
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 10 August 2021
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.706463
This article is part of the Research TopicOmics and Fish NutritionView all 12 articles
 Yongkang Chen1
Yongkang Chen1 Shuyan Chi1,2,3
Shuyan Chi1,2,3 Shuang Zhang1,2,3
Shuang Zhang1,2,3 Xiaohui Dong1,2,3
Xiaohui Dong1,2,3 Qihui Yang1,2,3
Qihui Yang1,2,3 Hongyu Liu1,2,3
Hongyu Liu1,2,3 Beiping Tan1,2,3*
Beiping Tan1,2,3* Shiwei Xie1,2,3*
Shiwei Xie1,2,3*The present study evaluated the effects of the dietary black soldier fly larvae meal (BSF) on growth performance, intestinal health, and susceptibility to Vibrio parahaemolyticus in the Pacific white shrimp Litopenaeus vannamei. The basal diet was formulated to contain 25% fish meal (FM), and then the FM was replaced with BSF for 10, 20, and 30% of the FM protein in the experimental diets, which are referred to as FM, BSF10, BSF20, and BSF30, respectively. Four hundred and eighty healthy and uniform-sized shrimp (~0.88 g) were distributed among four groups of three replicates, each with 40 shrimp in a 300-L tank and they were fed four times daily for 7 weeks. The results showed that the growth performance did not change significantly in shrimp fed with BSF10 and BSF20 diets, but significantly decreased in those fed with BSF30 diet compared to the ones fed with FM diet. After feeding trial and sampling, a V. parahaemolyticus infection challenge trial was conducted on shrimp. The results showed that the survival rate of shrimp fed with BSF10 was significantly higher than those fed with FM. The results of the midgut histology showed that the width and height of intestinal mucosal folds decreased significantly in shrimp fed with BSF20 and BSF30, and the early signs of apoptosis in the intestinal cells were found in shrimp fed with BSF30. The mRNA levels of non-specific immune-related genes dorsal and relish were downregulated in shrimp fed with BSF20 and BSF30 diets. The mRNA levels of antimicrobial peptides–related genes alf (anti-lipopolysaccharide factor) were upregulated in shrimp fed with BSF10 but downregulated in shrimp fed with BSF30. The mRNA level pen3 (penaeidins 3) was upregulated in shrimp fed with BSF10 and BSF20 diets. The intestinal bacterial communities on operational taxonomic unit levels among groups were not significantly differentiated according to the beta diversity analysis. At the genus levels, a decrease in Vibrio, Photobacterium, and Candidatus_Bacilloplasma, as well as the increase in Bacillus and Pseudoalteromonas abundance, indicated the improvement of intestinal microbiota in shrimp fed with dietary BSF. Therefore, the use of BSF in shrimp diet should be controlled at a dosage of 20% of the FM, which can improve the intestinal microbiota without causing any negative effects.
Pacific white shrimp Litopenaeus vannamei has become an important species in global aquaculture due to the advantages of its rapid growth and strong stress resistance (FAO, 2020). However, some gastrointestinal diseases, such as white feces syndrome (WFS) (Hou et al., 2018) and early mortality syndrome (EMS) (Zorriehzahra and Banaederakhshan, 2015) have caused significant losses to shrimp farming in recent years. As a vital organ for nutrient uptake and immune defense, the intestinal structure and biochemical barrier are closely related to the health status of the shrimp (Suo et al., 2017).
The intestinal structure and the biochemical barrier refer to the integrity of the histological structure of the intestine. The midgut of shrimp consists of the outer epidermis, muscular layers, protruding mucosal folds, and the inner epidermis, and an intact intestinal structure forms the basis for the functioning of the intestine (Xie et al., 2020). In addition, the immune function of the gut should be considered, as pathogens in the external environment can pass through the intestine (Pilotto et al., 2020). The antimicrobial peptides such as alf (anti-lipopolysaccharide factor), cru (crustins), and pen3 (penaeidins 3) are regulated by secreted cytokines and immune signaling pathways, such as the Toll and IMD (immune deficiency). When Toll and IMD pathways are activated in early response to pathogens, the expression of downstream factors dorsal and relish can regulate pathways activities (Qiu et al., 2014). Intestinal microbiota are an important part of the intestinal biochemical barrier (Qi et al., 2017). The intestinal microbiota have been extensively studied and shown to benefit aquatic animals with its functions in immune response, nutrient uptake, and homeostasis maintenance (Li et al., 2018), which is mainly influenced by environmental and dietary factors (Egerton et al., 2018). Although close contact with the aquatic environment may influence the early gut colonization (Roeselers et al., 2011), feed causes a continuous effect on the intestinal microbiota composition over a longer culture period (Ringø et al., 2016).
In shrimp diets, fish meal (FM) is the most commonly used protein source, usually accounting for 25–35% of the formulation (Xie et al., 2016). The depletion and shortage of fishery resources and the high cost of FM have encouraged researchers to look for alternatives (Cai et al., 2020). In the last decade, experimental studies on feeding of insect meal–based diets have been carried out in L. vannamei, which has been conducted on mealworm (Tenebrio molitor), silkworm pupae (Bombyx mori L.), as well as fly maggots (Cao et al., 2012; Choi et al., 2018; Rahimnejad et al., 2019). Besides, the black soldier fly is a saprophytic insect (Hermetia illucens) commonly used for the sustainable recycling of animal waste and other organic wastes. Its larvae are rich in proteins and lipids, and also it is considered as a potential protein source for aquatic animals (Barragan-Fonseca et al., 2017). Several studies have been conducted to evaluate the effectiveness of BSF as an FM replacement in the diets of L. vannamei. In general, most growth responses could be obtained if the amount of BSF replacement FM is limited to 25% of the ratio without changing the nutrition levels of the diet (Cummins et al., 2017). Another study showed that BSF substitution of <30% FM in diets did not negatively affect the growth performance of L. vannamei (Hu et al., 2019). Also, dietary BSF has been shown to affect the intestinal health status; however, it causes different effects on different animals. In previous studies, a total replacement of the FM with BSF did not impair the intestinal health of Atlantic salmon (Salmo salar L.) (Li et al., 2020). However, replacement of 75% FM with defatted BSF led to significant pathological changes in the intestinal wall of Jian carp (Cyprinus carpio var. Jian) (Li et al., 2017). It is still unclear how dietary BSF affects the intestinal health of L. vannamei and our experiments were conducted to study the effect of BSF on the growth performance, intestinal structure, and immunity as well as the intestinal microbiota communities of L. vannamei.
This study was carried out following the recommendations of Care and Use of Laboratory Animals in China, Animal Ethical and Welfare Committee of China Experimental Animal Society. The protocol was approved by the Animal Ethical and Welfare Committee of Guangdong Ocean University, Guangdong, China.
The black soldier fly larvae meal used in this experiment was provided by (Guangzhou Fishtech Biotechnology Co., Ltd, Guangdong, China) and contained 35.17% crude protein and 32.60% crude lipid. The basal diet was formulated to contain 25% FM and then the FM was replaced with BSF for 10, 20, and 30% of the FM protein, which we referred to as FM, BSF10, BSF20, and BSF30, respectively. With the increase of BSF addition, the level of fish oil, soybean oil, and soybean lecithin was gradually reduced to balance the crude lipid levels among the four diets. Several essential amino acids were supplemented to the BSF diets to obtain similar amino acid profiles as the FM diet. Diets were processed as follows: all the ingredients were smashed and sifted out with an 80-mesh sieve. Then, all the ingredients were weighed, combined, and then mixed to homogeneity (M-256, South China University of Technology, Guangdong, China). It is important to note that BSF and soybean meal were mixed (1:1) and then crushed and sieved before being mixed with other ingredients. The 1.0- and 1.5-mm diameter pellets were extruded using a pelletizer (Institute of Chemical Engineering, South China University of Technology, Guangdong, China) and then ripened in an electric oven at 60°C for 30 min, and stored frozen (−20°C) before use. Formulation and proximate composition of the diets are given in Table 1.
Juvenile L. vannamei were obtained from the nursery base of Guangdong Yuehai Seed Co., Ltd (Zhanjiang, China). The shrimp were fed with commercial feed (Guangdong HAID Group, crude protein 48.0%, crude fat 8.0%) and acclimatized to the experimental conditions for a week. After starvation treatment for 24 h, healthy and uniform-sized juvenile L. vannamei (~0.88 g) were distributed among four groups with triplicate fiberglass tanks (300 L) per group and 40 shrimp in each tank. Shrimp were fed to apparent satiation four times daily at 7:20, 11:20, 16:00, and 21:00 h for 7 weeks. In the first 4 weeks, shrimp were fed 1.0 mm diameter feed, while they were fed 1.5 mm diameter feed in the last 3 weeks. During the experiment, 60% of the water was exchanged each day by seawater disinfected with chlorine dioxide to maintain water quality. Water temperature and salinity were measured every day in a range of 27–31°C and 25–30%, respectively.
Diets of each group were collected for moisture, crude protein, crude lipid, ash, amino acid analysis, and fatty acid profiles. Crude protein was determined by the Dumas Nitrogen method with a Primacs100 analyzer (Skalar, Dutch); crude lipid was determined by the ether extraction method with an XT15 extractor (Ankom, United States); moisture was determined by oven drying at 105°C. Amino acid composition was determined according to the standard of GB/T 18246-2019 in the national standard of P. R. China (Table 2). Samples were hydrolyzed in 6 N HCl at 110°C for 22 h, then separated by the ion exchange column and reaction with ninhydrin solution, and the concentrations of amino acids were obtained through spectrophotometry. Fatty acids composition of diets and ingredients were determined by GB/T 5009.168-2016 in the national standard of the P. R. China with the assistance of Willtest Technology Co. Ltd., Sichuan, China (Table 3).
After the feeding trial, the shrimp in each tank were counted and weighed. Ten shrimp from each tank were randomly selected and the intestines were removed on ice, immediately placed in liquid nitrogen, and transferred to −80°C for RNA extraction and intestinal microbiota analysis.
Prior to the pathogen challenge trial, the shrimp fed with commercial diets (mentioned above, crude protein 48.0%, crude fat 8.0%) and raised under the same conditions were divided into four subgroups (n = 10), injected with graded concentrations of Vibrio parahaemolyticus (106, 107, 108, and 109 CFU/ml for 40 μl) and continually monitored for 1 week to determine the 50% lethal concentration. After the feeding trial, 10 shrimp from each tank were injected with 40 μl V. parahaemolyticus (5 × 108 CFU/ml) for the challenge trial. The challenge trial continued for 12 days after injection to finalize the survival rate.
In each tank, midguts from three shrimp were fixed in Bouin's fixative solution for 24 h and then transferred to 70% ethanol. Then gut samples were dehydrated in a graded series of ethyl alcohol and embedded in paraffin. Sections were stained with H&E and observed under a microscope (Nikon Ni-U, Japan). Another three midguts were collected for transmission electron microscopy (TEM) examination. The method was described by Xie et al. (2020), briefly, samples were fixed in 2.5% glutaraldehyde solution (4°C) for 24 h, post-fixed in 1% osmium tetroxide (OsO4) for 1 h, dehydrated in a graded series of ethyl alcohol, and finally embedded with resin. Ultrathin sections (90 nm) were placed on copper grids and were stained with saturated uranyl acetate solution for 30 min, rinsed with distilled water, and post stained with lead citrate for 30 min. Ultrathin sections were screened and observed with a TEM (Hitachi HT7700 TEM, Japan). The statistical study of intestinal histology was carried out by measuring the height of microvilli, height and width of mucosal folds, as well as the thickness of muscle layer by ImageJ software. Each index was randomly measured six times in one section, and one-way ANOVA followed by Tukey's multiple-range test was used to determine significant differences among groups. The probability value of P < 0.05 was deemed to be statistically significant.
Total RNA of intestine was extracted from four shrimp as a pooled sample in each tank using the TransZol Up Plus RNA kit (TransGen Biotech Co., Ltd, China). Agarose gel electrophoresis and spectrophotometric analysis (Nanodrop 2000, Thermo, USA) were used to assess the RNA quality and concentration. cDNA was synthesized using a PrimeScript RT reagent kit with gDNA Eraser (Takara, Japan), according to the instructions of the manufacturer. Briefly, oligo dT primers and random 6-mers were used to reverse transcribe 1,000 ng RNA in the presence of PrimeScript RT enzyme Mix I, 5×PrimeScript buffer, and RNase-free H2O at 37°C for 15 min, following inactivation at 85°C for 5 s.
Real-time PCR for the target genes was performed using a SYBR Green Premix Pro Taq HS qPCR Kit II (Accurate Biotechnology Hunan Co., Ltd, China) and quantified on the LightCycler 480 (Roche Applied Science, Switzerland) using the following program: 0.5 μM of forward- and reverse-specific primers, 5 μl of 2×SYBR Green Pro Taq HS Premix II, 10 ng of cDNA template and nuclease-free water to a final volume of 10 μl, denaturation step at 95°C for the 30 s, followed by 40 amplification cycles of 5 s denaturation at 95°C, 30 s annealing at 60°C, followed by a melt-curve analysis, and cooling to 4°C. β-actin was used as the reference gene according to Xie et al. (2019). Primers of relish and dorsal were referred to a previous study (Qiu et al., 2014), while other primers were designed by Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) (Table 4).
Microbial DNA of the intestine from six shrimp as two pooled samples in each tank was extracted using E.Z.N.A. stool DNA Kit (Omega Bio-tek, Inc., United States) following the protocols of the manufacturer. To analyze the microbial population, the V3–V4 variable region of the 16S ribosomal RNA gene was amplified by the primers 341F (5′-CCTACGGGNGGCWGCAG-3′) and 806R (5′-GGACTACHVGGGTATCTAAT-3′) where the barcode is an eight-base sequence unique to each sample. The reactions were carried out in triplicate 50 μl mixture containing 100 ng of template DNA, 5 μl of 10 × KOD Buffer, 5 μl of 2.5 mM dNTPs, 1.5 μl of each primer (5 μM), and 1 μl of KOD polymerase. The PCR was run as follows: initial denaturation at 94°C for 2 min, followed by 30 cycles of denaturation at 98°C for 10 s, 62°C for 30 s, and 68°C for 30 s, followed by a final extension at 68°C for 5 min.
High-throughput sequencing of the purified PCR products was carried out using Illumina NovaSeq 6000 sequencing system. Amplicons were extracted from 2% agarose gels and purified using the AMPure XP Beads (Beckman Agencourt, United States) following the protocols of the manufacturer and quantified using ABI StepOnePlus Real-Time PCR System (Life Technologies, Foster City, United States). Purified amplicons were pooled in equimolar and paired-end sequenced (PE250) in the Illumina platform according to the standard protocols. After sequencing, the raw reads containing more than 10% of unknown nucleotides or containing less than 50% of bases with quality (Q-value) > 20 were removed, and the paired-end clean reads were merged as raw tags using FLASH (version 1.2.11) (Magoč and Salzberg, 2011). Raw tags were filtered following Bokulich et al.'s (2013) condition to obtain high-quality clean tags. The clean tags were clustered into operational taxonomic units (OTUs) of ≥97% similarity using UPARSE (version 9.2.64) pipeline (Edgar, 2013). All chimeric tags were removed using UCHIME algorithm and finally obtained effective tags for further analysis. The tag sequence with the highest abundance was selected as a representative sequence within each cluster. The representative OTU sequences were classified into organisms using RDP classifier based on SILVA database (version 132), with the confidence threshold value of 0.8. Among groups, Venn analysis was performed in R project Venn Diagram package (version 1.6.16) to identify unique and common species of OTUs. The stacked bar plot of the community composition on phylum level was visualized in the R project ggplot2 package (version 2.2.1). Heatmap of genus abundance was plotted using pheatmap package (version 1.0.12) in R project. Alpha diversity analysis, including ACE, Chao1, Simpson, and Shannon were calculated in QIIME (version 1.9.1). The alpha index comparison among groups was computed by Tukey's HSD test in SPSS 21.0. The principal coordinates analysis (PCoA) of weighted-UniFrac distances of multivariate statistical techniques and analysis of similarities (Anosim) test of Bray–Curtis distances performed on OTU level were calculated by the R project Vegan package (version 2.5.3) (Oksanen et al., 2013) and plotted in the R project ggplot2 package.
The growth performance was calculated as follows:
Weight gain (WG, %)
= 100 × (final body weight - initial body weight)/initial body weight
Specific growth rate (SGR, % per day)
= 100 × (Ln final body weight - Ln initial body weight)/t
Survival (%)
= 100 × (final number of shrimp)/ (initial number of shrimp)
Feed conversion rate (FCR)
= feed consumed (g)/ (final body weight - initial body weight)
where t is the experimental duration in days.
The results were presented as the means with SEM. All data were subjected to ANOVA followed by Tukey's HSD test to determine significant differences among treatments using SPSS 21.0 (SPSS, Chicago, IL, United States). A probability value of P < 0.05 was deemed to be statistically significant.
As shown in Table 5, the final body weight (FBW), weight gain (WG), and the specific growth rate (SGR) decreased with the increasing BSF addition, and FBW, WG, and SGR of shrimp fed with BSF30 were significantly lower than those fed with FM (P < 0.05). Feed conversion rate (FCR) first decreased and then increased with the increasing dietary BSF levels, and FCR of shrimp fed with BSF20 diet was significantly lower than those fed with BSF30 (P < 0.05). The results of the challenge trial are shown in Figure 1. The survival rate of shrimp fed with BSF10 significantly increased compared with those fed with FM after infection for 12 d (P < 0.05).
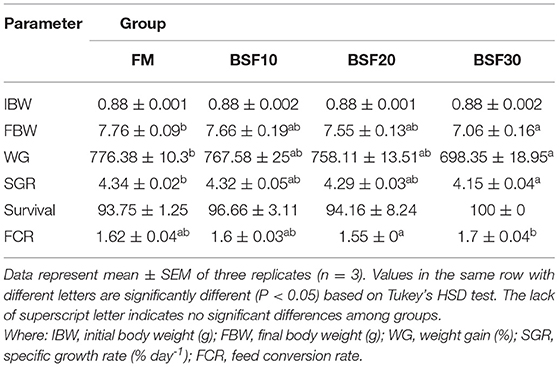
Table 5. The effect of black soldier fly larvae meal (BSF) on growth performance of Litopenaeus vannamei.
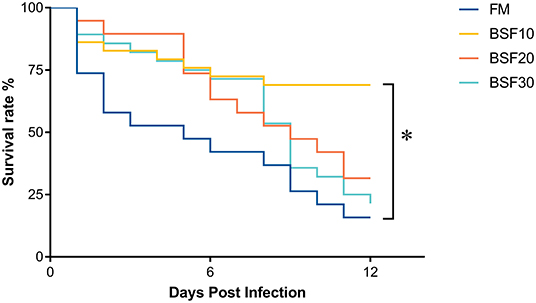
Figure 1. Effect of black soldier fly larvae meal (BSF) on the survival rate of Litopenaeus vannamei after infection of Vibrio parahaemolyticus. Asterisk (*) indicates significantly different (P < 0.05) among groups based on Tukey's HSD test.
The statistical results of intestinal histology are shown in Table 6. Results showed that the height of the microvilli significantly increased in shrimp fed with BSF10 diet, but decreased in those fed with BSF20 diet (P < 0.05). The height of the mucosal folds significantly decreased with the increasing BSF addition (P < 0.05). The width of the mucosal folds of shrimp fed with FM and BSF10 were significantly more than those fed with BSF20 and BSF30 (P < 0.05). The thickness of the muscle layer of shrimp fed with BSF10 was significantly higher than the other three groups (P < 0.05). As shown in Figure 2, the results of TEM revealed that shrimp fed with BSF20 and BSF30 showed obvious swelling of the nuclear membrane, and in particular, the chromatin of shrimp fed with BSF30 was abnormally agglutinated and distributed along the nuclear membrane. Mitochondrial cristae of shrimp fed with BSF20 were irregular and the mitochondrial structure of shrimp fed with BSF30 was disrupted. The cystic lumen of the endoplasmic reticulum of shrimp fed with BSF30 swelled and a large number of vesicles appeared in the cytoplasm.
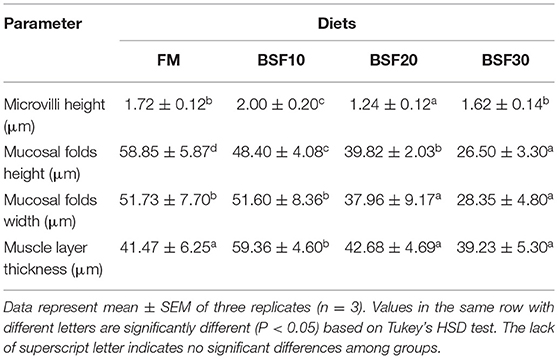
Table 6. The effect of black soldier fly larvae meal (BSF) on intestinal histology of Litopenaeus vannamei.
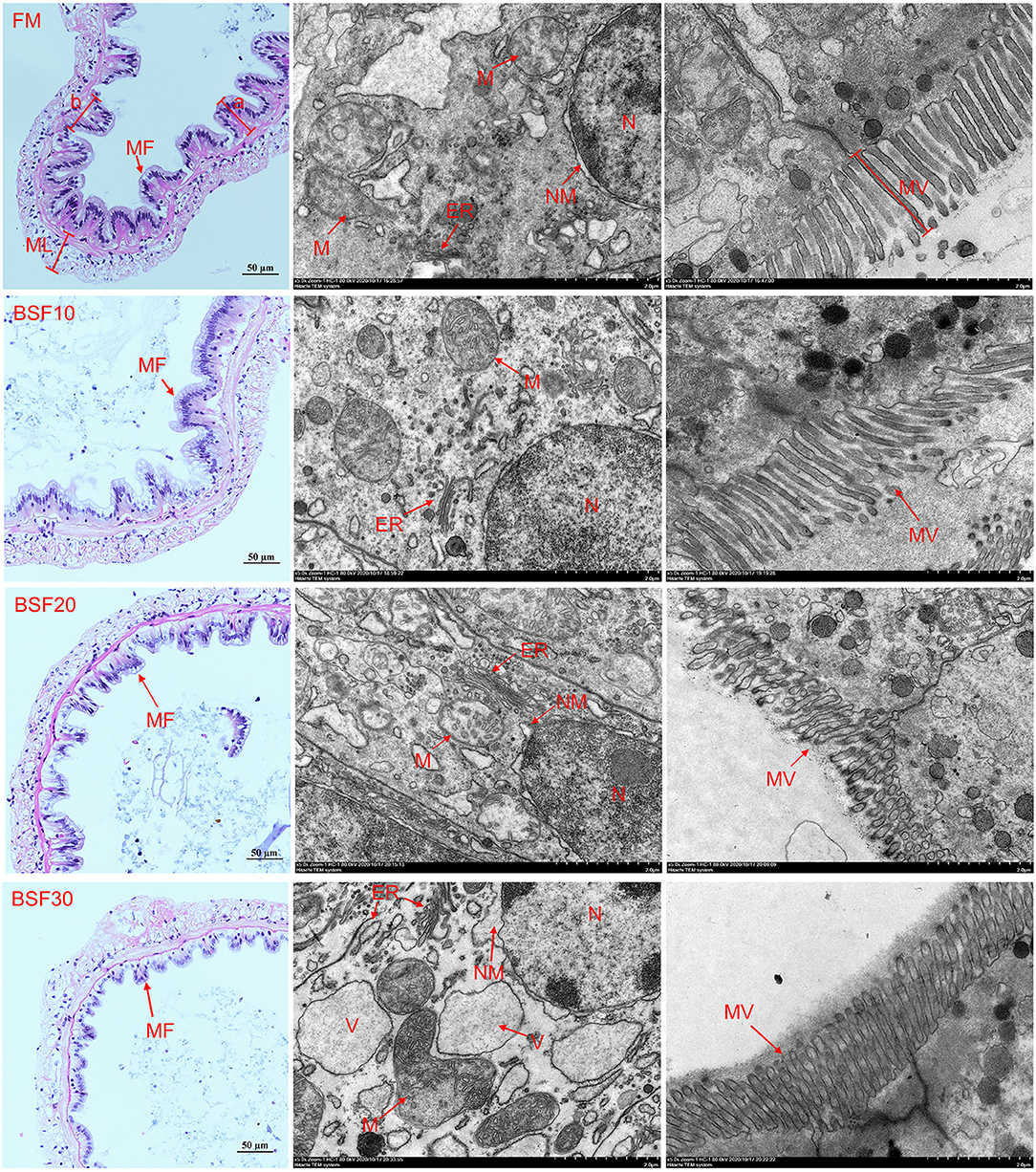
Figure 2. Effect of BSF on midgut histology of L. vannamei. Where: ER, endoplasmic reticulum; N, cell nucleus; NM, nucleus membrane; MF, mucosal folds; ML, muscle layer; M, mitochondria; MV, microvilli; V, Vesicles. a shows height of mucosal folds; and b shows width of mucosal folds. Pictures in the same row are considered as the same group.
As shown in Figure 3, mRNA expression of dorsal and relish significantly decreased in shrimp fed with BSF20 and BSF30, while no significant change was found in shrimp fed with BSF10 compared to those fed with FM (P < 0.05). The mRNA expression of alf (anti-lipopolysaccharide factor) significantly increased in shrimp fed BSF10 but decreased in shrimp fed BSF30 compared to those fed FM (P < 0.05). The mRNA expression of cru (crustins) showed no significant difference between the shrimp fed FM and BSF diets (P > 0.05). The mRNA expression of pen3 (penaeidins 3) significantly increased in shrimp fed with BSF10 and BSF20 (P < 0.05), but no significant changes were observed in shrimp fed with BSF10 compared to those fed with FM (P > 0.05).
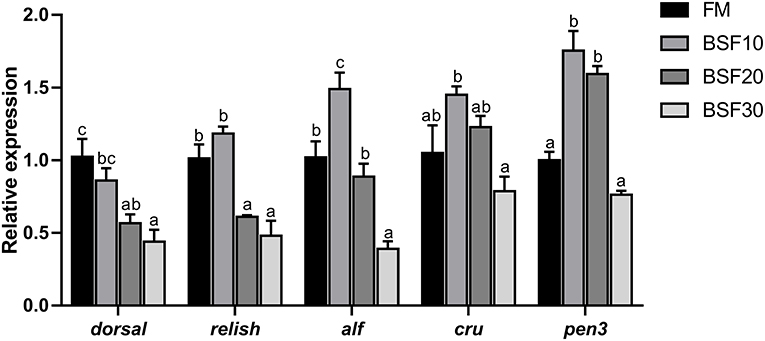
Figure 3. Effect of BSF on mRNA levels of dorsal, relish, alf (anti-lipopolysaccharide factor), cru (crustins), and pen3 (penaeidins 3) in the intestine of L. vannamei. Vertical bars represent the mean ± SEM (n = 3). Data marked with different letters differ significantly (P < 0.05) among groups based on Tukey's HSD test.
A total of 998,755 raw tags were obtained from the gut samples, with an average of 110,972.8 raw tags per sample (ranging from 102,809 to 116,331 sequences). After sequence analysis and filtering, we finally got a total of 942,176 effective tags with an average of 104,686.2 effective tags per sample (ranging from 100,932 to 111,704 sequences). Sequences with more than 97% similarity were clustered into OTUs. As shown in Figure 4A, 224 OTUs were overlapped among the three groups. To be specific, the core OTU numbers in the shrimp fed with FM (104) were the highest, followed by the shrimp fed with BSF30 (104) and BSF20 (51). The PCoA based on weighted-UniFrac distances and analysis of similarities (Anosim) using the Bray–Curtis distance matrix on OUT levels were performed to assess the modification in microbiota composition after the feeding trial. As shown in Figure 4B, the composition of the intestine microbiota from the FM-fed group was clustered closer to BSF30-fed group rather than BSF20-fed group. However, the results of R > 0 and P > 0.05 in Anosim test indicated that the differences in microbiota composition among FM-, BSF20-, and BSF30-fed groups were not significant (P > 0.05) (Supplementary Figure 1). As for the alpha diversity indices, there were no significant differences in bacterial richness parameters (Chao1 and Ace) and bacterial diversity parameters (Shannon and Simpson) among the three groups (P > 0.05) (Table 7). After the feeding trial, the microbial composition of the intestinal microbiota in L. vannamei was altered by the BSF replacement. As shown in Figure 5, Proteobacteria, Bacteroidetes, Firmicutes, Actinobacteria, Verrucomicrobia, Planctomycetes, Cyanobacteria, Tenericutes, Chloroflexi, and Gemmatimonadetes were the top 10 phyla in L. vannamei. The heatmap of the top 20 abundance intestinal microbiota is shown in Figure 6. The abundance of genus Vibrio, Photobacterium, Haloferula, ZOR0006, Rubritalea, Candidatus_Bacilloplasma, Marivita, and Hoppeia increased in shrimp fed with FM diet. The abundance of Ruegeria, Halocynthiibacter, Pseudoalteromonas, Tenacibaculum, Tamlana, Pir4_lineage, Marivita, Ilumatobacter, Hoppeia, and Blastopirellula increased in shrimp fed with BSF20, while the abundance of Bacillus, Acinetobacter, and ZOR0006 increased in shrimp fed with BSF30.
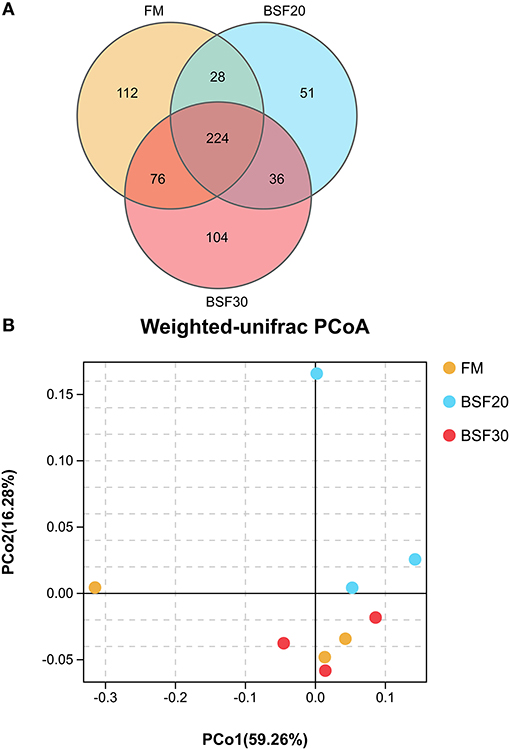
Figure 4. Intestine microbiota responses of L. vannamei fed with diets containing different contents of BSF. (A) Venn diagrams of OTUs among groups; (B) Principal coordinates analysis (PCoA) based on weighted-UniFrac analysis of bacterial profiles.
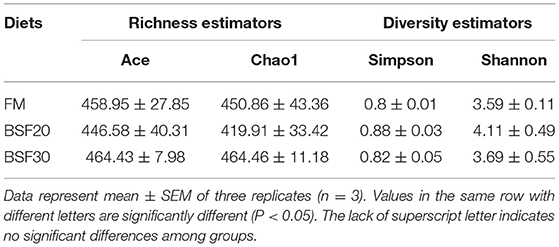
Table 7. The effect of black soldier fly larvae meal (BSF) on alpha diversity indices in the intestinal microbiota of Litopenaeus vannamei.
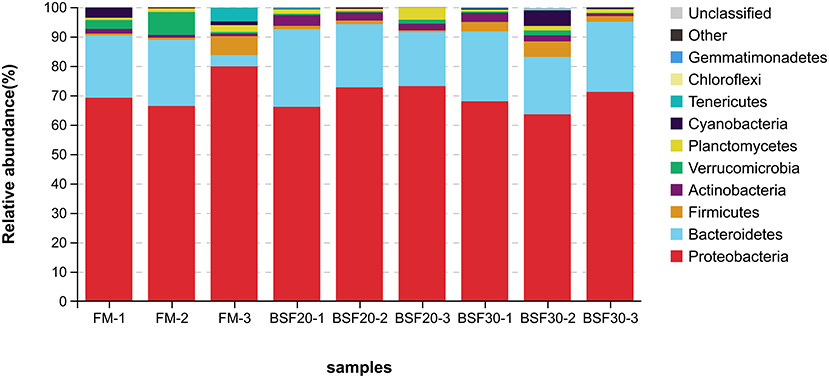
Figure 5. Relative abundance comparison of intestinal microbiota in L. vannamei fed with different diets at the phylum level of the taxonomy (n = 3).
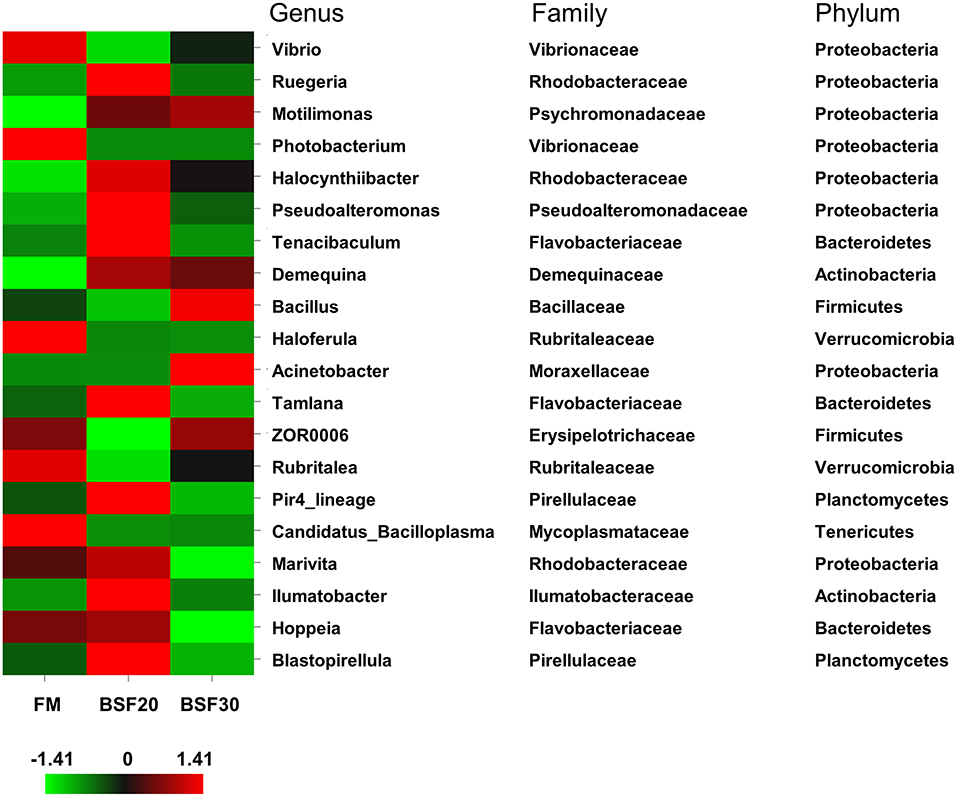
Figure 6. Heatmap of top 20 abundance intestinal microbiota in L. vannamei fed with different diets at the genus level. The color bar of each genus is average of three samples in each group. The taxonomy of the genus (family and phylum) is depicted on the right.
Although differences in the metamorphic stage of insects (larvae and pupae) (Huyben et al., 2019), nutrient composition caused by different food sources (Barroso et al., 2017), and processing techniques (Larouche et al., 2019) may lead to distinguishing experimental results, many studies have demonstrated the potential of BSF for application in aquatic animal feeds (Li et al., 2017; Renna et al., 2017; Wang et al., 2019). Similar to the previous studies, the present study showed that better growth performance was obtained when 20% of FM was replaced with BSF, but replacing 30% of FM significantly reduced the final body weight, weight gain, and specific growth rate of the shrimp (Cummins et al., 2017; Hu et al., 2019). Due to the high lipid content (10–40%), BSF is often processed by oil separation methods into protein-rich components to minimize the risk of fat oxidation and compositional changes. The use of defatted BSF in shrimp feed has also been studied, and defatted BSF can replace 60% of FM without negatively affecting the growth performance and antioxidant and immune capacity of shrimp (Wang et al., 2021). This suggests that the inhibition of growth performance by the addition of high levels of BSF to shrimp diets may be due to the fatty acid composition in BSF. Under the present experimental conditions, the fatty acid composition of BSF was rich in lauric acids, but very low in EPA and DHA, compared to FM. Lipids are not only a major source of energy and carriers of fat-soluble vitamins but also serve as components of cell membranes and precursors for many important metabolites (Zhou et al., 2019; Xie et al., 2020). Replacement of FM with BSF in the diet reduced the highly unsaturated fatty acid contents in the diet, and the hepatocyte disruption was observed in L. vannamei (Wang et al., 2021) and Cyprinus carpio var. Jian (Li et al., 2017). In addition to BSF, other insect proteins have been also studied in shrimp diet, such as silkworms (Bombyx mori L.). Replacing 75% of FM with defatted silkworm meal did not affect the growth performance of L. vannamei but higher levels of silkworm meal would lead to hepatopancreas tubular damage (Rahimnejad et al., 2019). However, the effect of dietary BSF on the intestinal health remains unknown. Excessive replacement of FM may lead to impaired nutritional sensitivity and metabolism (Xu et al., 2016; Shao et al., 2020). Besides, intestinal histology is directly related to the digestion and absorption of nutrients by the shrimp, thus affecting growth.
In addition to the growth status of shrimp, issues related to animal welfare need to be considered, which includes the effects of immune status, oxidative status, and diet on the intestinal structure, directly affecting the digestive function and immune response of shrimp (Bruni et al., 2018). The intestinal structural barrier separates the internal from the external environment and is the first line of defense against invasion by pathogens (Rahimnejad et al., 2018). In the results of HE-staining sections, it was clear that the width and height of intestinal mucosal folds decreased with increasing BSF replacement. The shrinkage of intestinal mucosal folds will reduce the contact area between the intestine and the food of shrimp, which is not conducive to food digestion and nutrient absorption. Besides, results of TEM showed that shrimp fed with BSF30 showed early signs of apoptosis: chromatin consolidation, separation and distribution along the nuclear membrane, swelling of the endoplasmic reticulum vesicles, and abnormal mitochondrial structures, demonstrating that BSF replacement for 30% FM can damage the intestinal epithelial cell structure of shrimp and may explain the reduction of growth performance (Xie et al., 2018). In a previous study, the reduction of FM in diets upregulated the expression of endoplasmic reticulum stress-related genes, resulting in severe endoplasmic reticulum stress in the intestine (Xie et al., 2020). The content of highly unsaturated fatty acids in the diet decreases with the proportion of FM and fish oil in the diet, which negatively affects the biophysical properties of cell membranes and may lead to apoptosis (O'shea et al., 2009).
The non-specific immune system of the shrimp plays an important role in the resistance to pathogen invasion (Gui et al., 2019). To evaluate the effect of BSF on resistance to V. Parahaemolyticus infection, a challenge trial was conducted to compare the disease resistance of shrimp fed diets containing different levels of BSF. Colonizing in the intestine, V. parahaemolyticus would result in EMS, also known as acute hepatopancreatic necrosis disease (Zorriehzahra and Banaederakhshan, 2015; Qi et al., 2017). In this study, the survival rate of shrimp fed with BSF10 was significantly higher than those fed with FM after infection, indicating that BSF replacement of 10% FM could improve the resistance of L. vannamei to V. Parahaemolyticus.
Furthermore, the effect of BSF on the non-specific immune gene expression in the intestine was investigated. dorsal and relish are Rel/NF-κB transcription factors, the former factor activating antifungal and antimicrobial responses in the Toll pathway through a cascade amplification effect, and the latter one activating antimicrobial peptide gene expression in the IMD pathway (Li and Xiang, 2013). The Toll and IMD pathways induce immune-related gene expression during pathogen invasion, thereby regulating the early stages of the immune response (Jin et al., 2018). The results showed that intestinal dorsal and relish mRNA levels were maintained at the control level in the shrimp fed with BSF10 but downregulated in those fed with BSF20 and BSF30. Furthermore, antimicrobial peptides are products of the immune response and are capable of directly killing or removing pathogens (Bachère, 2003; Wang et al., 2007). Anti-lipopolysaccharide factor has been reported in L. vannamei (De La Vega et al., 2008), Fenneropenaeus chinensis (Li et al., 2013), and Penaeus monodon (Supungul et al., 2004), which showed a broad-spectrum of antimicrobial activity against Gram-negative bacteria and endotoxins (Silveira et al., 2018). Crustins are antimicrobial peptides prevalent in crustaceans with antibacterial activity against Gram-positive bacteria, as well as inhibition of fungi and white spot syndrome virus (Shockey et al., 2009; Chen et al., 2019). Penaeidins 3 is an antimicrobial peptide isolated from L. vannamei (Destoumieux et al., 1997) with antibacterial activity against Gram-positive bacteria and fungi (Takuji, 2007). In the present study, mRNA level of alf upregulated in shrimp fed with BSF10, while downregulated in those fed with BSF30, which was consistent with the results of the challenge trial, indicating that dietary BSF replacing 10% FM can improve intestinal immunity in shrimp.
In addition to the immune defense of shrimp, the gut microbiota has been found to have a remarkable effect on animal health, growth, and survival and is of increasing interest to researchers and breeders (He et al., 2017; Li et al., 2018). To date, the effect of BSF on the intestinal microbiota has been studied on aquatic animals, such as rainbow trout (Terova et al., 2019), zebrafish (Danio rerio) (Osimani et al., 2019), rice field eel (Monopterus albus) (Hu et al., 2020), marron (Cherax cainii) (Foysal et al., 2019), and other animals. Since there was no significant difference in the growth performance of shrimp fed with BSF10 and BSF20, we explored the intestinal microbiota of shrimp fed with FM, BSF20, and BSF30 diets. Results showed that there were no significant differences in Anosim tests of beta diversity analysis, indicating that the intestinal microbiota community was not separated in shrimp fed with FM, BSF20, and BSF30 diets. At the phylum level, Proteobacteria was the most common bacteria in the shrimp gut, followed by Bacteroidetes, Firmicutes, and Actinobacteria, which was in line with the results of a previous study (Amoah et al., 2020). Proteobacteria do not change in abundance with environment or diet and are the most stable bacteria in L. vannamei, while the abundance of the other major bacterial phyla (Firmicutes, Bacteroidetes, Actinobacteria) may vary with developmental stage, diet, and environmental factors (Li et al., 2018). At the genus level, Vibrio and Photobacterium are known as opportunistic pathogens and have been reported in shrimp diseases (Avendaño-Herrera et al., 2006; Liu and Qiu, 2007; Wang et al., 2020). Tenacibaculum and Acinetobacter have also been reported as bacterial pathogens, while the former was identified in marine fish, which causes an ulcerative disease (Avendaño-Herrera et al., 2006) as well as the latter was identified as a potential pathogen of red leg disease in the Pacific white shrimp (Huang et al., 2020). Moreover, Candidatus_Bacilloplasma may contribute to WFS in L. vannamei (Hou et al., 2018). In the present study, the reduction of some harmful bacteria in shrimp fed with BSF20 may help improve intestinal health. On the other hand, some beneficial bacteria also differed among FM, BSF20, and BSF30. Haloferula has been reported in sea cucumbers (Apostichopus japonicus) and was reduced by the high levels of selenium and vitamin C supplementation, indicating that Haloferula is related to the antioxidant capacity of the gut (Zeng et al., 2020). Moreover, Pseudoalteromonas has been shown to produce extracellular antibacterial compounds, which could enhance the resistance of shrimp to V. parahaemolyticus (Wang et al., 2018). Bacillus is a non-pathogenic Gram-positive bacterium and is beneficial to the growth performance and immunity of the Pacific white shrimp (Wen et al., 2011; Zokaeifar et al., 2012; Tang et al., 2015). The increase of Pseudoalteromonas in BSF20-fed group and Bacillus in BSF30-fed group may enhance the intestinal health. According to the present study, the increase of beneficial bacteria and the reduction of harmful bacteria suggest that BSF20 diet may improve the intestinal bacterial communities of L. vannamei.
The present study evaluated the effects of BSF on growth, intestinal health, and susceptibility to V. parahaemolyticus of L. vannamei. Results showed that BSF replacement of 10% FM did not negatively affect the growth performance and intestinal histology of the shrimp L. vannamei, but improved the survival rate after infection of the bacteria V. parahaemolyticus. When the replacement of FM was increased to 20%, there was no negative effect on the intestinal integrity and promoted a positive modulation in the intestinal microbiota. However, FM replacement up to 30% promoted intestinal cell apoptosis and degeneration and was deleterious to the shrimp.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA729583.
This animal study was reviewed and approved by the Animal Ethical and Welfare Committee of Guangdong Ocean University.
YC and SX designed the experiments. YC carried out the experiments and drafted the manuscript. SC, SZ, XD, QY, and HL: accountable for some aspects (such as ingredients and sites) of the study in ensuring that the experiment can be carried out properly. SX and BT reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the fund of the National Key R&D Program of China (2019YFD0900200), the National Natural Science Foundation of China (32002402), and the Guangdong Basic and Applied Basic Research Foundation (2019A1515011970, 2021A1515010428).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are grateful to Guangzhou Genedenovo Biotechnology Co., Ltd (Guangdong, China) for assisting in sequencing and bioinformatics analysis (http://www.omicsmart.com).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.706463/full#supplementary-material
Supplementary Figure 1. Analysis of similarities (Anosim) test of Bray–Curtis distances performed on OTU level.
Amoah, K., Dong, X., Tan, B., Zhang, S., Chi, S., Yang, Q., et al. (2020). Administration of probiotic Bacillus licheniformis induces growth, immune and antioxidant enzyme activities, gut microbiota assembly and resistance to Vibrio parahaemolyticus in Litopenaeus vannamei. Aquacult. Nutr. 26, 1604–1622. doi: 10.1111/anu.13106
Avendaño-Herrera, R., Toranzo, A. E., and Magariños, B. (2006). Tenacibaculosis infection in marine fish caused by Tenacibaculum maritimum: a review. Dis. Aquat. Org. 71, 255–266. doi: 10.3354/dao071255
Bachère, E. (2003). Anti-infectious immune effectors in marine invertebrates: potential tools for disease control in larviculture. Aquaculture 227, 427–438. doi: 10.1016/S0044-8486(03)00521-0
Barragan-Fonseca, K. B., Dicke, M., and Van Loon, J. J. (2017). Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed–a review. J. Insects Food Feed 3, 105–120. doi: 10.3920/JIFF2016.0055
Barroso, F. G., Sánchez-Muros, M.-J., Segura, M., Morote, E., Torres, A., Ramos, R., et al. (2017). Insects as food: enrichment of larvae of Hermetia illucens with omega 3 fatty acids by means of dietary modifications. J. Food Composition Anal. 62, 8–13. doi: 10.1016/j.jfca.2017.04.008
Bokulich, N. A., Subramanian, S., Faith, J. J., Gevers, D., Gordon, J. I., Knight, R., et al. (2013). Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 10, 57–59. doi: 10.1038/nmeth.2276
Bruni, L., Pastorelli, R., Viti, C., Gasco, L., and Parisi, G. (2018). Characterisation of the intestinal microbial communities of rainbow trout (Oncorhynchus mykiss) fed with Hermetia illucens (black soldier fly) partially defatted larva meal as partial dietary protein source. Aquaculture 487, 56–63. doi: 10.1016/j.aquaculture.2018.01.006
Cai, L., Wang, L., Song, K., Lu, K., Zhang, C., and Rahimnejad, S. (2020). Evaluation of protein requirement of spotted seabass (Lateolabrax maculatus) under two temperatures, and the liver transcriptome response to thermal stress. Aquaculture 516:734615. doi: 10.1016/j.aquaculture.2019.734615
Cao, J., Yan, J., Wang, G., Huang, Y., Zhang, R., Zhou, T., et al. (2012). Effects of replacement of fish meal with housefly maggot meal on digestive enzymes, transaminases activities and hepatopancreas histological structure of Litopenaeus vannamei. South China Fish. Sci. 8, 72–79. doi: 10.3969/j.issn.2095-0780.2012.05.011
Chen, Y., Lian, Y., He, H., Yuan, K., Zhang, C., Yue, G., et al. (2019). Functional characterization of an ER-stress responding Crustin gene in Litopenaeus vannamei. Fish Shellfish Immunol. 84, 541–550. doi: 10.1016/j.fsi.2018.10.047
Choi, I.-H., Kim, J.-M., Kim, N.-J., Kim, J.-D., Park, C., Park, J.-H., et al. (2018). Replacing fish meal by mealworm (Tenebrio molitor) on the growth performance and immunologic responses of white shrimp (Litopenaeus vannamei). Acta Scientiarum Anim. Sci. 40:e39077. doi: 10.4025/actascianimsci.v40i1.39077
Cummins, V. C., Rawles, S. D., Thompson, K. R., Velasquez, A., Kobayashi, Y., Hager, J., et al. (2017). Evaluation of black soldier fly (Hermetia illucens) larvae meal as partial or total replacement of marine fish meal in practical diets for Pacific white shrimp (Litopenaeus vannamei). Aquaculture 473, 337–344. doi: 10.1016/j.aquaculture.2017.02.022
De La Vega, E., O'leary, N. A., Shockey, J. E., Robalino, J., Payne, C., Browdy, C. L., et al. (2008). Anti-lipopolysaccharide factor in Litopenaeus vannamei (LvALF): a broad spectrum antimicrobial peptide essential for shrimp immunity against bacterial and fungal infection. Mol. Immunol. 45, 1916–1925. doi: 10.1016/j.molimm.2007.10.039
Destoumieux, D., Bulet, P., Loew, D., Van Dorsselaer, A., Rodriguez, J., and Bachere, E. (1997). Penaeidins, a new family of antimicrobial peptides isolated from the shrimp Penaeus vannamei (Decapoda). J. Biol. Chem. 272, 28398–28406. doi: 10.1074/jbc.272.45.28398
Edgar, R. C. (2013). UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998. doi: 10.1038/nmeth.2604
Egerton, S., Culloty, S., Whooley, J., Stanton, C., and Ross, R. P. (2018). The gut microbiota of marine fish. Front. Microbiol. 9:873. doi: 10.3389/fmicb.2018.00873
Foysal, M. J., Fotedar, R., Tay, C.-Y., and Gupta, S. K. (2019). Dietary supplementation of black soldier fly (Hermetica illucens) meal modulates gut microbiota, innate immune response and health status of marron (Cherax cainii, Austin 2002) fed poultry-by-product and fishmeal based diets. PeerJ 7:e6891. doi: 10.7717/peerj.6891
Gui, L., Mai, H., Chi, S., Zhou, W., Li, Y., Tan, B., et al. (2019). Effects of yeast culture on growth performance, hematological parameters, immunity and disease resistance of Litopenaeus vannamei. J. Guangdong Ocean Univ. 39, 30–37. doi: 10.3969/j.issn.1673-9159.2019.03.005
He, Y., Chi, S., Tan, B., Zhang, H., Dong, X., Yang, Q., et al. (2017). Effect of yeast culture on intestinal microbiota of Litopenaeus vannamei. J. Guangdong Ocean Univ. 37, 21–27. doi: 10.3969/j.issn.1673-9159.2017.04.004
Hou, D., Huang, Z., Zeng, S., Liu, J., Wei, D., Deng, X., et al. (2018). Intestinal bacterial signatures of white feces syndrome in shrimp. Appl. Microbiol. Biotechnol. 102, 3701–3709. doi: 10.1007/s00253-018-8855-2
Hu, Y., Huang, Y., Tang, T., Zhong, L., Chu, W., Dai, Z., et al. (2020). Effect of partial black soldier fly (Hermetia illucens L.) larvae meal replacement of fish meal in practical diets on the growth, digestive enzyme and related gene expression for rice field eel (Monopterus albus). Aquacult. Rep. 17:100345. doi: 10.1016/j.aqrep.2020.100345
Hu, J., Wang, G., Huang, W., Zhao, H., Mo, W., and Huang, Y. (2019). Effects of fish meal replacement by black soldier fly (Hermetia illucens) larvae meal on growth performance, body composition, serum biochemical indexes and antioxidant ability of juvenile Litopenaeus vannamei. Chin. J. Animal Nutr. 31, 5292–5300. doi: 10.3969/j.issn.1006-267x.2019.11.046
Huang, X., Gu, Y., Zhou, H., Xu, L., Cao, H., and Gai, C. (2020). Acinetobacter venetianus, a potential pathogen of red leg disease in freshwater-cultured whiteleg shrimp Penaeus vannamei. Aquacult. Rep. 18:100543. doi: 10.1016/j.aqrep.2020.100543
Huyben, D., Vidaković, A., Hallgren, S. W., and Langeland, M. (2019). High-throughput sequencing of gut microbiota in rainbow trout (Oncorhynchus mykiss) fed larval and pre-pupae stages of black soldier fly (Hermetia illucens). Aquaculture 500, 485–491. doi: 10.1016/j.aquaculture.2018.10.034
Jin, M., Xiong, J., Zhou, Q., Yuan, Y., Wang, X., and Sun, P. (2018). Dietary yeast hydrolysate and brewer's yeast supplementation could enhance growth performance, innate immunity capacity and ammonia nitrogen stress resistance ability of Pacific white shrimp (Litopenaeus vannamei). Fish Shellfish Immunol. 82, 121–129. doi: 10.1016/j.fsi.2018.08.020
Larouche, J., Deschamps, M.-H., Saucier, L., Lebeuf, Y., Doyen, A., and Vandenberg, G. W. (2019). Effects of killing methods on lipid oxidation, colour and microbial load of black soldier fly (Hermetia illucens) larvae. Animals 9:182. doi: 10.3390/ani9040182
Li, E., Xu, C., Wang, X., Wang, S., Zhao, Q., Zhang, M., et al. (2018). Gut microbiota and its modulation for healthy farming of Pacific white shrimp Litopenaeus vannamei. Rev. Fish. Sci. Aquacult. 26, 381–399. doi: 10.1080/23308249.2018.1440530
Li, F., and Xiang, J. (2013). Recent advances in researches on the innate immunity of shrimp in China. Dev. Compar. Immunol. 39, 11–26. doi: 10.1016/j.dci.2012.03.016
Li, S., Ji, H., Zhang, B., Zhou, J., and Yu, H. (2017). Defatted black soldier fly (Hermetia illucens) larvae meal in diets for juvenile Jian carp (Cyprinus carpio var. Jian): growth performance, antioxidant enzyme activities, digestive enzyme activities, intestine and hepatopancreas histological structure. Aquaculture 477, 62–70. doi: 10.1016/j.aquaculture.2017.04.015
Li, S., Zhang, X., Sun, Z., Li, F., and Xiang, J. (2013). Transcriptome analysis on Chinese shrimp Fenneropenaeus chinensis during WSSV acute infection. PLoS ONE 8:e58627. doi: 10.1371/journal.pone.0058627
Li, Y., Kortner, T. M., Chikwati, E. M., Belghit, I., Lock, E.-J., and Krogdahl, Å. (2020). Total replacement of fish meal with black soldier fly (Hermetia illucens) larvae meal does not compromise the gut health of Atlantic salmon (Salmo salar). Aquaculture 520:734967. doi: 10.1016/j.aquaculture.2020.734967
Liu, W., and Qiu, D. (2007). Effect of Vibrio alginolyticus on nitric oxide and oxygen free radical in serum of Penaeus vannamei Boone. J. Guangdong Ocean Univ. 27, 60–63. doi: 10.3969/j.issn.1673-9159.2007.03.013
Magoč, T., and Salzberg, S. L. (2011). FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963. doi: 10.1093/bioinformatics/btr507
Oksanen, J., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P. R., O'Hara, R., et al. (2013). Package ‘vegan'. Community ecology package, version 2, 1–295.
O'shea, K. M., Khairallah, R. J., Sparagna, G. C., Xu, W., Hecker, P. A., Robillard-Frayne, I., et al. (2009). Dietary ω-3 fatty acids alter cardiac mitochondrial phospholipid composition and delay Ca2+-induced permeability transition. J. Mol. Cell. Cardiol. 47, 819–827. doi: 10.1016/j.yjmcc.2009.08.014
Osimani, A., Milanović, V., Roncolini, A., Riolo, P., Ruschioni, S., Isidoro, N., et al. (2019). Hermetia illucens in diets for zebrafish (Danio rerio): a study of bacterial diversity by using PCR-DGGE and metagenomic sequencing. PLoS ONE 14:e0225956. doi: 10.1371/journal.pone.0225956
Pilotto, M. R., Argenta, N., Forte, J. M., Hostins, B., Menezes, F. G. R., Maggioni, R., et al. (2020). Environmental rearing conditions are key determinants of changes in immune gene expression patterns in shrimp midgut. Dev. Compar. Immunol. 106:103618. doi: 10.1016/j.dci.2020.103618
Qi, C., Wang, L., Liu, M., Jiang, K., Wang, M., Zhao, W., et al. (2017). Transcriptomic and morphological analyses of Litopenaeus vannamei intestinal barrier in response to Vibrio paraheamolyticus infection reveals immune response signatures and structural disruption. Fish Shellfish Immunol. 70, 437–450. doi: 10.1016/j.fsi.2017.09.004
Qiu, W., Zhang, S., Chen, Y., Wang, P., Xu, X., Li, C., et al. (2014). Litopenaeus vannamei NF-κB is required for WSSV replication. Dev. Compar. Immunol. 45, 156–162. doi: 10.1016/j.dci.2014.02.016
Rahimnejad, S., Hu, S., Song, K., Wang, L., Lu, K., Wu, R., et al. (2019). Replacement of fish meal with defatted silkworm (Bombyx mori L.) pupae meal in diets for Pacific white shrimp (Litopenaeus vannamei). Aquaculture 510, 150–159. doi: 10.1016/j.aquaculture.2019.05.054
Rahimnejad, S., Yuan, X., Wang, L., Lu, K., Song, K., and Zhang, C. (2018). Chitooligosaccharide supplementation in low-fish meal diets for Pacific white shrimp (Litopenaeus vannamei): effects on growth, innate immunity, gut histology, and immune-related genes expression. Fish Shellfish Immunol. 80, 405–415. doi: 10.1016/j.fsi.2018.06.025
Renna, M., Schiavone, A., Gai, F., Dabbou, S., Lussiana, C., Malfatto, V., et al. (2017). Evaluation of the suitability of a partially defatted black soldier fly (Hermetia illucens L.) larvae meal as ingredient for rainbow trout (Oncorhynchus mykiss Walbaum) diets. J. Anim. Sci. Biotechnol. 8, 1–13. doi: 10.1186/s40104-017-0191-3
Ringø, E., Zhou, Z., Vecino, J. G., Wadsworth, S., Romero, J., Krogdahl, Å., et al. (2016). Effect of dietary components on the gut microbiota of aquatic animals. A never-ending story? Aquacult. Nutr. 22, 219–282. doi: 10.1111/anu.12346
Roeselers, G., Mittge, E. K., Stephens, W. Z., Parichy, D. M., Cavanaugh, C. M., Guillemin, K., et al. (2011). Evidence for a core gut microbiota in the zebrafish. ISME J. 5, 1595–1608. doi: 10.1038/ismej.2011.38
Shao, J., Wang, L., Shao, X., and Liu, M. (2020). Dietary different replacement levels of fishmeal by fish silage could influence growth of Litopenaeus vannamei by regulating mTOR at transcriptional level. Front. Physiol. 11:359. doi: 10.3389/fphys.2020.00359
Shockey, J. E., O'leary, N. A., De La Vega, E., Browdy, C. L., Baatz, J. E., and Gross, P. S. (2009). The role of crustins in Litopenaeus vannamei in response to infection with shrimp pathogens: an in vivo approach. Dev. Compar. Immunol. 33, 668–673. doi: 10.1016/j.dci.2008.11.010
Silveira, A. S., Matos, G. M., Falchetti, M., Ribeiro, F. S., Bressan, A., Bachère, E., et al. (2018). An immune-related gene expression atlas of the shrimp digestive system in response to two major pathogens brings insights into the involvement of hemocytes in gut immunity. Dev. Compar. Immunol. 79, 44–50. doi: 10.1016/j.dci.2017.10.005
Suo, Y., Li, E., Li, T., Jia, Y., Qin, J. G., Gu, Z., et al. (2017). Response of gut health and microbiota to sulfide exposure in Pacific white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 63, 87–96. doi: 10.1016/j.fsi.2017.02.008
Supungul, P., Klinbunga, S., Pichyangkura, R., Hirono, I., Aoki, T., and Tassanakajon, A. (2004). Antimicrobial peptides discovered in the black tiger shrimp Penaeus monodon using the EST approach. Dis. Aquat. Org. 61, 123–135. doi: 10.3354/dao061123
Takuji, O. (2007). Effects of lipopolysaccharide on gene expression of antimicrobial peptides (penaeidins and crustin), serine proteinase and prophenoloxidase in haemocytes of the Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 22, 68–76. doi: 10.1016/j.fsi.2006.03.013
Tang, J., Huang, Y., Cai, J., Lu, Y., Wu, Z., and Jian, J. (2015). Effects of a probiotics combined with chinese herbal medicine on growth performance, water quality and resistance to diseases for Litopenaeus vannamei. J. Guangdong Ocean Univ. 35, 47–52. doi: 10.3969/j.issn.1673-9159.2015.06.009
Terova, G., Rimoldi, S., Ascione, C., Gini, E., Ceccotti, C., and Gasco, L. (2019). Rainbow trout (Oncorhynchus mykiss) gut microbiota is modulated by insect meal from Hermetia illucens prepupae in the diet. Rev. Fish Biol. Fish 29, 465–486. doi: 10.1007/s11160-019-09558-y
Wang, G., Peng, K., Hu, J., Mo, W., Wei, Z., and Huang, Y. (2021). Evaluation of defatted Hermetia illucens larvae meal for Litopenaeus vannamei: effects on growth performance, nutrition retention, antioxidant and immune response, digestive enzyme activity and hepatic morphology. Aquacult. Nutr. 27, 986–997. doi: 10.1111/anu.13240
Wang, G., Peng, K., Hu, J., Yi, C., Chen, X., Wu, H., et al. (2019). Evaluation of defatted black soldier fly (Hermetia illucens L.) larvae meal as an alternative protein ingredient for juvenile Japanese seabass (Lateolabrax japonicus) diets. Aquaculture 507, 144–154. doi: 10.1016/j.aquaculture.2019.04.023
Wang, H., Wang, C., Tang, Y., Sun, B., Huang, J., and Song, X. (2018). Pseudoalteromonas probiotics as potential biocontrol agents improve the survival of Penaeus vannamei challenged with acute hepatopancreatic necrosis disease (AHPND)-causing Vibrio parahaemolyticus. Aquaculture 494, 30–36. doi: 10.1016/j.aquaculture.2018.05.020
Wang, Y., Chang, P., and Chen, H. (2007). Tissue expressions of nine genes important to immune defence of the Pacific white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 23, 1161–1177. doi: 10.1016/j.fsi.2007.04.004
Wang, Z., Shi, C., Wang, H., Wan, X., Zhang, Q., Song, X., et al. (2020). A novel research on isolation and characterization of Photobacterium damselae subsp. damselae from Pacific white shrimp, Penaeus vannamei, displaying black gill disease cultured in China. J. Fish Dis. 43, 551–559. doi: 10.1111/jfd.13153
Wen, C., Xiu, M., Zhang, L., Li, X., and Wu, Y. (2011). Detection of Bacillus preparations used for aquaculture. J. Guangdong Ocean Univ. 31, 39–44. doi: 10.3969/j.issn.1673-9159.2011.01.008
Xie, S., Liu, Y., Tian, L., Niu, J., and Tan, B. (2020). Low dietary fish meal induced endoplasmic reticulum stress and impaired phospholipids metabolism in juvenile Pacific white shrimp, Litopenaeus vannamei. Front. Physiol. 11:1024. doi: 10.3389/fphys.2020.01024
Xie, S., Liu, Y., Zeng, S., Niu, J., and Tian, L. (2016). Partial replacement of fishmeal by soy protein concentrate and soybean meal based protein blend for juvenile Pacific white shrimp, Litopenaeus vannamei. Aquaculture 464, 296–302. doi: 10.1016/j.aquaculture.2016.07.002
Xie, S., Wei, D., Yin, P., Zheng, L., Guo, T., Liu, Y., et al. (2019). Dietary replacement of fish-meal impaired protein synthesis and immune response of juvenile Pacific white shrimp, Litopenaeus vannamei at low salinity. Compar. Biochem. Physiol. Part B Biochem. Mol. Biol. 228, 26–33. doi: 10.1016/j.cbpb.2018.11.002
Xie, S., Zheng, L., Wan, M., Niu, J., Liu, Y., and Tian, L. (2018). Effect of deoxynivalenol on growth performance, histological morphology, anti-oxidative ability and immune response of juvenile Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 82, 442–452. doi: 10.1016/j.fsi.2018.08.053
Xu, D., He, G., Mai, K., Zhou, H., Xu, W., and Song, F. (2016). Postprandial nutrient-sensing and metabolic responses after partial dietary fishmeal replacement by soyabean meal in turbot (Scophthalmus maximus L.). Br. J. Nutr. 115, 379–388. doi: 10.1017/S0007114515004535
Zeng, F., Rabbi, M. H., Hu, Y., Li, Z., Ren, X., Han, Y., et al. (2020). Synergistic effects of dietary selenomethionine and vitamin C on the immunity, antioxidant status, and intestinal microbiota in sea cucumber (Apostichopus japonicus). Biol. Trace Elem. Res. 9, 1–13. doi: 10.1007/s12011-020-02483-3
Zhou, W., Rahimnejad, S., Lu, K., Wang, L., and Liu, W. (2019). Effects of berberine on growth, liver histology, and expression of lipid-related genes in blunt snout bream (Megalobrama amblycephala) fed high-fat diets. Fish Physiol. Biochem. 45, 83–91. doi: 10.1007/s10695-018-0536-7
Zokaeifar, H., Balcázar, J. L., Saad, C. R., Kamarudin, M. S., Sijam, K., Arshad, A., et al. (2012). Effects of Bacillus subtilis on the growth performance, digestive enzymes, immune gene expression and disease resistance of white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 33, 683–689. doi: 10.1016/j.fsi.2012.05.027
Keywords: Litopenaeus vannamei, black soldier fly larvae meal, intestinal histology, non-specific immune, intestinal microbiota
Citation: Chen Y, Chi S, Zhang S, Dong X, Yang Q, Liu H, Tan B and Xie S (2021) Evaluation of the Dietary Black Soldier Fly Larvae Meal (Hermetia illucens) on Growth Performance, Intestinal Health, and Disease Resistance to Vibrio parahaemolyticus of the Pacific White Shrimp (Litopenaeus vannamei). Front. Mar. Sci. 8:706463. doi: 10.3389/fmars.2021.706463
Received: 07 May 2021; Accepted: 15 July 2021;
Published: 10 August 2021.
Edited by:
Kang-le Lu, Jimei University, ChinaCopyright © 2021 Chen, Chi, Zhang, Dong, Yang, Liu, Tan and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiwei Xie, eHN3enNkeEAxNjMuY29t; Beiping Tan, YnB0YW5AMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.