
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 28 May 2021
Sec. Marine Biology
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.677024
Bartholomea annulata is a facultative host of the A. armatus species complex. In the Mexican Caribbean it is commonly found in cracks and crevices located where the vertical walls meet the sandy bottom or on large coral patches away from the sand. To protect themselves from predators, anemones often contract their hydraulic body into a cavernous den and extend the stinging tentacles toward the entrance. The high sediment dynamics of the region, however, result in a permanent risk of animal shelters to be obstructed by sand. By both analysing field data and conducting laboratory experiments with artificial shelters, the present study explored the den cleaning behaviour widely extended amongst alpheid shrimp, and its role in the alpheid-anemone symbiotic interaction. Videorecordings showed that den cleaning was composed of three main behaviours: digging, tossing and tamping. It commenced as soon as 7.2 ± 10.5 min after anemones were recognised by alpheids, and behaviours were displayed systematically amongst all 12 replicates. Despite being completely burrowed in sand, Alpheus spp. were capable of finding the anemone and liberating the entrance of the artificial shelters in less than 2.5 h. In addition, manipulative experiments showed that anemones confronted with shelters that were obstructed with sediment had a 25% probability of fully retracting when Alpheus spp. were absent, compared to a 75% probability when shrimps had cleaned the shelter’s entry and internal passage. The analysis of field data indicated that the presence of alpheid shrimps as symbionts of B. annulata was 30% higher amongst anemones in close contact with sandy bottoms than when inhabiting crevices on the top or lateral walls of hard substrates, away from the sediment. Overall, our study concludes that den cleaning constitutes a quick and effective mechanism to assure the anemone’s full retraction into their den, and by keeping the sediment away, alpheids provide the necessary conditions that serve both the anemone’s and the shrimp’s higher chances of acquiring maximum protection from predators. This advantageous exchange in protection partially explains why alpheids are more frequently present in B. annulata in interface microhabitats where the benefit of the interaction is maximised.
Coral reefs are heterogeneous environments, featuring a great variety of optimal and sub-optimal habitats (Richardson, 1999). The main variables that affect the community structure in coral reefs are luminosity, water currents flow, water temperature, intra and inter-specific competition, predation, and sediment transport (Glynn and Enochs, 2011; Alvarez-Filip et al., 2013). Anemones are an essential component of coral reefs’ benthic fauna in the Caribbean Sea and are typically attached to hard substrates, such as coral rubbles, rocks and live or dead coral on which the pedal disc can be firmly fixed (Ellis, 1767; Le Sueur, 1817; Duchassaing de Fonbressin and Michelotti, 1860; Duerden, 1897; Manjarrés, 1977; González-Muñoz et al., 2013). Other species, such as Actinostella flosculifera or Phymanthus crucifer, are found in sandy patches and also attach to hard structures but maintain the column covered with sediment (Le Sueur, 1817; González-Muñoz et al., 2012).
Partnership and common objectives amongst individuals of different species are one way to survive against natural selective pressures (Bauer, 2004). Symbiosis could be the difference between life and death for dissimilar organisms living together (Baeza, 2015). The cost and benefit of the symbiotic interaction (i.e., parasitism, mutualism or commensalism) and the degree of dependence (i.e., facultative versus obligate) vary widely (Thiel and Baeza, 2001), and have been recognised as an important speciation mechanism that explains and maintains the biodiversity patterns found in underwater ecosystems (Watson and Pollack, 1999; Crandall et al., 2008; Dáttilo and Rico-Gray, 2018; Pérez-Botello and Simões, 2021).
Sea anemones are host to a wide variety of invertebrates, amongst which crustaceans, and more specifically shrimps, dominate (Herrnkind et al., 1976; Silbiger and Childress, 2008). Symbiotic relationships can act in two directions altering both symbiont and host fitness (Crawford, 1992; Roopin and Chadwick, 2009). Without exception, this symbiotic interaction is facultative for the anemones, hence the occurrence of anemones both with and without symbiotic crustacea. For the crustacea, however, the level of dependence varies amongst interactions, with some species being obligate anemone-symbionts. Such is the case of shrimps Ancylomenes pedersoni, Periclimenes yucatanicus, Alpheus armatus, and Thor amboinensis, whereas such as the crabs Stenorynchus seticornis and Mithraculus cinctimanus are facultative guests of anemones (Manjarrés, 1977; Silbiger and Childress, 2008; González-Muñoz et al., 2012; Hurt et al., 2013).
Interactions between anemones and alpheid shrimp have demonstrated benefits for both symbiotic species. Anemones obtain protection against benthic predators that are immune to cnida-toxins through the snapping water jet made by their alpheid guests (Smith, 1977; McCammon and Brooks, 2014). It has also been argued that the particles that alpheids discard after feeding constitute a source of nitrogen supply for the anemone’s zooxanthellae (Spotte, 1996), increasing the number and efficiency of associated photosynthetic cells (Kropp, 1987; Day, 1994; LaJeunesse, 2002; Khan et al., 2003). In exchange, alpheids obtain a safe refuge from their pelagic predators derived from the defensive mechanism of the anemones’ toxic sting (Romey et al., 1976; Vincent et al., 1980; Cestele, 2000; Sanchez-Rodriguez, 2001; McCammon, 2010; Mascaró et al., 2012).
The advantage of the anemone’s chemical protection is restricted to shrimps that can acclimate to the host’s toxins (Mascaró et al., 2012). These guests, however, may also benefit from other anemone defensive strategies that are not directly dependent of the chemical power of nematocysts. Anemones have three distinct defence strategies that do not directly involve cnida: (i) releasing the pedal disc from the substrate and swimming away from the hazard; (ii) sticking the tentacle to protect the oral disc and column; and (iii) contracting its hydraulic body and dodging any possible danger (Rosin, 1969; Edmunds et al., 1974; Harris and Howe, 1979; Shick, 1979; González-Muñoz et al., 2012). If body contraction occurs within a burrow, the defence efficiency of toxic cnida could be increased: anemones cluster the tentacles with the deadly nematocysts at the burrow’s opening, while keeping vulnerable parts of the body sheltered and secure. By remaining close to the column and pedal disk when the anemone retracts into the burrow, alpheids may take advantage of the cover provided by the protective tentacles.
The shrimp genus Alpheus is a diverse and highly radiated group of fully benthic shrimp, distributed throughout all shallow tropical and subtropical marine regions of the world (0–50 m deep) (NMNH, 2001; Anker et al., 2006; CRED, 2008; Massuti et al., 2020). These shrimps are common in coral reefs, seagrass beds, mangroves, estuaries, and mixed sand-rock bottoms (Anker, 2001; Duarte et al., 2014). Some species are hard-substrates drillers, and others specialise on excavating soft substrates; but in every case digging behaviour influences the alpheid community structure and dynamics (Palomar et al., 2005; Anker et al., 2006). A remarkable behaviour amongst alpheid shrimp that are obligate symbionts of goby fish consists on building dens for their fish host and keeping them clear of sediment, an interaction that has been extensively documented in recent literature on crustacean symbiosis (Karplus, 1979, 1987, 2014; Karplus et al., 2011; Bauer, 2004). In this symbiotic relationship, the digging behaviour constitutes the primary shrimp behaviour on which the interaction is sustained (Karplus, 1987; Karplus et al., 2011). Whilst the goby fish watch over, the snapping shrimps dig, construct and maintain clean the burrows that goby fish will use for breeding and shelter (Karplus, 1987, 2014; Karplus et al., 2011; Henmi et al., 2017). This association is reported to exist in all five oceans and across sediment types, ranging from silty mud to coral rubble (Karplus, 1987), suggesting that digging behaviour amongst alpheid shrimp is not only common within the group but also geographically widespread.
The sea anemone Bartholomea annulata has a symbiotic multi-specific interaction with several crustacea, such as A. pedersoni, P. yucatanicus, T. amboinensis and specially three alpheid species of the Alpheus armatus species complex (i.e., A. armatus, A. immaculatus, and A. polystictus) (Knowlton and Keller, 1983, 1985; Silbiger and Childress, 2008; McCammon, 2010; Hurt et al., 2013). Bartholomea annulata is distributed in the lagoon and back-reef zones of the Gulf of Mexico and the Caribbean inside caves and crevices of rocks and coral rubble with only its tentacles exposed into the water column (González-Muñoz et al., 2012; Rodríguez et al., 2014). It is common between 1 and 15 m deep, but has been observed as deep as 20 m (González-Muñoz et al., 2012). In contrast with other anemones common in tropical waters that are found attached to surfaces relatively distant of the sea bed, such as Condylactis gigantea, B. annulata is frequently found in the interface between the hard and soft substrate, a microhabitat exposed to abundant sediment and high wave intensity (Barrios-Suárez et al., 2002; González-Muñoz et al., 2012; Santos et al., 2020).
The A. armatus species complex are obligate symbionts of anemones that are occasionally found as one shrimp, yet most commonly are found as a pair of male-female reproductive shrimps occupying a large anemone or anemone cluster (Hurt et al., 2013). Because alpheids are markedly territorial (Schein, 1975; Schmitz and Herberholz, 1998; Rahman et al., 2004), groups of more than two reproductive adults are never found (Smith, 1977). The species common to the North Western Atlantic Ocean are A. armatus and A. immaculatus, distributed in the Gulf of Mexico and the Caribbean Sea from 1 to 25 m deep (Hurt et al., 2013). Whilst Alpheus armatus typically occurs at depths lower than 10 m, whereas A. immaculatus is generally found at depths between 13 and 25 m (Knowlton and Keller, 1983, 1985; Hurt et al., 2013). Both species are extremely similar morphologically and occur microsympatrically (Knowlton and Keller, 1983). They also occupy the anemone’s column and protect their body by sharing their host’s burrow (Huebner et al., 2019). The occurrence of A. armatus species complex is therefore restricted to the distribution of available of host anemones (Hurt et al., 2013; Huebner et al., 2019).
While the alpheid behaviour of building and maintaining the dens of goby fishes is well known, the contribution of similar digging behaviours to the symbiotic interaction with anemones has not been studied. If den cleaning behaviour is a common feature of alpheid shrimp that are obligate symbionts of B. annulata and the advantage for the anemone is confirmed, then symbiotic associations between alpheids and B. annulata should be most frequent in those microhabitats where the benefit of the interaction is maximised. To support this hypothesis the present study aimed at describing the distribution of B. annulata with and without A. armatus complex (sensu Knowlton and Keller, 1983, 1985) in different types of microhabitats within the reefs of the Mexican Caribbean from Isla Contoy to Punta Xcalac. Laboratory experiments were then conducted to characterise the den cleaning behaviour of A. armatus complex in the presence of B. annulata and provide evidence that this behaviour improves the retraction capability of anemones within artificial shelters built for this purpose.
Information related to the type of substrate, structure and the position in which individuals of B. annulata were observed was extracted from Campos-Salgado (2009) field sampling reports. The study reported a total 179 immersions in 87 different sites along 7 regions in the Mexican Caribbean from Isla Contoy (21.6633°, −87.1000°) to Punta Xcalak (18.1500°, −87.8430°; Figure 1 and Table 1). At each site two independent divers performed visual census of B. annulata and Condylactis gigantea and their crustacean symbionts following each a 45 min transect. Anemones, including B. annulata, were identified using the Reef Creatures identification book (Humann and DeLoach, 2002) and the micro-habitat in which they were found was noted. Micro-habitats were originally described as crevices, caves or small holes on coral fragments, sand, limestone massif, platforms or rocks; and in positions that varied from on the top or lateral surface of hard structures or at the interface between these hard structures and the sandy bottom. Each anemone was carefully inspected for crustacean symbionts and the number of individuals and species found was noted.
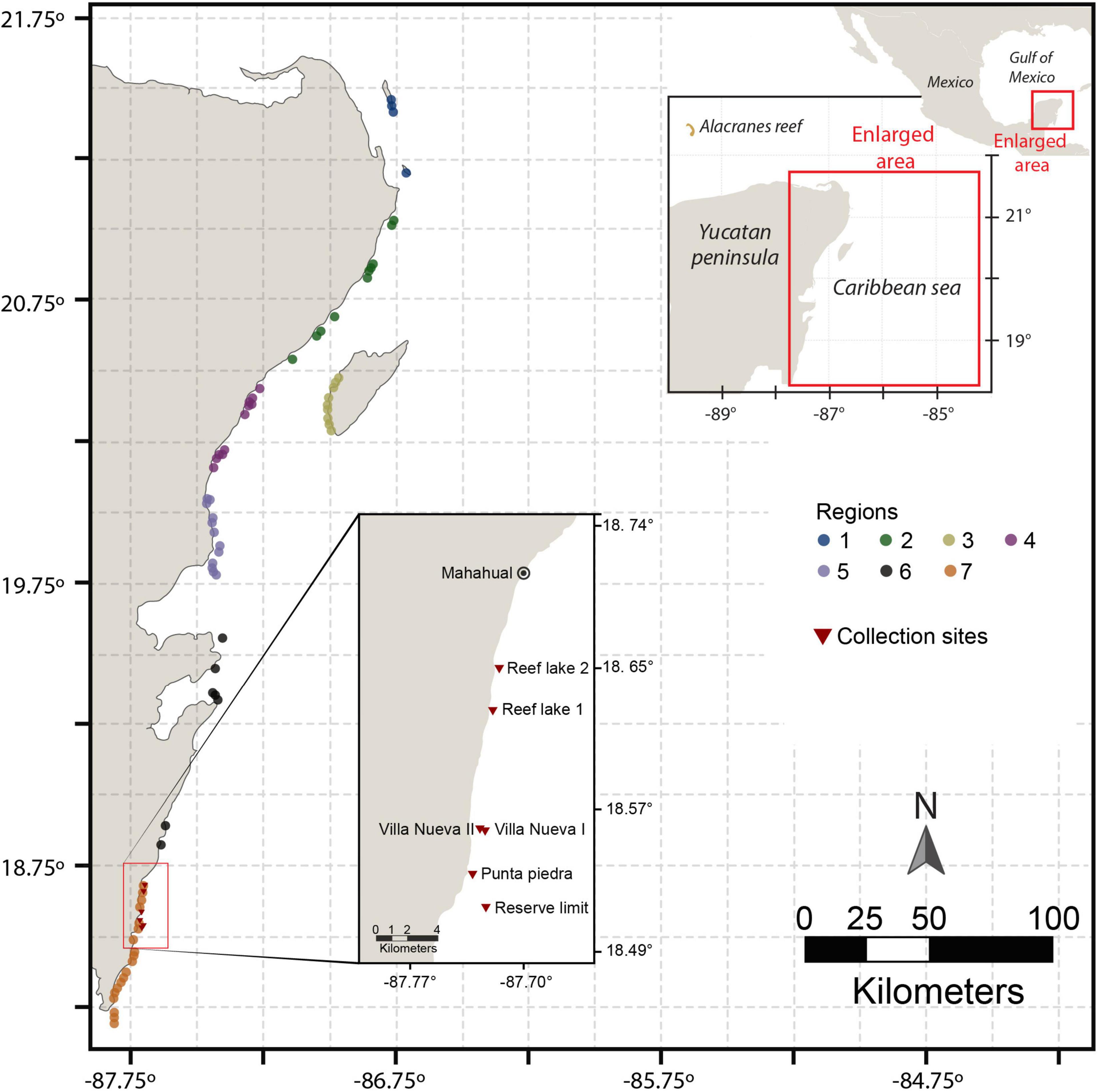
Figure 1. Location of sites off the Mexican Caribbean coast of Quintana Roo, where immersions to cense populations of Bartholomea annulata and Condylactis gigantea and their crustacean symbionts took place. The study area was subdivided in seven regions (for details see Campos-Salgado, 2009). Sites where experimental animals were collected are also shown.
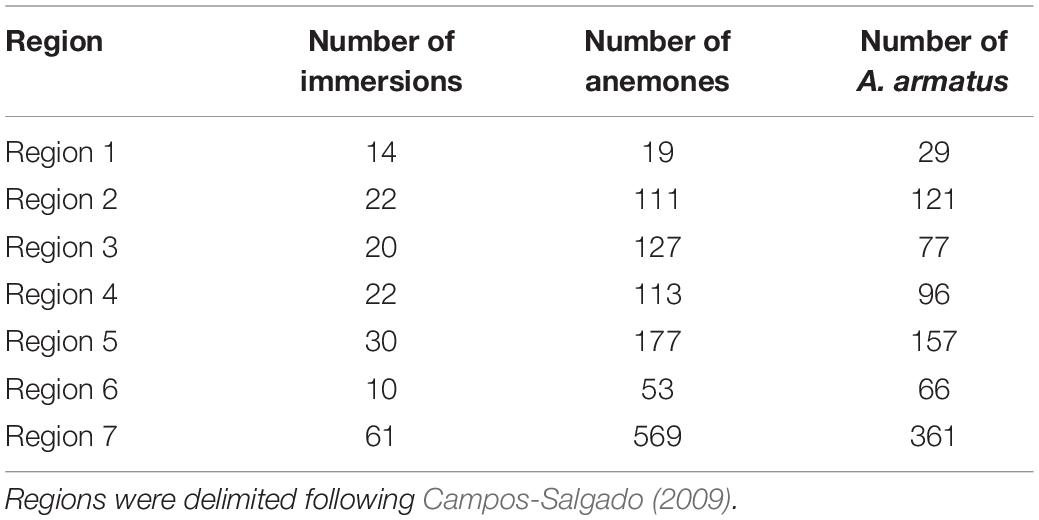
Table 1. The number of scuba-diving immersions performed in sites distributed throughout seven regions off the Mexican Caribbean coast of Quintana Roo and the total number of anemones B. annulata and associated A. armatus found in each region.
From this data base, we retrieved the number of B. annulata observed at each site and the position in which each individual anemone was found: i) in crevices, caves or small holes located on the top of lateral walls of coral fragments, limestone massif and rocks (Figure 2A); or ii) at the interface between hard substrate and the sandy bottom (Figure 2B). In addition, each anemone was classified as the host of none or at least one individual of the A. armatus species complex (Knowlton and Keller, 1983, 1985).
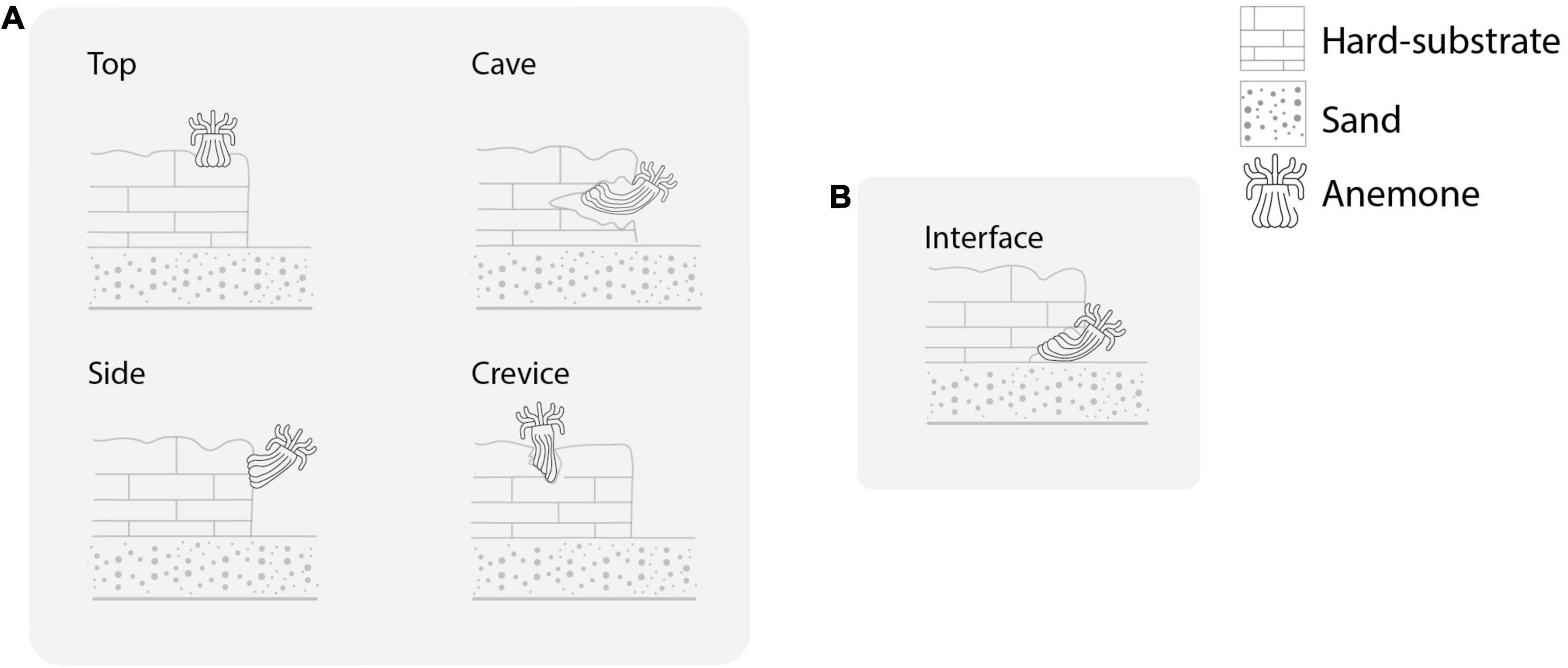
Figure 2. Diagrams showing the positions in which Bartholomea annulata was found during surveys off the Mexican Caribbean coast: in crevices, caves or small holes located (A) on the top or lateral walls of coral fragments, limestone massif and rocks; (B) at the interface between hard substrate and the sandy bottom.
To test the hypothesis that the proportion of anemones with alpheid shrimps of the total number of anemones was higher in microhabitats with a sandy interface than amongst those found elsewhere, a logistic regression with the logit link function and the binomial distribution was applied to the field data (Powers and Xie, 1999). The frequency of anemones was transformed to the odds of finding an anemone with at least one alpheid shrimp present (against those without a shrimp) in each habitat and region. The odds (O) are defined as the ratio of the probability of one outcome (p) to another (1 − p)
where p is the probability of finding an anemone with at least one alpheid shrimp. The underlying general lineal model (GLM) had “habitat” (with 2 levels: Interface and Elsewhere) and “region” (with 7 levels) as additive terms. To assess the effect of habitat and region on the response variable, a hypothesis testing procedure that compared the residual deviance of a full versus a reduced model by means of a χ2-test was applied for each term (Zuur et al., 2007). If the term “habitat” was significant, it would constitute evidence that the occurrence of shrimp-anemone associations differed between the two habitats more than expected by chance alone; and that these differences were irrespective of region. If the term “region” was significant, it would constitute evidence that the occurrence of shrimp-anemone associations, in either interface habitats or elsewhere, varied amongst regions.
A total of 27 sea anemones and 33 snapping shrimps of two cryptic species, Alpheus armatus and A. immaculatus, in the same study area where previous field work took place (Region 7; Figure 1). Because both species occupy a similar ecological niche (Knowlton and Keller, 1983), they were treated as a single taxonomic unit also known as A. armatus species complex throughout experiments despite the ability to identify each one. Anemones were carefully removed from the substrata with a hammer and chisel, avoiding any damage to the column or the pedal disc, while snapping shrimps were collected using a plastic slurp pump. Both anemones and shrimp were then transported to laboratory facilities at the Unidad Multidisciplinaria de Docencia e Investigación of the Universidad Nacional Autónoma de México at Sisal, Yucatan, where experimental trials took place.
Experimental animals were placed in a system consisting of Perspex aquaria (length 26.5 × width 20 × depth 20 cm; 10.6 L) with a continuous supply of recirculating sea water treated with mechanical (25, 10, and 5 μm), biological (bio-balls) and UV filtration. Aquaria were equipped with an artificial shelter to provide anemones with a hard substrate for attachment and shrimps a safe place to hide. These rigid shelters were made of a mixture of sand and cement and had an arched entrance leading to a halfpipe gallery, thereby mimicking natural dens (Figure 3). Anemones were left to attach their foot to the end furthest from the shelter’s entrance, resulting in the full extension of the column, oral disc, and the tentacles (Figure 3). Apart from the artificial shelter, no substrate was added to aquaria during maintenance.
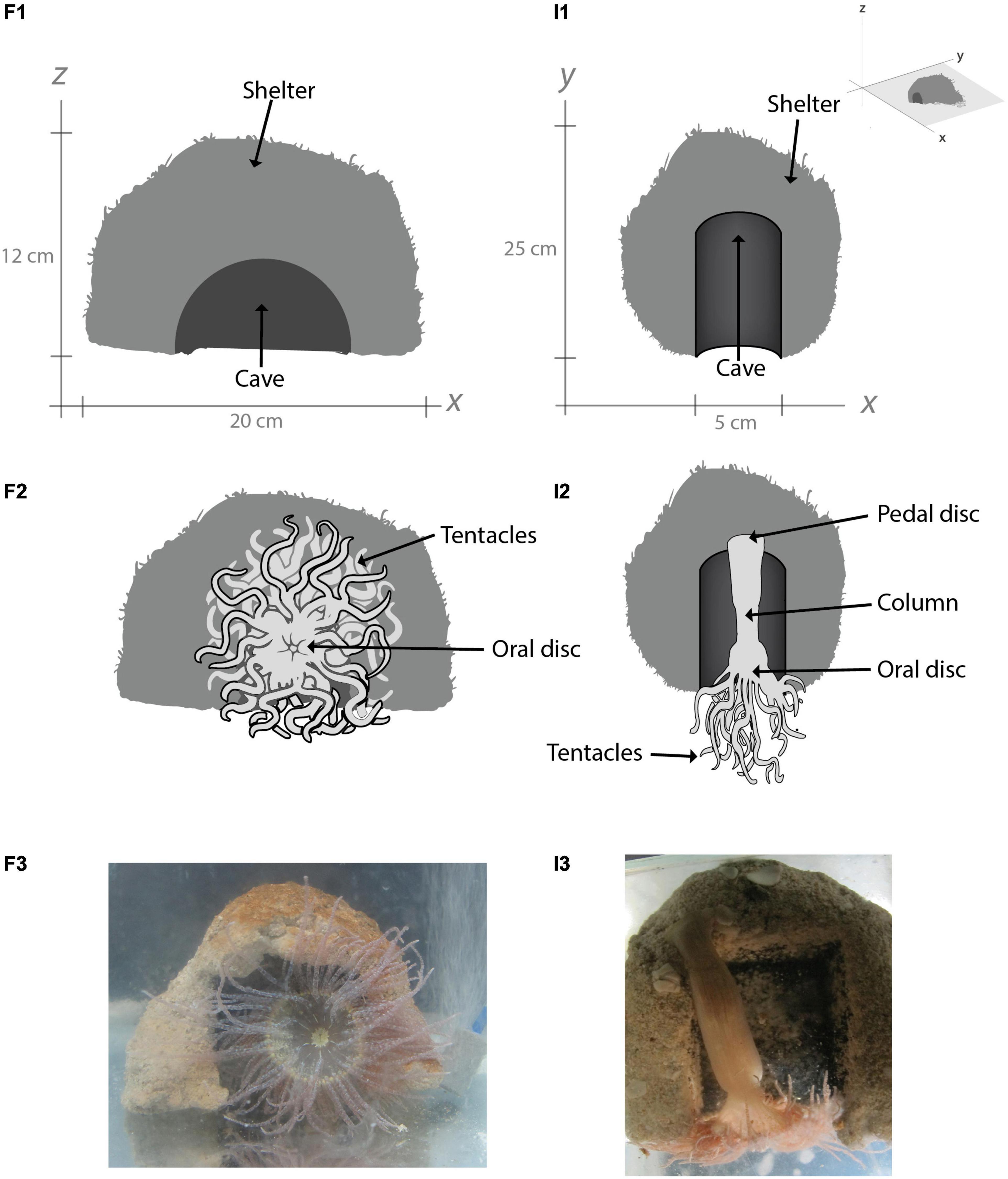
Figure 3. Diagrams of artificial shelters made of a mixture of sand and cement for anemones to attach and find refuge. View from the front with (F1) and without an anemone (F2); view from the interior gallery with (I1) and without an anemone (I2). Photographic registers of the frontal (F3) and interior (I3) views of the artificial shelter holding a Bartholomea annulata during maintenance.
Female and male shrimps of the A. armatus species complex were paired into 15 couples thereafter treated as experimental units. An attempt was made to keep naturally bonded pairs as they were originally found in the field, but new stable pairs were formed when this was not possible. Because male and female shrimp were not in equal numbers, three organisms were discarded from subsequent experimental trials. Stability in the newly formed couples was achieved by presenting pairs of individuals through controlled choice procedures, in which escalating agonistic interactions were interrupted and animals presented with an alternative partner until positive coexistence was achieved. Each couple was then placed with one sea anemone in an aquarium forming 15 distinct triads. The remaining anemones were kept in shelters without snapping shrimps. All experiments were conducted with couples of shrimps in order to keep conditions as similar as possible to those occurring naturally in reefs.
During maintenance (2–3 weeks), anemones were fed three times a day with 25 g of defrosted brine shrimp uniformly distributed amongst holding aquaria. Alpheids were fed twice a day a mixture of 5 g of mussel gonad and 20 g of squid cubes (9:00 a.m.) and 25 g of squid cubes. Uneaten food was extracted daily by syphon and water levels re-established to keep sea water conditions constant. Water in the aquaria was kept at 26 ± 0.5°C (mean ± standard deviation), salinity 37 ± 1 ups and gentle aeration. A 12:12 light:darkness period was kept throughout both maintenance and experimental trials.
Experiments took place in an isolated room where noise and other sources of interference were kept to a minimum. Four glass aquaria (length 23 × width 23 × depth 23 cm; 12 L) were filled with sea water from the maintenance system and fully exchanged at the end of each trial. Two of the aquaria were covered with a 5 cm layer of sand collected from Sisal, Yucatan, Mexico, and sieved through a 0.95 mm mesh to standardise particle size (<1 mm). The other two aquaria were kept free of sand throughout experiments.
A video recording system was implemented inside the room to register the behaviours of both anemones and snapping shrimp displayed throughout experiments. This system consisted of a closed-circuit television (CCTV) and a hand-held video camera (Canon Vixia Hf R11 Full HD). The CCTV was used to record activity continuously, and the handheld video camera was used to capture high-quality videos of specific behaviours of alpheid shrimp.
To investigate the effect of the den cleaning behaviour of snapping shrimp on the retraction response of B. annulata, a manipulation experiment was designed using three different treatments: (i) anemone in a shelter with sand and a pair of A. armatus shrimps (BSA), (ii) anemone in a shelter with sand but without A. armatus shrimp (BS0), and (iii) anemone alone in its shelter, i.e., without shrimp or sand (B00). The 27 anemones, together with their artificial shelters, were transported four at a time to the experimental room and placed individually in an aquarium with or without sand depending on the treatment they would be receiving (BSA: n = 9; BS0: n = 8; B00: n = 10). Shelters in the BSA and BS0 treatments were manually buried with the sand available in the aquarium, partially covering the anemone. A 24 h period was allowed for anemones to habituate to the aquaria and for shrimp to remove the sand from the shelters before initiating trials. To simulate an aggression and trigger the anemone’s defence behaviour of retracting the body into the shelter, a quick jet of seawater was then fired at the anemones’ oral disk using a 3–5 ml hand pipette. The defence response was subsequently video recorded during 30 s by means of the hand-held video camera. In each case, the success or failure of the anemones’ full contraction was noted. Only results for the first contraction response were considered.
A defence response was considered successful (p) when the anemone was able to fully retract both the column and the oral disc into the shelter, whereas a defence failure (q) was considered when the anemone could not retract the column or the oral disc either partially or totally within the shelter. This defence behaviour has been reported as the primary and immediate strategy used by B. annulata to avoid predation (Harris and Howe, 1979; González-Muñoz et al., 2013). Focus was directed to the protection of the oral disc and column as they constitute vital structures to the anemone and its exposure entails a high risk to survival (Ottaway, 1977; Harris and Howe, 1979). Finally, to compare the probability of successful defence responses between treatments, a χ2-test was performed on a 3 × 2 contingency table (Zar, 2010). A higher probability of successful retractions by the anemone in treatments with the presence of alpheids would be considered evidence in support of our hypothesis.
Once the manipulation experiment was terminated, the video recording of 12 triads of animals was performed to describe the cleaning behaviour. Shrimps were removed from triads in BSA treatment 24 h prior to the beginning of observations. Shelters were then manually buried with the sand available in the aquaria to assure all anemones were similarly covered when shrimp were introduced. Two additional A. armatus. couples were placed in aquaria that contained an artificial shelter and sand, but no anemone. Activity was recorded during the first 12 h of light immediately after shrimp pairs had been introduced into the aquaria.
Preliminary observations allowed to clearly identify three stages of A. armatus complex behaviour that took place chronologically and were operationally defined with the following criteria:
(1) Habituation: From the moment A. armatus complex shrimps were introduced in the aquaria to the moment both individuals ceased movement and remained still on the bottom.
(2) Searching: From the moment habituation ended to the moment both shrimp had recognised the anemone. Anemone/shelter recognition normally occurred by chemical stimuli and was characterised by shrimp moving directly toward the anemone, making physical contact with the pedal disc, column or tentacles and remaining in close contact with the host.
(3) Cleaning and maintenance: From the moment both A. armatus complex recognised the anemone/shelter to the end of the recordings. Four distinct behaviours states were displayed by shrimp during this stage and are categorised according to Palomar et al. (2005):
(a) Sweeping: vigorous fanning of pleopods resulting in loose and suspended sand being pushed from under the abdomen toward the rear (Figure 4A).
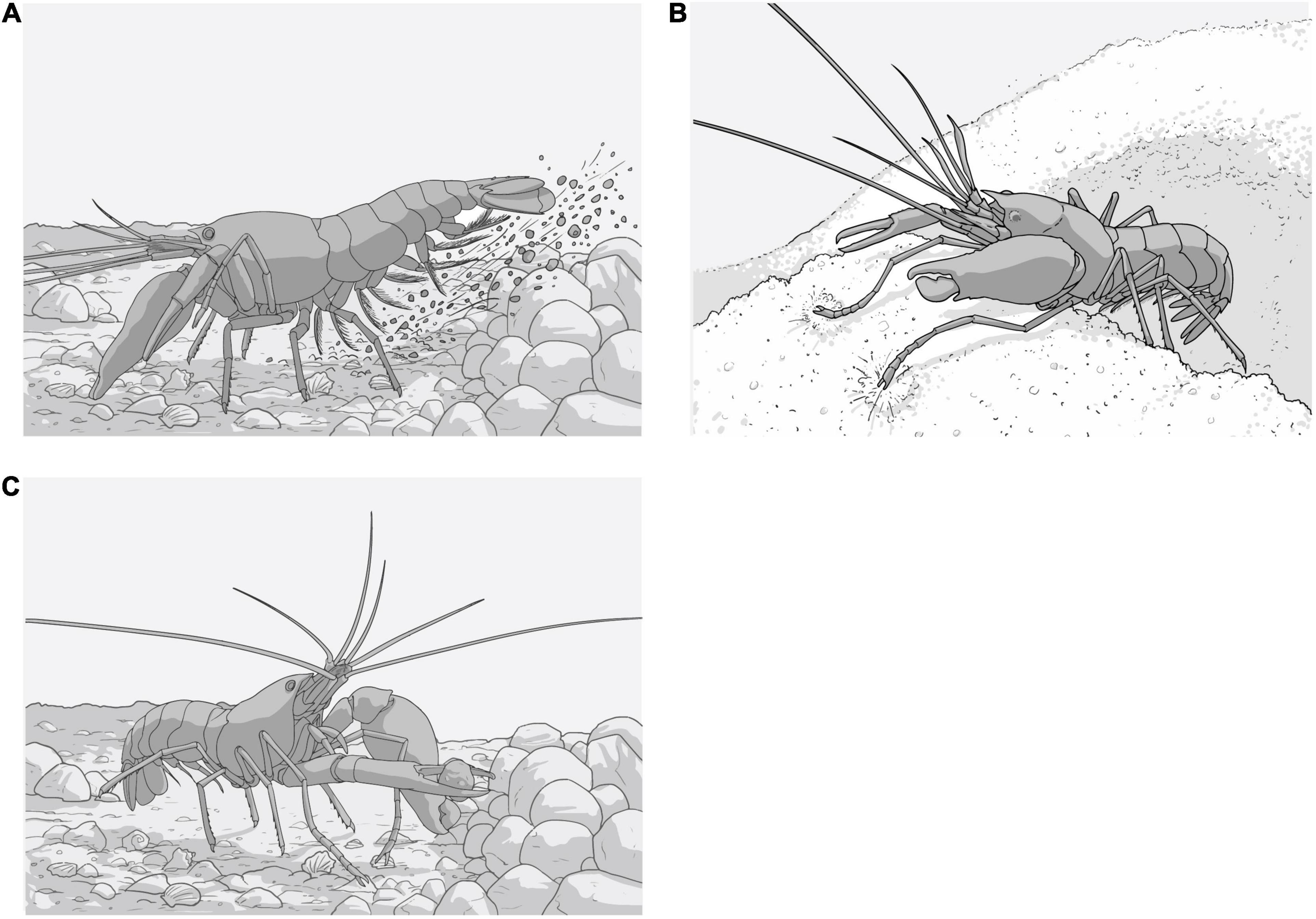
Figure 4. Diagrams representing the body posture, movement and appendices used by Alpheus armatus complex when displaying three den cleaning behaviours during observations in experimental conditions. (A) Sweeping: shrimp used their pleopods remove the sand from under the abdomen toward the rear. (B) Tamping: shrimps tamped the surrounding sediment down firmly using the second pair of chelated pereiopods. (C) Dumping: shrimp used the first minor chela to lift larger sediment particles and move them away from the shelter.
(b) Tamping: shrimps tamped the surrounding sediment down firmly using the second pair of chelated pereiopods (Figure 4B).
(c) Dumping: shrimps used the first minor chela to lift larger sediment particles and move them away from the shelter (Figure 4C).
(d) Resting: shrimps displayed no activity, entered the shelter or wandered shortly away in strolls that never lasted more than 15 s.
Video recordings were analysed to measure the duration of each stage and the latency of anemone/shelter recognition by A. armatus (i.e., the time elapsed from the moment shrimp were introduced into the aquaria to the moment both shrimp had recognised the anemone/shelter). Once anemone recognition had occurred, the number of times that at least one shrimp was observed sweeping, dumping, tamping or resting were registered during 10 min every 0.5 h. This produced the frequency distribution of all four behaviours in 24 consecutive moments throughout the observation period. Central tendency and dispersion parameters (mean ± standard deviation) were calculated for measures of duration and latency to add quantitative descriptors to the qualitative characterisation of the shrimps’ cleaning behaviour.
The distribution of both anemones Bartholomea annulata and individuals of the Alpheus armatus species complex in the seven regions of the Mexican Caribbean is shown in Table 1. Overall, slightly more than half of the total number of individuals of this species (627 of 1169; 53.6%) hosted at least one shrimp, whereas 542 (46.4%) had no alpheid shrimps associated. There were 634 (54.2%) anemones observed inhabiting the interface between the sandy bottom and hard structures, such as coral fragments, limestone massif and rocks, whilst 535 (45.8%) were found in crevices and fractures on the horizontal or lateral surface of hard substrates, but distant from the sand.
Results of the hypothesis testing procedure showed that both the “habitat” and “region” terms significantly contributed to explain the total deviance estimated by the GLM. The odds of finding a B. annulata with an alpheid shrimp as a symbiont differed significantly depending on the microhabitat in which anemones occurred (χ2 = 5.25; p < 0.05), and were 30% higher in interface microhabitats than elsewhere irrespective of the region. The odds of finding a B. annulata with an alpheid shrimp in any type of microhabitat also varied significantly amongst regions (χ2 = 115.95; p < 0.001), the highest being in region 6, followed by regions 1 and 2, 4 and 5; regions 3 and 7 has the lowest odds. Despite the variability throughout sampling regions (Figure 5A), the data revealed a pattern that was remarkably consistent: anemones with alpheid shrimps were more frequent when anemones were in close contact with sandy bottoms than when inhabiting crevices on the top or lateral walls of hard substrates (Figure 5B). The statistical analysis showed that these differences were larger than those expected by chance alone.
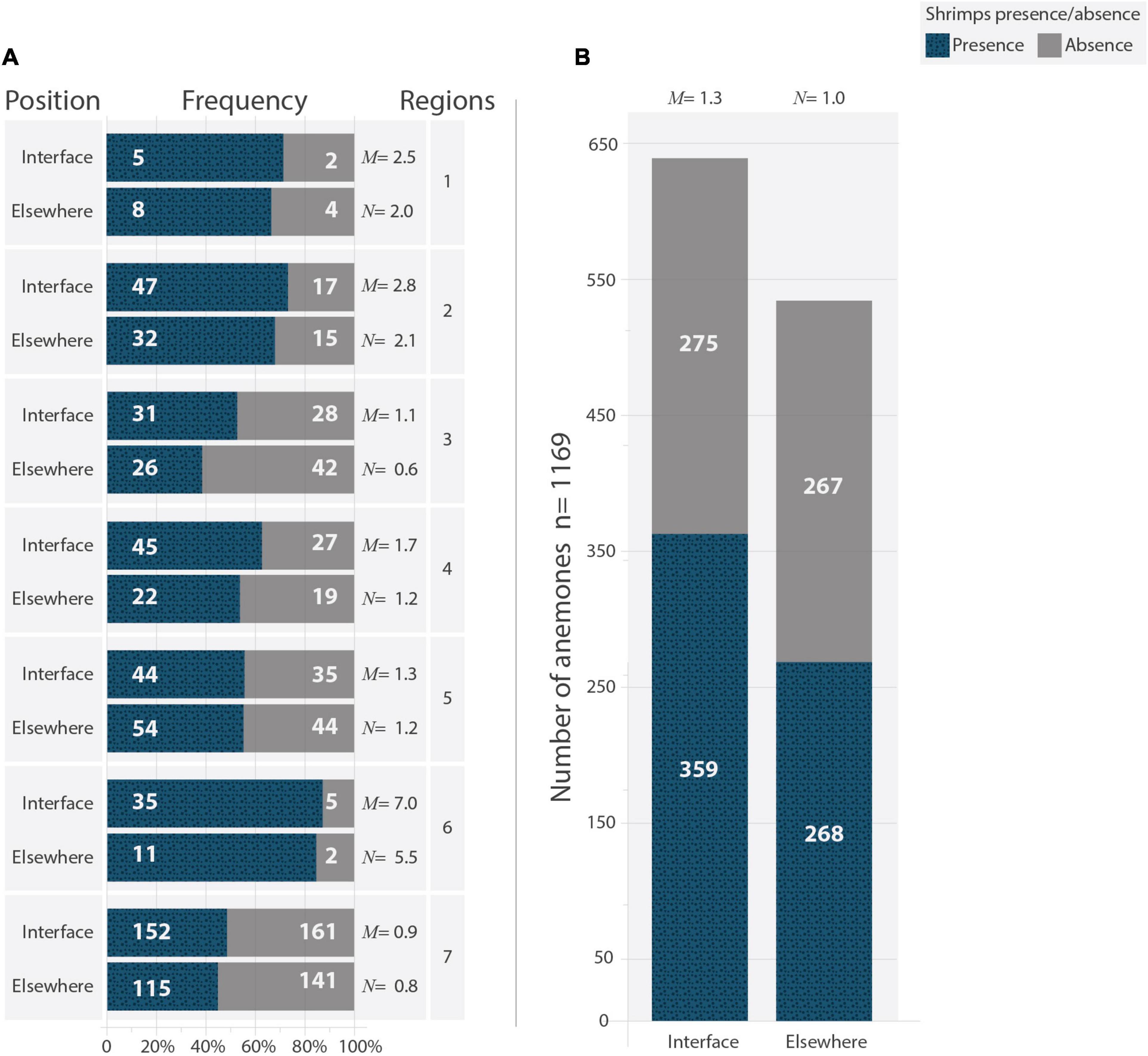
Figure 5. Occurrence of Bartholomea annulata in the interface between hard substrates and the sandy bottom (Interface) or other microhabitats (Elsewhere), and were associated with at least one (blue) or without (gray) A. armatus complex. (A) Relative frequency in seven regions off the Mexican Caribbean coast of Quintana Roo. (B) Absolute frequency of pooled data of all seven regions. The odds of finding a B. annulata with at least one Alpheus shrimp in the interface (M) and elsewhere (N) are given for each region and for the pooled data. The numbers of anemones falling in each of the four categories is also given.
Results of the experiment showed that the absence of snapping shrimps reduced the ability of Bartholomea annulata to retract successfully when sediment was obstructing the entry and/or free passage inside the shelter. When both alpheid shrimps and sand where absent from the aquaria (B00), all 10 anemones retracted fully within the shelter after receiving the jet of sea water (Supplementary Material 1; video recording). By contrast, in treatments where sand obstructed the free movement within the shelter, the success of retraction from stimuli was strongly associated to the presence of alpheid shrimps: 8 (88.8%) successful retractions were observed in treatment with alpheid shrimp (BSA), but only 2 (25%) were observed in those where shrimps were absent (BS0; Supplementary Materials 2, 3, respectively).
The frequency of successful retractions relative to those that failed varied significantly between treatments (χ2 = 15.34; p < 0.01; Figure 6). Anemones confronted with shelters that were obstructed with sediment had only a 25% probability of fully retracting when Alpheus armatus complex were absent, compared to a 75% probability when snapping shrimps kept the shelter’s entry and passage free of sediment.
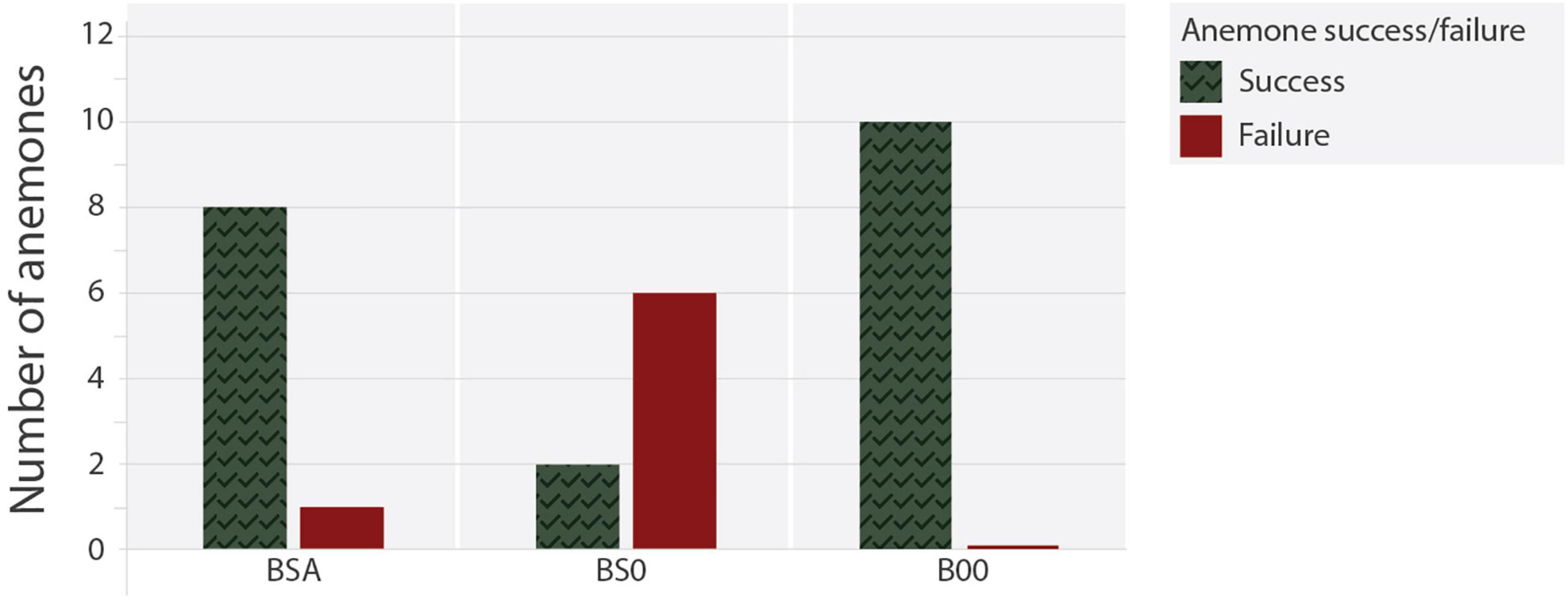
Figure 6. Number of anemones Bartholomea annulata that displayed successful or failed retraction response to a simulated aggression in each of three experimental treatments: (i) anemone in a shelter with sand and two snapping shrimps (BSA); (ii) anemone in a shelter with sand but without shrimp (BS0) and (iii) anemone alone in its shelter, i.e., without shrimp or sand (B00).
As soon as they were placed in the observation aquaria, the Aalpheus armatus complex displayed frantic swimming in all directions in the water column and/or walked swiftly on the bottom sediment. This hectic behaviour often resulted in A. armatus colliding with the aquarium walls, with one another, or with the anemone or its shelter. Collisions with the anemone, however, did not appear to trigger host recognition immediately, and the shrimps kept moving until they eventually came to a complete standstill. The habituation stage had a mean duration of 3.9 ± 8.7 min (mean ± standard deviation; Figure 7A), but both shrimp in triads had completely ceased motion in the first 2.1 min since observation trials began. The sole exception was the case in which shrimps ceased motion after 30.9 min had passed.
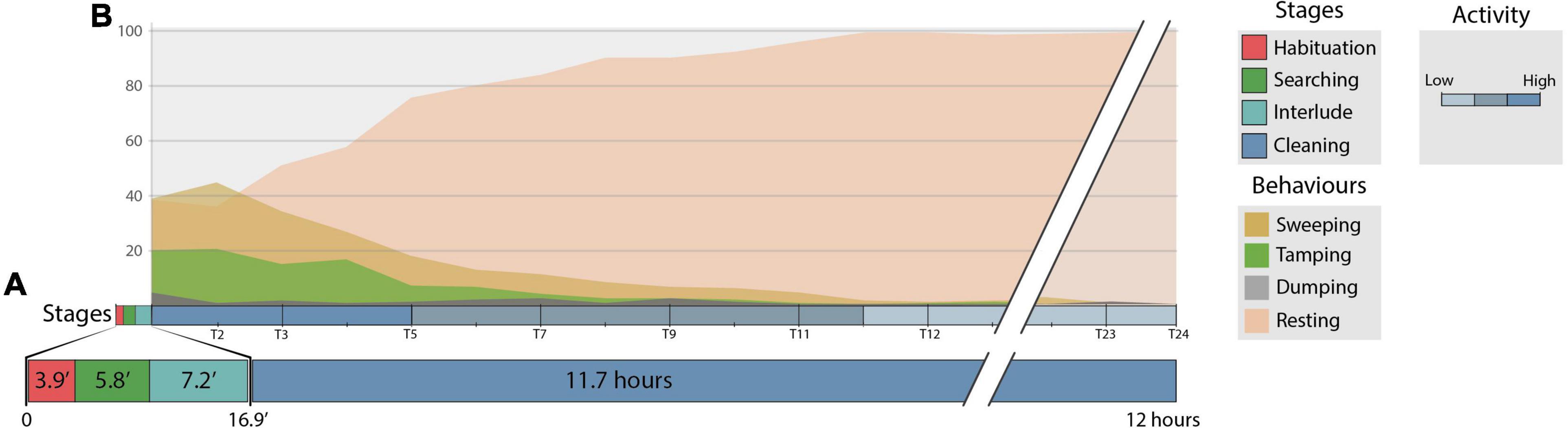
Figure 7. (A) Mean duration (minutes) of the habituation, searching, interlude and cleaning stages of den cleaning behaviour displayed by snapping shrimps during observation experiments under controlled laboratory conditions. (B) Relative frequency of four behaviours (sweeping, tamping, dumping and resting) exhibited by snapping shrimps during the cleaning stage. T1–24 represent the 30-min intervals throughout the 12-h observation period. Shades of blue indicate high, medium and low cleaning activity as the relative frequency of resting increased. Calculations were made for n = 12 independent replicates.
Searching shrimps explored the surroundings by probing with the antennae, antennule and chelate pleopods as they moved in progressively widening stretches throughout the bottom of the aquaria. Movement was characterised as short, forward and backwards jabs as shrimp slowly advanced in their trajectories. Alpheus armatus often encountered each other during searching and would touch using the antennae and antennule. When this occurred, physical interactions lasted no more than 1.2 min and searching would immediately be resumed. The searching stage had a mean duration of 5.8 ± 4.6 min and concluded when at least one shrimp in each pair had recognised the presence of the anemone (Figure 7A). After 17 ± 8.2 min, 27 shrimps had found protection with the anemone, and only 3 individuals buried in the sand near an aquarium corner. All snapping shrimps were near the anemone and its shelter when 38.7 min had elapsed.
Den cleaning occurred 7.2 ± 10.5 min after anemone recognition and commenced by snapping shrimps clearing of the internal gallery as they made their way toward the shelter’s entrance. This period was identified as an interlude and represented the latency of the first cleaning behaviours (Figure 7A). Shrimp mainly used “sweeping” to move most of the sediment and unearth the entrance to the den (Figure 4A). By removing the sediment in this manner, shrimp created a semi-circular depression in front of the shelter’s entrance that was surrounded by a slowly increasing mound of accumulated material. Second most frequently, they used “tamping” (Figure 4C), where they crawled toward the top of the mound and compacted the sediment with the second pair of chelated pereiopods. Shrimp occasionally used “dumping” to expel the larger particles away from the den’s entrance (Figure 4B).
The frequency in which den cleaning behaviours were alternated with “resting” changed throughout the observation period (Figure 7B). Shrimp were most active during the first 2 h, when the frequency of “sweeping”, “tamping” and “dumping” was 35.6, 17.6 and 1.5 times every 10 min. The external appearance of the shelter was transformed markedly during this period (Figure 8, 2 hours), but was thereafter almost unchanged (Figure 8, 5.5–12 hours) and (Supplementary Material 4). Correspondingly, the three main cleaning behaviours became more sporadic as shrimp advanced in the clearance of the shelter’s gallery and entrance, and the anemone was free to expand its column and tentacles without obstruction. After 5.5 h an increase in the frequency of “resting” to 98.4 times every 10 min was observed (Figure 8, 12 hours). Thereafter, the frequency of “sweeping” and “tamping” decreased to 1 and 0.3 every 10 min, respectively. Despite its rareness, shrimps never stopped cleaning the den and intermittently displayed these behaviours throughout the 12-h observation period (Figure 7B). No differences in the frequency, duration or latency of behaviours were observed amongst naturally bonded pairs and those formed at their arrival at the laboratory.
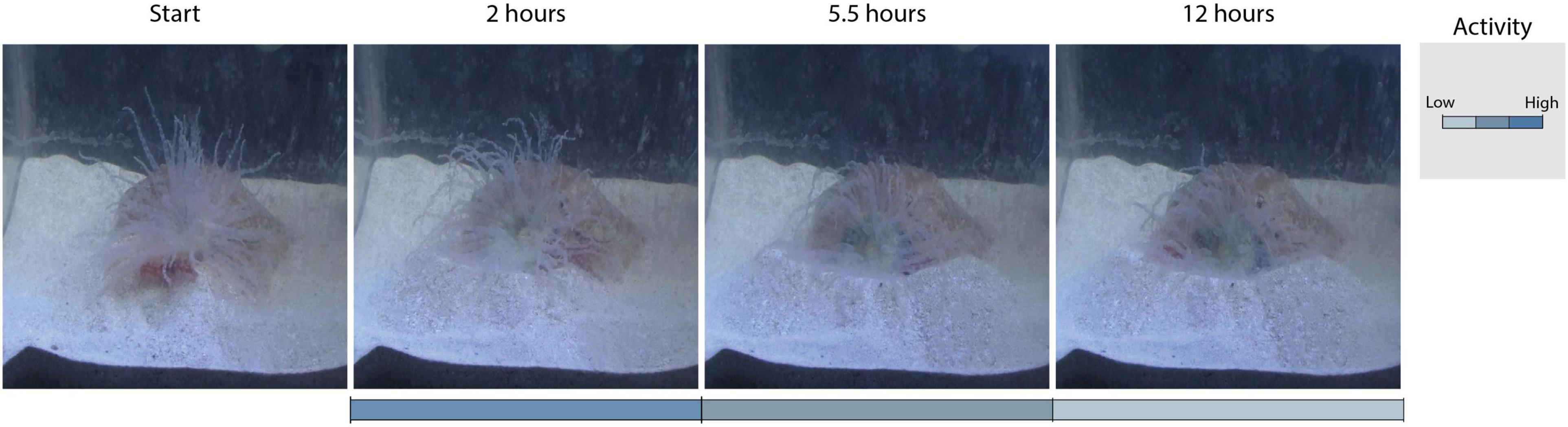
Figure 8. Photographic registers showing the external appearance of anemones B. annulata and their artificial shelters at different moments throughout observation experiments: Start (before shrimp were introduced), after 2, 5.5, and 12 h of den cleaning by Alpheus spp. Shades of blue indicate high, medium and low cleaning activity as the relative frequency of resting increased and sweeping, tamping and dumping increased. Note that differences are only clearly visible within the first 2 h of observation. Thereafter, the external appearance of the entrance remained almost unchanged (Supplementary Material 4).
Observations of snapping shrimp in the absence of an anemone showed a marked similarity in the body position and movement of shrimp during the habituation and searching stages. The habituation stage had less duration compared with previous observations (1 ± 0.1 min). The searching stage ended after 29.7 ± 40.8 min with shrimp full covered with sediment away from the artificial shelter, where they remained until the end of the observation period. It is important to note that despite that shrimps frequently encountered the empty shelters (25 times maximum), they never displayed any of the cleaning behaviours described previously.
The frequencies in which Bartholomea annulata is found in optimal and sub-optimal microhabitats within the reef is determined by their availability, as well as by the set of advantages and risks brought to the anemone from inhabiting such places. Whilst B. annulata does not need shrimp to survive, A. armatus complex is highly territorial and occurs only in association with this anemone species (Smith, 1977; Knowlton and Keller, 1983, 1985; McCammon and Brooks, 2014). This asymmetric interaction implies that the occurrence of B. annulata will depend on the availability of microhabitats with a varying degree of quality for anemone growth and survival, whereas shrimp occurrence will be primarily driven by the abundance of unoccupied anemones. The field data analysed herein showed variations in the abundance of both anemones and snapping shrimps amongst the seven regions in the Mexican Caribbean (Table 1), indicating a varying availability of optimal and suboptimal microhabitats for B. annulata throughout the study area. Previous studies on the distribution of B. annulata in the Caribbean have explained variations in the abundance within geographic areas in terms of the, exposure to wave dynamics, microhabitat accessibility, photosynthetic efficiency and food availability (Cornell and Lawton, 1992; Amarasekare, 2002; Begon et al., 2009; Briones-Fourzán et al., 2012; Chesson, 2020).
Despite this environmental heterogeneity, the frequency of B. annulata hosting at least one alpheid shrimp was consistently higher when inhabiting interface microhabitats then in those distant from the sandy bottom (Figure 5), and these differences were statistically distinguishable from random noise. Caves and crevices located in the interface between hard coral or rock massifs and the soft sandy may constitute ideal places for anemones to attach because they can take advantage of both microhabitats. The hard substrate protects the anemone from ocean energy and natural predators (Knowlton and Keller, 1986; Barrios-Suárez et al., 2002), whilst the soft sediment is rich in a variety of food sources made easily available through nutrient re-suspension (Chapman and Tolhurst, 2004, 2007). Such sources of food for suspension feeders have a central role in oligotrophic environments (Hobson and Lorenzen, 1972; Aguirre and Salmerón, 2015) increasing the value of microhabitats where these sources are plentiful. In the present study, the odds of finding B. annulata in interface microhabitats compared to elsewhere varied from 0.58 to 3.08, suggesting anemones do not actively select the former. Whilst differences in anemone survival amongst different microhabitats were not examined the present study, if demonstrated such differences could offer a plausible explanation of the observed field patterns, particularly if survival was positively associated to the presence of shrimp.
The pattern revealed by the field data could be explained if the benefit of hosting a snapping shrimp varies amongst microhabitats and the interaction with A. armatus provides B. annulata an advantage in occupying interface microhabitats more frequently than others within the same reef. A mechanism explaining this advantage is provided by the results of the manipulation and observation experiments herein, in which full retraction by anemones into the den was successful only when snapping shrimps kept it clean. If den cleaning by alpheid shrimps is related to the effectiveness of the anemones’ defence mechanism from predation, then this symbiotic interaction will prevail in those microhabitats where the benefits of such behaviour confer some ecological value; i.e., places where sediment may obstruct the den and impede the anemone’s full retraction, increasing the risk of predation. To choose habitats that are less preferred by other species, reduces interspecific competition and promotes positive interactions such as facilitation and mutualism (Bertness and Leonard, 1997; Chong et al., 2000; Levin et al., 2001; Halpern et al., 2007). Whilst the quality of the interface microhabitat by itself may not differ compared to others, it is the symbiotic interaction that improves the ability of anemones to withdraw before danger.
Proximity to the sediment can pose certain dangers to sessile animals or those with low mobility, since it increases the possibility of scoured tissue from abrasive erosion or accidentally being buried by sedimentation (Chapman and Tolhurst, 2007; Irving and Witman, 2009; Stewart et al., 2013). The Caribbean Sea has high sediment dynamics created by seasonal winds and waves, prevalent marine currents and occasional but recurrent pulses of high activity due to hurricanes and tropical storms (Vázquez-Lule and Díaz-Gallegos, 2009; Miloslavich et al., 2010). These hydrometeorological conditions result in a high and permanent risk of reef structures to be buried beneath the sand and animal shelters obstructed. The high occurrence of B. annulata in crevices located in the interface between hard structures and sand could be related to the symbiotic relation between this anemone and A. armatus complex. To protect themselves from predators, anemones need an obstacle-free, cavernous den into which they can retract the column and pedal disc whilst fully extending the tentacles toward the source of danger. When threatened, symbiotic A. armatus complex will benefit from the defensive display of anemones by hiding near the column or pedal disc behind the stinging nematocysts (the preferred zone anemone for the Alpheus armatus complex; Huebner et al., 2019). Thus, by keeping the sediment away, snapping shrimps provide the necessary conditions within the den that serve both the anemone’s and the shrimp’s higher chances of survival.
In the B. annulata—A. armatus complex. interaction, the anemone benefits from the snapping shrimp defence against benthic predators (Smith, 1977). This shrimp defence behaviour consists of a water jet capable of seriously harming the targeted animal (Lohse et al., 2001; McCammon and Brooks, 2014). Nonetheless, if the alpheid’s first defence line breaks down, the last resource for the anemone is to retract its hydraulic body into the shelter, block the den’s entrance, and facilitate a quick snapping response from the shrimp to repel the attack from the predator. Our results of the manipulative experiment show clear evidence that the anemone’s full retraction into the den is only possible when its interior is free of sediment (Figure 7). Moreover, only by maintaining the entrance to the den clear from occlusion, do shrimps enable the full expansion of the anemone’s tentacles toward exterior perils. This defence position enhances the protection offered by the battery of nematocysts, whilst the shrimp seeks refuge close to the column and pedal disc.
Video recordings of the den cleaning behaviour displayed by A. armatus complex in the present study showed it constitutes a quick and effective mechanism to assure the anemone’s full retraction. Despite being completely covered with sand, A. armatus complex were capable of finding the anemone and liberating the entrance of the artificial shelters in less than 2.5 h. In addition, the sweeping, tamping and dumping behaviours were displayed systematically by repeating them in a consistent order, duration and frequency amongst all experimental replicates. These features together with the velocity of this response suggest that den cleaning behaviour is common and effective under natural conditions and provides a true selective advantage for both the anemones and the shrimps. The mutual beneficial nature of this behaviour resides in that by keeping the den clean either through sweeping or otherwise moving sediment away from the entrance, A. armatus complex increases its chances of taking refuge when the anemone retracts. This idea is further supported by the fact that in in the two replicate trials where anemones were absent, shrimp did not delay to bury in the sediment.
After 5.5 h of the initial cleaning, shrimp intermittently removed particles and compacted the sediment surrounding the den’s entrance throughout the duration of observations. It is interesting to note that shrimps continued to provide maintenance to their hosts’ refuge in such a persistent manner, suggesting it constitutes a recurrent feature of their daily activity. Whilst snapping in A. armatus complex is a more conspicuous defence behaviour (Smith, 1977; McCammon and Brooks, 2014), den cleaning is more consistent and ubiquitous in the shrimp’s behavioural repertoire. Furthermore, results herein demonstrate that anemones without shrimps will not be able to use full retraction as a last resource defence mechanism.
Overall, these findings describe a new, undervalued alpheid shrimp behaviour that contributes to cleaning the shelter’s entry and internal passage, and experimentally demonstrate its potential relation to enhanced predator avoidance, enabling the anemone to fully retract into its den. Such behaviour may constitute an important element of trade in the symbiotic interaction between A. armatus complex and its host anemone.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
AP-B, MM, and NS contributed to conception and design of the study. AP-B and NS did field work observations. AP-B conducted the experiments, organised the database, produced the videos and figures, and wrote the first draft of the manuscript. MM and AP-B performed the statistical analysis and wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Project CONACyTCB-2012-01-177293 provided financial support assigned to NS. This Project and AP-B was supported by the Unidad Multidisciplinaria de Docencia e Investigación—Sisal, Facultad de Ciencias, Universidad Nacional Autónoma de México (UMDI Sisal-FC-UNAM), and by CONACyT doctoral fellowship 2019-000037-02NACF through the Posgrado en Ciencias de la Biológicas, Facultad de Ciencias, Universidad Nacional Autónoma de México (PCB-FC-UNAM).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to M.P. Guadarrama for dry laboratory coordination; P.M. Balam, and I.G. Palomino-Albarrán for live food supply; G. Martínez for wet laboratory coordination and life-support systems; A.I. Campos-Salgado, for the extraordinary fieldwork, visual census, and data collection for the anemones distribution; N. Soledad-Calvo and Y. Dávila, for their help in animal collection, maintenance, and exchanging interesting and original ideas; to R. González-Muñoz, for his input in generating ideas and his constructive criticism on the project; and to D. Ortigosa for her comments on an earlier version of the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.677024/full#supplementary-material
Supplementary Material 1 | Video recordings examples of the behaviours observed on each of the treatments depicted in Figure 6. BOO, Bartholomea annulata, no Alpheus spp., no sediment/sand.
Supplementary Material 2 | Video recordings examples of the behaviours observed on each of the treatments depicted in Figure 6. BSO, Bartholomea annulata, sediment/sand, no shrimp.
Supplementary Material 3 | Video recordings examples of the behaviours observed on each of the treatments depicted in Figure 6. AOS, Alpheus spp., no anemone Bartholomea annulata, sediment/sand.
Supplementary Material 4 | Time-lapse of the den cleaning behaviour by Alpheus spp. showing the external appearance of artificial shelter of Bartholomea annulata.
Supplementary Material 5 | Photograph of the anemone and shrimp in the field for communication purposes.
Aguirre, G. R., and Salmerón, G. O. (2015). Characterization of the western Caribbean Sea waters through in vivo chlorophyll fluorescence Caracterización de las aguas del Mar Caribe occidental mediante clorofila por fluorescencia in vivo. Rev. Mar. Cost. 7, 9–26. doi: 10.15359/revmar.7.1
Alvarez-Filip, L., Carricart-Ganivet, J. P., Horta-Puga, G., and Iglesias-Prieto, R. (2013). Shifts in coral-assemblage composition do not ensure persistence of reef functionality. Sci. Rep. 3:3486. doi: 10.1038/srep03486
Amarasekare, P. (2002). Interference competition and species coexistence. Proc. Biol. Sci. 269, 2541–2550. doi: 10.1098/rspb.2002.2181
Anker, A. (2001). Two new species of snapping shrimps from the Indo-Pacific, with remarks on colour patterns and sibling species in alpheidae (Crustacea: Caridea). Raffles Bull. Zool. 49, 57–72.
Anker, A., Ahyong, S. T., Noël, P. Y., and Palmer, A. R. (2006). Morphological phylogeny of alpheid shrimps: parallel preadaptation and the origin of a key morphological innovation, the snapping claw. Evolution 60:2507. doi: 10.1554/05-486.1
Baeza, J. A. (2015). “Crustaceans as symbionts: an overview of their diversity, host use and life styles,” in Lifestyles and Feeding Biology: The Natural History of the Crustacea, eds M. Thiel and W. Les (New York, NY: Oxford University Press), 163–189.
Barrios-Suárez, L., Reyes, J., Navas, G., and García, C. (2002). Distribution of anemones (Anthozoa: Actiniaria and Corallimorpharia) in the area of Santa Marta, Colombian Caribbean. Ciencias Mar. 28, 37–48. doi: 10.7773/cm.v28i1.207
Bauer, R. T. (2004). Remarkable Shrimps: Adaptations and Natural History of the Carideans (Animal Natural History Series). Oklahoma: Oklahoma University Press.
Begon, M., Mortimer, M., and Thompson, D. J. (2009). Population Ecology: A Unified Study of Animals and Plants, Third Edn. Oxford: Blackwell Science Ltd. doi: 10.1002/9781444313765
Bertness, M. D., and Leonard, G. H. (1997). The role of positive interactions in communities: lessons from intertidal habitats. Ecology 78:1976. doi: 10.2307/2265938
Briones-Fourzán, P., Pérez-Ortiz, M., Negrete-Soto, F., Barradas-Ortiz, C., and Lozano-Álvarez, E. (2012). Ecological traits of Caribbean sea anemones and symbiotic crustaceans. Mar. Ecol. Prog. Ser. 470, 55–68. doi: 10.3354/meps10030
Campos-Salgado, A. I. (2009). Distribución Geográfica y Abundancia de las Anémonas (Cnidaria: Anthozoa) Condylactis gigantea, Bartholomea annulata y sus Camarones Simbiontes Periclimenes pedersoni, P. yucatanicus, Decapoda: Caridea) en la costa del Caribe mexicano. Ph. D. thesis, Mexico: Universidad Nacional Autonma de México.
Cestele, S. (2000). Molecular mechanism of neurotoxin action on voltagegated sodium channels. Biochimie 82, 883–892. doi: 10.1016/s0300-9084(00)01174-3
Chapman, M. G., and Tolhurst, T. J. (2004). The relationship between invertebrate assemblages and bio-dependant properties of sediment in urbanized temperate mangrove forests. J. Exp. Mar. Bio. Ecol. 304, 51–73. doi: 10.1016/j.jembe.2003.11.019
Chapman, M. G., and Tolhurst, T. J. (2007). Relationships between benthic macrofauna and biogeochemical properties of sediments at different spatial scales and among different habitats in mangrove forests. J. Exp. Mar. Bio. Ecol. 343, 96–109. doi: 10.1016/j.jembe.2006.12.001
Chesson, P. (2020). Species Competition and Predation, ed. R. A. Meyers (New York, NY: Springer New York). doi: 10.1007/978-1-4939-2493-6
Chong, C. T., Larned, S. T., Covich, A. P., and Kinzie, R. A. (2000). Species interactions between estuarine detritivores: inhibition or facilitation? Hydrobiologia 434, 11–16. doi: 10.1023/A:1004098425855
Cornell, H. V., and Lawton, J. H. (1992). Species interactions, local and regional processes, and limits to the richness of ecological communities: a theoretical perspective. J. Anim. Ecol. 61:1. doi: 10.2307/5503
Crandall, E., Jones, M., Muñoz, M., Akinronbi, B., Erdmann, M., and Barber, P. (2008). Comparative phylogeography of two seastars and their ectosymbionts within the Coral Triangle. Mol. Ecol. 17, 5276–5290. doi: 10.1111/j.1365-294X.2008.03995.x
Crawford, J. A. (1992). Acclimation of the shrimps, Periclimenes anthophilus, to the giant sea anemone, Condylactis gigantea. Bull. Mar. Sci. 50, 331–341.
CRED (2008). Coral Reef Ecosystem Division (CRED), NOAA Pacific Island Fisheries Science Center, 2008-05-08, CRED Rapid Ecological Assessment of Invertebrate in the Pacific Ocean, from 2002 to 2008. Available online at: http://ipt.obis.org/nonode/ (accessed February 3, 2021).
Dáttilo, W., and Rico-Gray, V. (eds). (2018). Ecological Networks in the Tropics. Cham: Springer International Publishing. doi: 10.1007/978-3-319-68228-0
Day, R. (1994). Algal symbiosis in Bunodeopsis: sea anemones with “auxiliary” structures. Biol. Bull. 186, 182–194. doi: 10.2307/1542052
Duarte, J., Hermoso-Salazar, M., Anker, A., and Simões, N. (2014). New records of alpheid shrimps (Crustacea: Decapoda: Alpheidae) from the southern Gulf of Mexico. Mar. Biodivers Rec. 7, 1–7. doi: 10.1017/S1755267214000773
Duchassaing de Fonbressin, P., and Michelotti, G. (1860). Mémoire sur les coralliaires des Antilles. Turin: De l’imprimerie royale. doi: 10.5962/bhl.title.11388
Duerden, J. E. (1897). I.— The actiniarian family aliciidæ. Ann. Mag. Nat. Hist. 20, 1–15. doi: 10.1080/00222939708680594
Edmunds, M., Potts, G., Swinfer, R., and Waters, V. (1974). Defensive behavior of sea anemones in response to predation by the opisthobranch mollusc Aeolidia papillosa. Mar. Biol. Assoc. UK 56, 65–83. doi: 10.1017/S0025315400020440
Ellis, J. (1767). An account of the Actinia sociata, or clustered animal-flower, lately found on the sea-coasts of the new-ceded islands: in a letter from John Elllis, Esquire, F. R. S. to the Right Honourable the Earl of Hillsborough, F. R. S. Philos. Trans. R. Soc. Lond. 57, 428–437. doi: 10.1098/rstl.1767.0043
Glynn, P., and Enochs, I. (2011). “Invertebrates and their roles in coral reef ecosystems,” in Coral Reefs: An Ecosystem in Transition, eds Z. Dubinsky and N. Stambler (Dordrecht: Springer), 273–325. doi: 10.1007/978-94-007-0114-4_18
González-Muñoz, R., Simões, N., Sanchez-Rodriguez, J., Rodríguez, E., and Segura-Puertas, L. (2012). First inventory of sea anemones (Cnidaria: Actiniaria) of the Mexican Caribbean. Zootaxa 38, 1–38. doi: 10.11646/zootaxa.3556.1.1
González-Muñoz, R., Simões, N., Tello-Musi, J. L., and Rodríguez, E. (2013). Sea anemones (Cnidaria, Anthozoa, Actiniaria) from coral reefs in the southern Gulf of Mexico. Zookeys 106, 77–106. doi: 10.3897/zookeys.341.5816
Halpern, B. S., Silliman, B. R., Olden, J. D., Bruno, J. P., and Bertness, M. D. (2007). Incorporating positive interactions in aquatic restoration and conservation. Front. Ecol. Environ. 5:153–160.
Henmi, Y., Fujiwara, C., Kirihara, S., Okada, Y., and Itani, G. (2017). Burrow morphology of alpheid shrimps: case study of Alpheus brevicristatus and a review of the genus. Zoolog. Sci. 34, 498–504. doi: 10.2108/zs170055
Harris, L., and Howe, N. (1979). An analysis of the defensive mechanisms observed in the anemone Anthopleura elegantissima in response to its nudibranch predator Aeolidia papillosa. Biol. Bull. 157, 138–152. doi: 10.2307/1541083
Herrnkind, W., Stanton, G., and Conklin, E. (1976). Initial characterization of the commensal complex associated with the anemone, Lebrunia danae, at Grand Bahama. Bull. Mar. Sci. 26, 65–71.
Hobson, L. A., and Lorenzen, C. J. (1972). Relationships of chlorophyll maxima to density structure in the Atlantic Ocean and gulf of Mexico. Deep. Res. Oceanogr. Abstr. 19, 297–306. doi: 10.1016/0011-7471(72)90023-X
Huebner, L. K., Shea, C. P., Schueller, P. M., Terrell, A. D., Ratchford, S. G., and Chadwick, N. E. (2019). Crustacean symbiosis with Caribbean sea anemones Bartholomea annulata: occupancy modeling, habitat partitioning, and persistence. Mar. Ecol. Prog. Ser. 631, 99–116.
Humann, P., and DeLoach, N. (2002). Reef Creature Identification Florida Caribbean Bahamas. Jacksonville, FL: New World Publications, Inc.
Hurt, C., Silliman, K., Anker, A., and Knowlton, N. (2013). Ecological speciation in anemone-associated snapping shrimps (Alpheus armatus species complex). Mol. Ecol. 22, 4532–4548. doi: 10.1111/mec.12398
Irving, A. D., and Witman, J. D. (2009). The effects of sedimentation on rocky coast assemblages. J. Ecol. 97, 827–833. doi: 10.1007/978-1-4615-7007-3_36
Karplus, I. (1979). The tactile communication between Cryptocentrus steinitzi (Pisces, Gobiidae) and Alpheus purpurilenticularis (Crustacea, Alpheidae). Zeitschrift Tierpsychol. 49, 173–196. doi: 10.1111/j.1439-0310.1979.tb00286.x
Karplus, I. (1987). The association between gobiid fishes and burrowing alpheid shrimps. Oceanogr. Mar. Biol. An Annu. Rev. 25, 507–562.
Karplus, I., Patzner, R. A., Van Tassell, M., and Kovacie, and Kapoor, B. G (2011). “The Partnership between gobiid fishes and gurrowing alpheid shrimps,” in The Biology of Gobies, eds R. A. Patzner, J. L. Van Tassell, M. Kovacie, and B. G. Kapoor (Boca Raton, FL: CRC Press), 183. doi: 10.1201/b11397-29
Karplus, I. (2014). Symbiosis in Fishes, ed. I. Karplus (Oxford: Wiley-Blackwell). doi: 10.1002/9781118759769
Khan, R. N., Becker, J. H. A., Crowther, A. L., and Lawn, I. D. (2003). Sea anemone host selection by the symbiotic saddled cleaner shrimp Periclimenes holthuisi. Mar. Freshw. Res. 54, 653–656. doi: 10.1071/MF02121
Knowlton, N., and Keller, B. D. (1983). A new, sibling species of snapping shrimp associated with the Caribbean sea anemone Bartholomea annulata. Bull. Mar. Sci. 33, 353–362.
Knowlton, N., and Keller, B. D. (1985). Two more sibling species of Alpheid shrimps associated with the caribbean sea anemona Bartholomea annulata and Heteractis lucida. Coral Reefs 37, 893–904.
Knowlton, N., and Keller, B. D. (1986). Larvae which fall far short of their potential: highly localized recruitment in an alpheid shrimp with extended larval development. Bull. Mar. Sci. 39, 213–223.
Kropp, R. K. (1987). Descriptions of some endolithic habitats from snapping shrimp (Alpheidae) in mricronesia. Bull. Mar. Sci. 41, 204–213.
LaJeunesse, T. (2002). Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar. Biol. 141, 387–400. doi: 10.1007/s00227-002-0829-2
Le Sueur, C. A. (1817). Observations on several species of the genus Actinia; illustrated by figures. J. Acad. Nat. Sci. Philadelphia 1, 149–154,169–189.
Levin, L. A., Boesch, D. F., Covich, A., Dahm, C., Erséus, C., Ewel, K. C., et al. (2001). The function of marine critical transition zones and the importance of sediment biodiversity. Ecosystems 4, 430–451. doi: 10.1007/s10021-001-0021-4
Lohse, D., Schmitz, B., and Versluis, M. (2001). Snapping shrimp make flashing bubbles. Nature 413, 477–478. doi: 10.1038/35097152
Manjarrés, G. A. (1977). Contribución al conocimiento de las actinias en la region de Santa Marta. An. del Inst. Investig. Mar. Punta Betín 9, 91–104. doi: 10.25268/bimc.invemar.1977.9.0.522
Mascaró, M., Rodríguez-Pestaña, L., Chiappa-Carrara, X., and Simoes, N. (2012). Host selection by the cleaner shrimp Ancylomenes pedersoni: do anemone host species, prior experience or the presence of conspecific shrimp matter? J. Exp. Mar. Bio. Ecol. 413, 87–93. doi: 10.1016/j.jembe.2011.11.026
Massuti, E., García, C., Guijarro, B., Quetglas, A., de Sola, L. G., and Instituto Español de Oceanografía (IEO), Spain (2020). Demersal and mega-benthic species from the MEDITS (Mediterranean International Bottom Trawl Survey) program at the Spanish continental shelf and upper slope between 1994 and 2009. Available online at: http://ipt.vliz.be/eurobis/ (accessed February 3, 2021).
McCammon, A. M. (2010). Snapping Shrimp Protect Host Anemones From Predators. master’s thesis. Boca Raton, FL: Florida Atlantic University.
McCammon, A. M., and Brooks, W. R. (2014). Protection of host anemones by snapping shrimps: a case for symbiotic mutualism? Symbiosis 63, 71–78.
Miloslavich, P., Díaz, J. M., Klein, E., Alvarado, J. J., Díaz, C., Gobin, J., et al. (2010). Marine biodiversity in the Caribbean: regional estimates and distribution patterns. PLoS One 5: e11916. doi: 10.1371/journal.pone.0011916
NMNH (2001). National Museum of Natural History, Smithsonian Institution NMNH Invertebrate Zoology Collection Database. National Museum of Natural History, Smithsonian Institution, 10th and Constitution Ave. N.W., Washington, DC 20560-0193, 2001, Version 3.2.04 (0802221). New Delhi: National Museum of Natural History.
Palomar, N. E., Juinio-Meñez, M. A., and Karplus, I. (2005). Behavior of the burrowing shrimp Alpheus macellarius in varying gravel substrate conditions. J. Ethol. 23, 173–180. doi: 10.1007/s10164-005-0149-3
Pérez-Botello, A. M., and Simões, N. (2021). Sponge-dwelling fauna: a review of known species from the Northwest Tropical Atlantic coral reefs. Biodivers. Data J. 9:e63372. doi: 10.3897/BDJ.9.e63372
Powers, D. A., and Xie, Y. (1999). Statistical Methods for Categorical Data Analysis, 2nd Edn. Cambridge, MA: Academic Press.
Rahman, N., Dunham, D., and Govind, C. K. (2004). Mate choice in the big-clawed snapping shrimp, Alpheus heterochaelis say, 1818. Crustaceana 77, 95–111. doi: 10.1163/156854004323037919
Richardson, D. (1999). Correlates of environmental variables with patterns in the distribution and abundance of two anemonefishes (Pomacentridae: Amphiprion) on an eastern Australian sub-tropical reef system. Environ. Biol. 55, 255–263. doi: 10.1023/A:1007596330476
Rodríguez, E., Barbeitos, M. S., Brugler, M. R., Crowley, L. M., Grajales, A., Gusmão, L., et al. (2014). Hidden among sea anemones: the first comprehensive phylogenetic reconstruction of the order Actiniaria (Cnidaria, Anthozoa, Hexacorallia) reveals a novel group of hexacorals. PLoS One 9:e96998. doi: 10.1371/journal.pone.0096998
Romey, G., Abita, J., Schweitz, H., Wunderer, G., and Lazdunski, M. (1976). Sea anemone toxin: a tool to study molecular mechanism of nerve conduction and excitation-secretion coupling. Proc. Natl. Acad. Sci. U.S.A. 11, 4055–4089. doi: 10.1073/pnas.73.11.4055
Roopin, M., and Chadwick, N. E. (2009). Benefits to host sea anemones from ammonia contributions of resident anemonefish. J. Exp. Mar. Bio. Ecol. 370, 27–34. doi: 10.1016/j.jembe.2008.11.006
Rosin, R. (1969). Escape response of the sea-anemone Anthopleura nigrescens (Verrill) to its predatory solid nudibranch Herviella baba spec. nov. Veliger 12, 74–77.
Sanchez-Rodriguez, J. (2001). Cutaneous stings from Bartholomea annulata. Contact 44, 314–315. doi: 10.1034/j.1600-0536.2001.440511-7.x/
Santos, M. E. A., Faria-Junior, E., Aued, A. W., Peluso, L., Kitahara, M. V., Pires, D. O., et al. (2020). Benthic Cnidaria community in the oceanic archipelago of Trindade and Martin Vaz, Southwestern Atlantic Ocean. Reg. Stud. Mar. Sci. 33:100895. doi: 10.1016/j.rsma.2019.100895
Schein, H. (1975). Aspects of the aggressive and sexual behaviour of Alpheus heterochaelis say. Mar. Behav. Physiol. 3, 83–96. doi: 10.1080/10236247509378
Schmitz, B., and Herberholz, J. (1998). Snapping behaviour in intraspecific agonistic encounters in the snapping shrimp (Alpheus heterochaelis). J. Biosci. 23, 623–632. doi: 10.1007/BF02709175
Shick, J. M. (1979). The limits to indeterminate growth: an optimal size model applied to passive suspension feeders. Am. Zool. 19, 699–713. doi: 10.2307/1937045
Silbiger, N. J., and Childress, M. J. (2008). Interspecific variation in anemone shrimp distribution and host selection in the Florida Keys (USA): implications for marine conservation. Bull. Mar. Sci. 83, 329–345.
Smith, W. L. (1977). Beneficial behavior of a symbiotic shrimp to its host anemone. Bull. Mar. Sci. 27, 343–346. doi: 10.1007/s13199-014-0289-8
Spotte, S. (1996). Supply of regenerated nitrogen to sea anemones by their symbiotic shrimp. J. Exp. Mar. Bio. Ecol. 198, 27–36. doi: 10.1016/0022-0981(95)00169-7
Stewart, H. L., Price, N. N., Holbrook, S. J., Schmitt, R. J., and Brooks, A. J. (2013). Determinants of the onset and strength of mutualistic interactions between branching corals and associate crabs. Mar. Ecol. Prog. Ser. 493, 155–163. doi: 10.3354/meps10525
Thiel, M., and Baeza, J. A. (2001). Factors affecting the social behaviour of crustaceans living symbiotically with other marine invertebrates: a modelling approach. Symbiosis 30, 163–190.
Vázquez-Lule, A., and Díaz-Gallegos, J. (2009). Caracterización del sitio de manglar El Palmar. En: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO). Sitios de Manglar Con Relevancia Biológica y Con Necesidades de Rehabilitación Ecológica. Mexico City: CONABIO.
Vincent, J., Balerna, M., Fosset, M., and Lazdunski, M. (1980). Binding of sea anemone toxin to a receptor site associated with gating system of sodium channel in synaptic nerve endings in vitro. Proc. Natl. Acad. Sci. U.S.A. 77, 1646–1650. doi: 10.1073/pnas.77.3.1646
Watson, R. A., and Pollack, J. B. (1999). How symbiosis can guide evolution. Adv. Artif. Life 1674, 29–38. doi: 10.1007/3-540-48304-7_7
Keywords: symbiosis, ecological interactions, coral reefs, animal behaviour, Alpheus armatus, Alpheus immaculatus, snapping shrimps, Bartholomea annulata
Citation: Pérez-Botello AM, Mascaró M and Simões N (2021) The Importance of Home Cleaning: Sediment Transport by Alpheid Shrimps Provides a Competitive Advantage to Their Host Anemones. Front. Mar. Sci. 8:677024. doi: 10.3389/fmars.2021.677024
Received: 07 March 2021; Accepted: 30 April 2021;
Published: 28 May 2021.
Edited by:
Francesca Porri, South African Institute for Aquatic Biodiversity, South AfricaReviewed by:
Gyo Itani, Kôchi University, JapanCopyright © 2021 Pérez-Botello, Mascaró and Simões. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nuno Simões, bnNAY2llbmNpYXMudW5hbS5teA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.