
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 03 November 2021
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.764042
This article is part of the Research TopicNew Progresses of Fish Meal Replacement in Aquatic AnimalsView all 16 articles
The efficacy of a single cell protein (SCP) methanotroph (Methylococcus capsulatus, Bath) bacteria meal (FeedKind®, Calysta, Menlo Park, CA, United States), in Pacific white shrimp (Penaeus vannamei) diets was studied to determine growth performance, survival rate and disease resistance against Vibrio parahaemolyticus causing Acute Hepatopancreatic Necrosis Disease (AHPND). The growth trial was assigned in a completely randomized design (CRD) with four treatments and 5 replicates of each, T1: a fishmeal-based control containing 15% fish meal and 3 diets with graded levels of methanotroph bacteria meal, namely T2: 5% methanotroph bacteria meal, T3: 10% methanotroph bacteria meal, and T4: 15% methanotroph bacteria meal. Shrimp were fed ad libitum for 6 weeks on trial diets to assess growth. Subsequent to the growth trial, three replicates of the same groups were exposed to V. parahaemolyticus by a single bath challenge and held for a further 15 days on the same diets as the growth study to assess survival and resistance. No significant differences (p > 0.05) in survival or in growth performance, including final weight, weight gain, specific growth rate, feed consumption or feed conversion ratio of white shrimp fed feeds containing methanotroph bacteria meal or control diets for 6 weeks. Immune markers such as hemocyte counts, phenoloxidase, superoxide dismutase and lysozyme activity were similar across all groups after the 6-week feeding trial. In a V. parahaemolyticus challenge, methanotroph bacteria meal in the diet significantly promoted the survival rate, and the reduction of Vibrio sp. in the hepatopancreas of white shrimp. Hemocyte count and phenoloxidase activity showed no significant differences (p > 0.05) between diet treatment groups, but hemolymph protein was significantly higher (p < 0.05) in shrimp fed diets containing 15% methanotroph bacteria meal after challenge. The Vibrio colony counts from hepatopancreas in the treatment groups were all significantly lower than the control (p < 0.05). The findings show that methanotroph bacteria meal can entirely replace fishmeal in white shrimp diets and the 15% inclusion of methanotroph bacteria meal in shrimp diet shows no adverse effects on growth performance, feed utilization and survival rate. In addition, shrimp fed methanotroph bacteria meal diets exhibited improved survival rates to an AHPND challenge.
The increase in aquaculture in recent years has led to a concomitant increase in demand for fishmeal with approximately 75% of the global production of fishmeal in 2018 utilized in aquaculture (FAO, 2018). Fishmeal is used in aquaculture feeds because it has all the nutritional requirements aquatic animals need (Swick et al., 1995; Samocha et al., 2004; Tacon et al., 2009). However, it can be an expensive ingredient because of high demand and inelastic supply (Samocha et al., 2004) and thus one option for feed mills is to substitute fishmeal with alternative proteins, but these frequently do not match the nutritional requirements of shrimp and can have variable or unbalanced nutritional profiles (Malcorps et al., 2019).
Animal sources of protein such as meat and bone meal, and poultry by-product meal, are used to replace fishmeal; but growth may be lower because of low lipid quality and differing nutritional profiles (Tan et al., 2005; Gamboa-Delgado et al., 2014). Swine meat meal has been used to replace 35% of the protein contribution of fishmeal but, at higher levels, caused nutritional imbalances in Penaeus vannamei (Hernández et al., 2008). Alternative proteins such as yeast, fungi, microalgae, and bacteria can serve as single cell protein sources for animals and several studies have investigated the utility of these proteins in a variety of animal feeds (Qui and Davis, 2017a,b; Qui et al., 2017; Linder, 2019; Glencross et al., 2020; Jones et al., 2020); their use in aquaculture species have been recently reviewed by Glencross et al. (2020).
Methanotrophs are gram-negative bacteria that use methane as their sole source of carbon and energy and have been identified for their potential use as a protein source in feeds for a variety aquaculture species (Berge et al., 2005; Øverland et al., 2006; Aas et al., 2007; Øverland et al., 2011; Biswas et al., 2020; Chen et al., 2021). Whilst studies have investigated the use of bacterial derived single cell protein feed additives from Corynebacterium ammoniagenes (Hamidoghli et al., 2019) and purple non-sulfur bacteria Rhodobacter sphaeroides and Afifella marina (Chumpol et al., 2018) in shrimp diets, to date only one published study has evaluated the use of Methylococcus capsulatus as an alternative protein source for shrimp feed formulations with up to 45% of the fish meal replaced with FeedKind® followed by exposure to Vibrio parahaemolyticus by injection (Chen et al., 2021). The current study builds on the results obtained by Chen et al. (2021) by replacing up to 100% of the fish meal in the diet with M. capsulatus-derived protein and by exposing shrimp to the AHPND bacterium through a more natural, bath route.
FeedKind® (Calysta, Menlo Park, CA, United States) is a bacterial biomass composed primarily of M. capsulatus produced via a continuous aerobic fermentation. Other bacterial strains present in the culture population that support the growth of M. capsulatus include Ralstonia sp. (DB3), Aneurinibacillus sp. (DB4), and Brevibacillus agri (DB5). The harvested biomass is centrifuged, heat inactivated, and spray dried. The nutrient content and amino acid profile of methanotroph bacteria meal, FeedKind®, is comparable to traditional proteins used in animal feeds such as fishmeal (Biswas et al., 2020) and has been shown to be a viable protein source for popular species of aquaculture fish including rainbow trout (Oncorhynchus mykiss), Atlantic salmon (Salmo salar) and yellowtail (Seriola quinqueradiata) (Berge et al., 2005; Øverland et al., 2006; Biswas et al., 2020). An additional benefit of producing methanotroph bacteria meal, is that it utilizes less than 0.01% of the land and around 10% of blue water compared with that used to produce soy protein, enhancing its sustainability credentials. Recently, Chen et al. (2021) reported that replacement of fishmeal with methanotroph bacteria meal did not negatively impact the growth or feed conversation rates of shrimp. Chen et al. (2021) also found that at higher dietary levels of methanotroph bacteria meal, shrimp increased oxidation levels in the hepatopancreas and increased the height of the mucosal folds in the gut, improved gut microbiota, and increased resistance to a V. parahaemolyticus challenge via intraperitoneal injection.
Several problematic bacterial diseases of shrimp have been reported, some of which have become globally widespread via the rapid expansion of shrimp farming (Thitamadee et al., 2016). Whilst Vibrio species are common in the normal microbiota of shrimp ponds, many are pathogenic to shrimp (Yang et al., 2014; Anandaraja et al., 2017), including V. parahaemolyticus, Vibrio harveyi, Vibrio owensii, Vibrio campbellii, and Vibrio alginolyticus (Soto-Rodriguez et al., 2012, 2015; Kondo et al., 2015; Liu et al., 2015; Dong et al., 2017; Wu et al., 2019).
Acute Hepatopancreatic Necrosis Disease (AHPND), also known as Early Mortality Syndrome (EMS), is a major concern for shrimp production caused by the bacterium V. parahaemolyticus with outbreaks occurring routinely in Southeast Asia shrimp farms in which mortality rates exceed 70% and global annual losses are estimated at more than US$1 billion (Zorriehzahra and Banaederakhshan, 2015). V. parahaemolyticus is transmitted orally and infects the digestive organs and hepatopancreas of shrimp (Lightner et al., 2012). China detected the first outbreak of EMS in 2009 with the disease subsequently identified in Vietnam and Malaysia in 2010 and 2011, respectively (Tran et al., 2013). Following the first outbreak recorded in Thailand in 2011, the country has continued to be impacted by outbreaks since (Chucherd, 2013; Putth and Polchana, 2016). In the past antibiotics were commonly used to treat EMS and other Vibrio diseases, but these have been widely prohibited (Liu et al., 2017). To enhance shrimp health, many farmers “top-dress” feeds at the farm with additives to improve health outcomes, but this practice is of limited efficacy and is problematic (FAO, 2019). On the other hand, methanotroph bacteria meal is blended in the feed during manufacture at the feed mill, providing a potentially more efficient method for delivering an immune stimulant.
This study was designed to determine the effects of methanotroph bacteria meal (FeedKind®) on growth performance, survival rate and resistance to V. parahaemolyticus challenge of Pacific white shrimp, P. vannamei. As an inactivated gram-negative bacterial biomass, it was theorized that FeedKind® may impart an immune response in shrimp.
All research was conducted at the Nutrition and Aquafeed Laboratory, Department of Aquaculture, Faculty of Fisheries, Kasetsart University, Bangkok, Thailand.
Shrimp feed ingredients were ground to 150–250 μm, mixed, and then water was added at 25% before passing through a Hobart mincer to form feed pellets. Pellets were then placed in a hot air oven at 60°C for 8–10 h to dry. The proximate compositions of trial feed such as moisture, protein, lipid, fiber, ash, energy, calcium, and phosphorus were analyzed as described by Association of Official Analytical Chemists (AOAC) (2000). All diets were sieved to suitable particle sizes of 1.5–2 mm for use in the trial. Samples of every batch of feed were kept at room temperature to be used later for verifications if needed. The formulations for each treatment diet are shown in Table 1. Soybean and poultry meal levels were adjusted accordingly to ensure crude protein levels were equivalent across all treatments.
Shrimp were obtained from a private farm in the Samutsongkarm province, Thailand, acclimated under trial conditions for 10 days, and fed three times daily with a commercial feed containing 35% CP followed by a further 7–8 days feeding with the trial diet prior to the start of the study. Five hundred individual shrimp were randomly allocated in batches of 25 to one of twenty 500 L tanks containing 300 L of brackish water (15–20 PSU). Twenty percent of the water in the static tanks was replaced every 2 days to maintain water quality in the range pH 7–8, DO > 5.0 mg/L, temperature 27–31°C, salinity 15–20 PSU, alkalinity >100 mg/L, and ammonia <1.0 mg/L. Shrimp were fed to satiation three times daily; 2 h after feeding, any uneaten feed was siphoned out of the tank, collected using a plankton net, dried at 65°C for 24 h before being weighed to calculate feed intake. Shrimp were fed with experimental diet as described above containing either 15% fishmeal (T1: Control) or methanotroph bacteria meal (FeedKind®) to replace fishmeal at 33% (T2: 5% FeedKind®), 66% (T3: 10% FeedKind®), or 100% (T4: 15% FeedKind®) with five replicates for each treatment. The diets were blinded to the technicians and randomly assigned to individual tanks. The amounts fed to each treatment tank were recorded. Shrimp were fed test diets for 6 weeks. At the end of the 6-week trial, material was collected for histological analysis, for immune measurements and for Vibrio spp. counts as described below.
After 6 weeks of the experimental feeding trial, thirty shrimp from each treatment in the growth trial were moved to 100L challenge test aquariums to form three replicates, each containing 10 shrimp for each diet to give a total of 120 shrimp used in the challenge trial. The V. parahaemolyticus (AHPND) virulent strain from Faculty of Veterinary Medicine, Kasetsart University, Kamphaeng Saen, Nakhon Pathom, Thailand was cultured on tryptic soy agar (Difco) supplemented with 1.5% NaCl (w/v) for 24 h at 35°C. After 24 h of growth, bacterial colonies were transferred to tryptic soy broth (Difco) supplemented with 1.5% NaCl and incubated for 24 h at 35°C. Next, the bacterial culture was centrifuged at 1,000 rpm for 15 min at room temperature. The supernatant was removed, and the bacterial pellet was resuspended in saline solution and then added to the tanks on Day 0 to reach a concentration of 5.8 × 104 CFU/ml in the water column of each challenge test aquarium. The concentration used was based on prior results obtained in our laboratory for this strain. A negative control (unchallenged) group was included in the study. Challenged shrimp were immersed in the Vibrio solution and continued to be fed test diets for 15 days before termination when haemolymph was taken for immune measurements and hepatopancreas taken for V. parahaemolyticus assessment as described below.
Measurements of performance parameters during the experimental period were recorded. To determine growth performance factors, the live weight of shrimp was taken to determine average shrimp weight at day 0 and at days 14, 28, and 42, to determine overall weight gain in the growth trials. Specific growth rate (SGR) for each replicate was determined using the formula:
where W6wk is the average weight of the shrimp at week 6 and W0wk is the initial average weight of shrimp in each group. Feed conversion ratio (FCR) was determined by dividing total feed consumption by total weight gain for each treatment. Survival rate for each treatment group was also calculated.
The hemolymph of fifty shrimp per experimental treatment (ten shrimp per replicate) randomly selected at the end of the growth trial and all shrimp per treatment at the end of the V. parahaemolyticus trial were collected using 10 percent (w/v) sodium citrate as an anticoagulant. The hemolymph were taken from the pericardial cavity using a 1-mL syringe, pooled, and stored at −20 until analysis. Measurements including total hemocyte count, hemolymph protein level, and phenol oxidase activity were determined according to the method of Encarnacion et al. (2012), lysozyme activity by turbidity method as described by Shugar (1952), superoxide dismutase enzyme activity and total glutathione using Sigma-Aldrich Assay Kits (19160-1KT-F and CS0260-1KT, respectively).
Histological examinations of hepatopancreas health were conducted using five shrimp from each tank (replicate) in the growth trial. Shrimp were randomly collected and immersed in ice water for stunning before removal of small portions of hepatopancreas tissue, injected with Davidson’s fixative and then transferred to 70% ethanol before being processed to wax blocks, then sectioned using a microtome, with sections being stained with hematoxylin and eosin (H&E) according to Bell and Lightner (1988). The number of B-cells, R-cells, and other cell types were counted from up to 10 tubules per slide.
Vibrio bacteria counts from hepatopancreas of shrimp after 42 days of the growth trial and 15 days after V. parahaemolyticus challenge were conducted. Two shrimp from each replicate of each treatment were randomly sampled and then surface body sterilized with 70% ethanol, scarified to collect the hepatopancreas which was then homogenized in 0.85 saline water. The solution was spread on the TCBS Agar (Difco) with 1.5% NaCl (w/v) for 24 h at 35°C. After 24 h of growth, bacterial colonies were counted and recorded.
This study was conducted in completely randomized design (CRD). All data were analyzed by one-way analysis of variance (ANOVA). If an ANOVA resulting from the analyses was significant then least square means were used to test pairwise differences among treatment. The Duncan’s Multiple Range Test was used to determine the significant difference test. Statistical tests were considered significant at an alpha = 0.05 and the alphabetical notation was used to mark the differences at these significance levels. Residuals were checked for normality using quantile to quantile plots and Bartlett tests were used to assess the homogeneity of variances. The data in percentage of mortality among methanotroph bacterial meal replaced for fishmeal in the growth trial and challenge assays were transformed following the method of Sokal and Rohlf (1995) and then subjected to ANOVA. Differences were considered statistically significant if p < 0.05.
The initial average weight of pacific white shrimp was 1.17 ± 0.09 g. The growth performance of white shrimp fed different levels of methanotroph bacteria meal for 6 weeks showed no significant differences (p > 0.05) in terms of final weight, weight gain, specific growth rate, feed consumption, and feed conversion ratio when compared to the control diet (see Table 2). Survival rate was not significantly different (p > 0.05) among treatments.
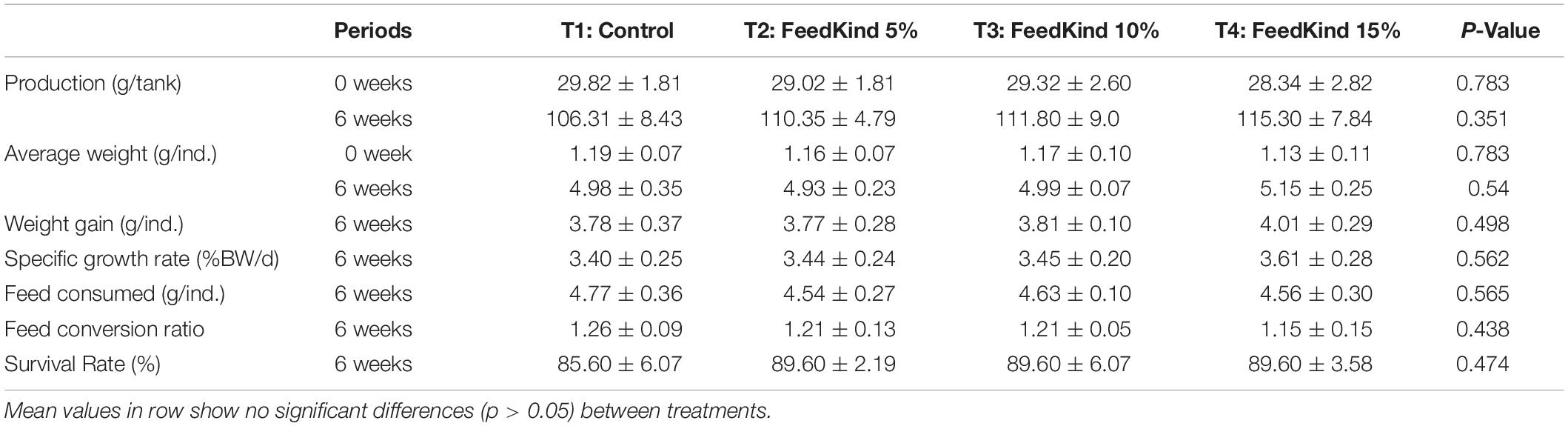
Table 2. Growth performance of white shrimp (n = 25 for each replicate and each time period) fed different level of methanotroph bacteria meal for 6 weeks.
The immune parameters of white shrimp fed different levels of methanotroph bacteria meal or control diet are presented in Table 3. No significant differences (p > 0.05) were noted in hemocyte counts, hemolymph protein, phenoloxidase activity, lysozyme activity, superoxide dismutase activity, and amount of total glutathione between treatments. However, Vibrio sp. counts in the hemolymph and in the hepatopancreas following plate cultures were significantly reduced in shrimp fed methanotroph bacteria meal compared with control diet prior to challenge with V. parahaemolyticus (Table 3).
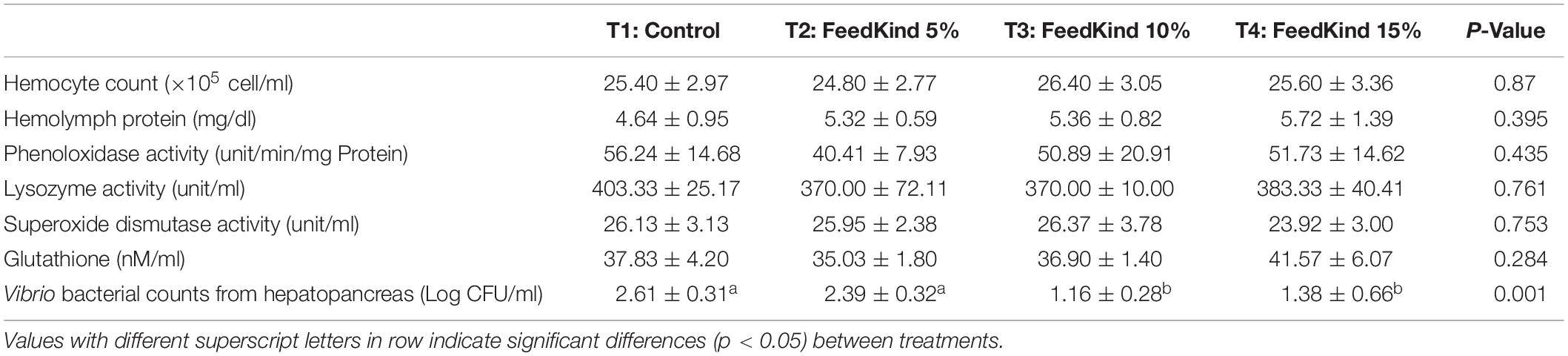
Table 3. Immune parameters (n = 10 per replicate) and Vibrio counts (n = 2 per replicate) of white shrimp fed different level of methanotroph bacteria meal for 6 weeks under pre-challenge conditions.
Histological sections of hepatopancreases of shrimp fed diets containing different concentrations of methanotroph bacteria meal, showed that blister-like cells (B-cell) percentage was highest in shrimp fed diet T2 (5% FeedKind) but lowest in those fed the control diet. Resorptive-cells (R-cell) percentages were highest in shrimp fed the control diet and in shrimp fed diet T4 (15% FeedKind) (Table 4).
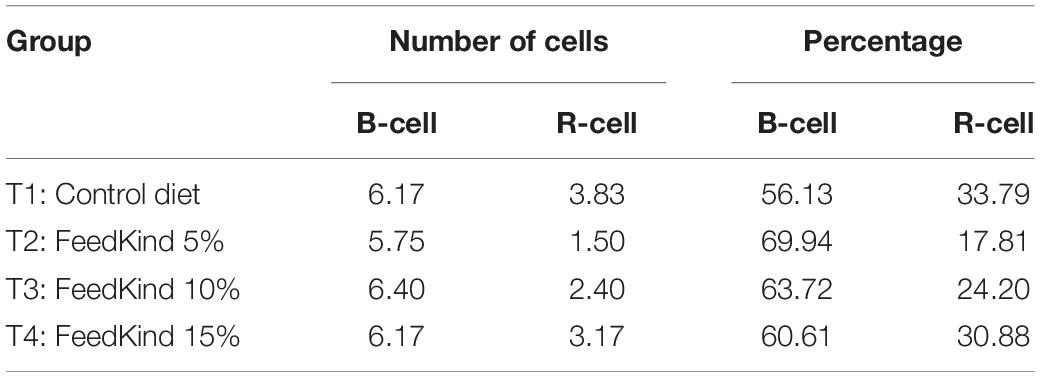
Table 4. Number and percentage of B-cell and R-cell from hepatopancreas of shrimp fed different levels of methanotroph bacteria meal for 6 weeks under pre-challenge conditions.
B-cell size was largest in the control group compared to other groups. Degeneration and change of lumen structure (note the star-like shaped seen is normal) were found in groups fed diets T2 and T3 in which 33 and 66%, respectively, of the fishmeal was replaced with methanotroph bacteria meal. Representative histological images are shown in Figure 1.
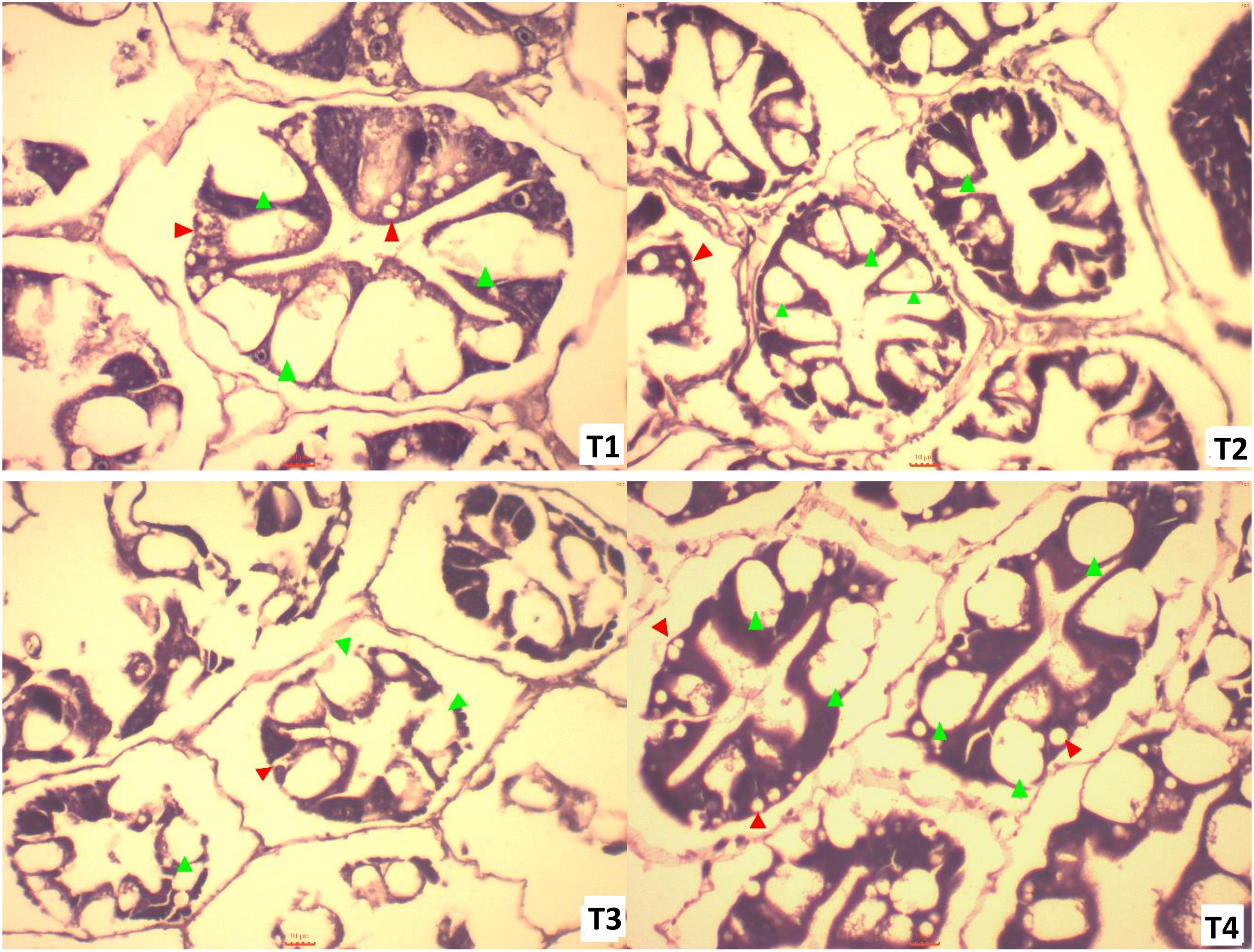
Figure 1. Light micrograph of hepatopancreas of shrimp fed different concentration of methanotroph bacteria meal for 6 weeks under pre-challenge conditions (T1: Control diet, T2: FeedKind 5%, T3: FeedKind 10%, T4: FeedKind 15%; green arrow = B-cell, red arrow = R-cell; scale bar = 10 μm, ×400).
The survival rate of shrimp immersed in V. parahaemolyticus EMS 5.8 × 104 CFU/ml for 15 days after being fed feeds containing different levels of single cell protein for 6 weeks are presented in Table 5. Mortalities were noted in the control diet group at day 9 post-infection. The results showed that on day 9 to day 15 after immersion, shrimp fed methanotroph bacteria meal at 10 and 15% in diets T3 and T4 had a significantly higher survival rate (p < 0.05) than diets T2 and T1 control, respectively. No mortalities were noted in shrimp fed diets containing 15% methanotroph bacteria meal.
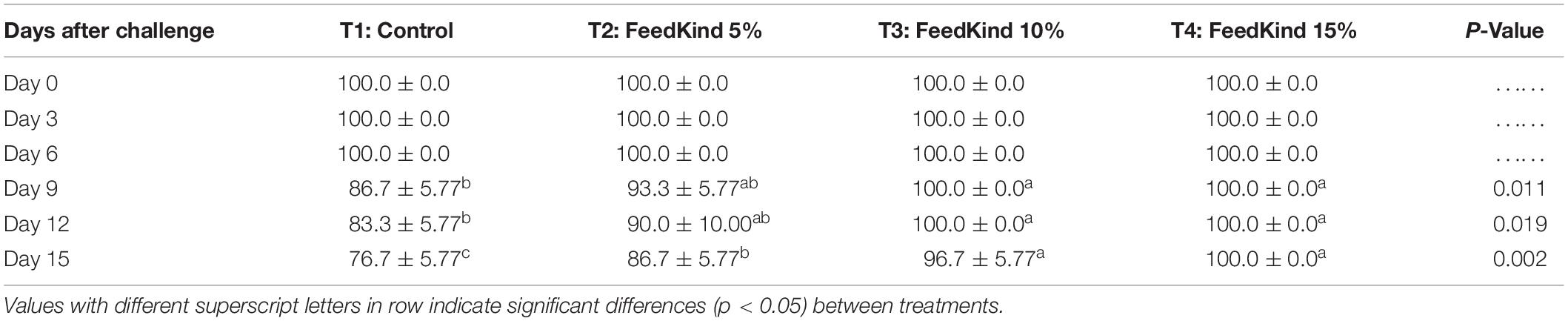
Table 5. Survival rate of white shrimp following immersion challenge by V. parahaemolyticus (AHPND) at 5.8 × 104 CFU/ml for 15 days after being fed different levels of methanotroph bacteria meal diets for 6 weeks.
The measured immune parameters of white shrimp collected after the 15-day challenge with V. parahaemolyticus are presented in Table 6. Hemocyte count and phenoloxidase activity showed no significant differences (p > 0.05) between diet treatment groups, but hemolymph protein was significantly higher (p < 0.05) in shrimp fed diet T4 containing 15% methanotroph bacteria meal. The Vibrio colony counts from hepatopancreas in the treatment groups were all significantly lower than the control (p < 0.05), and the colony counts from the group fed diet T4 were further reduced and significantly different from diet T2.
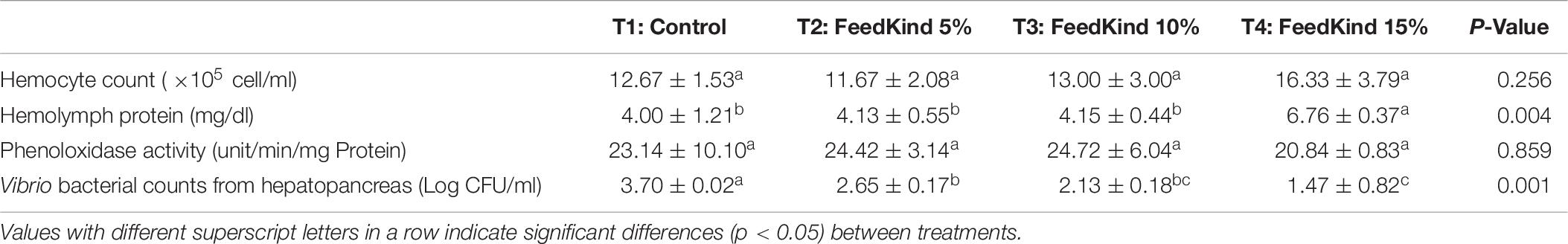
Table 6. Immune parameters in white shrimp fed different levels of methanotroph bacteria meal after 15 days of immersion by Vibrio parahaemolyticus.
This study has successfully shown that Pacific white shrimp fed diets where fishmeal is replaced with the single cell protein methanotroph bacteria meal, FeedKind, for 6 weeks shows comparable levels of growth and survival to shrimp fed standard feeds containing 15% fishmeal (T1). Specifically, feed conversion ratios, specific growth rates and overall increases in weight are greater in shrimp fed FeedKind compared with those fed standard diets. Importantly, methanotroph bacteria meal reduces Vibrio spp. loads in the hepatopancreas and improves the survival rate when shrimp are exposed to a V. parahaemolyticus challenge. In agreement with Chen et al. (2021), this study has shown the substitution of fishmeal with methanotroph bacteria meal did not affect uptake of feed nor have any other detrimental effects such as antinutritional properties or impacts on a range of immune measures.
These results are in broad agreement with studies showing dietary supplementation of fishmeal with single cell protein shows no significant differences in growth and feed efficiency in fish species such as Atlantic salmon (Berge et al., 2005), Atlantic halibut (Aas et al., 2007), and Japanese yellowtail (Biswas et al., 2020). Other research on single cell protein as replacement of fishmeal in shrimp diets has shown they can be used to replace fishmeal, partially or fully, e.g., purple non-sulfur bacteria (Chumpol et al., 2018), C. ammoniagenes (Hamidoghli et al., 2019), and KnipBio Meal (Tlusty et al., 2017). Previous studies have found that disease resistance parameters can be promoted in shrimp by using nutrition supplements such as biofloc feed (Ekasari et al., 2014) and organic acids and essential oils (He et al., 2017). The positive effect on the immune capacity, disease resistance and gut microbiota of P. vannamei have also been shown to be improved by using single cell protein (Chumpol et al., 2018; Chen et al., 2021). Furthermore, shrimp fed methanotroph bacteria meal have been shown to have increased oxidation levels in the hepatopancreas, increased mucosal fold height in the gut, improved gut microbiota, and an overall improvement in general disease resistance factors (Chen et al., 2021).
The digestive gland or hepatopancreas of crustaceans is used for monitoring cultured shrimp health and serves as a sensitive indicator for metabolism, ecdysis phase, nutritional status, and disease state in various shrimp species. The hepatopancreas is the site of digestion, nutrient absorption, reserve nutrient storage, and synthesis and secretion of digestive enzymes and is composed of numerous blinded tubules, with each tubule consisting of different epithelial cell types, i.e., E-cell (embryonic), R-cell (resorptive), F-cell (fibrillary), and B-cell (blister like). Histological analysis of the hepatopancreas has been used as a practical means for assessing the nutritional condition in the shrimp culture (Díaz et al., 2010; Vogt, 2020). In the current study, B-cell types are more numerous and smaller in shrimp fed FeedKind compared with those fed control diets suggesting B-cell types may be produced in greater numbers in shrimp fed FeedKind® diets. The increased number of these secretory cells that function as primary producers of digestive enzymes and antioxidants, suggests that FeedKind®-fed shrimp are better able to accumulate nutrients from the diet and to transport digested material compared with those fed control diets. Conversely, R-cells which function as the main site for lipid and glycogen storage, do not show a clear pattern of size distribution between treatments suggesting that diet has little impact on these cells.
In the current study, improved survival rates of shrimp fed diets with total or partial replacement of fishmeal with methanotroph bacteria meal for 6 weeks followed by a V. parahaemolyticus bath challenge was demonstrated. Specifically, no mortality was observed in the diet with 15% FeedKind (replacement of 100% of fishmeal) while only 76% of shrimp challenged with V. parahaemolyticus and fed the control fishmeal-based diet survived. In comparison, only 50% of Pacific shrimp fed a diet containing up to 10.5% FeedKind® and 13.75% fishmeal survived a challenge with V. parahaemolyticus by injection compared to 25% survival when fed control diets without bacterial meal (Chen et al., 2021). The differences observed in shrimp survival between the current study and that of Chen et al. (2021) can be partly explained by the challenge method (IP vs. bath) and by the challenge dose. Using the bath challenge method in the current study is a more natural route of exposure and is more comparable to infection processes on the farm. Comparison between doses used in different studies and by different exposure routes is rarely considered. However, Joshi et al. (2014) exposed P. vannamei to 108 CFU/ml V. parahaemolyticus by the bath route or to 103 CFU per shrimp by injection with mortality outcomes being similar, although characteristic disease was not induced in shrimp exposed via the IP route. Unlike Chen et al. (2021), typical clinical signs of AHPND were noted in shrimp exposed to V. parahaemolyticus in the current study, supporting the view that a bath exposure route is preferable to demonstrate the impact of AHPND on the host. In addition, the control diets used by Chen et al. (2021) differed significantly from those in the present study. Whereas the prior work included peanut meal as a source of protein, this ingredient is not commonly used in shrimp feeds outside of China, and therefore was not included in the current study. Similarly, the prior study included brewer’s yeast, which, as a single cell product containing beta glucans and nucleic acids, may have also influenced the shrimp immune response alongside the methanotroph bacteria meal (Chen et al., 2021).
It is recognized that feeding shrimp with diets containing different levels of methanotroph bacteria meal for 6 weeks prior to a V. parahaemolyticus challenge led to a substantial reduction in Vibrio spp. numbers in the hepatopancreas as well as lower numbers of V. parahaemolyticus post-challenge leading to improved survival. Reduced Vibrio spp. levels and increased expression of anti-lipopolysaccharide factor (ALF) have been identified in the intestine of shrimp fed diets containing bacterial protein meal (Chen et al., 2021). The authors speculate that bacterial protein meal may stimulate toll-like receptors (TLRs) present in the shrimp digestive tract, which then activate an innate immune response and up-regulate the production of antimicrobial peptides or other enzymes increasing the ability of white shrimp to resist a bacterial pathogen. ALFs are known to bind to gram-negative bacterial cell walls and disrupt cellular function causing cell death and lysis (Zhan et al., 2015) and thus may play an important role in reducing the impact of V. parahaemolyticus in shrimp fed diets containing FeedKind. Further study is necessary to validate this hypothesis and possibly to identify the specific pathway and antimicrobial function stimulated by methanotroph bacteria meal protein. In the current study, although Vibrio sp. counts in the hemolymph and in the hepatopancreas were significantly reduced in shrimp fed methanotroph bacteria meal compared with control diet, no significant differences were noted in hemocyte counts, hemolymph protein, phenoloxidase activity, lysozyme activity, superoxide dismutase activity or amount of total glutathione between treatments suggesting that these measures are not sufficiently sensitive in detecting differences in immune responses prior to a disease challenge. However, in shrimp challenged with V. parahaemolyticus and fed a diet where 100% of the fish meal was replaced with FeedKind, haemolymph protein was significantly higher compared with other groups. Although the current study did not attempt determine which haemolymph proteins were amplified, it is known that a wide range of these antimicrobial peptides, which perform a number of functions on host defense, have been reported in crustaceans (Fredrick and Ravichandran, 2012). It is likely that the decrease in V. parahaemolyticus and improved survival rates in animals fed FeedKind® is a direct result of the noted increase in haemolymph proteins.
The current study has shown that fish meal can be entirely replaced in shrimp diets with limited impact on growth and survival of shrimp; additional studies should validate the results of the current study under field conditions to confirm that complete replacement of fishmeal with methanotroph bacteria meal protein, followed by a natural V. parahaemolyticus challenge leads to improved survival.
The efficacy of single cell protein, methanotroph bacteria meal protein in Pacific white shrimp diets was studied to determine growth performance, survival rate and disease resistance against V. parahaemolyticus. Trials of this novel protein source for replacing fishmeal in shrimp diets have shown that the protein does not affect the growth feed efficiency, or survival of shrimp reared under experimental conditions. Additionally, shrimp in this study demonstrated an increased tolerance to disease when challenged with V. parahaemolyticus, the causative agent of Early Mortality Syndrome (EMS), indicating methanotroph bacteria meal protein, FeedKind® protein, may help promote a robust immune response.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
OJ: study design, conception, data analysis, and writing manuscript. SC and ST: data collection, data analysis, and writing manuscript. AL and JS: study design, conception, and writing manuscript.
Calysta, Inc. funded this research and contributed to the drafting of this manuscript.
AL and JS are employed by and own company stock in Calysta, Inc., the supplier of the bacterial protein meal FeedKind used in these studies.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aas, T. S., Hatlen, B., Grisdale-Helland, B., Terjesen, B. F., Penn, M., Bakke-McKellep, A. M., et al. (2007). Feed intake, growth and nutrient utilization in Atlantic halibut (Hippoglossus hippoglossus) fed diets containing a bacterial protein meal. Aquac. Res. 38, 351–360. doi: 10.1111/j.1365-2109.2007.01672.x
Anandaraja, R., Sridhar, R., Balachandran, C., Palanisammi, A., Ramesh, S., and Nagarajan, K. (2017). Pathogenicity profile of Vibrio parahaemolyticus in farmed pacific white shrimp, Penaeus vannamei. Fish Shellfish Immunol. 67, 368–381. doi: 10.1016/j.fsi.2017.06.020
Association of Official Analytical Chemists (AOAC) (2000). Official Methods of Analysis of AOAC International, 17th Edn. Gaithersburg, MD: AOAC International.
Bell, T. A., and Lightner, D. V. (1988). A Handbook of Normal Penaeid Shrimp Histology: World Aquaculture Society. Louisiana: World Aquaculture Society.
Berge, G. M., Baeverfjord, G., Skrede, A., and Storebakken, T. (2005). Bacterial protein grown on natural gas as protein source in diets for Atlantic salmon, Salmo salar, in saltwater. Aquaculture 244, 233–240. doi: 10.1016/j.aquaculture.2004.11.017
Biswas, A., Takakuwa, F., Yamada, S., Matsuda, A., Saville, R. M., LeBlanc, A., et al. (2020). Methanotroph (Methylococcus capsulatus, Bath) bacteria meal as an alternative protein source for Japanese yellowtail, Seriola quinqueradiata. Aquaculture 529:735700. doi: 10.1016/j.aquaculture.2020.735700
Chen, M., Chen, X.-Q., Tian, L.-X., Liu, Y.-J., and Niu, J. (2020). Enhanced intestinal health, immune responses and ammonia resistance in Pacific white shrimp (Litopenaeus vannamei) fed dietary hydrolyzed yeast (Rhodotorula mucilaginosa) and Bacillus licheniformis. Aquac. Rep. 17:100385. doi: 10.1016/j.aqrep.2020.100385
Chen, Y., Chi, S., Shuang, Z., Dong, X., Yang, Q., Liu, H., et al. (2021). Replacement of fishmeal with methane-utilizing bacteria products in the diets of Pacific white shrimp (Litopenaeus vannamei). Aquaculture 541:736801. doi: 10.1016/j.aquaculture.2021.736801
Chumpol, S., Kantachote, D., Nitoda, T., and Kanzaki, H. (2018). Administration of purple non-sulfur bacteria as single cell protein by mixing with shrimp feed to enhance growth, immune response and survival in white shrimp (Litopenaeus vannamei) cultivation. Aquaculture 489, 85–95. doi: 10.1016/j.aquaculture.2018.02.009
Díaz, A. C., Sousa, L. G., and Petriella, A. M. (2010). Functional cytology of the hepatopancreas of Palaemonetes argentines (Crustacea, Decapoda, Caridea) under osmotic stress. Brazilian Arch. Biol. Technol. 53, 599–608. doi: 10.1590/S1516-89132010000300013
Dong, X., Wang, H., Xie, G., Zou, P., Guo, C., Liang, Y., et al. (2017). An isolate of Vibrio campbellii carrying the pirVP gene causes acute hepatopancreatic necrosis disease. Emerg. Microbes Infect. 6, 1–3. doi: 10.1038/emi.2016.131
Ekasari, J., Azhar, M. H., Surawidjaja, E. H., Nuryati, S., De Schryver, P., and Bossier, P. (2014). Immune response and disease resistance of shrimp fed biofloc grown on different carbon sources. Fish Shellfish Immunol. 41, 332–339. doi: 10.1016/j.fsi.2014.09.004
Encarnacion, A. B., Fagutao, F., Jintasataporn, O., Worawattanamateekul, W., Hirono, I., and Ohshima, T. (2012). Application of ergothioneine-rich extract from an edible mushroom Flammulina velutipes for melanosis prevention in shrimp, Penaeus monodon and Litopenaeus vannamei. Food Res. Internatl. 45, 232–237. doi: 10.1016/j.foodres.2011.10.030
FAO (2018). The State of World Fisheries and Aquaculture 2018: Meeting the Sustainable Development Goals. Italy: Food and Agriculture Organization of the United Nations. 210.
FAO (2019). Report of the FAO Workshop on the on-Farm Feeding and Feed Management in Aquaculture. Rome: Food and Agriculture Organization of the United Nations., 8.
Fredrick, W. S., and Ravichandran, S. (2012). Hemolymph proteins in marine crustaceans. Asian Pac. J. Trop. Biomed. 2, 496–502. doi: 10.1016/S2221-1691(12)60084-7
Gamboa-Delgado, J., Castañeda-Solís, J. D., Nieto-López, M. G., Villarreal-Cavazos, D., and Cruz-Suárez, L. E. (2014). Isotopic evaluation of the nutritional contribution of poultry by-product meal and fishmeal to the growth of pacific white shrimp, Litopenaeus vannamei. J. World Aquac. Soc. 45, 430–438. doi: 10.1111/jwas.12134
Glencross, B. D., Huyben, D., and Schrama, J. W. (2020). The application of single-cell ingredients in aquaculture feeds—a review. Fishes 5:22. doi: 10.3390/fishes5030022
Hamidoghli, A., Yun, H., Won, S., Kim, S., Farris, N. W., and Bai, S. C. (2019). Evaluation of a single-cell protein as a dietary fishmeal substitute for whiteleg shrimp Litopenaeus vannamei. Fish. Sci. 85, 147–155. doi: 10.1007/s12562-018-1275-5
He, W., Rahimnejad, S., Wang, L., Song, K., Lu, K., and Zhang, C. (2017). Effects of organic acids and essential oils blend on growth, gut microbiota, immune response and disease resistance of Pacific white shrimp (Litopenaeus vannamei) against Vibrio parahaemolyticus. Fish Shellfish Immunol. 70, 164–173. doi: 10.1016/j.fsi.2017.09.007
Hernández, C., Olvera-Novoa, M. A., Aguilar-Vejar, K., González-Rodríguez, B., and de la Parra, I. A. (2008). Partial replacement of fishmeal by porcine meat meal in practical diets for Pacific white shrimp (Litopenaeus vannamei). Aquaculture 277, 244–250. doi: 10.1016/j.aquaculture.2008.02.016
Jones, S. W., Karpol, A., Friedman, S., Maru, B. T., and Tracy, B. P. (2020). Recent advances in single cell protein use as a feed ingredient in aquaculture. Curr. Opin. Biotechnol. 61, 189–197. doi: 10.1016/j.copbio.2019.12.026
Joshi, J., Srisala, J., Troung, V. H., Chen, I., Nuangsaeng, B., Suthienkul, O., et al. (2014). Variation in Vibrio parahaemolyticus isolates from a single Thai shrimp farm experiencing an outbreak of acute hepatopancreatic necrosis disease (AHPND). Aquaculture 42, 297–302. doi: 10.1016/j.aquaculture.2014.03.030
Kondo, H., Van, P. T., Dang, L. T., and Hirono, I. (2015). Draft genome sequence of non-Vibrio parahaemolyticus acute hepatopancreatic necrosis disease strain KC13.17.5, isolated from diseased shrimp in Vietnam. Genome Announcements 3:e978. doi: 10.1128/genomeA.00978-15
Lightner, D. V., Redman, R. M., Pantoja, C. R., Noble, B. L., and Loc, T. (2012). Early mortality syndrome affects shrimp in Asia. Global Aquac. Adv. 2012:40.
Linder, T. (2019). Making the case for edible microorganisms as an integral part of a more sustainable and resilient food production system. Food Security 11, 265–278. doi: 10.1007/s12571-019-00912-3
Liu, L., Xiao, J., Xia, X., Pan, Y., Yan, S., and Wang, Y. (2015). Draft genome sequence of vibrio owensii strain SH-14, which causes shrimp acute hepatopancreatic necrosis disease. Genome Announcements 3:e1395. doi: 10.1128/genomeA.01395-15
Liu, X., Steele, J. C., and Meng, X. Z. (2017). Usage, residue, and human health risk of antibiotics in Chinese aquaculture: a review. Environ. Poll. 223, 161–169. doi: 10.1016/j.envpol.2017.01.003
Malcorps, W., Kok, B., Land, M. V., Fritz, M., van Doren, D., Servin, K., et al. (2019). The sustainability conundrum of fishmeal substitution by plant ingredients in shrimp feeds. Sustainability 11:1212. doi: 10.3390/su11041212
Øverland, M., Borge, G. I., Vogt, G., Schøyen, H. F., and Skrede, A. (2011). Oxidative stability and sensory quality of meat from broiler chickens fed a bacterial meal produced on natural gas. Poultry Sci. 90, 201–210. doi: 10.3382/ps.2010-00784
Øverland, M., Romarheim, O. H., Hovin, M., Storebakken, T., and Skrede, A. (2006). Apparent total tract digestibility of unprocessed and extruded diets containing basic and autolyzed bacterial protein meal grown on natural gas in mink and rainbow trout. Animal Feed Sci. Technol. 129, 237–251. doi: 10.1016/j.anifeedsci.2005.12.017
Putth, S., and Polchana, J. (2016). “Current status and impact of early mortality syndrome (EMS)/acute hepatopancreatic necrosis disease (AHPND) and hepatopancreatic microsporidiosis (HPM) outbreaks on Thailand s shrimp farming,” in Addressing Acute Hepatopancreatic Necrosis Disease (AHPND) and Other Transboundary Diseases for Improved Aquatic Animal Health in Southeast Asia: Proceedings of the ASEAN Regional Technical Consultation on EMS/AHPND and Other Transboundary Diseases for Improved Aquatic Animal Health in Southeast Asia, eds R. V. Pakingking Jr., E. G. T. de Jesus-Ayson and B. O. Acosta (Iloilo: Aquaculture Department, Southeast Asian Fisheries Development Center), 79–87.
Qui, X., and Davis, D. A. (2017a). Effects of dietary phytase supplementation on growth performance and apparent digestibility coefficients of Pacific white shrimp Litopenaeus vannamei. Aquacu. Nutr. 23, 942–951.
Qui, X., and Davis, D. A. (2017b). Evaluation of flash dried yeast as a nutritional supplement in plant-based practical diets for Pacific white shrimp Litopenaeus vannamei. Aquac. Nutr. 23, 1244–1253. doi: 10.1111/anu.12499
Qui, X., Tian, H., and Davis, D. A. (2017). Evaluation of a high protein distiller’s dried grains product as a protein source in practical diets for Pacific white shrimp Litopenaeus vannamei. Aquaculture 480, 1–10. doi: 10.1016/j.aquaculture.2017.07.038
Samocha, T., Davis, D. A., Saoud, I. P., and DeBault, K. (2004). Substitution of fishmeal by co-extruded soybean poultry by-product meal in practical diets for the Pacific white shrimp, Litopenaeus vannamei. Aquaculture 231, 197–203. doi: 10.1016/j.aquaculture.2003.08.023
Shugar, D. (1952). The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochim. Biophys. Acta. 8, 302–309. doi: 10.1016/0006-3002(52)90045-0
Sokal, R. R., and Rohlf, F. J. (1995). Biometry: The Principles and Practice of Statistics in Biological Research, 3rd Edn. New York: W.H. Freeman and Co.
Soto-Rodriguez, S. A., Gomez-Gil, B., Lozano, R., del Rio-Rodríguez, R., Diéguez, A. L., and Romalde, J. L. (2012). Virulence of vibrio harveyi responsible for the “bright-red” syndrome in the pacific white shrimp Litopenaeus vannamei. J. Invertebr. Pathol. 109, 307–317. doi: 10.1016/j.jip.2012.01.006
Soto-Rodriguez, S. A., Gomez-Gil, B., Lozano-Olvera, R., Betancourt-Lozano, M., and Morales-Covarrubias, M. S. (2015). Field and experimental evidence of Vibrio parahaemolyticus as the causative agent of acute hepatopancreatic necrosis disease of cultured shrimp (Litopenaeus vannamei) in Northwestern Mexico. Appl. Environ. Microbiol. 81, 1689–1699. doi: 10.1128/AEM.03610-14
Swick, R. A., Akiyama, D. M., Boonyaratpalin, M., and Creswell, D. C. (1995). Use of Soybean Meal and Synthetic Methionine in Shrimp Feed. St. Louis, MO: American Soybean Association, Technical Bulletin, AQ43–AQ1995.
Tacon, A. G. J., Metian, M., and Hasan, M. R. (2009). Feed Ingredients and Fertilizers for Farmed Aquatic Animals. Sources and Composition. FAO Fisheries and Aquaculture technical paper, 540. Italy: FAO.
Tan, B., Mai, K., Zheng, S., Zhou, Q., Liu, L., and Yu, Y. (2005). Replacement of fishmeal by meat and bone meal in practical diets for the white shrimp Litopenaeus vannamei (Boone). Aquac. Res. 36, 439–444.
Thitamadee, S., Prachumwat, A., Srisala, J., Jaroenlak, P., Salachan, P. V., Sritunyalucksana, K., et al. (2016). Review of current disease threats for cultivated penaeid shrimp in Asia. Aquaculture 452, 69–87. doi: 10.1016/j.aquaculture.2015.10.028
Tlusty, M., Rhyne, A., Szczebak, J. T., Bourque, B., Bowen, J. L., Burr, G., et al. (2017). A transdisciplinary approach to the initial validation of a single cell protein as an alternative protein source for use in aquafeeds. PeerJ 5:e3170. doi: 10.7717/peerj.3170
Tran, L., Nunan, L., Redman, R. M., Mohney, L. L., Pantoja, C. R., Fitzsimmons, K., et al. (2013). Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis. Aquat. Orgs. 105, 45–55. doi: 10.3354/dao02621
Vogt, G. (2020). Cytopathology and immune response in the hepatopancreas of decapod crustaceans. Dis. Aquat. Orgs. 138, 41–88. doi: 10.3354/dao03443
Wu, C. C., Lin, C. L., Huang, C. Y., Hsieh, S., Liu, C. H., and Hsieh, S. L. (2019). α-Phellandrene enhances the immune response and resistance against Vibrio alginolyticus in white shrimp (Litopenaeus vannamei). Fish Shellfish Immunol. 84, 1108–1114. doi: 10.1016/j.fsi.2018.11.013
Yang, Y. T., Chen, I. T., Lee, C. T., Chen, C. Y., Lin, S. S., Hor, L. I., et al. (2014). Draft genome sequences of four strains of Vibrio parahaemolyticus, three of which cause early mortality syndrome/acute hepatopancreatic necrosis disease in shrimp in China and Thailand. Genome Announcements 2:e816. doi: 10.1128/genomeA.00816-14
Zhan, W., He, L., Wei, X., Wang, X., and Tang, X. (2015) An anti-lipopolysaccharide factor in Litopenaeus vannamei participates in the immune defense against WSSV and Vibrio anguillarum. J. Crust. Biol. 35, 670–675. doi: 10.1163/1937240x-00002364
Keywords: single cell protein (SCP), Penaeus vannamei, Vibrio parahaemolyticus (AHPND), FeedKind, functional feeds
Citation: Jintasataporn O, Chumkam S, Triwutanon S, LeBlanc A and Sawanboonchun J (2021) Effects of a Single Cell Protein (Methylococcus capsulatus, Bath) in Pacific White Shrimp (Penaeus vannamei) Diet on Growth Performance, Survival Rate and Resistance to Vibrio parahaemolyticus, the Causative Agent of Acute Hepatopancreatic Necrosis Disease. Front. Mar. Sci. 8:764042. doi: 10.3389/fmars.2021.764042
Received: 24 August 2021; Accepted: 14 October 2021;
Published: 03 November 2021.
Edited by:
Xiang-Jun Leng, Shanghai Ocean University, ChinaCopyright © 2021 Jintasataporn, Chumkam, Triwutanon, LeBlanc and Sawanboonchun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Orapint Jintasataporn, ZmZpc29yYUBrdS5hYy50aA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.