
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 26 November 2021
Sec. Marine Pollution
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.765256
This article is part of the Research Topic Challenges in Marine Pollution Diagnosis View all 6 articles
 Giuseppe d'Errico1†
Giuseppe d'Errico1† Alessandro Nardi1†
Alessandro Nardi1† Maura Benedetti1
Maura Benedetti1 Marica Mezzelani1
Marica Mezzelani1 Daniele Fattorini1
Daniele Fattorini1 Marta Di Carlo1
Marta Di Carlo1 Lucia Pittura1
Lucia Pittura1 Maria Elisa Giuliani1
Maria Elisa Giuliani1 Simona Macchia2
Simona Macchia2 Valentina Vitiello2
Valentina Vitiello2 Davide Sartori2
Davide Sartori2 Alice Scuderi2
Alice Scuderi2 Lorenzo Morroni2
Lorenzo Morroni2 Gianluca Chiaretti2
Gianluca Chiaretti2 Stefania Gorbi1
Stefania Gorbi1 David Pellegrini2
David Pellegrini2 Francesco Regoli1,3*
Francesco Regoli1,3*The use of multidisciplinary investigations for the evaluation of aquatic ecosystems status is recommended by the European Directives, but it is still a challenging practice. In this study, we apply a quantitative weight of evidence (WOE) approach (Sediqualsoft) for the integration of extensive data obtained from different typologies of investigations, obtained over a 4-year monitoring study of dredging activities in the harbor of Leghorn (Italy). During different phases of such operations, selected sites have been characterized in terms of levels of trace metals and polycyclic aromatic hydrocarbons in sediments, bioaccumulation of contaminants, and a wide battery of biomarkers in transplanted mussels, ecotoxicological effects of sediments through a battery of bioassays (algal growth inhibition, bioluminescence inhibition, and embryotoxicity tests), and the status of benthic communities. Each typology of data, line of evidence (LOE), has been initially elaborated through dedicated logical flowcharts and algorithms providing specific hazard indices, followed by their overall integration based on different weights assigned to each LOE. This approach allowed to summarize more than 10,000 results, reaching robust conclusions on environmental impact during various phases of dredging operations. This approach was confirmed as a useful tool for monitoring the risk, supporting a “site-oriented” decision making process by providing stakeholders simple interpretation of complex data.
Sources of anthropogenic stress have dramatically increased over the past decades, especially in terms of inputs and typologies of chemicals that reach the marine environment through a variety of pathways, i.e., accidental discharge, riverine effluents, urban sewers, and atmospheric transport (European Environment Agency, 2019). Marine organisms cope with complex mixtures of organic and inorganic pollutants, which can produce biological impacts, ranging from cellular and physiological processes up to populations dynamics and ecosystem functioning (Regoli et al., 2019). This is particularly evident in coastal ecosystems, where anthropogenic activities and socio-economic opportunities must be harmonized with environmental protection issues aiming at a sustainable ocean economy (Winther et al., 2020).
Harbor areas are of elevated strategic and economic importance but often subjected to accumulation of relevant loads of contaminants in sediments due to the limited hydrodynamic and water renewal conditions (Renzi et al., 2009; Bebianno et al., 2015; Luna et al., 2019). Dredging activities are thus periodically required to maintain proper depths, posing serious environmental concerns depending on the quantity and quality of removed sediments. In addition to possible resuspension and bioavailability of contaminants during the operations, the more appropriate management options and the destination of dredged material need to be addressed (DelValls et al., 2004; Eggleton and Thomas, 2004).
In such a complex scenario, monitoring plans are accurately designed and developed aiming to assess the impact of dredging and disposal activities. The concept of monitoring has evolved from the original approach focused on the assessment of chemicals in abiotic matrices toward a more integrated procedure which includes the evaluation of effects on biota at various levels of biological organization (Linkov et al., 2009; Bruce et al., 2020; Fonseca et al., 2021). The use of multidisciplinary integrative strategies based on both chemical and biological approaches is recommended by several international agencies, such as OSPAR, HELCOM, MEDPOL, and ICES, and strongly encouraged by European Directives such as the Marine Strategy Framework Directive (MSFD, Directive 2008/56/EC) and the Water Framework Directive (WFD, Directive 2000/60/EC). The first example of integrated characterization of sediment quality was the TRIAD approach (Chapman, 2007), based on the analysis of sediment chemistry, ecotoxicological bioassays, and benthic communities elaborated through a weight of evidence (WOE) procedure, assigning a different weight to various lines of evidence (LOEs). The relevant advantage of such multidisciplinary characterization is derived from the improved ability to interpret and describe alterations of environmental conditions (Regoli et al., 2019). Although the quantification of chemicals in abiotic matrices is a key-procedure, by itself it does not provide information on bioavailability and biological effects of detected pollutants (Fent, 2004; Benedetti et al., 2012). Batteries of ecotoxicological bioassays on sediments allow to quantify acute biological effects on taxa of different trophic positions and life-stages exposed to mixtures of chemicals acting in synergy (Morroni et al., 2020; Broccoli et al., 2021). The third LOE of the original TRIAD provided information on long-term, ecologically relevant effects of polluted sediments; investigations on benthic communities composition have been gradually revised with the introduction of new quantitative ecological quality status descriptors (WFD 2000/60/EC). The last Italian normative on sediments quality classification (DM, 173/2016) has introduced the legal requirement to integrate chemical and ecotoxicological characterization of these materials for defining the allowed management option. An innovative aspect of this regulation is the adoption of weighted criteria to elaborate analytical results, thus abandoning the pass-to-fail approach based on comparison of individual threshold values for chemicals or the worst result for ecotoxicological hazard. Results are now elaborated on the basis of number, typology, and magnitude of contaminants exceeding specific thresholds, and also on the overall variations of ecotoxicological responses weighting the different sensitivity of tested species, relevance of biological endpoints, and assay conditions. Environmental monitoring and management of dredged sediments can be further enhanced by the introduction of additional LOEs, which have been recently applied to various environmental scenarios improving the robustness of WOE approach and allowing a more accurate discernment of the links between chemicals in the environment and their effects on biota. Bioaccumulation of chemicals in wild or transplanted organisms provides information on bioavailability, whereas measuring sublethal biological effects allows a sensitive early-warning sight at the molecular, cellular, and functional level, highlighting alterations and mechanisms of action prognostic for the onset of adverse effects at higher levels of biological organization (Broeg and Lehtonen, 2006; Moore et al., 2006; Regoli and Giuliani, 2014; Benedetti et al., 2015). Such integration of chemicals in abiotic matrices, their bioavailability, biomarkers, bioassays, and benthic communities have been developed in recent years through the quantitative WOE model Sediqualsoft, applied in various environmental risk assessment case-studies (Benedetti et al., 2014; Bebianno et al., 2015; Mestre et al., 2017; Lehtonen et al., 2019; Regoli et al., 2019; Morroni et al., 2020; Manfra et al., 2021). Different LOEs are independently elaborated through specific and weighted criteria, which provide both quantitative and qualitative hazard indices before their final integration in a WOE assessment (Piva et al., 2011; Regoli et al., 2019).
The use of standardized procedures is crucial for integrating data especially in complex monitoring scenarios based on multidisciplinary investigations. The elaboration must guarantee scientific relevance and robustness, allowing at the same time an easy communication of environmental risks to policy-makers and non-expert stakeholders (Linkov et al., 2009). In this context, the present study aimed to demonstrate the applicability of WOE approach as a practical tool in the environmental impact assessment of dredging activities. Using the harbor of Leghorn as a model case study, a multidisciplinary program was conducted across different areas and years, integrating chemical characterization of sediments, bioaccumulation and biological effects in transplanted mussels, sediments toxicity testing, and benthic communities composition. The WOE elaboration of such an extensive dataset was aimed to demonstrate the feasibility of multidisciplinary approaches and weighted criteria in the management of dredging activities, combining scientific soundness with easy interpretation of results, and choice of appropriate destination options by stakeholders.
The study followed the main phases of the dredging activities and construction of the new confined disposal facility (CDF) for sediments of the harbor of Leghorn. Samplings carried out in summer 2012 correspond to the ante-operam phase, whereas those of summer 2013 reflect the maximum intensity of dredging activities (completed in 2015) and construction of the new CDF; in summer 2015 and 2017, the monitoring plan was included in the management of the CDF and aimed to assess the possible impact of disposed sediments. Overall, five areas were selected as representative of geographical sectors of the harbor differently influenced by the dredging and disposal activities (Figure 1): CTL, supposed to be not impacted by the harbor activities and used as control (site VN4); NB near the new CDF (sites VN2 and VN3); OB near an old CDF (sites VE4 and VE5); IH in the inner harbor, and focus of the dredging of sediments that were to be confined in CDF (sites VE7, VE8, and DP) and OH outside the harbor and influenced by marine traffic (sites VN1 and VE1). Coordinates of different sites are given in Supplementary Materials (SM1). During each sampling, sediments samples were collected for chemical characterization, toxicity testing, and benthic communities evaluation. Mussels transplants were also performed for chemicals bioaccumulation and biomarkers responses: Mediterranean mussels (Mytilus galloprovincialis, 6.0 ± 0.5 cm shell length) was obtained from a shellfish farm in Lerici (La Spezia), immediately transported to the harbor of Leghorn, deployed in net bags (80 cm height × 25 cm diameter, mesh size 1.5 × 3 cm), at 5 m depth, and recovered after 4–6 weeks.
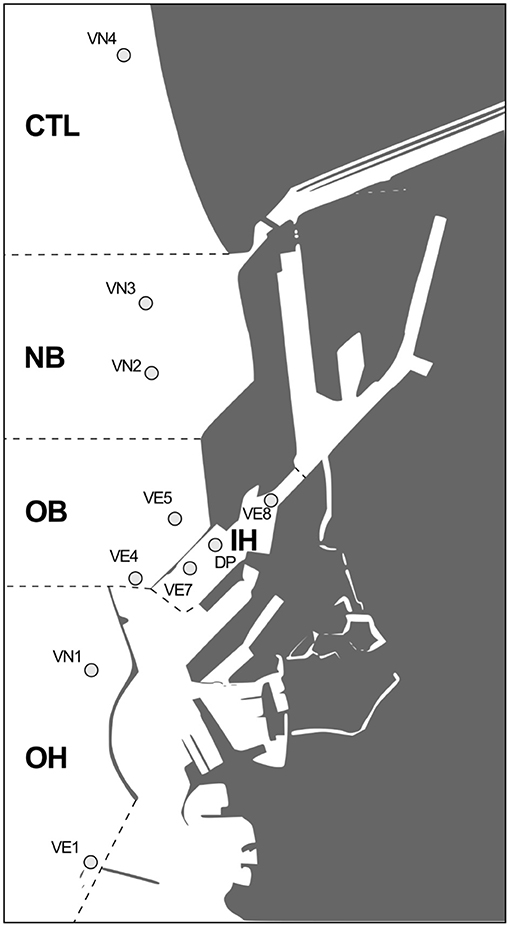
Figure 1. Study area, with detail of each investigated section within the area and sites within. CTL, control area, VN4 site; NB, new CDF area, VN2 and VN3 sites; OB, old CDF area, VE4 and VE5 sites; IH, inner harbor area, source of dredged materials, VE7, VE8 and DP sites; OH, outer harbor area, VN1 and VE1 sites.
At the end of each transplant, five pools, each constituting of whole soft tissues of 10 organisms, were prepared and stored at −20°C for bioaccumulation analyses. In addition, 30 organisms were dissected from each site, digestive glands excised, and haemolymph withdrawn from the adductor muscle, pooled in 10 samples per tissue (each with three specimens tissues), frozen in liquid nitrogen, and stored at-80°C until the analyses of biomarker; aliquots of haemolymph were further collected from 15 specimens, pooled in five samples, and immediately used for immune parameters and genotoxicity; for histological analyses, the digestive glands were rapidly excised from five mussels, placed on cork chucks, frozen in n-hexane precooled to −70°C in liquid nitrogen, and maintained at −80°C.
Analyses of the trace metals, such as arsenic (As), cadmium (Cd), chromium (Cr), copper (Cu), lead (Pb), mercury (Hg), nickel (Ni), and zinc (Zn) and polycyclic aromatic hydrocarbons (PAHs) in sediments and organisms were carried out with standardized procedures (Regoli et al., 2019) through atomic absorption spectrophotometry and high-performance liquid chromatography with diode array and fluorimetric detection. All the analytical determinations were performed by analyzing five replicates, carefully checking for accuracy, precision, and recovery by testing a series of blank solutions (reagents only), reference standards, and selected certified standard materials. Detailed procedures have been described in Supplementary Material 1.
Validated protocols, detailed in Supplementary Material 1, were applied for the analysis of the following biochemical, cellular, and histological biomarkers in mussels tissues. Metallothioneins levels (MT), activities of acyl-CoA-oxidase (AOX), total glutathione levels (TGSH), activities of catalase (CAT), glutathione S-transferase (GST), glutathione reductase (GR), glutathione peroxidases (Se-dependent and total forms, Se-dep. GPx and total GPx, respectively), total oxyradical scavenging capacity (TOSC) toward peroxyl and hydroxyl radicals (ROO• and HO•, respectively), malondialdehyde levels (MDA), lipofuscin, and neutral lipids were measured in mussels digestive gland; lysosomal membrane stability (LMS), acetylcholinesterase activity (AChE) and the onset of genotoxic damage in terms of DNA fragmentation and micronuclei frequency (MN) were measured in mussels hemocytes. Due to the limited availability of samples, biomarker analyses could not be performed on all the sites within various areas.
The battery of ecotoxicological bioassays used to test sediment toxicity included the bioluminescence inhibition in the bacterium Aliivibrio fischeri (ISO, 2019, solid phase), the algal growth inhibition of Phaeodactylum tricornutum (ISO, 2016), and the embryotoxicity of sea urchin Paracentrotus lividus (ISPRA, 2007). Preparation of samples, elutriates test conditions, and standardized procedures for bioassays have been detailed elsewhere (Regoli et al., 2019; Morroni et al., 2020).
Sieved sediment samples were sorted at the stereomicroscope, and the principal animal taxa were generally classified at the species level. For each species, whenever possible, the corresponding biocenosis was identified. All taxa were identified and classified to the lowest taxonomic level possible. In this study, AZTI' Marine Biotic Index (AMBI), which is based on the relative proportion of taxa classified into five ecological groups depending on their tolerance to perturbation, was used as an ecological status indicator (Borja et al., 2000).
Results were elaborated within the Sediqualsoft model (Regoli et al., 2014, 2019). Logical flow charts (LOEs) based on expert judgment and legislative constraints provide specific hazard indices for each typology of data, including sediment chemistry (LOE-1), bioavailability of chemicals (LOE-2) and biomarkers (LOE-3) in transplanted mussels, ecotoxicological bioassays (LOE-4), and benthic communities in sediments (LOE-5); elaboration of individual LOEs are therefore integrated into the final WOE assessment. Scientific criteria, validation of weights and thresholds, and specific flow-charts for each LOE have been validated elsewhere (Piva et al., 2011; Benedetti et al., 2012, 2014; Regoli et al., 2019) and summarized below.
The evaluation of chemical hazard (LOE-1) is initially based on the calculation for each pollutant of the ratio to reference (RTR), i.e., the ratio between concentration measured in sediments and those indicated by a sediment quality guideline (SQG); in the present investigation, threshold limits were those indicated by the SQG-L2 of the Italian decree (DM, 173/2016) for determining quality class and management options for dredged marine sediments. The RTR is corrected by a factor (w) which depends on the typology of chemicals (i.e., nonpriority w = 1, priority w = 1.1, priority and hazardous pollutants w = 1.3). In the calculation of the specific hazard quotient (HQC), an average RTRw is obtained for all of the parameters with RTR ≤ 1 (i.e., values below the SQG), while for those with RTR >1, the RTRw are individually added into the summation Σ:
The values of HQC are assigned to one of the six classes of chemical hazard, namely absent, negligible, slight, moderate, major, and severe depending on the number, typology, and magnitude of chemicals exceeding the thresholds (Regoli et al., 2019).
The results on bioaccumulation of chemicals in tissues of mussels (LOE-2) are elaborated calculating, for each parameter, the increase of concentration compared to organisms transplanted at the control site (CTL), corrected for the typology of pollutant, and the statistical significance of the difference. The cumulative bioavailability hazard (HQBA) does not consider parameters with RTRw <1.3, calculates the average for those with RTRw ranging between 1.3 and 2.6, and adds the summation of all those with RTRw ≥ 2.6:
The HQBA is assigned to one of the five classes of hazard for bioavailability, from absent to severe (Regoli et al., 2019).
The module for the elaboration of biomarkers (LOE-3) contains a wide battery of responses, each assigned with a weight (depending on the relevance of biological endpoint) and a threshold indicative of changes of biological relevance (both induction and inhibition), which consider the possibility of biphasic responses. For each biomarker, the measured variation is compared with organisms transplanted in the control site (CTL), corrected for statistical significance and importance of biomarker (weight), and assigned to one of the–five classes of effect, which are then differently weighted in the calculation of cumulative HQBM.
According to the percentage distribution of biomarkers in the five classes of effect, the level of cumulative HQBM is assigned to one of the five classes of hazard; all the relevant information are given in the model output (Regoli et al., 2019).
Weighted criteria to elaborate results from sediment ecotoxicological bioassays (LOE-4) are based on specific thresholds and weights assigned to each bioassay depending on the biological endpoint, tested matrix, time of exposure, and the possibility of hormetic responses (Regoli et al., 2019).
The cumulative hazard quotient (HQBattery) is obtained by the summation (Σ) of the weighted effects (Ew), i.e., the variations measured for each test compared with specific thresholds, corrected for the statistical significance of the difference (w), and biological importance of the endpoint and exposure conditions (w2):
The HQBattery is normalized to a scale ranging from 0 to 10, where 1 is the battery threshold (when all the measured bioassays exhibit an effect equal to the threshold, 10 when all the assays exhibit 100% of effect). The HQBattery is then assigned to one of the five classes of hazard, from absent to severe (Regoli et al., 2019).
The benthic communities module (LOE-5) elaborates the list of identified species in several available univariate and multivariate indices for the classification of ecological quality (Borja et al., 2000; Muxika et al., 2005, 2007). Among all the available indices, the AMBI index was chosen in this work for the integration with other LOEs in the final WOE elaboration of ecological risk.
The extensive datasets of results elaborated within individual LOEs are finally integrated through a WOE approach. The quantitative hazard quotients (HQs) obtained for each of the five LOEs have been normalized to a common scale and given a different weight according to the ecological relevance. In this study, weights given to each LOE were as follows 1.0 for chemical characterization of sediments (LOE-1), 1.2 for the bioavailability of chemicals in mussels, 1.0 for sublethal effects on biomarkers (LOE-3), 1.2 for the ecotoxicological results of the battery of bioassays (LOE-4), and 1.3 for the composition of benthic communities (LOE-5). An overall WOE level of risk is thus calculated and assigned to one of the 5 classes of risk from absent to severe (Piva et al., 2011).
Chemical analyses of PAHs and trace metals in sediment samples produced ~1,000 results, which are shown in Supplementary Tables 1–4. The weighted elaboration of results summarized a chemical hazard quotient (HQC) ranging from “Absent” to “Moderate” in various sites and periods (Table 1).
In the ante-operam campaign (2012), all investigated sites had a “Slight” chemical hazard, with the exception of VN2, VE7, VE8, DP, and VN1, where As was the main parameter causing a “Moderate” classification (Table 1; Supplementary Table 1).
In 2013, during the dredging activities and CDF construction, all the sites had a chemical hazard “Slight” (VN3, VE5, VE4 and VE1) or “Moderate” (VN2, VE7, VE8, DP), except for VN4 (CTL) and VN1 (OH) in which the hazard was “Negligible” and “Absent,” respectively. Pb was the exceeding parameter which contributed more (or exclusively) to HQC in all the investigated sediments (Table 1; Supplementary Table 2).
In the 2015 campaign, a net reduction of the chemical hazard was observed, resulting in “Absent” for all sites, except for VE7 and DP (“Slight”) and VN1 (“Negligible”). Parameters exceeding the thresholds were Ni in VE7 and DP, and As in VN1 (Table 1; Supplementary Table 3).
The chemical hazard reduction was even more evident in the 2017 campaign, when all investigated sites had an “Absent” classification, with no exceeding parameter (Table 1; Supplementary Table 4).
Bioavailability of chemicals in transplanted mussels produced ~3,000 results shown in Supplementary Tables 5–8. The weighted elaboration of these results provided a bioavailability hazard index that ranged from “Absent” to “Severe,” with area- and site-specificities highlighted across the different campaigns (Table 2).
In the 2012 campaign, the bioavailability hazard ranged from “Absent” in CTL area to “Moderate” in DP site (IH area), whereas all the other sites showed a “Sight” classification. The parameters determining the HQBA in DP site were benzo(b)fluoranthene, benzo(k)fluoranthene, and chrysene (Table 2; Supplementary Table 5).
In the 2013 campaign, the HQBA ranged from “Absent” in CTL area to “Major” or “Severe” in IH area, where mussels transplanted in VE7, VE8, and DP, exhibited a marked accumulation of high molecular weight PAHs congeners and of anthracene (Table 2; Supplementary Table 6). All the other areas and sites showed a “Slight” HQBA.
In the 2015 campaign, the three IH sites exhibited a “Major,” bioavailability hazard mostly related to the accumulation of high molecular weight PAHs congeners of anthracene and of acenaphthylene (Table 2; Supplementary Table 7). In the other sites, the HQBA ranged from “Absent” (CTL, NB, OH) to “Slight” (OB).
In the 2017 campaign, the bioavailability hazard was “Absent” for the CTL or “Slight” in all the areas with the exception of HI sites (VE7, VE8, and DP) that exhibited a HQBA “Major” or “Moderate” due to high molecular weight PAHs (Table 2; Supplementary Table 8).
Biomarkers analyzed included 17 recognized biological parameters, resulting in a total of more than 1,000 results to interpret. Variations of different parameters occurred in various areas during each campaign ( Supplementary Tables 9–12), and the overall significance of obtained results, elaborated through weighted criteria, has been summarized in Table 3. Overall, the hazard index for biomarkers ranged from “Absent” to “Slight,” in different years and areas, with only a “Moderate” classification in 2017 for organisms transplanted in the NB area near the new CDF.
The contribution of investigated parameters revealed a “Moderate” disturbance of LMS, lipid metabolism, and peroxidation in 2012, of LMS and antioxidants in 2013, of AOX activity, LMS, MN frequency and MDA in 2015, of NL, Se-dep. GPx, and GST activities, and AOX in 2017 (Table 3; Supplementary Tables 9–12).
Ecotoxicological characteristics of sediment samples, including results of three bioassays (A. fischeri, S. costatum, and P. lividus) are detailed in Supplementary Tables 13–16, while weighted elaboration of the whole battery is detailed in Table 4.
In 2012, the weighted elaboration revealed a “Moderate” toxicity for CTL, OB areas, and VN1 site (OH area) and “Major” in the inner harbor (IH). The HQBattery was classified as “Absent” for NB area and VE1 site, within OH (Table 4; Supplementary Table 13).
In 2013, the ecotoxicological classification was similar to 2012 (Table 4; Supplementary Table 14) with only a few changes for VN2 and VN3 (“Moderate” and “Slight”), VE5 and VN1 (“Absent”), and VE1 (“Moderate”).
In 2015, with the exception of the sites DP (IH) and VN1 (OH) that showed a “Major” ecotoxicological hazard level, lower HQBattery was obtained for all the other investigated sites, namely: “Absent” or "Slight” for CTL, NB and OB areas, “Moderate” for two sites within IH (VE7 and VE8), and within OH for VE1 (Table 4; Supplementary Table 15).
In the 2017 campaign, a “Moderate” ecotoxicological hazard was obtained in IH area and the site VN3 (NB), whereas all the other investigated sites were assigned to the class “Absent” (Table 4 and Supplementary Table 16).
The data for the benthic communities, available for CTL, NB, and OB areas, allowed to identify 125 taxonomic groups (Supplementary Table 17). The AMBI index, selected as the most appropriate for the investigated area, provided a hazard generally “Absent” for the CTL area, and “Slight” for the NB and OB areas (Table 5).
The overall elaboration, integrating the Hazard Quotients from each LOEs, summarized a total of about 10,000 analytical results in a WOE index, allowing to provide a synthetic classification for each area during the various campaigns from 2012 to 2017 (Figure 2; Table 6).
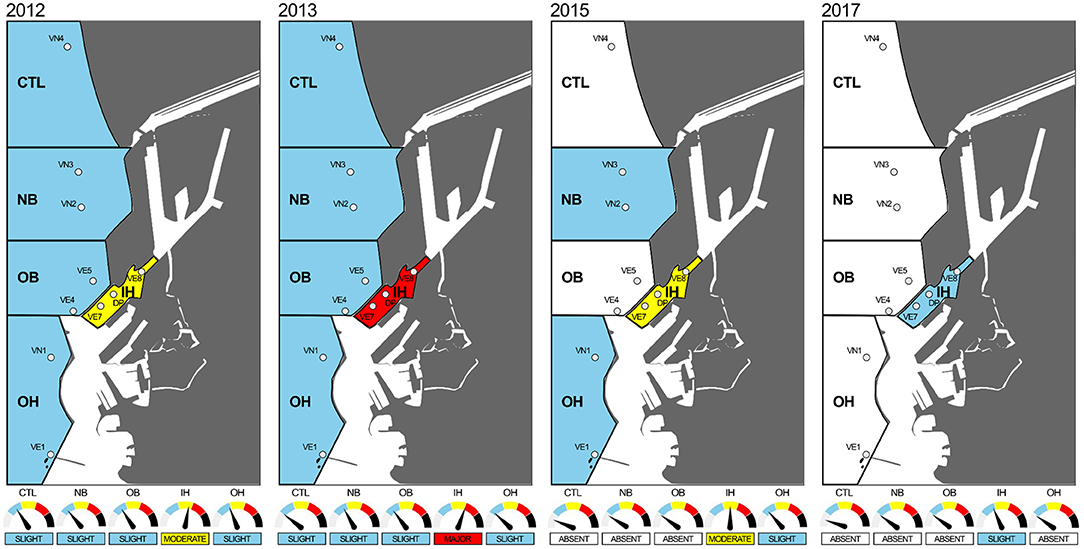
Figure 2. Graphical representation of weighted elaboration. For each area during each campaign, colors are used to highlight risk class as follow: absent (white), slight (blue), moderate (yellow), major (red), and severe (black).
In 2012, all the areas were assigned a “Slight” risk, apart from the inner harbor area IH (“Moderate”). In 2013, the inner harbor area showed a worsening with the WOE risk classified as “Major.” In 2015, the integrated risk decreased in all investigated areas, being “Absent” in CTL and OB, “Slight” in NB and OH, and “Moderate” in IH area. The last campaign in 2017 revealed a consistent reduction of the WOE risk, which was “Slight” in the inner harbor area and “Absent” in all the others.
The assessment of environmental impacts in complex systems requires multidisciplinary and holistic approaches, integrating diverse typologies of data ranging from chemical characterization to their effects at various levels of biological organization (Hylland, 2006; Vethaak et al., 2017; Martínez-Gómez and Vethaak, 2019; Gambardella et al., 2021). Among the different integrative tools nowadays available, the WOE approach has gained a relevant consensus, given the scientific reliability and transparency of the process and the “user-friendly” format to easily understand the meaning of numerous data from different typologies of investigations (Regoli et al., 2019).
The present study aimed to demonstrate the effectiveness of integrative methodologies on complex and long-term monitoring scenarios, applying such an approach during a multiannual (2012–2017) investigation of dredging activities and disposal of sediments in the Leghorn harbor. The proposed weighted, quantitative and scientifically-sound integrative procedure provided synthetic hazard indices from an extensive dataset, allowing to easily transfer the knowledge on complex environmental scenarios from environmental scientists to policymakers and nonexpert stakeholders (Piva et al., 2011; Regoli et al., 2019).
Management of harbor dredged materials has been often based on “pass-to-fail” methodology, where even a single parameter slightly above or below a threshold determines their classification. In this study, a detailed evaluation of the chemical quality of sediments is obtained by using synthetic hazard indices based on the number, typology, and magnitude of chemicals exceeding specific thresholds (Regoli et al., 2019). Such an integrated approach allows easier comparison between different conditions, including sites and/or sampling periods. In our study, the overall evaluation of chemicals in sediments allowed to compare across different phases of activities the synthetic elaborations produced by ~1,000 results. The chemical hazard in sediments (LOE-1) revealed a similar trend in the ante-operam phase (2012) and during the maximum intensity of dredging activities and new CDF construction (2013), with a “Moderate” class of hazard attributed to the IH area and VN2 site, influenced by the disposal of dredged materials. The chemical classification improved in the following years in the entire study area. The chemical hazard was mostly driven by trace metals with concentrations exceeding the limits indicated by the Italian legislation for harbor-dredged sediments (L2 values, DM, 173/2016) in 2012 (mainly As) and 2013 (mainly Pb); levels of PAHs were always lower than the thresholds and comparable with values of other harbor areas (Soclo et al., 2000; Sprovieri et al., 2007).
Chemical characterization of sediments was integrated with the assessment of bioavailability in transplanted mussels, which is recognized as fundamental to assess the transfer of hazardous compounds from abiotic matrices to organisms (Benedetti et al., 2012; Bebianno et al., 2015). Also for this LOE, the use of weighted criteria allows to overcome the intrinsic limits of the few EQS available for organisms, which do not take into account key issues like seasonal variability, species-specific characteristics, and local geochemical characteristics, to cite a few (Regoli et al., 2019). Despite that, in our study, the chemical hazard of harbor sediments was primarily related to trace metals, and the weighted elaboration of bioaccumulation analyses (LOE-2) highlighted an increased bioavailability of high molecular weight PAHs. Mussels transplanted in the inner harbor areas exhibited a hazard ranging from “Major” to “Severe” in 2013 and 2015 during dredging activities, which decreased in the 2017 campaign. The discrepancy between the chemicals influencing the HQ in sediments (LOE-1) and in organisms (LOE-2) highlights the complexity of bioavailability, confirming the need of integrated approaches for a more realistic assessment of pollutants associated with and released from sediments (Regoli and Orlando, 1994; Bocchetti et al., 2008a; Bebianno et al., 2015).
In such a context, investigation on sublethal alterations in organisms provides early warning signals of disturbance, revealing the onset of stressful conditions before these progress toward higher levels of biological organization (Regoli et al., 2004; Benedetti et al., 2012). Although the benefits of evaluating sublethal effects in transplanted mussels are now widely recognized, their application is still limited to research purposes and has not yet been included in the normative regulating monitoring programs due to the difficulties in summarizing the effects and communicating the biological significance of observations (Lehtonen et al., 2019). In this study, the elaboration of biomarkers evaluated in transplanted mussels during the different campaigns allowed to synthesize ~1,000 results obtained from 17 biological processes, revealing low hazard at cellular level arising from steady fluctuations of certain markers. Overall, the negligible transfer of trace metals from sediments to organisms was confirmed by the levels of metallothioneins, which were always comparable among areas and in line with those of organisms from reference and unpolluted areas (Bocchetti and Regoli, 2006; Bocchetti et al., 2008b). On the other hand, the accumulation of PAHs in mussels transplanted at IH area did not elicit harsh alterations but produced pin-point effects moderately modifying lysosomal membranes stability and lipid accumulation, metabolism, and peroxidation processes. In any case, the number and the magnitude of these alterations were higher in the early phases of the activities and decreased along their progression, suggesting improvement in the health condition of mussels and low hazard of sublethal effects.
The weighted elaboration of the battery of bioassays revealed a higher ecotoxicological hazard (classified as “Major”) in the inner area of the harbor in 2012 and 2013, generally decreasing to “Moderate” in the following years. Also in the other areas, the HQ from “Moderate” to “Absent” reflects the decline in hazard observed with time for all the LOEs. Among the three bioassays, the embryotoxicity of P. lividus was the most sensitive (Morroni et al., 2018; Broccoli et al., 2021), followed by the bioluminescence inhibition of A. fischeri.
The analyses of benthic communities composition were carried out close to the previous and the new CDF, revealing neglectable variations of the AMBI index; the elaboration of these results confirmed a limited disturbance of dredging and disposal activities for benthic biodiversity in these areas, increasing the ecological relevance of the investigation.
The hazard quotients provided by each of the five LOEs were ultimately integrated providing a comprehensive assessment of the environmental impact of dredging and disposal activities in different harbor areas. The synthetic risk index indicated a “Major” impact in the inner harbor area during the period of maximum intensity of dredging activities. The risk was progressively reduced with the proceeding of the activities reaching a “Slight” level in the last monitoring campaign. The other harbor areas, in particular, that in front of the new CDF, highlighted a limited risk associated with different phases of dredging and disposal activities, which did not elicit marked environmental impacts.
Besides the specific results of the presented case-study, the application of the WOE approach provided a straightforward risk index that allowed to compare the evolution of impacts across the different areas and phases of activities, and to easily communicate risk to policy makers and non-expert stakeholders. The multidisciplinary approach used herein and the elaboration through weighted criteria should therefore be considered a gold standard when facing complex scenarios in environmental impact assessments.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Gd'E: data elaboration and original draft writing. AN: data collection (biomarker analyses), data elaboration, and original draft writing. MB: conceptualization and design of the study. MM, MEG, and LP: data collection (biomarker analyses). DF and MDC: data collection (chemicals analyses of PAHs). SM: samplings coordination, data collection (chemical analyses of trace metals). VV, GC, and DS: data collection (ecotoxicological bioassays). AS: samplings coordination and data collection (chemical analyses of trace metals). LM: data collection (ecotoxicological bioassays, benthic communities composition). SG: conceptualization and design of the study, original draft writing, and final draft revision. DP: conceptualization and design of the study and project coordination. FR: conceptualization and design of the study, final draft revision, and project coordination. All authors contributed to manuscript revision, read, and approved the submitted version.
This work was founded by the North Tyrrhenian Sea Ports System Authority for the monitoring of dredging activities.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors wish to thank the colleague Dr. Paolo Tomassetti (CN-LAB, ISPRA) for his support on sample analyses.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.765256/full#supplementary-material
Bebianno, M. J., Pereira, C. G., Rey, F., Cravo, A., Duarte, D., D'Errico, G., et al. (2015). Integrated approach to assess ecosystem health in harbor areas. Sci. Total Environ. 514, 92–107. doi: 10.1016/j.scitotenv.2015.01.050
Benedetti, M., Ciaprini, F., Piva, F., Onorati, F., Fattorini, D., Notti, A., et al. (2012). A multidisciplinary weight of evidence approach for classifying polluted sediments: integrating sediment chemistry, bioavailability, biomarkers responses and bioassays. Environ. Int. 38, 17–28. doi: 10.1016/j.envint.2011.08.003
Benedetti, M., Giuliani, M. E., and Regoli, F. (2015). Oxidative metabolism of chemical pollutants in marine organisms: Molecular and biochemical biomarkers in environmental toxicology. Ann. N. Y. Acad. Sci. 1340, 8–19. doi: 10.1111/nyas.12698
Benedetti, M., Gorbi, S., Fattorini, D., D'Errico, G., Piva, F., Pacitti, D., et al. (2014). Environmental hazards from natural hydrocarbons seepage: Integrated classification of risk from sediment chemistry, bioavailability and biomarkers responses in sentinel species. Environ. Pollut. 185, 116–126. doi: 10.1016/j.envpol.2013.10.023
Bocchetti, R., Fattorini, D., Pisanelli, B., Macchia, S., Oliviero, L., Pilato, F., et al. (2008a). Contaminant accumulation and biomarker responses in caged mussels, Mytilus galloprovincialis, to evaluate bioavailability and toxicological effects of remobilized chemicals during dredging and disposal operations in harbour area. Aquatic Toxicol. 89, 257–266. doi: 10.1016/j.aquatox.2008.07.011
Bocchetti, R., Lamberti, C. V., Pisanelli, B., Razzetti, E. M., Maggi, C., Catalano, B., et al. (2008b). Seasonal variations of exposure biomarkers, oxidative stress responses and cell damage in the clams, Tapes philippinarum, and mussels, Mytilus galloprovincialis, from Adriatic sea. Mar. Environ. Res. 66, 24–26. doi: 10.1016/j.marenvres.2008.02.013
Bocchetti, R., and Regoli, F. (2006). Seasonal variability of oxidative biomarkers, lysosomal parameters, metallothioneins and peroxisomal enzymes in the Mediterranean mussel Mytilus galloprovincialis from Adriatic Sea. Chemosphere 65, 913–921. doi: 10.1016/j.chemosphere.2006.03.049
Borja, A., Franco, J., and Pérez, V. (2000). A marine Biotic Index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments. Mar. Pollut. Bull. 40, 1100–1114. doi: 10.1016/S0025-326X(00)00061-8
Broccoli, A., Morroni, L., Valentini, A., Vitiello, V., Renzi, M., Nuccio, C., et al. (2021). Comparison of different ecotoxicological batteries with WOE approach for the environmental quality evaluation of harbour sediments. Aquatic Toxicol. 237:105905. doi: 10.1016/j.aquatox.2021.105905
Broeg, K., and Lehtonen, K. K. (2006). Indices for the assessment of environmental pollution of the Baltic Sea coasts: integrated assessment of a multi-biomarker approach. Mar. Pollut. Bull. 53, 508–522. doi: 10.1016/j.marpolbul.2006.02.004
Bruce, P., Sobek, A., Ohlsson, Y., and Bradshaw, C. (2020). Risk assessments of contaminated sediments from the perspective of weight of evidence strategies–a Swedish case study. Hum. Ecol. Risk Assess. 27, 1366–1387. doi: 10.1080/10807039.2020.1848414
Chapman, P. M. (2007). Determining when contamination is pollution—weight of evidence determinations for sediments and effluents. Environ. Int. 33, 492–501. doi: 10.1016/j.envint.2006.09.001
DelValls, T. A., Andres, A., Belzunce, M. J., Buceta, J. L., Casado-Martinez, M. C., Castro, R., et al. (2004). Chemical and ecotoxicological guidelines for managing disposal of dredged material. TrAC—Trends Anal. Chemistr. 23, 819–828. doi: 10.1016/j.trac.2004.07.014
DM (173/2016). Ministero dell'Ambiente e della Tutela del Territorio e del Mare Supplemento ordinario alla Gazzetta Ufficiale, n. 208 del 6 settembre 2016-Serie generale. Regolamento recante modalità e criteri tecnici per l'autorizzazione all'immersione in mare dei materiali di escavo di fondali marini.
Eggleton, J., and Thomas, K. V. (2004). A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events. Environ. Int. 30, pp. 973–980. doi: 10.1016/j.envint.2004.03.001
European Environment Agency. (2019). The European environment—state and outlook 2020. Knowl. Transit. Sustain. Europe. 90:9. doi: 10.2800/96749
Fent, K. (2004). Ecotoxicological effects at contaminated sites. Toxicology 205, pp. 223–240. doi: 10.1016/j.tox.2004.06.060
Fonseca, M. F., Ferreira, F. C., Choueri, R. B., and Fonseca, G. (2021). M-Triad: An improvement of the sediment quality triad. Sci. Total Environ. 770:145245. doi: 10.1016/j.scitotenv.2021.145245
Gambardella, C., Leggio, O., Montarsolo, A., Harriague, A. C., Del Core, M., Faimali, M., et al. (2021). An integrated approach to characterize deep sediment toxicity in Genoa submarine canyons (NW Mediterranean). Environ. Sci. Pollut. Res. 15:807. doi: 10.1007/s11356-021-15807-0
Hylland, K. (2006). Biological effects in the management of chemicals in the marine environment. Mar. Pollut. Bull. 53, 614–619. doi: 10.1016/j.marpolbul.2006.08.010
ISO (2016). Water quality—Marine algal growth inhibition test with Skeletonema sp. and Phaeodactylum tricornutum. 10253:2016.
ISO (2019). Water quality–Determination of the inhibitory effect of water samples on the light emission of Vibrio fischeri (Luminescent bacteria test). 11348–11353.
ISPRA (2007). Saggio di fecondazione e saggio di sviluppo embrionale con il riccio di mare Paracentrotus lividus (Lamarck) (Echinodermata: Echinoidea). ISPRA:Rome.
Lehtonen, K. K., d'Errico, G., Korpinen, S., Regoli, F., Ahkola, H., Kinnunen, T., et al. (2019). Mussel Caging and the Weight of Evidence Approach in the Assessment of Chemical Contamination in Coastal Waters of Finland (Baltic Sea). Front. Marine Sci. 6:688. doi: 10.3389/fmars.2019.00688
Linkov, I., Loney, D., Cormier, S., Satterstrom, F. K., and Bridges, T. (2009). Weight-of-evidence evaluation in environmental assessment: Review of qualitative and quantitative approaches. Sci. Total Environ. 407, 5199–5205. doi: 10.1016/j.scitotenv.2009.05.004
Luna, G. M., Manini, E., Turk, V., Tinta, T., D'Errico, G., Baldrighi, E., et al. (2019). Status of faecal pollution in ports: a basin-wide investigation in the Adriatic Sea. Mar. Pollut. Bull. 147, 219–228. doi: 10.1016/j.marpolbul.2018.03.050
Manfra, L., Maggi, C., D'Errico, G., Rotini, A., Catalano, B., Maltese, S., et al. (2021). A weight of evidence (Woe) approach to assess environmental hazard of marine sediments from adriatic offshore platform area. Water 13:1691. doi: 10.3390/w13121691
Martínez-Gómez, C., and Vethaak, A. D. (2019). Understanding the impact of chemicals on marine fish populations: the need for an integrative approach involving population and disease ecology. Curr. Opin. Environ. Sci. Health 11, 71–77. doi: 10.1016/j.coesh.2019.08.001
Mestre, N. C., Rocha, T. L., Canals, M., Cardoso, C., Danovaro, R., Dell'Anno, A., et al. (2017). Environmental hazard assessment of a marine mine tailings deposit site and potential implications for deep-sea mining. Environ. Pollut. 228, 169–178. doi: 10.1016/j.envpol.2017.05.027
Moore, M. N., Icarus Allen, J., and McVeigh, A. (2006). Environmental prognostics: An integrated model supporting lysosomal stress responses as predictive biomarkers of animal health status. Mar. Environ. Res. 61, 278–304. doi: 10.1016/j.marenvres.2005.10.005
Morroni, L., d'Errico, G., Sacchi, M., Molisso, F., Armiento, G., Chiavarini, S., et al. (2020). Integrated characterization and risk management of marine sediments: the case study of the industrialized Bagnoli area (Naples, Italy). Marine Environ. Res. 160:104984. doi: 10.1016/j.marenvres.2020.104984
Morroni, L., Giuliani, S., Pellegrini, D., and Sartori, D. (2018). In situ embryo toxicity test with sea urchin: development of exposure chamber for test execution. Chemosphere 196, pp. 354–360. doi: 10.1016/j.chemosphere.2017.12.174
Muxika, I., Borja, A., and Bald, J. (2007). Using historical data, expert judgement and multivariate analysis in assessing reference conditions and benthic ecological status, according to the European Water Framework Directive. Mar. Pollut. Bull. 55, 16–29. doi: 10.1016/j.marpolbul.2006.05.025
Muxika, I., Borja, Á., and Bonne, W. (2005). The suitability of the marine biotic index (AMBI) to new impact sources along European coasts. Ecol. Indicat. 5, 19–31. doi: 10.1016/j.ecolind.2004.08.004
Piva, F., Ciaprini, F., Onorati, F., Benedetti, M., Fattorini, D., Ausili, A., et al. (2011). Assessing sediment hazard through a weight of evidence approach with bioindicator organisms: A practical model to elaborate data from sediment chemistry, bioavailability, biomarkers and ecotoxicological bioassays. Chemosphere 83, 475–485. doi: 10.1016/j.chemosphere.2010.12.064
Regoli, F., d'Errico, G., Nardi, A., Mezzelani, M., Fattorini, D., Benedetti, M., et al. (2019). Application of a weight of evidence approach for monitoring complex environmental scenarios: The case-study of off-shore platforms. Front. Marine Sci. 6:377. doi: 10.3389/fmars.2019.00377
Regoli, F., Frenzilli, G., Bocchetti, R., Annarumma, F., Scarcelli, V., Fattorini, D., et al. (2004). Time-course variations of oxyradical metabolism, DNA integrity and lysosomal stability in mussels, Mytilus galloprovincialis, during a field translocation experiment. Aquatic Toxicol. 68, 167–178. doi: 10.1016/j.aquatox.2004.03.011
Regoli, F., and Giuliani, M. E. (2014). Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 93, 106–117. doi: 10.1016/j.marenvres.2013.07.006
Regoli, F., and Orlando, E. (1994). Seasonal variation of trace metal concentrations in the digestive gland of the Mediterranean mussel Mytilus galloprovincialis: Comparison between a polluted and a non-polluted site. Arch. Environ. Contam. Toxicol. 27, 36–43. doi: 10.1007/BF00203885
Regoli, F., Pellegrini, D., Cicero, A. M., Nigro, M., Benedetti, M., Gorbi, S., et al. (2014). A multidisciplinary weight of evidence approach for environmental risk assessment at the costa concordia wreck: integrative indices from mussel watch. Mar. Environ. Res. 96, 92–104. doi: 10.1016/j.marenvres.2013.09.016
Renzi, M., Perra, G., Guerranti, C., Mariottini, M., Baroni, D., Volterrani, M., et al. (2009). Assessment of environmental pollutants in ten southern Italy harbor sediments. Toxicol. Ind. Health 25, 351–363. doi: 10.1177/0748233709104868
Soclo, H. H., Garrigues, P., and Ewald, M. (2000). Origin of polycyclic aromatic hydrocarbons (PAHs) in coastal marine sediments: Case studies in Cotonou (Benin) and Aquitaine (France) Areas. Mar. Pollut. Bull. 40, 387–396. doi: 10.1016/S0025-326X(99)00200-3
Sprovieri, M., Feo, M. L., Prevedello, L., Manta, D. S., Sammartino, S., Tamburrino, S., et al. (2007). Heavy metals, polycyclic aromatic hydrocarbons and polychlorinated biphenyls in surface sediments of the Naples harbour (southern Italy). Chemosphere 67, 998–1009. doi: 10.1016/j.chemosphere.2006.10.055
Vethaak, A. D., Davies, I. M., Thain, J. E., Gubbins, M. J., Martínez-Gómez, C., Robinson, C. D., et al. (2017). Integrated indicator framework and methodology for monitoring and assessment of hazardous substances and their effects in the marine environment. Mar. Environ. Res. 124, 11–20. doi: 10.1016/j.marenvres.2015.09.010
Keywords: dredging activities, risk assessment, WOE integration, multidisciplinary approaches, harbor areas, ecotoxicology
Citation: d'Errico G, Nardi A, Benedetti M, Mezzelani M, Fattorini D, Di Carlo M, Pittura L, Giuliani ME, Macchia S, Vitiello V, Sartori D, Scuderi A, Morroni L, Chiaretti G, Gorbi S, Pellegrini D and Regoli F (2021) Application of a Multidisciplinary Weight of Evidence Approach as a Tool for Monitoring the Ecological Risk of Dredging Activities. Front. Mar. Sci. 8:765256. doi: 10.3389/fmars.2021.765256
Received: 26 August 2021; Accepted: 18 October 2021;
Published: 26 November 2021.
Edited by:
Mónica Noemí Gil, CONICET Centro de Estudios de Sistemas Marinos (CESIMAR), ArgentinaReviewed by:
Kari Lehtonen, Finnish Environment Institute (SYKE), FinlandCopyright © 2021 d'Errico, Nardi, Benedetti, Mezzelani, Fattorini, Di Carlo, Pittura, Giuliani, Macchia, Vitiello, Sartori, Scuderi, Morroni, Chiaretti, Gorbi, Pellegrini and Regoli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Regoli, Zi5yZWdvbGlAdW5pdnBtLml0
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.