- 1Institute of Environmental and Marine Sciences, Silliman University, Dumaguete, Philippines
- 2Center for Research and Engagement, University of St. La Salle, Bacolod, Philippines
Global marine mammal research is disproportionately lacking compared to terrestrial mammal research and is strongly biased toward populations in Europe, North America, New Zealand, and Australia. With high extinction risks facing marine mammals in the tropics, we sought to identify potential drivers of research effort and extinction risk evaluations for marine mammals in the Philippines as a model for tropical island nations with limited resources and research capacity. Using a bibliographic approach, we compiled all materials on marine mammal research in the Philippines from 1991 to 2020, which we categorized into eight thematic areas of research focus. We reviewed all materials based on their research focus to assess the current scientific knowledge of local marine mammal populations. Using a simple metric to calculate research effort allocation, we found that all marine mammal species in the Philippines receive inadequate research attention. Using generalized linear models, we analyzed the relationship of potential factors that drive research effort. The model with the lowest Akaike Information Criterion value suggests that frequency of marine mammal stranding incidents may influence an increase in research effort on marine mammals by providing access to biological specimens that would normally be difficult to obtain. Strandings are unfortunate events with often unclear causes, but they provide an opportunity to collect data from behaviorally cryptic animals in areas where financial constraints often hamper scientific progress. We also determined that a national Red List evaluation was predicted by increased research effort. Maximizing local research using all materials from strandings and building research capacity may be an alternative to expensive field-based methods to increase knowledge on local marine mammal populations.
Introduction
Among all the biogeographical regions in the world, the tropics contain the highest levels of biodiversity, as well as high levels of conservation threats (Myers et al., 2000; Brooks et al., 2002). As such, they present the most pressing need for conservation efforts. Southeast Asia in particular has some of the highest number of species at risk of extinction due to various compounding factors (Sodhi et al., 2010; Hughes, 2017). This biodiversity extends beyond the terrestrial ecosystem. The complex geological history of Southeast Asia (Hall, 1998) gave rise to an extensive and diverse marine environment with the highest global diversity of marine organisms (Hughes et al., 2002; Carpenter and Springer, 2005) that encompass a dynamic food web from planktons in the deep scattering layer to large predators such as marine mammals (Field et al., 1998; Tucker and Rogers, 2014; Trites, 2019).
Conservation is an evidence-based discipline (Sutherland et al., 2004) but recent global reviews have identified a mismatch between conservation priorities and evidence-generating research effort (Wilson et al., 2016; Lindegren et al., 2018; Willer et al., 2019). Moreover, in regions of top conservation priority such as Southeast Asia, there is a substantial lack of research effort to address knowledge gaps which are fundamental in addressing escalating global biodiversity loss (Sodhi et al., 2010). Currently, global marine mammal research is disproportionately lacking compared to terrestrial mammals (Schipper et al., 2008) and is strongly biased toward populations in Europe and North America (Jarić et al., 2015) as well as New Zealand and Australia.
To date, there are 28 confirmed marine mammals found in the Philippines, though up to 30 might be present in the country’s waters. A comprehensive review of the conservation status of 26 species is detailed in Alava et al. (2012) which assessed the extinction risk of all known species at the time of publication through the IUCN Red List Assessment (RLA) process. Twenty out of 26 species (77%) that were assessed were classified as “Data Deficient” and the remaining six were either “Vulnerable” or “Critically Endangered.” Since then, two beaked whale species in Philippine waters have been verified and added to the list. A ginkgo-toothed beaked whale (Mesoplodon ginkgodens) stranded in 2010 (SEAMAMIII, 2015) and the 8th specimen of the rare Deraniyagala’s beaked whale (Mesoplodon hotaula) was confirmed from a stranded individual through molecular analyses (Lacsamana et al., 2015). An additional two species from stranding records have been added to the list, but could not be verified by the authors. For this review, we maintain a provisional list of 30 species and subspecies of marine mammals from two orders (Cetacea and Sirenia) documented within Philippine waters. Except for historical whaling records (Townsend, 1935; Slijper et al., 1964) and a documentation of a whale stranding in 1925 (Herre, 1925), the earliest peer-reviewed journal publication on marine mammals in the Philippines was not produced until 1992 (Leatherwood et al., 1992). The extinction risk assessments are certainly alarming given the lack of species- and population-specific information. Filling these critical information gaps on basic biological and ecological processes is needed for a complete picture of a species’ overall population health and conservation outlook.
This review quantifies all available publications, including peer-reviewed articles, conference proceedings, and technical reports on marine mammal research in the Philippines from 1991 to 2020. We categorized the publications into eight thematic areas: (1) biodiversity and distribution, (2) biology and ecology, (3) fisheries and bycatch, (4) conservation, (5) taxonomy and systematics, (6) socio-politics and governance, (7) reviews, and (8) health and ecotoxicology. Using a bibliographic approach, we modeled the relationship of research effort with various parameters to identify potential drivers of research and identify areas that can facilitate more research attention, and areas that can benefit from increased research effort. We also identified species that required more research attention. This review offers insights into major research trends in relation to marine mammals in the Philippines and identifies conservation and knowledge gaps that can be used to outline priorities for future marine mammal research.
Materials and Methods
Data Sources
We compiled all available published materials including peer-reviewed articles, conference proceedings, and technical reports that contain relevant datasets on marine mammals in the Philippines. The materials were obtained from authors’ personal collections, experts’ self-archived works in ResearchGate1, university libraries, laboratory websites, and personal communications with researchers working on Philippine marine mammals. A bibliographic search of SCOPUS-indexed publications (Elsevier) and Google Scholar2 were also performed to complement the above. We used the following keyword search combination (Philippines OR Luzon OR Visayas OR Mindanao) AND (“marine mammals” OR cetaceans OR dolphin OR dolphins OR whale OR whales OR dugong OR dugongs) to come up with a list of publications. The materials were categorized according to type, year of publication, and research focus (Table 1).

Table 1. Different thematic areas of research on marine mammals in the Philippines identified for the purpose of this review.
We also gleaned data from peer-reviewed literature and readily available secondary sources (i.e., online databases and field guides) to collect data on life history characteristics, and conservation-based assessments for each of the 30 species in the provisional list of marine mammals occurring in Philippine waters. Life history traits such as age at sexual maturity and maximum lengths were obtained from Jefferson et al. (2015); Perrin et al. (2008), and Myers et al. (2020). Data on group sizes were from Dolar et al. (2006); Acebes et al. (2007), de la Paz (2012), and Tiongson and Karczmarski (2016). Stranding data were obtained from Aragones et al. (2017) and Aragones and Laggui (2019).
SREA
We applied the Species-Research Effort Allocation (SREA) metric developed by Tanalgo and Hughes (2018) to identify species that received adequate attention in the 30-year period covered in this review. The SREA is expressed as
where: SREA, Species-Research Effort Allocation; x, species; R∘, number of times species (x) was recorded from publications; y, number of years covered by the review.
An SREA value equal to 1.00 indicates that the species received average attention in (y) relative to all other species. An SREA value > 1.00 indicates higher research effort dedicated to the species in question whereas a value < 1.00 indicates low effort.
Model Selection
We performed data exploration, using graphical and descriptive statistics, to reveal general patterns in research effort on different species and research themes across 30 years. We classified species according to their preferred habitat types (i.e., coastal and offshore).
We employed model selection using generalized linear models (GLMs) for overdispersed data with number of publications for each species as the response variable and age at sexual maturity, maximum length (m), group size, habitat, and total strandings as explanatory variables. Figure 1 illustrates the life history and behavioral characteristics of marine mammals that may potentially influence their observability and data collection in the field. Since population sizes, morphology, and behavior influence detectability of animals in the field, we used average age at sexual maturity for both females and males to represent population dynamics. Maximum lengths of both sexes were averaged and used to represent morphology. Gregariousness, denoted as group size, was used to represent group dynamics. Species which prefer coastal habitats have higher chances of being observed compared to offshore species, which may skew research focus toward coastal species. We include preferred habitat type as a covariate, represented as the distance of a species’ preferred habitat from shore. Data on the number of stranded individuals of each species from 2005 to 2018 were compiled to represent accessibility of specimens for research.
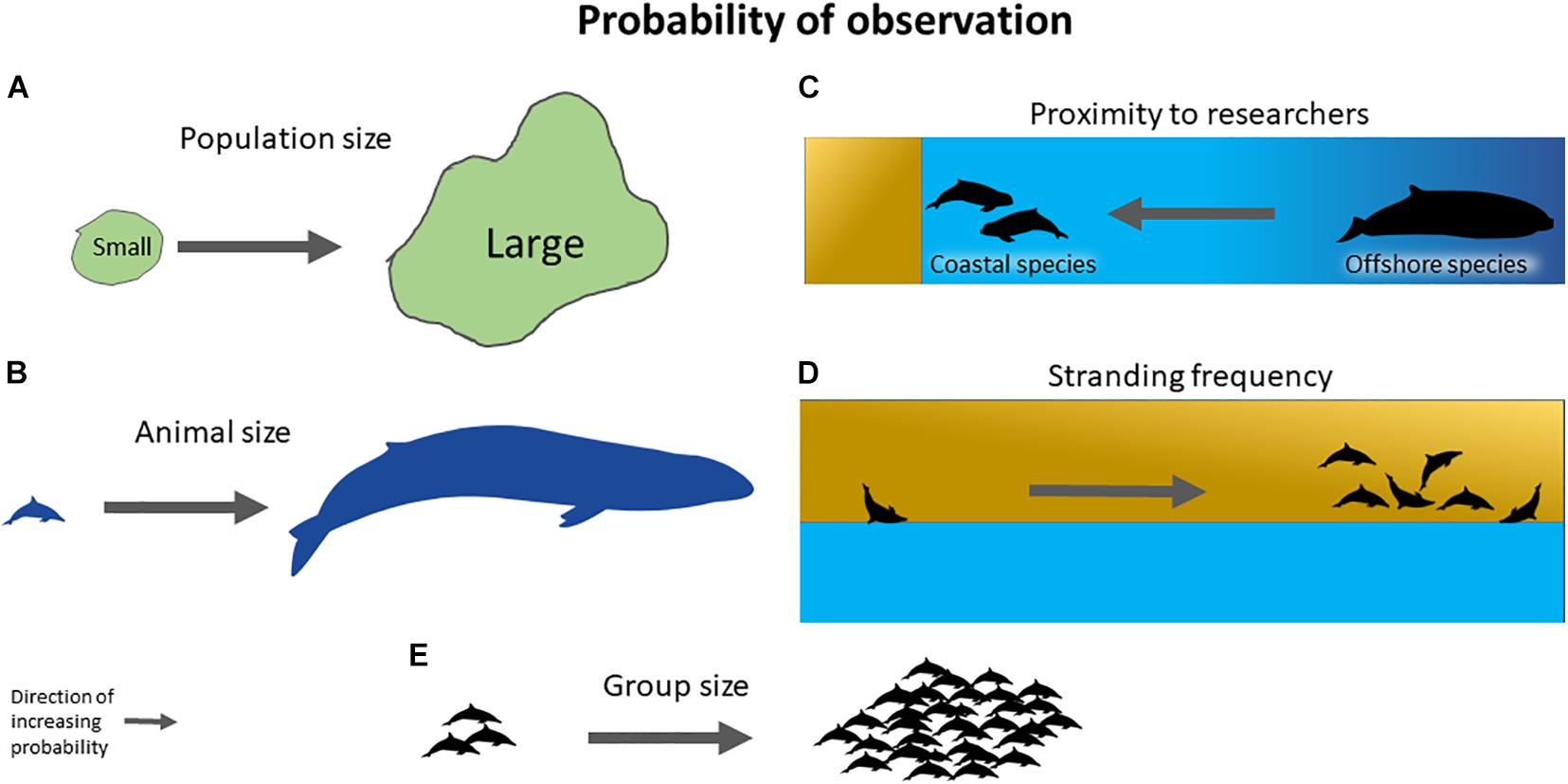
Figure 1. The probability of observation and data collection hinges on the theoretical framework that (A) species with larger population sizes will have more chances of being observed in the field, (B) larger species may be easier to find than smaller species, (C) coastal species may be easier to find and observe than offshore species, (D) species that strand more often may provide accessible data for research that are otherwise difficult to obtain, and (E) species that form larger group sizes may have higher chances of being found than species that are solitary or form smaller groups. The arrows indicate direction of increased probability. Marine mammal silhouettes were accessed through Phylopic 2019 via a Creative Commons Attribution-ShareAlike 3.0 Unported license by Chris Huh.
To identify outliers that could potentially influence the models, we used Cleveland dotplots to visually inspect the data. We also plotted a correlation matrix of the covariates using pairplots to examine collinearity. We measured the degree of collinearity by calculating the variance inflation factors (VIFs) for each pair of covariates using the function vif() in the CAR Rpackage (Fox and Weisberg, 2019).
We performed a negative binomial GLM with logarithmic link function to account for overdispersion of the response variable using the glm.nb() function in the MASS Rpackage (Venables and Ripley, 2002). An automatic backward stepwise selection procedure based on Akaike Information Criterion (AIC) values using the command stepAIC() in the MASS package was used to compare the best-fit model out of 16 possible models. The model with the smallest AIC value was selected as the most parsimonious model after comparing the models using the AICcmodavg Rpackage (Mazerolle, 2020).
Extinction Risk
We examined if the number of publications influenced whether a species was locally evaluated in the National Red List Assessment (NRLA) (Alava et al., 2012). We used the NRLA to reflect local extinction risks. We defined NRLA evaluation as a binary factor, with species that were evaluated as 1, and species that were not evaluated as 0. Species that were evaluated as “Data Deficient” were categorized as not evaluated. We classified the species into five taxonomic groups (i.e., superfamily). We performed a binomial generalized linear mixed effects model (GLMM) by maximum likelihood (Laplace Approximation) (LA) using glmer() function in lme4 R package (Bates et al., 2015) with NRLA evaluation as the response variable and the number of publications as the fixed variable and superfamily as the random effect. All analyses were performed in R (R Core Team, 2020).
Results
Data Sources
The document compilation returned 174 documents, 60% of which are unpublished research such as thesis and dissertations, grant reports, and conference presentation abstracts. The remaining 40% consisted of books, book chapters, conference proceedings, and journal articles. Out of the published documents, we identified 54 published peer-reviewed research papers on marine mammals (96% journal articles, 4% conference proceedings) to use in our analyses. There has been a steady increase in the number of publications since the earliest peer-reviewed publication in 1992 (Figure 2), with a disproportionate output on diversity and distribution studies (n = 13, accounting for 24.5% of publications) and biology and ecology (n = 16, or 30%). Publications on fisheries and by-catch (n = 6), health and ecotoxicology (n = 5), conservation (n = 3), socio-politics and governance (n = 2), taxonomy and systematics (n = 3), and review papers (n = 1) account for the remaining 45.3%. Annual publication rate range between 0 and 5 ( = 1.77 ± 1.38) with no significant interannual differences although an apparent doubling can be noted in the last 5 years (Figure 3).
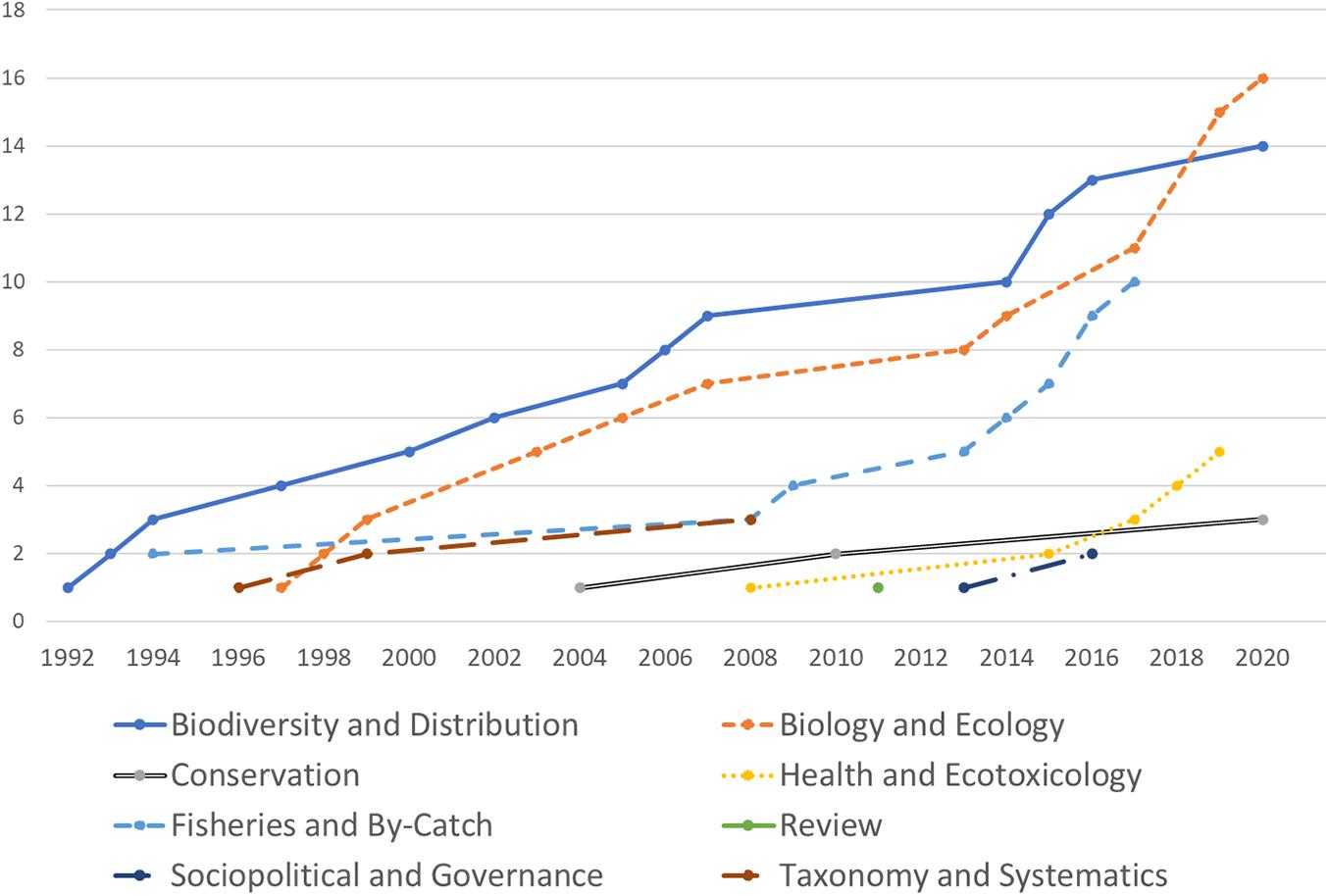
Figure 2. Cumulative number of marine mammal research in the Philippines from 1991 to 2020 based on peer-reviewed journal articles and conference proceedings.
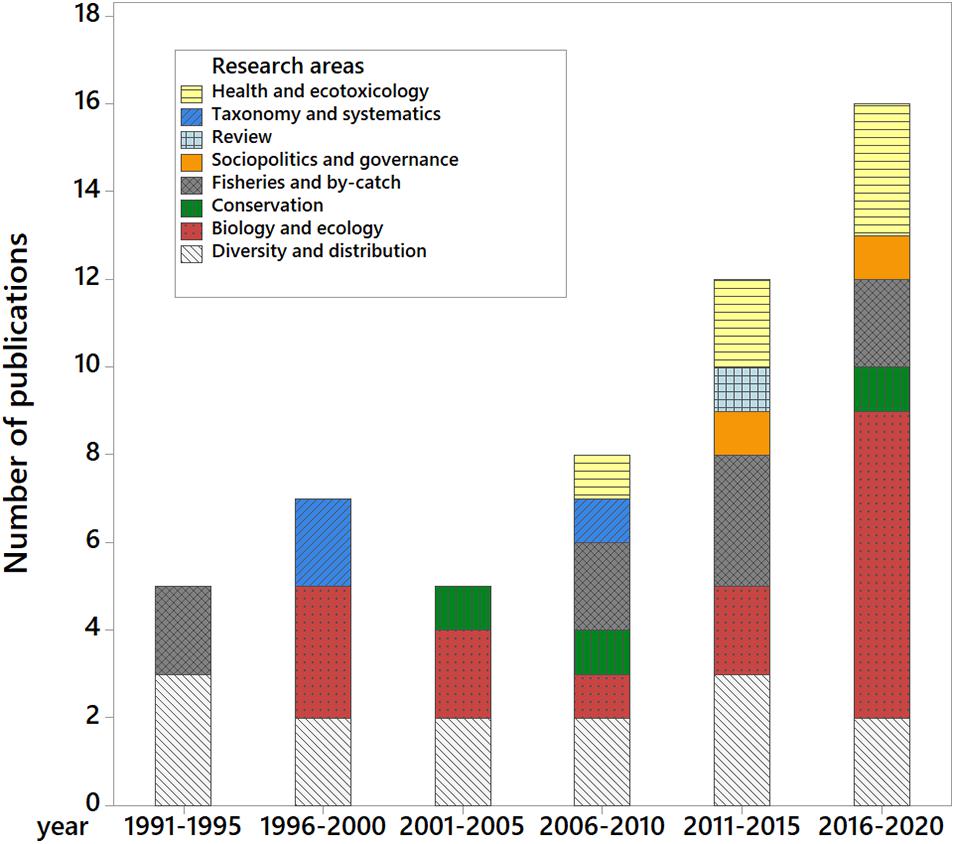
Figure 3. Summary of peer-reviewed journal articles and conference proceedings on Philippine marine mammals from 1991 to present in 5-year periods. Annual publication rate range between 0 and 5 papers a year ( = 1.77 ± 1.38).
SREA
All species were well below the threshold of 1.00, suggesting that all Philippine marine mammals are receiving inadequate research attention. For simplicity, we will refer to species by their common names from this point and refer the reader to Table 2 for the binomial names. The species with the highest SREA (0.63) is the spinner dolphin, which is the most widely distributed species with the highest relative abundance. The spinner dolphin is mentioned in 19 publications out of 53, followed by Fraser’s dolphin, which is mentioned in 15 publications (SREA = 0.5). All the remaining species have an SREA of 0.37 or below (Table 2). All six NRLA evaluated species have a mean SREA of 0.37 compared to an SREA of 0.16 for all unassessed species.
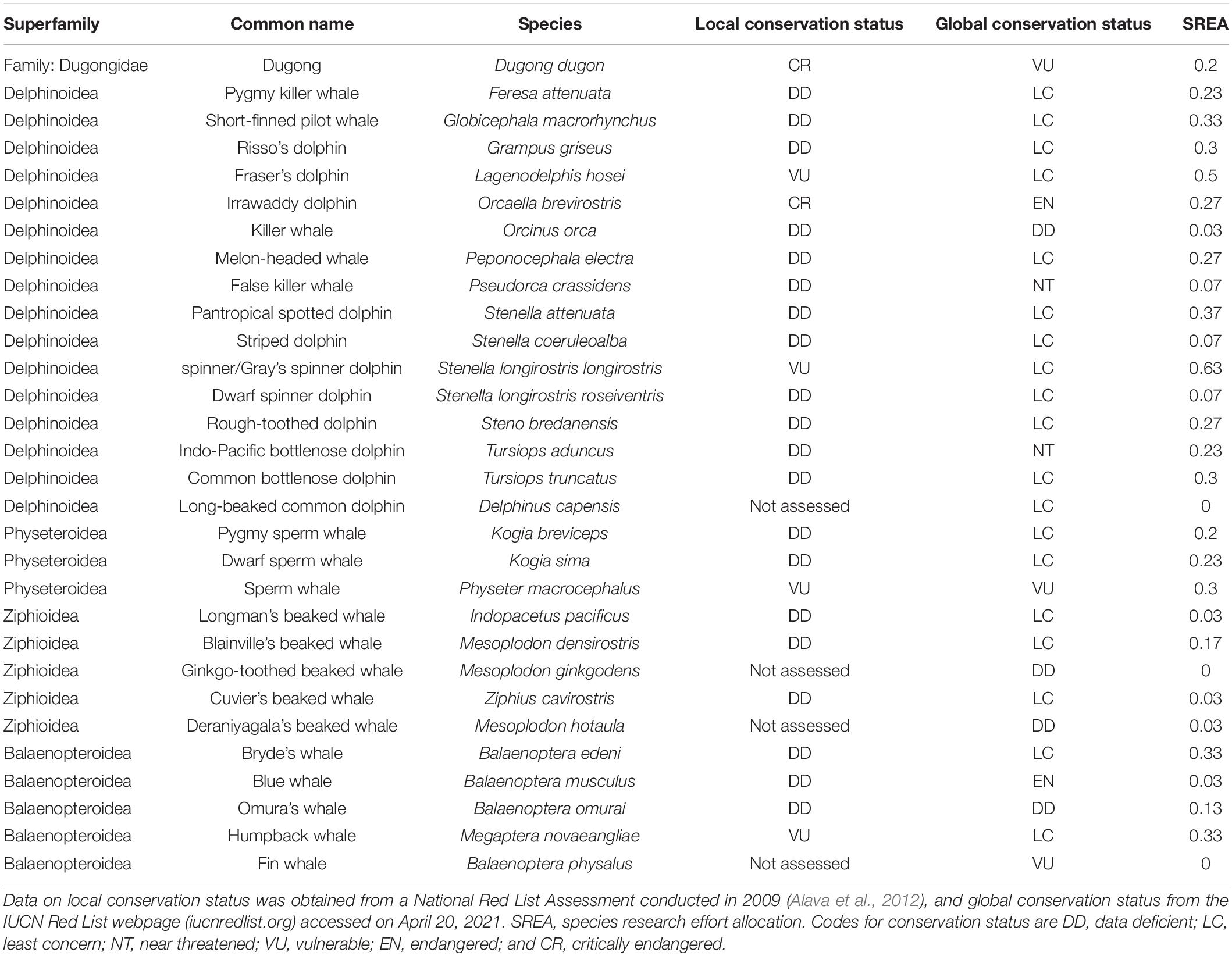
Table 2. Research allocation of 30 marine mammal species provisionally listed to occur in Philippine waters.
Model Selection
Missing information for some life history parameters, particularly for beaked whales, were removed from further analysis. The VIF values of the explanatory variables were all below the threshold of 3 (Table 3) suggesting non-collinearity among covariates (Figure 4). Except for number of strandings which showed a positive relationship, the explanatory variables show no obvious patterns in relation to the response variable (Figure 5). The best-fit model based on corrected AIC for small sample sizes, with a 49% explanatory power (AICc weight = 0.49), included frequency of strandings in the model only. Given that the best model is not strongly weighted and there were two models with ΔAIC < 2 (all models had ΔAIC < 10) which indicates model uncertainty, a full-model averaging was preferred. The model-averaged values suggest that stranding frequency may directly influence an increase in publications of marine mammals by providing access to biological specimens of what normally would be difficult to obtain from behaviorally cryptic animals. The relationship between research effort and variables such as maximum length, age at sexual maturity, and group size were not supported (Figure 5).
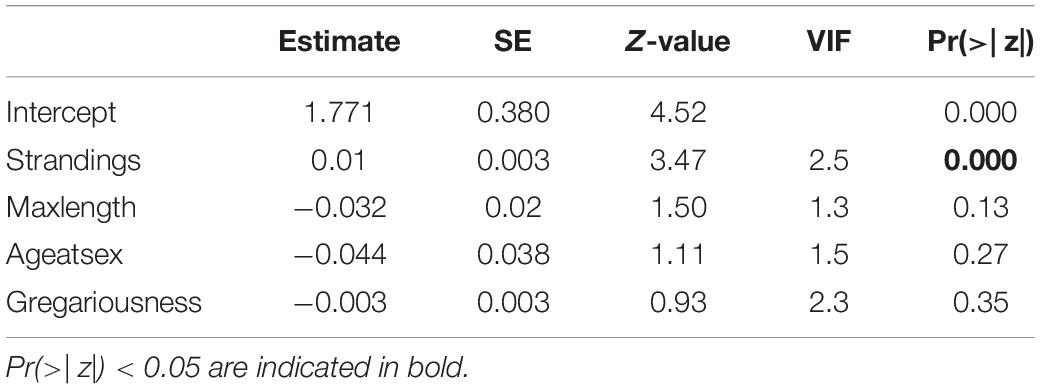
Table 3. Summary of model-averaged coefficients from negative binomial GLM to model the relationships of research effort (response variable) of Philippine marine mammal species and their life history parameters and data sources (predictor variables).
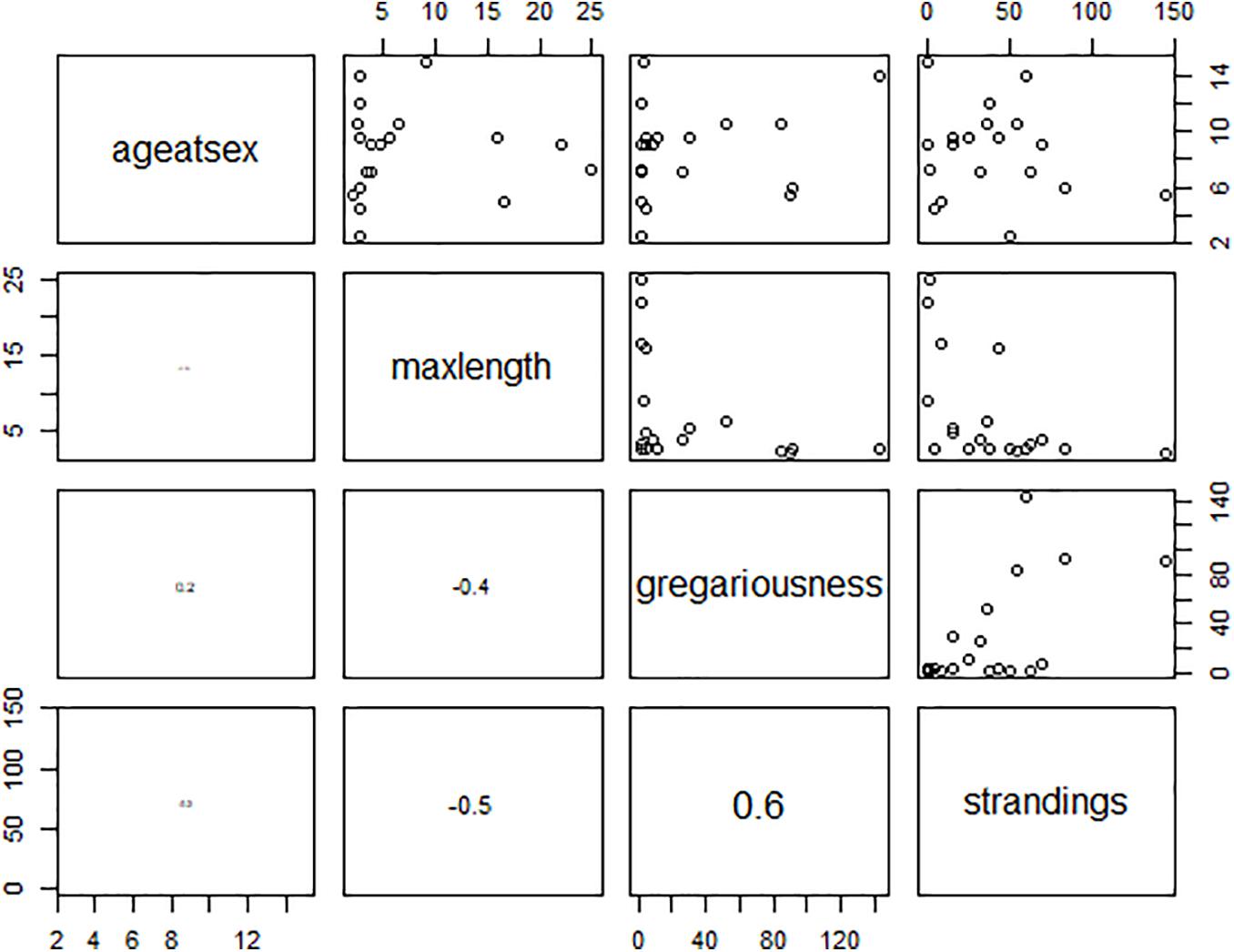
Figure 4. Correlation matrix of the explanatory variables using pairplots. The lower panels show the Pearson pairwise correlation with font size proportional to correlation coefficient. Variance inflation factors of the covariates are all below the threshold of 3. Diagonal text panels are ageatsex = age at sexual maturity; maxlength = maximum length; gregariousness = group sizes; strandings = frequency of strandings.
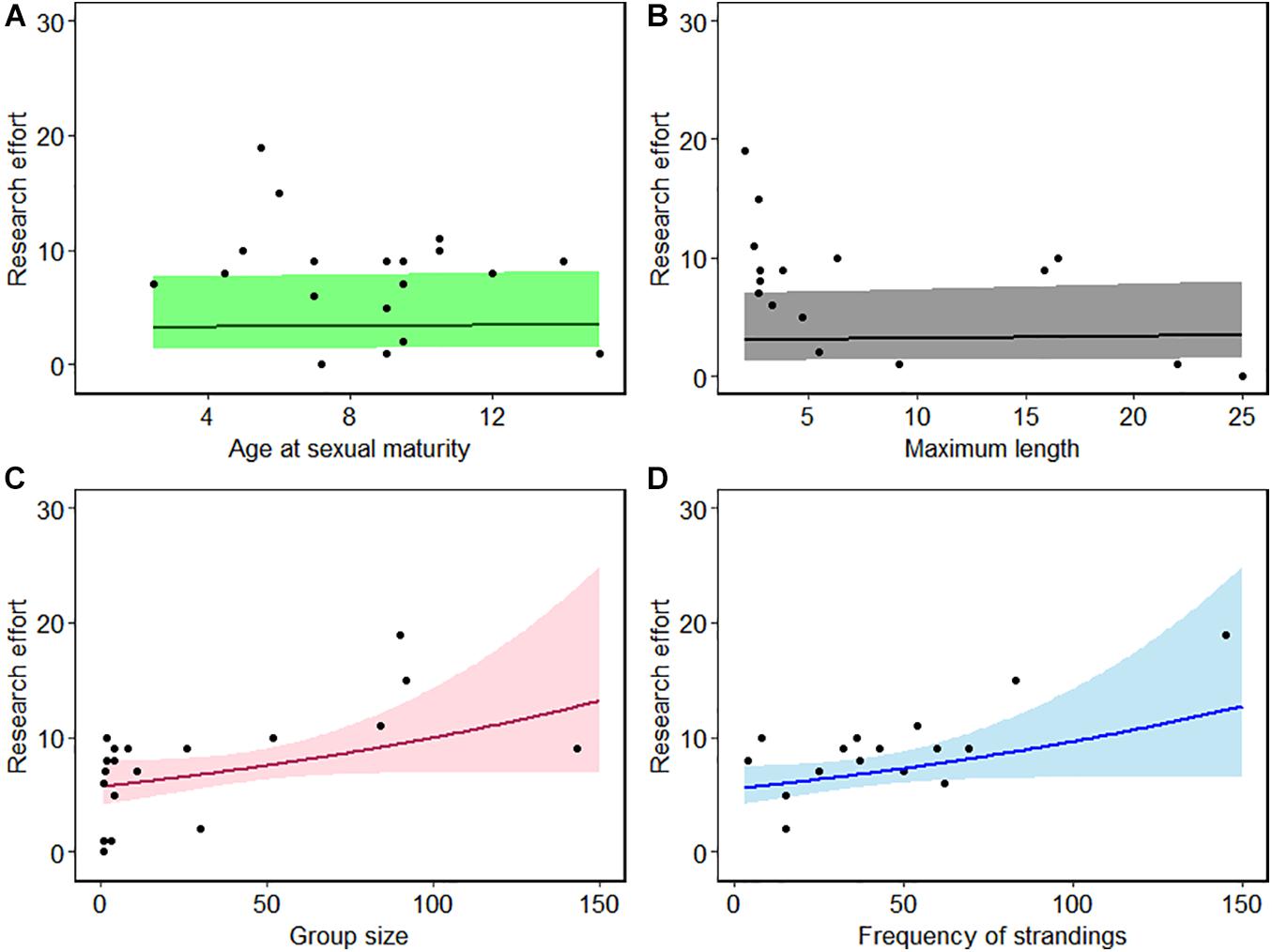
Figure 5. Mean fitted number of publications with 95% confidence interval against (A) age at sexual maturity (years), (B) maximum length (m), (C) gregariousness, and (D) stranding frequency.
Extinction Risk
National Red List Assessment evaluation was predicted by research effort, whereby the likelihood that a species has been evaluated increased as more species information was published. The underlying effect of unknown life history traits was controlled for by modeling taxonomic group (i.e., superfamily level) as a random variable. Six out of a possible 30 species have been NRLA evaluated with the remaining mainly assessed as “Data Deficient.” Three of these evaluated belong to superfamily Delphinoidea (Table 2), two of which (spinner dolphin and Fraser’s dolphin) are also mentioned the most in published research (Figure 6). The third delphinid species (Irrawaddy dolphin) has one of the smallest population sizes globally. The three other evaluated marine mammal species are the sperm whale, humpback whale, and dugong.
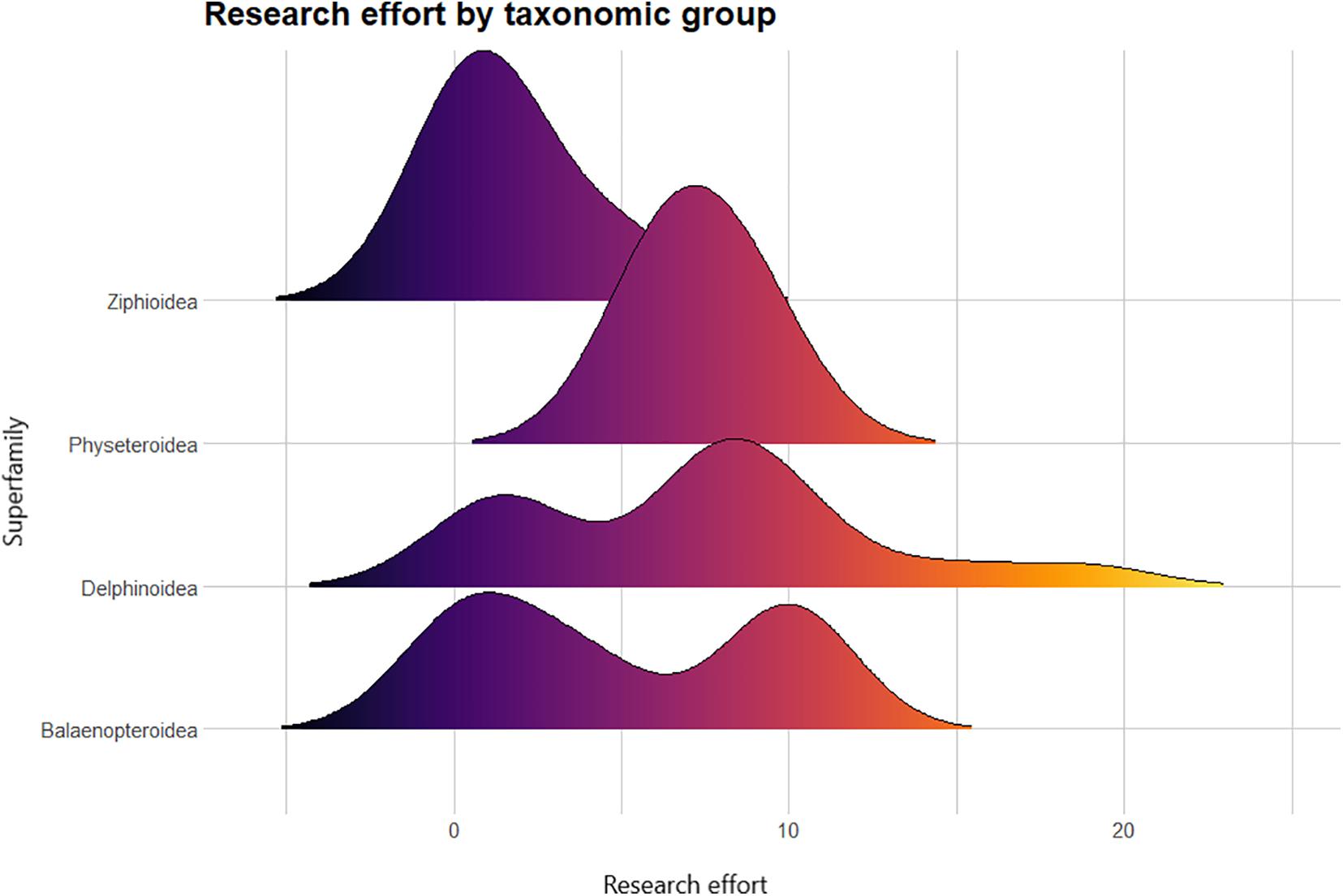
Figure 6. A density distribution of research effort on marine mammal taxa in the Philippines from 1991 to 2020. The color gradient indicates the research effort, with warmer colors indicating more research output. This figure demonstrates that a substantial number of species have not been mentioned in a published research (particularly true for most ziphiids). The panel for dolphins shows two peaks and a skewed tail signifying some species receiving more research attention than others. The second peak for baleen whales is due to dedicated research on the humpback whales. The Family: Dugongidae is removed from the panel because of having only one representative species.
Discussion
Biodiversity and Distribution
Systematic documentation of marine mammal diversity and distribution in the Philippines did not start until 1990, when Leatherwood et al. (1992) started compiling records from American and European whaling logbooks, conducted interviews with scientists and fishers, investigated direct and indirect fisheries capture of marine mammals, and launched surveys in various areas within the Philippines’ Exclusive Economic Zone (EEZ). These surveyed areas include Batangas Bay, Verde Island Passage, the Sibuyan Sea, Bohol Sea, Cebu Strait, Sulu Sea, Tañon Strait, Visayan Sea, and Camotes Sea. These initial efforts resulted to the documentation of 17 species of marine mammals including dugongs. Prior to this, marine mammal records in the Philippines were sparse, despite these animals being an important fisheries resource in certain local communities (Dolar et al., 1994; Acebes, 2014).
Of the 30 species known to occur in the Philippines, three are based solely on stranded specimens or stranding reports and have no live records: striped dolphins, reported in Bicol and Calabarzon regions (Aragones and Laggui, 2019); a Deraniyagala’s beaked whale, found in Davao Gulf and confirmed using genetic analysis (Lacsamana et al., 2015); and four specimens of Longman’s beaked whales stranded in Davao in 2004, General Nakar, Quezon in 2016, and in Cagayan Province in 2018 (Acebes et al., 2005, 2019). Omura’s whale was described as a species only very recently (Wada et al., 2003), and skeletons collected from indigenous fishery in Pamilacan and Camiguin Islands in the Bohol Sea which were previously thought to be Bryde’s whale have now been confirmed to be Balaenoptera omurai (Cerchio et al., 2019). Three species previously listed in the Philippine Red Data Book in 1997 were removed by Alava et al. (2012) due to lack of valid or current confirmation. The Indo-Pacific humpback dolphin (Sousa chinensis) was only recorded from a single stranding incident in the Turtle Islands, Tawi-Tawi and could have belonged to populations from northeastern Borneo (Perrin et al., 2002). Similarly, there have been no confirmed sightings of finless porpoises (Neophocaena phocaenoides), as reports of this species from northwestern Palawan in 1995 were never validated. The fin whale (Balaenoptera physalus) was only recorded once during a survey in Dumaran Island, Palawan (Dolar et al., 2006) and was also supposed to be removed from the list due to lack of further verification. However, a recent stranding was reported in Sorsogon (Aragones et al., 2017), although we could not verify this.
Dugongs used to be widely distributed all over the Philippine archipelago (Perrin et al., 2002), but current data show their limited distribution in fragmented seagrass habitats in Palawan, Romblon, Guimaras, Negros, Fuga, and Tawi-Tawi Islands, as well as other parts of Mindanao Island. Their current distribution is estimated to have declined by 63% from its former extent and has been attributed to a number of threats, such as direct hunting, bycatch, dynamite fishing, and the destruction of seagrass habitats caused by major coastal development and reclamation projects (Alava et al., 2012).
The distribution of marine mammals in the Philippines spread across a diverse range of habitats: from deep drop-offs, oceanic shelves, coastal seas, semi-enclosed gulfs, and shallow estuaries. Species distributions are strongly influenced by the movement of their prey, which are also influenced by depth and sea surface temperature ranges (Dolar et al., 2006). Many pelagic odontocetes such as the sperm whale, beaked whales, pilot whale, and Fraser’s dolphin have been reported in deep, off-shore habitats where they feed on mesopelagic fish and cephalopods. On the other hand, coastal cetaceans have more restricted distribution: Bryde’s whale, for example, has only been reported in northeastern Palawan, Balabac Strait (Dolar et al., 2006), southeastern Siquijor, Bohol, and Panay Gulf (Leatherwood et al., 1992). Migratory humpback whales are also known to occur only around Babuyan Islands and Isabela coasts in northern Luzon during the breeding season (Acebes et al., 2007). Similarly, Irrawaddy dolphin is restricted to shallow, highly turbid estuarine habitats in Malampaya Sound and Quezon in Palawan (Dolar et al., 2002; Whitty, 2015) and Iloilo and Guimaras Straits in the Visayas (de la Paz et al., 2020). Other rare cetaceans include the rough-toothed dolphin, which has only been documented in the Babuyan Islands (Nakagun et al., 2013), the blue whale in the Bohol Sea (Acebes et al., 2021), and beaked whales, of which there have been only sporadic sightings. In contrast to habitat-restricted species, some odontocetes such as spinner, pantropical spotted, and bottlenose dolphins appear to be more cosmopolitan, being found in wider depth ranges and with more flexible in habitat preferences (Dolar et al., 2006; Tiongson and Karczmarski, 2016).
Of the thirty species provisionally listed in the Philippines, only nine species have abundance estimates. The most abundant and widespread species is the spinner dolphin, found throughout the archipelago. Abundance for populations in eastern Sulu Sea and Tañon Strait were estimated at 31,512 (CV = 27%) and 3,489 (CV = 26%) respectively from surveys conducted in 1994 and 1995 (Dolar et al., 2006).
As of 2002, only approximately 40% of Philippine marine waters have been explored for marine mammals (SEAMAMIII, 2015). The eastern seaboard of the Philippines, as well as the northwestern side of Luzon, western side of Palawan, and most waters surrounding Mindanao Island and the Tawi-Tawi group of islands still need to be investigated. Despite the number of publications made on marine mammal diversity and distribution in the Philippines relative to other themes, there is currently little to no information on the abundance estimates and movement patterns of many other species, especially large whales. Such information is important to assess the impacts of threats and whether strandings and bycatch are within sustainable limits. Likewise, research on endangered species (i.e., dugong and Irrawaddy dolphin) need to be updated to monitor whether conservation programs and policies have been effective.
Biology and Ecology
Studies on the biology of Philippine marine mammals range from investigations of external physical variation and cranial morphometric comparison between sexes (Jefferson et al., 1997; Perrin et al., 2003) to measuring hematological values (Dolar et al., 1999). The first external physical description of the dwarf spinner dolphin was photographed in Balabac Channel in the Philippines providing important reference characteristics to discern the subspecies from the nominal form in the wild (Perrin et al., 2007). Studies on feeding ecology provided important baselines and comparative data across other global populations on the diet of pelagic dolphins. Dolar et al. (2003) used the depth distribution of their mesopelagic prey species from stomach contents of spinner and Fraser’s dolphins caught from a tuna gillnet fishery as a proxy to estimate the depth at which they may forage. Analyses of myoglobin content of different muscle groups from the same specimens also gave insights into the physiological adaptations to foraging in deep waters between the two species (Dolar et al., 1999). In contrast, gut analysis of coastal Irrawaddy dolphin revealed a diet of mainly demersal fish species such as conger eels (Conger japonicus) and pony fish (Eubleekeria splendens) (Postrado et al., 2019). The scope of the remaining studies assessed in this theme focused on behavioral ecology. Migration patterns of the western North Pacific stock of humpback whales wintering in the Babuyan Islands of the northern Philippines have been elucidated through a transnational compilation of photo-identification catalogs (Silberg et al., 2013). A subpopulation of Irrawaddy dolphin, on the other hand, appears to have an exceedingly small, restricted core habitat in Guimaras Strait (de la Paz et al., 2020). Three studies explored bioacoustics with one experimenting on a captive spinner dolphin (Smith et al., 2019) and the other two on humpback whale songs. Songs recorded in the Babuyan Islands were compared to songs from other stocks across the North Pacific revealing interesting patterns of convergence and divergence (Darling et al., 2014, 2019). The increasing trend of ecological studies is a welcome development. There is an appreciable diversity in terms of research topics, but there is also a lack of studies on population processes and size estimates which are crucial to estimating survivability and extinction risk.
Conservation
Most of the research in the Philippines contributes to conservation efforts by providing much needed scientific information. But studies explicitly designed to address species conservation are few and far between. Wide-ranging and migratory species such as the humpback whale face different conservation challenges from coastal and range-restricted species. The northern Philippines is a known wintering ground for humpback whales of the western North Pacific stock. Cross-catalog matching of humpback whale flukes between Japan, Russia, and the Philippines revealed inter- and intra-annual movements (Silberg et al., 2013; Titova et al., 2018; Nakagun et al., 2020) suggesting conservation challenges across varying socio-political contexts.
In contrast, Smith et al. (2004) quantified the population status and recommended conservation actions for the Malampaya Sound subpopulation of Irrawaddy dolphins. The population estimate has since decreased, and unless drastic measures to mitigate bycatch are applied, this subpopulation will almost certainly face extirpation (Smith et al., 2004; Whitty, 2016). Research on range-restricted Irrawaddy dolphin subpopulations in the Philippines has been critical for their extinction risk evaluations. Their restricted habitat preference close to human habitation, small population size, and competition with fisheries increases their risk of extinction potentially beyond recovery. Furthermore, plans for a large-scale bridge over Irrawaddy dolphin habitat in Iloilo and Guimaras Straits may exacerbate this species’ chances of extinction (Tiongson et al., 2020). A similar level of risk applies to dugongs, another coastal species. Moreover, spinner and Fraser’s dolphins face increased extinction risk because of their interactions with commercial fisheries in their offshore environment, while large whales were historically hunted and their populations are still undergoing recovery.
Fisheries and Bycatch
Historically, marine mammals were taken in large numbers as targeted catch and bycatch (Dolar, 1994; Dolar et al., 1994) and artisanal whaling communities existed in some islands (Acebes, 2009). Bycatch assessments (Dolar, 1994) became the impetus for the Bureau of Fisheries and Aquatic Resources (BFAR) to enact Fisheries Administrative Order (FAO) 185 in 1992, which outlined rules and regulations for the protection and conservation of all dolphin species; this was later amended (FAO 185-1) to include all whales. Species with the highest number of individuals caught in tuna gillnet fisheries were the spinner and Fraser’s dolphin; both notable for also being the most frequently mentioned species in research publications and in stranding records. Although no dedicated bycatch studies in commercial fisheries have been conducted following the issuance of FAOs 185 and 185-1, anecdotal reports suggest that bycatch has been reduced or driven underground (SEAMAMIII, 2015). Impacts of small-scale fisheries are also major threats to marine mammals particularly coastal species (Whitty, 2015). Bycatch is globally identified as a major threat to marine mammal populations given the intensity of fishing pressure where marine mammals are found, and the Philippines is no exception.
Whaling and its history in the Philippines is perhaps one of the topics that has been most extensively researched. In brief, artisanal whaling communities were known to have existed since the 16th century (Acebes, 2011) and there was a short stint in commercial whaling in the 19th century (Acebes, 2014). American and British whaling records during the 1800s charted the Sulu and Celebes Seas as primary hunting grounds for sperm whales, but they were also hunted in south and western Palawan, west of Manila Bay and Mindoro, Sibuyan Sea, Bohol Sea, and Philippine Sea (Townsend, 1935). All forms of whaling stopped following the implementation of FAOs 185 and 185-1. While it is possible that whaling and trade of marine mammal meat and by-products may still occur in secret and through the black market, there now exists a database that citizens can contribute reports to. The database accepts records of live sightings of wild animals as well as reports of strandings and sale at markets. The database is maintained by the non-profit organization Marine Wildlife Watch of the Philippines, which aims to raise public awareness and aid government agencies in monitoring lawful compliance. For an extensive account of Philippine whaling, see Acebes (2011, 2014).
Reviews
There is a global consensus that climate change will have negative effects on marine mammals. One common response to changing water temperatures is a shift in their distribution. Tropical species have been posited to benefit from an expansion of their distributional range (Learmonth et al., 2006; MacLeod, 2009). However, Dolar and Sabater (2013) dedicated a review on the impacts of climate change to marine mammal populations in southeast Asia and made two very critical points to consider: (1) species with limited distributions such as the coastal and riverine Irrawaddy dolphin will have a decrease in their ranges, and (2) changes in human behavior in response to climate change, such as increased hunting pressure on cetaceans, and secondary effects of climate change (e.g., droughts, increased sea level, storm frequency, and severity) will potentially have more impacts on their abundance, distribution, and survivability. This echoes the idea that changes in human behavior in response to the effects of climate change will have an equal or even greater negative impact than direct effects of climate change on cetacean populations worldwide (Alter et al., 2010). Dolar and Sabater (2013) further emphasized that six species and one subspecies in southeast Asia might be severely impacted by climate change, five of which can be found in the Philippines (Omura’s whale, Indo-Pacific bottlenose dolphin, Irrawaddy dolphin, dwarf spinner dolphin, and dugong).
Socio-Politics and Governance
Eco-tourism in the Philippines has steadily increased over the years, but only a few sites have dedicated tours for dolphin- and whale-watching. In 2004, a Joint Administrative Order (JAO-1) between BFAR and the Department of Tourism (DOT) establishing guidelines for responsible tourism interactions with dolphins and whales was implemented. Compliance to the JAO-1 in two islands in the Bohol Marine Triangle in April–May 2009 was studied by Sorongon et al. (2010), with results suggesting that a decrease in cetacean sightings was due to avoidance behavior linked to untrained boat operators and high density of boats in the area. Similarly, a social study of the dolphin watching industry in southern Tañon Strait from 2004 to 2012 identified pivotal challenges such as increasing number of tour boats, misconceptions about dolphin interactions with fisheries, and lack of a taxa-specific management plan, which led to recommendations for a limit on the number of operating vessels and establishment of a centralized monitoring organization that would ensure prioritization of the cetaceans’ welfare (Aragones et al., 2013). A larger study employing a similar non-ecological approach to data collection assessed the potential impacts of this industry to selected sites all over southeast Asia, including two sites in the Philippines, and found a median risk to the welfare of dolphins in the Philippine sites (Mustika et al., 2017).
Health and Ecotoxicology
Focused research on the health of marine mammals, especially in wild populations, remains low and largely opportunistic even though more studies have been published in the last decade. Skin nodular lesions have been observed in wild populations of humpback whale and Irrawaddy dolphin during boat-based surveys in the Babuyan Islands and Guimaras Strait, respectively. However, published research on cetacean health has been limited to ecotoxicological studies from stranding events (n = 5). The earliest of these studies documented aspergillosis in a melon-headed whale calf that was initially rescued alive and brought to an oceanarium for rehabilitation (Torno et al., 2008); however, the calf eventually died due to respiratory failure resulting from severe bronchopneumonia caused by the fungus Aspergillus fumigatus.
The parasite Toxoplasma gondii and bacteria of the genus Giardia were found in some samples collected from single stranding events of 30 individuals representing 11 species that were documented from January 2012 to March 2013 (Obusan et al., 2015). From these same stranding events, nine individuals that underwent rehabilitation were screened to test for bacterial susceptibility patterns to antibiotics (Obusan et al., 2018); these individuals represented five species including the rough-toothed dolphin, which exhibited development of some antibiotic resistance. In a similar study of samples collected from stranding events that occurred between October 2016 to August 2018 (n = 40), T. gondii and bacteria of the genus Leptospira were also detected (Obusan et al., 2019). Opportunistic hematological, macroscopic and microscopic studies of a Blainville’s beaked whale and dwarf sperm whale that stranded in Davao City in April and July 2014 were also undertaken (Bondoc et al., 2017), which reported severe iron deficiency in the former.
Taxonomy and Systematics
Twenty-nine of the world’s 44 Omura’s whale skull specimens are stored in the Whale Bone Museum at Silliman University’s Institute of Environmental and Marine Sciences (IEMS) in the Philippines (Cerchio et al., 2019), making it the largest collection of Omura’s whale osteological specimens globally. Species identification was confirmed from several cumulative studies (Perrin et al., 1996; Yamada et al., 2008) that culminated in the identification of a new species record in the country. This confirmation suggests that the Philippines may have historically been one of the hotspots of Omura’s whales and thus have massive global implications in its conservation, especially considering that it was a formerly targeted species in local artisanal whaling. The effects of historical whaling on the population size of Omura’s whales in the Philippines has never been quantified on account of its recent known occurrence in the country. However, some indications suggest that their current numbers are exceptionally low.
A comparison of spinner dolphin skeletal specimens at IEMS collected from a tuna gillnet fishery and with other specimens stored globally revealed that specimens from the Gulf of Thailand, other parts of southeast Asia, and northern Australia were significantly smaller with fewer teeth and vertebrae, suggesting a completely different ecotype of spinner dolphin while those from Philippine internal waters were similar to the nominal form (Perrin et al., 1999).
Conservation and Research Gaps and Priorities
In general, marine mammals benefit from redundant levels of protection in the Philippines enforced at different spatial scales and across multiple national and municipal laws. Alava et al. (2012) summarized all existing conservation measures for each species, from an outright ban of catching, owning, and possessing any marine mammal and their byproducts, to place-based protection in the form of protected seascapes, parks, and marine protected areas designed specifically around the conservation of marine mammals. Despite these measures, all marine mammal species still face many threats. An improved understanding of the dynamics of populations in Philippine waters may provide better insights into their management in the face of future uncertainty.
Strandings are unfortunate events, but we find from this review that these events may provide research opportunities that are otherwise difficult to achieve under normal circumstances. An increasing trend of research based on data collected from stranded specimens has been observed (Aragones et al., 2010), but we find that there is still some wasted opportunity in the use of such specimens and suggest further expanding studies on strandings. We also not that there has been no concerted effort to collate all specimens from strandings – a quandary resulting from a lack of coordination between research institutions, volunteer groups, and BFAR. We also argue that modern molecular techniques are an underutilized tool, and this may be due to a shortage of local expertise and capacity, lack of institutional support, and lack of long-term programs. Some research methods that can be used to collect data from strandings are included in Table 4.
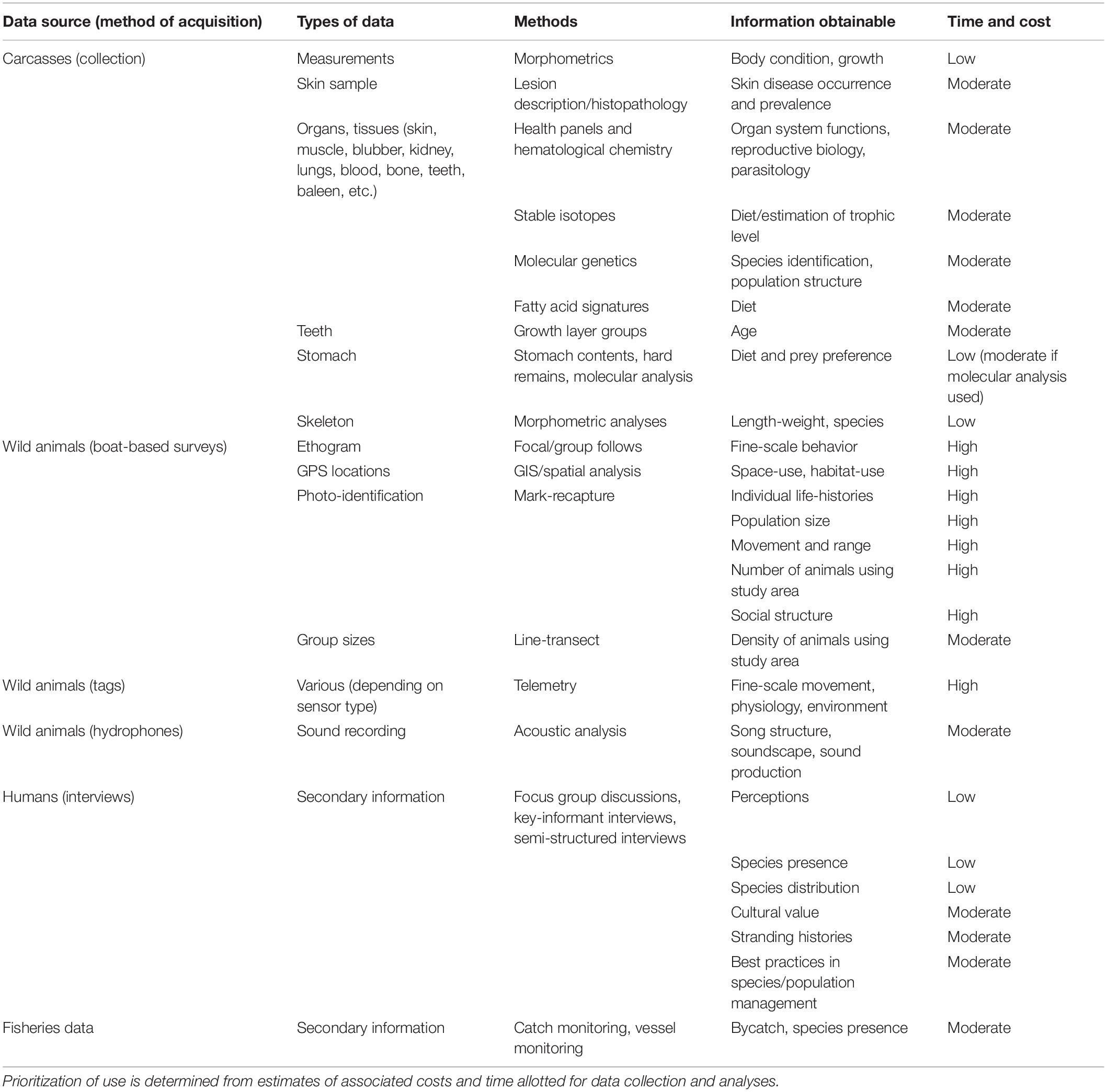
Table 4. Summary of marine mammal research methods that are underutilized and/or recommended for future use.
In view of these gaps, we recommend the inventory of all available data and material on marine mammals to establish a database of whole specimens, tissue and osteological samples from BFAR and other sources (e.g., local government units, academic institutions, museums, and laboratories), and that this database be easily accessible to researchers for coordination and collaboration. Accessing these materials from all over the archipelago could potentially help understand how the populations are structured in different water bodies, which may lead to defining population units with management implications.
Conclusion
Marine mammal research in the Philippines is relatively young, and much has been learned about these animals since the 1990s. Yet, this study highlights a critical need for more research studies on each marine mammal species to evaluate their risk of extinction in the face of climate change and diverse, ongoing threats. Specifically, there is a dearth of knowledge on the populations and ecology of most marine mammals in the Philippines. We know that there is evidence of threats of different magnitudes impacting marine mammals but how much these affect the overall survival of these populations is largely undetermined. Continued monitoring of populations has never been undertaken since the implementation of FAOs 185 and 185-1, leaving much unknown regarding their effectiveness. Moreover, blanket bans of capture, trade, and possession are also not necessarily enough to assist in the conservation of marine mammals (Pavone, 2019) and thus, we advise caution in interpreting the effectivity of FAOs 185 and 185-1. It is important that consequences of conservation actions are documented, otherwise the considerable efforts and investments in conservation may end up achieving very little (Sutherland et al., 2004).
The standard methods in population studies often require substantial funding to collect data on wild populations through photo-identification mark-recapture studies. However, molecular techniques with samples taken from stranded animals may be a good alternative that could significantly reduce the financial constraints of expensive fieldwork. The importance of museum collections should also be emphasized, as exemplified in the studies elucidating the existence of Omura’s whale in the Philippines. With sufficient funding, field studies and other methods such as photo-identification mark-recapture, focal follows, biopsies, necropsies, and telemetry could also provide more information on individual life histories, social interactions, biology, behavior, population size, and movement among others (Hammond et al., 1990; Mann, 1999; Croft et al., 2008; Hussey et al., 2015; Seber and Schofield, 2019). Detailed studies following these methods provide the opportunity to ask questions from a broader perspective. A summary of recommended methods that can be used to enhance current research practices is provided in Table 4.
Despite a sustained interest in marine mammals, further research is still hampered by the difficulty of securing funding and accessing resources to collect robust datasets for studying population and ecological processes of wild marine mammal populations. The Philippines, with its island ecosystem, coastal features, and deep waters close to shore, has a high potential as a natural laboratory for investigating movement and range, population trends and interchange, and cross-disciplinary research topics such as consequential impacts of globalization and development on marine mammals. As early career researchers, we want to encourage more research opportunities that are financially accessible, and which maximize use of different analytical tools that are locally available, to address the knowledge gaps that plague overall conservation of marine mammals in the Philippines.
Author Contributions
AT conceptualized the study and conducted the analyses. AT, JU, and MP collected the data and wrote and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Jo Marie V. Acebes for reviewing an earlier draft of this manuscript and the reviewers for their comments to improve the manuscript. We gratefully recognize all marine mammal researchers in the Philippines who have dedicated extra time and effort from their full-time jobs to contribute to our growing knowledge of marine mammals. We also appreciate the efforts of Tara Sayuri Whitty to further improve the manuscript. We would also like to thank the Center for Research and Engagement of the University of St. La Salle for supporting the publication of this review article.
Footnotes
References
Acebes, J. M. V. (2009). Historical Whaling in the Philippines?: Origins of ‘ Indigenous Subsistence Whaling’, Mapping Whaling Grounds and Comparison With Current Known Distribution?: A HMAP Asia Project Paper. Available online at: https://cran.r-project.org/web/packages/AICcmodavg/index.html (accessed October, 2009).
Acebes, J. M. V. (2011). “Local whaling in the Philippines?: community adaptations and resilience,” in Whaling and History IV, ed. J. E. Ringstad (Sandefjord: Whaling Museum).
Acebes, J. M. V. (2014). “A history of whaling in the Philippines: a glimpse of the past and current distribution of whales,” in Historical Perspectives of Fisheries Exploitation in the Indo-Pacific, eds J. Christensen and M. Tull (Dordrecht: Springer), 83–105. doi: 10.1007/978-94-017-8727-7_5
Acebes, J. M. V., Bautista, A. L., Yamada, T., Dolar, M. L. L., and Perrin, W. F. (2005). “Stranding of Indopacetus pacificus in Davao, Philippines,” in Poster Presented at the 24th Biennial Conference on the Biology of Marine Mammals, San Diego, CA.
Acebes, J. M. V., Bautista-Barcelona, A. L., Yamada, T., Santos, M. D., Dolar, M. L. L., and Tan, J. M. L. (2019). “Strandings of longman’s beaked whale (Indopacetus pacificus) in the Philippines,” in Poster Presented at the 15th National Symposium on Marine Science, Banga.
Acebes, J. M. V., Darling, J. D., and Yamaguchi, M. (2007). Status and distribution of humpback whales (Megaptera novaeangliae) in northern Luzon. Philippines. J. Cetacean Res. Manag. 9, 37–43.
Acebes, J. M. V., Silberg, J. N., Gardner, T. J., Sabater, E. R., Tiongson, A. J. C., Dumandan, P., et al. (2021). First confirmed sightings of Blue Whales Balaenoptera musculus Linnaeus, 1758 (Mammalia: Cetartiodactyla: Balaenopteridae) in the Philippines since the 19th century. J. Threat. Taxa 13, 17875–17888. doi: 10.11609/jott.6483.13.3.17875-17888
Alava, M. N. R., Dolar, M. L. L., Sabater, E. R., Aquino, M. T., and Santos, M. D. (2012). Red List Status of Marine Mammals in the Philippines. Metro Manila: National Fisheries Research and Development Institute.
Alter, S. E., Simmonds, M. P., and Brandon, J. R. (2010). Forecasting the consequences of climate-driven shifts in human behavior on cetaceans. Mar. Policy 34, 943–954. doi: 10.1016/j.marpol.2010.01.026
Aragones, L. V., Laggui, H. L., and Amor, A. K. (2017). The Philippine Marine Mammal Strandings From 2005 to 2016. Quezon City: University of the Philippines. A PMMSN Publication. Technical Report No. 1.
Aragones, L. V., and Laggui, H. L. M. (2019). Marine Mammal Strandings in the Philippines From 2017 to 2018: Initial Biennial Analysis. Quezon City: University of the Philippines. A PMMSN Publication. Technical Report No. 2.
Aragones, L. V., Roque, M. A. A., Flores, M. B., Encomienda, R. P., Laule, G. E., Espinos, B. G., et al. (2010). The philippine marine mammal strandings from 1998 to 2009: animals in the philippines in peril? Aquat. Mamm. 36, 219–233. doi: 10.1578/AM.36.3.2010.219
Aragones, L. V., Talaue-Mcmanus, L., Roque-Borigas, M. A. A., Amor, A. K. S., and Keith, E. O. (2013). Dolphin watching in the southern tañon strait protected seascape, Philippines Issues and challenges. Sci. Diliman. 25, 1–33.
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Bondoc, J. L., Aragones, L. V., and Masangkay, J. S. (2017). Hematological, macroscopic and microscopic findings in two stranded whales (Mesoplodon densirostris and Kogia sima) and possible causes of deaths. Phil. J. Vet. Med. 54, 63–69.
Brooks, T. M., Mittermeier, R. A., Mittermeier, C. G., Da Fonseca, G. A. B., Rylands, A. B., Konstant, W. R., et al. (2002). Habitat loss and extinction in the hotspots of biodiversity. Conserv. Biol. 16, 909–923. doi: 10.1046/j.1523-1739.2002.00530.x
Carpenter, K. E., and Springer, V. G. (2005). The center of the center of marine shore fish biodiversity: the Philippine Islands. Environ. Biol. Fishes 72, 467–480. doi: 10.1007/s10641-004-3154-4
Cerchio, S., Yamada, T. K., and Brownell, R. L. (2019). Global distribution of Omura’s whales (Balaenoptera omurai) and assessment of range-wide threats. Front. Mar. Sci. 6:67. doi: 10.3389/fmars.2019.00067
Croft, D. P., James, R., and Krause, J. (2008). Exploring Animal Social Networks. New Jersey: Princeton University Press.
Darling, J. D., Acebes, J. M. V., Frey, O., Jorge Urbán, R., and Yamaguchi, M. (2019). Convergence and divergence of songs suggests ongoing, but annually variable, mixing of humpback whale populations throughout the North Pacific. Sci. Rep. 9:7002. doi: 10.1038/s41598-019-42233-7
Darling, J. D., Acebes, J. M. V., and Yamaguchi, M. (2014). Similarity yet a range of differences between humpback whale songs recorded in the Philippines. Japan and Hawaii in 2006. Aquat. Biol. 21, 93–107. doi: 10.3354/ab00570
de la Paz, M. E. L. (2012). Behavior and area use of Irrawaddy dolphins (Orcaella brevirostris, Gray 1886) in the coastal waters of Bago and Pulupandan, Negros Occidental, Philippines. Master’s Thesis, Philippines: Silliman University.
de la Paz, M. E. L., Palomar-Abesamis, N., Sabater, E., Señoron, J. A., and Dolar, M. L. (2020). Habitat use and site fidelity of Irrawaddy dolphins (Orcaella brevirostris) in the coastal waters of Bago-Pulupandan, negros occidental, Philippines. Raffles Bull. Zool. 68, 562–573. doi: 10.26107/RBZ-2020-0072
Dolar, M. L. L. (1994). Incidental takes of small cetaceans in fisheries in Palawan, Central Visayas and northern Mindanao in the Philippines. Rep. Int. Whal. Comm. Spec. Issue 15, 355–363.
Dolar, M. L. L., Leatherwood, S. J., Wood, C. J., Alava, M. N. R., Hill, C. L., and Aragones, L. V. (1994). Directed fisheries for cetaceans in the Philippines. Rep. Int. Whal. Comm. 44, 439–449.
Dolar, M. L. L., Perrin, W. F., Pres Gaudiano, J., Yaptinchay, A. A. S. P., and Tan, J. M. L. (2002). Preliminary report on a small estuarine population of Irrawaddy dolphins Orcaella brevirostris in the Philippines. Raffles Bull. Zool. 10, 155–160.
Dolar, M. L. L., Walker, W. A., Kooyman, G. L., and Perrin, W. F. (2003). Comparative feeding ecology of spinner dolphins (Stenella longirostris) and Fraser’s dolphins (Lagenodelphis hosei) in the Sulu Sea. Mar. Mammal. Sci. 19, 1–19. doi: 10.1111/j.1748-7692.2003.tb01089.x
Dolar, M. L. L., Perrin, W. F., Taylor, B. L., Kooyman, G. L., and Alava, M. N. R. (2006). Abundance and distributional ecology of cetaceans in the central Philippines. J. Cetacean Res. Manag. 8, 93–111.
Dolar, M. L. L., and Sabater, E. R. (2013). Potential impact of cliamte change on marine mammal biodiversity in Southeast Asia: a review. Silliman J. 52, 91–113.
Dolar, M. L. L., Suarez, P., Ponganis, P. J., and Kooyman, G. L. (1999). Myoglobin in pelagic small cetaceans. J. Exp. Biol. 202, 227–236. doi: 10.1242/jeb.202.3.227
Field, C. B., Behrenfeld, M. J., Randerson, J. T., and Falkowski, P. (1998). Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281, 237–240. doi: 10.1126/science.281.5374.237
Fox, J., and Weisberg, S. (2019). An R Companion to Applied Regression, 3rd Edn. Thousand Oaks, CA: SAGE.
Hall, R. (1998). “The plate tectonics of Cenozoic SE Asia and the distribution of land and sea,” in Biogeography and Geological Evolution of SE Asia, eds R. Hall and J. D. Holloway (Leiden: Backhuys Publishers), 99–131.
Hammond, P. S., Mizroch, S. A., and Donovan, G. P. (1990). Individual recognition of cetaceans: use of photo-identification and other techniques to estimate population parameters. Rep. Int. Whal. Comm. Spec. Issue 12, 325–333.
Hines, E., Ponnampalam, L., Jamal Hisne, F., Tara, S., Jackson-Ricketts, J., Kuit, S., et al. (2015). Report of the Third Southeast Asian Marine Mammal Symposium (SEAMAM III). Bonn: UNEP / CMS Secretariat.
Hughes, A. C. (2017). Understanding the drivers of Southeast Asian biodiversity loss. Ecosphere 8:e01624. doi: 10.1002/ecs2.1624
Hughes, T. P., Bellwood, D. R., and Connolly, S. R. (2002). Biodiversity hotspots, centres of endemicity, and the conservation of coral reefs. Ecol. Lett. 5, 775–784. doi: 10.1046/j.1461-0248.2002.00383.x
Hussey, N. E., Kessel, S. T., Aarestrup, K., Cooke, S. J., Cowley, P. D., Fisk, A. T., et al. (2015). Aquatic animal telemetry: a panoramic window into the underwater world. Science 348:6240. doi: 10.1126/science.1255642
Jarić, I., Knežević-Jarić, J., and Gessner, J. (2015). Global effort allocation in marine mammal research indicates geographical, taxonomic and extinction risk-related biases. Mamm. Rev. 45, 54–62. doi: 10.1111/mam.12032
Jefferson, T., Pitman, R., Leatherwood, S., and Dolar, M. (1997). Developmental and sexual variation in the external appearance of Fraser’s dolphins (Lagenodelphis hosei). Aquat. Mamm. 23, 145–154.
Jefferson, T. A., Webber, M. A., Pitman, R. L., and Gorter, U. (2015). Marine Mammals of the World: A Comprensive Guide to Their Identificaton, 2nd Edn. Amsterdam: Elsevier, doi: 10.1016/B978-0-7216-7618-0.50002-X
Lacsamana, J. K. M., Ventolero, M. F. H., Blatchley, D., and Santos, M. D. (2015). First record of a rare beaked whale Mesoplodon hotaula in the Philippines. Mar. Biodivers. Rec. 8:e77. doi: 10.1017/S1755267215000457
Learmonth, J. A., Macleod, C. D., Santos, M. B., Pierce, G. J., Crick, H. Q. P., and Robinson, R. A. (2006). Potential effects of climate change on marine mammals. Oceanogr. Mar. Biol. 44, 431–464. doi: 10.1201/9781420006391.ch8
Leatherwood, S., Dolar, M. L. L., Wood, C. J., Aragones, L. V., and Hill, C. (1992). Marine mammal species confirmed from Philippine waters. Silliman J. 36, 65–86.
Lindegren, M., Holt, B. G., MacKenzie, B. R., and Rahbek, C. (2018). A global mismatch in the protection of multiple marine biodiversity components and ecosystem services. Sci. Rep. 8, 1–8. doi: 10.1038/s41598-018-22419-1
MacLeod, C. D. (2009). Global climate change, range changes and potential implications for the conservation of marine cetaceans: a review and synthesis. Endanger. Species Res. 7, 125–136. doi: 10.3354/esr00197
Mann, J. (1999). Behavioral sampling methods for cetaceans: a review and critique. Mar. Mammal Sci. 15, 102–122. doi: 10.1111/j.1748-7692.1999.tb00784.x
Mazerolle, M. J. (2020). AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c). R Package Version 2.3-0. Available online at: https://cran.r-project.org/web/packages/AICcmodavg/index.html (accessed August 26, 2020).
Mustika, P. L. K., Welters, R., Ryan, G. E., D’Lima, C., Sorongon-Yap, P., Jutapruet, S., et al. (2017). A rapid assessment of wildlife tourism risk posed to cetaceans in Asia. J. Sustain. Tour. 25, 1138–1158. doi: 10.1080/09669582.2016.1257012
Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B., and Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858. doi: 10.1038/35002501
Myers, P., Espinosa, R., Parr, C. S., Jones, T., Hammond, G. S., and Dewey, T. A. (2020). Animal Diversity Web (online). Accessed online at: https://animaldiversity.org (accessed June 06, 2020).
Nakagun, S., Acebes, J. M. V., and Ponzo, A. (2013). “Steno bredanensis in the Babuyan Islands, Northern Luzon, Philippines,” in Poster Presented at the 20th Biennial Conference on the Biology of Marine Mammals, Dunedin.
Nakagun, S., Smoll, L. I., Sato, T., Layusa, C. A. A., and Acebes, J. M. V. (2020). Interchange of humpback whales (Megaptera novaeangliae) between northern Philippines and Ogasawara, Japan, has implications for conservation. Pacific Conserv. Biol. 26, 378–383. doi: 10.1071/pc19003
Obusan, M. C. M., Aragones, L. V., Rivera, W. L., and Siringan, M. A. T. (2018). Antibiotic susceptibility patterns of bacteria isolated from cetaceans stranded in the Philippines. Aquat. Mamm. 44, 558–569. doi: 10.1578/AM.44.5.2018.558
Obusan, M. C. M., Aragones, L. V., Salibay, C. C., Siringan, M. A. T., and Rivera, W. L. (2015). Occurrence of human pathogenic bacteria and Toxoplasma gondii in cetaceans stranded in the philippines: providing clues on ocean health status. Aquat. Mamm. 41, 149–166. doi: 10.1578/AM.41.2.2015.149
Obusan, M. C. M., Villanueva, R. M. D., Siringan, M. A. T., Rivera, W. L., and Aragones, L. V. (2019). Leptospira spp. and Toxoplasma gondii in stranded representatives of wild cetaceans in the Philippines. BMC Vet. Res. 15:372. doi: 10.1186/s12917-019-2112-5
Pavone, I. R. (2019). Is banning enough? The intricacy inherent to marine mammal conservation. Ger. Law J 20, 587–613. doi: 10.1017/glj.2019.52
Perrin, W. F., Aquino, M. T., Dolar, M. L. L., and Alava, M. N. R. (2007). External appearance of the dwarf spinner dolphin Stenella longirostris roseiventris. Mar. Mammal Sci. 23, 464–467. doi: 10.1111/j.1748-7692.2007.00117.x
Perrin, W. F., Dolar, M. L., and Ortega, E. (1996). Osteological comparison of Bryde’s whales from the Philippines with specimens from other regions. Rep. Int. Whal. Comm. 46:409413.
Perrin, W. F., Dolar, M. L. L., Amano, M., and Hayano, A. (2003). Cranial sexual dimorphism and geographic variation in Fraser’s dolphin, Lagenodelphis hosei. Mar. Mammal Sci. 19, 484–501. doi: 10.1111/j.1748-7692.2003.tb01316.x
Perrin, W. F., Dolar, M. L. L., and Robineau, D. (1999). Spinner dolphins (Stenella longirostris) of the western Pacific and Southeast Asia: pelagic and shallow-water forms. Mar. Mammal Sci. 15, 1029–1053. doi: 10.1111/j.1748-7692.1999.tb00876.x
Perrin, W. F., Reeves, R. R., Dolar, M. L. L., Jefferson, T. A., Marsh, H., Wang, J. Y., et al. (2002). Report of the Second Workshop on The Biology and Conservation of Small Cetaceans and Dugongs of South-East Asia. Available online at: https://www.cms.int/siberian-crane/sites/default/files/publication/tech_series_no9_seamam_3_0_0.pdf (accessed May 06, 2020).
Perrin, W. F., Wursig, B., and Thewissen, J. G. M. (2008). Encyclopedia of Marine Mammals, 2nd Edn. Amsterdam: Elsevier.
Postrado, J. F., Jover, K. M. A., Mabulac, H. J., Velasco, J. S., de la Paz, M. E., Pereda, J. M., et al. (2019). Identification of fish prey of an irrawaddy dolphin (Orcaella brevirostris) using Mitochondrial Cytochrome c Oxidase 1 Sequence Analysis. Philipp. J. Fish 26, 1–7. doi: 10.31398/tpjf/26.1.2019-0001
Schipper, J., Chanson, J. S., Chiozza, F., Cox, N. A., Hoffmann, M., Katariya, V., et al. (2008). The status of the world’s land and marine mammals: Diversity, threat, and knowledge. Science 322, 225–230. doi: 10.1126/science.1165115
Seber, G. A. F., and Schofield, M. R. (2019). Capture-Recapture?: Parameter Estimation for Open Animal Populations. Berlin: Springer.
Silberg, J. N., Acebes, J. M. V., Burdin, A. M., Mamaev, E. G., Dolan, K. C., Layusa, C. A., et al. (2013). New insight into migration patterns of western North Pacific humpback whales between the Babuyan Islands, Philippines and the Commander Islands, Russia. J. Cetacean Res. Manag. 13, 53–57.
Slijper, E. J., Van Utrecht, W. L., and Naaktgeboren, C. (1964). Remarks on the distribution and migration of whales, based on observations from Netherlands ships. Bijdragen tot de Dierkunde 34, 3–93.
Smith, A. B., Pacini, A. F., Nachtigall, P. E., Laule, G. E., Aragones, L. V., Magno, C., et al. (2019). Transmission beam pattern and dynamics of a spinner dolphin (Stenella longirostris). J. Acoust. Soc. Am. 145, 3595–3605. doi: 10.1121/1.5111347
Smith, B. D., Beasley, I., Buccat, M., Calderon, V., Evina, R., Lemmuel de Valle, J., et al. (2004). Status, ecology and conservation of Irrawaddy dolphins (Orcaella brevirostris) in Malampaya Sound, Palawan, Philippines. J. Cetacean Res. Manag. 6, 41–52.
Sodhi, N. S., Posa, M. R. C., Lee, T. M., Bickford, D., Koh, L. P., and Brook, B. W. (2010). The state and conservation of Southeast Asian biodiversity. Biodivers. Conserv. 19, 317–328. doi: 10.1007/s10531-009-9607-5
Sorongon, P. M. E., Acebes, J. M. V., Dolar, M. L. L., Hilomen, V. V., and Palomares, M. L. D. (2010). “The effect of tourism on cetacean populations in southern Philippines,” in Marine Biodiversity in Southeast Asian and Adjacent Seas Part 1, eds M. L. D. Palomares and D. Pauly (Vancouver, BC: University of British Columbia), 78–96.
Sutherland, W. J., Pullin, A. S., Dolman, P. M., and Knight, T. M. (2004). The need for evidence-based conservation. Trends Ecol. Evol. 19, 305–308. doi: 10.1016/j.tree.2004.03.018
Tanalgo, K. C., and Hughes, A. C. (2018). Bats of the Philippine Islands—A review of research directions and relevance to national-level priorities and targets. Mamm. Biol. 91, 46–56. doi: 10.1016/j.mambio.2018.03.005
Tiongson, A. J. C., and Karczmarski, L. (2016). The Indo-Pacific bottlenose dolphin (Tursiops aduncus) in Tañon Strait, central Philippines. Mar. Biodivers. Rec. 9:85. doi: 10.1186/s41200-016-0088-4
Tiongson, A. J. C., Utzurrum, J., and de la Paz, M. E. L. (2020). Critically endangered subpopulation of Irrawaddy dolphin Orcaella brevirostris in central Philippines may lose its habitat to large-scale development project. Oryx 54, 761–762.
Titova, O. V., Filatova, O. A., Fedutin, I. D., Ovsyanikova, E. N., Okabe, H., Kobayashi, N., et al. (2018). Photo-identification matches of humpback whales (Megaptera novaeangliae) from feeding areas in Russian Far East seas and breeding grounds in the North Pacific. Mar. Mammal Sci. 34, 100–112. doi: 10.1111/mms.12444
Torno, C. S., Buccat, M. C., and Masangkay, J. S. (2008). Aspergillosis in a melon-headed whale (Peponocephala electra). Philipp. J. Vet. Med. 45, 49–57.
Townsend, C. H. (1935). The distribution of certain whales as shown by logbook records of American Whaleships. Zoologica 19, 1–50.
Trites, A. W. (2019). “Marine mammal trophic levels and trophic interactions,” in Encyclopedia of Ocean Sciences, 3rd Edn, Vol. 2, eds J. Kirk Cochran, H. J. Boluniewicz, and P. L. Yager (Amsterdam: Elsevier Ltd), doi: 10.1016/B978-0-12-409548-9.11618-5
Tucker, M. A., and Rogers, T. L. (2014). Examining predator-prey body size, Trophic level and body mass across marine and terrestrial mammals. Proc. R. Soc. B Biol. Sci. 281, 1–9. doi: 10.1098/rspb.2014.2103
Venables, W. N., and Ripley, B. D. (2002). Modern Applied Statistics with S, 4th Edn. Berlin: Springer. ISBN 0-387-95457-0.
Wada, S., Oishi, M., and Yamada, T. K. (2003). A newly discovered species of living baleen whale. Nature 426, 278–281. doi: 10.1038/nature02101
Whitty, T. S. (2015). Governance potential for cetacean bycatch mitigation in small-scale fisheries: a comparative assessment of four sites in Southeast Asia. Appl. Geogr. 59, 131–141. doi: 10.1016/j.apgeog.2015.01.003
Whitty, T. S. (2016). Multi-methods approach to characterizing the magnitude, impact, and spatial risk of Irrawaddy dolphin (Orcaella brevirostris) bycatch in small-scale fisheries in Malampaya Sound, Philippines. Mar. Mammal Sci. 32, 1022–1043. doi: 10.1111/mms.12322
Willer, D. F., Smith, K., and Aldridge, D. C. (2019). Matches and mismatches between global conservation efforts and global conservation priorities. Front. Ecol. Evol. 7:297. doi: 10.3389/fevo.2019.00297
Wilson, K. A., Auerbach, N. A., Sam, K., Magini, A. G., Moss, A. S. L., Langhans, S. D., et al. (2016). Conservation research is not happening where it is most needed. PLoS Biol. 14:e1002413. doi: 10.1371/journal.pbio.1002413
Keywords: marine mammals, Philippines, extinction risk, conservation, research effort
Citation: Tiongson AJC, Utzurrum JA and de la Paz MEL (2021) Patterns of Research Effort and Extinction Risk of Marine Mammals in the Philippines. Front. Mar. Sci. 8:607020. doi: 10.3389/fmars.2021.607020
Received: 16 September 2020; Accepted: 12 May 2021;
Published: 07 June 2021.
Edited by:
Karen A. Stockin, Massey University, New ZealandReviewed by:
Mark Peter Simmonds, University of Bristol, United KingdomRob Harcourt, Macquarie University, Australia
Copyright © 2021 Tiongson, Utzurrum and de la Paz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angelico Jose C. Tiongson, YW5nZWxpY29jdGlvbmdzb25Ac3UuZWR1LnBo
 Angelico Jose C. Tiongson
Angelico Jose C. Tiongson Jean Asuncion Utzurrum
Jean Asuncion Utzurrum Manuel Eduardo L. de la Paz
Manuel Eduardo L. de la Paz