
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 20 April 2021
Sec. Deep-Sea Environments and Ecology
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.567438
This article is part of the Research Topic 7th International Symposium on Deep-Sea Corals View all 22 articles
 David Alonso1*
David Alonso1* Martha Vides-Casado1
Martha Vides-Casado1 Francisco Arias-Isaza1
Francisco Arias-Isaza1 Hernando Zambrano2,3
Hernando Zambrano2,3 Emilio Rodriguez3
Emilio Rodriguez3 Venus Rocha-Gutierrez1
Venus Rocha-Gutierrez1 Pilar Herron1
Pilar Herron1 Anny Castillo4
Anny Castillo4Deep-water environments make up 64% of the world’s oceans (nearly 202 million km2). In the past, the belief that this environment represented one of the most stable and unproductive ecosystems on the planet has been refuted by scientific research and the interest of potential productive sectors evaluating seabed resources. Human activities that threaten the health of deep-sea threats are uncontrolled and unregulated fishing, deep-sea mining, oil spills, marine litter, and climate change. With recent advances in technology, the study of deep-sea coral communities is a growing subject. The deep-sea corals are long-lived, slow-growing, and fragile systems, making them especially vulnerable to physical damage. In the last 40 years, Colombia has discovered these communities’ existence scarcely distributed in its territorial waters. A representative and irreplaceable sample of deep-sea coral formations triggered in 2013 the establishment of the Corales de Profundidad National Natural Park, a Marine Protected Area (MPA), which holds 40% of the marine biodiversity known in the Colombian Caribbean continental shelf-slope break. The MPA’s essential ecological value is the Madracis myriaster species’ presence as a primary habitat-forming organism, a unique habitat for the Caribbean and the world. Here we describe the MPA creation process in three phases. Firstly, in the provisioning phase, three main threats from human activities are identified. Secondly, in the preparation phase, the area’s conservation objectives and management category are defined, and the negotiation process with the fishing, communications, and oil and gas economic sectors is described. Lastly, in the designation phase, three MPA scenario proposals were evaluated, assessing the minimum distance, the possible effects of activities in the area as the main criteria for the buffer zone and the management of possible future impacts. As a result, the most extended boundary was adopted, guaranteeing these communities’ conservation despite the limited information to carry out a complete planning process. The MPA designation is considered the first experience of deep communities in the Southern Caribbean and an example that it is possible to have effective conservation agreements with economic sectors.
Deep-sea corals have been known and commercially exploited (e.g., jewellery making) since the 18th century. For decades, technological advances in the exploration of deep-sea environments have made it possible to locate them and understand their distribution on a global scale (Freiwald et al., 2004). Today, we know that deep-sea coral communities distribute throughout the ocean, forming genuine biodiversity hot-spots (Roberts and Hirshfield, 2004; Roberts et al., 2006).
There is a hypothesis establishing greater species diversity in deep-sea coral communities than in tropical shallow reef communities (Roberts et al., 2009). Despite this, most of these communities have not yet adequately been mapped or studied. Deep-sea benthic communities are underrepresented in most countries marine protected systems (Lourie and Vincent, 2004), becoming one of the most significant challenges worldwide, as deep-sea ecosystems have large gaps in conservation (Fischer et al., 2019; Gownaris et al., 2019; Stratoudakis et al., 2019).
Deep habitat exploration began in the 1970s along the Colombian Caribbean, with two expeditions carried out by the Rosenstiel School of Marine and Atmospheric Science aboard R.V. Oregon and RV Pillsbury. They continued in 1995 with a joint expedition between the Marine and Coastal Research Institute (INVEMAR), National Navy Oceanographic and Hydrographic Research Center and the Smithsonian Institution aboard the R.V. Ancon (Figure 1). INVEMARs research reached continental biodiversity margin was up to 900 m in 1998. Among the most remarkable results of the last expedition (2000) was the discovery of three deep-sea azooxanthellate coral formations (DSCF) (Figure 2). The first one, dominated by Cladocora debilis species, is located at La Guajira’s peninsula at 70 m deep. The second is positioned north off-coast the city of Santa Marta at 200 m depth, accounting for 12 species of scleractinian corals, associated mainly with Madracis myriaster. In front of the San Bernardo Archipelago at 150 m depth, the third area is M. myriaster dominant, accompanied by 19 scleractinian coral species (Reyes et al., 2005).
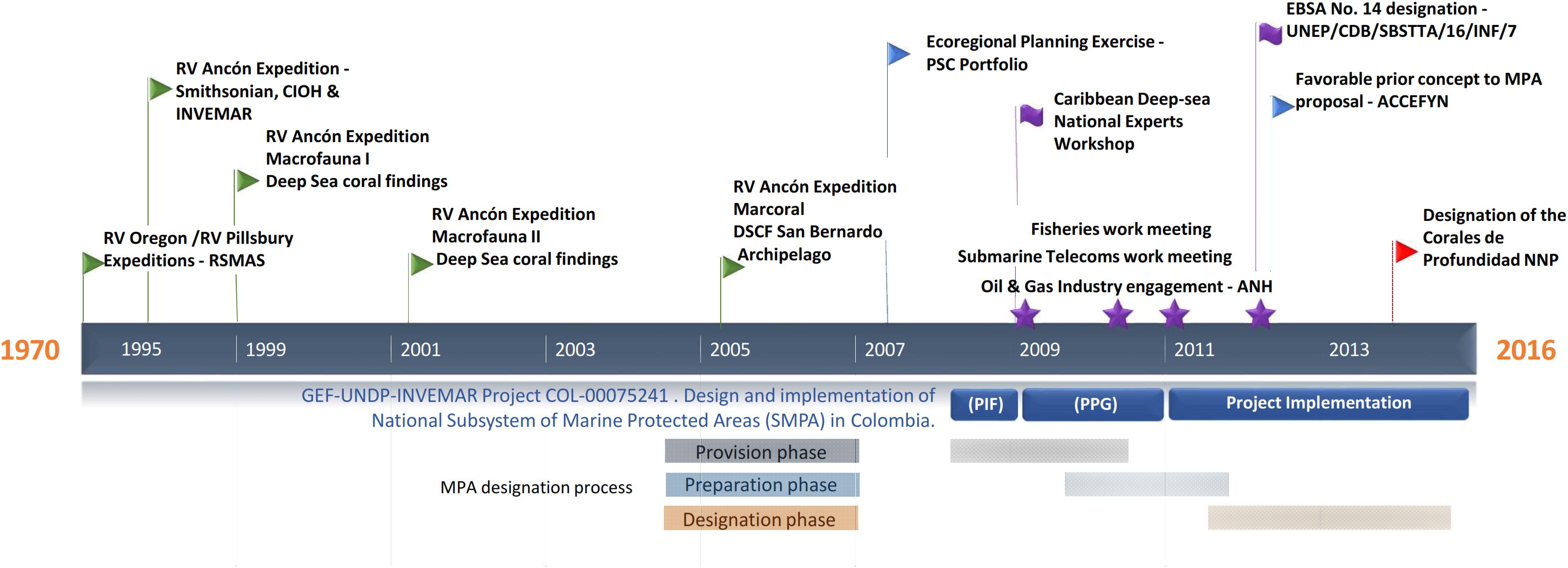
Figure 1. Timeline for the designation process of the Corales de profundidad National Natural Park in Colombia.
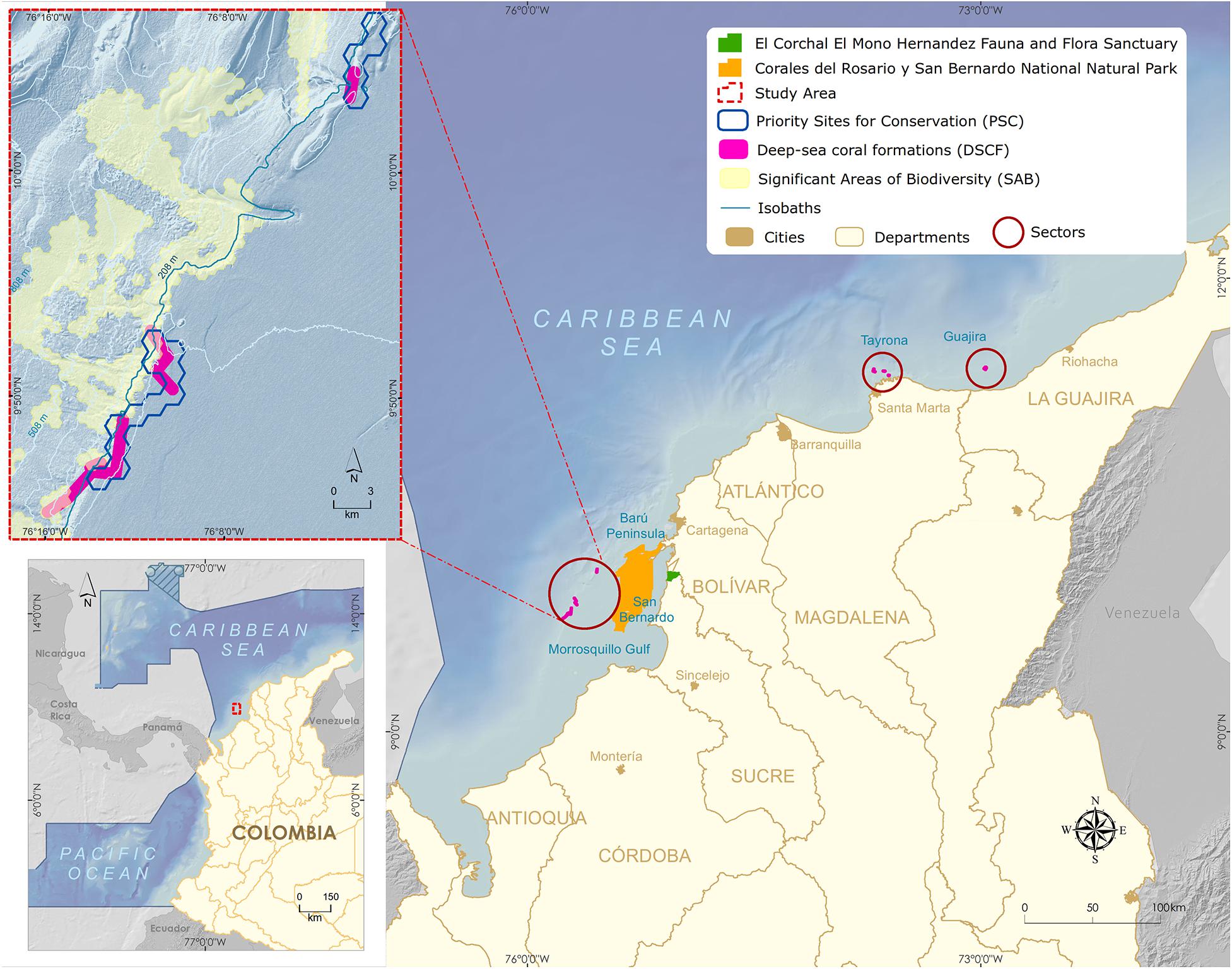
Figure 2. Map of the Colombian Caribbean with the three main sectors of Deep-sea coral formations (Guajira, Tayrona, and San Bernardo).
Deep-sea azooxanthellate coral formations (DSCF) have long life cycles and are adapted to more stable conditions than shallow environments, making them very vulnerable to physical damage and environmental disturbance considered fragile environments (Roberts and Hirshfield, 2004; Ramirez-Llodra et al., 2011). Among the anthropogenic factors that threaten these formations are demersal fisheries’ bottom trawling (Pusceddu et al., 2014; Huvenne et al., 2016), submarine cables dredging installation, deep-sea mining, and offshore oil and gas exploration (Freiwald et al., 2004). After uncontrolled and unregulated fishing, offshore hydrocarbon exploration is considered the second most crucial threat worldwide to the conservation of deep-sea coral communities (Roberts et al., 2006; Davies et al., 2007). Offshore oil and gas activities can have detrimental direct and indirect environmental effects during the main phases of exploration, production, and decommissioning phases (Cordes et al., 2016).
Colombia’s commitments to the United Nations Convention on Biological Diversity (United Nations CBD) and contributing to the Aichi Target 11 comply with conserving at least 10% of marine environments worldwide by 2020 (CBD, 2010). Between 2010 and 2015, accessed financial resources from the Global Environmental Facility-GEF through the United Nations Development Program for the identification, preparation, and implementation of the project “Design and implementation of the Subsystem of Marine Protected Areas (SMPA) as part of the National System of Protected Areas in Colombia (SINAP, from its Spanish acronym) (Figure 1). Within the framework of the project, the designation of new MPA’s was pursued. This paper describes the new MPA’s provisioning, preparation, and designations phases for the inclusion of DSCFs as a new conservation target, improving SMPA representativeness, and describing the negotiation process with the main economic sectors involved in the conservation strategy.
One of the discovered deep-sea azooxanthellate coral formations sits in southeastern Colombian Caribbean’s, between the continental platform’s shelf edge and slope, facing the Gulf of Morrosquillo and the San Bernardo Archipelago (Figure 2). The DSCF is outlined at 32 km from the nearest continental point and 12 km from the Corales del Rosario y San Bernardo National Natural Park. The area is made up of the shallow coral formations of the greatest geomorphological and structural development in the Colombian continental Caribbean (Díaz et al., 2000). It is a dynamic interaction area between three tectonic plates, Nazca, South American, and the Caribbean, whose displacements generate compression and shearing phenomena rising the San Jacinto and Sinú belts. Mud diapirism is a predominant geomorphology factor, allowing the establishment of shallow coral communities (Vernette, 1985; Vernette et al., 1992) and deep-sea coral formations (Santodomingo et al., 2007).
The DSCF shows a clear association with some geo forms. On soft sandy-muddy bottoms, branched corals like Madracis spp. communities are dominant. On hard bottoms, communities contain octocorals (soft corals), black corals (antipathia), anthozoans, and corals of the genus Madrepora. Sponge communities grow on dead fragments of the Halimeda limestone algae (Santodomingo et al., 2007). Madracis myriaster is the dominant structuring species, found at depths of between 150 and 160 m (Reyes et al., 2005), although recent studies have extended its distribution to 202 m depth (Cedeño-Posso et al., 2017).
The MPA design followed three phases under the technical guide for the declaration of new areas and extensions in the National Protected Areas System in Colombia (SINAP) (Pérez and Zambrano, 2009): provision, preparation, and declaration (Figure 1). Two governmental authorities led the entire process, the National Natural Parks System of Colombia (PNNC, from its Spanish acronym) and the Ministry of Environment and Sustainable Development (MADS). INVEMAR provided technical and scientific support to the entire process.
In the beginning, a broad review of various information sources and fieldwork data was intended to support the MPA’s designation and to justify its contribution to the SINAP’s conservation objectives.
Priority conservation site portfolios were the basis for conducting a representativeness gap analysis within the SINAP. The verification of DSCF communities as part of ecoregional planning exercises within the country and abroad was established. Also, identifying potential threats to the main economic activities in the area could be detrimental to the future viability of the MPA. For this site, industrial and artisanal fishing, telecommunications for installing submarine cables, and the exploration and exploitation of oil and gas in the area were previously identified. Possible threats from ocean acidification due to climate change were also considered.
We used cartographic information provided by the Marine Environmental Information System of Colombia1, the National Land Map (exploration blocks) of the National Hydrocarbons Agency (ANH)2, and the HMN Technologies map for submarine cables3. Furthermore, information was supplied by the General Maritime Directorate and the National Environmental Licensing Authority. All the spatial information was analyzed with ArcGIS 9.3.1 software.
In this phase, the biophysical and socio-economic information was consolidated. Information was requested from sectoral Government entities on oil and gas exploration and exploitation projects, development of communications infrastructure (submarine cables), and the identification of industrial and artisanal fishing zones. We identify and describe the main ecological criteria supporting the importance of the area. The ecological criteria were (1) richness and uniqueness, (2) representativeness, (3) ecosystem services, (4) ecological connectivity, (5) research and, (6) vulnerable habitats and human threats. Identifying conservation objectives and goals (natural values of the area) determined the site’s delimitation factors. Goals, criteria, guidelines, and procedures regulate the System’s different categories according to the conservation objectives of the protected areas of SINAP (Decree 2372,2010). One of the main results of this phase is the design limits of the MPA. Three scenario proposals for negotiation within buffer zones reduced telecommunications’ economic activities, the fishing, and oil and gas industry effects.
As a resulting product phase, a summary document containing all the information collected and analyzed during the previous stages set out priority strategic actions that must be taken in the MPA’s future. The Colombian Academy of Exact Physical and Natural Sciences required a favorable prior concept for designing a protected area within the national management scope of the PNNC. Once this was done, MADS published the country’s official administrative act through a designation Resolution.
The inclusion of DSCF in SINAP was supported by the results of a representativeness gap analysis in the National Natural Parks System of Colombia (Segura-Quintero et al., 2012), wherein no MPA included these communities. Despite three distinct zones (Guajira, Tayrona, and San Bernardo) had already been described (Reyes et al., 2005; Figure 2) and selected as a priority site for the conservation of coastal and marine biodiversity (Alonso et al., 2007) and significant biodiversity area of the continental margin (Alonso et al., 2010), the San Bernardo Archipelago DSCF was selected to advance the MPA designation process, given the rare presence of the species Madracis myriaster as the primary habitat-forming organism (Santodomingo et al., 2007), becoming a global research reference (CBD, 2012; Secretariat of the Convention on Biological Diversity, 2014).
Threats from major economic activities and ocean acidification due to the climate change process were identified in the area. Origins, causes, and possible effects documented in scientific literature within these communities in other regions of the world (Table 1) before the designation of MPAs describe the context of threats results according to the area’s review of information.
Within the Colombian Caribbean, industrial trawling is prohibited, and artisanal exclusive areas for fishing are regulated. The areas currently used as the leading industrial shrimp trawl fishing grounds (∼70 m) do not overlap with the DSCF (Figure 3A). However, due to the overexploitation and depletion of the shallow-water shrimp stock, the National Fisheries Authority of Colombia has carried out in alliance with the national research group’s bioprospection to determine the potential of the fishery resources of deeper-water. The results show that between 100–600 m, there is a high abundance of three shrimp species (giant red, pink and royal red) and a deep-sea lobster, especially in the northern Caribbean, where a seasonal up-welling occurs (Paramo and Saint-Paul, 2012b; Paramo et al., 2012). Also, the presence of artisanal and industrial deep-sea fishing longlines or hooks located near the DSCF can cause mechanical damage to coral colonies and affect fish populations associated with these formations (Figure 3B; Roberts and Hirshfield, 2004).
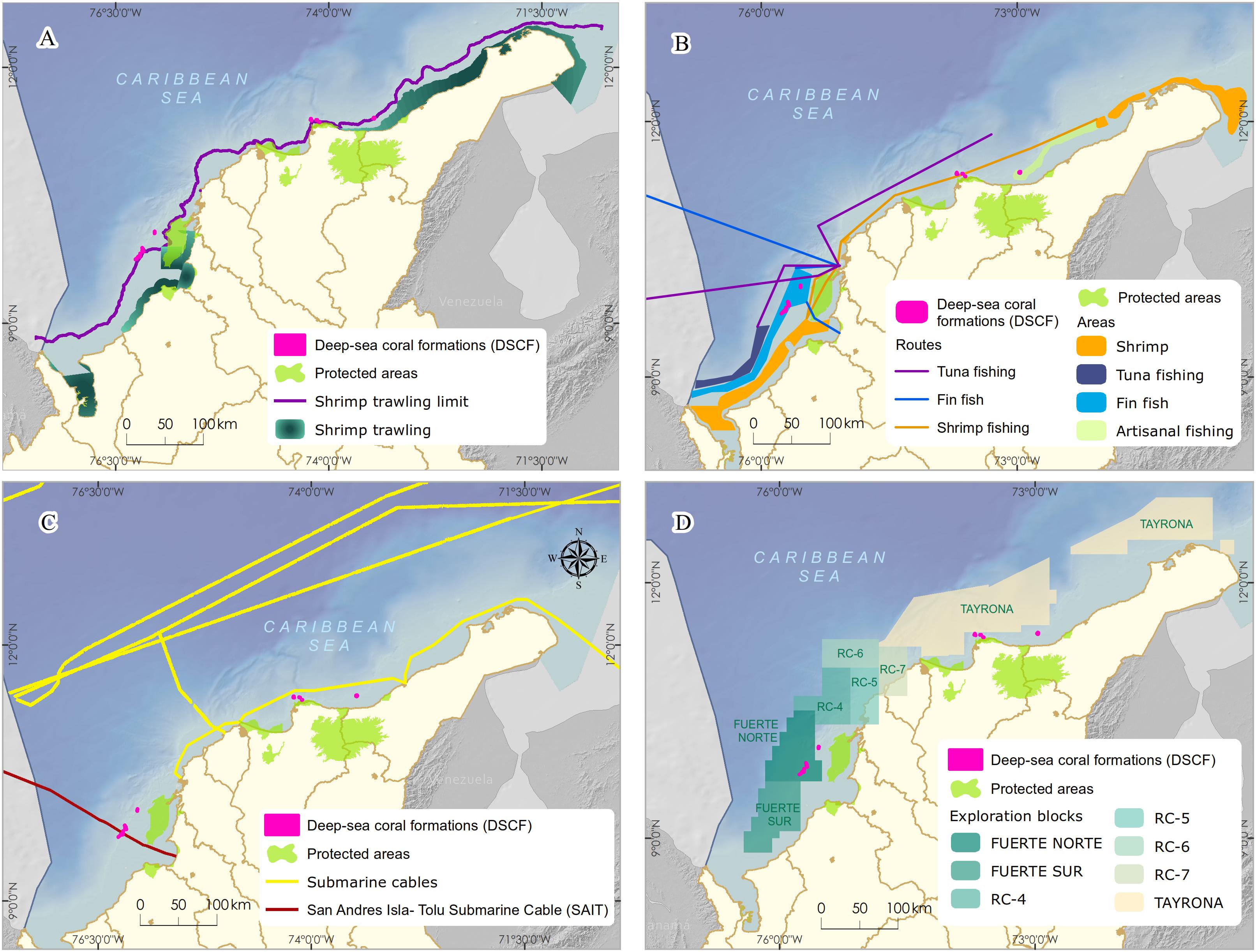
Figure 3. Main economic activities developed in the sectors with the presence of Deep-sea corals formations in the Colombian Caribbean. (A,B) commercial bottom trawling and other bottom fishing, (C) presence of submarine cables for telecommunications, (D) oil and gas exploration blocks.
According to the geospatial information analysis, the DSCF overlaps the southwest end with the underwater fiber optic cable4 (Figure 3C), which runs 826 km from the municipality of Tolú to the island of San Andrés. This cable was installed between 2009 and 2010 and is now managed by the Ministry of Information Technology and Telecommunications. In the process of placing a submarine cable, it buries in shallow areas. Maintenance or repair activities can generate mechanical damage to benthic marine organisms, resulting in the loss of habitat and mortality of specific populations (Austin et al., 2004). However, this impact is localized in time and space because the cable’s diameter varies between 2 and 5 cm; the affected area is a strip of a maximum of 8 m wide. Once the line lais on the bottom, maintenance approved every 10 or 15 years, and repairs become less common thanks to advances in fiber optic technology (Carter, 2009).
In the last decade, Colombia has been undertaking one of the most ambitious bets exploring offshore oil and gas, mainly focusing on the Caribbean basin. The Government’s decision and companies in the sector bet on offshore projects opened a new demand for marine environmental studies (DNP, 2011). By crossing cartographic information, it was possible to observe that the location of the DSCF overlapped with exploration areas called Fuerte Norte and Fuerte Sur. These areas are reserved and assigned since 2006 for hydrocarbon exploration by the company ECOPETROL S.A. through the National Hydrocarbon Agency (Figure 3D). Therefore, it was necessary to carry out a negotiation process and agreement under MADS coordination, which will be discussed later.
Among the ocean environment’s alterations associated with climate change, deep-sea coral reefs could be affected primarily by ocean acidification (Table 1). Rising atmospheric carbon dioxide levels may alter the global ocean’s chemical balance by reducing pH (Davies et al., 2007). The distribution of deep-sea scleractinian corals could be limited, partially by the depth of the aragonite saturation horizon (ASH) in the world’s oceans (Guinotte et al., 2006). Reduced growth rates have been observed in deep-sea coral Lophelia pertusa (Maier et al., 2009), supporting the findings that aragonite regulates their distribution. For calcification rates, the synergistic effect of acidification, heating of water, and dissolved oxygen (hypoxia) produce systemic stress in organisms. Therefore, metabolism (acid-base balance, respiration, and photosynthesis) and growth and reproductive processes are affected (Hofmann et al., 2010; Barry et al., 2011). Since the stress caused by climate change and acidification has global sources and causes, the MPA’s designation in itself will not solve the issue. Still, it can help provide adaptation and resilience for the habitats involved by controlling other local stress sources.
Today the most remarkable diversity of species is recognized in the deep-sea coral communities compared to tropical shallow scleractinian coral communities (Roberts et al., 2006). Nineteen species of scleractinian corals have been reported in the DSCF across San Bernardo Archipelago. It is estimated that they include approximately 40% of the Colombian Caribbean biodiversity of the continental shelf (Reyes et al., 2005), with nearly 142 invertebrate and fish species with high levels of diversity, richness, and uniformity compared to other neighboring areas (Urriago et al., 2011). The species Madracis myriaster is the primary azooxanthellate coral in the Colombian Caribbean deep-sea communities. This species has not been reported previously in the Caribbean region nor other places in the world as the primary-habitat forming organism of deep-sea corals waters (Lutz and Ginsburg, 2007). This characteristic makes it a unique habitat not only in the Caribbean region but also in the world.
Protected areas’ ecological representativeness is an adequate sample of biodiversity at different biological organization levels (genes, species, communities, and ecosystems), guaranteeing ecological processes and their long-term viability (Stevens, 2002; Dudley and Parish, 2006; Barr et al., 2011). If a particular kind of habitat has not yet been protected, it should have a high rating (Salm et al., 2000; Stevens, 2002). San Bernardo Archipelago holds 64% of the total DSCF coverage known in the Colombian Caribbean. The inclusion of these communities in an MPA would make it possible to fill the identified conservation gap (Segura-Quintero et al., 2012) and advance in fulfilling commitments to the United Nations Convention on Biological Diversity (CBD).
Deep-sea coral communities have been identified as the habitat for certain commercially important fish and crustacean species (Freiwald et al., 2004). In Florida, a close relationship has been found between the abundance of species of fishery importance (grouper, snapper, and mackerel) and the conservation status of deep coral colonies of the genus Oculina (Koenig et al., 2005; Reed et al., 2007). In the northern Colombian Caribbean, fishing exploitation statistics suggest a correlation between sites identified as fishing grounds with high volumes of commercially important fish catch (snapper and horse mackerel) and areas with deep-sea corals (Majarres, 2004). Additionally, the high biodiversity associated with DSCF in the Colombian Caribbean offers a refuge for many deep-sea species (Reyes et al., 2005). This diversity has an intrinsic value, with a significant potential for bioprospecting in the production of secondary metabolites. It has been studied in scleractinian coral species as Lophelia pertusa and Madrepora oculata (Mancini et al., 1999; Skropeta, 2008).
Connectivity is an essential criterion for MPA and network MPA design (Roberts et al.,2003a,b). It is described as the linkage of spatially distinct populations, communities, ecosystems, or habitats to exchanging genes, organisms, nutrients, and energy (Almany et al., 2009; Botsford et al., 2009). In this way, the community’s composition regulates, and the ecosystem’s capacity to recover from a disturbance increases (Gaines et al., 2007). Although connectivity processes in this DSCF have not been evaluated, five common fish species associated with shallow coral reefs from the Corales del Rosario and San Bernardo National Natural Park (Reyes et al., 2005) suggest a possible ecological relationship with these reefs. Thus, it is possible to hypothesize about the possible use of DSCF as stepping-stones sites in the population dispersing processes considered exclusive to shallow environments. Understanding the links between shallow and deep coral fish communities will depend on future studies that obtain spatial and temporal coverage of these habitats’ use and the specific mechanisms that mediate their distribution and abundance (Auster, 2007).
There are considerable knowledge gaps about DSCF to be addressed through multidisciplinary research. These include distribution models, geology, biology, ecology, and human impact assessment (Freiwald et al., 2004). The main characteristic of this area is the presence of Madracis myriaster as a structuring coral, where it has been evidenced that they could be relatively new communities (on a wide time scale) given the absence of lithification processes (Lutz and Ginsburg, 2007) conditioned to diapirism and gas emission events, and subduction from tectonic activity (Duque-Caro, 1984; Vernette, 1985). The main research fields arising from this DSCF would be studying reproductive biology and larval dispersal considered molecular genetics. These communities’ longevity and slow growth allow for a very high-resolution record to generate historical reconstructions of climatic and oceanographic conditions. Their global distribution also allows comparisons on long time and space scales (Roberts et al., 2006). These qualities make them highly valuable for climate research and modeling.
Intact habitats that can easily be damaged or changed by human activities increase MPA’s priority (Roberts et al., 2003b). Due to their biological characteristics and oceanographic environment, DSCF are particularly vulnerable to physical damage. Vast areas of these communities have disappeared in a short time due to fishing activities in different parts of the world (Davies et al., 2007; Ramirez-Llodra et al., 2011). The information collected on current and potential uses show a high risk of threat to the conservation of the San Bernardo DSCF due to the growth of the oil and gas industry (DNP, 2011), the increasing technology of fishing gear to access ever deeper fishing grounds (Paramo and Saint-Paul, 2012a) and the increase in the installation of submarine cables. Additionally, of the nine species included in the “Identification Manual of marine invertebrates of Colombia” for the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) (Reyes and Santodomingo, 2002), seven are present in the Colombian Caribbean DSCF. There are reports from Colombia as an exporting country before CITES for their value in elaborating jewels (Lutz and Ginsburg, 2007), although the sites and depths of exploitation of this resource are unknown.
The conservation objectives of a protected area are the starting and ending point toward planning actions guiding the definition of management priorities (Salm et al., 2000). Based on the ecological criteria described above and the selection of the DSCF as the principal conservation target of the site, two conservation objectives were defined for the MPA: (1) to preserve the DSCF located at the shelf-edge and the upper slope, as an expression of ecosystem representativeness and uniqueness and essential habitat for marine diversity, and (2) to contribute to the supply of ecosystemic goods and services provided by the DSCF, especially considering their potential connectivity with other marine ecosystems and their role in the dispersion of diverse species of benthic habitats.
Considering the current management categories of SINAP (Decree 622,1977; Decree 2372,2010), the category that best fits the conservation objectives proposed for the DSCF area is the Natural National Park (NNP). The NNP is equivalent to Category II- IUCN National Park (Dudley, 2008). For a definition of NNP, see Decree 622 (1977).
The activities allowed in NNP are preservation, restoration, recreation, research, education, and culture. In the case of the San Bernardo Archipelago, all these activities are viable. However, recreational and cultural activities are limited because it is a remote deep water area. Still, the option of underwater tourism could be explored in the future.
The NNP design began with the integration of technical information and national consensus. It was oriented to incorporate the conservation targets (Deep-sea coral formations) with local planning perspectives by the different institutional actors and productive sectors. Three scenarios with varying boundaries of NNP were proposed and discussed with stakeholders (Figure 4). These conditional factors were taken into account to define the limit: (1) include a 100% coverage of the DSCF; (2) establish the minimum distance where the possible effects of activities in the area (i.e., bottom fishing, oil, and gas) did not threaten the viability of conservation objectives, and (3) the limits facilitate monitoring and control by the environmental authority.
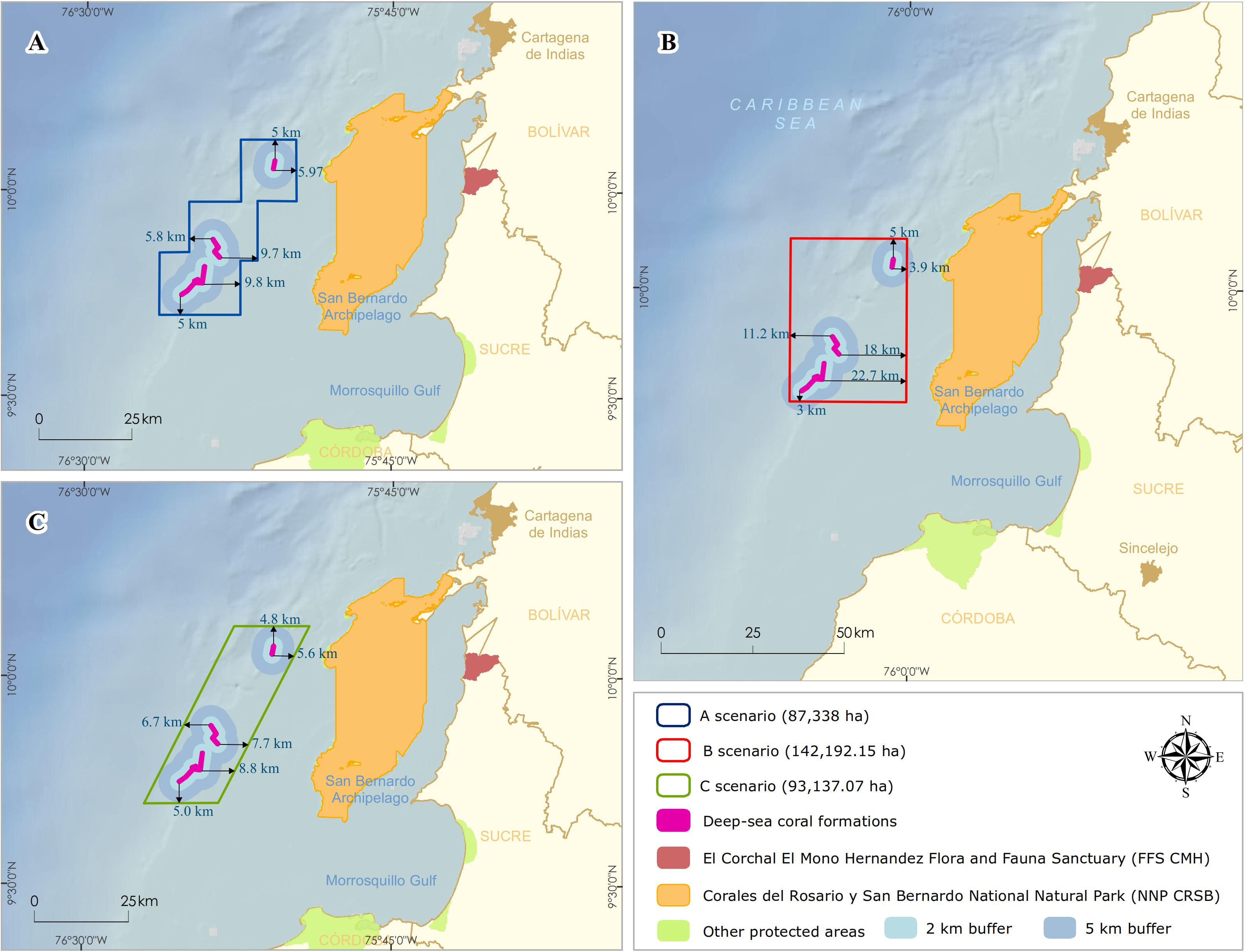
Figure 4. Delimitation of the polygons for the possible scenarios of the designation of the marine protected area. Distances of deep-sea coral formations edges (km) in each design (A) staircase, (B) rectangular, and (C) rhomboid.
For scenario A, a staircase polygon was proposed with 12 georeferenced vertex points covering an area of 87,338 ha (Figure 4A). Scenario B proposed a square polygon with an area of 142,192 ha (Figure 4B). For scenario C, a rhomboid was proposed with an area of 93,137 ha (Figure 4C). Of the three scenarios, scenario B has the largest area, both on the continental shelf (50,771 ha) and the continental slope (70,986 ha), and reaching the highest depth (1,234 m) (Table 2).
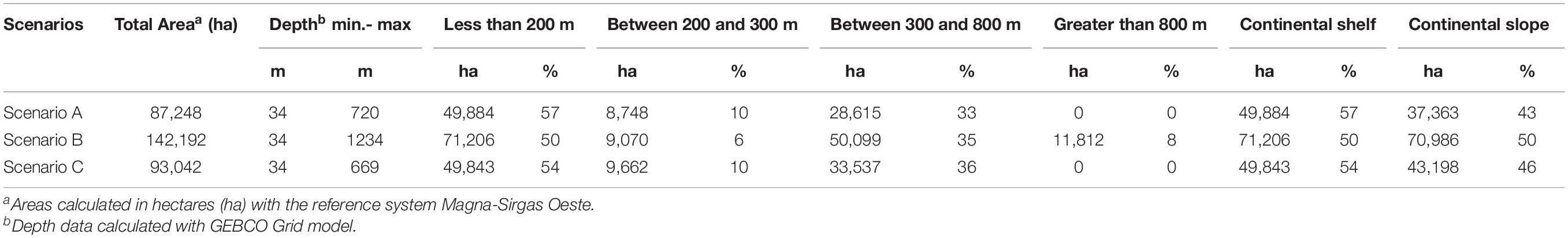
Table 2. Area coverage and depths of the three scenarios proposed for the delimitation of the Marine Protected Area.
All three scenarios meet the first conditional factor. For future monitoring, control, and surveillance activities of NNP, scenarios B and C best fit this requirement as they cover only four vertices. However, only scenario B was adjusted to parallel and meridians (i.e., latitude and longitude), assuming that, at sea, these actions with updated GPS equipment and nautical charts can be more easily performed with this System of geographical coordinates and exercise better management of the area.
On the other hand, to evaluate the second condition, there is scientific evidence on the direct physical and chemical impacts that the oil and gas sector generates (e.g., anchor chains, drill cuttings, and drilling fluids) on benthic communities in the various steps of the exploration and production process (Olsgard and Gray, 1995; Sammarco et al., 2004; Gass and Roberts, 2006; Netto et al., 2009; Larsson and Purser, 2011; Jones et al., 2012; Purser and Thomsen, 2012; White et al., 2012). Due to the drilling of multiple wells in the exploration phase, the predominant discharges during drilling would be the cuttings. The deposition of the cuttings on the seabed depends mainly on local oceanographic conditions and the depth they are discharged (Breuer et al., 2004). Physical burial of natural seabeds shows detrimental effects at distances between 100 and 500 m from the well (Santos et al., 2009; Jones et al., 2012), where recovery is likely to be slow. Lesser biological effects are detected in community parameters at distances of 1,000 m or in areas with stronger currents and at depths of 2,000 m from the drilling platform and where elevated hydrocarbon levels can extend up to approximately 4–5 km in the direction of the direct current (Olsgard and Gray, 1995; Lincoln, 2002). Based on the above, the three scenarios were designed with minimum distances to the DSCF of between 3 and 5 km, with scenario B having the longest (∼ 18 km) and shortest distances (∼ 3 km) (Figure 4B).
Scenarios proposal for the new NNP were discussed with each of the economic sectors in separate meetings to avoid alienating these interest groups with marginal adverse effects (Armstrong and van den Hove, 2008).
With the fishing sector meetings through the National Fisheries Authority, the restrictions on carrying out activities in an NNP were exposed. Scenarios A and C were compared, and unlike scenario B, they did not guarantee a sufficiently large and safe buffer area for positioning and operation of fishing gear (e.g., bottom and longline fishing) invading the NNP boundaries. This argument was supported by experiences such as that of Gully MPA in Canada and MPA Darwin Mounds in the United Kingdom, both with the presence of the deep-sea coral of Lophelia pertusa (Linnaeus, 1758) and where fishing activities were prohibited in 2002 and 2003, respectively, due to the damage caused to these communities (Breeze and Fenton, 2007; De Santo and Jones, 2007).
According to fishing statistics in the Colombian Caribbean, a sharp decrease in fish catches began in 2007, with 47% only in the 2009–2010 period (Rueda et al., 2013). Furthermore, the catch sizes of the leading commercially important fish species in the Caribbean (i.e., grouper, snapper, and mackerel) were below the average size at sexual maturity (MADR–CCI, 2010). For the fishing sector, it was essential to describe the existing scientific evidence in more than 100 areas of the world on the direct and indirect benefits of the designation of marine reserves (MPA where fishing is restricted) (PISCO, 2008). These studies demonstrate the increase in the abundance, diversity, and size of the marine species that live within the MPA and the biomass they export, maximizing fish spill-over (Babcock et al., 2010; Murillo et al., 2011). Based on the above, the fisheries sector welcomed the establishment of this new NNP with the limits of scenario B as a complementary strategy to recover and preserve commercial fisheries since DSCF are strategic sites in their life cycles.
Telecommunications is an exponentially growing industry worldwide, facing the need to connect continents and islands through fiber optic cables across the ocean becoming increasingly common due to new technology (Carter, 2009). In the first meetings to approach the sector about the future declaration of the NNP, the lack of coordination between the environmental sector and the communications sector was indisputable. Despite the evidence from the DSCF, since 2005 in the area where the submarine cable was laid, there was no mapping of these communities’ spatial distribution when the project was presented to the environmental authority in 2010. Therefore the project was made viable in the Environmental Impact Assessment. Therefore, the proposal for scenario B for the PNN was accepted, given the importance of this area in planning the country’s conservation priorities and the consolidation of SINAP (DNP, 2010).
Furthermore, in areas where there are submarine cables in Colombian jurisdictional waters (EEZ), the anchoring of any boat and trawling is prohibited, and any maritime activity that maintains complete or partial contact with the sea bottom. In those cases, parallel safety zones of 1/4 nautical mile (500 m) are established on each side of the cable, duly marked on the nautical charts (Resolution 204,2012). This Colombian regulation strengthens the management measures within the new NNP.
Due to the exponential increase in demand and the depletion of hydrocarbon reserves in more accessible locations, deep-waters oil and gas exploitation is a growing industry worldwide (Freiwald et al., 2004). During the Design and designation of this NNP, the mining and energy sector (e.g., offshore gas and oil) constituted one of the five main economic development strategies in the National Development Plan (NDP) 2010–2014 (DNP, 2011). Thus, seismic exploration activity in Colombian territory and mainly in Caribbean waters between 2009–2011, was the highest in the last three decades, concentrating on offshore areas and licensing exploration blocks at an unprecedented rate (ANH, 2011), which would continue increasing as proposed, for oil and gas supply and demand scenarios in Colombia in 2030 (UPME, 2012).
The negotiation on the selection of the best scenario of the NNP limit to the industry required a much broader negotiation time, given the presence of the DSCF within two hydrocarbon exploration blocks reserved and assigned by the ANH to the ECOPETROL S.A. (Fuerte Norte Block and Fuerte Sur Block; Figure 3D).
The areas had two exploration and production contract actives since 2006 with BHP Billiton Petroleum5. The industry started from the premise of subtracting a minimum area from the blocks that would allow the conservation of these communities and the continuity of oil and gas exploration operations. The above was encouraged by the NDP 2010–2014, which for the first time in Colombia, prohibited the development of mining activities, exploration, exploitation of hydrocarbons, aquaculture, industrial trawling, as well as the extraction of coral components for the elaboration of handicrafts in coral reefs (Law 1450 of 2011: article 207, paragraph 1). Furthermore, within the two oil and gas exploration contracts, there was the possibility of making “reductions in assigned areas” if the competent Environmental Authority adopts the extension of reserved, excluded, protected, or restricted areas after the contract is signed. Likewise, suppose new such areas are established, the extension of which corresponds partially to an assigned exploitation area. In that case, the contractor irrevocably undertakes to fully respect the conditions and regulations to which the area is subject and to comply with the obligations and requirements.
Therefore, the sector was more inclined to select scenarios A or C since they specifically met a buffer area of at least 5 km and areas of smaller polygons (Figure 3), which generated a balanced proposal for both parties. The strategy of minimizing the alignment of proposals with the three sectors in a discussion meeting, having achieved a prior consensus of scenario B with the two previous sectors, allowed the first significant advance in negotiations with the oil and gas sector. As part of the technical support, the recently published results on the impacts of the Deepwater Horizon oil spill in the Gulf of Mexico in April 2010 were evaluated. These effects impacted the deep-sea coral, reaching a depth of 1,370 m and a distance of 11 km to the Macondo well (White et al., 2012). This threat in marine areas could translate into severe impacts on these communities characterized by low metabolism, growth, and reproduction rates (Freiwald et al., 2004; Ramirez-Llodra et al., 2011).
For this reason, a polygon with a larger area was designed with the precautionary principle strengthened, in the face of a possible future accident, as the NNP is adjacent to the two exploration blocks. A study on the individual size, frequency, and total amount of oil spilled in the United States MPAs showed that although fewer spills within the MPAs, there are no significant differences in the effects within and outside (Dalton and Jin, 2010). Therefore, having a much larger buffer area, such as scenario B, will allow clear rules and policies to be developed within the NNP management plan that reduces vulnerability to potential oil spills.
Furthermore, the area where the DSCF was identified in 2012 as part of an ecologically or biologically significant marine area (EBSA), during the Wider Caribbean and Midwest Atlantic regional workshop to facilitate the description of EBSA, in Recife, Brazil (CBD, 2012; Secretariat of the Convention on Biological Diversity, 2014). This recognition generated international anticipation to protect these communities with a characteristic of singularity or rarity and strengthen the national position to preserve this area’s ecological integrity.
There is a knowledge gap of the physical (i.e., deep currents) and biological processes that control these communities. Presence, distribution, and connectivity processes are not well understood within and outside of NNP. The sector accepted scenario B’s proposal as the best option based on the precautionary principle.
Once the negotiation process was over, in 2012, the NNP proposal was presented to the Colombian Academy of Exact Physical and Natural Sciences under “Corales de Profundidad National Natural Park.” The designation of the NNP was carried out in 2013 through an administrative act signed by the MADS (Resolution 339,2013). This MPA became the first Colombian underwater marine national park to conserve the DSCF found on the edge of the continental shelf and the upper slope as an expression of ecosystem representativeness, uniqueness, and essential habitat for a great diversity of marine species. It was also the first MPA in the Latin American and Caribbean region to conserve these DSCF. This new area became the 14th MPA of the System of National Natural Parks as part of SINAP with an area of 142,192.15 ha (Figure 4B), contributing to Colombia’s international commitments to the 11th Aichi Target for Biological Diversity of the CBD.
Including the DSCF as a conservation target in the designation of a new MPA for Colombia was a process that took 40 years to build, if we take into account the first scientific evidence found of these communities in Colombian waters and the subsequent expeditions between 1995 and 2005 (Reyes et al., 2005; Santodomingo et al., 2007, 2013; Alonso et al., 2015).
The secondary information reviewed in the provisioning phase allowed justifying the urgency of establishing an in situ conservation strategy, since these communities are a conservation priority in the country (DNP, 2010), following other countries in the region such as Brazil, Chile, and Venezuela (Chatwin, 2007), and other Marine Ecoregions of the World (Álvarez-Romero et al., 2018). The designation of the NNP allowed conserving 64.7% of the currently estimated area covered of these communities in the Colombian Caribbean and improving the representativeness of the System of National Natural Parks (Alonso and Corredor-Rubiano, 2020), contributing to the conservation of these communities in recent decades by including them in countries such as France (Fabri et al., 2014), Spain (Serrano et al., 2017), United States (Auster et al., 2020) and future designations for discoveries such as in the northern Red Sea waters of Saudi Arabia (Qurban et al., 2020).
The uniqueness criterion conferred by the Madracis myriaster species as the primary-habitat forming organism supports this NNP designation’s ecological importance in the Caribbean and worldwide. However, although the lack of scientific information for designation, recent research within the NNP has expanded and confirmed the ecological importance of recording mesophotic coral communities (Sánchez and Andrade, 2014; Sánchez et al., 2016). The presence of 80 fish species as large snapper and groupers (Chasqui-Velasco and González-Corredor, 2019) highlights the black grouper’s presence Hyporthodus nigritus, captured at a depth of 200 m (Bustos-Montes et al., 2013). Recent campaigns have advanced in the knowledge of the geomorphology of the marine bottom of the NNP (Morales-Giraldo et al., 2017) and expanded the distribution of the DSCF (Cedeño-Posso et al., 2017).
Although the designation of the NNP was based on the potential ecosystem services provided by the area, a recent study made the first approach to capture the economic value of the ecosystem services provided by this DSCF, concluding that the NNP has an essential value associated with its existence (non-uses values), where people are willing to pay a high value that would be equivalent to around the United States $ 95 million annually (Maldonado and Cuervo, 2016).
The National Natural Park category limits the three economic activities identified as significant threats to the area. The exclusion of future industrial fishing activities, especially bottom trawling, should avoid physical damage to these deep-sea communities, which continue to report losses and damages worldwide (Pusceddu et al., 2014; Clark et al., 2015). On the other hand, given the industry’s growth offshore oil and gas in Colombia (DNP, 2019), it is crucial to consider the potential threat that would have lead to a potential oil spill in the adjacent exploration areas.
During the last decade, after the Deepwater Horizon oil spill accident in the Gulf of Mexico (Fisher et al., 2014), the need to fill information gaps regarding the response of deep-water corals to oil and chemical dispersant exposure (DeLeo et al., 2016), growth rates and ages of deep-sea corals impacted (Prouty et al., 2016), and the importance of studying the physical oceanography of the area to predict the movement of water and the hydrocarbons they carry in future scenarios blowout (Joye et al., 2016). These research questions unresolved in the NNP should be strengthened with the industry of oil and gas to obtain baseline information required for a solid understanding of these systems’ ecology and interpretation of the results of monitoring, both local and regional scale (Cordes et al., 2016).
In general, fishing and oil and gas activities outside the NNP will continue, so the area management plan must be integrated into a broader marine management strategy such as integrated coastal zone management (MMA, 2000). Likewise, to marine spatial planning schemes that are being advanced in the area (Afanador-Franco et al., 2019), where the articulation of these planning tools is recommended to obtain the success in the effectiveness of the MPA (IOC-UNESCO, 2017). This harmonization of planning instruments is a challenge ensuring that the governance of the NNP is based on shared visions and goals.
To design limits of the NNP was the most critical step in the designation process. One of the most significant weaknesses was the limited information of the area and the few other world regions’ experiences. Uncertainty about the scope of potential threats was always present in the negotiation process; however, the country’s political will was clear from the beginning, managing to lay the minimum technical and scientific bases to support this conservation strategy actors involved. Describing the behind-the-scenes process of designation and simplifying discussions and decisions was not an easy task. However, it will serve as a case study to contribute to the process of building the global framework for biodiversity post-2020, currently under negotiation (CBD, 2018), and demonstrate that it is possible to generate spaces for consultation and agreements between the productive and environmental sectors to protect marine biodiversity in this type of deep environment.
This first experience in the designation of marine protected areas of such nature allowed the acquisition of some lessons learned: (1) to follow a system based on using the best available scientific evidence precautionary approach, where scientific discourse’s transparency reinforced confidence in knowledge. (2) To promote actions to strengthen decision-making and the private sector’s commitment to achieving the country’s conservation objectives as a signatory of the Convention on Biological Diversity and the Aichi Target 11. (3) To encourage the harmonization of Government and economic sectors with the marine protected area’s management, differentiated to avoid alienating the stakeholders by seeking solutions with marginal adverse effects. (4) Promote cooperation of the oil and gas industry, fisheries, and communications with scientific research to manage future environmental impact.
The NNP designation opens up future opportunities in various actions. One of the most important in the short term is bringing different stakeholders and the general public closer to these communities’ existence and fragility through education and communication campaigns (for example, photos, multimedia videos). These actions should be thought of and directed at discrete and defined audiences. The latter will allow a better understanding and acceptance of the need and importance of protecting these invisible resources for society.
Finally, it will be essential to promote cooperation between government entities to reduce surveillance and control costs over the NNP. Likewise, strengthen strategic alliances with nationally and internationally recognized academic and research institutions to direct future research and monitoring programs.
The original contributions presented in the study are included in the manuscript/supplementary material, further inquiries can be directed to the corresponding author/s.
DA wrote, edited, and revised the manuscript. MV-C, VR-G, FA-I, and PH revised the text and contributed analysis and figures. DA, FA-I, HZ, ER, and PH contributed to researching and conceiving the manuscript. AC for serving as support in the workshops during the process. All authors contributed to the article and approved the submitted version.
This work was made possible by GEF-UNDP-INVEMAR Project COL-00075241, PIMS # 3997 Design and Implementation of Subsystem of Marine Protected Areas (SMPA) in Colombia, co-funding by 14 national partners.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Thanks to the Institute of Marine and Coastal Research-INVEMAR, especially to Paula Sierra-Correa for support investigation from the GEF coordination project, Carolina Segura, as part of the Information Systems Laboratory’s research team. Likewise, thanks to Julia Miranda Londoño, General Director of National Natural Parks of Colombia, and her team, especially Adriana Perez, for her support throughout the designation process. Lastly, thanks to Boris Navarro, who facilitated the process from the ANH with the Environmental Authorities. Contribution No. 1297 of the Marine and Coastal Research – INVEMAR.
Afanador-Franco, F., Molina Jiménez, M., Pusquin Ospina, L. T., Escobar Olaya, G. A., and Castro Mercado, I. (2019). Conflictos de uso en el proceso de ordenamiento marino costero: visión de autoridad marítima. Departamento De Bolívar - Colombia Boletín Científico 38, 27–40. doi: 10.26640/22159045.2019.507
Almany, G. R., Connolly, S. R., Heath, D. D., Hogan, J. D., Jones, G. P., McCook, L. J., et al. (2009). Connectivity, biodiversity conservation and the design of marine reserve networks for coral reefs. Coral Reefs 28, 339–351. doi: 10.1007/s00338-009-0484-x
Alonso, D., and Corredor-Rubiano, I. (2020). Contribution of the marine protected areas of the national natural park system to the ecosystems representativeness of Colombia. Bull. Mar. Coast. Res. 49, 275–288.
Alonso, D., Ramirez, L., Diaz, J. M., Segura-Quintero, C., Castillo, P., and Chatwin, A. (2007). “Coastal and marine conservation priorities in colombia,” in Priorities for Coastal and MarineConservation in South America, ed. A. Chatwin (Arlington: The Nature Conservancy-TNC), 30–39.
Alonso, D., Segura-Quintero, C., Torres, P., Rozo-Garzón, D., Espriella, J., Bolaños, J., et al. (2010). “Áreas significativas para la biodiversidad,” in Biodiversidad del Margen Continental del Caribe Colombiano, ed. En: Invemar (Santa Marta: INVEMAR), 393–419. Available online at: http://www.invemar.org.co/redcostera1/invemar/docs/8868Version_Final_Libro_Invemar-ANH.pdf
Alonso, D., Vides, M., Cedeño-Posso, C., Marrugo, M., Henao, A., Sanchez, J. A., et al. (2015). Parque Nacional Natural Corales de Profundidad: Descripción de Comunidades Coralinas y Fauna Asociada. Santa Marta: Serie de Publicaciones Generales del Invemar.
Althaus, F., Williams, A., Schlacher, T. A., Kloser, R. J., Green, M. A., Barker, B. A., et al. (2009). Impacts of bottom trawling on deep-coral ecosystems of seamounts are long-lasting. Mar. Ecol. Prog. Ser. 397, 279–294. doi: 10.3354/meps08248
Álvarez-Romero, J. G., Mills, M., Adams, V. M., Gurney, G. G., Pressey, R. L., Weeks, R., et al. (2018). Research advances and gaps in marine planning: towards a global database in systematic conservation planning. Biol. Conserv. 227, 369–382. doi: 10.1016/j.biocon.2018.06.027
Armstrong, C. W., and van den Hove, S. (2008). The formation of policy for protection of cold-water coral off the coast of Norway. Mar. Policy 32, 66–73. doi: 10.1016/j.marpol.2007.04.007
Auster, P. J., Hodge, B. C., McKee, M. P., and Kraus, S. D. (2020). A scientific basis for designation of the Northeast Canyons and seamounts marine national monument. Front. Mar. Sci. 7:566.
Austin, S., Wyllie-Echeverria, S., and Groom, M. J. (2004). A comparative analysis of submarine cable installation methods in Northern Puget Sound, Washington. J. Mar. Environ. Eng. 7, 173–183.
Babcock, R. C., Shears, N. T., Alcala, A. C., Barrett, N. S., Edgar, G. J., Lafferty, K. D., et al. (2010). Decadal trends in marine reserves reveal differential rates of change in direct and indirect effects. Proc. Natl. Acad. Sci. 107, 18256–18261. doi: 10.1073/pnas.0908012107
Barr, L. M., Pressey, R. L., Fuller, R. A., Segan, D. B., Mcdonald-madden, E., and Possingham, H. P. (2011). A new way to measure the world ’ s protected area coverage. PLoS One 6:0024707.
Barry, J. P., Widdicombe, S., and Hall- Spencer, J. M. (2011). “Effects of ocean acidification on marine biodiversity and ecosystem function,” in Ocean Acidification: a National Strategy to Meet the Challenges of a Changing Ocean, ed. J. Gattuso (Oxford: Oxford University Press), 192–209.
Botsford, L. W., White, J. W., Coffroth, M.-A., Paris, C. B., Planes, S., Shearer, T. L., et al. (2009). Connectivity and resilience of coral reef metapopulations in marine protected areas: matching empirical efforts to predictive needs. Coral Reefs 28, 327–337. doi: 10.1007/s00338-009-0466-z
Breeze, H., and Fenton, D. (2007). Designing management measures to protect cold-water corals off Nova Scotia, Canada. Bull. Mar. Sci. 81, 123–133.
Breuer, E., Stevenson, A. G., Howe, J. A., Carroll, J., and Shimmield, G. B. (2004). Drill cutting accumulations in the Northern and central North sea: a review of environmental interactions and chemical fate. Mar. Pollut. Bull. 48, 12–25. doi: 10.1016/j.marpolbul.2003.08.009
Bustos-Montes, D., Viaña-Tous, J., Arturo, A. P., Pardo, E. R., Garrido, M., Rueda, M., et al. (2013). Registro de un mero negro adulto, hyporthodus nigritus (Perciformes: Ephinephelidae), en un arrecife profundo del caribe colombiano. Bol. Investig. Mar. Costeras 42, 413–419.
Carter, L., Burnett, D., Drew, S., Marle, G., Hagadorn, L., Bartlett-McNeil, D., et al. (2009). “Submarine cables and the oceans – connecting the World,” in ICPC/UNEP/UNEP-WCMC, ed. UNEP-WCMC Biodiversity Series No. 31. Available online at: http://www.unep-wcmc.org/resources/publications/%0AUNEP_WCMC_bio_series/31.aspx (accessed May 9, 2020).
CBD (2010). The Strategic Plan for Biodiversity 2011-2020 and the Aichi Biodiversity Targets. Available online at: https://www.cbd.int/decisions/cop/10/2 [Accessed January 23, 2012]
CBD (2012). Report of the Wider Caribbean and Western Mid-Atlantic Regional Workshop to Facilitate the Description of Ecologically or Biologically Significant Marine Areas. Available online at: https://www.cbd.int/kb/record/meetingDocument/87392?Event=SBSTTA-16 (accessed May 3, 2020).
CBD (2018). Decision Adopted by the Conference of the Parties to the Convention on Biological Diversity 14/8. Protected areas and other effective area-based conservation measures. Available online at: https://www.cbd.int/doc/decisions/cop-14/cop-14-dec-08-en.pdf (accessed January 23, 2021).
Cedeño-Posso, C., Alonso, D., Ballesteros, D., Yepes- Narvaez, V., Rocha-Martinez, V., Cardenas, A., et al. (2017). “Mapeo de la distribución del ensamblaje de Madracis spp., en el Parque Nacional Natural Corales de Profundidad,” in Informe Técnico Final, Santa Marta: Invemar.
Chasqui-Velasco, L. H., and González-Corredor, J. D. (2019). Peces registrados en ambientes mesofóticos de Bajo Frijol, la porción más somera del parque nacional natural corales de profundidad, usando buceo técnico CCR. Bull. Mar. Coast. Res. 48, 89–101.
Chatwin, A. (2007). Priorities for Coastal and MarineConservation in South America. Arlington: The Nature Conservancy-TNC.
Clark, M. R., Althaus, F., Schlacher, T. A., Williams, A., Bowden, D. A., and Rowden, A. A. (2015). The impacts of deep-sea fisheries on benthic communities: a review. ICES J. Mar. Sci. 73, i51–i69.
Clark, M., Tittensor, D., Rogers, A., Brewin, P., Schlacher, T., Rowden, A., et al. (2006). Seamounts, Deep-Sea Corals and Fisheries: Vulnerability of Deep-Sea Corals to Fishing on Seamounts Beyond Areas of National Jurisdiction. UNEP Regional Seas Report and Studies No 183. Cambridge: UNEP-WCMC.
Cordes, E. E., Jones, D. O. B., Schlacher, T. A., Amon, D. J., Bernardino, A. F., Brooke, S., et al. (2016). Environmental impacts of the deep-water oil and gas industry: a review to guide management strategies. Front. Environ. Sci. 4:58.
Dalton, T., and Jin, D. (2010). Extent and frequency of vessel oil spills in US marine protected areas. Mar. Pollut. Bull. 60, 1939–1945. doi: 10.1016/j.marpolbul.2010.07.036
Davies, A. J., Roberts, J. M., and Hall-Spencer, J. (2007). Preserving deep-sea natural heritage: emerging issues in offshore conservation and management. Biol. Conserv. 138, 299–312. doi: 10.1016/j.biocon.2007.05.011
De Santo, E., and Jones, P. (2007). The Darwin Mounds: from undiscovered coral to the development of an offshore marine protected area regime. Bull. Mar. Sci. 81, 147–156.
Decree 2372 (2010). Diario Oficial de la Republica de Colombia. Colombia: Ministerio de Ambiente y Desarrollo Sostenible.
Decree 622 (1977). Diario Oficial de la Republica de Colombia. Colombia: Ministerio de Ambiente y Desarrollo Sostenible.
DeLeo, D. M., Ruiz-Ramos, D. V., Baums, I. B., and Cordes, E. E. (2016). Response of deep-water corals to oil and chemical dispersant exposure. Deep. Res. II Top. Stud. Oceanogr. 129, 137–147. doi: 10.1016/j.dsr2.2015.02.028
Díaz, J. M., Barrios, L. M., Cendales, M. H., Garzón-Ferreira, J., Geister, J., López-Victoria, M., et al. (2000). Áreas Coralinas de Colombia. Santa Marta: INVEMAR.
DNP (2010). Lineamientos Para la Consolidación del Sistema Nacional de Areas Protegidas de Colombia- SINAP. Bogota: Departamento Nacional de Planeacion.
DNP (2011). Plan Nacional de Desarrollo 2010-2014 -Prosperidad Para Todos. Bogota: Departamento Nacional de Planeacion.
DNP (2019). Plan Nacional de Desarrollo 2019-2022 Pacto por Colombia, Pacto Por la Equidad. Bogota: Departamento Nacional de Planeacion.
Dudley, B. Y. N., and Parish, J. (2006). Closing the Gap. Creating Ecologically Representative Protected Area Systems: a Guide to Conducting the Gap Assessments of Protected Area Systems for the Convention on Biological Diversity. Montreal: Secretariat of the Convention on Biological Diversity.
Duque-Caro, H. (1984). Estilo estructural, diapirísmo y episodios de acrecionamiento del terreno Sinú-San Jacinto en el noroccidente de Colombia. Boletín Geológico 27, 1–29.
Fabri, M. C., Pedel, L., Beuck, L., Galgani, F., Hebbeln, D., and Freiwald, A. (2014). Megafauna of vulnerable marine ecosystems in French mediterranean submarine canyons: spatial distribution and anthropogenic impacts. Deep. Res. II Top. Stud. Oceanogr. 104, 184–207. doi: 10.1016/j.dsr2.2013.06.016
Fischer, A., Bhakta, D., Macmillan-Lawler, M., and Harris, P. (2019). Existing global marine protected area network is not representative or comprehensive measured against seafloor geomorphic features and benthic habitats. Ocean Coast. Manag. 167, 176–187. doi: 10.1016/j.ocecoaman.2018.10.001
Fisher, C. R., Hsing, P.-Y., Kaiser, C. L., Yoerger, D. R., Roberts, H. H., Shedd, W. W., et al. (2014). Footprint of deepwater horizon blowout impact to deep-water coral communities. Proc. Natl. Acad. Sci. U. S. A. 111, 11744–11749. doi: 10.1073/pnas.1403492111
Freiwald, A., Fosså, J. H., Grehan, A., Koslow, T., and Roberts, J. M. (2004). Cold-Water Coral Reefs: Out of Sight - No Longer Out of Mind. Cambridge: UNEP-WCMC.
Gaines, S., Gaylord, B., Gerber, L., Hastings, A., and Kinlan, B. (2007). Connecting places. Oceanography 20, 90–99.
Gass, S. E., and Roberts, J. M. (2006). The occurrence of the cold-water coral Lophelia pertusa (Scleractinia) on oil and gas platforms in the North Sea: colony growth, recruitment and environmental controls on distribution. Mar. Pollut. Bull. 52, 549–559. doi: 10.1016/j.marpolbul.2005.10.002
Gownaris, N. J., Santora, C. M., Davis, J. B., and Pikitch, E. K. (2019). Gaps in protection of important ocean areas: a spatial meta-analysis of ten global mapping initiatives. Front. Mar. Sci. 6:650.
Guinotte, J. M., Orr, J., Cairns, S., Freiwald, A., Morgan, L., and George, R. (2006). Will human-induced changes in seawater chemistry alter the distribution of deep-sea scleractinian corals? Front. Ecol. Environ. 4, 141–146. doi: 10.1890/1540-9295(2006)004[0141:whcisc]2.0.co;2
Hofmann, G. E., Barry, J. P., Edmunds, P. J., Gates, R. D., Hutchins, D. A., Klinger, T., et al. (2010). The effect of ocean acidification on calcifying organisms in marine ecosystems: an organism-to-ecosystem perspective. Annu. Rev. Ecol. Evol. Syst. 41, 127–147. doi: 10.1146/annurev.ecolsys.110308.120227
Huvenne, V. A. I., Bett, B. J., Masson, D. G., Le Bas, T. P., and Wheeler, A. J. (2016). Effectiveness of a deep-sea cold-water coral marine protected area, following eight years of fisheries closure. Biol. Conserv. 200, 60–69. doi: 10.1016/j.biocon.2016.05.030
IOC-UNESCO (2017). The 2nd International Conference on Marine/Maritime Spatial Planning. Workshop Reports Series, 279. Paris: IOC-UNESCO..
Jones, D. O. B., Gates, A. R., and Lausen, B. (2012). Recovery of deep-water megafaunal assemblages from hydrocarbon drilling disturbance in the Faroe-shetland channel. Mar. Ecol. Prog. Ser. 461, 71–82. doi: 10.3354/meps09827
Joye, S. B., Bracco, A., Özgökmen, T. M., Chanton, J. P., Grosell, M., MacDonald, I. R., et al. (2016). The gulf of Mexico ecosystem, six years after the Macondo oil well blowout. Deep. Res. II Top. Stud. Oceanogr. 129, 4–19. doi: 10.1016/j.dsr2.2016.04.018
Koenig, C. C., Florida, S. U., Shepard, A. N., Reed, J. K., Coleman, F. C., Brooke, S. D., et al. (2005). Habitat and fish p opulations in the deep-sea fish populations oculina cor al ecosystem of the western Atlantic. Am. Fish. Soc. Symp. 41, 795–805.
Larsson, A. I., and Purser, A. (2011). Sedimentation on the cold-water coral Lophelia pertusa: cleaning efficiency from natural sediments and drill cuttings. Mar. Pollut. Bull. 62, 1159–1168. doi: 10.1016/j.marpolbul.2011.03.041
Lincoln, D. (2002). Sense and Nonsense- The Environmental Impacts of Exploration on Marine Organisms Offshore Cape Breton. Available online at: www.scribd.com/document/424201682/Sense-and-NonsenseThe-Environmental-Impacts-of-Exploration-Marine-Offshore-Cape-Breton (accessed May 19, 2020).
Lourie, S. A., and Vincent, A. C. J. (2004). using biogeography to help set priorities in marine conservation. Conserv. Biol. 18, 1004–1020. doi: 10.1111/j.1523-1739.2004.00137.x
Lutz, S. J., and Ginsburg, R. N. (2007). State of Deep Coral Ecosystems in the Caribbean Region: Puerto Rico and the U.S. Virgin Islands. Washington, DC: NOAA.
MADR–CCI (2010). Pesca y Acuicultura Colombia 2009. Bogotá D.C. Available online at: http://sepec.aunap.gov.co/Home/VerPdf/11 (Accessed January 23, 2020).
Maier, C., Hegeman, J., Weinbauer, M. G., and Gattuso, J.-P. (2009). Calcification of the cold-water coral Lophelia pertusa, under ambient and reduced pH. Biogeosciences 6, 1671–1680. doi: 10.5194/bg-6-1671-2009
Majarres, L. (2004). Pesquerías Demersales del Área Norte del Mar Caribe de Colombia y Parámetros Biológico-Pesqueros y Poblacionales del Recurso Pargo. Santa Marta: U. del Magdalena
Maldonado, J., and Cuervo, R. (2016). Valoración económica del parque nacional natural corales de profundidad. Boletín de Investigaciones Marinas Costeras 45, 99–121.
Mancini, I., Guerriero, A., Guella, G., Bakken, T., Zibrowius, H., and Pietra, F. (1999). Novel 10-hydroxydocosapolyenoic acids from deep-water scleractinian corals. Helv. Chim. Acta 82, 677–684. doi: 10.1002/(sici)1522-2675(19990505)82:5<677::aid-hlca677>3.0.co;2-0
MMA (2000). Política Nacional Ambiental Para el Desarrollo Sostenible de los Espacios Oceánicos y las Zonas Costeras e Insulares de Colombia Colombia: INVEMAR.
Morales-Giraldo, D. F., Rocha-Gutiérrez, V. L., and Posada-Posada, B. O. (2017). Geomorfología de los fondos submarinos del parque nacional natural corales de profundidad, mar caribe colombiano. Bol. Investig. Mar. Costeras 46, 73–90.
Morgan, L., Tsao, C.-F., and Guinotte, J. (2007). Ecosystem-based management as a tool for protecting deep-sea corals in the USA. Bull. Mar. Sci. 81, 39–48.
Murillo, F. J., Durán Muñoz, P., Altuna, A., and Serrano, A. (2011). Distribution of deep-water corals of the flemish cap, flemish pass, and the grand banks of newfoundland (Northwest Atlantic Ocean): interaction with fishing activities. ICES J. Mar. Sci. 68, 319–332. doi: 10.1093/icesjms/fsq071
Netto, S. A., Gallucci, F., and Fonseca, G. (2009). Deep-sea meiofauna response to synthetic-based drilling mud discharge off SE Brazil. Deep. Res. II Top. Stud. Oceanogr. 56, 41–49. doi: 10.1016/j.dsr2.2008.08.018
Olsgard, F., and Gray, J. S. (1995). A comprehensive analysis of the effects of offshore oil and gas exploration and production on the benthic communities of the Norwegian continental shelf. Mar. Ecol. Prog. Ser. 122, 277–306. doi: 10.3354/meps122277
OSPAR Comission (2008). Background Document on Potential Problems Associated With Power Cables Other Than Those for Oil and Gas Activities. Available online at: www.ospar.org/documents?v=7128 (accessed May 3, 2020).
Paramo, J., and Saint-Paul, U. (2012a). Deep-sea shrimps Aristaeomorpha foliacea and Pleoticus robustus (Crustacea: Penaeoidea) in the colombian caribbean sea as a new potential fishing resource. J. Mar. Biol. Assoc. U. K. 92, 811–818. doi: 10.1017/s0025315411001202
Paramo, J., and Saint-Paul, U. (2012b). Spatial structure of the Caribbean lobster (Metanephrops binghami) in the Colombian Caribbean sea. Helgol. Mar. Res. 66, 25–31. doi: 10.1007/s10152-011-0243-6
Paramo, J., Wolff, M., and Saint-Paul, U. (2012). Deep-sea fish assemblages in the Colombian Caribbean sea. Fish. Res. 12, 87–98. doi: 10.1016/j.fishres.2012.02.011
Pérez, A., and Zambrano, H. (2009). Ruta para la Declaratoria de Nuevas Áreas y Ampliaciones en el Sistema Nacional de Areas Protegidas. Bogota: PNN-WWF.
PISCO (2008). The Science of Marine Reserves. Available online at: www.piscoweb.org/outreach/pubs/reserves (accessed January 23, 2020).
Prouty, N. G., Fisher, C. R., Demopoulos, A. W. J., and Druffel, E. R. M. (2016). Growth rates and ages of deep-sea corals impacted by the deepwater horizon oil spill. Deep. Res. II Top. Stud. Oceanogr. 129, 196–212. doi: 10.1016/j.dsr2.2014.10.021
Purser, A., and Thomsen, L. (2012). Monitoring strategies for drill cutting discharge in the vicinity of cold-water coral ecosystems. Mar. Pollut. Bull. 64, 2309–2316. doi: 10.1016/j.marpolbul.2012.08.003
Pusceddu, A., Bianchelli, S., Martín, J., Puig, P., Palanques, A., Masqué, P., et al. (2014). Chronic and intensive bottom trawling impairs deep-sea biodiversity and ecosystem functioning. Proc. Natl. Acad. Sci. 111, 8861–8866. doi: 10.1073/pnas.1405454111
Qurban, M. A., Krishnakumar, P. K., Joydas, T. V., Manikandan, K. P., Ashraf, T. T. M., and Sampath, G. (2020). Discovery of deep-water coral frameworks in the northern Red sea waters of Saudi Arabia. Sci. Rep. 10: 15356.
Ramirez-Llodra, E., Tyler, P. A., Baker, M. C., Bergstad, O. A., Clark, M. R., Escobar, E., et al. (2011). Man and the last great wilderness: human impact on the deep sea. PLoS One 6:e22588. doi: 10.1371/journal.pone.0022588
Reed, J. K., Koenig, C. C., and Shepard, A. N. (2007). Impacts of bottom trawling on a deep-water oculina coral ecosystem off Florida. Bull. Mar. Sci. 81, 481–496.
Resolution 204 (2012). Diario Oficial de la República de Colombia. Colombia: Direccion General Maritima.
Resolution 339 (2013). Diario Oficial de la Republica de Colombia. Colombia: Ministerio de Ambiente y Desarrollo Sostenible.
Reyes, J., and Santodomingo, N. (2002). Manual de Identificación Cites de Invertebrados Marinos de Colombia. Colombia: INVEMAR.
Reyes, J., Santodomingo, N., Gracia, A., Borrero-Pérez, G., Navas, G., Mejía-Ladino, L. M., et al. (2005). “Southern caribbean azooxanthellate coral communities off colombia,” in Cold-Water Corals and Ecosystems. Erlangen Earth Conference Series, eds A. Freiwald and J. M. Roberts (Berlin: Springer).
Roberts, C. M., Andelman, S., Branch, G., Bustamante, R. H., Castilla, J. C., Dugan, J., et al. (2003a). Ecological criteria for evaluating candidate sites for marine reserves. Ecol. Appl. 13, 199–214. doi: 10.1890/1051-0761(2003)013[0199:ecfecs]2.0.co;2
Roberts, C. M., Branch, G., Bustamante, R. H., Castilla, J. C., Dugan, J., Halpern, B. S., et al. (2003b). Application of ecological criteria in selecting marine reserves and developing reserve networks. Ecol. Appl. 13, 215–228. doi: 10.1890/1051-0761(2003)013[0215:aoecis]2.0.co;2
Roberts, J. M., Wheeler, A. J., and Freiwald, A. (2006). Reefs of the deep: the biology and geology of cold-water coral ecosystems. Science 312, 543–547. doi: 10.1126/science.1119861
Roberts, J. M., Wheeler, A., Freiwald, A., and Cairns, S. (2009). Cold-Water Corals: the Biology and Geology of Deep-Sea Coral Habitats. Cambridge: Cambridge University Press.
Roberts, S., and Hirshfield, M. (2004). Deep-sea corals: out of sight, but no longer out of mind. Front. Ecol. Environ. 2, 123–1302. doi: 10.1890/1540-9295(2004)002[0123:dcoosb]2.0.co;2
Rueda, M., Gómez, J., Viloria, E., Santos-Acevedo, M., Mármol, D., Bustos, D., et al. (2013). Causas y Tensores del Cambio en los Ecosistemas Marinos y Costeros y sus Servicios: Indicadores de Presión. Report no 3. Colombia: INVEMAR
Salm, R. V., Clark, J., and Siirila, E. (2000). Marine and Coastal Protected Areas: a Guide for Planners and Managers. Washington D.C: IUCN.
Sammarco, P. W., Atchison, A. D., and Boland, G. S. (2004). Expansion of coral communities within the Northern Gulf of Mexico via offshore oil and gas platforms. Mar. Ecol. Prog. Ser. 280, 129–143. doi: 10.3354/meps280129
Sampaio, I., Braga-Henriques, A., Pham, C., Ocaña, O., De Matos, V., Morato, T., et al. (2012). Cold-water corals landed by bottom longline fisheries in the Azores (north-eastern Atlantic). J. Mar. Biol. Assoc. U. K. 92, 1547–1555. doi: 10.1017/s0025315412000045
Sánchez, A., and Andrade, J. (2014). Informe de las Primeras Observaciones Realizadas Mediante Buceo de Circuito Cerrado “Rebreather” (CCR) en el PNN Corales de Profundidad, Caribe colombiano. Bogota: BioMar.
Sánchez, J. A., Dueñas, L. F., González, F., Gómez, M., and Andrade, J. (2016). Primeras Exploraciones en los Arrecifes Mesofóticos del PNN Corales de Profundidad Mediante Recirculadores (CCR-Trimix), Caribe Colombiano. Bogota: BioMar.
Santodomingo, N., Reyes, J., Flórez, P., Chacón-Gómez, I. C., van Ofwegen, L. P., and Hoeksema, B. W. (2013). Diversity and distribution of azooxanthellate corals in the Colombian Caribbean. Mar. Biodivers. 43, 7–22. doi: 10.1007/s12526-012-0131-6
Santodomingo, N., Reyes, J., Gracia, A., Martínez, A., Ojeda, G., and García, C. (2007). Azooxanthellate Madracis coral communities off San Bernardo and Rosario Islands (Colombian Caribbean). Bull. Mar. Sci. 81, 273–287.
Santos, M. F. L., Lana, P. C., Silva, J., Fachel, J. G., and Pulgati, F. H. (2009). Effects of non-aqueous fluids cuttings discharge from exploratory drilling activities on the deep-sea macrobenthic communities. Deep. Res. II Top. Stud. Oceanogr. 56, 32–40. doi: 10.1016/j.dsr2.2008.08.017
Secretariat of the Convention on Biological Diversity (2014). Ecologically or Biologically Significant Marine Areas (EBSAs). Special Places in the world’s oceans, Vol 2: Wider Caribbean And Western Mid-Atlantic Region. New York: Secretariat of the Convention on Biological Diversity.
Segura-Quintero, C., Alonso, D., and Ramírez, L. (2012). Gap representativeness analysis in the marine protected areas of the national system of natural parks of Colombia. Bol. Investig. Mar. Costeras 41, 299–322.
Serrano, A., González-Irusta, J. M., Punzón, A., García-Alegre, A., Lourido, A., Ríos, P., et al. (2017). Deep-sea benthic habitats modeling and mapping in a NE Atlantic seamount (Galicia Bank). Deep. Res. I Oceanogr. Res. Pap. 126, 115–127. doi: 10.1016/j.dsr.2017.06.003
Skropeta, D. (2008). Deep-sea natural products. Nat. Prod. Rep. 25, 1131–1166. doi: 10.1039/b808743a
Stevens, T. (2002). Rigor and representativeness in marine protected area design. Coast. Manag. 30, 237–248. doi: 10.1080/08920750290042183
Stratoudakis, Y., Hilário, A., Ribeiro, C., Abecasis, D., Gonçalves, E. J., Andrade, F., et al. (2019). Environmental representativity in marine protected area networks over large and partly unexplored seascapes. Glob. Ecol. Conserv. 17:e00545. doi: 10.1016/j.gecco.2019.e00545
Thresher, R. E., Tilbrook, B., Fallon, S., Wilson, N. C., and Adkins, J. (2011). Effects of chronic low carbonate saturation levels on the distribution, growth and skeletal chemistry of deep-sea corals and other seamount megabenthos. Mar. Ecol. Prog. Ser. 442, 87–96. doi: 10.3354/meps09400
Turley, C. M., Roberts, J. M., and Guinotte, J. M. (2007). Corals in deep-water: will the unseen hand of ocean acidification destroy cold-water ecosystems? Coral Reefs 26, 445–448. doi: 10.1007/s00338-007-0247-5
Urriago, J. D., Santodomingo, N., and Reyes, J. (2011). Deep-sea corals formations: biologic criteria for the establishment of marine protected areas on a continental margin (100-300 m) in the Colombian Caribbean. Bol. Investig. Mar. Costeras 40, 89–113.
Vernette, G. (1985). La Plateforme continentale Caräibe de Colombie (du debouché du Magdalena au golfe du Morrosquillo). Importance du diapirisme argileux sur la morphologie et la sedimentation. TheÌse de doctorat, University of Bordeaux: Bordeaux
Vernette, G., Mauffret, A., Bobier, C., Briceno, L., and Gayet, J. (1992). Mud diapirism, fan sedimentation and strike-slip faulting, Caribbean Colombian margin. Tectonophysics 202, 335–349. doi: 10.1016/0040-1951(92)90118-p
Keywords: deep-sea corals, Madracis myriaster, marine protected area, Caribbean, Colombia, marine conservation
Citation: Alonso D, Vides-Casado M, Arias-Isaza F, Zambrano H, Rodriguez E, Rocha-Gutierrez V, Herron P and Castillo A (2021) Behind the Scenes for the Designation of the Corales de Profundidad National Natural Park of Colombia. Front. Mar. Sci. 8:567438. doi: 10.3389/fmars.2021.567438
Received: 29 May 2020; Accepted: 22 March 2021;
Published: 20 April 2021.
Edited by:
Juan Armando Sanchez, University of Los Andes, Colombia, ColombiaReviewed by:
Jorge Maldonado, University of Los Andes, Colombia, ColombiaCopyright © 2021 Alonso, Vides-Casado, Arias-Isaza, Zambrano, Rodriguez, Rocha-Gutierrez, Herron and Castillo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Alonso, ZGF2aWQuYWxvbnNvQGludmVtYXIub3JnLmNv
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.