
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Mar. Sci., 29 November 2021
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.766038
 Khor Waiho1,2,3
Khor Waiho1,2,3 Hanafiah Fazhan1,3*
Hanafiah Fazhan1,3* Alexander Chong Shu-Chien2,4
Alexander Chong Shu-Chien2,4 Muyassar H. Abualreesh5
Muyassar H. Abualreesh5 Hongyu Ma3,6
Hongyu Ma3,6 Mohammad Syahnon1
Mohammad Syahnon1 Ghazali Azmie1
Ghazali Azmie1 Nurul Jannah Razman1
Nurul Jannah Razman1 Mhd Ikhwanuddin1,3*
Mhd Ikhwanuddin1,3*Spiny lobsters of the genus Panulirus are economically important and support local fishing communities. However, mud spiny lobster Panulirus polyphagus is among the least known species within this genus in terms of their biological information. This study relates to the size distribution, length-weight relationship, and size at morphometric maturity of P. polyphagus in the Johor Strait. Within the year 2010, 300 specimens were collected off the coast of Johor Strait, Malaysia. There was no significant difference in body size (cephalothorax length, CL) and body weight (BW) between sexes. CL and BW were highly correlated and males of P. polyphagus displayed positive growth allometry, whereas the opposite was observed in females. Based on the merus (ML) and carpus length (CPL) of the third right walking leg, the piecewise linear regression analysis estimated that the size at maturity for male was 6.58 cm CL (based on ML) and 7.58 cm CL (based on CPL), whereas it was 8.18 cm CL (based on ML) and 6.75 cm (based on CPL) for females. Two discriminant functions of high classification and revalidation rates (> 98.6% in males and > 98.7% in females) that can discern maturation status in males and females of P. polyphagus were derived using the discriminant function analysis. Biological information derived from this study serves as an essential baseline for future fishery management and conservation of P. polyphagus.
The genus Panulirus White, 1847 includes various species of spiny lobsters in the family Palinuridae, which are most economically significant and support coastal fisheries. They differ from clawed lobsters in the family Nephropidae in terms of reproductive structures. Specifically, Panulirus do not possess sperm receptacle on the sternum of females and pleopods of males and are not involved in sperm transfer (George, 1995). There are 24 recognized species/subspecies in the genus Panulirus, making them the most diverse genus within Palinuridae compared to the remaining 11 genera (Briones-Fourzán, 2014). Most of the spiny lobster species are sturdy, exhibit attractive coloration, are huge (generally with a body length of up to 60 cm), and inhabit a wide range of habitats and depths. Due to their benthic nature, spiny lobsters have important ecological roles, functioning as predators to other benthic organisms such as bivalves, gastropods, and other crustaceans (Castañeda-Fernández-de-Lara et al., 2005; Mashaii et al., 2010) and prey to other predators including octopus, sharks, rays, and snappers (Smith and Herrkind, 1992).
Panulirus polyphagus (Herbst, 1793) is a marine demersal carnivorous crustacean species often associated with muddy coastal marine environment, thus the common name mud spiny lobster (Damodaran et al., 2017). It is widely distributed in the tropical Indo-Pacific region, ranging from India (Murugan et al., 2005; Kotiya and Vadher, 2021) to Malaysia (Fatihah et al., 2016, 2017; Chen and Fatihah, 2018) and Indonesia (Tewfik, 2014; Wahyudin et al., 2017). They often form the bycatch of trawlers due to their preferred benthic habitat along the muddy coastal zones. However, unlike other Panulirus species such as scalloped spiny lobster P. homarus (Linnaeus, 1758) and ornate spiny lobster P. ornatus (Fabricius, 1798) that are often associated with coastal rocky reefs (Kulmiye and Mavuti, 2005), not much is known about the general biology, physiology, and ecology aspects of P. polyphagus. Nonetheless, all Panulirus species are high-value potential aquaculture candidate species (Anh and Jones, 2015).
The length-weight relationship is essential for understanding and assessing the general growth characteristic, population health, and dynamics of a species (Miyasaka et al., 2007; Fazhan et al., 2021a). Therefore, the length-weight relationship of a species serves as an essential biometric parameter that would subsequently influence conservation efforts and fisheries management. In general, a higher mass at a given length is preferable as it implies better health conditions (Waiho et al., 2016; Jist et al., 2018). Since the length-weight relationship relies on body length and weight measurements, it is influenced by various biotic and abiotic factors such as sex, season, food availability, genetic, and fishing pressure (Al Nahdi et al., 2016; Li et al., 2016; Jist et al., 2018).
Sexual maturity marks the transition of immature juveniles to mature adults capable of reproducing offspring and is characterized by a series of abrupt morphological, physiological, and behavioral changes (Comeau and Savoie, 2002; Waiho et al., 2017). The onset of sexual maturity can be further divided into morphometric maturity, based on the abrupt changes in the allometric growth of specific morphometric characters, physiological maturity, based on the maturation of gonadal tissues, and functional maturity, based on the functional ability to copulate (Corgos and Freire, 2006; Waiho et al., 2017). Although depending on the species, there can be slight discrepancies between these three types of maturities (Waiho et al., 2016, 2017), morphometric maturity is more commonly used as it does not require the sacrifice of the studied species. Morphometric maturity is often estimated by analyzing the growth rate of specific body dimensions that will undergo rapid changes upon reaching sexual maturity in relation to body dimensions that exhibit a constant growth rate (Hartnoll, 1974). Size at morphometric maturity has been estimated for various species in the genus Panulirus (Jayakody, 1989; Hogarth and Barratt, 1996; Robertson and Butler, 2003; Kulmiye et al., 2006; Melville-Smith and de Lestang, 2006). However, this knowledge has not been extended to P. polyphagus. In Malaysia, although it could fetch up to USD50/kg in the wet market, the capture volume of P. polyphagus is largely undocumented due to their seasonal small-sized landings. Due to the lack of biological information (e.g., length and weight distribution and size at sexual maturity), size regulation and resource management of P. polyphagus are yet to be established. The first report of biological features of P. polyphagus was by Ikhwanuddin et al. (2014). Apart from the brief description of size distribution based on sexes, the estimation of length-weight relationship and size at maturity were rudimentary, whereby the authors did not fit their data with regression equation and method potency equation during the estimation of the former, and the assignment of maturity was purely based on the value of second and third merus lengths of the longest right walking leg with cephalothorax length (CL) (Ikhwanuddin et al., 2014). Therefore, this study aimed to recharacterize the length-weight relationship and size at morphometric maturity P. polyphagus population in the southern coast of South China Sea using sound statistical methods. We incorporated detailed analyses to warrant as a new publication, including regression equations of length-weight relationship, determination of size at morphometric maturity using the piecewise linear regression analysis, reclassification of maturity status using the principal component analysis, and providing two equations for the identification of maturity status in P. polyphagus based on the discriminant analysis. The results of this study serve as essential baseline information for future fishery and conservation management of this economically important crustacean species.
The specimens analyzed were sampled between July and December 2010 in the Johor Strait, along the coastal waters of Southeast Johor, Malaysia (Supplementary Figure 1). The locations (Teluk Ramunia, 1°21′52″N, 104°14′48″E, Sungai Rengit, 1°20′45″N, 104°13′8″E, and Kampung Jawa, 1°20′24″N, 104°7′12″E) were chosen based on the anecdotal accounts of the local fishing community. Spiny lobsters P. polyphagus were randomly obtained from local fishermen, and the measurements were acquired in situ right after their landing using gill nets. All live lobsters were returned to the fishermen after the measurement. As P. polyphagus is a commercial species, permit is not required for its acquisition. There is also no regulation on the capturing of immature and berried females of P. polyphagus in Malaysia. The specimens were identified based on the identification keys provided in the FAO Species Catalog (Holthuis, 1991). Three body dimensions, namely, cephalothorax length (CL)—the distance along the dorsal midline from the transverse ridge between the supraorbital horns to the posterior extremity of the cephalothorax, merus length (ML), and carpus length (CPL) of the longest (third) right walking leg were obtained using vernier caliper to the nearest 0.01 cm. Wet weight (BW, g) was measured using a digital electronic balance. All the measurements were reported as mean ± standard error (SE) unless stated otherwise. Raw data used in this study (Supplementary Material 1) were based on the study by Ikhwanuddin et al. (2014).
Length (CL) and weight (BW) between sexes were compared using the Welch’s t-test on the IBM SPSS Statistics Version 25 (IBM Corp, United States) due to the unequal sample size. The length–weight (CL–BW) relationship of P. polyphagus was estimated using the method potency equation, W = aLb, with W as BW, L as CL, and a and b are constants. The linear regression analysis was performed onto the log-transformed values of BW and CL. The allometric coefficient b represents the slope of the regression equation and b = 3 represents the isometric growth; b < 3 represents the negative allometric growth; and b > 3 represents the positive allometric growth (Cusba and Páramo, 2017).
To estimate the size at morphometric maturity, CL was designated as the independent variable due to their growth consistency upon reaching morphometric maturity. The dimensions of ML and CPL were used as dependent variables as leg dimensions are known to exhibit distinct changes in relative growth from immature juveniles to mature adults (Waiho et al., 2016). According to sex, the piecewise linear regression analysis was performed between CL and dependent variables (i.e., ML and CPL) using the statistical software Origin Lab Pro 2019b (OriginLab Corporation, United States). Independent variables were clustered into two groups (i.e., two regression lines) using the least square method, and this further allowed for the estimation of the breakpoint between the two regression lines representing the immature and mature morphometric growth. The breakpoint represents the abrupt change in growth allometry between immature and mature specimens along with size increment. The “gap” between regression lines was minimized during fitting-dependent variables to the piecewise linear regression algorithm, thereby minimizing SE. The F-test was used to determine the significance of the two regression slopes obtained by the piecewise linear regression analysis, and the reduced chi-square (χ2) and adjusted coefficient of determination (adj. R2) were recorded.
To characterize the relative growth pattern of ML and CPL before and after morphometric maturity, individuals were classified as juveniles and adults based on the method of Corgos and Freire (2006). In brief, the principal component analysis (PCA) was used to divide individuals into two groups (i.e., immature and mature) based on their log-transformed-dependent variables. Subsequently, each individual was assigned as either immature or mature using hierarchical k-means clustering, during which the intragroup variance was minimized, but the between-group variance was maximized. Based on the assigned maturity status, the log-transformed values of CL, ML, and CPL were subjected to the stepwise discriminant function analysis to generate a discriminant function and assess the misclassification rate.
A total of 300 P. polyphagus were sampled in this study, of which 231 were males and 69 were females. The average CL and BW (average CLmale: 7.51 ± 0.09 cm, average CLfemale: 7.39 ± 0.22 cm; BWmale: 189.57 ± 8.16 g; BWfemale: 186.98 ± 14.63 g) of males and females were similar, and most males (84.0%) were concentrated at a size range of 5.0–8.9 cm (Figure 1A). The CL and BW did not differ significantly between sexes (Welch’s t-test, FCL 93.92: 0.477, p = 0.635; FBW 113.69: 0.154, p = 0.878).
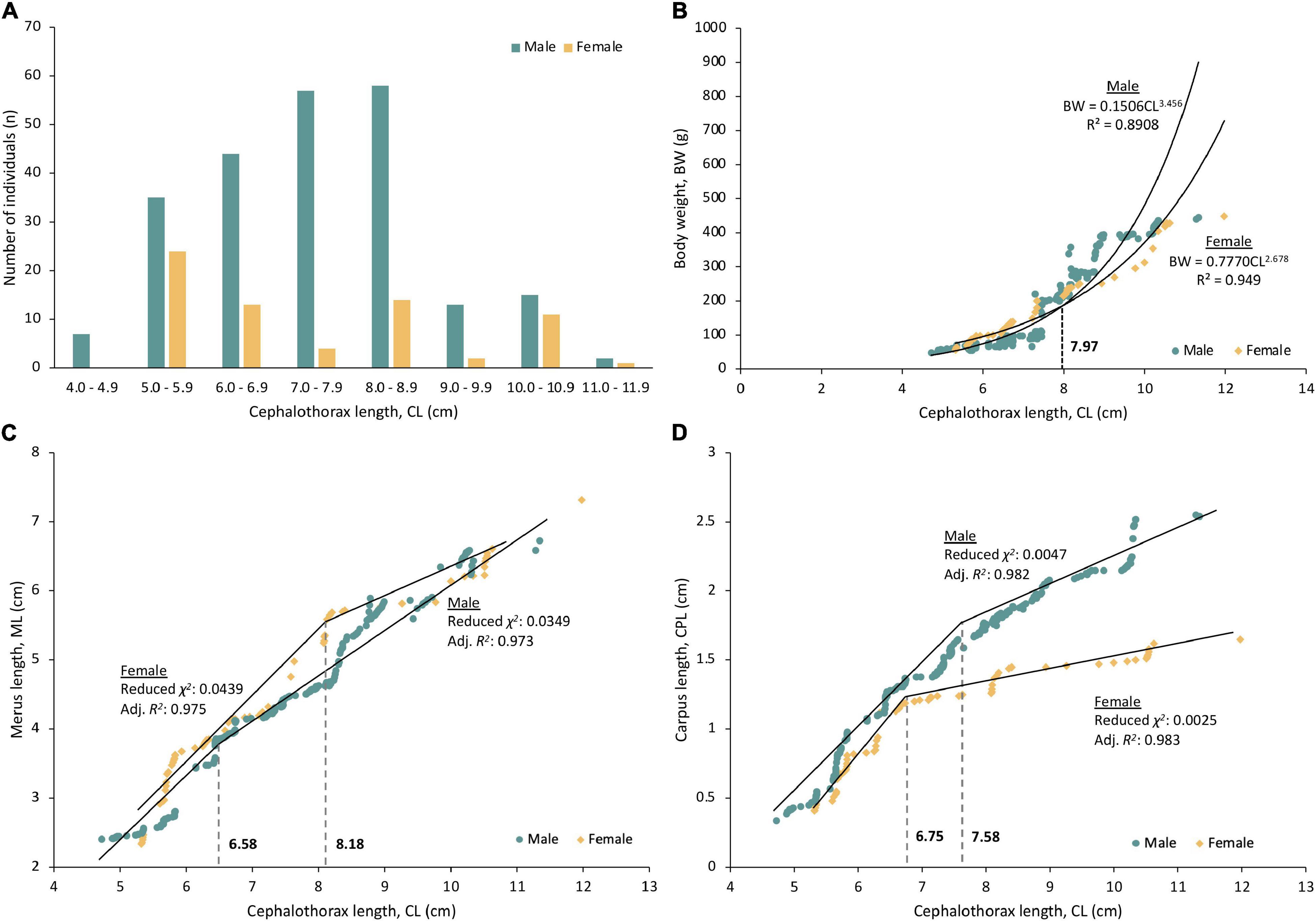
Figure 1. The (A) Size distribution, (B) Length-weight relationship, (C) Estimated size at morphometric maturity based on merus length (ML), and (D) Estimated size at morphometric maturity based on carpus length (CPL) of the third walking leg of mud spiny lobster. Fitting of ML and CPL with CL in both sexes was successful (F-test: all p < 0.001), and the estimated size at morphometric maturities was indicated using gray dotted line between the two regression lines of immature and mature specimens.
There is a strong correlation between CL and BW of P. polyphagus (r > 0.94, Table 1). The classical positive growth allometry in males and negative growth allometry in females were observed, as revealed by the regression coefficient b in Table 1. The weight of females was heavier than males at smaller body sizes (CL < 7.97 cm), but the trend gradually reversed after the intersecting point at 7.97 cm CL (Figure 1B).
Fitting of ML and CPL with CL in both sexes was successful (F-test: all p < 0.001). Based on the segmented regression lines produced using the piecewise linear regression analysis, the onset of morphometric maturity ranged from 6.58 to 8.18 cm CL (Figures 1C,D). The significant changes in the slope of regression lines were observed between immature and mature P. polyphagus. Overall, immature P. polyphagus, regardless of sex, showed higher regression slopes in both ML and CPL compared to their mature counterparts (Figures 1C,D). The estimated morphometric size at maturity for males was 6.58 cm CL (based on ML) and 7.58 cm CL (based on CPL), whereas it was 8.18 cm CL (based on ML) and 6.75 cm (based on CPL) for females.
The clustering of specimens into mature and immature was successful (F-test, all p < 0.001), with males being categorized into 43 immature and 188 mature individuals, females being categorized into 30 immature and 39 mature individuals, respectively. The stepwise discriminant analysis was significant in males (Wilks’ λ = 0.146, χ2 = 441.20, df = 3, eigenvalue = 5.838, canonical correlation = 0.924, p < 0.001) and females (Wilks’ λ = 0.129, χ2 = 134.30, df = 3, eigenvalue = 6.771, canonical correlation = 0.933, p < 0.001). Based on the coefficients of the canonical discriminant function, the discriminant equations of maturity status in males and females of P. polyphagus were derived (Table 2). The equations of both sexes achieved high classification and revalidation rates, with a percentage of 98.6% in males and 98.7% in females. This showed that the maturity status could be estimated based on the ML and CPL measurements of the third right walking leg of P. polyphagus.
The biological data of the mud spiny lobster P. polyphagus are comparatively less than other Panulirus species. Nonetheless, the size range (4.72–11.98 cm CL) found in this study coincides with that reported in P. polyphagus at the Arabian Sea (Gujarat, India; size interval of 3.5–11.0 cm CL) (Kizhakudan and Patel, 2010). The positive growth allometry in males and negative growth allometry in females of P. polyphagus based on the deviation of regression coefficient b from 3 are expected and commonly observed in crustaceans of good population health (Fazhan et al., 2021a), including other lobster species (Hossain et al., 1987; Steinback et al., 2008). In crustaceans, males would channel most of their energy into the somatic growth to increase their chances in territorial defense and mating, whereas females would focus more on maximizing reproductive outputs (Elner and Campbell, 2009; Waiho et al., 2015). Similarly, along the southern coastal region of Sri Lanka, Panulirus homarus females exhibited negative growth allometry, whereas males exhibited isometric growth patterns (Senevirathna et al., 2014). Such pattern, positive/isometric growth in males and negative growth in females, however, is dependent on various factors, including health conditions (Datta et al., 2013), sample size (Fazhan et al., 2021a), and environmental factors (Al Nahdi et al., 2016). The size concentration of male P. polyphagus (5.0–8.9 cm size range) around the estimated size at sexual maturity (6.58 cm CL [based on ML] and 7.58 cm CL [based on CPL]) is expected. A similar pattern was also found in other crustaceans (Little and Watson, 2005; Waiho et al., 2016) and is closely linked with the reproduction potential of a species. For example, the mode of production of the female egg coincided with the mode of size distribution of females in spiny lobster Palinurus elephas (Goñi et al., 2003).
Size at the onset of sexual maturity is an essential parameter that can be used in the assessment and management of fishery stock, including the setting up of a suitable minimum landing size (MLS) (Little and Watson, 2005; Waiho et al., 2017). Based on a 7-year length–frequency data, Kagwade (1987) showed that in P. polyphagus, the growth was significantly faster in males than in females after reaching sexual maturity. Kagwade (1988) further estimated that P. polyphagus reaches sexual maturity from hatching in a period of 3 years compared to less than 3 years in other Panulirus species (Skewes et al., 1994). Compared to the size at morphometric maturities found in this study, P. polyphagus from the Saurashtra coast, Gujarat, India, population had smaller size at morphometric maturities, with males maturing in the range of 51–55 mm CL, while females maturing between 51 and 60 mm CL (Kizhakudan and Patel, 2010) (Table 3). Various factors could contribute to the difference in size at maturity between populations, especially if the populations being compared are geographically distinct, e.g., at different latitudes (Bakke et al., 2018). These include female recruitment (Orensanz et al., 2007), temperature (Aguilar-Alberola and Mesquita-Joanes, 2014), food supply (Pollock, 1995), fishing pressure (Little and Watson, 2005), and genotypic variation (Pollock, 1995).
Another potential factor that could contribute to the variation in size at morphometric maturity between populations is the morphometric characters selected during its estimation. Aside from ML and CPL used in this study, other studies have used the second leg length, and the meropodite lengths of walking legs to estimate morphometric maturity (Table 3), and all research showed that estimates of size at morphometric maturity differed according to the characters used. Such variation implies that the abrupt changes in growth might occur at an interval for different body segments of crustaceans. Therefore, it is important to consider the measured morphological characters when estimating and comparing size at sexual maturity in crustaceans. The same criterion as earlier studies should be used in a future study to allow an accurate comparison of the estimated size at sexual maturity for a designated species from a specific location. In addition, it is also worth exploring other morphological characters that are proven to be useful in estimating morphometric size at sexual maturity in other shrimp or lobster species, such as the abdominal segment length (Cusba and Páramo, 2017; Pacheco et al., 2021).
In crustaceans, discriminant functions are useful tools for sex classification (Mantel and Dudgeon, 2005; Parvizi et al., 2017), species identification (Fazhan et al., 2020, 2021b), determination of maturity (Mura et al., 2005), and health monitoring (Fazhan et al., 2018). Due to the minimal biological information available for P. polyphagus population in the equatorial region, the discriminant functions (Table 2) derived in this study are useful for the non-invasive determination of its sexual maturity. This is especially useful for the involvement of local coastal communities, in particular fishermen, as citizen scientists come across P. polyphagus they would only be required to record three measurements (CL, CPL, ML) in comparison to the more complicated method of assessing gonadal condition (Kizhakudan and Patel, 2010). The use of ML and CPL to estimate size at sexual maturity is common in Panulirus lobsters (Table 3) and other crustacean species (Waiho et al., 2016). However, due to the lack of measured morphometric characters, we acknowledged that the use of the same variables (ML and CPL) in the maturation group clustering and discriminant analysis of P. polyphagus might be biased. To strengthen its feasibility and accuracy, the derived model of determination of sexual maturity of P. polyphagus obtained in this study could be validated in the future by testing on P. polyphagus specimens with confirmed maturation status based on physiological characters (e.g., gonadal maturation). Such initiative of constant monitoring by the local communities would not only aid in the population health monitoring of P. polyphagus but also contribute to the efforts of management of fishery in future.
Essential biological information such as size distribution, length-weight relationship, and size at sexual maturity of mud spiny lobster P. polyphagus in the South China Sea was made available in this study. These data generated will be useful for developing appropriate regulations to manage the fishery practices of P. polyphagus in this region. Furthermore, the development of two maturation stage-specific discriminant functions based on the sex of P. polyphagus would enhance future data collection as they could easily be employed by the public owing to their simplicity and non-invasive characteristics.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
KW: methodology, investigation, formal analysis, writing – original draft, and writing – review and editing. HF and MI: conceptualization, methodology, investigation, formal analysis, writing – review and editing, and supervision. AS-C: investigation, formal analysis, and writing – review and editing. MA and HM: validation and writing – review and editing. MS, GA, and NJR: investigation and validation. All authors contributed to the article and approved the submitted version.
This study was supported by the Ministry of Higher Education, Malaysia, under the Higher Centre of Excellence (HICoE) program, Malaysia, accredited to the Institute of Tropical Aquaculture and Fisheries, Universiti Malaysia Terengganu (Vot Nos. 63933 and 56046).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
An adjunct Academic Fellow position from USM to KW is acknowledged.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.766038/full#supplementary-material
Aguilar-Alberola, J. A., and Mesquita-Joanes, F. (2014). Breaking the temperature-size rule: thermal effects on growth, development and fecundity of a crustacean from temporary waters. J. Therm. Biol. 42, 15–24. doi: 10.1016/j.jtherbio.2014.02.016
Al Nahdi, A., Farcia de Leaniz, C., and King, A. J. (2016). Spatio-temporal variation in length-weight relationships and condition of the ribbonfish Trichiurus lepturus (Linnaeus, 1758): Implications for fisheries management. PLoS One 11:e0161989. doi: 10.1371/journal.pone.0161989
Al-Marzouqi, A., Chesalin, M., Al-Shajibi, S., Al-Hadabi, A., and Al-Senaidi, R. (2015). Changes in the scalloped spiny lobster, Panulirus homarus biological structure after a shift of the fishing season. J. Aquac. Mar. Biol. 3:00056.
Anh, T. L., and Jones, C. (2015). “Lobster seed fishing, handling and transport in Vietnam,” in Spiny lobster aquaculture development in Indonesia, Vietnam and Australia. Proceedings of the International Lobster Aquaculture Symposium held in Lombok, Indonesia, 22-25 April 2014, Chap. 2, ed. C. M. Jones (Canberra: Australian Centre for International Agricultural Research), 31–35.
Bakke, S., Larssen, W. E., Woll, A. K., Søvik, G., Gundersen, A. C., Hvingel, C., et al. (2018). Size at maturity and molting probability across latitude in female Cancer pagurus. Fish. Res. 205, 43–51. doi: 10.1016/j.fishres.2018.03.024
Briones-Fourzán, P. (2014). Differences in life-history and ecological traits between co-occurring Panulirus spiny lobsters (Decapoda, Palinuridae). Zookeys 457, 289–311. doi: 10.3897/zookeys.457.6669
Castañeda-Fernández-de-Lara, V., Serviere-Zaragoa, E., Hernández-Vázquez, S., and Butler, M. J. I. V. (2005). Feeding ecology of juvenile spiny lobster, Panulirus interruptus, on the Pacific coast of Baja California Sur, Mexico. New Zeal. J. Mar. Fresh. Res. 39, 425–435.
Chang, Y.-J., Sun, C.-L., Chen, Y., Yeh, S.-Z., and Chiang, W.-C. (2007). Reproductive biology of the spiny lobster, Panulirus penicillatus, in the southeastern coastal waters off Taiwan. Mar. Biol. 151, 553–564.
Chen, C.-A., and Fatihah, S. N. (2018). A preliminary study on the distribution of spiny lobster (Panulirus spp.) in Labuan Island, Malaysia. Borneo J. Mar. Sci. Aquac. 2, 60–63.
Comeau, M., and Savoie, F. (2002). Maturity and reproductive cycle of the female American lobster, Homarus americanus, in the Southern Gulf of St. Lawrence, Canada. J. Crust. Biol. 22, 762–774.
Corgos, A., and Freire, J. (2006). Morphometric and gonad maturity in the spider crab Maja brachydactyla: a comparison of methods for estimating size at maturity in species with determinate growth. ICES J. Mar. Sci. 63, 851–859. doi: 10.1016/j.icesjms.2006.03.003
Cusba, J., and Páramo, J. (2017). Morphometric relationships and size at sexual maturity of the deep-sea Caribbean lobster Metanephrops binghami (Decapoda: Nephropidae) in the Colombian Caribbean. Univ. Sci. 22, 145–160.
Damodaran, D., Koya, K. M., Mojjada, S. K., Lalaji, C. D., Dash, G., Vase, V. K., et al. (2017). Optimization of the stocking parameters for mud spiny lobster Panulirus polyphagus (Herbst, 1793) capture-based aquaculture in tropical open sea floating net cages. Aquac. Res. 49, 1080–1086.
Datta, S. N., Kaur, V. I., Dhawan, A., and Jassal, G. (2013). Estimation of length-weight relationship and condition factor of spotted snakehead Channa punctata (Bloch) under different feeding regimes. SpringerPlus 2:436. doi: 10.1186/2193-1801-2-436
Elner, R. W., and Campbell, A. (2009). Force, function and mechanical advantage in the claw of the American lobster Homarus americanus (Decapoda: Crustacea). J. Zool. 193, 269–286. doi: 10.1111/j.1469-7998.1981.tb03444.x
Ernawati, T., Priatna, A., and Satria, F. (2019). Biological reference points of painted spiny lobster Panulirus versicolor (Latreille, 1804) in Karimunjawa waters, Indonesia. Indones. Fish. Res. J. 25, 91–101.
Fatihah, S. N., Jaasmani, S., Abol-Munafi, A. B., Noorbaiduri, S., Muhd-Farouk, H., and Ikhwanuddin, M. (2016). Development of a sperm cryopreservation protocol for the mud spiny lobster, Panulirus polyphagus. Aquaculture 462, 56–63. doi: 10.1016/j.aquaculture.2016.04.025
Fatihah, S. N., Muhd-Farouk, H., Amin-Safwan, A., Mahsol, K. H., and Ikhwanuddin, M. (2017). Histological characteristics on the testes of mud spiny lobster, Panulirus polyphagus (Herbst, 1793). Pakist. J. Biol. Sci. 20, 365–371. doi: 10.3923/pjbs.2017.365.371
Faye, Y. P. W., Kantoussan, J., Diedhiou, F., Ba, A. O., and Thiaw, O. T. (2020). Reproductive biology in green spiny lobster, Panulirus regius (De Brito Capello 1864), from the Petite Cote of Senegal, West Africa. Internat. J. Adv. Res. 8, 182–195. doi: 10.21474/ijar01/11841
Fazhan, H., Waiho, K., Al-Hafiz, I., Kasan, N. A., Ishak, S. D., Afiqah-Aleng, N., et al. (2021a). Composition, size distribution, length-weight relationship of sympatric mud crab species (Scylla) and the case of presumed hybrids. Est. Coast. Shelf Sci. 250:107154. doi: 10.1016/j.ecss.2020.107154
Fazhan, H., Waiho, K., Fujaya, Y., Rukminasari, N., Ma, H., and Ikhwanuddin, M. (2021b). Sexual dimorphism in mud crabs: a tale of three sympatric Scylla species. PeerJ. 9:e10936. doi: 10.7717/peerj.10936
Fazhan, H., Waiho, K., Quinitio, E., Baylon, J. C., Fujaya, Y., Rukminasari, N., et al. (2020). Morphological descriptions and morphometric discriminant function analysis reveal an additional four groups of Scylla spp. PeerJ. 8:e8066. doi: 10.7717/peerj.8066
Fazhan, H., Waiho, K., Wee, H. B., Surzanne, M. A., Ma, H., and Ikhwanuddin, M. (2018). Predicting the sacculinid Sacculina beauforti infection status of the orange mud crab Scylla olivacea by discriminant analysis. Aquaculture 491, 128–134. doi: 10.1016/j.aquaculture.2018.03.009
George, R. W. (1995). Comparative morphology and evolution of the reproductive structures in spiny lobsters, Panulirus. New Zeal. J. Mar. Fresh. Res. 39, 493–501. doi: 10.1080/00288330.2005.9517328
Goñi, R., Quetglas, A., and Reñones, O. (2003). Size at maturity, fecundity and reproductive potential of a protected population of the spiny lobster Palinurus elephas (Fabricius, 1787) from the western Mediterranean. Mar. Biol. 143, 583–592. doi: 10.1007/s00227-003-1097-5
Hartnoll, R. G. (1974). Variation in growth pattern between some secondary sexual characters in crabs (Decapoda: Brachyura). Crustaceana 27, 131–136. doi: 10.1163/156854074x00334
Hogarth, P. J., and Barratt, L. A. (1996). Size distribution, maturity and fecundity of the spiny lobster Panulirus penicillatus (Olivier 1791) in the Red Sea. Trop. Zool. 9, 399–408. doi: 10.1080/03946975.1996.10539319
Holthuis, L. B. (1991). FAO Species Catalogue. Vol. 13. Marine lobsters of the world. An annotated and illustrated catalogue of species of interest to fisheries known to date. FAO Fish. Synop. 125:292.
Horsford, I., Simon, H., Archibald, M., Webber, J., and Joseph, T. (2013). “Biology, status, and current management of the Caribbean spiny lobster (Panulirus argus) in Antigua and Barbuda,” in Proceedings of the 66th Gulf and Caribbean Fisheries Institute, November 4-8, 2013 (Corpus Christi).
Hossain, M. A., Hartnoll, R. G., and Mohamedeen, H. (1987). The length-weight relationship and flesh production of the Norway lobster, Nephrops norvegicus (L.) (Decapoda, Astacidea). Crustaceana 52, 40–46. doi: 10.1163/156854087x00042
Ikhwanuddin, M., Fatihah, S. N., Nurul, J. R., Zakaria, M. Z., and Abol-Munafi, A. B. (2014). Biological features of mud spiny lobster, Panulirus polyphagus (Herbst, 1793) from Johor coastal water of Malaysia. World Appl. Sci. J. 31, 2079–2086.
Jayakody, D. S. (1989). Size at onset of sexual maturity and onset of spawning in female Panulirus homarus (Crustacea: Decapoda: Palinuridae) in Sri Lanka. Mar. Ecol. Prog. Ser. 57, 83–87. doi: 10.3354/meps057083
Jist, N., Younes, G., Sukhn, C., and El-Dakdouki, M. H. (2018). Length-weight relationships and relative condition factor of fish inhabiting the marine area of the Eastern Mediterranean city, Tripoli-Lebanon. Egypt. J. Aquat. Res. 44, 299–305.
Kagwade, P. V. (1987). Morphological relationships and conversion factors in spiny lobster Panulirus polyphagus (Herbst) from the north-west coast of India. Indian J. Fish. 34, 348–352.
Kagwade, P. V. (1988). Fecundity in the spiny lobster Panulirus polyphagus (Herbst). J. Biolog. Assoc. India 30, 114–120.
Kizhakudan, J. K., and Patel, S. K. (2010). Size at maturity in the mud spiny lobster Panulirus polyphagus (Herbst, 1793). J. Mar. Biol. Assoc. India 52, 170–179.
Kotiya, A. S., and Vadher, K. H. (2021). Comparing the most preferred raw feed for Mud Spiny Lobster Panulirus polyphagus growth in pit culture at intertidal area of Akatariya (Mahuva) coast. J. Surv. Fish. Sci. 7, 143–160.
Kulmiye, A. J., and Mavuti, K. M. (2005). Growth and moulting of captive Panulirus homarus Homarus in Kenya, western Indian Ocean. New Zeal. J. Mar. Freshw. Res. 39, 539–549. doi: 10.1080/00288330.2005.9517332
Kulmiye, A. J., Mavuti, K. M., and Groeneveld, J. C. (2006). Size at onset of maturity of spiny lobsters Panulirus homarus homarus at Mambrui, Kenya. Afr. J. Mar. Sci. 28, 51–55. doi: 10.2989/18142320609504133
Li, Y., Zhou, F., Ma, Z., Huang, J., Jiang, S., Yang, Q., et al. (2016). Length–weight relationship and condition factor of giant tiger shrimp, Penaeus monodon (Fabricius, 1798) from four breeding families. SpringerPlus 5:1279. doi: 10.1186/s40064-016-2979-6
Little, S. A., and Watson, W. H. III (2005). Differences in the size at maturity of female American lobsters, Homarus americanus, captured throughout the range of the offshore fishery. J. Crust. Biol. 25, 585–592. doi: 10.1651/c-2552.1
Mantel, S. K., and Dudgeon, D. (2005). Reproduction and sexual dimorphism of the palaemonid shrimp Macrobrachium homaruse in Hong Kong streams. J Crust. Biol. 25, 450–459. doi: 10.1651/c-2541
Mashaii, N., Rajabipour, F., and Shakouri, A. (2010). Feeding habits of the scalloped spiny lobster, Panulirus Homarus (Linnaeus, 1758) (Decapoda: Palinuridae) from the South East Coast of Iran. Turkish J. Fish. Aquat. Sci. 11, 45–54.
Melville-Smith, R., and de Lestang, S. (2006). Spatial and temporal variation in the size at maturity of the western rock lobster Panulirus cygnus George. Mar. Biol. 150, 183–195. doi: 10.1007/s00227-006-0349-6
Miyasaka, H., Genkai-Kato, M., Goda, Y., and Omori, K. (2007). Length-weight relationships of two varunid crab species, Helice tridens and Chasmagnathus convexus, in Japan. Limnology 8, 81–83. doi: 10.1007/s10201-006-0195-8
Mura, M., Orrù, F., and Cau, A. (2005). Size at sexual maturity of the spider crab Anamathia rissoana (Decapoda, Majoidea) from the Sardinian Sea. J. Crust. Biol. 25, 110–115. doi: 10.1651/c-2520
Murugan, T. S., Remany, M. C., Leema, T. M., Kumar, J. H. A. D., Santhanakumar, J., Vijayakumaran, M., et al. (2005). Growth, repetitive breeding, and aquaculture potential of the spiny lobster, Panulirus ornatus. New Zeal. J. Mar. Freshw. Res. 39, 311–315. doi: 10.1080/00288330.2005.9517311
Orensanz, J. M., Ernst, B., and Armstrong, D. A. (2007). Variation of female size and stage at maturity in snow crab (Chionoecetes opilio) (Brachyura: Majidae) from the eastern Bering Sea. J. Crust. Biol. 27, 576–591. doi: 10.1651/s-2790.1
Pacheco, C., Cusba, J., Paramo, J., Queirolo, D., and Pérez, D. (2021). Spatial structure and morphometric relationships of the deep-sea shrimp Solenocera acuminata (Decapoda, Solenoceridae) in the Colombian Caribbean. ZooKeys 1040, 1–24. doi: 10.3897/zookeys.1040.61005
Parvizi, E., Naderloo, R., Keikhosravi, A., and Schubart, C. D. (2017). Life history traits and patterns of sexual dimorphism in the freshwater crab Potamon ibericum (Bieberstein, 1809) (Decapoda: Brachyura: Potamidae) from the western Alborz Mountains, Iran. J. Crust. Biol. 37, 323–331. doi: 10.1093/jcbiol/rux029
Pollock, D. E. (1995). Notes on phenotypic and genotypic variability in lobsters. Crustaceana 68, 193–202. doi: 10.1163/156854095x00098
Robertson, D. N., and Butler, M. J. I. V. (2003). Growth and size at maturity in the spotted spiny lobster, Panulirus guttatus. J. Crust. Biol. 23, 265–272. doi: 10.1651/0278-0372(2003)023[0265:gasami]2.0.co;2
Senevirathna, J. D. M., Thushari, G. G. N., and Munasinghe, D. H. N. (2014). Length-weight relationship of spiny lobster, Panulirus homarus population inhabiting southern coastal region of Sri Lanka. Internat. J. Sci. Eviron. 3, 607–614.
Skewes, T., Pitcher, C. R., and Trendall, J. (1994). Changes in the size structure, sex ratio and molting activity of a population of ornate rock lobsters, Panulirus ornatus, caused by an annual maturation molt and migration. Bull. Mar. Sci. 54, 38–48.
Smith, K. N., and Herrkind, W. F. (1992). Predation on early juvenile spiny lobsters Panulirus argus (Latreille): influence of size and shelter. J. Exp. Mar. Biol. Ecol. 157, 3–18. doi: 10.1016/0022-0981(92)90070-q
Steinback, S. R., Allen, R. B., and Thunberg, E. (2008). The benefits of rationalization: the case of the American Lobster fishery. Mar. Resource Econ. 23, 37–63. doi: 10.1086/mre.23.1.42629601
Tewfik, A. (2014). The influence of waves on landing patterns within a diverse Sumatran spiny lobster (Panulirus spp.) fishery. New Zeal. J. Mar. Freshw. Res. 48, 245–255. doi: 10.1080/00288330.2014.895391
Thangaraja, R., and Radhakrishnan, E. V. (2017). Reproductive biology and size at onset of sexual maturity of the spiny lobster Panulirus homarus homarus (Linnaeus, 1758) in Khadiyapatnam, southwest coast of India. J. Mar. Biol. Associat. India, 59, 19–28. doi: 10.6024/jmbai.2017.59.2.1938-03
Wahyudin, R. A., Wardiatno, Y., Boer, M., Farajallah, A., and Hakim, A. A. (2017). A new distribution record of the mud-spiny lobster, Panulirus polyphagus (Herbst, 1793) (Crustacea, Achelata, Palinuridae) in Mayalibit Bay, West Papua, Indonesia. Biodiversitas 18, 780–783. doi: 10.13057/biodiv/d180248
Waiho, K., Fazhan, H., Baylon, J. C., Madihah, H., Noorbaiduri, S., Ma, H., et al. (2017). On types of sexual maturity in brachyurans, with special reference to size at the onset of sexual maturity. J. Shellfish Res. 36, 807–839. doi: 10.2983/035.036.0330
Waiho, K., Fazhan, H., and Ikhwanuddin, M. (2016). Size distribution, length-weight relationship and size at the onset of sexual maturity of the orange mud crab, Scylla olivacea, in Malaysian waters. Mar. Biol. Res. 12, 726–738. doi: 10.1080/17451000.2016.1200726
Keywords: mud spiny lobster, Panulirus, length-weight relationship, discriminant functions, Johor Strait
Citation: Waiho K, Fazhan H, Shu-Chien AC, Abualreesh MH, Ma H, Syahnon M, Azmie G, Razman NJ and Ikhwanuddin M (2021) Size Distribution, Length-Weight Relationship, and Size at Morphometric Maturity of the Mud Spiny Lobster Panulirus polyphagus (Herbst, 1793) in the Johor Strait. Front. Mar. Sci. 8:766038. doi: 10.3389/fmars.2021.766038
Received: 28 August 2021; Accepted: 22 October 2021;
Published: 29 November 2021.
Edited by:
Huang Wei, Ministry of Natural Resources, ChinaReviewed by:
Julia Ramos Miranda, Universidad Autónoma de Campeche, MexicoCopyright © 2021 Waiho, Fazhan, Shu-Chien, Abualreesh, Ma, Syahnon, Azmie, Razman and Ikhwanuddin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hanafiah Fazhan, ZmF6aGFuQHVtdC5lZHUubXk=; Mhd Ikhwanuddin, aWtod2FudWRkaW5AdW10LmVkdS5teQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.