
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mar. Sci. , 16 June 2021
Sec. Marine Evolutionary Biology, Biogeography and Species Diversity
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.657651
This article is part of the Research Topic Benthic Biodiversity of the Indian Ocean View all 14 articles
The present study is the first completed and taxonomically validated literature review of the biodiversity of barnacles (Cirripedia) in India. A total of 144 species in 75 genera and 19 families have been recorded in India. The highest number of species has been recorded from the Bay of Bengal province, located on the eastern side of the Indian Peninsula, comprising the Eastern India ecoregion (76 species) and Northern Bay of Bengal ecoregion (34 species). The West and South India Shelf province has fewer species (Western India ecoregion: 29 species; South India and Sri Lanka ecoregion: 40 species; and Maldives ecoregion: 10 species) compared to the Bay of Bengal province. The Andaman province is composed of the Andaman and Nicobar Islands, and contains 65 species. Most of the coral-associated barnacles (family Pyrgomatidae) have been recorded in the corals reefs of the Andaman and Nicobar Islands (7 species), Eastern India (6 species), and Northern Bay of Bengal ecoregions (5 species). Sponge-associated barnacles (mostly in the subfamily Acastinae) were recorded in the Eastern India ecoregion, Southern India and Sri Lanka, and Andaman and Nicobar Islands ecoregions. Deepwater species were recorded the most extensively in the Andaman and Nicobar Islands ecoregion (21 species), followed by the South India and Sri Lanka ecoregion (9 species) and Eastern India ecoregion (7 species). Six Atlantic/boreal cold water species previously reported in India were removed due to incorrect identification, and some incorrectly identified species were validated and corrected.
India is one of the 12 mega-biodiversity countries and has 25 biodiversity hotspots, containing a considerable number of endangered species (Myers et al., 2000; Venkataraman and Wafar, 2005; Venkataraman and Raghunathan, 2015). India supports a rich diversity of marine habitats and invertebrate fauna (Aneesh et al., 2018; Trivedi et al., 2018; Kottarathil et al., 2019; Shih et al., 2019; Aneesh and Kappalli, 2020). India contains 7516.6 km of coastline—5422.6 km in the Indian Peninsula and 2094 km in islands (Andaman and Nicobar Islands: 1962 km; Lakshadweep Islands: 132 km) (Ahmad, 1972; Kumar et al., 2006; Trivedi et al., 2018).
More than 1400 species of barnacle were recorded from different oceanic regions across the world (Innocenti, 2006; Chan et al., 2009). Barnacles occur in almost all marine environments, from intertidal zones to the deepest parts of the oceans (Chan and Høeg, 2015). The greatest diversity of barnacles is observed in the tropical Indo-Pacific region (Newman and Abbott, 1980). Taxonomic studies of barnacles date back to the 16th century, when barnacles were identified as eggs of barnacle geese (Anderson, 1994). Burmeister (1834) was the first naturalist to classify barnacles into cirripedes which later attracted the attention of numerous taxonomists (Anderson, 1994). Darwin (1851, 1854) monographs on barnacles, written shortly after Burmeister’s publication, became one of the major contributions to barnacle taxonomy.
Taxonomic studies on Indian barnacle fauna came out of the systemic work from one of these Darwin (1854) monographs, which included several species of barnacles from the Indian waters. Thomas Nelson Annandale, the first Director of the Zoological Survey of India, published a series of taxonomy papers (Annandale, 1905; Annandale, 1906a; Annandale, 1906b; Annandale, 1907; Annandale, 1909Annandale, 1910a; Annandale, 1910b; Annandale, 1911; Annandale, 1913; Annandale, 1914; Annandale, 1916; Annandale, 1924;) based on the barnacle specimens deposited into the Indian Museum. Annandale (1906a) examined stalked barnacles collected from the R.I.M.S. (Royal India Marine Survey Ship) “Investigator” and described 11 new species. Later, Annandale (1909); (Annandale, 1910a,b) described eight new species and one new family (Poecilasmatidae; Annandale, 1909) of barnacles from Indian waters. Annandale (1924) published a list of cirripedia associated with Indian corals and described two new species. Sundararaj (1927) recorded five species of barnacles from the Krusadai Islands (Tamil Nadu). Nilsson-Cantell (1938) carried out a taxonomic study on barnacles of the Bay of Bengal, Arabian Sea and Indian Ocean, and listed a total of 139 species, 36 of which had a wide distribution beyond these three regions. Augustine Daniel of the Zoological Survey of India studied barnacle diversity of the Tamil Nadu state and recorded 42 species (Daniel, 1956). Daniel (1981) listed 15 species of barnacles collected from estuaries in both the east and south-west coasts of India. Fernando (2006) later published a monograph on Indian barnacles that contained more extensive records and listed 70 species.
Scientists at institutes and universities across Indian have contributed to our understanding of the taxonomy, diversity and biology of barnacles occurring in Indian waters (Gray, 1831; Gruvel, 1907; Hoek, 1913; Kemp, 1915; Pillai, 1958; Bhatt and Bal, 1960; Karande and Palekar, 1963, 1966; Daniel and Chakrabarathi, 1967; Prem-Kumar and Daniel, 1968; Balakrishnan, 1969; Wagh and Bal, 1969, 1974; Devaraj and Bennet, 1974; Karande, 1974; Desai and Senthilkumar, 1975; Fernando, 1978; Venkateswaran and Fernando, 1982; Wagh and Sawant, 1982; Newman and Killingley, 1984; Sudakaran and Fernando, 1987; Rao and Balaji, 1988; Frazier, 1989; Nandakumar, 1990; Rajaguru and Shantha, 1992; Ramakrishna and Talukdar, 2003; Karuppiah et al., 2004; Singh et al., 2004; Mitra and Misra, 2006; Sanjeeva Raj, 2006; Krishnamoorthy, 2007; Kumaravel et al., 2009; Pati et al., 2009; Mitra et al., 2010; Swami et al., 2011; Namboothri and Fernando, 2012; Patro, 2012; Ramamoorthy et al., 2012; Trivedi et al., 2015; Sahadevan, 2016; Roy and Rath, 2017; Desai et al., 2018; Parmar et al., 2018; Mondal et al., 2019). In addition to diversity research, there are a considerable number of studies focusing on the larval biology and settlement ecology of the common Indian intertidal barnacle Amphibalanus amphitrite (Gaonkar and Anil, 2010, 2012, 2013a,b; Gaonkar et al., 2012). In Goa and Kochi, on the west coast of India, settlement of cypris larvae have been investigated for their responses to diatom exopolymers (Patil and Anil, 2005), conspecific cues (Khandeparker and Anil, 2011), specific bacteria associating with the barnacle shells (De Gregoris et al., 2012), and sponge extracts (Mol et al., 2010). The reproductive cycle and population dynamics of A. mphitrite in Goa, India is affected by the interactions among monsoons, temperature and chlorophyll a concentration in seawater (Desai and Anil, 2005; Desai et al., 2006). The breeding and settlement patterns of A. mphitrite in Tamil Nadu, southeast coast of India are affected by tidal patterns, temperature and phytoplankton abundance (Karuppaiyan and Raja, 2007; Satheesh and Wesley, 2009). To date, there has not been a complete literature review on those extensive records of barnacle diversity in India or their accuracy. The objective of the present study is to provide the most taxonomically updated, validated and complete diversity records on barnacles in India. Species identities were validated based on morphological identifications using relevant taxonomic references, and supported by recent results of molecular taxonomic studies of barnacles in the Indo-Pacific waters.
According to the definitions in Spalding et al. (2007), the coastline of India is comprised of four marine biogeographical provinces and six ecoregions (Figure 1). The West and South Indian Shelf province comprises the Western India ecoregion (from Kachchh district of Gujarat state to Ashtamudi Lake of Kerala state) and the South India ecoregion (Ashtamudi Lake of Kerala state to Karaikal, Puducherry of Tamil Nadu state) (Figure 1). The Central Indian Ocean Islands province includes the Maldives ecoregion, comprising Lakshadweep Islands and Minicoy. The Bay of Bengal province in India covers the Eastern India ecoregion (from Karaikal, Pondicherry of Tamil Nadu state to Konark of Odisha state) and the Northern Bay of Bengal ecoregion (Konark of Odisha state to Bidyadhari River Delta of West Bengal state). The Andaman province in India includes Andaman and Nicobar Islands ecoregion, which covers the Andaman and Nicobar Islands (Figure 1; Spalding et al., 2007).
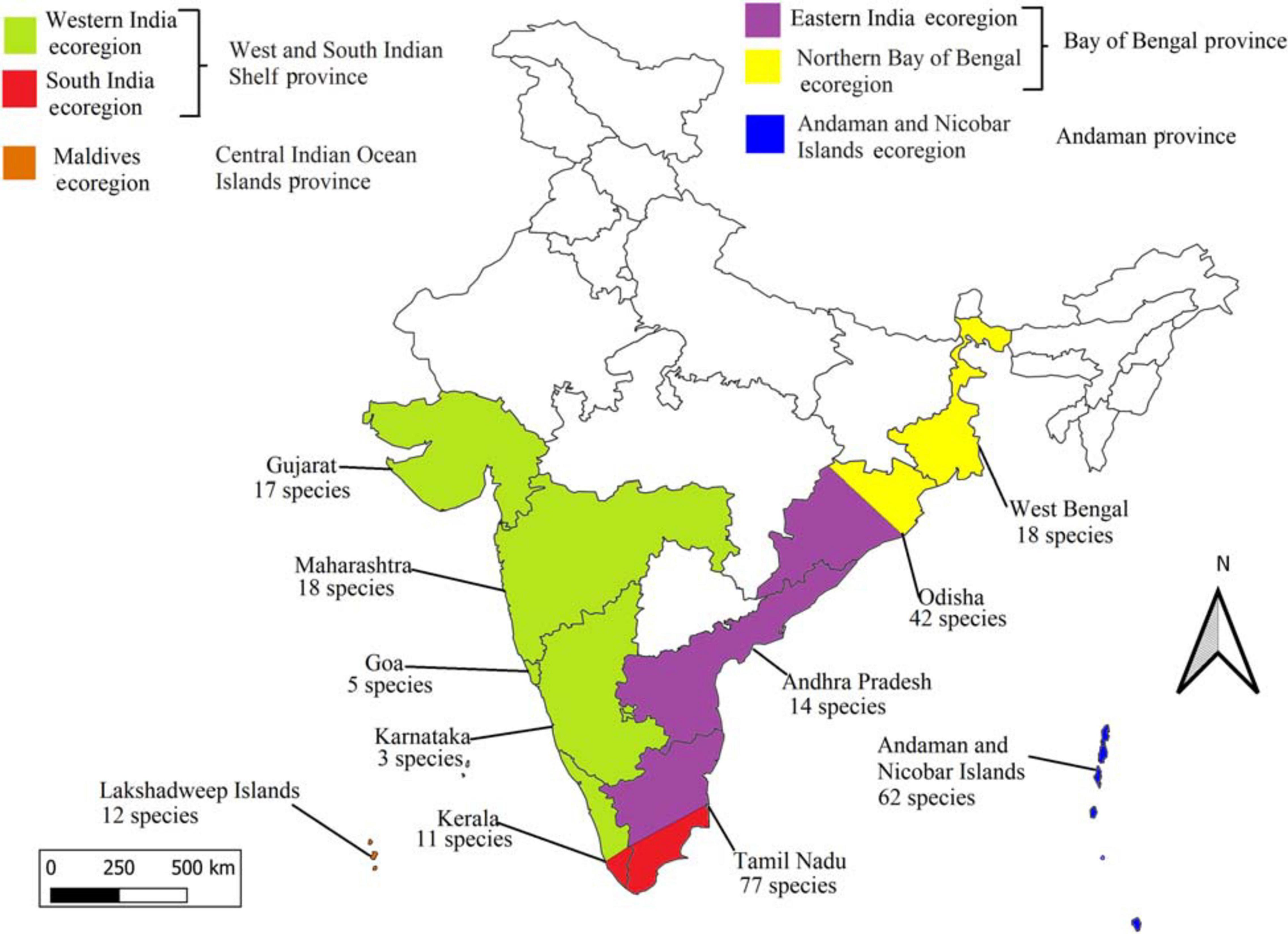
Figure 1. Map of India (Sri-Lanka was not included) showing the states as well as ecoregions and provinces as defined by Spalding et al. (2007).
The data on the barnacle fauna of India in the present study are based on peer-reviewed published literature and/or deposited material in marine collections. Only species present in Indian waters were recorded in the present study.
The collection site of each species is classified based on the Indian state and relevant marine provinces and ecoregions from Spalding et al. (2007). Species reported from adjacent countries that formed a part of the former British Indian empire (Sri Lanka, Pakistan, Bangladesh, Maldives, and Myanmar) were excluded. A few old records without clearly stated collection sites were omitted. The synonyms, nomenclatural changes and classification of different species were validated based on Southward and Newman (2003), Chan et al. (2009), Jones and Hosie (2016).
Based on what is present and absent in the list of all recorded barnacle species, multivariate analysis (using species as variables) was performed using the PRIMER package (v6, Plymouth Routine in Multivariate Analysis, PRIMER-E Ltd; Clarke and Gorley, 2006) to examine variations in species assemblages among the ecoregions in India. Similarities among the ecoregions based on species data were calculated using the Sorensen similarity index. Non-metric Multidimensional Scaling (nMDS; Clarke, 1993) was conducted to generate two-dimensional plots on the species composition in all the ecoregions.
A total of 155 barnacle species belonging to 75 genera and 19 families were reported from India (Supplementary Appendix Table 1). Of these, 40 species were described from Indian waters, of which 35 are valid species (Table 1) and five were synonymized: Platylepas multidecorata Daniel, 1962—described from Little Andaman, Andaman and Nicobar Islands—is now treated as a synonym of Platylepas decorata (Darwin, 1854). Balanus longirostrum var. krusadaiensis Daniel (1956), described from Tamil Nadu, is now treated as a synonym of Membranobalanus longirostrum (Hoek, 1913). Balanus (Membranobalanus) roonwali Prem-Kumar and Daniel (1968)—described from Chennai, Tamil Nadu—is now treated as a synonym of M. longirostrum. Balanus amphitrite var. cochinensis Nilsson-Cantell (1938)—described from Ernakulam, Cochin, Kerala—is now treated as a synonym of Amphibalanus amphitrite (Darwin, 1854). Pollicipes polymerus madrasensis Daniel, 1953—described from Royapuram, Chennai, Tamil Nadu—is now treated as a synonym of Pollicipes polymerus Sowerby (1833).
At the family level, the highest number of species were reported from the family Poecilasmatidae Annandale (1909) (mostly epibiotic on crustaceans; 27 species, 8 genera), followed by Archaeobalanidae Newman and Ross (1976) (epibiotic in various organisms; 25 species, 12 genera); Balanidae Leach (1817) (mostly intertidal and subtidal, 21 species, 6 genera); Pyrgomatidae Gray (1825) (coral-associated, 15 species, 12 genera); Scalpellidae Pilsbry (1907) (deepwater; 15 species, 7 genera); Lepadidae Darwin (1852) (pelagic; 8 species, 4 genera); Tetraclitidae Gruvel (1903) (8 species, 4 genera); Verrucidae Darwin (1854) (deepwater; 6 species, 4 genera); Chthamalidae Darwin (1854) (intertidal; 6 species, 3 genera); Calanticidae Zevina (1978) (deepwater; 5 species, 3 genera); Heteralepadidae Nilsson-Cantell (1921) (deepwater; 5 species, 3 genera); Platylepadidae Newman and Ross (1976) (epibiotic on turtles; 5 species, 2 genera); Chelonibiidae Pilsbry (1916) (epibiotic on turtles; 2 species, 1 genus); and Oxynaspididae Pilsbry (1907) (epibiotic on corals; 2 species, 1 genus). The following families each contain only one species in one genus: Coronulidae Leach (1817) (on cetaceans); Iblidae Leach (1825) (intertidal); Lithotryidae Gruvel (1905) (intertidal); Pollicipedidae Leach (1817) (intertidal); and Sacculinidae Lilljeborg (1861) (parasitic).
State-wide data revealed that the highest number of species were reported from Tamil Nadu (77 species, 39 genera, 15 families) followed by Andaman and Nicobar Islands (62 species, 40 genera, 15 families), Odisha (42 species, 26 genera, 12 families), Maharashtra (18 species, 12 genera, 8 families), West Bengal (18 species, 11 genera, 6 families), Gujarat (17 species, 11 genera, 7 families), Andhra Pradesh (14 species, 8 genera, 5 families), Lakshadweep Islands (12 species, 8 genera, 7 families), Kerala (11 species, 9 genera, 7 families), Goa (5 species, 4 genera, 3 families), and Karnataka (3 species, 3 genera, 2 families) (Figure 1 and Supplementary Appendix Table 2).
The Eastern India ecoregion has the highest number of species (76 species), followed by Andaman and Nicobar Islands ecoregion (65), South India and Sri Lanka ecoregion (40), Northern Bay of Bengal ecoregion (34), Western India ecoregion (29), and Maldives ecoregion (11) (Figure 2A and Supplementary Appendix Table 1). The low number of species recorded in the Maldives ecoregion is probably due to the low number of studies conducted there.
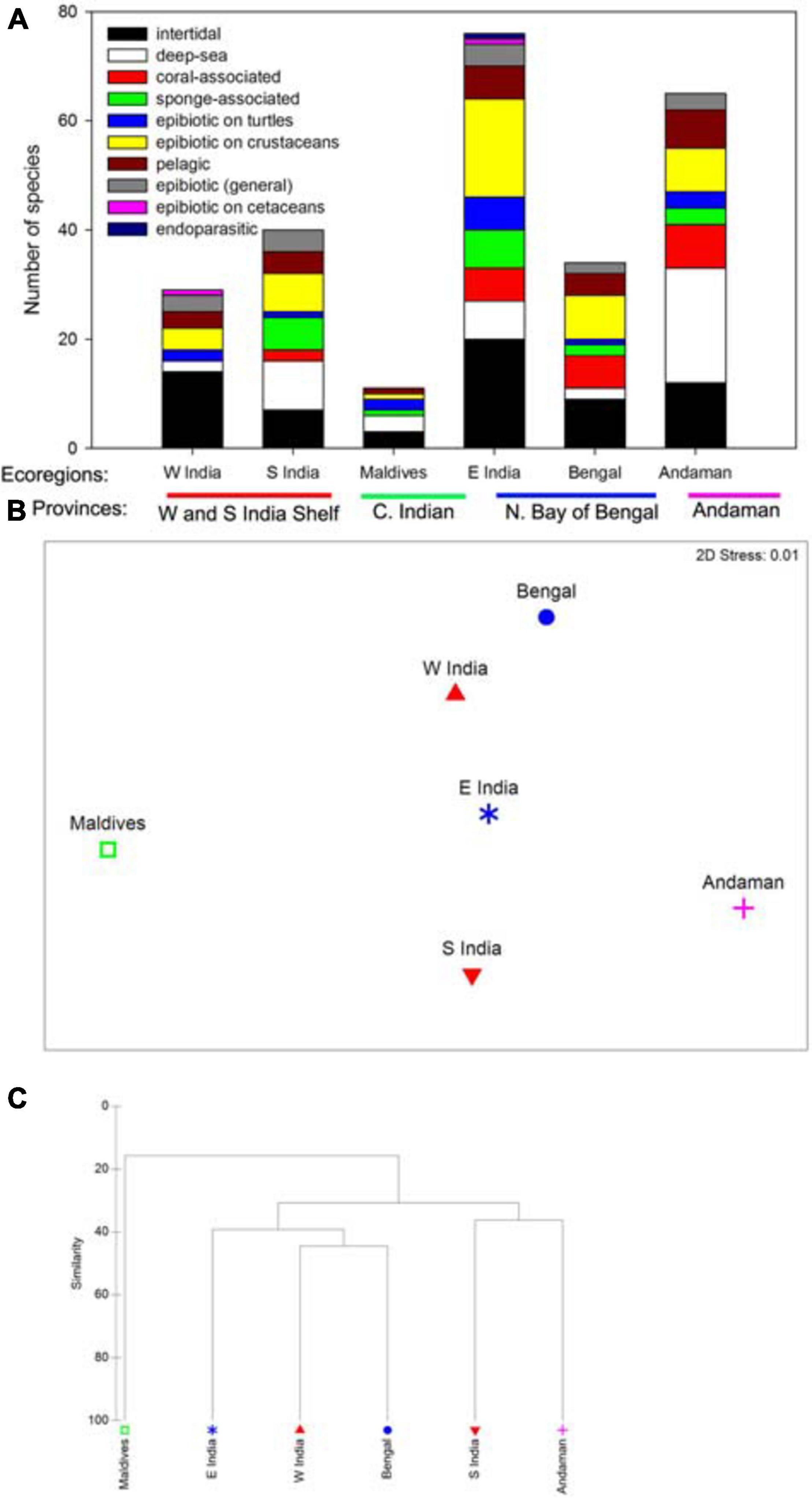
Figure 2. (A) Species richness of barnacles from different life styles/habitats in different ecoregions and provinces. For clarity, names of ecoregions were abbreviated. W India – Western India Ecoregion, S India – Southern India and Sir Lanka ecoregion, Maldives – Maldives ecoregion, Bengal – Bay of Bengal ecoregion, Andaman – Andaman and Nicobar Islands ecoregion. (B) nMDS plot on the ordination of ecoregions based on species assemblage data. (C) Cluster analysis on the similarity of species assemblages among ecoregions.
Most of the coral-associated barnacles (family Pyrgomatidae) were recorded in the Andaman and Nicobar Islands ecoregion (7 species), Eastern India ecoregion (6 species) and Northern Bay of Bengal ecoregion (5 species), which contain a great diversity of coral reefs (Venkataraman and Wafar, 2005; Venkataraman and Raghunathan, 2015; Figure 2A). The Maldives ecoregion covers the Lakshadweep Islands, which have extensive coral reefs; however, due to a lack of studies conducted in this ecoregion, only two coral barnacle species were recorded here. No coral-associated barnacles were recorded in the Western Indian ecoregion, the coastlines of which are characterized by mangroves and soft-bottomed shores and rocky shores in the urbanized Mumbai. There are, however, coral reefs in the Gulf of Kachchh of Gujarat state, but no detailed coral barnacle studies have been conducted there so far. Sponge-associated barnacles (mostly in the subfamily Acastinae) were recorded in the Eastern India, Southern India and Sri Lanka, and Andaman and Nicobar Islands ecoregions. Sponge diversity appears to be high in these three regions, supporting such diversity in barnacles (Ubare and Mohan, 2018; Figure 2A).
The deepwater scalpellid, calanticid and verrucid species were relatively extensively recorded in the Andaman and Nicobar Islands ecoregion (Figure 2A and Supplementary Appendix Table 1), followed by the Eastern India ecoregion (21 species) and the South India and Sri Lanka ecoregion (9 species). These three ecoregions contain deep-sea ecosystems and are often reported to contain deepwater crustaceans (Macpherson et al., 2020; Padate et al., 2020).
Based on the multivariate analysis, the distribution of the ecoregion clusters does not reflect clear separations among the provinces (Figure 2B). The Eastern India, Western India, and Northern Bay of Bengal ecoregions were located in the same cluster with 40% similarity (Figure 2C). The Andaman ecoregion is closer to the South India ecoregion. The Maldives ecoregion is separate from the other ecoregions (Figures 2B,C).
Some species are specific to certain ecoregions. Chthamalus barnesi, Tetraclita ehsani are Arabian species and are only recorded in the Western India ecoregion. The stalked barnacle Lithotrya nicobarica, which lives in the burrows of calcareous rocks, is only recorded in the Andaman and Nicobar Islands ecoregion, where the intertidal rocks are mostly coral formations and calcareous (Bandopadhyay and Carter, 2017). Tetraclita squamosa, which has green shells, is only recorded in the mid intertidal shore of the Eastern India ecoregion and Andaman and Nicobar Islands ecoregion; it is absent from the Western India ecoregion, the mid intertidal shore of which instead harbors Tetraclita ehasani and Tetraclitella karandei. These two species were only reported in the Gujarat state in the Western India ecoregion (Trivedi et al., 2021). Differences in diagnostic species between the South India Shelf province (Western India ecoregion) and the Northern Bay of Bengal provinces may be a result of differences in major oceanographic currents between these two provinces. The Bay of Bengal is affected by monsoon gyres. Large anti-cyclonical gyres are generated in the Bay of Bengal during the winter months and weaken in summer (Potemra et al., 1991). On the other hand, the western coast of India faces the Arabian Sea, where the hydrography is affected by the counter flows of the northeast Monsoon Current and the Indian Monsoon Current during different seasons. This resulted in Northern Bay of Bengal province is different from South India Shelf province from the cluster analysis (Figures 2B,C).
There are several records of Atlantic cold-water species reported in India that are apparently misidentifications or have taxonomic ambiguities. Daniel (1956) and Krishnamoorthy (2007) recorded Semibalanus balanoides (Linnaeus, 1767) in Tamil Nadu. This species is a boreal intertidal barnacle in the Atlantic and Arctic waters (Southward, 2008), and therefore cannot live in the tropical Indian waters. Subsequently, Daniel (1958, 1962) described two new species from Tamil Nadu, namely Semibalanus madrasensis (Daniel, 1958) and S. sinnurensis (Daniel, 1962), and it is suggested that S. balanoides might be either one of these species. Ramamoorthy et al. (2012) identified Balanus balanus (Linnaeus, 1758), a boreal-arctic subtidal barnacle in the northern Atlantic (Southward, 2008), from a coral reef invertebrate survey on Pirotan Island, Gujarat, western India. This record, too, may be a misidentification, and hence was not considered as a valid record.
Daniel (1956) identified Perforatus perforatus (Bruguière, 1792), another Atlantic species, in Tamil Nadu, but its presence in the Indian waters has not been confirmed. A recent survey in Korea reported this to be an invader in southern Korean waters, probably through ballast water, that has established itself as an ecologically important species (Choi et al., 2013; Kim et al., 2020a,b). Another doubtful record is that of Balanus glandula Darwin (1854) from shrimp ponds in Odisha (Nayak and Berkes, 2014; Nayak, 2017); the species is an inhabitant of the temperate northeast and North West Pacific, so we considered the record to be invalid in the present study.
The high shore barnacle Chthamalus challengeri Hoek (1883)—a cold-water inhabitant of the West Pacific, including Japan and far-east Russia—was reported by Bhatt and Bal (1960) and Daniel (1972) from Maharashtra. However, a recent review of the genus Chthamalus Ranzani (1817) by Southward and Newman (2003) refutes the above record. Only C. malayensis was recorded from Tranquebar and Mumbai by Southward and Newman (2003). The presence of C. malayensis Pilsbry (1916) along the eastern and western coasts of India was further confirmed from molecular evidence (Tsang et al., 2012; Figures 3A,B). Based on the above, the record of C. challengeri in Maharashtra is believed to actually be C. malayensis. In Mumbai, another chthamalid barnacle, Microeuraphia withersi (Figures 3E,F), was also recorded. Microeuraphia have three teeth on their mandible, whereas Chthamalus have four teeth on their mandible. It is therefore not possible that Daniel (1972) identified Microeuraphia withersi to C. challengeri due to such big morphological differences in mandibles between the two species.

Figure 3. Indian coastlines and barnacles. (A) Mamahariprum, eastern coast of India. Most of the hard substrates are large boulders. (B) Chthamalus malayensis is the dominant intertidal species on eastern coast of India. (C) Tranquebar, the remnants of Danish fort on coastline. (D) Coastline of Tranquebar is composed of large brick walls deteriorated from the Danish fort and large boulders. (E) Mumbai, large extent of rocky shores just below the urbanized city. (F) Microeuraphia withersi on the Mumbai coastline. (G) Diu, Gujarat showing the sandstone rocky shores. (H) Tetraclita ehsani in northwest India. (I) Tetraclitella karandei at Diu, Gujarat.
Another chthamalid barnacle, Chthamalus stellatus (Poli, 1791), was recorded from Gujarat, North West India by Parmar et al. (2018) and is an intertidal species in the Atlantic Ocean and Mediterranean Sea (Pannacciulli et al., 1997). The record of C. stellatus in Gujarat appears to be erroneous. A recent examination of Chthamalus from Diu, Gujarat revealed that only C. barnesi Achituv and Safriel, 1980 is present on the rocky intertidal zone in this region (Trivedi et al., 2021). Geographical distribution of C. barnesi range from North Western India to the Persian Gulf (Shahdadi and Sari, 2011). The record of C. stellatus from Gujarat should be C. barnesi (Supplementary Appendix Table 1). Similarly, records of Chthamalus malayensis in Maharashtra, Gujarat (Karande and Palekar, 1963; Daniel, 1972; Wagh and Bal, 1974) should also be C. barnesi (Supplementary Appendix Table 1).
The stalked barnacle Pollicipes polymerus Sowerby (1833) is common in the intertidal regions of the northeast Pacific coast (Newman and Abbott, 1980). However, Daniel (1953b) recorded eight specimens of this temperate species attached to floating wood in Tamil Nadu as Pollicipes polymerus madrasensis. Newman and Abbott (1980) tried to investigate why such a temperate species is present in India by analyzing variations in the oxygen-18 stable isotope at different positions on its shell plates relative to the basal margin of the type specimen of P. polymerus madrasensis. Oxygen-18 stable isotope on shells can reflect the growth pattern of barnacle shells from different climatic environments. Results of the isotope analysis found that the type specimens of Pollicipes polymerus madrasensis found in India grew up in cold temperate waters, meaning that this species is not native to India. The present study did not consider this record to be an Indian record.
Tetraclita Schumacher (1817) is a common acorn barnacle on mid-intertidal shores of tropical and subtropical regions (Chan et al., 2007a). In India, several species of Tetraclita have been recorded, but a few of these records need clarification. In the Eastern India ecoregion, the green Tetraclita squamosa (Bruguière, 1792) was identified by Bruguière (1792) from Tranquebar, east coast of India (Figures 3C,D), which was a Danish colony in the 17th century. The illustration of T. squamosa in Bruguière (1792) shows an empty external shell without opercular plates. Bruguière (1792) probably collected an empty shell of Tetraclita that had washed onshore. The identification of Tetraclita could not be confirmed based on the external shell as identification requires the shapes of the scutum and tergum, and cirral morphology (Chan et al., 2007a). The third author (BKKC) visited several seashores in Tamil Nadu, including Pondicherry and Tranquebar, in 2007 (Figures 3A–D) and attempted to collect specimens of Tetraclita squamosa from its type locality for comparative studies, but he did not find any. Fernando (2006, 77) commented that T. squamosa “occurs not so commonly in Tranquebar and it takes about an hour to locate a single specimen,” thereby suggesting that it occurs in very low abundance and is not common on the Eastern coast of India. The above on-field observations and published reports suggest that the species identity of T. squamosa in eastern India is still uncertain.
In the Western Indian ecoregion, the pink T. rufotincta Pilsbry (1916) was recorded in Gujarat (Wagh and Bal, 1969; Daniel, 1972; Fernando, 2006; Parmar et al., 2018). However, a detailed revision of Tetraclita in the West Indian Ocean using a molecular approach revealed that T. rufotincta is distributed in the Red Sea and the Persian Gulf; Tetraclita ehsani Chan et al., 2011 is common in the Gulf of Oman and Arabian Sea (Shahdadi et al., 2011; Tsang et al., 2012). In India, T. ehsani and T. karandei are only present in Gujarat, and absent south of Gujarat (Trivedi et al., 2021; Figures 3G–I).
In the Andaman and Nicobar Islands ecoregion, the pink Tetraclita japonica formosana (Hiro, 1939) was recorded in the Andaman and Nicobar Islands (Malakar et al., 2015). Tetraclita japonica formosana is only common along the east coast of Taiwan and the Pacific coast of Japan (Chan et al., 2007b; Tsang et al., 2007). The records from Andaman and Nicobar Islands need further investigation and clarification.
The present study revealed that most of the species reported from India are intertidal and epibiotic species of crustaceans and common fishery catches in India. More specialized species, including coral-associated barnacles, remain understudied. Previous coral barnacle records in India were mainly from old publications dating back as much as from 48 to 96 years ago (Annandale, 1924; Nilsson-Cantell, 1938; Daniel, 1972). No coral-associated barnacles were collected from the extensive corals in the Lakshadweep Islands and Minicoy or the Gulf of Kachchh of Gujarat state. The diversity of coral-associated barnacles in Indian waters is clearly a knowledge gap that requires more attention. There are presently no records of barnacles in the superorder Acrothoracica, and only one in the parasitic superorder Rhizocephala (Supplementary Appendix Table 1). More research is needed to understand the species diversity of these two superorders.
The Indian Exclusive Economic Zone (EEZ) is characterized by deepwater basins, including the Bay of Bengal to the east and the Arabian Basin to the west. The deepwater barnacle fauna of the Indian EEZ and the Indian Ocean are less studied than their Pacific counterparts. Recent deep-sea sampling in the Indian Ocean revealed the first deep-sea hydrothermal vent barnacles from the region (Watanabe et al., 2018; Chan et al., 2020). Long-term dedicated exploration of the deepwater basins in the Indian EEZ waters would certainly offer a richer perspective on the species composition of deep-sea barnacles in the region.
JT, KP, BKKC, and VP wrote the manuscript. All authors involved in collection of barnacle specimens and literature review.
BKKC was supported by the Academia Sinica Career Development Award and Senior Investigator Award.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors are thankful to Mr. Dhaval Bhatt, Ms. Pooja Patel, and Ms. Dimple Thacker for their technical support. Thanks to Noah Last, the Third Draft Editing for editing the English of the present manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.657651/full#supplementary-material
Achituv, Y., and Safriel, U. N. (1980). A new Chthamalus (CRUSTACEA: CIRRIPEDIA) from intertidal rocks of the red sea. Isr. J. Zool. 29, 99–109. doi: 10.1080/00212210.1980.10688487
Achituv, Y., and Simon-Blecher, N. (2006). Pyrgopsella (Cirripedia: Balanomorpha: Pyrgomatidae) is not a sponge-inhabiting barnacle. Zootaxa 1319, 29–42. doi: 10.11646/zootaxa.1319.1.3
Achituv, Y., and Simon-Blecher, N. (2014). The Rise and Fall of Pyrgopsella youngi-Rediscovery of a Lost Species. J. Crust. Biol. 34, 663–670. doi: 10.1163/1937240X-00002257
Anderson, D. T. (1994). Barnacles: Structure, Function, Development and Evolution. Berlin: Springer.
Aneesh, P. T., and Kappalli, S. (2020). Protandrous hermaphroditic reproductive system in the adult phases of Mothocya renardi (Bleeker, 1857) (Cymothoidae: Isopoda: Crustacea) - light and electron microscopy study. Zool. Stud. 59:61. doi: 10.6620/ZS.2020.59-61
Aneesh, P. T., Sudha, K., Helna, A. K., and Anilkumar, G. (2018). Agarna malayi Tiwari 1952 (Crustacea: Isopoda: Cymothoidae) parasitizing the marine fish, Tenualosatoli (Clupeidae) from India: re-description/description of parasite life cycle and patterns of occurrence. Zool. Stud. 57:25.
Anil, A. C., and Kurian, J. (1996). Influence of food concentration, temperature and salinity on the larval development of Balanus amphitrite. Mar. Biol. 127, 115–124. doi: 10.1007/BF00993651
Annandale, N. (1905). Malaysian barnacles in the Indian Museum, with a list of the Indian Pedunculata. Mem. Asiatic Soc. Bengal. 1, 73–83.
Annandale, N. (1906a). Natural history notes from the R.I.M.S. INVESTIGATOR, Captain T. H. Heming, R. N., commanding. Series III, No. 12. Preliminary report on the Indian Stalked Barnacles. Ann. Mag. Nat. Hist. 7, 389–400. doi: 10.1080/00222930608562544
Annandale, N. (1906b). Report on the Cirripedia collected by Professor Herdman, at Ceylon, in 1902. Rep. Gov. Ceylon Pearl Oyster Fish. Gulf Mannar. 5, 137–150.
Annandale, N. (1909). An account of the Indian Cirripedia Pedunculata. Part I-Family Lepadidae (s. str.). Mem. Indian. Mus. 2, 51–137. doi: 10.1086/bblv230n1p51
Annandale, N. (1910a). Description of a new species of Scalpellum from the Andaman Sea. Rec. Ind. Mus. 5, 115–116.
Annandale, N. (1910b). The Indian barnacles of the subgenus Smilium, with remarks on the classification of the genus Scalpellum. Rec. Ind. Mus. 5, 145–155.
Annandale, N. (1911). Note on a rhizocephalous crustacean from fresh water and on some specimens of the order from Indian Seas. Rec. Indian. Mus. 6, 1–4. doi: 10.5962/bhl.part.21325
Annandale, N. (1914). New and interesting Pedunculate cirripedes from Indian seas. Rec. Indian Mus. 10, 273–280. doi: 10.5962/bhl.part.5628
Annandale, N. (1916). Three plates to illustrate the Scapellidae and Iblidae of Indian Seas with synonymy and notes. Mem. Indian Mus. 6, 127–131.
Annandale, N. (1924). Cirripedes associated with Indian corals of the families Astraidae and Fungidae. Mem. Ind. Mus. 8, 61–68.
Aurivillius, C. W. S. (1892). Neue Cirripeden aus dem Atlantischen, Indischen und Stillen Ocean. Ofversigr. Kongl. Vet Aka. Forh. 3, 123–135.
Aurivillius, C. W. S. (1894). Studien uber Cirripeden. Kun. Svensk. Vet Akad. Handl. 26, 1–107. doi: 10.1007/978-3-662-40029-6_1
Balakrishnan, K. P. (1969). Observations on the occurrence of Conchoderma virgatum (Spengler) (Cirripedia) on Diodon hystrix Linnaeus (Pisces). Crustaceana 16, 101–103. doi: 10.1163/156854068X00269
Bandopadhyay, P. C., and Carter, A. (2017). “Introduction to the geography and geomorphology of the Andaman-Nicobar Islands,” in The Andaman-Nicobar Accretionary Ridge: Geology, Tectonics and Hazards, Vol. 47, eds P. C. Bandopadhyay and A. Carter (London: Geological Society), 9–18. doi: 10.1144/M47.2
Bhatt, Y. M., and Bal, D. V. (1960). New records of barnacles from Bombay shores. Curr. Sci. 29, 439–440.
Borradaile, L. A. (1903). “Marine crustaceans. Part VII. The barnacles (Cirripedia),” in The Fauna and Geography of the Maldive and Laccadive Archipelagoes, Vol. 2, ed. J. S. Gardiner (Cambridge: The University Press), 440–443.
Broch, H. (1922). Studies on Pacific Cirripedes. Paper Presented at the Dr Th. Mortensen’s Pacific Expedition 1914-1916 (10). Videnskabelige. Meddelelser. Fra. Dansk. Naturhistorisk. Forening, Vol. 73, København, 215–358.
Broch, H. (1927). Report on the Crustacea Cirripedia. Cambridge expedition to the Suez Canal, 1924. Trans. Zool. Soc. 2, 133–138.
Broch, H. (1931). Indomalayan Cirripedia. Papers from Dr Th. Mortensen’s Pacific Expedition 1914-1916, LVI. Vidensk. Medd. Naturhist. Køben. 91, 1–146.
Bruguière, M. (1792). Encyclopédie Méthodique; Histoire Naturelle des Vers, de Lamarck, Continuées par GP Deshayes, Vol. 1. Paris: Chez Panckoucke.
Buhl-Mortensen, L., and Newman, W. A. (2004). A new pedunculate barnacle (Cirripedia: Heteralepadidae) from the Northwest Atlantic. Proc. Biol. Soc. Wash. 117, 385–397.
Burmeister, H. (1834). Beiträge zur Naturgeschichte der Rankenfüsser (Cirripedia). Berlin: G. Reimer.
Chan, B. K. K., and Høeg, J. T. (2015). “Diversity of lifestyles, sexual systems, and larval development patterns in sessile crustaceans,” in Lifestyles and Feeding Biology, The Natural History of the Crustacea, Vol. 2, eds M. Thiel and L. Watling (Oxford: Oxford University Press), 14–34.
Chan, B. K. K., Ju, S. J., Kim, D. S., and Kim, S. J. (2020). First discovery of the sessile barnacle Eochionelasmus (Cirripedia: Balanomorpha) from a hydrothermal vent field in the Indian Ocean. J. Mar. Biol. Assoc. 100, 1–9. doi: 10.1017/S0025315420000466
Chan, B. K. K., Prabowo, R. E., and Lee, K. S. (2009). Crustacean Fauna of Taiwan: barnacles, Volume I-Cirripedia: Thoracica excluding the Pyrgomatidae and Acastinae. Keelung: National Taiwan Ocean University.
Chan, B. K. K., Tsang, L. M., and Chu, K. H. (2007a). Morphological and genetic differentiation of the acorn barnacle Tetraclita squamosa (Crustacea, Cirripedia) in East Asia and description of a new species of Tetraclita. Zool. Scr. 36, 79–91. doi: 10.1111/j.1463-6409.2007.00260.x
Chan, B. K. K., Tsang, L. M., Ma, K. Y., Hsu, C. H., and Chu, K. H. (2007b). Taxonomic revision of the acorn barnacles Tetraclita japonica and Tetraclita formosana (Crustacea: Cirripedia) in East Asia based on molecular and morphological analyses. Bull. Mar. Sci. 81, 101–113.
Choi, K. H., Choi, H. W., Kim, I. H., and Hong, J. S. (2013). Predicting the invasion pathway of Balanus perforatus in Korean Seawaters. OPR 35, 63–68. doi: 10.4217/OPR.2013.35.1.063
Clarke, K. R. (1993). Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 18, 117–143. doi: 10.1111/j.1442-9993.1993.tb00438.x
Conrad, T. A. (1837). Descriptions of new marine shells from upper California, collected by Thomas Nuttal, Esq. J. Aca. Nat. Sci., Series 17, 227–268.
Costa, O. G. (1838). Di alcuni balanidi appartenti al Regno di Napoli. Atti. Della. Reale. Accademia Delle. Scienze. e Belle-Lettere. di Napoli 5, 17–70.
Daniel, A. (1952). XLV. A new barnacle, Lepas bengalensis, from Madras. Ann. Mag. Nat. Hist. 5, 400–403. doi: 10.1080/00222935208654307
Daniel, A. (1953a). Conchoderma indicum n. sp. a pedunculate cirripede from Krusadi Islands. J. Zool. Soc. India 5, 235–238.
Daniel, A. (1953b). XXV. On a new barnacle, Pollicipes polymerus madrasensis, subsp. nov., in Madras. Ann. Mag. Nat. Hist. 6, 286–287. doi: 10.1080/00222935308654423
Daniel, A. (1956). The Cirripedia of the madras Coast. Bull. Madras Gov. Mus. Nat. Hist. Sec. 6, 1–40.
Daniel, A. (1958). On Platylepas indicus n. sp. a new barnacle from the Madras coast of India. Ann. Mag. Nat. Hist. Ser. 13 1, 755–757. doi: 10.1080/00222935808651151
Daniel, A. (1962). On a new species of operculate barnacle (Cirripedia: Crustacea) from the Gastropod Mollusc, Murex sp. from Porto Novo, Madras State. Ann. Mag. Nat. Hist. Sere. 13, 193–197. doi: 10.1080/00222936208651234
Daniel, A. (1971). A new species of pedunculate barnacle of the genus Lepas (Cirripedia, Thoracica) from the Eastern Indian Ocean. J. Mar. Biol. Assoc. India 13, 82–85.
Daniel, A. (1972). Marine intertidal barnacles in the Indian Ocean. Proc. Ind. Nat. Sci. Acad. U.S.A. 38, 179–189.
Daniel, A. (1981). Distribution pattern of barnacles (Crustacea: Cirripedia) in estuarine systems of India. Bull. Zool. Sur. India 4, 173–179.
Daniel, A., and Chakrabarathi, P. K. (1967). Notes on a collection of barnacles from east coast of India. J. Bombay Nat. Hist. Soc. 63, 772–777.
Daniel, A., and Ghosh, A. (1963). A new cirripede of the subgenus Megabalanus from the stomatopod (Squilla sp.) from Madras. Ann. Mag. Nat. Hist. 6, 477–479. doi: 10.1080/00222936308651385
Darwin, C. (1852). A Monograph on the Sub-Class Cirripedia with Figures of all Species. The Lepadidae or Pedunculated Barnacles. London: Royal Society, doi: 10.5962/bhl.title.2104
Darwin, C. (1854). A Monograph on the Sub-Class Cirripedia, with Figures of all the Species. The Balanidae, (or sessile cirripedes); the Verrucidae, etc. London: The Ray Society.
De Gregoris, T. B., Khandeparker, L., Anil, A. C., Mesbahi, E., Burgess, J. G., and Clare, A. S. (2012). Characterisation of the bacteria associated with barnacle, Balanus amphitrite, shell and their role in gregarious settlement of cypris larvae. J. Exp. Mar. Biol. Ecol. 413, 7–12. doi: 10.1016/j.jembe.2011.11.014
Desai, D. V., and Anil, A. C. (2005). Recruitment of the barnacle Balanus amphitrite in a tropical estuary: implications of environmental perturbation, reproduction and larval ecology. J. Mar. Biol. Assoc. UK 85, 909–920. doi: 10.1017/S0025315405011884
Desai, D. V., Anil, A. C., and Venkat, K. (2006). Reproduction in Balanus amphitrite Darwin (Cirripedia: Thoracica): influence of temperature and food concentration. Mar. Biol. 149, 1431–1441. doi: 10.1007/s00227-006-0315-3
Desai, D. V., Krishnamurthy, V., and Anil, A. C. (2018). Biofouling Community Structure in a Tropical Estuary of Goa on the West Coast of India. AJSTD 35, 37–42. doi: 10.29037/ajstd.471
Desai, K. M., and Senthilkumar, S. A. (1975). The neurosecretory control over spawning in the barnacles of the Veraval coast. Ann. Zool. 11, 27–40.
Devaraj, M., and Bennet, S. P. (1974). Occurrence of Xenobalanus globicipitis (Steenstrup) on the finless black porpoise, Nemerisph ocoenoides in Indian seas. Ind. J. Fish. 21, 575–579.
Dhandapani, K., and Fernando, S. A. (1994). “Fecundity of some sessile barnacles with emphasis on fugitive forms from Porto Novo, South India,” in Recent Developments in Biofouling Control, eds M. F. Thompson, R. Nagabhushanam, R. Sarojini, and M. Fingerman (India: Oxford and IBH), 133–140.
Dinamani, P. (1964). Variation in form, orientation and mode of attachment of the cirripede, Octolasmis stella (Ann.), symbiotic on the gills of lobster. J. Anim. Ecol. 33, 357–362. doi: 10.2307/2636
Ellis, J. (1758). An account of several rare species of barnacles. Philos. Trans. R. Soc. London 50, 845–855. doi: 10.1098/rstl.1757.0114
Fabricius, J. C. (1798). Tillaeg-til Conchylie-Slaegterne Lepas, Pholas, Mya og Solen. Skr. Naturhist. Selsk. Kiob. 4, 34–51.
Fernando, S. A. (1978). Studies on the Biology of Barnacles (Crustacea: Cirripedia) of Porto-Novo region, South India. Ph. D. thesis. Chidambaram: Annamalai University.
Fernando, S. A. (2006). Monograph on Indian Barnacles. Kochi: Ocean Sciences and Technology Cell, Department of Marine Biology, Microbiology and Biochemistry, School of Marine Sciences, Cochin University of Science & Technology.
Frazier, J. (1989). Observations on stranded green turtles, Chelonia mydas, in the Gulf of Kutch. J. Bombay. Nat. Hist. Soc. 86, 250–252.
Gaonkar, C. A., and Anil, A. C. (2010). What do barnacle larvae feed on? Implications in biofouling ecology. J. Mar. Biol. Assoc. U.K. 90, 1241–1247. doi: 10.1017/s0025315409991238
Gaonkar, C. A., and Anil, A. C. (2012). Gut fluorescence analysis of barnacle larvae: an approach to quantify the ingested food. Estu. Coast. Shel. Sci. 111, 147–150. doi: 10.1016/j.ecss.2012.07.005
Gaonkar, C. A., and Anil, A. C. (2013a). Settlement and recruitment of the barnacle Balanus amphitrite from a tropical environment influenced by monsoons. J. Mar. Biol. Asso. U.K. 93, 1335–1349. doi: 10.1017/s0025315412001865
Gaonkar, C. A., and Anil, A. C. (2013b). Stable isotopic analysis of Barnacle larvae and their faecal pellets to evaluate the ingested food. J. Exp. Mar. Biol. Ecol. 441, 28–32. doi: 10.1016/j.jembe.2013.01.007
Gaonkar, C. A., Samiksha, S. V., George, G., Aboobacker, V. M., Vethamony, P., and Anil, A. C. (2012). Numerical simulations of barnacle larval dispersion coupled with field observations on larval abundance, settlement and recruitment in a tropical monsoon influenced coastal marine environment. J. Mar. Syst. 94, 218–231. doi: 10.1016/j.jmarsys.2011.12.002
González, S., Scarabino, F., Ortega, L., Martínez, A., Fabiano, G., Abreu, M., et al. (2014). Dosima fascicularis (Cirripedia: Lepadidae) in Uruguayan waters: the southernmost western Atlantic presence of the ‘blue goose barnacle’. Mar. Biod. Rec. 7, 1–8. doi: 10.1017/S1755267214000748
Gould, A. A. (1841). A Report on the Invertebrata of Massachusetts, Comprising the Mollusca, Crustacea, Annelida and Radiata. Cambridge: Folsom, Wells and Thurston.
Gray, J. E. (1825). A synopsis of the genera of Cirripedes arranged in natural families, with a description of some new species. Ann. Philos. 10, 97–107.
Gray, J. E. (1831). “Description of two new species of Serpula, and an undescribed coral barnacle discovered by Sammuel Pearce Pratt, Esq,” in The Zoological Miscellany. To be Continued Occasionally, ed. J. E. Gray (London: Treuttel, Wurtz and Co.), 1–86. doi: 10.5962/bhl.title.113722
Gruvel, A. (1902). Cirripedes. Expeditions Scientifiques du Travailleur et du Talisman Pendant les Annees 1880, 1881, 1882 and 1883, Vol. 7. Paris: G. Masson.
Gruvel, A. (1903). Revision des Cirrhipedes appartenant a la collection du Museum D’Histoire Naturelle. Nouv. Arc. Mus. d’Hist. Nat. Paris 4, 95–170.
Gruvel, A. (1905). Monographie des Cirrhipèdes ou Thécostracés. Paris: Libraires de l’Académie de Médecine.
Gruvel, A. (1906). Sur uneforme nouvelle de Cirrhipèdeoperculé (Pyrgopsis annandalei, n. g., n. sp.). Comptes Rendus Hebdomad. Acad. Sci. 142, 1558–1559. doi: 10.5962/bhl.part.13806
Gruvel, A. (1907). Cirrhipèdesoperculés de l’Indian Muséum de Calcutta. Mem. As. Soc. Bengal. 2, 1–10.
Hayashi, R. (2013). A checklist of turtle and whale barnacles (Cirripedia: Thoracica: Coronuloidea). Marine Biological Association of the United Kingdom. J. Mar. Biol. Assoc. U.K. 93, 143–182. doi: 10.1017/S0025315412000847
Henry, D. P., and McLaughlin, P. A. (1975). The barnacles of the Balanus amphirite complex (Cirripedia, Thoracica). Zoologische. Verhandelingen. 141, 1–254.
Hiro, F. (1939). Studies on the Cirripedian Fauna of Japan. IV. Cirripeds of Formosa (Taiwan), with some geographical and ecological remarks on the littoral forms. Mem. Coll. Sci. Koyoto Imper. Univ. Ser. B 15, 245–284.
Hoek, P. P. C. (1883). Report on the Cirripedia collected by HMS Challenger during the years 1873-1876. Rep. Sci. Results Explor. Voyag. HMS Challeng. Zool. 8, 1–169.
Hoek, P. P. C. (1907). The Cirripedia of the Siboga Expedition. A. Cirripedia Pedunculata. Siboga Expeditie Monogr. 31a, 1–127.
Hoek, P. P. C. (1913). The Cirripedia of the Siboga Expedition. B. Cirripedia Sessilia. Siboga Expeditie Monogr. 31b, 129–275.
Innocenti, G. (2006). Collections of the Natural History Museum, zoological section “La Specola” of the University of Florence. XXIII. Crustacea, Class Maxillopoda, Subclass Thecostraca, Infraclass Cirripedia. Atti. Della. Soc. Tosc. Sci. Nat. 113, 1–11.
Jones, D. S., and Hosie, A. M. (2016). A checklist of the barnacles (Cirripedia: Thoracica) of Singapore and neighbouring waters. Raff. Bull. Zool. 34, 241–311.
Karande, A. (1974). Development of Pedunculate barnacle Ibla cumingi Darwin. Ind. J. Mar. Sci. 3, 173–177.
Karande, A. A., and Gaonkar, S. N. (1977). Histology and histochemistry of cement glands of Balanus kondakovi. Proc. Ind. Acad. Sci. Sect. B 86, 409–416. doi: 10.1007/BF03051371
Karande, A. A., and Palekar, V. C. (1963). On a shore barnacle Chthamalus malayensis Pilsbry from Bombay, (India). Ann. Mag. Nat. Hist. Ser. 6, 231–234. doi: 10.1080/00222936308651346
Karande, A. A., and Palekar, V. C. (1966). The sessile Barnacles (Cirripedia) of the Bombay Coast. J. Bombay Nat. Hist. Soc. 63, 139–151.
Karuppaiyan, M., and Raja, K. (2007). New Records of Two Asteroids Calliaster children Gray, 1840 and Pentaceraster horridus (Gray, 1840) from the Bengal. Int. J. Zool. Res. 3, 227–230. doi: 10.3923/ijzr.2007.227.230
Karuppiah, S., Subramanian, A., and Obbard, J. P. (2004). The barnacle, Xenobalanus globicipitis (Cirripedia, Coronulidae), attached to the bottle-nosed dolphin, Tursiops truncatus (Mammalia, Cetacea) on the south-eastern coast of India. Crustaceana 77, 879–882. doi: 10.1163/156854004774248753
Khandeparker, L., and Anil, A. C. (2011). Role of conspecific cues and sugars in the settlement of cyprids of the barnacle, Balanus amphitrite. J. Zool. 284, 206–214. doi: 10.1111/j.1469-7998.2011.00794.x
Kim, H. K., Chan, B. K. K., Kang, C. B., Kim, H. W., and Kim, W. (2020a). How do whale barnacles live on their hosts? Functional morphology and mating-group sizes of Coronula diadema (Linnaeus, 1767) and Conchoderma auritum (Linnaeus, 1767) (Cirripedia: Thoracicalcarea). J. Crust. Biol. 40, 808–824. doi: 10.1093/jcbiol/ruaa075
Kim, H. K., Chan, B. K. K., Lee, S., and Kim, W. (2020b). Biogeography of intertidal and subtidal native and invasive barnacles in Korea in relation to oceanographic current ecoregions and global climatic changes. J. Mar. Biol. Ass. U.K. 100, 1079–1091. doi: 10.1017/S0025315420001009
Kolbasov, G. A. (1993). Revision of the genus Acasta Leach (Cirripedia: Balanoidea). Zool. J. Linn. Soc. 109, 395–427. doi: 10.1111/j.1096-3642.1993.tb00307.x
Kottarathil, H. A., Sahadevan, A. V., Kattamballi, R., and Kappalli, S. (2019). Norileca indica (Crustacea: Isopoda, Cymothoidae) infects Rastrelliger kanagurta along the Malabar Coast of India - seasonal variation in the prevalence and aspects of host-parasite interactions. Zool. Stud. 58, 35.
Krishnamoorthy, P. (2007). “Cirripedes,” in Fauna of Chennai Coast, eds K. Venkataraman, J. T. Jothinayagam, and P. Krishnamoorthy (Kolkata: Zoological Survey of India), 57–60.
Kumar, S. V., Pathak, K. C., Pednekar, P., Raju, N. S. N., and Gowthaman, R. (2006). Coastal processes along the Indian coastline. Curr. Sci. 91, 530–536.
Kumaravel, K., Ravichandran, S., and Rameshkumar, G. (2009). Distribution of barnacle Octolasmis on the gill region of some edible crabs. Acad. J. Ent. 2, 36–39.
Lamarck, J. B. P. A. (1818). Histoire Naturelle des Animaux Sans Vertèbres, Vol. 5. Deterville: Paris.
Lanchester, W. F. (1902). On the Crustacea collected during the Skeat Expedition to the Malay Peninsula. Proc. Zool. Soc. Lond. 2, 363–381.
Lazarus, R. J., Reddy, S. R. P., and Thangavelu, R. (1987). Epizoic fauna on the flathead locust lobster, Thenusorientalis (Lund). Ind. J. Fish. 34, 464–466.
Leach, W. E. (1817). Distribution, systématique de la class Cirripèdes: par la même. J. Phys. Chim. Hist. Nat. Paris 85, 67–69.
Leach, W. E. (1818). “Cirripedes,” in Supplement to the Fourth, Fifth, and Sixth Editions of the Encyclopaedia Britannica. With Preliminary Dissertations on the History of the Sciences, Vol. 3, ed. M. Napier (Edinburgh: Archibald Constable), 168–171. doi: 10.1017/s0016756800195238
Leach, W. E. (1825). A tabular view of the genera composing the Class Cirripedes, with descriptions of the species of Otion, Cineras and Clyptra. Zool. J. 2, 208–215.
Lilljeborg, W. (1861). Supplément au mémoire sur les genres Liriope et Peltogaster, H. Rathke. Nov. Acta. Reg. Soc. Sci. Ups. Seriei. Tertiae 3, 74–102.
Linnaeus, C. (1758). Systema Naturae Per Regna tria Naturae, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis, Vol. 1. Stockholm: Laurentii Salvii.
Linnaeus, C. (1767). Systema Naturae: Per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis, 12th Edn, Vol. 1. Stockholm: Laurentii Salvii.
MacDonald, J. D. (1869). On an apparently new genus of minute parasitic cirripede between Lepas and Dichelaspis. Proc. Zool. Soc. Lond. 1869, 440–444. doi: 10.1111/j.1469-7998.1869.tb07353.x
Macpherson, E., Chan, T.-Y., Kumar, A. B., and Rodríguez-Flores, P. C. (2020). On some squat lobsters from India (Decapoda, Anomura, Munididae), with description of a new species of Paramunida Baba, 1988. ZooKeys 965, 17–36. doi: 10.3897/zookeys.965.55213
Malakar, B., Venu, S., Ram, B. S., and Prabhakaran, M. P. (2015). A study on the biodiversity of snake island in South Andaman. J. Biodiver. Environ. Sci. 6, 205–214.
Mishra, J. K., Patro, S., Adhavan, D., and Mishra, A. (2010). Biodiversity of rock pool organisms and their adaptive zonation along the coast of port blair. J. Coast. Environ. 1, 159–167.
Mitra, S., and Misra, A. (2006). A report on intertidal macrofauna of Talssari (Balasore: Orissa). Rec. Zool. Surv. India 106, 131–141.
Mitra, S., Misra, A., and Pattanayak, J. G. (2010). Intertidal macrofauna of Subarnarekha Estuary (Balasore: Orissa). Rec. Zool. Surv. India. Occ. Paper 313, 1–135.
Mol, V. P. L., Raveendran, T. V., Abilash, K. R., and Parameswaran, P. S. (2010). Inhibitory effect of Indian sponge extracts on bacterial strains and larval settlement of the barnacle, Balanus amphitrite. Int. Biodeter. Biodegrad. 64, 506–510. doi: 10.1016/j.ibiod.2010.06.003
Mondal, S., Maitra, A., Bose, K., Goswami, P., Bardhan, S., and Mallick, S. (2019). Substrate-controlled naticid gastropod drilling predation on recent barnacles from Chandipur, India and its Palaeontological implications. Hist. Biol. doi: 10.1080/08912963.2019.1677639
Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B., and Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858. doi: 10.1038/35002501
Namboothri, N., and Fernando, S. A. (2012). “Intertidal distribution of the coral-boring barnacle Lithotrya nicobarica Reinhardt, 1850 in the Great Nicobar Island,” in Ecology of Faunal Communities on the Andaman and Nicobar Islands, eds K. Venkataraman, C. Raghunathan, and C. Sivaperuman (Berlin: Springer), 49–57. doi: 10.1007/978-3-642-28335-2_3
Nandakumar, K. (1990). Studies on Energy Content of Fouling Organisms with Special Reference to Sessile Barnacles. Ph.D. thesis. Goa: Goa University.
Nayak, P. K. (2017). Fisher communities in transition: understanding change from a livelihood perspective in Chilika Lagoon, India. Marit. Stud. 16, 1–33. doi: 10.3233/jcc-160001
Nayak, P. K., and Berkes, F. (2014). Linking global drivers with local and regional change: a social-ecological system approach in Chilika Lagoon, Bay of Bengal. Reg. Environ. Chang. 14, 2067–2078. doi: 10.1007/s10113-012-0369-3
Newman, W. A., and Abbott, D. P. (1980). “Cirripedia: the barnacles,” in Intertidal Invertebrates of California, Vol. 200, eds R. H. Morris, D. P. Abbott, and E. C. Haderlie (Stanford: Stanford University Press), 504–535.
Newman, W. A., and Killingley, J. S. (1984). The north-east Pacific intertidal barnacle Pollicipes polymerus in India? A biogeographical enigma elucidated by 18O fractionation in barnacle calcite. J. Nat. Hist. 19, 1191–1196. doi: 10.1080/00222938500770741
Newman, W. A., and Ross, A. (1976). Revision of the balanomorph barnacles; including a catalogue of the species. Mem. San. Diego. Soc. Nat. Hist. 9, 1–108.
Nilsson-Cantell, C. A. (1921). Cirripedien Studien. Zur Kenntnis der Biologie, Anatomie und Systematik dieser Gruppe. Zool. Bid. Uppsala 7, 75–390. doi: 10.21248/contrib.entomol.56.1.75-103
Nilsson-Cantell, C. A. (1929). Cirripedien des Genus Verruca der Deutschen Tiefsee- Expedition auf dem Dampfer “Valdiva” 1898-1899. Zool. Jahrb. Abt. Syst. Okol. Geog. Tier. 58, 459–480.
Nilsson-Cantell, C. A. (1938). Cirripedes from the Indian ocean in the collection of the Indian Museum, Calcutta. Mem. Ind. Mus. 14, 1–81. doi: 10.5962/bhl.part.10537
Owen, R. (1830). Catalogue of the contents of the Museum of the Royal College of Surgeons Part IV. Fasciculus I. Comprehending the first division of the preparations of Natural History in spirit. Catal. Mus. Royl. Coll. Surg. Invertebr. 1, 1–14.
Padate, V. P., Baragi, L. V., and Rivonker, C. U. (2009). Biological aspects of sea snakes caught incidentally by commercial trawlers off Goa, west coast of India. J. Thr. Taxa 23, 609–616. doi: 10.11609/JoTT.o2253.609-16
Padate, V. P., Cubelio, S. S., and Jayachandran, K. V. (2020). Description of a new species of deep-water crab of the genus Homolodromia A. Milne-Edwards, 1880 from the northern Indian Ocean (Crustacea: Decapoda: Brachyura: Homolodromiidae). Mar. Biol. Res. 16, 1–10. doi: 10.1080/17451000.2020.1735641
Pannacciulli, F. G., Bishop, J. D. D., and Hawkins, S. J. (1997). Genetic structure of populations of two species of Chthamalus (Crustacea: Cirripedia) in the north-east Atlantic and mediterranean. Mar. Biol. 128, 73–82. doi: 10.1007/s002270050070
Parmar, H. H., Joshi, D. M., Salvi, H., and Kamboj, R. D. (2018). Diversity and distribution of Cirripedia from Gujarat Coast, India. IJRBS 5, 25–29. doi: 10.26438/ijsrbs/v5i5.2529
Pati, S. K., Mahapatro, D., Singhsamant, B., and Panigrahy, R. C. (2009). Intertidal benthic fauna of Gopalpur, east coast of India. Flora Fauna 15, 310–316.
Patil, J., and Anil, A. C. (2005). Influence of diatom exopolymers and biofilms on metamorphosis in the barnacle Balanus amphitrite. Mar. Ecol. Prog. Ser. 301, 231–245. doi: 10.3354/meps301231
Patro, S. (2012). Studies on larval Settlement Process of the Barnacle, Balanus amphitrite and their Inhibition Mechanism. Ph. D Thesis. Andaman and Nicobar Islands: Pondicherry University.
Perreault, R. T. (2016). Acasta sulcata akanthosa, new replacement name for Acasta sulcata spinosa. Zootaxa. 4169:374.
Pillai, N. K. (1958). Development of Balanus amphirite, with a note on the early larvae of Chelonibia testudinaria. Bull. Cen. Res. Inst. Kerala 6, 117–130.
Pilsbry, H. A. (1907). The barnacles (Cirripedia): contained in the collections of the US National museum (No. 60). Bull. U.S. Natl. Mus. 60, 1–122. doi: 10.5479/si.03629236.93.1
Pilsbry, H. A. (1910). Stomatolepas, a barnacle commensal in the throat of the loggerhead turtle. Am. Nat. 44, 304–306. doi: 10.1086/279142
Pilsbry, H. A. (1912). Diagnoses of new barnacles from the Philippine Archipelago and China Sea. Proc. U.S. Natl. Mus. 42, 291–294. doi: 10.5479/si.00963801.1904.291
Pilsbry, H. A. (1916). The sessile barnacles (Cirripedia) contained in the United States National Museum; including a monograph of the American species. Bull. U.S. Natl. Mus. 93, 1–366.
Pitombo, F. B. (2003). Phylogenetic analysis of the Balanidae (Cirripedia, Balanomorpha). Zool. Scr. 33, 261–276. doi: 10.1111/j.0300-3256.2004.00145.x
Poli, G. S. (1791). Testacea Utriusque Siciliae eorumque historia et anatome tabulis aeneis illustrata, Vol. 1. Parmae: Ex Regio Typographeio, 1–73.
Poli, G. S. (1795). Testacea Utriusque Siciliae eorumque historia et anatome Tabulis Aeneis illustrata, Vol. 2. Parmae: Ex Regio Typographeio, 75–264.
Potemra, J. T., Luther, M. E., and O’Brien, J. J. (1991). The seasonal circulation of the upper ocean in the Bay of Bengal. J. Geophy. Res. 96, 12667–12683. doi: 10.1029/91jc01045
Pradeep, H. D., Shirke, S. S., and Devi, S. M. (2016). Report of epizootic cirripede, Conchoderma virgatum (Spengler, 1790) on Pennella instructa (Wilson, 1917) parasitic on Indo-Pacific sailfish Istiophorus platypterus caught from Andaman Sea. J. Ent. Zool. Stud. 4, 1208–1210.
Prasanth, R. M., and Sureshkumar, P. (2020). Species composition and distribution of marine foulers along the southeast coast of Tamilnadu, India. Proc. Int. Acad. Ecol. Env. Sci. 10, 1–7.
Prem-Kumar, V. K., and Daniel, A. (1968). A new species of operculate barnacle of the subgenus Membranobalanus (Cirripedia, Thoracica) from sponges in the Indian seas. Crustaceana 14, 147–150. doi: 10.1163/156854068x00520
Raj, P. J. S., Tilak, J. L., and Kalaimani, G. (2002). Experiments in restoration of benthic biodiversity in Pulicat Lake, south India. J. Mar. Biol. Ass. India 44, 37–45.
Rajaguru, A., and Shantha, G. (1992). Association between the sessile barnacle Xenobalanus globicipitis (Coronulidae) and the bottlenose dolphin Tursiops truncatus (Delphinidae) from the Bay of Bengal, India, with a summary of previous records from cetaceans. Fish. Bull. 90, 197–202.
Ramakrishna, S. J., and Talukdar, S. (2003). Marine invertebrates of Digha coast and some recommendations on their conservation. Rec. Zool. Surv. India 101, 1–23.
Ramamoorthy, K., Sankar, G., and Sakkaravarthi, K. (2012). Assessment of reef associated biota in the Pirotan Island, Gulf of Kachchh, Gujarat, India. Eur. J. Exp. Biol. 3, 551–561.
Ranzani, C. (1817-1818). Osservazioni su i Balanidi. Opusc. Sci. 3, 63–93. pp. 195-202; II (1817), pp. 269-276; III (1818).
Rao, K. S., and Balaji, M. (1988). “Biological fouling at port Kakinada, Godavari estuary, India,” in Marine Biodeterioration— Advanced Technique Applicable to the Indian Ocean, eds M. F. Thompson, R. Sarojini, and R. Nagabhushanam (New Delhi: Oxford & IBH publishing Ltd), 551–574.
Reinhardt, J. T. (1850). Om Slaegten Lithotryas Evne til at bore sig ind Steenblokke. Vidensk. Meddel. Naturhist. Foren. Kjøbenhavn. 1, 1–8.
Rosell, N. C. (1991). Crustacea Cirripedia Thoracica: MUSORSTOM 3 Philippines collection. Mém. Mus. Nat. Hist. Nat. 9, 9–61.
Ross, A. (1971). Studies on the Tetraclitidae (Cirripedia: Thoracica). A new tetraclitellan from India. Trans. San. Diego. Soc. Nat. Hist. 16, 215–224. doi: 10.5962/bhl.part.15459
Roy, M. D., and Rath, S. (2017). An inventory of crustacean fauna from Odisha Coast, India. J. Env. Soc. 14, 49–112.
Sahadevan, P. (2016). Diversity of fishes, crustaceans and molluscs of Puthuvypeen of Ernakulam District, Kerala, South India. Int. J. Fish. Aqua. Stud. 4, 101–107.
Sanjeeva Raj, P. J. (2006). Macro Fauna of Pulicat Lake, NBA Bulletin No. 6. Chennai: National Biodiversity Authority.
Satheesh, S., and Wesley, S. G. (2009). Breeding biology of the barnacle Amphibalanus amphitrite (Crustacea: Cirripedia): influence of environmental factors in a tropical coast. J. Mar. Biol. Assoc. U.K. 89, 1203–1208. doi: 10.1017/S0025315409000228
Satpathy, K. K., Mohanty, A. K., Sahu, G., Biswas, S., Prasad, M. V. R., and Slvanayagam, M. (2010). “Biofouling and its control in seawater cooled power plant cooling water system-a review,” in Nuclear Power, ed. V. T. Pavel (Croatia: Intech), 191–242.
Schumacher, C. F. (1817). Essai d’un nouveau Système des Habitations des vers TESTACÉS; avec XXII Planches. Copenhagen: Schultz.
Shahdadi, A., Chan, B. K. K., and Sari, A. (2011). Tetraclita ehsani sp. n. (Cirripedia, Tetraclitidae), a common intertidal barnacle from the Gulf of Oman, Iran. ZooKeys 136, 1–12. doi: 10.3897/zookeys.136.1772
Shahdadi, A., and Sari, A. (2011). Chthamalid barnacles (Cirripedia: Thoracica) of the Persian Gulf and Gulf of Oman, Iran. J. Mar. Biol. Asso. U.K. 91, 745–753. doi: 10.1017/s0025315410001803
Shih, H. T., Ng, P. K. L., Ravichandran, S., and Prema, M. (2019). Resurrection of Gelasimus variegatus Heller, 1862, a fiddler crab closely related to Austruca bengali (Crane, 1975) and A. triangularis (A. Milne-Edwards, 1873) (Decapoda, Brachyura, Ocypodidae), from the Bay of Bengal, Indian Ocean. Zool. Stud. 58:12.
Singh, H. S., Pandey, C. N., Yennawar, P., Asari, R. J., Patel, B. H., Tatu, K., et al. (2004). The Marine Natinal Park and Sanctuary in the Gulf of Kachchh (A Comprehensive Study on biodiversity and management issues). Gandhinagar: GEER Foundation.
Southward, A. J. (2008). Barnacles. Keys and Notes for the Identification of British Species, Synopses of the British Fauna. Shrewsbury: Linnean Society of London and the Estuarine and Coastal Sciences Association, Field Studies Council.
Southward, A. J., and Newman, W. A. (2003). A review of some common Indo-Malayan and western Pacific species of Chthamalus barnacles (Crustacea: Cirripedia). J. Mar. Biol. Asso. U.K. 83, 797–812. doi: 10.1017/S0025315403007835h
Sowerby, G. B. (1823). The Genera of Recent and Fossil Shells, for the Use of Students in Conchology and Geology, with Original Plates by James Sowerby (nos. i-xvii), Continued by J. de C. Sowerby (nos. xviii-xlii). London: GB Sowerby.
Sowerby, G. B. (1833). Pollicipes ruber and polymerus n. sp. Proc. Zool. Soc. Lond. 1, 1–74. doi: 10.1007/978-3-319-23534-9_1
Spalding, M. D., Fox, H. E., Allen, G. R., Davidson, N., Ferdaña, Z. A., Finlayson, M., et al. (2007). Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience 57, 573–583. doi: 10.1641/b570707
Spengler, L. (1780). Beschreibung zweier neuev Gattung Meereicheln (Lepades) nebst der Islandischen Kammuschel (Ostrea islandica) mit Abbildungen. Schr. Berlin Ges. Naturf. Foren. 1, 101–111.
Spengler, L. (1790). Beskrivelse og Oplysing over den hidindtil lidet udarbeide Slaegt af mangeskallede Konchylier, som Linnaes har daldet Lepas, med tilfoiede nye og ubeskrevne Arter. (Om. Conchylie-Slaegten Lepas). Skr. Naturhist. Selsk. 1, 158–212.
Steenstrup, J. (1852). Om Xenobalanus globicipitis, en ny Cirriped-Slaegt af Coronula familien. Vidensk. Medd. Naturhist. Foren 1852, 62–64.
Sudakaran, E., and Fernando, S. A. (1987). Studies on Octolasmis Gray, 1825 (Cirripedia: Pedunculata) the gill infesting barnacles of crabs of Porto Novo. Mar. Biol. Assoc. India. 29, 201–207.
Sundararaj, B. (1927). The littoral fauna of the Krusadai Islands in the Gulf of Mannar. Bull. Madras. Govt. Mus. 1, 12–128.
Swami, B. S., Udhayakumar, M., and Gaonkar, S. N. (2011). Biodiversity in fouling species at Karanja Jetty (Mumbai), west coast of India. J. Mar. Biol. Ass. India. 53, 242–250.
Tarasov, N. I., and Zevina, G. B. (1957). Cirripedia. Fauna SSSR. Zool. Inst. Akad. Nauk. SSSR 69, 1–268.
Thompson, J. V. (1836). Natural history and metamorphosis of an anomalous crustaceous parasite of Carcinus maenas, the Sacculina carcini. Entomol. Mag. 3, 452–456.
Trivedi, D. J., Trivedi, J. N., Soni, G. M., Purohit, B. D., and Vachhrajani, K. D. (2015). Crustacean fauna of Gujarat state of India: a review. Int. J. Env. Sci. 8, 23–31.
Trivedi, J. N., Doshi, M., Patel, K. J., and Chan, B. K. K. (2021). Diversity of intertidal epibiotic and fouling barnacles (Cerripedia: Thoracica) from Gujarat, Northwest India. Zookeys 1026, 143–178. doi: 10.3897/zookeys.1026.60733
Trivedi, J. N., Trivedi, D. J., Vachhrajani, K. D., and Ng, P. K. (2018). An annotated checklist of the marine brachyuran crabs (Crustacea: Decapoda: Brachyura) of India. Zootaxa 4502, 1–83. doi: 10.11646/zootaxa.4502.1.1
Tsang, L. M., Achituv, Y., Chu, K. H., and Chan, B. K. K. (2012). Zoogeography of intertidal communities in the West Indian Ocean as determined by ocean circulation systems: patterns from the Tetraclita barnacles. PLoS One 7:e45120. doi: 10.1371/journal.pone.0045120
Tsang, L. M., Chan, B. K. K., Ma, K. Y., Hsu, C. H., and Chu, K. H. (2007). Lack of mtDNA and morphological differentiation between two acorn barnacle Tetraclita japonica and T. formosana differing in parietes colours and geographical distribution. Mar. Biol. 151, 147–155. doi: 10.1007/s00227-006-0460-8
Ubare, V. V., and Mohan, P. M. (2018). New records and range extensions of some marine sponges (Porifera: Demospongiae and Homoscleromorpha) from the Andaman Islands, India; part of the Indo-Burma biodiversity hotspot. Zool. Stud. 57:3. doi: 10.6620/ZS.2018.57-03
Utinomi, H. (1958). A new stalked cirriped (Pisiscalpellum withersi n. gen. et n. sp.) from Sagami Bay and a discussion on its phylogeny. Odokon 12, 113–122.
Utinomi, H. (1967). Comments on some new and already known cirripeds with emended taxa, with special reference to the parietal structure. Publ. Seto. Mar. Biol. Lab. 15, 199–237. doi: 10.5134/175466
Van Syoc, R. J., Fernandes, J. N., Carrison, D. A., and Grosberg, R. K. (2010). Molecular phylogenetics and biogeography of Pollicipes (Crustacea: Cirripedia), a Tethyan relict. J. Exp. Mar. Biol. Ecol. 392, 193–199. doi: 10.1016/j.jembe.2010.04.024
Van Syoc, R. J., and Newman, W. A. (2010). Morphology and evolutionary ecology of a sponge-barnacle symbiosis: four new genera of barnacles (Archaeobalanidae, Bryozobiinae). J. Exp. Mar. Biol. Ecol. 392, 65–88. doi: 10.1016/j.jembe.2010.04.011
Venkataraman, K., and Raghunathan, C. (2015). “Coastal and marine biodiversity of India,” in Marine Faunal Diversity in India, eds K. Venkataraman and C. Sivaperuman (Cambridge, MA: Academic Press), 303–348. doi: 10.1016/B978-0-12-801948-1.00019-7
Venkataraman, K., and Wafar, M. (2005). Coastal and marine biodiversity of India. Ind. J. Mar. Sci. 34, 57–75.
Venkateswaran, K., and Fernando, S. A. (1982). Distribution and variation in form of the epizoic cirriped, Octolasmis cor (Aurivillius, 1892). Ind. J. Mar. Sci. 11, 243–246.
Wagh, A. B., and Bal, D. V. (1969). New records of intertidal barnacles from India. Cur. Sci. 38, 344–345.
Wagh, A. B., and Bal, D. V. (1974). Observation on systematics of sessile barnacles from West Coast of India. J. Bombay Nat. Hist. Soc. 71, 109–123.
Wagh, A. B., and Sawant, S. S. (1982). Observations on marine biofouling on electroplated metallic surfaces in Goa waters. Mahasagar 15, 183–188.
Watanabe, H. K., Chen, C., Marie, D. P., Takai, K., Fujikura, K., and Chan, B. K. K. (2018). Phylogeography of hydrothermal vent stalked barnacles: a new species fills a gap in the Indian Ocean ‘dispersal corridor’ hypothesis. R. Soc. Open. Sci. 5:172408. doi: 10.1098/rsos.172408
Weltner, W. (1894). Zwei neu Cirripedien aus dem Indischen Ozean (Scalpellum, Megalasma). Berlin: Sitzungber. Ges Naturf. Freunde, 80–87.
Withers, T. H. (1935). Catalogue of Fossil Cirripedia in the Department of Geology: British Museum (Natural History): Cretaceous. London: British Museum.
Wood, W. (1815). General Conchology; or a Description of Shells Arranged According to the Linnean System. London: John Booth.
Zevina, G. B. (1978). A new classification of the family Scalpellidae Pilsbry (Cirripedia, Thoracica). 1. Subfamilies Lithotryinae, Calanticinae, Pollicipinae, Scalpellinae, Brochiinae and Scalpellopsinae. Zool. Zhurnal. 57, 998–1007.
Keywords: India, checklist, cirripedia, barnacles, biodiversity
Citation: Trivedi J, Patel K, Chan BKK, Doshi M and Padate V (2021) Diversity of Indian Barnacles in Marine Provinces and Ecoregions of the Indian Ocean. Front. Mar. Sci. 8:657651. doi: 10.3389/fmars.2021.657651
Received: 23 January 2021; Accepted: 06 April 2021;
Published: 16 June 2021.
Edited by:
Mandar Nanajkar, National Institute of Oceanography, Council of Scientific and Industrial Research, IndiaCopyright © 2021 Trivedi, Patel, Chan, Doshi and Padate. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benny K. K. Chan, Y2hhbmtrQGdhdGUuc2luaWNhLmVkdS50dw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.