Curasub activity produced records of 68 shallow species (25% of the that group) and 35 (85%) of the deep-reef species. At both sites, the diversity of shallow species recorded by DROP was skewed toward demersal forms (relative to their diversity in the fauna as a whole) whereas the deep species included similar abundances of demersal and different benthic forms, in proportions reflective of their proportions in the entire site fauna.
New records of species by DROP represent 85–90% of the known deep-reef fish inventory at each of Curacao, Roatan and Statia, and over 90% of the combined total (Figure 3). New species previously unknown to science that were discovered by DROP at those three islands account for about 40% of the deep-reef species recorded by that research at each island separately and in combination. Further, those three sites each harbor significant numbers of species not known from the other two sites (Figure 3). Those unshared new DROP records represent 19–46% of that group of species at each island. While the large proportion of such species at Curacao, which received the great majority of DROP submersible research, reflects the much larger known deep-reef fauna at that island, the faunas at Roatan and Statia, which are 50–60% of the size of the Curacao fauna, each have significant numbers of unshared species. That pattern also applies to new species collected by DROP that were not previously known to science at each island, as 31–39% of them are unshared. Collectively those 19 unshared “new species” represent 19% of all the deep reef fishes known from the three sites and 21.6% of the new records obtained by DROP.
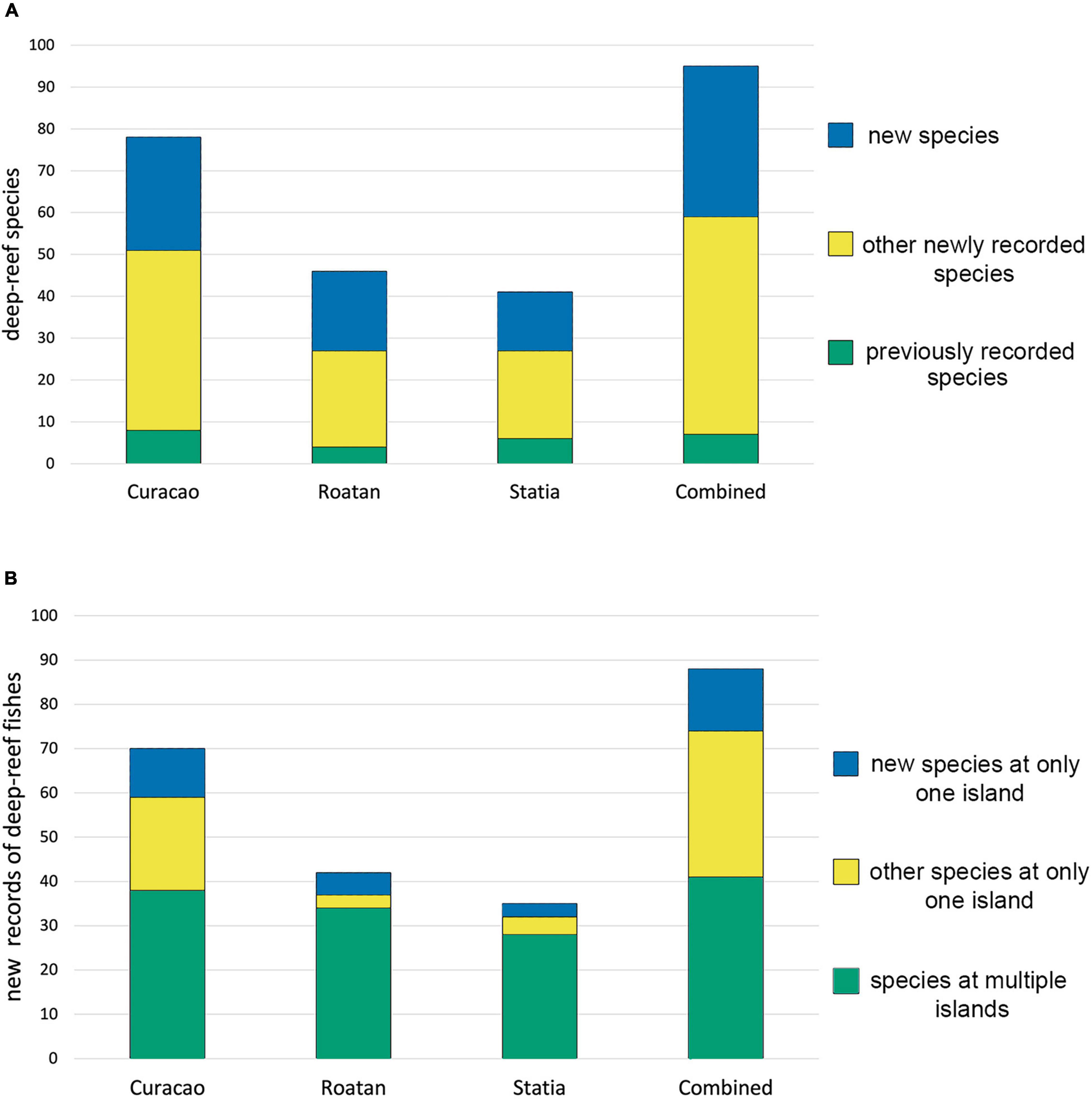
Figure 3. Contribution of DROP submersible research to knowledge of the deep-reef faunas at three sites. (A) Numbers of species recorded by DROP that were not recorded by other research, and the number of those that are new (i.e., previously unknown) species. (B) Percent of new and already named species present at only one of the three islands vs. those at multiple islands.
The 50 year era of submersible research on reef fishes in the Greater Caribbean started in the second half of the 1960s and was preceded by about 15 years when SCUBA came into use for studying reef fishes. In the ∼120 years between the first description of a species that is part of the Greater Caribbean deep-reef fish fauna and the start of the SCUBA era traditional methods of collection (trawls, nets, dredges, grabs, fish floating at the surface, stomach contents of predators, and unknown) led to the description of one third of the deep-reef fishes known from the region that were described based on type specimens collected within that area. Deep SCUBA diving accounted for one third of the new deep-reef species added during the ∼15 year SCUBA era. During the first 45 year of the submersible/CCR era submersible collections produced about one fifth of the new additions and CCR ∼2%. Subsequently, the 9 years of the DROP program produced types of 38.5 new species, 32% of all those ever collected within the region, and almost 90% of those collected anywhere during that period. Overall, submersible discovery accounted for 35.6% of the types of deep-reef species collected in the Greater Caribbean and 60% of all species types collected there during the submersible era.
The rate of accumulation of new-species descriptions of deep-reef fishes based on type specimens collected in the region was relatively low before the advent of SCUBA, i.e., before 1960. It roughly doubled during the SCUBA and JSL periods (1960s–2010), then increased about sixfold due to DROP collections during the last decade (Figure 4).

Figure 4. The historical dynamics of discovery of deep-reef fishes known from the Greater Caribbean. (A) Number of non-pelagic species discovered (i.e., first type specimens collected) per decade. (B) Cumulative discovery of those three classes of deep-reef fishes.
Collection of type specimens of deep-reef fishes by non-traditional methods, including SCUBA, CCR and submersible, accounted for 37.6% of all non-pelagic deep-reef fishes known from the Greater Caribbean, with 84% of those by submersibles (Supplementary Table 4). The great majority of type specimens collected by submersibles were small, cryptobenthic species belonging to CCRFs. In contrast, other, traditional methods produced most of the types of demersal species and much lower proportions of CCRF and, particularly, small species. The numbers of species attributable to diver collections (SCUBA and CCR) are small but include similar proportions of ecotypes to those collected by submersibles (Supplementary Table 4). The bias toward small species and CCRF forms was present among type specimens of deep-reef species collected within the Greater Caribbean, but not among those collected outside the region (Table 2), almost all of which were collected by traditional methods. Even though traditional methods produced almost twice as many type specimens as did submersibles, submersibles were overwhelmingly important in producing type specimens of small and CCRF species, accounting for double the proportion of those obtained by traditional methods. That difference was achieved despite the much greater difference in time over which traditional methods have produced types (>190 years) vs. the duration of submersible operations (51 years) (Figure 4).
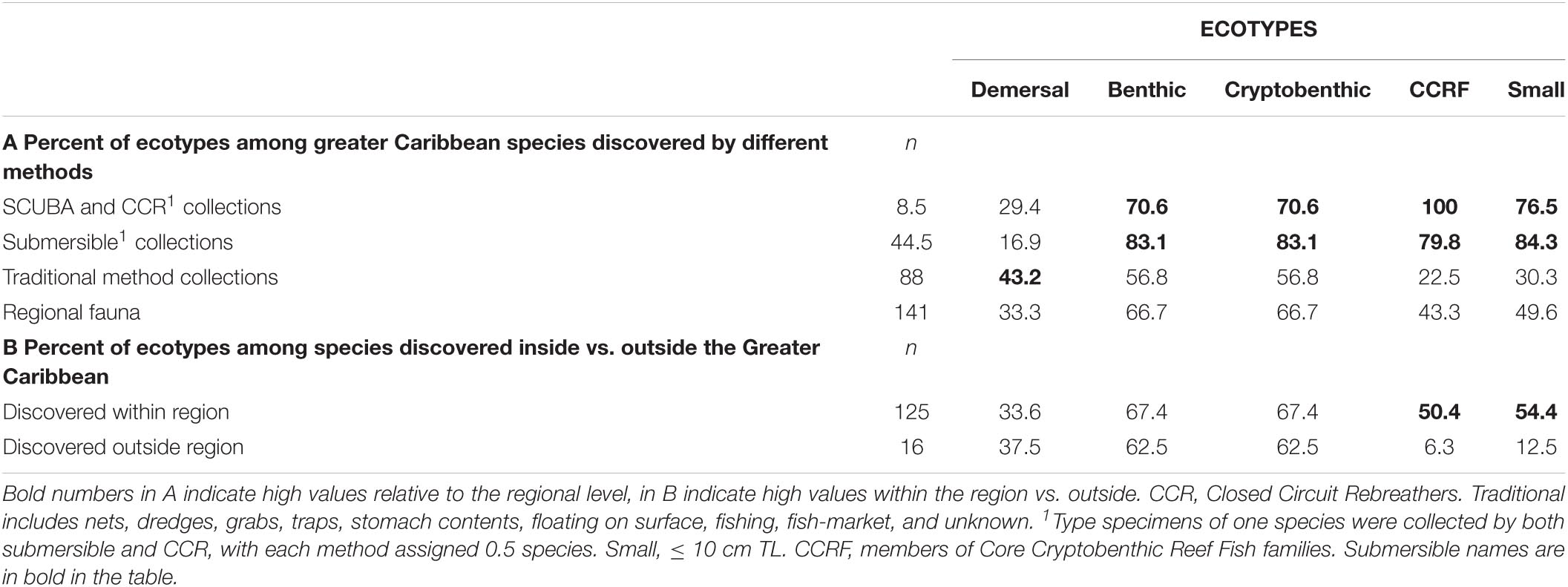
Table 2. Relative abundance of different ecotypes of non-pelagic Greater Caribbean deep-reef fishes collected by different methods in and outside the Greater Caribbean region.
Collectively the reef-fish faunas of the six sites include the great majority (almost 80%) of the regional fauna and only slightly smaller proportions of its different ecotypic faunal components. Thus, they provide a comprehensive overview of the regional GC fauna of reef fishes. Some of the 1.6-fold variation in the size of the known fauna at the six study sites can be attributed to variation in sampling effort. Statia, where there has been no ichthyocide collecting of shallow reef fishes, has a deficit in numbers of small, shallow cryptobenthic species that such collecting produces (Robertson et al., 2020). Because many such species were detected by such sampling on Saba Bank, only 20 km from the Statia shelf (Williams et al., 2010), Statia likely has a larger fauna of small, shallow cryptobenthic species. Bermuda, with a fauna very similar in total size to that of Statia, has a much larger habitat area and greater habitat diversity than Statia. Bermuda also has a much more extensive history of research, much of it involving rotenone sampling, on its marine fishes (Table 1; Smith-Vaniz et al., 1999; Smith-Vaniz and Collette, 2013). The relatively depauperate nature of the Bermuda reef-fish fauna is well known (Smith-Vaniz et al., 1999) and is attributable to a combination of its high-latitude location (it is the world’s most northerly coral reef) producing low-temperature limitation of tropical species, isolation limiting dispersal to the island from the rest of the GC, reduced habitat diversity and extinction of temperature-sensitive species during cold periods of glacial peaks (Smith-Vaniz et al., 1999).
Among the remaining four sites the sizes of the entire and shallow faunas of non-pelagic species are within 10% of each other (Figure 2). All those sites have been extensively sampled for shallow reef fishes, particularly small cryptobenthic forms, and similarities in their diversity and proportional abundances indicate that those sites have been effectively sampled. From this, we expect that any well sampled shallow site in the Greater Caribbean that has high habitat diversity will have a fauna about half the size of the regional fauna, with shallow benthic ecotypes predominating.
In contrast, the deep-reef faunas of the three DROP sites were 1.6–4.3 times the absolute size and 2–4 times the relative size of those at the non-DROP sites (Figure 2). Thus, while the shallow faunas may have been well sampled in all sites except Statia, the absolute and relative sizes of the deep-reef component of the site faunas was only revealed by DROP sampling. The fact that the deep fauna at Curacao is almost twice the size of those currently known from Roatan and Statia can be largely attributed to the level of DROP submersible research at Curacao being 6–10 times greater than at those other two sites. Among the three sites lacking DROP research, Bermuda, despite its relatively small entire and shallow faunas, has the largest deep-reef fauna (Figure 2), likely a reflection of more submersible research there than at either Alligator reef or St. Croix.
The ecotypic composition of the deep faunas at the six sites also reflect levels of submersible activity: the three DROP sites have much higher numbers and proportions of benthic, cryptobenthic, CCRF, and small species than the non-DROP sites, due to a focus of research by DROP on those hard-to-see and hard-to-sample groups that are major contributors to both the diversity and productivity of reef-fish assemblages (Brandl et al., 2018, 2019).
Usage of both submersible and CCR research on deep-reef fishes at two sites allows a rough comparison of their results, the only situation in which it is possible to directly compare research production by those methods in the Greater Caribbean. Submersible research at Bermuda (Smith-Vaniz et al., 1999) produced new records of 31% of the known deep-reef species. CCR studies there that were aimed at establishing the depth-distributions of readily visible (largely demersal) species produced very few deep species of any type and no new records of identifiable named deep-reef species. The CCR studies were limited to a maximum depth of 80–100 m, and thus the vast majority of the species included in those studies were shallow types, and almost all were demersal, readily visible species. However, one CCR study (Goodbody-Gringley et al., 2019) did note the presence of unidentified deep-living gobies and an apogonid, which are cryptobenthic types.
CCR work at Curacao (Pinheiro et al., 2016) reached substantially greater depths than at Bermuda (130 vs. 80 m). It recorded 13.0% of the known deep component. As at Bermuda, that study focused on demersal, readily visible forms, of both shallow and deep faunas. DROP’s Curasub work there recorded > 90% of the deep reef fauna, with 90% of them new records. Submersible records of shallow species on deep reefs at both Bermuda and Curacao were similar to the CCR results and also skewed strongly toward demersal species. DROP submersible dives at Roatan and Statia produced records of 17 and 25% of the shallow species and 89 and 85% of the known deep species, respectively. As at Curacao, the diversity of shallow species recorded by DROP at Statia and Roatan was skewed toward demersal forms (relative to their diversity in the fauna as a whole) whereas the deep species included similar proportional abundances of demersal and benthic forms to those in the entire site faunas.
In general then, shallow species found on deep reefs by both CCR and submersibles were mainly demersal, readily visible forms although proportions of benthic and cryptobenthic were somewhat higher among the submersible records at two of three islands. This likely reflects (i) differences in the amounts of time spent on deep reefs by the studies using time-limited CCR vs. submersibles and (ii) differences in the aims of the studies in which they were used: rapid assessments of change in community composition with depth in the CCR studies, vs. detailed assessment of diversity and ecotypes of fishes across deep reefs as well as observations on depth-distributions in the submersible studies. However, submersibles are at least as effective as CCR diving at revealing the diversity of species and ecotypes among both shallow- and deep-reef fishes and do so too much greater depths than CCR is capable of.
The onset of the submersible era of research on Greater Caribbean deep reefs in the mid-1960s produced large changes in the dynamics of discovery of deep-reef fishes. The 45 year of activity of submersibles prior to the advent of DROP produced about one fifth of the new species discovered in the region during that period (Figure 4). The 9 years of DROP research, which was focused on revealing the diversity of those fishes, resulted in almost 90% of the new species discovered during that period and almost one third of all deep-reef species ever discovered to date within the Greater Caribbean. In contrast to research in the Indo-Pacific (e.g., Pyle, 2000; Pinheiro et al., 2019b; Shepherd et al., 2020; Tea et al., 2020), submersibles have been much more active than CCRs in the process of diversity discovery in the Greater Caribbean. Again, to some extent, this reflects differences in the aims of studies using those two techniques in the GC, although large differences in maximum effective working depths and bottom times of submersibles vs. CCR are also involved.
The rate of discovery of new species of deep-reef fishes within the Greater Caribbean roughly doubled over the historical level following the advent of SCUBA research (Figure 4). While that rate did not increase during the period of JSL activity, there was a huge (∼6 fold) jump in the number of species discovered during the recent, much shorter period of DROP’s submersible research. This provides a very clear demonstration of the lack of information about such fishes before that work began, and the impact on the level of knowledge by submersible collecting aimed at comprehensively documenting the taxonomic and ecotypic diversity of deep-reef fishes.
Information about collection of type specimens by traditional methods (nets, traps, fishing etc.) and submersibles have produced information about the diversity of different ecotypes of deep-reef fishes useful for the assessment of the value and effectiveness of submersible research (Supplementary Table 4). Traditional methods produced most of the types of demersal species, which are more accessible to capture by such methods. In contrast, the great majority of types collected by submersibles have been small, cryptobenthic species, most of which belong to CCRFs. That collecting has clearly demonstrated that assemblages of deep living reef fishes have a similar structure in terms of the relative abundance of different ecotypes of demersal and benthic forms to assemblages of shallow living species. However, while the two assemblages may be derived from the same families, most of the genera of fishes that have deep-reef species also lack shallow-reef species. Thus, the deep-reef fauna has special taxonomic characteristics at both the species- and higher taxonomic levels.
Submersible research by DROP has greatly expanded knowledge of deep-reef fish faunas, not only at Roatan, Statia, and (especially) Curacao, but also in the region as a whole. New records of species by DROP represent ∼90% of the known deep-reef fish inventory at each island (Figure 3). New species that were discovered by that DROP research account for about 40% of the deep-reef species recorded by that research at each island separately and the three islands in combination. Distributing DROP’s research effort between these three widely dispersed Caribbean islands demonstrates that, even though one intensively studied island (Curacao) hosts a substantial percentage of the regional fauna, one island alone cannot provide a clear understanding of the regional diversity of deep-reef fishes.
The intensively sampled Curacao fauna includes large percentages of the major components of the regional fauna: 52% of the non-pelagic shallow-reef fauna and 55% of the deep-reef fauna. At present, the regional fauna of reef fishes is known to include 15.4% deep species. The deep-reef fauna at Curacao represents 16.3% of the entire local fauna, slightly greater than that regional level. There are various reasons to believe that the regional deep-reef fauna is distinctly larger than 15%, the larger relative size of the Curacao deep-reef fauna among them.
First, local assemblages like those at individual islands are unlikely to contain as high a percentage of deep species as the regional fauna because the latter represents an aggregate of many local faunas. Because many species have small geographic ranges, each local fauna will include only a portion of the region’s deep-reef species that have small ranges. Based on the data at hand on geographic distributions, about 1/3 of shallow reef fishes have small ranges, as do a similar proportion of deep-reef species. For CCRFs, which DROP sampling has made increasingly clear represent a major component of the regional deep reef fauna, those rates are 50% or more for both shallow and deep species. Thus, although the geographical extent of sampling for deep-reef species is much less than it is for shallow reef fishes, many deep-reef species may well have small ranges and are not present in many local faunas. Hence, the aggregated proportion of the regional reef-fish fauna represented by deep species should be substantially greater than the Curacao level of 16.3%.
Second, among 25 of the 82 families that have reef-fish representatives there are 122 deep-living species that currently are not known to associate with reefs (see Robertson and Tornabene, 2021). The lack of a reef association in some of those species may well reflect inadequate sampling, due to the difficulty of studying reefs, deep or shallow, by traditional methods such as trawls and dredges. Among those 122 species, ∼40% have small known ranges, reducing the possibility of detection of any reef association without more widespread sampling than currently is the case.
The shallow component of the Greater Caribbean regional reef-fish fauna is much better defined than the deep component. This is attributable to the greater intensity and much wider distribution of sampling for shallow reef fishes in different parts of the region, vs. the geographically much more constrained, and typically much less intensive sampling for deep reef fishes. DROP sampling at Curacao, Roatan and Statia detected significant proportions of new species at each site that are not shared with either of the other two sites. Even Roatan and Statia, which have received much less DROP sampling and have much smaller known deep-reef faunas than Curacao have substantial proportions of new deep species that are not known from the much better sampled Curacao fauna (Figure 3). Moreover, even at heavily sampled Curacao, DROP is still discovering new deep-reef species after more than 100 research dives at that same discovery site (Tornabene et al., 2016a). The numbers of new deep species discovered at those three sites, the extent to which different examples are present at only one of those sites, the proportions of deep-reef species that have small ranges, and the numbers of deep species that currently are not known to associate with reefs but could do so, all collectively point to the deep-reef fish fauna of the Greater Caribbean being considerably larger than its current known size and the proportional representation of the regional deep fauna being distinctly larger than its present 15%.
The age of submersible research on deep-reef fishes began with observations in 1965 from the submersible Asherah down to 192 m in Hawaii by Strasburg et al. (1968). Research on deep-reef fishes in the Greater Caribbean (GC) using submersibles started in 1968, with observations of depth ranges and depth distributions similar to those made in the central Pacific (Colin, 1974, 1976; Starck and Colin, 1978). Colin (1974, 1976) also observed but did not collect four “unknown” species, a pomacentrid, two grammatids and a goby. Three of those were subsequently described (Chromis vanbebberae, Gramma linki, and Lipogramma evides), while the goby most likely is Priolepis hipoliti, which is found on both shallow and deep reefs. Early submersible research in the GC included the first collection of type specimens of a new reef fish by lock-out divers supplied with mixed-gas through an umbilicus from a submersible (Cook, 1982), as was done from the Deep Diver by Starck and Colin (1978). In 1982 the two Johnson Sea Link submersibles (JSL-I and JSL-II) were equipped with an effective fish-catching device that combined the ability to inject liquid ichthyocide into reef substrata to flush out specimens with a suction tube that drew the immobilized fish into a container attached to the submersible (Gilmore, 2016).
Research on a broad range of geological and biological phenomena involved many thousands of dives made by the JSLs over three decades at widely scattered sites in the Greater Caribbean, as well as the Galapagos, the Mediterranean and Brazil (HBOI, 2021). The JSL program ended in 2010 when the JSL-II and its support ship were sold (Gaskill, 2011; Reed and Frank, 2011). JSL research on deep-reef organisms, including fishes in some cases, in the GC occurred in the southeastern US (Parker and Ross, 1986; Gilmore and Jones, 1992; Quattrini et al., 2004; Reed et al., 2005a,b; Ross and Quattrini, 2009), the Gulf of Mexico (Shipp et al., 1986; Quattrini et al., 2004; Sulak et al., 2007; Locker et al., 2016), the Bahamas (Gilmore and Jones, 1988), Caribbean Mexico (Tyler et al., 1992), Belize (Gilmore, 1997), Cuba, Jamaica, Puerto Rico, and the US Virgin Islands (García-Sais, 2005), Barbados, Bequia, Mustique, Union Island, St Lucia (Tyler et al., 1992), and Bonaire and Curacao (Reed and Pomponi, 2000).
Observations have also been made on depth distributions of GC deep-reef fishes using various other submersibles as well as the JSLs. This included studies in the Bahamas by the Deep Diver in 1968 (Starck and Colin, 1978) and the Alvin in 1975 (Colin, 1976), by the Nekton Gamma off Jamaica and the Nekton Beta off Belize in 1972 (Colin, 1974), as well as the Nekton Gamma (and JSL-II) off the southeast United States in 1980 (Parker and Ross, 1986) and the PC-8 at Jamaica in 1984 (Itzkowitz et al., 1991). In the early 2000s the submersibles Deepworker 2000 and Deep Rover were used for observational research on deep-reef fishes in the northwest Gulf of Mexico and Dry Tortugas in the southeast of that gulf (Weaver et al., 2006a,b). Submersible research at Bermuda that provided information on occurrences of deep-reef fishes began with that by the Geo in 1983 and the SDL-1 in 1997, with the latter collecting a few fish specimens (Smith-Vaniz et al., 1999). Collection of data by DROP on depth distributions of deep-reef fishes at Curacao led to the definition of the rariphotic zone (130–300 m) below the mesophotic (40–130 m), with both those zones having distinctive faunas (Baldwin et al., 2018). In 2015, the submersibles Nemo and Nomad were used to study depth distributions of members of reef-fish assemblages at depths between 150 and 300 m at Bermuda, which, in conjunction with collection of data in fishes in shallower water by technical divers and BRUVs, confirmed the existence of a rariphotic zone there (Goodbody-Gringley et al., 2019; Stefanoudis et al., 2019).
ROVs feed video images to surface operators through a tether from depths as great as 4,000 m (e.g., Weaver et al., 2002; Quattrini et al., 2017). They have been extensively used for observational studies involving deep-reef fishes in the southeast United States, the Gulf of Mexico, Cuba, the Puerto Rico plateau and St Croix, and Saba Bank, particularly during the late 1990s and 2000s (Weaver et al., 2002; Reed et al., 2005a,b; Quattrini and Ross, 2006, Quattrini et al., 2015, 2017; Toller et al., 2008; Bryan et al., 2013; Reed et al., 2015, 2017, 2018). This technology has also been used to study mesophotic fishes in Brazil (Magalhães et al., 2015). An ROV equipped with a fish-catcher equivalent to that used on the JSL submersibles was used to collect deep-reef fishes in the northern Gulf of Mexico in 1997 (Weaver et al., 2002). However, no further collecting by ROVs occurred until a similarly equipped unit recently captured deep-reef fishes in the western Pacific (Chaloux et al., 2021).
Early discovery of deep-reef fishes in the GC began with deep SCUBA diving (Randall, 1963; Robins and Colin, 1979) but was subsequently replaced with the use of safer CCRs (reviewed by Pyle, 2000). These units allow divers to conduct research down to ∼160–180 m (Coleman et al., 2018), although use in reef-fish studies typically occurs above 130 m. CCR research has been extensively used to define depth distributions of species on Mesophotic Coral Ecosystems (MCEs) and demonstrate the existence of distinct assemblages of fishes concentrated in various mesophotic depth zones down to ∼150 m (Rocha et al., 2018). Research on reef fishes in the Greater Caribbean using CCR began in the late 1960s, with shallow-water studies of reef fishes in the US Virgin Islands (Collette, 1996), followed, in 1968, by the collection of a new reef fish on deep reefs off Mexico (Starck and Colin, 1978). However, there has been much less usage of CCR for research on deep-reef fishes in the GC than in other tropical areas. Only two substantial studies have employed that technique to quantitatively examined the distributions of fishes across much of the depth range commonly associated with MCEs (30–150 m). Those used CCR observations to assess changes in taxonomic and ecological composition in reef-fish assemblages down to 100 m depth at Bermuda (Pinheiro et al., 2016; Goodbody-Gringley et al., 2019; Stefanoudis et al., 2019), and 130 m at Curacao (Pinheiro et al., 2016). CCRs also have been used to characterize changes in species presence, diversity and ecological groupings of shallow and upper-mesophotic reef fishes at Puerto Rico (Bejarano et al., 2010, 2014; Garcia-Sais, 2010) and record depth ranges of fishes down to 130 m in the Bahamas (Pinheiro et al., 2019a) and conduct exploratory work to mid-mesophotic depths at San Andres Island (Chasqui et al., 2020). In the central and western Pacific CCRs are commonly used to record quantitative depth-distribution data throughout most of the mesophotic zone of reefs (e.g., Fukunaga et al., 2017; Coleman et al., 2018; Pinheiro et al., 2019b) and also to collect specimens of new species of deep-reef fishes (e.g., Pyle, 2000; Pinheiro et al., 2019b; Shepherd et al., 2020; Tea et al., 2020). However, there has been little actual collecting of reef fishes using this technique in the Greater Caribbean (Starck and Colin, 1978; Sparks and Gruber, 2012), with somewhat more collecting activity in Brazil (Pimentel et al., 2020).
BRUVs have been used to capture video of both shallow and deep-reef fishes on tropical reefs down to 240 m. BRUV usage, although it has provided data for studies of deep-reef fishes in the Indo-west Pacific (Colton and Swearer, 2010; Sih et al., 2017, 2019; Abesamis et al., 2020; Andradi-Brown et al., 2021; Cure et al., 2021) has occurred at relatively low levels in the Greater Caribbean. Goodbody-Gringley et al. (2019) provided the most comprehensive depth coverage to date by BRUVs at any Greater Caribbean site, while Andradi-Brown et al. (2016) used BRUVs in shallow mesophotic depths. The recent Bermuda research combined submersible surveys in the deeper zones with shallower CCR and BRUV surveys (Goodbody-Gringley et al., 2019; Stefanoudis et al., 2019). Recent research at an oceanic island in Brazil also has involved combined usage of SCUBA, CCR, BRUVs, and submersibles to examine the depth-distributions of reef fishes between 0 and 600 m (Pinheiro et al., 2020).
Each of the different technologies used in deep-reef fish studies has its advantages and disadvantages. Variation in cost is one major factor. A 7-day research expedition using submersibles like Curasub or Idabel would cost ∼$30,000 for dives immediately adjacent to the submersible’s land base and > $200,000 using the RV Chapman to transport and act as a base for Curasub at distant locations. Newer small research submersibles are available,1 but are expensive to buy and, since they have yet to be used for any major fish-related research program, research costs are unclear. Small recreation-grade ROVs costing < $1,000 are very flexible in where they can operate. However, they require boat support, are limited to shallow mesophotic depths and are sensitive to water currents. A week-long expedition with a small industrial ROV capable of working to 300 m that was operated from a small fishing vessel likely would cost > $40,000. Large industrial ROVs that require research-vessel support have costs higher than those for Idabel and Curasub. CCR, which is limited to mesophotic depths, is arguably the most mobile technology, allowing dives with minimal boat support anywhere suitable gases and support services are available. For CCR, the combination of startup costs of equipment and training, together with upkeep and usage costs are on the order of $1,000/dive/diver for the first 50 dives. BRUVs are the cheapest technology (∼$25,000 for multiple units to run deep-reef surveys) and can be used at sites wherever there is boat support. However, since they rely on baits that differentially tend to attract carnivorous fishes, the data they collect are not directly comparable to data produced by other methods.
Besides the Greater Caribbean the tropical area with most extensive research on deep-reef fishes using submersibles is the Hawaiian Archipelago. The Asherah research in the 1960s considerably expanded the known depth ranges of many shallow-water reef fishes in Hawaii. Extensive submersible research at depths down to 2,000 m in the Hawaiian Archipelago and nearby Johnston Island between 1982 and 1992 eventually provided comprehensive coverage of the taxonomic and biogeographic composition and depth ranges of members of the deep-sea fish fauna (Randall et al., 1985; Chave and Mundy, 1994). During this submersible activity specimens of reef fishes, including species new to science, were obtained using ichthyocide deployed from a submersible and collected using a suction device on the submersible. In addition, a 1982 study by Thresher and Colin (1986) of the depth variation in distributions of reef fishes between 30 and 300 m at Enewetak atoll in the central Pacific recorded substantial increases in the known depth ranges of various shallow reef-fishes, and change-with-depth in the taxonomic- and ecological composition of the reef-fish fauna and in the numerical abundance of individual species and assemblages of species. Those authors also observed a substantial number of unidentifiable deep-reef fishes likely new to science. During the 2000s the submersibles Pisces IV and V were used, in combination with other methods, to collect data on mesophotic fishes in Hawaii that were analyzed to compare the ecological structure of MCE fish assemblages with that of fishes on shallow reefs (Boland et al., 2020). The large amounts of biodiversity information generated about fishes and other deep-reef organisms by submersible research in the Hawaiian Islands, Enewetak and Greater Caribbean amply demonstrate the value of this tool. The Hawaiian submersible research comprises the most comprehensive published assessment anywhere to date of a regional-level tropical fauna of deep-living marine fishes based on submersible research.
Small research submersibles are relatively large, not very agile devices that are expensive to buy, maintain and support from the surface, usually by research vessels. They are at the upper extreme of the range in terms of cost of use by different techniques. However, they offer various advantages for research on and collections of deep-reef fishes. They can operate at greater depths than techniques such as CCR, which, due to human physiological limitations at great depth, and design features of many rebreathers, typically are limited to water shallower than ∼100–150 m. CCR requires extended periods of decompression: six hours for a 10-min dive at 150 m, which would increase for deeper dives and make them impracticable (R. Pyle pers. com. to DRR, July 2021). There are no such decompression down-time requirements with submersibles. Hence, research time per dive is maximized when using a submersible and can occupy many hours at maximum depths occupied by reef-fish assemblages. Although CCR divers can effectively collect specimens in the shallow 1/3–1/2 of the deep-reef depth range, and likely are more effective than submersibles at collecting rapidly moving demersal fishes and at working in structurally complex microhabitats, that capacity has been little used in the Greater Caribbean. Our analysis shows that submersibles can be as effective as CCR for visual sampling of the diversity and depth-distributions of shallow species living on deep reefs. BRUVs, which can provide data on a broad range of ecotypes of fishes (e.g., Cure et al., 2021), have been used in the IWP to generate useful observational data across the entire deep-reef depth range, but not for collecting specimens. ROVs can operate to the same depths as small research submersibles and do so for longer periods. However, they lack the visual capabilities of submersibles that support multiple researchers inside a transparent dome with a panoramic view. While ROVs in a very few instances have been equipped with suction fish-catchers equivalent to those used by submersibles there has been no comparative evaluation of the capabilities and effectiveness of those devices on ROVs vs. submersibles.
There is no apparent lower limit to the size of fishes than can be collected by submersibles. DROP researchers have used two different submersibles to collect adult specimens of numerous new species that are ∼2 cm total length (Baldwin and Robertson, 2013; Baldwin et al., 2016; Tornabene et al., 2016a,b, 2018), which is close to the minimum known size for vertebrates. Submersibles equipped with spears, nets and claws can and do catch large fish, which ROVs are not (yet) capable of doing (see Gilmore, 2016). Thus, the research described here amply demonstrates that small research submersibles are versatile, highly effective visual samplers and collectors of specimens of all types of deep-reef fishes, including speciose, small cryptobenthic forms, across the full depth range of deep reefs. No other technique currently has all of those abilities.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://zenodo.org/record/5590602#.YXG-kBrMKUk and DOI: 10.5281/zenodo.5592149.
The animal study was reviewed and approved by the Smithsonian Animal Care and Use Committee (ACUC). Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
DR, CB, and LT collected the data and specimens from submersibles. DR delineated the study and analyzed the data. DR, LT, CL, and CB wrote and edited the manuscript. LT and CB organized funding for this work. All authors contributed to the article and approved the submitted version.
Funding for DROP was provided internally by NMNH Research Programs to CB, the Consortium for Understanding and Sustaining a Biodiverse Planet to CB, the Competitive Grants for the Promotion of Science Program to CB and DR, the Herbert R. and Evelyn Axelrod Endowment Fund for Systematic Ichthyology to CB, and from STRI General Research Funds to DR. External funding was obtained from the National Geographic Society’s Committee for Research and Exploration to CB (Grant #9102-12) and the Prince Albert II of Monaco Foundation (Grant #1801 and gift funds). Fieldwork in Roatan was funded in part by the University of Washington School of Aquatic and Fishery Sciences, and by the Burke Museum Ichthyology Excellence Fund.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Mark Vermeij (voucher photographs of fishes from Curacao, habitat information about Curacao); Carlos and Allison Estape (voucher photographs of shallow fishes from Statia, Roatan, and St Croix); Mickey Charteris (voucher photographs of shallow fishes from Roatan); and Ernesto Peña for general preparation of databases used here. We thank Katherine Maslenikov and Rachel Manning for assistance in the field at Roatan. We thank Emily McFarland and Jenna Barrett for help with submersible videos. Karl Stanley and Thomas Trudel facilitated fieldwork aboard the submersible Idabel. We thank Bruce Brandt, Barry Brown, Cristina Castillo, Amy Driskell, Tico Christiaan, Tommy Devine, Rob Loendersloot, Laureen Schenk, Adriaan Schrier, Barbara van Bebber, and Lee Weigt for assistance with various aspects of DROP including submersible diving using the Curasub. Guidelines for the Use of Fishes in Research established by the American Society of Ichthyologists and Herpetologists (http://static1.squarespace.com/static/618bf11a71fcdf5398996eda/t/618fbed1f40e6c713dfa71ee/1636810449675/asf-guidelines-use-of-fishes-in-research-2013.pdf) were followed for all field-collecting activities. Fish-specimen collecting at Curacao and Sint Eustatius were done so under Smithsonian Animal Care and Use Committee (ACUC) approval to CB (ACUC #2011–07 and #2014–13. Richard Pyle provided much useful information about the costs, technical characteristics and utility of CCR for research on deep-reef fishes. Comments in a review by Hudson Pinheiro were useful for improving the manuscript. This is Ocean Heritage Foundation/Curaçao Sea Aquarium/Substation Curaçao contribution OHF/SCA/SC#51.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.800250/full#supplementary-material
Abesamis, R. A., Utzurrum, J. A. T., Raterta, L. J. J., and Russ, G. R. (2020). Shore-fish assemblage structure in the central Philippines from shallow coral reefs to the mesophotic zone. Mar. Biol. 167:185. doi: 10.1007/s00227-020-03797-5
Ackerman, J. L., and Bellwood, D. R. (2000). Reef fish assemblages: a re-evaluation using enclosed rotenone stations. Mar. Ecol. Prog. Ser. 206, 227–237. doi: 10.3354/meps206227
Alzate, A., Zapata, F. A., and Giraldo, A. (2014). A comparison of visual and collection-based methods for assessing community structure of coral reef fishes in the Tropical Eastern Pacific. Rev. Biol. Trop. 62(Suppl. 1), 359–371. doi: 10.15517/rbt.v62i0.16361
Andradi-Brown, D. A., Beer, A. J. E., Colin, L., Hastuti, Head, C. E. I., Hidayat, N. I., et al. (2021). Highly diverse mesophotic reef fish communities in Raja Ampat, West Papua. Coral Reefs 40, 111–130. doi: 10.1007/s00338-020-02020-7
Andradi-Brown, D. A., Macaya-Solis, C., Exton, D. A., Gress, E., Wright, G., and Rogers, A. D. (2016). Assessing Caribbean shallow and mesophotic reef fish communities using baited-remote underwater video (BRUV) and diver-operated video (DOV) survey techniques. PLoS One 11:e0168235. doi: 10.1371/journal.pone.0168235
Baker, E. K., Puglise, K. A., Harris, P. T., and Grid–Arendal (2016). Mesophotic Coral Ecosystems: A Lifeboat for Coral Reefs?. Nairobi: United Nations Environment Programme and GRID-Arendal.
Baldwin, C. C., Robertson, D. R., Nonaka, A., and Tornabene, L. (2016). Two new deep-reef basslets (Teleostei, Grammatidae, Lipogramma), with comments on the eco-evolutionary relationships of the genus. ZooKeys 638, 45–82. doi: 10.3897/zookeys.638.10455
Baldwin, C. C., Tornabene, L., and Robertson, D. R. (2018). Below the mesophotic. Sci. Rep. 8:4920. doi: 10.1038/s41598-018-23067-1
Baldwin, C., and Robertson, R. (2013). A new Haptoclinus blenny (Teleostei, Labrisomidae) from deep reefs off Curaçao, southern Caribbean, with comments on relationships of the genus. ZooKeys 306, 71–81. doi: 10.3897/zookeys.306.5198
Bejarano, I., Appeldoorn, R. S., and Nemeth, M. (2014). Fishes associated with mesophotic coral ecosystems in La Parguera, Puerto Rico. Coral Reefs 33, 313–328. doi: 10.1007/s00338-014-1125-6
Bejarano, I., Nemeth, M., and Appeldoorn, R. S. (2010). “Use of mixed-gas rebreathers to access fish assemblages in mesophotic coral ecosystems (MCE) off La Parguera shelf-edge, Puerto Rico (San Juan, Puerto Rico,” in Proceedings of the 63rd Gulf and Caribbean Fisheries Institute. (San Juan), 130–133.
Boland, R. C., Hyrenbach, K. D., DeMartini, E. E., Parrish, F. A., and Rooney, J. J. (2020). Comparing mesophotic and shallow reef fish assemblages in the ‘Au’au Channel, Hawaii: fish size, feeding guild composition, species richness, and endemism. Bull. Mar. Sci. 96, 577–592. doi: 10.5343/bms.2019.0031
Brandl, S. J., Goatley, C. H. R., Bellwood, D. R., and Tornabene, L. (2018). The hidden half: ecology and evolution of cryptobenthic fishes on coral reefs. Biol. Rev. Camb. Philos. Soc. 93, 1846–1873. doi: 10.1111/brv.12423
Brandl, S. J., Tornabene, L., Goatley, C. H. R., Casey, J. M., Morais, R. A., Côté, I. M., et al. (2019). Demographic dynamics of the smallest marine vertebrates fuel coral reef ecosystem functioning. Science 364, 1189–1192. doi: 10.1126/science.aav3384
Bryan, D. R., Kilfoyle, K., Gilmore, R. G. Jr., and Spieler, R. E. (2013). Characterization of the mesophotic reef fish community in south Florida, USA. J. Appl. Ichthyol. 29, 108–117. doi: 10.1111/j.1439-0426.2012.02055.x
Chaloux, N., Phillips, B. T., Gruber, D. F., Schelly, R. C., and Sparks, J. S. (2021). A novel fish sampling system for ROVs. Deep Sea Res. Part Oceanogr. Res. Pap. 167:103428. doi: 10.1016/j.dsr.2020.103428
Chasqui, L., Mejía-Quintero, K., and González, J. D. (2020). Biodiversity and ecological units of the mesophotic coral ecosystems in San Andrés Island, Seaflower biosphere reserve. Front. Mar. Sci. 7:559273. doi: 10.3389/fmars.2020.559273
Chave, E. H., and Mundy, B. C. (1994). Deep-sea benthic fish of the Hawaiian Archipelago, cross seamount, and Johnston Atoll. Pac. Sci. 48, 367–409.
Coleman, R. R., Copus, J. M., Coffey, D. M., Whitton, R. K., and Bowen, B. W. (2018). Shifting reef fish assemblages along a depth gradient in Pohnpei, Micronesia. PeerJ 6:e4650. doi: 10.7717/peerj.4650
Colin, P. L. (1974). Observation and collection of deep-reef fishes off the coasts of Jamaica and British Honduras (Belize). Mar. Biol. 24, 29–38. doi: 10.1007/BF00402844
Colin, P. L. (1976). Observations of deep-reef fishes in the tongue-of-the-ocean, Bahamas. Bull. Mar. Sci. 26, 603–605. doi: 10.1111/gcb.15732
Collette, B. B. (1996). “Results of the Tektite Program: ecology of coral-reef fishes,” in Proceedings of the American Academy of Underwater Sciences Scientific Diving Symposium. Methods and Techniques of Underwater Research, eds M. A. Lang and C. C. Baldwin (Washington, DC: Smithsonian Institution). Available online at: https://irma.nps.gov/DataStore/DownloadFile/564485 (accessed October 15, 2021).
Colton, M., and Swearer, S. (2010). A comparison of two survey methods: differences between underwater visual census and baited remote underwater video. Mar. Ecol. Prog. Ser. 400, 19–36. doi: 10.3354/meps08377
Cook, R. W. (1982). “Technological advancements of lock. Out submersibles,” in Proceedings of the Underwater Operations and Techniques: Conférence Internationale, Paris, 6-8 Décembre 1982. (Paris: Association Technique Maritime et Aéronautique), 267–277.
Cure, K., Currey-Randall, L., Galaiduk, R., Radford, B., Wakeford, M., and Heyward, A. (2021). Depth gradients in abundance and functional roles suggest limited depth refuges for herbivorous fishes. Coral Reefs 40, 365–379. doi: 10.1007/s00338-021-02060-7
Depczynski, M., and Bellwood, D. R. (2003). The role of cryptobenthic reef fishes in coral reef trophodynamics. Mar. Ecol. Prog. Ser. 256, 183–191. doi: 10.3354/meps256183
Fukunaga, A., Kosaki, R. K., and Wagner, D. (2017). Changes in mesophotic reef fish assemblages along depth and geographical gradients in the Northwestern Hawaiian Islands. Coral Reefs 36, 785–790. doi: 10.1007/s00338-017-1569-6
García-Sais, J. R. (2005). “Inventory and Atlas of Corals and Coral Reefs, with emphasis on deep-water coral reefs from the U. S. Caribbean EEZ,” in Final Report Submitted to Caribbean Fishery Management Council. San Juan, Puerto Rico. Available online at: https://fdocuments.in/document/inventory-and-atlas-of-corals-and-coral-reefs-with-emphasis-on-deep-water.html (accessed October 15, 2021).
Garcia-Sais, J. R. (2010). Reef habitats and associated sessile-benthic and fish assemblages across a euphotic–mesophotic depth gradient in Isla Desecheo, Puerto Rico. Coral Reefs 29, 277–288. doi: 10.1007/s00338-009-0582-9
Gaskill, M. (2011). End of an era for research subs. Nature Available online at: http://www.nature.com/news/2011/110822/full/news.2011.488.html (accessed August 22, 2011)
Gilmore, R. G. (1997). Lipogramma robinsi, a new basslet from the tropical western Atlantic, with descriptive and distributional notes on L. flavescens and L. anabantoides (Perciformes: Grammatidae). Bull. Mar. Sci. 60, 782–788.
Gilmore, R. G. Jr. (2016). You can’t catch a fish with a robot. Gulf Caribb. Res. 27, ii–xiv. doi: 10.18785/gcr.2701.11
Gilmore, R. G., and Jones, R. S. (1988). Lipogramma Flavescens, a new Grammid fish from the Bahama Islands, with descriptive and distributional notes on L. Evides and L. Anabantoides. Bull. Mar. Sci. 42, 435–445.
Gilmore, R. G., and Jones, R. S. (1992). Color variation and associated behavior in the Epinepheline groupers, Mycteroperca microlepis (Goode and Bean) and M. Phenax Jordan and Swain. Bull. Mar. Sci. 51, 83–103.
Goodbody-Gringley, G., Noyes, T., and Smith, S. R. (2019). “Bermuda,” in Mesophotic Coral Ecosystems Coral Reefs of the World 12, eds Y. Loya, K. A. Puglise, and T. C. L. Bridge (Cham: Springer International Publishing), 31–45. doi: 10.1007/978-3-319-92735-0_2
HBOI (2021). JSL and Clelia Dive Logs_1971 to 2011 All Dives. Harbor Branch Oceanographic Submersible Dives Access Database, Dives (Archive).mdb. Fort Pierce, FL: Harbor Branch Oceanographic Institution.
Itzkowitz, M., Haley, M., Otis, C., and Evers, D. (1991). A reconnaissance of the deeper jamaican coral reef fish communities. Northeast Gulf Sci. 12, 25–34. doi: 10.18785/negs.1201.03
Locker, S. D., Reed, J. K., Farrington, S., Harter, S., Hine, A. C., and Dunn, S. (2016). Geology and biology of the “Sticky Grounds”, shelf-margin carbonate mounds, and mesophotic ecosystem in the eastern Gulf of Mexico. Cont. Shelf Res. 125, 71–87. doi: 10.1016/j.csr.2016.06.015
Magalhães, G. M., Amado-Filho, G. M., Rosa, M. R., de Moura, R. L., Brasileiro, P. S., De Moraes, F. C., et al. (2015). Changes in benthic communities along a 0–60 m depth gradient in the remote St. Peter and St. Paul Archipelago (Mid-Atlantic Ridge, Brazil). Bull. Mar. Sci. 91, 377–396. doi: 10.5343/bms.2014.1044
Nelson, W. R., and Appeldoorn, R. S. (1985). A Submersible Survey of the Continental Slope of Puerto Rico and the U.S. Virgin Islands, October 1-23, 1985. Cruise Report R/V Seward Johnson Pascagoula. Silver Spring, MD: Mississippi Laboratories: National Marine Fisheries Service, 76.
Parker, R. O. Jr., and Ross, S. (1986). Observing reef fishes from submersibles off North Carolina. Gulf Mex. Sci. 8, 31–49. doi: 10.18785/negs.0801.03
Pimentel, C. R., Rocha, L. A., Shepherd, B., Phelps, T. A. Y., Joyeux, J.-C., Martins, A. S., et al. (2020). Mesophotic ecosystems at Fernando de Noronha Archipelago, Brazil (South-western Atlantic), reveal unique ichthyofauna and need for conservation. Neotropical Ichthyol. 18:e200050. doi: 10.1590/1982-0224-2020-0050
Pinheiro, H. T., Shepherd, B., Castillo, C., Abesamis, R. A., Copus, J. M., Pyle, R. L., et al. (2019b). Deep reef fishes in the world’s epicenter of marine biodiversity. Coral Reefs 38, 985–995. doi: 10.1007/s00338-019-01825-5
Pinheiro, H. T., Eyal, G., Shepherd, B., and Rocha, L. A. (2019a). Ecological insights from environmental disturbances in mesophotic coral ecosystems. Ecosphere 10:e02666.
Pinheiro, H. T., Goodbody-Gringley, G., Jessup, M. E., Shepherd, B., Chequer, A. D., and Rocha, L. A. (2016). Upper and lower mesophotic coral reef fish communities evaluated by underwater visual censuses in two Caribbean locations. Coral Reefs 35, 139–151. doi: 10.1007/s00338-015-1381-0
Pinheiro, H. T., Macena, B. C. L., Francini-Filho, R. B., Ferreira, C. E. L., Albuquerque, F. V., Bezerra, N. P. A., et al. (2020). Fish biodiversity of Saint Peter and Saint Paul’s Archipelago, Mid-Atlantic Ridge, Brazil: new records and a species database. J. Fish Biol. 97, 1143–1153. doi: 10.1111/jfb.14484
Pyle, R. L. (2000). Assessing undiscovered fish biodiversity on deep coral reefs using advanced self-contained diving technology. Mar. Technol. Soc. J. 34, 82–91. doi: 10.4031/MTSJ.34.4.11
Quattrini, A. M., and Ross, S. W. (2006). Fishes associated with North Carolina shelf-edge hardbottoms and initial assessment of a proposed marine protected area. Bull. Mar. Sci. 79, 137–163.
Quattrini, A. M., Demopoulos, A. W. J., Singer, R., Roa-Varon, A., and Chaytor, J. D. (2017). Demersal fish assemblages on seamounts and other rugged features in the northeastern Caribbean. Deep Sea Res. Part Oceanogr. Res. Pap. 123, 90–104. doi: 10.1016/j.dsr.2017.03.009
Quattrini, A. M., Ross, S. W., Sulak, K. J., Necaise, A. M., Casazza, T. L., and Dennis, G. D. (2004). Marine fishes new to continental United States waters, North Carolina, and the Gulf of Mexico. Southeast. Nat. 3, 155–172.
Quattrini, A. M., Nizinski, M. S., Chaytor, J. D., Demopoulos, A. W., Roark, E. B., France, S., et al. (2015). Exploration of the canyon-incised continental margin of the northeastern United States reveals dynamic habitats and diverse communities. PLoS One 10:e0139904. doi: 10.1371/journal.pone.0139904
Randall, J. E. (1963). Three new species and six new records of small Serranoid fishes from Curaçao and Puerto Rico. Stud. Fauna Curaçao Caribb. Isl. 19, 77–110.
Randall, J. E., Lobel, P. S., and Chave, E. H. (1985). Annotated checklist of the fishes of Johnston Island. Pac. Sci. 39, 24–80. doi: 10.11646/zootaxa.4379.1.2
Reed, J. K., and Frank, T. M. (2011). Johnson-Sea-Link Submersibles Tools for Research and Discovery: Summary of Users, Publications, Documentaries and Testimonials. Harbor Branch Oceanographic Institution Technical Report. Available online at: https://fau.digital.flvc.org/islandora/object/fau%3A2731 (accessed October 15, 2021).
Reed, J. K., and Pomponi, S. A. (2000). Final Cruise Report. Submersible and SCUBA Collections in the Netherlands Antilles (Curacao, Bonaire) and Aruba: Biomedical and Biodiversity Research of the Benthic Communities with Emphasis on the Porifera and Gorgonacea. Ft. Pierce, FL: Harbor Branch Oceanographic Institution.
Reed, J. K., Farrington, S., Moe, H., Harter, S., Hanisak, M., David, A., et al. (2017). Characterization of Mesophotic Coral/Sponge Habitats and Fish Assemblages in the Regions of Pulley Ridge and Tortugas from ROV Dives during R/V Walton Smith Cruises of 2012 to 2015. (NOAA CIOERT Cruise Report). Submitted to NOAA-NOS-NCCOS, NOAA Office of Ocean Exploration and Research. Available online at: https://www.semanticscholar.org/paper/Characterization-of-Mesophotic-Coral%2FSponge-and-in-Reed-Farrington/a277eee2d42176e89fa51b9a4256e66c1ab7bab9 (accessed October 15, 2021).
Reed, J. K., Farrington, S., Pomponi, S. A., and Price, M. (2015). Preliminary Cruise Report. 2015 HBOI-FAU Cruise. Mesophotic and deepwater reef ecosystems- ROV/AUV surveys of SW Florida Shelf including Northern Pulley Ridge and Howell Hook regions. Report to NOAA OE, NOAA DSCRP, and NOAA Mesophotic Program: Harbor Branch Oceanographic Institution Technical Report 162. Available online at: https://fau.digital.flvc.org/islandora/object/fau%3A2731 (accessed October 15, 2021).
Reed, J. K., González-Díaz, P., Busutil, L., Farrington, S., Martínez- Daranas, B., Cobián Rojas, D., et al. (2018). Cuba’s mesophotic coral reefs and associated fish communities. Rev. Investig. Mar. 38, 60–129.
Reed, J. K., Pomponi, S. A., Weaver, D., Paull, C. K., and Wright, A. E. (2005a). Deep-water sinkholes and bioherms of South Florida and Pourtales Terrace – habitat and fauna. Bull. Mar. Sci. 77, 267–296.
Reed, J. K., Shepard, A., Koenig, C., Scanlon, K., and Gilmore, R. G. Jr. (2005b). “Mapping, habitat characterization, and fish surveys of the deep-water Oculina coral reef Marine Protected Area: a review of historical and current research,” in Cold-Water Corals and Ecosystems, eds A. Freiwald and J. M. Roberts (Berlin: Springer-Verlag), 443–465. doi: 10.1007/3-540-27673-4_22
Robertson, D. R., and Tornabene, L. (2021). Reef-Associated Bony Fishes of the Greater Caribbean: A Checklist (Version 4). Zenodo Repository. Geneva: CERN. doi: 10.5281/zenodo.5592149
Robertson, D. R., Estapé, C. J., Estapé, A. M., Peña, E., Tornabene, L., and Baldwin, C. C. (2020). The marine fishes of St Eustatius Island, northeastern Caribbean: an annotated, photographic catalog. ZooKeys 1007, 145–180. doi: 10.3897/zookeys.1007.58515
Robins, R. C., and Colin, P. L. (1979). Three new grammid fishes from the Caribbean Sea. Bull. Mar. Sci. 29, 41–52.
Rocha, L. A., Pinheiro, H. T., Shepherd, B., Papastamatiou, Y. P., Luiz, O. J., Pyle, R. L., et al. (2018). Mesophotic coral ecosystems are threatened and ecologically distinct from shallow water reefs. Science 361, 281–284. doi: 10.1126/science.aaq1614
Ross, S. W., and Quattrini, A. M. (2009). Deep-sea reef fish assemblage patterns on the Blake Plateau (Western North Atlantic Ocean). Mar. Ecol. 30, 74–92. doi: 10.1111/j.1439-0485.2008.00260.x
Shepherd, B., Pinheiro, H. T., Phelps, T. A. Y., Easton, E. E., Perez-Matus, A., and Rocha, L. A. (2020). A new species of Chromis (Teleostei: Pomacentridae) from mesophotic coral ecosystems of Rapa Nui (Easter Island) and Salas y Gómez, Chile. Copeia 108, 326–332. doi: 10.1643/CI-19-294
Shipp, R. L., Tyler, W. A., and Jones, R. S. (1986). Point count censusing from a submersible to estimate reef fish abundance over large areas. Northeast Gulf Sci. 8, 83–89. doi: 10.18785/negs.0801.06
Sih, T. L., Cappo, M., and Kingsford, M. J. (2017). Deep-reef fish assemblages of the Great Barrier Reef shelf-break (Australia). Sci. Rep. 7:10886. doi: 10.1038/s41598-017-11452-1
Sih, T. L., Daniell, J. J., Bridge, T. C. L., Beaman, R. J., Cappo, M., and Kingsford, M. J. (2019). Deep-Reef fish communities of the Great Barrier Reef shelf-break: trophic structure and habitat associations. Diversity 11:26. doi: 10.3390/d11020026
Smith-Vaniz, W. F., and Collette, B. B. (2013). Fishes of Bermuda. Aqua Int. J. Ichthyol. 19, 165–186.
Smith-Vaniz, W. F., Collette, B. B., and Luckhurst, B. E. (1999). Fishes of Bermuda: History, Zoogeography, Annotated Checklist, and Identification Keys. American Society of Ichthyologists and Herpetologists Special Publication 4. Available online at: https://pubs.er.usgs.gov/publication/70162457 (accessed October 15, 2021).
Smith-Vaniz, W. F., Jelks, H. L., and Rocha, L. A. (2006). Relevance of cryptic fishes in biodiversity assessments: a case study at Buck Island Reef National Monument, St. Croix. Bull. Mar. Sci. 79, 17–48.
Sparks, J. S., and Gruber, D. F. (2012). A new mesophotic clingfish (Teleostei: Gobiesocidae) from the Bahamas. Copeia 2012, 251–256. doi: 10.2307/23273218
Starck, W. A., and Colin, P. L. (1978). Gramma linki: a new species of Grammid Fish from the tropical Western Atlantic. Bull. Mar. Sci. 28, 146–152.
Stefanoudis, P. V., Gress, E., Pitt, J. M., Smith, S. R., Kincaid, T., Rivers, M., et al. (2019). Depth-Dependent structuring of reef fish assemblages from the shallows to the rariphotic zone. Front. Mar. Sci. 6:307. doi: 10.3389/fmars.2019.00307
Strasburg, D. W., Jones, E. C., and Iversen, R. T. B. (1968). Use of a small submarine for biological and oceanographic research. ICES J. Mar. Sci. 31, 410–426. doi: 10.1093/icesjms/31.3.410
Sulak, K. J., Brooks, R. A., Luke, K. E., Norem, A. D., Randall, M., Quaid, A. J., et al. (2007). Demersal fishes associated with Lophelia pertusa coral and hard-substrate biotopes on the continental slope, northern Gulf of Mexico. Bull. Mar. Sci. 81, 65–92.
Tea, Y.-K., Pyle, R. L., and Rocha, L. A. (2020). A new species of fairy wrasse (Teleostei: Labridae: Cirrhilabrus) from mesophotic coral ecosystems of the Verde Island Passage, Philippines. Copeia 108, 91–102. doi: 10.1643/CI-19-297
Thresher, R. E., and Colin, P. L. (1986). Trophic structure, diversity and abundance of fishes of the deep reef (30-300m) at Enewetak, Marshall Islands. Bull. Mar. Sci. 38, 253–272.
Toller, W., Lundvall, S., and Hoetjes, P. C. (2008). Some Observations Made From ROV on Mid-Depth Habitats and Reef Fish Communities of Saba Bank, Netherlands Antilles. Report for the Department of Environment of the Netherlands Antilles. Available online at: https://www.dcbd.nl/document/some-observations-made-rov-mid-depth-habitats-and-reef-fish-communities-saba-bank (accessed October 15, 2021).
Tornabene, L., Robertson, D. R., and Baldwin, C. C. (2016a). Varicus lacerta, a new species of goby (Teleostei, Gobiidae, Gobiosomatini, Nes subgroup) from a mesophotic reef in the southern Caribbean. ZooKeys 596, 143–156. doi: 10.3897/zookeys.596.8217
Tornabene, L., Robertson, D. R., and Baldwin, C. C. (2018). A new species of Lipogramma from deep reefs of Roatan, Honduras (Teleostei, Grammatidae). ZooKeys 809, 79–95. doi: 10.3897/zookeys.809.29280
Tornabene, L., Van Tassell, J. L., Gilmore, R. G., Robertson, D. R., Young, F., and Baldwin, C. C. (2016b). Molecular phylogeny, analysis of character evolution, and submersible collections enable a new classification of a diverse group of gobies (Teleostei: Gobiidae: Nes subgroup), including nine new species and four new genera. Zool. J. Linn. Soc. 177, 764–812. doi: 10.1111/zoj.12394
Tyler, J. C., Robins, C. R., Smith, C. L., and Gilmore, R. G. (1992). Deepwater populations of the Western Atlantic Pearlfish Carapus bermudensis (Ophidiiformes: Carapidae). Bull. Mar. Sci. 51, 218–223.
Weaver, D. C., Dennis, G. D., and Sulak, K. J. (2002). Northeastern Gulf of Mexico Coastal and Marine Ecosystem Program: Community Structure and Trophic Ecology of Demersal Fishes on the Pinnacles Reef Tract; Final Synthesis Report. U.S. Department of the Interior, Geological Survey, USGS BSR-2001-0008 and Minerals Management Service Gulf of Mexico OCS Region, New Orleans, LA, OCS Study MMS 2002-034. Available online at: https://pubs.er.usgs.gov/publication/bsr010008 (accessed October 15, 2021).
Weaver, D. C., Hickerson, E. L., and Schmahl, G. P. (2006a). “Deep reef fish surveys by submersible on Alderdice, McGrail, and Sonnier Banks in the Northwestern Gulf of Mexico,” in Emerging Technologies for Reef Fisheries Research and Management. NOAA Professional Paper NMFS,5), ed. J. C. Taylor (Seattle, WA: NOAA), 69–87.
Weaver, D. C., Naar, D. F., and Donahue, B. T. (2006b). “Deepwater reef fishes and multibeam bathymetry of the Tortugas South Ecological Reserve, Florida Keys National Marine Sanctuary, Florida,” in Emerging Technologies for Reef Fisheries Research and Management. NOAA Professional Paper NMFS 5, ed. J. C. Taylor (Seattle, WA: NOAA), 48–68.
Keywords: Caribbean, mesophotic, deep-reef fishes, submersible, diversity, species discovery, depth distribution, biogeography
Citation: Robertson DR, Tornabene L, Lardizabal CC and Baldwin CC (2022) Submersibles Greatly Enhance Research on the Diversity of Deep-Reef Fishes in the Greater Caribbean. Front. Mar. Sci. 8:800250. doi: 10.3389/fmars.2021.800250
Received: 22 October 2021; Accepted: 28 December 2021;
Published: 31 January 2022.
Edited by:
Wei-Jen Chen, National Taiwan University, TaiwanReviewed by:
Hudson Tercio Pinheiro, California Academy of Sciences, United StatesCopyright © 2022 Robertson, Tornabene, Lardizabal and Baldwin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: D. Ross Robertson, Um9iZXJ0c29uZHJAc2kuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.