- Department of Marine Biology, Texas A&M University at Galveston, Galveston, TX, United States
The Cassiopea genus is an emergent focus for behavioral, ecological, and genetic research. Cassiopea ephyrae, a key intermediate in the life cycle of this benthic jellyfish, have been left out of much work on the genus. Here we investigate the Cassiopea xamachana ephyra response to six combinations of light and feeding regimes. We show that zero light condition results in fast bleaching of ephyrae and significant reductions in bell size and predation success. We also show that ephyrae starved in sub-compensation level light experienced only meager reductions in size over 42 days, and those starved in zero light were still largely recoverable at 28 days. Developmental trajectories on various metrics of Cassiopea xamachana ephyrae were significantly impacted by both light and feeding level in the first 42 days of life.
Introduction
Much work has been done on the invasive potential of species of the phylum Cnidaria, especially scyphozoans and hydrozoans (Graham and Bayha, 2007; Brotz and Pauly, 2012; Morandini et al., 2017). Cnidarians have spread throughout the world in a pattern largely consistent with ship traffic (Holland et al., 2004; Purcell, 2012). Much of this spread is considered a result of extremely hardy early stages, including planula, polyps, podocysts, and planulocysts (Lotan et al., 1992; Miller and Graham, 2012). Podocysts, polyps and planulocysts are known to accommodate survival in extreme conditions (Frolova and Miglietta, 2020). These present a clear invasion concern as potential foulers on ship hulls and other surfaces. Beyond these, other life stages should be considered as well, including motile ephyrae. For example, ephyrae of the semaeostome Aurelia spp. are known to persist for months (as long as 100 days) without feeding (Fu et al., 2014). Starvation tolerance and robustness in the motile ephyra stage may contribute to a species’ capability to survive long journeys in cargo ships or along coastlines and thus to its potential as an invasive species.
Cassiopea is a rhizostome genus of Scyphozoa, with an epibenthic medusa form that has successfully spread throughout the tropics and subtropics of the world (Holland et al., 2004; Morandini et al., 2017). This spread includes a large natural distribution and an even larger invasive distribution (Morandini et al., 2017). Cassiopea medusae live in shallow waters and rely on a combination of nutrition from Symbiodiniaceae cells housed within their tissues and active consumption of prey material. Light is an essential environmental parameter for Cassiopea, given its reliance on Symbiodiniaceae’s photosynthetic capacity (Verde and Mccloskey, 1998; Mammone et al., 2021). The known compensation light level for Cassiopea medusae is 50 μmol/m2/s PAR in adults (Welsh et al., 2009). However, recent work has shown that these medusae exhibit remarkable capacity for photosynthetic plasticity (Mammone et al., 2021). Despite Cassiopea’s long-term presence in aquaria and more recent spotlight as a model system and a potential food source (Ohdera et al., 2018), little work exists on the ecology and development of its ephyrae and the role of light on their development.
Scyphozoans are phenotypically plastic in response to external conditions (Swift et al., 2016). While it is evident that starvation induces aberrant morphologies in Aurelia jellyfish, many other stressors that impact jellyfish developmental trajectories are poorly understood (Skikne et al., 2009; Fu et al., 2014). This work aims to understand how development and survivorship in non-clonal Cassiopea xamachana are affected by starvation and sub-compensation light conditions. Development was analyzed in one hundred and eight newly released ephyrae, split into low-light and darkness groups. Within both low-light and darkness treatments, multiple feeding levels were implemented to investigate the survival rates of the ephyrae and their morphological and developmental response to these regimes. Growth, zooxanthellae concentration, and aberrant body form are used as indicators of the hardiness of a species from a known invasive genus during one of its most motile stages.
Materials and Methods
Animal Sourcing, Care, and Experimental Parameters
Eight adult Cassiopea were acquired from the Florida Keys through an independent fisherman as breeding stock. Polyps that rooted along the sides of the tank were transferred to a polyp holding tank.
Polyp culture was raised at 22°C in 35 ppt in a low light (30 μmol/m2/s PAR) 5-gallon tank in the sea-life facility at Texas A&M University at Galveston. This culture was fed twice weekly, with water filtered continuously. To induce strobilation, indomethacin dissolved in DMSO was introduced gradually to the polyp culture over the course of 1 week to reach a peak concentration of 40 mM (Cabrales-Arellano et al., 2017). One week after exposure, the first visible indications of strobilation occurred. Water was carefully exchanged each day following exposure to DMSO, and ephyrae released in the 24 h prior were removed. Individuals released from April 3, 2021 to April 7, 2021 were isolated. On April 3, 4, and 5, all ephyrae between 2 and 5 mm diameter were included in the experiment (1, 4, and 25 ephyrae, respectively). On April 6th and 7th, 60 and 18 ephyrae between 2 and 5 mm were used, all excess individuals were removed. At intake, each ephyra was moved to clean artificial seawater, then photographed using Leica Acquire. After photographing, all ephyrae were placed in inverted individual clear plastic containers (Taral Plastics 6-70-CPSr) of 150 mL of artificial seawater and placed within an incubator held stable at 24.5°C for light hours and 23°C for dark hours. Viparspectra P-1000 full-spectrum lights were shaded and adjusted such that the mean PAR within a container was 41 μmol/m2/s with a deviation of 15 μmol/m2/s PAR (as measured with an Apogee MQ-500 PAR meter). Lights were on from 7 a.m. to 7 p.m. each day. To maintain a mean of 41 μmol/m2/s PAR lighting and even heating, containers were rotated through positions throughout each week. Each day, each ephyra was individually removed from the holding container and placed in a petri dish for photography (image capture completed using Leica MDG41 microscope and Leica MC170 camera using Leica Acquire application version 3.4.1 running on Mac computer). While each ephyra was on the microscope stage, 50 mL of artificial seawater were removed from the holding container and replaced with new artificial seawater, along with the relevant treatment dose of 18-h Artemia shrimp. Before water removal, all remaining living and dead Artemia accounting for greater than 50% of an Artemia body were counted, recorded, and removed individually. Smaller fragments of Artemia were removed but not counted. Ephyra was then carefully returned to the container with a pipette, the container was inverted slowly and returned to the incubator. During each photography session, ambient room conditions were kept at 22–23°C with 7 μmol/m2/s PAR, with the exception of the 0.5–1 min spent on the light stage, where light was between 10 and 79 μmol/m2/s PAR. Zero-light treatment individuals (see below for treatment descriptions) were held under blackout conditions within the incubation chamber except during photography and food introduction. Every 7 days, a complete 150 mL water change was done with surface cleaning and wiping. The above procedure was repeated each day from days 1 to 40. Upon day 41, all procedures continued as described above but without feedings, in order to reduce sample contamination in day 42 processing.
All feedings and photography took place daily during daylight hours between 8 a.m. and 12 p.m.
Dark Starvation Control
On April 7, 10 newly released ephyrae were placed in zero light conditions. These ephyrae were removed only for water change and photography as a double negative control for light and prey. Ephyrae were counted and photographed daily to monitor survival rate and body modifications. Body measurements (see below) were performed from pictures using ImageJ software. After 4 weeks (28 days), these individuals were moved to low light conditions and provided 8 Artemia/day. The move occurred after the mean bell size for living individuals fell below 2.2 mm. This size threshold represented a one third reduction in size from day 0. Ephyrae were followed for the remaining 14 days to determine growth after 28 days of complete starvation. Given the change in treatment at day 28, statistics for this group are reported separately from the other treatments. For this group, measurements of bell diameter were taken instead of rhopaliar radius measurements. As the ephyrae shrank, their rhopalia became increasingly difficult to differentiate from the surrounding tissue, and the center of the manubrium was often indistinguishable from the bell. Bell diameter was well correlated (r = 0.89) with weight in other treatment groups, and as such, the replacement was deemed acceptable.
Treatments
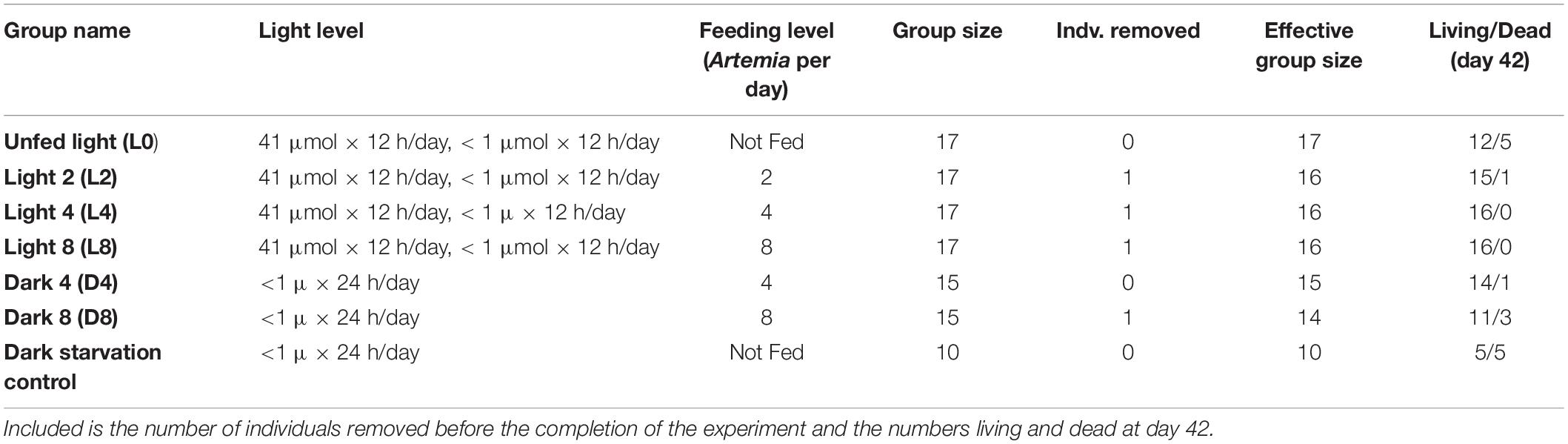
Ninety-eight ephyrae (<24 h old) were divided randomly into six experimental groups (Table 1). Four treatment groups of 17 individuals each placed in 41 μmol/m2/s light for 12 h/day (blackout conditions for the remaining 12 h). Within this group, an Unfed Light group (L0) was not given Artemia; the Light 2 group (L2) was given two 18-h Artemia nauplii per day; the Light 4 group (L4) was given four 18-h Artemia nauplii per day; the Light 8 group (L8) was given eight 18-h Artemia nauplii per day.
Two treatment groups of 15 individuals each were held in zero-light. The Dark 4 (D4) treatment group was held in blackout conditions 23.5 h/day (the last half hour representing a generous estimate of the time to feed, photograph, and change water each day) and fed four 18-h Artemia nauplii per day. The Dark 8 (D8) treatment group was fed 8 18-h Artemia nauplii per day.
L0 operates as a control for size increase from feeding, and D8 group operates as a control for light. The Dark Starvation control group experienced declines too intense for one-to-one comparisons with other groups and were not included in downstream analyses with other groups.
Each ephyra in each treatment was photographed daily following the protocol described in section “Animal Sourcing, Care, and Experimental Parameters.”
Processing
At the end of each treatment (on day 42), oral and aboral photographs of each surviving ephyrae were taken. Individuals were then processed for wet weight and zooxanthellae concentrations following a modified protocol from Zamoum and Furla (2012). First, each individual was dried lightly with a Kimtech wipe on the oral and aboral sides for 2 s. Next, each ephyra was placed in a weigh boat, weighed to the nearest tenth of a milligram (±0.1 mg), and then vivisected to remove a complete radial segment, weighing roughly 10 mg (between half and an eighth of the medusa in most cases). The segment was placed directly into 500 μL of 4 M NaOH. The remaining tissue was weighed separately and placed directly into 500 μL 100% EtOH for storage. Tissue placed in NaOH was incubated at 37°C for 60 min, vigorously vortexed for 15 s every 15 min. After 1 h, the sample was vortexed again, and 40 μL of fluid was immediately drawn from the vial and placed on a Hausser Scientific hemacytometer for cell counting. Cell counts were standardized by original tissue volume in each sample for comparability (Zamoum and Furla, 2012).
Twelve adults between 8 and 31 mm in diameter from the same culture, held in 350 PAR standard coral tank light conditions with daily uncontrolled feeding, were clipped for tissue samples. Tissue samples were processed following the same zooxanthellae extraction protocols as above. Five random day-zero ephyrae frozen at 80°C and pooled for zooxanthelle extraction as well.
Removal of Damaged and Dead Ephyra
Ninety-eight specimens were initially included in this experiment. All individuals in the fed treatment groups that failed to consume a minimum of one Artemia nauplii within the first 7 days were removed from the experiment. Each of these individuals died within 10 days of release. One individual was removed from the experiment due to an error that led to bell damage of this specimen. In total, 4 out of 98 ephyrae were removed from the experiment.
All ephyrae that died during the experiment were flash-frozen and stored for later use. Given the difficulty of diagnosing the actual point of death in Cassiopea ephyrae, removal due to death required meeting two of the following three conditions: (1) bell diameter reduction to below 1.6 mm, (2) cessation of pulsing, and (3) significant deterioration of bell margin features. While we recognize there may be a slim chance that ephyrae in these states could recover, we deemed it unlikely. Once two criteria were met, individuals were frozen and eliminated from the experiment.
Measurements and Data Analyses
A total of 4,587 photographs were produced during the experiment. All photographs associated with this project were labeled by specimen and day. ImageJ was used to measure bell diameter, rhopaliar radius, average oral arm or manubrium length (referred to together as oral arm length), and number of oral arm appendage buds in each photograph and compiled for all specimens (see example measurements in Figure 1). These measurements represented the quantitative factors that can be gleaned from images of living non-anesthetized ephyrae. Bell diameter and oral arm length fluctuated each day as no chemical relaxant was used on ephyrae, and level of relaxation and tension in each ephyra varied by day. Predation success was averaged by week and measured as the number of Artemia nauplii provided minus Artemia nauplii collected after each feeding session. While not a meaningful metric for comparison between individuals, color changes within individuals were noted over the course of the experiment.
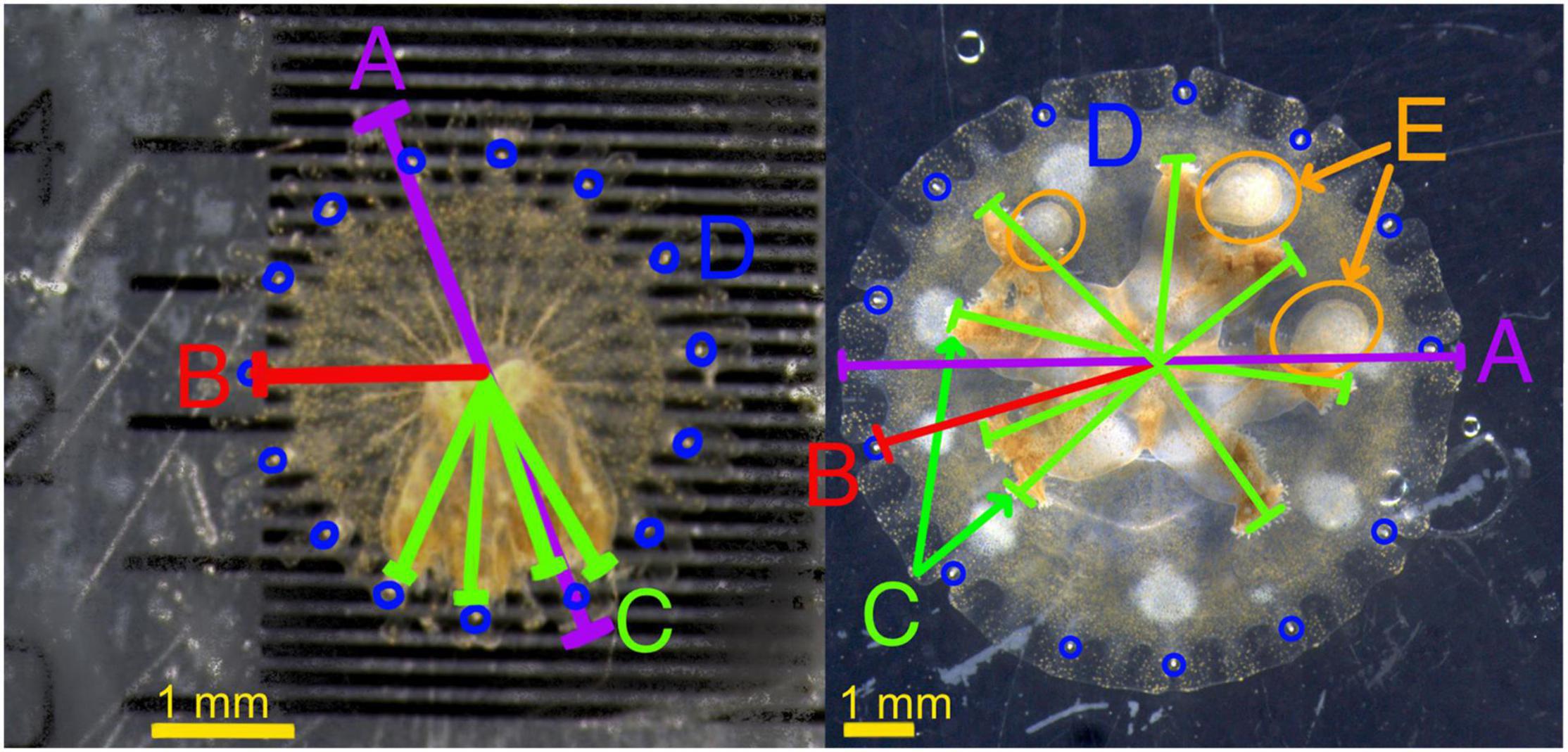
Figure 1. Measurement examples on two example ephyrae, one at 1 week and one at 4 weeks. (A) (Purple) Bell diameter, (B) (red) Rhopaliar radius, (C) (green) oral arm length, reported as average of all oral arms, (D) (blue) Rhopalia, added together for rhopaliar number, (E) oral arm appendages.
Correlations between bell diameter, rhopaliar radius, average oral arm length and wet weight were calculated for each photographed medusa. Rhopaliar radius was used as the standardized size proxy in all analyses as it was well correlated at day 42 with wet weight (r = 0.91) and less variable over time within the same individual than bell diameter (see Supplementary Figure 1).
Survivorship significance testing was done using the “survival” R package using the “survdiff” function for Kaplan-Meier survivorship. Growth curves were fitted using AICc comparisons under linear, logarithmic and polynomial growth models. Westfall-adjusted Tukey comparisons of means were run using R. Difference in growth by day and group was computed using the lme package and adjusted using Bonferroni adjustments for all against all. Planned comparisons were run on consumption, growth and oral arm appendage development between lit and unlit groups, and planned comparisons were run on growth between groups within lit treatment groups.
Terminology
Throughout the text, the terms “ephyra” and “medusa,” and “oral arms” and “manubrium,” are used interchangeably. During the first 6 weeks post-strobilation, the ephyrae morph into small medusae and manubrium morphs into oral arms. For each individual, these transitions happened at different times, or in steps too subtle or difficult to measure within the bounds of the experiment. The term “oral arms” refers to the tissue around the gastrovascular opening, homologous to the manubrium in the newest ephyrae. “Ephyra” will be used preferentially given the youth of the study organisms and the difficulty of determining a date of cross over to medusa. The oral arm appendages here are a term used for the early version of one of the heavily pigmented floating appendages (also known as palps in adult medusae).
Results
Mortality Rates in Treatments
Four of the six experimental groups (L0, L2, D4, D8) experienced some mortality. Survival was impacted by group identity within the experiment (chi-squared:12.3, df: 5, p = 0.03). Survivorship in the L0 treatment group (70.6%) was the lowest, however, ephyrae held in the dark and provided eight nauplii/day (D8) were also below 80% survivorship (78.6%). The four other treatment groups all had survivorship of greater than 90% (L2: 93.8%, L4: 100%, L8: 100%, D4: 93.3%). No tested random factors (date of addition, starting container position, rhopaliar number, coloring at strobilation) except for starting bell width explained mortality. As there was no significant difference in starting bell size among groups, this was not a confounding factor. Seven of the 10 deaths were in individuals with a starting bell size of under 3.2 mm in diameter (mean starting bell size overall was 3.65 mm). The mortality rate of L0 was significantly higher than that of the fed groups (p = 0.01). No other groups were significantly different than all others at an p = 0.1 threshold. The average death date for the 10 ephyrae who died before day 42 was day 28.3, with a range of days 15–37. For all further analyses, only surviving medusae were used.
Growth
Changes in rhopaliar radius per day were distinct between most groups and were best explained by polynomial (Figure 2) growth curves. All groups except L0 experienced an increase in size over the course of the experiment.

Figure 2. Change in rhopaliar radius from days 0 to 42 in millimeters. Bolded lines with crosses are averages of each group, accompanied by polynomial trend lines.
From days 0 to 42, L0 saw a decrease of rhopaliar radius of 19%. D4 and D8 saw an average growth of 40 and 86%, respectively. The fed groups held in light saw roughly a 60% increase in growth relative to one another with each doubling of food availability (L2 = 58% growth, L4 = 115% growth, L8 = 179% growth). As these rates all leveled off before day 42, size at day 42 likely represents the maximum size sustainable under each set of conditions. While L0 experienced slight size reduction, oral arm tissue shrank in by one quarter from the day 0 average (1.11 mm vs. 0.83.mm) and was visibly less distinct (see Supplementary Materials). Periods of increasing size were commensurate with feeding conditions, and growth lasted longest in the L8 and D8 groups (Figure 2) as proxied by point of polynomial inversions. D4 and L0 groups had extremely short growth periods, both averaging 11 days of growth (in the case of L0 very weak growth) before slight size reduction and stabilization. However, individual trajectories within every group varied widely.
Figure 3 shows the average increase in rhopaliar radius between days 0 and 42. Only L4 and L8 groups grew enough for Tukey comparison of means confidence intervals to exclude a 1x ratio (no change in size). The 6-week growth means of D4 and D8 groups were both significantly different from that of the light at the same feeding level (L4 and L8) (D4: 1.40 vs. L4: 2.15, p = 0.0015 and D8: 1.86 vs. L8: 2.79, p = 8.28e-05) (Figure 3). While the mean of growth of L2 was significantly higher than L0 (1.58 ± 0.59 vs. 0.81 ± 0.27, p = 0.0006), D4 had a non-significant increase in growth over L0 and a non-significant difference from D8 (L0: 0.81 ± 0.27, D4:1.40 ± 1.04, D8: 1.86 ± 0.97, p = > 0.1). Dark groups were most comparable in growth to the L2 light group, despite the dramatic range of feeding levels between these three (Figure 3).
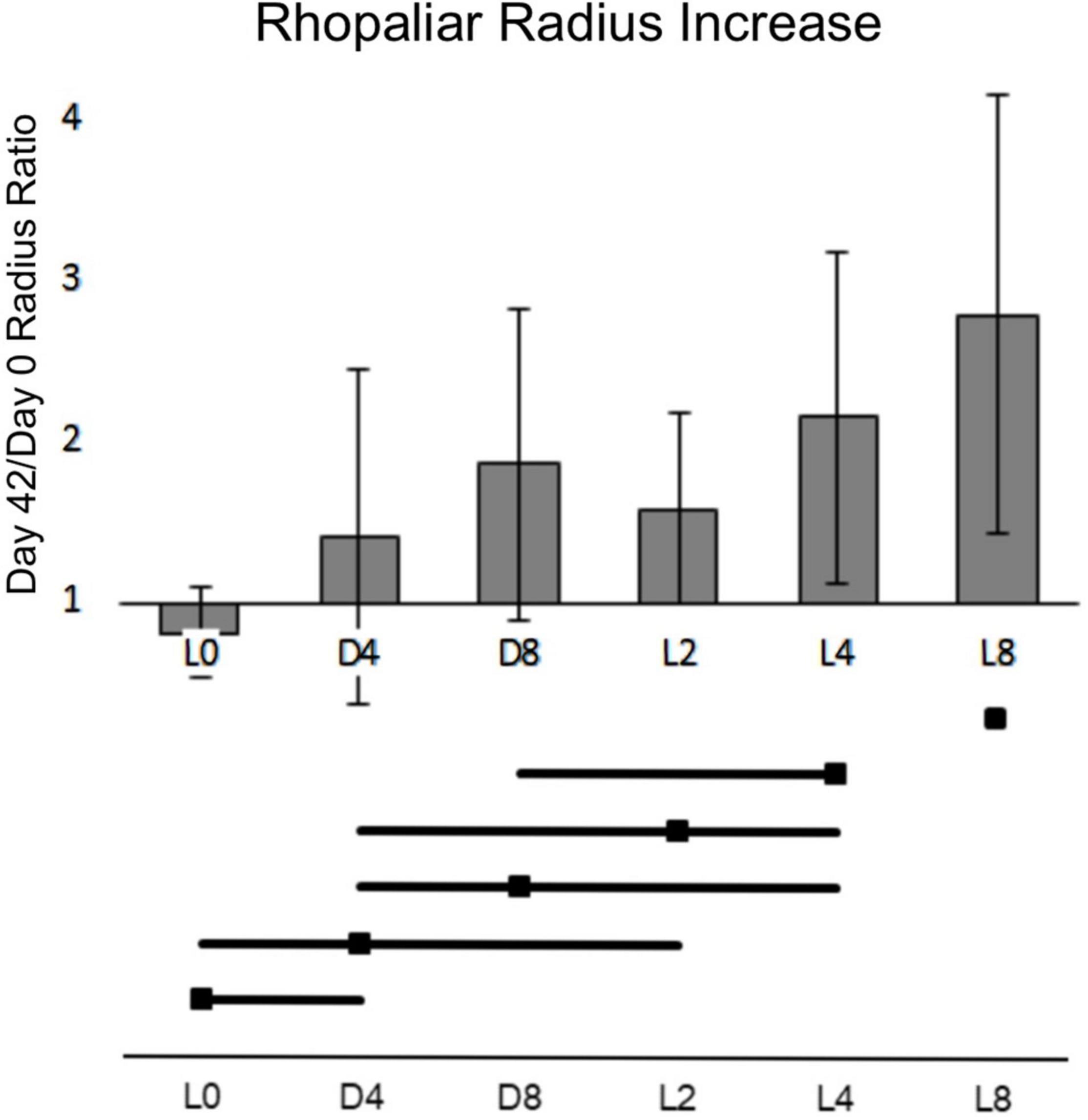
Figure 3. Ratio of days 42 to 0 rhopaliar radius averaged by group. X-axis represents growth multiplier in radius from days 0 to 42. Error bars represent 95% confidence interval. Horizontal bars represent non-significance of comparisons of the means at a = 0.05.
There was a highly significant interaction between Light and Feeding levels in growth (p = 1.7e-8), linked to a reduction in predation success in groups held in the dark. This trend is visible in this clustering of individuals by averaged real consumption (Figure 4).
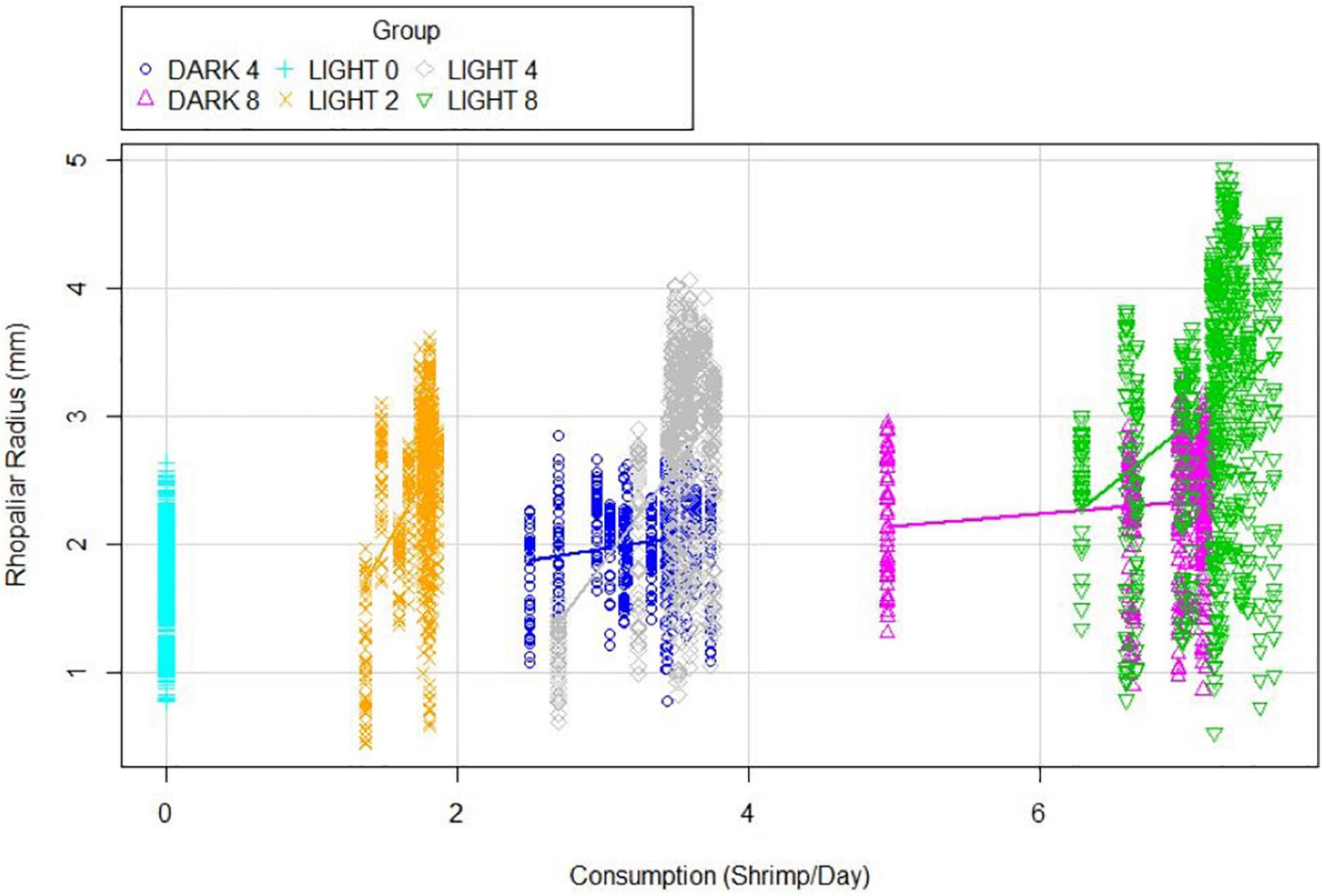
Figure 4. Rhopaliar radius explained Consumption averaged over the course of the experiment. Each direct vertical represents a single individual, all groups are color-coded.
L2 consumed an average of 1.72 ± 0.27 Artemia per day. L4 consumed 3.52 ± 0.51 Artemia per day, slightly higher than D4. D4 individuals averaged 0.259 fewer Artemia nauplii consumed per day than their lit counterparts, while D8 averaged 0.347 fewer per day than L8. Within every group (except for L0), an increase in real consumption was associated with a trend toward higher average rhopaliar radiuses and higher maximum rhopaliar radiuses. Within the L4 group, a linear model of real consumption was significantly associated with realized growth (adj. R2: 0.672, p = 6.117e-05). This was true for D4 (adj. R2: 0.578, p = 0.0009637) and D8 (adj. R2: 0.518, p = 0.007564) as well, but explained less than 5% of variability and was statistically insignificant in L2 and L8 groups. Oral arm to bell ratio showed no significant difference by group, and average oral arm length tracked fairly closely with rhopaliar radius.
Mass
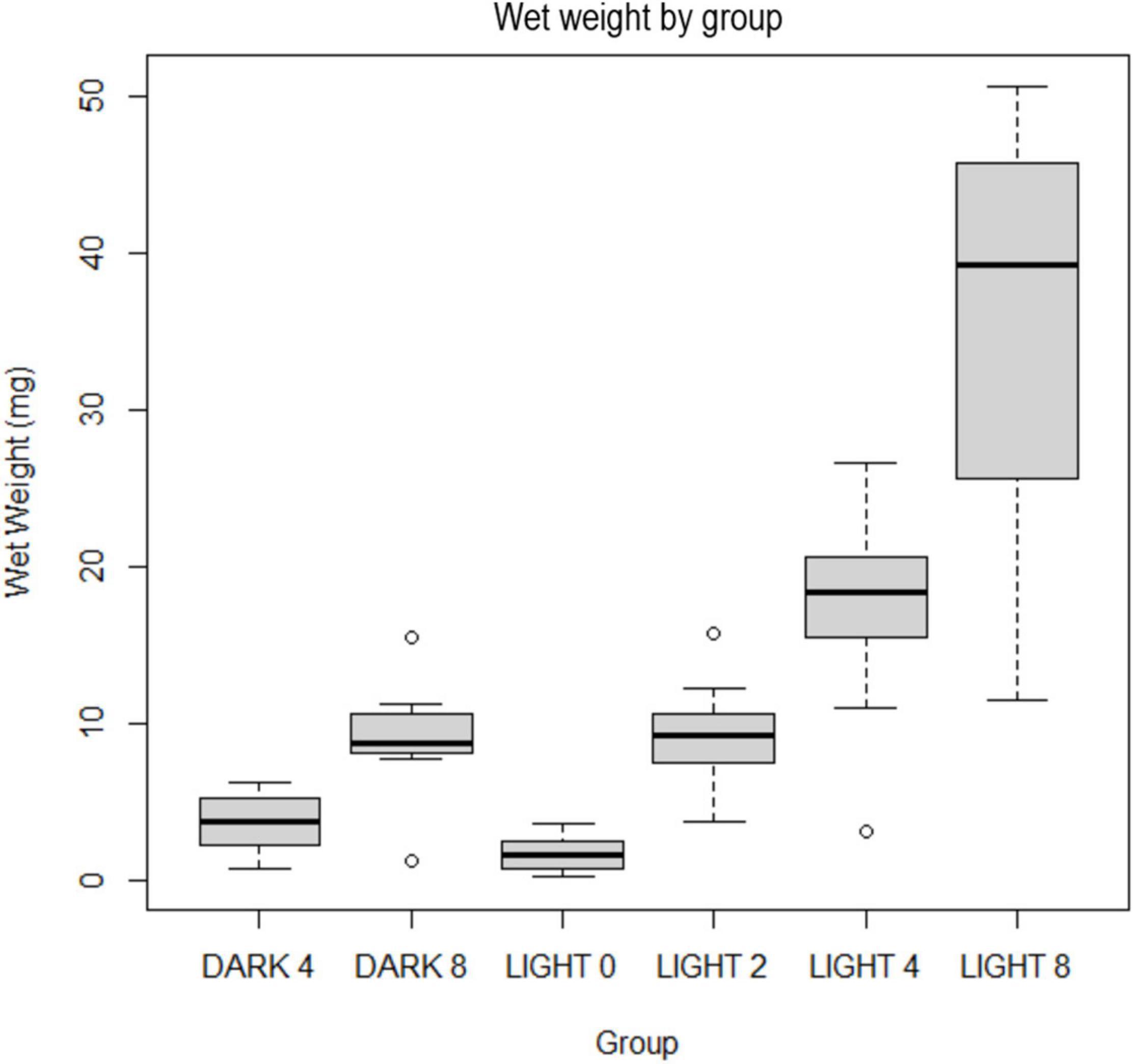
Final weights ranged from 0.2 to 50.6 mg of tissue with a heteroscedastic distribution of weights. The median weight was 9.7 mg. Average weights were 1.66 mg (L0); 9.04 mg (L2); 17.48 mg (L4); 36.30 mg (L8); 3.74 mg (D4); 9.12 mg (D8). The weight range for L8 was far larger than all other groups (11.7–50.6 mg) (Figure 5). The single lowest weight (0.2 mg) and the lowest average weight (1.7 mg) were in the L0 group.
Symbionts
Symbiont cell densities were normalized by tissue mass and were not statistically significant between experimental groups within the same light conditions. However, differences were significant (p < 2e-16) between light and dark groups (Figure 6). All dark individuals (25) had below 1,400 cells/mg, and the majority (15/25) had too few for symbiont cells to be present in the hemacytometer sampling (recorded as zero). These individuals were all visibly blue. As the day-zero pooled ephyrae sample had a zooxanthelle concentration of 27,400 cells/mg tissue, the low zooxanthellae counts in bleached ephyrae likely represent a loss in cells, not a maintenance of low cell density.
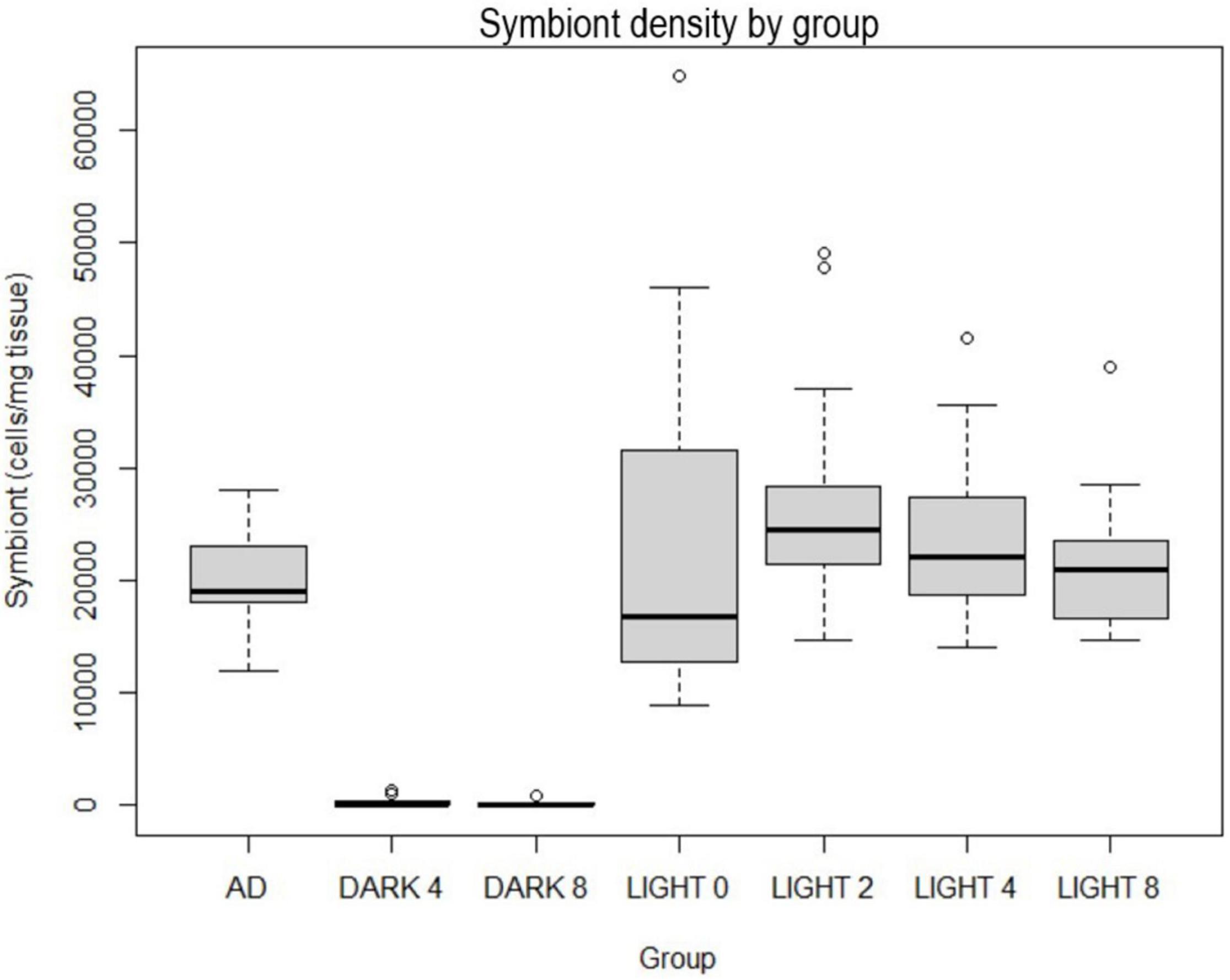
Figure 6. Symbiont cell densities per mg tissue, “AD” group represents digested segments from 12 adults between 8 and 31 mm in diameter from the same polyp culture held in standard tank light conditions (350 PAR).
In the D groups, shifts toward bleached coloration within the bell began within the first week of life and continued throughout the experiment. The average time to first reduction in color intensity was 6.5 days in the D4 group and 5.6 days in the D8 group. About half of the 25 surviving individuals in the D groups started bleaching between days 4 and 6. The earliest bleaching involved a D4 group ephyra that had little coloration at day 0 and was entirely devoid by day 5. The latest was a D4 group ephyra that did not begin losing pigmentation until day 34. Four D4 individuals and one D8 individual were not bleached by day 42. The average time to plateau (the point at which visible reduction in pigmentation stopped, even if a small number of visible spot clusters remained) was 33 days, with 50% plateauing between 26.1 and 39 days.
Six out of 98 individuals had exceptionally little pigmentation at day zero (entirely transparent oral arms). Bleaching at day zero was observed in 4 (out of 29) ephyra in the D treatments and 2 (of 65) in L treatment. The two bleached or near bleached ephyrae in the light group slowly gained full coloration over the course of the experiment. There was no increased mortality associated with the bleaching (one of the six died) or any difference in growth rates compared to their peers.
Oral Arm Appendage Development
In the analysis of variance comparisons, the number of oral arm appendages relative to size was far higher in analysis of variance comparisons in the D groups relative to their lit counterparts (D8 vs. L8 and D4 vs. L4, p = < 2e-16) (Figure 7). Over the 6 weeks of the study, tissue nodules resembling small versions of the floating appendages usually found on adult Cassiopea developed on almost all individuals (in D and L treatments), except those in the unfed group (L0). New tissue nodules generally appeared between days 9 and 15 and expanded in size or shrank as the medusae expanded or shrank. However, some may be the beginning stages of the oral arm compartments holding cassiosomes (free-floating nematocyst-laden stinging structures) in adults (Ames et al., 2020). When controlling for rhopaliar radius, the number of oral arm appendages per mm radius was significantly higher in zero-light groups than their counterparts in low light.

Figure 7. Oral arm appendage density (number of OAAs per mm rhopaliar radius) averaged by group over the 42 days.
Starvation Dark Control
To determine Cassiopea tolerance to starvation and absence of light, 10 additional ephyrae were placed under completely lightless conditions without feeding for 4 weeks. They were then moved into lit conditions with high food availability (8 Artemia/day) to determine whether they were recoverable. Two out of the 10 individuals died during starvation (days 13 and 18), and three individuals died during the recovery (days 36, 36, 39). Generally, individuals who fell close to or below 1.5 mm bell diameter were unable to consume Artemia nauplii and/or began disintegrating.
Half of the individuals (5/10) survived starvation and recovery. The surviving ephyrae gained back more than their lost size within 2 weeks post-starvation (surviving ephyrae were 20% larger at day 42 than day 0). The surviving ephyrae had an average diameter of 3.46 mm on day 0. The nadir in average diameter for this group was 2.17 mm on day 29, less than two-thirds their size at release. After a 2-week recovery, the average diameter rose to 4.17 mm, nearly double their size 2 weeks prior (Figure 8).
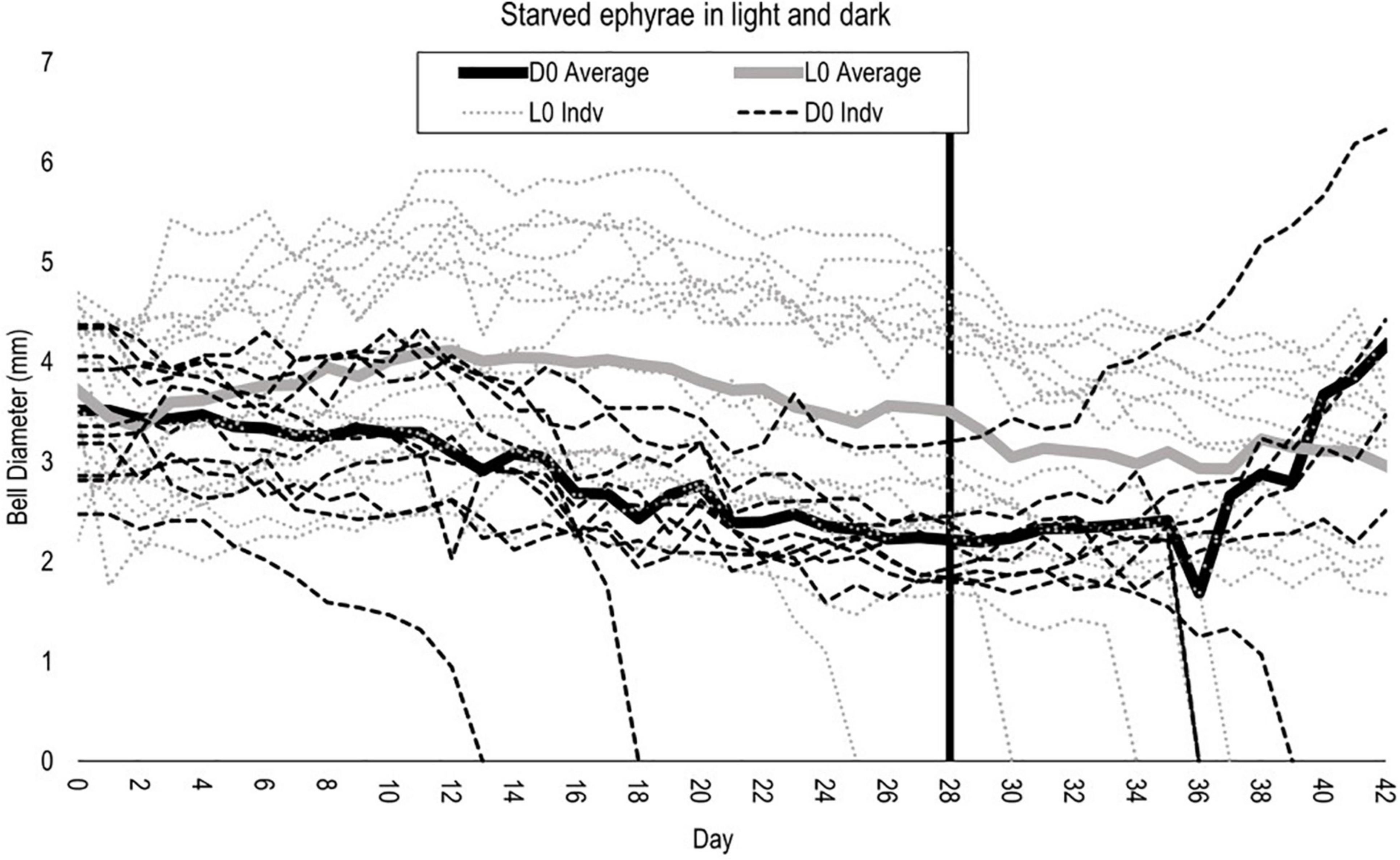
Figure 8. Bell diameter of starved light (gray) and dark (black) individuals. Vertical line at removal from starvation (day 28) for dark individuals only. Continuous lines are averages for each group.
Aberrant Body Forms
Four individuals (two in L2, one in L4, and one in L8) developed oversized oral arms relative to bell size. The two individuals in the L2 also displayed inverted bells in the first 2 weeks of the experiment. Nine individuals across different treatments regularly had tensed bells in the first 14 days of the experiment, causing them to be laid on their sides during daily recording. These individuals mostly returned to normal pulsing patterns within 10 days. All three individuals were removed early for failure to feed (see section “Removal of Damaged and Dead Ephyra”) and had some visible abnormality by day 3. Specimen 3 (L4) and 26 (L2) tightly contracted their bells into a near sphere, while specimen 66 (D8) inverted its bell before ceasing to pulse altogether. Specimen 17 (D4) developed two especially large oral arm appendages, extending well over the bell margin despite relatively small oral arms. All aberrant body forms and positionings can be seen in Supplementary Photographs.
Discussion
We observed 108 ephyrae of Cassiopea respond to 7 combinations of light conditions and food regimes for 43 days. While starved Aurelia ephyrae show a reduction in bell size (Fu et al., 2014), Cassiopea ephyrae’s response to starvation in light (L0 group) is a reduction in the size of the manubrium with a statistically insignificant reduction in bell size. Starved Cassiopea ephyrae kept at below compensation light levels (treatment L0) were able to survive 6 weeks, with only a few showing a reduction to the size at which ability to feed is hindered (<2 mm diameter). Starved Cassiopea ephyrae in dark conditions (D0) were also able to survive for 28 days and showed remarkable recovery capability when feeding and light exposure was reinstated. Our results on survivability in the dark and absence of food are overall similar to those observed in Aurelia ephyra (Cassiopea: 62.5% recovery at 28 days starvation in 24°C, Aurelia: 60% advancement at 33 days in 15°C) (Fu et al., 2014).
Starvation work on Cassiopea is not new, and many of the changes observed in early authors on the subject holdshold true here. The mouthparts of the starved ephyrae experienced clear retreat and many stressed individuals did experience bell inversion as reported by Mayer (1914). Hatai (1917) and Mayer (1994) both report severe shrinking in small starved Cassiopea, so it is both possible that these works signal a difference between adults and ephyrae, and that these works occurred in darker environments than 41 PAR.
As documented in corals, marginally sub-compensation level light was not an impetus for bleaching in Cassiopea ephyra, but near-zero light exposure was (D4 and D8) (Figure 7; Titlyanov et al., 2001). Growth in zero-light ephyrae was significantly reduced compared to their unbleached counterparts kept at a light level of ∼41 uE/m2/s for 12 h/day (L0, L2, L4, L8). All ephyra in light treatments (L0, L2, l4, L8) maintained similar symbiont densities to those of healthy adults, while zero-light groups had an average symbiont density less than 1.5% the average symbiont density of their peers in light groups, most with densities too low to be detected (Figure 7). The variability in photosynthesis to respiration rates reported by past work on Cassiopea may be in part due to the large differences in Symbiodinium cell counts between individuals—while the Symbiodinium densities reported here for day zero and adult controls and all light groups are in overlapping ranges, they include individuals with double the cell/mg zooxanthellae density of others (Cates, 1975). Within light conditions (Dark or Light), final weight was correlated with the amount of food, with weight increasing from L0 to L8 and from D4 to D8. Ephyrae in L8 weighed on average 21.9 times more than L0. The mean weight of ephyrae kept in the dark and fed with four nauplii/day was less than half (41%) that of ephyra kept in lit conditions and fed with two nauplii/day. In Cassiopea specifically, bleaching has been suggested to have an impact on wet weight in adults (McGill and Pomory, 2008). Within this study we see a marked differential in individuals allowed light access and not, likely exacerbated by the starting condition of the individuals (i.e., newly released, less tissue). The weight of the ephyra kept in the dark and fed eight nauplii a day was nearly indistinguishable from the weight of ephyra kept in lit conditions and fed with two nauplii/day (9.11 vs. 9.04 mg). Weight comparison between ephyrae kept at similar feeding regimes but with or without light (D8 vs. L8 and D4 vs. L4) indicates that the presence of light was responsible for a fourfold increase in weight.
Oral arm appendages developed early (week 2) in most ephyrae and in larger numbers (relative to size) in those fed in the dark (D4 and D8). However, when not controlling for ephyra size, D8/L8 and D4/L4 had similar trajectories, possibly indicating that oral arm appendage development is influenced by consumption regardless of ephyra size. While the exact reason is unclear, the higher number of appendages represents the only morphological difference, beyond bleaching itself, observed between ephyrae kept in dark and light conditions.
The ephyrae photographed demonstrated high variability between individuals in coloration, number of rhopalia, and growth outcomes. A sizable number developed irregularly as well. Beyond enlarged oral arms, our photographs also captured irregular coloration development, oral arm structure changes, bell patterning, and other features of growth not enumerated here. We encourage the full exploration and use of the over four thousand photographs available in Supplementary Materials. A time series of one individual from each group is pictured below (Figure 9).
In summary, we show that ephyrae of Cassiopea xamachana are tolerant of food and light stress and may be a sturdy life cycle stage with potential for species introduction. Furthermore, we show that they may survive in environments that are suboptimal in both light and food requirements, and that Cassiopea ephyrae can retain stable zooxanthellae levels in response to changes in feeding regime.
While polyps, planula, podocysts and planulocysts, are still the more likely long-distance spreading life cycle stages, our data join a recent body of knowledge that shows ephyrae have a remarkable ability to withstand extreme conditions and can possibly survive weeks-long journeys in ballast water or on currents to new locations.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI (accession: MZ343250). All photographs are available on Figshare under project “Cassiopea Light and Development”, available at: https://figshare.com/projects/Cassiopea_Light_and_Development/123538.
Author Contributions
KM and MM contributed to conception and design of the study. KM and JA collected and processed data. KM performed the statistical analysis and wrote the first draft of the manuscript. JA wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was funded by Texas A&M University Galveston in the form of supplies and space grants to the primary and secondary authors.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the Sea Life Facility at Texas A&M Galveston for housing polyps and adults of our Cassiopea cultures. We would like to thank Evomar 2020 for the opportunity to present the preliminary data for this work.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.783876/full#supplementary-material
References
Ames, C. L., Klompen, A. M. L., Badhiwala, K., Muffett, K., Reft, A. J., Kumar, M., et al. (2020). Cassiosomes are stinging-cell structures in the mucus of the upside-down jellyfish Cassiopea xamachana. Commun. Biol. 3:67. doi: 10.1038/s42003-020-0777-8
Brotz, L., and Pauly, D. (2012). Jellyfish populations in the mediterranean sea. Acta Adriat. 53, 211–230.
Cabrales-Arellano, P., Islas-Flores, T., Thomé, P. E., and Villanueva, M. A. (2017). Indomethacin reproducibly induces metamorphosis in Cassiopea xamachana scyphistomae. PeerJ 2017, 1–11. doi: 10.7717/peerj.2979
Cates, N. (1975). Productivity and organic consumption in Cassiopea and Condylactus. J. Exp. Mar. Biol. Ecol. 18, 55–59. doi: 10.1016/0022-0981(75)90016-7
Frolova, A., and Miglietta, M. P. (2020). Insights on bloom forming jellyfish (class: scyphozoa) in the gulf of mexico: environmental tolerance ranges and limits suggest differences in habitat preference and resistance to climate change among congeners. Front. Mar. Sci. 7:93. doi: 10.3389/fmars.2020.00093
Fu, Z., Shibata, M., Makabe, R., Ikeda, H., and Uye, S. I. (2014). Body size reduction under starvation, and the point of no return, in ephyrae of the moon jellyfish Aurelia aurita. Mar. Ecol. Progr. Ser. 510, 255–263. doi: 10.3354/meps10799
Graham, W. M., and Bayha, K. M. (2007). Biological Invasions by Marine Jellyfish. Berlin Heidelberg: Springer, 239–255. doi: 10.1007/978-3-540-36920-2_14
Hatai, S. (1917). On the composition of the medusa Cassiopea xmachana the changes in it after starvation. Proc. Nat. Acad. Sci. U.S.A. 3:22. doi: 10.1073/pnas.3.1.22
Holland, B. S., Dawson, M. N., Crow, G. L., and Hofmann, D. K. (2004). Global phylogeography of Cassiopea (scyphozoa: rhizostomeae): molecular evidence for cryptic species and multiple invasions of the Hawaiian Islands. Mar. Biol. 145, 1119–1128. doi: 10.1007/s00227-004-1409-4
Lotan, A., Ben-Hillel, R., and Loya, Y. (1992). Life cycle of Rhopilema nomadica: a new immigrant scyphomedusan in the mediterranean. Mar. Biol. 112, 237–242.
Mammone, M., Ferrier-Pages, C., Lavorano, S., Rizzo, L., Piraino, S., and Rossi, S. (2021). High photosynthetic plasticity may reinforce invasiveness of upside-down zooxanthellate jellyfish in mediterranean coastal waters. PLoS One 16:e0248814. doi: 10.1371/journal.pone.0248814
Mayer, A. G. (1914). Laws governing loss of weight in starving Cassiopea. Carnegie Instit. Washington Public. 6, 55–82.
McGill, C. J., and Pomory, C. M. (2008). Effects of bleaching and nutrient supplementation on wet weight in the jellyfish Cassiopea xamachana (bigelow) (Cnidaria: Scyphozoa). Mar. Fresh. Behav. Physiol. 41, 179–189.
Miller, M. E. C., and Graham, W. M. (2012). Environmental evidence that seasonal hypoxia enhances survival and success of jellyfish polyps in the northern gulf of mexico. J. Exp. Mar. Biol. Ecol. 432–433, 113–120.
Morandini, A. C., Stampar, S. N., Maronna, M. M., and Da Silveira, F. L. (2017). All non-indigenous species were introduced recently? The case study of Cassiopea (cnidaria: scyphozoa) in Brazilian waters. J. Mar. Biol. Assoc. U.K. 97, 321–328.
Ohdera, A. H., Abrams, M. J., Ames, C. L., Baker, D. M., Suescún-Bolívar, L. P., Collins, A. G., et al. (2018). Upside-down but headed in the right direction: review of the highly versatile Cassiopea xamachana system. Front. Ecol. Evolu. 6:1–15. doi: 10.3389/fevo.2018.00035
Purcell, J. E. (2012). Jellyfish and ctenophore blooms coincide with human proliferations and environmental perturbations. Ann. Rev. Mar. Sci. 4, 209–235. doi: 10.1146/annurev-marine-120709-142751
Skikne, S. A., Sherlock, R. E., and Robison, B. H. (2009). Uptake of dissolved organic matter by ephyrae of two species of scyphomedusae. J. Plankton Res. 31, 1563–1570. doi: 10.1093/plankt/fbp088
Swift, H. F., Gómez Daglio, L., and Dawson, M. N. (2016). Three routes to crypsis: stasis, convergence, and parallelism in the Mastigias species complex (scyphozoa, rhizostomeae). Mol. Phylogenet. Evolu. 99, 103–115. doi: 10.1016/j.ympev.2016.02.013
Titlyanov, E. A., Titlyanova, T. V., Yamazato, K., and Van Woesik, R. (2001). Photo-acclimation dynamics of the coral Stylophora pistillata to low and extremely low light. J. Exp. Mar. Biol. Ecol. 263, 211–225. doi: 10.1016/S0022-0981(01)00309-4
Verde, E. A., and Mccloskey, L. R. (1998). Production, respiration, and photophysiology of the mangrove jellyfish Cassiopea xamachana symbiotic with zooxanthellae: effect of jellyfish size and season. Mar. Ecol. Prog. Ser. 168, 147–162.
Welsh, D. T., Dunn, R. J. K., and Meziane, T. (2009). Oxygen and nutrient dynamics of the upside down jellyfish (Cassiopea sp.) and its influence on benthic nutrient exchanges and primary production. Hydrobiologia 635, 351–362. doi: 10.1007/s10750-009-9928-0
Keywords: Cassiopea, ephyra, zooxanthellae, medusa, development
Citation: Muffett KM, Aulgur J and Miglietta MP (2022) Impacts of Light and Food Availability on Early Development of Cassiopea Medusae. Front. Mar. Sci. 8:783876. doi: 10.3389/fmars.2021.783876
Received: 27 September 2021; Accepted: 06 December 2021;
Published: 25 January 2022.
Edited by:
Maria Vittoria Modica, Zoological Station Anton Dohrn, ItalyReviewed by:
Shin-ich Uye, Hiroshima University, JapanAki Ohdera, California Institute of Technology, United States
Edgar Gamero-Mora, Centro de Investigación en Alimentación y Desarrollo, Consejo Nacional de Ciencia y Tecnología (CONACYT), Mexico
Copyright © 2022 Muffett, Aulgur and Miglietta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaden McKenzie Muffett, a21tdWZmZXR0QHRhbXUuZWR1
 Kaden McKenzie Muffett
Kaden McKenzie Muffett Joleen Aulgur
Joleen Aulgur Maria Pia Miglietta
Maria Pia Miglietta