
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Mar. Sci. , 03 August 2021
Sec. Marine Biology
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.702492
This article is part of the Research Topic Biological Invasions in the Mediterranean Sea View all 13 articles
Herbivory has long been considered an important component of biotic resistance against macroalgae invasions in marine habitats. However, most of the studies on herbivory of invasive algae refer only to consumption by strictly herbivorous organisms, whereas consumption by omnivorous species has been largely ignored and rarely quantified. In this study, we assess whether the commonest omnivorous sparid species in the Mediterranean Sea are consuming the highly invasive alga, Caulerpa cylindracea, and determine both, its importance in their diet and their electivity toward it as a source of food. Our results confirm that three of the four fish species studied regularly consume C. cylindracea, but in most cases, the importance of C. cylindracea in the diet is low. Indeed, the low electivity values indicate that all species avoid feeding on the invasive alga and that it is probably consumed accidentally. However, despite animals and detritus being the main food for these sparid species, several individual specimens were found to have consumed high amounts of C. cylindracea. This suggests a potential role that these fish species, being really abundant in shallow rocky bottoms, may play in controlling, to some extent, the abundance of the invader.
Invasive macroalgae are one of the most successful and conspicuous groups of invaders in marine systems (Schaffelke et al., 2006; Thomsen et al., 2016). These organisms, once established, contribute to the homogenization of marine habitats and affect the structure of native assemblages by reducing both native species biomass and the overall assemblage diversity (Williams and Smith, 2007; Schaffelke and Hewitt, 2008; Thomsen et al., 2009, 2016). In the receiving community, the establishment and persistence of invasive algae can be reduced and affected by biotic and abiotic factors (Dunstan and Johnson, 2007; Catford et al., 2009; Thomsen et al., 2009; Kimbro et al., 2013; Papacostas et al., 2017), thus determining the invasive success of the invader. Among biotic factors, herbivory has long been considered as a potential biotic resistance mechanism and many studies have been conducted worldwide to assess the role of this mechanism on invasive macroalgae success (see references within Kimbro et al., 2013; Papacostas et al., 2017). Until now, assessments on the role of herbivory as a limiting factor for macroalgae invasion have mainly considered strictly herbivorous species (e.g., Lyons and Scheibling, 2008; Britton-Simmons et al., 2011; Enge et al., 2017; Noè et al., 2017; Caselle et al., 2018), reporting contrasting results depending on the assemblage and the invasive species considered (e.g., Sumi and Scheibling, 2005; Steinarsdóttir et al., 2009; Tomas et al., 2011b; Nejrup et al., 2012; Enge et al., 2017; Caselle et al., 2018). Actually, considering that omnivores have been reported to influence macroalgae and phytobenthos abundance in different ecosystems around the globe (Bellwood et al., 2006; Mendes et al., 2015, 2019; Tebbett et al., 2020), and that they can act as important functional groups, for instance, in the reversal of community phase-shifts in coral reefs (Bellwood et al., 2006; Tebbett et al., 2020), we pose that they could also potentially consume invasive alga species and influence their abundance. Therefore, to improve our understanding on the ecological role of omnivorous fishes and to assess whether they can contribute to the resistance of native habitats against invaders, it is necessary to assess invasive alga consumption by these species.
In the Mediterranean Sea, the most successful and widespread invasive macroalga is C. cylindracea (Klein and Verlaque, 2008; Katsanevakis et al., 2016), a green alga native to the Southwestern coast of Australia that was first detected in Mediterranean waters in Libya in 1990 (Nizamuddin, 1991). Since then, it has colonized marine communities throughout the entire Mediterranean basin (Piazzi et al., 2005; Klein and Verlaque, 2008), where it can exert strong detrimental effects on native communities (Piazzi et al., 2001; Klein and Verlaque, 2008; Bulleri et al., 2016, 2017). However, despite its rampant success, several C. cylindracea meadows have suffered sudden steep declines in abundance some years after the onset of the invasion (Klein and Verlaque, 2008; García et al., 2016; Santamaría et al., 2021), which may indicate the existence of certain resistance mechanisms against this invasive species. Among these, herbivory on C. cylindracea has been described and assessed mainly in relation to the strictly herbivorous species present in the Mediterranean Sea, such as the fishes Sarpa salpa and Siganus luridus (Azzurro et al., 2004; Ruitton et al., 2006; Tomas et al., 2011b; Santamaría et al., 2021), and the sea urchins Paracentrotus lividus, Sphaerechinus granularis and Arbacia lixula (Ruitton et al., 2006; Bulleri et al., 2009; Cebrian et al., 2011; Tomas et al., 2011a). Nevertheless, there are omnivorous fish species, such as Diplodus sargus, Boops boops, and Spondyliosoma cantharus, that have been occasionally observed feeding on C. cylindracea before (Ruitton et al., 2006; Box et al., 2009; Terlizzi et al., 2011). Unfortunately, information is scarce on whether C. cylindracea is a common food source for omnivorous fish species, or whether these fish actively elect to feed on it. Since some of these species are the dominant fish in the shallow, infralittoral rocky habitats of the Mediterranean Sea (García-Rubies, 1997; Sala and Ballesteros, 1997; García-Charton et al., 2004), information on their consumption of C. cylindracea is needed to assess whether they can potentially contribute to limit the abundance of the invader.
In this study, the diets of four of the most abundant omnivorous sea bream species (Sparidae) were examined in order to determine (i) whether they feed on the invasive alga C. cylindracea, (ii) whether C. cylindracea is important in their diet, and (iii) whether they actively select or avoid C. cylindracea as a source of food.
The samples for this study were collected in the Cabrera Archipelago National Park (North-Western Mediterranean Sea; 39°12′21″ N, 2°58′44″ E) (Supplementary Figure 1) in 2008. This marine-terrestrial protected area was established in 1991 and since then it has maintained an exceptional conservation status for its marine habitats (Sala et al., 2012; Coll et al., 2013; Guidetti et al., 2014). C. cylindracea was recorded for the first time in the National Park in 2003 at a depth of 30–35 m and since then its distribution has expanded to cover most of its benthic communities at depths of between 0 and 65 m (Cebrian and Ballesteros, 2009).
To determine whether non-strictly herbivorous fish species consume C. cylindracea, specimens for this study were captured by artisanal long-lines and gillnets once a month during June and July 2008, at different sites across the Archipelago, at Ses Rates and Foradada Islets (Supplementary Figure 1). These two sites where chosen because they are representative areas for the Cabrera Archipelago and have similar benthic community composition and abundances of sparid fishes (Reñones et al., 1997). Fishing campaigns were performed in the summer because it corresponds to the period of the year when the activity of the targeted fishes is higher. The main fishes targeted belong to the family Sparidae: white sea bream (D. sargus), annular sea bream (Diplodus annularis), two-banded sea bream (Diplodus vulgaris) and black sea bream (S. cantharus). These species were chosen because they are common representatives of the fish assemblages found in the Western Mediterranean, they are not herbivorous but can feed on macroalgae (Sala and Ballesteros, 1997) and some of them have been observed feeding on C. cylindracea before (Box et al., 2009; Terlizzi et al., 2011). These four species have different abundances within the National Park, being S. cantharus the least abundant and D. vulgaris the most abundant (Reñones et al., 1997; Coll, 2020). Specifically, in a 250 m2 area, 1.7 S. cantharus, 1.9 D. annularis, 5.3 D. sargus, and up to 42 D. vulgaris individuals, were found.
The long-lines and gillnets, two gears commonly used in artisanal fishing, were deployed in the morning at depths of between 10 and 30 m. Gillnets were 300 m long, had a mess size of 40 mm, and were left in the water for 4 h; whereas artisanal long-lines were 250 m long, had a total of 150 hooks, and were left in the water for 4 h. In total, two fishing events, one in June and the other in July, were performed at each location and with each gear. Every time a targeted fish species was hauled in, it was gutted and its stomach was stored and preserved in buffered 4% formaldehyde-seawater solution for later analysis of its content. Once in the laboratory, the species composition and abundance of the food items in each fish stomach was determined under a Stemi 2000-C stereomicroscope (Carl Zeiss, Berlin, Germany). The content of each stomach was spread onto a reticulated Petri dish and the food items were classified to the lowest taxonomic level possible. Both surface area and weight measurements can reflect the dietary contribution of food items (Hyslop, 1980; Macdonald and Green, 1983), but in this case, and to avoid biases that could be derived from the small quantities present in the stomach contents, surface area measurements were preferred over weight measurements to quantify the dietary contribution of each food item. As such, the abundance of a particular food item was estimated as the percentage cover on the reticulated fields of the Petri dish in relation to the cover of the whole stomach content. When a species had a minimal presence and its cover could not be determined, a value of 0.1% of relative coverage was assigned.
When calculating the relative measures of prey quantity (RMPQ), the stomach contents were divided into the following five food categories: C. cylindracea, Other algal content, Seagrasses, Animal content and Organic detritus. Subsequently, for each fish species, the percentage frequency of occurrence of each food category (FO) was calculated as:
where Si is the number of stomachs containing the food category and St is the total number of stomachs analyzed for that particular fish species. The FOi value is a measure of the consistency with which a species selects a given food category and was used to calculate two dietary indices that allow to compare the diets between species: the Combined Index (Q) and the Geometric Index of Importance (GII).
The combined index, Q, was chosen to assess the relative importance of each food category for each fish species. This index standardizes the abundance of each category and increases the importance of frequent smaller items while reducing the importance of occasional larger items (Nilssen et al., 2019). It was calculated as:
where Vi refers to the percentage surface of a food category, FOi refers to the frequency of occurrence of the given food category, and m is the total number of food categories.
On the other hand, the Geometric Index of Importance, GII, represents the degree of feeding specialization on a particular food type (Assis, 1996; Preti et al., 2001) and allows us to classify the food categories as: “Primary prey,” “Secondary prey,” and “Occasional prey” in relation to the larger discontinuities in a decreasing sequence of values (Assis, 1996; Tripp-Valdez et al., 2015). It was calculated as:
Finally, the degree to which the four fish species tend to elect to feed on C. cylindracea, was assessed by Ivlev’s electivity Index (E) (Ivlev, 1961). This Index was determined by:
where di = % of C. cylindracea in the stomach content and ai = % of C. cylindracea available in the environment (see following section). The values of the Ivlev’s Index (E) can range from −1 (complete avoidance of the food item) to +1 (exclusive selection of the item), with positive values indicating that the food item is selected and eaten more than it is encountered by chance in the environment (Ivlev, 1961).
The abundance of C. cylindracea at the sampling sites where fish specimens were captured was assessed by means of scuba diving, also in the summer 2008. At each site, a perpendicular transect to shore was done, at depths of 10–30 m, so as to cover the same bathymetric range as that of the fishing gear used to collect fish samples. To estimate C. cylindracea abundance, a total of 30 quadrats measuring 25 cm × 25 cm were randomly positioned within each 10 m-depth range (total of 90 quadrats per sampling site). These quadrats were divided into 25 subquadrats of 5 cm × 5 cm and the number of subquadrats where C. cylindracea appeared was used as the unit of abundance (Sala and Ballesteros, 1997; Cebrian and Ballesteros, 2004; Sant et al., 2017). Subsequently, the average C. cylindracea abundance for the study area was calculated and this value was used in the calculation of the Ivlev’s electivity Index.
Additionally, to characterize the benthic macroalgae community, the quadrat methodology was also used. In this case, at each site and at each depth stratum (10, 20, and 30 m depth), six 25 cm × 25 cm quadrats were randomly positioned and the abundance of each algal species was calculated as a function of the number of subquadrats where each species appeared. Subsequently, as there were no differences in the benthic composition between sites, the average abundance per alga species at the study area was calculated.
To evaluate sampling sufficiency, prey accumulation curves for each of the sampled species were generated using the package BiodiversityR (Kindt and Coe, 2005) in the R environment (R Core Team, 2018). Two curves per species were fitted, one to the number of prey taxa and the other to the number of food categories, to assess whether the number of stomachs analyzed was adequate for the diet characterization of each species.
Differences in the specific composition of stomach contents between fish species were assessed through multivariate techniques such as non-metric multi-dimensional scaling plots (NMDS plots), analysis of similarities (ANOSIM) and similarity percentage analysis (SIMPER). All of these techniques were performed in R, within the vegan package (Oksanen et al., 2018). First, in order to visualize and represent stomach content composition, a NMDS (Clarke and Warwick, 1994; Cox and Cox, 2000) based on the Bray-Curtis dissimilarity matrix of the square root transformed data was plotted and the most important species that determine the least stressful ordination were detected using the envfit function within the vegan package. Then, the statistical differences in the food items consumed by the fish species were assessed using ANOSIM (Clarke, 1993), applied to the Bray-Curtis dissimilarity matrix, with fish species as a fixed factor. Additionally, a pairwise ANOSIM was performed by modifying the pairwise.adonis function1 and the R-values obtained were used as an indication of diet similarity, with values near 1 indicating separation in diet composition and values near 0 indicating diet similarity (Rogers et al., 2012). Finally, a SIMPER analysis based on the Bray-Curtis dissimilarity index was used to assess the relative contribution of each food item to the overall differences between fish species diets.
During the sampling events, a total of 93 fishes were captured, with D. sargus being the most abundant (n = 51) followed by S. cantharus (n = 22), D. vulgaris (n = 13), and D. annularis (n = 7). All the stomachs examined contained ingested material of some kind, which, as a whole, was composed of a high diversity of taxonomic groups, with 73 different prey items identified, 32 of them to the species level (Supplementary Table 1).
According to the prey accumulation curves (Supplementary Figure 2), sampling sufficiency was a little low to quantitatively characterize the diet of the sampled species using the full resolution of the data (lowest taxonomic level), as all the curves were only beginning to plateau but did not reach the asymptote. However, asymptotes were reached for all the species when prey taxa was combined in food categories, which suggests that our data is adequate to investigate the quantitative contribution of these categories to the diet of the sampled species.
Differences in stomach content were observed between species in terms of the dominant prey categories, although organic detritus and animal content were certainly prominent in all four species (Table 1). In this sense, the Combined Index (Q) and the Geometric Index of Importance (GII), identified the category “Organic detritus” as the primary food item for S. cantharus, while the category “Animal content” was the primary prey for the other three fish species (Figures 1, 2). Despite this predominance for “Animal” and “Organic detritus” items, algae and seagrasses were found in all the species, being the stomach contents of both D. sargus and D. annularis particularly rich in algae, with values of around 18 and 30%, respectively (Table 1).
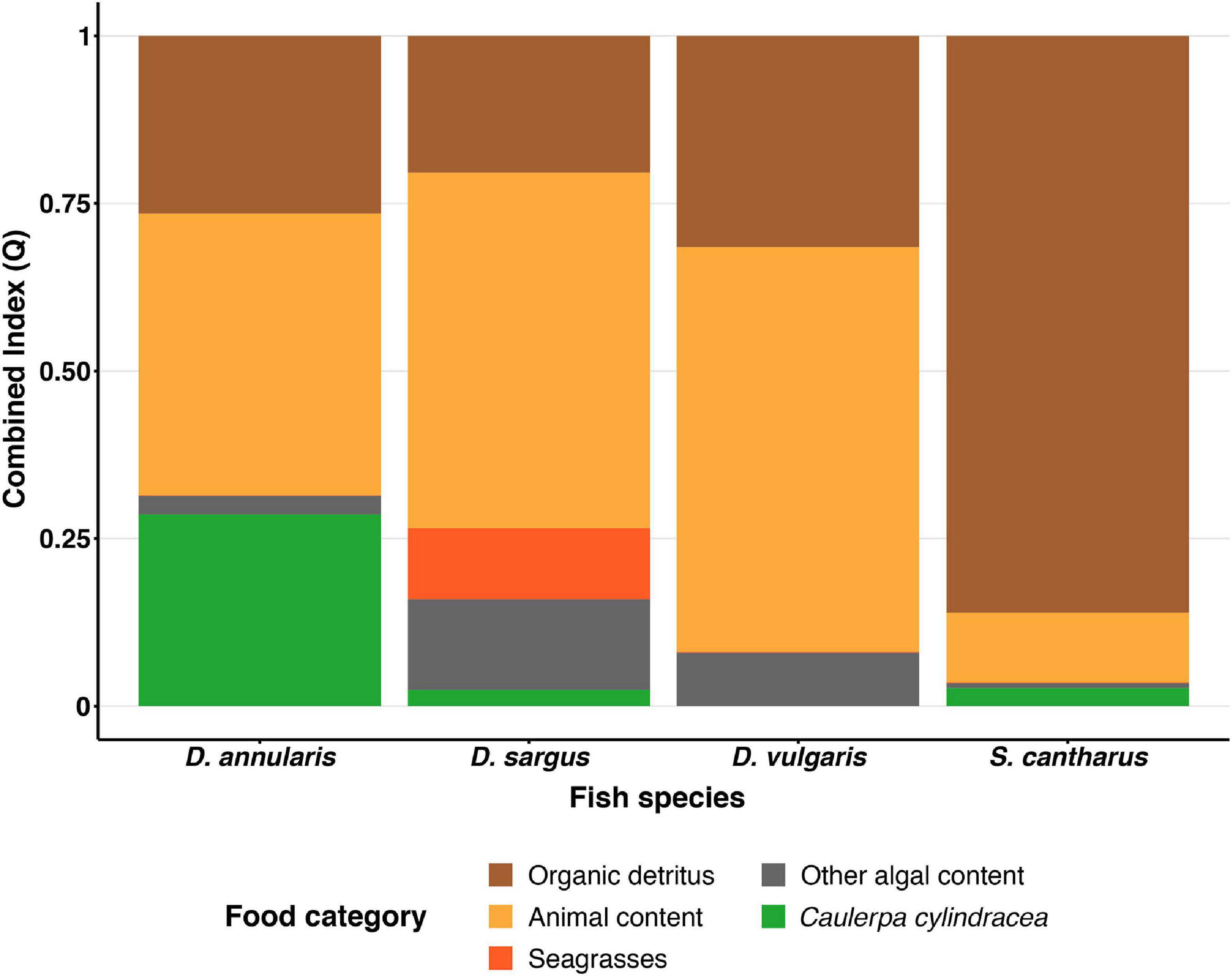
Figure 1. Combined Index (Q) for each fish species. Each color represents one of five food categories (Organic detritus, Animals, Seagrasses, Other algae and Caulerpa).
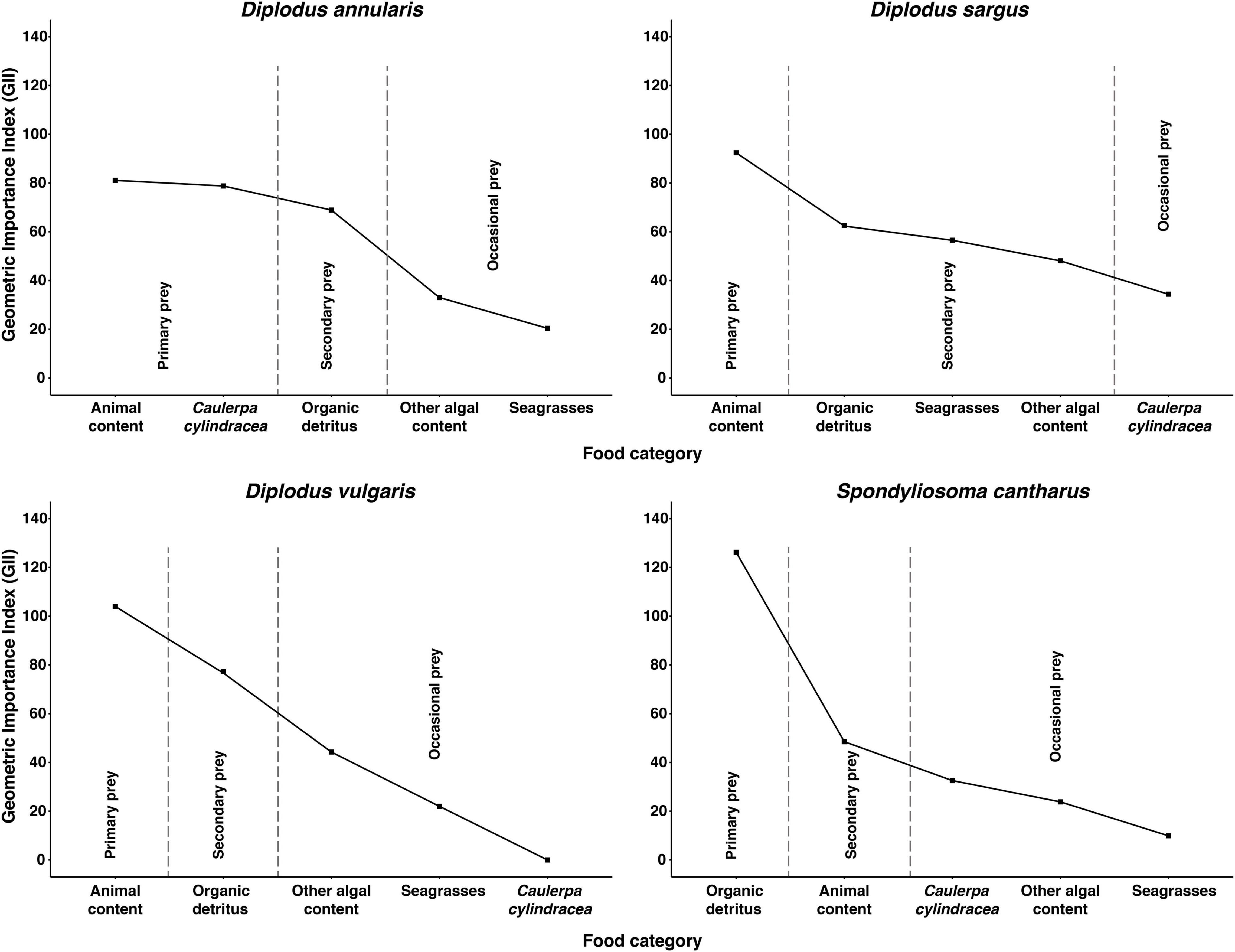
Figure 2. Geometric Importance Index (GII) for each fish species. Food items are classified as: “Primary prey,” “Secondary prey,” or “Occasional prey” according to their relative importance to the diet of each fish species. The line connecting the points was added to help interpretation of the figure.
Regarding the consumption of the invasive alga C. cylindracea, 81, 45, and 41% of the D. annularis, D. sargus and S. cantharus specimens contained C. cylindracea in their stomachs, respectively; whereas D. vulgaris was the only fish species that had not consumed the invader. However, the contribution of C. cylindracea to the total stomach content was generally low, except for D. annularis, in which 26% of the stomach content corresponded to the invasive species (Table 1). Actually, both dietary indexes, Q and GII, classified C. cylindracea as a primary food item for D. annularis, being the second most common food category in the stomachs and having values similar to animal content (Figures 1, 2). In contrast to this, C. cylindracea was classified as an occasional food item for both D. sargus and S. cantharus (Figures 1, 2), but most of the algae content in the stomachs of S. cantharus corresponded to the invasive species (≈74%; Table 1). However, even with the high proportion of C. cylindracea found in some of the studied species, the negative values obtained for the Ivlev’s Electivity Index suggest that C. cylindracea is generally avoided by all the species (Table 2), despite C. cylindracea being the dominant species in the benthic community (Supplementary Table 1).

Table 2. Mean ± S.E. values for Ivlev’s electivity Index (E), as a measure of the electivity of the four sparid fish species studied towards the invasive alga C. cylindracea.
Considering the whole diet of the four sampled sea bream species, the graphical ordination suggested that there might be some overlap between diets (Supplementary Figure 3), yet the ANOSIM detected significant differences in the stomach content composition between all fish species (ANOSIM; p < 0.05; Supplementary Table 2), with two exceptions: D. sargus—D. vulgaris and D. sargus—D. annularis (p > 0.05; Supplementary Table 2). In this sense, the greatest dissimilarities in diet were found between S. cantharus and two of the Diplodus species, D. annularis and D. vulgaris (R = 0.63 and R = 0.47 respectively, Supplementary Table 2), since the diet of S. cantharus was more homogeneous (Supplementary Figure 2) and it was dominated by organic detritus rather than by animal content (Table 1 and Figures 1, 2). Actually, the SIMPER analysis identified “organic detritus” as the biggest contributor to the diet dissimilarities between the four fish species, with values ranging from 19 to 40% (Supplementary Table 3). Remarkably, C. cylindracea was one of the species most strongly influencing the graphical ordination (Supplementary Figure 3) and the SIMPER analysis identified the invasive alga as the second most important food item in terms of explaining the diet dissimilarities between D. annularis and the other fish species (Supplementary Table 3).
Stomach content analysis of some of the commonest species in the shallow rocky infralittoral communities in the western Mediterranean Sea, revealed that three of the four studied fish species have consumed the invasive alga C. cylindracea. Actually, the invader was identified as the most prominent algae for two of the fish species, D. annularis and S. cantharus, and was a primary food item for D. annularis. On the contrary, it was only an occasional food item for both S. cantharus and D. sargus. Complementary, despite these species having a diet predominately based on animal and detritus content (Sala and Ballesteros, 1997; Gonçalves and Erzini, 1998; Pita et al., 2002; Box et al., 2009; Terlizzi et al., 2011; Felline et al., 2012, 2017), our results show that they can also feed on considerable amounts of algae, with values exceeding those previously reported for non-strictly herbivorous sea bream species (Sala and Ballesteros, 1997; Sánchez-Jerez et al., 2002; Leitão et al., 2007; Box et al., 2009).
In general, being C. cylindracea a widespread and regionally abundant alga (Klein and Verlaque, 2008; Katsanevakis et al., 2016), our findings clearly suggest that it can potentially become a recurrent food item for omnivorous fish species, similarly to what has happened with the herbivorous fish S. salpa, which has adopted the invader as a preferred food item (Tomas et al., 2011b). Actually, several studies involving lower sample sizes, had previously reported the ability of D. sargus, S. cantharus, and D. vulgaris to occasionally feed on C. cylindracea on other locations in the Mediterranean Sea (Box et al., 2009; Terlizzi et al., 2011; Felline et al., 2012, 2017; Gorbi et al., 2014). In our assessment, however, C. cylindracea consumption seems frequent, as a high proportion of the sampled individuals (41%) was found with the invader in the stomach. Interestingly, our findings are, to our knowledge, the first evidence of D. annularis feeding on C. cylindracea in the Mediterranean Sea, but given that almost all the fished individuals (86%) had consumed the invader and that they had high amounts of C. cylindracea in their stomachs, this fish species should be included in subsequent assessments to further elucidate whether this feeding behavior is common across the Mediterranean region. Unfortunately, it should be taken into account that sample sizes, mainly for D. vulgaris and D. annularis, were low (Supplementary Figure 2), so the findings reported here should be interpreted as a first exploration. Therefore, to fully characterize the contribution of C. cylindracea to the diet of the different seabream species and to thoroughly quantify C. cylindracea consumption, the analysis of more stomach contents is definitively needed. Despite this, however, our data clearly shows that the invader has become a recurrent food source for at least three of the four assessed species, according to C. cylindracea being found consistently in the stomach contents of these species.
Concurrently, despite previous studies reporting the consumption of C. cylindracea by omnivorous fishes, none of them reports the availability of C. cylindracea in the environment, making it impossible to assess whether the omnivorous fishes are actually targeting the invader. In this sense, in our study area, C. cylindracea was found to be the dominant species in the benthic communities where the fish species were fished, with mean coverage values close to 55% (Supplementary Table 1). However, electivity of the fish species for C. cylindracea suggests that all the species were avoiding feeding on the invader, although laboratory experiments should be considered to have a more detailed interpretation on the avoidance/preference for the invader. Therefore, the low electivity suggests that the consumption by the omnivorous fishes was more occasional than that of the strictly herbivorous fishes, which show a positive electivity for the invader (Tomas et al., 2011b). Surprisingly, despite this general avoidance of C. cylindracea, high amounts of the invader were found in the stomach contents of some individuals, with values reaching over 35%. This suggests that the invasive alga is probably consumed accidentally when the fish are trying to feed on other prey living within the dense meadows of C. cylindracea. In fact, polychaetes, mollusks, and decapods—which are the preferential prey for most of the sea breams studied (Bauchot and Hureau, 1986; Sala and Ballesteros, 1997; Gonçalves and Erzini, 1998; Pita et al., 2002; Leitão et al., 2007)—have been found to be very abundant under the stolons of C. cylindracea (Carriglio et al., 2003; Galil, 2007; Box, 2008; Klein and Verlaque, 2008). Furthermore, the suggestion that consumption of the alga is accidental is also supported by the low assimilation of C. cylindracea in the stomach contents, as in most cases it was found intact and undigested (Supplementary Figure 4). However, taking into account that some of the sea breams considered here have small home ranges and show strong site fidelity (D’Anna et al., 2011; March et al., 2011; Alós et al., 2012; Di Lorenzo et al., 2014), it cannot be ruled out that they might also be forced to feed on C. cylindracea in heavily colonized areas, and this might have a negative impact on the physiology of the fish species. In fact, previous evidence relates C. cylindracea consumption to a decrease in certain essential fatty acids in fish tissues and liver (Felline et al., 2014), to an increase in the levels of antioxidants and in pro-oxidant effects (Box et al., 2009; Terlizzi et al., 2011; Felline et al., 2012), to a decrease in the condition factor (Terlizzi et al., 2011) and to a decrease in the gonadosomatic index (Felline et al., 2012), all of which may negatively affect the fish fitness. It is not yet clear what causes these physiological responses, although they could be caused by the accumulation of some of the compounds produced by C. cylindracea, such as caulerpenyne, a toxic, secondary metabolite that has herbivore-deterrent properties (Paul et al., 2007). However, considering that Caulerpa prolifera, a native species in the Mediterranean Sea, has much higher caulerpenyne concentrations than C. cylindracea (Box et al., 2010) and that sea breams can often consume the native Caulerpa species (Supplementary Table 1; Chaouch et al., 2013, 2014; Marco-Méndez et al., 2017), it is likely that the fish might have developed a certain tolerance and effective detoxification pathways for the toxic metabolites, as other herbivores do (Cornell and Hawkins, 2003; Sotka and Whalen, 2008; Sotka et al., 2018). In any case, more studies are needed to understand the possible long-term consequences of C. cylindracea consumption on the health of fish assemblages and whether these consequences could propagate throughout the food web, potentially affecting the functioning of the ecosystem through cascading effects.
Overall, our findings confirm that the invasion of C. cylindracea in the Mediterranean Sea has the potential to influence the feeding habits of the omnivorous fish species as it has already done with some of the strictly herbivorous organisms (Azzurro et al., 2004; Ruitton et al., 2006; Cebrian et al., 2011; Tomas et al., 2011a,b). In this sense, the assessment of C. cylindracea consumption by omnivorous (i.e., non-strict herbivores) fish presented here is noteworthy since most of the previous research into the effects of herbivory on invasive algae has focused only on the strict herbivores, disregarding the effect that omnivorous organisms might have (e.g., Davis et al., 2005; Sumi and Scheibling, 2005; Ruitton et al., 2006; Wikström et al., 2006; Lyons and Scheibling, 2008; Vermeij et al., 2009; Britton-Simmons et al., 2011; Cebrian et al., 2011; Tomas et al., 2011a,b; Nejrup et al., 2012; Hammann et al., 2013; Enge et al., 2017; Caselle et al., 2018). Furthermore, while some of the previous studies have highlighted the contribution of some of these herbivores to limit the abundance of invasive algae, our findings suggest that non-strict herbivores could also potentially have a similar, albeit less important contribution. Therefore, these findings provide another example on the important role that omnivorous fish species might have on marine ecosystems, where they have been reported to play a key role on the coral-algae equilibrium in coral reefs from several regions (Bellwood et al., 2006; Tebbett et al., 2020), and to become an important link between primary producers and higher trophic levels in rocky reefs (Mendes et al., 2015, 2019). In our case, given the high amounts of C. cylindracea found in some specimens and considering that omnivorous sparid fishes dominate the shallow rocky infralittoral habitats in the Mediterranean Sea (García-Rubies, 1997; Sala and Ballesteros, 1997; García-Charton et al., 2004; Coll et al., 2013) with abundances of up to 60 individuals per 250 m2 and biomasses of more than 40 g/m2 (García-Rubies and Zabala, 1990; Sala and Ballesteros, 1997; Di Franco et al., 2009; Guidetti et al., 2014; Coll, 2020), it seems that they could potentially have some impact on the abundance of C. cylindracea. Therefore, we suggest that the lower impact exerted by omnivorous fish could complement the higher impact exerted by strictly herbivorous organisms (Santamaría et al., 2021) and that, taken together, they might significantly reduce the abundance of C. cylindracea in shallow habitats. Still, considering our limited sample sizes, we pose that more in depth assessments are needed to better quantify the impact that omnivorous fish might have on the success of C. cylindracea. Finally, considering that sea breams are highly targeted by fisheries and have already suffered important declines in the Mediterranean basin (Sala et al., 1998; Coll et al., 2004; Sala, 2004; Morales-Nin et al., 2005; Guidetti, 2006; Lloret et al., 2008), places that promote their recovery, such as well-enforced marine protected areas (MPAs) (Mosquera et al., 2000; Micheli et al., 2005; Claudet et al., 2006; Guidetti, 2006; Guidetti and Sala, 2007; Guidetti et al., 2008, 2014; Sala et al., 2012; Coll et al., 2013), might also foster the strength of this complementary control mechanism on the abundance of C. cylindracea.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the animal study because the sampling was performed during 2008 in the framework of a big project carried out by the National Park and the Autonomic Government. The sampled species are commercial species usually fished in the area. For our study we received only the stomach of the fishes already sampled and we did not took part on the manipulation of the species.
FT and EC conceived the ideas and the experimental design. FT, EB, and EC collected the data. JS, FT, EB, and EC analyzed the data. JS and EC drafted the manuscript. All authors contributed substantially to revisions and accepted the final version before submission.
Funding for this project was obtained from the Spanish Ministry Project ANIMA (CGL2016-76341-R, MINECO/FEDER, UE), the Spanish Autonomous Organism of National Parks through the project BIGPARK (project 2017-2462), and the Padi Foundation to FT (CGA App #: 5134). JS has received funding from “La Caixa” Foundation (ID 100010434), under agreement LCF/BQ/DE17/11600001.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Xisco Riera, Biel Morey, and Oliver Navarro for help during the fishing campaigns, Paula Molina for help with laboratory work, the authorities and staff of the Cabrera Archipelago National Park for sampling permissions and logistical support, and the Directorate General for Fisheries and Marine Resources of the Balearic Islands Government for helping with fishing logistics. This is a contribution from the Consolidated Research Group “Medrecover; www.medrecover.org,” SGR2017-1521 (Catalan Government).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.702492/full#supplementary-material
Alós, J., Cabanellas-Reboredo, M., and March, D. (2012). Spatial and temporal patterns in the movement of adult two-banded sea bream Diplodus vulgaris (Saint-Hilaire, 1817). Fish. Res. 115–116, 82–88. doi: 10.1016/j.fishres.2011.11.025
Assis, C. A. (1996). A generalised index for stomach contents analysis in fish. Sci. Mar. 60, 385–389.
Azzurro, E., Fanelli, E., and Andaloro, F. (2004). Preliminary data on feeding habits of dusky spinefoot Siganus luridus in the Sicily Channel (central Mediterranean). Biol. Mar. Mediterr. 11:145.
Bauchot, M. L., and Hureau, J. C. (1986). “Sparidae,” in Fishes of the North-eastern Atlantic and the Mediterranean, Vol. 3, eds P. J. P. Whitehead, M. L. Bauchot, J. C. Hureau, J. Nielsen, and E. Tortonese (Paris: UNESCO), 883–907.
Bellwood, D. R., Hughes, T. P., and Hoey, A. S. (2006). Sleeping functional group drives coral-reef recovery. Curr. Biol. 16, 2434–2439. doi: 10.1016/j.cub.2006.10.030
Box, A. (2008). Ecología de Caulerpales: Fauna y Biomarcadores. Ph.D. thesis, Balearic Islands University, Palma.
Box, A., Deudero, S., Sureda, A., Blanco, A., Alòs, J., Terrados, J., et al. (2009). Diet and physiological responses of Spondyliosoma cantharus (Linnaeus, 1758) to the Caulerpa racemosa var. cylindracea invasion. J. Exp. Mar. Bio. Ecol. 380, 11–19. doi: 10.1016/j.jembe.2009.08.010
Box, A., Sureda, A., Tauler, P., Terrados, J., Marbà, N., Pons, A., et al. (2010). Seasonality of caulerpenyne content in native Caulerpa prolifera and invasive C. taxifolia and C. racemosa var. cylindracea in the western Mediterranean Sea. Bot. Mar. 53, 367–375. doi: 10.1515/BOT.2010.034
Britton-Simmons, K. H., Pister, B., Sánchez, I., and Okamoto, D. (2011). Response of a native, herbivorous snail to the introduced seaweed Sargassum muticum. Hydrobiologia 661, 187–196. doi: 10.1007/s10750-010-0523-1
Bulleri, F., Badalamenti, F., Iveša, L., Mikac, B., Musco, L., Jaklin, A., et al. (2016). The effects of an invasive seaweed on native communities vary along a gradient of land-based human impacts. Peer J. 4:e1795. doi: 10.7717/peerj.1795
Bulleri, F., Benedetti-Cecchi, L., Ceccherelli, G., and Tamburello, L. (2017). A few is enough: a low cover of a non-native seaweed reduces the resilience of Mediterranean macroalgal stands to disturbances of varying extent. Biol. Invas. 19, 2291–2305. doi: 10.1007/s10530-017-1442-0
Bulleri, F., Tamburello, L., and Benedetti-Cecchi, L. (2009). Loss of consumers alters the effects of resident assemblages on the local spread of an introduced macroalga. Oikos 118, 269–279. doi: 10.1111/j.1600-0706.2008.16955.x
Carriglio, D., Sandulli, R., Deastis, S., Gallo D’Addabbo, M., and Grimaldi de Zio, S. (2003). Caulerpa racemosa spread effects on the meiofauna of the Gulf of Taranto. Biol. Mar. Mediterr. 10, 509–511.
Caselle, J. E., Davis, K., and Marks, L. M. (2018). Marine management affects the invasion success of a non-native species in a temperate reef system in California, USA. Ecol. Lett. 21, 43–53. doi: 10.1111/ele.12869
Catford, J. A., Jansson, R., and Nilsson, C. (2009). Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Divers. Distrib. 15, 22–40. doi: 10.1111/j.1472-4642.2008.00521.x
Cebrian, E., and Ballesteros, E. (2004). Zonation patterns of benthic communities in an upwelling area from the Western Mediterranean (La Herradura, Alboran Sea). Sci. Mar. 68, 69–84. doi: 10.1016/0377-8398(88)90006-0
Cebrian, E., and Ballesteros, E. (2009). Temporal and spatial variability in shallow- and deep-water populations of the invasive Caulerpa racemosa var. cylindracea in the Western Mediterranean. Estuar. Coast. Shelf Sci. 83, 469–474. doi: 10.1016/j.ecss.2009.04.026
Cebrian, E., Ballesteros, E., Linares, C., and Tomas, F. (2011). Do native herbivores provide resistance to Mediterranean marine bioinvasions? A seaweed example. Biol. Invas. 13, 1397–1408. doi: 10.1007/s10530-010-9898-1
Chaouch, H., Hamida, O. B. A. B. H., Ghorbel, M., and Jarboui, O. (2013). Diet composition and food habits of Diplodus puntazzo (Sparidae) from the Gulf of Gabès (Central Mediterranean). J. Mar. Biol. Assoc. U.K. 93, 2257–2264. doi: 10.1017/S0025315413000805
Chaouch, H., Hamida, O. B. A. B. H., Ghorbel, M., and Jarboui, O. (2014). Feeding habits of the annular Seabream, Diplodus annularis (Linnaeus, 1758) (Pisces: Sparidae), in the Gulf of Gabès (Central Mediterranean). Cah. Biol. Mar. 55, 13–19.
Clarke, K. R. (1993). Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 18, 117–143. doi: 10.1111/j.1442-9993.1993.tb00438.x
Clarke, K. R., and Warwick, R. M. (1994). Similarity-based testing for community pattern: the two-way layout with no replication. Mar. Biol. 118, 167–176. doi: 10.1007/BF00699231
Claudet, J., Pelletier, D., Jouvenel, J. Y., Bachet, F., and Galzin, R. (2006). Assessing the effects of marine protected area (MPA) on a reef fish assemblage in a northwestern Mediterranean marine reserve: identifying community-based indicators. Biol. Conserv. 130, 349–369. doi: 10.1016/j.biocon.2005.12.030
Coll, J. (2020). Seguiment de les Comunitats Marines d’Espais Naturals Protegits de les Illes Balears. Cens de Poblacions de Peixos (PN Cabrera). Informe Tècnic per a la Direcció General d’Espais Naturals i Biodiversitat del Govern de les Illes Balears.
Coll, J., Garcia-Rubies, A., Morey, G., Reñones, O., Álvarez-Berastegui, D., Navarro, O., et al. (2013). Using no-take marine reserves as a tool for evaluating rocky-reef fish resources in the western Mediterranean. ICES J. Mar. Sci. 70, 578–590. doi: 10.1038/srep04684
Coll, J., Linde, M., García-Rubies, A., Riera, F., and Grau, A. M. (2004). Spear fishing in the Balearic Islands (west central Mediterranean): species affected and catch evolution during the period 1975-2001. Fish. Res. 70, 97–111. doi: 10.1016/j.fishres.2004.05.004
Cornell, H. V., and Hawkins, B. A. (2003). Herbivore responses to plant secondary compounds: a test of phytochemical coevolution theory. Am. Nat. 161, 507–522. doi: 10.1086/368346
D’Anna, G., Giacalone, V. M., Pipitone, C., and Badalamenti, F. (2011). Movement pattern of white seabream, Diplodus sargus (L., 1758) (Osteichthyes, Sparidae) acoustically tracked in an artificial reef area. Ital. J. Zool. 78, 255–263. doi: 10.1080/11250000903464059
Davis, A. R., Benkendorff, K., and Ward, D. W. (2005). Responses of common SE Australian herbivores to three suspected invasive Caulerpa spp. Mar. Biol. 146, 859–868. doi: 10.1007/s00227-004-1499-z
Di Franco, A., Bussotti, S., Navone, A., Panzalis, P., and Guidetti, P. (2009). Evaluating effects of total and partial restrictions to fishing on Mediterranean rocky-reef fish assemblages. Mar. Ecol. Prog. Ser. 387, 275–285. doi: 10.3354/meps08051
Di Lorenzo, M., D’Anna, G., Badalamenti, F., Giacalone, V. M., Starr, R. M., and Guidetti, P. (2014). Fitting the size of no-take zones to species movement patterns: a case study on a Mediterranean seabream. Mar. Ecol. Prog. Ser. 502, 245–255. doi: 10.3354/meps10723
Dunstan, P. K., and Johnson, C. R. (2007). Mechanisms of invasions: can the recipient community influence invasion rates? Bot. Mar. 50, 41–52. doi: 10.1515/9783110211344
Enge, S., Sagerman, J., Wikström, S. A., and Pavia, H. (2017). A review of herbivore effects on seaweed invasions. Oceanogr. Mar. Biol. Annu. Rev. 55, 421–440. doi: 10.1201/b21944
Felline, S., Caricato, R., Cutignano, A., Gorbi, S., Lionetto, M. G., Mollo, E., et al. (2012). Subtle effects of biological invasions: cellular and physiological responses of fish eating the exotic pest Caulerpa racemosa. PLoS One 7:e38763. doi: 10.1371/journal.pone.0038763
Felline, S., Mollo, E., Cutignano, A., Grauso, L., Andaloro, F., Castriota, L., et al. (2017). Preliminary observations of Caulerpin accumulation from the invasive Caulerpa cylindracea in native Mediterranean fish species. Aquat. Biol. 26, 27–31. doi: 10.3354/ab00671
Felline, S., Mollo, E., Ferramosca, A., Zara, V., Regoli, F., Gorbi, S., et al. (2014). Can a marine pest reduce the nutritional value of Mediterranean fish flesh? Mar. Biol. 161, 1275–1283. doi: 10.1007/s00227-014-2417-7
Galil, B. S. (2007). Loss or gain? Invasive aliens and biodiversity in the Mediterranean Sea. Mar. Pollut. Bull. 55, 314–322. doi: 10.1016/j.marpolbul.2006.11.008
García, M., Weitzmann, B., Pinedo, S., Cebrian, E., and Ballesteros, E. (2016). First report on the distribution and impact of marine alien species in coastal benthic assemblages along the Catalan coast. Handb. Environ. Chem. 43, 249–270. doi: 10.1007/698-2015-411
García-Charton, J. A., Pérez-Ruzafa, Á, Sánchez-Jerez, P., Bayle-Sempere, J. T., Reñones, O., and Moreno, D. (2004). Multi-scale spatial heterogeneity, habitat structure, and the effect of marine reserves on Western Mediterranean rocky reef fish assemblages. Mar. Biol. 144, 161–182. doi: 10.1007/s00227-003-1170-0
García-Rubies, A. (1997). Estudi ecològic de les Poblacions de Peixos Litorals Sobre Substrat Rocós a la Mediterrània Occidental: Efecte de la Fondària, el Substrat, l’Estacionalitat i la Protecció.
García-Rubies, A., and Zabala, M. (1990). Effects of total fishing prohibition on the rocky fish assemblages of Medes Islands marine reserve (NW Mediterranean). Sci. Mar. 54, 317–328.
Gonçalves, J. M. S., and Erzini, K. (1998). Feeding habits of the two-banded sea bream (Diplodus vulgaris) and the black sea bream (Spondyliosoma cantharus) (Sparidae) from the south-west coast of Portugal. Cybium 22, 245–254.
Gorbi, S., Giuliani, M. E., Pittura, L., D’Errico, G., Terlizzi, A., Felline, S., et al. (2014). Could molecular effects of Caulerpa racemosa metabolites modulate the impact on fish populations of Diplodus sargus? Mar. Environ. Res. 96, 2–11. doi: 10.1016/j.marenvres.2014.01.010
Guidetti, P. (2006). Marine reserves reestablish lost predatory interactions and cause community changes in rocky reefs. Ecol. Appl. 16, 963–976.
Guidetti, P., Baiata, P., Ballesteros, E., Di Franco, A., Hereu, B., Macpherson, E., et al. (2014). Large-scale assessment of Mediterranean marine protected areas effects on fish assemblages. PLoS One 9:e91841. doi: 10.1371/journal.pone.0091841
Guidetti, P., Milazzo, M., Bussotti, S., Molinari, A., Murenu, M., Pais, A., et al. (2008). Italian marine reserve effectiveness: does enforcement matter? Biol. Conserv. 141, 699–709. doi: 10.1016/j.biocon.2007.12.013
Guidetti, P., and Sala, E. (2007). Community-wide effects of marine reserves in the Mediterranean Sea. Mar. Ecol. Prog. Ser. 335, 43–56. doi: 10.3354/meps335043
Hammann, M., Wang, G., Rickert, E., Boo, S. M., and Weinberger, F. (2013). Invasion success of the seaweed Gracilaria vermiculophylla correlates with low palatibility. Mar. Ecol. Prog. Ser. 486, 93–103. doi: 10.3354/meps10361
Hyslop, E. J. (1980). Stomach contents analysis - a review of methods and their application. J. Fish Biol. 17, 411–429. doi: 10.1111/j.1095-8649.1980.tb02775.x
Ivlev, V. S. (1961). Experimental Ecology of the Feeding of Fishes. New Haven, CT: Yale University Press.
Katsanevakis, S., Tempera, F., and Teixeira, H. (2016). Mapping the impact of alien species on marine ecosystems: the Mediterranean Sea case study. Divers. Distrib. 22, 694–707. doi: 10.1111/ddi.12429
Kimbro, D. L., Cheng, B. S., and Grosholz, E. D. (2013). Biotic resistance in marine environments. Ecol. Lett. 16, 821–833. doi: 10.1111/ele.12106
Kindt, R., and Coe, R. (2005). Tree Diversity Analysis. A Manual and Software for Common Statistical Methods for Ecological and Biodiversity Studies. Nairobi: World Agroforestry Centre.
Klein, J., and Verlaque, M. (2008). The Caulerpa racemosa invasion: a critical review. Mar. Pollut. Bull. 56, 205–225. doi: 10.1016/j.marpolbul.2007.09.043
Leitão, F., Santos, M. N., and Monteiro, C. C. (2007). Contribution of artificial reefs to the diet of the white sea bream (Diplodus sargus). ICES J. Mar. Sci. 64, 473–478. doi: 10.1093/icesjms/fsm027
Lloret, J., Zaragoza, N., Caballero, D., Font, T., Casadevall, M., and Riera, V. (2008). Spearfishing pressure on fish communities in rocky coastal habitats in a Mediterranean marine protected area. Fish. Res. 94, 84–91. doi: 10.1016/j.fishres.2008.07.002
Lyons, D. A., and Scheibling, R. E. (2008). Context-dependant survival of the invasive seaweed Codium fragile ssp. tomentosoides in kelp bed and urchin barren habitats off Nova Scotia. Aquat. Biol. 2, 17–27. doi: 10.3354/ab00035
Macdonald, J. S., and Green, R. H. (1983). Redundancy of variables used to describe importance of prey species in fish diets. Can. J. Fish. Aquat. Sci. 40, 635–637. doi: 10.1139/f83-083
March, D., Alós, J., Grau, A., and Palmer, M. (2011). Short-term residence and movement patterns of the annular seabream Diplodus annularis in a temperate marine reserve. Estuar. Coast. Shelf Sci. 92, 581–587. doi: 10.1016/j.ecss.2011.02.015
Marco-Méndez, C., Ferrero-Vicente, L. M., Prado, P., and Sánchez-Lizaso, J. L. (2017). Epiphytes and nutrient contents influence Sarpa salpa herbivory on Caulerpa spp. vs. Seagrass species in Mediterranean meadows. Estuar. Coast. Shelf Sci. 184, 54–66. doi: 10.1016/j.ecss.2016.11.005
Mendes, T. C., Cordeiro, C. A. M. M., and Ferreira, C. E. L. (2015). An experimental evaluation of macroalgal consumption and selectivity by nominally herbivorous fishes on subtropical rocky reefs. J. Exp. Mar. Bio. Ecol. 471, 146–152. doi: 10.1016/j.jembe.2015.06.001
Mendes, T. C., Quimbayo, J. P., Bouth, H. F., Silva, L. P. S., and Ferreira, C. E. L. (2019). The omnivorous triggerfish Melichthys niger is a functional herbivore on an isolated Atlantic oceanic island. J. Fish Biol. 95, 812–819. doi: 10.1111/jfb.14075
Micheli, F., Benedetti-Cecchi, L., Gambaccini, S., Bertocci, I., Borsini, C., Osio, G. C., et al. (2005). Cascading human impacts, marine protected areas, and the structure of Mediterranean reef assemblages. Ecol. Monogr. 75, 81–102. doi: 10.1890/03-4058
Morales-Nin, B., Moranta, J., García, C., Tugores, M. P., Grau, A. M., Riera, F., et al. (2005). The recreational fishery off Majorca Island (western Mediterranean): some implications for coastal resource management. ICES J. Mar. Sci. 62, 727–739. doi: 10.1016/j.icesjms.2005.01.022
Mosquera, I., Côté, I. M., Jennings, S., and Reynolds, J. D. (2000). Conservation benefits of marine reserves for fish populations. Anim. Conserv. 3, 321–332. doi: 10.1017/S1367943000001049
Nejrup, L. B., Pedersen, M. F., and Vinzent, J. (2012). Grazer avoidance may explain the invasiveness of the red alga Gracilaria vermiculophylla in Scandinavian waters. Mar. Biol. 159, 1703–1712. doi: 10.1007/s00227-012-1959-9
Nilssen, K. T., Lindstrøm, U., Westgaard, J. I., Lindblom, L., Blencke, T. R., and Haug, T. (2019). Diet and prey consumption of grey seals (Halichoerus grypus) in Norway. Mar. Biol. Res. 15, 137–149. doi: 10.1080/17451000.2019.1605182
Noè, S., Badalamenti, F., Bonaviri, C., Musco, L., Fernández, T. V., Vizzini, S., et al. (2017). Food selection of a generalist herbivore exposed to native and alien seaweeds. Mar. Pollut. Bull. 129, 469–473. doi: 10.1016/j.marpolbul.2017.10.015
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., et al. (2018). Vegan: Community Ecology Package. R Packag. Version 2.4-6.
Papacostas, K. J., Rielly-Carroll, E. W., Georgian, S. E., Long, D. J., Princiotta, S. D., Quattrini, A. M., et al. (2017). Biological mechanisms of marine invasions. Mar. Ecol. Prog. Ser. 565, 251–268. doi: 10.3354/meps12001
Paul, V., Arthur, K. E., Ritson-Williams, R., Ross, C., and Sharp, K. (2007). Chemical defenses: from compounds to communities. Biol. Bull. 213, 226–251.
Piazzi, L., Ceccherelli, G., and Cinelli, F. (2001). Threat to macroalgal diversity: effects of the introduced green alga Caulerpa racemosa in the Mediterranean. Mar. Ecol. Prog. Ser. 210, 149–159. doi: 10.3354/meps210149
Piazzi, L., Meinesz, A., Verlaque, M., Akçali, B., Antolić, B., Argyrou, M., et al. (2005). Invasion of Caulerpa racemosa var. cylindracea (Caulerpales, Chlorophyta) in the Mediterranean Sea: an assessment of the spread. Cryptogam. Algol. 26, 189–202.
Pita, C., Gamito, S., and Erzini, K. (2002). Feeding habits of the gilthead seabream (Sparus aurata) from the Ria Formosa (southern Portugal) as compared to the black seabream (Spondyliosoma cantharus) and the annular seabream (Diplodus annularis). J. Appl. Ichthyol. 18, 81–86. doi: 10.1046/j.1439-0426.2002.00336.x
Preti, A., Smith, S. E., and Ramon, D. A. (2001). Feeding habits of the common thresher shark (Alopias vulpinus) sampled from the California-based drift gill net fishery, 1998-1999. Calif. Coop. Ocean. Fish. Investig. Rep. 42, 145–152.
Reñones, O., Moranta, J., Coll, J., and Morales-Nin, B. (1997). Rocky bottom fish communities of Cabrera Archipelago National Park (Mallorca, Western Mediterranean). Sci. Mar. 61, 495–506.
Rogers, P. J., Huveneers, C., Page, B., Hamer, D. J., Goldsworthy, S. D., Mitchell, J. G., et al. (2012). A quantitative comparison of the diets of sympatric pelagic sharks in the gulf and shelf ecosystems off southern Australia. ICES J. Mar. Sci. 69, 1382–1393. doi: 10.2307/4451315
Ruitton, S., Verlaque, M., Aubin, G., and Boudouresque, C. F. (2006). Grazing on Caulerpa racemosa var. cylindracea (Caulerpales, Chlorophyta) in the Mediterranean Sea by herbivorous fishes and sea urchins. Vie Milieu 56, 33–41.
Sala, E. (2004). The past and present topology and structure of Mediterranean subtidal rocky-shore food webs. Ecosystems 7, 333–340. doi: 10.1007/s10021-003-0241-x
Sala, E., and Ballesteros, E. (1997). Partitioning of space and food resources by three fish of the genus Diplodus (Sparidae) in a Mediterranean rocky infralittoral ecosystem. Mar. Ecol. Prog. Ser. 152, 273–283. doi: 10.3354/meps152273
Sala, E., Ballesteros, E., Dendrinos, P., Di Franco, A., Ferretti, F., Foley, D., et al. (2012). The structure of Mediterranean rocky reef ecosystems across environmental and human gradients, and conservation implications. PLoS One 7:e32742. doi: 10.1371/journal.pone.0032742
Sala, E., Boudouresque, C. F., and Harmelin-Vivien, M. (1998). Fishing, trophic cascades, and the structure of algal assemblages: evaluation of an old but untested paradigm. Oikos 82, 425–439. doi: 10.2307/3546364
Sánchez-Jerez, P., Gillanders, B. M., Rodríguez-Ruiz, S., and Ramos-Esplá, A. A. (2002). Effect of an artificial reef in Posidonia meadows on fish assemblage and diet of Diplodus annularis. ICES J. Mar. Sci. 59, 59–68. doi: 10.1006/jmsc.2002.1213
Sant, N., Chappuis, E., Rodríguez-Prieto, C., Real, M., and Ballesteros, E. (2017). Cost-benefit of three different methods for studying Mediterranean rocky benthic assemblages. Sci. Mar. 81:129. doi: 10.3989/scimar.04463.04a
Santamaría, J., Tomas, F., Ballesteros, E., Ruiz, J. M., Bernardeau-Esteller, J., Terrados, J., et al. (2021). The role of competition and herbivory in biotic resistance against invaders: a synergistic effect. Ecology doi: 10.1002/ecy.3440
Schaffelke, B., and Hewitt, C. L. (2008). Impacts of introduced seaweeds. Seaweed Invas. Synth. Ecol. Econ. Leg. Imp. 50, 77–97.
Schaffelke, B., Smith, J. E., and Hewitt, C. L. (2006). Introduced macroalgae - a growing concern. J. Appl. Phycol. 18, 529–541. doi: 10.1007/s10811-006-9074-2
Sotka, E. E., Jormalainen, V., and Poore, A. G. B. (2018). “The evolution of marine herbivores in response to algal secondary metabolites,” in Chemical Ecology: The Ecological Impacts of Marine Natural Products, eds M. P. Puglisi and M. A. Becerro (Milton Park: Taylor & Francis Group), 193.
Sotka, E. E., and Whalen, K. E. (2008). “Herbivore offense in the sea: the detoxification and transport of secondary metabolites,” in Algal Chemical Ecology, ed. C. D. Amsler (Berlin: Springer), 203–228. doi: 10.1007/978-3-540-74181-7_10
Steinarsdóttir, M. B., Ingólfsson, A., and Ólafsson, E. (2009). Trophic relationships on a fucoid shore in south-western Iceland as revealed by stable isotope analyses, laboratory experiments, field observations and gut analyses. J. Sea Res. 61, 206–215. doi: 10.1016/j.seares.2008.11.004
Sumi, C. B. T., and Scheibling, R. E. (2005). Role of grazing by sea urchins Strongylocentrotus droebachiensis in regulating the invasive alga Codium fragile ssp. tomentosoides in Nova Scotia. Mar. Ecol. Prog. Ser. 292, 203–212. doi: 10.3354/meps292203
Tebbett, S. B., Hoey, A. S., Depczynski, M., Wismer, S., and Bellwood, D. R. (2020). Macroalgae removal on coral reefs: realised ecosystem functions transcend biogeographic locations. Coral Reefs 39, 203–214. doi: 10.1007/s00338-019-01874-w
Terlizzi, A., Felline, S., Lionetto, M. G., Caricato, R., Perfetti, V., Cutignano, A., et al. (2011). Detrimental physiological effects of the invasive alga Caulerpa racemosa on the Mediterranean white seabream Diplodus sargus. Aquat. Biol. 12, 109–117. doi: 10.3354/ab00330
Thomsen, M. S., Wernberg, T., South, P. M., and Schiel, D. R. (2016). “Non-native seaweeds drive changes in marine coastal communities around the world,” in Seaweed Phylogeography: Adaptation and Evolution of Seaweeds Under Environmental Change, eds Z. M. Hu and C. Fraser (Dordrecht: Springer), 147–185. doi: 10.1007/978-94-017-7534-2
Thomsen, M. S., Wernberg, T., Tuya, F., and Silliman, B. R. (2009). Evidence for impacts of nonindigenous macroalgae: a meta-analysis of experimental field studies1. J. Phycol. 45, 812–819. doi: 10.1111/j.1529-8817.2009.00709.x
Tomas, F., Box, A., and Terrados, J. (2011a). Effects of invasive seaweeds on feeding preference and performance of a keystone Mediterranean herbivore. Biol. Invas. 13, 1559–1570. doi: 10.1007/s10530-010-9913-6
Tomas, F., Cebrian, E., and Ballesteros, E. (2011b). Differential herbivory of invasive algae by native fish in the Mediterranean Sea. Estuar. Coast. Shelf Sci. 92, 27–34. doi: 10.1016/j.ecss.2010.12.004
Tripp-Valdez, A., Galván-Magaña, F., and Ortega-García, S. (2015). Food sources of common dolphinfish (Coryphaena hippurus) based on stomach content and stable isotopes analyses. J. Mar. Biol. Assoc. U.K. 95, 579–591. doi: 10.1017/S0025315414001842
Vermeij, M. J. A., Smith, T. B., Dailer, M. L., and Smith, C. M. (2009). Release from native herbivores facilitates the persistence of invasive marine algae: a biogeographical comparison of the relative contribution of nutrients and herbivory to invasion success. Biol. Invas. 11, 1463–1474. doi: 10.1007/s10530-008-9354-7
Wikström, S. A., Steinarsdóttir, M. B., Kautsky, L., and Pavia, H. (2006). Increased chemical resistance explains low herbivore colonization of introduced seaweed. Oecologia 148, 593–601. doi: 10.1007/s00442-006-0407-2
Keywords: invasive species, fish-alga interaction, grazing, biotic control, Mediterranean Sea, Sparidae, Caulerpa cylindracea
Citation: Santamaría J, Tomas F, Ballesteros E and Cebrian E (2021) Herbivory on the Invasive Alga Caulerpa cylindracea: The Role of Omnivorous Fishes. Front. Mar. Sci. 8:702492. doi: 10.3389/fmars.2021.702492
Received: 29 April 2021; Accepted: 12 July 2021;
Published: 03 August 2021.
Edited by:
Periklis Kleitou, Marine and Environmental Research Lab (MER), CyprusReviewed by:
Alvaro Anibal Israel, University of Haifa, IsraelCopyright © 2021 Santamaría, Tomas, Ballesteros and Cebrian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emma Cebrian, ZW1tYUBjZWFiLmNzaWMuZXM=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.