
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mar. Sci., 09 February 2021
Sec. Marine Conservation and Sustainability
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.608155
This article is part of the Research TopicAdvances in the Biology, Aquaculture, and Conservation of Threatened Marine Species and their Application in Human Health and NutritionView all 27 articles
 Peng Xu1†
Peng Xu1† Haiwei Bai1†
Haiwei Bai1† Xiaoyong Xie2
Xiaoyong Xie2 Chun-Chieh Wang3
Chun-Chieh Wang3 Xing Huang4
Xing Huang4 Xueping Wang4
Xueping Wang4 Mingming Zhang1
Mingming Zhang1 Zhenyu Ye1
Zhenyu Ye1 Junhua Zhu1
Junhua Zhu1 Wenquan Zhen1
Wenquan Zhen1 Siu Gin Cheung5
Siu Gin Cheung5 Paul K. S. Shin5
Paul K. S. Shin5 Kit Yue Kwan1*
Kit Yue Kwan1*As a well-known example of “living fossil,” horseshoe crabs are ecologically significant macroinvertebrates in coastal and estuarine ecosystems. The tri-spine horseshoe crab, Tachypleus tridentatus, has been widely utilized for Tachypleus amebocyte lysate production and food consumption since the 1980s, which led to considerable population declines along the west coast of the Pacific Ocean. The declining horseshoe crab population is expected to have ecological and social impacts. Stock enhancement through captive rearing of juveniles is cited as an important alternative to repopulate the native T. tridentatus, which in turn supports sustainable resource utilization and research activities. The hatchery production techniques for this species have gradually developed following the mass culture efforts in Japan since the late 1980s. However, the previous studies have primarily concerned the feed types and husbandry conditions to maximize the growth and survival of the juveniles. Little is known about the practicability and effectiveness of releasing large numbers of hatchery-bred individuals through releasing programs. In this review, we (1) summarize the available captive breeding and rearing techniques, (2) discuss the release strategies that could potentially improve the survival of released juveniles, and (3) identify the future opportunities and challenges in establishing technical frameworks to support responsible stock enhancement programs for T. tridentatus. The information should benefit future horseshoe crab fisheries management efforts in the attempt to restore the severely depleted populations.
Current global fisheries production is severely threatened by overfishing, climate change, and their interactions (Brander, 2007). However, with the advance in aquaculture techniques, this opens up the possibility to release hatchery-reared juveniles to help restore depleted global fishery stocks (Bartley and Bell, 2008; Le Vay et al., 2008; Lorenzen et al., 2010; Han et al., 2016; Taylor et al., 2017). Uses of hatchery releases with different management objectives are referred to as restocking (“to restore depleted spawning biomass to a level where it can once again provide regular, substantial yield”), stock enhancement (“to augment a natural supply of juveniles and optimize harvest by overcoming recruitment limitation”), and sea ranching (“to harvest at a larger size in put-grow-take operations”) (Bell et al., 2008). Yet, few releasing programs have claimed their management interventions successful to produce a measurable impact on fisheries stock abundance and yield. Challenges such as producing cost-effective juveniles, developing optimal release strategies and searching for appropriate monitoring and evaluation methods, have been identified (Molony et al., 2005; Wada et al., 2010). A set of elements aimed at developing a responsible approach for releasing programs has been proposed, which emphasizes the equal weight to the dynamics of biological and human components (Blankenship and Leber, 1995; Lorenzen et al., 2010). Lorenzen et al. (2010) also identified common weaknesses in most stock enhancement efforts in coastal systems, including lack of fishery stock assessments and modeling, ignorance in governance framework establishment, rare involvement of stakeholders in program development, and poor integration of adaptive management into stock enhancement plans. Compared to finfish fisheries, some coastal invertebrates may have a higher potential for effective sea ranching and stock enhancement due to their sessile or sedentary characteristic and are more likely to create self-replenishing populations within a relatively localized region (Bell et al., 2006; Gomez and Mingoa-Licuanan, 2006).
A case in point is the potential application of stock enhancement through captive rearing of juveniles as a proactive management tool to restore the dwindling population of the tri-spine horseshoe crab, Tachypleus tridentatus, worldwide. Horseshoe crabs are an ancient group of macroinvertebrates that use multiple inshore and estuarine habitats throughout their life cycles: adults spawn on sheltered intertidal flats near the high tide mark, the newly hatched larvae emerge from sediment and enter the sea as plankton, the larvae/juveniles settle and grow on the same or adjacent beaches, and gradually migrate into subtidal areas to reach adulthood (Figure 1; Chen et al., 2015; Laurie et al., 2019). Therefore, horseshoe crabs are referred to as the potential sentinel species to indicate the general health status of coastal and estuarine ecosystems (Kwan et al., 2018b).
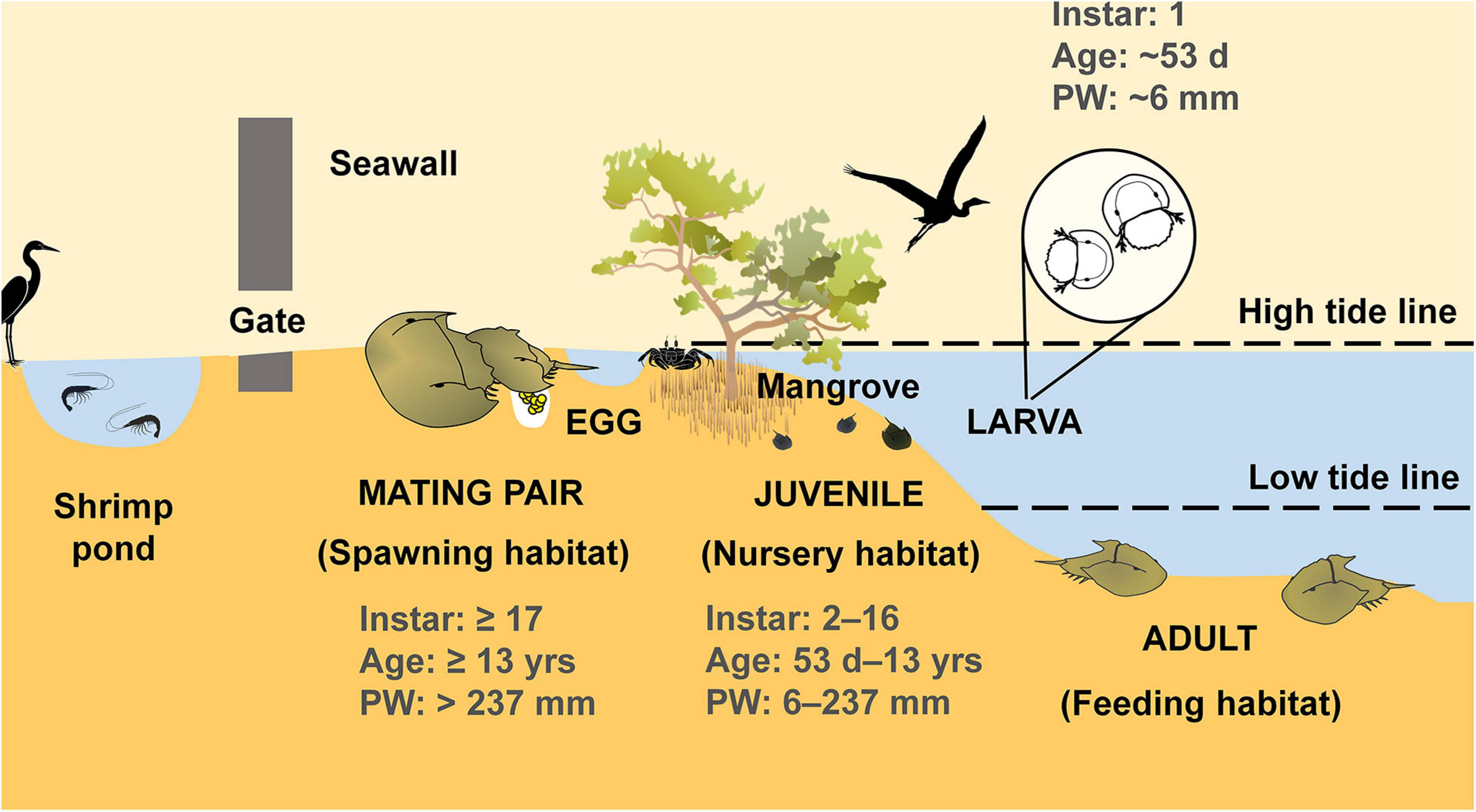
Figure 1. Life cycle and the associated habitats for T. tridentatus. PW, prosomal width (size); d, day; yrs, years.
Horseshoe crabs have a lengthy maturity period. For example, T. tridentatus become mature at age 13–14 (instar stage: 17–18) after undergoing 16–17 molts (Figure 1; Chen et al., 2010; Hu et al., 2015, for detailed information on instar, age and size). Apart from that, horseshoe crabs also exhibit low dispersal rates, long life span, small home range, and delayed reproductive development (Kwan et al., 2015b, 2020). These life-history features render them vulnerable to overharvesting and habitat loss (John et al., 2018; Laurie et al., 2019). T. tridentatus has also been heavily harvested since the 1980s for the production of Tachypleus amebocyte lysate (TAL), a substance that quantitatively detects the presence of bacterial endotoxins or fungal contamination in drugs and biomedical devices (Liao and Li, 2001; Liao et al., 2019a). T. tridentatus is recently listed as “Endangered (EN)” on the IUCN Red List (Laurie et al., 2019). Considering the increasing demand for pharmaceuticals and biomedical applications in emerging markets, including China, India, and Brazil, mass harvest for wild T. tridentatus resources will not cease.
The global decline in T. tridentatus populations has attracted increasing interest in searching for appropriate management tools to revert the situation. Despite the introduction of marine protected areas and the establishment of new environmental legislation can be effective, stock enhancement initiative in some cases is considered as a more proactive approach to populate the exploited stocks (Carmichael and Brush, 2012; Wang et al., 2020; Zhu et al., 2020). Releasing programs using hatchery-bred T. tridentatus juveniles, mostly the newly hatched first-instars or second-instars, and occasionally adults have been attempted or are underway along the Chinese coast of Fujian, Guangdong, and Guangxi provinces in the Mainland and Taiwan (Hong, 2011; Zhu et al., 2020). In total, more than 7,450,000 juveniles and 2,500 adults of T. tridentatus have been used for sea ranching and stock enhancement during 2010–2020, in which 42% of these programs released more than 100,000 individuals each time (Supplementary Table 1). Apart from that, every year by joining a conservation education program in Hong Kong, students from 30 selected secondary schools also release the laboratory-reared juveniles under their care at Ha Pak Nai, the most important nursery habitat for the local T. tridentatus population (Kwan et al., 2017b). These programs, however, are unlikely to have the desired result given a lack of proper formulation of release strategies, dependable evaluation, and understanding of the ecosystem.
In this review, we summarize the available aquaculture technologies for hatchery production of juvenile T. tridentatus. Based on our best knowledge regarding biological and ecological characteristics of the species, release strategies that could potentially improve the survival of released T. tridentatus were discussed. Future opportunities and challenges in establishing technical frameworks to support responsible stock enhancement for T. tridentatus were also identified. Relevant articles and references were searched and identified mostly through databases and search engines, including Web of Science, Scopus, China National Knowledge Infrastructure, Scholarly and Academic Information Navigator, Japan, and Google Scholar. To include as many studies as possible, reference searching using local names of T. tridentatus in varying languages was also conducted. The ultimate goal is to establish effective and economically viable methods for the mass release of environmentally fit juveniles in the attempt to restore the severely depleted T. tridentatus populations.
There is considerable demand in the development of hatchery production technology for rearing horseshoe crab larvae and juveniles due to the decline in horseshoe crab populations (Carmichael and Brush, 2012). However, the capacity of hatcheries to producing a sufficient number of high-quality juveniles in a cost-effective manner is the prerequisite of any releasing activity. Culturing T. tridentatus in the laboratory was initiated by the Japanese scientist, Koichi Sekiguchi in 1972 from artificially fertilized eggs with an aim to study the growth and development of juveniles (Sekiguchi et al., 1988). A broader scale of T. tridentatus culture efforts has later been attempted in China (e.g., Liang, 1987) and Japan (e.g., Tsuchiya, 2009) since the 1980s. While most previous studies concerned how diet compositions and water quality variables affect the survival and growth of horseshoe crab embryos and juveniles (Carmichael and Brush, 2012; Hu et al., 2013a, 2014, 2018; Liao et al., 2019b), the mass production of cost-effective, high-quality seedstocks has presented a bottleneck in the releasing programs for T. tridentatus (Kwan et al., 2014; Zhu et al., 2020). We compiled and discussed the available rearing techniques in maintaining T. tridentatus embryos and juveniles in hatcheries with the ultimate goal in defining effective culture conditions (Figure 2).
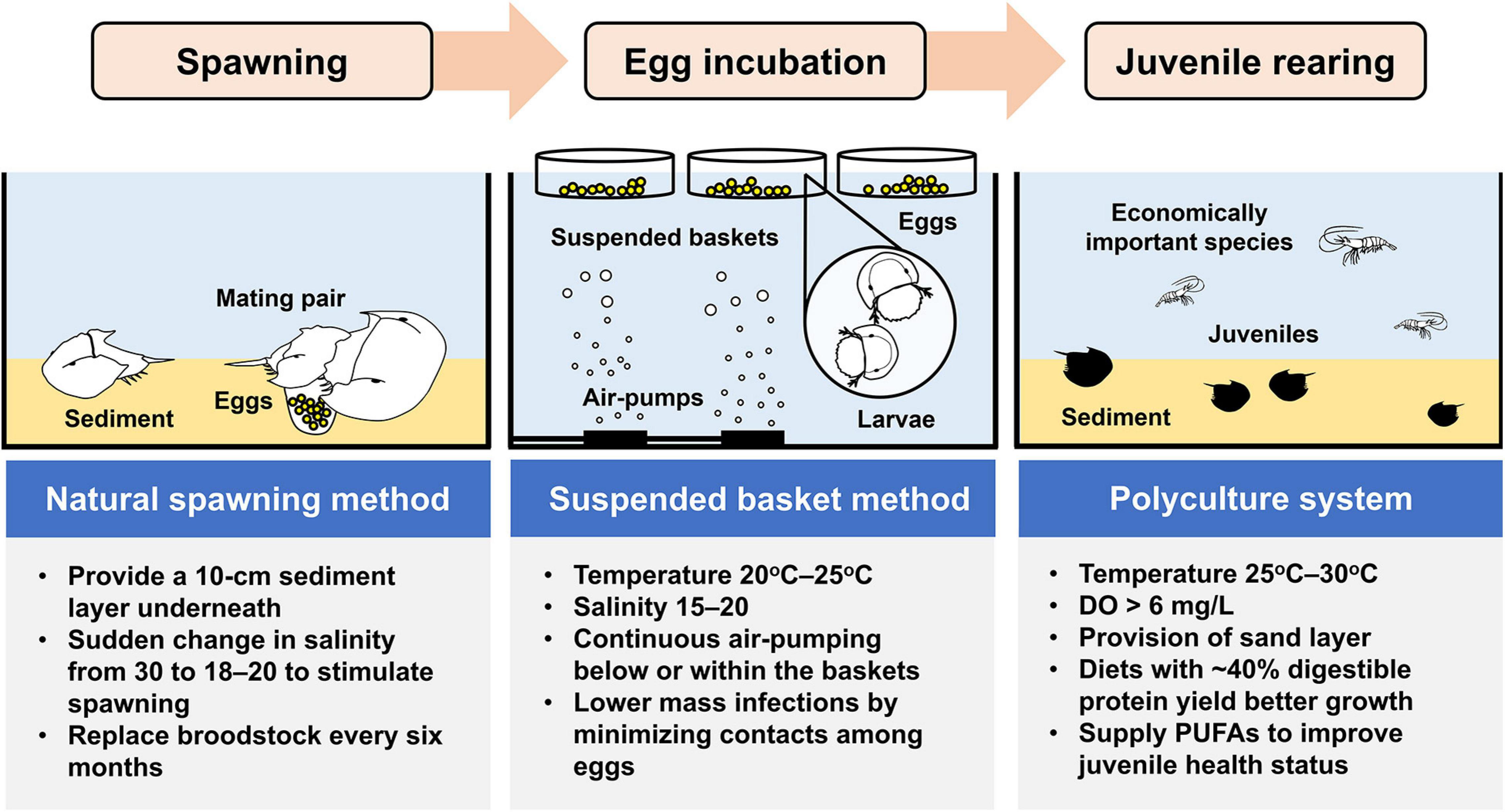
Figure 2. Important techniques and operating procedures on artificial breeding as well as larval and juvenile culture for T. tridentatus.
The development of T. tridentatus aquaculture has been enabled by breakthroughs in artificial breeding. Earlier in the 1940s, Oka (1943) reported his trial on artificial insemination following Patten (1894) to obtain T. tridentatus embryos for developmental mechanism study. Sekiguchi et al. (1988) further improved the fertilization rate by spreading the diluted sperm evenly over the eggs after a shorter preparation procedure. The sperm and eggs were collected by cutting the soft tissue on the ventral sides of the prosoma of male and female T. tridentatus from the appendage base toward the lateral margin (Chen et al., 2010). Nonetheless, this invasive method would further deplete the remaining population of T. tridentatus. Non-invasive electrical stimulation for egg and sperm collection was applied in the Atlantic counterpart, Limulus polyphemus. By adopting a similar method, however, a very limited amount of eggs/sperm could be collected from T. tridentatus (Sekiguchi et al., 1988; Li, 2008). Alternatively, the natural spawning method has been adopted by keeping amplexed mating pairs of horseshoe crabs in tanks with approximately 10-cm sediment layer underneath (Liang, 1987; Chen and Xia, 2002). The authors also claimed that the released eggs obtained from natural spawning were mostly mature, which can considerably enhance the fertilization rate (Liang, 1987), compared to that from electrical stimulation or artificial insemination.
However, there is relatively little information available regarding the effects of diet and culture conditions on adult T. tridentatus survival, breeding, and cellular health. Chen and Xia (2002) reported that natural spawning occurred in breeding tanks during May–July when the mating pairs were fed with shrimp and fish meat, and maintained at water temperature 23–26°C, salinity 25–30, and pH 7.8–8.2. Chow (2019) observed stimulation of T. tridentatus spawning when the water salinity was gradually lowered from 30 to 18–20. A recent study demonstrated that the addition of copper supplements into the feed for adult T. tridentatus could improve their immune enzyme activity and hemolymph quality (Xu et al., 2020). Future efforts should be concentrated on developing prototypical nutritional and aquaculture standards for adult horseshoe crab husbandry to support long-term aquaculture management (Tinker-Kulberg et al., 2020a,b, c). Otherwise, the broodstock should be replaced every 6 months. Sustained aquaculture in the facilities has been linked to low survival rates, possibly due to poor nutritional conditions and water quality which would increase their susceptibility to bacterial and parasitic infections (Nolan and Smith, 2009).
To maintain relatively stable environmental conditions, the culture of T. tridentatus embryos was mostly confined in indoor husbandries or laboratories. In most cases, the fertilized eggs were extracted from the sediment several days after the spawning events, and transferred to an individual culture system for egg incubations. Many authors reported generally higher survival rates (>60%) of fertilized eggs under the following incubation conditions: water temperature 20–35°C, salinity 15–35, pH 8.1–8.3 under continuous aeration with water renewal once or twice a day (Sekiguchi et al., 1988; Li et al., 1999; Li, 2008; Hong et al., 2009, 2011; Chen et al., 2010; Wu et al., 2014; Carmichael and Brush, 2012; Miao et al., 2020).
Despite the fact that most previous studies overlooked the effect of sole environmental factor on fertilization, survival, and hatching of T. tridentatus eggs (Table 1), Li et al. (1999) found surprisingly high fertilization (>95%) and survival (>98%) rates when the eggs were cultured under salinity ranged 16–33. Li (2008), however, observed interactive effects between temperature and salinity, in which the egg survivorship was deleteriously affected (<30%) at salinity 25 and 30 when the water temperature was kept at 32°C. No hatching was noted during the 90-day experiment in all treatment groups with salinity at 15 (Li, 2008).

Table 1 | Effects of various environmental factors on fertilization and survival of T. tridentatus embryos in hatcheries (–, no data).
Bacterial/fungal infections and dissolved oxygen deficiencies seem to be the most prominent issue in the rearing of horseshoe crab eggs. Faizul et al. (2015) identified four species of bacteria (Shewanella putrefaciens, Bacillus cereus, Corynebacterium sp., and Enterococcus faecalis) and fungi (Aspergillus niger, Aspergillus sp., Penicillium sp., and Gliocladium sp.), respectively, in infected horseshoe crab eggs. The authors suggested that S. putrefaciens and A. niger possess primary threats to egg survival and development. While possible treatments to the infections are largely lacking, a 3- to 12-min freshwater bath, and/or addition of chlorine or other disinfectant agents, may be useful in removing the pathogens in hatcheries (Li, 2008; Nolan and Smith, 2009; Shinn et al., 2015). To lower the spread of diseases, it is also important to maintain an appropriately low density of eggs or embryos within each incubator, even though the optimal density information is currently unknown.
Dissolved oxygen (DO) plays a critical role in promoting horseshoe crab embryonic developments (Penn and Brockmann, 1994; Vasquez et al., 2015). Consequently, many attempts were made to maximize the DO concentrations in the culture system for T. tridentatus eggs and embryos. Suspended culture, which involves hanging baskets from the surface of culture water, was the most widely reported method to be efficient in egg hatching (Liao, 2011, 2015; Liao et al., 2015). Continuous air-pumping below or within the baskets where the eggs housed provides more DO. The vigorous airflow can also agitate the eggs constantly to avoid mass infections due to the prolonged contact among eggs. Keeping the eggs/embryos within a moist and sterile sediment layer in husbandries can also be another alternative to be attempted.
The success of sea ranching and stock enhancement programs is predominantly determined by rearing technologies in the mass production of seedstocks and juveniles for release. However, since most T. tridentatus individuals in the releasing programs were the newly hatched first-instar larvae (Zhu et al., 2020; Supplementary Table 1), the development of improved culture techniques to produce environmentally fit, older juveniles has been greatly impeded. To date, most available rearing results were derived from laboratory experiments (Table 2), in which their practicability in large-scale seedstock production is virtually unknown.
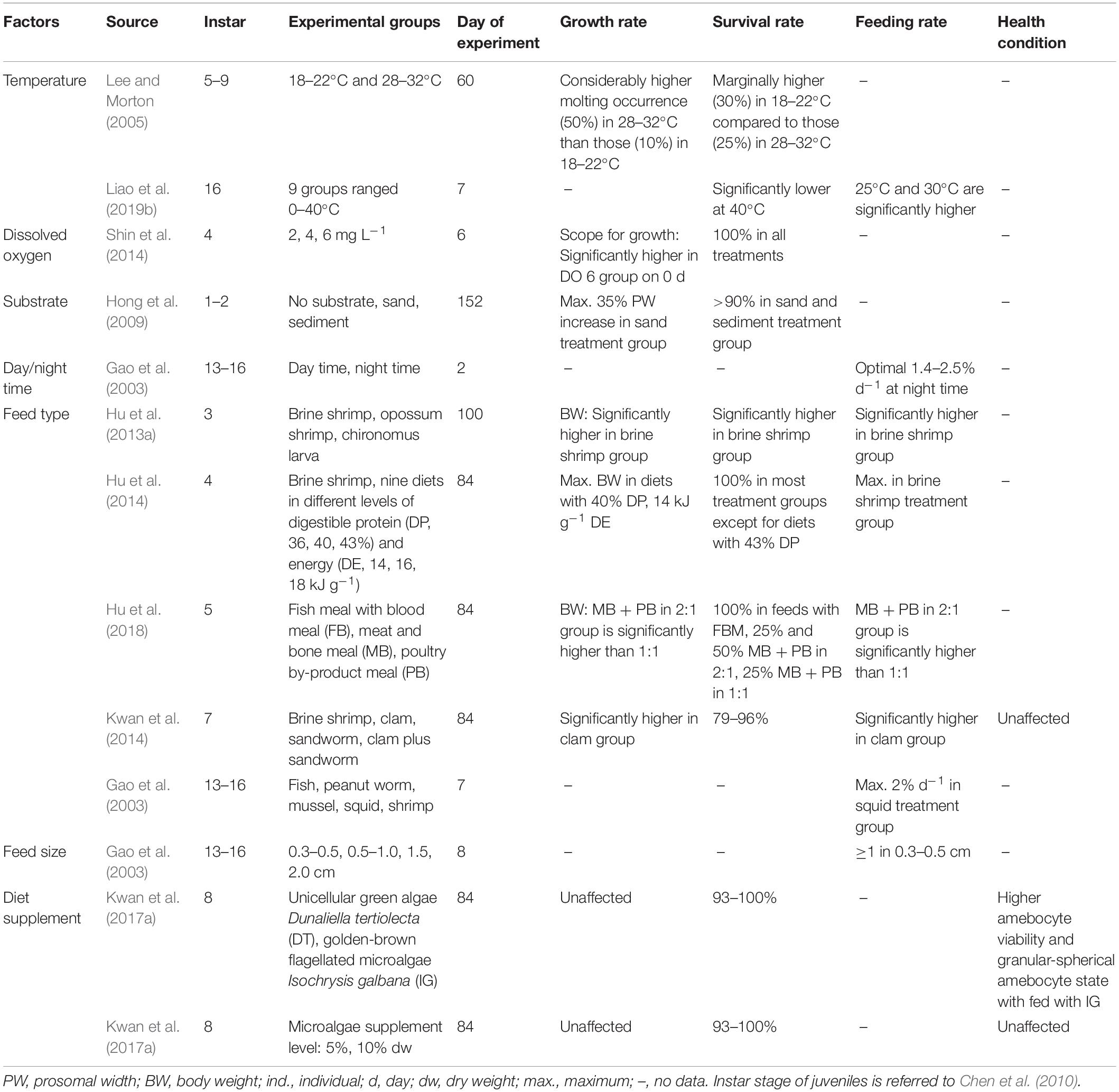
Table 2 | Effects of various environmental factors and diet conditions on optimal growth, survival and health status of juvenile T. tridentatus in culture environments.
In the culture environment, the first-instar larvae were not fed until after the first post-hatch molt (Carmichael et al., 2009; Schreibman and Zarnoch, 2009; Chen et al., 2010) since the previous studies suggested that the larval digestive system was rather incomplete and appeared to subsist on the yolk of the embryos for nutrition (Botton et al., 1992; Carmichael et al., 2009). For juvenile culture, many studies concerned their growth and survival under varying dietary conditions, except a small amount of research investigated their responses toward different environmental variables such as temperature, DO, light-dark cycle, and substrate type (Table 2). Among frozen food sources commonly available for aquarium fish, hatchery-bred first-year juveniles (instars 1–6) fed with brine shrimp, Artemia sp. had higher growth and survival rates due to the increased consumption rate (Hu et al., 2013a). Biswal et al. (2016) provided small, chopped earthworms to the second-instar juvenile coastal horseshoe crab, Tachypleus gigas, and observed most molted into the fourth-instar within 90 days from the hatching. For older juveniles, clam meat, which has higher crude protein, can significantly promote their growth, compared to sandworms, Marphysa sanguinea and brine shrimp that contain indigestible chaete and exoskeleton, respectively (Kwan et al., 2014). Feed size is another vital factor determining juvenile feeding rates and prey sources (Gao et al., 2003). Gaines et al. (2002) found that the prey selection of L. polyphemus in varying growth stages depends largely on their mouth size, in which the younger juveniles (instars 2–7) mainly forage on benthic and suspended particulate organic matters (POM), whereas instars 8 or older juveniles shifted their diets to a combination of POM and small benthic invertebrates such as crustaceans and polychaetes. Nonetheless, feeding cultured juveniles with natural diets is costly, and is difficult to be tailored to fulfill the different nutritional requirements in accordance with the ontogenetic development of T. tridentatus.
In the search of the optimal diets for hatchery-reared juvenile T. tridentatus, Hu et al. (2014) formulated nine artificial diet treatments in different levels of digestible protein and energy. While the fifth-instar juveniles fed with brine shrimp had the highest feeding rate, the formulated diet containing 40% digestible protein yielded the best growth and feed utilization. The authors suggested that excessively high digestible protein diets such as brine shrimp, fish and clam meat, could reduce juvenile feed consumption and pollute the culture environment. Providing the juveniles with artificial feeds using poultry by-products (PB) as well as meat and bone meals (MB) to replace the traditional fish meal were also attempted to lower the production cost (Hu et al., 2018). The experimental results are promising that a mixture of MB and PB in 2:1 resulted in similar growth performance to that fed with fish meals. However, a longer acclimation period may be required with these dry, hard artificial feeds as horseshoe crabs prefer softer food sources in both natural habitats and captive environments (Zhou and Morton, 2004; Kwan et al., 2015a; Razali et al., 2020).
Since the production of high-quality juveniles is essential in any releasing activity, technological development of seed production has gradually shifted its focus from quantity to quality (e.g., Fushimi, 2001). Concerning the health status of cultured juvenile T. tridentatus, Kwan et al. (2014) observed a general decline in juvenile hemolymph quality during a 12-week feeding experiment, even though high-protein diets were provided. To further verify the presence of captivity-related stress, Kwan (2016) kept wild juvenile T. tridentatus in the laboratory for 3 months under the best husbandry practices. Significant declines in health indicators, including amebocyte density, amebocyte morphological states, and plasma protein level, were consistently found. Such a decrease in juvenile health status may indicate deficiencies of essential diet compositions, movement constraints and/or absence of tidal rhythms in the culture systems (Kwan et al., 2014). To address the problems, Kwan et al. (2018a) invented a double-layered water-circulating enclosure, allowing the simulation of the daily ebb and flow tidal conditions. A high survival rate was obtained, as the circulating system can keep good water quality for at least 3 months and prevent disturbance to the juveniles since frequent water renewal is not required. The system also enabled the juvenile horseshoe crabs to accommodate natural feeding activities under low tides. Supplementing diets with microalgal powder with high polyunsaturated fatty acids, notably eicosapentaenoic acids, also improved the hemolymph quality of juvenile T. tridentatus (Kwan et al., 2017a).
In terms of environmental variables (Table 2), juvenile T. tridentatus can survive in a broad range of rearing temperatures (15–30°C), except for the significantly higher mortality that occurred at 40°C (Liao et al., 2019b). It seems that T. tridentatus population in the higher latitudes such as that in Japan, is better adapted to the lower temperatures (Shuster and Sekiguchi, 2009; Liao et al., 2019b). The optimal water temperature for growth and survival was observed within the range of 25–30°C. Deficiencies in DO (2 or 4 mg L–1) lowered the scope of growth in juvenile T. tridentatus, but their physiological performance can be recovered to the normal level once the short-term hypoxic conditions were ceased on day 4 (Shin et al., 2014). Gao et al. (2003) showed higher juvenile feeding activities during the night, which is consistent with previous studies, suggesting that horseshoe crabs are nocturnally active (Rudloe, 1979; Wada et al., 2016). Provision of a sand layer in the culture environment that simulates the nursery habitat conditions of juveniles can also effectively enhance their growth and survival by 10% and 42%, respectively (Hong et al., 2009).
The current market demand for mass production of hatchery-bred juvenile T. tridentatus is mainly for releasing programs organized by local governments and research institutes (Supplementary Table 1; Zhu et al., 2020). In hatcheries, large numbers of fertilized eggs are incubated using suspended baskets or other similar methods within indoor cement ponds (Figure 2; Liao, 2011, 2015; Liao et al., 2015). For the third-instar or older juveniles, a very limited amount has been produced from laboratories to serve research (Chen et al., 2010; Kwan et al., 2019) and conservation educational purposes (Kwan et al., 2017b). The production of adults for TAL biomedical industry through captive rearing is potentially technically feasible (Tinker-Kulberg et al., 2020a,b,c). Such an approach could not only reduce the need to harvest wild horseshoe crabs for such bleeding practices, but would also provide a cost-effective indirect approach for the production of seedstock, including the polyculture of juveniles for a limited amount of time for subsequent release for stock enhancement purposes. Such captivity studies have been recently reported with replacement, reduction and refinement as important drivers of change for horseshoe crab conservation (Gorman, 2020). To the best of our knowledge, however, no study has successfully reared the early instar T. tridentatus juveniles till adulthood. Sekiguchi et al. (1988) obtained the 10th-instar (prosomal width, PW: 48.4 mm) juveniles after 7 years of laboratory culture, whereas Huang and Tsai (2011) spent only 4 years to successfully rear three 10th-instar juveniles from a total of 12,000 first-instar larvae in research facilities.
To produce more cost-effective juvenile T. tridentatus for releasing programs, Chen et al. (2016) proposed to adopt a polyculture system to rear the juveniles with other economically important aquaculture species in husbandries. In this case, the juveniles can be a “by-product” from other rearing activities without the additional investments in husbandry management and maintenance. To examine the practicability of such production mode, a trial on mixed cultivation of juvenile T. tridentatus (instars 1–2; 203,000 individuals) with a scavenging species, juvenile spotted Babylon snail, Babylonia areolata (338,000 individuals) was conducted in outdoor cement ponds for 150 days (Chen et al., 2016). Despite the fact that the temperature maintenance system was unavailable during the winter and spring, about half of the juvenile T. tridentatus molted into the second- or third-instar with an acceptably high survival rate of 33%. Meanwhile, 87% of juvenile B. areolata survived through the entire experiment. Chen et al. (2016) explained that the outdoor culture system had provided larger movement space and a relatively stable rearing environment to the juveniles, compared to the typically small experimental tanks within the research facilities. The juveniles can feed on overgrown benthic algae in the outdoor ponds, which are important food sources for these young individuals (Gaines et al., 2002; Carmichael et al., 2009). The polyculture with juvenile B. areolata also can enhance the growth performance of juvenile T. tridentatus by providing better water quality and culture environment due to their complementing feeding niches. Rearing juvenile T. tridentatus with shrimp and other mollusks is also worth to be attempted to identify more practical, cost-effective methods for seedstock production.
The important techniques and operating procedures on artificial breeding, as well as larval and juvenile culture for T. tridentatus are summarized in Figure 2. All in all, the experimental findings suggest that the optimal rearing techniques of juvenile T. tridentatus should be based on their biology and ecology. Therefore, an improved understanding of the released species and ecosystems can compensate for the limitations in providing older captive-bred juveniles and increase the potential success of sea ranching and stock enhancement. While captivity-related and other health issues are largely unsolved, mass production of the juveniles using more natural, ecologically relevant methods, e.g., outdoor ponds with sediment substrates, may be more promising.
The success of stock enhancement programs depends largely on the survival of the hatchery-bred juveniles to natural habitat conditions. The mortality of released juveniles, particularly those of invertebrates, is typically higher than 60% within the first month (Oliver et al., 2005; Dixon et al., 2006; Purcell and Simutoga, 2008). Thus, potential reasons that can cause high mortality of released juveniles, including predation, habitat conditions, environmental stress, and extreme weather, should be addressed (Lorenzen, 1996; Lorenzen, 2000; Hines et al., 2008; Ceccarelli et al., 2018; Poh et al., 2018). Of these, Bartley and Bell (2008) perceived predation as the key obstacle to the survival of released juveniles. While relevant experimental results in developing optimal release strategy for juvenile T. tridentatus are largely lacking, we discuss these based on our understanding of the biology and ecology of juvenile T. tridentatus in the estuarine ecosystem.
Horseshoe crabs have specific spawning and nursery requirements related to a combination of beach topography and physico-chemical parameters such as dissolved oxygen, chlorophyll a content, total sulfide content, and sediment grain size (Hsieh and Chen, 2009; Vasquez et al., 2015; Cheng et al., 2016; Xie et al., 2020). Therefore, the identification of optimal nursery habitats for releases is the first step. Among the 14 nursery sites distributed along the northern Beibu Gulf, China, a high density of wild juveniles was found in the intertidal areas along the outer fringe of mangrove forest, especially near the outflows of tidal creeks (Xie et al., 2020). In addition, at the nursery site where seagrass patches are available, areas with higher seagrass coverage and coarser sediment had more abundant juvenile T. tridentatus individuals (Xie et al., 2020). Most wild juveniles were found forage actively in areas with surface water with 1–10 mm depth during ebb tides to keep the juvenile book gills wet and enable respiration while feeding under high surface temperatures (Lee and Morton, 2009; Kwan et al., 2020). Based on the above findings, releasing hatchery-produced juveniles at the existing nursery sites on the intertidal areas along the outer fringe of mangroves or near the seagrass patches seems to enhance their survival rate.
Chiu and Morton (2004) demonstrated that juvenile T. tridentatus buries in the sediment and remains inactive when the water temperature drops below 20°C. In such cases, decisions related to factors linked to the ecological requirements of the juveniles, including microhabitat, season and timing play critical roles for the release success. To improve the survival of released juveniles, they should be placed on the intertidal areas during low tides in summer when the surface water temperature exceeds 20°C. This can also mimic the spawning period of T. tridentatus typically recorded in late spring (Cai et al., 1984) and would coincide with the summer peak in juvenile production.
If there are problems in locating any actively utilized nursery habitats for T. tridentatus for releases, the programs can be conducted at any suitable intertidal habitats where the juveniles were known to occur in the past but are now extirpated. However, it is important to ensure the current state of the intertidal areas fits the macrohabitat requirements of the juveniles, e.g., mangroves, seagrass beds, and tidal creeks. For microhabitat conditions, juvenile T. tridentatus tends to concentrate in regions where sediment comprised medium-sized (median particle: 0.1–0.5 mm) sand with relatively higher chlorophyll a content (1–4 μg/g) and/or total organic content (0.1–0.7%) (Hsieh and Chen, 2009; Xie et al., 2020), which presumably indicate a higher abundance of food from the marine algae-derived particulate organic matter sources (Gaines et al., 2002). For optimal surface water quality, salinity should range between 12 and 34, dissolved oxygen content 7–9 mg/L, and pH 7.3–7.9 (Hsieh and Chen, 2009; Xie et al., 2020), although Xie et al. (2020) found that sediment physico-chemical conditions, rather than surface water parameters, can better explain the distribution pattern of wild juvenile T. tridentatus population.
To date, however, no study has looked specifically at the survival, growth, and behavioral responses of hatchery-reared juvenile T. tridentatus at the release sites. During a release trial with 60,000 individuals of cultured first-instar larvae (∼53-day old) in mangrove and seagrass areas at Yuzhouping, Guangxi region, China (Zhu et al., 2020), an actively utilized nursery habitat for T. tridentatus, none of the released larvae had been recovered during monthly belt-transect population survey in the following 4 months, which was possibly due to the high predation rates. Size at release is, therefore, another critical component to be considered prior to the releases.
Size at release is one of the key elements in a successful stock enhancement. Natural mortality rates within natural fish (possibly also invertebrate) populations are approximately inversely proportional to their lengths (Lorenzen, 1996). Meanwhile, the predation risk at the released site is inversely related to their age and size. Carmichael et al. (2003) found that only very few L. polyphemus in Cape Cod, Massachusetts, United States survived their first year (instars 1–6), in which the cumulative mortality rate can be as high as 99%. Conversely, most juveniles survived to reach adulthood given that they survived through the first year (Carmichael et al., 2003). While there is very limited information regarding key predators of juvenile horseshoe crabs (Botton, 2009), releasing using hatchery-bred juveniles at their sixth instar or older seems to be an optimal decision to minimize potential predation by fish and hermit crabs, and to increase the success of releases.
Releasing undersized juveniles, e.g., the first-instar larvae, is unlikely to be effective, but the effectiveness of releases at small sizes can be increased significantly through the selection of optimal habitats and times as well as acclimation and soft release strategy (refer to sections “Selection of Release Sites, Season and Timing” and “Stress Management”). Apart from that, the fitness of juveniles under prolonged captivity is another area of concern. Despite the development of rearing technologies for Asian horseshoe crabs has been initiated in the 1980s, Kwan et al. (2014) suggested that the present culture models may not guarantee the continuing fitness of juveniles. To maximize the survival rate of released juveniles, it is important to identify the captivity-related issues such as deficiencies in essential dietary components (Kwan et al., 2017a) that are widely present in the current hatchery systems. Nevertheless, it is also important to account for ecological differences between wild and hatchery-produced juveniles (Lorenzen, 2005). Natural mortality rates of hatchery-reared juveniles tend to be substantially higher than those of wild conspecifics of similar size (Lorenzen, 2000; Fleming and Petersson, 2001). Reproductive success of captive-bred salmonids, for example, is generally higher than that of their wild conspecifics (Fleming and Petersson, 2001). However, no strong evidence on their differences in growth was reported (Svåsand et al., 2000; Fleming and Petersson, 2001).
The development of cost-effective tagging methods is necessary to identify the released individuals from the wild stock to evaluate the effectiveness of stock enhancement programs (Bartley and Bell, 2008). In fact, little research has focused on selecting appropriate tags and markers for juvenile horseshoe crabs. No tagging experiment was currently conducted on the most commonly released size, the first-instar larvae. For other released size, only physical tags have been attempted. Hu et al. (2013b) marked 300 individuals of the second-instar T. tridentatus (∼134-day old; prosomal width, PW ∼8 mm) using visible implant elastomer (VIE, i.e., colored tag; Northwest Marine Technology Inc., WA, United States) and found the 22-d survival rate was 96%. Nonetheless, no information on the juvenile survival rate at the released site was provided. Tag retention is another important consideration when injecting the implants of fluorescent colors under the juvenile carapace. Although the tag retention time in juvenile horseshoe crabs is virtually unknown, Brennan et al. (2005) observed such tag loss in 2% of tagged snook Centropomus undecimalis after 6 months. Kwan et al. (2015b) examined the recovery and movement of a total of 150 individuals of the 7th- to 10th-instar T. tridentatus (1–2-year old; PW 31–59 mm) with colored plastic tape, passive integrated transponder tag (PIT; 134.2 kHz, Biomark, WA, United States) and their combinations. The mortalities induced by the three kinds of tags were minimal, and the cumulative recovery rates of the released juveniles within 2 months in summer were ranged from 70 to 82% (Kwan et al., 2015b). The PIT tag seems not to affect the juvenile foraging activity as their utilization distribution area using these three tagging methods was statistically similar.
More tagging studies were conducted in adult horseshoe crabs to investigate their migratory, spawning, and post-bleeding behavior (James-Pirri et al., 2005; Anderson et al., 2013; Bopp et al., 2019). Disk tags were developed by U.S. Fish and Wildlife Service, in which the tagging involves drilling a small hole through the lower back corner of horseshoe crab prosoma. Mattei et al. (2011) found no mortality in tagged adult Atlantic horseshoe crab, L. polyphemus, and reported an 11–20% recapture rate within the 44-day field observation. James-Pirri et al. (2005) determined movement patterns of spawning L. polyphemus during 2000–2002 with a double T-bar anchor tag (model SHD, Floy Tag Inc., WA, United States). However, the recovery of these conventional passive tags can be labor-intensive and time-consuming, particularly when only a small number of tagged individuals were released to an open area of the appropriate habitat. Ultrasonic transmitters, which allow wider coverage of detection in submerged habitat, were useful in tracking the distribution and movement of adult horseshoe crabs at varying temporal and spatial scales (Schaller et al., 2010; Wada et al., 2016; Owings et al., 2019). Since the transmitter size is much larger and heavier compared to that of conventional physical tags, telemetry technologies are only suitable for adult horseshoe crabs. However, restocking with the adults, should not be encouraged, except for those bled or confiscated from illegal wildlife trade. In fact, all captive adult horseshoe crabs are harvested from the wild stocks because of their lengthy life histories, and thereby impractical to rear them till adulthood.
The search for optimal tagging methods for the first-year juveniles (instar 1–6) is urgently needed, despite the fact that their natural mortality rates are too high (Carmichael et al., 2003) and should not be used for releasing programs. As mentioned earlier, since the selection of release size is a balance between survival rates and production costs, we predict that the use of the first-instar larvae would still be predominant in the releasing programs, unless the hatchery techniques have been largely improved and the production costs can be lowered to an acceptable level. The application of physical tags to these juveniles would be challenging due to their minute body size (PW < 30 mm, wet weight < 2 g), frequent molting (50–290 days for each instar stage), and burying behavior during high tides. The development of biological tags such as genetic markers and some visible morphological characters for the early instar juveniles is worth attempting. Non-invasive genetic markers are developed based on the selection of a rare DNA variant that distinguishes the released batch from the native stock (Romana-Eguia, 2006). Maternally inherited mitochondrial DNA markers and biparentally inherited nuclear DNA markers (such as microsatellite DNA and allozyme electrophoresis) are commonly used and effective for assessing the success of releasing activities and possible loss of genetic variability in both released and wild stocks. Nevertheless, the use of genetic markers can be ineffective when the genetic variation within and between stocks is negligible.
Proper management of stress derived from handling and transport can also improve the survival of released juveniles. Purcell et al. (2006) found that prolonged transport duration (i.e., 12 and 24 h) suppressed the normal sand burrowing behavior of juvenile sea cucumber, Holothuria scabra. In addition to transport duration, inappropriate storage temperature and medium, as well as juvenile density during transport can elevate both lethal and non-lethal stress of released invertebrates, which in turn, increase their predation risk upon release (Heasman et al., 2004; Purcell et al., 2006). Unfortunately, to the best of our knowledge, no study has focused on the effects of handling and transport stress on juvenile T. tridentatus before release. However, previous studies suggested that health conditions of horseshoe crabs would be affected by hypoxia (Shin et al., 2014), increased temperature (Coates et al., 2012), and aerial exposure originated from transport and holding procedure of biomedical bleeding (Hurton et al., 2009). The changes in water chemistry in transport containers can also be relevant to the survival of released juveniles. Heasman et al. (2004) found increasing temperature, in the combination with elevated ammonia and lowered dissolved oxygen in transport containers, has resulted in substantial mortality of juvenile blacklip abalone, Haliotis rubra, during the transport to the release site. Keeping juvenile T. tridentatus at cooler and constant temperatures during the transport can be attempted to reduce their metabolic activities, which was proven to be effective in the transport of hatchery-reared grouper, Epinephelus larvae (Estudillo and Duray, 2003). Further examinations of an optimal temperature and juvenile density during transport are also necessary for T. tridentatus.
Burying behavior is an important measure for juvenile horseshoe crabs to avoid predation. During high tides when large numbers of diurnal predators can access the upper intertidal zones, juvenile horseshoe crabs tend to bury themselves into sediments. Lee and Morton (2009) observed that less than 5% of juvenile T. tridentatus emerged from sediments during simulated high tides in a laboratory experiment. In such a case, transport with a thin sediment layer prior to release would be an important approach to lower the transport stress. A similar approach has been undertaken by Juinio-Meñez et al. (2012) showing that sand-conditioned juvenile H. scabra took a shorter time burying themselves into the sediment at the released site, which possibly improves their survival in the wild. Alternatively, the juvenile horseshoe crabs can be placed in acclimation cages at the release site for 48 h before the release, in which the technique has been demonstrated successful in improving post-release survival, growth and site fidelity in many fish species such as juvenile winter flounder, Pseudopleuronectes americanus (total length: 130–175 mm, Fairchild et al., 2009) and juvenile common snook, Centropomus undecimalis (fork length: 76–251 mm, Brennan et al., 2006). Gil et al. (2014) demonstrated that hatchery-produced juvenile meagres, Argyrosomus regius had adverse body conditions due to starvation during the first few days after the release, which could cause a high mortality rate.
Genetic factors deserve serious considerations in releasing programs. A study of natural population genetic structure should be conducted once stock enhancement programs have been considered. A systematic review by Kitada (2018) found clear evidence of substantial gene flow from hatcheries, in which the magnitude was attributed to the numbers of broodstock and stocking intensity. Half of 38 empirical studies on marine stock enhancement initiatives demonstrated a reduction in genetic diversity and changes in the population structure of wild populations (Kitada, 2018).
However, no study has investigated the impact of releasing initiatives on the genetic variability of wild horseshoe crab stocks. Sugawara et al. (1988) found no genetic differences among T. tridentatus populations from Japanese and Chinese waters. The recent application of amplified fragment length polymorphism on T. tridentatus populations from the three coastal provinces in southern China also drew a similar conclusion (Xu et al., 2011). In contrast, at a finer scale, several previous studies also suggested the existence of genetically distinct subdivisions between that on Kinmen Island and Magong Island in Taiwan Strait (Yang et al., 2007), between Ninghai and Danzhou along the Chinese coast (Weng et al., 2013), as well as between eastern and western shores around Kyushu, Japan (Nishida and Koike, 2009).
For L. polyphemus, a clear isolation-by-distance model was evident, in which six major zones of genetic discontinuity were identified, including the Gulf of Maine, Mid-Atlantic, Southeast, Florida Atlantic, Northeast Gulf, and Yucatán Peninsula of Mexico (Smith et al., 2017). Within each zone, considerable gene flow was found to occur between geographically proximate localities. The genetic variation patterns may be due to the limited potential of female migration between embayments (King et al., 2005; Swan, 2005) and larval dispersal (Botton and Loveland, 2003).
While the population genetic structure of T. tridentatus is insufficiently clear at present, the wild populations are likely to have very low genetic diversity due to their considerably low population sizes and densities. Mass release of seedstocks from a small number of breeders in the hatchery may have substantial effects on the genetic diversity of wild horseshoe crab populations. A large number of parents from geographically proximate estuaries as “genetic repositories” is generally required for the larger stocking rates to maintain genetic diversity in populations (Kitada et al., 2009). Releasing multiple cohorts of juveniles from mating pairs or replacing the breeders regularly can be the alternative way to avoid genetic degradation caused by inbreeding from mass release. Interactions between wild and released stocks in the environment are worth to be investigated in the future.
The development of hatchery and stock enhancement technology for horseshoe crabs is new. Compared to other Asian species, there are relatively larger numbers of studies focused on the growth and survival of juvenile T. tridentatus under varying husbandry conditions as well as their distribution pattern and habitat characteristics, all of which would benefit the development of the responsible stock enhancement programs. We summarize the important findings from previous studies into a technical framework for guiding the increasing releasing initiatives for the exploited T. tridentatus population, especially along the Chinese coast (Figure 3).
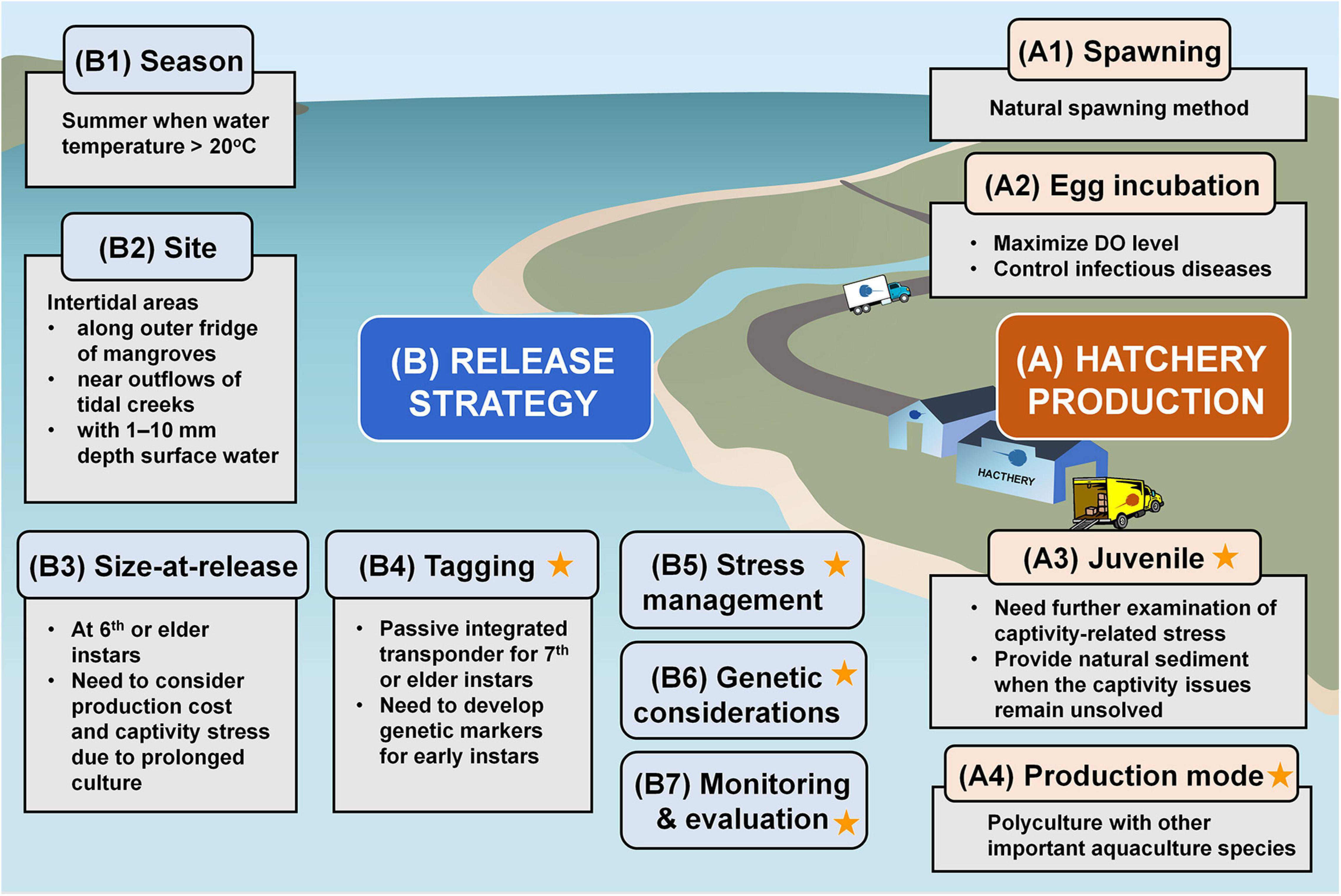
Figure 3. Technical framework for developing the responsible stock enhancement programs for T. tridentatus conservation. The star symbols indicate the research gaps which should be focused in future studies. The sources of information are summarized in Supplementary Table 2.
Despite the recent progress in aquaculture, the mass production of environmentally fit juveniles for releases has been largely impeded by the presence of unidentifiable captivity-related issues due to the knowledge gaps in understanding the growth requirements and complex life histories of T. tridentatus in their nursery habitats. The deficiencies in essential fatty acids and tidal rhythms have been identified to contribute to the lowered immune competence under prolonged culture. Other rearing alternatives such as the use of natural sediment from their nursery habitats (with unknown minor essential nutrients and minerals) to replace the commonly applied aquarium sand can be helpful in maintaining the good health and growth performance of juveniles in culture facilities. Further research is also required to explore other possible culture modes, including polyculture with other economically important aquaculture species, to produce T. tridentatus seedstocks cost-effectively (Figure 3).
Overall, the success of stock enhancement programs must be guided by enhanced knowledge on horseshoe crab biology and ecology, so that the released juveniles can ultimately contribute to spawning biomass. The effectiveness of releases can be increased significantly through responsible release strategies and pre-release acclimation. Release strategies involving the selection of optimal habitats (intertidal areas near mangrove fringes) and timing (during low tides with water temperature exceeds 20°C) with thorough genetic consideration would greatly improve the survival of released juveniles, particularly those at early instar stages. The use of acclimation cages at the release site for the first 48 h or sand conditioning in hatcheries or during the transport would be important to lower natural mortality after releases. Future research should also include the identification of key predators to avoid immediate predation on hatchery-produced juveniles at release sites. Given that efficient tagging methods for the first-year juvenile horseshoe crabs are currently lacking, the development of non-invasive tagging approaches such as genetic markers should be prioritized to evaluate the effectiveness of releasing programs (Figure 3). Follow-up monitoring and assessment after the releases are the prerequisite to optimizing the current stock enhancement protocol.
Apart from the releasing program itself, interventions with other management measures, including nursery habitat recovery, fishing pressure reduction and habitat use restriction during peak spawning, would also allow more promising conservation outcomes. Identification, mitigation and elimination of human-mediated disturbances at the release site must be addressed prior to the releases. Other institutional, socio-economic and legal factors should also receive equal emphasis. The involvement of local communities and other stakeholders in the releasing programs can reduce the ingrained apathy toward the environment, which often contradicts the community’s established practices to consume horseshoe crabs for food and traditional medicine. While there are uncertainties in the potential role of releasing programs, we can expect to gain further insights from continuous research and trial-and-error experience in developing a responsible stock enhancement framework to meet conservation needs for T. tridentatus.
PX and HB led the writing and prepared the original draft. MZ and ZY compiled and clustered data from the published studies. XX, C-CW, XH, XW, JZ, and WZ provided input to different writing sections. SC and PS developed the overall review framework and methods. KK supervised, reviewed and edited the final draft, which was proof-read and approved by all authors. All authors contributed to the article and approved the submitted version.
This research was supported by the Guangxi BaGui Youth Scholars Programme, Beibu Gulf Ocean Development Research Centre under Key Research Base of Humanities and Social Sciences in Guangxi Universities, Guangxi Recruitment Program of Hundred Global Experts, the National Natural Science Foundation of China (32060129), the Guangxi Training Program of Innovation and Entrepreneurship for Undergraduates (202011607133), and Central Public-interest Scientific Institution Basal Research Fund, South China Sea Fisheries Research Institute, CAFS (2019TS21).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank to the students from the College of Marine Sciences, Beibu Gulf for collecting relevant literature for the review. Constructive comments and suggestions from reviewers are much appreciated.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.608155/full#supplementary-material
Anderson, R. L., Watson, W. H. III, and Chabot, C. C. (2013). Sublethal behavioral and physiological effects of the biomedical bleeding process on the American horseshoe crab, Limulus polyphemus. Biol. Bull. 225, 137–151. doi: 10.1086/BBLv225n3p137
Bartley, D. M., and Bell, J. D. (2008). Restocking, stock enhancement, and sea ranching: Arenas of progress. Rev. Fish. Sci. 16, 357–365. doi: 10.1080/10641260701678058
Bell, J. D., Bartley, D. M., Lorenzen, K., and Loneragan, N. R. (2006). Restocking and stock enhancement of coastal fisheries: potential, problems and progress. Fish. Res. 80, 1–8. doi: 10.1016/j.fishres.2006.03.008
Bell, J. D., Leber, K. M., Blankenship, H. L., Loneragan, N. R., and Masuda, R. (2008). A new era for restocking, stock enhancement and sea ranching of coastal fisheries resources. Rev. Fish. Sci. 16, 1–9. doi: 10.1080/10641260701776951
Biswal, G. C., Andia, B. N., Pati, S., and Dash, B. P. (2016). “Conservation of Indian horseshoe crab, Tachypleus gigas through captive rearing,” in Frontiers in Life Sciences, eds R. L. Behera, E. Kariali, and S. K. Sahu (Delhi: Excel India Publishers), 179–185.
Blankenship, H. L., and Leber, K. M. (1995). A responsible approach to marine stock enhancement. Am. Fish. Soc. Symp. 15, 167–175.
Bopp, J. J., Sclafani, M., Smith, D. R., McKown, K., Sysak, R., and Cerrato, R. M. (2019). Geographic-specific capture–recapture models reveal contrasting migration and survival rates of adult horseshoe crabs (Limulus polyphemus). Estuar. Coasts 42, 1570–1585. doi: 10.1007/s12237-019-00595-1
Botton, M. L. (2009). “The ecological importance of horseshoe crabs in estuarine and coastal communities: a review and speculative summary,” in Biology and Conservation of Horseshoe Crabs, eds J. T. Tanacredi, M. L. Botton, and D. R. Smith (Boston: Springer), 45–63. doi: 10.1007/978-0-387-89959-6_3
Botton, M. L., and Loveland, R. E. (2003). Abundance and dispersal potential of horseshoe crab (Limulus polyphemus) larvae in the Delaware estuary. Estuaries 26, 1472–1479. doi: 10.1007/BF02803655
Botton, M. L., Loveland, R. E., and Jackobson, T. R. (1992). Overwintering by trilobite larvae of horseshoe crab Limulus polyphemus on sandy beach of Delaware Bay (New Jersey, USA). Mar. Ecol. Prog. Ser. 88, 289–292.
Brander, K. M. (2007). Global fish production and climate change. Proc. Natl. Acad. Sci. U.S.A. 104, 19709–19714. doi: 10.1073/pnas.0702059104
Brennan, N. P., Darcy, M. C., and Leber, K. M. (2006). Predator-free enclosures improve post-release survival of stocked common snook. J. Exp. Mar. Biol. Ecol. 335, 302–311. doi: 10.1016/j.jembe.2006.04.001
Brennan, N. P., Leber, K. M., Blankenship, H. L., Ransier, J. M., and DeBruler, R. Jr. (2005). An evaluation of coded wire and elastomer tag performance in juvenile common snook under field and laboratory conditions. N. Am. J. Fish. Manage. 25, 437–445. doi: 10.1577/M04-003.1
Cai, X. Y., Lin, Q. W., and Huang, J. Y. (1984). Spawning behavior and early embryonic development of Tachypleus tridentatus. Acta Oceanol. Sin. 6, 663–671.
Carmichael, R. H., and Brush, E. (2012). Three decades of horseshoe crab rearing: a review of conditions for captive growth and survival. Rev. Aquac. 4, 32–43. doi: 10.1111/j.1753-5131.2012.01059.x
Carmichael, R. H., Gaines, E., Sheller, Z., Tong, A., Clapp, A., and Valiela, I. (2009). “Diet composition of juvenile horseshoe crabs: implications for growth and survival of natural and cultured stocks,” in Biology and Conservation of Horseshoe Crabs, eds J. T. Tanacredi, M. L. Botton, and D. R. Smith (Boston: Springer), 521–534. doi: 10.1007/978-0-387-89959-6_33
Carmichael, R. H., Rutecki, D., and Valiela, I. (2003). Abundance and population structure of the Atlantic horseshoe crab Limulus polyphemus in Pleasant Bay, Cape Cod. Mar. Ecol. Progr. Ser. 246, 225–239. doi: 10.3354/meps246225
Ceccarelli, D. M., Logan, M., and Purcell, S. W. (2018). Analysis of optimal habitat for captive release of the sea cucumber Holothuria scabra. Mar. Ecol. Progr. Ser. 588, 85–100. doi: 10.3354/meps12444
Chen, B., and Xia, H. (2002). Tachypleus tridentatus and artificial breeding techniques. China Aquac. 6, 55–56.
Chen, C., Chen, R., Chen, P., Liu, H., and Hsieh, H. Y. (2016). Intermediate culture of juvenile horseshoe crab (Tachypleus tridentatus) mixed with juvenile spotted babylon (Babylonia areolata) for restocking horseshoe crab populations. Aquac. Aquar. Conserv. Legis. 9, 623–633.
Chen, C. P., Yang, M. C., Fan, L. F., Qiu, G., Liao, Y. Y., and Hsieh, H. L. (2015). Co-occurrence of juvenile horseshoe crabs Tachypleus tridentatus and Carcinoscorpius rotundicauda in an estuarine bay, southwestern China. Aquat. Biol. 24, 117–126. doi: 10.3354/ab00641
Chen, Y., Lau, C. W., Cheung, S. G., Ke, C. H., and Shin, P. K. S. (2010). Enhanced growth of juvenile Tachypleus tridentatus (Chelicerata: Xiphosura) in the laboratory: a step towards population restocking for conservation of the species. Aquat. Biol. 11, 37–46. doi: 10.3354/ab00289
Cheng, H., Chabot, C. C., and Watson, W. H. (2016). Influence of environmental factors on spawning of the American horseshoe crab (Limulus polyphemus) in the Great Bay Estuary, New Hampshire, USA. Estuar. Coasts 39, 1142–1153. doi: 10.1007/s12237-015-0044-2
Chiu, H. M., and Morton, B. (2004). The behaviour of juvenile horseshoe crabs, Tachypleus tridentatus (Xiphosura), on a nursery beach at Shui Hau Wan, Hong Kong. Hydrobiologia 523, 29–35. doi: 10.1023/B:HYDR.0000033085.71861.63
Chow, C. W. (2019). “The feasible methodology of stimulating the spawning and incubating the eggs under secondary school environment,” in Presented in the 4th International Workshop on the Science and Conservation of Horseshoe Crabs, Qinzhou.
Coates, C. J., Bradford, E. L., Krome, C. A., and Nairn, J. (2012). Effect of temperature on biochemical and cellular properties of captive Limulus polyphemus. Aquaculture 334, 30–38. doi: 10.1016/j.aquaculture.2011.12.029
Dixon, C. D., Day, R. W., Huchette, S. M., and Shepherd, S. A. (2006). Successful seeding of hatchery-produced juvenile greenlip abalone to restore wild stocks. Fish. Res. 78, 179–185. doi: 10.1016/j.fishres.2005.11.023
Estudillo, C. B., and Duray, M. N. (2003). Transport of hatchery-reared and wild grouper larvae, Epinephelus sp. Aquaculture 219, 279–290. doi: 10.1016/S0044-8486(02)00413-1
Fairchild, E. A., Rennels, N., and Howell, H. (2009). “Using telemetry to monitor movements and habitat use of cultured and wild juvenile winter flounder in a shallow estuary,” in Tagging and Tracking of Marine Animals with Electronic Devices, eds J. L. Nielsen, H. Arrizabalaga, N. Fragoso, A. Hobday, M. Lutcavage, and J. Sibert (Dordrecht: Springer), 5–22. doi: 10.1007/978-1-4020-9640-2_1
Faizul, M. I. M., Eng, H. T., Christianus, A., and Abdel-Hadi, Y. M. (2015). “Bacteria and fungi identified on horseshoe crabs, Tachypleus gigas and Carcinoscorpius rotundicauda in the laboratory,” in Changing Global Perspectives on Horseshoe Crab Biology, Conservation and Management, eds R. H. Carmichael, M. L. Botton, P. K. S. Shin, and S. G. Cheung (Cham: Springer), 303–311. doi: 10.1007/978-3-319-19542-1_17
Fleming, I. A., and Petersson, E. (2001). The ability of released, hatchery salmonids to breed and contribute to the natural productivity of wild populations. Nord. J. Freshwat. Res. 75, 71–98.
Fushimi, H. (2001). Production of juvenile marine finfish for stock enhancement in Japan. Aquaculture 200, 33–53. doi: 10.1016/S0044-8486(01)00693-7
Gaines, E. F., Carmichael, R. H., Grady, S. P., and Valiela, I. (2002). Stable isotopic evidence for changing nutritional sources of juvenile horseshoe crabs. Biol. Bull. 203, 228–230. doi: 10.2307/1543412
Gao, F., Liao, Y., and Ye, F. (2003). Feed study of the Tachypleus tridentatus juvenile. Mar. Sci. Bull. 22, 92–96.
Gil, M. D. M., Palmer, M., Grau, A., Deudero, S., Alconchel, J. I., and Catalán, I. A. (2014). Adapting to the wild: the case of aquaculture-produced and released meagres Argyrosomus regius. J. Fish Biol. 84, 10–30. doi: 10.1111/jfb.12241
Gomez, E. D., and Mingoa-Licuanan, S. S. (2006). Achievements and lessons learned in restocking giant clams in the Philippines. Fish. Res. 80, 46–52. doi: 10.1016/j.fishres.2006.03.017
Gorman, R. (2020). Atlantic horseshoe crabs and endotoxin testing: perspectives on alternatives, sustainable methods, & the 3Rs (replacement, reduction and refinement). Front. Mar. Sci. 7:582132. doi: 10.3389/fmars.2020.582132
Han, Q., Keesing, J. K., and Liu, D. (2016). A review of sea cucumber aquaculture, ranching, and stock enhancement in China. Rev. Fish. Sci. Aquacult. 24, 326–341. doi: 10.1080/23308249.2016.1193472
Heasman, M., Chick, R., Savva, N., Worthington, D., Brand, C., Gibson, P., et al. (2004). Enhancement of Populations of Abalone in NSW Using Hatchery-Produced Seed. NSW Fisheries Final Report Series. Available online at: https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0005/545648/FFRS-62_Heasman-et-al-2004.pdf (accessed August 22, 2020).
Hines, A. H., Johnson, E. G., Young, A. C., Aguilar, R., Kramer, M. A., Goodison, M., et al. (2008). Release strategies for estuarine species with complex migratory life cycles: stock enhancement of Chesapeake blue crabs (Callinectes sapidus). Rev. Fish. Sci. 16, 175–185. doi: 10.1080/10641260701678090
Hong, S., Zhang, X., Zhao, Y., Xie, Y., Zhang, Y., and Xu, H. (2009). “Effect of sediment type on growth and survival of juvenile horseshoe crabs (Tachypleus tridentatus),” in Biology and Conservation of Horseshoe Crabs, eds J. T. Tanacredi, M. L. Botton, and D. R. Smith (Boston: Springer), 535–540. doi: 10.1007/978-0-387-89959-6_34
Hong, S. G. (2011). “Artificial breeding and releasing juveniles back to ocean,” in Biology of Horseshoe Crabs Tachypleus tridentatus, ed. S. G. Hong (Xiamen: Xiamen University Press), 229–232.
Hong, S. G., Liao, S. M., Sun, S. H., and Xue, W. Y. (2011). Artificial Culture Method for Horseshoe Crabs. China Patent No ZL201110367455.3. Beijing: National Intellectual Property Administration.
Hsieh, H. L., and Chen, C. P. (2009). “Conservation program for the Asian horseshoe crab Tachypleus tridentatus in Taiwan: characterizing the microhabitat of nursery grounds and restoring spawning grounds,” in Biology and Conservation of Horseshoe Crabs, eds J. T. Tanacredi, M. L. Botton, and D. R. Smith (Boston: Springer), 417–438. doi: 10.1007/978-0-387-89959-6_26
Hu, M., Kwan, B. K. Y., Wang, Y., Cheung, S. G., and Shin, P. K. S. (2015). “Population structure and growth of juvenile horseshoe crabs Tachypleus tridentatus and Carcinoscorpius rotundicauda (Xiphosura) in southern China,” in Changing Global Perspectives on Horseshoe Crab Biology, Conservation and Management, eds R. H. Carmichael, M. L. Botton, P. K. S. Shin, and S. G. Cheung (Cham: Springer), 167–180. doi: 10.1007/978-3-319-19542-1_8
Hu, M., Shin, P. K., Cheung, S. G., Yan, M., and Wang, Y. (2018). Growth performance and feed utilization of low-cost artificial feeds for juvenile Asian horseshoe crab culture. J. Shellfish Res. 37, 581–589. doi: 10.2983/035.037.0311
Hu, M., Wang, Y., Cheung, S. G., and Shin, P. K. S. (2013a). Comparison of different frozen natural foods on survival and growth of juvenile Chinese horseshoe crab Tachypleus tridentatus (Leach, 1819): implications on laboratory culture. Aquac. Res. 44, 567–573. doi: 10.1111/j.1365-2109.2011.03059.x
Hu, M., Wang, Y., Cheung, S. G., and Shin, P. K. S. (2014). Digestible dietary protein and energy requirements of juvenile Asian horseshoe crabs, Tachypleus tridentatus and Carcinoscorpius rotundicauda. Aquacult. Res. 45, 1621–1633. doi: 10.1111/are.12109
Hu, M., Wu, F., Li, Q., Gan, H., Gong, Z., and Wang, Y. (2013b). Habitat selection of released juvenile Chinese horseshoe crab Tachypleus tridentatus tagged with visible implant elastomer. Mar. Environ. Sci. 32, 907–910.
Huang, T. S., and Tsai, W. S. (2011). “Artificial breeding of Tachypleus tridentatus,” in Epic of Horseshoe Crabs, ed. C. W. Chang (Taiwan: National Museum of Marine Biology and Aquarium), 49–54.
Hurton, L., Berkson, J., and Smith, S. (2009). “The effect of hemolymph extraction volume and handling stress on horseshoe crab mortality,” in Biology and Conservation of Horseshoe Crabs, eds J. T. Tanacredi, M. L. Botton, and D. R. Smith (Boston: Springer), 331–346. doi: 10.1007/978-0-387-89959-6_21
James-Pirri, M. J., Tuxbury, K., Marino, S., and Koch, S. (2005). Spawning densities, egg densities, size structure, and movement patterns of spawning horseshoe crabs, Limulus polyphemus, within four coastal embayments on Cape Cod, Massachusetts. Estuaries 28, 296–313. doi: 10.1007/BF02732863
John, B. A., Nelson, B. R., Sheikh, H. I., Cheung, S. G., Wardiatno, Y., Dash, B. P., et al. (2018). A review on fisheries and conservation status of Asian horseshoe crabs. Biodivers. Conserv. 27, 3573–3598. doi: 10.1007/s10531-018-1633-8
Juinio-Meñez, M. A., de Peralta, G. M., Dumalan, R. J. P., Edullantes, C. M., and Catbagan, T. O. (2012). “Ocean nursery systems for scaling up juvenile sandfish (Holothuria scabra) production: ensuring opportunities for small fishers,” in Asia–Pacific Tropical Sea Cucumber Aquaculture, eds C. A. Hair, T. D. Pickering, and D. J. Mills (New Caledonia: ACIAR), 57–62.
King, T. L., Eackles, M. S., Spidle, A. P., and Brockmann, H. J. (2005). Regional differentiation and sex-biased dispersal among populations of the horseshoe crab Limulus polyphemus. Trans. Am. Fish. Soc. 134, 441–465. doi: 10.1577/T04-023.1
Kitada, S. (2018). Economic, ecological and genetic impacts of marine stock enhancement and sea ranching: a systematic review. Fish Fish. 19, 511–532. doi: 10.1111/faf.12271
Kitada, S., Shishidou, H., Sugaya, T., Kitakado, T., Hamasaki, K., and Kishino, H. (2009). Genetic effects of long-term stock enhancement programs. Aquaculture 290, 69–79. doi: 10.1016/j.aquaculture.2009.02.011
Kwan, B. K. Y., Chan, A. K. Y., Cheung, S. G., and Shin, P. K. S. (2014). Hemolymph quality as indicator of health status in juvenile Chinese horseshoe crab Tachypleus tridentatus (Xiphosura) under laboratory culture. J. Exp. Mar. Biol. Ecol. 457, 135–142. doi: 10.1016/j.jembe.2014.04.011
Kwan, B. K. Y., Chan, A. K. Y., Cheung, S. G., and Shin, P. K. S. (2017a). Marine microalgae as dietary supplements in the culture of juvenile Chinese horseshoe crabs, Tachypleus tridentatus (Xiphosura). Aquacult. Res. 48, 3910–3924. doi: 10.1111/are.13218
Kwan, B. K. Y., Cheung, J. H. Y., Law, A. C. K., Cheung, S. G., and Shin, P. K. S. (2017b). Conservation education program for threatened Asian horseshoe crabs: a step towards reducing community apathy to environmental conservation. J. Nat. Conserv. 35, 53–65. doi: 10.1016/j.jnc.2016.12.002
Kwan, B. K. Y., Cheung, S. G., and Shin, P. K. S. (2015a). A dual stable isotope study for diet composition of juvenile Chinese horseshoe crab Tachypleus tridentatus (Xiphosura) on a seagrass-covered intertidal mudflat. Mar. Biol. 162, 1137–1143. doi: 10.1007/s00227-015-2647-3
Kwan, B. K. Y., Fu, Y., Liao, Y., Wu, Z., Zhou, Z., Cheng, A., et al. (2018a). Ecologically-Simulated Culture System for Juvenile Tachypleus Tridentatus. China Patent No ZL201821334591.6. Beijing: National Intellectual Property Administration.
Kwan, B. K. Y., Hu, M., Wang, Y., Cheung, S. G., and Shin, P. K. S. (2019). Fatty acids from controlled feeding as dietary markers of juvenile Chinese horseshoe crab, Tachypleus tridentatus. J. Mar. Biolog. Assoc. U. K. 99, 421–428. doi: 10.1017/S0025315418000279
Kwan, B. K. Y., Shin, P. K. S., and Cheung, S. G. (2015b). “Preliminary home range study of juvenile Chinese horseshoe crabs, Tachypleus tridentatus (Xiphosura), using passive tracking methods,” in Changing Global Perspectives on Horseshoe Crab Biology, Conservation and Management, eds R. H. Carmichael, M. L. Botton, P. K. S. Shin, and S. G. Cheung (Cham: Springer), 149–166. doi: 10.1007/978-3-319-19542-1_7
Kwan, B. K. Y., Un, V. K., Cheung, S. G., and Shin, P. K. (2018b). Horseshoe crabs as potential sentinel species for coastal health: juvenile haemolymph quality and relationship to habitat conditions. Mar. Freshw. Res. 69, 894–905. doi: 10.1071/MF17210
Kwan, K. Y. (2016). Ecological and Health Perspectives of Juvenile Asian Horseshoe Crabs. doctoral dissertation, City University of Hong Kong, Hong Kong.
Kwan, K. Y., Wong, W. T., Lam, P. Y., Chan, H. K., Lo, H. S., and Cheung, S. G. (2020). Effects of rubble zones from oyster cultivation on habitat utilization and foraging behaviour of the endangered tri-spine horseshoe crab: an implication for intertidal oyster cultivation practices. J. Environ. Manage. 271:110925. doi: 10.1016/j.jenvman.2020.110925
Laurie, K., Chen, C. P., Cheung, S. G., Do, V., Hsieh, H., John, A., et al. (2019). Tachypleus tridentatus. The IUCN Red List of Threatened Species 2019: e.T21309A149768986. Available online at: https://dx.doi.org/10.2305/IUCN.UK.2019-1.RLTS.T21309A149768986.en (accessed August 22, 2020).
Le Vay, L., Lebata, M. J. H., Walton, M., Primavera, J., Quinitio, E., Lavilla-Pitogo, C., et al. (2008). Approaches to stock enhancement in mangrove-associated crab fisheries. Rev. Fish. Sci. 16, 72–80. doi: 10.1080/10641260701727285
Lee, C. N., and Morton, B. (2005). Experimentally derived estimates of growth by juvenile Tachypleus tridentatus and Carcinoscorpius rotundicauda (Xiphosura) from nursery beaches in Hong Kong. J. Exp. Mar. Biol. Ecol. 318, 39–49. doi: 10.1016/j.jembe.2004.12.010
Lee, C. N., and Morton, B. (2009). “Emergence behavior of juvenile Tachypleus tridentatus under simulated tidal conditions in the laboratory and at two different sediment temperatures,” in Biology and Conservation of Horseshoe Crabs, eds J. T. Tanacredi, M. L. Botton, and D. R. Smith (Boston: Springer), 275–283. doi: 10.1007/978-0-387-89959-6_17
Li, F., Liao, Y., and Dong, X. (1999). The influence of salinity upon embryogeny of Tachypleus tridentatus. J. Zhanjiang Ocean Univ. 28, 621–629.
Li, H. Y. (2008). The Conservation of Horseshoe Crabs in Hong Kong. master’s thesis, City University of Hong Kong, Hong Kong.
Liang, G. Y. (1987). The research of artificial incubation of Chinese horseshoe crab. Mar. Sci. 1, 10–47.
Liao, S. H. (2011). “Culture and restocking of Tachypleus tridentatus,” in Epic of Horseshoe Crabs, ed. C. W. Chang (Taiwan: National Museum of Marine Biology and Aquarium), 41–48.
Liao, Y. (2015). Improved Artificial Culture Method for Juvenile Tachypleus Tridentatus. China Patent No 201510220337.8. Beijing: National Intellectual Property Administration.
Liao, Y., Hsieh, H. L., Xu, S., Zhong, Q., Lei, J., Liang, M., et al. (2019a). Wisdom of Crowds reveals decline of Asian horseshoe crabs in Beibu Gulf, China. Oryx 53, 222–229. doi: 10.1017/S003060531700117X
Liao, Y., Liu, J., Lei, J., Wang, P., Wang, P., and Li, W. F. (2015). Improved Incubation Basket for Tachypleus Tridentatus. China Patent No ZL201520858265.5. Beijing: National Intellectual Property Administration.
Liao, Y., Liu, K., Wu, H., Xu, Y., Huang, H., Xu, S., et al. (2019b). How survival and food intake of tri-spine horseshoe crabs, Tachypleus tridentatus respond to thermal variation: implications for understanding its distribution limit. J. Nat. Hist. 53, 1951–1960. doi: 10.1080/00222933.2019.1679268
Liao, Y. Y., and Li, X. M. (2001). Present situation of horseshoe crab resources in the sea area of China and tactics of preservation. Resour. Sci. 23, 55–59.
Lorenzen, K. (1996). The relationship between body weight and natural mortality in fish: a comparison of natural ecosystems and aquaculture. J. Fish Biol. 49, 627–647. doi: 10.1111/j.1095-8649.1996.tb00060.x
Lorenzen, K. (2000). Allometry of natural mortality as a basis for assessing optimal release size in fish stocking programmes. Can. J. Fish. Aquat. Sci. 57, 2374–2381. doi: 10.1139/f00-215
Lorenzen, K. (2005). Population dynamics and potential of fisheries stock enhancement: practical theory for assessment and policy analysis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360, 171–189. doi: 10.1098/rstb.2004.1570
Lorenzen, K., Leber, K. M., and Blankenship, H. L. (2010). Responsible approach to marine stock enhancement: an update. Rev. Fish. Sci. 18, 189–210. doi: 10.1080/10641262.2010.491564
Mattei, J. H., Beekey, M. A., Potter, H. R., Bond, C. S., Woronik, A. R., Roberts, J. A., et al. (2011). Estimation of short-term tag-induced mortality in horseshoe crabs Limulus polyphemus. Trans. Am. Fish. Soc. 140, 954–958. doi: 10.1080/00028487.2011.601223
Miao, F., Zhao, Z., Li, Q., Song, J., Wang, Y., and Hu, M. (2020). Impact of initial feeding and molting on Tachypleus tridentatus gut microbiota. Curr. Microbiol. 77, 2847–2858. doi: 10.1007/s00284-020-02108-x
Molony, B. W., Lenanton, R., Jackson, G., and Norriss, J. (2005). Stock enhancement as a fisheries management tool. Rev. Fish. Sci. 13, 409–432. doi: 10.1007/s11160-005-1886-7
Nishida, S., and Koike, H. (2009). “Genetic structure of Japanese populations of Tachypleus tridentatus by mtDNA AT-rich region sequence analysis,” in Biology and Conservation of Horseshoe Crabs, eds J. T. Tanacredi, M. L. Botton, and D. R. Smith (Boston: Springer), 183–196. doi: 10.1007/978-0-387-89959-6_11
Nolan, M. W., and Smith, S. A. (2009). “Clinical evaluation, common diseases, and veterinary care of the horseshoe crab, Limulus polyphemus,” in Biology and Conservation of Horseshoe Crabs, eds J. T. Tanacredi, M. L. Botton, and D. R. Smith (Boston: Springer), 479–499. doi: 10.1007/978-0-387-89959-6_30
Oka, H. (1943). Recherches sur l’embryologie causale du Limule. II. Sci. Rep. Tokyo Bunrika Daigaku Sec. B 6, 87–127.
Oliver, M. D., Stewart, R., Mills, D., Macdiarmid, A. B., and Gardner, C. (2005). Stock enhancement of rock lobsters (Jasus edwardsii): timing of predation on naive juvenile lobsters immediately after release. New Zeal. J. Mar. Freshw. Res. 39, 391–397. doi: 10.1080/00288330.2005.9517320
Owings, M., Chabot, C., and Watson, W. III (2019). Effects of the biomedical bleeding process on the behavior of the American horseshoe crab, Limulus polyphemus, in its natural habitat. Biol. Bull. 236, 207–223. doi: 10.1086/702917
Patten, W. (1894). Artificial modification of the segmentation and blastoderm of Limulus polyphemus. Zool. Anz. 9, 72–78.
Penn, D., and Brockmann, H. J. (1994). Nest-site selection in the horseshoe crab, Limulus polyphemus. Biol. Bull. 187, 373–384. doi: 10.2307/1542294
Poh, B., Tweedley, J. R., Chaplin, J. A., Trayler, K. M., and Loneragan, N. R. (2018). Estimating predation rates of restocked individuals: the influence of timing-of-release on metapenaeid survival. Fish. Res. 198, 165–179. doi: 10.1016/j.fishres.2017.09.019
Purcell, S. W., Blockmans, B. F., and Agudo, N. N. (2006). Transportation methods for restocking of juvenile sea cucumber, Holothuria scabra. Aquaculture 251, 238–244. doi: 10.1016/j.aquaculture.2005.04.078
Purcell, S. W., and Simutoga, M. (2008). Spatio-temporal and size-dependent variation in the success of releasing cultured sea cucumbers in the wild. Rev. Fish. Sci. 16, 204–214. doi: 10.1080/10641260701686895
Razali, F. N., Ismail, N., Fisal, A., Tuan Zainazor, T. C., Mohamad, F., and Shamsuddin, A. (2020). Growth performance and feeding utilization of alternative feed for adult horseshoe crabs Carcinoscorpius rotundicauda kept in captivity. Aquac. Res. 51, 1523–1532. doi: 10.1111/are.14500
Romana-Eguia, M. R. R. (2006). “Application of DNA-based markers in stock enhancement programs,” in Proceedings of the Regional Technical Consultation on Stock Enhancement for Threatened Species of International Concern, eds J. H. Primavera, E. T. Quinitio, and M. R. R. Romana-Eguia (Iloilo: Southeast Asian Fisheries Development Center), 7–15.
Rudloe, A. (1979). Locomotor and light responses of larvae of the horseshoe crab, Limulus polyphemus (L.). Biol. Bull. 157, 494–505. doi: 10.2307/1541033
Schaller, S. Y., Chabot, C. C., and Watson, W. H. III (2010). Seasonal movements of American horseshoe crabs Limulus polyphemus in the Great Bay estuary, New Hampshire (USA). Curr. Zool. 56, 587–598. doi: 10.1093/czoolo/56.5.587
Schreibman, M. P., and Zarnoch, C. B. (2009). “Aquaculture methods and early growth of juvenile horseshoe crabs (Limulus polyphemus),” in Biology and Conservation of Horseshoe Crabs, eds J. T. Tanacredi, M. L. Botton, and D. R. Smith (Boston: Springer), 501–511. doi: 10.1007/978-0-387-89959-6_31
Sekiguchi, K., Yamamichi, Y., Seshimo, H., and Sugita, H. (1988). “Normal development,” in Biology of Horseshoe Crabs, ed. K. Sekiguchi (Tokyo: Science House Co. Ltd), 375–382.
Shin, P. K. S., Chan, C. S. K., and Cheung, S. G. (2014). Physiological energetics of the fourth instar of Chinese horseshoe crabs (Tachypleus tridentatus) in response to hypoxic stress and re-oxygenation. Mar. Pollut. Bull. 85, 522–525. doi: 10.1016/j.marpolbul.2013.10.023
Shinn, A. P., Mühlhölzl, A. P., Coates, C. J., Metochis, C., and Freeman, M. A. (2015). Zoothamnium duplicatum infestation of cultured horseshoe crabs (Limulus polyphemus). J. Invert. Pathol. 125, 81–86. doi: 10.1016/j.jip.2014.12.002
Shuster, C. N., and Sekiguchi, K. (2009). “Basic habitat requirements of the extant species of horseshoe crabs (Limulacea),” in Biology and Conservation of Horseshoe Crabs, eds J. T. Tanacredi, M. L. Botton, and D. R. Smith (Boston: Springer), 115–129. doi: 10.1007/978-0-387-89959-6_7
Smith, D. R., Brockmann, H. J., Beekey, M. A., King, T. L., Millard, M. J., and Zaldívar-Rae, J. (2017). Conservation status of the American horseshoe crab (Limulus polyphemus): a regional assessment. Rev. Fish Biol. Fish. 27, 135–175. doi: 10.1007/s11160-016-9461-y
Sugawara, K., Yonekawa, H., Tagashima, Y., and Sekiguchi, K. (1988). “Mitochondrial DNA polymorphisms,” in Biology of Horseshoe Crabs, ed. K. Sekiguchi (Tokyo: Science House Co. Ltd), 375–382.
Svåsand, T., Kristiansen, T. S., Pedersen, T., Salvanes, A. G. V., Engelsen, R., Naevdal, G., et al. (2000). The enhancement of cod stocks. Fish Fish. 1, 173–205. doi: 10.1046/j.1467-2979.2000.00017.x
Swan, B. L. (2005). Migrations of adult horseshoe crabs, Limulus polyphemus, in the middle Atlantic bight: a 17-year tagging study. Estuaries 28, 28–40. doi: 10.1007/BF02732751
Taylor, M. D., Chick, R. C., Lorenzen, K., Agnalt, A.-L., Leber, K. M., Blankenship, H. L., et al. (2017). Fisheries enhancement and restoration in a changing world. Fish. Res. 186, 407–412. doi: 10.1016/j.fishres.2016.10.004
Tinker-Kulberg, R., Dellinger, A., Brady, T. E., Robertson, L., Goddard, M. K., Bowzer, J., et al. (2020a). Effects of diet on the biochemical properties of Limulus amebocyte lysate from horseshoe crabs in an aquaculture setting. Front. Mar. Sci. 7:541604. doi: 10.3389/fmars.2020.541604
Tinker-Kulberg, R., Dellinger, A., Gentit, L. C., Fluech, B. A., Wilder, C. A., Spratling, I. L., et al. (2020b). Evaluation of indoor and outdoor aquaculture systems as alternatives to harvesting hemolymph from random wild capture of horseshoe crabs. Front. Mar. Sci. 7:568628. doi: 10.3389/fmars.2020.568628
Tinker-Kulberg, R., Dellinger, K., Brady, T., Robertson, L., Levy, J. H., Abood, S. K., et al. (2020c). Horseshoe crab aquaculture as a sustainable endotoxin testing resource. Front. Mar. Sci. 7:153. doi: 10.3389/fmars.2020.00153
Tsuchiya, K. (2009). “The history of horseshoe crab research and conservation in Japan,” in Biology and Conservation of Horseshoe Crabs, eds J. T. Tanacredi, M. L. Botton, and D. R. Smith (Boston: Springer), 559–570. doi: 10.1007/978-0-387-89959-6_36
Vasquez, M. C., Johnson, S. L., Brockmann, H. J., and Julian, D. (2015). Nest site selection minimizes environmental stressor exposure in the American horseshoe crab, Limulus polyphemus (L.). J. Exp. Mar. Biol. Ecol. 463, 105–114. doi: 10.1016/j.jembe.2014.10.028
Wada, T., Mitsushio, T., Inoue, S., Koike, H., and Kawabe, R. (2016). Movement patterns and residency of the critically endangered horseshoe crab Tachypleus tridentatus in a semi-enclosed bay determined using acoustic telemetry. PLoS One 11:e0147429. doi: 10.1371/journal.pone.0147429
Wada, T., Yamada, T., Shimizu, D., Aritaki, M., Sudo, H., Yamashita, Y., et al. (2010). Successful stocking of a depleted species, spotted halibut Verasper variegatus, in Miyako Bay, Japan: evaluation from post-release surveys and landings. Mar. Ecol. Progr. Ser. 407, 243–255. doi: 10.3354/meps08553
Wang, C. C., Kwan, K. Y., Shin, P. K. S., Cheung, S. G., Itaya, S., Iwasaki, Y., et al. (2020). Future of Asian horseshoe crab conservation under explicit baseline gaps: a global perspective. Glob. Ecol. Conserv. 2020:e01373. doi: 10.1016/j.gecco.2020.e01373
Weng, Z. H., Xie, Y. J., Xiao, Z. Q., Wang, Z. Y., and Gui, J. F. (2013). Microsatellite and mitochondrial DNA analysis of the genetic structure of Chinese horseshoe crab (Tachypleus tridentatus) in southeast China coast. Afr. J. Biotechnol. 12, 2088–2099. doi: 10.5897/AJB12.1912
Wu, T. J., Gan, H., Luo, B., Nong, Z., Chen, X. L., Gong, Z. L., et al. (2014). Effective Artificial Breeding Method for Horseshoe Crabs from Fertilized Eggs to the Second-Instar Juveniles. China Patent No 201410450199.8. Beijing: National Intellectual Property Administration.
Xie, X., Wu, Z., Wang, C. C., Fu, Y., Wang, X., Xu, P., et al. (2020). Nursery habitat for Asian horseshoe crabs along the northern Beibu Gulf, China: implications for conservation management under baseline gaps. Aquat. Conserv. Mar. Freshw. Ecosyst. 30, 260–272. doi: 10.1002/aqc.3259
Xu, Q. A., Chen, F., Shin, P. K. S., Cheung, S. G., Chen, Y., and Ke, C. H. (2011). AFLP analysis of genetic variation among three natural populations of horseshoe crab Tachypleus tridentatus along Chinese coast. Chinese J. Oceanol. Limnol. 29, 284–289. doi: 10.1007/s00343-011-0066-y
Xu, Z., Wang, Y., Gul, Y., Li, Q., Song, J., and Hu, M. (2020). Effects of copper supplement on the immune function and blood-chemistry in adult Chinese horseshoe crab Tachypleus tridentatus. Aquaculture 515:734576. doi: 10.1016/j.aquaculture.2019.734576
Yang, M. C., Chen, C. A., Hsieh, H. L., and Chen, C. P. (2007). Population subdivision of the tri-spine horseshoe crab, Tachypleus tridentatus, in Taiwan Strait. Zool. Sci. 24, 219–224. doi: 10.2108/zsj.24.219
Zhou, H., and Morton, B. (2004). The diets of juvenile horseshoe crabs, Tachypleus tridentatus and Carcinoscorpius rotundicauda (Xiphosura), from nursery beaches proposed for conservation in Hong Kong. J. Nat. Hist. 38, 1915–1925. doi: 10.1080/0022293031000155377
Keywords: captive rearing, release strategy, optimal diet, size at release, tagging
Citation: Xu P, Bai H, Xie X, Wang C-C, Huang X, Wang X, Zhang M, Ye Z, Zhu J, Zhen W, Cheung SG, Shin PKS and Kwan KY (2021) Tri-Spine Horseshoe Crab Aquaculture, Ranching and Stock Enhancement: Perspectives and Challenges. Front. Mar. Sci. 8:608155. doi: 10.3389/fmars.2021.608155
Received: 19 September 2020; Accepted: 13 January 2021;
Published: 09 February 2021.
Edited by:
Rochelle Diane Seitz, College of William & Mary, United StatesReviewed by:
Neil R. Loneragan, Murdoch University, AustraliaCopyright © 2021 Xu, Bai, Xie, Wang, Huang, Wang, Zhang, Ye, Zhu, Zhen, Cheung, Shin and Kwan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kit Yue Kwan, a2l0eXVla3dhbkBiYmd1LmVkdS5jbg==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.