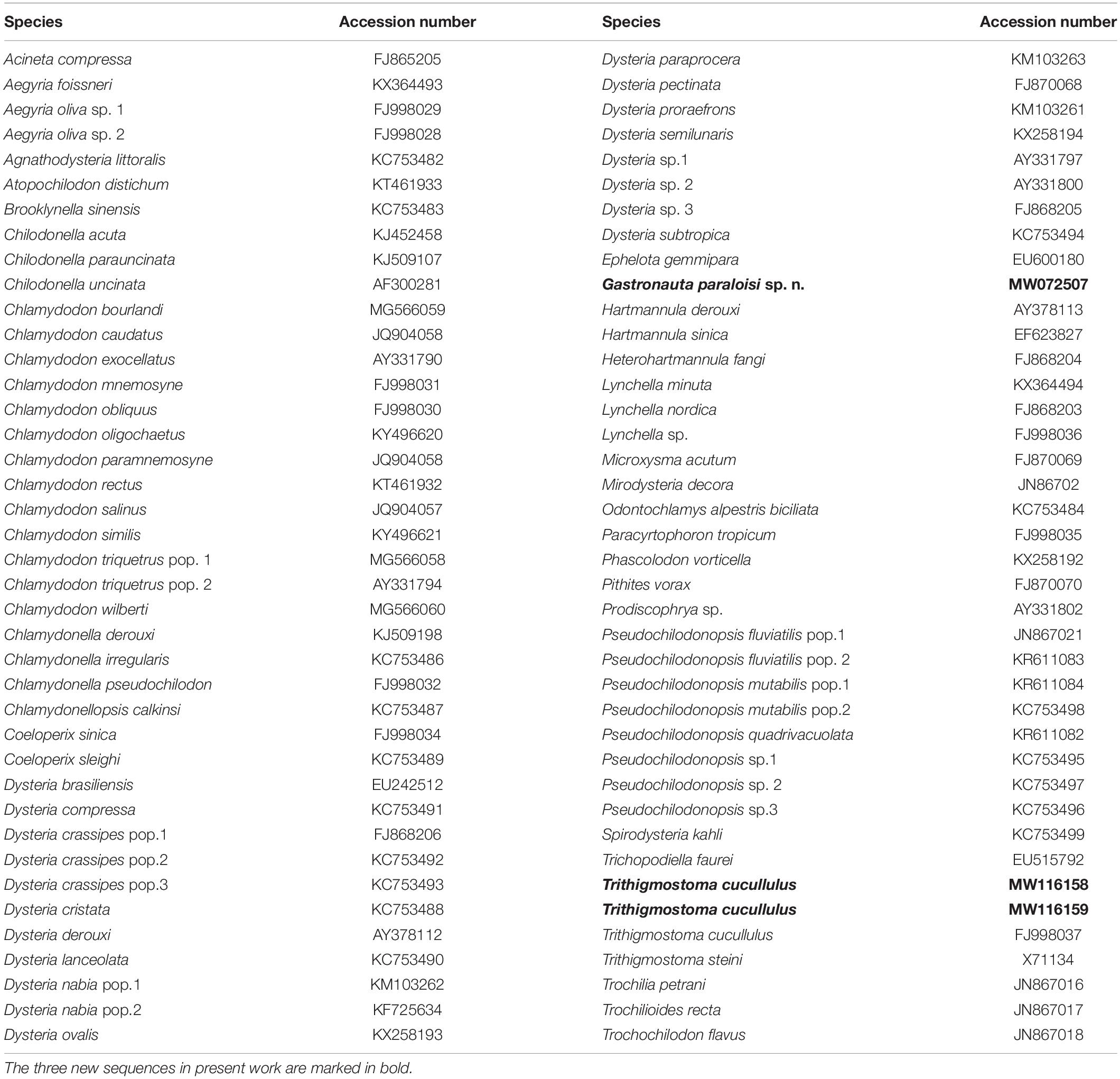
- 1Key Laboratory of Mariculture, Ministry of Education, Institute of Evolution and Marine Biodiversity, Ocean University of China, Qingdao, China
- 2Department of Ecology, Technische Universität Kaiserslautern, Kaiserslautern, Germany
- 3Shanghai Universities Key Laboratory of Marine Animal Taxonomy and Evolution, Shanghai Ocean University, Shanghai, China
Studies on cyrtophorian ciliates (Cyrtophoria) have accumulated much knowledge on morphological taxonomy and molecular phylogeny, and the general classification and phylogenetic relationships of most families have thereby been revealed. However, the phylogenetic position of the family Gastronautidae Deroux, 1994 remains uncertain. This is due to the presence of specialized characteristics (in particular a circumoral kinety in a closed circle), and most importantly, the lack of molecular data of this family. In addition, Trithigmostoma Jankowski, 1967 holds a special position among genera in Chilodonellidae Deroux, 1976 due to its divergent characteristics. In the present work, we studied a new gastronautid, Gastronauta paraloisi sp. n., and three populations of Trithigmostoma cucullulus (Müller, 1786) Jankowski, 1967, using integrative methods. Species identifications were confirmed by morphological research. We also obtained SSU rDNA sequences, which included the first available sequence of Gastronautidae. The following SSU rDNA-inferred phylogenetic analyses showed that the establishment of the family Gastronautidae is necessary, and Gastronautidae and Trithigmostoma may represent intermediate evolutionary links in the order Chlamydodontida.
Introduction
Subclass Cyrtophoria Fauré-Fremiet in Corliss, 1956 is a group of highly specialized and divergent ciliated protistans. Most cyrtophorian ciliates constitute important components of the aquatic periphytic community and can be found in various habitats like freshwater, marine and brackish waters (Kahl, 1931; Deroux, 1976a,b; Foissner et al., 1991; Petz et al., 1995; Jankowski, 2007; Song et al., 2009; Xu et al., 2016; Pan et al., 2017). The morphology-based taxonomy and classification of the cyrtophorians have been studied intensively in the last five decades, and a comprehensive knowledge has accumulated (e.g., Deroux, 1976a,b, 1994; Gong et al., 2002, 2007; Gong and Song, 2004; Pan et al., 2013, 2016; Qu et al., 2017, 2018a; Chen et al., 2018; Wang et al., 2019). Over the past 20 years, with the employment of phylogenetic analyses inferred from small-subunit ribosome gene (SSU rDNA) sequences, the basic systematic relationship of Cyrtophoria has gradually been revealed. It has generally been in accordance with the morphological classification (Snoeyenbos-West et al., 2004; Lynn, 2008; Gao et al., 2012; Chen et al., 2016; Wang et al., 2017; Qu et al., 2018b; Hu et al., 2019). The relatively well-matching molecular phylogeny and morphological traits make cyrtophorian ciliates potential candidates for evolutionary research. However, only a few studies have looked at the evolutionary relationships among cyrtophorian ciliates based on both molecular phylogeny and morphological data (Gao et al., 2012; Chen et al., 2016). In addition, a lack of data on some key taxa largely restrains the evolutionary resolution and makes many details obscure (Chen et al., 2016).
The main obstacles faced by the cyrtophorian taxonomists are the same as those with other ciliate groups: (1) The sampling is insufficient inferring that a number of species still remain undiscovered, especially from “extreme” environments like hypersaline waters and hot springs. (2) Incomplete and vague descriptions of some known species make the species identification difficult. (3) A lack of molecular data for some intermediate taxa and even some well-known type species largely obstructs the phylogenetic and evolutionary analysis. Therefore, massive sampling along with a combined study tool of morphological observation and gene marker analysis is required to address these issues (Warren et al., 2017).
During a faunal investigation targeting cyrtophorian ciliates in China, we isolated a ciliate belonging to a rarely studied genus, Gastronauta Engelmann in Bütschli, 1889, and three populations of Trithigmostoma cucullulus (Müller, 1786) Jankowski, 1967. Gastronauta is a highly specialized but species-poor cyrtophorid genus. Its most diagnostic characteristic is its elongated oral slit surrounded by a circle of kinetids, which makes it unique among all cyrtophorians (for example, Bütschli, 1889; Kahl, 1931; Nie & Ho, 1943; Wilbert, 1971; Deroux, 1976b; Song and Wilbert, 1989; Foissner et al., 1991; Blatterer & Foissner, 1992; Oberschmidleitner & Aescht, 1996; Xu et al., 2016). The highly specialized oral structure, as well as the combination of several intermediate characteristics, obscures the systematic assignment of Gastronauta, but also indicates its potentially special phylogenetic position. Unfortunately, the SSU rDNA sequence of this genus is unavailable yet. Thus, this family has not been included in phylogenetic analyses. In this paper, we describe a new species, G. paraloisi sp. n., and Trithigmostoma cucullulus morphologically. More importantly, we also provide three SSU rDNA sequences, with a focus on that of Gastronautidae, and analyze their phylogenetic and evolutionary positions. The results indicate that the establishment of the family Gastronautidae by Deroux (1994) is necessary, and Gastronautidae and Trighigmostoma present as intermediate groups in evolution, within the order Chlamydodontida.
Materials and Methods
Sampling and Cultivation
Gastronauta paraloisi sp. n. and a population of Trithigmostoma cucullulus were isolated from a water sample taken from the surface of a freshwater river in Shenzhen, China (22°33′59.57″N, 114°6′52.80″E). The river lays in the inner city and influxes into a sea gulf approximately 10 kilometers away. This river receives household sewage water, and is thus nutrient-rich. The second population of T. cucullulus was isolated from an artificial freshwater lake in Qingdao, China (36°3′49.66″N, 120°20′42.86″E). The third population of T. cucullulus was found in a puddle near the coast in Zhanjiang, China (21°16′3.80″N, 110°25′31.08″E). The water salinity was about 3‰, and the sample was taken by scratching the substrate that contained diatoms. Immediately after the sampling, the ciliates were maintained by adding rice grains to the originally collected indigenous water in bottles. The attempt to obtain the pure culture with distilled water or Volvic water and rice grains failed. Instead, raw cultures were established by adding rice grains to Petri dishes filled with indigenous water (filtered through 0.65 μm-membranes to maintain indigenous bacteria). The rice grains supported the growth of bacteria as a food source for ciliates.
Morphological Studies
Living cells collected from raw cultures were observed using bright field and differential interference contrast microscopy (Olympus BX53). Wilbert’s (1975) protargol-staining method was applied to reveal the ciliature pattern and nuclear apparatus. Morphometric measurement was mainly conducted on specimens after protargol preparation. Illustrations of live specimens were based on photomicrographs and notes, while those of protargol-stained cells were made with the aid of a drawing device and photomicrographs. Terminology mainly follows Foissner (2000), and classification system refers to Lynn (2008).
DNA Extraction, Amplification, and Sequencing
For each of the ciliates, one single cell was picked from the raw culture under a stereomicroscope (SZH-ILLD-200, Olympus, Japan) and washed three times with distilled water in order to remove eukaryotic contaminants. The cell was then immediately transferred into a 1.5 ml-tube with 60 μl ATL buffer (Qiagen, Hilden, Germany). Genomic DNA was then extracted using the DNeasy Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions for animal tissues. Fifty ng of DNA (measured with a NanoDrop2000, peQLab, Biotechnology GmbH, Germany) from the extract were used for the subsequent amplification of the 18S rDNA. Q5 Hot Start High-Fidelity DNA Polymerase (New England Biolabs Inc., United States) and the primer pair EukA and EukB (Medlin et al., 1988) were used for PCR. Obtained amplicons were purified with the MinElute kit (Qiagen, Germany) and then sent directly for sequencing. Bi-directional Sanger sequencing was done to obtain a near-complete 18S rDNA sequence (Pan et al., 2019). The NCBI accession numbers of the assembled sequences of Gastronauta paraloisi sp. n., as well as the Qingdao and Zhanjiang populations of Trithigmostoma cucullulus are MW072507, MW116158, and MW116159, respectively. Sequence similarities were calculated using BioEdit 7.0.9.0.
Phylogenetic Analyses
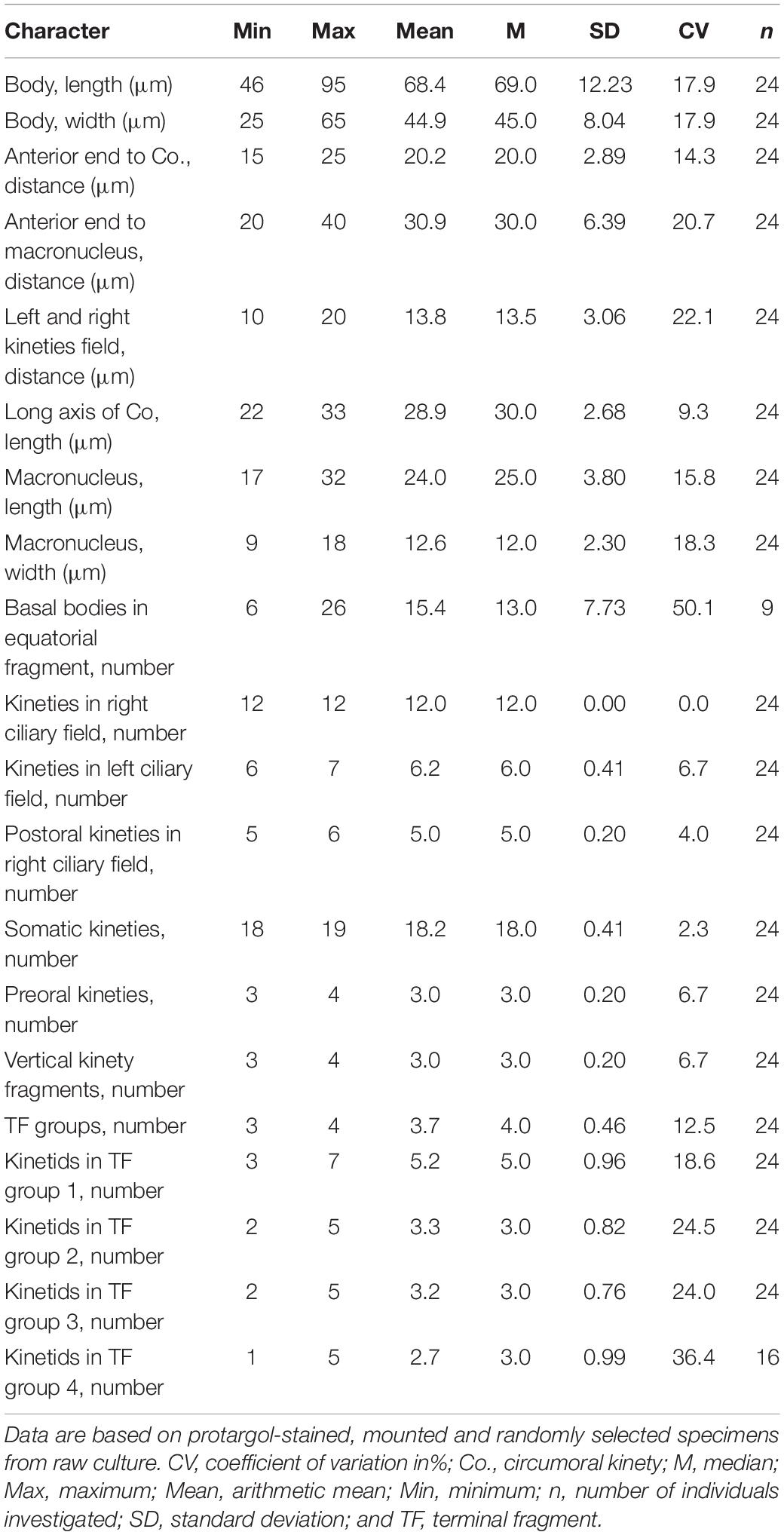
With the newly obtained 18S rDNA sequences included in the present work, a total of 80 SSU rDNA sequences were used for the phylogenetic analyses (accession numbers shown in Table 1). Three Suctoria species, Acineta compressa, Ephelota gemmipara and Prodiscophrya sp., were selected as outgroups, as they are phylogenetically close to Cyrtophoria. Sequences were aligned using the MUSCLE package on the European Bioinformatics Institute website1. The resulting alignment was then manually edited by trimming both ends. This resulted in a consensus matrix of 1,721 nucleotide positions. Two phylogenetic tools were used. A maximum-likelihood (ML) analysis was constructed with 1,000 bootstrap replicates using RAxML-HPC2 v. 8.2.12 (Stamatakis, 2014) online (CIPRES Science Gateway; Miller et al., 2010), with the evolutionary model GTR + I + Γ. A Bayesian inference (BI) analysis was run using the MrBayes on XSEDE 3.2.7a package (Ronquist and Huelsenbeck, 2003) on the CIPRES Science Gateway; optional model was GTR + I + Γ, selected using MrModeltest 2.2 with the criterion of Akaike Information Criterion (Nylander, 2004). Markov chain Monte Carlo simulations were run for ten million generations with a sample frequency of every 100th generations; the first 10% of the simulations were discarded as burn-in. The topology of the best ML tree was visualized using MEGA 5.0 (Tamura et al., 2011).
Results
Taxonomy and Morphological Description of the New Species
Phyllopharyngea de Puytorac et al., 1974
Cyrtophoria Fauré-Fremiet in Corliss, 1956
Chlamydodontida Deroux, 1976
Gastronautidae Deroux, 1994
Gastronauta Engelmann in Bütschli, 1889
Gastronauta paraloisi sp. n.
Synonym: Gastronauta membranaceus sensu da Silva and da Silva-Neto, 2001
Diagnosis
Cell size 50–120 × 30–80 μm in vivo. Body oval to ellipsoid with both ends broadly rounded. Macronucleus positioned at mid-body or slightly below. Two contractile vacuoles in diagonal positions. 18 or 19 kineties on postoral area, including 12 right and six or seven left kineties; two innermost right kineties anteriorly curved leftward, and the anterior ends of innermost right and left kinety almost confluent; outermost left kinety interrupted by oral slit. Three arc kineties above oral slit. Usually three vertical fragments, three or four terminal fragments. Freshwater and saprobic habitat.
Type Locality
The surface layer of a freshwater river in Shenzhen, China (22°33′59.57″N, 114°6′52.80″E), which receives household sewage water.
Type Material
One holotype slide with protargol-stained specimens was deposited in the Laboratory of Protozoology, Ocean University of China, with the accession number QZS2015120202.
Etymology
The species-group name paraloisi is a composite of the Greek prefix par- (closely related, beside) and the species-group name aloisi, indicating that the new species morphologically resembles Gastronauta aloisi Oberschmidleitner & Aescht (1996).
ZooBank Accession Number of the New Species
urn:lsid:zoobank.org:act:61698C4E-0FC8-4F45-A4FE-9B26AACFE4B3.
Morphological Description (Figures 1, 2 and Table 2)
Cell size 60–120 × 30–80 μm in vivo, and on average 68.4 × 44.9 μm after protargol-staining (n = 24). Body oval to ellipsoid with both ends broadly rounded (Figures 1A,B,D,E, 2G,H); dorsoventrally compressed, dorsal side conspicuously humped and ventral side concave. Cell acontractile but flexible. Anterior periphery thin and more transparent. Oral slit about 30 μm in width, slightly obliquely oriented and located in anterior third of cell, extending from approximate left margin to right of midline on ventral side (Figures 1A,D,E); oral membrane lamellar in vivo; pharyngeal fibers visible after protargol-staining, but individual nematodesmal rods not recognizable. Centrally heteromeric macronucleus about 25 × 15 μm in vivo (Figure 1A) and about 24 × 13 μm after protargol-staining (Figures 1I, 2A–F), positioned in mid-body or slightly below equator. Micronucleus not detected in vivo, but can be seen attached to macronucleus after protargol-staining, about 4 μm across (Figure 1I). Two contractile vacuoles in ordinary diagonal positions (Figures 1A,B,D,E, 2H), each approximately 10 μm across, pulsating at an interval of 10–30 s (n = 2); excretory pores recognizable after protargol-stating, anterior pore between second and third inner right kineties and posterior pore between fourth and fifth left kineties (Figure 1I). Cytoplasm transparent and colorless to grayish, containing many small (about 1–2 μm across) greenish to yellowish algae and colorless food vacuoles. Usually crawling slowly on substrate, and adhering to substrate firmly on concaved ventral side when disturbed; seldom swimming in water.
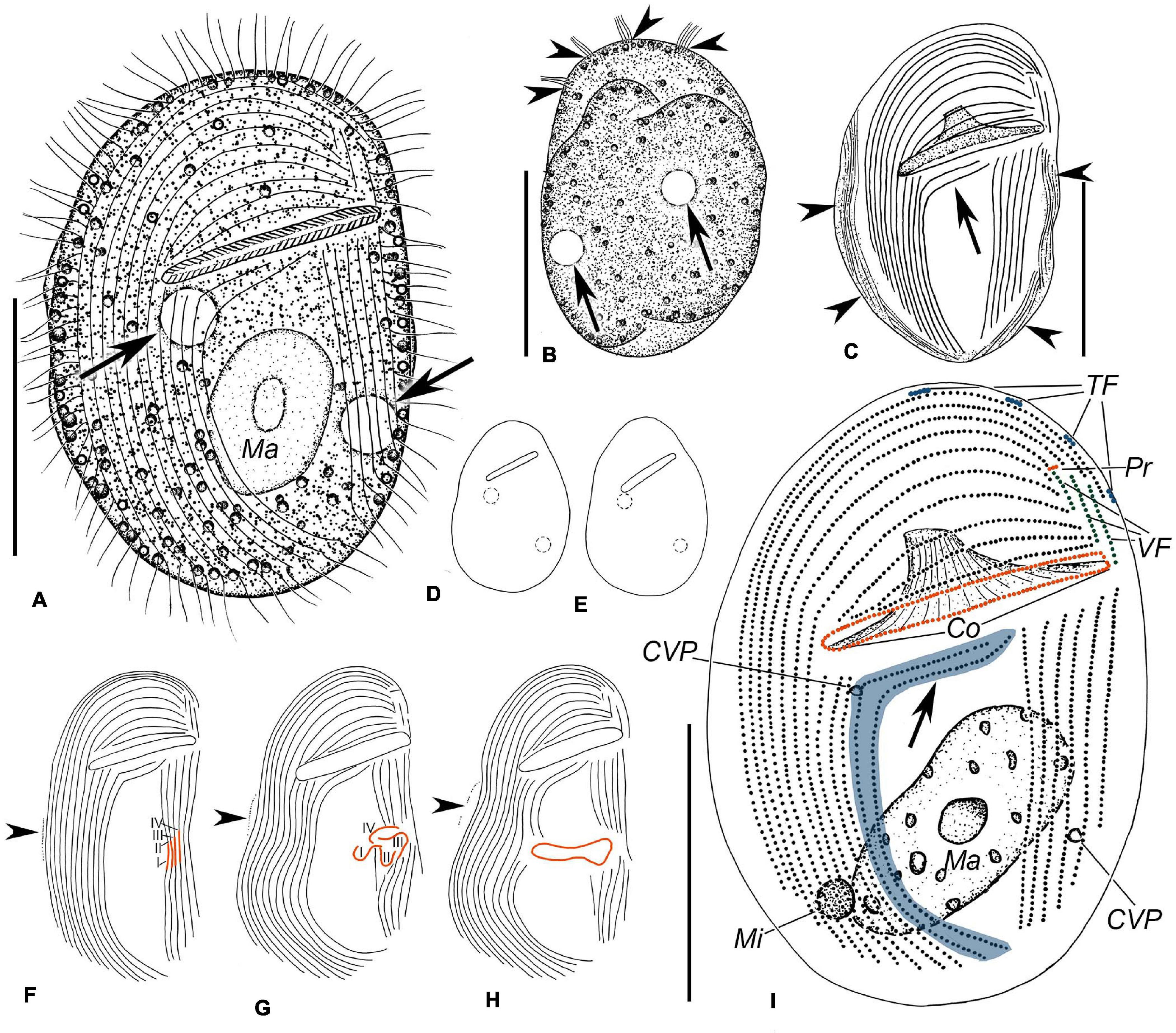
Figure 1. Illustrations of Gastronauta paraloisi sp. n. from life (A,B,D,E) and after protargol-staining (C,F–I). (A) Ventral view of a representative specimen. Arrows mark contractile vacuoles. (B) Dorsal view. Arrows indicate contractile vacuoles and arrowheads point to terminal fragments. (C) Ventral view of general ciliature; arrowheads denote the fibers along the cell margin after protargol-staining, and arrow shows the two curved postoral right kineties. (D,E) Body shape variations. (F–H) Several stages of morphogenesis. (F) Is an early stage, (G) is a middle stage, and (H) represents a late stage. Arrowheads indicate the equatorial fragment. I–IV represent anlagen I–IV. (I) Ventral overview of the ciliature. Arrow shows the two anteriorly curved right kineties, and special structures of Gastronauta are highlighted with different colors. Co, circumoral kinety; CVP, contractile vacuole pore; Ma, macronucleus; Mi, micronucleus; Pr, preoral kinety; TF, terminal fragments; and VF, vertical fragments. Scale bars: 30 μm.
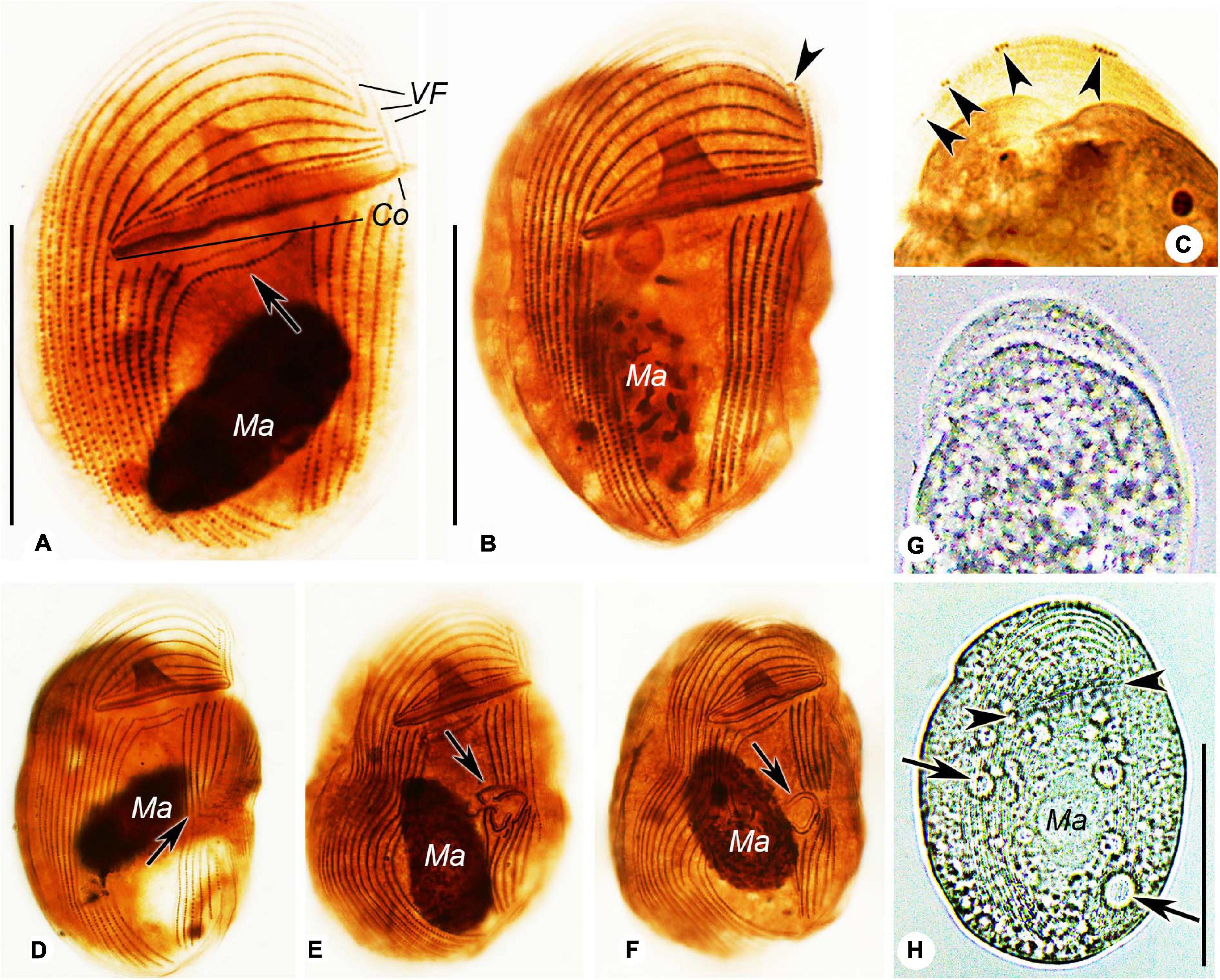
Figure 2. Photomicrographs of Gastronauta paraloisi sp. n. after protargol-staining (A–F) and from life (G,H). (A) Ventral view of general ciliature. Arrow shows the two curved postoral right kineties. (B) Ventral view of another individual. Arrowhead marks preoral kinety. (C) Dorsal view of anterior cell. Arrowheads point to terminal fragments. (D–F) Several stages of morphogenesis. (D) is an early stage, (E) is a middle stage, and (F) is a late stage. Arrows indicate the forming process of oral ciliature (stomatogenesis). (G) Dorsal view. (H) Ventral view of a slightly compressed individual. Arrows mark contractile vacuoles and arrowheads mark the oral slit. Abbreviations: Co, circumoral kinety; Ma, macronucleus; and VF, vertical fragments. Scale bars: 30 μm.
Ciliary pattern as shown in Figures 1C,I, 2A–C. In total, 18 or 19 (mostly 18) kineties on postoral (below oral slit) area, including 12 right and six or seven left kineties; a conspicuous barren gap (10–20 μm wide) present between right and left kineties. Usually seven outermost right kineties exceeding the level of oral slit and bending leftward anteriorly; remaining five (one out of 24 specimens contains six) right kineties commencing anteriorly below oral slit (postoral right kineties), of which two innermost ones anteriorly curved leftward and the innermost joining the innermost left kinety anteriorly; almost all right kineties bending slightly leftward posteriorly and terminating sub-caudally. Left kineties anteriorly commencing below oral level and posteriorly shortened from right to left; outermost left kinety interrupted by oral slit Three or four terminal fragments (dorsal brush; cilia about 6 μm long) along left anterior margin of cell, each consisting of three to five basal bodies.
Oral ciliature composed of one circumoral kinety, which is closed and surrounds oral slit, as shown in Figures 1I, 2A,B. Three arc kineties shortened from the outside in above oral slit; Usually three short vertical kineties (one of 24 specimens observed contained four) to left anterior of oral slit, extending from anterior ends of fourth to second right kinety to left of circumoral kinety; the innermost one slightly shorter. One short transverse fragment (preoral kinety) in front of vertical kineties, consisting of only three or four basal bodies. Fibers along the cell margins recognizable after protargol-staining (Figure 1C).
Morphogenesis
Several stages of morphogenesis were observed in protargol-stained specimens. In an early divider (Figures 1F, 2D), the middle portions of the innermost four left kineties become thickened and curved inward, forming the opisthe’s oral primordia; meanwhile, an equatorial fragment occurs to the right of right kineties. In a middle stage (Figures 1G, 2E), equatorial fragment is elongated to form the anlage of opisthe’s terminal fragments (dorsal brush). The four oral primordia then dispatch from their original left kineties: anlage IV drifts in an anti-clockwise direction followed by anlagen III, II and I in succession. The fifth left kinety is interrupted in the middle. The middle part of right kineties concave to the left. In a late divider (Figures 1H, 2F), the four parts of the oral primordia merge to form the circumoral kinety of the opisthe. The outermost left kinety and the innermost two right kineties are interrupted in the middle. The anlage of terminal fragments develops into terminal fragments of the opisthe.
Taxonomy and Morphological Description of Trithigmostoma cucullulus (Müller, 1786) Jankowski, 1967
Chilodonellidae Deroux, 1970
Trithigmostoma Jankowski, 1967
Trithigmostoma cucullulus (Müller, 1786) Jankowski, 1967
Voucher Slides
Slides of the Qingdao, Zhanjiang and Shenzhen population of Trithigmostoma cucullulus were deposited in the Laboratory of Protozoology, Ocean University of China, with accession numbers QZS2015050601, QZS2015040301, and QZS2015120401, respectively.
Morphological Description of Qingdao Population (Figures 3A,B,D–F, 4C–E and Table 3)
Cell size 68–80 × 32–40 μm in vivo, and approximately 80 × 35 μm after protargol-staining. Body ellipsoid-shaped, both ends broadly rounded, inconspicuous protrusion located at left anterior end of cell (Figures 3A,B, 4E); dorsoventrally compressed with a width: thickness ratio of about 2:1 (Figure 3C). Cell acontractile but flexible. Anterior body end transparent. Cytostome in anterior 1/4 to 1/3, surrounded by 11 to 13 (on average 12) nematodesmal rods (Figures 3A,B,E). Approximately eight to 12 contractile vacuoles (diameter about 3 μm) counted from live cells, irregularly distributed on ventral side (Figure 3A), pulsating at an interval of 5–10 s; three to eight excretory pores detected in protargol-stained specimens (Figures 3E,F, 4C). Macronucleus in mid-body or slightly below, about 22 × 10 μm in vivo and 23 × 10 μm after staining; not (or inconspicuously) centrally heteromeric (Figures 3A,B,E,F, 4C). Cytoplasm usually containing many diatoms (Figures 3A,D, 4E,E). Usually crawling slowly on substrate, occasionally swimming in water; strongly thigmotactic.
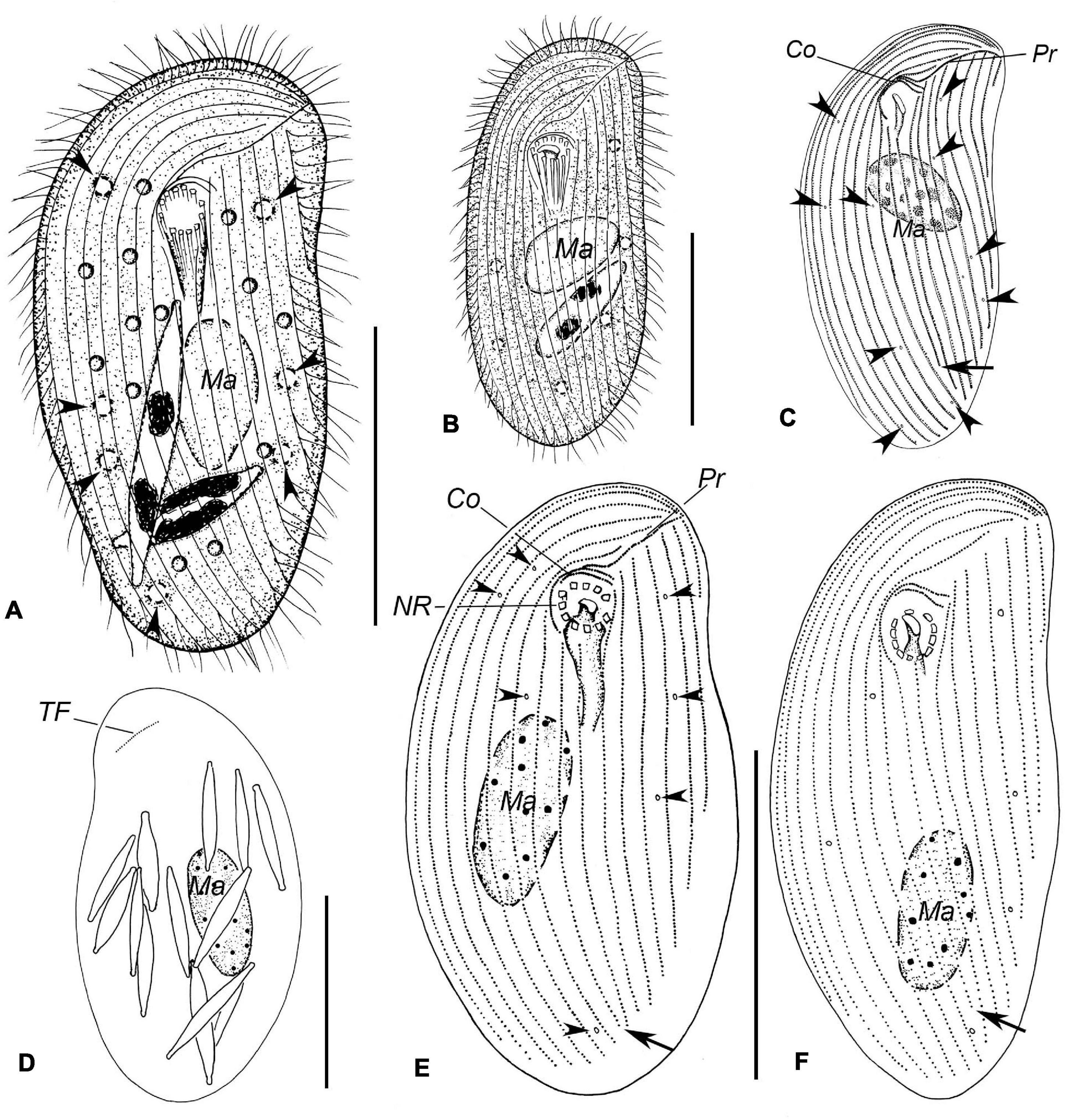
Figure 3. Illustrations of Trithigmostoma cucullulus, Qingdao (A,B,D–F) and Zhanjiang populations (C). (A,B) Ventral view of individuals in different body shapes. Arrowheads denote contractile vacuoles. (C,E,F) Ventral ciliature pattern. Arrows point to the end of the right postoral kinety, and arrowheads show the contractile vacuole pores. (D) Dorsal side, showing the terminal fragment as well as the food diatoms. Co, circumoral kineties; Ma, macronucleus; NR, nematodesmal rods; Pr, preoral kinety; and TF, terminal fragment. Scale bars: 40 μm.
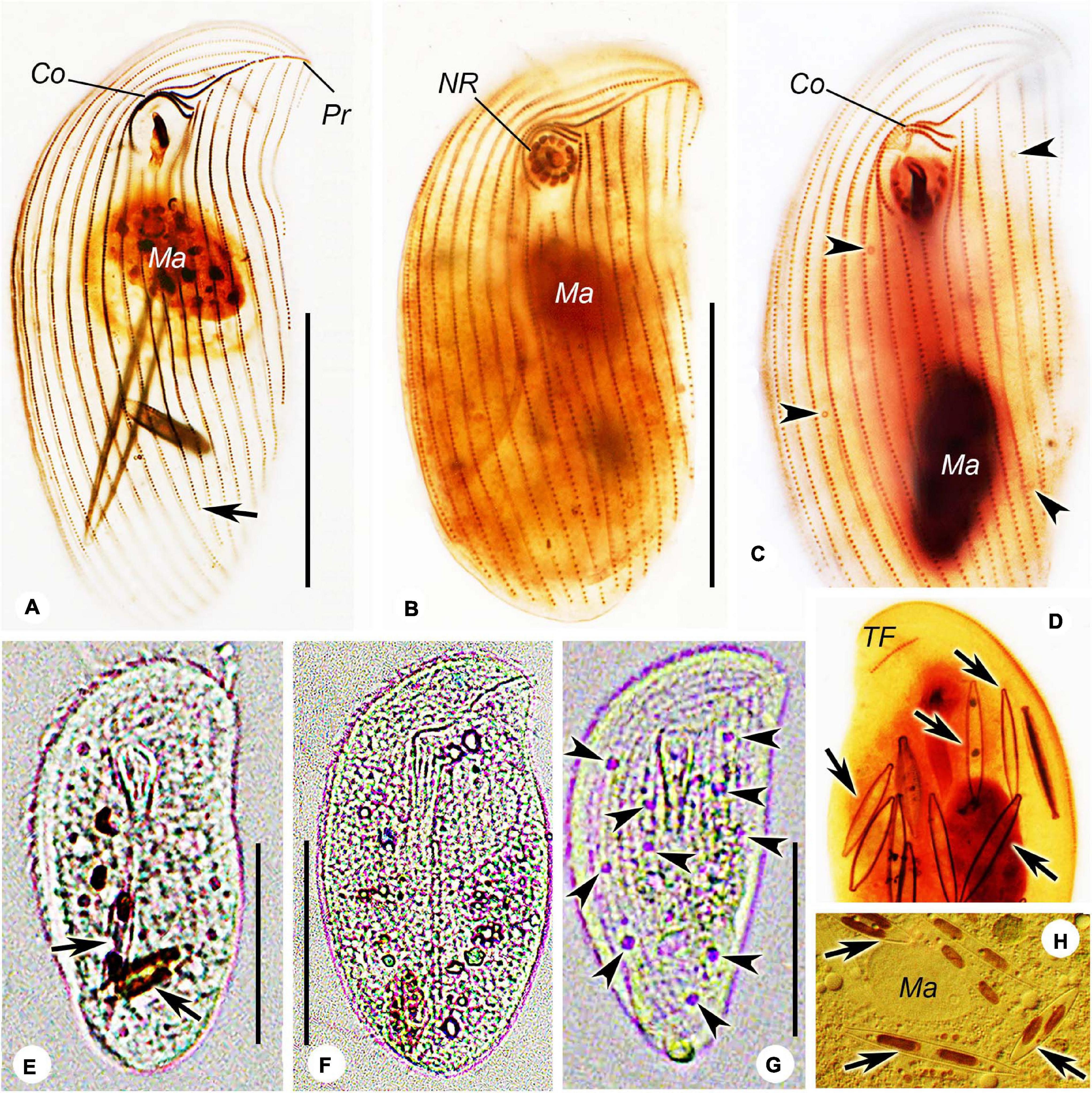
Figure 4. Photomicrographs of Trithigmostoma cucullulus, Zhanjiang (A,F–H), Shenzhen (B), and Qingdao populations (C,D,E). (A–C) Ventral view of protargol-stained specimens, arrowheads mark contractile vacuole pores, and arrow points to the end of the right postoral kinety. (D) Dorsal side of a stained individual, arrows denote the food diatoms. (E,F) Ventral view of cells from life. Arrows show food diatoms. (G) Ventral view of a starved cell, arrowheads show contractile vacuoles. (H) Arrow shows the food diatoms. Co, circumoral kineties; Ma, macronucleus; NR, nematodesmal rods; Pr, preoral kinety; and TF, terminal fragment. Scale bars: 40 μm.
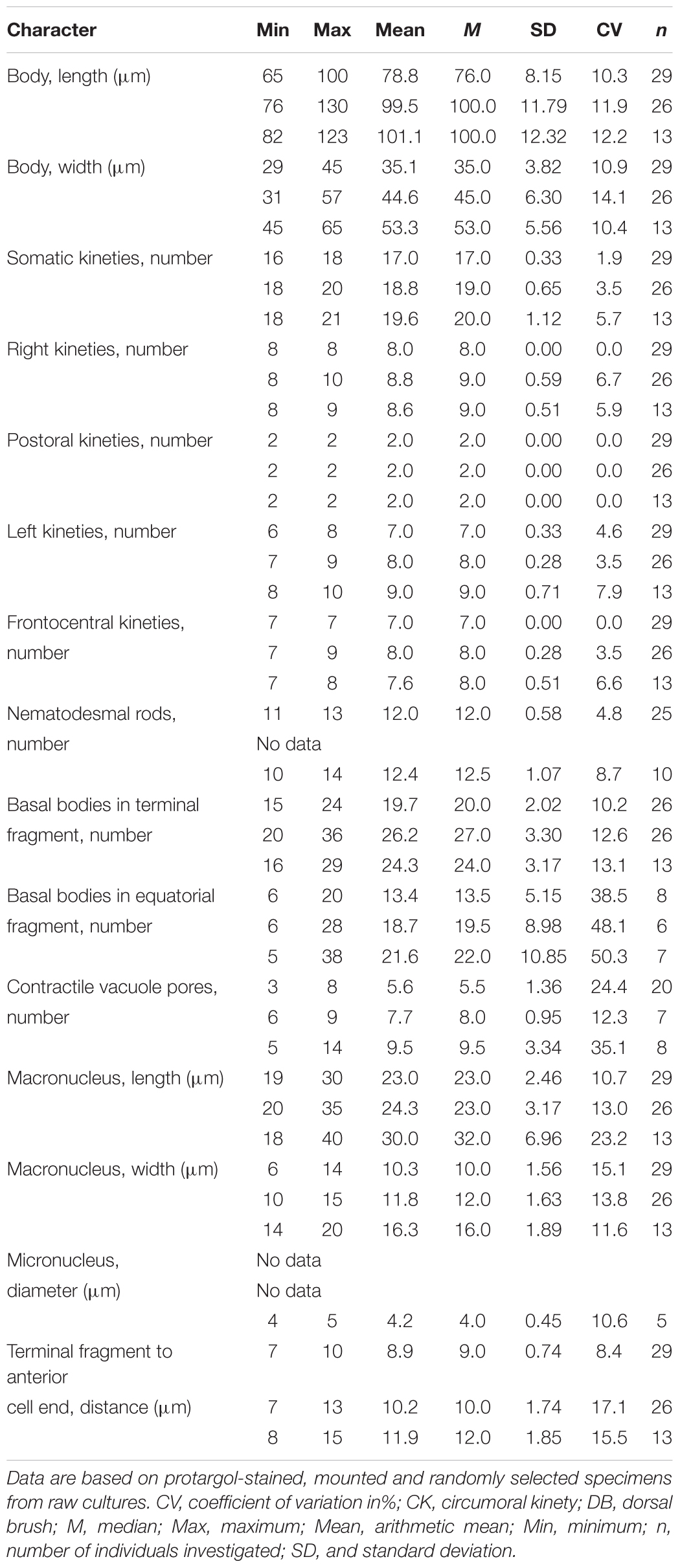
Table 3. Morphometric data of Trithigmostoma cucullulus from Qingdao (upper rows), Zhanjiang (middle rows), and Shenzhen (lower rows) populations.
Sixteen to 18 somatic kineties composed of three parts: right, postoral, and left kineties (Figures 3E,F, 4C). Constantly eight right kineties, all but the innermost one extending to left anterior cell end; posteriorly extending to rear end of cell. Two postoral kineties, ending at posterior 1/6 of cell, right one usually shortened (Figures 3E,F, 4C). Six to eight left kineties, all but the innermost one commences from preoral kinety, posteriorly shortened from right to left; the innermost left kinety usually starts at the level of cytostome; the outermost left kinety very short, only comprising 20 to 25 basal bodies.
Two circumoral kineties parallel to each other, slightly above cytostome, with the outer one about twice the length of the inner one; preoral kinety obliquely arranged ahead of cytostome and left kinety (Figures 3E,F, 4C). Single and oblique terminal fragment subapically located on dorsal side, comprising 15 to 24 basal bodies (Figures 3D, 4D). Equatorial fragment seldom detected, containing six to 20 basal bodies.
For Shenzhen and Zhanjiang populations, only illustrations, microphotographs and morphometric data are provided (Figures 3C, 4A,B,F–H and Table 3).
Sequence Similarity and Molecular Phylogeny
Three SSU rDNA sequences were obtained. The sequence of Gastronauta paraloisi sp. n. is highly divergent from available reference sequences in the GenBank database. By pairwise comparison, its closest relative, with a sequence similarity of 92.8%, is Phascolodon vorticella KX258192. The two Trithigmostoma cucullulus populations in the present work (Qingdao population, T. cucullulus QD; Zhanjiang population, T. cucullulus ZJ) have a similarity of 99.31%. The result of pairwise comparison of related sequences is shown in Figure 6F.
In the phylogenetic trees inferred from SSU rDNA sequences (Figure 5), four Trithigmostoma sequences form a monophyletic clade with full support. Gastronauta paraloisis sp. n. clusters with Trithigmostoma species with moderate support values (ML/BI, 80/0.87). This branch is then sister to the clade formed by other Chilodonellidae taxa represented by genera Chilodonella, Odontochlamys, Phascolodon, and Pseudochilodonopsis, with full support. The Gatronautidae/Chilodonellidae clade is located within the order Chlamydodontida. As in previous studies (Gao et al., 2012; Chen et al., 2016; Qu et al., 2017; Wang et al., 2017), both Chlamydodontidae and Lynchellidae are monophyletic (arrows in Figure 5 indicate their monophylies).
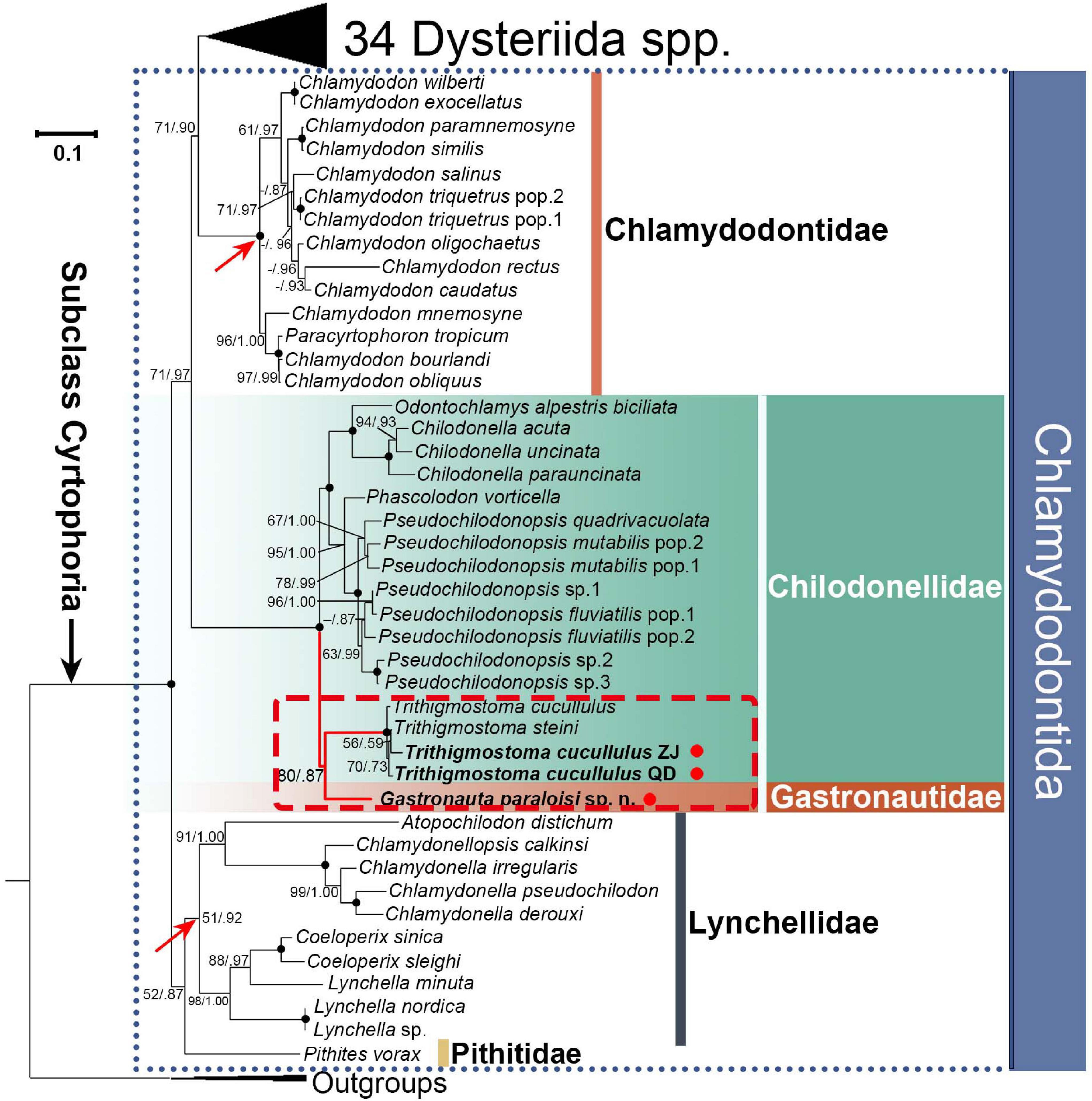
Figure 5. ML tree inferred from SSU rDNA sequences. Support values from ML and posterior probabilities of BI are provided at branching point (ML/BI). Bold dots at branching point mean full support from both analyses. “-” indicates discrepancy between topologies of ML and BI trees, or support values < 50%/0.5 (ML/BI). Red arrows denote the monophyly of Chlamydodontidae and Lynchellidae, and red dots indicate the three sequences from present work. QD and ZJ represent Qingdao and Zhanjiang populations of Trithigmostoma cucullulus, respectively. The scale bar represents one substitution per ten nucleotide positions.
Discussion
Brief Review of Family Gastronautidae
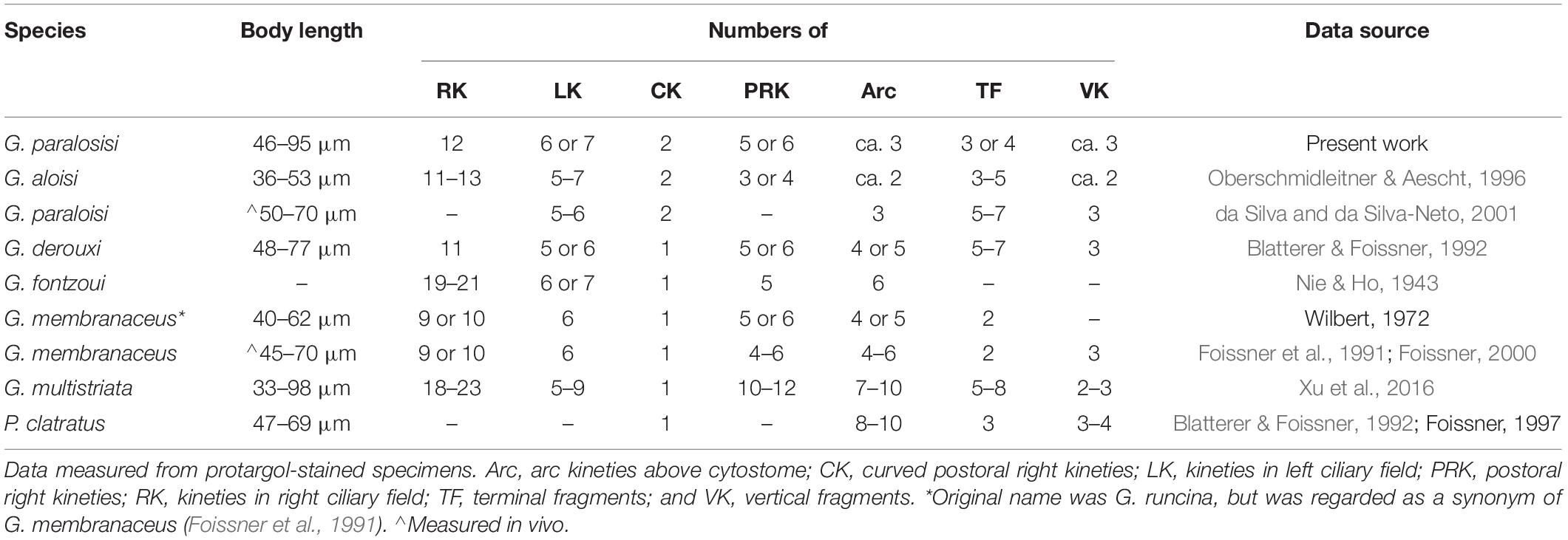
The subclass Cyrtophoria contains two orders, Dysteriida and Chlamydodontida, which are mainly characterized by the patterns of flattened body (dorsoventrally or laterally flattened), and the presence or absence of adhesive organelle (podite or secretory organelle) (Lynn, 2008). The dorsoventrally flattened body and the absence of adhesive organelle confirm that Gastronauta belongs to the order Chlamydodontida. By this criterion, Kahl (1931) put Gastronauta under the family Chlamydodontidae. The range of “Chlamydodontidae” by Kahl equates to order Chlamydodontida in today’s classification. While, the special structures, for example, a closed circular circumoral kinety and short vertical fragments in the left anterior body, make Gastronauta unique among all families in Chlamydodontida. Corliss (1979) tended to assign it to the family Lynchellidae because they both contain short arc kineties before cytostome (a large portion of kineties interrupted by cytostome). But their differences are also clear: (1) Gastronauta has a complete circular oral kinety, whereas Lynchellidae has parallel oral kinety fragments; and (2) Gastronauta has a centrally heteromeric macronucleus, while Lynchellidae species possess a parallelly heteromeric macronucleus. Thus, Deroux (1994) established the family Gastronautidae for the genus. Foissner (2000) also briefly discussed the familial assignment of Gastronauta, and he agreed with Deroux (1994)’s classification. On top of that, Foissner split the genus into two, based on the presence/absence of the postoral barren gap between right and left kineties: Gastronauta has the gap, while Paragastronauta does not. Lynn (2008) also adopted Gastronautidae, under subclass Chlamydodontida. Gastronauta is currently comprised of six species (including the new species), namely: G. membranaceus Engelmann in Bütschli, 1889 (type); G. fontzoui Nie & Ho, 1943; G. derouxi Blatterer & Foissner, 1992; G. aloisi Oberschmidleitner & Aescht, 1996; G. multistriata Xu et al., 2016, and G. paraloisi sp. n. Paragastronauta contains only one species, P. (Gastronauta) clatratus (Deroux, 1976) Foissner, 2000. The key morphometric characteristics of gastronautid species are shown in Table 4. A key to the identification of gastronautids based on morphological characteristics is provided below.
| 1 Barren gap present between right and left kineties | 2 Gastronauta |
| 1′ Barren gap absent between right and left kineties | Paragastronauta clatratus |
| 2 Two curved postoral right kineties | 3 |
| 2′ One curved postoral right kinety | 4 |
| 3 Two arc kineties above oral slit | G. alosi |
| 3′ Three arc kineties above oral slit | G. paraloisi sp. n. |
| 4 No more than 12 right kineties | 5 |
| 4′ More than 17 right kineties | 6 |
| 5 Two terminal fragments | G. membranaceus |
| 5′ Five to seven terminal fragments | G. derouxi |
| 6 Five postoral right kineties | G. fontzoui |
| 6′ Ten to twelve postoral right kineties | G. multistriala |
Morphological Comparison of Gastronauta paralosisi sp. n. With Its Congeners
Based on the overall living morphology and the general ciliary pattern, especially the number of kineties and the two leftward curved innermost right kineties, one could be inclined to assign the new ciliate to Gastronauta aloisi. However, the new species can be clearly distinguished from the latter by the combination of the following characteristics: (1) body size. Our form is generally larger than G. aloisi (60–120 μm in vivo and about 68 × 45 μm after staining vs. 50–70 μm in vivo and 45 × 35 μm in G. aloisi); (2) number of arc kineties above oral slit (three vs. two); (3) number of vertical kinety fragments (three or four vs. two); and (4) the position of the anterior end of the innermost right kinety (almost joining the innermost left kinety vs. slightly left to the middle of the barren gap between right and left kineties) (Oberschmidleitner & Aescht, 1996). Further, the new species can be clearly distinguished from other Gastronauta species mainly by the two curved innermost right kineties (morphometric comparison see the identification key above and Table 4).
Foissner (2016) synonymized a Brazilian population of Gastronauta membranaceus with G. aloisi mainly because of the feature of the two curved postoral right kineties. However, this form has three arc kineties and the anterior end of the innermost right kinety connects the innermost left kinety (da Silva and da Silva-Neto, 2001), indicating it should be conspecific with the current new form. However, the Brazilian population is slightly different from our form by the position of the macronucleus (in anterior body vs. in mid-body or slightly below) and the number of terminal fragments (5–7 vs. 3–4), which could be considered as intraspecific variations.
Occurrence of Gastronauta paraloisi sp. n
da Silva and da Silva-Neto (2001) isolated a population of Gastronauta paraloisi sp. n. (see section “Discussion”) from an experimental reactor of an activated sludge sewage treatment plant. Although the environmental parameters were not measured in our study, our population was isolated from a similar habitat. The sampling river is part of the sewer system of the inner city, and it seems saprobic, especially in rush hours in the morning and evening. Right after the sampling, the food vacuoles were checked, and many small algae (diameter about 1–2 μm) were found. When the raw culture was established with rice grains added to the indigenous water, G. paraloisi sp. n. thrived and cells tended to gather around the grains (bacteria-rich), indicating it is omnivorous (feeding on bacteria and small algae). As demonstrated by Šimek et al. (2019), unselective grazers that feed on a broader size spectrum from bacteria to small algae may have a considerable competitive advantage in hypertrophic environments, which explains the occurrence of a high abundance of G. paraloisi sp. n. in a saprobic environment. The fact that the attempt to establish the pure culture solely with rice grains and instilled water failed shows that the cultivation of this species may not be monoxenic.
Brief Review of Trithigmostoma and Identification of T. cucullulus
Jankowski (1967) established the genus Trithigmostoma from Chilodonella, mainly based on the lack of a barren gap between right and left kineties. The type is Trithigmostoma cucullulus. Foissner (1987, 1988) then summarized and made new combinations for some Chilodonella species. So far, the genus is comprised of five species, namely: T. bavariensis (Kahl, 1931) Foissner, 1987; T. chattoni (Mac Dougall, 1936) Foissner, 1988; T. cucullulus (Müller, 1786) Jankowski, 1967; T. srameki (Sramek-Husek, 1952) Foissner, 1988; and T. steini (Blochmann, 1895) Foissner, 1988. Details about the species identification refer to Foissner (1987, 1988) and Blatterer & Foissner (1992). Three other genera, Odontochlamys Certes (1891), Thigmogaster Deroux, 1976 and Pseudochilodonopsis Foissner, 1979 were also separated from Chilodonella (Qu et al., 2015), and together with Trithigmostoma, are now assigned to the family Chilodonellidae. However, characteristics such as a lack of barren gap between right and left kineties, almost all right kineties reaching posterior cell end, and many irregularly distributed contractile vacuoles, make Trithigmostoma very special in Chilodonellidae.
Trithigmostoma cucullulus is one of the earliest identified ciliates. It was originally reported by Müller (1786), using the name Kolpoda cucullulus (Aescht, 2001). Because of its wide distribution and relatively high abundance, Trithigmostoma cucullulus has been recorded worldwide and its morphology is well studied. Since the original report, the generic assignment of this species (species name) has been changed several times, for example to Chilodon cucullulus (Müller, 1786) Klein, 1927, Chilodonella cucullulus (Müller, 1786) Kahl, 1931 and Trithigmostoma cucullus (Müller, 1786) Foissner (1988)Jankowski, 1967 (spelling mistake was corrected by Foissner, 1988). Foissner et al., 1991 summarized the morphology of the species in detail. Our populations correspond well with the previous populations as well as with a recent estuarine population from China (salinity 4.3‰; Chen et al., 2018). Although reported many times in history, T. cucullulus demonstrates very stable morphological features. In addition, considering its worldwide distribution, it might be a cosmopolitan species.
In the present phylogenetic analyses, three sequences of Trithigmostoma cucullulus did not form a monophyletic group, but formed a clade within which T. steini X71134 (Leipe et al., 1994) nested. In general living morphology, T. cucullulus is very similar to T. steini. The main differences are the number of somatic kineties (18–22 in T. cucullulus vs. 25–33 in T. steini) and the number of contractile vacuole pores (2–11 vs. 10–40), which are mainly detectable after staining (Foissner et al., 1991; Chen et al., 2018; and data from the present work). Therefore, it is likely that T. steini X71134 (morphological data not available) was misidentified, or that the morphological discrepancy of these two species could not be shown by the molecular phylogeny inferred from SSU rDNA sequences.
Intermediate Position of Gastronautidae and Trithigmostoma
The special positions of Gastronautidae (Gastronauta) and Trithigmostoma are also revealed by phylogeny derived from the SSU rDNA sequences. They cluster but also branch away from the core Chilodonellidae genera of the family Chilodonellidae. As mentioned above, Trithigmostoma has a special position within Chilodonellidae. The familial assignment to Chilodonellidae is beyond doubt, but its special characteristics also make it close to Chlamydodontidae and Lynchellidae. Chen et al. (2016) briefly discussed the status of this genus using phylogeny from SSU rDNA sequences, and demonstrated that it is a peripheral genus within Chilodonellidae. In another analysis inferred from mitochondrial small subunit ribosome DNA, the genus is also revealed to have an uncertain position in Chilodonellidae (Wang et al., 2017). Chen et al. (2018) concluded that Trithigmostoma is a basal genus in Chilodonelliae. Thus, based on previous studies and our own we agree that Trithigmostoma represents a basal group in Chilodonellidae, and it may be closely related to the common ancestor (still undiscovered or extinct) of Chilodonellidae, Chlamydodontidae and Lynchellidae.
Gastronautidae has more specialized and intermediate characteristics among the order Chlamydodontida (Figure 6). As mentioned above, both Gastronautidae and Lynchellidae have kineties interrupted by cytostome, thus there is no clear suture formed between right and left kineties in the left anterior body (violet dashes in Figure 6). Gastronautidae, Lynchellidae and Chilodonellidae usually have two (or sometimes several in Chilodonellidae) regularly distributed contractile vacuoles (vs. many irregularly distributed in Chlamydodontidae). Gastronautidae and Chilodonellidae have a centrally heteromeric macronucleus (sometimes inconspicuous in Trithgmostoma) (marked green in Figure 6) and usually well-separated right and left kineties (except for Trithigmostoma and Paragastronauta) (red double arrows in Figure 6), while the other two families have a parallelly heteromeric macronucleus and unseparated right and left kineties (Atopochilodon in Lynchellidae has only unobvious separation). In addition, Gastronautidae’s multiple terminal fragments make it close to Chlamydodontidae and Lynchellidae but not to Chilodonellidae (blue fragments in Figure 6). More importantly, Gastronautidae can be clearly separated from others by its unique, closed circular oral kinety (marked orange in Figure 6), which confirms the rationality of the establishment of Gastronautidae. Thus, we assume Gastronautidae represents an evolutionary link among Chlamydodontida ciliates. However, the poor-matching of morphology of Gastronauta and Trithigmostoma, along with the grouping of these two genera in SSU rDNA phylogeny, indicates that data concerning the key evolutionary link(s) of cyrtophorian ciliates are still missing. But up to date, there is only one sequence of Gastronauta available and the molecular representative of the type species, G. membranaceus, is still lacking, which largely constrains the phylogenetic resolution. Further study with high phylogenetic resolution needs to be conducted with more morphological and molecular data from Gastronautidae and other related groups.
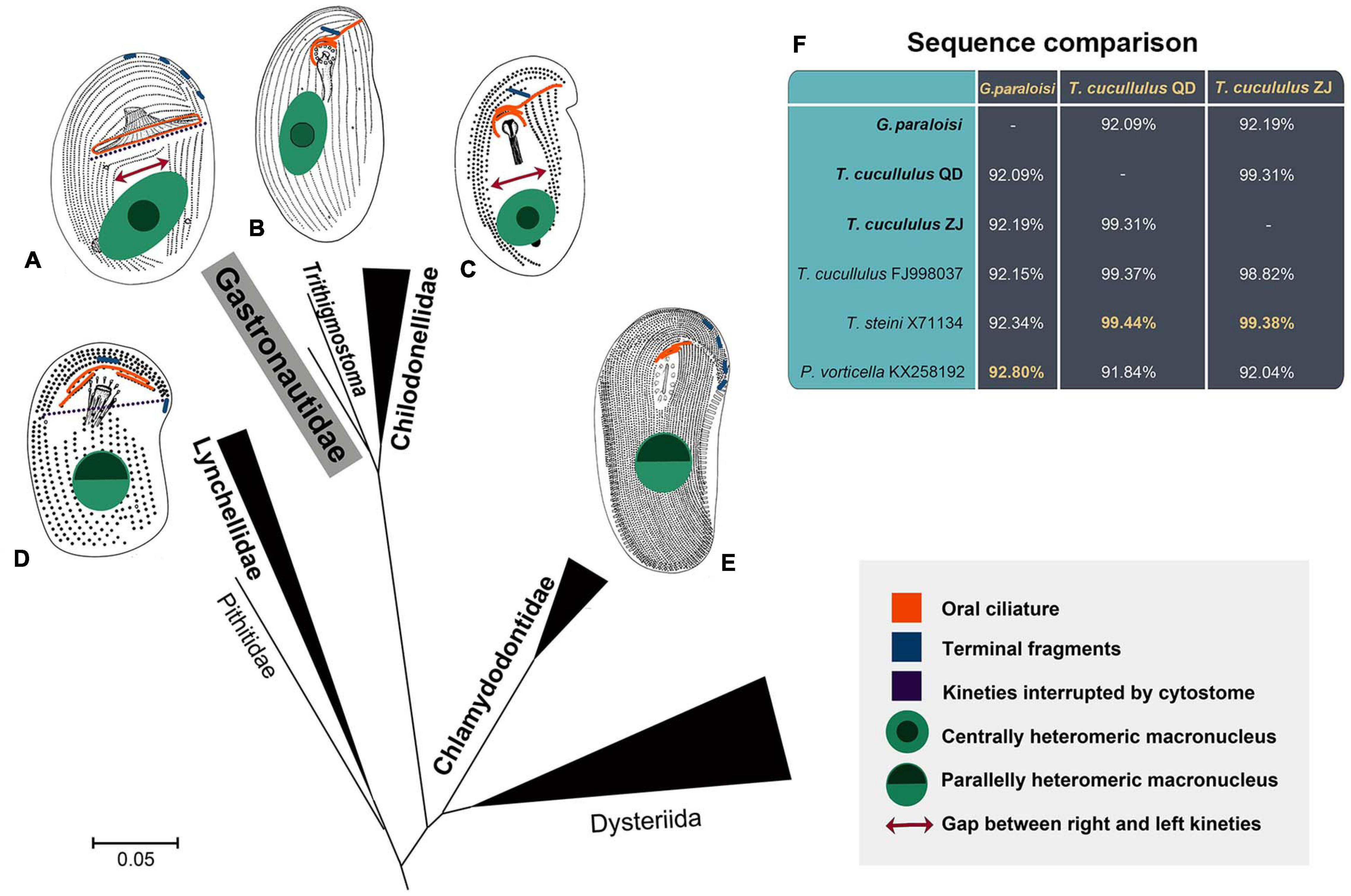
Figure 6. The illustrations display key morphological characteristics (indicated by different colors and shapes) of Chlamydodontida families (A–E). The scale bar represents 5 substitutions per 100 nucleotide positions. (A) Gastronautidae (Gastronauta). (B) Trithigmostoma. The heteromeric centralization of the macronucleus is sometimes inconspicuous. (C) Chilodonellidae (Chilodonella). (D) Lynchellidae (Lynchella). (E) Chlamydodontidae (Chlamydodon). (F) Comparison of closely related sequences. The greatest similarities to the three sequences in the present work are highlighted.
Author’s Note
This article is registered in ZooBank under:urn:lsid:zoobank.org:pub:384B19A2-DCE5-4CB8-8C5D-379BF9A04EB2.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author Contributions
ZQ conducted the field and lab work, morphological and molecular analyses, and wrote and revised the draft. HP contributed to the taxonomy and phylogenetic analyses, and revised the draft. CW conducted phylogenetic analyses and figure visualization. HM revised the draft. TS contributed to sections, revised the draft, and improved the language. XH conceived and designed the study, supervised the work, and revised the draft. All authors have read and agreed to be accountable for the content of the work.
Funding
This work was financially supported by the National Natural Science Foundation of China (projects numbers: 41976086, 32070432, and 32030015).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors gratefully acknowledge Prof. Weibo Song, Ocean University of China, for his valuable comments on drafting the manuscript.
Footnotes
References
Aescht, E. (2001). Catalogue of the generic names of ciliates (Protozoa, Ciliophora). Denisia 1, 1–350. doi: 10.1007/bf00017483
Blatterer, H., and Foissner, W. (1992). Morphology and infraciliature of some cyrtophorid ciliates (Protozoa, Ciliophora) from freshwater and soil. Arch. Protistenk. 142, 101–118. doi: 10.1016/S0003-9365(11)80075-8
Bütschli, O. (1887–1889). “Erster band protozoa. III. Abtheilung. Infusoria und system der radiolaria,” in Klassen und Ordnungen des Thier-Reichs, Vol. I, ed. H. G. Bronn (Leipzig: Winter’sche Verlagshandlung), 1089–2035.
Chen, X., Li, L., Al-Farraj, S. A., Ma, H., and Pan, H. (2018). Taxonomic studies on Aegyria apoliva sp. nov. and Trithigmostoma cucullulus (Müller, 1786) Jankowski, 1967 (Ciliophora, Cyrtophoria) with phylogenetic analyses. Eur. J. Protistol. 62, 122–134. doi: 10.1016/j.ejop.2017.12.005
Chen, X., Pan, H., Huang, J., Warren, A., Al-Farraj, S. A., and Gao, S. (2016). New considerations on the phylogeny of cyrtophorian ciliates (Protozoa, Ciliophora): expanded sampling to understand their evolutionary relationships. Zool. Scr. 45, 334–348. doi: 10.1111/zsc.12150
Corliss, J. O. (1979). The Ciliated Protozoa. Characterization, Classification, and Guide to the Literature, 2nd Edn. New York, NY: Pergamon Press
da Silva, S. B. A., and da Silva-Neto, I. D. (2001). Morfologia dos pro-tozoários ciliados presentes em um reator experimental detratamento de esgoto por processo de lodos ativados. Rev. Bras. Zoociências Juiz de Fora 3, 203–229.
Deroux, G. (1976a). Le plan cortical des Cyrtophorida unité d’expression et marges de variabilité. I. Le cas des Plesiotrichopidae, fam. nov., dans la nouvelle systématique. Protistologica 12, 469–481.
Deroux, G. (1976b). Le plan cortical des Cyrtophorida unité d’expression et marges de variabilité. II. Cyrtophorida a thigmotactisme ventral généralise. Protistologica 12, 483–500.
Deroux, G. (1994). “Sous-classe des cyrtophoria fauré-fremiet in Corliss, 1956,” in Traité de Zoologie. Anatomie, Biologie. Infusoires Ciliés, Fasc. 2 Systématique, Vol. II, ed. P. de Puytorac (Paris: Masson), 401–431.
Foissner, W. (1987). Miscellanea nomenclatorica ciliatea (Protozoa. Ciliophora). Arch. Protistenk. 133, 219–235. doi: 10.1016/S0003-9365(87)80054-4
Foissner, W. (1988). Taxonomie und ökologie einiger ciliaten (Protozoa, Ciliophora) des Saprobiensystems. II. Familie Chilodonellidae. Hydrobiologia 162, 21–45. doi: 10.1007/BF00014331
Foissner, W. (2000). Revision of the genera Gastronauta Engelmann in Bütschli, 1889 and Paragastronauta nov. gen. (Ciliophora: Gastronautidae). Protozool. Monogr. 1, 63–101.
Foissner, W. (2016). Protists as bioindicators in activated sludge: identification, ecology and future needs. Eur. J. Protistol. 55, 75–94. doi: 10.1016/j.ejop.2016.02.004
Foissner, W., Blatterer, H., Berger, H., and Kohmann, F. (1991). Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems. Band I: cyrtophorida, oligotrichida, hypotrichia, colpodea. Informationsberichte des bayer. Landesamt. Wasserwirtschaft 1/91, 478.
Gao, S., Huang, J., Li, J., and Song, W. (2012). Molecular phylogeny of the cyrtophorid ciliates (Protozoa, Ciliophora, Phyllopharyngea). PLoS One 7:e33198. doi: 10.1371/journal.pone.0033198
Gong, J., and Song, W. (2004). Description of a new marine cyrtophorid ciliate, Dysteria derouxi nov. spec., with an updated key to 12 well-investigated Dysteria species (Ciliophora, Cyrtophorida). Eur. J. Protistol. 40, 13–19. doi: 10.1016/j.ejop.2003.07.002
Gong, J., Song, W., and Warren, A. (2002). Redescriptions of two marine cyrtophorid ciliates, Dysteria cristata (Gourret and Roeser, 1888) and Dysteria monostyla (Ehrenberg, 1838) Kahl, 1931 (Protozoa, Ciliophora, Cyrtophorida), from China. Eur. J. Protistol. 38, 213–222. doi: 10.1078/0932-4739-00862
Gong, J., Song, W., Warren, A., Lin, X., and Roberts, D. M. (2007). Microscopical observations on four marine Dysteria species (Ciliophora, Cyrtophorida). Eur. J. Protistol. 43, 147–161. doi: 10.1016/j.ejop.2007.01.002
Hu, X., Lin, X., and Song, W. (2019). Ciliate Atlas: Species Found in the South China Sea. Beijing: Science Press, doi: 10.1007/978-981-13-5901-9
Jankowski, A. W. (1967). Taxonomy of the genus Chilodonella and a new proposed genus Trithigmostoma gen. nov. Zool. Zh. 46, 1247–1250.
Jankowski, A. W. (2007). “Phylum Ciliophora Doflein, 1901,” in Protista. Part 2. Handbook on Zoology, ed. A. F. Alimov (St. Petersburg: Russian Academy of Sciences, Zoological Institute), 371–993.
Kahl, A. (1931). Urtiere oder Protozoa I: wimpertiere oder ciliata (Infusoria) 2. Tierwelt Dtl. 21, 181–398.
Leipe, D. D., Bernhard, D., Schlegel, M., and Sogin, M. L. (1994). Evolution of 16S-like ribosomal RNA genes in the ciliophoran taxa litostomatea and phyllopharyngea. Eur. J. Protistol. 30, 354–361. doi: 10.1016/S0932-4739(11)80083-0
Lynn, D. H. (2008). The Ciliated Protozoa. Characterization, Classi?cation, and Guide to the Literature, 3rd Edn. Dordrecht: Springer, doi: 10.1007/978-1-4020-8239-9
Medlin, L., Elwood, H. J., Stickel, S., and Sogin, M. L. (1988). The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 71, 491–499. doi: 10.1016/0378-1119(88)90066-2
Miller, M. A., Pfeiffer, W., and Schwartz, T. (2010). Creating the CIPRES science gateway for inference of large phylogenetic trees. Paper presented at the 2010 Gateway Computing Environments Workshop (GCE), (New Orleans, LA: IEEE), doi: 10.1109/GCE.2010.5676129
Müller, O. F. (1786). Animalcula Infusoria Fluviatila et Marina, quae Detexit, Systematice Descripsit et ad Vivum Delineari Curavit. Hauniae: Typis Nicolai Mölleri.
Nie, D., and Ho, Y. (1943). Notes on some epizoic infusoria from the freshwater shrimp, Palaemon nipponensis. Sinensia 14, 143–149.
Nylander, J. (2004). MrModeltest v2. Program Distributed by the Author. Uppsala: Uppsala University.
Oberschmidleitner, R. A., and Aescht, E. (1996). Taxonomische untersuchungen über einige ciliaten (Ciliophora, Protozoa) aus Belebtschlämmen oberösterreichischer Kläranlagen. Beitr. Naturk. Oberösterreichs 4, 3–30.
Pan, B., Chen, X., Hou, L., Zhang, Q., Qu, Z., Warren, A., et al. (2019). Comparative genomics analysis of ciliates provides insights on the evolutionary history within “Nassophorea-Synhymenia-Phyllopharyngea” assemblage. Front. Microbiol. 10:2819. doi: 10.3389/fmicb.2019.02819
Pan, H., Jiang, J., Fan, X., Al-Farraj, S. A., and Gao, S. (2017). Phylogeny and taxonomy of five poorly known species of cyrtophorian ciliates (Protozoa, Ciliophora, Phyllopharyngea) from China seas. Zool. J. Linn. Soc. 180, 475–492. doi: 10.1093/zoolinnean/zlw006
Pan, H., Li, L., Al-Rasheid, K. A. S., and Song, W. (2013). Morphological and molecular description of three new species of the cyrtophorid genus Chlamydodon (Ciliophora, Cyrtophoria). J. Eukaryot. Microbiol. 60, 2–12. doi: 10.1111/jeu.12001
Pan, H., Wang, L., Jiang, J., and Stoeck, T. (2016). Morphology of four cyrtophorian ciliates (Protozoa, Ciliophora) from Yangtze Delta, China, with notes on the phylogeny of the genus Phascolodon. Eur. J. Protistol. 56, 134–146. doi: 10.1016/j.ejop.2016.08.005
Petz, W., Song, W., and Wilbert, N. (1995). Taxonomy and ecology of the ciliate fauna (Protozoa, Ciliophora) in the endopagial and pelagial of the Weddell sea, Antarctica. Stapfia 40, 1–223.
Qu, Z., Li, L., Lin, X., Stoeck, T., Pan, H., Al-Rasheid, K. A., et al. (2018a). Diversity of the cyrtophorid genus Chlamydodon (Protista, Ciliophora): its systematics and geographic distribution, with taxonomic descriptions of three species. Syst. Biodivers 16, 497–511. doi: 10.1080/14772000.2018.1456493
Qu, Z., Ma, H., Al-Farraj, S. A., Lin, X., and Hu, X. (2017). Morphology and molecular phylogeny of Aegyria foissneri sp. n. and Lynchella minuta sp. n. (Ciliophora, Cyrtophoria) from brackish waters of southern China. Eur. J. Protistol. 57, 50–60. doi: 10.1016/j.ejop.2016.10.003
Qu, Z., Pan, H., Hu, X., Li, J., Al-Farraj, S. A., Al-Rasheid, K. A. S., et al. (2015). Morphology and molecular phylogeny of three cyrtophorid ciliates (Protozoa, Ciliophora) from China, including two new species, Chilodonella parauncinata sp. n. and Chlamydonella irregularis sp. n. J. Eukaryot. Microbiol. 62, 267–279. doi: 10.1111/jeu.12175
Qu, Z., Pan, H., Lin, X., Li, L., Aleidan, A. M. A., Al-Farraj, S. A., et al. (2018b). A contribution to the morphology and phylogeny of Chlamydodon, with three new species from China (Ciliophora, Cyrtophoria). J. Eukaryot. Microbiol. 65, 236–249. doi: 10.1111/jeu.12472
Ronquist, F., and Huelsenbeck, J. P. (2003). MrBayes 3: bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574. doi: 10.1093/bioinformatics/btg180
Šimek, K., Grujčić, V., Nedoma, J., Jezberová, J., Šorf, M., Matoušů, A., et al. (2019). Microbial food webs in hypertrophic fishponds: omnivorous ciliate taxa are major protistan bacterivores. Limnol. Oceanogr. 64, 2295–2309. doi: 10.1002/lno.11260
Snoeyenbos-West, O., Cole, J., Campbell, A., Coats, D. W., and Katz, L. A. (2004). Molecular phylogeny of phyllopharyngean ciliates and their group I introns. J. Eukaryot. Microbiol. 51, 441–450. doi: 10.1111/j.1550-7408.2004.tb00392.x
Song, W., Warren, A., and Hu, X. (2009). Free-living Ciliates in the Bohai and Yellow Seas. Beijing. Science Press.
Song, W., and Wilbert, N. (1989). Taxonomische untersuchungen an aufwuchsciliaten (Protozoa, Ciliophora) im Poppelsdorfer Weiher, Bonn. Lauterbornia 3, 2–221.
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., and Kumar, S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739. doi: 10.1093/molbev/msr121
Wang, C., Qu, Z., and Hu, X. (2019). Morphology and SSU rDNA sequences of four cyrtophorian ciliates from China, with description of a new species (Protista, Ciliophora, Phyllopharyngea). Zootaxa 4664, 206–220. doi: 10.11646/zootaxa.4664.2.3
Wang, P., Wang, Y., Wang, C., Zhang, T., Al-Farraj, S. A., and Gao, F. (2017). Further consideration on the phylogeny of the ciliophora: analyses using both mitochondrial and nuclear data with focus on the extremely confused class phyllopharyngea. Mol. Phylogenet. Evol. 112, 96–106. doi: 10.1016/j.ympev.2017.04.018
Warren, A., Patterson, D. J., Dunthorn, M., Clamp, J. C., Achilles-Day, U. E. M., Aescht, E., et al. (2017). Beyond the “code”: a guide to the description and documentation of bio-diversity in ciliated protists (Alveolata, Ciliophora). J. Eukaryot. Microbiol. 64, 539–554. doi: 10.1111/jeu.12391
Wilbert, N. (1971). Morphologie und Ökologie einiger neuer ciliaten (Holotricha, Cyrtophorina) des Aufwuchses. Protistologica 7, 357–363.
Wilbert, N. (1975). Ein verbesserte technik der protargolimprägnation für ciliaten. Mikrokosmos 64, 171–179.
Keywords: ciliate, Gastronauta, Gastronautidae, morphology, phylogeny, Trithigmostoma
Citation: Qu Z, Pan H, Wang C, Ma H, Stoeck T and Hu X (2021) Gastronautidae Deroux, 1994 and Trithigmostoma Jankowski, 1967: Evolutionary Links Among Cyrtophorian Ciliates (Protista, Ciliophora, and Phyllopharyngea). Front. Mar. Sci. 8:625644. doi: 10.3389/fmars.2021.625644
Received: 03 November 2020; Accepted: 09 February 2021;
Published: 26 February 2021.
Edited by:
Zhijun Dong, Yantai Institute of Coastal Zone Research (CAS), ChinaReviewed by:
Jae-Ho Jung, Gangneung–Wonju National University, South KoreaMing Li, Institute of Hydrobiology (CAS), China
Copyright © 2021 Qu, Pan, Wang, Ma, Stoeck and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaozhong Hu, eGlhb3pob25naHVAb3VjLmVkdS5jbg==
 Zhishuai Qu
Zhishuai Qu Hongbo Pan
Hongbo Pan Congcong Wang1
Congcong Wang1 Thorsten Stoeck
Thorsten Stoeck