
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 15 June 2021
Sec. Deep-Sea Environments and Ecology
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.662899
This article is part of the Research Topic Deep-sea Sponge Ecosystems: Knowledge-based Approach Towards Sustainable Management and Conservation View all 29 articles
Cold-water coral reefs (CWC) are known to be biodiversity hotspots, however, the sponge assemblages found to dwell within these habitats haven not been studied in depth to date in the Mediterranean Sea. The present article provides the first insight on the associated sponge fauna of the recently discovered CWC communities on the Catalan Margin and, to a lesser extent, the Cabliers Coral Mound Province, while also reviewing the current knowledge of the sponge fauna dwelling in all the Mediterranean CWC provinces. In regards to the studied areas, some rare species are cited for the first time in the Mediterranean or redescribed, while two of them, Hamacantha (Hamacantha) hortae sp. nov. and Spongosorites cabliersi sp. nov. are new to science. At a basin scale, Mediterranean CWC appear as poriferan biodiversity hotspots, yet current diversity values on each site rather represent a small fraction of its actual fauna. Additionally, the existence of an endemic sponge fauna exclusively dwelling on CWC is refuted. Nonetheless, the sponge fauna thriving in Mediterranean CWC appears to be unique, and different from that of other Atlantic regions. Finally, with the current knowledge, the sponge fauna from the Mediterranean CWC is grouped in three distinguishable clusters (Alboran Sea, Western and Eastern Mediterranean), which appears to be determined by the basins water circulation, specially the Levantine Intermediate Water and the Atlantic Water following a western-eastern pattern from the Strait of Gibraltar to the Adriatic Sea. Overall, sponge living in Mediterranean CWC are still poorly explored in most areas, yet they appear to be good candidates for biogeographical studies.
Zoobank Registration: LSID urn:lsid:zoobank.org:pub:E58A3DFF-EDC5-44FC-A274-1C9508BF8D15.
Coral dominated ecosystems are amongst the most exceptional and endangered benthic habitats all over the world (Hughes et al., 2002; Roberts et al., 2002). From a functional point of view, they play a major role in benthic-pelagic coupling processes and biogeochemical cycles (Wild et al., 2008) by creating a flow between water-column productivity and the benthic realm (Lesser, 2006). From a structural point of view, reef-building corals act as ecosystem engineers (sensu Jones et al., 1994), forming complex biogenic frameworks and providing niches, nursery grounds or shelter for a wide variety of organisms (Jensen and Frederiksen, 1992; Plaisance et al., 2009; Messmer et al., 2011). As so, they result in a heterogeneity increase in the abundance and functional diversity of the associated fauna (Buhl-Mortensen et al., 2010; Linley et al., 2017), giving them the status of biodiversity hotspots (Hughes et al., 2002; Roberts et al., 2002).
The cold-water corals (CWC) Desmophyllum pertusum (Linnaeus 1758) (hereon referred as Lophelia pertusa) and Madrepora oculata Linnaeus 1758 are slow growing, reef-forming species, with an almost worldwide distribution (Roberts et al., 2009b). Both species can build reefs several hundreds of meters tall and, due to their three-dimensional structure, they alter the water flux and sedimentation rates across their bodies, creating a myriad of different microhabitats within the reef (Roberts et al., 2009b; Buhl-Mortensen et al., 2016 and references within both). Like their shallow waters counterparts, their complex configuration provides suitable habitat and nursery ground for other species (Rogers, 1999; Henry and Roberts, 2007; Henry et al., 2013), even commercial fish (Baillon et al., 2012; Corbera et al., 2019), which has led to the consideration of CWC as deep-sea biodiversity hotspots (Roberts et al., 2009b; Henry and Roberts, 2016), as they greatly enhance the biodiversity of the areas where they occur (Jensen and Frederiksen, 1992). This, paired with their long lifespan (Buhl-Mortensen et al., 2010) and susceptibility to anthropogenic impacts (Rogers, 1999; Orejas et al., 2009), has prompted their inclusion as Vulnerable Marine Ecosystems (VMEs) by United Nations General Assembly (2007) (UNGA Resolution 61/105), the Food and Agriculture Organization (FAO, 2008; General Fisheries Commission for the Mediterranean (GFCM S.A.C.), 2008) and the OSPAR commission (OSPAR, 2009, 2010). Nevertheless, and despite the rapid increase of information regarding the associated CWC reef biodiversity over the last two decades, considerable knowledge gaps still exist, specially outside of the North Atlantic area (Henry and Roberts, 2016). In this regard, studies focusing on the fauna associated with CWC habitats consistently note the high proportion of suspension and filter feeders such as sponges, cnidarians, bryozoans, crinoids, ophiuroids or ascidians, which are suspected to benefit from shelter and elevated feeding positions (Henry and Roberts, 2007; Roberts et al., 2009b; Buhl-Mortensen et al., 2016; Corbera et al., 2019). In this sense, studies and projects focusing on North-Atlantic CWC habitats have reported more than 1.300 associated species (Roberts et al., 2006), representing a 30% increase from less than a decade ago (Rogers, 1999), yet it is expected that this number will continue growing as further work to characterize the high faunal diversity of NE Atlantic cold-water coral reefs is on-going (Roberts et al., 2006). In this regard, CWC-associated sponges in the North Atlantic might be amongst the globe’s most well-characterized, with studies focusing partially or fully on their biodiversity, distribution and spatial and trophic ecology having been undertaken during the past decades (e.g., Henry and Roberts, 2007; van Soest et al., 2007; Roberts et al., 2009a; van Soest and Beglinger, 2009; Kazanidis and Witte, 2016; Kazanidis et al., 2016). Amongst others, these studies have highlighted the vast diversity of CWC-associated Porifera (van Soest and De Voogd, 2015), which is often underrepresented (Henry and Roberts, 2016), and started unveiling their ecological importance for the reefs, with sponges enhancing the reef’s biodiversity (Kazanidis et al., 2016), and being key actors in the biogeochemical cycles (Rix et al., 2016, 2017; Bart et al., 2021) and eroding processes (Beuck and Freiwald, 2005; Beuck et al., 2007, 2010) within CWC reefs.
On the contrary, despite a surging interest and research focus on CWC ecosystems in the Mediterranean Sea (Evans et al., 2019), less than 500 species have been recorded so far associated with CWC reefs (Rueda et al., 2019). Currently, from all their known accompanying fauna, Porifera stands out as one of the most diverse groups associated with Mediterranean CWC, with ca. 90 species recorded so far (Rueda et al., 2019), yet it is still far beyond Atlantic records for the same phyla (van Soest and Lavaleye, 2005; van Soest et al., 2007; van Soest and De Voogd, 2015). Additionally, while CWC ecosystems are known to occur across the whole Mediterranean basin, almost all data regarding Mediterranean CWC associated sponges comes from the Italian margin (Bertolino et al., 2019) and the Gulf of Lions (Vacelet, 1969), with information for the rest of the basin being almost anecdotal and limited to just a few species (e.g., Aguilar et al., 2011; Boury-Esnault et al., 2015; Fabri et al., 2017; Santín et al., 2020b). As so, Mediterranean CWC might be considered as suitable environments for the discovery of new or rare species (Cardone et al., 2019; Santín et al., 2020b). Furthermore, while CWC support diverse associated fauna, most of it seems to be rather facultative than endemic to CWC, yet this is still to be assessed (Rueda et al., 2019). Finally, prior works on Atlantic CWC have suggested that geographical distance between CWC and water currents might be amongst the most important factors shaping the diversity and composition of CWC sponge associated fauna (van Soest et al., 2007; van Soest and De Voogd, 2015). Regarding the later, invertebrates’ larvae might act as passive particles in a given water mass, thus being their transport tied to their said water mass movements (Shanks et al., 2002). The Mediterranean Sea, being a semi-enclosed basin (Robinson et al., 2001), with a single connection to the Atlantic through the Strait of Gibraltar, poses a perfect setting to test such hypotheses. In this regard, the Mediterranean is a concentration basin (evaporation exceeds precipitation and runoff), which circulation is mainly marked by two water masses: the inflowing Atlantic Water (AW) and the Levantine Intermediate Water (LIW) (Menna and Poulain, 2010). In this sense, the LIW is formed at the eastern Mediterranean by cooling and evaporation of the surface mixed layer, yet the exact process of the LIW formation and the exact locality where it occurs is still subject of debate (Hayes et al., 2019). After its formation, the LIW flows westwards at a depth of 200 – 600 m toward the Adriatic Sea and the Sicily Channel, from which it enters the western Mediterranean Sea, eventually exiting the basin through the Strait of Gibraltar as the Western Intermediate Water (WIW) (Robinson et al., 2001; Menna and Poulain, 2010). Flowing on opposing direction is the Atlantic Water (AW), which enters the Mediterranean Sea through the Strait of Gibraltar, down to 200 m depth, to compensate for the water’s evaporation net loss across the basin. It flows throughout the western part of the basin subjected to cyclonic anticyclonically mesoscale phenomena, gradually becoming the Modified Atlantic Water (MAW), which flows into the eastern part of the basin through the Strait of Sicily, where it transforms into the Levantine Surface Water (LSW), ultimately taking part in the formation of the LIW (Robinson et al., 2001). Currently, Mediterranean CWC’ distribution is believed to be marked by the Levantine Intermediate Water (LIW) (Chimienti et al., 2019), thus it would also be expected to have a major influence in the growth and distribution of CWC associated fauna.
During the ABIDES (Assessment of Bottom-trawling Impacts in Deep-sea Sediments, September, 2017) project, CWCs ecosystems were discovered at several locations within the Blanes Canyon (north-western Mediterranean Sea), always associated with the presence of exposed rocky outcrops. While the presence of CWC in the area was known from incidental Agassiz trawl data (Zabala et al., 1993; Rosell and Uriz, 2002; Aymà et al., 2019), the occurrence of living CWCs reefs in this canyon had not been documented before, and represented an unexpected finding, especially considering that they occur in a heavily trawled area (De Leo et al., 2019; Puig et al., 2019). During the ABIDES-ROV oceanographic cruise, it was possible to recollect a few pieces of M. oculata from a canyon wall, which proved to be rich in sponges (Santín et al., 2018a). Additionally, during the MELCOR (MELilla CORals, June 2012) Cruise, the Cabliers Coral Mound in the Alboran Sea, the largest coral mound found alive in the Mediterranean Sea up to date (Corbera et al., 2019), was characterized and sampled for the first time. Again, a diverse associated sponge fauna was detected in CWC fragments (Costa et al., 2018), yet it still needs to be studied in depth.
In this context, this paper aims to (i) provide the first insight into the associated poriferan fauna of the recently discovered CWCs in the Blanes Canyon and in the Cabliers Coral Mound, (ii) testing whether or not a Mediterranean CWC endemic sponge fauna exists, (iii) gaining insight on the diversity and possible relationships between the associated sponge fauna of CWC provinces within the Mediterranean (iv) exploring the possible role of the Mediterranean water masses circulation in the distribution of CWC sponge fauna across the Mediterranean basin.
Cold-water coral reefs provinces are typically defined as areas with large coral growth and colony density (Taviani et al., 2011), yet the term is not without controversy (Chimienti et al., 2019; Orejas and Jiménez, 2019). Originally termed for CWCs occurring in the Atlantic (Taviani et al., 2011), currently there are eight CWC provinces recognized in the Mediterranean basin so far (Taviani et al., 2017; Chimienti et al., 2019; Angeletti et al., 2020), being: the “Eastern Alboran CWC province (eA),” the “Gulf of Lions CWC province (GoL),” the “South Sardinia CWC province (sS),” the “South Malta CWC province (M),” the “Santa Maria di Leuca CWC province (SMdL),” the “Bari Canyon CWC province (BC),” all as defined in Chimienti et al. (2019), and the recently discovered “Corsica Channel CWC province (CC),” as defined by Angeletti et al. (2020). For the purpose of the present paper, three additional sites are considered for analysis despite not being defined as CWC provinces: the “Catalan Margin (CM)” the “Strait of Gibraltar-western Alboran (SoG-wA)” and the “Albanian subfossil reefs (AL).”
Spanning from the Ligurian Sea to the northern area of the Catalan margin, the Gulf of Lions’ continental margin displays one of the highest canyon densities of the world (Würtz, 2012), with the Cap de Creus and Lacaze-Duthiers canyons being its westernmost representatives. South to the aforementioned canyons, we found the Palamós (also known as La Fonera) and the Blanes canyons, which are classified alongside the Gulf of Lions as an Ecologically or Biologically Significant Area (EBSA) by the Regional Activity Center for Specially Protected Areas (Gabrie et al., 2012). While until recently all known CWC communities in the Catalan coast were restricted to the Cap de Creus Canyon (Orejas et al., 2009), recent explorations in the Palamós and the Blanes canyons have started reporting the occurrence of living CWC communities in both areas (Lastras et al., 2016; Puig and Gili, 2019; Santín et al., 2020a,b). In its most recent revision, Chimienti et al. (2019) excluded La Fonera canyon from the Gulf of Lions CWC province, yet it acknowledged the presence of CWC reefs communities in the area. As so, both the Palamós and the Blanes canyons are here treated as a separate entity from the Gulf of Lions CWC province, yet further discussion regarding whether they should be considered a new CWC province, or part of the Gulf of Lions’ one, falls of the scope of the present paper.
The existence of dead CWC communities in the Western Alboran Sea has been long documented (Margreth et al., 2011; Palomino et al., 2011), yet the actual presence of living reefs is restricted to La Línea and Guadiaro canyons (Vázquez et al., 2015), with additional sporadic observations of isolated colonies (Palomino et al., 2011), and also being suspected to occur in the deep-sea area around Ceuta (Pardo et al., 2011). Regarding the Strait of Gibraltar, it was not until recent years that the presence of living CWC banks was reported in the area (Álvarez-Pérez et al., 2005). In this sense, the Strait of Gibraltar and its surrounding areas subjected to a series of particular oceanographic conditions with an outflow of Mediterranean Water (40 – 900 m depth) toward the Atlantic and the inflow of superficial Atlantic water on its inner area, which could justify its consideration as a distinct extra-Mediterranean CWC province (Chimienti et al., 2019). Additionally, while still scarce, a few articles have reported the presence of sponges attached to coral rubble in the Gibraltar Straits and the Ceuta area (Topsent, 1928; Boury-Esnault et al., 1994). As so, while information is scant, the uniqueness of the area justifies its inclusion as the easternmost CWC site considered for the study.
The presence of subfossil CWC reefs in Albanian waters had long been known by fishermen, yet it was not until recent years that their presence was properly documented (Nasto et al., 2018), quickly followed by the discovery of isolated, yet alive, M. oculata and L. pertusa colonies (Angeletti et al., 2014). While all data regarding its sponge fauna comes from a single sampling of the subfossil reefs (Nasto et al., 2018), the Albanian waters represent a unique CWC site, as it is one of the only known south-eastern Adriatic CWC communities, and it has been suggested that it might play a major role on the connectivity of two of the Adriatic and Ionian Sea CWC communities (Chimienti et al., 2019), which, objectively, makes it worthy of inclusion. Finally, the area is also part of the Ionian Sea EBSA area (Gabrie et al., 2012), established in part due to the exceptional presence of CWC in the area.
The ABIDES-ROV cruise took place from the 9th to 19th of September 2017, on board of the R/V ‘Sarmiento de Gamboa,’ using the Remotely Operated Vehicle (ROV) ‘Liropus 2000.’ The main goal of this cruise was to evaluate the impacts of bottom trawling activities on submarine canyon flanks of the Catalan continental margin (north-western Mediterranean Sea). During the exploration of the Blanes submarine canyon (Figure 1A) a vertical wall expanding from 670 to 860 m depth was surveyed (Supplementary Material 1). The wall was densely covered by colonies of the reef building scleractinians L. pertusa (Figure 2A), M. oculata (Figures 2B–F), the solitary coral Desmophyllum dianthus (Esper, 1794) (Figure 2F), found along with scattered colonies of the antipatharians Parantipathes larix (Esper, 1788) and the gorgonian Muriceides lepida Carpine and Grasshoff, 1975.
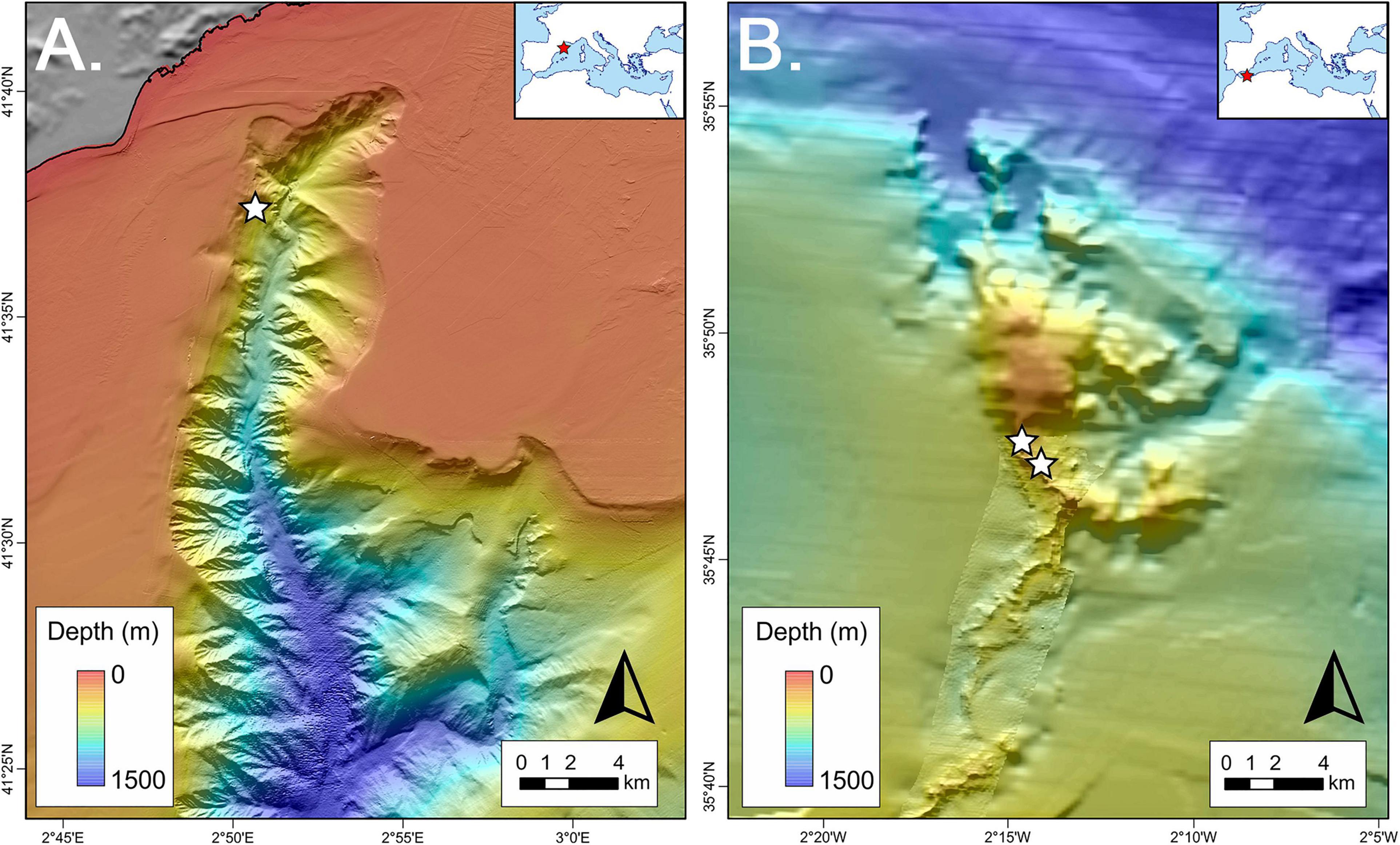
Figure 1. (A) Location of the collection site (white star) in the Blanes Canyon, north-western Mediterranean Sea). (B) Location of the collection site (white stars) in the Cabliers Coral Mound, in the Alboran Sea. Projected view [UTM Zone 31N (WGS84)] with geographic (WGS84) coordinates indicated for reference.
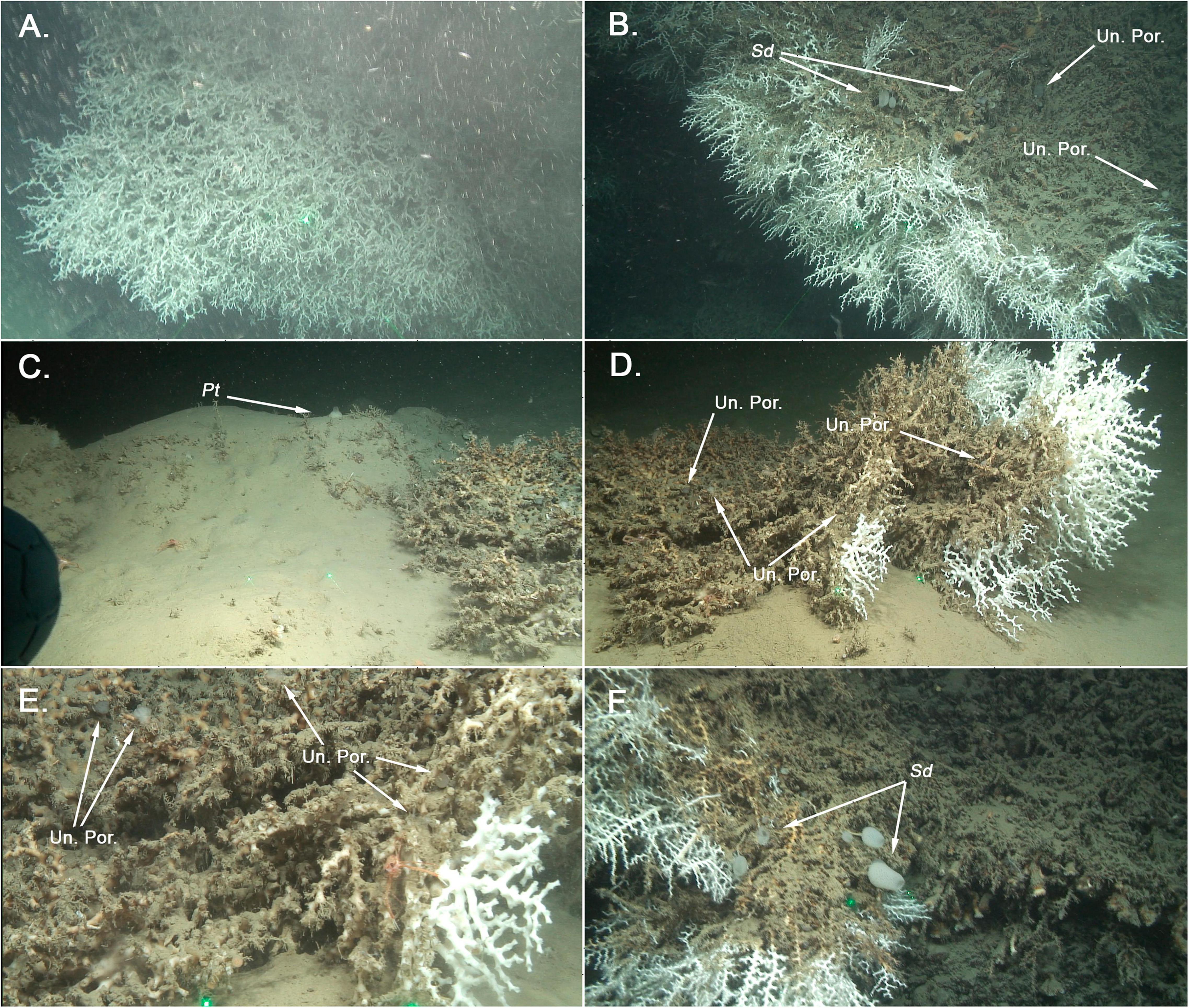
Figure 2. (A) Colony of Lophelia pertusa growing on a vertical wall on the Blanes Canyon at ca. 840 m depth (41°34′872′′N 02°50′986′′E). (B) Partially death colony of Madrepora oculata, growing on a vertical wall at ca. 600 m depth (41°37′642′′N 02°51′4265′E). Sd signals de presence of the Sponge Sympagella delauzei, whereas Un. Por. correspond to unidentified sponges. (C) Partially buried colony of M. oculata (rear). Pt signals an individual of Polymastia tissieri growing amidst the sand/rubble matrix at ca. 680 m depth at the eastern flank of the canyon (41°30′338′′N 02°56′04′′E). (D) Partially buried colony of M. oculata (front). Un. Por. correspond to unidentified sponges. (E) Close up of the partially buried colony, where a Munida sp. (reddish crab) can be seen alongside unidentified sponges (Un. Por.). (F) Sympagella delauzei (Sd) individuals growing onto the dead skeleton of a M. oculata colony at ca. 600 m depth (41°37′642′′N 02°51′4265′E).
The “MELCOR” cruise took place from the 25th May to 1st of June 2012, on board the R/V ‘Garcia del Cid’ (Lo Iacono, 2012). During the cruise, the Cabliers Coral Mound was entirely mapped for the first time through the acquisition of multibeam swath bathymetry (Figure 1B). The Cabliers Coral Mound Province develops on a volcanic substrate within a depth of 290 – 700 m, and consists of a system of linear ridge-like Cold-Water Coral mounds, up to 140 m tall, aligned for up to 20 km along an NNE-SSW direction (Lo Iacono et al., 2014). The top of the mound is characterized by thriving cold-water coral reefs, mainly composed by the framework building scleractinian M. oculata and L. pertusa, whose colonies reach larger dimensions compared to similar reefs of the Mediterranean (Corbera et al., 2019). The reefs are accompanied by several species including the glass sponge Asconema setubalense Kent, 1870, the scleractinian Dendrophyllia cornigera (Lamarck, 1816), the black corals Phanopathes cf. rigida (Pourtalès, 1880) and P. larix (Corbera et al., 2019). Martin-Rauschert dredges were collected on the dead coral frameworks and coral rubble deposits surrounding the above described living reefs (Supplementary Material 1).
During the ABIDES-ROV cruise, a few pieces of L. pertusa and M. oculata were retrieved from the Blanes Canyon, north-western Mediterranean Sea (41°37′642′′N 02°51′426′E), at ca. 600 m depth, by means of the articulated arm of the ROV. The coral rubble samples consisted of three small, broken colony fragments and a single medium-sized piece of M. oculata, (ca. 23 – 23 cm wide) which had been originally collected to estimate the age of the reef. Thus, the sample was not preserved neither in formaldehyde nor in alcohol, but was let to dry instead. A few additional sponge specimens were found attached to a living piece of M. oculata, being preserved in absolute ethanol (see Supplementary Material 2).
During the “MELCOR” cruise, several fragments of coral rubble composed by both L. pertusa and M. oculata were sampled by means of a Martin Rauscher epibenthic sledge, at a depth range between 340 and 350 m depth (Figure 1B, being fixed in 4% formaldehyde and posteriorly transferred at 70% ethanol). Part of this samples were sent to the Università degli Studi di Genova (UNIGE) for further study (Costa et al., 2018), while a single piece of Madrepora and a few isolated sponge individuals remained at the Institut de Ciències del Mar (ICM-CSIC), having those been used for the present study (see Supplementary Material 2).
Once back in the laboratory, the largest sponge samples (>1 cm2) were detached from the coral rubble and individualized in Eppendorf containers, whereas smaller ones (<1 cm2). Provisional identifications were made by examination of teased preparations of fragments, made permanent by mounting in Canada balsam. To obtain spicule preparations for both optical and scanning electron microscopy (SEM) fragments of the sponges were dissolved with nitric acid (HNO3) following the procedures described in Cristobo et al. (1993) and Uriz et al. (2017). The SEM observation was conducted through a HITACHI TM3000 TableTop scanning electron microscope from the Centre d’Estudis Avançats de Blanes (CEAB) and a HITACHI S-3500 N scanning electron microscope from the Institut de Ciències del Mar (ICM-CSIC), both at 5 Kv. Spicule dimensions are given as maximum and minimum length and width for each spicule category with the average values being given in between in italics followed by ±the Standard Deviation (i.e., MIN. – MEAN ± SD – MAX.). Unless stated, all spicule measurements were based on 40 spicules.
All the material examined has been labeled and deposited in the Museu de Ciències Naturals de Barcelona (MZB), following the reference number specified in the species’ examined material (Supplementary Material 2). Species classification has followed the current proposed classification for sponges in the World Porifera Database (van Soest et al., 2021).
In order to be able to compare between the selected CWC sites (as defined in section “Definition of the Mediterranean Cold-Water Coral Provinces”), a careful examination of the current bibliography dealing with CWC in the Mediterranean was conducted, in search for any relevant data regarding the occurrence of sponges in the aforementioned CWC coral sites. To such aim, a systematic literature search was conducted using the biographic database Web of Science (WoS) in September 2020. Specifically, an advanced search was confined by the combination of the terms “sponge” or “Porifera,” “Mediterranean” and a third term, “cold-water coral,” “Lophelia” or “Madrepora.” Published articles until September 2020, including scientific papers, book chapters and workshop and congress proceeding, were screened and included in the analysis if they contained information regarding the presence of sponge species growing onto CWC in the Mediterranean Sea. Additionally, ‘historical papers’ susceptible of containing relevant information were checked regardless of whether or not they were retrieved by the search engine, as WoS is known to offer an incomplete coverage of old literature (Marx, 2012). Finally, the reference lists of all articles were checked for any additional relevant studies. Due to the widely varying procedence and quality of the information, which included presence/absence, semiquantitative and quantitative data, incidence data was selected as the most appropriate approach to establish comparisons between areas, thus simplifying information available into a presence/absence matrix. Basic diversity estimates were then calculated, including number of species per area, shared species between areas, and percentage of exclusive Mediterranean endemism and percentage of North Atlantic sponge fauna on each area. Additionally, graphics were produced regarding (i) the total number of sponge species identified vs. total publications involving sponges’ in Mediterranean CWC through time, both at a basin scale and for each area individually, (ii) the total number of species identified based on collected material and/or ROV per area and (iii) a randomized species accumulation curve to estimate the total number of species at a basin scale. Finally, a Jaccard dissimilarity matrix was generated for the 10 evaluated CWC sites defined in section “Definition of the Mediterranean Cold-Water Coral Provinces,” which was later represented as dendrogram (average linkage) and a non-metric multidimensional scaling (nMDS) for visual exploration by means of the package vegan (Oksanen et al., 2019), available for the R software platform (R Core Team, 2019). After an initial visual assessment of the plots, a 25% similarity threshold was applied for cluster delimitation. Later on, a one-way PERMANOVA (Anderson, 2001) was applied to test for differences in species composition between the clusters using the adonis function (999 permutations) of the vegan package (Oksanen et al., 2019). Posteriorly, additional tests were applied to elucidate whether or not clusters might be influenced by the geographical distance between CWC sites. To such aim, the geographic distances between all 10 sites were calculated and transformed into Euclidean distance matrix, which was then confronted with the Jaccard dissimilarity matrix by means of a Mantel tests (999 permutations) using the mantel function of the vegan package (Oksanen et al., 2019). Finally, a Person correlation was applied between the geographical linear distance between sites and the Jaccard dissimilarity matrix using the cor.test function, available on the stats package, and graphically represented using the tidyverse (Wickham et al., 2019) and ggpubr (Kassambara, 2020) packages, all available for the R software platform (R Core Team, 2019).
A total of 184 sponge specimens could be detected within the analyzed material, accounting for a total of 46 species that could be identified at least to genus level, with 39 corresponding to the Blanes Canyon and 7 to the Cabliers Coral Mound (Table 1, Figure 3, and Supplementary Material 2). In both areas, three out of the four Porifera classes (Calcarea, Demospongiae, Hexactinella) were present in the samples, only lacking Homoscleromorpha representatives. Calcarea and Hexactinellida were represented by three species and four species respectively, while all the others corresponded to Demospongiae, with Poecilosclerida being the most diverse order present. From those, Poecillastra taviani (Tetractinellida; Figure 3D) and Acanthancora schmidti (Poecilosclerida) were the most abundant, with 72 and 21 individuals, respectively.
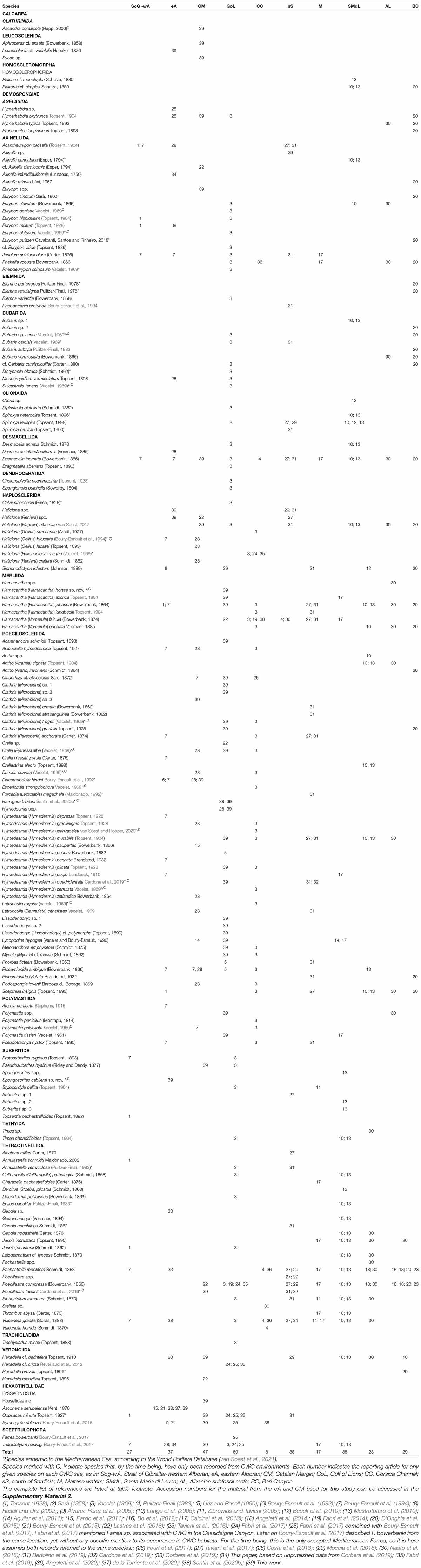
Table 1. Comparative table including all known sponge species that have been recorded within CWC provinces in the Mediterranean Sea.
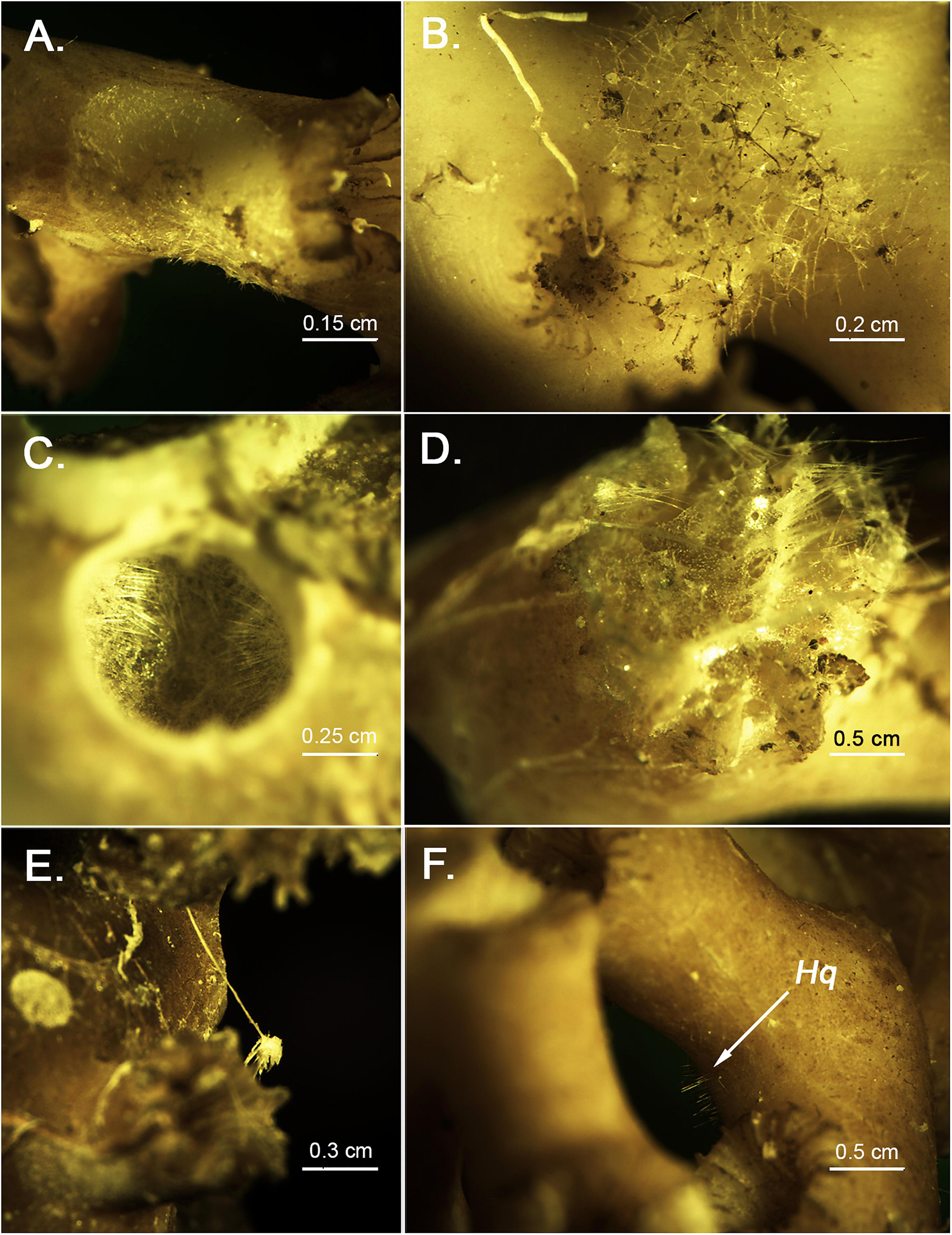
Figure 3. Close up of several sponge species growing onto the Madrepora rubble (A) holotype of Hamacantha (Hamacantha) hortae sp. nov. (B) Individual of Tetrodictyum reiswigi. (C) Sphincter caused by the boring sponge Siphonodictyon infestum, known to erode the calcareous skeleton of several CWC species. (D) Poecillastra taviani individual growing inside a dead polyp. Interestingly enough, and despite the huge number of individuals encountered (over 70), practically all individuals dwelled exclusively inside the polyps, which might be interpreted as a niche preference for cavities and other burrows. (E) Individual of the carnivorous sponge Lycopodina hypogea, which had been previously recorded in the area associated with marine debris (Santín et al., 2020a). (F) Encrusting individual of Hymedesmia (Hymedesmia) quadridentata, with a clear hispidation visible to the naked eye.
Below, a morphological description is provided for those species deemed rare, faunistically relevant or new to science, and from which enough material was collected to allow for their proper identification and description. Several other potential new or rare species were collected, yet the scarcity of the material did not allow for a proper taxonomical characterization of the samples. Acronyms used for the examined material correspond with those on the Supplementary Material 1, were additional information is provided for each specimen.
Class DEMOSPONGIAE Sollas, 1885
SubClass Heteroscleromorpha Cárdenas et al., 2012
Order HAPLOSCLERIDA Topsent, 1928
Family CHALINIDAE Gray, 1867
Genus Haliclona Gray, 1841
Haliclona (Flagellia) hiberniae van Soest, 2017
Material examined: H. (F.) hiberniae: Hh_1; Hh_2; Hh_3; Hh_4; Hh_5; Hh_ 6. Haliclona (Reniera) sp.: HRsp_1. Haliclona sp. Hsp_1.
Additional comparative material: Haliclona (Flagellia) sp., Cap de Creus, north-western Mediterranean Sea (42°20′37.5″N 3°19′50.8″E), ca. 120 m depth, collected by dredging by local fishermen attached to a rock, Summer 2019.
Diagnosis:
Small, beige, cushion-shaped sponges, growing attached to coral rubble. They usually present a roundish body (ca. 10 mm × 5 mm) with a clear osculum more or less located at the center of the sponge, which might be absent or inconspicuous in smaller individuals. The sponge is rather lax, with a clearly visible reticulation of interconnected fibers and a hairy appearance due to projecting spicules.
Skeleton: Rather confused ascending paucispiculate tracks of megascleres connected by single spicules (Figure 4), with sponging being only visible at the nodes. Microscleres are numerous, arranged without any discernible pattern, yet mostly concentrating toward the spicular tracts.

Figure 4. Haliclona (Flagellia) hiberniae spicular set and skeletal arrangement as seen with an optical microscope. The skeleton is formed by paucispiculated tracts of oxeas, with abundant sigmas and flagelosigmas (F1 and F2), both in two categories. Scale bars: General, 100 μm; F1, 30 μm and F2, 50 μm.
Spicules: Oxeas: mostly straight or slightly curved, with acerate ends (330 – 384 ± 30.7 – 434.4 × 6 – 11.1 ± 1.3 – 14 μm). Flagellosigma I (Figure 4, F1): Clearly asymmetrical, with one side being larger than the other, yet the degree of it is subjected to individual variability. Additionally, the larger shaft tends to be more straight compared to the shortest side, which describes a more pronounced curvature. The tips are both recurved, with the shortest end’s tip being gently curved compared with the larger tip ending, which is more abruptly recurved, as if it were a hook (86 – 95.8 ± 4.9 – 105.2 μm). Flagellosigma II (Figure 4, F2): similar to flagellosigma I, the main difference being their size (27.5 – 53 ± 8.4 – 76.2 μm). Sigma I: symmetrical, describing a gentle curvature (51 – 59.1 ± 2.7 – 64.4 μm). Sigma II: smaller than sigma I, and describing a deeper curve when compared (20.6 – 32.9 ± 6.9 – 41 μm).
Remarks:
The subgenus Flagellia van Soest, 2017 was recently erected to encompass all Haliclona species which shared the possession of flagellosigmas, a unique sigma-like spicule with asymmetrical ends and a more or less ovoid shape (van Soest, 2017). From all their current accepted species, only three are present in the North Atlantic area (Table 2). From those, H. (F.) xenomorpha has two categories of oxeas and stout flagellosigmas (Dinn, 2020), whereas H. (F.) porosa possess a single flagellosigma and sigma categories as opposed to H. (F.) hiberniae (van Soest, 2017), which possess two of each (Table 2). As so, while H. (F.) xenomorpha is easily told apart from its North Atlantic counterparts and the current material (Dinn, 2020), both H. (F.) porosa and H. (F.) hiberniae have been described from CWC environments, and the size range of H. (F.) porosa flagellosigmas’ encompasses both flagellosigma categories in H. (F.) hiberniae, being told apart from subtle differences in overall shape and tip curvature (van Soest, 2017). Nevertheless, in H. (F.) porosa sigmas appear in a single category and are consistently rare (van Soest, 2017), whereas in the examined specimens they are abundant and clearly divisible in two categories (Figure 4), for which the examined specimens are here assigned to H. (F.) hiberniae.
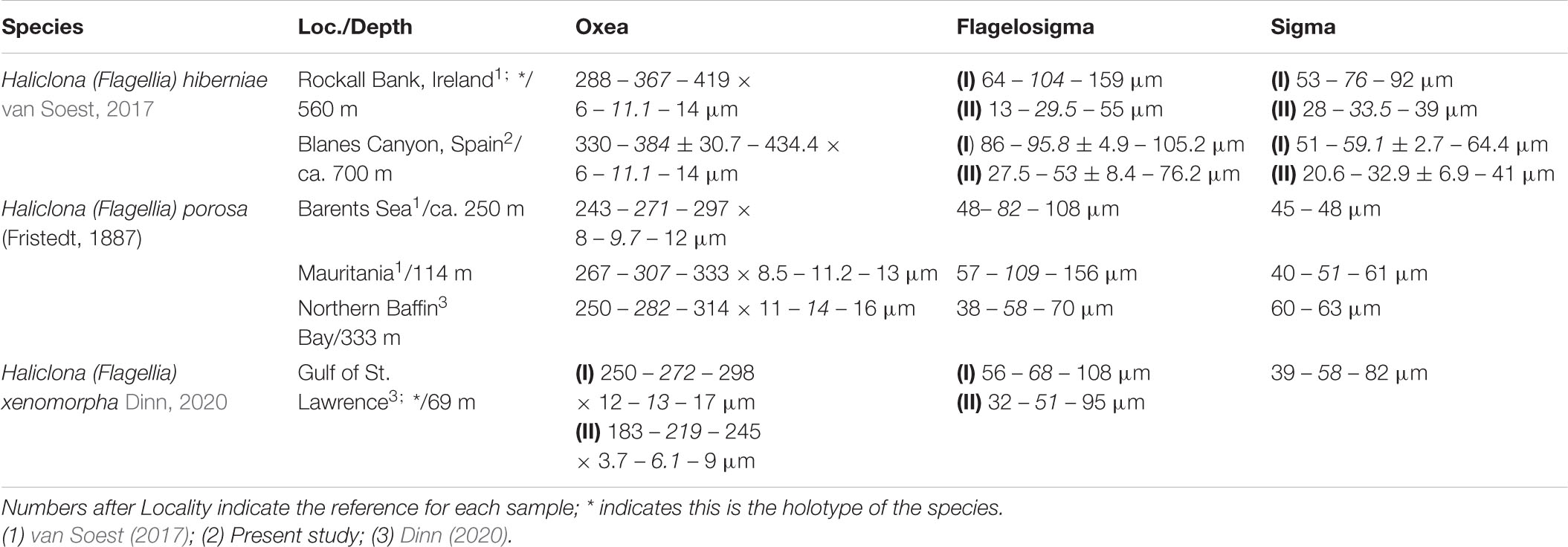
Table 2. Comparative table between the North Atlantic Haliclona (Flagellia) species, including the locality (Loc.) and depth of the sample, as well as the measurement of their spicular complement.
While recently described, H. (F.) hiberniae had long been recorded from the Atlanto-Mediterranean region under the name of ‘Gellius flagilifer’ and ‘Gellius vagabundus’ (Babiç, 1922; Vacelet, 1969), and it is plausible that several Mediterranean records for said species correspond in fact to H. (F.) hiberniae (van Soest, 2017). While the current specimens represent the first confirmed records for the Spanish Mediterranean waters, ‘Gellius flagilifer’ had been previously recorded from the Alboran Sea (Sitjà and Maldonado, 2014), the Catalan coast (Bibiloni, 1981), and the Balearic Archipelago (Bibiloni, 1990), with the Alboran Sea records already being suspected to correspond to H. (F.) hiberniae (van Soest, 2017). Regarding the Catalan and Balearic records, both seem to fit well within H. (F.) hiberniae, with two clear sigma and flagellosigma categories, all within size range (Bibiloni, 1981, 1990). Nevertheless, an additional Haliclona (Flagellia) sp. sample examined from the Catalan continental shelf, while also possessing two categories of sigma and flagelosigmas, presented differences with the H. (F.) hiberniae samples from the CWC environments, mainly in shape (heavily encrusting and spinny, vs. cushion shaped bodies) and stouter, larger oxeas (ca. 500 × 15 μm). As so, older records for G. flagilifer and G. vagabundus from shallow Mediterranean areas might correspond to a yet undescribed Haliclona (Flagellia) species, close to H. (F.) hiberniae, for which their assignment to the later should be taken with caution until further data can be obtained.
Finally, in the Atlantic the species has been mostly reported from CWC communities, were it seems to be fairly abundant (van Soest, 2017). Nevertheless, the species seems to be less abundant in the Mediterranean CWC communities, with only four other records, three as G. flagilifer (Vacelet, 1969; Longo et al., 2005; D’Onghia et al., 2015) and one as H. (F.) cf. hiberniae (Bertolino et al., 2019) reported. Nevertheless, it is possible that more records will surface in other CWC communities across the Mediterranean once their accompanying fauna is properly inventoried.
Order MERLIIDA Vacelet, 1979
Family HAMACANTHIDAE Gray, 1872
Genus Hamacantha Gray, 1867
Hamacantha (Hamacantha) hortae Santín, Grinyó, Uriz, and Gili sp. nov.
Material examined: H. (H.) hortae sp. nov.: Holotype – MZB 2020-0967 – Blanes Canyon, north-western Mediterranean Sea (41°37′642′′N 02°51′426′E), ‘ABIDES’ survey, ca. 600 m depth, 2018; MZB 2020-0927 – slide of the holotype. Paratypes – MZB 2020-0968 – 1 individual; MZB 2020-0927 – microscopic slide of MZB 2020-0968; MZB 2020-0977 – 2 individuals; all attached to Madrepora rubble, same location as the holotype. Hamacantha (Hamacantha) azorica Topsent, 1904: Ha_1; Ha_2; Ha_3. Hamacantha (Hamacantha) johnsoni (Bowerbank, 1864): Hj_1; Hj_2; Hj_3; Hj_4.
Diagnosis:
Small encrusting sponge (Figure 3A; all examined species less than ca. 1 cm2), with a smooth, sparsely lumpy surface. No oscula could be observed. The ectosome is translucent, which allows to easily distinguish the megascleres at naked eye. Color bright white when dry.
Skeleton: The ectosome is frail, translucent and easily detachable, consisting on a loose tangential reticulation of oxeas, with the occasional presence of diancistras. On the contrary, megascleres on the choanosome are arranged in tracts, which occur both parallel and perpendicular to the ectosome, occasionally traversing it. Tylostyles are present in the form of exotyles on the perpendicular tracts. Diancistras occur along the choanosomal tracts without any discernible patter. All samples had low spicular density, with huge spaces between tracts.
Spicules: Tylostyles (Figures 5, 6A): Robust, straight with cleat marked tyles and an acerate end, their width slightly decreases while approaching the acerate tip. They occur in low numbers and exclusively in the choanosomal tracks, mostly perpendicular to the sponge’s surface, with the tyle point toward it, becoming exotyles (369.7 – 452 ± 60 – 573.4 × 4.9 – 5.7 ± 0.9 – 7.3 μm). Oxea I (Figures 5, 6B): Robust, with acerate ends, on very rare occasions, telescoped. They are usually slightly bent at two thirds of its length (348.2 – 410.3 ± 55 – 567.2 × 4.9 – 6.3 ± 0.8 – 8.7 μm). Oxea II (Figures 5, 6C): Smaller and slender than oxea I, with a somewhat more prominent bending that the later. The least abundant of the two oxea categories, could only be found in the holotype and two out of three of the paratypes (91.9 – 188.2 ± 49.9 – 235.2 × 2.9 – 3.7 ± 0.6 – 4.4 μm). Diancistra I (Figures 5, 6D,E,G): Typical of the genus, with and stout, straight shaft, with recurved hook-like endings. One of the endings presents a gentler curvature, with a clear rounded notch and a slightly bigger hook-like end, whereas the other presents a more acute angle, with a smaller, elongated notch, and a slightly slimmer hook-like end. The inner side of the shaft presents a subtle depression on the middle, with fimbriae on both sides, with the fimbriae closer to the rounded-notch end usually resembling a blade. There is a certain degree of variation on said pattern, as diancistras with both ends “rounded-notched” occasionally occur. Finally, both ends are not on the same plane as the shaft, but usually bent (ca. 45° in opposing directions; Figure 6G) in regards to it (123 – 139 ± 11.1 – 159.2 μm). Diancistra II (Figures 5, 6F): With a strongly bent shaft (boomerang-like), with asymmetrical, recurved acerate ends. One of them is “sigma-like,” with a gentle curvature and a long, acerate tip, while the other one is considerably smaller, describing a markedly narrow curvature. They are considerably scarce, with no more than 20 observed per specimen (33.8 – 35.8 ± 1.9 – 37.5 μm).
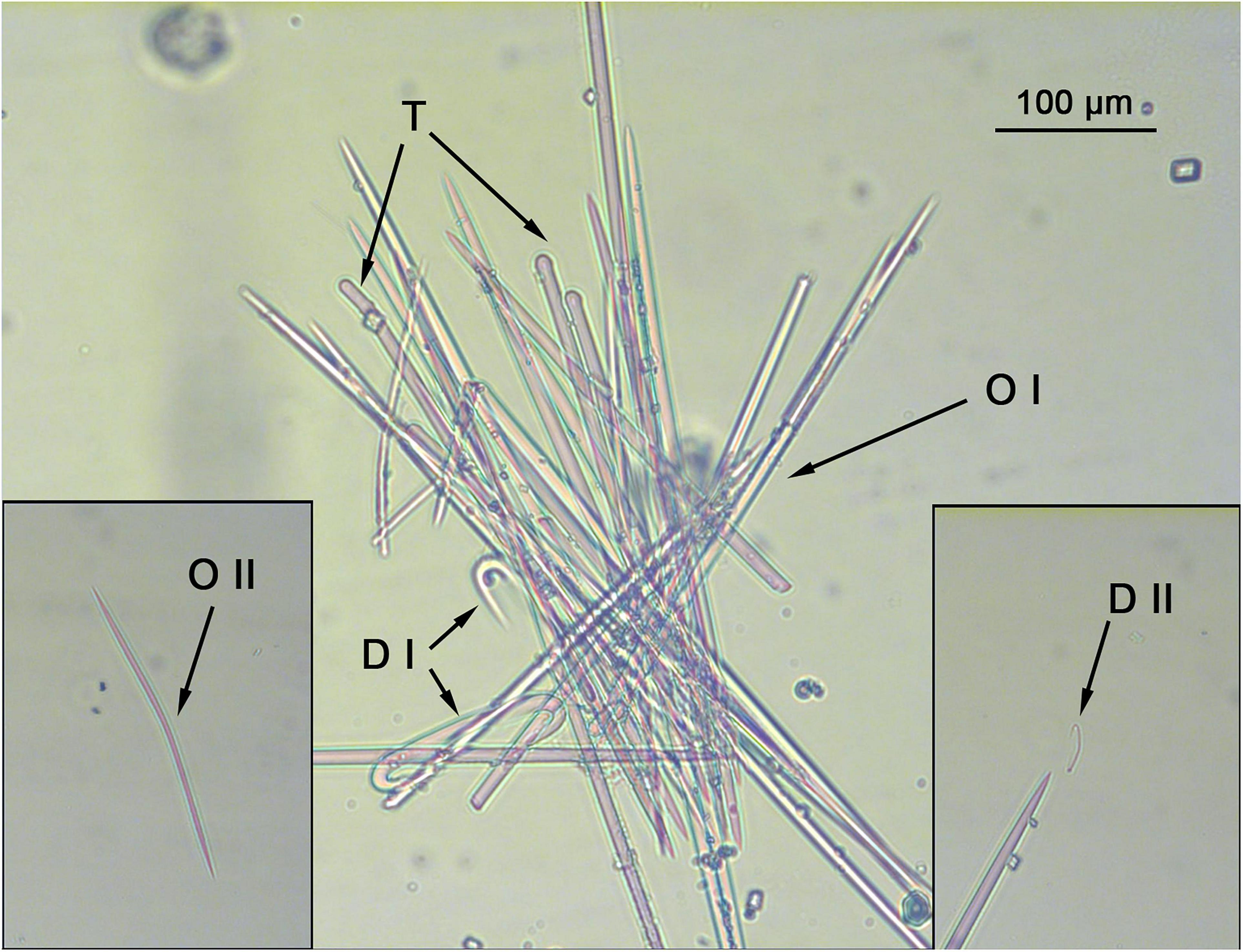
Figure 5. Hamacantha (Hamacantha) hortae sp. nov. spicular set. The skeleton is formed by loose tracts of tylostyles (T) and oxeas in two size categories (O I and O II), with diancistras in two size categories (D I and D II) scattered around. Scale bar, 100 μm.
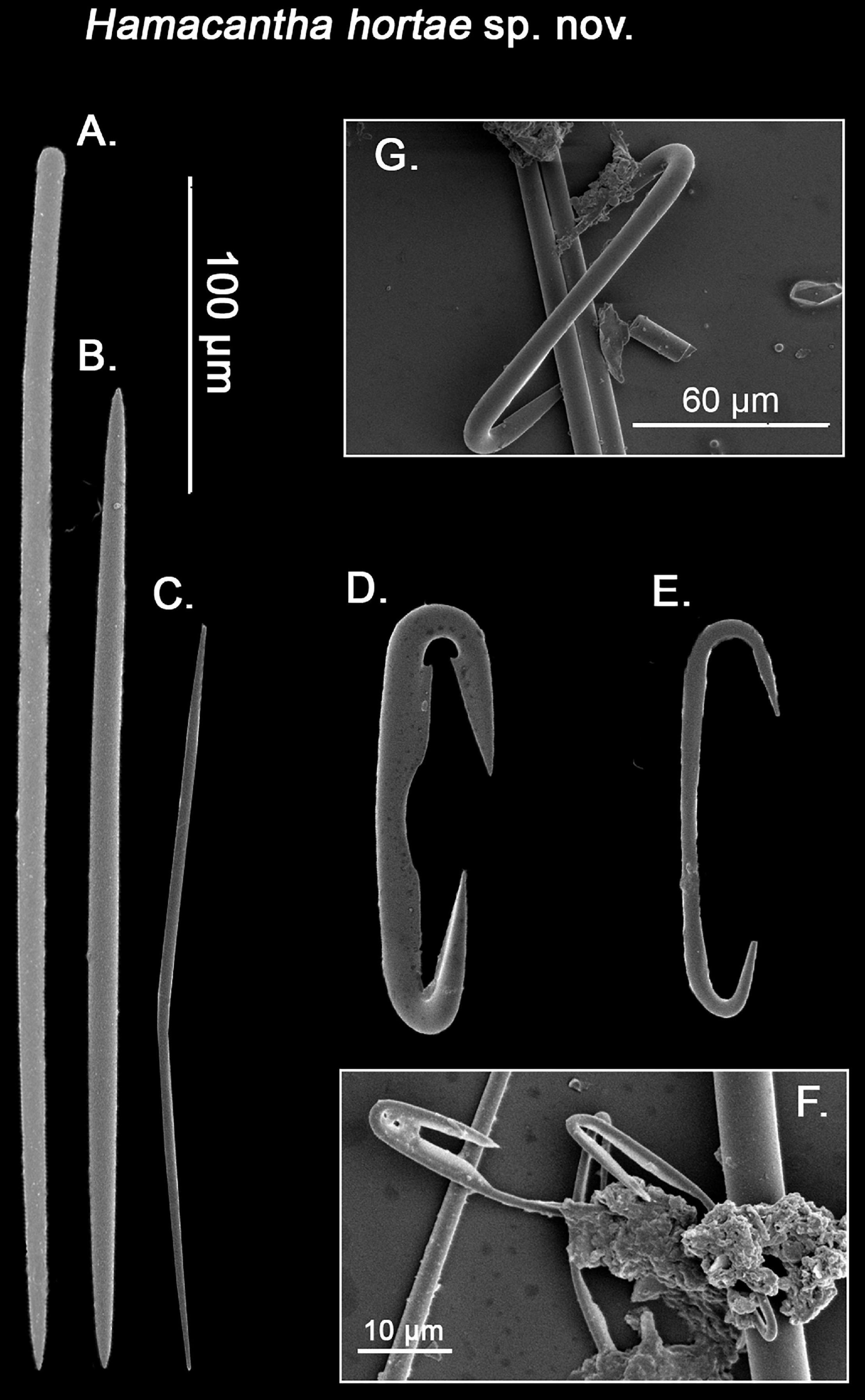
Figure 6. SEM imagining of Hamacantha (Hamacantha) hortae sp. nov. spicular set. (A) Tylostyle; (B) Oxea I; (C) Oxea II; (D) Diancistra I; (E) developmental form of diancistra I, still lacking the hook-like expansions; (F) Diancistra II and (G) Lateral view of Diancistra I. Scale bar for (A–E) 100 μm; scale bar for (F) 20 μm; and scale bar for (G) 60 μm.
Etymology:
The name hortae is chosen in honor of the neighborhood of Horta (Catalonia, Spain). Known at least since the early X century, it was an independent village until 1904, when it got annexed to Barcelona. Yet, it still retains nowadays the aura of being a village within a city, exemplified by one of its popular mottos “Horta no és ni serà mai Barcelona” (Horta is not and will never be Barcelona).
Remarks:
Characterized by the possession of diancistras, the genus Hamacantha is easily identifiable and relatively common within deep-sea and other dark habitats around the world (Hajdu and Castello-Branco, 2014; Hajdu et al., 2015; Ise et al., 2019). Currently, the genus is classified in three subgenera, Hamacantha (Hamacantha), Hamacantha (Vomerula), and Hamacantha (Zygherpe), which are told apart by their main megascleres (Oxea vs. Style vs. Tylostyle). In this sense, H. (H.) hortae sp. nov. would fit within the Hamacantha subgenus, where it is easily distinguishable from all other known species of Hamacantha (Hamacantha) due to its lack of sigmas and the possession of two categories of oxeas, and the additional presence of tylostyles as megascleres (Table 3). While H. (H.) hortae sp. nov. has been here assigned to Hamacantha (Hamacantha) as oxeas are its most abundant megasclere category, the species represents a crack on the current Hamacantha subgeneric classification (Hajdu, 2002), as the presence of tylostyles is the diagnostic feature for the subgenus Zygherpe (Hajdu et al., 2015). In this sense, tylostyle bearing Hamacantha species are considerably rare, with only two species known so far, both occurring in the Pacific (Laubenfels, 1932; Hajdu et al., 2015). Yet, their tylostyles resemble those of H. (H.) hortae sp. nov., which might suggest a certain degree of relationship between species. Nevertheless, Hamacantha subgenus was erected based on classification convenience and do not necessarily represent a monophyletic clade (Hajdu, 2002; Hajdu et al., 2015). While currently there are no molecular data for any Hamacantha species, H. (H.) hortae sp. nov. seems to reinforce such view and, additionally, would support a closer relationship between Hamacantha (Zygherpe) and Hamacantha (Hamacantha) species than with Hamacantha (Vomerula), despite the later possessing styles as main megascleres.
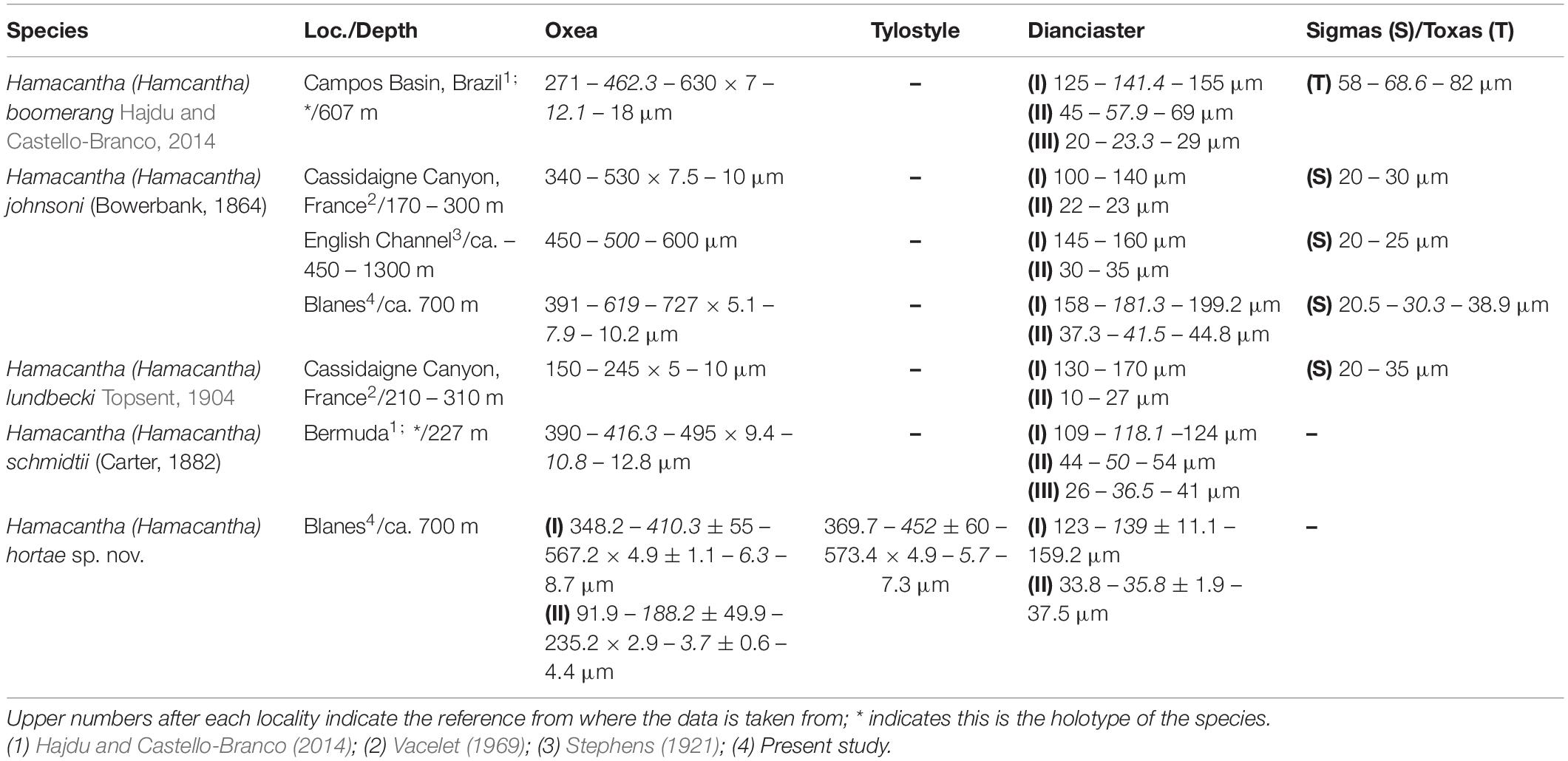
Table 3. Comparative table between the North Atlantic Hamacantha (Hamacantha) species, including the locality (Loc.) and depth of the sample, as well as the measurement of their spicular complement.
Order POECILOSCLERIDA Topsent, 1928
Family CLADORHIZIDAE Dendy, 1922
Genus Cladorhiza Sars, 1872
Cladorhiza cf. abyssicola Sars, 1872
Material examined: C. cf. abyssicola: Ca_1
Diagnosis:
Stipitate sponge with branching lateral processes. Color in spirit is translucent yellow.
Skeleton: The sponges’ body and branches are formed by a dense axis of mycalostyles, whereas anchorate anisochelae and sigmas are found scattered across the whole body of the sponge, with sigmas being less abundant that chelae.
Spicules: Mycalostyles (Figure 7A): Almost straight, usually in the form of styles with a blunt end. (290.1 – 329.3 ± 32.1 – 390 × 8.9 – 10.2 ± 2.1 – 15.3 μm). Anisochelae (Figure 7B): chelae with clear unequal ends, the bigger one with well-formed acerate alae and visible fimbriae attached to the shaft, whereas the other end consists of five spaced underdeveloped alae, in the form of claw-like appendages. (12.5 – 21.4 ± 1.5 – 23.9 μm). Sigmas (Figure 7C): Large sigmas, with a C-like contour and acerate tips (72.3 – 85.3 ± 7.7 – 95.4 μm).
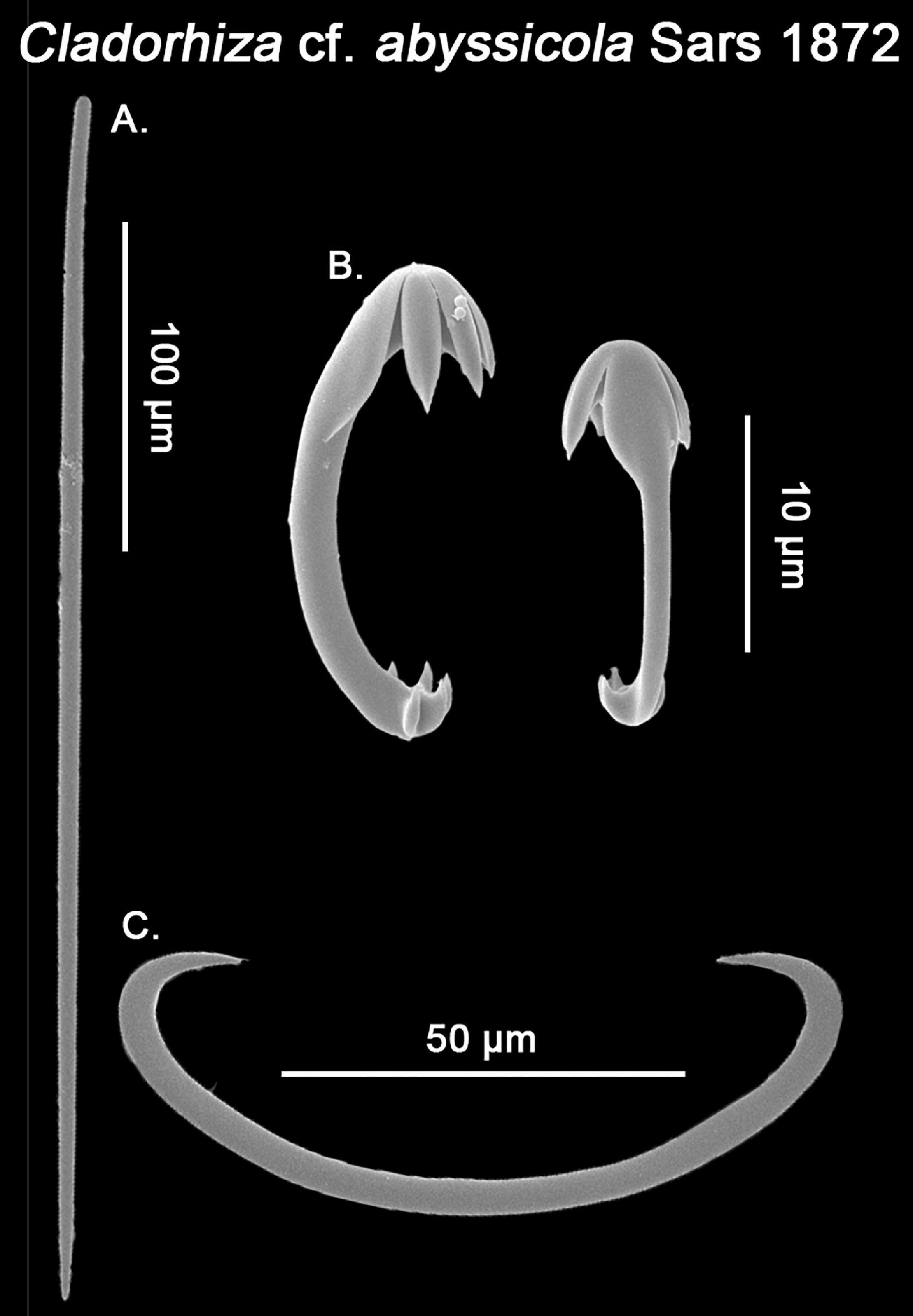
Figure 7. SEM imagining of Cladorhiza cf. abyssicola spicular set. (A) Mycalostyes; (B) Anchorate anisochelae; (C) Sigma. Scale bar for (A) 100 μm; scale bar for (B) 10 μm; and scale bar for (C) 50 μm.
Remarks:
While currently there are ca. 200 carnivorous sponge species accepted worldwide (van Soest et al., 2021), only two have been recorded in the Mediterranean so far; Lycopodina hypogea (Vacelet and Boury-Esnault, 1996) and the North Atlantic C. abyssicola, easily told apart from each other based on their external appearance and spicular set. Cladorhiza abyssicola is far less abundant than L. hypogea, having only been found five times in the Mediterranean prior to this study (Babiç, 1922; Vacelet, 1969; Boury-Esnault et al., 1994; Fourt et al., 2017; Hestetun et al., 2017), being this its first report for the Catalan coast. Nevertheless, other North Atlantic Cladorhiza are very close to or practically indistinguishable from C. abyssicola (Göcke et al., 2016; Hestetun et al., 2017), with the possibility of ‘cryptic Cladorhiza’ within Mediterranean records having already been noted (Fourt et al., 2017). Overall, a ‘rare’ species record for the Mediterranean basin, which suggests further investigation including molecular techniques.
Family HYMEDESMIIDAE Topsent, 1928
Genus Acanthancora Gray, 1867
Acanthancora schmidti (Topsent, 1898)
Material examined: A. schmidti: As_1; As_2; As_3; As_4; As_5; As_6; As_7; As_8; As_9; As_10; As_11; As_12; As_13; As_14; As_15; As_16; As_17; As_18; As_19; As_20; As_21.
Diagnosis:
Small smooth encrusting sponge (ca. 1 cm2), without apparent oscula or any other distinguishing external characteristics. Color dull white, “bone-like” when dry.
Skeleton: The ectosome consists of a tangential layer of strongyles, without a clear distinguishable organization, alongside a relatively dense layer of chelae. The choanosome consists of erect acanthostyles in a single category attached to a basal layer of spongin.
Spicules: Strongyles (Figure 8A): Tornotes modified into strongyles, with a thin, straight shaft with polytylote processes and rounded ends. Styloid modifications are rare, but present in some individuals (167.2 – 203.2 ± 10.1 – 240 × 2.5 – 2.9 ± 0.8 – 4.2 μm). Acanthostyles (Figure 8B): Small, stout acanthostyles, with spines all over the shaft and head, and an acerate end. The head’s spines are robust and round-ended, whereas the shaft’s ones have a hook-like appearance, with the hook always pointing toward the head. Two categories could eventually be distinguished, yet there are no morphological distinctive traits between bigger and smaller acanthostyles (105 – 125.5 ± 23.6 – 253 × 12.2 – 14.3 ± 1.7 – 15.1 μm). Chelae (Figure 8C): Peculiar, strongly acanthose chelae, with spines terminating in rounded to acerate ends, with a prevalence for the former. They possess four teeth, with the central two possessing two considerably developed rounded alae each, whereas the two lateral teeth are reduced to curved expansions with a thinner shaft and short, small rounded alae in comparison. The chelae’s shaft is robust and heavily spinned on its external side, with spine morphology varying from smaller, rounded wart-like ones to wide, stout spines with almost acerate ends. Very regular in size (31 – 35.1 ± 0.5 – 35.9 μm). Chiastosigmas (Figure 8D): small sigma derivative, with 4 – 6 rays, typically 5, protruding from a central disk. The shafts are curved as in forming a sphere, giving the chiastosigmas the appearance of an empty ball when looked from the side. In 4-rayed morphs, the shafts are symmetrical with each other, whereas in 5- and 6- rayed morphs, this symmetry is lost. The central disk varies from a flat, ovoid plate to a subtle sphere. Finally, the shafts inner face is almost entirely serrulated except for its basal area, while also ending with heavily recurved tips, as in a claw (5 – 6.3 ± 0.7 – 7.4 μm).
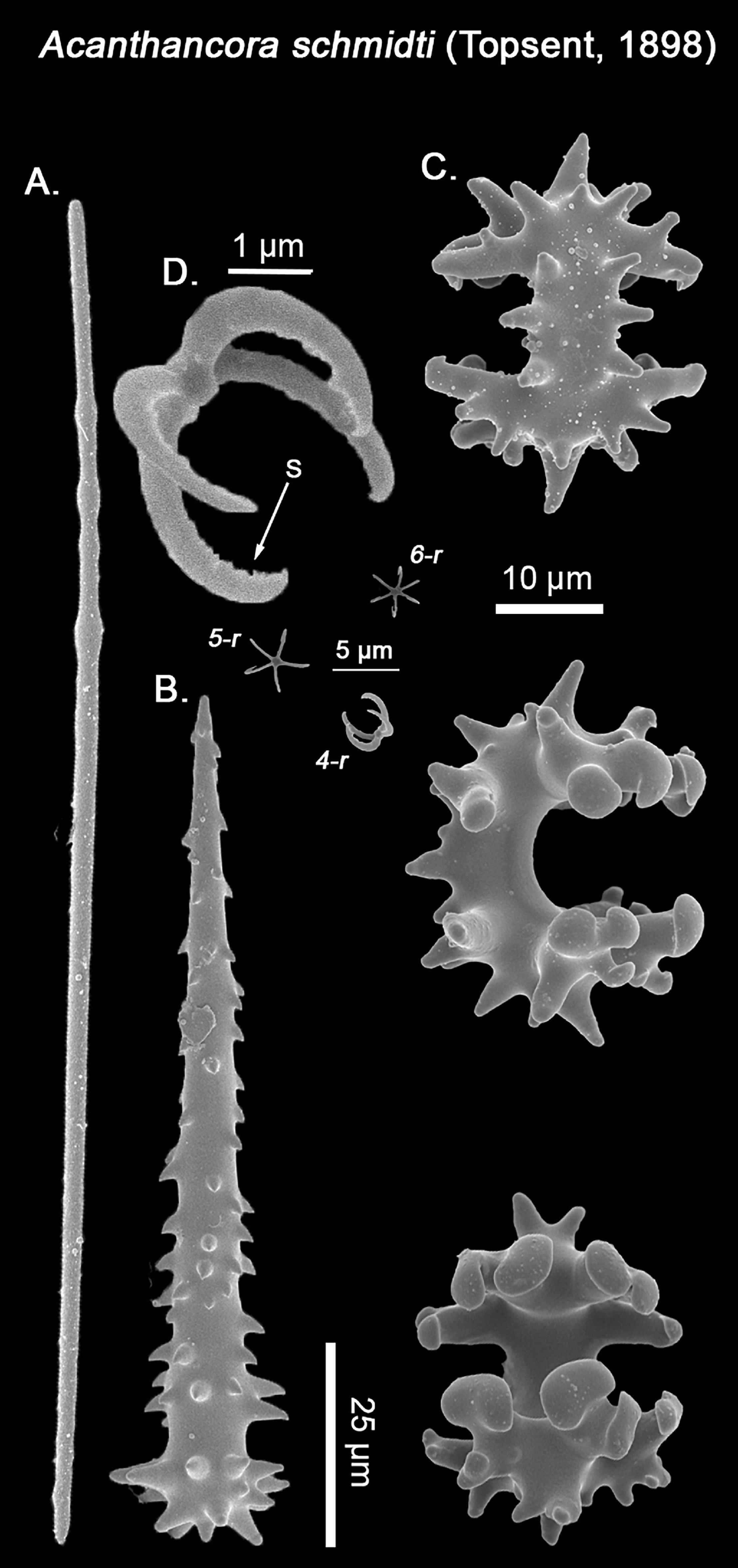
Figure 8. SEM imagining of Acanthancora schmidti spicular set. (A) Strongyle with polytylote processes; (B) Acanthostyle. (C) Rear, lateral and frontal view of the acanthose chelae; (D). Four-rayed (4-r), five-rayed (5-r), and six-rayed (6-r) chiastosigmas, with a close up of a four-rayed chiastosigma, with visible serrulated shafts (S). Scale bar for (A–C) 25 μm; Scale bar for (D), close up chiastosigmas 1 μm, and scale bar for (D), general view of the chiastosigmas types, 5 μm.
Remarks:
The genus Acanthancora is easily recognizable due to their peculiar acanthose chelae, a characteristic only shared with the ill-known Pseudohalichondria. Nevertheless, the latter refers to massive specimens with plumose skeleton and lacking acanthostyles, whereas Acanthancora possess a hymedesmid skeletal organization and acanthostyles, easily telling them apart (Van Soest, 2002).
In his original and subsequent descriptions, Topsent noted the presence of chiastosigmas as a diagnostic character for Acanthancora (Topsent, 1904, 1928), yet in the rexamination of Acanthancora type material no chiastosigmas could be found (van Soest, 2002). After careful examination of the current material, no chiastosigmas could be observed with an optic microscope but become apparent with SEM imaging, being almost as abundant as the chelae. Thus, chiastosigmas do appear to be proper for the species, yet a re-examination of the type material of all other known Acanthancora would be necessary to determine if it is a shared trait for the genus, or just species-specific. While this represents the first record for Acanthancora in the Mediterranean, two other species are known to occur in North Atlantic waters. From those, A. schmidti is distinguished from Acanthancora aenigma (Lundbeck, 1910) due to the bigger size of the ectosomal tornotes and lack of chiastosigmas in the later, yet the species remains insufficiently described (Lundbeck, 1910) and its synonymy with A. schmidti cannot be ruled out in the future. Concerning A. schmidti and Acanthancora clavatancora Topsent, 1927, Topsent (1928) mainly distinguished A. clavatancora as possessing polytylote styles and chelae with rounded spines, whereas, in contrast, A. schmidti possessed ectosomal polytylote strongyles and chelae with acerate spines, with otherwise all spicules in a similar size-range category (Table 4). Nevertheless, the analyzed material possessed both ectosomal polytylote strongyles, rarely styloids, and chelae with rounded spines, blurring the separation between both species. In light of these findings, it seems there is no longer any clear difference that would justify both being considered separate species, as already suggested by Van Soest (2002).
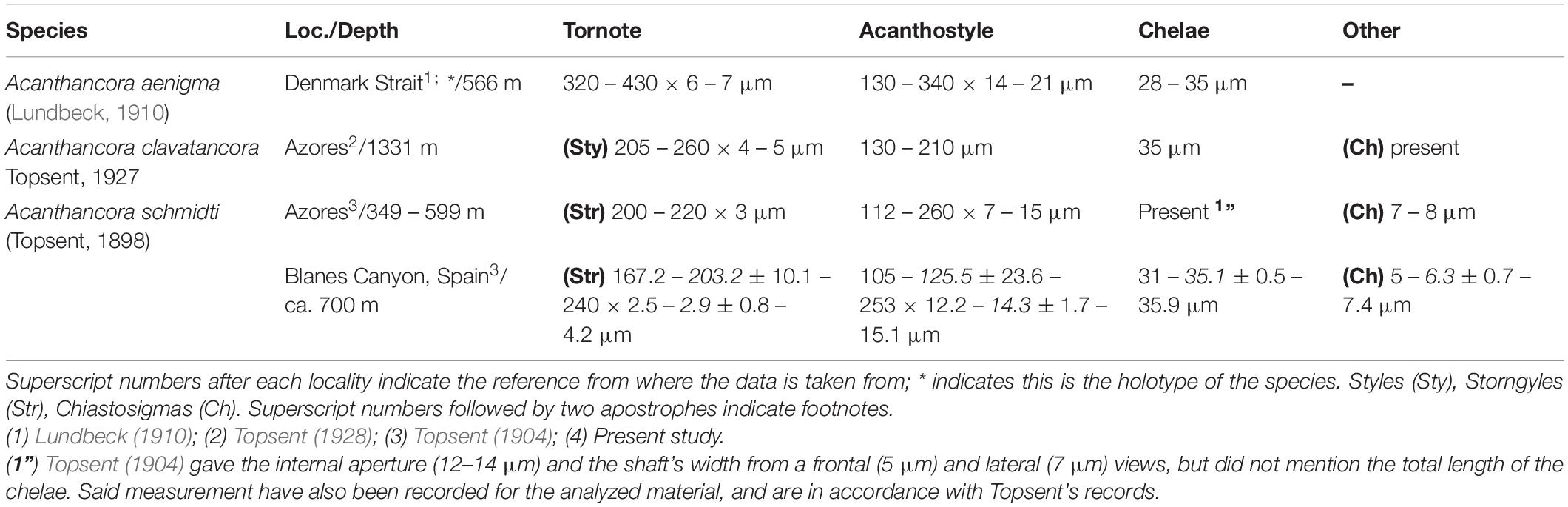
Table 4. Comparative table between the North Atlantic Acanthancora, including the locality (Loc.) and depth of the sample, as well as the measurement of their spicular complement.
Genus Hymedesmia Gray, 1858
Hymedesmia (Hymedesmia) quadridentata Cardone et al., 2019.
Material examined: H. (H.) quadridentata: Hq_1; Hq_2; Hq_3; Hq_4. Hymedesmia sp.: Hsp_1; Hsp_2; Hsp_3; Hsp_4. Hymedesmia (Hymedesmia) cf. mutabilis (Topsent, 1904): Hm_1; Hm_2; Hymedesmia (Hymedesmia) cf. plicata Topsent, 1928: Hp_1.
Diagnosis:
Heavily encrusting sponge, extremely hispid due to projecting acanthostyles protruding the sponge (Figure 3F). Color gray-white when dry. Smaller individuals might appear as translucent-gray stains, slightly hispid/velvety at the binocular microscope, which can be easily overlocked as discolorations of the skeletal framework if it were not by the apparent hispidation.
Skeleton: Typical from the genus (Figure 9), with acanthostyles single erect on the substrate, with tylostyles as ectosomal spicules, arranged parallel to the sponge surface. Chelae and sigmas concentrate toward the base of the sponge.
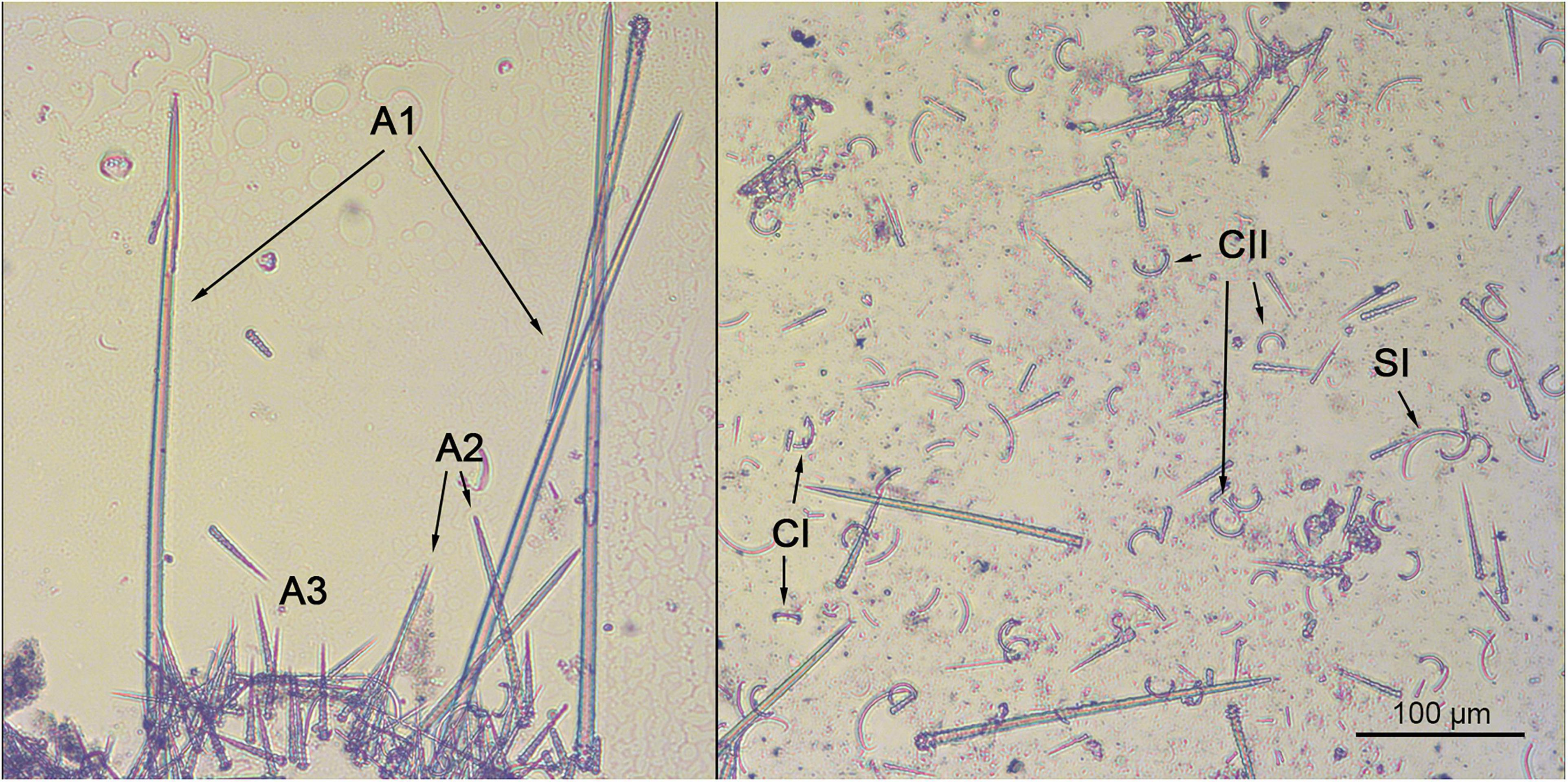
Figure 9. Hymedesmia (Hymedesmia) quadridentata skeletal arrangement and spicular set. Left: Hymedesmoid skeleton, typical of the genus, with three acanthostyle categories (A1, A2, A3) erect on the substrate. Right: General view of H. (H.) quadridentata microscleres, including two categories of sigmas (SI and SII) and two categories of chelae (CI and CII), one of them with four unguliferous alae (CII). Scale bar 100 μm.
Spicules: Tylostyles: Slightly curved, with asymmetrical inflated ends. They appear to be scarce, as less than 10 could be measured (188 – 195.2 ± 17.6 – 215 × 2.4 – 3.9 ± 1.7 – 6.7 μm). Acanthostyles I (Figure 9): mostly straight, with an acerate end. They possess round, spinose heads, with most spines appearing blunt or with roundish terminations. Contrary to the head, the shaft and acerate tip are almost devoid of spines (368.5 – 600.5 ± 95.2 – 799.2 × 11.6 – 17.2 ± 2.4 – 19.4 μm). Acanthostyles II (Figure 9): smaller in size than the acanthostyles I and, contrary to these, spines are common from the head to the middle of the shaft (124.3 – 185.7 ± 42.9 – 319.2 × 8.7 – 11.2 ± 2.9 – 17.5 μm). Acanthostyles III (Figure 9): the smallest and most abundant acanthostyle category, identical in shape to the other two, but contrary to these they are completely spinned (55 – 74.3 ± 22.1 – 151.5 × 6.3 – 8 ± 1.2 – 10.7 μm). Chelae I (Figure 9): typical arcuate isochelae, with a curved shaft and three free alae (22.2 – 25.2 ± 1.7 – 28 μm). Chelae II (Figure 9): “C” shaped unguliferate chelae, with a curved shaft and four small unguliferous teeth on each end (21.8 – 25.1 ± 2.4 – 30.8 μm). Sigmas I (Figure 9): “C” shaped sigmas, rarely “S” shaped, with recurved ends. They are slightly asymmetrical, giving the impression that they don’t bent on the middle of the shaft, but at 2/3 of its length (50 – 59.5 ± 5.6 – 75.5 μm). Sigma II (Figure 9): pretty much identical to Sigmas I, but smaller (7.3 – 12.5 ± 3.8 – 22.8 μm).
Remarks:
The genus Hymedesmia is amongst the most diverse within Porifera, and it is also recognized as one of the most abundant taxa in CWC environments (Goodwin et al., 2011). Nevertheless, from all known Hymedesmia, the presence of unguliferous chelae is a rare feature, shared by just a few species worldwide (Cardone et al., 2019). In the North Atlantic only three species, H. (H.) mucronata, H. (H.) zetlandica, and H. (H.) quadridentata, possess unguliferous chelae. From these, H. (H.) mucronata is only known from its type locality in the Azores, and widely differs from the present material in terms of spicular size while, at the same time, it only possesses a single category of sigmas (Topsent, 1904). On the other hand, both H. (H.) zetlandica and H. (H.) quadridentata are present in the Mediterranean CWC communities (Bertolino et al., 2019), yet H. (H.) zetlandica possesses a single category of sigmas and considerably smaller acanthostyles (van Soest, 2002), which clearly tells it apart from H. (H.) quadridentata. Contrary to H. (H.) quadridentata’s original description (Cardone et al., 2019) three acanthostyles categories could be differentiated in the present material, yet they would fit in range of the original two acanthostyles categories described, and wouldn’t justify erecting a new species. Interestingly enough, H. (H.) zetlandica, possessing unguliferous chelae, is the type species of the genus Hymedesmia, yet barely no more than half-a-dozen other species share such characteristic. Additionally, the unguliferous chelae morphology widely varies between those Hymedesmia possessing such, with teeth ranging from two to four, and, in the case of H. zetlandica, being additionally spinned (Table 5). Currently, the genus Hymedesmia is mainly built around the shared possession of an “hymedesmiid skeleton,” yet such character is believed to be linked with the encrusting habitus of most Hymedesmia rather than a real synapomorphy for the genus, which is already known to be polyphyletic (Redmond et al., 2013; Ríos et al., 2020). As so, the new discovery or rediscovery of more of these “deviant” Hymedesmia, specially once molecular data of such can be obtained, might eventually led to a complete reorganization of the genus.
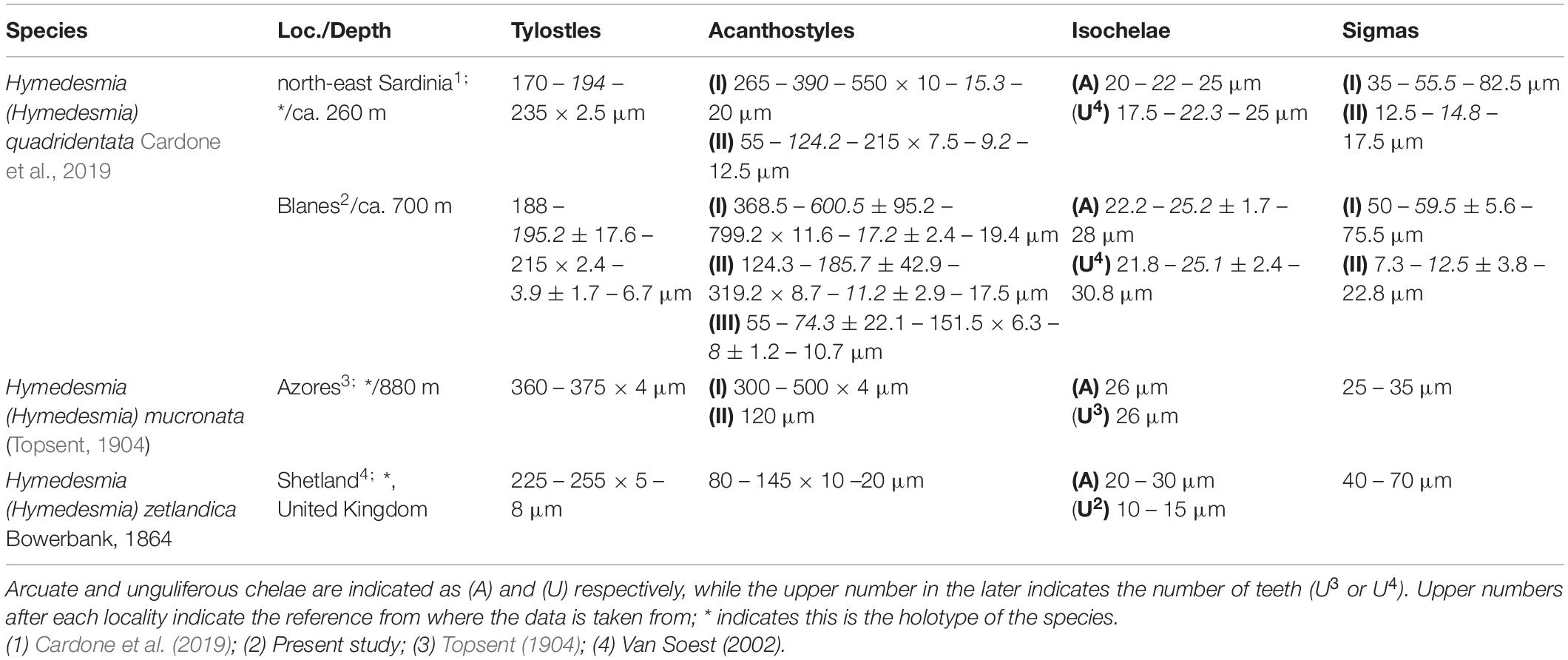
Table 5. Comparative table between the North Atlantic Hymedesmia (Hymedesmia) species with unguliferous chelae, including the locality (Loc.) and depth of the sample, as well as the measurement of their spicular complement.
Order SUBERITIDA Chombard and Boury-Esnault, 1999
Family HALICHONDRIIDAE Gray, 1867
Genus Spongosorites Topsent, 1896
Spongosorites cabliersi Santín, Grinyó and Lo Iacono sp. nov.
Material examined: S. cabliersi sp. nov.: Holotype – MZB 2020-0936 – Cabliers Coral Mound, western Alboran Sea (35°47′58′′N 2°15′17′′ W), ‘MELCOR’ survey, 340 m depth, 2012. Paratype – MZB 2020-0937 – Cabliers Coral Mound, western Alboran Sea (35°47′58′′N 2°15′17′′ W), ‘MELCOR’ survey, 340 m depth, 2012.
Diagnosis:
Both the holotype and paratype consist of fragments of a massive, lumpy sponge, with a smooth appearance. The sponge possesses a thin, translucid ectosome, firmly attached to the choanosome, and can only be peeled off as flakes. The choanosome is cavernous, with several openings of varying size. The sponge is hardly compressible. Color in spirit, orange to brown.
Skeleton: The ectosome is made of loose reticulation of strongyloxeas, parallel to the sponge’s surface and without a clear organization. The choanosome on the other hand is of an ‘halichlonoid’ nature, densely packed with strongyleoxeas which occur in a rather confused manner, without a clear direction.
Spicules: Strongyleoxeas (Figure 10): Robust oxeas with blunt terminations (Figure 10A), which gives them appearance of strongyleoxeas, with a ‘cigar-shaped’ appearance. They are mostly straight, with a slight or subtle central bending, sometimes showing a subtle double bending toward the tips instead. They possess the same width across the spicule except for its terminations, which end in a characteristic short tapering, with either a blunt or a mucronate tip, typically with 1 to 2 tapering points (Figure 10B). Albeit very rare, true style or strongyle modifications might occur (194 – 349.2 ± 52.2 – 388 × 9.8 – 15.2 ± 4.2 – 19.4 μm). It is to be noted however, that while smaller spicules can be found, most of them fall between 320 – 360 μm size range.
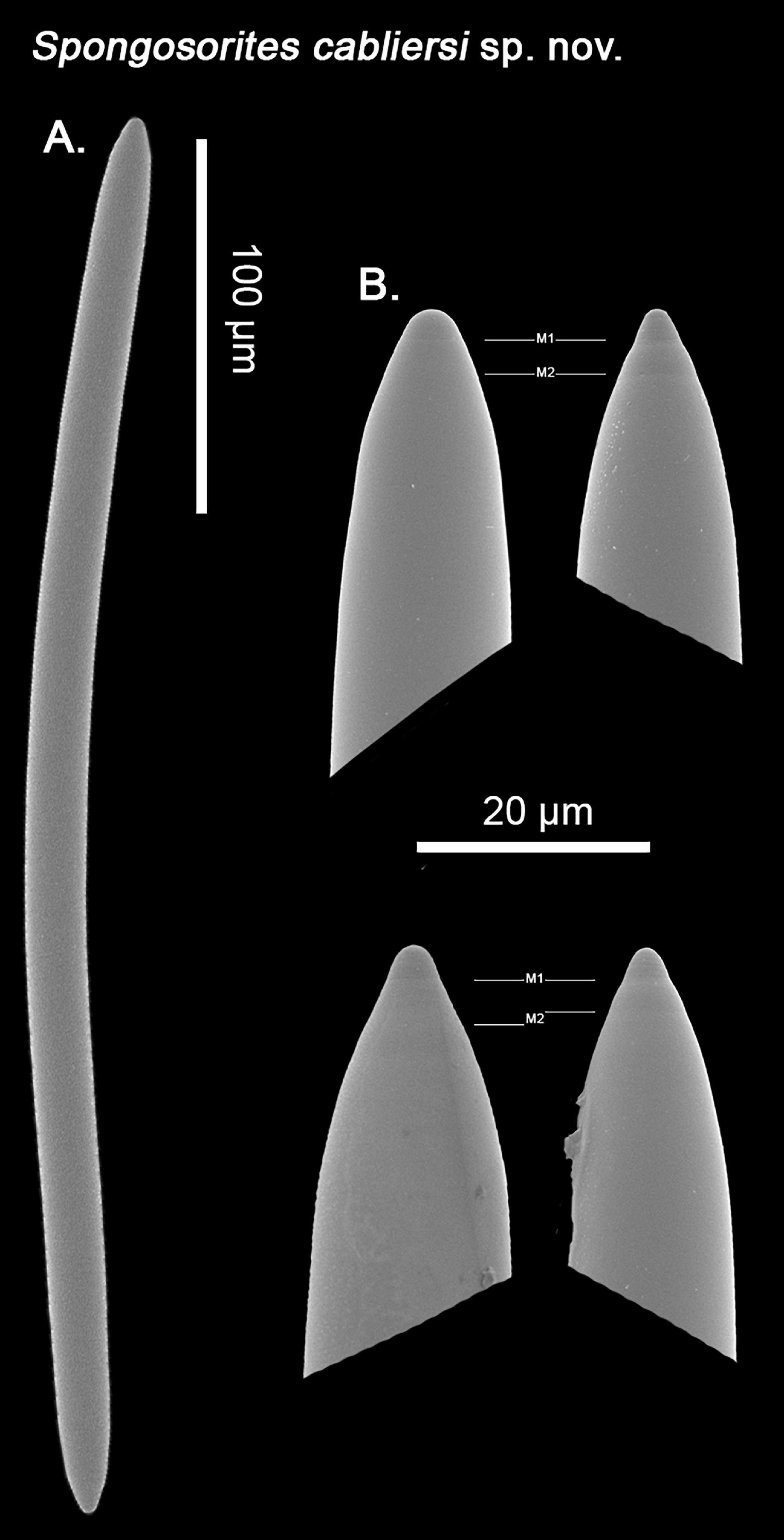
Figure 10. SEM imagining of Spongosorites cabliersi sp. nov. spicular set. (A) General view of the strongyleoxeas. (B) Close up of the strongyleoxea’s heads, with a more or less mucronated end, preceded by more two tapering points (M1 and M2), which might be more or less marked between individual spicules. Scale bar for (A) 100 μm; scale bar for (B) 20 μm.
Etymology:
The name cabliersi refers to the area in which the sample was collected, the Cabliers Coral Mound. This CWC reef is of special ecological relevance because of the large extension (at least 5.2 km) of coral communities found in exceptional thriving conditions and for their pristine state of conservation (Corbera et al., 2019).
Remarks:
The genus Spongosorites is closely aligned with Topsentia, with which it shares several diagnostic characters (Bertolino et al., 2015). In this sense, the current material has been assigned to Spognosorites due to the possession of bent oxeas and a smooth surface, with its only oxea category being smaller than 600 μm. Nevertheless, species differentiation with Spongosorites is hazardous, as it mostly relies on subtle differences in the spicules (Picton and Goodwin, 2007). In this sense, from all the described Spongosorites in the North Atlantic (Table 6), Spongosorites cabliersi sp. nov. differs from most of them in the possession of a relatively small single oxea category, with only another four species possessing a single oxea category in the same size range. From those, Spongosorites calcicola and Spongosorites flavens would be the closest to the current material, with Spongosorites difficilis and Spongosorites coralliophaga being told apart due to their possession of oxeas with long, accerate tampering ends (Lundbeck, 1902; Stephens, 1915). Spongosorites calcicola is so far only known to occur in shallow Irish muddy bottoms, and possesses oxeas with closely resemble those of S. cablersi sp. nov. being mostly straight or slightly bent, with blunt or mucronate endings (Picton and Goodwin, 2007). Nevertheless, they are described as possessing a proportional width to length ratio, whereas in S. cabliersi sp. nov. this is not the case, being of the same width regardless of the length of the spicules with, additionally, S. cabliersi sp. nov. strongyloxeas doubling in width those of S. calcicola. Finally, S. calcicola is said to possess numerous large oscula and a bright lemon color not lost when after preservation, none of which appears to be shared with S. cabliersi sp. nov. On the other hand, S. flavens is an ill-known species, being mostly known from Italian and Adriatic coasts (Pulitzer-Finali, 1983) and the Alboran Sea (Maldonado, 1992, 1993), thus co-occurring with S. cabliersi sp. nov. in the later. Nevertheless, its oxeas are defined as slightly fusiform, with frequent tetratological modifications (Pulitzer-Finali, 1983; Maldonado, 1993) not observed in S. cabliersi sp. nov. while, at the same time, they are also considerably thinner (Table 6). As so, S. cabliersi sp. nov. is characterized by a single straight to slightly bent strongyloxea size category with a constant, robust width, and blunt to mucronate, short-tapering endings, which tells it apart from all other North Atlantic Spongosorites described so far.
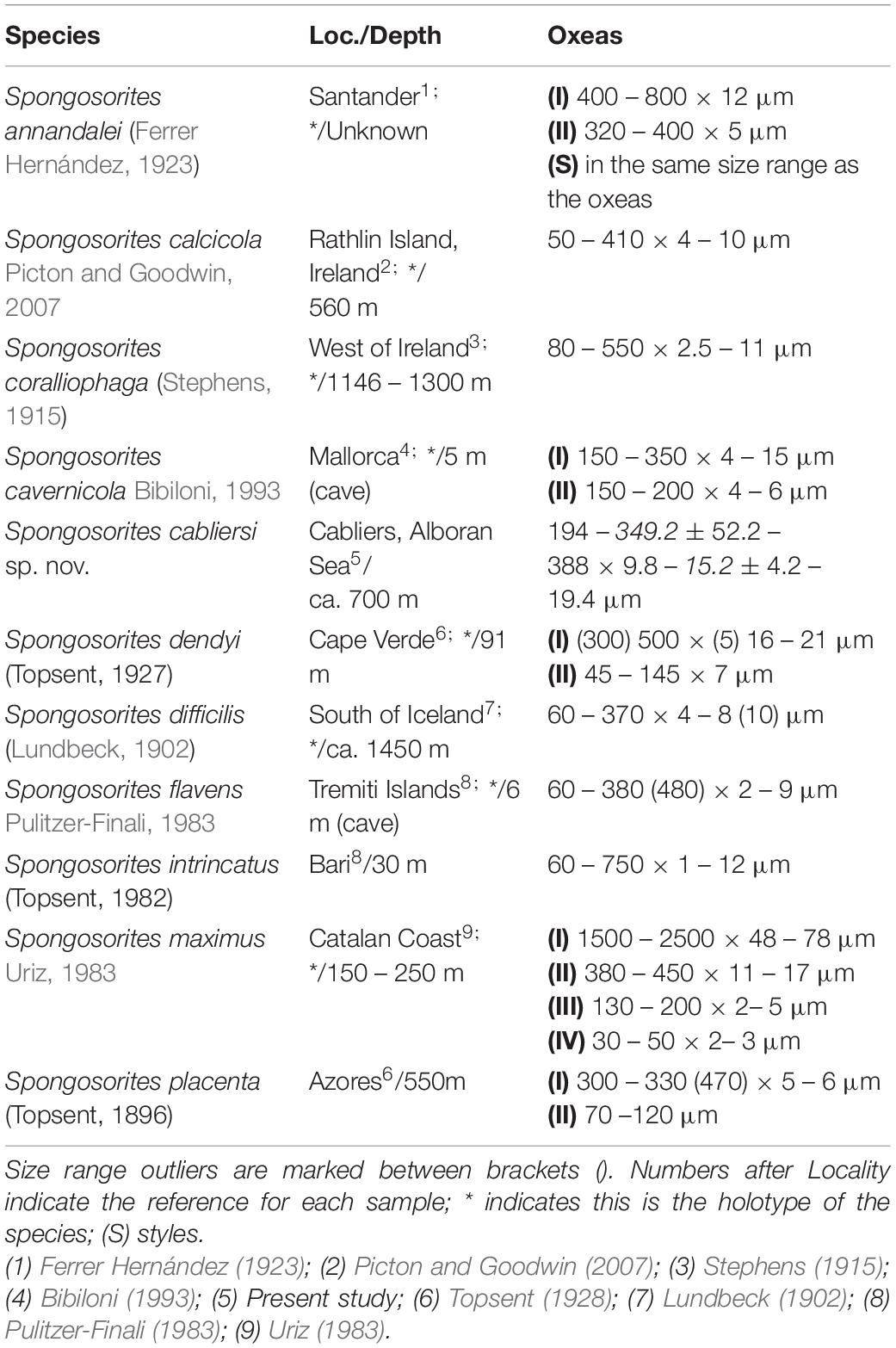
Table 6. Comparative table between the Eastern North Atlantic Spongosorites species, including the locality (Loc.) and depth of the sample, as well as the measurement of their spicular complement.
The bibliographical search reported 37 papers or conference presentations which included data regarding the presence of sponge species associated with Mediterranean CWC frameworks, with approximately 2 – 4 papers per CWC site (Figure 11 and Tables 1, 7). While the oldest paper dates back almost a century (Topsent, 1928), it was not until 2010–2015 that the Mediterranean CWC sponge fauna began to be properly inventoried (Figure 11A). While, overall, a clear increment in number of publications and recorded species has been occurring yearly since then at a basin scale (Figure 11A), this is not the case for all the evaluated CWC sites (Figure 11B). As an example, the Gulf of Lions, which corresponds to the best studied area so far (Tables 1, 7 and Supplementary Materials 3, 4), has barely increased in the total number of recorded species since the early 70s (Vacelet, 1969) despite the continuous exploration of the CWC communities in the area (Fabri et al., 2014, 2017, 2019; Fourt et al., 2017). In this sense, most sites follow a similar trend, with a single publication each representing a sharp increase in the total number of sponge species recorded (Figure 11B), while most other publications barely contribute to expand the total pool of identified species. Additionally, ROV based publications account for a relatively low number of the total species identified on each area (ca. 3 – 5 per area), with a maximum of just 8 species identified for the GoL (Figure 11C).
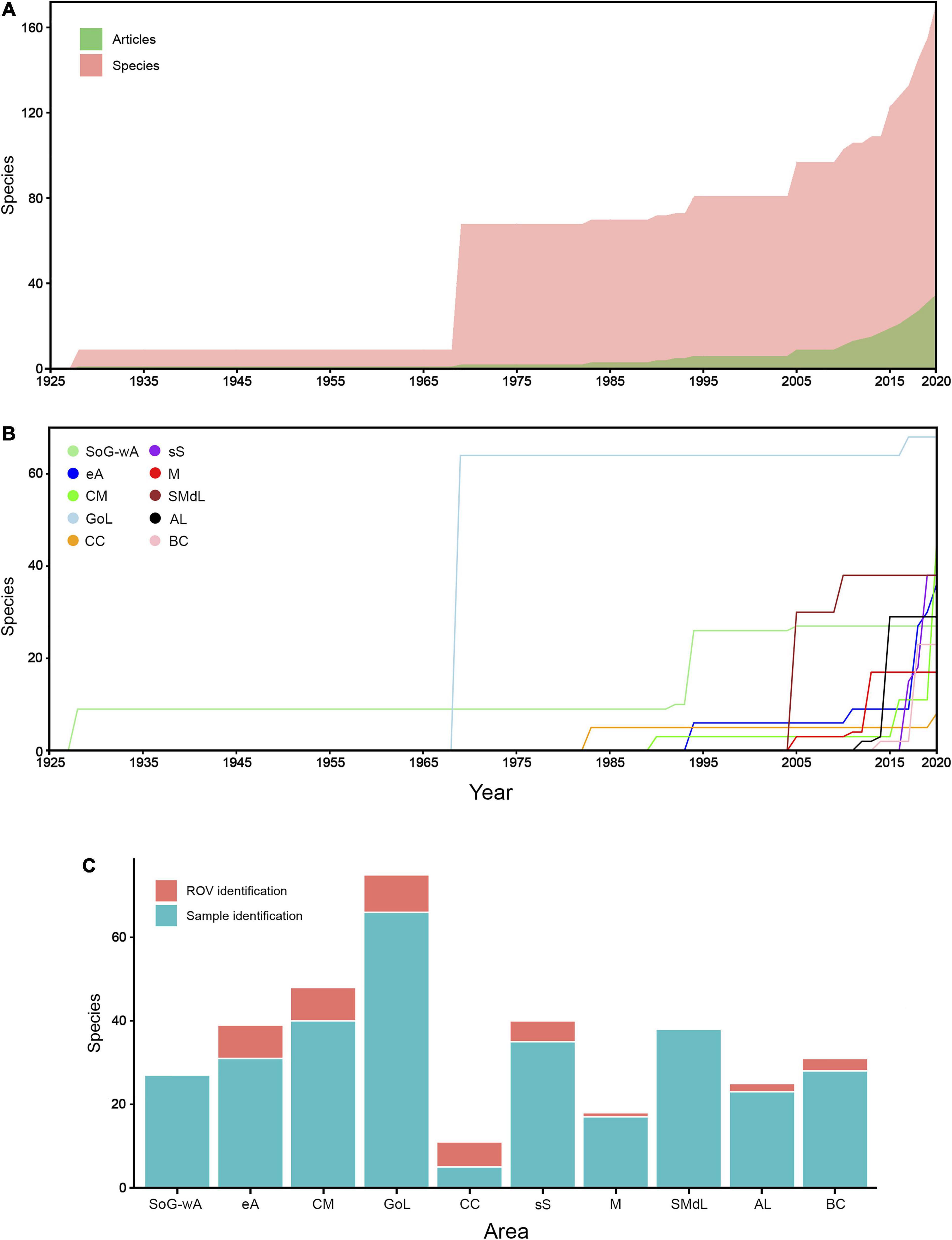
Figure 11. (A) Cumulative timeline of the total number of species (light red) and total number of publications (light green) including data regarding sponge living within Mediterranean CWC. (B) Cumulative timeline of the total number of sponge species associated with CWC on each one of the evaluated areas: SoG-wA (light green); eA (navy blue); CM (bright green); GoL (sky blue); CC (orange); sS (purple); M (red); SMdL (carmine); AL (black); BC (pink). (C) Stacked barplots showing the total number of sponge species identified on each area based on sample identification (duck blue) and/or ROV based identification (red). Note that the sum of both might be higher than the total amount of identified species per area, as the same species might have been identified by both sample and ROV.
The total number of sponge species associated with CWC was 172, with 143 (83%) having been identified to species level (Tables 1, 7). Most sites had between 25 and 35 species per area except for the Gulf of Lions, the Maltese waters and the Corsica Channel, which reported 69, 17, and 8 species, respectively (Tables 1, 7). Due to the low number of reported species in the Corsica Channel, records from nearby coral rubble in the Gulf of Liguria (four species; Sarà, 1958; Pulitzer-Finali, 1983) were included as part of this area given their proximity. The percentage of local endemism for each site was considerably low (Table 7), with only two out of 10 areas having endemic species and representing, in both cases, less than a 10% of the total sponge species on each area. Nevertheless, and despite that most of the encountered species corresponded to North Atlantic fauna, Mediterranean endemic sponges consistently represented 10–20% of the total sponge fauna on most sites (Table 7), except for the Corsica Channel, the Maltese waters and Albanian waters, where no Mediterranean endemics were present. However, those three areas are amongst the least explored of all Mediterranean CWC sites, and the presence of Mediterranean endemic sponges should be expected in all of them once sampling effort increases. Regarding their distribution, only one species sponges, only, Desmacella inornata (Bowerbank, 1866), is currently present at all the evaluated sites (Table 1), but it is likely that other common species, such as Poecillastra compressa (Bowerbank, 1866), Pachastrella monilifera Schmidt, 1868 or Hamacantha (Hamacantha) johnsoni (Bowerbank, 1864) might be present at all or most of the CWC sites, but haven’t yet been recorded everywhere due to a lack of sampling effort. In this sense, several species that appear to be limited to the western Mediterranean, such as Oopsacas minuta Topsent, 1927, are in fact known from other deep-sea environments on the eastern Mediterranean (Bakran-Petricioli et al., 2007), thus their absence might also be a results of the currently insufficient sampling effort rather than ecological requirements.
The beta diversity revealed a low number of shared species between CWC sites, rarely exceeded more than 20% (Supplementary Material 3). Similarly, when paired together, none of the investigated areas accounted for more than a 40% of the total diversity of sponge in the basin (Supplementary Material 4). This is in concurrence with the randomized species accumulation curve (Supplementary Material 5), which is far from reaching an asymptote, and shows high standard deviation values of across the curve, implying that the current knowledge on the Mediterranean poriferan diversity is still far from its peak.
Finally, the relationship between the different evaluated CWC sites was represented by means of a dendrogram and nMDS (Figure 12). With a threshold of 25% similarity four groups could be clearly distinguished, which were also significantly different amongst each other (PERMOANOVA, F 1.4741, p-value 0.042∗). In this sense, clusters appeared to partially reflect geographic regions within the Mediterranean Sea, with both Alboran sites grouped together, as well as all western (Catalan Margin, Gulf of Lions and the south of Sardinia) and eastern (Santa Maria di Leuca, Bari Canyon and the Albanian coasts) Mediterranean sites (Figures 12, 13). The fourth cluster did not follow any geographical pattern, including the Corsica Channel (western Mediterranean) and the Maltese waters (eastern Mediterranean). Yet this final cluster is most likely an artifact due to the low number of species recorded in both sites (Tables 1, 7), which are insufficiently characterized when compared to all other the other Mediterranean CWC provinces. As so, we excluded both the CC and M sites from the PERMANOVA analysis, and ran it again (F 2.4428, p-value 0.005∗∗), resulting in a stronger differentiation between clusters. Regarding the possible influence of geographical distance between CWC sites and its associated sponge fauna composition, the Mantel test proved statistically significant (rM 0.4474, p-value 0.006∗∗), thus indicating that geographical distance was at least partially responsible for the CWC sponge communities’ composition. In this sense, the Person correlation also proved statistically significant (n = 45 pairwise comparisons, R 0.48, t 3.4861 p-value 0.001∗∗∗), with closer sites being more similar than distant sites (Supplementary Material 6), supporting the nMDS and dendrogram clustering.
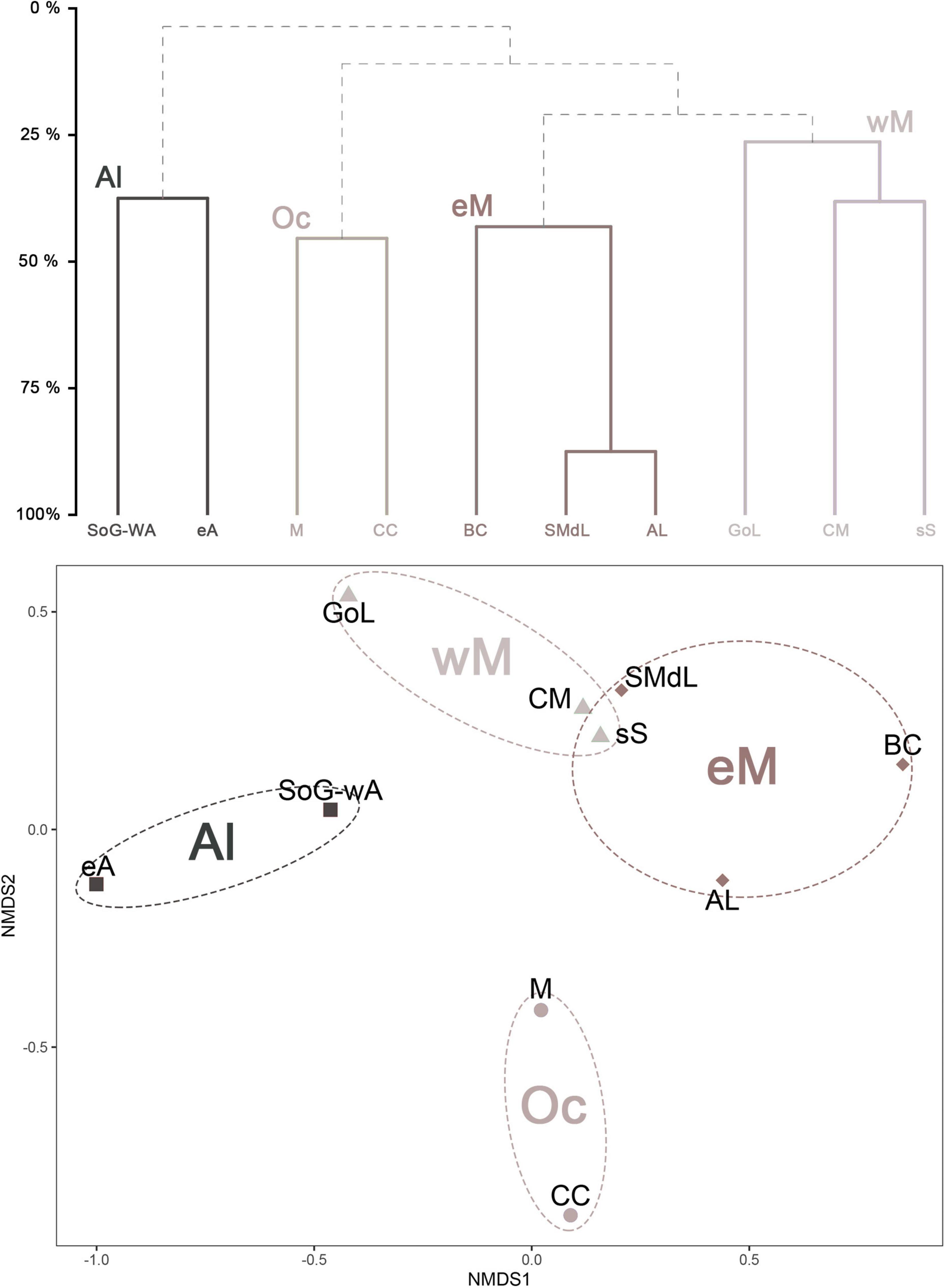
Figure 12. Top: dendrogram representing the sample clustering based on a Bray–Curtis dissimilarity matrix. A 25% similarity threshold was used for clustering. Clusters are differently shaded on a gray-scale to ease visual group identification. Bottom: non-metric multidimensional scaling (nMDS) ordination plot. CWC provinces are represented based on a presence/absence matrix of their associated sponge assemblages. A stress estimate of 0.048 was obtained, indicating a good fit. Clusters are differently shaded on a gray-scale to ease visual group identification, as in the dendrogram. Strait of Gibraltar and western Alboran (SoG-wA); eastern Alboran (eA); Catalan Margin (CM); Gulf of Lions (GoL); South of Sardinia (sS); Corsica Channel (CC); Maltese waters (M); Santa Maria di Leuca (SMdL); Albanian coasts (AL); Bari Canyon (BC); Alboran cluster (Al); western Mediterranean cluster (wM); eastern Mediterranean cluster (eM); Outlier cluster (Oc).
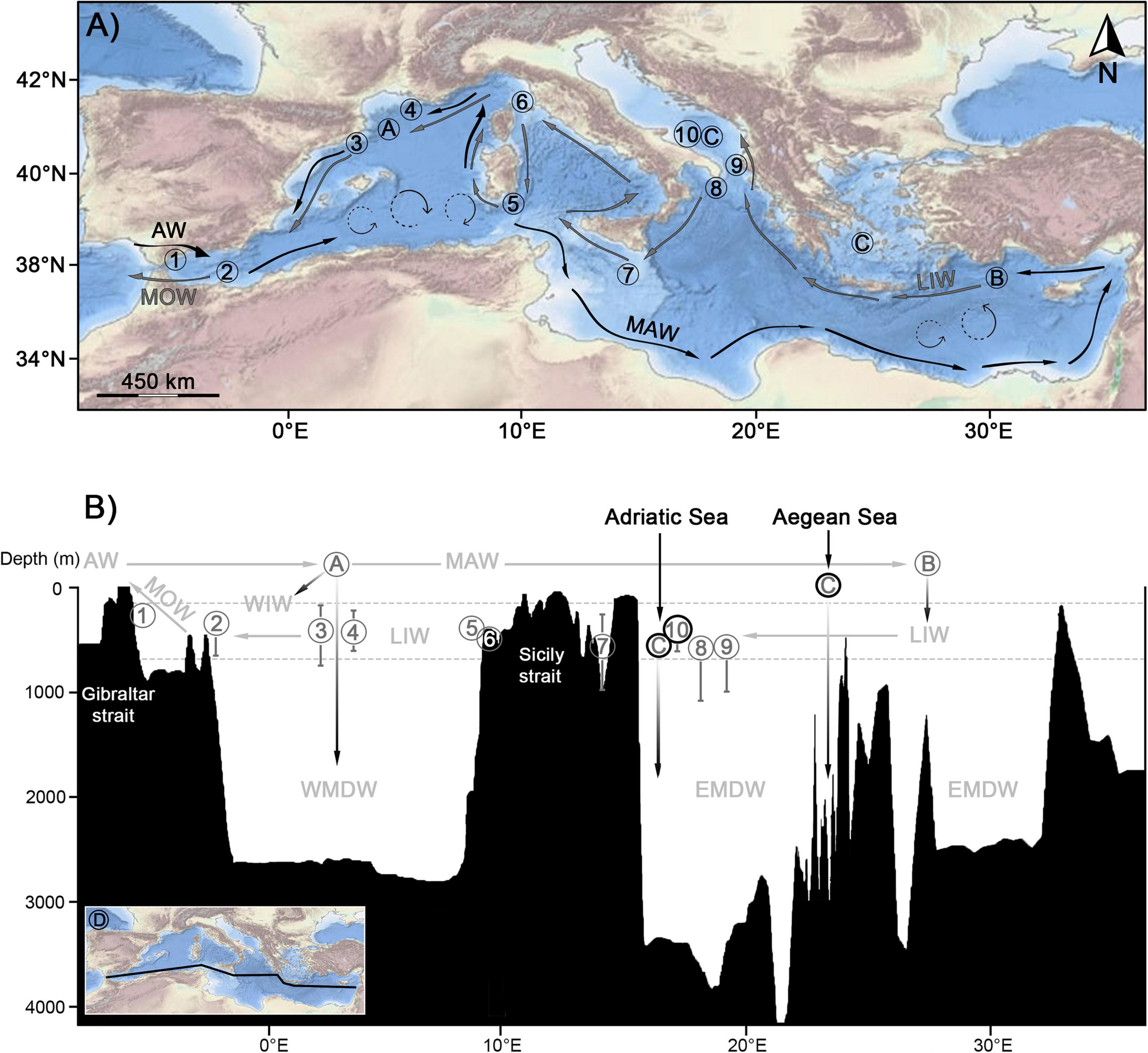
Figure 13. (A) Bathymetric map of the Mediterranean Sea, with all CWC provinces (numbers) evaluated represented alongside a simplified schematic representation of its water masses circulation and formation (Letters). (B) Vertical profile of the Mediterranean Sea with all CWC provinces depth ranges represented alongside a simplified schematic representation of its water masses circulation (arrows) and formation (letters). The whiskers on each number represent the depth range of occurrence for each CWC province according to Chimienti et al. (2019) with additional data for the Corsica Channel from Angeletti et al. (2020) and for the Catalan Margin from the De Leo et al. (2019) and Puig et al. (2019). 1: Strait of Gibraltar and western Alboran (SoG-wA); 2: eastern Alboran (eA); 3: Catalan Margin (CM); 4: Gulf of Lions (GoL); 5: South of Sardinia (sS); 6: Corsica Channel (CC); 7: Maltese waters (M); 8: Santa Maria di Leuca (SMdL); 9: Albanian coasts (AL); 10: Bari Canyon (BC). Water masses are abbreviated as follows: AW, Atlantic Water; MAW, Modified Atlantic Water; LIW, Levantine Intermediate Water; WIW, Winter Intermediate Water; WDMW, Western Mediterranean Deep Water; EMDW, Eastern Mediterranean Deep Water; MOW, Mediterranean Outflow Water. A, area of formation of the WIW and WDMW; B, suspected area of formation of the LIW based on Hayes et al. (2019); C, areas of formation of the EMDW; D, Approximate geographical representation of the area represented on the vertical profile. The bathymetric metadata and Digital Terrain Model data products have been obtained and modified from the EMODnet Bathymetry portal – http://www.emodnet-bathymetry.eu.
In general terms, the sponge fauna associated with CWC Mediterranean communities did not differ much from their Atlantic counterparts, both being dominated by Demospongiae from the orders Poecilosclerida and Tetractinellida, as it has already been described by van Soest and De Voogd (2015). Hexactinellida, while not as numerous, are still common in most of the basin, specifically its western side as stated in previous studies (Boury-Esnault et al., 2015, 2017) whereas, on the contrary, Calcarea and Homoscleromorpha have been seldomly recorded, and their presence is currently testimonial.
Currently, over 172 potential poriferan species have been found to occur within Mediterranean CWC provinces (Table 1), well behind data published for CWC in the North Atlantic (ca. 260 potential species; van Soest and De Voogd, 2015), yet almost doubling those from the last review on Mediterranean CWC sponge fauna (ca. 90 species; Rueda et al., 2019). On the other hand, when considered individually, almost none of the CWC sites accounted for more than ca. 20% of the total sponge diversity observed for the basin (Table 7). Moreover, the species accumulation curve for the basin is far from asymptotic (Supplementary Material 5), altogether suggesting that the poriferan fauna associated with CWC in the Mediterranean is still underrepresented both, at a local (CWC sites) and global (whole Mediterranean basin) scale, despite the increasing effort to map it.
In this sense, sampling effort and taxonomic resolution seems like the most likely explanation for the current diversity values observed. Regarding sampling effort, CWC are known to be highly heterogeneous substrates (Buhl-Mortensen et al., 2010), thus sponge diversity and abundance widely varies between areas of the reef, with higher sponge abundance and diversity on dead coral frameworks or mixed substrates than living CWC (van Soest et al., 2007), which in turn means sampling effort heavily constraints the pool of species to be encountered. As an example, van Soest et al. (2007) identified over 150 sponge species from over 126 boxcores within three different CWC mount off the coast of Ireland, with sponges’ diversity per core ranging between 0 and 57 species. Yet, even so, total poriferan diversity was estimated to be underrepresented in the area (van Soest and Lavaleye, 2005; van Soest et al., 2007). Currently, while CWC sponge research in the Mediterranean has considerably increased during the past decade (Rueda et al., 2019), this still mostly comes from sparse, fragmentary CWC material, usually no more than a few pieces of coral per study (Longo et al., 2005; Calcinai et al., 2013; Bertolino et al., 2019; present study). Considering this in more detail, the East Alboran CWC province contains, amongst other CWC sites, the Cabliers Coral Mound, which extends for over 25 km, and has an average high between 70 and 140 m (Corbera et al., 2019). In contrast, our knowledge of its associated sponge fauna comes from just a few pieces of dead coral, less than 0.04 m2 (Costa et al., 2018; present study) and 5 megafaunal species observed by ROV (Boury-Esnault et al., 2015; Corbera et al., 2019), a trend that is extensive to all other CWC provinces of the Mediterranean.
Finally, while CWC research in the Mediterranean dates back to 1970s (Figure 11A), it is not until the first decade of the 21th century that biological and ecological studies flourish, thanks to the development and advances in deep-sea robotics and other deep-sea imaging techniques (Orejas and Jiménez, 2019). Nevertheless, while video footage has boosted our knowledge on deep-sea environments (Aguilar et al., 2011; Bo et al., 2012; D’Onghia et al., 2015; Taviani et al., 2017; Chimienti et al., 2019; Fabri et al., 2019), the taxonomic resolution of these techniques widely varies between phyla (Fabri et al., 2014, 2019). As an example, Bertolino et al. (2019) in their study of the sponges associated with CWC in the South of Sardinia CWC province, they could only identify three species by ROV [Poecillastra compressa (Bowerbank, 1866), Pachastrella monilifera Schmidt, 1868, Phakellia robusta (Bowerbank, 1866)], whereas a total of 28 could be identified from the collected CWC rubble studied. Yet, the analysis of collected material is not extent of taxonomic resolution hazards. Porifera is generally considered a rather difficult to identify phylum (Reveillaud et al., 2011; van Soest et al., 2012), whose proper identification usually requires of qualified experts (van Soest et al., 2012) and the combined used of morphological and molecular tools (Reveillaud et al., 2010, 2011) and, even so, sometimes species identification cannot be reached (van Soest, 2009). This is exacerbated with deep-sea samples, as the encrusting nature of most species, alongside the paucity of the material might compromise species identification. Furthermore, the cryptic nature of several encrusting sponges living in coral reefs ecosystems (van Soest, 2009), as well as poriferan groups with few distinctive morphological traits (e.g., Halichondrida or Haplosclerida; van Soest, 2009; van Soest, 2017), has led researchers to assign specimens to the closest available names, creating ‘complex species,’ which ultimately leads to the underestimation of their diversity until those are properly resolved (Reveillaud et al., 2010, 2011).
As already mentioned, the current knowledge on the Mediterranean CWC sponge fauna is still fragmentary and underrepresented (Figure 11 and Table 7), but potentially as diverse as in the Atlantic region. Currently, estimates for sponge diversity in Atlantic CWC would be in range of that of other ecosystems on the same region (van Soest and De Voogd, 2015). Contrarily, while at a basin scale Mediterranean CWC appear to be hotspots of sponge diversity (Tables 1, 7 and Supplementary Materials 3, 4), at a local scale (CWC province), diversity values trail behind other deep and shallow Mediterranean habitats. In this sense, sponge grounds along the Mediterranean continental shelves and upper slopes (Sitjà and Maldonado, 2014; Bertolino et al., 2015; Santín et al., 2018b, 2019) or even shallow circalittoral environments (Pulitzer-Finali, 1983; Bibiloni, 1990; Maldonado, 1993; Bertolino et al., 2013) or caves (Gerovasileiou and Voultsiadou, 2012) show diversity values (50 – 100 species per article) exceeding those of the evaluated CWC provinces individually, yet this is likely to be a result of the higher sampling effort in shallower environments.
While at a basin scale CWC appear as sponge hotspots, there are still knowledge gaps regarding the main drivers for CWC sponges’ diversity (van Soest and De Voogd, 2015) as well as their ecology (Kazanidis and Witte, 2016; Rix et al., 2016; Kazanidis et al., 2018) and their role as biological structures (Buhl-Mortensen et al., 2010; Kazanidis et al., 2016). In this sense, it is known that depth is strongly linked with changes in the composition of sponge assemblages (Santín et al., 2018b, 2019), including CWC associated sponges (Longo et al., 2005; van Soest and De Voogd, 2015), yet it is suspected it might just be a proxy of other abiotic factors in play, such as temperature or food availability (van Soest and De Voogd, 2015). In fact, this concurs with previous observations of vertical sponge diversity, which showed low correlation with temperature and salinity values over a 500 m depth range (Maldonado and Young, 1996). Contrary to other areas of the world, the Mediterranean deep-sea benthic fauna, including sponges, is known to be mostly eurybathic (Bouchet and Taviani, 1992), with some species even occurring from the shallow littoral areas to over 1000 m depth (Santín et al., 2020b). In this sense, the Mediterranean Sea, contrary to the Atlantic, is known to be homothermic (ca. 13°C) below 200 m, where all CWC provinces occur, whereas in the Atlantic, water temperature decreases gradually until 3000 m (Bouchet and Taviani, 1992). As so, while temperature might play a major role in shaping sponge diversity in the Atlantic, this would not seem to be the case for Mediterranean CWC. On the contrary, other factors such as substrate availability (Maldonado and Young, 1996; Sitjà and Maldonado, 2014; Santín et al., 2018b), food supply (van Soest and De Voogd, 2015; Robertson et al., 2017), the presence of predators (Robertson et al., 2017) or historical factors and large-scale phenomena (Gage, 2004) might be or have been theorized to play a major role in shaping Mediterranean deep-sea sponge communities, including those in CWC.
Cold-water coral reefs in the Mediterranean are typically known to occur in submarine canyons (Puig and Gili, 2019), where the topography of the area creates localized circulation patterns which might induce larvae retention within the reef, limit species’ dispersal, and potentially enhance rates of speciation within the reef (Roberts et al., 2006). Nevertheless, levels of endemism in CWC sponge fauna seems to be really low, with only 10% of all known species having been recorded exclusively from CWC ecosystems across their distribution (Tables 1, 7). In this sense, Porifera is a highly plastic phylum (van Soest et al., 2012), which might allow them to adapt to a wide myriad of environments, helping them expand their vertical distribution (Bertolino et al., 2019). Most sponge species found in Mediterranean CWC are also known to occur in other hard-substrate ecosystems (Bertolino et al., 2019), a trend that also occurs in the North Atlantic (van Soest and Lavaleye, 2005; van Soest et al., 2007). As so, Mediterranean CWC sponge fauna seems to support the view that sponges living on CWC are mostly facultative, and not settling preferentially on CWC over other hard substrates.
Yet, present results might suggest the existence, to a certain level, of a Mediterranean CWC sponge fauna, different from that on the Atlantic. In this sense, the Mediterranean Sea is characterized by low endemism and diversity values when compared to the fauna of the Northeast Atlantic, a fact mostly linked to the Messinian Salinity Crisis (Bouchet and Taviani, 1992; Emig and Geistdoerfer, 2004). During this crisis, it is thought that the Mediterranean was almost completely desiccated, leaving to a massive extinction of the basin’s fauna (Hsü et al., 1973). Nevertheless, the view of a completely desiccated Mediterranean has recently been challenged (Krijgsman et al., 2018), since there are evidences supporting that the connection with the Atlantic was not lost completely during the crisis (Gili et al., 1998; Krijgsman et al., 2018), as there is speculation on the existence of areas with almost normal salinity values during this period (Por, 1989). This seems to be supported by the existence of Mediterranean Tethyan relicts, which refers to species whose current distribution dates back to the closing of the Indo-Pacific-Mediterranean connection (Hou and Li, 2018). In this sense, several deep-sea Porifera have been proposed as possible Tethyan relicts (Boury-Esnault et al., 1992, 1994; Maldonado and Uriz, 1995; Pisera et al., 2018; Santín et al., 2020b), supporting the view that the component of Tethyan relicts on the deep-sea fauna of the Mediterranean Sea and neighboring Atlantic areas might be higher than originally thought. Thus, the current Mediterranean deep-sea fauna would be a mix between the survivors of the Messinian Salinity Crisis and the restocking fauna from the Atlantic (Emig and Geistdoerfer, 2004). This would concur with the current observed trend, with the larger fraction of sponges found in all sites also being present in the Atlantic (Table 7), yet with a consistent ca. 20% of sponge species in all sites being Mediterranean endemics (Table 7). As so, while endemic CWC Porifera appear to be very rare, it could be concluded that Mediterranean CWC sponge fauna is unique, and differing from that of other Atlantic regions.
As seen, the Mediterranean CWC provinces are a hotspot of sponge diversity, which composition is the result of a myriad of intertwined biotic and abiotic factors. Sponges have long been proven to be an excellent tool for a biogeography approach, as they are a major component of most benthic ecosystems (Maldonado and Uriz, 1995; Carballo et al., 1997; Xavier and Van Soest, 2012) with overall low dispersal capabilities (Mariani et al., 2006), which in turn translates into clear geographical diversity patters (Xavier and Van Soest, 2012; Roberts et al., 2021). Nevertheless, so far only shallow species had been considered for such type of approach, with deep-sea sponges rarely ever being considered until recently (Sitjà, 2020; Sitjà et al., 2020; Roberts et al., 2021).
Clustering of the evaluated sites supports the presence of at least three different CWC sponge clusters in the Mediterranean Sea (Figure 12), with a 4th one mostly likely being an artifact due to insufficient data (see Section “Diversity Estimates Amongst CWC Provinces”). In this sense, while there is a current sampling bias for all the considered areas (see Section “Diversity estimates, sampling effort and identification bias”), previous studies in CWC communities have highlighted that the sponge fauna dwelling on these reefs tends to be represented by a few, very abundant species, whilst all other occur in relatively low numbers (van Soest and Lavaleye, 2005; van Soest et al., 2007; van Soest and De Voogd, 2015), allowing for an exploratory comparison between sites, assuming that the most common sponges on each site have already been sampled.
From a biogeographical point of view, two main water masses might be suspected to be the drivers behind any possible pattern of sponge diversity within Mediterranean CWC: The Atlantic Water (AW) and the Levantine Intermediate Water (LIW) with, additionally, the Strait of Gibraltar and the Strait of Sicily being the most likely geographical barrier for dispersal (Sitjà, 2020). Assuming that sponge assemblage’s composition is dependent on larval supply (Reveillaud et al., 2010), and considering the main currents occurring in the Mediterranean Sea (Figures 13A,B), several explanations can be proposed to explain the current pattern observed in the basin.
First of all, it is known that the Strait of Gibraltar is both the door and the biggest barrier for benthic species dispersal between the Atlantic and Mediterranean populations, including sponges (Maldonado and Uriz, 1995; Carballo et al., 1997). Yet, the Strait is not a clear-cut barrier, but a rather complex buffer zone including the Gulf of Cadiz and the Alboran Sea (Carballo et al., 1997; Sitjà et al., 2020). As so, species with Mediterranean affinities can exist outside the basin in the Gulf of Cadiz waters under the Mediterranean Outflow Water (MOW) influence (Sitjà et al., 2020), whereas Atlantic species might find their limit of distribution at the Alboran Sea (Carballo et al., 1997). This could explain the grouping of both Alboran CWC sites, which would include the presence of Atlantic species which does not occur in the Mediterranean Sea far beyond the area of major influence of the AW, even if the exact limiting factors are not well-understood (e.g., Asconema setubalense Kent, 1870; Pardo et al., 2011; Sitjà and Maldonado, 2014; Boury-Esnault et al., 2015). The two remaining groups reflect a western-eastern pattern, separated by the Strait of Sicily (Figures 12, 13). Regarding the western Mediterranean cluster, the CM and the GoL are located at the area of influence of the Northern Current, which flows southwards from the Gulf of Liguria to the Catalan coast (Robinson et al., 2001). This surface current is influenced by the MAW, which in the Gulf of Lions is known to be subjected to up and downwelling processes, as well as being a major component of the deep-water formation (Millot, 1990; Palanques and Puig, 2018). As so, the sinking of the Northern Current might bring larvae down to the CWC occurring in the Gulf of Lions and nearby areas (Figure 13B), much like upwelling events have been hypothesized to maintain the connectivity and larval flux between deep-sea and cave environments in the zone (Vacelet et al., 1994). Finally, the sS site is located in the LIW flow toward the GoL and CM areas (Figures 13A,B), so a passive transport of larvae from one to the others seems feasible. This would be supported by the present finding of high abundances of H. (H.) quadridentata and P. tavianii in the CM area, which were recently described from the sS CWC province (Cardone et al., 2019) and haven’t been found anywhere else so far.
The third group would include all CWC sites east of the Strait of Sicily (SMdL, AL, and BC) except for the Maltese waters, which clusters with the Corsica Channel area due to a major lack of data in both compared to all other CWC sites. Santa Maria di Leuca is located on the near the entrance of the Adriatic Sea (Figure 13A), were the LIW enters the Adriatic to form the Eastern Mediterranean Deep Water (EMDW). In this sense, the Strait of Sicily (Figure 13B) would pose as a second geographical barrier for any Atlantic migrant (Pansini, 1987), further reducing the pool of species that might make it to the eastern section of the Mediterranean when compared to its western counterpart (Bouchet and Taviani, 1992). As so, it could be hypothesized that the sponge fauna from the AL and BC might come from the pool of species found at the SMdL, rather than directly from the Atlantic or any other CWC province in the Mediterranean (Taviani et al., 2011; Angeletti et al., 2014; Chimienti et al., 2019). In this sense, while genetic studies are still scarce, there are evidences than gene flow between Lopehlia and Madrepora reefs at both sides of the Strait of Gibraltar is currently relatively low (Boavida et al., 2019), which would also seem to be the case for its associated fauna (e.g., the polychaete Eunice norvegica; Boavida et al., 2019), and it would not be unreasonable to expect a similar pattern for species leaving at both sides of the strait of Sicily. Finally, this western-eastern pattern would be supported by the correlation between dissimilarity in species composition vs. geographical distance between sites (Supplementary Material 6), which suggests that the closer to CWC sites area, the more their sponge fauna is shared, thus supporting the view that closer CWC provinces are to have a higher connectivity and shared number of species than distant ones.
Thus, the connectivity between CWC Mediterranean sponge populations could be explained by passive larval dispersal enhanced by LIW and AW currents and limited by the existence of geographical barriers (Gary et al., 2020) and which would be facilitated by the existence of intermediate or stepping-stones populations, as theorized for other sponge species (Padua et al., 2018). In this sense, it is also worth noticing the importance of stochasticity and asexual reproduction might have for the configuration of CWC sponge assemblages. It is well documented that stochastic settlement of larvae plays a major role in the composition of benthic communities, both in term of abundance and diversity (Edwards and Stachowicz, 2011), most likely including those living in association with CWC. Given the aforementioned limitations for larval dispersal between CWC, it should be expected that, if reproductive, self-recruitment and asexual reproduction would be more important than external larval supply in sustaining the Mediterranean CWC sponges’ populations, especially considering that (i) water circulation around CWC favors a semi-enclosed dynamic (Roberts et al., 2006) and (ii) the generally poor-swimming capabilities of sponge larvae (Mariani et al., 2006) and (iv) the frequent occurrence of asexual reproduction in sponges populations (e.g., Teixidó et al., 2006). In this sense, P. taviani and A. schmidti [the first recently described by Cardone et al. (2019), the latter by Topsent (1904, 1928) a century ago, and just recently reencountered in the Cantabrian Sea, Heres et al. (2014)], were the most abundant species within the Catalan Margin CWC (Supplementary Material 1) whilst, prior to this finding, they were both considered rare species. While it could be argued that their abundance might indicate a higher degree of adaptation to their environmental setting than other sponges, it is hard to believe that they might be much better adapted than any of the other small encrusting sponges encountered, their abundance most likely being the result of stochastic reproductive events rather than favourability.
As so, much as their shallow counterparts (Jones et al., 2009 and references within), CWC could be theorized to be semi-enclosed ecosystems for sponges, with limited external larval input and mostly sustained by self-recruiting and asexual reproduction of its existing fauna, fueled by the larval retention caused by local hydrodynamics around the reefs (Roberts et al., 2006) and, thus, critically affected by stochastic events (e.g., first arrival, settlement success, mortality events) that have occurred during the reef’s history. Nevertheless, this remains speculative, as currently, no studies have evaluated the genetic connectivity between and within CWC reef sponges while, the same time, our current knowledge on the behavior, ecology and physiology of sponge remains scant (Mariani et al., 2006; Bo et al., 2012; Robertson et al., 2017; Santín et al., 2019), even more for those occurring in deep-sea ecosystems (Maldonado et al., 2020).
The present article provided the first insight on the associated sponge fauna of the recently discovered CWC communities on the Catalan Margin and, to a lesser extent, the Cabliers Coral Mound Province, with the description of several new or rare species. From a global point of view, the present article highlights, despite an overall lack of knowledge, the role of CWC in general, and of Mediterranean CWC in particular, as biodiversity hotspots for sponges, the major bullet points being:
– The observed poriferan diversity values on Mediterranean CWC currently represent a small fraction of its actual fauna rather than reflecting the real diversity on each area, which is suspected to be highly underrepresented.
– The existence of an endemic CWC sponge fauna is refuted, yet the Mediterranean CWC sponge fauna appears to be unique and different from that of other Atlantic regions, having being shaped by the basin’s geological and hydrological history, and consisting of a mixture of Atlantic migrants and pre-Messinian relict fauna.
– The sponge assemblage at a specific CWC province cannot be explained by a single or reduced group of factors, but it might be the result of a complex interaction between species’ evolutionary history, their life traits and both abiotic and biotic factors as well as the reef’s own long-term history.
– The current composition of the Mediterranean CWC sponge assemblages seems to be determined by the basin’s water circulation, specially the Levantine Intermediate Water and the Atlantic Water, with a western-eastern pattern from the Strait of Gibraltar to the Adriatic Sea.
– Cold-water corals might act as semi-enclosed ecosystems for sponges, which might be rather sustained by asexual reproduction and self-recruitment than external larval supply.
– Overall, sponge living in CWC appear as good candidates for biogeographical studies, and might help understand the connectivity between CWC sites, yet there is still a current major lack of knowledge on the topic.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: ZooBank (urn:lsid:zoobank.org:pub:E58A3DFF-EDC5-44FC-A274-1C9508BF8D15).
AS, JG, JMG, and MU conceived of the presented idea. PP and CL devised the framework projects from which the samples came from. JG, PP, and CL attended the oceanographic surveys and collected the samples. AS and MU performed the SEM imaging and other analyses. AS wrote the manuscript with inputs from all other authors, whom provided critical feedback and helped shape the research, analysis and manuscript. All the authors contributed to the article and approved the submitted version.
Samples from the Blanes Canyon were collected during the ABIDES project (Ref. CTM2015-65142-R) and analyzed during the ABRIC project (Ref. RTI2018-096434-B-I00), both funded by the Spanish Ministry of Science and Innovation and granted to PP. Samples from the Cabliers Coral Mound were collected during the MELCOR Cruise in the frame of the FP7 EU Marie Curie ProjectGeo-Habit (GA29874) granted to CL and the SHAKE Project (Ref. CGL2011-30005-C02-02) funded by the Spanish Ministry of Science and Innovation. Finally, AS was the recipient of the 2019 Young Scientist Best Paper Award of the Department of Marine Biology and Oceanography at the Institute of Marine Sciences, which provided funding for the SEM imaging. With the institutional support of the ‘Severo Ochoa Centre of Excellence’ accreditation (CEX2019-000928-S).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the crew of the R/V Sarmiento de Gamboa and the crew of the ROV ‘Liropus2000’ pilots. We also thank the entire crew of the R/V Garcia del Cid, in particular the captain Eduardo Otal, and the UTM-CSIC technicians Marcos Pastor, Cristina Alvarez, and Joel Sanz for their help during the MELCOR oceanographic survey; Ruth Durán for her help creating the maps; Carlota Ruiz for listening to endless conjecturations and her aid with bibliography; José Manuel Fortuño (ICM-CSIC) for his technical assistance during SEM image acquisition and finally, Francesc Uribe, curator of the MZB, for his technical assistance.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.662899/full#supplementary-material
Aguilar, R., López Correa, M., Calcinai, B., Pastor, X., De La Torriente, A., and Garcia, S. (2011). First records of Asbestopluma hypogea Vacelet and Boury-Esnault, 1996 (Porifera, Demospongiae Cladorhizidae) on seamounts and in bathyal settings of the Mediterranean Sea. Zootaxa 2925, 33–40. doi: 10.11646/zootaxa.2925.1.3
Álvarez-Pérez, G., Busquets, P., De Mol, B., Sandoval, N. G., Canals, M., and Casamor, J. L. (2005). “Deep-water coral occurrences in the Strait of Gibraltar,” in Cold-water corals and ecosystems, eds A. Freiwald and J. M. Roberts (Berlin: Springer), 207–221. doi: 10.1007/3-540-27673-4_10
Anderson, M. J. (2001). A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 26, 32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x
Angeletti, L., Castellan, G., Montagna, P., Remia, A., and Taviani, M. (2020). The “Corsica Channel Cold-Water Coral Province”(Mediterranean Sea). Front. Mar. Sci. 7:661. doi: 10.3389/fmars.2020.00661
Angeletti, L., Taviani, M., Canese, S., Foglini, F., Mastrototaro, F., Argnani, A., et al. (2014). New deep-water cnidarian sites in the southern Adriatic Sea. Med. Mar. Sci. 15, 263–273. doi: 10.12681/mms.558
Aymà, A., Aguzzi, J., Canals, M., Company, J. B., Lastras, G., Mecho, A., et al. (2019). “Occurrence of living Cold-Water Corals at large depths within submarine canyons of the northwestern Mediterranean Sea”, in Mediterranean Cold-Water Corals: Past, Present and Future, eds C. Orejas and C. Jiménez (Springer International Publishing AG, part of Springer Nature). Coral Reefs World Ser. 9, 271–284. doi: 10.1007/978-3-319-91608-8_26
Babiç, K. (1922). Monactinellida und Tetractinellida des Adriatischen Meeres. Zool. Jahrb. Abt. Syst. Geog. Biol. Tiere 46, 217–302.
Baillon, S., Hamel, J. F., Wareham, V. E., and Mercier, A. (2012). Deep cold-water corals as nurseries for fish larvae. Front. Ecol. Environ. 10, 351–356. doi: 10.1890/120022
Bakran-Petricioli, T., Vacelet, J., Zibrowius, H., Petricioli, D., Chevaldonné, P., and Raa, T. (2007). New data on the distribution of the ‘deep-sea’ sponges Asbestopluma hypogea and Oopsacas minuta in the Mediterranean Sea. Mar. Ecol. 28, (Suppl. 1), 10–23. doi: 10.1111/j.1439-0485.2007.00179.x
Bart, M. C., Hudspith, M., Rapp, H. T., Verdonschot, P. F., and De Goeij, J. M. (2021). A deep-sea sponge loop? sponges transfer dissolved and particulate organic carbon and nitrogen to associated fauna. Front. Mar. Sci. 8:229. doi: 10.3389/fmars.2021.604879
Bertolino, M., Bo, M., Canese, S., Bavestrello, G., and Pansini, M. (2015). Deep sponge communities of the Gulf of St Eufemia (Calabria, southern Tyrrhenian Sea), with description of two new species. J. Mar. Biolog. Assoc. U K. 95, 1371–1387. doi: 10.1017/s0025315413001380
Bertolino, M., Cerrano, C., Bavestrello, G., Carella, M., Pansini, M., and Calcinai, B. (2013). Diversity of Porifera in the Mediterranean coralligenous accretions, with description of a new species. ZooKeys 336, 1–37.
Bertolino, M., Ricci, S., Canese, S., Cau, A., Bavestrello, G., Pansini, M., et al. (2019). Diversity of the sponge fauna associated with white coral banks from two Sardinian canyons (Mediterranean Sea). J. Mar. Biolog. Assoc. U K. 99, 1735–1751. doi: 10.1017/s0025315419000948
Beuck, L., and Freiwald, A. (2005). “Bioerosion patterns in a deep-water Lophelia pertusa (Scleractinia) thicket (Propeller Mound, northern Porcupine Seabight,” in Cold-water Corals and Ecosystems, eds A. Freiwald and J. M. Roberts (Berlin: Springer-Verlag), 915–936. doi: 10.1007/3-540-27673-4_47
Beuck, L., Freiwald, A., and Taviani, M. (2010). Spatiotemporal bioerosion patterns in deep-water scleractinians from off Santa Maria di Leuca (Apulia, Ionian Sea). Deep Sea Res. Part II Top. Stud. Oceanogr. 57, 458–470. doi: 10.1016/j.dsr2.2009.08.019
Beuck, L., Vertino, A., Stepina, E., Karolczak, M., and Pfannkuche, O. (2007). Skeletal response of Lophelia pertusa (Scleractinia) to bioeroding sponge infestation visualised with micro-computed tomography. Facies 53, 157–176. doi: 10.1007/s10347-006-0094-9
Bibiloni, M. A. (1981). Estudio sistemático de orden Poecilosclerida (Demospongia) de la Costa Brava (Gerona). Bol. Inst. Esp. Océano. 6, 103–154.
Bibiloni, M. A. (1990). Fauna de esponjas de las islas Baleares: Variación cualitativa y cuantitativa de la población de esponjas en un gradiente batimétrico: comparación Baleares-costa catalana. Ph. D. Thesis. Barcelona: Universitat de Barcelona, 483.
Bibiloni, M. A. (1993). Some new or poorly known sponges of the Balearic Islands (western Mediterranean). Scien. Mar. 57, 307–318.
Bo, M., Bertolino, M., Bavestrello, G., Canese, S., Giusti, M., Angiolillo, M., et al. (2012). Role of deep sponge grounds in the Mediterranean Sea: a case study in southern Italy. Hydrobiologia 687, 163–177. doi: 10.1007/978-94-007-4688-6_14
Boavida, J., Becheler, R., Addamo, A. M., Sylvestre, F., and Arnaud-Haond, S. (2019). “Past, Present and Future Connectivity of Mediterranean Cold-Water Corals: Patterns, Drivers and Fate in a Technically and Environmentally Changing World”. in Mediterranean Cold-Water Corals: Past, Present and Future, eds C. Orejas and C. Jiménez (Springer International Publishing AG, part of Springer Nature). Coral Reefs World Ser. 9, 357–372. doi: 10.1007/978-3-319-91608-8_31
Bouchet, P., and Taviani, M. (1992). The Mediterranean deep-sea fauna: pseudopopulations of Atlantic species? Deep Sea Res. Pt. A Oceanogr. Res. Pap. 39, 169–184.
Boury-Esnault, N., Pansini, M., and Uriz, M. J. (1992). — Discorhabdella hindei n.sp. (Porifera, Demospongiae), a new Tethyan relict of pre-Messinian biota? J. Nat. History 26, 1–7. doi: 10.1080/00222939200770011
Boury-Esnault, N., Pansini, M., and Uriz, M. J. (1994). Spongiaires bathyaux de la mer d’Alboran et du golfe ibéro-marocain. Mém. Mus. Natl. Hist. Nat. 160, 1–174.
Boury-Esnault, N., Vacelet, J., Dubois, M., Goujard, A., Fourt, M., Perez, T., et al. (2017). New hexactinellid sponges from deep Mediterranean canyons. Zootaxa 4236, 118–134. doi: 10.11646/zootaxa.4236.1.6
Boury-Esnault, N., Vacelet, J., Reiswig, H. M., Fourt, M., Aguilar, R., and Chevaldonné, P. (2015). Mediterranean hexactinellid sponges, with the description of a new Sympagella species (Porifera, Hexactinellida). J. Mar. Biolog. Assoc. U K. 95, 1353–1364. doi: 10.1017/s0025315414001891
Buhl-Mortensen, L., Vanreusel, A., Gooday, A. J., Levin, L. A., Priede, I. G., Buhl-Mortensen, P., et al. (2010). Biological structures as a source of habitat heterogeneity and biodiversity on the deep ocean margins. Mar. Ecol. 31, 21–50. doi: 10.1111/j.1439-0485.2010.00359.x
Buhl-Mortensen, P., Buhl-Mortensen, L., and Purser, A. (2016). ““Trophic Ecology and Habitat Provision in Cold-Water Coral Ecosystems”,” in Marine Animal Forests, The Ecology of Benthic Biodiversity Hotspots, eds S. Rossi, L. Bramanti, A. Gori, and C. Orejas (Switzerland: Springer), 919–944. doi: 10.1007/978-3-319-21012-4_20
Calcinai, B., Moratti, V., Martinelli, M., Bavestrello, G., and Taviani, M. (2013). Uncommon sponges associated with deep coral bank and maërl habitats in the Strait of Sicily (Mediterranean Sea). Ita. J. Zool. 80, 412–423. doi: 10.1080/11250003.2013.786763
Carballo, J. L., Naranjo, S., and Garcia-Gómez, J. C. (1997). Where does the Mediterranean Sea begin? Zoogeographical affinities of the littoral sponges of the Straits of Gibraltar. J. Biogeogr. 24, 223–232. doi: 10.1046/j.1365-2699.1997.00082.x
Cárdenas, P., Pérez, T., and Boury-Esnault, N. (2012). Sponge systematics facing new challenges. Adv. Mar. Biol. 61, 79–209.
Cardone, F., Pansini, M., Corriero, G., and Bertolino, M. (2019). Two new species of deep-sea sponges (Porifera, Demospongiae) from submarine canyons of the Sardinian continental margin (western Mediterranean Sea). Zootaxa 4688, 407–419. doi: 10.11646/zootaxa.4688.3.7
Chimienti, G., Bo, M., Taviani, M., and Mastrototaro, F. (2019). “Occurrence and Biogeography of Mediterranean Cold-Water Corals,” in Mediterranean Cold-Water Corals: Past, Present and Future, eds C. Orejas and C. Jiménez (Berlin: Springer International Publishing AG).
Chombard, C., and Boury-Esnault, N. (1999). “Good congruence between morphology and molecular phylogeny of Hadromerida, or how to bother sponge taxonomists,” in Proceedings of the 5th International Sponge Symposium 1998, Vol. 44, ed. J. N. A. Hooper (Brisbane, OLD: Memoirs of the Queensland Museum), 100.
Corbera, G., Lo Iacono, C., Gràcia, E., Grinyó, J., Pierdomenico, M., Huvenne, V. A., et al. (2019). Ecological characterisation of a Mediterranean cold-water coral reef: Cabliers Coral Mound Province (Alboran Sea, western Mediterranean). Prog. Oceanog. 175, 245–262. doi: 10.1016/j.pocean.2019.04.010
Costa, G., Bo, M., Grinyó, J., Lo Iacono, C., Pansini, M., and Bertolino, M. (2018). “A first preliminary study of the sponge fauna associated to white coral banks from the Alboran Sea,” in 4th International Workshop on Taxonomy of Atlanto-Mediterranean Deep-Sea and Cave Sponges 2018, eds C. T. Pérez, N. Boury-Esnault, P. Cárdenas, P. Chevaldonné, A. Ereskovsky, C. Ruiz, et al. (IWTAM: Marseille), 32.
Cristobo, F. J., Urgorri, V., Solórzano, M. R., and Ríos, P. (1993). “Métodos de recogida, estudio y conservación de las colecciones de poríferos,” in Simposio Internacional sobre Preservación y Conservación de Colecciones de Historia Natural (Comunicaciones Sobre la Situación, Preservación y Conservación de Colecciones de Historia Natural), Vol. 2, eds B. Thomas, F. Palacios, and M. C. Martínez-López 277–287.
de la Torriente, A., Aguilar, R., González-Irusta, J. M., Blanco, M., and Serrano, A. (2020). Habitat forming species explain taxonomic and functional diversities in a Mediterranean seamount. Ecol. Indic. 118:106747. doi: 10.1016/j.ecolind.2020.106747
De Leo, F., Puig, P., Lo Iacono, C., Durán, R., Grinyó, J., Ambroso, S., et al. (2019). “First observations of Living Cold Water Corals surrounded by fishing grounds in Blanes Canyon (NW Mediterranean),” in 7th Internation Symposium on Deep-Sea Corals, July 29 – August 2, 2019, Cartagena, Colombia (Colombia: ISDSC).
Dendy, A. (1922). “Report on the sigmatotetraxonida collected by H.M.S.‘Sealark’ in the Indian Ocean,” in Reports of the Percy Sladen Trust Expedition to the Indian Ocean in 1905, Vol. 7, (London: Transactions of the Linnean Society of London), 1–18.
Dinn, C. (2020). A new species of Haliclona (Flagellia) Van Soest, 2017 (Porifera, Demospongiae, Heteroscleromorpha) from the Gulf of St. Lawrence, Canada. Zootaxa 4778, 3910–3395.
D’Onghia, G., Capezzuto, F., Cardone, F., Carlucci, R., Carluccio, A., and Chimienti, et al. (2015). Macro- and megafauna recorded in the submarine Bari Canyon (southern Adriatic, Mediterranean Sea) using different tools. Med. Mar. Sci. 16, 180–196. doi: 10.12681/mms.1082
Edwards, K. F., and Stachowicz, J. J. (2011). Spatially stochastic settlement and the coexistence of benthic marine animals. Ecology 92, 1094–1103. doi: 10.1890/10-1332.1
Emig, C. C., and Geistdoerfer, P. (2004). The Mediterranean deep-sea fauna: historical evolution, bathymetric variations and geographical changes. Carnets Géol. 2004:3230.
Evans, J., Knittweis, L., Borg, J. A., and Schembri, P. J. (2019). “Cold-Water Corals in the Mediterranean: A History of Discovery,” in Mediterranean Cold-Water Corals: Past, Present and Future, eds C. Orejas and C. Jiménez (Berlin: Springer International Publishing AG).
Fabri, M. C., Bargain, A., Pairaud, I., Pedel, L., and Taupier-Letage, I. (2017). Cold-water coral ecosystems in Cassidaigne Canyon: an assessment of their environmental living conditions. Deep Sea Res. Part II Top. Stud. Oceanogr. 137, 436–453. doi: 10.1016/j.dsr2.2016.06.006
Fabri, M. C., Pedel, L., Beuck, L., Galgani, F., Hebbeln, D., and Freiwald, A. (2014). Megafauna of vulnerable marine ecosystems in French Mediterranean submarine canyons: Spatial distribution and anthropogenic impacts. Deep Sea Res. Part II Top. Stud. Oceanogr. 104, 184–207. doi: 10.1016/j.dsr2.2013.06.016
Fabri, M. C., Vinha, B., Allais, A. G., Bouhier, M. E., Dugornay, O., Gaillot, A., et al. (2019). Evaluating the ecological status of cold-water coral habitats using non-invasive methods: An example from Cassidaigne canyon, northwestern Mediterranean Sea. Prog. Oceanog. 178:102172. doi: 10.1016/j.pocean.2019.102172
FAO (2008). Report of the Technical Consultation on International Guidelines for the Management of Deep-sea Fisheries in the High Seas. Rome, 4–8 February and 25–29 August 2008. FAO Fisheries and Aquaculture Report, No. 881. Rome: FAO, 1–42.
Ferrer Hernández, F. (1923). Más datos para el conocimiento de las esponjas de las costas españolas. Boletín Pescas 7, 247–272.
Fourt, M., Goujard, A., Pérez, T., and Chevaldonné, P. (2017). Guide de la faune profonde de la mer Méditerranée. Explorations des roches et des canyons sous-marins des côtes françaises. Patrimoines naturels. Publicat. Sci. Museum Natl. d’Histoire Nat. Paris 75, 1–184.
Gabrie, C., Lagabrielle, E., Crochelet, E., Meola, B., Webster, C., Claudet, J., et al. (2012). The status of marine protected areas in the Mediterranean Sea. MedPAN and RAC/SPA. Gland: IUCN, 256.
Gage, J. D. (2004). Diversity in deep-sea benthic macrofauna: the importance of local ecology, the larger scale, history and the Antarctic. Deep Sea Res. Part II Top. Stud. Oceanogr. 51, 1689–1708. doi: 10.1016/j.dsr2.2004.07.013
Gary, S. F., Fox, A. D., Biastoch, A., Roberts, J. M., and Cunningham, S. A. (2020). Larval behaviour, dispersal and population connectivity in the deep sea. Scient. Rep. 10, 1–12.
General Fisheries Commission for the Mediterranean (GFCM, S.A.C.) (2008). Criteria for the identification of sensitive habitats of relevance for the management of priority species, General Fisheries Commission for the Mediterranean- Scientific Advisory Committee –Sub –Committee on Marine Environment and Ecosystems (SCMEE), Eleventh Season, Marrakech, Morocco, 1–5 December 2008. Rome: General Fisheries Commission for the Mediterranean.
Gerovasileiou, V., and Voultsiadou, E. (2012). Marine Caves of the Mediterranean Sea: A Sponge Biodiversity Reservoir within a Biodiversity Hotspot. PLoS One 7:e39873. doi: 10.1371/journal.pone.0039873
Gili, J. M., Bouillon, J., Pagès, F., Palanques, A., Puig, P., and Heussner, S. (1998). Origin and biogeography of the deep-water Mediterranean Hydromedusae including the description of two new species collected in submarine canyons of Northwestern Mediterranean. Sci. Mar. 62, 113–134.
Göcke, C., Hestetun, J. T., Uhlir, C., Freiwald, A., Beuck, L., et al. (2016). Cladorhiza corallophila sp. nov., a new carnivorous sponge (Cladorhizidae, Demospongiae) living in close association with Lophelia pertusa and Madrepora oculata (Scleractinia). Zootaxa 4168, 512–524. doi: 10.11646/zootaxa.4168.3.4
Goodwin, C. E., Picton, B. E., and van Soest, R. W. M. (2011). Hymedesmia (Porifera: Demospongiae: Poecilosclerida) from Irish and Scottish cold-water coral reefs, with a description of five new species J. Mar. Biolog. Assoc. U K. 91, 979–997. doi: 10.1017/s0025315411000014
Hajdu, E. (2002). “Family Hamacanthidae Gray, 1872,” in Systema Porifera. A guide to the classification of sponges, Vol. 1, eds J. N. A. Hooper and R. W. M. van Soest (New York, NY: Kluwer Academic), 665–668. doi: 10.1007/978-1-4615-0747-5_72
Hajdu, E., and Castello-Branco, C. (2014). Hamacantha (Hamacantha) boomerang sp. nov. from deep-sea coral mounds at Campos Basin, SW Atlantic, and redescription of H. (H.) schmidtii (Carter, 1882) (Hamacanthidae, Poecilosclerida, Demospongiae). Zootaxa 3753, 384–390. doi: 10.11646/zootaxa.3753.4.7
Hajdu, E., Hooker, Y., and Willenz, P. (2015). New Hamacantha from Peru and resurrection of Zygherpe as subgenus (Demospongiae, Poecilosclerida, Hamacanthidae). Zootaxa 3926, 87–99. doi: 10.11646/zootaxa.3926.1.3
Hayes, D. R., Schroeder, K., Poulain, P.-M., Testor, P., Mortier, L., Bosse, A., et al. (2019). “Review of the Circulation and Characteristics of Intermediate Water Masses of the Mediterranean: Implications for Cold-Water Coral Habitats,” in Mediterranean Cold-Water Corals: Past, Present and Future, eds C. Orejas and C. Jiménez (Berlin: Springer International Publishing AG).
Henry, L. A., and Roberts, J. M. (2016). “Global biodiversity in cold-water coral reef ecosystems,” in Marine Animal Forests, The Ecology of Benthic Biodiversity Hotspots, eds S. Rossi, L. Bramanti, A. Gori, and C. Orejas (Berlin: Springer), 235–256. doi: 10.1007/978-3-319-21012-4_6
Henry, L. A., Navas, J. M., Hennige, S. J., Wicks, L. C., Vad, J., and Roberts, J. M. (2013). Cold-water coral reef habitats benefit recreationally valuable sharks. Biol. Cons. 161, 67–70. doi: 10.1016/j.biocon.2013.03.002
Henry, L.-A., and Roberts, J. M. (2007). Biodiversity and ecological composition of macrobenthos on cold-water coral mounds and adjacent off-mound habitat in the bathyal Porcupine Seabight, NE Atlantic. Deep Sea Res. Part I Oceanogr. Res. Pap. 54, 654–672. doi: 10.1016/j.dsr.2007.01.005
Heres, P., Cristobo, J., and Ríos, P. (2014). “Acanthancora schmidti (Topsent, 1898) (Porifera, Poecilosclerida), new record in the Cantabrian Sea,” in XVIII Simposio Ibérico de Estudios de Biología Marina, eds P. Ríos, L. A. Suárez, and J. Cristobo (Gijón: Centro Oceanográfico de Gijón).
Hestetun, J. T., Tompkins-Macdonald, G., and Rapp, H. T. (2017). A review of carnivorous sponges (Porifera: Cladorhizidae) from the Boreal North Atlantic and Arctic. Zool. J. Linn. Soc. 181, 1–69. doi: 10.1093/zoolinnean/zlw022
Hsü, K. J., Ryan, W. B. F., and Cita, M. B. (1973). Late Miocene desiccation of the Mediterranean. Nature 242, 240–244. doi: 10.1038/242240a0
Hughes, T. P., Bellwood, D. R., and Connolly, S. R. (2002). Biodiversity hotspots, centres of endemicity, and the conservation of coral reefs. Ecol. Lett. 5, 775–784. doi: 10.1046/j.1461-0248.2002.00383.x
Ise, Y., Woo, S. P., Tan, S. H., and Fujita, T. (2019). First record of Hamacantha (Porifera, Demospongiae, Merliida, Hamacanthidae) from Japan, with description of two new species. Zootaxa 4657, 474–482. doi: 10.11646/zootaxa.4657.3.3
Jensen, A., and Frederiksen, R. (1992). The fauna associated with the bank-forming deepwater coral Lophelia pertusa (Scleractinaria) on the Faroe shelf. Sarsia 77, 53–69. doi: 10.1080/00364827.1992.10413492
Jones, C. G., Lawton, J. H., and Shachak, M. (1994). “Organisms as ecosystem engineers,” in Ecosystem management, eds F. B. Samson and F. L. Knopf (New York, NY: Springer), 130–147. doi: 10.1007/978-1-4612-4018-1_14
Jones, G. P., Almany, G. R., Russ, G. R., Sale, P. F., Steneck, R. S., Van Oppen, M. J. H., et al. (2009). Larval retention and connectivity among populations of corals and reef fishes: history, advances and challenges. Coral Reefs 28, 307–325. doi: 10.1007/s00338-009-0469-9
Kassambara, A. (2020). ggpubr: ‘ggplot2’ Based Publication Ready Plots. R package version 0.4.0. Berlin: R Core Team.
Kazanidis, G., and Witte, U. F. (2016). The trophic structure of Spongosorites coralliophaga-coral rubble communities at two northeast Atlantic cold water coral reefs. Mar. Biol. Res. 12, 932–947. doi: 10.1080/17451000.2016.1216569
Kazanidis, G., Henry, L. A., Roberts, J. M., and Witte, U. F. (2016). Biodiversity of Spongosorites coralliophaga (Stephens, 1915) on coral rubble at two contrasting cold-water coral reef settings. Coral Reefs 35, 193–208. doi: 10.1007/s00338-015-1355-2
Kazanidis, G., van Oevelen, D., Veuger, B., and Witte, U. F. (2018). Unravelling the versatile feeding and metabolic strategies of the cold-water ecosystem engineer Spongosorites coralliophaga (Stephens, 1915). Deep Sea Res. Part I Oceanogr. Res. Pap. 141, 71–82. doi: 10.1016/j.dsr.2018.07.009
Krijgsman, W., Capella, W., Simon, D., Hilgen, F. J., Kouwenhoven, T. J., Meijer, P. T., et al. (2018). The Gibraltar corridor: Watergate of the Messinian salinity crisis. Mar. Geol. 403, 238–246. doi: 10.1016/j.margeo.2018.06.008
Lastras, G., Canals, M., Ballesteros, E., Gili, J. M., and Sanchez-Vidal, A. (2016). Cold-water corals and anthropogenic impacts in La Fonera submarine canyon head, Northwestern Mediterranean Sea. PLoS One 11:e0155729. doi: 10.1371/journal.pone.0155729
Laubenfels, M. W. (1932). The marine and fresh-water sponges of California. Proc. U S. Natl. Mus. 81, 1–140. doi: 10.5479/si.00963801.81-2927.1
Lesser, M. P. (2006). Benthic–pelagic coupling on coral reefs: feeding and growth of Caribbean sponges. J. Exp. Mar. Biol. Ecol. 328, 277–288. doi: 10.1016/j.jembe.2005.07.010
Linley, T. D., Lavaleye, M., Maiorano, P., Bergman, M., Capezzuto, F., Cousins, N. J., et al. (2017). Effects of cold-water corals on fish diversity and density (European continental margin: Arctic, NE Atlantic and Mediterranean Sea): Data from three baited lander systems. Deep Sea Res. Part II Top. Stud. Oceanogr. 145, 8–21. doi: 10.1016/j.dsr2.2015.12.003
Lo Iacono, C. (2012). MELCOR (MELillaCORals). Geomorphologic, Ecologic and Environmental Study of CWC Mounds in the Mediterranean Moroccan Margin. Cruise Report. United Kingdom: National Oceanography Centre.
Lo Iacono, C., Gràcia, E., Ranero, C. R., Emelianov, M., Huvenne, V. A., Bartolomé, R., et al. (2014). The West Melilla cold water coral mounds, Eastern Alboran Sea: Morphological characterization and environmental context. Deep Sea Res. Part II Top. Stud. Oceanogr. 99, 316–326. doi: 10.1016/j.dsr2.2013.07.006
Longo, C., Mastrototaro, F., and Corriero, G. (2005). Sponge fauna associated with a Mediterranean deep-sea coral bank. J. Mar. Biolog. Assoc. U K. 85, 1341–1352. doi: 10.1017/s0025315405012518
Lundbeck, W. (1902). Porifera. (Part I.) Homorrhaphidae and Heterorrhaphidae. Danish Ingolf-Expedit. 6, 1–108.
Maldonado, M. (1992). Demosponges of the red coral bottoms from the Alboran Sea. J. Nat. Hist. 26, 1131–1161. doi: 10.1080/00222939200770661
Maldonado, M. (1993). Demosponjas litorales de Alborán: faunística y biogeografía. Ph. D Thesis. Barcelona: Universitat de Barcelona, 496.
Maldonado, M., and Uriz, M. J. (1995). Biotic affinities in a transitional zone between the Atlantic and the Mediterranean: a biogeographical approach based on sponges. J. Biogeog. 22, 89–110. doi: 10.2307/2846075
Maldonado, M., and Young, C. M. (1996). Bathymetric patterns of sponge distribution on the Bahamian slope. Deep Sea Res. Part I Oceanogr. Res. Pap. 43, 897–915. doi: 10.1016/0967-0637(96)00042-8
Maldonado, M., López-Acosta, M., Beazley, L., Kenchington, E., Koutsouveli, V., and Riesgo, A. (2020). Cooperation between passive and active silicon transporters clarifies the ecophysiology and evolution of biosilicification in sponges. Sci. Adv. 6:eaba9322. doi: 10.1126/sciadv.aba9322
Margreth, S., Gennari, G., Rüggeberg, A., Comas, M. C., Pinheiro, L. M., and Spezzaferri, S. (2011). Growth and demise of cold-water coral ecosystems on mud volcanoes in the West Alboran Sea: The messages from the planktonic and benthic foraminifera. Mar. Geol. 282, 26–39. doi: 10.1016/j.margeo.2011.02.006
Mariani, S., Uriz, M. J., Turon, X., and Alcoverro, T. (2006). Dispersal strategies in sponge larvae: integrating the life history of larvae and the hydrologic component. Oecologia 149, 174–184. doi: 10.1007/s00442-006-0429-9
Mastrototaro, F., d’Onghia, G., Corriero, G., Matarrese, A., Maiorano, P., Panetta, P., et al. (2010). Biodiversity of the white coral bank off Cape Santa Maria di Leuca (Mediterranean Sea): An update. Deep Sea Res. Part II Top. Stud. Oceanogr. 57, 412–430. doi: 10.1016/j.dsr2.2009.08.021
Menna, M., and Poulain, P. M. (2010). Mediterranean intermediate circulation estimated from Argo data in 2003–2010. Ocean Sci. 6, 331–343. doi: 10.5194/os-6-331-2010
Messmer, V., Jones, G. P., Munday, P. L., Holbrook, S. J., Schmitt, R. J., and Brooks, A. J. (2011). Habitat biodiversity as a determinant of fish community structure on coral reefs. Ecology 92, 2285–2298. doi: 10.1890/11-0037.1
Millot, C. (1990). The gulf of Lions’ hydrodynamics. Cont. Shelf Res. 10, 885–894. doi: 10.1016/0278-4343(90)90065-t
Moccia, D., Cau, A., Alvito, A., Canese, S., Cannas, R., Bo, M., et al. (2018). New sites expanding the “Sardinian cold-water coral province” extension: A new potential cold-water coral network? Aquat. Conserv. 29, 153–160. doi: 10.1002/aqc.2975
Nasto, I., Cardone, F., Mastrototaro, F., Panetta, P., Rosso, A., Sanfilippo, R., et al. (2018). Benthic invertebrates associated with subfossil cold-water coral frames and hardgrounds in the Albanian deep waters (Adriatic Sea). Turk. Zool. Derg. 42, 360–371. doi: 10.3906/zoo-1708-44
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., et al. (2019). vegan: Community Ecology Package. R package version 2.5-6. Vienna: R Core Team.
Orejas, C., and Jiménez, C. (2019). “An Introduction to the Research on Mediterranean Cold-Water Corals,” in Mediterranean Cold-Water Corals: Past, Present and Future, eds C. Orejas and C. Jiménez (Berlin: Springer International Publishing AG).
Orejas, C., Gori, A., Iacono, C. L., Puig, P., Gili, J. M., and Dale, M. R. (2009). Cold-water corals in the Cap de Creus canyon, northwestern Mediterranean: spatial distribution, density and anthropogenic impact. Mar. Ecol. Prog. Ser. 397, 37–51. doi: 10.3354/meps08314
OSPAR (2009). Background Document for Lophelia pertusa reefs. Luxembourg: OSPAR commission Publication.
Padua, A., Cunha, H. A., and Klautau, M. (2018). Gene flow and differentiation in a native calcareous sponge (Porifera) with unknown dispersal phase. Mar. Biod. 48, 2125–2135. doi: 10.1007/s12526-017-0742-z
Palanques, A., and Puig, P. (2018). Particle fluxes induced by benthic storms during the 2012 dense shelf water cascading and open sea convection period in the northwestern Mediterranean basin. Mar. Geol. 406, 119–131.
Palomino, D., Vázquez, J. T., Ercilla, G., Alonso, B., López-González, N., and Díaz-del-Río, V. (2011). Interaction between seabed morphology and water masses around the seamounts on the Motril Marginal Plateau (Alboran Sea, Western Mediterranean). Geo-Mar. Lett. 31, 465–479. doi: 10.1007/s00367-011-0246-y
Pansini, M. (1987). “Littoral demosponges from the banks of the Strait of Sicily and the Alboran Sea.”, in Taxonomy of Porifera from the N.E. Atlantic and Mediterranean Sea eds J., Vacelet, and N. Boury-Esnault, (Springer-Verlag: Berlin, Heidelberg). NATO ASI Ser. G 13, 149–185. doi: 10.1007/978-3-642-70892-3_8
Pardo, E., Rubio, R. A., García, S., and Ubero, J. (2011). Documentación de arrecifes de corales de agua fría en el Mediterráneo occidental (Mar de Alborán). Chronica Nat. 2011, 20–34.
Picton, B. E., and Goodwin, C. E. (2007). Sponge biodiversity of Rathlin Island, Northern Ireland. J. Mar. Biolog. Assoc. U K. 87, 1441–1458. doi: 10.1017/s0025315407058122
Pisera, A., Łukowiak, M., Fromont, J., and Schuster, A. (2018). First record of the genus Vetulina Schmidt, 1879 (Porifera: Demospongiae: Sphaerocladina) from the Indian Ocean with the description of two new species: biogeographic and evolutionary significance. Mar. Biod. 48, 1529–1539. doi: 10.1007/s12526-017-0658-7
Plaisance, L., Knowlton, N., Paulay, G., and Meyer, C. (2009). Reef-associated crustacean fauna: biodiversity estimates using semi-quantitative sampling and DNA barcoding. Coral Reefs 28, 977–986. doi: 10.1007/s00338-009-0543-3
Por, F. D. (1989). “Postface: The Legacy of Tethys—Or the Guise of a Conclusion,” in The Legacy of Tethys, ed. F. D. Por (Dordrecht: Springer), 165–168. doi: 10.1007/978-94-009-0937-3_7
Puig, P., and Gili, J. M. (2019). “Submarine Canyons in the Mediterranean: A Shelter for Cold-Water Corals,” in Mediterranean Cold-Water Corals: Past, Present and Future, eds C. Orejas and C. Jiménez (Berlin: Springer).
Puig, P., Lo Iacono, C., Durán, R., Grinyó, J., Ambroso, S., and De Leo, et al. (2019). “Living Cold Water Corals surrounded by fishing grounds in Blanes Canyon (NW Mediterranean),” in GeoHab 2019 Annual Conference (St. Petersburg: GeoHab).
Pulitzer-Finali, G. (1983). A collection of Mediterranean Demospongiae (Porifera) with, in appendix, a list of the Demospongiae hitherto recorded from the Mediterranean Sea. Ann. Mus. Civ. Stor. Nat. Giacomo Doria 84, 445–621.
Redmond, N. E., Morrow, C. C., Thacker, R. W., Diaz, M. C., Boury-Esnault, N., Cárdenas, P., et al. (2013). Phylogeny and systematics of Demospongiae in light of new small-subunit ribosomal DNA (18S) sequences. Integr. Comp. Biol. 53, 388–415. doi: 10.1093/icb/ict078
Reveillaud, J., Remerie, T., van Soest, R., Erpenbeck, D., Cárdenas, P., Derycke, S., et al. (2010). Species boundaries and phylogenetic relationships between Atlanto-Mediterranean shallow-water and deep-sea coral associated Hexadella species (Porifera, Ianthellidae). Mol. Phylogenet. Evol. 56, 104–114. doi: 10.1016/j.ympev.2010.03.034
Reveillaud, J., van Soest, R., Derycke, S., Picton, B., Rigaux, A., and Vanreusel, A. (2011). Phylogenetic relationships among NE Atlantic Plocamionida Topsent (1927) (Porifera, Poecilosclerida): under-estimated diversity in reef ecosystems. PLoS One 6:e16533. doi: 10.1371/journal.pone.0016533
Ríos, P., Riesgo, A., Taboada, S., and Cristobo, J. (2020). A new species of Isodictya (Porifera: Poecilosclerida) from the Southern Ocean. Polar Biol. 43, 523–533. doi: 10.1007/s00300-020-02654-x
Rix, L., De Goeij, J. M., Mueller, C. E., Struck, U., Middelburg, J. J., Van Duyl, F. C., et al. (2016). Coral mucus fuels the sponge loop in warm-and cold-water coral reef ecosystems. Sci. Rep. 6, 1–11. doi: 10.1007/978-3-319-17001-5_6-1
Rix, L., de Goeij, J. M., van Oevelen, D., Struck, U., Al-Horani, F. A., Wild, C., et al. (2017). Differential recycling of coral and algal dissolved organic matter via the sponge loop. Funct. Ecol. 31, 778–789. doi: 10.1111/1365-2435.12758
Roberts, C. M., McClean, C. J., Veron, J. E., Hawkins, J. P., Allen, G. R., McAllister, D. E., et al. (2002). Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 295, 1280–1284. doi: 10.1126/science.1067728
Roberts, E. M., Bowers, D. G., Meyer, H. K., Samuelsen, A., Rapp, H. T., and Cárdenas, P. (2021). Water masses constrain the distribution of deep-sea sponges in the North Atlantic Ocean and Nordic Seas. Mar. Ecol. Prog. Ser. 659, 75–96. doi: 10.3354/meps13570
Roberts, J. M., Davies, A. J., Henry, L. A., Dodds, L. A., Duineveld, G. C. A., Lavaleye, M. S. S., et al. (2009b). Mingulay reef complex: an interdisciplinary study of cold-water coral habitat, hydrography and biodiversity. Mar. Ecol. Prog. Ser. 397, 139–151. doi: 10.3354/meps08112
Roberts, J. M., Wheeler, A. J., and Freiwald, A. (2006). Reefs of the deep: the biology and geology of cold-water coral ecosystems. Science 312, 543–547. doi: 10.1126/science.1119861
Roberts, J., Wheeler, A. J., Freiwald, A., and Cairns, S. (2009a). Cold-Water Corals. The Biology and Geology of Deep-Sea Coral Habitats. Cambridge: Cambridge University Press.
Robertson, L. M., Hamel, J. F., and Mercier, A. (2017). Feeding in deep-sea demosponges: Influence of abiotic and biotic factors. Deep Sea Res. Part I Oceanogr. Res. Pap. 127, 49–56. doi: 10.1016/j.dsr.2017.07.006
Robinson, A. R., Leslie, W. G., Theocharis, A., and Lascaratos, A. (2001). “Mediterranean sea circulation,” in 1st edition of the Encyclopedia of Ocean Sciences, eds J. H. Steele, S. A. Thorpe, and K. K. Turekian (Amsterdam: Elsevier Ltd).
Rogers, A. D. (1999). The Biology of Lophelia pertusa (Linnaeus 1758) and Other Deep-Water Reef-Forming Corals and Impacts from Human Activities. Int. Rev. Hydrobiol. 84, 315–406. doi: 10.1002/iroh.199900032
Rosell, D., and Uriz, M. J. (2002). Excavating and endolithic sponge species (Porifera) from the Mediterranean: species descriptions and identification key. Organisms Divers. Evolut. 2, 55–86. doi: 10.1078/1439-6092-00033
Rueda, J. L., Urra, J., Aguilar, R., Angeletti, L., Bo, M., García-Ruiz, C., et al. (2019). “Cold-Water Coral Associated Fauna in the Mediterranean Sea and Adjacent Areas,” in Mediterranean Cold-Water Corals: Past, Present and Future, eds C. Orejas and C. Jiménez (Berlin: Springer).
Santín, A., Grinyó, J., Ambroso, S., Uriz, M. J., Dominguez-Carrió, C., et al. (2019). Distribution patterns and demographic trends of demosponges at the Menorca Channel (Northwestern Mediterranean Sea). Prog. Oceanog. 173, 9–25. doi: 10.1016/j.pocean.2019.02.002
Santín, A., Grinyó, J., Ambroso, S., Uriz, M. J., Gori, A., Dominguez-Carrió, C., et al. (2018a). Sponge assemblages on the deep Mediterranean continental shelf and slope (Menorca Channel, Western Mediterranean Sea). Deep Sea Res. Part I Oceanogr. Res. Pap. 131, 75–86. doi: 10.1016/j.dsr.2017.11.003
Santín, A., Grinyó, J., Bilan, M., Ambroso, S., and Puig, P. (2020a). First report of the carnivorous sponge Lycopodina hypogea (Cladorhizidae) associated with marine debris, and its possible implications on deep-sea connectivity. Mar. Poll. Bull. 159:111501. doi: 10.1016/j.marpolbul.2020.111501
Santín, A., Grinyó, J., Uriz, M. J., Gili, J. M., and Puig, P. (2020b). First deep-sea Hamigera (Demospongiae: Porifera) species associated with Cold-Water Corals (CWC) on antipodal latitudes of the world. Deep Sea Res. Part I Oceanogr. Res. Pap. 164:103325. doi: 10.1016/j.dsr.2020.103325
Santín, A., Uriz, M. J., and Puig, P. (2018b). “Cryptic and rare sponges associated with Cold-Water Corals of the Blanes Canyon (northwestern Mediterranean Sea),” in 4th International Workshop on Taxonomy of Atlanto-Mediterranean Deep-Sea and Cave Sponges 2018, eds C. T. Pérez, N. Boury-Esnault, P. Cárdenas, P. Chevaldonné, A. Ereskovsky, C. Ruiz, et al. (Marseille: MBE).
Sarà, M. (1958). Contributo all consoscenza dei Poriferi del Mar Ligure. Ann. Mus. Civ. Stor. Nat. Giacomo Doria 70, 207–244.
Shanks, A. L., Largier, J., Brink, L., Brubaker, J., and Hooff, R. (2002). Observations on the distribution of meroplankton during a downwelling event and associated intrusion of the Chesapeake Bay estuarine plume. J. Plankton Res. 24, 391–416. doi: 10.1093/plankt/24.4.391
Sitjà, C. (2020). The bathyal connections between the Mediterranean Sea and the Northeastern Atlantic Ocean: an assessment using deep-water sponges as a case study. Ph. D. thesis. Barcelona: Universitat de Barcelona, 360.
Sitjà, C., and Maldonado, M. (2014). New and rare sponges from the deep shelf of the Alboran Island (Alboran Sea, Western Mediterranean). Zootaxa 3760, 141–179. doi: 10.11646/zootaxa.3760.2.2
Sitjà, C., Maldonado, M., Farias, C., and Rueda, J. L. (2020). Export of bathyal benthos to the Atlantic through the Mediterranean outflow: Sponges from the mud volcanoes of the Gulf of Cadiz as a case study. Deep Sea Res. Part I Oceanogr. Res. Pap. 163:103326. doi: 10.1016/j.dsr.2020.103326
Stephens, J. (1915). Sponges of the Coasts of Ireland. I.- The Triaxonia and part of the Tetraxonida. Fisher. Ireland Sci. Investigat. 1914, 1–43.
Stephens, J. (1921). Sponges of the Coasts of Ireland. II. The Tetraxonida (concluded). Scientific Investigations of the Fisheries Branch. Depart. Agricult. Ireland 1920, 1–75.
Taviani, M., Angeletti, L., Antolini, B., Ceregato, A., Froglia, C., Lopez Correa, M., et al. (2011). “Geo-biology of Mediterranean deep-water coral ecosystems,” in Marine research at CNR. Dipartamento Terra e Ambiente, eds E. Brugnoli, G. Cavaretta, S. Mazzola, F. Trincardi, M. Ravaioli, and R. Santoleri (Roma: Consiglio Nazionale delle Ricerche), 705–720.
Taviani, M., Angeletti, L., Canese, S., Cannas, R., Cardone, F., Cau, A., et al. (2017). The “Sardinian cold-water coral province” in the context of the Mediterranean coral ecosystems. Deep Sea Res. Part II Top. Stud. Oceanogr. 145, 61–78. doi: 10.1016/j.dsr2.2015.12.008
Teixidó, N., Gili, J. M., Uriz, M. J., Gutt, J., and Arntz, W. E. (2006). Observations of asexual reproductive strategies in Antarctic hexactinellid sponges from ROV video records. Deep Sea Res. Pt. II Top. Stud. Oceanogr. 53, 972–984.
Topsent, E. (1904). Spongiaires des Açores. Résultats des campagnes scientifiques accomplies par le Prince Albert I. Monaco 25, 1–280.
Topsent, E. (1928). Spongiaires de l’Atlantique et de la Méditerranée provenant des croisières du Prince Albert ler de Monaco. Résultats des campagnes scientifiques accomplies par le Prince Albert I. Monaco 74, 1–376.
United Nations General Assembly (2007). Resolution 61/105, Sustainable fisheries, including through the 1995 Agreement for the Implementation of the Provisions of the United Nations Convention on the Law of the Sea of 10 December 1982 relating to the Conservation and Management of Straddling Fish Stocks and Highly Migratory Fish Stocks, and related instruments, A/RES/61/105. New York, NY: United Nations General Assembly.
Uriz, M. J. (1983). Monografía I. Contribución a la fauna de esponjas (Demospongia) de Cataluña. Anal. Sección Ciencias Colegio Univers. Gerona 7, 1–220.
Uriz, M. J., Garate, L., and Agell, G. (2017). Molecular phylogenies confirm the presence of two cryptic Hemimycale species in the Mediterranean and reveal the polyphyly of the genera Crella and Hemimycale (Demospongiae: Poecilosclerida). PeerJ 5:e2958.
Uriz, M. J., and Rosell, D. (1990). Sponges from bathyal depths (1000–1750 m) in the Western Mediterranean Sea. J. Nat. Hist. 24, 373–391. doi: 10.1080/00222939000770281
Vacelet, J. (1969). Eponges de la Roche du Large et de l’étage bathyal de Méditerranée (Récoltes de la soucoupe plongeante Cousteau et dragages). Mém. Mus. Natl. Hist. Nat. A Zool. 59, 145–219.
Vacelet, J. (1979). “Descriptions et affinités d’une éponge sphinctozoaire actuelle,” in Biologie des Spongiaires. Sponge Biology, Vol. 291, eds C. Lévi and N. Boury-Esnault (Paris: Colloques Internationaux du Centre National de la Recherché Scientifique), 483–493.
Vacelet, J., Boury-Esnault, N., and Harmelin, J. G. (1994). Hexactinellid cave, a unique deep-sea habitat in the scuba zone. Deep Sea Res. Part I Oceanogr. Res. Pap. 41, 965–973. doi: 10.1016/0967-0637(94)90013-2
van Soest, R. W. (2009). New sciophilous sponges from the Caribbean (Porifera: Demospongiae). Zootaxa 2107, 1–40. doi: 10.11646/zootaxa.2107.1.1
van Soest, R. W. M. (2002). “Family Hymedesmiidae,” in Systema Porifera. A guide to the classification of sponges, eds J. N. A. Hooper and R. W. M. van Soest (New York, NY: Kluwer Academic).
van Soest, R. W. M. (2017). Flagellia, a new subgenus of Haliclona (Porifera, Haplosclerida). Eur. J. Taxon. 351, 1–48. doi: 10.1007/978-3-319-23534-9_1
van Soest, R. W. M., and Beglinger, E. J. (2009). New bioeroding sponges from Mingulay coldwater reefs, north-west Scotland. J. Mar. Biolog. Assoc. U K. 89, 329–335. doi: 10.1017/s0025315408002725
van Soest, R. W. M., and De Voogd, N. J. (2015). Sponge species composition of north-east Atlantic cold-water coral reefs compared in a bathyal to inshore gradient. J. Mar. Biolog. Assoc. U K. 95, 1461–1471. doi: 10.1017/s0025315413001410
van Soest, R. W. M., and Lavaleye, M. S. S. (2005). Diversity and abundance of sponges in bathyal coral reefs of Rockall Bank, NE Atalantic, from boxcore samples. Mar. Biol. Res. 1, 338–349. doi: 10.1080/17451000500380322
van Soest, R. W. M., Boury-Esnault, N., Hooper, J. N. A., Rützler, K., de Voogd, N. J., Alvarez, B., et al. (2021). World Porifera Database. Ostend: World Register of Marine Species.
van Soest, R. W. M., Boury-Esnault, N., Vacelet, J., Dohrmann, M., Erpenbeck, D., De Voogd, N. J., et al. (2012). Global diversity of sponges (Porifera). PLoS One 7:e35105. doi: 10.1371/journal.pone.0035105
van Soest, R. W. M., Cleary, D. F. R., de Kluijver, M. J., Lavaleye, M. S. S., Maier, C., and van Duyl, F. C. (2007). Sponge diversity and community composition in Irish bathyal coral reefs. Contrib. Zool. 76, 121–142. doi: 10.1163/18759866-07602005
Vázquez, J. T., Ercilla, G., Alonso, B., Juan, C., Rueda, J. L., Palomino, D., et al. (2015). “Submarine Canyons and related features in the Alboran Sea: continental margins and major isolated reliefs,” in Submarine canyon dynamics in the Mediterranean and tributary seas: An integrated geological, oceanographic and biological perspective, ed. F. Briand (Monaco: CIESM Publisher).
Wickham, H., Averick, M., Bryan, J., Chang, W., McGowan, L. D. A., François, R., et al. (2019). Welcome to the Tidyverse. J. Open Source Softw. 4:1686. doi: 10.21105/joss.01686
Wild, C., Jantzen, C., Struck, U., Hoegh-Guldberg, O., and Huettel, M. (2008). Biogeochemical responses following coral mass spawning on the Great Barrier Reef: pelagic–benthic coupling. Coral Reefs 27, 123–132. doi: 10.1007/s00338-007-0298-7
Würtz, M. (2012). “Submarine canyons and their role in the Mediterranean ecosystem,” in Mediterranean Submarine Canyons: Ecology and Governance, ed. M. Würtz (Switzerland: IUCN), 11–26.
Xavier, J. R., and Van Soest, R. W. (2012). Diversity patterns and zoogeography of the Northeast Atlantic and Mediterranean shallow-water sponge fauna. Hydrobiologia 687, 107–125.
Zabala, M., Maluquer, P., and Hermelin, J. G. (1993). Epibiotic bryozoans on deep-water seleractinian corals from the Catalonia slope (western Mediterranean, Spain, France). Sci. Mar. 57, 65–78.
Zibrowius, H., and Taviani, M. (2005). “Remarkable sessile fauna associated with deep coral and other calcareous substrates in the Strait of Sicily, Mediterranean Sea,” in Cold-water Corals and Ecosystems, eds A. Freiwald and J. M. Roberts (Berlin: Springer-Verlag), 807–819. doi: 10.1007/3-540-27673-4_42
Keywords: sponges (Porifera), cold water coral (CWC), new species, biogeography, remotely operated vehicles (ROV), Mediterranean Sea
Citation: Santín A, Grinyó J, Uriz MJ, Lo Iacono C, Gili JM and Puig P (2021) Mediterranean Coral Provinces as a Sponge Diversity Reservoir: Is There a Mediterranean Cold-Water Coral Sponge Fauna? Front. Mar. Sci. 8:662899. doi: 10.3389/fmars.2021.662899
Received: 01 February 2021; Accepted: 12 May 2021;
Published: 15 June 2021.
Edited by:
Shirley A. Pomponi, Florida Atlantic University, United StatesReviewed by:
Sergi Taboada, Autonomous University of Madrid, SpainCopyright © 2021 Santín, Grinyó, Uriz, Lo Iacono, Gili and Puig. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andreu Santín, c2FudGluQGljbS5jc2ljLmVz
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.