- 1Department of Estuarine and Delta Systems, NIOZ Royal Netherlands Institute for Sea Research, Yerseke, Netherlands
- 2Department of Physical Geography, Faculty of Geosciences, Utrecht University, Utrecht, Netherlands
Abundant research has shown that macrobenthic species are able to increase sediment erodibility through bioturbation. So far, however, this has been at the level of individual species. Consequently, we lack understanding on how such species effects act on the level of bioturbator communities. We assessed the isolated and combined effects of three behaviorally contrasting macrobenthic species, i.e., Corophium volutator, Hediste diversicolor, and Limecola balthica, at varying densities on the critical bed shear stress for sediment resuspension (τcr). Overall, the effect of a single species on sediment erodibility could be described by a power function, indicating a relatively large effect of small bioturbator densities which diminishes toward higher individual density. In contrast to previous studies, our results could not be generalized between species using total metabolic rate, indicating that metabolic rate may be only suitable to integrate bioturbation effects within and between closely related species; highly contrasting species require consideration of species-specific bioturbation strategies. Experiments at the benthic community level revealed that the ability of a benthic community to reduce τcr is mainly determined by the species that has the largest individual effect in reducing τcr, as opposed to the species that is dominant in terms of metabolic rate. Hence, to predict and accurately model the net effect of bioturbator communities on the evolution of tidal flats and estuaries, identification of the key bioturbating species with largest effects on τcr and their spatial distribution is imperative. Metabolic laws may be used to describe their actual activity.
Introduction
Muddy tidal flats are living landscapes, shaped not only by feedbacks between hydrodynamics, sediment transport, and morphology but also by the presence and behavior of benthic organisms that live within or on top of the sediment (Widdows et al., 2000; Widdows and Brinsley, 2002; Brückner et al., 2021). Erosion occurs when the forces that stabilize the sediment, expressed as the critical bed shear stress (τcr), are exceeded by the shear stress induced by flow or waves. Microphytobenthos can protect the mud from erosion through the creation of biofilms that increase τcr. Macrobenthic species generally decrease τcr, as they disrupt muddy sediments and biofilms through their motility and feeding behavior (Herman et al., 2001; Le Hir et al., 2007), albeit that some species are able to stabilize sediment under specific conditions (e.g., Meadows and Tait, 1989). Consequently, the overall erodibility of tidal flat sediments is determined by complex interactions within and between benthic communities, causing significant spatiotemporal variations in sediment stability (Widdows et al., 2000; Le Hir et al., 2007; Montserrat et al., 2008). The influence of bioturbation on sediment erodibility is strongest in cohesive sediments, as bioturbation reduces the sediment stabilizing properties of the mud fraction (Li et al., 2017; Cozzoli et al., 2020). The destabilizing effect of macrobenthic species is particularly important on intertidal flats, as they mediate sediment export from tidal flats toward the estuary and can induce a redistribution of fines at the landscape level (Pearson and Rosenberg, 1978; Nilsson and Rosenberg, 2000; Orvain et al., 2012; Brückner et al., 2020, 2021).
Distinct benthic species communities can be observed along the salinity and tidal gradients of dynamic estuaries as a result of hydrodynamic stresses, salinity, and sediment properties of the bed (Ysebaert and Herman, 2002; Thrush et al., 2003, 2005; Ysebaert et al., 2003; Cozzoli et al., 2013, 2014). Moreover, species interactions and species-specific life-cycles and behavior influence species occurrence and community structure (Wilson and Parker, 1996). This includes the capacity of single organisms to modify their environment through, for instance, bioturbation, which creates new habitat for co-existing species additionally affecting species distributions (Crooks, 2002; Brückner et al., 2021). Given the diversity of macrobenthic species and their behavior, generalization of bioturbation effects across species is challenging (Nowell and Jumars, 1984; Le Hir et al., 2007). This knowledge gap hampers our understanding of the morphological evolution of intertidal flats and our capability to parameterize biogeomorphic models that represent species communities.
Biogeomorphological modeling is becoming increasingly important given that tidal flats are globally decreasing (Murray et al., 2019) and threatened by drowning under climate change (Leuven et al., 2019). Recently, total metabolic rate has been proposed as a method to scale the effects of bioturbation on sediment erodibility and to integrate this across species (Cozzoli et al., 2018). Many studies have confirmed the destabilizing potential of individual macrobenthic species (see, e.g., Le Hir et al., 2007 for a review; Cozzoli et al., 2019 for species distinction). However, the effect of multiple co-existing macrobenthic species has so far been neglected in experimental studies. It is possible that sediment erodibility varies under various compositions of the macrobenthic communities. However, current understanding of how co-occurring species facilitate or impede the activity of co-existing neighbors and consequently determine total resuspension rates remains limited. Hence, we aim to quantitatively compare the effect of single species against the combined effects of multiple species on the erodibility of muddy sediments defined in terms of critical bed shear stress (τcr).
To gain insight in how to upscale macrobenthos effects on sediment resuspension from an individual species to a community, we measured the effect of bioturbation on the critical bed shear stress of mud for three behaviorally highly contrasting macrobenthic species, both at the single species level and in mixed communities. To be able to assess many combinations, we used a newly developed mobile Oscillatory-Channel Resuspension flume (OsCaR). We selected three key species that are abundant on the tidal flats along the brackish-marine part of the Western Scheldt estuary (Netherlands) and represent distinct macrobenthic activities, including biofilm grazing, pellet formation, and burrow construction (Queirós et al., 2013): Corophium volutator, Hediste diversicolor, and Limecola balthica. We assessed whether total metabolic rate is an applicable measure to generalize bioturbation effects across behaviorally contrasting benthic communities, or whether sediment erodibility is dominated by one single species or by non-linear effects due to interactions between species.
Materials and Methods
Experimental Design and Sediment Preparation
Sediment was collected from Groot Buitenschoor, a muddy tidal flat near the Antwerp harbor, Belgium (51°21′50″ N; 4°14′48″ E, Figure 1). The sediment had a median grain size (D50) of 45 μm and an average mud content of 62% (measured with a Malvern laser diffractor), which is representative for the habitat of the selected species, and higher than typically used in erodibility studies (Table 1). There were only small, soft-tissue benthic invertebrates present in the sediment. Hence, in order to maintain the natural water content, the sediment was not sieved but instead frozen for a period of at least 48 h for defaunation and then thawed and mixed thoroughly. Any remaining larger items in the sediment, such as plant or shell fragments, were removed manually. The defaunated sediment was carefully placed in sediment cores (l × w × d = 22.5 cm × 15 cm × 5 cm) and left to consolidate, while covered with a thin layer of seawater to prevent desiccation, for at least 24 h before introducing the macrobenthic organisms.
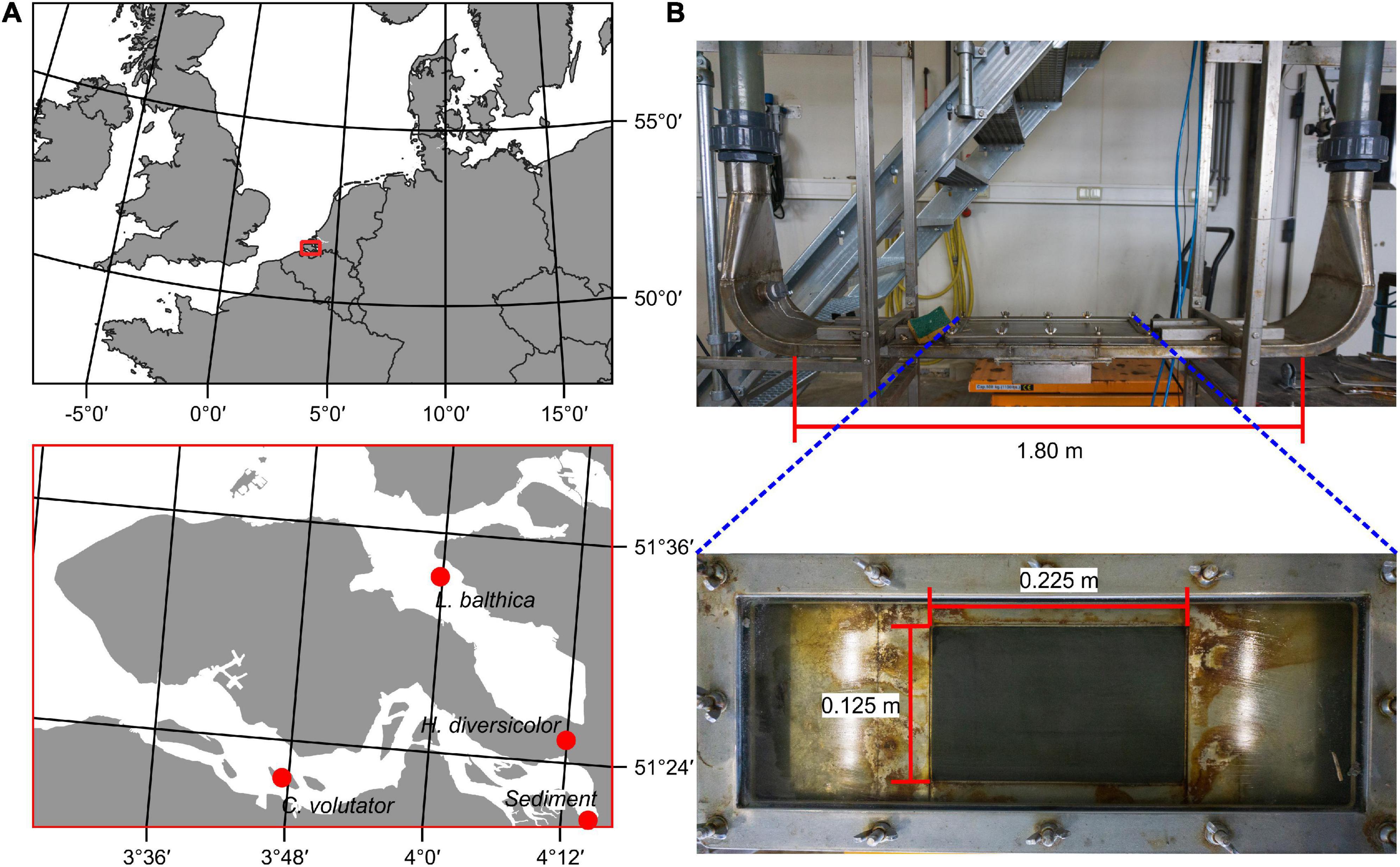
Figure 1. (A) Sampling locations of selected species and sediment in the Scheldt estuary, Netherlands. (B) The OsCaR flume. A piston in one of the vertical tubes drives an oscillatory flow over the sediment core, which is pushed into the flume channel from below.
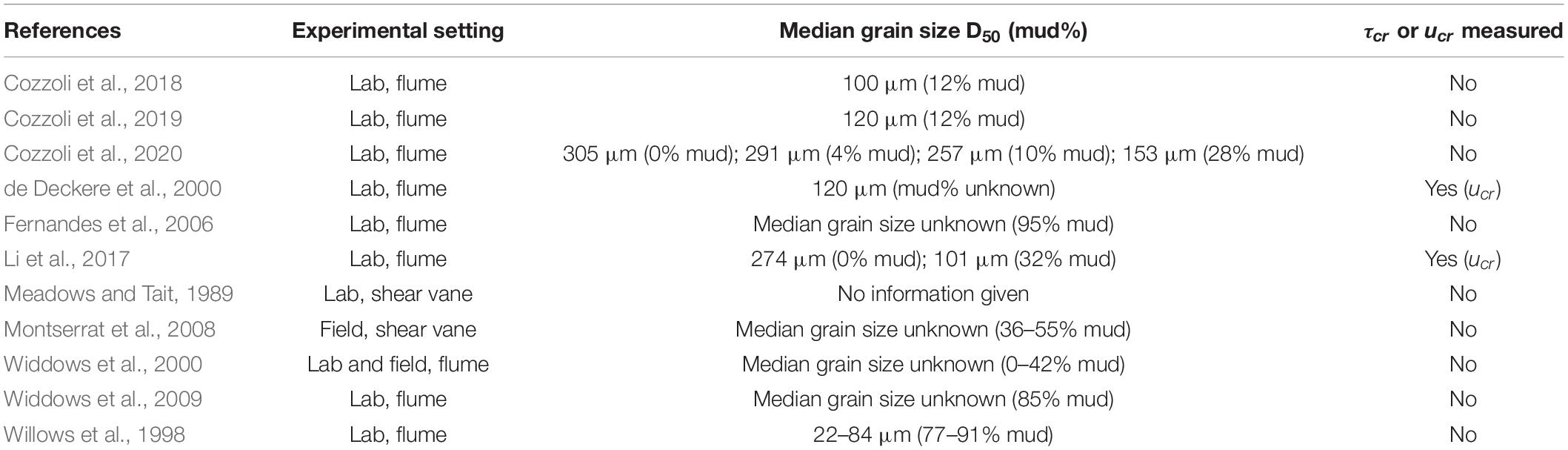
Table 1. Experimental setting and sediment composition of experimental studies on sediment erodibility by species selected in this study.
Three key species were identified that commonly co-occur on the muddy intertidal flats of temperate estuaries in high numbers, but which are strongly contrasting in motility and feeding behavior:
• Hediste diversicolor is an intermediate to deep burrowing polychaete, which builds a complex network of galleries up to a depth of 15 cm, though most individuals can be found in the top 8 cm of the bed during summer (Davey, 1994). Typical densities of H. diversicolor range between 100 and 3,000 individuals per m2 (Ysebaert and Herman, 2002). This species can be a biostabilizer in low densities under calm hydrodynamic conditions, as the building of galleries compacts the adjacent sediment and the secretion of mucus further fixates the sediment (Meadows and Tait, 1989). Under higher hydrodynamic stresses, however, i.e., shear stresses near τcr of the sediment, the created galleries and added surface roughness of H. diversicolor result in an increase of the erodibility of intertidal sediments (Fernandes et al., 2006).
• Limecola balthica (also commonly called Macoma balthica) is an intermediate burrowing bivalve, which feeds by scraping the surface sediment with its siphons and deposits pseudofaeces, thereby increasing sediment erodibility (Willows et al., 1998; Van Prooijen et al., 2011). It typically occurs in densities ranging from 100 to 2,000 individuals per m2 (Ysebaert and Herman, 2002). In this study, juveniles (0.02 m mean shell length) were used in order to keep the total biomass and total metabolic rate in the same order of magnitude as H. diversicolor.
• Corophium volutator is an intermediate burrowing amphipod which can occur in very high densities of up to 100,000 individuals per m2 but typically occurs in densities ranging from 1,000 to 15,000 in m–2 (Flach, 1992; Ysebaert and Herman, 2002). It creates U-shaped burrows of approximately 5 cm depth. As a filter-feeder during high tide, C. volutator pumps large amounts of water through its burrows, enhancing sediment resuspension (De Backer et al., 2011). During low tide, it can also act as a deposit feeder, in which case it scrapes detritus of the sediment surface using its two antennae (Meadows and Reid, 1966).
In the flume experiments, we tested various densities for each species (Table 2) based on reported characteristic low and high individual densities (Ysebaert and Herman, 2002). Species community experiments were conducted using a low individual density only, as combining multiple species with high individual density yields unrealistically high total individual densities and total metabolic rates compared to those reported in literature.
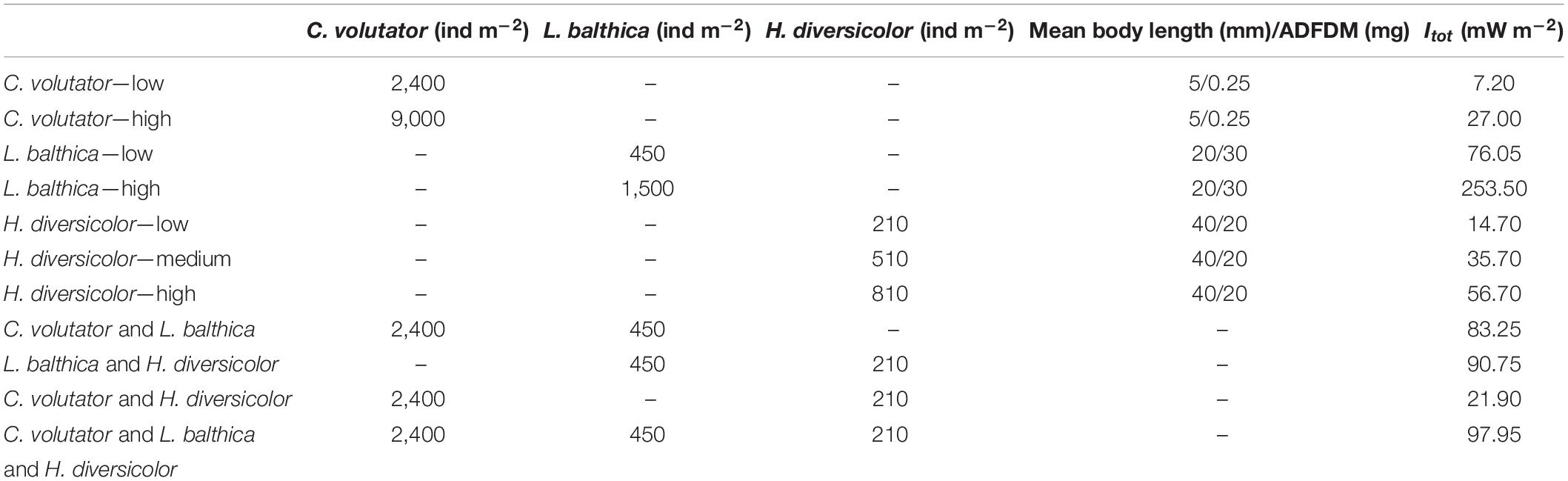
Table 2. Number of individuals and total metabolic rate for the density and species community treatments.
The macrobenthic organisms were collected between June and August 2019 from various locations in the Eastern and Western Scheldt, The Netherlands (Figure 1). After being introduced to the sediment cores, they were acclimatized in a tidal mesocosm for 2 days (cf. Cozzoli et al., 2019) under natural conditions (18°C water temperature, 30 ppt salinity, 16–8 h light–dark cycle, and a semidiurnal tide with 50% inundation frequency). In order to retain all macrobenthic organisms, the sediment cores were covered with a 0.2-mm mesh size net. The 2-day acclimatization period was chosen based on results of a pilot experiment that had shown negligible differences in sediment erodibility between 2, 3, and 4 days of acclimatization. Hence, longer acclimatization periods were avoided as the absence of waves and currents in the tidal mesocosm can cause the uninterrupted accumulation of loose bioturbated sediment at the sediment surface, potentially leading to an unrealistically low erosion threshold. Prior to the erodibility measurements, pictures were taken of the sediment cores with benthos in order to compare bioturbation patterns visually. Quantifiable observations of bioturbation patterns created by benthic organisms were conducted using ImageJ 1.53a.
Calculation of Total Metabolic Rate
To quantify the effect of both density and species on sediment erodibility, and to directly compare the different benthic communities, the total benthic metabolic rate (Itot, mW m–2) was calculated as the product of individual metabolic rate (Iind) and species density of each experiment. The metabolic rate is a measure for all benthic activity. This makes it a suitable single predictor to characterize benthic activity across different species and especially species communities, while accounting for various types of bioturbation activities such as increasing bed roughness or altering bulk sediment properties. Iind was determined from the mean individual ash-free dry weight using the empirical model of Brey (2010), which also accounts for age in case of juvenile L. balthica. The ash-free dry mass (AFDM) of L. balthica, H. diversicolor, and C. volutator (Table 2) was estimated from the mean body length using empirical relations from Eklöf et al. (2017); Galasso et al. (2018), and Boates and Smith (1979), respectively:
Where AFDM is the ash-free dry mass in mg, and l is the body length in mm.
Flume Description and Experimental Procedure
Sediment erodibility measurements were conducted in the Oscillatory-Channel Resuspension flume [OsCaR, Figure 1 (de Smit et al., 2021)]. The OsCaR flume is an oscillatory (U-tube) flow channel, which imposes the near-bed hydrodynamic conditions under natural waves and can be used to measure the critical bed shear stress (τcr) of the sediment (cf. Jonsson and Carlsen, 1976). The flume consists of two vertical PVC tubes of which one contains a piston driving the oscillatory flow through the flow channel (l × w × h = 1.5 m × 0.21 m × 0.03 m). The sediment core (l × w = 0.225 m × 0.15 m) is pushed from below into an open measurement section (l × w = 0.2 m × 0.125 m) in the center of the flow channel. The placement of the core can be adjusted precisely, creating a smooth transition between the stainless-steel flume bottom and the sediment core. Subsequently, the flume is gradually filled with saline water (30 ppt salinity, 18°C). Oscillatory flow velocity was gradually increased from 10 cm s–1 in small increments in the order of 5 cm s–1 up to the point of sediment resuspension, which depending on species and density was between 22 and 69 cm s–1. A pressure sensor (Drück 1800 series) placed in the open-ended tube measures the water level at 25 Hz. Given that the cross-sectional area of the flume is equal between the tubes and flow channel, the first derivative of the water level signal (m) is used to determine the flow velocity over the sediment core (m s–1). The suspended sediment concentration was measured with a turbidity sensor at 25 Hz (Turner Cyclops 7). This sensor was placed sideways through a small hole located 0.2 m away from the edge of the sediment core in order not to disrupt the oscillatory flow over the sediment core.
We specifically chose to measure τcr, as it is applicable in quantitative modeling approaches used to upscale the biogeomorphological interactions investigated here. τcr of the sediment was measured rather than that of the fluff layer, as the thickness of the latter is generally in the order of mm, while erosion on tidal flats during storms can be in the order of centimeters (de Vet et al., 2020). This is distinctly different from annular flume measurements (cf. Willows et al., 1998; Amos et al., 2010; Cozzoli et al., 2018, 2019), where equilibrium suspended sediment concentrations at a given shear stress are measured. As an indication, τcr is proportional to the bed shear stress step at which the largest increase in equilibrium sediment concentration is observed (Cozzoli et al., 2020). In order to quantify τcr of the sediment, rather than that of flocs, it is important to distinguish between resuspension of loose bed material and the cohesive sediments underneath. The erosion threshold was primarily defined based on a critical sediment resuspension rate. Secondarily, it was identified visually as the moment when resuspension of the cohesive sediment was observed (c.f. van Rijn, 2020), in order to ensure that turbidity observations were not skewed by edge scouring at the flow channel–sediment core transition. We specifically defined a critical sediment resuspension rate rather than a suspended sediment concentration, as the latter is influenced by the presence and amount of loose sediment and the active pumping, by e.g., C. volutator. The sediment resuspension rate was determined from the first derivative of the turbidity signal. Based on tests comparing visual observations of τcr against the corresponding turbidity signal for a variety of sediments ranging from sand to 70% mud concentration, a sediment resuspension rate of 0.06 g m–2 s–1 was defined as the threshold for the onset of sediment transport (de Smit et al., 2021). The erosion of loose bed material and erosion of cohesive sediments are well distinguishable in the resuspension rate signal, as loose flocculated material yields a much lower turbidity than well-dispersed resuspended sediments. Therefore, τcr as measured in this study corresponds to the onset of type 1b or type 2 erosion (Amos et al., 1992, 2010). Once sediment resuspension was observed, the velocity setting was kept constant for at least 1 minute to include a sufficient number of waves for determining the peak orbital velocity.
The critical peak orbital velocity (ucr, m s–1) was calculated as the mean of all velocity peaks during the 1-minute time frame that sediment resuspension was observed. The critical bed shear stress (τcr, N m–2) was calculated from the critical peak orbital velocity as (Van Rijn, 1993):
Where ρ is the water density (1,025 kg m–3 for 30 ppt salinity water at 18°C) and fw is a friction factor (-) to account for the properties of the wave boundary layer. Normally, fw is calculated from the bed roughness. However, given that the sediment core only makes up a small part of the flume channel, the wave boundary layer inside the OsCaR flume is formed by the metal flume bottom rather than the sediment core. As a result, the bed roughness is not appropriate for calculating fw. Therefore, fw was back calculated from erodibility measurements using a non-cohesive sediment with a known τcr of 0.2 N m–2 following the Shields curve. ucr of this sediment was 0.15 m s–1, yielding an fw of 0.017 following Equation 4. Assuming that fw is proportional to the orbital excursion to the power of −0.52 (Soulsby, 1997), this yielded the following flume specific friction factor (de Smit et al., 2021):
where Amax is the orbital excursion (m). Equation 5 translates to a bed roughness of the sediment of 1.35 mm. If this roughness is significantly exceeded due to the presence of bioturbation patterns on the sediment core, fw may be higher. However, by assuming a constant bed roughness instead, such bioturbation effects are included directly as a species property rather than indirectly via the bed roughness (Cozzoli et al., 2019).
Statistical Analyses
Erodibility measurements were replicated five times except for low-density H. diversicolor, which was replicated three times due to practical reasons. Five control measurements using defaunated sediment were conducted for each set of species experiments to account for potential changes in sediment properties between the sets of flume runs, which could have an effect on τcr. However, there was no significant change in τcr of control runs between the sets (p = 0.23), hence we did not account for this is in any subsequent analyses and considered all control measurements as a single group. Data were tested for normality using Shapiro–Wilks tests, and homogeneity of variance was tested using Bartlett tests. If these assumptions were met, ANOVAs and Tukey-HSD post hoc tests were used. These assumptions were, however, not met for the erodibility measurements, due to the stochastic nature of the onset of erosion. Therefore, statistical comparisons on erodibility were done using Kruskal–Wallis tests and Wilcoxon rank sum tests.
Results
Emerging Visual Differences in Bioturbation Patterns
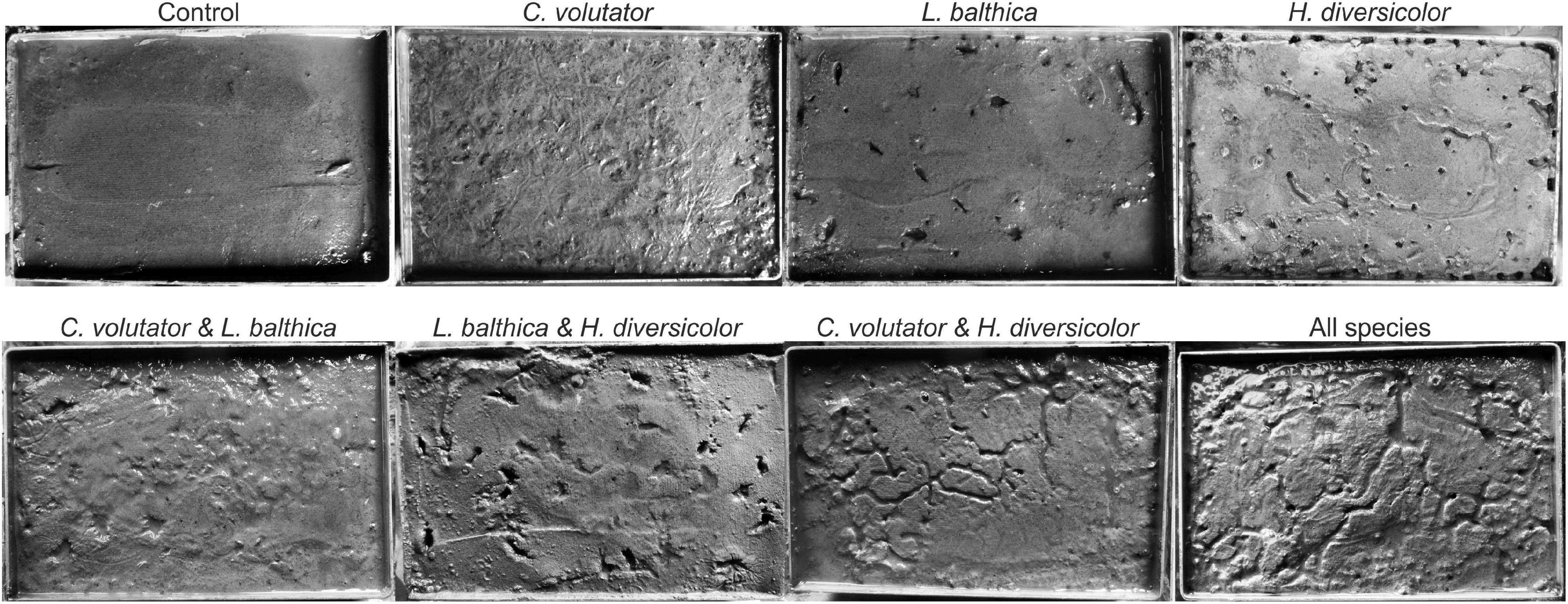
Clear visual differences in bioturbation patterns emerged at the sediment surface prior to the erodibility measurements (Figure 2). In the single species experiments, L. balthica immediately burrowed into the sediment and remained immobile during the acclimatization period, leaving clear holes on the sediment surface as there was no flow or wave action in the tidal mesocosm. In contrast, the mobile species C. volutator and H. diversicolor continuously bioturbated the sediment during the acclimatization period, leaving a large number of small holes and gullies at the sediment surface. In addition, C. volutator promoted the formation of a thin layer of fine loosened mud on top of the sediment as a result of their filter-feeding behavior.
In the community experiments, the traces on the sediment surface of C. volutator and L. balthica did not differ visually from the single species experiments. However, the number of holes by H. diversicolor was lower when combined with L. balthica (Figure 3, p < 0.01). In the communities where C. volutator was added, the number of holes was similarly reduced (Figure 3, p < 0.01), but the total length of the gullies at the sediment surface created by H. diversicolor was higher (Figure 3, p = 0.01). Combining all species yielded an intermediate effect on the number of holes and gully length of H. diversicolor (Figure 3). This indicates that the composition of the benthic community influences the bioturbating behavior of H. diversicolor.
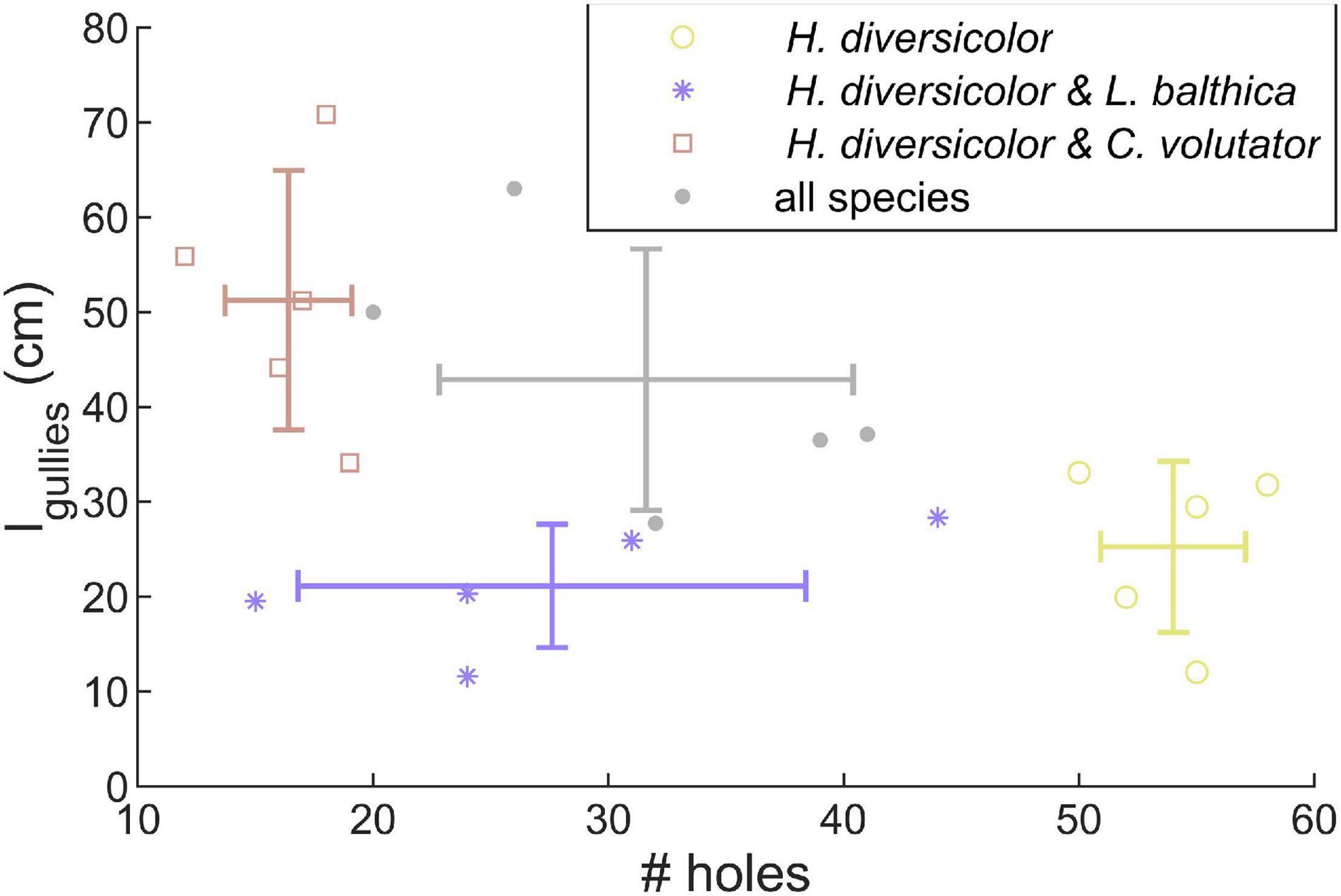
Figure 3. Number of holes and total length of gullies created by H. diversicolor depend on the presence of other species. Lines show means ± SE.
Single Species Experiments; Effects of Species and Individual Density on τcr
When macrobenthos were introduced to the sediment, the erosion threshold decreased by a factor of two to three (Figure 4). This reduction in τcr depended strongly on the specific species (p < 0.01) and on the individual density (p < 0.01), albeit that a higher individual density generally caused a much smaller reduction in τcr compared to adding a species to defaunated sediment. Interestingly, while having the lowest total metabolic rate of all tested species, C. volutator caused the largest reduction in τcr, followed by L. balthica and H. diversicolor, respectively (Figure 4). The effect of individual density on τcr reduction was largest for H. diversicolor, followed by C. volutator and L. balthica, respectively (Figure 4). Overall, the observed reduction in τcr could be explained by a power function:
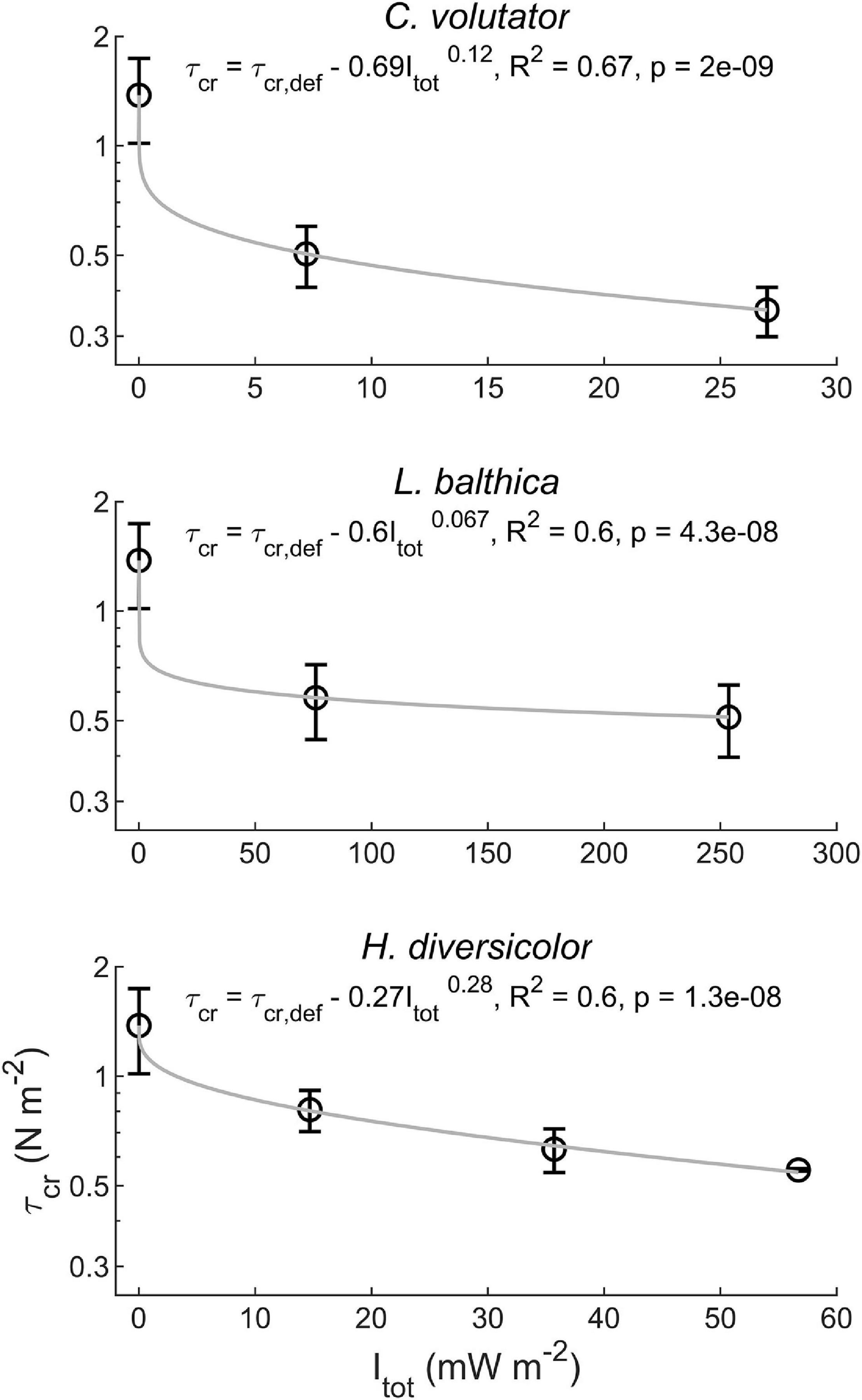
Figure 4. Reduction of τcr depending on the total metabolic rate (Itot) for the single species treatments.
where τcr,def is the erodibility of the defaunated sediment, and a and b are species-specific coefficients. a is related to the potential of a species to reduce τcr. A higher a value indicates a higher potential of the species to reduce τcr at low individual density. b is related to the effect of individual density on τcr reduction. A higher b value indicates a larger dependence of τcr reduction on individual density. Given the significant variation in a and b between the tested species, total metabolic rate could not be used to integrate bioturbation effects on τcr across these highly contrasting species.
Species Community Experiments
Overall, the results from the benthic community experiments indicate that the erodibility of natural sediments is dominated by the species that has the largest individual effect on sediment erodibility, here primarily C. volutator or L. balthica when combined with H. diversicolor only (Figure 5 and Table 3), rather than the species that is dominant in terms of total metabolic rate. That is, the addition of C. volutator to a single species or a benthic community caused a significant reduction in τcr compared to benthic communities where C. volutator was absent (Figure 5, p < 0.01). As a result, τcr of the sediments with a benthic community containing C. volutator was similar to the values of τcr induced by low densities of C. volutator in the single species scenarios, showing that the addition of H. diversicolor, L. balthica, or both to C. volutator did not cause a significant change in τcr (Figure 5 and Table 3, p = 0.22, 0.69, and 1, respectively). Even though the bioturbation activity of H. diversicolor was visually observed to increase once C. volutator was added to the sediment (Figures 2, 3), this did not have measurable effects on sediment erodibility. These findings indicate that the addition of an extra species to a benthic communities does not necessarily induce a significant further reduction of τcr.
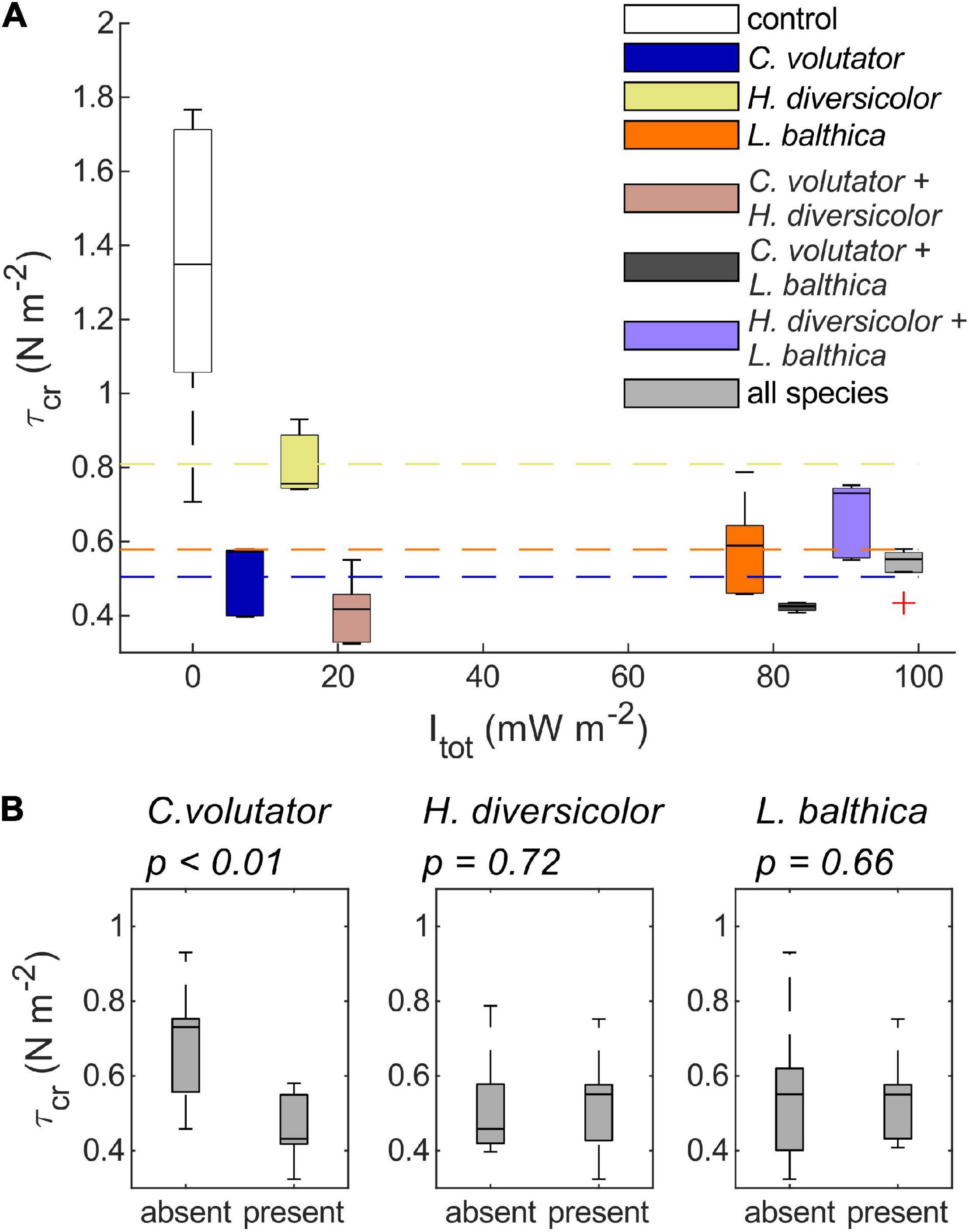
Figure 5. (A) boxplots of τcr of low-density single species treatments and τcr of combined species treatments against Itot. Dashed horizontal lines indicate the mean τcr for the single species treatments. (B) Boxplots of τcr measurements grouped based on the absence or presence of each species.
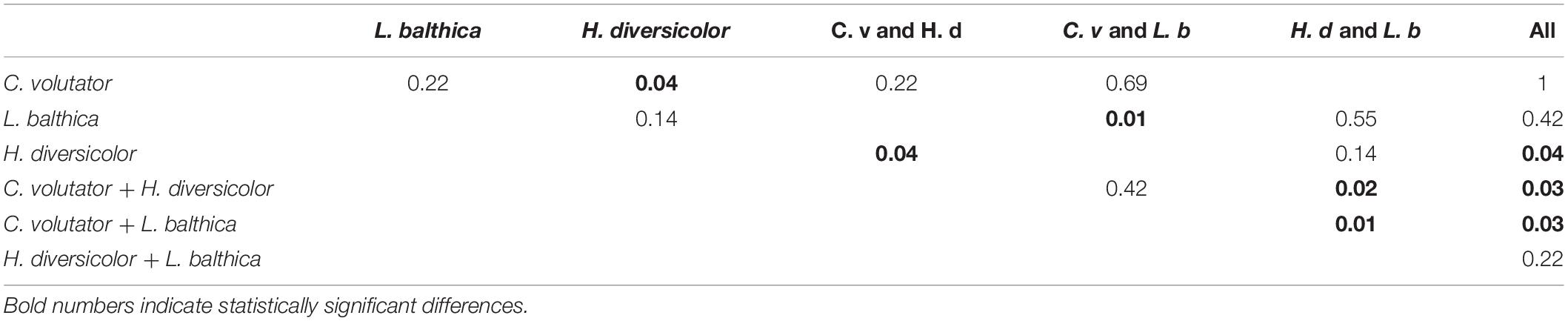
Table 3. Wilcoxon rank sum test results from individual comparisons between low-density individual species and species combinations.
Discussion
This study shows that the presence of bioturbating benthic species leads to a strong reduction in the critical bed shear stress (τcr) of muddy sediments, depending mainly on the species present and to a lesser extent on individual density and community structure. In general, measured τcr of the experiments with bioturbators is high compared to previously reported values. This can be attributed to the high mud concentration used in this study (Table 1), given that measured τcr of the defaunated sediment corresponds well with theory (Wu et al., 2018). Also, the variability in τcr of the control sediments was higher than that of the sediments with bioturbators. This may be caused by the increased likelihood of bed failure and subsequent mass erosion at higher bed shear stresses. While the direct effects of bioturbation may be smaller for less compacted sediments with lower τcr, the results presented here clearly show that in benthic communities the sediment erodibility is determined by the key bioturbating species, i.e., the species that individually causes the largest reduction in τcr, as opposed to the species which is dominant in terms of total metabolic rate.
Effects of Individual Bioturbation on Sediment Erodibility: Species Behavior and Individual Density
The net erodibility of the sediment under bioturbation is mainly the result of species behavior rather than individual density. Behavior characterizes how a species moves within or on top of the sediment and includes (i) their activity in modifying the sediment surface by creating tracks and burrows and (ii) the mobility of the organisms either locally or across larger areas. We found that the effect of increasing individual density of Corophium volutator and Limecola balthica minimally affected τcr after an initial reduction by the smallest number of individuals. Instead, we found a larger variation in sediment erodibility between species. This observation suggests that the species-specific behavior of these species controls the sediment properties. C. volutator is both a highly active and mobile species that diligently mobilizes the sediment surface through its feeding behavior (De Backer et al., 2011). Consequently, the effect of C. volutator on τcr is high even under low individual densities, as they are able to fully rework the sediment surface (de Deckere et al., 2000), albeit that future measurements of τcr at very low individual densities are needed for further validation of Equation 6.
Although L. balthica also actively modifies the sediment surface, it is largely immobile (Queirós et al., 2013), which limits the reworked sediment area by an individual to its direct surroundings. As a result, increasing individual densities rework an increasing area of surface sediment until individual feeding areas become overlapping (Van Prooijen et al., 2011). Although this affects the availability of erodible sediments at a given bed shear stress (Willows et al., 1998), we did not observe a significant effect of L. balthica individual density on τcr. A similar trend was observed by Li et al. (2017) and Cozzoli et al. (2020) for Cerastoderma edule, a bivalve similar to L. balthica, suggesting that τcr may be determined by local sediment destabilization, while sediment availability is determined by an average sediment stability over the inhabited area.
Hediste diversicolor showed a larger effect of individual density on τcr than the other tested species. While being highly mobile, H. diversicolor only passively reworks the sediment by burrowing (Queirós et al., 2013) and creating holes and gullies at the sediment surface. Therefore, increased sediment erodibility due to H. diversicolor bioturbation activity is likely a result of added bed roughness, rather than active destabilization of the sediment surface (Widdows et al., 2009). However, even for H. diversicolor, the additional decrease in τcr with increasing individual density was much smaller than the initial reduction in τcr upon adding the smallest number of individuals.
Present results therefore indicate that the effect of benthic macrofauna on sediment erodibility (τcr) is mainly affected by the presence or absence of a species, rather than by their abundance. Previous studies also reported either no or little influence of individual density on sediment erodibility at environmentally relevant individual densities (see, e.g., de Deckere et al., 2000 for C. volutator; Widdows et al., 2009 for H. diversicolor; Van Prooijen et al., 2011 for L. balthica). Our results modify the current postulate that metabolic rate scales with morphological modification proposed in Cozzoli et al. (2018). While metabolic rate may be useful for generalizing single species effects on sediment availability, it appears to be insufficient for generalizing bioturbation effects as an alteration to τcr across highly contrasting species. This is consistent with Cozzoli et al. (2019), who observed that total metabolic rate is sufficient for describing maximum suspended sediment concentrations at high bed shear stress, but found a significant species effect on the bed shear stress at which sediment resuspension rate peaked. The latter is proportional to the critical bed shear stress (Cozzoli et al., 2020).
Effect of Benthic Communities on τcr
It has been both hypothesized and shown experimentally that benthic communities may have either cumulative or averaging effects on bioturbation activity when compared to the effects of each species individually, with the outcome depending on community structure and species interactions (Meadows and Tait, 1989; Cozzoli et al., 2019). It is therefore surprising that previous studies that directly measured the effect of bioturbation on sediment erosion in an experimental setting have so far been limited to single species (Table 1). We show for the first time how the community structure of bioturbating benthic macrofauna alters the net erodibility of muddy sediments on intertidal flats compared to single species effects. When several organisms are present, the key bioturbator with the strongest effects on sediment erodibility determines the net erodibility of mud on tidal flats as opposed to species that are dominant in total metabolic rate. This key bioturbator modifies the sediment matrix and thereby seems to overwrite the lower bioturbation effect of the co-existing organisms. As a result, specific key species with a high potential to reduce τcr of muddy sediments, even if present in small numbers, may dominate the morphological evolution of estuaries instead of high densities of less efficient bioturbators (Brückner et al., 2021). This implies that a functional group approach may be more appropriate to describe the biogeomorphological effect of a benthic community rather than using total metabolic rate only.
Implications for Estuarine Biogeomorphological Processes and Modeling
Our findings have important implications for the morphological evolution of tidal flat-channel systems and the establishment of salt marshes. As sediment dynamics on tidal flats increase exponentially with increasing sediment erodibility, certain key species may have a disproportionate effect on the morphodynamics of tidal systems (Figure 6). Potentially enhanced sediment transport rates from the tidal flats toward the deeper channels of the estuary will affect local mud content of the bed and mud availability in other reaches of the estuary (Widdows and Brinsley, 2002; Townend et al., 2011; Brückner et al., 2021). The resulting changes in bed composition and elevation potentially affect the establishment and erosion of salt marshes through local bed level changes at the marsh edges (Hughes, 1999; Bouma et al., 2016; Brückner et al., 2020). In comparison with direct interactions between macrobenthic activity and saltmarshes, such as seed(ling) harvesting (Emmerson, 2000; Zhu et al., 2016a) that inhibits saltmarsh establishment or seed burial promoting saltmarsh germination (Zhu et al., 2016b), these indirect effects can play a significant role on the success of vegetation colonization (Volkenborn et al., 2007). Hence, the presence of key bioturbators on tidal flats may have important ramifications for the establishment and development of salt marshes.
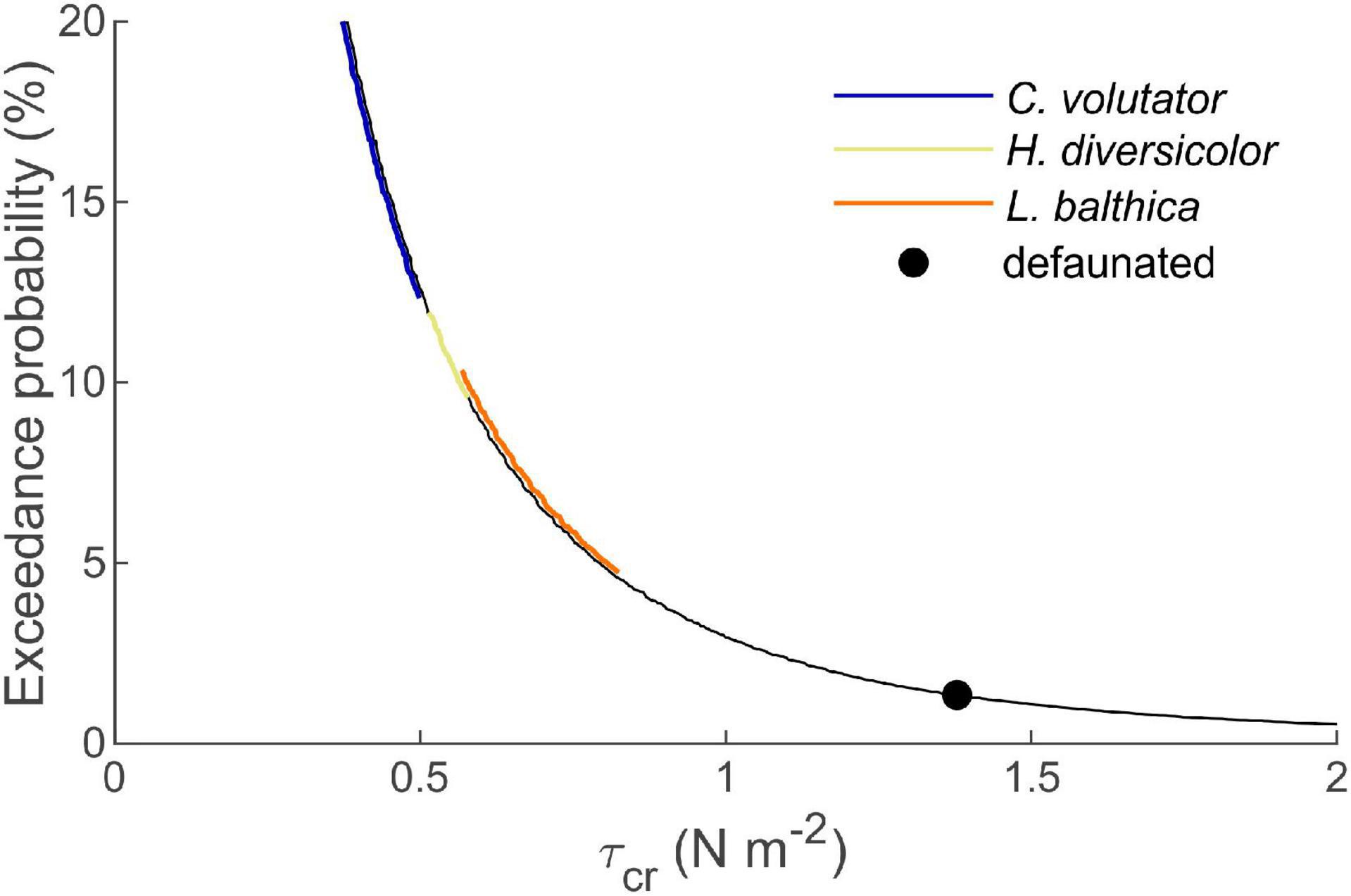
Figure 6. Exceedance probability of a given wave-induced τcr for a typical tidal flat in the Western Scheldt estuary. Colored lines (shifted horizontally for visualization purposes) correspond to the τcr range of the indicated species. As exceedance probability increases exponentially with reducing τcr by, e.g., bioturbation, tidal flat morphodynamics are dominated by key benthic species causing the largest τcr reduction, as these have a disproportionate effect on τcr exceedance probability.
To quantify the impact of benthic infauna on the sediment stability at the scale of tidal flats or even entire estuaries, there has been a growing interest in implementing bioturbation effects in morphodynamic models (e.g., Borsje et al., 2008; Van Prooijen et al., 2011; Brückner et al., 2021). Generally, this is implemented as an alteration to the critical bed shear stress and a calibration factor describing the erosion rate at a given bed shear stress. Calculated erosion rates are highly sensitive to τcr (Van Prooijen and Winterwerp, 2010; Van Prooijen et al., 2011). Hence, upscaling bioturbation effects to the ecosystem scale using models requires parameterization of bioturbation effects on τcr. Here we show that there are species-specific relations between total metabolic rate and τcr, which can be quantified, and that within benthic communities, τcr can be approximated as the minimum τcr imposed by the key species present.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below (when published): 4TU repository, doi: 10.4121/14790936.
Author Contributions
JS, MB, MK, and TB: conceptualization. JS, MB, KM, and TB: experimental design. JS, MB, and KM: data curation and data analysis. JS and MB: manuscript writing. JS, MB, MK, KM, and TB: manuscript review and editing. All authors contributed to the article and approved the submitted version.
Funding
KM was supported by the Utrecht University - NIOZ student work experience program.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Tom Ysebaert and Natalie Steiner for their inputs during the conceptualization of the research. We would also like to thank Lennart van IJzerloo and Chiu Cheng for their contributions with the fieldwork.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.726238/full#supplementary-material
References
Amos, C. L., Grant, J., Daborn, G. R., and Black, K. (1992). Sea Carousel-A benthic, annular flume. Estuar. Coast. Shelf Sci. 34, 557–577. doi: 10.1016/S0272-7714(05)80062-9
Amos, C. L., Umgiesser, G., Ferrarin, C., Thompson, C. E. L., Whitehouse, R. J. S., Sutherland, T. F., et al. (2010). The erosion rates of cohesive sediments in Venice lagoon, Italy. Cont. Shelf Res. 30, 859–870. doi: 10.1016/j.csr.2009.12.001
Boates, J. S., and Smith, P. C. (1979). Length-weight relationships, energy content and the effects of predation on Corophium volutator (pallas) (crustacea: amphipoda). Proc. Nov. Scotian Inst. Sci. 29, 489–499.
Borsje, B. W., de Vries, M. B., Hulscher, S. J. M. H., and de Boer, G. J. (2008). Modeling large-scale cohesive sediment transport affected by small-scale biological activity. Estuar. Coast. Shelf Sci. 78, 468–480. doi: 10.1016/j.ecss.2008.01.009
Bouma, T. J., van Belzen, J., and Balke, T. (2016). Short-term mudflat dynamics drive long-term cyclic salt marsh dynamics. Limnol. Oceanogr. 61, 2261–2275. doi: 10.1002/lno.10374
Brey, T. (2010). An empirical model for estimating aquatic invertebrate respiration. Methods Ecol. Evol. 1, 92–101. doi: 10.1111/j.2041-210x.2009.00008.x
Brückner, M. Z. M., Braat, L., Schwarz, C., and Kleinhans, M. G. (2020). What Came First, Mud or Biostabilizers? Elucidating Interacting Effects in a Coupled Model of Mud, Saltmarsh, Microphytobenthos, and Estuarine Morphology. Water Resour. Res. 56:2019wr026945. doi: 10.1029/2019wr026945
Brückner, M. Z. M., Schwarz, C., Coco, G., Baar, A., Boechat Albernaz, M., and Kleinhans, M. G. (2021). Benthic species as mud patrol - modelled effects of bioturbators and biofilms on large-scale estuarine mud and morphology. Earth Surf. Process. Landforms 2021, 1–17. doi: 10.1002/esp.5080
Cozzoli, F., Bouma, T. J., Ottolander, P., Lluch, M. S., Ysebaert, T., and Herman, P. M. J. (2018). The combined influence of body size and density on cohesive sediment resuspension by bioturbators. Sci. Rep. 8, 1–12. doi: 10.1038/s41598-018-22190-3
Cozzoli, F., Bouma, T. J., Ysebaert, T., and Herman, P. M. J. (2013). Application of non-linear quantile regression to macrozoobenthic species distribution modelling: Comparing two contrasting basins. Mar. Ecol. Prog. Ser. 475, 119–133. doi: 10.3354/meps10112
Cozzoli, F., Eelkema, M., Bouma, T. J., Ysebaert, T., Escaravage, V., and Herman, P. M. J. (2014). A mixed modeling approach to predict the effect of environmental modification on species distributions. PLoS One 9:15–17. doi: 10.1371/journal.pone.0089131
Cozzoli, F., Gjoni, V., and Pasqua, M. Del (2019). A process based model of cohesive sediment resuspension under bioturbators’ influence. Sci. Total Environ. 670, 18–30. doi: 10.1016/j.scitotenv.2019.03.085
Cozzoli, F., Gomes da Conceição, T., and Van Dalen, J. (2020). Biological and physical drivers of bio-mediated sediment resuspension: A flume study on Cerastoderma edule. Estuar. Coast. Shelf Sci. 241:106824. doi: 10.1016/j.ecss.2020.106824
Crooks, J. A. (2002). Characterizing ecosystem-level consequences of biological invasions: The role of ecosystem engineers. Oikos 97, 153–166. doi: 10.1034/j.1600-0706.2002.970201.x
Davey, J. T. (1994). The architecture of the burrow of Nereis diversicolor and its quantification in relation to sediment-water exchange. J. Exp. Mar. Bio. Ecol. 179, 115–129. doi: 10.1016/0022-0981(94)90020-5
De Backer, A., Van Coillie, F., Montserrat, F., Provoost, P., Van Colen, C., Vincx, M., et al. (2011). Bioturbation effects of Corophium volutator: Importance of density and behavioural activity. Estuar. Coast. Shelf Sci. 91, 306–313. doi: 10.1016/j.ecss.2010.10.031
de Deckere, E. M. G. T., van de Koppel, J., and Heip, C. H. R. (2000). The influence of Corophium volutator abundance on resuspension. Hydrobiologia 426, 37–42. doi: 10.1023/A:1003978714382
de Smit, J. C., Kleinhans, M. G., Gerkema, T., and Bouma, T. J. (2021). Quantifying natural sediment erodibility using a mobile oscillatory flow channel. Estuar. Coast. Shelf Sci. 107574., doi: 10.1016/j.ecss.2021.107574
de Vet, P. L. M., van Prooijen, B. C., Colosimo, I., Steiner, N., Ysebaert, T., Herman, P. M. J., et al. (2020). Variations in storm-induced bed level dynamics across intertidal flats. Sci. Rep. 10, 1–15. doi: 10.1038/s41598-020-69444-7
Eklöf, J., Austin, Å, Bergström, U., Donadi, S., Eriksson, B. D. H. K., Hansen, J., et al. (2017). Size matters: Relationships between body size and body mass of common coastal, aquatic invertebrates in the Baltic Sea. PeerJ 2017, 1–14. doi: 10.7717/peerj.2906
Emmerson, M. (2000). Remedial habitat creation: Does Nereis diversicolor play a confounding role in the colonisation and establishment of the pioneering saltmarsh plant, Spartina anglica? Helgol. Mar. Res. 54, 110–116. doi: 10.1007/s101520050009
Fernandes, S., Sobral, P., and Costa, M. H. (2006). Nereis diversicolor effect on the stability of cohesive intertidal sediments. Aquat. Ecol. 40, 567–579. doi: 10.1007/s10452-005-8000-z
Flach, E. C. (1992). The influence of four macrozoobenthic species on the abundance of the amphipod Corophium volutator on tidal flats of the Wadden Sea. Netherlands J. Sea Res. 29, 379–394. doi: 10.1016/0077-7579(92)90077-R
Galasso, H. L., Richard, M., Lefebvre, S., Aliaume, C., and Callier, M. D. (2018). Body size and temperature effects on standard metabolic rate for determining metabolic scope for activity of the polychaete Hediste (Nereis) diversicolor. PeerJ 6:e5675. doi: 10.7717/peerj.5675
Herman, P. M. J., Middelburg, J. J., and Heip, C. H. R. (2001). Benthic community structure and sediment processes on an intertidal flat: Results from the ECOFLAT project. Cont. Shelf Res. 21, 2055–2071. doi: 10.1016/S0278-4343(01)00042-5
Hughes, R. G. (1999). Saltmarsh erosion and management of saltmarsh restoration; the effects of infaunal invertebrates. Aquat. Conserv. Mar. Freshw. Ecosyst. 9, 83–95. doi: 10.1002/(sici)1099-0755(199901/02)9:1<83::aid-aqc323>3.0.co;2-9
Jonsson, I. G., and Carlsen, N. A. (1976). Experimental and theoretical investigations in an oscillatory turbulent boundary layer. J. Hydraul. Res. 14, 45–60. doi: 10.1080/00221687609499687
Le Hir, P., Monbet, Y., and Orvain, F. (2007). Sediment erodability in sediment transport modelling: Can we account for biota effects? Cont. Shelf Res. 27, 1116–1142. doi: 10.1016/j.csr.2005.11.016
Leuven, J. R. F. W., Pierik, H. J., van der Vegt, M., Bouma, T. J., and Kleinhans, M. G. (2019). Sea-level-rise-induced threats depend on the size of tide-influenced estuaries worldwide. Nat. Clim. Chang. 9, 986–992. doi: 10.1038/s41558-019-0608-4
Li, B., Cozzoli, F., Soissons, L. M., Bouma, T. J., and Chen, L. (2017). Effects of bioturbation on the erodibility of cohesive versus non-cohesive sediments along a current-velocity gradient: A case study on cockles. J. Exp. Mar. Bio. Ecol. 496, 84–90. doi: 10.1016/j.jembe.2017.08.002
Meadows, P. S., and Reid, A. (1966). The behaviour of Corophium Volutator (Crustacea: Amphipoda). J. Zool. 150, 387–399. doi: 10.1111/j.1469-7998.1966.tb03013.x
Meadows, P. S., and Tait, J. (1989). Modification of sediment permeability and shear strength by two burrowing invertebrates. Mar. Biol. 101, 75–82. doi: 10.1007/BF00393480
Montserrat, F., Van Colen, C., Degraer, S., Ysebaert, T., and Herman, P. M. J. (2008). Benthic community-mediated sediment dynamics. Mar. Ecol. Prog. Ser. 372, 43–59. doi: 10.3354/meps07769
Murray, N. J., Phinn, S. R., and DeWitt, M. (2019). The global distribution and trajectory of tidal flats. Nature 565, 222–225. doi: 10.1038/s41586-018-0805-8
Nilsson, H. C., and Rosenberg, R. (2000). Succession in marine benthic habitats and fauna. Mar. Ecol. 197, 139–149. doi: 10.3354/meps197139
Nowell, A. R. M., and Jumars, P. A. (1984). Flow environments of aquatic benthos. Annu. Rev. Ecol. Syst. Vol. 15, 303–328. doi: 10.1146/annurev.es.15.110184.001511
Orvain, F., Le Hir, P., Sauriau, P. G., and Lefebvre, S. (2012). Modelling the effects of macrofauna on sediment transport and bed elevation: Application over a cross-shore mudflat profile and model validation. Estuar. Coast. Shelf Sci. 108, 64–75. doi: 10.1016/j.ecss.2011.12.036
Pearson, T. H., and Rosenberg, R. (1978). Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Ocean. Mar. Bio. Ann. Rev. 16, 229–331.
Queirós, A. M., Birchenough, S. N. R., and Bremner, J. (2013). A bioturbation classification of European marine infaunal invertebrates. Ecol. Evol. 3, 3958–3985. doi: 10.1002/ece3.769
Soulsby, R. (1997). Dynamics of Marine Sands: A Manual for Practical Applications. London: Thomas Telford Ltd.
Thrush, S. F., Hewitt, J. E., Herman, P. M. J., and Ysebaert, T. (2005). Multi-scale analysis of species-environment relationships. Mar. Ecol. Prog. Ser. 302, 13–26. doi: 10.3354/meps302013
Thrush, S. F., Hewitt, J. E., Norkko, A., Nicholls, P. E., Funnell, G. A., and Ellis, J. I. (2003). Habitat change in estuaries: predicting broad- scale responses of intertidal macrofauna to sediment mud content. Mar. Ecol. Prog. Ser. 263, 101–112. doi: 10.3354/meps263101
Townend, I., Fletcher, C., Knappen, M., and Rossington, K. (2011). A review of salt marsh dynamics. Water Environ. J. 25, 477–488. doi: 10.1111/j.1747-6593.2010.00243.x
Van Prooijen, B. C., and Winterwerp, J. C. (2010). A stochastic formulation for erosion of cohesive sediments. J. Geophys. Res. Ocean. 115, 1–15. doi: 10.1029/2008JC005189
Van Prooijen, B. C., Montserrat, F., and Herman, P. M. J. (2011). A process-based model for erosion of Macoma balthica-affected mud beds. Cont. Shelf Res. 31, 527–538. doi: 10.1016/j.csr.2010.12.008
Van Rijn, L. C. (1993). Principles of sediment transport in rivers, estuaries and coastal seas, Aqua publications. Blokzijl: Aqua publications.
van Rijn, L. C. (2020). Erodibility of Mud–Sand Bed Mixtures. J. Hydraul. Eng. 146:04019050. doi: 10.1061/(asce)hy.1943-7900.0001677
Volkenborn, N., Hedtkamp, S. I. C., van Beusekom, J. E. E., and Reise, K. (2007). Effects of bioturbation and bioirrigation by lugworms (Arenicola marina) on physical and chemical sediment properties and implications for intertidal habitat succession. Estuar. Coast. Shelf Sci. 74, 331–343. doi: 10.1016/j.ecss.2007.05.001
Widdows, J., and Brinsley, M. (2002). Impact of biotic and abiotic processes on sediment dynamics and the consequences to the structure and functioning of the intertidal zone. J. Sea Res. 48, 143–156. doi: 10.1016/S1385-1101(02)00148-X
Widdows, J., Brinsley, M. D., and Pope, N. D. (2009). Effect of Nereis diversicolor density on the erodability of estuarine sediment. Mar. Ecol. Prog. Ser. 378, 135–143. doi: 10.3354/meps07864
Widdows, J., Brinsley, M., Salkeld, P., and Lucas, C. (2000). Influence of biota on spatial and temporal variation in sediment erodability and material flux on a tidal flat (Westerschelde, The Netherlands). Mar. Ecol. Prog. Ser. 194, 23–37. doi: 10.3354/meps194023
Willows, R. I., Widdows, J., and Wood, R. G. (1998). Influence of an infaunal bivalve on the erosion of an intertidal cohesive sediment: A flume and modeling study. Limnol. Oceanogr. 43, 1332–1343. doi: 10.4319/lo.1998.43.6.1332
Wilson, W. H., and Parker, K. (1996). The life history of the amphipod, Corophium volutator: The effects of temperature and shorebird predation. J. Exp. Mar. Bio. Ecol. 196, 239–250. doi: 10.1016/0022-0981(95)00133-6
Wu, W., Perera, C., Smith, J., and Sanchez, A. (2018). Critical shear stress for erosion of sand and mud mixtures. J. Hydraul. Res. 56, 96–110. doi: 10.1080/00221686.2017.1300195
Ysebaert, T., and Herman, P. M. J. (2002). Spatial and temporal variation in benthic macrofauna and relationships with environmental variables in an estuarine, intertidal soft-sediment environment. Mar. Ecol. Prog. Ser. 244, 105–124. doi: 10.3354/meps244105
Ysebaert, T., Herman, P. M. J., Meire, P., Craeymeersch, J., Verbeek, H., and Heip, C. H. R. (2003). Large-scale spatial patterns in estuaries: Estuarine macrobenthic communities in the Schelde estuary, NW Europe. Estuar. Coast. Shelf Sci. 57, 335–355. doi: 10.1016/S0272-7714(02)00359-1
Zhu, Z., Cozzoli, F., Chu, N., Salvador, M., Ysebaert, T., Zhang, L., et al. (2016b). Interactive effects between physical forces and ecosystem engineers on seed burial: A case study using Spartina anglica. Oikos 125, 98–106. doi: 10.1111/oik.02340
Keywords: bioturbation, sediment resuspension, benthic communities, tidal flat, flume experiment
Citation: de Smit JC, Brückner MZM, Mesdag KI, Kleinhans MG and Bouma TJ (2021) Key Bioturbator Species Within Benthic Communities Determine Sediment Resuspension Thresholds. Front. Mar. Sci. 8:726238. doi: 10.3389/fmars.2021.726238
Received: 16 June 2021; Accepted: 27 August 2021;
Published: 04 October 2021.
Edited by:
Simon Marius Mudd, University of Edinburgh, United KingdomReviewed by:
Charlie Emma Louise Thompson, University of Southampton, United KingdomYining Chen, Second Institute of Oceanography, Ministry of Natural Resources, China
Copyright © 2021 de Smit, Brückner, Mesdag, Kleinhans and Bouma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jaco C. de Smit, amFjby5kZS5zbWl0QG5pb3oubmw=
†Present address: Muriel Z. M. Brückner College of Life and Environmental Sciences, University of Exeter, Exeter, United Kingdom
 Jaco C. de Smit
Jaco C. de Smit Muriel Z. M. Brückner
Muriel Z. M. Brückner Katherine I. Mesdag2
Katherine I. Mesdag2 Maarten G. Kleinhans
Maarten G. Kleinhans