- 1Evolution and Ecology Research Centre, School of Biological Earth and Environmental Sciences, University of New South Wales, Sydney, NSW, Australia
- 2Centre for Marine Science and Innovation, School of Biological Earth and Environmental Sciences, University of New South Wales, Sydney, NSW, Australia
There are multiple blue whale acoustic populations found across the Southern Hemisphere. The different subspecies of blue whales feed in separate areas, but during their migration to lower-latitude breeding areas each year, Antarctic blue whales become sympatric with pygmy and Chilean blue whales. Few studies have compared the degree of this overlap of the Southern Hemisphere blue whale subspecies across ocean basins during their migration. Using up to 16 years of acoustic data, this study compares the broad seasonal presence of Antarctic blue whales, Chilean blue whales, and Southeast Indian Ocean (SEIO) pygmy blue whales across the Pacific and Indian Oceans. Antarctic blue whales were sympatric with the other two blue whale subspecies during the migrating season of every year. Despite this overlap, Chilean and pygmy blue whale detections peaked earlier during the austral autumn (April–May) while Antarctic blue whale detections peaked later during the austral winter (June). Chilean (Pacific Ocean) and SEIO (Indian Ocean) pygmy blue whales showed similar seasonal patterns in detections despite occurring in different ocean basins. Though we have shown that Antarctic blue whales have the potential to encounter other blue whale subspecies during the breeding season, these distinct groups have remained acoustically stable through time. Further understanding of where these whales migrate will enable a better insight as to how these subspecies continue to remain separate.
Introduction
Sympatric populations are groups of conspecifics that coexist spatially with limited to no gene flow between them. Yet, unlike allopatric populations, there are no physical barriers separating sympatric groups. Out of all the modes of speciation, sympatry is arguably the rarest (Bolnick and Fitzpatrick, 2007). Evidence of sympatric populations includes the highly diverse cichlid species found in the East African Great Lakes and the Midas cichlid species complex found in Nicaraguan crater lakes (Schliewen et al., 1994; Barluenga et al., 2006). A more recent example of sympatric speciation in the last 200 years is found in the apple maggot, Rhagoletis pomonella (Filchak et al., 2000). This species of fruit fly originally colonized wild hawthorn in the eastern parts of North America, but with the introduction of apples in the mid-1800 s, R. pomonella was able to adapt to a new host. Today there are two distinct groups of R. pomonella, one living on hawthorn and the other living on apple.
Sympatric speciation is not limited to terrestrial systems but has also been observed in marine systems. In the ocean, there are few physical barriers separating different species and populations, especially for highly mobile pelagic species. For example, killer whales (Orcinus orca) form different ecotypes (ecologically specialized lineages) globally, each with distinct morphology and behaviors (Hoelzel and Dover, 1991; Saulitis et al., 2000). In the eastern North Pacific, orcas have diverged into separate groups known as residents and transients. Occupying the same waters, these two groups have little to no interaction with each other and differ in diet, morphology, vocal behavior, and social structures (Hoelzel and Dover, 1991; Saulitis et al., 2000). While the resident orcas use echolocation to feed on salmon, transients do not, as they feed on marine mammals with good underwater hearing, including seals and porpoises. This hunting behavior is likely passed on from generation to generation through social learning, resulting in the further divergence of these two sympatric groups so that despite the proximity and overlap in habitat, the populations remain separate from one another. Sympatric speciation in orcas has been well studied for many decades, but little is known about its existence in other cetacean species, especially baleen whales.
Different blue whale acoustic populations produce distinctive songs. In fact, worldwide there are more than ten region-specific blue whale song types, referred to as blue whale “acoustic populations” or “stocks” (McDonald et al., 2006; Stafford et al., 2011). Nine of these acoustic populations are found in the Southern Hemisphere, including the Antarctic (Ljungblad et al., 1998; Širović et al., 2004; Rankin et al., 2005); Southeast Indian Ocean (McCauley et al., 2000); Southwest Indian Ocean (Ljungblad et al., 1998; Samaran et al., 2010b); Northern Indian Ocean (Alling et al., 1991); Southeast Pacific Ocean (Cummings and Thompson, 1971; Buchan et al., 2014, 2018); Southwest Pacific Ocean (Kibblewhite et al., 1967; Miller et al., 2014); and the Solomon Island (Frank and Ferris, 2011) call types. Recently, two new blue whale call types have been identified in the Indian Ocean, the Oman call type in the northwest Indian Ocean (Cerchio et al., 2020) and the Chagos call type in the Central Equatorial Indian Ocean (Leroy et al., 2021). Despite the high diversity in acoustic groups, only two subspecies of blue whales are currently recognized in the Southern Hemisphere based on morphology (i.e., length at sexual maturity, dimensions of baleen plates, and length of the tail region; Mackintosh and Wheeler, 1929; Ichihara, 1966; Branch and Mikhalev, 2008) and genetic differences (LeDuc et al., 2007, 2017). These are the Antarctic blue whale (Balaenoptera musculus intermedia) found in the Southern Ocean around Antarctica, and the pygmy blue whale (B. m. brevicauda) found in Subantarctic and temperate waters in the Indian and Southwest Pacific Ocean during the austral summer. A potential third subspecies has also been described: the Southeast Pacific (SEP) or Chilean acoustic population of blue whale (Branch et al., 2007a; Pastene et al., 2020). Though these subspecies are known to overlap spatially, very few studies have compared their migratory patterns and almost none have done so across different oceans or over long periods of time.
Chilean blue whales are found in the eastern parts of the South Pacific Ocean, off the coast of Chile. Evidence suggests they belong to a different subspecies, as their body length is intermediate to the pygmy and Antarctic blue whales (Branch et al., 2007a; Leslie et al., 2020). Chilean blue whales have been observed feeding during the austral summer and autumn in southern Chilean waters (Cummings and Thompson, 1971; Gilmore, 1971; Hucke-Gaete et al., 2004; Cabrera et al., 2005; Galletti Vernazzani et al., 2012), while sightings (Abramson and Gibbons, 2010; Försterra and Häussermann, 2012) and acoustic detections (Buchan et al., 2015) have been reported off southern Chile extending into the austral winter (June–August). Photo-identification surveys over 9 years show that individual Chilean blue whales have a high degree of site fidelity to specific regions such as the Isla de Chiloé (Galletti Vernazzani et al., 2012). A proportion of Chilean blue whales migrate up to the eastern tropical Pacific near the Galápagos Islands during the winter months (Hucke-Gaete et al., 2018).
The Southeast Indian Ocean (SEIO) pygmy blue whale acoustic population spends the austral summer and autumn feeding in the subtropical convergence zone in the southern Indian Ocean (Garcia-Rojas et al., 2018) as well as in productive upwelling areas off the south coast of Australia (Gill, 2002; Rennie et al., 2009; Gill et al., 2011). SEIO pygmy blue whales are rarely found east of Bass Strait, where the Southwest Pacific or New Zealand acoustic population is more commonly found (Balcazar et al., 2015). In the central Indian Ocean, SEIO pygmy blue whales have been detected as far west as Amsterdam Island (Samaran et al., 2013). Satellite tracking of individual blue whales off western Australia revealed a migration route toward the Banda Sea near Indonesia where the whales possibly overwinter and breed (Double et al., 2014).
Antarctic blue whales are sympatric with the Chilean and SEIO pygmy blue whale acoustic populations, during the autumn and winter months when they migrate into the Indian and Pacific Oceans. Antarctic blue whales have been detected acoustically in the central and eastern parts of the Indian Ocean (Stafford et al., 2004; Samaran et al., 2013; Balcazar et al., 2017); the eastern and western Pacific Ocean (Stafford et al., 2004; Balcazar et al., 2017), and eastern and central Atlantic Ocean (Mackintosh and Wheeler, 1929; Thomisch et al., 2016; Samaran et al., 2019). During the austral summer, they are found primarily in the Southern Ocean, feeding south of the Antarctic convergence (Branch et al., 2007b). Partial migration is observed in Antarctic blue whales where some individuals do not migrate and are observed in the Southern Ocean year-round (Širović et al., 2004; Branch et al., 2007b; Širović et al., 2009). It is unknown whether individual whales exhibit site fidelity and return to the same breeding locations or venture into other oceans in different years. There is also a lack of information on the exact locations where these whales migrate.
Here we compare the seasonal presence of the three blue whale acoustic populations described above across two ocean basins at similar latitudes. We identify blue whale presence through the detection of acoustic signals using year-round passive-acoustic data from bottom-mounted hydrophones located off the coasts of central Chile in the Southeast Pacific Ocean and Western Australia in the Indian Ocean.
Specifically, we:
a) identify the timing of the presence of sympatric blue whale subspecies in the Southeast Pacific Ocean (Chilean and Antarctic blue whales) and Southeast Indian Ocean (SEIO pygmy and Antarctic blue whales);
b) compare the difference in seasonal timing of Chilean and SEIO pygmy blue whale presence across ocean basins; and
c) compare the temporal differences in Antarctic blue whale presence across the Pacific and Indian Oceans.
Materials and Methods
Study Area
Acoustic data was obtained from the Comprehensive Nuclear Test Ban Treaty Organization (CTBTO) hydroacoustic network. Data from the Cape Leeuwin (H01) and Juan Fernández (H03) sites were analyzed. These two recording sites are located to the southwest of Cape Leeuwin (34.9° S, 114.2° E) in the Indian Ocean and the north side of Juan Fernández Island (33.4° S, 78.9° E) in the Pacific Ocean (Figure 1). Each array consisted of three hydrophones, but only data from a single hydrophone from each location was analyzed in this study.
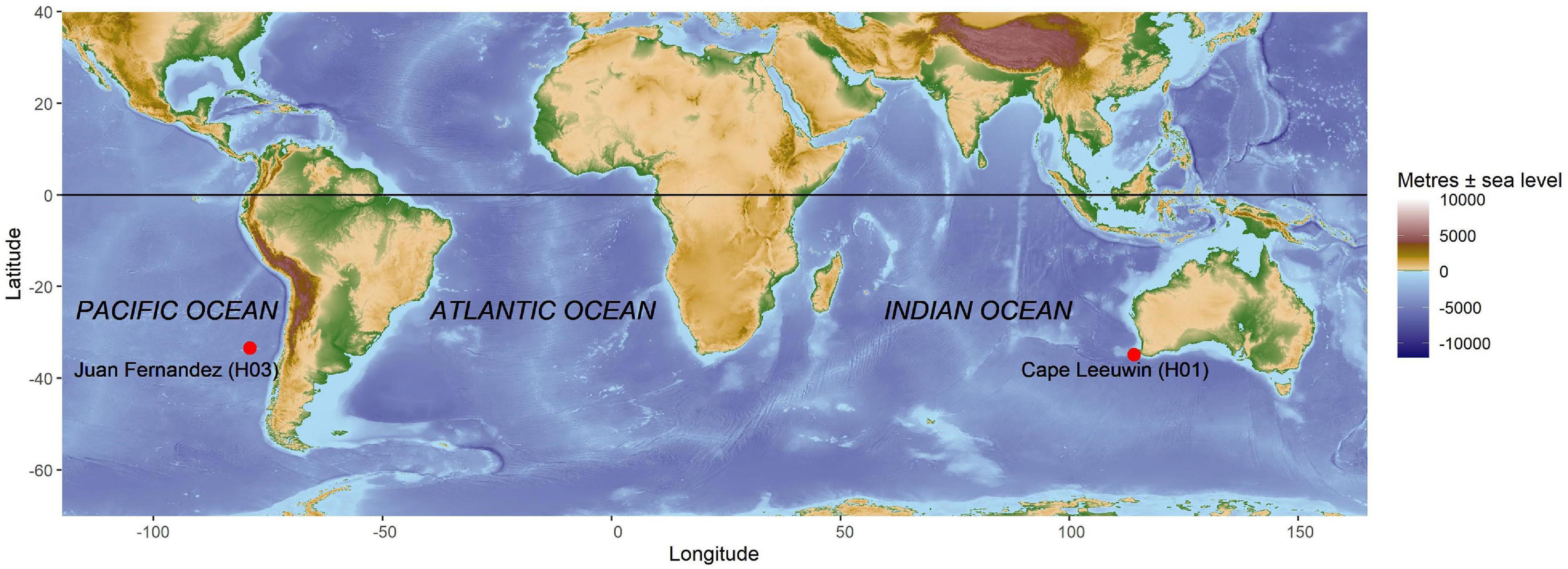
Figure 1. Locations of the two hydroacoustic stations indicated by the red circles. Cape Leeuwin (H01) is located off the southwest corner of Western Australia in the Southeast Indian Ocean and Juan Fernández (H03) is located off the coast of central Chile in the Southeast Pacific Ocean.
Data Collection
The hydrophones used in this study were situated in the Sound Fixing and Ranging (SOFAR) channel. The sampling rate of the hydrophones was 250 Hz with a sensitivity of ≤ 60 dB per μPa (1-Hz band) ≤ 81 dB per μPa (wide band) with a frequency range of 1–100 Hz (Coyne et al., 2012). Acoustic data from Cape Leeuwin was collected between January 2003 and December 2018 from the H01W1 hydrophone situated at a depth of 1,063 m. Data from Juan Fernández was collected between July 2003 and December 2018 from the H03N1 hydrophone at a depth of 1,413 m. An earthquake and a subsequent tsunami in 2010 caused extensive damage to the Juan Fernández station. As a result, there is missing data from 2010 to 2013 until the site was restored in 2014. The data from 2003 to 2006 at Juan Fernández was excluded from further analysis due to large amounts of missing data.
Call Detection
The Chilean blue whale population produces two distinct call types, the SEP1 (Figure 2A) and SEP2 calls (Figure 2B). Previous studies have shown that although the two calls are produced at different rates, they are both heard throughout the year in similar proportions (Buchan et al., 2015), so the energy ratio detector we used in Ishmael v3.0 (Mellinger et al., 2018) did not distinguish between them. The SEP call detector targeted the high-intensity band between 19 and 21 Hz of the first units of the SEP1 and SEP2 calls and triggered a detection when the energy ratio between this band and a lower frequency band exceeded a 0.2 threshold. The duration of the detection was set between 12 and 20 s. The bandwidth of the detector was designed to account for a shift of approximately 0.10–0.11 Hz per year for SEP calls (Malige et al., 2020). The SEP detector was only used for the Juan Fernández dataset, as Chilean blue whales do not occur at the Cape Leeuwin site.
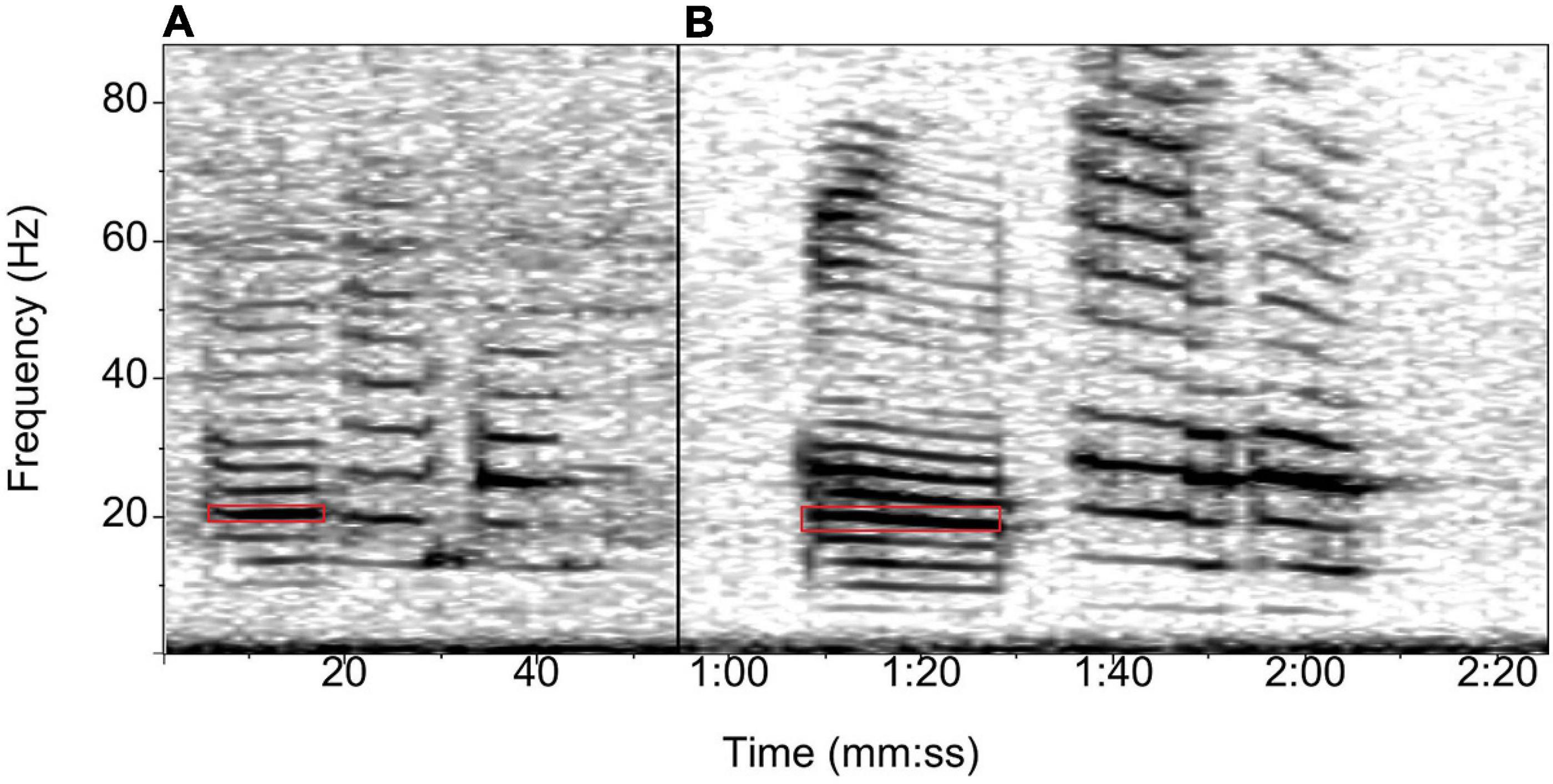
Figure 2. Spectrogram of the Chilean or Southeast Pacific (SEP) blue whale calls: (A) SEP1 call and (B) SEP2 call. The red box shows the part of the call targeted by the automated energy-ratio detector in Ishmael. Spectrogram parameters: Hamming window, 1,024 points FFT, 50% overlap.
To detect the SEIO pygmy blue whale, we used the three-part tonal call (Figure 3) described by McCauley et al. (2000). This call is approximately 120 s in length and has an inter-call interval of approximately 78 s and has been referred to as the “Southeast Indian Ocean” or “Australian blue whale” call in previous studies (McDonald et al., 2006; Gavrilov et al., 2011; Stafford et al., 2011; Samaran et al., 2013; Balcazar et al., 2015; Tripovich et al., 2015). Detector templates were created in Ishmael v3.0 (Mellinger et al., 2018), based on the third harmonic of the second unit as this component has a high signal-to-noise ratio as described in Balcazar et al. (2015). We used a broad-range detector that sampled over 66–71 Hz to account for the yearly decrease in the frequency of this part of the call (e.g., 0.35 ± 0.3 Hz/year, Gavrilov et al., 2011). The SEIO detector was used only for the Cape Leeuwin dataset, as SEIO pygmy blue whales do not typically occur at the Juan Fernández site.
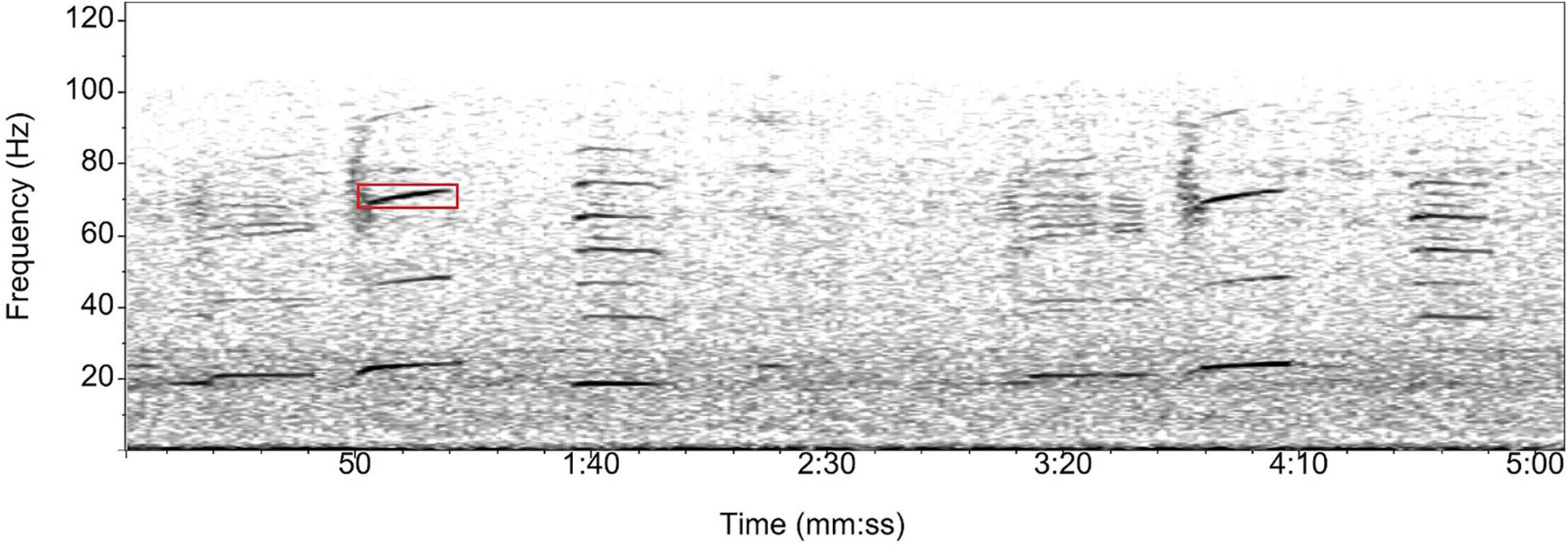
Figure 3. Spectrogram of the Southeast Indian Ocean (SEIO) pygmy blue whale call. The red box shows the third harmonic of the second unit targeted by the automated spectrogram correlation detector in Ishmael. Spectrogram parameters: Hamming window, 1,024 points FFT length, 50% overlap.
The Antarctic blue whale emits highly stereotypical calls of low frequency and strong intensity known as z-calls due to their z-shape in the time-frequency domain (Figure 4). The z-call comprises of three units: the first is a tonal unit around 25–28 Hz, lasting about 8–12 s; the second is a 1 s frequency down sweep; and the third is a second tonal unit around 18–20 Hz, lasting about 8–12 s (Ljungblad et al., 1998; Stafford et al., 2004; Rankin et al., 2005). These calls are repeated with regular intervals (approx. 60 s) in patterned sequences (or songs) lasting minutes to hours. For the z-call detector, we chose a 2 Hz interval frequency band focused on the first unit of the call. To account for the decrease in frequency of 0.14–0.16 Hz per year for Antarctic blue whale calls (McDonald et al., 2009), the frequency band for the detector was shifted slightly over the 16 years. Initially, the interval was set at 26–28 Hz between 2002 and 2007. The band was shifted down to 25–27 Hz from 2007 to 2015 and shifted again to 24–26 Hz from 2015 onward. These shifts were implemented to detect the highest number of calls for each given year. The Antarctic detector was used on the Juan Fernández and Cape Leeuwin datasets.
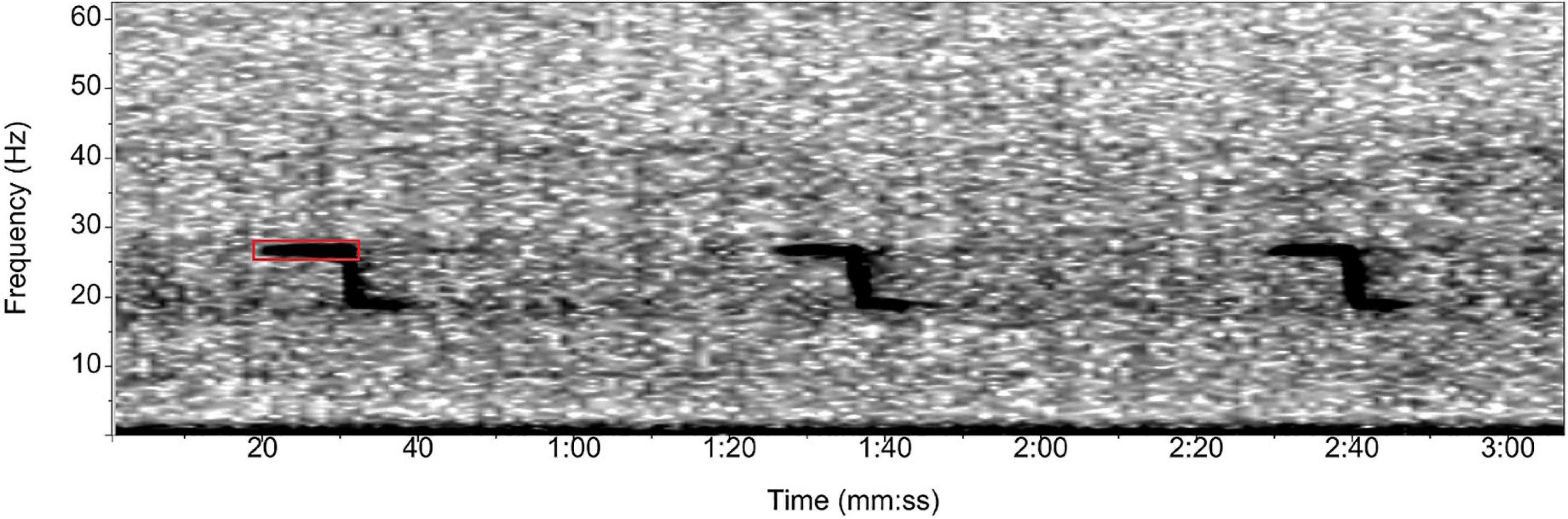
Figure 4. Spectrogram of a series of Antarctic blue whale z-calls. The first unit of the z-call is highlighted by the red box. This unit was targeted by the automated energy-ratio detector in Ishmael. Spectrogram parameters: Hamming window, 1,024 points FFT length, 50% overlap.
As this study compared calls across time, to ensure that there was a comparable proportion of detections with time thus we took a very conservative approach. To do this we aimed to target calls in the near field (i.e., close to the hydrophone). To aim for a very stable detection rate we then had a very high missed detection rate. This also minimized our rate of false detections. To do this we detected calls only of higher signal-to-noise ratio, the detection threshold was set relatively high: at 0.2 for the SEP and SEIO detector and 0.4 for the z-call detector. This resulted in a relatively high number of missed detections (approximately 80%, comprising of faint distant calls) and a relatively low false detection rate (Table 1). All detections were checked manually using Raven Pro 1.6 to verify detections and remove false detections. Spectrogram parameters used included 1,024 points Fast Fourier Transform and 50% overlap, Hamming window with a sampling rate of 250 Hz.
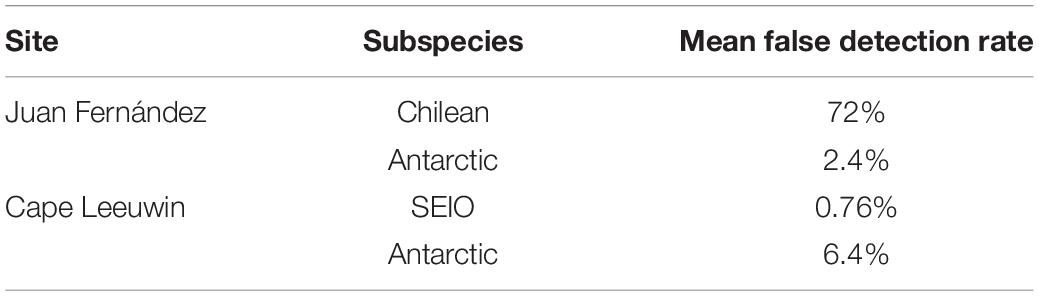
Table 1. Mean false detection rates for the blue whale subspecies at Juan Fernández and Cape Leeuwin.
Statistical Analysis of Seasonal Presence
To calculate seasonal patterns and to account for differing number of detections across years and sites, monthly detections were standardized as a percentage of total detections per year. An average was taken for the same month across all available years to calculate the overall seasonal pattern. Seasonal patterns of occurrences for each acoustic population were compared using Spearman’s rank correlation coefficient in R version 4.0.2. This is a non-parametric test which examines whether a monotonic relationship exists between two variables—in this case whale calls. Spearman’s rank correlation coefficient (ρ) measures the strength and direction of association between two ranked variables.
Results
Chilean Blue Whale Detections
A total of 1,526 Chilean blue whale calls were positively identified from the 86,938 h of recordings from Juan Fernández. 627 (41%) of the calls were detected in 2003 even though data was only available for 6 months of that year. Only three calls were detected in 2015, while the other years ranged from 28 to 265 detections. Most of the Chilean calls were detected between April and June, with very few calls detected between August and December (Figure 5). The detections show a clear seasonal pattern, with the peak in detected calls occurring during the austral autumn/early winter.
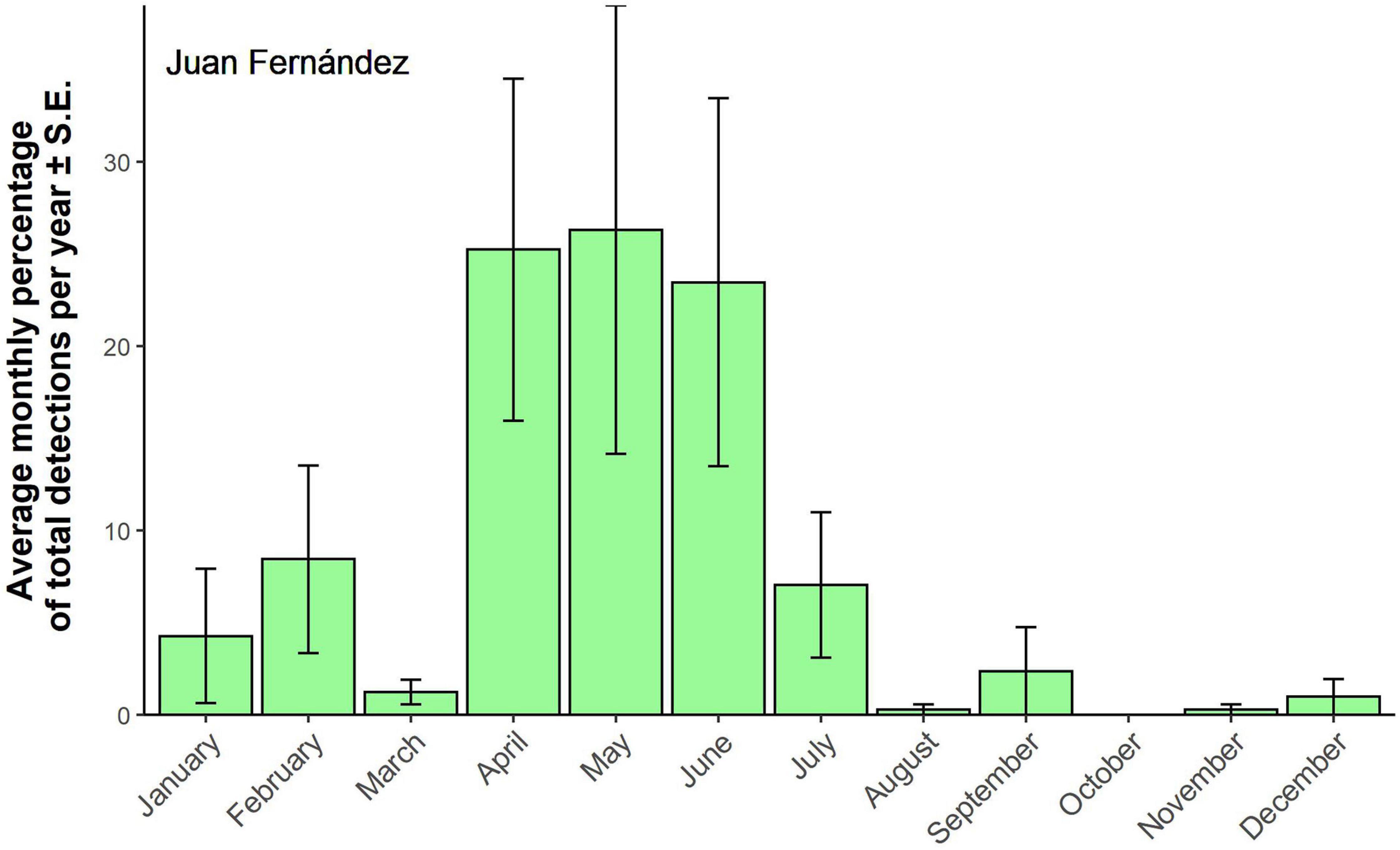
Figure 5. Chilean blue whale detections at Juan Fernández. Averaged monthly detections ± SE. Monthly detections have been standardized by calculating the monthly percentage of total detections per year. Monthly detections were averaged over 10 years of available data.
Southeast Indian Ocean Blue Whale Detections
A total of 74,812 SEIO pygmy blue whale calls were identified from Cape Leeuwin from 134,790 h of acoustic data taken between 2003 and 2018. 2003 recorded the fewest number of calls, with 257 detections and 2008 recorded the highest number with 13,118 detections. Most of the SEIO calls were detected between March and May, while very few to no calls were detected from July to October (Figure 6). A second, but smaller peak was observed during December. The detections show a clear seasonal pattern with a peak in detected calls occurring during the austral autumn.
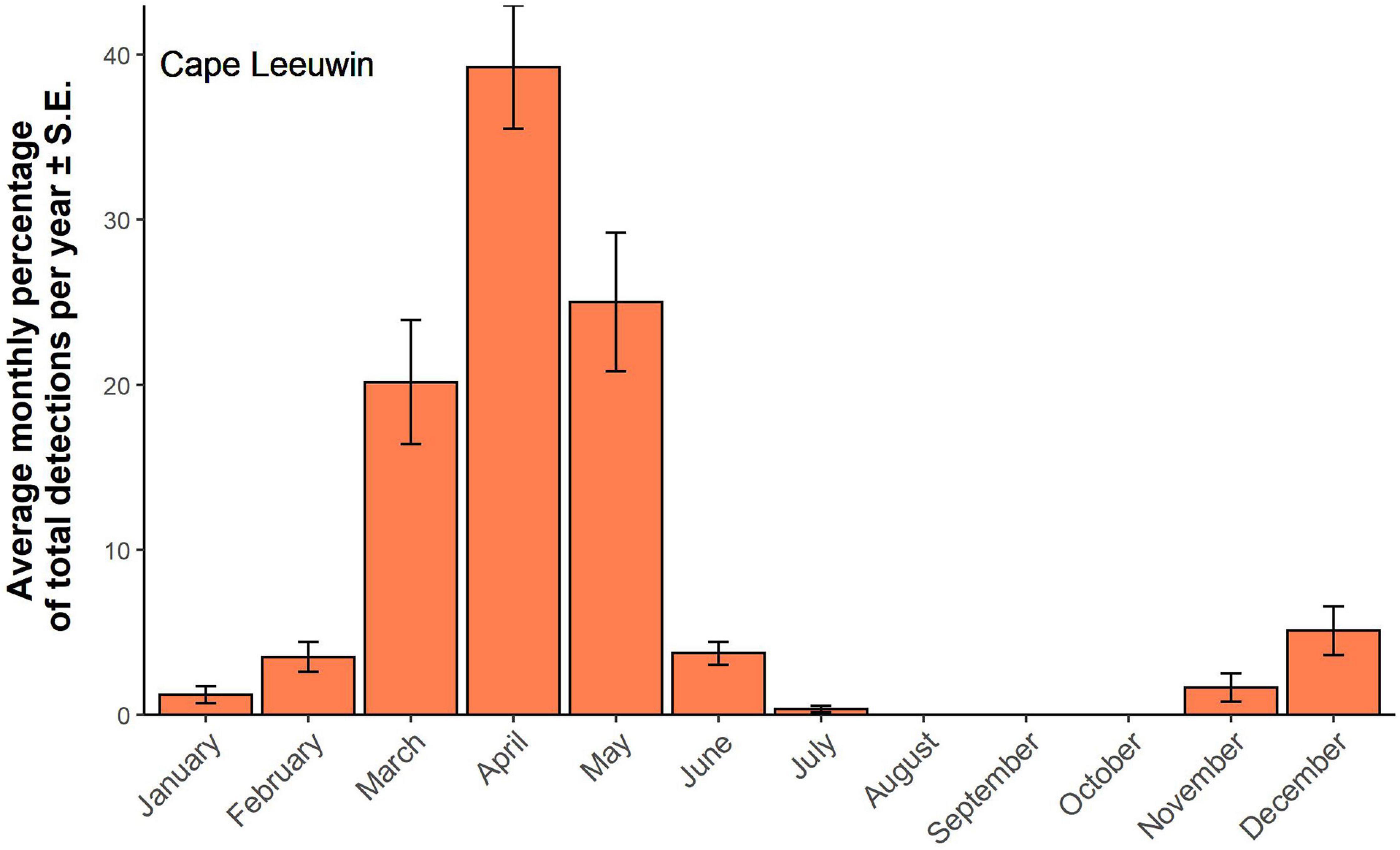
Figure 6. SEIO call detections at Cape Leeuwin. Averaged monthly detections ± SE. Monthly detections have been standardized by calculating the monthly percentage of total detections per year. Monthly detections were averaged over 16 years of available data.
Antarctic Blue Whale Detections
A total of 20,451 z-calls were detected at Juan Fernández. The lowest number of calls detected was 394 in 2005 and the highest was 4,870 in 2018, with an average of 1,859 detections per year. Antarctic calls at Juan Fernández were detected almost year-round, with most of the calls occurring between April and October, corresponding to the austral autumn to spring. The peak in detections was observed in June (Figure 7).
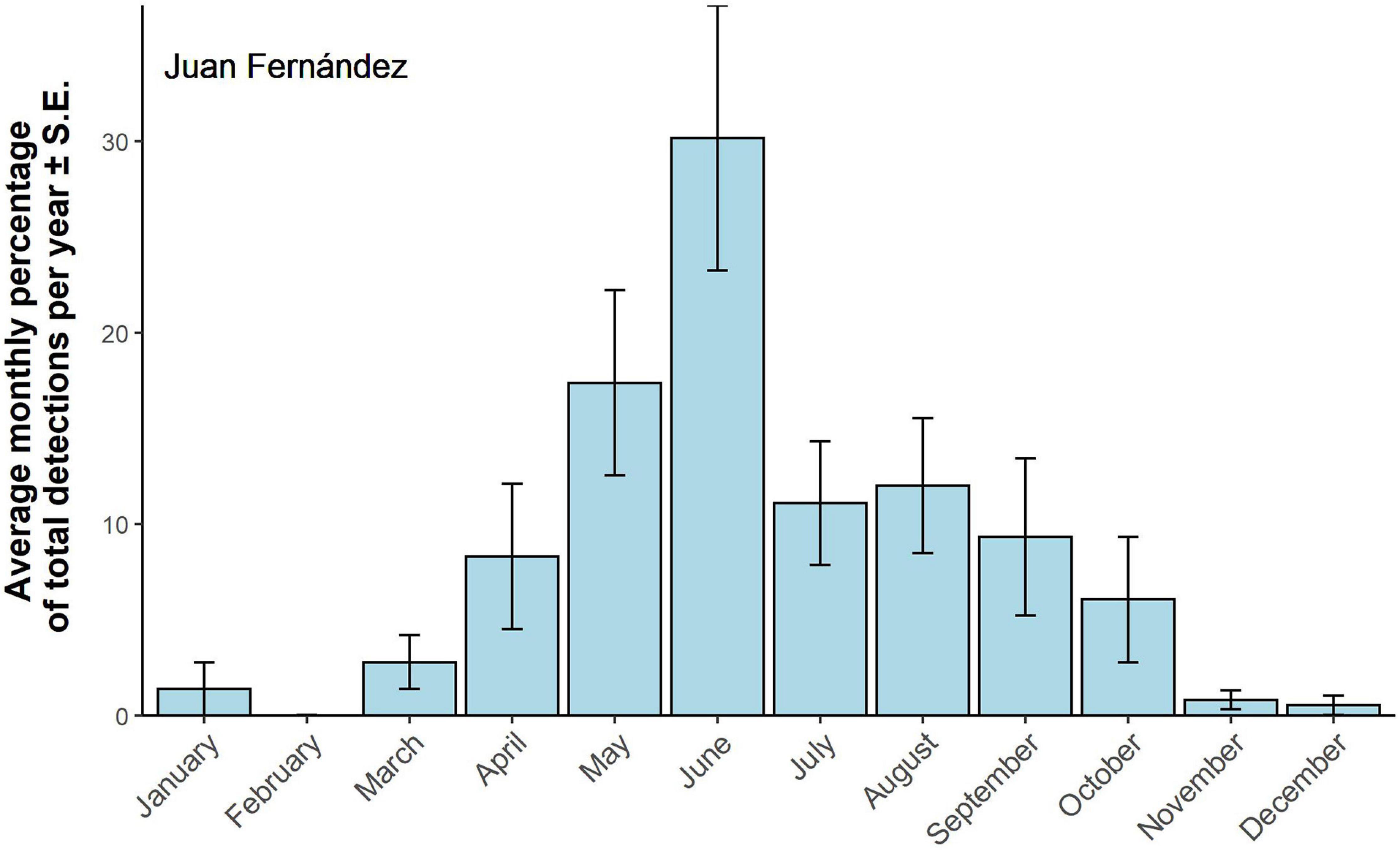
Figure 7. Antarctic Blue whale detections at Juan Fernández. Averaged monthly detections ± SE. Monthly detections have been standardized by calculating the monthly percentage of total detections per year. Monthly detections were averaged over 10 years of available data.
A total of 13,525 z-calls was detected at Cape Leeuwin. The lowest number of calls detected at Cape Leeuwin was 50 in 2004 and highest was 1,587 in 2012, with an average of 795 calls per year. Most Antarctic calls were detected from May to August, corresponding to the late austral autumn to late winter. The peak in detections was observed in June, although July and August show a similar percentage of detections (Figure 8).
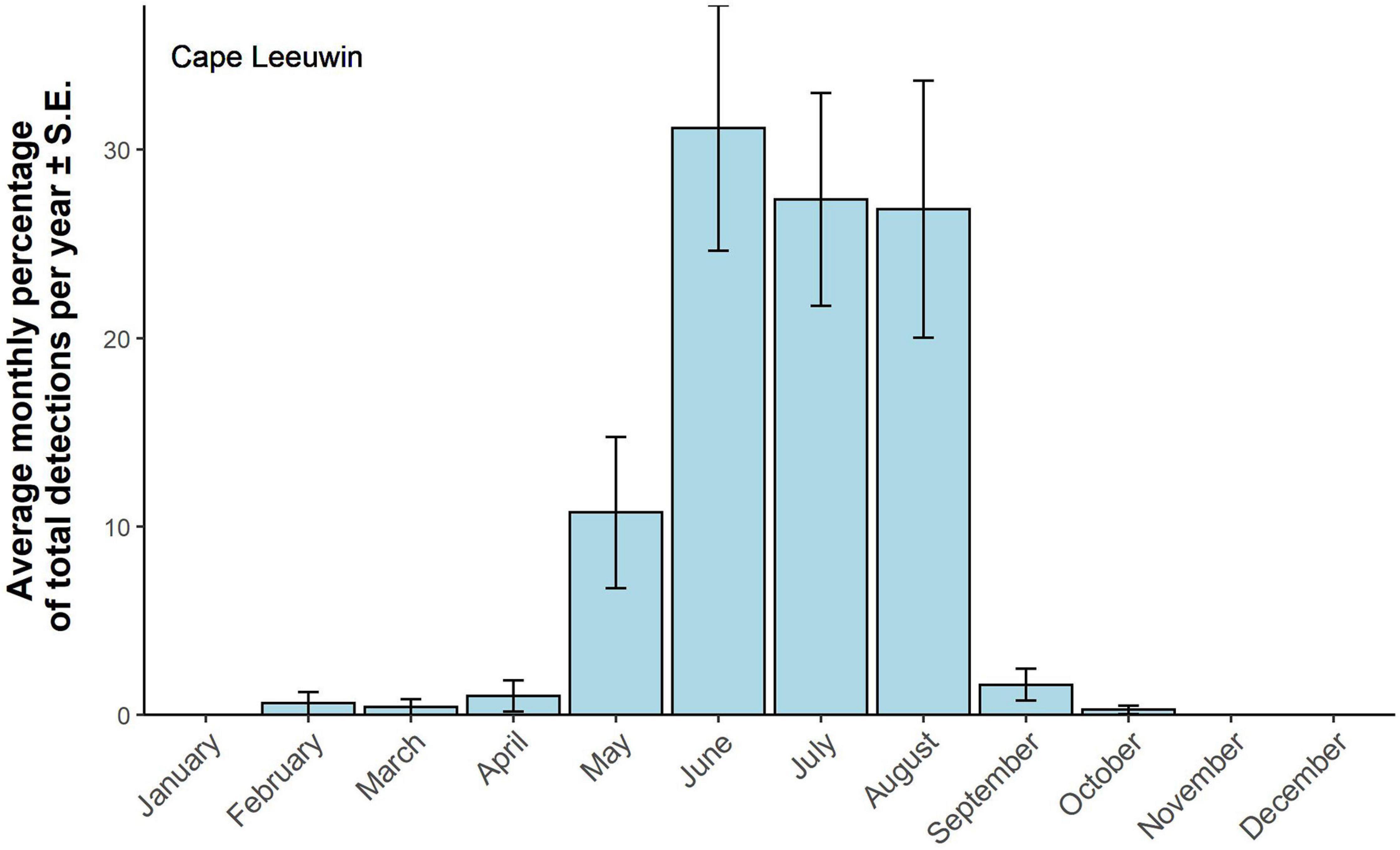
Figure 8. Antarctic Blue whale detections at Cape Leeuwin. Averaged monthly detections ± SE. Monthly detections have been standardized by calculating the monthly percentage of total detections per year. Monthly detections were averaged over 16 years of available data.
Comparison of Seasonal Patterns
Spearman’s rank correlation test showed that the Chilean blue whales and SEIO pygmy blue whales had similar seasonal patterns (ρ = 0.63 P = 0.029). Antarctic blue whales also had similar seasonal patterns across the oceans (ρ = 0.87, P < 0.001), despite having a longer migration period in the Pacific Ocean. When comparing the Antarctic blue whales to their smaller counterparts, Antarctic blue whales did not have a similar seasonal pattern to the Chilean (ρ = 0.49, P = 0.106) and SEIO pygmy blue whales (ρ = 0.03, P = 0.931).
Discussion
Our results show that blue whales living in temperate waters (Chilean and SEIO pygmy acoustic populations) had similar seasonal migration patterns, and differed from the Antarctic blue whales. We also show that Antarctic blue whale presence is similar between the Pacific and Indian Oceans.
Temperate-Water Blue Whales Have Similar Seasonal Presence at the Same Latitudes in the Pacific and Indian Oceans
Although they occur in different ocean basins, the Chilean blue whales and SEIO pygmy blue whales have similar patterns of seasonal presence at similar latitudes. For both acoustic populations there were relatively few songs detected toward the beginning of the year. This timing corresponds with the feeding season when the whales of the two populations are believed to be feeding in productive areas further south, i.e., for the Chilean blue whale in the Chiloe Archipelago (Hucke-Gaete et al., 2004; Cabrera et al., 2005; Galletti Vernazzani et al., 2012) which is approximately 1,160 km southeast of our recording location and for the SEIO pygmy blue whales in the Bonney upwelling approximately 2,200 km to the east (Gill, 2002; Gill et al., 2011), the subtropical convergence zone to the south (Garcia-Rojas et al., 2018), and the Perth Canyon (Rennie et al., 2009) approximately 300 km to the north of the Cape Leeuwin recording site. The number of whale song detections increased toward the austral autumn, peaking between April and June for the Chilean blue whales and March and May for the SEIO pygmy blue whales. This peak in song detections likely coincides with the migration of most of the population north to their breeding grounds in warmer tropical waters. After the peak there is a steep decline in song detections, with very few to no detections between August and October, indicating that majority of migrating whales have migrated past the hydrophone locations. The lack of detections between August and October for Chilean and SEIO pygmy blue whales corresponds to the time when the two subspecies presumably overwinter in their breeding grounds before returning to feeding areas south of the recording site, indicated by a second smaller peak in song detections in November and December. These results are in line with other studies of Chilean blue whales along the coast of South America. Chilean blue whales have been detected further north, in the eastern tropical Pacific, through winter (Buchan et al., 2015), while further south, off the Chiloe Archipelago, they are detected in high numbers during late summer/autumn with a peak in detections in April (Buchan et al., 2015).
Antarctic and Temperate-Water Blue Whales Have Different Seasonal Presence at the Same Latitudes Across Ocean Basins
In the austral autumn-winter, Antarctic blue whales are sympatric with Chilean blue whales in the eastern Pacific Ocean and with SEIO pygmy blue whales in the western Indian Ocean. In both oceans, the peak in Antarctic blue whale detections was later than their smaller counterparts. In the eastern Pacific, off the coast of Juan Fernández, Antarctic blue whales and Chilean blue whales were detected together from April to June. The peak in detections for the Chilean blue whales was in April and for the Antarctic blue whales the peak was in June-July, indicating the Chilean blue whales migrated past Juan Fernández earlier than the Antarctic blue whales. This is likely due to Chilean blue whale feeding areas being closer to the hydrophone location such as off the Corcovado coast in Southern Chile (Hucke-Gaete et al., 2004). As they cover less distance than the Antarctic blue whales, they are detected earlier. In the Indian Ocean, Antarctic blue whales and SEIO pygmy blue whales were detected together in May and June. The peak in detections for the SEIO pygmy population occurred in June, 1 month earlier than the peak of Antarctic blue whale detections. Again, this is most likely explained by the SEIO pygmy blue whales feeding in areas closer to the hydrophone location such as the Perth Canyon and the Great Australia Bight (Gill, 2002; Rennie et al., 2009). Tripovich et al. (2015) found a similar temporal segregation of SEIO pygmy and Antarctic blue whales from a hydrophone off Portland, Victoria. Their 15-month data set showed that Antarctic blue whales were detected more frequently from June to October, while SEIO pygmy blue whales were detected more frequently from March to June.
Satellite tracking of individuals has indicated the potential migration routes of both subspecies, with most of the tagged SEIO pygmy blue whales passing close to the Cape Leeuwin recording location (Double et al., 2014). However, tagged Chilean blue whales showed a wider range of migration paths (Hucke-Gaete et al., 2018). This could explain why the detection of Chilean blue whale songs at Juan Fernández was highly variable and inconsistent, with fewer detections. Any individuals that migrated close to the continent or far offshore would most likely be outside the detection range of the hydrophones. We have not modeled the detection range here, but it is influenced by factors including bathymetry, source level, ambient noise level, and frequency bandwidth (Samaran et al., 2010a). Samaran et al. (2010a) found that maximum detection range for Antarctic and pygmy blue whales in the Indian Ocean varied throughout the year but was around 150–180 km which was similar to the detection range of SEIO pygmy blue whales at Cape Leeuwin estimated by Gavrilov and McCauley (2013). A study on fin whale acoustic presence at Juan Fernández estimated the detection range of fin whales to be approximately 186 km which would be similar for the blue whales detected at this site (Buchan et al., 2019).
Antarctic Blue Whales Have Similar Seasonal Presence at the Same Latitudes Between the Pacific and Indian Ocean
Seasonal migration patterns of Antarctic blue whales were similar between the Pacific and Indian Ocean sites, although with some differences. There were very few song detections at the beginning of the year for the Antarctic blue whales, as most of the population would be feeding in the krill-rich waters around Antarctica (Corkeron et al., 1999; Andrews-Goff et al., 2013). In the eastern Pacific, at Juan Fernández, Antarctic blue whale songs were detected over a longer period, from March to October, with the peak in detections in June. In the eastern Indian Ocean, at Cape Leeuwin, most (>90%) of the Antarctic blue whale songs were detected between May and August. Very few Antarctic blue whale songs were detected at Cape Leeuwin after August, while songs are detected well into October at Juan Fernández. That is, the migrating season is extended in the eastern Pacific Ocean. This could be related to the relatively small distance between the Antarctic Peninsula and the South American continent, while the larger distance between Antarctica and Australia meant the migrating season in the Indian Ocean is narrower. One possible explanation is that the Antarctic blue whales in the eastern Pacific are staggering their migration as observed in humpback whales (Clapham, 1996), while the Antarctic blue whales in the Indian Ocean may not stagger their migration to the same extent.
Conclusion
We show that in every year of this 16-year study, Antarctic blue whales had the potential to encounter other blue whale subspecies in what is presumed to be their breeding season, the austral autumn through winter. Yet these different acoustic groups have remained stable through time, meaning that there must be some mechanism maintaining their distinction. A potential driver could be cultural learning within the acoustic populations which has been observed in humpback whales, orcas, and sperm whales (Deecke et al., 2000; Noad et al., 2000; Rendell and Whitehead, 2003). Cultural learning has not been documented in blue whales, but if it exists may take place during the summer when the groups are separated. Alternatively, the whales may only be sympatric during migration to different breeding locations whereupon interbreeding and gene flow between the groups becomes limited, although their breeding sites remain unknown. Our recording sites in this study were not within breeding areas, they are located in regions along migration routes. A better understanding of blue whale breeding locations will assist the conservation efforts and recovery of this species and provide some insight to how different blue whale acoustic groups remain distinct from one another.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the animal study because acoustic data was obtained passively with no interaction with wild animals.
Author Contributions
GT conducted blue whale acoustic analyses, statistical analysis, and wrote the first draft of the manuscript. Both authors conceived and designed the project, revised, and approved final manuscript.
Funding
GT was funded by an Australian Research Training Program Scholarship and a grant from the Winnifred Scott Foundation.
Author Disclaimer
The findings and conclusion in this manuscript are those of the authors and do not necessarily represent the views of the CTBTO.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Anna Lewis for her constructive feedback and editing of the manuscript. We also would like to thank members of the Mammal Lab for their comments on the manuscript. Data was made available via a vDEC contract with the CTBTO.
References
Abramson, J., and Gibbons, J. (2010). New records of blue whales Balaenoptera musculus (Linnaeus, 1758) in winter season in the inlet waters of Chiloé continental – Chile. Anales Instituto Patagonia, (Chile) 38, 107–109. doi: 10.4067/S0718-686X2010000200010
Alling, A., Dorsey, E., and Gordon, J. (1991). Blue whales (Balaenoptera musculus) off the northeast coast of Sri Lanka: distribution, feeding and individual identification. UNEP Mar. Mamm. Tech. Rep. 3, 247–258.
Andrews-Goff, V., Olson, P. A., Gales, N. J., and Double, M. C. (2013). “Satellite telemetry derived summer movements of Antarctic blue whales,” in Paper SC/65a/SH03 presented to the IWC Scientific Committee, Korea.
Balcazar, N. E., Klinck, H., Nieukirk, S. L., Mellinger, D. K., Klinck, K., Dziak, R. P., et al. (2017). Using calls as an indicator for Antarctic blue whale occurrence and distribution across the southwest Pacific and southeast Indian Oceans. Mar. Mamm. Sci. 33, 172–186. doi: 10.1111/mms.12373
Balcazar, N. E., Tripovich, J. S., Klinck, H., Nieukirk, S. L., Mellinger, D. K., Dziak, R. P., et al. (2015). Calls reveal population structure of blue whales across the southeast Indian Ocean and the southwest Pacific Ocean. J. Mammal. 96, 1184–1193. doi: 10.1093/jmammal/gyv126
Barluenga, M., Stölting, K. N., Salzburger, W., Muschick, M., and Meyer, A. (2006). Sympatric speciation in Nicaraguan crater lake cichlid fish. Nature 439, 719–723. doi: 10.1038/nature04325
Bolnick, D. I., and Fitzpatrick, B. M. (2007). Sympatric speciation: models and empirical evidence. Annu. Rev. Ecol. Evol. Syst. 38, 459–487. doi: 10.1146/annurev.ecolsys.38.091206.095804
Branch, T. A., Abubaker, E. M. N., Mkango, S., and Butterworth, D. S. (2007a). Separating southern blue whale subspecies based on length frequencies of sexually mature females. Mar. Mamm. Sci. 23, 803–833. doi: 10.1111/j.1748-7692.2007.00137.x
Branch, T. A., and Mikhalev, Y. A. (2008). Regional differences in length at sexual maturity for female blue whales based on recovered Soviet whaling data. Mar. Mamm. Sci. 24, 690–703. doi: 10.1111/j.1748-7692.2008.00214.x
Branch, T. A., Stafford, K. M., Palacios, D. M., Allison, C., Bannister, J. L., Burton, C. L. K., et al. (2007b). Past and present distribution, densities and movements of blue whales Balaenoptera musculus in the Southern Hemisphere and northern Indian Ocean. Mamm. Rev. 37, 116–175. doi: 10.1111/j.1365-2907.2007.00106.x
Buchan, S. J., Gutierrez, L., Balcazar-Cabrera, N., and Stafford, K. M. (2019). Seasonal occurrence of fin whale song off Juan Fernandez, Chile. Endanger. Species Res. 39, 135–145. doi: 10.3354/esr00956
Buchan, S. J., Hucke-Gaete, R., Rendell, L., and Stafford, K. M. (2014). A new song recorded from blue whales in the Corcovado Gulf. Southern Chile, and an acoustic link to the Eastern Tropical Pacific. Endanger. Species Res. 23, 241–252. doi: 10.3354/esr00566
Buchan, S. J., Hucke-Gaete, R., Stafford, K. M., and Clark, C. W. (2018). Occasional acoustic presence of Antarctic blue whales on a feeding ground in southern Chile. Mar. Mamm. Sci. 34, 220–228. doi: 10.1111/mms.12441
Buchan, S. J., Stafford, K. M., and Hucke-Gaete, R. (2015). Seasonal occurrence of southeast Pacific blue whale songs in southern Chile and the eastern tropical Pacific. Mar. Mamm. Sci. 31, 440–458. doi: 10.1111/mms.12173
Cabrera, E., Carlson, C., and Vernazzani, B. G. (2005). Presence of blue whale (Balaenoptera musculus) in the northwestern coast of Chiloé Island, southern Chile. Lat. Am. J. Aquat. Mamm. 4, 73–74. doi: 10.5597/lajam00073
Cerchio, S., Willson, A., Leroy, E. C., Muirhead, C., Al Harthi, S., Baldwin, R., et al. (2020). A new blue whale song-type described for the Arabian Sea and Western Indian Ocean. Endanger. Species Res. 43, 495–515. doi: 10.3354/esr01096
Clapham, P. J. (1996). The social and reproductive biology of humpback whales: an ecological perspective. Mamm. Rev. 26, 27–49. doi: 10.1111/j.1365-2907.1996.tb00145.x
Corkeron, P. J., Ensor, P., and Matsuoka, K. (1999). Observations of blue whales feeding in Antarctic waters. Polar Biol. 22, 213–215. doi: 10.1007/s003000050412
Coyne, J., Bobrov, D., Bormann, P., Duran, E., Grenard, P., Haralabus, G., et al. (2012). “CTBTO: goals, networks, data analysis and data availability,” in New Manual of Seismological Observatory Practice 2 (NMSOP-2), ed. P. Bormann (Potsdam: Deutches GeoForschungsZentrum), 1–41. doi: 10.2312/GFZ.NMSOP-2_ch15
Cummings, W. C., and Thompson, P. O. (1971). Underwater sounds from the blue whale. Balaenoptera musculus. J. Acoust. Soc. Am. 50, 1193–1198. doi: 10.1121/1.1912752
Deecke, V. B., Ford, J. K., and Spong, P. (2000). Dialect change in resident killer whales: implications for vocal learning and cultural transmission. Anim. Behav. 60, 629–638. doi: 10.1006/anbe.2000.1454
Double, M. C., Andrews-Goff, V., Jenner, K. C. S., Jenner, M.-N., Laverick, S. M., Branch, T. A., et al. (2014). Migratory movements of pygmy blue whales (Balaenoptera musculus brevicauda) between Australia and Indonesia as revealed by satellite telemetry. PLoS One 9:e93578. doi: 10.1371/journal.pone.0093578
Filchak, K. E., Roethele, J. B., and Feder, J. L. (2000). Natural selection and sympatric divergence in the apple maggot Rhagoletis pomonella. Nature 407, 739–742. doi: 10.1038/35037578
Försterra, G., and Häussermann, V. (2012). Report on blue whales sightings (Balaenoptera musculus. Linnaeus, 1758) in a narrow fjord during autumn-winter in southern Chile. Spixiana 35, 237–245.
Frank, S. D., and Ferris, A. N. (2011). Analysis and localization of blue whale vocalizations in the Solomon Sea using waveform amplitude data. J. Acoust. Soc. Am. 130, 731–736. doi: 10.1121/1.3605550
Galletti Vernazzani, B., Carlson, C., Cabrera, E., and Brownell, Jr, R. L (2012). Chilean blue whales off Isla Grande de Chiloe, 2004-2010: distribution, site-fidelity and behaviour. J. Cetacean Res. Manag. 12, 353–360.
Garcia-Rojas, M. I., Jenner, K. C. S., Gill, P. C., Jenner, M. N. M., Sutton, A. L., and McCauley, R. D. (2018). Environmental evidence for a pygmy blue whale aggregation area in the Subtropical Convergence Zone south of Australia. Mar. Mamm. Sci. 34, 901–923. doi: 10.1111/mms.12494
Gavrilov, A. N., and McCauley, R. D. (2013). Acoustic detection and long-term monitoring of pygmy blue whales over the continental slope in southwest Australia. J. Acoust. Soc. Am. 134, 2505–2513. doi: 10.1121/1.4816576
Gavrilov, A. N., McCauley, R. D., Salgado-Kent, C., Tripovich, J., and Burton, C. (2011). Vocal characteristics of pygmy blue whales and their change over time. J. Acoust. Soc. Am. 130, 3651–3660. doi: 10.1121/1.3651817
Gill, P. C. (2002). A blue whale (Balaenoptera musculus) feeding ground in a southern Australian coastal upwelling zone. J. Cetacean Res. Manag. 4, 179–184.
Gill, P. C., Morrice, M. G., Page, B., Pirzl, R., Levings, A. H., and Coyne, M. (2011). Blue whale habitat selection and within-season distribution in a regional upwelling system off southern Australia. Mar. Ecol. Prog. Ser. 421, 243–263. doi: 10.3354/meps08914
Gilmore, R. M. (1971). Observations on marine mammals and birds off the coast of southern and central Chile, early winter 1970. Antarct. U.S. 6, 10–11.
Hoelzel, A. R., and Dover, G. A. (1991). Genetic differentiation between sympatric killer whale populations. Heredity 66, 191–195. doi: 10.1038/hdy.1991.24
Hucke-Gaete, R., Bedrinana-Romano, L., Viddi, F. A., Ruiz, J. E., Torres-Florez, J. P., and Zerbini, A. N. (2018). From Chilean Patagonia to Galapagos. Ecuador: novel insights on blue whale migratory pathways along the Eastern South Pacific. PeerJ 6:e4695. doi: 10.7717/peerj.4695
Hucke-Gaete, R., Osman, L. P., Moreno, C. A., Findlay, K. P., and Ljungblad, D. K. (2004). Discovery of a blue whale feeding and nursing ground in southern Chile. Proc. R. Soc. Lond. B Biol. Sci. 271 (suppl_4), S170–S173. doi: 10.1098/rsbl.2003.0132
Ichihara, T. (1966). “The pygmy blue whale, Balaenoptera musculus brevicauda, a new subspecies from the Antarctic,” in Whales, Dolphins, and Porpoises, ed. K. S Norris (Berkeley, CA: University of California Press), 79–111.
Kibblewhite, A., Denham, R., and Barnes, D. (1967). Unusual low-frequency signals observed in New Zealand waters. J. Acoust. Soc. Am. 41, 644–655. doi: 10.1121/1.1910392
LeDuc, R., Archer, F., Lang, A., Martien, K., Hancock-Hanser, B., Torres-Florez, J., et al. (2017). Genetic variation in blue whales in the eastern pacific: implication for taxonomy and use of common wintering grounds. Mol. Ecol. 26, 740–751. doi: 10.1111/mec.13940
LeDuc, R., Dizon, A., Goto, M., Pastene, L., Kato, H., Nishiwaki, S., et al. (2007). Patterns of genetic variation in Southern Hemisphere blue whales and the use of assignment test to detect mixing on the feeding grounds. J. Cetacean Res. Manage. 9:73.
Leroy, E. C., Royer, J. Y., Alling, A., Maslen, B., and Rogers, T. L. (2021). Multiple pygmy blue whale acoustic populations in the Indian Ocean: whale song identifies a possible new population. Sci. Rep. 11, 1–21. doi: 10.1038/s41598-021-88062-5
Leslie, M. S., Perkins-Taylor, C. M., Durban, J. W., Moore, M. J., Miller, C. A., Chanarat, P., et al. (2020). Body size data collected non-invasively from drone images indicate a morphologically distinct Chilean blue whale (Balaenoptera musculus) taxon. Endanger. Species Res. 43, 291–304. doi: 10.3354/esr01066
Ljungblad, D., Clark, C. W., and Shimada, H. (1998). SC/49/SH17 a comparison of sounds attributed to pygmy blue whales (Balaenoptera musculus brevicauda) recorded south of the Madagascar Plateau and those attributed to’true’ blue whales (Balaenoptera musculus) recorded off Antarctica. Rep. Int. Whal. Comm. 48, 439–442.
Mackintosh, N. A., and Wheeler, J. F. G. (1929). Southern blue and fin whales. Discov. Rep. 1, 257–540.
Malige, F., Patris, J., Buchan, S. J., Stafford, K. M., Shabangu, F., Findlay, K., et al. (2020). Inter-annual decrease in pulse rate and peak frequency of Southeast Pacific blue whale song types. Sci. Rep. 10, 1–11. doi: 10.1038/s41598-020-64613-0
McCauley, R. D., Jenner, C., Bannister, J. L., Cato, D. H., and Duncan, A. (2000). “Blue whale calling in the Rottnest trench, Western Australia, and low frequency sea noise,” in In Australian Acoustical Society Conference, Joondalup, Australia, 15–17.
McDonald, M. A., Hildebrand, J. A., and Mesnick, S. (2009). Worldwide decline in tonal frequencies of blue whale songs. Endanger. Species Res. 9, 13–21. doi: 10.3354/esr00217
McDonald, M. A., Mesnick, S. L., and Hildebrand, J. A. (2006). Biogeographic characterization of blue whale song worldwide: using song to identify populations. J. Cetacean Res. Manage. 8, 55–65.
Miller, B. S., Collins, K., Barlow, J., Calderan, S., Leaper, R., McDonald, M., et al. (2014). Blue whale vocalizations recorded around New Zealand: 1964–2013. J. Acoust. Soc. Am. 135, 1616–1623. doi: 10.1121/1.4863647
Noad, M. J., Cato, D. H., Bryden, M. M., Jenner, M. N., and Jenner, K. C. S. (2000). Cultural revolution in whale songs. Nature 408, 537–537. doi: 10.1038/35046199
Pastene, L. A., Acevedo, J., and Branch, T. A. (2020). Morphometric analysis of Chilean blue whales and implications for their taxonomy. Mar. Mamm. Sci. 36, 116–135. doi: 10.1111/mms.12625
Rankin, S., Ljungblad, D., Clark, C., and Kato, H. (2005). Vocalisations of Antarctic blue whales. Balaenoptera musculus intermedia, recorded during the 2001/2002 and 2002/2003 IWC/SOWER circumpolar cruises, Area V, Antarctica. J. Cetacean Res. Manage. 7, 13–20.
Rendell, L. E., and Whitehead, H. (2003). Vocal clans in sperm whales (Physeter macrocephalus). Proc. R. Soc. Lond. B Biol. Sci. 270, 225–231. doi: 10.1098/rspb.2002.2239
Rennie, S., Hanson, C., McCauley, R., Pattiaratchi, C., Burton, C., Bannister, J., et al. (2009). Physical properties and processes in the Perth Canyon, Western Australia: links to water column production and seasonal pygmy blue whale abundance. J. Mar. Syst. 77, 21–44. doi: 10.1016/j.jmarsys.2008.11.008
Samaran, F., Adam, O., and Guinet, C. (2010a). Detection range modeling of blue whale calls in Southwestern Indian Ocean. Appl. Acoust. 71, 1099–1106. doi: 10.1016/j.apacoust.2010.05.014
Samaran, F., Adam, O., and Guinet, C. (2010b). Discovery of a mid-latitude sympatric area for two Southern Hemisphere blue whale subspecies. Endanger. Species Res. 12, 157–165. doi: 10.3354/esr00302
Samaran, F., Berne, A., Leroy, E. C., Moreira, S., Stafford, K. M., Maia, M., et al. (2019). Antarctic blue whales (Balaenoptera musculus intermedia) recorded at the Equator in the Atlantic Ocean. Mar. Mamm. Sci. 35, 641–648. doi: 10.1111/mms.12559
Samaran, F., Stafford, K. M., Branch, T. A., Gedamke, J., Royer, J., Dziak, R. P., et al. (2013). Seasonal and geographic variation of southern blue whale subspecies in the Indian Ocean. PLoS One 8:e71561. doi: 10.1371/journal.pone.0071561
Saulitis, E., Matkin, C., Barrett-Lennard, L., Heise, K., and Ellis, G. (2000). Foraging strategies of sympatric killer whale (Orcinus orca) populations in Prince William Sound, Alaska. Mar. Mamm. Sci. 16, 94–109. doi: 10.1111/j.1748-7692.2000.tb00906.x
Schliewen, U. K., Tautz, D., and Pääbo, S. (1994). Sympatric speciation suggested by monophyly of crater lake cichlids. Nature 368, 629–632. doi: 10.1038/368629a0
Širović, A., Hildebrand, J. A., Wiggins, S. M., and Thiele, D. (2009). Blue and fin whale acoustic presence around Antarctica during 2003 and 2004. Mar. Mamm. Sci. 25, 125–136. doi: 10.1111/j.1748-7692.2008.00239.x
Širović, A., Hildebrand, J. A., Wiggins, S. M., McDonald, M. A., Moore, S. E., and Thiele, D. (2004). Seasonality of blue and fin whale calls and the influence of sea ice in the Western Antarctic Peninsula. Deep Sea Res. 2 Top. Stud. Oceanogr. 51, 2327–2344. doi: 10.1016/j.dsr2.2004.08.005
Stafford, K. M., Bohnenstiehl, D. R., Tolstoy, M., Chapp, E., Mellinger, D. K., and Moore, S. E. (2004). Antarctic-type blue whale calls recorded at low latitudes in the Indian and eastern Pacific Oceans. Deep Sea Res.2 Oceanogr. Res. Pap. 51, 1337–1346. doi: 10.1016/j.dsr.2004.05.007
Stafford, K. M., Chapp, E., Bohnenstiel, D. R., and Tolstoy, M. (2011). Seasonal detection of three types of “pygmy” blue whale calls in the Indian Ocean. Mar. Mamm. Sci. 27, 828–840. doi: 10.1111/j.1748-7692.2010.00437.x
Thomisch, K., Boebel, O., Clark, C. W., Hagen, W., Spiesecke, S., Zitterbart, D. P., et al. (2016). Spatio-temporal patterns in acoustic presence and distribution of Antarctic blue whales Balaenoptera musculus intermedia in the Weddell Sea. Endanger. Species Res. 30, 239–253. doi: 10.3354/esr00739
Keywords: sympatric populations, long-term data, bioacoustics, subspeciation, blue whales, passive acoustic monitoring
Citation: Truong G and Rogers TL (2021) Seasonal Occurrence of Sympatric Blue Whale Subspecies: the Chilean and Southeast Indian Ocean Pygmy Blue Whales With the Antarctic Blue Whale. Front. Mar. Sci. 8:671145. doi: 10.3389/fmars.2021.671145
Received: 23 February 2021; Accepted: 24 August 2021;
Published: 16 September 2021.
Edited by:
Bob Dziak, National Oceanic and Atmospheric Administration (NOAA), United StatesReviewed by:
Robert McCauley, Curtin University, AustraliaSamara Haver, Oregon State University, United States
Copyright © 2021 Truong and Rogers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gary Truong, Zy50cnVvbmdAdW5zdy5lZHUuYXU=; orcid.org/0000-0001-5308-8745
 Gary Truong
Gary Truong Tracey L. Rogers
Tracey L. Rogers