- 1Fisheries and Oceans Canada, Freshwater Institute, Winnipeg, MB, Canada
- 2Higdon Wildlife Consulting, Winnipeg, MB, Canada
- 3Greenland Institute of Natural Resources, Nuuk, Greenland
- 4Fisheries and Oceans Canada, Pacific Biological Station, Nanaimo, BC, Canada
Bowhead whales (Balaena mysticetus L., 1758) of the Eastern Canada-West Greenland population have been hunted by Inuit for millennia. Significant commercial harvests, conducted by European and American whalers for about 400 years, ended ca. 1915. A small co-managed subsistence harvest from this population has occurred inconsistently in Canada and Greenland, since 1996 and 2009, respectively. Since near extirpation from commercial whaling, population size has increased and the Inuit subsistence hunt now requires a harvest management framework that incorporates knowledge of abundance trends, population dynamics, and carrying capacity. Here, we use a model estimate of pre-commercial exploitation abundance to approximate carrying capacity and develop a management framework with reference points and corresponding stock status zones. When applied to recent abundance estimates, our framework indicates that the population is likely within the healthy (N50–N70) zone. Thus, an appropriate management objective is to support continued population increase, with concurrent marginal harvesting, while maintaining the population level above the target reference point (N70) of ca 12,000 whales. However, there remains large uncertainty about current population size and growth rate. The resulting data gaps require a plan for future research to monitor this population in the context of climate changes.
Introduction
Sound wildlife management is based on clearly defined objectives and prioritized actions that can incorporate new stock assessment information as it becomes available. For example, biological reference points can inform management objectives and in turn guide short-term management decisions (Punt, 2006; Curtis et al., 2015). International agreements, e.g., the “Rio Declaration,” the “Cancun Summit,” and the “United Nations Agreement on Straddling Fish Stocks and Highly Migratory Fish Stocks,” have established that the Precautionary Approach (PA) should be applied to management of the environment and of fisheries (Hilborn et al., 2006). A number of government agencies, including Fisheries and Oceans Canada (DFO), have adopted PA policies including an ecosystem-based approach to fisheries management. The North Atlantic Marine Mammal Commission (NAMMCO) and the International Whaling Commission (IWC) have similarly worked to develop international cooperation on conservation and management of cetaceans (Williams et al., 2014).
Implementation of the internationally accepted PA concept requires the adoption of corrective measures when there is a significant risk of damage to a population, even when this risk cannot be scientifically proven. The PA requires countries to cooperate by preventing environmental deterioration while considering scientific uncertainty. For example, the impact of climate change on population persistence has to be taken into consideration as it has important implications for conservation and management (Simmonds and Isaac, 2007; IWC, 2009a; MacLeod, 2009; Lambert et al., 2014). The PA is a tool to deal with uncertainty, and requires international cooperation as well as the implementation of mechanisms that guarantee efforts aimed at protecting the species from possible threats (González-Laxe, 2005). The development of a harvest management framework is of importance to whale management in Canada where domestic legislation (e.g., Fisheries Act, Oceans Act, and Species at Risk Act) outlines the use of a PA framework with conservation reference limits (Hammill and Stenson, 2007). This approach was developed recognizing that fishery management requires clear decision-making rules to guide managers under cases of scientific uncertainty (Butterworth and Punt, 1999). There is a need to apply this formal approach more broadly so that managers can make decisions based on multiple objectives for marine mammal species (Stenson et al., 2012).
Bowhead whales (Balaena mysticetus L., 1758) are characterized by a long life (>200 years), late sexual maturity (ca 25 years), and long inter-birth interval (ca 5 years), combining to result in an overall low fecundity (George et al., 1999, 2021c). These particular life-history traits are adaptations to high latitude environments and thus make this species more vulnerable to the effects of a warming planet (Ferguson and Higdon, 2013). In addition, bowhead whales now contend with increasing killer whale (Orcinus orca) predation (Higdon and Ferguson, 2009) and the impacts of human activities (e.g., shipping, mining, oil and gas field exploitation), especially those occurring near or within feeding or nursery areas (COSEWIC, 2009; Dueck and Ferguson, 2009). The Eastern Canada-West Greenland (ECWG) bowhead population is distributed from the Canadian Arctic Archipelago to the west Greenland coast (Figure 1; NWMB, 2000; Heide-Jørgensen et al., 2003; Chambault et al., 2018). Within this range, individuals travel extensively while following the seasonal growth and ablation of sea ice (Eschricht and Reinhardt, 1866; Reeves et al., 1983; Ferguson et al., 2010; Nielsen et al., 2015). During the late summer and late autumn periods, feeding is concentrated on large Arctic copepods most commonly along the outlets of fiords of eastern Baffin Island (Richardson et al., 1995; Chambault et al., 2018; Fortune et al., 2020b).
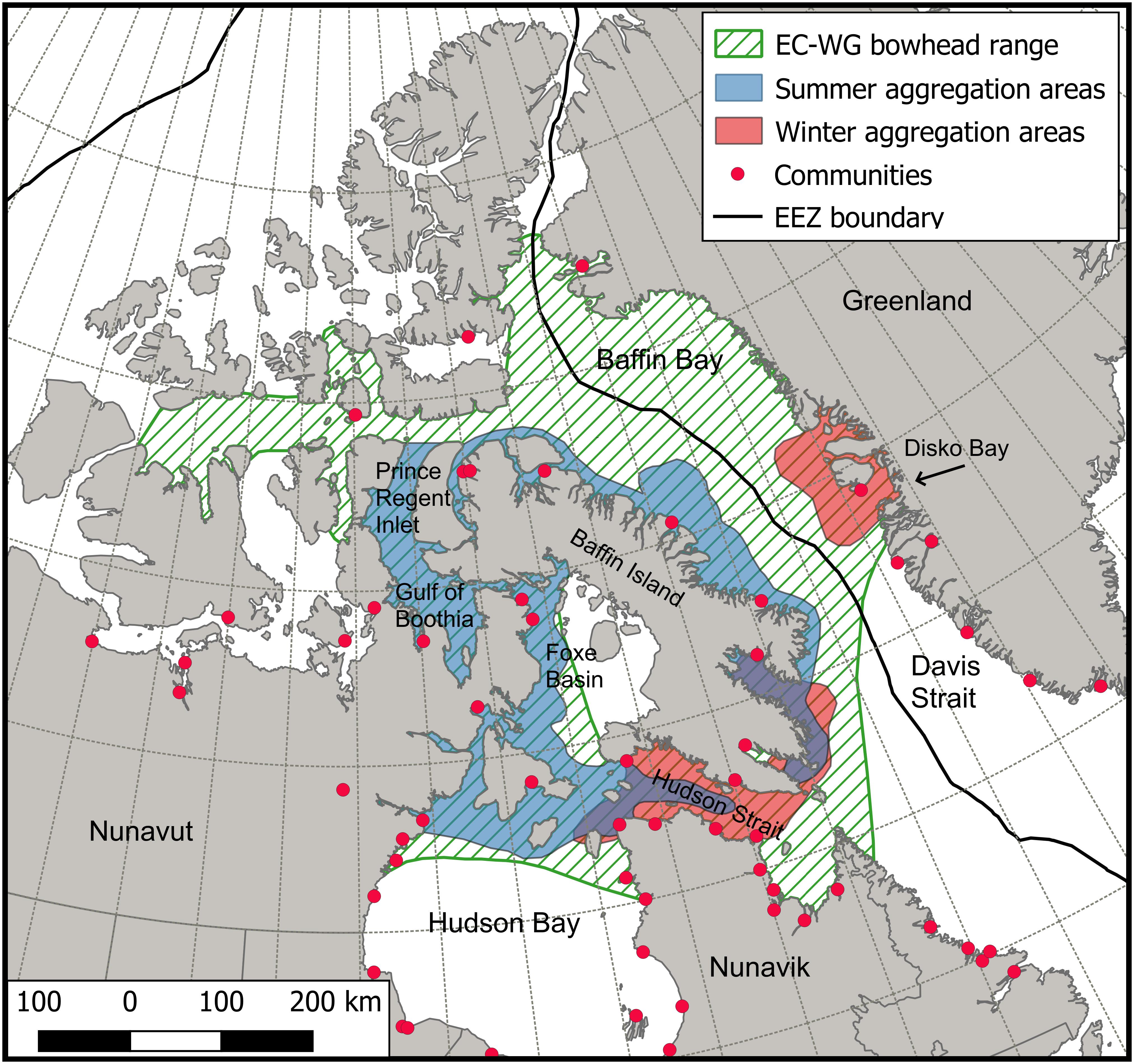
Figure 1. Approximate geographic range of the Eastern Canada-West Greenland bowhead whale population, with important aggregation areas during the open-water (“summer”) and ice-covered (“winter”) seasons. Community locations are shown, and Table 2 indicates those communities (and their locations) where bowhead whale subsistence hunts have occurred. Geographic range and summer and winter aggregation areas modified from NWMB (2000), COSEWIC (2009), Heide-Jørgensen et al. (2021), DFO, unpubl. data, and GINR, unubl. data.
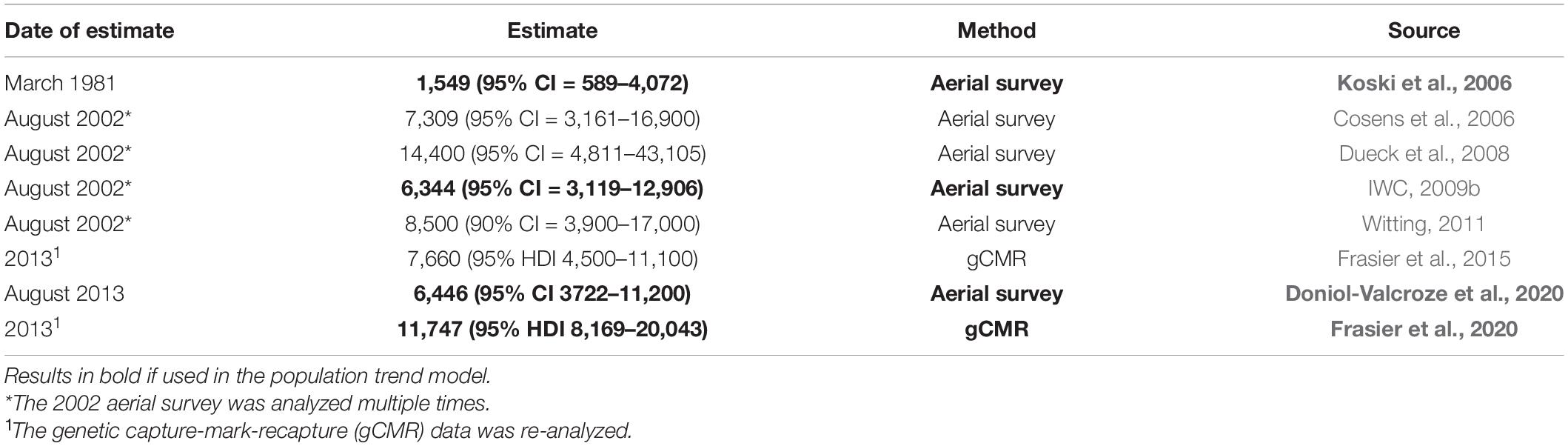
Table 1. Abundance estimates of the ECWG bowhead whale stock using data that covered most of the range of the stock.
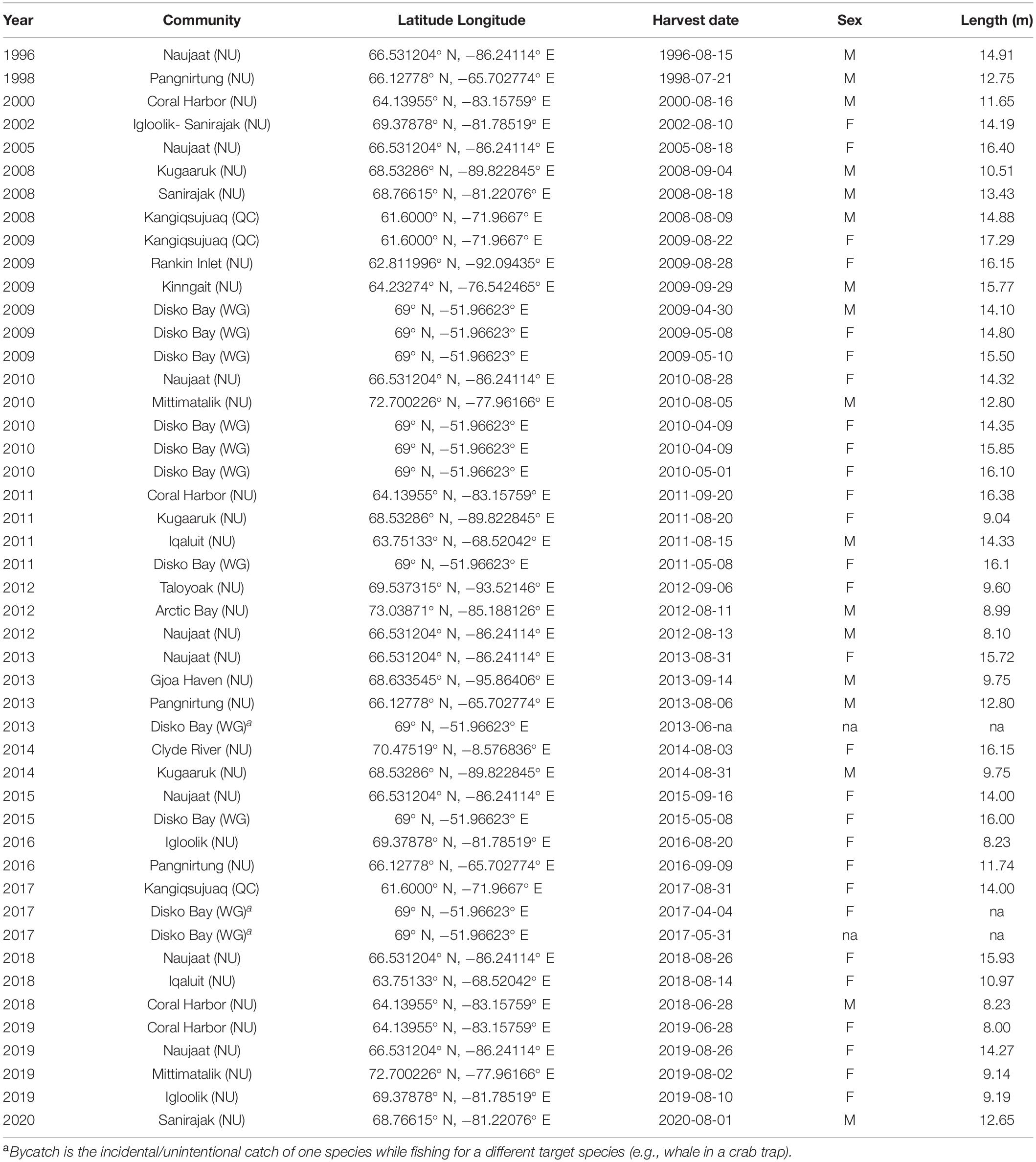
Table 2. Inuit subsistence harvests of ECWG bowhead whales in Nunavut (NU), Nunavik (QC), and West Greenland (WG) since 1996.
Indigenous bowhead whale hunting in Greenland and eastern Canada dates as far back as 4000 years, but at low levels. Higdon (2010) estimated that Inuit harvested 11,435 whales between 1200 and 1529 AD (an average of 36/year between 1200 and 1400 AD period of maximum bowhead whale utilization), based on archeological data from winter houses. While there is some evidence that at least parts of the Thule/Inuit harvest series are underestimated (e.g., see Savelle, 2010 for additional archeological data that was not available to Higdon, 2010), Indigenous whaling occurred at sustainable levels compared to later overharvesting by commercial whalers (Higdon, 2010; Seersholm et al., 2016).
Small, regulated subsistence harvests of ECWG Bowhead whales by Nunavut Inuit resumed in 1996, and by Inuit of Nunavik (N Quebec) in 2008. In Greenland, government-authorized Inuit subsistence hunts resumed in 2009 (Heide-Jørgensen et al., 2012). In each country, subsistence bowhead hunts must be licensed by the corresponding regulatory agency (i.e., Fisheries and Ocean Canada, Greenland Self-Government).
Greenland is a party to the International Whaling Commission (IWC), and the domestic ECWG bowhead fishery is managed by the Greenland Institute of Natural Resources, using sustainable catch advice from the IWC Aboriginal Subsistence Whaling management scheme.1 Canada is not a party to the IWC, and has implemented a wildlife co-management framework in areas where Indigenous Land Claims Agreements have been settled. The seasonal range of the ECWG bowhead population encompasses the Nunavut Settlement Area and the Nunavik Marine Region. The Nunavut Agreement and the Nunavik Inuit Land Claims Agreement each include a formal process by which wildlife management decisions are agreed between Inuit and Government. Fisheries and Oceans Canada is responsible for marine fisheries, and departmental scientists have used the Potential Biological Removal method (PBR: Wade, 1998) to calculate sustainable harvest advice for consideration by the co-management organizations.
The majority of the seasonal range of the ECWG population occurs in Canadian waters, including overwintering habitat (Hudson Strait), calf rearing areas (Foxe Basin and Gulf of Boothia), and the region of maximum feeding in autumn (east coast of Baffin Island) (Figure 1). In Greenland, an aggregation of predominantly feeding females gather in Disko Bay during spring, presumably to mate and regain fat deposits for the next calving period (Rekdal et al., 2015; Heide-Jørgensen et al., 2021). Changes to habitat, prey availability, and increased natural mortality may lead to changes in bowhead abundance, distribution and stock structure (Laidre et al., 2008; Pomerleau et al., 2011). Loss of Arctic sea ice will likely result in increased marine vessel activity (Hauser et al., 2018), associated with tourism and industrial development (e.g., commercial fishing, mining and hydrocarbon exploration; Halliday et al., 2018). Increased vessel traffic may cause acoustic disturbance to bowhead whales, negatively impact critical habitat areas, and increase frequency of vessel strikes in bowhead migration corridors (e.g., Hudson Strait, Lancaster Sound, Davis Strait, Baffin Bay). Bowhead whales are specialized filter feeders, and may be at physiological risk if baleen plates are fouled by fuel oil or become entangled in nets or debris (Lambertson et al., 2005). Also, the warming of the Arctic is expected to favor smaller and leaner zooplankton species from more temperate waters, thus changing the food web which may result in bowhead whales, as zooplankton specialists, having to cope with changes in food quality and availability (Pomerleau et al., 2012; Fortune et al., 2020a).
The ECWG bowhead whale population has increased considerably since its near extirpation (Doniol-Valcroze et al., 2020; Rekdal et al., 2015), and now requires a harvest management framework that provides subsistence value to Inuit communities. Using the 2013 aerial survey estimate of 6,446 whales (Doniol-Valcroze et al., 2020) and a recovery factor of 0.5 in the PBR formulation, calculates that the ECWG bowhead whale population can support a total human-induced mortality of 52 whales annually from direct losses (harvest) and indirect losses (whales struck and lost, fishing gear entanglements, and vessel strikes) (DFO, 2015).
However, the use of a single abundance estimate to calculate a PBR threshold does not allow all of the available information to be included and constrains the choice of management strategies (Lonergan, 2011). Recognizing that different levels of uncertainty and different management objectives exist among stocks, alternative approaches have been proposed to allow the best use of available information (e.g., Brandon et al., 2017). Here, we apply a Precautionary Approach (PA) framework to the ECWG bowhead whale population that, unlike PBR, incorporates knowledge of abundance trends, population dynamics, and carrying capacity. Using historical catch data, ECWG bowhead population abundance trends were modeled over the past 400+ years to estimate an historical population level of bowhead whales (i.e., pre-commercial exploitation or carrying capacity). From this we develop reference points and corresponding zones in the PA framework that can be incorporated into a formal decision making process for population management in Canada.
Materials and Methods
The stock assessment information available for the ECWG bowhead whale population included: (1) historical harvest levels including an estimate of struck and lost (https://archive.iwc.int/?r=272&k=cde0e1f3a5: Higdon, 2010), (2) three aerial surveys and one genetic mark-recapture analysis provided the four abundance estimates used to assess recent trends (Table 1), and (3) information on fecundity and/or survival, available from the genetically similar Bering-Chukchi-Beaufort bowhead whale population (Nerini et al., 1984). Previously, Indigenous and commercial harvest data were used to estimate a plausible historical population size (Higdon and Ferguson, 2016) that we used to delineate reference levels.
Over the last 4 decades, the different survey approaches used to estimate ECWG bowhead whale abundance have made comparisons difficult and trend assessment problematic (Higdon and Ferguson, 2016). The first aerial survey in March 1981 estimated 1,549 (95% CI = 589–4,529) whales (Koski et al., 2006). The second aerial survey occurred in August 2002 and resulted in a number of estimates due to different analyses but the best estimate considered here was conducted by the IWC (2009b) of 6,344 (95% CI = 3,119–12,906) whales. The most recent aerial survey, conducted in 2013, used distance-sampling and double platform with mark-recapture methods and estimates were corrected for availability bias using analysis of satellite-linked time depth recorders produced a fully corrected abundance estimate of 6,446 (95% CI: 3,838–10,827) (Doniol-Valcroze et al., 2020). However, all aerial surveys are considered to provide conservative estimates because they do not include all of the known range of the population. A Bayesian analyses of genetic capture-mark-recapture using biopsies and samples from hunted whales that took into consideration unsampled locations to infer individuals that were missed in these locations estimated a total population abundance of 11,747 (95% HDI 8,169–20,043) (Frasier et al., 2020).
We used a discrete time, theta-logistic growth model, as used by the IWC and is also the underlying population model in PBR (Wade, 1998):
where:
Pt = total population size during year t,
Rmax = intrinsic rate of increase,
K = carrying capacity, assumed to be equal to abundance before exploitation (i.e., Pt = 0),
γ = density-dependent shape parameter or the size of the population, relative to K, at the maximum sustainable yield level (MSY level is the population abundance that allows for the greatest net annual increment in population numbers with reproduction and/or growth less losses due to natural mortality),
Ct = the recorded catch in terms of numbers of whales during year t, and,
Ω = correction for whales struck and lost (inferred to have subsequently died from their injuries).
We used the catch-history deterministic model for the ECWG bowhead whale population that previously estimated carrying capacity (K) at 18,500 animals as the pre-commercial exploitation population abundance (Higdon and Ferguson, 2016). We estimated variation from 10,000 Monte Carlo simulations of the logistic population growth model and assessed model sensitivity to changing parameter values. Removals were estimated using historical catch data up to 2009 and a struck and lost correction factor that ranged from 1.10 to 1.20 (uniform distribution). Models included a uniform distribution for Rmax that ranged from 0.035 to 0.045. Population growth rate of the ECWG bowhead whale population is unknown. However, the Bering-Chukchi-Beaufort (BCB) population growth has been estimated as 3.4%, the IWC (2013) has endorsed a growth rate of 3.7%, and a theoretical maximum population growth rate for cetaceans has been provided of 4.0% (Wade, 1998). We ran the deterministic model and visually assessed model fit relative to current population abundance estimates from aerial surveys and genetics (i.e., the model was not directly fit to those data).
Direct estimation of the maximum rate of population growth, Rmax, is rarely feasible for large marine mammals. In addition to estimates of survival and fecundity, Rmax requires a long time series of abundance estimates for a growing population recovering from a depleted state. Alternative methods to estimate Rmax include; (1) using default values such as 0.04 for Rmax of most cetaceans under the United States Marine Mammal Protection Act (Wade, 1998), or; (2) using values from other populations of the same species or other species within similar taxa (e.g., Wallace et al., 2011), or; (3) estimating Rmax from vital rate parameters derived indirectly from life history or allometric models (Žydelis et al., 2009). For example, meta-analysis incorporating life-history and evolutionary theory can provide robust estimates of Rmax for data-poor species (Fagan et al., 2013).
We assessed model sensitivity and assumptions relative to the historical population trends and current trajectory. In one example, we assessed the effect of the delay in female bowhead whale age of sexual maturity on the timing of population growth rate. Bowhead whales have the longest delay in age of sexual maturity of all mammal species with a median of about 26 years-of-age (Rosa et al., 2013) and this delay might explain a delay in exponential population growth. We implemented this delay in our surplus production model to account for age at maturity. We recognize that age at maturity is considered a component of Rmax in the Euler-Lotka equation (Hutchings et al., 2012). However, the Euler-Lotka equation assumes a population at equilibrium in terms of age structure and the small number of bowhead whales that survived the peak commercial whaling might represent an unbalanced age structure biased toward younger whales. Commercial whalers very rarely reached into Foxe Basin and only into the northern half of Prince Regent Inlet during the later years of the fishery (Ross, 1979; Reeves et al., 1983), areas that currently support a higher proportion of young whales during the ice-free season (Fortune et al., 2020c).
We also conducted sensitivity testing of the density-dependent shape parameter (γ) to achieve a slower population recovery that was suggested by the recent survey abundance estimates. We used a γ of 1.0 (Maximum Net Productivity at 50% K), 2.39 (60%), and 5.0 (80%) to mimic a maximum sustainable yield level in a range, as often assumed in density-regulated models of baleen whales (Witting, 2013).
Next, we used the population carrying capacity estimate to develop a PA framework that determined the appropriate population levels at which the reference points and corresponding stock status zones should be set (Punt and Smith, 2001; DFO, 2006). The use of a predetermined level of decline in abundance to trigger management action has been recommended in other jurisdictions (e.g., Mace and Gabriel, 1999). The primary components of the generalized framework are:
• The healthy zone lower boundary is set at 50% (N50) of the pre-commercial exploitation population size.
• The cautious zone is bounded by 30% (N30) and 50% (N50) of the pre-commercial exploitation population size, and
• The critical zone is below 30% (N30) of the pre-commercial exploitation population size.
A corresponding Target Reference Point set at 70% (N70), is an approximate abundance target that closely matches the maximum sustainable yield estimates of 60% of carrying capacity commonly used for large whales (Baker and Clapham, 2004). The Removal reference is the maximum acceptable removal rate for the stock and includes all mortality from all types of harvesting. The target reference point (N70) identifies a level within the healthy zone below which risk-averse management control rules should apply. When population abundance exceeds the target reference point (N70), management goals based on other considerations, such as ecosystem impacts and/or socio-economic benefits, can be considered. The N50 reference point (50%) identifies an “unsafe” population status range (cautious zone) when a hunt is subjected to limited harvesting. Lastly, the limit reference point (N30; 30%) represents the estimated abundance at which continued anthropogenic removals will cause serious and irreversible harm to the population. This approach provides clearly defined benchmarks that link to magnitudes of changed abundance from a population level at carrying capacity (NK). Last, we used the deterministic model estimate (10,968 whales; Higdon and Ferguson, 2016) and the most recent genetic abundance estimate (11,747 whales; Frasier et al., 2020) to determine where the current ECWG population lies with the PA framework; thereby recommending management advice for the population.
Results
Current removals included all sources of anthropogenic mortality (e.g., harvest, struck and lost, fishing gear entanglements and vessel strikes; Table 2). Since 1996, with the resumption of local hunting, 50 whales have been killed, 11 in West Greenland and 35 in Canada, including 3 bycatch from crab traps and 4 struck and lost whales. Recent harvest levels have been 3.3 whales/year between 2008 and 2019; lower than the PBR recommended sustainable harvest of 52.
The trend in abundance estimated by the deterministic model (Rmax is 3.8–4.0 and the struck and lost factor is 1.10–1.15) followed the catch history data and provided a reasonable pre-commercial whaling estimate of ca. 18,500 whales that could be used to develop a PA framework (Figure 2A). Some model trajectories went “extinct” at zero abundance and therefore, we re-ran the model with those outcomes removed since we know that the population survived (Figure 2B). Overall, the population trajectory over the past 50 years did not fit the recent abundance estimates from aerial surveys and genetic mark-recapture.
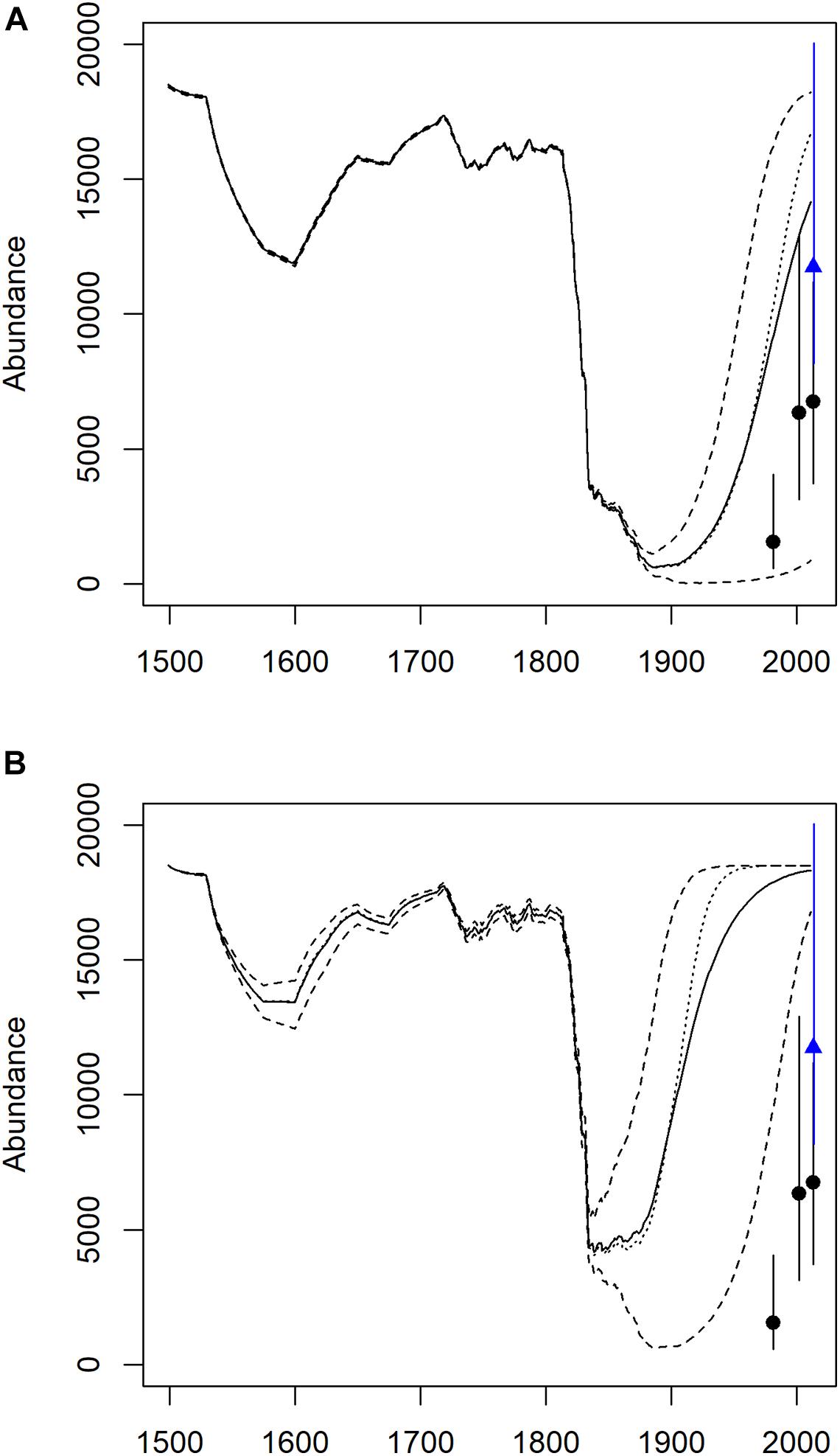
Figure 2. Mean (solid line) and 95% confidence intervals (dashed line) of ECWG bowhead whale population trajectory estimated from 10,000 Monte Carlo simulations of a logistic population growth model and removals estimated using historical catch data and a struck and lost correction factor. Carrying capacity (K), defined as the starting population size prior to exploitation, was set at 18,500 animals. Models included a uniform distribution for struck and lost correction that ranged from 1.10 to 1.20, and a uniform distribution for Rmax that ranged from 0.035 to 0.045. Recent population estimates are shown for comparison, from aerial surveys (circle symbols) in 1981 (Koski et al., 2006), 2002 (IWC, 2009b) and 2013 (Doniol-Valcroze et al., 2020) and from genetic Capture-Mark-Recapture analysis (2013), blue triangle symbol (Frasier et al., 2020)]. Upper panel (A) is model results that include zero values and lower panel (B) is model results that exclude zero population abundance values.
After the reduction in population numbers in the late 1800s and low numbers in the early 1900s, the model projections described a faster rate of increase than observed by the series of population abundance estimates. We re-ran the model under different scenarios to try to understand this mismatch. One explanation was the long delay in the onset of first reproduction by bowhead whales. Applying this delay did delay the recovery (Figure 3A), and thus better fit the estimates.
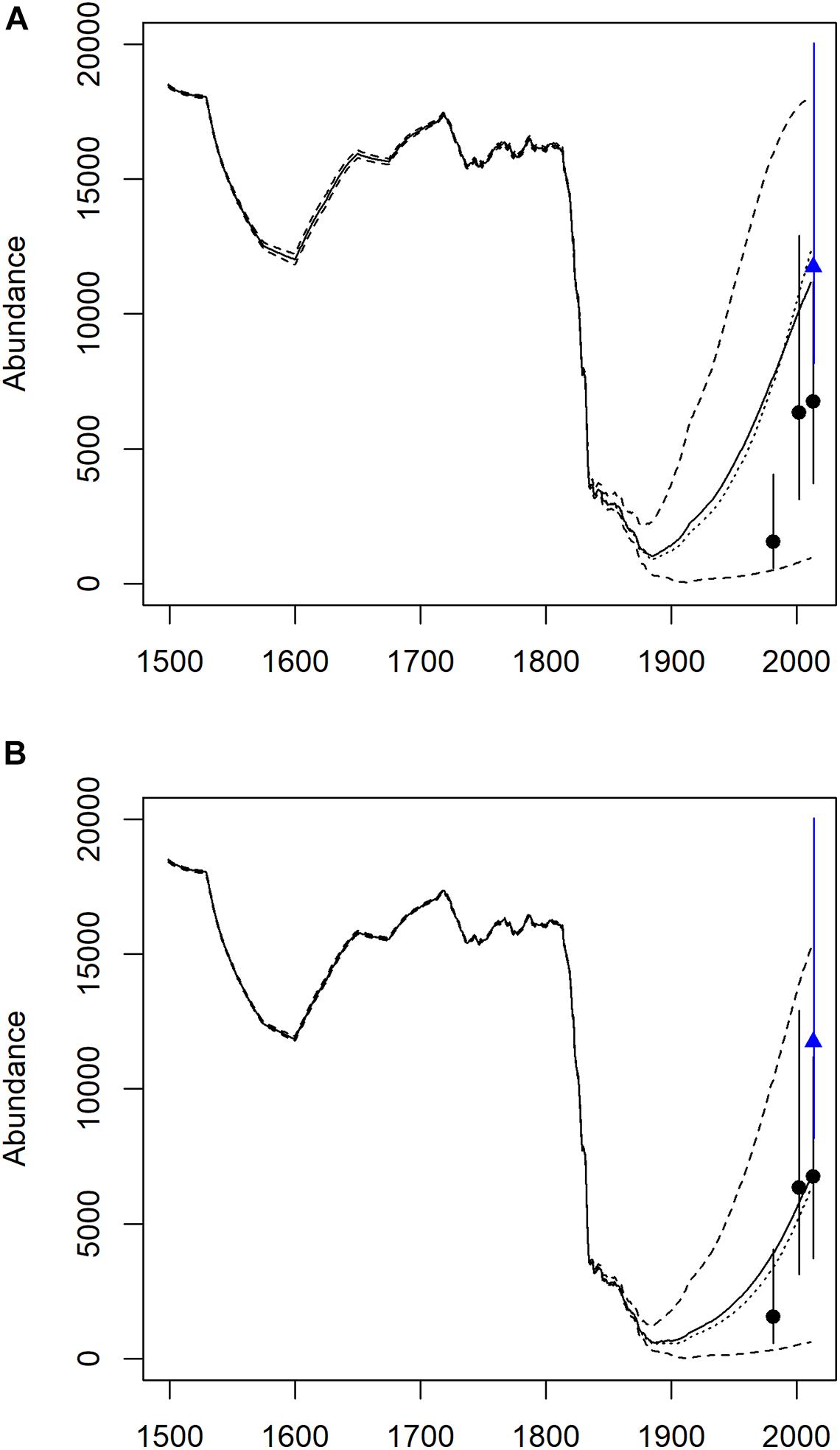
Figure 3. ECWG bowhead whale population trajectory from a logistic population growth model estimated using historical catch data. Upper panel (A) is model results (solid line mean and dashed 95% confidence intervals) with a 26 y delay to represent bowhead whale age of maturation. Lower panel (B) is model results with a modified density-dependent shape parameter (γ) constrained between 1.8 and 1.9 and with Rmax constrained between 3.8 and 4.0.
We found that the deterministic model was sensitive to the range of parameter adjustments; however, they did not affect the historical abundance/K value. Indeed, there was only a very narrow range of historical abundance values (between 18,000 and 19,000) that allowed the population trajectories to survive the heavy removals of the industrial whaling period and yet not overshoot the recent abundance estimates. Changing the γ for the density-dependent relationship (γ constrained between 1.8 and 1.9), allowed us to cause a slower recovery that better matched the available abundance estimates (Figure 3B). However, the choice of an appropriate density-dependent shape parameter is subjective.
Using the model results, we calculated the following stock status zones for consideration in ongoing sustainable management of ECWG bowhead whales (Table 3):
(1) Healthy zone between N50 (9,250) and NK (18,500),
(2) The target reference point (N70) of 12,000 whales,
(3) Cautious zone between N30 (5,550) and N50 (9,250), and
(4) Critical zone below N30 (5,550) where all removals should be avoided.
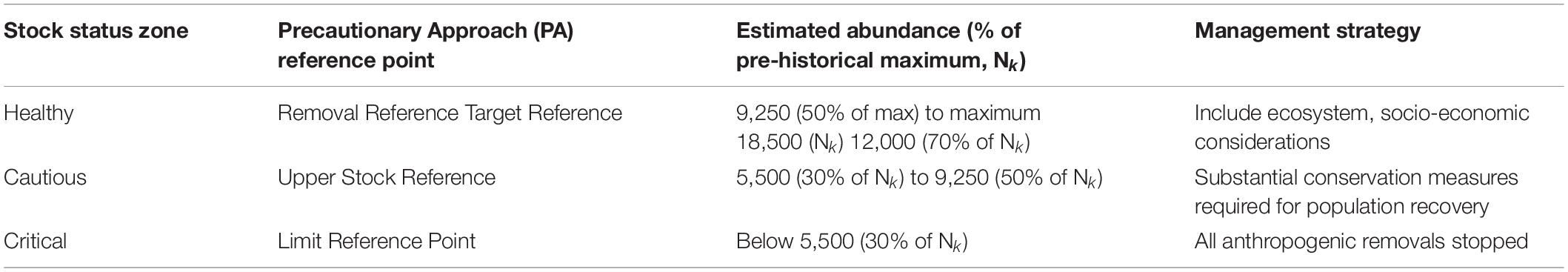
Table 3. Stock status zones and reference points proposed for a Precautionary Approach to co-management of the Eastern Canada-West Greenland bowhead whale population, based on historical (pre-commercial exploitation) population abundance (Higdon and Ferguson, 2016).
Applying model results to the precautionary approach indicated that the ECWG bowhead population abundance in 2013 was above N50 and within the N50 to N70 zone (i.e., Healthy).
Discussion
We defined reference points for the ECWG bowhead whale population by using the estimated historical population size as a measure of carrying capacity. To inform the PA framework, we identified the transition between different abundance (stock status) zones and their corresponding management objectives. Population status reference points have often been developed using Nmsy, the maximum sustainable yield level (Curtis et al., 2015); however, this has generally not been the case for marine mammals that are harvested at low rates (Barber, 1988). Here, we set short-term management goals by modeling catch statistics and relate results to a time series of abundance estimates that can project reference points for consideration by the decision making process (Garcia et al., 2000). Using this approach, we consider it likely that the current ECWG bowhead population estimate is within the N50 to N70 zone.
Estimates of removal numbers, population abundance, and demographic rates are often highly uncertain. For most species it is extremely difficult to estimate reference points based on population trend; even more so for large whales like bowhead (Witting, 2013). The most recent ECWG aerial survey was conducted in 2013 and produced an abundance estimate of 6,745 (95% CI 3,722–11,200) whales (Doniol-Valcroze et al., 2020). This estimate is conservative, because it does not include the summer aggregation areas of Foxe Basin and Repulse Bay. A Bayesian analyses of genetic capture-mark-recapture data collected over a 19-year period (1995–2013) provided an estimate of 11,747 (Frasier et al., 2020). The genetic estimate of population size is similar to the deterministic model estimate (Figure 2). Regardless of which abundance estimate is used, the ECWG bowhead whale population should continue to increase to the N70 target reference point (above 12,000) over the next 10 years if subsistence hunts take most of the 52 whales estimated using current PBR calculations. Caution is necessary, however, as this mortality rate would include not only losses from harvest and struck and lost whales, but also net entanglements and vessel strikes. For example, mortality from bowhead whale net entanglements due to fisheries activities have been reported (Citta et al., 2014) and similar to their closest relative, the right whale, bowhead whales are susceptible to mortality from marine vessel strikes (George et al., 2021b).
Modeled population growth after the cessation of commercial whaling suggested a faster rate of increase in the population than was realized based on survey abundance estimates. Witting (2013) had already observed that the estimated density-regulated growth following whaling was slower than expected. One explanation for this discrepancy may be the delayed population growth from the continued commercial whaling in the early 1900s when few whales were available. Another explanation for this mismatch is that the three aerial survey abundance estimates used in our trend analysis were all negatively biased due to missing areas known to have bowhead whales (Koski et al., 2006; Dueck et al., 2008; Doniol-Valcroze et al., 2020). Another explanation was the uncommonly long delay in sexual maturity of females, longer than any other whale species (Ferguson and Higdon, 2013), resulted in a delay in exponential population growth. Other possible explanations that could be explored include an Allee effect, perhaps due to difficulties in finding suitable mating partners (Frasier et al., 2007). The logistic model used did not incorporate Allee effects; however, it could be incorporated into future versions (Fujiwara and Caswell, 2001). Following the low population abundance from commercial whaling, whales were likely few and far between and prevented from reproducing at their full Rmax. Another possibility is that the large commercial whale mortality resulted in a social/cultural deficit to the population from the loss of their experienced members that held knowledge of how to navigate through sea ice and/or find food during atypical years. Killer whales were reasonably abundant in the Baffin Bay-Davis Strait area during the commercial whaling era (Reeves and Mitchell, 1988), as whalers did not start pursuing them until the mid-1900s (Jourdain et al., 2019). Killer whale predation is typically included in natural mortality but our modeling approach did not allow for changes in natural mortality over time. Additional data would be needed to inform such changes, including an increase in killer whale predation (Ripple et al., 2014). Last, “pre-commercial exploitation” carrying capacity may be unreachable given the large-scale environmental changes associated with ice loss (Overland and Wang, 2010; Punt and Wade, 2012).
Bowhead whales are also among the largest animals on earth and the amount of body fat is extremely high, up to 60% body mass (George et al., 2021a). Due to their large body mass and fat composition, bowhead whales are expected to have high energetic requirements. Although bowhead whale numbers are recovering from overharvesting by commercial whaling activities in eastern Canadian waters, concern remains regarding their future because of anthropogenic factors such as climate change that are expected to alter ice conditions and the quantity and quality of prey available to them. Possible replacement of high-lipid Arctic species of zooplankton by lower-energy temperate species may strongly affect the foraging success of bowhead whales. Although, bowhead whale diet and foraging behavior is well understood (e.g., Fortune et al., 2020a), the possible demographic impact of environmental change has not been investigated.
Bowhead whales face multiple threats in addition to harvest mortality and managing catch using the PA framework should account for additional anthropogenic impacts, e.g., pollution, climate warming, vessel strikes, and noise (Halliday et al., 2021; Kochanowicz et al., 2021). Ultimately, cumulative impacts should be incorporated into the decision-making process (Murray et al., 2021). Recent advances in techniques for data-limited populations can address some of these challenges (Fagan et al., 2013). Another concern is the model assumption of constant carrying capacity which is unrealistic with a warming climate and associated greater environmental variability. The approach we have used here is to define population thresholds that provide managers with guidelines to maintain sustainable harvest and thereby avoid the carrying capacity concept (McLeod, 1997); however, the approach will need to be monitored using adaptive management and updated and improved as more population information becomes available (McCarthy and Possingham, 2007). For example, simulation modeling may help to assess uncertainty estimated from probability distributions for vital rates, abundance, and true catch (e.g., struck and lost). Simulation can consider different scenarios, such as density dependent growth and age- or sex-dependent harvest mortality (Dillingham et al., 1990).
The PA framework can be used to determine management goals for the ECWG population. At the upper conservation zone, management intervention is important, whereas at the lower zone, management intervention is required. Following this approach, if the population is below N70 and above N50, management is required and harvest control rules would come into effect. Among the different management modeling approaches that could be investigated are incorporating more uncertainty with Bayesian approaches, defining alternative population growth models (e.g., Allee effects), and incorporating different population thresholds (e.g., maintaining abundance above 0.70 K). With more data, a population model can be developed to evaluate the probability that a given harvest scenario will meet stated management objectives, thus replacing harvest advice based on PBR (Hammill et al., 2015). Future research can incorporate ecosystem considerations and provide more biological data on population productivity, e.g., reproductive cycle and age, to improve estimation of catch reference points.
Management Considerations
We recommend that the most recent 2013 population abundance estimate for ECWG bowhead whales be updated. We propose that this update be based on the Mark-Recapture genetic model (Frasier et al., 2020) and include more recent genetic samples from biopsy sampling programs in Canada and Greenland. With a new genetic Mark-Recapture estimate, a population model incorporating bowhead catch history, historical population size, vital rates, and trend in population abundance can provide sustainable harvest advice in Canada, and assist with conservation efforts to maintain population health. The limit-reference point framework outlined here is consistent with the internationally adopted PA and should provide simple management guidelines while ensuring the growth and health of the ECWG bowhead whale population. The ECWG bowhead whale population range includes the jurisdictional waters of two countries that harvest whales, requiring political considerations between a non-IWC member state (Canada) and an IWC member state (Greenland, via Denmark). Canada and Greenland/Denmark are a party to the United Nations Convention on the Law of the Sea (UNCLOS). Concerning marine mammals within their 200 mile zone, Article 65 of UNCLOS provides that “States shall cooperate with a view to the conservation of marine mammals and in the case of cetaceans shall in particular work through the appropriate international organizations for their conservation, management and study.” To assist this collaboration on transboundary cetacean management, we recommend a joint management process between Canada and Greenland following in the footsteps of the management of walruses, narwhals and belugas that all have co-shared populations subjective to local hunts in both countries.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: Higdon, J. W. (2010) Commercial and subsistence harvests of bowhead whales (Balaena mysticetus) in eastern Canada and West Greenland. J. Cetacean Res. Manag. 11, 185–216.
Author Contributions
SF and JH designed the study. SF, JH, and TD-V conducted the modeling. SF wrote the first draft of the manuscript. JH, PH, RH, and TD-V contributed to the writing and reviewing of drafts. All authors provided the data, contributed to the article, and approved the submitted version.
Funding
This project was funded by Fisheries and Oceans Canada.
Conflict of Interest
JH is employed by Higdon Wildlife Consulting.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This research has benefited from the process to develop an Integrated Fishery Management Plan (IFMP) for the Eastern Canada-West Greenland (ECWG) population of Bowhead Whales within Canadian territorial waters. The IFMP is under collaborative development by Fisheries and Oceans Canada, the Nunavut Wildlife Management Board (NWMB), the Nunavik Marine Region Wildlife Board (NMRWB), the Regional Wildlife Organizations (RWO) in Nunavut and the Regional Nunavimmi Umajulirijiit Katujjiqatigiinninga (RNUK) in Nunavik. In addition, Nunavut Tunngavik Incorporated (NTI) and Makivik Corporation have participated in its development, as the primary Designated Inuit Organizations established under the Nunavut and Nunavik Inuit Land Claims Agreements, respectively. Fruitful discussions with the DFO National Marine Mammal Peer Review Committee have assisted with developing the reference points concepts and their application to bowhead whales. We acknowledge the assistance with figures by William Halliday, critical review of an earlier version of the manuscript by K. Martin, as well reviews by RR and an anonymous reviewers that together helped to considerably improve the manuscript.
Footnotes
References
Baker, C. S., and Clapham, P. J. (2004). Modelling the past and future of whales and whaling. Trends Ecol. Evol. 19, 365–371. doi: 10.1016/j.tree.2004.05.005
Barber, W. E. (1988). Maximum sustainable yield lives on. North Am. J. Fish. Manag. 8, 153–157. doi: 10.1577/1548-8675(1988)008<0153:msylo>2.3.co;2
Brandon, J. R., Punt, A. E., Moreno, P., and Reeves, R. R. (2017). Toward a Tier System Approach for Calculating Limits on Human-caused Mortality of Marine Mammals. ICES J. Mar. Sci. 74, 877–887. doi: 10.1093/icesjms/fsw202
Butterworth, D. S., and Punt, A. E. (1999). Experiences in the evaluation and implementation of management procedures. ICES J. Mar. Sci. 56, 985–998. doi: 10.1006/jmsc.1999.0532
Chambault, P., Albertsen, C. M., Patterson, T. A., Hansen, R. G., Tervo, O., Laidre, K. L., et al. (2018). Sea surface temperature predicts the movements of an Arctic cetacean: the bowhead whale. Sci. Rep. 8:9658. doi: 10.1038/s41598-018-27966-1
Citta, J. J., Burns, J. J., Quakenbush, L. T., Vanek, V., George, J. C., Small, R. J., et al. (2014). Potential for bowhead whale entanglement in cod and crab pot gear in the Bering Sea. Mar. Mamm. Sci. 30, 445–459. doi: 10.1111/mms.12047
Cosens, S. E., Cleator, H., and Richard, P. (2006). Number of Bowhead Whales (Balaena mysticetus) in the eastern Canadian Arctic, based on aerial surveys in 2002, 2003 and 2004. DFO Can. Sci. Advis. Sec. Res. Doc. 2006, iv–21.
COSEWIC (2009). COSEWIC assessment and update status report on the Bowhead Whale Balaena mysticetus, Bering–Chukchi–Beaufort population and Eastern Canada–West Greenland population, in Canada. Ottawa: Committee on the Status of Endangered Wildlife in Canada, 49.
Curtis, K. A., Moore, J. E., Boyd, C., Dillingham, P. W., Lewison, R. L., Taylor, B. L., et al. (2015). Managing catch of marine megafauna: Guidelines for setting limit reference points. Mar. Policy 61, 249–263. doi: 10.1016/j.marpol.2015.07.002
DFO (2006). A Harvest Strategy Compliant with the Precautionary Approach. DFO Can. Sci. Advis. Sec. Sci. Advis. Rep. 2006:023.
DFO (2015). Harvest advice for the eastern Canada-West Greenland bowhead whale population. DFO Can. Sci. Advis. Sec. Sci. Advis. Rep. 2015:052.
Dillingham, P. W., Moore, J. E., Fletcher, D., Cortés, E., Curtis, K. A., James, K., et al. (1990). Improved estimation of intrinsic growth rmax: integrating matrix models and allometry. Ecol. Appl. 1990:1. doi: 10.1890/14-1990.1
Doniol-Valcroze, T., Gosselin, J.-F., Pike, D. G., Lawson, J. W., Asselin, N. C., Hedges, K. J., et al. (2020). Distribution and Abundance of the Eastern Canada – West Greenland Bowhead Whale Population Based on the 2013 High Arctic Cetacean Survey. NAMMCO Sci. Publicat. 2020:11. doi: 10.7557/3.5315
Dueck, L., and Ferguson, S. H. (2009). Habitat use by bowhead whales (Balaena mysticetus) of the Eastern Canadian Arctic. DFO Can. Sci. Advis. Sec. Res. Doc. 2009:28.
Dueck, L., Richard, P., and Cosens, S. E. (2008). A review and re-analysis of Cosens et al. (2006) aerial survey assessment of bowhead whale abundance for the eastern Canadian Arctic. Can. Sci. Adv. Res. Secretar. Res. Doc. 2007:33.
Eschricht, D. F., and Reinhardt, L. (1866). On the Greenland right-whale (Balaena mysticetus, Linn.) with especial reference to its geographical distribution and migrations in times past and present, and to its external and internal characteristics. London: The Royal Society, 150.
Fagan, W. F., Pearson, Y. E., Larsen, E. A., Lynch, H. J., Turner, J. B., Staver, H., et al. (2013). Phylogenetic prediction of the maximum per capita rate of population growth. Proc. R. Soc. B 280:20130523. doi: 10.1098/rspb.2013.0523
Ferguson, S. H., and Higdon, J. W. (2013). Grouping world cetaceans according to life-history characteristics indicates two divergent strategies. Can. Wildl. Biol. Manage. 2, 51–66.
Ferguson, S. H., Dueck, L., Loseto, L. L., and Luque, S. P. (2010). Bowhead whale Balaena mysticetus seasonal selection of sea ice. Mar. Ecol. Prog. Series 411, 285–297. doi: 10.3354/meps08652
Fortune, S., Ferguson, S. H., Trites, A., Hudson, J., and Baumgartner, M. (2020a). Bowhead whales use two foraging strategies in response to fine-scale differences in zooplankton vertical distribution. Sci. Rep. 2020:9. doi: 10.1038/s41598-020-76071-9
Fortune, S., Ferguson, S. H., Trites, A., LeBlanc, B., LeMay, V., Hudson, J. M., et al. (2020b). Seasonal diving and foraging behaviour of Eastern Canada-West Greenland bowhead whales. MEPS 643, 197–217. doi: 10.3354/meps13356
Fortune, S. M., Young, B. G., and Ferguson, S. H. (2020c). Age-and sex-specific movement, behaviour and habitat-use patterns of bowhead whales (Balaena mysticetus) in the Eastern Canadian Arctic. Polar Biol. 43, 1725–1744. doi: 10.1007/s00300-020-02739-7
Frasier, T. R., Hamilton, P. K., Brown, M. W., Conger, L. A., Knowlton, A. R., Marx, M. K., et al. (2007). Patterns of male reproductive success in a highly promiscuous whale species: the endangered North Atlantic right whale. Mole. Ecol. 16, 5277–5293. doi: 10.1111/j.1365-294x.2007.03570.x
Frasier, T. R., Petersen, S. D., Postma, L., Johnson, L., Heide-Jørgensen, M. P., and Ferguson, S. H. (2015). Abundance estimates of the Eastern Canada-West Greenland bowhead whale (Balaena mysticetus) population based on genetic capture-mark-recapture analyses. DFO Can. Sci. Advis. Sec. Res. Doc. 2015, iv–21.
Frasier, T. R., Petersen, S. D., Postma, L., Johnson, L., Heide-Jørgensen, M. P., and Ferguson, S. H. (2020). Abundance estimation from genetic mark-recapture data when not all sites are sampled: an example with the bowhead whale. Glob. Ecol. Conserv. 22:e00903. doi: 10.1016/j.gecco.2020.e00903
Fujiwara, M., and Caswell, H. (2001). Demography of the endangered North Atlantic right whale. Nature 414, 537–541. doi: 10.1038/35107054
Garcia, S., Staples, D., and Chesson, J. (2000). The FAO guidelines for the development and use of indicators for sustainable development of marine capture fisheries and an Australian example of their application. Ocean Coastal Manag. 43, 537–556. doi: 10.1016/s0964-5691(00)00045-4
George, J. C., Bada, J., Zeh, J., Scott, L., Brown, S. E., O’Hara, T., et al. (1999). Age and growth estimates of Bowhead Whales (Balaena mysticetus) via aspartic acid racemization. Can. J. Zool. 77, 571–580. doi: 10.1139/cjz-77-4-571
George, J. C., Horstmann, L., Fortune, S., Sformo, T. L., Elsner, R., and Follmann, E. (2021a). Thermoregulation and energetics in The Bowhead Whale. Cambridge, MA: Academic Press, 237–260.
George, J. C., Sheffield, G., Tudor, B. J., Stimmelmayr, R., and Moore, M. (2021b). Fishing gear entanglement and vessel collisions in The Bowhead Whale. Cambridge, MA: Academic Press, 577–590.
George, J. C., Thewissen, J. G. M., Von Duyke, A., Breed, G. A., Suydam, R., Sformo, T. L., et al. (2021c). Life history, growth, and form. In The Bowhead Whale. Cambridge, MA: Academic Press, 87–115.
González-Laxe, F. (2005). The precautionary principle in fisheries management. Mar. Policy 29, 495–505. doi: 10.1016/j.marpol.2004.09.002
Halliday, W. D., Pine, M. K., Citta, J. J., Harwood, L., Hauser, D. D., Hilliard, R. C., et al. (2021). Potential exposure of beluga and bowhead whales to underwater noise from ship traffic in the Beaufort and Chukchi Seas. Ocean Coastal Manag. 204:105473. doi: 10.1016/j.ocecoaman.2020.105473
Halliday, W. D., Têtu, P. L., Dawson, J., Insley, S. J., and Hilliard, R. C. (2018). Tourist vessel traffic in important whale areas in the western Canadian Arctic: Risks and possible management solutions. Mar. Policy 97, 72–81. doi: 10.1016/j.marpol.2018.08.035
Hammill, M. O., and Stenson, G. B. (2007). Application of the precautionary approach and conservation reference points to management of Atlantic seals. ICES Mar. Sci. 64, 702–706. doi: 10.1093/icesjms/fsm037
Hammill, M. O., Stenson, G. B., Doniol-Valcroze, T., and Mosnier, A. (2015). Conservation of northwest Atlantic harp seals: Past success, future uncertainty? Biolog. Conserv. 192, 181–191. doi: 10.1016/j.biocon.2015.09.016
Hauser, D. D., Laidre, K. L., and Stern, H. L. (2018). Vulnerability of Arctic marine mammals to vessel traffic in the increasingly ice-free Northwest Passage and Northern Sea Route. Proc. Natl. Acad. Sci. 115, 7617–7622. doi: 10.1073/pnas.1803543115
Heide-Jørgensen, M. P., Garde, E., Nielsen, N. H., Andersen, O. N., and Hansen, S. H. (2012). A note on biological data from the hunt of bowhead whales in West Greenland 2009–2011. J. Cetacean Res. Manag. 12, 329–333.
Heide-Jørgensen, M. P., Hansen, R. G., and Shpak, O. V. (2021). Distribution, migrations, and ecology of the Atlantic and the Okhotsk Sea Populations. In The Bowhead Whale. Cambridge, MA: Academic Press, 57–75.
Heide-Jørgensen, M. P., Laidre, K. L., Wiig, Ø, Jensen, M. V., Dueck, L., Maiers, L. D., et al. (2003). From Greenland to Canada in ten days: Tracks of Bowhead Whales, Balaena mysticetus, across Baffin Bay. Arctic 56, 21–31.
Higdon, J. W. (2010). Commercial and subsistence harvests of bowhead whales (Balaena mysticetus) in eastern Canada and West Greenland. J. Cetacean Res. Manag. 11, 185–216.
Higdon, J. W., and Ferguson, S. H. (2009). Loss of Arctic sea ice causing punctuated change in sightings of killer whales (Orcinus orca) over the past century. Ecol. Appl. 19, 1365–1375. doi: 10.1890/07-1941.1
Higdon, J. W., and Ferguson, S. H. (2016). Historical abundance of Eastern Canada - West Greenland (ECWG) bowhead whales (Balaena mysticetus) estimated using catch data in a deterministic discrete-time logistic population model. DFO Can. Sci. Advis. Sec. Res. Doc. 2016:26.
Hilborn, R., Maguire, J.-J., Parma, A. M., and Rosenberg, A. A. (2006). The Precautionary Approach and risk management: can they increase the probability of successes in fishery management? Can. J. Fish. Aquat. Sci. 2001, 99–107. doi: 10.1139/f00-225
Hutchings, J. A., Myers, R. A., García, V. B., Lucifora, L. O., and Kuparinen, A. (2012). Life−history correlates of extinction risk and recovery potential. Ecol. Appl. 22, 1061–1067. doi: 10.1890/11-1313.1
IWC. (2009a). Draft Report of the Workshop on Cetaceans and Climate Change. Cambridge, MA: Scientific Committee of the International Whaling Commission.
IWC. (2013). Annual report of the International Whaling Commission, Aug 2012-Aug 2013. Cambridge, UK: International Whaling Commission.
Jourdain, E., Ugarte, F., Víkingsson, G. A., Samarra, F. I., Ferguson, S. H., Lawson, J., et al. (2019). North Atlantic killer whale Orcinus orca populations: a review of current knowledge and threats to conservation. Mammal Rev. 49, 384–400. doi: 10.1111/mam.12168
Kochanowicz, Z., Dawson, J., Halliday, W. D., Sawada, M., Copland, L., Carter, N. A., et al. (2021). Using western science and Inuit knowledge to model ship-source noise exposure for cetaceans (marine mammals) in Tallurutiup Imanga (Lancaster Sound), Nunavut, Canada. Mar. Policy 130:104557. doi: 10.1016/j.marpol.2021.104557
Koski, W. R., and Ferguson, S. H. (2012). Review of Methods for Eastern Canada-Western Greenland Bowhead Whale (Balaena mysticetus) Population Abundance Estimation. DFO Can. Sci. Advis. Sec. Res. Doc. 2012:23.
Koski, W. R., Heide-Jorgensen, M. P., and Laidre, K. (2006). Winter abundance of bowhead whales, Balaena mysticetus, in the Hudson Strait, March 1981. J. Cetac. Res. Manag. 8, 139–144.
Laidre, L. L., Stirling, I., Lowry, L. F., Wiig, Ø, Heide-Jørgensen, M. P., and Ferguson, S. H. (2008). Quantifying the sensitivity of Arctic marine mammals to climate induced habitat change. Ecolog. Appl. 18, 297–S125.
Lambert, E., Pierce, G. J., Hall, K., Brereton, T., Dunn, T. E., Wall, D., et al. (2014). Cetacean range and climate in the eastern North Atlantic: future predictions and implications for conservation. Glob. Change Biol. 20, 1782–1793. doi: 10.1111/gcb.12560
Lambertson, R. H., Rasmussen, K. J., Lancaster, W. C., and Hintz, R. J. (2005). Functional morphology of the mouth of the bowhead whale and its implications for conservation. J. Mammal. 86, 342–352. doi: 10.1644/ber-123.1
Lonergan, M. (2011). Potential biological removal and other currently used management rules for marine mammal populations: a comparison. Mar. Policy 35, 584–589. doi: 10.1016/j.marpol.2011.01.020
Mace, P. M., and Gabriel, W. L. (1999). “Evolution, scope, and current applications of the precautionary approach in fisheries,” in Proc. Fifth Natl. NMFS Stock Assess. Workshop NOAA Tech Memo NMFS-FSPO-40, ed. V. R. Restrepo (Washington, D.C: U.S. Department of Commerce), 65–73.
MacLeod, C. D. (2009). Global climate change, range changes and potential implications for the conservation of marine cetaceans: a review and synthesis. Endang. Species Res. 7, 125–136. doi: 10.3354/esr00197
McCarthy, M. A., and Possingham, H. P. (2007). Active adaptive management for conservation. Conserv. Biol. 21, 956–963. doi: 10.1111/j.1523-1739.2007.00677.x
McLeod, S. R. (1997). Is the concept of carrying capacity useful in variable environments? Oikos 1997, 529–542. doi: 10.2307/3546897
Murray, C. C., Hannah, L., Doniol-Valcroze, T., Wright, B., Stredulinsky, E., Nelson, J. C., et al. (2021). A cumulative effects for population trajectories of resident killer whales in the Northeast Pacific. Biolog. Conserv. 257:109124. doi: 10.1016/j.biocon.2021.109124
Nerini, M. K., Braham, H. W., Marquette, W. M., and Rugh, D. J. (1984). Life history of the bowhead whale, Balaena mysticetus (Mammalia: Cetacea). J. Zool. Lon. 204, 443–468. doi: 10.1111/j.1469-7998.1984.tb02381.x
Nielsen, N., Laidre, K., Stenbak Larsen, R., and Heide-Jørgensen, M. P. (2015). Identification of Potential Foraging Areas for Bowhead Whales in Baffin Bay and Adjacent Waters. Arctic 68:4488.
NWMB. (2000). Final report of the Inuit Bowhead Knowledge Study. Iqaluit, NU: Nunavut Wildlife Management Board, 90.
Overland, J. E., and Wang, M. (2010). Large-scale atmospheric circulation changes are associated with the recent loss of Arctic sea ice. Tellus A 62, 1–9. doi: 10.1111/j.1600-0870.2009.00421.x
Pomerleau, C., Lesage, V., Ferguson, S. H., Winkler, G., Petersen, S. D., and Higdon, J. W. (2012). Prey assemblage isotopic variability as a tool for assessing diet and the spatial distribution of bowhead whale Balaena mysticetus foraging in the Canadian eastern Arctic. Mar. Ecol. Prog. Series 469, 161–174. doi: 10.3354/meps10004
Pomerleau, C., Patterson, T. A., Luque, S., Lesage, V., Heide-Jørgensen, M. P., Dueck, L. L., et al. (2011). Bowhead whale Balaena mysticetus diving and movement patterns in the eastern Canadian Arctic: implications for foraging ecology. Endang. Species Res. 15, 167–177.
Punt, A. E. (2006). The FAO precautionary approach after almost 10 years: have we progressed towards implementing simulation−tested feedback−control management systems for fisheries management? Nat. Resour. Model. 19, 441–464. doi: 10.1111/j.1939-7445.2006.tb00189.x
Punt, A. E., and Smith, D. M. (2001). “The gospel of maximum sustainable yield in fisheries management: birth, crucifixion and reincarnation,” in Conservation of Exploited Species, eds J. D. Reynolds, G. M. Mace, K. H. Redford, and J. G. Robinson (Cambridge, MA: Cambridge University Press) 41–66.
Punt, A. P., and Wade, P. R. (2012). Population status of the eastern North Pacific stock of gray whales in 2009. J Cetacean. Res. Manage. 12, 15–28.
Reeves, R. R., and Mitchell, E. (1988). Killer whale sightings and takes by American pelagic whalers in the North Atlantic. Rit. Fiskideildar. 11, 7–23.
Reeves, R. R., Mitchell, E., Mansfield, A., and McLaughlin, M. (1983). Distribution and migration of the Bowhead Whale. Balaena mysticetus, in the eastern North American Arctic. Arctic 36, 5–64.
Rekdal, S., Hansen, R. G., Borchers, D., Bachmann, L., Laidre, K., and Nielsen, N. (2015). Trends in bowhead whales in West Greenland: Aerial surveys vs. genetic capture−recapture analyses. Mar. Mammal. Sci. 31:12150. doi: 10.1111/mms.12150
Richardson, W. J., Greene, C. R., Hanna, J. S., Koski, W. R., Miller, G. W., Patenaude, N. J., et al. (1995). Acoustic effects of oil production activities on Bowhead and white whales visible during spring migration near Pt. Barrow, Alaska – 1991 and 1994 phases. OCS Study MMS 95-0051; LGL Rep. TA954. Rep. from LGL Ltd., King City, Ont., for U.S. Herndon, VA: Minerals Manage. Serv, B98–B107667.
Ripple, W. J., Estes, J. A., Beschta, R. L., Wilmers, C. C., Ritchie, E. G., Hebblewhite, M., et al. (2014). Status and ecological effects of the world’s largest carnivores. Science 343:6167.
Rosa, C., Zeh, J., Craig George, J., Botta, O., Zauscher, M., Bada, J., et al. (2013). Age estimates based on aspartic acid racemization for bowhead whales (Balaena mysticetus) harvested in 1998–2000 and the relationship between racemization rate and body temperature. Mar. Mam. Sci. 29, 424–445.
Ross, W. G. (1979). The annual catch of Greenland (bowhead) whales in waters north of Canada 1719–1915: a preliminary compilation. Arctic 32, 91–121.
Savelle, J. M. (2010). Cumulative bowhead whale (Balaena mysticetus) harvest estimates by prehistoric Thule Inuit in the Canadian Arctic 1200-1500 A.D.: Implications for bowhead whale population modeling and Thule demography. Bull. Natl. Museum Ethnol. 34, 593–618.
Seersholm, F., Pedersen, M., Søe, M., et al. (2016). DNA evidence of bowhead whale exploitation by Greenlandic Paleo-Inuit 4,000 years ago. Nat. Commun. 7:13389. doi: 10.1038/ncomms13389
Simmonds, M. P., and Isaac, S. J. (2007). The impacts of climate change on marine mammals: early signs of significant problems. Oryx 41, 9–26.
Stenson, G. B., Hammill, M., Ferguson, S., Stewart, R., and Doniol-Valcroze, T. (2012). Applying the Precautionary Approach to Marine Mammal Harvests in Canada. DFO Can. Sci. Advis. Sec. Res. Doc. 2012:15.
Wade, P. R. (1998). Calculating limits to the allowable human-caused mortality of cetaceans and pinnipeds. Mar. Mamm. Sci. 14, 1–37. doi: 10.1111/j.1748-7692.1998.tb00688.x
Wallace, B. P., DiMatteo, A. B., Bolten, A. B., Chaloupka, M. Y., Hutchinson, B. J., Abreu-Grobois, F. A., et al. (2011). Global conservation priorities for marine turtles. PLoS One 6:e24510.
Williams, R., Kelly, N., Boebel, O., Friedlaender, A. S., Herr, H., Kock, K. H., et al. (2014). Counting whales in a challenging, changing environment. Sci. Rep. 4, 1–6.
Witting, L. (2011). On Population Dynamics of Eastern Canada-West Greenland Bowhead Whales. Unpublished Paper SC/63/AWMP3, Presented to the IWC Scientific Committee. (Available by request from the International Whaling Commission).
Witting, L. (2013). Selection-delayed population dynamics in baleen whales and beyond. Populat. Ecol. 55, 377–401. doi: 10.1007/s10144-013-0370-9
Keywords: commercial whaling, deterministic model, subsistence hunting, limit reference points, precautionary principle, historical abundance, Inuit
Citation: Ferguson SH, Higdon JW, Hall PA, Hansen RG and Doniol-Valcroze T (2021) Developing a Precautionary Management Approach for the Eastern Canada-West Greenland Population of Bowhead Whales (Balaena mysticetus). Front. Mar. Sci. 8:709989. doi: 10.3389/fmars.2021.709989
Received: 14 May 2021; Accepted: 18 August 2021;
Published: 07 September 2021.
Edited by:
Asha de Vos, The Sri Lankan Blue Whale Project and Oceanswell, Sri LankaReviewed by:
Randall Reeves, Okapi Wildlife Associates, CanadaRussell Fielding, Coastal Carolina University, United States
Copyright © 2021 Ferguson, Higdon, Hall, Hansen and Doniol-Valcroze. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steven H. Ferguson, c3RldmUuZmVyZ3Vzb25AZGZvLW1wby5nYy5jYQ==; Patricia A. Hall, cGF0cmljaWEuaGFsbEBkZm8tbXBvLmdjLmNh
 Steven H. Ferguson
Steven H. Ferguson Jeff W. Higdon
Jeff W. Higdon Patricia A. Hall1*
Patricia A. Hall1* Thomas Doniol-Valcroze
Thomas Doniol-Valcroze