
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 18 March 2021
Sec. Marine Conservation and Sustainability
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.603229
Adaptation is a biological mechanism by which organisms adjust physically or behaviorally to changes in their environment to become more suited to it. This is a report of free-ranging bottlenose dolphins’ behavioral adaptations to environmental changes from coastal construction in prime habitat. Construction was a 5-year bridge removal and replacement project in a tidal inlet along west central Florida’s Gulf of Mexico coastline. It occurred in two consecutive 2.5-year phases to replace the west and east lanes, respectively. Lane phases involved demolition/removal of above-water cement structures, below-water cement structures, and reinstallation of below + above water cement structures (N = 2,098 photos). Data were longitudinal (11 years: 2005–2016, N = 1,219 surveys 2–4 times/week/11 years, N = 4,753 dolphins, 591.95 h of observation in the construction zone, 126 before-construction surveys, 568 during-construction surveys, 525 after-construction surveys). The dependent variable was numbers of dolphins (count) in the immediate construction zone. Three analyses examined presence/absence, total numbers of dolphins, and numbers of dolphins engaged in five behavior states (forage-feeding, socializing, direct travel, meandering travel, and mixed states) across construction. Analyses were GLIMMIX generalized linear models for logistic and negative binomial regressions to account for observation time differences as an exposure (offset) variable. Results showed a higher probability of dolphin presence than absence before construction began, more total dolphins before construction, and significant decreases in the numbers of feeding but not socializing dolphins. Significant changes in temporal rhythms also revealed finer-grained adaptations. Conclusions were that the dolphins adapted to construction in two ways, by establishing feeding locations beyond the disturbed construction zone and shifting temporal rhythms of behaviors that they continued to exhibit in the construction zone to later in the day when construction activities were minimized. This is the first study to suggest that the dolphins learned to cope with coastal construction with variable adjustments.
The accelerating pace and breadth of marine coastal development threatens marine mammals with cumulative effects from unsustainable human practices and pollution (Williams et al., 2015; Gomez et al., 2016; Shannon et al., 2016; Avila et al., 2018). Potentially unsustainable human activities include vessel strikes (Laist et al., 2001; Panigada et al., 2006), whaling, other direct harvesting, by-catch (Read, 2008; Mannocci et al., 2012; Nabe-Nielsen et al., 2014; Allen et al., 2019); entanglement, debris ingestion (Harcourt et al., 1994; Page et al., 2004), petroleum exploration, underwater shipping noise (Williams et al., 2015), military seismic sonar (Frantzis, 1998; Sivle et al., 2015; Southall et al., 2016), other sources of anthropogenic disruption (Christiansen et al., 2013), and noise pollution (Miller et al., 2015).
Noise pollution is an issue because cetaceans (Tyack, 2008) and many species of fish (Popper and Hastings, 2009) depend on sound to navigate, communicate with conspecifics, avoid predators, and search for prey (Williams et al., 2015; Gomez et al., 2016; Shannon et al., 2016; Carroll et al., 2017; Wenger et al., 2017), which makes them particularly vulnerable to noise pollution that interferes with these behaviors. Noise drowns out or masks vocalizations, forcing animals to wait for noise pollution to subside or to invest more energy in communication, i.e., the Lombard effect (Jensen et al., 2008; May-Collado and Quiñones-Lebrón, 2014; Erbe et al., 2016; Gomez et al., 2016). Because they depend on sound, cetaceans may suffer more stress from noise pollution than most terrestrial species (Wright et al., 2007), but the impact of noise pollution varies by species. Cuvier’s beaked whales (Ziphius cavirostris, Frantzis, 1998), harbor porpoises (Phocoena phocoena, Dahne et al., 2013; Tougaard et al., 2015; Graham et al., 2019), finless porpoises (Neophocaena sp., Tougaard et al., 2015), and northern bottlenose whales (Hyperoodon ampullatus, Miller et al., 2015) are notably affected by noise pollution; two examples of notable impact are fatal stranding and acute avoidance. A review of experimental exposures to sonar by Southall et al. (2016) provided causal evidence that even controlled doses of noise pollution (controlled to minimize long-term impacts) changed free-ranging cetaceans’ current activities to avoidance (DeRuiter et al., 2013; Goldbogen et al., 2013; Isojunno et al., 2016).
Pile driving and dredging are common reasons why cetaceans temporarily avoid or show longer-term displacement from the vicinity of coastal construction in cetacean habitat. Pile driving displaces harbor porpoises (P. phocoena, Carstensen et al., 2006; Thompson et al., 2010, 2013a,b; Brandt et al., 2011, 2018), fish (Popper and Hastings, 2009), and bottlenose dolphins (Tursiops sp., Paiva et al., 2015; Weaver, 2015). Pile driving is the only documented anthropogenic sound, other than explosives, that causes fish kills in the wild (Popper and Hastings, 2009). Odontocetes depend on fish, so fish recovery from noise-based depletions influences cetacean recovery directly (Nabe-Nielsen et al., 2014). Dredging, which is the process of excavating and relocating sediment from waterways, is also a typical component of most coastal construction projects (Wenger et al., 2017). Dredging is usually fatal to fish eggs and larva (Todd et al., 2015; Wenger et al., 2017) and displaces adult fish (Popper and Hastings, 2009), which changes the local ecology and leads to displacement of cetaceans from previous foraging grounds (Thompson et al., 2010; Marley et al., 2017).
Avoidance manifests as displacement and may be short- or long-term. Among cetaceans, short-term avoidance is expressed as temporary, localized reactions that typically revert to baseline behavior in short order (Buckstaff et al., 2013). For example, killer whales (Orcinus orca) and long-finned pilot whales (Globicephala sp.) avoided an area for at least 5 h following controlled experimental exposure to sonar (Miller et al., 2012). Short-term avoidance includes several types of behavior changes, including changing directions to avoid boats (Nowacek et al., 2001; Lusseau, 2004, 2005, 2006; Bejder et al., 2006b; Carrera et al., 2008; Steckenreuter et al., 2011; Parsons, 2012; Pirotta et al., 2015; Cribb and Seuront, 2016), changing acoustic behavior in noisy environments (Buckstaff, 2004; Carstensen et al., 2006; Nowacek et al., 2007; de Souza Albuquerque and da Silva Souto, 2013; May-Collado and Quiñones-Lebrón, 2014; van Ginkel et al., 2017), and increased travel or decreased foraging (Piwetz, 2019). The consequences of cumulative effects of short-term displacements on cetacean behavior are unknown.
Long-term displacement from prolonged exposure to single or cumulative impacts of construction lasts months to years (Graham et al., 2019; Kreb et al., 2020). Vessel traffic related to construction of an underwater gas pipeline displaced gray seals (Halichoerus grypus) and minke whales (Balaenoptera sp.) for 3 years, but not bottlenose dolphins (Anderwald et al., 2013). Construction of a gas pipeline displaced harbor porpoises (P. phocoena), gray seals (H. grypus), and minke whales (Balaenoptera sp.) for 6 years (Culloch et al., 2016). Bridge construction displaced female bottlenose dolphins for 8 years that had previously shown high-site fidelity but did not displace male bottlenose dolphins that had also shown high-site fidelity (Weaver, 2015). Harbor porpoises’ presence declined significantly during and after wind farm construction, and had not fully recovered 10 years later (Teilmann and Carstensen, 2012).
High-resolution tag and passive acoustic monitoring systems (Tyack et al., 2011; Nowacek et al., 2016) have permitted the detection of short changes in cetacean foraging (Teilmann and Carstensen, 2012; Williams et al., 2015), traveling (Miller et al., 2014), and resting (Miller et al., 2009) indicative of avoiding anthropogenic activities (Miller et al., 2012; DeRuiter et al., 2013; Goldbogen et al., 2013; Isojunno et al., 2016). However, brief impact studies on unidentified individuals are of limited value in demonstrating the repercussions of longer-term changes in cetacean behavior budgets concurrent with coastal construction (Bejder et al., 2009). Moreover, anthropogenic impacts on cetaceans and measures of cetacean responses vary tremendously (Gomez et al., 2016). As a result of these variations, predicting the biological consequences of cumulative impacts on disrupted behavior requires data based on several elements: careful interpretation of the contexts of exposure, the nature and duration of displacements, how disturbance manifested over time, mitigating roles of nearby alternative quality habitat, and whether displaced individuals returned or adjusted, ideally from a longitudinal perspective (Bejder et al., 2006a,b; Ellison et al., 2012; Williams et al., 2015; Gomez et al., 2016; Shannon et al., 2016; Bossley et al., 2017; Hawkins et al., 2017; Avila et al., 2018).
The accelerating pace of urban development makes coastal zones the most ecologically imperiled and noisy ecosystems on earth (Sekovski et al., 2012; Ramesh et al., 2015; Williams et al., 2015; Gomez et al., 2016; Shannon et al., 2016). Development exposes 47% of coastal marine mammal species and 51% of core marine mammal habitats to pervasive anthropogenic disturbance (Avila et al., 2018); offshore populations are typically less affected or unaffected by coastal construction. Despite relative accessibility to researchers, data on the impacts of coastal construction on coastal cetaceans are too often inadequate (Hawkins et al., 2017) despite the fact that they are urgently needed (Sekovski et al., 2012; Ramesh et al., 2015; Weaver, 2015; Williams et al., 2015; Gomez et al., 2016; Shannon et al., 2016; Avila et al., 2018; Kreb et al., 2020).
Most bottlenose dolphins show strong site fidelity to coastal habitats, which makes them particularly vulnerable to anthropogenic pressures because it exposes them to the greatest number of cumulative threats (Appendix K maps, Avila et al., 2018). Bridge removal and replacement is a widespread form of coastal construction in some bottlenose dolphin habitats. An 11-year bridge replacement project in west central Florida provided the opportunity to conduct a longitudinal case study evaluating the impact of bridge replacement on individually identified bottlenose dolphins (Weaver, 2015). Because non-experimental field observations can only show that anthropogenic activities correlate with, rather than cause, concurrent changes in cetacean behavior, any evidence of dolphin behavior change in the immediate vicinity of construction provided the opportunity to examine, although not establish, causation. Therefore, the behavior of individual dolphins was observed before, during, and after bridge construction in the immediate vicinity of construction. This is the first study to report how free-ranging bottlenose dolphins show variable adjustments to immediate exposure to long-term coastal construction.
In this analysis, “behavior” only refers to dolphins in the immediate vicinity of the construction zone. The construction zone (Figure 1A) was located in the middle of the southern half of the study area (Figure 1B). The John’s Pass construction zone was geographically localized over a narrow (183 m wide) and shallow (12 m) tidal inlet connecting the Gulf of Mexico and Intracoastal Waterways (27.782679°−82.782705°).
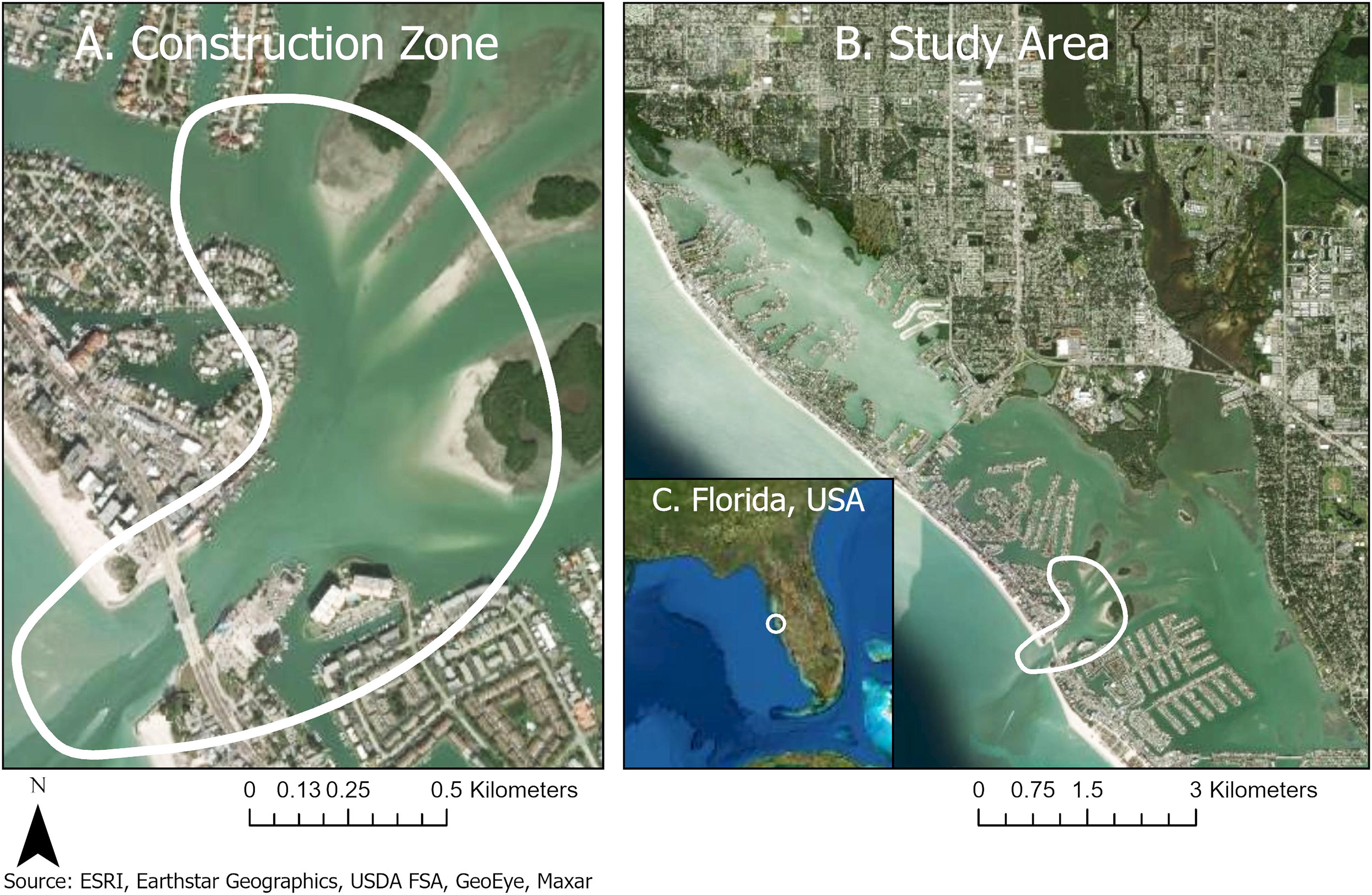
Figure 1. A coastal construction project to replace a double-leaf bascule bridge exposed prime bottlenose dolphin feeding and socializing habitat to 58 months of noise pollution and habitat degradation. (A) John’s Pass construction zone (27.782679°, –82.782705°). (B) Entire study area in the Intracoastal Waterway or ICW (north end: 27.831986° –82.830557°, south end: 27.771542° –82.753089°) from Redington Shores to Treasure Island, Pinellas Country, Florida. (C) State of Florida. John’s Pass dolphins are part of the Tampa Bay, Florida stock.
Construction involved removing the old bridge and replacing it with a new 14,750 m2 double-leaf bascule bridge. Construction lasted 58 consecutive months and construction activities were photo-documented weekly, N = 2,098 photos. Construction activities (Figure 2) took place in two consecutive phases, first removing and replacing the west or Gulf side of the bridge (during-construction W lane: January 2006 through approximately mid-summer 2008) and then removing and replacing the east or ICW side of the bridge (during-construction E lane: mid-summer 2008 through October 2010). The during-construction W lane and during-construction E lane phases exposed the construction zone habitat to a 3-part series of similar environmental impacts. The first part mainly involved above-water demolition and extraction of the cement roadway, lane supports, and portions of lift towers (Figure 3). The second part mainly involved below-water demolition, dredging, and removal of submerged cement roadway supports and portions of lift towers sunken deep into the sea floor for stability (Figures 4–9). The third part of the 3-part series of environmental impacts from construction mainly involved above- and below-water activities for replacing caissons, lift towers, bascule leaves to span the inlet, and roadway lanes.

Figure 2. View of the construction zone from the ICW. Construction activities occurred in two consecutive sets to maintain one lane open to vehicular traffic. The first set involved removing and replacing the Gulf or W lane of the bridge (January 2006 through approximately mid-summer 2008). The second set involved removing and replacing the ICW or E lane of the bridge (mid-summer 2008 through October 2010). Each step consecutively exposed the construction zone habitat to three sets of impacts.

Figure 3. The first set of impacts was mainly destruction and removal of above-water cement structures. Shown here is the partially dismantled NW lift tower housing before the bascule leaf and lift mechanisms were removed.

Figure 4. Bridge replacement included four separate explosions (dynamite) to remove below-water cement structures, with commensurate habitat destruction and personal observations of extensive fish kills. This image shows demolition of the submerged portions of the lift tower on the NW portion of the W lane.

Figure 5. The second set of impacts was mainly related to below-water activity. This included demolition, dismantling, dredging, and removing submerged portions of cement roadway supports and lift towers sunken deep into the sea floor for stability, followed by drilling new pipes of various diameters into the seabed as supports for new traffic lanes and for caissons supporting lift towers.

Figure 6. The construction site experienced regular percussive pile driving, bored pile driving, and extensive dredging across construction. This is a photo of a dredging bit, used to churn up the seafloor and suck substrate into a pipe for delivery onto a barge for off-site dumping.

Figure 7. Barges were regularly loaded with rubble, ranging in size from peas to cars, from old cement structures extracted from above and below seafloor. Rubble was transported to off-shore dump sites throughout construction.

Figure 8. The third set of impacts was mainly related to below + above water activity to rebuild the new bridge. Shown here is the NW portion of the construction of new W lanes and cement debris being broken into smaller-sized rubble for transport.

Figure 9. Noise and vibrational pollution from multiple barges, tugboats, small vessels, and cranes operated throughout construction added to environmental impacts.
Below-water demolition included four detonations of submerged portions of the bridge. Demolition dates for the W lane were 8/3/2006 and 8/24/2006 and for the E lane 11/20/2008 and 12/16/2008 (Figure 4). The company that conducted the construction and detonations was Flatiron Construction, a heavy infrastructure civil contractor headquartered in Broomfield, Colorado, United States. Flatiron Construction builds infrastructure for the transportation, energy, and water sectors, specializing in large-scale infrastructure projects. The precise identity and quantity of dynamite and related explosive materials was unavailable to this researcher. Florida Fish and Wildlife Conservation Commission personnel, this researcher AW, and other members of the local stranding network were stationed in John’s Pass on land and in boats outside of the impact zone and in helicopters to spot wildlife moving into the area during the time that the detonations were scheduled. Only one detonation (8/24/2006) was delayed because five dolphins and a manatee entered John’s Pass and swam through it to the Gulf of Mexico. The detonation was delayed 45 min. The other three detonations were conducted as scheduled because wildlife did not appear.
Throughout, construction-related activities included 24-h illumination and 24-h noise pollution from increased vessel presence, running tugboat(s), compressor, and crane engines (Figure 8) although nighttime activities were not as constant as daytime activities. Dolphin tour boats moved through the area 2–4 times/day. Types and received levels of noise pollution and fish communities were not measured due to the extended time frame and logistics of construction in a tightly constricted area. Regular percussive pile driving, bored pile driving, and dredging extensively altered the construction zone habitat (Figures 6–8).
The study area (Figure 1B) is a narrow 10.5-km section of the contiguous Boca Ciego Intracoastal Waterway (north end: 27.831986°−82.830557°, south end: 27.771542°−82.753089°). The survey route is a line transect between channel markers with good visual access to both shorelines. The sea floor is a patchwork of hard-packed sand, muddy sand, oyster bars, and seagrass beds. Water depth averages 1–3 m deep except in John’s Pass, which is 6–12 m deep. Boat-based surveys of the study area started within 2 h after dawn (19’ Proline boat, Yamaha 115 hp outboard).
Each survey began at John’s Pass and included coverage of the entire study area (included on the map, Figure 1B). A complete survey took the following route: (1) from the home dock (72.785173°, −82.771513°), (2) John’s Pass (27.782679°, −82.782705°), (3) west of the John’s Pass tidal deltas to the north end of the study area, (4) retraced the route half way but east of John’s Pass tidal deltas to the southern end of the study area, and (5) ended back at the home dock, covering an average distance of 20–25 km. Survey time depended on whether dolphins were sighted or not. When dolphins were not sighted (3% of all surveys, Weaver and Kuczaj, 2016), surveys lasted an average of 2 h. When dolphins were sighted (97% of all surveys), surveys lasted up to 8 h. Surveys typically ended between 11 am and 2 pm and occasionally ran longer as dictated by dolphins sightings (e.g., an 8-h survey that started at 7 am ended around 3 pm) or shorter as dictated by weather (time of day was taken into account as a covariate in these analyses). Sampling was homogeneous in that the entire study area was surveyed (total study area approximately 25 km2). The exceptions were surveys aborted by bad weather; those data were excluded from this report. This study used a sequential research design by collecting observations across the entire study area to compare at multiple points of time (Bejder et al., 2009). The process of searching for dolphins in the study area involved traveling at average speeds of about 9.65–12.87 km/hr between channel markers with three observers scanning 360 degrees for dolphins. AW’s onboard role was to sight dolphins, identify individual dolphins, and to identify and dictate behavior state to a second observer whose role was to sight dolphins, record data, and collect photo ID photos. The boat driver served as a third observer for sighting dolphins. Environmental data were recorded at the onset of each survey in John’s Pass and included tidal currents, moon phase, Beaufort sea state, cloud cover, water temperature, and salinity. The study area is a narrow stretch of ICW (Figure 1B); binoculars are unnecessary for sighting dolphins.
The observational protocol was as follows. Dolphins sighted along the survey route were initially approached to about 18 m (three boat lengths) to assess current behavior state. Subsequent observations were typically made from a distance of 7–10 m or less. Observations were made during focal group follows (Mann, 1999) and lasted as long as required to identify behavior state(s) and obtain reasonable photos to identify individual dolphins (photo-identification photos); the protocol did not limit the number of behavior states that might be observed or observation time. However, because social behavior is central to this study, this led to differences in sampling rules for ending a sighting to resume the survey. The sampling rule for ending a sighting of non-social behavior was after 15 min if the behavior state had not changed, group composition had not changed, and photo-ID photos had been obtained. When behavior state changed, an additional 15-min observation period was added to observe subsequent behavior. The sampling rule for ending a sighting of social behavior was to continue observations until the social bout reached an apparent natural end.
A group was defined as all of the visible dolphins behaving in a coordinated manner. The data collection procedure defined changes in group composition (either when some of the dolphins left the group during fission events or new dolphins joined the group during fusion events) as a new sighting because the new group was composed of a new set of individual dolphins. The observational protocol was repeated. After a fission event, the group with the least recent data was the new focal group. However, because social behavior is of central interest to this study, observations of socializing dolphins were given priority and continued until they stopped socializing.
Behavior data were behavior states measured continuously during focal group follows (Mann, 1999) to calculate state frequency and duration for individual dolphins so that the predominant group behavior could be assessed for analysis. If the visible dolphins showed one behavior state for half or more of an observation, that state was identified as the predominant behavior state. If visible dolphins showed two or more behavior states without a clearly predominant state, the observation was coded as mixed states for the entire group.
AW interpreted behavior states and dictated data to a second observer, who recorded data on an Excel spreadsheet fitted with behavior codes and who also took photo-ID photos. A third person drove the boat and helped to detect animals. Behavior states were interpreted with the following criteria. Spatial formation categorized alignment within and between groups (swimming side-by-side, head-to-tail, or no discernible formation). Proximity categorized distance between dolphins within groups with body length (2.3 m) as close, loose, or wide proximity, and between groups with boat lengths (6 m). Orientation within and between groups was recorded as same or different headings. Matched movements were categorized as synchronous (same as unison), closely coordinated (syncopated), or asynchronous. Predictability of next surfacing location was loosely based on whether observers could correctly anticipate where the dolphin would surface next given the heading of its most recent submergence. Behavior units were used from an 11-category ethogram of 105 behavior units visible at the water surface (Müeller et al., 1998; Weaver and Kuczaj, 2016; Weaver, 2020). Swim speeds were estimated from the boat’s speed while moving parallel to dolphins during photo-ID efforts.
Five behavior states were recorded for lone dolphins and for groups of dolphins: forage-feeding, socializing, direct travel, meandering travel, and rest. Forage-feeding was characterized as lone or highly dispersed dolphins in no discernible formation exhibiting steep or tailout dives, sudden lunges or accelerations, extended submergences, considerable water movement, and unpredictable surfacing locations, and often holding, carrying, or tossing fish. Foraging and feeding often took place under feeding sea birds and/or in waters showing fish on sonar. Socializing occurred when dolphins made direct physical contact (Weaver and Kuczaj, 2016; Weaver, 2020), followed each other persistently at close proximity in contentious (Weaver, 2003, 2020) or affiliative (playful or explicitly sexual) contexts (Weaver and Kuczaj, 2016; Weaver, 2020). Travel involved coordinated dolphins swimming at variable speeds without evidence of other behavior states. Direct travel was steady water movement in a specific direction with predictable surfacing locations exhibited by synchronized or syncopated dolphins in close proximity ranks in the same orientation at variable speeds of 3.2–9.6 + km/h. Meandering travel was non-directional movement around a defined patch of water at very slow to moderate speeds with variable heading changes and unpredictable surfacing locations. It appears to be a form of stationing behavior. Rest was very slow water movement at speeds 3.2 km/h or less with predictable surfacing locations among synchronized or syncopated dolphins in close proximity ranks in the same orientation. The behavior of groups of dolphins that were engaged in different behavior states was labeled as mixed states.
Dolphins were identified by naturally occurring and human-based dorsal fin notches and scars, and documented with mark-recapture photo ID (Hammond et al., 1990) with high-speed Canon digital cameras and 70–200 mm digital zoom lens. AW and a second observer sorted dorsal fin photos by eye and quality (Urian et al., 1999) and did not use software to assist with matching. The best photos of each dolphin were dated, labeled, and stored by survey, N = 418 identified dolphins to date logged with the Gulf of Mexico Dolphin Identification System (GoMDIS OBIS-SEAMAP portal); left and right sides of dorsal fins were photographed and linked to each other. This study took place under NOAA GA permits 1088-1815, 16299, and 16299-2.
There were three research questions. RQ1: Did the presence (versus absence) of dolphins in the construction zone before construction change during or after construction? RQ2: Did the numbers of dolphins using the construction zone before construction change during or after construction? RQ3: Did the numbers of dolphins engaged in specific behaviors in the construction zone before construction change during or after construction? For RQ1, presence/absence data were binary and examined with logistic regression. For RQ2 and RQ3, the numbers of dolphins present per sighting were collected by identifying individual dolphins and later comparing photos to the photo-ID catalog. Numbers of dolphins present were counts with a lower bound of 0, no theoretical upper bound, and were only meaningful as whole number integers. Count data were therefore examined with negative binomial regressions that adjusted for differences in minutes of observation time per sighting (exposure) by transforming exposure into ln hours and entering it as the offset. The offset accounted for differences in survey effort before, during, and after construction. Counts are data that are generated over a period of time or an area of space or volume. To compare outcomes when the outcome is a count, exposure (i.e., time, space, or volume) must be equal. If all elements have the same exposure, it can be excluded from the model. Otherwise, an offset (exposure) is specified. It is not a parameter or predictor in the model (i.e., not estimated) but is included to account for differences in exposure. Observations less than 15 min were excluded. The independent variable was construction phase (four levels: before (baseline): dates 3/1/2005–1/22/2006, N = 126 surveys; during W lane: dates 1/25/2006–7/18/2008, N = 330 surveys; during E lane: dates 7/20/2008–10/9/2010, N = 238 surveys; after: dates 10/10/2010–2/28/2016, N = 525 surveys).
Continuously scaled environmental variables were tested as potential covariates: time of day (temporal effect: measured as minutes after sunrise for consistency across daylight savings times), water temperature, current direction and speed (ebb tidal currents indicated by negative numbers), lunar phase (percent moon visible), and season (1 = spring March–May, 2 = summer June–August, 3 = fall September–November, 4 = winter December–February consecutive). The only significant environmental covariate was time of day so marginal means on Figures 10–14 reflect emmeans numbers of dolphins after taking minutes after sunrise into account. Power analysis (run with GPower 3.1 construction effect pη2 = 0.44, Weaver, 2015; α = 0.05, power = 0.80) estimated that a minimum of N = 77 surveys per construction phase were needed (Table 1 shows that this criterion was met). Analyses were conducted with SAS software, significance alpha = 0.05.
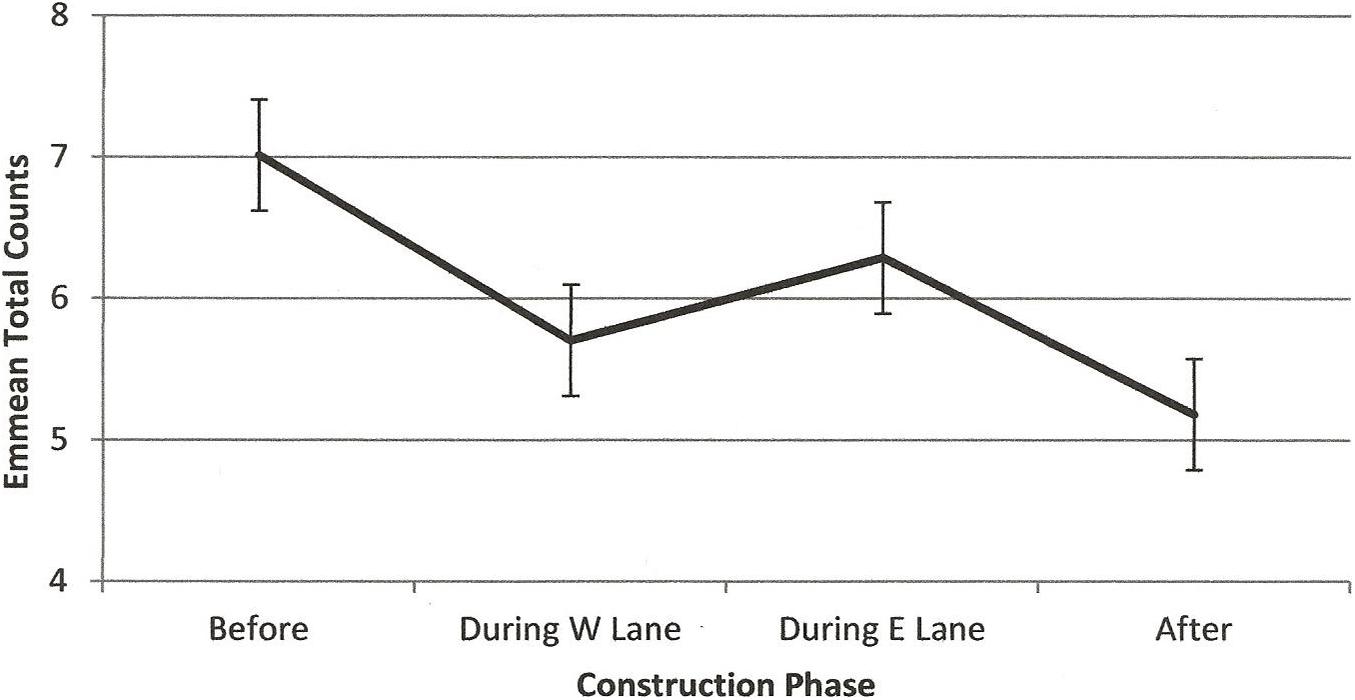
Figure 10. Estimated marginal means of total counts of dolphins in the construction zone before, during, and after construction.
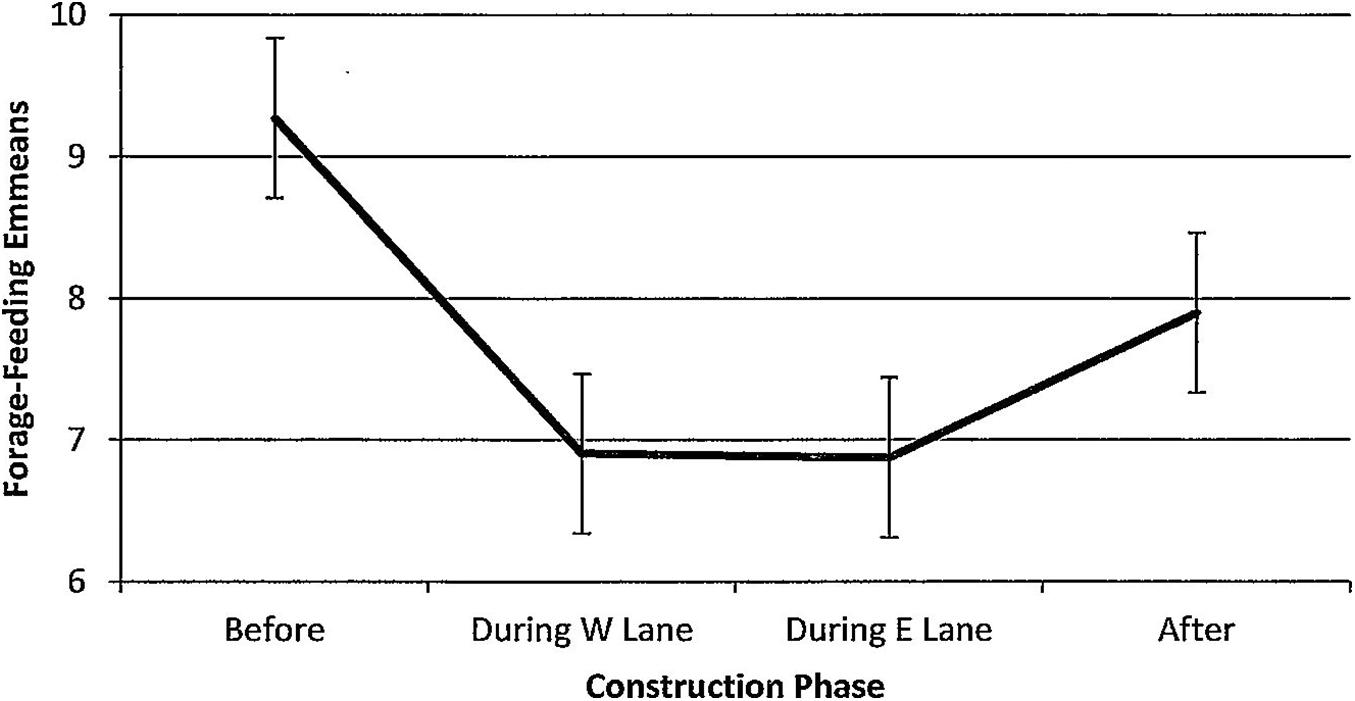
Figure 11. Estimated marginal means of numbers of dolphins forage-feeding in the construction zone before, during, and after construction.
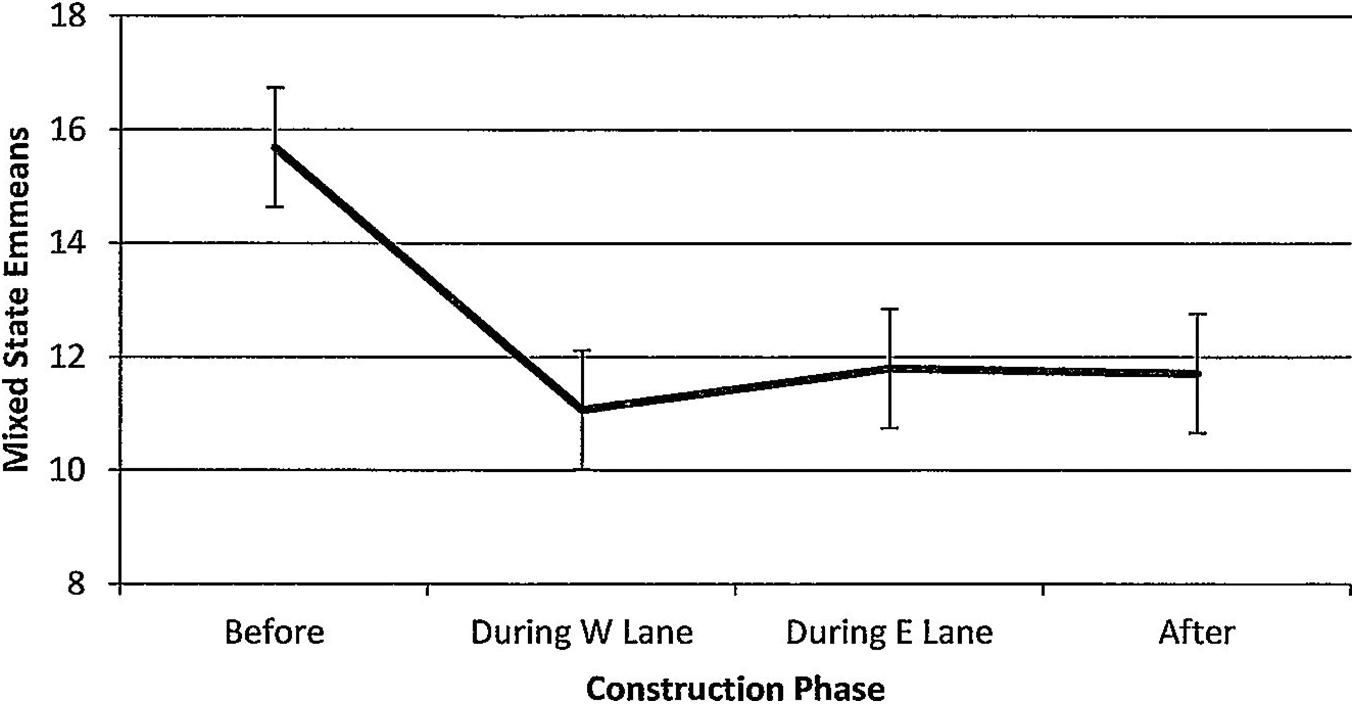
Figure 12. Estimated marginal means of numbers of dolphins engaged in mixed states in the construction zone before, during, and after construction.
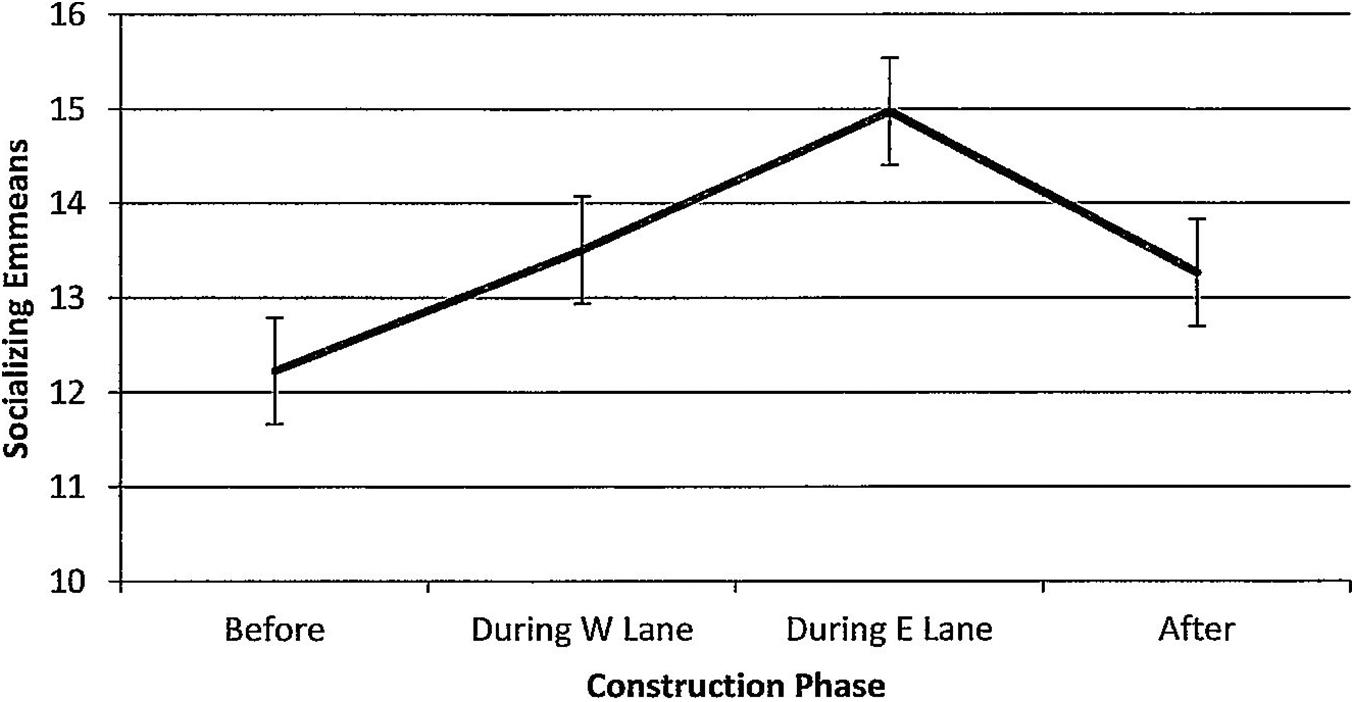
Figure 13. Estimated marginal means of numbers of dolphins socializing in the construction zone before, during, and after construction.
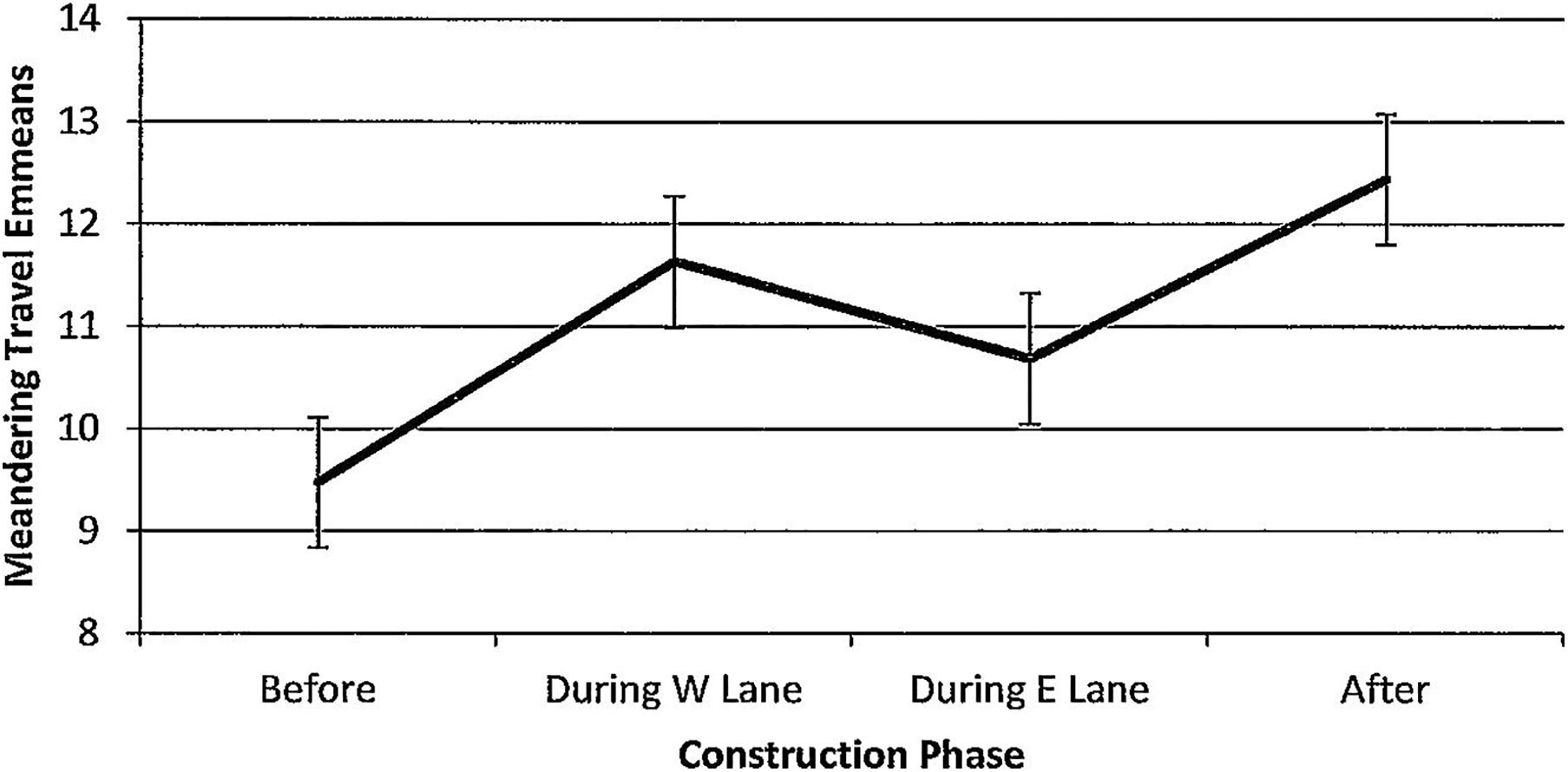
Figure 14. Estimated marginal means of numbers of dolphins engaged in meandering travel around the construction zone before, during, and after construction.
The entire study area, starting with the construction zone, was surveyed 2–4 times a week from 2005 to 2016, subtotals of 94–136 surveys/year, a total of N = 1,219 surveys, N = 4,753 dolphins observed in the construction zone, and a total N = 15,456 dolphins observed in the entire study area. Table 1 shows the observation effort per construction phase across the 11 years of study. Survey effort reflects the time frame of each construction phase, i.e., 1 year of before-construction data, 2.5 years each of during-construction data, and 5 years of after-construction data.
RQ1 was, Did the presence (vs. absence) of dolphins in the construction zone before construction change during or after construction? Overall, dolphins were present on more surveys (N = 635) than absent (N = 584). Presence probabilities were influenced by a significant construction phase × season interaction (F(9,1202) = 3.09, p = 0.001). Probabilities of dolphin presence by season were the most diverse before construction, coalesced to comparable values during W lane construction, and began to differentiate during E lane construction and after. A significant main effect of construction (F(3,1202) = 4.23, p = 0.005) showed that dolphin presence probabilities before construction (M = 0.61) were higher than both phases during construction (W lane M = 0.53, p = 0.029; E lane M = 0.58, p = 0.033), and also after construction (M = 0.48, p = 0.022). Season also had a significant main effect on dolphin presence (F(3,1202) = 6.64, p = 0.0002) with winter and spring probabilities the most affected, in that winter and spring probabilities before construction were p = 0.8 and p = 0.9, respectively, but during the during construction phases dropped to p = 0.56 and p = 0.55, respectively. Minutes after sunrise also exerted a significant effect on dolphin presence (F(1,1202) = 67.30, p < 0.0001). Dolphins tended to be present later in the day (M = 264.23 min after sunrise, SD = 226.88) compared to survey times without dolphins (absence M = 160.36 min, SD = 146.99). This time difference was significant (t(1217) = −9.43, p < 0.001, 95% CI diff [82, 125]).
RQ2 was, Did the numbers of dolphins using the construction zone before construction change during or after construction? Figure 10 illustrates a steady decline in emmeans per hour of observation across construction. All paired differences were significant except the numbers in the two during- construction phases. Results of the negative binomial regression showed significant main effects of construction phase (F(3,1211) = 2.85, p = 0.036), season (F(3,1211) = 3.58, p = 0.013), and minutes after sunrise (F(1,1211) = 95.17, p < 0.001) on numbers of dolphins in the construction zone, accounting for an average parameter estimate of 1–3 fewer dolphins observed per hour over the course of construction.
RQ3 was, Did the numbers of dolphins engaged in specific behavior states in the construction zone before construction change during or after construction? Dolphins were observed resting in the construction zone only 15 times in 15 years so resting behavior was excluded from behavior state analysis. Behavior states are presented in order of descending impact.
For forage-feeding, numbers declined steadily per hour of observation time (Figure 11). Overall differences were non-significant (F(3,188) = 1.86, p = 0.138) with multiple comparisons revealing significantly lower numbers during W lane (p = 0.032) and a clear trend toward significance during E lane (p = 0.079) phases. Forage-feeding was unaffected by season (F(3,188) = 1.06, p = 0.368). Minutes after sunrise had a significant influence (F(1,188) = 8.88, p = 0.003); correlations with numbers of forage-feeding dolphins revealed that the relationship was strongest during E lane construction (r before(26) = −0.01, p = 0.996; r during W lane(50) = 0.21, p = 0.143; r during E lane (23) = 0.36, p = 0.08; r after (89) = −0.04, p = 0.684).
For mixed states, numbers of dolphins also declined steadily per hour of observation time (Figure 12). The overall significant trend (F(3,139) = 2.26, p = 0.084) showed that numbers were significantly higher before construction than during W lane (p = 0.018), during E lane (p = 0.033), and after (p = 0.029). Mixed states were unaffected by season (F(3,139) = 1.83, p = 0.148). Minutes after sunrise had significant influence (F(1,139) = 8.43, p = 0.004); correlations with numbers of dolphins engaged in mixed states revealed that the relationship was strongest during E lane construction (r before(19) = 0.07, p = 0.775; r during W lane(30) = −0.06, p = 0.740; r during E lane (40) = 0.42, p = 0.006; r after (50) = 0.13, p = 0.372).
For socializing, numbers of dolphins per hour of observation time did not change (Figure 13, F(3,104) = 0.53, p = 0.665) and were unaffected by seasons (F(3,104) = 0.94, p = 0.423). Minutes after sunrise had a significant influence on socializing (F(1,104) = 7.18, p = 0.008); correlations with numbers of socializing dolphins revealed that the relationship was strongest during E lane construction (r before(11) = 0.11, p = 0.721; r during W lane(42) = 0.06, p = 0.706; r during E lane (24) = 0.38, p = 0.058; r after (27) = 0.22, p = 0.256).
For meandering travel, numbers of dolphins per hour of observation time did not vary (F(3,78) = 0.84, p = 0.478, Figure 14) and were unaffected by season (F(3,78) = 0.40, p = 0.752). Minutes after sunrise had a significant influence (F(1,78) = 8.88, p = 0.005) with an unusual pattern; correlations with dolphin numbers revealed an inverse relationship before construction that reversed and strengthened across construction (r before(7) = −0.30, p = 0.428; r during W lane(14) = 0.12, p = 0.667; r during E lane (22) = 0.28, p = 0.28; r after (35) = 0.31, p = 0.06).
For direct travel, numbers of dolphins per hour of observation time did not change (construction phase F(3,71) = 0.81, p = 0.493) and were unaffected by season (F(3,71) = 1.46, p = 0.234) or minutes after sunrise (F(1,71) = 0.91, p = 0.344).
This research provides a longitudinal perspective on the cumulative impacts of coastal construction. Construction involved bridge replacement over John’s Pass, a narrow tidal inlet in west central Florida connecting the Gulf of Mexico and Intracoastal Waterway, which served as prime bottlenose dolphin habitat by providing passage between major water bodies and rich feeding grounds supported by strong tidal currents. Measurements of dolphin presence versus absence, total numbers of dolphins, and numbers of dolphins engaged in five behavior states before construction began were compared to the same measures during and after construction, based on 11 years of direct observational data. Results showed that construction initially disrupted established rhythms of dolphin behavior patterns in John’s Pass, which confirmed numerous studies that cetaceans avoid many anthropogenic activities, including construction (Frantzis, 1998; Teilmann and Carstensen, 2012; Anderwald et al., 2013; DeRuiter et al., 2013; Goldbogen et al., 2013; Miller et al., 2015; Tougaard et al., 2015; Weaver, 2015; Cribb and Seuront, 2016; Isojunno et al., 2016; Southall et al., 2016; Graham et al., 2019; Kreb et al., 2020).
However, this is the first study to suggest that the dolphins learned to cope with coastal construction by adapting in two ways: establishing feeding locations outside of the construction zone and shifting the temporal rhythm of behaviors that they continued to exhibit in the construction zone to later in the day when construction activities were minimized. The first form of apparent adaptation was that the dolphins used John’s Pass for feeding less and less. However, observations of the continued presence of dolphins foraging and feeding elsewhere in the rest of the study area suggested that they shifted their feeding activities outside of the construction zone to more viable locations. The significant decrease in numbers of foraging and feeding dolphins in the construction zone remained low across the 5 years after construction had ended (Figure 11). Feeding behavior in the construction zone did not recover.
Significant decreases in foraging and feeding were paralleled by significant reductions in numbers of dolphins engaged in mixed states (Figure 12). Before construction, the future construction zone was a common location for large congregations of as many as 60 dolphins “up and doing” by exhibiting complex co-occurrences of group fissions and fusions, foraging, feeding, a range of social interactions, and other activities lasting hours (to reinforce the importance of John’s Pass to dolphin gatherings before construction, gatherings of 40–60 dolphins is a notable 10-fold increase over the average 4–6 dolphins/group across the entire study area, calculated from 9,551 sightings across 8 years of study (1/2006–12/2013, Weaver and Kuczaj, 2016). Significant reductions in the numbers of dolphins engaged in mixed states also failed to recover during the 5 years after construction had ended. This finding suggested how the impacts of construction-related habitat degradation may ripple through the behavior budget with impacts on social organization.
Cetacean displacements from previous foraging grounds are well-established responses implicating the dispersive effects of construction-related noise pollution, dredging, and pile driving (Carrera et al., 2008; Miller et al., 2009, 2012; Thompson et al., 2010; Pirotta et al., 2013, 2015; Paiva et al., 2015; Marley et al., 2017; Wisniewska et al., 2018; Agrelo et al., 2019; Kreb et al., 2020). Significant reductions in foraging and feeding in this study confirmed reports that construction displaces cetaceans from previous foraging grounds (Thompson et al., 2010; Marley et al., 2017). Viewed as a proxy for food availability, significant reductions in the number of forage-feeding dolphins in John’s Pass implicated construction-related habitat degradation.
Regular pile driving was documented with construction photos, directly indicated by crane operations and indirectly indicated by pilings regularly appearing in different locations across the construction zone for 5 years (Figures 2–9). Pile driving accounted for a substantial proportion of noise pollution and direct environmental degradation in John’s Pass.
Pile driving is a major international concern because coastal cetaceans and many fresh and marine fishes of commercial value reside in shallow waters (Popper and Hastings, 2009; Carroll et al., 2017). Sounds from pile driving emanate from rapid releases of energy in several directions (Popper and Hastings, 2009). Sound from the impact of a hammer on a pile radiates into the air. Transverse stress waves in the pile walls radiate additional sound into the air and water. Transient stress waves propagate down the length of the pile that can transmit sound waves through bottom sentiments outward some distance from the pile, such that received sound levels can be higher further from the pile than closer to it. Environmental assessments for offshore construction projects (e.g., wind farms) generally identify pile driving as the activity with the greatest potential to impact local cetacean populations (Carstensen et al., 2006; Diederichs et al., 2008; Thompson et al., 2010).
Pile driving is the only documented anthropogenic sound source, besides direct explosions, that causes fish kills in the wild (Popper and Hastings, 2009). Like cetaceans, fish obtain information from listening to abiotic and biotic sounds from conspecifics, other fish species, prey, and predators (which include marine mammals). This auditory scene provides crucial information beyond vision and even non-fatal interference with it can impact fish significantly (Wenger et al., 2017). Fish startle in response to noise pollution (Skalski et al., 1992). They are injured by temporary to permanent hearing loss by exposure to pile driving noise (Schaffeld et al., 2020). A limitation in the current study, however, was that noise levels could not be measured in the narrow construction zone so it was not possible to examine the real possibility of hearing loss in local dolphins as well.
Intensive dredging in the construction zone was also documented photographically. Steady numbers of barges were loaded with cement rubble from demolished sections of the old bridge for transport to dump sites (Figures 6, 7). Rubble ranged in size from peas to cars (Figures 4, 5, 7, 8). Dredging is such an essential component of marine infrastructure coastal construction that massive hydraulic dredges are generally required to sustain high production rates (Wenger et al., 2017). Dredging has at least two main sites of impact, the dredge site and the dredged material disposal site.
Fish communities are chiefly vulnerable to dredging (Figures 6–8; Todd et al., 2015; Wenger et al., 2017). Dredging-related loss of prey (McCook et al., 2015), habitat loss, and smothering results from the release of toxic contaminants from disturbed substrate, hydraulic entrainment, sediment settlement, and suspended sentiment plumes exacerbated by the stress from noise and vibration pollution (McCook et al., 2015). Depending on the quantities and grain-size composition of the dredged material and local hydrodynamic conditions, sediment plumes can extend several kilometers from the dredge site (Fisher et al., 2015). The fast currents of the John’s Pass construction zone (Krock, 2005) were capable of dispersing sediment plumes widely. Wenger et al.’s (2017) and Todd et al.’s (2015) substantive reviews show that dredging-related impacts are the most likely to have lethal impacts on early life stages (eggs and larvae) and behavioral impacts on later life stages (juvenile and adult fishes).
The second form of apparent adaptations to cope with construction was that the dolphins altered their temporal rhythms. This was evident in presence-absence patterns. Before construction, there was a higher probability of dolphin presence in winter and spring compared to summer and fall. Like most coastal bottlenose dolphin communities (Mann et al., 2000), John’s Pass dolphins migrate out of the shallow ICW study area to spend the colder months in deeper waters offshore, return to the study area late winter through spring, and by summer have scattered across the ICW where they remain through the fall, followed by resumption of the next offshore migration cycle. During the first construction phase, dolphin presence in the vital passage between the Gulf and ICW dropped significantly and showed no resemblance to before-construction probabilities. However, a shift back to before-construction probability patterns emerged during the second construction phase. This finding suggested that the dolphins had learned to cope with construction during the first phase, which implied a new type of short-term adaptation to anthropogenic disturbance by the second phase.
Further evidence of apparent adaptation emerged from temporal changes in four out the five major behaviors. Adaptation took the form of shifting the timing of dolphin forage-feeding, socializing, meandering travel, and mixed states in the construction zone to later in the day. These shifts tended to coincide with times when construction activities such as vessel traffic, pile driving, and dredging were minimized. Temporal adaptations in behavior states also emerged during the second half of construction. These shifts also suggested that the dolphins had learned to cope with construction during the first phase and implied other new types of short-term adaptations to anthropogenic disturbance by the second phase across major behavior patterns. Adaptation also suggested that familiarity with construction promoted some tolerance, as described by Bejder et al. (2009).
The nature of behavioral adaptations drew attention to the idea that dolphin behavior states differ in their reliance on environment quality. For example, John’s Pass dolphins shifted out of the construction zone to alternative feeding grounds. Foraging and feeding are clearly contingent on specific environmental conditions (i.e., available food). Because fish communities are vulnerable to construction-related habitat degradation, dolphin feeding behavior may be more susceptible to and show more obvious changes in response to anthropogenic disturbances than other dolphin activities. Alternatively, socializing is primarily contingent on the quality of the social environment (i.e., available dolphins willing to socialize). John’s Pass dolphins continued to use the construction zone for socializing and stationing as they had before construction. These findings suggested that social behavior in its many forms may be less dependent on quieter seas than is feeding or in other ways perhaps more resilient to construction-related habitat degradation. If so, the presence of socializing dolphins in construction zones can mislead observers about the true impacts of construction. The implication is that differential behavioral adaptations ought to be taken into account in future studies that attempt to measure the impact of coastal construction projects on coastal cetaceans.
Recommendations for reducing impact are: (1) Monitor-mitigate and, when possible, develop 2-part construction plans to give dolphins a chance to adapt. (2) Include mandatory legislation that requires a compulsory monitor-mitigation protocol to provide adequate funding for study sufficient to understand how the impacted marine mammal and fish communities use the habitat before construction-related habitat degradation occurs and as it occurs. (3) Use studies of before-construction behavior to identify and safeguard suitable alternative habitat in the area and protect it from anthropogenic disturbance to provide the animals with a safe haven from dredging, pile driving, and explosions. (4) Schedule construction activities to give marine mammals a chance to become familiar with activities and time to adapt accordingly.
In conclusion, the loss of some 1–3 dolphins over the study period is concerning and suggests that dolphin communities may be significantly compromised, especially given the scale of ongoing bridge construction projects across Florida (i.e., some 4,000 projects; Weaver, 2015). This matter deserves more attention if we wish to maintain these dolphin populations.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This animal study was reviewed and approved by the National Oceanic and Atmospheric Administration.
AW obtained the federal permits, collected the data, conducted statistical analyses, wrote this manuscript, and agreed to be accountable for the content of the work.
AW was employed by Jaasas Academic Press.
AW gratefully acknowledges Casey Hamel, Marie Dahlberg, Amy Walters, and Kirsten Smail for help with data collection, photography, and image labeling; John Heidemann for boat driving and maintenance; Amy Sophie White for rendering the study area map; and Brianna Seay, Shauna Kebert, and Beth Brady for early reviews of the manuscript.
Agrelo, M., Daura-Jorge, F. G., Bezamat, C., Silveira, T. C. L., de Castilho, P. V., Pires, J. S. S., et al. (2019). Spatial behavioural response of coastal bottlenose dolphins to habitat disturbance in southern Brazil. Aquat. Conserv. Mar. Freshw. Ecosyst. 29, 1949–1958. doi: 10.1002/aqc.3188
Allen, S. J., Tyne, J. A., Kobryn, H. T., Bejder, L., Pollock, K. H., and Loneragan, N. R. (2019). Patterns of dolphin bycatch in a North-Western Australian trawl fishery. PLoS One 9:e93178. doi: 10.1371/journal.pone.0093178
Anderwald, P., Brandecker, A., Coleman, M., Collins, C., Denniston, H., Haberlin, M. D., et al. (2013). Displacement responses of a mysticete, an odontocete, and a phocid seal to construction-related vessel traffic. Endanger. Species Res. 21, 231–240. doi: 10.3354/esr00523
Avila, I. C., Kaschner, K., and Dormann, C. F. (2018). Current global risks to marine mammals: taking stock of the threats. Biol. Conserv. 221, 44–58. doi: 10.1016/j.biocon.2018.02.021
Bejder, L., Samuels, A., Whitehead, H., and Gales, N. (2006a). Interpreting short-term behavioural responses to disturbance within a longitudinal perspective. Anim. Behav. 72, 1149–1158. doi: 10.1016/j.anbehav.2006.04.003
Bejder, L., Samuels, A., Whitehead, H., Finn, H., and Allen, S. (2009). Impact assessment research: use and misuse of habituation, sensitisation and tolerance in describing wildlife responses to anthropogenic stimuli. Mar. Ecol. Prog. Ser. 395, 177–185. doi: 10.3354/meps07979
Bejder, L., Samuels, A., Whitehead, H., Gales, N., Mann, J., Connor, R., et al. (2006b). Decline in relative abundance of bottlenose dolphins exposed to long-term disturbance. Conserv. Biol. 20, 1791–1798. doi: 10.1111/j.1523-1739.2006.00540.x
Bossley, M. I., Steiner, A., Rankin, R. W., and Bejder, L. (2017). A long-term study of bottlenose dolphins (Tursiops aduncus) in an Australian industrial estuary: increased sightings associated with environmental improvements. Mar. Mamm. Sci. 2017, 277–290. doi: 10.1111/mms.12368
Brandt, M. J., Dragon, A. C., Diederichs, A., Bellmann, M. A., Wahl, V., Piper, W., et al. (2018). Disturbance of harbour porpoises during construction of the first seven offshore wind farms in Germany. Mar. Ecol. Prog. Ser. 596, 213–232. doi: 10.3354/meps12560
Brandt, M., Diederichs, A., Betke, K., and Nehls, G. (2011). Responses of harbour porpoises to pile driving at the Horns Rev II offshore wind farm in the Danish North Sea. Mar. Ecol. Prog. Ser. 421, 205–216. doi: 10.3354/meps08888
Buckstaff, K. C. (2004). Effects of watercraft noise on the acoustic behavior of bottlenose dolphins, Tursiops truncatus, in Sarasota Bay, Florida. Mar. Mamm. Sci. 2004, 709–725. doi: 10.1111/j.1748-7692.2004.tb01189.x
Buckstaff, K. C., Wells, R. S., Gannon, J. G., and Nowacek, D. P. (2013). Responses of bottlenose dolphins (Tursiops truncatus) to construction and demolition of coastal marine structures. Aquat. Mamm. 2013, 174–186. doi: 10.1578/AM.39.2.2013.174
Carrera, M. L., Favaro, E. G. P., and Souto, A. (2008). The response of marine tucuxis (Sotalia fluviatilis) towards tourist boats involves avoidance behaviour and a reduction in foraging. Anim. Welf. 17, 117–123.
Carroll, A. G., Przeslawski, R., Duncan, A., Gunning, M., and Bruce, B. (2017). A critical review of the potential impacts of marine seismic surveys on fish & invertebrates. Mar. Pollut. Bull. 114, 9–24. doi: 10.1016/j.marpolbul.2016.11.038
Carstensen, J., Henriksen, O. D., and Teilmann, J. (2006). Impacts of offshore wind farm construction on harbour porpoises: acoustic monitoring of echolocation activity using porpoise detectors (T-PODs). Mar. Ecol. Prog. Ser. 321, 295–308. doi: 10.3354/meps321295
Christiansen, F., Rasmussen, M., and Lusseau, D. (2013). Inferring activity budgets in wild animals to estimate the consequences of disturbances. Behav. Ecol. 24, 1415–1425. doi: 10.1093/beheco/art086
Cribb, N., and Seuront, L. (2016). Changes in the behavioural complexity of bottlenose dolphins along a gradient of anthropogenically-impacted environments in South Australian coastal waters: implications for conservation and management strategies. J. Exp. Mar. Biol. Ecol. 482, 118–127. doi: 10.1016/j.jembe.2016.03.020
Culloch, R. M., Anderwald, P., Brandecker, A., Haberlin, D., McGovern, B., Pinfield, R., et al. (2016). Effect of construction-related activities and vessel traffic on marine mammals. Mar. Ecol. Prog. Ser. 549, 231–242. doi: 10.3354/meps11686
Dahne, M., Gilles, A., Lucke, K., Peschko, V., Adler, S., Krugel, K., et al. (2013). Effects of pile-driving on harbour porpoises (Phocoena phocoena) at the first offshore wind farm in Germany. Environ. Res. Lett. 8:025002. doi: 10.1088/1748-9326/8/2/025002
de Souza Albuquerque, N., and da Silva Souto, A. (2013). Motorboat noise can potentially mask the whistle sound of estuarine dolphins (Sotalia guianensis). Ethnobiol. Conserv. 2, 1–15. doi: 10.15451/ec2013-8-2.5-1-15
DeRuiter, S. L., Southall, B. L., Calambokidis, J., Zimmer, W. M. X., Sadykova, D., Falcone, E. A., et al. (2013). First direct measurements of behavioral responses by Cuvier’s beaked whales to midfrequency active sonar. Biol. Lett. 9, 1–5. doi: 10.1098/rsbl.2013.0223
Diederichs, A., Nehis, G., Dahne, M., Adler, S., Koschinski, S., and Verfuss, U. K. (2008). Methodologies for Measuring and Assessing Potential Changes in Marine Mammal Behaviour, Abundance or Distribution from Construction, Operation and Decommissioning of Offshore Windfarms. BioConsult SH Report 2008, to COWRIE Ltd. London: COWRIE Ltd.
Ellison, W. T., Southall, B. L., Clark, C. W., and Frankel, A. S. (2012). A new context-based approach to assess marine mammal behavioral responses to anthropogenic sounds. Conserv. Biol. 26, 21–28. doi: 10.1111/j.1523-1739.2011.01803.x
Erbe, C., Reichmuth, C., Cunningham, K., Lucke, K., and Dooling, R. (2016). Communication masking in marine mammals: a review and research strategy. Mar. Pollut. Bull. 103, 15–38. doi: 10.1016/j.marpolbul.2015.12.007
Fisher, R., Stark, C., Ridd, P., and Jones, R. (2015). Spatial patterns in water quality changes during dredging in tropical environments. PLoS One 10:e0143309. doi: 10.1371/journal.pone.0143309
Goldbogen, J. A., Southall, B. L., DeRuiter, S. L., Calambokidis, J., Friedlaender, A. S., Hazen, E. L., et al. (2013). Blue whales respond to simulated mid-frequency military sonar. Proc. R. Soc. B Biol. Sci. 280:20130657. doi: 10.1098/rspb.2013.0657
Gomez, C., Lawson, J. W., Wright, A. J., Buren, A. D., Tollit, D., and Lesage, V. (2016). A systematic review on the behavioural responses of wild marine mammals to noise: the disparity between science and policy. Can. J. Zool. 94, 801–819. doi: 10.1139/cjz-2016-0098
Graham, I. M., Merchant, N. D., Farcas, A., Barton, T. R., Cheney, B., Bono, S., et al. (2019). Harbour porpoise responses to pile-driving diminish over time. R. Soc. Open Sci. 6:190335. doi: 10.1098/rsos.190335
Hammond, P. S., Mizroch, S. A., and Donovan, G. P. (1990). Individual Recognition of Cetaceans: Use of Photo-Identification and Other Techniques to Estimate Population Parameters. Reports of the International Whaling Commission 1990, Special Issue 12. Cambridge: International Whaling Commission.
Harcourt, R., Aurioles, D., and Sanchez, J. (1994). Entanglement of California sea lions at Los islotes, Baja California Sur, México. Mar. Mamm. Sci. 10, 122–125. doi: 10.1111/j.1748-7692.1994.tb00399.x
Hawkins, E. R., Harcourt, R., Bejder, L., Brooks, L. O., Grech, A., Christiansen, F., et al. (2017). Best practice framework and principles for monitoring the effect of coastal development on marine mammals. Front. Mar. Sci. 4:59. doi: 10.3389/fmars.2017.00059
Isojunno, S., Curé, C., Kvadsheim, P. K., Lam, F. P. A., Tyack, P. L., Wensveen, P., et al. (2016). Sperm whales reduce foraging effort during exposure to 1-2 kHz sonar and killer whale sounds. Ecol. Appl. 26, 77–93. doi: 10.1890/15-0040
Jensen, F. H., Wahlberg, M., Bejder, L., and Madsen, P. (2008). Noise levels and masking potential of small whale watching and research vessel around two delphinid species. Bioacous 17, 166–168. doi: 10.1080/09524622.2008.9753803
Kreb, D., Lhota, S., Porter, L., Redman, A., Susanti, I., and Lazecky, M. (2020). Long-term population and distribution dynamics of an endangered Irrawaddy dolphin population in Balikpapan Bay, Indonesia in response to coastal development. Front. Mar. Sci. 7:555197. doi: 10.3389/fmars.2020.533197
Krock, J. R. (2005). Historical Morphodynamics of John’s Pass, West Central Florida. Ph.D. thesis, University of South Florida, Scholar Commons. Completion date 6/1/2005.
Laist, D., Knowlton, A. R., Mead, J. C., Collet, A. S., and Podesta, M. (2001). Collisions between ships and whales. Mar. Mamm. Sci. 17, 35–75. doi: 10.1111/j.1748-7692.2001.tb00980.x
Lusseau, D. (2004). The hidden cost of tourism: detecting long-term effects of tourism using behavioral information. Ecol. Soc. 9, 1–15. doi: 10.5751/ES-00662-0902r01
Lusseau, D. (2005). Residency pattern of bottlenose dolphins Tursiops spp. in Milford Sound, New Zealand, is related to boat traffic. Mar. Ecol. Prog. Ser. 295, 265–272. doi: 10.3354/meps295265
Lusseau, D. (2006). The short-term behavioral reactions of bottlenose dolphins to interactions with boats in Doubtful Sound, New Zealand. Mar. Mamm. Sci. 22, 802–818. doi: 10.1111/j.1748-7692.2006.00052.x
Mann, J. (1999). Behavioral sampling methods for cetaceans: a review and critique. Mar. Mamm. Sci. 15, 102–122. doi: 10.1111/j.1748-7692.1999.tb00784.x
Mann, J., Connor, R. C., Tyack, P. L., and Whitehead, H. (eds) (2000). Cetacean Societies. Chicago: University of Chicago Press, 433.
Mannocci, L., Dabin, W., Augeraud-Véron, E., Dupuy, J., Barbraud, C., and Ridoux, V. (2012). Assessing the impact of bycatch on dolphin populations: the case of the common dolphin in the eastern North Atlantic. PLoS One 7:e32615. doi: 10.1371/journal.pone.0032615
Marley, S. A., Salgado Kent, C. P., and Erbe, C. (2017). Occupancy of bottlenose dolphins (Tursiops aduncus) in relation to vessel traffic, dredging, and environmental variables with a highly urbanized estuary. Hydrobiologia 792, 234–263. doi: 10.1007/s10750-016-3061-7
May-Collado, L. J., and Quiñones-Lebrón, S. G. (2014). Dolphin changes in whistle structure with watercraft activity depends on their behavioral state. J. Acoust. Soc. Am. 135, 193–198. doi: 10.1121/1.4869255
McCook, L. J., Schaffelke, B., Apte, S. C., Brinkman, R., Brodie, J., Erftemeijer, P., et al. (2015). Synthesis of Current Knowledge of the Biophysical Impacts of Dredging and Disposal on the Great Barrier Reef: Report of an Independent Panel of Experts. Townsville, QLD: Great Barrier Reef Marine Park Authority 2015.
Miller, P. J. O., Antunes, R. N., Wensveen, P. J., and Samarra, F. I. (2014). Dose-response relationships for the onset of avoidance of sonar by free-ranging killer whales. J. Acoust. Soc. Am. 135, 975–993. doi: 10.1121/1.4861346
Miller, P. J. O., Kvadsheim, P. H., Lam, F. P. A., and Wensveen, P. J. (2012). The severity of behavioral changes observed during experimental exposures of killer (Orcinus orca), long-finned pilot (Globicephala melas), and sperm (Physeter macrocephalus) whales to naval sonar. Aquat. Mamm. 38, 362–401. doi: 10.1578/AM.38.4.2012.362
Miller, P. J. O., Kvadsheim, P. H., Lam, F. P. A., Tyack, P. L., Curé, C., DeRuiter, S. L., et al. (2015). First indications that northern bottlenose whales are sensitive to behavioral disturbance from anthropogenic noise. R. Soc. Open Sci. 2:140484. doi: 10.1098/rsos.140484
Miller, P. J., Johnson, M. P., Madsen, P. T., Biassoni, N., Quero, M., and Tyack, P. L. (2009). Using at-sea experiments to study the effects of airguns on the foraging behavior of sperm whales in the Gulf of Mexico. Deep Sea Res. I 56, 1168–1181. doi: 10.1016/j.dsr.2009.02.008
Müeller, M., Boutiere, H., Weaver, A., and Candelon, N. (1998). Ethogram of the bottlenose dolphin, with special reference to solitary and sociable dolphins. Vie Milieu 48, 89–104.
Nabe-Nielsen, J., Sibly, R. M., Tougaard, J., Teilmann, J., and Sveegaard, S. (2014). Effects of noise and by-catch on a Danish harbour porpoise population. Ecol. Model. 272, 242–251. doi: 10.1016/j.ecolmodel.2013.09.025
Nowacek, D. P., Christiansen, F., Bejder, L., Goldbogen, J. A., and Friedlaender, A. S. (2016). Studying cetacean behaviour: new technological approaches and conservation applications. Special issue: conservation behaviour. Anim. Behav. 120, 235–244. doi: 10.1016/j.anbehav.2016.07.019
Nowacek, D. P., Thorne, L. H., Johnston, D. W., and Tyack, P. L. (2007). Responses of cetaceans to anthropogenic noise. Mamm. Rev. 37, 81–115. doi: 10.1111/j.1365-2907.2007.00104.x
Nowacek, S. M., Wells, R. S., and Solow, A. R. (2001). Short-term effects of boat traffic on bottlenose dolphins, Tursiops truncatus, in Sarasota Bay, Florida. Mar. Mamm. Sci. 17, 673–688. doi: 10.1111/j.1748-7692.2001.tb01292.x
Page, B., McKenzie, J., McIntosh, R., Baylis, A., Morrissey, A., Calvert, N., et al. (2004). Entanglement of Australian sea lions and New Zealand fur seals in lost fishing gear and other marine debris before and after government and industry attempts to reduce the problem. Mar. Pollut. Bull. 49, 33–42. doi: 10.1016/j.marpolbul.2004.01.006
Paiva, E. G., Salgado Kent, C. P., Gagnon, M. M., McCauley, R., and Finn, H. (2015). Reduced detection of Indo-Pacific bottlenose dolphins (Tursiops aduncus) in an inner harbor channel during pile driving activities. Aquat. Mamm. 41, 455–468. doi: 10.1578/AM.41.4.2015.455
Panigada, S., Pesante, G., Zanardelli, M., Capoulade, F., Gannier, A., and Weinrich, M. (2006). Mediterranean fin whales at risk from fatal ship strikes. Mar. Pollut. Bull. 52, 1287–1298. doi: 10.1016/j.marpolbul.2006.03.014
Parsons, E. C. M. (2012). The negative impacts of whale-watching. J. Mar. Biol. 12, 1–9. doi: 10.1155/2012/807294
Pirotta, E., Laesser, B. E., Hardaker, A., Riddoch, N., Marcoux, M., and Lusseau, D. (2013). Dredging displaces bottlenose dolphins from an urbanised foraging patch. Mar. Pollut. Bull. 74, 396–402. doi: 10.1016/j.marpolbul.2013.06.020
Pirotta, E., Merchant, N., Thompson, P., Barton, T., and Lusseau, D. (2015). Quantifying the effect of boat disturbance on bottlenose dolphin foraging activity. Biol. Conserv. 181, 82–89. doi: 10.1016/j.biocon.2014.11.003
Piwetz, S. (2019). Common bottlenose dolphin (Tursiops truncatus) behavior in an active narrow seaport. PLoS One 14:e0211971. doi: 10.1371/journal.pone.0211971
Popper, A. N., and Hastings, M. C. (2009). The effects of anthropogenic sources of sound on fishes. J. Fish. Biol. 75, 455–489. doi: 10.1111/j.1095-8649.2009.02319.x
Ramesh, R., Chen, Z., Cummins, V., Day, J., D’Elia, C., Dennison, B., et al. (2015). Land–ocean interactions in the coastal zone: past, present & future. Anthropocene 2015, 85–98. doi: 10.1016/j.ancene.2016.01.005
Read, A. J. (2008). The looming crisis: interactions between marine mammals and fisheries. J. Mamm. 89, 541–548. doi: 10.1644/07-MAMM-S-315R1.1
Schaffeld, T., Schnitzler, J. G., Ruser, A., Woelfing, B., Baltzer, J., and Siebert, U. (2020). Effects of multiple exposures to pile driving noise on harbor porpoise hearing during simulated flights—an evaluation tool. J. Acoust. Soc. Am. 147, 685–697. doi: 10.1121/10.0000595
Sekovski, I., Newton, A., and Dennison, W. C. (2012). Megacities in the coastal zone: using a driver-pressure-state-impact-response framework to address complex environmental problems. Estuar. Coast. Shelf Sci. 96, 48–59. doi: 10.1016/j.ecss.2011.07.011
Shannon, G., McKenna, M. F., Angeloni, L. M., Crooks, K. R., Fristrup, K. M., Brown, E., et al. (2016). A synthesis of two decades of research documenting the effects of noise on wildlife. Biol. Rev. 91, 982–1005. doi: 10.1111/brv.12207
Sivle, L. D., Kvadsheim, P. H., Curé, C., Isojunno, S., Wensveen, P. J., Lam, F. P. A., et al. (2015). Severity of expert-identified behavioural responses of humpback whale, minke whale and northern bottlenose whale to naval sonar. Aquat. Mamm. 41, 469–502. doi: 10.1578/AM.41.4.2015.469
Skalski, J. R., Pearson, W. H., and Malme, C. I. (1992). Effects of sounds from a geophysical survey device on catch-per-unit-effort in a hook-and-line fishery for rockfish (Sebastes spp.). Can. J. Fish. Aquat. Sci. 1992, 1357–1365. doi: 10.1139/f92-151
Southall, B. L., Nowacek, D. P., Miller, P. J. O., and Tyack, P. L. (2016). Experimental field studies to measure behavioral responses of cetaceans to sonar. Endanger. Species Res. 31, 293–315. doi: 10.3354/esr00764
Steckenreuter, A., Harcourt, R., and Möller, L. (2011). Distance does matter: close approaches by boats impede feeding and resting behaviour of Indo-Pacific bottlenose dolphins. Wildl. Res. 38, 455–463. doi: 10.1071/WR11048
Teilmann, J., and Carstensen, J. (2012). Negative long term effects on harbour porpoises from a large scale offshore wind farm in the Baltic—evidence of slow recovery. Environ. Res. Lett. 7:045101. doi: 10.1088/1748-9326/7/4/045101
Thompson, P. M., Brookes, K. L., Graham, I. M., Barton, T. R., Needham, K., Bradbury, G., et al. (2013a). Short-term disturbance by a commercial two-dimensional seismic survey does not lead to long-term displacement of harbour porpoises. Proc. R. Soc. B 280:20132001. doi: 10.1098/rspb.2013.2001
Thompson, P. M., Hastie, G. D., Nedwell, J., Barham, R., Brookes, K. L., Cordes, L. S., et al. (2013b). Framework for assessing impacts of pile-driving noise from offshore wind farm construction on a harbor seal population. Environ. Impact Assess. Rev. 43, 73–85. doi: 10.1016/j.eiar.2013.06.005
Thompson, P. M., Lusseau, D., Barton, T., Simmons, D., Rusin, J., and Bailey, H. (2010). Assessing the responses of coastal cetaceans to the construction of offshore wind turbines. Mar. Pollut. Bull. 60, 1200–1208. doi: 10.1016/j.marpolbul.2010.03.030
Todd, V. L. G., Todd, I. B., Gardiner, J. C., Morrin, E. C. N., MacPherson, N. A., DiMarzio, N. A., et al. (2015). A review of impacts of marine dredging activities on marine mammals. ICES J. Mar. Sci. 72, 328–340. doi: 10.1093/icesjms/fsu187
Tougaard, J., Wright, A. J., and Madsen, P. T. (2015). Cetacean noise criteria revisited in the light of proposed exposure limits for harbour porpoises. Mar. Pollut. Bull. 90, 196–208. doi: 10.1016/j.marpolbul.2014.10.051
Tyack, P. L. (2008). Implications for marine mammals of large-scale changes in the marine acoustic environment. J. Mamm. 89, 549–558. doi: 10.1644/07-MAMM-S-307R.1
Tyack, P. L., Zimmer, W. M. X., Moretti, D., Southall, B. L., and Claridge, D. E. (2011). Beaked whales respond to simulated and actual navy sonar. PLoS One 6:e17009. doi: 10.1371/journal.pone.0017009
Urian, K. W., Hohn, A. A., and Hansen, L. J. (1999). Status of the Photo-Identification Catalog of Coastal Bottlenose Dolphins of the Western North Atlantic: Report of a Workshop of Catalog Contributors. NOAA Technical Memorandum 1999, NMFS-SEFSC-425. Beaufort, NC: U.S. Department of Commerce, 24.
van Ginkel, C., Becker, D. M., Gowans, S., and Simard, P. (2017). Whistling in a noisy ocean: Bottlenose dolphins adjust whistle frequencies in response to real-time ambient noise levels. Bioacous 27, 1–15. doi: 10.1080/09524622.2017.1359670
Weaver, A. (2003). Conflict and reconciliation in captive bottlenose dolphins, Tursiops truncatus. Mar. Mamm. Sci. 19, 836–846. doi: 10.1111/j.1748-7692.2003.tb01134.x
Weaver, A. (2015). Sex differences in bottlenose dolphin sightings during a long-term bridge construction project. Anim. Behav. Cogn. 2, 1–13. doi: 10.12966/abc.02.01.2015
Weaver, A., and Kuczaj, S. (2016). Neither toy nor tool: grass-wearing behavior among free-ranging bottlenose dolphins in western Florida. Int. J. Comp. Psychol. 29:31885.
Wenger, A. S., Harvey, E., Wilson, S., Rawson, C., Newman, S. J., Clarke, D., et al. (2017). A critical analysis of the direct effects of dredging on fish. Fish Fish. 18, 967–985. doi: 10.1111/faf.12218
Williams, R., Wright, A. J., Ashe, E., Blight, L. K., Bruintjes, R., Canessa, R., et al. (2015). Impacts of anthropogenic noise on marine life: publication patterns, new discoveries, and future directions in research and management. Ocean Coast. Manag. 115, 17–24. doi: 10.1016/j.ocecoaman.2015.05.021
Wisniewska, D. M., Johnson, M., Teilmann, J., Siebert, U., Galatius, A., Dietz, R., et al. (2018). High rates of vessel noise disrupt foraging in wild harbour porpoises (Phocoena phocoena). Aquat. Conserv. Mar. Freshw. Ecosyst. 23, 222–232. doi: 10.1098/rspb.2017.2314
Keywords: coastal construction, anthropogenic disturbance, conservation management, free-ranging bottlenose dolphins, Tursiops sp., behavior states
Citation: Weaver A (2021) An Ethology of Adaptation: Dolphins Stop Feeding but Continue Socializing in Construction-Degraded Habitat. Front. Mar. Sci. 8:603229. doi: 10.3389/fmars.2021.603229
Received: 05 September 2020; Accepted: 22 February 2021;
Published: 18 March 2021.
Edited by:
John A. Cigliano, Cedar Crest College, United StatesReviewed by:
Mark Peter Simmonds, University of Bristol, United KingdomCopyright © 2021 Weaver. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ann Weaver, YW5uc3RhdHM1NEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.