- 1Laboratory of Protozoology, Institute of Evolution and Marine Biodiversity, Ocean University of China, Qingdao, China
- 2Zoology Department, College of Science, King Saud University, Riyadh, Saudi Arabia
- 3Department of Life Sciences, Natural History Museum, London, United Kingdom
The morphology of four trachelocercid ciliates, Paratrachelocerca typica gen. nov., spec. nov., Trachelolophos monocaryon (Dragesco, 1965) comb. nov. (original combination: Tracheloraphis monocaryon Dragesco, 1965), Tracheloraphis katzae spec. nov., and Tracheloraphis colubis (Kahl, 1933) Xu et al., 2011 were studied in live and protargol-stained specimens. All samples were isolated from the intertidal zone of sandy beaches at Qingdao, China. The new genus Paratrachelocerca can be distinguished from other trachelocercid genera mainly by the three circumoral kineties each composed of a row of dikinetids and the absence of a brosse or ciliary tuft in the oral cavity. The detailed investigation on the poorly described Tracheloraphis monocaryon (Dragesco, 1965) reveals that its oral infraciliature includes one uninterrupted circumoral kinety and a conspicuous ciliary tuft in the center of the oral cavity, which is consistent with the genus Trachelolophos rather than Tracheloraphis. Therefore, this species is transferred to Trachelolophos as Trachelolophos monocaryon (Dragesco, 1965) comb. nov. Tracheloraphis katzae spec. nov. can be recognized by the combination of its minute brownish cortical granules and 9–15 somatic kineties. The small subunit (SSU) rDNA of each species was sequenced for the first time. Phylogenetic analyses of the SSU rDNA show that Paratrachelocerca typica gen. nov., spec. nov. clusters with Apotrachelocerca arenicola (Kahl, 1933) Xu et al., 2011 in a group that is sister to all other trachelocercids.
Introduction
The family Trachelocercidae Kent, 1881 is the largest family within the class Karyorelictea Corliss, 1974 and all its members inhabit marine sandy sediments in intertidal zones (Al-Rasheid, 1996, 1997, 1998, 2001; Foissner and Dragesco, 1996a; Al-Rasheid and Foissner, 1999; Song et al., 2009; Hu et al., 2019). Since the first species was described over 200 years ago, about 80 nominal species of trachelocercids have been reported (Carey, 1992; Xu et al., 2011a, 2014; Yan et al., 2015, 2016). However, only a limited number of detailed studies of trachelocercids have been carried out using modern methods such as protargol staining, scanning electron microscopy and phylogeny analyses (Foissner and Dragesco, 1996a,b; Dragesco, 1997, 1999; Foissner, 1997; Xu et al., 2012; Yan et al., 2019) and most species are known only from live observations (Dragesco, 1960; Raikov et al., 1975; Wilbert, 1986; Carey, 1992). Moreover, the molecular data of trachelocercids remain scarce.
In the present study, the morphological data of four trachelocercids isolated from marine coastal habitats at Qingdao, China, are documented based on observations of specimens in vivo and following protargol staining. In addition, the SSU rDNA sequence of each species is provided and phylogenetic analyses are performed to assess their evolutionary relationships. Paratrachelocerca gen. nov. is assigned to the family Trachelocercidae based on both morphological and molecular information.
Materials and Methods
Sample Collection, Observation, and Identification
Paratrachelocerca typica gen. nov., spec. nov. and Tracheloraphis colubis (Kahl, 1933) Xu et al., 2011 were collected from the intertidal zone of Silver Beach, Qingdao (35°55′09″N, 120°11′55″E) on March 11, 2013 and May 27, 2019, respectively. The water temperature on each day of sampling was 11 and 23°C, respectively, and the salinity was about 27‰. Trachelolophos monocaryon (Dragesco, 1965) comb. nov. and Tracheloraphis katzae spec. nov. were both collected on June 24, 2019, from the intertidal zone of the No. 1 Bathing beach, Qingdao (36°03′24″N, 120°20′32″E) where the water temperature was 26°C and the salinity was about 30‰ (Figure 1). For each sample, the top 5 cm of sand or sediment along with seawater from the site was collected. Ciliates were extracted from the sediment using the method from Uhlig (1968). In brief, the sand or sediment was placed in a plastic tube (4.5 cm diameter and 10 cm long, at one end of which was a tightly fitting nylon gauze (mesh size 80–90 μm)). The depth of the sediment in the tube was 5 cm. Finely crushed ice was added to fill the remainder of the tube. A glass culture dish containing about 20 ml filtered seawater was placed under the tube so that the nylon gauze was barely in contact with the seawater surface. Ciliates in the sand/sediment sample migrating downwards to escape the advancing front of meltwater were collected in the culture dish.
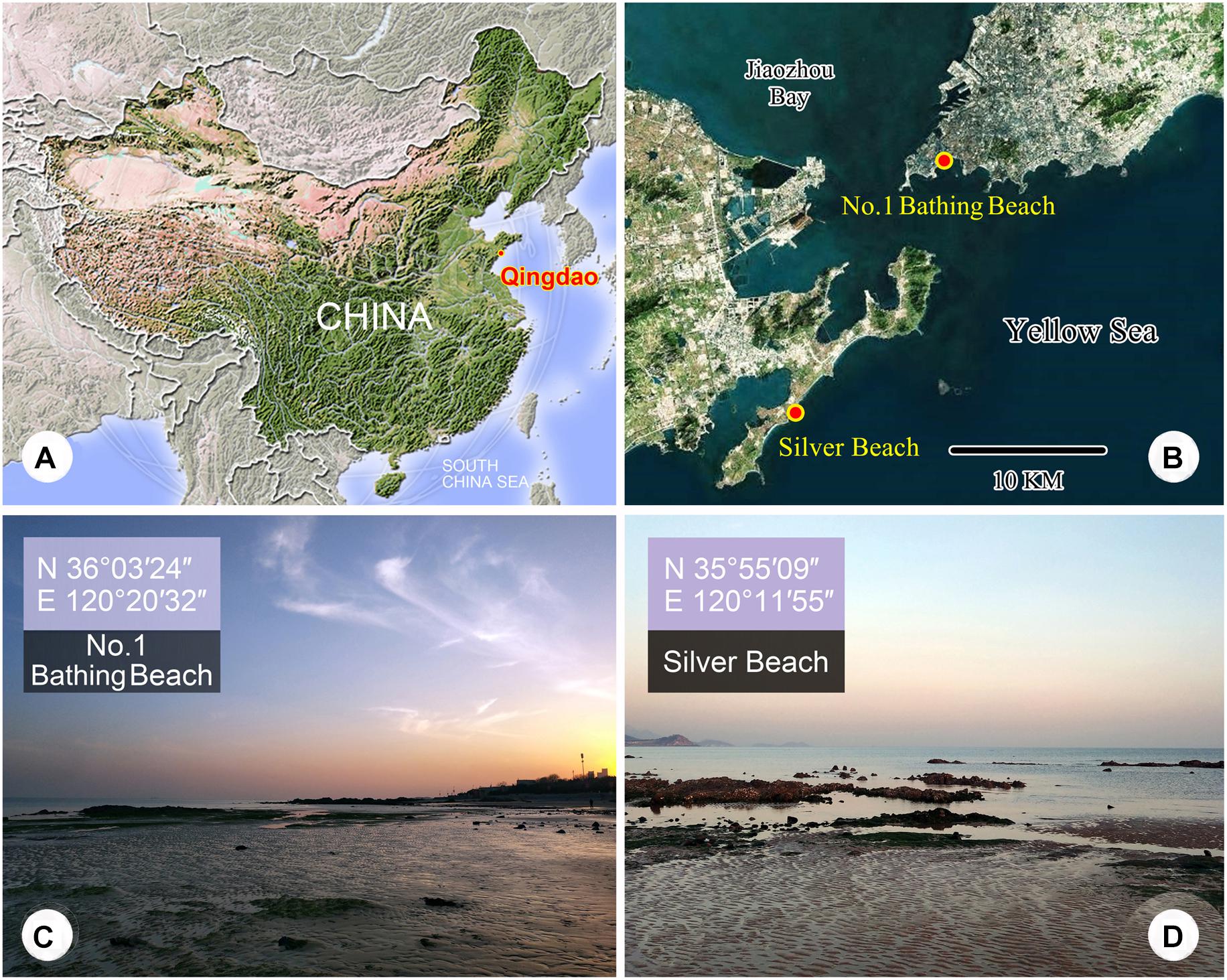
Figure 1. (A–D) Maps and photographs of the sample sites. (A) Location of Qingdao. (B) The dots indicates the locations of the two sample sites. (C) The intertidal zone of the No. 1 Bathing Beach. (D) The intertidal zone of Silver Beach.
Cells were isolated and observed in vivo using bright field and differential interference contrast (DIC) microscopy (Olympus BX 53). The infraciliature was revealed using the protargol staining method (Wilbert, 1975). Counts, measurements, and drawings of stained specimens were performed at 1,000 × magnification. Terminology and systematics mainly follow Foissner (1996) and Lynn (2008), respectively.
DNA Extraction, PCR Amplification, and Gene Sequencing
DNA extraction, PCR amplification, and SSU rDNA sequencing of the four species were performed according to Wang et al. (2020). For each species, we extracted total genomic DNA from single cells using the DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany). The primers 82F (5′-GAA ACT GCG AAT GGC TC-3′) (Jerome et al., 1996), 18S-F (5′-AAC CTG GTT GAT CCT GCC AGT-3′), and 18S-R (5′-TGA TCC TTC TGC AGG TTC ACC TAC-3′) were used to amplify the SSU rDNA (Medlin et al., 1988). PCR amplification was carried out according to Wang et al. (2019, 2020) and PCR products were then sequenced bidirectionally by the Tsingke Biological Technology Company (Beijing, China).
Phylogenetic Analyses
Using MAFFT implemented in GUIDANCE1 with default parameters (Penn et al., 2010), the newly generated SSU rDNA sequences of the four species were aligned with 54 other sequences of karyorelictean and heterotrich (outgroup) species downloaded from NCBI GenBank. The resulting alignments were manually refined by trimming both ends with Bioedit v.7.0.5 (Hall, 1999) and the final alignments were 1,802 bp.
Maximum likelihood (ML) and Bayesian inference (BI) analyses were both carried out using the CIPRES Science Gateway v.3.32 (Stamatakis, 2014). ML bootstrapping analysis was performed online with 1,000 replicates using RAxML-HPC2 on XSEDE v.8.2.12 (Stamatakis et al., 2008) and the GTRGAMMA model. BI analysis was performed with MrBayes on XSEDE v.3.2.7a (Ronquist et al., 2012) using the GTR + I + G model selected by MrModeltest v.2.2 (Nylander, 2004). The chain length for our analysis was 1,000,000 generations with trees sampled every 100 generations, the first 25% of which were discarded as burn-in. MEGA v.5.0 (Tamura et al., 2011) was used to visualize the phylogenetic tree topology.
Topology Testing
The phylogenetic relationships among different taxa within Karyorelictea were assessed using the approximately unbiased (AU) test (Shimodaira, 2002). Two constrained ML trees were generated by RAxML v8.2.10 (Stamatakis, 2014) with the enforced constraints (Table 1) and their topologies were compared with those of the best unconstrained ML trees implemented in CONSEL (Shimodaira and Hasegawa, 2001). Internal relationships in the constrained group and among the remaining taxa were unspecified.
ZooBank Registration
ZooBank registration number of present work: urn:lsid:zoobank.org:pub:913C263C-56A7-40AC-9885-E06DC530A6D8.
Results and Discussion
Class Karyorelictea Corliss, 1974
Order Protostomatida Small & Lynn, 1985
Family Trachelocercidae Kent, 1881
Genus Paratrachelocerca gen. nov.
Diagnosis
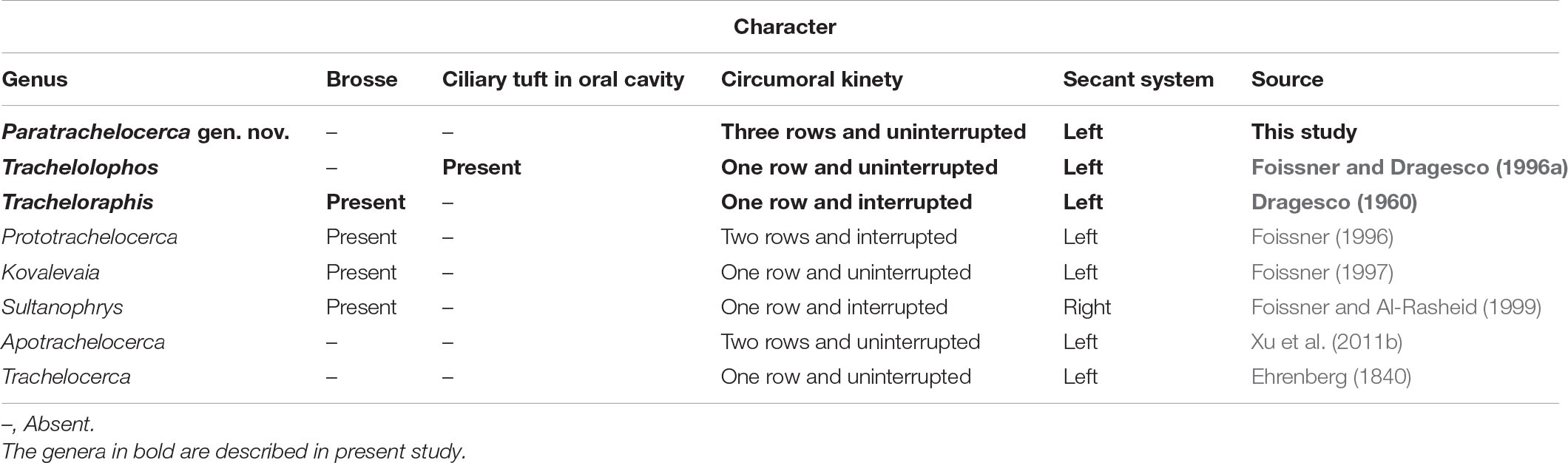
Trachelocercidae with three circumoral kineties each composed of a row of dikinetids. No brosse or ciliary tuft in oral cavity. Marine habitat.
Etymology
The new genus name is dedicated to the eminent ciliatologist, Prof. Wilhelm Foissner, Universität Salzburg, Austria, in recognition of his significant contributions to the study of ciliates.
Type Species
Paratrachelocerca typica spec. nov.
Discussion
Paratrachelocerca gen. nov. possesses all the diagnostic characters of trachelocercids, i.e., elongate body shape with distinct “head” and “neck” and somatic ciliature covering the body apart from longitudinal glabrous zone that is bordered by a “bristle-like” kinety. Therefore, Paratrachelocerca gen. nov. undoubtedly belongs to the family Trachelocercidae. The shape and structure of the oral ciliature is the main character for generic classification (Foissner and Dragesco, 1996a,b; Foissner and Al-Rasheid, 1999). Paratrachelocerca gen. nov. possesses three circumoral kineties each composed of a row of dikinetids. Consequently, it can be easily separated from its most closely related genera such as Apotrachelocerca Xu et al., 2011, which has two rows of uninterrupted circumoral kineties, Trachelocerca Ehrenberg, 1840, which has a single uninterrupted circumoral kinety composed of dikinetids, and Prototrachelocerca Foissner, 1996 has two rows of circumoral kineties interrupted by short brosse kineties (Table 2). It is noteworthy that no anterior or posterior secant system is present in Paratrachelocerca typica gen. nov. spec. nov., which is similar to Apotrachelocerca arenicola (Kahl, 1933) Xu et al., 2011. However, the presence/absence of the anterior or posterior secant system is not considered as a genus-level character for trachelocercid classification. Paratrachelocerca typica spec. nov. (Figures 2, 3 and Table 3)
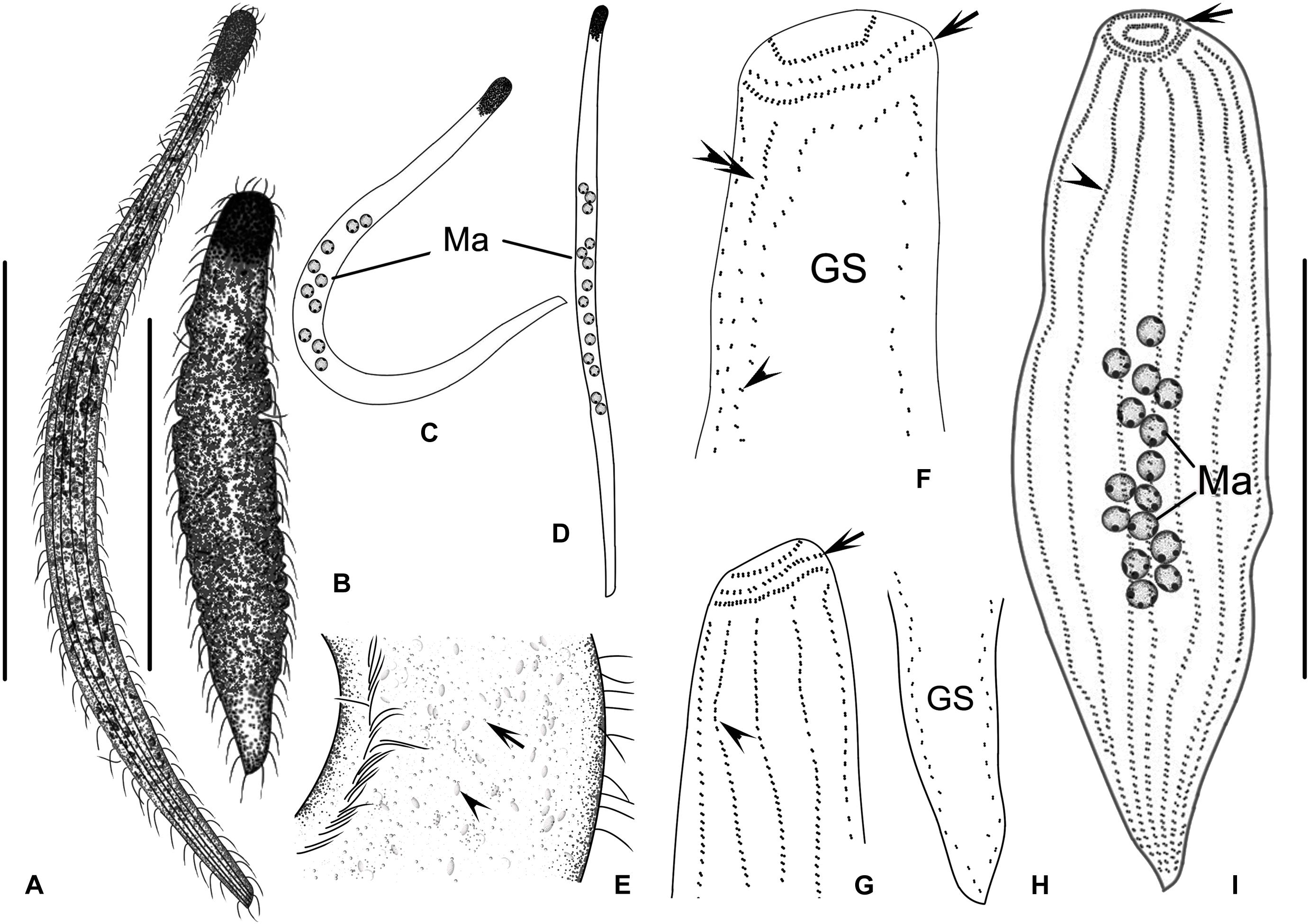
Figure 2. (A–I) Paratrachelocerca typica gen. nov., spec. nov. in vivo (A–E) and after protargol staining (F–I). (A) Typical individual. (B) An extremely contracted cell. (C–D) Different body shapes. (E) Distribution of cortical granules in glabrous stripe (arrow). Arrowhead points to the granules. (F,G) Detailed ciliature of left (F) and right (G) side of anterior body region, showing three circumoral kineties each composed of a row of dikinetids (arrow), glabrous stripe, somatic kineties (double arrowheads in F and arrowhead in G) and bristle kinety (arrowhead in F). (H) Ciliary pattern of tail region showing glabrous stripe. (I) Overview showing ciliary pattern and multiple macronuclei of holotype specimen, three circumoral kineties each composed of a row of dikinetids (arrow) and somatic kineties (arrowhead). GS, glabrous stripe; Ma, macronuclei. Scale bars = 300 μm (A,B); 80 μm (I).
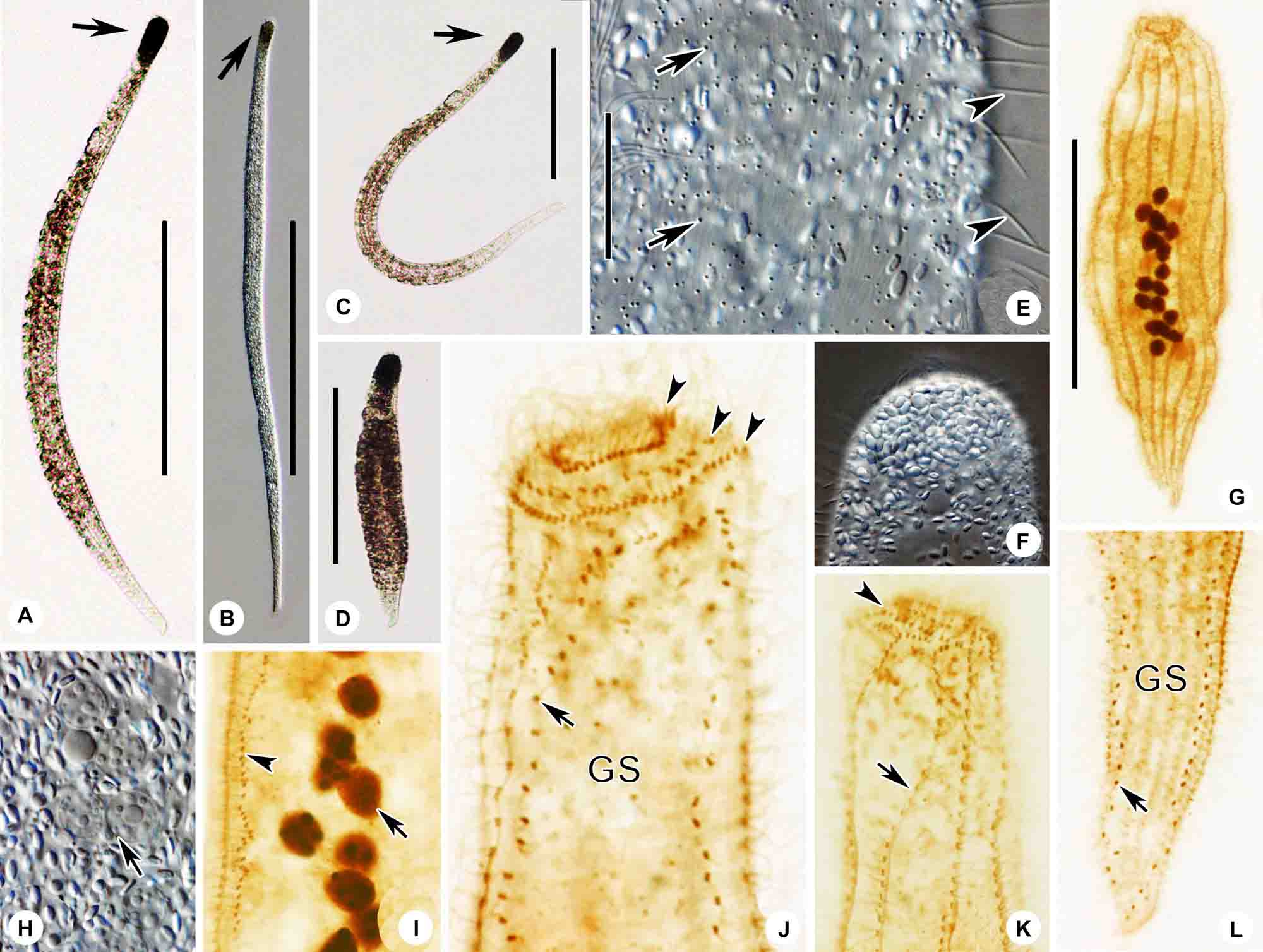
Figure 3. (A–I) Paratrachelocerca typica gen. nov., spec. nov. in vivo (A–F,H) and after protargol staining (G,I–L). (A–C) Typical individuals. Arrow showing the head of the cell. (D) An extremely contracted cell. (E) Distribution of cortical granules in glabrous stripe (arrows) and spine-like bristle cilia (arrowheads). (F) Showing head of cell packed with ellipsoidal granules. (G) Right side view of the holotype specimen showing the ciliary pattern and multiple macronuclei. (H) Showing globular macronuclei. (I) Left margin of mid-body showing macronucleus (arrow) and bristle kinety (arrowhead). (J,K) Detailed ciliature of left (J) and right (K) side of anterior body region, three circumoral kineties each composed of a row of dikinetids (arrowheads), glabrous stripe, somatic kineties (arrow in K), and bristle kinety (arrow in J). (L) Ciliary pattern of tail region showing glabrous stripe and bristle kinety (arrow). GS, glabrous stripe. Scale bars = 300 μm (A–D); 30 μm (E); 80 μm (G).
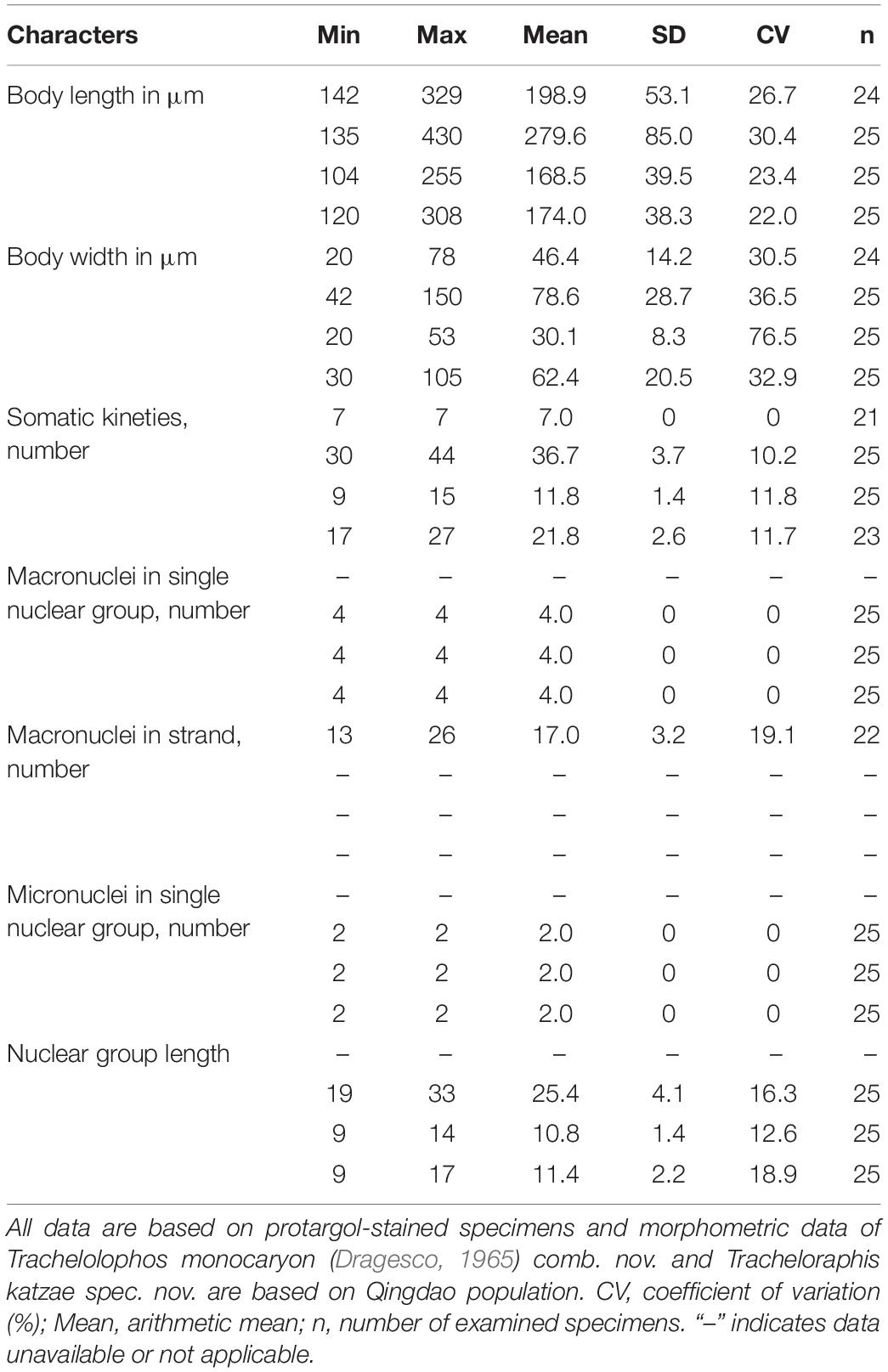
Table 3. Morphometric data for Paratrachelocerca typica gen. nov., spec. nov. (first line), Trachelolophos monocaryon (Dragesco, 1965) comb. nov. (second line), Tracheloraphis katzae spec. nov. (third line), and Tracheloraphis colubis (fourth line).
Diagnosis
Extended cells in vivo about 400–800 μm × 15–25 μm; body ribbon-like and flattened; head dominant and black in color, distinguished from trunk; usually 13–24 macronuclei; 7 ciliary rows on right side of cell; left side unciliated except for bristle kinety; glabrous stripe as wide as trunk; no anterior or posterior secant system on either side of glabrous stripe; cortical granules minute and colorless.
Type Locality
The intertidal zone of Silver Beach, Qingdao (35°55′09″N, 120°11′55″E), China (Figure 1).
Type Specimens
A protargol-stained slide containing the holotype specimen marked with an ink circle is deposited in the Laboratory of Protozoology, Ocean University of China, Qingdao, China (No. YY2013031107).
Etymology
This species-group name typica means that it is the representative species of this genus.
Morphological Description
Cells in vivo 400–800 μm × 15–25 μm, flexible and contractile (Figures 2A,B, 3A–D); ribbon-like flattened (up to 3:1) including oral area (Figures 2A,C,D, 3A–C). Width almost constant throughout anterior three-quarters of cell; head region dominant; no distinct neck; posterior quarter of body narrowed (Figures 2A,C,D, 3A–C). At low magnification, anterior of cell often dark grayish due to multiple inclusions (Figure 3F). Cortical granules colorless, about 0.5 μm in diameter, sparsely scattered between ciliary rows and in glabrous stripe (Figures 2E, 3E). Thirteen to 26 macronuclei, 6–9 μm in diameter, arranged in a longitudinally oriented group in mid-body region (Figures 2C,D, 3G and Table 3). Macronuclei containing many large chromatin aggregates, possibly nucleoli (Figures 2I, 3H,I).
Locomotion by gliding sluggishly along bottom of Petri dish.
Infraciliature consists of dikinetids (Figure 2F–I). Only right side ciliated with seven somatic kineties. Left side occupied by glabrous stripe. Cilia about 8 μm long (Figure 3G). No anterior or posterior secant system on either side of glabrous stripe (Figures 2F,H, 3I,J,L). Glabrous stripe bordered by a bristle kinety composed of one row of dikinetids (Figures 2F,H, 3I,J,L). Three circumoral kineties each composed of an uninterrupted row of obliquely oriented and narrowly spaced dikinetids (Figures 2F,G, 3J,K).
Genus Trachelolophos Foissner & Dragesco, 1996
Trachelolophos monocaryon (Dragesco, 1965) comb. nov. (Figures 4, 5 and Table 3)
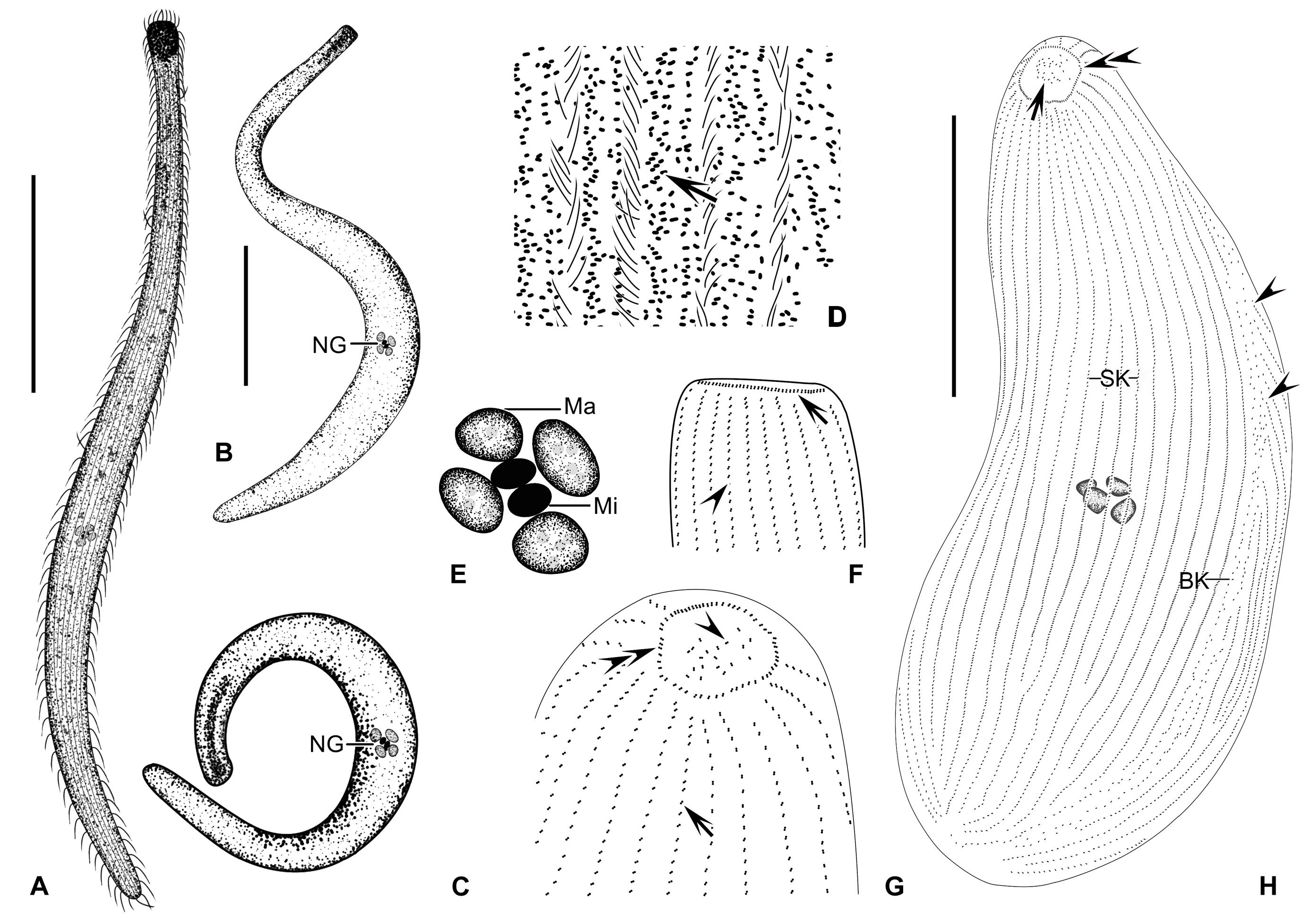
Figure 4. (A–H) Trachelolophos monocaryon (Dragesco, 1965) comb. nov. in vivo (A–D) and after protargol staining (E–H). (A,B) Typical individual. (C) An extremely curved cell. (D) Distribution of cortical granules (arrow) between somatic kineties. (E) Nuclear group. (F) Lateral view of head showing circumoral kinety (arrow) and somatic kineties (arrowhead). (G) Apical view of head, showing the ciliary tuft (arrowhead), somatic kineties (arrow), and circumoral kinety (double arrowheads). (H) Overview showing the multiple macronuclei, ciliary tuft (arrow), circumoral kinety (double arrowheads), and secant system (arrowheads). BK, bristle kinety; Ma, macronuclei; Mi, micronuclei; NG, nuclear group; SK, somatic kineties. Scale bars = 200 μm (A,B); 80 μm (H).
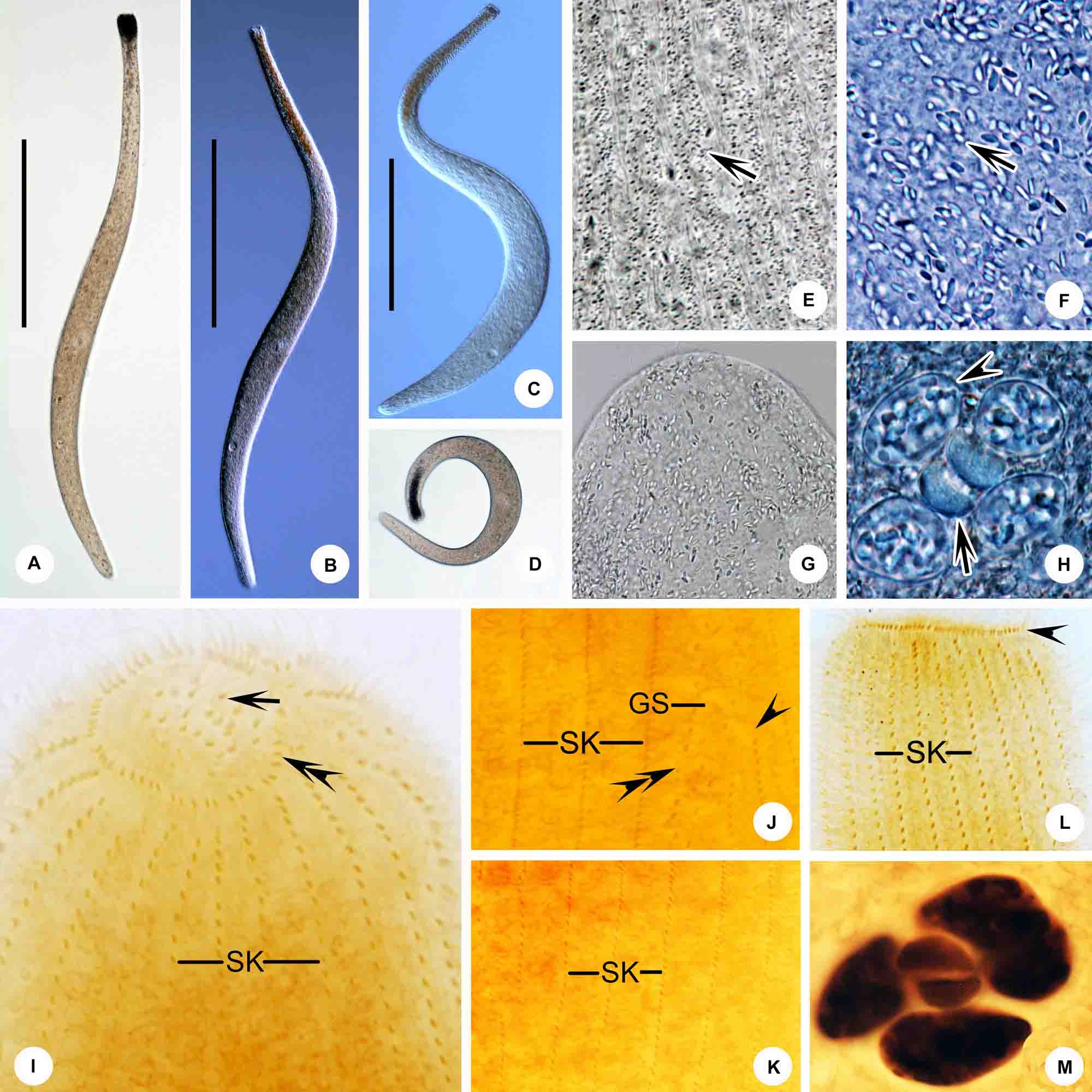
Figure 5. (A–M) Trachelolophos monocaryon (Dragesco, 1965) comb. nov. in vivo (A–H) and after protargol staining (I–M). (A–C) Typical individual. (D) An extremely curved cell. (E) Distribution of cortical granules (arrow) between somatic kineties. (F) Showing ellipsoidal granules (arrow). (G) Showing the head full of ellipsoidal granules. (H) Nuclear group, arrow showing micronuclei, arrowhead showing crystals in the macronuclei. (I) Anterior region of cell showing the ciliary tuft (arrow) and circumoral kinety (double arrowheads). (J) Detail of trunk showing the glabrous stripe with secant system on left side (arrowheads), bristle kinety (double arrowheads), and somatic kineties. (K) Showing the somatic kineties. (L) Anterior of cell. arrowhead shows circumoral kinety. (M) Nuclear group. GS, glabrous stripe; SK, somatic kineties. Scale bars = 300 μm (A–C).
(original combination: Tracheloraphis monocaryon Dragesco, 1965)
Dragesco (1965) described this species under the name of Tracheloraphis monocaryon, based solely on live observations. Thus, this form remained largely unknown for over half a century due to the lack of knowledge of the infraciliature. Observations of both live and silver-stained specimens of the present isolate indicates that this taxon should be assigned to the genus Trachelolophos. Therefore, a new combination is suggested and a redescription and improved diagnosis are supplied.
Improved Diagnosis
Extended cells 500–1,000 μm × 40–70 μm in vivo with inconspicuous dark head and a rounded posterior end. Thirty to 44 somatic kineties. Glabrous stripe narrow, about the width occupied by 2–3 somatic kineties. Four macronuclei and two micronuclei in a single group. Cortical granules grayish and ellipsoidal, ca. 0.5 μm × 0.8 μm, densely distributed.
Deposition of Voucher Materials
A voucher slide with protargol-stained specimens has been deposited in the Laboratory of Protozoology, Ocean University of China, Qingdao, China (registration number: MMZ2019062406).
Redescription
Extended cells 500–1,000 μm × 40–70 μm in vivo; body cylindrical or rod-like, flexible and contractile (Figures 4A–C, 5A–D); 30–44 somatic kineties; neck and tail indistinctly separated from trunk, head triangular, posterior end of tail rounded (Figures 4A–C,H, 5A–D and Table 3). Cortical granules ellipsoidal, ca. 0.5 μm × 0.8 μm, grayish in bright field at high magnifications; densely packed (but non-grouped) between somatic kineties and in glabrous stripe (Figures 4D, 5E). Cytoplasm colorless and transparent, packed with cytoplasmic granules, ellipsoidal, 1–3 μm long and colorless (Figures 5F,G).
Locomotion by gliding on substrate, winding between sand grains and organic debris.
Entire infraciliature consisting of dikinetids (Figures 4H, 5K). Cilia about 10 μm long in vivo and arranged in longitudinal rows. Oral infraciliature consists of a single uninterrupted circumoral kinety and a conspicuous ciliary tuft located in center of oral cavity (Figures 4F–H, 5I,L). Glabrous stripe very narrow, width about equal to the gap between two adjacent somatic kineties, bordered by irregularly spaced bristle kinety (Figures 4H, 5J). Anterior and posterior secant systems formed on left side of glabrous stripe and some kineties also abut to bristle kinety (Figures 4H, 5J). Four macronuclei (in which crystals are sometimes present) and two micronuclei in a single group (Figures 4E, 5H,M).
Discussion
This organism was first reported by Dragesco (1965) under the name Tracheloraphis monocaryon and the original description was based solely on a rather schematic figure and a short description of the cell in vivo. The Qingdao population corresponds closely with the original population in several key characters such as the narrow glabrous stripe, the single nuclear group composed of four macronuclei and two micronuclei and the possession of about 40 somatic kineties. Therefore, we consider them to be conspecific. Before transferring this species to Trachelolophos, we compare the Qingdao isolate to all four species of Trachelolophos.
The four congeners can all be clearly distinguished from the new isolate by body shape, body length, the number of somatic kineties and the number of nuclei or nuclear groups. Trachelolophos filum (Dragesco & Dragesco-Kernéis, 1986) Foissner & Dragesco, 1996 differs from the new isolate in having fewer somatic kineties on the trunk (26–35 vs. 30–44) and 4–16 nuclear groups, each with usually two macronuclei and a micronucleus, whereas the Qingdao isolate has only one nuclear group consisting of four macronuclei and two micronuclei (Dragesco and Dragesco-Kernéis, 1986; Foissner and Dragesco, 1996a). Trachelolophos gigas Foissner & Dragesco, 1996 differs from T. monocaryon in possessing a much longer body (2,000 μm vs. 500–1,000 μm), more somatic kineties on the trunk (52–71 vs. 30–44), and in the number and arrangement of its macronuclei (17–33 forming a strand vs. four in one nuclear group) (Foissner and Dragesco, 1996a). Trachelolophos binucleatus Yan et al., 2016 differs from T. monocaryon in having fewer somatic kineties on the trunk (17–26 vs. 30–44) and fewer macronuclei (2 vs. 4) (Foissner and Dragesco, 1996a). Trachelolophos quadrinucleatus Yan et al., 2016 differs from T. monocaryon in possessing a longer body (1,100–1,400 μm vs. 500–1,000 μm) and a wedge-shaped (vs. rounded) posterior body end (Yan et al., 2016). Given these distinctions, the validity of T. monokaryon as a distinct species within the genus Trachelolophos is strongly supported.
Genus Tracheloraphis Dragesco, 1960
Tracheloraphis katzae spec. nov. (Figures 6, 7 and Table 3)
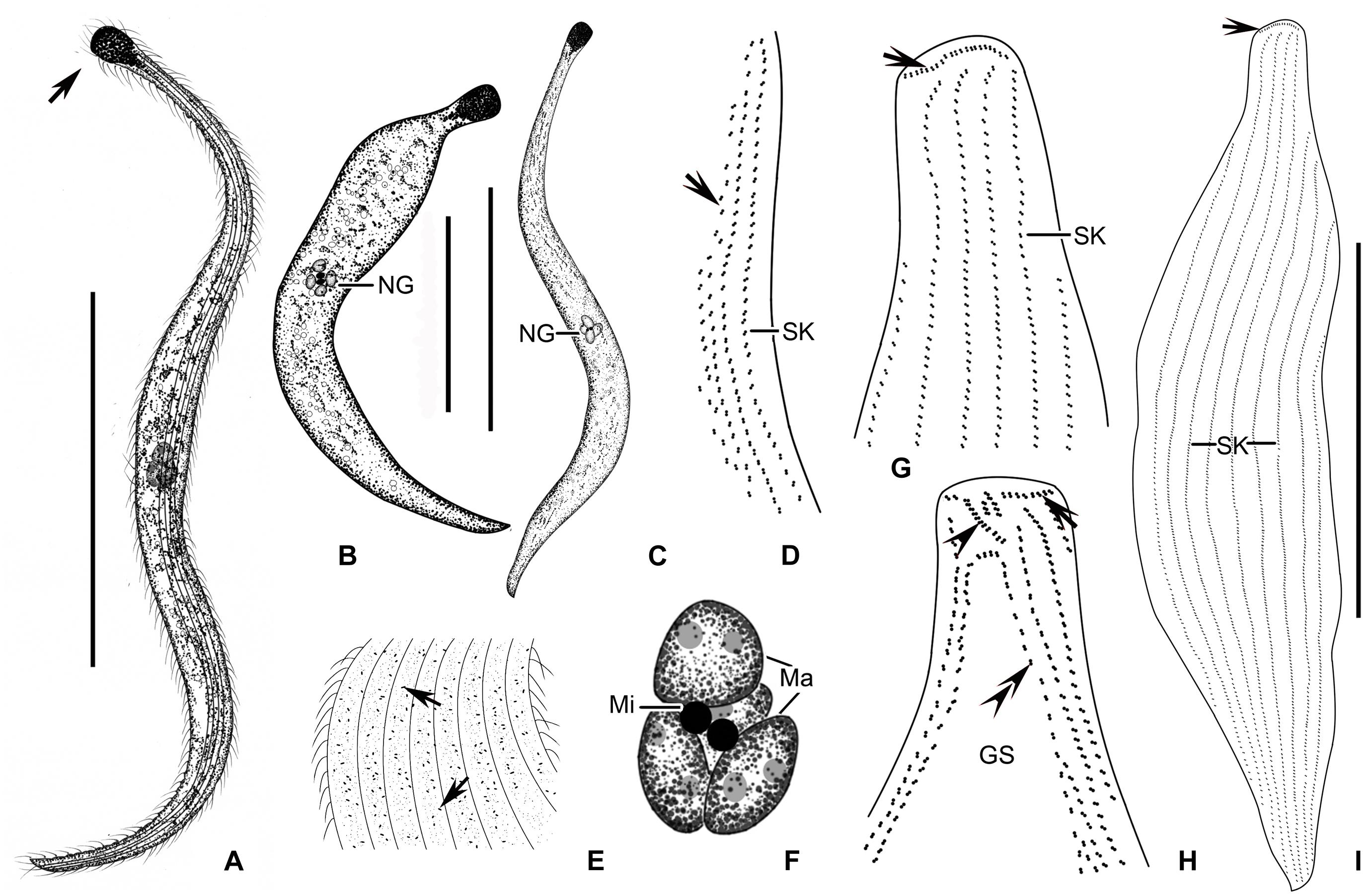
Figure 6. (A–I) Tracheloraphis katzae spec. nov. in vivo (A–C,E) and after protargol staining (D,F–I). (A–C) Typical individual. Arrow showing the head of the cell. (D) Ciliary pattern of bristle kinety (arrow) and somatic kinety. (E) Distribution of cortical granules (arrows) between somatic kineties. (F) Nuclear group. (G,H) Detailed ciliature of left (H) and right (G) side of anterior body region. Arrowhead in (H) showing brosse, arrow showing circumoral kinety, double arrowheads showing bristle kinety. Arrowhead in (G) showing circumoral kinety. (I) Right view of the holotype specimen showing the ciliary pattern, arrow depicts circumoral kinety. GS, glabrous stripe; SK, somatic kineties. Scale bars = 200 μm (A–C); 70 μm (I).
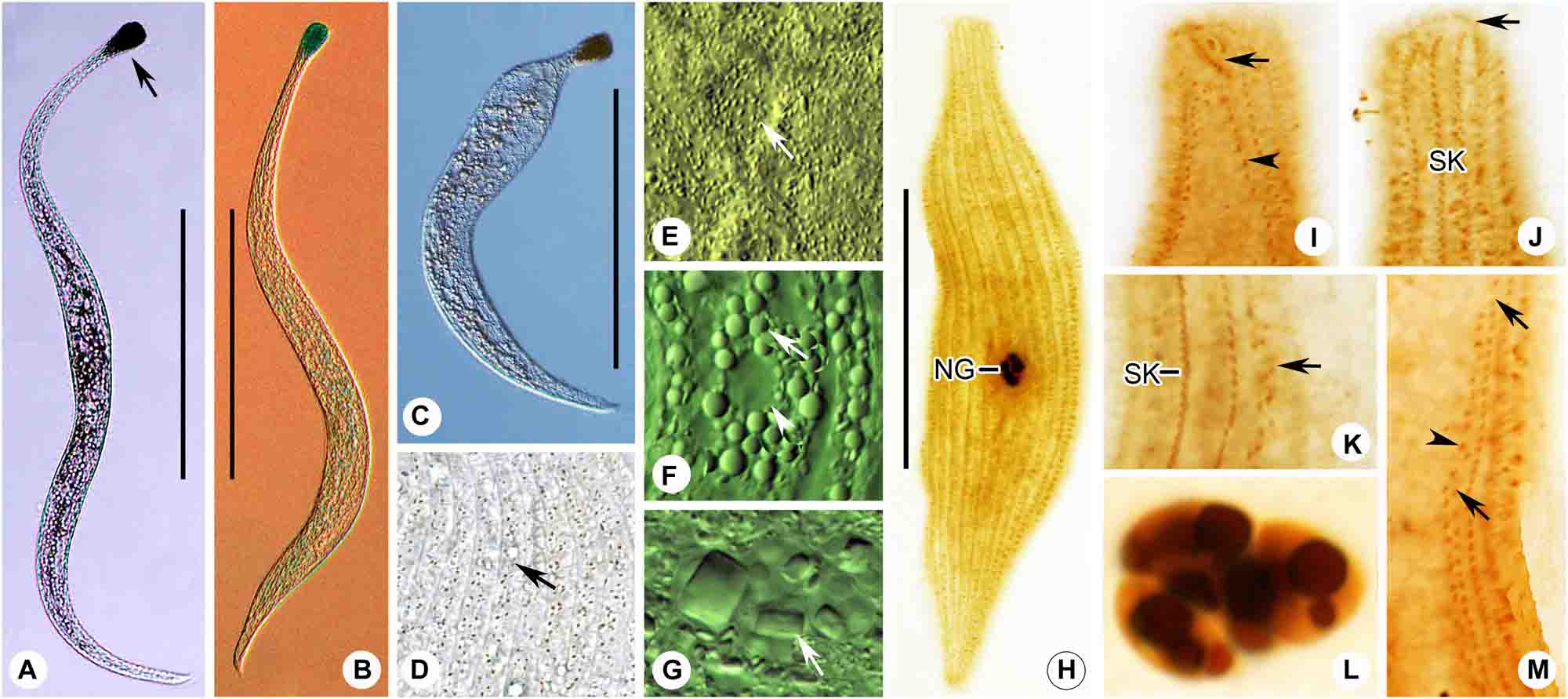
Figure 7. (A–M) Tracheloraphis katzae spec. nov. in vivo (A–G) and after protargol staining (H–M). (A–C) Typical individual. Arrow showing the head of the cell. (D) Distribution of cortical granules (arrow) between somatic kineties. (E) Distribution of cortical granules in glabrous stripe (arrow). (F) Arrow showing globular granules in cell. Arrowhead showing a vacuole. (G) Nuclear group. Arrow showing crystals in the macronuclei. (H) Right view of the holotype specimen showing the ciliary pattern and multiple macronuclei. (I,J) Detailed ciliature of left (I) and right (J) side of anterior body region. Arrow in I shows brosse, arrowhead showing bristle kinety. Arrow in (J) showing circumoral kinety. (K) Arrow showing somatic kineties. (L) Nuclear group. (M) Arrows showing secant system on left side of glabrous stripe. Arrowhead showing bristle kinety. NG, nuclear group; SK, somatic kineties. Scale bars = 200 μm (A–C); 70 μm (H).
Diagnosis
Size in vivo 400–800 μm × 20–30 μm; 9–15 somatic kineties on trunk; head dark and conspicuous, trunk widened, tail narrowed; single nuclear group composed of four macronuclei and two micronuclei; glabrous stripe as wide as trunk; cortical granules brownish, ellipsoidal, about 0.5 μm × 0.2 μm, densely distributed.
Type Locality
The intertidal zone of the No. 1 Bathing beach, Qingdao (36°03′24″N, 120°20′32″E), China, where the water temperature was 26°C and the salinity was about 30‰ (Figure 1).
Type Specimens
A protargol slide containing the holotype specimen marked with an ink circle is deposited in the Laboratory of Protozoology, Ocean University of China, Qingdao, China (No. MMZ2019062407).
Etymology
We dedicate this new species to our eminent colleague, Prof. Laura Katz, Smith College, United States, in recognition of her great contributions to ciliate research.
Description
Fully extended cells about 600 μm × 25 μm in vivo; body flexible and flattened, ribbon-like, with claviform head and pointed tail, trunk region conspicuously widened (Figures 6A–C, 7A–C). Trunk dark at low magnification, neck and tail portions transparent due to lack of inclusions (Figures 6A–C, 7A). Globular granules clustering in mid-body region (Figure 7F). Single nuclear group located in center of trunk, containing four macronuclei, 5–7 μm in diameter, in which there are some crystals (Figure 7G), and two micronuclei, 2–3 μm in diameter (Figures 6F, 7G,L). Brownish cortical granules, ellipsoidal, ca. 0.2 μm × 0.5 μm, densely distributed between ciliary rows and in glabrous stripe (Figures 6E, 7D,E).
Locomotion by gliding between sand grains and organic debris. Cells surface densely ciliated (Figures 6I, 7H). Glabrous stripe about as wide as trunk (Figures 6H, 7I). Entire infraciliature consisting of dikinetids with cilia ca. 10 μm long (Figures 6A,E,I). Five to ten somatic kinetics on head, 9–15 on trunk. Anterior and posterior secant systems on left side of glabrous stripe, some kineties abut to bristle kinety (Figures 6D,H, 7I,K,M). Oral ciliature composed of single-rowed circumoral kinety, interrupted by two inserted brosses (Figures 6G,H, 7I,J).
Discussion
As shown in Figure 8 and Table 4, the current new species should be compared with its most similar congeners (Figure 8 and Table 4). Tracheloraphis prenanti Dragesco, 1960 and T. phoenicopterus (Cohn, 1866) Dragesco, 1960 resemble T. katzae spec. nov. in body shape and the width of the glabrous stripe. They can be distinguished from the latter, however, by their greater number of somatic kineties (14–26, 19–27, vs. 9–15) and macronuclei in the nuclear group (6–12, 4–10, vs. 4). Features of cortical granules can further separate three species. Cortical granules of T. prenanti are colorless, globular and about 0.5 μm in diameter, and in T. phoenicopterus they are ellipsoidal, yellowish, and 0.6 μm × 1.2 μm, whereas, in T. katzae spec. nov. they are ellipsoidal, brownish, and 0.2 μm × 0.5 μm (Figures 8A,G; Foissner and Dragesco, 1996b; Ma et al., 2020).
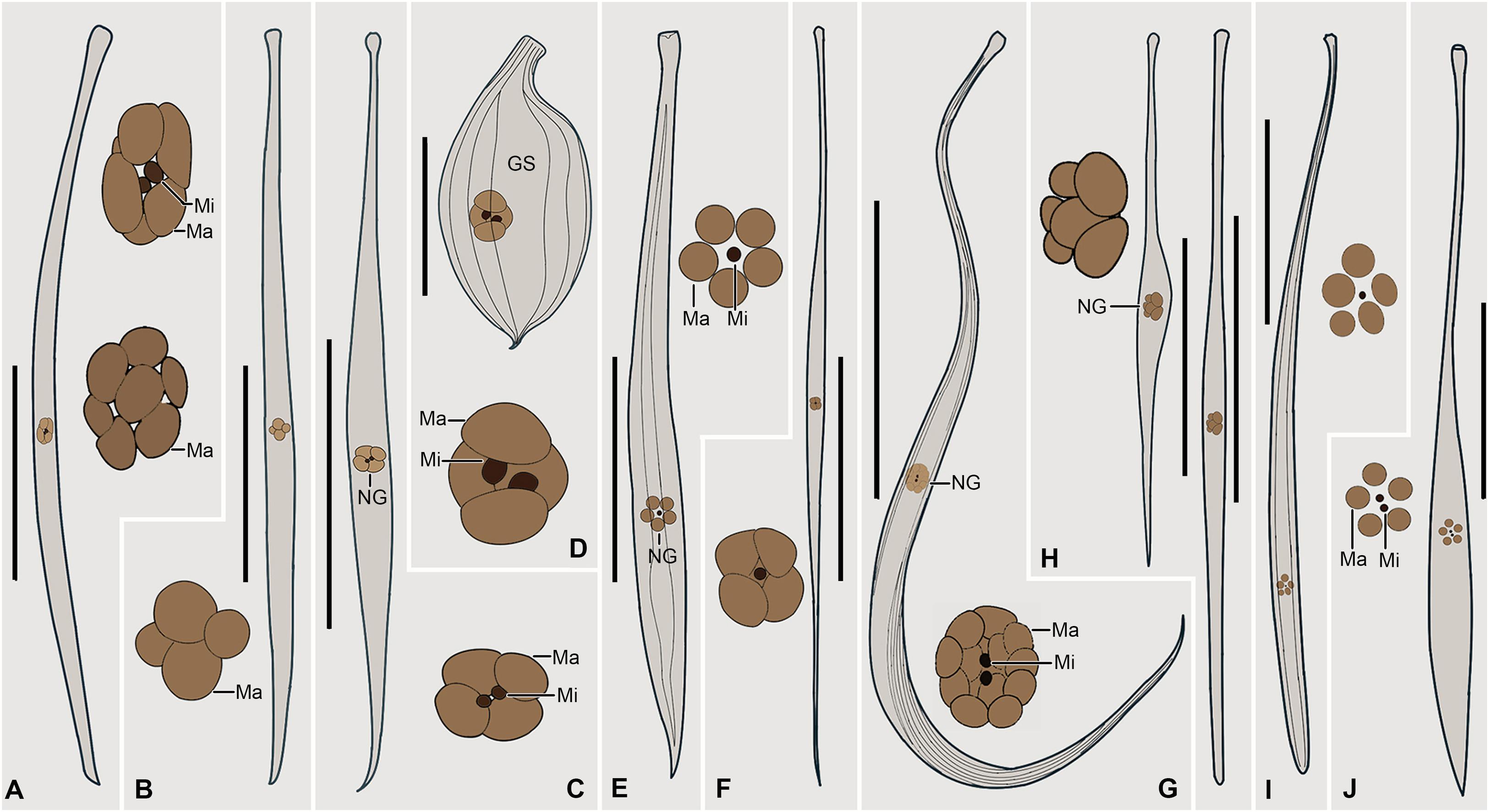
Figure 8. (A–J) Selected Traceloraphis species that are morphologically similar to T. katzae spec. nov. A. Tracheloraphis prenanti (after Ma et al., 2020). B. T. dragescoi (after Xu et al., 2014). C. T. africanus (after Dragesco and Dragesco-Kernéis, 1986). D. T. striatus (after Raikov, 1962). E. T. remanei (after Dragesco, 1960). F. Trachelocerca schulzei (after Dragesco, 1960). G. T. phoenicopterus (after Foissner and Dragesco, 1996b). H. T. hamatus (after Dragesco, 2002). I. T. gracilis (after Dragesco, 1960). J. T. enigmaticus (after Dragesco, 1960). GS, glabrous stripe; Ma, macronuclei; Mi, micronuclei; NG, nuclear group. Scale bars = 200 μm (A–C,E–J); 60 μm (D).

Table 4. Comparison of Tracheloraphis katzae spec. nov. (in bold) with morphologically similar congeners.
Tracheloraphis dragescoi Xu et al., 2014 has a similar body size and number of macronuclei as T. katzae spec. nov., but it can be separated from the latter by its oval cortical granules (colorless, about 0.2 μm × 1 μm vs. brownish, about 0.2 μm × 0.5 μm) and in having 14–22 (vs. 9–15) somatic kineties (Figure 8B; Xu et al., 2014).
Tracheloraphis hamatus Wright, 1982 resembles the novel form in body size, width of the glabrous stripe and the number of macronuclei. It can be distinguished from the latter, however, by having a different type of cortical granules (globular, less than 0.5 μm in diameter vs. ellipsoidal, about 0.2 μm × 0.5 μm) (Figure 8H).
Tracheloraphis africanus Dragesco, 1965 resembles T. katzae spec. nov. in body size and the number of macronuclei. However, it differs from the latter by the absence (vs. presence) of cortical granules although according to Dragesco (1965) the 3 μm long rod-shaped “granules” scattered in the superficial cytoplasm may be bacteria (Figure 8C).
Tracheloraphis gracilis Dragesco, 1960 has a similar number of macronuclei compared to Tracheloraphis katzae spec. nov. and both species have oval cortical granules, but it can be separated from the latter by its tail which has a rounded (vs. wedge-shaped) end (Figure 8I; Dragesco, 1960).
Based on the original illustration, the nuclear apparatus of Tracheloraphis enigmaticus Dragesco, 1960 consists of five macronuclei and two micronuclei, but these are not clustered in a nuclear group as they are in T. katzae spec. nov. Furthermore, the figure of T. enigmaticus shows that it has short subuliform tail, whereas the new species has a narrow tail with a wedge-shaped end (Figure 8J; Dragesco, 1965).
The original descriptions of Tracheloraphis striatus Raikov, 1962 and T. remanei Dragesco, 1960 are based solely on stained specimens, and no information on their live morphology is available. Nevertheless, both can be separated from T. katzae spec. nov. by having a narrower glabrous stripe (about double distance between two adjacent somatic kineties, and one-third of the body width, respectively vs. about as wide as the body) (Figures 8D,E; Dragesco, 1960; Raikov, 1962).
Although no information on the infraciliature of Trachelocerca schulzei Dragesco, 1960 is available, based on the original illustration, it can be separated from T. katzae spec. nov. by its larger ratio of body length to body width (about 35:1 vs. about 20:1) in fully extended cells (Figure 8F; Dragesco, 1960).
Tracheloraphis colubis (Kahl, 1933) Xu et al., 2011; Figures 9, 10 and Table 3)
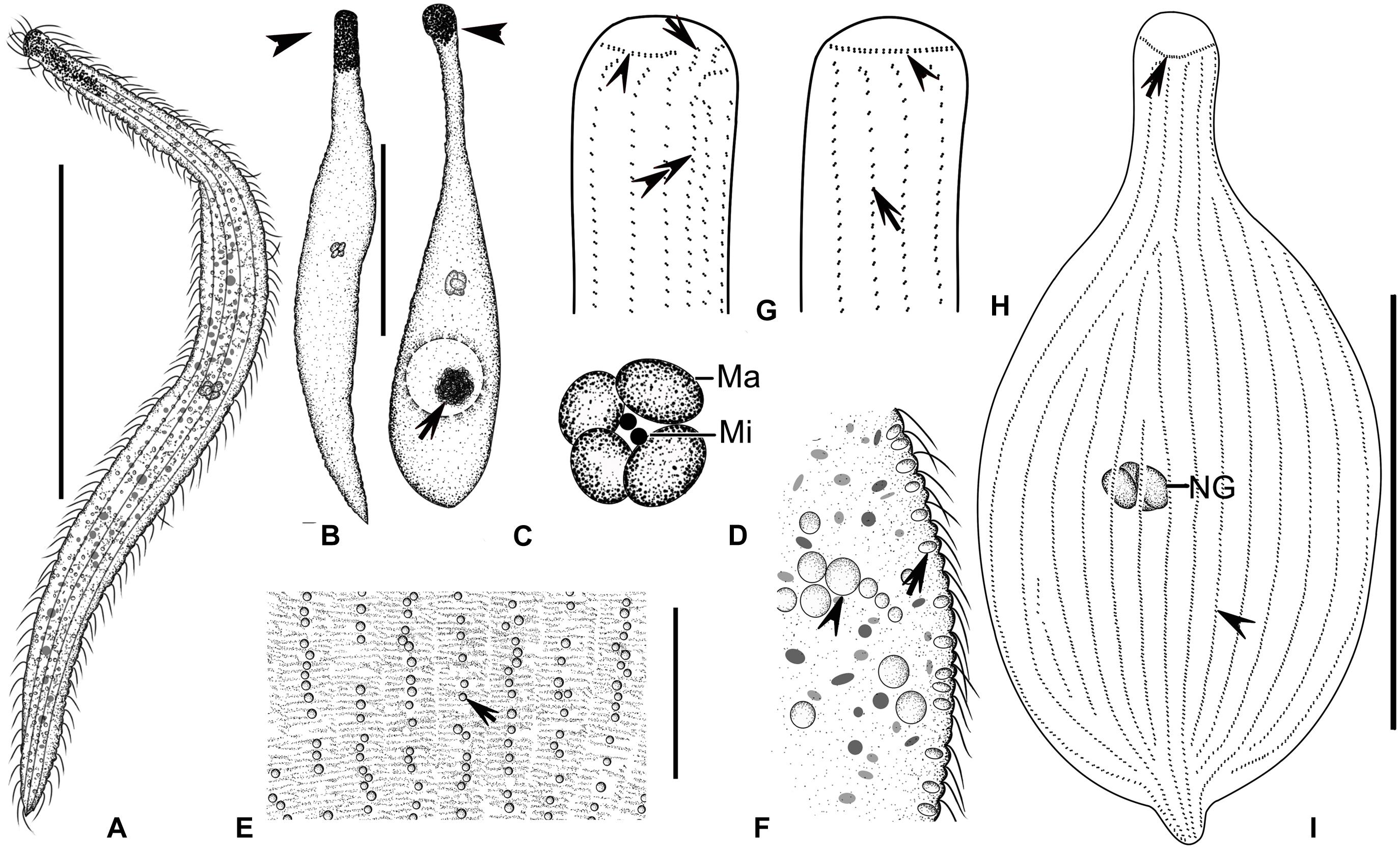
Figure 9. (A–I) Tracheloraphis colubis in vivo (A–F) and after protargol staining (G–I). (A,B) Typical individuals. Arrowhead showing the head of cell. (C) An extremely contracted cell, arrow showing algae in the food vacuole, arrowhead showing the head of cell. (D) Nuclear group. (E) Distribution of cortical granules between somatic kineties (arrow). (F) Lateral view, showing the elliptical cortical granules at the cell margin (arrow). Arrowhead showing the spherical inclusions. (G,H). Detailed ciliature of left (G) and right (H) side of anterior body region. Arrowhead in (G) showing circumoral kinety, arrow showing brosse, double arrowheads showing bristle kinety. Arrowhead in (H) showing circumoral kinety, arrow showing somatic kinety. (I) Overview showing ciliary pattern. Arrow showing circumoral kinety, arrowhead showing somatic kinety. Ma, macronuclei; Mi, micronuclei; NG, nuclear group. Scale bars = 300 μm (A); 200 μm (B,C); 60 μm (E); 100 μm (I).
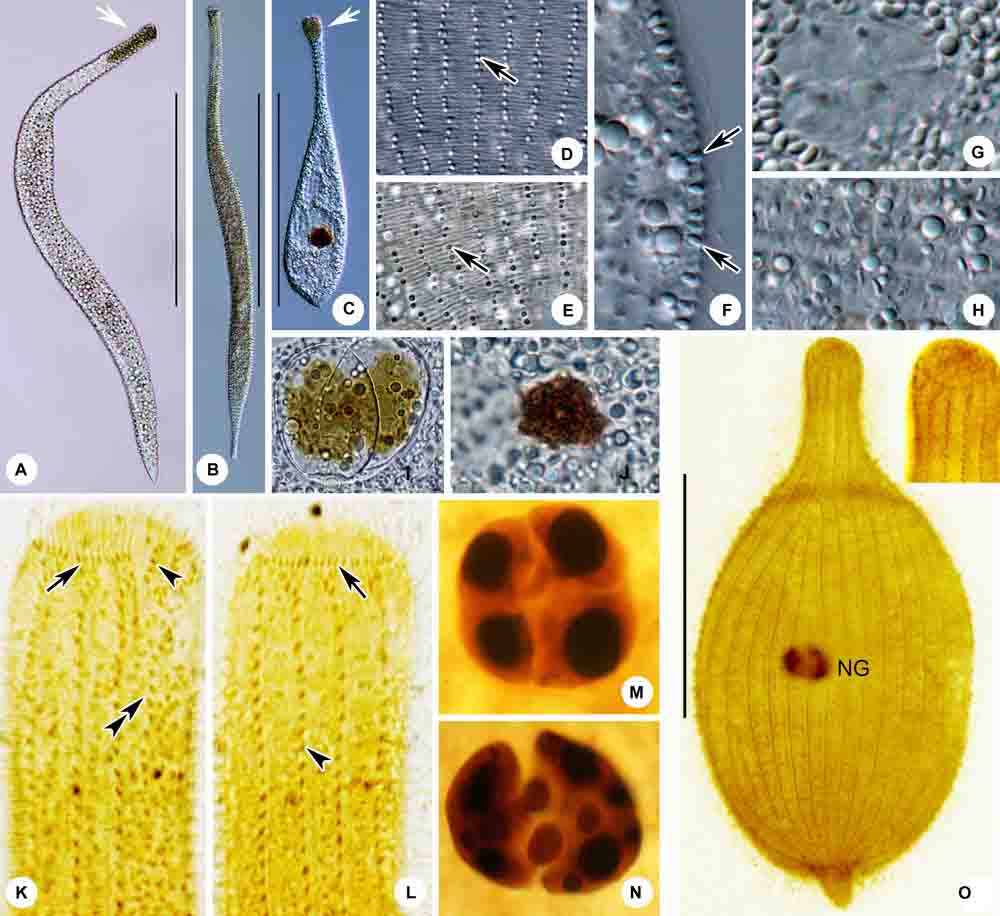
Figure 10. (A–O) Tracheloraphis colubis in vivo (A–J) and after protargol staining (K–O). (A,B) Typical individuals. Arrowhead showing the head of cell. (C) An extremely contracted cell. Arrowhead showing the head of cell. (D,E) Distribution of cortical granules between somatic kineties (arrow). (F) Lateral view, showing the elliptical cortical granules at the cell margin (arrows). (G) Nuclear group. (H) Showing granules in cell. (I,J) Showing algae in the food vacuole. (K,L) Detailed ciliature of left (K) and right (L) side of anterior body region. Arrow in (K) showing circumoral kinety, arrowhead showing brosse, double arrowheads showing bristle kinety. Arrow in (L) showing circumoral kinety, arrowhead showing somatic kinety. (M,N) Nuclear group. (O) Overview showing ciliary pattern. NG, nuclear group. Scale bars = 300 μm (A–C); 100 μm (O).
This species was originally reported by Kahl (1933) and redescribed in detail by Xu et al. (2011a). Our population matches both descriptions very well, therefore redescription and an improved diagnosis based on the present and previous populations are provided here.
Improved Diagnosis
Extended cells 250–1,000 μm × 20–50 μm in vivo; claviform tail. Seventeen to thirty-one somatic kineties. Glabrous stripe narrow, width about equal to gap between two adjacent somatic kineties. Four macronuclei in a single group. Cortical granules circular in outline when viewed from above, elliptical in lateral view 1.5–2 μm × 2.5 μm, colorless.
Redescription Based on Qingdao Population
Extended cells 250–600 μm × 30–50 μm in vivo; body flattened about 3:1, flexible and contractile (Figures 9A–C, 10A–C); neck and tail indistinctly separated from trunk, head triangular and conspicuous, tail claviform (Figures 9A–C, 10A–C). Cortical granules ellipsoidal, 1.5–2 μm × 2.5 μm, colorless in bright field at high magnification; round when viewed from above, elliptical in lateral view; arranged in rows between somatic kineties and sparsely distributed in glabrous stripe (Figures 9E,F, 10D–F). Cytoplasm colorless and transparent, packed with cytoplasmic granules, ellipsoidal, 1–3 μm long and colorless (Figure 10H). Some food vacuoles containing algae (Figures 9C, 10C,I,J).
Locomotion by gliding, winding between sand grains and organic debris.
Entire infraciliature consisting of dikinetids (Figures 9I, 10O). Somatic cilia about 10 μm long in vivo and arranged in longitudinal rows. Usually one brosse kinety (Figures 9G,H, 10K,L). Glabrous stripe very narrow, width about equal to gap between two adjacent somatic kineties, and bordered by irregularly spaced bristle kinety (Figures 9G, 10K). Seventeen to 27 somatic kineties. Anterior and posterior of secant system formed on left side of glabrous stripe, some kineties abut to bristle kinety (Figures 9G, 10K). Four macronuclei and two micronuclei in a single group (Figures 9D, 10G,M,N).
Discussion
There have been several redescriptions of Tracheloraphis colubis since it was first reported (Kahl, 1933; Raikov, 1963; Xu et al., 2011a). Kahl (1933) and Raikov (1963) both assigned this species to the genus Trachelocerca due to its curved posterior end and narrow glabrous stripe. However, the shape and structure of the oral ciliature are the most important characters for generic classification (Foissner and Dragesco, 1996a,b; Foissner and Al-Rasheid, 1999). Therefore, Xu et al. (2011a) assigned this species to the genus Tracheloraphis. The present population matches the population described by Xu et al. (2011a), so we have no doubt that these two populations are conspecific.
Molecular Phylogeny Based on SSU rDNA Sequence Data (Figure 11)
The length (bp), GC content and Genbank accession numbers of the new SSU rDNA sequences of the four isolates are as follows: Paratrachelocerca typica gen. nov., spec. nov.—1,557, 48.62%, MT777541; Trachelolophos monocaryon (Dragesco, 1965) comb. nov.—1,473, 48.47%, MT795961; Tracheloraphis katzae spec. nov.—1,520, 47.89%, MT795962; Tracheloraphis colubis—1,597, 47.65%, MT777539.
The ML and BI trees have similar topologies, therefore only the ML tree is presented (Figure 11). The family Trachelocercidae is a well-supported monophyletic group (89% ML, 1.00 BI) that is sister to the family Loxodidae (65% ML, 1.00 BI). Within Trachelocercidae there are four main clades (Clades I, II, III, and IV). Tracheloraphis colubis is sister to several Tracheloraphis and Trachelocerca species. Tracheloraphis katzae spec. nov. groups with T. dragescoi within Clade I. Prototrachelocerca fasciolata shows a close relationship with Tracheloraphis similis which together occupy the basal position within Clade I. Tracheloraphis species interdigitate with those of Trachelocerca resulting in the non-monophyly of both genera, which is consistent with previous studies (Xu et al., 2014; Yan et al., 2016). Clade II consists of three Tracheloraphis species. Two Trachelolophos species group together forming a sister branch to Clades I and II. Clade III comprises Trachelolophos monokaryon and T. quadrinucleatus. Kovalevaia sulcata is sister group to the assemblage of Clades I–III. Clade IV comprises Paratrachelocerca typica and two species of Apotrachelocerca.
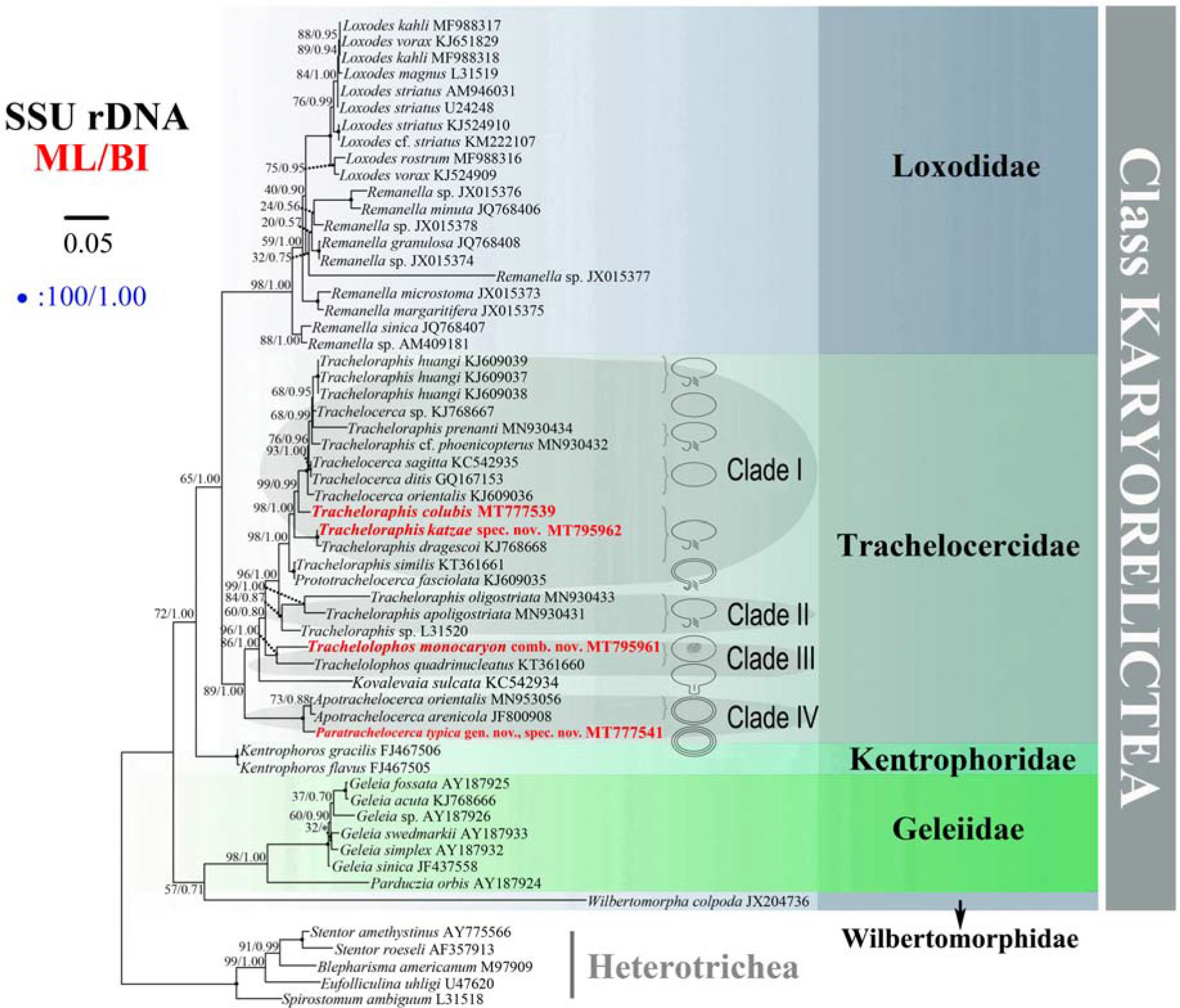
Figure 11. Maximum likelihood (ML) tree inferred from the small subunit rDNA sequences showing the positions of the four newly sequenced Trachelocercidae isolates (in red bold). Nodal support for branches in the ML and BI trees marked in order. Black circles indicate full support in both analyses. Clades with a different topology between the two analyses are shown by an asterisk. Heterotrichous species are the outgroup. The line diagrams show the oral structure of different genera in Trachelocercidae. All branches are drawn to scale. The scale bar corresponds to five substitutions per 100 nucleotides positions.
Based on the combination of morphological features and molecular evidence, Yan et al. (2016) suggested that Apotrachelocerca, which possesses two uninterrupted rows of circumoral kineties, is the closest relative to the common ancestor of trachelocercids, followed by Kovalevaia, Trachelolophos and Prototrachelocerca and that Trachelocerca, Tracheloraphis and possibly Sultanophrys derived from Prototrachelocerca. Our results mainly support this hypothesis except that Kovalevaia forms a sister branch with Clades I–III (of which Clade III comprises two Trachelolophos species) rather than grouping directly with Trachelolophos (Figure 11). In addition, the early branching of Paratrachelocerca gen. nov. within Clade IV provides further evidence that multiple rows of uninterrupted circumoral kineties is probably an ancestral feature.
Data Availability Statement
The datasets generated for this study can be found in the online repositories. The names of the repository/repositories and accession number(s) are as follows: https://www.ncbi.nlm.nih.gov/genbank/, MT777541; http://zoobank.org/, urn:lsid:zoobank.org:pub:913C263C-56A7-40AC-9885-E06DC530A6D8; https://www.ncbi.nlm.nih.gov/genbank/, MT7 95961; https://www.ncbi.nlm.nih.gov/genbank/, MT795962; https://www.ncbi.nlm.nih.gov/genbank/, MT777539.
Author Contributions
YY and YW conceived to the study. MM, YL, and YY carried out the live observation, protargol staining, DNA extraction, and data analyses. All authors contributed to the writing of the manuscript. YY, MM, and AW contributed to the revision and all authors approved the final version.
Funding
The work was financially supported by the National Natural Science Foundation of China (Project Nos. 32030015 and 31801984) and the Researchers Supporting Project (RSP-2020/10) of the King Saud University, Saudi Arabia.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Many thanks are given to Prof. Weibo Song (OUC) for kind suggestions during drafting the manuscript. We thank Dr. Helmut Berger (Consulting Engineering Office for Ecology, Salzburg, Austria) for alerting us to the fact that Foissnerella Ma et al., 2021 is a junior primary homonym and for his helpful suggestions.
Footnotes
References
Al-Rasheid, K. A. S. (1996). Records of free-living ciliates in Saudi Arabia. I. Marine interstitial ciliates of the Arabian Gulf islands of Al-Bātinah and Abū Ali. Arab Gulf J. Sci. Res. 14, 747–765.
Al-Rasheid, K. A. S. (1997). Records of free-living ciliates in Saudi Arabia. III. Marine interstitial ciliates of the Arabian Gulf island of Tarut. Arab Gulf J. Sci. Res. 15, 733–766.
Al-Rasheid, K. A. S. (1998). Records of marine interstitial karyorelictid ciliates from Jubail Marine Wildlife Sanctuary in the Gulf-shore of Saudi Arabia. Arab Gulf J. Sci. Res. 16, 595–610.
Al-Rasheid, K. A. S. (2001). New records of interstitial ciliates (Protozoa Ciliophora) from the Saudi coasts of the Red Sea. Trop. Zool. 14, 133–156. doi: 10.1080/03946975.2001.10531148
Al-Rasheid, K. A. S., and Foissner, W. (1999). Apical feeding in the karyorelictids (Protozoa, Ciliophora) Sultanophrys arabica and Tracheloraphis sp. J. Eukaryot. Microbiol. 46, 458–463. doi: 10.1111/j.1550-7408.1999.tb06061.x
Dragesco, J. (1960). Ciliés mésopsammiques littoraux. Systématique, morphologie, écologie. Trav. Stn. Biol. Roscoff. 12, 1–356.
Dragesco, J. (1997). Infraciliature et morphométrie des cinq espèces deciliés mésopsammiques méditerranéens. Cah. Biol. Mar. 37, 261–293.
Dragesco, J. (1999). Revision des Geléiides (Ciliophora, Karyorelictea). Stapfia 66, 1–91. doi: 10.1016/s0003-4339(99)80006-9
Dragesco, J. (2002). Infraciliature de quinze especes de cilies mesopsammiques marins comprenant Trachelocerca stephani comb. nova, T. bodiani comb. nova, Tracheloraphis filiformis spec. nova, T. exilis spec. nova, et Sathrophilus arenicolus spec. nova. Linz. Biol. Beitr. 34, 1545–1626.
Dragesco, J., and Dragesco-Kernéis, A. (1986). Ciliés Libres de l’ Afrique Intertropicale Faune Tropicale, Vol. 26. Paris: Édition de l’ ORSTOM. doi: 10.1016/S0932-4739(88)80062-2
Ehrenberg, C. G. (1840). Das grössere Infusorienwerk (Diagnosen von 274 neuen Infusorien). Ber. Verh. K. Preuss. Akad. Wiss. Berl. 1840, 197–219.
Foissner, W. (1996). Updating the trachelocercids (Ciliophora, Karyorelictea). II. Prototrachelocerca nov. gen. (Prototrachelocercidae nov. fam.), with a redescription of P. fasciolata (Sauerbrey, 1928) nov. comb. and P. caudata (Dragesco & Raikov, 1966) nov. comb. Eur. J. Protistol. 32, 336–355. doi: 10.1016/s0932-4739(96)80058-7
Foissner, W. (1997). Updating the trachelocercids (Ciliophora, Karyorelictea). V. Redescription of Kovalevaia sulcata (Kovaleva, 1966) gen. n., comb. n. and Trachelocerca incaudata Kahl, 1933. Acta Protozool. 36, 197–219.
Foissner, W., and Al-Rasheid, K. A. S. (1999). Updating the trachelocercids (Ciliophora, Karyorelictea). VI. A detailed description of Sultanophrys arabica nov. gen., nov. spec. (Sultanophryideae nov. fam.). Eur. J. Protistol. 35, 146–160. doi: 10.1016/s0932-4739(99)80032-7
Foissner, W., and Dragesco, J. (1996a). Updating the trachelocercids (Ciliophora, Karyorelictea). I. A detailed description of the infraciliature of Trachelolophos gigas n. g., n. sp. and T. filum (Dragesco and Dragesco-Kernéis, 1986) n. comb. J. Eukaryot. Microbiol. 43, 12–25. doi: 10.1111/j.1550-7408.1996.tb02467.x
Foissner, W., and Dragesco, J. (1996b). Updating the trachelocercids (Ciliophora, Karyorelictea). III. Redefinition of the genera Trachelocerca Ehrenberg and Tracheloraphis Dragesco, and evolution in trachelocercid ciliates. Arch. Protistenkd. 147, 43–91. doi: 10.1016/s0003-9365(96)80007-8
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 41, 95–98. doi: 10.1021/bk-1999-0734.ch008
Hu, X. Z., Lin, X. F., and Song, W. (2019). Ciliate Atlas: Species Found in the South China Sea. Beijing: Science Press, doi: 10.1007/978-981-13-5901-9
Jerome, C. A., Lynn, D. H., and Simon, E. M. (1996). Description of Tetrahymena empidokyrea n. sp., a new species in the Tetrahymena pyriformis sibling species complex (Ciliophora, Oligohymenophorea), and an assessment of its phylogenetic position using small-subunit rRNA sequences. Can. J. Zool. 74, 1898–1906. doi: 10.1139/z96-214
Kahl, A. (1933). “Ciliata Libera et Ectocommensalia,” Die Tierwelt der Nord-und Ostsee, Leipzig, eds G. Grimpe and E. Wagler Lief. 23 (Teil II, c3), 29–146.
Lynn, D. H. (2008). The Ciliated Protozoa: Characterization, Classification, and Guide to the Literature. Berlin: Springer.
Ma, M. Z., Xu, Y., Yan, Y., Li, Y. Q., Warren, A., and Song, W. B. (2020). Taxonomy and molecular phylogeny of four karyorelictid species belonging to the genera Apotrachelocerca and Tracheloraphis (Protozoa: Ciliophora), with descriptions of two new species. Zool. J. Linn. Soc. 64, (Accepted).
Medlin, L., Elwood, H. J., Stickel, S., and Sogin, M. L. (1988). The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 71, 491–499. doi: 10.1016/0378-1119(88)90066-2
Penn, O., Privman, E., Ashkenazy, H., Landan, G., Graur, D., and Pupko, T. (2010). GUIDANCE: a web server for assessing alignment confidence scores. Nucl. Acids Res. 38(Suppl. 2), W23–W28. doi: 10.1093/nar/gkq443
Raikov, I. B. (1962). Les cilié mésopsammiques du littoral de la Mer Blanche (U.R.S.S.) avec une description de quelques espèces nouvellesou peu connues. Cah. Biol. Mar. 3, 325–361.
Raikov, I. B. (1963). Ciliates of the mesopsammon of the Ussuri Gulf (Japan Sea). Zool. Zh. 42, 1753–1767.
Raikov, I. B., Gerassimova-Matvejeva, Z. P., and de Puytorac, P. (1975). Cytoplasmic fine structure of the marine psammobiotic ciliate Tracheloraphis dogieli Raikov. I. Somatic infraciliature and cortical organelles. Acta Protozool. 14, 17–42.
Ronquist, F., Teslenko, M., Van Der Mark, P., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Shimodaira, H. (2002). An approximately unbiased test of phylogenetic tree selection. Syst. Biol. 51, 492–508. doi: 10.1080/10635150290069913
Shimodaira, H., and Hasegawa, M. (2001). Consel: for assessing the confidence of phylogenetic tree selection. Bioinformatics 17, 1246–1247. doi: 10.1093/bioinformatics/17.12.1246
Song, W. B., Warren, A., and Hu, X. Z. (2009). Free-living Ciliates in the Bohai and Yellow Seas. Beijing: Science Press.
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Stamatakis, A., Hoover, P., and Rougemont, J. (2008). A rapid bootstrap algorithm for the RAxML web-servers. Systematic Biol. 75, 758–771. doi: 10.1080/10635150802429642
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., and Kumar, S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739. doi: 10.1093/molbev/msr121
Uhlig, G. (1968). Quantitative methods in the study of interstitial fauna. Trans. Am. Microsc. Soc. 87, 226–232. doi: 10.2307/3224446
Wang, Y. R., Jiang, Y. H., Liu, Y. Q., Li, Y., Katz, L. A., Gao, F., et al. (2020). Comparative studies on the polymorphism and copy number variation of mtSSU rDNA in ciliates (Protista, Ciliophora): implications for phylogenetic, environmental, and ecological research. Microorganisms 8:316. doi: 10.3390/microorganisms8030316
Wang, Y. R., Wang, C. D., Jiang, Y. H., Katz, L. A., Gao, F., and Yan, Y. (2019). Further analyses of variation of ribosome DNA copy number and polymorphism in ciliates provide insights relevant to studies of both molecular ecology and phylogeny. Sci. China Life Sci. 62, 203–214. doi: 10.1007/s11427-018-9422-5
Wilbert, N. (1975). Eine verbesserte Technik der Protargolimprägnation für Ciliaten. Mikrokosmos 64, 171–179.
Wilbert, N. (1986). Die orale infraciliature von Tracheloraphis dogieli Raikov, 1957 (Ciliophora, Gymnostomata, Karyorelictida). Arch. Protistenkd. 132, 191–195. doi: 10.1016/S0003-9365(86)80020-3
Xu, Y., Esaulov, A., Lin, X., Mazei, Y., Hu, X., Al-Rasheid, K. A. S., et al. (2011a). Morphological studies on five trachelocercids from the Yellow Sea coast of China, with a description of Tracheloraphis huangi spec. nov. (Ciliophora, Karyorelictea). Acta Protozool 50, 205–218. doi: 10.1016/j.proenv.2011.10.118
Xu, Y., Li, J., Gao, F., Hu, X., and Al-Rasheid, K. A. S. (2011b). Apotrachelocerca arenicola (Kahl, 1933) n. g., comb. n. (Protozoa, Ciliophora, Trachelocercidae): morphology and phylogeny. J. Eukaryot. Microbiol. 58, 504–510. doi: 10.1111/j.1550-7408.2011.00578.x
Xu, Y., Miao, M., Warren, A., and Song, W. (2012). Diversity of the karyorelictid ciliates: Remanella (Protozoa, Ciliophora, Karyorelictida) inhabiting intertidal areas of Qingdao, China, with descriptions of three species. Syst. Biodivers. 10, 207–219. doi: 10.1080/14772000.2012.681713
Xu, Y., Yan, Y., Li, L., Al-Rasheid, K. A. S., Al-Farraj, S. A., and Song, W. (2014). Morphology and phylogeny of three karyorelictean ciliates (Protista, Ciliophora), including two novel species, Trachelocerca chinensis sp. nov. and Tracheloraphis dragescoi sp. nov. Int. J. Syst. Evol. Microbiol. 64, 4084–4097. doi: 10.1099/ijs.0.068783-0
Yan, Y., Gao, F., Xu, Y., Al-Rasheid, K. A. S., and Song, W. (2015). Morphology and phylogeny of three trachelocercid ciliates, with description of a new species, Trachelocerca orientalis spec. nov. (Ciliophora, Karyorelictea). J. Eukaryot. Microbiol. 62, 157–166. doi: 10.1111/jeu.12154
Yan, Y., Maurer-Alcalá, X. X., Knight, R., Pond, S. L. K., and Katz, L. A. (2019). Single cell transcriptomics reveal a correlation between genome architecture and gene family evolution in ciliates. mBio 10:e2524-19. doi: 10.1128/mBio.02524-19
Yan, Y., Xu, Y., Al-Farraj, S. A., Al-Rasheid, K. A. S., and Song, W. (2016). Morphology and phylogeny of three trachelocercids (Protozoa, Ciliophora, Karyorelictea), with description of two new species and insight into the evolution of the family Trachelocercidae. Zool. J. Linn. Soc. 177, 306–319. doi: 10.1111/zoj.12364
Keywords: infraciliature, SSU rDNA, ciliates, Karyorelictea, Trachelocercidae
Citation: Ma M, Li Y, Ma H, Al-Rasheid KAS, Warren A, Wang Y and Yan Y (2021) Morphology and Molecular Phylogeny of Four Trachelocercid Ciliates (Protozoa, Ciliophora, Karyorelictea) Found in Marine Coastal Habitats of Northern China, With Description of a New Genus, Two New Species and a New Combination. Front. Mar. Sci. 7:615903. doi: 10.3389/fmars.2020.615903
Received: 10 October 2020; Accepted: 03 December 2020;
Published: 13 January 2021.
Edited by:
Hongbo Pan, Shanghai Ocean University, ChinaReviewed by:
Xumiao Chen, Institute of Oceanology (CAS), ChinaJun Gong, Sun Yat-sen University, China
Copyright © 2021 Ma, Li, Ma, Al-Rasheid, Warren, Wang and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yurui Wang, d2FuZ3l1cnVpMjAxMUAxNjMuY29t; Ying Yan, eWFueWluZ0BvdWMuZWR1LmNu
†These authors have contributed equally to this work
 Mingzhen Ma
Mingzhen Ma Yuqing Li1†
Yuqing Li1† Khaled A. S. Al-Rasheid
Khaled A. S. Al-Rasheid Yurui Wang
Yurui Wang Ying Yan
Ying Yan