
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 13 April 2021
Sec. Ocean Observation
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.629643
This article is part of the Research TopicInnovation and Discoveries in Marine Soundscape ResearchView all 20 articles
Soundscape ecology is a relatively new field that can provide insights into the structure and health of marine habitats. Though this field is growing, the acoustics of many marine habitats, including the giant kelp forests off Southern California, remain poorly studied. Here, we examine the diel and seasonal periodicity of kelp forest soundscapes within a protected and unprotected site off San Diego, CA. Singular value decomposition was used to identify frequency bands of interest, enabling tracking of these bands through seasons to examine their variability. Four frequency bands were identified: (1) 60–130 Hz, which encompassed a putative fish chorus, (2) 300–500 Hz, which encompassed a different putative fish chorus, (3) a band that encompassed humming generated by Plainfin Midshipmen Poricthys notatus (fundamental frequency: 85–95 Hz, and two subharmonics 175–185 Hz and 265–275 Hz), and (4) a band that encompassed the snaps of snapping shrimps from 2.5 to 7.5 kHz. Overall, kelp forest soundscapes exhibited diel and seasonal variability. In particular, the two putative fish choruses dominated the dusk soundscapes during late spring and summer, and the Midshipmen hums persisted throughout nights in summer. Snapping shrimp sounds exhibited stereotypic crepuscular activity, with peaks in acoustic energy in the 2.5–7.5 kHz band occurring at dusk and dawn. In addition, vessel noise was identified and found to exhibit strong seasonal and spatial variation. Vessel noise was greatest during August and September at the protected site and was generally lower during the winter and spring months. These findings help establish reference acoustic indices for the kelp forests off Southern California, within and outside of a protected area, and can provide resource managers with information on how well a marine reserve protects a species of interest, as well as the putative human visitation of these protected areas.
Sound sources of biological, geophysical, and anthropogenic origin all contribute to an underwater soundscape (Pijanowski et al., 2011). Marine soundscapes, like the habitats from which they emanate, are dynamic and vary over time and space (Radford et al., 2008; McWilliam and Hawkins, 2013; Monczak et al., 2019), and even adjacent habitats can exhibit distinct soundscapes (Radford et al., 2010, 2014; Butler et al., 2016). Physical properties of the environment (e.g., structural habitat complexity or day length) influence a habitat’s soundscape (Lammers et al., 2008; Kennedy et al., 2010), as do the density and diversity of species within each habitat (Kennedy et al., 2010; Kaplan et al., 2015; Nedelec et al., 2015; Buscaino et al., 2016; Merchant et al., 2016; Pieretti et al., 2017; Wilson et al., 2020). Although sound has the potential to propagate over long distances in seawater, the soundscapes emanating from reefs have been linked to small-scale variation (e.g., on the order of tens of meters) in reef fish communities (Kaplan et al., 2015; Nedelec et al., 2015). Thus, habitat-associated soundscapes (i.e., a soundscape that emanates from a specific habitat) can provide information about the quality of a habitat and its inhabitants (Kennedy et al., 2010; Radford et al., 2014; Lyon et al., 2019).
Fish are important contributors to marine soundscapes, and many fish produce sounds during courtship, spawning, and feeding activities (Connaughton and Taylor, 1996; Gilmore, 2002; Mann et al., 2009; Wilson et al., 2020). Fish have evolved the most diverse array of sound producing organs of all vertebrates (Ladich and Fine, 2006). Many fish species produce sounds by vibrating their swim bladders via sonic muscles (e.g., Oyster Toadfish, Opsanus tau Skoglund, 1961) and Red Drum, Sciaenops ocellatus (Parmentier et al., 2014), whereas others vibrate their pectoral girdle (e.g., Sculpin, Myoxocephalus octodecimspinus; Barber and Mowbray, 1956), pluck enlarged fin tendons (e.g., Croaking Gourami, Trichopsis vittatus; Kratochvil, 1978), or stridulate pharyngeal teeth [e.g., grunts (Haemulidae); Tavolga, 1971]. Furthermore, some fishes will aggregate into large groups and call persistently, creating underwater choruses and adding substantial acoustic energy to the marine soundscape (Cato, 1978; McCauley, 2012; Erbe et al., 2015).
Although there is growing evidence that habitat-associated soundscapes underpin various ecological processes, the soundscapes of many coastal habitats have yet to be characterized including patterns of temporal and spatial variation. For example, a Web of Science search for literature regarding soundscapes of specific coastal habitats in June 2020 revealed 40 studies of coral reef soundscapes, yet only 11 studies on seagrass soundscapes, five studies of mangrove soundscapes, and despite a long history of kelp forest research dating back to Darwin (1839), only one kelp forest soundscape study (Rossi et al., 2017). In addition, Radford et al. (2010) discuss the soundscape of “macroalgal-dominated reefs” off the coast of New Zealand, and Gottesman et al. (2020) discuss kelp soundscapes of the Channel Islands.
Kelp forests are ecologically and economically important coastal habitats of temperate waters worldwide. The macroalgal species that dominate these habitats (often Macrocystis pyrifera off the coast of Southern California) are highly productive (Jackson, 1987) and create three-dimensional structure in the environment, with some individuals reported growing from the seafloor upwards of 60 m to the ocean’s surface (Shiel and Foster, 2015). This structure provides habitat and shelter for hundreds of species of algae, invertebrates, fishes, and mammals (North, 1971; Foster and Schiel, 1985) in Southern California kelp forests, and many of these species are obligate kelp forest inhabitants (e.g., Surfperch, Cymatogastor aggregata, Kelp Crab, Pugettia producta, and Umbrella Crab, Cryptolythoides stichensis; Graham, 2004).
Among the species found in kelp forest communities, many produce sounds. White Seabass, Atractoscion nobilis, form spawning aggregations in Southern California and produce pulse trains, drumrolls and thuds at low frequencies (around 70–80 Hz) during courtship (Aalbers and Drawbridge, 2008). Giant Sea Bass, Stereolepis gigas, produce “boom” calls in association with aggressive behavior toward other Giant Sea Bass (Clark and Allen, 2018). Multiple different choruses of unknown fish species have recently been reported offshore of kelp forests in Southern California (Pagniello et al., 2019). Many of the fish in the kelp forests, such as the iconic Garibaldi, Hypsypops rubicundus, and Barred Sand Bass, Paralabrax nebulifer, are members of the Pomacentridae and Serranidae—families with many sound producing members (e.g., Rice and Lobel, 2003; Parmentier and Diogo, 2006; Mann et al., 2009; Nelson et al., 2011). Anecdotal evidence from SCUBA divers suggests that these species may produce calls supporting further research efforts into the identification of biological sources of kelp forest sounds.
Unfortunately, the proximity of kelp forests to the coast exposes them to direct human impacts, including pollution, increased sedimentation, and overfishing (Konar and Roberts, 1996; Jackson et al., 2001; Foster and Schiel, 2010). For example, two kelp forests, one offshore of Los Angeles, California and the other offshore of San Diego, California, collapsed likely due to the confluence of extreme environmental factors and rapid coastal urbanization (Foster and Schiel, 2010). Increased regulations on sewage outflows and better treatment techniques have facilitated the recovery of some forests, yet few have returned to their full extent (Shiel and Foster, 2015). In addition to land-based pollution, historical overfishing of coastal ecosystems can lead to trophic imbalances that allow herbivores to overgraze primary producers (e.g., the near-extirpation of sea otters from the Aleutian Islands allowed sea urchins to graze away kelp forests; Estes and Palmisano, 1974). These and other anthropogenic effects on kelp forests will likely be exacerbated as global oceanic temperatures rise and the concomitant decrease in nutrients available for growth. These changes are of particular concern along the coast of Southern California where kelp forests are stressed by decadal and secular changes in temperature and associated nutrient conditions (Parnell et al., 2010).
As continued research reveals the interplay between marine soundscapes and ecological processes (e.g., larval recruitment, Lillis et al., 2013 and community dynamics, Nedelec et al., 2015), it is becoming increasingly important to monitor and conserve coastal soundscapes—particularly the soundscapes of ecologically and economically significant communities such as kelp forests. Thus, the goals of the present study were to examine the spatial and temporal variability in soundscapes of two kelp forest areas, one inside and the other outside of a Marine Protected Area (MPA), off the coast of La Jolla, CA to address the following questions:
(1) What sounds dominate the kelp forest soundscape?
(2) How do kelp forest soundscapes vary temporally and spatially?
(3) Can these soundscapes be used to estimate anthropogenic use in the kelp forest?
Our study was in the La Jolla kelp forest, off San Diego, California, United States. The nearshore habitat is dominated by a hard-bottom substratum scattered with sand and cobble patches (Parnell, 2015). The kelp forest varies in size over time, but its fullest extent is ∼8 km long and ∼1.5 km wide (Parnell et al., 2006). Many species of algae compose the kelp forest community; however, the dominant biogenic engineer of vertical structure is the giant kelp Macrocystis pyrifera, which occurs from ∼8 to 24 m depth in the La Jolla kelp forest (Dayton et al., 1992). The kelp forest is bounded to the north, south, and offshore edges by sand (Parnell et al., 2006). The extreme northern portion of the forest (∼0.08 km2) is protected by the San Diego-La Jolla Ecological Reserve (Parnell et al., 2006), and the southern half of the kelp forest is protected by the South La Jolla State Marine Reserve (SLJSMR).
Two sites were selected along the offshore edge of the kelp forest at ∼25 m depth. The northern site (Site A) lies north of the SLJSMR on a flat pavement reef that rises out of sand from ∼28 m depth, whereas the southern site (Site B) lies approximately 2 km south of Site A within the SLJSMR in an area of rocky rubble reef (Figure 1). At each site, a Wildlife Acoustics SM3M passive acoustic recorder (Wildlife Acoustics, Inc., Maynard, MA) was deployed to record the ambient soundscape. Each unit had an HTI-96-MIN omnidirectional hydrophone (High Tech, Inc., Long Beach, MS; sensitivity: −165 dB re: 1 V/μPa, frequency response: 2 Hz–30 kHz) and recorded uncompressed WAV files at 48 kHz, 16-bit depth. Recorders were calibrated using pure sine wave signals from a signal generator across the range of recording frequencies. The response was flat (67.15 ± 1.02 dB re: 1 μPa) over the 50 Hz–20 kHz frequency range. SCUBA divers deployed acoustic recorders from May 2015 to December 2017 at each site. Hydrophone recorders were programmed to record continuously during deployments. Recorders were deployed six times at Site A (totaling 183 days of recording) and eight times at Site B (totaling 198 days of recording).
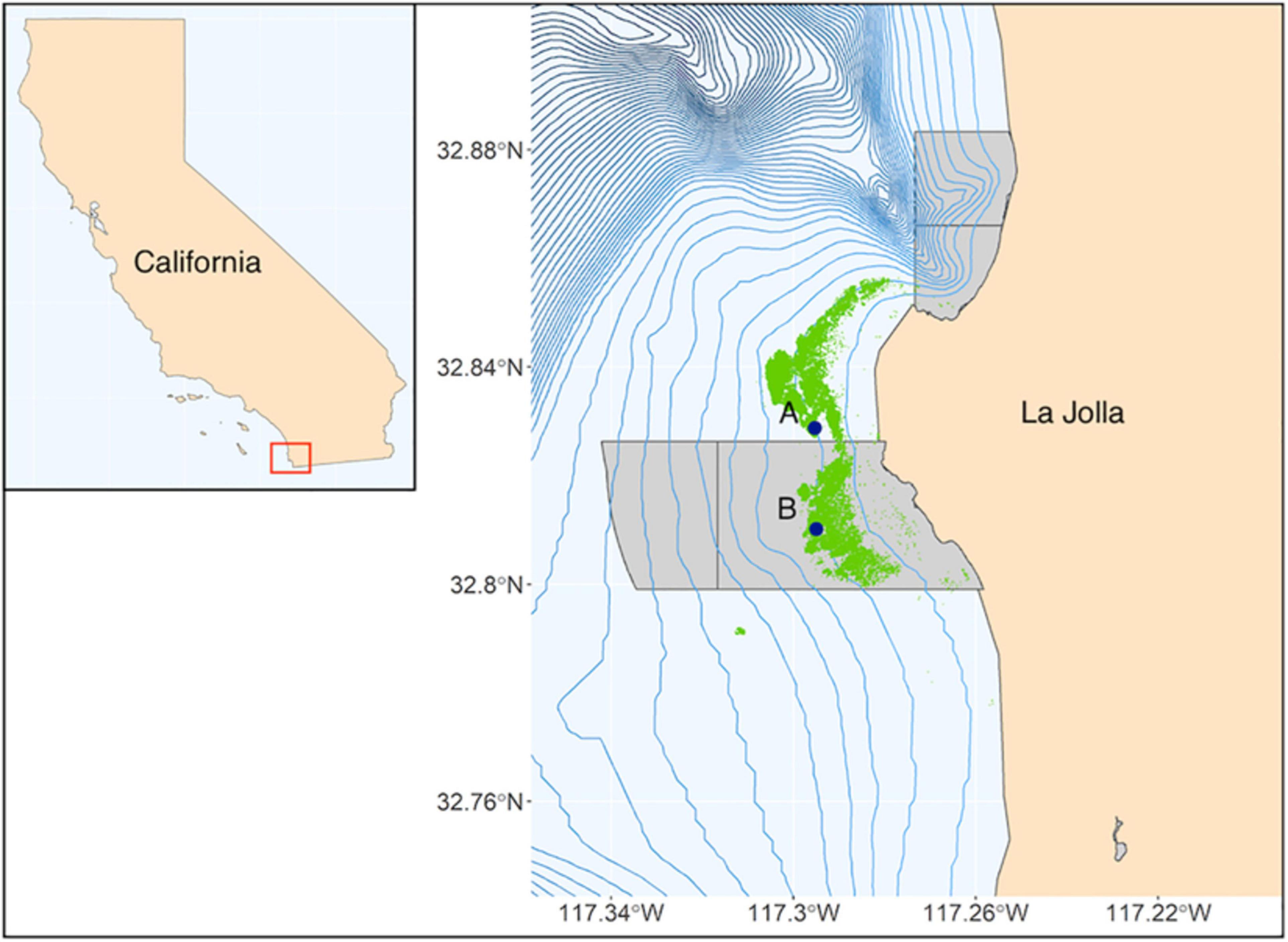
Figure 1. State of California (inset) with study area highlighted by red box. Site locations (indicated by dark blue points) off the coast of Southern California. Marine protected areas are denoted by light gray polygons; blue lines are 10-m isobaths; kelp forest extent is indicated in green.
Digital acoustic recordings were analyzed in MATLAB 2016b (Mathworks, Inc., Natick, MA) using the Triton software package (Wiggins et al., 2010) and other custom scripts. We created long-term spectral averages (LTSAs) from each deployment’s data by calculating 1 min spectral averages with a 1 Hz frequency resolution over the duration of the deployment (Figure 2). These LTSAs were used to visualize the multi-terabyte acoustic dataset, as well as to identify frequency bands of interest and to identify vessel noise that occurred within the recordings.
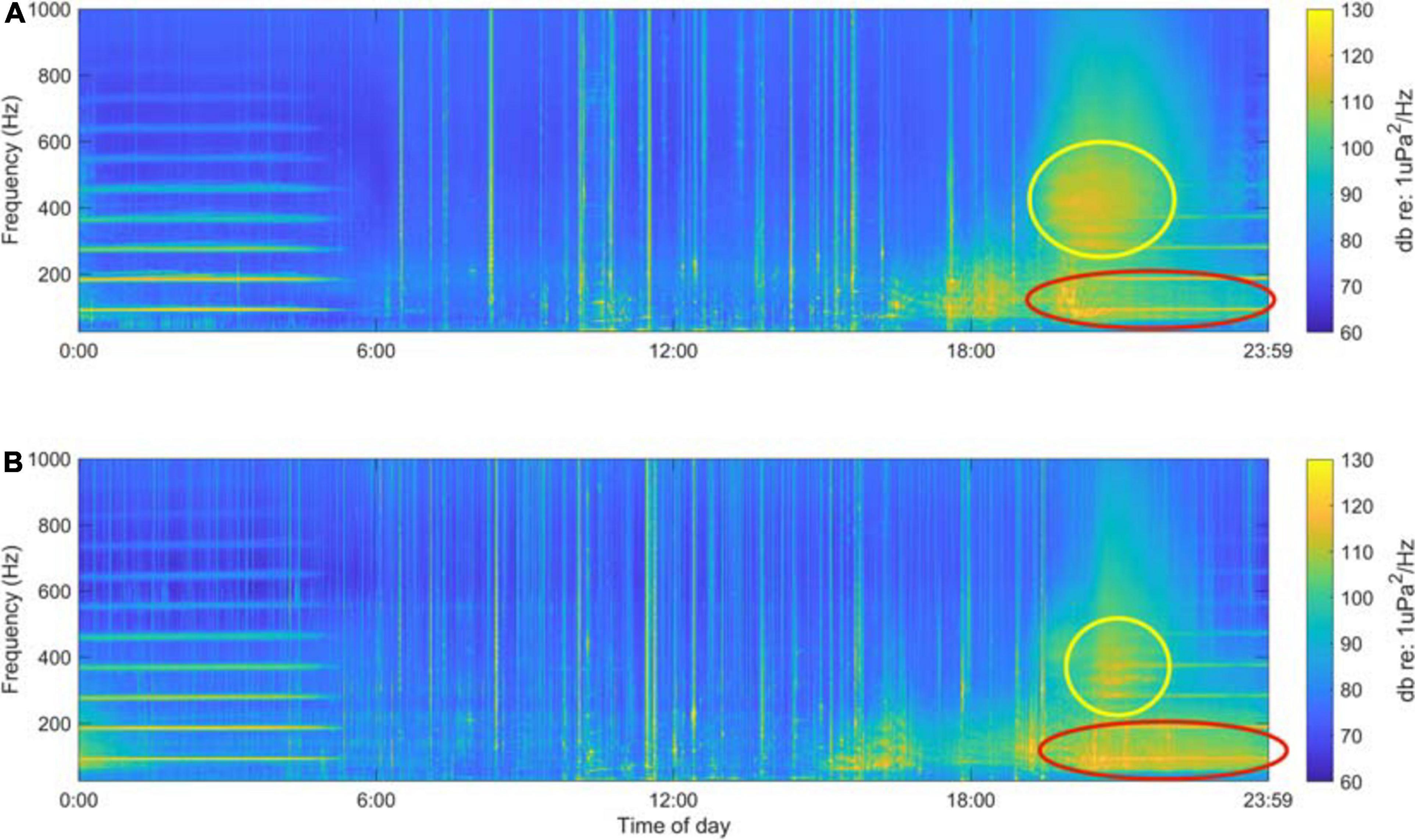
Figure 2. Example long-term spectral average plots for a 24 h period (midnight August 21st to midnight August 22nd, 2017) at Site A (A) and Site B (B). The two putative fish choruses are highlighted by the yellow and red ovals.
To identify frequency bands of interest from the LTSAs, each LTSA was treated as a large matrix, where the columns of the matrix represent each minute of a recorder deployment, each row represents a specific frequency, and each cell value (e.g., the value found in the first row and first column) represents the spectral average at that minute and frequency. These matrices (one matrix for each deployment) were arranged together to create a single, large data matrix to allow for comparison of seasonal variability across both sites. Singular value decomposition (SVD) was used to decompose this data matrix into three distinct matrices: its left singular matrix (U), its singular value matrix (S), and its right singular matrix (V). After decomposition, the original data can be recovered by multiplying U∗S∗VT. Lower rank approximations (SVD components) that highlight important elements of the original data can be generated by multiplying a singular column in U (ui) by the singular value in S (si) by the transpose of the singular column in V (vi). For these analyses, the data reconstruction from the first four SVD components explained > 97% of the variation and were used to identify elements of interest within the LTSA data (Figure 3).
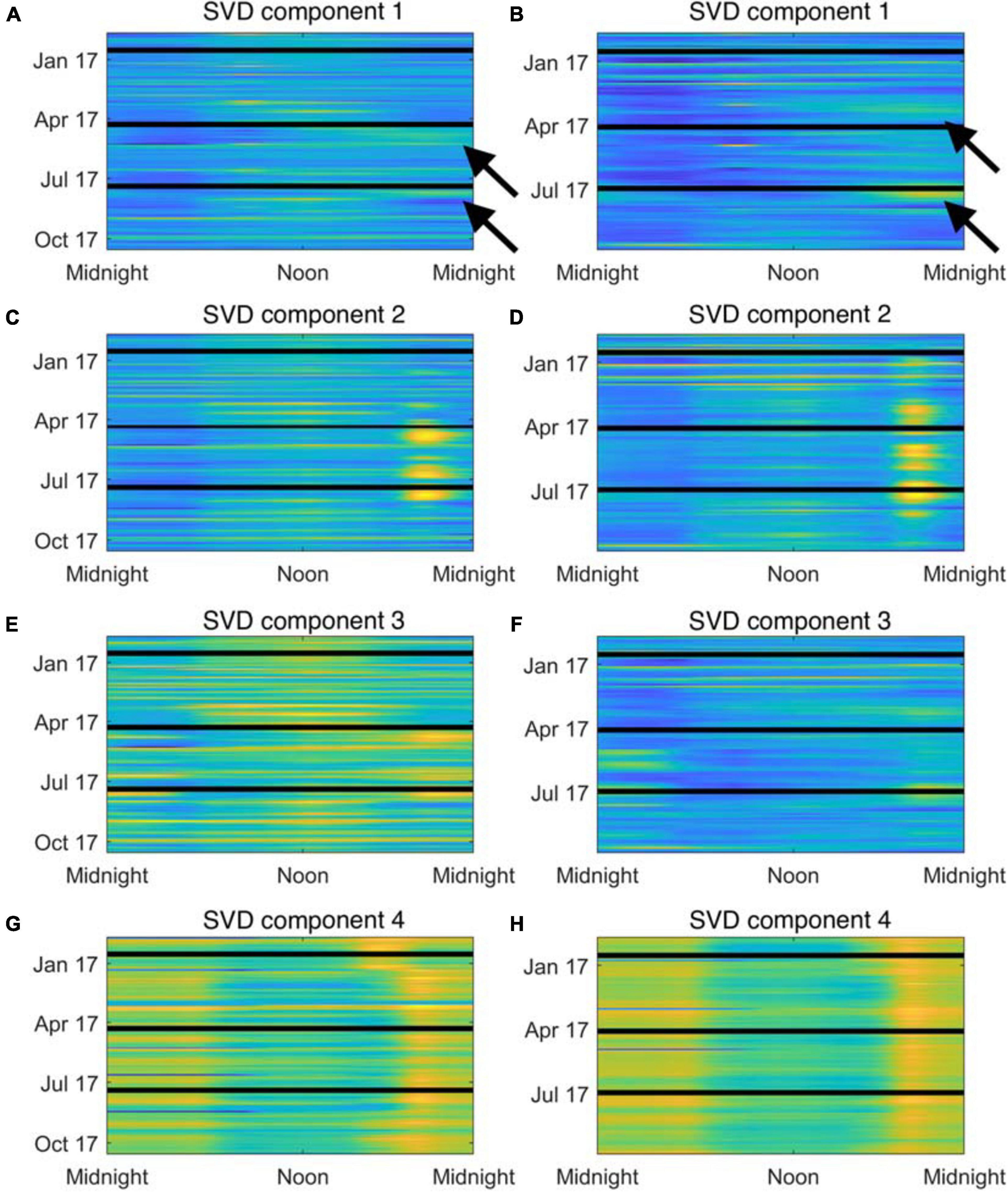
Figure 3. Reconstructed time series from acoustic recordings at Site A (A,C,E,G) and Site B (B,D,F,H) using the first four SVD components. Time-of-day is indicated along the x-axis, and calendar day is along the y-axis. Color indicates standardized power spectral density, where cooler colors are quieter and warmer colors are louder. Black lines indicate breaks in deployments. (A,B) Show SVD component 1 that corresponds to the 60–130 Hz frequency bands (choruses are highlighted by arrows). (C,D) Show SVD component 2 that corresponds to the 300–500 Hz frequency band. (E,F) Show SVD component 3 that corresponds to the midshipman frequency band. (G,H) Show SVD component 4 that corresponds to the snapping shrimp frequency band.
From the decomposition of the LTSA data, each SVD component was visually examined to determine which sounds were dominant in that component, and four frequency bands of interest were identified for further analysis. Two bands of interest were associated with putative fish calls and spanned the 60–130 Hz and 300–500 Hz ranges. The third frequency band was associated with long bouts of a tonal sound focused in the 85–95 Hz range, and its harmonics in the 175–185 Hz and 265–275 Hz ranges produced by Plainfin Midshipman, Poricthys notatus (Ibara et al., 1983). The fundamental frequency component of this band was removed from the previous 60-130 Hz band for these analyses. Dissimilar to the previous three frequency bands of interest, the final band of interest was associated with the crackle and pop of snapping shrimp sounds. In our recordings, the primary energy of snapping shrimp snaps occurred between 2.5 and 7.5 kHz. For each frequency band, acoustic energy across the band was root-mean-square averaged for each minute and then z-score standardized to produce a time series for that frequency band for the duration of the deployment of its standardized spectral levels. These time series allow for direct comparison of temporal variation (e.g., diel and seasonal variation) and spatial variation (i.e., differences between Sites A and B) within a frequency band, as well as comparison among the various frequency bands over space and time. To quickly discern patterns within these time series, the time series were rearranged into a matrix, where each row represents a single day and each column represents a 1 min interval within the day (1,440 total 1 min intervals within 1 day). SVD was again used to decompose this matrix and reconstruct lower rank SVD components to explore how these bands of interest vary. For each frequency band, the coefficient of deviation (CD) was calculated to compare the variability of the different frequency bands of interest by dividing the median absolute deviation (MAD) of the first two left singular columns of the decomposition by the absolute value of the median of the first two left singular columns (|median|) (Marmolejo-Ramos and Ospina, 2019), and the CD was used to determine the variability of the frequency bands. The decompositions of these data were visualized by plotting the first two columns of the left singular matrix as scatterplots (i.e., U1 on the x-axis versus U2 on the y-axis), and k-means clustering via gap analysis was performed to identify any clusters within the data related to site (A or B) or month.
In addition to these spectral analyses, vessel noise occurrence at each site was manually logged from the LTSAs and acoustic recordings (Supplementary Figure 1). The LTSAs were used to efficiently scan through the acoustic data to find vessel noise; once an occurrence of vessel noise was found, a spectrogram of the acoustic timeseries was created (4,096-point Blackman window; 4,096-point FFT; 90% window overlap) to more accurately estimate the duration of the noise event. All vessel noise logging was performed in MATLAB using the Triton software package with Logger feature (Wiggins et al., 2010). The start and end times of each occurrence were marked within the spectrogram and logged into an Excel database. Counts of vessel noise occurrence for each day during deployments were calculated for each site, and a generalized linear model with a gamma distribution was used to model the relationship between these counts and the following factors: site (A or B), month, and their interaction in R (R Project for Statistical Computing). Graphs of residuals versus fitted values and quantile-quantile plots were visually inspected to ensure model assumptions were met. Throughout this text, the term “sound” is used to refer to sounds produced by animals, whereas the term “noise” is used to refer to signals emanating from human activity.
Singular value decomposition analysis of the LTSAs generated for each deployment revealed a complex kelp forest soundscape that varied spatially and temporally, containing acoustic components of both biotic and anthropogenic origin. The 60–130 Hz band described the occurrence of short “drum beat” calls consisting of either a single beat or a few repeated beats. These calls became so frequent that they overlapped, creating a biological chorus in the spring and summer months that dominated the 60–130 Hz frequency range (red oval, Figure 2), and on days when this chorus occurred it persisted 6–12 h beginning in the afternoon hours and extending well into night. The time series of this frequency band from both sites exhibited broad dusk peaks (Figure 4) during the summer months. The dusk spectral peaks for this frequency band were absent from the late fall to early spring acoustic recordings. There were higher standardized spectral levels and increased chorus duration of the 60–130 Hz band at Site B, indicating that the chorusing fish are likely closer to the recorder at Site B (and thus possibly within the SLJSMR no-take area).
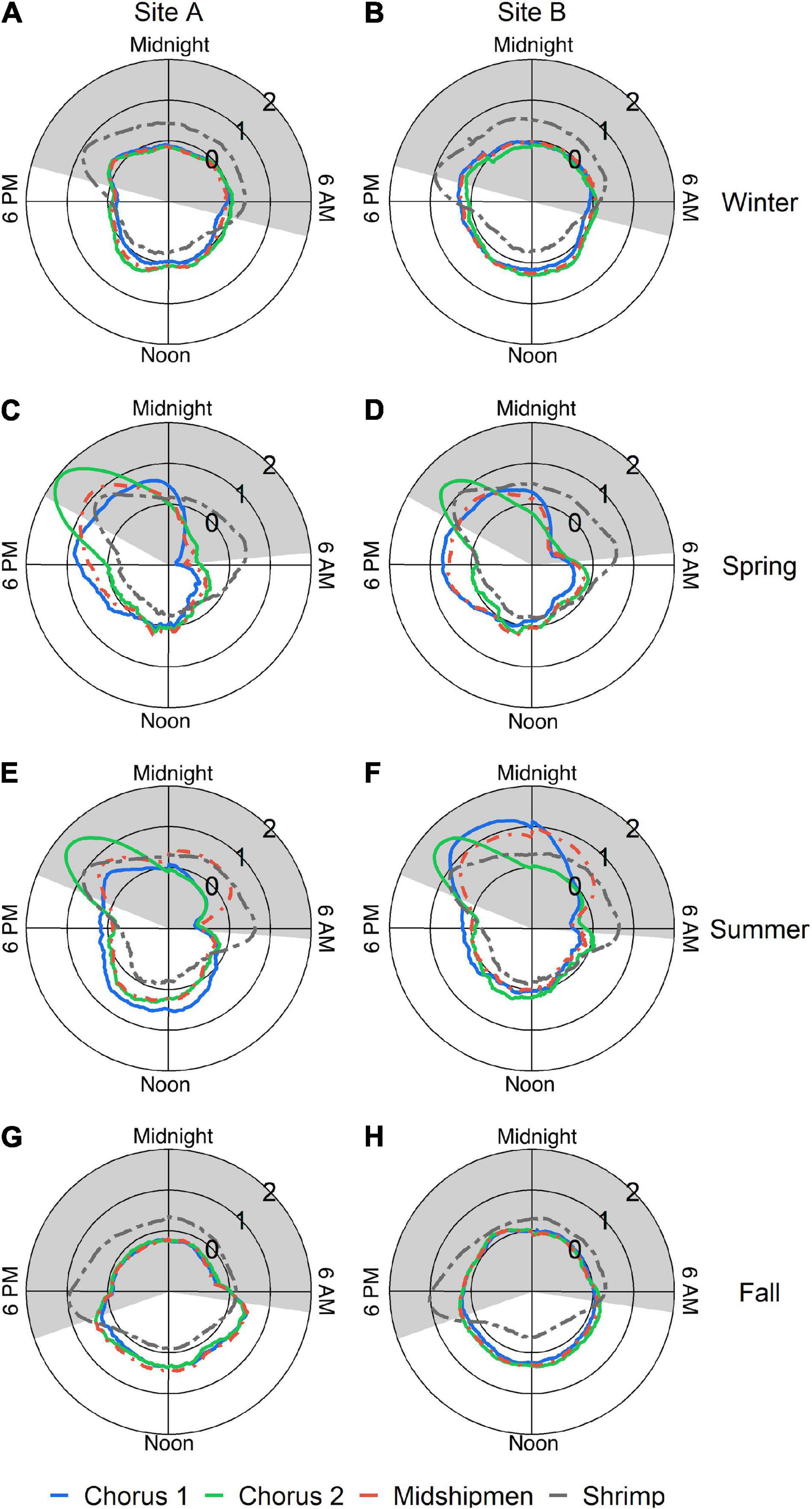
Figure 4. Canonical time series of the four frequency bands of interest created from the LTSAs during different seasons (November 2016–August 2017). (A,C,E,G) represent Site A, and (B,D,F,H) represent Site B. Each frequency band of interest is represented within each panel by color (blue: 60–130 Hz chorus, green; 300–500 Hz chorus, orange: midshipmen band, and gray: snapping shrimp band). Gray arcs represent nighttime (sunset to sunrise). Radial scale is z-score standardized spectrum levels for each band.
The 300–500 Hz band measured occurrence of fish calls that exhibited pulsed calls with peak energy between 300 and 400 Hz, with less energy extending up to 500 Hz. Similar to the 60–130 Hz band, these calls formed a biological chorus that dominated the dusk soundscape in this frequency band (yellow circle, Figure 2). Though these choruses occurred almost every evening for up to 2 weeks during the late spring and summer, they were relatively short in duration, lasting only 2–3 h and peaking at sunset. Time series from both Site A and Site B exhibited quick-onset dusk peaks (Figure 4), and a short-lived energy increase was easily discernible in the LTSAs on nights when chorusing occurred (Figure 2). These dusk choruses were absent from acoustic recordings from the late fall to early spring, and when present in the late spring and summer they did not appear to vary with lunar phase.
In addition to the two putative fish choruses above, the long tonal bouts, likely calls produced by the Plainfin Midshipman (Ibara et al., 1983), occurred primarily from May through August and typically began around sunset, persisted throughout the night, and ended around sunrise (Figure 4). The time series of spectral levels from snapping shrimp snaps displayed strong crepuscular periodicity, rising steeply to a dusk peak, then decreasing slightly to a level above that of daytime, and often rising again to a smaller peak at dawn (Figure 4). The diel periodicity of snapping shrimp was consistent across months.
The singular value decomposition of the timeseries of these frequency bands revealed that the 300–500 Hz frequency band exhibited the highest coefficient of deviation (CD = 17.94), indicating a high degree of variability in the acoustic energy within this band. The 60–130 Hz frequency band and the frequency band likely associated with midshipman calling exhibited lower CDs (3.46 and 4.10, respectively), whereas the frequency band associated with snapping shrimp snaps exhibited much tighter grouping with less variability (CD = 1.85; Figure 5), which indicates a consistent pattern in the acoustic energy recorded within this band and is represented by a tighter cluster of points with less spread. K-means clustering did not reveal any groups within the data related to site or month, which is supported by the scatterplots of the first and second columns of the left singular matrix; there are no clear groupings related to season (indicated by color of the points on the plots).
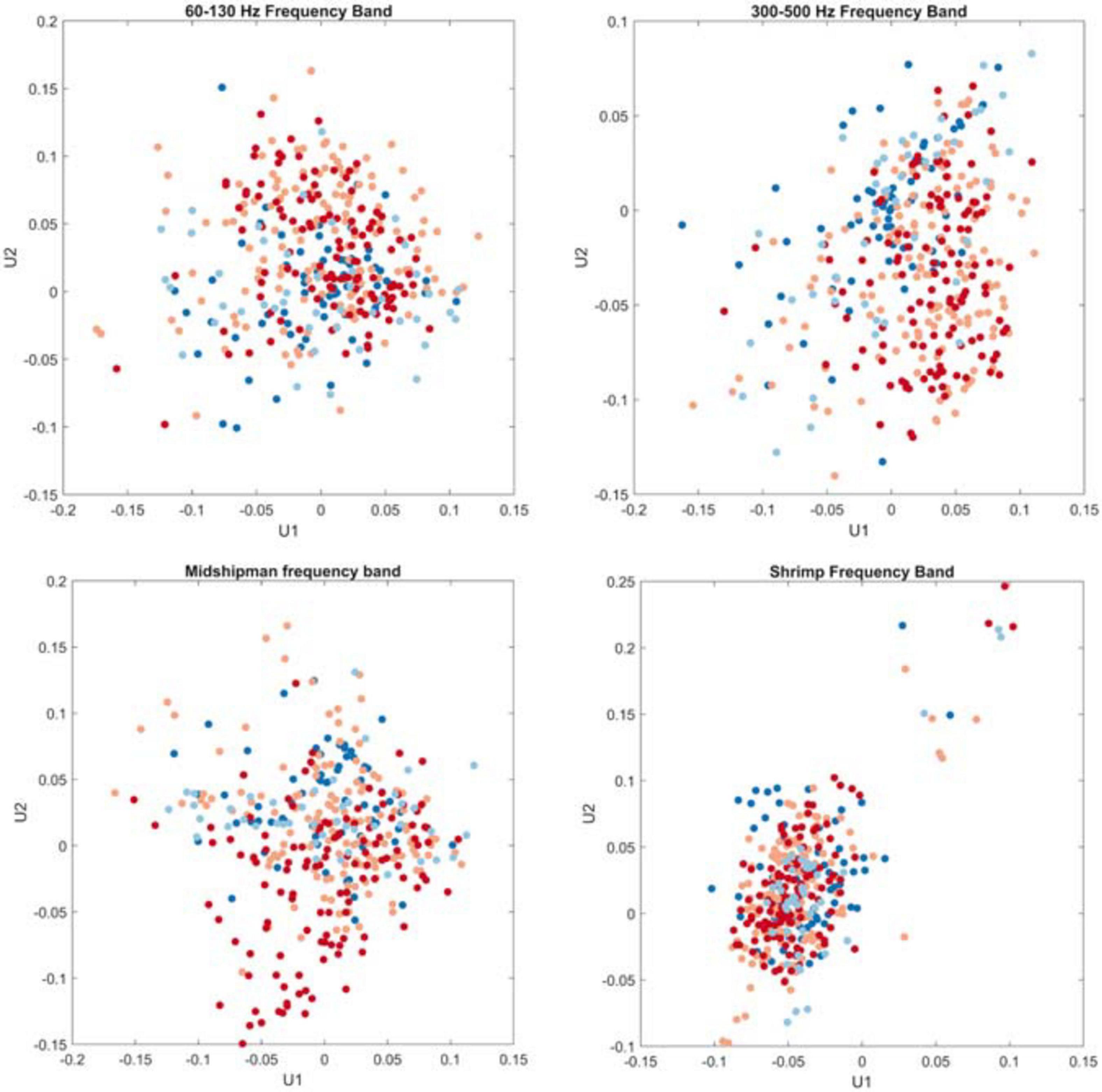
Figure 5. Ordination plot of the four frequency bands of interest based on singular value decomposition. X-axes are the first singular column of the left singular matrix, and y-axes are the second column of the left singular matrix. Colors denote seasons (fall: light blue, winter: dark blue, spring: light red, summer: dark red).
Overall, 13,424 occurrences of vessel noise were counted over 465 days of hydrophone deployments; of those 6,007 vessel noise occurrences were at Site A, and 7,417 at Site B. The maximum count of vessel noise occurrences in 1 day was 138 at Site B on August 25th 2017, and the minimum count of a single vessel per day happened three times, all at Site B: June 12th 2017, October 29th 2017, and November 30th 2017. Only 3% (181 instances) of vessel noise occurred during night at Site A, and only 1.5% (114 instances) of vessel noise occurred at night at Site B. The generalized linear regression of daily vessel noise occurrence showed a significant interaction between site and month [F(9, 445) = 5.04, p < 0.001], as well as a significant effect of month [F(9, 445) = 15.89, p < 0.001]; however, site did not have a significant effect [F(1, 445) = 0.38, p = 0.54] (Table 1). Counts of vessel noise occurrence at both sites followed the same trend; counts were lower during fall and winter months, they gradually rose throughout the spring, and peaked during the summer months (Figure 6). Counts of vessel noise occurrence at Site B during August, September, and October were significantly higher than counts of vessel noise occurrence at Site A during those months (Figure 6).
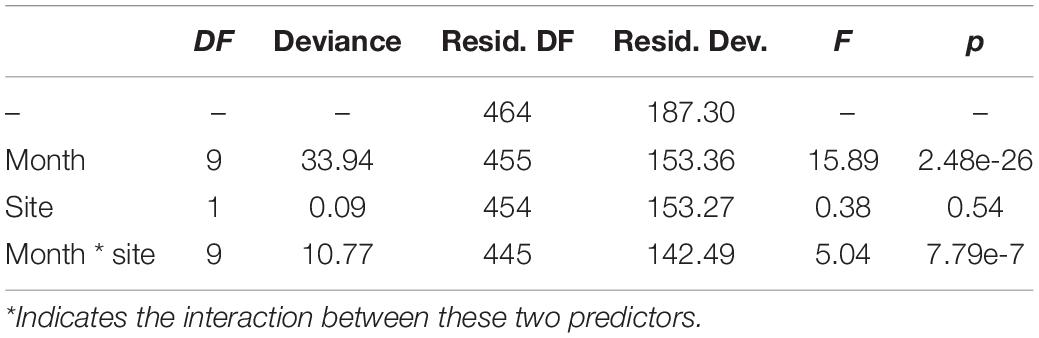
Table 1. Analysis of deviance of the generalized linear regression model describing the relationship between month and site on vessel noise occurrence within acoustic recordings.
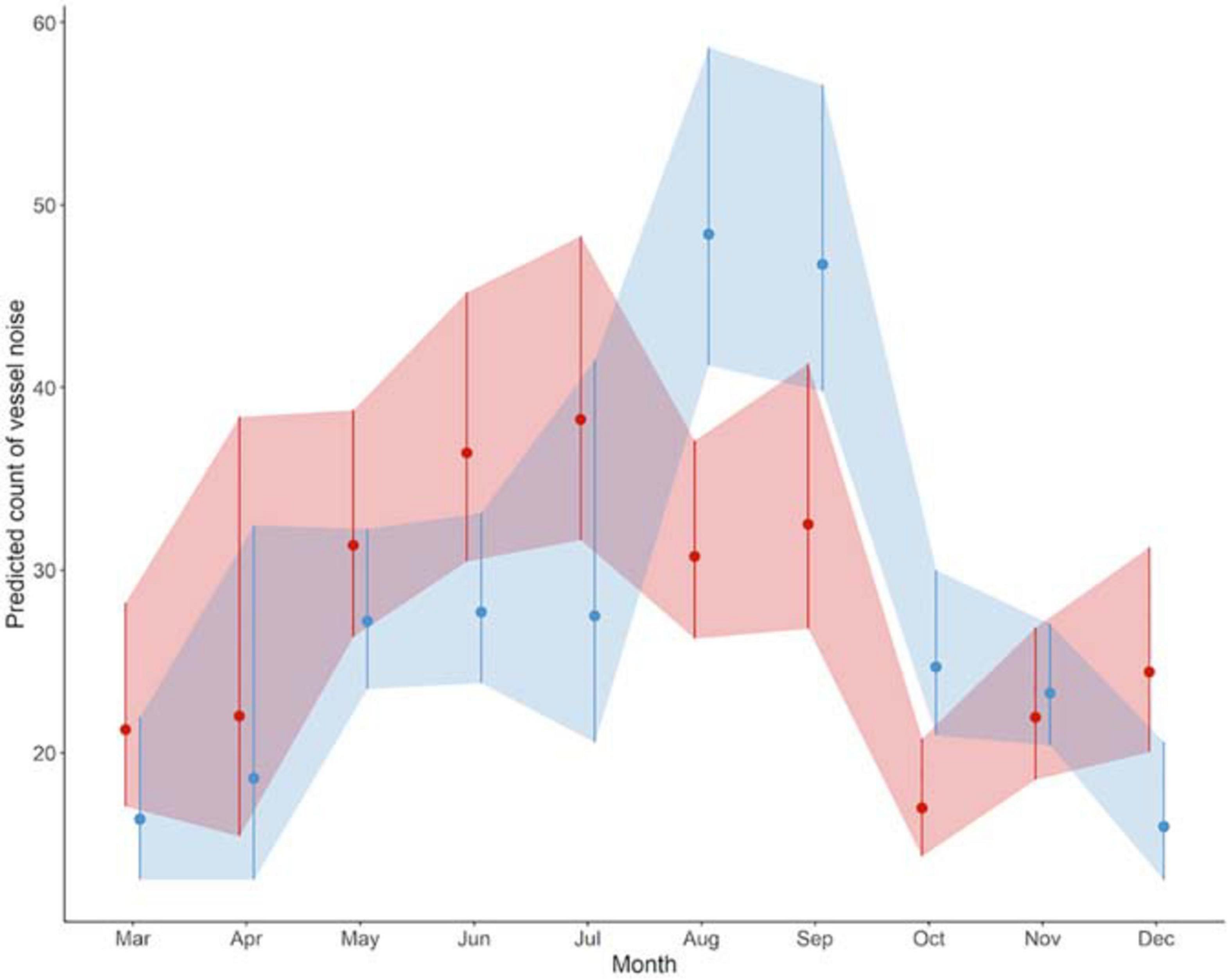
Figure 6. Daily counts of vessel noise occurrence during each month predicted by the gamma regression model. Color denotes sites (Site A: red, Site B: blue). Points represent values (±SD shaded region) predicted by the regression model.
Though site did not significantly affect the daily counts of vessel noise occurrence, the duration of noise events (i.e., how long a single vessel noise occurrence could be heard within the recordings) was greater at Site A (8.11 ± 0.13 min; mean ± st. err) than at Site B (7.77 ± 0.19 min). This was particularly evident during August when the average vessel noise duration at Site A was 13.15 ± 0.28 min, whereas vessel noise duration at Site B was 8.15 ± 0.08 min.
This study represents the first multi-season study of kelp forest soundscape dynamics to our knowledge. The results showcase the complex and dynamic nature of kelp forest soundscapes. During the late spring and summer, choruses of two putative fish calls dominated the dusk soundscapes. One chorus, with much of its energy between 300 and 500 Hz, was short-lived each night, lasting only about 2 h around dusk. This chorus matches descriptions reported in Johnson (1948) that described a chorus of harsh “croaks” that built to peak just after sunset and lasted for 2–3 h just off the Scripps Institution of Oceanography pier and reported in Gottesman et al. (2020) from kelp soundscapes off the Channel Islands, CA. This chorus was likely produced by a croaker species found off the coasts of Southern California (Black Croaker, Sciaena saturna; Spotfin Croaker, Roncadro stearnsi; or, Yellowfin Croaker, Umbrina roncador). The lower frequency chorus, with most of its energy between 60 and 130 Hz, persisted much longer throughout days it was present, beginning in the afternoon and lasting well into night. Anecdotal evidence by divers in the kelp forest near our sites suggest that the Lingcod, Ophiodon elongatus, may produce these calls. These two choruses appear to be the same as Chorus I and Chorus IV reported by Pagniello et al. (2019) in the same area.
In addition to these two putative fish choruses, long bouts of calling likely produced by the Plainfin Midshipman were present in our recordings. The timing of these bouts was consistent with their mating season. During the late spring and summer, the times when we recorded their long nighttime calling bouts, male Plainfin Midshipmen prepare nests in burrows under rocks, where they shelter and produce long “hums” to attract females; once a mate is found, female Plainfin Midshipman attaches eggs inside the burrow and leaves the male to care for and guard the brood (Ibara et al., 1983).
Snapping shrimp in the family Alpheidae are common throughout the world’s tropical and temperate waters and are perhaps the most ubiquitous sources of underwater sound in shallow waters (Au and Banks, 1998; Radford et al., 2008; Bohnenstiehl et al., 2016). These shrimps possess enlarged chelae, which, when rapidly closed, create a cavitation bubble that produces a loud “pop” upon its collapse (Versluis et al., 2000). In our acoustic data, snapping shrimp sound displayed the stereotypic crepuscular periodicity, peaking at dawn and dusk, as seen in other regions worldwide (Lammers et al., 2008; Radford et al., 2008; Bohnenstiehl et al., 2016; Butler et al., 2016). However, unlike some previous studies (e.g., Bohnenstiehl et al., 2016), we did not observe much seasonal variation in acoustic energy in the snapping shrimp frequency band (i.e., 2.5–7.5 kHz). Previous studies on snapping shrimp snap rate (i.e., counts of snapping shrimp snap over a given time period) and acoustic energy from snapping shrimp have correlated those metrics with water temperature, length of day, and dissolved oxygen (Watanabe et al., 2002; Bohnenstiehl et al., 2016), so our results could indicate more constancy in the environmental conditions during our deployments. For example, divers who deployed the acoustic recorders at our sites reported water temperatures near the sea floor varied between 8 and 15°C, whereas Bohnenstiehl et al. (2016) reported water temperatures between 10 and 30°C that influenced snapping shrimp snap rate and sound pressure levels. Thus, the lower variability in water temperatures near the sea floor around our sites might reduce the variability in snapping shrimp sounds as well.
We also found extensive anthropogenic noise within our acoustic data. Noise from small vessels pervaded the soundscapes of both sites. The kelp forests off Southern California support thriving recreational and commercial fisheries (e.g., Spiny Lobster Panulirus interruptus fishing from late September to late March), and their proximity to a dense human population along the coast makes them vulnerable to anthropogenic stress (Foster and Schiel, 2010). The infusion of anthropogenic sounds within natural soundscapes could have deleterious consequences on the ecological community. For example, Nichols et al. (2015) found that Giant Kelpfish, Heterostichus rostratus, exhibited an acute stress to the playback of small vessel noise, and Bruintjes and Radford (2013) found that playback of small vessel noise altered the social behaviors of the cooperatively breeding Cichlid Neolamprologus pulcher. Furthermore, vessel noise has been found to alter orientation and settlement behaviors in coral reef fish larvae (Holles et al., 2013), as well as prevent coral planulae from selecting preferred settlement habitat (Lecchini et al., 2018). Though one of our sites was within a protected area, the occurrence of vessel noise was greater at this site during late summer months—likely due to its proximity to the inlet to Mission Bay, a busy harbor in San Diego County. Thus, the noise from small vessels still has the potential to affect the ecology of the kelp forest in subtle ways, even within protected areas.
Ecoacoustics, and its many subdisciplines like soundscape ecology, is a developing field of marine ecological research whose goal is to explore how underwater soundscapes vary over space and through time (Radford et al., 2008, 2010; Staaterman et al., 2014; Butler et al., 2016), how animals use underwater sounds for orientation and navigation (Stanley et al., 2012; Lillis et al., 2013; Chapuis et al., 2019), and how anthropogenic contributions to marine soundscapes alter the soundscapes themselves, as well as other ecological processes relying on soundscapes (Holles et al., 2013; Marley et al., 2017; Lecchini et al., 2018). Recently, “ocean sound” has been designated as an essential ocean variable by the Global Ocean Observing System committee (Tyack, 2017), and passive acoustic monitoring stations are collecting baseline measurements for and monitoring changes in marine soundscapes (e.g., Haver et al., 2018). Our study employed passive acoustic monitoring and multivariate data analysis techniques (namely, singular value decomposition) to explore the soundscape of the kelp forests off La Jolla, CA. These data and techniques can be used as baseline measures for these kelp habitats for comparison to future studies on how coastal ecosystems are changing.
As the need to protect marine resources and ecosystems increases with human exploitation and climate change, more governing entities such as local or state governments and fisheries management councils have begun implementing area closures and usage regulations to conserve species or habitats of concern. Recognizing the imperative to protect its economically and ecologically valuable marine resources, the state of California passed the Marine Life Protection Act in 1999 and established a network of marine protected areas (MPAs) in 2012, which protects roughly 1,920 km2 (16%) of California’s jurisdictional waters (Marine Protected Area Monitoring Action Plan, Oct. 2018). Long-term passive acoustic recorders used in this study helped us catalog the soundscapes within the South La Jolla State Marine Reserve (part of California’s MPA network), as well as the soundscape just outside the reserve. This reserve was established in 2012 to protect a portion of the most diverse habitat in the southern coastal region of California [California Dept. of Fish & Wildlife (CDFW 2016)], and these recordings can serve as reference indicators of biophony and vessel activity to which future acoustic efforts can be compared.
The two putative fish choruses that occurred in our recordings during the late spring and summer months are likely related to spawning. Future acoustic monitoring efforts can explore whether the protection provided by the marine reserve has led to increased chorusing (and, thus, possibly increased spawning), and also how these choruses respond to long-term environmental changes or physical processes (e.g., the timing of the choruses might move earlier in the year as coastal waters warm). The anecdotal evidence of possible species producing the 60–130 Hz chorus—Lingcod—was identified as species likely to benefit from the implementation of the marine reserve. Lingcod have traditionally supported recreational and for-hire commercial fisheries, however, fishery-dependent and fishery-independent data analysis indicated significant population declines (CDFW Status of the Fisheries Report 2008). These declines led to the enactment of new size and bag limits for Lingcod in 2019. In addition, though the Croaker species typically inhabit nearshore sandy bottom along the Southern California coast, evidence suggests that these species move to deeper water to form spawning aggregations in the summer months (Pondella et al., 2008; Williams et al., 2011).
Studies of the life histories of these fish have provided fisheries managers useful information for their future management, as well as suggested that marine protected areas might work well to bolster their stocks. Lingcod exhibit spawning behavior that lends itself to protection via spatial closures. Male lingcod defend territories in rocky reef areas, where a female will lay a nest of up to 500,000 eggs in rock crevices or under rocks (King and Withler, 2005). Females leave the spawning area, but males remain and actively defend their nests. Additionally, male Lingcod exhibit extreme nest site fidelity, reusing the exact same territory and nest site through multiple years. Therefore, if protecting Lingcod spawning locations is a management priority, then establishing an MPA in an area where spawning has previously occurred is crucial.
Though passive acoustic methods show promise as a non-invasive method to monitor ecosystem health (e.g., Parks et al., 2014; Mooney et al., 2020), they are not without limitations. Though sound has the potential to convey biologically relevant information long distances through seawater (Urick, 1983), only soniferous species will occur in the acoustic record. Furthermore, sounds for only a small portion of fish species that occur in the world’s oceans have been cataloged (Rountree et al., 2006). Studies that have cataloged fish sounds have generally recorded fish housed within tanks (e.g., Ibara et al., 1983; Hawkins and Amorim, 2000; Širović and Demer, 2009; Allen et al., 2020); however, linking recorded sounds with the behavioral context of the fish that produced them is difficult to do both in laboratory settings (due to behavioral changes of fishes within tanks) and in situ. Recent studies have begun pairing passive acoustic recording with time-lapse photography or videography (e.g., Pagniello et al., 2018; Mouy et al., 2020). Associating specific underwater sounds, the species that produces those sounds, and the behavioral context in which those sounds are made will create, in essence, a database to allow efficient determination of species that are present within recordings and their behavioral state (Hastings and Širović, 2015).
In addition to the limited number of marine sounds attributed to individual fish species, another challenge is application of long-term acoustic recordings to abundance or density estimation, rather than solely presence/absence. For example, most ecological studies that employ passive acoustic recorders use only a single element (i.e., a single hydrophone). Studies using multi-element arrays can leverage the physics of acoustic propagation to determine sound source locations (Spiesberger and Fristrup, 1990; Au and Hastings, 2008) and are becoming increasingly common in the ecological literature to locate soniferous fishes (e.g., Locascio and Mann, 2011; Putland et al., 2018; Wilson et al., 2019) and marine mammals (e.g., Adam et al., 2006, among many others). Information about location of sources of underwater sounds can be used to estimate species density (e.g., Marques et al., 2013) and spatial use patterns (e.g., Rowell et al., 2015). Employing multi-element arrays in future studies assessing the efficacy of marine reserves can help managers determine whether the reserve was placed in areas used by target species. For example, anecdotal evidence in this study suggests that Croaker and Lingcod may produce the two choruses described here. Lingcod, in particular, exhibit spawning spatial constraint (i.e., high interannual nest site fidelity; King and Withler, 2005). If choruses were produced by these species, acoustic data could inform on whether the marine reserve was placed in the appropriate area to protect sites used by these species.
Monitoring biodiversity is essential to tracking the effects of human-exacerbated global climate change (Tittensor et al., 2014), and the use of passive acoustics as a cost-effective, non-invasive way to monitor biodiversity and ecosystem health is increasing in both the terrestrial and marine realms (e.g., the U.S. National Parks Service is actively monitoring and managing soundscapes in terrestrial and marine parks). Though cost-effective and generally easy-to-use, monitoring of soundscapes via passive acoustics yields large datasets that preclude manual analysis. To investigate ecological patterns emerging from environmental sounds and to create rapid assessments of biodiversity and ecosystem health, several ecoacoustics indices have been developed [e.g., Acoustic Complexity Index (ACI); Pieretti et al., 2011, Acoustic Richness (AR) and Acoustic Entropy Index (H); Sueur et al., 2008, among others]. However, care needs to be taken when applying these indices as monitoring tools, as their link to the actual biodiversity present in habitats can break down for various reasons (Fuller et al., 2015; Harris et al., 2016). For example, Gasc et al. (2013) and Fuller et al. (2015) found that the ACI is not sensitive to constant, sustained acoustic signals. Therefore, it would not serve well to detect the nocturnal humming of the Plainfin Midshipman within our recordings, and also was not well suited for the forests of New Caledonia and eastern Australia. Harris et al. (2016) formalized a set of four criteria that would make an acoustic index successful in marine environments and tested ACI, AR, and H against visual surveys of reef fish abundance in New Zealand, finding that ACI and H could be appropriate for use on temperate reefs to monitor fish diversity. However, Lyon et al. (2019) found that in tropical back-reef environments ACI did not correlate with habitat complexity or fish community structure.
In the present study, we did not implement ecoacoustic indices, but rather created LTSAs of the acoustic timeseries, and then decomposed these LTSAs via singular value decomposition (SVD). Sattar et al. (2016) had proposed the use of robust principle components analysis, which uses SVD, and multiresolution acoustic features (MRAF) to automatically classify fish sounds within large, marine acoustic datasets. In their study, Sattar et al. (2016) employed robust principle components analysis to decompose their multiresolution acoustic features dataset into lower-rank approximations of the data to aid in feature selection to train a multiclass support vector machine classifier based on manually annotated data. In our study, however, we used it as an approach to rapidly determine frequency bands of interest to track through time in an objective manner.
Though decomposition of our acoustic dataset identified frequencies of interest, k-means clustering of SVD components was unable to discriminate site and temporal patterns of these frequency bands (Figure 5). For example, the putative fish choruses in the 300-500 Hz frequency band exhibited higher acoustic energy at dusk during the spring and summer and lower energy during fall and winter (Figures 3C,D), yet these groupings did not separate via k-means clustering (top right panel, Figure 5). This could be an artifact of the root-mean-square averaging and z-score standardization of our acoustic energy time series; that is, the variability in the raw acoustic energy might have allowed the k-means clustering algorithm to identify distinct groupings of data, whereas the z-score standardization constrained this variability and possibly limited the effectiveness of the k-means clustering algorithm. Singular value decomposition underpins many multivariate analysis techniques and harnessing some of those techniques to extend the use of SVD and k-means clustering to the types of analyses presented in this study could aid the clustering techniques in discriminating distinct groupings. For example, future research could explore whether multivariate distance or dissimilarity transforms (e.g., Bray-Curtis; Ricotta and Podani, 2017) aid discriminating groups among times-of-day, seasons, and sites.
In addition, future studies of kelp forest soundscapes need to further explore the spatial variability of these soundscapes. The sites we selected for our study were relatively close together (only ∼2 km apart) and exhibited similar diel and seasonal variability. Our study employed single-hydrophone recorders, yet future studies could use arrays of two or more hydrophones to allow sound source localization (e.g., Locascio and Mann, 2011) and restrict the analysis of spatial differences in soundscapes to sounds that occur within a set distance from the hydrophones. This technique would allow researchers to explore small-scale spatial variability in underwater sounds, while also exploring where those sounds were produced and whether there is a relationship between sounds and occurrence of sound-producers and the habitat.
Still, this technique can aid biodiversity monitoring efforts to identify robust features of interest to monitor over time that relate to the biology of species targeted for conservation or the ecology of imperiled habitats. Though ecoacoustic indices such as ACI or AR can be rapidly applied to large acoustic datasets, care should be taken when applying ecoacoustic indices to large datasets from disparate and distinct marine environments. Many factors can influence ecoacoustic metrics (e.g, sound source levels, spatial and temporal scales), thus the end user must be aware of how these factors might affect the relationship between acoustic and biological diversity (Mooney et al., 2020). Augmenting ecoacoustic indices with objective acoustic features that show clear links to the unique ecology of habitats or areas of concern can help monitoring efforts and ultimately aid resource managers to more accurately assess the health of ecosystems and track their responses to disturbance as the world’s oceans continue to change.
Here, we explored how the soundscapes emanating from two sites off La Jolla, CA, vary spatially and temporally. We applied singular value decomposition and k-means clustering to identify acoustic bands of interest in the data. Two fish choruses dominated the dusk soundscape during the late spring and summer, and the din of snapping shrimp exhibited stereotypic crepuscular peaks. Vessel noise was also often heard in the kelp soundscapes, and our analysis indicated seasonal trends in vessel activity, peaking in summer months and declining to winter. These analyses revealed a complex and highly variable soundscape, comprised of biological and anthropogenic sounds, and provide valuable baseline for researchers and natural resource managers for future comparisons.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
JJ, PEP, and AŠ designed the experiment and secured funding. JB, CP, and PEP conducted field work. JB and CP performed analyses with guidance from JJ, PEP, and AŠ. JB wrote the manuscript, with significant input from CP, JJ, PEP, and AŠ. All authors contributed to the article and approved the submitted version.
This research was funded by the California Sea Grant #NA14OAR4170075 awarded to JJ, PEP, and AŠ to monitor kelp forest soundscapes and develop an acoutic-optical monitoring device.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank the cadre of divers who helped with deployment and recovery of acoustic equipment, including Rich Walsh, Christian McDonald, Phil Zerofski, Ashleigh Palinkas, Katherine Wilson, and Mohammad Sederat, as well as Addison Sherwood for his help identifying and logging vessel noise.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.629643/full#supplementary-material
Supplementary Figure 1 | Example spectrogram of vessel noise occurring within acoustic recordings of kelp forests off Southern California.
Aalbers, S. A., and Drawbridge, M. A. (2008). White seabass spawning behavior and sound production. Trans. Am. Fish. Soc. 137, 542–550. doi: 10.1577/t04-058.1
Adam, O., Motsch, J.-F., Desharnais, F., Dimarzio, N., Gillespie, D., and Gisiner, R. C. (2006). Overview of the 2005 workshop on detection and localization of marine mammals using passive acoustics. Appl. Acoust. 67, 1061–1070. doi: 10.1016/j.apacoust.2006.05.013
Allen, L. G., Ladin, E. S., and Rowell, T. J. (2020). Sound production and mechanism in the Giant Sea bass, Stereolepis gigas (Polyprionidae). Copeia 108, 809–814.
Au, W. W., and Banks, K. (1998). The acoustics of the snapping shrimp Synalpheus parneomeris in Kaneohe Bay. J. Acoust. Soc. Am. 103, 41–47. doi: 10.1121/1.423234
Barber, S. B., and Mowbray, W. H. (1956). Mechanism of sound production in the sculpin. Science 124, 219–220. doi: 10.1126/science.124.3214.219
Bohnenstiehl, D. R., Lillis, A., and Eggleston, D. B. (2016). The curious acoustic behavior of estuarine snapping shrimp: temporal patterns of snapping shrimp sound in sub-tidal oyster reef habitat. PLoS One 11:e0143691. doi: 10.1371/journal.pone.0143691
Bruintjes, R., and Radford, A. N. (2013). Context-dependent impacts of anthropogenic noise on individual and social behaviour in a cooperatively breeding fish. Anim. Behav. 85, 1343–1349. doi: 10.1016/j.anbehav.2013.03.025
Buscaino, G., Ceraulo, M., Pieretti, N., Corrias, V., Farina, A., Filiciotto, F., et al. (2016). Temporal patterns in the soundscape of the shallow waters of a Mediterranean marine protected area. Sci. Rep. 6:34230.
Butler, J., Stanley, J. A., and Butler, M. J. IV (2016). Underwater soundscapes in near-shore tropical habitats and the effects of environmental degradation and habitat restoration. J. Exp. Mar. Biol. Ecol. 479, 89–96. doi: 10.1016/j.jembe.2016.03.006
Cato, D. H. (1978). Marine biological choruses observed in tropical waters near Australia. J. Acoust. Soc. Am. 64, 736–743. doi: 10.1121/1.382038
Chapuis, L., Collin, S. P., Yopak, K. E., Mccauley, R. D., Kempster, R. M., Ryan, L. A., et al. (2019). The effect of underwater sounds on shark behaviour. Sci. Rep. 9:6924.
Clark, B., and Allen, L. G. (2018). Field observations on courtship and spawning behavior of the Giant Sea Bass, Stereolepis gigas. Copeia 106, 171–179. doi: 10.1643/ce-17-620
Connaughton, M. A., and Taylor, M. H. (1996). Drumming, courtship, and spawning behavior in captive weakfish, Cynoscion regalis. Copeia 1996, 195–199. doi: 10.2307/1446957
Dayton, P. K., Tegner, M. J., Parnell, P. E., and Edwards, P. B. (1992). Temporal and spatial patterns of disturbance and recovery in a kelp forest community. Ecol. Monogr. 62, 421–445. doi: 10.2307/2937118
Erbe, C., Verma, A., Mccauley, R., Gavrilov, A., and Parnum, I. (2015). The marine soundscape of the Perth Canyon. Prog. Oceanogr. 137, 38–51. doi: 10.1016/j.pocean.2015.05.015
Estes, J. A., and Palmisano, J. F. (1974). Sea otters: their role in structuring nearshore communities. Science 185, 1058–1060. doi: 10.1126/science.185.4156.1058
Foster, M. S., and Schiel, D. R. (1985). The Ecology of Giant Kelp Forests in California: A Community Profile. Washington, DC: US Fish and Wildlife Service.
Foster, M. S., and Schiel, D. R. (2010). Loss of predators and the collapse of Southern California kelp forests (?): alternatives, explanations and generalizations. J. Exp. Mar. Biol. Ecol. 393, 59–70. doi: 10.1016/j.jembe.2010.07.002
Fuller, S., Axel, A. C., Tucker, D., and Gage, S. H. (2015). Connecting soundscape to landscape: which acoustic index best describes landscape configuration? Ecol. Indic. 58, 207–215. doi: 10.1016/j.ecolind.2015.05.057
Gasc, A., Sueur, J., Pavoine, S., Pellens, R., and Grandcolas, P. (2013). Biodiversity sampling using a global acoustic approach: contrasting sites with microendemics in New Caledonia. PLoS One 8:e65311. doi: 10.1371/journal.pone.0065311
Gilmore, R. G. Jr. (2002). “Passive acoustic transects: mating calls and spawning ecology in East Florida Sciaenids. Listening to fish,” in Proceedings of an International Workshop on the Applications of Passive Acoustics in Fisheries Cambridge, MA, 33–42.
Gottesman, B. L., Sprague, J., Kushner, D. J., Bellisario, K., Savage, D., McKenna, M. F., et al. (2020). Soundscapes indicate kelp forest condition. Mar. Ecol. Prog. Ser. 654, 35–52. doi: 10.3354/meps13512
Graham, M. H. (2004). Effects of local deforestation on the diversity and structure of Southern California giant kelp forest food webs. Ecosystems 7, 341–357.
Harris, S. A., Shears, N. T., and Radford, C. A. (2016). Ecoacoustic indices as proxies for biodiversity on temperate reefs. Methods Ecol. Evol. 7, 713–724. doi: 10.1111/2041-210x.12527
Hastings, P. A., and Širović, A. (2015). Soundscapes offer unique opportunities for studies of fish communities. Proc. Natl. Acad. Sci. U.S.A. 112, 5866–5867. doi: 10.1073/pnas.1505897112
Haver, S. M., Gedamke, J., Hatch, L. T., Dziak, R. P., Van Parijs, S., Mckenna, M. F., et al. (2018). Monitoring long-term soundscape trends in US waters: the NOAA/NPS ocean noise reference station network. Mar. Policy 90, 6–13. doi: 10.1016/j.marpol.2018.01.023
Hawkins, A. D., and Amorim, M. C. P. (2000). Spawning sounds of the male haddock, Melanogrammus aeglefinus. Environ. Biol. Fish. 59, 29–41. doi: 10.1023/a:1007615517287
Holles, S., Simpson, S. D., Radford, A. N., Berten, L., and Lecchini, D. (2013). Boat noise disrupts orientation behaviour in a coral reef fish. Mar. Ecol. Prog. Ser. 485, 295–300. doi: 10.3354/meps10346
Ibara, R. M., Penny, L. T., Ebeling, A. W., Van Dykhuizen, G., and Cailliet, G. (1983). “The mating call of the plainfin midshipman fish, Porichthys notatus,” in Predators and Prey in Fishes. Developments in Environmental Biology of Fishes, Vol. 2, eds D. L. G. Noakes, D. G. Lindquist, G. S. Helfman, and J. A. Ward (Dordrecht: Springer), 205–212. doi: 10.1007/978-94-009-7296-4_22
Jackson, G. (1987). Modelling the growth and harvest yield of the giant kelp Macrocystis pyrifera. Mar. Biol. 95, 611–624. doi: 10.1007/bf00393105
Jackson, J. B., Kirby, M. X., Berger, W. H., Bjorndal, K. A., Botsford, L. W., Bourque, B. J., et al. (2001). Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–637. doi: 10.1126/science.1059199
Johnson, M. W. (1948). Sound as a tool in marine ecology, from data on biological noises and the deep scattering layer. J. Mar. Res. 7, 443–458.
Kaplan, M. B., Mooney, T. A., Partan, J., and Solow, A. R. (2015). Coral reef species assemblages are associated with ambient soundscapes. Mar. Ecol. Prog. Ser. 533, 93–107. doi: 10.3354/meps11382
Kennedy, E., Holderied, M., Mair, J., Guzman, H., and Simpson, S. (2010). Spatial patterns in reef-generated noise relate to habitats and communities: evidence from a Panamanian case study. J. Exp. Mar. Biol. Ecol. 395, 85–92. doi: 10.1016/j.jembe.2010.08.017
King, J. R., and Withler, R. E. (2005). Male nest site fidelity and female serial polyandry in lingcod (Ophiodon elongatus, Hexagrammidae). Mol. Ecol. 14, 653–660. doi: 10.1111/j.1365-294x.2005.02438.x
Konar, B., and Roberts, C. (1996). Large scale landslide effects on two exposed rocky subtidal areas in California. Bot. Mar. 39, 517–524.
Kratochvil, H. (1978). Der bau des lautapparates vom knurrenden gurami (Trichopsis vittatus Cuvier & Valenciennes)(Anabantidae, Belontiidae). Zoomorphologie 91, 91–99. doi: 10.1007/bf00994156
Ladich, F., and Fine, M. L. (2006). “Sound-generating mechanisms in fishes: a unique diversity in vertebrates,” in Communication in Fishes, eds F. Ladich, S. P. Collin, P. Moller, and B. G. Kapoor (Washington, DC: Science Publisher), 1–43. doi: 10.1007/978-3-642-69150-8_1
Lammers, M. O., Brainard, R. E., Au, W. W., Mooney, T. A., and Wong, K. B. (2008). An ecological acoustic recorder (EAR) for long-term monitoring of biological and anthropogenic sounds on coral reefs and other marine habitats. J. Acoust. Soc. Am. 123, 1720–1728. doi: 10.1121/1.2836780
Lecchini, D., Bertucci, F., Gache, C., Khalife, A., Besson, M., Roux, N., et al. (2018). Boat noise prevents soundscape-based habitat selection by coral planulae. Sci. Rep. 8:9283.
Lillis, A., Eggleston, D. B., and Bohnenstiehl, D. R. (2013). Oyster larvae settle in response to habitat-associated underwater sounds. PLoS One 8:e79337. doi: 10.1371/journal.pone.0079337
Locascio, J. V., and Mann, D. A. (2011). Localization and source level estimates of black drum (Pogonias cromis) calls. J. Acoust. Soc. Am. 130, 1868–1879. doi: 10.1121/1.3621514
Lyon, R. P., Eggleston, D. B., Bohnenstiehl, D. R., Layman, C. A., Ricci, S. W., and Allgeier, J. E. (2019). Fish community structure, habitat complexity, and soundscape characteristics of patch reefs in a tropical, back-reef system. Mar. Ecol. Prog. Ser. 609, 33–48. doi: 10.3354/meps12829
Mann, D. A., Locascio, J. V., Coleman, F. C., and Koenig, C. C. (2009). Goliath grouper Epinephelus itajara sound production and movement patterns on aggregation sites. Endanger. Species Res. 7, 229–236. doi: 10.3354/esr00109
Marley, S. A., Erbe, C., Salgado Kent, C. P., Parsons, M. J., and Parnum, I. M. (2017). Spatial and temporal variation in the acoustic habitat of bottlenose dolphins (Tursiops aduncus) within a highly urbanized estuary. Front. Mar. Sci. 4:197. doi: 10.3389/fmars.2017.00197
Marmolejo-Ramos, F., and Ospina, R. (2019). Performance of some estimators of relative variability. Front. Appl. Math. Stat. 5:43. doi: 10.3389/fams.2019.00043
Marques, T. A., Thomas, L., Martin, S. W., Mellinger, D. K., Ward, J. A., Moretti, D. J., et al. (2013). Estimating animal population density using passive acoustics. Biol. Rev. 88, 287–309. doi: 10.1111/brv.12001
McCauley, R. (2012). “Fish choruses from the Kimberley, seasonal and lunar links as determined by long term sea noise monitoring,” in Proceedings of the Acoustical Society of Australia, Fremantle, WA.
McWilliam, J. N., and Hawkins, A. D. (2013). A comparison of inshore marine soundscapes. J. Exp. Mar. Biol. Ecol. 446, 166–176. doi: 10.1016/j.jembe.2013.05.012
Merchant, N. D., Pirotta, E., Barton, T. R., and Thompson, P. M. (2016). “Soundscape and noise exposure monitoring in a marine protected area using shipping data and time-lapse footage,” in The Effects of Noise on Aquatic Life II. Advances in Experimental Medicine and Biology, Vol. 875, eds A. Popper and A. Hawkins (New York, NY: Springer), 705–712. doi: 10.1007/978-1-4939-2981-8_85
Monczak, A., Mueller, C., Miller, M. E., Ji, Y., Borgianini, S. A., and Montie, E. W. (2019). Sound patterns of snapping shrimp, fish, and dolphins in an estuarine soundscape of the southeastern USA. Mar. Ecol. Prog. Ser. 609, 49–68. doi: 10.3354/meps12813
Mooney, T. A., Di Iorio, L., Lammers, M., Lin, T. H., Nedelec, S. L., Parsons, M., et al. (2020). Listening forward: approaching marine biodiversity assessments using acoustic methods. R. Soc. Open Sci. 7:201287. doi: 10.1098/rsos.201287
Mouy, X., Black, M., Cox, K., Qualley, J., Mireault, C., Dosso, S., et al. (2020). FishCam: a low-cost open source autonomous camera for aquatic research. HardwareX 8:e00110. doi: 10.1016/j.ohx.2020.e00110
Nedelec, S. L., Simpson, S. D., Holderied, M., Radford, A. N., Lecellier, G., Radford, C., et al. (2015). Soundscapes and living communities in coral reefs: temporal and spatial variation. Mar. Ecol. Prog. Ser. 524, 125–135. doi: 10.3354/meps11175
Nelson, M. D., Koenig, C. C., Coleman, F. C., and Mann, D. A. (2011). Sound production of red grouper Epinephelus morio on the West Florida Shelf. Aquat. Biol. 12, 97–108. doi: 10.3354/ab00325
Nichols, T. A., Anderson, T. W., and Širović, A. (2015). Intermittent noise induces physiological stress in a coastal marine fish. PLoS One 10:e0139157. doi: 10.1371/journal.pone.0139157
North, W. J. (1971). The Biology of Giant Kelp Beds Macrocystis in California. Lehre, RFA: J Cramer Publisher.
Pagniello, C. M. L. S., Butler, J., D’Spain, G. L., Jaffe, J. S., Parnell, P. E., and Sirovic, A. (2018). Searching for the FishOASIS: using passive acoustics and optical imaging to identify a chorusing species of fish. J. Acoust. Soc. Am. 144:1692. doi: 10.1121/1.5067519
Pagniello, C. M. L. S., Cimino, M. A., and Terrill, E. (2019). Mapping fish chorus distributions in Southern California using an autonomous wave glider. Front. Mar. Sci. 6:526. doi: 10.3389/fmars.2019.00526
Parks, S. E., Miksis-Olds, J. L., and Denes, S. L. (2014). Assessing marine ecosystem acoustic diversity across ocean basins. Ecol. Inform. 21, 81–88. doi: 10.1016/j.ecoinf.2013.11.003
Parmentier, E., and Diogo, R. (2006). Evolutionary trends of swimbladder sound mechanisms in some teleost fishes. Commun. Fish. 1, 43–68.
Parmentier, E., Tock, J., Falguière, J.-C., and Beauchaud, M. (2014). Sound production in Sciaenops ocellatus: preliminary study for the development of acoustic cues in aquaculture. Aquaculture 432, 204–211. doi: 10.1016/j.aquaculture.2014.05.017
Parnell, P. E., Dayton, P. K., Lennert-Cody, C. E., Rasmussen, L. L., and Leichter, J. J. (2006). Marine reserve design: optimal size, habitats, species affinities, diversity, and ocean microclimate. Ecol. Appl. 16, 945–962. doi: 10.1890/1051-0761(2006)016[0945:mrdosh]2.0.co;2
Parnell, P. E., Miller, E. F., Cody, C. E. L., Dayton, P. K., Carter, M. L., and Stebbins, T. D. (2010). The response of giant kelp (Macrocystis pyrifera) in Southern California to low-frequency climate forcing. Limnol. Oceanogr. 55, 2686–2702. doi: 10.4319/lo.2010.55.6.2686
Parnell, P. E. (2015). The effects of seascape pattern on algal patch structure, sea urchin barrens, and ecological processes. J. Exp. Mar. Biol. Ecol. 465, 64–76. doi: 10.1016/j.jembe.2015.01.010
Pieretti, N., Farina, A., and Morri, D. (2011). A new methodology to infer the singing activity of an avian community: the acoustic complexity index (ACI). Ecol. Indic. 11, 868–873. doi: 10.1016/j.ecolind.2010.11.005
Pieretti, N., Martire, M. L., Farina, A., and Danovaro, R. (2017). Marine soundscape as an additional biodiversity monitoring tool: a case study from the Adriatic Sea (Mediterranean Sea). Ecol. Indic. 83, 13–20. doi: 10.1016/j.ecolind.2017.07.011
Pijanowski, B. C., Villanueva-Rivera, L. J., Dumyahn, S. L., Farina, A., Krause, B. L., Napoletano, B. M., et al. (2011). Soundscape ecology: the science of sound in the landscape. BioScience 61, 203–216.
Pondella, D. J. II, Froeshke, J. T., Wetmore, L. S., Miller, E., Valle, C. F., and Madeiros, L. (2008). Demographic parameters of Yellowfin Croaker, Umbrina roncador (Perciformes: Sciaenidae), from the Southern California Bight. Pac. Sci. 62, 555–568. doi: 10.2984/1534-6188(2008)62[555:dpoycu]2.0.co;2
Putland, R. L., Mackiewicz, A., and Mensinger, A. F. (2018). Localizing individual soniferous fish using passive acoustic monitoring. Ecol. Inform. 48, 60–68. doi: 10.1016/j.ecoinf.2018.08.004
Radford, C., Stanley, J., and Jeffs, A. (2014). Adjacent coral reef habitats produce different underwater sound signatures. Mar. Ecol. Prog. Ser. 505, 19–28. doi: 10.3354/meps10782
Radford, C., Stanley, J., Tindle, C., Montgomery, J., and Jeffs, A. (2010). Localised coastal habitats have distinct underwater sound signatures. Mar. Ecol. Progr. Ser. 401, 21–29. doi: 10.3354/meps08451
Radford, C. A., Jeffs, A. G., Tindle, C. T., and Montgomery, J. C. (2008). Temporal patterns in ambient noise of biological origin from a shallow water temperate reef. Oecologia 156, 921–929. doi: 10.1007/s00442-008-1041-y
Rice, A. N., and Lobel, P. S. (2003). The pharyngeal jaw apparatus of the Cichlidae and Pomacentridae: function in feeding and sound production. Rev. Fish Biol. Fish. 13, 433–444. doi: 10.1007/s11160-004-8794-0
Ricotta, C., and Podani, J. (2017). On some properties of the Bray-Curtis dissimilarity and their ecological meaning. Ecol. Complex. 31, 201–205. doi: 10.1016/j.ecocom.2017.07.003
Rossi, T., Connell, S. D., and Nagelkerken, I. (2017). The sounds of silence: regime shifts impoverish marine soundscapes. Landsc. Ecol. 32, 239–248. doi: 10.1007/s10980-016-0439-x
Rountree, R. A., Gilmore, R. G., Goudey, C. A., Hawkins, A. D., Luczkovich, J. J., and Mann, D. A. (2006). Listening to fish: applications of passive acoustics to fisheries science. Fisheries 31, 433–446. doi: 10.1577/1548-8446(2006)31[433:ltf]2.0.co;2
Rowell, T. J., Nemeth, R. S., Schärer, M. T., and Appeldoorn, R. S. (2015). Fish sound production and acoustic telemetry reveal behaviors and spatial patterns associated with spawning aggregations of two Caribbean groupers. Mar. Ecol. Prog. Ser. 518, 239–254. doi: 10.3354/meps11060
Sattar, F., Cullis-Suzuki, S., and Jin, F. (2016). Acoustic analysis of big ocean data to monitor fish sounds. Ecol. Inform. 34, 102–107. doi: 10.1016/j.ecoinf.2016.05.002
Shiel, D. R., and Foster, M. S. (2015). The Biology and Ecology of Giant Kelp Forests. Oakland, CA: University of California Press.
Širović, A., and Demer, D. A. (2009). Sounds of captive rockfishes. Copeia 2009, 502–509. doi: 10.1643/cp-08-121
Skoglund, C. (1961). Functional analysis of swim-bladder muscles engaged in sound production of the toadfish. J. Cell Biol. 10, 187–200. doi: 10.1083/jcb.10.4.187
Spiesberger, J. L., and Fristrup, K. M. (1990). Passive localization of calling animals and sensing of their acoustic environment using acoustic tomography. Am. Nat. 135, 107–153. doi: 10.1086/285035
Staaterman, E., Paris, C. B., Deferrari, H. A., Mann, D. A., Rice, A. N., and D’alessandro, E. K. (2014). Celestial patterns in marine soundscapes. Mar. Ecol. Prog. Ser. 508, 17–32. doi: 10.3354/meps10911
Stanley, J. A., Radford, C. A., and Jeffs, A. G. (2012). Location, location, location: finding a suitable home among the noise. Proc. R. Soc. Lond. B Biol. Sci. 279, 3622–3631. doi: 10.1098/rspb.2012.0697
Sueur, J., Pavoine, S., Hamerlynck, O., and Duvail, S. (2008). Rapid acoustic survey for biodiversity appraisal. PLoS One 3:e4065. doi: 10.1371/journal.pone.0004065
Tavolga, W. N. (1971). “Sound production and detection,” in Fish Physiology, eds W. S. Hoar and D. J. Randall (New York, NY: Elsevier), 135–205. doi: 10.1016/s1546-5098(08)60047-3
Tittensor, D. P., Walpole, M., Hill, S. L., Boyce, D. G., Britten, G. L., Burgess, N. D., et al. (2014). A mid-term analysis of progress toward international biodiversity targets. Science 346, 241–244.
Tyack, P. L. (2017). Developing an essential ocean variable for the acoustic environment. J. Acoust. Soc. Am. 141, 3525–3525. doi: 10.1121/1.4987432
Versluis, M., Schmitz, B., Von Der Heydt, A., and Lohse, D. (2000). How snapping shrimp snap: through cavitating bubbles. Science 289, 2114–2117. doi: 10.1126/science.289.5487.2114
Watanabe, M., Sekine, M., Hamada, E., Ukita, M., and Imai, T. (2002). Monitoring of shallow sea environment by using snapping shrimps. Water Sci. Technol. 46, 419–424. doi: 10.2166/wst.2002.0772
Wiggins, S. M., Roch, M. A., and Hildebrand, J. A. (2010). TRITON software package: analyzing large passive acoustic monitoring data sets using MATLAB. J. Acoust. Soc. Am. 128, 2299–2299. doi: 10.1121/1.3508074
Williams, J. P., Claisse, J. T., Pondella, D. J. II, Medeiros, L., Valle, C. F., and Shane, M. A. (2011). Patterns of life history and habitat use of an important recreational fishery species, Spotfin Croaker, and their potential fishery implications. Mar. Coast. Fish. 4, 71–84. doi: 10.1080/19425120.2012.661392
Wilson, K. C., Semmens, B. X., Gittings, S. R., Pattengill-Semmens, C., and Širović, A. (2019). “Development and evaluation of a passive acoustic localization method to monitor fish spawning aggregations and measure source levels,” in Oceans 2019 MTS/IEEE Seattle, (Seattle, WA: IEEE), 1–7. doi: 10.1007/978-94-007-1980-4_1
Keywords: marine soundscapes, kelp forests, boat noise, fish chorus, soundscape metrics, marine bio-acoustics
Citation: Butler J, Pagniello CMLS, Jaffe JS, Parnell PE and x0160;irović A (2021) Diel and Seasonal Variability in Kelp Forest Soundscapes Off the Southern California Coast. Front. Mar. Sci. 8:629643. doi: 10.3389/fmars.2021.629643
Received: 15 November 2020; Accepted: 19 March 2021;
Published: 13 April 2021.
Edited by:
Eric Delory, Oceanic Platform of the Canary Islands, SpainReviewed by:
Almo Farina, University of Urbino Carlo Bo, ItalyCopyright © 2021 Butler, Pagniello, Jaffe, Parnell and Širović. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jack Butler, SmFjay5CdXRsZXJAbXlmd2MuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.