
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 26 October 2021
Sec. Marine Ecosystem Ecology
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.731675
This article is part of the Research TopicThe Philippine Seas: Biodiversity and Ecological Impacts of Natural and Anthropogenic stressors in Tropical Reef SystemsView all 14 articles
Overfishing and destructive fishing practices are major threats to marine biodiversity in the Philippines, where over 1.9 million small-scale fishers are supported by these biodiverse marine communities. Nearly 50% of all marine fish capture in the Philippines is from artisanal fisheries, with much of it not reported or under-reported. Marine reserves, where fishing is prohibited have been created in many local government units to help restore and preserve this biodiversity. The success of these marine reserves is still under serious debate with effective management still representing a significant challenge. The lack of a governance system to centrally managed marine reserves has resulted in non-governmental organizations developing community-based management schemes. Using independent fisheries data from Rare’s Fish Forever program, we applied PERMANOVA, SIMPER and biodiversity index analyses to evaluate the community structure of reef fish in 20 sites within the Philippines. We analyzed the differences in fish assemblage inside and outside of these marine reserves, before and after community-based management had been implemented. We provide evidence that: (i) fish community structure changes within marine reserves after community-based management strategies are implemented; and (ii) community-based management of marine fisheries resources protects and promotes biodiversity inside, and in some cases, outside marine reserves. Variability across sites suggests that other social or ecological factors may be influencing the ability of marine reserves to fully protect biodiversity and marine resources. Small-scale fishers in the Philippines participate in mixed-catch harvests and depend on biodiversity and reef community structure for their livelihoods. Thus, this work has implications on how community-based management strategies for marine reserves and adjacent waters may be beneficial for the sustainability of small-scale fishers.
Destructive fishing habits, overfishing, and pollution have impacted coral reef systems and fisheries (Pastorok and Bilyard, 1985; McManus and Reyes, 1997; Wenger et al., 2015; Graham et al., 2017). Unsustainable fishing techniques as well as sediment and nutrient pollution can transition coral reef ecosystems from oligotrophic complex living coral reef structures with high biodiversity to eutrophic, macro-algae algae covered structures with reduced biodiversity (Mumby et al., 2007). Overfishing or destructive fishing practices exist in commercial or industrial fishing fleets as well as small-scale fishing (SSF) (Mora, 2008; Alfaro-Shigueto et al., 2010; Shester and Micheli, 2011; Selgrath et al., 2018; Muallil et al., 2019). Small-scale fishing, generally defined by small, man- or low-powered vessels, makes up at least 30% of global catch (Pauly and Zeller, 2016). In the Philippines, approximately 50% of catch is harvested by over 1.9 million small-scale fishers (FAO, 2014) and 68% of fisheries have been found to be unsustainable (Muallil et al., 2014b). Additionally, small-scale fishers are growing in number in the Philippines, increasing the total annual fishing pressure (Selgrath et al., 2018). Rural coastal communities, where much of small-scale fishing occurs, rely on subsistence fishing and are uniquely vulnerable to fishery collapse and environmental changes, such as sea-level rise or ocean acidification (World Bank, 2012). Thus, it continues to be important to focus on sustainable management strategies for small-scale fishing.
In many parts of the world, top-down management approaches, such as catch limits and gear restrictions, are the most common type of commercial fisheries management (Hilborn and Ovando, 2014). For these traditional approaches to be successful, they typically rely on centralized governance, limited targeted species and large quantities of biological data. In emergent countries with a high proportion of small-scale fishing, these top-down approaches are challenging due to lack of infrastructure for monitoring and enforcement (Brownman et al., 2004). In order to overcome these challenges, governments and non-governmental organizations (NGOs) have been focusing on management methods to create more sustainable fishing practices for small-scale fishers and create resilience for fishing communities (FAO, 2018).
Rare, one such international NGO, has been working with local communities in the Philippines to address overfishing. Rare, with the Environmental Defense Fund and University of California Santa Barbara, developed the Fish Forever Program using Pride Campaigns to inspire behavior change to reduce illegal fishing in marine reserves and increase effectiveness. Beginning in 2011, the program paired managed access with marine reserves hypothesizing that the combination would provide benefits to both fish, fishers, and the broader marine ecosystem (Rare, 2018). Importantly, local communities manage both the marine reserves and the managed access areas to reduce illegal fishing of the marine reserve and reduce destructive fishing habits outside the reserves.
Marine reserve areas, where fishing is prohibited, can be a powerful management tool for protection of an essential habitat and also benefit fishers through increased catch (Guidetti, 2006; Kerwath et al., 2013; Strain et al., 2019). However, marine reserves will only be beneficial to both if they are effectively managed (Mora, 2008; Strain et al., 2019). The Philippines is often cited as a success story for marine reserves because of how early they were implemented (since the 1970s) and how many were delineated (1,800 as of 2014) (Cabral et al., 2014). However, only 2% of reefs are under protection, many of them small, and only 10–30% are effectively managed (Campos and Aliño, 2008; Weeks et al., 2010; Arceo et al., 2013). As a part of the Convention on Biological Diversity, the Philippines had agreed to protect 10% of the country’s marine resources by 2020 (Cabral et al., 2014), a target that was not achieved (Marine Conservation Institute, 2021).
One way to potentially increase effectiveness of marine reserves is to implement or strengthen community-based management (CBM) of those areas, which addresses the need for multi-species management and enforcement or compliance of fishing regulations (Smallhorn-West et al., 2019). Community-based management of natural resources has been a way for users to self-enforce and monitor those resources (Pinkerton, 1989; Pomeroy, 1995; Ostrom, 2000; Kearney et al., 2007). The Philippines government has decentralized fishery regulations, moving toward participatory approaches through the Local Government Code in 1991 and Fisheries code in 1998, which allow local governments or municipalities to manage fishery resources (Pomeroy and Courtney, 2018). Since then, the Philippines has used CBM in marine reserves with varying success (Aliño et al., 2000; Campos and Aliño, 2008; Arceo et al., 2013; Rohrer, 2017). Notably, Apo Island, one of the best studied marine reserves, has demonstrated that important fish species increase in both biomass and catch (Russ and Alcala, 1996; Maypa et al., 2002; Russ et al., 2003). Other studies have found that CBM marine reserves maintain fish abundance and diversity within the reserves, but not in the surrounding reefs (Christie et al., 2002).
Many of the existing marine reserves already established in the Philippines were “paper parks,” protected in name only (Campos and Aliño, 2008). One of the issues with small-scale fishing is that top-down governance structures frequently lack enforcement and therefore are ineffective at reducing fishing pressure (Brownman et al., 2004). Managing these fisheries on a local level may increase enforcement and compliance of the marine reserves (McClanahan et al., 2006). Depending on the location and ecosystem, designation of a no-take marine reserves is not enough to protect the ecosystem and does not show significant regeneration of coral reef habitat or fishes, such as in parrotfish in Belize (Cox et al., 2017). Community-based management has been identified as a key component of effective marine reserves where increases in biomass of fished species is observed (Kearney et al., 2007; Guidetti and Claudet, 2010; Smallhorn-West et al., 2019).
In the Philippines, biomass of fish has increased both inside and outside CBM marine reserves (Russ et al., 2003; Rare, 2018). While fish biomass is higher in marine reserves, the stocks themselves are generally overfished (Muallil et al., 2019). In addition to biomass for evaluating management strategies, fish community structure is also needed because total biomass does not account for the diversity of species contributing to that biomass. Additionally, the increase of one species may not be as ecologically significant as the increase of all species across the community.
Researchers have repeatedly concluded that marine reserves will not lead to an increase in fishery resources if they are not effectively managed or designed (Mora, 2008; Gaines et al., 2010; Rife et al., 2013; Muallil et al., 2019). Here we investigated the impact of implementing community-based management on fish community structure and biodiversity in marine reserves and open access areas across the Philippines.
Fisheries independent data, such as species name, abundance, and estimated length were collected from 20 sites in the Philippines between the years of 2011 and 2017 as part of Rare’s Fish Forever Program (Figure 1). The data were collected using five 50 m transects and two swimmers at each site, who would visually identify species, count, and estimate total lengths of each fish. In each of the sites, abundance data were collected inside and outside the marine reserve. Reserve implementation occurred as early as 1989 and as late as 2012 (Table 1). Rare facilitated the implementation of community based management in 2014 at all of these sites.
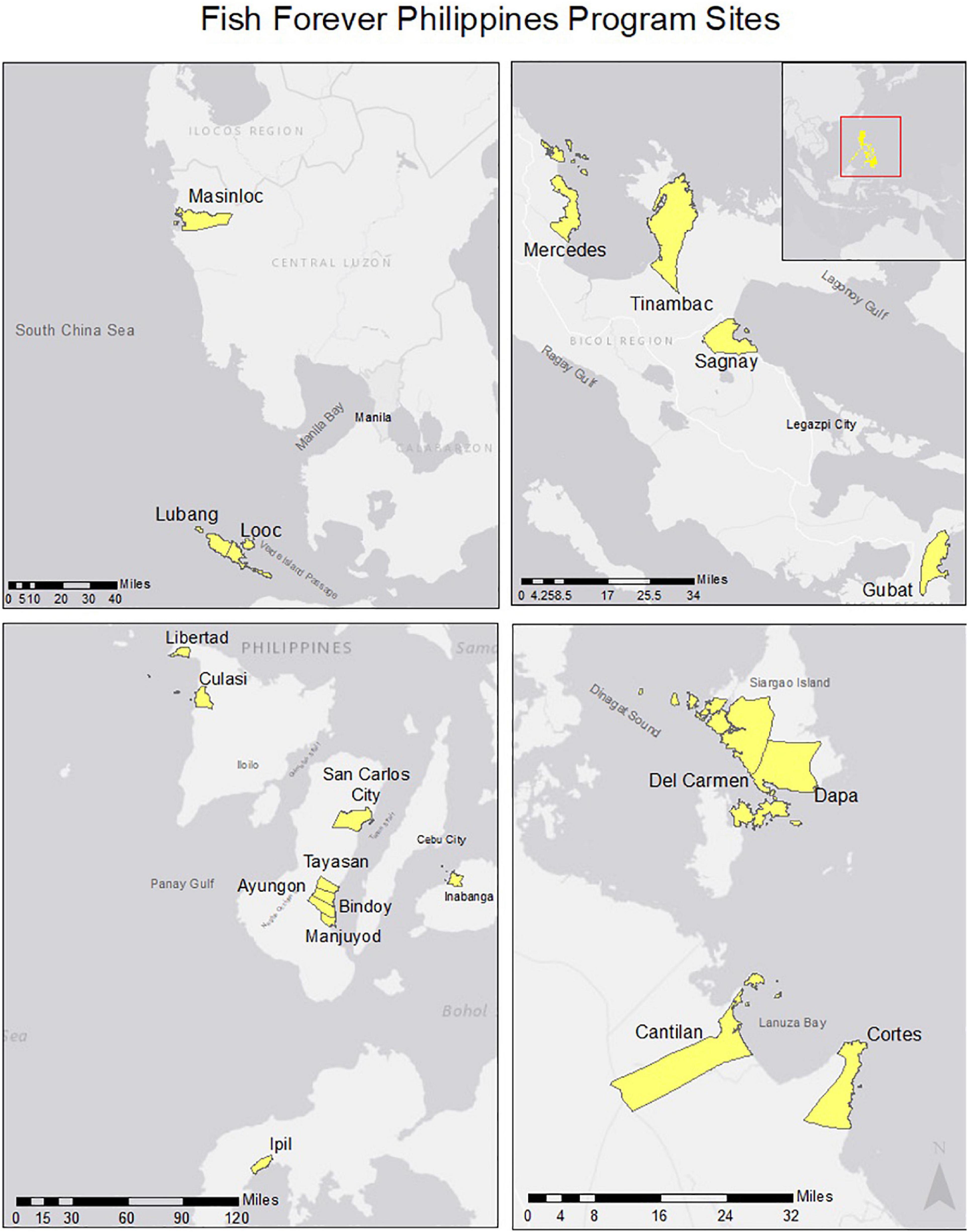
Figure 1. Site Map of the 20 Fish Forever program sites where marine reserves are located. Data Source: GADM, Spatial Reference: GCS WGS 1984.
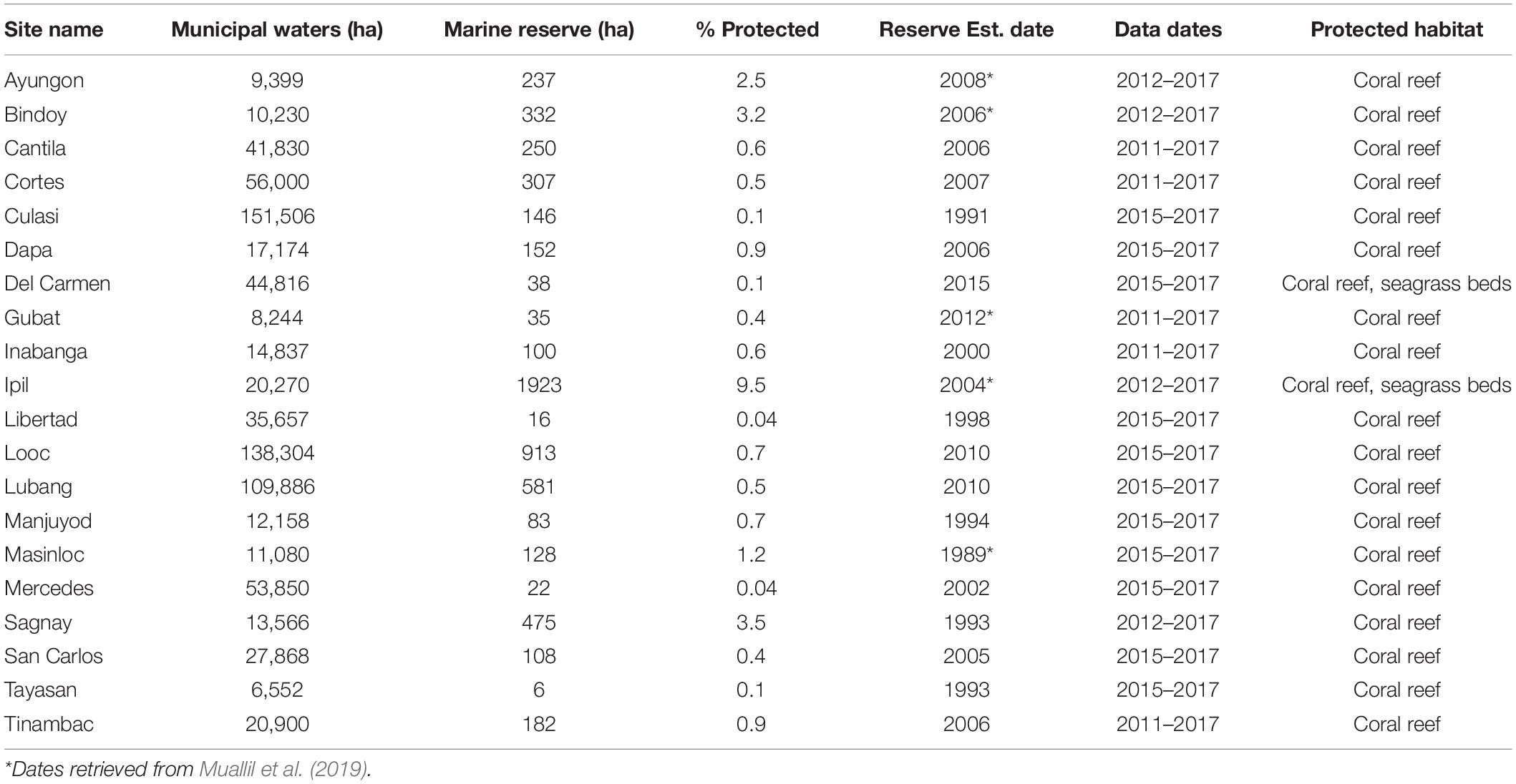
Table 1. Description of marine reserve area, dates of establishment, and range of dates for data collection for each site.
To determine the reef fish communities inside and outside the marine reserves before and after CBM was implemented, one-way PERMANOVA and SIMPER analyses were run using PRIMER-e (Clarke and Gorley, 2006). Both analyses were completed on a combination factor of whether the samples were inside or outside the marine reserve and if they were before or after CBM. Of all the combinations we used pair-wise tests within the PERMANOVA to compare four of the combinations: (1) “inside before” and “inside after” (2) “outside before” and “outside after” (3) “inside before” and “outside before” and (4) “inside after” and “outside after”. Fish abundance data were transformed using a 4th-root transformation and then a Hellinger similarity matrix was applied. Fish community structure was then visualized using non-metric multidimensional scaling (nMDS). The nMDS allows us to see similarities of species composition between treatment groups. PERMANOVAs were performed on each site to test the significance of difference between fish community structures, using the interaction of marine reserves status and before and after implementation of CBM. The PERMANOVAs ran up to 10,000 permutations. Where significant differences were found, SIMPER analyses, using the same combinations, were conducted to determine which fish families and species were the main contributors to the differences between treatment groups. Special attention was given to important fishery families jacks (Carangidae), fusiliers (Caesionidae), wrasses (Labridae), breams (Lethrinidae and Nemipteridae), rabbitfish (Siganidae), snapper (Lutjanidae), goatfish (Mullidae), grunts (Haemulidae), hogfish (Bodianinae), grouper (Serranidae, specifically Epinephelinae), parrotfish (Scaridae), surgeonfish (Acanthuridae), and ponyfish (Leiognathidae) (Muallil et al., 2014a; Fish Forever, 2020). We investigated CBM implementation effects on all sites aggregated as well as each site separately to observe overall patterns of fish community change and site level dynamics.
Biodiversity was assessed by calculating a Shannon Index on each transect and testing the interactions at the site, reserve status, and CBM level. The Shannon Index calculates both the species richness and evenness in an area, giving weight to rarer species. This type of diversity index is useful for areas where overexploitation of fishing resources may have resulted in more rare species, and accounting for the differences in abundance of these rare species is relevant to the study. Kruskal-Wallis tests were then performed on the Shannon Index values to test if CBM implementation resulted in significant differences. Biodiversity analyses were performed using the R package vegan and rstatix (R Core Team, 2013; Kassambara, 2020; Oksanen et al., 2020).
When all site data were aggregated, there is no clear clustering of fish communities between those inside marine reserves and outside marine reserves, but instead a strong clustering by site (Figure 2). However, when each site was analyzed separately, the percent of sites that had significant differences between fish community structure inside and outside marine reserves increased from 65% of sites to 85% of sites after the implementation of CBM (Table 2). Additionally, shifts in fish community structures were observed after CBM was implemented both inside marine reserves (40% of sites had a significant difference) and outside marine reserves (35% of sites had a significant difference). Due to the large total number of fish species observed (over 600), no single species makes up a large percentage of the dissimilarity between factors (inside versus outside the marine reserve before CBM, and inside versus outside the marine reserve after CBM) for any site in the SIMPER analyses. The SIMPER analyses performed after aggregating the data to the family level revealed that increases of abundance of many important fishery families contributed to the differences seen in community composition after the CBM was implemented.
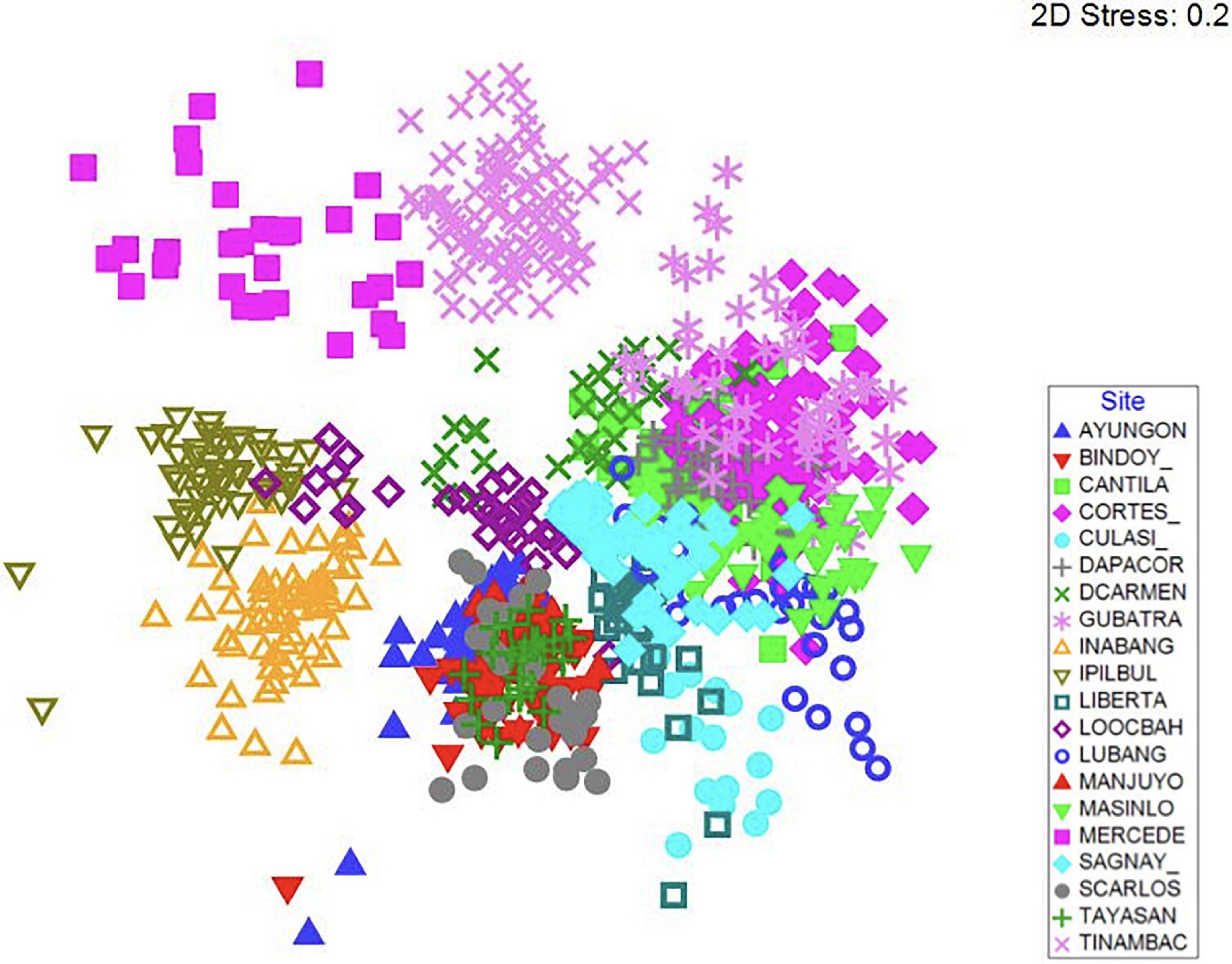
Figure 2. Non-metric multidimensional scaling (nMDS) plot of fish species community structure of all sites. Symbols indicate site.
Some sites, such as Lubang, San Carlos City, Bindoy, and Looc, had clearly defined clusters (P < 0.05) for fish communities inside and outside the marine reserve, but no significant differences for changes before and after the implementation of CBM (Figure 3 and Supplementary Figures 1–4). For example, in Bindoy an increase of important fishery families (snappers, wrasses, jacks, goatfish, groupers, parrotfish, and breams) made up 25.9% of dissimilarity between inside and outside the marine reserves before CBM (Figure 4). However, not every important fishery family increased uniformly at each site. For the same location (Bindoy) and treatment (inside and outside reserve before CBM implementation), a decrease in fusiliers, rabbitfish, hogfish, and surgeonfish was responsible for 12.3% of the dissimilarity. Additionally, when comparing the differences inside and outside the marine reserves after CBM was implemented the increase of important families (snappers, wrasses, jacks, goatfish, groupers, parrotfish, breams, fusiliers, surgeonfish, and rabbitfish) contributed to 34.5% of the dissimilarity. Notably, there were increases in fusiliers and rabbitfish contributing to 6.8% of dissimilarity between inside and outside marine reserves after CBM was implemented.
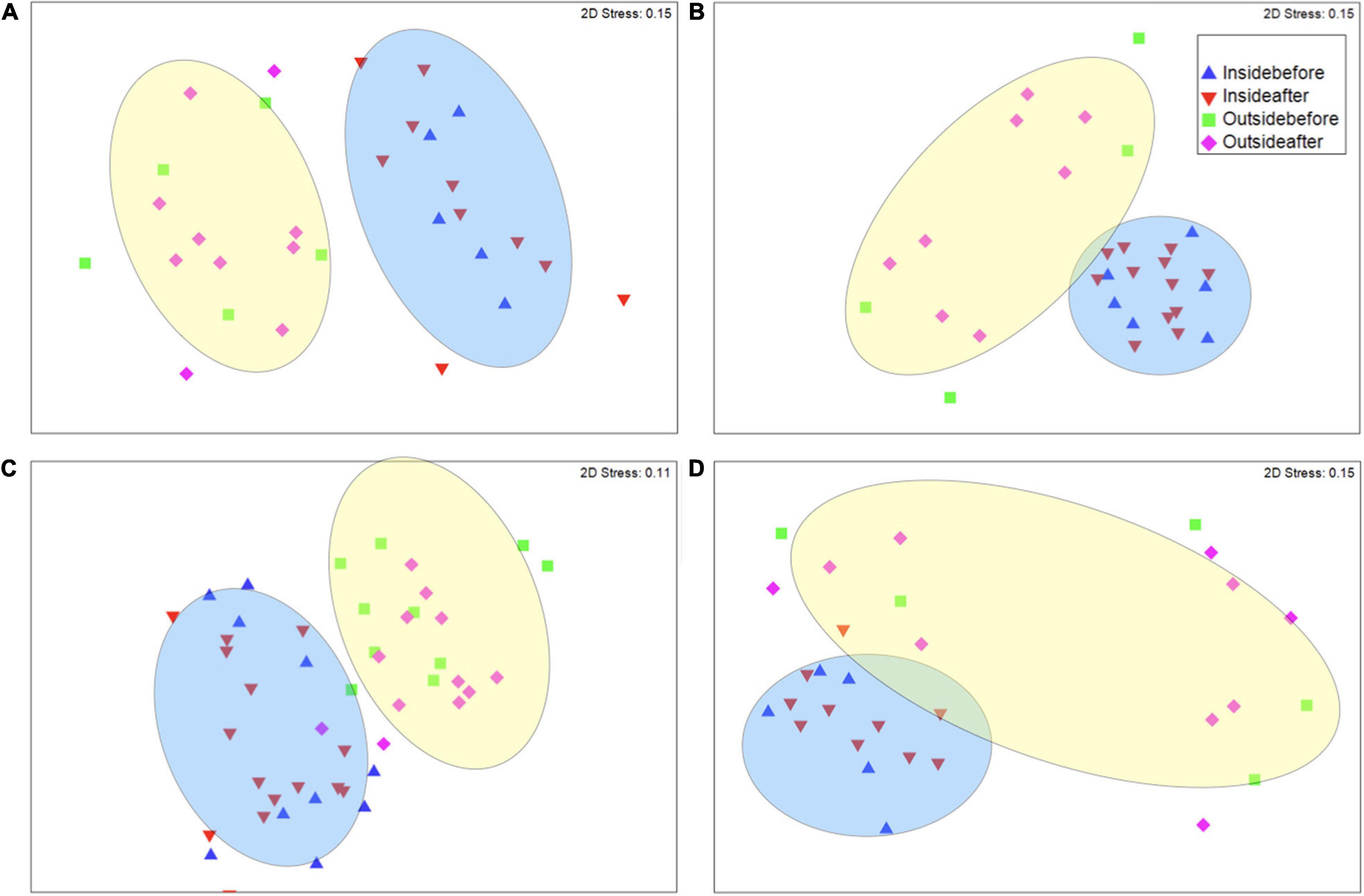
Figure 3. Non-metric multidimensional scaling plot of fish species community structure similarities of (A) Lubang, (B) San Carlos City, (C) Bindoy, and (D) Looc. Triangles denote the community structure inside marine reserve [blue indicates before and red indicates after community-based management (CBM) implementation], and squares and diamonds outside of marine reserves, with squares indicating before, and diamonds indicating after CBM implementation. The ellipses indicate significant clusters (P < 0.05).
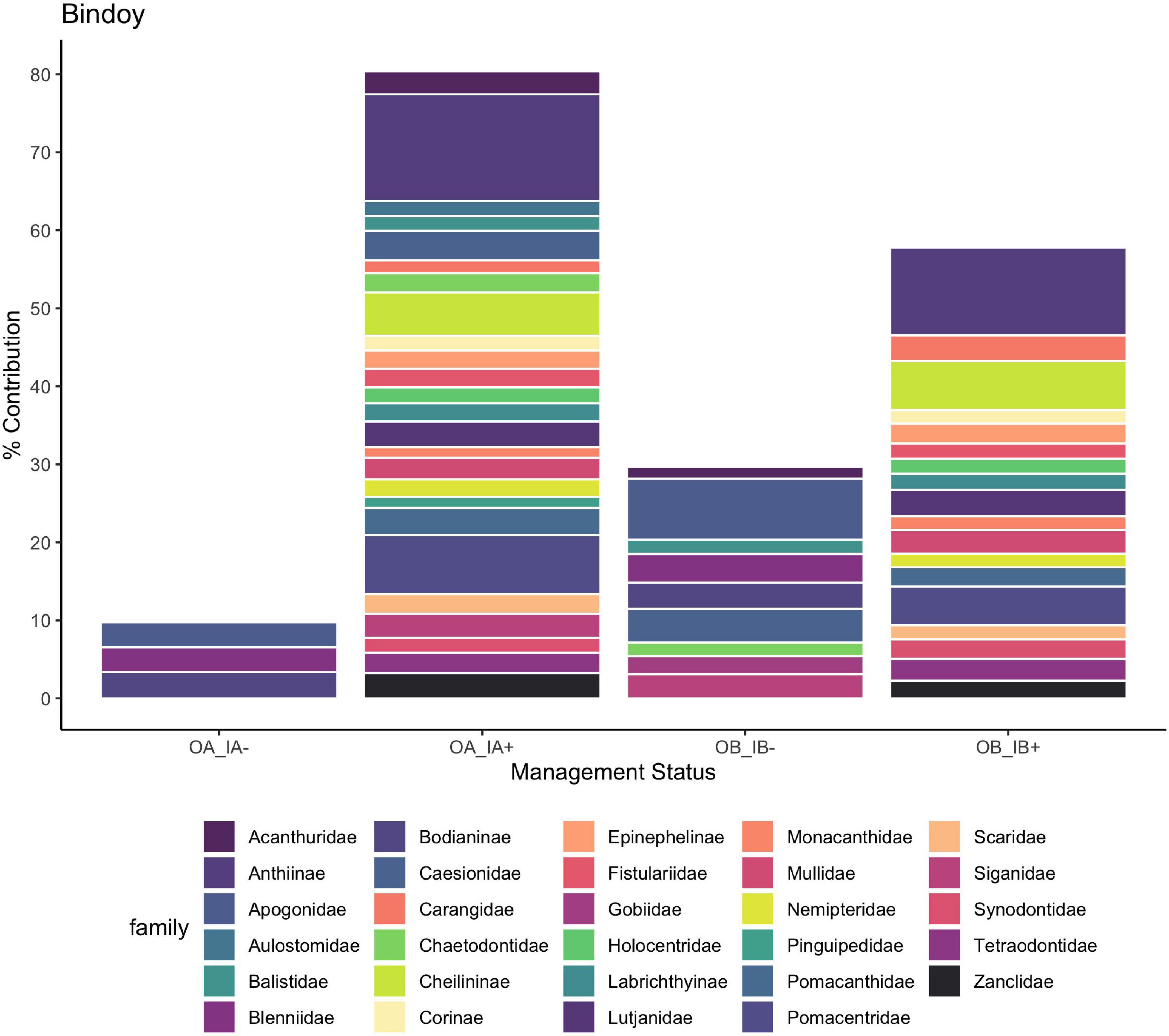
Figure 4. Percent contribution to the dissimilarity between inside and outside before (average dissimilarity 30.36) and after (average dissimilarity 34.82) CBM implementation in Bindoy, aggregated by family. OA, outside marine reserve; after CBM implementation; OB, outside marine reserve; before CBM implementation; IA, inside marine reserve; after CBM implementation; IB, inside marine reserve; before CBM implementation. + means that there was higher abundance after CBM, – means there was lower abundance after CBM.
Other sites, such as Cantilan, Tinambac, and Cortes, had significant clusters (P < 0.05) for each interaction of reserve and CBM status (Figure 5 and Supplementary Figures 5, 6). For these three sites, the general effect of CBM implementation for both inside and outside the reserve was increasing abundances of most important fishery families. Tinambac outside the reserves after CBM was implemented was an exception, where a decrease in abundance of fusiliers, rabbitfish, wrasses, and parrotfish were responsible for 23% of dissimilarity (Figure 6). For Tinambac, both outside and inside the marine reserve after CBM was implemented, fusiliers decreased in abundance.
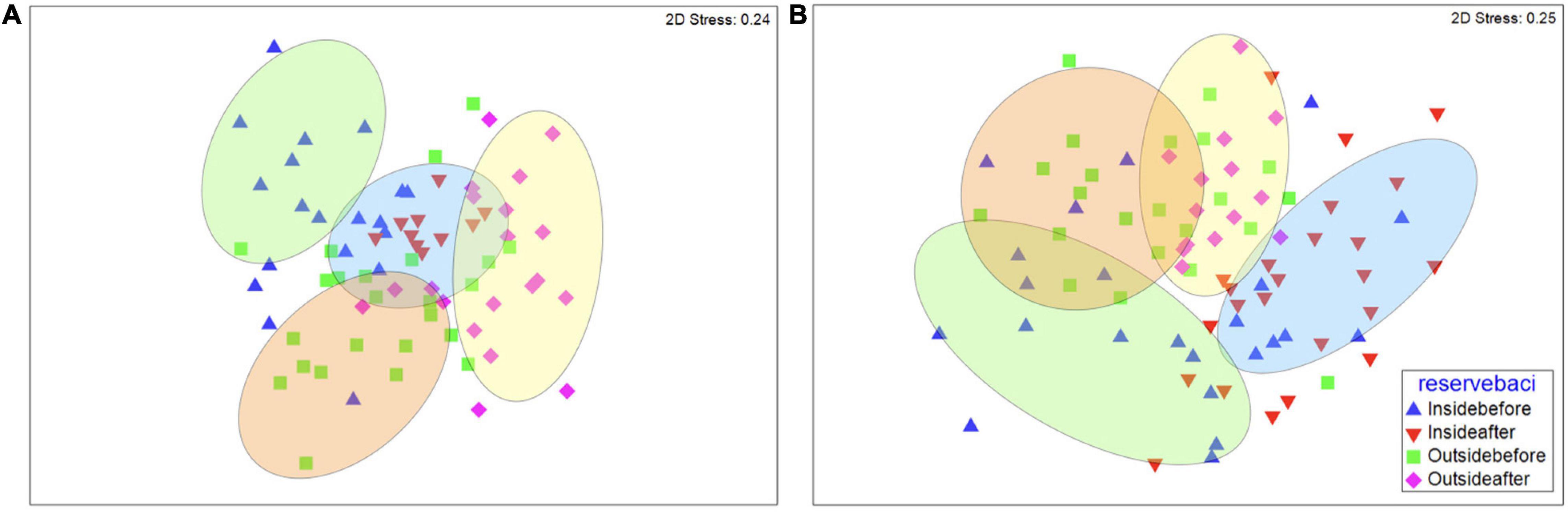
Figure 5. Non-metric multidimensional scaling plot of fish species community structure similarities of (A) Cantila and (B) Cortes. Triangles denote the community structure inside marine reserve (blue indicates before and red indicates after CBM implementation), and squares and diamonds outside of marine reserves, with squares indicating before, and diamonds indicating after CBM implementation. The ellipses indicate significant clusters (P < 0.05).
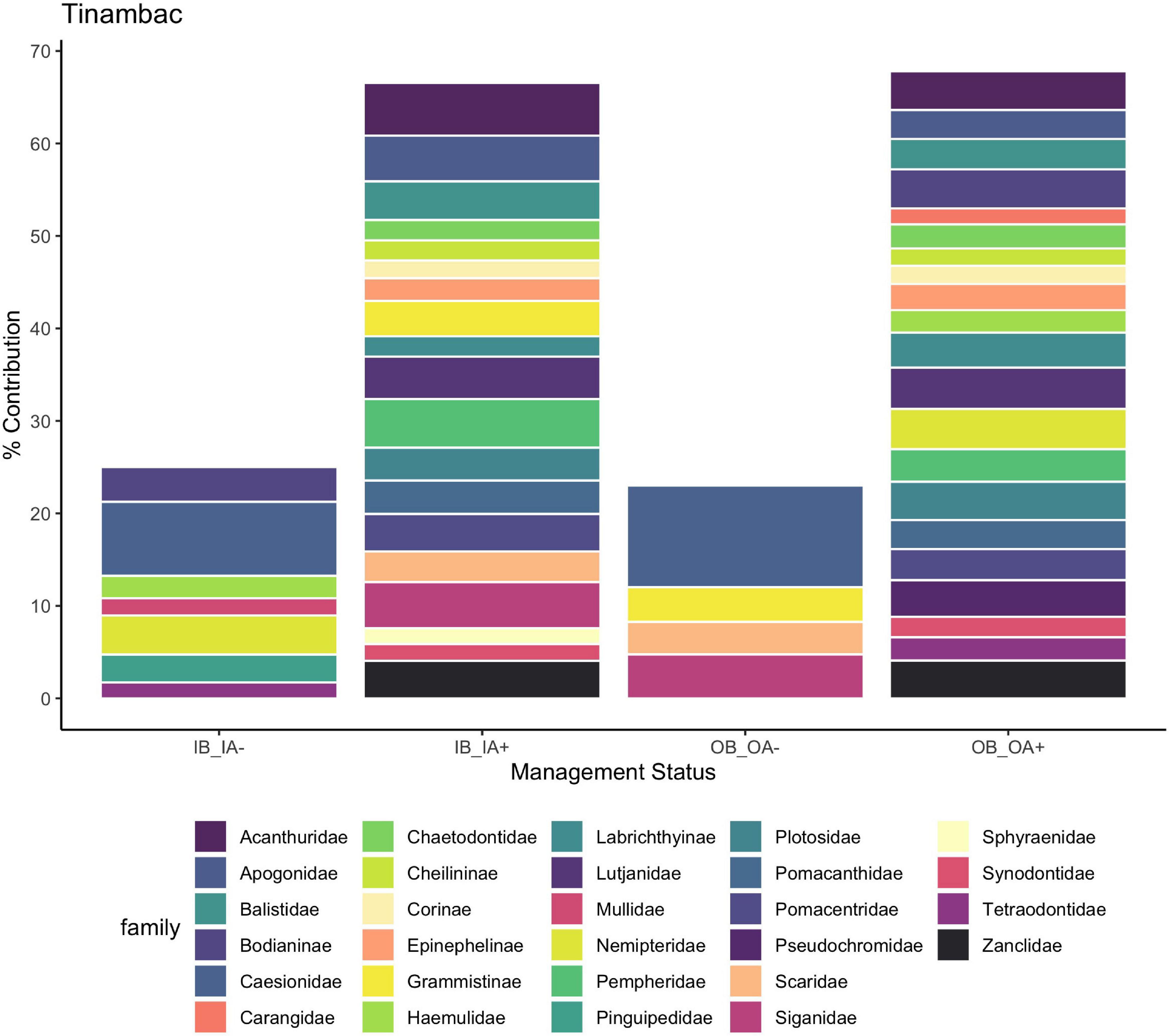
Figure 6. Percent contribution to the dissimilarity between inside before and after (average dissimilarity 31.86) and outside marine reserves before and after (average dissimilarity 31.80) CBM in Tinambac, aggregated by family. OA, outside marine reserve; after CBM implementation; OB, outside marine reserve; before CBM implementation; IA, inside marine reserve; after CBM implementation; IB, inside marine reserve; before CBM implementation. + means that there was higher abundance after CBM, – means there was lower abundance after CBM.
Four sites, Manjuyod, Culasi, Tayasan, and Gubat, had no difference in fish community structure before CBM inside and outside marine reserves, however, after CBM was implemented, there was a significant shift in community structure inside marine reserves (Figure 7 and Supplementary Figures 7–10). This was also accounted for in the SIMPER analysis for species where prior to CBM there was a lower dissimilarity (56.68) between inside the marine reserve and outside the marine reserve. However, after CBM implementation the average dissimilarity increased both within the reserve (69.71) and in comparison, to outside the reserve (64.63). The SIMPER analysis of Gubat revealed that an increase of snappers, goatfish wrasse, fusiliers, hogfish, and rabbitfish contributed to 23% of the dissimilarity between inside the marine reserve before and after CBM was implemented, indicating the application of CBM marine reserves for fisheries, not just conservation (Figure 8). Similarly to the previous sites, not all important fishery species had an increase of relative abundance after CBM, approximately 8% of the dissimilarity inside the reserves after CBM was due to the decrease of surgeonfish and parrotfish. Finally, two sites, Del Carmen and Sagnay had no community structure changes after CBM was implemented either inside or outside the marine reserve.
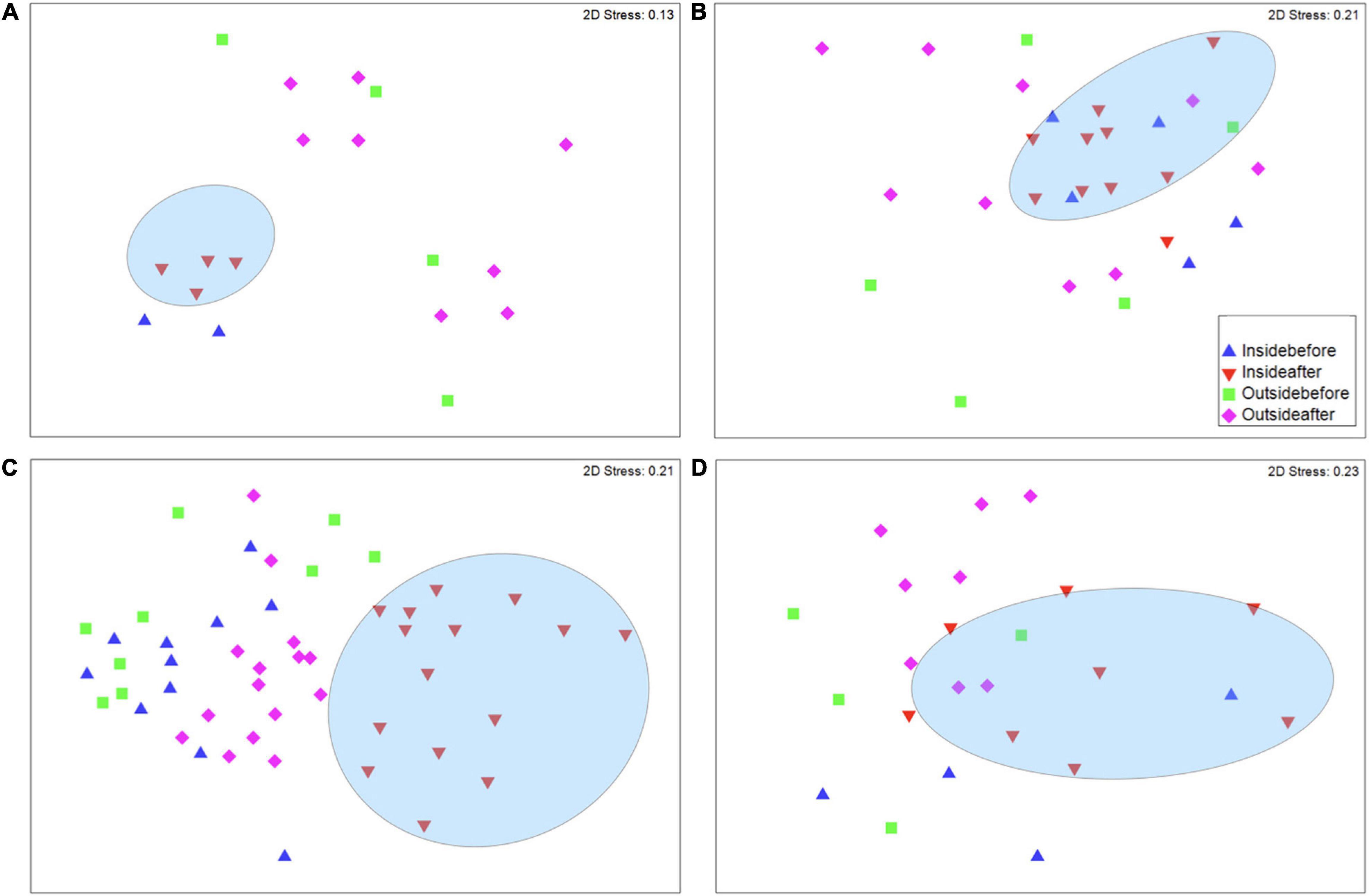
Figure 7. Non-metric multidimensional scaling of community structure similarities for (A) Culasi, (B) Manjuyod, (C) Gubat, and (D) Tayasan. Triangles denote the community structure inside marine reserve and squares and diamonds outside of marine reserves. Triangles denote the community structure inside marine reserve (blue indicates before and red indicates after CBM implementation), and squares and diamonds outside of marine reserves, with squares indicating before, and diamonds indicating after CBM implementation. The ellipses indicate significant clusters (P < 0.05).
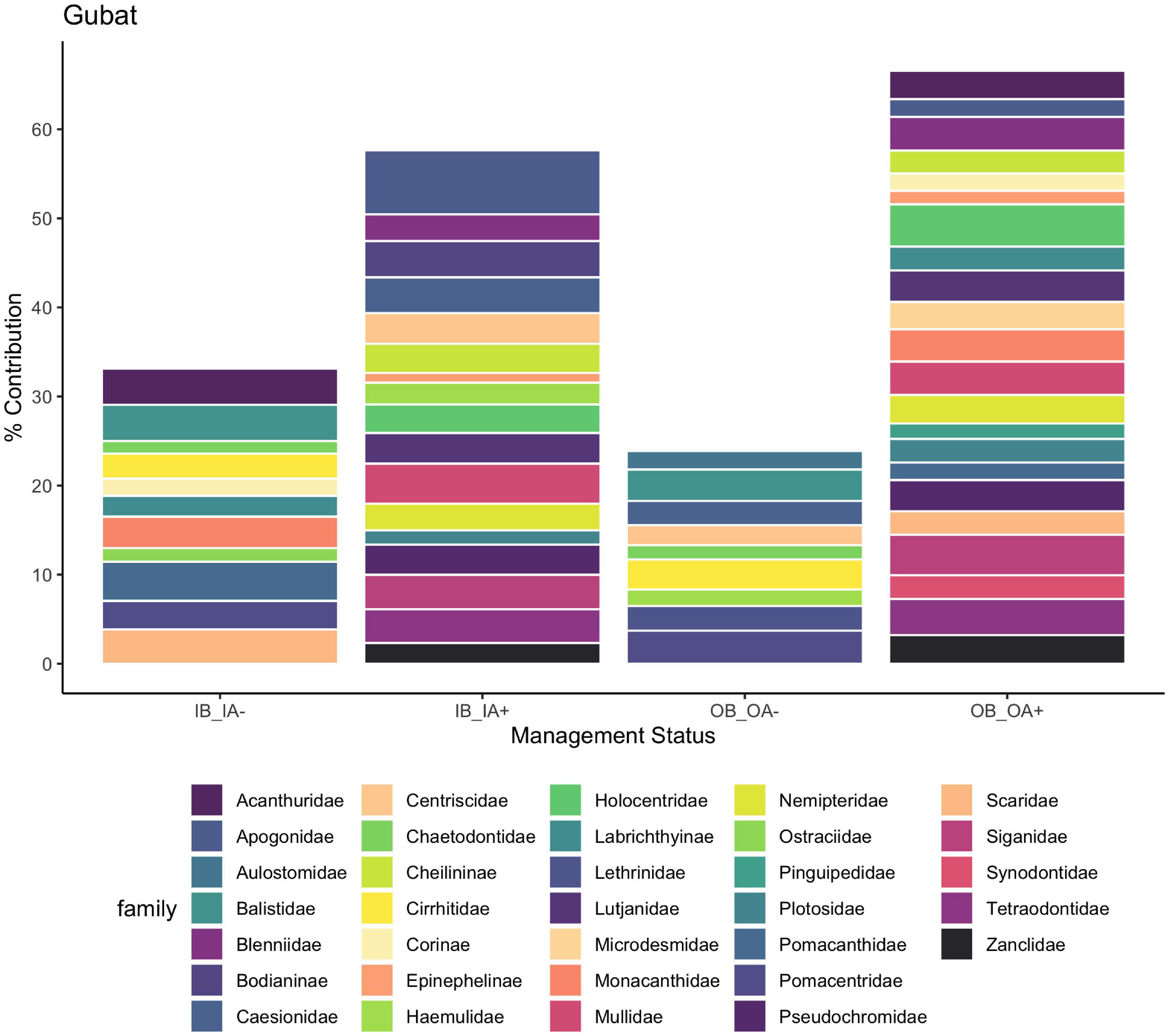
Figure 8. Percent contribution to the dissimilarity between inside the marine reserves before and after CBM, inside and outside marine reserves before CBM, and inside and outside after CBM. OA, outside marine reserve; after CBM implementation; OB, outside marine reserve; before CBM implementation; IA, inside marine reserve; after CBM implementation; IB, inside marine reserve; before CBM implementation. + means that there was higher abundance inside the reserve or after CBM, – means there was lower abundance inside the reserve or after CBM.
At the site level, six sites (30%) had significant differences in biodiversity inside or outside marine reserves and five (25%) had differences before and after community-based management. Cortes and Libertad had similar biodiversity inside and outside marine reserves, but after CBM was implemented were significantly different, with high diversity inside the reserve than outside after implementation (Figure 9). Additionally, while some sites (Cantila, Masinloc, and Tinambac) had no significant differences in biodiversity inside and outside the marine reserves, biodiversity significantly increased in both areas after CBM was implemented. One site, Del Carmen, decreased in biodiversity overall after CBM, though the remaining sites increased in biodiversity. While there is some overlap between sites that had significant community structure changes and biodiversity changes there is no overall pattern.
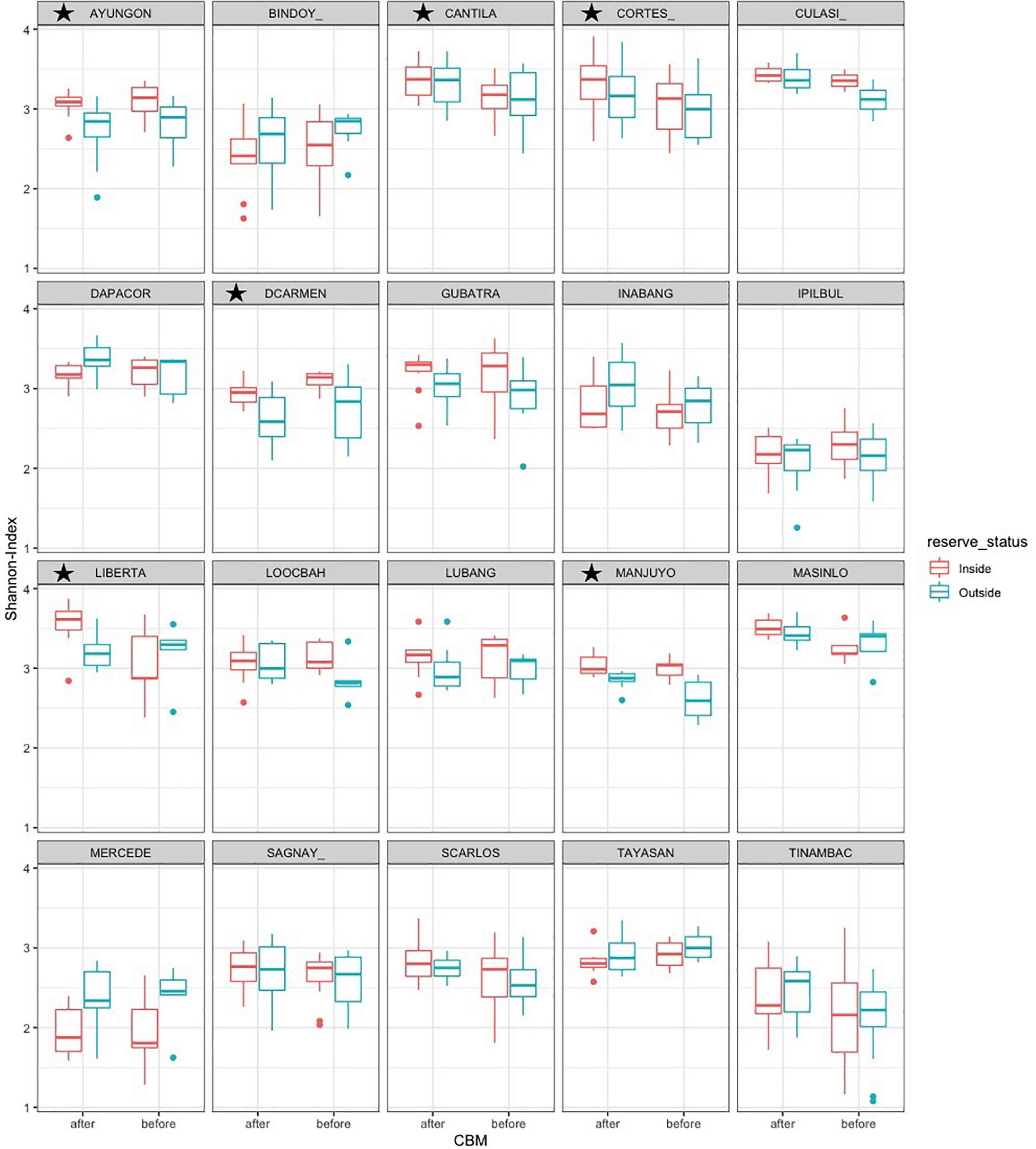
Figure 9. Biodiversity index inside and outside marine reserves before and after CBM. * Indicates the interaction between CBM and reserve status at each site is P < 0.05.
Well-managed marine reserves in the Philippines have the potential to increase abundance of important species and biodiversity. Typically when evaluating the indicators of success for marine reserves, age and size of the reserve are important (Halpern, 2003; Vandeperre et al., 2011). While many of the marine reserves at our study sites have been established for many years (x = 18 years; SD = 7.5), it is possible they had little enforcement and were considered “paper parks” (Campos and Aliño, 2008). It is likely that there was little effect on fish and fisheries from the marine reserves prior to the implementation of managed access and CBM governance structure in 2014. Other studies have demonstrated that reduction of fishing pressure can result in changes in fish communities, such as an increase of high trophic level fish after fishing effort was reduced (Graham et al., 2017). Our study found that when looking at site-specific community composition, shifts in community structure occurred inside the marine reserve after community-based management was implemented.
There are large numbers of reef fish that are important for small-scale fisheries in the Philippines contributing to differences in communities after CBM was implemented. Fishers rely on a wide variety of fish species, but fusiliers (Casionidae.), rabbitfish (Siganidae), and groupers (Serranidae) make up the top ten fished species at these sites (Fish Forever, 2020). Because of this, their increased abundance after CBM implementation, as was seen in our study, is significant to the communities who depend on them. One target species, the leopard coral grouper (Plectropomus leopardus), was previously listed as “Near-Threatened” by the International Union for Conservation of Nature (IUCN), citing population declines in the Philippines (Choat and Samoilys, 2018). In our study, the leopard coral grouper did contribute to the dissimilarity between communities inside marine reserves before and after community-based management, though with mixed results. In Bindoy, groupers, such as P. leopardus, decreased in relative abundance inside marine reserves after CBM (0.55% contribution to dissimilarity), but in Looc, they increased in abundance (0.56% contribution to dissimilarity). Plectropomus leopardus increased in abundance inside marine reserves after CBM in other sites such as Cantilan and Tinambac as well (contributing to 0.61 and 1.12%, respectively). Though the IUCN status has recently been updated to “Least Concern”, populations are still declining and effectively managed marine reserves may be key to their continued recovery, especially since groupers have smaller home ranges and their populations generally respond well to marine reserves (Kramer and Chapman, 1999; Lowe et al., 2003).
In addition to groupers increasing in abundance inside the marine reserve after CBM implementation, other families of fishes displayed interesting movements. For example, in Tinambac rabbitfish (Siganidae) and parrotfish (Scaridae) decreased in abundance outside of the reserve but showed relative increases inside the reserves. One explanation for this is that these fish are fished more heavily outside the reserve after CBM implementation. Another explanation is that fish leave fished areas and move into protected areas (Pittman et al., 2014). While this would appear as an increase of abundance inside the marine reserves, fish movement as opposed to fish reproduction is not a net increase of fish for the fishery. Though, the ability for fish to have refuge from fishing gives them a better chance for growth and reproduction in the future.
Biodiversity is typically used as an indicator of ecosystem health in relation to conservation, additionally, biodiversity is also correlated with higher catch and biomass, which is vital to small-scale fishers (Micheli et al., 2014). Marine reserves can assist in achieving both conservation and fishery goals if designed and enforced effectively (Cinner et al., 2020). While previous studies at the Fish Forever sites could not link the presence of the Fish Forever Pride Campaigns to increased biodiversity in marine reserves (Veríssimo et al., 2018), the implementation of community-based management investigated in this study does affect biodiversity in Philippine reefs. Overall, biodiversity increased after the implementation of CBM across all aggregated sites, which is a change from previous studies that did not find significant differences in biodiversity inside and outside marine reserves (Muallil et al., 2015). CBM at the sites in this study increased the effectiveness of the reserves and fostered higher biodiversity. A reason for this may be that Muallil et al. (2015) used 1 year of data (2012–2013), while our study uses a time series that allows for more time for populations to grow after fishing ceases. Additionally, our study tests biodiversity inside and outside marine reserves after CBM implementation, which occurred in 2014. Though, following the trends of the community structure analyses in this study, not all biodiversity indices increased significantly after the implementation of CBM for all sites. Our analyses reveal that sites do not all follow the same patterns, which leads to additional questions about why these fish communities differ.
In the Philippines, there are still instances of fishing occurring within marine reserves, which counteracts the ecological protections for the fish resulting in a lack of sustainability of the fishery (Muallil et al., 2014b). To increase the ecological function and protection of the reserves some suggest that marine reserves need to be larger (Muallil et al., 2014b). If community-based management does lead to reduced fishing pressure inside the marine reserves and more thoughtful fishing outside the marine reserves, we should expect greater biodiversity, abundance, and different fish communities. Our results suggest some sites performed better after CBM was implemented but not uniformly, approximately 30%. Some hypothesize that small marine reserves do not offer enough protection to impact fish community changes or biodiversity differences (Halpern, 2003; Friedlander et al., 2017), though the sites in our study that did show evidence of increase of biodiversity and abundance after community-based management were smaller reserves under 100 ha. The sites that had a significant difference in biodiversity in the interaction between reserves and CBM ranged from 16 to 307 ha in size. Even Tayasan, the smallest of the marine reserves at six hectares, and Gubat, a recently established marine reserve (in 2015), showed a significant change in community structure, showing increases of important fishery species, after CBM, which indicates that even small and recently established well-managed reserves can positively affect community structure for fisheries. It is also possible that some sites that did not show differences after CBM was implemented were already functioning and managed well prior to CBM. Of the sites where there were no community structure differences inside and outside reserves before CBM, 57% of them had differences after CBM. These sites indicate there is promise in well-designed CBM programs. Even though, the “after” dataset only encompassed 3 years, previous studies have shown that marine reserve impacts are seen within 1–3 years of implementation (Halpern and Warner, 2002; Micheli et al., 2004). Longer timeframes of data may allow for representation in the data of fish reproduction, spill-over, and growth, as well as for rules to be fully established and enforced, which may result in future changes to community structure and biodiversity (Russ and Alcala, 1996; Halpern et al., 2009; Friedlander et al., 2017).
Coastal fisheries exist in a complex socio-ecological system that involves many factors that determine the success of a particular management regime, but two major factors are compliance with fishery regulations and availability of nursery habitat. We provide evidence that community-based management leads to ecological changes that could benefit fisheries in the Philippines. However, our study is limited in answering “why” communities respond differently to CBM. We intend to further explore precise reasons why CBM may not be effective across all communities and address other impacts on fish communities, abundance, and biodiversity to improve the function of CBM of marine reserves. For example, marine reserves tend to be more effective when there is higher compliance within CBM (Pollnac et al., 2010). Though, there are many other indicators that also lead to increases in abundance of fish and biodiversity in CBM marine reserves such as residential community size, individual perceptions of fish populations, alternative livelihoods, trust in the marine reserve, and high participation in decision making (Pollnac et al., 2001; Quintana et al., 2021). These socio-ecological factors are dynamic, each heavily linked to the next, creating challenges in identifying a singular predictor for CBM marine reserve success. Negative perceptions on fish populations may result in the belief that the marine reserve is failing, thus that CBM is not working and create a feedback loop resulting in fewer rules for the marine reserves (Quintana et al., 2021). Additionally, external actors, such as fishers from other communities, may influence resources within the community fishing boundaries. Many municipalities in the Philippines have reported commercial fishers illegally fishing in municipal waters, who may have no regard nor knowledge of local regulations (Mullil 2014). The presence of the commercial fishers may be impacting the success of these marine reserves to no fault of the local communities managing them. Future research will include interviewing fishers to clarify levels of compliance of regulations and what types of effects external commercial fishers have on the resources where CBM has not shown to have significant effects on fish biodiversity and community structure.
This study examined the fishery independent data in the context of marine reserves and the implementation of community-based management of those reserves. The implementation of these strategies does not necessarily result in compliance of the regulations. Additionally, marine reserves assessed in this study protect the near-shore reef, but not adjoining habitats. Many species of fish in this region, especially parrotfish and snappers, which are both commercially important species, undergo ontogenetic migration from nursery habitats onto the reef (Nagelkerken et al., 2000; Mumby, 2006; Unsworth et al., 2008; Jones et al., 2010). Mangroves and seagrasses provide much of that nursery habitat that many fish depend on (Honda et al., 2013). Mangroves have declined rapidly, with 80% percent loss in the Philippines in the last 100 years (Primavera, 2000). The loss of mangrove habitat has been linked to declines in fishery stock (Melana et al., 2005; Tran and Fischer, 2017), which may occur regardless of protection of reefs. Decline of mangroves may also negatively impact corals by the increase of turbidity and sedimentation on reefs (Manson et al., 2005). When the reef structure and nursery habitat is threatened, continued production of important fishery species can also be vulnerable (Manson et al., 2005). Additional research will examine the habitat availability and quality at these sites as part of the suite of variables that may contribute to why sites responded differently to CBM implementation.
Protecting coastal coral reef areas and managing fishing access through marine reserves are important steps in sustainability of fishery resources, however, if critical nursery habitat or habitat that provides ecological functions for corals is not protected, then the efforts of marine reserves may be in vain. Thus, small-scale fishery management through community-based management needs to fit into a broader social-ecological systems model, considering both the compliance of the fishing communities and the relationship of nursery habitats.
Fish communities have a high site fidelity, supporting the notion that marine reserves and fishery management strategies need to be evaluated by site. Our study highlights the importance of site level dynamics in the success of community-based management. While CBM implementation resulted in positive changes of biodiversity in 25% of the sites and fish community structure increasing from 65 to 85% of the sites, further research is needed to investigate the reasons why some sites successfully increased fish biodiversity and abundance and others did not. Understanding the variability across sites, enabling conditions, and drivers of success will promote better design and implementation of CBM marine reserves. We suggest that resource managers explore interactions occurring between social and ecological factors within reef fishing communities to tailor interventions for each locality and increase potential for success when implementing community-based management.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
SM conceived the presented idea, performed analysis, and wrote the manuscript. CC, RA, RM, and DA coordinated the data collection and management. KM contributed to the design and implementation of research as well as meaningful contributions to the manuscript. All authors provided critical feedback and helped shape the research and manuscript.
Funding for data collection was provided by Rare.
CC, RA, RM, and DA work for Rare, which facilitated the implementation of the community-based management at these sites in the Philippines. To minimize the potential for a conflict of interest, Rare colleagues did not participate in the data analysis or interpretation used for this manuscript.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors want to acknowledge Rare and the partners in the Philippines for collecting and providing the data, which without this research would not be possible. S. Sanghera for advisement and thoughtful feedback on data analysis.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.731675/full#supplementary-material
Alfaro-Shigueto, J., Mangel, J. C., Pajuelo, M., Dutton, P. H., Seminoff, J. A., and Godley, B. J. (2010). Where small can have a large impact: structure and characterization of small-scale fisheries in Peru. Fish. Res. 106, 8–17. doi: 10.1016/j.fishres.2010.06.004
Aliño, P. M., Palomar, N. E., Arceo, H. O., and Uychiaoco, A. T. (2000). Challenges and opportunities for Marine Protected Area (MPA) management in the Philippines. Proc. Coral Reef Symp. 2, 635–640.
Arceo, H. O., Cazalet, B., Aliño, P. M., Mangialajo, L., and Francour, P. (2013). Moving beyond a Top-down fisheries management approach in the Northwestern Mediterranean: some lessons from the Philippines. Mar. Policy 39, 29–42. doi: 10.1016/j.marpol.2012.10.006
Brownman, H., Cury, P. M., Lotze, H. K., Jennings, S., Hilborn, R., Mace, P., et al. (2004). Perspectives on ecosystem-based approaches to the management of marine resources. Mar. Ecol. Prog. Ser. 274, 269–303. doi: 10.3354/meps274269
Cabral, R. B., Aliño, P. M., Balingit, A. C. M., Alis, C. M., and Arceo, H. O. Nañola Jr, C. L., et al. (2014). The Philippine marine protected area (MPA) database. Philipp. Sci. Lett. 7, 300–308.
Campos, W. L., and Aliño, P. M. (2008). Recent advances in the management of marine protected areas in the Philippines. Kuroshio Sci. 2, 29–34.
Christie, P., White, A., and Deguit, E. (2002). Starting point or solution? Community-based marine protected areas in the Philippines. J. Environ. Manag. 66, 441–454. doi: 10.1006/jema.2002.0595
Choat, J. H., and Samoilys, M. (2018). Plectropomusleopardus. The IUCN Red List of Threatened Species 2018: e.T44684A100462709. doi: 10.2305/IUCN.UK.2018-2.RLTS.T44684A100462709.en
Cinner, J. E., Zamborain-Mason, J., Gurney, G. G., Graham, N. A. J., MacNeil, M. A., Hoey, A. S., et al. (2020). Meeting fisheries, ecosystem function, and biodiversity goals in a human-dominated World. Science 368, 307–311. doi: 10.1126/science.aax9412
Clarke, K. R., and Gorley, R. N. (2006). PRIMER v6: User Manual/Tutorial. Plymouth: PRIMER-E, 1–190.
Cox, C., Valdivia, A., McField, M., Castillo, K., and Bruno, J. F. (2017). Establishment of marine protected areas alone does not restore coral reef communities in Belize. Mar. Ecol. Prog. Ser. 563, 65–79. doi: 10.3354/meps11984
FAO (2014). Fishery and Aquaculture Country Profiles. Philippines. Country Profile Fact Sheets. In: FAO Fisheries and Aquaculture Department [online]. Rome. Available Online at: http://www.fao.org/fishery/
FAO (2018). State of the World’s Fisheries and Aquaculture: Meeting Sustainable Development Goals. Rome: FAO.
Fish Forever (2020). Philippines Fisheries Data. Rare. Avaialable Online at: https://portal.rare.org/en/tools-and-data/fisheries-data/ [Accessed: 06/05/2021].
Friedlander, A. M., Golbuu, Y., Ballesteros, E., Caselle, J. E., Gouezo, M., Olsudong, D., et al. (2017). Size, age, and habitat determine effectiveness of Palau’s marine protected areas. PloS One 12:e0174787. doi: 10.1371/journal.pone.0174787
Gaines, S. D., White, C., Carr, M. H., and Palumbi, S. R. (2010). Designing marine reserve networks for both conservation and fisheries management. Proc. Natl. Acad. Sci. 107, 18286–18293. doi: 10.1073/pnas.0906473107
Graham, N. A. J., McClanahan, T. R., MacNeil, M. A., Wilson, S. K., Cinner, J. E., Huchery, C., et al. (2017). Human disruption of coral reef trophic structure. Curr. Biol. 27, 231–236. doi: 10.1016/j.cub.2016.10.062
Guidetti, P. (2006). Marine reserves reestablish lost predatory interactions and cause community changes in rocky reefs. Ecol. Appl. 16, 963–976. doi: 10.1890/1051-0761(2006)016[0963:MRRLPI]2.0.CO;2
Guidetti, P., and Claudet, J. (2010). Comanagement practices enhance fisheries in marine protected areas. Conserv. Biol. 24, 312–318. doi: 10.1111/j.1523-1739.2009.01358.x
Halpern, B. S. (2003). The impact of marine reserves: Do reserves work and does reserve size matter? Ecol. Appl. 13, 117–137. doi: 10.1890/1051-0761(2003)013[0117:TIOMRD]2.0.CO;2
Halpern, B. S., Lester, S. E., and Kellner, J. B. (2009). Spillover from marine reserves and the replenishment of fished stocks. Environ. Conserv. 36, 268–276. doi: 10.1017/S0376892910000032
Halpern, B. S., and Warner, R. R. (2002). Marine reserves have rapid and lasting effects. Ecol. Lett. 5, 361–366. doi: 10.1046/j.1461-0248.2002.00326.x
Hilborn, R., and Ovando, D. (2014). Reflections on the success of traditional fisheries management. ICES J. Mar. Sci. 71, 1040–1046. doi: 10.1093/icesjms/fsu034
Honda, K., Nakamura, Y., Nakaoka, M., Uy, W. H., and Fortes, M. D. (2013). Habitat use by fishes in coral reefs, seagrass beds and mangrove habitats in the Philippines. PLoS ONE 8:e65735. doi: 10.1371/journal.pone.0065735
Jones, D. L., Walter, J. F., Brooks, E. N., and Serafy, J. E. (2010). Connectivity through Ontogeny: fish population linkages among mangrove and coral reef habitats. Mar. Ecol. Prog. Ser. 401, 245–258. doi: 10.3354/meps08404
Kassambara, A. (2020). rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R package version 0.6.0. Available Online at: https://CRAN.R-project.org/package=rstatix.
Kearney, J., Berkes, F., Charles, A., Pinkerton, E., and Wiber, W. (2007). The role of participatory governance and community-based management in integrated coastal and ocean management in Canada. Coastal Manage. 35, 79–104. doi: 10.1080/10.1080/08920750600970511
Kerwath, S. E., Winker, H., Götz, A., and Attwood, C. G. (2013). Marine protected area improves yield without disadvantaging fishers. Nat. Commun. 4:2347. doi: 10.1038/ncomms3347
Kramer, D. L., and Chapman, M. R. (1999). Implications of fish home range size and relocation for marine reserve function. Environ. Biol. Fishes 55, 65–79. doi: 10.1023/A:1007481206399
Lowe, C. G., Topping, D. T., Cartamil, D. P., and Papastamatiou, Y. P. (2003). Movement patterns, home range, and habitat utilization of adult kelp bass paralabrax clathratus in a temperate no-take marine reserve. Mar. Ecol. Prog. Ser. 256, 205–216. doi: 10.3354/meps256205
Manson, F. J., Loneragan, N. R., Skilleter, G. A., and Phinn, S. R. (2005). An evaluation of the evidence for linkages between mangroves and fisheries: A synthesis of the literature and identification of research directions. Oceanogr. Mar. Biol. 43, 493–524.
Marine Conservation Institute (2021). Marine Protection Atlas: Philippines. Avaialable Online at: https://mpatlas.org/countries/PHL [Retrieved February 28, 2021].
Maypa, A. P., Russ, G. R., Alcala, A. C., and Calumpong, H. P. (2002). Long-term trends in yield and catch rates of the coral reef fishery at Apo Island, Central Philippines. Mar. Freshwater Res. 53:207. doi: 10.1071/MF01134
McClanahan, T. R., Marnane, M. J., Cinner, J. E., and Kiene, W. E. (2006). A comparison of marine protected areas and alternative approaches to coral-reef management. Curr. Biol. 16, 1408–1413. doi: 10.1016/j.cub.2006.05.062
McManus, J. W., and Reyes, R. B. Jr. (1997). Effects of some destructive fishing methods on coral cover and potential rates of recovery. Environ. Manage. 21, 69–78. doi: 10.1007/s002679900006
Melana, D. M., Melana, E. E., and Mapalo, A. M. (2005). Mangroves Management and Development in the Philippines. [Book chapter]. Aquaculture Department, Southeast Asian Fisheries Development Center. Available online at: http://hdl.handle.net/10862/712
Micheli, F., Halpern, B. S., Botsford, L. W., and Warner, R. R. (2004). Trajectories and correlates of community change in No-Take marine reserves. Ecol. Appl. 14, 1709–1723. doi: 10.1890/03-5260
Micheli, F., Mumby, P. J., Brumbaugh, D. R., Broad, K., Dahlgren, C. P., Harborne, A. R., et al. (2014). High vulnerability of ecosystem function and services to diversity loss in caribbean coral reefs. Biol. Conserv. 171, 186–194. doi: 10.1016/j.biocon.2013.12.029
Mora, C. (2008). A clear human footprint in the coral reefs of the Caribbean. Proc. R. Soc. B Biol. Sci. 275, 767–773. doi: 10.1098/rspb.2007.1472
Muallil, R. N., Deocadez, M. R., Martinez, R. J. S., Mamauag, S. S., Nañola, C. L., and Aliño, P. M. (2015). Community assemblages of commercially important coral reef fishes inside and outside marine protected areas in the Philippines. Reg. Stud. Mar. Sci. 1, 47–54. doi: 10.1016/j.rsma.2015.03.004
Muallil, R. N., Deocadez, M. R., Martinez, R. J. S., Campos, W. L., Mamauag, S. S., Nañola, C. L., et al. (2019). Effectiveness of small locally-managed marine protected areas for coral reef fisheries management in the Philippines. Ocean Coastal Manage. 179:104831. doi: 10.1016/j.ocecoaman.2019.104831
Muallil, R. N., Mamauag, S. S., Cababaro, J. T., Arceo, H. O., and Aliño, P. M. (2014a). Catch trends in Philippine small-scale fisheries over the last five decades: The Fishers× Perspectives. Mar. Policy 47, 110–117. doi: 10.1016/j.marpol.2014.02.008
Muallil, R. N., Mamauag, S. S., Cabral, R. B., Celeste-Dizon, E. O., and Aliño, P. M. (2014b). Status, trends and challenges in the sustainability of small-scale fisheries in the Philippines: Insights from FISHDA (Fishing Industries’ Support in Handling Decisions Application) model. Mar. Policy 44, 212–221. doi: 10.1016/j.marpol.2013.08.026
Mumby, P. J. (2006). Connectivity of reef fish between mangroves and coral reefs: algorithms for the design of marine reserves at seascape scales. Biol. Conserv. 128, 215–222. doi: 10.1016/j.biocon.2005.09.042
Mumby, P. J., Harborne, A. R., Williams, J., Kappel, C. V., Brumbaugh, D. R., Micheli, F., et al. (2007). Trophic cascade facilitates coral recruitment in a marine reserve. Proc. Natl. Acad. Sci. 104, 8362–8367. doi: 10.1073/pnas.0702602104
Nagelkerken, I., van der Velde, G., Gorissen, M. W., Meijer, G. J., Van’t Hof, T., and den Hartog, C. (2000). Importance of mangroves, seagrass beds and the shallow coral reef as a nursery for important coral reef fishes, using a visual census technique. Estuar. Coast. Shelf Sci. 51, 31–44. doi: 10.1006/ecss.2000.0617
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., et al. (2020). vegan: Community Ecology Package. R package version 2.5-7. Available Online at: https://CRAN.R-project.org/package=vegan.
Ostrom, E. (2000). Collective action and the evolution of social norms. J. Econ. Perspec. 14, 137–158. doi: 10.1257/jep.14.3.137
Pastorok, R. A., and Bilyard, G. R. (1985). Effects of sewage pollution on coral-reef communities. Mar. Ecol. Prog. Ser. 21, 175–189. doi: 10.3354/meps021175
Pauly, D., and Zeller, D. (2016). Catch reconstructions reveal that global marine fisheries catches are higher than reported and declining. Nat. Commun. 7:10244. doi: 10.1038/ncomms10244
Pinkerton, E. (1989). “Introduction: attaining better fisheries management through co-management-prospects, problems and propositions,” in Cooperative Management of Local Fisheries, ed. E. Pinkerton (Vancouver, Canada: University of British Columbia Press).
Pittman, S. J., Monaco, M. E., Friedlander, A. M., Legare, B., Nemeth, R. S., Kendall, M. S., et al. (2014). Fish with chips: tracking reef fish movements to evaluate size and connectivity of caribbean marine protected areas. PLoS One 9:e96028. doi: 10.1371/journal.pone.0096028
Pollnac, R., Christie, P., Cinner, J. E., Dalton, T., Daw, T. M., Forrester, G. E., et al. (2010). Marine reserves as linked social-ecological systems. Proc. Natl. Acad. Sci. 107, 18262–18265. doi: 10.1073/pnas.0908266107
Pollnac, R. B., Crawford, B. R., and Gorospe, M. L. G. (2001). Discovering factors that influence the success of community-based marine protected areas in the Visayas, Philippines. Ocean Coastal Manage. 44, 683–710. doi: 10.1016/S0964-5691(01)00075-8
Pomeroy, R., and Courtney, C. A. (2018). The Philippines context for marine tenure and small-scale fisheries. Mar. Policy 95, 283–293. doi: 10.1016/j.marpol.2018.05.030
Pomeroy, R. S. (1995). Community-based and Co-management institutions for sustainable coastal fisheries management in Southeast Asia. Ocean Coastal Manage. 27, 143–162. doi: 10.1016/0964-5691(95)00042-9
Primavera, J. H. (2000). Development and conservation of Philippine Mangroves: institutional issues. Ecol. Econ. 35, 91–106. doi: 10.1016/S0921-8009(00)00170-1
Quintana, A. C. E., Giron-Nava, A., Urmy, S., Cramer, A. N., Domínguez-Sánchez, S., Rodríguez Van Dyck, S., et al. (2021). Positive social-ecological feedbacks in community-based conservation. Front. Mar. Sci. 8:652318. doi: 10.3389/fmars.2021.652318
R Core Team (2013). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
Rare (2018). Stemming the Tide of Coastal Overfishing Fish Forever Program Results 2012–2017. Arlington, VA: Rare, 1–64.
Rife, A. N., Erisman, B., Sanchez, A., and Aburto-Oropeza, O. (2013). When good intentions are not enough Insights on Networks of ‘Paper Park’ marine protected areas: Concerns regarding marine ‘Paper Parks. Conserv. Lett. 6, 200–212. doi: 10.1111/j.1755-263X.2012.00303.x
Rohrer, J. M. (2017). Effectiveness of Locally Managed Marine Protected Areas in the Visayas, Negros Oriental Province, Philippines. Master’s thesis. Lisboa: Instituto Universitário de Ciências Psicológicas, Sociais e da Vida (ISPA).
Russ, G. R., and Alcala, A. C. (1996). Do marine reserves export adult fish biomass? Evidence from Apo Island, Central Philippines. Mar. Ecol. Prog. Ser. 132, 1–9. doi: 10.3354/meps132001
Russ, G. R., Alcala, A. C., and Maypa, A. P. (2003). Spillover from marine reserves: The Case of Naso Vlamingii at Apo Island, the Philippines. Mar. Ecol. Prog. Ser. 264, 15–20. doi: 10.3354/meps264015
Selgrath, J. C., Gergel, S. E., and Vincent, A. C. J. (2018). Shifting gears: diversification, intensification, and effort increases in small-scale fisheries (1950-2010). PLoS One 13:e0190232. doi: 10.1371/journal.pone.0190232
Shester, G. G., and Micheli, F. (2011). Conservation challenges for small-scale fisheries: bycatch and habitat impacts of traps and gillnets. Biol. Conserv. 144, 1673–1681. doi: 10.1016/j.biocon.2011.02.023
Smallhorn-West, P. F., Bridge, T. C. L., Malimali, S., Pressey, R. L., and Jones, G. P. (2019). Predicting impact to assess the efficacy of community-based marine reserve design. Conserv. Lett. 12:e12602. doi: 10.1111/conl.12602
Strain, E. M. A., Edgar, G. J., Ceccarelli, D., Stuart-Smith, R. D., Hosack, G. R., and Thomson, R. J. (2019). A global assessment of the direct and indirect benefits of marine protected areas for coral reef conservation. Diver. Distrib. 25, 9–20. doi: 10.1111/ddi.12838
Tran, L. X., and Fischer, A. (2017). Spatiotemporal changes and fragmentation of mangroves and its effects on fish diversity in Ca Mau province (Vietnam). J. Coastal Conserv. 21, 355–368. doi: 10.1007/s11852-017-0513-9
Unsworth, R. K. F., De León, P. S., Garrard, S. L., Jompa, J., Smith, D. J., and Bell, J. J. (2008). High connectivity of indo-pacific seagrass fish assemblages with mangrove and coral reef habitats. Mar. Ecol. Prog. Se. 353, 213–224. doi: 10.3354/meps07199
Vandeperre, F., Higgins, R. H., Sánchez-Meca, J., Maynou, F., Goñi, R., Martín-Sosa, P., et al. (2011). Effects of No-Take area size and age of marine protected areas on fisheries yields: A meta-analytical approach: MPA size and age: effects on fisheries. Fish Fish. 12, 412–426. doi: 10.1111/j.1467-2979.2010.00401.x
Veríssimo, D., Bianchessi, A., Arrivillaga, A., Cadiz, F. C., Mancao, R., and Green, K. (2018). Does it work for biodiversity? Experiences and challenges in the evaluation of social marketing campaigns. Soc. Market. Q. 24, 18–34. doi: 10.1177/1524500417734806
Weeks, R., Russ, G. R., Alcala, A. C., and White, A. T. (2010). Effectiveness of marine protected areas in the Philippines for biodiversity conservation. Conserv. Biol. 24, 531–540. doi: 10.1111/j.1523-1739.2009.01340.x
Wenger, A., Fabricius, K., Jones, G., and Brodie, J. (2015). “Effects of Sedimentation, Eutrophication, and Chemical Pollution on Coral Reef Fishes,” in Ecology of Fishes on Coral Reefs, ed. C. Mora (Cambridge, England: Cambridge University Press), 145–153. doi: 10.1017/CBO9781316105412.017
Keywords: marine reserve, small scale fisheries, biodiversity, community-based management (CBM), community structure
Citation: Marriott SE, Cox C, Amolo RC, Apistar D, Mancao RH and de Mutsert K (2021) Implications of Community-Based Management of Marine Reserves in the Philippines for Reef Fish Communities and Biodiversity. Front. Mar. Sci. 8:731675. doi: 10.3389/fmars.2021.731675
Received: 28 June 2021; Accepted: 04 October 2021;
Published: 26 October 2021.
Edited by:
Yehuda Benayahu, Tel Aviv University, IsraelReviewed by:
Jan Vanaverbeke, Royal Belgian Institute of Natural Sciences, BelgiumCopyright © 2021 Marriott, Cox, Amolo, Apistar, Mancao and de Mutsert. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara E. Marriott, c2FyYS5tYXJyaW90dEB1c20uZWR1; c2FyYWVpc2xlcjkxQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.