Use of Synthetic Salmon GnRH and Domperidone (Ovaprim®) in Sharks: Preparation for ex situ Conservation
- 1College of Veterinary Medicine and Research Institute for Veterinary Science, Seoul National University, Seoul, South Korea
- 2Hanwha Marine Biology Research Center, Hanwha Hotels & Resorts Co., Ltd., Seoul, South Korea
- 3Department of Marine Industry and Maritime Police, College of Ocean Science, Jeju National University, Jeju, South Korea
A Corrigendum on
Use of Synthetic Salmon GnRH and Domperidone (Ovaprim®) in Sharks: Preparation for ex situ Conservation
by Kim, S. W., Hong, W. H., Han, S. J., Kwon, J., Ko, H., Lee, S. B., et al. (2020). Front. Mar. Sci. 7:571741. doi: 10.3389/fmars.2020.571741
In the original article, there was an error. We have used the whitetip reef shark (Triaenodon obesus) for this study but wrote the species' name incorrectly as the Oceanic whitetip shark (Carcharhinus longimanus).
Corrections have been made to the main text, Table, and Figures, and the supplementary material, simply substituting ‘Carcharhinus longimanus' to ‘Triaenodon obesus', ‘C. longimanus' to ‘T. obesus', and ‘whitetip shark' to ‘whitetip reef shark'.
Detailed corrections are as follows.
Corrections have been made to the Abstract:
Shark populations are constantly decreasing owing to environmental destruction and overfishing; thus, sharks are now at a risk of extinction, with 27.9% of shark species classified as endangered on the International Union for Conservation of Nature's Red List. Sharks are apex predators and a keystone species in balancing the marine food chain; their extinction will create an imbalance of the entire marine ecosystem. Assisted reproductive technology is the last resort for protecting animals facing severe extinction. Here, as a proactive effort toward building a hormone-induced artificial insemination protocol for endangered wild sharks, we identified the possibility of germ cell maturation by administration of Ovaprim®, a commercially produced synthetic salmon gonadotropin-releasing hormone, and calculated its optimum dosage and injection timing. The experiment was conducted on two shark species—Triakis scyllium and Triaenodon obesus. We found that intramuscular injections of 0.2 mL/kg of Ovaprim® for male T. scyllium and T. obesus, 0.2 mL/kg + 0.5 mL/kg at a 24 h interval for female T. scyllium, and 0.2 mL/kg + 0.2 mL/kg or 0.2 mL/kg + 0.3 mL/kg at a 24 h interval for female T. obesus were optimal dose protocols. These doses effectively induced the maturation and ovulation of oocytes and the release of semen. Our results confirm that Ovaprim® is a suitable tool for shark hormone-induced artificial insemination and indicate that this method may enable the conservation of the endangered shark species.
Corrections have been made to the Introduction, 6th, 7th, and 8th paragraphs:
Ovaprim® is composed of a salmon gonadotropin-releasing hormone analog (20 μg/mL) and domperidone (10 mg/mL)1. The salmon gonadotropin-releasing hormone analog elicits the release of gonadotropins from the pituitary and domperidone acts as a Dopamine D2 receptor antagonist, negating other mechanisms of GnRH release inhibition (Yanong et al., 2009). Ovaprim® facilitates gonadotropin release and eventually induces maturation and release of germ cells, which is conducive to artificial insemination. However, since there is no official record of Ovaprim® application to elasmobranchs yet, it is not known whether Ovaprim® will work on sharks. To confirm whether it works, experiments using two shark species: the banded houndshark (Triakis scyllium) and the whitetip reef shark (Triaenodon obesus) were performed.
Triakis scyllium and T. obesus belong to the order Carcharhiniformes (the former to the family Triakidae and the latter, to Carcharhinidae). Triakis scyllium inhabits the Northwest Pacific Ocean and shows aplacental viviparity with internal fertilization (Compagno, 1984). They are relatively easier to handle by aquarists when bred in aquariums, thanks to their small body size (less than 1.5 m). Triakis scyllium is classified into the group of “least concern” by the IUCN Red List2 and is one of the most accessible shark species in the Republic of Korea. Triaenodon obesus lives in the warm waters of the Indo-Pacific Ocean. This species also fertilizes its gametes internally but shows placental viviparity. Although the IUCN Red List classifies this species into the “near threatened” group3, it is also a species commonly seen in aquariums. We chose these two species owing to their accessibility, abundance in the aquarium, and their smaller size in which both are thought to be sexually mature if the body length is longer than ~1 m. Another important reason is that both show a synchronous reproduction strategy, which makes it meaningful to induce reproduction at the desired time point. A group of sharks showing reproductive synchrony have same stage of the reproduction cycle at a time, manifesting seasonal breeding (Castro, 2009).
Herein, to the best of our knowledge, we administered Ovaprim® for the first time to T. scyllium and T. obesus, confirming its effect on oocyte maturation, ovulation, and semen production. Based on this, a platform where artificial insemination in endangered sharks becomes practically possible, was constructed.
Corrections have been made to Materials and Methods, Sharks for the Experiment, 1st and 4th paragraphs:
As a result of selecting sharks with a total body length of approximately 90 centimeters or more, a total of five male and seven female T. scyllium, and three male and three female T. obesus individuals were collected from Hanwha Aqua Planet Jeju, Jeju-do, Republic of Korea. We searched for more adult sharks in large aquariums and fish markets in Korea for a sufficiently large experimental population. There were many immature sharks, but fully-grown ones were not available. In other words, the sharks used in this experiment were virtually the best cohort available in the country. Triakis scyllium males were named M001–M005 and T. obesus WM001–WM003. Females were named F001–F007 and WF001–WF003, respectively. For identification, punch biopsy (Kai Medical, Japan) was done at the tip of the left pectoral fin of the sharks using a binary numbering system.
The sharks' breeding history was examined and according to it the T. scyllium have shown irregular pregnancy regardless of the season, and the T. obesus did not show any pregnancy at all throughout the exhibition. Since there was little change in water temperature and circadian rhythm throughout the year in the facility, their loss of seasonality is explainable (Schaller, 2006; George et al., 2017).
Corrections have been made to Materials and Methods, Dose Optimization of Ovaprim® and Its Effects in Male T. scyllium, 1st and 2nd paragraphs:
The final purpose of this study was to determine if Ovaprim® could draw biological reactions through the shark's hypothalamus-pituitary-gonadal axis, and if confirmed, to find out the optimum dose for performing artificial insemination in the sharks. In the case of male sharks, the criterion of judgment was quantity and quality of collected semen. For females, the administered dose should induce follicular maturation and ovulation but should not result in egg dropping. This is described schematically in Figure 1, and the doses falling under the shaded area indicate the optimum range for female sharks. Too high a dose will result in egg dropping, and too little cannot induce follicular maturation. Throughout this study, diverse doses were tested and according to their biological reactions in the tested sharks, the experiments were marked on the schematic graph (Figure 1). Optimized doses were assessed through this process for T. scyllium and T. obesus.
After testing the basic hormone level of the sharks, dose optimization experiments were performed using T. scyllium, which had a larger population size than T. obesus. The experiment was first performed on male sharks. Female doses were tested in another experiment, based on the determined male dose.
Corrections have been made to Materials and Methods, Dose Optimization of Ovaprim® and Its Effects in T. obesus, 1st and 2nd paragraphs:
Based on the experimental outcome in T. scyllium, 0.2 mL/kg Ovaprim® was administered to T. obesus males. As the biological reaction to the injection was thought to be appropriate, three replicate experiments were performed without further dose optimization for males. The concentration of estradiol, progesterone, and testosterone was assessed in blood drawn 10 min pre-injection and 12, 24, 36, and 48 h post-injection. Semen collection was performed at 15 min, and 1, 12, 24, 36, and 48 h post-injection.
In females, 0.2 mL/kg Ovaprim® was administered twice at a 24 h interval for the first experiment, extrapolating the results of female T. scyllium and male T. obesus. Two experimental doses were administered in the female group with more than a month interval between the trials. Administered doses were as follows: First: 0.2 mL/kg + 0.2 mL/kg; Second: 0.2 mL/kg + 0.3 mL/kg at a 24 h interval. Blood collection for hormone analyses, ovary ultrasonography for follicular growth measurement, and egg drop checking were carried out 10 min pre-injection and at 12, 24, 36, and 48 h post-injection, using the same methodologies that were applied to T. scyllium.
Corrections have been made to Results, Reproductive Hormone Levels Before Ovaprim® Administration:
Results of blood hormone levels are shown in Table 1. In females, it was possible to distinguish between pregnant and non-pregnant individuals based on their estradiol concentration. An average estradiol level of 9159.80 pg/mL for the pregnant group, and 1018.68 pg/mL for the non-pregnant group was recorded, showing more than a nine-fold difference between the two (Table 1). There were evident interspecific differences, especially in estradiol and testosterone concentrations in females and males, respectively. Male T. obesus showed a more than six times higher testosterone level than that in male T. scyllium. Female T. scyllium, however, showed a more than 400 times higher estradiol concentration than that in female T. obesus. This suggests that the baseline data must be established separately for each species. As there is no previously published information on hormone levels for both T. scyllium and T. obesus, the data presented in Table 1 merits academic use in other studies and by clinicians.
Corrections have been made to Results, Dose Optimization of Ovaprim® and Its Effects in T. obesus, 1st paragraph:
Changes in blood hormone levels after administration of 0.2 mL/kg Ovaprim® in males are as shown in Figure 3D. Overall, testosterone levels after salmon gonadotropin-releasing hormone analog administration were significantly higher in T. obesus than those in T. scyllium, although these decreased at 24 h post-injection. Progesterone, however, showed peak concentrations at 24 h compared to at 12 h in T. scyllium, thus showing different increments between species. Estradiol remained constant at very low levels. Unlike T. scyllium, semen was not sampled at both 1 h and 12 h post-injection. The sampling time point was further reduced to 15 min post-injection and as a result, sufficient amounts of semen could be collected with an average volume of 6.5 mL. Semen sampling was found to be effective if carried out almost immediately after Ovaprim® administration in T. obesus. Semen sampling at later stages could result in the loss of semen to the surrounding environment.
Corrections have been made to Discussion, Biological Reactions Following Ovaprim® Administration in Sharks, 1st paragraph:
For seasonally breeding sharks, natural ovarian follicular maturation occurs gradually throughout the year concomitant with a gradual increase in follicular diameter (Sulikowski et al., 2007; George et al., 2017). Therefore, the follicular maturation that we observed over 1 or 2 days in the current study is not a natural phenomenon. We found that follicular diameters increased by more than 1.5–2.0 cm within only 48 h in response to the administration of Ovaprim®, thereby confirming that this salmon gonadotropin-releasing hormone can effectively induce follicular maturation in both T. scyllium and T. obesus.
Corrections have been made to Discussion, Necessity and Possibility of Ovaprim® Usage for Hormone-Induced Artificial Insemination in Wild Shark Conservation, 6th paragraph:
As a proactive step toward the development of a hormone-induced artificial insemination protocol for endangered wild sharks, we used Ovaprim® to induce germ cell maturation and established suitable dosages and an injection protocol. We found that a single 0.2 mL/kg Ovaprim® dose induced semen release in the males of both study species and that the administration of two 0.2 mL/kg + 0.5 mL/kg doses (at a 24 h interval) in T. scyllium, and 0.2 mL/kg + 0.2 mL/kg or 0.3 mL/kg (at a 24 h interval) in T. obesus females induced follicular maturation without egg dropping. Accordingly, given that the basic conditions for hormone-induced artificial insemination can be established using Ovaprim®, we can reasonably expect that hormone-induced reproduction can be achieved in sharks. Compared with the development of hormone-induced artificial insemination technology in other fish species, the research reported herein is in its infancy; however, the findings of the present study, which to the best of our knowledge, is the first to have demonstrated successful hormone-induced reproduction in sharks, can serve to guide the direction of further related studies (Nandeesha et al., 1990; Khan et al., 2006; Brzoska and Adamek, 2008; Olumuji and Mustapha, 2012; Dhara and Saha, 2013). Considering the importance of hormone-induced reproduction techniques in the ex-situ conservation of wild sharks, we believe that the findings of the present study will make a potentially important contribution to the conservation of endangered shark species.
Corrections have been made to Discussion, Study Limitations and Future Perspectives, 2nd paragraph:
Nevertheless, even given such limitations, we believe that the findings of the present study provide a clear direction for further research on shark hormone-induced artificial insemination. Ovaprim® was found to induce biological reactions in the shark, and as expected, the induced reactions were confirmed to be related to germ cell maturation and release. We were able to establish a general dose range and determined an appropriate administration protocol, and although fewer experiments were carried out for T. obesus than for T. scyllium, the desired biological responses could be induced, thereby indicating that the protocol developed in this study has potentially broad applicability in sharks.
In the original article, there was a mistake in the legends for Table 1, Figure 3, Figure 4, Figure 5, Supplementary Table S1, and Supplementary Table S2 as published. We have used the whitetip reef shark (Triaenodon obesus) for this study but wrote the species' name incorrectly as the Oceanic whitetip shark (Carcharhinus longimanus). The correct legends appear below.
Table 1. Hormone concentrations of Triakis scyllium and Triaenodon obesus prior to Ovaprim® administration.
Figure 3. Blood hormone level changes after Ovaprim® injection in Triakis scyllium and Triaenodon obesus. (A) average hormone concentration changes in male T. scyllium. Ovaprim® was injected at 0.2 mL/kg dose at the orange arrowed time point. (B) Average hormone concentration changes of female T. scyllium. Ovaprim® was injected at 0.6 mL/kg dose at the orange arrowed time points. (C) Average hormone concentration changes of T. scyllium (Female #3; F003). Ovaprim® was injected at 0.6 mL/kg dose at the orange arrowed time point. (D) Average hormone concentration changes of male T. obesus. Ovaprim® was injected at 0.2 mL/kg dose at the orange arrowed time point. (E) Average hormone concentration changes in female T. obesus. Ovaprim® was injected at 0.2 mL/kg and 0.3 mL/kg doses in serial at the orange arrowed time points.
Figure 4. Ovarian follicle size change after Ovaprim® injection in Triakis scyllium and Triaenodon obesus. (A) Ovarian follicle size changes after Ovaprim® injection in female T. scyllium. Axis length and area values were measured directly on ultrasonography. Eccentricity was calculated with the long and short axis values. The orange arrows indicate the time points of Ovaprim® administration. (B) Ultrasonography of left ovary before Ovaprim® injection in T. scyllium (F003). The orange arrows indicate the same follicle in (C) at different time point. Size of the follicle with the orange arrow was 3.7 cm × 1.9 cm (5.45 cm2). (C) Ultrasonography of left ovary after Ovaprim® injection in T. scyllium (F003). Follicles showed distinct size increase. The orange arrows indicate the same follicle in (B) at different time point. Size of the follicle with the orange arrow was 4.9 cm × 2.0 cm (7.60 cm2). (D) Ovarian follicle size changes after Ovaprim® injection in female T. obesus. Axis length and area values were measured directly on ultrasonography. Eccentricity was calculated with the long and short axis values. The orange arrows indicate the time points of Ovaprim® injection. (E) Ultrasonography of left ovary before Ovaprim® injection in WF002 (T. obesus). The orange arrows indicate the same follicle in (F) at different time point. Size change of the follicles can be recognized by ultrasonography. Size of the follicle with the orange arrow was 4.4 cm × 3.0 cm (10.27 cm2). (F) Ultrasonography of left ovary after Ovaprim® injection in WF002 (T. obesus). The orange arrows indicate the same follicle in (E) at different time point. Size of the follicle with the orange arrow was 7.4 cm × 3.7 cm (21.77 cm2).
Figure 5. Schematic graph of dose optimization in Triakis scyllium and Triaenodon obesus. A conceptual graph showing the correlation between biological phenomena (x-axis) and artificial insemination suitability (y-axis) that change depending on the total dose of Ovaprim® administered. The shaded area denotes mature oocytes after follicle growth but prior to egg dropping, which is the targeted area for females in this study. (A) Ovaprim® experimental doses for female T. scyllium. TS①: 0.6 mL/kg + 0.6 mL/kg, 6 h time gap; TS②: 0.2 mL/kg + 0.4 mL/kg, 12 h time gap; TS③: 0.2 mL/kg + 0.6 mL/kg, 24 h time gap; TS④: 0.2 mL/kg + 0.5 mL/kg, 24 h time gap. (B) Ovaprim® experimental doses for female T. obesus. CL①: 0.2 mL/kg + 0.2 mL/kg, 24 h time gap; CL②: 0.2 mL/kg + 0.3 mL/kg, 24 h time gap.
Supplementary Table S1. Body measurement traits of Triakis scyllium and Triaenodon obesus. C: calcified; NC: not calcified; E: elongated, longer than pelvic fin; NE: not elongated, not longer than pelvic fin; B: bending; NB: not bending; R: rhipidion formed; RN: rhipidion not formed.
Supplementary Table S2. Average blood test results of Triakis scyllium and Triaenodon obesus. CBC: complete blood count; WBC: white blood cell; RBC: red blood cell; mPCV: manual packed cell volume; BUN: blood urea nitrogen; ALKP: alkaline phosphatase; AST: aspartate transaminase; ALT: alanine transferase; GGT: gamma-glutamyl transferase; CK: creatinine kinase; LDH: lactate dehydrogenase; TCO2: total CO2; T: temperature; NT: not tested.
In the original article, there was a mistake in Table 1, Figure 3, Figure 4, Figure 5, Supplementary Table S1, and Supplementary Table S2 as published. We have used the whitetip reef shark (Triaenodon obesus) for this study but wrote the species' name incorrectly as the Oceanic whitetip shark (Carcharhinus longimanus). The corrected Table and Figures appears below. The corrected Supplementary Tables have been published.
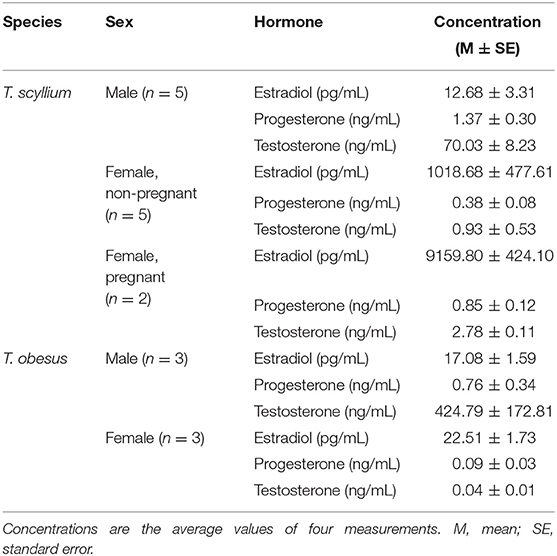
Table 1. Hormone concentrations of Triakis scyllium and Triaenodon obesus prior to Ovaprim® administration.
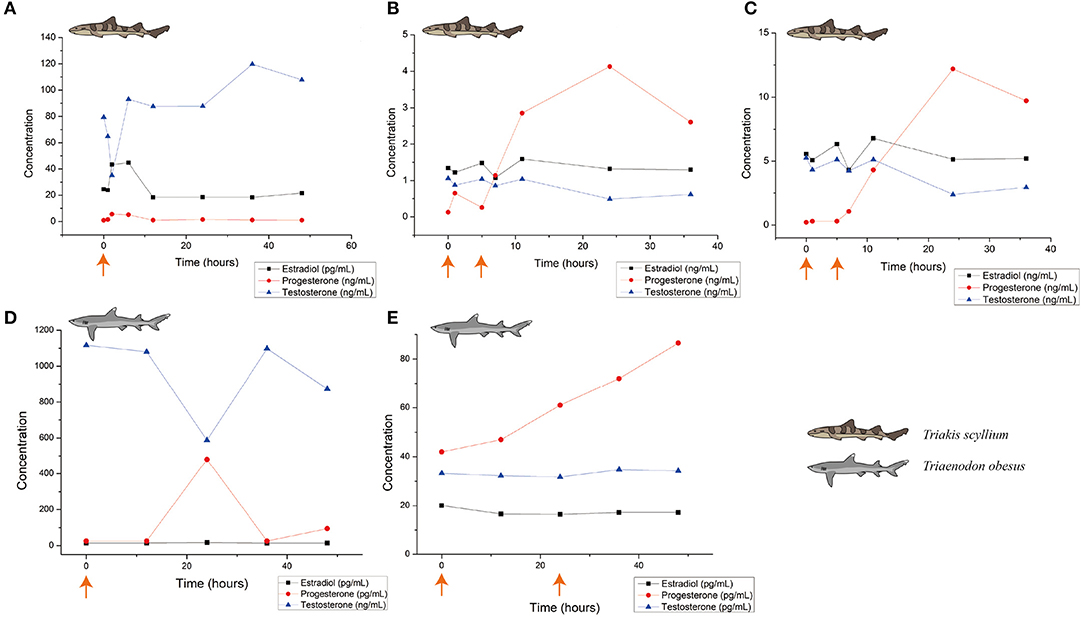
Figure 3. Blood hormone level changes after Ovaprim® injection in Triakis scyllium and Triaenodon obesus. (A) average hormone concentration changes in male T. scyllium. Ovaprim® was injected at 0.2 mL/kg dose at the orange arrowed time point. (B) Average hormone concentration changes of female T. scyllium. Ovaprim® was injected at 0.6 mL/kg dose at the orange arrowed time points. (C) Average hormone concentration changes of T. scyllium (Female #3; F003). Ovaprim® was injected at 0.6 mL/kg dose at the orange arrowed time point. (D) Average hormone concentration changes of male T. obesus. Ovaprim® was injected at 0.2 mL/kg dose at the orange arrowed time point. (E) Average hormone concentration changes in female T. obesus. Ovaprim® was injected at 0.2 mL/kg and 0.3 mL/kg doses in serial at the orange arrowed time points.
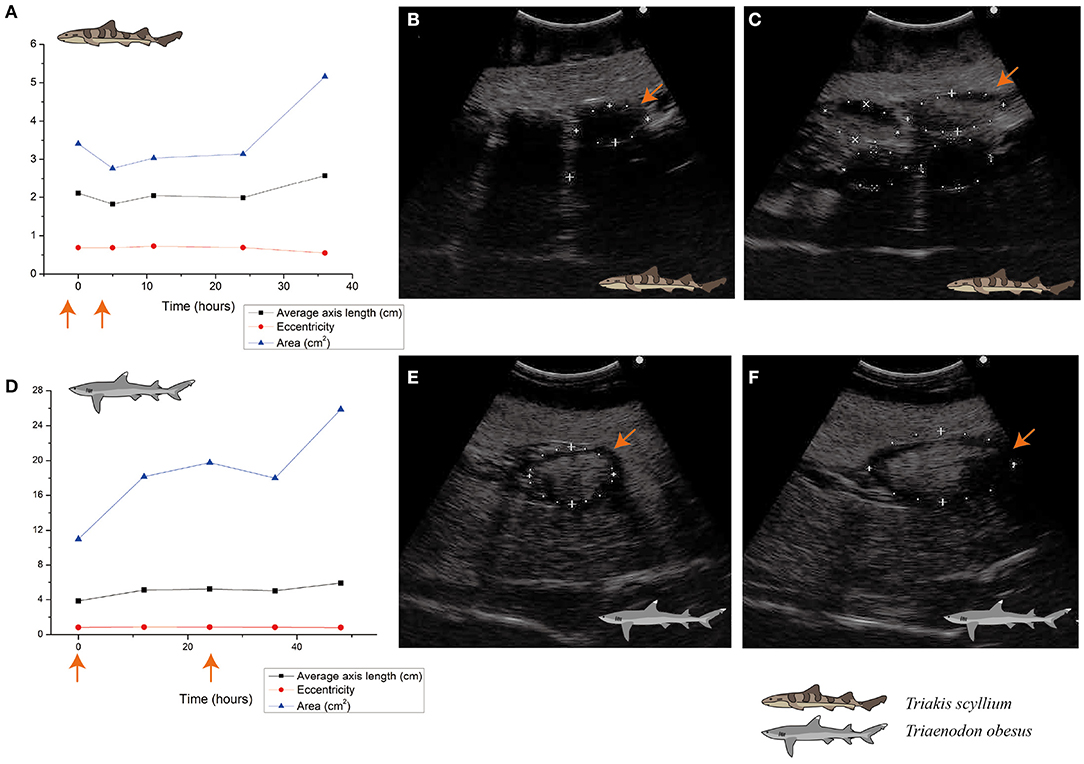
Figure 4. Ovarian follicle size change after Ovaprim® injection in Triakis scyllium and Triaenodon obesus. (A) Ovarian follicle size changes after Ovaprim® injection in female T. scyllium. Axis length and area values were measured directly on ultrasonography. Eccentricity was calculated with the long and short axis values. The orange arrows indicate the time points of Ovaprim® administration. (B) Ultrasonography of left ovary before Ovaprim® injection in T. scyllium (F003). The orange arrows indicate the same follicle in (C) at different time point. Size of the follicle with the orange arrow was 3.7 cm × 1.9 cm (5.45 cm2). (C) Ultrasonography of left ovary after Ovaprim® injection in T. scyllium (F003). Follicles showed distinct size increase. The orange arrows indicate the same follicle in (B) at different time point. Size of the follicle with the orange arrow was 4.9 cm × 2.0 cm (7.60 cm2). (D) Ovarian follicle size changes after Ovaprim® injection in female T. obesus. Axis length and area values were measured directly on ultrasonography. Eccentricity was calculated with the long and short axis values. The orange arrows indicate the time points of Ovaprim® injection. (E) Ultrasonography of left ovary before Ovaprim® injection in WF002 (T. obesus). The orange arrows indicate the same follicle in (F) at different time point. Size change of the follicles can be recognized by ultrasonography. Size of the follicle with the orange arrow was 4.4 cm × 3.0 cm (10.27 cm2). (F) Ultrasonography of left ovary after Ovaprim® injection in WF002 (T. obesus). The orange arrows indicate the same follicle in (E) at different time point. Size of the follicle with the orange arrow was 7.4 cm × 3.7 cm (21.77 cm2).
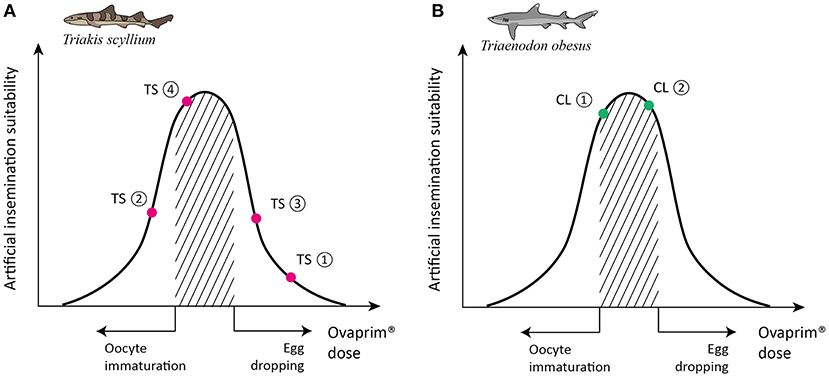
Figure 5. Schematic graph of dose optimization in Triakis scyllium and Triaenodon obesus. A conceptual graph showing the correlation between biological phenomena (x-axis) and artificial insemination suitability (y-axis) that change depending on the total dose of Ovaprim® administered. The shaded area denotes mature oocytes after follicle growth but prior to egg dropping, which is the targeted area for females in this study. (A) Ovaprim® experimental doses for female T. scyllium. TS①: 0.6 mL/kg + 0.6 mL/kg, 6 h time gap; TS②: 0.2 mL/kg + 0.4 mL/kg, 12 h time gap; TS③: 0.2 mL/kg + 0.6 mL/kg, 24 h time gap; TS④: 0.2 mL/kg + 0.5 mL/kg, 24 h time gap. (B) Ovaprim® experimental doses for female T. obesus. CL①: 0.2 mL/kg + 0.2 mL/kg, 24 h time gap; CL②: 0.2 mL/kg + 0.3 mL/kg, 24 h time gap.
In the original article, there was an error in the keywords. We have used the whitetip reef shark (Triaenodon obesus) for this study but wrote the species' name incorrectly as the Oceanic whitetip shark (Carcharhinus longimanus). Corrections have been made to the Keywords: “Carcharhinus longimanus” has been updated to “Triaenodon obesus”.
The authors apologize for these errors and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.638335/full#supplementary-material
Footnotes
1. ^U.S.FDA. FOI Summary Ovaprim. https://www.fda.gov/animal-veterinary/minor-useminor-species/foi-summary-ovaprim (accessed September 9, 2020).
2. ^IUCN Red List of Threatened Species 2019. Banded houndshark (Triakis scyllium). https://www.iucnredlist.org/species/161395/5413845 (accessed March 16, 2020).
3. ^IUCN Red List of Threatened Species 2019. Whitetip reef shark (Triaenodon obesus). https://www.iucnredlist.org/species/39384/10188990 (accessed March 16, 2020).
References
Brzoska, E., and Adamek, J. (2008). Artificial spawning of European catfish, Silurus glanis L.: stimulation of ovulation using LHRH-a, Ovaprim and carp pituitary extract. Aquac. Res. 30, 59–64. doi: 10.1046/j.1365-2109.1999.00301.x
Castro, J. I. (2009). Observations on the reproductive cycles of some viviparous North American sharks. Aqua, Int. J. Ichthyol. 15, 4–15.
Compagno, L. J. V. (1984). Sharks of the World: An Annotated and Illustrated Catalogue of Shark Species Known to Date, Vol. 4, FAO Species Catalogue. Rome: FAO, 432.
Dhara, K., and Saha, N. C. (2013). Controlled breeding of Asian catfish Clarias batrachus using pituitary gland extracts and Ovaprim at different temperatures, latency periods and their early development. J. Aquac. Res. Dev. 4:186. doi: 10.4172/2155-9546.1000186
George, R. H., Steeil, J., and Baine, K. (2017). “Diagnosis and treatment of common reproductive problems in elasmobranchs,” in The Elsmobranch Husbandry Manual II: Recent Advances in the Care of Sharks, Rays and their Relatives, eds M. Smith, D. Warmolts, D. Thoney, R. Hueter, M. Murray, and J. Ezcurra (Columbus, OH: The Ohio State University Printing Servicies), 363–374.
Khan, A. M., Shakir, H. A., Ashraf, M., and Ahmad, Z. (2006). Induced spawning of Labeo rohita using synthetic hormones. Punjab Univ. J. Zool. 21, 67–72.
Nandeesha, M. C., Rao, K. G., Jayanna, R. N., Parker, N. C., Varghese, T. J., Keshavanath, P., et al. (1990). “Induced spawning of Indian major carps through single application of Ovaprim-C,” in The Second Asian Fisheries Forum, eds R. Hirano and I. Hanvu (Manila: Asian Fisheries Society), 581–585.
Olumuji, O. K., and Mustapha, M. K. (2012). Induced breeding of African mud catfish, Clarias gariepinus (Burchell 1822), using different doses of normal saline diluted Ovaprim. J. Aquac. Res. Dev. 3:133. doi: 10.4172/2155-9546.1000133
Schaller, P. (2006). Husbandry and reproduction of Whitetip reef sharks at Steinhart Aquarium. San Francisco. Int. Zoo Yearb 40, 232–240. doi: 10.1111/j.1748-1090.2006.00232.x
Sulikowski, J. A., Driggers, W. B. III., Ford, T. S., Boonstra, R. K., and Carlson, J. K. (2007). Reproductive cycle of the blacknose shark Carcharhinus acronotus in the Gulf of Mexico. J. Fish Biol. 70, 428–440. doi: 10.1111/j.1095-8649.2007.01314.x
Keywords: Triakis scyllium, Triaenodon obesus, synthetic salmon GnRH, Ovaprim, hormone-induced artificial insemination
Citation: Kim SW, Hong WH, Han SJ, Kwon J, Ko H, Lee SB, Giri SS, Kim SG, Kim BY, Jang G, Lee BC, Kim DW and Park SC (2021) Corrigendum: Use of Synthetic Salmon GnRH and Domperidone (Ovaprim®) in Sharks: Preparation for ex situ Conservation. Front. Mar. Sci. 7:638335. doi: 10.3389/fmars.2020.638335
Received: 06 December 2020; Accepted: 16 December 2020;
Published: 22 January 2021.
Edited and reviewed by: Bahram Falahatkar, University of Guilan, Iran
Copyright © 2021 Kim, Hong, Han, Kwon, Ko, Lee, Giri, Kim, Kim, Jang, Lee, Kim and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Se Chang Park, cGFya3NlYyYjeDAwMDQwO3NudS5hYy5rcg==
†These authors have contributed equally to this work
 Sang Wha Kim
Sang Wha Kim Won Hee Hong1,2†
Won Hee Hong1,2† Sib Sankar Giri
Sib Sankar Giri Goo Jang
Goo Jang Byeong Chun Lee
Byeong Chun Lee Se Chang Park
Se Chang Park