- 1Institute of Marine Affairs and Resource Management, National Taiwan Ocean University, Keelung, Taiwan
- 2Center of Excellence for the Oceans, National Taiwan Ocean University, Keelung, Taiwan
Precious corals are a fishery resource of cultural and religious importance. Because of their high commercial value, precious corals have been exploited for several centuries in the Mediterranean and for almost one century in the Northwest Pacific. Taiwanese fishing fleets have harvested precious corals since the 1920s; however, management regulations have only been promulgated since January 2009, when the catch and effort data of fisheries began to be collected. This study examined spatiotemporal variation in the catch composition and abundance of precious corals Corallium, Hemicorallium, and Pleurocorallium spp. around Taiwan using fishery data from 2009 to 2018 and discussed its implications for fisheries management. Licenses are issued for 60 vessels annually, and the annual total catch was 2.9–3.5 t between 2009 and 2018, peaking in 2015 and then decreasing sharply in 2016. Because of the use of non-selective fishing gear, dead and fossilized colonies were included in the total catches of the fishery. Fossilized colonies were predominant (average 78.5%) in the total catches, whereas the proportion of live colony catches accounted for less than 5%. Pink coral (Momo) was predominant in the total and live colony catches during the 10-year period. The Taiwanese precious coral fishing fleets are restricted to harvesting precious corals in five designated fishing grounds (DFGs; A–E). The fishing effort (vessel⋅day) was mainly concentrated in DFG-A (average 56.6%), which accounted for an average of 63.9% of the total catches. However, the live colony catches were largest in DFG-E (average 39.9%) and DFG-A (average 39.6%). The annual catch rates of live colonies decreased in two major fishing grounds (DFGs-A and DFG-B), whereas it increased in two minor fishing grounds (DFGs-C and DFG-D). The temporal variation in occurrence rates of live colonies decreased between 2015 and 2018, indicating a declining trend for precious coral populations around Taiwan. These results indicate that an unsustainable condition may occur in the near future if the precious corals continue to be harvested at the current scale. Revised regulations for the Taiwanese precious coral fishery should contain proposals on fishing gear modifications, a rotational harvesting scheme, or both; such measures can contribute to the conservation of precious coral populations. Regional cooperation in fisheries management is necessary to achieve the sustainable development of precious corals and their fisheries in the Northwest Pacific.
Introduction
Sustainable marine resources exploitation is a central goal in fisheries management and the United Nations 2030 Agenda for Sustainable Development (FAO, 2018). However, most management measures focus on “typical fishery,” targeting marine resources characterized by free movement and supporting essential food security for a rising human population (Jennings et al., 2001; FAO, 2018). By contrast, several fisheries, such as precious coral fisheries, target marine resources with limited movement or sessile organisms that do not represent an essential food source for humans; however, these marine resources play a substantial role in ecosystem function (Bruckner, 2009; Tsounis et al., 2010). Marine resources, particularly precious corals, have warranted attention for their vulnerability to commercial exploitation; for example, studies have highlighted their slow growth rates, high commercial values (Collette et al., 2011), and critical functions in ecosystems (Tsounis et al., 2010). Conservation and management measures for these resources and relevant fisheries require specific and distinct approaches from those of typical fisheries to achieve sustainable development (Carugati et al., 2020).
Precious corals are a group of coral species belonging to various orders, such as black corals Antipathes spp. from Antipatharia, blue corals Heliopora spp. from Helioporacea, gold corals Gerardia spp. from Zoanthidae, and red corals Corallium, Hemicorallium, and Pleurocorallium spp. from Gorgonacea (Bruckner, 2009). Of these, red corals from the family Corallidae are the most well-known and valuable precious corals in the world (Bruckner, 2014). Precious corals are non-reef building corals that do not live endosymbiotically with zooxanthellae, and thus may inhabit areas deeper than the euphotic zone (Grigg, 1984). Precious corals, in contrast to other exploited marine resources, are characterized by sessile behavior and slow growth rates (Garrabou and Harmelin, 2002; Santangelo et al., 2003, 2004; Bramanti et al., 2005; Torrents et al., 2005; Tsounis et al., 2006b) that increase their vulnerability to commercial exploitations (Tsounis et al., 2010). The harvesting of precious corals in live or dead colonies substantially affect their ecosystem functions, and consequently, other organisms.
A high proportion of fossilized colonies has been reported in the total catches of precious corals around Taiwan (Huang and Ou, 2010; Chen, 2012); however, the causal mechanism underlying the formation of fossilized colonies has yet to be investigated. Therefore, a conservative management approach, which may include precautionary measures (reducing the number of vessels or catch quota) and adaptive measures (reviewing the effectiveness of management every 1–2 years), must be implemented for precious coral fisheries to ensure the sustainability of precious coral populations.
Fisheries management following the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) may play a critical role in marine resources conservation (Vincent et al., 2014). However, proposals to include red corals Coralliidae in Appendix II listing of the CITES did not meet sufficient support (in terms of votes) at the respective Conference of Parties in meetings in 2007 and 2009. At these meetings, various concerns were raised including species identification difficulties (for materials and products) and a lack of information on stock status that must be clarified in future proposals (Bruckner, 2014). Furthermore, researchers have suggested the improvement of the local management of precious coral fisheries, rather than trade restrictions through the CITES (Tsounis et al., 2013; Bruckner, 2014). The biology and ecology of red corals has been comprehensively studied, although these works have largely centered on Corallium rubrum in the Mediterranean region (Santangelo et al., 2003; Torrents et al., 2005; Tsounis et al., 2006a,b,c; Santangelo et al., 2007; Rossi et al., 2008; Bavestrello et al., 2014; Cattaneo-Vietti et al., 2016; Garrabou et al., 2017; Montero-Serra et al., 2018). Few studies have explored the biology and ecology of red corals in the Northwest Pacific region, where red corals typically dwell in depths of more than 150 m (Iwasaki et al., 2012). Fishery-dependent data may provide population status (or abundance index) information for exploited fish stock, while relevant fishing efforts should be deliberately considered and standardized (Maunder and Punt, 2004).
The harvesting of precious corals in the waters around Taiwan has been documented since the early 1920s, with systematic development of the precious coral fishery initiated by the fisheries authority in the 1960s (Huang and Ou, 2010). Nevertheless, at that time, relevant data and studies on precious coral populations around Taiwan were limited. Amended management regulation for the precious coral fishery, which included actions on input controls, output controls and technical measures, were implemented in January 2009 when associated fisheries data began to be collected (Huang and Ou, 2010). Several studies have examined the development of the fishery, catch composition, management regulations, and taxonomy of precious corals on the basis of collected data (Huang and Ou, 2010; Chen, 2012; Chang et al., 2013; Tu et al., 2015). However, updated information on the catch composition (by category) and abundance status of the precious corals and management practices are necessary to serve as a reference for the future revision of management regulations.
This study examined the composition and abundance of the precious corals [based on abundance indices of catch rate (CR) and occurrence rate (OR) of live colonies] around Taiwan using 10-year (from 2009 to 2018) catch-effort data from the Taiwanese precious coral fishery. Variation in precious coral abundance was analyzed, and challenges for the management practices of the fishery were identified. The results provide fundamental information regarding the stock status of precious corals around Taiwan and have substantial implications for the development of comprehensive conservation and management measures for precious coral fisheries in the Northwest Pacific.
Materials and Methods
Fishery Data
Logbook data of the Taiwanese precious coral fishery were analyzed in this study. Logbooks have been a requirement for each fishing vessel involved in the Taiwanese precious coral fishery since January 2009. Within 3 days after landing from every trip, logbooks must be submitted to the local fishers’ association, who then collect and submit them to the Fisheries Agency of Taiwan every month. Recorded items in logbooks include the vessel registered number, operation date, operation location (latitude and longitude), and catch in weight (by commercial category and species). According to the management regulations, fishing vessels of the Taiwanese precious coral fishery must be equipped with a vessel monitoring system and report their locations hourly for the duration of the trip. This fishery’s observer program was implemented in 2009 and records the catches of precious corals as well as relevant by-catches and biological measurements. The observed trips numbered 4 to 50 annually between 2009 and 2018. The logbook data were compared with the observer data from 2012 to 2018. The annual deviation of catch (logbook data minus observer data) was low (between 70 and –299 g), although high variations were noted in some years (Supplementary Figure 1).
Harvested precious corals are classified by the coral colony condition (commercial category) and sold at distinct prices. The categories are as follows: (1) live colony, in which the colony was alive (with polyps) when harvested; (2) dead colony, in which the colony had been dead (fallen) for some time when harvested; and (3) fossilized colony, in which the colony had been dead (fallen) for a long time and contained apparent cavities. The three categories follow the common names used by the fishery and jewelry industries; thus, fossilized colonies do not indicate real fossils in this study. The precious coral species around Taiwan are identified by the coral colony colors and have been assigned common names, sometimes referring to the color in the Japanese language, including Aka coral (deep red coral, Corallium japonicus), Miss coral (angel skin coral, Hemicorallium sulcutum), Momo coral (pink coral, Pleurocorallium. elatius), and Shiro coral (white coral, P. konojoi; Tu et al., 2015).
Similar fishing gear and methods are used for precious coral fisheries in the Northwest Pacific region such as in Japan and Taiwan. The fishing gear, also known as tangle or coral net, comprises a cobblestone for weighing and four to five nets for collecting precious corals by tangling and twisting the colonies. Each vessel is equipped with 6–12 sets of fishing gear, depending on the number of winches (rollers) onboard. In total, 56 vessels were issued licenses in 2009; however, since 2010, the maximum number of allowable vessels (and licenses issued) has been 60.
According to management regulations, the Taiwanese precious coral fishing fleets are restricted to harvesting precious corals in five designated fishing grounds (DFGs; A–E) and to land the catches at one of three designated fishing ports (Nanfangao, Magung and Cijin; Figure 1). The five DFGs have varying ranges (areas) and are characterized by different oceanographic environments. The DFG areas were revised by the Fisheries Agency of Taiwan in 2014 to account for enforcement facilities at sea and seabed topology. The boundaries of the revised DFGs have almost parallel longitudes and latitudes and contours of 200 m in depth. The revised areas were 1.37 times greater than the previous DFG areas. The area effect on precious coral catches following the 2014 revision was temporally neglected in this study; however, a conversion factor of 1.37 for DFG areas was noted.
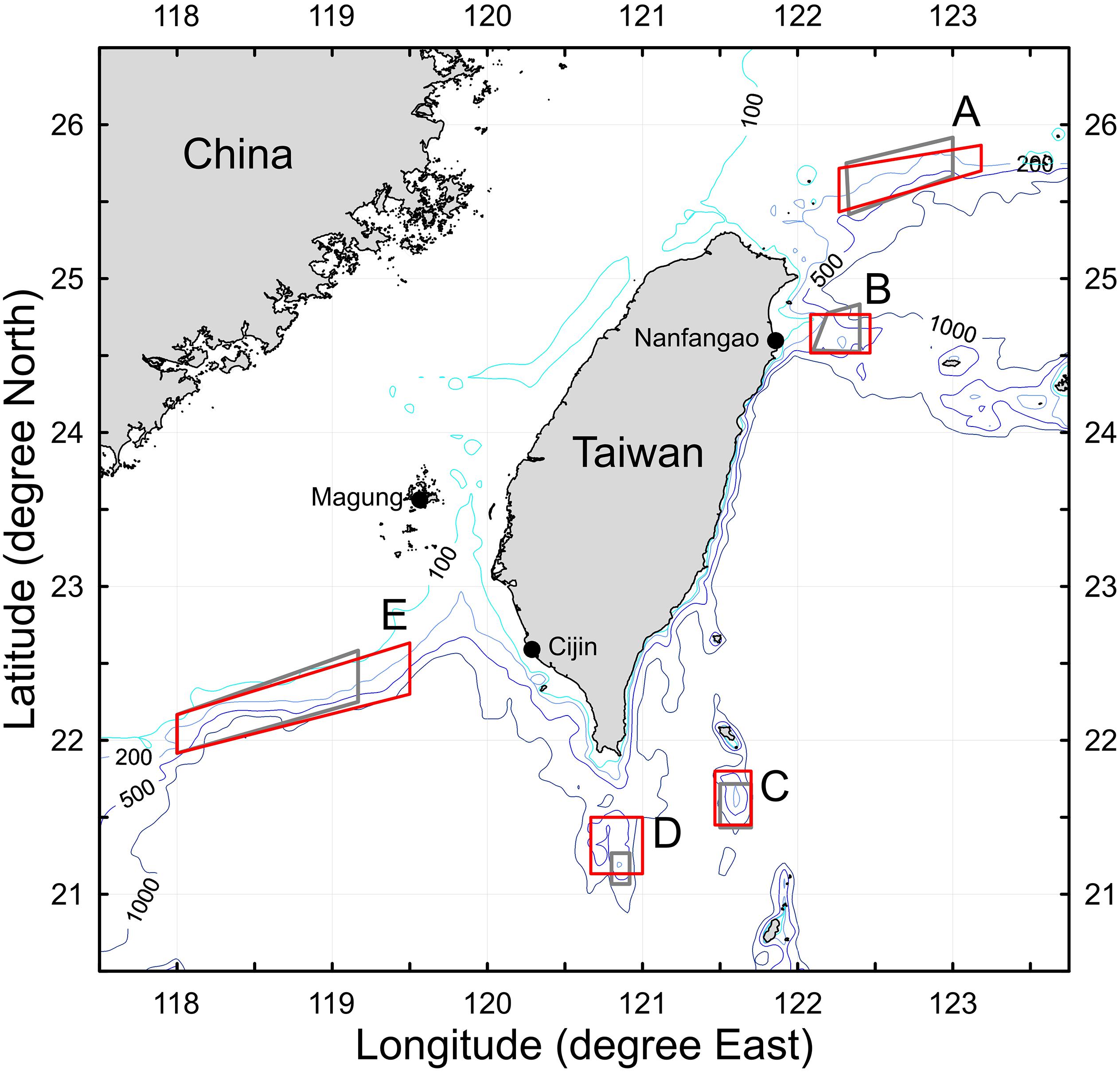
Figure 1. Map depicting the five designated fishing grounds (DFGs, A–E) around Taiwan of the Taiwanese precious coral fishery [gray lines, delimited in 2009; red lines, revised in 2014; three designated landing ports (black circles) are also indicated].
Data Analysis
The catch rate (CR), representing nominal catch per unit effort (CPUE), of precious corals by total catch and live colony catch served as a relative abundance index for the entire resource of precious corals (included live and dead colonies) and their populations (live colonies), respectively. The CR was calculated as followed,
where CR is the catch rate of precious corals, i.e., weight of total catch (or live colony catch) of precious corals per vessel per day (kg vessel–1 day–1); Cij is the catch of precious corals for vessel i on day j (kg); and Ei is the fishing effort of vessel I, which was calculated as total fishing days for vessel i (vessel ⋅ day). The total fishing effort was calculated as total fishing days for all vessels. The CR was calculated by year and by region.
The Taiwanese precious coral fishing fleet comprises vessels similar in size (20 to 99.9 GRT), number of fishermen (four to six persons), and amount of fishing gear (6–12 sets). Fishing operations were conducted during daytime hours between 5 a.m. and 9 p.m. The fishing power of the vessels in the fleet was also relatively homogenous. Thus, the CR of live colonies can serve as a reasonable abundance index of precious corals.
Annual total and live colony CRs were standardized using the 10-year average and standard deviation (SD). The standardized values (CRs) were calculated as followed,
where CRs is the standardized value; CRi is the original value; and σ are the average and SD of the original value between 2009 and 2018, respectively.
The occurrence rate (OR) of live colonies of precious corals was obtained to reflect the population status of exploited precious corals in this study. To examine the live colony OR around Taiwan, catch data were compiled in a geographic reference format with a unit statistical grid of 0.125° (latitude by longitude). The monthly live colony OR was calculated as followed,
where ORi is the live colony OR (%) in month i; Ai is the number of grids in which live colonies were harvested in month i; Gi is the number of grids in which the vessels operated in month i.
To examine the effects of DFG modification on the fishery, differences in the total catch, fishing effort and live colony catch between two periods (2009–2013 and 2014–2018) were compared using a nonparametric Mann-Whitney U test. The statistical analyses were performed using R version 4.0.0, and the statistical significance for the tests was at the 5% level.
Results
The annual total catch of the Taiwanese precious coral fishery ranged from 2.6 to 3.6 t, with an average of 3.0 t, between 2009 and 2018 (Figure 2), peaking in 2015 and then decreasing sharply in 2016. The total catch remained at a low level (compared with the long-term average) during the last 3 years of the study period. The annual fishing effort of the fishery ranged from 6664 to 8584 vessel ⋅ days, with an average of 7356 vessel ⋅ days, between 2009 and 2018, peaking in 2015 and, to a slightly lesser degree, in 2014. The fishing effort remained at a medium level (compared with the long-term average) during the last 3 years of the study period.
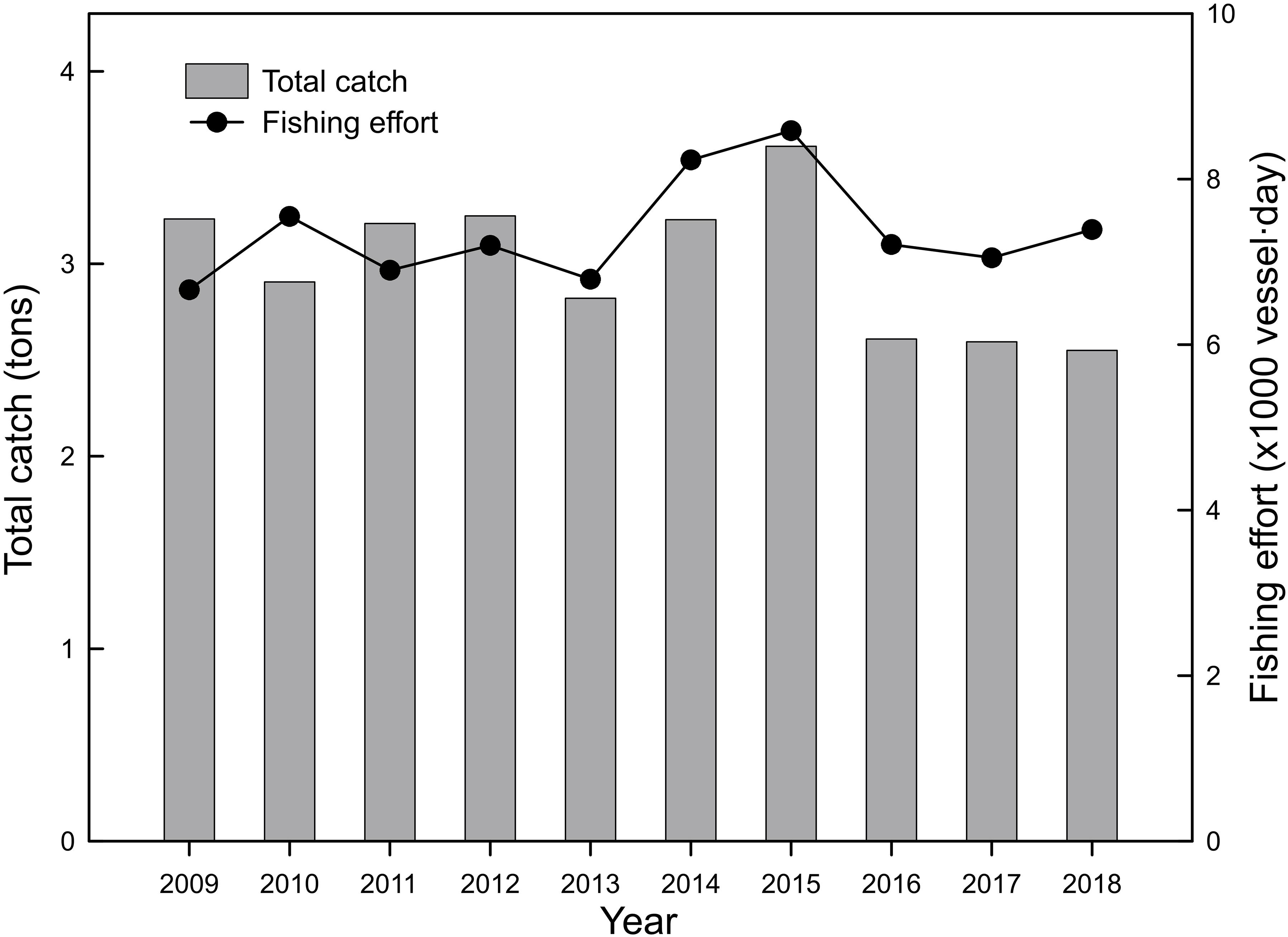
Figure 2. Annual variation in the total catches and fishing efforts (vessel ⋅ day) of the Taiwanese precious coral fishery between 2009 and 2018.
Composition of Precious Coral Catch
The harvested precious corals were classified into three categories according to their colony condition: namely live, dead, and fossilized colonies. The total catches of the fishery were from predominantly fossilized colonies, with an average of 79.2% (57.0–86.5%), between 2009 and 2018 (Figure 3), whereas live colonies comprised 2.9% (1.8–4.6%) of the catches on average. The proportion of live colonies peaked in 2009 (4.6%), decreased to a low level in 2011 (1.9%), and then peaked again in 2016 (4.1%) before decreasing to a low level in 2018 (1.8%). Few fossilized colonies were recorded in the 2011 total catch (Figure 3), although this may be attributable to a modification in the logbook format in January 2011, when a relatively high proportion of miscellaneous (non-categorized) colonies (28.5%) was noted. A similar situation, with a high proportion of miscellaneous (non-categorized) colonies (8.9%) and low proportion of fossilized colonies (77.9%), was observed in 2018 when a new logbook format was applied in September of that year.
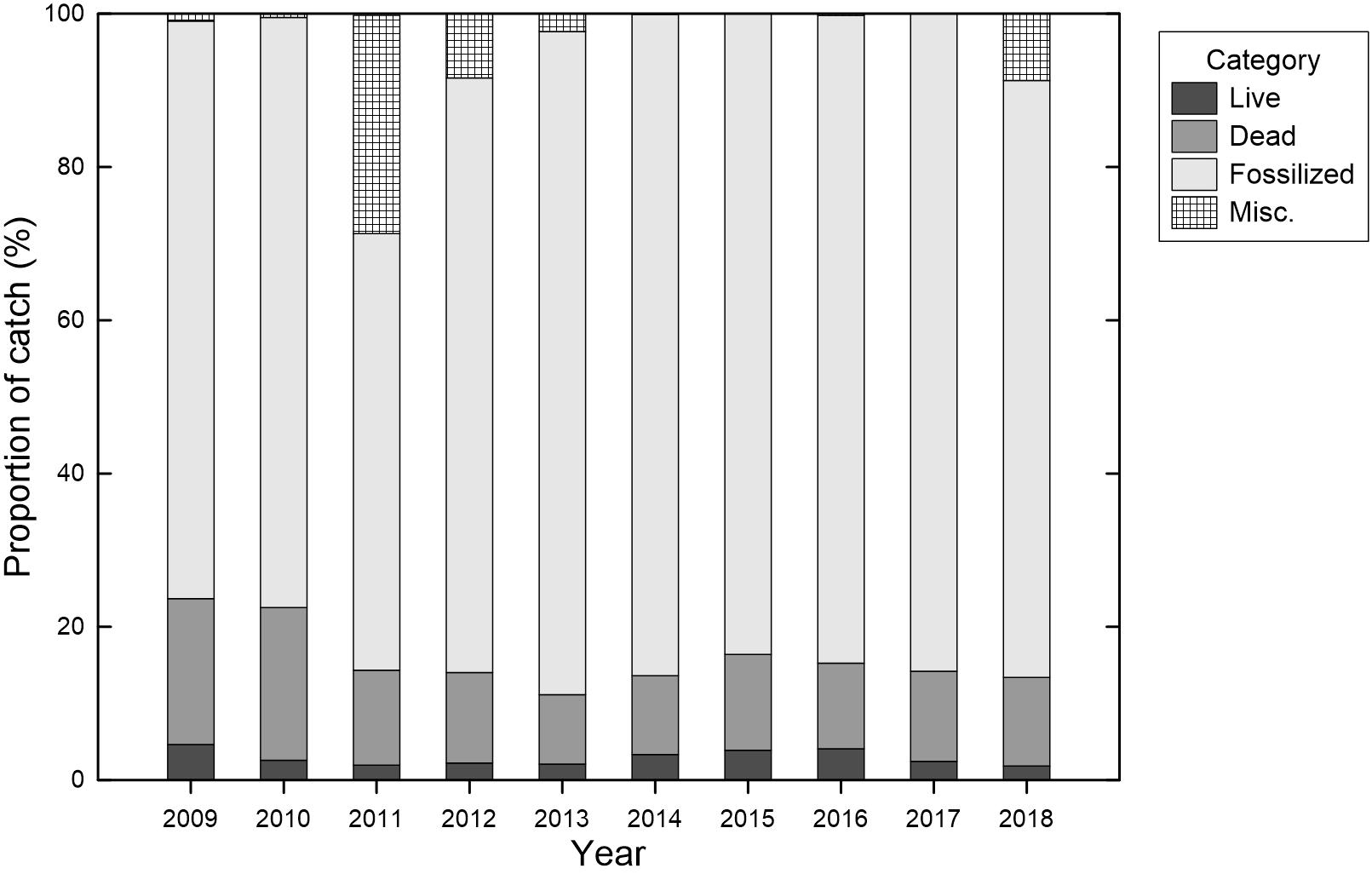
Figure 3. Annual proportion (%) of the total catches categorized by the commercial category (live, dead, fossilized colonies, or miscellaneous) of precious corals of the Taiwanese precious coral fishery between 2009 and 2018.
The Taiwanese precious coral fishery predominantly caught pink coral (Momo), representing an average of 83.2% (63.2–93.9%) of the total catch between 2009 and 2018 (Figure 4A). The proportion of pink coral in the total catch of the fishery was stable during these 10 years, although a low proportion (63.2%) was observed in 2011, which is concurrent with a modification in the logbook and documentation of a high proportion of other corals (unidentified species, 29.3%). The live colony catches of the fishery were predominantly pink coral, with an average of 72.9% (58.2–89.2%), followed by deep red coral (Aka), with an average of 15.1% (3.0–32.6%), between 2009 and 2018 (Figure 4B). The proportion of pink coral in live colony catches was stable over these 10 years, peaking in 2011 and 2014. By contrast, the annual proportion of deep red coral in live colony catches was highly variable, with a marked peak in 2016.
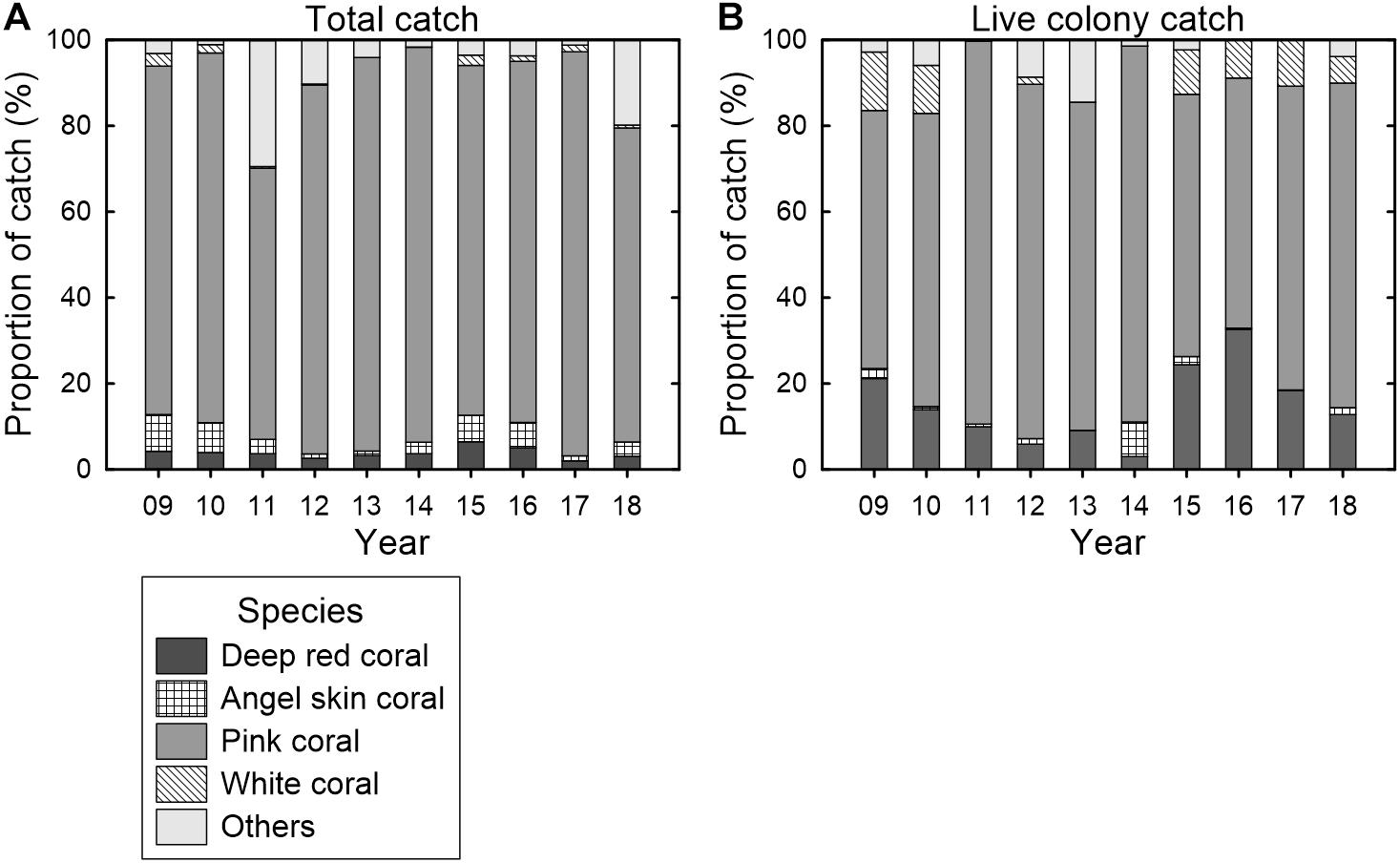
Figure 4. Annual proportion (%) of (A) the total catches and (B) live colony catches categorized by the species composition of precious corals of the Taiwanese precious coral fishery between 2009 and 2018.
Spatial Distribution in Fishing Effort and Catch
The fishing effort (vessel ⋅ day) of the Taiwanese precious coral fishing fleet was highest in DFG-A (56.6% ± 9.5% SD), followed by DFG-B (24.5% ± 7.8% SD) and DFG-E (8.7% ± 6.3% SD; Figure 5A). The fishing effort in these three areas accounted for approximately 90% of the total fishing effort. The total catches of precious corals exhibited a similar spatial distribution pattern to that of the fishing effort, in that they were largely harvested from DFG-A (63.9% ± 7.3% SD), followed by DFG-B (18.4% ± 6.0% SD) and DFG-E (10.2% ± 5.5% SD; Figure 5B). The total catches of precious corals harvested from these three areas accounted for approximately 92% of the total catches. However, the live colony catches exhibited a different spatial pattern and were largely harvested from DFG-E (39.9% ± 15.5% SD) and DFG-A (39.6% ± 9.5% SD; Figure 5C). The highest average proportion of live colony catches was from DFG-E, even though a high variation (±15.5%) was noted.
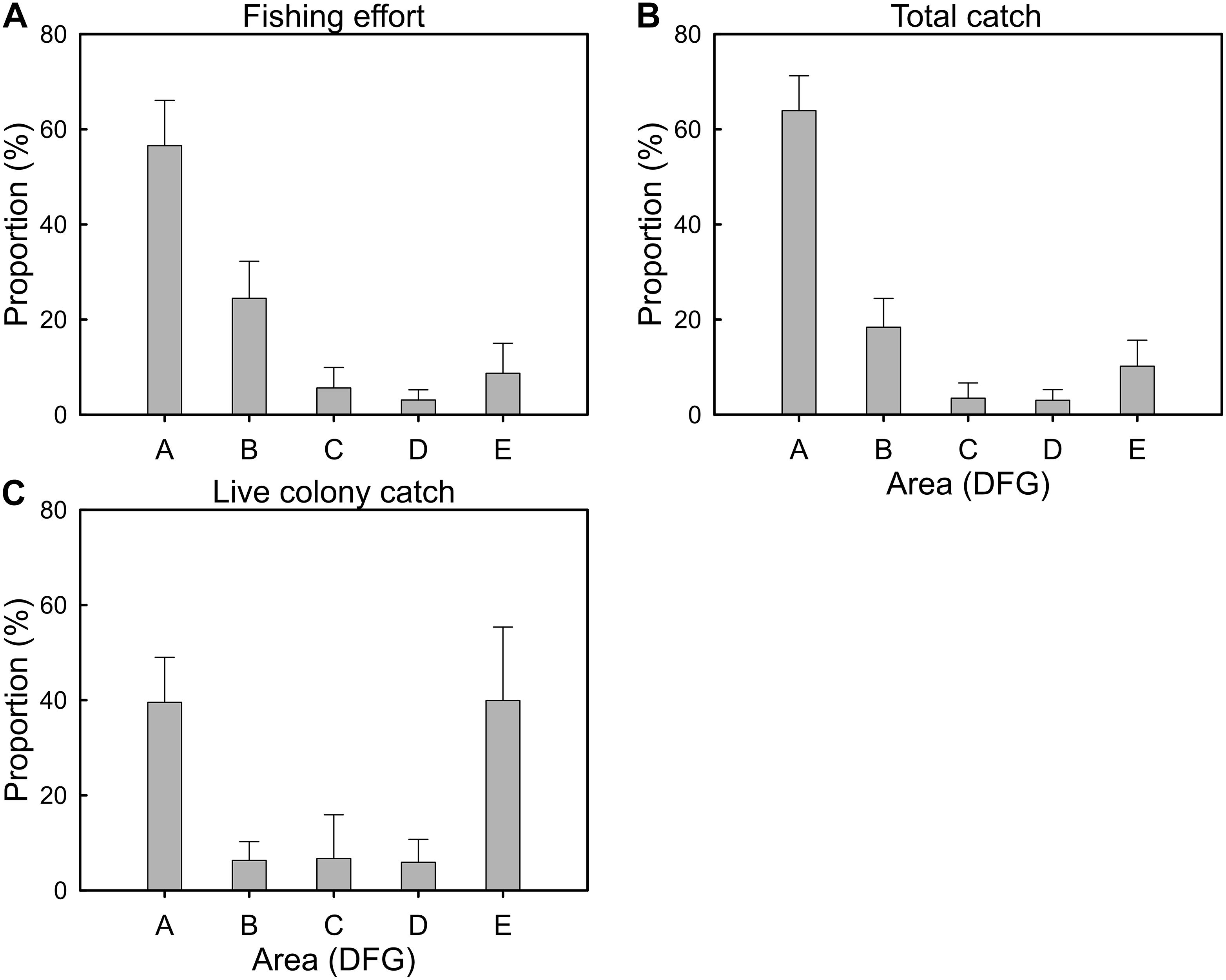
Figure 5. Average annual proportion (%) of the five DFGs for the (A) fishing efforts, (B) total catches, and (C) live colony catches of the Taiwanese precious coral fishery between 2009 and 2018 (error bar, standard deviation).
DFG areas for the Taiwanese precious coral fishery were revised in January 2014. The spatial distribution patterns of the fishery were compared for the period before (2009–2013) and after (2014–2018) the DFG revision. The fishing effort decreased in DFG-A and DFG-E but increased in DFG-B, DFG-C and DFG-D (Figure 6A). The differences in fishing effort were statistically significant in DFG-B, DFG-C and DFG-E between the two periods (Mann-Whitney test, DFG-A: U = 7, P = 0.7540; DFG-B: U = 0, P = 0.0090; DFG-C: U = 0, P = 0.0090; DFG-D: U = 10, P = 0.6015; DFG-E: U = 0, P = 0.0090). A similar shift was observed in the spatial distribution pattern of precious coral total catches (Figure 6B), with significant differences in total catches observed in DFG-C between the two periods (Mann-Whitney test, DFG-A: U = 7, P = 0.7540; DFG-B: U = 6, P = 0.1745; DFG-C: U = 3, P = 0.0472; DFG-D: U = 5, P = 0.1172; DFG-E: U = 6, P = 0.1745). By contrast, the live colony catches decreased in DFG-E but increased in DFG-A, DFG-B, DFG-C and DFG-D (Figure 6C). The live colony catches in DFG-C and DFG-E differed significantly between the two periods (Mann-Whitney test, DFG-A: U = 11, P = 0.7540; DFG-B: U = 11, P = 0.7540; DFG-C: U = 3, P = 0.0472; DFG-D: U = 7, P = 0.2506; DFG-E: U = 1, P = 0.0163).
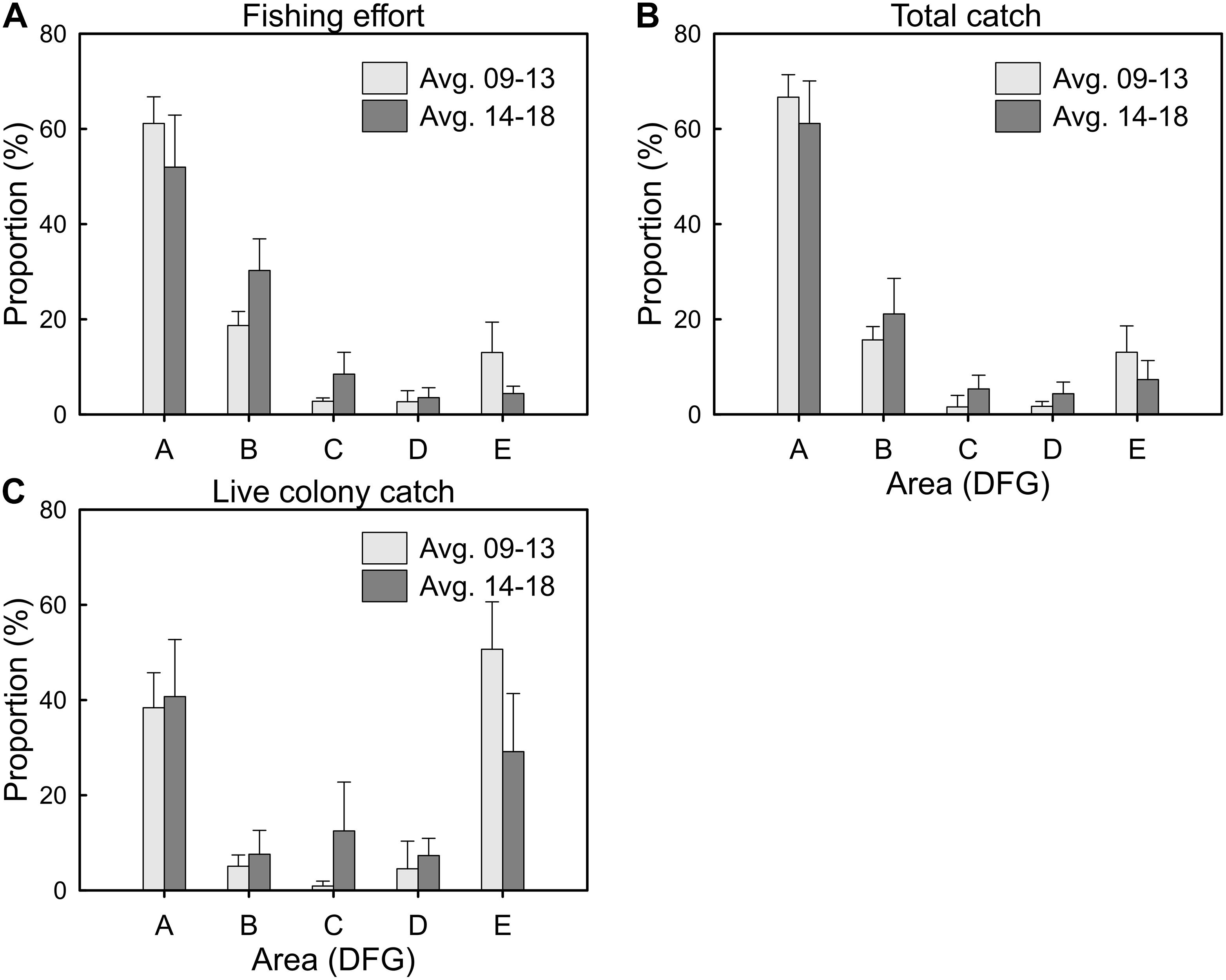
Figure 6. Average annual proportion (%) of the DFGs for the (A) fishing efforts, (B) total catches, and (C) live colony catches of the Taiwanese precious coral fishery from 2009 to 2013 and 2014 to 2018 (error bar, standard deviation).
Temporal Variation in Catch Rate
Annual total and live colony CRs were standardized using the 10-year average and SD. The CR trends were similar between DFG-A and DFG-B. However, for DFG-A, the total CR decreased between 2011 and 2018 (Figure 7A). The live colony CR decreased from 2009 to 2013, increased between 2014 and 2016, and then decreased again between 2017 and 2018 (Figure 7A). For DFG-B, the total CR also decreased between 2013 and 2018 (Figure 7B), the live colony CR decreased from 2009 to 2013, increased in 2014, but decreased again after 2014 (Figure 7B).
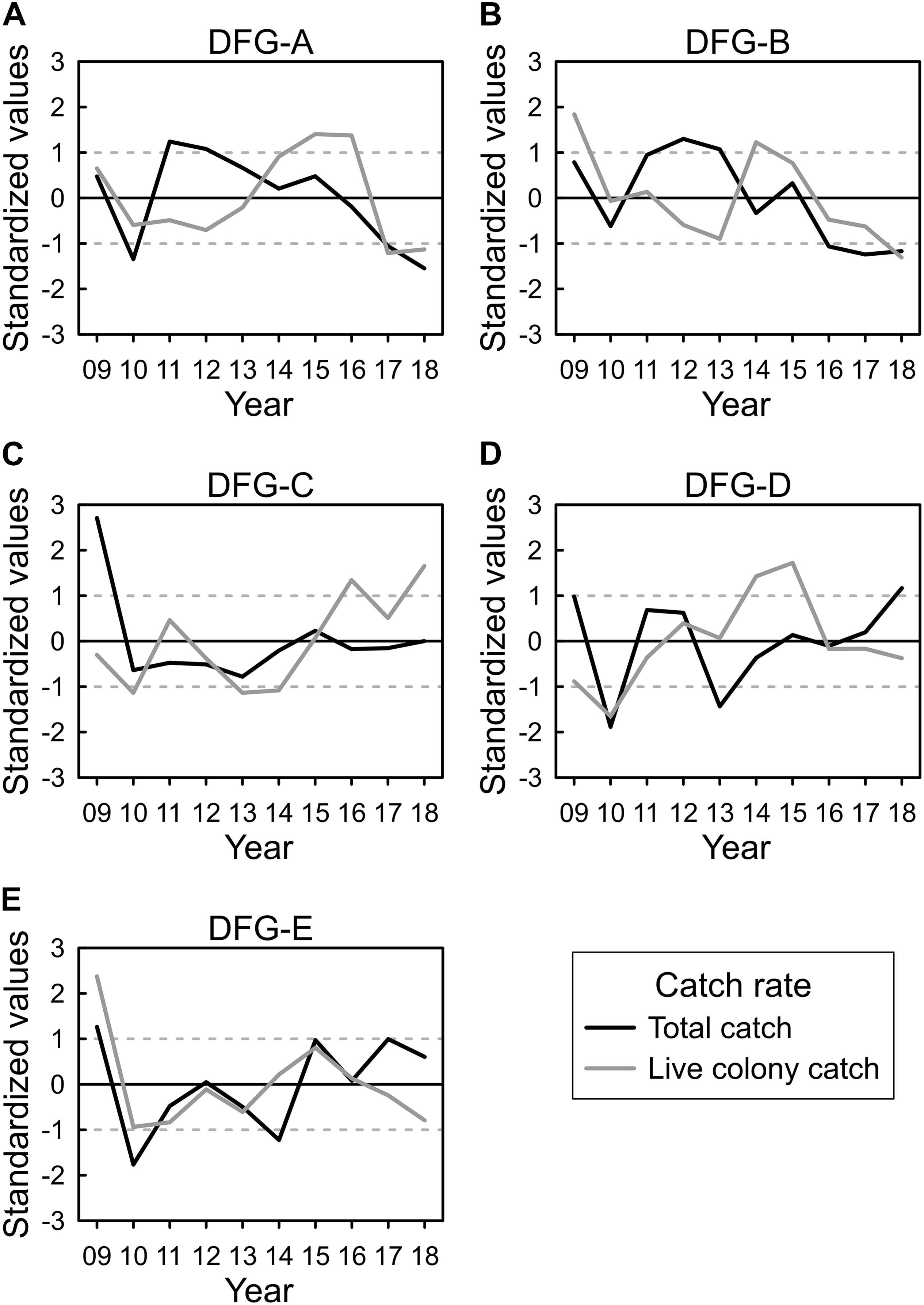
Figure 7. (A–E) Annual total and live colony catch rates (standardized using the average and standard deviation) in the five DFGs of the Taiwanese precious coral fishery between 2009 and 2018 (dashed lines: ±1 standard deviation).
The CR trends were similar between DFG-C and DFG-D. For DFG-C, the total CR remained below average from 2010 to 2014 but reached the average level from 2015 to 2018 (Figure 7C). The live colony CR fluctuated between 2009 and 2014 but increased between 2015 and 2018 (Figure 7C). For DFG-D, the total CR fluctuated from 2009 to 2013 and then steadily increased from 2014 to 2018 (Figure 7D). The live colony CR increased between 2009 and 2015 and decreased after this period (Figure 7D).
For DFG-E, the total CR fluctuated and then gradually increased from 2010 to 2018 (Figure 7E), whereas the live colony CR increased from 2010 to 2015 and then decreased between 2016 and 2018 (Figure 7E).
Monthly Live Colony Occurrence Rate
The monthly live colony OR was calculated using the proportion of grids (0.125° by latitude and longitude) in which live colonies were harvested to the total grids in which vessels operated. The live colony OR provides an approximately reflection of the population status of exploited precious corals. The average monthly live colony OR between 2009 and 2018 was 26.7% around Taiwan (Figure 8). The monthly live colony OR remained high (25–34%) between 2012 and 2015, but decreased in later years (18–22%; Figure 8).
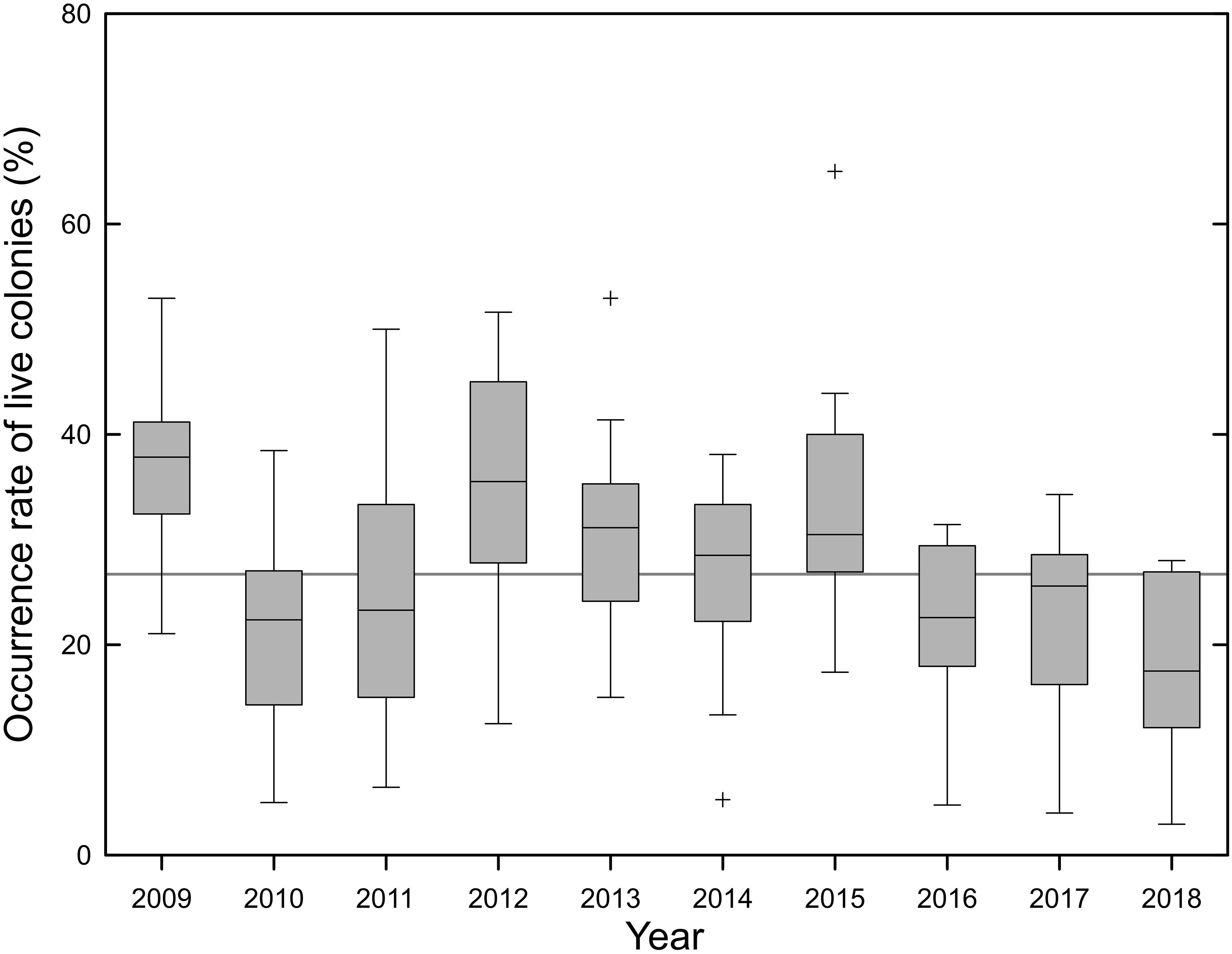
Figure 8. Annual variation in the monthly live colony occurrence rate of precious corals around Taiwan of the Taiwanese precious coral fishery between 2009 and 2018 (box range, first and third quartiles; band, median; whiskers, 1.5 interquartile; cross, outliers. The 26.7% overall average monthly live colony occurrence rate is indicated, gray line).
Discussion
Taiwanese fishermen have harvested previous corals in the Northwest Pacific for approximately 100 years, and the fishery was previously one of the largest producers of precious corals globally. However, intensive management, regulation enforcement, and scientific studies have been conducted since 2009. The current study examined spatiotemporal variations in the catch composition and abundance of precious corals around Taiwan based on 10-year fishery data. The live colony CR and OR were low in the last 3 years of the study period, which may be a result of the non-selective fishing gear that typically limits the harvesting of precious coral live colonies by chance. However, this may also indicate low levels of stock of precious corals around Taiwan. Management regulations underwent several revisions over the 10-year study period, although effective conservation measures for precious corals were not prioritized. The goals of precious corals and fishery sustainability can only be achieved when more comprehensive measures for precious coral conservation are included in management regulations.
Status of Precious Coral Populations Around Taiwan
The low proportion of live colonies in the total precious coral catches around Taiwan may be a consequence of long-term harvesting in areas with relatively low precious corals population density (Iwasaki et al., 2012; Montero-Serra et al., 2015; Cau et al., 2016) or from the high catch uncertainty inherent in the use of non-selective fishing gear that typically harvests at depths of 200 m (Chen, 2012). Moreover, the harvesting (or collecting) of fossilized colonies, which is similar to a “coal mining” process (Tsounis et al., 2010), may obstruct potential ecosystem functions and affect the benthos; however, fossilized colonies can be sold for higher prices compared with other fishery products around Taiwan (Chang, 2015). Accordingly, relevant management measures regulating the catch of fossilized colonies, such as a catch control or fixed proportion of a colony category, must be implemented. Further studies are necessary to explore the potential causes of precious coral colony death, which leads to the formation of fossilized colonies. The effects of long-term harvesting by fisheries and frequent earthquake events in the Northwest Pacific have also been noted; for example, earthquakes have damaged live colonies of precious corals in Sciacca, Italy, and led to the formation of the sub-fossil red coral in the Sicily Channel (Lodolo et al., 2017; Bavestrello et al., 2021).
In this study, two indices from the fishery-dependent data were calculated to assess the status of precious coral, namely the live colony CR and OR. The results revealed that the live colony CR decreased in DFG-A and DFG-B but remained stable in DFG-C, DFG-D, and DFG-E (Figure 7). A significant increase was observed for the live colony CR in DFG-A and DFG-B in 2014 after a revision of the DFG area in January of that year in which their areal extensions were increased 1.37-fold. The live colony ORs of precious corals were adjusted according to the grid proportions (of 0.125° by latitude and longitude) of live colonies harvested each month. Despite considerable uncertainty regarding the effects of the fishing gear used in fisheries, the live colony OR from the fishery reflected the average density of live colonies in this area to a certain degree. The long-term (2009–2018) average monthly live colony OR was 26.7% around Taiwan (Figure 8), although no baseline information for precious coral populations was available for reference (Garrabou et al., 2017). The highest average live colony OR was 37.5% in 2009 when the management regulations were promulgated, and fishing vessels could only harvest precious corals in five DFGs (Huang and Ou, 2010; Chen, 2012). Nevertheless, the live colony OR of precious corals around Taiwan decreased in later years (Figure 8), indicating a declining trend for precious coral populations around Taiwan. Such a situation is not unexpected for the Taiwanese precious coral fishery, whose fishing vessels have targeted sessile animals and harvested them within the same restricted areas for 10 years. However, additional and more detail fishing effort data such as number of operations per day and operational hours, which could have affected the abundance variations of the precious corals, must be comprehensively examined in future studies.
Although this study lacked biomass estimates for precious coral populations measured with traditional stock assessment models, two abundance indices were calculated and provide useful information on the abundance variations of the precious coral populations in these regions. Nevertheless, a decreasing trend in live colony CRs in DFG-A and DFG-B, and a low level in live colony ORs were noted for the most recent 3 years. These results indicated that an unsustainable condition may occur in the near future if precious corals continue to be harvested at the current scale, particularly in DFG-A and DFG-B. Alternative management measures, such as controlling total allowable effort by region, controlling for size through the use of selective gear [e.g., remotely operated vehicles (ROVs)] or initiating rotational harvesting must be considered in the future revision of regulations (Caddy, 1993; Tsounis et al., 2010).
Management Measures for Precious Coral Fisheries in Taiwan
The management regulations for the Taiwanese precious coral fishery have been amended several times since 2009 and were specifically revised in terms of precious coral and fishery sustainability objectives. However, some additional factors must be addressed to achieve this sustainability.
Conservation and management measures of precious corals must take their population status (abundance and structure) and ecosystem functions (coverage) into account. Similar to measures in the Mediterranean, Taiwanese management measures can apply colony size limitations that restrict the harvesting of colonies that surpass a minimum size, thus maintains the reproductive potential of precious corals (Tsounis et al., 2007). Furthermore, the plenter wood system employed in forestry management to sustain population structures and ecosystem functions was suggested (Tsounis et al., 2010). However, although these management measures are suitable for fisheries that use selective fishing gear, they have rarely been implemented by precious coral fisheries in Northwest Pacific regions, where the habitats of precious corals are typically located at depths exceeding 200 m. Harvesting precious corals through the use of selective fishing gears and ROVs constitutes an adequate approach that would assist the sustainable development of resources and fisheries (Carugati et al., 2020). However, the high cost and technical operation of ROVs (at depths exceeding 200 m) may limit their applicability in the fishery. Moreover, further research examining the potential effects of the used of tangle nets on precious corals, including measurement of live colony mortality and gear efficiency, would provide valuable information for future conservation and management measures.
Alternative measures for the conservation of precious corals, such as rotational harvest schemes, have been proposed in other studies (Caddy, 1993; Tsounis et al., 2010; Iwasaki et al., 2012). The size (scale) of unit areas and rotation cycle schedule are key factors in the development of a rotational harvest scheme (Caddy, 1993). The size determination of unit areas must account for population density, fraction of the population (the exploited populations to the entire population), and recovery period, for which considerable information on population parameters of precious corals, locally and globally, is required (Caddy, 1993). Rotation cycles ranging from 5 to 25 years have been implemented in several countries and regions (Grigg, 1989). However, the optimal rotation cycle schedule for high-value precious coral fisheries must be structured in relation to the biological factors, such as population parameters, and economic factors, such as the value of harvestable colonies and prevention of illegal fishing in restricted areas (Caddy, 1993). Comprehensive research on the spatial distribution and population connectivity of precious corals is essential for robust rotational harvest schemes (Caddy, 1993; Plagányi et al., 2015). Nevertheless, uncertainties in the practice of rotational harvesting and its effects in regards to sustainability remain and must be considered when formulating management regulations.
Perspectives for the Fisheries Management of Precious Corals
Precious corals Corallium, Hemicorallium, and Pleurocorallium spp. in the Northwest Pacific region are distributed along shelf-slope breaks from southern Japan to the northern South China Sea (Nonaka and Muzik, 2009; Iwasaki et al., 2012; Bruckner, 2016). Precious corals, with potential habitats crossing the territorial waters or exclusive economic zones (EEZs) of countries (e.g., Japan and Taiwan), are straddling fish stock. Taiwan has enforced strict management regulations for the precious coral fishery around Taiwan since 2009 (Huang and Ou, 2010). The Fishery Agency of Japan also issued technical advice to the local authorities for the management of precious coral fisheries in 2015 (Cannas et al., 2019). Nevertheless, parts of the rich fishing grounds for precious corals are located in the East China Sea or South China Sea, where fishing is the subject of economic conflict (e.g., marine resources) and political disputes (Schofield and Townsend-Gault, 2011; Lee et al., 2017; Teh et al., 2017; Kao and Pearre, 2018). Precious coral fisheries are strictly prohibited in China, although data regarding the production of precious corals in China were included in FAO Global Production Statistics. In addition, four precious corals produced in China were requested to be listed in the CITES Appendix III, and concerns on taxonomy and species distribution were raised (Cannas et al., 2019). The problems of the precious coral industry in China extends to the product supply chain and potential incentives for illegal, unreported, and unregulated (IUU) fishing activities (Chang, 2015).
The North Pacific Fisheries Commission (NPFC) was established in 2015. Fishing for cold-water corals (e.g., Alcyonacea, Antipatharia, Gorgonacea, Scleractinia), which are indicator species for vulnerable marine ecosystems, is prohibited in the convention area (North Pacific Fisheries Commission, 2018). International cooperation is essential for fisheries comprising international fishing fleets to address resource distribution across the territorial boundaries of various countries (straddling stock). The status and structure of entire populations of exploited resource must be considered, and the potential influences of international fishing fleets evaluated (Tsounis et al., 2010). The management of precious coral fisheries in Northwest Pacific regions is implemented in the territorial waters or EEZ of countries within the region, such as Japan and Taiwan (Huang and Ou, 2010; Cannas et al., 2019). The establishment of a regional fisheries management organization specifically for precious corals or a working group within an existing organization, such as the NPFC, is an appropriate approach to the effective conservation of precious corals and management of fisheries as well as the minimization of IUU fishing activities in the areas beyond national jurisdiction.
Conclusion
Although further studies are required for confirmation, precious corals constitute a renewable marine resource with regeneration times that are potentially longer than those of traditional fishery resources. Because of their life-history traits and sessile nature, the exploitation of precious corals can be compared to that of forests, which is managed under robust regulations (harvest plan) with rigorous monitoring and surveillance and based on comprehensive scientific research. The Taiwanese precious coral fishery has operated under strict regulations since 2009, including those on catch control, effort control, and relevant technical measures (e.g., DFGs). The results of this study (catch composition and abundance indices) indicated that unsustainable conditions may occur in the near future if precious corals continue to be harvested at the current scale. After 10-year of harvesting in the DFGs, a revision of the management regulations of the Taiwanese precious coral fishery is warranted and must include appropriate conservation measures, such as selective fishing gears and rotational harvesting. In addition, regional cooperation in fisheries management for precious corals in the Northwest Pacific can be used to effectively implement conservation and management measures in the region. Future studies using fishery-dependent or -independent data could focus on the population ecology (abundance and distribution) and connectivity of precious corals, effects of fisheries on ecosystems, and economic influences of product manufacturing and the jewelry industry.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
C-SC contributed to conception and design of the study, performed the statistical analysis, wrote the first draft of the manuscript, and read and approved the submitted version.
Funding
This study was funded by the Fisheries Agency, Council of Agriculture, Executive Yuan of the Republic of China (Taiwan), through grants 105AS-11.2.1-FA-F1, 106AS-10.2.1-FA-F1, and 107AS-9.2.1-FA-F1.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The author thanks the Association of Precious Coral Fishery Promotion of Yilan for their assistance in catch data collection. The author also thanks J.-Y. Chen and H.-T. Chang at the Institute of Marine Affairs and Resource Management, National Taiwan Ocean University for their assistance in data maintenance and administration. Constructive comments and suggestions from the reviewers and the editor are greatly appreciated.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.716157/full#supplementary-material
Supplementary Figure 1 | Annual deviation in the catch record between logbook and observer data (per trip) of the Taiwanese precious coral fishery between 2012 and 2018. (A) Box-whisker plot of the deviation (box range, first and third quartiles; band, median; whiskers, 1.5 interquartile; symbol, outliers), (B) mean (1 standard error) and median of the deviation.
References
Bavestrello, G., Bo, M., Calcagnile, L., Canessa, M., D’Elia, M., Quarta, G., et al. (2021). The sub-fossil red coral of sciacca (Sicily Channel, Mediterranean Sea): colony size and age estimates. Facies 67:13.
Bavestrello, G., Bo, M., Canese, S., Sandulli, R., and Cattaneo-Vietti, R. (2014). The red coral populations of the gulfs of Naples and Salerno: human impact and deep mass mortalities. Ital. J. Zoolog. 81, 552–563. doi: 10.1080/11250003.2014.950349
Bramanti, L., Magagnini, G., DeMaio, L., and Santangelo, G. (2005). Recruitment, early survival and growth of the Mediterranean red coral Corallium rubrum (L 1578), a 4-year study. J. Exp. Mar. Biol. Ecol. 314, 69–78. doi: 10.1016/j.jembe.2004.08.029
Bruckner, A. W. (2009). Rate and extent of decline in Corallium (pink and red coral) populations: existing data meet the requirements for a CITES Appendix II listing. Mar. Ecol. Prog. Ser. 397, 319–332. doi: 10.3354/meps08110
Bruckner, A. W. (2014). Advances in management of precious corals in the family Coralliidae: are new measures adequate? Curr. Opin. Environ. Sustain. 7, 1–8. doi: 10.1016/j.cosust.2013.11.024
Bruckner, A. W. (2016). “Advances in management of precious corals to address unsustainable and destructive harvest techniques,” in The Cnidaria, Past, Present and Future, eds S. Goffredo and Z. Dubinsky (Switzerland: Springer International Publishing), 747–786. doi: 10.1007/978-3-319-31305-4_46
Caddy, J. F. (1993). Background concepts for a rotating harvesting strategy with particular reference to the Mediterranean red coral, Corallium rubrum. Mar. Fish. Rev. 55, 10–18.
Cannas, R., Follesa, M. C., Cau, A., and Friedman, K. (2019). Global Report on the Biology, Fishery and trade of Precious Corals. FAO Fisheries and Aquaculture Circular No. 1184. Rome: Food and Agriculture Organization.
Carugati, L., Cau, A., Follesa, M. C., Melis, R., Moccia, D., Porcu, C., et al. (2020). Onboard scientific observers provide a realistic picture of harvesting and management priorities for the precious red coral (Corallium rubrum L.). Front. Mar. Sci. 7:482.
Cattaneo-Vietti, R., Bo, M., Cannas, R., Cau, A., Follesa, C., Meliadò, E., et al. (2016). An overexploited Italian treasure: past and present distribution and exploitation of the precious red coral Corallium rubrum (L., 1758) (Cnidaria: Anthozoa). Ital. J. Zoolog. 83, 443–455. doi: 10.1080/11250003.2016.1255788
Cau, A., Bramanti, L., Cannas, R., Follesa, M. C., Angiolillo, M., Canese, S., et al. (2016). Habitat constraints and self-thinning shape Mediterranean red coral deep population structure: implications for conservation practice. Sci. Rep. 6:23322.
Chang, S.-K. (2015). Precious corals become more precious in the northwestern Pacific: urgent need for integrated policy. Mar. Pol. 52, 103–107. doi: 10.1016/j.marpol.2014.10.020
Chang, S.-K., Yang, Y.-C., and Iwasaki, N. (2013). Whether to employ trade controls or fisheries management to conserve precious corals (Coralliidae) in the Northern Pacific Ocean. Mar. Pol. 39, 144–153. doi: 10.1016/j.marpol.2012.09.007
Chen, C.-S. (2012). Management of the precious coral fishery in Taiwan: progress and perspectives. Mar. Pol. 36, 623–629. doi: 10.1016/j.marpol.2011.10.016
Collette, B. B., Carpenter, K. E., Polidoro, B. A., Juan-Jordá, M. J., Boustany, A., Die, D. J., et al. (2011). High value and long life–double jeopardy for tunas and billfishes. Science 333, 291–292. doi: 10.1126/science.1208730
FAO (2018). The State of World Fisheries and Aquaculture 2018Meeting the Sustainable Development Goals. Rome: Food and Agriculture Organization.
Garrabou, J., and Harmelin, J. G. A. (2002). 20-year study on life-history traits of a harvested long-lived temperate coral in the NW Mediterranean: insights into conservation and management needs. J. Anim. Ecol. 71, 966–978. doi: 10.1046/j.1365-2656.2002.00661.x
Garrabou, J., Sala, E., Linares, C., Ledoux, J. B., Montero-Serra, I., Dominici, J. M., et al. (2017). Re-shifting the ecological baseline for the overexploited Mediterranean red coral. Sci. Rep. 7, 42404.
Grigg, R. W. (1984). Resource management of precious corals: a review and application to shallow water reef building corals. Mar. Ecol. 51, 57–74. doi: 10.1111/j.1439-0485.1984.tb00307.x
Grigg, R. W. (1989). “Precious coral fisheries of the pacific and mediterranean,” in Marine Invertebrate Fisheries: Their Assessment and Management, ed. J. F. Caddy (New York: John Wiley & Sons), 637–645.
Huang, M.-H., and Ou, C.-H. (2010). Precious coral fisheries management in TaiwanPast, present and future. Mar. Pol. 34, 1002–1009. doi: 10.1016/j.marpol.2010.02.007
Iwasaki, N., Fujita, T., Bavestrello, G., and Cattaneo-Vietti, R. (2012). Morphometry and population structure of non-harvested and harvested populations of the Japanese red coral (Paracorallium japonicum) off Amami Island, southern Japan. Mar. Freshw. Res. 63, 468–474. doi: 10.1071/mf11254
Jennings, S., Kaiser, M. J., and Reynolds, J. D. (2001). Marine Fisheries Ecology. Oxford: Blackwell Science Ltd.
Kao, S.-M., and Pearre, N. S. (2018). Regional cooperation in the post-South China Sea arbitration era: potential mechanism and cooperative areas. Coast. Manage. 46, 103–122. doi: 10.1080/08920753.2018.1426377
Lee, S., Park, Y. K., and Park, H. (2017). The complex legal status of the current fishing pattern zone in the east China Sea. Mar. Pol. 81, 219–228. doi: 10.1016/j.marpol.2017.03.021
Lodolo, E., Sanfilippo, R., Rajola, G., Canese, S., Andaloro, F., Montagna, P., et al. (2017). The red coral deposits of the graham bank area: constraints on the Holocene volcanic activity of the Sicilian Channel. GeoResJ 13, 126–133. doi: 10.1016/j.grj.2017.04.003
Maunder, M. N., and Punt, A. E. (2004). Standardizing catch and effort data: a review of recent approaches. Fish. Res. 70, 141–159. doi: 10.1016/j.fishres.2004.08.002
Montero-Serra, I., Linares, C., Doak, D. F., Ledoux, J. B., and Garrabou, J. (2018). Strong linkages between depth, longevity and demographic stability across marine sessile species. Proc. R. Soc. B 285:20172688. doi: 10.1098/rspb.2017.2688
Montero-Serra, I., Linares, C., García, M., Pancaldi, F., Frleta-Velić, M., Ledoux, J.-B., et al. (2015). Harvesting effects, recovery mechanisms, and management strategies for a long-lived and structural precious coral. PLoS ONE 10:e0117250. doi: 10.1371/journal.pone.0117250
Nonaka, M., and Muzik, K. (2009). Recent harvest records of commercially valuable precious corals in the Ryukyu Archipelago. Mar. Ecol. Prog. Ser. 397, 269–278. doi: 10.3354/meps08396
North Pacific Fisheries Commission (2018). Conservation and Management Measure for Bottom Fisheries and Protection of Vulnerable Marine Ecosystems in the Northwestern Pacific Ocean. CMM 2018-05. Tokyo: North Pacific Fisheries Commission.
Plagányi, ÉE., Skewes, T., Murphy, N., Pascual, R., and Fischer, M. (2015). Crop rotations in the sea: increasing returns and reducing risk of collapse in sea cucumber fisheries. Proc. Natl. Acad. Sci. U.S.A. 112, 6760–6765. doi: 10.1073/pnas.1406689112
Rossi, S., Tsounis, G., Orejas, C., Padrón, T., Gili, J.-M., Bramanti, L., et al. (2008). Survey of deep-dwelling red coral (Corallium rubrum) populations at Cap de Creus (NW Mediterranean). Mar. Biol. 154, 533–545. doi: 10.1007/s00227-008-0947-6
Santangelo, G., Bramanti, L., and Iannelli, M. (2007). Population dynamics and conservation biology of the over-exploited Mediterranean red coral. J. Theor. Biol. 244, 416–423. doi: 10.1016/j.jtbi.2006.08.027
Santangelo, G., Carletti, E., Maggi, E., and Bramanti, L. (2003). Reproduction and population sexual structure of the overexploited Mediterranean red coral Corallium rubrum. Mar. Ecol. Prog. Ser. 248, 99–108. doi: 10.3354/meps248099
Santangelo, G., Maggi, E., Bramanti, L., and Bongiorni, L. (2004). Demography of the overexploited Mediterranean red coral (Corallium rubrum L. 1758). Sci. Mar. 68, 199–204. doi: 10.3989/scimar.2004.68s1199
Schofield, C. H., and Townsend-Gault, I. (2011). Choppy waters ahead in “a sea of peace cooperation and friendship”? Slow progress towards the application of maritime joint development to the East China Sea. Mar. Pol. 35, 25–33. doi: 10.1016/j.marpol.2010.07.004
Teh, L. S. L., Witter, A., Cheung, W. W. L., Sumaila, U. R., and Yin, X. (2017). What is at stake? Status and threats to South China Sea marine fisheries. Ambio 46, 57–72. doi: 10.1007/s13280-016-0819-0
Torrents, O., Garrabou, J., Marschal, C., and Harmelin, J. G. (2005). Age and size at first reproduction in the commercially exploited red coral Corallium rubrum (L.) in the Marseilles area (France, NW Mediterranean). Biol. Conserv. 121, 391–397. doi: 10.1016/j.biocon.2004.05.010
Tsounis, G., Rossi, S., Aranguren, M., Gili, J.-M., and Arntz, W. (2006a). Effects of spatial variability and colony size on the reproductive output and gonadal development cycle of the Mediterranean red coral (Corallium rubrum L.). Mar. Biol. 148, 513–527. doi: 10.1007/s00227-005-0100-8
Tsounis, G., Rossi, S., Bramanti, L., and Santangelo, G. (2013). Management hurdles for sustainable harvesting of Corallium rubrum. Mar. Pol. 39, 361–364. doi: 10.1016/j.marpol.2012.12.010
Tsounis, G., Rossi, S., Gili, J.-M., and Arntz, W. (2006b). Population structure of an exploited benthic cnidarian: the case study of red coral (Corallium rubrum L.). Mar. Biol. 149, 1059–1070. doi: 10.1007/s00227-006-0302-8
Tsounis, G., Rossi, S., Gili, J.-M., and Arntz, W. E. (2007). Red coral fishery at the Costa Brava (NW Mediterranean): case study of an overharvested precious coral. Ecosystem 10, 975–986. doi: 10.1007/s10021-007-9072-5
Tsounis, G., Rossi, S., Grigg, R., Santangelo, G., Bramanti, L., and Gili, J.-M. (2010). The exploitation and conservation of precious coral. Oceanogr. Mar. Biol. Annu. Rev. 48, 161–212. doi: 10.1201/ebk1439821169-3
Tsounis, G., Rossi, S., Laudien, J., Bramanti, L., Fernandez, N., Gili, J.-M., et al. (2006c). Diet and seasonal prey capture rates in the Mediterranean red coral (Corallium rubrum L.). Mar. Biol. 149, 313–325. doi: 10.1007/s00227-005-0220-1
Tu, T.-H., Dai, C.-F., and Jeng, M.-S. (2015). Phylogeny and systematics of deep-sea precious corals (Anthozoa: Octocorallia: Coralliidae). Mol. Phylogen. Evol. 84, 173–184. doi: 10.1016/j.ympev.2014.09.031
Keywords: precious coral fishery, red coral, Coralliidae, fisheries management, regional cooperation, Northwest Pacific
Citation: Chen C-S (2021) Spatiotemporal Variation in the Catch Composition and Abundance of Precious Corals Around Taiwan: Implications for Fisheries Management. Front. Mar. Sci. 8:716157. doi: 10.3389/fmars.2021.716157
Received: 28 May 2021; Accepted: 03 September 2021;
Published: 04 October 2021.
Edited by:
Tomaso Fortibuoni, Istituto Superiore per la Protezione e la Ricerca Ambientale (ISPRA), ItalyReviewed by:
Georgios Tsounis, California State University, Northridge, United StatesGiorgio Bavestrello, University of Genoa, Italy
Copyright © 2021 Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chih-Shin Chen, Y3NjaGVuQG1haWwubnRvdS5lZHUudHc=
 Chih-Shin Chen
Chih-Shin Chen