
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Mar. Sci. , 19 July 2021
Sec. Coral Reef Research
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.657429
This article is part of the Research Topic Stony Coral Tissue Loss Disease in the Caribbean View all 50 articles
 Karen L. Neely1*
Karen L. Neely1* Cynthia L. Lewis2*
Cynthia L. Lewis2* Keri O’Neil3
Keri O’Neil3 Cheryl M. Woodley4
Cheryl M. Woodley4 Jennifer Moore5
Jennifer Moore5 Zach Ransom6
Zach Ransom6 Amelia Moura7
Amelia Moura7 Ken Nedimyer8
Ken Nedimyer8 David Vaughan9
David Vaughan9As stony coral tissue loss disease (SCTLD) swept through the Florida Reef Tract, one of the most severely impacted species was the iconic pillar coral, Dendrogyra cylindrus. As the species’ population experienced a precipitous decline, a collaborative rescue project collected colony fragments for safekeeping at onshore and offshore nursery facilities. Between 2015 and 2019, a total of 574 fragments representing 128 genotypes were collected. These are currently dispersed among five facilities where they continue to provide opportunities to (1) refine best husbandry practices for D. cylindrus, (2) develop treatment options for SCTLD that have been adapted for use on other species, (3) maximize growth and fragmentation to provide the live tissue needed for eventual restoration, and (4) provide a source of parent colonies for assisted sexual reproduction and new genetic diversity. With the wild pillar coral population in Florida at the end of 2020 at less than 6% of its known 2014 population and continuing to decline, the rescued ex situ colonies represent the entirety of the restoration potential for this species in Florida.
The pillar coral Dendrogyra cylindrus is an uncommon but conspicuous scleractinian coral species throughout the Caribbean. It is unique in its taxonomy as the only species of its genus, unique in its structure as the only columnar coral in the Atlantic, and unique in its behavior as a species with tentacles extended both day and night. The population in Florida is genetically distinct from other Caribbean regions (Chan et al., 2019), and the dispersed nature of the colonies has likely rendered it reproductively extinct for decades (Neely et al., 2021).
Beginning in 2013–2014, baseline surveys of all known D. cylindrus colonies on the Florida Reef Tract identified chronic white plague as a substantial ongoing stressor to the population (Neely et al., 2021). Back-to-back thermal bleaching events in 2014 and 2015 further negatively impacted the population (Lewis et al., 2019; Neely et al., 2021). Recognizing that the population was on a negative trajectory and unlikely to be capable of unassisted recovery, a small-scale D. cylindrus rescue program was initiated to bring in fragments of colonies to onshore nurseries for protection from thermal extremes and ongoing disease.
Less than a year later, the impact of a newly emerging threat termed stony coral tissue loss disease (SCTLD) became apparent. Originating near Miami in 2015, the disease began rapidly spreading throughout the Florida Reef Tract (Precht et al., 2016) affecting over 20 stony coral species. One of the hardest hit species was D. cylindrus, which suffered catastrophic losses as SCTLD moved through the region (Neely et al., 2021). As a result of this new stressor, which has threatened the D. cylindrus population in Florida with regional extinction, rescue efforts were scaled up by collecting fragments of as many remaining genotypes as possible.
As the threat of SCTLD to the Florida D. cylindrus population became more apparent, the urgency and methodology of collections changed. Initial collections were exclusively of unattached pillars that had been separated from their parent colonies by storms, boat damage, or other physical impacts. These collections were opportunistic, and federal permitting for removal of these fragments was predicated on observations that most such broken fragments perished rather than cemented to the substrate for regrowth (K Neely and C Lewis, pers obs). However, as disease prevalence increased from 2016 onward, collections became more targeted with the goal of systematically collecting fragments from as many genotypes as possible by allowing for the removal of fragments still attached to the skeleton (Figure 1a). The following categorical guidelines helped to direct the collections of each genotype:
(1) Prioritize the collection of fragments separated from other areas of live tissue by a region of dead skeleton. If possible, avoid cutting into live tissue so as not to create new potential entry points for infection.
(2) Within a genotype, prioritize collection from a colony that does not show signs of disease. Genotypes were distinguished by either previous genetic sampling (Chan et al., 2019), or by distance from other colonies (Neely et al., 2021).
(3) If only diseased colonies are available within a genotype, prioritize tissue isolates that do not show signs of disease.
(4) As a final option, harvest any representative fragment from the parent colony in order to preserve the genotype.

Figure 1. Collection, preparation, and care of Dendrogyra cylindrus rescue fragments. Fragments were collected from the reef using hammer and chisel (a). Excess skeleton was trimmed (b) and fragments were mounted on tiles (c). Early fragments in offshore nurseries were suspended from trees (d), but all offshore holding eventually moved to mounted fragments (e). Onshore fragments were held in a variety of aquarium systems (f). Development of gemmae on rescue fragments appeared as an attached growth of soft tissue (g) with internal skeletal fragments (h).
The type of fragments collected depended strongly on geographic region and timeframe. Collections from colonies that could be visited before the arrival of the progressing SCTLD front to an area were generally from the first category. Collections from colonies that were within areas affected by SCTLD fell into the latter categories. In many cases, rescue efforts occurred just in time to collect the very last fragments of tissue that were actively dying of SCTLD.
Collections occurred on most known colonies within the Florida Keys National Marine Sanctuary and Dry Tortugas National Park. Some collections were done in Biscayne National Park and the Southeast Florida region north of Biscayne, but many of these colonies perished before collections began and were not able to be saved (Figure 2). In total, 574 fragments representing 128 genotypes were collected and transported to holding facilities. When possible, clonal fragments were dispersed among multiple facilities to spread the risk of genotype loss.
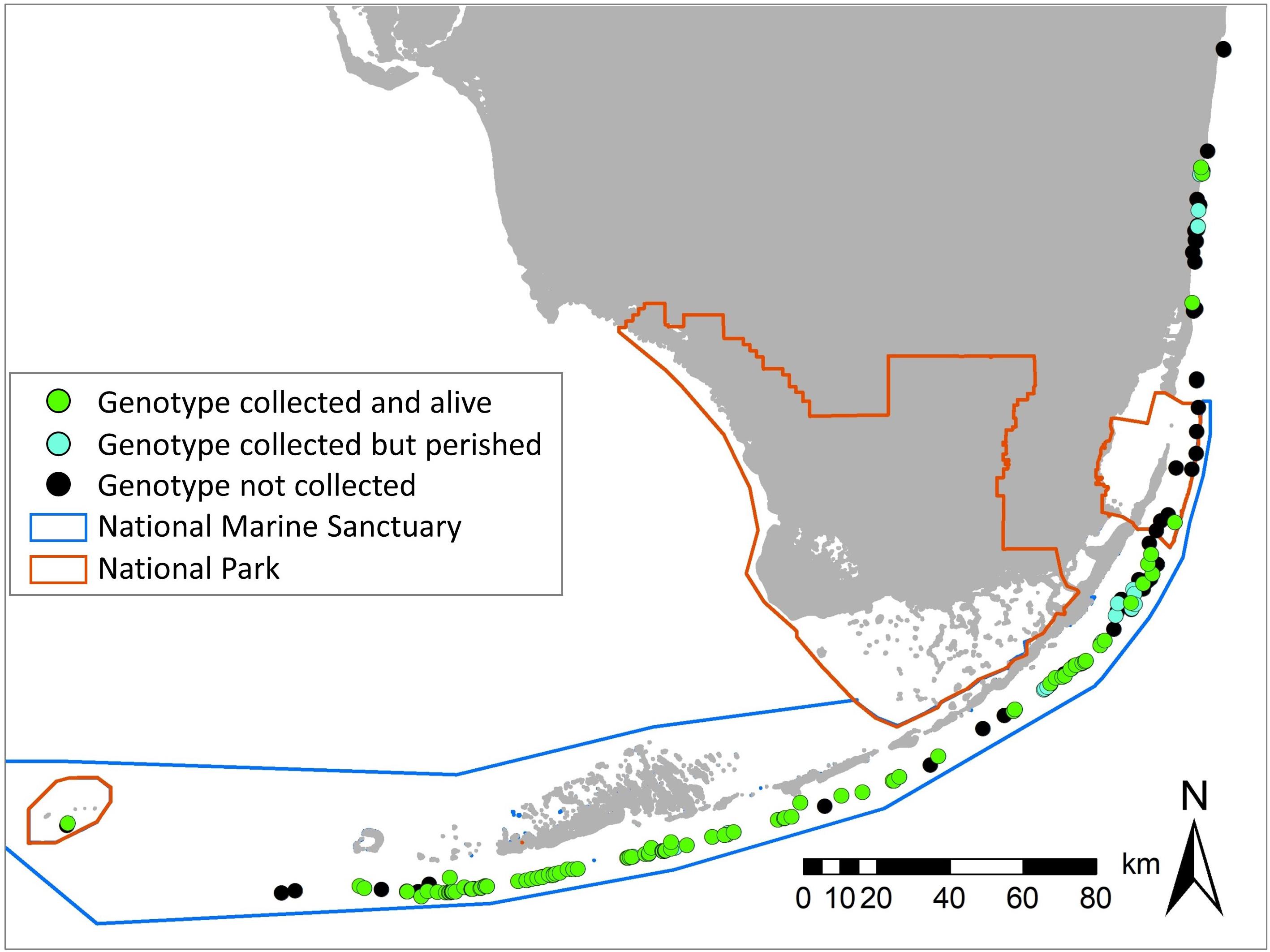
Figure 2. Location of all known Dendrogyra cylindrus colonies on the Florida Reef Tract (black), overlaid with the genotypes collected. Most collected genotypes have survived and are held in rescue facilities (green); a few were collected but perished (blue).
Prior to these rescue efforts, D. cylindrus had never been cultured in aquarium facilities, and partners overcame many challenges to learn husbandry best practices. Facilities ranged from in-water nurseries to outdoor greenhouses to indoor closed-recirculating and lighted systems (Figures 1e,f). Water sources included artificial seawater, saltwater wells, and locally sourced seawater. Regardless of the holding location, the species proved to be more particular about environmental conditions than many other coral species. Ultimately, high light levels and high water flow were deemed essential for D. cylindrus survival and growth. Comparative experiments were not conducted, but successful growth was achieved with filtration rates at 5–10 times the system volume per hour as well as in-water pumps creating flow rates 15–55 times the system volume per hour. Improved growth was noted under light levels greater than 250 μmol m–2 s–1 and up to at least 400 μmol m–2 s–1, although some genotypes were prone to bleaching under these upper limits. Fragments at indoor facilities seemed to also respond well to a red-shift in spectrum compared to other coral species.
It took time to learn the proper care for the individuals, and some setbacks included early tissue recession in suboptimal conditions, an outbreak of “brown jelly syndrome” in one tank, and the loss of corals in two locations due to facility damage and extended loss of power as a result of Hurricane Irma. Survival (through 2020) of the fragments collected in 2016 was only 25%. Nevertheless, general husbandry and disease management improved rapidly, and corals collected in 2018–2019 had survival rates through 2020 of over 92%, a value which is particularly impressive considering the active SCTLD infections that many of these later acquisitions were experiencing at the time of collection.
Most collected fragments were maintained in their original orientation and size, though trimming of excess skeleton was adopted as standard practice (Figure 1b). A few fragments in offshore nurseries were hung on in-water “trees” using monofilament line (Figure 1d) as is commonly done for branching corals. These showed little growth, and all were subsequently moved onto flat surfaces mounted to the trees (Figure 1e). Although slower growing than most other massive coral species, the potential for in-water nursery propagation is promising, but requires more research and development to customize and optimize methods for this unique species.
In land-based facilities, all corals were mounted on tiles or molded cement bases (Figure 1c) to elevate them from the aquarium floor, facilitate care and cleaning, and provide a substrate for growth. In some cases, collected isolates were fragmented to facilitate care or to attempt growth stimulation. Results of such fragmentation experiments are preliminary but show promise that fragmented pieces can survive and start growing new tissue.
Fragments with active or potential SCTLD were sent primarily to the Coral Disease Health Program’s facility in Charleston, SC. Various methods of treatment were trialed; these included traditional water changes and antiseptic dips, but also novel antibiotic water dosing which proved highly effective at halting active disease lesions (Miller et al., 2020). The facility subsequently developed a topical antibiotic-infused paste that could be applied directly to SCTLD lesions in aquarium settings to arrest tissue loss and prevent mortality. These successes prompted the wider-scale development of a topical antibiotic paste that could be applied to SCTLD-infected corals in the wild (Neely et al., 2020b). In partnership with CoreRx/Ocean Alchemists, this product is now being used on over 20 coral species throughout the affected regions of the Caribbean to save SCTLD-affected colonies.
The D. cylindrus colonies held at genetic rescue facilities continue to grow and create new material for eventual restoration needs. Currently (November 2020), 505 live fragments representing 128 genotypes are being cared for. The estimated amount of D. cylindrus tissue within the rescue program exceeds 124 square meters. Most colonies have demonstrated appreciable growth once they are stabilized and ideal conditions are met, and new fragments have been created through intentional fragmentation as well as the development of gemmae [for the initial description of gemmae as well as additional imagery from other species, please see Weil (2000)]. A gemma first appears as a mass of soft tissue on a rapidly growing colony (Figure 1g). Within the tissue mass, skeletal structures (separate from the main colony skeleton) develop and weigh down the gemma (Figure 1h). As the gemma grows, it pinches off and separates from the soft tissue of the parent colony, thus creating an asexual bud. Using a small bead of superglue on the protruding septa, gemmae can be attached to substrate tiles where they begin laying down skeleton and developing into new fragments. Such asexual reproduction is known from a variety of coral species held ex situ, but is rare although documented on D. cylindrus and other species in the wild (Weil, 2000).
Of the genotypes held as part of the Dendrogyra rescue project, only 2% of them remain healthy in the wild as of this writing (end of 2020). Eighty-three percent are already dead, and the remaining 15% are currently SCTLD-infected or reduced to less than 2% of their 2013 baseline population (Neely et al., 2021). Any hopes for maintaining the existing genetic diversity of the Florida population and providing the material for future restoration of the species rests heavily on the corals contained within the ex situ population established by this project.
Great strides have been made in using rescued corals in sexual reproduction to create new individuals and enhance genetic diversity. Rescued D. cylindrus fragments held in outdoor facilities during the summers of 2017 and 2018 were first used in assisted reproduction (Neely et al., 2020a); eggs and sperm from spawning individuals were mixed for fertilization and larval development. Juveniles from those efforts are alive and growing today. Subsequently, rescued D. cylindrus colonies held at the Florida Aquarium’s Center for Conservation were the first Caribbean corals to be acclimated to artificially mimicked abiotic cues in order to control coordinated and synchronous spawning (O’Neil et al., 2021). Ex situ reproduction events also provide opportunities to refine cryopreservation techniques, promoting long-term genetic banking and future assisted reproduction of the species. With each step and each year of experience, the process of promoting high rates of fertilization, larval development, settlement, and juvenile survivorship improves. The ability to spawn a large number of genotypes in a controlled environment is clearly the future of the reproductive capacity of this Florida D. cylindrus population.
The evolution of the Dendrogyra rescue project was driven by rapid population declines, rapid lessons learned in husbandry practices, and the collaborative actions of field biologists, aquarium professionals, and coral disease scientists. A large proportion of these efforts were conducted as “value added” components of other projects as the partners recognized the need for immediate action, but key funding sources also came in at critical junctures to provide support. Collection efforts were occurring simultaneously with the progression of SCTLD, and the development of husbandry practices and disease treatment protocols were conducted rapidly with little margin for failure. Lessons learned and novel protocols have contributed substantially to the development of disease treatment options for other species in aquaria and in situ, to collection and husbandry protocols being implemented for other species and regions, and to the development of novel and successful propagation methodologies that are likely to significantly impact restoration work for decades to come.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
KLN and CL initiated the rescue project, coordinated holding partners, and managed resulting databases. KLN conducted collections primarily with assistance from other partners. KO, CW, ZR, AM, KN, and DV provided rescue facilities and advanced husbandry practices. CW, KO, KLN, and CL advanced SCTLD treatment options. KO, provided information on gemmae. KO, KLN, CL, and DV coordinated and advanced onshore spawning efforts. JM assisted with external funding and some field coordination. KLN prepared the manuscript with assistance and editing from all authors.
Substantial funding and resources were provided by all of the agencies and institutions of the authors. This included a National Science Foundation RAPID response research grant (1503483) to M. Rodriguez-Lenetty at Florida International University IMaGeS Lab. Two additional grants from NOAA-CRCP (NA18NOS4820206) and NOAA Office of Protected Resources (NA15NMF4720280) to KLN included funding for field collections and holding facilities.
The scientific results and conclusions, as well as any views or opinions expressed herein, are those of the author(s) and do not necessarily reflect those of NOAA or the Department of Commerce.
Five authors were employed by non-governmental or academic companies: KO (Florida Aquarium), ZR (Phillip and Patricia Frost Museum of Science), DV (Plant a Million Corals), AM (Coral Restoration Foundation), and KN (Reef Renewal).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to the many people who helped with collections and care of fragments. These include collection teams from Nova Southeastern University, Florida’s Fish and Wildlife Research Institute, Keys Marine Laboratory, Florida Aquarium’s Center for Conservation, Mote Marine Lab, Coral Restoration Foundation, and the National Park Service. Extensive coral care was conducted by teams at Florida Aquarium, Frost Museum of Science, NOAA NOS Coral Health and Disease Program (particularly Carl Miller and Zachary Moffitt), Florida Institute of Oceanography at Keys Marine Laboratory, Coral Restoration Foundation, and Mote Marine Lab. Collections were conducted and corals held under Florida Keys National Marine Sanctuary permits FKNMS-2015-156 and FKNMS-2016-121, and by Florida permit SAL-19-2142-SCRP. This is contribution #283 from the Coastlines and Oceans Division of the Institute of Environment at Florida International University.
Chan, A. N., Lewis, C. L., Neely, K. L., and Baums, I. B. (2019). Fallen Pillars: the Past, Present, and Future Population Dynamics of a Rare, Specialist Coral–Algal Symbiosis. Front. Mar. Sci. 6:218. doi: 10.3389/fmars.2019.00218
Lewis, C., Neely, K., and Rodriguez-Lanetty, M. (2019). Recurring Episodes of Thermal Stress Shift the Balance From a Dominant Host-Specialist to a Background Host-Generalist Zooxanthella in the Threatened Pillar Coral, Dendrogyra cylindrus. Front. Mar. Sci. 6:5. doi: 10.3389/fmars.2019.00005
Miller, C., May, L., Moffitt, Z., and Woodley, C. (2020). Exploratory treatments for stony coral tissue loss disease: pillar coral (Dendrogyra cylindrus). Charleston: NOAA Technical Memorandum NOS NCCOS 245 and CRCP 37.
Neely, K. L., Lewis, C. L., Lunz, K. S., and Kabay, L. (2021). Rapid Population Decline of the Pillar Coral Dendrogyra cylindrus Along the Florida Reef Tract. Front. Mar. Sci. 8:656515. doi: 10.3389/fmars.2021.656515
Neely, K. L., Lewis, C. L., and Macaulay, K. A. (2020a). Disparities in spawning times between in situ and ex situ pillar corals. Front. Mar. Sci. 7:643. doi: 10.3389/fmars.2020.00643
Neely, K. L., Macaulay, K. A., Hower, E. K., and Dobler, M. A. (2020b). Effectiveness of topical antibiotics in treating corals affected by Stony Coral Tissue Loss Disease. PeerJ 8:e9289. doi: 10.7717/peerj.9289
O’Neil, K. L., Serafin, R. M., Patterson, J. T., and Craggs, J. R. (2021). Repeated ex situ spawning in two highly disease susceptible corals in the family Meandrinidae. Front. Mar. Sci. 8:669976. doi: 10.3389/fmars.2021.669976
Precht, W. F., Gintert, B. E., Robbart, M. L., Fura, R., and Van Woesik, R. (2016). Unprecedented Disease-Related Coral Mortality in Southeastern Florida. Sci. Rep. 6:31374. doi: 10.1038/srep31374
Keywords: pillar coral, Dendrogyra cylindrus, coral rescue, stony coral tissue loss disease, gemma, propagation, restoration
Citation: Neely KL, Lewis CL, O’Neil K, Woodley CM, Moore J, Ransom Z, Moura A, Nedimyer K and Vaughan D (2021) Saving the Last Unicorns: The Genetic Rescue of Florida’s Pillar Corals. Front. Mar. Sci. 8:657429. doi: 10.3389/fmars.2021.657429
Received: 22 January 2021; Accepted: 16 June 2021;
Published: 19 July 2021.
Edited by:
Sarah Annalise Gignoux-Wolfsohn, Smithsonian Environmental Research Center (SI), United StatesReviewed by:
Rebecca Certner, National Oceanic and Atmospheric Administration (NOAA), United StatesCopyright © 2021 Neely, Lewis, O’Neil, Woodley, Moore, Ransom, Moura, Nedimyer and Vaughan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karen L. Neely, a25lZWx5MEBub3ZhLmVkdQ==; Cynthia L. Lewis, Y3ludGhpYWxld2lzQHVzZi5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.