
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 11 June 2021
Sec. Marine Megafauna
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.674134
This article is part of the Research TopicSmall Cetacean Conservation: Current Challenges and OpportunitiesView all 51 articles
The establishment of marine protected areas (MPAs) requires a thorough assessment of the abundance, distribution, and habitat preferences of a variety of marine species. Small cetacean spatial distribution and abundance were examined in the Pacific waters of Guatemala to provide this information. Boat surveys were conducted for 38 months between January 2008 and June 2012. A total of 64,678 cetaceans in 505 sightings from nine Delphinidae species were recorded. Three species, referred to as common species, accounted for 90% (n = 456) of all sightings. They included Tursiops truncatus (56%, n = 278), Stenella attenuata (29%, n = 143), and Stenella longirostris (7%, n = 35). Group size was significantly different among the common species (p < 0.001). S. longirostris had the largest group size (444 ± 75 dolphins), followed by S. attenuata (28 ± 5 dolphins), and T. truncatus (15 ± 2 dolphins). T. truncatus was the most common in the study area (0.02 ± 0.002 sightings/km of survey effort), and S. attenuata (0.37 ± 0.16 dolphins/km) and S. longirostris (1.62 ± 0.41 dolphins/km) were the most abundant in the neritic (≤200 m depth) and oceanic zones (≥200 m depth), respectively. The wide-ranging distribution of T. truncatus overlapped with the distribution of S. attenuata in the neritic zone and S. longirostris in the oceanic zone. Little overlap was observed in the distribution of S. attenuata and S. longirostris. Most hot spots (∼66%) were in the oceanic zone and no hot spots were near or in the MPAs. Hot spots were identified along the 200 m isobath, the Middle America trench, and the San José Canyon. These could be areas of high productivity where dolphins concentrate to feed. To the north of the San José Canyon, five species of small cetaceans were observed in a stretch of the neritic zone including three MPAs. No other section of this zone had such high diversity. Results need to be taken with caution given the small sample size. Our results suggest that the protection of small cetaceans needs to consider the creation of oceanic MPAs that should be integrated into the existing network.
The effective management of wild animal populations depends on a strong foundation of knowledge regarding their distribution and abundance. A thorough understanding is necessary of the areas used by a population to enable effective prioritization of the conservation or management approaches to be used. In many Latin American countries, baseline information on cetaceans is sparse or absent. However, cetacean populations that inhabit the waters of those countries face extensive problems such as habitat degradation and bycatch from numerous types of fishing operations. According to the IUCN “Dolphins, Whales and Porpoises: 2002–2010 Conservation Action Plan for the World’s Cetaceans” (Reeves et al., 2003), these problems pose serious threats to many dolphin populations. Populations of northeastern offshore spotted dolphins (Stenella attenuata attenuata) and eastern spinner dolphins (Stenella longirostris orientalis) have been reduced due to the dolphin bycatch of the purse-seine fishery for yellowfin tuna in the eastern tropical Pacific waters (Gerrodette and Forcada, 2005), although their populations were reported to be increasing in 2006 (Gerrodette et al., 2008). In countries such as Guatemala, Chile, Colombia, Panama, Peru, and Venezuela, dolphin meat has been used as bait for a range of fishing practices (Goodall et al., 1988; Vidal et al., 1994; Culik, 2004; Alfaro-Shigueto et al., 2008; Ávila et al., 2008; Loch et al., 2009; Mangel et al., 2010; Quintana-Rizzo, 2011a; Mel and Fisher, 2016; Mintzer et al., 2018; Campbell et al., 2020). Furthermore, in Guatemala, some fishermen harpoon dolphins because they consider them to be a threat and fishing competitor (Quintana-Rizzo, 2011a). Baseline information on population numbers and distribution could be extremely helpful for assessing the status of local populations and developing appropriate conservation and management decisions.
In Guatemala, baseline information on species abundance and distribution is particularly relevant as the government is currently in the process of expanding four existing coastal protected areas to include a marine zone and creating a new area that will include both terrestrial and marine zones in the Pacific Ocean. This initiative is part of the 2012–2022 Guatemalan National Strategy for Biological Diversity and Plan of Action that seeks to promote the sustainable use and conservation of at least 10% of local coastal–marine ecosystems (CONAP, 2013). Marine spatial planning efforts began in 2006 with the identification of gaps in the Guatemalan System for Protected Areas and the proposal of priority areas for marine conservation (CONAP and MARN, 2009). The proposals are currently being reviewed by the National Council of Protected Areas (in Spanish: Consejo Nacional de Áreas Protegidas, CONAP), the government agency whose mission is the conservation of biological diversity and sustainable use of protected areas of the country. Although this initiative is an encouraging step by the government toward the protection of marine resources, the effort needs to be supported by a solid foundation of scientific information that incorporates a wide range of marine megafauna species. The proposed network of coastal marine protected areas (MPAs) was based on characteristics such as substrate type, geology, topography, and depth; human pressures and threats to ecosystems; and biological elements including wetlands, mangroves, sea turtles, and birds (Hoyt, 2011). The presence of cetaceans was recognized in the review process but there were no assessments of the species abundance, distribution, habitat preferences, or identification of critical habitats. These aspects are critical in the early steps of establishing MPAs (Hoyt, 2011). More than 15 species of cetaceans have been identified in the Pacific waters of the country (Quintana-Rizzo and Gerrodette, 2009; Cabrera et al., 2014).
Various levels of information exist for the different cetacean species found off the Pacific coast of Guatemala. The lack of comprehensive data makes it impossible to determine the status of the different species or develop appropriate conservation and management strategies. Most studies have focused on particular species (e.g., Megaptera novaeangliae: Godoy Aguilar et al., 2009; Quintana-Rizzo, 2019. Tursiops truncatus, S. longirostris, and Delphinus delphis: Ortiz, 2011. S. attenuata and S. longirostris: Cabrera and Ortíz, 2012), and have been conducted in localized areas to different extents (Cabrera and Ortíz, 2010; Dávila, 2011; Cabrera et al., 2012; Quintana-Rizzo, 2019). However, one literature review identified 13 species of small cetaceans belonging to the Delphinidae and Kogiidae families (Cabrera et al., 2014). Information at a large scale is available from studies conducted in the 1990s in the eastern tropical Pacific (e.g., Wade and Gerrodette, 1993), which includes Guatemala, and describes species at the regional level (e.g., Reilly and Thayer, 1990; Escorza-Treviño et al., 2005; Chivers et al., 2007). Those studies reported two endemic subspecies of small cetaceans (S. attenuata graffmani and S. longirostris centroamericana) in the region (Dizon et al., 1994).
Many factors can influence the spatial and temporal distributions of cetaceans. They include physiographic characteristics such as water depth and seafloor slope (Cañadas et al., 2002; Baumgartner, 2006; Cubero-Pardo, 2007; Gómez de Segura et al., 2008; Azzellino et al., 2012), environmental factors such as sea surface temperature and salinity (Reilly, 1990), and biological factors such as prey distribution and breeding areas (Palacios et al., 2013). Important habitats require areas for feeding, breeding, socializing, and calving, as well as nursing and raising calves (Hoyt, 2011). Identifying areas essential for the day-to-day survival of species is a necessary component of an effectively designed spatial plan of prioritized conservation measures.
The effective protection of cetaceans could mean the protection of many marine organisms living in the ecosystem and of the ecosystem itself (Prideaux, 2003). This is because cetaceans typically live in large areas where, if effective protection measures are established, numerous other species could be conserved and protected, as well as their ecosystems and ecosystem processes. Isolated MPAs may serve to protect a certain species at a given time and place (Maxwell et al., 2014), but cetaceans are highly mobile. The protection of highly mobile species will necessitate the protection of areas that provide connectivity between critical habitats through a network of MPAs (Hyrenbach et al., 2000) and complementary management and mitigation measures.
Here we present an extensive analysis of the distribution and abundance of small cetaceans that have been observed in the Pacific waters of Guatemala. The analysis was based on data collected during boat surveys between 2008 and 2012. This is a unique dataset because of its sampling frequency and the spatial extent of the field effort within the Guatemalan Exclusive Economic Zone (EEZ). We have identified hot spot areas for all species of small cetaceans combined and for each of the most common species across the sampling area. The relationship between physiographic characteristics and social parameters such as group size was also examined. The results represent a vital contribution to marine spatial planning in Guatemala and fill a critical gap in knowledge to ensure that reserve designs are based primarily on the information obtained for species of interest (Hyrenbach et al., 2000).
The study area covers approximately 41,365 km2 of the Pacific EEZ of Guatemala (Figure 1). It includes the continental shelf and oceanic plate. The Pacific continental shelf is an area of about 14,700 km2, which extends from the coastline to 200 m depth and has an average width of 60 km (URL-IIA, 2004). The shelf edge is relatively straight, except for a major embayment on the east side, associated with the San José canyon (McMillen et al., 1982). This canyon is a major feature on the shelf and slope of the Guatemalan EEZ that drops from 200 to 2,000 m (Ladd and Schroder, 1985) and extends out into the Middle America Trench (von Huene et al., 1985). The Middle America trench is located within the oceanic plate at about 100 km from shore and reaches depths up to 6,400 m (Fisher, 1961).
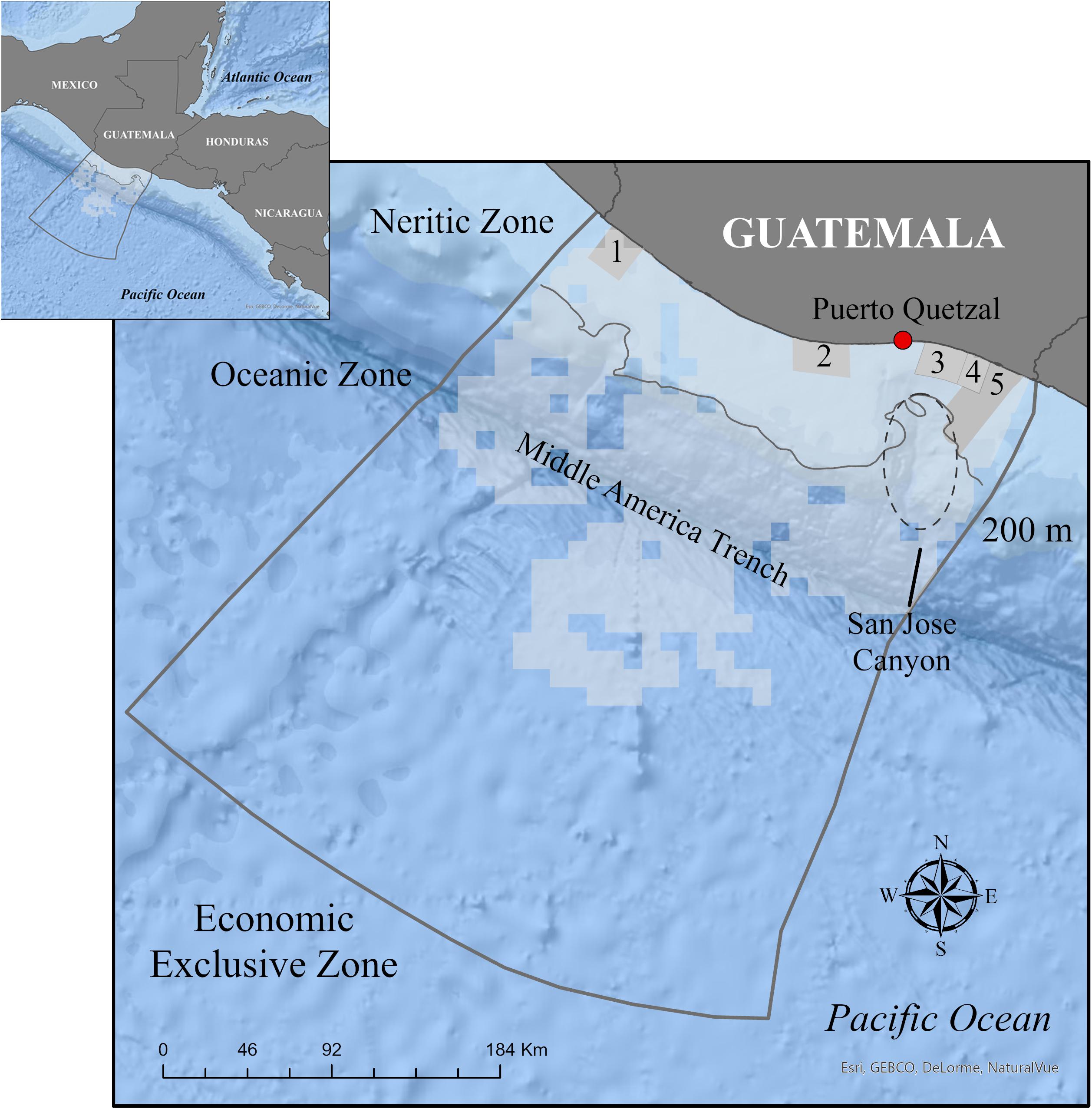
Figure 1. Study area (light gray) inside the Guatemalan Pacific Economic Exclusive Zone including the neritic and oceanic zones, 200 m isobath, and coastal marine protected areas (medium gray color). Clear sections inside the study area represent sections that were not surveyed. The coastal protected areas from west to east are: (1) Manchon-Guamuchal, (2) Sipacate-Naranjo, (3) Monterrico, (4) Hawaii, and (5) Las Lisas.
The Guatemalan basin is part of the warm pool of the eastern tropical Pacific (>27.5°C), which results from a seasonally large net heat flux and weak wind mixing. The center of the warm pool is along the coast of southwestern Mexico and Guatemala (Fiedler and Talley, 2006). The area also includes the polygons of five proposed MPAs but the extent, size, and shape are under review (PNUD, 2018). From west to east, the MPAs are (Figure 1): (1) Manchón-Guamuchal (marine zone: 463.32 km2), (2) Sipacate-Naranjo (marine zone: 543.90 km2), (3) Monterrico (marine zone: 430.46 km2), (4) Hawaii (marine zone: 239.76 km2), and (5) Las Lisas (marine zone: 1018.48 km2) (DIPESCA/MAGA/PNUD/TNC, 2018).
Boat surveys were conducted nearly every month from January 2008 to June 2012. Surveys were planned according to good weather conditions using two types of vessels: 6.7–7.62 m long fishing boats driving at 11–16 knots and the Guatemalan Coast Guard vessel (∼20 m long) driving at 7–8 knots. The latter towed a small vessel (∼7 m long) used to approach dolphin groups in which species confirmation was needed. All boat surveys were conducted under sea state conditions of Beaufort Sea State ≤ 3. Survey tracks were recorded in a Garmin GPSmap76, GPSmap 76S, or GPS Garmin Vista. Field time varied between 7 and 12 h/day.
Once a group of dolphins was spotted, the species identification, the number of individuals, and the presence or absence of calves were recorded, resuming sailing shortly after. Species identification with confidence levels of definite (high confidence in species identification) and probable (moderate confidence) were included in the analysis. A group was defined as all individuals displaying the same general activity within a 100 m distance (Wells et al., 1987), except in the case of groups of hundreds of individuals, which tended to spread over larger distances. A calf was defined as a dolphin up to about 75% of the presumed mother’s length, which consistently traveled alongside the presumed mother, in baby position (Wells et al., 1987; Smolker et al., 1992; Urian and Wells, 1996). Each dolphin encountered was considered a sighting (Quintana-Rizzo and Wells, 2001). For each sighting, standard forms were completed with information about the time, geographical location, and environmental conditions.
Statistical analyses were performed on the species that had at least 20 sightings to satisfy the minimum sample size of statistical comparisons (Ott, 1994). Thus, sightings from multiple years were pooled together. In the case of the bathymetric zone comparisons, statistical analyses only involved the species with at least 20 sightings in each zone (see below). An “all species” category was included in the abundance and spatial distribution analyses. Statistical tests were performed using the SPSS 26.0 package (2019) at a significance level of 0.05.
The study area was divided into the two marine zones based on bathymetry (Hedgpeth, 1957): neritic (<200 m depth) and oceanic (>200 m depth) to examine the relationship between cetacean abundance, distribution, and physiography (see below). Neritic and oceanic zones include ecosystems of the continental shelf and slope, respectively. Neritic waters lie over the continental shelf, beyond which the seafloor begins to descend more sharply. This zone division also allowed us to examine the significance of the current and planned protected areas since all of them are in the neritic zone. The study area included approximately 12,856 km of the neritic zone and 28,509 km of the oceanic zone.
Cetacean sightings and track lines were plotted in ArcGIS Pro 2.7.0. The area for data analysis was divided into 10 km × 10 km grid cells resulting in 434 cells. For each species, three values were calculated within each grid cell: the total number of dolphins, the total number of sightings, and the total length of survey tracks. If no sightings occurred in a grid cell that was surveyed, the grid cell was attributed a value of zero but the cell was considered part of the survey effort. Two indices were calculated: (1) relative abundance of dolphins defined as individuals per unit effort (IPUE), and (2) sightings per unit effort (SPUE), where effort represents the distance surveyed in km. The latter was also a commonality index because a specie can be commonly sighted without being abundant or vice versa. IPUE and SPUE were calculated for all species combined and for each of the most common species in the neritic and oceanic zones. The two indices were compared between zones using a Mann–Whitney U test.
A hot spot analysis was used to delineate the spatial occurrence or clustered distribution of dolphins (IPUE) and sightings (SPUE) of each of the most common species and all cetacean species combined. A hot spot analysis test for statistically significant spatial clustering of IPUE and SPUE using the Getis-Ord Gi∗ statistic (Getis and Ord, 1992), which determines the spatial clustering of grid cell values that are higher (hot spot) or lower (coldspot) than is expected by random distribution. It performs significant tests between nearby cells in the surrounding neighborhood area using a z-score (Getis and Ord, 1992). The recommended fixed distance band was used to ensure each feature has a neighbor within a specified distance that was objectively calculated within ArcGis Pro 2.7.1 (Queiroz et al., 2016; Yurkowski et al., 2019). Distributional maps were created at three levels of confidence (99, 95, and 90%), and all clusters that were within the 90% confidence level were considered hot spots. For the gap analysis, the spatial and percentage overlap (km2 and % area, respectively) of species diversity hot spots of the study area and protected areas were calculated in ArcGis Pro 2.7.1.
Mean group size and the percentage of groups with calves were calculated for all the small cetacean species. Group size among the most common species was compared using a two-tailed Kruskal–Wallis test.
The analysis of group size and sighting relationship to depth included three components. First, the group size of the most common species was compared between the neritic and the oceanic zones using a two-tailed Mann–Whitney test. Since the zones are characterized by different bathymetry, they are a proxy to evaluate the relationship between group size and depth. Second, we examined if depth explained variation in group size by fitting a linear regression. The variables were log-transformed to ensure the data conformed to the assumptions of linear regression. Third, sighting depth among the most common species was compared using a two-tailed Kruskal–Wallis test. Depth was extracted using the bathymetry raster of the Guatemalan EEZ generated by DIPESCA/MAGA/PNUD/TNC (2018) in ArcGIS Pro 2.7.0.
A total of 171 surveys, covering 24,112 km, were conducted between January 2008 and June 2012 (Table 1). During the surveys, 64,678 cetaceans were sighted and 505 sightings were recorded. Species identification was confirmed in 98% (n = 495) of the cases. Nine species of small cetaceans, all belonging to the Delphinidae family, were identified. Three species accounted for 90% (n = 456) of all sightings (Table 2) and were referred to as the common species, which included: T. truncatus (56%, n = 278), S. attenuata (29%, n = 143), and S. longirostris (7%, n = 35). Two subspecies of Stenella were identified based on their morphological characteristics in some sightings. Of the 143 sightings of S. attenuata, 31 sightings (20%) were classified at the subspecies level. Of these 31 sightings, 26 were identified as S. attenuata graffmani and five were identified as S. attenuata attenuata. In the case of S. longirostris, eight (23%) out of the 35 sightings were classified at the subspecies level: four were identified as S. longirostris centroamericana and another four were identified as S. longirostris orientalis. Six species have the IUCN status of “Least Concern,” two species have the status of “Data Deficient,” and one species has the status of “Near Threatened” (Table 2). Sightings of seven species of small cetaceans included calves (Table 2).
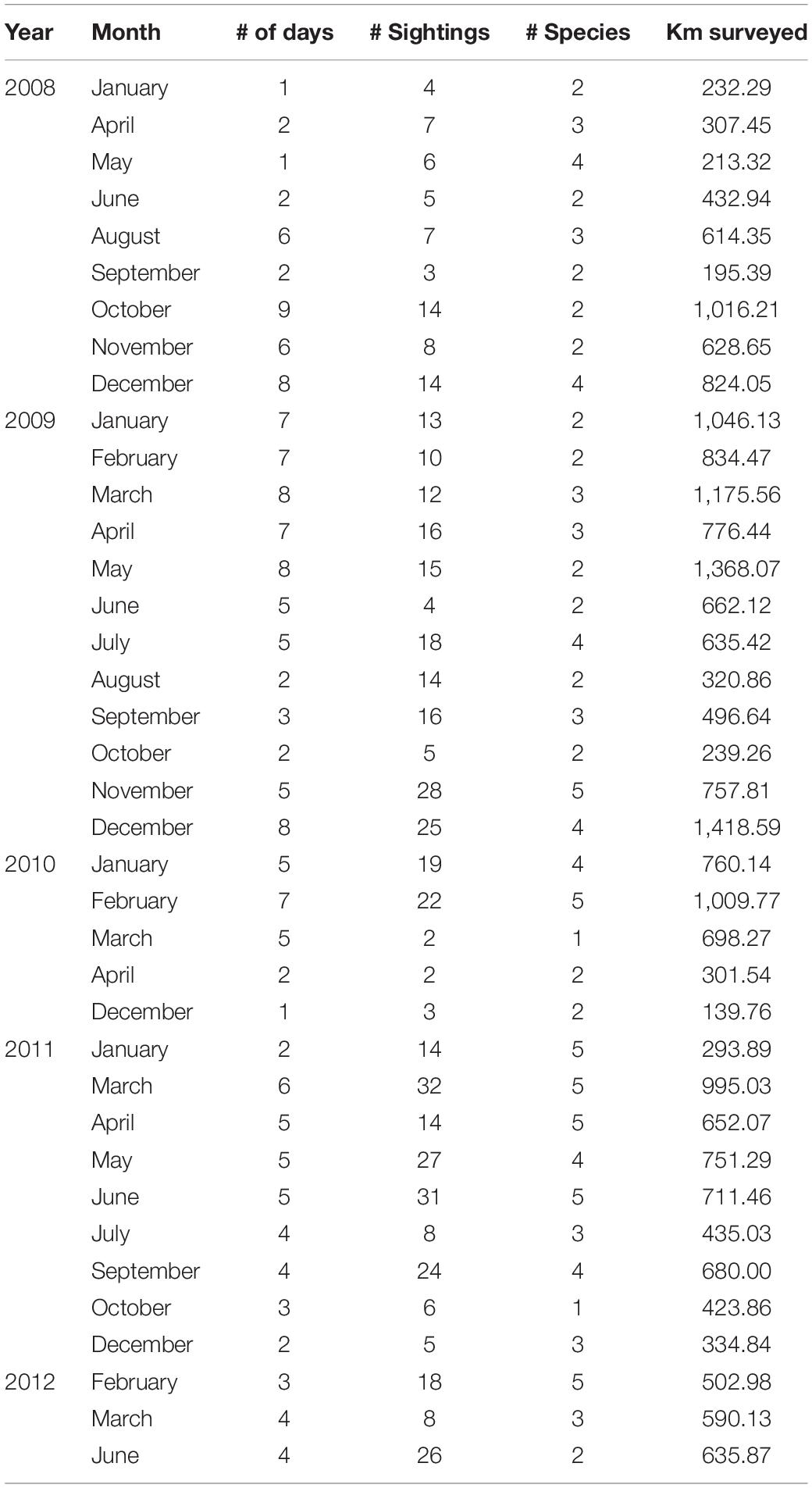
Table 1. Survey effort including the number of days, sightings, species, and kilometers surveyed in the Pacific Ocean of Guatemala.
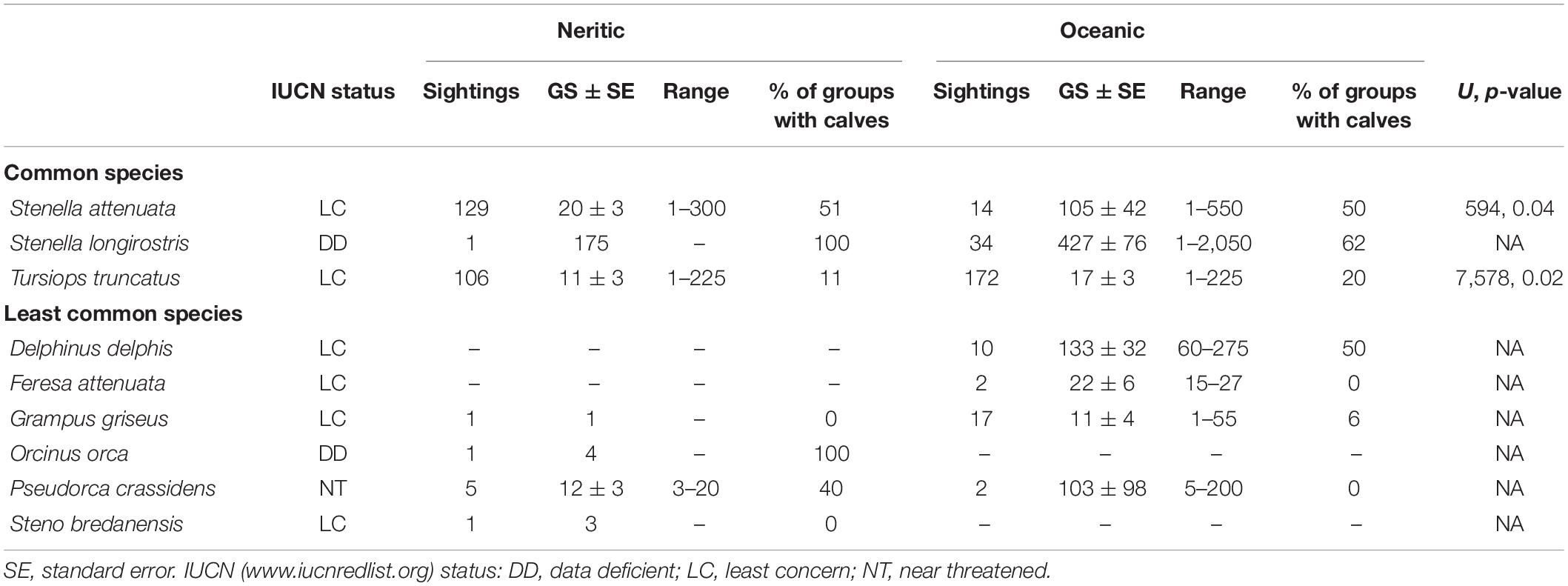
Table 2. Summary of small cetacean species sighted of the Pacific waters of Guatemala including the number of sightings, mean and range of group size, percentage of groups with calves, and Mann–Whitney U test (U) results of group size comparisons between the neritic (<200 m depth) and oceanic (>200 m depth) zones.
Small cetaceans were sighted throughout the study area including the neritic and oceanic zones (Figure 2). However, the proportion of sightings in each zone varied among them. Ninety percent of the sightings of S. attenuata occurred in the neritic zone while 97 and 62% of the sightings of S. longirostris and T. truncatus, respectively, occurred in the oceanic zone (Table 2). The sighting frequency for the least common species was Grampus griseus (4%), D. delphis (2%), Pseudorca crassidens (1%), Feresa attenuata (0.40%), Orcinus orca (0.20%), and Steno bredanensis (0.20%). G. griseus and P. crassidens were sighted in the two zones, O. orca and S. bredanensis were only sighted in the neritic zone, while D. delphis and F. attenuata were only sighted in the oceanic zone (Table 2).
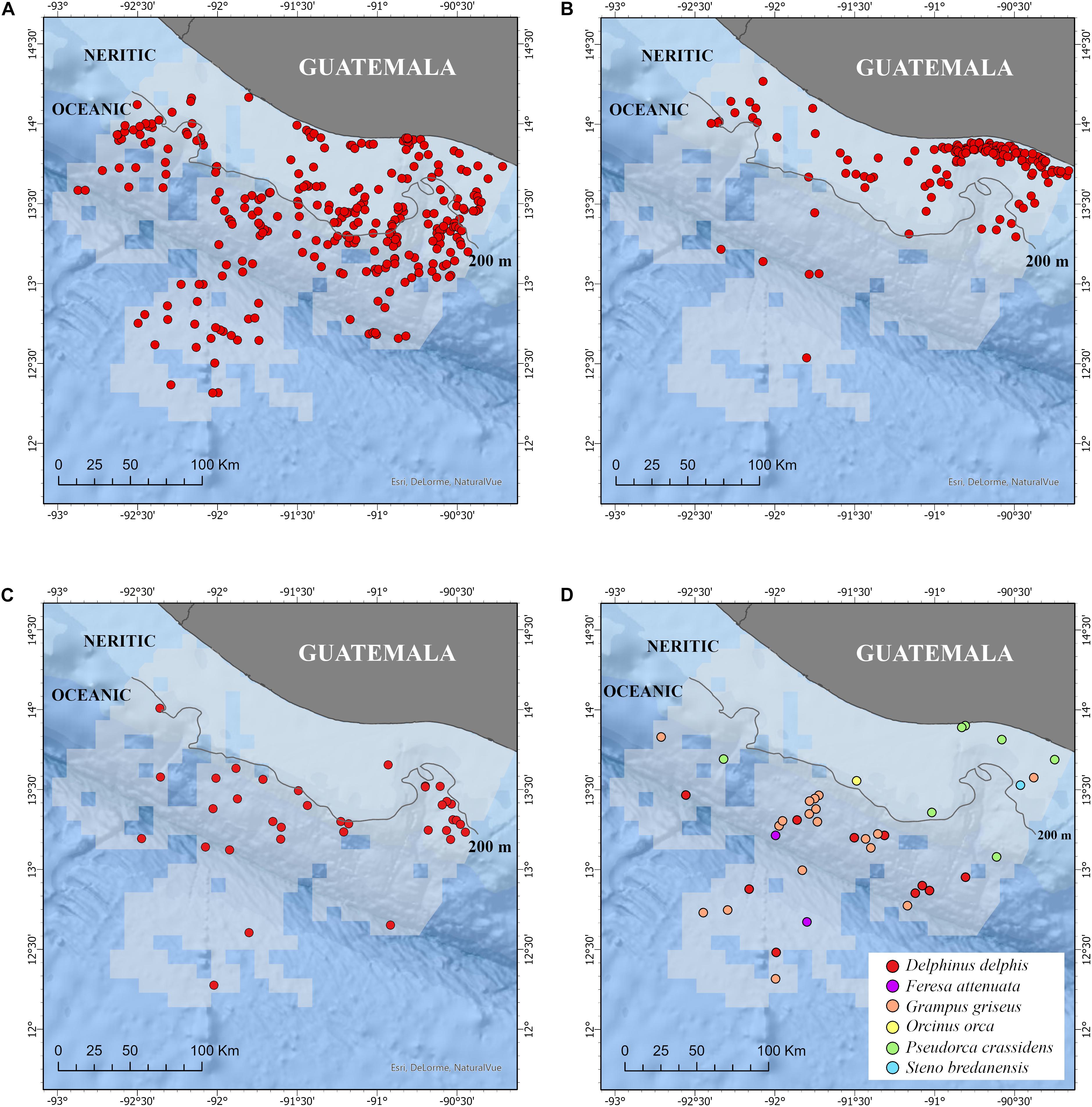
Figure 2. Sightings of the three most common species: (A) Tursiops truncatus, (B) Stenella attenuata, and (C) Stenella longirostris, and (D) of the least common species in the study area (light gray) in the Pacific waters of Guatemala. Neritic and oceanic zones, 200 m isobath, and coastal marine protected areas (medium gray color) are also included. Additional details of the study area are shown in Figure 1.
Relative abundance (IPUE and SPUE) was estimated for the entire sampling area, as well as for the neritic and oceanic zones. For the entire sampling area, mean IPUE was calculated at 2.14 dolphins/km, and mean SPUE was calculated at 0.04 sightings/km. No statistically significant difference was found between the IPUE and SPUE of all species combined between zones (p > 0.05, Table 3). However, higher numbers of individuals and sightings were detected in the individual grid cells in the oceanic zone than in the neritic zone.
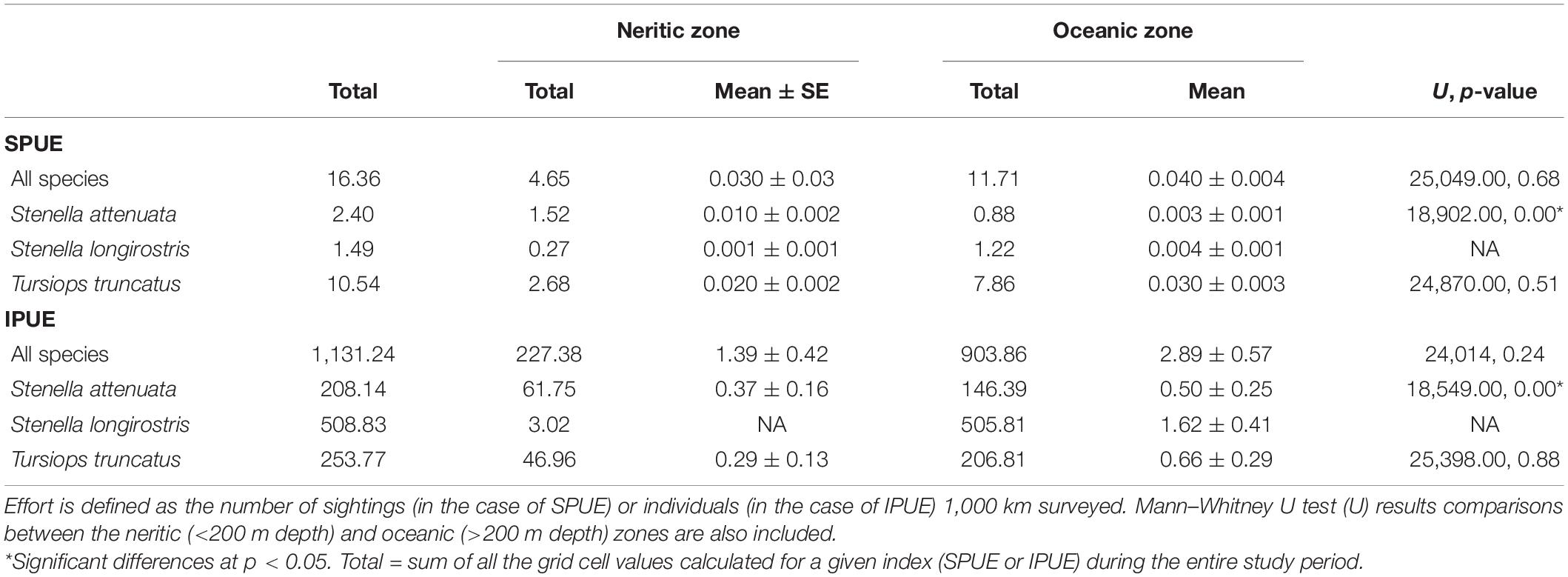
Table 3. Sightings per unit effort (SPUE) and dolphins per unit effort (IPUE) for all small cetacean species and the most common species sighted of the Pacific waters of Guatemala.
The distribution of small cetaceans was more fragmented in the oceanic zone than in the neritic zone (Figures 3, 4). A hot spot analysis showed eight IPUE clusters throughout the oceanic zone, particularly near the 200 m bathymetry line, the San José Canyon, and the Middle America Trench. In contrast, only one IPUE cluster of small cetaceans was found in the neritic zone, which was located on the northwestern side and near the 200 m bathymetry line (Figure 5). There were more SPUE than IPUE clusters in the oceanic (n > 10) and neritic (n = 4), including three large SPUE clusters (≥300 km each) in the San José canyon (Figures 5, 6).
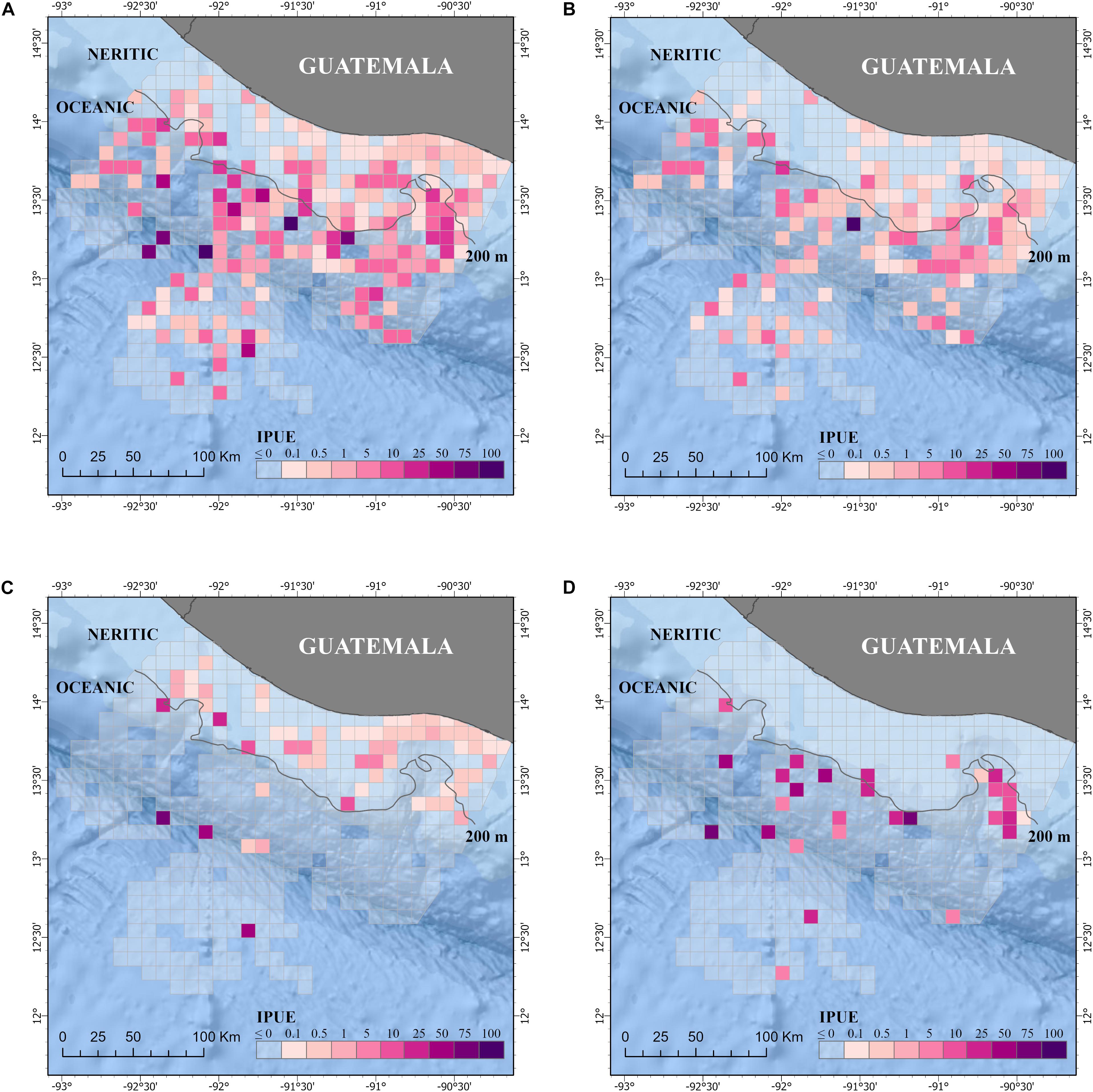
Figure 3. Relative abundance is defined as individuals per survey effort (IPUE) of all small cetacean species (A) and the three most common species: (B) Tursiops truncatus, (C) Stenella attenuata, and (D) Stenella longirostris in the Pacific waters of Guatemala. Effort is km of survey summarized by 10 km × 10 km grid cells. Neritic (<200 m depth) and ocean (>200 m depth) zones are also included. Additional details of the study area are shown in Figure 1.
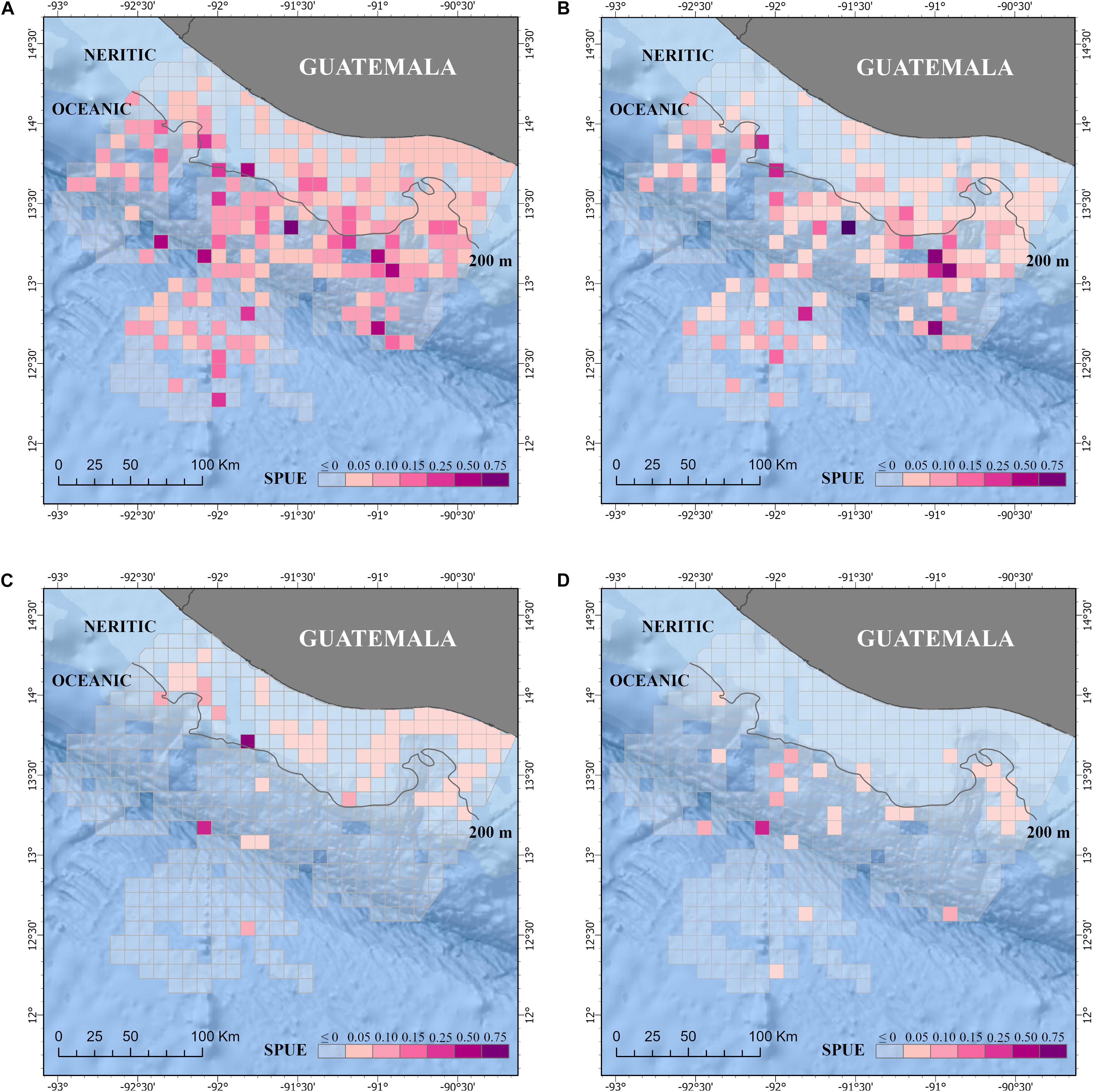
Figure 4. Sightings per unit effort (SPUE) of all cetacean species (A) and the three most common species: (B) Tursiops truncatus, (C) Stenella attenuata, and (D) Stenella longirostris in the Pacific waters of Guatemala. Effort is km of survey summarized by 10 km × 10 km grid cells. Neritic (<200 m depth) and ocean (>200 m depth) zones are also included. Additional details of the study area are shown in Figure 1.
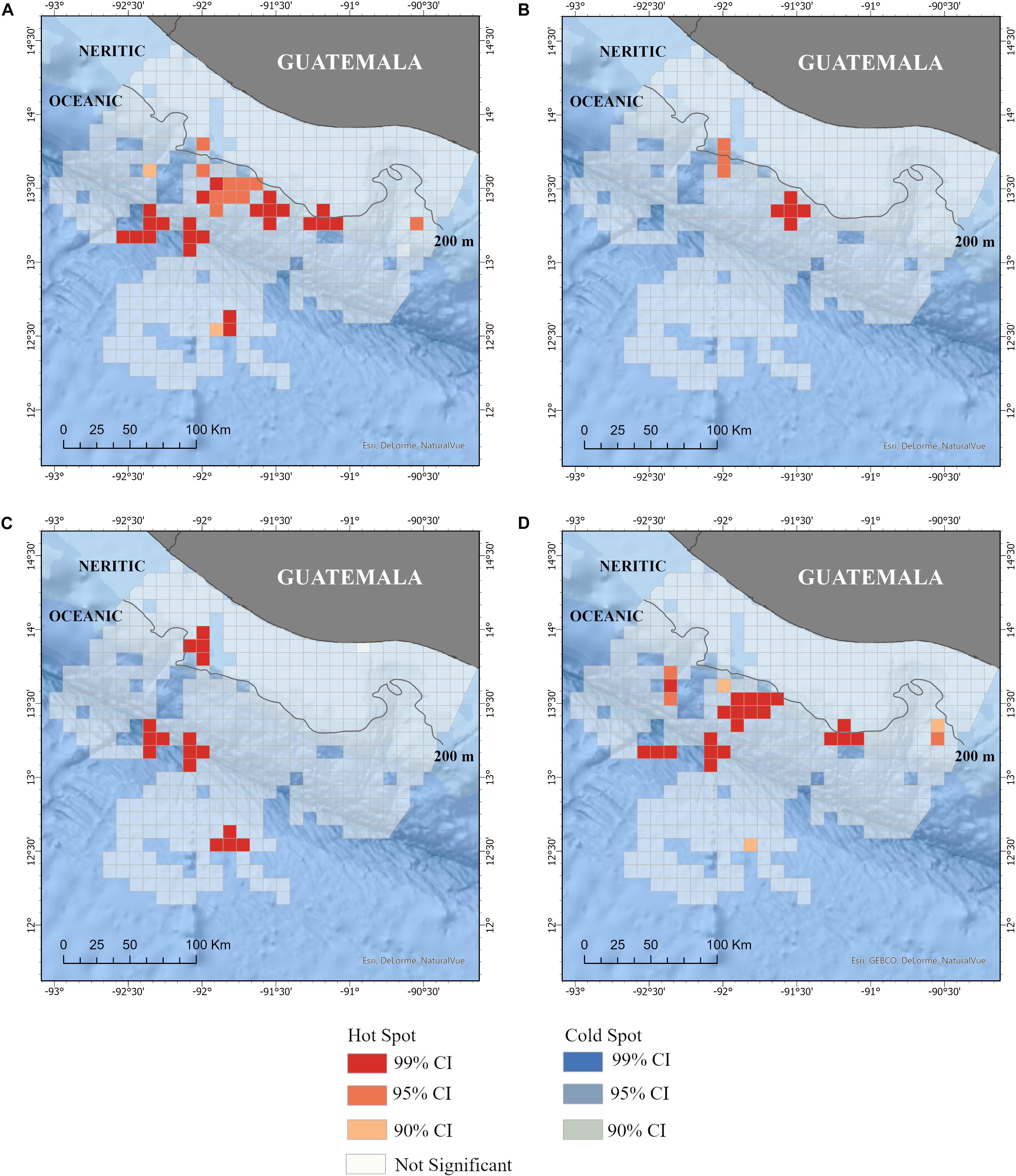
Figure 5. Hot spots of distribution of all cetacean species (A) and the three most common species: (B) Tursiops truncatus, (C) Stenella attenuata, and (D) Stenella longirostris in the Pacific waters of Guatemala. No cold spots were identified. Neritic (<200 m depth) and ocean (>200 m depth) zones are also included. Additional details of the study area are shown in Figure 1.
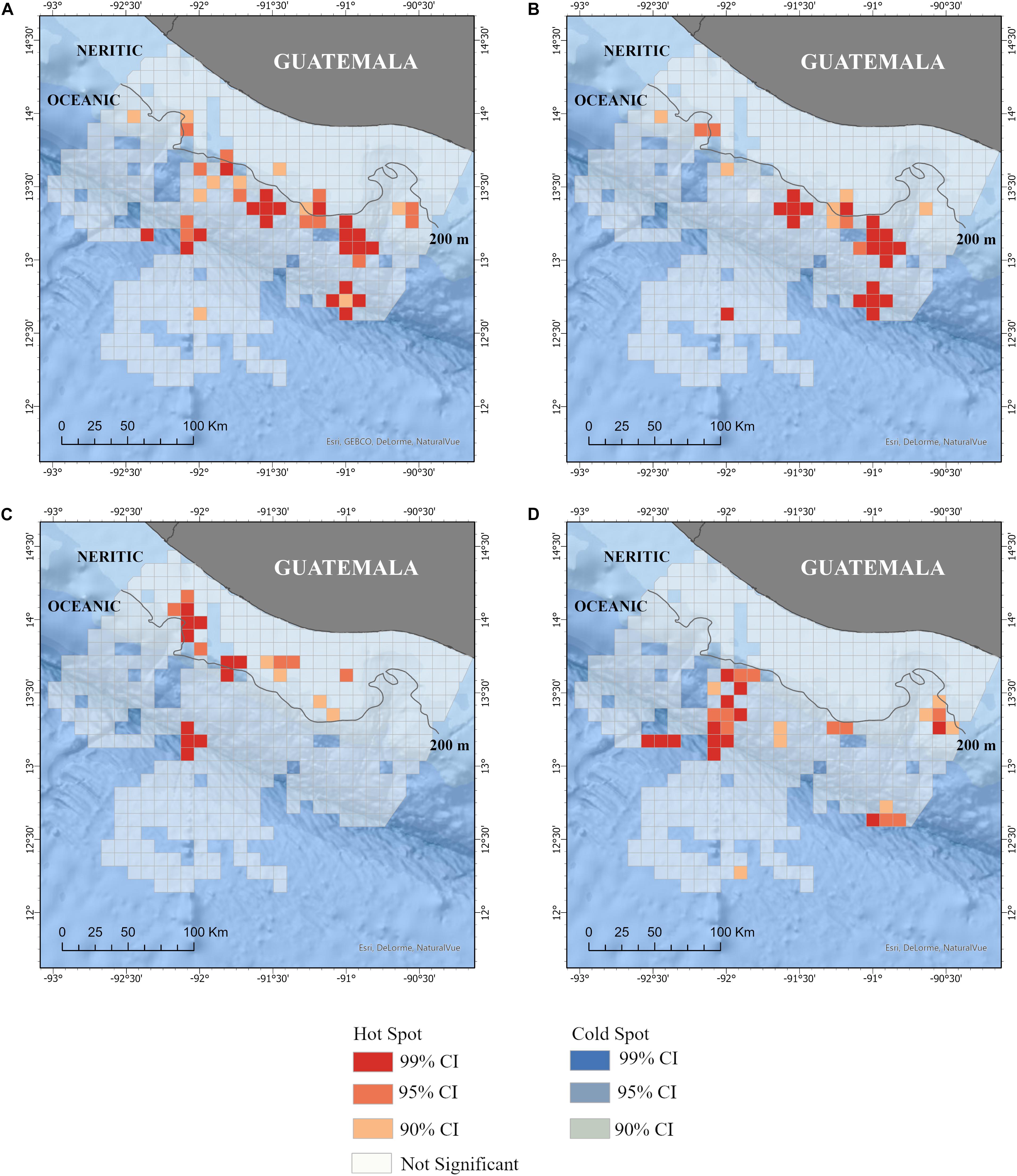
Figure 6. Hot spots of sightings of all cetacean species (A) and the three most common species: (B) Tursiops truncatus, (C) Stenella attenuata, and (D) Stenella longirostris in the Pacific waters of Guatemala. No cold spots were identified. Neritic (<200 m depth) and ocean (>200 m depth) zones are also included. Additional details of the study area are shown in Figure 1.
The mean IPUE of T. truncatus was calculated at 0.50 dolphin/km during the study. Total SPUE was 0.02 sightings/km (Table 3). No statistically significant difference was found in the IPUE and SPUE of this species between zones (Table 3). In the neritic zone, the sightings were concentrated on the eastern part of the zone, but in the oceanic zone they were more evenly distributed. Similar to the pattern observed in the “all cetacean species” category, the individual grid cells with sighting data of T. truncatus had a slightly higher abundance in the oceanic zone than in the neritic zone. However, when the overall area of the surveys was taken into account, the relative abundance of the IPUE and SPUE indexes in these two zones was not statistically different (Table 3). Mean SPUE was ≤0.03 sightings/km in both zones. Expectedly, IPUE showed more variation with a mean value of 0.29 dolphins/km in the neritic zone and 0.66 dolphins/km in the oceanic zone (Table 3). Based on the hot spot analysis, two IPUE clusters were identified: one small cluster extended from the neritic to the oceanic zone and a second large cluster in the oceanic zone. Most of the SPUE clusters were in the oceanic zone where six clusters were identified near the 200 m bathymetry line and the San José canyon (Figure 6B).
IPUE of S. attenuata was calculated at 0.004 dolphin/km and SPUE was determined to be 0.01 sightings/km (Table 3). IPUE and SPUE between zones were statistically different, but SPUE were higher in the neritic zone while IPUE were higher in the oceanic zone (Table 3). This means that a higher number of sightings/km of S. attenuata was recorded in the neritic zone while a higher number of dolphins/km was recorded in the oceanic zone (Table 3). A higher sighting density of S. attenuata was detected on the eastern side of the coast, which encompasses three of the MPAs. Based on the hot spot analysis, significant clusters of SPUE were identified in deeper waters, near the 200 m isobath (Figure 6C). In the oceanic zone, three IPUE clusters were identified, two of which were along the Middle America Trench and the other farther offshore at nearly 200 km from the coast (Figure 5C).
Stenella longirostris had a mean IPUE of 1.07 dolphins/km. Except for one sighting, all sightings of S. longirostris occurred within the oceanic zone where the mean IPUE was 1.62 dolphins/km and the mean SPUE was 0.004 sightings/km (Table 3). Three general areas were identified by the hot spot analyses for S. longirostris, the San José Canyon, the central region of the continental slope, and the central region of the Middle America Trench. The hot spots identified were consistent between IPUE and SPEU (Figures 5D, 6D).
Group size was variable among small cetacean species (Supplementary Figure 1), although general conclusions were difficult to make for species with a small number of observations (n < 5). In the case of the two least common species with greater than five sightings, the mean group size was 133 for D. delphis and 11 for G. griseus. Among the most three common species, group size was significantly different (Kruskal–Wallis test = 96.03, df = 2, p < 0.001). Group size was the largest in S. longirostris with a mean of 444 dolphins, followed by S. attenuata with a mean of 28 dolphins, and T. truncatus with a mean of 15 dolphins (S. longirostris vs. S. attenuata U = 637.50, p < 0.001; S. longirostris vs. T. truncatus U = 924.00, p < 0.001; S. attenuata vs. T. truncatus U = 12343.50, p < 0.001).
The percentage of groups with calves was also variable. In the case of the most common species, S. attenuata and S. longirostris had the highest proportion of groups with calves, which corresponded to at least 50% and 60% of the groups, respectively (Table 2). Groups with calves of S. attenuata were sighted in the two bathymetric zones. The percentage of groups of T. truncatus with calves was less than ≤20% regardless of zone type (Table 2). Groups with calves were also detected in D. delphis, G. griseus, O. orca, and P. crassidens. In the case of P. crassidens, groups with calves were only seen in the neritic zone, even though two groups were sighted overall in the oceanic zone (Table 2).
Group size varied between bathymetric zones (see Table 2). Depth did not always explain the variation in group size, although it accounted for a very small percentage of the variance in some cases. Groups were larger in the oceanic zone than in the neritic zone for S. attenuata and T. truncatus. In the case of S. attenuata, groups varied between 1 and 550 dolphins ( = 105) in the oceanic zone and between 1 and 300 dolphins ( = 20) in the neritic zone (Table 2). Further, there was a significant linear relationship, though weak positive relationship, between the logarithm of group size of S. attenuata and the logarithm of depth (F1,141 = 13.82, p < 0.001; R2 = 0.09). In the case of T. truncatus, groups varied between 1 and 225 dolphins in both zones, however, the mean group size was only 17 in the oceanic zone and 11 in the neritic zone (Table 2). For this species, a significant linear and weak positive relationship was also found between the logarithm of group size and the logarithm of depth (F1,276 = 4.88, p = 0.03; R2 = 0.02). A zone comparison was not possible for S. longirostris because all but one of the sightings occurred in the oceanic zone. The largest group of S. longirostris had 2,050 dolphins (Table 2). For this species, no significant linear relationship between the logarithm of group size and the logarithm of depth was identified (F1,33 = 1.14, p = 0.29).
Dolphin sightings occurred at a wide range of depths. Sighting depth was significantly different among the three most common species (Kruskal–Wallis test = 142.53, df = 2, p < 0.001). Sightings of S. longirostris and T. truncatus occurred in deeper waters than those of S. attenuata (S. longirostris vs. S. attenuata U = 260.00, p < 0.001; S. longirostris vs. T. truncatus U = 3666.50, p = 0.02; S. attenuata vs. T. truncatus U = 6838.50, p < 0.001). Mean depth of all the sightings of T. truncatus was 1,346 m (range = 5–6,031 m), of S. longirostris was 1,773.74 (range = 78–6,002 m), and of S. attenuata was 269 m (range = 18–5,988 m) (Figure 7).
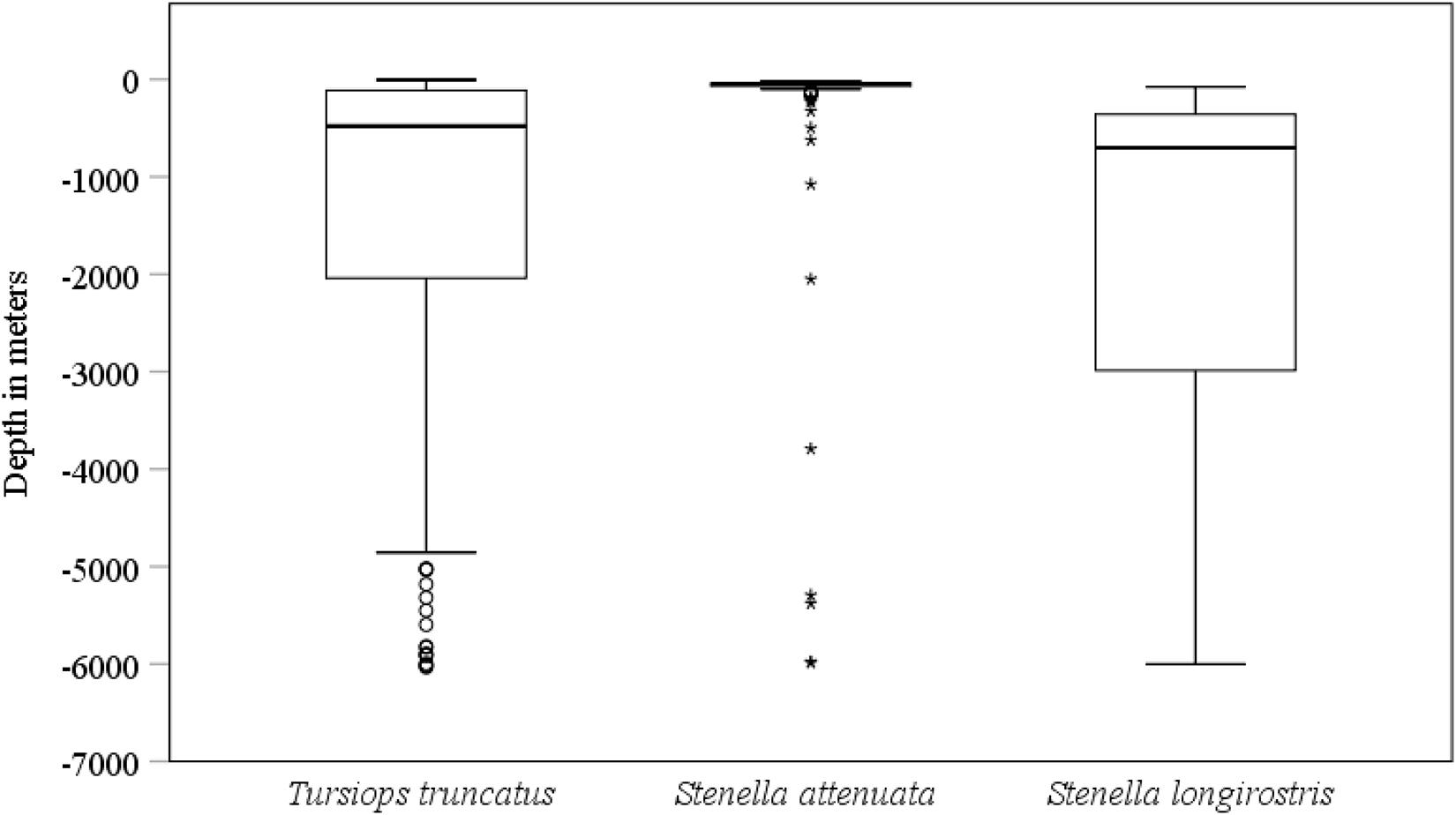
Figure 7. Central tendency and variability of group size of the most common species of small cetaceans identified in the Pacific waters of Guatemala. The solid line drawn across each box represents the median group size. The lower boundary is the 25th percentile and the upper boundary is the 75th percentile of a box. The lines on top and bottom of each box represent the largest and smallest group size, respectively, excluding outliers (o) and extreme values (*).
Nine species of small cetaceans all belonging to the Delphinidae family were identified during the study. Those species corresponded to nine out of 13 species of small cetaceans confirmed for the Guatemalan Pacific EEZ during the most extensive survey effort conducted by the National Oceanic and Atmospheric Administration, Southwest Fisheries Science Center surveys (NOAA-SWFSC; Quintana-Rizzo and Gerrodette, 2009). Differences between the two studies are related to differences in the number of pelagic species that were identified. The NOAA-SWFSC surveys covered the entire Guatemalan oceanic zone where those species are typically found, while this study sampled only about a third of the same zone; thus, reducing the probability of encountering pelagic species. Also, some species are gregarious by nature and might be more difficult to spot and identify (Sahri et al., 2020). The total number of small cetacean species identified in this study was smaller than that reported for the neighboring country of Mexico (15 species; Rosales-Nanduca et al., 2011), and other countries of the eastern tropical Pacific including Costa Rica (12 species; May-Collado et al., 2005) and Colombia (12 species; Palacios et al., 2012). Those studies included data collected by the NOAA-SWFSC surveys in their corresponding EEZ.
Tursiops truncatus was the most commonly sighted species in the entire study area as indicated by the high SPUE estimates. However, the species was not often sighted inside of 10 km along most of the coastline (Figure 2A). This was surprising considering that T. truncatus is usually a coastal species in many parts of the world (Würsig, 1978; Wells et al., 1980; Irvine et al., 1981; Quintana-Rizzo and Wells, 2001; Oviedo et al., 2005; Gamboa-Poveda and May-Collado, 2006) and it can typically be found within 1 km from shore in coastal communities (Wells et al., 1980, 1987; Wells, 2003), some of which are in the Pacific Ocean (Defran et al., 1999). In Guatemala, the behavior of T. truncatus suggests that more research is needed to understand its habitat use. Along the eastern half of the coast, the species displayed a characteristic avoidance behavior toward the artisanal fishing boats used for the surveys (EQR unpublished data). When the survey boat tried to approach the dolphins during a sighting, they typically fled the area at a high speed. Although it is natural for some dolphins to avoid being followed during a sighting, this repeated and widespread reaction might not be. The behavior could be a response to the uncontrolled dolphin-watching activities of the area, although those activities tend to use a different type of vessel and they do not occur in half of this area. This reactive behavior could also be a learned behavior related to previous experiences that dolphins have had with artisanal fishing vessels (Wells et al., 1980, 1987; Wells, 2003). Habitat differences including the lack of protected areas such as bays and rivers; prey availability and abundance, intraspecific competition, among others, could also play a role on its distribution. Quintana-Rizzo (2011a,b) documented that some fishermen in this region of the coast harpoon the species to use its meat as shark bait, but it is unclear how common the practice continues to be.
In addition to T. truncatus, S. attenuata and S. longirostris were the other most common species detected during this study. S. attenuata had the highest IPUE estimate in the neritic zone while S. longirostris had the highest IPUE estimate in the oceanic zone. S. attenuata and S. longirostris also had the highest proportion of groups with calves. Previous studies reported the same three species as common in Guatemala (Quintana-Rizzo and Gerrodette, 2009; Cabrera, 2011; Cabrera et al., 2014; Ortiz, 2019) and the Pacific coast of Central America (e.g., Costa Rica; Rodríguez and Rodríguez-Fonseca, 2004). Each of these three species showed a distinctive spatial distribution indicating that they have different habitat preferences. T. truncatus was found throughout most of the study area, whereas 90% of the sightings of S. attenuata were recorded in the neritic zone, and all but one sighting of S. longirostris were recorded in the oceanic zone.
The wide-ranging distribution of T. truncatus across zones overlapped with the distribution of S. attenuata in the neritic zone and S. longirostris in the oceanic zone. However, there was little overlap in the distribution of S. attenuata and S. longirostris in the study area. This latter pattern is inconsistent with the observations of Au and Perryman (1985), who stated that the Central American Bight, which includes the coastal waters from Guatemala to Ecuador, is the most important area of overlap for S. attenuata and S. longirostris. Their study was based on data collected during the NOAA-SWFSC surveys which, as previously mentioned, were more spatially comprehensive and oceanic-focused. They did not report any sightings in the neritic zone of Guatemala, but in the oceanic zone of the eastern tropical Pacific they reported the offshore forms of the two species (S. attenuata attenuata, S. longirostris orientalis), which use tropical surface waters with temperatures over 25°C and a shallow mixed layer, shoal, and sharp thermocline at 50 m. In the same area, it was reported that S. attenuata attenuata commonly occurred in large mixed groups with S. longirostris and yellowfin tuna (Thunnus albacares) (Au and Perryman, 1985; Perrin and Hohn, 1994; Ballance et al., 2006). On the other hand, in the present study, the most sighted form of S. attenuata was probably the coastal form, S. attenuata graffmani, since those sightings occurred in the neritic zone and this subspecies does not overlap with S. longirostris orientalis. S. attenuata graffmani occurs in a narrow coastal band along the Pacific coasts of southern Mexico to south Peru (Perrin, 2009).
Ecological factors play a role in determining group size (Gomez-Salazar et al., 2012; Markham et al., 2015; Casari and Tagliapietra, 2018; Moura et al., 2019). This was evident in the group size differences between zones. In the shallow waters of the neritic zone, large numbers of small groups of the common species were observed, while in the deep waters of the oceanic zone large groups of the same species were more frequently sighted. Group size differences between habitats are an ecological strategy to avoid predation and a foraging strategy to adapt to diverse prey items (Connor, 2000; Krause and Ruxton, 2002; Markham et al., 2015). Specifically, small offshore cetaceans tend to form large groups as a social mechanism to minimize the risk of predation by individual dolphins (dilution effect) and to deal with larger and more irregular patches of prey availability (Connor, 2000; Bearzi, 2005; Silva et al., 2008). For example, S. longirostris longirostris forms large groups that prey on unpredictable mesopelagic fishes and squids in the eastern and western Pacific (Perrin and Gilpatrick, 1994; Dolar et al., 2003). In contrast, coastal cetacean species tend to form smaller groups as the predation risk is comparatively small and prey resources are generally more predictable (Moors-Murphy, 2014; Acevedo-Gutiérrez, 2018).
In the neritic zone of Guatemala, S. attenuata was the most commonly detected species. The mean group size (20 ± 3 dolphins) was comparable to that of the neritic zones of the Pacific near Costa Rica (mean = 10, range = 1–50 dolphins; May-Collado and Forcada, 2012) and Panama (mean = 23, range = 1–50 dolphins; Garcia and Dawson, 2003). Oceanic groups were slightly smaller in Guatemala (105 dolphins) than in the rest of eastern tropical Pacific (mean = 120; Ferguson et al., 2006) but larger than in the Philippines (mean = 84, range = 1–540 dolphins; Dolar et al., 2006).
Other habitat differences included the number of hot spots. Less than five SPUE and IPUE hot spots were identified for all species combined in the neritic zone, and no hot spots were identified near or within the MPAs. The low number could be because the analysis identifies areas of high spatial clustering, which were uncommon in this zone. In the neritic zone, several hot spots (≥10) were identified, but the results need to be taken with caution given the small sample size. However, some general patterns were observed. The hot spots were located along the 200 m isobath (near the continental shelf edge), the Middle America trench, and the San José Canyon. These are likely areas of high productivity where dolphins concentrate to feed. The offshore waters of Guatemala are characterized by seasonal eddies that act as retention mechanisms for planktonic organisms, which serve as food sources for first-order consumers and consequently generate food for higher trophic predators (Ehrhardt and Fitchett, 2006; Acosta-Pachón et al., 2017). The accumulation of members of the pelagic food web near the continental shelf edge and over the shelf break is a recognized phenomenon throughout the world ocean. Marine mammals, birds (Certain et al., 2007), fish (e.g., Uriarte and Lucio, 2001), and phytoplankton (Lampert et al., 2002) benefit from inorganic nutrients supplied by physical forcing (New and Pingree, 1990). Submarine canyons, such as the San José Canyon, serve as conduits for the transport of deep, nutrient-rich waters to the continental shelf waters of coastal ecosystems (Fernandez-Arcaya et al., 2017; Santora et al., 2018). Canyons support high levels of biodiversity and serve as feeding grounds for many species (De Leo et al., 2014; Moors-Murphy, 2014; Santora et al., 2018). In the San José Canyon, swordfish (Carey and Robinson, 1981; Carey, 1983) and hundreds of sea turtles (Brittain, 2016) have been sighted, and several hot spots of S. longirostris and T. truncatus were identified in this study.
Five species of small cetaceans (S. attenuata, T. truncatus, P. crassidens, G. griseus, and Steno bredanensis) were sighted to the north of the San José Canyon, in a stretch of approximately 30 km of the neritic zone that includes three of the MPAs (Monterrico, Hawaii, and Las Lisas) and waters off Puerto (port) Quetzal (Figure 1). S. attenuata was particularly common in this section as indicated by IPUE and SPUE estimates. Additionally, some of these dolphins exhibited a degree of residency to the area (Quintana-Rizzo, 2011b); thus, suggesting that the habitat provided resources needed for their survival. No other section of the Guatemalan neritic zone had such high diversity or such a continuous presence of small cetaceans.
In the Puerto Quetzal-Monterrico-Las Lisas section, the all species IPUE estimate varied from 0.1 to 1 dolphin/km whereas the SPUE estimate was more consistent at 0.05 sightings/km. Other sections of the coast had either zero sightings or sightings of either T. truncatus or S. attenuata. The frequent presence of small cetaceans in the Puerto Quetzal-Monterrico-Las Lisas section could be due to a “spillover effect” from the San José canyon, where currents bring nutrient-rich waters to the nearby coastal areas; thus, attracting several cetacean species. It could also be an effect of sampling effort but a similar pattern has been observed in recent surveys (EQR unpublished data).
Our results suggest that the protection of small cetaceans needs to consider the creation of oceanic MPAs. Those areas should be integrated into the current network of MPAs to ensure habitat connectivity because protected areas in coastal habitats alone might provide little safety to highly mobile cetaceans (Dinis et al., 2016). A highly mobile species is O. orca, and a group of this species that was sighted in Guatemala was confirmed through photographic identification to have been seen in Cabo Corriente, Mexico, 11 years earlier (Cabrera et al., 2012). This shows that some species move across hundreds of miles in the eastern tropical Pacific and MPAs can serve as a tool to provide connectivity among habitats. Other offshore species deserve close attention because millions of dolphins died since the 1960’s as bycatch in tuna nets in the eastern tropical Pacific Ocean (Gerrodette and Forcada, 2005; Wade et al., 2007). The management of oceanic MPAs could be challenging due to the financial burden required to patrol offshore sites and/or maintain the proper law enforcement presence in a vast area. It is already difficult for the country to manage its local coastal marine resources, which in theory are easier to protect due to their proximity to shore. Still, there are important international tools that could be used such as the Convention on the Conservation of Migratory Species of Wild Animals and the United Nations Convention on the Law of the Sea for increased offshore habitat protection (Hoyt, 2011).
Other conservation actions are greatly needed for the protection of small cetaceans. Dolphin-watching activities need to be managed, especially as they are becoming more popular along the Puerto Quetzal-Monterrico-Las Lisas section. The guidelines for those activities have been effective for some years but their control is non-existent. This is worrisome because the local activities seem to affect the behavior of large cetaceans such as humpback whales (Quintana-Rizzo, 2019), and it is unclear how they could impact small cetaceans. Research is needed to study their potential effects on dolphins. Additionally, the bycatch of small cetaceans in commercial fishing operations needs to be investigated. This is a common threat in Latin America (Van Waerebeek and Reyes, 1994; Palacios and Gerrodette, 1996; Reeves et al., 2003; Ávila et al., 2008) and other parts of the world (Palacios and Gerrodette, 1996). Further, boat collisions and the effects of commercial shipping need to be examined. Puerto Quetzal is one of the main commercial ports of Guatemala and due to its proximity to the MPAs, ship traffic, noise, and pollution near the port need to be evaluated and proper management and conservation measurements need to be established. This port moved nearly a quarter of a million cargo tons in 2018 (UN-ECLAC, 2018), and is one of the top 30 busiest ports of 118 ports in Latin America and the Caribbean according to the United Nations Economic Commission for Latin American and the Caribbean (UN-ECLAC, 2018).
Conservation is a complex task. It requires an integrated ecosystem approach to be successful, and our research has shown that oceanographic features, and not the political boundaries, are most likely to affect the distribution of small cetaceans. Conservation management should also focus on protecting wildlife habitat linked to important activities such as feeding, resting, breeding, and caring for young (Smith et al., 2016). They should also take into consideration the presence of different subspecies since they will probably require different measures of management (e.g., S. attenuata: coastal subspecies forms small groups in the neritic zone, and the offshore subspecies forms groups of hundreds of individuals in the oceanic zone). Biological, ecological, and oceanographic information should be used to identify MPAs that reflect the needs of mobile marine species in order to be relevant to those species that they intend to protect.
The full raw datasets used for this study are available on request to the corresponding author. Data collected by AAC, J-OW, and VD are available as Supplementary Table 1.
During this study the following research permits were obtained: Guatemalan Government National Council for Protected Areas (CONAP) 053/2009 and 007/2011.
EQ-R conceived the original idea for this manuscript. EQ-R, AAC, JO-W, and VD expanded and agreed on the details of the publication. EQ-R (lead), VD, and AAC analyzed the data. JO-W reviewed the format of the bibliography. EQ-R led the writing with contributions from all authors who discussed the results and commented on the manuscript. All authors conducted fieldwork and collected data.
This research was published with the financial support of the Wildlife Conservation Society (WCS) Marine Program and its Marine Protected Areas (MPA) Project, funds from the University of Florida to prof. José Miguel Ponciano, Project #00099235, and the University of Florida, Tropical Conservation and Development Program, Center for Latin American Studies. Surveys conducted by EQ-R were made possible with funding support of various institutions including Fondo Nacional de Ciencia Tecnología, awarded by the Consejo Nacional de Ciencia y Tecnología, through the Secretaria Nacional de Ciencia y Tecnología (Fodecyt 85-2007 Project), Cetacean Society International, Sarasota Dolphin Research Institute; PADI Foundation; Idea Wild, and Defensores de la Naturaleza Foundation. Surveys conducted by AAC and JO-W were made possible with funding support of various institutions including Fondo Nacional para la Conservación de la Naturaleza (FONACON) (F10/2008/A and F16/2010 FONACON Projects), Dirección General de Investigación (DIGI), Universidad de San Carlos de Guatemala (USAC) (4.8.63.7.08 DIGI Project), Cetacean Society International, and Western Hemisphere Migratory Species Initiative (WHMSI).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank Raquel Sigüenza for facilitating information and data related to the marine protected areas in the Pacific coast; and Roan McNab, Christian Barrientos, José Moreira, José Miguel Ponciano Castellanos, Bette Loiselle, Alejandro Arrivillaga, and Milton Cabrera for their support in the publication of this work. Their contributions were invaluable and are greatly appreciated. This work was possible with an army of volunteers and field assistants. EQ-R would like to acknowledge several key people including L. Girón, G. Méndez, and J. Morales, and the enthusiasm and support from P. Negreros, V. García, L. Palmieri, S. Rosales, and O. Zamora. AAC and JO-W would like to thank all the volunteers and people that made the study and fieldwork possible, J. Romero, J. Yee, R. Jimenez, J. Conde, J. del Cid, and F. Herrera. They would also like to thank the valuable support of Comando Naval del Pacifico de Guatemala (CONAPAC) for providing the boats and crew for the surveys. VD would like to thank the following projects, institutions, and people: FODECYT 106-2006 Aves Pelágicas de la Costa Pacífica de la República de Guatemala; Escuela de Biología, Universidad de San Carlos de Guatemala; AGROCYT 033-2006 Seguimiento Oceanográfico al Fenómeno del Niño y su impacto en los rendimientos de las pesquerías del Pacífico de Guatemala, Centro de Estudios del Mar y Acuicultura, Universidad de San Carlos de Guatemala; and specially to P. Velásquez, M. Ixquiac, R. Rodas, C. Tejeda, and A. López. Two reviewers provided useful comments to improve the manuscript. This work is dedicated to the conservation efforts of marine megafauna species in Guatemala.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.674134/full#supplementary-material
Acevedo-Gutiérrez, A. (2018). “Group behavior,” in Encyclopedia of Marine Mammals, eds B. Wursig, J. G. M. Thewissen, and K. M. Kovacs (California: Elsevier Academic Press), 428–435.
Acosta-Pachón, T. A., Martínez-Rincón, R. O., and Hinton, M. G. (2017). Habitat preferences of striped marlin (Kajikia audax) in the eastern Pacific Ocean. Fish. Oceanogr. 26, 615–624. doi: 10.1111/fog.12220
Alfaro-Shigueto, J., Mangel, J. C., and Van Waerebeek, K. (2008). Small cetacean captures and CPUE estimates in artisanal fisheries operating from a port in northern Perú, 2005-2007 SC/60/SM19. Int. Whal. Comm. Sci. Meet. 60, 1–13.
Au, D. W. K., and Perryman, W. L. (1985). Dolphin habitats in the eastern tropical Pacific. Fish. Bull. 83, 623–643.
Ávila, I. C., Garca, C., and Bastidas, J. C. (2008). A note on the use of dolphinsas bait in the artisanal fisheries off Baha Solano, Choc, Colombia. J. Cetacean Res. Manag. 10, 179–182.
Azzellino, A., Panigada, S., Lanfredi, C., Zanardelli, M., Airoldi, S., and Notarbartolo, G. (2012). Predictive habitat models for managing marine areas: Spatial and temporal distribution of marine mammals within the Pelagos Sanctuary (Northwestern Mediterranean Sea). Ocean Coast Manag. 67, 63–74. doi: 10.1016/j.ocecoaman.2012.05.024
Ballance, L. T., Pitman, R. L., and Fiedler, P. C. (2006). Oceanographic influences on seabirds and cetaceans of the eastern tropical Pacific: a review. Prog. Oceanogr. 69, 360–390. doi: 10.1016/j.pocean.2006.03.013
Baumgartner, M. F. (2006). The distribution of Risso’s dolphin (Grampus griseus) with respect to the physiography of the northern Gulf of Mexico. Mar. Mamm. Sci. 13, 614–639. doi: 10.1111/j.1748-7692.1997.tb00087.x
Bearzi, M. (2005). Aspects of the ecology and behaviour of bottlenose dolphins (Tursiops truncatus) in Santa Monica Bay, California. J. Cetacean Res. Manag. 7, 75–83.
Brittain, R. (2016). Assessing Interactions Between Marine Megavertebrates and Small-Scale Fisheries on the Pacific Coast of Guatemala. Master’s thesis. London: University of Exeter.
Cabrera, A. (2011). Distribución y Selección De Hábitat De Cetáceos En El Pacífico Este De Guatemala. Licenciatura thesis. Guatemala: Universidad de San Carlos de Guatemala.
Cabrera, A., and Ortíz, J. (2010). Cetáceos de la Costa Pacífica de Guatemala. Parte I: Pacífico Este. Documento Técnico 75 (1-2010). Guatemala: Consejo Nacional de Áreas Protegidas, CONAP.
Cabrera, A., and Ortíz, J. (2012). Distribución espaciotemporal y patrones de comportamiento del delfín manchado (Stenella attenuata) y el delfín tornillo (Stenella longirostris) en el Pacífico centro-este de Guatemala. Ciencia Conservación 3, 5–14.
Cabrera, A., Ortíz, J., and Romero, J. (2012). Cetáceos de la Costa Pacífica de Guatemala. Parte II: Pacífico central. Documento Técnico 102 (1-2012). Guatemala: Consejo Nacional de Áreas Protegidas.
Cabrera, A., Ortíz-Wolford, J. S., Corona-Figueroa, M. F., and Gudiel-Corona, V. M. (2014). Cetáceos del Pacífico de Guatemala: Cincuenta años de historia. Ciencia Tecnol. Salud 1, 51–63.
Campbell, E., Pasara-Polack, A., Mangel, J. C., and Alfaro-Shigueto, J. (2020). Use of small cetaceans as bait in small-scale fisheries in Peru. Front. Mar. Sci. 7:534507. doi: 10.3389/fmars.2020.534507
Cañadas, A., Sagarminaga, R., and García-Tiscar, S. (2002). Cetacean distribution related with depth and slope in the Mediterranean waters off southern Spain. Deep Sea Res. Part I Oceanogr. Res. Pap. 49, 2053–2073. doi: 10.1016/S0967-0637(02)00123-1
Carey, F., and Robinson, B. (1981). Daily patterns in the activities of swordfish, Xiphias gladius, observed by acoustic telemetry. Fish. Bull. 79, 277–292.
Carey, F. G. (1983). “Experiments with free-swimming fish,” in Oceanography, ed. P. G. Brewer (New York, NY: Springer), 57–68. doi: 10.1007/978-1-4612-5440-9_4
Casari, M., and Tagliapietra, C. (2018). Group size in social-ecological systems. Proc. Natl. Acad. Sci. U.S.A. 115, 2728–2733. doi: 10.1073/pnas.1713496115
Certain, G., Bellier, E., Planque, B., and Bretagnolle, V. (2007). Characterizing the temporal variability of the spatial distribution of animals: an application to seabirds at sea. Ecography 30, 695–708. doi: 10.1111/j.2007.0906-7590.05197.x
Chivers, S. J., Baird, R. W., McSweeney, D. J., Webster, D. L., Hedrick, N. M., and Salinas, J. C. (2007). Genetic variation and evidence for population structure in the eastern North Pacific false killer whales (Pseudorca crassidens). Can. J. Zool. 85, 783–794. doi: 10.1139/z07-059
CONAP (2013). Política nacional de Diversidad Biológica (Acuerdo Gubernativo 220 -2011) Estrategia nacional de Diversidad Biológica y Su Plan de Acción (Resolución 01-16-2012) La Década de la Vida y el Desarrollo. Políticas, Programas y Proyectos No. 03(01-2013). Guatemala: CONAP.
CONAP and MARN (2009). Biodiversidad Marina de Guatemala: Análisis de Vacíos y Estrategias Para Su Conservación. Documento Técnico 69 (02-2009). Guatemala: Consejo Nacional de Áreas Protegidas, Ministerio de Ambiente y Recursos Naturales, The Nature Conservancy.
Connor, R. C. (2000). “Group living in whales and dolphins,” in Cetacean Societies: Field Studies of Dolphins and Whales, eds J. Mann, R. C. Connor, P. L. Tyack, and H. Whitehead (Chicago: The University of Chicago Press), 199–218.
Cubero-Pardo, P. (2007). Environmental factors governing the distribution of the bottlenose (Tursiops truncatus) and the spotted dolphin (Stenella attenuata) in Golfo Dulce, South Pacific, off Costa Rica. Invest. Mar. 35, 15–23.
Culik, B. M. (2004). Review of Small Cetaceans: Distribution, Behavior, Migration and Threats. Marine Mammal Action Plan. Regional Seas Reports and Studies No. 177. Germany: Convention on the Conservation of Migratory Species of Wild Animals.
Dávila, V. (2011). Diversidad y Abundancia de la Megafauna Pelágica (Ballenas, Delfines, Tortugas Marinas, Peces Pico y Rayas) Presente en el Pacífico de Guatemala. Licenciatura thesis. Guatemala: Universidad de San Carlos de Guatemala.
De Leo, F. C., Vetter, E. W., Smith, C. R., Rowden, A. A., and McGranaghan, M. (2014). Spatial scale-dependent habitat heterogeneity influences submarine canyon macrofaunal abundance and diversity off the Main and Northwest Hawaiian Islands. Deep Sea Res. Part II Top. Stud. Oceanogr. 104, 267–290. doi: 10.1016/j.dsr2.2013.06.015
Defran, R. H., Weller, D. W., Kelly, D. L., and Espinosa, M. A. (1999). Range characteristics of pacific coast bottlenose dolphins (Tursiops truncatus) in the southern California bight. Mar. Mamm. Sci. 15, 381–393. doi: 10.1111/j.1748-7692.1999.tb00808.x
Dinis, A., Carvalho, A., Alves, F., Nicolau, C., Ribeiro, C., Kaufmann, M., et al. (2016). Spatial and temporal distribution of bottlenose dolphins, Tursiops truncatus, in the Madeira archipelago, NE Atlantic. Arqu. Life Mar. Sci. 33, 45–54.
DIPESCA/MAGA/PNUD/TNC (2018). Cartografía de la Caracterización Biológica y Oceanográfica del Pacfico de Guatemala. Guatemala: Proyecto Conservación y uso Sostenible de la Biodiversidad en Áreas Protegidas Marino-Costeras (APM) (MARN-CONAP/PNUD-GEF).
Dizon, A. E., Perrin, W. F., and Akin, P. A. (1994). Stocks of Dolphins (Stenella spp. and Delphinus delphis) in the Eastern Tropical Pacific: a Phylogeographic Classification. NOAA Technical Memorandum-NMFS 119. Settle, WA: Scientific Publications Office, National Marine Fisheries Service, National Oceanic and Atmospheric Administration.
Dolar, M. L. L., Perrin, W. F., Taylor, B. L., Kooyman, G. L., and Alava, M. N. R. (2006). Abundance and distributional ecology of cetaceans in the central Philippines. J. Cetacean Res. Manage. 8, 93–111.
Dolar, M. L. L., Walker, W. A., Kooyman, G. L., and Perrin, W. F. (2003). Comparative feeding ecology of spinner dolphins (Stenella longirostris) and Fraser’s dolphins (Lagenodelphis hosei) in the Sulu Sea. Mar. Mamm. Sci. 19, 1–19. doi: 10.1111/j.1748-7692.2003.tb01089.x
Ehrhardt, N. M., and Fitchett, M. D. (2006). On the seasonal dynamic characteristics of the sailfish, Istiophorus platypterus, in the eastern Pacific Off Central America. Bull. Mar. Sci. 79, 589–606.
Escorza-Treviño, S., Archer, F. I., Rosales, M., Lang, A., and Dizon, A. E. (2005). Genetic differentiation and intraspecific structure of eastern tropical Pacific spotted dolphins, Stenella attenuata, revealed by DNA analyses. Conserv. Genet. 6, 587–600. doi: 10.1007/s10592-005-9013-9
Ferguson, M. C., Barlow, J., Fiedler, P., Reilly, S. B., and Gerrodette, T. (2006). Spatial models of delphinid (family Delphinidae) encounter rate and group size in the eastern tropical Pacific Ocean. Ecol. Model. 193, 645–662. doi: 10.1016/j.ecolmodel.2005.10.034
Fernandez-Arcaya, U., Ramirez-Llodra, E., Aguzzi, J., Allcock, A. L., Davies, J. S., Awantha, D., et al. (2017). Ecological role of submarine canyons and need for canyon conservation: a review. Front. Mar. Sci. 4, 1–26. doi: 10.3389/fmars.2017.00005
Fiedler, P. C., and Talley, L. D. (2006). Hydrography of the eastern tropical Pacific: a review. Prog. in Oceanogr. 69, 143–180. doi: 10.1016/j.pocean.2006.03.008
Fisher, R. L. (1961). Middle America Trench: topography and structure. GSA Bull. 72, 703–719. doi: 10.1130/0016-7606(1961)72[703:mattas]2.0.co;2
Gamboa-Poveda, M., and May-Collado, L. J. (2006). Insights on the occurrence, residency, and behavior of two coastal dolphins from Gandoca-Manzanillo, Costa Rica: Sotalia guianensis and Tursiops truncatus. Cambridge: The International Whaling Commission, 1–9.
Garcia, C., and Dawson, S. M. (2003). Distribution of pantropical spotted dolphins in Pacific coastal waters of Panama. LAJAM 2, 29–38. doi: 10.5597/lajam00028
Gerrodette, T., and Forcada, J. (2005). Non-recovery of two spotted and spinner dolphin populations in the eastern tropical Pacific Ocean. Mar. Ecol. Prog. Ser. 291, 1–21. doi: 10.3354/meps291001
Gerrodette, T., Watters, G., Perryman, W., and Balance, L. (2008). Estimates of 2006 Dolphin Abundance in the Eastern Tropical Pacific, With revised estimates from 1986-2003. NOAA Technical Memorandum-NMFS-SWFSC 422. La Jolla, CA: U.S. Department of Comerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service.
Getis, A., and Ord, J. K. (1992). The analysis of spatial association by use of distance statistics. Geog. Anal. 24, 189–206. doi: 10.1111/j.1538-4632.1992.tb00261.x
Godoy Aguilar, P., Aguilar Calderón, C., Urban, J., and Calambokidis, J. (2009). “Research on humpback whales (Megaptera novaeangliae) in Guatemala,” in Proceeding of the 18th Biennial Marine Mammal Conference, Quebec, Canada.
Gómez de Segura, A., Hammond, P. S., and Raga, J. A. (2008). Influence of environmental factors on small cetacean distribution in the Spanish Mediterranean. J. Mar. Biolog. Assoc. U.K. 88, 1185–1192. doi: 10.1017/S0025315408000386
Gomez-Salazar, C., Trujillo, F., and Whitehead, H. (2012). Ecological factors influencing group sizes of river dolphins (Inia geoffrensis and Sotalia fluviatilis). Mar. Mam. Sci. 28, 1–19. doi: 10.1111/j.1748-7692.2011.00496.x
Goodall, R. N. P., Galleazzi, A. R., and Ladiana, S. J. (1988). Exploitation of small cetaceans off Argentina 1979–1986. Rep. Int. Whaling Comm. 38, 7–410.
Hoyt, E. (2011). Marine Protected Areas for Whales, Dolphins and Porpoises: A World Handbook for Cetacean Habitat Conservation and Planning. London: Earthscan.
Hyrenbach, D., Forney, K., and Dayton, P. (2000). Marine protected areas and ocean basin management. Aquat. Conserv. 10, 437–458. doi: 10.1002/1099-0755(200011/12)10:6<437::AID-AQC425<3.0.CO;2-Q
Irvine, A. B., Scott, M. D., Wells, R. S., and Kaufmann, J. H. (1981). Movements and activities of the Atlantic bottlenose dolphin, Tursiops truncatus, near Sarasota, Florida. Fish. Bull. 79, 671–688.
Ladd, J., and Schroder, S. (1985). Seismic Stratigraphy of the Continental Shelf Offshore Guatemala: Implications for Vertical Tectonics Related to Subduction. New York, NY: Lamont-Doherty Geological Observatory of Columbia University, doi: 10.2973/DSDP.PROC.84.140.1985
Lampert, L., Quéguiner, B., Labasque, L., Pichon, A., and Lebreton, N. (2002). Spatial variability of phytoplankton composition and biomass on the eastern continental shelf of the Bay of Biscay (north-east Atlantic Ocean). Evidence of a bloom of Emiliania huxleyi (Prymnesiophyceae) in spring 1998. Cont. Shelf Res. 22, 1225–1247. doi: 10.1016/S0278-4343(01)00103-0
Loch, C., Marmontel, M., and Simões-Lopes, P. C. (2009). Conflicts with fisheries and intentional killing of freshwater dolphins (Cetacea: Odontoceti) in the Western Brazilian Amazon. Biodivers. Conserv. 18, 3979–3988. doi: 10.1007/s10531-009-9693-4
Mangel, J. C., Alfaro-Shigueto, J., Van Waerebeek, K., Cáceres, C., Bearhop, S., Witt, M. J., et al. (2010). Small cetacean captures in Peruvian artisanal fisheries: high despite protective legislation. Biol. Conserv. 143, 136–143. doi: 10.1016/j.biocon.2009.09.017
Markham, A. C., Gesquiere, L. R., Alberts, S. C., and Altmann, J. (2015). Optimal group size in a highly social mammal. Proc. Natl. Acad. Sci. U.S.A. 112, 14882–14887. doi: 10.1073/pnas.1517794112
Maxwell, S., Ban, N., and Morgan, L. (2014). Pragmatic approaches for effective management of pelagic marine protected areas. Endanger. Species Res. 26, 59–74. doi: 10.3354/esr00617
May-Collado, L., Gerrodette, T., Calambokidis, J., Rasmussen, K., and Sereg, I. (2005). Patterns of cetacean sighting distribution in the pacific exclusive economic zone of costa rica based on data collected from 1979-2001. Rev. Biol. Trop. 53, 249–263.
May-Collado, L. J., and Forcada, J. (2012). Small-scale estimation of relative abundance for the coastal spotted dolphins (Stenella attenuata) in Costa Rica: the effect of habitat and seasonality. Rev. Biol. Trop. 60, 133–142. doi: 10.15517/rbt.v60i2.19997
McMillen, K. J., Enkeboll, R. H., Moore, J. C., Shipley, K. H., and Ladd, J. W. (1982). Sedimentation in different tectonic environments of the middle America Trench, Southern Mexico and Guatemala. Geol. Soc. London Special Publi. 10, 107–119. doi: 10.1144/gsl.Sp.1982.010.01.07
Mel, A., and Fisher, S. (2016). The utilization of aquatic bushmeat from small cetaceans and manatees in South America and West Africa. Front. Mar. Sci. 3:163. doi: 10.3389/fmars.2016.00163
Mintzer, V. J., Diniz, K., and Frazer, T. K. (2018). The Use of aquatic mammals for bait in global fisheries. Front. Mar. Sci. 5, 1–26. doi: 10.3389/fmars.2018.00191
Moors-Murphy, H. B. (2014). Submarine canyons as important habitat for cetaceans, with special reference to the Gully: a review. Deep Sea Res. II Top. Stud. Oceanogr. 104, 6–19. doi: 10.1016/j.dsr2.2013.12.016
Moura, J. F., Pivari, D., and Pagliani, B. (2019). Environmental factors related to group size and habitat use of Guiana dolphins from São Marcos Bay, Amazon coast. Trop. Ecol. 60, 426–432. doi: 10.1007/s42965-019-00041-0
New, A. L., and Pingree, R. D. (1990). Evidence for internal tidal mixing near the shelf break in the Bay of Biscay. Deep Sea Res. 37, 1783–1803. doi: 10.1016/0198-0149(90)90078-a
Ortiz, J. (2011). Comportamientos de Socialización y Alimentación de Tres Especies de Delfines (Tursiops truncatus, Stenella longirostris y Delphinus delphis) en el Cañón de San José y la Fosa Centroamericana - Pacífico Este de Guatemala. Licenciatura thesis. Guatemala: Universidad de San Carlos de Guatemala.
Ortiz, J. (2019). “Familia delphinidae en guatemala: avances en el conocimiento sobre su distribución y comportamiento,” in en Perspectivas de Investigación Sobre Mamíferos Silvestres de Guatemala, eds C. Kraker, A. P. Calderón, and A. A. Cabrera (Guatemala: Asociación Guatemalteca de Mastozoólogos), 204–249.
Ott, R. L. (1994). An Introduction to Statistical Methods and Data Analysis. Duxbury: Press, Wadsworth Publishing Company.
Oviedo, L., Silva, N., Bermudez, L., and Odell, D. (2005). Distribution of bottlenose bolphins (Tursiops truncatus) on the east coast of isla margarita and the los frailes archipelago, Venezuela. Aquat. Mamm. 31, 442–446. doi: 10.1578/AM.31.4.2005.442
Palacios, D. M., Baumgartner, M. F., Laidre, K. L., and Gregr, E. J. (2013). Beyond correlation: integration environmentally and behaviourally mediated processes in models of marine mammal distributions. Endang. Species Res. 22, 191–203. doi: 10.3354/esr00558
Palacios, D. M., Herrera, J. C., Gerrodette, T., Garcia, C., Soler, G. A., Avila, S. C., et al. (2012). Cetacean distribution and relative abundance in Colombia’s Pacific EEZ from survey cruises and platforms of opportunity. J. Cetacean Res. Manag. 12, 45–60.
Palacios, D. T., and Gerrodette, T. (1996). Potential Impact of Gillnet Fisheries on Small Cetacean Populations in the Eastern Tropical Pacific. Administrative Report LJ-96-11. Southwest Fisheries Science Center.
Perrin, W. F. (2009). “Pantropical spotted dolphins,” in Encyclopedia of Marine Mammals, eds W. Perrin, B. Wursig, and J. G. M. Thewissen (Elsevier), 819–821.
Perrin, W. F., and Gilpatrick, J. W. Jr. (1994). “Spinner dolphin Stenella longirostris (Gray, 1828),” in Handbook of Marine Mammals, eds S. H. Ridgway and R. Harrison (San Diego: Academic Press), 99–128.
Perrin, W. F., and Hohn, A. A. (1994). “Spotted dolphin Stenella attenuata,” in Handbook of Marine Mammals, eds S. H. Ridgway and R. Harrison (San Diego, CA: Academic Press), 71–98.
PNUD. (2018). Aportes del Proyecto Conservación y uso Sostenible de la Biodiversidad en Áreas Protegidas Marino-Costeras (APMs). Guatemala: Programa de las Naciones Unidas para el Desarrollo (PNUD), Ministerio de Ambiente y Recursos Naturales (MARN).
Prideaux, M. (2003). Beyond the State: Building Regimes for Species Protection in all Oceans. Adelaide, SA: Hawke Research Institute, University of South Australia, Australia. doi: 10.13140/RG.2.1.3633.2569
Queiroz, N., Humphries, N. E., Mucientes, G., Hammerschlag, N., Lima, F. P., Scales, K. L., et al. (2016). Ocean−wide tracking of pelagic sharks reveals extent of overlap with longline fishing hotspots. Proc. Natl. Acad. Sci. U.S.A. 113, 1582–1587. doi: 10.1073/pnas.1510090113
Quintana-Rizzo, E. (2011a). Harpooning and entanglement of wild dolphins in the Pacific coast of Guatemala. Lat. Am. J. Aquat. Mamm. 9, 179–182. doi: 10.5597/lajam00187
Quintana-Rizzo, E. (2011b). “Resighting patterns of coastal spotted dolphins (Stenella attenuata graffmani) along the Pacific coast of Guatemala,” in Proceeding of the 19th Biennial Marine Mammal Conference, November 26-December 2, 2011, Tampa, Florida.
Quintana-Rizzo, E. (2019). “Distribución y abundancia de las ballenas en Guatemala con énfasis en el comportamiento de la ballena jorobada (Megaptera novaeangliae),” in Perspectivas de Investigación Sobre Mamíferos Silvestres de Guatemala, eds C. Kraker, A. P. Calderón, and A. A. Cabrera (Guatemala: Asociación Guatemalteca de Mastozoólogos), 247–261.
Quintana-Rizzo, E., and Gerrodette, T. (2009). First Study of the Abundance and Distribution of Cetaceans in the Guatemalan Exclusive Economic Zone. Report. Guatemala: Chicago Board of Trade Endangered Species Fund, Chicago Zoological Society.
Quintana-Rizzo, E., and Wells, R. S. (2001). Resighting and association patterns of bottlenose dolphins (Tursiops truncatus) in the Cedar Keys, Florida: insights into social organization. Can. J. Zool. 79, 447–456. doi: 10.1139/cjz-79-3-447
Reeves, R. R., Smith, B. D., Crespo, E. A., and Notarbartolo di, S. G. (2003). Dolphins, Whales and Porpoises: 2002-2010. Conservation Action Plan for the World’s Cetaceans. Cambridge: IUCN/SSC Cetacean Specialist Group.
Reilly, S. (1990). Seasonal changes in distribution and habitat differences among dolphins in the eastern tropical Pacific. Mar. Ecol. Prog. Ser. 66, 1–11. doi: 10.3354/meps066001
Reilly, S. B., and Thayer, V. G. (1990). Blue whale (Balaenoptera musculus) distribution in the eastern tropical Pacific. Mar. Mam. Sci. 6, 265–277. doi: 10.1111/j.1748-7692.1990.tb00357.x
Rodríguez, K., and Rodríguez-Fonseca, J. (2004). Avistamientos del delfín manchado, Stenella attenuata (Cetacea: Delphinidae) en Bahía Culebra, Costa Rica, 1999-2000. Rev. Biol. Trop. 52, 189–194.
Rosales-Nanduca, H., Gerrodette, T., Urbán, R. J., Cárdenas-Hinojosa, G., and Medrano-González, L. (2011). Macroecology of marine mammal species in the Mexican Pacific Ocean: diversity and distribution. Mar. Ecol. Prog. Ser. 431, 281–291. doi: 10.3354/meps09120
Sahri, A., Mustika, P. L. K., Purwanto, P., Murk, A. J., and Scheidat, M. (2020). Using cost-effective surveys from platforms of opportunity to assess cetacean occurrence patterns for marine park management in the heart of the Coral Triangle. Front. Mar. Sci. 7:569936. doi: 10.3389/fmars.2020.569936
Santora, J. A., Zeno, R. A., Dorman, J. G., and Sydeman, W. (2018). Submarine canyons represent an essential habitat network for krill hotspots in a large marine ecosystem. Sci. Rep. 8:7579. doi: 10.1038/s41598-018-25742-9
Silva, M. A., Prieto, R., Magalhaes, S., Seabra, M. I., Santos, R. S., and Hammond, P. S. (2008). Ranging patterns of bottlenose dolphins living in oceanic waters: implications for population structure. Mar. Biol. 156, 179–192. doi: 10.1007/s00227-008-1075-z
Smith, H., Frère, C., Kobryn, H., and Bejder, L. (2016). Dolphin sociality, distribution and calving as important behavioural patterns informing management. Anim. Conserv. 19, 462–471. doi: 10.1111/acv.12263
Smolker, R. A., Richards, A. F., Connor, R. C., and Pepper, J. W. (1992). Sex differences in patterns of association among Indian Ocean bottlenose dolphins. Behaviour 123, 38–69. doi: 10.1163/156853992x00101
UN-ECLAC. (2018). Statistical Data of the Port Activity Report of Latin America and the Caribbean 2018. https://www.cepal.org/en/notes/port-activity-report-latin-america-and-caribbean-2018 (accessed February 23, 2021)
Urian, K. W., and Wells, R. S. (1996). Bottlenose Dolphin Photo-Identification Workshop: March 21–22, 1996, Charleston, South Carolina. NOAA Tech. Mem. NMFS-SEFSC-393. Charleston, SC: U.S. Department of Comerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service.
Uriarte, A., and Lucio, P. (2001). Migration of adult mackerel along the Atlantic European shelf edge from a tagging experiment in the south of the Bay of Biscay in 1994. Fish. Res. 50, 129–139. doi: 10.1016/S0165-7836(00)00246-0
URL-IIA. (2004). Perfil Ambiental de Guatemala: Informe sobre el Estado del Ambiente y Bases Para su Evaluación Sistemática. Guatemala: URL-IARNA-IIA (Universidad Rafael Landívar - Instituto de Agricultura, Recursos Naturales y Ambiente - Instituto de Incidencia Ambiental).
Van Waerebeek, K., and Reyes, J. C. (1994). Interactions between small cetaceans and Peruvian fisheries in 1988/1989 and analysis of trends. Rep. Int. Whal. Commn. 15, 495–502.
Vidal, O., Van Waerebeek, K., and Findley, L. T. (1994). Cetaceans and gillnet fisheries in Mexico, Central America and the Wider Caribbean: a preliminary review. Rep. Int. Whal. Commn. 15, 221–233.
von Huene, R., Friesen, W., and Blome, C. (1985). Igneous and Sedimentary Rocks Recovered During Deep Sea Drilling Project Sites off Guatemala. Initial Report, Deep Sea Drilling Project 84. Washington, D.C: US Government Printing Office, 619–624.
Wade, P., and Gerrodette, T. (1993). Estimates of cetacean abundance and distribution in the Eastern tropical Pacific. SC/44/O 18. Rep. Int. Whal. Commn. 43, 477–494.
Wade, P. R., Watters, G. M., Gerrodette, T., and Reilly, S. B. (2007). Depletion of spotted and spinner dolphins in the eastern tropical Pacific: modeling hypotheses for their lack of recovery. Mar. Ecol. Prog. Ser. 343, 1–14. doi: 10.3354/meps07069
Wells, R. S. (2003). “Dolphin social complexity: lessons from the long-term study and life history,” in Animal Social Complexity, eds F. B. De Waal and P. L. Tyack (Harvard University Press), 32–56. doi: 10.4159/harvard.9780674419131.c4
Wells, R. S., Irvine, A. B., and Scott, M. D. (1980). “The social ecology of inshore odontocetes,” in Cetacean Behavior: Mechanisms and Functions, ed. L. M. Herman (New York, NY: Wiley & Sons).
Wells, R. S., Scott, M. D., and Irvine, A. B. (1987). “The social structure of free-ranging bottlenose dolphins,” in Current Mammalogy, Vol.I, ed. H. H. Genoways (New York, NY: Plenum Press), 247–305. doi: 10.1007/978-1-4757-9909-5_7
Würsig, B. (1978). Occurence and group organization of Atlantic bottlenose porpoises (Tursiops truncatus) in an Argentine Bay. Biol. Bull. 154, 348–359. doi: 10.2307/1541132
Keywords: odontocete, spatial analysis, boat surveys, eastern tropical Pacific, hot spots
Citation: Quintana-Rizzo E, Cabrera AA, Ortiz-Wolford J and Dávila V (2021) Spatial Distribution and Abundance of Small Cetaceans in the Pacific Waters of Guatemala. Front. Mar. Sci. 8:674134. doi: 10.3389/fmars.2021.674134
Received: 28 February 2021; Accepted: 26 April 2021;
Published: 11 June 2021.
Edited by:
Karen A. Stockin, Massey University, New ZealandReviewed by:
David Ainley, H. T. Harvey & Associates, United StatesCopyright © 2021 Quintana-Rizzo, Cabrera, Ortiz-Wolford and Dávila. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ester Quintana-Rizzo, dGV0ZXF1aW50YW5hQGNvbWNhc3QubmV0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.