- 1Key Laboratory of Freshwater Aquatic Genetic Resources, Ministry of Agriculture, Shanghai Ocean University, Shanghai, China
- 2Shanghai Engineering Research Center of Aquaculture, Shanghai Ocean University, Shanghai, China
- 3National Demonstration Centre for Experimental Fisheries Science Education, Shanghai Ocean University, Shanghai, China
To investigate the growth and feeding conditions of the Chinese mitten crab Eriocheir sinensis under different feeding modes: traditional (mainly consisting of wheat, bran, and soybean meal), formulated, and mixed feeds (1:1 mixture of traditional and formulated feeds) were fed in different crab breeding ponds in this study. During the experiment, the stomach contents of juvenile crabs under the different feeding modes were collected. The main potential eukaryotic food components were studied using 18S ribosomal DNA sequencing, and the contribution of different feeding modes to the feeding source of juvenile crabs were analyzed using C and N stable isotopes. The terminal weight and weight gain rate of crabs under the formulated feeding mode were significantly higher than those in the traditional and mixed feeding modes (P < 0.05). No differences were observed in the diversity and abundance of the main potential eukaryotic feed components of male and female crabs under different feeding modes (P > 0.05). Thirty-four phyla, composed mainly of benthic organisms, were identified, with Arthropoda (mainly including Malacostraca, 30.25–51.48%), Phragmoplastophyta (mainly including Embryophyta and Trebouxiophyceae, 5.08–24.74%), and Diatomea (3.13–8.43%) being the most abundant. The δ13C and δ15N values of the feeding sources and muscle of crabs ranged from −34.45 to −22.21‰, and from 0.27 to 5.66‰, respectively, varying greatly among the three feeding modes and δ15N value of muscle under formulated feeding mode was significantly higher than that in traditional feeding mode (P < 0.05). The proportion of particulate organic matter (11.92–17.50%) is similar to Alternanthera philoxeroides (11.24–16.03%) in three feeding modes. There was no significant difference in feeding habits between male and female crabs under the same and different feeding modes. Juvenile crabs feed on both plant- and animal-based feeds in an aquaculture pond, but they are not complete predators and selectively feed on animal or plant feeds as supplements of that which is deficient, in addition to their main feed.
Introduction
The Chinese mitten crab, Eriocheir sinensis, which belongs to the phylum Arthropoda, subphylum Crustacea, order Decapoda, and family Graspidae, is one of the major economically important farmed crab species in China (Huang et al., 2019). In 2020, its production from aquaculture reached 775 887 tons (MOAC, 2020). It is mainly produced using the pond culture method (Wang and Wang, 2013), and its breeding cycle usually takes 2 years, with the first year involving juvenile crab breeding from the big-eyed larvae to juvenile crabs weighing 5–10 g (Cheng et al., 2008) and the second year involving adult crab breeding until gonad maturation (Cheng et al., 2008). Que et al. (2012) found that the survival rate, weight gain rate, and first molting time of crabs in the second year could be improved if they were fed with high-quality bait at the latter stage of juvenile culture. Therefore, it is necessary to understand the nutrient requirements of crabs in the juvenile culture stage. At present, three feeding modes for juvenile crab culture are prevalent in China: whole-course formulated feeds, traditional feeds, and mixed feeds (1:1 mixture of traditional and formulated feeds) (Fu et al., 2014). The traditional feeding mode, which plays a leading role in the culture of juvenile E. sinensis in China, mainly contains soybean meal, bran, rapeseed meal and wheat (Wang and Wang, 2013; He et al., 2014). This feeding mode is cheaper, but will lead to problems such as early maturity of crab species, poor quality of adult crab and deterioration of water quality, which seriously restricts the green and sustainable development of the culture industry of E. sinensis (Pan et al., 2016). Consequently, nutritionally balanced artificial formulated feed is increasingly being adopted to facilitate sustainable development of industry (Han et al., 2021). However, the food composition of E. sinensis under these different feeding modes and the contribution of the different feed sources to its growth have not been reported yet.
Chinese mitten crab is an omnivorous animal (Lafontaine and Veilleux, 2002). Using the stomach content analysis to study the feeding habits of different life stages of wild E. sinensis, Rudnick and Resh (2005) showed that the zoea and megalopa stages mainly feed on phytoplankton and zooplankton; the juvenile crab feeds mainly on aquatic plants (Rudnick et al., 2000); and the second instar adult crab often feeds on plants, shrimp, shellfish, and aquatic insects, being more inclined to a carnivorous diet (Chen et al., 1989). There are related reports on the feeding habits of E. sinensis, but owing to technical limitations, there is a lack of accurate quantitative data on its feeding habits. In recent years, with the development of molecular techniques, DNA barcoding has been widely used in the analysis of aquatic animal feeding habits. At present, there are three methods of DNA barcoding for stomach content analysis, including the mitochondrial cytochrome-oxidase I (COI) gene (Bade et al., 2014), internal transcribed spacer (ITS) (Bachy et al., 2013), and 18S ribosomal DNA (rDNA) (Riemann et al., 2010). However, the amplified fragments of COI and ITS are longer than those of 18S rDNA, and the variation is higher. Therefore, the amplified fragments of COI and ITS are suitable for species with a single feeding level. The 18S rDNA can be applied to the analysis of the feeding habits of aquatic animals (Wang X. F. et al., 2017), including the main feed source of organisms (Zhou et al., 2020), change in feeding habits during the transformation of growth (Zeng, 2010), environment (Wang X. F. et al., 2017), and even the discovery of feed sources that have not been found in previous studies (O’Rorke et al., 2012). For example, Arthropoda was the most dominant phylum in the gut of Carcinus maenas, followed by Ochrophyta and Mollusca (Cordone et al., 2020). The feeding habits of Ostrea gigas Thunberg did not change significantly before and after the Enteromorpha transit, and the dominant species belonged to the genus Streptophyta (Wang X. F. et al., 2017).
After the determination of feed sources, the contribution of different feed sources to the growth of aquatic animals should be quantitatively studied, and stable isotopes have been developed as a relatively advanced technique (Lin, 2013). The simultaneous use of 13C and 15N stable isotopes can determine the contribution ratio of different feed sources (Sun, 2012) under natural conditions in the wild (Lan et al., 2020) as well as during artificial breeding (Li et al., 2018). For example, under natural conditions, shellfish is the main feed for Oratosquilla oratoria, with an average contribution rate of 38.6%, which is significantly higher than the average contribution rate of fish (only 8.9%) (Ning et al., 2016). Paddy-farmed Procampus clarkii were mainly fed with feed (18.86–44.17%), followed by particulate organic matter POM (16.77–23.2%). Therefore, the combination of 18S rDNA and stable isotope analysis can be used for improved study of the feeding habits of crabs.
It is necessary to understand the changes in the intestinal content and food composition under different feeding modes to develop a feed formula more conducive to the growth of juvenile crab. To date, the effect of these three feeding modes on the diet of crabs has not been evaluated. In this study, 18S rDNA sequencing and stable isotope analysis were used to determine the effects of three different feeding modes on the growth of juvenile crabs, the composition and contribution of the main potential feed sources in the stomach of juvenile crabs, and to compared the feeding habits of male and female crabs. Understanding pond aquaculture nutrition ecology in the process of juvenile crab breeding is of great significance and can provide a theoretical basis for feeding ecology for the research and development of high-efficiency formulated feed for E. sinensis in artificial culture.
Materials and Methods
Breeding Management
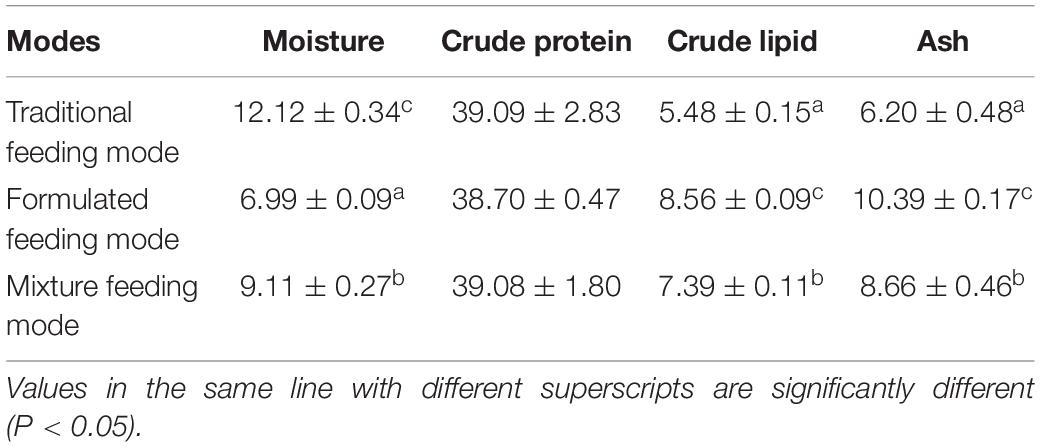
The experiment was carried out in nine mud aquaculture ponds with length, width, and depth of 70, 30, and 1.5 m, respectively, in Dongtan Aquaculture Base at Shanghai Daohong Aquaculture Technology Co., Ltd (31.62° N, 121.40° E) from July to November 2018. To begin the experiment, 6,000 juvenile crabs weighing between 1 and 2g were placed in each aquaculture pond. Alternanthera philoxeroides was planted in the center of the pond, and the surrounding area was a “large rectangle embedded with a small rectangle.” To provide shelter and clean water, A. philoxeroides was transplanted evenly in the circular groove of the pond and accounted for 75% of the experimental pond, and the area of hollow algal species in each pond was strictly controlled, with these species being removed when exceeding this area or supplemented in case of being insufficient. The outside of the pond was surrounded by a 40 cm-high fence to prevent crabs from escaping. The crabs were fed under three different feeding modes: traditional (consisting of wheat, bran, and soybean meal), formulated (Zhejiang Aohua Feed Co., Ltd., Jiaxing, China), and mixed (1:1 mixture of traditional and formulated feeds) feeds. The conventional biochemical composition of the different feeding modes is shown in Table 1. Each feeding mode was applied in three replicates. During the breeding period, the feeding ratio of each feeding mode was consistent, and the amount fed was approximately 1–3% of the total body weight of the crabs, at approximately 16:00 h every day.
Sample Collection and Data Determination
A Hach HQd portable water quality analyser (HQd, Hach Water Analysis Instrument Co., Ltd., Shanghai, China) was used to measure the temperature, dissolved oxygen, and pH of the water at 9–10 am in each pond. Water quality changes in the breeding process are shown in Figure 1. During the experiment, 1–2 g of formulated feed was collected, freeze-dried, crushed (particle size < 0.1 mm), and then cryopreserved at −40°C for subsequent analysis. The water sample collected using a water collector was passed through a 100 μm sieve, and the water samples removed from zooplankton were filtered using a glass fiber membrane (GF/F Whatman, Little Chalfont, United Kingdom; 47 mm diameter, 0.7 μm aperture) to obtain POM, which was wrapped in aluminum foil and frozen for subsequent analysis. At the end of the experiment, a fresh A. philoxeroides sample was collected from the crab breeding pond and washed slowly and repeatedly with double distilled water to remove soil on its surface and then dried, freeze-dried, and crushed (particle size < 0.1 mm) before storing it at −20°C for subsequent stable isotope analysis. In October, three male and three female crabs with sound appendages and good vitality in each pond were collected and selected for the experiment, and three samples were combined into one sample according to gender. The collected crabs were anesthetized on ice, and the surface was disinfected with 70% ethanol. The specimens were then dissected, and their stomach contents were extracted and transferred to 2.0 mL labeled cryopreservation tubes. The tubes were immersed into a liquid nitrogen tank and then stored in a refrigerator at −80°C for subsequent 18S rDNA analysis. The three E. sinensis specimens collected in each pond in November were anesthetized on ice, and the surface was disinfected with 70% ethanol. The shell was separated from the body along the side of the shell, and the hepatopancreas, head breastplate, appendages, and gills were removed to get body. Then, the muscle of the crab was freeze-dried, crushed (particle size < 0.1 mm), and cryopreserved at −40°C for subsequent stable isotope analysis.
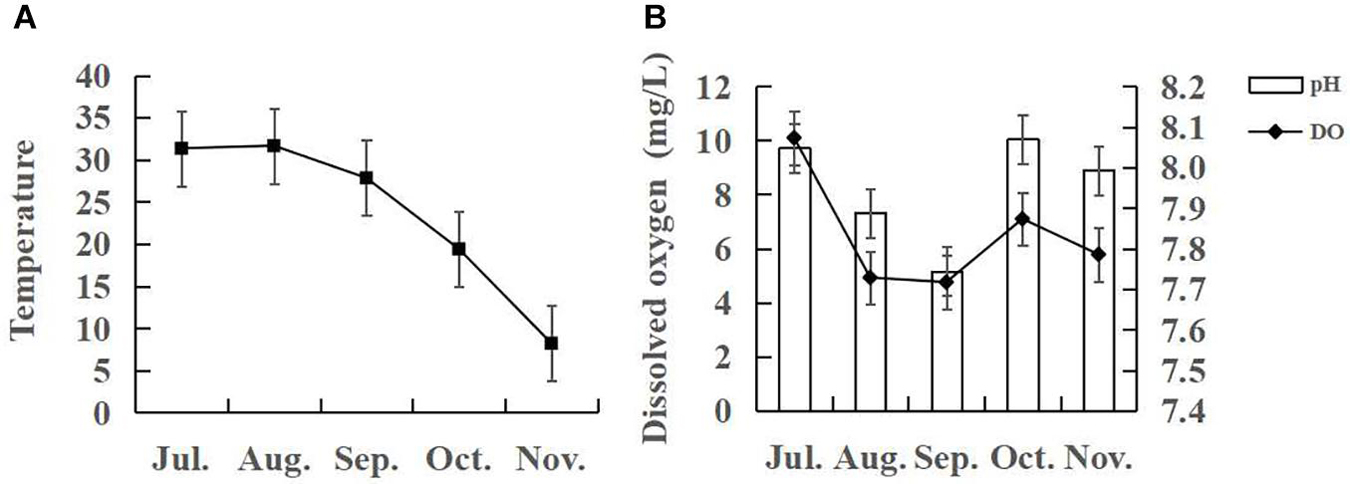
Figure 1. Trends in (A) temperature and (B) dissolved oxygen and pH in pond water used to culture juvenile E. sinensis from July to November, 2018.
Twenty male and twenty female crabs were randomly taken from each pond in the middle of each month to understand their growth during the experiment. The crabs were dried with a dry towel and accurately weighed on an electronic balance (accurate to 0.01g). The weight gain rate (WGR) of each juvenile E. sinensis was calculated as follows:
where, Wt and Wn are the average body weight (g) of crabs in months t and n, respectively.
Genomic DNA Extraction, Polymerase Chain Reaction Amplification, Construction of Illumina PE Library, and Computer Sequencing
Genomic DNA from the stomach contents of E. sinensis was extracted using the E.Z.N.A. SOIL DNA Kit (Omega Bio-Tek, Norcross, GA, United States). The diluted genomic DNA was used as a template to amplify the target gene with specific primers. The primer sequences were TAREF (5′-CCAGCASCYGCGGTAATTCC-3′) and TAREF (5′-AC TTTCGTTCTTGATYRA-3′). The ABI Geneamp® 9700 PCR (Applied Biosystems, Foster City, CA, United States) instrument was used for PCR using TransGen AP221-02: TransStart FastPFU DNA polymerase. Using high-efficiency and high-fidelity enzymes for PCR can ensure amplification efficiency and accuracy (Bokulich and Mills, 2013). The PCR amplification was carried out in a total volume of 20 μL containing 4 μL of 5× FastPFU Buffer, 0.8 μL of each primer (5 μmol/L), 0.4 μL of FastPFU Polymerase, 10 ng of template DNA, and 2 μL of 2.5 mmol/L dNTPs. The following cycling program was used: initial denaturation at 95°C for 2 min; 25 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s; and a final extension at 72°C for 5 min. The PCR products were detected using 2% agarose gel electrophoresis and quantified using the QuantifluorTM-ST Blue Fluorescence Quantitative System (Promega, Madison, WI, United States) according to the preliminary quantitative results of electrophoresis. The PCR products were mixed and purified using 2% agarose gel electrophoresis in 1× TAE buffer, followed by gel extraction. PCR products with the main band size between 300 and 400 bp were selected, recovered by gel-elution using the AxyPrep DNA Gel Recovery Kit (Axygen Biosciences, Union City, CA, United States), and eluted with Tris-HCl.
The purified PCR products were quantified on a Qubit 3.0 fluorometer (Life Technologies, Invitrogen, Carlsbad, CA, United States), and all 24 barcoded amplicons were evenly mixed and used to construct an Illumina antithesis library, according to the preparation procedure of the Illumina genomic DNA library. Sequencing was performed on the Illumina MiSeq platform (Shanghai Biozeron Co., Ltd., Shanghai, China) (2 × 250).
Stable Isotope Determination
The crab samples were small and comprised a mix of three individual samples. After freeze-drying, the processed samples were ground, and screened using an 80-mesh sieve. The stable isotopes of the samples were determined at the Laboratory of Ingestion Ecology, Shanghai Ocean University. The samples were covered with aluminum foil, and the muscle of E. sinensis, POM, A. philoxeroides, and feed samples weighed 1.00, 5.00, 2.00, and 1.50 mg, respectively. A stable isotope mass spectrometer (ISOPRIME100, Isoprime Corporation, Cheadle, United Kingdom) was used to measure the stable isotope (δ13C and δ15N) values of crab samples, international standard material (PBD), and purified atmospheric N2 as reference standards; δ13C and δ15N were calculated using the following formula:
where, X is 13C or 15N; Rsample is the sample’s isotope ratio of 13C/12C or 15N/14N; and Rstandard is the isotope ratio of the standard (Caut et al., 2009). To ensure the accuracy of the experimental results and the stability of the instrument, one standard sample was inserted every ten samples for calibration. The analysis accuracies of the δ13C and δ15N values of the samples were 0.05 and 0.06‰, respectively.
Data Analysis
Sequences were classified as operational taxonomic units (OTUs) at 97% sequence similarity level using UPASE (version 7.1), and UCHime was used to identify and delete chimeric sequences (Edgar, 2010) for OTU clustering and species classification analysis based on the valid data, so as to obtain the corresponding species information and abundance distribution. In addition, species composition and alpha diversity were analyzed to obtain the composition and richness information of eukaryotes in river crabs (Bokulich and Mills, 2013; Mueller et al., 2014).
The software program SPSS 26.0 (SPSS, Chicago, IL, United States) was used to analyze the obtained data. One-way analysis of variance was used to compare the differences in δ13C or δ15N in feeding sources under different feeding modes. To determine homogeneity of variance, inverse sine or square root processing was performed on the percentage data. One-way analysis of variance was then applied, using the Tukey’s-b (K) method for multiple comparisons. If homogeneity of variance was not satisfied after data conversion, then multiple comparisons were performed with Games-Howell Parametric tests. P < 0.05 was considered significant. To assess the contribution of different feed sources to the isotopic characteristics of E. sinensis, the stable isotope mixing model “Stable Isotope Analysis in R” (SIAR; Parnell and Jackson, 2013) package (version 4.2) was used in R 3.3.2 (R Development Core Team, 2016). The SIAR model used the δ13C and δ15N values of consumers and their feed sources to estimate the potential contribution of each feed source that consumers prey on. The model was run in R, allowing variability to be included in the stable isotope ratios of predators and potential feed sources (Parnell et al., 2010). The SIAR model was used to identify the stomach contents and obtain stable isotopic values according to the study of Caut et al. (2009), who found that the δ15N fractionation factor of E. sinensis, i.e., the Δ value, was 2.75 ± 0.01 ‰, and the fractionation factor of δ13C was 0.75 ± 0.11 ‰.
Results
Growth Performance of Juvenile Eriocheir sinensis Under Three Feeding Modes
Changes in the average weight and WGR of juvenile crabs during culture under three feeding modes are shown in Figure 2. As shown in Figure 2A, the average weight of juvenile E. sinensis in all three feeding modes gradually increased as the crabs matured to the breeding stage. At the end of the breeding stage (November), there was no significant difference in the average weight between the traditional feeding mode (8.43 ± 2.13) and the mixed feeding mode (8.36 ± 2.39) (P > 0.05), which was significantly lower than the average weight of the juvenile crabs in the formulated feeding mode (10.52 ± 2.60) (P < 0.05). As shown in Figure 2B, there is no significant difference in the WGR between the traditional feeding mode (593.23 ± 15.29) and the mixed feeding mode (615.91 ± 38.09) (P > 0.05), and was significantly lower than that in the formulated feeding mode (593.23 ± 15.29) (P < 0.05).
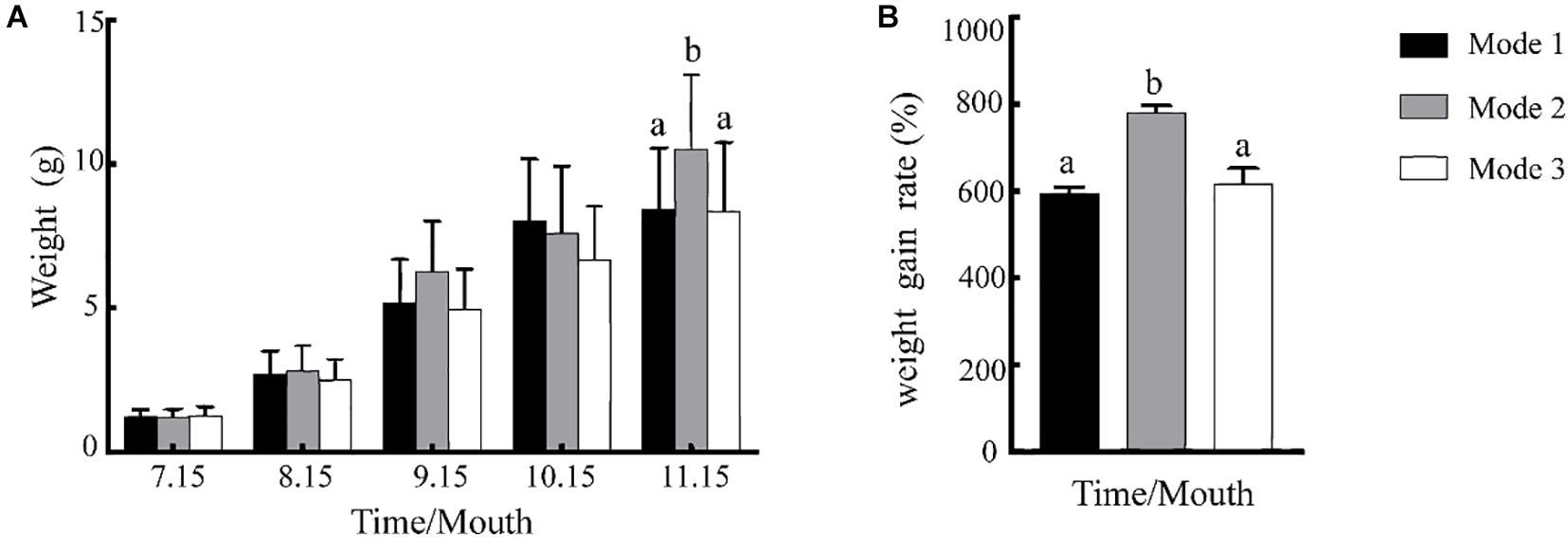
Figure 2. Changes of body weight (A) and comparison of juvenile crab weight gain rates (B) during juvenile crab breeding under three feeding modes. The values on the bar graph represent the average; the error bars represent the standard deviation (n = 40). Letters above the bars of the same series indicate significant differences between treatments (P < 0.05). Mode 1, 2, and 3 represent traditional, formulated, and mixture feeding modes, respectively.
Diversity Index and Abundance Index of the Stomach Contents of Eriocheir sinensis Under Three Feeding Modes
The alpha diversity of the stomach contents of male and female crabs under the three feeding modes is shown in Figure 3. The dilution (Figure 3A) and Shannon–Wiener diversity index curves (Figure 3B), which were based on the number of OTUs, tend to saturation.
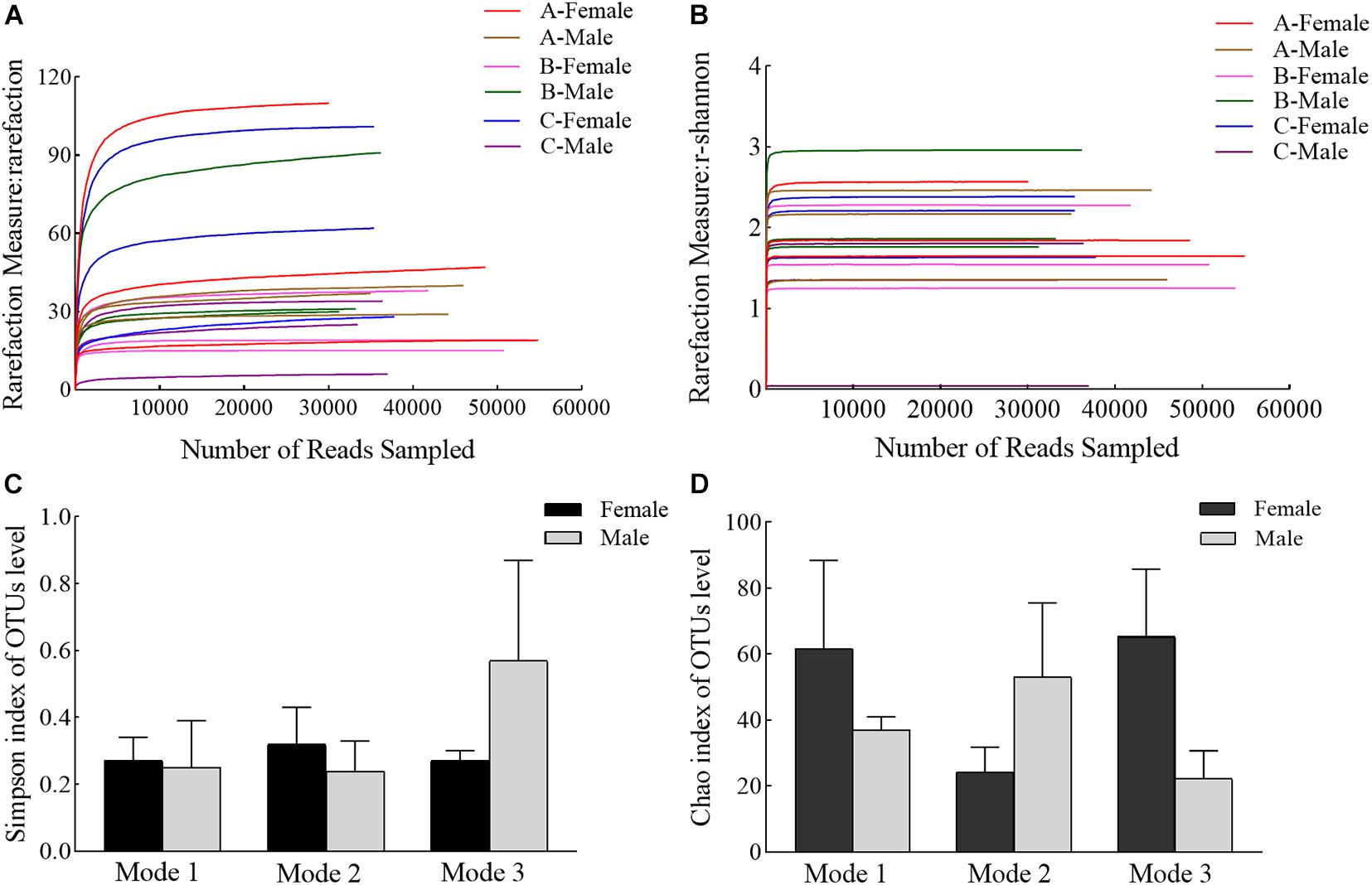
Figure 3. Alpha diversity comparisons of the stomach contents of juvenile Eriocheir sinensis reared under different feeding modes. (A) Rarefaction curve, (B) Shannon-Wiener curve using Shannon’s index as the metric for crab fed with various diets, (C) Simpson’s diversity indices in different feeding modes, and (D) Chao’s species richness indices in different feeding modes. Values on the bar graph represent the average; error bars represent the standard deviation (n = 9). Mode 1, 2, and 3 represent traditional, formulated, and mixture feeding modes, respectively.
The Simpson’s diversity indices of the stomach contents of female crabs under traditional, formulated, and mixed feeding modes were 0.27 ± 0.07, 0.32 ± 0.11, and 0.27 ± 0.03, respectively, indicating no differences between any two groups (Figure 3C; P > 0.05), and the corresponding Simpson’s diversity indices of the stomach contents of male crabs were 0.25 ± 0.14, 0.24 ± 0.09, and 0.57 ± 0.30, respectively, also indicating no differences between any two groups (Figure 3C; P > 0.05). There were no differences in Simpson’s diversity indices between male and female crabs under the same feeding mode (P > 0.05).
The Chao’s species richness indices of the stomach contents of female crabs under traditional, formulated, and mixed feeding modes were 61.67 ± 26.77, 24.33 ± 7.42, and 65.33 ± 20.50, respectively, indicating no differences between any two groups (Figure 3D; P > 0.05). Similarly, the Chao’s species richness indices of the stomach contents of male crabs under traditional, formulated, and mixed feeding modes are 37.00 ± 4.04, 53.00 ± 22.50, and 22.33 ± 8.41, respectively, indicating no differences between any two groups (Figure 3D; P > 0.05). There were no differences in the Chao’s species richness indices between male and female crabs under the same feeding mode (P > 0.05). The abundance of the main potential eukaryotes in the stomach of male and female crabs was not different under the different feeding modes, but the compositions of their stomach contents were different.
Analysis of the Main Potential Eukaryotic Components in the Stomach of Eriocheir sinensis Under Three Feeding Modes
The species with the highest OTU abundance under the three feeding modes are shown in Table 2. A total of 34 phyla were identified in the stomach contents of crabs. The number of phyla identified in the stomach contents of crabs reared under traditional, formulated, and mixed feeding modes were 24, 24, and 29, respectively.
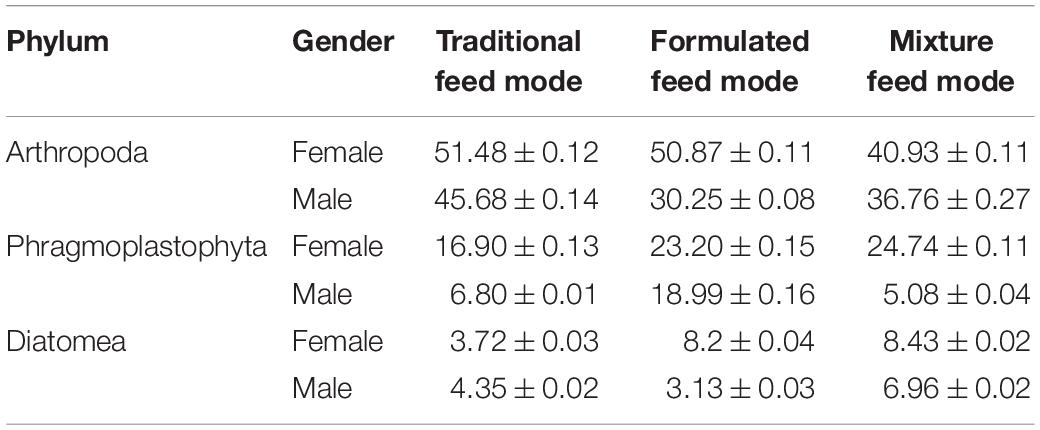
Table 2. Operational taxonomic units (OTUs) of stomach content abundance exceeding 5% in crab samples reared under three feeding modes (%).
No differences (P > 0.05) in the abundance of stomach phyla were observed between crabs reared under the three feeding modes, with Arthropoda showing the highest overall phylum abundance in the stomach contents of crabs reared under the three feeding modes. The content of Arthropoda in the stomach of male (45.68 ± 0.14%) and female (51.48 ± 0.12) crabs was the highest under the traditional feeding mode, but the opposite was true under the formulated feeding mode. The results showed that the contents of Phragmoplastophyta in the stomach of male (23.20 ± 0.15%) and female (18.99 ± 0.16%) crabs was the highest under the formulated feeding mode, and the contents of Diatom in the stomach of male (8.43 ± 0.02%) and female (6.96 ± 0.02%) crabs was the highest under the mixed feeding mode, while the contents of Phragmoplastophyta (in male crabs abundance accounted for 6.80 ± 0.01%; in female crabs abundance accounted for 16.90 ± 0.13%) and Diatom (male crabs abundance accounted for 4.35 ± 0.02%; in female crabs abundance accounted for 3.72 ± 0.03%) in the stomach of crabs were the lowest under the traditional feeding mode.
The class level of abundance of the main potential feed sources of crabs under the different feeding modes is shown in Figure 4, in which Malacostraca has the highest overall abundance in the stomach contents of crabs under the three feeding modes, with the lowest being 71.02% (formulated feeding mode), the highest 61.84% (mixed feeding mode), and an average of 67.44%. The proportions of Malacostraca, Embryophyta, Trebouxiophyceae, Bivalvia, Chlorophyceae, Insecta, and Intramacronucleata decreased successively to 69.45, 9.01, 8.34, 4.34, 3.30, 3.18, and 2.39%, respectively, under the traditional feeding mode. The proportions of Malacostraca, Trebouxiophyceae, Embryophyta, Chlorophyceae, Intramacronucleata, Insecta, and Bivalvia decreased successively to 71.02, 13.39, 8.95, 6.15, 0.48, 0.1, and 0.08%, respectively, under the formulated feeding mode. The proportions of Malacostraca, Embryophyta, Trebouxiophyceae, Chlorophyceae, Intramacronucleata, Insecta, and Bivalvia decreased successively to 61.84, 12.91, 5.45, 3.5, 0.29, 0.1, and 0.09%, respectively, under the mixed feeding mode. The taxa with different feeding patterns at different class levels are the same but have different proportions (Figure 4). Among the three feeding modes, the top three taxa were Malacostraca, Embryophyta, and Trebouxiophyceae.
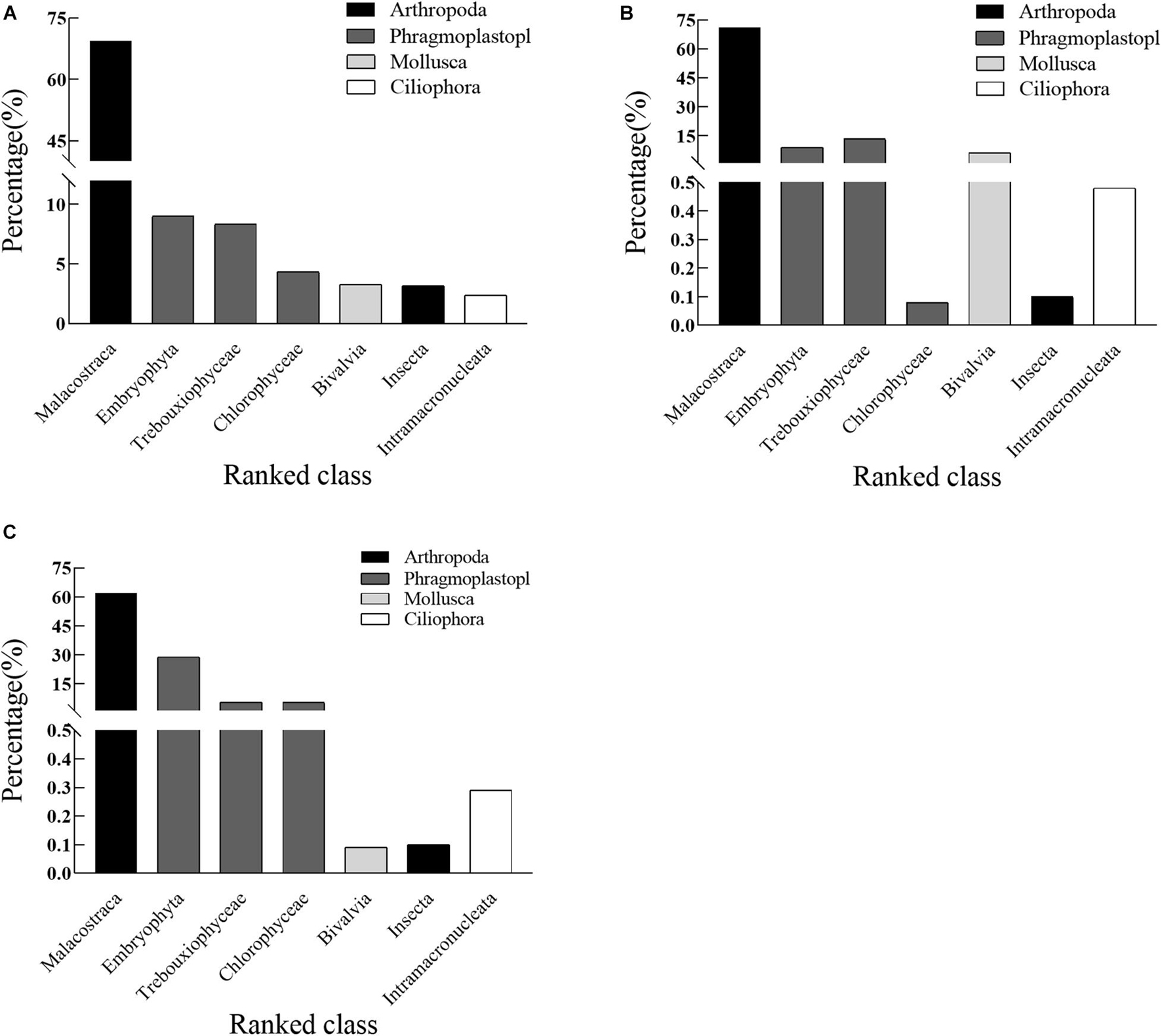
Figure 4. Rank abundance of the main potential prey reads of juvenile Eriocheir sinensis crabs at the class level under (A) traditional, (B) formulated, and (C) mixture feeding modes based on 18S rDNA. Values on the bar graph represent the average.
δ13C and δ15N Stable Isotopic Characteristics of Eriocheir sinensis Under the Three Feeding Modes
The δ13C and δ15N values of E. sinensis juvenile crab muscle under three different feeding modes are shown in Table 3. The muscle δ13C values (‰) of juvenile crabs under the formulated, mixed, and traditional feeding modes were −24.02 ± 0.72, −24.45 ± 1.22, and −23.31 ± 1.19, respectively, indicating no difference between the three feeding modes (P > 0.05). The muscle δ15N values (‰) of juvenile crabs in the formulated, mixed, and traditional feeding modes were 3.37 ± 0.64, 3.08 ± 0.37, and 2.13 ± 0.57, respectively, and that of the formulated feeding mode was significantly higher than that of the traditional feeding mode (P < 0.05).
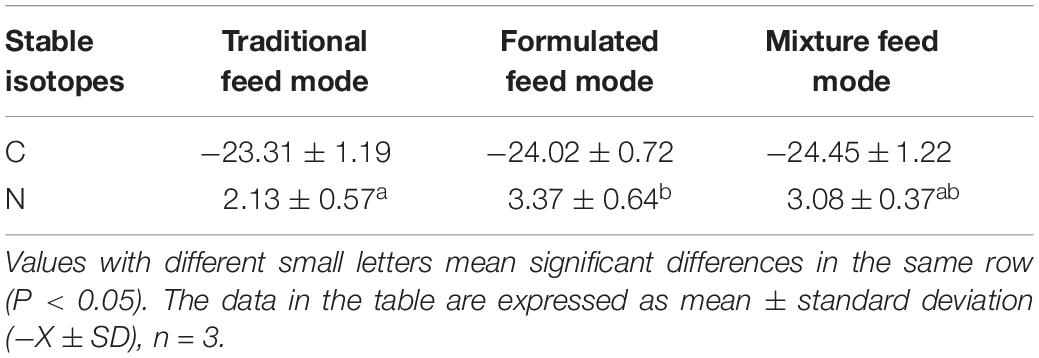
Table 3. Analysis of variance of 13C and 15N isotope contents in muscle of Chinese mitten crab in different feeding modes (‰).
δ13C and δ15N Stable Isotopic Characteristics of Potential Feed Sources of Eriocheir sinensis
The overall distribution of δ13C and δ15N stable isotopes of the potential feeding sources of crabs under the three feeding modes is shown in Figure 5. The δ13C values ranged from −33.90 ± 0.54‰ to −23.19 ± 0.11‰, and the δ15N values ranged from 0.49 ± 0.02‰ to 5.63 ± 0.01‰. Among them, the δ13C variation ranges of the potential feed sources of crabs under the traditional, formulated, and mixed feeding modes were –33.43 ± 0.71‰ to –26.04 ± 0.19‰, –32.98 ± 0.93‰ to –23.19 ± 0.12‰, and –33.90 ± 0.54‰ to –24.47 ± 0.05‰, which changed substantially, like the corresponding δ15N variation under the three feeding modes (0.49 ± 0.02–5.63 ± 0.01‰, 2.01 ± 0.07–5.63 ± 0.01‰, and 1.09 ± 0.21–5.63 ± 0.01‰, respectively).
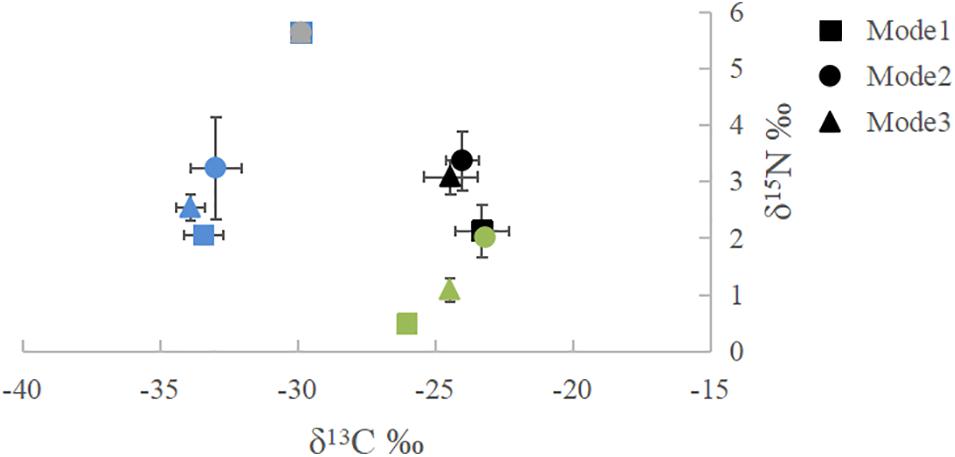
Figure 5. Isotopic compositions of 13C and 15N stability of muscles and possible feed sources under the traditional, formulated, and mixture feeding modes. Error bars are ± SD. Mode1, 2, and 3 represent traditional, formulated, and mixted feeding modes, respectively. Color of symbol denotes different sources: black are muscle samples; gray are Alternanthera philoxeroides samples; bule are particulate organic matter (POM) samples; green are feed samples.
Potential Feed Source Composition of Eriocheir sinensis Under Three Feeding Modes Based on Stable Isotope Analysis
The average feed contribution rate in E. sinensis was analyzed using SIAR (Figure 6). Under the traditional feeding mode, the main diet of E. sinensis was a traditional feed, with an average contribution of 71.89%, followed successively by POM and A. philoxeroides, with average contributions of 16.66 and 11.46%, respectively. Under the formulated feeding mode, the main diet of crabs was formulated feed, with an average contribution of 66.48%, followed successively by A. philoxeroides and POM, with average contributions of 17.50% and 16.03%, respectively. Under the mixed feeding mode, the main diet of crabs was a mixed feed, with an average contribution of 76.84%, followed by POM (11.92%) and A. philoxeroides (11.24%), whose average contribution rates were not significantly different.
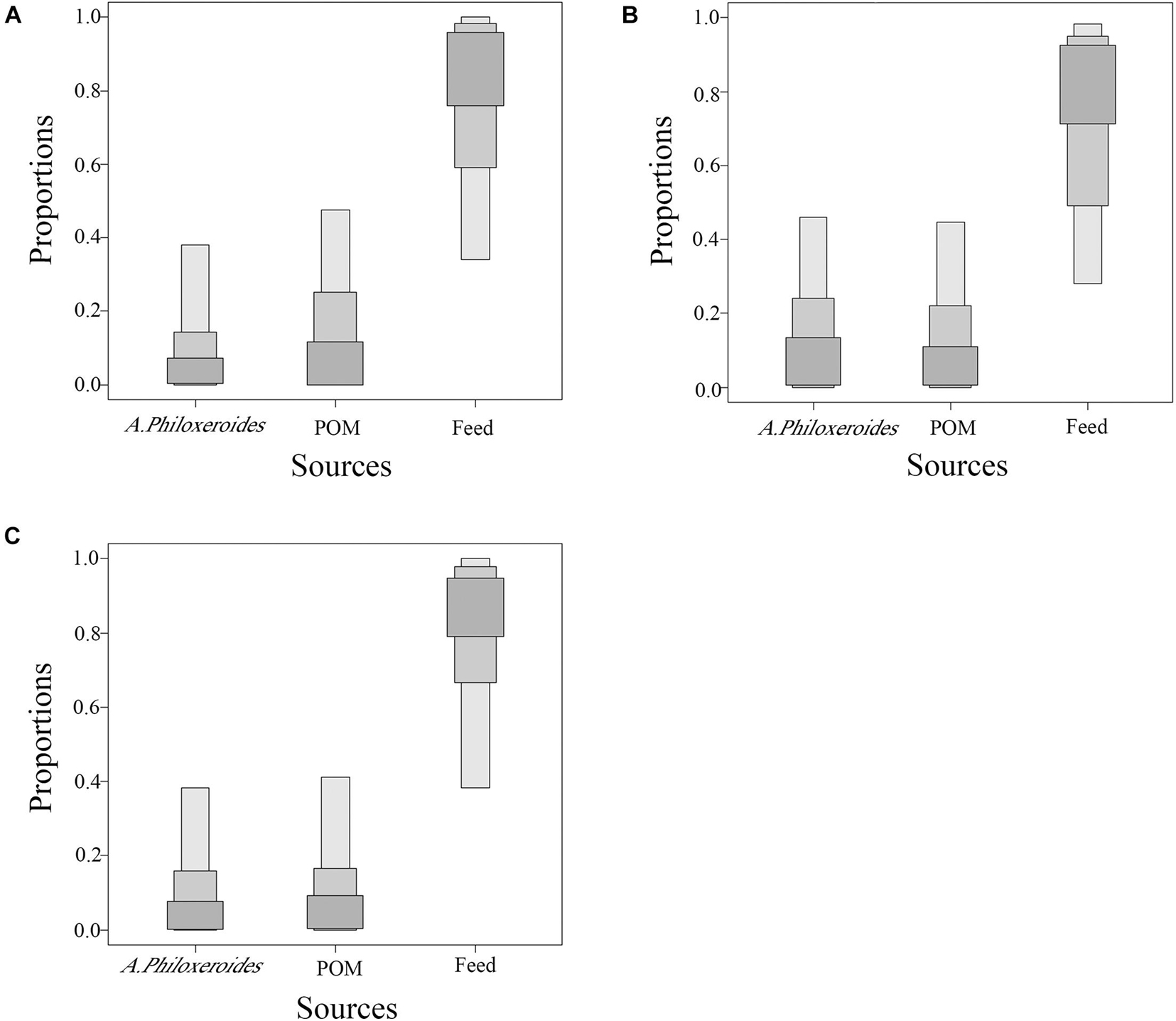
Figure 6. Feed contribution rate of juvenile Eriocheir sinensis based on the stable isotope analysis in R package (SIAR) analysis. (A) Traditional feeding mode, (B) Formulated feeding mode, and (C) Mixture feeding mode.
Discussion
Differences in the Growth and Dietary Composition of Eriocheir sinensis Under the Three Feeding Modes
In this study, 18S rDNA was used to analyze the stomach feed composition of male and female juvenile crabs under traditional, formulated, and mixed feeding modes. The flat curve of Rarefaction index and Shannon–Wiener curve indicated that the sequencing data covering the feeding source species of juvenile crabs and were sufficient to reflect the feed source diversity of crabs under different feeding modes. The Simpson and Chao indexes represented the diversity and abundance of food composition in the stomach of the crabs, respectively. The higher the Simpson index, the lower the diversity index, while the Chao index of abundance showed a contrary trend. The results showed that there was no significant difference among the three feeding modes (P > 0.05), but the terminal body weight and WGR under the formulated feeding mode were significantly higher than those under traditional feeding mode and mixed feeding mode. This result may be due to the traditional, formulated, and mixed feeds made up the majority of the crabs’ diet, so the diversity and abundance of other feed items were different but not significantly so. But the growth of E. sinensis is affected by factors such as feed and culture environment (Shao et al., 2013, 2014), which can reflect the quality of feed (Yang et al., 2014). The formulated feed containing high protein and fat (Yang et al., 2011), which is conducive to the growth of juvenile crabs. However, the traditional feeding mode had fewer nutrients, so the above indexes of juvenile crabs fed with formulated feed alone were significantly higher than those of the other two feeding modes.
The most representative phyla reported for the sampling site (Arthropoda, Phragmoplastophyta, and Diatomea) also showed the highest occurrence in the dietary samples analyzed. The phylum Arthropoda, with the highest proportion, may be composed of arthropods within the feeding space of crabs, including Malacostraca (injured or newly molting crabs) (Li, 2006) and Insecta (aquatic nymphal insects) (Chen et al., 1989). This is different from the results of Jin et al. (2003), who studied the feeding habits of the second instar adult E. sinensis, and can be attributed to the different ecological niches and living environments of the crabs. The animal feed content of Malacostraca and Bivalvia in the stomach of juvenile crabs reared under the traditional feeding mode was the highest among the three feeding modes because the traditional feed is a plant-based feed mainly including bran, wheat, and soybean meal, which are insufficient to meet the nutritional needs of juvenile crabs (Veilleux and de Lafontaine, 2007), thus driving them to consume more animal feed. Under the formulated feeding mode, the plant feed content of Embryophyta and Trebouxiophyceae in the juvenile crab stomach was the highest among the three feeding modes, mainly because the formulated feed is mainly composed of animal feed, and to achieve nutritional balance (Chen et al., 1989), which are entwined and attached to the other feed sources of crabs (Vizzini and Mazzola, 2003). Under the mixed feeding mode, the animal and plant feed contents in the crab stomach were the lowest between the three feeding modes because the mixed feed contained both animal and plant feeds and provided balanced nutrition to crabs (Qian and Zhu, 1999).
Compared with zooplankton, fish, and macroinvertebrates in the near waters of Gouqi Island, the variation in the values of δ13C and δ15N were relatively large (Jiang et al., 2014), indicating that crabs have a wide range of feed sources. Previous studies have shown that the crab’s δ13C and δ15N changes depending on what it eats (Zeng, 2010) and the δ13C value of consumers is usually close to that of their feed, the δ13C value of individuals does not change much in the transmission of the whole feed chain (Lin, 2013), which is in accordance with the results of this study. Animals reject δ15N during exudation of substances; therefore, the higher the trophic level, the higher the content of δ15N (McCutchan et al., 2010). However, the δ15N values of E. sinensis crabs under traditional (2.13 ± 0.57‰), formulated (3.37 ± 0.64 ‰), and mixed feeding (3.08 ± 0.37 ‰) modes in the present study were lower than those of A. philoxeroides (5.63 ± 0.37‰), and this may be because δ15N is poorly conserved and easily modified by biogeochemical processes, and an increase in the N content in the environment may increase the N content in plants (Lin, 2013; Yu et al., 2014).
Previous studies have shown that juvenile crabs with better growth performance can be obtained by feeding high quality feed (Wu et al., 2009), the content of fat and ash in formulated feed was higher than that of two other feeding modes indicating that formulated feed had higher energy level, this may be the reason why the growth performance of the crab fed with formulated feed is better. Under the traditional feeding mode, crabs mainly consumed POM as a feed supplement in addition to the traditional feed, which is consistent with the results of Paning (1934) and Rudnick et al. (2000), who reported that the stomach contents of E. sinensis contain a large amount of POM. POM is the substrate of complex biological communities, formed by the decomposition and mineralization of dead animals and plants, humus, and feces by microorganisms (Liu, 1999), and some of its components are the main feed sources for benthic invertebrates (Vizzini and Mazzola, 2003). Under the formulated feeding mode, apart from the formulated feed, the proportion of A. philoxeroides was the highest. In the presence of sufficient animal feed, crabs will still choose A. philoxeroides as a feed supplement (Li et al., 2018). This is because A. philoxeroides is rich in cellulase, which helps to improve the cellulase activity (Yang et al., 2014) and digestive ability of crabs. The mixed feed containing plant- and animal-based feeds had a balanced nutritional ratio (Qian and Zhu, 1999) and high palatability, and the proportion of mixed feed consumed by E. sinensis was the highest, resulting in the lowest feeding proportion of POM and A. philoxeroides between the three feeding modes. But the size, shape and palatability of the two diets were significantly different, and the physical difference of the mixed feeding group made the crab unable to make full use of the feed (Wang S. M. et al., 2017). Therefore, there was no significant difference in the weight change and WGR of juvenile crabs under the mixed feeding mode and the traditional feeding mode. The results of the stable isotope technique were consistent with those of the 18S rDNA analysis. Juvenile crabs feed on both plant- and animal-based feeds in an aquaculture pond, but they are not complete predators and selectively feed on animal or plant feeds as supplements of that which is deficient, in addition to their main feed (Jin, 2003).
Difference in Stomach Content Composition Between Male and Female Eriocheir sinensis
Various studies have shown that there are no differences in feed composition between male and female crabs, and they are, therefore, ecological equivalents (Spooner et al., 2007; Young and Elliott, 2020). In the present study, the diversity and abundance of female crabs were generally higher than those of male crabs, and female crabs had a higher rate of feeding on available feed sources in the pond, this may be because female crabs increase their feeding rate during October to November every year to meet their growth and development needs (Chen et al., 1989), while promoting gonad maturation and transformation (Xu et al., 2019). However, no significant differences were observed in Simpson’s diversity and Chao’s abundance indices of male and female crabs under the same feeding mode as well as in the abundance of the three main feeding sources, Arthropoda, Phragmoplastophyta, and Diatomea (Table 2), indicating that male and female crabs showed similar feed composition under the same feeding mode. Cordone et al. (2020) studied the feeding habits of C. maenas and found that different living water depths and a wide range of available feed sources lead to differences in feed composition between male and female crabs, which is different from the results of this study. This may be because the ponds mainly contained artificial feeds for arthropods (including shrimp), mollusks (including snail), cultivated A. philoxeroides, Phragmoplastophyta dominated by Trebouxiophyceae species, and diatoms dominated by Chlorella (Lu et al., 2013). The pond was shallow, and the crabs can eat less feed than wild crabs, but the difference was minor.
Conclusion
The three different feeding modes and different genders had no effect on the feeding habits of crabs. But we concluded that feeding formulated feed is more favorable to the growth of juvenile crabs. The identified benthic organisms were the main food in the stomach contents of crabs under different feeding modes. In addition, the proportion of POM is similar to Alternanthera philoxeroides in three feeding modes.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov, BioProject accession PRJNA759104.
Ethics Statement
The animal study was reviewed and approved by Animal Ethics Committee, Shanghai Ocean University.
Author Contributions
ZL and YS designed the experiments of this study. CX assisted ZL to complete several animal experiments. ZL performed results analysis and manuscript writing. YS reviewed and edited the manuscript. YS and YC provided the funding resources. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 31802320) and the China Agriculture Research System (No. CARS-48).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bachy, C., Dolan, J. R., López-García, P., Deschamps, P., and David, M. (2013). Accuracy of protist diversity assessments: morphology compared with cloning and direct pyrosequencing of 18S rRNA genes and ITS regions using the conspicuous tintinnid ciliates as a case study. ISME J. 7, 244–255. doi: 10.1038/ismej.2012.106
Bade, L. M., Balakrishnan, C. N., Pilgrim, E. M., McRae, S. B., and Luczkovich, J. J. (2014). A genetic technique to identify the diet of cownose rays, Rhinoptera bonasus: analysis of shellfish prey items from North Carolina and Virginia. Environ. Biol. Fishes 97, 999–1012. doi: 10.1007/s10641-014-0290-3
Bokulich, N. A., and Mills, D. A. (2013). Improved selection of internal transcribed spacer-specific primers enables quantitative, ultra-high-throughput profiling of fungal communities. Appl. Environ. Microbiol. 79, 2519–2526. doi: 10.1128/AEM.03870-12
Caut, S., Angulo, E., and Courchamp, F. (2009). Variation in discrimination factors (Δ15N and Δ13C): the effect of diet isotopic values and applications for diet reconstruction. J. Appl. Ecol. 46, 443–453. doi: 10.1111/j.1365-2664.2009.01620.x
Chen, B., Du, N. S., and Ye, H. F. (1989). Analysis on the feeding habit of the mitten crab Eriocheir Sisensis. Fish. Sci. Technol. Inf. 01, 2–5. doi: 10.16446/j.cnki.1001-1994.1989.01.001
Cheng, Y. X., Wu, X. G., Yang, X. Z., and Anson, H. H. (2008). Current trends in hatcherytechniques and stock enhancement for Chinese mitten crab, Eriocheir japonica sinensis. Rev. Fish. Sci. 16, 377–384. doi: 10.1080/10641260701681698
Cordone, G., Lozada, M., Vilacoba, E., Thalinger, B., Bigatti, G., Lijtmaer, D., et al. (2020). Metabarcoding, direct stomach observation and stable isotope analysis reveal a highly diverse diet for the invasive green crab in Atlantic Patagonia. Ecol. Environ. Conserv. 489. doi: 10.1101/2020.08.13.249896
Edgar, R. C. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461. doi: 10.1093/bioinformatics/btq461
Fu, L. L., Li, Y. H., Lu, Q. P., Ding, S. Y., and Deng, Y. F. (2014). Comparative analysis of several breeding modes of 1st age crab species. Aquaculture 035, 39–42. doi: 10.3969/j.issn.1004-2091.2014.12.014
Han, W. F., Sun, Y. F., Liu, J., Zhang, Y., and Cheng, Y. X. (2021). Effect of different feeding modes on the growth, biochemical composition, and living environment of the juvenile Chinese mitten crab Eriocheir sinensis. Aquaculture 1:736687. doi: 10.1016/J.AQUACULTURE.2021.736687
He, J., Wu, X. G., Li, J. Y., Huang, Q., Huang, Z. F., and Cheng, Y. X. (2014). Comparison of the culture performance and profitability of wild-caught and captive pond-reared Chinese mitten crab (Eriocheir sinensis) juveniles reared in grow-out ponds: implications for seed selection and genetic selection programs. Aquaculture 434, 48–56.
Huang, J., Norgbey, E., Li, G., Wang, J., Rainizafy, M., Takyi-Annan, G. E., et al. (2019). Unraveling the feeding dynamics of chinese mitten crab-based ecosystems using carbon and nitrogen stable isotope techniques. J. Consum. Prot. Food Saf. 14, 251–261. doi: 10.1007/s00003-019-01220-w
Jiang, R. J., Wang, K., Zhou, X. J., and Zhao, J. (2014). Stable isotope analysis of food web in coastal waters of Gouqi Island. Chin. J. Ecol. 33, 930–938.
Jin, G. (2003). Advances in crab ecology. J. Shenzhen Vocat. Tech. Coll. 01, 28–34. doi: 10.3969/j.issn.1672-0318.2003.01.008
Jin, G., Xie, P., and Li, Z. J. (2003). Feeding habits of the second instar Eriocheir sinensis released from lakes. Acta Hydrobiologica Sin. 02, 29–35. doi: 10.3321/j.issn:1000-3207.2003.02.007
Lafontaine, Y. D., and Veilleux, É (2002). Biological synopsis of the Chinese mitten crab (Eriocheir sinensis). Fish. Sci. China 2812, 1–45. doi: 10.3724/SP.J.1011.2010.01351
Lan, S. Q., Zhang, L. W., Yi, H. P., Xu, C. L., Lu, F., Feng, G. H., et al. (2020). Food source and feeding habit of Helice tientsinensis from the common reed vegetation in high marsh of Yellow River Delta, China. Chin. J. Appl. Ecol. 31, 319–325. doi: 10.13287/j.1001-9332.202001.034
Li, C., Cheng, Y. X., Guan, Q. Z., Xi, Y. W., and Li, J. Y. (2018). Analysis of rice shrimp system by stable isotope technique. J. Fish. China 42, 1778–1786.
Lu, D., Cheng, G. P., and Qin, M. L. (2013). Biological composition and dynamic changes of algae in different aquaculture ponds. World Econ. Yearb. Chin. Bus. Urban Rural Constr. 003, 336–336.
McCutchan, J. H., Lewis, W. M., Kendall, C., and Mcgrath, C. C. (2010). Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102, 378–390. doi: 10.1034/j.1600-0706.2003.12098.x
Mueller, R. C., Paula, F. S., Mirza, B. S., Rodrigues, J. L. M., Bohannan, B. J. M., and Nusslein, K. (2014). Links between plant and fungal communities across a deforestation chronosequence in the Amazon rainforest. ISME J. 8, 1548–1550. doi: 10.1038/ismej.2013.253
Ning, J. J., Du, F. Y., Wang, X. H., Gu, Y. G., Wang, G. L., and Li, Y. F. (2016). Analysis of feeding habits of mantis shrimps based on stable isotopes. J. Fish. China 6, 903–910. doi: 10.11964/jfc.20151110177
O’Rorke, R., Lavery, S., Chow, S., Takeyama, H., Tsai, P., Beckley, L. E., et al. (2012). Determining the diet of larvae of western rock lobster (Panulirus cygnus) using high-throughput DNA sequencing techniques. PLoS One 7:e42757. doi: 10.1371/journal.pone.0042757
Pan, J., Wu, X. G., Zhao, H. L., He, J., Jiang, X. D., Wang, Y. P., et al. (2016). Effects of three feeding modes on the culture performance of adult pond-reared Chinese mitten crab (Eriocheir sinensis) during the second year culture. Freshw. Fish. 46, 87–93.
Parnell, A., and Jackson, A. (2013). Stable Isotope Analysis in R, Vol 4.2. Vienna: R Foundation for Statistical Computing.
Parnell, A. C., Inger, R., Bearhop, S., and Jackson, A. L. (2010). Source partitioning using stable isotopes: coping with too much variation. PloS One 5:e9672. doi: 10.1371/journal.pone.0009672
Qian, G. Y., and Zhu, Q. H. (1999). The appropriate content of protein, fat and cellulose in the compound diet of chinese mitten crab. J. Fish. Sci. China 03, 61–65.
Que, Y. Q., Yang, Z. G., Ji, L. Y., He, J., Shao, L. C., Wang, C., et al. (2012). Effects of formulated dietary replacement of trash fish on growth performance, body composition and fatty acid composition of Eriocheir sinensis. J. Fish. Sci. China 036, 1612–1623. doi: 10.3724/SP.J.1231.2012.27912
R Development Core Team (2016). R: A Language and Environment for Statistical Computing, Vol 3.3.2. Vienna: R Foundation for Statistical Computing.
Riemann, L., Alfredsson, H., Hansen, M. M., Als, T. D., Nielsen, T. G., Munk, P., et al. (2010). Qualitative assessment of the diet of European eel larvae in the Sargasso Sea resolved by DNA barcoding. Biol. Lett. 6, 819–822. doi: 10.1098/rsbl.2010.0411
Rudnick, D., and Resh, V. (2005). Stable isotopes, mesocosms and gut content analysis demonstrate trophic differences in two invasive decapod crustacea. Freshw. Biol. 50, 1323–1336. doi: 10.1111/j.1365-2427.2005.01398.x
Rudnick, D. A., Halat, K. M., and Resh, V. H. (2000). Distribution, Ecology and Potential Impacts of the Chinese Mitten Crab (Eriocheir sinensis) in San Francisco Bay. Berkeley: University of California Water Resources Center, 206.
Shao, L. C., Wang, C., and He, J. (2013). Hepatopancreas and gonad quality of Chinese mitten crabs fattened with natural and formulated diets. J. Food Qual. 36, 217–227. doi: 10.1111/jfq.12030
Shao, L. C., Wang, C., He, J., Wu, X. G., and Cheng, Y. X. (2014). Meat quality of Chinese mitten crabs fattened with natural and formulated diets. J. Aquat. Food Prod. Technol. 23, 59–72. doi: 10.1080/10498850.2012.694583
Spooner, E. H., Coleman, R. A., and Attrill, M. J. (2007). Sex differences in body morphology and multitrophic interactions involving the foraging behaviour of the crab Carcinus maenas. Mar. Ecol. 28, 394–403. doi: 10.1111/j.1439-0485.2007.00186.x
Sun, Z. L. (2012). Study on the Metabolic Process of Main Nutrient Elements in the Culture of Sea Cucumber. Ph.D. thesis. Qingdao: Ocean University of China.
Veilleux, E., and de Lafontaine, Y. (2007). Biological synopsis of the Chinese mitten crab (Eriocheir sinensis). Can. Manusc. Rep. Fish. Aquat. Sci. 2812:45. doi: 10.3724/SP.J.1011.2010.01351
Vizzini, S., and Mazzola, A. (2003). Seasonal variations in the stable carbon and nitrogen isotope ratios (13C/12C and 15N/14N) of primary producers and consumers in a western Mediterranean coastal lagoon. Mar. Biol. 142, 1009–1018. doi: 10.1007/s00227-003-1027-6
Wang, S. M., Lu, J., and Wang, P. P. (2017). A comparative experiment on the effect of whole-process feed feeding and traditional breeding method for Eriocheir sinensis. Aquaculture 38, 7–9.
Wang, W., and Wang, C. H. (2013). Ecological Culture of River Crab, 2nd Edn. Beijing: China Agriculture Press.
Wang, X. F., Lin, C. G., Xu, Q., Song, X. Y., Zhang, H. J., Ru, S. G., et al. (2017). Analysis of feeding changes of Crassostrea gigas during the transit of Enteromorpha green tide using 18S rDNA molecular method. Oceanol. Limnol. Sin. 048, 1362–1370.
Wu, X., Cheng, Y., Zeng, C., Sui, L., Southgate, P. C., Zhou, G., et al. (2009). Reproductive performance and offspring quality of Chinese mitten crab Eriocheir sinensis (H. Milne-Edwards) females fed an optimized formulated diet and the razor clam Sinonovacula constricta. Aquac. Res. 40, 1335–1349. doi: 10.1111/j.1365-2109.2008.02121.x
Xu, J. J., Geng, Z., and Feng, G. P. (2019). Feeding habits and feeding intensity of female crab E.sinensis in the Changjiang Estuary. Mar. Fish. 41, 397–407. doi: 10.13233/j.cnki.mar.fish.2019.04.002
Yang, L. L., Yang, X. Z., Zhao, L. L., Fan, P., Liang, P., and Cheng, Y. X. (2011). Effects of frozen fish and compound diet on growth, digestive enzyme activities and blood cells of juvenile Chinese mitten crab (Eriocheir sinensis). J. Fudan Univ. 50, 619–624.
Yang, X., Ye, J. Y., Zhang, Y. X., Wu, C. L., Liu, P., and Wang, W. (2014). Effects of replacement of fish meal by common cottonseed meal and fermented cottonseed meal on growth performance, body composition and hepatopancreas digestive enzyme activities of juvenile Chinese mitten crab (Eriocheir sinensis). Chin. J. Anim. Nutr. 26, 683–693. doi: 10.3969/j.issn.1006-267x.2014.03.018
Young, A. M., and Elliott, J. A. (2020). Life history and population dynamics of green crabs (Carcinus maenas). Fishes 5:4. doi: 10.3390/fishes5010004
Yu, H. Y., Yu, Z. M., Song, X. X., Liu, L. L., Cao, X. H., and Yuan, Y. Q. (2014). Seasonal distribution of stable nitrogen isotopes and key biogeochemical processes of suspended particulate organic matter in the Yangtze River estuary. Acta Oceanol. Sin. 36, 16–22. doi: 10.3969/j.issn.0253-4193.2014.02.002
Zeng, W. T. (2010). Studies on the Growth, Feed Sources and Nutritional Niche of Eriocheir sinensis. Ph.D. thesis. Beijing: University of Chinese Academy of Sciences.
Keywords: Eriocheir sinensis, juvenile crab, feeding mode, 18S rDNA, stable isotope analysis
Citation: Lu Z, Sun Y, Xiao C and Cheng Y (2021) Dietary Analysis Based on 18S rDNA, and Stable Carbon and Nitrogen Isotopes in Juvenile Eriocheir sinensis Crabs Reared Under Three Feeding Modes. Front. Mar. Sci. 8:741780. doi: 10.3389/fmars.2021.741780
Received: 15 July 2021; Accepted: 30 August 2021;
Published: 22 September 2021.
Edited by:
Kang-Le Lu, Jimei University, ChinaReviewed by:
Song Yang, Sichuan Agricultural University, ChinaBin Xia, Qingdao Agricultural University, China
Ce Shi, Ningbo University, China
Copyright © 2021 Lu, Sun, Xiao and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongxu Cheng, eXhjaGVuZ0BzaG91LmVkdS5jbg==
†These authors have contributed equally to this work
 Zhenzhen Lu1,2,3†
Zhenzhen Lu1,2,3† Yunfei Sun
Yunfei Sun