- 1Department of Life Sciences, University of Trieste, Trieste, Italy
- 2Department of Biological, Geological and Environmental Sciences, University of Catania, Catania, Italy
Canopy-forming brown algae support highly productive ecosystems whose decline has been attributed to the interplay of several anthropogenic disturbances. Climate change could have disruptive effects on the biology of these species, but the role of temperature in the development of early life stages is poorly understood. The aim of this study was to assess the response of Ericaria giacconei, a winter-reproducing Southern–Mediterranean endemic species, to thermal stress by testing five temperatures (12, 15, 18, 24, and 28°C) on adults and early stages. Chlorophyll a fluorescence of adult plants was measured at 0, 24, 72, and 120 h on nine fronds in each of the three aquaria per treatment. To assess egg release, zygote settlement, and embryo growth rate, approximately 1,200 receptacles were cultured on six Petri dishes per temperature treatment, and 10 random subsections of 2 ×2 mm were examined in three Petri dishes at 0, 20, 44, and 92 h after fertilization. Adult plants showed a plastic physiological response, and thermal stress had no significant effect on PSII efficiency. Embryos fully developed only at 12 and 15°C. Mortality increased at 18 and 24°C, and no zygotes survived at 28°C. In a scenario of further increasing temperatures, the effects of warming could affect the recruitment of E. giacconei and increase its vulnerability to further stresses. These effects on the survival of early stages, which are the bottleneck for the long-term survival of the species, should be taken into account in conservation and restoration measures to maintain canopy-forming macroalgal populations and associated biodiversity and ecosystem services.
Introduction
Canopy-forming algae of the order Fucales and Laminariales (Phaeophyceae) are among the most ecologically and socio-economically valuable marine species in temperate waters (Steneck et al., 2002; Smale et al., 2013; Bennett et al., 2015). They provide a structural and trophic framework that supports rich biodiversity by providing food, shelter, and habitat for other associated species (Bustamante et al., 2017; Teagle et al., 2017), and are responsible for nutrient cycling and CO2 storage (Krause-Jensen and Duarte, 2016; Filbee-Dexter and Wernberg, 2020).
Macroalgal forests are undergoing major regressions worldwide due to a combination of multiple natural and anthropogenic sources of disturbance (Steneck et al., 2002; Strain et al., 2014; Mineur et al., 2015; Krumhansl et al., 2016). In recent years, an increasing number of studies have reported changes in the distribution and abundance of these macroalgal populations as a result of ocean warming and thermal anomalies (especially marine heat waves, MHWs) (e.g., Smale, 2020 and the references therein; Bevilacqua et al., 2019; Savonitto et al., 2021; Verdura et al., 2021). Populations at the edge of their range appear to be particularly affected by this trend (e.g., Viejo et al., 2011; Nicastro et al., 2013; Araújo et al., 2014; Álvarez-Losada et al., 2020; Gurgel et al., 2020). Thermal anomalies may affect the phenology and physiology of these species, impairing their performance, increasing their vulnerability to other stressors, and eventually leading to population declines and local extinction events (Wernberg et al., 2010, 2016; Gouvêa et al., 2017; de Bettignies et al., 2018). These events could also lead to changes in associated species and their interactions (Vergés et al., 2016; Wernberg et al., 2016; Provost et al., 2017), which may ultimately result in detrimental cascading effects on ecosystem functions and the resulting provision of goods and services (Smale et al., 2013; Vergés et al., 2014; Straub et al., 2019). To date, most works addressing the effects of heat stress on the physiology and biology of brown algae have been manipulative and laboratory-based, focusing on kelps, with the ‘sporophyte phase’ being the most studied (e.g., Wilson et al., 2015; Burdett et al., 2019; Nepper-Davidsen et al., 2019; Fernández et al., 2020; Hereward et al., 2020; Diehl et al., 2021; Umanzor et al., 2021). As for the Fucales, the genus Fucus is the most extensively studied, and works on adults predominate over those on early life stages (e.g., Strömgren, 1977; Pearson et al., 2009; Jueterbock et al., 2014; Nielsen et al., 2014; Graiff et al., 2015; Mota et al., 2015; Smolina et al., 2016; Rothäusler et al., 2018; Figueroa et al., 2019). Overall, the trend that emerges is a high sensitivity in the early life stages and a relative ability of adults to grow and survive over broader temperature ranges and to physiologically compensate for thermal stress.
In the Mediterranean Sea, macroalgal forests are dominated by Cystoseira sensu lato (s.l.) species (Fucales, Phaeophyceae). In recent decades, they have declined or become locally extinct due to anthropogenic pressure (e.g., Thibaut et al., 2005, 2015; Falace et al., 2010; Perkol-Finkel and Airoldi, 2010; Blanfuné et al., 2016). To date, there is little evidence of natural recovery of damaged Cystoseira populations (e.g., Munda, 2000; Iveša et al., 2016; Orlando-Bonaca and Rotter, 2018; Medrano et al., 2020), because once losses have occurred, recovery from nearby populations tends to be difficult due to the short dispersal of eggs/zygotes and low connectivity of populations (e.g., Soltan et al., 2001; Buonomo et al., 2017; Capdevila et al., 2018). There is evidence that thermal anomalies and warming can alter the reproductive phenology, germling growth, and viability of Cystoseira s.l. species (Celis-Plá et al., 2017; Capdevila et al., 2018; Savva et al., 2018; Bevilacqua et al., 2019; Cáliz et al., 2019; Mancuso et al., 2019; Savonitto et al., 2021; Verdura et al., 2021). As the Mediterranean Sea is warming faster than the oceans and thermal anomalies occur with increasing intensity, frequency, and duration (Diffenbaugh et al., 2007; Vargas-Yáñez et al., 2008; IPCC, 2019; Pastor et al., 2020; Pisano et al., 2020), examining the response of Cystoseira s.l. species to temperature may provide useful insights into their potential future fate under global warming.
The present study focuses on Ericaria giacconei Serio et G. Furnari (= Cystoseira hyblaea Giaccone), a species endemic to the Sicily Channel (Central Mediterranean Sea) that lives in the intertidal and upper sublittoral at depths of 0.2–1.5 m on semi-exposed and exposed rocky shores. Maximum vegetative and reproductive development occurs in winter, from January to March, when mean seawater temperature at 1 m depth ranges from 14.7 to 16.1°C. This species was described at Punta D’Aliga (southern coast of Sicily, Italy) (Giaccone, 1985), where it is locally extinct (Cormaci et al., 2012). Its current range is fragmented and restricted to two localities: Cap Bon (Kelibia) along the Northern Tunisian coast (Bouafif et al., 2016) and Portopalo di Capo Passero (Isola delle Correnti) in Southern Italy (present study). Its disappearance from the type locality, its limited range, and the fact that it lives in shallow waters raise concerns about the possible fate of E. giacconei in the current warming regime. This species, like other Cystoseira s.l. species, is listed in some international agreements (e.g., Barcelona Convention, Directive 92/43/EEC), but these are not legally binding.
The objective of this study is to determine the thermal tolerance of both early developmental stages and adults of E. giacconei. Adult photosynthetic efficiency and egg release, zygote settlement, and embryo development were examined at five temperature treatments from 12 to 28°C. Evidence for the likely response of this species to projected climate change is provided, together with a thorough discussion on its conservation status. Another outcome of this work is the embryology of E. giacconei, which has never been described before.
Materials and Methods
Sampling Site
Samples were collected from a semi-exposed rocky shoreline on the southern coast of Sicily (Sicily Channel, Mediterranean Sea: 36°38′49″ N; 15°04′45″ E). On the seabed, sandy substrates covered by Posidonia oceanica (L.) Delile alternate with shallow rocky reefs dominated by dense and well-structured stands of E. giacconei in the upper subtidal. This species also occurs in the intertidal, replacing the typical fringe of Ericaria amentacea (C. Agardh) Molinari et Guiry as previously described by Giaccone (1985) at the type locality.
The mean seawater temperature on the Ionian coast of Sicily at 1 m depth is 15.6°C in winter, with values ranging from 15.1 to 16.7°C, and 25.5°C in summer, with values ranging from 22.0 to 27.5°C (Clementi et al., 2019).
Experimental Set-up
Approximately 6000 receptacles and 135 primary branches (approximately 10 cm long) of E. giacconei were collected in March 2020. Samples were wrapped in aluminum foil, stored at 4°C in the dark and transported to the Phycological Laboratory, University of Trieste, within 24 h after collection. At the laboratory, the receptacles were stored at 4°C for 36 h, while the adult fronds were acclimatized at 18°C for 48 h.
Five temperature treatments were replicated in environmentally controlled rooms: 12°C, i.e., the lowest temperature the species can be exposed to in winter; 15°C, i.e., the average daily seawater temperature during the reproductive period; 18°C, i.e., the average daily temperature in early winter (December); 24 and 28°C, i.e., temperatures the species is normally exposed to in summer. Light intensity was set to 125 μmol photons m–2 s–1 supplied by LED lamps (AM366 Sicce USA Inc., Knoxville, TN, United States) and measured with a LI-COR LI-190/R Photometer (LICOR-Biosciences, Lincoln, NE, United States); photoperiod was set to 12:12 h light:dark.
For each heat treatment, three aquaria were filled with 10 l of filtered seawater (0.22 μm filter membrane), and each aquarium contained nine adult primary branches. Pumps (Sicce Syncra Nano, Sicce S.r.l., Pozzoleone, IT) were placed at the bottom of each aquarium, to ensure that the medium was properly oxygenated. The experiment on adult fronds lasted 120 h (Figure 1).
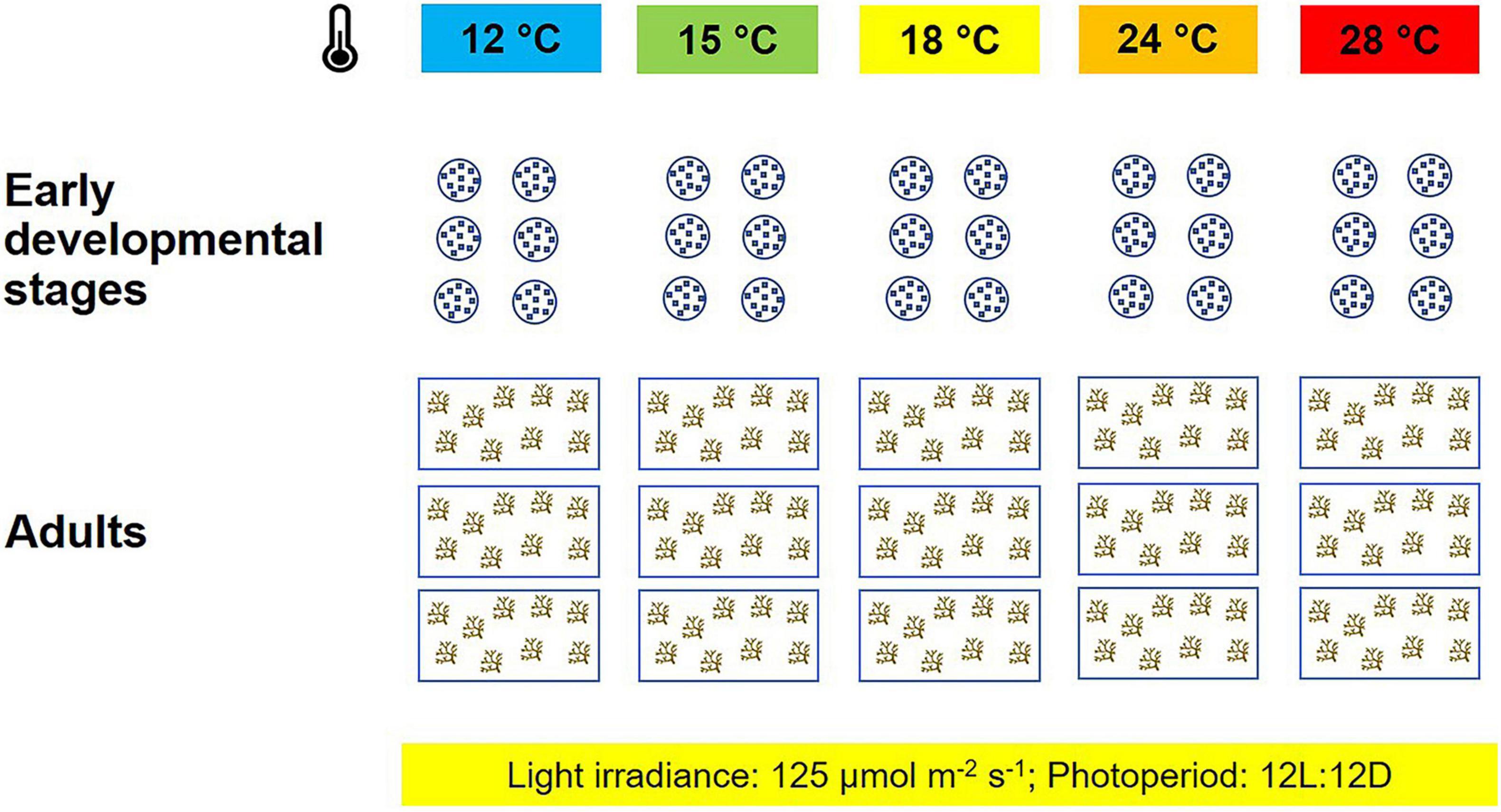
Figure 1. Experimental setup: for each temperature treatment (°C), six Petri dishes and three aquaria were used to test the thermal tolerance of early life stages and adults of Ericaria giacconei.
Early life stages, up to the end of the embryonic stage (i.e., the fall of apical hair; Nienburg, 1931; Galun and Torrey, 1969; Savonitto et al., 2019), were studied for 92 h (Figure 1). Six replicate Petri dishes per treatment were filled with 10 ml of filtered seawater (0.22 μm filter membrane) and incubated at the five temperatures listed above. Each Petri dish was seeded with approximately 200 receptacles.
To counteract evaporation, additional aquaria filled with filtered seawater were kept at the same temperatures to refill the experimental aquaria and Petri dishes.
Response Variables
Adult Plants
Chlorophyll a fluorescence (ChlaF) of each adult specimen was measured at the end of acclimation (t0) and after 24 h (t1), 72 h (t2) and 120 h (t3) using a Photosynthetic Efficiency Analyzer Fluorimeter Handy-PEA (Hansatech, King’s Lynn, United Kingdom). Measurements were taken after a 30 min dark adaptation using the standard Handy-PEA clip. A saturating red-light pulse of 3500 μmol photons m–2 s–1 for 0.8 s was emitted to obtain the 0JIP fluorescence transient, i.e., the time resolved Kautsky induction, and hence Fm (transient maximum ChlaF level). F0 (minimum ChlaF level), needed to calculate Fv (variable ChlaF level, i.e., Fm–F0) and thus Fv/Fm (maximum quantum yield of PSII photochemistry), was measured 50 μs after the onset of illumination. The performance index (PIabs) was also calculated from the analysis of the ChlaF transient from F0 to Fm, the so-called JIP test (Strasser et al., 2000; Bussotti et al., 2010). PIabs is calculated from three independent expressions related to (a) the density of reaction centers, (b) the maximum quantum yield of primary photochemistry, and (c) the efficiency of the electron transport chain between PSII and PSI (Strasser et al., 2000). PIabs is commonly used to test the effects of environmental factors such as temperature, salinity and high intensities of visible and UV-light on the viability and efficiency of the photosynthetic apparatus (Misra et al., 2001).
Early Developmental Stages
Receptacles were removed from Petri dishes after fertilization (AF; 30 h after seeding). To avoid experimental bias and to ensure that the receptacles of all thermal treatments had the same reproductive potential (RP), it was estimated as follows:
The number of conceptacles per receptacle was counted under a stereomicroscope (Leica MZ 6, Leica Microsystems, Wetzlar, Germany). Receptacles were then dried at 70°C for 48 h.
To quantify egg release and zygote settlement at different temperatures, 10 subareas of 0.2 × 0.2 cm2 in three Petri dishes were randomly selected per treatment and photographed under a stereomicroscope with a Nikon Coolpix 4500 camera (Nikon Corporation, Tokyo, Japan) at each sampling time. To reduce stress on the algae, photographs were taken within a few minutes. Three Petri dishes were randomly selected to assess egg release and the remaining three were used to assess zygote settlement. Photographic sampling was carried out at the time of fertilization (i.e., 30 h after seeding) and 20 h AF. The digital images were analyzed to count the number of specimens in each subarea. The counts were then extrapolated to the entire culture area (i.e., 23.76 cm2). Release (RE) and settlement (SE) efficiencies were calculated as follows:
Embryo growth was assessed by taking digital images of 10 randomly selected subareas (0.2 × 0.2 cm2) under an inverted microscope (Leica DM IL LED, Leica Microsystems, Wetzlar, Germany) using a Canon Powershot G9 camera (Canon Inc., Tokyo, Japan) at 20, 44, and 92 h AF. In each subarea, the percentage of unfertilized eggs (= stage 0), zygotes (= stage 1), two-celled embryos (= stage 2), multicellular embryos (= stage several), multicellular embryos with rhizoids (= stage rhizoids), dead embryos (= stage dead), deformed dead embryos (= stage deformed dead), and deformed living embryos (= stage deformed living) were counted (Supplementary Figure 1).
To describe the embryo development, additional dedicated slides were seeded with receptacles at 15°C (i.e., the average seawater temperature during the reproductive period) and observed over time under an inverted microscope.
Statistical Analysis
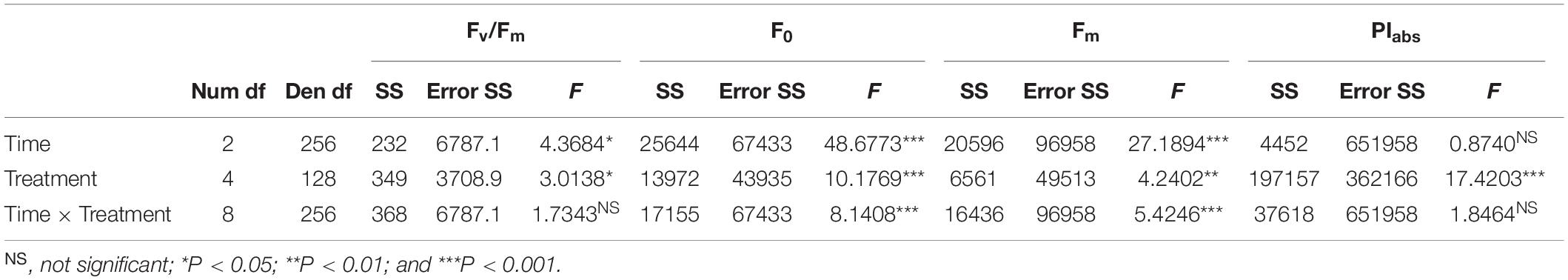
Repeated-measures ANOVA was used to test the effects of temperature (five levels: 12, 15, 18, 24, and 28°C) and time (three levels: t1, t2, and t3) on F0, Fm, Fv/Fm, and PIabs (n = 27). The assumption of normality of response variables was tested with the Shapiro–Wilk test. In all cases, the assumption of normal distribution was fulfilled. Tuckey’s HSD post hoc test was used to examine pairwise significant differences between treatment combinations.
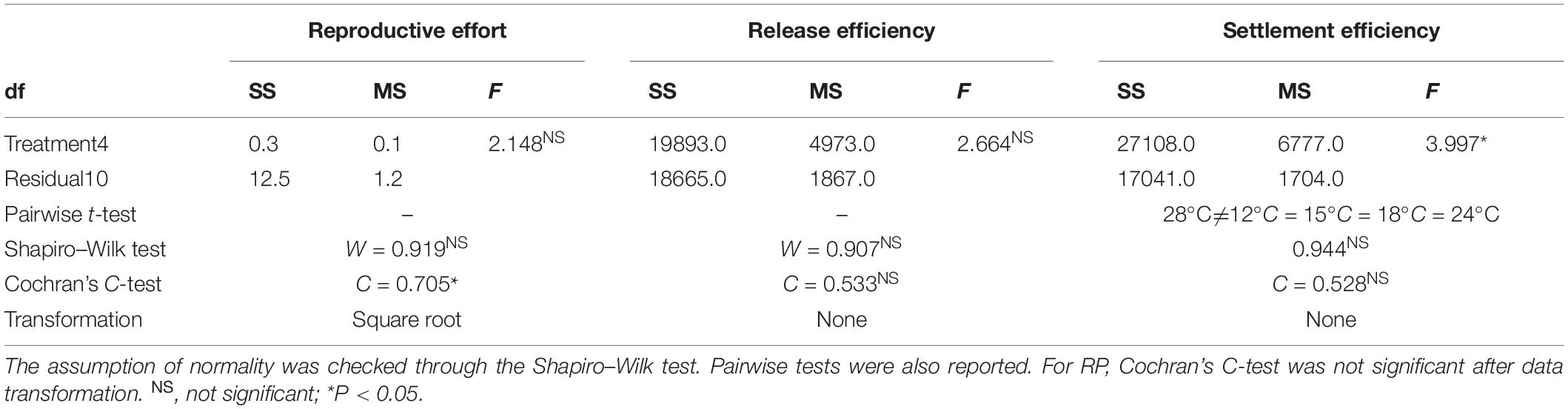
One-way ANOVA was performed to test for differences between temperature treatments on RP, RE, and SE. The assumption of normality of response variables was tested with the Shapiro–Wilk test. In all cases, the assumption of normal distribution was fulfilled. Significant terms were examined by performing a post hoc pairwise t-test to compare the different treatments. Cochran’s C-test (Underwood, 1997) was used to test the assumption of homogeneity of variances prior to analysis. For RP, data were square root-transformed to remove heterogeneous variances. To explain the observed bell-shaped patterns, a quadratic regression model was fitted to RE and SE against temperature.
Distance-based permutational multivariable analysis of variance (PERMANOVA, Anderson, 2001) was used to test for differences in temporal patterns of embryonic development between treatments. Data from treatments at 28°C were not included in the analysis since the number of settled zygotes at 20 h AF was extremely low (mean 0.7 zygotes/subarea ± 0.1 SE), and zygote mortality at later sampling times was 100%. The analysis was based on Bray–Curtis dissimilarities (Bray and Curtis, 1957) on untransformed data, and each term in the analysis was tested by 5,000 random permutations. The design for the analysis included two crossed factors: Treatment (Tr, four levels, and fixed) and Time (Ti, three levels, and fixed), with n = 3. Non-metric multidimensional scaling ordination (nMDS) of the Tr × Ti centroids was used to represent the multivariate patterns.
Results
After acclimation, the adult primary branches of E. giacconei had Fv/Fm values ranging from 0.606 to 0.768, attesting the viability and good physiological status of the photosynthetic apparatus of the samples.
Fv/Fm values were stable throughout the experiment, although slight but significant changes were observed as a function of temperature and time (Figure 2A, Table 1, and Supplementary Table 1). Specifically, at 12°C Fv/Fm statistically increased over time by 5.3% (Supplementary Table 1). The interaction between temperature and time had a significant effect on F0 and Fm (Table 1 and Supplementary Table 1); from t1 to t3, both parameters were stable at 18 and 24°C, whereas F0 significantly decreased in samples at 12 and 15°C and Fm at 15 and 28°C (Figures 2B,C and Supplementary Table 1). PIabs was only affected by temperature (Table 1 and Supplementary Table 1): it was highest at 28°C and gradually decreased from 24 to 15°C, with the lowest values at 12°C at t3 (Table 1, Figure 2D, and Supplementary Table 1).
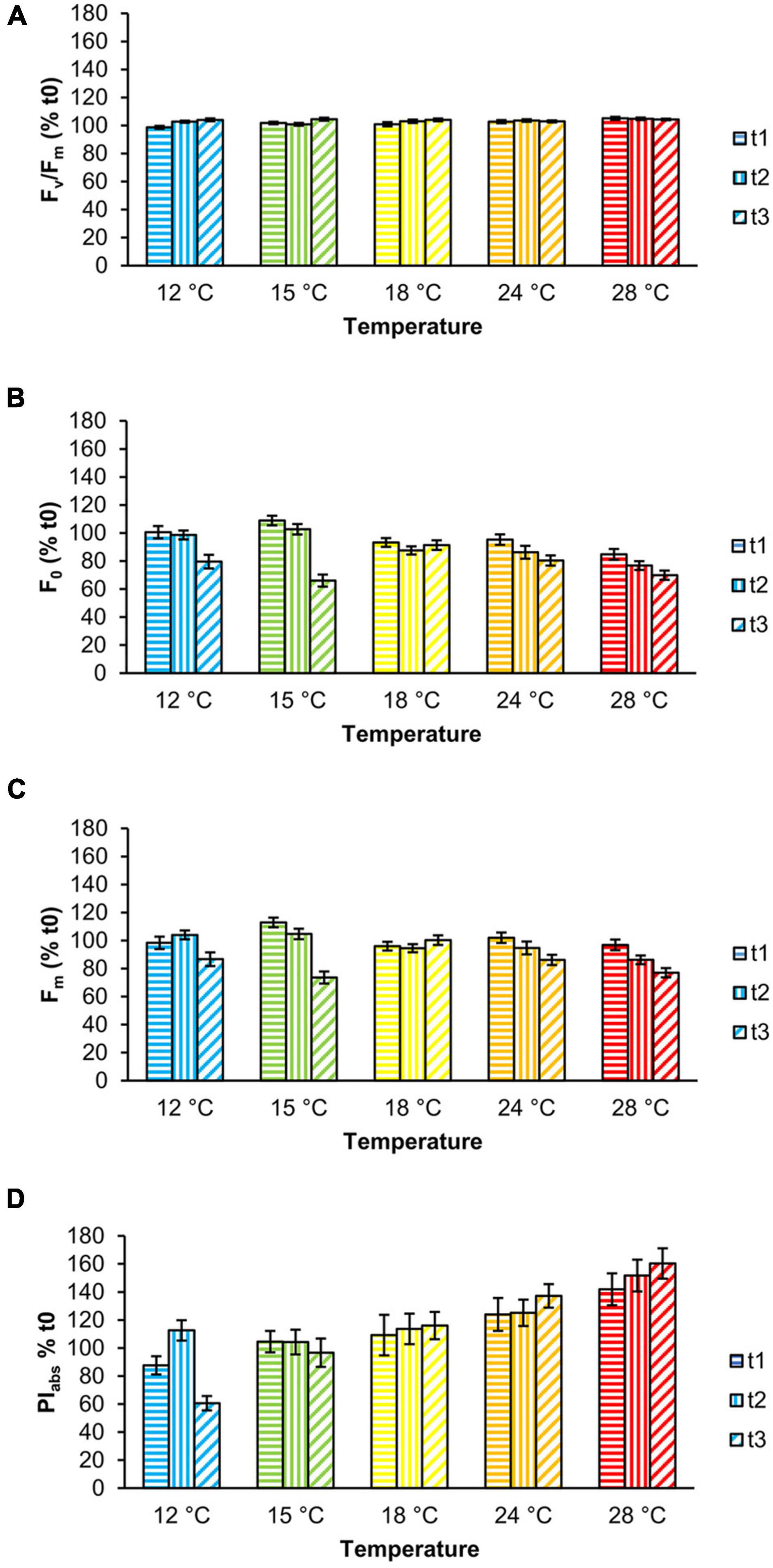
Figure 2. Chlorophyll a fluorescence parameters of Ericaria giacconei adults as a function of temperature: Fv/Fm (A), F0 (B), Fm (C), and PIabs (D) (color-coded as in Figure 1). Fronds were exposed for 24 (t1), 72 (t2), and 120 h (t3) to the tested temperatures. Values (mean ± SE; n = 27) are expressed as percentage (%) of the mean value at t0.
Ericaria giacconei has branched pigmented antheridia and ovoid oospheres retained in the conceptacle (Supplementary Figure 2). The following embryological traits were observed: the first and second division of the zygote are parallel to each other and the third division is perpendicular to the previous ones. The embryo development takes place directly on the substratum: four primary rhizoids are formed fixing it (Supplementary Figure 3).
The RP did not differ significantly among thermal treatments, making them comparable at the beginning of the experiment (Table 2 and Figure 3A).
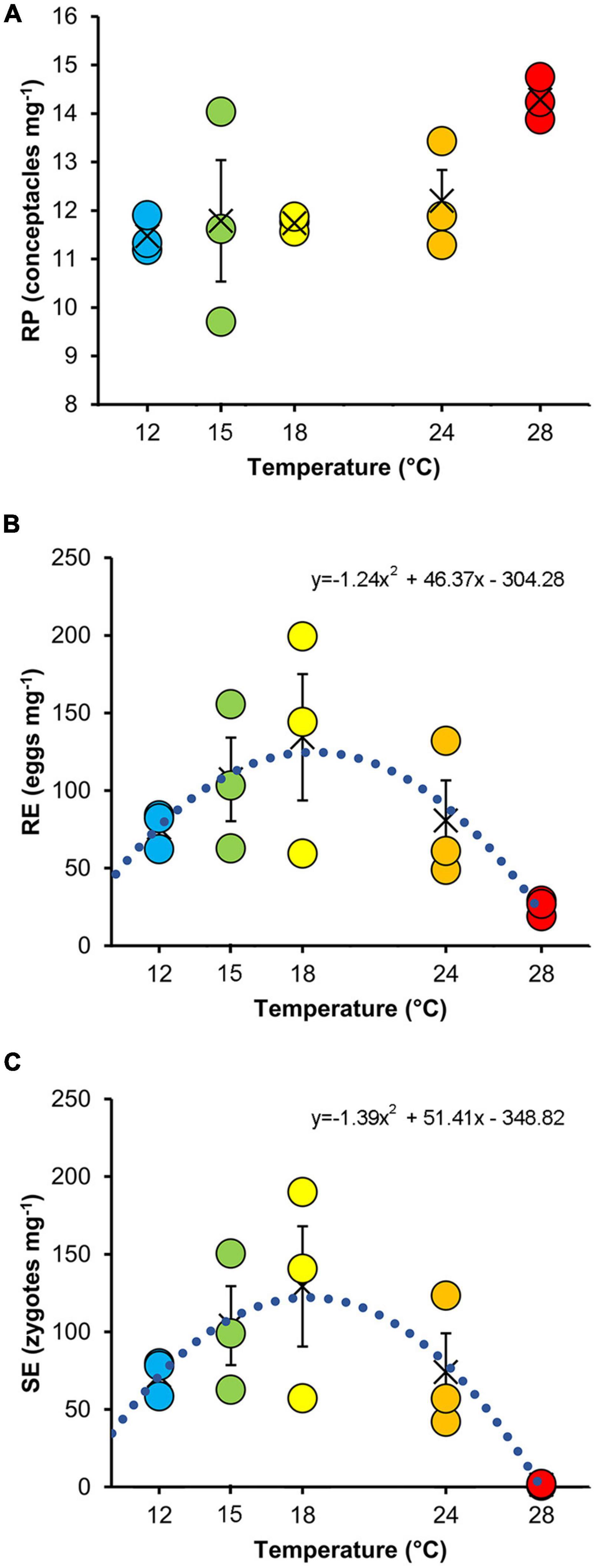
Figure 3. Mean values (±SE) of reproductive potential (A), release efficiency (B), and settlement efficiency (C) at the different temperatures. The values of each replicate are also indicated (color-coded as in Figure 1). The dotted curves show the quadratic model fitted to the data (see Table 3).
No significant effects of temperature were detected on RE (Figure 3B and Table 2). In contrast, temperature significantly affected SE (Figure 3C and Table 2). Specifically, SE at 28°C was lower than all other treatments. RE (Figure 3B) and SE (Figure 3C) showed a bell-shaped response to temperature, and the quadratic model fitted to the data explained 51 and 61% of the variability for RE and SE, respectively (Table 3).
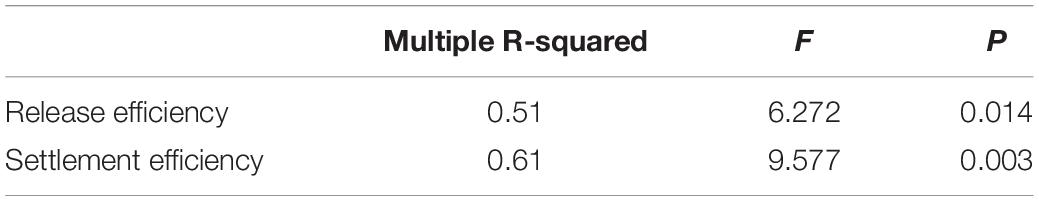
Table 3. Summary of quadratic regression fitted to data of release and settlement efficiency against temperature.
PERMANOVA on embryo status revealed a significant Tr × Ti interaction (Table 4), indicating that temporal patterns of embryonic development differed significantly between temperature treatments. These differences were evident in the nMDS ordination of Tr × Ti centroids (Figure 4). The centroids of 12 and 15°C clustered alongside those of 18 and 24°C, the latter also showing marked separation between 20 and 44–92 h AF. These differences were mainly due to the fact that at 20 h AF a higher percentage of eggs, zygotes, or two-celled embryos were found in the treatments at 18 and 24°C than in those at 12 and 15°C (Figure 4A). In contrast, multicellular embryos or rhizoids were found in the treatments at 12 and 15°C in each time interval (Figure 4B), suggesting that the development rate was faster at lower temperatures. In addition, embryo mortality was consistently higher at 18 and 24°C than at 12 and 15°C, with the highest percentage of dead embryos recorded at 24°C (Figure 4C).
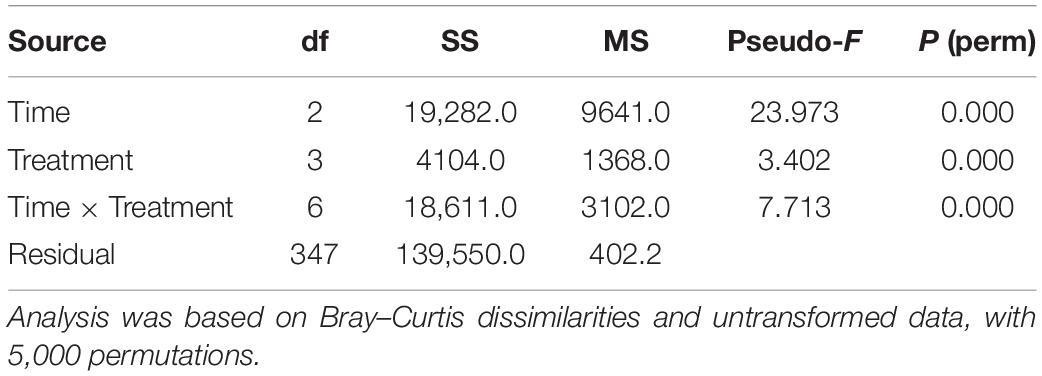
Table 4. PERMANOVA testing for differences in the proportion of different developmental stages of embryos at varying times and temperature treatments after fertilization.
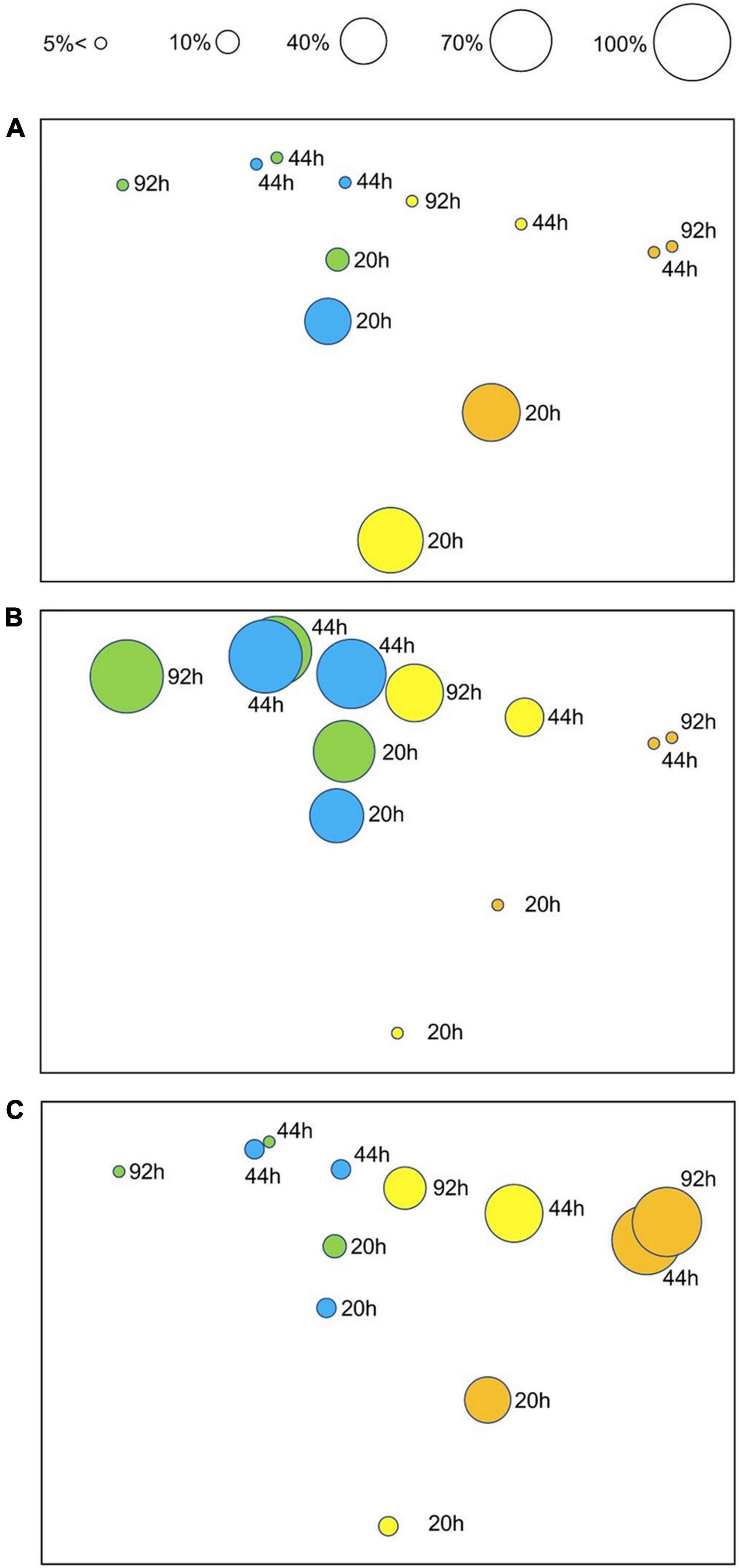
Figure 4. nMDS ordination of Tr × Ti centroids (stress: 0.04) based on Bray–Curtis dissimilarities (untransformed embryo development data). The ordination plot is presented in three versions highlighting three developmental stages, with superimposed bubbles, indicating the corresponding percentage of embryos in earlier (cumulative for stages 0, 1, and 2) (A) and later (cumulative for stages “several” and “rhizoid”) (B) developmental stages, and dead (cumulative for stages “dead” and “deformed dead”) embryos (C) for each time point (20, 44, and 92 h AF) and treatment (color-coded as in Figure 1 for 12, 15, 18, and 24°C).
Discussion
Climate change, coupled with multiple anthropogenic and natural stressors occurring in coastal ecosystems, poses a major threat to the long-term survival of marine forests. From this perspective, studying species vulnerability to temperature stress can provide relevant insights that can be used to make more robust and integrated predictions for marine forest conservation and management.
In our experiment, adults of E. giacconei were not negatively affected by temperatures, indicating an expected ability to acclimatize to a wide range of temperatures typical of the Mediterranean Sea and especially the intertidal zone. All temperatures to which thalli were exposed had a statistically significant, but not physiologically relevant effect on Fv/Fm (max increase +5% at 12°C, from 0.659 to 0.694; max decrease −1% at 28°C, from 0.731 to 0.725), which remained generally steady and within the range of values indicative of a healthy PSII, i.e., >0.6 (e.g., Celis-Plá et al., 2014; Smolina et al., 2016; Falace et al., 2018b; Savva et al., 2018; Cáliz et al., 2019; Verdura et al., 2021). In contrast, temperatures above 18°C caused an almost equal decrease in F0 and Fm (Figures 2B,C). Photosystem II is considered the most heat-sensitive component of the photosynthetic apparatus, especially at the level of the oxygen-evolving complex (Oukarroum et al., 2016). Impairment of this component leads to a progressive decrease in electrons entering the electron transport chain from PSII until its complete inactivation (Allakhverdiev et al., 2008). Several parameters of the fast ChlaF transients, such as the maximal and basal fluorescence (Fm and F0) and the derived maximum quantum yield (Fv/Fm), are the most appropriate tools for detecting early effects of heat stress, as they have been shown to correlate with heat sensitivity/tolerance (Allakhverdiev et al., 2008). In particular, the increase in F0 is closely related to the temperature at which PSII is inactivated (Yamane et al., 2000). For the aforementioned reasons, this could be interpreted as a transient adaptation of the photosynthetic apparatus to the temperature change rather than heat stress.
Several works reporting the effects of temperature on photosynthetic efficiency of brown algae have shown that adults are generally tolerant of temperature fluctuations. For instance, ChlaF of E. selaginoides adults was not affected after exposure to temperatures up to 28°C for 15 days (Cáliz et al., 2019). Savva et al. (2018) reported that Fv/Fm of Cystoseira compressa exposed from 12 to 34°C maintained values close to the optimum in the range of 19.2–30.9°C. Similarly, Mancuso et al. (2019) observed an increase in Fv/Fm in the field up to 28°C when the algae were submerged, and a marked decrease during tidal emersion only when air temperature exceeded 28°C. Accordingly, populations of Fucus serratus from southern areas of North Atlantic showed a decrease in PIabs only when temperatures ranged from 28 to 36°C (Jueterbock et al., 2014), although F. serratus is a cold-affine species. In our case, adults of E. giacconei showed higher PIabs at the upper extreme of the tested temperature range (Figure 2D), suggesting that they have better PSII efficiency in warm seasons. Negative effects on ChlaF parameters were observed in Fucus distichus only when thalli were exposed to temperatures 10–15° above their optimum (Smolina et al., 2016), and in E. selaginoides when dissolved CO2 and nutrients were also altered (Celis-Plá et al., 2017).
The tolerance of adult thalli of E. giacconei and the other intertidal Cystoseira s.l. species to temperatures up to 28°C might be related to an adaptation to the highly dynamic habitat they colonize. Indeed, the intertidal is characterized by large temperature fluctuations due to tidal cycles, especially during the warmer months. Notably, during summer tidal cycles, at the site where E. giacconei was sampled, these algae can experience temperatures ranging from 28°C (seawater temperature) at 1 m depth at high tide to 41°C (air temperature) at low tide within a few hours (Servizio Informativo Agrometereologico Siciliano, 1995; Clementi et al., 2019). In contrast, species that are not adapted to such extreme environmental changes might be more sensitive to temperature increases. For example, Verdura et al. (2021) reported that adults of the subtidal species Ericaria crinita showed a marked decrease in biomass, Fv/Fm, and C:N ratio during a 30-day period at 28°C. Similarly, Sato et al. (2020) observed a decrease in PSII efficiency in the subtidal kelp Saccharina sculpera maintained at temperatures ≥28°C, while the optimal range for the tested population was 22–24°C.
Despite the high tolerance of Cystoseira s.l. adults, especially of intertidal species, to temperature fluctuations, little is known about the possible effects on early developmental stages and developmental processes. Apart from the oldest embryological studies (e.g., Guern, 1962; Colombo et al., 1982; Gil-Rodríguez et al., 1988; Motta et al., 1988; Alongi et al., 1999), the embryogenesis of many Cystoseira s.l. species is still poorly known (Falace et al., 2018a; Savonitto et al., 2019). Based on reproductive traits and zygote division sequence, E. giacconei fits into the first embryological group described by Guern (1962), which includes most Cystoseira s.l. species (e.g., Ericaria mediterranea, Gongolaria elegans, and E. selaginoides).
Regarding the effect of seawater temperature on early developmental stages, we found that the eggs’ release efficiency did not vary significantly among the tested temperatures. However, greater exudate production was observed at higher temperatures (Supplementary Figure 4). Exudates, typically phlorotannins, are released by macroalgae under stress conditions (Sieburth and Jensen, 1969; Kroes, 1970; Abdala-Díaz et al., 2006). The settlement efficiency of the zygotes of E. giacconei increased from 12 to 18°C, but no statistically significant difference was found, then it started to decrease (24°C) and dropped significantly at 28°C. Remarkably, the extremely low settlement efficiency at 28°C was due to the fact that eggs and zygotes had undergone cell lysis and clustered together (Supplementary Figure 5).
The detrimental effect of heat was even more pronounced during germling development. Embryos were able to fully develop only at 12 and 15°C, while mortality increased sharply at 18°C and all germlings died at 28°C. The highest development rate observed at 15°C (highest percentage of embryos with rhizoids already after 20 h AF) suggests that this temperature represents the thermal optimum for reproduction and development of the early life stages. Actually, it corresponds to the mean seawater temperature during the winter months when the species reproduces.
To date, very few studies have investigated the potential effects of warming on the early life stages and in adults of Cystoseira s.l. species (e.g., Cáliz et al., 2019; Capdevila et al., 2019; Verdura et al., 2021). These studies focused specifically on the effects of high temperatures on the settlement and survival of recruits, showing that higher temperatures lead to embryo death. In particular, a tolerance threshold of 24°C was found in Ericaria zosteroides (as. C. zosteroides), a deep-sea species (Capdevila et al., 2019), and 28°C in Ericaria selaginoides (as C. tamariscifolia) (Cáliz et al., 2019) and Ericaria crinita (Verdura et al., 2021), two species from shallower waters. These results are only partially consistent with ours, as almost all germlings in this study failed to settle or survive at 28°C. However, in contrast to previous studies, we tested a broader temperature range and found that although E. giacconei is an intertidal to upper sublittoral species endemic to the southern Mediterranean, and thus hypothetically adapted to high temperatures, its thermal optimum is at much lower temperatures (12–15°C) than the other Cystoseira s.l. species examined.
Our findings suggest that E. giacconei is a stenothermic, cold-adapted macroalga that requires an extremely narrow range of low temperatures for embryonic development and survival. These results support the findings of Bouafif and Langar (2019) who, by modeling the potential spatial distribution of Cystoseira s.l. species in Tunisia, reported that E. giacconei occurs only in the colder waters of northern Tunisia. Sites where E. giacconei thrives could represent climatic refugia where the species still survives (e.g., Lourenço et al., 2016; Abelson et al., 2020; Verdura et al., 2021). The Sicilian Channel is characterized by a surface current called “Modified Atlantic Water” (MAW), forming two flows: one along the Sicilian shelf and the other off the Tunisian coast (Robinson et al., 1999; Béranger et al., 2004; Jouini et al., 2016). The complex bathymetry, as well as the water circulation, favor a semi-permanent upwelling regime, which is enhanced by local winds (e.g., Mistral) along the southern coast of Sicily. Therefore, the interplay of surface currents and upwelling provides lower sea surface temperatures along the coast (Raffa et al., 2017), but cannot prevent the occurrence of adverse climatic conditions.
Exceptionally high temperatures for several consecutive days during the reproductive season (e.g., Supplementary Figure 6) may actually lead to massive mortality of zygotes/embryos, thus defeating the reproductive efforts of the species. Furthermore, the negative effects of warming on recruitment could be exacerbated by other stressors that have been shown to negatively affect the early developmental stages of Cystoseira s.l., such as herbicides and pollutants (de Caralt et al., 2020).
From this point of view, the recruitment of new individuals seems to be the real bottleneck for the population dynamics of E. giacconei, as recruitment failures, if they occur over several years, can lead to lower population densities, ultimately affecting their long-term survival.
The stenothermic nature of the early life stages and the warmer sea areas that evenly surround the few localities with favorable conditions make this species a dotted endemism (Giaccone and Di Martino, 1996). Consequently, E. giacconei may become extinct if climate change continues with the current pattern. In the Sicilian Channel, several studies have already reported the disappearance of infralittoral stenoecious species of Cystoseira s.l. due to the increase in sea surface temperature and changes in deep circulation (Alongi et al., 2004; Catra et al., 2006; Serio et al., 2006), further evidence of the tropicalization process caused by climate change affecting the Mediterranean Sea (Boero et al., 2008; Furnari and Cormaci, 2009; Marbà et al., 2015).
Together with all Mediterranean species of Cystoseira s.l. (except C. compressa), E. giacconei is included in the “List of Threatened or Endangered Species” of Barcelona Convention (modified Annex II of the “Protocol on Specially Protected Areas and Biological Diversity”; United Nations Environment Agency, 2019; Verlaque et al., 2019), but its conservation status has not yet been defined by the IUCN (like the fucoid Sargassum, see Thibaut et al., 2016). In our opinion, E. giacconei should be included in the IUCN Red List of Species (International Union for Conservation of Nature, 2021) and classified as Critically Endangered due to its limited distribution and high vulnerability. As a conservation strategy, the climate refugia that ensure the persistence of E. giacconei should receive the highest level of protection.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
AF, MT, and GA conceived the ideas and designed the methodology. GM and GA collected samples in the field. AF, GS, and MS performed the experiments in aquaria. FC and SB performed the statistical analysis. AF led the writing of the manuscript. AF, GM, GS, MS, FC, SB, and MT contributed significantly to the draft of the manuscript and approved the submitted version. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the LIFE financial instrument of the European Community, the ROC-POP-LIFE project (LIFE16 NAT/IT/000816). Further support came from the University of Catania through grants under “Piano di incentivi per la ricerca di Ateneo 2020/2022 (Pia.ce.ri.) – Ricerca Dipartimentale.”
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Luca Giuseppe Costanzo for his helpful assistance in sampling and Marco Peplis for his valuable contribution to the laboratory work.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.760637/full#supplementary-material
References
Abdala-Díaz, R. T., Cabello-Pasini, A., Pérez-Rodríguez, E., Álvarez, R. C., and Figueroa, F. L. (2006). Daily and seasonal variations of optimum quantum yield and phenolic compounds in Cystoseira tamariscifolia (Phaeophyta). Mar. Biol. 148, 459–465. doi: 10.1007/s00227-005-0102-6
Abelson, A., Reed, D. C., Edgar, G. J., Smith, C. S., Kendrick, G. A., Orth, R. J., et al. (2020). Challenges for restoration of coastal marine ecosystems in the anthropocene. Front. Mar. Sci. 7:544105. doi: 10.3389/fmars.2020.544105
Allakhverdiev, S. I., Kreslavski, V. D., Klimov, V. V., Los, D. A., Carpentier, R., and Mohanty, P. (2008). Heat stress: an overview of molecular responses in photosynthesis. Photosynth. Res. 98, 541–550. doi: 10.1007/s11120-008-9331-0
Alongi, G., Catra, M., and Cormaci, M. (1999). Observations sur Cystoseira susanensis (Cystoseiraceae, Phaeophyta): une espèce méditerranéenne rare et peu connue. Cryptogam. Algol. 20, 25–33.
Alongi, G., Catra, M., Cormaci, M., Furnari, G., and Serio, D. (2004). Spring marine vegetation on rocky substrata of Pantelleria Island (the Straits of Sicily. Italy). Nova Hedwigia 79, 447–478. doi: 10.1127/0029-5035/2004/0079-0447
Álvarez-Losada, O., Arrontes, J., Martinez, B., Fernandez, C., and Viejo, R. M. (2020). A regime shift in intertidal assemblages triggered by loss of algal canopies: a multidecadal survey. Mar. Environ. Res. 160:104981. doi: 10.1016/j.marenvres.2020.104981
Anderson, M. J. (2001). A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26, 32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x
Araújo, R. M., Serrão, E. A., Sousa-Pinto, I., and Åberg, P. (2014). Spatial and temporal dynamics of Fucoid populations (Ascophyllum nodosum and Fucus serratus): a comparison between central and range edge populations. PLoS One 9:e92177. doi: 10.1371/journal.pone.0092177
Bennett, S., Wernberg, T., Arackal Joy, B., de Bettignies, T., and Campbell, A. H. (2015). Central and rear-edge populations can be equally vulnerable to warming. Nat. Commun. 6:10280. doi: 10.1038/ncomms10280
Béranger, K., Mortier, L., Gasparini, G. P., Gervasio, L., Astraldi, M., and Crépon, M. (2004). The dynamics of the Sicily Strait: a comprehensive study from observations and models. Deep Sea Res. Part II Top. Stud. Oceanogr. 51, 411–440. doi: 10.1016/j.dsr2.2003.08.004
Bevilacqua, S., Savonitto, G., Lipizer, M., Mancuso, P., Ciriaco, S., Srijemsi, M., et al. (2019). Climatic anomalies may create a long-lasting ecological phase shift by altering the reproduction of a foundation species. Ecology 100:e02838. doi: 10.1002/ecy.2838
Blanfuné, A., Boudouresque, C. F., Verlaque, M., and Thibaut, T. (2016). The fate of Cystoseira crinita, a forest-forming fucale (Phaeophyceae, Stramenopiles), in France (Northwestern Mediterranean Sea). Estuar. Coast. Shelf Sci. 181, 196–208. doi: 10.1016/j.ecss.2016.08.049
Boero, F., Féral, J., Azzurro, E., Cardin, V., Riedel, B., Despalatović, M., et al. (2008). Climate warming and related changes in Mediterranean marine biota. CIESM Workshop Monogr. 35, 5–21.
Bouafif, C., and Langar, H. (2019). “Predicting Cystoseira species distribution in Tunisia using maximum entropy method (MAXENT),” in Proceedings of the 6th Mediterranean Symposium on Marine Vegetation (Antalya, Turkey, 14-15 January 2019), (Antalya),
Bouafif, C., Verlaque, M., and Langar, H. (2016). New Contribution to the Knowledge of the Genus Cystoseira C. Agardh in the Mediterranean Sea, with the Reinstatement of Species Rank for C. schiffneri Hamel. Cryptogam. Algol. 37, 133–154. doi: 10.7872/crya/v37.iss2.2016.133
Bray, J. R., and Curtis, J. T. (1957). An ordination of the upland forest communities of Southern Wisconsin. Ecol. Monograph. 27, 325–349. doi: 10.2307/1942268
Buonomo, R., Assis, J., Fernandes, F., Engelen, A. H., Airoldi, L., and Serrão, E. A. (2017). Habitat continuity and stepping-stone oceanographic distances explain population genetic connectivity of the brown alga Cystoseira amentacea. Mol. Ecol. 26, 766–780. doi: 10.1111/mec.13960
Burdett, H. L., Wright, H., and Smale, D. A. (2019). Photophysiological responses of canopy-forming kelp species to short-term acute warming. Front. Mar. Sci. 6:516. doi: 10.3389/fmars.2019.00516
Bussotti, F., Desotgiu, R., Pollastrini, M., and Cascio, C. (2010). The JIP test: a tool to screen the capacity of plant adaptation to climate change. Scand. J. For. Res. 25, 43–50. doi: 10.1080/02827581.2010.485777
Bustamante, M., Tajadura, J., Díez, I., and Saiz-Salinas, J. I. (2017). The potential role of habitat-forming seaweeds in modeling benthic ecosystem properties. J. Sea Res. 130, 123–133. doi: 10.1016/j.seares.2017.02.004
Cáliz, A. C., Fernández, A. N., de Pedro, R. S., Flores-Moya, A., and Bañares-España, E. (2019). “Physiological responses of adults and juveniles of Cystoseira tamariscifolia to projected warming scenarios along Alboran sea populations,” in Proceedings of the II International Congress of Young Marine Researchers. Book of Abstracts, (Malaga, ES: Fundación CEIÁMar), 426–430.
Capdevila, P., Hereu, B., Salguero-Gómez, R., Rovira, G., Medrano, A., Cebrian, E., et al. (2019). Warming impacts on early life stages increase the vulnerability and delay the population recovery of a long-lived habitat-forming macroalga. J. Ecol. 107, 1129–1140. doi: 10.1111/1365-2745.13090
Capdevila, P., Linares, C., Aspillaga, E., Riera, J. L., and Hereu, B. (2018). Effective dispersal and density-dependence in mesophotic macroalgal forests: insights from the Mediterranean species Cystoseira zosteroides. PLoS One 13:e0191346. doi: 10.1371/journal.pone.0191346
Catra, M., Giaccone, T., Giardina, S., and Nicastro, A. (2006). Il patrimonio naturale marino bentonico della Timpa di Acireale (Catania). Boll. Acc. Gioenia Sci. Nat. 39, 129–158.
Celis-Plá, P. S., Korbee, N., Gómez-Garreta, A., and Figueroa, F. L. (2014). Seasonal photoacclimation patterns in the intertidal macroalga Cystoseira tamariscifolia (Ochrophyta). Sci. Mar. 78, 377–388. doi: 10.3989/scimar.04053.05A
Celis-Plá, P. S., Martínez, B., Korbee, N., Hall-Spencer, J. M., and Figueroa, F. L. (2017). Photoprotective responses in a brown macroalgae Cystoseira tamariscifolia to increases in CO2 and temperature. Mar. Environ. Res. 130, 157–165. doi: 10.1016/j.marenvres.2017.07.015
Clementi, E., Pistoia, J., Escudier, R., Delrosso, D., Drudi, M., Grandi, A., et al. (2019). Mediterranean Sea analysis and forecast (CMEMS MED-Currents, EAS5 system) [Data set]. Copernicus Monitoring Environment Marine Service (CMEMS).
Colombo, P., Curcio, M. F., and Giaccone, G. (1982). Biologia dello sviluppo di un endemismo mediterraneo del genere Cystoseira (Phaeophyceae, Fucales): Cystoseira sedoides C. Agardh. Naturalista Sicil. 6, 81–93.
Cormaci, M., Furnari, G., Catra, M., Alongi, G., and Giaccone, G. (2012). Flora marina bentonica del Mediterraneo: phaeophyceae. Boll. Acc. Gioenia Sci. Nat. 45, 1–508.
de Bettignies, T., Wernberg, T., and Gurgel, C. F. (2018). Exploring the influence of temperature on aspects of the reproductive phenology of temperate seaweeds. Front. Mar. Sci 5:218. doi: 10.3389/fmars.2018.00218
de Caralt, S., Verdura, J., Vergés, A., Ballesteros, E., and Cebrian, E. (2020). Differential effects of pollution on adult and recruits of a canopy-forming alga: implications for population viability under low pollutant levels. Sci. Rep. 10:17825. doi: 10.1038/s41598-020-73990-5
Diehl, N., Roleda, M. Y., Bartsch, I., Karsten, U., and Bischof, K. (2021). Summer heatwave impacts on the European kelp Saccharina latissima across its latitudinal distribution gradient. Front. Mar. Sci. 8:695821.
Diffenbaugh, N. S., Pal, J. S., Giorgi, F., and Gao, X. (2007). Heat stress intensification in the Mediterranean climate change hotspot. Geophys. Res. Lett. 34:L11706. doi: 10.1029/2007GL030000
Falace, A., Alongi, G., Cormaci, M., Furnari, G., Curiel, D., Cecere, E., et al. (2010). Changes in the benthic algae along the Adriatic Sea in the last three decades. Chem. Ecol. 26, 77–90. doi: 10.1080/02757541003689837
Falace, A., Tamburello, L., Guarnieri, G., Kaleb, S., Papa, L., and Fraschetti, S. (2018b). Effects of a glyphosate-based herbicide on Fucus virsoides (Fucales, Ochrophyta) photosynthetic efficiency. Environ. Pollut. 243, 912–918. doi: 10.1016/j.envpol.2018.08.053
Falace, A., Kaleb, S., De La Fuente, G., Asnaghi, V., and Chiantore, M. (2018a). Ex situ cultivation protocol for Cystoseira amentacea var. stricta (Fucales, Phaeophyceae) from a restoration perspective. PLoS one 13:e0193011.
Fernández, P. A., Gaitán-Espitia, J. D., Leal, P. P., Schmid, M., Revill, A. T., and Hurd, C. L. (2020). Nitrogen sufficiency enhances thermal tolerance in habitat-forming kelp: implications for acclimation under thermal stress. Sci. Rep. 10:3186. doi: 10.1038/s41598-020-60104-4
Figueroa, F. L., Celis-Plá, P. S., Martínez, B., Korbee, N., Trilla, A., and Arenas, F. (2019). Yield losses and electron transport rate as indicators of thermal stress in Fucus serratus (Ochrophyta). Algal Res. 41:101560. doi: 10.1016/j.algal.2019.101560
Filbee-Dexter, K., and Wernberg, T. (2020). Substantial blue carbon in overlooked Australian kelp forests. Sci. Rep. 10:12341. doi: 10.1038/s41598-020-69258-7
Furnari, G., and Cormaci, M. (2009). Floristic changes in the Mediterranean macroalgal flora. Bocconea 23:85.
Galun, E., and Torrey, J. G. (1969). Initiation and suppression of apical hairs of Fucus embryos. Develop. Biol. 19, 447–459. doi: 10.1016/0012-1606(69)90082-7
Giaccone, G. (1985). Una nuova specie mediterranea del genere Cystoseira C. Agardh (Phaeophyta, Fucales): C. hyblaea G. Giaccone, con osservazioni critiche su alcune entità tassonomiche poco note o imperfettamente descritte. Boll. Acc. Gioenia Sci. Nat. 18, 429–442.
Giaccone, G., and Di Martino, V. (1996). Flora, vegetazione marina e stato dell’ambiente nell’area iblea. Boll. Acc. Gioenia Sci. Nat. 29, 359–391.
Gil-Rodríguez, M. C., Afonso Carrillo, J., Sansón, M., Chacana, M. E., Reyes, J., and Wildpret de la Torre, W. (1988). “Embriogénesis en Cystoseira abies-marina (Gmelin) C. Agardh (Pheaeophyta). Importancia biosistemática,” in Actes del Simposi Internacional de Botanica Pius Font i Quer, eds J. A. Conesa and J. Recasens (Lleida, ES: Institut d’estudis Llerdencs), 123–127.
Gouvêa, L. P., Schubert, N., Martins, C. D. L., Sissini, M., Ramlov, F., Rodrigues, E. R. D. O., et al. (2017). Interactive effects of marine heatwaves and eutrophication on the ecophysiology of a widespread and ecologically important macroalga. Limnol. Oceanogr. 62, 2056–2075. doi: 10.1002/lno.10551
Graiff, A., Liesner, D., Karsten, U., and Bartsch, I. (2015). Temperature tolerance of western Baltic Sea Fucus vesiculosus growth, photosynthesis and survival. J. Exp. Mar. Biol. Ecol. 471, 8–16. doi: 10.1016/j.jembe.2015.05.009
Guern, M. (1962). Embryologie de quelques espèces du genre Cystoseira Agardh 1821 (Fucales) [Embryology of some species of the genus Cystoseira Agardh 1821 (Fucales)]. Vie Milieu 13, 649–679.
Gurgel, C. F. D., Camacho, O., Minne, A. J. P., Wernberg, T., and Coleman, M. A. (2020). Marine heatwave drives cryptic loss of genetic diversity in underwater forests. Curr. Biol. 30, 1199–1206. doi: 10.1016/j.cub.2020.01.051
Hereward, H. F., King, N. G., and Smale, D. A. (2020). Intra-annual variability in responses of a canopy forming kelp to cumulative low tide heat stress: implications for populations at the trailing range edge. J. Phycol. 56, 146–158. doi: 10.1111/jpy.12927
International Union for Conservation of Nature (2021). The International Union for Conservation of Nature’s Red List of Threatened Species. Available online at: https://www.iucnredlist.org/ (accessed November 12, 2021).
IPCC (2019). IPCC Special Report on the Ocean and Cryosphere in a Changing Climate (IPCC, 2019). Geneva: IPCC.
Iveša, L., Djakovac, T., and Devescovi, M. (2016). Long-term fluctuations in Cystoseira populations along the west Istrian Coast (Croatia) related to eutrophication patterns in the northern Adriatic Sea. Mar. Poll. Bull. 106, 162–173. doi: 10.1016/j.marpolbul.2016.03.010
Jouini, M., Béranger, K., Arsouze, T., Beuvier, J., Thiria, S., Crépon, M., et al. (2016). The Sicily Channel surface circulation revisited using a neural clustering analysis of a high-resolution simulation. J. Geophys. Res. Oceans 121, 4545–4567. doi: 10.1002/2015JC011472
Jueterbock, A., Kollias, S., Smolina, I., Fernandes, J. M. O., Coyer, J. A., Olsen, J. L., et al. (2014). Thermal stress resistance of the brown alga Fucus serratus along the North-Atlantic coast: acclimatization potential to climate change. Mar. Genomics 13, 27–36. doi: 10.1016/j.margen.2013.12.008
Krause-Jensen, D., and Duarte, C. M. (2016). Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci. 9, 737–742. doi: 10.1038/ngeo2790
Kroes, H. W. (1970). Excretion of mucilage and yellow-brown substances by some brown algae from the intertidal zone. Bot. Mar. 13, 107–110.
Krumhansl, K. A. K., Okamoto, D. D. K. D., Rassweiler, A., Novak, M., Bolton, J. J., Cavanaugh, K. C., et al. (2016). Global patterns of kelp forest change over the past half-century. PNAS 113, 13785–13790. doi: 10.1073/pnas.1606102113
Lourenço, C. R., Zardi, G. I., McQuaid, C. D., Serrao, E. A., Pearson, G. A., Jacinto, R., et al. (2016). Upwelling areas as climate change refugia for the distribution and genetic diversity of a marine macroalga. J. Biogeogr. 43, 1595–1607. doi: 10.1111/jbi.12744
Mancuso, F. P., Messina, C. M., Santulli, A., Laudicella, V. A., Giommi, C., Sarà, G., et al. (2019). Influence of ambient temperature on the photosynthetic activity and phenolic content of the intertidal Cystoseira compressa along the Italian coastline. J. Appl. Phycol. 31, 3069–3076. doi: 10.1007/s10811-019-01802-z
Marbà, N., Jorda, G., Agusti, S., Girard, C., and Duarte, C. M. (2015). Footprints of climate change on Mediterranean Sea biota. Front. Mar. Sci. 2:56.
Medrano, A., Linares, C., Aspillaga, E., Capdevila, P., Montero-Serra, I., Pagès-Escolà, M., et al. (2020). Long-term monitoring of temperate macroalgal assemblages inside and outside a no take marine reserve. Mar. Environ. Res. 153:104826. doi: 10.1016/j.marenvres.2019.104826
Mineur, F., Arenas, F., Assis, J., Davies, A. J., Engelen, A. H., Fernandes, F., et al. (2015). European seaweeds under pressure: consequences for communities and ecosystem functioning. J. Sea Res. 98, 91–108. doi: 10.1016/j.seares.2014.11.004
Misra, A. N., Srivastava, A., and Strasser, R. J. (2001). Utilization of fast chlorophyll a fluorescence technique in assessing the saltion sensitivity of mung bean and Brassica seedlings. J. Plant Physiol. 158, 1173–1181. doi: 10.1078/S0176-1617(04)70144-3
Mota, C. F., Engelen, A. H., Serrao, E. A., and Pearson, G. A. (2015). Some don’t like it hot: microhabitat-dependent thermal and water stresses in a trailing edge population. Funct. Ecol. 29, 640–649. doi: 10.1111/1365-2435.12373
Motta, G., Cormaci, M., and Giaccone, G. (1988). Osservazioni in coltura sui primi stadi di segmentazione dello zigote di alcune specie del genere Cystoseira C. Agardh (Fucales, Phaeophyta). Boll. Acc. Gioenia Sci. Nat. 21, 199–208.
Munda, I. M. (2000). Long-term marine floristic changes around Rovinj (Istrian coast, North Adriatic) estimated on the basis of historical data from Paul Kuckuck’s field diaries from the end of the 19th century. Nova Hedwigia 71, 1–36.
Nepper-Davidsen, J., Andersen, D. T., and Pedersen, M. F. (2019). Exposure to simulated heatwave scenarios causes long-term reductions in performance in Saccharina latissima. Mar. Ecol. Prog. Ser. 630, 25–39. doi: 10.3354/meps13133
Nicastro, K. R., Zardi, G. I., Teixeira, S., Neiva, J., Serrão, E. A., and Pearson, G. A. (2013). Shift happens: trailing edge contraction associated with recent warming trends threatens a distinct genetic lineage in the marine macroalga Fucus vesiculosus. BMC Biol. 11:6. doi: 10.1186/1741-7007-11-6
Nielsen, S. L., Nielsen, H. D., and Pedersen, M. F. (2014). Juvenile life stages of the brown alga Fucus serratus L. are more sensitive to combined stress from high copper concentration and temperature than adults. Mar. Biol. 161, 1895–1904. doi: 10.1007/s00227-014-2471-1
Nienburg, W. (1931). Die Entwicklung der Keimlinge von Fucus vesiculosus und ihre Bedeutung für die Phylogenie der Phaeophyceen. Wiss. Meer. Ab. Kiel. 1, 52–62.
Orlando-Bonaca, M., and Rotter, A. (2018). Any signs of replacement of canopy-forming algae by turf-forming algae in the northern Adriatic Sea? Ecol. Ind. 87, 272–284. doi: 10.1016/j.ecolind.2017.12.059
Oukarroum, A., El Madidi, S., and Strasser, R. J. (2016). Differential heat sensitivity index in barley cultivars (Hordeum vulgare L.) monitored by chlorophyll a fluorescence OKJIP. Plant Physiol Biochem 105, 102–108. doi: 10.1016/j.plaphy.2016.04.015
Pastor, F., Valiente, J. A., and Khodayar, S. (2020). A warming Mediterranean: 38 years of increasing sea surface temperature. Remote Sens. 12:2687. doi: 10.3390/rs12172687
Pearson, G. A., Lago-Leston, A., and Mota, C. (2009). Frayed at the edges: selective pressure and adaptive response to abiotic stressors are mismatched in low diversity edge populations. J. Ecol. 97, 450–462. doi: 10.1111/j.1365-2745.2009.01481.x
Perkol-Finkel, S., and Airoldi, L. (2010). Loss and recovery potential of marine habitats: an experimental study of factors maintaining resilience in subtidal algal forests at the Adriatic sea. PLoS One 5:e10791. doi: 10.1371/journal.pone.0010791
Pisano, A., Marullo, S., Artale, V., Falcini, F., Yang, C., Leonelli, F. E., et al. (2020). New evidence of Mediterranean climate change and variability from sea surface temperature observations. Remote Sens. 12:132. doi: 10.3390/rs12010132
Provost, E. J., Kelaher, B. P., Dworjanyn, S. A., Russell, B. D., Connell, S. D., Ghedini, G., et al. (2017). Climate-driven disparities among ecological interactions threaten kelp forest persistence. Glob. Change Biol. 23, 353–361. doi: 10.1111/gcb.13414
Raffa, F., Ludeno, G., Patti, B., Soldovieri, F., Mazzola, S., and Serafino, F. (2017). X-band wave radar for coastal upwelling detection off the southern coast of Sicily. J. Atmos. Ocean. Technol. 34, 21–31. doi: 10.1175/JTECH-D-16-0049.1
Robinson, A. R., Sellschopp, J., Warn-Varnas, A., Leslie, W. G., Lozano, C. J., Haley, P. J., et al. (1999). The Atlantic Ionian stream. J. Mar. Syst. 20, 129–156.
Rothäusler, E., Rugiu, L., and Jormalainen, V. (2018). Forecast climate change conditions sustain growth and physiology but hamper reproduction in range-margin populations of a foundation rockweed species. Mar. Environ. Res. 141, 205–213. doi: 10.1016/j.marenvres.2018.09.014
Sato, Y., Kozono, J., Nishihara, G. N., and Terada, R. (2020). Effect of light and temperature on photosynthesis of a cultivated brown alga, Saccharina sculpera (Laminariales), from Japan. Phycologia 59, 375–384. doi: 10.1080/00318884.2020.1777384
Savonitto, G., Alongi, G., and Falace, A. (2019). Reproductive phenology, zygote embryology and germling development of the threatened Carpodesmia barbatula (= Cystoseira barbatula) (Fucales, Phaeophyta) towards its possible restoration. Webbia 74, 317–323. doi: 10.1080/00837792.2019.1692594
Savonitto, G., De La Fuente, G., Tordoni, E., Ciriaco, S., Srijemsi, M., Bacaro, G., et al. (2021). Addressing reproductive stochasticity and grazing impacts in the restoration of a canopy-forming brown alga by implementing mitigation solutions. Aquat. Conserv. 31, 1611–1623. doi: 10.1002/aqc.3555
Savva, I., Bennett, S., Roca, G., Jordà, G., and Marbà, N. (2018). Thermal tolerance of Mediterranean marine macrophytes: vulnerability to global warming. Ecol. Evol. 8, 12032–12043. doi: 10.1002/ece3.4663
Serio, D., Alongi, G., Catra, M., Cormaci, M., and Furnari, G. (2006). Changes in the benthic algal flora of Linosa Island (Straits of Sicily. Mediterranean Sea). Bot. Mar. 49, 135–144. doi: 10.1515/BOT.2006.018
Servizio Informativo Agrometereologico Siciliano (1995). Climatologia della Sicilia. Available online at: http://www.sias.regione.sicilia.it/pdf/Climatologia_sicilia.pdf (accessed November 12, 2021).
Sieburth, J. M., and Jensen, A. (1969). Studies on algal substances in the sea. II. The formation of gelbstoff (humic material) by exudates of Phaeophyta. J. Exp. Mar. Biol. Ecol. 3, 275–289.
Smale, D. A. (2020). Impacts of ocean warming on kelp forest ecosystems. New Phytol. 225, 1447–1454. doi: 10.1111/nph.16107
Smale, D. A., Burrows, M. T., Moore, P., O’Connor, N., and Hawkins, S. J. (2013). Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecol. Evol. 3, 4016–4038. doi: 10.1002/ece3.774
Smolina, I., Kollias, S., Jueterbock, A., Coyer, J. A., and Hoarau, G. (2016). Variation in thermal stress response in two populations of the brown seaweed, Fucus distichus, from the Arctic and subarctic intertidal. R. Soc. Open Sci. 3:150429. doi: 10.1098/rsos.150429
Soltan, D., Verlaque, M., Boudouresque, C. F., and Francour, P. (2001). Changes in macroalgal communities in the vicinity of a Mediterranean sewage outfall after the setting up of a treatment plant. Mar. Pollut. Bull. 42, 59–70. doi: 10.1016/S0025-326X(00)00116-8
Steneck, R. S., Graham, M. H., Bourque, B. J., Corbett, D., Erlandson, J. M., Estes, J. A., et al. (2002). Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ. Conserv. 29, 436–459. doi: 10.1017/S0376892902000322
Strain, E. M. A., Thomson, R. J., Micheli, F., Mancuso, F. P., and Airoldi, L. (2014). Identifying the interacting roles of stressors in driving the global loss of canopy-forming to mat-forming algae in marine ecosystems. Glob. Change Biol. 20, 3300–3312. doi: 10.1111/gcb.12619
Strasser, R. J., Srivastava, A., and Tsimilli-Michael, M. (2000). “The fluorescence transient as a tool to characterize and screen photosynthetic samples,” in Probing Photosynthesis: Mechanism, Regulation and Adaptation, eds M. Yunus, U. Pathre, and P. Mohanty (London: Taylor and Francis), 443–480.
Straub, S. C., Wernberg, T., Thomsen, M. S., Moore, P. J., Burrows, M. T., Harvey, B. P., et al. (2019). Resistance, extinction, and everything in between – The diverse responses of seaweeds to marine heatwaves. Front. Mar. Sci. 6:763. doi: 10.3389/fmars.2019.00763
Strömgren, T. (1977). Short-term effects of temperature upon the growth of intertidal Fucales. J. Exper. Mar. Biol. Ecol. 29, 181–195.
Teagle, H., Hawkins, S. J., Moore, P. J., and Smale, D. A. (2017). The role of kelp species as biogenic habitat formers in coastal marine ecosystems. J. Exp. Mar. Biol. Ecol. 492, 81–89. doi: 10.1016/j.jembe.2017.01.017
Thibaut, T., Blanfuné, A., Boudouresque, C. F., and Verlaque, M. (2015). Decline and local extinction of Fucales in the French riviera: the harbinger of future extinctions? Mediterr. Mar. Sci. 16, 206–224. doi: 10.12681/mms.1032
Thibaut, T., Blanfuné, A., Verlaque, M., Boudouresque, C. F., and Ruitton, S. (2016). The Sargassum conundrum: very rare, threatened or locally extinct in the NW Mediterranean and still lacking protection. Hydrobiologia 781, 3–23. doi: 10.1007/s10750-015-2580-y
Thibaut, T., Pinedo, S., Torras, X., and Ballesteros, E. (2005). Long-Term decline of the populations of Fucales (Cystoseira spp. and Sargassum spp.) in the Albères Coast (France, North-Western Mediterranean). Mar. Pollut. Bull. 50, 1472–1489. doi: 10.1016/j.marpolbul.2005.06.014
Umanzor, S., Sandoval-Gil, J., Sánchez-Barredo, M., Ladah, L. B., Ramírez-García, M. M., and Zertuche-González, J. A. (2021). Short-term stress responses and recovery of giant kelp (Macrocystis pyrifera, Laminariales, Phaeophyceae) juvenile sporophytes to a simulated marine heatwave and nitrate scarcity. J. Phycol. 57, 1604–1618. doi: 10.1111/jpy.13189
Underwood, A. J. (1997). Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variance. Cambridge: Cambridge University Press.
United Nations Environment Agency (2019). Resolution 73/284: United Nations Decade on Ecosystem Restoration (2021–2030). Available online at: https://undocs.org/A/RES/73/284 (accessed November 12, 2021).
Vargas-Yáñez, M., Garcia, M. J., Salat, J., Garcia-Martinez, M. C., Pascual, J., and Moya, F. (2008). Warming trends and decadal variability in the Western Mediterranean shelf. Glob. Planet. Change 63, 177–184. doi: 10.1016/j.gloplacha.2007.09.001
Verdura, J., Santamaría, J., Ballesteros, E., Smale, D., Cefalì, M. E., Golo, R., et al. (2021). Local-scale climatic refugia offer sanctuary for a habitat-forming species during a marine heatwave. J. Ecol. 109, 1–16. doi: 10.1111/1365-2745.13599
Vergés, A., Doropoulos, C., Malcolm, H. A., Skye, M., Garcia-Pizá, M., Marzinelli, E. M., et al. (2016). Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. PNAS 113, 13791–13796. doi: 10.1073/pnas.1610725113
Vergés, A., Steinberg, P. D., Hay, M. E., Poore, A. G. B., Campbell, A. H., Ballesteros, E., et al. (2014). The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proc. R. Soc. B 281:20140846. doi: 10.1098/rspb.2014.0846
Verlaque, M., Boudouresque, C. F., and Perret-Boudouresque, M. (2019). Mediterranean seaweeds listed as threatened under the Barcelona Convention: a critical analysis. Sci. Rep. Port Cros natl. Park 33, 179–214.
Viejo, R. M., Martínez, B., Arrontes, J., Astudillo, C., and Hernández, L. (2011). Reproductive patterns in central and marginal populations of a large brown seaweed: drastic changes at the southern range limit. Ecography 34, 75–84. doi: 10.1111/j.1600-0587.2010.06365.x
Wernberg, T., Thomsen, M. S., Tuya, F., Kendrick, G. A., Staehr, P. A., and Toohey, B. D. (2010). Decreasing resilience of kelp beds along a latitudinal temperature gradient: potential implications for a warmer armer future. Ecol. Lett. 13, 685–694. doi: 10.1111/j.1461-0248.2010.01466.x
Wernberg, T. S., Bennett, R. C., Babcock, T., de Bettignies, K., Cure, M., Depczynski, F., et al. (2016). Climate-driven regime shift of a temperate marine ecosystem. Science 353, 169–172. doi: 10.1126/science.aad8745
Wilson, K. L., Kay, L. M., Schmidt, A. L., and Lotze, H. K. (2015). Effects of increasing water temperatures on survival and growth of ecologically and economically important seaweeds in Atlantic Canada: implications for climate change. Mar. Biol. 162, 2431–2444. doi: 10.1007/s00227-015-2769-7
Keywords: thermal stress, early life stages, photosynthetic efficiency, marine forest, climate change, conservation
Citation: Falace A, Marletta G, Savonitto G, Candotto Carniel F, Srijemsi M, Bevilacqua S, Tretiach M and Alongi G (2021) Is the South-Mediterranean Canopy-Forming Ericaria giacconei (= Cystoseira hyblaea) a Loser From Ocean Warming? Front. Mar. Sci. 8:760637. doi: 10.3389/fmars.2021.760637
Received: 18 August 2021; Accepted: 02 November 2021;
Published: 23 November 2021.
Edited by:
Cataldo Pierri, University of Bari Aldo Moro, ItalyReviewed by:
Carlos Sangil, University of La Laguna, SpainValentina Asnaghi, University of Genoa, Italy
Ignacio Gestoso, Center for Marine and Environmental Sciences (MARE), Portugal
Copyright © 2021 Falace, Marletta, Savonitto, Candotto Carniel, Srijemsi, Bevilacqua, Tretiach and Alongi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Annalisa Falace, ZmFsYWNlQHVuaXRzLml0
 Annalisa Falace
Annalisa Falace Giuliana Marletta
Giuliana Marletta Gilda Savonitto
Gilda Savonitto Fabio Candotto Carniel
Fabio Candotto Carniel Marina Srijemsi
Marina Srijemsi Stanislao Bevilacqua
Stanislao Bevilacqua Mauro Tretiach1
Mauro Tretiach1 Giuseppina Alongi
Giuseppina Alongi