- ICAR-Central Marine Fisheries Research Institute, Cochin, India
Pomfrets (genus Pampus), a highly commercial fishery resource distributed in the Indo-Western Pacific that includes Lessepsian migrants, have witnessed a series of systematic reforms. In this study, based on comprehensive sampling spanning type localities and coevals in the Northern Indian Ocean, the cryptic and valid species Stromateus griseus is resurrected from the synonymy and re-described as Pampus griseus (New Combination) based on 35 specimens from the Bay of Bengal, corroborated by a molecular analysis, which indicated a confined distribution of the species. The Bayesian phylogeny of the genus was reconstructed, incorporating redressed barcodes (582 nucleotides) and concatenated mitochondrial gene sequence data (1,822 nucleotides) generated from the recorded species P. candidus, P. chinensis and the neophyte along with sequences from GenBank entrusting the latest literature. The phylograms differed in topology as for seven valid species, and the one predicated on the concatenated data erected a highly supported polytomous clade for the P. cinereus complex (P. griseus, P. cinereus, and P. candidus) which shares synapomorphies. Pampus argenteus and P. minor, together, formed a sister clade to the rest. Climate-driven vicariant events during glacial epochs and the Indo-Pacific Barrier effect can be the drivers behind the Indian and Pacific Ocean sister lineages in P. chinensis. A multivariate analysis isolated the cryptic species from its congeners. This article portrays the systematics revision of genus Pampus with an integrative taxonomic approach compiling distinctive molecular, morphological, and anatomical features, revised key for species identification, taxonomic archives of Indian stromateids, and winds up with specific remarks.
Introduction
The genus Pampus (family: Stromateidae), commonly known as pomfret, first proposed by Bonaparte (1837), characterized by an oval to diamond-shaped body, falcate median fins with a series of 5–10 small blade-like spines protruding ahead of it, and absence of ventral spine on the pelvic bone (Haedrich, 1967), is widely distributed in the Indo-Western Pacific Oceans renowned for species diversity (Manel et al., 2020), with possible Lessespian migrants in the Mediterranean (Sami et al., 2014). These medium-sized schooling fishes, at times invading estuaries and frequently caught in trawls (Haedrich, 1984; Last, 1997), are recognized as one of the most valuable and relished table fishes in both internal and export markets because of their soft and tender flesh, few bones, good taste, high protein and fat content (Gupta, 2020). There are reports on their utilization in Chinese medicines (Tang, 1987). High value and perpetual demand make the genus one of the most sought after in commercial targeted fisheries in its native ranges, such as India (CMFRI, 2020),1 countries bordering the seas of China, the maritime subregion of the Bay of Bengal (“BOB”), Persian Gulf countries, and Pakistan (Froese and Pauly, 2020). Despite this, the taxonomy of the genus Pampus has been uncertain and is still debated because of synapomorphies (Yin et al., 2019), and, conversely, the taxonomic literature hinted that many of the known species probably represented misidentified ones, leading to the occurrence of synonyms (Li et al., 2019c).
Latest global reports indicate the presence of the following seven valid species in the genus including the cryptic species described herein: Pampus argenteus, Pampus minor, Pampus punctatissimus, Pampus chinensis, Pampus cinereus, Pampus candidus, and Pampus sp. In 2017, Jawad and Jig, based on detailed comparative osteology of the axial skeleton, identified eight valid species in this genus, such as Pampus liuorum (Liu and Li, 2013) and Pampus nozawae (Ishikawa, 1904), which were found to be invalid by previous and subsequent researchers who considered them as synonyms of P. cinereus (Liu et al., 2013a; Li et al., 2019c; Yin et al., 2019). The existence of a strong geographic genetic structure in P. argenteus from the Indo-Western Pacific area was indicated (Sun et al., 2013), and a COI-based analysis of Pampus specimens by Divya et al. (2017) revealed the presence of seven distinct but taxonomically indecisive clades with two putative species viz. Pampus sp.1 from the Arabian Sea and the BOB, and Pampus sp.2 from the BOB. Subsequently, the Pampus sp.1 was re-described as P. candidus by Divya et al. (2019). They hinted that the Pampus sp.2 is a second valid species that was previously designated as Pampus sp. nov by Li et al. (2016), and recommended further morphological investigation to arrive at a taxonomic conclusion. Later, Li et al. (2019c) reiterated seven species in the genus based on COI analysis and prepared a key for six, although it turned out to be inconclusive. In the same year, Yin et al. (2019) provided a robust classification of Pampus based on ample nuclear markers and detailed morphological re-examination that provided a simplified identification key for the following five species: P. argenteus, P. punctatissimus, P. chinensis, P. minor, and P. cinereus. Their study confirmed that P. argenteus and P. cinereus are two distinct species and that Pampus echinogaster and Pampus liuorum are invalid being synonyms of P. argenteus and P. cinereus, respectively. They considered the three clades in P. cinereus that were grouped according to sampling localities as the P. cinereus complex.
Historically, five species of pomfrets (Pampus) were described from Indian waters in Cuvier and Valenciennes (1833): Stromateus candidus from Malabar (Kerala) and Pondicherry (Puducherry), Stromateus securifer from Bombay (Mumbai), Stromateus griseus from Pondicherry, and Stromateus albus and Stromateus atous from Visakhapatnam. The last two were synonymized to P. chinensis by Haedrich (1967). Later, S. candidus, in synonymy with P. argenteus, was resurrected to Pampus candidus (Cuvier, in Cuvier and Valenciennes, 1833), and S. securifer was synonymized with it by Divya et al. (2019), who indicated that P. candidus, so far treated as P. argenteus in India, appears to be the most common species in Indian waters, and that the latter is totally absent along the Indian coastline. Divya et al. (2019) further indicated that the name Pampus griseus (Cuvier and Valenciennes, 1833) is valid if the BOB counterpart (Pampus sp.2) turns to be distinct.
Inaccurate identification of cryptic fish species and use of ambiguous names in landing reports will lead to undesired consequences in long-term sustainable fishery management plans (Fischer, 2013), where collection of species-specific information is vital. Meanwhile, similarity in external morphological traits among pomfrets, in general, poses problems in proper identification, resulting in numerous controversies regarding species classification, associated nomenclatures, and numerous erroneous GenBank records (Li et al., 2019c). Pampus candidus, misidentified as P. argenteus, formed a regular export commodity from Veraval, a major port in India, to Chinese, Middle East, and EU markets, with reports of 10–15 containers (∼26 tons/container) during peak fishing season (September to December) (Source: Export Inspection Council, Veraval). Precise information on species forms the basis for international trade, consumer safety, biodiversity research, and prevention of fraudulence (Fischer, 2013). Traceability is being applied within seafood supply chains to ensure the legality and sustainability of products (Lewis and Boyle, 2017). However, conventional fish identification methods based on morphology may, at times, lead to misidentification, especially in the case of cryptic and recently diverged species. Furthermore, this cannot be fully relied on when only a part of the body is available; therefore, mislabeling can happen at any point (Willette et al., 2017). In such situations, DNA-based approaches can be successfully applied as an alternative tool for seafood authentication, even on partially or fully processed fishes when important morphological characters are lost.
Traditional taxonomic tools cannot provide a stand-alone platform to solve the taxonomic perplexity of pomfrets; hence, an integrative taxonomic approach that combines molecular, morphological, and ecological characters, which is successful in detecting cryptic species (Guimarães et al., 2020) and resolving the systematics (Katwate et al., 2020), was adopted. This study aimed at the following objectives, viz., (i) establish the identity of the cryptic congener hitherto indicated in the literature as Pampus sp. nov (Li et al., 2016)/Pampus sp.2 (Divya et al., 2017, 2019)/Pampus sp. (Li et al., 2019c) in comparison with its contemporaries P. candidus and P. chinensis sympatric in Indian waters (ii) re-evaluate Pampus spp. of the Indo-Western Pacific for phylogenetic resolution (iii) revise the systematics, and (iv) prepare a field identification key for all the species. Classical taxonomic tools, viz., morphology, multivariate analysis, gill raker shape, sagittal otolith morphology, vertebral count, morphology of transverse occipital canal of the lateral line, and fishery information, have been integrated into this study with molecular data for all the species fished along the Indian coast, and with global literature and database for inference.
Materials and Methods
Taxon Sampling
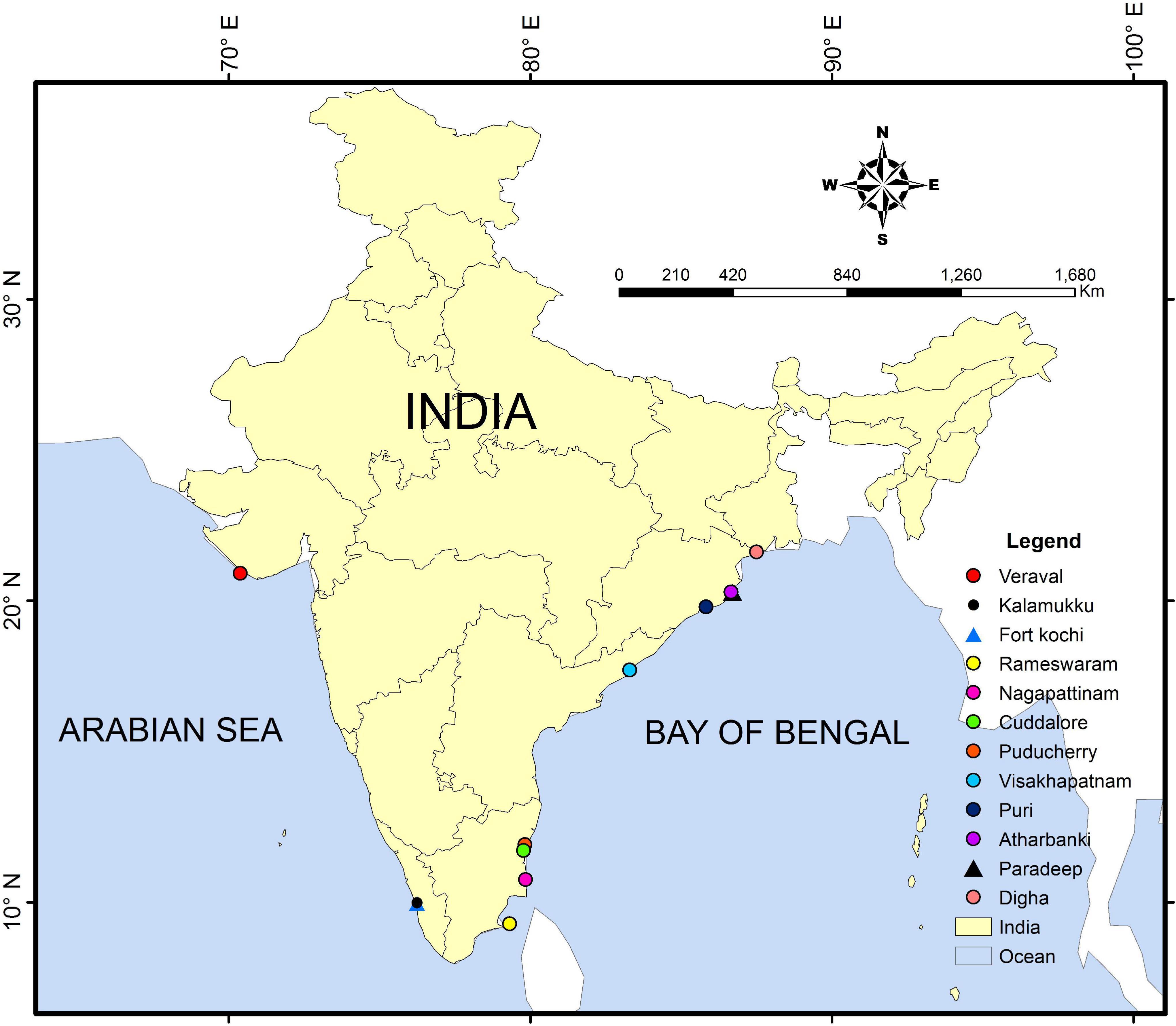
One hundred thirteen fresh specimens of Pampus spp. were collected from various locations such as type localities along the east coast in the BOB (West Bengal, Odisha, Andhra Pradesh, and Tamil Nadu) and the west coast (Gujarat and Kerala) in the Arabian Sea from 2019 to 2020 (Figure 1). The specimens were sampled directly from fishing vessels and fish landing sites. The collected specimens were transported to the laboratory in crushed ice for detailed taxonomic investigation and photographed immediately to capture their original color and pigmentation. The species were tentatively identified based on original descriptions and recent keys (Divya et al., 2019; Li et al., 2019c; Yin et al., 2019).
Materials Examined
Pampus candidus/Pampus sp. (n = 69)
Six ex., 132–167 mm SL, Paradeep fishing harbor (20°17.345′N, 086°42.422′E), Jagatsingpur, Odisha, trawl, 40–70 m, sandy silt, 24 Jan. 2020; 9 ex., 117–177 mm SL, Atharabanki landing center (20°17′28.2264″N, 86°39′18.738″E), Jagatsingpur, Odisha, India, gillnet, 30 m, sandy silt, June 9 and October 3, 2020; 2 ex., 87–96 mm SL, Fort Kochi (9°58′6.1572″N, 76°14′35.6856″E), Kerala, gillnet, 10–15 m, muddy, June 29, 2020; 13 ex., 100–161 mm SL, Veraval fishing harbor (20°54′19.23″N, 070°22′52.03″E), Gujarat, gillnet, 30 m, muddy, July 14, 2020; 4 ex., 180–190.7 mm SL, Rameshwaram fish landing center (9.281146°N, 79.315055°E), Palk Bay, Tamil Nadu, trawl, 15–20 m, muddy, August 13, 2020; 7 ex., 70.6–120.5 mm SL, Puri north landing center (19°47′43.062″N, 85°49′38.5788″E), Puri, Odisha, shore seine, 3–5 m, sandy silt, August 18, 2019; 1 ex., 177 mm SL, Veraval fishing harbor (20°54′19.23″N, 070°22′52.03″E), Gujarat, trawl, 30 m, muddy, August 24 2020; 4 ex., 102–115 mm SL, Visakhapatnam Fishing Harbor (17.696°N, 83.301°E), Andhra Pradesh, trawl, 35–40 m, sandy silt, September 2, 2020; 7 ex., 130–203 mm SL, Nagapattinam fishing harbor (10°45′03.8″N, 79°50′24.0″E), Tamil Nadu, trawl, 30–100 m, rocky, September 11, 2020; 5 ex., 120–140 mm SL, Fish Market, Kochi, Kerala, India, September 7, 2020; 4 ex., 83–110 mm SL, Digha (21°36′58.3272″N, 87°29′56.8644″E), West Bengal, shore seine, 3.5 m, sandy silt, September 12, 2020; 2 ex., 146–150 mm SL, Kalamukku (9°59′18.006″N, 76°14′34.836″E), Kochi, Kerala, trawl, 30–40 m, muddy, September 15, 2020; 2 ex., 150–170 mm SL, Puducherry (Pondicherry) fishing harbor (16°3′17.964″N, 78°14′47.49″E), trawl, sandy, Tamil Nadu, October 10, 2020; 3 ex., 150–170 mm SL, Cuddalore Harbor (11°42′52″N, 79°46′31″E), Tamil Nadu, Multiday trawl, 15 m, sandy, October 2020.
Pampus chinensis (n = 44)
Five ex., 86–110 mm SL, Digha (21°36′58.3272″N, 87°29′56.8644″E), West Bengal, shore seine, 3.5 m, sandy silt, September 12, 2020; 6 ex., 87.2–152.5 mm SL, Puri North landing center (19°47′43.062″N, 85°49′38.5788″E), Puri, Odisha, gillnet, 10–12 m, sandy silt, July 24, 2019; 14 ex., 67–216.3 mm SL, Paradeep fishing harbor (20°17.345′N, 086°42.422′E), Jagatsingpur, Odisha, India, trawl, 40–70 m, sandy silt, September 8, 2020; 3 ex., 114–129 mm SL, Visakhapatnam fishing harbor (17.696°N, 83.301°E), Andhra Pradesh, India, trawl, 35–40 m, sandy silt, September 2, 2020; 4 ex., 163–203 mm SL, Veraval fishing harbor (20°54′19.23″N, 070°22′52.03″E), Gujarat, India, trawl, 60–70 m, muddy, August 24, 2020; 7 ex., 132–252 mm SL, Nagapattinam fishing harbor (10°45′03.8″N 79°50′24.0″E), Tamil Nadu, India, trawl, 30–100 m, rocky, September 11, 2020; 2 ex., 160–170 mm SL, Puducherry fishing harbor (16°3′17.964″N, 78°14′47.49″E), Tamil Nadu, India, trawl, October 2020; 3 ex., 200–210 mm SL, Cuddalore Harbor (11°42′52″N, 79°46′31″E), Tamil Nadu, Single day trawl, 9 m, sandy, October 2020.
Museum Specimens Examined
Two specimens of Pampus from the Marine Biodiversity Referral Museum of ICAR-Central Marine Fisheries Research Institute (CMFRI), Kerala, India, and two digital images of Pampus from the Museum National D’histoire Naturelle, Paris were examined (Supplementary Figure 1).
Polymerase Chain Reaction and Sequencing
Genomic DNA was extracted using the phenol-chloroform method from ethanol-preserved fin clips of specimens (65 nos) specified in materials examined. Later, an additional sample of 18 nos. were added to maintain a proper sample size (Supplementary Table 1). Partial fragments of the COI gene were amplified using universal primer pairs Fish F2 (5′ TCGACTAATCATAAAGA TATCGGCAC 3′) and Fish R2 (5′ ACTTCAGGGTGACCGAAGAATCAGAA 3′) (Ward et al., 2005). Cyt b, ATP6/8, and 16S rRNA genes were amplified using the universal primers L14724 (5′ GACTTGAAAAACCACCGTTG 3′) and H15915 (5′ CTCC GATCTCCGGATTACAAGAC 3′) (Xiao et al., 2001); ATP8. 2L8331 (5′ AAA GCRTYRGCCTTTTAAGC 3′), and COIII.2H9236 (5′ GTTAGTGGTCAKGGGCTTGGRTC 3′) (Sivasundar et al., 2001); and 16 Sar (5′ CGCCT GTTTATCAAAAACAT 3′) and 16 Sbr (5′ CCGGTC TGAACTCAGATCACGT 3′) primers (Palumbi et al., 1991). Polymerase chain reaction (PCR) amplifications were performed in 25 μl using TaKaRa Emerald Amp GT PCR Master Mix, primer pairs, and template DNA. The PCR profiles were as follows: 4 min at 94°C for initial denaturation followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 50–55°C depending on the genes for 30 s, elongation at 72°C for 45 s, and a final extension at 72°C for 10 min. The amplified products were purified and sequenced. Complementary sequences were edited and assembled into consensus using BioEdit v7.1.9.
Confirmation of Nucleotide Sequences
Sequence similarities were determined using the NCBI nBLAST tool, and the haplotype data generated for COI, Cyt b,16S rRNA, and ATPase 8/6 genes from this study were deposited in GenBank (Supplementary Table 1; accession numbers: MW422591–MW422604, MW421904–MW421919, MW343690–MW343704, MW366881–MW366888, MW419276–MW419288, MW337238–MW337245, MW3434 60–MW343467, MW332295–MW332304, MW260616–MW260 617, MW343468–MW343475, MW447297, MW447300–MW447 301, and MW456736). Representative sequences of the Cyt b (1,137 nucleotides (“nt”) and ATPase8/6 genes (835 nt) from previous population genetic studies (Sun et al., 2012; Divya et al., 2015) were also reanalyzed integrating sequences from this study to affirm the genetic distinctness of the cryptic species.
Phylogenetic Trees and Genetic Divergence
Separate phylogenetic analyses were carried out with two data sets, i.e., concatenated mitochondrial sequences (1,822 nt; Supplementary Table 2) and curated COI barcodes alone (582 nt; Supplementary Table 3), because of global data availability. The veracity of COI sequences from GenBank was confirmed, and misidentifications were corrected as per the latest references. Consequently, representative COI haplotypes were sampled for all the six valid species and Pampus sp. distributed in the Indo-Pacific Oceans, giving due weightage to their type localities and geographical origins. For concatenation of the noncoding (16S rRNA) and coding (COI and Cyt b) mitochondrial sequences, data available either from the same individuals or complete mitogenome data were used. In absentia, sequences from the same geographic areas were pooled after validation. The concatenation consisted of 470 nt of 16S, 582 nt of COI, and 770 nt of Cyt b with no introns or indels in the latter two. Stromateus stellatus from the paraphyletic genus Stromateus and the carangid Parastromateus niger were used as outgroups. The two datasets were separately aligned using Clustal W with a default setting in MEGA X (Kumar et al., 2018). The net genetic distance between species groups was also calculated in MEGA X under uniform rates using a Kimura two-parameter (K2P) model (Kimura, 1980) for 51 concatenated sequences eliminating P. minor 2, 4, 5, and 6 because of insufficient characters for the Cyt b region. Appropriate partition strategies and evolutionary models for each partition in concatenated data were selected with PartitionFinder v.1.1.1. A Bayesian analysis was implemented with MrBayes v.3.2 using the best model, i.e., GTR+G. The final consensus tree was visualized and edited in FigTree v.1.4.3.2 A Generalized Mixed Yule Coalescent (GMYC) model (Pons et al., 2006; Fujisawa and Barraclough, 2013), used for species delimitation analysis of concatenated multilocus data (Luo et al., 2018), was created by uploading the trees generated in BEAST1.8.0 (Drummond et al., 2012) with default settings. The maximum credibility tree was used as input for GMYC analysis with the SPLITS package in R (Ezard et al., 2009; Fujisawa and Barraclough, 2013), and results were visualized with R v.3.5.2 (R Core Team, 2018).
Morphometry and Otolith Comparison
Morphometric measurements and meristic counts were recorded according to Haedrich (1967) and Liu et al. (2013a,b). Total vertebrae were counted as the number of precaudal plus the number of caudal vertebrae, including the urostyle from the X-radiographs according to Jawad and Jig (2017). Sex and the gonad maturity stage of the specimens were determined to the possible extent based on the macroscopic observation of the gonads. The morphometric characters measured using a digital Vernier caliper (0.1 mm accuracy) were given as percentages of standard length (SL) for size-independent comparison with the other available species under this genus (Table 1). To remove the size component from the shape component, the morphometric measurements were subjected to allometric transformation, as suggested by Elliott et al. (1995). The standardization function was:
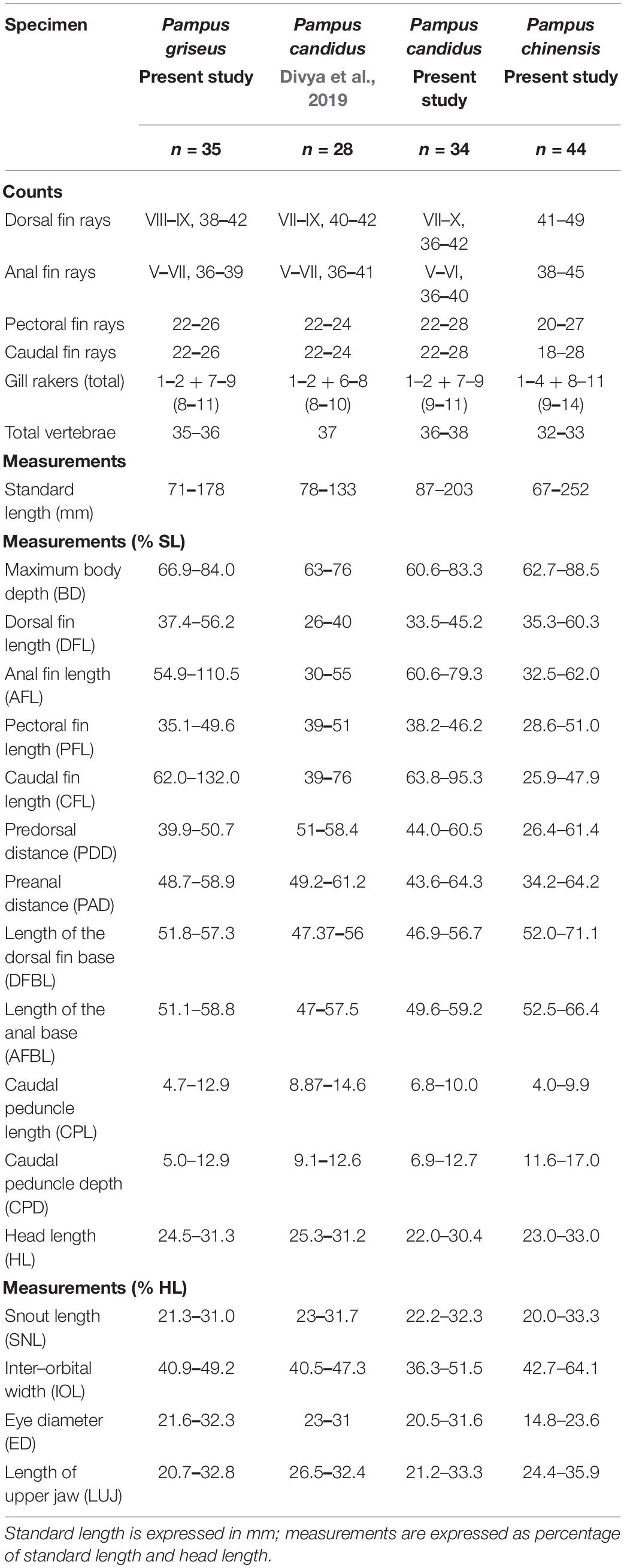
Table 1. Morphometric and meristic data for Pampus griseus, Pampus candidus, and Pampus chinensis from Indian waters.
Ms = Mo(SLm/SLo)b, where Ms is the standardized morphometric variable, Mo is the observed variable, SLm is the overall mean of the standard length (scaling variable), SLo is the observed standard length, and b is the within-group (here species) regression slope of Log Mo (Y-axis) on Log SLo (X-axis). The transformed data were subjected to principal component analysis (PCA) using the FactoMinerR package in R v.3.5.2 (R Core Team, 2018). Sagittal otoliths were extracted, cleaned in distilled water to remove the dirt and remnant tissue, air-dried, and stored in plastic vials. Images of the medial face of the otoliths were captured using a stereomicroscope (Nikon SMZ1270) fitted with a camera. As both the otoliths were found symmetrical in shape and size, only the left otoliths were photographed and used for comparison. The otoliths were assessed and compared based on gross morphology following Zhang et al. (2017). The key characteristics, like overall shape, presence/absence of crenulations, shape of anterior and posterior regions, type and shape of sulcus, ostium, and cauda, were compared across species for similarity or dissimilarity. Furthermore, we have also collected otoliths across different size ranges (juvenile, subadult, and adult) to account for any ontogenic changes as the species grows in size, which was lacking in descriptions of Zhang et al. (2017). Voucher specimens of whole fish of each species were fixed in 10% formalin, transferred to 70% ethanol, and then stored for future reference.
Results and Discussion
Nucleotide Sequence Characteristics
Three species, viz., P. chinensis, P. candidus, and Pampus sp., were identified based on BLAST results and further curated with the latest literature. P. chinensis does not pose a problem in detection and showed >99% identity with NCBI sequences, while morphological similarity of the other two species necessitated the assistance of COI barcodes for identification. The Indian Ocean representatives of P. chinensis followed a pattern similar to that of previous submissions from the Arabian Sea (FJ226529–FJ226530; KP410329) and exhibited a COI divergence of 0.6–1% from the Pacific. The COI data generated from this study and previous ones were summarized for analysis in Supplementary Table 3. Barcodes of the cryptic species exhibited a complete identity with records from the BOB sub-region in South Asia covering North-eastern Indian Ocean (KF373011–KF373012 and KX530942–KX53094), Bangladesh (KX455908), Myanmar (DQ107597–DQ107598 and DQ107600), Myanmar and Thailand (JN202078–JN202087 and JN202090–JN202092), and Vietnam (DQ107599). The Cyt b sequences of the same showed >99.7% similarity with sequences from BOB populations of Thailand and Burma (JF790230–790250) submitted by Sun et al. (2012), while ATPase showed a ∼100% identity with the sequences (JX293029–JX293030) submitted by Divya et al. (2015) from West Bengal, India. Thus, the COI barcodes and other gene sequences implied that Pampus sp. may be the Pampus griseus indicated by Divya et al. (2019). Review of Cyt b data from the Indo-Pacific (Sun et al., 2012) and ATPase 8/6 gene data (Supplementary Table 4; Divya et al., 2015) indicated K2P divergence values of 3.86% and 5.3%, respectively, between P. candidus and P. griseus (Supplementary Figures 2, 3).
Phylogenetic Interrelationships Among Pampus Species
Although there is an overall similarity, conflicts could be noted in the tree topologies derived from BI analyses of the two data sets (Figure 2 and Supplementary Figure 4), which visualized seven monophyletic clades representing seven species. However, a Bayesian Inference (BI) tree derived from concatenated nucleotide data supported the major clades with high posterior probability values (BI PP > 0.95). Hence, discussions are mainly based on the BI tree (Figure 2) derived from the concatenated data unless otherwise stated. In this phylogram, one major clade contained the sister groups P. argenteus and P. minor. The second major clade had two sub-clades in which P. chinensis and P. punctatissimus formed sister groups to each other in one clade, whereas P. griseus, P. candidus and P. cinereus grouped in another clade. P. chinensis bifurcated into two representing the Pacific and Indian Ocean lineages. Interestingly, the haplotypes at Veraval were unique and were not shared with any other region. The net evolutionary divergence value between the two major clades was 8.1%, while it was 6.9% between the sister clades of the second major clade. The intraspecific divergence ranged from 0 to 0.009, and inter-specific genetic distance spanned from 1.6 to 15.5% (Table 2). The major clades were not well-defined in the COI-based species tree (Supplementary Figure 4), particularly with reference to P. minor and P. argenteus.
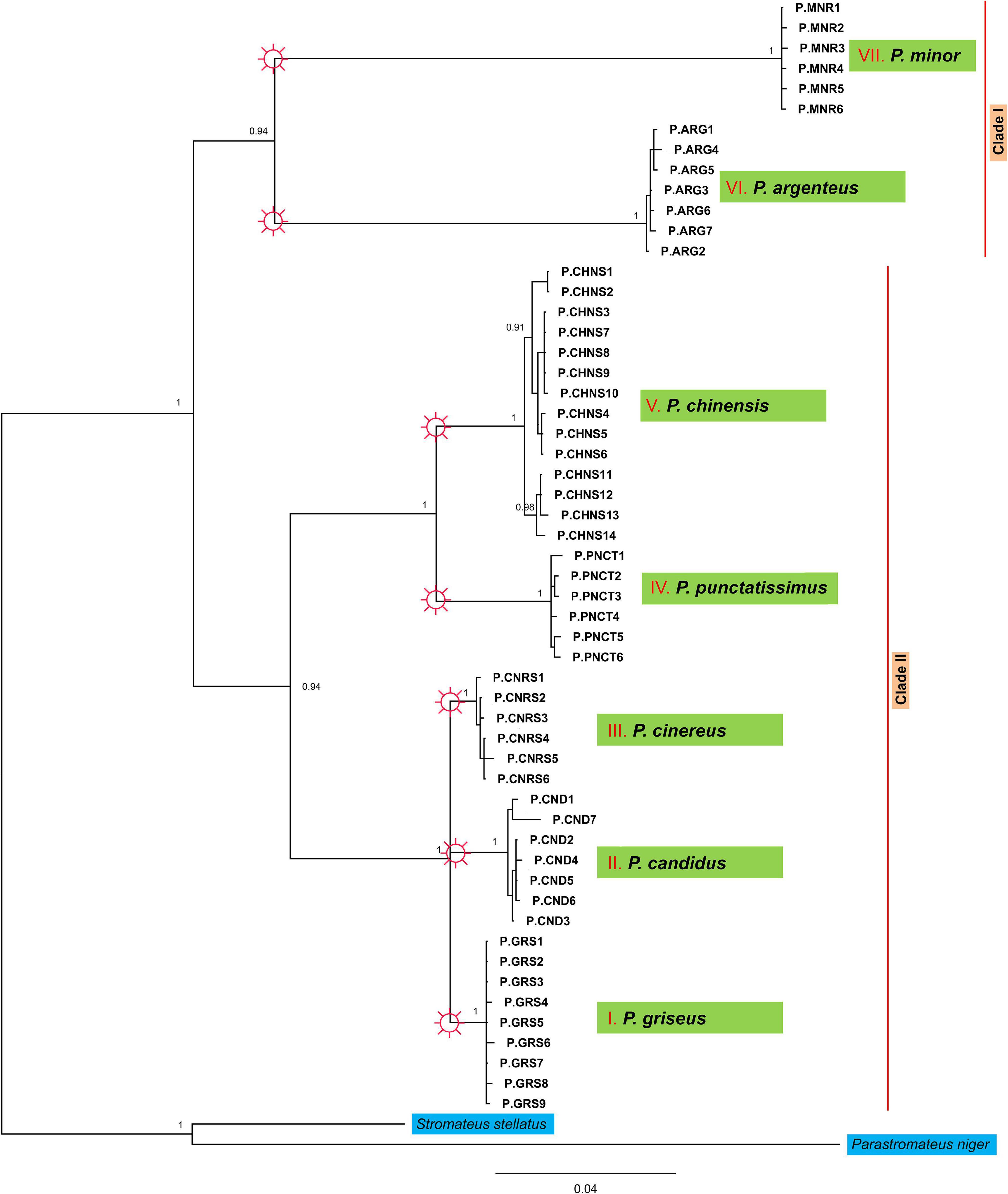
Figure 2. Bayesian inference (BI) tree of genus Pampus based on concatenated dataset of 16S, COI, and Cyt b partial sequences (Supplementary Table 2). Support values on branches indicate Bayesian posterior probability (BPP) values. Internal branch values are not represented in the Figure. Species identified from Generalized Mixed Yule Coalescent (GMYC) model is shown with the symbol on the branches.
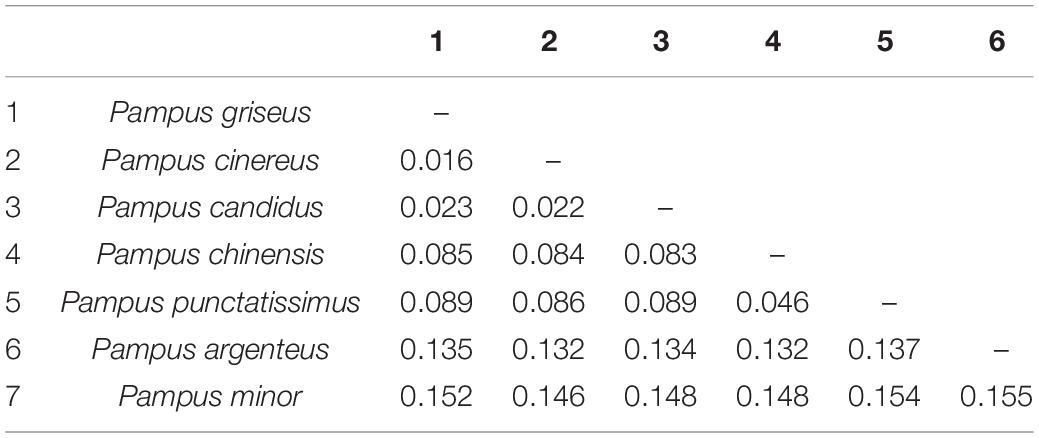
Table 2. Genetic distance based on K2P model (Kimura, 1980) among the seven species of Pampus in the Indo-Western Pacific Oceans based on the concatenated data (1,822 nt).
Bayesian analysis of phylogenetic interrelationships based on the concatenated gene tree proved to give a stable output compared to the previous individual gene genealogy with differing topologies (Cui et al., 2010, 2011; Divya et al., 2017). From our study, P. cinereus and P. griseus do not form a separate cluster in the BI trees and lead to polytomy unlike previous phylograms (Li et al., 2019c; Yin et al., 2019). The NJ tree in Li et al. (2019c), which is concordant with the COI-based tree in this study, received a comparatively low Bayesian probability value in this node, probably because of the insufficient character sampling in single-gene trees. The overall tree topology derived from the concatenated data matches with the robust gene tree built on the conserved nuclear coding markers outlined in Yin et al. (2019) but differs in the clustering of species in the P. cinereus complex which does not include exact species designations. Phylograms from previous studies indicating exact species designations (highlighted) based on results from our study incorporating latest revisions (Divya et al., 2019; Yin et al., 2019) are given in Supplementary Figure 5. The species delimitation analysis indicated the occurrence of nine taxonomic units with two morphospecies (P. chinensis and P. candidus) subdivided into two GMYC species (Supplementary Figure 6). The maximum intraspecific K2P distance in the concatenated data (51 sequences) was 0.7% while the minimum intergroup distance was 1.6% (Supplementary Table 5). Hence, the two GMYC units in P. chinensis and P. candidus that differ by 0.7% should be considered as the same species, thus setting up only seven independently evolving species in the genus.
The integrated data in this study, although discordant with the assumption of a possible species complex in P. chinensis (Divya et al., 2017; Li et al., 2019c), support the existence of heterogeneous populations forming a vicariant sister lineage pair in the Indo-Pacific Oceans. The lineage observed in P. chinensis in this study supports previous findings by Li et al. (2019a) who hypothesized the lack of genetic admixture of refuge populations of the Pacific and Indian Ocean to the geographic barrier, the Malay Peninsula, a part of Sundaland during past glacial periods (Hall and Morley, 2004). The effect of the Indo-Pacific Barrier resulted in a curbed gene flow that became prominent during Pleistocene sea-level variations, resulting in the sympatric distribution of ichthyofaunal lineages in the Indian and Pacific Oceans (Gaither and Rocha, 2013). The presence of non-shared haplotypes in Veraval indicates the probability of a distinct genetic stock in P. chinensis. Although there are reports of another lineage in P. minor from Malaysia (Li et al., 2019b), we could not include representative sequences in genetic distance calculation because of unavailability of data. The genetic distance values in this study lie in the range reported by Li et al. (2019c) with a comparatively higher divergence and bootstrap support in the P. cinereus–P. griseus–P. candidus complex. The evolutionary history of the recently diverged species may be the probable cause for the <2% heuristic threshold divergence (1.6%) between P. cinereus and P. griseus, as suggested by Divya et al. (2017). However, this value is higher compared to the single gene-based phylogenetic inference (Divya et al., 2017; Li et al., 2019c), indicating the efficiency of multilocus-based gene trees.
Our results agree that the Pampus sp. collected from the Indian Ocean by Divya et al. (2017) was closely related to the P. cinereus redescribed by Liu et al. (2013a) from China. The clustering of the three sister species, P. griseus, P. candidus, and P. cinereus indicated a convergent evolution characterized by the elongation of dorsal, caudal, and anal fins, a shared characteristic among the three (Yin et al., 2019). The tree topology discriminated each species into separate clades with no shared haplotypes, concordant to observed morphological divergence.
Multivariate Comparison of Pampus Species From India
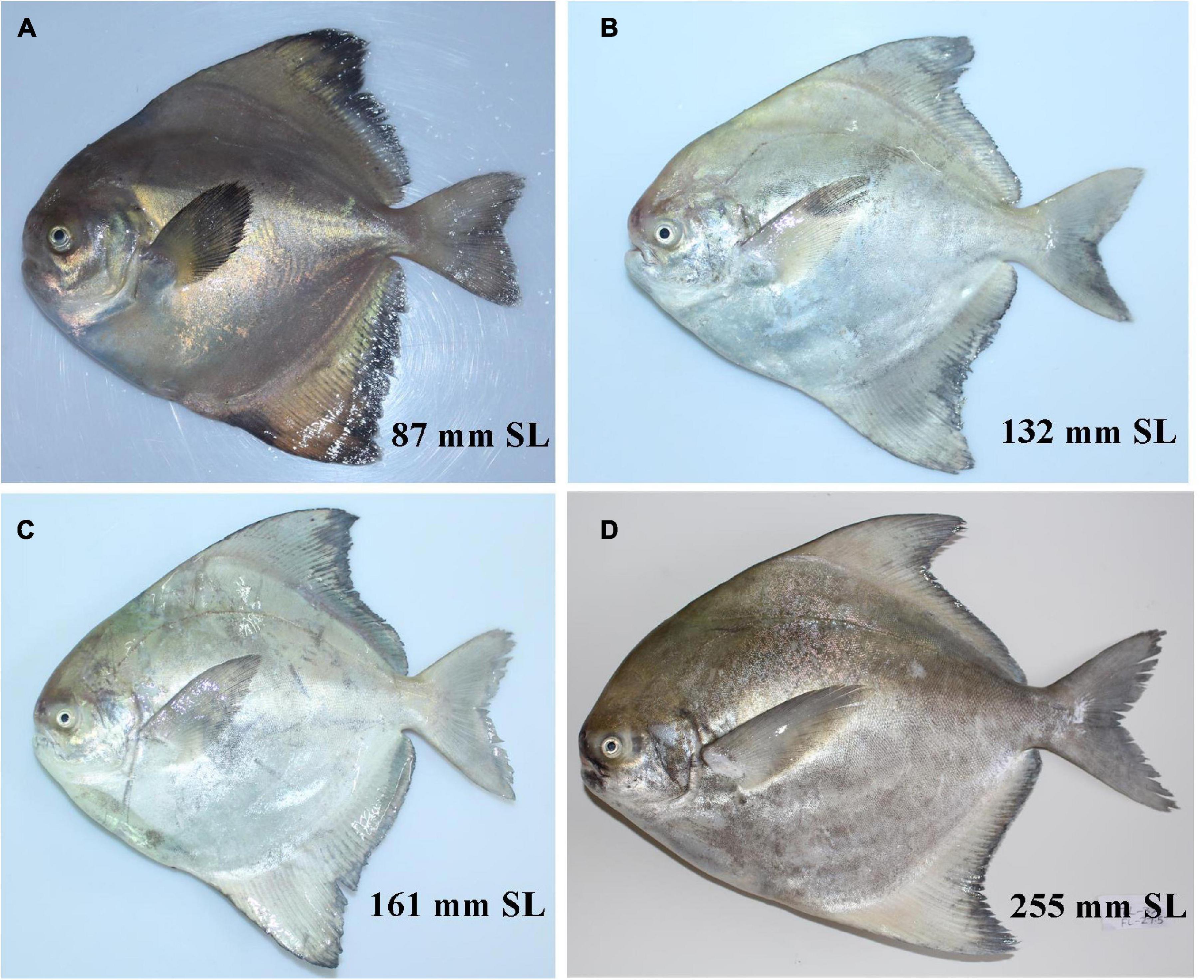
Multivariate analysis has been widely performed for species identification and discrimination (Takacs, 2012; Marramà and Kriwet, 2017; Behera et al., 2020). The first three principal components or dimensions accounted for 71.7% of the variability in the data as reflected by the eigenvalues of the principal components. The first principal component (Dim 1) separates the species P. chinensis from the two other species (P. griseus and P. candidus). Morphometric variables, such as caudal fin length (CFL), anal fin length (AFL), length of the dorsal fin base (DFBL), length of the anal fin base (AFBL), inter-orbital width (IOL), and length of upper jaw (LUJ) were found to have significantly higher loadings on the PC1 or Dim 1 and, hence, have higher discrimination power in the separation of P. chinensis from the other two species. The other two closely resembling species, P. griseus and P. candidus, showed a significant overlap and were not separated along PC1. The second dimension (PC2 or Dim 2) was able to achieve significant separation from these two species in a multivariate space with some overlap. Morphometric variables, such as caudal peduncle length (CPL), pre-dorsal distance (PDD), pectoral fin length (PFL), and eye diameter (ED), were important morphometric measurements capable of separating P. candidus and P. griseus (Figure 3). The marginal overlap in the two groups suggested their cryptic nature and, hence, is misidentified as single species (P. candidus) until now.
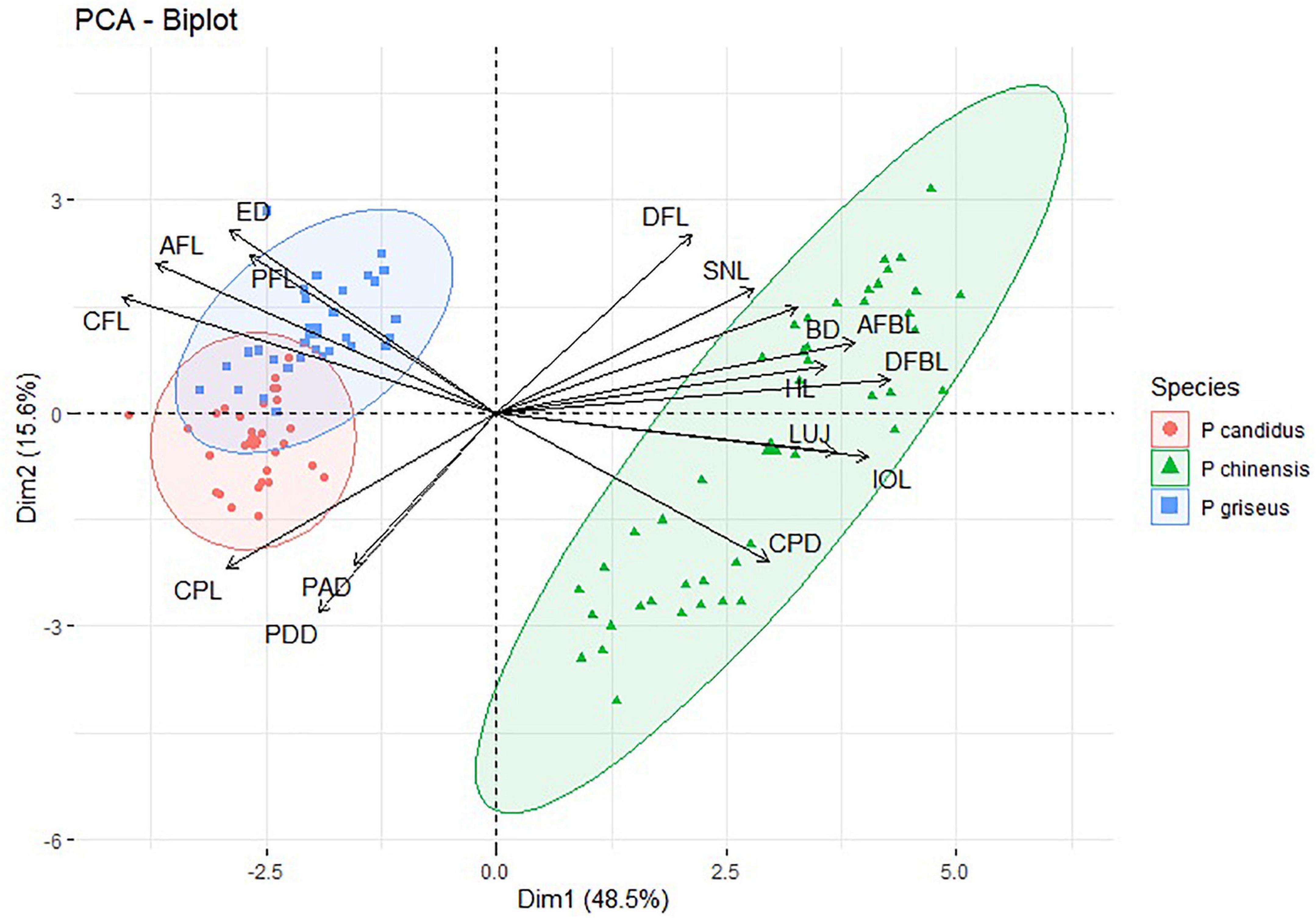
Figure 3. Bi-plot showing the variables and individuals (samples) oriented along the first two principal components with eclipses (species). The Dim1 and Dim2 are the first and second principal component extracted in PCA and the notation in graphs refers to the morphometric variables mentioned in Table 1.
Thus, by molecular and multivariate analyses, it is concluded that the specimens in the materials examined (P. candidus/Pampus sp.) collected from Kerala, Veraval (Gujarat), Rameshwaram (Tamil Nadu), and Nagapattinam (Tamil Nadu) represented P. candidus, while the rest of the samples were of P. griseus.
Comparison of Otoliths
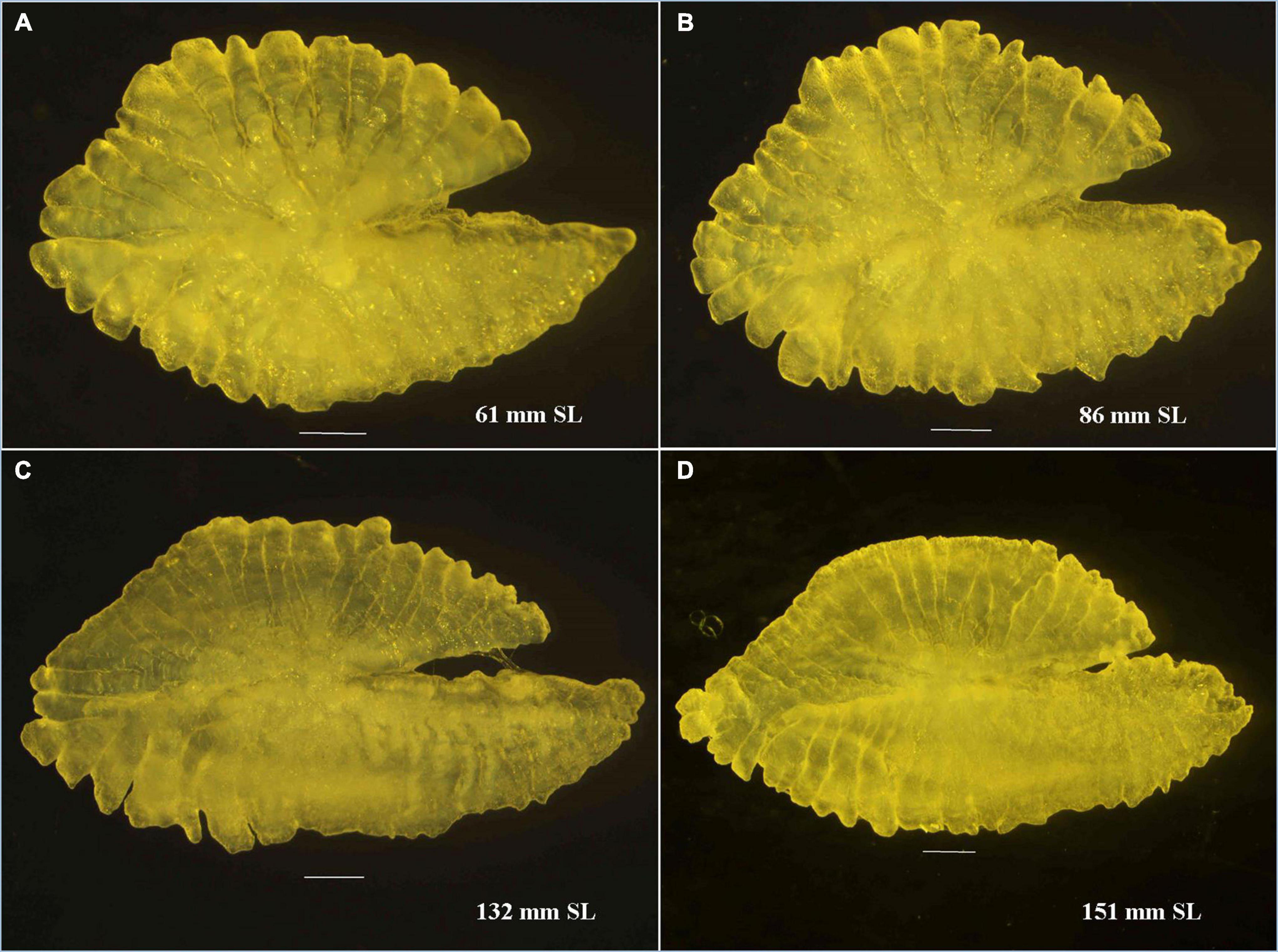
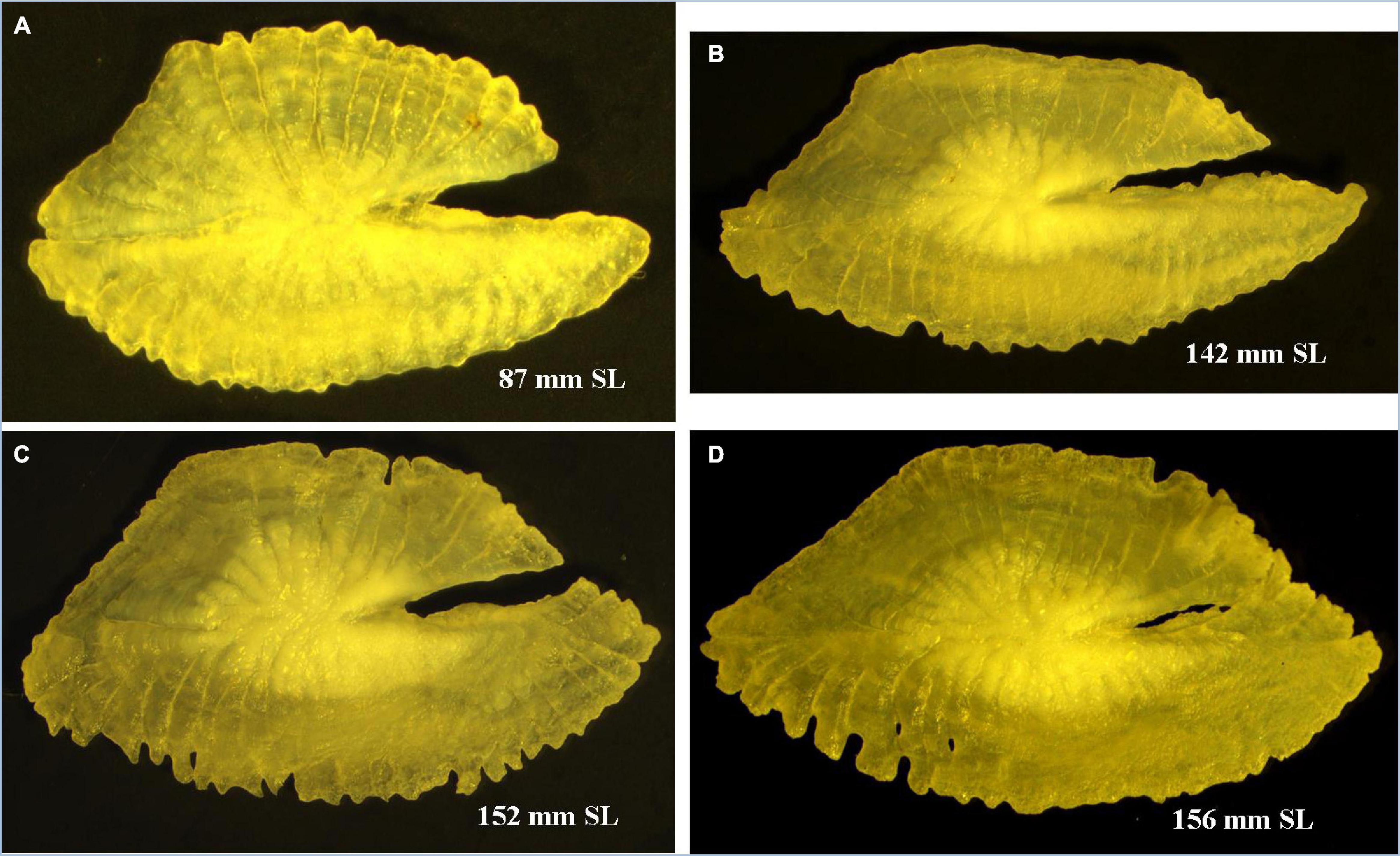
Otoliths are depicted to have high morphological variability with particular characteristics across species and genera (Koken, 1884; Platt and Popper, 1981), and such species-specific characteristics have been introduced in taxonomy (Schmidt, 1969; Nolf, 1985). The sagittal otolith morphology of five Pampus species from the Chinese coast was described by Zhang et al. (2017). The Pampus sp. mentioned in their study can be P. argenteus or P. echinogaster, which are currently synonymized to P. argenteus (Yin et al., 2019). A comparison of the sagittal otolith morphology of seven valid species of Pampus, such as P. griseus (Figure 4), P. candidus (Figure 5), and P. chinensis (Figure 6), from Indian waters, and four species from Chinese waters (Table 3 and Supplementary Figure 7) revealed that the overall gross morphology of the sagittal otolith of P. griseus is more similar to that of P. candidus, P. cinereus, P. chinensis, and P. punctatissimus than that of the others (see Figure 2 in Zhang et al., 2017). Furthermore, the subtle variations across different size ranges (as observed in this study) reduce its taxonomic utility to differentiate P. griseus from closely resembling congeneric species, especially when used exclusively.
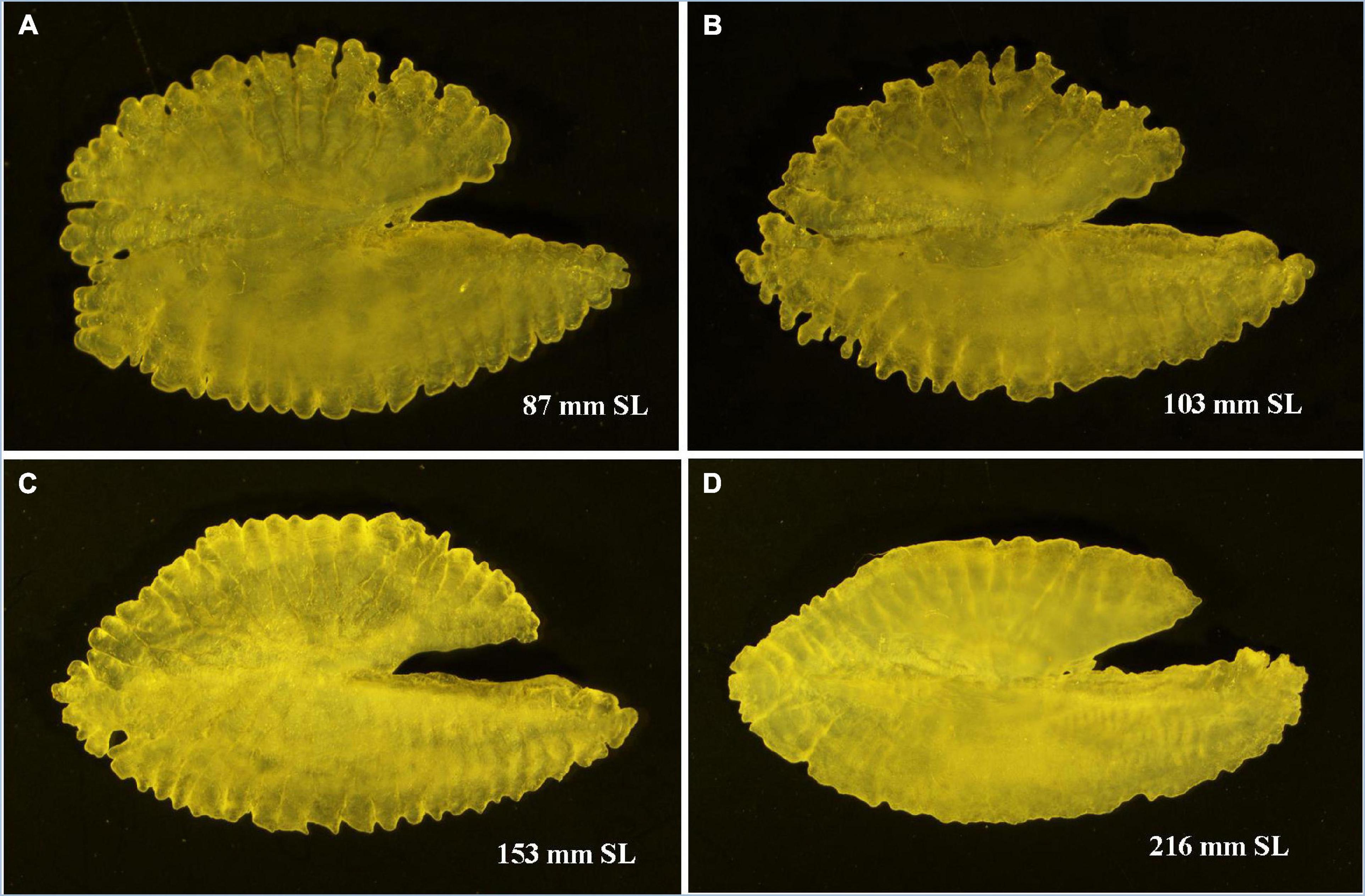
Figure 6. Pampus chinensis. (A–C) Sagittal otolith of juvenile and subadult, and (D) adult specimens.
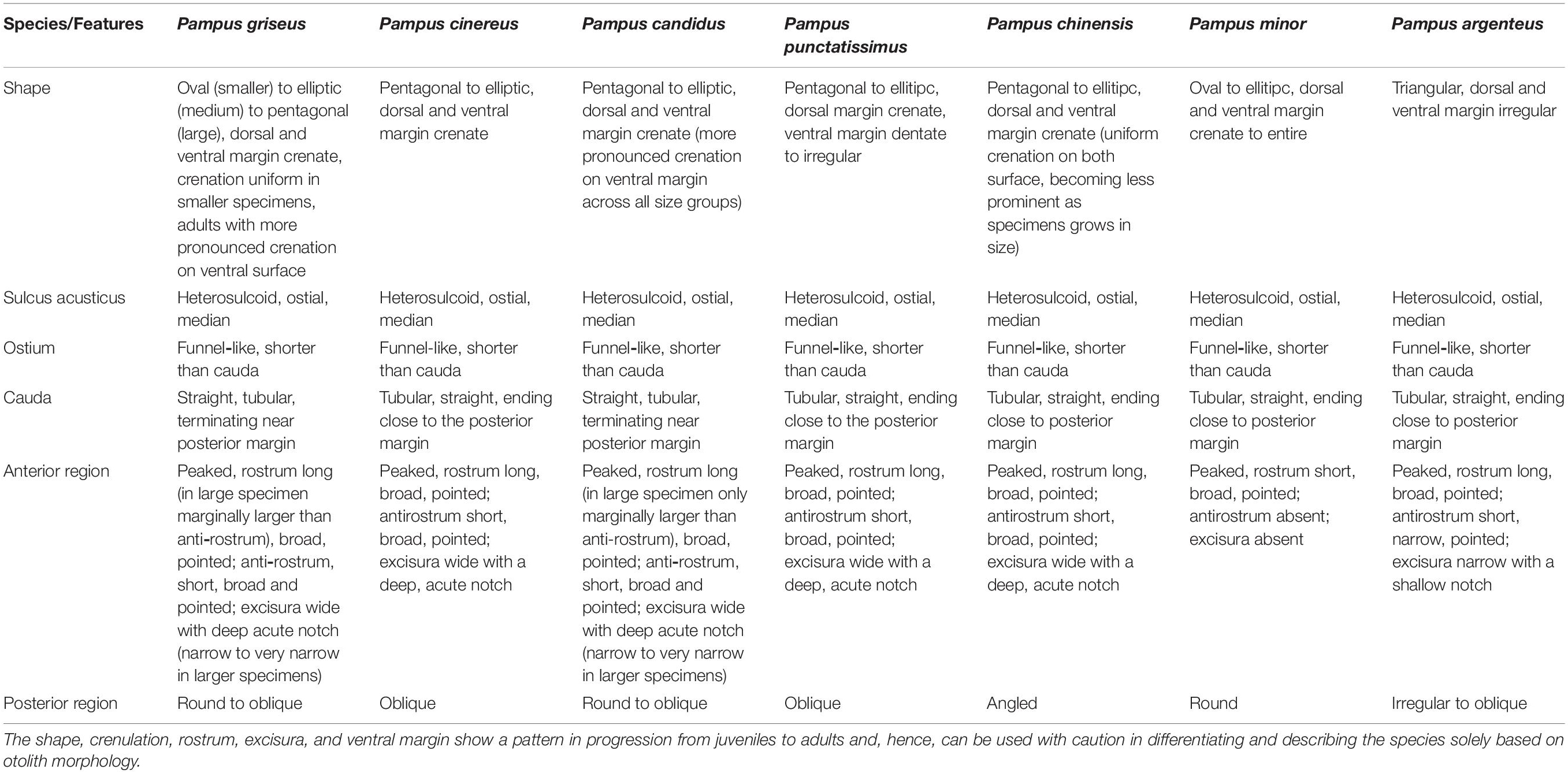
Table 3. Gross description of the sagittal otolith morphology of seven Pampus species from the Indo-Western Pacific Oceans based on Zhang et al. (2017) and this study.
Revision of Systematics
Order Perciformes (Bleeker, 1863).
Family Stromateidae (Rafinesque, 1810).
Genus Pampus (Bonaparte, 1834).
Pampus cinereus Species Complex
In the Pampus genus, the first species complex, “Pampus nozawae species complex”, recognized by Cheng and Zheng (1987), consisted of P. nozawae, P. punctatissimus, and P. cinereus. Later, Liu and Li (2013) recognized another species complex, “Pampus punctatissimus species complex,” which included P. punctatissimus, P. cinereus, and P. liuorum. However, both these complexes, characterized by greatly extended anterior rays of the anal fin, were not approved by subsequent researchers. In the first complex, P. nozawae, described by Ishikawa (1904), was proved to be an invalid species that was synonymized with P. punctatissimus and P. cinereus (Yamada et al., 2007; Liu et al., 2013a). It is to be added that P. nozawae, reported by Huang et al. (2016), from Daya Bay, China indeed represents P. cinereus. Similarly, P. liuorum of the second species complex described by Liu and Li (2013) from Zhuhai fish market, China, re-validated by Jawad and Jig (2017), was recently synonymized with P. cinereus (Yin et al., 2019). The molecular analysis in this study could effectively delineate the three species of the P. cinereus complex mentioned in Table 1 of Yin et al. (2019) as P. griseus (collection from Bangladesh, voucher nos CL1315-2, CL1317, CL1318-1, CL1316-2, CL1320-1, CL1320-2, CL2010, and CL2009), P. candidus (collection of uncertain origin, voucher nos CL1975-1, CL1943-1, CL1975-2, CL1975-3, CL2012, and CL1943-2), and P. cinereus (South and East China Seas; voucher nos CL1286-3, CL1303-1, and CL1287-2). The genetically close species P. cinereus, P. argenteus, and Pampus sp. identified by Li et al. (2019c) were validated as P. cinereus, P. candidus, and Pampus sp. respectively, the last of which is re-described as Pampus griseus (new combination) in this communication. We propose that “Pampus cinereus species complex” consists of P. griseus, P. candidus, and P. cinereus, and that this complex is characterized by oval body, long pectoral fin, greatly extended anal fin, slightly to greatly extended lower-lobe of caudal fin, 7–12 short and tubercular-like spinules gill rakers, absence of groove on lower ridge of gill cover, transverse occipital canals of ventral branches of lateral line equal to or slightly longer than dorsal branches, and 36–38 total vertebrae (see Table 4).
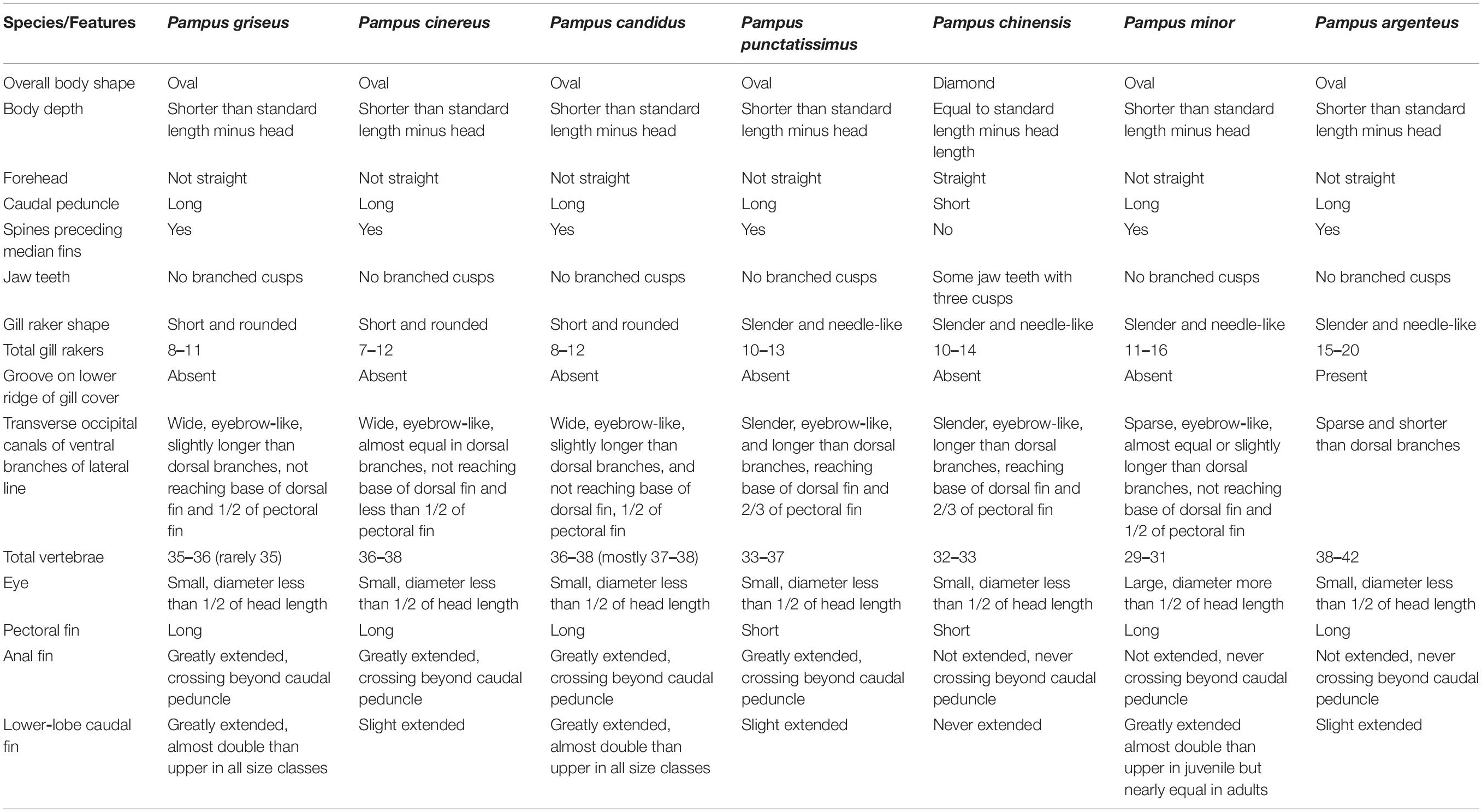
Table 4. Comparison of the morphological characteristics of seven species of Pampus from the Indo-Western Pacific Oceans.
Resurrection and Re-description of Pampus griseus
New proposed English name: Bengal silver pomfret (Tables 1, 3, 4, Figures 4, 7, and Supplementary Figures 7–11).
Stromateus griseus Cuvier and Valenciennes, 1833: 391 (Pondichéry, Tamil Nadu, India).
Material Examined From India (n = 35)
Four ex., 83–110 mm SL, Digha (21°36′58.3272″N, 87°29′56.8644″E), West Bengal, shore seine, 3.5 m, sandy silt, September 12, 2020; 7 ex., 70.6–120.5 mm SL, Puri north landing center (19°47′43.062″N, 85°49′38.5788″E), Puri, Odisha, shore seine, 3–5 m, sandy silt, Aug. 18, 2019; 6 ex., 132–167 mm SL, Paradeep fishing harbor (20°17.345′N, 086°42.422′E), Jagatsingpur, Odisha, trawl, 40–70 m, sandy silt, January 24, 2020; 9 ex., 117–177 mm SL, Atharabanki landing center (20°17′28.2264″N, 86°39′18.738″E), Jagatsingpur, Odisha, India, gillnet, 30 m, sandy silt, June 9 and October 3, 2020; 4 ex., 102–115 mm SL, Visakhapatnam Fishing Harbor (17.696°N, 83.301°E), Andhra Pradesh, trawl, 35–40 m, sandy silt, September 2, 2020; 2 ex., 150–170 mm SL, Puducherry (Pondicherry) fishing harbor (16°3′17.964″N, 78°14′47.49″E), trawl, sandy, Tamil Nadu, October 10, 2020; 3 ex., 150–170 mm SL, Cuddalore Harbor (11°42′52″N, 79°46′31″E), Tamil Nadu, Multiday trawl, 15 m, sandy, October 2020.
Museum Specimens Identified as Pampus griseus (Cuvier, in Cuvier and Valenciennes, 1833)
Lectotype (Present Designation)
MNHN-IC-A-5479, 157 mm SL (stuffed), Pondichéry, Tamil Nadu, India (Supplementary Figure 1A); GB.31.145.1.1, 157.2 mm SL, Visakhapatnam, Andhra Pradesh, India, 5–110 m, January 2005, labeled as P. argenteus (Supplementary Figure 1C); GB. 31.145.1.8, 136.1 mm SL, West Bengal, India, 60 m, July 24, 2015, labeled as P. griseus (Supplementary Figure 1D).
Diagnosis
A species of Pampus with the following combination of characters: oval-shaped body, dorsal fin rays VIII-IX 38–42, anal fin rays V-VII 36–39, dorsal fin produced into distinct falcate lobe and anterior lobe of anal fin greatly extended crossing beyond caudal peduncle (54.9–103.2% of SL), preceded by 5–9 small blade-like spines embedded in skin and not prominent in larger specimens (adults); long pectoral fin, 35.1–49.6% of SL; deeply forked caudal fin, lower lobe larger than the upper and greatly extended almost double in all size classes (62–132% of SL); short and tubercular-like spinules gill rakers, 1–2 + 7–9 (8–11); absent groove on the lower ridge of gill cover; ventral transverse occipital canals of lateral line wide, eyebrow-like, slightly longer than dorsal branches, not reaching base of dorsal fin and almost ½ of pectoral fin; sagittal otolith pentagonal to elliptical with uniform crenulation along both dorsal and ventral margin; 35–36 total vertebrae (rarely 35), such as 13–15 precaudal and 21–23 caudal vertebrae.
Description
Counts and proportional measurements of specimens of P. griseus are given as percentage of SL in Table 1. D: VIII–IX 38–42; A. V-VII 36–39; P. 22–26; C. 22–26; Gr. 1–2 + 7–9 (8–11); vertebrae 13–15 + 21–23 = 35–36 (rarely 35). Body deep, compressed and oval shape, shorter than the standard length minus head, covered with very small and deciduous cycloid scales; head compressed, dorsal profile strongly keeled, being more prominent behind the eye; small eye its diameter lesser than head length; small mouth, terminal; mouth slit curved downward posteriorly, reaching the middle of the eye; upper jaw not movable, covered with skin; minute teeth on the jaws, in a single row narrowed from the sides without branched cusps; teeth absent on the vomer and palatine; presence of papillae in both halves of pharyngeal sacs; branchiostegal membrane fused with isthmus; gill membranes joined to belly, gill slit longer, its lower margin below the level of pectoral-fin base; short and tubercular-like spinules gill rakers with 8–11 (total) on first first-gill arch (Supplementary Figure 8); both dorsal and anal fin lobe-like anteriorly, preceded by 8–9 and 5–7 small, blade-like spines, embedded in skin in larger specimens, respectively; anterior rays (lobe) of anal fin always greatly extended, crossing beyond the caudal peduncle in all size classes (Figure 7); long pectoral fins; almost equal dorsal and anal fin base lengths; deeply forked caudal fin, lower lobe larger than the upper and always greatly extended in all size classes, almost double than upper lobe (Figure 7); absence of groove on the lower ridge of gill cover (Supplementary Figure 9); transverse occipital canals and dorsal branches of the lateral-line canal on top of the head with a truncated rear edge; wide ventral branches, eyebrow-like and slightly longer than the upper branches, not reaching the origin of the dorsal fin, and nearly ½ of the pectoral fin length (Supplementary Figure 10); lateral line is high, following dorsal profile to caudal peduncle.
Sagittal Otolith Morphology
The sagittal otolith of P. griseus showed a marginal transformation in its shape with an increase in size of the fish (61–151 mm SL). The major transformation was evident in depth (dorsal-ventral axis) to length (anterior-posterior axis) ratio, which was found smaller in the case of otoliths of larger specimens (Figure 4). The otolith, in general, can be characterized by a pentagonal to elliptical shape with uniform crenulation along both the dorsal and ventral margins (Supplementary Figure 7). The depth of incision in crenulation tends to decrease as the size of the otolith increases. The heterosulcoid otolith with funnel-shaped ostia is shorter than the tubular and straight cauda. The rostrum is long and pointed in contrast to anti-rostrum, which is shorter but pointed. The excisura major was deep with acute oblique notch (Table 3).
Habitat
Inhabits inshore shallow waters of various bottom types: sandy silt, rocky, and turbid muddy bottoms; usually moves in large schools; juveniles found in the depth gradient of 3–30 m, whereas subadults and adults mostly at 30–70 m. Specimens were observed and obtained from various locations along the east coast of India (Bay of Bengal) from different fishing gears: ring seines (bunt mesh size 10 mm, wing and shoulder mesh size 15 mm) affirmed at 12–18 m water depth; shore seines (bag mesh size 5–10 mm, wing mesh size 15–20 mm) operated at 3–5 m water depth; trawls (cod-end mesh size 40 mm) operated at 30–100 m water depth; and gill nets (mesh size 45–58 mm) operated at 10–30 m water depth.
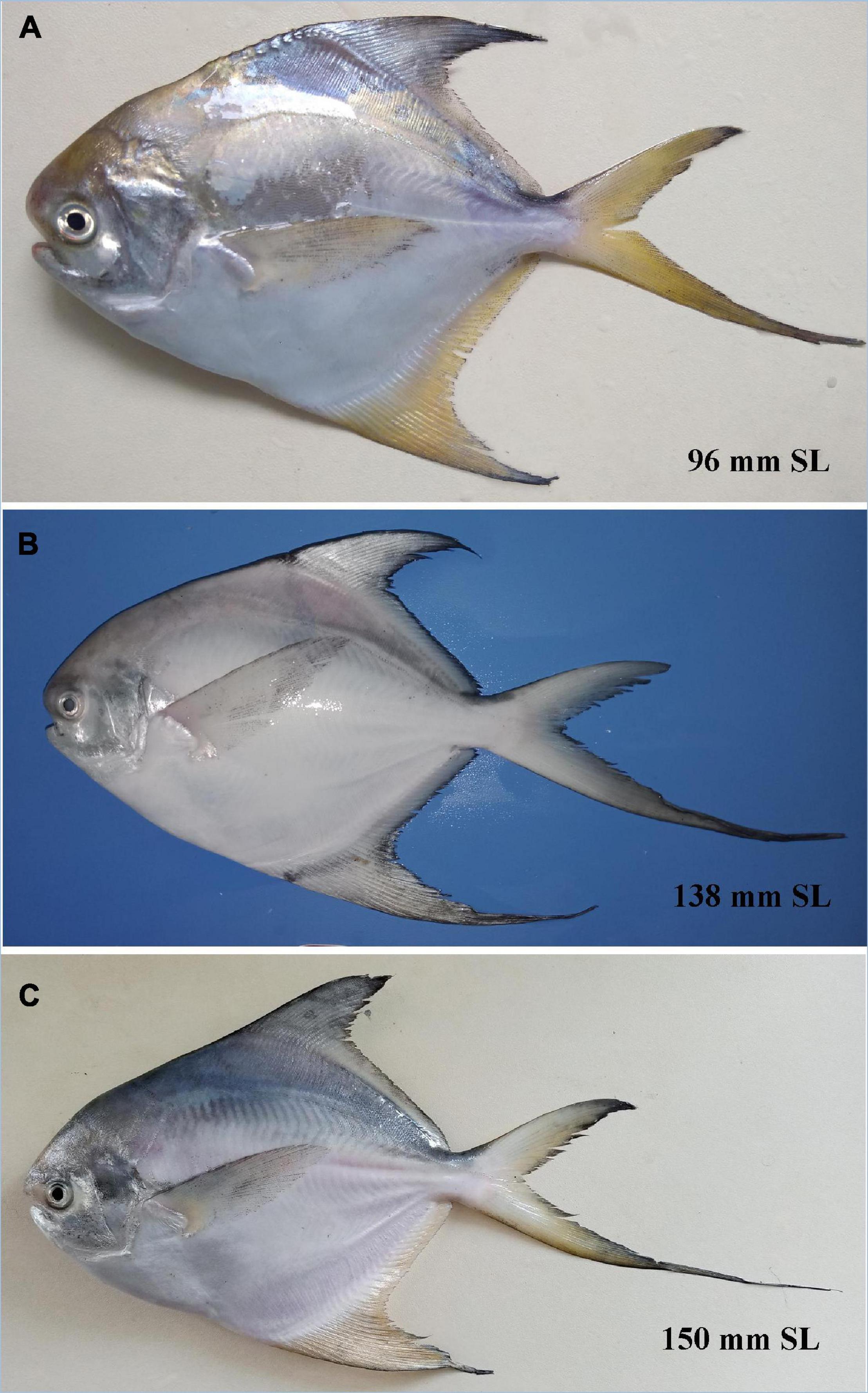
Color of Live Specimens (Supplementary Figure 12)
Color description is based on the live specimens observed in the ring seines and shore seines. The dorsal and lateral surfaces of head and body above pectoral fin aquamarine blue; silvery on ventral side below pectoral fin area; dorsal fin grayish silvery with blackish outer margin; anal fin light to dark yellowish with blackish outer margins; pectoral fin translucent and light yellowish or hyaline; caudal fin light yellowish with blackish outer margin and silver-blue tinge toward the tip of both lobes; minute black dots covered almost entire the body, which generally got removed or disappeared during fishing and post-harvest handling; faint blackish spot on the upper part of the opercle; some specimens, mostly young ones, are much darker than the adults, and all the fins nearly blackish or grayish in appearance.
Color of Formalin-Preserved Specimens
Dorsal and lateral surfaces of the body and head brownish tan, fading to pale creamy or brownish toward ventral sides. All fins are yellowish-brown toward the base with dusky margin posteriorly.
Geographic Distribution
This study and the GenBank records (COI, Cyt b, ATP6/8, and 16s rRNA sequences) suggest that P. griseus has a restricted distribution in the Bay of Bengal (East coast of India, Bangladesh, Myanmar) and Southeast Asia (Thailand, Vietnam, and Malaysia) (Supplementary Tables 2–4 and Supplementary Figure 2).
Etymology
The species was named “griseus” in the original description with reference to the gray color of the body. The new English name “Bengal silver pomfret” is proposed for this species since its type locality falls in the Bay of Bengal and it forms the major component in pomfret fishery of the region.
Pampus candidus (Cuvier, in Cuvier and Valenciennes, 1833)
New proposed English name: Indian silver pomfret (Tables 1, 3, 4, Figures 5, 8, and Supplementary Figures 7–11).
Pampus candidus was recently resurrected from the synonymy with P. argenteus and re-described as a valid species by Divya et al. (2019) based on fresh specimens from the Arabian Sea and the Bay of Bengal, and a lectotype was designated in the absence of type specimen. Their study provided the count D. VII–IX 40–42, A. V–VII 36–41, P. 22–24, C. 22–24, Gr. 1–2 + 6–8 = 8–10, and vertebrae 16 + 21 = 37. Similar counts with some variation were observed by Li et al. (2019c) where they misidentified P. candidus to P. argenteus (validated in this study); with counts concurrent to this study, i.e., D., D. VII–X, 36–42, A. V–VI, 36–40, P. 22–28, C. 22–28, Gr. 1–2 + 7–9 = 9–11 and vertebrae 36–38 (mostly 37–38, rarely 36) such as 14 precaudal and 22–24 caudal vertebrae. Divya et al. (2019) had stated the presence of extended lower lobe of caudal fin in subadults that progressively shortens with age to lobes of almost equal length in specimens over 100 mm SL, contrary to the observation of this study, wherein the extended lower lobe for caudal fin could be observed in all size groups (see Figure 8). It may be summarized that P. candidus is distributed in the Persian Gulf (Iran, Iraq, and Kuwait), Oman Sea, Arabian Sea (Pakistan and west coast of India), Bay of Bengal (West Bengal and Tamil Nadu), China Sea (Xiamen, Taiwan, and Beibu Bay), Southeast Asia (Malaysia and Indonesia), sporadically occurring in the Southern Pacific (Fowler, 1938), Adriatic Sea, North Sea, and the Mediterranean Sea (Dulčić et al., 2004; Piper, 2010; Sami et al., 2014). A new English name “Indian silver pomfret” is also proposed for this species, since it represents the predominant species in the Indian Ocean and is originally described as Stromateus candidus Cuvier and Valenciennes (1833) from Indian waters.
Pampus cinereus (Bloch, 1795)
Gray pomfret (Tables 3, 4 and Supplementary Figures 7–11).
Pampus cinereus was believed to be a common species distributed in the Indo-Western Pacific Oceans. The species was originally described by Bloch (1795) as Stromateus cinereus based on a single stuffed specimen without any information on type locality, whereas in a later publication, Bloch and Schneider (1801) mentioned it as Tranquebar (Tharangambadi, Tamil Nadu). However, recently, Li et al. (2019c) mentioned Malaysia as the type locality for the species. The only diagnostic characteristics provided in the original description of the species are greatly extended anal fin and distinctly long pectoral fins. In subsequent publications, P. cinereus was considered as a synonym of P. argenteus (Haedrich, 1967; Lindberg and Krasyukova, 1975; Parin and Piotrovsky, 2004; Li et al., 2013, 2017; Sun, 2015), but it was later accepted as a valid species through morphological (Regan, 1902; Wu, 1985; Cui et al., 2010, 2011; Liu et al., 2013a; Jawad and Jig, 2017; Zhang et al., 2017) and molecular studies (Divya et al., 2017, 2019; Li et al., 2019c; Yin et al., 2019). Due to insufficient information in Bloch’s original description and loss of the original type specimen, Liu et al. (2013a) re-described P. cinereus based on fresh specimens from Guangdong, South China Sea, and a neotype was designated. Their study provides the counts of D. VIII–X 37–41, A. V–VII 36–41, P. 20–22, C. 22–24, Gr. 1–2 + 6–8 = 7–10 and vertebrae 15 + 21 = 36. Later, Jawad and Jig (2017) confirmed the identity of P. cinereus based on osteology where they counted vertebrae. Zhang et al. (2017) also retained the species status of P. cinereus based on sagittal otolith morphology. Li et al. (2019c) provided the vertebrae counts (37–38) with some variations from Liu et al. (2013a). Yin et al. (2019) recorded 36–37 vertebrae for P. cinereus and 36–38 for P. liuorum, and considered the latter to be a synonym. However, their vertebral count of P. cinereus could be ambiguous, since they considered the three species (P. candidus, P. griseus, and P. cinereus) as complex. Based on the available data in GenBank, and the recent studies (Li et al., 2019c; Yin et al., 2019), we concluded that P. cinereus is completely absent in India and distributed in the western Pacific Ocean throughout the waters south of Taiwan Strait and those extending southward toward the Malaysian peninsula (Supplementary Table 3).
Pampus chinensis (Euphrasen, 1788)
Chinese silver pomfret (Tables 1, 3, 4, Figures 6, 9, and Supplementary Figures 7–11).
Pampus chinensis is a commercially important species, widely distributed in the Indo-Western Pacific Oceans. The species was originally described as Stromateus chinensis by Euphrasen (1788) from “Castellum Chinese Bocca Tigris,” Humen, Guangdong province, China. Since then, the species has been renamed and described in various names: Stromateus sinensis (Forster, 1795), S. atous, and S. albus (Cuvier and Valenciennes, 1833), Stromateoides atous (Richardson, 1846), S. atokoia (Bleeker, 1851), S. atoukoia (Bleeker, 1852), S. sinensis (Regan, 1902), and Pampus chinensis (Beaufort and Chapman, 1951; Haedrich, 1967). Currently, all the names are considered as synonyms of the accepted P. chinensis. The diagnostic characteristics, such as vertebral count 33; no spines preceding the median fins; fins never deeply falcate and the fin rays gradually and uniformly diminishing in length posteriorly, were provided in the Euphrasen’s original description of P. chinensis. Recently, several authors have also affirmed the identity of the species based on morphological examination and molecular analyses. Li et al. (2019c) provided the counts D. V–VI 41–46, A. IV–VI 40–41, P. 20–22, C. 22–24, Gr. 2–3 + 9–10 = 11–13 and vertebrae 32–33. However, their dorsal and anal fin ray counts were erroneous, and they seem to include separately some anterior smaller rays as spine, which is completely absent in P. chinensis. Our study counted D. 41–49, A. 38–45, P. 20–27, C. 18–28, Gr. 1–4 + 8–11 = 9–14 and total vertebrae 32–33 (rarely 32), such as 12–14 precaudal and 19–21 caudal vertebrae. Overlapping counts for vertebrae were provided by Jawad and Jig (2017) and Yin et al. (2019). Comparative COI (Divya et al., 2017; Li et al., 2019c) and multiple marker-based studies indicated that P. chinensis from the Indian Ocean (Arabian Sea) formed a distinct lineage from the Pacific (Li et al., 2019a), and a detailed study covering entire areas of species distribution is recommended. The Chinese silver pomfret population, distributed along India, does not differ significantly from that in the Pacific in morphology or other features covered in this study. The overall analysis led to the assumption that the species is mainly distributed in the Arabian Sea, the Bay of Bengal, and in waters south of the East China Sea, South China Sea, and that it may also be distributed throughout the coastal areas of Southeast Asian countries, such as Malaysia (Liu et al., 2002; Yamada et al., 2009; Divya et al., 2017; Li et al., 2019a,c; Yin et al., 2019; present study).
Pampus punctatissimus (Temminck and Schlegel, 1845)
New proposed English name: Japanese silver pomfret (Tables 3, 4 and Supplementary Figures 7–11).
Pampus punctatissimus is an economically important species, widely distributed in the Western Pacific. The species was originally described as Stromateus punctatissimus by Temminck and Schlegel (1845) based on two specimens (whose fins were damaged) from Nagasaki, Japan. Later, the species was synonymized with P. argenteus by some authors (Bleeker, 1852; Haedrich, 1967) because of their morphological similarities. The original description lacks some of the important diagnostic characteristics, such as gill raker and vertebra count; hence, Liu and Li (1998b) re-described P. punctatissimus in detail based on specimens collected from Chinese coastal waters and compared its morphology with that of P. argenteus to resolve the taxonomic ambiguity of the species. In subsequent publications, the identity of the species was confirmed based on detailed osteology, sagittal otolith morphology, and integrative taxonomy (Dolganov et al., 2007; Jawad and Jig, 2017; Zhang et al., 2017; Li et al., 2019c; Yin et al., 2019). Li et al. (2019c) gave counts D. V–VII 39–48, A. V–VII 32–42, P. 22–25, C. 23–26, Gr. 2–3 + 9–10 = 11–13, and vertebrae 33–35 for P. punctatissimus. Overlapping counts for vertebrae were provided by several authors: 34–37 (Dolganov et al., 2007), 34 (Jawad and Jig, 2017), and 35 (Yin et al., 2019). The available information suggests that P. punctatissimus is distributed in the western Pacific Ocean, namely, the Sea of Japan, Pacific coast of Japan, Korean Peninsula, Yellow Sea, the East China Sea, and South China Sea, and that its distribution might even extend southward toward the Indonesian Islands. A new English name “Japanese silver pomfret” is proposed for this species in commemoration of its original description from the Japanese waters.
Pampus argenteus (Euphrasen, 1788)
Silver pomfret (Tables 3, 4 and Supplementary Figures 7–11).
Pampus argenteus was believed to be the most widely distributed Pampus in the Indo-Western Pacific and the most commercially important of all, remained the most controversial until recent past. The species was first described as Stromateoides argenteus by Euphrasen (1788) based on a single specimen from “Castellum Chinese Bocca Tigris,” Guangdong province, China. Most species of Pampus were considered to be synonyms of P. argenteus for several years before they were described, re-described, and resurrected (Liu and Li, 1998a,b; Liu et al., 2013a,b; Divya et al., 2019; This study). Because of difficulty in locating the type specimen and ambiguity in Euphrasen’s original description, Liu et al. (2013b) re-described P. argenteus based on the specimens collected from the type locality, and a neotype was designated. Another species, Pampus echinogaster, was described as Stromateoides echinogaster by Basilewsky (1855) from the Gulf of Chihli (Bohai), Beijing, China. Later, the validity of the species was ascertained by several authors (Haedrich, 1967; Jawad and Jig, 2017; Li et al., 2017, 2019c). The original description of P. echinogaster did not include meristics, and modern ichthyologists could not locate its type specimen. Therefore, Li et al. (2017) re-described P. echinogaster and validated the identity of the species based on specimens collected from China and Japan, and was again confirmed by Li et al. (2019c). However, during the same year, Yin et al. (2019), through an extensive study, established that P. echinogaster is not a valid species but a synonym of P. argenteus based on examinations of specimens from the South China Sea, type locality of P. argenteus and the Bohai Sea, and type locality of P. echinogaster. Their study concluded that Euphrasen’s original morphologic description of P. argenteus and P. echinogaster, described by Haedrich (1967), is P. argenteus according to the number of its fin rays and gill raker count, but that Haedrich’s P. argenteus is potentially another species. In re-description of P. argenteus, Liu et al. (2013a) provided overlapping counts with P. echinogaster and distinguished both the species mainly based on vertebral count (40 vs 40–41). Jawad and Jig (2017) reported the vertebral count, P. echinogaster (39) vs. P. argenteus (41), whereas Yin et al. (2019) gave the vertebral count, 41 vs 39–40. The above-mentioned studies support that the two species should be treated as same as “P. argenteus.” The current knowledge affirms that P. argenteus is completely absent in the Indian Ocean and is primarily distributed in the Western Pacific: Pacific coast of Japan, Sea of Japan (East Sea), Korean Peninsula, South China Sea, East China Sea, Bohai Sea, and Yellow Sea.
Pampus minor Liu and Li, 1998
Southern lesser pomfret (Tables 3, 4 and Supplementary Figures 7–11).
Pampus minor is a small-sized pomfret widely distributed in the Western Pacific. The species was originally described by Liu and Li (1998a) based on 32 specimens from the coastal waters of the South China Sea and the continental coast of the Taiwan Strait. Because of the smaller size of adult fish, P. minor was previously mistaken as the juvenile or larvae of P. argenteus and P. cinereus (Liu and Li, 1998a). Later on, the species was confirmed based on morphological and molecular data (Guo et al., 2010; Jawad and Jig, 2017; Zhang et al., 2017; Li et al., 2019c; Yin et al., 2019; Liu et al., 2020). Li et al. (2019c) and Liu et al. (2020) provided the counts D. VII–IX 34–39, A. V–VII 35–39, P. 22–24, C. 18–20; Gr. 3–4 + 8–10 = 11–14; and vertebrae 29–31 with some variations in gill raker and vertebra counts in comparison with the original description. Overlapping vertebra counts were provided by Jawad and Jig (2017), 30, and Yin et al. (2019), 29, for P. minor. Based on the literature (Li et al., 2019b,c; Yin et al., 2019; Liu et al., 2020), we confirmed that P. minor is mainly distributed in the southern part of the Taiwan Strait and the northern part of the South China Sea and Beibu Gulf, with its northernmost distribution reaching the coastal waters of Wenzhou, China (East China Sea) and coastal areas of Southeast Asian countries, such as Malaysian waters.
Key to Species Identification
For morphological synapomorphies, identification of species in Pampus is at times difficult. Yin et al. (2019) developed a convenient identification key for five species, whereas Li et al. (2019c) provided a key for six species, such as P. echinogaster, from the Western Pacific. However, both the keys are incomplete, as they lack representation of P. candidus and P. griseus, which form the major fishery in the Indian subcontinent, and correctly identifying them is important. The seven species of pomfrets (P. griseus, P. candidus, P. cinereus, P. chinensis, P. punctatissimus, P. argenteus, and P. minor) discussed in this study can be distinguished based on the diagnostic characteristics given in Table 4. A simple key for all the known congeners adapted from Li et al. (2019c); Yin et al. (2019), and this study is provided below.
1a Body has a diamond shape, very deep equal to standard length minus head length, forehead almost straight, short caudal peduncle, some jaw teeth having three cusps, caudal fin deeply forked and both upper and lower lobes equal in length, anal fin and caudal fin not extended, and no spines preceding the median fins………………………………………………………………P. chinensis (Euphrasen, 1788).
1b Body has an oval shape, body depth smaller than standard length minus head length, forehead not straight, long caudal peduncle, jaw teeth without branched cusps, caudal fin deeply forked and lower lobe larger than the upper, anal fin and caudal fin slight to greatly extended, and spines preceding the median fins……………………………………………… 2
2a Gill rakers short and tubercular-like spinules………………………………………… 3
2b Gill rakers slender and needle-like…………………………………. 5
3a Transverse occipital canals of the ventral branches of lateral line almost equal to the dorsal branches and less than 1/2 of pectoral fin length; caudal fin lower lobe slight extended……………………………………………………. P. cinereus (Bloch, 1795).
3b Transverse occipital canals of the ventral branches of lateral line longer than dorsal branches, not reaching base of dorsal fin and 1/2 of pectoral fin; caudal fin lower lobe greatly extended…………………………………………………………….. 4
4a Total vertebrae, 36–38 (mostly 37–38)………………………… P. candidus (Cuvier, in Cuvier and Valenciennes, 1833).
4b Total vertebrae, 35–36 (rarely 35)…………………………P. griseus (Cuvier, in Cuvier and Valenciennes, 1833).
5a Groove present on the lower ridge of gill cover, gill rakers 15–20, vertebrae 38–42, transverse occipital canals of ventral branches of lateral line sparse and shorter than dorsal branches…………………………P. argenteus (Euphrasen, 1788).
5b Groove absent on the lower ridge of gill cover; gill rakers 10–16, vertebrae 29–37, transverse occipital canals of ventral branches of lateral line equal or longer than dorsal branches………………………………………………………………………… 6
6a Transverse occipital canals of the ventral branches of lateral line longer than dorsal branches, reaching base of dorsal fin and 2/3 of pectoral fin; vertebrae: 33–37; pectoral fin short; eye small, diameter less than 1/2 of head length……………………………………………………………….P. punctatissimus (Temminck and Schlegel, 1845).
6b Transverse occipital canals of the ventral branches of lateral line almost equal or slightly longer than dorsal branches, not reaching base of dorsal fin and 1/2 of pectoral fin; vertebrae: 29–31; pectoral fin long; eye large, diameter more than 1/2 of head length…………………………………………………..P. minor (Liu and Li, 1998).
Taxonomic Archives of Indian Stromateids
The taxonomy of pomfrets (Pampus) in Indian waters dates back to 1803 when Russell described four species under the Stromateus genus from Visakhapatnam (Visagapatanam) on the Coromandel Coast of India (Russell, 1803): Stromateus argenteus (Stromateus with squamous rhomboidal body, Tella Sandawa); S. niger (Stromateus with squamous ovate body, Nala Sandawah); Stromateus with body nearly orbicular, covered with small scales (Atoo Koia); and Stromateus with rhomb-form body, without scales (Sudi Sandawah). Subsequently, five species of stromateids were described in Cuvier and Valenciennes (1833): S. candidus, S. securifer, S. griseus, S. albus, and S. atous. Day (1876) recorded three species of pomfrets (S. cinereus, S. sinensis, and S. niger) from the Indian seas. Of the species of Stromateus mentioned above, Russell’s Atoo Koia (a species similar to Pampus chinensis) and Sudi Sandawah (based on description of an immature specimen) are native names and, thus, were not used by subsequent authors [see Plate-XLIV and Plate-V in Russell (1803)]. Haedrich (1967) synonymized the four species, S. candidus, S. griseus, S. securifer, and S. cinereus, to Pampus argenteus and two species, S. albus and S. atous, described from Indian waters to P. chinensis. However, recently, many species of Pampus were resurrected and re-described, like P. cinereus (Liu et al., 2013a) and P. candidus (Divya et al., 2019). While examining the Pampus species from India, our study revealed that the specimens from the east coast (West Bengal, Odisha, Andhra Pradesh and Tamil Nadu) in the Bay of Bengal are sufficiently distinct from the closely related congener P. candidus from India and P. cinereus from the South China Sea both at morphological and molecular levels.
Pampus griseus is originally described as Stromateus griseus by Cuvier and Valenciennes (1833), with counts D. 7–1/40, A. 5–1/38, C. 28, P.23 and caudal fin divided into two acute lobes, lower one nearly double in length than the upper, matches well with Russell’s Sudi Sandawah [see descriptions and Plate-XLV in Russell (1803)], the second valid species in this study (Table 1 and Figure 7). The type locality of both S. candidus and S. griseus was mentioned as Puducherry in the original description; therefore, the authors revisited, collected, and examined several specimens from the type locality (Puducherry) and nearby areas, such as Rameshwaram (Palk Bay), Nagapattinam, and Cuddalore, along the coast of Tamil Nadu in order ascertain the species. All the specimens examined from these areas were confirmed and identified to be either P. candidus or P. griseus. Also, there exist GenBank records of P. candidus from Karaikal (KX530944–KX530948) and Chennai (KF373001–KF373002) by Divya et al. (2017). S. griseus is represented by two syntypes (MNHN-IC-A-5479 and MNHN-IC-A-5512) at the Muséum National d’Histoire Naturelle (Fricke et al., 2020). Examination of the digital images of syntypes revealed that both the specimens are under dried and poor conditions, which makes it impossible to extract the correct morpho-meristic measurements to compare with the present fresh materials (source:https://science.mnhn.fr/institution/mnhn/collection/ic/item/a-5512 and https://science.mnhn.fr/institution/mnhn/collection/ic/item/a-5479). MNHN-IC-A-5479 (Supplementary Figure 1A) may be probably the specimen of P. griseus collected from Pondicherry (Puducherry), Tamil Nadu on the east coast of India, whereas MNHN-IC-A-5512 is obviously a specimen of Parastromateus niger (Supplementary Figure 1B). Examination of the drawing and description of Sudi Sandawah and Tella Sandawa from Visakhapatnam revealed that Sudi Sandawah is identical to the immature specimens examined in this study in that it has greatly extended anal and caudal fins (Supplementary Figure 13A); Tella Sandawa is also identical to the subadult and adult specimens examined in this study in that it has similar morphology of transverse occipital canal but differs because of having short anal and caudal fins (Supplementary Figure 13B). Russell’s Tella Sandawa might have been drawn from a specimen with broken caudal and lower lobe caudal fins (personal observation). The authors also revisited and examined the specimens from Visakhapatnam (type locality) and nearby areas (Odisha and West Bengal) and confirmed it as the second valid species. Sudi Sandawah is the native name and, thus, cannot be retained as species name, and the Tella Sandawa described as P. argenteus may be probably the specimen of the second valid species in the Bay of Bengal. Therefore, we prefer to retain a valid species status by providing the earlier described Indian species as Stromateus griseus from Pondicherry resurrecting it from the synonymy of P. argenteus and re-described as Pampus griseus (Cuvier, in Cuvier and Valenciennes, 1833) in the Bay of Bengal based on an integrative taxonomic approach. A lectotype (MNHN-IC-A-5479) was designated for Stromateus griseus to identify the species and fix the name. Additionally, two preserved specimens, GB. 31.145.1.8 (136.1 mm SL), labeled as P. griseus from West Bengal (Supplementary Figure 1D), and GB.31.145.1.1 (157.2 mm SL), labeled as P. argenteus from Visakhapatnam, Andhra Pradesh (Supplementary Figure 1C) deposited in the Marine Biodiversity Referral Museum of ICAR-Central Marine Fisheries Research Institute (CMFRI), Cochin, Kerala, India were re-examined and presumed to be P. griseus.
Remarks on Phenotypic Similarities and Distribution
In overall body appearance, P. griseus is similar to P. candidus and P. cinereus, which form a closely related complex (Table 4 and Supplementary Figure 10). Anal fin, greatly extended in P. griseus and P. candidus crossing beyond the caudal peduncle, was observed in this study (Figures 7, 8). Conversely, Liu et al. (2013a) provided an opposite character where the extended anal fin reaches behind the caudal peduncle, as reflected in Bloch’s original drawings (see Figure 1B in Liu et al., 2013a). However, after a thorough examination of a colored photograph of P. cinereus (see Figure 1A in Liu et al., 2013a), it was evident that the tip of the anal fin would have been broken but crossing beyond the caudal peduncle, a feature that was overlooked by Liu et al. (2013a). The long deeply forked caudal fin, with the lower lobe longer than upper and extended almost double in length is a feature in all size classes of P. griseus and P. candidus (Figures 7, 8), which is only slightly extended in P. cinereus (see Figure 1A in Liu et al., 2013a). This study refutes the observation of shortening of lower caudal fin lobe with age (Divya et al., 2019), and assumes that this feature may not be considered as an important character to distinguish species. The thin and fragile extended fins in most pomfret species frequently get damaged/cut either during fishing operation and post-harvest handling, or sometimes because of predator attacks (personal observation; Almatar and Chen, 2010), and urges the need for utmost care during taxonomic investigation of specimens, which can easily be overlooked by researchers.
This study that covered the Indian coastline suggested that the three species sympatric in the Indian Ocean vary in range of distribution and abundance; P. chinensis has a wider distribution compared to P. candidus, which occurs in Arabian Sea and certain areas of the BOB (Rameshwaram, Karaikal, Nagapattinam, and Chennai of Tamil Nadu), while P. griseus is distributed exclusively in Cuddalore (Tamil Nadu), Puducherry (type locality), Andhra Pradesh, Odisha, and West Bengal in the BOB. It is interesting to note that although both the species are present in Tamil Nadu (BOB), only P. griseus could be traced from the fishing harbors of the type locality and regions adjacent to Cuddalore. This study hinted P. griseus as the dominant species in fishing harbors of West Bengal and Andhra Pradesh, as evidenced by the ATPase sequences submitted by Divya et al. (2015). However, the presence of two COI barcodes (KF373009–KF373010; Divya et al., 2017) of P. candidus from West Bengal cannot be ignored and hinted at the rare chance of its occurrence in this area.
However, sporadic catches of Pampus argenteus beyond its native ranges (Indo-Western Pacific Oceans) such as Adriatic Sea, Southern Pacific, North Sea, and Mediterranean Sea (Fowler, 1938; Dulčić et al., 2004; Piper, 2010; Sami et al., 2014) were reported. Such records, except for the Southern Pacific, may be attributed to the Lessepsian migration of pomfrets through the Suez Canal and then to the Adriatic Sea and the northeastern Atlantic, consistent with the hypothesis that pomfret followed a slow-moving vessel or maintained an association with pelagic medusa (Sami et al., 2014). The morphological similarities of this complex group, coupled with absence of detailed morphological and molecular data, create ambiguity in establishing the occurrence of true “P. argenteus” in the indicated areas. The re-description of P. candidus and reported absence of P. argenteus from the Indian Ocean (Divya et al., 2019) affirm that P. argenteus is endemic to western Pacific Oceans. Thus, the reports from the Adriatic, North, and Mediterranean Seas can probably represent P. candidus or potentially another species that needs to be validated.
Conclusion
The classification of the genus Pampus has long been a subject of research with persistent controversies, and the results have struggled to be consistent and uniform, which imparted little scope to morphological distinction among some taxa. Therefore, classical taxonomy alone may not be suitable to resolve the systematics of the genus. Erroneous submissions in the GenBank database indicated that nominations based only on monogenic barcodes can be jeopardous. An integrative approach coupling DNA-based analysis and traditional taxonomy is the most authentic and informative tool used for delineating novel species and revitalizing taxonomy (Rajpoot et al., 2016). In this study, we have successfully employed this method to effectively resurrect the cryptic and valid species “Pampus griseus”. Misidentifications of sequences in the NCBI database were corrected based on reference sequences and recent literature to reconstruct the Bayesian phylogeny of the genus, which resulted in seven clades representing the seven species distributed in the Indo-Pacific, of which three (P. chinensis, P. candidus, and P. griseus) having distribution in the Indian Ocean. We confirmed that Pampus chinensis from Indian and Pacific Oceans represent two distinct lineages of single species unlike reported earlier. Major diagnostic characteristics were identified, systematics of genus Pampus was reviewed, and an easy field identification key was provided based on a combination of features. A future study on its distributional ranges is obligatory to have detailed information on fishery, biology, and population parameters of this species, which will help fishery managers for sustainable exploitation and proper management.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, MW422591–MW422604, MW421904–MW421919, MW343690–MW343704, MW366881–MW366888, MW419276–MW419288, MW337238–MW337245, MW343460–MW343467, MW332295–MW332304, MW260616–MW260617, MW343468–MW343475, MW447297, MW447300–MW447301, and MW456736.
Author Contributions
SRo and NJ conceptualized the work, analyzed the morphological and molecular data respectively, and wrote the complete manuscript in concurrence. ShR and RK provided specimens, fishery, and export information of P. candidus from Gujarat, and described otolith morphology. SuR carried out the molecular lab works. RV sampled from Tamil Nadu (East Coast of India). SG arranged specimens from Visakhapatnam. PR and SG critically reviewed and corrected the write up. AG co-ordinated the work. All authors contributed to the article and approved the submitted version.
Funding
This work has been carried by the institution fund available with ICAR-CMFRI.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the director of ICAR-CMFRI, Kerala, India for providing facilities and support during the study period. We are grateful to Divya P. R, NBFGR, Cochin for sharing the information on Pampus spp. We are thankful to Jinkoo Kim for providing the supplementary data of Pampus species (images and radiographs). The help rendered by the staff of Puri Field Centre of ICAR-CMFRI, Odisha in the field and laboratory is acknowledged. We also thank the following staff of CMFRI: Retheesh TB from Cochin, Pradeep from Puducherry, Ramesh from Nagapattinam, and Simon from the Department of Fisheries, Puducherry for collecting and providing pomfret samples from respective localities during this crisis time of the pandemic. Sincere thanks to K. M. David, STA (Artist) of ICAR-CMFRI for the line drawings included in this article.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.778422/full#supplementary-material
Footnotes
- ^ https://www.zauba.com/export-frozen+pomfret+fish-hs-code.html
- ^ http://tree.bio.ed.ac.uk/software/figtree/
References
Almatar, S., and Chen, W. (2010). Deformities in silver pomfret Pampus argenteus caught from Kuwait waters. Chin. J. Oceanol. Limnol. 28, 1227–1229. doi: 10.1007/s00343-010-0082-3
Basilewsky, S. (1855). Ichthyographia Chinae borealis. Nouv. Mém. Soc. Imp. Mosc. 10, 215–263. Pls. 1-9.
Beaufort, L. F., and Chapman, W. H. (1951). The fishes of Indo-Australian Archipelago. Leiden 9, 85–95. doi: 10.5962/bhl.title.12497
Behera, P. R., Jishnudev, M. A., Saravanan, R., Roul, S. K., Ghosh, S., Mahesh, V. U., et al. (2020). Redescription of the enigmatic jellyfish, Crambionella annandalei (Cnidaria: Scyphozoa) from Indian waters. J. Mar. Biol. Assoc. U.K. 100, 691–699. doi: 10.1017/S0025315420000703
Bleeker, P. (1851). Over eenige nieuwe geslachten en soorten van Makreelachtige visschen van den Indischen Archipel. Natuurkd. Tijdschr. Ned. Indië 1, 341–372.
Bleeker, P. (1852). Bijdrage tot de kennis der Makreelachtige visschen van den Soenda-Molukschen Archipel. Verh. Bat. Gen. 24, 1–93.
Bloch, M. E., and Schneider, J. G. (1801). Systema Ichthyologiae Iconibus ex Illustratum. Post Obitum Auctoris opus Inchoatum Absolvit, Correxit, Interpolavit. Jo. Gottlob Schneider. Berolin: Sanderiano Commissum, 584.
Bonaparte, C. L. (1837). ). Iconografia Della Fauna Italica, per le Quattro Classi Degli Animali Vertebrati. Tome III, Part 2, Pesces. Roma: Tipografia Salviucci, 90.
Cheng, Q. T., and Zheng, B. S. (1987). Systematic Synopsis of Chinese Fishes. Beijing: Science Press.
Cui, Z., Liu, Y., Li, C. P., and Chu, K. H. (2011). Species delineation in Pampus (Perciformes) and the phylogenetic status of the Stromateoidei based on mitogenomics. Mol. Biol. Rep. 38, 1103–1114. doi: 10.1007/s11033-010-0207-y
Cui, Z., Liu, Y., Liu, J., and Luan, W. (2010). Molecular identification of Pampus fishes (Perciformes, Stromateidae). Ichthyol. Res. 57, 32–39. doi: 10.1007/s10228-009-0119-9
Cuvier, G., and Valenciennes, M. (1833). Histoire Naturelle des Poisons, Vol. 9. Paris: Nabu Press, 552.
Day, F. (1876). The Fishes of India; being a Natural History of Fishes known to Inhabit the Seas and Freshwaters of India, Burma and Ceylon, Part 2. London: William Dawson & Sons Ltd, 247.
Divya, P. R., Gopalakrishnan, A., Basheer, V. S., Swaminathan, R., Mohitha, C., Joy, L., et al. (2015). Mitochondrial ATPase 6/8 genes to infer the population genetic structure of silver pomfret fish Pampus argenteus along the Indian waters. Mitochondrial DNA 26, 189–194. doi: 10.3109/19401736.2013.879655
Divya, P. R., Kumar, R. G., Mohitha, C., Rajool Shani, C. P., Bineesh, K. K., Basheer, V. S., et al. (2019). Resurrection and re-description of Pampus candidus (Cuvier), silver pomfret from the northern Indian Ocean. Zool. Stud. 58, 1–10. doi: 10.6620/ZS.2019.58-07
Divya, P. R., Mohitha, C., Rahul, G. K., Shanis, C. R., Basheer, V. S., and Gopalakrishnan, A. (2017). Molecular based phylogenetic species recognition in the genus Pampus (Perciformes: Stromateidae) reveals hidden diversity in the Indian Ocean. Mol. Phylogenetics Evol. 109, 240–245. doi: 10.1016/j.ympev.2016.12.030
Dolganov, V. N., Kharin, V. E., and Zemnukhov, V. V. (2007). Species composition and distribution of butterfishes (Stromateidae) in waters of Russia. J. Ichthyol. 47, 579–584. doi: 10.1134/S0032945207080048
Drummond, A. J., Suchard, M. A., Xie, D., and Rambaut, A. (2012). Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 29, 1969–1973. doi: 10.1093/molbev/mss075
Dulčić, J., Jardas, I., Pallaoro, A., and Lipej, L. (2004). On the validity of the record of silver pomfret Pampus argenteus (Stromateidae) from the Adriatic Sea. Cybium 28, 69–71.
Elliott, N. G., Haskard, K., and Koslow, J. A. (1995). Morphometric analysis of orange roughy (Hoplostethus atlanticus) off the continental slope of southern Australia. J. Fish Biol. 46, 202–220. doi: 10.1111/j.1095-8649.1995.tb05962.x
Euphrasen, B. A. (1788). Beskrifning po trenne fiskar. Vetensk. Akad. Nya Hand.l Stockholm 9, 49–55.
Ezard, T., Fujisawa, T., and Barraclough, T. G. (2009). SPLITS: Species’ Limits by Threshold Statistics. R Package Version, 1.0- 11/r29.
Fischer, J. (2013). Fish Identification Tools for Biodiversity and Fisheries Assessments: Review and Guidance for Decision-Makers. FAO Fisheries and Aquaculture Technical Paper No. 585. Rome: FAO.
Forster, J. R. (1795). Zoologia Indica, Sistens I. Descriptiones Animalium Selectorum Tabulis Aeneis delineatorum; II. Observationes de Finibus et Indole Aëris, Soli, Marisque Indici; Denique III. Faunam Indicam Quantum Fieri Licuit Perfectissimam. Zweyte Sehr Vermehrte Auflage (Second Edition). 4 Unnum. pp. + i-iv + 2 + pp. 1-42 (main text) + p. 138 (Faunula indica), Pls. I-XII + 3 unnumb. pls, 2nd Edn. Halle.
Fowler, H. W. (1938). The fishes of the george vanderbilt south pacific expedition, 1937. Monogr. Acad. Nat. Sci. Phila. 2, 1–349.
Fricke, R., Eschmeyer, W. N., and Van der Laan, R. (eds) (2020). Eschmeyer’s Catalog of Fishes: Genera, Species. Available online at: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed May 22, 2020)
Froese, R., and Pauly, D. (2020). FishBase. World Wide Web Electronic Publication. Los Baños: FishBase. (accessed May 2020).
Fujisawa, T., and Barraclough, T. G. (2013). Delimiting species using single-locus data and the generalized mixed Yule coalescent approach: a revised method and evaluation on simulated data sets. Syst. Biol. 62, 707–724. doi: 10.1093/sysbio/syt033
Gaither, M. R., and Rocha, L. A. (2013). Origins of species richness in the Indo-Malay-Philippine biodiversity hotspot: evidence for the centre of overlap hypothesis. J. Biogeogr. 40, 1638–1648. doi: 10.1111/jbi.12126
Guimarães, E. C., de Brito, P. S., Bragança, P. H. N., Santos, J. P., Katz, A. M., Carvalho Costa, L. F., et al. (2020). Integrative taxonomy reveals two new cryptic species of Hyphessobrycon Durbin, 1908 (Teleostei: Characidae) from the Maracaçumé and middle Tocantins River basins, Eastern Amazon region. Eur. J. Taxon. 723, 77–107. doi: 10.5852/ejt.2020.723.1145
Guo, E., Liu, Y., Liu, J., and Cui, Z. (2010). DNA barcoding discriminates Pampus minor (Liu et al., 1998) from Pampus species. Chin. J. Ocean. Limnol. 28, 1266–1274. doi: 10.1007/s00343-010-9917-1
Gupta, S. (2020). Reviews on the biology and culture of Silver Pomfret, Pampus argenteus (Euphrasen, 1788). Int. J. Aquat. Biol. 8, 228–245.
Haedrich, R. L. (1967). The stromateoid fishes: systematics and a classification. Bull. Museum. Compar. Zool. 135, 31–139.
Haedrich, R. L. (1984). “Stromateidae,” in FAO Species Identification Sheets for Fishery Purposes. Western Indian Ocean (Fishing Area 51), eds W. Fischer and G. Bianchi (Rome: FAO).
Hall, R., and Morley, C. K. (2004). “Sundaland basins,” in Continent-Ocean Interactions within East Asian Marginal Seas, eds P. Clift, P. Wang, W. Kuhnt, and D. Hayes (Washington, DC: American Geophysical Union), 55–85. doi: 10.1029/149gm04
Huang, W., Xu, X., Xie, Z., Li, Y., Wang, D., Wang, X., et al. (2016). The complete mitochondrial genome of the Pampus nozawae (Perciformes: Stromateidae). Mitochondrial DNA Part A 27, 988–989. doi: 10.3109/19401736.2014.926518
Ishikawa, C. (1904). Notes on some new or little known fishes of Japan. Proc. Dep. Nat. Hist. Tokyo Imp. Mus. 1, 8–9.
Jawad, L. A., and Jig, L. (2017). Comparative osteology of the axial skeleton of the genus Pampus (Family: Stromateidae, Perciformes). J. Mar. Biol. Assoc. U.K. 97, 277–287. doi: 10.1017/S0025315416000369
Katwate, U., Knight, J. M., Anoop, V. K., Raghavan, R., and Dahanukar, N. (2020). Three new species of filament barbs of the genus Dawkinsia (Teleostei: Cyprinidae) from the Western Ghats of India. Vertebr. Zool. 70, 207–233. doi: 10.26049/VZ70-2-2020-08
Kimura, M. (1980). A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16, 111–120. doi: 10.1007/BF01731581
Koken, E. (1884). Ueber Fisch-Otolithen, insbesondere über diejenigen der norddeutschen Oligocän-Ablagerungen. Z. Dtsch. Geol. Gesellschaft. 36, 500–565.
Kumar, S., Stecher, G., Li, M., Knyaz, C., and Tamura, K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. doi: 10.1093/molbev/msy096
Last, P. R. (1997). “Stromateidae. Butterfishes, silver pomfrets,” in FAO Identification Guide for Fishery Purposes, eds K. E. Carpenter and V. Niem (Rome: FAO). The Western Central Pacific.
Lewis, S. G., and Boyle, M. (2017). The expanding role of traceability in seafood: tools and key initiatives. J. Food Sci. 82, A13–A21. doi: 10.1111/1750-3841.13743
Li, Y., Gao, T., Zhou, Y., and Lin, L. (2019a). Spatial genetic subdivision among populations of Pampus chinensis between China and Pakistan: testing the barrier effect of the Malay Peninsula. Aquat. Living Resour. 32:8. doi: 10.1051/alr/2019004
Li, Y., Song, N., Khan, F. S., Yanagimoto, T., and Gao, T. X. (2013). New evidence of morphological characters and DNA barcoding of Pampus argenteus (Euphrasen, 1788). J. Fish China 37, 1601–1608. doi: 10.3724/SP.J.1231.2013.38824
Li, Y., Zhang, L., Loh, K., Feng, J., Zheng, X., Song, P., et al. (2019b). Genetic diversity comparison of Pampus minor between Chinese and Malaysian populations inferred from mtDNA Cytb. Pakistan J. Zool. 51, 149–157. doi: 10.17582/journal.pjz/2019.51.1.149.157
Li, Y., Zhang, Y., Gao, T., Han, Z., Lin, L., and Zhang, X. (2017). Morphological characteristics and DNA barcoding of Pampus echinogaster (Basilewsky, 1855). Acta Oceanol. Sin. 36, 18–23. doi: 10.1007/s13131-017-1124-x
Li, Y., Zhang, Z., Song, N., and Gao, T. (2016). Characteristics of complete mitogenome of Pampus sp. nov (Perciformes: Stromateidae). Mitochondrial DNA Part A 27, 1640–1641. doi: 10.3109/19401736.2014.958707
Li, Y., Zhou, Y., Li, P., Gao, T., and Lin, L. (2019c). Species identification and cryptic diversity in Pampus species as inferred from morphological and molecular characteristics. Mar. Biodivers. 49, 2521–2534. doi: 10.1007/s12526-019-00976-6
Lindberg, G. U., and Krasyukova, Z. V. (1975). Fishes of the Sea of Japan and the Adjacent Areas of the Sea of Okhotsk and Yellow Sea part 4. Akad. Leningrad: Nauk SSSR, 332.
Liu, C., Yang, Z., Liu, P., Ye, S., Siyal, F. K., Zhu, G., et al. (2020). First record of Pampus minor (Actinopterygii: Perciformes: Stromateidae) from the coastal waters of Wenzhou, China. Aquat. Living Resour. 33:5. doi: 10.1051/alr/2020006
Liu, J., and Li, C. S. (1998a). A new pomfret species, Pampus minor sp. nov. Chin. J. Oceanol. Limnol. 16, 280–285. doi: 10.1007/BF02848735
Liu, J., and Li, C. S. (1998b). Redescription of a stromateoid fish Pampus punctatissimus and comparison with Pampus argenteus from Chinese coastal waters. Chin. J. Ocean Limnol. 16, 161–166. doi: 10.1007/BF02845182
Liu, J., and Li, C. S. (2013). A new species of the genus Pampus (Perciformes, Stromateidae) from China. Acta Zootaxonomica Sin. 38, 885–890.
Liu, J., Li, C. S., and Li, X. S. (2002). Studies on Chinese pomfret fishes of the genus Pampus (Pisces: Stromateidae). Stud. Mar. Sin. 44, 240–252.
Liu, J., Li, C. S., Li, X. S., and Ning, P. (2013a). A redescription of grey pomfret Pampus cinereus (Bloch and Schneider, 1801) with the designation of a neotype (Teleostei: Stromateidae). Chin. J. Oceanol. Limnol. 31, 140–145. doi: 10.1007/s00343-013-2039-9
Liu, J., Li, C. S., Li, X. S., and Ning, P. (2013b). Identity of silver pomfret Pampus argenteus (Euphrasen, 1788) based on specimens from its type locality, with a neotype designation (Teleostei: Stromateidae). Acta Zootaxonomica Sin. 38, 171–177.
Luo, A., Ling, C., Ho, S. Y., and Zhu, C. D. (2018). Comparison of methods for molecular species delimitation across a range of speciation scenarios. Syst. Biol. 67, 830–846. doi: 10.1093/sysbio/syy011
Manel, S., Guerin, P. E., Mouillot, D., Blanchet, S., Velez, L., Albouy, C., et al. (2020). Global determinants of freshwater and marine fish genetic diversity. Nat. Commun. 11:692. doi: 10.1038/s41467-020-14409-7
Marramà, G., and Kriwet, J. (2017). Principal component and discriminant analyses as powerful tools to support taxonomic identification and their use for functional and phylogenetic signal detection of isolated fossil shark teeth. PLoS One 12:e0188806. doi: 10.1371/journal.pone.0188806
Nolf, D. (1985). “Otolithi piscium,” in Handbook of Paleoichthyology, Vol. 10, ed. H. P. Schultze (Stuttgart: Gustav Fischer Verlag), 1–145.
Palumbi, S. R., Martin, A. P., Romano, S., Mcmilan, W. O., Stice, L., and Grabowski, G. (1991). A Simple Fool’s Guide to PCR. Honolulu, HI: University of Hawaii Press.
Parin, N. V., and Piotrovsky, A. S. (2004). Stromateoid fishes (suborder Stromateoidei) of the Indian Ocean (species composition, distribution, biology, and fisheries). J. Ichthyol. 44, (Suppl. 1), 33–62.
Piper, R. (2010). Re-occurrence of silver pomfret Pampus argenteus in the North Sea. Mar. Biodivers. Rec. 3:e102. doi: 10.1017/S175526721000093X
Platt, C., and Popper, A. N. (1981). “Fine structure and function of the ear,” in Hearing and Sound Communication in Fishes, eds W. N. Tavolga, A. N. Popper, and R. R. Fay (New York, NY: Springer-Verlag), 3–38.
Pons, J., Barraclough, T. G., Gomes-Zurita, J., Cardoso, A., Duran, D. P., Hazell, S., et al. (2006). Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Syst. Biol. 55, 595–609. doi: 10.1080/10635150600852011
R Core Team (2018). R: A Language and Environment for Statistical Computing. Vienna: R foundation for statistical computing.
Rajpoot, A., Kumar, V. P., Bahuguna, A., and Kumar, D. (2016). DNA barcoding and traditional taxonomy: an integrative approach. Int. J. Curr. Res. 8, 42025–42031. doi: 10.1139/gen-2015-0167
Richardson, S. J. (1846). Report on the ichthyology of the seas of China and Japan. Rep. Br. Assoc. Adv. Sci. 15, 187–320. doi: 10.5962/bhl.title.59530
Russell, P. (1803). Descriptions and Figures of Two Hundred Fishes Collected at Vizagapatam on the Coast of Coromandel. London: W. Bulmer, 1.
Regan, C. T. (1902). A review of fishes of the family Stromateidae. Ann. Mag. Nat. Hist. Ser. 7:205. doi: 10.1007/s11230-017-9717-5
Sami, M., Rym, E., Othman, J., and Hechmi, M. (2014). First record of Pampus argenteus (Euphrasen, 1788) (Osteichthyes: Stromateidae) in Tunisian coast (Mediterranean sea). J. Mar. Biol. Oceanogr. 3:1. doi: 10.4172/2324-8661.1000123
Schmidt, W. (1969). “The otolith as a means for differentiation between species of fish of very similar appearance,” in Proceedings of the Symposium on the Oceanography and Fisheries Resources of the Tropical Atlantic, (Abidjan: UNESCO), 393–396.
Sivasundar, A., Bermingham, E., and Ortí, G. (2001). Population structure and biogeography of migratory freshwater fishes (Prochilodus: Characiformes) in major South American rivers. Mol. Ecol. 10, 407–417. doi: 10.1046/j.1365-294x.2001.01194.x
Sun, P. (2015). Comparison of genetic difference in cultured Pampus populations between China and Kuwait based on COI partial sequences. Mar. Sci. 39, 53–58.
Sun, P., Shi, Z., Yin, F., and Peng, S. (2012). Population genetic structure and demographic history of Pampus argenteus in the Indo-West Pacific inferred from mitochondrial cytochrome b sequences. Biochem. Syst. Ecol. 43, 54–63. doi: 10.1016/j.bse.2012.02.028
Sun, P., Yin, F., Shi, Z., and Peng, S. (2013). Genetic structure of silver pomfret (Pampus argenteus (Euphrasen, 1788)) in the Arabian Sea, Bay of Bengal, and South China Sea as indicated by mitochondrial COI gene sequences. J. Appl. Ichthyol. 29, 733–737. doi: 10.1111/jai.12130
Takacs, P. (2012). Morphometric differentiation of gudgeon species inhabiting the Carpathian Basin. Ann. Limnol. Int. J. Limnol. 48, 53–61. doi: 10.1051/limn/2011058
Temminck, C. J., and Schlegel, H. (1845). “Pisces,” in Fauna Japonica, Sive Descriptio Animalium quae Initinere per Japoniam suscepto Annis 1823–30 Collegit, Notis Observationibus et Adumbrationibus Illustravit P. F. de Siebold. Parts 7–9: 113–172, Pls. 1–143 + A.
Ward, R. D., Zemlak, T. S., Innes, B. H., Last, P. R., and Hebert, P. D. (2005). DNA barcoding Australia’s fish species. Philos. Trans. R. Soc. B. Biol. Sci. 360, 1847–1857. doi: 10.1098/rstb.2005.1716
Willette, D. A., Simmonds, S. E., Cheng, S. H., Esteves, S., Kane, T. L., Nuetzel, H., et al. (2017). Using DNA barcoding to track seafood mislabelling in Los Angeles restaurants. Conserv. Biol. 31, 1076–1085. doi: 10.1111/cobi.12888
Wu, H. L. (1985). “Stromateidae,” in The Fishes of Fujian Province, Vol. 2, ed. Y. T. Chu (Fuzhou: Fujian Science and Technology Press), 431–433.
Xiao, W., Zhang, Y., and Liu, H. (2001). Molecular systematics of Xenocyprinae (Teleostei: Cyprinidae): taxonomy, biogeography, and coevolution of a special group restricted in East Asia. Mol. Phylogenetics Evol. 18, 163–173. doi: 10.1006/mpev.2000.0879
Yamada, U., Tokimura, M., Horikawa, H., and Nakabo, T. (2007). Fishes and Fisheries of the East China and Yellow Seas. Tokyo: Tokai University Press.
Yamada, U., Tokimura, M., Hoshino, K., Deng, S., Zheng, Y., Li, S., et al. (2009). Name and Illustrations Offish from the East China Sea and the Yellow Sea: Japanese, Chinese, Korean. Tokyo: Overseas Fishery Cooperation Foundation of Japan, 525–528.
Yin, G., Pan, Y., Sarker, A., Baki, M. A., Kim, J. K., Wu, H., et al. (2019). Molecular systematics of Pampus (Perciformes: Stromateidae) based on thousands of nuclear loci using target-gene enrichment. Mol. Phylogenetics Evol. 140:106595. doi: 10.1016/j.ympev.2019.106595
Keywords: Bay of Bengal, phylogenetic trees, species delimitation, multivariate analysis, sagittal otolith, integrative taxonomy
Citation: Roul SK, Jeena NS, Kumar R, Vinothkumar R, Rahangdale S, Rahuman S, Ghosh S, Rohit P and Gopalakrishnan A (2021) Postulating the Modality of Integrative Taxonomy in Describing the Cryptic Congener Pampus griseus (Cuvier) and Systematics of the Genus Pampus (Perciformes: Stromateidae). Front. Mar. Sci. 8:778422. doi: 10.3389/fmars.2021.778422
Received: 16 September 2021; Accepted: 09 November 2021;
Published: 24 December 2021.
Edited by:
Christian Marcelo Ibáñez, Andres Bello University, ChileReviewed by:
Claudio Oliveira, São Paulo State University, BrazilMariana Díaz-Santana-Iturrios, Andres Bello University, Chile
Copyright © 2021 Roul, Jeena, Kumar, Vinothkumar, Rahangdale, Rahuman, Ghosh, Rohit and Gopalakrishnan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Subal Kumar Roul, c3ViYWxyb3VsQGdtYWlsLmNvbQ==; orcid.org/0000-0001-7146-955X
†These authors have contributed equally to this work and share first authorship
 Subal Kumar Roul
Subal Kumar Roul N. S. Jeena†
N. S. Jeena† Rajan Kumar
Rajan Kumar Shubhadeep Ghosh
Shubhadeep Ghosh Achamveetil Gopalakrishnan
Achamveetil Gopalakrishnan