
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 24 December 2021
Sec. Global Change and the Future Ocean
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.762086
This article is part of the Research Topic Seagrasses under Times of Change View all 19 articles
 Alyson Lowell1*
Alyson Lowell1* Eduardo Infantes2,3
Eduardo Infantes2,3 Laura West4
Laura West4 Lauren Puishys5
Lauren Puishys5 Claudia E. L. Hill6,7
Claudia E. L. Hill6,7 Kirti Ramesh8
Kirti Ramesh8 Bradley Peterson1
Bradley Peterson1 Just Cebrian9
Just Cebrian9 Sam Dupont8
Sam Dupont8 T. Erin Cox5,10*
T. Erin Cox5,10*
Elevated partial pressure of carbon dioxide (pCO2) as a concomitant of global climate change may facilitate the establishment of future seagrass meadows and subsequently its benefit could be incorporated into techniques to increase restoration success. In five manipulative experiments, we determined how increased CO2 affects the maturation of flowers, and the development of seeds and seedlings for the foundation species Zostera marina. Experiments tested the development from both seeds collected from non-treated flowering shoots (direct) and seeds harvested from flowering shoots after CO2 exposure (parental carryover). Flowering shoots were collected along the western coast of Sweden near the island of Skafto. The seeds produced were used in experiments conducted at Kristineberg, Sweden and Dauphin Island, AL, United States. Experiments varied in temperature (16, 18°C) and salinity (19, 33 ppt), as well as duration and magnitude of elevated CO2 exposure. Flowering maturation, spathe number, seed production, and indicators of seed quality did not appear to be affected by 39–69 days of exposure to CO2 conditions outside of natural variability (pCO2 = 1547.2 ± 267.60 μatm; pHT = 7.53 ± 0.07). Yet, seeds produced from these flowers showed twofold greater germination success. In another experiment, flowering shoots were exposed to an extreme CO2 condition (pCO2 = 5950.7 ± 1,849.82 μatm; pHT = 6.96 ± 0.15). In this case, flowers generated seeds that demonstrated a fivefold increase in an indicator for seed viability (sinking velocity). In the latter experiment, however, germination appeared unaffected. Direct CO2 effects on germination and seedling production were not observed. Our results provide evidence of a parental CO2 effect that can benefit germination or seed viability, but early benefits may not lead to bed establishment if other environmental conditions are not well suited for seedling development. Outcomes have implications for restoration; CO2 can be supplied to flowering shoot holding tanks to bolster success when the purpose is to redistribute seeds to locations where beds are extant and water quality is adequate.
Seagrass meadows serve as important ecosystem engineers hallmarked for their ability to sequester carbon and provide habitat for a diversity of marine organisms (Heck and Orth, 1980; Hemminga and Duarte, 2000; Hernán et al., 2017). Seagrasses are in decline due to anthropogenic stressors, like eutrophication and ocean warming, which have accelerated global decline in coverage while simultaneously increasing susceptibility to natural stressors like disease (Orth et al., 2006; Waycott et al., 2009; Zimmerman et al., 2015). Loss of highly productive seagrass beds represents a significant disruption to global carbon cycling. In the context of changing ocean carbonate chemistry, seagrasses serve as valuable carbon sinks utilizing carbon dioxide for photosynthesis and burying carbon in their root and rhizome mass. Efforts to restore seagrass beds serves as a possible adaptive strategy for protecting coastal systems from disruptions in marine carbonate chemistry (Unsworth et al., 2012).
The ocean absorbs carbon dioxide (CO2) from the atmosphere increasing seawater (SW) concentrations of inorganic carbon [DIC] and CO2 while simultaneously decreasing pH in a process referred to as ocean acidification (OA). Carbon in its dissolved form [DIC] can exist as one of three species: aqueous carbon dioxide [CO2 (aq)], bicarbonate (HCO3–), and carbonate (CO32–) (Doney et al., 2009). OA has resulted in bicarbonate rather than carbonate dominating in the water column; reducing the amount of carbonate available for marine calcifers (Sabine et al., 2004; Caldeira and Wickett, 2005). The reduction in carbonate is also linked to an excess release of H+ decreasing pH and leading to OA (Doney et al., 2009; Hall-Spencer and Harvey, 2019). Surface ocean average pH has decreased by 0.1 units since the beginning of the industrial revolution with a further decline (0.06–0.32 units) projected over the next century (Ciais et al., 2013).
Under current ocean conditions HCO3– is already widely available and CO2 is the smallest pool of DIC. CO2 will have the greatest percent increase in the next century (Koch et al., 2013). Seagrasses can use bicarbonate, but preferentially use CO2 for photosynthesis with rates experimentally increasing in response to elevated partial pressure CO2 (pCO2) (Invers et al., 1997; Jiang et al., 2010; Koch et al., 2013; Cox et al., 2015). Indirect responses to elevated pCO2 have been described as increases in above and below ground biomass (Zimmerman et al., 1997; Russell et al., 2013) as well as increases in flowering frequency (Palacios and Zimmerman, 2007). Increasing pCO2 may counteract the negative impacts of rising sea surface temperature (e.g., reduced photosynthesis, reduced growth, increased physiological stress, increased rate of mortality). Although the mechanism is unclear, Zimmerman et al. (2015) hypothesize that elevated pCO2 provides additional carbon reserves necessary to promote physiological repair despite thermal stress, thus inferring that increased pCO2 may improve landscape level resiliency (Zimmerman et al., 2015, 2017). For climax species like Zostera marina, it is still unclear how short-term changes in plant performance under high pCO2 fit into long-term carbon budgets or how elevated pCO2 will integrate into the life history of the plant (Campbell and Fourqurean, 2013; Takahashi et al., 2015; Cox et al., 2016). OA has the potential to stimulate seagrass parental investment into the production and quality of seeds to ultimately facilitate seedling establishment and increase population growth.
Remarkably little is known about how conditions experienced during the flowering stage influence phenotypic and genetic diversity of seagrass populations at large (Höckerstedt et al., 2021). A handful of published studies indicate a positive effect of OA on flowering frequency, seed quality, germination, and seedling carbon gains (Palacios and Zimmerman, 2007; Burnell et al., 2014; Hernán et al., 2016). Seeds generated at a high pCO2 (>1,550 μatm) may have higher carbon content and stored sucrose; thus, allowing an advantage in establishment and growth (Hernán et al., 2016). Additionally, when Ruppia maritima seeds were passed through fish digestive tracks (pH < 7), germination was facilitated. Therefore, low pH may trigger the rupture of the seed coat (Agami and Waisel, 1988). This is also observed in terrestrial species where low sediment pH (<7) induces germination for species like Stylosanthes humilis (Pelacani et al., 2005). Although low pH may enhance germination, studies focused on this topic are largely confined to the terrestrial realm with limited published material available for marine angiosperms.
Initiatives using seeds for seagrass restoration have primarily focused on small scale dispersal efforts except for the Chesapeake Bay, United States where large scale efforts resulted in the most successful restoration effort worldwide (Orth and McGlathery, 2012). Recent evidence suggests an upswing in bed surface coverage for some regions in Europe and southeast Florida, United States (Tomasko et al., 2018; de los Santos et al., 2019). Increases in coverage are associated with improved water quality and may be indirectly linked to alterations in carbonate chemistry as result of global change. More available (DIC) decreases dependency of improved water quality and provides more substrate available for photosynthesis (Zimmerman et al., 1997; Invers et al., 2002). Carbon enrichment may provide additional below ground carbon stores to bolster response to thermal stress (Zimmerman et al., 2015, 2017; Wilson and Lotze, 2019). This, in combination with hypothesized increases in parental investment, may serve to bolster restoration efforts and facilitate the establishment of meadows in locations now extant.
The aims of this study were twofold: (1) determine how OA influences flowering, seed production, seed quality, and seedling development of Zostera marina and (2) develop novel techniques geared toward rearing healthier, more viable seeds. We hypothesize that flowering shoots exposed to a short-term exposure of elevated pCO2 will increase spathe production and maturation, seed production, and investment into seed quality to ultimately enhance seedling development. Short term exposure to elevated pCO2 was used as a preliminary step to understand how low pH-high pCO2 will influence germination and seedling development as well as to determine if it is a feasible restoration technique. Secondly, we tested whether low pH-high pCO2 has direct effects on germination and seedling development. Previous evidence demonstrates the efficacy of low pH/salinity shocks to stimulate germination (Pelacani et al., 2005). We hypothesize an overall positive effect of increased pCO2 on the production and development of seedlings.
Five experiments were done to test for CO2 effects on flowering maturation, seed production and quality, and seedling development. An overview of experimental setup, images, and conditions can be found in Figure 1, Table 1, and Supplementary Figure 1.
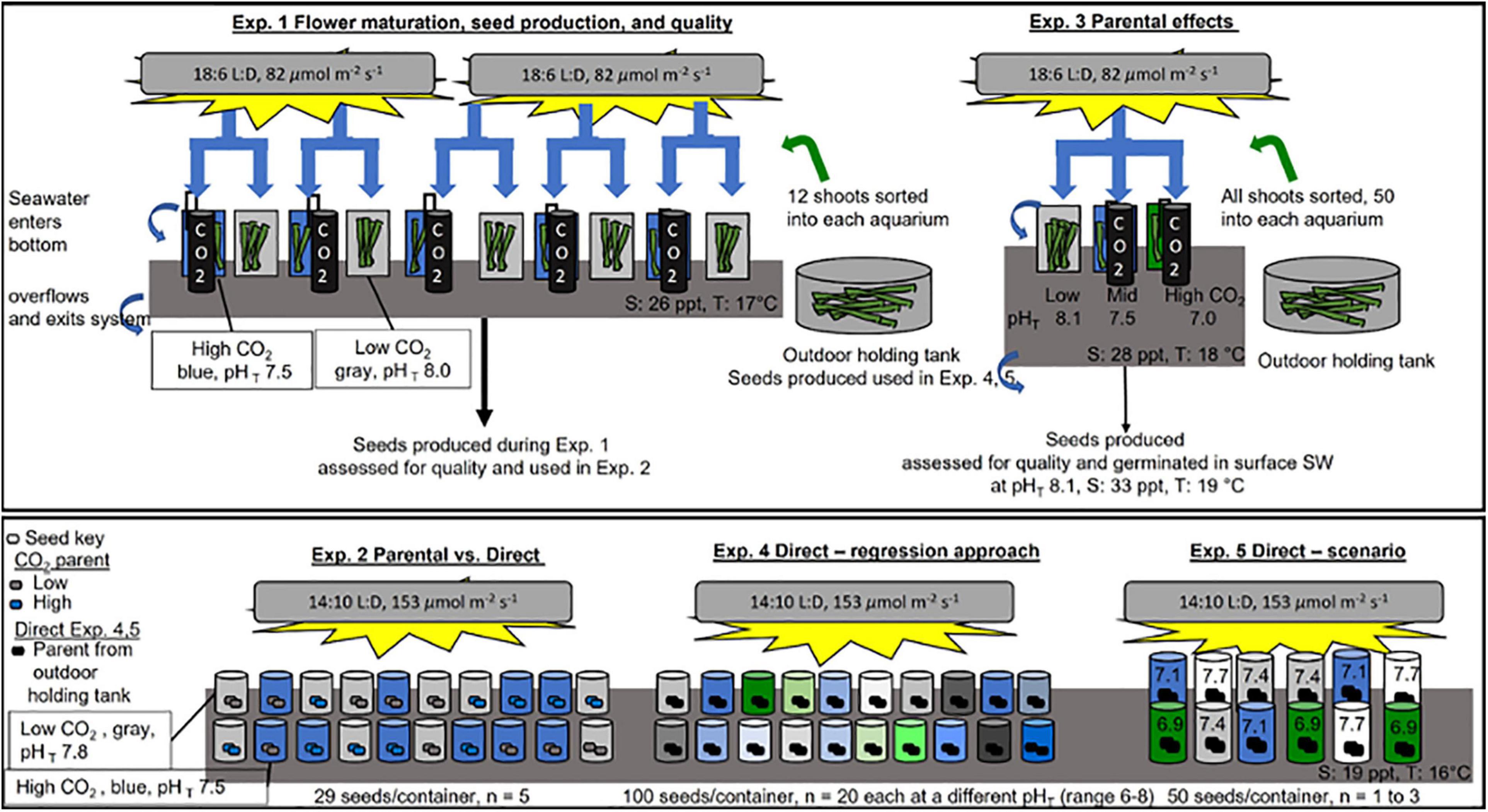
Figure 1. Schematic of experimental design for experiments (Exp.) 1–5. See Table 1 and see section “Materials and Methods” for more information. Exp 1 and 3 (top box) were done at Kristineberg Marine Research Station under a light bank, within a flow through system where surface supplied seawater (SW) entered the bottom of the containers, over flowed and exited the system (water flow shown by blue arrows). Flowering shoots of Zostera marina were collected on 17 July 2017 (Exp 1) and 9 August 2019 (Exp 3) near the station. Shoots were held overnight in an outdoor holding tank and then immediately sorted into replicate containers. Containers were held at appropriate CO2 treatments with a controller that sensed pH (black line) and regulated bubbling of CO2 (black cylinders) into seawater. Exps. 2, 4, and 5 (bottom box) were done at Dauphin Island Sea Lab in closed system placed within a cold room (16 C), under a light bank. SW at appropriate treatment levels was replaced in containers every 1–2 days.
Reproductive shoots of Zostera marina were harvested from two bays located on the West coast of Sweden, Gåsö (58°13′48.3N 11°23′43.7) on 17-July-2017 and Skallhavet (58°11′58.3N 11°26′34.6) on 9-August-2018. After harvesting, shoots were transported to the Kristineberg Marine Research Station. Shoots were used for two main tasks, (i) to test the effect CO2 on flowering maturation and seed development and (ii) to later test the effect of CO2 on seed germination and seedling development.
Shoots harvested for seed collection developed outdoors in a seawater (SW) flow-through 1,500 L tank until September (pHT 7.97 ± 0.06, temperature: 25.77°C ± 1.51; salinity: 21.96 ppt ± 3.98). The tank was aerated with compressed air to increase water mixing and prevent hypoxia. Shoots were covered with a PVC frame to submerge shoots just below the water to avoid desiccation [see Infantes and Moksnes (2018) for full description]. In September of both years, seeds were siphoned from the bottom of the tank and stored in the dark at 4°C, 32 ppt. Studies conducted at the Kristineberg Marine Research Station have shown that these conditions prevent germination (Infantes et al., 2016). Additional shoots, harvested to test the effect of elevated pCO2 on seed development, were held overnight and immediately used in laboratory experiments.
The effect of CO2 was tested by exposing flowering shoots to two treatments (n = 5): a low (∼380 μatm; pHT = 8.1) and elevated pCO2 (∼1,800 μatm; pHT = 7.5). In the fjords of Sweden, pH can have a diel variability of 0.9 units (minimum pHT ∼ 7.6) (Dorey et al., 2013). The low pH treatment is therefore outside the present range of variability and within range projected for 2,100 [−△0.3 pH units relative to the present natural variability following the RCP 8.5 scenario (Schwalm et al., 2020)].
One hundred and twenty flowering shoots were randomly distributed across ten 3-L containers with each container populated by 12 shoots. Shoots had an overall mean length of 61 ± 15.8 cm. The ten containers were arranged into pairs (n = 5) under a light bank (18:6 h light: dark cycle, 82 μmol m–2 s–1 at the canopy) with temperature ranging from 16.1 to 18.3°C and salinity ranging from 24–32 ppt. Light was reduced 20% from field observations, but this reduction was compensated for by using an elongated growing period (Björk et al., 2021). Seawater passed through a 500 μm filter entering the bottom of each container and continuously overflowed averaging a minimum of 5 turnovers per hour. Air was bubbled into both ambient and treated containers to prevent hypoxia and enhance water mixing. For clarity, individual flowers are referred to as spathes. Like seeds, spathes go through stages of development with earlier stages (1–3) being comparatively immature to later stages (5–6) (Infantes and Moksnes, 2018; Supplementary Figure 2). Prior to pH manipulation, spathes in stages 4 and 5 (seeds that are mature or have been released) were removed to ensure that the flowers matured, and seeds developed under experimental conditions for 69 days. Spathes continue to develop and drop seeds through September in temperate environments, therefore we carried the experiment through until all seeds developed and dropped (Infantes and Moksnes, 2018).
Five AquaMedic pH stat systems (controller, probe, CO2 tank) were utilized for each carbon treatment and controlled the delivery of pure CO2 into the containers. pHT was measured twice weekly in containers using a glass potentiometric probe (Metrohm 827 pH Meter) calibrated on total scale with TRIS and AMP buffers (Université de Liege) at a salinity of 35 ppt. The stat system was adjusted at each measuring interval to maintain targeted experimental conditions. At weekly timepoints, 80 mL of seawater was collected from each mesocosm, filtered through a Whatman G/F filter, and refrigerated. Samples were analyzed for Total Alkalinity (AT) using an SI Analytics TitroLine alpha plus. AT and pHT were used to calculate the carbonate chemistry of the system using CO2SYS v2.1 with dissociation constants set to Mehrbach et al. (1973) refit by Dickson and Millero (1987) (Lewis and Wallace, 2006).
Measurements of flowering maturation were initiated 1 week after the commencement of the experiment and assessed on nine to 10-day intervals for a total of 39 days following the methods of Infantes and Moksnes (2018). Under normal conditions, spathe development occurs at approximately one stage per week, thus our measuring intervals correspond to spathes naturally moving through development (Infantes and Moksnes, 2018). Number of spathes and number of developing and mature seeds within each spathe were measured on every flowering shoot. Flowering stage was also recorded for all spathes from each shoot as described by De Cock (1980). Flowering stages were classified as: (1) styles are erect from the spadix, (2) styles bent back after pollination, (3) pollen released from the anthers, (4) seed maturation commencing at 4–5 weeks, and (5) seeds are released (see Supplementary Figure 2). Any seeds observed at the bottom of each container were collected and counted. Seeds were held within the container of origin, inside a labeled submerged open-top watch glass. Because seeds fell on different dates, we maintained each batch in separate open-top watch glasses labeled with the date of collection.
Experimentally raised seeds were tested for viability using the “squeeze” method. Viable seeds develop a hard seed coat and do not compress with a pinch from tweezers. We also estimated seed quality from sinking velocities. A large 500 mL Erlenmeyer flask was filled with SW, marked, and then seeds were dropped one by one into the flask. A handheld stopwatch was used to time seed fall from the mark to the bottom (30 cm). Seeds with a falling velocity greater than 5.5 cm s–1 have a 95% chance of germination (Infantes and Moksnes, 2018). Seed quality may be related to seed size or mass. A microscope or zooscan was used to image all seeds at high resolution (105 pixels to mm). A scale bar was included in each image. These images were analyzed in ImageJ using a calibrated line and measure tool to determine seed length and diameter (width). Repeated measures on the same seed by data collectors showed reproducibility within 0.05 mm. Seed volume was calculated based on the volume of an ellipsoid π /6 L D2 following methods of Delefosse et al. (2016). Seeds not used in other experiments were placed in a drying oven at 60°C until dry and weighed to the nearest milligram on a microbalance.
A two-level factorial experiment was designed to evaluate the impact of seed seawater treatment (low vs. high CO2) and parental seawater treatment (low vs. high CO2) on seedling production. Seeds developed from the previous experiment were pooled into two groups (high and low CO2) and shipped to the Dauphin Island Sea Lab (DISL) in Mobile, Alabama (United States).
Large volumes of seawater from Mobile Bay were coarse filtered, aerated, and held in a cold room (16°C) until use in experiment. The mean pHT of this seawater for the area was 7.84 ± 0.05 with a salinity of 19.19 ppt. For clarity, the control treatment in this experiment was not modified from natural seawater carbonate chemistry found in Mobile Bay. In the carbon enriched seed treatment, a CO2 gas cylinder bubbled pure gas into seawater until the target pHT of 7.5 was reached. pH was measured using a glass potentiometric probe (InLab Routine Pro) calibrated on total scale using Dickson Certified Reference Tris (Batch 30) at a salinity of 33.4 ppt. After calibration, pHT and temperature was measured every other day prior to water replacement. Two 120 mL aliquots of seawater were collected from the holding tank, filtered on a Whatman GF/F filter (Riebesell et al., 2011), and immediately inoculated with 72 μL of 33% saturated mercuric chloride solution (HgCl2) and stored until analyzed for AT. A standard provided by Dickson (Batch 157) was used to check precision and accuracy (AT, 3.9 and 0.1 μmol kg–1, respectively; n = 7). The carbonate chemistry was calculated using pHT and AT using CO2SYS v2.1 with dissociation constants set to Mehrbach et al. (1973) refit by Dickson and Millero (1987) (Lewis and Wallace, 2006).
Seeds were sorted. An equal number of seeds (n = 29) that developed early, mid, or later in time were placed into 20–125 mL clear glass jars filled with seawater (19 ppt) and sealed. The jars were divided with respect to flowering treatment and carbonate chemistry (n = 5). The experiment continued for 32 days, and seedling development was assessed at the end (see Supplementary Figure 3). Seeds need approximately 30 days to germinate and move through development (Xu et al., 2016).
To isolate how parental investment influences germination and seedling development, flowering shoots were kept at three CO2 conditions and resulting seeds were allowed to develop under ambient pCO2. One hundred and fifty flowering shoots (see section “Materials and Methods,” Flowering shoot and ambient seed collection) were assigned to three 50 L containers at Kristineberg Marine Research Station. Before assignment, spathes in development stages 4 and 5 were cut from the rhipidia ensuring all seeds developed under treatment conditions. Each container was populated with 50 intact shoots. These plants were arranged, held, and maintained within the same flow-through system as described in the first experiment. Containers with submerged plants were exposed to one of three distinct CO2 seawater treatments: high, mid, and low CO2 condition corresponding to a pHT of 6.5, 7.4, 7.9 for 55 days. Two AquaMedic pH stat systems (see first experiment) were used to maintain pHT in the mid and high CO2 treatments, however, pCO2 conditions fluctuated in the low treatment (ambient) in the seawater flow-through system. The pHT was monitored and water was collected from containers for AT determinations at weekly intervals using the same protocols as in experiment 1. Titrations for AT and calculations for carbon speciation were done using the same protocol in experiment 1.
Seeds were collected from the bottom of each of the tank and tested for viability. Seeds were then stored separately at 4°C for 120 days to simulate a period of winter dormancy (Infantes et al., 2016; Infantes and Moksnes, 2018). Seeds were sorted into fifteen 1-L jars (13 seeds per jar) resulting in 5 replicates per parental CO2 condition. Seeds placed in containers developed for 30 days under ambient flow-through conditions under a saturating light field (18:6 h light: dark cycle, 82 μmol m–2 s–1 at the canopy) at 14°C (Dennison and Alberte, 1982). Seed germination and seedling development were assessed weekly and unlike earlier experiments, viability of ungerminated seeds was assessed at each time point using the squeeze method. Remaining seeds not used in experimentation were tested for viability by assessing sinking velocities.
To test the effect of high pCO2/low pH on germination, control seeds (developed in flow-through, outdoor holding tanks) were transported from Kristineberg Marine Research Station to DISL and randomly sorted into jars filled with seawater (19 ppt) and sealed. Twenty jars (125 mL) each received 100 seeds. Four targeted CO2 levels were selected: 900, 1,800, 4,800, and 7,700 μatm (corresponding to a pHT of 7.8, 7.5, 7.1, 6.9) with 5 replicates each. However, biological respiration in experimental units lowered pH from targets leading to differences between replicates. pH was monitored rigorously (up to twice daily), and water changes were frequent (1–2x daily), but the carbonate chemistry did vary through time so that each jar had different pH-pCO2 environment. Therefore, the response of seeds/seedlings in each jar (n = 20, no longer n of 5 at selected levels) with its corresponding measured carbon enriched conditions was used in a regression approach. Seeds were sorted on December 18, 2017, and experimental conditions lasted for 41 days. Development was assessed on four intervals: day 9, 17, 29, 40.
The experiment was conducted using 12–250 mL jars with each replicate receiving 50 seeds. The larger volume and reduced seed numbers were used to carefully control pH and robustly test for direct effects of CO2 on germination and seedling development. The same CO2 conditions as in experiment 4 were targeted (corresponding to pHT 7.7, 7.4, 7.1, 6.9) with 3 replicates each. Carbon enriched conditions are not representative of ocean acidification projections within the next century but were selected to examine replicable physiological responses for enhancing restoration. Furthermore, the experiment was started December 23, 2017, and continued for 27 days. Development was assessed at day 22.
For both experiments testing direct pH effects (experiment 4 and 5), seeds were kept in a closed system (jars) at 16°C under a fluorescent light bank (153.7 ± 89.4 μmol m2 s–1 as measured across replicate jars) on a 14:10 h light: dark cycle. These light conditions were selected to mimic spring conditions at a shallow depth (∼1.5 m) in the fjords where flowering shoots were collected. To remove any location bias, jars were haphazardly rotated in position within a cold room daily. seawater at the specified pHT was replaced in jars once to twice daily to ensure treatment conditions were maintained and water remained oxygenated. Seawater (19 ppt) was collected at DISL mesocosm facility from Mobile Bay and manipulated using the system described above. pHT was measured daily within the jars. Aliquots of seawater (n = 1–5 per measuring interval) were collected periodically from the mesocosm facility holding tanks, filtered, dosed with HgCl2, and used for AT determinations at end of study. AT and carbonate chemistry calculations follow the protocol outlined in experiment 2.
At each interval, the stage (0–6) in seedling development was also noted for each seed or seedling in a replicate jar. We followed the schematic outlined by Xu et al. (2016) which can be observed in Supplementary Figure 3: stage 0: intact seed coat; stage 1: germination as the emergence of the cotyledon and hypocotyl; stage 2: pre-seedling stage with no true leaf or adventitious roots, but the cotyledon and hypocotyl are elongated; stage 3: seedling stage reached with the emergence of first true leaf; stage 4: development of adventitious root; stage 5: development of second true leaf; and finally stage 6: intact seedling lacking cotyledonary blade and develops a third leaf. The length of each seedling (stages 3–6), from root tip to longest leaf height was also assessed to the nearest mm.
Prior to all parametric analyses, residuals were plotted and examined for normality. Prior to all t-tests and ANOVAs, data were also tested for homogeneity of variance using the Levene’s test.
Data on sinking velocity from experiment 1 were natural log transformed to meet parametric requirements prior to use. Data were averaged to produce one value per replicate experimental unit. To analyze the dataset from the first experiment (flower maturation and spathe production), a series of repeated measures two-way ANOVAs with the container as a random effect and date and treatment (and their interaction) as fixed factors were used to test for differences in (1) the number of spathes in development stages (1–5), (2) the number of spathes produced, and (3) the number of mature seeds in the spathes. Holm–Sidak multiple comparison procedure was used to identify pairwise differences when a main effect was found. A series of t-tests were applied to examine seed quality (viability with squeeze method, sinking velocity, volume, and dried weight) and quantity of seeds produced. In addition, a t-test was used to test for differences in percentage of population with reduced chance of germination (sinking velocity below 5.5 cm/s).
A series of two-way ANOVAs tested whether parental condition and the water condition surrounding seeds and seedlings, and the interaction, contributed to (1) the number of seeds which germinated, (2) maximum stage reached in seedling development, and (3) the total number of seedlings (stages 3–6) produced at the end of the study. Differences in seedling height between treatments could not be robustly tested because of the limited replicates within the same stage (4, 5, or 6) at the end of the study.
For the dataset generated from experiment 3, differences in germination success (percentage of viable seeds entering stage 1) and germination rate among the three treatments were examined using a two-way repeated measures ANOVA with time (weekly sampling interval) and treatment as fixed factors with container as a random subject factor. Lastly, we considered each seed a replicate from one of the three container populations when testing for the effects on sinking velocity. Holm–Sidak multiple comparison test was used to determine pairwise differences when a main effect was found. One-way ANOVA (on ranks) due to failing the assumptions of normality was then used to test for differences in sinking velocity among the three CO2 treatments, followed by a Dunn’s multiple comparison procedure to determine pairwise differences.
To test the direct effects of CO2 on seed germination, seedling production, and seedling size, a series of orthogonal least squares linear regressions were done with pH to ease data visualization. The mean pHT and lowest pHT measured for jars from the start of the experiment to the sampling interval (1, 2, and 3) was regressed with the non-cumulative count of seeds that germinated (stage 1) between intervals. This approach was taken to account for the cumulative pH-CO2 conditions that seeds experienced and possible delays in effects on germination. At the end of the study (interval 4), the mean pH and lowest pH of jars during the entire course of study was used to predict (separately) the cumulative number of germinated seeds, and seedlings produced. Separate general linear models with seedling stage as a covariate was used to test the effects of CO2 (mean pHT and lowest pHT from initiation of experiment through interval 4) on seedling height at the end of study.
In experiment 5, using larger volumes and fewer seeds to maintain chemistry, separate one-way ANOVAs were used to test for differences in the cumulative number of germinated seeds and the total seedlings produced at the end of the study. To ensure treatments were discrete, when the deviation in pH overlapped with another treatment, the data from that replicate was removed prior to any analyses. This resulted in an n of 1–3 for each CO2 level. Differences in seedling height were not analyzed because of the few numbers of replicate jars with seedlings within the same developmental stage by the end of the experiment duration.
Low (pHT = 7.97 ± 0.06, pCO2 = 508.4 ± 86.46 μatm) and high (pHT = 7.53 ± 0.07, pCO2 = 1547.2 ± 267.60 μatm) CO2 conditions were maintained for 69 days and monitored for the first 39 days (Supplementary Table 1). The low CO2 condition has a carbonate chemistry within the present range of variability reported in local surface waters (Dupont et al., 2013). All values are reported as means (±SD).
Flowering maturation, number of spathes produced, and the number of seeds in development within spathes did not differ significantly between CO2 conditions (Table 2 and Figures 2, 3). The flowers developed though time following a typical maturation pattern (Figure 2 and Table 2). The number of spathes in stage 1 was greatest on day 1 (low CO2 condition: 51.8 ± 8.2, high CO2 condition: 53.8 ± 13.2) and declined through time, while the number of spathes in stage 3 was highest on day 9 (low CO2 condition: 56.0 ± 12.3, high CO2 condition: 54.4 ± 9.9). The highest number of spathes in stage 4 (low CO2 condition: 101.4 ± 18.5, high CO2 condition: 98.6 ± 26.5) and in stage 5 (low CO2 condition: 77.2 ± 16.8, high CO2 condition: 66.2 ± 11.9) occurred on day 19 and 39, respectively.
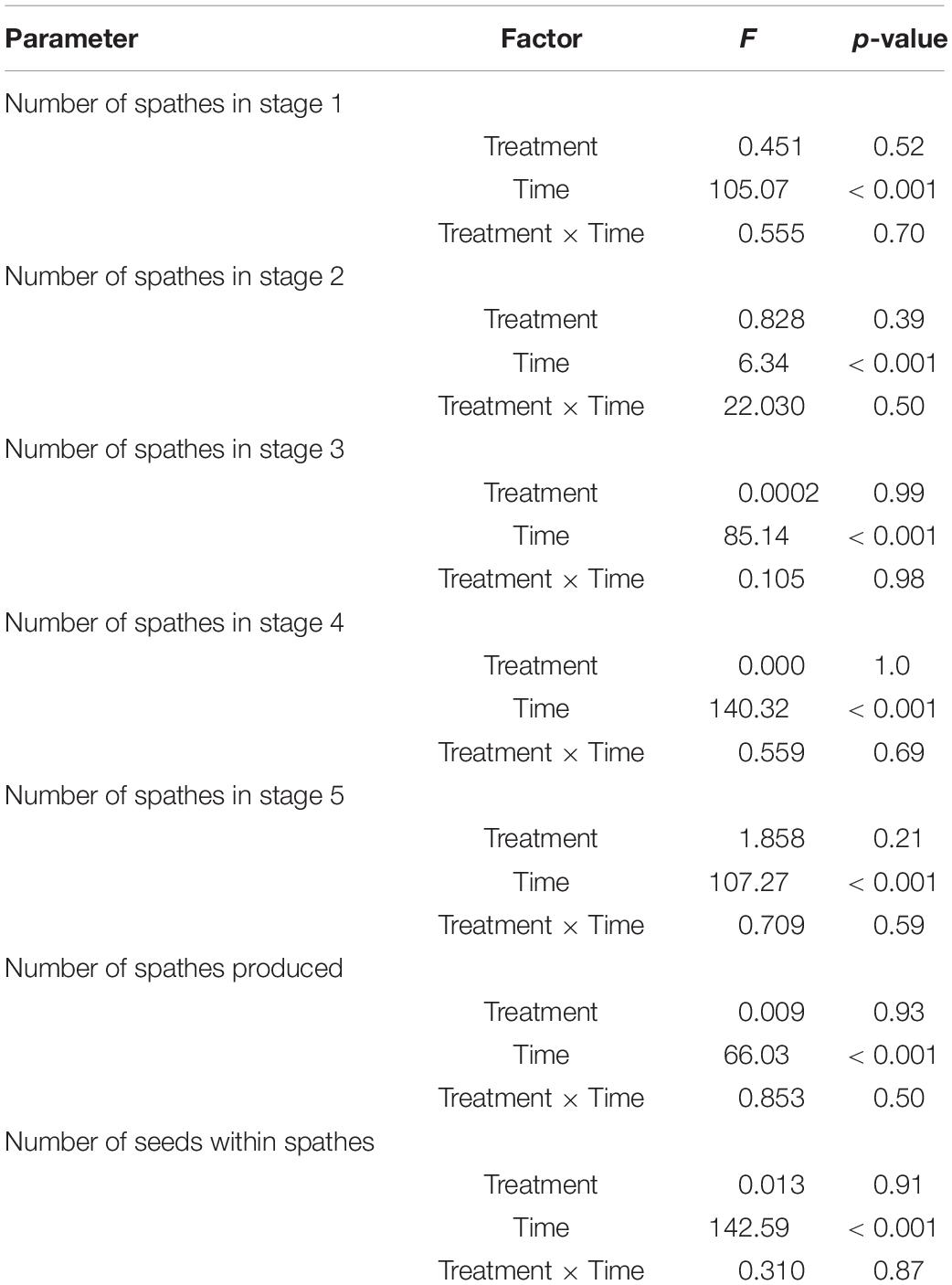
Table 2. Results of repeated measures two-way ANOVA testing for the effects of treatment condition (degrees of freedom, df = 1), time (df = 4), and their interaction (df = 4) on flower maturation (tested for differences in stages 1–5) and, spathe and seed production (Experiment 1).
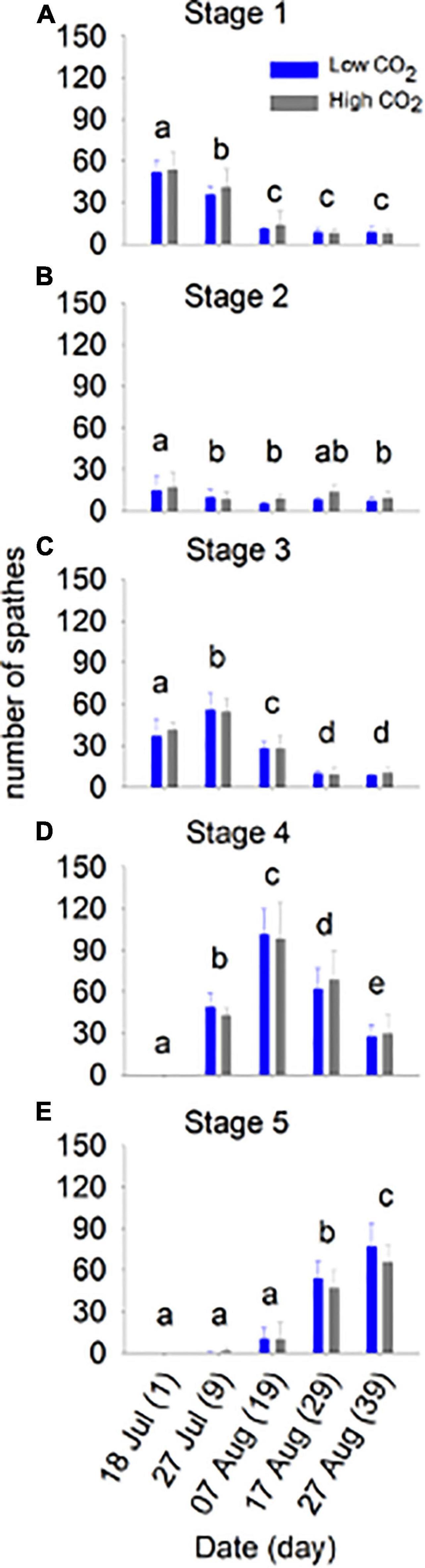
Figure 2. Flower maturation at low and high CO2 conditions for 39 days (Experiment 1). The number of spathes (mean ± SD) in stages 1–5 (top to bottom) are shown for each day (n). The different letters above bars represent statistical differences between times as specified by the results of a Holm–Sidak pairwise comparison test following a repeated measured two-way ANOVA, Table 2.
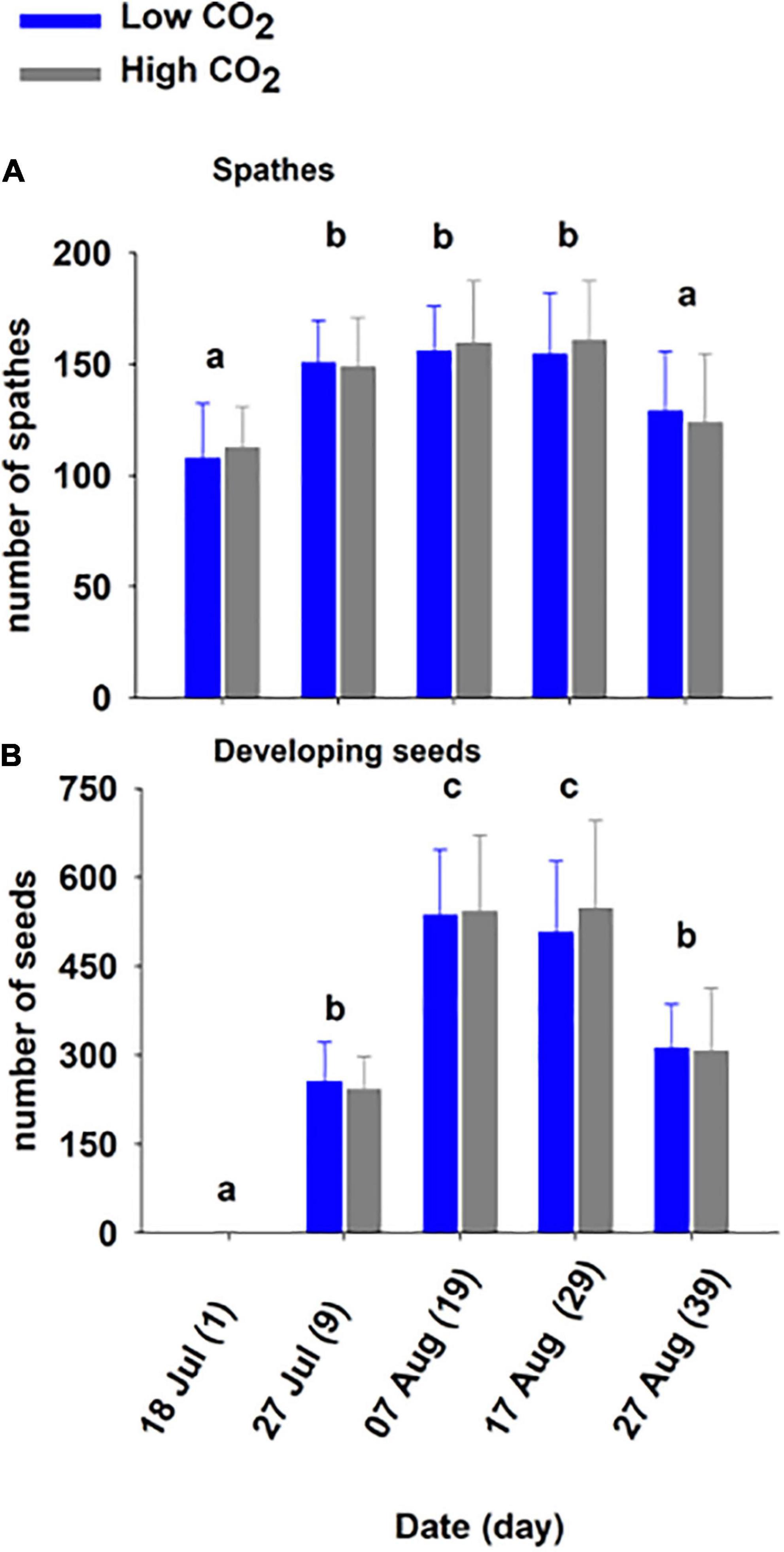
Figure 3. Mean (± SD) spathes (A) and seed number (B) on shoots held at low and high CO2 condition for 39 days (Experiment 1). The different letters above bars represents statistical differences between time as specified by the results of a Holm–Sidak pairwise comparison test following a repeated measure two-way ANOVA, Table 2.
The number of spathes also increased significantly through time (Figure 3A and Table 2). The number of spathes produced increased from day 1 (low CO2 condition: 107.8 ± 24.7, high CO2 condition: 112.4 ± 18.6) to day 9 (low CO2 condition:150.8 ± 19.0, high CO2 condition: 148.8 ± 22.1) and declined in number from day 29 (low CO2 condition: 154.6 ± 27.5, high CO2 condition: 160.8 ± 26.5) to day 39 (low CO2 condition: 129.0 ± 26.9, high CO2 condition: 123.8 ± 31.0). This is congruent with the observation that many spathes became brittle and broke off from shoots in later stages of development and were not counted.
The number of seeds developing reflected flowering maturation (Figure 3B) and was significantly impacted by time (p < 0.001; Table 2). The number increased from July—day 1 and 9 (low CO2 condition: 256.0 ± 66.1, high CO2 condition: 242.2 ± 55.9) to early August—day 19 and 29 (low CO2 condition: 536.4 ± 110.9, high CO2 condition: 542.4 ± 128.4) and the mean number (low CO2 condition: 311.6 ± 74.6, high CO2 condition: 306.8 ± 106.5) was lower at the end of August—day 39, as seeds matured and were released.
After 69 days a pooled total of 2,017 seeds were released from plants and 226 of these seeds were deemed non-viable using the “squeeze” method. Plants from the high and low CO2 conditions produced a similar number of seeds (Table 3). Furthermore, all measured metrics (number non-viable, sinking velocity, volume, and dried weight) indicated seeds formed under high CO2 conditions were no different in quality than those developed at low CO2 conditions. Overall seeds (n = 1,903) sunk to 30 cm in 4.5 ± 0.6 s with a mean velocity of 6.67 cm s–1, seed length and width (n = 2,010) were 4.42 ± 0.377 and 1.96 ± 0.217 mm, respectively, and seed dried weight (n = 1,001) was 3.136 ± 0.853 mg.
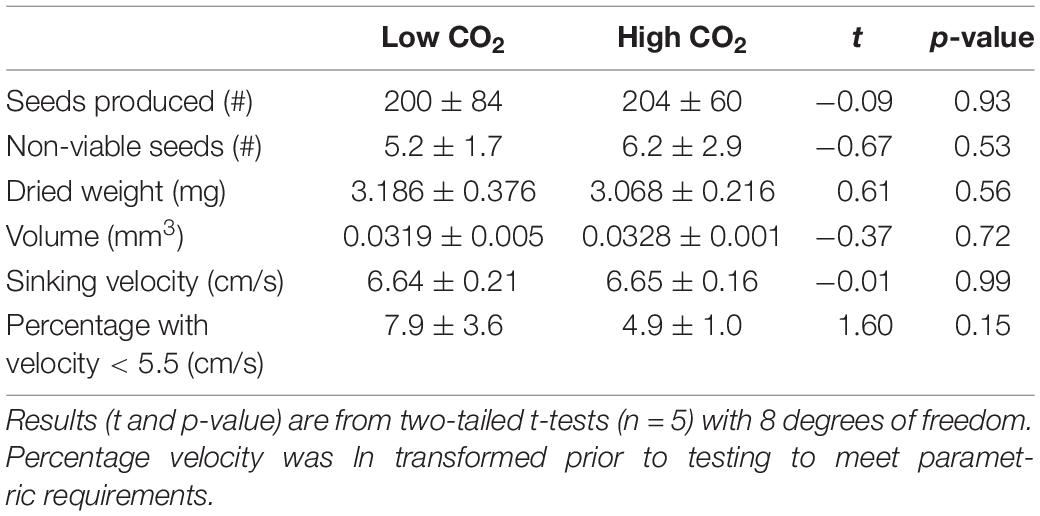
Table 3. Mean (± SD) of seed quantity and metrics of seed quality produced from flowering shoots maintained in low and high CO2 conditions for 69 days (Experiment 1).
Seawater chemistry was maintained for the entirety of the experiment (32 days): low CO2 condition (pHT = 7.84 ± 0.05; pCO2 = 714.85 ± 67.28 μatm) and high CO2 condition (pHT = 7.55 ± 0.05; pCO2 = 1381.9 ± 125.50 μatm) (for parental CO2 condition, see Supplementary Table 1; for more complete carbonate chemistry, see Supplementary Table 2). Overall germination success (mean 6.9% ± 5.5) and seedling production (total of 18 seedlings out of 580 total seeds) was low within the 32-day period. Despite low values, seed germination was significantly (∼2x) greater when the parental generation was from the high CO2 condition (3.2 ± 1.7 vs. 1.5 ± 1.0, Figure 4 and Table 4). Greater germination success translated into 2x greater mean number of seedlings (high CO2 parents 1.2 ± 0.9 vs. low CO2 parents 0.6 ± 0.7) and an increased mean maturation as gauged by maximum stage reached at end of the study (high CO2 parents 3.7 ± 1.9 vs. low CO2 parents 2.3 ± 1.8, Figure 3). However, there was high variation among replicate jars and these metrics (i.e., seedlings produced and maturation) were not found to be statistically different between treatments. There was also no indication of an effect of CO2 on germination, nor an interaction of parent and seed water condition, on germination success, seedlings produced, and maximum stage reached (Table 4). It is anecdotal, but worth nothing that at the end of the study, seedlings in stage 5 and 6 within high CO2 condition were taller (Stage 5 = 3.7 cm; Stage 6 = 4.3 cm) than those in the low CO2 condition (Stage 5 = 2.2 cm; Stage 6 = 3.5). Statistical analysis could not be performed due to limited number of replicate seedlings within the same stage of development (Supplementary Figure 3).
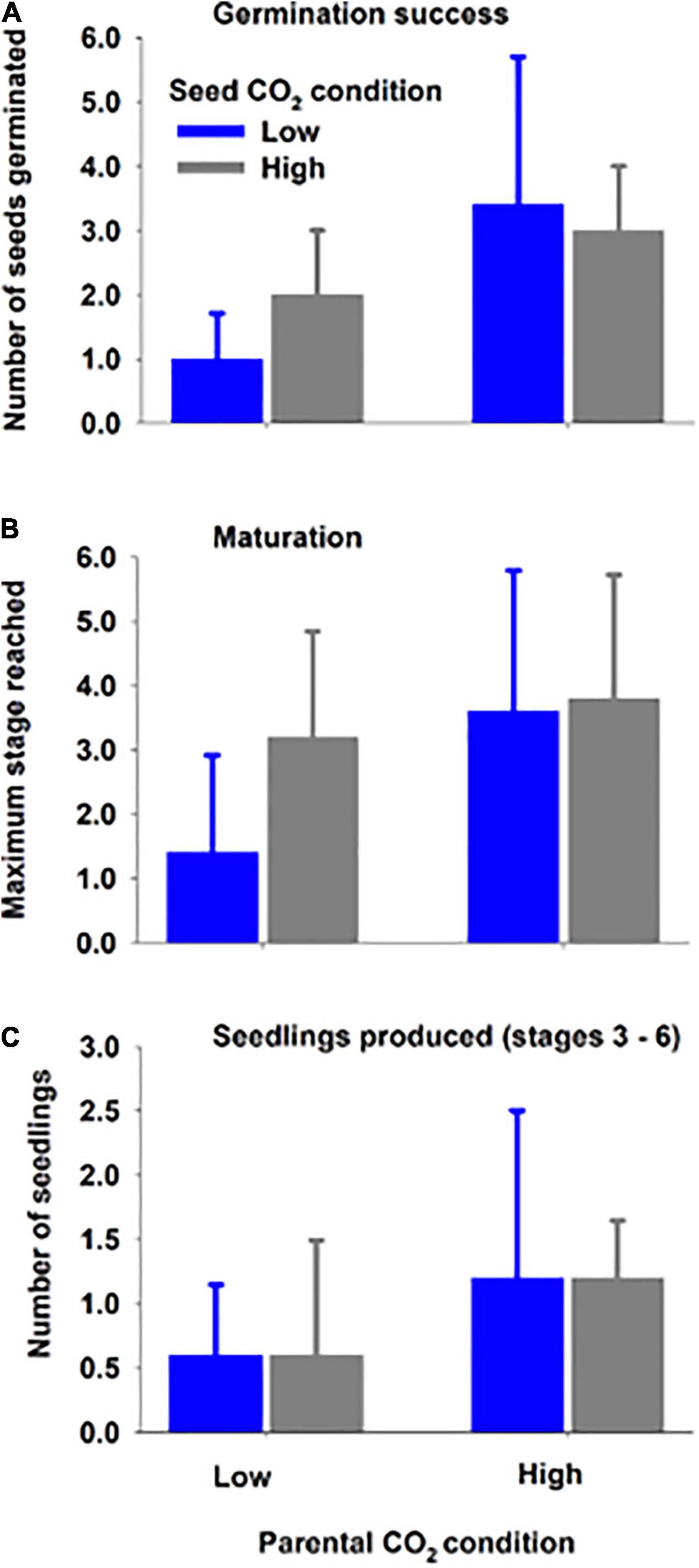
Figure 4. Mean (± SD) germination success (A), seedling maturation (B), and seedlings produced (C) at the end of study for seeds developed (parent) under high or low CO2 condition and placed (seed) within water with high or low CO2 condition for 32 days (Experiment 2). Results of two-way ANOVAs (Table 4) indicate that the high parental CO2 condition resulted in greater germination success.
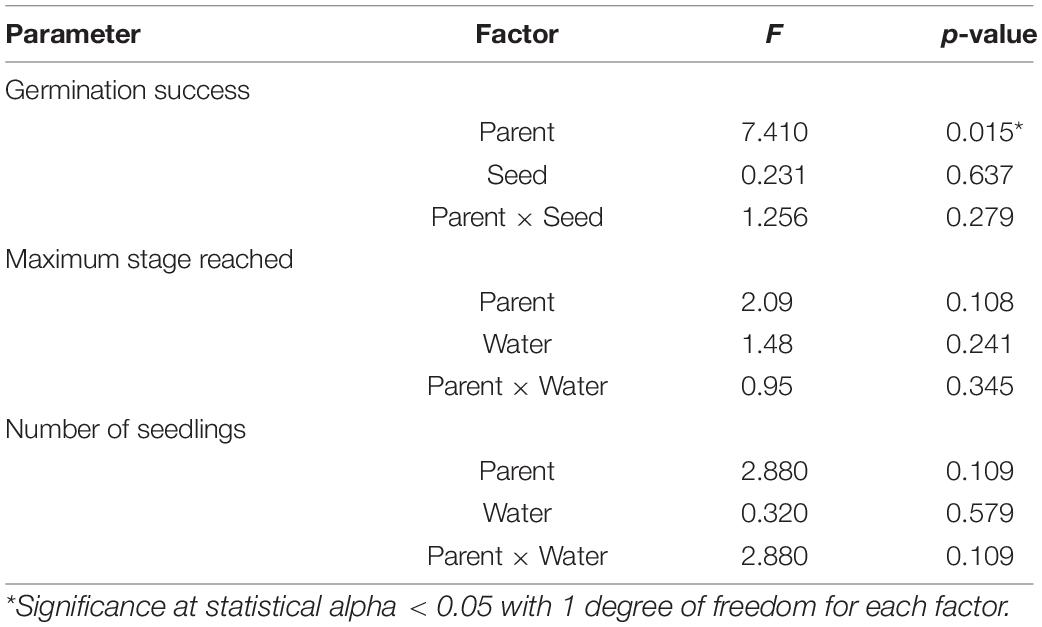
Table 4. Results of two-way ANOVAs testing for parental (parent) and direct (seed) CO2 effects on germination success, seedling maturation (as maximum stage reached), and number of seedlings produced (Experiment 2).
Three treatments corresponding to high, mid, and low parental CO2 condition (pHT = 6.96 ± 0.15, 7.54 ± 0.24, 8.13 ± 0.08) were maintained for 55 days (additional parameters are reported in Supplementary Table 3). Once seeds were harvested and transferred for germination, ambient water (pHT 8.1 ± 0.06) from Gullmarsfjord was used (Supplementary Table 4). There were 102 seeds produced from flowering shoots (45, 35, and 27 seeds from the high, mid, and low parental CO2 treatments, respectively). Seeds sunk at a velocity of 6.12 ± 1.3, 5.6 ± 1.6, 3.7 ± 1.8 cm s–1 for the populations, respectively (Figure 5). 75.0, 68.2, and 14.3 % of the seed population from the high, mid, and low parental CO2 condition sunk at a velocity greater than 5.5 cm s–1 a cutoff indicating greater than 95% chance of germination. There was a significant effect of parental condition on sinking velocity (ANOVA: df = 2, H = 16.0, p < 0.001). Thus, low parental CO2 condition produced a seed population with distinctively slower sinking velocities compared to the other two with carbon enriched parents (p < 0.05). Yet, percent germinated out of viable seeds (Figure 6) did not vary by parental CO2 condition (two-way ANOVA: df = 2, F = 1.24, p = 0.32, treatment × time: df = 8, F = 0.39, p = 0.32). Instead, germination increased through time (ANOVA: df = 4, F = 36.1, p < 0.001). A greater number of seeds germinated on day 23 and day 30 than on day 1, 8, and 16. Out of the total seeds that germinated (25.6 % ± 9.5) only 2 seeds developed and survived into seedling stage 3 by the end of the experimental period.
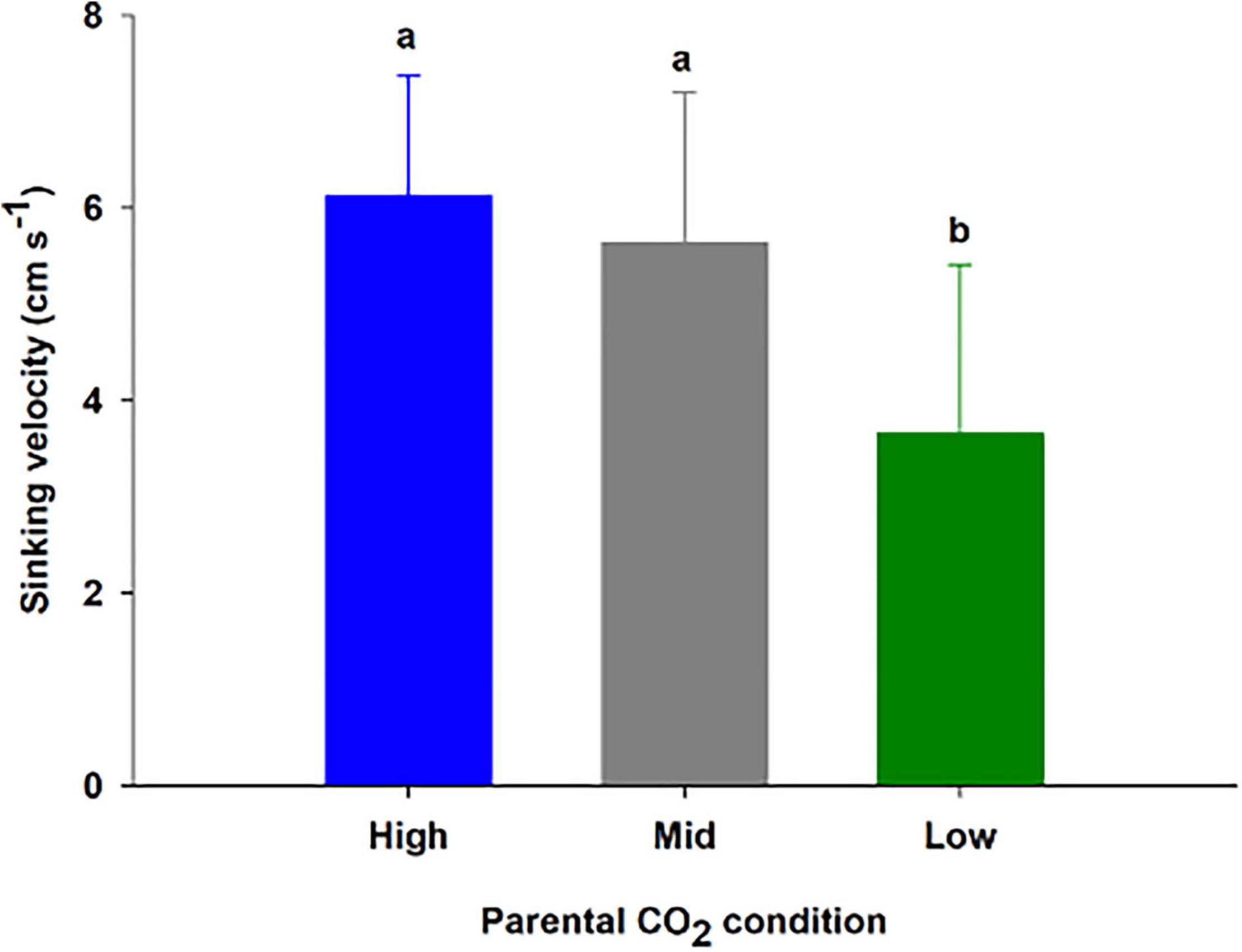
Figure 5. Sinking velocity (mean ± SD) of 14–24 seeds produced from flowering shoots kept at three CO2 conditions (high, mid and low) for 55 days (Experiment 3). Conditions correspond to a pHT of 6.96, 7.54, 8.13 (Supplementary Table 3). The different letters above bars represent statistical differences between conditions as specified by the results of a Tukey’s pairwise comparison test following significance from a one-way ANOVA.
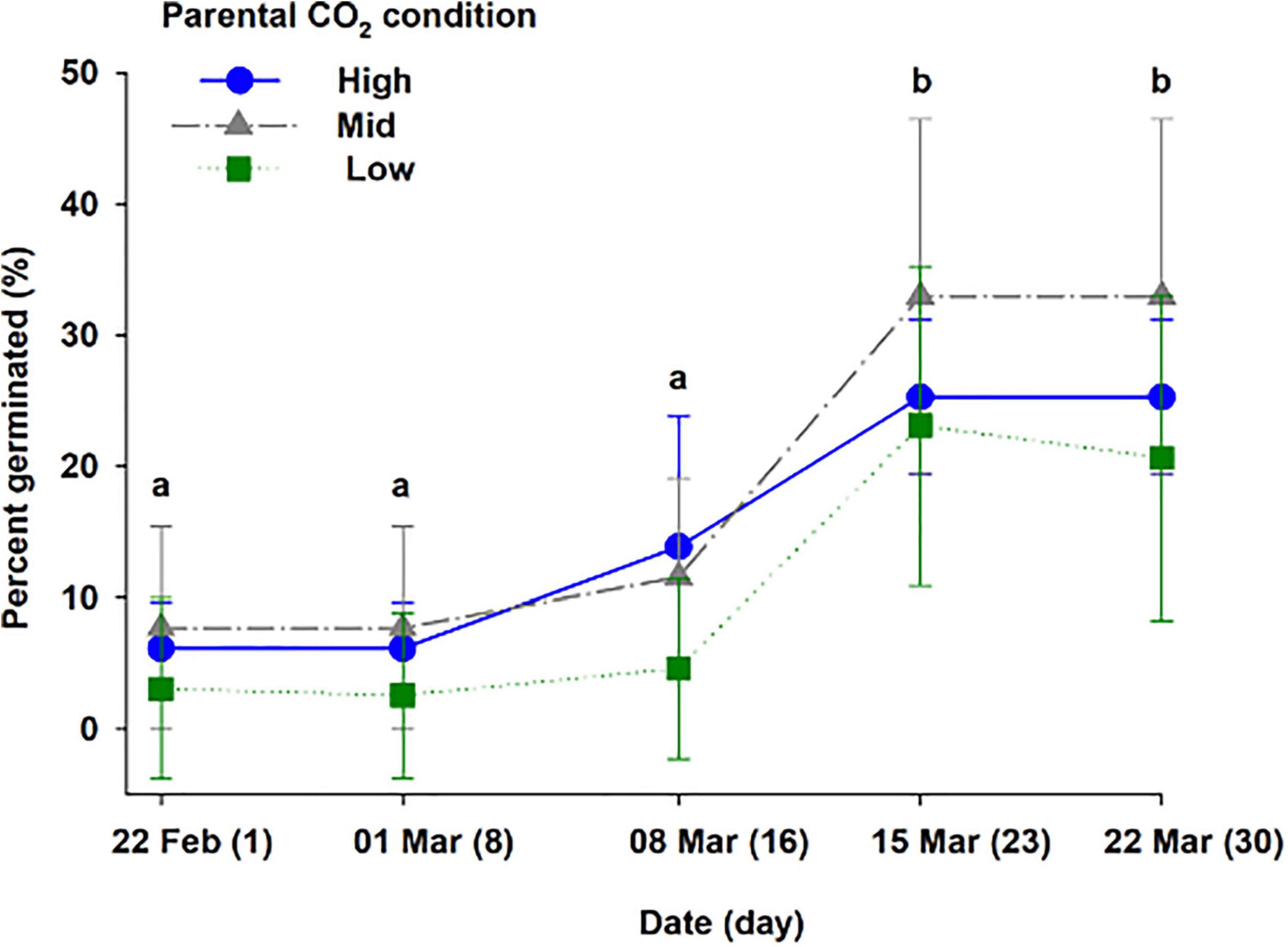
Figure 6. Percent germinated out of viable seeds harvested from flowering shoots kept at three CO2 conditions (high, mid, and low), corresponding to a pHT of 6.96, 7.54, 8.13 (Experiment 3, Supplementary Table 3). The different letters above bars represents statistical differences between days of study as specified by the results of a Holm–Sidak pairwise comparison test following a repeated measure two-way ANOVA. The ANOVA model indicated a main effect for time (p < 0.001) but, not for treatment level nor for an interaction of time × treatment.
On average 15.2 % ± 4.1 of the seeds in jars (100 per jar) germinated to produce a total of 191 seedlings (out of 2,000 used) in 41 days. Seawater treatments were reported in intervals (see Supplementary Tables 5, 6) and integrated to capture the dynamic carbonate chemistry driven by biological activity experienced by the seeds and seedlings over the course of the experiment The pHT of the experiment ranged from 6.00–7.99 across experimental units for the duration of the experiment (Supplementary Tables 5, 6). The target pH for each treatment (which negatively correlated with mean pCO2) repeatedly failed to predict number of germinated seeds (intervals 1–3), the cumulative number germinated (at end of study), and the number of seedlings produced at the end of the study. This was evident from the high, non-significant p-values (0.29–0.77) and very low R2 values (ranging from 0.0 to 7.9%) reported from orthogonal least squares linear regression models (Supplementary Table 7 and Supplementary Figure 4). Germination and seedlings produced were also not related to the maximum pCO2 (minimum pHT) that seeds experienced (Supplementary Table 7 and Supplementary Figure 4). Furthermore, seedling height significantly differed with stage in development, yet it was not related to CO2 conditions (Supplementary Figure 5, ANCOVA mean pH, stage df = 1, F = 17.2, p < 0.001, pH df = 19, F = 1.3, p = 0.27; ANOVA lowest pH, stage df = 1, F = 13.11, p = 0.001, pH df = 15, F = 0.91, p = 0.56). Seedlings in stage 3 of development had a height of 2.60 ± 0.65 cm. Seedlings in stage 4, 5, and 6 of development were larger, increasing in size with development stage, 2.83 ± 0.42, 3.00 ± 0.66, and 3.42 ± 0.66 cm respectively.
Three out of twelve jars with variable conditions (pH ≥ 1.5 SD) were removed from this dataset (Supplementary Figure 6) to ensure the carbonate chemistry was tightly controlled and results could be contrasted with the earlier experiment where the carbonate chemistry was variable through time. It should be noted that the overall outcome and conclusions drawn from the full dataset are congruent to those reported with the reduced dataset. In the reduced dataset seawater treatments had minimal deviation: pHT 6.88 ± 0.01, 7.10 ± 0.00, 7.40 ± 0.02, 7.68 ± 0.15 (for a more complete characterization, see Supplementary Table 8). The total number of seeds germinated was 13.3 % ± 4.0 out of 50 seeds used and the mean number of seedlings produced was 3.2 ± 1.5 per jar. The number of seeds germinated statistically differed between treatments (Supplementary Figure 6, df = 2, F = 10.9, p = 0.015) yet, pairwise comparisons did not show any trend with increasing CO2 (Supplementary Figure 6). Furthermore, the number of seedlings produced did not differ between CO2 conditions (df = 2, F = 2.2, p = 0.21). Seedling height was plotted and can be found in Supplementary Figure 7.
Our results provide a better understanding of how increased CO2 affects three potential bottlenecks (seed production, germination, and seedling production) in eelgrass establishment from seed (Table 5). Firstly, results indicate that flowering shoots exposed to brief periods of high CO2 produce a greater proportion of viable seeds (experiment 3, Table 5). Secondly, results indicate that the germination success of Z. marina is greater in seeds produced by parents maintained in high CO2 (experiment 2 and 3, Table 5). Thirdly, results provide insight into the mechanism driving this response. Increased germination does not appear to be directly related to the conditions which surround the seed but rather stem from a carryover effect from parental exposure. A common practice for bed restoration is to harvest flowers, develop seeds, and then distribute seeds to the targeted location to allow for germination and bed development. Therefore, once flowering shoots have been collected and returned to laboratory settings, bubbling CO2 into the seawater holding tanks could help bolster germination success and facilitate bed recovery. However, we found that exposure to elevated CO2 conditions did not significantly translate into seedling success (experiment 2–5, Table 4). The small percentage of seedlings established in the present study highlight the vulnerability of this life stage. Our results give further evidence for the precarious nature of seed germination and seedling development. Efforts in the future must be made to standardize experimental conditions to make more direct comparisons and to clarify the role of other environmental metrics in these outcomes.
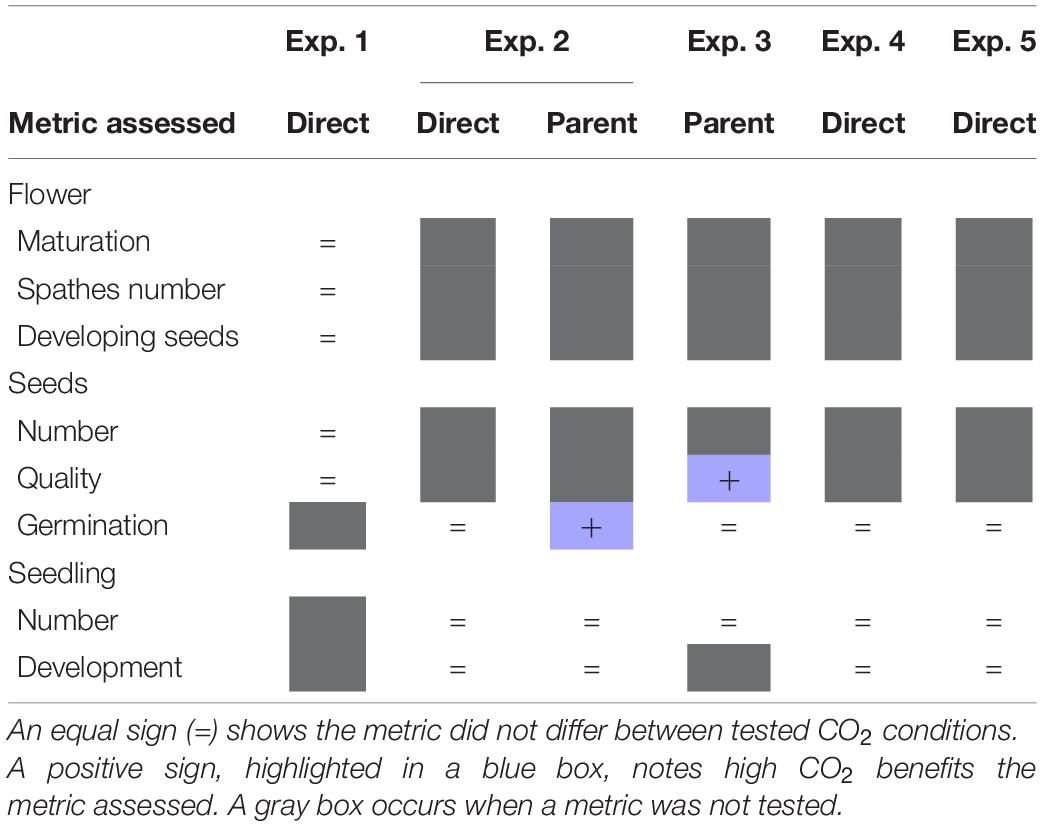
Table 5. Summary of outcomes for experiments (Exp.) 1–5 testing direct or parental CO2 effects on flower, seed, and seedling metrics.
Resource availability (e.g., light and ammonium) can influence spathe and seed production for Zostera marina (van Lent and Verschuure, 1994; Jackson et al., 2017; Johnson et al., 2017). A yearlong study of carbon enrichment found flowering to occur earlier, maturation to occur faster, and an increased percentage of flowering to vegetative shoots for Z. marina, with significant affects occurring at extreme OA (pHT = 6.4) treatment (Palacios and Zimmerman, 2007). Increased flowering production is also described as a response to environmental stressors (e.g., turbidity, temperature, salinity) (Phillips et al., 1983). Our study intended to test the use of elevated pCO2 as a dose injection to bolster seed production or seed quality to ultimately increase seedling biomass. We found flowering maturation patterns to reflect field observations (Infantes and Moksnes, 2018) and we did not find any statistical evidence supporting the effect of CO2 on flowering development or seed production. However, duration and magnitude of resource availability (e.g., light, ammonium) need to be considered since both variables are important in seagrass allocation from vegetative growth to sexual reproduction (Johnson et al., 2017; Qin et al., 2021). Indeed, in experiment 5, under a larger CO2 gradient, seed quality increased with increasing pCO2.
Positive parental CO2 effects for early seedling development were observed in two experiments, yet the metric affected (germination vs. sinking velocity) was different. A large part of the seed tissue is of maternal origin and thus, significant increases in seed mass (proxied by sinking velocity) are important indicators for increased investment in offspring (Wulff, 1995). Seeds developed at extreme CO2 condition (pCO2 > 1,600 μatm, experiment 3) had faster sinking velocities and provide evidence for greater parental investment (via increased rate of photosynthesis corresponding to more stored carbon) into seed quality. Increased germination was observed in experiment 2 when more moderate enrichment (pCO2 < 1,600 μatm) was applied to flowering shoots. Presumably this increased germination is also the result of greater parental allocation into seed quality, even though statistical testing indicated the metrics assessed (e.g., volume, weight, sinking velocity) were unaffected. These two experiments were conducted at different temperatures and salinities both of which are important environmental factors to consider for germination success (Agami and Waisel, 1988; Tanner and Parham, 2010). Experiments conducted at DISL were conducted at 19.19 ppt, 16°C and showed a significant increase in germination compared to experiments performed at the Kristineberg Marine Research Station where conditions were 33.25 ppt, 19.5°C. Orth and Moore (1983) report that experiments conducted in cooler, less haline environments have higher germination rates. An additional study conducted by Niu et al. (2012) demonstrated that the ideal temperature range for seedling development and establishment is between 16 and 17°C. Experiment 2 not only had the highest germination rate, but also the highest percentage of seeds developing into seedlings (62.8%). This is presumably due to ideal conditions for the early life stage of Z. marina.
Sinking velocity and other measured metrics of seed quality are not consistent indicators for germination. Maternal environment in conjunction with abiotic variables (e.g., pCO2, salinity, temperature) are powerful covariates in predicting germination success (Jarvis and Moore, 2015). Because of our comparatively small sample sizes, our results may also be attributed to Type II error. When germination success favored seeds from high CO2 parents (experiment 2), it corresponded to a greater mean proportion of seeds from low CO2 parents with sinking velocities below 5.5 cm s–1. Furthermore, in experiment 3, when sinking velocity differed among CO2 treatments, seeds from mid and high CO2 parents had a greater mean germination success than seeds from low CO2 parents. The content of seeds was not directly measured in the present study (dried seeds were lost in a laboratory fire) and we suggest future studies examine seed content to confirm the described mechanism benefitting germination. We also suggest future experiments standardize environmental conditions and repeat experiments using greater sample sizes to clarify disparate outcomes between predictors of germination and measured germination success.
Increased CO2 appears to have allowed shoots to allocate more carbon into seed content to facilitate viability and germination. Zostera marina seeds on average are composed of 52% starch, 13% protein and 4% minerals with variation in these ratios influencing development (Delefosse et al., 2016). Heavy seeds correlate to increased sinking velocities and contain more microgram per gram carbon in the form of starch to benefit germination (Delefosse et al., 2016; Jorgensen et al., 2019). Energy and nutrients stored within the hypocotyl is consumed during germination and higher energy reserves assist in the development and emergence of the radicle and leaf primordium (Kuo and Kirkman, 1992). Seeds with sinking velocities less than 4 cm s–1 do not have the energy or nutrients available to undergo germination. Current research demonstrates that 5.5 cm s–1 velocity is a threshold delineating heavier, viable seeds from light, nutrient poor seeds (Delefosse et al., 2016; Infantes and Moksnes, 2018). Our results can be used to explain increased bed establishment observed by Zimmerman et al. (2017). They found that following the reproductive season, vegetative shoots in carbon enriched treatments (pHNIST = 6.1, 6.5) nearly quadrupled compared to ambient conditions. We hypothesize that there were more viable seeds of better quality available to germinate and increase aboveground biomass.
Increases in seed quality and germination success are not density dependent and can scale up significantly to alter the landscape level (Orth et al., 2003). Field studies have recorded germination rates as remarkably low; averaging only 3.6% at the landscape level (Statton et al., 2017). In a typical meadow in Shinnecock Bay, New York (40.856678, −72.454090), an approximate calculation estimates the reproductive output of 1 m–2 of intact eelgrass at approximately 835 unaborted seeds (Parameters for dimensional analysis provided by Jackson et al., 2017).
If we are to use our results from experiment 5, 75% of the seeds raised at pHT = 6.9 will have > 95% chance of germination. Thus, if these same 40 shoots (40 shoots × 2.9 rhipidia × 2 spathes × 3.6 unaborted seeds × 75% viable seeds) are raised in carbon enriched conditions, it will generate an estimated 626 seeds. Whereas those same shoots (40 shoots × 2.9 rhipidia × 2 spathes × 3.6 unaborted seeds × 14% viable seeds) raised in low CO2 conditions (pHT = 7.9) may only produce as many as 120 viable seeds. This represents a fivefold increase in the production of viable seeds by altering only one parameter during development. This in combination with a twofold increase in germination may have substantial effects on the community at large. Successful sexual reproduction can increase genotypic variation, thereby increasing resiliency (McDonald et al., 2016). Beds of high genotypic diversity have been associated with greater stability and productivity (Hughes and Stachowicz, 2004; Macreadie et al., 2014; Jarvis and Moore, 2015; McDonald et al., 2016). Indeed, large scale improvements in seed quality will have demonstrable effects on in situ seed banks providing an additional bolster to erratic environmental conditions concurrent with climate change. Increasing genetic resiliency must be prioritized to ensure long term habitat sustainability.
The work conducted on the reproductive patterns of perennial meadows of Zostera marina have demonstrated the appreciable role of seeds in the configuration and expansion of established meadows (Furman et al., 2015). Viable seeds have a markedly higher chance of populating well beyond their natal patches and significant increases in viability will further increase colonization success (Sumoski and Orth, 2012). This is also particularly beneficial given that future ocean carbon scenarios predict that coastal acidification (a biogeochemical integration of eutrophication and ocean acidification) will result in more severe diurnal swings in pH with predictions fluctuating as much as 1 unit (Hofmann et al., 2010; Wallace et al., 2014; Pacella et al., 2018). Determining how coastal acidification will integrate into the reproductive history of Z. marina is critical to predicting long term ecological resilience.
Germination is a bottleneck for population growth from seeds, but our results also highlight the vulnerability of seedlings as they develop. Considering all five experiments, less than 10% of seeds developed into photosynthetically active seedlings (>stage 3). This limited seedling production reflects both the life history of Z. marina and emphasizes the complex interplay of biogeochemical interactions in influencing development (Probert and Brenchley, 1999; Valdemarsen et al., 2010). For example, the reproductive output of Zostera marina can be as high as 104 seeds m–2 with only a small fraction resulting in seedling establishment and survival (5–15%) (Harrison, 1993; Orth et al., 2003). In the present study, with typical germination success (enhanced by parental exposure to high CO2), only a fraction of the germinated seeds resulted in seedlings: 62.8, 4.0, 48.3, and 38.2 % of germinated seeds for experiments 2–5, respectively. When the seed coat lyses, the cotyledon acts as a proxy for the developing embryo to sense the outside environment. As the cotyledon elongates during the first and second stage of development, it not only prepares to unfurl the first primordial leaf, but it also serves as a siphon to harvest nutrients from the sediment and water column (Sugiura et al., 2009). Unfavorable environments can halt development and cause mortality. Indeed, for perennial meadows in mid-Atlantic, seedling establishment, not germination serves as the reproductive bottleneck for Z. marina (Churchill, 1983; Jarvis and Moore, 2015).
It should be noted that the small percentage of seedlings produced in experiment 3 (4% of seed germinated) is possibly an artifact of experimental duration. Development from germination to seedling establishment (stage 6) is reported to occur within 4–6 weeks (Alexandre et al., 2006; Salo and Pedersen, 2014). Therefore, the experimental durations in the present study should have allowed for the growth of seedlings into later stages (3–6). However, germination rates in experiment 3, greatly increased in the last two sampling intervals and we might have had greater seedling production with more time.
Once seedlings emerge, they are vulnerable to dynamic and sometimes adverse environmental conditions and thus survival rate varies widely among habitats (Churchill, 1983; Harrison, 1993; Greve et al., 2005; Boese et al., 2009). Hydrodynamic forces (Valdemarsen et al., 2010), light attenuation (from sediment burial, degraded water quality, and competition) (Hauxwell et al., 2001; Mills and Fonseca, 2003), as well as hypoxia (Hauxwell et al., 2003), are reported to cause seedling mortality (Valdemarsen et al., 2010; Salo and Pedersen, 2014). Other than mobilization of metabolic reserves in seeds, temperature, low dissolved oxygen, low organic matter, and burial depths are important predictive factors that correlate with increased seedling growth (Jarvis and Moore, 2015). Low salinity (>20 ppt) and lack of sediment used in the present study may have altered seedling investment in aboveground growth by failing to provide a source of additional nutrients and preventing the seedling from anchoring to its surroundings (Marion and Orth, 2010). Although low salinity significantly increases germination (Niu et al., 2012), seedlings are damaged by salinity less than 20 ppt due to osmotic stress generated by low salinity (Xu et al., 2016). Both salinity and temperature are confirmed factors influencing seedling aboveground morphology and biomass and must be considered for successful restoration.
A major concern for bed establishment from seeds is that CO2 availability increased germination but did not statistically increase seedling production nor survival. For Posidonia oceanica seedlings, CO2 enrichment increases the rate of the dark reaction in photosynthesis resulting in increased growth and storage capacity, carbon reserves in the below-ground tissues and lowered nitrogen content in leaves (Artika et al., 2021). Direct effects of CO2 may be masked by epiphytic growth, herbivory, or simultaneously co-occurring heat or light stress. Shading from epiphytic filamentous algae negates positive net growth associated with available [DIC] as both seedlings and algae benefit from increases in available metabolic substrate (Burnell et al., 2014). In the present study, we observed microscopic algae growing on developing seedlings presumably stunting development. Seedlings must also contend with how elemental changes to their leaf material will influence their palatability. P. oceanica seedlings grown under increased pCO2 shift their elemental leaf structure to favor carbon-based sucrose over nitrogen. Increased sugar production increases palatability to herbivores (Arnold et al., 2012; Hernán et al., 2016). Additionally, although there is a clear correlation between seagrass metabolism and temperature (Escolano-Molto et al., 2021), an emerging body of evidence suggests that seedlings are not as robust as adults and are therefore more vulnerable to heat stress (Guerrero-Meseguer et al., 2017; Zimmerman et al., 2017).
Much of our results are derived from highly variable conditions representing a potentially realistic portrayal of future variability. Although a potential cause for contention, our carbonate chemistry is reflective of the rapid biogeochemical restructuring of the Swedish waterways with areas along the Skagerrak Sea predicting pHT variations moving from present day values (8.7–7.6 units) to future conditions (8.3–7.2 units) in 2100 (Dorey et al., 2013). Our results infer germination response in the context of intense environment flux indicative of climate change, eutrophic events, sewage drainage and industrial effluence. Continued effort must be invested into determining how Z. marina will respond to the rapid restructuring of global oceans. Additional field experiments are needed to determine how sediment geochemistry, temperature and PAR influence germination and growth dynamics in situ.
Our data indicates that there is a significant and meaningful effect of CO2 at the parental level, and we accept our hypotheses. This research provides fundamental groundwork for laboratory-based adaptation strategies to increase parental investment and augment restoration success. Short-term injections of CO2 into flowering holding containers while seeds are developing is a feasible technique to bolster germination. Restoration strategies should be multitiered with site selection considering substrate (muddy sediment, >1% organic matter), light attenuation, and proximity to established meadows for both the adult plants but also the developing seedling (Bintz and Nixon, 2001; Jarvis and Moore, 2015). Carbon enriched seeds should be nested in burlap bags to anchor seeds in sediment while preventing from >5 cm burial depth (Jarvis and Moore, 2015). Using burlaps bags has resulted in nearly 100% retention of seed used for restoration (Harwell and Orth, 1999). Successful recovery of Z. marina in large systems is dependent upon seed production and dispersal and generating resilience, diverse eelgrass bed capable of withstanding impending environmental flux (Olesen and SandJensen, 1994).
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
AL collected and analyzed data as well as wrote the manuscript. EI and SD assisted with analysis and experimental design. LW, LP, CH, and KR assisted in data collection. BP and JC assisted in manuscript preparation. TC designed, analyzed, and assisted in writing the manuscript. All authors contributed to the article and approved the submitted version.
This research was funded by Kungl Vetenskaps-Akademien (KVA) travel grants in 2017, 2018 and 2019. This research was also supported by NOAA Sea Grant Project Number 1147060 and an additional grant (100985) from the University of New Orleans’s Office of Research and Sponsored Programs.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Laura Govers, Emory Wellman, Samantha Linhardt, and Jennifer Nguyen for assisting in data collection and Elena Tamarit for assisting with logistics and care for plants. We would also like to thank Laura Enzor (University of Hartford) for doing titrations for the Total Alkalinity and Jeanne Guimond for coordinating travel.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.762086/full#supplementary-material
Agami, M., and Waisel, Y. (1988). The role of fish in distribution and germination of seeds of the submerged macrophytes Najas marina L. and Ruppia maritima L. Oecologia 76, 83–88. doi: 10.1007/BF00379604
Alexandre, A., Cabaco, S., Santos, R., and Serrao, E. A. (2006). Timing and success of reproductive stages in the seagrass Zostera noltii. Aquat. Bot. 85, 219–223. doi: 10.1016/j.aquabot.2006.05.002
Arnold, T., Mealey, C., Leahey, H., Miller, A. W., Hall-Spencer, J. M., Milazzo, M., et al. (2012). Ocean acidification and the loss of phenolic substances in marine plants. PLoS One 7:e35107. doi: 10.1371/journal.pone.0035107
Artika, S., Ambo-Rappe, R., Samawi, M., Teichberg, M., Moreira-Saporiti, A., and Viana, I. G. (2021). Rising temperature is a more important driver than increasing carbon dioxide concentrations in the trait responses of Enhalus acoroides seedlings. Appl. Sci. 11:2730. doi: 10.3390/app11062730
Bintz, J., and Nixon, S. (2001). Responses of eelgrass Zostera marina seedlings to reduced light. Mar. Ecol. Prog. Ser. 223, 133–141. doi: 10.3354/MEPS223133
Björk, M., Asplund, M. E., Deyanova, D., and Gullström, M. (2021). The amount of light reaching the leaves in seagrass (Zostera marina) meadows. PLoS One 16:e0257586. doi: 10.1371/journal.pone.0257586
Boese, B. L., Kaldy, J. E., Clinton, P. J., Eldridge, P. M., and Folger, C. L. (2009). Recolonization of intertidal Zostera marina L. (eelgrass) following experimental shoot removal. J. Exp. Mar. Biol. Ecol. 374, 69–77. doi: 10.1016/j.jembe.2009.04.011
Burnell, O. W., Russell, B. D., Irving, A. D., and Connell, S. D. (2014). Seagrass response to CO2 contingent on epiphytic algae: indirect effects can overwhelm direct effects. Oecologia 176, 871–882. doi: 10.1007/s00442-014-3054-z
Caldeira, K., and Wickett, M. E. (2005). Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J. Geophys. Res. Oceans 110:C09S04.
Campbell, J. E., and Fourqurean, J. W. (2013). Effects of in situ CO2 enrichment on the structural and chemical characteristics of the seagrass Thalassia testudinum. Mar. Biol. 160, 1465–1475. doi: 10.1007/s00227-013-2199-3
Churchill, A. (1983). Field studies on seed germination and seedling development in Zostera marina L. Aqua Bot. 16, 21–29. doi: 10.1016/0304-3770(83)90048-7
Ciais, P., Sabine, C., Bala, G., Bopp, L., Brovkin, V., Canadell, J., et al. (2013). Carbon and Other Biogeochemical Cycles. Cambridge: Cambridge University Press.
Cox, T. E., Gazeau, F., Alliouane, S., Hendriks, I. E., Mahacek, P., Le Fur, A., et al. (2016). Effects of in situ CO2 enrichment on structural characteristics, photosynthesis, and growth of the Mediterranean seagrass Posidonia oceanica. Biogeosciences 13, 2179–2194. doi: 10.5194/bg-13-2179-2016
Cox, T. E., Schenone, S., Delille, J., Dìaz-Castañeda, V., Alliouane, S., Gattuso, J. P., et al. (2015). Effects of ocean acidification on Posidonia oceanica epiphytic community and shoot productivity. J. Ecol. 103, 1594–1609. doi: 10.1111/1365-2745.12477
De Cock, A. W. A. M. (1980). Flowering, pollination, and fruiting in Zostera marina L. Aquat. Bot. 9, 201–220. doi: 10.1016/0304-3770(80)90023-6
de los Santos, C., Krause-Jensen, D., Alcoverro, T., Marbà, N., Duarte, C. M., van Katwijk, M., et al. (2019). Recent trend reversal for declining European seagrass meadows. Nat. Commun. 10:3356. doi: 10.1038/s41467-019-11340-4
Delefosse, M., Povidisa, K., Poncet, D., Kristensen, E., and Olesen, B. (2016). Variation in size and chemical composition of seeds from the seagrass Zostera marina—ecological implications. Aquat. Bot. 131, 7–14. doi: 10.1016/j.aquabot.2016.02.003
Dennison, W. C., and Alberte, R. S. (1982). International association for ecology photosynthetic responses of Zostera marina L. (Eelgrass) to in situ Manipulations of light intensity. Oecologia 55, 137–144. doi: 10.1007/BF00384478
Dickson, A. G., and Millero, F. J. (1987). A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res. Part A Oceanogr. Res. Pap. 34, 1733–1743. doi: 10.1016/0198-0149(87)90021-5
Doney, S. C., Fabry, V. J., Feely, R. A., and Kleypas, J. A. (2009). Ocean acidification: the other CO2 problem. Ann. Rev. Mar. Sci. 1, 169–192.
Dorey, N., Lançon, P., Thorndyke, M., and Dupont, S. (2013). Assessing physiological tipping point of sea urchin larvae exposed to a broad range of pH. Glob. Change Biol. 19, 3355–3367. doi: 10.1111/gcb.12276
Dupont, S., Dorey, N., Stumpp, M., Melzner, F., and Thorndyke, M. (2013). Long-term and trans-life-cycle effects of exposure to ocean acidification in the green sea urchin strongylocentrotus droebachiensis. Mar. Biol. 160, 1835–1843. doi: 10.1007/s00227-012-1921-x
Escolano-Molto, A., Flecha, S., Vaquer-sunyer, R., Wesselmann, M., Marba, N., and Hendricks, I. (2021). Mediterranean seagrasses as carbon sinks: methodological and regional differences. Biogeosci. Discuss. [Preprint] 1–32.
Furman, B. T., Jackson, L. J., Bricker, E., and Peterson, B. J. (2015). Sexual recruitment in Zostera marina: a patch to landscape-scale investigation. Limnol. Oceanogr. 60, 584–599. doi: 10.1002/lno.10043
Greve, T. M., Krause-Jensen, D., Rasmussen, M. B., and Christensen, P. B. (2005). Means of rapid eelgrass (Zostera marina L.) recolonization in former dieback areas. Aquat. Bot. 82, 143–156. doi: 10.1016/j.aquabot.2005.03.004
Guerrero-Meseguer, L., Marín, A., and Sanz-Lázaro, C. (2017). Future heat waves due to climate change threaten the survival of P. oceanica seedlings. Environ. Pollut. 230, 40–45. doi: 10.1016/j.envpol.2017.06.039
Hall-Spencer, J. M., and Harvey, B. P. (2019). Ocean acidification impacts on coastal ecosystem services due to habitat degradation. Emerg. Topics Life Sci. 3, 197–206. doi: 10.1042/ETLS20180117
Harrison, P. G. (1993). Variations in demography of Zostera marina and Z. noltii on an intertidal gradient. Aquat. Bot. 45, 63–77. doi: 10.1016/0304-3770(93)90053-Y
Harwell, M. C., and Orth, R. J. (1999). Eelgrass (Zostera marina L.) seed protection for field ex- periments and implications for large-scale restoration. Aquatic Bot. 64, 51–61.
Hauxwell, J., Cebrian, J., Furlong, C., and Valiela, I. (2001). Macroalgal canopies contribute to eelgrass (Zostera marina) decline in temperate estuarine ecosystems. Ecology 82, 1007–1022. doi: 10.2307/2679899
Hauxwell, J., Cebrian, J., and Valiela, I. (2003). Eelgrass Zostera marina loss in temperate estuaries: relationship to land derived nitrogen loads and effect of light limitation imposed by algae. Mar. Ecol. Prog. Ser. 247, 59–73. doi: 10.3354/meps247059
Heck, K. L., and Orth, R. J. (1980). “Seagrass habitats: the roles of habitat complexity, competition and predation in structuring associated fish and motile macroinvertebrate assemblages,” in Estuarine Perspectives, ed. V. S. Kennedy (New York, NY: Academic Press), 449–464. doi: 10.1016/B978-0-12-404060-1.50043-5
Hernán, G., Ortega, M. J., Gándara, A. M., Castejón, I., Terrados, J., and Tomas, F. (2017). Future warmer seas: increased stress and susceptibility to grazing in seedlings of a marine habitat-forming species. Glob. Change Biol. 23, 4530–4543. doi: 10.1111/gcb.13768
Hernán, G., Ramajo, L., Basso, L., Delgado, A., Terrados, J., Duarte, C., et al. (2016). Seagrass (Posidonia oceanica) seedlings in a high-CO2 world: from physiology to herbivory. Sci. Rep. 6:38017. doi: 10.1038/srep38017
Höckerstedt, L., Susi, H., and Laine, A. (2021). Effect of maternal infection on progeny growth and resistance mediated by maternal genotype and nutrient availability. J. Ecol. 109, 1439–1451. doi: 10.1111/1365-2745.13568
Hofmann, G. E., Barry, J. P., Edmunds, P. J., Gates, R. D., Hutchins, D. A., Klinger, T., et al. (2010). The effect of ocean acidification on calcifying organisms in marine ecosystems: an organism-to-ecosystem perspective. Annu. Rev. Ecol. Evol. Syst. 41, 127–147. doi: 10.1146/annurev.ecolsys.110308.120227
Hughes, A. R., and Stachowicz, J. J. (2004). Genetic diversity enhances the resistance of a seagrass ecosystem to disturbance. Proc. Natl. Acad. Sci. U.S.A. 101, 8998–9002. doi: 10.1073/pnas.0402642101
Infantes, E., Eriander, L., and Moksnes, P.-O. (2016). Eelgrass (Zostera marina) restoration in the West coast of Sweden using seeds. Mar. Ecol. Prog. Ser. 545, 31–45. doi: 10.3354/meps11615
Infantes, E., and Moksnes, P. O. (2018). Eelgrass seed harvesting: flowering shoots development and restoration on the Swedish west coast. Aquat. Bot. 144, 9–19. doi: 10.1016/j.aquabot.2017.10.002
Invers, O., Romero, J., and Pérez, M. (1997). Effects of pH on seagrass photosynthesis: a laboratory and field assessment. Aquat. Bot. 59, 185–194. doi: 10.1016/S0304-3770(97)00072-7
Invers, O., Tomàs, F., Pérez, M., and Romero, J. (2002). Potential effect of increased global CO2 availability on the depth distribution of the seagrass Posidonia oceanica (L.) Delile: a tentative assessment using a carbon balance model. Bull. Mar. Sci. 71, 1191–1198.
Jackson, L. J., Furman, B. T., and Peterson, B. J. (2017). Morphological response of Zostera marina reproductive shoots to fertilized porewater. J. Exp. Mar. Biol. Ecol. 489, 1–6. doi: 10.1016/j.jembe.2017.01.002
Jarvis, J. C., and Moore, K. A. (2015). Effects of seed source, sediment type, and burial depth on mixed-annual and perennial Zostera marina L. seed germination and seedling establishment. Estuar. Coast. 38, 964–978. doi: 10.1007/s12237-014-9869-3
Jiang, Z. J., Huang, X. P., and Zhang, J. P. (2010). Effects of CO2 enrichment on photosynthesis, growth, and biochemical composition of seagrass Thalassia hemprichii (Ehrenb.) Aschers. J. Integr. Plant Biol. 52, 904–913. doi: 10.1111/j.1744-7909.2010.00991.x
Johnson, A. J., Moore, K. A., and Orth, R. J. (2017). The influence of resource availability on flowering intensity in Zostera marina (L.). J. Exp. Mar. Biol. Ecol. 480, 13–22. doi: 10.1016/j.jembe.2017.02.002
Jorgensen, M. S., Labouriau, R., and Olesen, B. (2019). Seed size and burial depth influence Zostera marina L. (eelgrass) seed survival, seedling emergence and initial seedling biomass development. PLoS One 14:e0215157. doi: 10.1371/journal.pone.0215157
Koch, M., Bowes, G., Ross, C., and Zhang, X. H. (2013). Climate change and ocean acidification effects on seagrasses and marine macroalgae. Glob. Change Biol. 19, 103–132. doi: 10.1111/j.1365-2486.2012.02791.x
Kuo, J., and Kirkman, H. (1992). Fruits, seeds and germination in the Seagrass Halophila ovalis (Hydrocharitaceae). Bot. Mar. 35, 197–204. doi: 10.1515/botm.1992.35.3.197
Lewis, P. D. E., and Wallace, D. W. R. (2006). MS Excel Program Developed for CO2 System Calculations. ORNL/CDIAC-105a. Oak Ridge, TN: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy. doi: 10.3334/CDIAC/otg.CO2SYS_XLS_CDIAC105a
Macreadie, P. I., York, P. H., and Sherman, C. D. H. (2014). Resilience of Zostera muelleri seagrass to small-scale disturbances: the relative importance of asexual versus sexual recovery. Ecol. Evol. 4, 450–461. doi: 10.1002/ece3.933
Marion, S. R., and Orth, R. J. (2010). Factors influencing seedling establishment rates in Zostera marina and their implications for seagrass restoration. Restorat. Ecol. 18, 549–559. doi: 10.1111/j.1526-100X.2010.00695.x
McDonald, A. M., Prado, P. P., Heck, K. L., Fourqurean, J. W., Frankovich, T. A., Dunton, K. H., et al. (2016). Seagrass growth, reproductive and morphological plasticity across environmental gradients over a large spatial scale. Aquat. Bot. 134, 87–97. doi: 10.1016/j.aquabot.2016.07.007
Mehrbach, C., Culberson, C. H., Hawley, J. E., and Pytkowicx, R. M. (1973). Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limn. Oce 18. doi: 10.4319/lo.1973.18.6.0897
Mills, K. E., and Fonseca, M. S. (2003). Mortality and productivity of eelgrass Zostera marina under conditions of experimental burial with two sediment types. Mar. Ecol. Prog. Ser. 255, 127–134. doi: 10.3354/MEPS255127
Niu, S., Zhang, P., Liu, P., Guo, D., and Zhang, X. (2012). The effects of temperature on the survival, growth, photosynthesis, and respiration of young seedlings of eelgrass Zostera marina L. Aquaculture 350-353, 98–108. doi: 10.1016/j.aquaculture.2012.04.010
Olesen, B., and SandJensen, K. (1994). Patch dynamics of eelgrass Zostera marina. Mar. Ecol. Prog. Ser. 106, 147–156.
Orth, R., Harwell, M., and Inglis, G. (2006). “Ecology of seagrass seeds and seagrass dispersal processes,” in Seagrasses: Biology, Ecology and Conservation, eds A. W. D. Larkum, R. J. Orth, and C. M. Duarte (Dordrecht: Springer), 111–133. doi: 10.1007/978-1-4020-2983-7_5
Orth, R. J., Fishman, J. R., Harwell, M. C., and Marion, S. R. (2003). Seed-density effects on germination and initial seedling establishment in eelgrass Zostera marina in the Chesapeake Bay region. Mar. Ecol. Prog. Ser. 250, 71–79. doi: 10.3354/meps250071
Orth, R. J., and McGlathery, K. J. (2012). Eelgrass recovery in the coastal bays of the Virginia Coast Reserve, USA. Mar. Ecol. Prog. Ser. 448, 173–176. doi: 10.3354/meps09596
Orth, R. J., and Moore, K. A. (1983). Seed germination and seedling growth of Zoster marina L. (Eelgrass) in the Chesapeake Bay. Aquat. Bot. 15, 117–131. doi: 10.1016/0304-3770(83)90023-2
Pacella, S. R., Brown, C. A., Waldbusser, G. G., Labiosa, R. G., Hales, B., and Karl, D. M. (2018). Seagrass habitat metabolism increases short-term extremes and long-term offset of CO2 under future ocean acidification. Proc. Natl. Acad. Sci. U.S.A. 115, 3870–3875. doi: 10.1073/pnas.1703445115
Palacios, S. L., and Zimmerman, R. (2007). Response of eelgrass Zostera marina to CO2 enrichment: possible impacts of climate change and potential for remediation of coastal habitats. Mar. Ecol. Prog. Ser. 344, 1–13. doi: 10.3354/meps7084
Pelacani, C. R., Barros, R. S., Ribeiro, D. M., and Frigeri, R. B. (2005). Breaking dormancy of Stylosanthes humilis seeds with low pH solutions. Acta Physiol. Plant. 27, 387–394. doi: 10.1007/s11738-005-0016-4
Phillips, R. C., Grant, W. S., and McRoy, C. P. (1983). Reproductive strategies of eelgrass (Zostera marina L.). Aquat. Bot. 16, 1–20. doi: 10.1016/0304-3770(83)90047-5
Probert, R. J., and Brenchley, J. L. (1999). The effect of environmental factors on field and laboratory germination in a population of Zostera marina L. from southern England. Seed Sci. Res. 9, 331–339. doi: 10.1017/S0960258599000343
Qin, L. Z., Suonan, Z., Kim, S. H., and Lee, K. S. (2021). Growth and reproductive responses of the seagrass Zostera marina to sediment nutrient enrichment. ICES J. Mar. Sci. 78, 1160–1173. doi: 10.1093/icesjms/fsab031
Riebesell, U., Fabry, V. J., Hansson, L., and Gattuso, J.-P. (eds) (2011). Guide to Best Practices for Ocean Acidification Research and Data Reporting (EUR 24872 EN) [Reprinted Edition Including Erratum]. Luxembourg: Publications Office of the European Union, 258. doi: 10.2777/66906
Russell, B. D., Connell, S. D., Uthicke, S., Muehllehner, N., Fabricius, K. E., and Hall-Spencer, J. M. (2013). Future seagrass beds: can increased productivity lead to increased carbon storage? Mar. Pollut. Bull. 73, 463–469. doi: 10.1016/j.marpolbul.2013.01.031
Sabine, C. L., Feely, R. A., Gruber, N., Key, R. M., Lee, K., Bullister, J. L., et al. (2004). The oceanic sink for anthropogenic CO2. Science 305, 367–371.
Salo, T., and Pedersen, M. F. (2014). Synergistic effects of altered salinity and temperature on estuarine eelgrass (Zostera marina) seedlings and clonal shoots. J. Exp. Mar. Biol Ecol. 457, 143–150. doi: 10.1016/j.jembe.2014.04.008
Schwalm, C. R., Spencer Glendon, S., and Duffy, P. B. (2020). RCP8.5 tracks cumulative CO2 emissions. Proc. Natl. Acad. Sci. U.S.A. 117, 19656–19657. doi: 10.1073/PNAS.2007117117
Statton, J., Montoya, L. R., Orth, R. J., Dixon, K. W., and Kendrick, G. A. (2017). Identifying critical recruitment bottlenecks limiting seedling establishment in a degraded seagrass ecosystem. Sci. Rep. 7:14786. doi: 10.1038/s41598-017-13833-y
Sugiura, H., Kawasaki, Y., Suzuki, T., and Maegawa, M. (2009). The structural and histochemical analyses and chemical characters of the cuticle and epidermal walls of cotyledon in ungerminated seeds of Zostera Marina L. Fish. Sci. 75, 369–377. doi: 10.1007/s12562-009-0064-6
Sumoski, S. E., and Orth, R. J. (2012). Biotic dispersal in eelgrass Zostera marina. Mar. Ecol. Prog. Ser. 471, 1–10. doi: 10.3354/meps10145
Takahashi, M., Noonan, S. H. C., Fabricius, K. E., and Collier, C. J. (2015). The effects of long-term in situ CO2 enrichment on tropical seagrass communities at volcanic vents. ICES. J. Mar. Sci. 73, 876–886. doi: 10.1093/icesjms/fsv157
Tanner, C. E., and Parham, T. (2010). Growing Zostera marina (eelgrass) from seeds in land-based culture systems for use in restoration projects. Rest. Ecol. 18, 527–537. doi: 10.1111/j.1526-100X.2010.00693.x
Tomasko, D., Alderson, M., Burnes, R., Hecker, J., Leverone, J., Raulerson, G., et al. (2018). Widespread recovery of seagrass coverage in Southwest Florida (USA): temporal and spatial trends and management actions responsible for success. Mar. Pol. Bull. 135, 1128–1137. doi: 10.1016/jmarpolbul.2018.08.049
Unsworth, R., Collier, C., Henderson, G., and McKenzie, L. (2012). Tropical seagrass meadows modify seawater carbon chemistry: implications for coral reefs impacted by ocean acidification. Environ. Res. Lett. 7:024026. doi: 10.1088/1748-9326/7/2/024026
Valdemarsen, T., Canal-Verges, P., Kristensen, E., Holmer, M., Kristiansen, M. D., and Flindt, M. R. (2010). Vulnerability of Zostera marina seedlings to physical stress. Mar. Ecol. Prog. Ser. 418, 119–130. doi: 10.3354/meps08828
van Lent, F., and Verschuure, J. M. (1994). Intraspecific variability of Zostera marina L. in the estuaries and lagoons of the southwestern Netherlands. Aquat. Bot. 48, 31–58. doi: 10.106/0304-3770(94)90072-8
Wallace, R. B., Baumann, H., Grear, J. S., Aller, R. C., and Gobler, C. J. (2014). Coastal ocean acidification: the other eutrophication problem. Estuar. Coast. Shelf Sci. 148, 1–13. doi: 10.1016/j.ecss.2014.05.027
Waycott, M., Duarte, C. M., Carruthers, T. J., Orth, R. J., Dennison, W. C., Olyarnik, S., et al. (2009). Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. U.S.A. 106, 12377–12381. doi: 10.1073/pnas.0905620106
Wilson, K. L., and Lotze, H. K. (2019). Climate change projections reveal range shifts of eelgrass Zostera marina in the Northwest Atlantic. Mar. Ecol. Prog. Ser. 620, 47–62. doi: 10.3354/meps12973
Wulff, R. D. (1995). “Environmental maternal effects on seed quality and germination,” in Seed Development and Germination, eds J. Kigel and G. Galili (New York, NY: Routledge).
Xu, S., Zhou, Y., Wang, P., Wang, F., Zhang, X., and Gu, R. (2016). Salinity and temperature significantly influence seed germination, seedling establishment, and seedling growth of eelgrass Zostera marina L. PeerJ 4:e2697. doi: 10.7717/peerj.2697
Zimmerman, R. C., Hill, V., Jinuntuya, M., Celebi, B., Ruble, D., Smith, M., et al. (2017). Experimental impacts of climate warming and ocean carbonation on eelgrass Zostera marina. Mar. Ecol. Prog. Ser. 566, 1–15. doi: 10.3354/meps1205
Zimmerman, R. C., Hill, V. J., and Gallegos, C. L. (2015). Predicting effects of ocean warming, acidification, and water quality on Chesapeake region eelgrass. Limnol. Oceanogr. 60, 1781–1804. doi: 10.1002/lno.10139
Keywords: CO2, parental investment, seedlings, seagrass restoration, seed viability
Citation: Lowell A, Infantes E, West L, Puishys L, Hill CEL, Ramesh K, Peterson B, Cebrian J, Dupont S and Cox TE (2021) How Does Ocean Acidification Affect the Early Life History of Zostera marina? A Series of Experiments Find Parental Carryover Can Benefit Viability or Germination. Front. Mar. Sci. 8:762086. doi: 10.3389/fmars.2021.762086
Received: 20 August 2021; Accepted: 02 December 2021;
Published: 24 December 2021.
Edited by:
Demian Alexander Willette, Loyola Marymount University, United StatesReviewed by:
Pablo P. Leal, Instituto de Fomento Pesquero (IFOP), ChileCopyright © 2021 Lowell, Infantes, West, Puishys, Hill, Ramesh, Peterson, Cebrian, Dupont and Cox. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alyson Lowell, QWx5c29uLnYubG93ZWxsQGdtYWlsLmNvbQ==; T. Erin Cox, dGVjb3hAdW5vLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.