
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mar. Sci. , 19 July 2021
Sec. Marine Pollution
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.642160
This article is part of the Research Topic Predicting Hydrocarbon Fate in the Ocean: Processes, Parameterizations, and Coupled Modeling View all 12 articles
 Antonietta Quigg1,2*
Antonietta Quigg1,2* Peter H. Santschi2,3
Peter H. Santschi2,3 Chen Xu3
Chen Xu3 Kai Ziervogel4
Kai Ziervogel4 Manoj Kamalanathan1
Manoj Kamalanathan1 Wei-Chun Chin5
Wei-Chun Chin5 Adrian B. Burd6
Adrian B. Burd6 Andrew Wozniak7,8
Andrew Wozniak7,8 Patrick G. Hatcher8
Patrick G. Hatcher8Microbes (bacteria, phytoplankton) in the ocean are responsible for the copious production of exopolymeric substances (EPS) that include transparent exopolymeric particles. These materials act as a matrix to form marine snow. After the Deepwater Horizon oil spill, marine oil snow (MOS) formed in massive quantities and influenced the fate and transport of oil in the ocean. The processes and pathways of MOS formation require further elucidation to be better understood, in particular we need to better understand how dispersants affect aggregation and degradation of oil. Toward that end, recent work has characterized EPS as a function of microbial community and environmental conditions. We present a conceptual model that incorporates recent findings in our understanding of the driving forces of MOS sedimentation and flocculent accumulation (MOSSFA) including factors that influence the scavenging of oil into MOS and the routes that promote decomposition of the oil post MOS formation. In particular, the model incorporates advances in our understanding of processes that control interactions between oil, dispersant, and EPS in producing either MOS that can sink or dispersed gels promoting microbial degradation of oil compounds. A critical element is the role of protein to carbohydrate ratios (P/C ratios) of EPS in the aggregation process of colloid and particle formation. The P/C ratio of EPS provides a chemical basis for the “stickiness” factor that is used in analytical or numerical simulations of the aggregation process. This factor also provides a relative measure for the strength of attachment of EPS to particle surfaces. Results from recent laboratory experiments demonstrate (i) the rapid formation of microbial assemblages, including their EPS, on oil droplets that is enhanced in the presence of Corexit-dispersed oil, and (ii) the subsequent rapid oil oxidation and microbial degradation in water. These findings, combined with the conceptual model, further improve our understanding of the fate of the sinking MOS (e.g., subsequent sedimentation and preservation/degradation) and expand our ability to predict the behavior and transport of spilled oil in the ocean, and the potential effects of Corexit application, specifically with respect to MOS processes (i.e., formation, fate, and half-lives) and Marine Oil Snow Sedimentation and Flocculent Accumulation.
One of the significant new insights from the large research effort launched after the Deepwater Horizon (DwH) oil spill in the Gulf of Mexico in 2010 is the information gained on the fate of oil and dispersants as they were transported to the seafloor (see e.g., reviews of Daly et al., 2016; Passow and Overton, 2021; Quigg et al., 2021a). In particular, it was found that the flocculent material observed in surface waters for several weeks after the incident formed as a result of microbial mucus production (Passow et al., 2012). This material contained significant fossil carbon as determined by the 13C technique (Passow, 2016). The term MOSSFA (Marine Oil Snow Sedimentation and Flocculent Accumulation) was coined to describe the combination of biological, chemical and physical processes that lead to the formation and sinking of this marine oil snow (MOS) material and its accumulation on the seafloor (Daly et al., 2016, 2020; Quigg et al., 2016, 2020; Burd et al., 2020). MOSSFA also includes the fate of oil and the biochemical signature left in exudates and sediments. It has since become clear that similar sedimentation events of oil-laden marine snow have also occurred during other spills, although they went largely undetected at the time (Vonk et al., 2015). Recent estimates suggest that 5–31% of oil reached the seafloor during DwH (Valentine et al., 2014; Chanton et al., 2015; Romero et al., 2015; Xu et al., 2018a, b). Interestingly, a similar proportion of oil (24%) reached the seafloor during the Ixtoc-I oil spill in the Bay of Campeche in 1979–1980 (Boehm and Fiest, 1980; Jernelöv and Lindén, 1981), emphasizing the importance of the MOSSFA process. Indeed, MOSSFA events are now recognized as a potential pathway for oil distribution in the marine environment, and the need to integrate this process into spill response planning has been recognized (Jacketti et al., 2020; Ross et al., 2021). Whereas aggregation between suspended sediments and oil [e.g., oil-sediment aggregations (OSA), also called oil-mineral-aggregates (OMAs), mineral-oil-aggregates (MOA), oil-particle aggregates (OPA) or oil-suspended-particulate-material-aggregates] form predominately via direct coagulation between oil droplets and particulates or sediments (Khelifa and Hill, 2006; Gong et al., 2014; Zhao et al., 2016), exopolymers released by bacteria and phytoplankton are an essential ingredient for the biologically mediated formation of marine snow and MOS (see recent reviews by Quigg et al., 2016; Burd et al., 2020; Santschi et al., 2020; Gregson et al., 2021).
Formation of marine (oil) snow is complex, depending on local conditions (light, UV, temperature, weathering, and minerals) and by a multitude of drivers spanning from molecular to organismal scales (Figure 1). This review examines the relationship between the microbial community, their exudates, oil, and dispersants in determining the major processes (dispersion, aggregation, and sinking), which in turn determine the fate of hydrocarbons in the ocean. It is known that most marine microbes, whether auto- or heterotrophic, bacteria or phytoplankton, are capable of producing mucilaginous substances that have varying functional roles and physical properties (Hoagland et al., 1993; Decho and Herndl, 1995; Verdugo, 2012; Decho and Gutierrez, 2017). This frequently leads to the formation of gelatinous mucus-like aggregates (Baelum et al., 2012; Passow et al., 2012; Ziervogel et al., 2012; Gutierrez et al., 2013a, b) such as biofilms and gels that facilitate the development of complex, interacting communities that are hotspots of microbial activity (Verdugo and Santschi, 2010; Doyle et al., 2018, 2020). However, it remains important to understand: (A) what are the general characteristics of EPS? (B) how do microbes respond to oil and dispersants? (C) what are the mechanisms by which EPS aids in the aggregation and/or dispersion of oil or oil plus dispersant, and (D) how does the presence of the resulting ternary system (oil-dispersant-EPS) modify the fate of the oil?
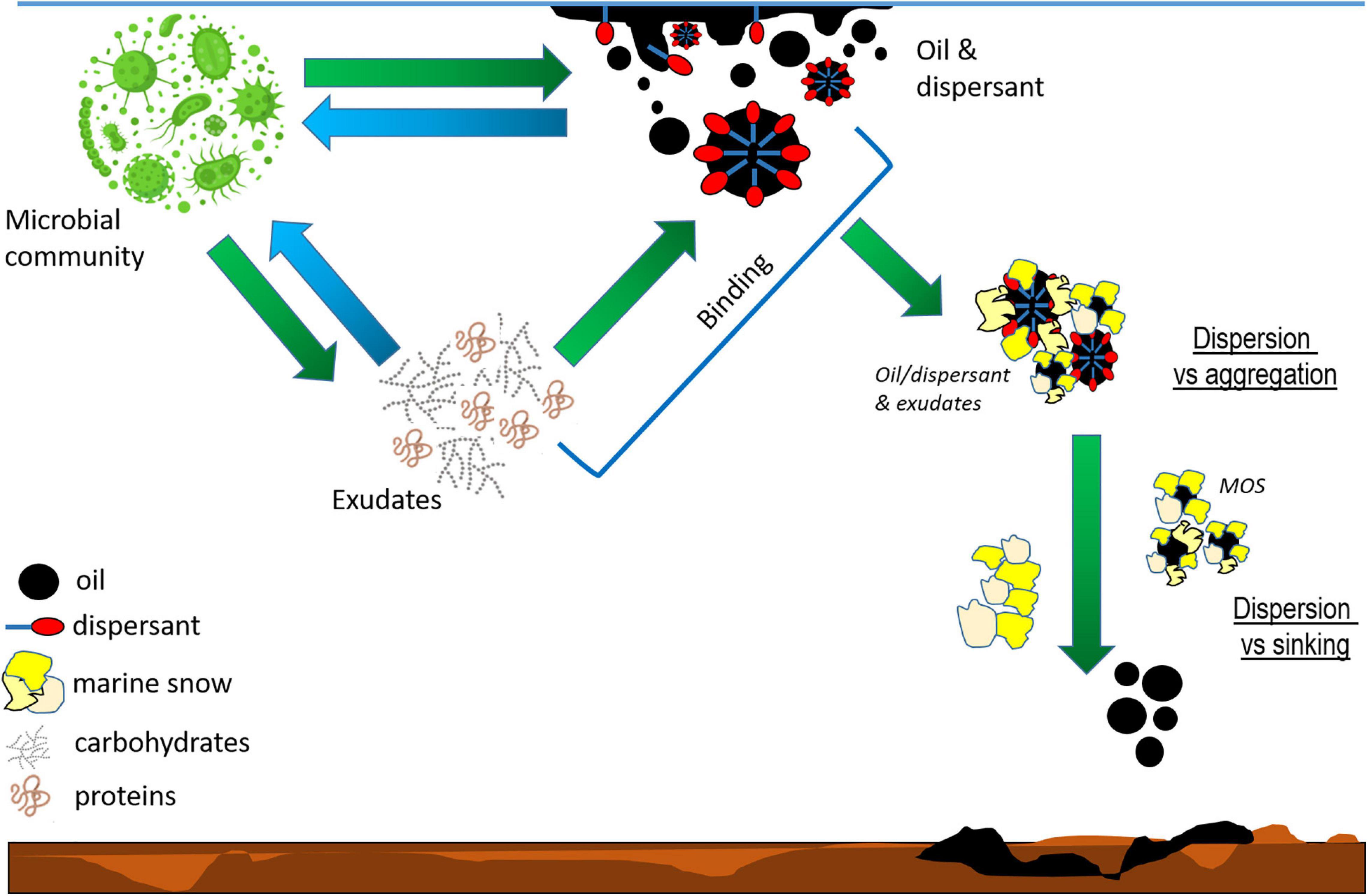
Figure 1. Schematic of potential interactions between the microbial community and the oil or oil plus dispersant. Microbial exudates are released in response to the oil and dispersant, which then triggers a cascade of reactions. The binding between exudates and oil or oil plus dispersant impact the oil’s fate (dispersion versus aggregation), which in turn determines its distribution between the water column and sediments (dispersion versus sinking), degradation and grazing rates. The microbial community composition depends on oil and dispersant availability, which in turn determines the characteristics of microbial exudates and dictates the method microbes interact with the oil (direct or via exudates).
The formation of marine snow and MOS are usually modeled using aggregation theory (Burd and Jackson, 2009; Dissanayake et al., 2018). Some models explicitly focus on the physical processes that bring particles together (e.g., Brownian motion, fluid shear, and differential sedimentation), the probability that particles adhere once a collision has occurred (stickiness), and the sinking of particles through the water column (Kiørboe et al., 1990; Burd and Jackson, 2009). Marine aggregates display fractal properties, and so models use fractal scaling to relate particle diameter to volume. Particle disaggregation is also important and occurs by fluid shear, turbulence, or fragmentation through animal-particle interactions. Although disaggregation models are less well developed, simplified representations of disaggregation are also often included in models which can be sensitive to how disaggregation is parameterized (Burd and Jackson, 2009; Dissanayake et al., 2018). Combining these factors together leads to a system of non-linear, coupled differential equations describing the evolution of the particle size distribution (Burd and Jackson, 2009). Other models, e.g., the “Brownian Pumping” model of Honeyman and Santschi (1989) express coagulation rates and extent as a non-linear function of particle concentration, to simulate the widely observed particle concentration effect on particle-water partition coefficients and kinetics of radioactive and stable metal sorption coupled to coagulation of colloids to particles. The Brownian Pumping model was also verified in controlled laboratory experiments using hematite particles (Honeyman and Santschi, 1991) and natural particle assemblages (Stordal et al., 1996; Wen et al., 1997).
In all formulations of marine snow formation, purely physicochemical processes are included in models only in the form of an empirical “stickiness” parameter (α) that takes a value between 0 and 1. This is usually regarded as a constant and depends on the amount of EPS particles (Passow, 2002). The aggregation rate (R(di,dj)) between particles of diameter di and dj is then written as:
Where β(di,dj) is the coagulation kernel and is the sum of the rates of the physical processes bringing particles together, and Ci is the concentration of particles of diameter di.
In this review, we propose that the stickiness parameter (α) should be modified to reflect the composition of EPS. In particular, we have suggested that α should be a function of the protein-to-carbohydrate ratio (ϑ) of the EPS which is in turn a function of microbial biomass and the concentration of oil and dispersant (Quigg et al., 2016; Santschi, 2018; Xu et al., 2019; Chen et al., 2020; Santschi et al., 2020). This modification will explicitly incorporate important biological and chemical contributions into the coagulation models so that the aggregation rate between particles becomes:
In this review, we will focus on the above four questions (A–D) as they lead us closer toward an understanding of the processes and pathways of MOS formation in determining the fate of hydrocarbons. Here in, we present a conceptual model that incorporates this information, which is critical to developing better predictions of hydrocarbon fate in the oceans. The reader is referred to the many excellent reviews published to learn more about other processes (e.g., role of zooplankton and food webs) which are equally critical but beyond the scope of the current paper in understanding MOS and MOSSFA (Beyer et al., 2016; Daly et al., 2016; Burd et al., 2020; Kujawinski et al., 2020; Quigg et al., 2020; 2021a, b; Passow and Overton, 2021; Ross et al., 2021). Nonetheless, additional studies would be invaluable to collecting data which could be used to develop flux measures between compartments and hence, the development of models to examine the fate of hydrocarbons.
Exopolymeric substances (EPS) represent a vast pool of reversibly self-assembling porous micro-gels released by microbes (bacteria and phytoplankton) that serve as a major source of dissolved organic matter in the oceans. Marine micro- and nano-gels are three-dimensional polymer networks reversibly formed from EPS (Verdugo et al., 2004; Verdugo and Santschi, 2010); they create microenvironments that interact with the surrounding media (Chin et al., 1998) and are thought to be the precursors to the more visible macro-gels. These high molecular weight exopolymers are thought to physically protect microbes (e.g., from heavy metals and pollutants) as well as aid in their attachment to different substrates by forming biofilms and gels (Verdugo and Santschi, 2010; Quigg et al., 2013, 2016; Decho and Gutierrez, 2017; Santschi, 2018; Santschi et al., 2020; and references therein). These exopolymers are composed mainly of polysaccharides and proteins, but also contain nucleic acids and lipids (Azam, 1998; Verdugo et al., 2005; Verdugo, 2007; Decho and Gutierrez, 2017; Santschi et al., 2020). Subclasses of EPS are named depending on the method of characterization (see review of Quigg et al., 2016). If alcian blue staining is used with Xanthan as the standard, the particulate fraction (retained on a filter) is named transparent exopolymer particles (TEP) (Passow, 1995; Bar-Zeev et al., 2011; Xu et al., 2011). If alginic acid is used as a standard, the particulate material is called acid polysaccharides (APS) (Hung et al., 2003). Coomassie Stained Particles (CSP) are proteinaceous particles made visible with Coomassie Blue staining (Nagasaki et al., 2004; Verdugo et al., 2008). It is important to note that often these different measurements are detecting different moieties of the same molecule(s). EPS, are therefore “operationally” defined based upon their characteristics, size(s), and methods of quantification (Bar-Zeev et al., 2015; Quigg et al., 2016). The majority of studies measure one, perhaps two of these operationally defined EPS. However, a recent study compared EPS, TEP, CSP, and microgels in seawater from the Gulf of Mexico (Xu et al., 2019). The study found that biopolymers making up EPS, TEP, and CSP consisted primarily of polysaccharides and proteins, most likely as proteoglycans and glycoproteins, and that overall, concentrations ranked in the order of [gels] > [TEP] > [particulate EPS]. This ranking was the same in the presence of a water accommodated fraction (WAF) of oil prepared with a Macondo surrogate oil. The EPS that was electrostatically held onto particle surfaces (operationally-defined as those extractable by 1% EDTA) accounted for a minor (∼4%) yet relatively constant proportion of TEP.
Polysaccharides, one of the major constituents of EPS, are mostly hydrophilic. Acid polysaccharides such as uronic acids contain carboxyl or sulfate groups and provide bidentate inner-sphere coordination sites for divalent (e.g., Ca2+ and Mg2+) or trivalent (e.g., Fe3+) ions, thus causing supra-macromolecular aggregation and Ca2+ bridging for structural stability (Verdugo et al., 2004). Proteins, another major EPS component, are amphiphilic and mediate the stability and aggregation of the 3-D networks of biopolymers, through hydrophobic and electrostatic interactions (Ding et al., 2008; Ortega-Retuerta et al., 2009; Song et al., 2015; Sun et al., 2020), as well as light-induced cross-linking (Sun et al., 2017, 2018, 2019, 2020). The hydrophobic domains of EPS (mainly consisting of proteins) were shown previously to absorb organic pollutants, such as petroleum hydrocarbons phenanthrene (Liu et al., 2001) and benzene (Spath et al., 1998). Particulate exudates, like TEP, may promote coagulation of marine particles and provide a matrix for marine snow (component particles > 0.5 mm) (Alldredge et al., 1993; Passow, 2002; Verdugo et al., 2004; Passow et al., 2012). The composition and characteristics of EPS can therefore lead to differences in the interactions with oil and/or dispersants, thus affecting petroleum hydrocarbons’ dispersion, degradation, and sedimentation pathways. Recent studies reveal that the hydrophobic interaction between proteins and hydrocarbons results from a selective partitioning with hydrophilic polysaccharides preferentially associating with sinking MOS (Xu et al., 2018a, b, 2019). Marine snow and MOS were found to be extremely sticky; with particles readily attaching in the water column. After losing their buoyancy, marine snow and MOS sink toward the seafloor (Ziervogel et al., 2011; Passow et al., 2012). Recent work by Ye et al. (2020) also shows that when oil droplets are combined with more cohesive bentonite clay, the aggregate density and settling velocity actually increase compared to the clay alone. This is because droplets no longer exist (observed microscopically) and oil is absorbed into the larger aggregate structure. Their observations were carried out at atypically high particle, EPS as Xanthan gum, and/or crude oil concentrations (e.g., 500 mg/L) and should be regarded with caution. However, these observations may be consistent with observations using individual diatom species exposed to a control and to WAF treatment in roller tanks by Passow et al. (2019) who found that measured settling velocities increased in the presence of oil.
The release of hydrocarbons as a result of a spill triggers a complex cascade of microbial responses (Figure 1), whereby not a single species dominates, but complex microbial consortia develop (Head et al., 2006; Baelum et al., 2012; Kleindienst et al., 2015, 2016; Doyle et al., 2018, 2020). The microbial response to oil-contamination and the fate of the oil varies, with some bacteria having genes for hydrocarbon and/or n-alkane degradation that can directly utilize components of the oil (Hazen et al., 2010; Valentine et al., 2010; Kessler et al., 2011; Lu et al., 2012; Redmond and Valentine, 2012; Ziervogel et al., 2014; Doyle et al., 2020). Several studies have confirmed that microbes (prokaryotes) indigenous to the Gulf of Mexico comprised largely of Gammaproteobacteria (Hazen et al., 2010; Baelum et al., 2012; Mason et al., 2012; Rivers et al., 2013; Kleindienst et al., 2016) played a significant role in the biodegradation of the oil during and post DwH oil spill (Lu et al., 2012; Mason et al., 2012, 2014). However, other microbial groups were also present (Gutierrez et al., 2013a, b, 2016; Yang et al., 2016; Kamalanathan et al., 2018, 2021) and have been shown to respond to oil spills in other areas (McKew et al., 2007; Prince et al., 2010; McGenity et al., 2012). Members of the genera Marinobacter, Alcanivorax, Cycloclasticus, Neptuniibacter, and others dominated the surface water column (Baelum et al., 2012; Kleindienst et al., 2015; Dombrowski et al., 2016; Doyle et al., 2018), while the order Oceanospirillales, and genus Colwellia dominated the deep sea plume responses (Redmond and Valentine, 2012; Kimes et al., 2013, 2014; Kleindienst et al., 2015, 2016). A change in microbial community structure was also observed across time, with groups such as Methylotrophs, Alteromonadales, Flavobacteria, Rhodobacter, and Deltaproteobacteria increasing in abundance following the succession of Cycloclasticus, Oceanospirillales, Colwellia, and Alcanivorax (Kimes et al., 2013, 2014). These shifts occur within a few hours of experiment initiation with surface water studies (Doyle et al., 2018, 2020) showing the microbial community response is also much faster in surface waters than those performed at depth (Baelum et al., 2012; Kleindienst et al., 2015). In mesocosms with oil (as a WAF), this manifested as an increase in community diversity due to the outgrowth of several aliphatic- and aromatic-hydrocarbon degrading species, including phytoplankton-associated taxa (Doyle et al., 2020). In contrast, microbial community diversity was reduced in microcosms containing oil (Meng et al., 2016; Kamalanathan et al., 2021) and dispersant (Meng et al., 2016), however, this effect has been shown to be temperature dependent (Techtmann et al., 2017).
Field samples from the site of DwH showed elevated levels of respiration accompanied by higher levels of expression of genes associated with chemotaxis, motility, hydrocarbon degradation pathways such as n-alkane and cyclohexane oxidation, and nutrient acquisition, especially denitrification in the prokaryotic microbial community (Edwards et al., 2011; Lu et al., 2012; Mason et al., 2012, 2014). Shifts in extracellular enzyme activities from β-glucosidase (associated with polysaccharide degradation) to lipase (often associated with hydrocarbon degradation) were also observed in mesocosm studies (Kamalanathan et al., 2021). At the sites of the DwH oil spill where both the sediments and their overlying water samples were taken, elevated levels of alkaline phosphatase were measured. This corresponded to a substantial biomass growth in the water column yet a non-significant biomass increase in the sediment (Edwards et al., 2011; Lu et al., 2012). Taken together, these findings indicate oil exposure shifted the metabolism toward hydrocarbon degradation which in-turn drove nitrogen uptake (Mason et al., 2014; Zhao et al., 2020).
Dramatic shifts in heterotrophs, photoautotrophs, and grazers altered the community structure in the water column with diatoms and certain dinoflagellates dominating after exposure to oil and/or oil plus dispersants in a variety of studies (Parsons et al., 2015; Almeda et al., 2018; Bretherton et al., 2019; Finkel et al., 2020; Quigg et al., 2021b). Some dinoflagellates have been shown to directly ingest oil (Almeda et al., 2014), however, the mechanism behind diatom oil tolerance remains unknown. Many physiological studies in the last decade have examined the response of the photosynthetic pathways, respiration, the reactive oxygen system, morphological features and toxin production (Bretherton et al., 2018, 2019, 2020; Kamalanathan et al., 2019) as well as other parameters (see review by Quigg et al., 2021b). Growth and photophysiological responses are often species specific with some showing a tolerance and others a sensitivity to oil or oil plus dispersants (Bretherton et al., 2018, 2020). Reactive oxygen species concentrations have been shown to be one of the major secondary factors leading to growth inhibition in sensitive phytoplankton (Ozhan et al., 2015; Quigg et al., 2021b). The presence of dispersant reduced the growth rate and increased the level of the toxin domoic acid in the diatom Pseudo-nitzschia (Bretherton et al., 2019). Nonetheless, a decrease in microbial interactions was also observed in microcosm studies in response to oil exposure, primarily due to a major change in the composition of eukaryotic phototrophs and a decrease in photosynthetic efficiency (Kamalanathan et al., 2021). Surprisingly, the same study found a significant decrease in heterotrophic EPS production with no changes to phototrophic EPS levels, suggesting an indirect impact on phototrophs and heterotroph interactions due to oil exposure.
Amongst the eukaryotes, the community composition shifted more toward fungi in the benthos (Bik et al., 2012) and salt marshes (Lumibao et al., 2018). This is not surprising as fungi have been shown to tolerate and degrade oil either directly (Davies and Westlake, 1979; Al-Nasrawi, 2012) or in conjunction with extracellular enzymes (Verdin et al., 2004; Asemoloye et al., 2018). There is also evidence that marine fungi play a role in oil degradation (Burd et al., 2020; Finkel et al., 2020). Fungi are well-known for their ability to metabolize biochemical compounds including lignin and complex carbohydrates (Hedges et al., 1985), and they have recently been shown to dominate biomass on marine snow in the deep sea (Bochdansky et al., 2017) suggesting their importance to marine organic matter degradation.
After the DwH oil spill, large, EPS-rich, marine snow, and MOS formed with elevated hydrolytic enzyme activities in association with the surface oil layer (Baelum et al., 2012; Passow et al., 2012; Ziervogel et al., 2012; Kleindienst et al., 2015; Kamalanathan et al., 2018). In addition to the above changes in microbial community activity, it was also found that the mucus-like EPS harbored a very distinct community of interacting microbes, with a specific functionality that was different from those persisting in the surrounding seawater (Ziervogel et al., 2012; Arnosti et al., 2015). These eukaryotic microbes and fungi can transform and degrade oil, sometimes in association with bacteria and dispersants including Corexit (Mishamandani et al., 2016; Severin and Erdner, 2019). Thus, marine snow is a hot spot for microbial activity (Azam, 1998; Arnosti et al., 2015; Doyle et al., 2018, 2020) and serves as a transport vehicle for hydrocarbons to the seafloor as well as the associated microbial communities (Kowalewska and Konat, 1997; Kowalewska, 1999; Ziervogel et al., 2012; Ziervogel et al., 2014, 2016; Arnosti et al., 2015). Collectively, these processes directly influence microbial community composition and structure, indirectly influence the quantity and quality of produced exudates, and ultimately influence both remineralization and mobilization of oil-derived carbon, by determining its fate (sinking versus dispersion versus aggregation) and transport (exudates).
Some data also indicates Corexit may serve as a microbial substrate (Baelum et al., 2012; Bacosa et al., 2018) while others show toxicity (Zahed et al., 2010; Hamdan and Fulmer, 2011; Paul et al., 2013). These differences may be due to a dissimilar experimental design, as Baelum et al. (2012) used deep-water samples, while Hamdan and Fulmer (2011) used hydrocarbon degrading isolates. Regardless, it is apparent that the impact of Corexit on microbial community function, in general, and on EPS production and function specifically, is still largely unconstrained. In addition, oceanic regions have distinctly different indigenous microbial communities than coastal regions (Doyle et al., 2020); these populations responded to oil/dispersant intrusion differently as also reflected in the EPS production and composition (Xu et al., 2019). For example, EPS produced by the natural consortia in the coastal seawater was generally more hydrophobic (with a higher P/C ratio, and thus higher α factor, see above) than that produced in the offshore waters. Nutrient status and particle concentrations in these oceanic regions (nearshore versus offshore) could have also been factors that directly affect EPS production and oil-laden aggregate formation (the latter serves as ballast). Such information needs to be taken into consideration when evaluating post-spill MOS formation and sedimentation.
Lastly, most of the research on microbial oil spill toxicology tend to highlight the most enriched microbial species, with less abundant ones grouped together as “others.” However, studies have shown that members belonging to order Burkholderiales, and Enterobacteriales, and phylum Planctomycetaceae, Hyphomonadaceae, Saprospiraceae, and Teridinibacter decreased in abundance in response to oil and dispersant exposure (Kleindienst et al., 2016; Meng et al., 2016; Kamalanathan et al., 2018). Amongst eukaryotes, Amoebozoa, dinoflagellates, diatoms such as Coscinodiscus, Thalassiosira, Stephanopyxis, and Thalassionema decreased in response to oil and dispersant exposure (Bretherton et al., 2018; Finkel et al., 2020). However, future studies with more emphasis on the oil and dispersant sensitive microbial species would be beneficial, e.g., using them as indicator species of unpolluted areas or areas that recovered post-spill.
Exopolymeric substances acting as biosurfactants can emulsify oil and its breakdown products (Head et al., 2006). For example, EPS produced by Halomonas sp. has amphiphilic properties, thereby interacting easily with hydrophobic substrates like hydrocarbons, leading to the solubilization and biodegradation of oil components (Gutierrez et al., 2013a). The exopolymer concentrations with entrained oil droplets grow to form networks that also act as an energy and carbon source to other members of the microbial community (Sinsabaugh et al., 2009; Kamalanathan et al., 2020). In addition, the production of EPS may facilitate attachment of specific microbes (e.g., Pseudomonas putida) to polycyclic aromatic hydrocarbons (McGenity et al., 2012). In this way, a complex network of microbes utilizing the different components of oil and the metabolites of the oil degraders will develop (McGenity et al., 2012). Such networks may enhance the formation of aggregates and biofilms (Gärdes et al., 2011).
Most petroleum hydrocarbons are insoluble in seawater so that biodegradation can only take place at the hydrocarbon-water interface. Dispersion or emulsification of oil increases the bioavailability of oil products to biodegradation as these processes introduce fine droplets of oil into the water column by wave action or sea turbulence and keep it dispersed. Given dispersants are designed to form emulsions to prevent oil droplets from coalescing and stabilize them in a suspension, Corexit and other dispersant products are a mixture of nonionic (∼48%) and anionic (∼35%) surfactants with enough solvent or petroleum distillate (∼17%) to make a homogeneous dispersant mixture of the surfactants and aid in their penetration onto and into the oily aggregate (John et al., 2016; Quigg et al., 2021b). As shown in Figure 2 (Phase 1), surfactants are long amphiphilic molecules that can arrange themselves at an interface such that the hydrophilic head interacts with the water and the hydrophobic tail shuns the water and crosses the interfacial boundary interacting with the air or another hydrophobic substance, such as oil. The presence of these molecules on the surface disrupts the cohesive energy and lowers the surface tension (Figure 2, Phase 2).
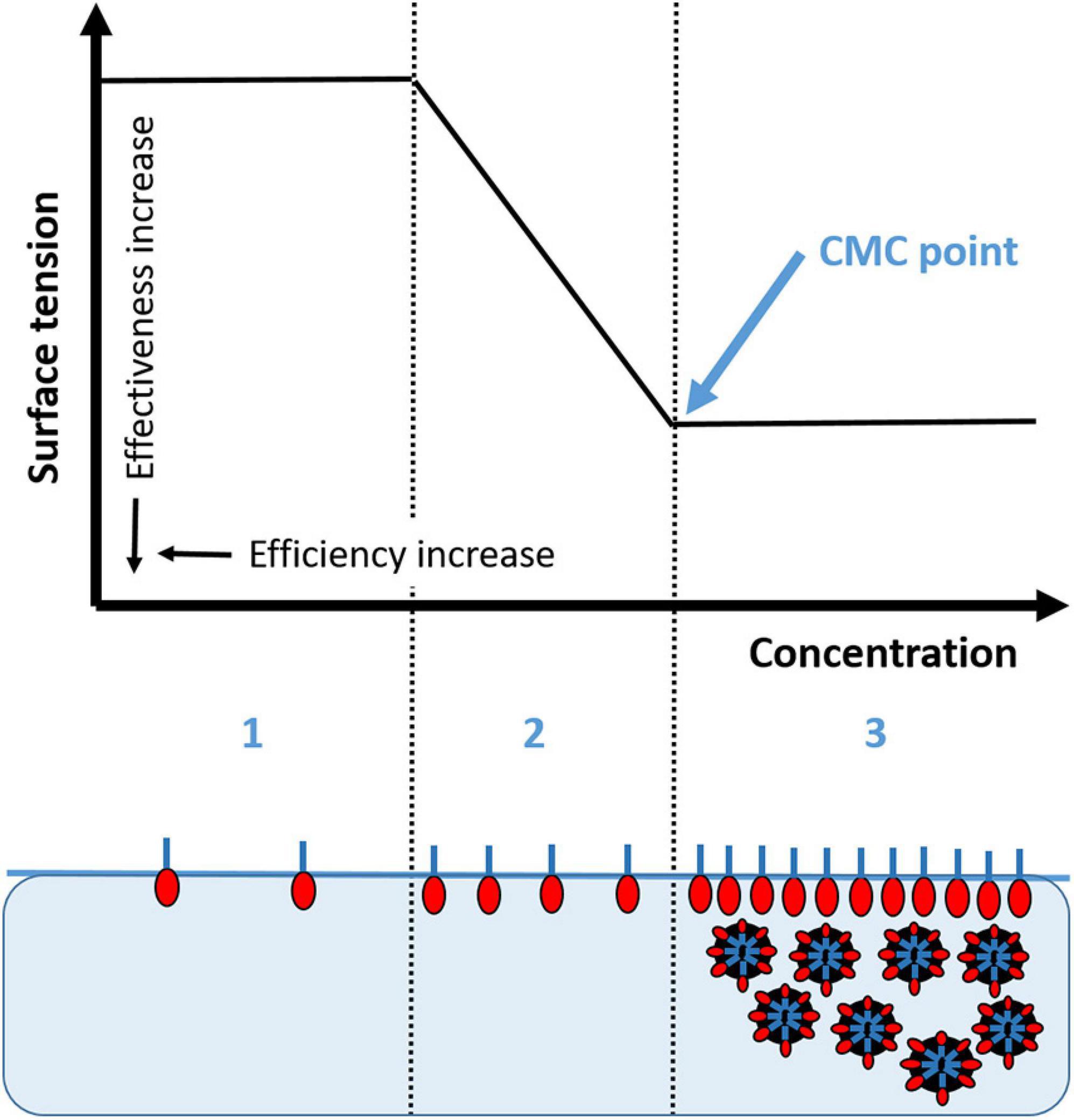
Figure 2. Surface tension as a function of surfactant concentration (logarithmic scale) in three separate phases. (1) low concentration of surfactant, with the round hydrophilic end of the molecule in the water and the long carbon chain or tail interacting with a hydrophobic substance, i.e., air or oil; (2) as the surfactant concentration increases, the interfacial surface tension decreases between the hydrophobic and hydrophilic moieties; and (3) when the concentration of surfactant increases to the critical micelle concentration (CMC) an emulsion is formed where the oil is entrained in the biosurfactant and can be dispersed or form networks or gel.
The purpose of dispersant application is to lower the oil/water interfacial tension to promote entrainment of oil droplets (<100 μm) into the water column (Chen and Yapa, 2007; Testa et al., 2016). The critical micelle concentration (CMC) is the concentration of dispersant at which the surfactant molecules form a uniform monolayer at the oil/water interface (Figure 2, Phase 3). The effectiveness of a dispersing agent increases the magnitude of the interfacial tension reduction to CMC (shown as the difference between the surface tension of Phase 1 and that of Phase 2, the greater the difference is, the higher the effectiveness). However, the efficiency is highest when the lowest concentration of dispersing agent is used after the CMC is reached (the surface tension of Phase 3, the lower the value, the higher the efficiency). Recent studies have tested the hypothesis that the exudates of bacteria and phytoplankton may also serve as biosurfactants (Quigg et al., 2016, 2020, Decho and Gutierrez, 2017; Ward et al., 2018). Schwehr et al. (2018) characterized EPS as biosurfactants by determining their effectiveness and efficiency through interfacial tension and CMC rheology measurements. They investigated mechanisms governing the self-assembly and phase separation for protein-polysaccharide-oil-dispersant interactions, thereby adding to our understanding of what is known about the CMC of oil/Corexit mixtures. With hydrophilic and hydrophopic moieties, EPS increases the bioavailability of certain oil components to the microbial community. In this sense, the biosurfactant properties of EPS are similar to those of dispersants used for oil spill remediation.
Exopolymeric substances with enhanced protein to carbohydrate (P/C) ratios (thus higher sticky factor α, see above) was present in oil and oil plus dispersant treatments, suggesting efficient bioemulsifying effects of proteins in the presence of Corexit (Schwehr et al., 2018; Xu et al., 2018a, b, 2019; Shiu et al., 2020). In addition, bovine serum albumin (used as a model protein) was found to slightly increase surface tension at low concentrations (<few mg/L), but decreased it at higher concentrations of >8 mg/L (Schwehr et al., 2018). These model molecules (including uronic acids such as glucuronic acid with carboxyl moieties and carrageenan with sulfate groups) appeared to be more efficient than Corexit in inducing the self-assembly of micelles in the seawater even when only very low concentrations were present. Schwehr et al. (2018) found Corexit is more effective, i.e., lowers the surface tension more than the EPS constituents, however, the EPS can emulsify oil at far lower concentrations, thus is more efficient (Figure 2). In several mesocosm experiments mimicking different environmental settings, the relative petro-carbon concentration (%, petro-carbon to total organic carbon) in the sinking MOS, as determined by 14C method, was found to be positively correlated with both the P/C ratio of colloidal EPS and sinking MOS-EPS (Xu et al., 2019). This result corroborates that EPS plays a similar role to that of Corexit in reducing the surface tension of oil but could have been more efficient than Corexit in forming oil-EPS micelles. This behavior was related to their relative hydrophilicity (i.e., P/C ratio). Passow et al. (2019) suggested that oil droplets may allow for tighter packaging of cells, which decreases porosity and results in faster sinking. Santschi et al. (2020) suggested using the data from Passow et al. (2019) which showed that decreased aggregate diameters and settling velocities were correlated with increased P/C ratios of EDTA extractable EPS to understand how P/C ratios affect stickiness. That is, the presence of oil not only seems to cause a tighter packaging of cells, it also facilitates the production of EPS with higher P/C ratios and incorporation of more hydrophobic biopolymers (shown as higher P/C ratio). Incorporation of these high P/C ratio EPS moieties into the aggregate resulted in a slower sinking velocity of aggregates. P/C ratios of EPS could be different from that of whole EPS (including attached EPS), while P/C ratios of non-attached EPS affect whole oil incorporation, aggregate porosity, buoyancy, and sinking velocity, but the exact relationships in the complex web of processes is not entirely clear.
In the last few years, considerable effort has been directed toward understanding how the ternary system (oil-dispersant-EPS) determines the fate and transport of oil (hydrocarbons) in the ocean, particularly considering the role of dispersants. Studies of oil, Corexit and organic matter, including exudates (EPS and TEP) may lead to conflicting observations as to the question of whether Corexit application promotes or hinders MOS formation and/or the subsequent sedimentation unless the findings are carefully dissected (Chiu et al., 2017; Passow et al., 2017, 2019). On one hand, Corexit application leads to significantly more oil droplets, thereby increasing the probability that oil is incorporated into marine snow. On the other hand, Corexit disperses whole oil-laden exudates, thus aggregation and subsequent sedimentation processes decrease (Passow, 2016, Passow et al., 2017). Also, Corexit application to oil appears to result in the formation of fewer but more oil-rich aggregates (Passow et al., 2017). If Corexit fully disperses oil-laden exudates more effectively compared to the scenario in which no Corexit is applied, all MOS formation would be inhibited and no sedimentation would be observed. In contrast to MOS, the formation of oil-sediment aggregates is independent of exudates, and not negatively affected by exudate dispersion (Henry et al., 2020). This finding was reflected in mesocosm studies that showed while the formation of oil-laden aggregates in the colloidal fraction increased, deposition of marine (oil) snow/particles decreased (Xu et al., 2018a, b). This is because each aggregate included higher concentrations of oil droplets thus increasing its buoyancy. In contrast to this, oil-sedimentation rates are less affected (over controls) when no dispersant is applied (Passow et al., 2012; Xu et al., 2018a).
The input of oil/dispersant fueled the microbial community by promoting EPS production and modifying their composition as observed by the positive correlations between EPS production and the reduction of oil/PAH/n-alkane when oil/dispersant was present in several mesocosm systems with various environmental settings (Xu et al., 2019). Some interesting patterns were observed: (1) more EPS preferably partitioned into suspended particulate matter (SPM) when dispersant was applied compared to treatments without oil/dispersant or only with oil; (2) riverine particle input in the coastal region enhanced EPS partitioning into the SPM fraction compared to the offshore water which has less terrestrial influence; (3) the coastal microbial communities tend to produce EPS with higher P/C ratio (thus higher α, see above) compared to those in offshore waters, indicating their potentially higher hydrophobicity and thus stronger tendency to form aggregates; (4) a contrasting pattern of consistently higher P/C ratio in sinking aggregate-EPS than in SPM-EPS in all treatments of the offshore region, with an opposite result in all treatments of the coastal water, suggesting EPS was from different sources in these two size fractions (SPM and sinking aggregates) and that different mechanisms regulated MOS formation and sedimentation in these two environmental settings.
Radiocarbon and 13C NMR results have further shown that the presence of dispersants enhances the amount of oil products incorporated into MOS (Hatcher et al., 2018; Xu et al., 2018b). These authors observed that after less than a week of mesocosm conditions replicating an oil spill, most of the chemically-dispersed oil preferentially partitioned into the colloidal and suspended particulate fractions rather than to the sinking MOS. Thus, the oil sedimentation efficiency in treatments with a dispersant was considerably lower than those in the control and oil only treatments, which is almost a universal observation irrespective of environmental settings (nutrients or algae particle addition, oceanic regions, etc.) (Xu et al., 2019). It was determined that 28–93% of sinking organic carbon consisted of petro-carbon in the oil plus dispersant treatment compared to a range of 17–42% for oil-alone treatment, yet the sedimentation efficiency was significantly and consistently lower in the oil plus dispersant treatment (0.1–8% of initial oil carbon) relative to the oil-alone treatment (1.4–27% of initial oil carbon) (Xu et al., 2019). However, in the long term (in a scale of >2-weeks), these above mentioned differences (relative petro-carbon concentration and petro-carbon sedimentation efficiency) of the sinking MOS in oil plus dispersant versus oil-alone treatments diminished indicating that dispersants simply postponed MOS sedimentation. In a parallel effort, estimated oil equivalents used as a proxy for changing oil concentrations in seawater unassociated with MOS, exponentially decreased at rates ranging from –0.013 to –0.027/h (Wade et al., 2017). These were slightly higher than the rate of –0.0066/h reported by Gearing et al. (1979), a MERL mesocosm study in which 12% of added oil was transported to the sediments (Wade and Quinn, 1980).
A clear signal for oil incorporation into MOS comes from a mesocosm study in which oil was observed in aggregate materials (Hatcher et al., 2018). MOS (as bottom particles) was analyzed by a solid-state 13C NMR CPMAS multi-pulse technique (Johnson and Schmidt-Rohr, 2014) modified to allow quantitative characterization of carbon functional groups (Hatcher et al., 2018). The latter study showed oil in chemically enhanced (with Corexit) WAF (CEWAF) treatments to have a very large peak in the aliphatic chemical shift region (Figure 3B) relative to controls (no oil added) (Figure 3A) indicative of inputs of highly aliphatic oil components. Extraction with dichloromethane (DCM), a solvent frequently used to extract oil, efficiently removed the aliphatic oil component from CEWAF MOS (Figures 3B,D); the control spectrum by contrast did not show appreciable changes (Figures 3A,C). A molecular mixing model adapted from Baldock et al. (2004) was then used to estimate the contributions of proteins, carbohydrates, lipids, and carbonyl carbon in the samples. After DCM extraction, control particles showed little to no change in the relative contributions of lipids suggesting that the losses in the operationally-defined lipid component for the oil treatment MOS could be attributed to the removal of oil (Δ lipid = oil contribution; Hatcher et al., 2018). Oil contributions were estimated to be 4, 27, and 26% for WAF, CEWAF, and diluted CEWAF (DCEWAF) treatments, respectively, using this technique. The 13C NMR-derived estimates for oil contributions to the sinking MOS matched well with relative oil contributions found in particles collected from the same mesocosm treatments as measured using a Δ14C tracer technique (Xu et al., 2018b). The agreement between these two approaches confirmed the ability to quantitatively extract oil from MOS using DCM for further characterization and assessment of oil degradation.
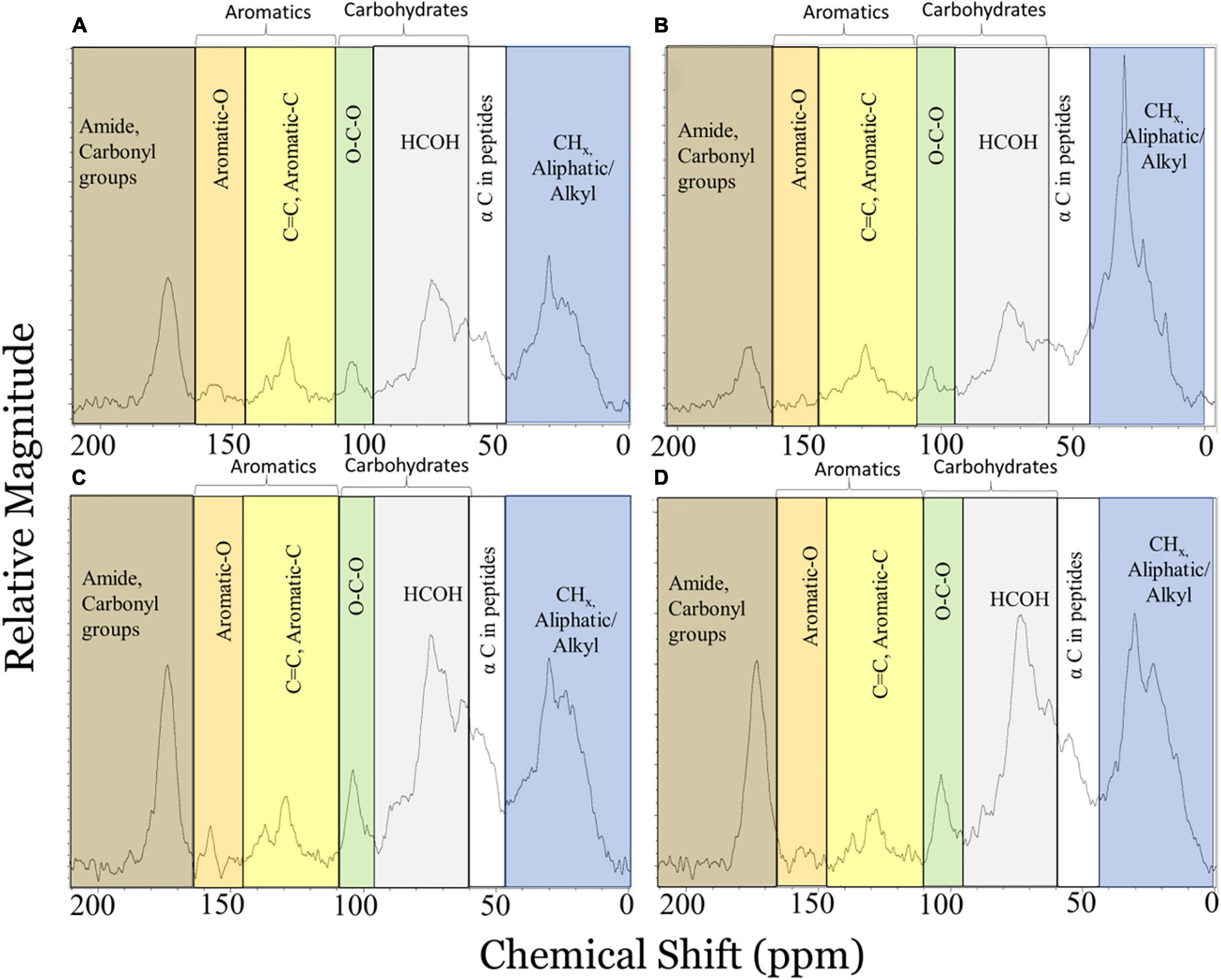
Figure 3. Solid-state 13C NMR spectra using a quantitative multiple cross polarization magic angle spinning pulse sequence for marine oil snow particles collected from the bottom of tanks in mesocosm control (A,C) and chemically-enhanced water accommodated fraction (B,D) treatments before (A,B) and after (C,D) extraction with dichloromethane to remove oil components. Shaded portions of the spectra represent chemical shift regions where signal for carbonaceous functional groups typically appear (blue: 0–45 ppm, aliphatic C; white: 46–60 ppm, α C in peptides; gray: 60–95 ppm, carbohydrate HCOH; green: 90–110 ppm, carbohydrate anomeric C; yellow: 110–145 ppm, Aromatic-C; orange: 145–165 ppm, Aromatic-O; brown: 165–215 ppm, Amide, Carbonyl C). Adapted from Hatcher et al. (2018).
Oil associated with marine snow appears to be rapidly oxidized (timescales of days rather than years) (Hatcher et al., 2018; Wozniak et al., 2019). DCM extracts of the sunken MOS showed the control treatment to have vastly different molecular characteristics from that in the DCEWAF treatments (Figure 4; Wozniak et al., 2019). The sunken MOS DCM extracts show unique formulas at relatively high O/C and low H/C ratios indicating the oxygenation and solubilization of oil components that are not found in either the control sample or the Macondo oil sample (Figure 4B) and more likely are produced via degradation processes in the mesocosms. After just 4 days of processing, the oil isolated from the DCEWAF and CEWAF treatments showed a pattern in their CHO-containing molecular formulas that was very different from the Macondo oil which showed most of the spectral signal for CHO formulas to be accounted by CHO2 formulas (Figure 4C). The DCEWAF and CEWAF CHO spectral signal shows reduced contributions from CHO2 formulas due to contributions from CHO3 and CHO4 formulas with increasingly smaller contributions from higher oxygenated formulas. The distribution of CHO formulas in the degraded MOS (CEWAF and DCEWAF) was very similar to oils exposed to weathering processes on Gulf of Mexico beaches for several months (Figures 4C,D; Chen et al., 2016; Wozniak et al., 2019). Similar results were found by Hatcher et al. (2018) using GC × GC Mass Spectrometry. Oil degradation has been demonstrated for surface slicks and oiled sands (Aeppli et al., 2012; Ruddy et al., 2014; Chen et al., 2016) but not within complex MOS aggregates. These results suggest that oil degradation in the mesocosm experiments was rapid and facilitated by the microbial community over time scales of hours to days. We also observed significant changes in the fingerprint of the oil in MOS including high relative abundance of oxygenated hydrocarbons (O = 2–4) with likely aliphatic and aromatic molecular structures (Wozniak et al., 2019). We were, however, not able to observe progressive stepwise changes in the oil fingerprint toward aromatic ring oxidation and enrichment in oxygen-containing molecules in polar fractions, changes analogous to those observed during oxidation influenced by air-exposure (Ruddy et al., 2014; Chen et al., 2016). Such information would be invaluable for understanding the rate of oil degradation in the marine environment.
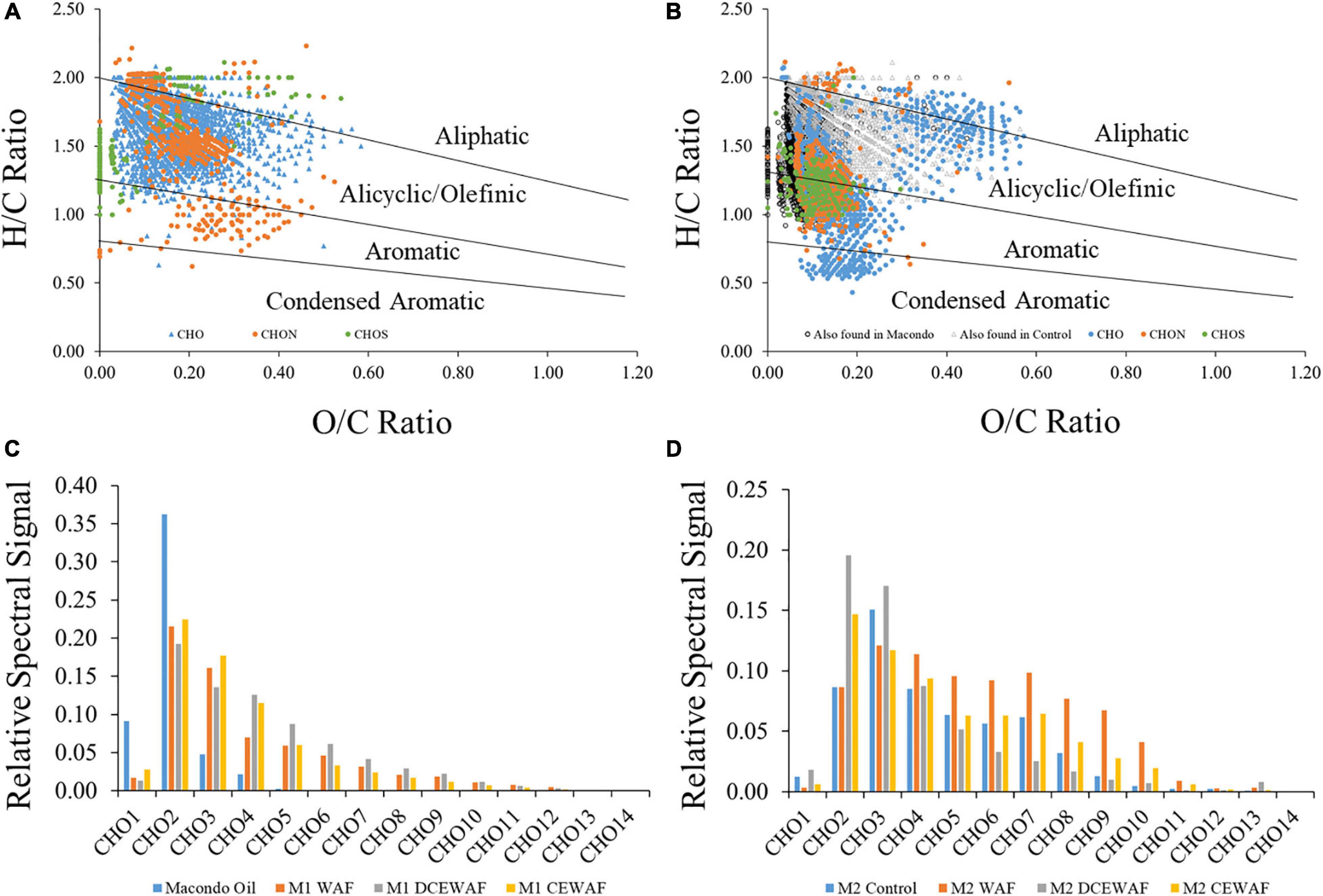
Figure 4. Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FT-ICR-MS) data for dichloromethane extracts of sinking particles collected from the bottom of tanks in mesocosm treatments displayed as van Krevelen diagrams (A,B) for control (A) and diluted chemically-enhanced water accommodated fraction (DCEWAF) treatments (B), and as isoabundance plots of oxygen (CHO1–14) (C,D) for Macondo oil and Mesocosm 1 water accommodated fraction treatments (C) and Mesocosm 2 control and water accommodated treatments (D). DCEWAF formulas also found in Macondo oil and the control treatments are noted as black circles and gray triangles, respectively, in panel (B). Adapted from Wozniak et al. (2019).
A comparison of PAH composition in MOS from laboratory roller table (Genzer et al., 2020) and mesocosm experiments [Bacosa et al., 2020; and GRIIDC dataset (10.7266/ER1EGZ8E)] with the PAH composition examined in the sediment samples of Gulf of Mexico (Romero et al., 2015), revealed interesting findings (Figure 5). Using non-metric multidimensional scaling (NMDS), the data points of both WAF and DCEWAF from roller table and mesocosm experiments clustered together with the sediment samples, suggesting some level of consistency in the PAHs that tend to remain in marine snow. While the DCEWAF samples from a mesocosm experiment clustered relatively closer to the sediment samples, a major fraction of the WAF samples from a mesocosm experiment clustered away, suggesting a slight effect (lower concentration of acenapthene) of the use of dispersant on the PAH composition of the marine snow leading to heterogeneity amongst marine snow composition. One clear pattern that emerged from this analysis, however, is the depletion of naphthalene and fluorene in nearly all the marine snow and the sediment samples relative to the composition of the original Macondo oil and the surrogate oil used in the roller table and mesocosm experiments. This is not surprising, as studies have shown rapid depletion and degradation of water soluble, light, volatile, low-molecular weight PAHs such as naphthalene and fluorene (Kappell et al., 2014; Bacosa et al., 2020). Overall, this analysis shows that the marine snow formed in the laboratory experiments such as roller table and mesocosm experiments closely mimicked the material found to be sedimenting in the field, further underscoring the role of MOSSFA phenomena during the DwH oil spill. It also shows that the use of dispersants might not have played a significant role in determining the PAH composition of the MOS.
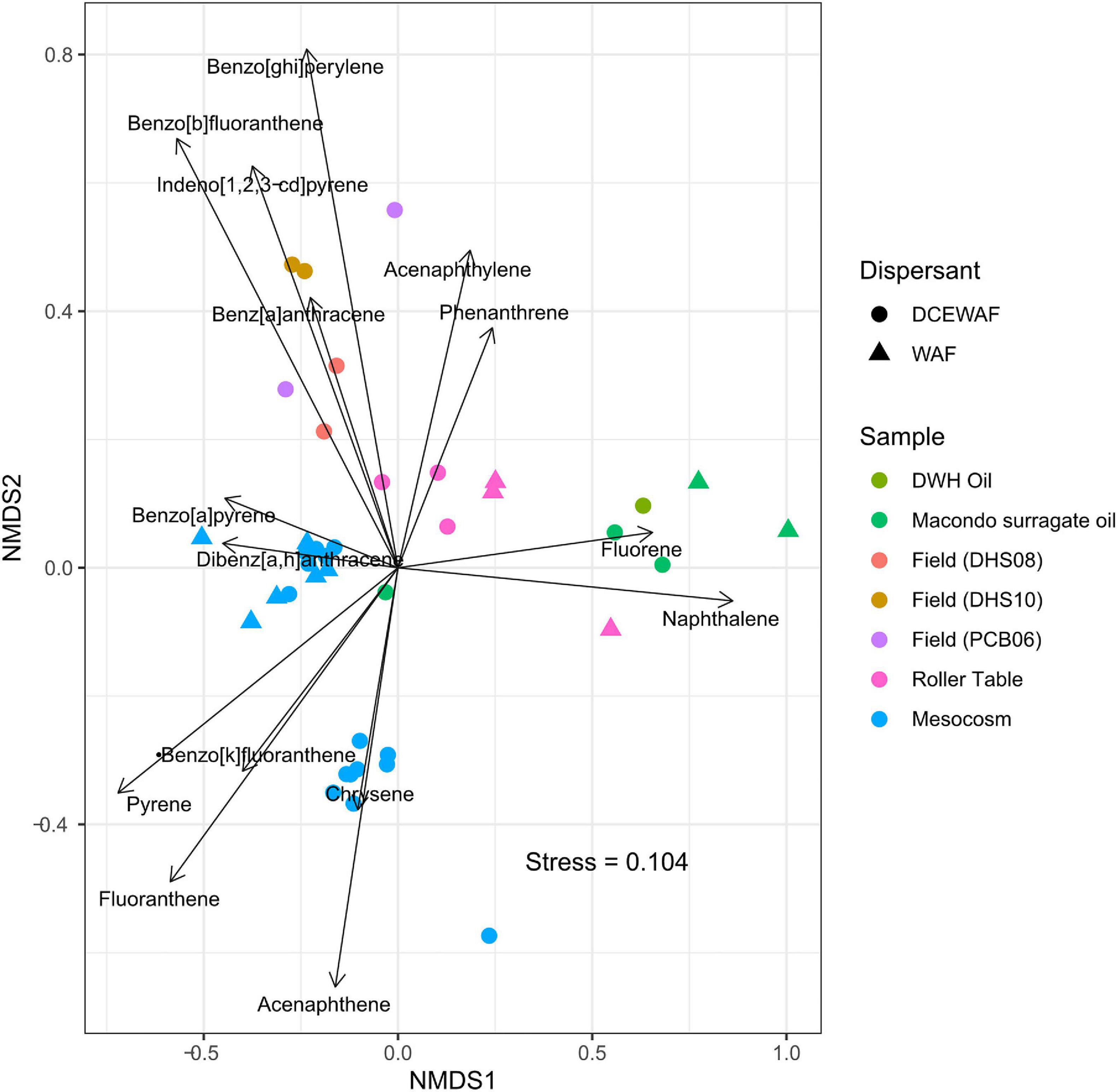
Figure 5. Non-metric multidimensional scaling (NMDS) plot showing clustering of marine snow samples from various mesocosm, roller table studies and field sediment samples from Gulf of Mexico based on PAH composition. Data points clustered to the right are sample from the original Deepwater Horizon (DWH) Macondo oil and the Day 1 sample from surrogate oil used in the laboratory mesocosm and roller table studies, whereas the points clustered largely to the left are marine snow samples from the field and laboratory experiments. Only PAHs such as Naphthalene, Acenaphthylene, Acenaphthene, Fluorene, Phenanthrene, Anthracene, Fluoranthene, Pyrene, Benz[a]anthracene, Chrysene, Benzo[b]fluoranthene, Benzo[k]fluoranthene, Benzo[a]pyrene, Indeno[1,2,3-cd]pyrene, Dibenz[a,h]anthracene, and Benzo[ghi]perylene were used for the analysis, with concentrations of each PAH normalized to percentage of total PAHs in each sample. More details on the PAH extraction, sample collection, field site and design of the roller table and mesocosm studies can be found in Genzer et al. (2020); Bacosa et al. (2020), Romero et al. (2015) and GRIIDC dataset (10.7266/ER1EGZ8E
The changing fingerprints of aliphatic and aromatic hydrocarbons documented preferential loss on n-alkanes (Morales-McDevitt et al., 2020) and lower molecular weight PAH (Shi et al., 2020) in mesocosm studies further indicating that biodegradation occurred within the first 24 h. In a recent mesocosm experiment designed to measure the rate of loss of n-alkanes over the time period of several days by GC × GC mass spectrometry found that the concentrations of nC17 and nC18 alkanes in extracts of MOS decreased with half-lives of 0.9 and 1.0 days, respectively. Such a rapid diminution of alkanes is remarkable but indicative of extremely rapid biodegradation of oil that becomes dispersed within the MOS and enhanced by the presence of Corexit that serves to finely disperse the oil within the MOS (Hatcher, pers. comm).
Predictive numerical models of MOS formation and fate balance processes, such as aggregation, (that produce MOS) and disaggregation (processes that alter and remove MOS, e.g., microbial respiration and sinking) have been attempted. A recent study adapted models of marine snow formation and fate to include oil (Dissanayake et al., 2018). They found that the fraction of oil reaching the seafloor in the model was most sensitive to two factors: the structure of the MOS particles (as measured using the fractal dimension) and the way in which particle stickiness was represented in the model. The values of stickiness of individual components (e.g., oil, phytoplankton, mineral particles, etc.) were held constant, but different ways of combining them to calculate the stickiness of an MOS aggregate were used.
To accurately model these processes requires realistic measures of (i) sinking velocities, (ii) stickiness, (iii) disaggregation kinetics of MOS, and (iv) processes that transform oil. There has been considerable work done over many decades to determine parameterizations of sinking velocities of marine snow (Alldredge and Gotschalk, 1998; Iversen and Lampitt, 2020; Laurenceau-Cornec et al., 2020; and the review by Silver, 2015). In contrast, measurements of stickiness and disaggregation are less ubiquitous (Alldredge et al., 1990; Engel, 2000; De La Rocha and Passow, 2007; Burd and Jackson, 2009).
Accurately modeling the settling velocity of marine snow and MOS is difficult. This is because there appears to be no clear, universal relationship between marine snow characteristics (e.g., size and density, etc.) and settling velocity. For example, recent laboratory experiments have suggested that Stokes’ Law modified using the particle fractal dimension provides a good parameterization (Laurenceau-Cornec et al., 2020), but even this parameterization does not account for the considerable scatter in measured settling velocities. Conversely, a recent in-situ field study of sinking particles concluded that there is no relationship between marine snow sinking velocity and particle size (Iversen and Lampitt, 2020). Compounding the confusion further, laboratory studies indicate that the incorporation of oil into marine snow can increase settling velocities even though the oil is positively buoyant, and this may be due to a restructuring of the aggregate via capillary bridging (Passow et al., 2019). Current models of MOS formation and fate use modified forms of Stokes’ Law to calculate particle settling velocities and take into account the overall density of the aggregate (Dissanayake et al., 2018) producing average settling velocities that are consistent with laboratory observations but do not reproduce the higher velocities seen in those studies.
Marine snow and MOS formation and fragmentation is strongly dependent on particle stickiness (see above). This is defined as the probability that particles adhere once they have collided, varies from 0 to 1, and depends on the chemical and physical nature of the particles. SterlingJr., Bonner et al. (2005) used a particle coagulation model combined with laboratory measurements to determine stickiness between oil and mineral particles, but no corresponding measurements exist for oil and marine snow. Stickiness is thought to be mainly dependent on the composition and concentration of EPS in the water column (Passow, 2002). This has led to the proposal to use the P/C ratio of EPS as a practical proxy for stickiness (Santschi, 2018; Chen et al., 2020; Santschi et al., 2020). The P/C ratio, and hence stickiness of EPS, varies with the community composition of organisms producing EPS and with the presence of dispersants such as Corexit (Shiu et al., 2020). For example, in mesocosm studies using both Corexit and oil, the P/C ratio of sinking MOS was found to be consistently lower than that of both SPM and colloidal particles. This suggested that Corexit and oil affect the partitioning of different components of EPS (e.g., polysaccharides versus protein) between the water column and sinking material (Xu et al., 2018a, b, 2019; Santschi et al., 2020). Oil-laden aggregates partition more strongly into the colloidal fraction because oil droplets increase the buoyancy of the resulting aggregates, thus temporarily slowing their sinking out of the water. Current models of marine snow and MOS formation use constant stickiness values for different types of particles, but it is not known how the stickiness of a heterogeneous aggregate depends on the stickiness of its components such as oil, mineral particles, and biological detrital material. The recent work by Chen et al. (2020), however, sheds more light and provides a novel magnetic tweezer technique to directly and quantitatively determine the relative stickiness of different EPS fractions to each other or to the solid substrates.
Marine particles can be eroded (small pieces being eroded from the surface of a larger particle), or fragmented (an aggregate breaking up into particles of a range of sizes), potentially affecting their sinking rate and their interactions with organisms in the water column. Adhesive forces between components of a single aggregate contribute to the strength of that aggregate and its ability to resist being broken up. Fragmentation of marine particles can result from turbulent fluid motion (Parker et al., 1972; Alldredge et al., 1990) and mechanical breakage arising from interactions between particles and zooplankton (Dilling and Alldredge, 2000). Indeed, a variety of physical and biological processes (Briggs et al., 2020) can result in particle breakup, including shear stress (Karl et al., 1988; Ruiz, 1997) as well as zooplankton swimming and sloppy feeding (Banse, 1995; Steinberg et al., 1997; Dilling and Alldredge, 2000; Goldtwhait et al., 2004; Giering et al., 2016). However, direct observations of aggregate fragmentation are limited to a few laboratory studies that demonstrate a relation between the particle type/composition and its physical strength under controlled small-scale turbulence (Alldredge et al., 1990; Rau et al., 2018). Conducting a turbulence experiment similar to the one by Alldredge et al. (1990), we found evidence that MOS is physically stronger compared to marine snow (Ziervogel, 2020). The presence of oil in marine snow aggregates will likely affect the particle’s cohesiveness (Passow et al., 2019), but this has yet to be quantified.
Existing models of marine snow and MOS fate use ad hoc representations of disaggregation, which can be parameterized for individual scenarios but which lack generality and mechanistic foundations (Burd and Jackson, 2009; Dissanayake et al., 2018). For example, the model used by Dissanayake et al. (2018) allows for particles to fragment when their size is greater than the Kolmogorov length scale and the fragmented particle produces two smaller, equally sized particles. This is an oversimplification because we have very little knowledge of the size distribution of fragmentation products and how the presence of oil and dispersants might affect them. A greater understanding of particle disaggregation and how it is affected by oil is needed in order to constrain the fraction of MOS lost during transit from the point of formation to the point of deposition in sediments, e.g., the fraction that reaches the seafloor, compared to the amount leaving the surface ocean. For example, understanding if the P/C ratio affects the cohesiveness of an aggregate and thereby its resistance to fragmentation would provide a more accurate model representation.
Lastly, as MOS sinks through the water column, it is affected by consumption and remineralization by organisms. Current models use only simple representations of microbial consumption of oil and particles (Dissanayake et al., 2018). To accurately model the fate of MOS will require the use of microbial respiration rates of oil and non-oil components and the corresponding changes in particle density. Models combining the aggregation processes with multiple particle types (e.g., mineral particles, biological particles, and oil) have the potential to synthesize our understanding of the fate of MOS. They also reveal where gaps remain in our understanding. Initial models are able to broadly reproduce evolution of MOS size distributions as material sinks from the surface to the deep ocean, but more work needs to be done to refine these results and be able to follow MOS from when oil is introduced into the system (e.g., from surface spill to deep-sea blow out) to when it settles on the seafloor. Recently developed in-situ methods that allow direct observations of the aggregation state of marine particles with respect to the aggregates’ physical strength (Ackleson and Rau, 2020) could fill the current data gap on aggregate fragmentation rates in the ocean.
Equation 2 is a mathematical formulation of the conceptual model given in Figures 1, 2. It expresses the idea that it should be possible, based on the current results, to relate the stickiness factor and aggregate formation rate to relevant chemical or biological processes. In this context it needs to be mentioned that EPS concentration, microbial biomass, as well as oil concentration are changing on time scales of hours to days. Thus, an improved formulation of these coupled processes would need to also include the kinetics of each of these processes. In addition, it is not clear which of the possible attachment processes are truly reversible (e.g., disaggregation is only occurring when fluid shear can overcome the binding strength). However, one could safely assume that hydrophobic interactions are less reversible than electrostatic interactions due to their stronger interaction forces (Chen et al., 2020). And, if conditions are favorable, hydrophobic moieties (e.g., in proteins, where they reside in the interior of the macromolecule) can also become exposed (e.g., in case of proteins, upon unfolding) and attach themselves more strongly to other hydrophobic surface moieties, mostly irreversibly, as has been reported recently by Chen et al. (2020). Thus, due to the complexity of the biochemical and biophysical interactions during aggregate formation, the use of a simple predictor of the stickiness or attachment strength of marine snow or MOS, e.g., the P/C ratio, is recommended, with the caveat that aggregate formation can occur in both the colloidal and particulate fractions, depending on their relative buoyancy or excess density.
Overall, this review highlights recent insights into the processes that control interactions between oil, dispersant, microbes, and microbially-produced EPS in producing MOS or dispersed gels that promote microbial degradation of oil compounds. The role of P/C ratios in the aggregation process of colloid and particle formation was also reviewed. We also discuss the factors affecting rapid oil oxidation and microbial degradation, such as indigenous microbes, especially the potential role of microbial succession through time and depth, possible role of nutrient limitation and changes to the physiology and community structure of eukaryotes including phytoplankton and fungi. Other phenomenon such as the processes that result in the sedimentation and preservation/degradation of oil in the sinking MOS are also discussed. This improved understanding has expanded our ability to predict the behavior and transport of released oil, and the potential effects of Corexit application, specifically with respect to MOS (i.e., formation, fate, and half-lives) and MOSSFA processes.
All authors contributed to the ideas and the development of this manuscript, from drafting the outline to the intellectual content. All authors provided approval for the publication of the content.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the editors of this special issue for the invitation to submit a paper. The research presented was made possible by grants from the Gulf of Mexico Research Initiative: Quigg, Santschi, Xu, Ziervogel, Kamalanathan, Chin, Wozniak, and Hatcher: Aggregation and Degradation of Dispersants and Oil by Microbial Exopolymers (ADDOMEx SA15-22 and SA18-13) and Burd: Oil-Marine Snow-Mineral Aggregate Interactions and Sedimentation during the 2010 Deepwater Horizon Oil-Spill. This review was made possible by the dedication of the entire ADDOMEx team (2015–2020), particular co-PI’s Terry Wade, Tony Knap, Jason Sylvan, Zoe Finkel, and Andrew Irwin. We also wish to remember our dear friend and colleague, David Hollander, who we lost unexpectedly in 2020. We will always remember his scientific endeavors that aggregated us together over many a conversation, in recent years, around especially the topic of MOSSFA. His insights, humor and good will be missed. We also thank the reviewers whose suggestion’s helped improve the final manuscript.
Ackleson, S. G., and Rau, M. J. (2020). A method to measure marine particle aggregate disruption in situ. Limnol. Oceanogr. Methods 18, 644–655.
Aeppli, C., Carmichael, C. A., Nelson, R. K., Lemkau, K. L., Graham, W. M., Remond, M. C., et al. (2012). Oil weathering after the Deepwater Horizon disaster led to the formation of oxygenated residues. Environ. Sci. Technol. 46, 8799–8807. doi: 10.1021/es3015138
Alldredge, A. L., and Gotschalk, C. (1998). In situ settling behavior of marine snow. Limnol. Oceanogr. 33, 339–351. doi: 10.4319/lo.1988.33.3.0339
Alldredge, A. L., Granata, T. C., Gotschalk, C. C., and Dickey, T. D. (1990). The physical strength of marine snow and its implications for particle aggregation in the ocean. Liminol. Oceanogr. 35, 1415–1428. doi: 10.4319/lo.1990.35.7.1415
Alldredge, A. L., Passow, U., and Logan, B. E. (1993). The abundance and significance of a class of large, transparent organic particles in the ocean. Deep Sea Res. 40, 1131–1140. doi: 10.1016/0967-0637(93)90129-Q
Almeda, R., Connelly, T. L., and Buskey, E. J. (2014). Novel insight into the role of heterotrophic dinoflagellates in the fate of crude oil in the sea. Nat. Sci. Rep. 4:7560. doi: 10.1038/srep07560
Almeda, R., Cosgrove, S., and Buskey, E. J. (2018). Oil spills and dispersants can cause the initiation of potentially harmful dinoflagellate blooms (“Red Tides”). Environ. Sci. Technol. 52, 5718–5724. doi: 10.1021/acs.est.8b00335
Al-Nasrawi, H. (2012). Biodegradation of crude oil by fungi isolated from Gulf of Mexico. J. Bioremed. Biodegrad. 3, 1–6. doi: 10.4172/2155-6199.1000147
Arnosti, C., Ziervogel, K., Yang, T., and Teske, A. (2015). Oil-derived marine aggregates – hot spots of polysaccharide degradation by specialized bacterial communities. Deep Sea Res. II 129, 179–186. doi: 10.1016/j.dsr2.2014.12.008
Asemoloye, M. D., Ahmad, R., and Jonathan, S. G. (2018). Transcriptomic responses of catalase, peroxidase and laccase encoding genes and enzymatic activities of oil spill inhabiting rhizospheric fungal strains. Environ. Poll. 235, 55–64. doi: 10.1016/j.envpol.2017.12.042
Azam, F. (1998). Microbial control of oceanic carbon flux: the plot thickens. Science 280, 694–696. doi: 10.1126/science.280.5364.694
Bacosa, H. P., Kamalanathan, M., Chiu, M. H., Tsai, S. M., Sun, L., Labonté, J. M., et al. (2018). Extracellular polymeric substances (EPS) producing and oil degrading bacteria isolated from the northern Gulf of Mexico. PLoS One 13:e0208406. doi: 10.1371/journal.pone.0208406
Bacosa, H. P., Kamalanathan, M., Cullen, J., Shi, D., Xu, C., Schwehr, K. A., et al. (2020). Marine snow aggregates are enriched in polycyclic aromatic hydrocarbons (PAHs) in oil contaminated waters; insights from a mesocosm study. J. Mar. Sci. Eng. 8:781. doi: 10.3390/jmse8100781
Baelum, J., Borglin, S., Chakraborty, R., Fortney, J. L., Lamendella, R., Mason, O. U., et al. (2012). Deep–sea bacteria enriched by oil and dispersant from the Deepwater Horizon spill. Environ. Microbiol. 14, 2405–2416. doi: 10.1111/j.1462-2920.2012.02780.x
Baldock, J. A., Masiello, C. A., Gelinas, Y., and Hedges, J. I. (2004). Cycling and composition of organic matter in terrestrial and marine ecosystems. Mar. Chem. 92, 39–64. doi: 10.1016/j.marchem.2004.06.016
Banse, K. (1995). Zooplankton—pivotal role in the control of ocean production. ICES J. Mar. Sci. 52, 265–277. doi: 10.1016/1054-3139(95)80043-3
Bar-Zeev, E., Berman, T., Rahav, E., Dishon, G., Herut, B., Kress, N., et al. (2011). Transparent exopolymer particle (TEP) dynamics in the eastern Mediterranean Sea. Mar. Ecol. Progr. Ser. 431, 107–118. doi: 10.3354/meps09110
Bar-Zeev, E., Passow, U., Romero-Vargas Castrill, S., and Elimelech, M. (2015). Transparent exopolymer particles (TEP): From aquatic environments and engineered systems to membrane biofouling. Environ. Sci. Technol. 49, 691–707. doi: 10.1021/es5041738
Beyer, J., Trannum, H. C., Bakke, T., Hodson, P. V., and Collier, T. K. (2016). Environmental effects of the Deepwater Horizon oil spill: a review. Mar. Poll. Bull. 110, 28–51.
Bik, H. M., Halanych, K. M., Sharma, J., and Thomas, W. K. (2012). Dramatic shifts in benthic microbial eukaryote communities following the Deepwater Horizon oil spill. PLoS One 7:e38550. doi: 10.1371/journal.pone.0038550
Bochdansky, A. B., Clouse, M. A., and Herndl, G. J. (2017). Eukaryotic microbes, principally fungi and labyrinthulomycetes, dominate biomass on bathypelagic marine snow. ISME J. 11, 362–373. doi: 10.1038/ismej.2016.113
Boehm, P. D., and Fiest, D. L. (1980). “Aspects of the transport of petroleum hydrocarbons to the offshore benthos during the Ixtoc-I blowout in the Bay of Campeche,” in Proceedings of the Symposium on the Preliminary Results from the September, 1979 Pierce/Research IXTOC-1Cruises, (Boulder, CO: NOAA).
Bretherton, L., Hillhouse, J., Bacosa, H., Setta, S., Genzer, J., Kamalanathan, M., et al. (2019). Growth dynamics and domoic acid production of Pseudo-nitzschia sp. in response to oil and dispersant exposure. Harmful Algae 86, 55–63. doi: 10.1016/j.hal.2019.05.008
Bretherton, L., Hillhouse, J., Kamalanathan, M., Finkel, Z. V., Irwin, A. J., and Quigg, A. (2020). Trait-dependent variability of the response of marine phytoplankton to oil and dispersant exposure. Mar. Poll. Bull. 153:110906. doi: 10.1016/j.marpolbul.2020.110906
Bretherton, L., Williams, A., Genzer, J., Hillhouse, J., Kamalanathan, M., Finkel, Z. V., et al. (2018). Physiological response of 10 phytoplankton species exposed to Macondo oil and the dispersant. Corexit. J. Phycol. 54, 317–328. doi: 10.1111/jpy.12625
Briggs, N., Dall’Olmo, G., and Claustre, H. (2020). Major role of particle fragmentation in regulating biological sequestration of CO2 by the oceans. Science 367, 791–793. doi: 10.1126/science.aay1790
Burd, A., Chanton, J. P., Daly, K. L., Gilbert, S., Passow, U., and Quigg, A. (2020). The science behind marine-oil snow and MOSSFA: past, present, and future. Prog. Oceanogr. 187:102398. doi: 10.1016/j.pocean.2020.102398
Burd, A. B., and Jackson, G. A. (2009). Particle aggregation. Ann. Rev. Mar. Sci. 1, 65–90. doi: 10.1146/annurev.marine.010908.163904
Chanton, J., Zhao, T., Rosenheim, B. E., Joye, S., Bosman, S., Brunner, C., et al. (2015). Using natural abundance radiocarbon to trace the flux of petrocarbon to the seafloor following the Deepwater Horizon oil spill. Environ. Sci. Technol. 49, 847–854. doi: 10.1021/es5046524
Chen, C.-S., Shiu, R.-F., Hsieh, Y.-Y., Xu, C., Vazquez, C. I., Cui, Y., et al. (2020). Stickiness of extracellular polymeric substances on different surfaces via magnetic tweezers. Sci. Total Environ. 757:143766. doi: 10.1016/j.scitotenv.2020.143766
Chen, F., and Yapa, P. D. (2007). Estimating the oil droplet size distributions in deep-water oil spills. J. Hydraulic Eng. 133, 197–207. doi: 10.1061/(asce)0733-9429(2007)133:2(197)
Chen, H., Hou, A., Corilo, Y. E., Lin, Q., Lu, J., Mendelssohn, I. A., et al. (2016). 4 Years after the Deepwater Horizon spill: Molecular transformation of Macondo well oil in Louisiana salt marsh sediments revealed by FT-ICR mass spectrometry. Environ. Sci. Technol. 50, 9061–9069. doi: 10.1021/acs.est.6b01156
Chin, W.-C., Orellana, M. V., and Verdugo, P. (1998). Spontaneous assembly of marine dissolved organic matter into polymer gels. Nature 391, 568–572. doi: 10.1038/35345
Chiu, M.-H., Garcia, S. G., Hwang, B., Claiche, D., Sanchez, G., Aldayafleh, R., et al. (2017). Corexit, oil and marine microgels. Mar. Poll. Bull. 122, 376–378. doi: 10.1016/j.marpolbul.2017.06.077
Daly, K. L., Passow, U., Chanton, J., and Hollander, D. (2016). Assessing the impacts of oil-associated marine snow formation and sedimentation during and after the Deepwater Horizon oil spill. Anthropocene 13, 18–33. doi: 10.1016/j.ancene.2016.01.006
Daly, K. L., Vaz, A. C., and Paris, C. B. (2020). “Physical processes influencing the sedimentation and lateral transport of MOSSFA in the NE Gulf of Mexico,” in Scenarios and Responses to Future Deep Oil Spills, ed. S. Murawski (Cham: Springer), 300–314. doi: 10.1007/978-3-030-12963-7_18
Davies, J. S., and Westlake, D. W. S. (1979). Crude oil utilization by fungi. Canadian J. Microbiol. 25, 146–156. doi: 10.1139/m79-023
De La Rocha, C., and Passow, U. (2007). Factors influencing the sinking of POC and the efficiency of the biological carbon pump. Deep Sea Res. II 54, 639–658. doi: 10.1016/j.dsr2.2007.01.004
Decho, A. W., and Gutierrez, T. (2017). Microbial extracellular polymeric substances (EPSs) in ocean systems. Front. Microbiol. 8:922. doi: 10.3389/fmicb.2017.00922
Decho, A. W., and Herndl, G. J. (1995). Microbial activities and the transformation of organic matter within mucilaginous material. Sci. Total Environ. 165, 33–42. doi: 10.1016/0048-9697(95)04541-8
Dilling, L., and Alldredge, A. L. (2000). Fragmentation of marine snow by swimming macrozoolankton: a new process impacting carbon cycling in the sea. Deep-Sea Res. I 47, 1227–1245. doi: 10.1016/s0967-0637(99)00105-3
Ding, Y.-X., Chin, W.-C., Rodriguez, A., Hung, C.-C., Santschi, P. H., and Verdugo, P. (2008). Amphiphilic exopolymers from Sagittula stellata induce DOM self-assembly and formation of marine microgels. Mar. Chem. 112, 11–19. doi: 10.1016/j.marchem.2008.05.003
Dissanayake, A. L., Burd, A. B., Daly, K. L., Francis, S., and Passow, U. (2018). Numerical modeling of the interactions of oil, marine snow, and riverine sediments in the ocean. J. Geophys. Res. Oceans 123:JC013790.
Dombrowski, N., Donaho, J. A., Gutierrez, T., Seitz, K. W., Teske, A. P., and Baker, B. J. (2016). Reconstructing metabolic pathways of hydrocarbon degrading bacteria from the Deepwater Horizon oil spill. Nat. Microbiol. 1:16057. doi: 10.1038/nmicrobiol.2016.57
Doyle, S. M., Lin, G., Morales-McDevitt, M., Wade, T. L., Quigg, A., and Sylvan, J. B. (2020). Niche partitioning between coastal and offshore shelf waters results in differential expression of alkane and PAH catabolic pathways. mSystems 5, e668–e620. doi: 10.1128/mSystems.00668-20
Doyle, S. M., Whitaker, E. A., De Pascuale, V., Wade, T. L., Knap, A. H., Santschi, P. H., et al. (2018). Rapid formation of microbe-oil aggregates and changes in community composition in coastal surface water following exposure to oil and corexit. Front. Microbiol. 9:689. doi: 10.3389/fmicb.2018.00689
Edwards, B. R., Reddy, C. M., Camilli, R., Carmichael, C. A., Longnecker, K., and Van Mooy, B. A. (2011). Rapid microbial respiration of oil from the Deepwater Horizon spill in offshore surface waters of the Gulf of Mexico. Environ. Res. Lett. 6:035301. doi: 10.1088/1748-9326/6/3/035301
Engel, A. (2000). The role of transparent exopolymer particles (TEP) in the increase in apparent particle stickiness (a) during the decline of a diatom bloom. J. Plankton Res. 22, 485–497. doi: 10.1093/plank/22.3.485
Finkel, Z. V., Liang, Y., Nanjappa, D., Bretherton, L., Brown, C. M., Quigg, A., et al. (2020). A ribosomal sequence-based oil sensitivity index for phytoplankton groups. Mar. Poll. Bull. 151:110798. doi: 10.1016/j.marpolbul.2019.110798
Gärdes, A., Iversen, M. H., Grossart, H.-P., and Passow, U. (2011). Diatom associated bacteria are required for aggregation of Thalassiosira weissflogii. ISME J. 5, 436–445. doi: 10.1038/ismej.2010.145
Gearing, J. N., Gearing, P. J., Wade, T., Quinn, J. G., McCarty, H. B., Farrington, J., et al. (1979). “The rates of transport and fates of petroleum hydrocarbons in a controlled marine ecosystem and a note on analytical variability,” in Proceedings of the 1979 Oil Spill Conference, (Washington, D.C: American Petroleum Institute), 555–564. doi: 10.7901/2169-3358-1979-1-555
Genzer, J. L., Kamalanathan, M., Bretherton, L., Hillhouse, J., Xu, C., Santschi, P. H., et al. (2020). Diatom aggregation when exposed to crude oil and chemical dispersants: potential impacts of ocean acidification. PLoS One 15:e0235473. doi: 10.1371/journal.pone.0235473
Giering, S. L. C., Sanders, R., Martin, A. P., Lindemann, C., Möller, K. O., Daniels, C. J., et al. (2016). High export via small particles before the onset of the North Atlantic spring bloom. J. Geophys. Res. Oceans 121, 6929–6945. doi: 10.1002/2016JC012048
Goldtwhait, S. A., Carlson, C. A., Henderson, G. K., and Alldredge, A. K. (2004). Effects of physical fragmentation on remineralization of marine snow. Mar. Ecol. Prog. Ser. 305, 59–65. doi: 10.3354/meps305059
Gong, Y., Zhao, X., Cai, Z., O’Reilly, S. E., Hao, X., and Zhao, D. (2014). A review of oil, dispersed oil and sediment interactions in the aquatic environment: Influence on the fate, transport and remediation of oil spills. Mar. Poll. Bull. 79, 16–33. doi: 10.1016/j.marpolbul.2013.12.024
Gregson, B. H., McKew, B. A., Holland, R. D., Nedwed, T. J., Princ, E. R. C., and McGenity, T. J. (2021). Marine oil snow, a microbial perspective. Front. Mar. Sci. 8:619484. doi: 10.3389/fmars.2021.619484
Gutierrez, T., Berry, D., Teske, A., and Aitken, M. D. (2016). Enrichment of Fusobacteria in sea surface oil slicks from the Deepwater Horizon oil spill. Microorganisms 4:24. doi: 10.3390/microorganisms4030024
Gutierrez, T., Berry, D., Yang, T., Mishamandani, S., McKay, L., Teske, A., et al. (2013a). Role of bacterial exopolysaccharides (EPS) in the fate of the oil released during the Deepwater Horizon oil spill. PLoS One 8:e67717. doi: 10.1371/journal.pone.0067717
Gutierrez, T., Singleton, D. R., Berry, D., Yang, T., Aitken, M. D., and Teske, A. (2013b). Hydrocarbon-degrading bacteria enriched by the Deepwater Horizon oil spill identified by cultivation and DNA-SIP. ISME J. 7, 2091–2104.
Hamdan, L., and Fulmer, P. (2011). Effects of COREXIT® EC9500A on bacteria from a beach oiled by the Deepwater Horizon spill. Aquat. Microb. Ecol. 63, 101–109. doi: 10.3354/ame01482
Hatcher, P. G., Obeid, W., Wozniak, A. S., Xu, C., Zhang, S., Santschi, P. H., et al. (2018). Identifying oil/marine snow associations in mesocosm simulations of the Deepwater Horizon Oil Spill event using solid-state 13C NMR spectroscopy and ion cyclotron resonance mass spectrometry. Mar. Pollut. Bull. 126, 159–165. doi: 10.1016/j.marpolbul.2017.11.004
Hazen, T. C., Dubinsky, E. A., DeSantis, T. Z., Andersen, G. L., Piceno, Y. M., Singh, N., et al. (2010). Deep-sea oil plume enriches indigenous oil-degrading bacteria. Science 330, 204–208. doi: 10.1126/science.1195979
Head, I. M., Jones, D. M., and Röling, W. F. M. (2006). Marine microorganisms make a meal of oil. Nat. Rev. Microbiol. 4, 173–182. doi: 10.1038/nrmicro1348
Hedges, J. I., Cowie, G. L., Ertel, J. R., Barbour, R. J., and Hatcher, P. G. (1985). Degradation of carbohydrates and lignins in buried woods. Geochim. Cosmochim. Acta 49, 701–711. doi: 10.1016/0016-7037(85)90165-6
Henry, I. A., Netzer, R., Davies, E., and Brakstad, O. G. (2020). The influences of phytoplankton species, mineral particles and concentrations of dispersed oil on the formation and fate of marine oil-related aggregates. Sci. Total Environ. 752, 141786. doi: 10.1016/j.scitotenv.2020.141786
Hoagland, K. D., Rosowsky, J. R., Gretz, M. R., and Reomer, S. C. (1993). Diatom extracellular polymeric substances: function, fine structure, chemistry, chemistry and physiology. J. Phycol. 29, 537–556. doi: 10.1111/j.0022-3646.1993.00537.x
Honeyman, B. D., and Santschi, P. H. (1989). A Brownian-pumping model for trace metal scavenging: evidence from Th isotopes. J. Mar. Res. 47, 950–995.
Honeyman, B. D., and Santschi, P. H. (1991). Coupling of trace metal adsorption and particle aggregation: kinetic and equilibrium studies using 59Fe-labeled hematite. Environ. Sci. Technol. 25, 1739–1747. doi: 10.1021/es00022a010
Hung, C.-C., Guo, L., Schultz, G., Pinckney, J. L., and Santschi, P. H. (2003). Production and fluxes of carbohydrate species in the Gulf of Mexico. Global Biogeochem. Cycles 17:1055. doi: 10.1029/2002GB001988
Iversen, M. H., and Lampitt, R. S. (2020). Size does not matter after all: No evidence for a size-sinking relationship for marine snow. Prog. Oceanogr. 189:102445. doi: 10.1016/j.pocean.2020.102445
Jacketti, M., Beegle-Krause, C. J., and Englehardt, J. D. (2020). A review on the sinking mechanisms for oil and successful response technologies. Mar. Poll. Bull. 160:111626. doi: 10.1016/j.marpolbul.2020.111626
Jernelöv, A., and Lindén, O. (1981). Ixtoc I: a case study of the world’s largest oil spill. AMBIO 10, 299–306.
John, V., Arnosti, C., Field, J., Kujawinski, E., and McCormick, A. (2016). The role of dispersants in oil spill remediation: fundamental concepts, rationale for use, fate, and transport issues. Oceanography 29, 108–117. doi: 10.5670/oceanog.2016.75
Johnson, R. L., and Schmidt-Rohr, K. (2014). Quantitative solid-state 13C NMR with signal enhancement by multiple cross polarization. J. Magnetic Resonance 239, 44–49. doi: 10.1016/j.jmr.2013.11.009
Kamalanathan, M., Chiu, M.-H., Bacosa, H., Schwehr, K., Tsai, S.-M., Doyle, S., et al. (2019). Role of polysaccharides in diatom Thalassiosira pseudonana and its associated bacteria in hydrocarbon presence. Plant Physiol. 180, 1898–1911. doi: 10.1104/pp.19.00301.pp.00301.2019
Kamalanathan, M., Doyle, S. M., Xu, C., Achberger, A. M., Wade, T. L., Schwehr, K., et al. (2020). Exoenzymes as a signature of microbial response to marine environmental conditions. mSystems 5, e00290–20. doi: 10.1128/mSystems.00290-20
Kamalanathan, M., Schwehr, K., Labonté, J., Taylor, C., Bergen, C., Patterson, N., et al. (2021). “The interplay of phototrophic and heterotrophic microbes under oil exposure-a microcosm study,” in Frontiers in Microbiology-Aquatic Microbiology, eds M. Kamalanathan, K. Schwehr, J. Labonté, C. Taylor, C. Bergen, N. Patterson, et al.
Kamalanathan, M., Xu, C., Schwehr, K., Bretherton, L., Beaver, M., Doyle, S. M., et al. (2018). Extracellular enzyme activity profile in a chemically enhanced water accommodated fraction of surrogate oil: toward understanding microbial activities after the Deepwater Horizon oil spill. Front. Microbiol. 9:798. doi: 10.3389/fmicb.2018.00798
Kappell, A. D., Wei, Y., Newton, R. J., Van Nostrand, J. D., Zhou, J., McLellan, S. L., et al. (2014). The polycyclic aromatic hydrocarbon degradation potential of Gulf of Mexico native coastal microbial communities after the Deepwater Horizon oil spill. Front. Microbiol. 5:205.
Karl, D. M., Knauer, G. A., and Martin, J. H. (1988). Downward flux of particulate organic matter in the ocean: a particle decomposition paradox. Nature 332, 438–441. doi: 10.1038/332438a0
Kessler, J. D., Valentine, D. L., Redmond, M. C., Du, M., Chan, E. W., Mendes, S. D., et al. (2011). A persistent oxygen anomaly reveals the fate of spilled methane in the deep Gulf of Mexico. Science 331, 312–315. doi: 10.1126/science.1199697
Khelifa, A., and Hill, P. S. (2006). Models for effective density and settling velocity of flocs. J. Hydraulic Res. 44, 390–401. doi: 10.1080/00221686.2006.9521690
Kimes, N. E., Callaghan, A. V., Aktas, D. F., Smith, W. L., Sunner, J., Golding, B. T., et al. (2013). Genomic analysis and metabolite profiling of deep-sea sediments from the Gulf of Mexico following the Deepwater Horizon oil spill. Front. Microbiol. 4:50. doi: 10.3389/fmicb.2013.00050
Kimes, N. E., Callaghan, A. V., Suflita, J. M., and Morris, P. J. (2014). Microbial transformation of the Deepwater Horizon oil spill—past, present, and future perspectives. Front. Microbiol. 5:603.
Kiørboe, T., Andersen, K. P., and Dam, H. G. (1990). Coagulation efficiency and aggregate formation in marine phyto–plankton. Mar. Biol. 107, 235–245. doi: 10.1007/bf01319822
Kleindienst, S., Grim, S., Sogin, M., Bracco, A., Crespo-Medina, M., and Joye, S. B. (2016). Diverse, rare microbial taxa responded to the Deepwater Horizon deep-sea hydrocarbon plume. ISME J. 10, 400–415. doi: 10.1038/ismej.2015.121
Kleindienst, S., Seidel, M., Ziervogel, K., Grim, S., Loftis, K., Harrison, S., et al. (2015). Chemical dispersants can suppress the activity of natural oil-degrading microorganisms. Proc. Natl. Acad. Sci. U.S.A. 112, 14900–14905. doi: 10.1073/pnas.1507380112
Kowalewska, G. (1999). Phytoplankton-the main factor responsible for transport of polynuclear aromatic hydrocarbons from water to sediments in the Southern Baltic ecosystem. ICES J. Mar. Sci. 56, 219–222. doi: 10.1006/jmsc.1999.0607
Kowalewska, G., and Konat, J. (1997). The role of phytoplankton in the transport and distribution of polynuclear aromatic hydrocarbons (PAHs) in the southern Baltic environment. Oceanologia 39:267.
Kujawinski, E. B., Reddy, C. M., Rodgers, R. P., Thrash, J. C., Valentine, D. L., and White, H. K. (2020). The first decade of scientific insights from the Deepwater Horizon oil release. Nat. Rev. Earth Environ. 1, 237–250. doi: 10.1038/s43017-020-0046-x
Laurenceau-Cornec, E. C., Le Moigne, F. A. C., Gallinari, M., Moriceau, B., Toullec, J., Iversen, M. H., et al. (2020). New guidelines for the application of Stokes’ models to the sinking velocity of marine aggregates. Limnol. Oceanogr. 65, 1264–1285. doi: 10.1002/lno.11388
Liu, A., Ahn, I. S., Mansfield, C., Lion, L. W., Shuler, M. L., and Ghiorse, W. C. (2001). Phenanthrene desorption from soil in the presence of bacterial extracellular polymer: Observations and model predictions of dynamic behavior. Water Res. 35, 835–843. doi: 10.1016/s0043-1354(00)00324-9
Lu, Z., Deng, Y., Van Nostrand, J. D., He, Z., Voordeckers, J., Zhou, A., et al. (2012). Microbial gene functions enriched in the Deepwater Horizon deep-sea oil plume. ISME J. 6, 451–460. doi: 10.1038/ismej.2011.91
Lumibao, C. Y., Formel, S., Elango, V., Pardue, J. H., Blum, M., and Van Bael, S. A. (2018). Persisting responses of salt marsh fungal communities to the Deepwater Horizon oil spill. Sci. Total Environ. 642, 904–913. doi: 10.1016/j.scitotenv.2018.06.077
Mason, O. U., Hazen, T. C., Borglin, S., Chain, P. S., Dubinsky, E. A., Fortney, J. L., et al. (2012). Metagenome, meta-transcriptome and single-cell sequencing reveal microbial response to Deepwater Horizon oil spill. ISME J. 6, 1715–1727. doi: 10.1038/ismej.2012.59
Mason, O. U., Scott, N. M., Gonzalez, A., Robbins-Pianka, A., Bælum, J., Kimbrel, J., et al. (2014). Metagenomics reveals sediment microbial community response to Deepwater Horizon oil spill. ISME J. 8, 1464–1475. doi: 10.1038/ismej.2013.254
McGenity, T., Folwell, B., McKew, B., and Sanni, G. (2012). Marine crude-oil biodegradation: a central role for interspecies interactions. Aquat Biosyst. 8:10. doi: 10.1186/2046-9063-8-10
McKew, B. A., Coulon, F., Osborn, A. M., Timmis, K. N., and McGenity, T. J. (2007). Determining the identity and roles of oil-metabolizing marine bacteria from the Thames estuary. UK. Environ. Microbiol. 9, 165–176. doi: 10.1111/j.1462-2920.2006.01125.x
Meng, L., Liu, H., Bao, M., and Sun, P. (2016). Microbial community structure shifts are associated with temperature, dispersants and nutrients in crude oil-contaminated seawaters. Mar. Poll. Bull. 111, 203–212. doi: 10.1016/j.marpolbul.2016.07.010
Mishamandani, S., Gutierrez, T., Berry, D., and Aitken, M. D. (2016). Response of the bacterial community associated with a cosmopolitan marine diatom to crude oil shows a preference for the biodegradation of aromatic hydrocarbons. Environ. Micro. 18, 1817–1833. doi: 10.1111/1462-2920.12988
Morales-McDevitt, M. E., Shi, D., Knap, A. H., Quigg, A., Sweet, S. T., Sericano, J. L., et al. (2020). Mesocosm experiments to better understand hydrocarbon half-lives for oil and oil dispersant mixtures. PLoS One 15:e0228554. doi: 10.1371/journal.pone.0228554
Nagasaki, K., Tomaru, Y., Katanozaka, N., Shirai, Y., Nishida, K., Itakura, S., et al. (2004). Isolation and characterization of a novel single-stranded RNA virus infecting the bloom-forming diatom Rhizosolenia. Appl. Environ. Microbiol. 70, 704–711. doi: 10.1128/aem.70.2.704-711.2004
Ortega-Retuerta, E., Passow, U., Duarte, C. M., and Reche, I. (2009). Effects of ultraviolet B radiation on (not so) transparent exopolymer particles. Biogeosciences 6, 3071–3080. doi: 10.5194/bg-6-3071-2009
Ozhan, K., Zahraeifard, S., Smith, A. P., and Bargu, S. (2015). Induction of reactive oxygen species in marine phytoplankton under crude oil exposure. Environ. Sci. Pollut. Res. 22, 18874–18884. doi: 10.1007/s11356-015-5037-y
Parker, D. S., Kaufman, W. J., and Jenkins, D. (1972). Floc breakup in turbulent flocculation processes. J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 98, 79–99. doi: 10.1061/jsedai.0001389
Parsons, M. L., Morrison, W., Rabalais, N. N., Turner, R. E., and Tyre, K. N. (2015). Phytoplankton and the Macondo oil spill: a comparison of the 2010 phytoplankton assemblage to baseline conditions on the Louisiana shelf. Environ. Pollut. 207, 152–160. doi: 10.1016/j.envpol.2015.09.019
Passow, U. (1995). A dye-binding assay for the spectrophotometric measurement of transparent exopolymer particles (TEP). Limnol. Oceanogr. 40, 1326–1335. doi: 10.4319/lo.1995.40.7.1326
Passow, U. (2002). Transparent exopolymer particles (TEP) in aquatic environments. Progr. Oceanogr. 55, 287–333. doi: 10.1016/S0079-6611(02)
Passow, U. (2016). Formation of rapidly-sinking, oil-associated marine snow. Deep Sea Res. II Top. Stud. Oceanogr. 129, 232–240. doi: 10.1016/j.dsr2.2014.10.001
Passow, U., and Overton, E. B. (2021). The complexity of spills: the fate of the Deepwater Horizon Oil. Ann. Rev. Mar. Sci. 13:1. doi: 10.1146/annurev-marine-032320-095153
Passow, U., Sweet, J., Francis, S., Xu, C., Dissanayake, A. L., Lin, Y.-Y., et al. (2019). Incorporation of oil into diatom aggregates. Mar. Ecol. Prog. Ser. 612, 65–86. doi: 10.3354/meps12881
Passow, U., Sweet, J., and Quigg, A. (2017). How Corexit Impacts the formation of sinking marine oil snow. Mar. Pollut. Bull. 125, 139–145. doi: 10.1016/j.marpolbul.2017.08.015
Passow, U., Ziervogel, K., Asper, V., and Diercks, A. (2012). Marine snow formation in the aftermath of the Deepwater Horizon oil spill in the Gulf of Mexico. Environ. Res. Lett. 7, 1–11. doi: 10.1088/1748-9326/7/3/035301
Paul, J. H., Hollander, D., Coble, P., Daly, K. L., Murasko, S., English, D., et al. (2013). Toxicity and Mutagenicity of Gulf of Mexico Waters during and After the Deepwater Horizon Oil Spill. Environ. Sci. Technol. 47, 9651–9659. doi: 10.1021/es401761h
Prince, R. C., Gramain, A., and McGenity, T. J. (2010). “Prokaryotic hydrocarbon degraders,” in Handbook of Hydrocarbon and Lipid Microbiology, ed. K. N. Timmis (Berlin: Springer), 1669–1692. doi: 10.1007/978-3-540-77587-4_118
Quigg, A., Chin, W.-C., Chen, C.-S., Zhang, S., Jiang, Y., Miao, A.-J., et al. (2013). Direct and indirect toxic effects of engineered nanoparticles on algae: role of natural organic matter. Special Issue Sustain. Nanotechnol. ACS Sustain. Chem. Eng. 1, 686–702. doi: 10.1021/sc400103x
Quigg, A., Farrington, J. W., Glibert, S., Murawski, S. A., and John, V. (2021a). A decade of GoMRI dispersant science: lessons learned and recommendations for the future. Accepted Oceanogr. 34, 98–111. doi: 10.5670/oceanog.2021.119
Quigg, A., Parsons, M., Bargu, S., Ozhan, K., Daly, K., Chakraborty, S., et al. (2021b). Marine phytoplankton responses to oil and dispersant exposures: Knowledge gained since the Deepwater Horizon Oil Spill. Mar. Poll. Bull. 164:112074. doi: 10.1016/j.marpolbul.2021.112074
Quigg, A., Passow, U., Chin, W. C., Xu, C., Doyle, S., Bretherton, L., et al. (2016). The role of microbial exopolymers in determining the fate of oil and chemical dispersants in the ocean. Limnol. Oceanogr. Lett. 1, 3–26. doi: 10.1002/lol2.10030
Quigg, A., Passow, U., Daly, K. L., Burd, A., Hollander, D. J., Schwing, P. T., et al. (2020). “Chapter 12: Marine Oil Snow Sedimentation and Flocculent Accumulation (MOSSFA) events: learning from the past to predict the future,” in Deep Oil Spills – Facts, Fate and Effects. Springer 608 pp. ISBN 978-3-030-11605-7, eds S. A. Murawski, C. Ainsworth, S. Gilbert, D. Hollander, C. B. Paris, M. Schlüter, et al. (Berlin: Springer), 199–224. doi: 10.1007/978-3-030-11605-7
Rau, M. J., Ackleson, S. G., and Smith, G. B. (2018). Effects of turbulent aggregation on clay floc breakup and implications for the oceanic environment. PLoS One 13:e0207809. doi: 10.1371/journal.pone.0207809
Redmond, M. C., and Valentine, D. L. (2012). Natural gas and temperature structured a microbial community response to the Deepwater Horizon oil spill. Proc. Natl. Acad. Sci. U.S.A. 109, 20292–20297. doi: 10.1073/pnas.1108756108
Rivers, A. R., Sharma, S., Tringe, S. G., Martin, J., Joye, S. B., and Moran, M. A. (2013). Transcriptional response of bathypelagic marine bacterioplankton to the Deepwater Horizon oil spill. ISME J. 7, 2315–2329. doi: 10.1038/ismej.2013.129
Romero, I. C., Schwing, P. T., Brooks, G. R., Larson, R. A., Hastings, D. W., Ellis, G., et al. (2015). Hydrocarbons in deep-sea sediments following the 2010 Deepwater Horizon blowout in the northeast Gulf of Mexico. PLoS One 10:e0128371. doi: 10.1371/journal.pone.0128371
Ross, J., Hollander, D., Burd, A. B., Gilbert, S., Saupe, S., and Quigg, A. (2021). Integrating marine oil snow sedimentation and flocculent accumulation (MOSSFA) events in oil spill response and damage assessment. Mar. Pollut. Bull. 165:112025. doi: 10.1016/j.marpolbul.2021.112025
Ruddy, B. M., Huette, M., Kostka, J. E., Lobodin, V. V., Bythell, B. J., McKenna, A. M., et al. (2014). Targeted petroleomics: analytical investigation of Macondo well oil oxidation products from Pensacola Beach. Energy Fuels 28, 4043–4050. doi: 10.1021/ef500427n
Ruiz, J. (1997). What generates daily cycles of marine snow? Deep Sea Res. I 44, 1105–1126. doi: 10.1016/s0967-0637(97)00012-5
Santschi, P. H. (2018). Marine colloids, agents of the self-cleansing capacity of aquatic systems: historical perspective and new discoveries. Mar. Chem. 207, 124–135. doi: 10.1016/j.marchem.2018.11.003
Santschi, P. H., Xu, C., Schwehr, K. A., Lin, P., Sun, L., Chin, W. C., et al. (2020). Can the protein/carbohydrate (P/C) ratio of exopolymeric substances (EPS) be used as a proxy for their ‘stickiness’ and aggregation propensity? Mar. Chem. 218:103734. doi: 10.1016/j.marchem.2019.103734
Schwehr, K. A., Xu, C., Chiu, M.-H., Zhang, S., Sun, L., Lin, P., et al. (2018). Protein/polysaccharide ratio in exopolymeric substances controlling the surface tension of seawater in the presence or absence of surrogate Macondo Oil with and without Corexit. Mar. Chem. 206, 84–92. doi: 10.1016/j.marchem.2018.09.003
Severin, T., and Erdner, D. L. (2019). The phytoplankton taxon-dependent oil response and its microbiome: correlation but not causation. Front. Microbiol. 10:385. doi: 10.3389/fmicb.2019.00385
Shi, D., Bera, G., Knap, A. H., Quigg, A., Al Atwah, I., Gold-Bouchot, G., et al. (2020). A mesocosm experiment to determine half-lives of individual hydrocarbons in simulated oil spill scenarios with and without dispersant. Mar. Poll. Bull. 151:110804. doi: 10.1016/j.marpolbul.2019.110804
Shiu, R.-F., Chiu, M.-H., Vazquez, C. I., Tsai, Y.-Y., Le, A., Kagiri, A., et al. (2020). Protein to carbohydrate (P/C) ratio changes in microbial extracellular polymeric substances induced by oil and Corexit. Mar Chem. 223:103789. doi: 10.1016/j.marchem.2020.103789
Silver, M. (2015). Marine snow: a brief historical sketch. Limnol. Oceanogr. Bull. 24, 5–10. doi: 10.1002/lob.10005
Sinsabaugh, R. L., Hill, B. H., and Shah, J. J. F. (2009). Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462, 795–798. doi: 10.1038/nature08632
Song, W. J., Zhao, C., Mu, S., Pan, X., Zhang, D., Al-Misned, F. A., et al. (2015). Effects of irradiation and pH on fluorescence properties and flocculation of extracellular polymeric substances from the cyanobacterium Chroococcus minutus. Colloids Surf B Biointerfaces 128, 115–118. doi: 10.1016/j.colsurfb.2015.02.017
Spath, R., Flemming, H. C., and Wuertz, S. (1998). Sorption properties of biofilms. Water Sci. Technol. 37, 207–210. doi: 10.2166/wst.1998.0623
Steinberg, D. K., Silver, M. W., and Pilskaln, C. H. (1997). Role of mesopelagic zooplankton in the community metabolism of giant larvacean house detritus in Monterey Bay. California, USA. Mar. Ecol. Prog. Ser. 147, 167–179. doi: 10.3354/meps147167
Sterling, M. C. Jr., Bonner, J. S., Ernest, A. N. S., Page, C. A., and Autenrieth, R. L. (2005). Application of fractal flocculation and vertical transport model to aquatic-sol systems. Wat. Res. 39, 1818–1830. doi: 10.1016/j.watres.2005.02.007
Stordal, M. C., Santschi, P. H., and Gill, G. A. (1996). Colloidal pumping: Evidence for the coagulation process using natural colloids tagged with 203Hg. Environ. Sci. Technol. 30, 3335–3340. doi: 10.1021/es9601806
Sun, L., Chin, W.-C., Chiu, H.-H., Xu, C., Lin, P., Schwehr, K. A., et al. (2020). Sunlight induced aggregation of protein-containing dissolved organic matter (DOM) in the coastal ocean. Mar. Chem. 654, 872–877.
Sun, L., Chin, W.-C., Chiu, M.-H., Xu, C., Lin, P., Schwehr, K. A., et al. (2019). Sunlight induced aggregation of protein-containing dissolved organic matter in the ocean. Sci. Total Environ. 654, 872–877.
Sun, L., Chiu, M.-H., Xu, C., Lin, P., Schwehr, K. A., Bacosa, H., et al. (2018). The effects of sunlight on the composition of exopolymeric substances affecting the subsequent aggregation process during oil spill and corexit exposure. Mar. Chem. 203, 49–54. doi: 10.1016/j.marchem.2018.04.006
Sun, L., Xu, C., Chin, W. C., Zhang, S., Lin, P., Schwehr, K. A., et al. (2017). Light-induced aggregation of microbial exopolymeric substances. Chemosphere 181, 675–681. doi: 10.1016/j.chemosphere.2017.04.099
Techtmann, S. M., Zhuang, M., Campo, P., Holder, E., Elk, M., Hazen, T. C., et al. (2017). Corexit 9500 enhances oil biodegradation and changes active bacterial community structure of oil-enriched microcosms. Appl. Environ. Microbiol. 83, e03462–16. doi: 10.1128/AEM.03462-16
Testa, J. M., Adams, E. E., North, E. W., and He, R. (2016). Modeling the influence of deep water application of dispersants on the surface expression of oil: a sensitivity study. J. Geophys. Res. Oceans 121, 5995–6008. doi: 10.1002/2015jc011571
Valentine, D. L., Fisher, G. B., Bagby, S. C., Nelson, R. K., Reddy, C. M., Sylva, S. P., et al. (2014). Fallout plume of submerged oil from Deepwater Horizon. Proc. Natl. Acad. Sci. U.S.A. 111, 15906–15911. doi: 10.1073/pnas.1414873111
Valentine, D. L., Kessler, J. D., Redmond, M. C., Mendes, S. D., Heintz, M. B., Farwell, C., et al. (2010). Propane respiration jump-starts microbial response to a deep oil spill. Science 330, 208–211. doi: 10.1126/science.1196830
Verdin, A., Sahraoui, A. L. H., and Durand, R. (2004). Degradation of benzo [a] pyrene by mitosporic fungi and extracellular oxidative enzymes. Intern. Biodeter. Biodegr. 53, 65–70. doi: 10.1016/j.ibiod.2003.12.001
Verdugo, P. (2007). Dynamics of marine biopolymer networks. Polym. Bull. 58, 139–143. doi: 10.1007/s00289-006-0615-2
Verdugo, P. (2012). Marine microgels. Annu. Rev. Mar. Sci. 4, 375–400. doi: 10.1146/annurev-marine-120709-142759
Verdugo, P., Alldredge, A. L., Azam, F., Kirchman, D. L., Passow, U., and Santschi, P. (2004). The oceanic gel phase: a bridge in the DOM-POM continuum. Mar. Chem. 92, 67–85. doi: 10.1016/j.marchem.2004.06.017
Verdugo, P., Alldredge, A. L., Azam, F., Kirchman, D. L., Passow, U., and Santschi, P. H. (2005). The oceanic gel phase: a bridge in the DOM-POM continuum. Mar. Chem. 92, 65–66.
Verdugo, P., Orellana, M. V., Chin, W. C., Petersen, T. W., van den Eng, G., Benner, R., et al. (2008). Marine biopolymer self-assembly: implications for carbon cycling in the ocean. Faraday Discuss 139, 393–398. doi: 10.1039/b800149a
Verdugo, P., and Santschi, P. H. (2010). Polymer dynamics of DOC networks and gel formation in seawater. Deep Sea Res II 57, 1486–1493. doi: 10.1016/j.dsr2.2010.03.002
Vonk, S. M., Hollander, D. J., and Murk, A. J. (2015). Was the extreme and widespread marine oil-snow sedimentation and flocculent accumulation (MOSSFA) event during the Deepwater Horizon blow-out unique? Mar. Pollut. Bull. 100, 5–12. doi: 10.1016/j.marpolbul.2015.08.023
Wade, T. L., Morales-McDevitt, M., Bera, G., Shi, D., Sweet, S., Wang, B., et al. (2017). A method for the production of large volumes of WAF and CEWAF for dosing mesocosms to understand marine oil snow formation. Heliyon 3, e00419. doi: 10.1016/j.heliyon.2017.e00419
Wade, T. L., and Quinn, J. G. (1980). Incorporation, distribution, and fate of saturated petroleum hydrocarbons in sediments from a controlled ecosystem. Mar. Environ. Res. 3, 15–33. doi: 10.1016/0141-1136(80)90033-1
Ward, C. P., Armstrong, C. J., Conmy, R. N., French-McCay, D. P., and Reddy, C. M. (2018). Photochemical oxidation of oil reduced the effectiveness of aerial dispersants applied in response to the Deepwater Horizon spill. Environ. Sci. Technol. Lett. 5, 226–231. doi: 10.1021/acs.estlett.8b00084
Wen, L. S., Santschi, P. H., and Tang, D. (1997). Interactions between radioactively labeled colloids and natural particles: Evidence for Colloidal Pumping. Geochim. Cosmochim. Acta 61, 2867–2878. doi: 10.1016/s0016-7037(97)00139-7
Wozniak, A. S., Prem, P., Obeid, W., Waggoner, D., Quigg, A., Xu, C., et al. (2019). Rapid degradation of oil in mesocosm simulations of marine oil snow events. Environ. Sci. Technol. 53, 3441–3450. doi: 10.1021/acs.est.8b06532
Xu, C., Chin, W.-C., Lin, P., Chen, H. M., Lin, P., Chiu, M.-C., et al. (2019). Marine gels, extracellular polymeric substances (EPS) and transparent exopolymeric particles (TEP) in natural seawater and seawater contaminated with a water accommodated fraction of Macondo oil surrogate. Mar. Chem. 215:103667. doi: 10.1016/j.marchem.2019.103667
Xu, C., Santschi, P. H., Hung, C. C., Zhang, S. J., Schwehr, K. A., Roberts, K. A., et al. (2011). Controls of (234)Th removal from the oligotrophic ocean by polyuronic acids and modification by microbial activity. Mar. Chem. 123, 111–126. doi: 10.1016/j.marchem.2010.10.005
Xu, C., Zhang, S., Beaver, M., Lin, P., Sun, L., Doyle, S., et al. (2018a). The role of microbially-mediated exopolymeric substances (EPS) in regulating Macondo oil transport in a mesocosm experiment. Mar. Chem. 206, 52–61. doi: 10.1016/j.marchem.2018.09.005
Xu, C., Zhang, S., Beaver, M., Lin, Y., Wade, T. L., Schwehr, K. A., et al. (2018b). Decreased sedimentation efficiency of petro- and non-petro-carbon caused by a dispersant for Macondo surrogate oil in a mesocosm simulating a coastal microbial community. Mar. Chem. 206, 34–43. doi: 10.1016/j.marchem.2018.09.002
Yang, T., Nigro, L. M., Gutierrez, T., D’Ambrosio, L., Joye, S. B., Highsmith, R., et al. (2016). Pulsed blooms and persistent oil-degrading bacterial populations in the water column during and after the Deepwater Horizon blowout. Deep Sea Res. Part II Top. Stud. Oceanogr. 129, 282–291. doi: 10.1016/j.dsr2.2014.01.014
Ye, L., Manning, A. J., and Hsu, T. J. (2020). Oil-mineral flocculation and settling velocity in saline water. Water Res. 173:115569. doi: 10.1016/j.watres.2020.115569
Zahed, M. A., Aziz, H. A., Isa, M. H., and Mohajeri, L. (2010). Effect of initial oil concentration and dispersant on crude oil biodegradation in contaminated seawater. Bull. Environ. Contamin. Toxic. 84, 438–442. doi: 10.1007/s00128-010-9954-7
Zhao, L., Boufadel, M. C., Geng, X., Lee, K., King, T., Robinson, B., et al. (2016). A-DROP: a predictive model for the formation of oil particle aggregates (OPAs). Mar. Poll. Bull. 106, 245–259. doi: 10.1016/j.marpolbul.2016.02.057
Zhao, Y., Chen, W., and Wen, D. (2020). The effects of crude oil on microbial nitrogen cycling in coastal sediments. Environ. Internat. 139, 105724. doi: 10.1016/j.envint.2020.105724
Ziervogel, K. (2020). Exopolymeric Substances (EPS) Mechanisms in Aggregation & Dispersion of Oil: Fragmentation of Skeletonema Grethae-775 snow – Disaggregation Dynamics. Distributed by: Gulf of Mexico Research Initiative Information and Data Cooperative (GRIIDC), Harte Research Institute, Texas A&M University–Corpus Christi. Corpus Christi TX: Texas A&M University–Corpus Christi, doi: 10.7266/n7-qzqh-bq48
Ziervogel, K., D’Souza, N., Sweet, J., Yan, B., and Passow, U. (2014). Natural oil slicks fuel microbial activities in surface waters above cold seeps in the Gulf of Mexico. Front. Aquat. Microbiol. 5:188. doi: 10.3389/fmicb.2014.00188
Ziervogel, K., Joye, S. B., and Arnosti, C. (2016). Microbial enzymatic activity and secondary production in sediments affected by the sedimentation pulse following the Deepwater Horizon oil spill. Deep Sea Res. Part II 129, 241–248. doi: 10.1016/j.dsr2.2014.04.003
Ziervogel, K., McKay, L., Rhodes, B., Osburn, C. L., Dickson-Brown, J., Arnosti, C., et al. (2012). Microbial activities and dissolved organic matter dynamics in oil contaminated surface seawater from the Deepwater Horizon oil spill site. PLoS One 7:e34816. doi: 10.1371/journal.pone.0034816
Keywords: marine oil snow, marine snow, exopolymers, MOSSFA, deepwater horizon, oil
Citation: Quigg A, Santschi PH, Xu C, Ziervogel K, Kamalanathan M, Chin W-C, Burd AB, Wozniak A and Hatcher PG (2021) Aggregation and Degradation of Dispersants and Oil by Microbial Exopolymers (ADDOMEx): Toward a Synthesis of Processes and Pathways of Marine Oil Snow Formation in Determining the Fate of Hydrocarbons. Front. Mar. Sci. 8:642160. doi: 10.3389/fmars.2021.642160
Received: 15 December 2020; Accepted: 24 June 2021;
Published: 19 July 2021.
Edited by:
Michael R. Stukel, Florida State University, United StatesReviewed by:
Tian Jian Hsu, University of Delaware, United StatesCopyright © 2021 Quigg, Santschi, Xu, Ziervogel, Kamalanathan, Chin, Burd, Wozniak and Hatcher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonietta Quigg, cXVpZ2dhQHRhbXVnLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.