
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 03 August 2021
Sec. Marine Conservation and Sustainability
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.669790
This article is part of the Research TopicSustainable Development Goal 14 - Life Below Water: Towards a Sustainable OceanView all 25 articles
A correction has been applied to this article in:
Corrigendum: Distribution of Cetaceans in the Canary Islands (Northeast Atlantic Ocean): Implications for the Natura 2000 Network and Future Conservation Measures
The waters of the Canary Islands are considered a hotspot for marine biodiversity, especially regarding cetacean species. Based on this fact, this study pays attention to the spatial distribution pattern of cetacean species and the conservation role of the Natura 2000 Network, a set of Special Areas of Conservation (SACs), which were defined mainly based on data compiled in 1996, under the framework of the European Habitats Directive. In recent years, the declaration of conservation areas for cetaceans between the Tenerife—La Gomera Islands by two global conservation programs, Mission Blue (“Hope Spots”) and Whale Heritage Site (“Whale Sanctuary”) sent clear signals of scientific and social interest to promote better protection of the cetacean species in the Canary Islands. The main aim of the designated SACs is the conservation of its biological and ecological diversity, ensuring the long-term survival of the target species in the waters around islands. In this case, the enactment of the SACs was based only on the sparse data available for the common bottlenose dolphin, Tursiops truncatus. This study shows that the spatial distribution of cetaceans in the Canary archipelago generated from a large database of cetacean sightings, from 2007 to 2018. The results obtained show the main marine areas where the different cetacean species are distributed around the different islands of the archipelago. The spatial distribution maps of the cetacean species, when compared with the existing SACs of the Natura 2000, show the need to extend these SACs into the open sea to include more cetacean species and a larger number of individuals for better conservation of the endangered marine mammals. As a consequence, some suggestions were proposed to improve and update the role of SACs in European Northeast Atlantic waters as a key environmental tool for cetacean conservation. The data supporting the recent declarations of these two new milestones the “Hope Spot” and the “Whale Sanctuary” enhance more keystone information to promote a large marine protected area in the Eastern Atlantic Ocean, such as the “Macaronesian Biodiversity and Ecological Migration Corridor for Cetaceans,” a conservation figure that has been already proposed in the scientific literature as a deserving candidate of governmental regulations and policies by Portugal and Spain; it would also require joint cross-border cooperation efforts for marine spatial planning.
The variations in the oceanographic, hydrological, and topographic features of the oceans create a wide heterogeneity of habitats, favoring the high diversity of cetaceans that is observed in the Northeast Subtropical Atlantic Ocean, especially in the waters around Canary Islands, an area considered a hotspot for marine biodiversity. At present, 30 species of cetaceans, out of the 90 described worldwide (Jefferson et al., 2015), have been recorded in these oceanic waters (23 odontocetes and 7 mysticetes; Supplementary Table 1). These species make the area one of the most diverse places for cetaceans; it is also the largest in Europe. The Canary Islands are an oceanic volcanic archipelago in the Northeast Subtropical Atlantic Ocean, comprising eight islands with a total surface area of 7,273 km2, a coastline of approximately 1,581 km, and an exclusive economic zone (EEZ) of approximately 494,192 km2.
The complex oceanographic characteristics of the Canary Islands are determined by a combination of factors, for example, the filaments—nutrient-rich waters—which originated in the upwelling system of the Northwestern African shores (Cape Juby, Cape Ghir, and Cape Bojador) that reach the Canary Islands. These filaments have an essential biological function, as they transport fish, cephalopods, and crustacean larvae—food for marine mammals—from the African coast to the coastal waters of the Canary Islands (Rodríguez et al., 1999, 2004; Bécognée et al., 2009; Landeira et al., 2017).
Although the waters of the Canary Islands are considered a hotspot for marine biodiversity, for cetaceans in particular, this does not exempt marine mammals from being subjected to pressure and threats. Some of these threats are due to natural causes, such as predation, but, for the most part, they are consequences of direct or indirect anthropogenic activities (Parsons, 2012), including by-catch, competition with fisheries, habitat degradation (Ruíz de la Rosa et al., 2015), marine pollution (Baulch and Perry, 2014; García-Álvarez et al., 2014, 2015; Puig-Lozano et al., 2018), acoustic/ noise disturbance (Aguilar de Soto, 2006; OSPAR, 2009), stranding (Tejedor and Carrillo, 2018; Puig-Lozano et al., 2020), and maritime traffic, including high-speed ferries—nearly 60% of sperm whale deaths are due to ship collisions in the Canaries (Arregui et al., 2019). These marine mammals are a highly mobile species; their distribution areas cover extensive oceanic areas, which pose a major challenge for their conservation. All cetaceans found in European Union waters receive protection under the Habitats Directive (Council Directive 92/43/EEC of 21 May 1992) and the Marine Strategy Framework Directive (Directive 2008/56/EC). These directives mandate both updating the conservation status and the monitoring of cetacean populations (e.g., distribution, abundance) as well as the adoption of conservation measures if the population status is considered unfavorable (Santos and Pierce, 2015).
Based on cetacean conservation and protection as per the Habitats Directive, this study pays attention to the Natura 2000 Network, a European network of natural areas whose aim is the conservation of the biological and ecological diversity of Europe, taking into account the economic, social, and cultural requirements of its different regions. Additionally, the main goal is to ensure the long-term survival of different species and habitat types in Europe, preventing the loss of biodiversity. The Natura 2000 Network is the main nature conservation instrument used by the European Union.
The Natura 2000 Network involves the natural habitats and species listed in Annexes I and II of the Habitats Directive (Council Directive 92/43/EEC of 21 May 1992), where only two species of cetaceans, the common bottlenose dolphin (Tursiops truncatus) and the harbor porpoise (Phocoena phocoena), as animals of community interest for whose conservation it is necessary to designate Special Areas of Conservation (SACs), and all other cetaceans as animals of community interest require strict protection. In December 2001, the European Commission approved the designation of 174 Sites of Community Importance (SCI) proposed by the Canary Islands Autonomous Community through the Spanish State. In 2011, as per the Order ARM/2417/2011 of 30 August, the 24 sites of marine community importance in the Macaronesian biogeographic region of the Natura 2000 Network were declared as SACs, and the corresponding management plans were approved, which included the conservation measures and regulation of uses and activities. Subsequently, in 2015 (BOE-A-2015-2329), with the results obtained through the studies carried out within the framework of the LIFE+ INDEMARES project (inventory and designation of the Natura 2000 Network in marine areas of the Spanish State), two new SCIs of the Natura 2000 Network were approved—the Conception Bank (ESZZ15001) and the marine area of the east and south of Lanzarote-Fuerteventura (ESZZ15002) (MITECO, 2019). The species of community interest considered to be declared as SACs are: a marine turtle, the loggerhead sea turtle (Caretta caretta), and only one cetacean, the common bottlenose dolphin (T. truncatus), following the indications described in Annex II of the Habitats Directive that was previously mentioned. The field data for the distribution pattern of the common bottlenose dolphin populations were collected before 1996; at that time, it was known that other cetacean species were present in these areas such as the short-finned pilot whale (Globicephala macrorhynchus), the Risso’s dolphin (Grampus griseus), the sperm whale (Physeter macrocephalus), the striped dolphin (Stenella coeruleoalba), and the spotted dolphin (Stenella frontalis). In the official enactment documents of those SACs, no records or distributional patterns were provided for the latter mentioned cetacean species; there was only a note stating that attention and consideration should also be given to their protection in these SACs. It is to be noted that the boundaries, the perimeter of the already approved SACs, have not taken into consideration the spatial distribution of these other cetacean species, with the exception of the common bottlenose dolphin; therefore, the delimited area of these SACs may not be sufficient to protect these animals. La Manna et al. (2020) presented similar results for Mediterranean waters, arguing that the extension of SACs was ineffective for the conservation efforts of these animals and, therefore, proposing to enlarge the borders of SACs for the effective protection of cetaceans in this sea basin.
In recent years, new milestones have contributed toward the protection of cetaceans in the Canary Islands waters. In November 2019, the waters near the Tenerife and La Gomera Islands were declared a “Hope Spot” site by the Mission Blue initiative1 due to its diversity of cetaceans. More recently, in January 2021, almost the same exact marine area has been nominated as a “Whale Sanctuary” by the global program Whale Heritage Sites2 as recognition of its outstanding cetacean species richness and the ecosystem services (ES) it may provide for local communities. Furthermore, in 2007 (Carrillo, 2007), considering the wide spatial distribution of cetaceans not only in the Canary Islands but also in the European Macaronesia (Azores, Madeira), the creation of a Macaronesian Biodiversity, Ecological and Cetacean migration corridor was proposed; it would greatly contribute to the conservation of these cetaceans, which are so important for the marine ecosystem. This would not be the first time, as there is already a “Mediterranean Cetacean Migration Corridor” (BOE-A-2018-9034).
Any advances are made in research about cetaceans every day, underlining the richness of these marine mammals in the waters of the Canary Islands (Aguilar de Soto et al., 2001; Pérez-Vallazza and Haroun, 2005; Pérez-Vallazza et al., 2008; Fernández et al., 2009; Carrillo and Ritter, 2010; Carrillo et al., 2010; Fais et al., 2016; Puig-Lozano et al., 2020) and in the Macaronesian region (Carrillo, 2007; Alves et al., 2015; Correia et al., 2019). Unfortunately, only a few research articles have been published on the spatial and temporal distribution of these animals, that too only for some specific areas in the waters of the Canary Islands; this study is the first in our knowledge to show the general spatial distribution of cetaceans in the waters of the whole Canary archipelago. This baseline knowledge is fundamental for further assessing the conservation status of the cetaceans with regards to their distribution and to manage the status of cetaceans in Canary Islands waters efficiently.
The aim of this work is to present the spatial distribution of cetaceans in the Canary archipelago, based on an extensive database of marine mammal sightings in recent years, from 2007 to 2018. This study also aims to identify the areas where the greatest number of individual cetaceans can be found, highlighting their relationship with the extant SACs from the Natura 2000 Network.
This study focuses on the waters around the Canary Islands, which is a Spanish autonomous region in the Northeast Subtropical Atlantic Ocean (from 29° 24′ 40″ N to 27° 38′ 16″ N and from 13° 19′ 54″ W to 18° 09′ 38″ W). This volcanic archipelago consists of eight islands (Figure 1) and is geographically split up into the eastern (La Graciosa, Lanzarote, and Fuerteventura), the central (Gran Canaria and Tenerife), and the western islands (La Gomera, La Palma, and El Hierro).
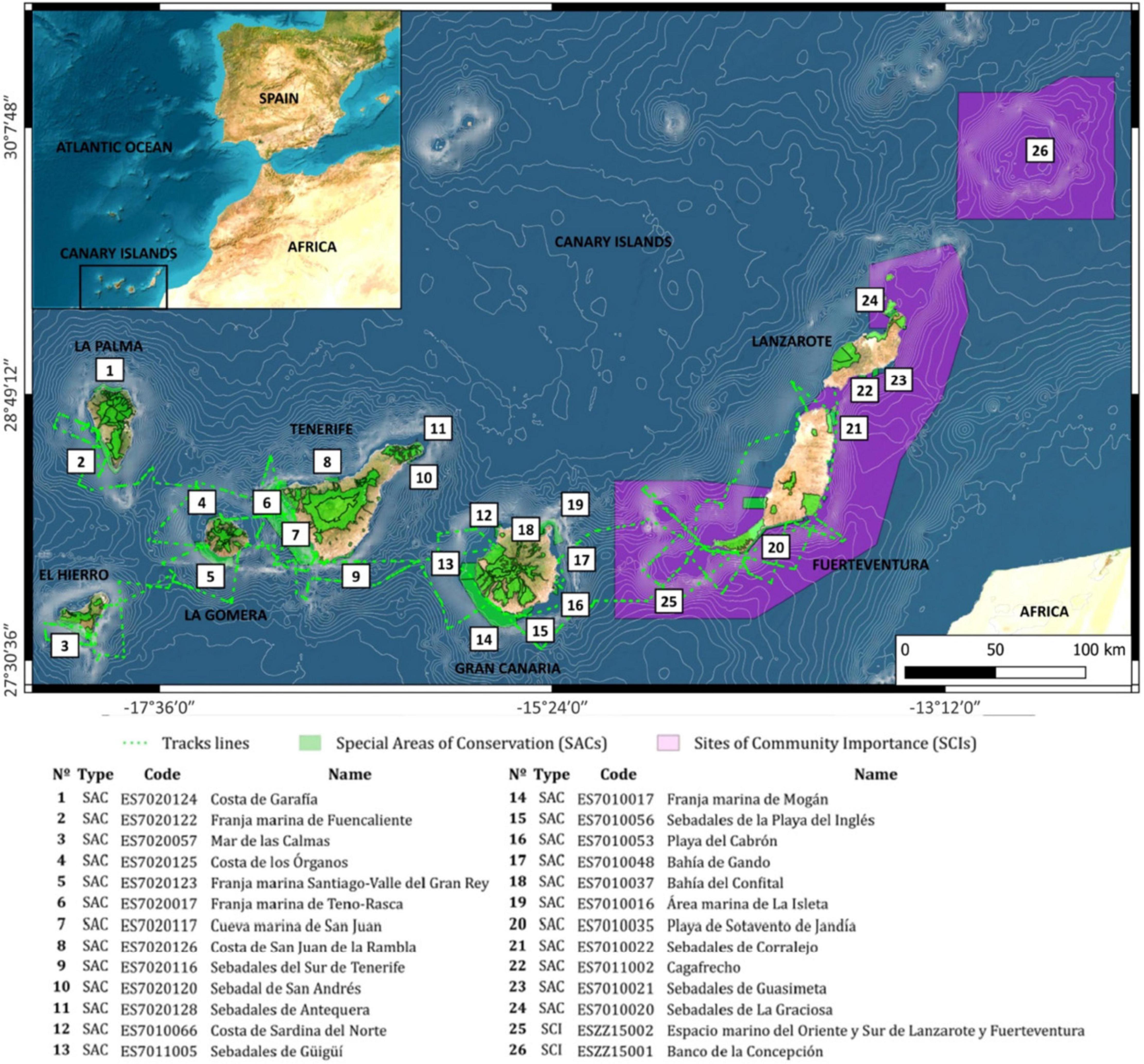
Figure 1. Map of the survey area location, Canary Islands Archipelago, including the Special Areas of Conservation (SACs; green color), the Sites of Community Importance (SCIs; pink color) and the surveyed spatial trajectory  tracks lines).
tracks lines).
The cetacean sightings datasets used to conduct this study are the result of enormous human efforts by various data sources, primarily the following ones: (1) Canarias Conservación: 2007–2018 in Fuerteventura, Gran Canaria, and Tenerife islands; and (2) Programa POSEIDON: 2013–2015 in Gran Canaria, Tenerife, and La Palma. A total of 1,688 cetacean sightings were observed between 2007 and 2018 (Supplementary Table 2), representing 12 years of robust scientific information on marine mammals in the waters of the Canary archipelago. The cetacean sightings were carried out in accordance with the code of conduct of the Government of the Canary Islands (Decree 178/2000; Decree 1727/2007). The data collected for each dataset included time, position, species identity, group size, the presence of calves, the coastal distance, and the spatial trajectory (tracks lines; Figure 1), among others parameters. The species were identified to the lowest taxonomic level possible from descriptions in field guides and scientific literature (Carwardine, 1995). Each dataset was obtained with the following singularities in its methodology:
(1) The Canarias Conservación dataset: Most of the data was collected by the marine environmental consulting company “Canarias Conservación”3 through visual identification, using programmed transects and opportunistic platforms. Cetacean surveys were carried out on three Canary Islands, namely, Fuerteventura, Gran Canaria, and Tenerife, with varying effort, from 2007 to 2018. If the weather conditions allowed—Beaufort scale 3—cruises were conducted along the edge of the island shelf in a 13-m speedboat with a flying bridge located 7 m above sea level, at an average survey speed of 6 knots (Carrillo et al., 2010). To establish standard and repeatable protocols for these site conditions, sighting data was collected following the standard method of line transects survey (Buckland et al., 1993; Heimlich-Boran, 1993; Dudzinski, 1999; Schwarz and Seber, 1999), together with the model previously designed by Carrillo et al. (2002) for Tenerife. In each survey, four expert observers were on-board the speedboat. Two of them observed with the naked eye from a platform that was 4.20 m above sea level, while a third person observed from the flight deck with 7 × 50 binoculars and the fourth person worked as a data recorder.
(2) The Programa POSEIDON dataset: The data for Gran Canaria, Tenerife, and La Palma were obtained through a citizen science tool, Programa POSEIDON,4 which was initiated by the Universidad de Las Palmas de Gran Canaria (ULPGC) to monitor marine biodiversity in the Canary Islands. This database was obtained through the sightings carried out by the whale-watching company “Spirit of the Sea”5 and Fancy II,6 both of which have good experience and expertise in cetacean species identification. In each survey, cetaceans were observed from the flight deck with 7 × 50 binoculars at a height of about 7 m during daylight hours, weather permitting (Beaufort scale 3). Each day, the sighting boats crossed the harbor by making random perpendicular transects to the coastline, depending on the route and the weather situation. Although the search pattern was not systematic, mainly regular zigzag transects were followed. The average speed was 6 knots, followed the methodology used by Carrillo et al. (2010).
The environmental information was mainly obtained from the Copernicus Marine Environment Monitoring Service (CMEMS7). The sea surface potential temperature (SST; °C) and chlorophyll-a (Chl-a; mg⋅m–3) were selected from among all the potential parameters, as they were the most relevant variables representing the oceanographic features of the Canarian waters (Figure 2). SST (daily L4 product IBI_MULTIYEAR_PHY_005_002) and Chl-a data (daily L4 product IBI_MULTIYEAR_BGC_005_003) was compiled by CMEMS. Daily data files, from January 2007 to December 2018 covering the Canary archipelago, were used with a spatial resolution of 0.083° × 0.083°. Data from 2017 was used as an example of SST and Chl-a values distribution because of the major effort put into conducting sightings during that year.
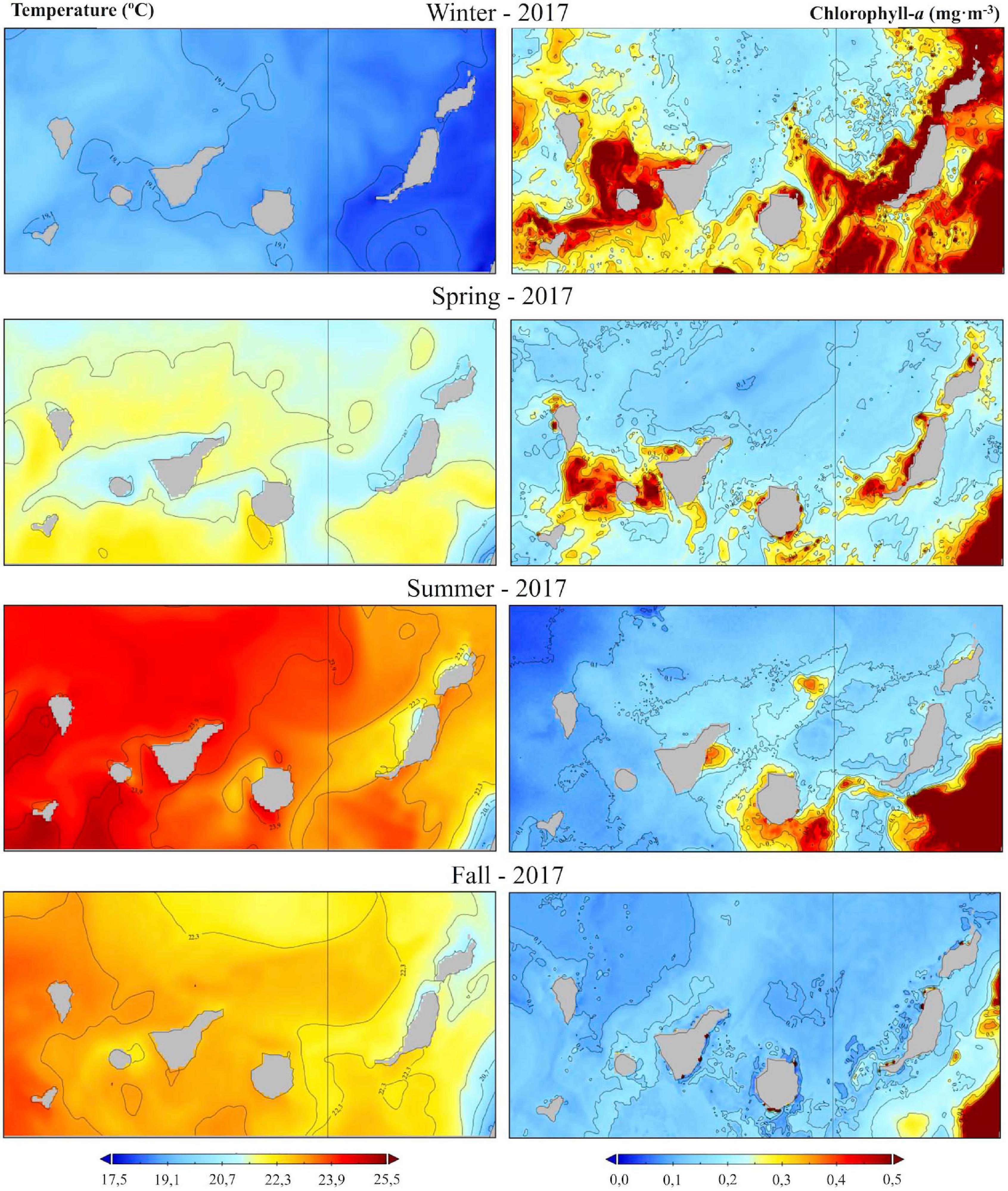
Figure 2. Environmental and biological variables. Left panels: sea surface potential temperature (SST, °C); right panels: chlorophyll-a (Chl-a, mg⋅m–3; an example from 2017 (http://marine.copernicus.eu/).
Once the information was collected, the sightings data was processed to characterize the different cetacean species identified in the Canary archipelago. To obtain the temporal distribution of each species, distinguishing between Odontoceti and Mysticeti, the frequency was estimated by directly counting the number of sightings. Then, this number was analyzed monthly, using the number of surveyed days. Therefore, the monthly sighting per unit effort (SPUE) was calculated by dividing the number of monthly sightings by the number of total surveyed days per month. The spatial distribution of each species was also presented on a map using the QGIS (3.16 Hannover) software. Sightings of the four most frequent species were represented individually on a map of the island, where SACs and SCIs were also included, to view their presence in waters under figures of conservation and protected areas.
From a total of 1,688 sightings, 18 species of cetaceans were recorded: 14 odontocetes and 4 mysticetes (Supplementary Table 2). The most frequently sighted species for the sub-order Odontoceti were Globicephala macrorhynchus, Tursiops truncatus, and Stenella frontalis, with sightings of 529, 492, and 243, respectively, forming 31.34%, 29.15%, and 14.40% of the total, respectively. The most frequently sighted species for the sub-order Mysticeti was Balaenoptera edeni, which was sighted on 86 occasions, representing 5.09% of the total number of cetacean sightings in the Canary Islands (Supplementary Table 2).
The temporal variation of the total cetacean sightings per unit effort (SPUE; sighting/surveyed days) is presented in Figure 3. The dataset allows us to determine that Odontoceti are the dominant cetacean species in Canarian waters throughout the period studied, presenting higher SPUE than Mysticeti. It is also evident that there is a constant increase in cetacean SPUE in the temporal variation for total cetaceans.
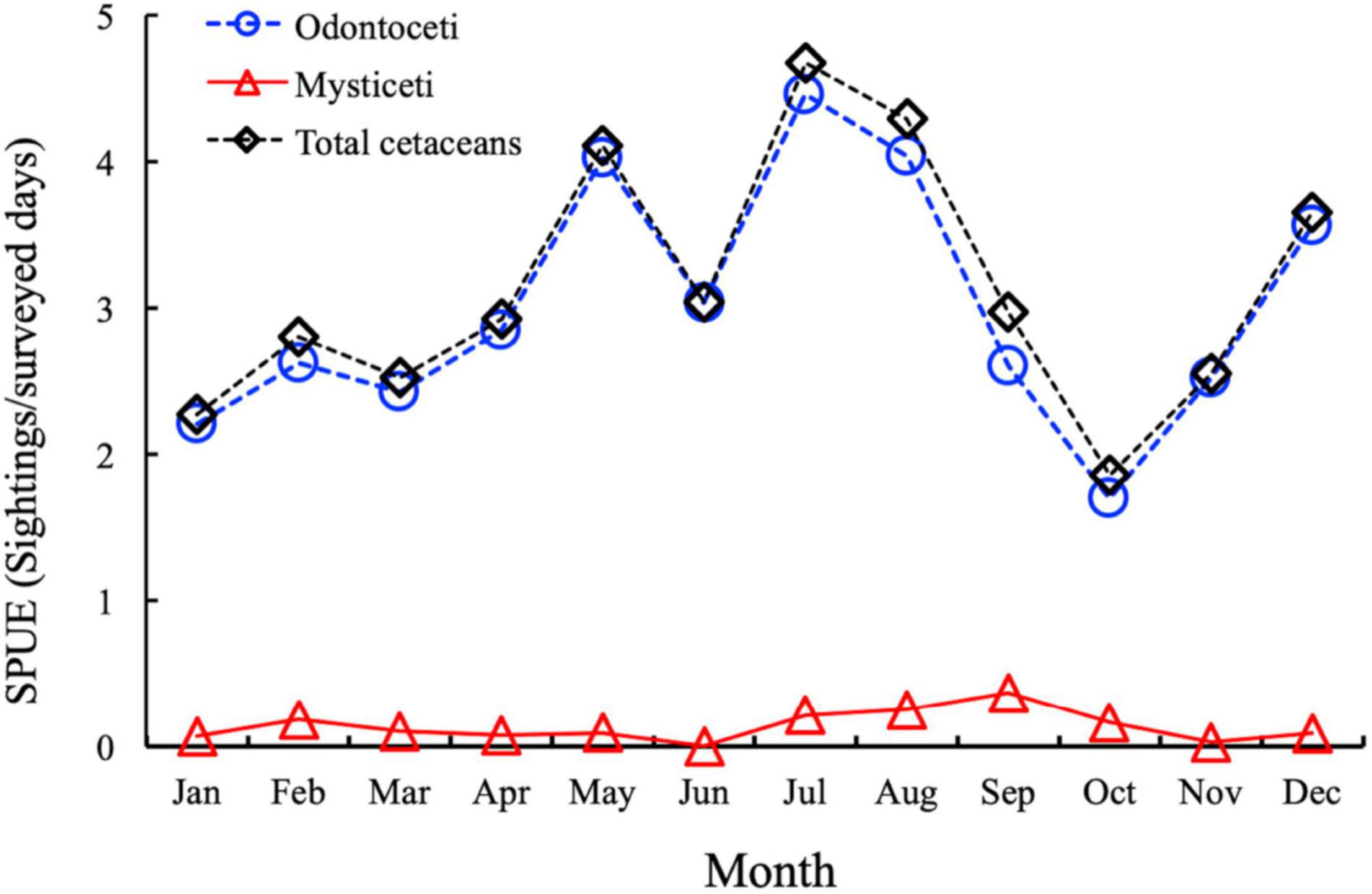
Figure 3. Temporal variation of total cetacean sightings per unit effort (SPUE; sighting/surveyed days) in the Canary Islands during the study period (Jan. 2007–Dec. 2018). Black line: Total cetacean sightings; blue line: Odontoceti; red line: Mysticeti.
The contribution of the different Odontoceti families to the total cetacean SPUE in the Canary Islands is presented in Figure 4. The Delphinidae (G. macrorhynchus, T. truncatus, and S. frontalis) is the most frequently sighted cetacean family, with a minor presence for species belonging to other Odontoceti families (Figure 4).
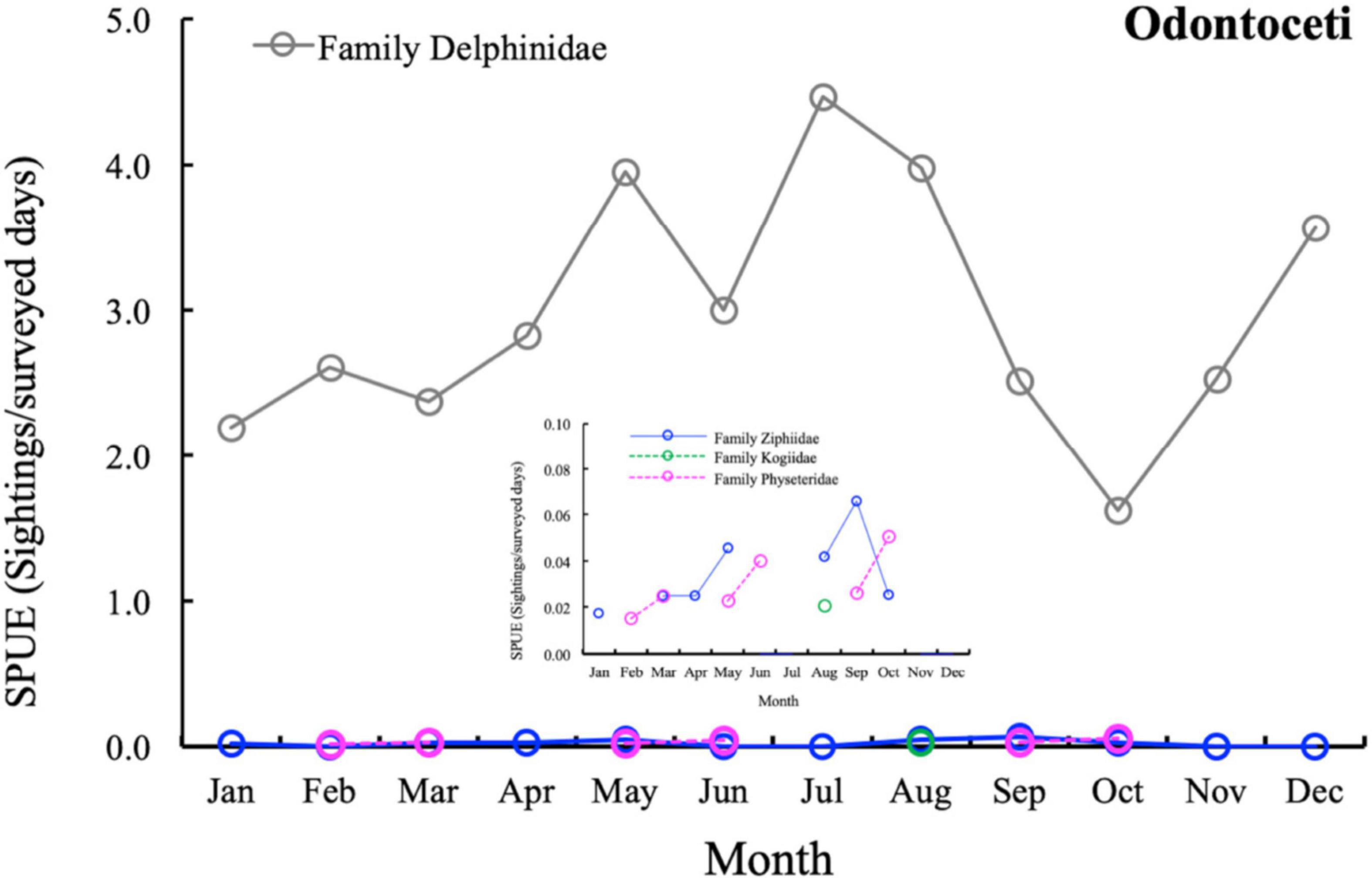
Figure 4. Temporal variation of total Odontoceti sightings per unit effort (SPUE; sighting/surveyed days) in the Canary Islands during the study period (Jan. 2007–Dec. 2018). Blue line: Family Zipphiidae; green line: Family Kogiiidae; pink line: Family Physeteridae; grey line: Family Delphinidae.
Regarding the SPUE of the sub-order Mysticeti in Canarian waters, only one family, Balaenopteridae, has been observed. In addition, very few species have been observed throughout the different months (Supplementary Table 3), Balaenoptera edeni being the main species observed with a high SPUE during the summer months (Figure 5 and Supplementary Table 3).
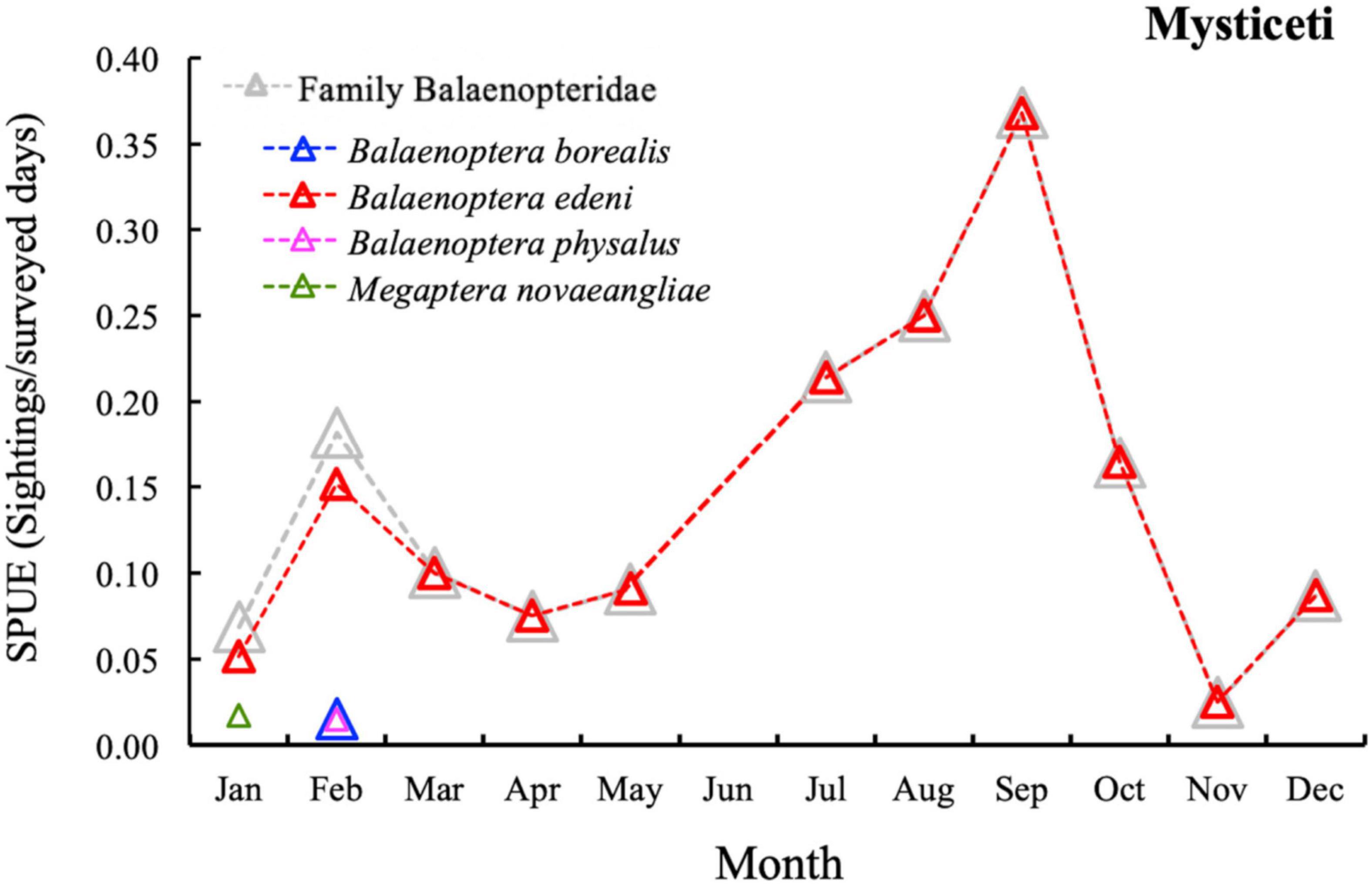
Figure 5. Temporal variation of total cetacean sightings per unit effort unit (SPUE; sighting/surveyed days) in the Canary Islands during the study period (Jan. 2007–Dec 2018). Grey triangle: Family Balaenopteridae; blue triangle: Balaenoptera borealis; red triangle: Balaenoptera edeni; pink triangle: Balaenoptera physalus; green triangle: Megaptera novaeangliae.
In terms of spatial distribution, 18 species of cetaceans are seen around the Canary Islands (Figure 6). These animals can be sighted frequently in areas close to the coast, mainly on the leeward side of the islands, coinciding with warmer temperatures (SST; °C) and high chlorophyll-a values (Chl-a; mg⋅m–3; Figure 2; an example from 2017). The SST and Chl-a time series from 2007 to 2018, for the Canary archipelago, is presented in Supplementary Figure 1.
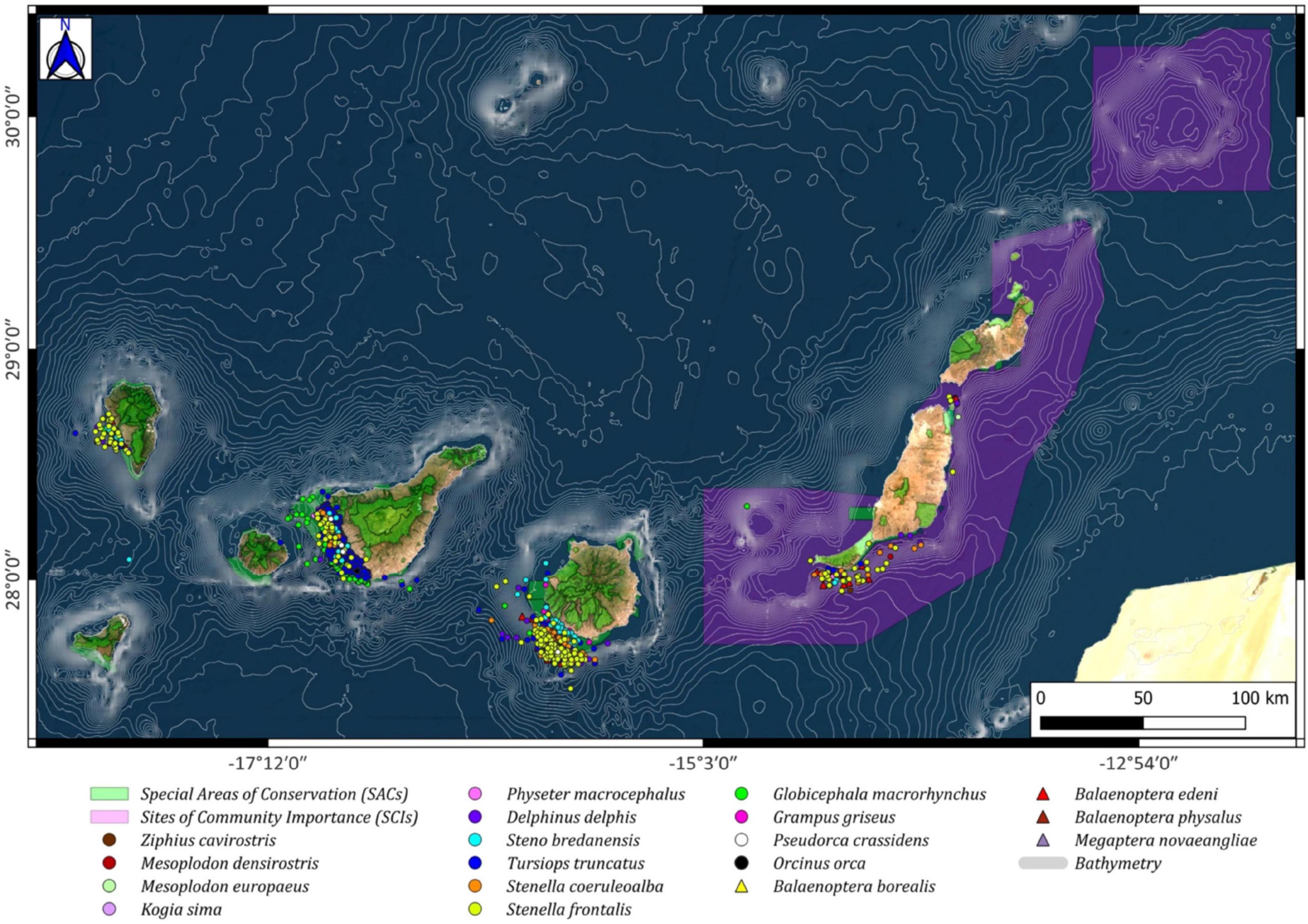
Figure 6. Cetacean spatial distribution around the Canary archipelago (Odontoceti: circles; Mysticeti: triangles), including the Special Areas of Conservation (SACs; green color) and the Sites of Community Importance (SCIs; pink color).
The four most frequently sighted cetacean species were three odontocetes (G. macrorhynchus, T. truncatus, and S. frontalis) and one Mysticeti (Balaenoptera edeni). Sighting information for each of these species individually is shown below, noting their spatial distribution with the SACs and SCIs defined in the Natura 2000 Network (Figure 1).
In the case of the short-finned pilot whale (Globicephala macrorhynchus), it was observed that this species is mainly distributed outside the Franja Marina of Mogán, whereas in the SAC Franja Marina de Teno-Rasca (southwest of Tenerife), most individuals were observed inside that protected area, and very few were sighted in the existing SACs of the southwestern coast of La Palma island. (Figure 7).
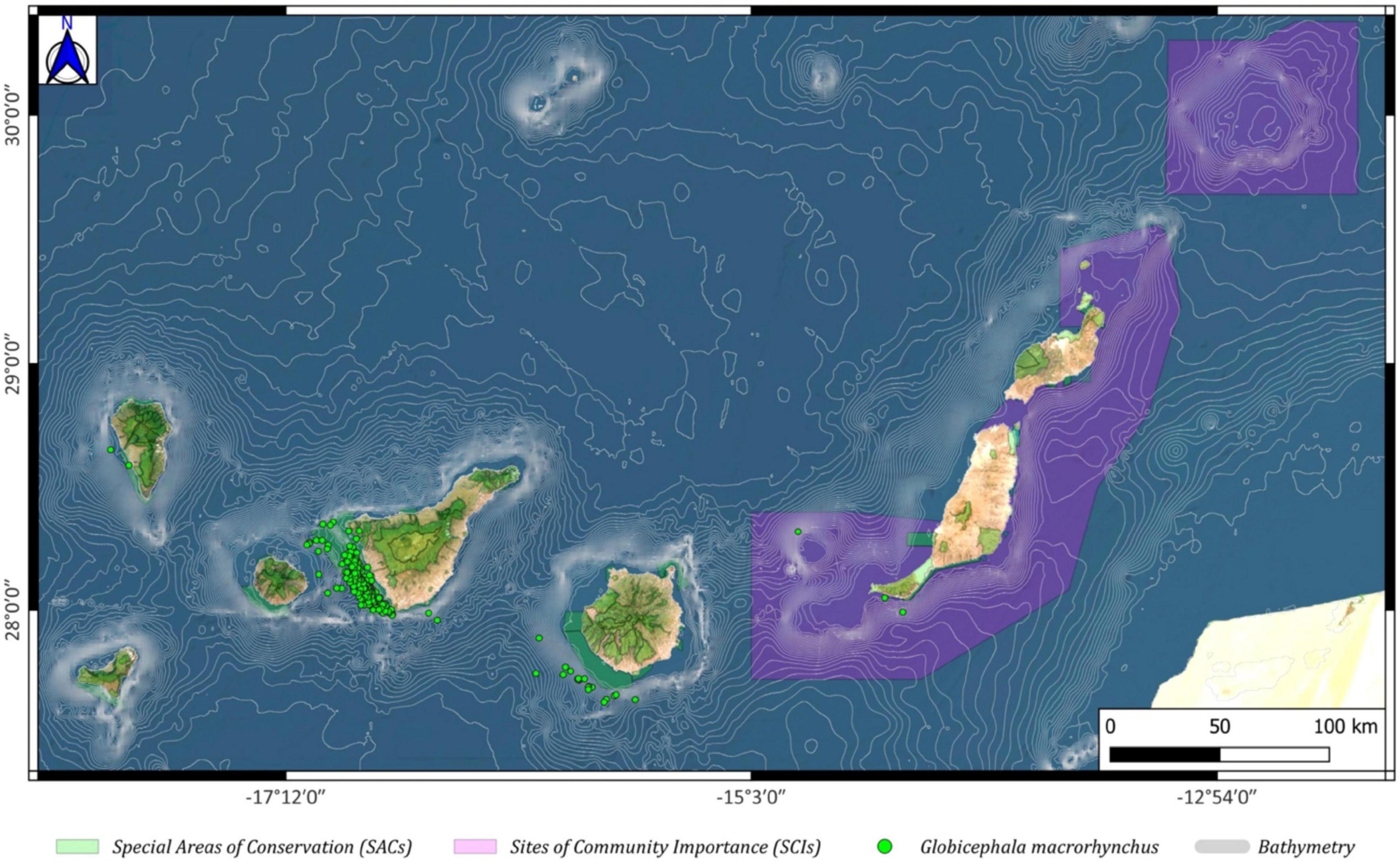
Figure 7. Globicephala macrorhynchus spatial distribution around the Canary archipelago (green circles), including the Special Areas of Conservation (SACs; green color) and the Sites of Community Importance (SCIs; pink color).
Tursiops truncatus (common bottlenose dolphin) sightings are concentrated in or around the SACs of Franja marina of Mogán and Sebadales de Güigüi to the southwest of Gran Canaria, in the Franja marina of Teno-Rasca (southwest Tenerife), and the Franja Marina of Fuencaliente to the west of La Palma (Figure 8).
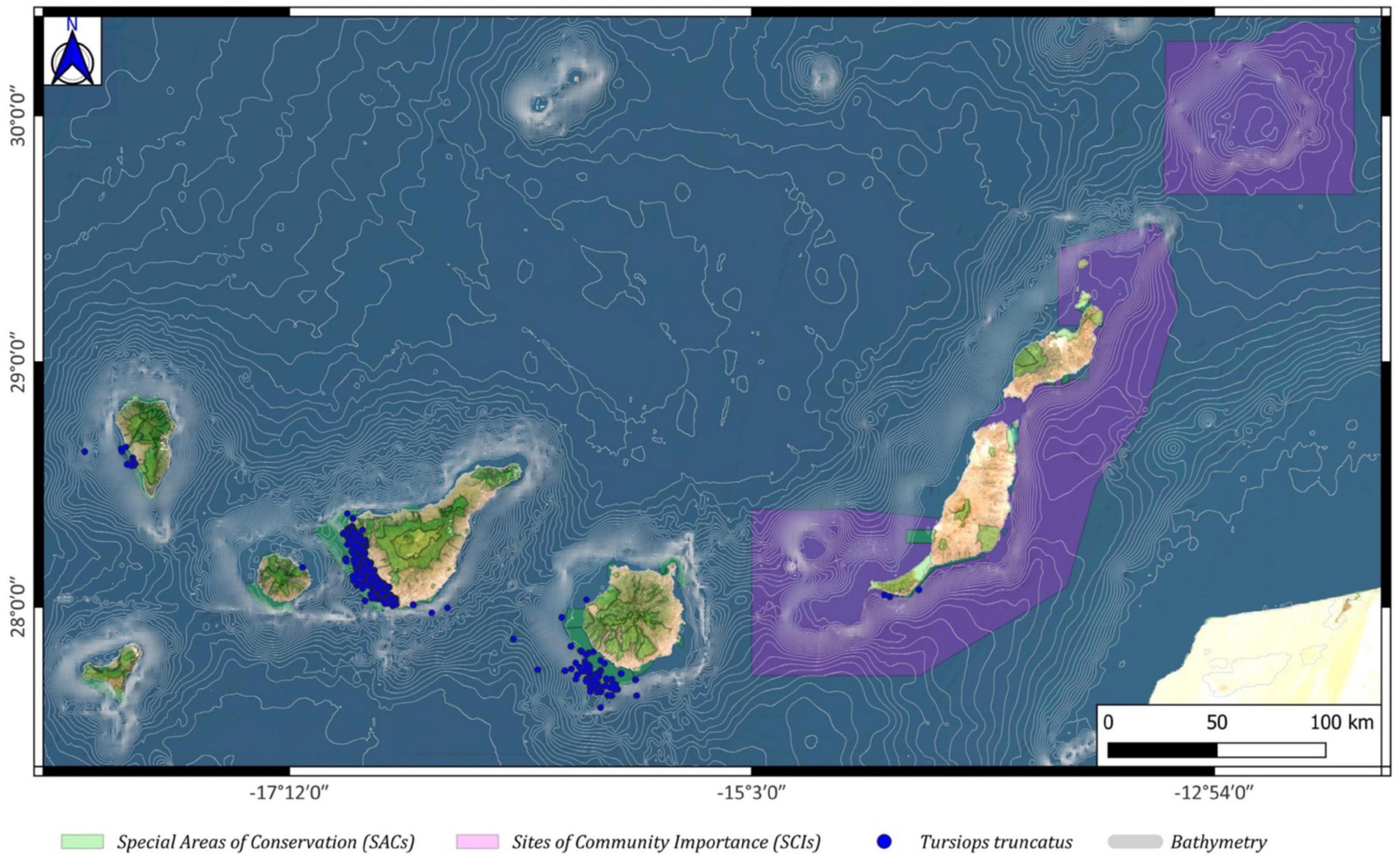
Figure 8. Tursiops truncatus spatial distribution around the Canary archipelago (blue circles), including the Special Areas of Conservation (SACs; green color) and the Sites of Community Importance (SCIs; pink color).
The presence of the Atlantic spotted dolphin (Stenella frontalis) was observed mostly to the southwest of the island of Gran Canaria, both inside and especially outside the SAC of Franja Marina of Mogán; they were also abundantly observed to the west of La Palma in and outside of the SAC of Franja Marina of Fuencaliente (Figure 9).
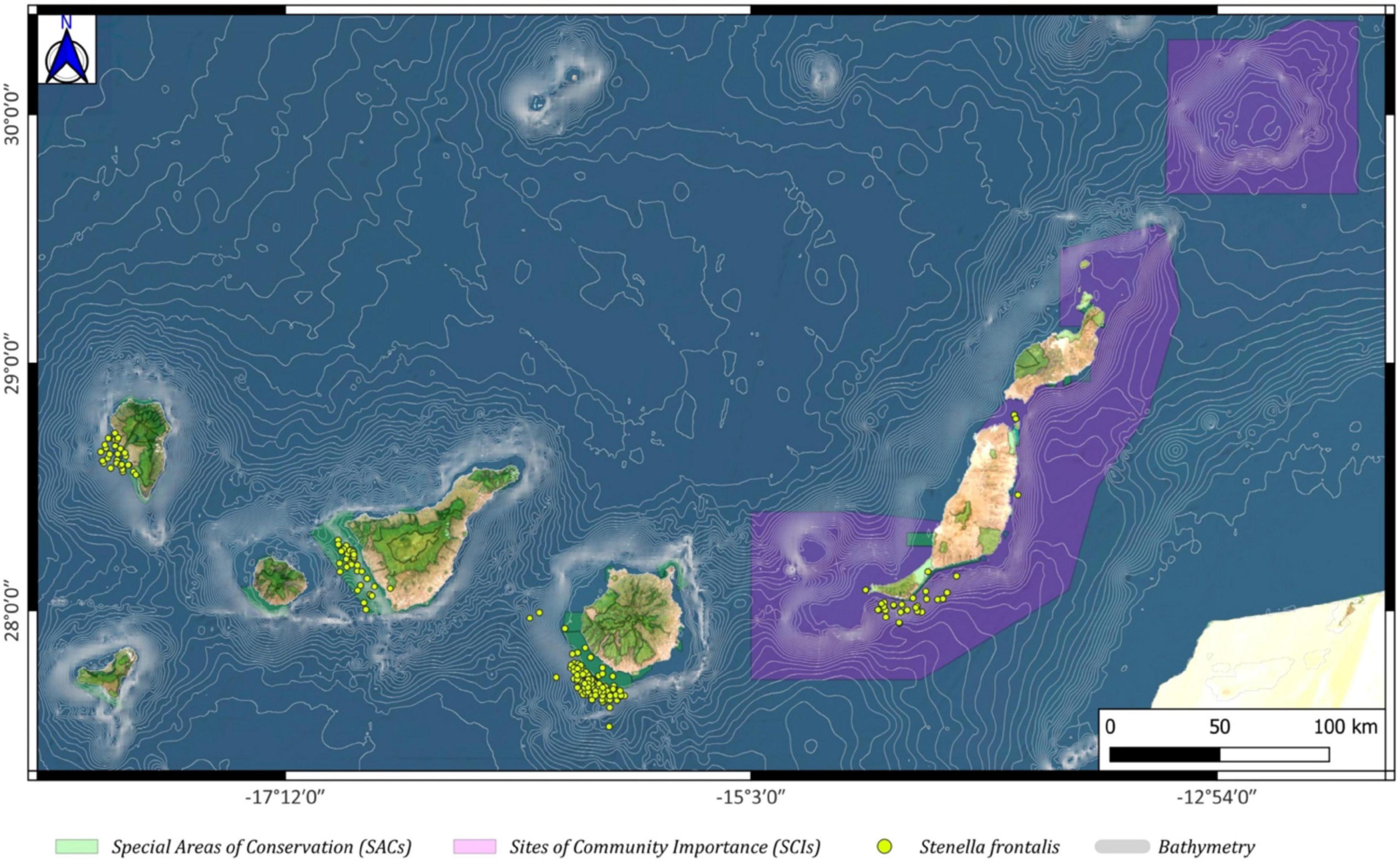
Figure 9. Stenella frontalis spatial distribution around the Canary archipelago (yellow circles), including the Special Areas of Conservation (SACs; green color) and the Sites of Community Importance (SCIs; pink color).
Balaenoptera edeni (Bryde’s whale) had a spatial distribution throughout the Canary archipelago. It was observed near the coast of different islands, namely, La Graciosa, Lanzarote, Fuerteventura, Tenerife, La Palma, and Gran Canaria, with a major presence in the last island mentioned. It was seen mainly outside the limits of the SAC of Franja Marina of Mogán (Figure 10).
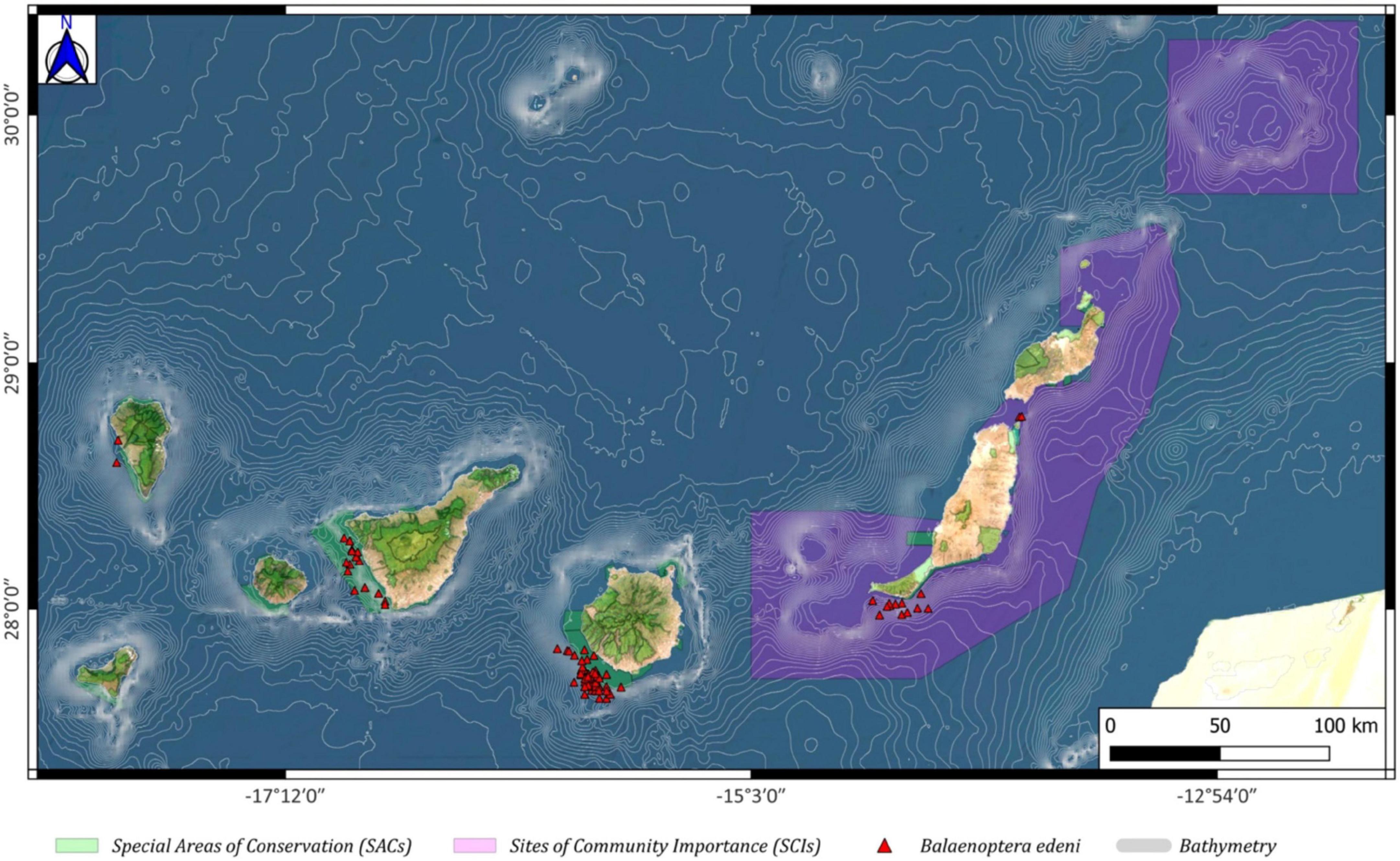
Figure 10. Balaenoptera edeni spatial distribution around the Canary archipelago (red triangles), including the Special Areas of Conservation (SACs; green color) and the Sites of Community Importance (SCIs; pink color).
Throughout the 12 years of marine mammals monitoring in different surveys, 18 species of cetaceans (14 odontocetes and 4 mysticetes) were recorded for the Canary Islands waters. Of these species, ten are labeled as “Least Concern,” four as “Data Deficient,” two as “Vulnerable,” one as “Near Threatened,” and one as “Endangered” in the International Union for Conservation of Nature (IUCN) red list (Supplementary Table 1; IUCN, 2020). Looking at the four most frequently sighted species in the Canary Islands waters, the odontocetes G. macrorhynchus, T. truncatus, and S. frontalis, and the mysticete Balaenoptera edeni are labeled as “Least Concern (LC), unlikely to become extinct in the near future” in the IUCN; however, the species T. truncatus and G. macrorhynchus, at the national level (Spain) and at the regional level (Canary Islands), are labeled as “Vulnerable.” Furthermore, S. frontalis and Balaenoptera edeni fall under the “special protection regime” (National Government: Royal Decree 139/2011, BOE N° 46, 23/02/2011; Regional Government: Decree 151/2001; BOC N° 097, 01/08/2001). The underlying scientific implication of these figures is the importance and relevance of these animals as key elements of ocean health and their role as major top-down regulators of marine ecosystem oceanic trophic chains (Reynolds et al., 2009; Giralt Paradell et al., 2019). Therefore, there is a clear need for further conservation efforts directed at the populations of these marine mammals in the Canary Islands. Additional studies related to better management and knowledge (e.g., distribution, abundance, feeding, threats, environmental variables) of these cetaceans in the Northeastern Atlantic Ocean, the Canary Islands, and nearby archipelagos such as Cape Verde, Madeira, and Azores are the need of the hour.
The results showed that all species of cetaceans concentrated in areas close to the coast had clear preferences for the leeward areas of the islands, where temperatures and concentrations of chlorophyll-a showed high values according to the environmental parameters obtained from the CMEMS. Other authors have observed that a large concentration of cetaceans seems to be connected to the environmental and biological characteristics (Perrin et al., 1994; Pérez-Vallazza et al., 2008). For instance, in the case of G. macrorhynchus, there is already a correlation between sea surface temperature and its distribution in the marine waters of Tenerife Island (Montero and Arechavaleta, 1996). Additionally, the high productivity in the Canary Islands waters, as described by various authors (Arístegui et al., 1997; Barton et al., 1998; Arístegui and Montero, 2005; Alonso-González et al., 2013), not only favors cetaceans but also their food/prey, such as cephalopods (Escánez et al., 2018), with large catches being observed in these areas, where the greatest influx of cetaceans (ICES, 2019) and small–medium pelagic fishes are found (Arístegui et al., 2009). Therefore, these areas, sheltered from the trade winds and rich in prey resources, seem to provide a suitable habitat for the cetaceans.
Moreover, of the 14 odontocetes observed during the 12 years of studies, three of them dominated the sightings (75.58% of the total sightings), namely, G. macrorhynchus, T. truncatus, and S. frontalis. The first two species have been well-studied in the Canary Islands (Morales-Herrera, 2015; Servidio et al., 2019), especially in the waters surrounding Tenerife (Carrillo and Peña, 2002; Carrillo et al., 2006, 2010; Pérez-Vallazza et al., 2008; Tobeña et al., 2014), showing similar spatial distribution in all years of the study. This suggests that environmental fluctuations do not affect the presence of those resident cetaceans. In the case of S. frontalis, unfortunately, this species has been less studied individually (Perrin et al., 1994). However, as shown by both our results and previous studies (Pérez-Vallazza et al., 2008; Carrillo et al., 2010; Morales-Herrera, 2015), it is an abundant species in the waters of the Canary Islands throughout the year.
The most frequently sighted mysticete species (and the sixth most frequently sighted cetacean species) was Balaenoptera edeni. Despite being a migratory species, its presence in the Canary Islands is quite marked (Aguilar de Soto, 2006; Carrillo et al., 2010; Morales-Herrera, 2015; Lado-Pedreda, 2018); it is found not only in these waters but also in nearby Macaronesian archipelagos, such as in Madeira (Alves et al., 2010) and the Azores (Steiner et al., 2008). Although robust information on the spatial and temporal distribution regarding the family Balaenopteridae and its habitats and/or feeding preferences is scarce, it is observed that all Balaenopteridae species found in the North Atlantic pass through the Canary Islands during certain months of the year. These animals have been monitored through observations coming from opportunity platforms (ferries navigating the inter-island waters), under the umbrella of the Red CetAvist project, where a wide spatial distribution of cetaceans of the family Balaenopteridae was observed in the waters of the Canary Islands (Lado-Pedreda, 2018). As in the case with other odontocetes species, it is assumed that their spatial distribution near the coast in the Canary Islands is also due to the high productivity in these waters (Arístegui et al., 1997) and may be related to their food preferences, in particular their affinity for schools of small pelagic fish, squid, and plankton (Tershy et al., 1993; Baines and Reichelt, 2014). In addition, the distance to the coast and the slope of the ocean floor (Tardin et al., 2017) may favor their feeding behavior; thus, it is common to find them near the Canary Islands’ western coasts.
As a consequence of rapid and mainly unplanned growth in the 80s and 90s, the Canary Islands are under constant threat of habitat degradation due to coastal land-use change, pollution by plastics and waste, maritime traffic, and, more recently, the negative effects of climate change, including rising sea levels and increasing seawater temperatures (IPCC, 2014; EEA, 2016). Climate change is having various negatives effects on the good marine environmental status worldwide. In recent years, a rising trend of an increase in temperature of 0.28°C per decade, from 1982 to 2013, was observed in the waters around the Canary Islands (Vélez-Belchí et al., 2015).
These increases in seawater temperature result in changes in the distribution of the cetacean preys in different trophic levels of the marine ecosystem (e.g., plankton, fish, cephalopods) (Evans et al., 2008). This directly affects the habits, diets, and behavior of whales and dolphins. Nowadays, scarce information is available on the effect of climate change on cetaceans in the Canary Islands and adjacent archipelagos (Madeira and Azores). This is due to the gaps in knowledge of geographical distribution, migration patterns, and diets in the Macaronesian biogeographic sub-region. However, the possible effects of climate change on cetaceans have already been described in other areas, with changes observed in the distribution patterns of these animals, mainly due to variations in the abundance or distribution of prey (Learmonth et al., 2006; Simmonds, 2016) and in the duration and timing of migration, as well as reproductive success rates (Leaper et al., 2006; Ramp et al., 2015). There is no doubt that studies of marine mammals are difficult, and trends, in their abundance and distribution, are inconclusive concerning the causal role of climate change (ICES, 2008). Therefore, it is necessary that decision-makers monitor and evaluate marine ecosystems, and include conservation plans adjusted to the current information on the ecosystems.
Since the conservation of cetaceans is of importance for regulatory bodies, the European Union has recognized the need for distribution maps of basin scales on a monthly basis (Habitats Directive 92/43/EEC, Marine Strategy Framework Directive: 2008/56/EC). Of the 18 species sighted during this study (2007–2018), we wanted to pay more attention to the three species already mentioned (G. macrorhynchus, T. truncatus, and S. frontalis), which reside and/or are present in the waters of the Canary Islands all year round (Morales-Herrera, 2015; Servidio et al., 2019). Regarding their spatial distribution, it is essential to highlight that most sightings for these species are found outside the external limits of the SACs included in the Natura 2000 Network. This spatial distribution has already been observed previously for Gran Canaria (SAC Franja Marina de Mogán) (Pérez-Vallazza and Haroun, 2005), Tenerife (SAC Franja Marina de Rasca-Teno) (Aguilar de Soto et al., 2001; Carrillo et al., 2010), La Palma (SAC Franja Marina de Fuencaliente) (Pérez-Vallazza et al., 2008), and El Hierro (Arranz et al., 2008; Amengual et al., 2015). In the marine waters of El Hierro, in addition to hosting the three species already mentioned, it should be noted that it is one of the few places in the world where the resident populations of Ziphiidae (deep-diving cetacean species) (Arranz et al., 2008) are known to exist. During the monitoring conducted between 2003 and 2007 on this island, more than 1,600 individuals of Ziphiidae were sighted in its waters (Figure 11, this study; Arranz et al., 2008; Amengual et al., 2015). Their local presence reinforces the role of the Canary Islands as a conservation hotspot for cetaceans, thus emphasizing the need to effectively protect populations of different species from any anthropogenic pressures.
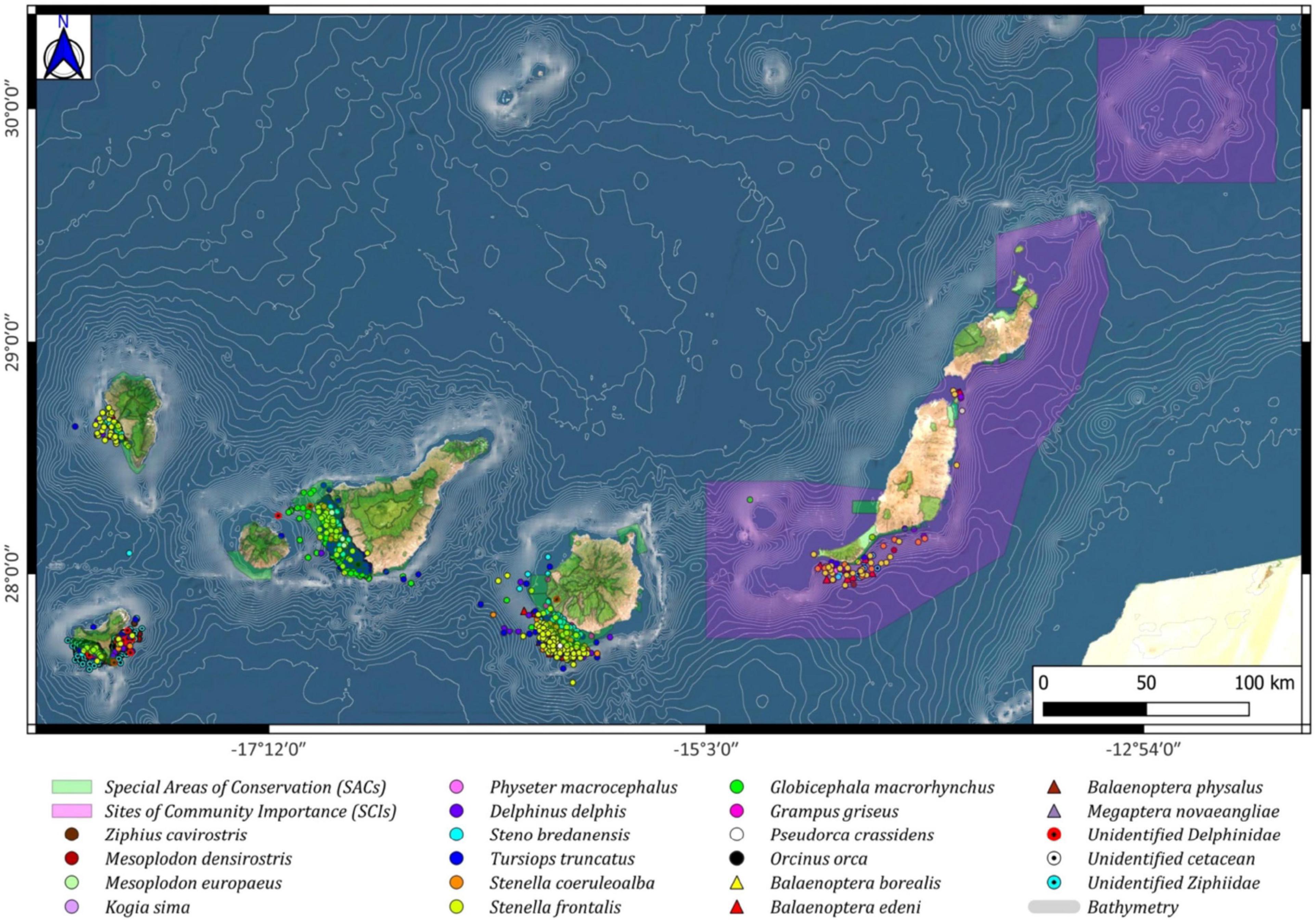
Figure 11. Cetacean spatial distribution around the Canary archipelago (Odontoceti: circles; Mysticeti: triangles), including the Special Areas of Conservation (SACs; green color) and the Sites of Community Importance (SCIs; pink color). Including sightings from El Hierro island.
After 12 years of scientific monitoring and sightings of cetacean populations around the Canary Islands, the present study shows that the current SACs are not large enough to effectively protect these endangered marine mammals; these keystone cetacean populations require large marine protected areas that include parts of their oceanic (offshore) habitat (Game et al., 2009). Currently, there are 24 SACs in the Canary Islands that have been designated for the conservation of two species of community interest, namely, the marine turtle C. caretta and the cetacean T. truncatus. However, there are other cetacean species in the marine waters surrounding the Canary Islands: the short-finned pilot whale (G. macrorhynchus), Risso’s dolphin (G. griseus), the sperm whale (P. macrocephalus), the striped dolphin (S. coeruleoalba), and the spotted dolphin (S. frontalis). These species also deserve to be included in the list for conservation and preservation in the already allocated SACs and SCIs, with special attention to their distribution, areas occupied, and the extents of these marine areas for future updates of management actions. This issue of the Canary Islands is not an isolated event; the same matter is observed in other areas such as the Mediterranean. A recent study by La Manna et al. (2020), which examined the relationships between oceanographic variables and the spatial distribution of the common bottlenose dolphin, showed the importance of updating and implementing current management and conservation instruments to extend the limits of SCIs while working on the reduction of the anthropogenic pressures that impact these marine mammals. The published results and those compiled in the present study show that the borders of several SACs and SCIs must be extended to effectively preserve the ecologically significant features sustaining the population of cetaceans found in the Canarian waters. These results should be considered in future conservation measures for the amendment and updating of management instruments, as well as the revisions of the limits of the Natura 2000 SACs. Thus, jurisdictional and administrative borders should be altered to better consider and preserve key ecological processes and functions that are ultimately responsible for the conservation of cetacean populations. To this end, the spatial distribution maps generated in the present study facilitate the consideration of the conservation of cetacean populations in the maritime spatial planning process.
New milestones in cetacean conservation also need to be considered for future measures. During the last (25th) United Nations Climate Change Conference (December 2019), Mission Blue had declared the Canary Islands as a “Hope Spot” for marine ecosystems, mainly for the cetacean populations, in the area between the islands of Tenerife and La Gomera. The objective was to support further protection of the area, in the spirit of the IUCN target of having 30% of the ocean officially protected by 2030, and to encourage the Spanish and Canarian governments to declare a large marine protected area in the waters of the islands, where humans and nature can thrive together. These Canarian waters are precisely where the greatest influx of cetaceans has been found during the study period of this work. In addition, between these two islands, there are several protected areas, SACs, that belong to the Natura 2000 Network. Considering all these features along with the ES that are generated by those animals (e.g., whale watching, scientific research, bequest, and spiritual value), currently, these waters have also been nominated as a “Whale Sanctuary” by the global Whale Heritage Sites program because of the great diversity and abundance of cetaceans found in these waters surrounding the islands of Tenerife and La Gomera. Cook et al. (2020) further describe the major ES linked to these protected cetaceans as inducing reasoned, rational compromises in decision-making, considering the various ES threats and trade-offs.
There is no doubt regarding the role played by cetaceans in the good environmental status (GES) of the ecosystems of the Canary Islands’ waters. They are considered valid indicators of the well-being status of marine ecosystems. Therefore, creating a network of marine protected areas, ecologically coherent with the biogeographical sub-region of Macaronesia, would favor the conservation of vulnerable ecological habitats and species of socio-economic interest. Thus, a wide marine protected area could be proposed for the European Macaronesia (Madeira, Azores, and Canary Islands), such as the creation of a Macaronesian Biodiversity, Ecological and Cetacean migration corridor (Carrillo, 2007). The focus of this proposal for marine protected species is on the design of an area for the conservation of vulnerable migratory species. A similar corridor has already been defined in the Mediterranean, the “Mediterranean Cetacean Migration Corridor,” as an important area for cetacean species with high ecological value (BOE-A-2018-9034). This marine protected area is included in the Natura 2000 – LIC-ESZZ16001 network and in the Important Marine Mammal Areas initiative (IMMAs8); managing and monitoring this area is a major activity of the Marine Mammal Protected Areas Task Force. The IMMAs are defined as discrete portions of habitat, important to marine mammal species, that have the potential to be delineated and managed for conservation and are identified to prioritize their consideration for conservation measures by governments, intergovernmental organizations, conservation groups, and the general public. Another example of an important conservation area is the “Alborán corridor IMMA”; this area represents a migratory corridor for vulnerable fin whales in the Northern Alborán Sea and Strait of Gibraltar (IUCN-MMPATF, 2017). In the European Macaronesia region, in an area located between Madeira and Desertas islands, there is already an established IMMA area for another marine mammal, the monk seal (Monachus monachus), which shares marine space with the common bottlenose dolphin (T. truncatus) (IUCN-MMPATF, 2018). Therefore, it is essential that monitoring and novel initiatives are undertaken to protect our waters and marine ecosystems, by identifying and enacting a “Macaronesian Biodiversity, Ecological and Cetacean Migration Corridor,” to enhance the protection of cetacean species along with their large distributional range in the Northeast Atlantic Ocean.
This study demonstrates that long-term monitoring can provide key information to identify areas of high marine mammal abundance as well as key data about their resident or migratory status in the Canarian waters. The areas with high abundance of cetaceans in many cases coincide with the designated Natura 2000 areas, SACs that were designated only to protect bottlenose dolphin populations and not for the conservation of the other cetacean species that are also present in the Canary Islands. In addition, the extent of the areas where cetacean species are distributed and inhabit extends beyond the outer limits of the designated SACs. These results suggest that improvements must be made to the current conservation measures, enabling the enlargement of the extant SACs limits to promote the efficient management of cetaceans, ecosystems, and GES in the waters of the Canary Islands. The recent declarations of two international conservation figures such as Tenerife-La Gomera “Hope Spot” by the Mission Blue Initiative or the Canary Islands “Whale Sanctuary” declaration by the program Whale Heritage Sites also pointed out the presence of cetacean species outside the extant SACs in Tenerife and La Gomera and further underpin the need of larger marine conservation areas in the Canarian archipelago. Moreover, the declaration of the Tenerife–La Gomera “Hope Spot” was considered as a starting point to inspire the Spanish and Canary governments for the enactment of a large marine protected area in the Spanish waters. In this sense, it seems essential to provide more extensive monitoring of oceanic waters around the Canary Islands and nearby archipelagos as well as promote novel initiatives to protect migratory routes and marine ecosystems, by identifying a “Macaronesian Biodiversity and Ecological Migration Corridor for Cetaceans,” a conservation figure that has been already proposed in the scientific literature but has not yet been accomplished through the proper measures and conservation policies by the Portuguese and Spanish Governments, which certainly will contribute to their international conservation commitment of an increase of marine protected areas in the framework of the European Biodiversity Strategy and the United Nations SD Goals. The enactment of the proposed marine biological corridor would require international cross-border cooperation efforts for assuring migratory pathways for diverse endangered marine species (such as cetaceans, seabirds, selected fish species and other), taking consideration of current marine spatial planning processes.
Our findings are also relevant as a contribution to population abundance estimates of cetaceans for the Canarian archipelago and its relationships with other nearby geographic areas in the Northeast Atlantic Ocean, such is the case of the archipelagos of Azores, Madeira, and Cape Verde, where some frequently sighted cetaceans may migrate at different times of the year. The connectivity or the genetic imprint of their populations is an interesting area of research for future funding and scientific efforts.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethical review and approval was not required for the animal study because this work presents information on the distribution of marine mammals collected through controlled observations in accordance with the code of conduct of the Government of the Canary Islands (Decree 178/2000; Decree 1727/2007).
IH and RH conceived the ideas. MC collected most of the data. MCdE did most of the graphics and maps. IH, MC, and RH processed the data. IH led the writing of the manuscript. RH did the fine-tuning of the final document. All authors contributed critically to the drafts and gave final approval for publication.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This study is a contribution of the project MarSP (EASME/EMFF/2016/1.2.1.6/03SI2.763106) supported by the European Maritime and Fisheries Fund (EMFF) and the European Commission. Thanks to the POSEIDON Program, run by the ULPGC and funded by Fundación Biodiversidad - Ministry of Ecological Transition & Demographic Challenge (MITECO). MCdE was supported by a PhD student fellowship granted by Agencia Canaria de Investigación Innovación y Sociedad de la Información (ACIISI) of the Canarian regional government. We thank the three reviewers whose comments/suggestions helped improve and clarify this manuscript. Due to unexpected and abrupt health problem, just after the submission of this article, we have experienced the loss of one of the co-authors: MC. May he rest in peace. We dedicate this work to his memory and his long-lasting efforts to protect marine mammals.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.669790/full#supplementary-material
Aguilar de Soto, N. (2006). Comportamiento Acústico y de Buceo del Calderón Globicephala macrorhynchus y del Zifio de Blainville Mesoplodon Densirostris en las Islas Canarias. Implicaciones Sobre los Efectos del Ruido Antrópico y Las Colisiones con Embarcaciones. Ph.D. thesis. San Cristóbal de La Laguna: Universidad de La Laguna.
Aguilar de Soto, N., Díaz, F., Carrillo, M., Brito, A., Barquín, J., Alayón, P., et al. (2001). Evidence of Disturbance of Protected Cetacean Populations in the Canary Islands. IWC SC/53/WW1. London: International Whaling Commission.
Alonso-González, I., Arístegui, J., Lee, C., Sánchez-Vidal, A., Calafat, A., Fabrés, J., et al. (2013). Carbon dynamics within cyclonic eddies: insights from a biomarker study. PLoS One 8:e82447. doi: 10.1371/journal.pone.0082447
Alves, F., Dinis, A., Cascao, I., and Freitas, L. (2010). Bryde’s whale (Balaenoptera brydei) stable associations and dive profiles: new insights into foraging behavior. Mar. Mamm. Sci. 26, 202–212. doi: 10.1111/j.1748-7692.2009.00333.x
Alves, F., Dinis, A., Nicolau, C., Ribeiro, C., Kaufmann, M., Fortuna, C., et al. (2015). Survival and abundance of short-finned pilot whales in the archipelago of Madeira, NE Atlantic. Mar. Mamm. Sci. 31, 106–121. doi: 10.1111/mms.12137
Amengual, P., Areces Maqueda, J. J., Centellas Bodas, A., Garay Zabala, J., and Serrada Hierro, J. (2015). Propuesta de Declaración del Parque Nacional Marino “Mar de Las Calmas” Documento Técnico. Ministerio de Agricultura, Alimentación y Medio Ambiente, Organismo Autonomo Parques Nacionales. (San Cristóbal de La Laguna: Fundación General Universidad de La Laguna), 162.
Arístegui, J., and Montero, M. F. (2005). Temporal and spatial changes in plankton respiration andbiomass in the Canary Islands region: the effect of mesoscale variability. J. Mar.Syst. 54, 65–82. doi: 10.1016/j.jmarsys.2004.07.004
Arístegui, J., Barton, E. D., Álvarez-Salgado, X. A., Santos, A. M. P., Figueiras, F. G., Kifani, S., et al. (2009). Sub-regional ecosystem variability in the Canary Current upwelling. Prog. Oceanogr. 83, 33–48. doi: 10.1016/j.pocean.2009.07.031
Arístegui, J., Tett, P., Hernández-Guerra, A., Basterretxea, G., Montero, M. F., Wild, K., et al. (1997). The influence of island-generated eddies on chlorophyll distribution: a study of mesoscale variation around Gran Canaria. Deep Sea Res. Part I Oceanogr. Res. Pap. 44, 71–96. doi: 10.1016/s0967-0637(96)00093-3
Arranz, P., Aguilar de Soto, N., Díaz, F., Domínguez, I., Aparicio, C., Brito, A., et al. (2008). Cetáceos Avistados en el Entorno de la Isla de El Hierro, en el Marco del Interreg “Bionatura”. Grupo de Investigación BIOECOMAC, Departamento de Biología Animal. (San Cristóbal de La Laguna: Universidad de La Laguna), 43.
Arregui, M., Bernaldo de Quirós, Y., Saavedra, P., Sierra, E., Suárez-Santana, C. M., Arbelo, M., et al. (2019). Fat embolism and sperm whale ship strikes. Front. Mar. Sci. 6:379. doi: 10.3389/fmars.2019.00379
Baines, M. E., and Reichelt, M. (2014). Upwellings, canyons and whales: an important winter habitat for balaenopterid whales off Mauritania, northwest Africa. J. Cetacean Res. Manage. 141, 57–67.
Barton, E. D., Arístegui, J., Tett, P., Cantón, M., García-Braun, J., Hernández-León, S., et al. (1998). The transition zone of the Canary Current upwelling region. Prog. Oceanogr. 41, 455–504. doi: 10.1016/s0079-6611(98)00023-8
Baulch, S., and Perry, C. (2014). Evaluating the impacts of marine debris on cetaceans. Mar. Pollut. Bull. 80, 210–221. doi: 10.1016/j.marpolbul.2013.12.050
Bécognée, P., Moyano, M., Almeida, C., Rodríguez, J. M., Fraile-Nuez, E., Hernández-Guerra, A., et al. (2009). Mesoscale distribution of clupeoid larvae in an upwelling filament trapped by a quasi-permanent cyclonic eddy off Northwest Africa. Deep Sea Res. Part I Oceanogr. Res. Pap. 56, 330–343. doi: 10.1016/j.dsr.2008.10.008
Buckland, S. T., Anderson, D. R., Burnham, K. P., and Laake, J. L. (1993). Distance Sampling: Estimating Abundance of Biological Populations. London: Chapman and Hall.
Carrillo, M. (2007). Cetaceans in the Macaronesia region (Eastern Central Atlantic Ocean) and Threats Faced in the Canary Islands. Western African Talk on Cetaceans and their Habitats. Convention on the conservation of Migratory Species of Wild Animals (CMS/ UNEP). WATCH 2007. Tenerife: Western African Talks on Cetaceans and their Habitats.
Carrillo, M., and Peña, A. (2002). Estudio de Distribución y Abundancia del Calderón de Aleta Corta en el sur de Tenerife. (Canary Government report), 240.
Carrillo, M., and Ritter, F. (2010). Increasing numbers of ship strikes in the Canary Islands: proposals for immediate action to reduce risk of vessel-whale collisions. J. Cetacean Res. Manage. 11, 131–138.
Carrillo, M., Pérez-Vallaza, C., and Álvarez-Vázquez, R. (2010). Cetacean diversity and distribution off Tenerife (Canary Islands). Mar. Biodivers. Rec. 3, 1–9.
Carrillo, M., Tejedor, M., and Ramos, J. J. (2006). Análisis del Estado de Conservación de las Costas y Áreas Marinas de la Isla de Tenerife. Proyecto de Voluntariado Ambiental Monachus. Resultados-2006. Fundación Global Nature–Tenerife Conservación. Área de Medio Ambiente y Paisaje. (Santa Cruz de Tenerife: Cabildo Insular de Tenerife), 80.
Carrillo, M., Tejedor, M., Peña, A., and González, G. (2002). Estudios Aplicados a la Conservación de las Poblaciones Cetáceos: Provincia de Santa Cruz de Tenerife. Informe Técnico. Dirección General de Política Ambiental. (Santa Cruz de Tenerife: Gobierno de Canarias), 254.
Carwardine, M. (1995). Whales, Dolphins, and Porpoises., 1st American Edn. London: Dorling Kindersley.
Cook, D., Malinauskaite, L., Davíđottir, B., Ögmundardóttir, H., and Roman, J. (2020). Reflections on the ecosystem services of whales and valuing their contribution to human well-being. Ocean Coast. Manage. 186:105100. doi: 10.1016/j.ocecoaman.2020.105100
Correia, A. M., Gandra, M., Liberal, M., Valente, R., Gil, A., Rosso, M., et al. (2019). A dataset of cetacean occurrences in the Eastern North Atlantic. Sci. Data 6:177. doi: 10.1038/s41597-019-0187-2
Dudzinski, K. M. (1999). An Investigation of Intraspecific Behavior in Bottle-Nose Dolphins Around Mikura Island. Final JSPS Fellowship Report. California, USA: Dolphin Communication Project Oxnard. Available online at: https://www.dolphincommunicationproject.org/pdf/FinalReport1999Japan.pdf
EEA (2016). Climate Change, Impacts and Vulnerability in Europe 2016 An Indicator-Based Report, EEA Report No 1/2017. Luxembourg: EEA. doi: 10.2800/66071
Escánez, A., Rodríguez, S., Riera, R., Rocha, F., and Brito, A. (2018). Octopods of the Canary Islands. new records and biogeographic relationships. Molluscan Res. 39, 1–12. doi: 10.1080/13235818.2018.1527970
Evans, P. G. H., Smeenk, C., and Van Waerebeek, K. (2008). “Cuvier’s beaked whale Ziphius cavirostris,” in Mammals of the British Isles, eds S. Harris and D. W. Yalden (London: Mammal Society), 690–692.
Fais, A., Lewis, T. P., Zitterbart, D. P., Álvarez, O., Tejedor, A., and Aguilar Soto, N. (2016). Correction: abundance and distribution of Sperm Whales in the Canary Islands: Can sperm whales in the archipelago sustain the current level of ship-strike mortalities? PLoS One 11:e0155199. doi: 10.1371/journal.pone.0155199
Fernández, R., Santos, M. B., Carrillo, M., Tejedor, M., and Pierce, G. (2009). Stomach contents of cetaceans stranded in the Canary Islands 1996-2006. J. Mar. Biol. Assoc. U.K. 89, 873–883. doi: 10.1017/s0025315409000290
Game, E. T., Grantham, H. S., Hobday, A. J., Pressey, R. L., Lombard, A. T., Beckley, L. E., et al. (2009). Pelagic protected areas: the missing dimension in ocean conservation. Trends Ecol. Evol. 24, 360–369. doi: 10.1016/j.tree.2009.01.011
García-Álvarez, N., Fernández, A., Boada, L. D., Zumbado, M., Zaccaroni, A., Arbelo, M., et al. (2015). Mercury and selenium status of bottlenose dolphins (Tursiops truncatus): a study in stranded animals on the Canary Islands. Sci. Total Environ. 536, 489–498. doi: 10.1016/j.scitotenv.2015.07.040
García-Álvarez, N., Martín, V., Fernández, A., Almunia, J., Xuriach, A., Arbelo, M., et al. (2014). Levels and profiles of POPs (organochlorine pesticides, PCBs, and PAHs) in free-ranging common bottlenose dolphins of the Canary Islands, Spain. Sci. Total Environ. 493, 22–31. doi: 10.1016/j.scitotenv.2014.05.125
Giralt Paradell, O., Díaz López, B., and Methion, S. (2019). Modelling common dolphin (Delphinus delphis) coastal distribution and habitat use: insights for conservation. Ocean Coast. Manage. 179:104836. doi: 10.1016/j.ocecoaman.2019.104836
Heimlich-Boran, J. R. (1993). Social Organisation of the Short-Finned Pilotwhale (Globicephala macrorhynchus), With a Special Reference to Thesocial Ecology of Delphinids. Ph.D. thesis. (Cambridge: Cambridge University), 154–157.
Herrera, I., Carrillo, M., and Haroun, R. (2020). Conservación de cetáceos y planificación del espacio marino en las Islas Canarias. Okeanos 10, 13–19.
ICES (2008). Advice on the Changes in the Distribution and Abundance of Marine Species in the OSPAR Maritime Area in Relation to Changes in Hydrodynamics and Sea Temperature. Section 1.5.5.1, 32.
ICES (2019). Interim Report of the Working Group on Cephalopod Fisheries and Life History (WGCEPH), 5–8 June 2018. Pasaia: ICES.
IPCC (2014). Summary for Policymakers, Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva: IPCC. doi: 10.1017/CBO9781107415324
IUCN (2020). The IUCN Red List of Threatened Species. Version 2020-1. Available online at: https://www.iucnredlist.org (accessed October 15, 2020).
IUCN-MMPATF (2017). Hellenic Trench IMMA. Full Accounts of Mediterranean IMMA Factsheet. IUCN Joint SSC/WCPA Marine Mammal Protected Areas Task Force. Gland: IUCN. (accessed October 15, 2020).
IUCN-MMPATF (2018). Hellenic Trench IMMA, Global Dataset of Important Marine Mammal Areas (IUCN-IMMA). December 2018. Made Available Under Agreement on Terms of Use by the IUCN Joint SSC/WCPA Marine Mammal Protected Areas Task Force and Made. Available online at: www.marinemammalhabitat.org/imma-eatlas. (accessed October 15, 2020).
Jefferson, T. A., Webber, M. A., and Pitman, R. L. (2015). Marine Mammals of the World: A Comprehensive Guide to Their Identification, 3rd Edn (San Diego, CA: Academic Press).
La Manna, G., Ronchetti, F., Sarà, G., Ruiu, A., and Ceccherelli, G. (2020). Common bottlenose dolphin protection and sustainable boating: species distribution modeling for effective coastal planning. Front. Mar. Sci. 7:542648. doi: 10.3389/fmars.2020.542648
Lado-Pedreda, L. (2018). Spatial Modelling of the Distribution of the Family Balaenopteridae in the Canary Islands Based on Data from Civic Science (Red CetAvist). Master’s thesis. (San Cristóbal de La Laguna: University of La Laguna), 46.
Landeira, J. M., Brochier, T., Mason, E., Lozano-Soldevilla, F., Hernández-León, S., and Barton, E. D. (2017). Transport pathways of decapod larvae under intense mesoscale activity in the Canary-African coastal transition zone: implications for population connectivity. Sci. Mar. 81, 299–315. doi: 10.3989/scimar.04599.06a
Leaper, R., Cooke, J., Trathan, P., Reid, K., Rowntree, V., and Payne, R. (2006). Global climate drives southern right whale (Eubalaena australis) population dynamics. Biol. Lett. 2, 289–292. doi: 10.1098/rsbl.2005.0431
Learmonth, J. A., MacLeod, C. D., Santos, M. B., Pierce, G. J., Crick, H. Q. P., and Robinson, R. A. (2006). Potential effects of climate change on marine mammals. Oceanogr. Mar. Biol. 44, 431–464.
Martín, V., and Carrillo, M. (in press). Cetáceos en Canarias: una perspectiva biológica. Rev. Acad. Canaria Cienc.
MITECO (2019). Estrategia Marina para la Demarcación Marina de Canarias: PARTE I. MARCO GENERAL.CARACTERÍSTICAS DE LA DEMARCACIÓN CANARIA, 1–13. Available online at: https://www.miteco.gob.es/es/costas/temas/proteccion-medio-marino/parteimarcogeneraldm can_tcm30-498330.pdf
Montero, R., and Arechavaleta, M. (1996). “Distribution Patterns: Relationships between Depths, Sea Surface Temperature, and Habitat Use of Short-finned Pilot Whales South-West of Tenerife. European Research on Cetaceans - 10,” in Proceedings of the 10th annual conference of the European Cetacean Society, Lisbon, 193–198.
Morales-Herrera, T. (2015). Cetacean Seasonal distribution in Canary Islands. Master’s thesis. (San Cristóbal de La Laguna: University of La Laguna), 62.
OSPAR (2009). Assessment of the Environmental Impact of Underwater Noise. Available online at: https://qsr2010.ospar.org/media/assessments/p00436_JAMP_Assessment_Noise.pdf (accessed November 07, 2020).
Pérez-Vallazza, C., Álvarez-Vázquez, R., Cardona, L., and Hernández-Brito, J. (2008). Cetacean diversity at the west coast of La Palma Island (Canary Islands). J. Mar. Biol. Assoc. U.K. 88, 1289–1296. doi: 10.1017/s0025315408001239
Pérez-Vallazza, C., and Haroun, R. (2005). Estudio de Comunidades de Cetáceos y Tortugas Marinas Enlos LIC Franja Marina de Mogán y Sebadales de Playa del Inglés. Caracterización, ordenación y gestión de Áreas Marinas Protegidas de la Macaronesia. PARQMAR [INTERREG IIIB: 03/MAC/4.2/M9]. 107.
Perrin, W. F., Caldwell, D. K., and Caldwell, M. C. (1994). ““Atlantic Spotted Dolphin Stenella frontalis” (G. Cuvier, 1829),” in Handbook of Marine Mammals. The First Book of Dolphins, Vol. 5, eds S. H. Ridgway and R. J. Harrison (London: Academic Press), 173–190.
Puig-Lozano, R., Bernaldo de Quirós, Y., Díaz-Delgado, J., García-Álvarez, N., Sierra, E., De la Fuente, J., et al. (2018). Retrospective study of foreign body-associated pathology in stranded cetaceans, Canary Islands (2000–2015). Environ. Pollut. 243, 519–527. doi: 10.1016/j.envpol.2018.09.012
Puig-Lozano, R., Fernández, A., Saavedra, P., Tejedor, M., Sierra, E., De la Fuente, J., et al. (2020). Retrospective study of traumatic intra-interspecific interactions in stranded cetaceans, Canary Islands. Front. Vet. Sci. 7:107. doi: 10.3389/fvets.2020.00107
Ramp, C., Delarue, J., Palsbøll, P. J., Sears, R., and Hammond, P. S. (2015). Adapting to a warmer ocean—seasonal shift of baleen whale movements over three decades. PLoS One 10:e0121374. doi: 10.1371/journal.pone.0121374
Reynolds, J. E. III, Marsh, H., and Ragen, T. J. (2009). Marine mammal conservation. Endang. Spec. Res. 7, 3–28. doi: 10.3354/esr00179
Rodríguez, J. M., Barton, E. D., Hernández-León, S., and Arístegui, J. (2004). The influence of mesoscale physical processes on the larval fish community in the Canaries CTZ, in summer. Prog. Oceanogr. 62, 171–188. doi: 10.1016/j.pocean.2004.07.006
Rodríguez, J. M., Hernández-León, S., and Barton, E. D. (1999). Mesoscale distribution of fish larvae in relation to an upwelling filament off Northwest Africa. Deep Sea Res. Part I Oceanogr. Res. Pap. 46, 1969–1984. doi: 10.1016/s0967-0637(99)00036-9
Ruíz de la Rosa, M., Tuya, F., Herrera, R., Moro-Abad, L., Espino, F., Haroun, R., et al. (2015). “Praderas de angiospermas marinas de las Islas Canarias,” in Atlas de las Praderas Marinas de España, eds J. M. Ruiz, J. E. Guillén, A. Ramos Segura, and M. M. Otero (Murcia: IEO), 425–487.
Santos, M. B., and Pierce, G. J. (2015). Marine mammals and good environmental status: science, policy and society; challenges and opportunities. Hydrobiologia 750, 13–41. doi: 10.1007/s10750-014-2164-2
Servidio, A., Pérez-Gil, E., Pérez-Gil, M., Cañadas, A., Hammond, P. S., and Martín, V. (2019). Site fidelity and movement patterns of short-finned pilot whales within the Canary Islands: evidence for resident and transient populations. Aquat. Conserv. Mar. Freshw. Ecosyst. 29, 227–241. doi: 10.1002/aqc.3135
Simmonds, M. P. (2016). “Impacts and effects of ocean warming on marine mammals,” in Explaining Ocean Warming: Causes, Scale, Effects and Consequences, eds D. Laffoley and J. M. Baxter (Gland: IUCN), 303–320.
Steiner, L., Silva, M. A., Zereba, J., and Leal, M. J. (2008). Bryde’s whales, Balaenoptera edeni, observed in the Azores: a new species record for the region. Mar. Biodivers. Rec. 1:e66.
Tardin, R. H., Chun, Y., Simão, S. M., and Alves, M. A. S. (2017). Modeling habitat use by Bryde’s whale Balaenoptera edeni off southeastern Brazil. Mar. Ecol. Prog. Ser. 576, 89–103. doi: 10.3354/meps12228
Tejedor, M., and Carrillo, M. (2018). Informe Cetáceos varados 2000-2018. Base de datos del Gobierno de Canarias. Arucas: ULPGC-UISA.
Tershy, B. R., Acevedo, G. A., Breese, D., and Strong, C. S. (1993). Diet and feeding behavior of fin and Bryde’s whales in the central Gulf of California, Mexico. Rev. Invest. Cient. 11, 1–4.
Tobeña, M., Escánez, A., Rodríguez, Y., López, C., Ritter, N., and Aguilar de Soto, N. (2014). Interisland movements of common bottlenose dolphins Tursiops truncatus among the Canary Islands: online catalogues and implications for conservation and management. Afr. J. Mar. Sci. 36, 137–141. doi: 10.2989/1814232X.2013.873738
Vélez-Belchí, P., González-Carballo, M., Pérez-Hernández, M. D., and Hernández-Guerra, A. (2015). “Open ocean temperature and salinity trends in the Canary Current Large Marine Ecosystem,” in Oceanographic and Biological Features in the Canary Current Large Marine Ecosystem, IOC Technical Series, Vol. 115, eds L. Valdés and I. Déniz-González (Paris: IOC-UNESCO), 299–308.
Keywords: dolphins, whales, East Atlantic Ocean, oceanographic features, abundance, conservation corridor, marine spatial planning
Citation: Herrera I, Carrillo M, Cosme de Esteban M and Haroun R (2021) Distribution of Cetaceans in the Canary Islands (Northeast Atlantic Ocean): Implications for the Natura 2000 Network and Future Conservation Measures. Front. Mar. Sci. 8:669790. doi: 10.3389/fmars.2021.669790
Received: 19 February 2021; Accepted: 30 June 2021;
Published: 03 August 2021.
Edited by:
Christian Lønborg, Aarhus University, DenmarkReviewed by:
Andrew M. Fischer, University of Tasmania, AustraliaCopyright © 2021 Herrera, Carrillo, Cosme de Esteban and Haroun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Inma Herrera, aW5tYS5oZXJyZXJhQGZwY3QudWxwZ2MuZXM=; aW5tYV9oZXJyaUBob3RtYWlsLmNvbQ==
†Deceased
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.