
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 21 July 2021
Sec. Marine Conservation and Sustainability
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.689089
Elasmobranchs are one of the most endangered vertebrate groups on the planet, but despite this situation the use of reproductive techniques in elasmobranch conservation strategies has been scarce. Among these techniques, sperm preservation is a potential tool for ex situ conservation and aquaria sustainability. However, there are no widespread preservation protocols for elasmobranch sperm, and shark sperm cryopreservation has never been achieved before. Here we present the establishment of successful cryopreservation protocols for elasmobranch sperm, tested in several species. We have formulated a sperm extender that can be used for different elasmobranch species, capable of maintaining sperm motility for several weeks. Additionally, we achieved the cryopreservation of sperm by previously diluting it in our extender and supplementing it with different combinations of cryoprotectants. The effects of methanol and dimethyl sulfoxide as permeating cryoprotectants were evaluated, as well egg yolk as a non-permeating cryoprotectant. Sperm quality was assessed by studying the motility and membrane integrity post-thawing, demonstrating its effectiveness in the 10 species tested, including two which are considered Critically Endangered. This is the first time that shark sperm cryopreservation has been reported, broadening our knowledge of the reproductive techniques that can be applied to elasmobranchs and laying the foundations for the first cryobanks for shark and ray sperm. Outcomes from this study will be useful for ex situ conservation efforts developed by public aquaria. A regular supply of frozen sperm will reduce the problems that result from the transport of specimens, inbreeding or lack of synchronized reproductive cycles in captivity.
Appearing almost 400 million years ago, elasmobranchs are nowadays an ecologically diverse vertebrate group that plays a key role in the regulation of the ecosystems they inhabit (Compagno, 1990; Stevens, 2000). But the extreme life histories of sharks (Superorder Selachiomorpha) and rays (Superorder Batoidea), with some species exhibiting long gestation periods, high maternal investment, and slow sexual maturity (Cortés, 2000) make this group extremely sensitive to elevated mortality from fishing (García et al., 2008; Dulvy and Forrest, 2010). Overfishing and habitat destruction are the main drivers for the decline in elasmobranch populations, and almost one quarter of the elasmobranch species are considered threatened according to the criteria of the IUCN (Dulvy et al., 2014).
Given this situation, the use of ex situ conservation breeding programs could be a strategy worthy of consideration in elasmobranch conservation plans. Ex situ conservation involves the protection of organisms, or their progeny, extracting or isolating them from their natural environment and keeping them in artificial conditions (Frankel and Soulé, 1981; IUCN/SSC, 2014). In that sense, in situ and ex situ conservation are two complementary and non-exclusive techniques that should benefit one another and increase the effectivity of each (Pritchard et al., 2012; Braverman, 2014).
However, to successfully develop this type of project, an essential step is to develop successful breeding programs in aquaria using reproductive techniques. These programs are common for a multitude of aquatic species, but not for elasmobranchs. In fact, until now, most public aquaria tend to rely on natural spontaneous mating events or captured wild specimens for the maintenance of their populations (Henningsen et al., 2004; Buckley et al., 2018). Nevertheless, some factors need to be considered when looking at reproduction in captivity of elasmobranchs, including suboptimal population structures and environmental factors (e.g., temperature and lighting cycles). Indeed, these factors have limited the success of the breeding programs in aquaria to date (Daly and Jones, 2017).
Among the reproductive techniques that can be used to deal with this situation, artificial insemination is one that is receiving increasing attention (Luer et al., 2008; Daly and Jones, 2017; Daochai et al., 2020). Nevertheless, to ensure the success of this technique a reliable supply of sperm is required, especially in the case of endangered species. At present, cryobanking is the most common way to guarantee the availability of viable sperm samples when necessary (Asturiano et al., 2017; Martínez-Páramo et al., 2017). The use of cryopreserved sperm for artificial insemination can avoid inbreeding and reduction of genetic variation in aquaculture companies and public aquaria, as well as solving the problem resultant of needing males and females with a synchronized reproductive cycle, and the need to transport sires from one aquarium to another for reproductive purposes.
While sperm cryopreservation is a well-established procedure used in other aquatic species (Martínez-Páramo et al., 2017; Beirão et al., 2019; Cabrita et al., 2021) information on elasmobranch sperm cryopreservation is very scarce, being limited to just two scientific publications. These publications offered information regarding three marine species: sparsely spotted stingaree Urolophus paucimaculatus (Daly et al., 2011), the Australian bull ray Myliobatis australis and the brownbanded bambooshark Chiloscyllium punctatum (Daly and Jones, 2017). While the authors reported successful results in the cryopreservation of both batoids (U. paucimaculatus and M. australis), no conclusive information was given about the bambooshark (C. punctatum) besides the toxic effect of the cryoprotectant used on the sperm. To date there is no reported information on successful shark sperm cryopreservation.
It must be noted that sperm cryopreservation results in a reduction of sperm quality because of the effects induced in the cell by the freezing conditions and the potential toxicity effects of the cryoprotectants (Horváth and Urbányi, 2020). To avoid these problems and improve sperm quality, the researchers studying cryobiology have been working for years with various procedures, technologies, cryoprotectants and specific supplements, adapted to different species (Martínez-Páramo et al., 2017; Horváth and Urbányi, 2020). But in the case of elasmobranchs research is still in its initial steps (Penfold and Wyffels, 2019). In addition, sperm cryopreservation requires the use of special equipment not always affordable for researchers, as well as supplies such as liquid nitrogen and cryoprotectants, which are not necessarily available when working in the field.
When cryopreservation is not possible, the alternative is to use techniques for the short-term storage of the sperm. Short-term storage methods are designed to preserve the integrity and quality of sperm for several days or weeks. In general, diluents are used to keep the sperm during storage at low temperatures (close to 0°C) in physio-chemical conditions like those in the seminal plasma, with the intention of maintaining high values of sperm viability (Tan-Fermin et al., 1999; Muñoz Gutiérrez, 2011) and even increase fertilization rates for some species (Tambasen-Cheong et al., 1995; Ohta and Izawa, 1996). In the few studies carried out on elasmobranchs to date, ionic solutions based on their biological fluids have been used to work with sperm, though not with the specific goal of using those solutions as short-term storage media. Jones et al. (1984) showed that sperm from the Port Jackson shark Heterodontus portusjacksoni was active after being kept in a phosphate buffer solution based on the ionic composition of the blood, and Minamikawa and Morisawa (1996) showed that sperm from the banded houndshark Triakis scyllium maintain motility in solutions based on blood and uterine conditions. Luer et al. (2008) used an extender based on a poultry semen extender modified to elasmobranch conditions through the addition of urea, TMAO and NaCl while working with the clearnose skate Raja eglanteria, and Daly et al. (2011) used a modified Ringer’s solution adapted to elasmobranchs. According to Daly and Jones (2017) it seems that the exact ionic composition of these solutions is not as important as the osmolality in which the sperm samples are kept. In fact, Wyffels et al. (2020) showed that the sperm of sand tiger shark (Carcharhinus taurus) remained active while diluted in sea water.
The sperm in elasmobranchs consists of a head with acrosome, a midpiece covered by a cytoplasmic sleeve and a slender flagellum. The external features of the head and midpiece show some variations depending on the species. Typically, the head is helical with a variable number of gyres (from 3 to 24) and longer (from 30 up to 93 μm) than the midpiece (from 6 to 21 μm). Spermatozoa in the seminal vesicle are grouped in sperm bundles, called spermatophores if are formed by encapsulated sperm, and spermatozeugmata if are formed by unencapsulated masses of naked sperm cells (Tanaka, 1995; Penfold and Wyffels, 2019).
As occurs with cryopreservation, in fish the main application of short-term storage for the sperm cells is in aquaculture and aquaria, to improve breeding management by synchronizing the production of the gametes of males and females (Gallego and Asturiano, 2019) while avoiding the complexity and cost of cryopreservation procedures.
The aim of the study was to advance in the development of strategies for ex situ conservation programs for elasmobranchs, through the use of short- and long-term conservation of their sperm. For this reason, the objectives were: (i) the development of a dilution medium (extender) to perform short-term sperm preservation in elasmobranchs, and (ii) the development of sperm cryopreservation protocols to be used as the foundations for the creation of the first cryobank for shark and ray sperm (Figure 1).
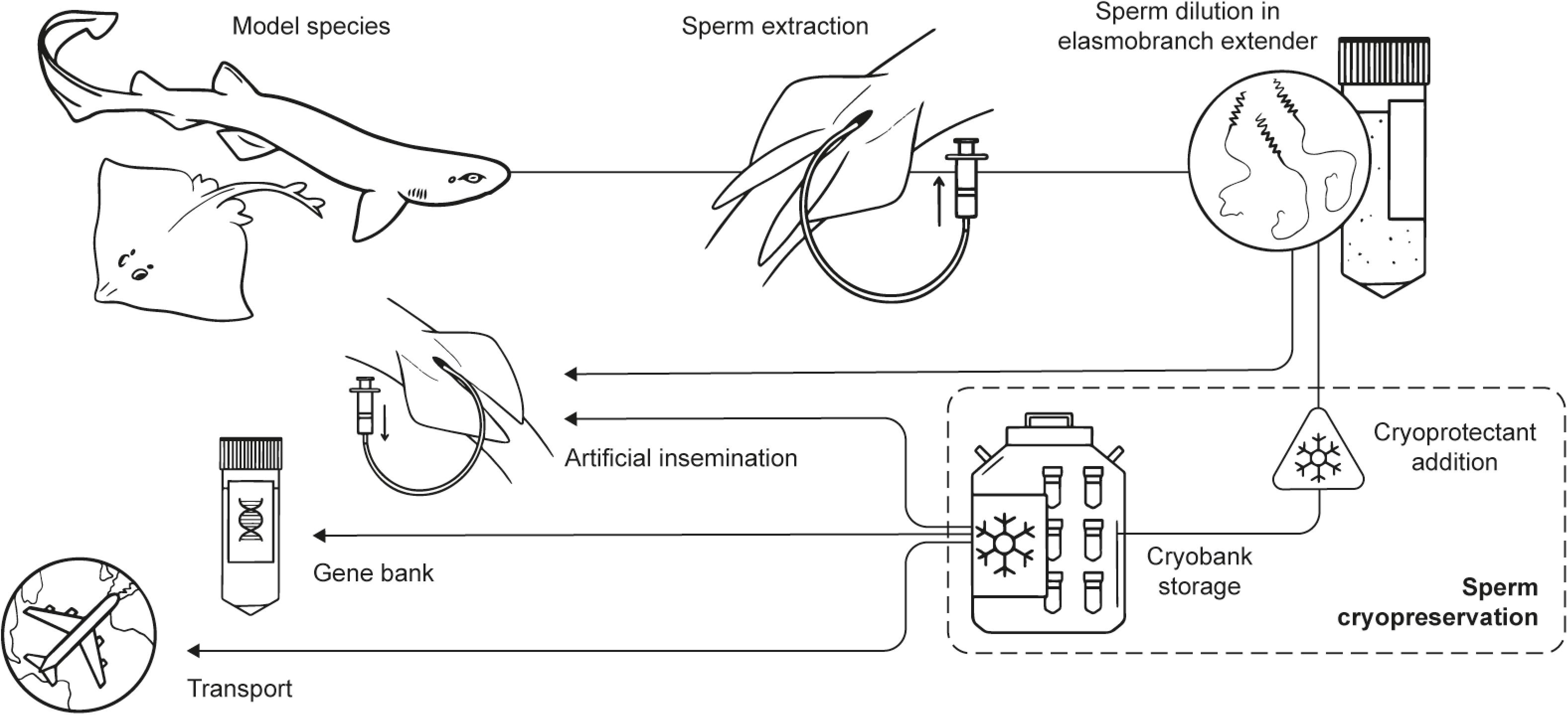
Figure 1. Experimental steps and potential applications. Scheme of the main experimental steps of the process (obtention of the specimens, sperm extraction, sperm dilution, cryopreservation, and storage) and potential uses derived from the outcomes of the study in ex situ conservation programs (sperm worldwide transport, gene bank, artificial insemination).
Sperm samples were obtained from either dead and alive mature individuals belonging to 10 different elasmobranch species with various conservation status (Table 1); 4 sharks (Selachimorpha) and 6 rays (Batoidea). Some of the species were regularly available in fish markets, while the availability of others was fortuitous. Maturity of the specimens was determined by their size according to bibliography, gonad development and clasper calcification (Ebert and Dando, 2020).
Dead specimens were obtained from fish markets, commercial fishing vessels that catch in the Gulf of Valencia, or from the Comunitat Valenciana Stranding Network. The animals from the fish markets were caught and kept in ice approximately 20 h before their acquisition. Animals from commercial fishing by-catch and stranded animals were dead for approximately 2 h when they were obtained. In all the situations, sperm was recovered immediately after the obtention of the specimen. Alive animals were obtained from a public aquarium (Oceanogràfic, València). In the latter case, 11 small spotted catsharks (Scyliorhinus canicula) and 5 nursehounds (Scyliorhinus stellaris) were kept separately in two 8,000 L tanks with recirculating sea water (temperature: 16–18°C; salinity: 35–37‰) and fed twice a day with herring, squid, and shrimps. Manipulation of animals kept in aquarium condition was authorized by the Ethic Committee of Fundación Oceanogràfic (Project reference: OCE-16–19) and the sperm extraction process was carried out under the supervision of their veterinary team.
Tonic immobility was induced prior to sperm extraction to minimize struggling and reduce stress during handling, as this technique carries fewer risks to animal health than the application of anesthetics (Henningsen, 1994; Kessel and Hussey, 2015). The procedure was carried out by holding the animals in an upside-down position while gentle pressure was applied to its snout. With the cloaca emerged, pressure on the abdominal area over the seminal vesicle was enough to make sperm flow through the urogenital papillae (Figures 2A,B). Sperm flowing form the urogenital papillae was immediately collected using a sterile syringe or pipette. Sperm was transferred to sterile tubes after collection.
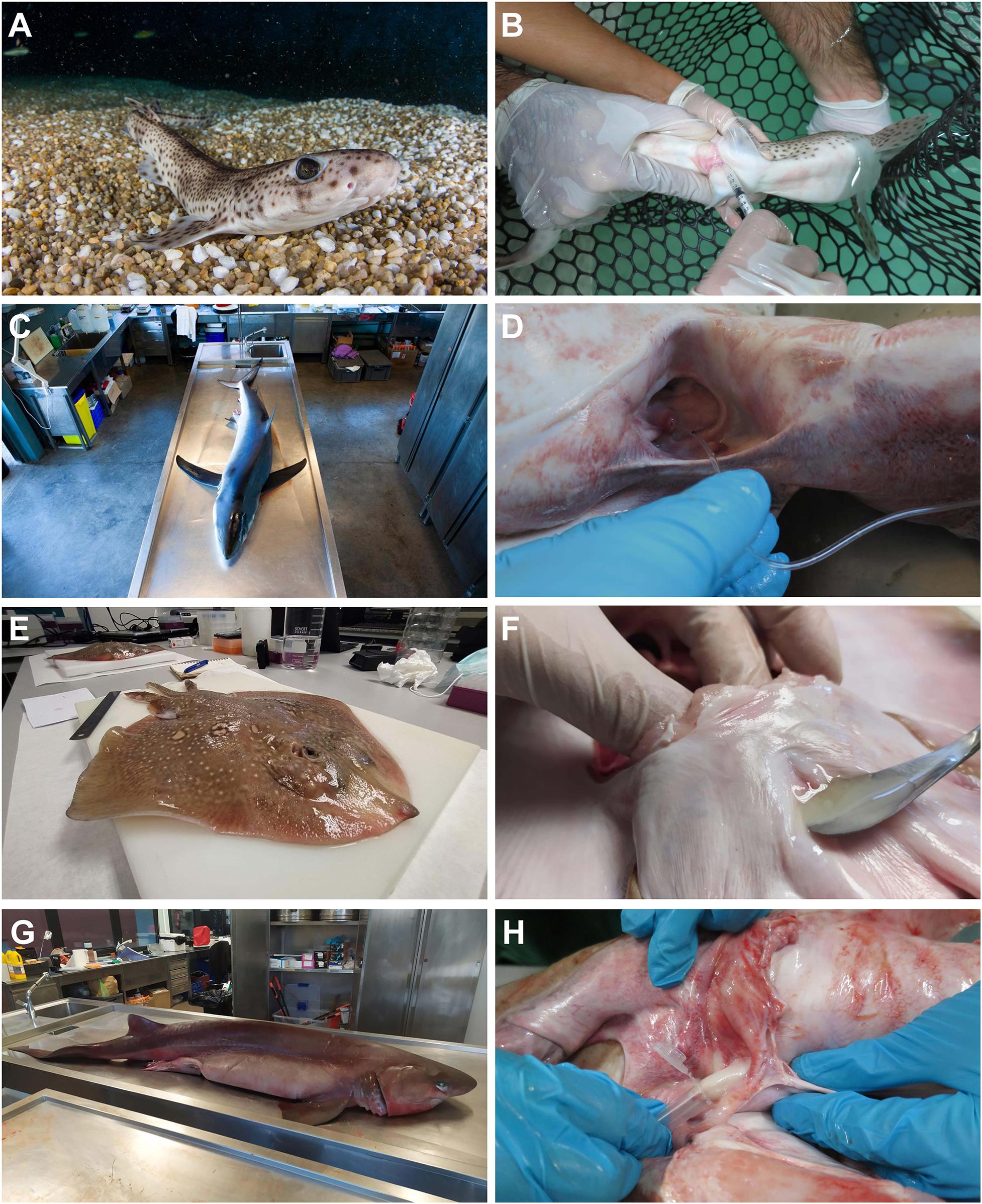
Figure 2. Sperm extraction procedures. Sperm obtained from: (A,B) live animals (Scyliorhinus canicula) by abdominal massage. (C,D) Stranded animals (Prionace glauca) by cannulation through the urogenital papilla. (E,F) Animals from commercial fisheries (Raja radula) by necropsy, and (G,H) animals obtained from by-catch (Hexanchus griseus) by necropsy.
The cloacal region of the dead animals was cleaned using sea water to remove mucus excess and other biological remains, such as blood or fishery residues. Sperm samples were obtained through the application of pressure to the abdominal area, over the seminal vesicle. In fish where the amount of sperm was scarce, samples were obtained through cannulation with a polyurethane cat catheter (BUSTER cat catheter, 1.0 × 130 mm, Kruuse) or a PVC nasogastric tube (Feeding Probe L/RX CH-05 2.67 × 50 mm) (Figures 2C,D). Sperm was obtained after necropsy through stripping directly over the seminal vesicle in the rough ray (Raja radula) (Figures 2E,F), the bull ray (Aetomylaeus bovinus), and the bluntnose sixgill shark (Hexanchus griseus) (Figures 2G,H). In both processes, the sperm flowing form the urogenital papillae was immediately collected using a sterile syringe, a steel medical spatula, or a pipette. After collection, the sperm was transferred to sterile tubes.
An artificial elasmobranch seminal plasma extender (EE) was formulated to be similar in composition (solutes, pH, and osmolality) to the inner fluids of marine elasmobranchs. An aliquot of sperm extracted in vivo from small spotted catshark (S. canicula), and post-mortem from rough skate (R. radula), common stingray (Dasyatis pastinaca), blue shark (Prionace glauca) and bull ray (A. bovinus) was centrifuged to obtain the seminal plasma and evaluate its pH and osmolality. EE solutes composition was concluded following Daly and Jones (2017), Minamikawa and Morisawa (1996), Robertson (1989), and Yancey (2015).
The components of the extender [in mM; 433 Urea, 376 NaCl, 120 Trimethylamine N-oxide (TMAO), 8.4 KCl, 50 Glucose, 7 CaCl2-2H2O, 3.5 NaHCO3, 0.08 Na2SO4, 1.4 MgSO4 (all the solutes were purchased from Sigma-Aldrich)] were kept in balance with physiological fluids by adjusting pH to 6.5 and the osmolality to 1,000 mOsm/kg. These values were obtained from the literature and confirmed by our own pH and osmolality measures.
TMAO can be considered a limiting factor due to its price or availability in some laboratories. Thus, an EE variant (EEV) was formulated without TMAO, and it was used to dilute sperm samples and to test its usefulness as short-term medium. In this case EEV osmolality was adjusted to 1,000 mOsm/kg by increasing the amount of NaCl and urea (for a final concentration in mM of; 500 Urea, 435 NaCl) to compensate the osmolality loss because of TMAO removal.
Artificial sea water (SW) was elaborated following manufacturer’s instructions (Tropic marine REEF salt) for a final osmolality of 1,000 mOsm/kg. Artificial sea water (SW) was elaborated following manufacturer’s instructions (Tropic marine REEF salt) for a final osmolality of 1,000 mOsm/kg (in mM: NaCl 354.7, MgCl2 52.4, CaCl2 9.9, Na2SO4, 28.2, KCl 9.4, in distilled water).
To test the effectiveness of different short-term preservation media, S. canicula sperm samples (n = 33) were diluted with EE, EEV, or SW (dilution ratio 1:9; sperm:dilution medium). Polypropylene 2 mL vials were filled with the different mixtures and stored closed in a refrigerator at 4°C. Vials were assessed after 5, 12, 19, 28, and 36 days. During each assessment, an aliquot of each treatment was obtained from the refrigerated samples and the percentage of sperm motility was evaluated twice (see Sperm motility evaluation below). The assessment was done at room temperature. Only sperm samples with an initial motility higher than 60% were considered for the trials.
A series of different combinations of cryoprotectants were tested according to 7 different protocols (Table 2). Three protocols used only permeating cryoprotectants [methanol (MET), dimethyl sulfoxide (DMSO) or the combination of both] for a final cryoprotectant concentration of 10%. Other three protocols used a mixture of the permeating cryoprotectants with the addition of a non-permeating cryoprotectant [fresh egg yolk (EGG)] for a final concentration of 20%. One last protocol only used the non-permeating cryoprotectant (EGG) on a final concentration of 20%. Methanol was obtained from VWR International Eurolab S.L, dimethyl sulfoxide was obtained from PanReac Química SLU.
Fresh sperm obtained from the specimens was diluted with EE at 4°C and the corresponding cryoprotectant (Table 2) inside 2 mL internal threaded polypropylene cryotubes. In all 7 protocols the final volume of mixture in each cryotube was 1.5 mL. The cryopreservation mixture was gently shaken and left for an equilibration period of 15 min at 4°C to ensure the correct performance of the cryoprotectant. Duplicate sperm samples were tested for each protocol in order to minimize the experimental error. A cryotube filled with 1.5 mL of fresh sperm diluted with EE (dilution ratio 1:9; sperm:EE) was used as a control.
Due to the small amount of sperm obtained from skates (Raja spp.) two protocols (DMSO + MET + EGG and EGG) were not tested on these species.
Only sperm samples with an initial motility higher than 60% were considered for the trials.
Freezing and thawing process:
A styrofoam box was partially filled with liquid nitrogen (LN) and kept closed until the temperature of the container was stable (the LN stopped boiling). After the equilibration process, cryotubes were placed over a metal mesh platform floating 1 cm over the LN, for a period of 15 min. Cryotubes were completely submerged into the LN after that time, where they remained for 5–10 min. For the thawing process, cryotubes were extracted from the LN and submerged for 75 s in a water bath at 70°C.
To evaluate spermatozoa motility, a 0.5 μL aliquot was collected from each sample and diluted in 4.5 μL of EE placed on a counting chamber ISAS Spermtrack 10 (Proiser R + D, S.L., Spain). The assessment of motility was done at room temperature. Samples were observed using a microscope with a 10x magnification lens (Nikon Eclipse 80i) and videos of the sample were recorded by a camera (Nikon Digital Sight DS-5M). The videos were then analyzed manually, and the percentage of motile (displacing, rotating, or actively beating flagella) and non-motile spermatozoa were recorded. A mean of 80–100 spermatozoa were individually counted per sample. Fresh (non-frozen) samples were also analyzed as a control.
To study the viability of the thawed spermatozoa their plasma membrane integrity was assessed using a fluorescence LIVE/DEAD Sperm Viability Kit (Thermo Fisher Scientific, MA, United States). The kit uses SYBR-14, which stains intact cells green, and propidium iodide (PI) that stains damaged cells red. For each thawed sample, a 50 μL aliquot was obtained and mixed with 1 μL PI (final concentration 12 μM) and 1.5 μL of SBYR-14 (final concentration 100 nM). After an incubation period of 10 min in darkness and at room temperature, the samples were observed under a fluorescence microscope (Nikon Eclipse 80i) at 10× magnification and pictures were taken. Pictures were analyzed to count the number of green and red spermatozoa heads. An average of 135 spermatozoa per sample were identified as live or dead. As mentioned above for the motility study, fresh (non-frozen) samples were also processed and analyzed.
Two species were selected as model species to perform the first preservation protocols: S. canicula and R. radula. Regarding the genus Raja, preliminary analysis showed that there were no significant differences in the motility and viability values of the species belonging to this genus (the Mediterranean starry ray Raja asterias, the thornback ray Raja clavata, the spotted ray Raja montagui and R. radula), thus a common group was established for statistical analysis.
Shapiro-Wilk and Levene tests were used to check data normality and variance homogeneity, respectively. To analyze the EE as a short-term preservation medium over time, a univariate General Linear Model (GLM) was used. A One-factor ANOVA was used to compare the different cryopreservation protocols as well the difference between EE, sea water and EEV. A Student-Newman-Keuls (SNK) post hoc test was used in all cases. All statistical analyses were carried out using the IBM SPSS Statistics 24 statistical software.
Although sperm extraction was achieved for 39 animals, the obtention of high-quality sperm was not feasible in all the specimens. This was particularly the case with adult skates obtained in fish markets (some of which possess a low sperm volume) and most of the S. stellaris kept in aquaria (which exhibited low sperm motility). Table 1 shows the species and the number of specimens (n = 28) whose sperm had enough motility (>60%) to be used in the trials. As an exception, bull ray (A. bovinus) sperm was used despite its low motility value (45%) because of its Critically Endangered conservation status.
The sperm obtained had a creamy white coloration and viscous consistency. Under the microscope, a mass of helical heads, typical of elasmobranchs, was clearly visible. The sperm mass disaggregated when the sample was diluted, allowing the spermatozoa to be seen individually. The spermatozoa concentration of the samples ranged from 2.27 × 108 to 4.22 × 108 cells per mL and the average pH value was 6.46 (SD: 0.15).
Short-term preservation was evaluated by analyzing the sperm motility of samples diluted with different solutions (EE, sea water, EEV) over time. The motility of the samples was checked on days 0, 5, 12, 19, 28, and 36. The results represented by Figure 3 (mean motility of each treatment after different times) showed that there was a continuous decrease in motility in all the samples over time. This reduction in motility was drastic in SW, while for EE and EEV it was more gradual and with no significant differences between the two treatments. Starting from an initial motility value of 85–90% for the three treatments, the motility of sperm samples diluted in EE (Figure 4A) and EEV (Figure 4B) decreased to values of 60–65% after 12 days, and 40% after 28 days. Then the sperm motility values of both treatments (EE and EEV) showed a progressive reduction until day 36 day, reaching values close to 10%. Overall, there were no significant differences between the two treatments (EE and EEV) in terms of motility. In contrast, sperm samples diluted in SW (Figure 4C) showed a significant decrease in the motility values after just 5 days, reaching values close to 30%. The sperm motility values were close to 0 on day 19 for sperm samples diluted in SW.
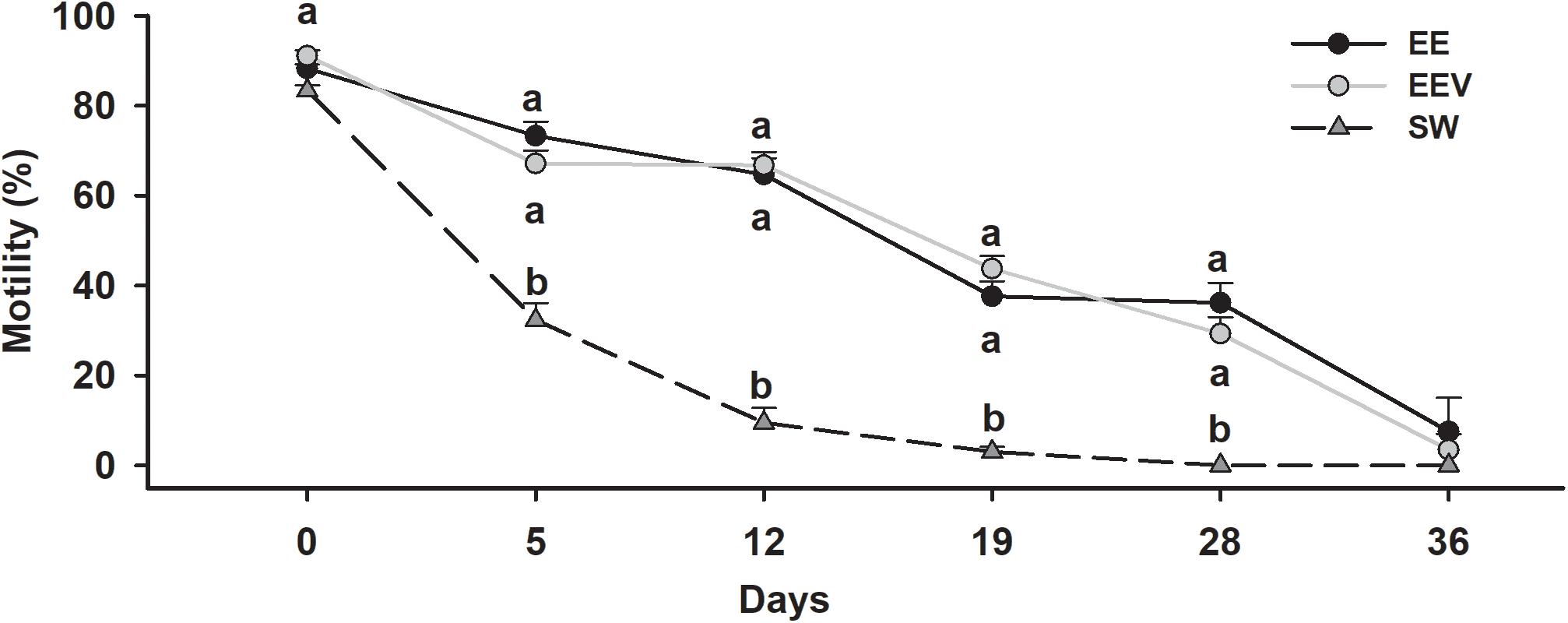
Figure 3. Short-term preservation. Motility variation over time in short-term preservation trials. Scyliorhinus canicula sperm was diluted 1:9 (sperm: dilution medium) with artificial elasmobranch seminal plasma extender (EE), artificial seminal plasma extender variant (EEV), or seawater (SW). Motility was assessed on different days (0, 5, 12, 19, 28, 36) after initial dilution. Lowercase letters represent statistical differences between treatments.
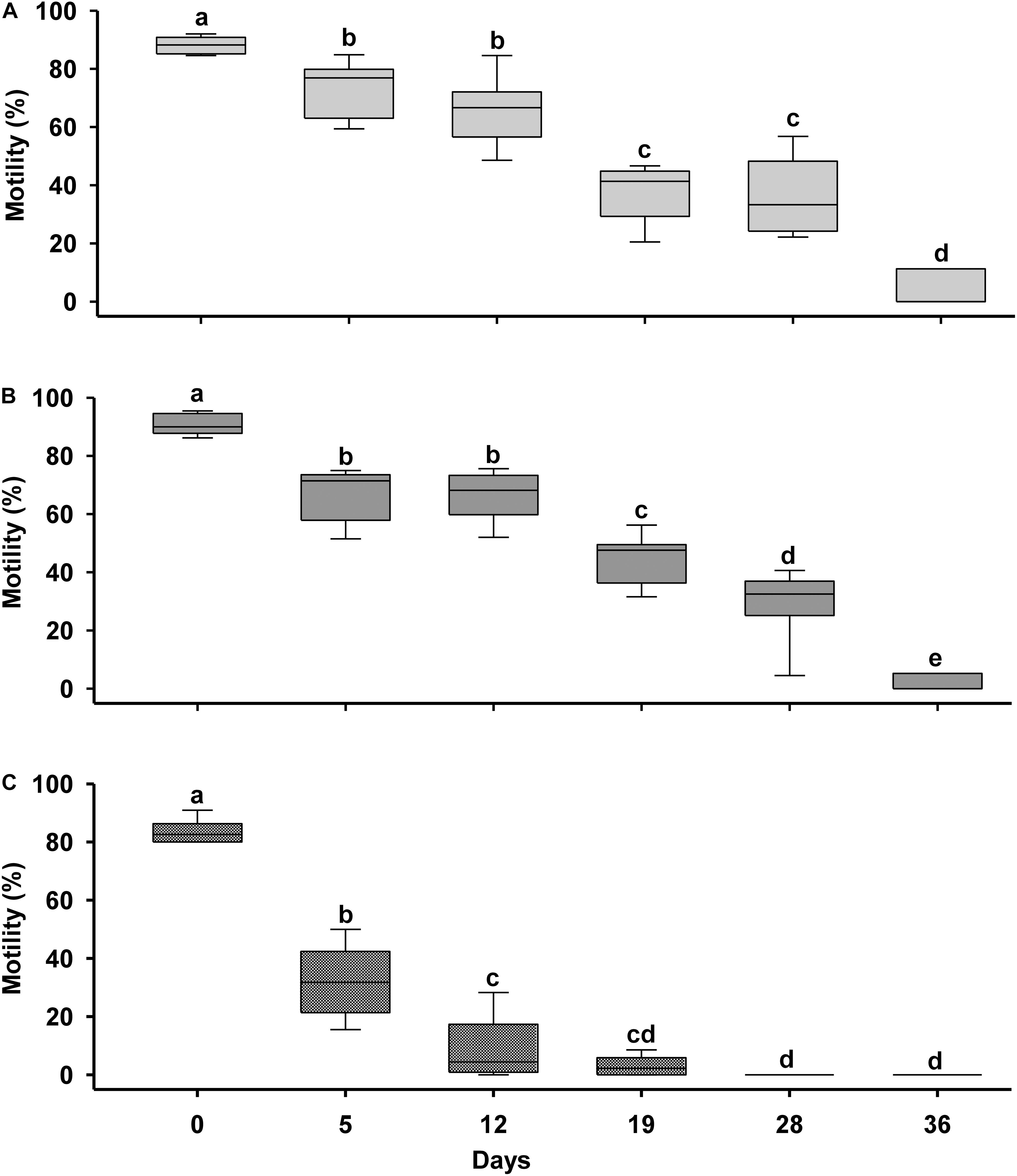
Figure 4. Media comparison for short-term preservation. Short-term preservation results after diluting 1:9 (sperm: dilution medium) Scyliorhinus canicula sperm with three diluents. (A) Artificial elasmobranch seminal plasma extender (EE); (B) artificial seminal plasma extender variant (EEV); (C) sea water (SW). Motility was assessed on different days (D) after initial dilution (CONTROL). Different letters mean significant motility differences between days into every treatment.
Cryopreserved samples showed significantly lower motility and viability values than the fresh samples, irrespective of the protocol used or the species from which the sperm came. In general, skates (Raja spp., Order Rajiformes) showed higher motility and viability results than sharks (Scyliorhinus spp. and P. glauca, Order Carcharhiniformes, and H. griseus, Order Hexanchiformes, which in turn showed higher values than stingrays (A. bovinus and D. pastinaca, Order Myliobatiformes).
In skates (Figure 5) the use of both 10% DMSO and 10% MET rendered motility values higher than 40%. However, the use of MET as a cryoprotectant caused a higher variability. The protocol combining of 5% DMSO plus 5% MET, obtained significantly lower results than the other protocols. The viability results were like those of motility, showing that the protocol using 10% DMSO produced higher values than the protocols which used 10% MET and the combination of MET plus DMSO.
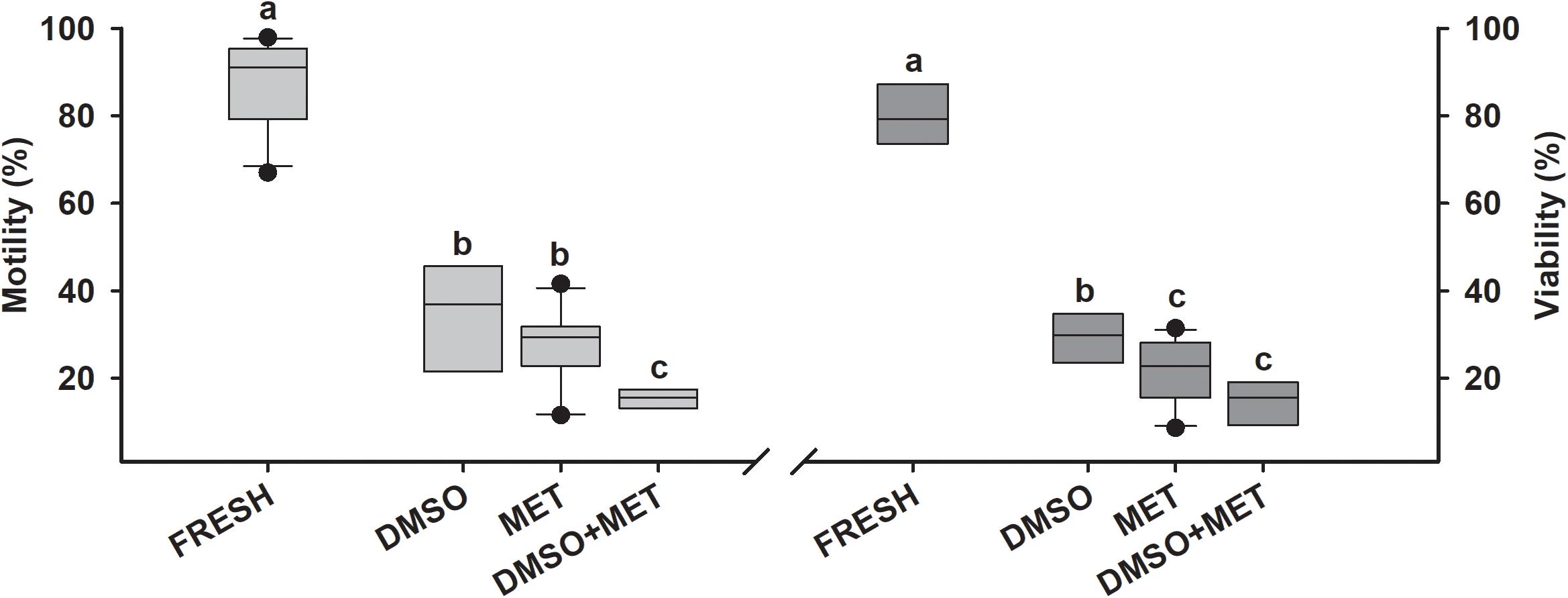
Figure 5. Cryopreservation results in rays. Motility and viability results for sperm cryopreservation protocols in skates (n = 11). Sperm obtained from Raja asterias, Raja clavata, Raja montagui, and Raja radula were diluted with artificial elasmobranch seminal plasma extender (EE) and frozen using different cryoprotectant combinations: dimethyl sulfoxide (DMSO), methanol (MET) or DMSO + MET. FRESH represents the motility and viability values in fresh sperm samples prior to freezing. Different letters mean significant differences between viability or motility values from each protocol.
In the case of the cryopreserved shark sperm samples (Figure 6 and Table 3) the best motility values were seen with protocols using 5% DMSO plus 5% MET and 10% egg yolk, or using 5% DMSO plus 5% MET, which induced average values close to 35%. Overall, the addition of egg yolk as non-permeating cryoprotectant increased the post-thawing motility values, by up to 42.1% in samples with initial motility values of 70%. As was the case of skates, in terms of viability sharks showed similar patterns to those found in motility (Figures 7A,B). Although after cryopreservation samples did not reach values higher than 40%, protocols using the combination of DMSO, MET, and EGG obtained significantly higher viability values (around 30%) than those obtained without this combination (10–20%). As a side note, the best motility and viability values after cryopreservation were obtained for H. griseus samples, reaching values of 74.3 and 73.2%, respectively, using the combination of 5% DMSO plus 5% MET and 10% egg yolk.
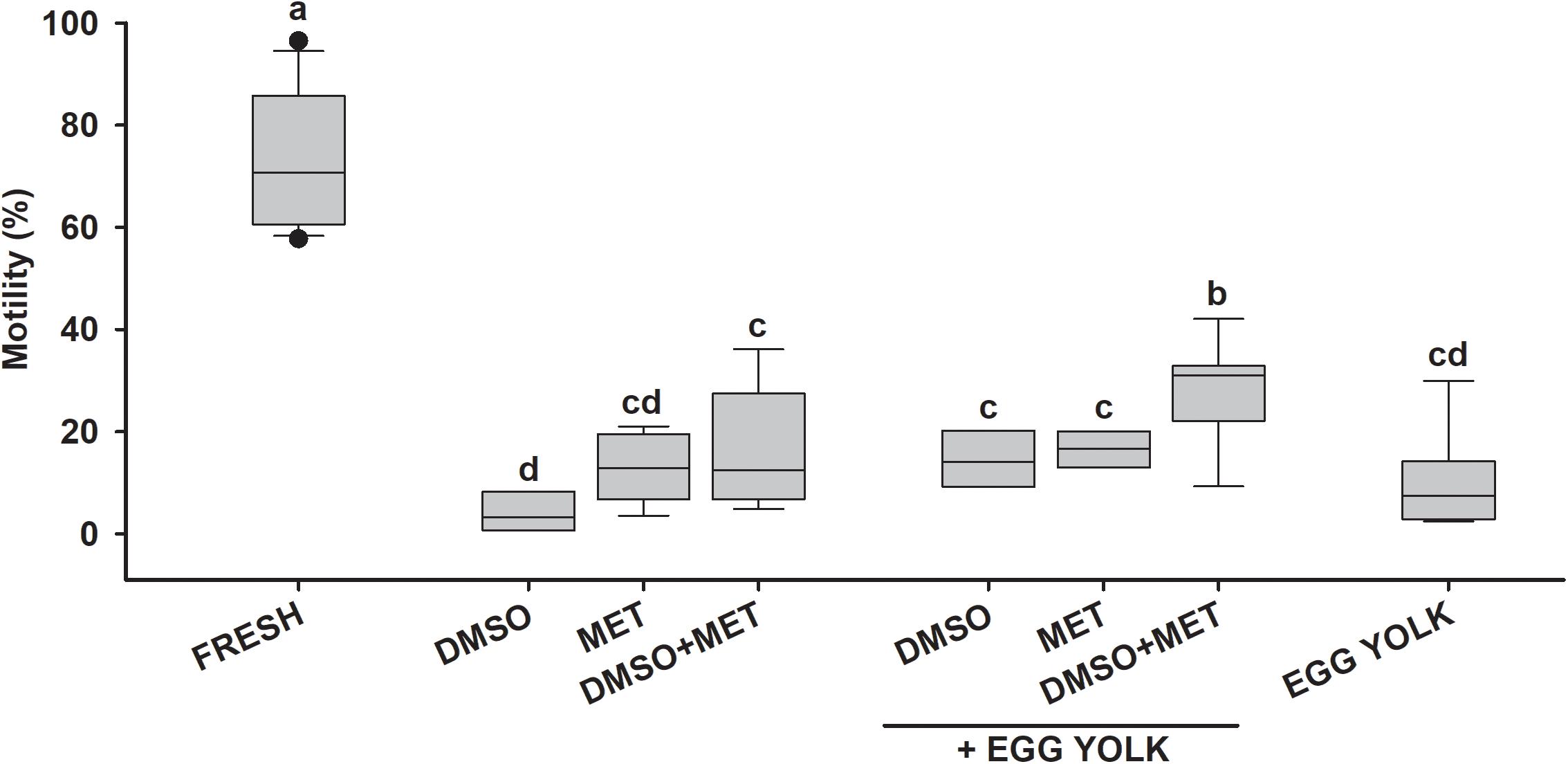
Figure 6. Motility after cryopreservation in sharks. Motility results for cryopreservation protocols in small spotted catshark (n = 11). Sperm obtained from Scyliorhinus canicula was diluted with artificial elasmobranch seminal plasma extender (EE) and frozen using different cryoprotectant combinations: dimethyl sulfoxide (DMSO), methanol (MET) and fresh egg yolk. FRESH represents the motility and viability values in fresh sperm samples prior to freezing. Different letters mean significant differences between motility values from each protocol.
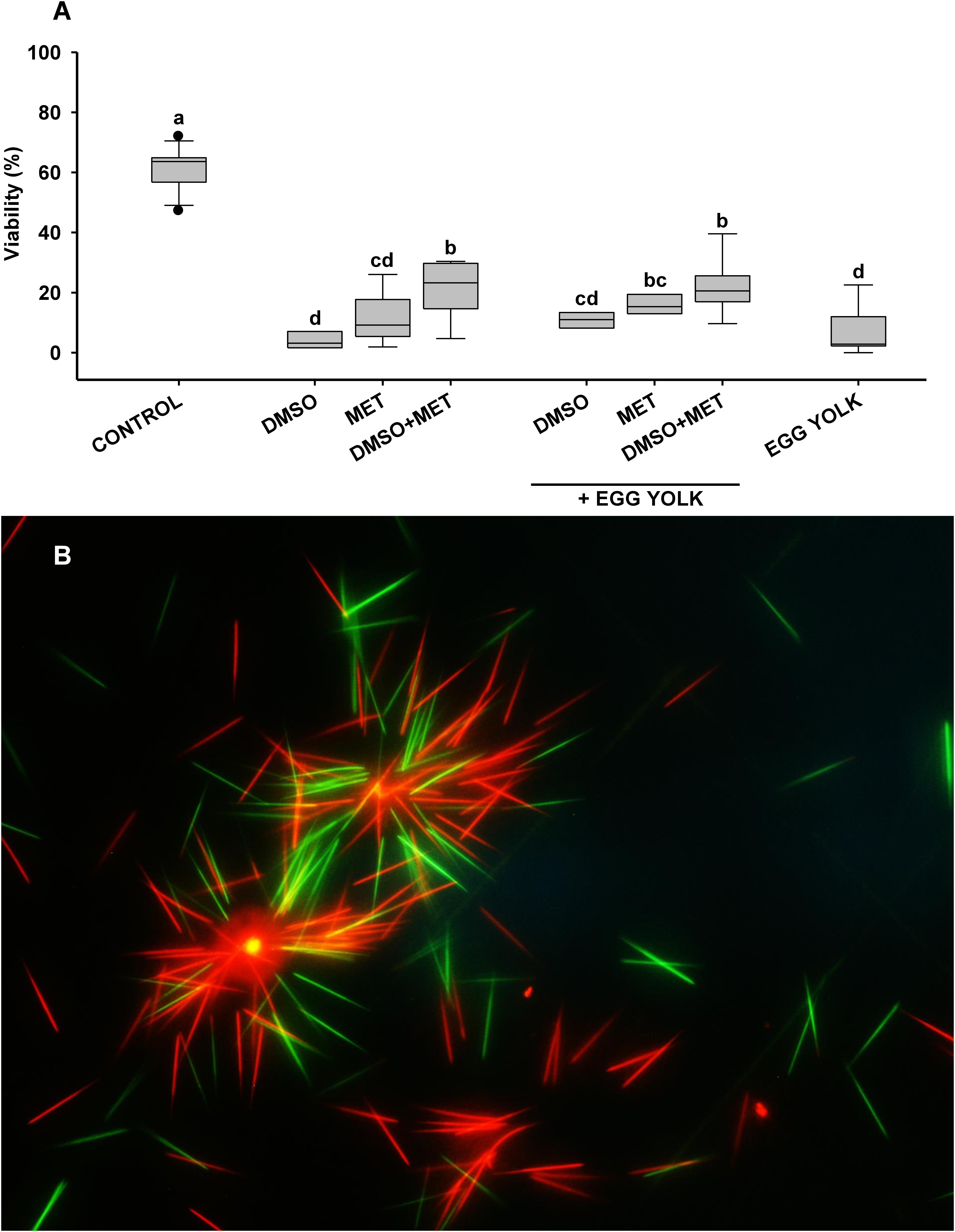
Figure 7. Viability after cryopreservation in sharks. (A) Viability results for cryopreservation protocols in small spotted catshark (n = 11). Sperm obtained from Scyliorhinus canicula was diluted with artificial elasmobranch seminal plasma extender (EE) and frozen using different cryoprotectant combinations: dimethyl sulfoxide (DMSO), methanol (MET) and fresh egg yolk. FRESH represents the motility and viability values in fresh sperm samples prior to freezing. Different letters mean significant differences between viability or motility values from each protocol. (B) Stained sperm to study the viability of the thawed spermatozoa by assessing their plasma membrane integrity. SYBR-14 stains intact cells green, and propidium iodide (PI) stains damaged cells red. Stained samples were observed under a fluorescence microscope (Nikon Eclipse 80i) at 10× magnification.
Only three stingrays were obtained from by-catch and stranding, two D. pastinaca and one A. bovinus. Although the number of animals was too small to perform a proper statistical analysis, some useful information was obtained (Table 3). For the common stingray (D. pastinaca) only protocols using 10% DMSO and 10% MET were used, because of the small volume of sperm obtained. The protocols used on D. pastinaca were not nearly as successful as those used in the other species tested, causing a maximum motility value post-thawing of 6.9%.
Unlike in the other species tested, the bull ray (A. bovinus) motility values were lower than their viability values. MET supplemented with egg yolk induced better results than the other protocols in both motility (17%) and viability (21.6%), from an initial motility of 45%.
In the present study, all the samples belonging to the control protocol (sperm diluted but no cryoprotectant added) showed motility and viability values lower than 0.5%.
Elasmobranch breeding attempts in public aquaria, to improve the sustainability of their zoological collections, or to carry out conservation programs are not as developed as those of other species. However, captive breeding programs are gaining importance, especially for threatened species (Daly and Jones, 2017). There are a wide variety of reproductive techniques that could be used on elasmobranchs, such as the monitoring of reproductive cycles, hormonal treatments, gamete collection, sperm cryopreservation and artificial insemination. These techniques have been well documented in many mammals, reptiles, birds, and bony fish, but their application in elasmobranchs has been anecdotal (Daly and Jones, 2017; Penfold and Wyffels, 2019). This study presents new contributions in this field, with the intention of serving as a basis for developing affordable preservation methods for elasmobranch sperm, without the need of expensive or complex equipment.
Short-term storage methods are designed to preserve sperm quality, by diluting the cells in an extender with physio-chemical conditions like those found in the seminal plasma. In the development of our dilution medium (EE), we considered the comments of Daly and Jones (2017) who proposed that while is true that osmotic pressure might be more important than ionic composition, most authors adapted their dilution media to the physiological characteristics of elasmobranchs. Thus, they included components present in this group such urea and NaCl. For this reason, we considered Minamikawa and Morisawa (1996) work on banded houndshark (T. scyllium) because of the description they provided on the ionic composition of the different fluids, and especially because of their comments on hexose. They observed that the duration of sperm movement was longer in a hexose-rich uterine fluid-like solution than in seawater, suggesting hexose plays an important role in maintaining sperm cell motility in the female reproductive tract. Thus, our proposed dilution medium has a high concentration of hexose in the form of glucose as a possible energy source for sperm motility. We also considered the work on osmotic constituents in S. canicula developed by Robertson (1989) and the review of Yancey (2015) about organic solutes in elasmobranchs, especially regarding the balance between the proportions of urea and TMAO used.
Our results show that samples diluted with EE maintained a motility > 70% for up to 12 days of storage, and up to 40% 28 days after dilution. It was from day 36 when the sperm motility drastically decreased. In contrast, the motility of samples stored after dilution in SW decreased rapidly: from an initial 80% to approximately 30% after 5 days of storage and falling to values under 10% after 12 days of storage. Samples diluted in EEV showed a similar motility pattern during the first few days to that of samples diluted in EE. Thus, for a up to 36 days storage there would be no need to include TMAO in the medium if the required osmolality is maintained. However, we chose EE as the extender for our cryopreservation trials for two reasons: (i) we wanted to use an extender with a composition like seminal plasma, and (ii) some authors considered that TMAO plays a key role in the physiology of marine animals by enhancing the binding ability and structural stability of proteins, thereby counteracting the negative effects of high urea and NaCl concentrations (Yancey et al., 2002). Additionally, these authors pointed out that it could play a role in protecting the tissues against other stressors, such as pressure and temperature. For these reasons, we believe that TMAO should not be discarded as a key element in the ionic composition of media for the short-term storage and the cryopreservation of elasmobranch sperm.
Sperm cryopreservation involves its dilution in a medium containing one or more cryoprotectants, which help to protect sperm from damage produced in the freezing and thawing processes. Cryoprotectant effectiveness depends on its toxicity to the spermatozoa, its concentration, the equilibration duration time, and the diluents used. DMSO is the most used cryoprotectant for the sperm of aquatic species such as cyprinids (Lujić et al., 2017), while MET, dimethylacetamide or glycerol are more effective in other groups such as salmonids (Labbé et al., 2013). In elasmobranchs, two successful cryopreservation trials with sparsely spotted stingaree (U. paucimaculatus) (Daly et al., 2011) and Australian bull ray (M. australis) (Daly and Jones, 2017) used DMSO or glycerol supplemented with egg yolk as non-permeating cryoprotectant. Non-permeating cryoprotectants in addition to permeating cryoprotectants can significantly increase the percentage of viable and motile spermatozoa post-thawing in different species (Gallego et al., 2017). In our study the best results for shark cryopreservation were obtained when adding egg yolk to the permeating cryoprotectants, suggesting the importance of including a non-permeating cryoprotectant in the freezing medium. The use of egg yolk for cryopreservation is common for teleost species reared in aquaculture, as it has a thermo-protective action, which is exercised by the lecithin and cephaline of its lipid fraction and a preservative action, given by the lipoproteic fraction (Robles et al., 2005). In fact, egg yolk has been used as an additive in many cryopreservation studies, and although positive effects have been observed in many cases, it may not be applicable in some species. For example, the addition of egg yolk increases fertilization times in common trout (Salmo trutta) after cryopreservation (Piironen, 1993) but has a negative effect on the sperm cryopreservation of asp (Leuciscus aspius) (Babiak et al., 1998).
In the present study, the cryopreserved sperm samples of sharks and skates showed post-thawing motility values of 30–40% depending on the species, with the notable exception of the bluntnose sixgill shark (H. griseus), with a motility value of 70%. This species can be found in depths up to 2,500 m where is adapted to cold deep-sea conditions (Ebert and Dando, 2020). There might be a connection between the high cryosurvival values and these adaptations (such as high levels of TMAO), that should be considered in future studies. The effects of DMSO also demonstrated the existence of species-dependant reactions to cryoprotectants. While 10% DMSO worked best for skates, it compromised post-thawed motility and viability of sharks and stingrays due to possible toxicity after prolonged exposure. Although not conclusive, 10% MET was the most effective protocol in A. bovinus sperm samples, with a post-thawing motility of 17%.
Even though the use of DMSO is highly recommended for the cryopreservation of fish sperm due to its rapid penetration into the cells, it can have negative effects by changing the osmolality of the medium (Garzón et al., 2007; Peñaranda et al., 2008). Also, DMSO can have a species-specific toxic effect (Arciniegas et al., 2005). Other studies, such as that carried out by Daly and Jones (2017), described the toxic effect of glycerol on brownbanded bambooshark (C. punctatum) sperm despite its protective effects in sparsely spotted stingaree (U. paucimaculatus) and Australian bull ray (M. australis) sperm. Thus, further research should be conducted to learn about the toxic effects of cryoprotectants in different species and whether certain aspects of the protocols should be specifically adjusted.
The present study expands the list of elasmobranch species in which cryopreserved sperm has been achieved. The use of two model species [the small spotted catshark (Scyliorhinus canicula) and the rough ray (Raja radula)] allowed us to refine protocols before testing them on other species. It is important to note that the source of the sperm samples covers almost every possible origin of elasmobranch gametes: aquaria collection, by-catch, fish markets and stranded animals, confirming the possible application of these protocols in all kinds of samples.
The protocols described here for short-term and long-term sperm preservation can be considered a complement to ex situ conservation projects for sharks and rays. In addition, to our knowledge this is the first time that cryopreserved shark sperm has been achieved, not only in our model species (S. canicula) but in a Nearly Threatened species, the nursehound (Scyliorhinus stellaris), a Critically Endangered species, the blue shark (Prionace glauca) and a deep-sea shark species, the bluntnose sixgill shark (Hexanchus griseus). Skate sperm has also been cryopreserved for the first time in four different species, including two Nearly Threatened species, the thornback ray (Raja clavata) and the Mediterranean starry ray (Raja asterias) and an Endangered endemism (Raja radula). Other threatened species such as D. pastinaca and the Critically Endangered A. bovinus expand the Myliobatiformes list where cryopreservation has been previously achieved. However, we were not able to design a universal protocol for elasmobranch sperm cryopreservation, so more research needs to be carried out to assess the different responses of different species. Our research can potentially help with the development of artificial insemination projects in elasmobranchs and has expanded our knowledge on the reproductive biology of this group of species.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The animal study was reviewed and approved by the Ethic Committee of Fundación Oceanogràfic (Project reference: OCE-16–19).
PG-S and VG got the samples from the different sources and carried out the experiments. JA supervised the experiments and was the responsible of funding management. Altogether wrote this manuscript. All authors contributed to the article and approved the submitted version.
Study partially funded by the Fundación Biodiversidad (PRCV00683). PG-S has a contract from the European Union through the Operational Program of the European Social Fund (ESF) of the Comunitat Valenciana 2014–2020 ACIF 2018 (ACIF/2018/147). VG has a postdoc contract from the MICIU, Programa Juan de la Cierva-Incorporación (IJCI-2017-34200).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Iraida Català for her assistance, and the Fundación Oceanogràfic, the Comunitat Valenciana Stranding Network and Jaime Penadés Suay and David Ruiz-García from the University of Valencia for allowing access to some of the samples.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.689089/full#supplementary-material
Arciniegas, S. P., Rodríguez, J. M., and Casallas, P. E. (2005). Ensayos preliminares sobre crioconservación de semen de bagre rayado Preliminary essays on crioconservation of semen of catfish “lined bagre”. Orinoquía 9, 28–37.
Asturiano, J. F., Cabrita, E., and Horváth, A. (2017). Progress, challenges and perspectives on fish gamete cryopreservation: a mini-review. Gen. Comp. Endocrinol. 245, 69–76. doi: 10.1016/j.ygcen.2016.06.019
Babiak, I., Glogowski, J., Kujawa, R., Kucharczyk, D., and Mamcarz, A. (1998). Cryopreservation of Sperm from Asp Aspius aspius. Progress. Fish-Culturist 60, 146–148. doi: 10.1577/1548-86401998060<0146:COSFAA<2.0.CO;2
Beirão, J., Boulais, M., Gallego, V., O’Brien, J. K., Peixoto, S., Robeck, T. R., et al. (2019). Sperm handling in aquatic animals for artificial reproduction. Theriogenology 133, 161–178. doi: 10.1016/j.theriogenology.2019.05.004
Braverman, I. (2014). Conservation without nature: the trouble with in situ versus ex situ conservation. Geoforum 51, 47–57. doi: 10.1016/j.geoforum.2013.09.018
Buckley, K. A., Crook, D. A., Pillans, R. D., Smith, L., and Kyne, P. M. (2018). Sustainability of threatened species displayed in public aquaria, with a case study of Australian sharks and rays. Rev. Fish Biol. Fish. 28, 137–151. doi: 10.1007/s11160-017-9501-2
Cabrita, E., Horváth, A., Marinović, Z., and Asturiano, J. F. (2021). “Technologies and strategies for ex situ conservation of aquatic organisms: the role of cryopreservation in long-term management,” in Cellular And Molecular Approaches In Fish Biology, eds I. Monzón and J. Fernandes (Berlin: Springer).
Compagno, L. J. V. (1990). Alternative life-history styles of cartilaginous fishes in time and space. Environ. Biol. Fishes 28, 33–75. doi: 10.1007/BF00751027
Cortés, E. (2000). Life history patterns and correlations in sharks. Rev. Fish. Sci. 8, 299–344. doi: 10.1080/10408340308951115
Daly, J., Holland, M., and Galloway, D. (2011). “Preliminary investigations on sperm cryopreservation of a stingray, the sparsely spotted stingaree,” in Cryopreservation in Aquatic Species, 2nd Edn, eds T. R. Tiersch and C. C. Green (Baton Rouge, LA: World Aquaculture Society), 337–344.
Daly, J., and Jones, R. (2017). “The use of reproductive technologies in breeding programs for elasmobranchs in aquaria,” in Elasmobranch Husbandry Manual II: Recent Advances in the Care of Sharks, Rays and their Relatives, eds M. Smith, D. Warmolts, D. Thorney, and R. Heuter (Columbus, O: Special Publication of the Ohio Biological Survey), 363–374.
Daochai, C., Keschumras, N., Chansue, N., and Haetrakul, T. (2020). Preliminary of intra-vagina artificial insemination using fresh semen in Ocellate river stingray (Potamotrygon motoro). Thai J. Vet. Med. 50, 383–385.
Dulvy, N. K., and Forrest, R. E. (2010). “Life histories, population dynamics and extinction risks in chondrichthyans,” in Sharks and Their Relatives II, eds J. C. Carrier, J. A. Musick, and M. R. Heithaus (Boca Raton, FL: CRC Press), 639–679. doi: 10.1201/9781420080483-c17
Dulvy, N. K., Fowler, S. L., Musick, J. A., Cavanagh, R. D., Kyne, P. M., Harrison, L. R., et al. (2014). Extinction risk and conservation of the world’s sharks and rays. Elife 3, 1–34. doi: 10.7554/eLife.00590
Ebert, D. A., and Dando, M. (2020). Field Guide to Sharks, Rays & Chimaeras of Europe and the Mediterranean. Princeton, NJ: Princeton University Press.
Gallego, V., and Asturiano, J. F. (2019). Fish sperm motility assessment as a tool for aquaculture research: a historical approach. Rev. Aquac. 11, 697–724. doi: 10.1111/raq.12253
Gallego, V., Cavalcante, S. S., Fujimoto, R. Y., Carneiro, P. C. F., Azevedo, H. C., and Maria, A. N. (2017). Fish sperm subpopulations: changes after cryopreservation process and relationship with fertilization success in tambaqui (Colossoma macropomum). Theriogenology 87, 16–24. doi: 10.1016/j.theriogenology.2016.08.001
García, V. B., Lucifora, L. O., and Myers, R. A. (2008). The importance of habitat and life history to extinction risk in sharks, skates, rays and chimaeras. Proc. R. Soc. B Biol. Sci. 275, 83–89. doi: 10.1098/rspb.2007.1295
Garzón, D. L., Peñaranda, D. S., Pérez, L., Marco-Jiménez, F., Espert, X., Müller, T., et al. (2007). Effects of pH, sodium bicarbonate, cryoprotectants and foetal bovine serum on the cryopreservation of European eel sperm. Reprod. Domest. Anim. 43, 99–105. doi: 10.1111/j.1439-0531.2007.00861.x
Henningsen, A. D. (1994). Tonic immobility in 12 elasmobranchs: use as an aid in captive husbandry. Zoo Biol. 13, 325–332. doi: 10.1002/zoo.1430130406
Henningsen, A. D., Smale, M. J., Gordon, I., Garner, R., Marin-Osorno, R., and Kinnunen, N. (2004). “Captive breeding and sexual conflict in elasmobranchs,” in Elasmobranch Husbandry Manual: Recent Advances in the Care of Sharks, Rays and their Relatives, eds M. Smith, D. Warmolts, D. Thorney, and R. Heuter (Columbus, O: Special Publication of the Ohio Biological Survey), 237–248.
Horváth, A., and Urbányi, B. (2020). “Sperm cryopreservation of aquatic species,” in Reproduction in Aquatic Animals: From Basic Biology to Aquaculture Technology eds M. Yoshida and J. F. Asturiano Singapore (Springer Singapore: Springer), 321–334. doi: 10.1007/978-981-15-2290-1_16
IUCN/SSC (2014). IUCN Species Survival Commission Guidelines on the Use of Ex Situ Management for Species Conservation. Version 2. 1–7. Available online at: https://portals.iucn.org/library/sites/library/files/documents/2014-064.pdf (accessed 07 December, 2020).
Jones, R. C., Jones, N., and Djakiew, D. (1984). Luminal composition and maturation of spermatozoa in the male genital ducts of the Port Jackson shark, Heterodontus portusjacksoni. J. Exp. Zool. 230, 417–426. doi: 10.1002/jez.1402300311
Kessel, S. T., and Hussey, N. E. (2015). Tonic immobility as an anaesthetic for elasmobranchs during surgical implantation procedures. Can. J. Fish. Aquat. Sci. 72, 1287–1291. doi: 10.1139/cjfas-2015-0136
Labbé, C., Robles, V., and Herráez, M. P. (2013). “Cryopreservation of gametes for aquaculture and alternative cell sources for genome preservation,” in Advances in Aquaculture Hatchery Technology, eds G. Burnell and G. Allan (Amsterdam: Woodhead Publishing Series), 76–116. doi: 10.1533/9780857097460.1.76
Luer, C. A., Walsh, C. J., Bodine, A. B., and Wyffels, J. T. (2008). “Normal embryonic development in the clearnose skate, Raja eglanteria, with experimental observations on artificial insemination,” in Biology of Skates, eds D. Ebert and J. Sulikowski (Dordrecht: Springer Netherlands), 133–149. doi: 10.1007/978-1-4020-9703-4_9
Lujić, J., Bernáth, G., Marinović, Z., Radojković, N., Simić, V., Ćirković, M., et al. (2017). Fertilizing capacity and motility of tench Tinca tinca (L., 1758) sperm following cryopreservation. Aquac. Res. 48, 102–110. doi: 10.1111/are.12865
Martínez-Páramo, S., Horváth, A., Labbé, C., Zhang, T., Robles, V., Herráez, M. P., et al. (2017). Cryobanking of aquatic species. Aquaculture 472, 156–177. doi: 10.1016/j.aquaculture.2016.05.042
Minamikawa, S., and Morisawa, M. (1996). Acquisition, initiation and maintenance of sperm motility in the shark, Triakis scyllia. Comp. Biochem. Physiol. - A Physiol. 113, 387–392. doi: 10.1016/0300-9629(95)02080-2
Muñoz Gutiérrez, M. E. (2011). Biotecnología aplicada en la reproducción de peces. Inf. Técnico 75, 66–72. doi: 10.23850/22565035.21
Ohta, H., and Izawa, T. (1996). Diluent for cool storage of the Japanese eel (Anguilla japonica) spermatozoa. Aquaculture 142, 107–118. doi: 10.1016/0044-8486(95)01246-X
Peñaranda, D. S., Pérez, L., Gallego, V., Barrera, R., Jover, M., and Asturiano, J. F. (2008). European eel sperm diluent for short-term storage. Reprod. Domest. Anim. 45, 407–415. doi: 10.1111/j.1439-0531.2008.01206.x
Penfold, L. M., and Wyffels, J. T. (2019). Reproductive science in sharks and rays. Adv. Exp. Med. Biol. 1200, 465–488. doi: 10.1007/978-3-030-23633-5_15
Piironen, J. (1993). Cryopreservation of sperm from brown trout (Salmo trutta m. lacustris L.) and Arctic charr (Salvelinus alpinus L.). Aquaculture 116, 275–285. doi: 10.1016/0044-8486(93)90014-P
Pritchard, D. J., Fa, J. E., Oldfield, S., and Harrop, S. R. (2012). Bring the captive closer to the wild: redefining the role of ex situ conservation. ORYX 46, 18–23. doi: 10.1017/S0030605310001766
Robertson, J. D. (1989). Osmotic constituents of the blood plasma and parietal muscle of Scyliorhinus canicula (L.). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 93, 799–805. doi: 10.1016/0300-9629(89)90504-5
Robles, V. M. M., Santamaría, Y. M. V., and Casallas, P. E. C. (2005). Aspectos generales de la crioconservación espermática en peces teleósteos. Rev. Colomb. Ciencias Pecu. 18, 34–48.
Stevens, J. (2000). The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J. Mar. Sci. 57, 476–494. doi: 10.1006/jmsc.2000.0724
Tambasen-Cheong, M. V. P., Tan-Fermin, J. D., Garcia, L. M. B., and Baldevarona, R. B. (1995). Milt-egg ratio in artificial fertilization of the Asian freshwater catfish, Clarias macrocephalus, injected salmon gonadotropin-releasing hormone analogue and domperidone. Aquat. Living Resour. 8, 303–307. doi: 10.1051/alr:1995031
Tanaka, S. (1995). “Comparative morphology of the sperm in chondrichthyan fishes,” in Advances in Spermatozoal Phylogeny and Taxonomy, eds B. G. M. Jamieson, J. Ausio, and L. Justine (Paris: Memoires du Museum National d’Histoire Naturelle), 313–320.
Tan-Fermin, J. D., Miura, T., Adachi, S., and Yamauchi, K. (1999). Seminal plasma composition, sperm motility, and milt dilution in the Asian catfish Clarias macrocephalus (Gunther). Aquaculture 171, 323–338. doi: 10.1016/S0044-8486(98)00402-5
Wyffels, J. T., George, R., Adams, L., Adams, C., Clauss, T., Newton, A., et al. (2020). Testosterone and semen seasonality for the sand tiger shark Carcharias taurus. Biol. Reprod. 102, 876–887. doi: 10.1093/biolre/ioz221
Yancey, P. H. (2015). Organic osmolytes in elasmobranchs. Fish Physiol. 34, 221–277. doi: 10.1016/B978-0-12-801286-4.00004-6
Keywords: fish reproduction, sperm cryobanking, sperm motility, ex situ conservation, reproductive technics assisted, aquarium management
Citation: García-Salinas P, Gallego V and Asturiano JF (2021) Development of Sperm Cryopreservation Protocols for Sharks and Rays: New Tools for Elasmobranch Conservation. Front. Mar. Sci. 8:689089. doi: 10.3389/fmars.2021.689089
Received: 31 March 2021; Accepted: 24 June 2021;
Published: 21 July 2021.
Edited by:
Lyne Morissette, M – Expertise Marine, CanadaReviewed by:
Yusuf Bozkurt, Iskenderun Technical University, TurkeyCopyright © 2021 García-Salinas, Gallego and Asturiano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan F. Asturiano, amZhc3R1QGRjYS51cHYuZXM=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.