
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 21 September 2021
Sec. Aquatic Physiology
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.740660
This article is part of the Research Topic New Progresses of Fish Meal Replacement in Aquatic Animals View all 16 articles
 Bin Yin1,2,3
Bin Yin1,2,3 Hongyu Liu1,2,3*
Hongyu Liu1,2,3* Beiping Tan1,2,3*
Beiping Tan1,2,3* Xiaohui Dong1,2,3
Xiaohui Dong1,2,3 Shuyan Chi1,2,3
Shuyan Chi1,2,3 Qihui Yang1,2,3
Qihui Yang1,2,3 Shuang Zhang1,2,3
Shuang Zhang1,2,3The supplementation of gossypol in excess is noted to cause detrimental effects such as the reduction of antioxidant enzymes and disruption of lipid metabolism in animals. Studies regarding the effects of different levels of gossypol are very rare; thus, this study was conducted to evaluate the effects of low and high dietary levels of gossypol and of supplementation with 0.13 % sodium butyrate (NaB) under high gossypol conditions on the growth performance and intestinal health of hybrid grouper (Epinephelus fuscoguttatus♀×Epinephelus lanceolatus♂). Four treatments were used: Feed containing 40% fish meal was used as the control group [fishmeal (FM)], the FM diet plus 0.03% gossypol acetic acid (abbreviated as gossypol) as the low-level gossypol group (gL), FM + 0.15% gossypol was used as the high-level gossypol group (gH), and FM+0.15 % gossypol with 0.13 % NaB as the repair group (gHNaB). All diets were isonitrogenous and isolipidic. The results showed that the gL treatment significantly increased specific growth rate (SGR) and feed utilization; upregulated mRNA levels of distal intestinal transforming growth factor-β1 (tgfβ1), jam, occludin, claudin3, and zo1; and downregulated mRNA levels of il8, ifnγ, and akt. The gH treatment significantly reduced SGR and feed utilization; increased distal intestinal total nitric oxide synthase (NOS) activity and nitric oxide (NO) content; upregulated mRNA levels of distal intestinal tnfα, il1β, il6, ifnγ, caspase2, caspase9, and akt; and downregulated mRNA levels of tgfβ1, jam, and zo1. NaB supplementation significantly increased distal intestinal total NOS activity and NO content; downregulated distal intestinal tnfα, il1β, ifnγ, pi3k p85, and akt mRNA levels; and increased distal intestinal tgfβ1, jam, occludin, and zo1 mRNA levels. Above all, low- and high-level gossypol exhibited positive and negative effects on growth performance, distal intestinal anti-inflammatory capacity, and tight junctions, respectively, in hybrid groupers. NaB supplementation improved distal intestinal anti-inflammatory capacity and tight junctions in hybrid groupers to a certain extent.
Hybrid grouper (♀Epinephelus fuscoguttatus × ♂Epinephelus lanceolatus) is a coastal warm-water, broad-salt fish that is widely cultured in southern China and has high economic value. In 2017, the Ministry of Agriculture included groupers in the National Technical System of the Marine Fish Industry. It has been reported that the feed protein content requirement of hybrid grouper can reach 50% (Rahimnejad et al., 2015; Jiang et al., 2016), and the content of fishmeal (FM) in feed is also up to 50% or more. The global shortage of FM resources has seriously limited the development of the grouper industry. Therefore, finding suitable protein sources to replace FM has become a long-term research focus in aquatic animal nutrition.
Cottonseed meal is a by-product obtained from cottonseed after oil extraction and is used as a protein source for aquatic animal feed because it contains up to 40–45 % crude protein (Kumar et al., 2021). However, intake of high levels of cottonseed meal can cause negative effects, such as reduced growth performance, organ damage, and reduced reproductive capacity in fish (Mbahinzireki et al., 2015). These negative effects may be related to the presence of antinutritional factors, including gossypol. Gossypol is a polyphenolic compound isolated from the pigment gland of cottonseed, a natural yellow pigment, and is the main antinutritional factor present in cottonseed meal (Krogdahl et al., 2010; Bian et al., 2017). The active hydroxyl group of free gossypol combines with lysine, and this complex reduces the availability of lysine in the cotton meal. Gossypol can also form complexes with protein, affecting animal growth, while the active aldehyde group can be combined with iron ions, interfering with the synthesis of hemoglobin. This gossypol/iron interaction reduces the number of red blood cells in the animal body, thereby causing iron deficiency anemia in animals (Cao et al., 2018).
In laying hens, diets containing high levels of gossypol led to the disruption of lipid metabolism and reduced antioxidant function (Hou, 2014). In the rumen, the 0.1 mg/g addition of gossypol significantly increased the number of total bacteria abundance and fiber-degrading bacteria and improved the degradation rate of dry matter (Hou, 2012). These results, across multiple livestock species, indicate that the effects of gossypol vary depending on the concentration and species. Similarly, diets high in cottonseed have negative effects on fish (Deng et al., 2014): This is mainly due to the presence of gossypol, which has phenolic and carbonyl groups that can react with acids and amines, thus causing anorexia, diarrhea, and eventually death (Rinchard et al., 2003). Studies have shown that high-level gossypol inhibits the growth performance of rainbow trout and causes damage to the liver and spleen (Herman, 1970). The addition of 900 mg/kg of gossypol to the feed significantly reduced the weight gain (WG) and feed utilization of channel catfish (Ictalurus puctatus) (Yildirim et al., 2003), while the growth performance of gibel carp (Carassius auratus gibelio) was significantly reduced when dietary gossypol went above 300 mg/kg, with gossypol residue in the tissues increased (Jiang, 2011); at levels above 900 mg/kg, the liver function of the gibel carp was also damaged. When cottonseed protein concentrate (CPC) was used to replace FM, the growth of hybrid grouper (Yin et al., 2018) and golden pompano (Trachinotus ovatus) (Shen et al., 2020) was significantly increased at low-level substitution. We suspect that this facilitation effect is related to the low-level gossypol contained in the CPC. However, the mechanism of this low-level gossypol or CPC addition has rarely been investigated.
Currently, the solution to reduced fish growth rates caused by high levels of dietary plant protein is usually to supplement the feed with additives. Sodium butyrate (NaB) has been widely reported as an alternative to antibiotics in promoting growth, nutrient absorption, and immunity in aquatic animals. NaB can significantly improve the growth performance of grass carp (Ctenopharyngodon idella) (Liu et al., 2017) and tilapia (Oreochromis niloticus) (Ahmed and Sadek, 2015), maintain the normal morphology of intestinal epithelial cells (Claus et al., 2007), and improve the antioxidant capacity of gilthead seabream (Sparus aurata) (Robles et al., 2013). However, it has not been reported whether the addition of NaB after high-level gossypol can have a positive effect on hybrid grouper.
The health of fish intestines is closely related to growth. Antimicrobial peptides, lysozyme, and immunoglobulins in the fish intestine form an immune barrier. In addition, tightly connected molecules in the intestine are important for maintaining normal intestinal structure and nutrient absorption. Therefore, a systematic and in-depth study of gossypol is necessary for the intestinal health of aquatic animals and the sustainable development of aquaculture.
In a previous study in our laboratory, we found that CPC as a replacement for FM had a nonlinear, parabolic effect on specific growth rate (SGR) in hybrid grouper (Yin et al., 2018), which we speculate is closely related to the residual gossypol in CPC. Currently, there is a gap in research on gossypol in hybrid grouper. Therefore, in the present study, we investigated the effects of gossypol on the growth performance, serum immunity, and intestinal health of hybrid grouper, as well as the restorative effect of NaB by supplementing low- and high-level gossypol, and supplementing the high-level gossypol with appropriate levels of NaB to provide a theoretical basis and reference for the efficient use of cottonseed meal in aquafeeds.
The composition of the basal diet is presented in Table 1. Gossypol-acetic acid (1 mg = 0.8962 mg gossypol, abbreviated as gossypol) used in this study was obtained from Ci Yuan Biotechnology Co., Ltd. Shannxi (purity = 98.02 %) (Wanga et al., 2018). The group with 0.00% gossypol and 0.00% NaB was used as the control group (FM). The control group was supplemented with 0.03% gossypol as the low-level gossypol group (gL); 0.15 % gossypol as the high-level gossypol group (gH); and 0.15 % gossypol and 0.13 % NaB as the repair group (gHNaB). Four groups of isonitrogenous (48 %) and isolipidic (10 %) experimental diets were prepared. Red FM, casein, and gelatin were used as the main protein sources; fish oil and soy lecithin were used as the main lipid sources, and wheat flour was used as the carbohydrate source. After passing all the solid raw materials through a 0.25-mm sieve, all the raw materials were weighed according to the percentage in Table 1 and mixed, and 30% of the mixture weight of water was added and mixed again. The final mixture was made into strips (2.0 mm diameter), naturally air-dried for 48 h, and then placed in a refrigerator at −20°C.
Hybrid grouper with an initial weight of 10.70 ± 0.09 g were purchased from a grouper hatchery in Zhanjiang, China. They were domesticated for 10 days in an outdoor concrete pond at the biological research base of Donghai Island, Guangdong Ocean University, China, using commercial feed to adapt to the base environment. A total of 480 hybrid grouper in healthy body condition and uniform size were randomly selected and divided into four treatment groups, each including four replicates with 30 fish per replicate. The culture experiments were conducted in 12 0.3-m3 fiberglass tanks for 8 weeks. Apparent satiation feeding was performed daily at 7:30 and 16:30 and adjusted according to feeding conditions. Water temperature was 30.00±1.59°C, salinity was maintained at 35.48±1.24 g/kg, dissolved oxygen was kept above 7 mg/L, pH was maintained at 7.8–8.1, and ammonia nitrogen was kept below 0.03 mg/L during the breeding period.
At the end of the feeding experiment, all fish were starved for 24 h. First, the fish in each tank were counted and weighed, and the data obtained were used to calculate the WG, SGR, and survival (SR) and combined with the weight of feed consumed to calculate the feed coefficient ratio (FCR) (Yin et al., 2021a). For the intestine, part of the intestine near the cloacal pore, accounting for one-third of the total intestine of two randomly selected fish from each replicate, was stored in 4% formalin solution and used as paraffin-embedded TUNEL-stained sections; the distal intestine from four randomly selected fish from each replicate was stored at −80°C and used for damage indicator determination, and the distal intestine from two randomly selected fish from each replicate was stored at −80°C and used as PCR samples.
The slices were placed in xylene I for 10 min; xylene II for 10 min; xylene II for 10 min; anhydrous ethanol I for 5 min; anhydrous ethanol II for 5 min; anhydrous ethanol III for 5 min; and distilled water wash. An immunohistochemistry pen was used to draw circles around the tissues, and proteinase K working solution (stock solution: PBS = 1:9) was added dropwise inside the circles and incubated for 22 min at 37°C. The samples were washed three times for 5 min each with PBS (pH 7.4). Next, 0.1 % Triton (stock solution: PBS = 1:1,000) was added to the circles, incubated for 20 min at room temperature, and again washed three times with PBS for 5 min each time. After shaking the sections dry, equilibration buffer was added dropwise to the circles and incubated for 10 min at room temperature. The mixture [V(TDT enzyme): V(Dutp): V(equilibration buffer) = 1:5:50] was added to the circles and incubated for 2 h at 37°C. The incubation was kept moist during the incubation. After washing three times with PBS for 5 min each time, DAPI staining solution was added to the circles and incubated for 10 min at room temperature away from light. The sections were washed again using PBS three times for 5 mins each, shaken dry, and sealed with anti-fade mounting medium. Finally, images were observed and acquired under an ortho-fluorescence microscope (Nikon Eclipse C1) with a camera (Nikon Eclipse Ci-L), and a 20× object lens (CFI, Plan Fluor, N.A. 0.17, W. D. 2.1 mm).
Nitric oxide (NO) (#A012-0-2) and total nitric oxide synthase (NOS) (#A014-2) were determined according to the instructions of the kits from Nanjing Jiancheng Institute of Biological Engineering. For NO, a standard curve was constructed using sodium nitrite. Equal volumes of double-distilled water, sodium nitrite standard solution, and intestinal supernatant samples were added to the blank, standard, and assay wells, respectively. The color developer agent was added to all wells and left for 15 min, and the OD was measured at 550 nm. For total NOS, equal volumes of double-distilled water and samples were added to the blank and assay wells, respectively. Then, the substrate buffer, accelerator, and color agent were added sequentially to all wells. A water bath at 37°C for 15 min was performed, followed by the addition of the wash solution and termination solution. After zeroing with double-distilled water, the OD value of each sample was determined using a UV-Vis spectrophotometer (UV-2450, Shimadzu, Japan) at 530 nm using a 1-cm optical diameter cuvette.
After the extraction of total RNA from the distal intestine using the traditional Trizol (TRI reagent solution, Invitrogen, Carlsbad, CA, United States), the quality and quantity of the total RNA were evaluated by 1% agarose gel electrophoresis with an electrophoresis instrument and A260: 280 values with a NanoDrop 2000 spectrophotometer (Thermo Fisher, United States), respectively. Total RNA was reverse-transcribed to cDNA using PrimeScriptTM RT-PCR Kit (Takara, Kusatsu, Japan) according to the manufacturer's instructions. Real-time quantitative PCR was performed using SYBR GreenPro Taq HS qPCR Kit II (Accurate Biology, China) on an Applied Biosystems 7500 Real-Time PCR System. The full-length transcriptome sequence of the distal intestine of hybrid grouper (Zhang et al., 2021) was the basis for designing the primers (Table 2) used in this experiment (accession nos.: PRJNA664623 and PRJNA664416). β-Actin was used as a housekeeping gene, and all the CT values were analyzed using the 2 −ΔΔCT method according to Livak and Schmittgen (2001).
To statistically analyze the data from this experiment, SPSS Statistics (v.22, SPSS Inc., Chicago, IL, United States) was used. After all the data were examined by joint hypotheses test to ensure that equal deviation standards were obtained, they were subjected to one-way ANOVA followed by Tukey's multiple range tests to determine significant differences among treatment groups using SPSS v. 22 (IBM, United States) at a significance level of P < 0.05. The results are presented as mean ± standard error (SEM).
The growth performance and feed utilization of the hybrid grouper are listed in Table 3. Compared with FM, WG and SGR were significantly higher in the gL and significantly lower in the gH treatments. NaB supplementation did not significantly increase WG and SGR, and WG and SGR in gHNaB were not significantly different from those in gH. The FCR was significantly decreased in the gL and significantly increased in the gH compared with the FM, and no significant difference was found between gH and gHNaB. For SR, there was no significant difference in SR among the four groups.
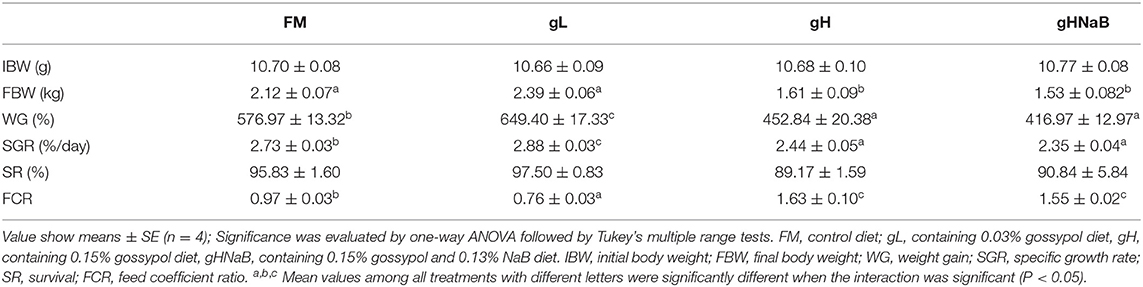
Table 3. Growth parameters and feed utilization of juvenile hybrid grouper fed the experimental diets for 8 weeks.
To investigate the effects of different levels of gossypol and supplementation of NaB under high-level gossypol conditions on the distal intestinal damage conditions, the NO level and total NOS activity were determined (Figure 1). Compared with FM, the addition of low-level gossypol had no significant effect on NO content or total NOS activity. The addition of high-level gossypol significantly increased NO content and total NOS activity. Compared with gH, the NO content and total NOS activity in gHNaB were significantly lower.
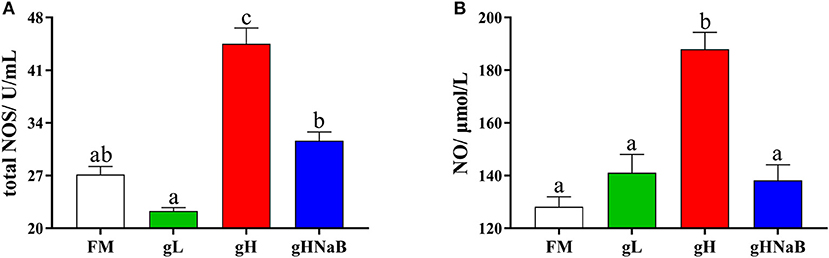
Figure 1. Effects of different treatments on the NO (A) content and total NOS (B) activity in the distal intestine of hybrid grouper. Values were means of eight replicates (n = 8), and were represented as mean ± standard error. a,b,c Mean values with unlike letters were significantly different (P < 0.05).
To investigate the effects of different levels of gossypol and supplementation of NaB under high-level gossypol condition on the distal intestinal inflammation and tight junction, the mRNA levels of tnfα, il1β, tgfβ1, hepcidin, il6, il8, ifnγ, jam, occludin, claudin3, claudin12, claudin15, and zo1 in the distal intestine of hybrid grouper were determined (Figure 2). Compared with the FM, the addition of low-level gossypol significantly downregulated the mRNA levels of il8 and ifnγ and significantly upregulated the mRNA levels of tgfβ1, jam, occludin, claudin3, and zo1. The addition of high-level gossypol significantly upregulated the mRNA levels of tnfα, il1β, il6, il8, and ifnγ and significantly downregulated the mRNA levels of tgfβ1, jam, and zo1. Compared with gH, the mRNA levels of tnfα, il1β, il8, and ifnγ were significantly downregulated, and jam, occludin, and zo1 were significantly upregulated in gHNaB. There was no significant difference in the mRNA levels of claudin12 or claudin15 among the four groups.
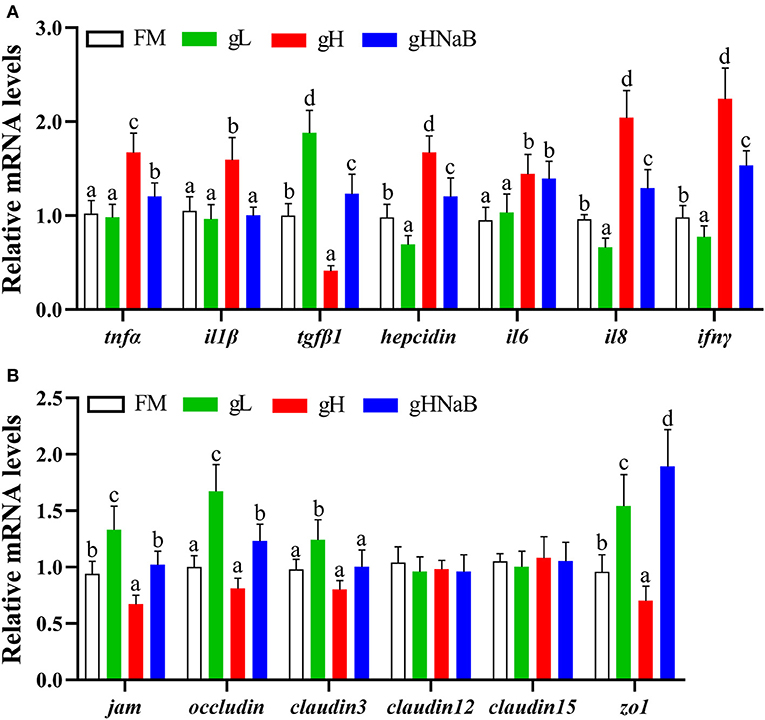
Figure 2. Effects of different treatments on the mRNA levels of immune-related genes (A), including tnfα, il1β, tgfβ1, hepcidin, il6, il8 and ifnγ, and tight junction-related genes (B), including jam, occludin, claudin3, claudin12, claudin15 and zo1 in the distal intestine of hybrid grouper. Values were means of eight replicates (n = 8), and were represented as mean ± standard error. a,b,c,d Mean values with unlike letters were significantly different (P < 0.05).
To investigate the effects of different levels of gossypol and supplementation of NaB under high-level gossypol condition on distal intestinal apoptosis, the TUNEL staining (Figure 3A) and mRNA levels of caspase2, caspase3, caspase6, caspase7, caspase8, caspase9, pi3k p85, 3pdk1, akt, foxo4, and fasl in the distal intestine of hybrid grouper were determined (Figure 3B). TUNEL staining showed no apoptotic cells in any of the four groups. Compared with FM, the addition of low-level gossypol significantly downregulated the mRNA levels of akt. The addition of high-level gossypol significantly upregulated the mRNA levels of caspase2, caspase3, caspase9, pi3k p85, and akt and significantly downregulated the mRNA levels of foxo4. Compared with gH, NaB supplementation significantly downregulated the mRNA levels of pi3k p85, 3pdk1, and akt. There were no significant differences among the four groups in the mRNA levels of caspase6, caspase7, caspase8, and fasl.
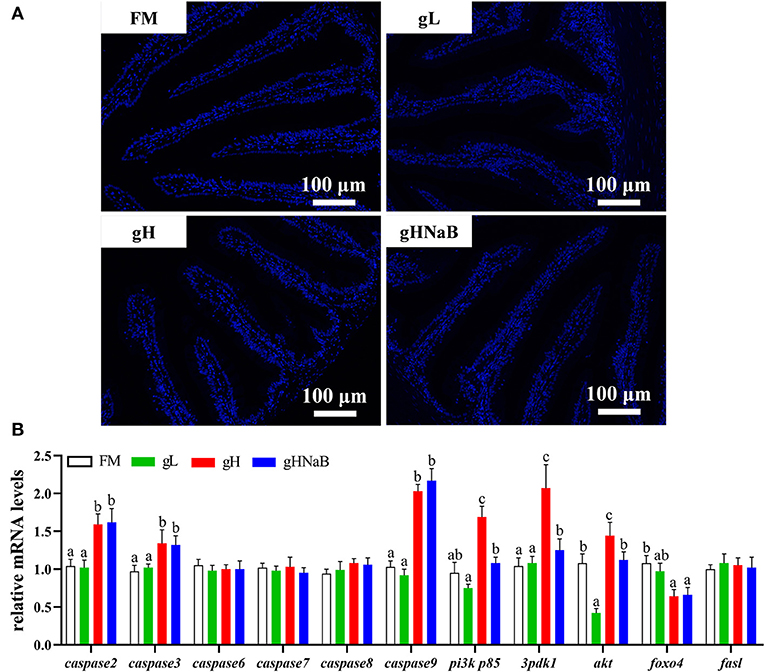
Figure 3. Effects of different treatments on the TUNEL staining (A) and mRNA levels of apoptosis-related genes (B), including caspase2, caspase3, caspase6, caspase7, caspase8, caspase9, pi3k p85, 3pdk1, akt, foxo4, and fasl in the distal intestine of hybrid grouper. Values were means of eight replicates (n = 8) and were represented as Mean ± standard error. a,b,c Mean values with unlike letters were significantly different (P < 0.05).
Cottonseed meal is a by-product of cottonseed oil extraction and has a high crude protein content and a rich variety of amino acids, which can effectively alleviate the shortage of protein feed resources in China (Mbahinzireki et al., 2015). However, cottonseed meal contains gossypol, which is toxic to aquatic animals at high levels, which greatly limits the utilization of cottonseed meal in aquatic feed. Therefore, in the present study, the effects of gossypol on hybrid grouper and the protective effect of NaB were investigated by exploring low- and high-level gossypol while supplementing NaB in the background of high-level gossypol.
The present study indicated that the addition of low-level gossypol to the feed significantly increased WG and SGR of hybrid grouper, similar to the experimental results for Nile tilapia (Oreochromis niloticus) (Lim et al., 2003). However, when high levels of gossypol were added, hybrid grouper showed the exact opposite growth performance to Nile tilapia. The reason for this difference may be related to fish species, as Nile tilapia may have a higher tolerance to gossypol than hybrid grouper. Different levels of tolerance to gossypol have been reported between fish species: The addition of gossypol at or above 1,175 mg/kg to the feed significantly reduced the SGR of grass carp (Wanga et al., 2018), while a study on channel catfish found that the addition of 1,500 mg/kg gossypol significantly reduced WG and feed utilization, similar to the results of the present experiment. Interestingly, the addition of gossypol had no significant effect on SR in either grass carp, channel catfish, or hybrid grouper, suggesting that gossypol might not directly affect fish mortality. To alleviate the negative effects on the growth of hybrid grouper caused by high-level gossypol, we added an appropriate dose of NaB under conditions of high-level gossypol addition. Unfortunately, NaB supplementation could not alleviate the negative effect of gossypol (Table 3).
The fish intestine is the main site where fish store food, digest, and absorb nutrients (Gu et al., 2013; Sun et al., 2021). Oxidative damage can destroy intestinal structural integrity (Wang et al., 2016). Maintaining normal antioxidant capacity is essential for maintaining normal intestinal cell function (Chen et al., 2018). Multiple organs in animals can be profoundly affected by the feed. To further verify whether this effect exists in the intestine, we determined the NO and total NOS in the distal intestine. NOS is a key enzyme in the process of NO synthesis, which is abundantly expressed in various inflammatory diseases, and it can promote inflammation and act as an inflammatory mediator (Al-Harbi et al., 2019). The results indicated that high-level gossypol may modulate the inflammatory process through total NOS. NaB supplementation reduced total NOS to the control level, which could be a side effect of the repairing effect of NaB on intestinal inflammation in hybrid grouper, which may be related to total NOS.
Nitric oxide reacts with superoxide anions to generate peroxynitrite anions, which are strong oxidants that cause damage to intestinal epithelial cells. We found that high-level gossypol significantly increased the distal intestinal NO content, suggesting that high-level gossypol induced intestinal damage and disrupted distal intestinal barrier function. NaB supplementation may alleviate this injury by reducing NO levels, which may be related to the fact that NaB can reduce NO production by inhibiting the NF-κB pathway (Liu et al., 2012). Since there are few studies on NOS and NO in aquatic animals, the modulation of NOS and NO in the distal intestine of hybrid grouper by gossypol and NaB still needs further investigation.
Fish intestinal health is closely related to intestinal structural integrity and immune barrier function, and harmful or toxic substances in feed would usually disrupt the intestinal structural integrity and the immune barrier. Fish intestinal inflammatory cytokines are closely related to intestinal health, and inflammatory cytokines play different roles in intestinal inflammation (Tian et al., 2017). Inflammatory factors are usually classified into two types: proinflammatory factors, such as tnfα, il1β, il8, and ifnγ (Li et al., 2018b; Carriero et al., 2020), whose upregulation further exacerbates inflammation, and anti-inflammatory factors, such as tgfβ1 and hepcidin (Li et al., 2017; Yan et al., 2019), whose upregulation enhances the intestinal anti-inflammatory capacity. In addition, hepcidin and il6, which often show high expression in intestinal inflammation, have different roles from proinflammatory factors in the progression of intestinal inflammation, and their upregulation is usually associated with a protective effect on the intestinal response to inflammation (Kuhn et al., 2018). Cotton seed meal can cause intestinal inflammation by upregulating the transcription of pro-inflammatory factors and downregulating anti-inflammatory factors (Liu et al., 2020). CPC substitution for FM upregulated intestinal tnfα and il1β expression in hybrid grouper (Yin et al., 2018) and downregulated TGF-β3 and IL-10 transcription in silver sillago (Sillago sihama Forsskál, 1775) (Liu et al., 2020). Studies in grass carp showed that excess gossypol upregulated the mRNA expression of tnfα, ifnγ2, il1β, and il6 in the intestines of grass carp (Wang et al., 2019). Few studies have examined the effects of low levels of gossypol on intestinal immunity in aquatic animals, and we speculate that the promotion of growth of hybrid grouper by low-level gossypol may be closely related to the upregulation of the anti-inflammatory factor tgfβ1 and the downregulation of the expression of the inflammatory factors il8 and ifnγ. Supplementation with high-level gossypol significantly reduced the immunity of the distal intestine of hybrid groupers and induced the development of intestinal inflammation; similar results were also found in grass carp (Wang et al., 2019). After further supplementation with NaB, we found that proinflammatory factors showed an overall downward trend, such as tnfα, il1β, il8, and ifnγ, while tgfβ1 was upregulated, suggesting that the supplementation of NaB in the feed could improve the distal intestinal anti-inflammatory capacity of hybrid grouper. Supplementation of 0.2 % NaB in the diet of turbot (Scophthalmus maximus L.) (Liu et al., 2019) can alleviate intestinal inflammation by decreasing the expression of tnfα, in line with the results of this experiment. However, after NaB supplementation, hybrid grouper still needed to express high-level il6, indicating that a certain degree of inflammation might still exist in the intestine, which also indicates that the repair of the intestine by NaB was incomplete.
In fish, intestinal health depends in part on intestinal barrier function, which is closely related to the structural integrity and tight junctions of the intestine (Al-Sadi and Ma, 2007; Al-Sadi et al., 2008; Yin et al., 2021b). Nutrient absorption interacts closely with the intestine to maintain the normal structure and nutrient absorption function of the intestine. Tight junction proteins, such as jam, claudins, occludin, and zonula occludens 1 (zo1), are the major membrane proteins of tight junctions that control the decellularization gap between epithelial cells, thus preventing the decellularization of intestinal bacteria and other antigens from spreading between epithelial cells (Zhao et al., 2014). Jam is a single transmembrane protein between epithelial cells and a protein that appears early in the formation of cell junctions and recruits other tight junctions (Hamazaki et al., 2002). The main function of occludin is to regulate tight junctions (Mclaughlin et al., 2004). claudin3 is involved in the formation of tight junction barriers and reduced expression of claudin3 results in reduced cell barrier function (Blasig et al., 2011). In this experiment, low-level gossypol promoted growth by upregulating the expression of jam, occludin, claudin3, and zo1 and improving the tight junctions of the distal intestine and the efficiency of nutrient absorption. In contrast, the expressions of jam, occludin, claudin3, and zo1 indicated that high-level gossypol disrupted the distal intestinal tight junctions of hybrid grouper. The expression of zo1, occludin, and claudin3c in the proximal, middle, and distal intestines of mid-growth grass carp was negatively affected by gossypol when the level of gossypol in the feed reached 243.94 mg/kg or higher (Wang, 2019), which was consistent with the results of the present study. The expression of jam, occludin, and zo1 was upregulated after supplementation with NaB, while the expression of claudin3 was not significantly changed, indicating that NaB was incomplete for the repair of tight junctions in the distal intestine of hybrid grouper, but still had a certain enhancement effect on tight junctions. Combined with the growth performance, we speculate that this incomplete repair effect may not be reflected in the growth performance, and therefore, the growth performance of the NaB group was not significantly improved. Gossypol was found to be the main cause of apoptosis induction in organ cells.
Gossypol can exacerbate apoptosis in grass carp intestinal epithelial cells by inducing DNA fragmentation (Wang, 2019) and induces apoptosis by interacting with mitochondrial caspases (Oliver et al., 2005). However, there are exceptions; as observed by TUNEL staining, the addition of 680 mg/kg gossypol to the feed did not cause apoptosis in carp hepatocytes (Zhang, 2019). There are no reports to date on the effect of gossypol on apoptosis in the intestinal tract of hybrid groupers. Therefore, we further verified the genes related to apoptosis in the distal intestine of hybrid groupers at the transcriptional level and observed apoptosis by TUNEL staining. The expression of genes associated with apoptosis revealed that low-level gossypol did not significantly affect apoptosis-related genes (Figure 4A). Apoptosis is regulated by multiple factors. The promoters of caspase2, caspase8, and caspase9, when tightly bound to pro-apoptotic signals, activate the downstream effectors caspase3, caspase6, and caspase7 to perform apoptotic functions (Degterev and Yuan, 2008; Fuchs and Steller, 2011; Kaufmann et al., 2012). pi3k/akt can regulate apoptosis not only directly through the promoters, but also by direct interaction with foxo4 and fasl, which promote or inhibit the onset of apoptosis (Kim et al., 2012; Li et al., 2018a; Zhang et al., 2020). There are several ways to regulate apoptosis, and inhibition of the pi3k/akt pathway can promote apoptosis to some extent (Zheng et al., 2012). Combined with the results of this experiment, apoptotic signals were transduced normally from pi3k/akt to promoters and effectors at high-level gossypol, yet high expression of pi3k/akt inhibited the expression of foxo4, blocking the transduction of apoptotic signals and inhibiting apoptosis (Figure 4B). Activated pi3k/akt could inhibit apoptosis mediated by FOXO transcription factors, and similarly, blocking pi3k/akt signaling can also activate FOXO-mediated apoptosis (Qi et al., 2020). This may suggest that this process may also be present in the intestine of hybrid grouper, but due to the inhibition of apoptosis by high expression of pi3k/akt via foxo4. Therefore, we speculate that no significant apoptosis occurred in the intestine of the hybrid grouper. When we supplemented with NaB, the expression of pi3k p85 and akt was downregulated, but the expression of promoter caspase2, caspase8, and caspase9, and effector caspase3, caspase6, and caspase7 were not significantly changed (Figure 4C). This incomplete transduction process may imply that the regulation of apoptosis by pi3k/akt through the promoter is blocked, and the exact reason needs to be further explored.
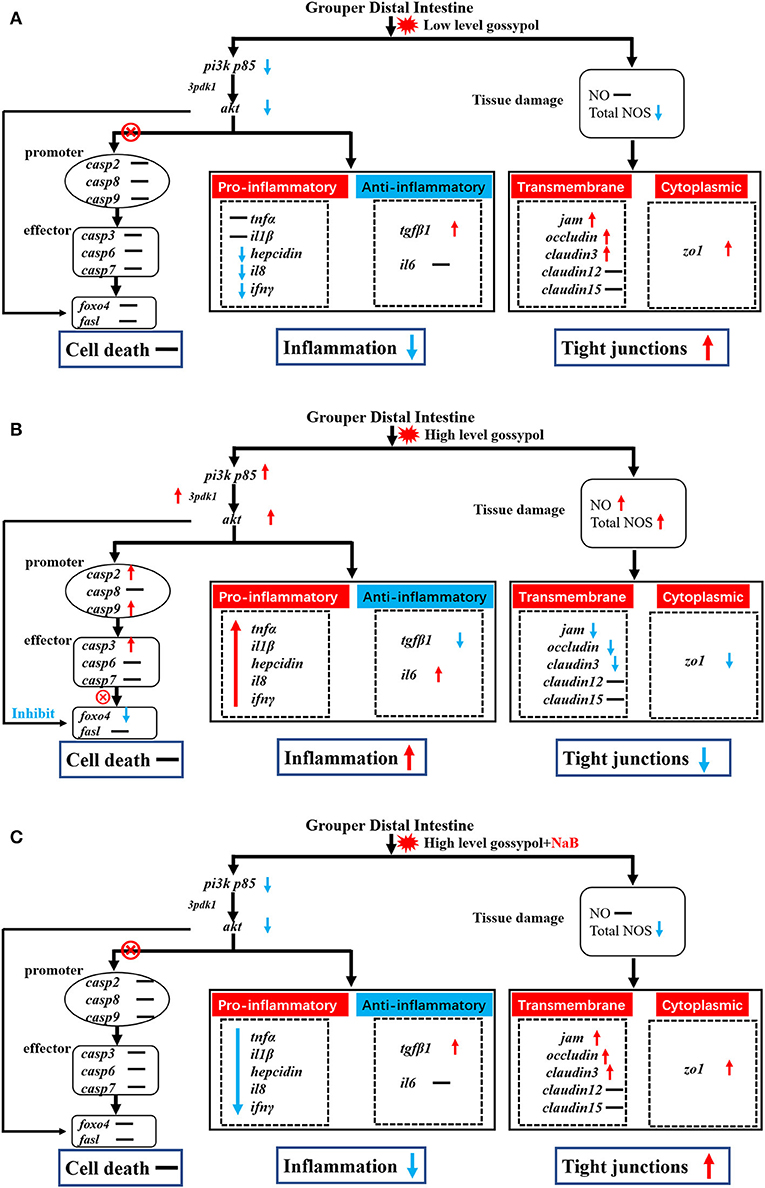
Figure 4. Potential signal transduction in the distal intestine of hybrid grouper. Casp: caspase. → , through; ⊗ → , not through; red arrow, upregulation; blue arrow, downregulation. (A) group gL; (B) group gH; (C) group gHNaB.
To visually verify the results regarding apoptosis at the transcriptional level, we further observed distal intestinal TUNEL staining of the hybrid grouper. From the TUNEL staining, it is interesting to note that we did not observe any apoptosis in any of the groups, suggesting that low- and high-level gossypol is not the cause of apoptosis in the intestine of the hybrid grouper. Few studies have reported the effects of gossypol on apoptosis in intestinal cells. However, studies in the macrophage cell line RAW264.7 cells found that gossypol induced the onset of apoptosis through a caspase-dependent mitochondrial signaling pathway (Deng et al., 2013). This difference in results may be due to species differences or may be caused by the different response mechanisms of different cells to gossypol.
In this study, appropriate low-level gossypol improved growth performance, distal intestinal anti-inflammatory capacity, and tight junctions, while high-level gossypol decreased the above indicators. NaB supplementation under exposure to high-level gossypol partially improved intestinal anti-inflammatory capacity and tight junctions; however, the positive effects of NaB could not be reflected in growth performance in hybrid grouper.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding authors.
The animal study was reviewed and approved by Ethics Review Board of Guangdong Ocean Unviersity.
HL and BT designed the study. BY conducted the study and analyzed the data. XD participated in the interpretation of the results. BY wrote the manuscript. QY, SC, and SZ purchased the reagent supplies. HL revised the manuscript. All authors have actively contributed to the study, read and approved the final manuscript.
This work was supported by the National Key R&D Program of China (2019YFD0900200), the National Natural Science Foundation of China (No. 31772864), and Natural Science Foundation of Guangdong Province (2018A030313154 and 2020A1515011129).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank the Shanghai Menon Animal Nutrition Technology Co., Ltd. (Shanghai, China) for kindly providing 30% microencapsulated NaB in this experiment.
Ahmed, H. A., and Sadek, K. (2015). Impact of dietary supplementation of sodium butyrate and/or protexin on the growth performance, some blood parameters, and immune response of Oreochromis niloticus. Int. J. Agric. Innov. Res. 3, 985–991. doi: 10.1016/0003-9969(77)90069-3
Al-Harbi, N. O., Imam, F., Al-Harbi, M. M., Al-Shabanah, O. A., Alotaibi, M. R., As Sobeai, H. M., et al. (2019). Rutin inhibits carfilzomib-induced oxidative stress and inflammation via the NOS-mediated NF-κB signaling pathway. Inflammopharmacology 27, 817–827. doi: 10.1007/s10787-018-0550-5
Al-Sadi, R., Ye, D. M., Dokladny, K., and Ma, T. Y. (2008). Mechanism of IL-1beta-induced increase in intestinal epithelial tight junction permeability. J. Immunol. 180, 5653–5661. doi: 10.4049/jimmunol.180.8.5653
Al-Sadi, R. M., and Ma, T. Y. (2007). IL-1 causes an increase in intestinal epithelial tight junction permeability. J. Immunol. 178, 4641–4649. doi: 10.4049/jimmunol.178.7.4641
Bian, F. Y., Jiang, H. W., Man, M. S., Mai, K. S., Zhou, H. H., Xu, W., et al. (2017). Dietary gossypol suppressed postprandial TOR signaling and elevated ER stress pathways in turbot (Scophthalmus maximus L.). Am. J. Physiol. Endocrinol. Metab. 312, E37–E47. doi: 10.1152/ajpendo.00285.2016
Blasig, I. E., Bellmann, C., Cording, J., Vecchio, G. D., Zwanziger, D., Huber, O., et al. (2011). Occludin protein family: oxidative stress and reducing conditions. Antioxid. Redox Signaling 15, 1195–1219. doi: 10.1089/ars.2010.3542
Cao, S. Y., Wang, G. M., Ge, F., Li, X. H., Zhu, Q. Q., Ge, R. S., et al. (2018). Gossypol inhibits 5α-reductase 1 and 3α-hydroxysteroid dehydrogenase: its possible use for the treatment of prostate cancer. Fitoterapia 133, 102–108. doi: 10.1016/j.fitote.2018.12.024
Carriero, M. M., Henrique-Silva, F., Meira, C. M., Gato, I. M. Q., Caetano, A. R., Lobo, F. P., et al. (2020). Molecular characterization and gene expression analysis of the pro-inflammatory cytokines IL-1β and IL-8 in the South American fish Piaractus mesopotamicus challenged with Aeromonas dhakensis. Genet. Mol. Biol. 43:e20200006. doi: 10.1590/1678-4685-gmb-2020-0006
Chen, J. L., Yu, B., Chen, D. W., Huang, Z. Q., Mao, X. B., Ping, Z., et al. (2018). Chlorogenic acid improves intestinal barrier functions by suppressing mucosa inflammation and improving antioxidant capacity in weaned pigs. J. Nutr. Biochem. 59, 84–92. doi: 10.1016/j.jnutbio.2018.06.005
Claus, R., Günthner, D., and Letzgub, H. (2007). Effects of feeding fat-coated butyrate on mucosal morphology and function in the small intestine of the pig. J. Anim. Physiol. Anim. Nutr. 91, 312–318. doi: 10.1111/j.1439-0396.2006.00655.x
Degterev, A., and Yuan, J. Y. (2008). Expansion and evolution of cell death programmes. Nat. Rev. Mol. Cell Biol. 9, 378–390. doi: 10.1038/nrm2393
Deng, J. M., Zhang, X., Long, X. W., Tao, L. L., Wang, Z., Niu, G. Y., et al. (2014). Effects of dietary cholesterol supplementation on growth and cholesterol metabolism of rainbow trout (Oncorhynchus mykiss) fed diets with cottonseed meal or rapeseed meal. Fish Physiol. Biochem. 40, 1827–1838. doi: 10.1007/s10695-014-9971-2
Deng, S. J., Yan, H., Yi, J. N., Lu, Y., Wei, Q., Guo, C. Z., et al. (2013). Gossypol acetic acid induces apoptosis in RAW264.7 cells via a caspase-dependent mitochondrial signaling pathway. J. Vet. Sci. 14, 281–289. doi: 10.4142/jvs.2013.14.3.281
Fuchs, Y., and Steller, H. (2011). Programmed cell death in animal development and disease. Cell 147, 742–758. doi: 10.1016/j.cell.2011.10.033
Gu, J. N., Krogdahl, Å., Sissener, N. H., Kortner, T. M., Gelencser, E., Hemre, G.-I., et al. (2013). Effects of oral Bt-maize (MON810) exposure on growth and health parameters in normal and sensitised Atlantic salmon, Salmo salar L. Br. J. Nutr. 109, 1408–1423. doi: 10.1017/S000711451200325X
Hamazaki, Y., Itoh, M., Sasaki, H., Furuse, M., and Tsukita, S. (2002). Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J. Biol. Chem. 277, 455–461. doi: 10.1074/jbc.M109005200
Herman, R. L. (1970). Effects of gossypol on rainbow trout Salmo guirdneri Richardson. J. Fish Biol. 2, 293–303. doi: 10.1111/j.1095-8649.1970.tb03288.x
Hou, C. Y. (2012). The Effect of Gossypol on Rumen Microbial Population and Fiber Degradability. Hohhot: Inner Mongolia Agricultural University.
Hou, N. N. (2014). Effects of Gossypol on Hepatic Lipid Metabolism and Antioxidant Function of Laying Hens. Tai'an: Shandong Agriculture University.
Jiang, C. Q. (2011). Study on rational utilizing rapeseed meal and cottonseed meal in Carassius auratus gibelio. Shanghai Ocean University.
Jiang, S., Wu, X., Luo, Y., Wu, M., Lu, S., Jin, Z., et al. (2016). Optimal dietary protein level and protein to energy ratio for hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) juveniles. Aquaculture 465, 28–36. doi: 10.1016/j.aquaculture.2016.08.030
Kaufmann, T., Strasser, A., and Post, P. J. (2012). Fas death receptor signalling: roles of Bid and XIAP. Cell Death Differ. 19, 42–50. doi: 10.1038/cdd.2011.121
Kim, J. H., Choi, J. S., and Lee, B. H. (2012). PI3K/Akt and MAPK pathways evoke activation of FoxO transcription factor to undergo neuronal apoptosis in brain of the silkworm Bombyx mori (Lepidoptera: Bombycidae). Cell Mol. Biol. 58, OL1780–1785. doi: 10.1170/209
Krogdahl, Å., Penn, M., Thorsen, J., Refstie, S., and Bakke, A. M. (2010). Important antinutrients in plant feedstuffs for aquaculture: an update on recent findings regarding responses in salmonids. Aquacult. Res. 41, 333–344. doi: 10.1111/j.1365-2109.2009.02426.x
Kuhn, K. A., Schulz, H. M., Regner, E. H., Severs, E. L., Hendrickson, J. D., Mehta, G., et al. (2018). Bacteroidales recruit IL-6-producing intraepithelial lymphocytes in the colon to promote barrier integrity. Mucosal Immunol. 11, 357–368. doi: 10.1038/mi.2017.55
Kumar, M., Tomar, M., Punia, S., Grasso, S., and Amarowicz, R. (2021). Cottonseed: a sustainable contributor to global protein requirements. Trends Food Sci. Technol. 111, 100–113. doi: 10.1016/j.tifs.2021.02.058
Li, S., Yuan, H., Liu, Y. Y., Zhao, Y. J., He, X. X., Zhang, L. L., et al. (2018a). Ipatasertib, a novel Akt inhibitor, induces transcription factor FoxO3a and NF-κB directly regulates PUMA-dependent apoptosis. Cell Death Dis. 9:911. doi: 10.1038/s41419-018-0943-9
Li, S. A., Jiang, W. D., Feng, L., Liu, Y., Wu, P., Jiang, J., et al. (2018b). Dietary myo-inositol deficiency decreased intestinal immune function related to NF-kappa B and TOR signaling in the intestine of young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 76, 333–346. doi: 10.1016/j.fsi.2018.03.017
Li, Y. X., Hu, H. B., Liu, J. T., Yang, P., Zhang, Y. J., Ai, Q. H., et al. (2017). Dietary soya allergen β-conglycinin induces intestinal inflammatory reactions, serum-specific antibody response and growth reduction in a carnivorous fish species, turbot Scophthalmus maximus L. Aquacult. Res. 48, 4022–4037. doi: 10.1111/are.13224
Lim, C., Aksoy, M., and Klesius, P. (2003). Levels of dietary gossypol affect growth and bacterial resistance of Nile tilapia. Global Aquacult. 6, 42–43.
Liu, H., Dong, X. H., Tan, B. P., Du, T., Zhang, S., Yang, Y. Z., et al. (2020). Effects of fish meal replacement by low-gossypol cottonseed meal on growth performance, digestive enzyme activity, intestine histology and inflammatory gene expression of silver sillago (Sillago sihama Forsskál) (1775). Aquacult. Nutr. 26, 1724–1735. doi: 10.1111/anu.13123
Liu, M. M., Guo, W., Wu, F., Qu, Q. C., Tan, Q. S., and Gong, W. B. (2017). Dietary supplementation of sodium butyrate may benefit growth performance and intestinal function in juvenile grass carp (Ctenopharyngodon idellus). Aquac. Res. 48, 4102–4111. doi: 10.1111/are.13230
Liu, T., Li, J., Liu, Y., Xiao, N., Suo, H., Xie, K., et al. (2012). Short-chain fatty acids suppress lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines through inhibition of NF-κB pathway in RAW264.7 cells. Inflammation 35, 1676–1684. doi: 10.1007/s10753-012-9484-z
Liu, Y., Chen, Z. C., Dai, J. H., Yang, P., Xu, W. Q., Ai, Q. H., et al. (2019). Sodium butyrate supplementation in high-soybean meal diets for turbot (Scophthalmus maximus L.): Effects on inflammatory status, mucosal barriers and microbiota in the intestine. Fish Shellfish Immunol. 88, 65–75. doi: 10.1016/j.fsi.2019.02.064
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Mbahinzireki, G. B., Dabrowski, K., Lee, K. J., and El-Saidy, D.R. W.E. (2015). Growth, feed utilization and body composition of tilapia (Oreochromis sp.) fed with cottonseed meal-based diets in a recirculating system. Aquacult. Nutr. 7, 189–200. doi: 10.1046/j.1365-2095.2001.00172.x
Mclaughlin, J., Padfield, P. J., Burt, J. P. H., and O'neill, C. A. (2004). Ochratoxin A increases permeability through tight junctions by removal of specific claudin isoforms. Am. J. Physiol. Cell Physiol. 287, 1412–1417. doi: 10.1152/ajpcell.00007.2004
Oliver, C. L., Miranda, M. B., Shangary, S., Land, S., Wang, S. M., and Johnson, D. E. (2005). (-)-Gossypol acts directly on the mitochondria to overcome Bcl-2- and Bcl-X(L)-mediated apoptosis resistance. Mol. Cancer Ther. 4, 23–31. doi: 10.1186/1476-4598-4-23
Qi, M., Gao, S. Q., Liu, J. Q., Han, Y. L., and Zheng, L. R. (2020). Inhibitory effects of Akt on neuronal FoxO4 activation after subarachnoid hemorrhage in vivo and in vitro. bioRxiv. doi: 10.1101/2020.01.20.912436
Rahimnejad, S., Bang, I.-C., Park, J.-Y., Sade, A., Choi, J., and Lee, S.-M. (2015). Effects of dietary protein and lipid levels on growth performance, feed utilization and body composition of juvenile hybrid grouper, Epinephelus fuscoguttatus × E. lanceolatus. Aquaculture 446, 283–289. doi: 10.1016/j.aquaculture.2015.05.019
Rinchard, J., Lee, K., Dabrowski, K., Ciereszko, A., Blom, J. H., and Ottobre, J. S. (2003). Influence of gossypol from dietary cottonseed meal on haematology, reproductive steroids and tissue gossypol enantiomer concentrations in male rainbow trout (Oncorhynchus mykiss). Aquacult. Nutr. 9, 275–282. doi: 10.1046/j.1365-2095.2003.00253.x
Robles, R., Lozano, A. B, Sevilla, A., Márquez, L., Nuez-Ortín, W., et al. (2013). Effect of partially protected butyrate used as feed additive on growth and intestinal metabolism in sea bream (Sparus aurata). Fish Physiol. Biochem. 39, 1567–1580. doi: 10.1007/s10695-013-9809-3
Shen, J. F., Liu, H. Y., Tan, B. P., Dong, X. H., Yang, Q. H., Chi, S. Y., et al. (2020). Effects of replacement of fishmeal with cottonseed protein concentrate on the growth, intestinal microflora, haematological and antioxidant indices of juvenile golden pompano (Trachinotus ovatus). Aquacult. Nutr. 26, 1119–1130. doi: 10.1111/anu.13069
Sun, C. Y., Liu, Y., Feng, L., Jiang, W. D., Wu, P., Jiang, J., et al. (2021). Xylooligosaccharide supplementation improved growth performance and prevented intestinal apoptosis in grass carp. Aquaculture 535:736360. doi: 10.1016/j.aquaculture.2021.736360
Tian, L., Zhou, X. Q., Jiang, W. D., Liu, Y., Wu, P., Jiang, J., et al. (2017). Sodium butyrate improved intestinal immune function associated with NF-kappa B and p38MAPK signalling pathways in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 66, 548–563. doi: 10.1016/j.fsi.2017.05.049
Wang, B., Feng, L., Chen, G. F., Jiang, W. D., Liu, Y., Kuang, S. Y., et al. (2016). Jian carp (Cyprinus carpio var. Jian) intestinal immune responses, antioxidant status and tight junction protein mRNA expression are modulated via Nrf2 and PKC in response to dietary arginine deficiency. Fish Shellfish Immunol. 51, 116–124. doi: 10.1016/j.fsi.2015.10.032
Wang, K. Z. (2019). Effects of Gossypol on the Intestinal Structure and Immune Barrier and the Related Mechanisms in Grass Carp (Ctenopharyngodon idella). Ya'an: Sichuan Agriculture University.
Wang, K. Z., Feng, L., Jiang, W. D., Wu, P., Liu, Y., Jiang, J., et al. (2019). Dietary gossypol reduced intestinal immunity and aggravated inflammation in on-growing grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 86, 814–831. doi: 10.1016/j.fsi.2018.12.014
Wanga, K. Z., Jianga, W. D., Pei, W., Yang, L., and Jiang, J. (2018). Gossypol reduced the intestinal amino acid absorption capacity of young grass carp (Ctenopharyngodon idella). Aquaculture 492, 46–58. doi: 10.1016/j.aquaculture.2018.03.061
Yan, L. C., Feng, L., Jiang, W. D., Wu, P. Y. L., et al. (2019). Dietary taurine supplementation to a plant protein source-based diet improved the growth and intestinal immune function of young grass carp (Ctenopharyngodon idella). Aquacult. Nutr. 25, 873–896. doi: 10.1111/anu.12907
Yildirim, M., Lim, C., Wan, P. J., and Klesius, P. H. (2003). Growth performance and immune response of channel catfish (Ictalurus puctatus) fed diets containing graded levels of gossypol–acetic acid. Aquaculture 219, 751–768. doi: 10.1016/S0044-8486(03)00062-0
Yin, B., Liu, H. Y., Tan, B. P., Dong, X. H., Chi, S. Y., Yang, Q. H., et al. (2018). Cottonseed protein concentrate (CPC)suppresses immune function in different intestinal segments of hybrid grouper ♀Epinephelus fuscoguttatus×♂Epinephelus. lanceolatu via TLR-2/MyD88 signaling pathways. Fish Shellfish Immunl. 81, 318–328. doi: 10.1016/j.fsi.2018.07.038
Yin, B., Liu, H. Y., Tan, B. P., Dong, X. H., Chi, S. Y., Yang, Q. H., et al. (2021a). MHC II-PI3K/Akt/mTOR signaling pathway regulates intestinal immune response induced by soy glycinin in hybrid grouper: protective effects of sodium butyrate. Front. Immunol. 11:615980. doi: 10.3389/fimmu.2020.615980
Yin, Z. Y., Liu, Q. D., Liu, Y. T., Gao, S. N. L. H. Y., et al. (2021b). Early life intervention using probiotic clostridium butyricum improves intestinal development, immune response, and gut microbiota in large yellow croaker (Larimichthys crocea) larvae. Front. Immunol. 12:640767. doi: 10.3389/fimmu.2021.640767
Zhang, J. J., Wang, J. Q., Xu, X. Y., Yang, J. Y., Wang, Z., Jiang, S., et al. (2020). Red ginseng protects against cisplatin-induced intestinal toxicity by inhibiting apoptosis and autophagy via the PI3K/AKT and MAPK signaling pathways. Food Function 11, 1–34. doi: 10.1039/D0FO00469C
Zhang, M. D. (2019). The Toxicity and Mechanism of Hepatotoxicity Induced by Gossypol Acetate in Cyprinus carpio. Henan: Henan Normal University.
Zhang, W., Tan, B. P., Deng, J. M., Dong, X. H., Yang, Q. H., Chi, S. Y., et al. (2021). The single-molecule long-read sequencing of intestine after soy meal-induced enteritis in juvenile pearl gentian grouper, Epinephelus fuscoguttatus♀×Epinephelus lanceolatus♂. Front. Mar. Sci. 8:688601. doi: 10.3389/fmars.2021.688601
Zhao, J., Feng, L., Liu, Y., Jiang, W. D., Wu, P., Jiang, J., et al. (2014). Effect of dietary isoleucine on the immunity, antioxidant status, tight junctions and microflora in the intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol. 41, 663–673. doi: 10.1016/j.fsi.2014.10.002
Keywords: hybrid grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂), gossypol, sodium butyrate, intestinal inflammation, tight junction
Citation: Yin B, Liu H, Tan B, Dong X, Chi S, Yang Q and Zhang S (2021) Effects of Low- and High-Level Gossypol and Sodium Butyrate Supplementation Under High-Level Gossypol Condition on Growth Performance and Intestinal Health of Hybrid Grouper (Epinephelus fuscoguttatus♀×Epinephelus lanceolatus♂). Front. Mar. Sci. 8:740660. doi: 10.3389/fmars.2021.740660
Received: 13 July 2021; Accepted: 24 August 2021;
Published: 21 September 2021.
Edited by:
Xiang-Jun Leng, Shanghai Ocean University, ChinaReviewed by:
Min Jin, Ningbo University, ChinaCopyright © 2021 Yin, Liu, Tan, Dong, Chi, Yang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongyu Liu, bGl1aHl1QGdkb3UuZWR1LmNu; Beiping Tan, YnB0YW5AMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.