- 1Center for Marine and Environmental Studies, University of the Virgin Islands, St. Thomas, VI, United States
- 2Division of Coastal Zone Management, U.S. Virgin Islands Department of Planning and Natural Resources, St. Thomas, VI, United States
- 3Caribbean Oceanic Restoration Education Foundation, St. Thomas, VI, United States
- 4CSS Inc., Under Contract to the National Oceanic and Atmospheric Administration Office for Coastal Management, Christiansted, VI, United States
Coral communities in the Caribbean face a new and deadly threat in the form of the highly virulent multi-host stony coral tissue loss disease (SCTLD). In late January of 2019, a disease with signs and characteristics matching that of SCTLD was found affecting a reef off the coast of St. Thomas in the U.S. Virgin Islands (USVI). Identification of its emergence in the USVI provided the opportunity to document the initial evolution of its spatial distribution, coral species susceptibility characteristics, and its comparative impact on coral cover at affected and unaffected coral reef locations. Re-assessments at sentinel sites and long-term monitoring locations were used to track the spread of the disease, assess species affected, and quantify its impact. The disease was initially limited to the southwest of St. Thomas for several months, then spread around the island and to the neighboring island of St. John to the east. Differences in disease prevalence among species were similar to reports of SCTLD from other regions. Highly affected species included Colpophyllia natans, Eusmilia fastigiata, Montastraea cavernosa, Orbicella spp., and Pseudodiploria strigosa. Dendrogyra cylindrus and Meandrina meandrites were also highly affected but showed more variability in disease prevalence, likely due to initial low abundances and the rapid loss of colonies due to disease. Siderastrea spp. were less affected and showed lower prevalence. Species previously reported as unaffected or data deficient that were found to be affected by SCTLD included Agaricia spp., Madracis spp., and Mycetophyllia spp. We also observed multi-focal lesions at SCTLD-affected sites on colonies of Porites astreoides, despite that poritids have previously been considered low or not susceptible to SCTLD. Loss of coral cover due to acute tissue loss diseases, which were predominantly SCTLD, was significant at several monitoring locations and was more impactful than previous mass bleaching events at some sites. There are no signs that the USVI SCTLD outbreak is abating, therefore it is likely that this disease will become widespread across the U.S. Caribbean and British Virgin Islands in the near future.
Introduction
Coral disease is a significant and increasing threat to Caribbean coral reef systems. This fact was highlighted by the appearance and rapid spread of stony coral tissue loss disease (SCTLD) along the southeast coast of Florida. SCTLD was first found affecting corals off of Miami in 2014, when it was identified as white plague (Precht et al., 2016), a previously described disease of stony corals that has been affecting reefs in the Caribbean for several decades (Sutherland et al., 2004). Faster rates of lesion expansion, higher prevalence of mortality, the specific set of species affected, and the continuous duration of the outbreak of the disease led researchers to identify this disease as distinct from white plague (Walton et al., 2018), and name it stony coral tissue loss disease (SCTLD Case Definition, 2018). SCTLD has since devastated coral reef communities throughout southeast Florida and has been reported from disparate areas around the Caribbean, including Mexico (Alvarez-Filip et al., 2019), Jamaica, the Turks and Caicos Islands, and many others1.
An important characteristic of SCTLD that differentiates it from other diseases of corals that cause acute tissue loss is its relentless geographic expansion. Originally limited to the area around the Port of Miami and Biscayne Bay, SCTLD has spread continuously north and south along nearly the entire Florida Reef Tract. Also, while other tissue loss diseases of corals have shown outbreaks that are temporally limited or follow seasonal cycles, SCTLD has shown no sign of abating in affected regions. In fact, sites in Florida where the disease first emerged in 2014–2015 continue to show new cases years after the initial outbreak (Muller et al., 2020). The continuous expansion of the disease in time and space are key factors that make SCTLD one of the gravest threats to modern Caribbean reefs.
Other key characteristics of SCTLD which distinguish it from other tissue loss diseases are the suite of species that it affects and lesion development patterns. According to the current case definition (SCTLD Case Definition, 2018), species first affected by SCTLD at a location include the pillar coral (Dendrogyra cylindrus), brain corals (Pseudodiploria strigosa, Pseudodiploria clivosa, Colpophyllia natans, and Diploria labyrinthiformis), and the maze coral (Meandrina meandrites). These are considered indicator species of the disease affecting a location (see text footnote 1). This is in contrast to white plague disease which tends to affect Orbicella spp. most severely (Smith et al., 2013; Williams et al., 2020). Morphological features of the disease also differ from white plague in that disease signs typically manifest as multi-focal expanding lesions that can appear on any part of the coral (SCTLD Case Definition, 2018). These lesions expand so rapidly that small bits of tissue can sometimes be seen hanging from the lesion edges. In contrast, white plague lesions can be multifocal and coalescing but originate from the base or margin of a coral (Sutherland et al., 2004). The expansion of white plague lesions is also less rapid than lesions associated with SCTLD which expand at rates several magnitudes faster (Aeby et al., 2019; Meiling et al., 2020; Williams et al., 2020).
These unique characteristics have made tracking the appearance of SCTLD in new regions fairly straightforward, however, there remains the question of whether SCTLD signs in different regions in fact represent a common etiology. Unfortunately, despite intensive efforts, no consistent etiological agent has been identified for SCTLD; however, candidate pathogens and pathogen indicators have been suggested (Meyer et al., 2019; Rosales et al., 2020). These candidates and the alleviation of disease signs with the application of antibiotics (Neely et al., 2020) suggest the pathogenic agent is likely bacterial or has some bacterial component, though investigations into associations with viruses and other organisms are ongoing.
In early 2019, a coral disease with characteristics consistent with SCTLD was identified affecting coral reefs off the southwest coast of St. Thomas in the U.S. Virgin Islands (USVI). Describing the impact of SCTLD as it spreads throughout the Caribbean is an important step in identifying how different coral assemblages and lineages respond through space and time. This study tracked the emergence of SCTLD signs at coral reef sites surrounding St. Thomas and St. John, VI, for the first 18 months after its initial sighting in the region. We provide documentation of the spread of the disease, species affected, and the impact on coral communities in this time frame. We provide summaries of data similar to that presented in Walton et al. (2018) and Alvarez-Filip et al. (2019) for consistency with other reports of this disease in the Caribbean.
Materials and Methods
Distinguishing Disease Signs
Identifying the emergence of SCTLD in situ is based on epidemiological characteristics, including which species are affected and when they become affected, since no specific pathogen has been identified that would allow for a confirmed diagnosis. Therefore, although it is possible to determine when SCTLD appears at a site, it is difficult to say for certain that acute tissue loss disease signs on an individual coral represent true SCTLD without following that coral through time. This is because recent mortality indicative of disease can be attributable to other acute tissue loss diseases, such as white plague. Whereas white plague lesions emerge at the base or margin of a colony, expand rapidly, but often do not coalesce and halt before full colony mortality (Brandt et al., 2013; Smith et al., 2013), SCTLD lesions appear as multi-focal lesions across a colony’s surface, expand rapidly, and often coalesce resulting in total colony mortality (Meiling et al., 2020). To be conservative, we combine and present data for all acute tissue loss (ATL) disease signs affecting corals in the survey data.
Transect Monitoring
The Territorial Coral Reef Monitoring Program (TCRMP) has been conducting annual coral and fish community assessments at locations across the USVI since 2002 (Ennis et al., 2019). Of the 34 total monitoring locations, 19 are within the northern USVI (St. Thomas and St. John), while 15 locations are distributed around St. Croix; however, SCTLD was not observed on St. Croix during the time period of this study and so St. Croix monitoring locations are not included here. The deepest (63 m) northern USVI location, Ginsburg’s Fringe, was also excluded since it is a mesophotic lettuce coral (Agaricia undata) reef making it ecologically distinct from the other TCRMP monitoring locations. Annual monitoring data at the other 18 St. Thomas and St. John TCRMP locations are presented here going back to 2005, with the exception of the Brewers Bay and Coral Bay locations, which begin in 2008 and 2011, respectively. In addition to these annual data, several monitoring locations were sampled outside of annual monitoring when SCTLD was first reported (Table 1).
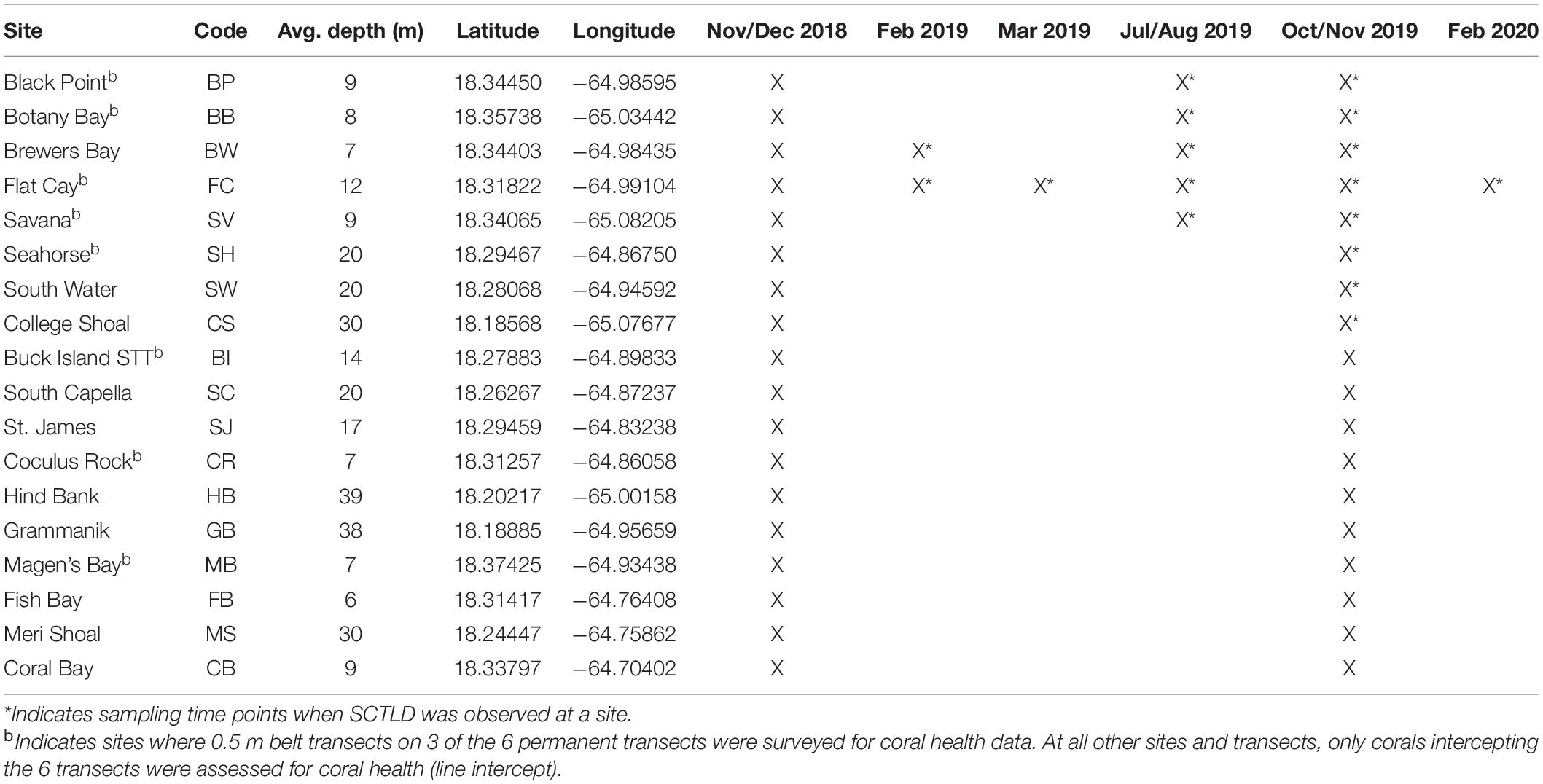
Table 1. Sampling time points at USVI TCRMP locations in the northern USVI (STT: St. Thomas, STJ: St. John).
The TCRMP methodology collects data on the benthic community composition at each location by surveying six marked 10 m transects annually using video methods detailed in Ennis et al. (2019) and summarized in Smith et al. (2013). A diver on SCUBA takes continuous video along each transect keeping the lens approximately 0.4 m above the substrate (a wand guide is used to maintain height). Twenty to fifty non-overlapping images are then captured from each transect’s video. Captured images represent an area of approximately 0.31 m2 (0.64 m × 0.48 m). Coral Point Count with Excel Extension software (Kohler and Gill, 2006; prior to 2019) or the “imager” (Barthelme, 2020) and “spatstat” (Baddeley et al., 2015) packages in R is then used to superimpose randomly located points on each image (20 points/image) and the substrate type under each point is identified to the most descriptive level possible.
The TCRMP methodology collects coral demographic and health metrics. Individual coral colonies along the same transects are assessed in situ for a number of coral health metrics focused on mortality, bleaching, and disease following modified AGGRA methodology (Ennis et al., 2019). As part of a project intended to understand coral juvenile abundances, three of the six transects at some sites (indicated byb in Table 1), were expanded in 2016 to include any coral that was within or intercepted a 10 m × 0.5 m belt transect on the left side of each transect. For each coral assessed, multiple characteristics are recorded. Those presented here include species and the presence of disease lesions and/or bleaching. Bleaching was defined as stark white tissue.
Roving Surveys
When SCTLD was first observed at Flat Cay (FC) in January of 2019, periodic roving surveys by trained observers were instituted on SCUBA or snorkeling at 59 sentinel monitoring locations surrounding St. Thomas ranging in depth from 3 to 40 m (Figure 1). These locations included the 18 long-term monitoring locations (indicated by stars in Figure 1 and methods described above) as well as 42 strategically placed locations along the potential path of disease spread. Identification of SCTLD was determined after evaluating the species affected, severity and description of lesions (supported with photographic evidence, see below), and in some cases locations were revisited within 1 week to further confirm disease progression and species susceptibility. The presence of SCTLD was confirmed if multifocal to coalescing lesions were found on highly susceptible species that included D. cylindrus, M. meandrites, P. strigosa, and D. labyrinthiformis. Roving surveys were completed at the sentinel locations approximately monthly to bi-monthly and were used to determine the first calendar month when the disease was reported at each location.
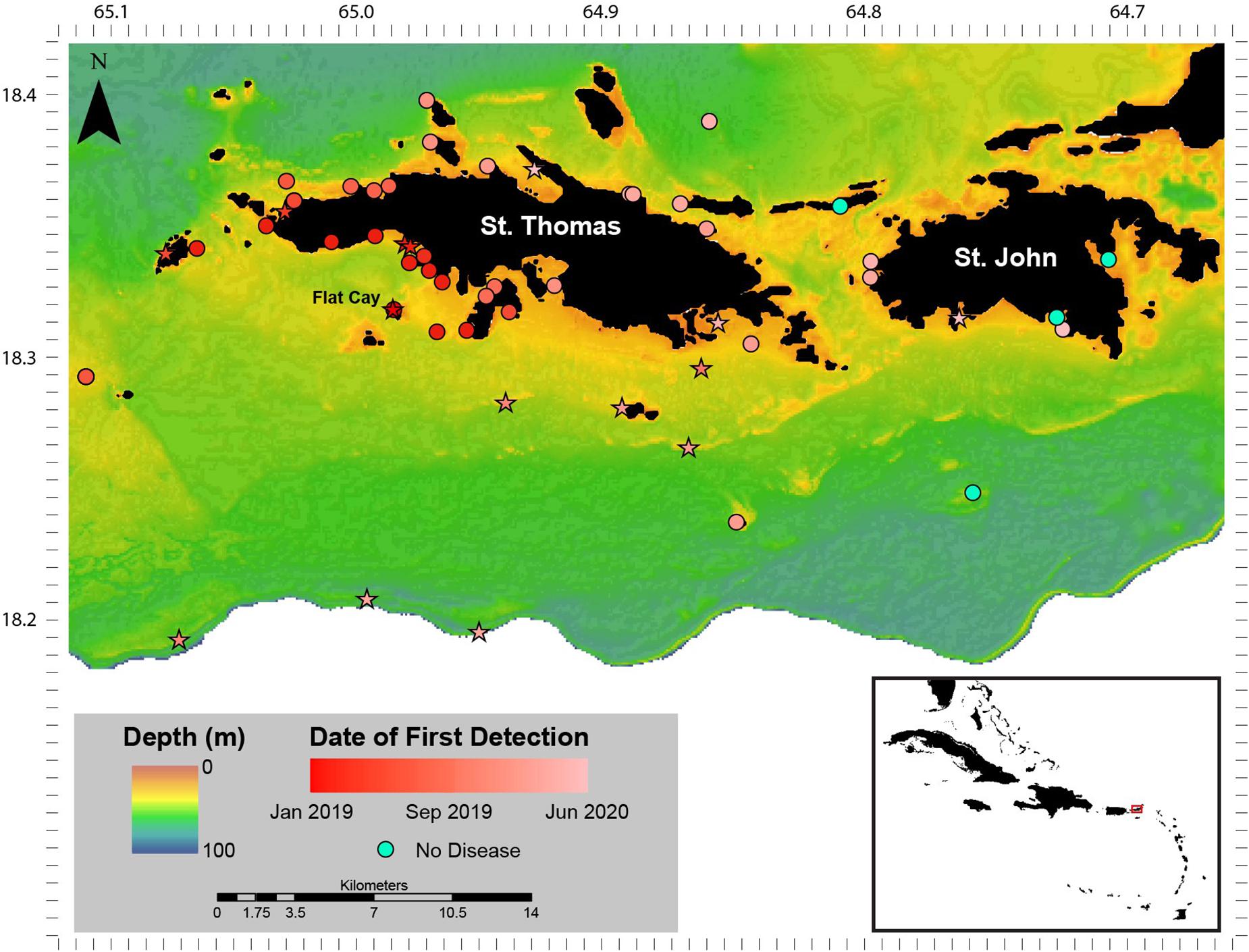
Figure 1. Stony coral tissue loss disease (SCTLD) distribution January 2019–June 2020. Sentinel monitoring locations and date of first detection of SCTLD around St. Thomas and St. John, U.S. Virgin Islands between January 2019 and June 2020. Sites that are long-term monitoring locations part of the Territorial Coral Reef Monitoring Program are indicated as stars. The Flat Cay (FC) monitoring location where SCTLD was first detected is indicated with a label. Inset: location of St. Thomas and St. John (red box) in the Caribbean region.
Data Analysis
To compare species susceptibility with studies from other regions, prevalence (% affected) of ATL diseases within species was calculated using corals assessed in TCRMP transects. Corals of each species were pooled across all transects and sites for surveys that occurred immediately after the first report of ATL indicative of SCTLD at a site (as indicated in Table 1). Differences in disease prevalence among species were tested using a likelihood ratio test. For FC, where there was repeated sampling of transects between February 2019 and February 2020, prevalence of ATL was calculated for all affected species for each sample time point in 2019 and 2020 (Table 1). ATL prevalence for these species at FC was also calculated for all transects pooled among all annual surveys between 2005 and 2018 for comparison. Differences in ATL prevalence through time for each species were analyzed using a likelihood ratio test. For two species, C. natans and D. cylindrus, a Fisher’s Exact test was used instead due to low sample sizes.
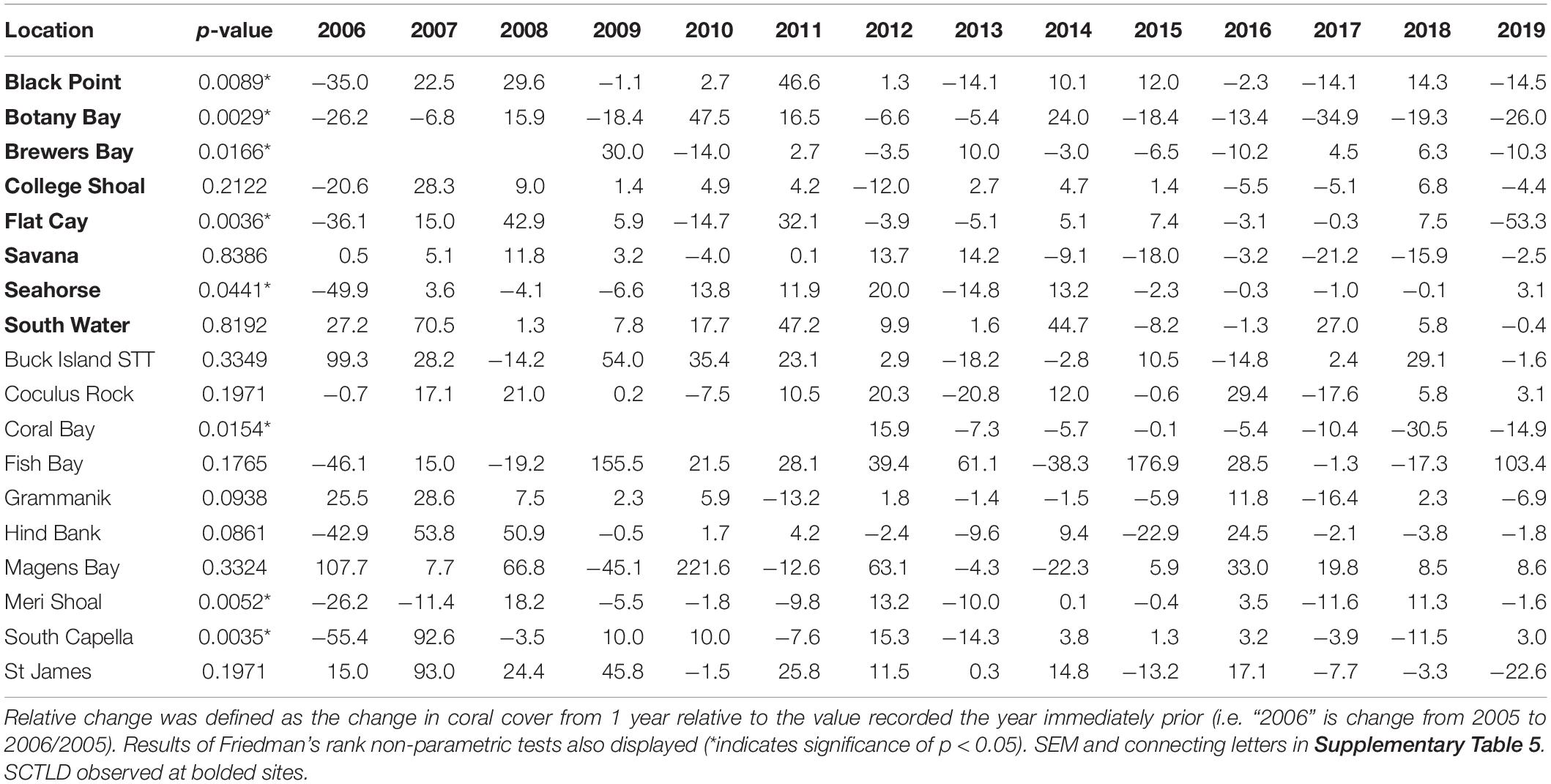
Metrics calculated at the overall coral community level included percent coral cover from video transects. Percent coral cover values were calculated for each transect at each time point as the number of randomly assigned points (described above) identified as living coral divided by the total number of identifiable points that landed on reef substrate. Relative change in coral cover was calculated as the difference between coral cover values in subsequent years divided by the previous year’s value.
Species richness, diversity (H’), and disease prevalence were also calculated for each transect at each time point from line intercept and belt transects. Disease prevalence (%) was calculated as the number of corals exhibiting ATL divided by the total number of corals assessed on that transect. Similarly, to Walton et al. (2018) and Muller et al. (2020), ATL was used instead of specifically SCTLD due to the difficulty in distinguishing between SCTLD and other acute tissue loss diseases such as white plague disease. However, it is likely that the majority of ATL lesions represented SCTLD, given the large difference in prevalence of ATL at sites considered SCTLD affected versus unaffected (detailed in results below). ATL lesions were distinguished from bleached tissue by the absence of living tissue.
To determine the impact of SCTLD on St. Thomas and St. John coral communities, percent coral cover, coral species richness, coral species diversity, and disease prevalence were each analyzed through time at monitoring locations using Friedman’s rank tests. Relative coral cover change was calculated as the change in coral cover from 1 year relative to the value recorded in the year immediately prior. For example, the relative change in coral cover for 2006 was calculated as coral cover in 2005 minus coral cover in 2006 divided by coral cover in 2005. Relative coral cover change was also analyzed through time for each location using Friedman’s rank tests. All statistical analyses were performed in R (v 3.5.1).
Results
Disease Spread
No SCTLD-like disease was found at long-term TCRMP monitoring locations surrounding St. Thomas and St. John in late 2018. However, a disease with characteristics described for SCTLD (i.e., multifocal large lesions) affecting species identified as highly and intermediately susceptible in the SCTLD Case Definition (2018) was found at the FC TCRMP monitoring site (depth 14 m) and on the shallow reefs of FC (depth 3–5 m) on 28 January 2019 (Figure 1). No ATL diseases were observed on monitored corals at the FC monitoring site one-month prior during normal annual TCRMP monitoring activities (see following data section), nor were any ATL disease signs noted on corals in observations outside of the transects in December 2018 (Brandt personal observation). In subsequent surveys of strategically placed sentinel sites around the island immediately following the first identification of SCTLD, SCTLD-affected reefs were found to be concentrated to the southwest of St. Thomas (Figure 1). SCTLD was not observed at sites to the southeast, east, or north of the island, or on reefs surrounding St. John.
Between the first identification of SCTLD in late January 2019 and June 2020, the disease spread west to the island of Culebra, Puerto Rico (first identified November 2019), and east to the British Virgin Islands (first identified May 2020, noted on vicoraldisease.org). Results from monitoring of the 59 sentinel sites located around St. Thomas and St. John indicated that the disease was concentrated on the southwest side of St. Thomas until April 2019 when it appeared on reefs on the northwest side of the island (Figure 1). The disease then remained concentrated on the west side of the island until the fall of 2019 when it began appearing on mesophotic (30–40 m depth) coral reefs sites (Supplementary Figure 1) at the shelf-edge as well as sites southeast of St. Thomas. In January 2020, the disease emerged at multiple locations on the east and northeast end of St. Thomas and appeared at locations on the west end of St. John. In the 6 months following that, the disease emerged at locations across St. John, though some embayments and locations were not yet affected by June 2020.
Species Affected
When all surveys from first observations of SCTLD at long-term monitoring locations were combined, prevalence of ATL due to disease showed differences among species (LR = 130.4, p < 0.0001; Figure 2). Highly susceptible species (i.e., species exhibiting >10% prevalence across all affected sites, as in Alvarez-Filip et al., 2019) showed overlap with species considered highly or intermediately susceptible in Florida and Mexico (Figure 2). These included P. strigosa, Montastraea cavernosa, D. cylindrus, C. natans, Eusmilia fastigiata, and Orbicella faveolata/franksi (Figures 3A–F). Species showing low prevalence (<10%) included the previously classified intermediately susceptible Orbicella annularis (Figure 3G) and Siderastrea spp. (Figure 3I). In addition, some species previously classified as unknown or not susceptible were found to have low prevalence of ATL, including Madracis spp. (Figure 3H), Agaricia spp. (Figure 3J), and Porites astreoides (Figure 3K). Species that did not show signs of disease during these initial surveys included Mycetophyllia spp., Stephanocoenia intersepta, branching Porites spp., and M. meandrites.
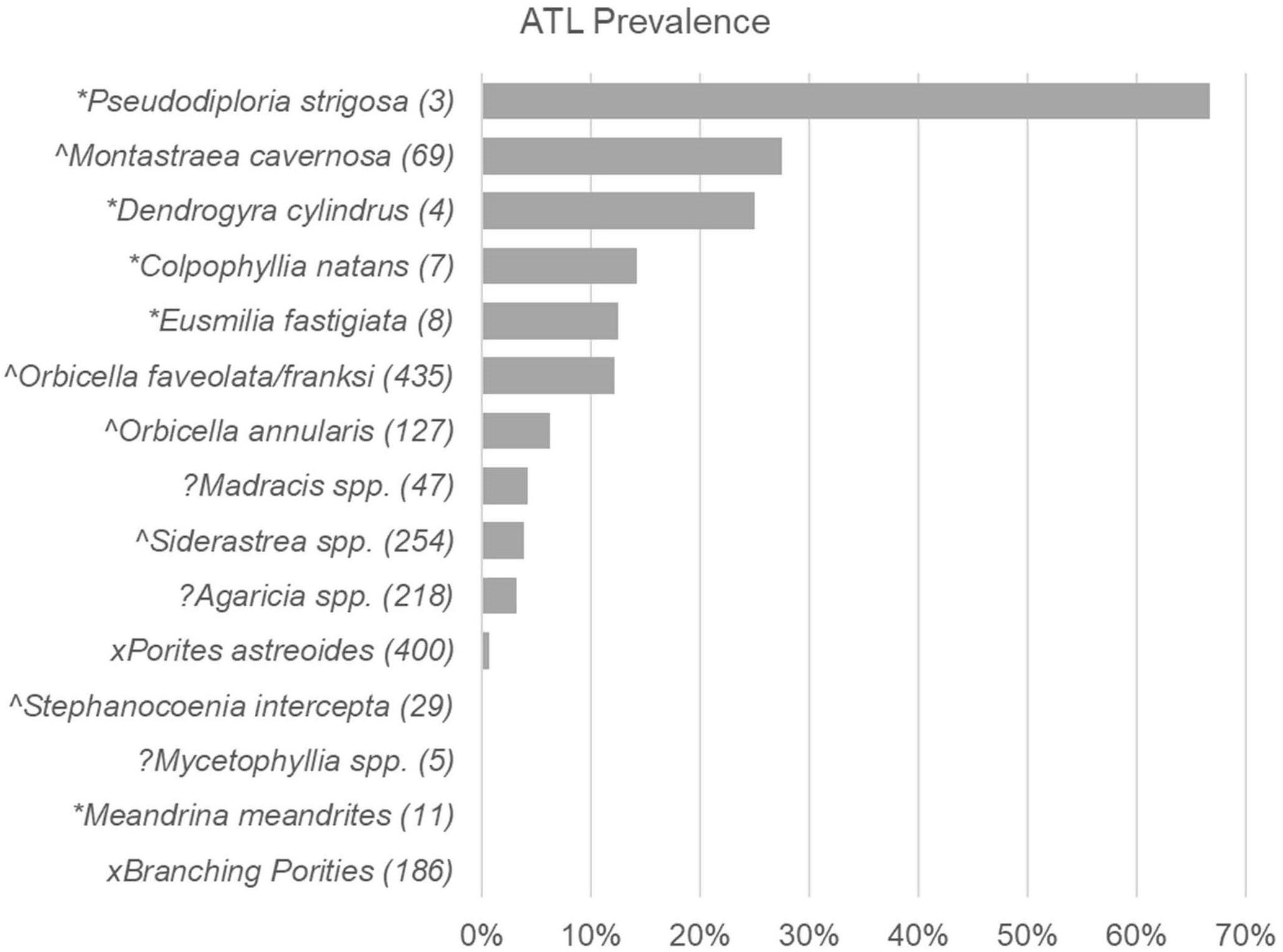
Figure 2. Acute tissue loss disease prevalence among coral species at first recording of SCTLD. Prevalence (% of corals) with Acute Tissue Loss (ATL) disease among different species of corals recorded in pooled Territorial Coral Reef Monitoring Program (TCRMP) surveys completed when disease was first reported at a site (sample time points of first report of SCTLD for sites indicated in Table 1). Species susceptibility as listed in the case description (SCTLD Case Definition, 2018) are indicated by symbols as follows: *, highly susceptible; ∧, intermediately susceptible; x, low or not susceptible; ?, Insufficient data to categorize. Numbers in parentheses indicate number of corals sampled (n).

Figure 3. Images of SCTLD-affected colonies of coral multiple species. Acute tissue loss disease signs consistent with SCTLD on coral species shown to be affected in first surveys at long-term monitoring locations around St. Thomas, VI. Species include: (A) Pseudodiploria strigosa, (B) Montastraea cavernosa, (C) Dendrogyra cylindrus, (D) Colpophyllia natans, (E) Eusmilia fastigiata, (F) Orbicella franksi, (G) Orbicella annularis, (H) Madracis spp., (I) Siderastrea siderea, (J) Agaricia spp., (K) Porites astreoides. Image credits: panle (A) J. Townsend, panel (B–K) M. Brandt.
When examining the time series of species affected at FC, species showed different levels of disease prevalence as well as changes in disease prevalence through time (Figure 4). Prior to 2019, ATL was very rare and found on only Orbicella spp. (3 of 684 corals assessed, 0.4%; Figure 4H) and C. natans (1 of 23 assessed, 4.3%; Figure 4B). All species except C. natans and D. cylindrus showed significant differences in ATL disease prevalence through time (Table 2).
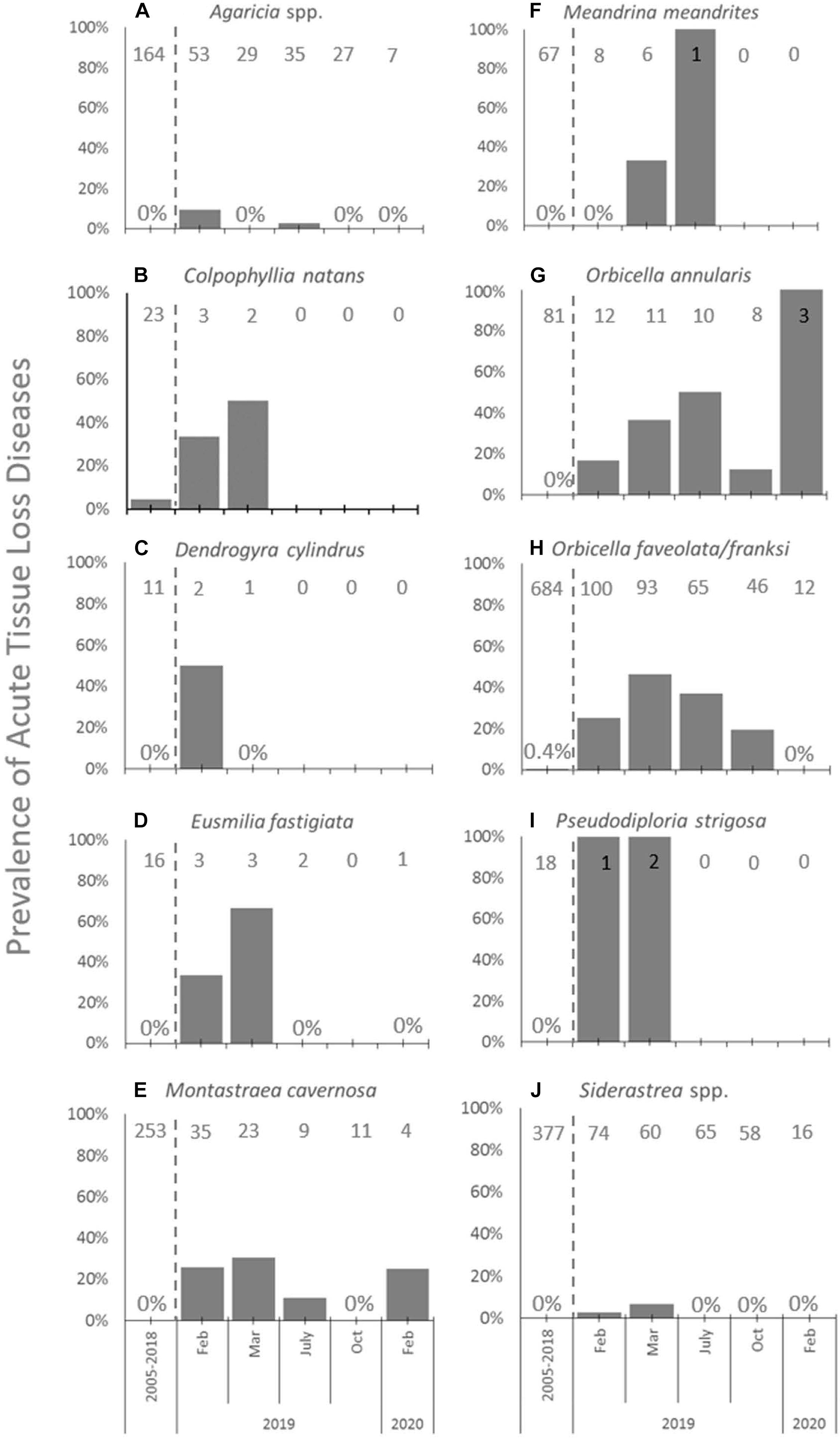
Figure 4. Acute tissue loss diseases among species at FC. Prevalence (%) of acute tissue loss diseases affecting the 10 most abundant coral species (A–J) from long-term monitoring transects at FC in the time period before SCTLD was identified (combined annual monitoring between 2005 and 2018, left of dashed lines), and during repeated sampling in 2019 and in February of 2020 (right of dashed lines). Sample sizes of corals at each time point indicated at top of each graph.
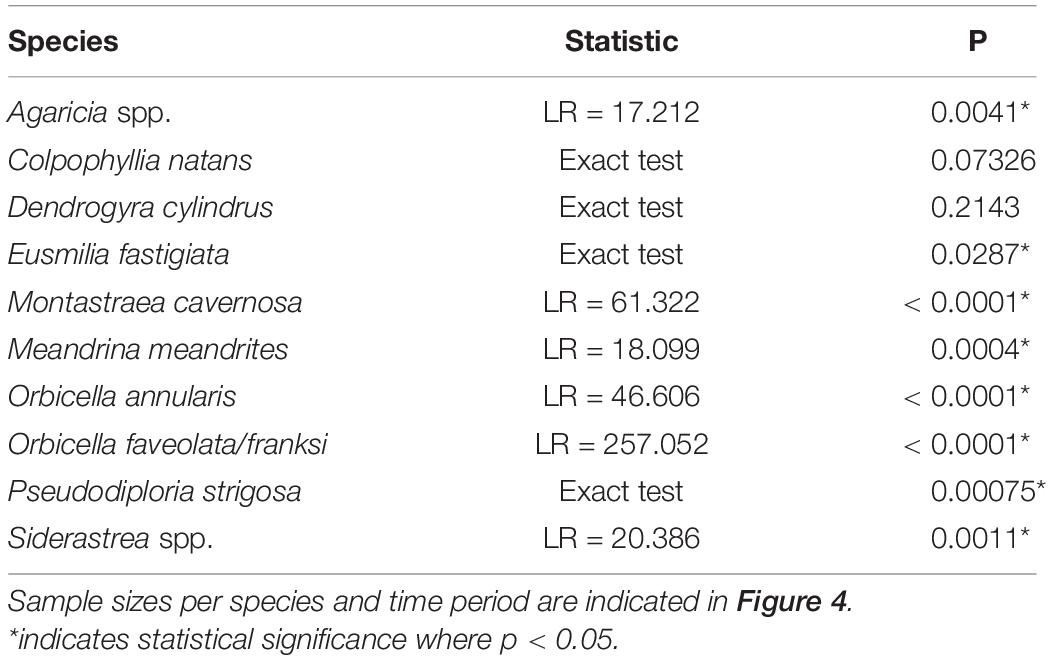
Table 2. Results of analysis of frequencies tests of diseased versus not disease colonies in time points between 2019 and 2020 and all surveys combined for 2005–2018 at FC.
At FC prior to the onset of SCTLD, the most abundant types of corals found in transects included Orbicella spp., Siderastrea spp., Agaricia spp., and M. cavernosa (Figures 4A,E,H,J, respectively). Of these, Orbicella spp. and M. cavernosa showed increasing or steady prevalence of ATL after SCTLD emerged and declining abundances until the last sampling in February 2020, when only 12% of combined O. franksi and O. faveolata, 30% of O. annularis, and 11% of M. cavernosa colonies counted 1 year prior remained. Agaricia spp. and Siderastrea spp. also declined to 13 and 21% of their abundances 1 year prior, respectively, but generally had low (<10%) ATL prevalence. Low prevalence among these corals may have been due to their small size; once affected by SCTLD these corals may die quickly, not allowing for active disease to have been picked up in the periodic surveys.
Other species that were in low abundance at FC (n < 4) yet were affected by disease included C. natans (Figure 4B), D. cylindrus (Figure 4C), E. fastigiata (Figure 4D), and P. strigosa (Figure 4I). At least one of colony of each of these species was affected early on, but then then no disease was recorded after March 2019, likely because individuals of these species died from disease and no longer appeared in the transects, save for one E. fastigiata. M. meandrites, a highly susceptible species and often used as an indicator of the onset of SCTLD at a site, was also low in abundance (n = 8) and showed no disease prevalence within transects at FC when first sampled after SCTLD emerged (Figure 4F). However, subsequent samplings showed disease prevalence within this species, and then the loss of the species from transects by October of 2019 (Figure 4F).
Disease Dynamics and Impact
Prevalence of ATL disease was significantly different through time at 6 of the 8 SCTLD-affected monitoring sites (Table 3). In 2019, ATL prevalence was statistically higher than previous sample year at five of these sites (Black Point, College Shoal, FC, Savana, Seahorse; Figure 5A and Supplementary Table 1 connecting letters) and was statistically equal to mass bleaching-related ATL disease outbreaks at one site (Botany Bay; Figure 5A, and Supplementary Table 1). Two SCTLD-affected sites, Botany Bay and South Water, showed elevated disease in 2019 but did not show statistically significant changes in disease prevalence through time (Table 3). Two additional sites that were unaffected by SCTLD (Hind Bank, Meri Shoal) showed significant changes in ATL disease prevalence through time (Table 3). These changes, however, were related to the mass bleaching events in 2005 and 2010 which immediately preceded white plague disease outbreaks the following year (Figure 5B and Supplementary Table 1, reported in Smith et al., 2013). All other sites unaffected by SCTLD showed little to no ATL prevalence through time (Figure 5B).
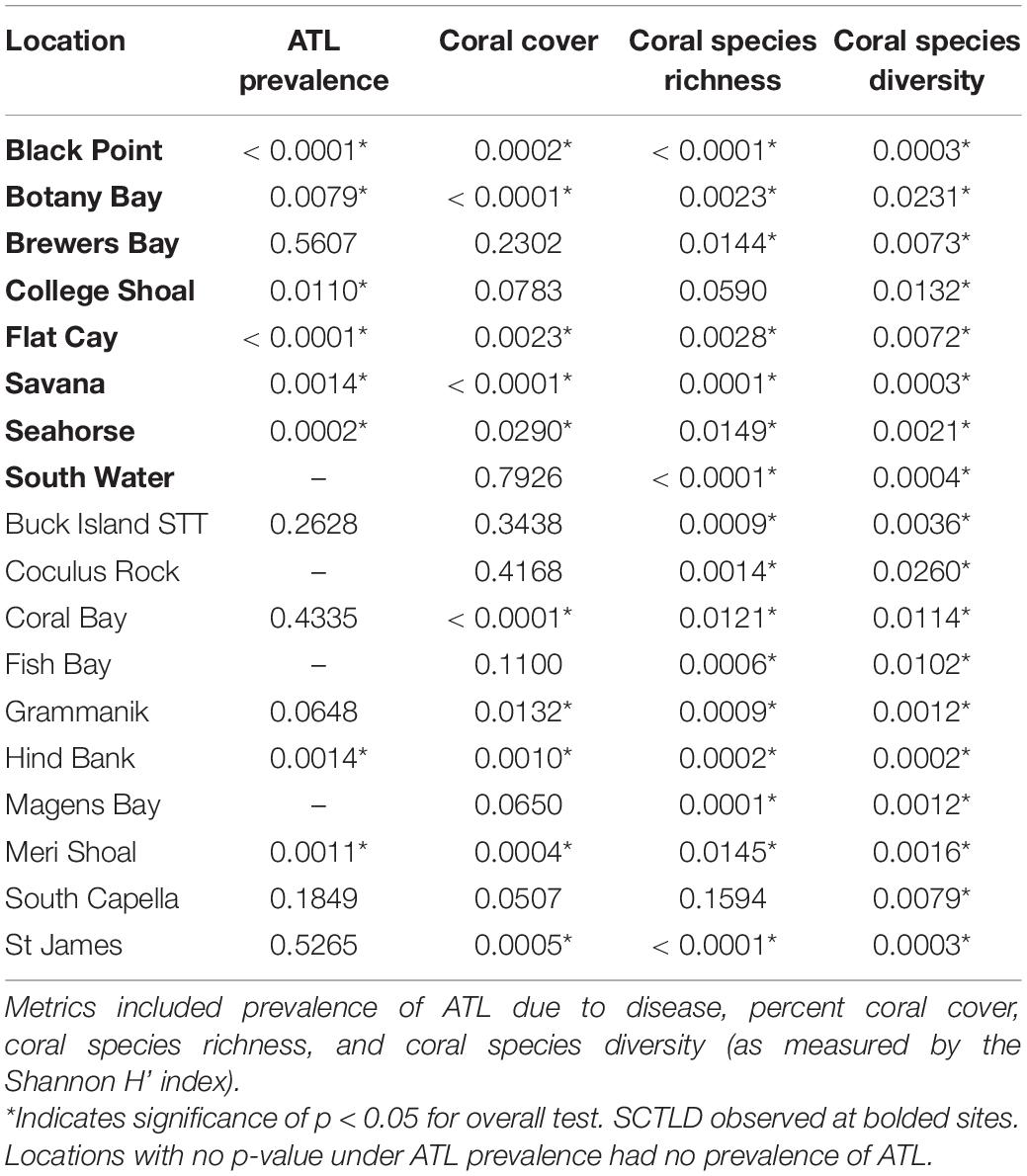
Table 3. Overall results of Friedman’s rank non-parametric tests examining different reef metrics at long-term TCRMP locations through time.
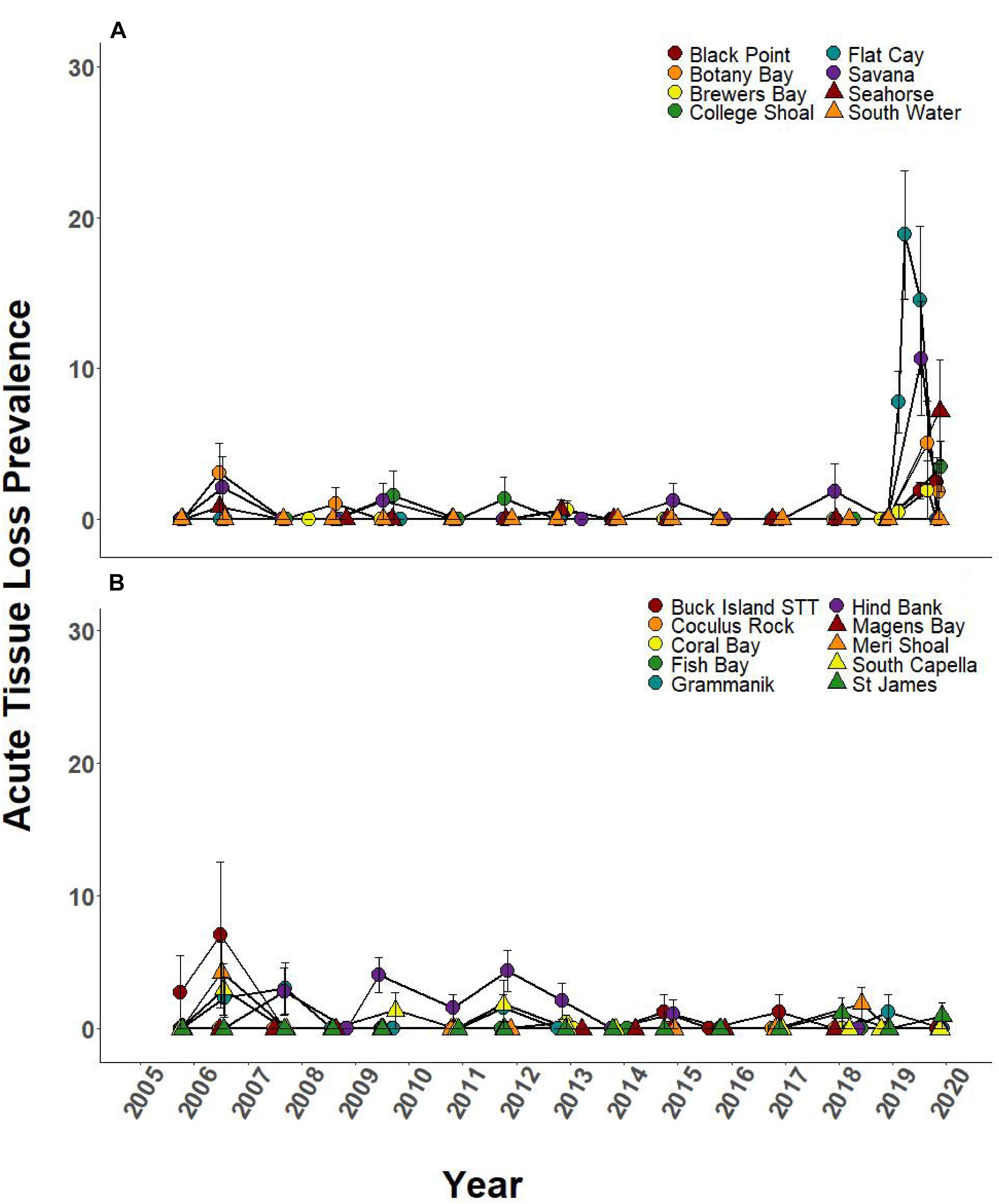
Figure 5. Prevalence of ATL due to disease (±SEM) at northern U.S Virgin Islands (USVI) TCRMP locations from 2005 to 2019. Acute tissue loss refers to colonies exhibiting large lesions consistent with disease. Prior to 2019, ATL was primarily due to white plague, while in 2019, ATL was almost exclusively due to SCTLD. (A) Locations with SCTLD present; (B) Locations with no SCTLD present as of December 2019.
Over the 2005–2019 monitoring period, 5 of the 8 SCTLD-affected sites showed significant changes in coral cover through time, but only FC, where SCTLD was first detected, saw a significant decline related to the emergence of SCTLD (Figure 6A, Table 3, and Supplementary Table 2 connecting letters). Coral cover did not show recent declines at sites unaffected by SCTLD (Figure 6B). Coral species richness and diversity declined at many sites around the 2005 bleaching event, but then slowly rebounded or surpassed their 2005 levels prior to the appearance of SCTLD in the USVI (Figure 7, Table 3, Supplementary Figure 2, and Supplementary Tables 3, 4). Species richness showed significant declines related to SCTLD at two sites, FC and Botany Bay (Figure 7A and Supplementary Table 3) and species diversity (as measured by the Shannon index) also declined significantly in relationship to the emergence of SCTLD for one site, Botany Bay (Supplementary Figure 2A and Supplementary Table 4).
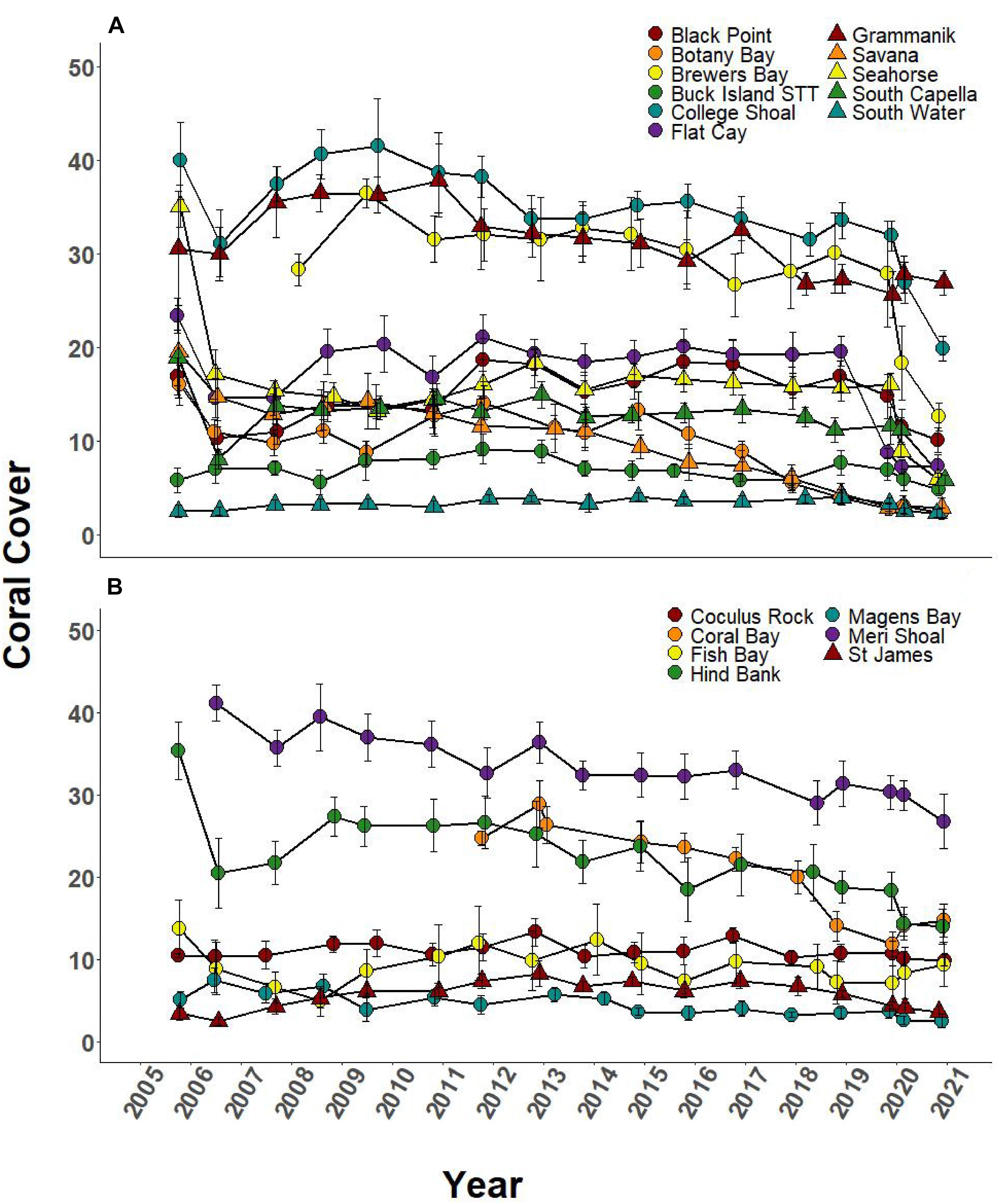
Figure 6. Temporal series of % coral cover (±SEM) at northern USVI TCRMP locations from 2001 to 2019. (A) Locations with SCTLD present; (B) Locations with no SCTLD present as of December 2019.
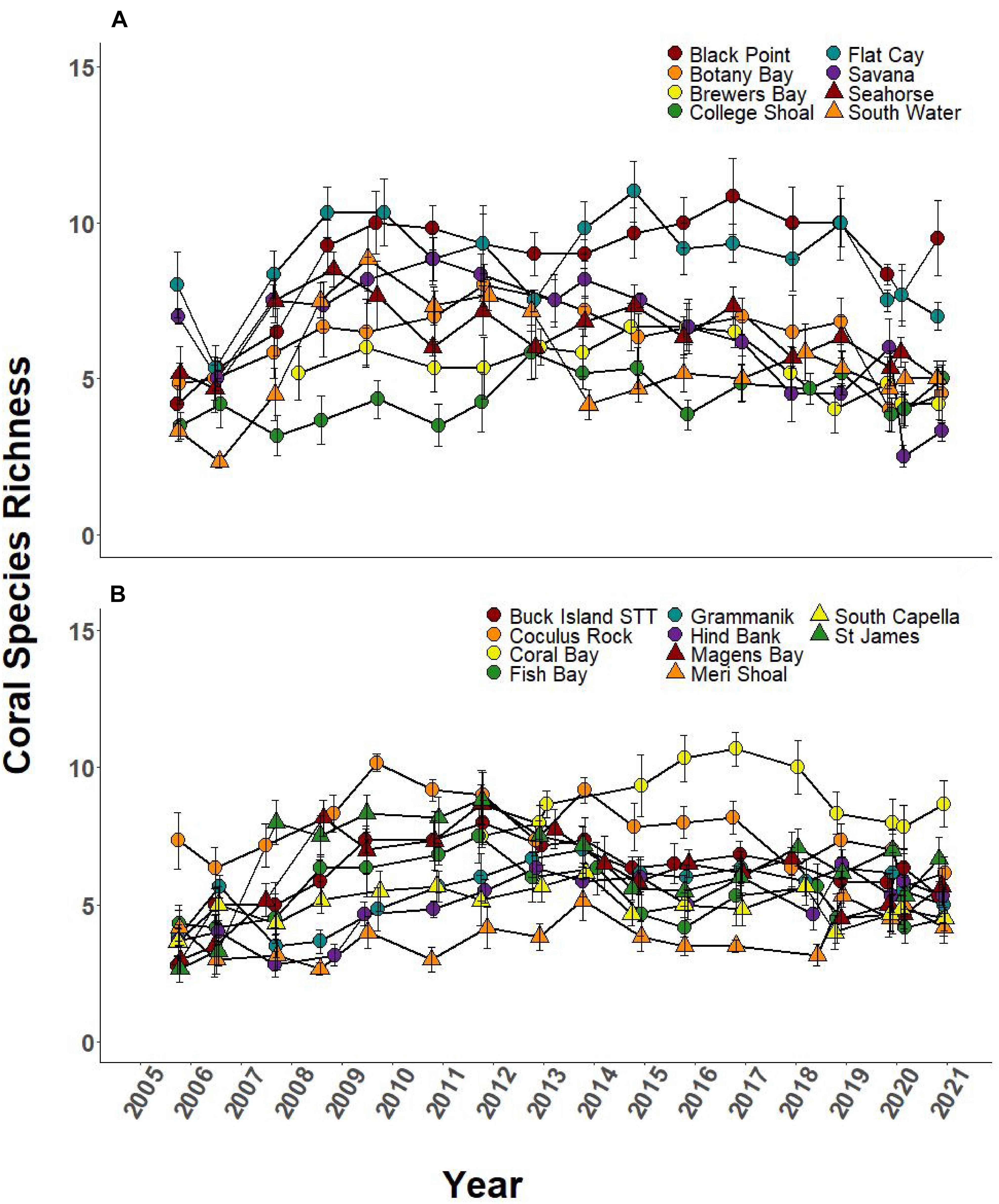
Figure 7. Species richness of coral community (±SEM) at northern USVI TCRMP locations from 2005 to 2019. (A) Locations with SCTLD present; (B) Locations with no SCTLD present as of December 2019.
Relative change in coral cover was significantly different through time at 5 of the 8 SCTLD-affected sites (Table 4). At those sites, the relative drop in coral cover in 2019 was as large or larger than that which occurred in 2006 due to the 2005 mass bleaching event. Flat Cay showed the most dramatic loss with an average relative drop of 53.3% from the previous year (Table 4). Three additional sites that were not affected by SCTLD showed significant changes in relative coral cover through time (Coral Bay, Meri Shoal, South Capella). For sites Meri Shoal and South Capella, these changes were due to losses from the 2005 mass bleaching event. At Coral Bay, significant change was seen in 2018, almost certainly related to the passage of Hurricanes Irma and Maria in 2017 (Irma tracked directly over St. John).
The higher frequency sampling at FC showed that percent cover of both highly and intermediately susceptible species dropped between the last SCTLD-free monitoring time point and the first survey when SCTLD was present (Figure 8). Highly susceptible species percent cover dropped again, but only slightly by the next sampling, and then remained approximately the same. Highly susceptible species at FC included primarily M. meandrites, E. fastigiata, D. labyrinthiformis, and P. clivosa (Supplementary Figure 3A). While the former three species showed large losses, P. clivosa remained approximately the same, suggesting that this species may be less susceptible at this location. However, percent cover of intermediately susceptible species dropped continuously across all samplings in 2019 and 2020. Intermediate species included Orbicella spp., M. cavernosa, Myceptophyllia spp., S. intersepta, and S. siderea. Of these the largest drops were observed in Orbicella spp. and M. cavernosa (Supplementary Figure 3B). Low susceptible species showed no large changes during the time period, and included Porites spp., Madracis spp. (Supplementary Figure 3D). ATL prevalence increased in early 2019 and remained high until mass bleaching was observed in October when no ATL was recorded. ATL was then once again observed in February 2020 corresponding with a drop in bleaching prevalence (Figure 8).
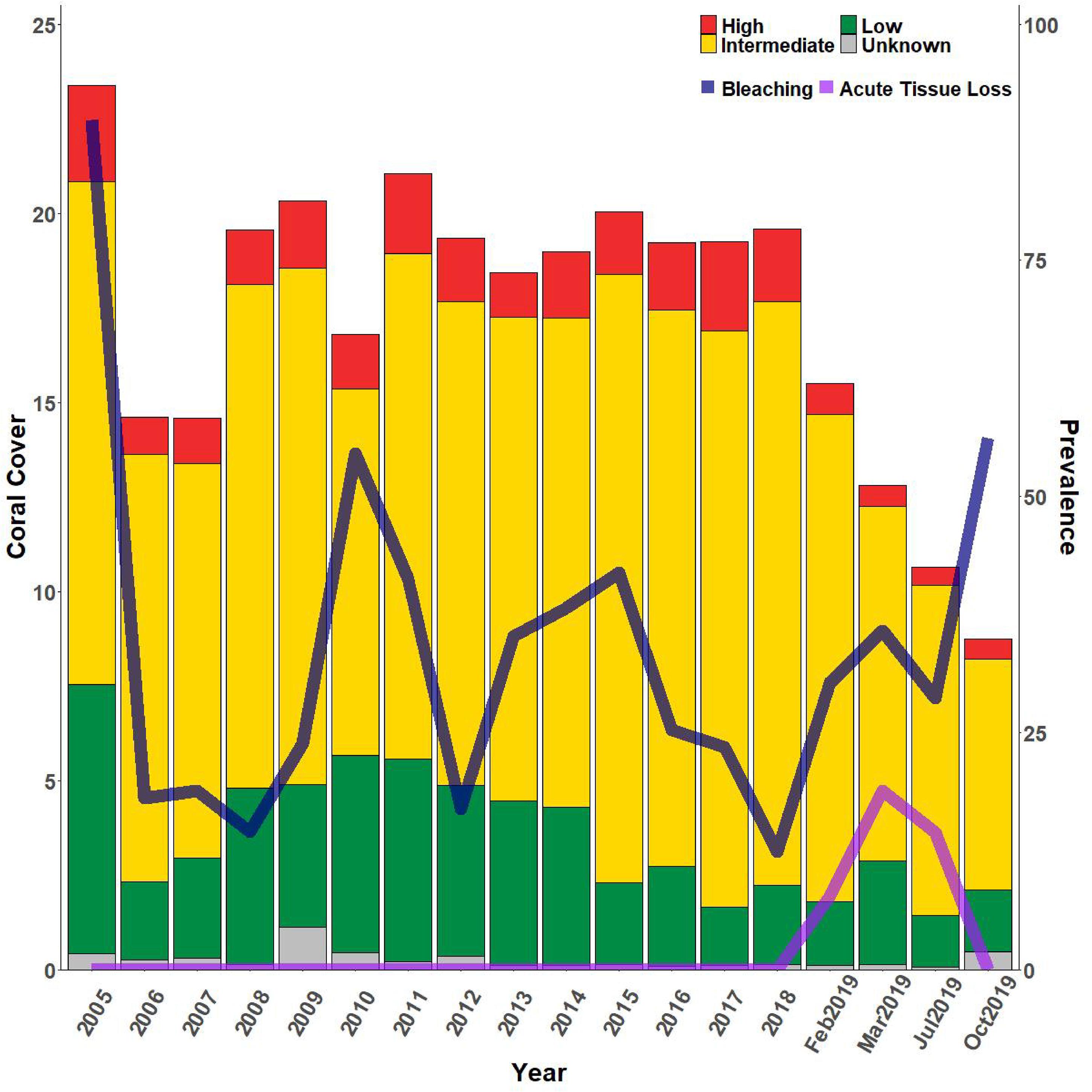
Figure 8. Relative percent coral cover changes at FC reef with bleaching and acute tissue loss disease. Bars indicate % cover of high, intermediate, low, and unknown SCTLD susceptibility corals where classifications of susceptibility are based on the NOAA SCTLD case description. Whole bars represent total coral cover. Lines indicate percent prevalence (# corals affected/total # corals) of acute tissue loss due to disease (light purple line) and bleaching (dark purple line). SCTLD was first observed at FC on January 29, 2019.
Discussion
The spread of SCTLD around the island of St. Thomas and to neighboring islands has been rapid and unrelenting. Previous outbreaks of acute tissue loss diseases in the USVI have in most cases been spatially limited or restricted to one or two affected species (Gladfelter, 1982; Grober-Dunsmore et al., 2006; Brandt et al., 2013). An exception to this was in 2005–2006, when thermal stress-driven mass coral bleaching in the fall of 2005 was followed by outbreaks of white plague disease on reefs throughout the USVI (Smith et al., 2013). This outbreak affected the majority of coral species (Whelan et al., 2007; Miller et al., 2009; Smith et al., 2013) and reef habitats (Smith et al., 2016b), and was also observed to impact other Caribbean locations (Eakin et al., 2010). However, all of these past disease outbreaks had a definitive end, typically coinciding with a change in environmental conditions, and subsequent years saw low prevalence of disease (Smith et al., 2013). In contrast, during this study SCTLD continued to emerge on new reefs over the one-year time frame, which included a change in seasons. SCTLD has continued to emerge on new reefs throughout the USVI even after the time frame of this study2.
Stony coral tissue loss disease in the USVI also appears to be spreading from its first point of detection at FC in a linear pattern from reef to reef through time. This type of spread is similar to what has been observed in Florida, where the temporal and spatial pattern of disease incidence on reefs indicated waterborne transmission along oceanic currents (Dobbelaere et al., 2020; Muller et al., 2020). Waterborne transmission of SCTLD has been demonstrated in laboratory settings (Aeby et al., 2019), including for SCTLD in the USVI (Meiling et al., 2021). The spread of SCTLD both within reefs and among reefs differs from observations of other acute tissue loss diseases, like white plague. Though white plague has been demonstrated to be transmissible through water in laboratory experiments (Williams et al., 2020; MacKnight et al., 2021) and its distribution within a reef shows significant clumping, there is limited evidence for it spreading from reef to reef (Muller and van Woesik, 2012). White plague has also been associated with environmental drivers including thermal stress and fragmentation (Brandt et al., 2013), rainfall and turbidity (Chaves-Fonnegra et al., 2021), and changes in macroalgal cover (Nugues et al., 2004; Brandt et al., 2012). SCTLD on the other hand, was not significantly associated with common environmental drivers of coral disease such as temperature and chlorophyll (Muller et al., 2020). A lack of relationship with environmental drivers, and its unrelenting spread suggests that SCTLD is novel to the coral reef habitats where it is emerging, including reefs of the USVI.
The majority of coral reef ecosystems that SCLTD has been found to affect have been located in shallow waters (<30 m). This may be due to the geomorphology of the regions where it has appeared combined with SCUBA diving limits. Therefore, a unique aspect of our study are the observations of SCTLD from multiple mesophotic coral reef sites, including a long-term monitoring location (College Shoal) and other sites surveyed in the sentinel monitoring component of this study (Supplementary Figure 1). These observations indicate that SCTLD is capable of affecting corals at depths up to at least 40 m. Mesophotic coral reef habitats have been proposed as possible refugia from many stressors, including thermal stress (Riegl and Piller, 2003) and anthropogenic impacts from coastal development (Smith et al., 2008). Mesophotic reef habitats in the USVI are extensive, encompassing a greater area than shallow reefs, and they share common species compositions with shallow reefs (Smith et al., 2016a, 2019). This study suggests that upper mesophotic reef habitats in the USVI do not represent a refuge from SCTLD. Therefore SCTLD has the potential to impose considerable damage on these reefs that represent a vast area of living coral and are habitat to essential fisheries. However, lower mesophotic habitats at depths below 65 have not been extensively surveyed. One 65 m depth Agaricia undata monitoring site of the TCRMP immediately below an affected reef at 30–40 m did not show disease signs on susceptible M. cavernosa as of April 2021 (T. Smith, unpublished observation). A further examination of the refuge potential of lower mesophotic reefs would be valuable.
Our data indicate that species susceptibilities in the USVI show similarities with that reported for Mexico (Alvarez-Filip et al., 2019) and Florida (SCTLD Case Definition, 2018; Walton et al., 2018). For example, based on observed high (>10%) disease prevalence levels, we found that C. natans, E. fastigiata, M. cavernosa, Orbicella spp., and P. strigosa were all highly susceptible. Other species that were highly affected but more variable in disease prevalence were D. cylindrus (Figure 3C) and M. meandrites (Supplementary Figure 4A). Observations across affected areas by the authors have shown numerous recently dead colonies of these species that were likely killed by SCTLD and finding living colonies, even where they were formerly abundant, has become rare. A species group showing low prevalence of disease was Siderastrea spp. However, SCTLD may be under-reported for this group as other studies have suggested Siderastrea spp. may present variable signs other than acute tissue loss, including dark patches (Walton et al., 2018). Species previously reported as unaffected or data deficient that were found to be affected by SCTLD in our data included species of the genera Agaricia (Figure 3J), Madracis (Figure 3H), and Mycetophyllia (Supplementary Figure 4B). Interestingly, poritids have been considered not susceptible to SCTLD in previous reports (Walton et al., 2018; Muller et al., 2020), but we observed multi-focal lesions on colonies of P. astreoides at affected sites (Figure 3K and Supplementary Figure 5), albeit at low levels. Some of our other work has suggested that these lesions may be SCTLD-related but often stop progressing (Meiling et al., 2020). Lesions were also observed on colonies of D. labyrinthiformis at monitoring locations (Supplementary Figure 4C), but this species was not recorded in monitoring transects due to its low abundance within habitats assessed by TCRMP.
The species groups that are most frequently affected by SCTLD make this disease distinct from other acute tissue loss diseases in the USVI region. In particular, Orbicella spp. have previously been identified as the most susceptible species group to white plague disease (Calnan et al., 2008; Brandt et al., 2013; Smith et al., 2013) as well as to an acute tissue loss syndrome affecting mesophotic corals (Smith et al., 2010). In addition, in two independent laboratory transmission experiments where multiple species were exposed to white plague disease, Orbicella spp. showed the highest prevalence and shortest times to infection when compared with the other species (Williams et al., 2020; MacKnight et al., 2021). In this study, Orbicella spp. showed high prevalence, but not the highest. Once affected, however, populations of this species group tended to show sustained prevalence. This may be because Orbicella spp. exhibit lower lesion progression rates (Meiling et al., 2020) and when combined with their large size distributions (Ennis et al., 2019), this results in disease activity that persists on colonies for a longer period of time. Other species, such as M. meandrites, that are on the smaller end of coral colony size distributions and show faster progression rates may “wink out” quickly.
Another key difference between SCTLD and white plague is the prevalence of SCTLD on M. cavernosa. This species is rarely affected by white plague in field studies (Calnan et al., 2008; Smith et al., 2013), and when challenged with the disease for 8 days showed no signs of lesions, while six other coral species exhibited lesions after 2–3 days (MacKnight et al., 2021). The resistance of this species to white plague and other diseases may be due to its immune activity which was found to be comparable to Porites species that tend to be disease resistant (Mydlarz and Palmer, 2011). M. cavernosa also showed less oxidative stress when compared with the Orbicella spp. (Mydlarz and Palmer, 2011). Therefore, the high prevalence of SCTLD on M. cavernosa may not be immunity-linked but may be the result of other physiological or ecological factors having to do with the coral host or pathogen virulence factors interacting with the host. Understanding why M. cavernosa shows high prevalence of SCTLD but no other common coral diseases may help in the identification of key characteristics of SCTLD transmission and virulence.
When compared with previous events producing losses in coral cover, SCTLD presents a unique and catastrophic threat. Coral bleaching events and subsequent disease outbreaks tend to be temporally restricted to several months or up to a year. Other events that can cause significant losses to coral cover include acute events like tropical storms or chronic stressors such as the continued development of coast lines. Tropical storms large enough to cause losses in coral cover are periodic and also tend to be spatially limited. In the USVI, the unprecedented passage of two category 5 hurricanes (Irma and Maria) within 2 weeks of each other in 2017 resulted in impacts to coral cover that were essentially limited to sites shallower than 10 m (Ennis et al., 2019). Coastal development, resulting in runoff of damaging sediments, has also caused declines in coral cover in the region (Nemeth and Sladek Nowlis, 2001) and coastal activities that affect water quality have led to chronic mortality on corals (Ennis et al., 2016). However, the impact of these activities also tends to be spatially limited to nearshore areas and there is a gradient of water quality and impact with distance from shore (Smith et al., 2008; Ennis et al., 2016), allowing for some coral reefs to remain un-impacted. Disturbingly, SCTLD has yet to show signs of “burn out” in affected regions, like other infectious coral diseases (Brandt et al., 2012), and it has spread throughout the entire range of the northern USVI, and most recently (September 2020) emerged on St. Croix (see text footnote 2). No temporal limitations or seasonal cycles in SCTLD have been observed to date; even the first sites in Florida where the disease emerged in 2014 continue to exhibit new cases (Muller et al., 2020).
Stony coral tissue loss disease is currently decimating what remains of many Caribbean coral reef ecosystems, which had already experienced significant declines in coral cover over the past 4 decades due to anthropogenic stressors (Gardner et al., 2003). In Florida, between 2014 and 2017, significant decreases in species diversity have occurred, as well as ∼50% decline in coral density (up to 95% decline in high susceptibility species), and ∼60% reduction in living coral tissue (Walton et al., 2018). If continued unabated, it is likely that SCTLD will have similar impacts in the USVI and surrounding regions. In a region where the economic and ecological benefits of living coral are so critical (Storlazzi et al., 2019), the level of coral loss caused by SCTLD is alarming and warrants immediate response.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://sites.google.com/site/usvitcrmp/available-data?authuser=0.
Author Contributions
MB, RE, and TS were involved with experimental design. MB, RE, SM, JT, KC, AG, JQ, VB, LH, and TS contributed to data collection. MB, RE, SM, and TS completed the statistical analyses. MB wrote the manuscript. RE, SM, JT, KC, AG, JQ, VB, LH, and TS assisted with editing the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Science Foundation (Biological Oceanography) award # 1928753 to MB and TS, the NOAA Coral Reef Conservation Program and USVI Department of Planning and Natural Resources funded Territorial Coral Reef Monitoring Program to TS (NA17NOS4820033), and VI EPSCoR (NSF #0814417). The writing of this manuscript was supported by the Expanding the Network of STEM Women of Color 2020 and 2021 Virtual Summer Writing Groups. Support for LH was provided by CSS, Inc. under NOAA contract 1305M218FNCNP0157.
Conflict of Interest
LH was employed by company CSS Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This work would not have been possible except for the hard work of the staff and students collecting data for the Territorial Coral Reef Monitoring Program. The Virgin Islands Coral Disease Advisory Committee was also essential in assisting with documenting the spatial spread of the disease. This is contribution #240 from the Center for Marine and Environmental Studies.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.715329/full#supplementary-material
Footnotes
References
Aeby, G. S., Ushijima, B., Campbell, J. E., Jones, S., Williams, G. J., Meyer, J. L., et al. (2019). Pathogenesis of a tissue loss disease affecting multiple species of corals along the Florida reef tract. Front. Mar. Sci. 6:678. doi: 10.3389/fmars.2019.00678
Alvarez-Filip, L., Estrada-Saldívar, N., Pérez-Cervantes, E., Molina-Hernández, A., and González-Barrios, F. J. (2019). A rapid spread of the stony coral tissue loss disease outbreak in the Mexican Caribbean. PeerJ 7:e8069. doi: 10.7717/peerj.8069
Baddeley, A., Rubak, E., and Turner, R. (2015). SpatialPoint Patterns: Methodology and Applications with R. London: CRC Press.
Barthelme, S. (2020). imager: Image Processing Library Based on “CImg”. R Package Version 0.42.1. Available online at: https://CRAN.R-project.org/package=imager (accessed January 20, 2021).
Brandt, M. E., Ruttenberg, B. I., Waara, R., Miller, J., Witcher, B., Estep, A. J., et al. (2012). Dynamics of an acute coral disease outbreak associated with the macroalgae Dictyota spp. in Dry Tortugas National Park, Florida, USA. Bull. Mar. Sci. 88, 1035–1050. doi: 10.5343/bms.2011.1104
Brandt, M. E., Smith, T. B., Correa, A. M. S., and Vega-Thurber, R. (2013). Disturbance driven coral fragmentation as a driver of a coral disease outbreak. PLoS One 8:e57164. doi: 10.1371/journal.pone.0057164
Calnan, J. M., Smith, T. B., Nemeth, R. S., Kadison, E., and Blondeau, J. (2008). Coral disease prevalence and host susceptibility on mid-depth and deep reefs in the United States Virgin Islands. Rev. Biol. Trop. 56(Suppl. 1), 223–234.
Chaves-Fonnegra, A., Panassiti, B., Smith, T. B., Brown, E., Clemens, E., Sevier, M., et al. (2021). Environmental and biological drivers of white plague disease on shallow and mesophotic coral reefs. Ecography 44, 1–15. doi: 10.1111/ecog.05527
Dobbelaere, T., Muller, E., Grameret, L. J., Holstein, D. M., and Hanert, E. (2020). Coupled epidemio-hydrodynamic modeling to understand the spread of a deadly coral disease in Florida. Front. Mar. Sci. 7:591881. doi: 10.3389/fmars.2020.591881
Eakin, C. M., Morgan, J. A., Heron, S. F., Smith, T. B., Liu, G., Alvarez-Filip, L., et al. (2010). Caribbean corals in crisis: record thermal stress, bleaching, and mortality in 2005. PLoS One 5:e13969. doi: 10.1371/journal.pone.0013969
Ennis, R. S., Brandt, M. E., Wilson Grimes, K. R., and Smith, T. B. (2016). Coral reef health response to chronic and acute changes in water quality in St. Thomas, United States Virgin Islands. Mar. Pollut. Bull. 111, 418–427. doi: 10.1016/j.marpolbul.2016.07.033
Ennis, R. S., Kadison, K., Heidmann, S., Brandt, M. E., Henderson, L., and Smith, T. B. (2019). The United States Virgin Islands Territorial Coral Reef Monitoring Program. 2019 Annual Report. St Thomas, USVI: University of the Virgin Islands, 295. doi: 10.13140/RG.2.2.21492.07047
Gardner, T., Cote, I., Gill, J., Grant, A., and Watkinson, A. (2003). Long-term region-wide declines in Caribbean corals. Science 301, 958–960. doi: 10.1126/science.1086050
Gladfelter, W. B. (1982). White-band disease in Acropora palmata: implications for the structure and growth of shallow reefs. Bull. Mar. Sci. 32, 639–643.
Grober-Dunsmore, R., Bonito, V., and Frazer, T. K. (2006). Potential inhibitors to recovery of Acropora palmata populations in St. John, US Virgin Islands. Mar. Ecol. Prog. Ser. 321, 123–132. doi: 10.3354/meps321123
Kohler, K., and Gill, S. M. (2006). Coral point count with excel extensions (CPCe): a visual basic program for the determination of coral and substrate coverage using random point count methodology. Comput. Geosci. 32, 1259–1269. doi: 10.1016/j.cageo.2005.11.009
MacKnight, N., Cobleigh, K., Lasseigne, D., ChavesFonnegra, A., Gutting, A., Dimos, B., et al. (2021). Microbial dysbiosis reflects disease resistance in diverse coral species. Commun. Biol. 4:679.
Meiling, S., Smith, T. B., Muller, E., and Brandt, M. E. (2020). 3D photogrammetry reveals dynamics of stony coral tissue loss disease (SCTLD) lesion progression across a thermal stress event. Front. Mar. Sci. 7:597643. doi: 10.3389/fmars.2020.597643
Meiling, S. S., Muller, E. M., Lassiegne, D. L., Rossin, A., Veglia, A. J., MacKnight, N., et al. (2021). Variable species responses to experimental stony coral tissue loss disease (SCTLD) exposure. Front. Mar. Sci. 8:670829. doi: 10.3389/fmars.2021.670829
Meyer, J. L., Castellanos-Gell, J., Aeby, G. S., Häse, C. C., Ushijima, B., and Paul, V. J. (2019). Microbial community shifts associated with the ongoing stony coral tissue loss disease outbreak on the Florida reef tract. Front. Microbiol. 10:2244. doi: 10.3389/fmicb.2019.02244
Miller, J., Muller, E. M., Rogers, C., Waara, R., Atkinson, A., Whelan, K., et al. (2009). Coral disease following massive bleaching in 2005 causes 60% decline in coral cover on reefs in the US Virgin Islands. Coral Reefs 28, 925–937. doi: 10.1007/s00338-009-0531-7
Muller, E. M., Sartor, C., Alcaraz, N. I., and van Woesik, R. (2020). Spatial epidemiology of the stony-coral-tissue-loss disease in Florida. Front. Mar. Sci. 7:163. doi: 10.3389/fmars.2020.00163
Muller, E. M., and van Woesik, R. (2012). Caribbean coral diseases: primary transmission or secondary infection? Glob. Chang. Biol. 18, 3529–3535. doi: 10.1111/gcb.12019
Mydlarz, L. D., and Palmer, C. (2011). The presence of multiple phenoloxidases in Caribbean reef-building corals. Comp. Biochem. Physiol. Part A 159, 372–378. doi: 10.1016/j.cbpa.2011.03.029
Neely, K. L., Macaulay, K. A., Hower, E. K., and Dobler, M. A. (2020). Effectiveness of topical antibiotics in treating corals affected by Stony Coral Tissue Loss Disease. PeerJ 8:e9289. doi: 10.7717/peerj.9289
Nemeth, R. S., and Sladek Nowlis, J. (2001). Monitoring the effects of land development on the near-shore reef environment of St. Thomas, USVI. Bull. Mar. Sci. 69, 759–775.
Nugues, M. M., Smith, G., van Hooidonk, R., and Seabra, M. I. (2004). Algal contact as a trigger for coral disease. Ecol. Lett. 7, 919–923. doi: 10.1111/j.1461-0248.2004.00651.x
Precht, W. F., Gintert, B. E., Robbart, M. L., Fura, R., and van Woesik, R. (2016). Unprecedented disease-related coral mortality in southeastern Florida. Sci. Rep. 6:31374.
Riegl, B., and Piller, W. E. (2003). Possible refugia for reefs in times of environmental stress. Int. J. Earth Sci. 92, 520–531. doi: 10.1007/s00531-003-0328-9
Rosales, S. M., Clark, A. S., Huebner, L. K., Ruzicka, R. R., and Muller, E. M. (2020). Rhodobacterales and Rhizobiales are associated with stony coral tissue loss disease and its suspected sources of transmission. Front. Microbiol. 11:681. doi: 10.3389/fmicb.2020.00681
SCTLD Case Definition (2018). Florida Coral Disease Response Research & Epidemiology Team. Available online at: https://floridadep.gov/sites/default/files/Copy%20of%20StonyCoralTissueLossDisease_CaseDefinition%20final%2010022018.pdf (accessed October 6, 2020).
Smith, T. B., Blondeau, J., Nemeth, R. S., Pittman, S. J., Calnan, J. M., Kadison, E., et al. (2010). Benthic structure and cryptic mortality in a Caribbean mesophotic coral reef bank system, the Hind Bank Marine Conservation District, U. S. Virgin Islands. Coral Reefs 29, 289–308. doi: 10.1007/s00338-009-0575-8
Smith, T. B., Brandt, M. E., Brandtneris, V., Ennis, R. S., Groves, S. H., Habtes, S., et al. (2019). “The United States Virgin Islands,” in Mesophotic Coral Ecosystems, Coral Reefs of the World, Vol. 12, eds Y. Loya, K. A. Puglise, and T. C. Bridge (Cham: Springer Nature), 131–147.
Smith, T. B., Brandt, M. E., Calnan, J. M., Nemeth, R. S., Blondeau, J., Kadison, E., et al. (2013). Convergent mortality responses of Caribbean coral species to seawater warming. Ecosphere 47:87.
Smith, T. B., Brandtneris, V., Canals, M., Brandt, M. E., Martens, J., Brewer, R. S., et al. (2016a). Potential structuring forces on a shelf edge upper mesophotic coral ecosystem in the US Virgin Islands. Front. Mar. Sci. 3:115. doi: 10.3389/fmars.2016.00115
Smith, T. B., Gyory, J., Brandt, M. E., Millier, W. J., Jossart, J., and Nemeth, R. S. (2016b). Caribbean mesophotic coral ecosystems are unlikely climate change refugia. Glob. Chang. Biol. 22, 2756–2765. doi: 10.1111/gcb.13175
Smith, T. B., Nemeth, R. S., Blondeau, J., Calnan, J. M., Kadison, E., and Herzlieb, S. (2008). Assessing coral reef health across onshore to offshore stress gradients in the US Virgin Islands. Mar. Pollut. Bull. 56, 1983–1991. doi: 10.1016/j.marpolbul.2008.08.015
Storlazzi, C. D., Reguero, B. G., Cole, A. D., Lowe, E., Shope, J. B., Gibbs, A. E., et al. (2019). Rigorously Valuing the Role of U.S. Coral Reefs in Coastal Hazard Risk Reduction: U.S. Geological Survey Open-File Report 2019–1027. Reston, VA: United States Geological Survey, 42. doi: 10.3133/ofr20191027
Sutherland, K. P., Porter, J. W., and Torres, C. (2004). Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Mar. Ecol. Prog. Ser. 266, 273–302. doi: 10.3354/meps266273
Walton, C. J., Hayes, N. K., and Gilliam, D. S. (2018). Impacts of a regional, multi-year, multi-species coral disease outbreak in southeast Florida. Front. Mar. Sci. 5:323. doi: 10.3389/fmars.2018.00323
Whelan, K. R. T., Miller, J., Sanchez, O., and Patterson, M. (2007). Impact of the 2005 coral bleaching event on Porites porites and Colpophyllia natans at Tektite Reef, US Virgin Islands. Coral Reefs 26, 689–693. doi: 10.1007/s00338-007-0241-y
Keywords: stony coral tissue loss disease, coral disease, susceptibility, Caribbean, coral reefs
Citation: Brandt ME, Ennis RS, Meiling SS, Townsend J, Cobleigh K, Glahn A, Quetel J, Brandtneris V, Henderson LM and Smith TB (2021) The Emergence and Initial Impact of Stony Coral Tissue Loss Disease (SCTLD) in the United States Virgin Islands. Front. Mar. Sci. 8:715329. doi: 10.3389/fmars.2021.715329
Received: 26 May 2021; Accepted: 20 July 2021;
Published: 18 August 2021.
Edited by:
Aldo Cróquer, The Nature Conservancy, Dominican RepublicReviewed by:
Rosa Elisa Rodríguez-Martínez, National Autonomous University of Mexico, MexicoLaurie Raymundo, University of Guam, Guam
Copyright © 2021 Brandt, Ennis, Meiling, Townsend, Cobleigh, Glahn, Quetel, Brandtneris, Henderson and Smith. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marilyn E. Brandt, bWJyYW5kdEB1dmkuZWR1
 Marilyn E. Brandt
Marilyn E. Brandt Rosmin S. Ennis
Rosmin S. Ennis Sonora S. Meiling
Sonora S. Meiling Joseph Townsend
Joseph Townsend Kathryn Cobleigh
Kathryn Cobleigh Adam Glahn1
Adam Glahn1 Viktor Brandtneris
Viktor Brandtneris Tyler B. Smith
Tyler B. Smith