- 1Fundación Dominicana de Estudios Marinos, Bayahibe, Dominican Republic
- 2Doctorado en Ciencias Biológicas y de la Salud, Universidad Autónoma Metropolitana, Ciudad de México, Mexico
- 3The Nature Conservancy, Caribbean Division, Punta Cana, Dominican Republic
- 4Laboratorio de Ecología Experimental, Departamento de Estudios Ambientales, Universidad Simón Bolívar, Caracas, Venezuela
Coral assisted fertilization, larval rearing and recruit propagation success in significant ecological scales, largely depend on scaling up and replicating these efforts in as many regions as possible. The Dominican Foundation for Marine Studies (FUNDEMAR) has become a pioneer of these efforts in the Dominican Republic, being the first institution to successfully implement coral sexual reproduction techniques in the country and establishing the first mobile larvae culturing facility. Here we share our perspective on three main components behind the success of FUNDEMAR’s program: (1) a self-sustainable program in alliance with local and international organizations, (2) the design and construction of the first Coral Assisted Reproduction Laboratory in the country, and a (3) clearly defined scalable structure for outcome performance. Two years after program implementation, FUNDEMAR has successfully produced an annual regional coral spawning prediction calendar, cultured seven coral species, and seeded over 4,500 substrates with more than 268,200 sexual coral recruits in approximately 1,880 m2 reef areas. Here, we provide a detailed description of a fully functional assisted coral reproduction program, including the lessons learned during its implementation as well as a series of specific solutions. We hope this work will help and inspire other countries and small institutions to replicate FUNDEMAR’s coral assisted reproduction program components and contribute to the expansion of sexual coral restoration efforts in the Caribbean.
Introduction
During the past few years, coral restoration efforts have increased globally (Boström-Einarsson et al., 2020) and across many Caribbean and Latin-American countries (Bayraktarov et al., 2020). Coral restoration has been proposed as a solution to decrease and/or ameliorate the impacts of local and global stressors as a useful tool to preserve reefs, recover populations of reef building corals or both (Calle-Triviño et al., 2018; Bayraktarov et al., 2020; Boström-Einarsson et al., 2020; Goergen et al., 2020; Shaver et al., 2020).
Coral restoration can be done through asexual and sexual propagation and other types of interventions to enhance substrate suitability for outplanted or gardened corals (Boström-Einarsson et al., 2020). While asexual fragmentation can rapidly increase coral tissue coverage, it does not directly increase genetic diversity in coral populations, making threatened populations potentially vulnerable to diseases and environmental stress (Barton et al., 2015). On the other hand, sexual propagation may increase genetic diversity and therefore resilience, however, low recruit survival after seeding to a reef is still a challenge for restoration practitioners (Baums et al., 2019). Regardless of the method or the approach used, restoration programs still face challenges often associated with the problem of scaling up efforts in space and time while preserving genetic diversity of wild coral populations (Boström-Einarsson et al., 2020).
Recent advances on larval enhancement techniques wouldn’t have been possible without the cumulative general knowledge of coral biology and ecology (Guest, 2010; Guest et al., 2014). Reproduction in corals consists of a sequence of events which include gametogenesis, spawning (for spawning species), fertilization, embryogenesis, planulation, dispersal, settlement, and recruitment (e.g., Harrison and Wallace, 1990; Baird et al., 2009; Harrison, 2011). These events have been described from different perspectives, including histological (e.g., Duerden, 1902; Fadlallah, 1983; Szmant, 1986; Richmond and Hunter, 1990; Soong, 1991; Steiner, 1998; Morales, 2006; Ritson-Williams et al., 2009; Weil and Vargas, 2010; Harrison, 2011; Soto and Weil, 2016), observational (e.g., Vermeij et al., 2003; Levitan et al., 2004; Van Woesik et al., 2006; Vize, 2006; Bastidas et al., 2012; Chamberland et al., 2016; Keith et al., 2016; Fogarty and Marhaver, 2019), and experimental (e.g., Morse et al., 1988; Webster et al., 2004; Kuffner et al., 2006, 2007; Nugues and Szmant, 2006; Vermeij et al., 2009; Ritson-Williams et al., 2010; Marhaver et al., 2015; Sharp et al., 2015) studies.
Early attempts to incorporate the concept of larval propagation for restoration purposes started two decades ago (Rinkevich, 1995; Petersen and Tollrian, 2001). In the Caribbean, first successful attempts to rear larvae in the laboratory were conducted by Szmant and Miller (2006) in the Florida Keys and by Randall and Szmant (2009) in Puerto Rico. Currently, Coralium lab at the National Autonomous University of Mexico, SECORE International and the Caribbean Research and Management of Biodiversity (CARMABI) are three leading institutions in the Caribbean on this subject. These institutions have played an important role in expanding larval propagation across geographies, improving capacity building, and developing cutting edge technology and protocols applied for coral restoration (e.g., Marhaver et al., 2015; Chamberland et al., 2016, 2017; Banaszak pers. comm.).
The Dominican Foundation for Marine Studies (FUNDEMAR) has been able to locally adapt these techniques integrating an assisted sexual coral reproduction program, the first one in the Dominican Republic (Calle-Triviño et al., 2018). Here, we provide our perspective explaining the success of FUNDEMAR’s program: (1) a self-sustainable program in alliance with local and international organizations, (2) the design and construction of the first Coral Assisted Reproduction Laboratory in the country, and (3) a clearly defined scalable structure for outcome performance.
Key Components Behind the Success of FUNDEMAR’s Program
FUNDEMAR’s Self-Sustainable Coral Restoration Program
FUNDEMAR was founded in 1991 with the mission to preserve marine ecosystems in the Dominican Republic. It is based in Bayahibe, a small town of fishermen which is also a hotspot for tourism activities in the country (Supplementary Figure 1). The early strategic alliance between FUNDEMAR and the tourism private sector was a key step for achieving a sustainable program. The first couple of alliances in our coral conservation program motivated and prompted other local stakeholders (hotels, resorts, dive centers, and the community) to get involved. In time, the stakeholders themselves became emotionally engaged with FUNDEMAR to preserve coral reefs resilience for its intrinsic value and for its services to local people.
This structure was consolidated not only with the support of the private sector (economically and in kind) but also by FUNDEMAR’s initiative to create permanent income mechanisms (such as hosting educational programs with international students amongst other fundraising strategies). As a result, since 2012 to date, FUNDEMAR has gradually scaled up the program as it was becoming more sustainable. The coral program relies on four pillars: (1) research and monitoring, (2) asexual (Calle-Triviño et al., 2020) and sexual restoration (Calle-Triviño et al., 2018; Supplementary Figure 2), (3) management, and (4) community integration and awareness. FUNDEMAR’s coral restoration program (Supplementary Figures 1,2) is only one of a series of interconnected marine conservation programs that complement each other for preserving coastal ecosystems within the Southeastern Reefs Marine Sanctuary.
In 2017, FUNDEMAR joined SECORE’s capacity building program as an implementation partner. Since, SECORE has provided restoration technology and training on larval prorogation to FUNDEMAR staff. This alliance and the partnership with The Nature Conservancy’s coral strategy, placed the Dominican Republic as a key location to scale up assisted fertilization and larval propagation efforts in the Caribbean, integrating SECORE’s unique Coral Rearing In Situ Basins (CRIBs, SECORE International, 2020; Supplementary Figures 2G,H) and substrate designs (Chamberland et al., 2017; Supplementary Figure 3), amongst other technologies.
Design and Construction of the First Coral Assisted Reproduction Laboratory in the Dominican Republic
In 2019, FUNDEMAR established a mobile ex situ coral assisted reproduction laboratory by adapting a storage container (12 m × 2.44 m × 2.6 m L, W, H) into a fully functioning laboratory for rearing corals (Supplementary Figures 2E,F). Inspired by the experience and knowledge gathered from trainings in Curaçao by SECORE and in Mexico by CORALIUM and SECORE, various FUNDEMAR partners helped design and build this facility for its intended goal. The main adaptations involved lining the walls with a thermal insulator, running electrical lines, plumbing for fresh and sea water flow and installing fans and air conditioning for temperature regulation. The wet lab itself consists of three major components: (1) a water catchment system, (2) a filtration system, and (3) an aquarium system. An in-depth description of the laboratory including design and system functioning is provided in the Supplementary Material (Supplementary Figure 4).
Furthermore, the CRIB technology allowed us to escalate the production of coral settlers. CRIBs consist of three main parts: (1) a floatable hydrodynamic ring that maintains the structure and is anchored to the bottom, (2) a canopy located above the floatable ring that protects the embryos from ultraviolet radiation as well as fresh water in case of rain, and (3) an underwater vertical enclosure with removable mesh windows (100 and 200 μm) to allow continuous water exchange (Supplementary Figures 2G,H).
Clearly Defined Scalable Structure for Outcome Performance
FUNDEMAR’s program structure has been capable of scaling up gradually based on clear annual goals defined in the institution’s restoration plan, which is annually evaluated. During the first 2 years of program implementation (2019–2020), efforts were mainly focused on the standardization of coral sexual culturing techniques as well as building capacity for FUNDEMAR staff and other coral restoration practitioners. Yet, we were able to scale up and enhance outcomes from 1 year to the next (presented below). We also focused on gathering enough spawning documentations to create our own coral spawning prediction calendar. The first calendar was available in 2019 and it is updated every year to increase reliability in gamete collection locally.
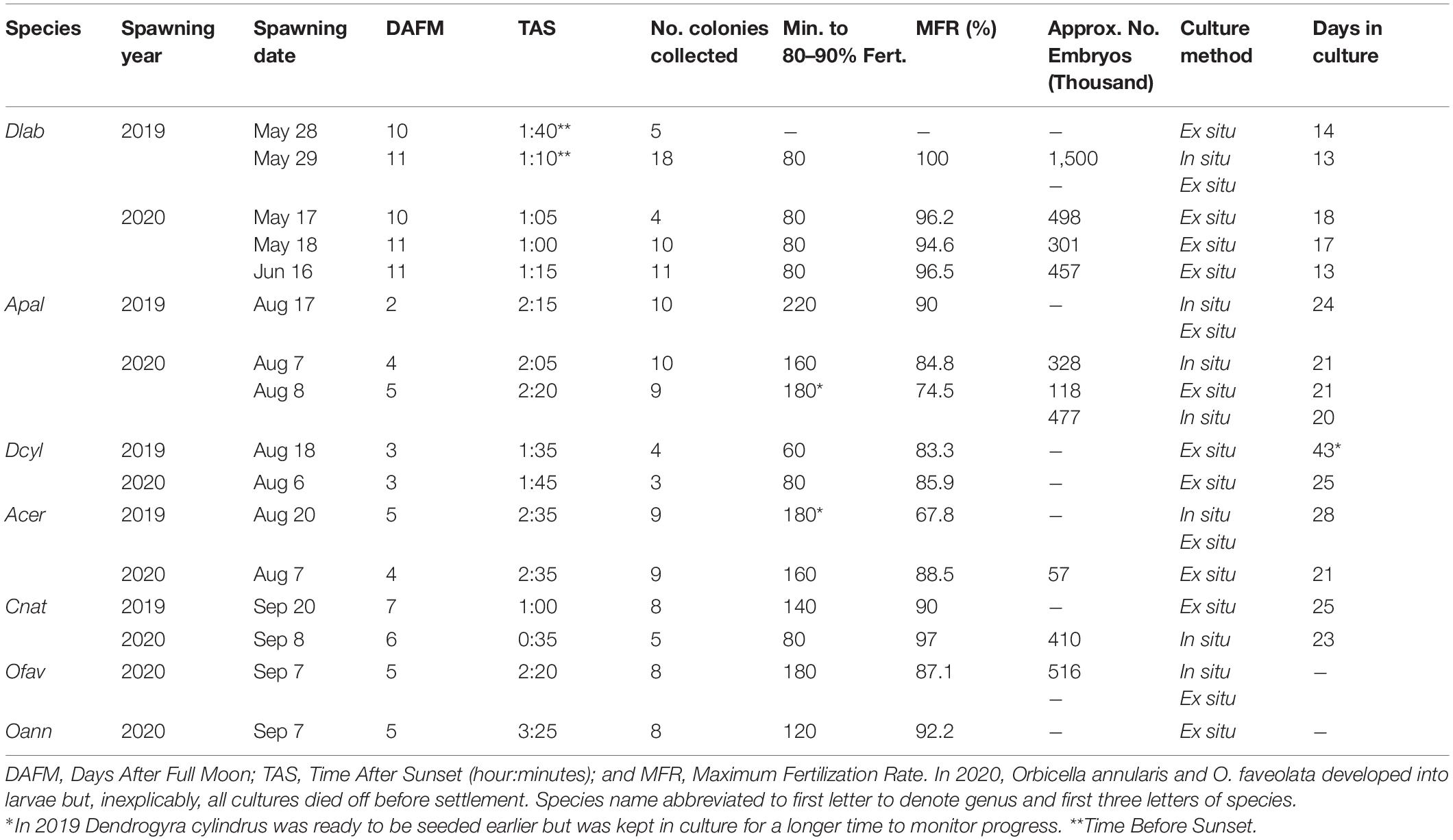
In Southeastern Dominican Republic, most massive coral spawning events (>25% of colonies spawning) occurred in May and June for Diploria labyrinthiformis, August for Acropora palmata, A. cervicornis and Dendrogyra cylindrus, and September for Colpophyllia natans, Orbicella annularis and O. faveolata. D. labyrinthiformis had the earliest spawning events while the acroporids and Orbicella spp. spawned the latest. For most species, a few colonies spawned a little the day before their massive spawning event. Spawning patterns were less stable through time and had longer windows for acroporids compared to other species (Supplementary Table 1).
From 2019 to 2020, gametes were collected, eggs fertilized, embryos cultivated, larvae settled, and recruits seeded from five different coral species: D. labyrinthiformis, D. cylindrus (Villalpando et al., 2021), A. palmata, A. cervicornis, and C. natans (Figure 1, Table 1, and Supplementary Table 2). In 2020, gametes from O. faveolata and O. annularis were also collected and successfully fertilized, however, the larvae culture died during the swimming stage in both in situ and ex situ cultures for unknown reasons.

Figure 1. Coral assisted sexual reproduction phases (1) Gamete collection (2) Fertilized embryos (3) Primary polyps and (4) Coral recruits after 6 to 12 months of seeding for the five reared coral species: (A) Diploria labyrinthiformis (B) Dendrogyra cylindrus (C) Acropora palmata (D) Acropora cervicornis (E) Colpophyllia natans. (Photo A3: Paul Selvaggio/PghZoo/SECORE).
The implementation of both in situ and ex situ culturing systems allowed for a high production of substrates with coral sexual recruits or seeding units (SUs: sensu Guendulain-Garcia et al., 2016; Chamberland et al., 2017). In 2019, 1,927 SUs with 28,900 coral settlers were seeded in 850 m2 of reef. In comparison, in 2020, 2,615 SUs with 239,300 settlers were seeded in 1,030 m2 of reef. In total for both years, over 4,500 SUs with approximately 268,200 sexual coral recruits were seeded in reef areas totaling around 1,880 m2 (Supplementary Table 2). These results were achieved with systems below their maximum capacity (i.e., ex situ system 1,000 substrates and CRIB ∼2,000).
In the middle to long term, we expect to produce a database to test specific hypotheses regarding which species and substrate type show higher recruit survival and which are more cost-effective to reproduce.
Discussion
In this paper we provide our perspective about a series of guidelines that are needed to implement a sexual propagation restoration program through assisted coral reproduction. We find these 3 points to be essential to create a robust and scalable program: (1) alliances with private and local stakeholders, (2) financial stability, and (3) adoption of novel technology supported by pertinent training to implement them. In our view, the balance between these components allowed FUNDEMAR to build the laboratory and produce results comparable to others in the Caribbean (Chamberland et al., 2016, 2017). Local engagement and key alliances between different stakeholders and the scientific community has been shown to be a strong pillar that supports conservation actions aimed to preserve coastal marine ecosystems (White and Vogt, 2000; Lundquist and Granek, 2005; Reyes-García et al., 2019).
In our perspective, the most valuable lessons learned during the implementation of our sexual propagation program are logistical, technical, and structural. These lessons are often interconnected and must be taken into account holistically as the restoration program is implemented.
From the logistic point of view, getting permits on time is a key step. FUNDEMAR has been able to obtain environmental permits to implement these actions, based on our solid reputation of contributing and supporting the national strategy for coral conservation in the Dominican Republic. Environmental hazards and catastrophes such as hurricanes may impose logistical problems, and in some cases, delay restoration efforts. The best way to cope with the problem is to create an action plan that can be carried out in various seasons and includes more than one species for collection and fertilization as well as diverse cultivation methods to reduce vulnerability to climatic, environmental or unforeseen events, always having a backup plan.
The combination of differing culturing facilities and settlement substrates designs was crucial to increase the production and upscaling the restoration effort. This allowed us to seed a substantial amount of SUs in larger reef areas. The use of the CRIBs represents a feasible strategy for mass production of SUs, nevertheless it is important to consider the local environmental conditions with this method. This type of system works well in relatively calm waters and having an emergency plan for adverse weather conditions is fundamental; in 2019 and 2020, we removed the CRIBs as a hurricane prevention measure, and sheltered the substrates in the laboratory as well as in underwater structures used for preconditioning substrates.
As for the technical lessons, initial time investment in monitoring local spawning events is essential to accurately forecast them, reducing costs in the long term by making time in the field more efficient. This in turn leads to better working plans and therefore a reduction of potential human errors that may compromise the success of gamete collection, fertilization, and culture in the laboratory. Also, the adoption of an experimental design framework and the standardization of protocols to assist coral reproduction is highly recommended. In terms of experimental design, having a clear formulation of hypothesis for the restoration experiment based on the comparison of variables measured in experimental, reference and control plots effectively estimates restoration success (Chapman, 1998; Croquer et al., 2019). Furthermore, sampling efforts should be established a priori based on power analysis.
It is critical to acknowledge that coral restoration by itself is not the solution for preventing local coral reef decline. Instead, coral propagation must be aligned with specific management strategies aimed at reducing local threats such as overfishing, water pollution, unsustainable coastal development and direct physical damage to the reef such as anchoring (Abelson et al., 2020). One of the advantages of FUNDEMAR’s coral restoration approach is the holistic strategy implemented to protect Southeastern Dominican reefs. Though local actions are essential, a coordinated international approach is necessary to reduce global threats to coral reefs such as mass coral bleaching and ocean acidification caused by climate change (Abelson et al., 2020).
To conclude, FUNDEMAR’s coral assisted sexual propagation program has become a robust program due to key alliances entailing the private sector, NGOs and governmental agencies, the engagement of the local community, the creation of job opportunities and training to become coral restoration technicians, and the creation of sources of income that make the program self-sustainable. The program relies on clear missions and objectives and integrates local stakeholders. Finally, the key of a successful and replicable program is to adapt the existing technologies and customize it to the reality of each country, the conditions of the site where it will be implemented and the technical and economic capacity of the institution.
FUNDEMAR is expected to continue growing in the near future and will continue sharing experiences with other NGOs in the Dominican Republic and the Caribbean region. We hope our story will be useful for others to design their programs and to replicate efforts across the region to scale up coral reef restoration efforts.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
RS-B conceived, designed and coordinated FUNDEMAR’s coral reproduction program, designed and led the Coral Assisted Reproduction Laboratory, acquired funds and resources and performed field work, supervised the data collection and execution of all activities, and contributed equally to the manuscript. MV led data collection, processing and analysis, elaborated the spawning prediction calendars, performed the field work, created figures and tables, and contributed equally to the manuscript. SG-G designed, led, adapted, and improved the construction of the Coral Assisted Reproduction Laboratory, performed field work, trained the technicians for the data collection, supported the creation of figures and tables, and contributed equally to the manuscript. AC contributed to the manuscript conception and structure, performed field work, provided support for the data analysis process, and contributed equally to the manuscript. All authors contributed to the article and approved the submitted version.
Funding
FUNDEMAR’s coral assisted sexual reproduction program was financially supported by the USAID and USFWS Grant No. F18AP00797, the German Embassy in Santo Domingo contract No. Gz: Wi 468.40 EKF AA46187491, and The Nature Conservancy Grant No: FY20-FUNDEMAR-CoralDR-032020; Award ID P118582-F105711.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We sincerely thank SECORE International, CORALIUM lab, and CARMABI for continually sharing their knowledge and providing training and guidance. Especially, we thank SECORE for the technology, trainings, and material contributions provided throughout the implementation of the program. We recognize the in-kind contributions provided by the Romana-Bayahibe private sector and local dive centers and appreciate the constant support of the government entities involved in this program. Lastly, we are exceptionally grateful to the FUNDEMAR team that made this possible, including Alido Luis, Yulissa Reyes, Juan Adrien, Andreina Valdez, and all of our volunteers and collaborators who have given above and beyond to this endeavor.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.669505/full#supplementary-material
References
Abelson, A., Reed, D. C., Edgar, G. J., Smith, C. S., Kendrick, G. A., Orth, R. J., et al. (2020). Challenges for restoration of coastal marine ecosystems in the Anthropocene. Front. Mar. Sci. 7:544105. doi: 10.3389/fmars.2020.544105
Baird, A. H., Guest, J. R., and Willis, B. L. (2009). Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu. Rev. Ecol. Evol. Syst. 40, 551–571. doi: 10.1146/annurev.ecolsys.110308.120220
Barton, J. A., Willis, B. L., and Hudson, K. S. (2015). Coral propagation: a review of techniques for ornamental trade and reef Restoration. Rev. Aquac. 0, 1–19. doi: 10.1111/raq.12135
Bastidas, C., Bone, D., Croquer, A., Debrot, D., Garcia, E., Humanes, A., et al. (2012). Massive hard coral loss after a severe bleaching event in 2010 at Los Roques, Venezuela. Rev. Biol. Trop. 60, 29–37. doi: 10.15517/rbt.v60i0.19843
Baums, I. B., Baker, A. C., Davies, S. W., Grottoli, A. G., Kenkel, C. D., Kitchen, S. A., et al. (2019). Considerations for maximizing the adaptive potential of restored coral populations in the western Atlantic. Ecol. App. 29:e01978. doi: 10.1002/eap.1978
Bayraktarov, E., Banaszak, A. T., Montoya Maya, P., Kleypas, J., Arias-González, J. E., Blanco, M., et al. (2020). Coral reef restoration efforts in Latin American countries and territories. PloS One. 15:e0228477. doi: 10.1371/journal.pone.0228477
Boström-Einarsson, L., Babcock, R. C., Bayraktarov, E., Ceccarelli, D., Cook, N., Ferse, S. C., et al. (2020). Coral restoration–A systematic review of current methods, successes, failures and future directions. PloS One. 15:e0226631. doi: 10.1371/journal.pone.0226631
Calle-Triviño, J., Cortés-Useche, C., Sellares-Blasco, R. I., and Arias-González, J. E. (2018). Assisted fertilization of threatened staghorn coral to complement the restoration of nurseries in Southeastern Dominican Republic. Reg. Stud. Mar. Sci. 18, 129–134. doi: 10.1016/j.rsma.2018.02.002
Calle-Triviño, J., Rivera-Madrid, R., León-Pech, M. G., Cortés-Useche, C., Sellares-Blasco, R. I., Aguilar-Espinosa, M., et al. (2020). Assessing and genotyping threatened staghorn coral Acropora cervicornis nurseries during restoration in southeast Dominican Republic. PeerJ. 8:e8863. doi: 10.7717/peerj.8863
Chamberland, V. F., Petersen, D., Guest, J. R., Petersen, U., Brittsan, M., and Vermeij, M. J. (2017). New seeding approach reduces costs and time to outplant sexually propagated corals for reef restoration. Sci. Rep. 7, 1–12. doi: 10.1038/s41598-017-17555-z
Chamberland, V. F., Petersen, D., Latijnhouwers, K. R. W., Snowden, S., Mueller, B., and Vermeij, M. J. (2016). Four-year-old Caribbean Acropora colonies reared from field-collected gametes are sexually mature. Bull. Mar. Sci. 92, 263–264. doi: 10.5343/bms.2015.1074
Chapman, M. G. (1998). Improving sampling designs for measuring restoration in aquatic habitats. J. Aquat. Ecosyst. Stress Recover. 6, 235–251. doi: 10.1023/A:1009987403481
Croquer, A., Sellares, R., Villalpando, M., Pollock, J., Escobar-Fadul, X., Reyes-Santana, Y., et al. (2019). “Grounding coral reef restoration in an experimental ecology framework: a case study in Bayahibe, Dominican Republic,” in Proceedings of the 72th GFCI Conference, Bayahibe, 60.
Fadlallah, Y. H. (1983). Sexual reproduction, development and larval biology in scleractinian corals. Coral Reefs 2, 129–150. doi: 10.1007/BF00336720
Fogarty, N. D., and Marhaver, K. L. (2019). Coral spawning, unsynchronized. Science. 365, 987–988. doi: 10.1126/science.aay7457
Goergen, E. A., Schopmeyer, S., Moulding, A. L., Moura, A., Kramer, P., and Viehman, T. S. (2020). Coral reef restoration monitoring guide: Methods to evaluate restoration success from local to ecosystem scales. Silver Spring, MD: NOAA Technical Memorandum NOS NCCOS 279, 145.
Guendulain-Garcia, S. G., Banaszak, A. T., Gómez-Campo, K., Mendoza-Quiroz, S., Avila-Pech, E. A., Schutter, M., et al. (2016). Seeding of early-stage sexual recruits of Acropora palmata for species and habitat rehabilitation. ICRS 13, 130–131.
Guest, J. (2010). “Rearing coral larvae for reef rehabilitation,” in Reef Rehabilitation Manual, ed. A. J. Edwards (St Lucia, Australia: The Coral Reef Targeted Research & Capacity Building for Management Program), 73–92.
Guest, J. R., Baria, M. V., Gomez, E. D., Heyward, A. J., and Edwards, A. J. (2014). Closing the circle: is it feasible to rehabilitate reefs with sexually propagated corals? Coral Reefs 33, 45–55. doi: 10.1007/s00338-013-1114-1
Harrison, P. L. (2011). “Sexual reproduction of scleractinian corals,” in Coral reefs: an ecosystem in transition, eds Z. Dubinsky and N. Stambler (Netherlands: Springer), 59–85.
Harrison, P. L., and Wallace, C. C. (1990). Reproduction, dispersal and recruitment of scleractinian corals. Ecosyst.World. 25, 133–207.
Keith, S. A., Maynard, J. A., Edwards, A. J., Guest, J. R., Bauman, A. G., Van Hooidonk, R., et al. (2016). Coral mass spawning predicted by rapid seasonal rise in ocean temperature. Proc. Biol. Sci. 283:20160011. doi: 10.1098/rspb.2016.0011
Kuffner, I. B., Brock, J. C., Grober-Dunsmore, R., Bonito, V. E., Hickey, T. D., and Wright, C. W. (2007). Relationships between reef fish communities and remotely sensed rugosity measurements in Biscayne National Park, Florida, USA. Environ. Biol. Fishes 78, 71–82.
Kuffner, I. B., Walters, L. J., Becerro, M. A., Paul, V. J., Ritson-Williams, R., and Beach, K. S. (2006). Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar. Ecol. Prog. Ser. 323, 107–117. doi: 10.3354/meps323107
Levitan, D. R., Fukami, H., Jara, J., Kline, D., McGovern, T. M., McGhee, K. E., et al. (2004). Mechanisms of reproductive isolation among sympatric broadcast-spawning corals of the Montastraea annularis species complex. Evolution 58, 308–323.
Lundquist, C. J., and Granek, E. F. (2005). Strategies for successful marine conservation: integrating socioeconomic, political, and scientific factors. Conserv. Biol. 19, 1771–1778. doi: 10.1111/j.1523-1739.2005.00279.x
Marhaver, K. L., Vermeij, M. J., and Medina, M. M. (2015). Reproductive natural history and successful juvenile propagation of the threatened Caribbean Pillar Coral Dendrogyra cylindrus. BMC Ecol. 15:9. doi: 10.1186/s12898-015-0039-7
Morales, J. A. (2006). Sexual reproduction in the Caribbean Coral genus Mycetophyllia, in La Parguera Puerto Rico [master’s thesis]. Puerto Rico: University of Puerto Rico.
Morse, D. E., Hooker, N., Morse, A. N., and Jensen, R. A. (1988). Control of larval metamorphosis and recruitment in sympatric agariciid corals. J. Exp. Mar. Biol. Ecol. 116, 193–217. doi: 10.1016/0022-0981(88)90027-5
Nugues, M. M., and Szmant, A. M. (2006). Coral settlement onto Halimeda opuntia: a fatal attraction to an ephemeral substrate? Coral Reefs 25, 585–591. doi: 10.1007/s00338-006-0147-0
Petersen, D., and Tollrian, R. (2001). Methods to enhance sexual recruitment for restoration of damaged reefs. Bull. Mar. Sci. 69, 989–1000.
Randall, C. J., and Szmant, A. M. (2009). Elevated temperature reduces survivorship and settlement of the larvae of the Caribbean scleractinian coral, Favia fragum (Esper). Coral Reefs 28, 537–545. doi: 10.1007/s00338-009-0482-z
Reyes-García, V., Fernández-Llamazares, Á, McElwee, P., Molnár, Z., Öllerer, K., Wilson, S. J., et al. (2019). The contributions of Indigenous Peoples and local communities to ecological restoration. Restor. Ecol. 27, 3–8. doi: 10.1111/rec.12894
Richmond, R. H., and Hunter, C. L. (1990). Reproduction and recruitment of corals: comparisons among the Caribbean, the tropical Pacific, and the Red Sea. Mar. Ecol. Prog. Ser. 60, 185–203. doi: 10.3354/meps060185
Rinkevich, B. (1995). Restoration strategies for coral reefs damaged by recreational activities: the use of sexual and asexual recruits. Restor. Ecol. 3, 241–251. doi: 10.1111/j.1526-100X.1995.tb00091.x
Ritson-Williams, R., Arnold, S. N., Fogarty, N. D., Steneck, R. S., Vermeij, M. J., and Paul, V. J. (2009). New perspectives on ecological mechanisms affecting coral recruitment on reefs. Smithson. Contrib. Mar. Sci. 38, 437–457. doi: 10.5479/si.01960768.38.437
Ritson-Williams, R., Paul, V. J., Arnold, S. N., and Steneck, R. S. (2010). Larval settlement preferences and post-settlement survival of the threatened Caribbean corals Acropora palmata and A. cervicornis. Coral Reefs 29, 71–81. doi: 10.1007/s00338-009-0555-z
SECORE International. (2020). Engineering Restoration, Coral Rearing In-Situ Basins- CRIBS. Available Online at: http://www.secore.org/site/our-work/detail/engineering-restoration.60.html [Accessed April 5, 2021]
Sharp, K. H., Sneed, J. M., Ritchie, K. B., Mcdaniel, L., and Paul, V. J. (2015). Induction of larval settlement in the reef coral Porites astreoides by a cultivated marine Roseobacter strain. Biol. Bull. 228, 98–107. doi: 10.1086/BBLv228n2p98
Shaver, E., Courtney, C., West, J., Maynard, J., Hein, M., Wagner, C., et al. (2020). A Manager’s guide to coral reef restoration planning and design. NOAA coral reef conservation program. NOAA Tech. Memoran. CRCP 36:120. doi: 10.25923/vht9-tv39
Soong, K. (1991). Sexual reproductive patterns of shallow-water reef corals in Panama. Bull. Mar. Sci. 49, 832–846.
Soto, D., and Weil, E. (2016). Sexual reproduction in the Caribbean coral genus Isophyllia (Scleractinia: Mussidae). PeerJ. 4:e2665. doi: 10.7717/peerj.2665
Steiner, S. C. C. (1998). La ultraestructura de espermatozoides y su valor en la sistemática de Scleractinia (Cnidaria: Antnozoa). Rev. Biol. Trop. 46, 127–135.
Szmant, A. M. (1986). Reproductive ecology of Caribbean reef corals. Coral Reefs. 5, 43–53. doi: 10.1007/BF00302170
Szmant, A. M., and Miller, M. W. (2006). Settlement preferences and post-settlement mortality of laboratory cultured and settled larvae of the Caribbean hermatypic corals Montastraea faveolata and Acropora palmata in the Florida Keys, USA. Proc. Int. Coral Reef Symp. 2, 43–49.
Van Woesik, R., Lacharmoise, F., and Köksal, S. (2006). Annual cycles of solar insolation predict spawning times of Caribbean corals. Ecol. Lett. 9, 390–398. doi: 10.1111/j.1461-0248.2006.00886.x
Vermeij, M. J. A., Sampayo, E., Bröker, K., and Bak, R. P. M. (2003). Variation in planulae release of closely related coral species. Mar. Ecol. Prog. Ser. 247, 75–84. doi: 10.3354/meps247075
Vermeij, M. J. A., Smith, J. E., Smith, C. M., Thurber, R. V., and Sandin, S. A. (2009). Survival and settlement success of coral planulae: independent and synergistic effects of macroalgae and microbes. Oecologia 159, 325–336. doi: 10.1007/s00442-008-1223-7
Villalpando, M. F., Croquer, A., and Sellares-Blasco, R. I. (2021). First report of in situ survival of laboratory-reared offspring of the vulnerable Dendrogyra cylindrus in the Caribbean. Bull. Mar. Sci. 97, 237–238.
Vize, P. D. (2006). Deepwater broadcast spawning by Montastraea cavernosa, Montastraea franksi, and Diploria strigosa at the Flower Garden Banks, Gulf of Mexico. Coral Reefs. 25, 169–171. doi: 10.1007/s00338-005-0082-5
Webster, N. S., Smith, L. D., Heyward, A. J., Watts, J. E. M., Webb, R. I, Blackall, L. L., et al. (2004). Metamorphosis of a scleractinian coral in response to microbial biofilms. Appl. Environ. Microbiol. 70, 1213–1231. doi: 10.1128/aem.70.2.1213-1221.2004
Weil, E., and Vargas, W. L. (2010). Comparative aspects of sexual reproduction in the Caribbean coral genus Diploria (Scleractinia: Faviidae). Mar. Biol. 157, 413–426. doi: 10.1007/s00227-009-1328-5
Keywords: coral restoration, coral reefs, sexual propagation, coral rearing, science-based solutions, Dominican Republic
Citation: Sellares-Blasco RI, Villalpando MF, Guendulain-García SD and Croquer A (2021) Assisted Coral Reproduction in the Dominican Republic: A Successful Story to Replicate in the Caribbean. Front. Mar. Sci. 8:669505. doi: 10.3389/fmars.2021.669505
Received: 18 February 2021; Accepted: 23 June 2021;
Published: 28 July 2021.
Edited by:
Baruch Rinkevich, National Institute of Oceanography, Israel Oceanographic and Limnological Research, IsraelReviewed by:
Sebastian Schmidt-Roach, University of Tasmania, AustraliaDouglas Fenner, Independent Researcher, Pago Pago, American Samoa
Copyright © 2021 Sellares-Blasco, Villalpando, Guendulain-García and Croquer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rita I. Sellares-Blasco, cnNlbGxhcmVzQGdtYWlsLmNvbQ==
 Rita I. Sellares-Blasco
Rita I. Sellares-Blasco Maria F. Villalpando
Maria F. Villalpando Sergio D. Guendulain-García
Sergio D. Guendulain-García Aldo Croquer
Aldo Croquer