- 1State Key Laboratory of Marine Pollution, Department of Chemistry, City University of Hong Kong, Hong Kong SAR, China
- 2The Swire Institute of Marine Science, School of Biological Sciences, The University of Hong Kong, Hong Kong SAR, China
- 3Department of Science and Environmental Studies, The Education University of Hong Kong, Hong Kong SAR, China
- 4Department of Aquatic Sciences and Assessment, Swedish University of Agricultural Sciences, Uppsala, Sweden
Trawl fisheries have been shown to cause overfishing and destruction of benthic habitats in the seabed. To mitigate these impacts, a trawling ban has been enforced in Hong Kong waters since December 31, 2012 to rehabilitate the ecosystem and enhance fisheries resources. Previous studies demonstrated that reduced trawling activities would increase the heterogeneity of benthic habitats, thereby enhancing species richness and abundance of benthic fauna and providing more prey resources for predatory fishes. This study aimed to test a hypothesis that the population and trophic dynamics of the Bartail flathead Platycephalus indicus, a heavily fished benthic predatory fish, at inner and outer Tolo Channel of Hong Kong (i.e., EI and EO) improved with increases in their body size, abundance, biomass, trophic niche, and trophic position after the trawl ban. Samples were collected from trawl surveys before and after the trawl ban to compare the pre-ban and post-ban populations of P. indicus from EI and EO. Body size, abundance, and biomass were assessed in 2004, 2013–2014, and 2015–2016, whereas trophic niche and trophic position were analyzed based on stable isotopes of fish samples collected in dry season of 2012, 2015, and 2018. Following the trawl ban, the abundance and biomass of P. indicus increased in EO, with body size increased in EI. Furthermore, as indicated by the results of stable isotope analysis (SIA) on their tissues and prey items, trophic niche, and trophic position of P. indicus increased in EI and EO, respectively. Our study demonstrated that the trawl ban had promoted the recovery of a predatory fish population through restoring size structure and trophic dynamics.
Introduction
Bottom fishing activities are considered to be the most widespread anthropogenic disturbances onto marine benthic ecosystems (Hiddink et al., 2017). In particular, fishing by bottom trawlers has increasingly intensified since 1960s and contributed to approximately 25% of global seafood catches during 2011 and 2013 (The Food and Agriculture Organization, 2016). In Hong Kong, bottom trawlers contributed more than 50% (i.e., ca. 12,000 tons) of the historic annual total fishery catch (Agriculture, Fisheries and Conservation Department, 2013), resulting in large-scale fishery resource depletion, sedimentary organic matter resuspension, and benthic habitat homogenization (Cheung and Sadovy, 2004; Thrush et al., 2006). Such fishing activities can also reduce the biomass and diversity of the demersal biological community (Hiddink et al., 2011; Collie et al., 2017), as well as altering the trophic structure and functioning of the marine ecosystem (Jennings et al., 2001). Apart from direct harvesting of demersal fishes and crustaceans, trawling can also reduce availability of prey via direct removal and habitat destruction, resulting in dietary shifts and reduced energy intake and body size of predatory fishes in frequently trawled areas (Hinz et al., 2009; Hiddink et al., 2011). Thus, benthic trawling, if not regulated properly, could seriously deteriorate the marine biological communities and ecosystem functions. To restore the benthic ecosystem and associated fishery resources, a territory-wide ban on all trawling activities has been implemented in Hong Kong waters since December 31, 2012 (Agriculture, Fisheries and Conservation Department, 2011).
Globally, trawl bans or time-limited fishing moratoriums are commonly applied for restoring marine biodiversity, food webs, and fishery resources that have been impaired by fishing activities (Murawski et al., 2000; Hamilton et al., 2014; Bergman et al., 2015). Such management interventions are expected to promote increases in abundance, biomass, body size, and trophic niche of marine fauna (Pipitone et al., 2000; Fiorentino et al., 2008; Hamilton et al., 2014). Yet, the ecological processes leading to these improvements after the reduction in fishing pressure are complex and usually involve concurrent changes in prey availability, community composition, and trophic pathway and interaction (e.g., inter- and intra-specific competitions) (Board and Council, 2006; Johnson et al., 2015; Hinz et al., 2017). Also, the time needed for full recovery of faunal populations to pre-impact states varies among taxa and trophic levels and depends on environmental conditions and historical fishing intensity (Hiddink et al., 2017; Kaiser et al., 2018; Sciberras et al., 2018).
Predatory fishes regulate the lower trophic levels through trophic cascades (Myers and Worm, 2005) and are important in maintaining the aquatic food web stability and structure (Quevedo et al., 2009; Kratina et al., 2012). However, many marine ecosystems worldwide have been degraded concurrently with the overexploitation of predatory fishes, loss of natural habitats due to reclamation, and pollution over the past decades (Jackson et al., 2001; Myers and Worm, 2003). Management interventions such as the implementation of trawl bans, establishment of marine protected areas and restriction of fishing effort are necessary to reduce fishing pressure. These aim to improve habitat quality and trophic linkages, facilitating ecosystem-wide and predatory fish population recovery (Serrano et al., 2011; Hamilton et al., 2014). As their population structure and trophic ecology are strongly related to the ecosystem’s health condition (Lau et al., 2017), recovery of predatory fishes can potentially reflect ecosystem recovery. Serrano et al. (2011) reported that predatory fishes, such as seabreams (Sparidae), catsharks and skates (Elasmobranchii), and red mullets (Mullidae), increased in biomass after 4 years of trawl exclusion in the Cantabrian Sea. After enforcement of regulations to reduce fishing pressure, the California sheephead (Semicossyphus pulcher: Labridae) had broadened trophic niches in kelp forests off San Nicolas Island in California, likely due to recovery of the prey diversity of larger individuals and an increase in the overall population size structure (Hamilton et al., 2014).
Widely distributed in Indo-West Pacific regions, the Bartail flathead, Platycephalus indicus (Linnaeus, 1758) (Platycephalidae), is a benthic predator of other fishes and crustaceans (Wu, 1984; Froese and Pauly, 2019). It is commercially and ecologically important in Asia, such as in Vietnam, India, Hong Kong, Mainland China, etc. (Eayrs et al., 2007; Mirzaei et al., 2017; Fish Marketing Organization, 2019). However, because of overexploitation, the flathead’s population declined by 80% in South China Sea over recent decades, with its annual landing in Hong Kong decreasing to 170 tons in 2006 (Agriculture, Fisheries and Conservation Department, 2006). Given their economic and ecological importance, it is of interest to assess whether the flathead’s population showed signs of recovery after the implementation of the trawl ban in Hong Kong.
As the trawl ban would reduce the physical disturbance and fishing mortality on benthic communities, it is expected that the habitat heterogeneity would increase, leading to further increases in the biomass and diversity of benthic community (Bergman et al., 2015; Wang et al., 2021). As such, it is anticipated that more diverse prey items would be available for predatory fishes after the trawl ban (Hamilton et al., 2014) that may promote the trophic niche and trophic position of the predatory fishes. Nevertheless, there is a global variation in the outcomes of trawl bans. Increases in species richness, abundance, biomass, animal size, and/or trophic position after trawl bans have been reported for the Gulf of Castellamares of Italy, the Scotian Shelf of Canada, and the Thermaikos Gulf of Greece (Fisher and Frank, 2002; Fiorentino et al., 2008; Dimarchopoulou et al., 2018). However, no positive changes in abundance or biomass were detected in St. Andrews Bay (Defew et al., 2012), and negative responses were recorded in the southern waters of Hong Kong after the implementation of the trawl ban (Tao et al., 2020), partly due to other anthropogenic disturbances after the intervention. To understand the effects of the trawl ban in Hong Kong waters, this study was designed to test two interrelated hypotheses: (1) the abundance, biomass, and body size of P. indicus would increase following the trawl ban due to release of fishing pressure on the population, and (2) trophic position and niche width of P. indicus, as reflected by stable isotope signatures, would also increase as a result of increased prey resources and recovery of their population structure. The results of this study can provide an invaluable baseline for evaluating the long-term effects of the trawl ban on marine ecosystems in Hong Kong waters and support the adoption of a trawl ban as an effective policy for sustainable fisheries management, especially in Southeast Asia.
Materials and Methods
Study Sites and Field Sampling
Hong Kong is influenced by oceanic currents from the western Pacific and South China Sea, resulting in two principal (i.e., wet and dry) monsoonal seasons (Morton and Morton, 1983). The eastern waters were a historically important fishing ground for fishes and shrimps, but fishery resources were depleted by intensive trawling activities over time (Environmental Resource Management, 1998). Riverine nutrient inputs cause regular eutrophication and harmful algal blooms that have further suppressed fishery resources in the Tolo area of Hong Kong (Environmental Protection Department, 2018).
As there were active trawling activities in all water regions of Hong Kong over the last decades before the trawl ban (Agriculture, Fisheries and Conservation Department, 2006), it was impossible to assign an area without trawling as a control site for this study. Thus, this study used a before-and-after comparison without control sites to test the hypotheses. Trawl surveys were conducted to collect samples of P. indicus in the inner Tolo Channel (EI) and outer Tolo Channel (EO) in northeast waters of Hong Kong, along two transects within each site (22°26′N–22°31′N, 114°14′E–114°23′E; Figure 1), before and after the implementation of the territory-wide trawl ban in Hong Kong, which was implemented on December 31, 2012. Monthly surveys were conducted in both sites from January to December 2004 (2004; before the ban), June 2013 to May 2014 (2013–2014; shortly after the ban), and June 2015 to May 2016 (2015–2016; 3.5 years after the trawl ban). Additionally, two trawl surveys were carried out, once in October 2012 and once in November 2012 (pre-ban), and 2015 and 2018 (post-ban), respectively, to collect P. indicus and its potential food sources to examine changes in trophic niche and trophic position of the fish based on stable isotopes. Water quality data such as turbidity (NTU), dissolved oxygen (DO; mg/L), pH, and chlorophyll-a (Chl-a) concentration (μg/L) from the bottom water layer (18.8 ± 2.9 m; mean ± SD) at the transect areas were extracted from the database of Environmental Protection Department (EPD) during 2004, 2013–2014, and 2015–2016 (Marine water quality data1).
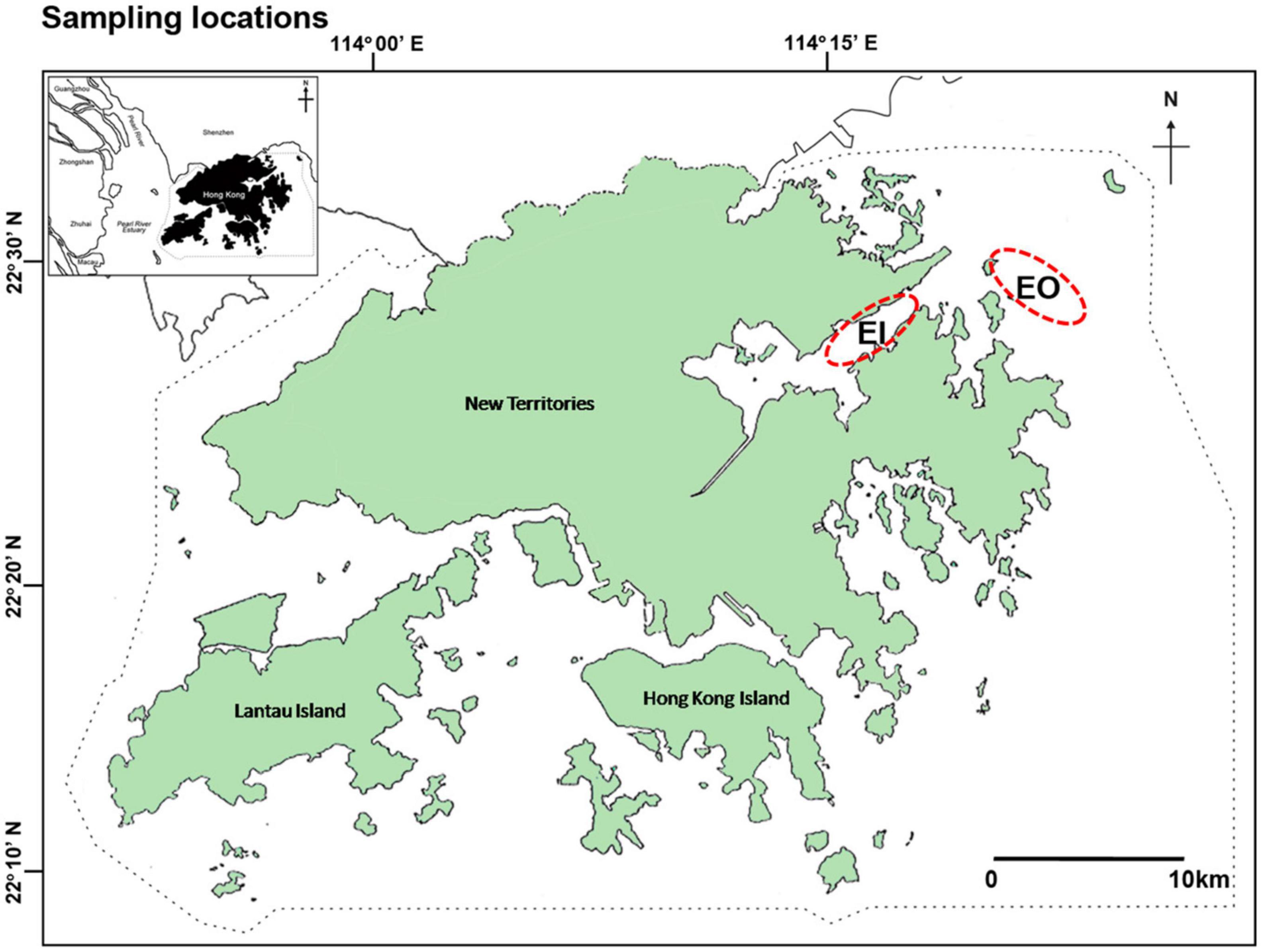
Figure 1. Sampling locations of benthic crustaceans in Hong Kong waters: inner Tolo Channel (EI) and outer Tolo (EO). Dotted line is Hong Kong jurisdictional boundary inside which all trawling-based fishing activities are banned.
A trawler (beam size: 2 m; stretched mesh size: 2 cm) with a 15-m outrigger and 10 replicate nets trawled along two transects of EI and EO for 30 min at a speed of 5–7 km/h, giving a total trawled area of 0.0375–0.0525 km2 per transect. All crustaceans and fishes were sorted on board, stored in ice, and then brought back to the laboratory. The samples were identified to the lowest possible taxonomic levels within 48 h of sampling, counted, and weighed (to the nearest 0.01 g in wet weight).
Stable Isotope Analysis
The diet of P. indicus included fish species from Apogonidae, Sciaenidae, Callionymidae, Clupeidae, Gobiidae, and Plotosidae and crustacean species from Portunidae, Peneaidea, Sergestidae, and Squillidae, according to Wu (1984) and gut content analysis of our samples. Thus, these taxa were used as potential food sources for P. indicus in stable isotope analysis (SIA) (Supplementary Table 1). Filter feeders (bivalve: Arcidae, Cardiidae, Mytilidae, Pinnidae, and Veneridae; gastropods: Turritellidae) were also collected from the same sampling periods and used as isotopic baselines for calculating the trophic positions of P. indicus (Tao et al., 2020). Only muscle tissues of the animals were used for SIA (dorsal muscles of fishes, abdominal muscles of shrimps and mantis shrimps, thoracic sternite muscles of crabs, and foot or adductor muscles of filter feeders). The dissected samples were rinsed with Milli-Q water, freeze-dried, and homogenized using a bead homogenizer (OMNI International, The Homogenizer Company). Then, 1.0 mg of individual samples were encapsulated in tin capsules and analyzed for stable carbon and nitrogen isotopes (δ13C and δ15N, measured in ‰) using a Nu Perspective Isotope Ratio Mass Spectrometer (Nu Instruments Ltd., Wrexham, United Kingdom) coupled with an Eurovector EA3028 Elemental Analyser (Isomass Scientific Inc., AB, Canada) at the Stable Isotope Laboratory in the University of Hong Kong. Two to 25 individuals for P. indicus and each prey taxon in each survey were analyzed. Because of the high lipid contents in fish muscles (usually C:N ratios > 4), δ13C values of fish samples were calibrated using the following equation (Mak, 2017):
However, δ13C values of mollusk and crustacean samples were not corrected as their lipid contents were low (C:N ratios < 4) (Tao et al., 2020).
Data Analysis
Biological Parameters
At each month and site, individuals of P. indicus were grouped into 3 cm classes (17 classes, from 4.9 to 53.8 cm total length; for example, 3 ≤ size class I < 6 cm, 6 ≤ size class II < 9 cm, etc.). To quantify the population structure, the monthly length frequency for each site was analyzed using Bhattacharya’s method of electronic length frequency analysis (ELEFAN; International Center for Living Aquatic Resources Management, the Philippines) implemented in FiSAT II (The FAO-ICLARM Stock Assessment Tools; version 1.2.2). The von Bertalanffy growth function was used to estimate the growth of P. indicus at each of the study sites:
where L∞ is the asymptotic length, Lt is the length at time t, t0 is the age at zero length, and K is the von Bertalanffy growth constant, which denotes the rate of growth as the curve approaches asymptote.
Total mortality (Z) was estimated using length-converted catch curve incorporated in the FISAT II, which is based on the negative exponential model:
where Ni is the number of individuals in length class i; Δti is the time needed to grow through length class i; ti is the age at mean length of class i, and Zt is the instantaneous mortality rate for age t.
Natural mortality rate (M) was calculated using Pauly (1980) equation along with the local annual mean sea surface local temperature from EPD of Hong Kong.
where T is the mean water temperature expressed in °C in each surveyed year.
The fishing mortality (F) was subsequently computed as F = Z − M.
Temporal Comparison of Abundance, Biomass, and Size
The abundance and biomass of P. indicus were compared before and after the trawl ban by Kruskal–Wallis test followed by Dunn’s test, due to the non-normal distribution and heterogeneity variance of the data. The mean values among transects at each month were treated as replicates. Same tests were also applied on individual weight and length of the fish using individual’s data as replicates.
Environmental Variables and Their Relationships With Biomass of Platycephalus indicus
The environmental variables including turbidity (Turb; NTU), DO (mg/L), pH, and Chl-a concentration (μg/L) in seawater samples of the sampling locations measured in 2004, 2013–2014, and 2015–2016 were compared using one-way analysis of variance (ANOVA) followed by post hoc Tukey tests to explore the yearly differences. The sampling year was used as a fixed factor, with the transects in each month as replicates.
Those environmental variables could potentially influence the abundance and/or biomass of benthic communities (Sciberras et al., 2018; Tao et al., 2018). Fish biomass was log10(x + 1) transformed to ensure normal distribution and homoscedasticity of data. Multiple linear regression models with stepwise forward selection, which chooses the best model by the Akaike information criterion (AIC), were used to examine the relationships between the environmental variables (i.e., turbidity, DO, pH, and Chl-a) and the biomass of P. indicus using R package “MASS.” The percentage of variance explained was calculated by dividing the sum of squares of individual factor with the total sum of squares of the model.
Trophic Position and Niche
At each site and sampling year, the mean δ15N among filter-feeding taxa was used as a baseline to estimate the trophic position of P. indicus individuals using the following equation:
where TP is the trophic position of P. indicus, δ15Nf is the δ15N of P. indicus, and δ15Nref is the mean δ15N among filter-feeder species. A trophic enrichment factor (TEF) of 2.9‰ was used, following the suggestion on muscle samples by McCutchan et al. (2003), whereas +2 is the assumed TP of the baseline (Post, 2002). The TPs of P. indicus before and after the trawl ban (i.e., 2012, 2015, and 2018) were compared using one-way ANOVA and the post hoc one-tailed Dunnett’s test (2012 as the control), with P. indicus individuals as replicates.
Isotopic niche of the P. indicus population at each site and sampling year (2012, 2015, and 2018) was quantified by using the Bayesian standard ellipse area (SEAB), which contains approximately 40% of the isotopic hull area of the fish. SEAB is unbiased with respect to samples size (Jackson et al., 2011). One-way ANOVA with the post hoc one-tailed Dunnett’s test was applied on SEAB to investigate whether any increase of the isotopic niche occurred over the study period. The Stable Isotope Bayesian Ellipses (SIBER 2.1.4) statistical package in R 3.6.0 was used to calculate SEAB (Jackson et al., 2011; R Core Team, 2016).
The relative contributions of individual prey taxa to the diet of P. indicus at each site and sampling year were analyzed using the MixSIAR package (Stock and Semmens, 2017) in R. MixSIAR is a Bayesian stable isotope mixing model that uses Markov Chain Monte Carlo method to identify the probability of contributions of food sources to the consumer, taking account of the variances in isotopic signatures of food sources, consumers, TEF, and source elemental concentrations (Parnell et al., 2013). Potential prey taxa were first grouped, and their isotopic values averaged, if they had both similar δ15N and δ13C (i.e., p > 0.05) based on the results from analysis of similarity (ANOSIM) and post hoc pairwise comparisons. The ANOSIM and pairwise comparisons were performed using PRIMER 6.1.5 with PERMANOVA+ (Clarke and Gorley, 2006). In MixSIAR, the TEFs of 0.5 ± 1.313 (mean ± SD) for δ13C and 2.3 ± 1.438 (mean ± SD) for δ15N for all potential prey taxa were used (McCutchan et al., 2003). Models were run for 100,000 iterations with the informative priors (Supplementary Table 2) for P. indicus diet based on Wu (1984) and gut content analysis of our samples.
Results
Growth and Mortality Parameters
Platycephalus indicus contributed to 0.2–5.0% and 1.4–13.9% of total fish abundance and biomass, respectively, in the eastern waters of Hong Kong (Table 1). The estimated ELEFAN parameters, L∞, K, and M for P. indicus varied among the sampling years in both EI and EO. Total mortality and fishing mortality for P. indicus did not differ between 2004 (pre-ban) and 2013–2014 (shortly after the ban) but were reduced in 2015–2016 (3.5 years post-ban) in both sites (Table 1).
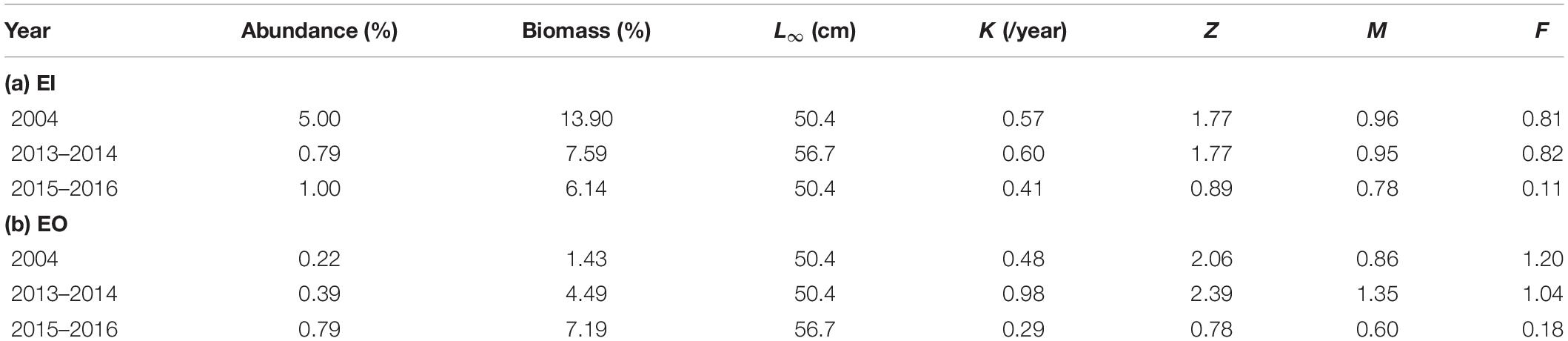
Table 1. Percentages of abundance and biomass (% over total fish abundance/biomass in the catch) and von Bertalanffy growth function (VBGF) growth parameters: asymptotic length (L∞), the growth rate (K), total mortality (Z), natural mortality (M), and fishing mortality (F) for Platycephalus indicus collected in the inner Tolo Channel (EI), outer Tolo Channel (EO) waters of Hong Kong from January 2004 to December 2004 (2004; before the trawl ban), June 2013 to May 2014 (2013–2014; immediately after the trawl ban), and June 2015 to May 2016 (2015–2016; 3.5 years after the trawl ban).
Abundance, Biomass, and Size
In EI, the abundance of P. indicus did not differ between years (Figure 2A). The fish biomass, however, significantly increased by 183.5% from 2004 to 2013–2014 (Figure 2B). Biomass of P. indicus in 2015–2016 was similar to that in the other sampling periods (Figure 2B). The fish weight and length increased significantly by 25.2% and 175.1% from 2004 to 2015–2016, respectively (Figures 2C,D).
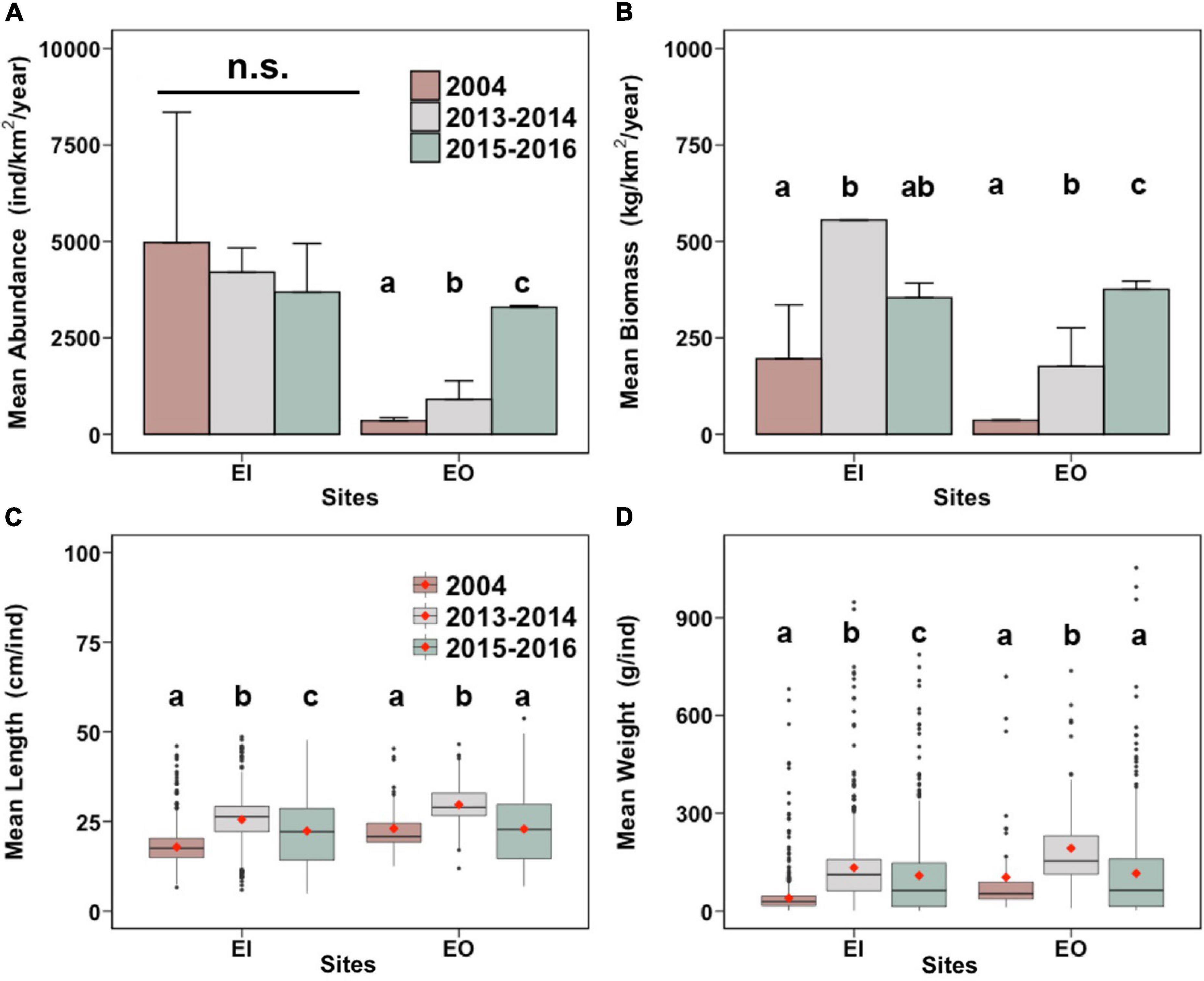
Figure 2. Total annual abundance [(A); mean + SEM; individuals/km2 per year] and total annual biomass [(B); mean + SEM; kg/km2 per year], as well as mean length of individuals [(C); cm/individual], and mean weight of individuals [(D); g/individual] of Platycephalus indicus collected in inner Tolo Channel (EI) and outer Tolo (EO) of Hong Kong from January to December 2004 (before trawling ban), June 2013 to May 2014 (2013–2014; after trawling ban), and June 2015 to May 2016 (2015–2016; after trawling ban). In panels (C,D), the vertical line shows the third quintile (Q) + 1.5 × interquartile range (IQR) to Q1 – 1.5 × IQR of the data. The black horizontal bars and red diamonds in the boxes indicate the medians and the means, respectively. In each panel and site, bars with different letters are significantly different (p < 0.05); n.s. denotes no significant difference between the mean values among the 3 years.
In EO, the abundance and biomass of P. indicus significantly increased over time after the trawl ban; that is, they were the highest in 2015–2016, which increased by 839.6% and 942.6%, respectively (Figures 2A,B). The length and weight of P. indicus also significantly increased by 28.9% and 85.6% after the ban (2013–2014), respectively, but were reduced in 2015–2016 and did not differ from values in 2004 (Figures 2C,D).
Environmental Variables and the Relationships With Biomass of Platycephalus indicus
The annual average turbidity significantly decreased in both EI and EO after the trawl ban (Figure 3A and Supplementary Figure 1). In EI, the average DO level was significantly reduced from 6.4 mg/L in 2004 to approximately 5.0 mg/L in 2015–2016 (Figure 3B). EI also had a slightly lower average pH in 2015–2016 (Figure 3C). Chl-a concentration did not differ among the sampling years (Figure 3D).
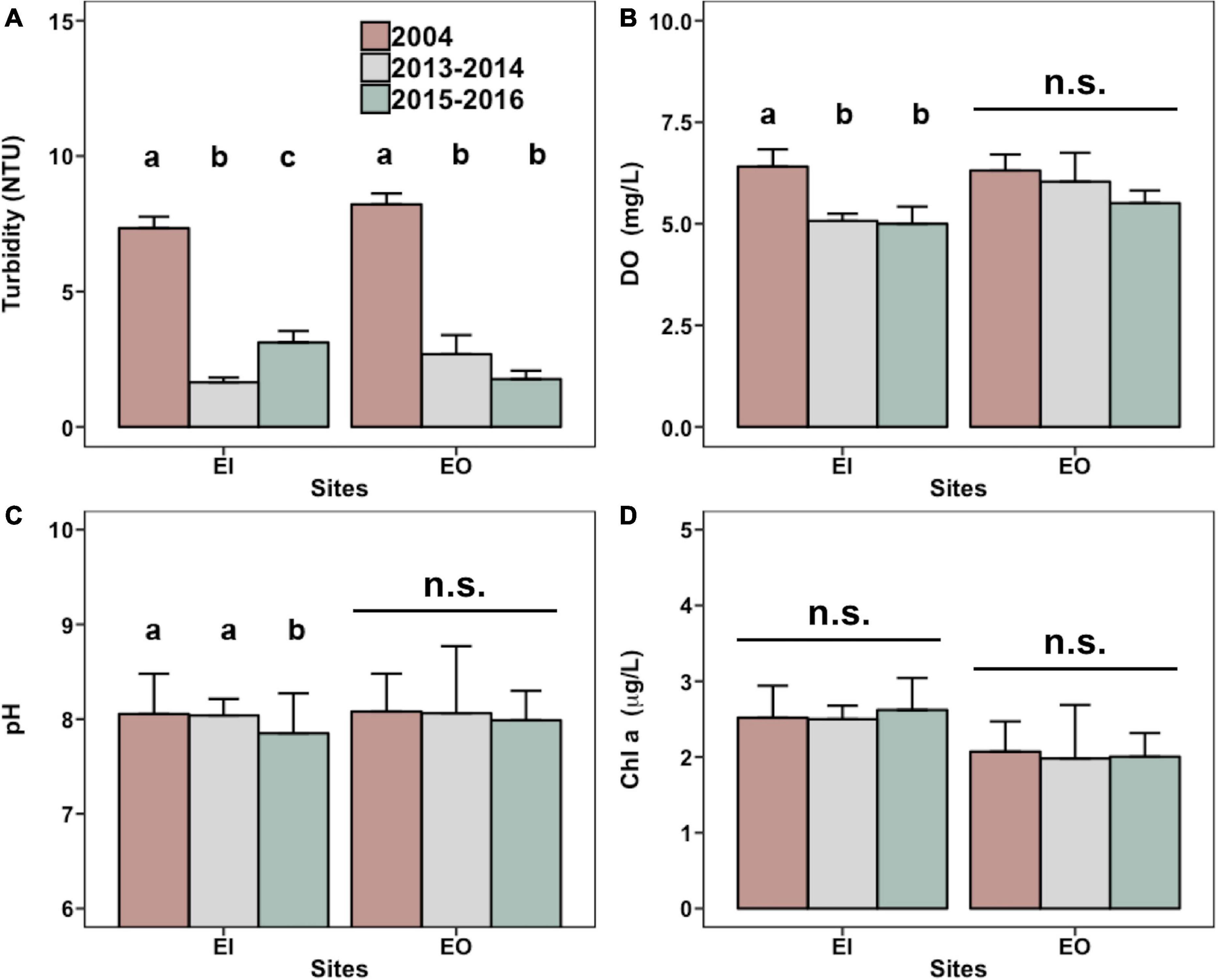
Figure 3. Mean levels (+SEM) of turbidity (A), dissolved oxygen [(DO; (B)], pH (C), and chlorophyll-a [Chl a; (D)] obtained from the Environmental Protection Department of Hong Kong for water samples that were collected in inner Tolo Channel (EI) and outer Tolo (EO) during January 2004 to December 2004 (2004; before the trawl ban), June 2013 to May 2014 (2013–2014; after the trawl ban), and June 2015 to May 2016 (2015–2016; after the trawl ban). In each panel and site, bars with different letters are significantly different (p < 0.05), whereas n.s. denotes no significant difference between the mean values among the 3 years.
Results of multiple linear regression analysis showed that the biomass of P. indicus had significant negative relationships with DO and turbidity in EI (Table 2). DO had an overall higher effect (percentage variance explained: 20.4%) than that of turbidity (percentage variance explained: 12.5%). In EO, the biomass of P. indicus had significant negative relationships with turbidity, DO, and Chl-a concentration, which explained 19.9%, 3.6%, and 5.0% of the total variation, respectively.
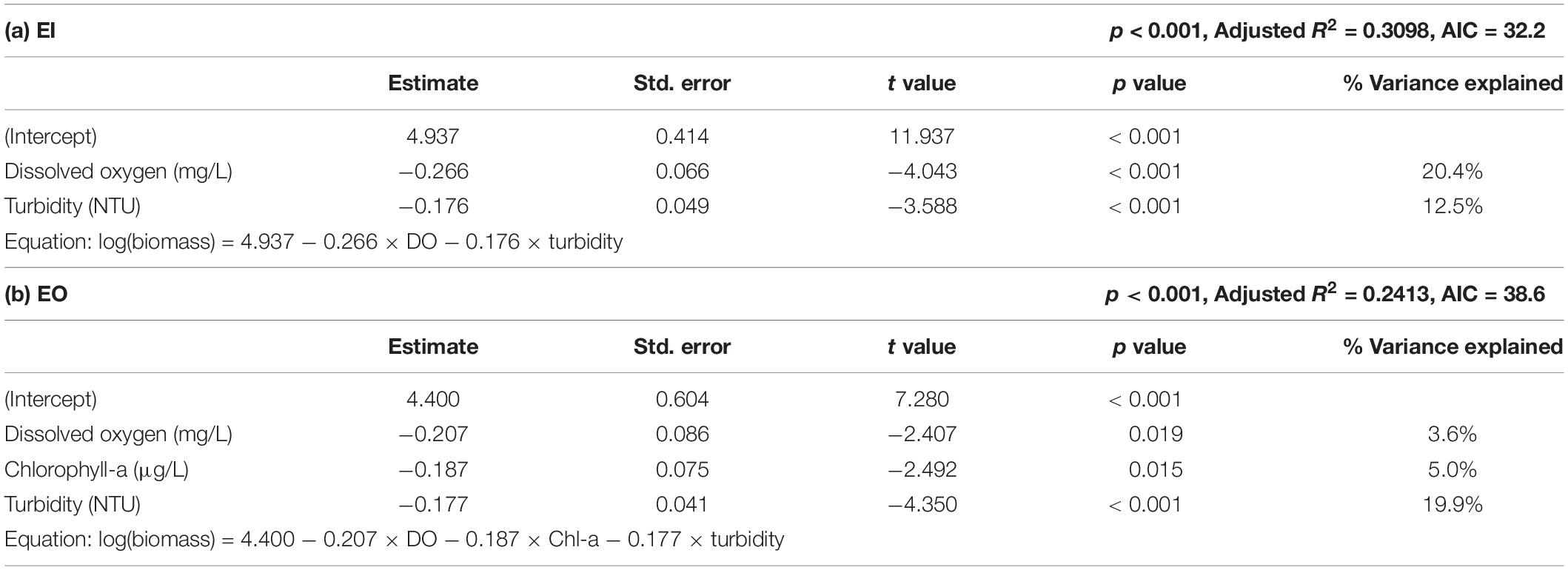
Table 2. Results of stepwise multiple linear regression analysis of Platycephalus indicus biomass (dependent variable) with the environmental parameters (independent variables) in the inner (a; EI) and the outer Tolo Channel (b; EO) of Hong Kong.
Trophic Metrics
The trophic niche of P. indicus in EI significantly expanded by 106% after the trawl ban. Similarly, in EO, trophic niche increased by 45% in 2015 compared to 2012 and then stabilized with no significant difference among 2012 and 2018 (Figures 4A,B). The trophic position of P. indicus in EO significantly increased by 11.5% and 4.1% in 2015 and 2018, respectively. Trophic position in EI was likewise higher in 2015 than in 2012 and 2018 (Figures 4C,D).
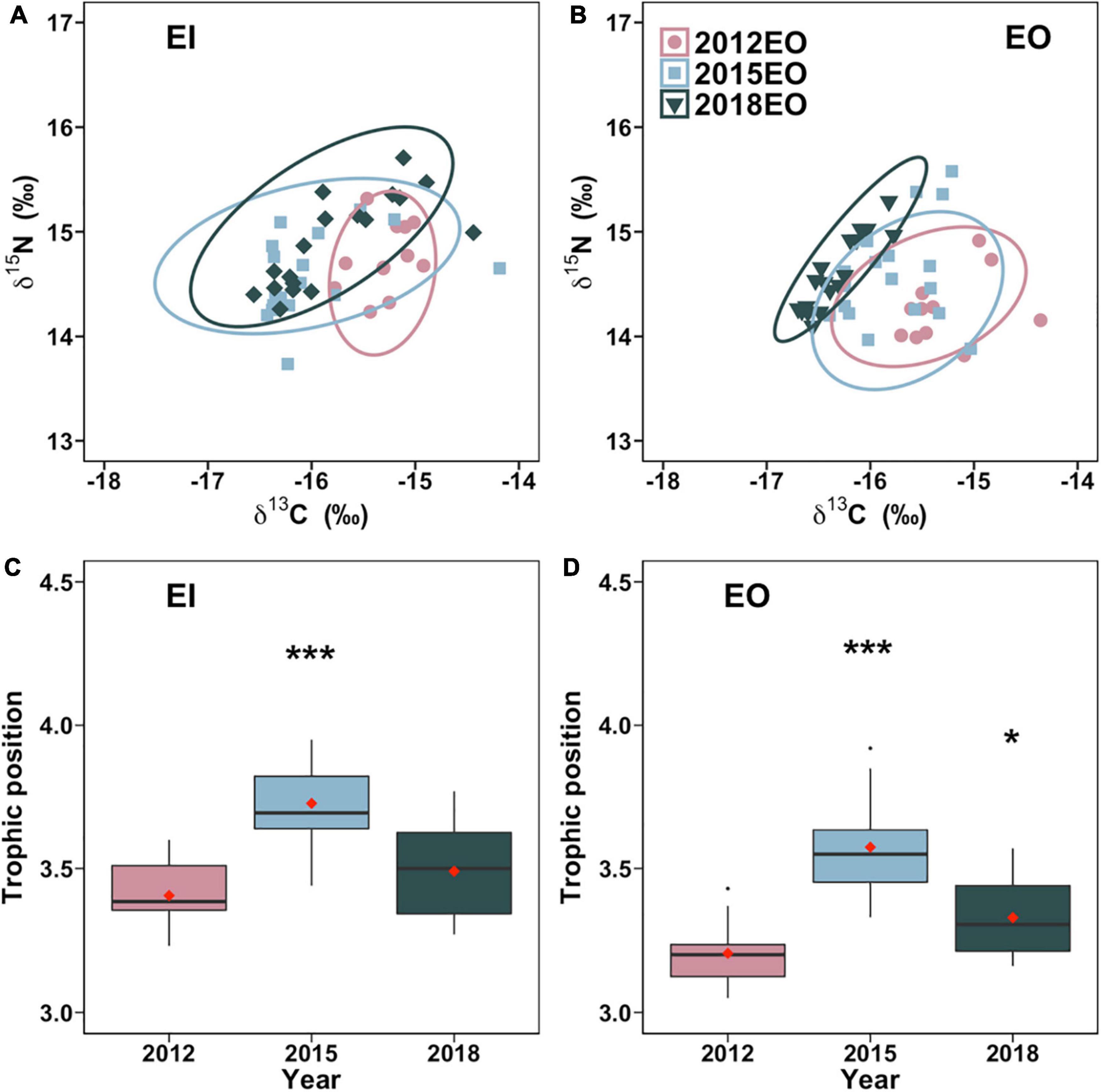
Figure 4. Trophic niche [SEAb; (A,B)] and trophic position (C,D) of Platycephalus indicus collected in inner Tolo Channel (EI) and outer Tolo (EO) of Hong Kong waters from dry season of 2012 (before the trawl ban), 2015 (3 years after the trawl ban), and 2018 (6 years after the trawl ban). Significant differences between 2012 and 2015 and between 2012 and 2018 are indicated by asterisks (*p < 0.05; ***p < 0.01).
The benthic community structure also changed after the trawl ban in both EI and EO, with varied δ13C and δ15N signals of P. indicus and its potential prey taxa among the sampling years (Figure 5). The abundance and biomass of prey species also fluctuated over time (Supplementary Figure 2). Results from the MixSIAR models showed that P. indicus shifted their diet from fishes to crustaceans in both EI and EO after the ban, and such a dietary shift was comparatively faster in P. indicus in EO than in EI (Figure 6). In EI, fishes from Callionymidae constituted the major diet of P. indicus in 2012 and 2015, whereas the diet contribution from the shrimp Metapenaeopsis species gradually increased after the ban and became the dominant food source of P. indicus in 2018 (Supplementary Figure 3). Similarly, P. indicus in EO shifted its diet from Callionymidae to Metapenaeopsis species after the ban (Supplementary Figure 3).
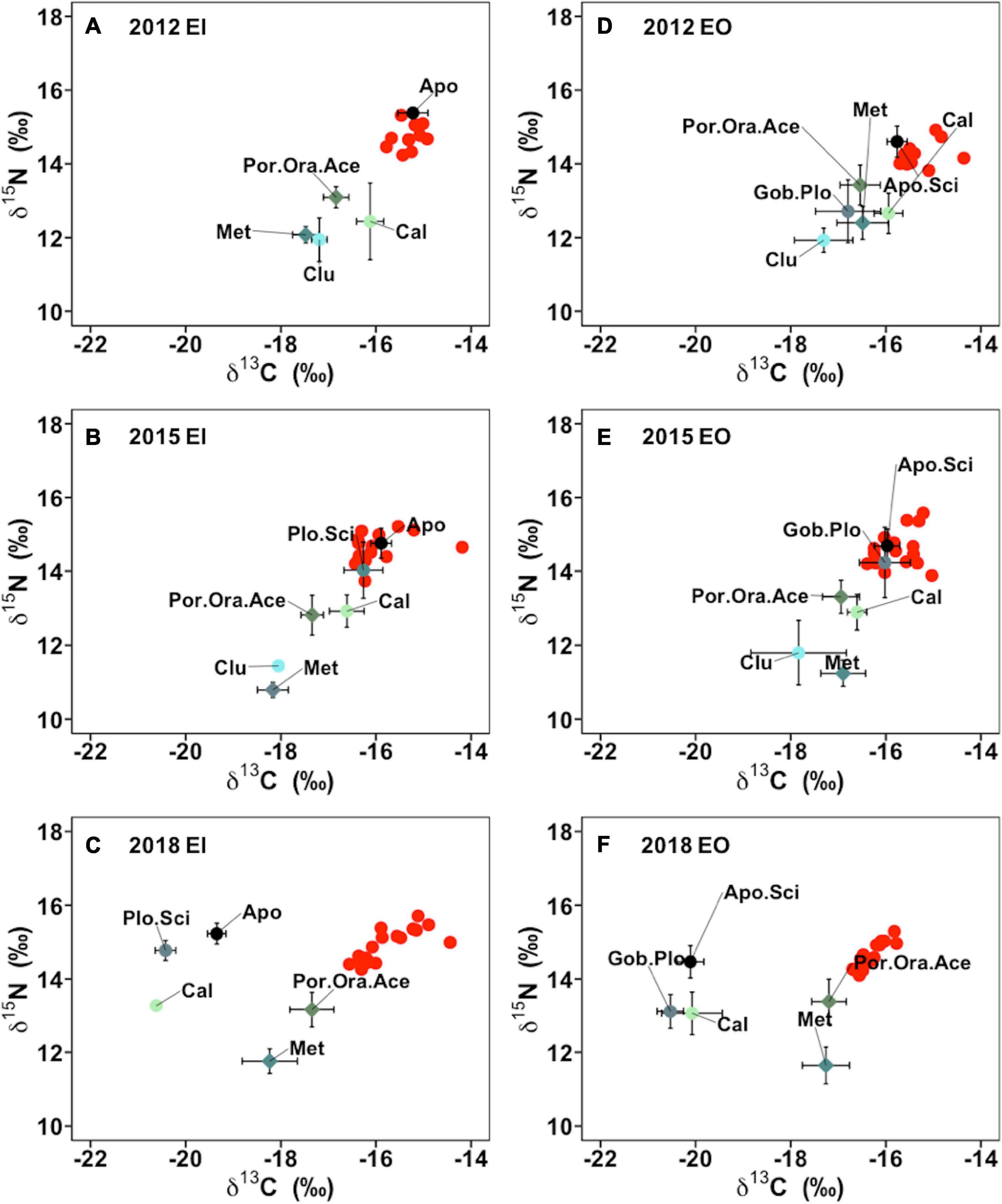
Figure 5. Isotope signatures of δ13C and of δ15N of Platycephalus indicus (red dots) and its potential food source (mean ± SEM) collected in inner Tolo Channel (EI) (A–C) and outer Tolo (EO) (D–F) from dry season of 2012, 2015, and 2018. Grouped species abbreviations in EI and EO: Apo, species from family Apogonidae; Apo.Sci, species from family Apogonidae and Sciaenidae; Cal, species from family Callionymidae; Clu, species from family Clupeidae; Gob.Plo, species from family Gobiidae and Plotosidae; Met, Metapenaeopsis sp.; Plo.Sci, species from family Plotosidae and Sciaenidae; Por.Ora.Ace, including species of Portunus dayawanensis, Portunus pseudohastatoides, Oratosquilla oratoria, and Acetes species.
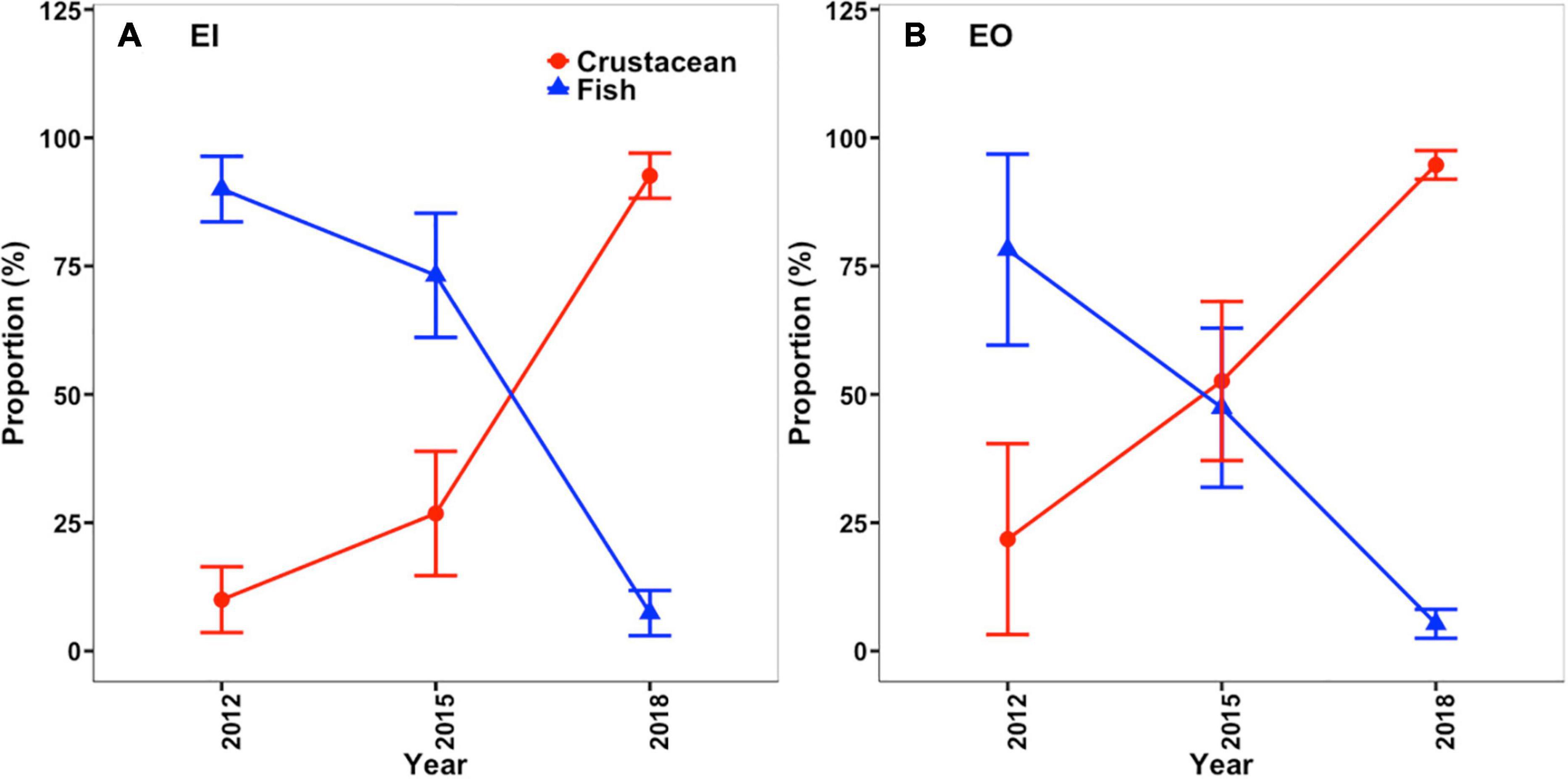
Figure 6. Changes in diet composition of Platycephalus indicus in terms the percentage of crustacean (red line) and fish (blue line) prey items in their diet in inner Tolo channel [EI; mean ± SD; (A)] and outer Tolo [EO; mean ± SD; (B)] before (2012) and after (2015 and 2018) the trawl ban, according to the values in the posterior distributions estimated using the MixSIAR models.
Discussion
Our results demonstrated that the population of P. indicus in eastern Hong Kong waters showed signs of recovery, and the benthic community structure also changed after the trawl ban. After the trawl ban, higher abundance, greater biomass, and broader isotopic niche were observed in outer Tolo area (EO), whereas P. indicus had a larger size and shifted to higher trophic positions in inner Tolo area (EI). Such changes were likely driven by reduction in fishing mortality, recovery of the lower trophic level species, and shifted dietary composition of P. indicus after the trawl ban.
Changes in Population Structure and Environmental Conditions After the Trawl Ban
At EI, the population growth rate (K), total mortality (Z), and natural mortality (M) were constant between 2004 and 2013–2014, but they decreased in 2015–2016. At EO, K, Z, and M increased in 2013–2014 and then declined in 2015–2016. These biological parameters were estimated based on the length frequency data which could be influenced by both fishing pressure and environmental factors.
Increases in abundance and biomass have been demonstrated in several fishes (Badalamenti et al., 2002; Fiorentino et al., 2008) and invertebrates (Kelly et al., 2000; Hoskin et al., 2011) after implementing a trawl ban. Similarly, increases in abundance and biomass of P. indicus in EO area of Hong Kong could be attributed to decreased fishing pressure due to the banning of trawling activities (Fiorentino et al., 2008; Fanelli et al., 2010). Such an explanation is evident in our study, which demonstrated decreases in fishing mortality on P. indicus after the trawl ban.
In this study, P. indicus generally increased in size in the study sites after the trawl ban, despite an insignificant increase in 2015–2016 in EO area. Similarly, the size of four predatory mantis shrimp species from the western waters of Hong Kong also increased significantly 3.5 years after the trawl ban (Tao et al., 2018), whereas the length of the red mullet Mullus barbatus increased after 14 years of closure to trawling in the Gulf of Castellammare in Italy (Fiorentino et al., 2008). In addition to trawl bans, body sizes and biomass of fishes were also found to be significantly increased after establishment of marine protected areas (Lester et al., 2009). Those findings, together with our study, advocate that some commercially important species can benefit from management interventions, such as establishing marine protected areas and a trawl ban policy.
As regular bottom trawling activities frequently stir up the sediment to increase turbidity and reduce organic matter to be retained in sediment, prohibition of trawling can lead to a substantial reduction of physical disturbance on the seabed (Fiorentino et al., 2008; Fanelli et al., 2010). This study detected that the trawl ban induced some positive changes in environmental conditions, such as significant decreases in water turbidity in both surveyed sites. A parallel study also confirmed that there were significant increases in total organic matter and chemical oxygen demand in surface sediment samples across the marine environment of Hong Kong after the trawl ban (Wang et al., 2021). Such changes in the turbidity and sedimentary organic matter clearly indicate a markedly reduction of physical disturbance on the benthic habitat. Past studies demonstrated that capture rates of predators decreased with an increase in turbidity (Ortega et al., 2020); however, piscivorous fishes tend to consume more prey and possess greater population sizes in low turbidity conditions (De Robertis et al., 2003; Wenger et al., 2013). Our study also concurred with the above findings by showing a negative relationship between the biomass of P. indicus and turbidity, suggesting that P. indicus improved their foraging efficiency under less turbid environment.
Unexpectedly, this study observed that the average DO level significantly decreased in EI area from 6.4 mg/L in 2004 to approximately 5.0 mg/L in 2015–2016 after the trawl ban, which might be attributable to unchanged abundance of P. indicus. The results of our multiple regression analysis also indicated that DO has an overall higher effect on the biomass of P. indicus in EI. It is postulated that the positive effects of the trawl ban on P. indicus recovery might be counteracted by the negative effects of decreased DO in EI. Marshall and Elliott (1998) found that the abundance of the common sole (Solea solea) was negatively correlated with DO. Similar findings were also recorded for the European eel (Anguilla anguilla) with a maximum abundance in the Elbe Estuary when the DO level was only 3.5 mg/L (Thiel et al., 1995). Some burrowing benthic infauna, such as polychaetes and shrimp species (e.g., Metapenaeopsis sp.), emerge from sediments to the bottom or upper water layer to obtain oxygen during hypoxia condition (Petersen and Pihl, 1995; Wei et al., 2009), thus increasing the chances of being preyed upon and enhancing food supply to benthic fishes (Breitburg, 2002). In Tolo Harbor and Channel, periodical hypoxia events annually occurred in June–September since 1980s as a result of severe organic pollution and harmful algal blooms (Wu, 1982; Lee and Arega, 1999; Supplementary Figure 1). Having experienced long-term low DO conditions, the Bartail flathead may have adapted to such a stressful environment by adjusting their feeding grounds and habits, but could not expand the population size via recruitment of juvenile fishes.
Changes in Trophic Metrics After the Trawl Ban
Imposing restrictions on fishing activities could lead to changes in trophic structure and function, such as increases in trophic levels of fish populations (Badalamenti et al., 2002; Fanelli et al., 2010) and of the entire fish community (Coleman et al., 2015). Our study observed an increase of trophic position of P. indicus in EO in both 2015 and 2018 (after the trawl ban), but such an increase was observed only in 2015 in EI. The elevated trophic position in this predatory fish species was likely linked to increased body size after the trawl ban, as large fish feed on large-sized prey items, leading to an increase in δ15N signature in their tissues. Concurrently, the trophic position of carnivorous crustaceans also increased significantly after the trawl ban in the western waters of Hong Kong (Tao et al., 2020). Hamilton et al. (2014) also demonstrated that a niche expansion and elevated δ15N in the California sheephead (S. pulcher) were attributable to the increases in its population size structure that the large sheepheads were able to feed on large-sized prey items. Likewise, our study also found expanded isotopic niche of P. indicus in EI after the trawl ban, whereas such an expansion was only prominent in 2015 in EO area. Nevertheless, such an expanded isotopic niche in P. indicus cannot be simply explained by shifting the dietary composition to consume more crustaceans (i.e., Metapenaeopsis sp.).
Two more possible pathways might also be attributable to the expanded isotopic niche in P. indicus, which were not revealed by MixSIAR model. First, cannibalism was observed in population of P. indicus in Tolo Harbor, important nursery and feeding grounds for P. indicus (Ho, 2005). As a result of increased size in EI and the inflated abundance and biomass in EO, the chance of cannibalism in P. indicus may have increased after the trawl ban (Claessen et al., 2004). However, the size range of the victim of P. indicus, potentially eaten by cannibals, is rarely known (Claessen et al., 2004) and thus taking the cannibalism into account in the MixSIAR model was impractical. Moreover, intraspecific competition in P. indicus after the trawl ban may have led to those less competitive individuals exploring less preferable food resources and thereby expanded their niches (Bolnick, 2001; Bolnick et al., 2007). Second, decreases in disturbance of the benthic floor and fishing mortality on benthic fauna after the trawl ban might have altered the energy flow, trophic structure, and prey availability of the ecosystem (McHugh et al., 2010; Hiddink et al., 2011; Hinz et al., 2017). The emergence of new abundant prey species in the food web (Post and Takimoto, 2007), which benefited from the trawl ban in the short term, such as polychaetes and crustaceans (Kaiser et al., 2006), was likely consumed more frequently by P. indicus. Our results confirmed that P. indicus preyed on more shrimps after the trawl ban. Badalamenti et al. (2002) also suggested that the trophic level of M. barbatus was higher in the trawl-ban area due to increased consumption of carnivorous polychaetes.
In the present study, the isotopic positions of Apogonidae and Sciaenidae, which competed for similar food sources with P. indicus (Froese and Pauly, 2019) and occupied similar isotopic positions in the food web, shifted to more depleted δ13C in 2018 comparing to that of 2012 in both study sites (Figure 5). Such niche differentiation and partitioning could alleviate interspecific competitions among Apogonidae, Sciaenidae, and P. indicus, likely governed by increased abundance and biomass of those fishes synergistically with increases in prey diversity after the trawl ban (Sánchez-Hernández et al., 2017; Eurich et al., 2018). Similarly, species from Plotosidae, Callionymidae, and Gobiidae also shifted their isotopic positions to more depleted δ13C, implying that the trophic structure of benthic community in the study area could have been reshaped after the trawl ban.
Implications for Management
Previous studies suggested that full protection from fishing (i.e., no-take zone) is considered as a more effective way to promote biodiversity and fishery resources (Roberts et al., 2005; Lester and Halpern, 2008; Sciberras et al., 2015). Espinosa-Andrade et al. (2020) also highlighted that restriction of all fishing efforts by establishing no-take zone would lead to greater benefits on the community than that of partial protection or without protection area. To fully restore the fisheries resources and biodiversity in Hong Kong water, supplementary fishery management policy, such as expanding the marine protected area in local waters, regulating non-selective fishing gears (e.g., trammel nets and snake cages), strengthening enforcement again illegal fishing, and enacting seasonal moratorium, will be needed (World Wildlife Fund, 2018). The Committee on Sustainable Fisheries of the Hong Kong SAR Government has proposed to designate Port Shelter and Tolo Harbor as fisheries protection areas, which will further reduce the fishing pressure and allow rehabilitation of predatory fish species such as P. indicus, and the Agriculture, Fisheries and Conservation Department is conducting a feasibility study for implementation of this proposal (Agriculture, Fisheries and Conservation Department, 2013). The results presented in this study would serve as a useful reference baseline to evaluate the effectiveness of the fisheries protection area at Tolo Harbor on the improvement of the population fitness of P. indicus in the future.
Conclusion
Our study demonstrated that the isotopic trophic metrics in tandem with population measurements provided insightful information to evaluate the effectiveness of the trawl ban for facilitating the recovery of the population of P. indicus in Hong Kong waters. Evidently, the trawl ban policy promoted the recovery of this population, as reflected by their larger size, higher biomass, elevated trophic position, and broader niches in inner and outer Tolo Channel of Hong Kong. Those changes could be associated with a dietary shift and arrival of new prey species in the ecosystem. This study highlights the benefits of a trawl ban policy in restoring benthic ecosystems and fisheries resources following intensive fishing activities. As a full recovery from the trawl ban is expected to take a decade (Lotze et al., 2011), long-term monitoring is required to fully ascertain the dynamics of the ecosystem recovery process.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the animal study because the trawling survey conducted in the present study was funded by a Research Grants Council of the Government of the Hong Kong Special Administrative Region (HKSAR) via a Collaborative Research Fund (CRF Project No. HKU5/CRF/12G and C7050-18EF) to KL for the purposes of investigating the effects of the trawl ban. According to Animals (Control of Experiments) Ordinance (Cap. 340) in Hong Kong (please see the attached link), which is the only regulation on the use of experimental animals, this study didn’t need to comply with the regulation. The definition for “experiment” in the regulation is “any experiment performed on an animal and calculated to give pain.” All samples utilized in this study were collected from trawl surveys granted by Agriculture, Fisheries and Conservation Department of Hong Kong Special Administrative Region (HKSAR) with a scientific research permit (R1710007 and R1710045). All the animals captured by trawl surveys had already been dead on board before being brought back to the lab for counting and measuring. Under such circumstances, we don’t have any guidelines and regulations to comply with Cap. 340 in Hong Kong: https://www.elegislation.gov.hk/hk/cap340!en?INDEX_CS=Nxpid=ID_1438403033682_004.
Author Contributions
KL and LT initiated and designed this study. LT, VH, YM, and RS conducted the experiments. LT drafted the main manuscript text. KL supervised the work, and contributed to the data analysis and interpretation, as well as manuscript preparation. TH, YM, RS, and DL improved on the methods of data analyses and result interpretation. All authors reviewed the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This research was substantially funded by the Research Grants Council of the Hong Kong SAR Government, Hong Kong, China via two grants from its Collaborative Research Fund scheme (CRF project numbers: HKU5/CRF/12G and C7050-18EF) to KL. VH, LT, and YM would like to thank the University of Hong Kong (HKU) for partially funding their research postgraduate study.
Acknowledgments
The authors would like to thank Helen Leung for her excellent technical support, and S. F. Leung, Director of Agriculture, Fisheries and Conservation Department for granting us a scientific research permit (R1710007 and R1710045) for conducting sampling using a shrimp trawler. The authors would also like to thank Archer Wong, Denis Chan, Kevin Ho, Kingsley Wong, Matthew Perkins, Racliffe Lai, Jason Yau, and Stella Wong for their assistance in the field survey and laboratory work. The authors would also further like to thank Thea Bradford for proofreading and improving the use of English on a draft of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.614219/full#supplementary-material
Footnotes
References
Agriculture, Fisheries and Conservation Department (2006). Port Survey 2006. Agriculture, Fisheries and Conservation Department, The Government of the Hong Kong Special Administrative Region, Hong Kong, China.
Agriculture, Fisheries and Conservation Department (2011). Legislative Proposals to Take Forward the Trawl Ban and Other Fisheries Management Measures. Available online at: https://www.afcd.gov.hk/english/fisheries/fish_cap/fish_cap_con/files/LEGOEng.pdf (accessed October 5, 2020)
Agriculture, Fisheries and Conservation Department (2013). Report of the Committee on Sustainable Fisheries. Available online at: https://www.afcd.gov.hk/english/fisheries/fish_cap/fish_cap_con/files/common/CSF_WP_10_01_2013Eng.pdf (accessed October 5, 2020)
Badalamenti, F., D’anna, G., Pinnegar, J. K., and Polunin, N. V. C. (2002). Size-related trophodynamic changes in three target fish species recovering from intensive trawling. Mar. Biol. 141, 561–570. doi: 10.1007/s00227-002-0844-3
Bergman, M. J. N., Ubels, S. M., Duineveld, G. C. A., and Meesters, E. W. G. (2015). Effects of a 5-year trawling ban on the local benthic community in a wind farm in the Dutch coastal zone. ICES J. Mar. Sci. 72, 962–972. doi: 10.1093/icesjms/fsu193
Board, O. S., and Council, N. R. (2006). Dynamic Changes in Marine Ecosystems: Fishing, Food Webs, and Future Options. Washington, DC: National Academy Press.
Bolnick, D. I. (2001). Intraspecific competition favours niche width expansion in Drosophila melanogaster. Nature 410, 463–466. doi: 10.1038/35068555
Bolnick, D. I., Svanbäck, R., Araújo, M. S., and Persson, L. (2007). Comparative support for the niche variation hypothesis that more generalized populations also are more heterogeneous. Proc. Natl. Acad. Sci. U.S.A. 104, 10075–10079. doi: 10.1073/pnas.0703743104
Breitburg, D. (2002). Effects of hypoxia, and the balance between hypoxia and enrichment, on coastal fishes and fisheries. Estuaries 25, 767–781. doi: 10.1007/bf02804904
Cheung, W. W. L., and Sadovy, Y. (2004). Retrospective evaluation of data-limited fisheries: a case from Hong Kong. Rev. Fish Biol. Fish. 14, 181–206. doi: 10.1007/s11160-004-5422-y
Claessen, D., De Roos, A. M., and Persson, L. (2004). Population dynamic theory of size-dependent cannibalism. Proc. R. Soc. B 271, 333–340. doi: 10.1098/rspb.2003.2555
Coleman, M., Bates, A., Stuart-Smith, R., Malcolm, H., Harasti, D., Jordan, A., et al. (2015). Functional traits reveal early responses in marine reserves following protection from fishing. Divers. Distrib. 21, 876–887. doi: 10.1111/ddi.12309
Collie, J., Hiddink, J. G., Van Kooten, T., Rijnsdorp, A. D., Kaiser, M. J., Jennings, S., et al. (2017). Indirect effects of bottom fishing on the productivity of marine fish. Fish Fish. 18, 619–637. doi: 10.1111/faf.12193
De Robertis, A., Ryer, C. H., Veloza, A., and Brodeur, R. D. (2003). Differential effects of turbidity on prey consumption of piscivorous and planktivorous fish. Can. J. Fish. Aquat. Sci. 60, 1517–1526. doi: 10.1139/f03-123
Defew, E., Wood, C., Bates, R., Wilson, L., and Wilson, J. (2012). An Assessment of the Potential Impact of No-take Zones upon Benthic Habitats: A Case Study from SE Scotland. London: The Crown Estate, 37.
Dimarchopoulou, D., Dogrammatzi, A., Karachle, P. K., and Tsikliras, A. C. (2018). Spatial fishing restrictions benefit demersal stocks in the northeastern Mediterranean Sea. Sci. Rep. 8, 5967.
Eayrs, S., Hai, N. P., and Ley, J. (2007). Assessment of a juvenile and trash excluder device in a Vietnamese shrimp trawl fishery. ICES J. Mar. Sci. 64, 1598–1602. doi: 10.1093/icesjms/fsm123
Environmental Protection Department (2018). Marine Water Quality in Hong Kong in 2017. Available online at: https://www.epd.gov.hk/epd/sites/default/files/epd/english/environmentinhk/water/hkwqrc/files/waterquality/annual-report/marinereport2017.pdf (accessed October 5, 2020)
Environmental Resource Management (1998). Fisheries Resources and Fishing Operations in Hong Kong Waters, Final Report. Hong Kong: Prepared for Agriculture and Fisheries Department, Hong Kong Special Administrative Region Government.
Espinosa-Andrade, N., Suchley, A., Reyes-Bonilla, H., and Alvarez-Filip, L. (2020). The no-take zone network of the Mexican Caribbean: assessing design and management for the protection of coral reef fish communities. Biodivers. Conserv. 29, 2069–2087. doi: 10.1007/s10531-020-01966-y
Eurich, J. G., Mccormick, M. I., and Jones, G. P. (2018). Direct and indirect effects of interspecific competition in a highly partitioned guild of reef fishes. Ecosphere 9:e02389.
Fanelli, E., Badalamenti, F., D’anna, G., Pipitone, C., and Romano, C. (2010). Trophodynamic effects of trawling on the feeding ecology of pandora, Pagellus erythrinus, off the northern Sicily coast (Mediterranean Sea). Mar. Freshw. Res. 61, 408–417. doi: 10.1071/mf09049
Fiorentino, F., Badalamenti, F., D’anna, G., Garofalo, G., Gianguzza, P., Gristina, M., et al. (2008). Changes in spawning-stock structure and recruitment pattern of red mullet, Mullus barbatus, after a trawl ban in the Gulf of Castellammare (central Mediterranean Sea). ICES J. Mar. Sci. 65, 1175–1183. doi: 10.1093/icesjms/fsn104
Fish Marketing Organization (2019). Wholesale Prices of Fresh Marine Fish. Available online at: https://www.fmo.org.hk/price?path=50 (accessed October 5, 2020)
Fisher, J.a.D, and Frank, K. T. (2002). Changes in finfish community structure associated with an offshore fishery closed area on the Scotian Shelf. Mar. Ecol. Prog. Ser. 240, 249–265. doi: 10.3354/meps240249
Froese, R., and Pauly, D. (2019). FishBase. World Wide Web Electronic Publication. Available online at: http://www.fishbase.org/ (accessed October 5, 2020)
Hamilton, S. L., Newsome, S. D., and Caselle, J. E. (2014). Dietary niche expansion of a kelp forest predator recovering from intense commercial exploitation. Ecology 95, 164–172. doi: 10.1890/13-0014.1
Hiddink, J. G., Jennings, S., Sciberras, M., Szostek, C. L., Hughes, K. M., Ellis, N., et al. (2017). Global analysis of depletion and recovery of seabed biota after bottom trawling disturbance. Proc. Natl. Acad. Sci. U.S.A. 114, 8301–8306. doi: 10.1073/pnas.1618858114
Hiddink, J. G., Johnson, A. F., Kingham, R., and Hinz, H. (2011). Could our fisheries be more productive? Indirect negative effects of bottom trawl fisheries on fish condition. J. Appl. Ecol. 48, 1441–1449. doi: 10.1111/j.1365-2664.2011.02036.x
Hinz, H., Moranta, J., Balestrini, S., Sciberras, M., Pantin, J. R., Monnington, J., et al. (2017). Stable isotopes reveal the effect of trawl fisheries on the diet of commercially exploited species. Sci. Rep. 7:6334.
Hinz, H., Prieto, V., and Kaiser, M. J. (2009). Trawl disturbance on benthic communities: chronic effects and experimental predictions. Ecol. Appl. 19, 761–773. doi: 10.1890/08-0351.1
Ho, C. M. (2005). Biology and Fishery of the Bartail Flathead, Platycephalus Indicus (Linnaeus, 1758), in the Northern South China Sea. [M.Phil. thesis]. Hong Kong: The University of Hong Kong.
Hoskin, M. G., Coleman, R. A., Von Carlshausen, E., and Davis, C. M. (2011). Variable population responses by large decapod crustaceans to the establishment of a temperate marine no-take zone. Can. J. Fish. Aquat. Sci. 68, 185–200. doi: 10.1139/f10-143
Jackson, A. L., Inger, R., Parnell, A. C., and Bearhop, S. (2011). Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 80, 595–602. doi: 10.1111/j.1365-2656.2011.01806.x
Jackson, J. B., Kirby, M. X., Berger, W. H., Bjorndal, K. A., Botsford, L. W., Bourque, B. J., et al. (2001). Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–637. doi: 10.1126/science.1059199
Jennings, S., Pinnegar, J. K., Polunin, N. V. C., and Warr, K. J. (2001). Impacts of trawling disturbance on the trophic structure of benthic invertebrate communities. Mar. Ecol. Prog. Ser. 213, 127–142. doi: 10.3354/meps213127
Johnson, A. F., Gorelli, G., Jenkins, S. R., Hiddink, J. G., and Hinz, H. (2015). Effects of bottom trawling on fish foraging and feeding. Proc. R. Soc. B 282:20142336. doi: 10.1098/rspb.2014.2336
Kaiser, M. J., Clarke, K. R., Hinz, H., Austen, M. C. V., Somerfield, P. J., and Karakassis, I. (2006). Global analysis of response and recovery of benthic biota to fishing. Mar. Ecol. Prog. Ser. 311, 1–14. doi: 10.3354/meps311001
Kaiser, M. J., Hormbrey, S., Booth, J. R., Hinz, H., and Hiddink, J. G. (2018). Recovery linked to life history of sessile epifauna following exclusion of towed mobile fishing gear. J. Appl. Ecol. 55, 1060–1070. doi: 10.1111/1365-2664.13087
Kelly, S., Scott, D., Macdiarmid, A. B., and Babcock, R. C. (2000). Spiny lobster, Jasus edwardsii, recovery in New Zealand marine reserves. Biol. Conserv. 92, 359–369. doi: 10.1016/s0006-3207(99)00109-3
Kratina, P., Lecraw, R. M., Ingram, T., and Anholt, B. R. (2012). Stability and persistence of food webs with omnivory: is there a general pattern? Ecosphere 3, 1–18.
Lau, D. C. P., Vrede, T., and Goedkoop, W. (2017). Lake responses to long−term disturbances and management practices. Freshwat. Biol. 62, 792–806. doi: 10.1111/fwb.12902
Lee, J. H. W., and Arega, F. (1999). Eutrophication dynamics of Tolo Harbour, Hong Kong. Mar. Pollut. Bull. 39, 187–192. doi: 10.1016/s0025-326x(99)00007-7
Lester, S. E., and Halpern, B. S. (2008). Biological responses in marine no-take reserves versus partially protected areas. Mar. Ecol. Prog. Ser. 367, 49–56. doi: 10.3354/meps07599
Lester, S. E., Halpern, B. S., Grorud-Colvert, K., Lubchenco, J., Ruttenberg, B. I., Gaines, S. D., et al. (2009). Biological effects within no-take marine reserves: a global synthesis. Mar. Ecol. Prog. Ser. 384, 33–46. doi: 10.3354/meps08029
Lotze, H. K., Coll, M., Magera, A. M., Ward-Paige, C., and Airoldi, L. (2011). Recovery of marine animal populations and ecosystems. Trends Ecol. Evol. 26, 595–605. doi: 10.1016/j.tree.2011.07.008
Mak, Y. K. Y. (2017). Effects of Trawl Ban on Demersal Fish Communities in the Marine Environment of Hong Kong, South China. [Ph.D thesis]. Hong Kong: The University of Hong Kong.
Marshall, S., and Elliott, M. (1998). Environmental influences on the fish assemblage of the Humber estuary, UK. Estuar. Coast. Shelf. Sci. 46, 175–184. doi: 10.1006/ecss.1997.0268
McCutchan, J. H., Lewis, W. M., Kendall, C., and Mcgrath, C. C. (2003). Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102, 378–390. doi: 10.1034/j.1600-0706.2003.12098.x
McHugh, P. A., Mcintosh, A. R., and Jellyman, P. G. (2010). Dual influences of ecosystem size and disturbance on food chain length in streams. Ecol. Lett. 13, 881–890. doi: 10.1111/j.1461-0248.2010.01484.x
Mirzaei, M. R., Azhang, B., and Kazemi, S. (2017). Evaluation of demersal trawl survey data for assessing the Biomass and Catch Per Unit Area (CPUA) of Platycephalidae. Res. Mar. Sci. 2, 59–64.
Morton, B., and Morton, J. (1983). The Sea Shore Ecology of Hong Kong. Hong Kong: Hong Kong University Press.
Murawski, S. A., Brown, R., Lai, H. L., Rago, P. J., and Hendrickson, L. (2000). Large-scale closed areas as a fishery-management tool in temperate marine systems: the Georges Bank experience. Bull. Mar. Sci. 66, 775–798.
Myers, R. A., and Worm, B. (2003). Rapid worldwide depletion of predatory fish communities. Nature 423, 280–283. doi: 10.1038/nature01610
Myers, R. A., and Worm, B. (2005). Extinction, survival or recovery of large predatory fishes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360, 13–20. doi: 10.1098/rstb.2004.1573
Ortega, J. C. G., Figueiredo, B. R. S., da Grace, W. J., Agostinho, A. A., and Bini, L. M. (2020). Negatve effect of turbidiy on prey capture for both visual and non-visual aquatic predators. J. Animal Ecol. 89, 2427–2439. doi: 10.1111/1365-2656.13329
Parnell, A. C., Phillips, D. L., Bearhop, S., Semmens, B. X., Ward, E. J., Moore, J. W., et al. (2013). Bayesian stable isotope mixing models. Environmetrics 24, 387–399.
Pauly, D. (1980). On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES J. Mar. Sci. 39, 175–192. doi: 10.1093/icesjms/39.2.175
Petersen, J. K., and Pihl, L. (1995). Responses to hypoxia of plaice, Pleuronectes platessa, and dab, Limanda limanda, in the south-east Kattegat: distribution and growth. Environ. Biol. Fishes 43, 311–321. doi: 10.1007/bf00005864
Pipitone, C., Badalamenti, F., D’anna, G., Whitmarsh, D., James, C., and Pickering, H. (2000). Trawling Ban in the Gulf of Castellammare: Effects on the Small Scale Fishery Economics and on the Abundance of Fish. Project Report. Castellammare del Golfo: CNR-IRMA.
Post, D. M. (2002). Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83, 703–718. doi: 10.1890/0012-9658(2002)083[0703:usitet]2.0.co;2
Post, D. M., and Takimoto, G. (2007). Proximate structural mechanisms for variation in food−chain length. Oikos 116, 775–782. doi: 10.1111/j.0030-1299.2007.15552.x
Quevedo, M., Svanbäck, R., and Eklöv, P. (2009). Intrapopulation niche partitioning in a generalist predator limits food web connectivity. Ecology 90, 2263–2274. doi: 10.1890/07-1580.1
R Core Team. (2016). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Comuputing.
Roberts, C. M., Hawkins, J. P., and Gell, F. R. (2005). The role of marine reserves in achieving sustainable fisheries. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360, 123–132. doi: 10.1098/rstb.2004.1578
Sánchez-Hernández, J., Gabler, H. M., and Amundsen, P. A. (2017). Prey diversity as a driver of resource partitioning between river-dwelling fish species. Ecol. Evol. 7, 2058–2068. doi: 10.1002/ece3.2793
Sciberras, M., Hiddink, J. G., Jennings, S., Szostek, C. L., Hughes, K. M., Kneafsey, B., et al. (2018). Response of benthic fauna to experimental bottom fishing: a global meta−analysis. Fish Fish. 19, 698–715. doi: 10.1111/faf.12283
Sciberras, M., Jenkins, S. R., Mant, R., Kaiser, M. J., Hawkins, S. J., and Pullin, A. S. (2015). Evaluating the relative conservation value of fully and partially protected marine areas. Fish Fish. 16, 58–77. doi: 10.1111/faf.12044
Serrano, A., Rodríguez-Cabello, C., Sánchez, F., Velasco, F., Olaso, I., and Punzón, A. (2011). Effects of anti-trawling artificial reefs on ecological indicators of inner shelf fish and invertebrate communities in the Cantabrian Sea (southern Bay of Biscay). J. Mar. Biol. Assoc. U.K. 91, 623–633. doi: 10.1017/s0025315410000329
Stock, B., and Semmens, B. (2017). MixSIAR GUI user Manual Version 3. 1. Available online at: https://github.com/brianstock/MixSIAR (accessed October 5, 2020).
Tao, L. S. R., Lau, D. C. P., Perkins, M. J., Hui, T. T. Y., Yau, J. K. C., Mak, Y. K. Y., et al. (2020). Stable-isotope based trophic metrics reveal early recovery of tropical crustacean assemblages following a trawl ban. Ecol. Indicators 117:106610. doi: 10.1016/j.ecolind.2020.106610
Tao, L. S. R., Lui, K. K. Y., Lau, E. T. C., Ho, K. K. Y., Mak, Y. K. Y., Sadovy de Mitcheson, Y., et al. (2018). Trawl ban in a heavily exploited marine environment: responses in population dynamics of four stomatopod species. Sci. Rep. 8:17876.
The Food and Agriculture Organization (2016). The State of World Fisheries and Aquaculture. Rome: FAO Fisheries and Aquaculture Department.
Thiel, R., Sepulveda, A., Kafemann, R., and Nellen, W. (1995). Environmental factors as forces structuring the fish community of the Elbe Estuary. J. Fish Biol. 46, 47–69. doi: 10.1111/j.1095-8649.1995.tb05946.x
Thrush, S. F., Gray, J. S., Hewitt, J. E., and Ugland, K. I. (2006). Predicting the effects of habitat homogenization on marine biodiversity. Ecol. Appl. 16, 1636–1642. doi: 10.1890/1051-0761(2006)016[1636:pteohh]2.0.co;2
Wang, Z., Leung, K. M. Y., Sung, Y. H., Dudgeon, D., and Qiu, J. W. (2021). Post-disturbance recovery of tropical marine benthos after a trawling ban: Linkage between abiotic and biotic changes. Commun. Biol. 4:212.
Wei, L., Zhang, X., Huang, G., and Li, J. (2009). Effects of limited dissolved oxygen supply on the growth and energy allocation of juvenile Chinese shrimp, Fenneropenaeus chinensis. J. World Aquacult. Soc. 40, 483–492. doi: 10.1111/j.1749-7345.2009.00269.x
Wenger, A. S., Mccormick, M. I., Mcleod, I. M., and Jones, G. P. (2013). Suspended sediment alters predator–prey interactions between two coral reef fishes. Coral Reefs 32, 369–374. doi: 10.1007/s00338-012-0991-z
World Wildlife Fund (2018). Sea For Future: Conservation priority sites for Hong Kong. Available online at: http://awsassets.wwfhk.panda.org/downloads/20180531_sff_mps_booklet.pdf (accessed October 5, 2020)
Wu, R. S. S. (1982). Periodic defaunation and recovery in a sub-tropical epibenthic community, in relation to organic pollution. J. Exp. Mar. Biol. Ecol. 64, 253–269. doi: 10.1016/0022-0981(82)90013-2
Keywords: management intervention, recovery, trophic niche, trophic level, stable isotope analysis, MixSIAR
Citation: Tao LSR, Mak YKY, Ho VCM, Sham RC-t, Hui TTY, Lau DCP and Leung KMY (2021) Improvements of Population Fitness and Trophic Status of a Benthic Predatory Fish Following a Trawling Ban. Front. Mar. Sci. 8:614219. doi: 10.3389/fmars.2021.614219
Received: 06 November 2020; Accepted: 09 June 2021;
Published: 13 July 2021.
Edited by:
Cornelia E. Nauen, Mundus Maris, BelgiumReviewed by:
Aylin Ulman, Independent Researcher, Izmir, TurkeyChongliang Zhang, Ocean University of China, China
Copyright © 2021 Tao, Mak, Ho, Sham, Hui, Lau and Leung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kenneth M. Y. Leung, a215bGV1bmdAY2l0eXUuZWR1Lmhr
†ORCID: Danny C. P. Lau, orcid.org/0000-0002-3246-7508
 Lily S. R. Tao
Lily S. R. Tao Yanny K. Y. Mak
Yanny K. Y. Mak Valerie C. M. Ho
Valerie C. M. Ho Ronia C.-t. Sham2,3
Ronia C.-t. Sham2,3 Tommy T. Y. Hui
Tommy T. Y. Hui Danny C. P. Lau
Danny C. P. Lau Kenneth M. Y. Leung
Kenneth M. Y. Leung