
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 05 May 2021
Sec. Marine Megafauna
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.619695
This article is part of the Research Topic Marine Conservation: Knowledge, Experience and Tools for Change View all 18 articles
 Shaili Johri1,2*
Shaili Johri1,2* Isabella Livingston2
Isabella Livingston2 Anjani Tiwari3
Anjani Tiwari3 Jitesh Solanki4
Jitesh Solanki4 Anissa Busch2
Anissa Busch2 Isabel Moreno5
Isabel Moreno5 Sam R. Fellows6
Sam R. Fellows6 Michael P. Doane7
Michael P. Doane7 Elizabeth A. Dinsdale7,2*
Elizabeth A. Dinsdale7,2*Chondrichthyes, an ancient and diverse class of vertebrates, are crucial to the health of marine ecosystems. Excessive demand for chondrichthyan products has increased fishing pressure, threatening ∼30% of species with extinction in recent decades. India is the second-largest shark landing nation globally and the province of Gujarat, is the largest contributor to its shark exports. Despite their significant contribution to global fish supplies, chondrichthyan fisheries in Gujarat remain understudied and many species, data deficient, posing challenges to the conservation of remaining populations in the region. Here, we report results from taxonomic assessment of elasmobranchs at four key landing sites in Gujarat. We identified thirty-one species of sharks and rays with a significant bias toward capture of females and juveniles by fisheries. Our data indicate the presence of nursery areas for species such as Sphyrna lewini and Rhynchobatus laevis in the neritic areas off Gujarat. Further, we discovered extensions of the current distribution range for three species -Torpedo sinuspersici, Carcharhinus sorrah, and Rhinobatos punctifer. Taxonomic identities for a subset of species were confirmed using genomic analyses conducted with portable DNA sequencing tools. We present assessments for six data deficient species in the region – Rhinobatos annandalei, Rhinoptera jayakari, Maculabatis bineeshi, Pateobatis bleekeri, T. sinuspersici, and Carcharhinus amboinensis. Our investigation underscores species with urgent conservation needs and reduces data deficiencies. These data will inform and pivot future scientific and conservation efforts to protect remaining populations of some of the most vulnerable Chondrichthyes in the Arabian Seas Region.
Chondrichthyes (sharks, rays, skates, and chimeras) have been extant for 420 million years and comprise one of the most diverse and ubiquitous group of vertebrates (Dulvy et al., 2014). Chondrichthyan species occupy diverse ecological niches and are selective of their habitats (Compagno, 1990; García et al., 2008). Most species play a crucial role as apex or meso predators in marine and freshwater ecosystems, by maintaining ecosystem health through regulation of population dynamics at all trophic levels (Dulvy et al., 2014). Chondrichthyes have slow life histories, long generation times, and low fecundity, because of which populations present a slow growth rate (Cortés, 2000; Stevens, 2000; García et al., 2008; Hutchings et al., 2012). Consequently, the population abundance of such species is less than that of lower trophic level organisms (Hutchings et al., 2012) and are extremely vulnerable to fishing pressure (Stevens, 2000). Incidental and targeted catch due to a growing demand for chondrichthyan products over the past few decades has increased fishing pressure and overexploitation of many species (Dulvy et al., 2014, 2017; Jabado et al., 2017, 2018; FAO, 2021). As a result, chondrichthyan populations have had significant declines, with some species showing declines of up to 90% (Jabado et al., 2017) and pushed to the brink of extinction. As such, an estimated 18% of chondrichthyan species are categorized as Critically Endangered, Endangered, or Vulnerable by IUCN (FAO, 2019, 2020). The decrease in abundance of Chondrichthyes as apex predators has led to damaging direct and indirect effects on oceanic ecosystems around the world (Bornatowski et al., 2014; Johri et al., 2019).
Globally, the decline of teleost fisheries in combination with technological advances in fishing methods have made elasmobranchs an attractive alternative resource for food and revenue (Lack and Sant, 2011; Dent and Clarke, 2015). Paradoxically, there is marginal investment in the management of elasmobranch stocks due to the presumably small proportion of elasmobranchs caught in fisheries and limited understanding of the ecology, distribution, and population health of the species (Rose, 1998; Castro et al., 1999; Musick et al., 2000; Barker and Schluessel, 2005; Dent and Clarke, 2015). A significant portion of elasmobranch landings are non-targeted fisheries catch and as a result, are often discarded, recorded as bycatch or unidentified shark/ray species, or not recorded at all (Barker and Schluessel, 2005). As a consequence elasmobranch landings are often underreported, making estimates of global catches difficult and inaccurate (Lack and Sant, 2011). A recent estimate places the actual number of catches as double that of the recorded value (Barker and Schluessel, 2005). Further, a majority of shark fishing nations do not report species composition of their catch to the World Food and Agriculture Organization of the United Nations (FAO) (Lack and Sant, 2011; Karnad et al., 2020), restricting assessments of stocks or fishing pressure on specific species and populations. The highly migratory nature of many chondrichthyan species places them in international territories, making country specific protections, when existent, only partially effective (Stevens, 2000). Additionally, national or international regulations protecting migratory species are difficult to enact when information on species distributions are limited or non-existent, as in the case of many Chondrichthyes (Dulvy et al., 2014, 2017). As a result of the many shortfalls in accountability listed above, chondrichthyan stocks are being depleted at a rampant rate while management policies are scarcely implemented or are often too inadequate to be effective. The absence of accurate and comprehensive datasets, management, and political will are significant barriers impeding the design and implementation of conservation measures for Chondrichthyes. This is reflected by the fact that only 13 of the 20 major shark fishing nations worldwide have developed a National Plan of Action for sharks (NPOA), and its implementation is extremely variable in each of the 13 nations with NPOAs (Bräutigam et al., 2020).
The Arabian Seas Region (ASR), bordered by 20 nations, is regarded as a global hotspot for marine biodiversity (Stein et al., 2018) and provides habitat for ∼15% of all chondrichthyan species (Jabado et al., 2017). While the ASR is prolific in fish resources, it is also one of the most over-exploited marine environments globally (Jabado and Spaet, 2017; Jabado et al., 2018). Several teleost species in the ASR have been over−exploited in the last two decades causing extreme threats to the teleost fisheries, with reported declines of 40–80% (FAO, 2007). At the same time demand for Chondrichthyes is growing primarily due to their high economic value in the fin trade and more recently, to suffice issues of food security through provision of animal protein from shark and ray meat (Lack and Sant, 2011; Dent and Clarke, 2015). Both targeted and incidental catches of elasmobranchs are being tapped to supply this demand (FAO, 2007; Henderson et al., 2016). The ASR is recognized for having the largest number of chondrichthyan fishers and traders in the world (Dent and Clarke, 2015; Dulvy et al., 2017; Jabado and Spaet, 2017). Within this region the top fishing nations are India, Iran, Pakistan, Oman, Yemen, Somalia, and Sri Lanka, respectively (Dent and Clarke, 2015; Jabado and Spaet, 2017). Regional reported landings of chondrichthyans in 2015 represented 9.62% of global landings, despite seven countries in the region not reporting their chondrichthyan catches (FAO, 2017). Despite the extreme pressures on fisheries and population declines of up to 90% in some elasmobranchs (Jabado et al., 2017, 2018), understanding of the extent of declines at the species level and the contributing factors remains poor. These knowledge gaps stem from the fact that approximately 19% of elasmobranch species in the ASR are data deficient (DD) (Dulvy et al., 2014).
India, the largest shark fishing nation in the ASR and second largest in the world (Dent and Clarke, 2015), contributes 74,000 metric tons of an estimated 831,460 metric tons of global chondrichthyan exports annually (FAO Yearbook, 2020). Chondrichthyan exports from India thus account for ∼ 9% of global and ∼ 93% of ASR exports of the species. While the FAO reports a 20% decline in global recorded landings of sharks and rays since 2003 (FAO, 2021), India has seen 20–60% declines in landings despite a simultaneous doubling of trawling effort during the same time period (Raje and Zacharia, 2009; Kizhakudan et al., 2015). Batoid landings in India have fared even worse with declines of up to 86% (Raje and Zacharia, 2009; Kyne et al., 2020b). The reduction in catch per unit effort (CPUE) has led to intensification of mechanized fishing efforts into off shore waters, further jeopardizing chondrichthyan populations previously protected from commercial fishing (Raje and Zacharia, 2009; Mohamed and Shettigar, 2016; Jabado and Spaet, 2017). Overexploitation in Indian fisheries has pushed 55% of its elasmobranch species to the brink of extinction with 3% categorized as Critically Endangered (CE), 5% as Endangered (EN), 26% as Vulnerable (VU), and 21% as Near Threatened (NT). In addition, 37% are data-deficient (DD) or not evaluated (Akhilesh et al., 2014).
The significantly high levels of threat and data deficiency in Chondrichthyes in India are a consequence of almost non-existent management measures for the species, and poor enforcement of existing measures at the state and national levels (Karnad et al., 2020). Chondrichthyan stock assessments remain absent, and as a result, catch limits on chondrichthyan landings are only imposed for species protected under Schedule I of the Wild Life (Protection) Act (1972) which includes the Whale shark Rhincodon typus, Pondicherry shark Carcharhinus hemiodon, and Giant guitarfish Rhynchobatus djiddensis (FAO, 2007; Karnad et al., 2020). Species-specific protections have repeatedly proven inadequate with the exception of whale sharks (Jabado et al., 2018; Karnad et al., 2020). India is the largest contributor to global seafood supplies and millions of fishermen livelihoods in India depend on commercial fisheries (Kizhakudan et al., 2015). The current rate of unsustainable shark fisheries in India is likely to cause further declines in commercial fisheries due to trophic effects. The lack of fisheries regulation in the Indian sub-continent, therefore not only threatens the diversity of Chondrichthyes, but could disrupt global food supply chains and diminish India’s national GDP (Jabado et al., 2017). India is ranked as the number one country, with the greatest need for conservation of sharks and rays, among the 20 largest shark fishing nations of the world (Dulvy et al., 2017).
The state of Gujarat, which accounts for 26% of fisheries landings (FAO, 2007) and 40% of all chondrichthyan landings (Kizhakudan et al., 2015) in India, has suffered significant declines in CPUE (Kizhakudan et al., 2015) and we have prioritized this region for our assessment. Gujarat comprises 1/5th of India’s total coastline, the longest in the country, and supports habitats such as mangroves, salt marshes, coral reefs, and seagrasses (Raje et al., 2007; Worldbank, 2020). A significant portion of Gujarat’s continental shelf area falls in the depth range of 0–50 m and supports commercially important species found at shallow depths including Carcharhinus limbatus, Carcharhinus falciformis, Rhizoprionodon acutus, Rhizoprionodon oligolinx, and Sphyrna lewini (Devadoss et al., 1989; Spaet and Berumen, 2015). Gujarat’s state economy relies heavily on fishing and comprises 15% of the total export economy of India (Devadoss et al., 1989). Despite a strong reliance of state and national economies on fisheries in Gujarat, it is lacking in adequate fisheries assessments by national or state agencies and is ranked 4th by the FAO among regions with the greatest need for research and conservation of Chondrichthyes (Dulvy et al., 2017; Nagle, 2019). The lack of scientific investigations of fisheries in Gujarat, suggest a lack of stock assessments and a subsequent lack of management measures to protect Chondrichthyes. Consequently, further declines in commercial fisheries can be expected with a potentially catastrophic effect on the state and national GDP, fishermen livelihoods and global seafood supply chain.
Gujarat’s significant contribution to Indian and global fisheries and its extreme paucity of chondrichthyan biodiversity assessments call for an urgent inquiry into chondrichthyan species distributions, ecology, and fishing practices in the area. Although excessive stretches of shallow coastal areas intermixed with mangrove and seagrass habitats along the coast of Gujarat are likely favorable habitats for juvenile sharks and rays (Spaet et al., 2012), local nursery areas remain unidentified within the region. A high number of recorded landings identified as immature elasmobranchs from the neighboring Red Sea region, suggest that juveniles are at a higher risk from fishing in the ASR (Spaet and Berumen, 2015), including in Gujarat. Because some species aggregate by age, sex, or reproductive state, their population numbers could be more vulnerable to fishing pressure than others (Barker and Schluessel, 2005), and habitats harboring these species, if present in Gujarat, should be identified and protected expediently. To address the knowledge gaps described here, we designed a fisheries dependent survey to assess elasmobranch biodiversity and fisheries in Gujarat. We hypothesized that fisher communities in Gujarat encounter a rich biodiversity of elasmobranchs as targeted or incidental catch, and that a survey of the fisheries will provide a proxy measure of elasmobranchs biodiversity and distribution, as well as fisheries’ catch composition in the area. We focused our studies in ports known to be the primary fishing and export hubs for elasmobranchs in Gujarat.
In the current report, we present assessments of seasonal elasmobranchs biodiversity obtained via fisheries dependent surveys at four major landing ports in Gujarat. Elasmobranch specimens were photographed at landing sites, fish markets and salting factories at the port cities of Veraval, Mangrol, Porbandar, and Okha in Gujarat. We report the identification and occurrence of 31 elasmobranchs species in Gujarat, including six data deficient species and three species reported for the first time in the region. In the current report, we therefore provide the first expansive assessment of elasmobranchs and their vulnerability to fisheries in Gujarat.
Four primary areas along the western coast of Gujarat, India -Veraval (20.9159° N, 70.3629° E), Mangrol (21.1172° N, 70.1158° E), Porbandar (21.6417° N, 69.6293° E), and Okha (22.4649° N, 69.0702° E) (Figure 1), were chosen as sites for sample collection due to their high volume of elasmobranch landings. Landing sites, fish markets, and salting factories were surveyed by a single researcher at Veraval (26 days), Mangrol (3 days), Porbandar (3 days), and Okha (6 days), for a total of 38 days over 5 months (April, May, August 2017, and March–May 2018) in 2017–2018. The number of sampling days in Veraval was highest due to increased sampling accessibility in markets facilitated by established relationships of the fisher communities with the College of Fisheries located in Veraval. Each day the researcher would enter the market, talk with fishers, and ask about the elasmobranchs that were caught, and each specimen would be photographed, including close-up photos of the head, mouth, eyes vulva/claspers, and body of the complete or dismembered elasmobranch specimen. Tissue samples were taken for genomic sequencing using methods described in Johri et al. (2019).

Figure 1. Sampling sites located along the western coast of Gujarat, India. The four sampling locations -Okha, Veraval, Mangrol, and Porbandar – were selected due to the high volume of elasmobranch landings reported previously in the region.
Photographic identification of each specimen was conducted following protocols described in Ebert et al. (2013) and Last et al. (2018). For each sample, photographs were uploaded to the species identification database iNaturalist1, where expert observers in the field assigned research-grade identifications for our samples, in parallel to our own taxonomic identification.
Genomic sequencing and phylogenetic assessment of select specimens was conducted following methods described by us previously in Johri et al. (2019, 2020a,b,c).
We searched for and downloaded relevant sequences from GenBank which are detailed in Supplementary Table 1 and aligned them using MUSCLE v.3.8.31 (Edgar, 2004). We constructed five alignments: one for the COI gene for genus Rhinobatos using Glaucostegus formosensis and Acroteriobatus annulatus as outgroups, two for genus Torpedo for COI and ND2 separately using Narcine brasiliensis and Narcine bancroftii as outgroups, one for the mitochondrial genome for the genera Mobula using Rhinoptera steindachneri as an outgroup, and one for the mitochondrial genome for genus Carcharhinus (inclusive of Prionace glauca) using Galeocerdo cuvier as an outgroup. We used the PartitionFinder2 v2.1.1 tool (Lanfear et al., 2016) on CIPRES (Miller et al., 2010) to assess partitioning schemes. We used best-fitting partitioning schemes identified by PartitionFinder2 to generate phylogenies in RAxML v8.2.12 (Stamatakis, 2014) and MrBayes v3.2.7a (Ronquist et al., 2012) on CIPRES. All RAxML runs used four starting trees, GTRGAMMA models for all partitions, and generated 1000 bootstrap replicates. All MrBayes runs were run in triplicate and used GTR + 4Γ models for all partitions and ran for 1,000,000,000 MCMC steps. Convergence of MrBayes runs was assessed by eye in Tracer v1.7.1 (Rambaut et al., 2018).
Sex was determined by assessing the presence of claspers or vulva for each intact specimen.
Morphological measurements -Total length (TL) for sharks, and TL or Disc Width (DW) for batoids, weight measurements (collected when available), and the presence of calcification of claspers in males and young or eggs in females’ uterus were used to identify maturity for each specimen. Specimens that were less than the gender specific measurements at maturity described for each species in the literature (Ebert et al., 2013; Last et al., 2018; Froese and Pauly, 2020; IUCN Red List, 2021; Pollerspöck and Straube, 2021; referenced in Table 1) were considered to be immature. Similarly, specimens greater than the gender specific measurements at maturity and showing hardened claspers and presence of young or eggs were assessed as mature.
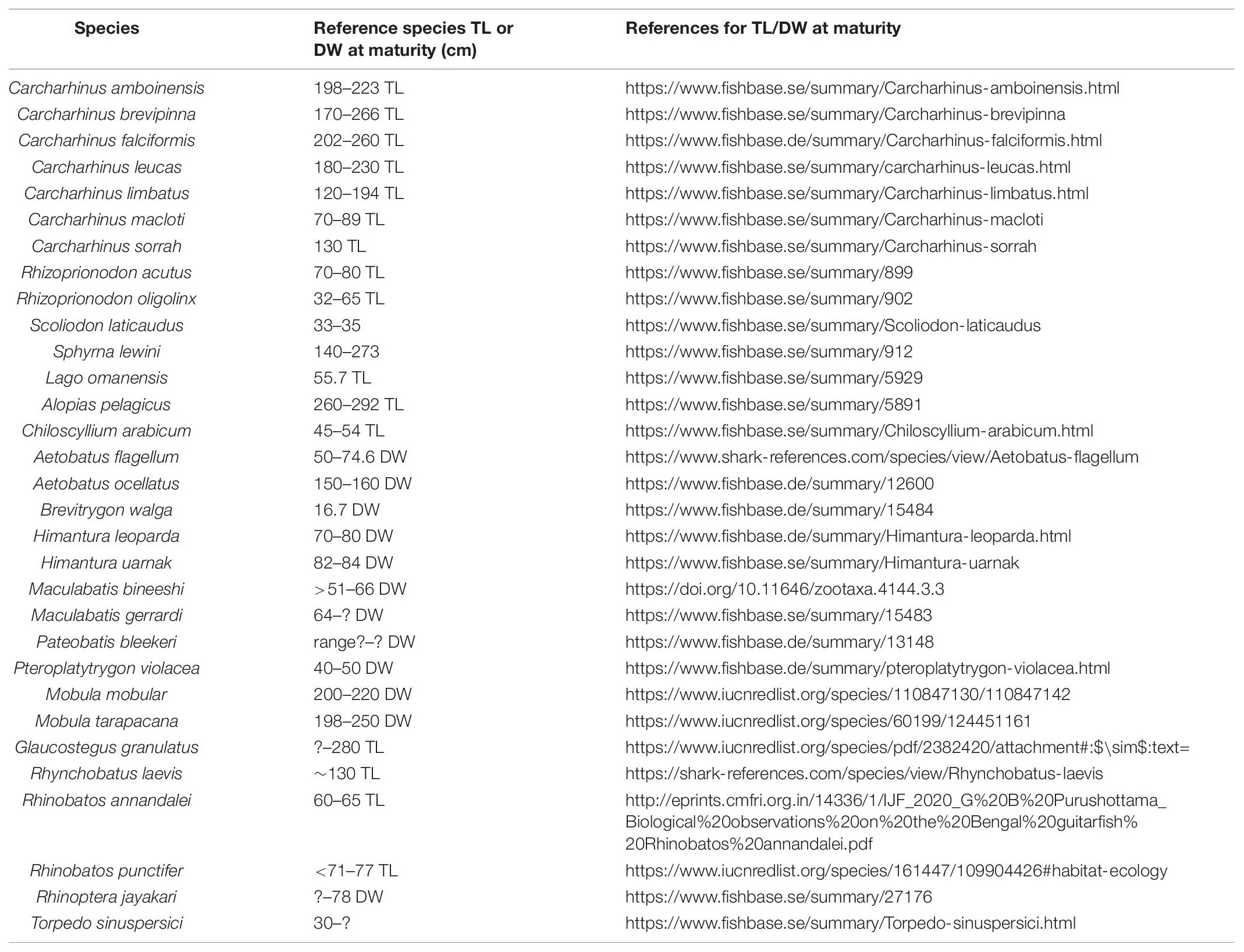
Table 1. Total Length (TL) or Disc Width (DW) (cm) threshold used to determine maturity for each species are listed below.
For Pateobatis bleekeri, DW at maturity was unavailable, and hence, maturity for the specimen could not be determined. Similarly, for Glaucostegus granulatus, species specific information on TL at maturity was unavailable. We therefore based our assessment of maturity on other taxa in the Glaucostegidae or Giant Guitarfish family (Last et al., 2018) and followed specifications for Rhinidae/Glaucostegidae maturity assessments (Rhinidae – IUCN Red List).
Conservation status of species was determined using the International Union for Conservation of Nature (IUCN) (IUCN Red List, 2021) to assess the impact of fisheries in Gujarat on priority concern species. Conservation categories defined by IUCN were used and include Critically Endangered (CE), Endangered (EN), Vulnerable (VU), Near Threatened (NT), Least Concern (LC), and Data deficient (DD).
The occurrence for each species at sampling locations was assessed to determine differences in species distribution across sampling sites. Range extensions were discovered by comparing the location of specimen landing site with geographic ranges previously reported for respective species on the IUCN Red List database (IUCN Red List, 2019).
We obtained depth ranges where species normally occur from the IUCN and FishBase databases (IUCN Red List, 2019; FishBase, 2020). We divided depth ranges into four categories by adopting the classical subdivision which considers 200 m of depth as a limit of the continental shelf, and considering that species which populate the continental shelf and beginning of the continental slope could be very different. The four depth categories are: 0–200 m, 201–600 m, >600 m, or no available information. We binned the species of sharks and rays’ samples into the depth categories listed above, based on published information about the species.
The surveys conducted at four fish markets and landing sites within Gujarat state (Figure 1) culminated in ∼1000 photographs of elasmobranchs. A total of 157 elasmobranchs were sampled opportunistically, including species from the superorder Selachimorpha (sharks) and Batoidea (rays). Within the superorder Selachimorpha, we identified fourteen species comprising three orders, five families, and seven genera (Table 2 and Figure 2), while within the superorder Batoidea we identified seventeen species, comprising four orders, eight families, and twelve genera (Table 2 and Figures 3, 4). Additional specimen photographs and research grade identifications for each specimen can be found at iNaturalist: https://www.inaturalist.org/observations?place_id=any&subview=grid&user_id=shaili&verifiable=any&view=species. Note that 71 specimens for which taxonomic identities remained unknown are not included in Table 2.
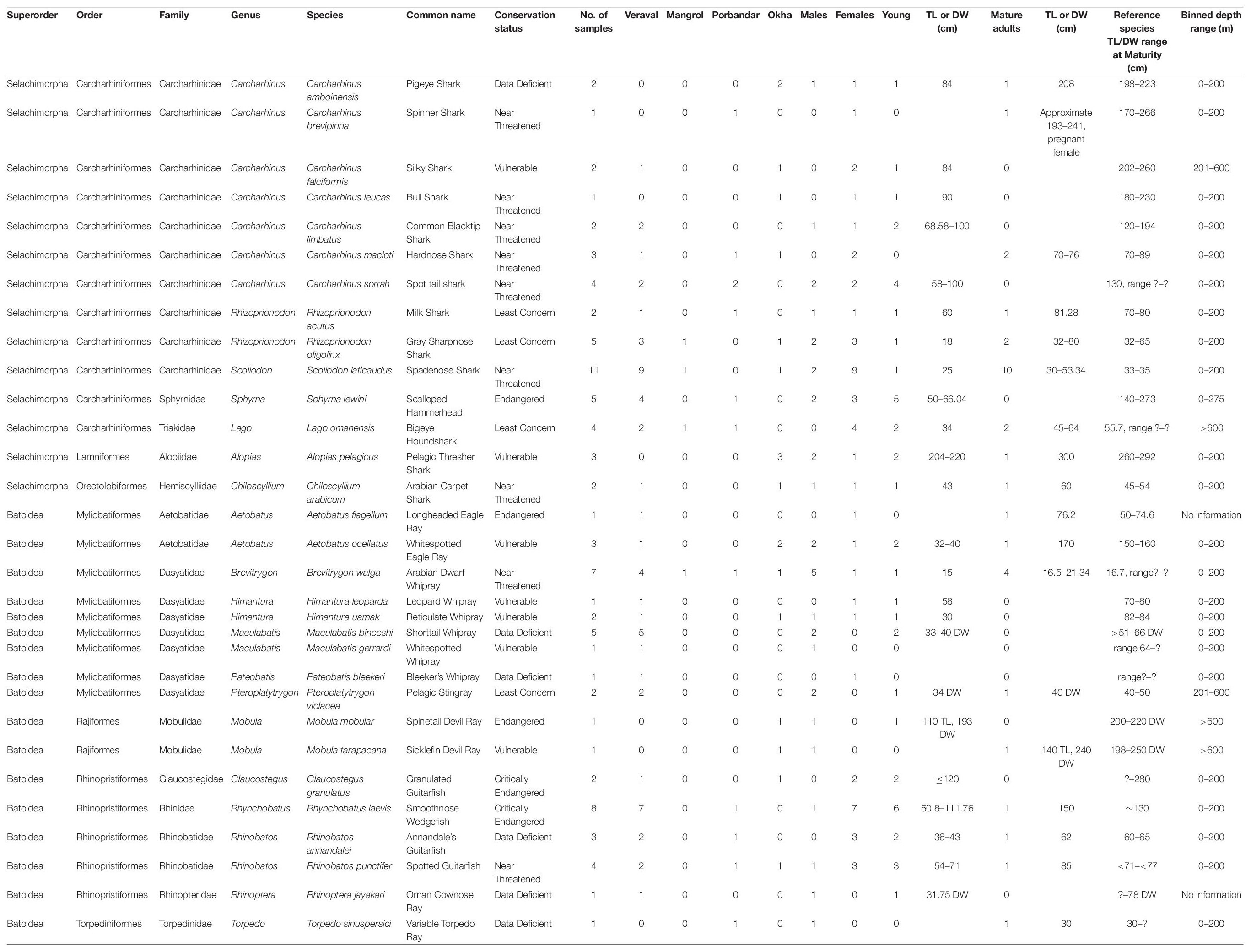
Table 2. Information about taxonomy, number of specimens, collection site, gender, maturity, TL/DW (cm) for mature and immature specimens, and binned depth range (in meters) for each species sampled.

Figure 2. Representative pictures of species sampled in the superorder Selachimorpha. (A) Chiloscyllium arabicum (Arabian carpetshark), (B) Iago omanensis (Bigeye houndshark), (C) Carcharhinus leucas (Bull shark), (D) Carcharhinus limbatus (Common Blacktip shark), (E) Rhizoprionodon oligolinx (Gray Sharpnose shark), (F) Carcharhinus macloti (Hardnose shark), (G) Rhizoprionodon acutus (Milk shark), (H) Alopias pelagicus (Pelagic Thresher shark), (I) Carcharhinus amboinensis (Pigeye shark), (J) Sphyrna lewini (Scalloped Hammerhead), (K) Carcharhinus falciformis (Silky shark), (L) Scoliodon laticaudus (Spadenose shark), (M) Carcharhinus brevipinna (Spinner shark), and (N) Carcharhinus sorrah (Spottail shark).

Figure 3. Representative pictures of species sampled in the superorder Batoidea. (A) Brevitrygon walga (Arabian Dwarf Whipray), (B) Pateobatis bleekeri (Bleeker’s Whipray), (C) Torpedo sinuspersici (Variable Torpedo ray), (D) Maculabatis gerrardi (Whitespotted Whipray), (E) Himantura leoparda (Leopard Whipray), (F) Aetobatus ocellatus (Whitespotted Eagle ray), (G) Mobula mobular (Spinetail Devil ray), (H) Mobula tarapacana (Sicklefin Devil ray), (I) Pteroplatytrygon violacea (Pelagic Stingray), (J) Aetobatus flagellum (Longheaded Eagle ray), (K) Rhinoptera jayakari (Oman Cownose Ray), (L) Himantura uarnak (Reticulate Whipray), and (M) Maculabatis bineeshi (Short-tail Whipray).
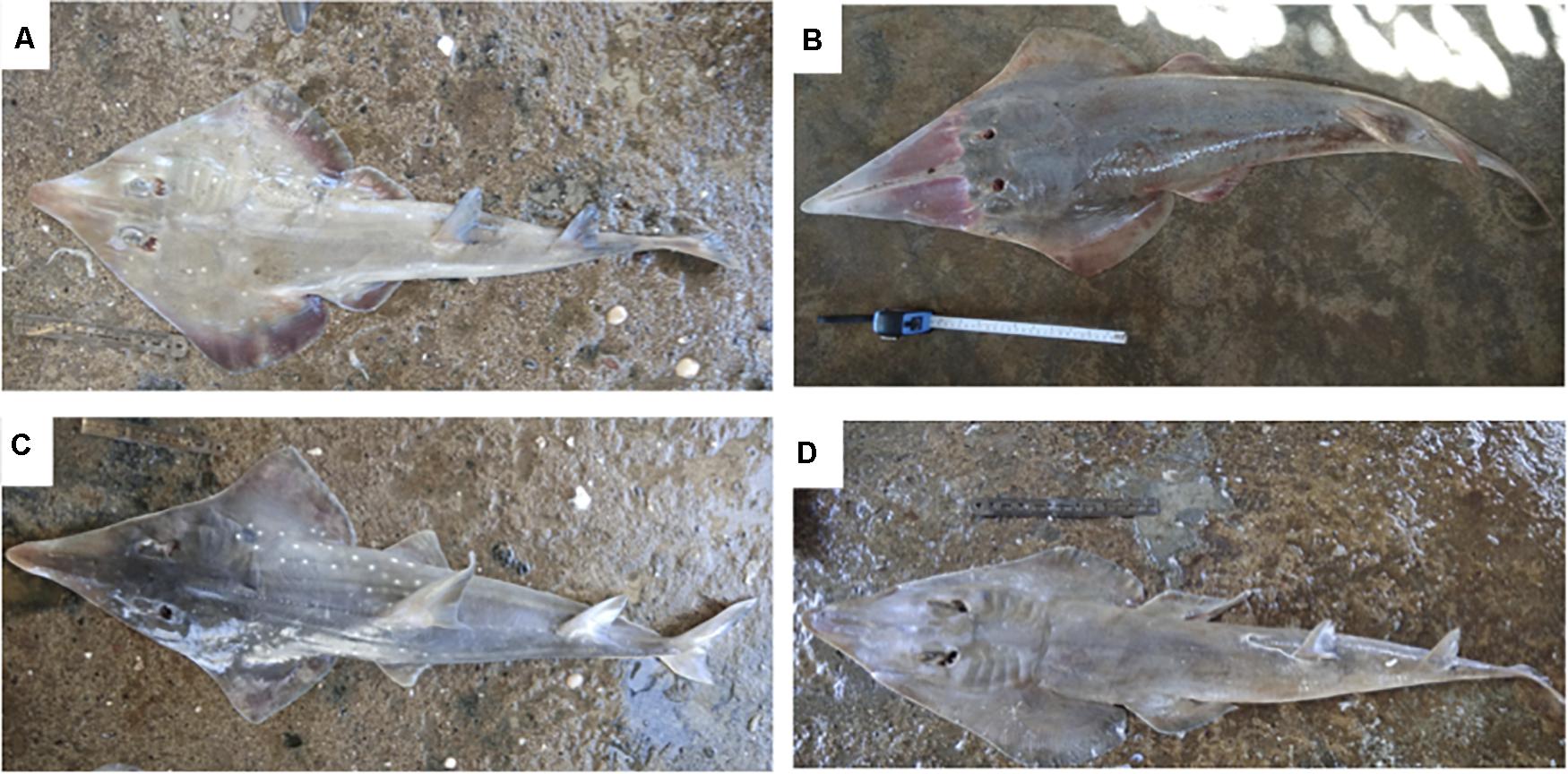
Figure 4. Representative pictures of species sampled in the order Rhinopristiformes. (A) Rhinobatos annandalei (Annandale’s guitarfish), (B) Glaucostegus granulatus (Granulated guitarfish), (C) Rhynchobatus laevis (Smoothnose wedgefish), and (D) Rhinobatos punctifer (Spotted guitarfish).
Taxonomic identity of specimens from five species were confirmed by sequencing and phylogenetic analyses in the current study. Phylogenetic analyses of the species are presented in Supplementary Figure 1 and the sequence data are available on GenBank using the listed accession numbers. We constructed four gene trees, one for each of the following genera of elasmobranchs: (1) 13 of 22 Rhinobatos species, (2) seven of 25 Torpedo species, (3) all 12 Mobula and Manta species, and (4) 14 of 34 Carcharhinus species. All Bayesian inference (BI) phylogenies converged within 1 billion steps. SRA Accession # SRR13587043 was placed within the Rhinobatos tree with 100/100 bootstrap support for Rhinobatos punctifer (Supplementary Figure 1A). SRA Accession # SRR13660201 was placed within Torpedo ML or BI trees, but relationship of our contig to any other known taxon was not resolved with high confidence and extremely short branch lengths occurred throughout the tree (Supplementary Figure 1B). SRA Accession # SRR13587044 was confidently placed with high confidence in both BI and ML Carcharhinus trees and identified as Carcharhinus sorrah (Supplementary Figure 1C). SRA Accession # SRR13587041 and SRA Accession # SRR13587042 were confidently placed in the Mobula/Manta trees identified as Mobula japanica + Mobula mobular and Mobula tarapacana, respectively (Supplementary Figure 1D).
There were differences in distribution of species across the four sampling locations. Scoliodon laticaudus was the most frequently sampled species (n = 11) among sharks and Rhynchobatus laevis was the most frequently sampled batoid (n = 8) (Supplementary Figure 2A). Alopias pelagicus, Carcharhinus amboinensis, Carcharhinus leucas, M. mobular, and M. tarapacana, were sampled only in Okha (Supplementary Figure 2A). Carcharhinus brevipinna and Torpedo sinuspersici were sampled only in Porbandar (Supplementary Figure 2A), whereas the Carcharhinus limbatus, Aetobatus flagellum, Pteroplatytrygon violacea, Himantura leoparda, Rhinoptera jayakari, Maculabatis gerrardi, and Pateobatis bleekeri were sampled only in Veraval (Supplementary Figure 2A). The difference in species sampled at the four locations may indicate distinct ecology and distribution patterns of the respective species, as well as distinct habitats in this part of the Arabian Sea region.
Elasmobranch species were identified across all conservation categories in the four sampling locations in Gujarat. Of the 31 species identified in our study, 12 (38.7%) are in the threatened categories of CE, EN, and VU (Table 2 and Supplementary Figure 2B), 9 (29%) are Near Threatened, 4 (13%) are Least concern and 6 (19%) are Data Deficient. A majority of shark species are in the Near Threatened category, whereas a majority of batoids sampled are either Threatened (CE, EN, VU) (n = 9) or Data deficient (n = 5) (Table 2 and Supplementary Figure 2B).
Of a total of 157 specimens, 127 samples were sufficiently intact for gender determination. Of these 127 samples, 63% were female (n = 80), and 37% were male (n = 47) (Figure 5A). There was a significant difference in the number of shark specimens that were female (68% or 48/71), compared to males (32% or 23/71) (Figure 5B, p = 0.03). For batoids, the difference was not significant with 57% or 32/55 female specimens and 43% or 23/55 male (Figure 5B). When gender ratios were compared across sampling locations, Veraval had a significantly higher proportion of female vs. male specimens (p = 0.02), whereas there was no significant difference between occurrence of male and female specimens at other locations (Figure 5C). Note that 41 specimens for which the taxonomic identities remained unknown, but gender was determined are included in the 127 male/female specimens. However these 41 specimens are not included in Table 2.
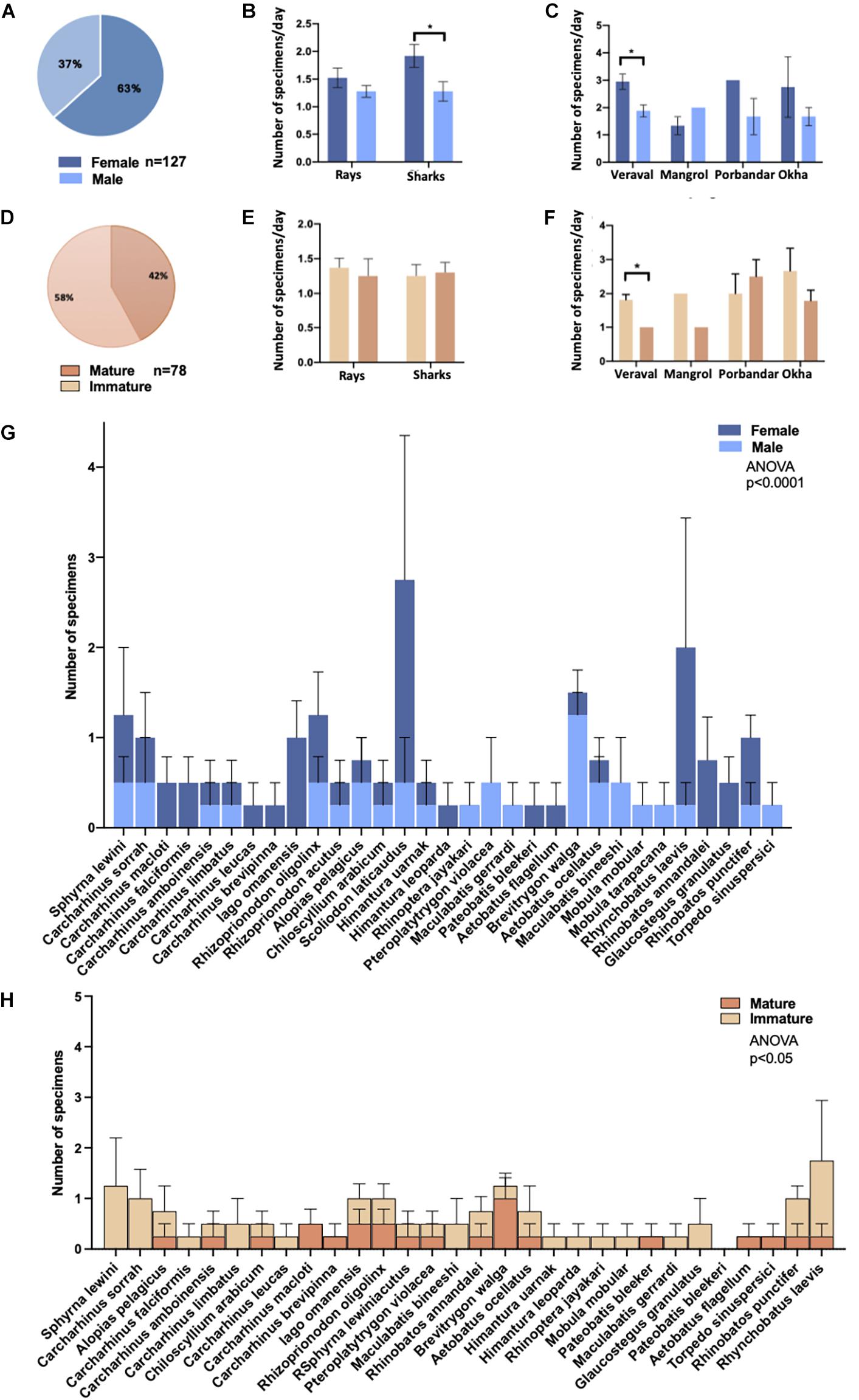
Figure 5. Frequency distribution of sharks and the rays by sex and maturity across sampling locations. Sex determination of (A) all samples (n = 117) in which determination could be made, including samples with no species identification, (B) by shark and ray specimens, and (C) by sampling location. A comparison of the ratio of young to mature adults (D) among all specimens (n = 77), (E) between sharks and rays, and (F) between sampling locations. Comparison of male and female specimens in all species in (G) and comparison of mature and immature specimens of each species except Scoliodon laticaudus in (H). Two-way ANOVA with multiple comparisons between the respective groups was performed in (B,C,E–H). Asterisk * denotes a significant difference (p≤0.05) and *** denotes a highly significant difference (p≤0.0005) between groups. We compared total population distribution of males vs. females in (G) and mature vs. immature specimens in (H). Distribution of males vs. females and of mature vs. immature individuals was significantly different as indicated by respective p values. S. laticaudus was identified as an outlier and removed from analyses in (H).
Morphological measurements were analyzed to determine the maturity of specimens. Of the 157 total specimens, sufficient data was available for 78 samples to determine maturity. Immature young comprised 58% (n = 45) of total specimens, while mature adults made up 42% (n = 32), as shown in Figure 5D. For all shark specimens with distinguishable life stages 51% (n = 22), were immature and 49% (n = 21) were mature adults (Figure 5E). Maturity was determined for 35 batoids of which, 65% were immature and 34% were mature adults (n = 23 and n = 12, respectively) (Figure 5E). There was no significant difference in the total number of mature vs. young specimens at the four sampling locations (Figures 5E,F).
For numerous species of sharks and batoids the gender ratio was skewed toward females. For instance, nine of eleven S. laticaudus (p = 0.05), seven of eight R. laevis, and all four Iago omanensis sampled were females (Table 2 and Figure 5G). Whereas for species like Brevitrygon walga the ratio was skewed towards males, with five of six total specimens being male (Table 2 and Figure 5G). Total distribution of males vs. females across all species had a significant difference (p < 0.0001, Figure 5G). Similarly, several species had a higher skew towards immature specimens including Sphyrna lewini, C. sorrah, R. laevis, and R. punctifer (Table 2 and Figure 5H). Conversely for S. laticaudus, 10 specimens were mature adults and only one was immature (Table 2). The total number of mature vs. immature specimens across all species (except S. laticaudus) was significantly different (p < 0.05, Figure 5H).
For Glaucostegus granulatus, species specific information on TL at maturity was unavailable. However, both specimens in our collection were female at ≤120 cm TL and 2–4 kg in weight. Since mature females in closely related species such as Glaucostegus typus and Glaucostegus cemiculus, are expected to have TL > 150 cm at maturity (Last et al., 2018, Rhinidae – IUCN Red List), we assessed G. granulatus specimens in our collection to be at an immature life history stage.
In our depth assessments, 78% (n = 24) of sampled species inhabited a depth range of 0–200 m, three species were in the range of 201–600 m and three species were in the >600 m range (Table 2 and Supplementary Figure 3). Coastal or neritic species found at 0–200 m were the most frequently sampled across all four locations (Supplementary Figure 3), suggesting that these species had a higher likelihood of capture in the sampled fisheries. For depth ranges between 0–200 m and 201–600 m, the frequency of shark and ray species was even, with 12 species each of sharks and rays at 0–200 m and 1 each at 201–600 m. At >600 m depth only one shark (I. omanensis) and two ray species (M. mobular and M. tarapacana) were found (Table 2). For the species A. flagellum and R. jayakari no depth information is available.
The high rate of data deficiencies and aggregated, non-species specific assessments among Chondrichthyes (Dulvy et al., 2014) mean that geographic ranges are not accurately documented for many elasmobranch species. Our sampling of fisheries, which catch mainly coastal elasmobranch species via targeted or incidental means, provided an opportunity to investigate the current geographic range of sampled coastal specimens. Of the 31 species, three were found at landing sites located outside of their previously reported ranges which are based on landing data (Dent and Clarke, 2015; IUCN Red List, 2020b; Park University, 2020). C. sorrah is listed as extant along the southwestern and eastern coast of India, in addition to its global distribution, as shown in Supplementary Figure 4A. We report four sightings of this species along the northwestern coast of India, specifically in Veraval (n = 2) and Porbandar (n = 2) (Supplementary Figure 4A), and taxonomic identity of these specimens was confirmed at species level through phylogenetic (Supplementary Figure 1C) and morphological assessments (Figure 2N). T. sinuspersici was reported to be found along the eastern coast of Africa, and Saudi Arabia based on landings data, and has not been reported in India previously (Jabado and Spaet, 2017; Kyne, 2019). We report its presence for the first time in Porbandar (n = 1), Gujarat, northwestern India, as shown in Supplementary Figure 4B and confirmed its taxonomic identity at the genus level phylogenetically (Supplementary Figure 1B) and at the species level morphologically (Figure 3C). A third species, R. punctifer, is known to be extant in the Arabian Seas Region from the northern Red Sea to the Sea of Oman (Ebert et al., 2017). Ours is the first study to report four specimens of the species landed in India, specifically, at the ports of Veraval (n = 2), Porbandar (n = 1), and Okha (n = 1) (Supplementary Figure 4C). The taxonomic identification of the specimens was confirmed to the species level through phylogenetic (Supplementary Figure 1A) and morphological assessments (Figure 4D).
Systematic evaluation of the distribution, population health and threats adversely affecting Chondrichthyes is vital to drive the management and conservation of remaining populations and limit extinctions in the Arabian Seas Region. Here we have conducted an elasmobranch biodiversity assessment in Gujarat, India, one of the most prolific fishing region in the ASR. Our assessment identified 31 species of elasmobranchs from 157 specimens collected in just 38 days. The survey complements global and regional (Jabado et al., 2017; FAO, 2019) efforts at chondrichthyan status assessments and the very few scientific investigations into elasmobranch biodiversity, distribution, and fisheries in India (Raje and Zacharia, 2009; Akhilesh et al., 2014; Bineesh et al., 2017; Jabado et al., 2018; Kumar et al., 2018; Pradeep et al., 2018), and Gujarat (Sutaria et al., 2015; Johri et al., 2019, 2020b, 2020c). Among the elasmobranch species sampled in our study ∼39% are in the ICUN Threatened categories and ∼20% in the data deficient category. We demonstrate that Gujarat is a biodiverse elasmobranch habitat and the fishery is exploiting (via targeted and incidental catch) ecologically important species at an alarming rate. We also alleviate the data deficiency of elasmobranchs (Dulvy et al., 2014; Jabado et al., 2017) by extension and confirmation of the geographic range of species and by documenting the catch of 31 different species with skewed gender and age ratios in the catch.
Our sequencing and phylogenetic analyses in the current study corroborates the taxonomic classification of five species (R. punctifer, T. sinuspersici, C. sorrah, M. mobular, and the M. tarapacana) in the sample set. Taxonomic identifications for Carcharhinus falciformis (Johri et al., 2019), Rhynchobatus laevis (Johri et al., 2020c), and Glaucostegus granulatus (Johri et al., 2020b) were confirmed previously by us. It should be noted that the low posterior probabilities or bootstrap support values in case of T. sinuspersici, Bayesian and maximum likelihood analyses are due to the lack of enough genetic data available for this clade of species in public databases. This again highlights the data deficiency with respect to genomic information on elasmobranchs and our current work is aimed at reducing these knowledge gaps.
A significantly high number of female and juvenile elasmobranchs are being captured by fisheries in Gujarat, suggesting the presence of a previously unknown nursery ground in coastal waters off Gujarat. S. lewini, R. laevis, C. sorrah, Rhinobatos annandalei, and Aetobatus ocellatus specimens were almost exclusively females (16 out of 26) and juveniles (21 out of 25). In Veraval, nearly all landings were females and juveniles. In addition, species found at shallow depths of 0–200 m were abundantly landed at all locations, with the exception of Okha and Porbandar which had a higher number of species found at deeper depths. Fishers in Veraval have high-capacity mechanized boats which are used for fishing in offshore waters several hundred nautical miles away from the coast (pers. comm. with fisher communities). However, at landing sites and fish markets we identified and sampled auctions of catch from smaller, coastal fishing vessels, which account for 25% of total fisheries catch in Veraval (pers. comm. with fisher communities). These fishers engage in daily fishing and landing activities in coastal and neritic zones as opposed to month long expeditions in offshore areas by larger vessels (pers. comm. with fisher communities). Consequently, catch from these fishers, was sampled heavily in the current study, represents species individuals caught close to shore. These data are indicative of the predominantly coastal fisheries in Veraval targeting coastal and neritic species and potentially nursery areas with a large proportion of females and juveniles for several species. Our findings support a potential nursery area for S. lewini, R. laevis, C. sorrah, R. annandalei, and A. ocellatus- all species with majority juvenile landings along the coast of Gujarat, which should be the target of future research.
Our results are consistent with observations of higher landing volumes for juvenile scalloped hammerhead sharks in other parts of the ASR (Henderson et al., 2016; FAO, 2021). S. lewini, a Critically Endangered, CITES (Pacoureau et al., 2020) listed species has had a >50% regional decline in the ASR (Jabado et al., 2017), in part due to its slow life history traits (Hazin et al., 2001; Harry et al., 2011). R. laevis which is a Critically Endangered, CITES (Peter Kyne, 2020) listed species, is similarly vulnerable to fishing pressure due to its slow life history traits (Compagno, 1990; García et al., 2008; Hutchings et al., 2012) and has suffered declines of 50–80% throughout the ASR (Kyne et al., 2020b; FAO, 2021). In general, the population growth rate of Chondrichthyes is limited by slow life history traits, long gestation periods, and low fecundity (Cortés, 2000; Hutchings et al., 2012). Overfishing of juveniles and sexually mature females as seen in our study, further exacerbates the recovery potential of overexploited species populations. Our findings thus identify priority concern species and their nursery grounds in the ASR and call for expedient management measures to conserve and protect remaining populations.
We extended the geographic ranges of three species that were previously unreported in northwest India or in two cases unreported in the Indian subcontinent. These species include T. sinuspersici, which is a Data Deficient species, and C. sorrah and R. punctifer, both of which are Near Threatened. Our observations are significant in establishing the geographic range of the species, understanding species ecology, and in evaluating species biodiversity of the Gujarat coast. Since each of these species has a coastal distribution range within 0–200 m, the specimens we report were potentially fished in coastal waters off of the respective landing sites in Gujarat. The fishing of these specimens in distant international/national coastal zones and subsequent landing in Gujarat is extremely unlikely due to national fishing restrictions within exclusive economic zones (EEZs) and provincial fishing restrictions within India. We are therefore confident that the range extensions reported here are accurate, but suggest dedicated sampling efforts are conducted to describe the distribution of the species in Gujarat, elsewhere in India and the ASR.
T. sinuspersici is a species complex (Dent and Clarke, 2015; Park University, 2020) and warrants investigation to resolve the component species. The spotted guitarfish R. punctifer, a second range extension, is commonly mistaken with the Annandale’s guitarfish R. annandalei, and thus its distribution in the area has been questionable (Ali et al., 2021). The spotted guitarfish was sampled four times in the current study confirming, for the first time, that the species is extant in India. The repeated landings also suggest extreme vulnerability of the species to capture by fisheries all along the coast of Gujarat and are concerning for a species with a declining population trend. The third species was the spottail shark C. sorrah, for which we identified four specimens caught at two different ports in Gujarat, thus extending the geographic range of the species to northwestern India. The species has been previously reported on the eastern coast and up to the southwestern coast of India, along with its wide range in the tropical Indo-West Pacific, the Indian Ocean, Southeast Asia, and Australia (IUCN Red List, 2020b). Our observations suggest a high vulnerability of the spottail shark to fisheries in Gujarat. Future genetic studies of the species are warranted and will assist in determining its population trend, which currently remains unknown. Our findings on the three species found in neritic marine zones significantly contribute to the current knowledge on species distributions, and will be instrumental in defining areas on the continental shelf that warrant protection from the prolific and exploitative fisheries in Gujarat.
Numerous species sampled in our study such as S. lewini, A. pelagicus, C. falciformis, C. brevipinna, C. leucas, C. limbatus, as well as M. mobular, M. tarapacana, and A. ocellatus are migratory in nature (The IUCN Red List, 2019). These species potentially cross international maritime boundaries with high frequency and call for special consideration by the Convention for Migratory Species (CMS), along with an international coalition for protection from fisheries, including those in India.
We have reduced the data deficiency of five batoids – Rhinobatos annandalei, R. jayakari, Maculabatis bineeshi, P. bleekeri, and T. sinuspersici. Batoid fisheries and trade receive even less attention and monitoring than true shark landings, and batoid specimens are often not reported (Bräutigam et al., 2020). Two of the sampled species, M. bineeshi and P. bleekeri have declined by >50% (Jabado et al., 2017) and have potentially undergone heavy exploitation by fisheries in Gujarat due to their habitation of in-shore coastal areas (Jabado et al., 2017). R. annandalei is frequently misidentified with the spotted guitarfish (R. punctifer), and therefore often goes unreported (Jabado et al., 2017). All three specimens of the species collected in Veraval and Porbandar were juvenile, suggesting nursery areas for the species close to the sampling sites. Carcharhinus amboinensis was the only DD shark species sampled in our study. Low fecundity of the species, deterioration in its habitat quality due to heavy coastal pollution and large scale development in Gujarat and elsewhere in the ASR (Jabado et al., 2017) are concerning for the species’ status which is expected to have a 30–50% decline in its population (IUCN Red List, 2020a). The high frequency of juvenile and female specimens captured from DD species, raises the concern that populations will be depleted through exploitation by fisheries, before we have an opportunity to enact conservation measures by assessing the species’ distribution and ecology. Our studies provide timely indicators of the species’ distribution, ecology and catch rate in fisheries, to enable specific investigations which will ultimately facilitate enactment of protective measures for the species.
There is an increasing trend toward mechanization of fishing vessels in the ASR (Raje and Zacharia, 2009; Jabado and Spaet, 2017). Mechanized fishing fleets explore deeper off shore environments as nearshore resources are depleted (Akhilesh et al., 2011; Jabado et al., 2018; Kyne et al., 2020b), thus expanding fishing capacity into territories with inadequate or absent oversight and management (Nagle, 2019). Chondrichthyes inhabiting deep water environments are adapted to colder and potentially resource limited environments, have slower than average growth rates and consequently are more vulnerable to fishing pressure than nearshore species (Nagle, 2019). However, an estimated 35% of chondrichthyan species are found in deep-water environments and are considered a low priority for fisheries management (Bräutigam et al., 2020). Thus, mechanization and offshore fishing exposes vulnerable species which were earlier protected in deep water refuges. Mechanized vessels found in the Indian EZZ use trawl nets, gill nets, and long line gear and are therefore likely to engage in indiscriminate fishing in the absence of observers or alternate monitoring mechanisms (Nagle, 2019). In addition offshore fisheries in India engage in overfishing of species which have a limited geographic range and extremely low fecundity, resulting in up to 99% declines in population abundance of species (Akhilesh et al., 2011; Kyne et al., 2020a; Pogonoski and Pollard, 2020; White, 2020). Thus, increased capacity for fishing in deeper waters in Gujarat is likely going to drive further species declines and extinctions, unless directed and expedient measure are taken to manage deep water fisheries at a national and regional level in the ASR.
Reporting of most fisheries occurs by aggregating species in higher groups such as orders and families, which masks declines of individual species (Dulvy et al., 2014; Nagle, 2019). Lack of species identification makes management of stock and protected species difficult or impossible. We provided species-specific assessments of landing sites along with the age or gender groups captured as targeted or incidental catch for 31 elasmobranch species in the fisheries of Gujarat. Thus, we provide critical information on priority species of concern impacted by fisheries in the area. While we acknowledge that the number of specimens recorded for each species was highly variable and very low in some cases, the data were obtained through opportunistic samplings of fish markets which offer no control on the type and number of specimens. However, we used these specimens to sequence the partial genome of a chondrichthyan species, C. falciformis (Johri et al., 2019), and reported the first mitogenomes for G. granulatus (Johri et al., 2020b), and R. laevis (Johri et al., 2020c), providing the first species specific assessments for these taxa in the ASR and demonstrating the power of molecular taxonomy in species assessments of wildlife trade and fisheries.
The ineffectiveness of protection and management measures in the ASR is represented by the decline in chondrichthyan stocks over the past few decades. To increase sustainability and effectiveness of conservation strategies, efforts should be directed at identification of priority concern species through ecological and threat assessments of chodnrichthyan populations in areas with high fishing volumes. Second, community partnerships should be forged to enact management measures to their full potential and to develop a sustainable conservation program with shared cross-sectoral responsibilities and beneficiaries. The current report bridges crucial knowledge gaps with regards to elasmobranch fisheries in India, the largest shark fishing nation in the ASR and second largest in the world. We expect that the data presented here will pivot the direction of conservation measures to protect priority concern species and underline the impending urgency of these efforts. We also expect to leverage community partnerships built during the current work to assist in co-designing and implementation of cross-sectoral management and conservation programs in India.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: BioProject: PRJNA698050 and at iNaturalist: https://www.inaturalist.org/observations?place_id=any&subview=grid&user_id=shaili&verifiable=any&view=species.
SJ conceptualized and designed the study, wrote the manuscript and contributed to sampling and DNA sequencing. IL contributed to writing and data analyses. AT conducted sampling and surveys. JS organized sampling trips and facilitated liaisons with fisher communities. AB and IM helped with data analyses, organization, outreach, and education materials. SF conducted phylogenetic analyses of samples. MD assisted with sampling. ED helped with study design and writing of the manuscript. All authors edited the manuscript.
This work was supported by the Society for Conservation Biology-Marine Section, University Grants Program-San Diego State University and the S. Lo and B. Billings’ Global Shark Research and Conservation Fund.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are immensely thankful to Prof. Asit Vyas Junagadh Agricultural University (JAU) and Prof. Sashikant Acharya (MSU) for connecting us with the community of students and faculty who contributed significantly to this work. We sincerely thank Prakash Doriya, Charan Kumar Paidi, and Farukhkha Husenkha from the Wildlife Trust of India (WTI) for their overwhelming support in forming community liaisons, arranging logistics and in sampling of specimens, which made possible the work presented here. We acknowledge continual support through this project from Prof. Ashit Desai, Dr. Prakash Parmar, and Dr. A. Khan at the College of Fisheries, Veraval, JAU, and we thank them for providing us with local contacts with the community of students and fishers in Veraval, and for space for us to conduct research in their laboratories. We are thankful to Arif Khan at the Fisheries Research Station, Okha (JAU) for their assistance in sampling at this location. We are grateful for the immense support we received from the fishing communities in Mangrol, Porbandar and Okha, and especially these individuals: Tulsi Keshav Gohel, Dhansukh Lal, Sunil Gohil, Chirag Junji, Sunil Bhaskar, Vishal Pithiya and Mohanbhai Lashkari. We sincerely thank Daniel Fernando, Akshay Tanna, and Gobi Raj at Blue Resources Trust Sri Lanka, Dr. Gavin Naylor (Director, Florida Program for Shark Research, University of Florida), Dr. Rima Jabado (Gulf Elasmo Project, Dubai, United Arab Emirates), Clinton Duffy (New Zealand Department of Conservation, iNaturalist), Diego Almendras (Universidad Católica del Norte, Chile, iNaturalist), and Alex Burton (iNaturalist) for their assistance with taxonomic identification of specimens. We are thankful to past and present members of the Dinsdale Lab for their assistance through this project.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.619695/full#supplementary-material
Supplementary Figure 1 | Bayesian and maximum likelihood estimates of phylogenetic relationships among elasmobranch species using mitochondrial protein coding genes for Rhinobatos punctifer (A), Torpedo sinuspersici (B), Carcharhinus sorrah (C), Mobula mobular and Mobula tarapacana (D). The unknown sequence contig for each of the five specimens clusters with their taxonomically assigned identities and thus confirms their taxonomic classification based on morphology. Numbers at nodes are posterior probabilities for BI trees and bootstrap support values are the numbers at nodes in ML trees.
Supplementary Figure 2 | A comparison of species distribution across sampling locations. (A) Frequency of species by sampling location, with 31 total species of elasmobranchs positively identified from 157 samples. (B) Percentage of species for sharks and rays sampled in each conservation category.
Supplementary Figure 3 | Depth range of 24 species sampled and described across all four sample locations.
Supplementary Figure 4 | Comparison of previously reported geographic ranges and landing site locations for three species from the current study indicated as: Okha (blue arrow), Porbandar (red arrow) and Veraval (black arrow). (A) Geographic range of Carcharhinus sorrah on the IUCN Redlist Database, and landing sites for the species in Porbandar and Veraval, (B) Geographic range of Torpedo sinuspersici on the IUCN Redlist Database, and landing site for the species in Porbandar, and (C) Geographic range of Rhinobatos punctifer on the IUCN Redlist Database, and landing sites for the species in Okha, Porbandar and Veraval.
Supplementary Table 1 | List of taxa, genetic loci and accession numbers used to assess phylogenetic relationships described in Supplementary Figure 1.
Akhilesh, K. V., Bineesh, K. K., Gopalakrishnan, A., Jena, J. K., Basheer, V. S., and Pillai, N. G. K. (2014). Checklist of chondrichthyans in Indian waters. J. Mar. Biol. Assoc. India 56, 109–120. doi: 10.6024/jmbai.2014.56.1.01750s-17
Akhilesh, K. V., Ganga, U., Pillai, N. G. K., Vivekanandan, E., Bineesh, K. K., Rajool Shanis, C. P., et al. (2011). Deep Sea fishing for chondrichthyan resources and sustainability concerns-a case study from Southwest Coast of India. Indian J. Geo Mar. Sci. 40, 347–355.
Ali, M., Akhilesh, K. V., and Khan, M. (2021). IUCN Red List of Threatened Species: Rhinobatos Punctifer. Available online at: https://www.iucnredlist.org/en (accessed February 9, 2021)
Barker, M. J., and Schluessel, V. (2005). Managing global shark fisheries: suggestions for prioritizing management strategies. Aquat. Conserv. Mar. Freshw. Ecosyst. 15, 325–347. doi: 10.1002/aqc.660
Bineesh, K. K., Gopalakrishnan, A., Akhilesh, K. V., Sajeela, K. A., Abdussamad, E. M., Pillai, N. G. K., et al. (2017). DNA barcoding reveals species composition of sharks and rays in the Indian commercial fishery. Mitochondr. DNA A DNA Mapp. Seq. Anal. 28, 458–472. doi: 10.3109/19401736.2015.1137900
Bornatowski, H., Navia, A. F., Braga, R. R., Abilhoa, V., and Corrêa, M. F. M. (2014). Ecological importance of sharks and rays in a structural foodweb analysis in Southern Brazil. ICES J. Mar. Sci. 71, 1586–1592. doi: 10.1093/icesjms/fsu025
Bräutigam, A., Callow, M., and Campbell, I. R. (2020). Global Priorities for Conserving Sharks and Rays?: A 2015-2025 Strategy. Available online at: https://portals.iucn.org/library/sites/library/files/documents/2016-007.pdf (accessed June 3, 2020).
Castro, J. I., Woodley, C. M., and Brudek, R. L. (1999). A Preliminary Evaluation of the Status of Shark Species. Rome: Food & Agriculture Organisation.
Compagno, L. J. V. (1990). Alternative life-history styles of cartilaginous fishes in time and space. Environ. Biol. Fishes 28, 33–75. doi: 10.1007/BF00751027
Cortés, E. (2000). Life history patterns and correlations in sharks. Rev. Fish. Sci. 8, 299–344. doi: 10.1080/10408340308951115
Devadoss, P., Kuthalingam, M. D. K., and Thiagarajan, R. (1989). The Present Status and Future Prospects of Elasmobranch Fishery in India. Sessions I&II. Kochi: Central Marine Fisheries Research Institute.
Dulvy, N. K., Fowler, S. L., Musick, J. A., Cavanagh, R. D., Kyne, P. M., Harrison, L. R., et al. (2014). Extinction risk and conservation of the World’s sharks and rays. eLife 3:e00590. doi: 10.7554/eLife.00590
Dulvy, N. K., Simpfendorfer, C. A., Davidson, L. N. K., Fordham, S. V., Bräutigam, A., Sant, G., et al. (2017). Challenges and priorities in shark and ray conservation. Curr. Biol. 27, R565–R572. doi: 10.1016/j.cub.2017.04.038
Ebert, D., Fowler, S., and Compagno, L. (2013). Sharks of the World: A Fully Illustrated Guide. Plymouth: Wild Nature Press Ltd.
Ebert, D. A., Khan, M., Ali, M., Akhilesh, K. V., and Jabado, R. W. (2017). Rhinobatos punctifer. The IUCN Red List of Threatened Species. Available online at: doi.org/10.2305/IUCN.UK.2017-2.RLTS.T161447A109904426.en (accessed December 6, 2019).
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. doi: 10.1093/nar/gkh340
FAO (2007). “Review of the state of world marine capture fisheries management. Pacific Ocean,” in FAO Fisheries Technical Paper, ed. C. De Young (Rome: Food and Agriculture Organization of the United Nations).
FAO (2017). FAO Fisheries & Aquaculture - FishStatJ - Software for Fishery and Aquaculture Statistical Time Series. Available online at: http://www.fao.org/fishery/statistics/software/fishstatj/en (accessed June 3, 2020)
FAO (2019). 2019 IUCN RL Update. Available online at: https://www.iucnssg.org/2019-iucn-rl-update.html (accessed February 5, 2021)
FAO (2020). 2020_03RedList. Available online at: https://www.iucnssg.org/2020_03redlist.html (accessed February 5, 2021)
FAO (2021). Overview of World Elasmobranch Fisheries. Available online at: http://www.fao.org/3/v3210e/V3210E01.htm#ch1. (accessed January 24, 2020)
FAO Yearbook (2020). Fishery and Aquaculture Statistics 2018/FAO Annuaire. Statistiques Des Pêches et de l’aquaculture 2018/FAO Anuario. Estadísticas de Pesca y Acuicultura 2018. Rome: FAO.
FishBase (2020). Search FishBase. Available online at: https://www.fishbase.in/search.php (accessed January 22, 2020)
Froese, R., and Pauly, P. (2020). FishBase. World Wide Web Electronic Publication. Available online at: https://www.fishbase.de/summary/citation.php (accessed February 24, 2021)
García, V. B., Lucifora, L. O., and Myers, R. A. (2008). The importance of habitat and life history to extinction risk in sharks, skates, rays and chimaeras. Proc. R. Soc. B Biol. Sci. 275, 83–89. doi: 10.1098/rspb.2007.1295
Harry, A. V., Macbeth, W. G., Gutteridge, A. N., and Simpfendorfer, C. A. (2011). The life histories of endangered hammerhead sharks (Carcharhiniformes, Sphyrnidae) from the East Coast of Australia. J. Fish Biol. 78, 2026–2051. doi: 10.1111/j.1095-8649.2011.02992.x
Hazin, F., Fischer, A., and Broadhurst, M. (2001). Aspects of reproductive biology of the scalloped hammerhead shark, Sphyrna Lewini, off Northeastern Brazil. Environ. Biol. Fishes 61, 151–159. doi: 10.1023/a:1011040716421
Henderson, A. C., Reeve, A. J., Jabado, R. W., and Naylor, G. J. P. (2016). Taxonomic assessment of sharks, rays and guitarfishes (Chondrichthyes: Elasmobranchii) from South-Eastern Arabia, using the NADH dehydrogenase subunit 2 (NADH2) gene. Zool. J. Linn. Soc. 176, 399–442. doi: 10.1111/zoj.12309
Hutchings, J. A., Myers, R. A., García, V. B., Lucifora, L. O., and Kuparinen, A. (2012). Life-history correlates of extinction risk and recovery potential. Ecol. Appl. 22, 1061–1067. doi: 10.1890/11-1313.1
IUCN Red List (2019). The IUCN Red List of Threatened Species. Available online at: https://www.iucnredlist.org/en (accessed December 17, 2019)
IUCN Red List (2020a). IUCN Red List of Threatened Species: Pigeye Shark. Available online at: https://www.iucnredlist.org/en (accessed June 11, 2020)
IUCN Red List (2020b). IUCN Red List of Threatened Species: Spottail Shark. Available online at: https://www.iucnredlist.org/ja (accessed June 7, 2020)
IUCN Red List (2021). The IUCN Red List of Threatened Species. Version 2020-1. Available online at: https://www.iucnredlist.org/en (accessed February 24, 2021)
Jabado, R. W., Kyne, P. M., and Pollom, R. A. (2017). The Conservation Status of Sharks, Rays, and Chimaeras in the Arabian Sea and Adjacent Waters. Bristol: Environment Agency.
Jabado, R. W., Kyne, P. M., Pollom, R. A., Ebert, D. A., Simpfendorfer, C. A., Ralph, G. M., et al. (2018). Troubled waters: threats and extinction risk of the sharks, rays and chimaeras of the arabian sea and adjacent waters. Fish Fish. 19, 1043–1062. doi: 10.1111/faf.12311
Jabado, R. W., and Spaet, J. L. Y. (2017). Elasmobranch fisheries in the Arabian seas region: characteristics, trade and management. Fish Fish. 18, 1096–1118. doi: 10.1111/faf.12227
Johri, S., Dunn, N., Chapple, T. K., Curnick, D., Savolainen, V., Dinsdale, E. A., et al. (2020a). Mitochondrial genome of the silvertip shark, carcharhinus albimarginatus, from the British Indian Ocean Territory. Mitochondr. DNA Part B 5, 2085–2086. doi: 10.1080/23802359.2020.1765210
Johri, S., Fellows, S. R., Solanki, J., Busch, A., Livingston, I., Mora, M. F., et al. (2020b). Mitochondrial genome to aid species delimitation and effective conservation of the sharpnose guitarfish (Glaucostegus granulatus). Meta Gene 24:100648. doi: 10.1016/j.mgene.2020.100648
Johri, S., Solanki, J., Cantu, V. A., Fellows, S. R., Edwards, R. A., Moreno, I., et al. (2019). ‘Genome skimming’ with the MinION hand-held sequencer identifies CITES-listed shark species in India’s exports market. Sci. Rep. 9:4476. doi: 10.1038/s41598-019-40940-9
Johri, S., Tiwari, A., Kerr, E. N., and Dinsdale, E. A. (2020c). Mitochondrial genome of the smoothnose wedgefish Rhynchobatus laevis from the Western Indian Ocean. Mitochondr. DNA Part B 5, 2083–2084. doi: 10.1080/23802359.2020.1765209
Karnad, D., Sutaria, D., and Jabado, R. W. (2020). Local drivers of declining shark fisheries in India. Ambio 49, 616–627. doi: 10.1007/s13280-019-01203-z
Kizhakudan, S. J., Zacharia, P. U., Thomas, S., Vivekanandan, E., and Muktha, M. (2015). Guidance on National Plan of Action for Sharks in India. Kochi: Central Marine Fisheries Research Institute.
Kumar, R., Sasidharan, V., Akhilesh, K. V., Bineesh, K. K., and Pt, R. (2018). First report of four deep-sea chondrichthyans (Elasmobranchii and Holocephali) from Andaman Waters, India with an updated checklist from the region. Acta Ichthyol. Piscat. 3, 289–301. doi: 10.3750/AIEP/02336
Kyne, P., Huveneers, C., Ebert, D., and Bigman, J. (2020a). IUCN Red List of Threatened Species: Western Gulper Shark. Available online at: https://www.iucnredlist.org/en (accessed June 8, 2020).
Kyne, P. M. (2019). Torpedo sinuspersici. The IUCN Red List of Threatened Species 2019. Available online at: https://dx.doi.org/10.2305/IUCN.UK.2019-3.RLTS.T60136A140820271.en. (accessed April 22, 2021).
Kyne, P. M., Jabado, R. W., Rigby, C. L., Dharmadi, Gore, M. A., Pollock, C. M., et al. (2020b). The thin edge of the wedge: extremely high extinction risk in Wedgefishes and Giant Guitarfishes. Aquat. Conserv. Mar. Freshw. Ecosyst. 30, 1337–1361. doi: 10.1002/aqc.3331
Lack, M., and Sant, G. (2011). The Future of Sharks: A Review of Action and Inaction. Cambridge: TRAFFIC Global Office.
Lanfear, R., Frandsen, P. B., Wright, M. A., Senfeld, T., and Calcott, B. (2016). PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 34, 772–773. doi: 10.1093/molbev/msw260
Last, P., White, W., Carvalho, M., Seret, B., Stehmann, M., and Naylor, G. (2018). Rays of the World, Peter Last, William White, Marcelo de Carvalho, Bernard Séret, Matthias Stehmann, Gavin Naylor, 9780643109131. Available online at: https://www.publish.csiro.au/book/7053/ (accessed September 14, 2018)
Miller, M., Pfeiffer, W. T., and Schwartz, T. (2010). “Creating the CIPRES science gateway for inference of large phylogenetic trees,” in Proceedings of the Gateway Computing Environments Workshop: 14 Nov 2010, Vol. 14, (New Orleans, LA), 1–8.
Mohamed, K., and Shettigar, V. (2016). How long does it take for tropical marine fish stocks to recover after declines?Case studies from the Southwest Coast of India. Curr. Sci. 110, 584. doi: 10.18520/cs/v110/i4/584-594
Musick, J. A., Burgess, G., Cailliet, G., Camhi, M., and Fordham, S. (2000). Management of sharks and their relatives (Elasmobranchii). American Fisheries Society, 25. Available online at: http://www.adfg.alaska.gov/static-sf/Region2/ground_fish/PDFs/AFSMarineStocksAtRisk31b_sharks.pdf (accessed March, 2000).
Nagle, C. (2019). Shark Fishing in the Indian Seas: A Quantitative Risk Assessment of the Impacts of Longline Fishing on the Sustainability of Regional Shark Populations. Ph. D. Thesis, Harvard Extension School, Cambridge, MA.
Pacoureau, N., Sherley, R., Liu, K.-M., Sul, R. B., Herman, K., Rigby, C., et al. (2020). IUCN Red List of Threatened Species: Scalloped Hammerhead. Available online at: https://www.iucnredlist.org/en (accessed June 11, 2020)
Park University (2020). (Charles D., Coordinator), A./ I.S.S.R. IUCN Red List of Threatened Species: Gulf Torpedo. Available online at: https://www.iucnredlist.org/en (accessed June 7, 2020)
Peter Kyne, R. J. (2020). IUCN Red List of Threatened Species: Smoothnose Wedgefish. Available online at: https://www.iucnredlist.org/en (accessed February 21, 2020)
Pogonoski, J. J., and Pollard, D. (2020). IUCN Red List of Threatened Species: Little Gulper Shark. Available online at: https://www.iucnredlist.org/en (accessed June 8, 2020)
Pollerspöck, J., and Straube, N. (2021). Bibliography Database. Version 2021. Available online at: https://www.shark-references.com/ (accessed February 24, 2021)
Pradeep, H., Shirke, S., Nashad, M., Sasidharan, V., Kumar, R., Sumitha, G., et al. (2018). First record and DNA barcoding of Oman Cownose Ray, Rhinoptera Jayakari Boulenger, 1895 from Andaman Sea, India. Zoosystema 40, 67–74. doi: 10.5252/zoosystema2018v40a4
Raje, S., and Zacharia, P. U. (2009). Investigations on fishery and biology of nine species of rays in Mumbai Waters. Indian J. Fish. 56, 95–101.
Raje, S. G., Sivakami, S., Mohanraj, G., Manojkumar, P. P., Raju, A., and Joshi, K. K. (2007). Atlas on the elasmobranch fishery resources of India. CMFRI Spec. Publ. 95, 1–253. doi: 10.7755/fb.114.1.1
Rambaut, A., Drummond, A. J., Xie, D., Baele, G., and Suchard, M. A. (2018). Posterior summarization in Bayesian Phylogenetics using tracer 1.7. Syst. Biol. 67, 901–904. doi: 10.1093/sysbio/syy032
Rhinidae – IUCN Red List. Supplementary Information for wedgefishes (Rhinidae) and giant guitarfishes (Glaucostegidae). Available online at: https://www.iucnredlist.org/species/pdf/2382420/attachment. (accessed March 01, 2021).
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Rose, D. A. (1998). Shark Fisheries and Trade in the Americas. Washington, DC: TRAFFIC North America.
Spaet, J. L. Y., and Berumen, M. L. (2015). Fish market surveys indicate unsustainable elasmobranch fisheries in the Saudi Arabian Red Sea. Fish. Res. 161, 356–364. doi: 10.1016/j.fishres.2014.08.022
Spaet, J. L. Y., Thorrold, S. R., and Berumen, M. L. (2012). A review of elasmobranch research in the Red sea. J. Fish Biol. 80, 952–965. doi: 10.1111/j.1095-8649.2011.03178.x
Stamatakis, A. (2014). RAxML Version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinform. Oxf. Engl. 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Stein, R. W., Mull, C. G., Kuhn, T. S., Aschliman, N. C., Davidson, L. N. K., Joy, J. B., et al. (2018). Global priorities for conserving the evolutionary history of sharks, rays and chimaeras. Nat. Ecol. Evol. 2, 288–298. doi: 10.1038/s41559-017-0448-4
Stevens, J. (2000). The effects of fishing on sharks, rays, and chimaeras (Chondrichthyans), and the implications for marine ecosystems. ICES J. Mar. Sci. 57, 476–494. doi: 10.1006/jmsc.2000.0724
Sutaria, D., Parikh, A., Barnes, A., and Jabado, R. W. (2015). First record of the Sandbar Shark, <span Class=”italic”>Carcharhinus Plumbeus</span>, (Chondrichthyes: Carcharhiniformes: Carcharhinidae) from Indian Waters. Mar. Biodivers. Rec. 8, e126. doi: 10.1017/S1755267215001025
The IUCN Red List (2019). The IUCN Red List of Threatened Species. Available online at: https://www.iucnredlist.org/en (accessed August 22, 2019)
White, W. T. (2020). IUCN Red List of Threatened Species: Leafscale Gulper Shark. Available online at: https://www.iucnredlist.org/en (accessed June 8, 2020)
Wild Life (Protection) Act. (1972). An Act to Provide for the Protection of Wild Animals, Birds and Plants and for Matters Connected Therewith or Ancillary or Incidental Thereto with a View to Ensuring the Ecological and Environmental Security of the Country. Available online at: http://indiacode.nic.in/handle/123456789/1726 (accessed September 9, 1972).
Worldbank (2020). Protecting India’s Coastline: Gujarat. Available online at: https://www.worldbank.org/en/news/feature/2012/10/11/protecting-indias-coastline-gujarat (accessed April 15, 2020)
Keywords: sharks, rays, fisheries, conservation, India, Arabian Sea, Gujarat, data-deficiency
Citation: Johri S, Livingston I, Tiwari A, Solanki J, Busch A, Moreno I, Fellows SR, Doane MP and Dinsdale EA (2021) Reducing Data Deficiencies: Preliminary Elasmobranch Fisheries Surveys in India, Identify Range Extensions and Large Proportions of Female and Juvenile Landings. Front. Mar. Sci. 8:619695. doi: 10.3389/fmars.2021.619695
Received: 12 November 2020; Accepted: 08 March 2021;
Published: 05 May 2021.
Edited by:
Emilio Sperone, University of Calabria, ItalyReviewed by:
Fabrizio Serena, Institute for Marine Biological Resources and Biotechnology (CNR), ItalyCopyright © 2021 Johri, Livingston, Tiwari, Solanki, Busch, Moreno, Fellows, Doane and Dinsdale. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaili Johri, c2hhaWxpakBzdGFuZm9yZC5lZHU=; Elizabeth A. Dinsdale, ZWxpemFiZXRoLmRpbnNkYWxlQGZsaW5kZXJzLmVkdS5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.